SPECIAL ANNIVERSARY EDITION II
Control & Therapy Series, June 2025 | Issue 319

SPECIAL ANNIVERSARY EDITION II
Control & Therapy Series, June 2025 | Issue 319

CELEBRATING 60 YEARS OF SERVICE TO THE VETERINARY PROFESSION IN 2025
Follow Tom Hungerford’s ‘goanna track to success’…

Guide Dogs use and trust Australia’s No.1 parasite protection and vaccine range for dogs*
Issue 319 | June 2025
Control & Therapy Series
PUBLISHER
Centre for Veterinary Education
Veterinary Science Conference Centre
Regimental Drive
The University of Sydney NSW 2006 + 61 2 9351 7979 cve.marketing@sydney.edu.au cve.edu.au
Print Post Approval No. 10005007
CVE Director
Associate Professor Kate Patterson kate.patterson@sydney.edu.au
EDITOR
Lis Churchward elisabeth.churchward@sydney.edu.au
EDITORIAL ASSISTANT
Dr Jo Krockenberger joanne.krockenberger@sydney.edu.au
VETERINARY EDITOR
Dr Richard Malik
DESIGNER
Samin Mirgheshmi
ADVERTISING
Lis Churchward elisabeth.churchward@sydney.edu.au
To integrate your brand with C&T in print and digital and to discuss new business opportunities, please contact: MARKETING & SALES MANAGER
Ines Borovic ines.borovic@sydney.edu.au
DISCLAIMER
All content made available in the Control & Therapy (including articles and videos) may be used by readers (You or Your) for educational purposes only.
Knowledge and best practice in this field are constantly changing. As new research and experience broadens our knowledge, changes in practice, treatment and drug therapy may become necessary or appropriate. You are advised to check the most current information provided (1) on procedures featured or (2) by the manufacturer of each product to be administered, to verify the recommended dose or formula, the method and duration of administration, and contraindications.
To the extent permitted by law You acknowledge and agree that:
I. Except for any non-excludable obligations, We give no warranty (express or implied) or guarantee that the content is current, or fit for any use whatsoever. All such information, services and materials are provided ‘as is’ and ‘as available’ without warranty of any kind.
II. All conditions, warranties, guarantees, rights, remedies, liabilities or other terms that may be implied or conferred by statute, custom or the general law that impose any liability or obligation on the University (We) in relation to the educational services We provide to You are expressly excluded; and
III. We have no liability to You or anyone else (including in negligence) for any type of loss, however incurred, in connection with Your use or reliance on the content, including (without limitation) loss of profits, loss of revenue, loss of goodwill, loss of customers, loss of or damage to reputation, loss of capital, downtime costs, loss under or in relation to any other contract, loss of data, loss of use of data or any direct, indirect, economic, special or consequential loss, harm, damage, cost or expense (including legal fees).
The Control & Therapy Series was established in 1969 by Director Dr Tom Hungerford. His aim was to publish uncensored and unedited material contributed by vets writing about:
...not what he/she should have done, BUT WHAT HE/SHE DID, right or wrong, the full details, revealing the actual “blood and dung and guts” of real practice as it happened, when tired, at night, in the rain in the paddock, poor lighting, no other vet to help.
The C&T forum gives a ‘voice’ to the profession and everyone interested in animal welfare. You don’t have to be a CVE Member to contribute an article or reply to a 'What's YOUR Diagnosis?'. We welcome contributions from Vets, Techs, Nurses, allied professionals and anyone interested in animal welfare—Non CVE Members included.
Submit your C&T article
A template and information about uploading high resolution images for print can be found here cve.edu.au/submit-article
Questions?
Please contact cve.marketing@sydney.edu.au
Join In!
The C&T is not a peer-reviewed journal. Rather, it is a unique forum allowing veterinary professionals to share their cases and experiences with their colleagues. We are keen on publishing short, pithy, practical articles (a simple paragraph is fine) that our members/readers can immediately relate to and utilise. Our editors will assist with English and grammar if required.
I enjoy reading the C&T more than any other veterinary publication.
-Terry King, Veterinary Specialist Services, QLD
Thank You to All Contributors & Advertisers
The C&T Series thrives due to your generosity.
Major Winner
Prize: A CVE$500 voucher
Winston Oakes page 3
Winners
Prize: A CVE$300 voucher
Samantha Coomes & Mikaela Green page 14
Lizzie Maher page 16
Bernie May page 33
Best Visuals
Prize: A CVE$300 voucher
Daniel Wilfred page 35
Best Answer C&T No 6060
Prize: A CVE$300 voucher
Jason Lenord page 9

Those of you in clinical practice will appreciate that the decision-making process when dealing with complicated cases is never straight forward. So many considerations feed into the matrix and include things like the clinical presentation, the owner’s wishes, and capacity to manage ongoing care, the welfare of the patient and the theoretical and tacit understanding of disease processes. In this issue, the excellent article written by Dr Winston Oakes highlights the nuance of decision making in practice, when the owner of the patient is also the veterinarian tasked with making the decisions! Reading this article then prompted a delightful side-path of discovery as I learned about American humourist Don Marquis and his satirical free-verse poems about Archy, a philosophical cockroach and Mehitabel, a cat in her ninth life.
Also in these pages, Dr Lizzie Maher describes an unexpected finding on abscess exploration in her (gorgeous) 8yo Cavoodle patient. It reminded me of an interesting and entertaining presentation by Dr Kate Worthing at the recent Australian Veterinary Association Conference Research Day where she posed the question “Who Ate All the Socks?”. It got me thinking – what has been the most unexpected foreign body finding you’re retrieved?! It would be interesting to hear your experiences and to showcase a collection of the most intriguing.
We continue to celebrate our 60th Anniversary and in this issue you’ll find a visual summary of the CVE history from 1965-2025. It was an enormous challenge to refine to just two A4 pages since there are so many stories and achievements to share. We are looking forward to highlighting more of these over the course of the year.
We hope you enjoy reading this 319th issue of C&T.

Associate Professor Kate Patterson Director
MAJOR Winner
The prize is a CVE$500 voucher
Made more difficult by a challenging owner, namely Me
Winston Oakes BVSc (Hons) BSc (Vet) MANCVS (Dentistry, Small Animal Internal Medicine)
Northside Veterinary Centre
2/51 Mort St
Braddon ACT 2612
t. (02) 6182 0111
e. winston.oakes@northsidevet.com.au
C&T No. 6068

Winston is a Canberra based small animal GP vet with Memberships of the ANZCVS in dentistry and medicine, who prefers a good cardiac case over a urinary tract infection any day.
Urinary tract infections are a common reason for dogs and cats to present to the vet. Unfortunately, antimicrobial resistance is also becoming increasingly common. Antimicrobial selection and dose should take in to account the likely causative organisms/s (ideally with culture and sensitivity information), drug delivery to the site of infection and, finally, best practice for antimicrobial stewardship (Australian Veterinary Prescribing Guidelines vetantibiotics.science.unimelb.edu.au).
Additionally, patient and owner compliance require consideration. What can initially seem to be a simple clinical problem can rapidly become a major intellectual challenge. The purpose of this case study is not to present the ideal management of a complex urinary tract infection, but rather to highlight the complexities of these type of cases that we sometimes consider as ‘simple’. To add to the complexity, the patient is owned by a vet, namely Me.

Mehitabel—18-year-old female desexed Siamese cross cat ( body weight 2.71 kg)
Pre-existing complaints include:
1. Chronic renal disease, IRIS stage 3
2. Osteoarthritis, managed with monthly frunevetmab (Solensia™)
3. Intermittent periuria, usually in times of stress or if litter hygiene not adequate
When she is well, Mehitabel can be very challenging to assess in the clinic and challenging to give oral medications to.
On the 21.6.24 she had an episode of periuria. At this time, she had a mild non-regenerative anaemia (HCT 30.0), isosthenuria (USG 1.018), azotaemia, haematuria (cystocentesis sample) and negative urine culture.
On the 29.8.24 she became quiet and inappetent. She had periuria, but also stranguria. Physical examination showed mild pyrexia and abdominal pain with weight loss.
Haematology (in-house IDEXX) showed worsening anaemia (HCT 23.9) with neutrophilia (with left shift), lymphocytosis, and monocytosis. Azotaemia was marginally increased (but difficult to directly compare due to samples being run on different laboratory equipment).
Due to stranguria, she had a very small bladder, so cystocentesis samples could not be collected. In-house urinalysis from free catch sample (squeezed from a towel) showed pyuria and bacteria.
A presumptive diagnosis of pyelonephritis was made and she was started on amoxycillin 50mg PO twice daily.

Culture from the free catch sample grew a heavy growth of E. coli and Enterococcus species both sensitive to amoxycillin.
She appeared to have a good clinical response to the amoxycillin. Her pyrexia and abdominal pain resolved, appetite improved, and she gained weight. The periuria improved, but did not resolve. During this time, she developed urinary incontinence (moderate volume of urine passed while sleeping). She was able to empty her bladder and her bladder was difficult to express. Amoxycillin was continued for 3 weeks.

Three days after the last dose of amoxycillin, urinalysis showed that the pyuria had resolved and urine culture was negative. Incontinence was still present but periuria had improved. Eight days after the last dose of amoxycillin, the incontinence and periuria worsened and the stranguria returned. Pyuria and bacteria were present. Ultrasound of her urinary tract showed no uroliths or bladder masses. Urine culture showed heavy growth of E. coli again, but no Enterococcus.
While waiting on this culture, she was started on 12.5mg marbofloxacin once daily with the thought that an antibiotic with better lipid solubility might have better penetration into bladder wall tissue and biofilms. Her clinical signs have improved significantly, including the incontinence signs although these have not completely resolved.

This case demonstrates many of the factors that make decision making for urinary tract infections in dogs and cats challenging.
Mehitabel had preexisting conditions that make recurrent UTIs likely (isosthenuria combined with short urethra) and she demonstrated that the infection was affecting her systemically (pyrexia and neutrophilia). Additional challenges included not being able to collect a sterile sample for culture on initial presentation, challenges around giving oral medications (for example, I considered oral Trimethoprim/sulfamethoxazole (TMS) likely to be impossible to give) and finally a demanding and highly emotional owner.
It also demonstrates the challenges of interpreting clinical signs in relation to UTIs in small animals. I am still unsure if her incontinence is a risk factor for infection, a clinical sign of her infection, or a consequence of the infection that will now not resolve.
Her periuria is also difficult to assess as she has demonstrated this sign without infection (based on urinalysis and negative culture). However, her periuria definitely worsened with infection. Given that antimicrobial stewardship dictates that we should use antibiotics only in the face of clinical signs (rather than bacteria only), being able to correlate clinical signs with infection is incredibly important.
It is unclear if the negative culture after the initial course of amoxicillin was due to a reinfection by E. coli, or failure to clear the original infection.
Possible causes of failure to clear the infection include
Susceptibility spectrum of the organism (although the original isolate was sensitive to amoxicillin)
The dose of amoxycillin (which was 18.5mg/kg in this case, and many authors suggest doses greater than 20mg/kg)
Host factors such as uroliths, or anatomical problems (none noted on ultrasound)
Poor tissue or biofilm penetration of amoxycillin compared to some other antibiotics
Virulence factors of the bacteria themselves. Studies on human E. coli UTIs have demonstrated highly virulent E. coli strains arising from meat (cidrap.umn.edu/antimicrobial-stewardship/studysuggests-e-coli-meat-could-be-causing-urinarytract-infections). This same process could lead to increasing numbers of dog and cat UTIs caused by E. coli with uropathological virulence factors.


I hope this case study helps to stimulate thought and discussion to help vets better manage such cases in clinical practice. With the benefit of hindsight, there are some changes I would have made to the initial management of this case (such as an increased dose of amoxycillin), but I am still unsure what an ideal management would look like.
Oral vaccines could provide relief for people who suffer regular UTIs. Here’s how they work cve.edu.au/ theconversation-oral-vaccines-utis Resource: theconversation.com

6 chances to Share the member benefits with your entire team: Small, Medium & Large Practice Memberships available
cve.edu.au/membership cve.edu.au/renew


The Post Graduate Foundation in Veterinary Science (PGF) established as the world’s first member-based organisation dedicated to postgraduate veterinary education
T G Hungerford Award established to recognise excellence in Continuing Veterinary Education
1987

CVE Distance Education Courses established. These popular and intensive courses continue to be recognised as world-class education for veterinarians at all stages of their career.
1991 1965
Control & Therapy (C&T) Series established and mailed to members

Innovative online courses expanded with TimeOnline courses developed
2004

1969


Dr Tom Hungerford OBE appointed as the first director. The Goanna Motif used by CVE comes from Tom’s learning philosophy where he promoted ‘follow the Goanna Track to success’.


Valentine Charlton left a generous bequest to the PGF towards extending research information by all means to veterinary practitioners on all matters concerning cats. Dr Richard Malik appointed VC Senior Lecturer in Feline Medicine in 1995.

