

The Murmur Initiative
Test Yourself VLAS module
When referral to a cardiologist is unavailable or declined, the Australasian Veterinary Cardiology Advisory Board (AVCAB)† recommend the use of vertebral left atrial size1 (VLAS) to assist in the diagnosis of Stage B2 MMVD2, to determine when it is appropriate to commence treatment with Vetmedin®.
This interactive VLAS module allows you to practice measuring the VLAS on six thoracic radiographs and to then compare your results to the experts.
Proudly developed with the assistance of:
Associate Professor Fiona Meyers
BVSc(Hons) PhD MANZCVS DACVIM, Specialist Veterinary Cardiologist;
Dr Brad Gavaghan
BVSc(Hons) MANZCVS FANZCVS, Specialist Veterinary Cardiologist;
Dr Christopher Lam
BA BVSc (Hons) Diplomate, ACVIM (Cardiology) Specialist Veterinary Cardiologist
† The Australasian Veterinary Cardiology Advisory Board: Dr Brad Gavaghan BVSc (Hons) MANZCVS FANZCVS (Cardiology), Dr Jacqui Huxley BVSc DVC MVM MRCVS,
Associate Professor Fiona Meyers BVSc (Hons) PhD MANZCVS Dip ACVIM (Cardiology), Dr Rita Singh BSc BVMS (Hons) Dip Vet Clin Stud FANZCVS Dip ACVIM (Cardiology), Dr Richard Woolley BVetMed Dipl ECVIM-CA (Cardiology).



Step 1:
Select the centre of the most ventral aspect of the carina.
Step 2:
Now select the most caudal aspect of the left atrium where it intersects with the dorsal border of the caudal vena cava
The line will be your VLAS measurement.
Step 3:
Select the cranial edge of T4 just ventral to the vertebral canal.
Step 4:
Use the virtual calipers to produce a line of equal length beginning at the cranial edge of T4 and extending caudally just ventral and parallel to the vertebral canal.
Step 5:
The VLAS is defined as the length of this line expressed in vertebral-body units to the nearest 0.1 vertebra. One vertebral-body unit extends from the cranial edge of one vertebra to the cranial edge of the next, and includes the inter-vertebral disc space.
Once you identify the VLAS measurement, enter it into the field opposite.
Simply go to: www.animalhealthacademy.com.au/murmurinitiative
When registering for Boehringer Ingelheim Animal Health Academy the first time, please use the access code: myAcademy
C&T
Issue 318 | March 2025
Control & Therapy Series
PUBLISHER
Centre for Veterinary Education
Veterinary Science Conference Centre Regimental Drive
The University of Sydney NSW 2006 + 61 2 9351 7979 cve.marketing@sydney.edu.au cve.edu.au
Print Post Approval No. 10005007
CVE Director
Associate Professor Kate Patterson kate.patterson@sydney.edu.au
EDITOR
Lis Churchward elisabeth.churchward@sydney.edu.au
EDITORIAL ASSISTANT
Dr Jo Krockenberger joanne.krockenberger@sydney.edu.au
VETERINARY EDITOR
Dr Richard Malik
DESIGNER
Samin Mirgheshmi
ADVERTISING
Lis Churchward elisabeth.churchward@sydney.edu.au
To integrate your brand with C&T in print and digital and to discuss new business opportunities, please contact: MARKETING & SALES MANAGER
Ines Borovic ines.borovic@sydney.edu.au
DISCLAIMER
All content made available in the Control & Therapy (including articles and videos) may be used by readers (You or Your) for educational purposes only.
Knowledge and best practice in this field are constantly changing. As new research and experience broadens our knowledge, changes in practice, treatment and drug therapy may become necessary or appropriate. You are advised to check the most current information provided (1) on procedures featured or (2) by the manufacturer of each product to be administered, to verify the recommended dose or formula, the method and duration of administration, and contraindications.
To the extent permitted by law You acknowledge and agree that:
I. Except for any non-excludable obligations, We give no warranty (express or implied) or guarantee that the content is current, or fit for any use whatsoever. All such information, services and materials are provided ‘as is’ and ‘as available’ without warranty of any kind.
II. All conditions, warranties, guarantees, rights, remedies, liabilities or other terms that may be implied or conferred by statute, custom or the general law that impose any liability or obligation on the University (We) in relation to the educational services We provide to You are expressly excluded; and
III. We have no liability to You or anyone else (including in negligence) for any type of loss, however incurred, in connection with Your use or reliance on the content, including (without limitation) loss of profits, loss of revenue, loss of goodwill, loss of customers, loss of or damage to reputation, loss of capital, downtime costs, loss under or in relation to any other contract, loss of data, loss of use of data or any direct, indirect, economic, special or consequential loss, harm, damage, cost or expense (including legal fees).
and Epileptic Seizures in Dogs
Engage With Your Profession
The C&T forum gives a ‘voice’ to the profession and everyone interested in animal welfare.
You don’t have to be a CVE Member to contribute an article or reply to a 'What's YOUR Diagnosis?'. We welcome contributions from Vets, Techs, Nurses, allied professionals and anyone interested in animal welfare—Non CVE Members included.
Submit your C&T article for publication
You can find a simple WORD style template to assist in setting out your article and also information about how to upload high resolution images required for print on this page of our website. cve.edu.au/submit-article
Questions?
Please contact cve.marketing@sydney.edu.au
Join In!
The C&T is not a peer-reviewed journal. Rather, it is a unique forum allowing veterinary professionals to share their cases and experiences with their colleagues.
We are keen on publishing short, pithy, practical articles (a simple paragraph is fine) that our members/readers can immediately relate to and utilise such as the possum box design article on page 37. Our editors will assist with English and grammar if required.
"I enjoy reading the C&T more than any other veterinary publication.
-Terry King, Veterinary Specialist Services, QLD
Thank You to All Contributors & Advertisers
The C&T Series thrives due to your generosity.
Major Winners
Prize: A CVE$500 voucher
When a Nailbed Lump Looks Nasty But Isn’t! Chantal Celindano
Emphysematous Cystitis with Coagulum Formation in a Dog Max Sleeman
Winners Prize: A CVE$100 voucher
Mycobacterium goodii Infection in a Cat Samantha Crothers
Systemic Amyloidosis in a Cat Presenting with Acute Haemoabdomen & Hepatic Rupture Alexander Teh
Best Visuals
Prize: A CVE$300 voucher
Possum Box Design Niko Clemente
What's YOUR Diagnosis? Replies
Prize: Vouchers
Robert Bird CVE$300
Kayla Hamilton & Tomas Gilead CVE$100 each