Tom Hungerford










PGF donated $1M of the Valentine Charlton bequest to build the bespoke Valentine Charlton Cat Clinic at USYD


PGF renamed the Centre for Veterinary Education to reflect it’s true nature and operation within the University of Sydney
CVE pioneers a whole-practice approach to CE with courses designed for Veterinary Nurses



In 2025


2003
Dr Bill Howey appointed as Director of the PGF
CVE expands online courses and pivots to virtual conferences during the COVID-19 pandemic
2020
Paul Gotis-Graham Memorial Award for Excellence in Small Animal Surgery established 2014

Together with 115 outstanding educators, CVE runs 98 unique continuing education courses and events

2009
Dr Michelle Cotton appointed Director of the PGF

CVE supports over 8300 members and remains not for profit, dedicated to empowering the veterinary profession globally through education: enhancing confidence, competence, wellbeing and welfare

CVE Clinical Competency Award established for Australiasian universities
Dr Simone Maher


2021 2008 2025
CVE Continuing Education Scholarship established





Your clinic likely sterilizes surgical instruments and implants using one or more of the following methods:
Single-use blue wraps around some form of basket
Single-use paper or sealable plastic pouches
Reusable cloth drapes
Reusable rigid sterilization containers
Single-use wraps (or blueys) are inexpensive and widely available. However, they are designed for one-time use and then discarded, contributing significantly to a practice’s waste stream, which ultimately ends up in landfills. Australians produce approximately 21.6 billion tonnes of landfill each year, with hospital single-use goods being a notable contributor.¹ Additionally, blueys cannot act as rigid barriers, and sharp instruments can perforate them, compromising kit sterility. If accidental perforation occurs, it’s unlikely you would even be aware of it. Wrapping a kit also takes time, which is something most nurses have in short supply.
Single-use paper or sealable plastic pouches are also inexpensive and commonly available. They allow small quantities of instruments to be contained and sterilized but also end up as landfill. Being non-rigid, they are also susceptible to accidental perforation.²
Reusable cloth drapes are relatively expensive and have been used for many decades. Although they are disposed of infrequently, they need to be washed and dried after each use. Consider the water, detergent, electricity, and nursing time required for each use.³
Reusable rigid sterilization containers are typically made of aluminium. While they require a larger upfront cost, their lifespan is incomparable, as rigid containers can last for more than a decade. If you calculate the processing cost per kit, a practice can see savings as soon as the second year of ownership, simply by comparing it to the cost of consumables that would have been used instead.⁴
References:
3. Exploring the Safety and Environmental Impact of Sterilization Techniques.
4. Sustainability | Reducing the Environmental Impact of Sterilization Packaging for Surgical Instruments in the Operating Room: A Comparative Life Cycle Assessment of Disposable versus Reusable Systems (mdpi.com)
Seen an intriguing case recently?
Send us a high resolution image/s to pose the Q. and an article on how you treated it to appear in the following issue.
Winner
Entitled to a CVE$300 voucher
C&T No. 6060
Jason Lenord BVSc (Hons) MANZCVS (Medicine, Radiology)
Balmain Vet Hospital
C&T No. 6069
Questions
1. Why is the aPTT prolonged?
APTT is prolonged when the intrinsic coagulation pathway is affected. In this case it may be potentially due to a number of contributing factors:
– Consumption of intrinsic coagulation factors from the intra- and extra-hepatic bleeding from the hepatic fractures present.
– Potentially from loss of antithrombin III. AT III naturally inhibits activation of the intrinsic coagulation pathway and thrombin formation. The cat had interstitial nephritis, so there is the potential AT III in the urine, creating a pro-coagulant environment initially and the formation of clots, resulting in consumption of intrinsic factors and then prolonged bleeding times.
– Reduced hepatic synthesis of clotting factors due to acute inflammation, plus production of acute phase reactant proteins being produced in abundance and inhibiting coagulation.
– Activation of complement due to the presence of antibody-antigen complexes.
– Vitamin K deficiency due to reduced hepatic recycling of Vitamin K from acute hepatitis. Initially this would affect Factors II, VII, IX and X (TV stations)—which are better tested using the Prothrombin time but then
Send in your answer to any of these articles.
The best and most complete will be published in the next issue.
The contributer will receive a CVE$300 Voucher.


have a follow-on effect on the intrinsic factors as factor X is also involved at the end stage of extrinsic and intrinsic pathways.
2. Why is the platelet count low?
Platelet count is low due the consumption of platelets during clot formation—there are blood clots within the liver and also within the abdomen. Platelets will be low if consumption exceeds production. The auto transfused blood will also have a dilutionary effect on the platelet concentration in the peripheral blood.
3. What additional measures might have been used to try to save this cat?
Fresh whole blood to provide red cells, (some) clotting factors, proteins and platelets. Intravenous fluids, medical treatment for any hypotension, oxygen therapy as required. The cat may also be septic so antimicrobial therapy would likely be warranted.
4. What is the treatment for hepatic amyloidosis?
There is no proven cure. Treatment consists of supportive care—in this case for the liver and kidney health. Medical therapy to reduce serum amyloid
A release such as colchicine. Hepatoprotectants— Ursodeoxycholic acid, S-adenosyl methionine, Silymarin. Dietary protein reduction if elevated renal parameters. Monitoring of haematology, liver and renal parameters, urine protein: creatinine ratio and managing this accordingly.
APTT is prolonged probably mainly due to consumption; we think liver production of clotting factors is likely adequate but recommend giving Vitamin K just in case.
The platelet count is low due to consumption— platelets are trying to seal lots of leaks.
Transfusion (fresh whole blood), belly wrap, and tranexamic acid (TXA) are additional measures which could have been tried. TXA is an antifibrinolytic drug that works by inhibiting the breakdown of blood clots. The median dose used in cats is typically around 10 mg/kg, often administered intravenously. Must be given slowly or causes vomiting.
Currently, treatment focuses on managing complications. To stop bleeding, TXA and the emerging area of interventional radiology—hepatic artery embolization—may be able to be performed to reduce high pressure liver perfusion.

Read the original article cve.edu.au/candt-6060 (Issue 318, March 2025) from Alexander Teh
Email your Question or Answer to us at cve.marketing@sydney.edu.au.
Authors of published Questions or Answers may use the voucher towards CVE course enrolment or membership.
C&T No.6070
Photos courtesy of Erica Romano and Patrizia Danesi
A young female Domestic Shorthaired cat was presented with multiple cutaneous nodules and oral lesions.
She had been treated with antibiotics and cortisone for 3 months at a previous vet clinic.





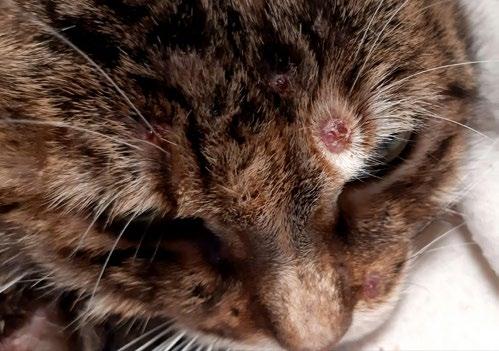

Fine needle aspirate cytology of the nodules is shown below


1. What is your diagnosis?
2. Explain the likely pathogenesis of the disease process
3. What is the significance if the disparate location of lesions?
Email your answer to cve.marketing@sydney.edu.au
April 2025 marked this major publishing milestone.
The C&T thrives due to its unique and altruistic nature: it’s a forum rather than an academic journal. Contributors share their experiences—warts and all—with colleagues to contribute to animal welfare everywhere.

On this special anniversary, the last word goes to its founder Tom Hungerford OBE BVSc FACVSc HAD who wanted a forum for uncensored and unedited material.
Tom wanted to get the clinicians writing:
Now every one of you who has done anything practical that’s not easily obtainable in all the common textbooks, write it up. Being local Australian* experience it’s invaluable.
*We welcome articles from everyone involved in the profession, worldwide. You don’t have to be a CVE member to contribute.
Email: cve.marketing@sydney.edu.au
Here’s to the next 56 years!
The C&T Team
Authors’ views are not necessarily those of the CVE
Maddy Reichstein
Southern Tablelands Veterinary Hospital
105 Robinson St Goulburn NSW 2580
e. southerntablelandsvets@gmail.com
Billie was a 6-week-old female entire chocolate Labrador puppy and on initial presentation, she was brought in with a singular 1-2mm scabbed lesion on the left dorsal eyelid, suspected to possibly be from a fight with a litter mate. The puppy was otherwise well at home and only the singular scab was noted.
The puppy then represented 6 days later with bilateral mucopurulent discharge, marked chemosis, periocular ulceration/crusting and multiple pustules on the inner pinna and muzzle. The submandibular lymph nodes were also enlarged. The puppy was quiet but still eating, drinking, and toileting well.
What’s Your Diagnosis and how would you treat this case?
Email your answer to cve.marketing@sydney.edu.au


B
C&T No. 6071 A C

Samantha Coomes BVSc MANZCVS (Small Animal Medicine)
Mikaela Green BVSc
4 Paws Veterinarians
383 Charles St Kirwan
Townsville QLD 4810
e. charlesstreetvet@hotmail.com
C&T No. 6072
Harry is a 2-year-old male entire Boxer acquired by the owners when the pup was approximately 10-weeks-old. Harry was brought to our clinic in September 2024 with a presenting complaint of stranguria for the past 2 to 3 days. The owners had noted penile extrusion, straining and when finally able to urinate, small amounts of urine came out in a spraying motion. No blood had been noted during urination and the owners had no other health concerns. Harry was noted to be eating his usual diet of pedigree and dog loaf with no changes and drinking and defecating as usual.
Of note, at 15 weeks-of-age Harry presented to our clinic for stranguria with urinalysis revealing a cocci-based urinary tract infection (UTI). The lower urinary tract signs resolved with a short course of amoxyclav. Harry has otherwise been a healthy dog and is up-to-date with preventative care.
Physical exam including neurological exam was unremarkable. On rectal exam, the prostate was small, symmetrical and not painful. After a discussion with the owners, the decision was made to hospitalise for further work up.
Harry was admitted to hospital for monitoring/ visualisation of urination and diagnostic imaging. Lateral radiographs were performed to assess the urethra and bladder for uroliths with no significant findings. Ultrasound of the bladder showed a small amount of hyperechoic mobile sediment within the bladder. Bladder volume was measured and estimated to be approximately 41 mLs. Otherwise, the prostate and kidneys were within normal limits.
A cystocentesis was also performed. Sediment examination showed 1+ cocci without white blood cells. There were no other abnormalities on urinalysis. The owners declined sending the urine for culture.
Following ultrasound, Harry was taken for a walk to observe urination. When urinating, Harry strained and his urine stream would stop and start (spurt). The bladder volume was re-evaluated after Harry’s urination attempt with a post void residual volume of 41.1mLs following urination, equating to 1.4mLs/kg residual urine volume. These findings raised suspicion of Idiopathic FOO, formerly known as Reflex Dyssynergia. A urinary catheter was later placed and passed easily, with no evidence of mechanical obstruction. The bladder was emptied prior to Harry being discharged home.
After discussion with the owners regarding our concerns for Idiopathic FOO, the decision was made to start a treatment trial.
The initial treatment administered was prazosin (an alpha-adrenoreceptor antagonist) at 2 mg/dog q12h PO to decrease internal urethral sphincter smooth muscle tone. The owners were advised to contact the clinic and discontinue prazosin immediately if there was any weakness, gastrointestinal upset or other concerns.
The owners were contacted 48 hours after prescription of Prazosin. The owners reported an initial improved response with Harry having one large urination without an interrupted stream, but unfortunately further urinations still had interrupted streams.
After reviewing the ACVIM consensus statement on diagnosis and management of urinary incontinence in dogs (2024), the benzodiazepine diazepam at 0.24 mg/kg/ day split q12h to be administered 20min prior to a walk was instituted for additional smooth muscle relaxation.
A further phone call follow up was performed 48 hours later. The owners reported that Harry was doing well and had full normal streams. The owners were also recommended to castrate Harry (see comments) but were strongly against castration.
It has been strongly recommended on multiple occasions that the owners bring Harry back for a day stay so we could observe urination and perform post-void residual volume measurements via ultrasound. Unfortunately, the owners have declined to perform these rechecks.
Idiopathic FOO is most common in young- to middleaged large and giant breed dogs. Labradors, Retrievers
and German Shepherds appear to have an increased risk of Idiopathic FOO (Mathews et al., 2023).
Idiopathic FOO results from a lack of coordination between detrusor contraction and urethral relaxation, with the urethra either pre-maturely contracting or not relaxing at all. In the majority of cases, a cause is not identified but lesions cranial to L2 spinal cord segment may be associated with the disease.
Several studies (Collins et al. 1986, Holt 1990, Lane et al. 2000, Coit et al. 2008, Haagsman et al. 2013) have suggested excitement and prostatic disease as possible causes of Idiopathic FOO; therefore, castration has been suggested to be included in the treatment protocol for Idiopathic FOO. Mathews et al., 2023 documented that 5/6 dogs showed improved outcomes when castration was included as part of their treatment protocol, with 3 of the dogs no longer requiring medications. These results supported a 2020 study by Stilwell et al. where 6/7 dogs had a good response to treatment with castration and 3 male entire dogs having no response to therapy where castration was not included in their treatment protocol.
Overall prognosis is good for those animals, like Harry, that respond to medical therapy which is often required lifelong. Detrusor atony may occur secondary to unmanaged Idiopathic FOO and this carries a poor prognosis.