Centre for Veterinary Education Est. 1965
Dear CVE Members and Readers
As the cover proudly announces, 2025 marks a significant milestone—the 60th anniversary of the Centre for Veterinary Education (CVE). We’re thrilled to celebrate this momentous occasion with our incredible CVE community. There’s so much to look forward to, and we’ll be sharing more details soon through our channels—so stay tuned!
This editorial marks my first as the new CVE Director, and I’m delighted to be honouring two exceptional individuals from our community. Dr Carolyn O’Brien is the 2025 Hungerford Award recipient and is recognised for her excellence in continuing veterinary education and her remarkable and enduring contributions to veterinary science. Dr Craig Kelly is the recipient of the inaugural CVE Scholarship and is recognised for his outstanding dedication and commitment to professional development. I know you will join me in congratulating them both for their respective achievements and exceptional dedication to the advancement of veterinary science.
A heartfelt thank you to all our contributors to the C&T Series. We know how busy life can get, yet you still make time to share valuable, practical insights with your peers. The quality of these articles is outstanding, and we wish we could reward everyone. This issue, we’re proud to present two contributors with the major CVE $500 voucher prize.
As we celebrate this milestone year, I encourage you to share your own stories and insights, especially in response to previous articles. The essence of CVE has always been about learning through experience, and I believe we can all gain so much from each other's journeys.
I look forward to connecting with many of you throughout the year and, as always, feel free to reach out anytime.

Associate Professor Kate Patterson Director
The CVE Continuing Education Scholarship
Congratulations to Dr Craig Kelly !

"Of Blaxland Veterinary Hospital, NSW, the winner of the inaugural CVE Continuing Education Scholarship in our 60th anniversary year.
Craig was selected from a very competitive cohort of over 50 applicants due to his demonstrated commitment to continuing veterinary education and professional development. He has extended himself through volunteering to broaden his veterinary skillset to treat a wide range of species outside his key expertise. His dedication to wildlife treatment and rehabilitation, his vision for the profession and efforts to enable other veterinarians to be supported in their pursuit of quality veterinary care and rehabilitation for wildlife is exemplary.
We are proud to be able to support you and to empower your learning journey.
I’m very honoured and humbled to be the recipient of the CVE Continuing Education Scholarship.
Wow! I feel like Charlie Bucket who has won The Golden Ticket. The proverbial kid in the candy store.
As an undergraduate, in 1991, I remember sitting in the Carslaw building, attending lectures for what was then the Post Graduate Foundation, now the CVE. I also remember going into the Pitt Street offices to buy Vade Mecums on a variety on species (very useful books for the new grad in the predigital age). I also had an arrangement with the director Doug Bryden to get audio tapes of the major conference each year to listen to while commuting between Canberra, Sydney and Newcastle.
Even now, I’m amazed how many WebinarLIVE!s and PLUSs fit my exact area of current ignorance. In short, the PGF/CVE has always been with me on my career fuelling my passion to learn more. And now rather than buying ‘10 cents of mixed lollies’ I can buy what feels like the whole lolly shop.
‘I’ll have three DE Anaesthesia’s and five exotics TimeOnlines please!’ I’m so grateful to be able to take a deep dive into these areas of Veterinary Education to get better each day because I still LOVE being a vet.
_Craig Kelly
2026 CVE CE Scholarship Applications
Open Wednesday 1 September 2025
Close Sunday 30 November 2025
This membership scholarship is valued at AU$8,000 and can be used toward enrolment into any CVE continuing education course(s) commencing in the year the scholarship is awarded. For eligibility, visit cve.edu.au/ce-scholarship
Kate, the CVE Director, presented the award to Craig at our feline conference in February.
The 2025 T. G. Hungerford Award
Congratulations to Dr Carolyn O’Brien!
We’re delighted to announce Carolyn as the 2025 recipient of this prestigious award for her profound and multifaceted contribution to continuing veterinary education, dedication to advancing feline medicine and research on zoonotic pathogens that has shaped the field globally.
Kate, the CVE Director presented the award to Carolyn (pictured left to right, respectively) at our recent highly successful ‘VC Feline Chronic Disease Management Conference’ in February.
The framing includes a silver coin featuring a Goanna—the CVE’s motif—in tribute to Tom’s famous philosophy: Follow the Goanna Track to Success.