1. Mathews, K., Toedebusch, C., Palm, C., Kendall, A. and Westropp, J.L. (2023). Idiopathic functional urinary outflow tract obstruction in dogs, a retrospective case series (2010‐2021): 31 cases. Journal of Veterinary Internal Medicine , 37(6), pp.2211–2218. doi:https://doi.org/10.1111/jvim.16843.
2. Stilwell C, Bazelle J, Walker D, Stanzani G, Florey J. Detrusor urethral dyssynergy in dogs: 35 cases (2007-2019). J Small Anim Pract. 2021;62:468-477.
In the recent 2024 ACVIM consensus guidelines of treating urinary incontinence, ‘6 of 8 (75%) SAIM panellists recommended the use of tamsulosin because of its wide safety margin and anecdotally better outcomes. Combined treatment with a skeletal muscle relaxant, either diazepam or alprazolam, is recommended initially or if initial response to an alpha-antagonist alone is poor. Six of 8 panellists recommended use of diazepam because of clinical experience and positive response.
Tamsulosin (Flomax) is an alpha blocker which works by relaxing the muscles in the prostate and bladder neck, facilitating easier urine flow; it has fewer side effects than Prazosin, a non-selective alphaadrenoceptor antagonist which causes hypotension in patients, and often other side effects.
Tadalafil—a phosphodiesterase type-5 (PDE5) inhibitor works by relaxing smooth muscle in the prostate and bladder neck which can help improve urinary flow and reduce symptoms such as difficulty urinating. A dose of 5 mg once a day is very helpful in people. This treatment has not been trialled in dogs but might also prove of benefit.
Lizzie Maher
Asquith Veterinary Hospital
1/53 Salisbury Rd
Asquith NSW 2077
e. avc@asquithvets.com
C&T No. 6073
An 8-year-old female spayed cavoodle presented for a mass over the right ribs and ongoing lethargy following a 2-week history of vomiting and diarrhoea.
Initial history included 2 weeks of intermittent vomiting, diarrhoea and tense abdomen, followed by pyrexia, treated with bland diet, probiotics, intravenous fluids, and a course of antibiotics. Blood tests indicated an inflammatory leukogram and monocytosis with a possible left shift. Ultrasound was recommended, but the owners declined. The patient was slowly improving on fluids and antibiotics so was discharged to home with oral medications.

The patient returned as she was still lethargic following the episodes of vomiting and diarrhoea, and the owner had noticed a large mass over the right lateral thorax the night prior to the appointment. She had finished a course of Noroclav a few days prior.
On presentation, the patient was tachycardic with a 2/6 left systolic murmur, had a temperature of 39.5°C and
mild non-repeatable reaction to palpation of the cranial/ mid abdomen. All other vitals were within normal limits. She had a 6cm x 6cm firm subcutaneous mass over the right ribs. The overlying skin had mild erythema and was warm to the touch. She had a moderate reaction to palpation. On FNA, blood-tinged purulent material was collected.
We admitted the patient for general anaesthesia and to drain/flush the abscess.
Under anaesthesia, the abscess was lanced, drained, and flushed with saline. On exploration, a blade of grass was located. On further exploration, a paddle pop stick was located, penetrating the abdominal wall. The paddle pop stick was left in situ, and the patient was prepped for an exploratory laparotomy.

On exploratory laparotomy, there was no peritoneal effusion noted. The paddle pop stick was located in the stomach, penetrating through the pylorus and abdominal wall into the abscess site. The stomach wall had adhered to the abdominal wall, walling off the infection from the abdominal cavity. The stomach wall was teased away from the abdominal wall to free it. The paddle pop stick was then pushed back into the stomach to allow for removal. The paddle pop stick was removed from the penetrating wound and the stomach closed. The abdominal wall wound was then closed and the abdomen flushed with warmed saline. A Penrose drain was placed in the abscess site and the abscess site was closed.

AThe patient recovered well from her anaesthetic and remained in hospital on fluids, pain relief and antibiotics for 2 days. During this time, she was slowly reintroduced to feeding through regular small meals and was then discharged with a further 7-day course of antibiotics.
On a 10-day post op recheck, the patient was much brighter, eating normally, had no further vomiting and was back to her normal, bouncy self (even following around the toddler trying to find some more food again!).
– Do you have a case of a foreign object behaving badly?
– Or an unusual foreign object case?
Email your case to cve.marketing@sydney.edu.au

Further reading
Perspective No. 47 ‘New’ and ‘Old’—Alimentary Foreign Bodies—in Dogs and Cats from Richard Malik with comments from Terry King covers a wide arrange of the weird and wonderful: oesophageal, nasopharyngeal and gastrointestinal foreign bodies.
cve.edu.au/New-and-Old

B

Terry King BVSc MANZCVS
e. terenceking70@gmail.com
C&T No. 6074
Tetrodotoxin is a neurotoxin, which results in paralysis of both the central and peripheral nervous systems, via blockade of all voltage-gated sodium channels.
The toxin is produced by as many as 21 species of endosymbionic bacteria, including E. coli, Vibrio spp. and Pseudomonas, which may be associated with multiple species of ‘Puffer fish’, such as toadfish, balloonfish, blowfish, globefish, porcupine fish, burrfish, molas, Fugu and Patkafish, as well as frogs, newts, crabs, octopus, starfish, flatworms and gastropods.
The clinical signs resulting from ingestion of the toxin include tingling / prickling sensation of the extremities, numbness of the lips, tongue and extremities, dryness of skin, subcutaneous haemorrhage and desquamation of the skin, nausea, vomiting, diarrhoea, dizziness, ataxia / incoordination, muscular twitching, tremour, muscular paralysis, respiratory distress, cyanosis, cardiovascular shock / arrest, and death.
Tetrodotoxicity occurs commonly in people throughout south-east Asia, China, and Japan, where despite its recognized ill-effects the consumption of ‘Puffer fish’ is considered a delicacy. Tetrodotoxicity is rarely documented in Australia, although as many as 11 cases of human poisoning have been recorded in New South Wales alone over a 16-month period, and several species of the offending marine species reside in Australian waterways.
Considering the geographical availability of ‘Puffer fish’ and the potentially fatal outcome if management is underestimated, tetrodotoxicity should be considered a differential diagnosis, along with tick paralysis, snake bite envenomation, ciguatera toxicity, botulism and polyradiculopathy (Coonhound paralysis), for any ascending flaccid paralysis.
This reports a single confirmed case of Puffer fish (tetrodotoxin) poisoning in a cat in south-east Queensland, detailing the clinical signs and successful treatment regime. Importantly, this case demonstrates that the initial neurological examination cannot be considered a reliable prognostic indicator, as the cat was completely unresponsive upon presentation, and had been artificially ventilated for over an hour prior to presentation yet was discharged home less than 72 hours later.
A 2-year-old, 4.0kg, male neutered Devon Rex was referred to a 24-hour emergency centre, for ongoing case management, following the ingestion of a Puffer fish. The cat had been treated that afternoon by the primary care veterinary clinic, but despite their care had continued to deteriorate. He had no pre-existing conditions and was last observed as normal earlier the same day.
Initial treatment, at the primary care veterinary clinic, consisted of intravenous (IV) fluid therapy with 0.9% NaCl at 70 mL/hour. Only 30 minutes later, however, as the cat’s condition continued to deteriorate, he was anaethetised, intubated and maintained on isoflurane at 2% and oxygen at 1.5 L/min. Atropine was administered (0.04mg/kg). The oral cavity was lavaged and a lubricant eye ointment placed across the corneas. Approximately 2 hours after admission, the cat respiratory arrested and then cardiac arrested. Positive pressure ventilation and external cardiac massage were immediately instituted. Intra-cardiac adrenalin was administered at 0.5 mg (1mg/ mL). A spontaneous cardiac rhythm was re-established, and subsequently maintained at 120 bpm. Spontaneous respiration failed to recommence, and positive pressure ventilation was continued. Hypothermia was observed, and active heating commenced.
Positive pressure ventilation and active heating were continued throughout the 35-minute transfer to the referral centre.
At presentation, the cat was moribund and remained in a state of respiratory arrest. On physical examination, the cat had a rectal temperature of 38.4˚C, a heart rate of 120 bpm, an artificial respiratory rate of 16 breaths per minute, pink mucous membranes, and a capillary refill time of less than 2 seconds.
Neurological examination revealed complete unresponsiveness to external stimuli, with no muscle tone, no motor, and no sensory function, as well as marked cranial nerve deficits, with symmetrically dilated and unresponsive pupils, absent menace, palpebral and corneal reflexes.
Results of haematological and biochemical analyses revealed mild haemoconcentration (high normal
RBC parameters and elevated TP and albumin), mild neutrophilia (14 x109/L), elevated ALT (121 U/L), elevated CPK (514 U/L), low urea (6.5mmol/L) and marked hypoglycaemia (1.9mmol/L). Venous blood gas results were indicative of respiratory acidosis.

The cat was established on a ventilator. A central venous catheter was placed. A Foley urinary catheter was placed. IV fluid therapy was recommenced with lactated Ringers Solution at 15 mL/hour. A single dose of 125 mg methylprednisolone was administered IV. An infusion of 20% Mannitol was administered at 12.5 mL over 30 minutes, and repeated q8hrly on another two occasions.
Throughout the night, ventilator parameters were constantly monitored, as well as SPO2. Clinical parameters, TPR were assessed hourly, urine output 2 hourly.
Six hours after admission to the referral centre, the cat commenced spontaneous respiration, and was extubated. The cat continued to improve throughout the night, becoming increasingly alert and responsive, vocalizing, etc.
By 10:00am the following morning, < 24hours postingestion, the cat was maintaining body temperature and oxygen saturation (measured using pulse oximetry).
Twenty-four hours post-admission, he was moving around, grooming, urinating voluntarily and was able to be discharged to his owners a further day later.
Discussion
There are multiple reports of tetrodotoxicity in humans, but a paucity of reported cases in the veterinary literature, including only one case report in Australasian veterinary medicine, published some 50 years ago—this involved two cases of toadfish poisoning in cats.


Tetrodotoxin is a neurotoxin, which results in paralysis of both the central and peripheral nervous system.
Tetrodotoxin is located in almost all the tissues of the fish, but the ovaries, roe, liver, intestine, and skin are considered to accumulate the greatest concentration of toxin. Toxicity is highest during the spawning period. A species may be toxic in one geographical region and not in another. A fatal dose in humans may be as little as 1 to 4 mg per adult. Humans may remain asymptomatic for 10 to 45 minutes, or as long as 3 hours.
Clinical signs reported include tingling / prickling sensation of the extremities, numbness of the lips, tongue and extremities, dryness of skin, subcutaneous haemorrhage and desquamation of the skin, nausea, vomiting, diarrhoea, dizziness, ataxia / incoordination, muscular twitching, tremour, muscular paralysis, respiratory distress, cyanosis. Fatality rates are high.
Similar clinical signs have been observed / reported in animals, including hypersalivation, vomiting, diarrhoea, ataxia, muscle weakness, with hindlimb flaccidity.
Cardiovascular shock and respiratory distress precede convulsions and death can occur within hours. In this case, the cat’s clinical signs commenced within 60 minutes. Neurological signs predominated, with subsequent effects on the cardiovascular system. Gastrointestinal signs were not observed, nor were haemorrhage or sloughing of the skin.
Based upon these clinical signs differential diagnoses include tick paralysis, snake bite, ciguatera.
1. There is no antidote—as Tetrodotoxin is a hydrophilic molecule, Intralipid therapy is unlikely to be of benefit.
2. Activated charcoal and gastric lavage.
3. Respiratory support may be as simple as fly-by or intra-nasal oxygen to intubation and mechanical ventilation.
4. Hypotension with vasopressors.
5. Entamiphylline has benefited some cases, in historical experimental laboratories.

Perspective No. 87, A Seavers & Comment S Page, How fishermen in a drought can inadvertently contribute to poisoning pets: Successful treatment of Toadfish intoxication in a cattle dog, Small Animal Poisons, C&T Series, June 2011, Issue 263, pp. 40-42.
cve.edu.au/toadfish
C&T No. 5361, R. Cope, Dec 2013, Beware the Bullrout: Small fish, big sting, excruciating pain, C&T Series, Dec 2013, Issue 273, pp. 30-31.
cve.edu.au/beware
C&T No. 5420, R. Cope & Dalefield R. Toxicology Brief: Stonefish Envenomation in Dogs, C&T Series, Sept 2014, Issue 276, pp.
cve.edu.au/stonefish


Randolph Baral
Paddington Cat Hospital
210 Oxford Street
Paddington NSW 2021
t. 0293806111
e. paddington@catvet.com.au
C&T No. 6075
This piece has several origins:
– The ISFM forum where a practitioner asked the maximum amount of dry food that can be fed to reduce the chance of recurrence of lower urinary tract diseases (LUTDs).
– My Science Week presentation last year where my clinical pathology centred talk showed the effect that dry food has on glycaemic load (then most of the discussion was more about nutrition than clinical pathology!).
– A recent weekend nutritional seminar where one speaker presented to the packed room that he wanted to ‘bust the myth’ that ‘cereal grains can cause food allergies’.
I have been in feline-only practice for almost 30 years. My experiences have echoed the growing body of evidence of the multiple problems caused by dry food in cats. Specific literature influences have been the Zoran ‘carnivore connection’ review,¹ the Mazzaferro et al diabetes paper² where nearly 2/3rds of cats had insulin discontinued after switching to a high protein canned diet and the Plantinga et al paper³ that assessed the nutrient profile of feral cat diets. These all point to feeding cats a diet consistent with what they have evolved to eat, which is ~70% water, with the remaining 30% dry matter comprising ~60% protein, 20-25% fat, ~12% total minerals (e.g. from bone) and next to no carbohydrate.
The difficulties that dry food has in achieving these metrics are:
1. Too low moisture content (after all, it is ‘dry’ food).
2. Too low protein content and too high carbohydrate content.
I will focus on these aspects as we explore them by clinical conditions:
Putting aside infection and neoplasia (that require specific therapeutic approaches), LUTDs can be considered as those associated with uroliths or crystal formation which can lead to obstructive disease (almost entirely in male cats) and idiopathic LUTDs; in both cases, wet food diets have been proven to result in better outcomes. It seems to me that many practitioners get concerned with urine pH (or magnesium or calcium content) and forget that the undisputed MAJOR factor associated with crystal and urolith formation is ‘relative supersaturation’.⁴ Wet food diets create more dilute urine,⁵ so we shouldn’t HAVE to prove that high moisture diets are less likely to result in less opportunity for crystals and uroliths to form, yet this has been demonstrated.6,7
Looking further at WHY wet food diets result in more dilute urine, it has been recognised for nearly 50 years (at least!) that cats eating a diet that contains >70% water barely drink.⁸ When cats eat dry food, they need to make up the deficit of water that they have evolved to ingest with their food. Consequently, a cat eating all dry food drinks 6-times as much as the same cat eating all wet food; yet, confoundingly, their total water intake from food and water is still considerably less than if they were eating all wet food.⁹ A lower total water intake results in more concentrated urine and thus provides the conditions to allow supersaturation to occur.⁴
A ‘small’ amount of crystalluria is normal in cats and so the mere presence of crystals does not necessarily cause clinical signs. However, we do know that large numbers of crystals can form a
matrix with inflammatory protein to result in urethral obstruction.10 One study assessed the effect of dry and wet versions of a prescription diet designed for lower urinary tract diseases. The study described the cats as having ‘idiopathic cystitis’ and is not clear as to how many cats had substantial crystalluria. Over a 12-month study duration, wet food diet resulted in only an 11% recurrence compared to 39% recurrence for the dry food diet group.⁵ As well as crystal reduction, it has been postulated that more dilute urine ‘may decrease the concentration of substances in urine that are irritating to the urinary bladder mucosa.’11
The studies noted above describing increased risk of urolith formation for cats eating dry food apply to uroliths generally and therefore upper urinary tract uroliths as well as those in the lower urinary tract.6,7 Additionally, dry food diets have proven to be a major risk factor for ureteral obstruction 12,13 and upper urinary tract uroliths specifically.13 One of these studies determined that cats eating predominantly dry food were 15.9 (!) times more likely to develop ureteral obstruction and even cats eating a mix of wet and dry food were 3.4 times more likely to get ureteral obstruction compared to predominantly wet food.12 The significant morbidity of the commonly resultant acute kidney injury as well as the high likelihood of subsequent chronic kidney disease,13 requiring lifelong management, can likely be significantly reduced by avoidance of dry food.
Chronic kidney disease (CKD) has been recognised in 52-73% of cats with upper urinary tract uroliths.12-16 This demonstrates that avoiding uroliths reduces a high chance of developing CKD, and, repeating that stated above, avoiding dry food greatly reduces the risk of upper urinary tract uroliths.
Kidneys have many functions, one of the most important (I would argue THE most important) being conservation of total body water. The reduced kidney function in cats with CKD means that they have reduced ability to maintain adequate water in their bodies. This is why a typical clinical sign is polyuria, and why our typical measures to diagnose CKD (increased creatinine and urea, i.e. azotaemia) are measures of dehydration. Polydipsia in CKD cats is an attempt to rehydrate. Even if dry food has not contributed to the initial development of CKD, as we’ve noted above, cats that eat dry food have a deficit of water that they have evolved to ingest with their food. With CKD, there is an additional water
deficit. Therapies such as nutrient enriched liquids (i.e. HydracareTM)17 or using subcutaneous fluids are based around reducing the water deficit to improve clinical signs. Feeding wet food instead of dry food is the most straightforward strategy to reduce water deficits.
There is much unknown about idiopathic hypercalcaemia, certainly the causes (as evidenced by the descriptor: idiopathic). We DO know that calcium-oxalate uroliths are a common consequence and this is borne out in papers that show ionized hypercalcemia in cats with uroliths,13,16 and we also know that many of the cats in these studies have eaten predominantly dry food diets. We DO know that fluid therapy with 0.9% NaCl is recommended for emergency treatment of hypercalcaemia18,19 since most calcium is excreted in the urine. We also know that diuresis is promoted by reducing USG with a high moisture diet and thus it is not surprising that wet food diets have been recommended to reduce idiopathic hypercalcaemia.19,20 Our experience at Paddington Cat Hospital is that this strategy is often, but not always effective on its own and other dietary strategies such as adding fibre21 may be additionally required. Of course, some cats require more therapy than diet change alone.
If you compare any brand of dry food to its equivalent form of wet food, for the same volume (say, 100g), the dry form has approximately 5 times as many calories. If you compare a ‘light’ or ‘diet’ form of a dry diet, that factor reduces to 4 times the calories of a maintenance wet food (and increases back to 5 times if compared to the equivalent diet wet food). I invite the reader to look up the nutritional data from pet food companies to confirm this. Given this large disparity in energy density, it is hardly surprising that multiple studies have identified dry food as a risk factor for obesity in cats.22-24 A large amount of this energy density disparity can be attributed to the water content which varies by a similar factor of approximately fivefold (in the opposite direction, of course). We know that feral cats’ diets of whatever raw prey they can find comprises approximately 70% moisture,³ and it is not unreasonable to conclude that cats evolved to consume this amount of moisture. Since it has been demonstrated in many species that satiety is, at least in part, from degree of gastric dilation,25,26 we could infer that cats evolved to meet
Authors’ views are not necessarily those of
their caloric requirements from a diet much less calorie dense than dry food.
Additional to simple caloric density, higher moisture content in food has been shown to promote increased activity,27,28 thus resulting in using more calories as well as ingesting less.
– Obesity: carbohydrate/protein proportions
Even dry food marketed for diabetics and obesity have 4 to 8 times the carbohydrate content as their wet food counterparts. Not only do cats have no dietary requirement for carbohydrate with glucose requirements (such as for the brain and red blood cells) being met by gluconeogenesis,29 they also lack effective means to fully metabolise carbohydrates.30,31 The precise amount that defines carbohydrate excess in cats has yet to be elucidated but one study assessing 12% carbohydrate as ‘low carbohydrate’ found significantly less glycaemic and insulin response than higher carbohydrate diets.32 Numerous dry diets marketed for diabetics and obesity have double this amount of carbohydrate. In any species, exceeding the ‘carbohydrate economy’ results in oxidation to synthesise lipid.33
Another consequence of high carbohydrate diets is that they inevitably result in a lower protein diet (since our only macronutrients are carbohydrate, protein, fat and moisture). High protein diets (>45% but preferably closer to 60%) not only help cats meet their high and specific amino acid requirements34 but also leads to significantly higher heat production and therefore increased energy expenditure.35
Regardless of if the driving factor of this aspect of obesity is high carbohydrate or resultant low protein or a combination of these, the macronutrient profile of dry food contributes to feline obesity.
HIGH CARBOHYDRATE
– Diabetes
I have already referred to the Mazzaferro et al diabetes paper² in which 11 of 18 cats went into diabetic remission switching to a high protein canned diet. This paper has had a profound effect at Paddington Cat Hospital and we now not only have a remission rate of approximately 70%, consistent with subsequent studies,36,37 but many of our new diabetics now come from other practices where there are not the same recommendations to avoid dry food from kittenhood as we recommend.
Dry food creates a double threat for diabetes since it provides excess carbohydrate that cats are unable


to efficiently metabolise30,31 as well as increasing the risk of obesity (as noted above) which creates insulin resistance.35,38
At Science Week last year, I presented blood glucose results from a now 13-year-old male desexed Burmese cat at separate visits over a 10-year period, in which definitive peaks and troughs can be seen.

I then superimposed the cat’s body weight and body condition score at each visit which enables us to see that the blood glucose peaks and troughs correlate quite closely with body weight and body condition score as we would expect from our knowledge of insulin resistance; however, there are some additional peaks towards the right that cannot be explained by body weight/BCS alone.

Adding this cat’s dietary history explains those additional peaks, as well as being closely associated with the cat’s weight gains (as expected).

I have been asked about the impact of stress which most definitely can elevate blood glucose results; however, the consistency of blood glucose results with body weight and dietary history over a 10-year period reduces the likelihood that stress is affecting blood glucose in this case. We cannot make overall statements from only one cat but this cat’s lack of glycaemic control when faced with the increased carbohydrate of dry food being even part of his diet is entirely consistent with published work.39
Gastrointestinal Diseases
Associations between high carbohydrate diets and gastrointestinal disease have also been made. Osmotic diarrhoea associated with carbohydrate dysfermentation31 and other ‘digestive disorders’30 have been recognised for over 30 years with dietary intake of >5g/kg body weight of sugars and starches respectively. High carbohydrate diets also reduce protein digestibility and incomplete carbohydrate fermentation by the small intestinal microbiota results in increased microbial fermentation in the large intestine.40 The small intestinal incomplete
microbial fermentation may alter small intestinal microbial diversity; independent to this, it has been shown that ‘the composition and number of mucosaassociated bacteria correlate with the presence and severity of inflammatory bowel disease in cats.’41 Food sensitivities demonstrated by gastroscopic food sensitivity tests as well as elimination-challenge trials found that wheat and corn gluten are among the most common allergens (as well as commercial dry diet in an additional 5 of 16 cats whose owners refused further testing).42 I am unaware of any assessment comparing efficacy of hydrolysed or novel protein diets to their wet food equivalents or nutritionist formulated home-made diets.
Dental disease is the biggest concern that clients and vets have when I advocate reduction or elimination of dry food from a cat’s diet. It has certainly been demonstrated in dogs that normal dry food offers no benefit to wet food for periodontal health43,44 and it is reasonable to assume that this hold for cats also. Specific dental diets with larger kibble size and texture to promote chewing and maximal contact with teeth have been demonstrated to help periodontal disease45 (again, at least for dogs; I was unable to find equivalent studies for cats).
However, in cats, the most common dental disease is tooth resorption46 for which no association of periodontal disease has been made.47 I am unaware of any epidemiologic studies but I am sure that I am not alone in having seen many cats eating dental-style biscuits yet still requiring dental work to attend to tooth resorption.
I would like to think that, when introduced in the 1940s-1950s, and through its growth in sales starting in the 1970s, that dry food was considered appropriate for cats, much in the same way that ‘sliced white bread’ used to be considered healthy for people. However, the widespread evidence that I have shared here of disease processes that have increased in prevalence as dry food sales increased from the 1970’s, exemplified by diabetes,48 has made me more and more certain that dry food for cats is an historical accident, far removed from how cats evolved to eat.
References cve.edu.au/dry-food-cats
Authors’ views are not necessarily those of
Vets1laser
e. aine@vets1laser.com
C&T No. 6076
This article reinforces the importance of ocular safety both for the vet patient and vet team to prevent complacency when utilising new technologies, such as laser surgery or laser/photobiomodulation medical therapy in the workplace.
Veterinary Medical Laser/Photobiomodulation Patient & Workplace Ocular
Medical Photobiomodulation (PBM) Laser therapy machines used correctly are some of the safest devices one can apply to a veterinary patient.
Unfortunately, that high level of safety can induce complacency around the use of these machines, especially because many of the therapeutic wavelengths in operation are not visible to the human eye.
Lasers outside the visible spectrum are especially dangerous because the eye does not have a natural aversion at these wavelengths. We do not blink fast enough to stop the damage from the beam.
Light, entering the eye from a collimated beam in the retinal hazard zone area, is concentrated by a factor of 100,000 times when it strikes the retina, so even a lowpower beam is a concern.
A medical laser beam hit can be 120 x hotter than a direct viewing of the sun.
On the electromagnetic spectrum, the greatest ocular hazards are:
– The 400 - 1400 nm wavelengths which can damage the retina
– Near-UV region and near-IR at certain wavelengths, the lens may be damaged.
– Far-UV and far-IR regions of the spectrum, the cornea will absorb the laser energy and be damaged.
– There remains therefore—where the adherence to Laser/PBM safety guidelines is substandard—a
constant concern of the risk of catastrophic ocular damage to any patient or staff inside the Nominal Ocular Hazard Zone (NOHZ).
The good news is that it is incredibly easy, simple, and cheap to maintain an appropriate level of safety in any and all veterinary laser/PBM workplace scenarios.

PLAN A
First and foremost is the use of ocular shielding devices for the Therapist and the Pet
Too many images abound on social media wherein the veterinary laser/PBM patient is without protective safety googles and hence left at an increased risk of ocular injury. The ‘I’m Not bothered’ attitude about the pet wearing proper eye protection in a laser session is simply not acceptable. This is a Safety Issue: No one gets to ignore the rules. There are NO situations where it is acceptable for a laser machine to be in use, yet for the animal not to be wearing some form of protective eye shielding.
The preference would be for the pet to be fitted with certified animal laser safety glasses and for all personnel in the room to be also wearing a set of laser safety glasses certified for humans.
– All laser therapy safety glasses supplied are specific to the wavelength of the machine supplied.
Your safety glasses must be marked with the exact wavelengths at which your therapy machine works, otherwise the glasses do not protect you. You cannot just commandeer your old welding machine shield and use it.
All replacements must comply with these wavelengths which are marked on the glasses.
– The marks ‘OD’ stands for ‘Optical Density’ the capability to block most of the laser light.
An OD of >/= 6 is Extreme blocking which is the rating of the human safety glasses with which most veterinary machines are supplied.
Whilst the OD range is 1-10, most medical therapy safety glasses stop at OD 6; otherwise, therapists cannot see properly in the darkness of 7 and beyond.
Beware of human safety glasses marked as the correct wavelengths but with an OD < 6.
– Dog safety glasses OD usually range from 4+ to 5+. Any darker and the dogs in particular start to reject the glasses.
– The glasses are there to protect from inadvertent accidental transient exposure to the laser.
The glasses do not protect against a laser beam being shone directly and persistently at the eye.
Tip: All laser glasses will have the safety numbers printed on the top edge of the glass lens. This is a handy way to know how to put the safety glasses on the correct way up on the pet!
PLAN A works very well with humans but sometimes our veterinary patients will simply not accept the pet safety glasses.
In those animals, for the therapy session to proceed, one must revert to protecting the pet by covering their eyes with materials that have the ability to block any stray laser beams.
If the pet will not accept the goggles, then think creatively.
Thanks to a wonderfully altruistic quantum physicist, I have some new unique data on the blocking ability of varied materials.
The material that proved the most useful result of all re blocking was the humble black towelling facecloth. I
chose this item because this cheap, towelling facecloth can easily be accessed or purchased at any housewares store. Experiments on this material showed it can block 7 W of a 20mm spot beam down to 1 W.
Whilst that is still not protective to the eyes as the dose is > 0.5W, we can work with that knowledge to improve the blocking ability of this facecloth. I recommend folding the facecloth over 4 times, before using it to shield the eyes.