I am deeply honoured and humbled to receive the TG Hungerford Award for 2025. To be recognised among such distinguished recipients is an extraordinary privilege.
My heartfelt gratitude goes to the Sydney School of Veterinary Science and the Centre for Veterinary Education. Their unwavering support, collaboration, and shared dedication to feline medicine have been invaluable throughout my journey. I have had the privilege of working alongside some of the most brilliant and passionate veterinarians, whose knowledge and dedication have both challenged and inspired me at every step.
In particular, I extend my deepest gratitude to my friend and mentor, Professor Richard Malik, whose encouragement and guidance have been instrumental in shaping my career.
It is truly heartening to reflect on how far we have come in advancing feline health. I remain excited for the future, eager to see the discoveries and innovations that will continue to improve the lives of cats and the people who care for them.
_Carolyn O'Brien
Carolyn joins the Honour Roll of past recipients of the Award, all giants in the profession:
2025 Carolyn O’Brien
2024
Linda Fleeman
2017 – 2023 Not awarded
2016
2015
Terry King
Stephen Page
2012 – 2014 Not awarded 2011
Stephen Holloway 2010
Robin Stanley 2009
Boyd Jones
2008
Karon Hoffmann 2007
Paul Canfield 2006
Wing Tip Wong & Glen Edwards 2005
Victor Menrath
Russell Mitten 2003 Not awarded 2002
Richard Malik
David Church
Jill Maddison 1995 – 1999 Not Awarded
D.C. Blood
Graeme Allan
Richard LeCouteur
Reuben Rose
Susan Shaw
Christopher Bellenger
Jakob Malmo
R.R. Pascoe
cve.edu.au/cve-awards
HUNGERFORD AWARD CITATION FOR 2025 Dr Carolyn O’Brien
The T.G. Hungerford Award was established on the retirement of Dr Tom Hungerford OBE who contributed significantly to the veterinary profession. The Hungerford Award honours the tradition of recognising excellence in continuing veterinary education.
“ So splendid is their performance; they adorn the awards for excellence now conferred upon them.
T.G. Hungerford OBE
Dr Carolyn O’Brien is a worthy recipient of the Hungerford Award for 2025 for her commitment to continuing veterinary education and her dedication to advancing feline medicine.
Dr O’Brien has transformed clinical practice and research in feline medicine. After graduating from the University of Melbourne in 1994, Dr O’Brien worked in general practice in Australia and the UK, developing a special interest in feline medicine. In 2000, she began a competitive feline medicine residency at the University of Sydney, marking the start of her career in feline health. Her professional development was underscored by her Membership and Fellowship status with the Australian and New Zealand College of Veterinary Scientists (ANZCVS) in Feline Medicine, establishing her as a leader in the field.
Dr O’Brien’s academic journey is impressive. She earned a master’s degree focused on the diagnosis and treatment of canine and feline cryptococcosis, a significant fungal infection. Recently, she completed a PhD at the University of Melbourne, where her research into mycobacteria in cats and Australian wildlife shed light on zoonotic infections and feline leprosy. Her work has advanced the understanding of feline mycobacterial infections, particularly those caused by Mycobacterium species, impacting veterinary and public health.
Dr O’Brien’s scholarly output is prolific. With nearly 1,900 citations and an h-index of 24, her work is widely recognised. Her 2010 publication, ‘A Major Role for Mammals in the Ecology of Mycobacterium ulcerans’ published in PLoS Neglected Tropical Diseases, has over 230 citations, highlighting the role of wildlife in zoonotic disease spread. This research has advanced the understanding of M. ulcerans, responsible for Buruli ulcer, affecting both humans and animals. Her retrospective study on cryptococcosis, published in Medical Mycology (2004) with over 160 citations, remains a comprehensive analysis of the condition in Australia. This study has guided therapeutic approaches and influenced clinical protocols. Additionally, her work on therapy outcomes for cats and dogs with cryptococcosis, published in the Australian Veterinary Journal (2006), has over 100 citations and serves as a reference for practitioners.
Dr O’Brien has made strides in understanding mycobacterial infections in cats and dogs, with her 2013 paper in Veterinary Dermatology cited over 70 times. Her contributions have enhanced diagnostic and therapeutic strategies and underscored the zoonotic potential of these infections. She is also the first to describe melioidosis in cats.
Dr O’Brien’s impact extends beyond publications. As the current Director and Feline Medicine Specialist at Melbourne Cat Vets, she provides expert care for feline patients and serves as an educator. She and her team have treated over 100 cats with FIP in the last two years, achieving an impressive success rate. For over 12 years, she has led the Centre for Veterinary Education’s Feline Medicine Distance Education program, inspiring the careers of many veterinarians.
This has created a strong relationship between the Centre for Veterinary Education (CVE) and International Cat Care. Dr O’Brien excels at simplifying complex clinical concepts into accessible content for veterinarians and students. She is also an engaging speaker at national and international events.
The Feline Medicine Distance Education program is one of the most successful continuing education programs provided by the CVE. Although initially developed by Dr Rob Labuc, it was extended by Dr O’Brien in collaboration with Dr Andy Sparkes from International Cat Care, with input from others. It involves up to eight tutors and attracts participants from Australia, New Zealand, the UK, Europe, North America, and Southeast Asia. It is a primary program for those preparing for the Membership of the ANZCVS in Medicine of Cats. Dr O’Brien has also been a keynote speaker at several feline conferences and initiated a journal club and case discussion forum for clinicians in Melbourne.
Her clinical interests span infectious diseases, cardiology, dermatology, feline behavior, and neurology. Dr O’Brien has contributed to understanding feline mycobacterial infections, and her research on zoonotic pathogens shapes the field. Her publications influence veterinary standards of care globally and foster collaboration between veterinarians and public health officials. Her leadership in feline medicine has advanced knowledge in critical areas like feline infectious peritonitis.
Dr O’Brien’s dedication to the profession, excellence in research and practice, and commitment to continuing education make her an exemplary recipient of the Hungerford Award for 2025. Her work has significantly influenced feline medicine and contributed to public health efforts through studies of zoonotic diseases.
Dr Carolyn O’Brien is a distinguished scholar, compassionate clinician, and passionate educator whose contributions deserve this prestigious honour.
The Centre for Veterinary Education of The University of Sydney

Have you ever examined how your practice handles its sterile goods?
Are you aware of the financial, environmental, or labour
impacts of these
processes?

Your clinic likely sterilizes surgical instruments and implants using one or more of the following methods:
Single-use blue wraps around some form of basket
Single-use paper or sealable plastic pouches
Reusable cloth drapes
Reusable rigid sterilization containers
Single-use wraps (or blueys) are inexpensive and widely available. However, they are designed for one-time use and then discarded, contributing significantly to a practice’s waste stream, which ultimately ends up in landfills. Australians produce approximately 21.6 billion tonnes of landfill each year, with hospital single-use goods being a notable contributor.¹ Additionally, blueys cannot act as rigid barriers, and sharp instruments can perforate them, compromising kit sterility. If accidental perforation occurs, it’s unlikely you would even be aware of it. Wrapping a kit also takes time, which is something most nurses have in short supply.
Single-use paper or sealable plastic pouches are also inexpensive and commonly available. They allow small quantities of instruments to be contained and sterilized but also end up as landfill. Being non-rigid, they are also susceptible to accidental perforation.²
Reusable cloth drapes are relatively expensive and have been used for many decades. Although they are disposed of infrequently, they need to be washed and dried after each use. Consider the water, detergent, electricity, and nursing time required for each use.³
Reusable rigid sterilization containers are typically made of aluminium. While they require a larger upfront cost, their lifespan is incomparable, as rigid containers can last for more than a decade. If you calculate the processing cost per kit, a practice can see savings as soon as the second year of ownership, simply by comparing it to the cost of consumables that would have been used instead.⁴
References:
3. Exploring the Safety and Environmental Impact of Sterilization Techniques.
4. Sustainability | Reducing the Environmental Impact of Sterilization Packaging for Surgical Instruments in the Operating Room: A Comparative Life Cycle Assessment of Disposable versus Reusable Systems (mdpi.com)
MAJOR Winner
The prize is a CVE$500 voucher
When a Nailbed Lump Looks Nasty But Isn’t!
Nodular Fibrovascular Proliferation, With Giant Cells and Osseous Metaplasia
Chantal Celindano
BVSc(Hons) MANZCVS (Medicine of Cats) ISFM AdvCert FB
Feline Fine Mobile Vet
e. meow@felinefinevet.com.au
w. felinefinevet.com.au
t. 0422 941 661
C&T No. 6055
Chantal Celindano BVSc(Hons) MANZCVS (Medicine of Cats) ISFM AdvCert FB has dedicated her 20 odd years in practice to feline medicine, working at cat hospitals in Melbourne, Sydney, Brisbane and London. She has obtained post graduate qualifications in Feline Medicine and Feline Behaviour and is an ISFM Advanced Practitioner and ANZCVS mentor. In her spare time, Chantal also runs a house-call clinic exclusively for cats called Feline Fine Mobile Vet in Brisbane Australia. Her passion is addressing the cat as a whole, within its environment as well as preventative medicine to give cats the highest quality of life possible, as every cat deserves. She is assisted by her vet-in-waiting daughter Vivi and her office admin assistant Meiko Nuggetini—a rescue Tonkinese vying for laziest cat alive.
Who is Roger?
Roger is a 10-year-old male desexed domestic shorthair cat. He weighs 4.8kg and is utterly adorable.
Roger presented with a painful toe on his third digit of his right foreleg on 9 May. He had been overgrooming it persistently but otherwise was his normal self.
Seemingly a straightforward case, there were of course complications due in part to being feline (so would not be reading the textbooks, thank you), owned by a veterinary nurse and also a tripod (having lost a hind leg as a juvenile at 9-months-old).
The toe had a swelling on the medial aspect of the nail bed which was fleshy in nature, dark pink in relation to the rest of the nail bed, ovoid and with a small granulomatous focus of infection present on the palmar surface. His nail could not be retracted. It did also