Some of my laser vet customers have sewn the fold permanently into the face cloth, embroidered their clinic logo on it and attached head ties.
The cloth shield is especially useful for guinea pigs, birds, or exotics. (That said, rabbits unfailingly accept the catsize commercial goggles).
One can also pre-spray the outer fold of the cloth with calming pheromones 30 minutes beforehand.
For larger animals, hand towels etc folded over multiple times can be used instead over the eyes or blinkers.
For skittish horses, have the owner or handler first hold the harness or mane, then drape a large heavy towel over their extended arm, forming a non-contact blocking shield between the horse’s eye and the laser beam.
Always face the animal’s head away from the beam and shield as much as possible from any stray beam etc.
Remove or cover bright reflective surfaces in the laser treatment zone.
Dark towels, dark denim or felt throws or blankets are ideal blocking covers.
– The leg of a pair of dark jeans, cut into various lengths and double folded over, makes for a great hood to place over the head midway to the nose to protect the eyes.
– A triangle-shaped piece of dark jean cloth can be draped around the head and eyes of a pet who does not want to put their face down into a hood.
– A Buster collar, made of 9mm silicone rubber, to wrap around the head also works.
Always make sure you are also operating the machine in a laser safe-zone, so there is no risk of environmental specular reflection back off shiny surfaces onto the animal’s eyes.
– Human Hand: Using your naked hand alone will not block enough of the beam to protect the patient’s eyes.
You are also exposing your own skin unnecessarily to a treatment beam your body does not need. First wrap/strap the folded facecloth onto the back of your hand, then place your covered hand over the patient’s eyes.
– Nylon, Viscose, or thin synthetic materials can ignite under a laser beam, so avoid using those materials. Instead, felt cloth or leather (minus any shiny metal pieces) make for good blocking materials.
There are far too many social media posts of veterinary patients not wearing protective goggles or coverings. If the animal truly doesn’t accept/fit the laser googles— which is very rare—then you should be using a range of eye cover protection alternatives similar to those described above. Pet Patient Eye safety is not negotiable.


AB C

Additional Reading
Woo SH, Chung P, Lee SJ, 2021, Safe Use of Medical Lasers. Medical Lasers; 10:68-75. https://doi.org/10.25289/ML.2021.10.2.68
DVM4 Student at SSVS at The University of Sydney e. xlin5798@uni.sydney.edu.au
C&T No. 6077
…the most significant motivating factor was a personal observation of stress reduction in animal patients
…difficulties in choosing and playing music that simultaneously caters to animal needs and staff preferences are a more major influence on music therapy implementation than scientific evidence
Despite the numerous barriers to music therapy, the majority of respondents who did not implement music therapy stated they were willing to consider it
Various studies have been conducted on the effects of music on animal physiology and behaviour, and the potential benefits of music as well as species differences have been reviewed.1, 2 In particular, the genre of classical music has been found to reduce stress-related behaviours in dogs3-5 and physiological responses in cats.⁶ Human-centric studies report similar positive effects, such as how classical music played at a low volume in a consultation room can improve pet owners’ satisfaction with their veterinary visit.⁷ Overall, these findings suggest that playing classical music in a veterinary clinic may be beneficial for both owners and animals.
This act of playing music with the intention of reducing stress is often termed ‘music therapy’. However, despite existing evidence, there are no published studies investigating the uptake of music therapy in veterinary hospitals, or veterinarians’ perception of music therapy. Therefore, we conducted a survey aiming to investigate how many veterinary hospitals in Australia are using music therapy, and what views are held by veterinarians on the use of music therapy in veterinary settings.
In total, there were 37 survey participants with valid responses. 51.4% (n=19) answered that they did implement music therapy at their workplace while 48.6% (n=18) did not. For those who implemented music therapy, the three most common motivating factors were ‘Stress reduction observed’ (n=10), ‘Personal preference’

Figure 1. Motivating factors for veterinarians (n=19) implementing music therapy in veterinary hospitals. From a survey of Australian veterinarians (n=37). Abbreviations: Personal preference=Personal preference/ taste in music; Past studies=Journal articles or past studies; Stress reduction observed=I have observed reduced stress behaviours in animals when using music therapy; Staff recommended=Staff at my workplace recommend music therapy; Online forums=Online forums or conferences recommend music therapy; Other=Other/typed answer.

Figure 2. Areas where music is played at the workplaces of veterinarians who implement music therapy in veterinary hospitals (n=19). From a survey of Australian veterinarians (n=37). Abbreviations: Other=Other/typed answer.
(n=7) and ‘Past studies’ (n=7) (Figure 1). Music was played most commonly in the hospital ward (n=15) and surgery theatre (n=8) (Figure 2)
For the respondents who did not implement music therapy, 94.4% (n=17) stated that they were willing to consider music therapy. They, alongside those who implemented music therapy, identified ‘Distracting for staff’ (n=8) and ‘Insufficient evidence’ (n=6) as major barriers to music therapy (Figure 3). Indeed, the one respondent who would not consider music therapy also chose ‘Distracting for staff’ as the reason for their decision. For the question regarding major barriers, a number of respondents (n=7) chose to type their own answers, and three of these answers stated a lack of awareness of or consideration for music therapy and three were associated with staff music preference (Figure 3, 4)
Authors’ views are not necessarily those of

Figure 3. Barriers against the implementation of music therapy, answered by veterinarians implementing music therapy (n=19) and veterinarians who do not but are willing to consider it (n=17). From a survey of Australian veterinarians (n=37). Abbreviations: Insufficient evidence=Not enough evidence for music therapy; Minimal effects= I have observed no effect after trying music therapy; Distracting for staff=It is distracting for veterinary staff; Client preferences=It does not cater to clients’ musical preferences; Vets do not decide music=I do not decide the kind of music played at my hospital; Evidence against music= I have seen/heard of evidence against it; Lack music technology= My hospital lacks music playing facilities/ technology; Other=Other/typed answer.
It was found that veterinarian age was the only significant predictor of music therapy implementation, with respondents aged 35-44 and 45-54 being significantly more likely to implement music therapy compared to those under 34 (p=0.03). None of the other demographic features—gender, years of experience and musical training background—were significant predictors.
For respondents who did implement music therapy, the most significant motivating factor was a personal observation of stress reduction in animal patients. It is unsurprising that a perception of positive outcomes is significant, as it is comparable to reward or extrinsic motivation.⁸ However, it should also be noted that reported observed positive effects of music on animals, such as the anecdotal answer provided for motivating factors (Figure 4), may not be due to the effect of the music on the animal per se, but the effect of the music in altering the demeanour and behaviour of persons interacting with or handling animals, which in turn influences the animal’s behaviour. Two previous studies conducted on dogs and their response to human behaviour showed that dogs can recognise different non-verbal cues as a form of communication and will behave differently depending on humans’ body language.9, 10 Furthermore, it has been suggested that canines’ stress response can mirror that of their owner’s, with regards to cortisol levels.11 This indirect effect may also be contributing to inconsistencies in the evidence involving music therapy, as those who strongly believe in the benefits of music therapy may behaviourally and physiologically exert a more calming effect than those who find it distracting.
One may expect research and existing evidence to be the most significant motivating factor, since veterinarians are encouraged to practise evidence-based medicine.12 Indeed, it was the second most frequently chosen motivating factor, albeit alongside personal preference. This may be due to the limited and conflicting evidence surrounding music therapy, supported by a perceived ‘lack of evidence’ in respondent answers regarding barriers to music therapy implementation.
For example, one study found that dogs showed signs of increasing stress during a mock veterinary visit, with no significant difference between those who were or were not exposed to classical music.13 The authors speculated that the salience of the stressor may have been strong enough to overcome the potential benefits of exposure to music. Another study found no significant difference in the mean Cat Stress Score between hospitalised cats listening to cat-specific music, classical music and no music.14 However, it has been suggested that features of each individual piece of music—the length of notes, the presence of pure tones and regular rhythms, and the tempo—can make a difference in the way it is perceived by animals,15 and thus there are expected variations between different pieces even if they are of the same genre. Furthermore, it must be considered that it is difficult to ‘measure’ stress, due to the subjective nature of recognising behavioural signs and a lack of universally approved guidelines and defined normal ranges of physiological stress markers.16 Thus, more research is needed to establish the definitive effects of music on animals.
However, the most frequently chosen barrier to music therapy was not a lack of evidence but ‘Distracting for staff’, followed by individually specified logistical reasons. Thus, difficulties in choosing and playing music that simultaneously caters to animal needs and staff preferences are actually a more major influence on the implementation of music therapy, rather than scientific evidence. This can be explained by the ‘Theory of Planned Behaviour’ by Ajzen,17 which suggests that behaviour is influenced by intention, and intention is influenced by one’s attitude toward the behaviour, the subjective norm, and the perceived behaviour control (or one’s ability to perform said behaviour). Hence, it is insufficient motivation for veterinarians to just be aware of music therapy and its potential benefits. This motivation can be outweighed by the aforementioned logistical difficulties, exacerbated by a lack of opportunity to make decisions on music or conflicting musical preferences with other staff. Furthermore, almost half of the survey respondents did not implement music therapy, so the implementation of music therapy itself is likely not a norm.
Despite the numerous barriers to music therapy, the majority of respondents who did not implement music therapy stated they were willing to consider it. Furthermore, none of the veterinarians who did not implement music therapy had seen or heard evidence against music therapy, nor did their workplace lack the
facilities or technology to play music (Figure 3). Thus, potential ways to mitigate the aforementioned barriers can include raising awareness of the potential benefits of music therapy for all veterinary staff, and further studies into the effects of music therapy to address conflicting evidence in current literature.