have a distinct malodourous air to it. Owing to Roger’s tripod status and impressive development of his foreleg muscles, it was postulated that he injured his nail pulling himself up onto a surface or doing a deadlift at the gym. The injury did not seem to hinder his mobility.
Initial Therapy
Initial therapy involved meloxicam at a 4kg dose (Metacam) and amoxycillin-clavulanic acid (Amoxyclav) (75mg twice daily) owing to the purulent discharge and swelling on presentation. No improvement was seen after 10 days. Purina Fortiflora was used post-antibiosis, and an e-collar was employed to reduce self-trauma.
Next Steps: X-ray & Debulking Surgery
Roger then underwent chest and foot radiology on the 21 May. No bone lysis or involvement was present on the digital lesion. Chest radiography dorsoventral and lateral were within normal limits, with no evidence of metastatic lesions (i.e. NO lung digit syndrome). At this point, the lump was debulked with a scalpel under IV sedation and local anaesthesia as Roger is the bestest boy. The lump was approximately 0.6cm x 0.4cm in size, and ovoid; it was sent for histopathology. It had a caseous 1mm area in the centre of the lesion when dissected in half.

Figure 1. The lesion near the nail bed
Figure 2. Roger and I—he tolerated his doughnut collar very well!


AHistopathology
NODULAR FIBROVASCULAR PROLIFERATION, WITH GIANT CELLS AND OSSEOUS METAPLASIA
These findings are compatible with those described for a novel nailbed lesion of cats as documented by Melanie Dobromylskyj et al (2017). This entity appears to be a distinct subset of digital lesions in cats, located either at or close to the nailbed epithelium. The clinical and histologic features are similar to giant cell reparative granulomas described at other sites and locations, and as such it is possible that trauma, infection, or other injury to the nailbed may contribute to the development.
These distinctive lesions are thought to be reactive/ reparative in nature but have potential for local recurrence (5/22 cases with follow-up in this study recurred post excision).
To my knowledge, metastasis has not been reported. Male cats appear to be predisposed and the second digit is most commonly affected. These fleshy, protuberant nodules are described as ranging from 1-20mm in size and are usually ulcerated and inflamed. There may be reactive bone changes but are not destructive. As seen in this case, histologically, these lesions are composed of proliferative fibroblast-like cells accompanied by multinucleated giant cells and frequently, regions of osseous metaplasia.
Recurrence of the Lump
The lump (now affectionately named Herman) continued to re-grow post operatively despite persistent use of meloxicam, daily Malaseb soaks and e-collar usage. I contacted Dr Malik (CVE) on 13 June.
Suggested courses of action
If it returned—amputation has been cited as curative (P2/P3 amputation)—but owing to Rogers distinct lack of digits at the time, and the painful recovery, we were focussed on avoiding this!
Employing a laser at a specialist such as a dermatologist to remove the nail bed rather than the entire digit
A second debulking surgery, with the use of cautery , laser +/- acid
Intralesional or topical trichloroacetic acid injections which had been documented to aid eyelid apocrine hidrocystoma therapy (Pigatto et al 2021)
Use of a high-speed dental drill in lieu of the above for more precision
Referring to Dr Melanie Dobromylskyj who had written a paper on this condition Clinical, histological and prognostic features of a novel nail-bed lesion of cats: 41 cases.
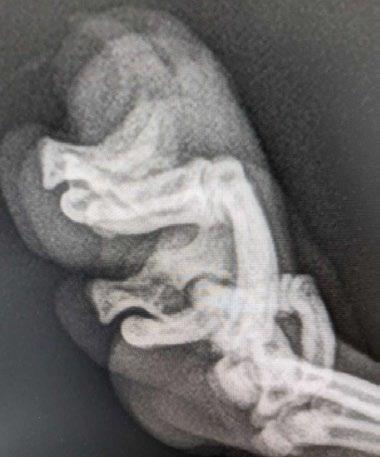
Figure 3 A B and C. Radiographs of the chest and distal limb


5 6



Figure 4. The lesion recurs. Damn!
Figure 5. Toe pre-op on 17 July
Figure 6. Toe during surgery after debulking
Figure 7a & 7b. Toe after cautery and formalin
7b


Figure 8. Post-op: swelling present
Figure 9. 7 August post-op
Figure 10. 17 September
Figure 11. October: 100% healed!
Figure 12. Roger 8 9


Getting The Diagnosis Verified
Dr Dobromylskyj confirmed that the photos of Roger’s lesion looked typical to those she had encountered and correlated with the histopathology report. In her experience, full excision could spare toe amputation.
Second Surgery
Unfortunately referral was not possible and trichloroacetic acid was only available in large quantities with long lead times. On 17 July, we made the decision to debulk with Dr Malik’s suggestion of a dental drill to aid precision and substituted trichloroacetic acid with formalin and cautery.
Roger continued to wear his fashionable collar and receive meloxicam daily with food.
Results
Though this method of therapy for this condition has not hereto been documented and is a GP’s version of hypothesised treatment modalities, the result has been favourable! Roger has had a full recovery and will live to see another day without the loss of a single toe-bean!

Comments Courtesy of Melanie Dobromylskyj
BSc Vet Path (Hons) BVSs PhD FRCPath MRCVS
Roger’s mass (‘Herman’) sounds like a classic example of this entity and it is so great to hear the treatment has been successful in his case. I first decided to study these lesions when I realised that pathologists received them all the time, but no-one was totally sure what they actually were! I love a weird feline lesion, so I decided to collect some cases together to try and work out whether these were actually tumours and, if so, did they have any metastatic or local recurrence potential, or were they just a strange reactive process. Given they contain multinucleate giant cells, areas of bone formation and proliferative spindle cells, down the microscope they can look quite scary, so I was really happy when we demonstrated that complete excision was curative and these appeared not to be a tumour at all.
As some of the possible differential diagnoses have a much poorer prognosis, it is very important to get the diagnosis correct. An osteosarcoma (of the multinucleate cell type), a nerve sheath tumour with osseous metaplasia (another weird thing cats like to do) and an undifferentiated pleomorphic sarcoma must be thoroughly excluded—from personal experience, I have diagnosed all of these entities at this site in cats.
One of the key features we found in our study was an absence of any underlying bone involvement on radiography—as seen in Roger’s case—and therefore radiographic assessment is important. This together with an exophytic growth pattern, the presence of ulceration
and of neutrophilic inflammation were also key histological features and, in some ways, they were the most consistent features in the cases we reviewed, as opposed to the mitotic count for example. This is one of those conditions where it is vital to have all the different pieces of information in order to reach the correct diagnosis—the gross appearance, the precise location, the radiographic findings and the histological features are all important pieces of the diagnostic puzzle.
In our study, we had a small number of cases with local recurrence, but with complete excision these masses are curable. We did not have any cases with more aggressive growth, nor any evidence of metastasis. We could only speculate as to possible causes, but we did suspect trauma could play a role at least in some cases—I hope Roger will look after his remaining toe beans a bit better from now on!