Figure 4. Typed responses under the ‘Other’ answer for survey questions regarding motivating factors and barriers to the implementation of music therapy. From a survey of Australian veterinarians (n=37). Abbreviations: Motivate=Motivating factors for music therapy implementation; Barrier=Barriers against music therapy implementation.
References
1. Alworth LC, Buerkle SC. The effects of music on animal physiology, behavior and welfare. Lab Animal 2013;42:54-61.
2. Dhungana S, Khanal D, Sharma M et al. Effect of music on animal behavior: a review. Nepalese Veterinary Journal 2018;35:142149.
3. Kogan LR, Schoenfeld-Tacher R, Simon AA. Behavioral effects of auditory stimulation on kenneled dogs. Journal of Veterinary Behavior-Clinical Applications and Research 2012;7:268-275.
4. Wells DL, Graham L, Hepper PG. The influence of auditory stimulation on the behaviour of dogs housed in a rescue shelter. Animal Welfare 2002;11:385-393.
5. Bowman A, Dowell FJ, Evans NP, Scottish S. ‘Four Seasons’ in an animal rescue centre; classical music reduces environmental stress in kennelled dogs. Physiology & Behavior 2015;143:70-82.
6. Mira F, Costa A, Mendes E, Azevedo P, Carreira LM. Influence of music and its genres on respiratory rate and pupil diameter variations in cats under general anaesthesia: contribution to promoting patient safety. Journal of Feline Medicine and Surgery 2016;18:150-159.
7. Engler WJ, Bain M. Effect of different types of classical music played at a veterinary hospital on dog behavior and owner satisfaction. Javma-Journal of the American Veterinary Medical Association 2017;251:195-200.
8. Hendijani R, Bischak DP, Arvai J, Dugar S. Intrinsic motivation, external reward, and their effect on overall motivation and performance. Human Performance 2016;29:251-274.
9. Soproni K, Miklósi Á, Topál J, Csányi V. Comprehension of human communicative signs in pet dogs (Canis familiaris) Journal of comparative psychology 2001;115:122.
10. Call J, Bräuer J, Kaminski J, Tomasello M. Domestic dogs (Canis familiaris) are sensitive to the attentional state of humans. Journal of comparative psychology 2003;117:257.
11. Sundman AS, Van Poucke E, Holm ACS et al. Long-term stress levels are synchronized in dogs and their owners. Scientific Reports 2019;9.
12. Holmes M, Cockcroft P. Evidence-based veterinary medicine 1. Why is it important and what skills are needed? In Practice 2004;26:28-33.
13. King T, Flint HE, Hunt AB, Werzowa WT, Logan DW. Effect of music on stress parameters in dogs during a mock veterinary visit. Animals 2022;12:187.
14. Paz JE, da Costa FV, Nunes LN, Monteiro ER, Jung J. Evaluation of music therapy to reduce stress in hospitalized cats. Journal of Feline Medicine and Surgery 2022;24:1046-1052.
15. McConnell P. New Research on Dogs and Music. 2013.
16. Kartashova I, Ganina K, Karelina E, Tarasov S. How to evaluate and manage stress in dogs–a guide for veterinary specialist. Applied Animal Behaviour Science 2021;243:105458.
17. Ajzen I. The Theory of Planned Behavior. Organizational Behavior and Human Decision Processes 1991.
Jeremy Rogers
Strathalbyn, SA.
C&T No. 6078
Introduction
From time-to-time, an unusual syndrome or unusual cases are reported from abattoirs or slaughterhouses by astute staff on the lookout for possible exotic diseases or new diseases. In this case, a mob of 50 lambs was submitted by a producer in the Adelaide plains area; one lamb had unusual skin lesions that were collected and sent for examination. Exotic disease was ruled out but a diagnosis of ‘follicular cysts’ was made. I have not heard of these in sheep before.
History
50 lambs of about 11 months-of-age were presented for slaughter. All lambs were healthy and in good condition. One lamb however had unusual lumps in the skin, the assumption being that these could be grass seed abscesses, but they did not have typical pus associated, and it was an unusual time of year for that.
The epidermis and follicular infundibulum is hyperplastic with orthokeratotic hyperkeratosis. Multifocally, there are intraepidermal or intracorneal abscesses and ballooning degeneration. No infectious agents are noted. In the dermis there is fibroadnexal dysplasia and a single or double follicular cysts lined by well-differentiated keratinising stratified squamous epithelium surrounded by a dense collagen. The cyst’s lumen is filled with lamellar keratin and scattered pale eosinophilic ghost cells. The epithelial keratinization contains a sparse granular layer with patchy areas of scant pigmentation. A few small sebaceous gland lobules are situated at the superficial aspect of the cysts.
Follicular cysts with epidermal hyperplasia and hyperkeratosis, epidermal and intracorneal abscesses.
Features are compatible with follicular cysts (epidermal inclusion cysts). There is no evidence of viral cytopathic effects or neoplastic transformation in these tissue sections. In general, follicular cysts represent a nonneoplastic follicular anomaly which is idiopathic (congenital) or acquired (as in this case) from occlusion
of follicular orifices due to local inflammation or scarring. Special stains were requested to screen for potential bacterial or fungal infectious agents that are not obvious on H & E.
Follicular cysts are often solitary but may be multiple either synchronously or sequentially in some animals. They typically present as intradermal or subcutaneous nodules, occasionally with a pore from which keratin/ grumous content may be expelled onto the skin surface. Gradual enlargement and eventual rupture releases keratin into the dermis which stimulates a granulomatous foreign body reaction with rapid increase in mass size.
Discussion
Sheep pox and Sheep scab (both exotic to Australia) were ruled out in this case and no further cases have been reported from this property. There appear to be very few reported cases of follicular cysts in sheep in the literature.¹
Reference
1. Follicular cysts in sheep. Oz HH, Foil CS, Memon MA, AlBagdadi FK Turk MA Sims D Journal of the American Veterinary Medical Association 01 Sep 1985, 187(5):502-503.

Peter Kerkenezov BM BVSc DipAppSc Cert Equine Surgery
e. equivet@nor.com.au
C&T No. 6079


Skeletal maturity of racehorses, particularly in regard to racing 2 and 3-year-olds, has been a controversial topic for a very long time. Horses are not skeletally mature until at least five years old. Reliable statistics claim 60% of the deaths in racehorses are due to fractures and 151 horses died on Australian racetracks last year (2023 – 2024), that we know about, so it should be a very real concern. Consensus has it, most causes of lameness are from the knee down. Why?
We know aberrant leg conformation and hoof capsule shape and structure, particularly of the front feet, can create imbalances and poor biomechanics with resultant pathological changes elsewhere, so why are the feet not getting closer scrutiny?
One apparent reason is that too much trust is being placed upon farriers. Poor farriery is so widespread it needs constant supervision by attending veterinarians.
Integrity and the welfare of racehorses are entwined to such a degree that any evidence—and there is plenty— of poor welfare of Australian Stud Book registered Thoroughbreds amounts to a failure of racing authority and veterinary regulatory legitimacy. The Five Domain Model as it is applicable to racehorses is contravened throughout the whole racehorse industry structure.
This dissertation is focused on one major aspect of racehorse welfare and that is racing ‘unfit’ horses due to locomotory dysfunction, hence this simplified overview of why preventable anatomical injury is the main cause of mortality, morbidity and horse ‘wastage’ in Australia.


Established in 2025, the aim is to assist a qualified veterinarian and loyal CVE Member to enhance their professional skills through continuing veterinary education and practical professional development.
You—or your practice—must be a current CVE Member with a record of continuous paid membership over the last 3 years, either as an individual member, a practice staff member, or a combination of both.
Staff working at a practice that has been a continuous CVE Practice Member for the last 3 consecutive years also qualify.
Renew your membership before the EOFY to retain eligibility.OFY 30 June 2025.
cve.edu.au/scholarship cve.edu.au/renew
Winner
Entitled to a CVE$300 voucher
Bernie May
m. 0412 457 440
t. bernardpmay@gmail.com
C&T No. 6080
For a few years, I have been using Clerapliq® from Silverglide in corneal ulcers. I tend to use it in the more serious ones. I am repeatedly amazed at how well it works, and how quickly. Many times I reckon it has saved eyes that would have been lost without it.
The product comes in single use sachets. I actually draw it into a 0.3 mL syringe to avoid using a whole dispensing pipette for each administration. I break the needle off after drawing it up and administer 1 or 2 drops from the syringe.
See ’before and after’ photos of a young goat seen recently (see Figure 1).
It was this case that prompted me to write for C&T. Unfortunately, the ‘before’ photo is head-on so hard to see the eyes. This goat was totally blind on presentation. The ‘after’ photos were 10 days later.
Clerapliq®
1. Does not seem to be as popular as it should be.
2. Is a heparan sulfate mimetic containing 4 mg/mL of OTR4120 (an alpha 1-6 poly-(carboxymethylsulfate)-glucose) and dextran in a 9 mg/mL sodium chloride solution.
3. Works in corneal ulcers by replacing destroyed heparan sulfate in the cornea, restoring the extracellular matrix (ECM) by organizing collagens in the matrix and promoting healing by protecting growth factors and cytokines.
4. Is a veterinary ophthalmic product designed to treat corneal ulcers and other ocular surface disorders in animals. It contains OTR4120, a heparan sulfate mimetic that belongs to the ReGeneraTing Agents (RGTA®) family.
5. It is typically administered as 1-2 eye drops 2-3 times a week, for one to several weeks.
6. Has shown success in treating various corneal issues, including superficial ulcers, bullous keratopathy, and nonhealing erosive ulcers.



BAReferences
Martinez JA, Chiappini F, Barritault D. Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs. Vet Sci. 2019 Dec 13;6(4):103. doi: 10.3390/vetsci6040103. PMID: 31847217; PMCID: PMC6958328.
Martinez, Jessica A., Franck Chiappini, and Denis Barritault.. Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs, Veterinary Sciences 2019 6, no. 4: 103. https://doi.org/10.3390/vetsci6040103
Aine Seavers MVB MRCVS
Vets1laser, Australia
e. aine@vets1laser.com
I totally agree with this article.
The only difference was we used up the whole vial once breached. I often had the owners apply it to the other eye on the basis of it did no harm and avoided waste; but often times that second eye is affected but not yet to a level we can visualise. So both eyes got the treatment.
The most beautiful case we had was a 2nd opinion 17-yearold pom with the most horrendous eye ulcer. The first vet had treated the eye very correctly but this eye was not responding, to the point where the dog was systemically affected by the pain and had almost shut down.


https://silverglide.com.au/clerapliq-2/
For want of a more technical description, I changed nothing except I ‘drowned’ both eyes in a vial of Clerapliq and sent the dog home over night to organise for referral to a specialist in the morning. The next morning I was presented with 2 elated owners and their perky pom.
The owners reported that the dog went home and slept the best sleep for 10 hours, then appeared bushytailed if not quite bright-eyed to share breakfast with the 3 other poms in the family.
After that case, I struggled to find an eye case wherein Clerapliq was ‘not’ indicated. I didn’t find any.
All clinics should stock this product and use it.
DO give the vial time to return to room temperature if it was in the fridge.
As discussed in a previous article on ocular ouch, cold eye medicines impart their own unique pain and hence opposition from the patient.