Figure 1. An overview at low power of a mass probably very similar to Roger’s, showing the exophytic nature of the mass, the extensive areas of ulceration (red arrows) and areas of bone formation (asterisks).

Figure 2. A high -power image showing some of the multinucleate giant cells (asterisk) with a background of proliferating spindle cells, one of which contains a mitotic figure (red arrow).
PetFAST
Pet owners deserve quality pet food which supports their pet’s health and wellbeing, but what happens when things go wrong? This is where PetFAST can help.
What is PetFAST?
PetFAST is a joint initiative that was introduced in 2012 by the Australian Veterinary Association (AVA) and The Pet Food Industry Association of Australia | PFIAA (PFIAA) and is managed by a vet from the AVA and the PFIAA Executive Officer.
Much like the APVMA online adverse experience reporting tool, PetFAST is a voluntary reporting system to track clinical cases in cats and dogs that have a suspected link to pet food (all formats, including treats, meat and supplements).
Only veterinarians can lodge a PetFAST report.
An adverse event can be described as an event where an unintended consequence (mild, moderate, severe or suspected) may be linked to consumption of pet food. All types of pet food are monitored through the PetFAST system, which collects reports from veterinarians throughout Australia.
These reports are logged, independently investigated by the PetFAST Team who identify trends and can recommend product withdrawals and recalls when necessary.
Veterinary professionals: partners in the reporting process
Veterinarians are an essential partner in the PetFAST system. Their medical expertise is needed to recognise the suspected adverse effect on pet health, to quantify the extent of any illness, and to conduct any diagnostic tests. Reporting veterinarians provide objective information about the pet, including mapping out its medical and dietary history, and offering a clear clinical opinion on any potential connections to the pet food.
Being ‘at the front line’ in managing animal health, assessing contributing factors and addressing pet owner concerns, vets also serve as a vital source of trusted advice. Taking an evidence-based approach and communicating clearly to ensure all concerns are addressed is important.
PetFAST focuses on the health of pets as a priority and the follow-up investigation of any suspected link with pet food. By submitting a report, the vet and the PetFAST team work together to investigate with the understanding that a PetFAST report is not evidence of a causal link, but a call-to-action to investigate.
What can veterinarians do?
Veterinarians assessing a cat or dog who they suspect has experienced an adverse event associated with pet food should submit a PetFAST report. Details of the information needed to complete a report can be found at Australian Veterinary Association | PetFAST Lodge Report .
What is expected of pet food businesses?
As a condition of their membership, PFIAA members must respond to and investigate all PetFAST reports they receive, providing an investigative report within 14 days. This request is also made of non-members; however, they are not answerable to PFIAA.
All reports are forwarded to the pet food business without the veterinarians' details. Only the details of those veterinarians who have given permission are shared separately.
The PetFAST team review and assess all investigative reports in accordance with the PetFAST Response Framework.
What PetFAST isn’t
PetFAST is not a portal for pet owners to log complaints and pet food issues. Responsible pet food manufacturers should be the first contact point for complaints and feedback. The manufacturer or distributor contact details should be on the packaging.
The PetFAST System is an important tool to safeguard the health and wellbeing of Australia’s pets. It’s a confidential and collaborative approach which combines the expertise of vets, industry experts and manufacturers, who pool their nutritional know-how to do what’s best for the nation’s pets.
For more information specifically about PetFAST, FAQs can be found at Australian Veterinary Association | PetFAST FAQs .
Potential New Threat to Pets!
Suspected Cases of Acute Change in Behaviour (‘Werewolf Syndrome’) and Epileptic Seizures in Dogs Fed Imported Rawhide Chews from China
Dakir Polidoro DVM MSc DECVN
EBVS European Specialist in Veterinary Neurology
AniCura Dierenartsenpraktijk Plantijn
Borsbeeksebrug 34, 2600 Antwerpen, Belgium
e. dakir.polidoro@anicura.be
C&T No. 6056

cve.edu.au/werewolf-syndrome
Ida is a 4-year-old neutered female Portuguese Waterdog, presented with acute signs of abnormal behaviour including extreme excitement, howling, panicking and attempts to run away from the owners. These episodes would last for a few minutes and could occur several times per day. The cause of these episodes remains unclear, but since Ida was fed imported rawhide chews previously to these clinical signs, ‘Werewolf syndrome’ was suspected. Owners were asked to stop feeding Ida these rawhide chews immediately and trazodone was administered. Owners were also advised to avoid any situation that could cause stress, like going out for a walk during a busy period of the day or places with too many people or too many dogs, prioritising walks during the evening, when its calmer and darker. Two days after treatment began, Ida made a full recovery.
Since mid-2024, veterinarians from some European countries, including Germany, Switzerland, France, Netherlands and Belgium, have reported a few cases of dogs presenting with acute and severe neurological symptoms, like behavioural changes resembling a ‘psychotic’ state, episodic ataxia, and, in some cases, generalized epileptic seizures in later stages. The initial signs include sudden and extreme excitement, panic attacks with howling, restlessness, screaming, and attempts to escape through windows or doors. It is particularly striking that several dogs are affected in some households.
In general, dogs affected become afraid, aggressive, run up the walls and would jump through a window if they could. They also howl like a werewolf, and for this reason, researchers from Germany have lately referred to this condition as ‘Werewolf syndrome’. It is a syndrome that we still know very little about, but we know that this is a very serious neurological condition, as some dogs have been already euthanized due to the severity of the clinical signs. In general, the course of this condition fluctuates over several weeks, with some patients showing gradual improvement after the symptoms have been treated. There is no specific treatment known at this moment, but there are reports of dogs improving when antidepressants, such as trazodone, are administered. In some cases, antiseizure drugs seem to help as well, including gabapentin, levetiracetam and phenobarbital.
There is still little information about the cause of this syndrome, but the symptoms are presumably signs of a poisoning that remains in the body for quite a long time. Some of these dogs have undergone full blood examinations and brain scans, but no abnormalities can be found. It is therefore very difficult to determine the exact cause. One thing in common in these cases is that all dogs were fed imported rawhide chews from China before showing these clinical signs. Some time ago, these same clinical signs were reported by veterinarians
in Sweden, which they believed was related to the consumption of specific rawhide chews. One theory is that these products may have been contaminated with a toxic substance during the manufacturing process. In previous publications, similar clinical signs were reported in dogs fed with agenized flour, which led to a similar syndrome termed as ‘canine hysteria’. When white flour is bleached with nitrogen trichloride, known as the ‘agene’ process, a toxic product is produced which will induce ‘canine hysteria’ when the flour is included in rations fed to dogs. Despite these theories, nothing has been proven at this time.
Even so, several food agencies in Europe, including the FAVV (Belgium) and the NVWA (Netherlands), have issued a safety warning about a possible link between neurological symptoms in dogs and the consumption of various imported rawhide chews. Most of these products are bought online, from international sellers.
Advice for owners is to immediately stop feeding their dogs with these rawhide chews, which have quite a long shelf life and may have been bought several months/ years ago, and report to their veterinarians in case their dogs present any abnormal behaviour. Advice for veterinarians is that they should be aware of these symptoms and if they believe they are facing a case of ‘Werewolf syndrome’, local authorities must be notified and rawhide chews samples should be submitted for analysis.
Veterinary neurologists from the University of Veterinary Medicine Hannover (TiHo) and the Ludwig Maximilian University in Munich are leading the research on the ‘Werewolf syndrome’. The aim of the current studies is to identify possible triggers or risk factors by comparing affected and unaffected dogs. As part of this research, a survey has been sent to owners and samples from rawhide chews given to dogs clinically affected are currently being analyzed. Although it is highly suspected that these symptoms are related to these imported rawhide chews, nothing has been proven so far.
At this point, this condition appears to be restricted to Europe, as there have been no other reported cases outside this continent. However, with the ease of purchasing these products online, authorities and veterinarians outside Europe should remain alert for possible cases of ‘Werewolf syndrome’ in dogs and its possible link to imported rawhide chews.
References (available in English)
1. tiho-hannover.de/universitaet/aktuelles-veroeffentlichungen/ pressemitteilungen/detail/studie-zum-werwolfsyndromschwere-neurologische-symptome-bei-hunden
2. pmc.ncbi.nlm.nih.gov/articles/PMC2055391/
3. nvwa.nl/documenten/waarschuwingen/2024/12/31/ veiligheidswaarschuwing-barkoo-kauwbotjes-voor-honden
4. favv-afsca.be/nl/news/favv-volgt-onderzoek-naarkauwbotjes-nauwgezet-op
5. abounderrattelser.fi/artikel/tuggben-av-market-hau-hauchampions-kan-ge-hundar-epilieptiska-anfall-produkten-harnu-aterkallats/
6. dognews.se/2024/08/27/varning-flera-hundar-har-insjuknattuggben-dar-ravaran-kommer-fran-kina/
Comments courtesy of
Linda Fleeman BVSc PhD MANZCVS
Animal Diabetes Australia
President of the Veterinary Endocrine Society of the Pacific and Asia (VESPA)
Sue Foster BVSc MVetClinStud FANZCVS
This is very concerning and we thank Dr Polidoro for sharing this information. It is critical that vets share and disseminate information worldwide and the importance of PetFAST in Australia cannot be overstated. Country of origin is not necessarily the issue with adverse pet food events. Suspicion should always be associated with the type of treat and the manufacturing process in order to avoid the false impression that treats will be safe if they are made somewhere other than China. There are a number of Australian foods, chews and treats that have been associated with, or caused, adverse pet food events also. Thus in Australia, it is always worth ensuring that any commercial PET FOOD or treats are manufactured by a member of the PFIAA as PFIAA members are required to RESPOND AND INVESTIGATE if there is a problem. Australia still has no legislation to mandate recalls and ensure other pet food safety processes.
Note: Pet food issues should be reported to the AVA or PFIAA.
PetFAST pfiaa.com.au
There have been no identified cases in Australia through PetFAST however it's possible veterinarians may see this combination of clinical signs and may not immediately make the connection to pet food.
Thanks to Dr Aine Seavers for connecting us with Dr Dakir Polidoro who kindly contributed this C&T article very promptly.
This is a similar situation that occured regarding the KraMar chicken treats product; read cve.edu.au/CnT-4940 from Drs Aine Seavers and Grahame Baker.
A tenacious and hardworking group of concerned and dedicated Vets including Drs Thompson, Fleeman and Foster (and others too numerous to name) worked hard to have this product banned in Australia. In particular, the courage of Dr Fleeman who appeared on the ABC’s 7.30 Report (in the face of legal threats from the company) and an anonymous Vet who leaked the name of the chicken treat to The Adelaide Advertiser saw the company recall the product.
Further reading
Thompson, M.; Fleeman, L.; Kessell, A.; Steenhard, L.; Foster, S. Acquired Proximal Renal Tubulopathy in Dogs Exposed to a Common Dried Chicken Treat: Retrospective Study of 108 Cases (2007–2009). Aust. Vet. J. 2013, 91, 368–373.
Safe Dog Treats
Aine Seavers
Vetlaser1
e. aine@vets1laser.com
After the KraMar toxic treats issue, I had a rule with my clients:
a. Buy Made in Australia and Product of Australia; both had to be on the label before I would allow a client to buy any as even Kangaroo gets shipped out overseas and sent back in. Heretical misrepresentation of an Aussie icon in my opinion.
b. Thanks to the superb article on salt content in commercial dog diets by then 5th year Vet student, Kathy Lin (see below), all salt levels had to be less than 1%.
c. Lin’s article was laminated and displayed in my practice; it helped heal so many animals suffering from ridiculous levels of salt being pumped into them by commercial treats.
That left very few commercial treats…
a. I allowed Liver treats if as (a) above; dosed out at no larger than a postage stamp/day. These treats were a gamechanger for health and safety in vet clinics because the dogs became easier to handle.
b. I didn’t like cheese as treats as I had such a huge cohort of food intolerant 2nd opinion dermatology cases; if allowed, it was hard to keep strict control of and became a slippery slope to anything else in the cupboard when no cheese was available.
c. The one exception I made was for Royal Canin Treats; low calorie, low salt, low allergen and the correct size
to demonstrate that treats should be the size of your trimmed fingernail not the size of your hand. I did a review for C&T on it – see below.
I could make the snappiest dachshund sell its soul to me once they knew I could access Educ.
For my own Dachs, he tried to bite my cleaning lady for 7 years until he realised she could open the Educ jar. He then let her in, walked her (in fact one day put his front legs on her calves and pushed her to the cupboard) wherein she had to count out 4 and give them to him. If she tried 3, he would not be happy. After 4, he stayed in his bed and she had open access to all of the house.
More information here:
Hypernatraemia: The importance of understanding labelling in commercial dog diets by Kathy Lin cve.edu.au/hypernatraemia
C&T No. 5810 The Frankie Lolly cve.edu.au/CnT-no-5810
C&T No. 5575 Alphabet Foods
Note: Currently unavailable in Australia cve.edu.au/alphabet-foods

Frankie
Emphysematous Cystitis with Coagulum Formation in a Dog
Max Sleeman
Castle Hill Veterinary Hospital
1 Francis St
Castle Hill NSW
e. maxsleeman66@gmail.com
t. 0296342712
C&T No. 6057

Dr Max Sleeman is a Partner and Director at Castle Hill Veterinary Hospital. Since graduating from the University of Sydney in 2014, he has been a dedicated member of the hospital’s team, providing high-quality veterinary care to the local community. Max has a particular interest in endocrinology and oncology and is also a keen surgeon, continually expanding his expertise in both orthopedic and soft tissue procedures. Despite these special interests, his true passion lies in general practice, where he values building strong relationships with clients and working collaboratively to ensure the best outcomes for their pets. Outside of work, he enjoys spending time with his wife, whom he is expecting his first child with later this year, staying active on the golf course or cricket pitch, and skiing during the winter months. At home, he is kept on his toes by his mildly obese but much-loved cat, who seems to have perfected the art of demanding attention.