Read Aine’s article from June 2019, Issue 295: ‘Ocular Ouch’: cve.edu.au/ocular-ouch
Winner - Best Visuals
Entitled to a a CVE$300 voucher
Daniel Wilfred DVM
Vet
Partners Malaysia
Jalan Serampang, 80050, Johor Bahru, Malaysia e. Danielwveterinaria@gmail.com
C&T No. 6081
Background
Dystocia is often used as a generalised term for eggbinding or ovostasis in reptiles. It can be broadly categorized into two types—dystocia caused by physiological problems or dystocia caused by anatomical abnormalities. In snakes it is often multifactorial.
Factors such as a poor diet prior to gravidity, genetic predisposition, female size relative to egg size, mating readiness, hydration status and egg clutch size are the primary causes of dystocia. There may be other contributing factors, such as tumours, infections, compression necrosis, egg adhesion and abnormal oviduct shapes; these factors often account for far fewer ovostasis cases compared to the former.3
It is, however, important to note that both anatomical and physiological factors can occur simultaneously.
A 4-year-old, 13.6 kg female Albino Reticulated Python ( Malayopython reticulatus), named ‘Lemon’ was presented with dystocia after laying 15 eggs. Of that total, 11 viable eggs and 4 unviable (slug) eggs were produced via natural egg laying. The viability of the eggs was determined by egg candling and were placed in an incubator. The patient had no prior history of illness or injury. She had been fed chickens, averaging 3 birds per week, but had been refusing food for approximately 2 months—while gravid. Most gravid pythons exhibit hyporexia/anorexia.
The patient was housed in a zoo enclosure with good sunlight penetration and ad libitum water supply and had never laid eggs before.
5 IU/kg of oxytocin was administered subcutaneously as soon as no eggs were produced for more than 6 hours after egg number 15. Another dose of 5 IU/kg of oxytocin was administered subcutaneously 12 hours after the first dose. With no progress 3 hours after the second dose, the patient was diagnosed with dystocia. External palpation revealed that there were 3 or more eggs in the oviduct; one particularly large egg was suspected to be the cause of dystocia and remained unmoving even after the oxytocin injections and gentle massage. Surgical removal of the eggs was determined to be the best option to preserve the oviduct as well as to minimize dystocia related complications.
Given that the patient had no previous history of being gravid and its fertility status was unknown prior to this lay, the patient was not given extra food to prepare for gravidity, which resulted in the patient having a less than desirable body condition score (2.5/5) during the egg-laying period. The patient guarded the clutch aggressively, as per usual nesting behavior. Dehydration rate was assessed at 3% (slightly dry skin with a skin tent of <3 seconds) post laying, she had pink mucous membranes, appeared responsive to external stimulus, tongue flicking was observed periodically pre- and post-egg-laying. The patient remained alert and did not show any signs of lethargy or sluggishness prior to the procedure. No other significant clinical findings were noted.
Radiographs were taken an hour prior to surgery. Five eggs were identified in the oviduct, with one large egg determined to be the source of obstruction. The radiograph was taken with the patient in the transport tub to minimise stress prior to the procedure.
Intubation was performed with local anaesthetic spray. Manual restraint comprised one hand at the base of the patient’s neck and one person holding the body. Given the aggressive nature of the patient, no manual opening of the mouth was required. The glottis was numbed with Lignoral (lidocaine) spray, and a size 3.5 endotracheal tube (lubricated with a small amount of saline) was inserted half of its full length into the glottis. The cuff was left uninflated. Isoflurane gas was administered at 5% and the Oxygen flowmeter was set to 1L/min to facilitate a quick induction.
A Jackson Reese anaesthetic circuit was used. Intermittent positive pressure ventilation (IPPV) by manual compression of the reservoir bag (500mLs) at 4 breaths/min was started as soon as the tube was inserted.
The patient was placed in dorsal recumbency on the operating table in a U-shape to maximise the body surface area contact to the heating pad. The heating pad was placed under a towel to prevent contact heat stress. Once depth of anaesthesia was determined satisfactory for surgery, the isoflurane gas was lowered to 3% and the endotracheal tube was pushed in almost to its full length into the trachea (excess of 2 inches). The base of the tube and mouth was then secured with micropore tape.
The patient breathed on its own occasionally, but IPPV was maintained at a steady pace throughout the whole procedure. Ultrasound was used to monitor an increase or decrease in heart rate throughout surgery. As the first incision was made, the patient showed a little movement in the upper body due to pain stimulus and isoflurane was increased to 3%. As soon as exposure of the oviduct was deemed satisfactory, the isoflurane was lowered to 2.5% for the rest of the surgery. No other stimulusinduced movement was noted. Once external suturing was complete and topical iodine applied, the isoflurane gas was turned off and the whole anaesthetic circuit was flushed with oxygen (detached from patient) to ensure no residual isoflurane remained in the circuit. IPPV was continued at 4 breaths/minute until the patient started ventilating regularly on its own, approximately 10 minutes after isoflurane flow was stopped.
To monitor self-ventilation, IPPV was stopped for 30 seconds every 2 minutes. Once inspiration was observed, IPPV was discontinued; if inspiration was not continuous, IPPV was resumed for another 2 minutes but at 2 breaths/minute. Subcutaneous tramadol (Analab 50mg/ vial) at a dosage of 10mg/kg and enrofloxacin (Baytril 5%) at a dosage 10mg/kg were administered as soon as the patient showed signs of regular self-ventilation. Subcutaneous 0.9% saline was administered at maximum flow rate during the post operative IPPV period, a total of 150mLs of saline was administered before the patient started moving. Once the patient showed increased movement and stable self-ventilation, it was extubated and placed in an open tub under sunlight with supervision. (Environmental temperature approximately 29-30°C) The patient was observed to be fully alert after 10 minutes of being placed in the sunlit tub.
Location of the incision site was estimated by physical palpation of the largest (obstructive) egg. In larger snakes, or snakes in which the obstruction is not
obviously palpable, radiograph assisted marking would be highly useful.
The surgical site was prepared with a 5% chlorhexidine scrub and sterile saline wash after the scrub. A povidone iodine and alcohol (ratio 1:1) mix was applied after drying the scrubbed area with a sterile towel.
The initial surgical incision was made with a size 20 scalpel blade cutting through scale and subcutaneous tissue. Given the thickness of the scales, the incision was facilitated by applying lateral traction on either side of the site with a thumb and index finger. Once the musculature was observed it was lifted and cut open with an iris scissor to prevent any unwanted puncture to the underlying structures below. Extension of the incision was done with Mayo scissors cutting all layers together. The final incision was 11 centimeters in length.
After cutting the parietal peritoneum, the ventral mesentery and oviduct were immediately observed. However, there was a large amount of vasculature surrounding the mesentery and one large blood vessel on ventral aspect of the oviduct itself. Rather than ligating the blood vessel, the blood vessel and fat was pulled to the left; excision was then done on a location where there was almost no vascularisation on the fascia.
The fascia was scraped off the surface of the egg, with a fresh surgical blade. Scraping is done instead of cutting to prevent the contents of the egg spilling into the coelom, especially in calcium deficient patients where the eggshell is relatively fragile.
The first egg was gently pushed out of the hole in the fascia. The following eggs were massaged downwards by an assistant and were able to be retrieved from the same site. Blood loss was estimated to be 10mLs 2.with the largest blood loss being from the muscle and subcutaneous blood vessels. No sutures were placed on the oviduct.
3/0 Polyglycolic acid (PGA) sutures were placed on the musculature with a modified continuous suture. Sutures were placed firmly but not taut to prevent suture or muscle breakdown. The skin was closed with 2/0 Nylon sutures in a simple interrupted pattern, including a small amount of subcutaneous tissue in each bite to add holding strength.
The skin sutures were spaced 3mm apart. Povidone iodine was applied to the surgical site. Aseptic surgical techniques were observed throughout this procedure.
The patient was transported back to the zoo and kept in quarantine for 5 days. Enrofloxacin at 10mg/kg once
daily was administered orally for 4 days. Tramadol was substituted with meloxicam 0.3mg/kg daily for 4 days, also administered subcutaneously. Treatment was halted as the patient ate a chicken offered 5 days post operation. The suture site was observed to be clean and devoid of any inflammation during feeding. Sutures should typically be removed 6-8 weeks after surgery in snakes. The patient has been feeding steadily since.
In some cases , early surgical intervention may be the best choice to both preserve the oviduct and ensure a healthy recovery by preventing sepsis from egg rupture or egg adhesion to the oviduct, which can arise from a prolonged laying period and extended oxytocin administration.
The surgery was performed on a relatively healthy patient allowing us to minimise anaesthesia-induced oxidative stress on the kidney and liver.
During any surgery involving reptiles, it is suggested to use separate instruments on the scales and the body cavity to maintain sterility, even after thoroughly scrubbing the skin/scales. The external flora on reptiles can become troublesome if introduced into the muscle or coelom.
We did not suture the oviduct as its fascia is extremely thin and since there were no contaminants in the oviduct (egg rupture or necrotic tissue), the introduction of additional foreign material i.e. sutures seemed counterintuitive. If there was any egg rupture/ infection, I would modify the surgery to an OVH without cutting into the oviduct.
Suturing the muscles can be done in various patterns with little to no difference in result. My preferred choice is a modified continuous suture—1 knot every 4 loops. I find it less time consuming than using a simple continuous pattern. For the scales I use a simple interrupted pattern as the external sutures will suffer the most friction and tension, especially since they are placed on the ventral aspect of the patient.
Regarding the fluid administration, while intra-cloacal fluid would have been a better option to preserve the sterility of the surgical site, the subcutaneous route was deemed to be a better choice in this situation. Given that the patient was only at 3% dehydration, it was deemed sufficient.
As no eggs were ruptured and no sign of infection was observed, we did not irrigate the body cavity.
If egg rupture or infection of the oviduct were suspected, the surgery should be modified to a hysterectomy to prevent post-operative complications.
It is important to note that in this case the patient was deemed to be a suitable candidate for surgery (the patient was alert, rapidly responsive to touch and had normal vital signs.)
It would be wise to always consider if the patient needs more time to lay or has other health related issues such as respiratory infections, severe ectoparasite or endoparasite infestation, advanced age, and any history of poor anesthetic induction or intolerance. Some conditions may need treatment prior to the procedure or may even make the patient unfit for surgery entirely. Measuring egg movement daily for up to four days post laying, either via palpation and/or radiography with external marking, can be a helpful tool to determine if the eggs are moving down the oviduct or have become static.
The following non-surgical treatments were attempted prior to surgery. Oxytocin injections with set intervals were given and the manual massage of the eggs was also attempted. Manual massage should be carried out with caution. Occasionally eggs will be adhered to the lining of the oviduct and excessive manipulation may lead to a uterine tear.
Aspiration of the egg via transcutaneous ovocentesis can also be attempted—this technique is often employed if there are only one or two eggs left in utero, they are close to the cloaca and manual massage is unsuccessful.
The technique involves aspiration of the egg contents via the abdomen with a large gauge needle, 20G and above, causing the egg to decompress and then hopefully be passed out naturally by the patient. Pythons should be given adequate pain relief, preferably sedated and sterile technique employed.
This surgery was fully sponsored by Vet Partners Malaysia. The patient was transported and cared for by the amazing team at A’Famosa Safari pre- and post-operatively. The patient has fully recovered and is healthy. I hope that future veterinarians can further streamline this guideline. The framework for this procedure has been built on the methods popularized by Prof Dr Zdenek Knotek.
References
1. J.N Stinner. Functional anatomy of the lung of the snake Pituophis melanoleucus, American Journal of Physiology September 1982; 243; 3; 251-257
2. Emran Ali Algadiem Abdulmohsen Ali Aleisa Huda
Ibrahim Alsubaie, Noora Radhi Buhlaiqah, Jihad Bagir
Algadeeb and Hussain Ali Alsneini, P. Blood loss estimation using gauze visual analogue, Trauma monthly , May 2016
3. Z. Knotek, World Small Animal Veterinary Association World Congress Proceedings , 2015





Figure 1. Dorso-ventral radiograph of the patient. Obstructed eggs are labelled number 2 to 5 and the obstructing egg is labelled number 1; the radiopaque structure to the right of number 1 is a faecal bolus.
Figure 2. Epiglottal opening of the patient
Figure 3. Tape securing the mouth and endotracheal tube
Figure 4. Visceral fat and mesenteric blood vessel
Figure 5. Displacing fat and blood vessels to scrape the fascia 1 4 5 2 3




Figure 6. External sutures
Figure 7. The eggs that were removed whole
Figure 8. Post operative radiograph to confirm no eggs were missed during surgery
Figure 9. Second image since the patient was not fully visualised in the first image (Figure 8)


AM BVSc MANZCVS (Feline Medicine) CertZooMed BA FAVA
Tutor for the CVE’s Reptile Medicine TimeOnline course
This case highlights the surgical treatment of a python, a reptile which is being treated more and more commonly in small animal practice. With respect to the anaesthesia and surgical principles employed, the principles do not differ greatly from those used in small animal mammalian practice; optimal pain relief, anaesthetic induction and maintenance are paramount as is sound surgical technique. Some things may differ; most reptiles breathhold so masked induction is useless, patients usually require ventilation during anaesthesia and wound healing takes a little longer with suture removal at 6-8 weeks post-op.
The author is to be congratulated in presenting this case. Some comments below:
– I would like to see more attention paid to pain relief and the use of injectable anaesthetic induction agents. Some medications are not easily available in Asia, especially ketamine and many opiates, so designing an appropriate anaesthetic protocol may be problematic. Intraoperative local anaesthesia is useful.
– I am not sure that a FBC and biochemistry is justified in an apparently healthy patient. The question that needs to be asked is how would any abnormal results be interpreted, and how would the treatment plan be changed to accommodate these factors?
– Multiple incisions often need to be made as the uterus is difficult to exteriorise due to firm mesenteric attachments.
– My skin suture pattern of choice in pythons is the forward interlocking or blanket suture. It is safe, secure and easy to do. Some clinicians advocate for an everting suture pattern which apparently results in better wound healing. In my opinion, an everting pattern is neither better nor worse than a simple continuous or forward interlocking pattern.
– Several texts have excellent descriptions of the surgical treatment of dystocia – see Reference list.
– Anaesthesia and analgesia notes Pain perception in non-mammalian vertebrates is likely to be analogous to that of mammals. For this reason alone, invasive, and painful procedures should always be accompanied by appropriate pain relief and anaesthesia. Although specific doses may not have been established in clinical trials, clinicians should
Authors’ views are not necessarily those of
attempt to provide reptiles with appropriate analgesia during painful procedures.
– Premedication
Midazolam (1.5-2mg/kg IM) is recommended for all reptile species. In reptiles, other than snakes, acute pain is best managed with mu-opioid agonists. However, in snakes there is limited evidence regarding the efficacy of opiates including mu-opioid agonists and mixed agonist and antagonists (e.g. butorphanol, buprenorphine). Some researchers have advocated the use of hydromorphone (0.5mg/kg IM), fentanyl, and tramadol (5mg/kg IM). Nonetheless, my standard premedication routine for snakes is midazolam IM and morphine (0.4-2mg/kg) IM given 30 minutes prior to induction.
– Anaesthetic induction
Alfaxalone administered intravenously via the ventral tail vein (5mg/kg) is a safe and effective induction agent in snakes, enabling easy handling and tracheal intubation.
– Analgesia
NSAIDs
Meloxicam 0.1-0.2mg/kg IM q24hrs
Ketoprofen 2mg/kg IM
Ensure that patients are adequately hydrated.
Local anaesthesia
Bupivacaine 1-2mg/kg (<4mg/kg maximum), applied topically.
References
Olsson A, Simpson M. Analgesia and Anaesthesia. In: Reptile Medicine and Surgery in Clinical Practice Doneley RT, Monks D, Johnson RSP, Carmel BP (eds.). John Wiley & sons, Oxford, 2018;16:369-381. https://doi. org/10.1002/9781118977705.ch27
Sladky KK, Mans C. Analgesia. In: Mader’s Reptile Medicine and Surgery, 3rd Edition SJ Divers and S Stahl (eds.). Elsevier Inc., 2019:465-474.