Initial Presentation
An 8-year-old, female neutered Cavoodle, presented for acute onset of large volume, hemorrhagic faecal/ urinary discharge and rapid weight loss. During the initial consultation the patient urinated a large amount of what appeared as frank blood.
Physical Examination
On examination the dog was quiet, alert, responsive and ambulatory. Heart rate was 140 beats per minute, respiratory rate was 40 breaths per minute. Rectal temperature was 37.1°C and body condition score was 3/5. Mucous membranes were pink and moist with a capillary refill time of 2 seconds. Cardiac auscultation was within normal limits, with strong synchronous pulses. Rectal exam revealed normal formed stools. On palpation the abdomen was firm and painful.
Investigation
Chest radiographs showed no significant abnormalities. Abdominal radiographs revealed a bladder that was markedly distended with a large amount of gas, which appeared to exist in a multitude of pocketed sections of the bladder ( Figure 1 ). There were also gas lines within the walls of the bladder. The rest of the abdomen showed no significant abnormalities.
Abdominal ultrasound revealed no free fluid in the abdomen. Imaging of the bladder sonographically proved difficult because the gas pockets hindered interrogation by the ultrasound beam. Gas in the wall of the bladder caused significant comet tail artifacts which prevented accurate interpretation of sonotexture of the bladder lumen. No significant findings were found in the rest of the abdomen.
The complete blood count showed an inflammatory leukogram with a left shift. The total white blood cell count was 33.4x109/L (RI 4.5-17x109/L). The neutrophil
Figure 1. Right lateral abdominal radiograph on the morning of initial presentation. Note the bizarre appearance of the bladder.

count was 28.7x109/L (RI 3.5-12x109/L). The band neutrophil count was 1.00 x109/L (RI 0.0-0.2x109/L). Red blood cell and platelet pathology were within normal limits.
The biochemical analysis revealed a severe hyperglycemia with a blood glucose of 37.1 mmol/L (RI 3.3-6.8mmol/L), hyperkalemia (potassium 6.2mmol/L; RI 3.9-5.9 mmol/L), hypochloremia, chloride (93 mmol/L ; RI 101-118mmol/L), bicarbonate 9/mmol/L (RI 12-26mmol/L) and β-hydroxybutyrate 8.1mmol/L (RI 0.0-0.5mmol/L) elevations consistent with diabetic ketoacidosis. Canine pancreatic lipase activity was >2000 ng/mL (RI <400 within normal limits; >400 consistent with pancreatitis).
The urinalysis revealed marked haematuria, marked leukocyturia, marked glucosuria and marked ketonuria and rod-shaped bacteria present. Urine culture returned a heavy growth of Proteus mirabilis susceptible to most tested antimicrobials except doxycycline (Table 1).
Assessment and the initial treatment plan
Based on available data the patient was diagnosed with diabetes mellitus, ketoacidosis, pancreatitis, urinary tract infection and emphysematous cystitis.
The patient was placed on intravenous NaCl 0.9% at 5mL/kg/hr. Protaphane human insulin was started at 0.5 IU/kg via subcutaneous injection every 12 hours. Esomeprazole was administered at 1 mg/kg intravenously (IV) every 24 hours. Antimicrobial therapy was initiated with amoxicillin/clavulanic acid 20 mg/ kg and enrofloxacin 10 mg/kg both administered via subcutaneous injection (SCI) every 24 hours.
Vomiting and diarrhoea began shortly after admission, thought to be due to pancreatitis. The patient was started on maropitant 1 mg/kg SCI every 24 hours. It was also noted that the suspected flatulence was actually pneumaturia which fitted with emphysematous cystitis.
Venous blood gasses were monitored daily during the initial stabilisation period. As blood glucose dropped below 10 mmol/L and the patient remained inappetent, the fluids were changed to 0.5% glucose in 0.9% sodium chloride at a maintenance rate of 3 mL/kg/hr.
A confusing and concerning clinical picture
Ketoacidosis initially worsened before beginning to improve. On Day 5 ketones were 0.5 mmol/L, blood glucose was stable between 5-15mmo/L and electrolytes had normalised. The spec canine pancreatic lipase had had dropped to 900 ng/mL.
The patient, however, was clinically deteriorating, with no improvement in haematuria. Despite best efforts from the nursing staff the patient remained inappetent. Repeat radiographs of the bladder revealed no improvement in the emphysematous cystitis.
Repeat urinalysis revealed rod bacteria remained in the urine and a complete blood count now revealed a neutrophil count of 70.43 x 109/L (RI 2.95-11.64x109/L).
A change in anti-microbial therapy
Deterioration in the clinical picture prompted concern for sepsis and ineffective antimicrobial regime. The patient was discontinued on amoxicillin/clavulanic acid and enrofloxacin and started on gentamicin 10 mg/ kg administered subcutaneously every 24 hours and trimethoprim with sulfadiazine 20mg/kg (combined) administered subcutaneously every 24 hours. The antibiotics were chosen based on their immediate availability and previous susceptibility data.

Figure 2. Free floating intraluminal bladder mass during surgery
Table 1. Urinalysis report on the morning of initial presentation