New in 2025, these valuable EmergencyLIVE! webinar courses are led by experienced emergency and critical care veterinarians and are FREE to CVE Members.
Each webinar focuses on practical, life-saving techniques—from patient triage and early case management to staying current with gold-standard protocols, including the RECOVER resuscitation guidelines.
With short, focused sessions and plenty of time for Q&A, you’ll walk away with tools you can apply in practice the very next day.
Take advantage of this new major member benefit by signing up to each webinar today.
Non-members very welcome!
cve.edu.au/emergencylive
-Tom Lonsdale
Pete Coleshaw
Jaffa’s Health Centre for Cats
52 St Francis Rd, Salisbury UK
e. jaffa@jaffavets.com
C&T No. 6082
Let me begin with a heartfelt plea: please read on!
Don’t dismiss this book, nor my review, out of hand. The author, Tom Lonsdale, has been vilified by the veterinary profession for decades, and the title itself is undeniably provocative—deliberately so, I suspect. The content, too, might challenge you, suggesting as it does that the veterinary profession has been complicit, knowingly or otherwise, with pet food manufacturers. Surely, we’re too intelligent for that?
Tom Lonsdale is a familiar name in the UK veterinary scene, having stood for the Royal College Council for over 30 years, persistently championing the message encapsulated in this book’s title. In Australia, I imagine he may be perceived as a zealous crank by some. However, this view isn’t shared by the tens of thousands of pet owners who see him as a crusading messiah. In the UK, this divide is striking. Pet owners are leading the way in advocating for biologically appropriate diets, while the profession often lags behind. Are these owners misled, victims of the ‘raw-meaty brigade’? Or is there more to their enthusiasm?
But why is good nutrition controversial? Why this apparent ‘us versus them’ divide? Before delving into the book, I’ll briefly share my own journey toward biologically appropriate feeding—an epiphany that started 30 years ago.
When rabbits began gaining popularity as pets, we in the profession realized how little we truly understood about their care. As veterinary students, we’d received no training on their unique needs. Rabbits were historically raised as meat animals, not as pets expected to live long, healthy lives.
A weekend course with two rabbit specialists changed my perspective entirely. I learned that a staggering 90%
of the medical problems we saw in rabbits were dietinduced. Dental abscesses? Diet-related. Diarrhoea? Often a failure to consume caecotrophs due to dental issues—again, diet-related. Flystrike? A downstream effect of ‘diarrhoea.’ It was a revelation. If nutrition was this pivotal for rabbits, why wouldn’t it be for other species?
This realization led me to explore rawfed.com—a light-bulb moment. I found logical, evidence-based explanations for ideas that had previously seemed implausible. For instance, we were taught that feeding bones to dogs was a recipe for disaster—risking bowel obstructions or worse. Yet, I’d seen farm dogs thrive on a diet of discarded stillborn calves. Foxhounds, too, prospered as waste-disposal units. Could the warnings we’d absorbed as students be overstated?
It was through rawfed.com that I discovered Tom Lonsdale and his seminal book, Raw Meaty Bones. My enlightenment continued—lagging 20 years behind Tom, but better late than never!
In Multi-Billion-Dollar Pet Food Fraud, Tom Lonsdale shares his journey from veterinary training to challenging the pet food industry’s pervasive influence. Having graduated within a decade of Tom, I found his experiences eerily familiar. Over time, we’ve both witnessed the pet food industry’s insidious integration into the veterinary profession. Today, Mars—not just a confectionery giant but a major pet food producer—is the largest owner of veterinary practices globally.
Tom’s arguments, particularly about dental disease in dogs, are compelling. He critiques veterinary dentists for their reluctance to address the root cause: inappropriate diets. Periodontitis is rampant, yet many cling to the belief that raw bones pose an unacceptable risk of tooth fractures. My own experience tells a different story—I’ve extracted far more teeth due to periodontal disease than I’ve seen fractured carnassials. My 13-year-old poodle-cross has a pristine mouth, thanks to raw meaty bones. The evidence is literally in the smile!
Tom’s writing is powerful, but it’s not without flaws. His language is often intemperate, and his tone may alienate some readers. After decades of whistleblowing with little acknowledgment, frustration is understandable—but it doesn’t always make for easy reading. Additionally, while the book is meticulously researched, a handful of statements haven’t aged well in light of new evidence. These, however, are exceptions that don’t detract from the book’s core message.
Tom invites readers to confront uncomfortable truths: that our profession receives scant nutritional training and is vulnerable to influence from pet food manufacturers. While it’s easy to dismiss such claims, consider this: corporations wouldn’t spend billions on advertising and sponsorship if these strategies weren’t effective. Are we really immune to the subtle (or not-so-subtle) effects of selective information?
Tom’s book is more than a critique; it’s a call to arms. It’s well-referenced, including shocking correspondence that illustrates the profession’s resistance to change. The connections he draws between the pet food industry’s tactics and the human food industry are particularly illuminating. For a broader context, I recommend pairing this book with Chris van Tulleken’s Ultra-Processed People: Why Do We Eat Stuff That Isn’t Food—And Can’t Stop?
Multi-Billion-Dollar Pet Food Fraud is not a comfortable read, but it’s an essential one. It challenges us to question entrenched beliefs, confront biases, and prioritise the health of our patients over convenience or convention. Let it change the way you think about pet food—and maybe even your own diet.

1



Paperback – 28 April 2023
2 3 "
For dogs’ sake, read Dr Tom Lonsdale’s books.
_The London Economic
Sarah Collins
C&T No. 6083
A physical examination is often a key part of a consultation and can allow us to glean important information about a cat’s physical health. However, how we prepare for and carry out a physical examination plays an important role in the quantity and quality of the information collected. In this article, we will look at what we can do (and what we should not do) during a physical exanimation.
It is important to always start with a hands-off approach by simply observing the cat from a distance; this allows us to gather information not only about the cat’s mobility, but also about its current emotional state. The cat should always have the choice to come out of its carrier and explore or remain inside it if it so wishes. Having a good understanding of feline emotions, body language and behavioural responses allows us to adapt our approach to each individual cat to help make the physical examination as stress-free as possible.
Make sure that everything required to aid you in your physical examination (e.g. stethoscope, blood pressure monitor, thermometer) is in the room before the cat enters. This removes the noise and stress associated with you leaving and re-entering the room.
As well as having everything to hand, make sure the room is safe (i.e. the cat cannot escape), quiet and clean. Use plug-in synthetic feline pheromone diffusers (keep them switched on all the time and replace them every 30 days) as well as synthetic feline pheromone sprays on blankets, etc.
Always allow cats to acclimatise to their environment before any physical contact is made.
The room should have features to allow the cat to express the behaviours required to cope with the situation it finds itself in, such as a place to hide (but somewhere that the cat can still be easily accessed) and a place to perch (e.g. shelving or windowsills). Aids to help monitor mobility, such as steps, can also be useful (Figure 1).
Allow cats to acclimatise to the environment. Cats that choose to voluntarily leave their carrier (or can be

DipAVN(Medical) RVN
VTS(ECC) Cert CFVHNut ISFM DipFN
Sarah Collins qualified as a veterinary nurse in 1995. Following 11 years in practice, she moved to the University of Bristol, UK, to work in the intensive care unit, where she obtained both the Diploma in Advanced Veterinary Nursing (Medical) and the Veterinary Technician Specialist in Emergency and Critical Care. She then worked as a veterinary marketing executive at Royal Canin before joining International Cat Care in 2017 as the learning organiser for the veterinary nurse course. As Veterinary Education Manager, Sarah is also Managing Editor of Feline Focus and looks after the Cat Friendly Clinic programme and all things nursing related within the charity.



tempted to come out with treats placed on the table [do not offer treats by hand as this can cause conflict for the cat]) should be given time to acclimatise to the room before any physical contact is made. Use this time to chat with the client and get a history/update as well as observe the cat’s body language, behaviours and movement.
Many cats will choose to remain in the relative safety of their carrier rather than voluntarily come out. With good client education, we can ensure that cats are presented to us in an appropriate carrier where the top can easily (and quietly) be removed, allowing the cat to remain in the base (Figure 2). Cats that choose to remain in their carrier are often trying to hide from the situation they find themselves in.
Gently draping a towel or blanket over them once the top of the carrier has been removed can help the cat feel more secure (Figure 3) This can then be gently moved to expose specific parts of the cat for examination one at a time.
Allow cats that are happy to leave their carrier to explore; let them jump to the floor or sit on shelves/ windowsills if they so wish. Examine the cat where it is most comfortable. It is up to us to adapt to the cat rather than expecting the cat to remain patiently on the table! This may mean sitting on the floor to conduct some or all the examination.
Allow the cat to sniff equipment: cats have a strong sense of smell, so allow them to acclimatise to any equipment used by allowing them to sniff it first.
Ask yourself – ‘is this cat in pain?’ If the cat is known or suspected to be in pain, then the most appropriate first line of action is to provide analgesia. Give this time to take effect before commencing the physical examination.
Approach the cat in a gentle, calm manner. Do not make direct eye contact; instead, blink slowly in the direction of the cat. Always move slowly and deliberately, and hold out a soft hand towards the cat; the cat can then choose to come to sniff the hand and interact further.
Remember the CAT acronym:
– C – Did I give the cat a CHOICE about whether it wanted to interact with me?
– A – Am I paying ATTENTION and looking out for any subtle signs that the cat is uncomfortable?
– T – Where am I TOUCHING the cat and does the cat want me to keep touching?
Start at the least invasive/painful part first. Beginning a physical examination by taking a rectal temperature is setting yourself up for failure! Know your patient and start with the areas that it is more comfortable with being touched, leaving the least preferred areas until the end of the examination. Most cats dislike touch over the caudal area, the abdomen and feet. Always monitor your patient’s body language and behaviour and stop if it gives you signals to do so.
Start with respiratory rate first—this can be carried out from a distance using a hands-off approach. Follow this with thoracic auscultation; most cats are accepting of a stethoscope placed gently on them, especially if no physical restraint is involved. This will provide you with key baseline information should the cat need to be sedated. Heart rate and murmurs can change during the examination, so it is useful to auscultate the thorax at various points during the examination to make sure nothing is missed. When auscultating the thorax, position yourself behind or to the side of the cat, and start along the lateral thorax. From the opposite side, slowly place your other hand under the sternum, gently lifting the cat slightly to facilitate moving the stethoscope over the heart at the sternum. Avoid unnecessary pressure to help prevent induced murmurs and be cautious with movement near the elbows of older cats, which may be arthritic.
Examine the ears and eyes if indicated. For otoscopic evaluation, holding the ear at the base of the pinna eases cone insertion and allows gentle canal extension for better tympanic visualisation (Figure 4).

Be cautious of face-to-face contact when performing ophthalmic examinations; always approach from an angle and avoid direct eye contact.
Take regular breaks—pausing between each element of the physical examination allows the cat to have a break and gives it the choice on what to do next. This is a dynamic process, and you must be prepared to adapt and slow down when required in order to complete the examination. Treats can be used as a distraction for confident cats that become frustrated during the examination.
Encourage positive emotions throughout the physical examination, and always aim to end on a positive note. If the final part of the examination is a negative experience (e.g., assessment of a painful area), try to leave the cat with a positive association of the visit by passively providing treats or other positive reinforcers.
After the examination, record in the patient’s notes what worked and what did not work, to help avoid protective behavioural responses and escalation at future visits. This alerts the wider team of the need to discuss with the owner the scheduling of appointments at a quieter time and/or of longer duration, and that preparation before the appointment may be required.
Don’t be noisy. Speak in a calm, quiet voice and make sure that there are no loud noises (including those coming from outside the room).
Don’t rush. Try to make sure all cat appointments are at least 15 mins (longer for procedures such as blood
pressure/sampling, or for cats known to be anxious/ fearful, etc). Take your time to get the information you need; less haste = more speed!
Don’t stare at cats. Direct eye contact is threatening to cats, so always direct your gaze away from their eyes. Always approach from an angle and avoid direct eye contact.
Don’t use surgical spirit or water to reduce excessive purring when auscultating the thorax. Instead, distract the cat by altering the lighting, providing visual access to a window, or using squeaky/chirpy toys. If necessary, reassess after any sample collection.
Don’t ramp up the restraint.
Avoid grabbing, rough handling or holding the cat down firmly – these generally increase fear–anxiety and/or frustration and will potentially make the cat struggle harder or resort to repelling behaviours leading to distress in the cat and injury to yourself. They also leave a lasting negative impression on the cat that may make future visits difficult as the cat may show the same repelling behaviours (and more) at the next visit.
Scruffing should never be used and certainly not for lifting. Grabbing and immediately scruffing is intimidating and leaves the cat no room for manoeuvre except for defensive behaviours. The same goes for ‘clipnosis’. Restraint equipment such as cat bags, gauntlets and muzzles should not be used. Instead, use items such as towels or blankets (Figure 5) that provide the patient with comfort, a sense of safety and a sense of choice along with positive distractions. With cats showing repelling behaviours, take a break and consider sending the cat home and prescribing anxiolytics or sedation.
Remember – there are no bad cats, just scared ones!

For otoscopic evaluation, holding the ear at the base of the pinna eases cone insertion and allows gentle canal extension for better tympanic visualisation.
For ophthalmic examinations, always approach from an angle and avoid direct eye contact.
Rodan I, Dowgray N, Carney HC, et al. 2022 AAFP/ISFM cat friendly veterinary interaction guidelines: approach and handling techniques. J Feline Med Surg 2022; 24: 1093–1132.
• Taylor S, St Denis K, Collins S, et al. 2022 ISFM/AAFP cat friendly veterinary environment guidelines. J Feline Med Surg 2022; 241; 133–1163.
This article was presented at the ISFM Congress Malta in June 2024.
The International Cat Care Veterinary Society (formerly ISFM) is the veterinary membership division of pioneering cat welfare charity International Cat Care (iCatCare), bringing together a global community of veterinary professionals dedicated to improving the lives of cats worldwide. Trusted by vets and nurses, it provides professional development and CPD through access to expert knowledge resources, including the Journal of Feline Medicine and Surgery (JFMS) The iCatCare website is also a trusted resource of information and guidance for veterinary professionals, cat owners and caregivers.
Welcome to Research Roundup, where we bring you summaries of the latest feline research. This month, we look at the importance of using the same laboratory if comparing samples, how insulating limbs and feet can help prevent heat loss under anaesthesia, the pressure on caregivers when looking after a cat with chronic kidney disease and some important results around faecal transplantation for cats with chronic enteropathy.

Read here: cve.edu.au/rr-june-25

Our extensive program offers you the chance to immerse yourself in a particular discipline in the company of like-minded peers. It is ideal as the first step towards the ANZCVS membership exams and veterinary excellence.
Learn at a steady pace—module by module—and implement new knowledge and expertise at your practice as you progress through the comprehensive and challenging course content.
Engage with your dedicated tutors and benefit from first-hand feedback on your assignments.
Share cases with your tutors and fellow participants on the online group discussion forum.
Benefit from individual and collective learning.
Choose from a wide range of Distance Education courses designed to deepen your expertise across core disciplines including Small Animal Medicine, Dermatology, Surgery, Dentistry, Anaesthesia, Cardiology, Diagnostic Imaging, Oncology, Ophthalmology, Emergency & Critical Care, and more.
CVE members save 10% on enrolments before 30 June 2025

This course presented by Taronga will support veterinary professionals and veterinary nurses to develop knowledge and skills in native wildlife triage, including first aid, initial treatment and emergency care.
• Supported by the NSW Government Department of Planning and Environment
Email: tarongprofvet@zoo.nsw.gov.au
Visit: https://taronga.org.au/vet-professional-training
• AVA and VNCA certified
• Online Course (20 CPD points)
• Hands-on Workshop (12 CPD Points) –available in NSW, QLD and Vic (see website for details)
• Subsidised positions available to eligible applicants nationally