Forty-eight hours after starting the new antimicrobial, the patient was eating, the haematuria had improved, and the urine no longer contained bacterial rods.
The patient was discharged 5 days after starting the new antibiotic regime. She went home on 4 IU of protaphane insulin subcutaneously every 12 hours, and trimethoprim/sulfadiazine given per os at 47 mg/kg once every 24 hours. The gentamicin was discontinued after 5 days and not continued at home.
At a one week recheck, the insulin dose was increased to 5 IU as there was polyuria and the in-house spot glucose test revealed a blood sugar of 24 mmol/L. The urine was cultured, and the trimethoprim/ sulfadiazine was discontinued. The culture was negative at this time.
One month later the patient had gained weight, was bright and free of haematuria. However, a large soft palpable tissue density remained in the bladder which was evident on ultrasound and radiographically. It was theorised that this was a resolving blood clot or a tumour. Repeat urine cultures were negative.
A second presentation of haematuria
Sixty-nine days post initial presentation the patient represented with severe haematuria. The urine once again had a dense population of rods present, and the patient was clinically unwell. Radiographically there was gas present in the bladder similar in nature to the first presentation.
The patient’s diabetes was stable and there was still a large soft tissue density in the bladder. A decision was made to take the patient to surgery. A midline laparotomy was performed to reveal a large distended firm bladder. A ventral cystotomy revealed a free-floating leathery mass measuring 105 x 75 x 45mm which was removed from the bladder lumen ( Figure 2 and 3 ). A biopsy of the bladder wall was taken, and the bladder was flushed and closed in
a single layer with 2/0 PDS and the abdomen was closed routinely.
The patient flourished after surgery and was discharged 2 days later. The histopathology on the mass was as follows:
'There are no neoplastic cells. The tissue is a coagulum of eosinophilic fibrillar with amorphous material which has large numbers of intermingled bacteria. Most of the bacteria are rods and are coated along the surface together with a scattering of neutrophils.'
'Diagnosis: Inflammatory coagulum with bacteria'
Histopathology of the bladder wall revealed a severe erosive to ulcerative neutrophilic cystitis (Dr David Taylor; Figure 4).
Three months post-surgery the patient is a well-managed diabetic dog with no signs of urinary tract disease.
Discussion
Both diabetes mellitus and bacterial cystitis are common conditions in general practice; however, emphysematous cystitis is a rare form of urinary tract infection. The most common risk factor for emphysematous cystitis is diabetes mellitus, although it has also been reported without diabetes in both humans and dogs. To the author’s knowledge, inflammatory coagulum formation in the urinary tract of a dog has not been described in the literature, although a dog from Mexico had a similar lesion which was a large blood clot. This case seemingly is a rare complication of common presenting diseases and it demonstrates the need for appropriate imaging in managing urinary tract disease, particularly with a concurrent comorbidity of diabetes mellitus.
Emphysematous cystitis is caused by gas producing bacteria in the urinary tract. The causative bacteria in this case was Proteus mirabilis, although a range of causative bacteria have been reported in the literature. Gas production is thought to be caused by fermentation of glucose in the urine caused by certain strains of bacteria. Glucose fermentation occurs in both aerobic and anaerobic environments and can be accelerated by these certain strains of bacteria. Glucose is converted into cellular energy, ethanol, carbon dioxide gas and often hydrogen gas.
It is the author’s belief that the original antibiotic selection was ineffective as the antibiotics were not penetrating deep enough into the initial blood clot which subsequently developed into a bacterial coagulum at the initiation of the course and resistance formed quickly. The second-choice combination of high-dose gentamicin and trimethoprim with sulfadiazine appeared
Figure 3. Mass removed from bladder lumen
to work more effectively and quickly; however, it is possible that the formation of the inflammatory coagulum had walled off pockets of bacteria within itself at this time. It is possible that the rupture of this mass led to the representation of severe haemorrhagic cystitis. It is not clear whether the inflammatory coagulum was present pre diagnosis of emphysematous cystitis or whether it formed as a result.
The level of gas present in the bladder at the time of presentation made imaging the lumen of the bladder a challenge. Computed tomography would have been ideal in this case and much of the human literature supports this as the gold standard imaging modality for emphysematous cystitis.
Conclusions
This case presented a number of significant challenges, including stabilising a critically ill diabetic ketoacidotic patient, managing sepsis and antibiotic failure, addressing large volumes of bacterially contaminated haemorrhagic urine, overcoming difficulties in bladder imaging, handling disease relapse, and performing anesthesia and surgery on a diabetic patient in poor condition. In addition, we had to work within the financial constraints of the owner.
Acknowledgements
It has been both a challenging and rewarding experience to be a part of this case. Special acknowledgement is due to the nursing team for their exceptional care and dedication to the patient, as well as the Vetnostics microbiology (Kristen Todhunter) and histopathology teams (David Taylor) for their timely and valuable contributions.

AB

C

D

Figure 4. (A): coagulum. (B): Rods on the surface of the coagulum and deeper. (C) and (D): Bladder mucosa, emphysematous, inflamed, haemorrhagic and ulcerated
Mycobacterium goodii
Infection
in a Cat
Dr Samantha Crothers BSc BVMS Dip ACVD
Veterinary Dermatology Specialists
305 Selby St North
Osborne Park WA 6017
e. info@vetdermspecialists.com.au
C&T No. 6058

History
2 May 2023
A female spayed Domestic Short-haired (DSH) 3-year-old cat presented to Veterinary Dermatology Specialists in Perth, Western Australia for assessment of chronic draining lesions on the ventral abdomen.
She initially presented to the referring vet in northern Western Australia 10-months prior for a traumatic injury on the ventral abdomen. Over the following weeks a subcutaneous swelling developed near the initial traumatic lesion. The cat was prescribed doxycycline (10mg/kg q 24 hours with food) for 4 months.
During the 4-month time period, the wound waxed and waned. She then presented to another referring vet in Perth for a second opinion in January 2023. A biopsy was performed, and the tissue was submitted for histopathology in February 2023. The wound was surgically debulked and debrided (see Figure 2 postsurgery). Bacterial tissue culture confirmed methicillin resistant Staphylococcus pseudointermedius (MRSP).
The owner had been instructed to apply topical mupirocin to the area which seemed to help improve the lesions but not resolve them.
Dermatological examination
Multiple draining tracts were present on the left ventral abdomen; firm subcutaneous masses (less than 1 cm in diameter) were palpable on the right side of the ventral abdomen.
Diagnostic tests performed at initial visit on 2nd May 2023
Cytology: Impression smear draining tracts on left ventral abdomen revealed 2+ neutrophils, occasional cocci.
Differential diagnosis
Rapidly growing Mycobacterium spp vs other deep bacterial infection vs deep fungal infection vs sterile inflammatory process.


Figure 1. The feline patient
Figure 2A & B. Appearance of the ventral abdomen postsurgical debulking in January 2023
Assessment
1. Secondary MRSP (confirmed on tissue culture in February 2023)
2. Non-healing wound on ventral abdomen—infectious vs sterile
A general anaesthetic was planned the following week to obtain fresh tissue for Mycobacteria PCR, bacterial and fungal culture and histopathology.
Management plan (patient weight 5.1 kg)
Continue doxycycline 50mg: give ½ tablet twice daily (4.90mg/kg q 12 hours) with food.
Discontinue mupirocin.
17 May 2023 (15 days later)
The owner had noticed that the wounds were getting worse. Her weight had reduced from 5.1kg to 5.0kg.
A general anaesthetic was declined so fine needle aspirates were obtained under sedation. These were taken from the largest subcutaneous (> 1cm) nodule on the right flank and submitted for Mycobacteria PCR assay. Minimal fluid was obtained during the sampling.
Management plan whilst awaiting PCR results:
Continued doxycycline 50mg: give ½ tablet twice daily (5mg/kg q 12 hours) with food.
Commence marbofloxacin 25mg once daily (5mg/kg q 24 hours) with food.
Weight management: Royal Canin Weight Reduction (half an 83 mL cup) and 1 pouch of Fancy Feast (half AM and half PM).
1 June 2023 (13 days later)
PCR assay confirmed Mycobacterium goodii.
Management plan (patient weight 4.9 kg)
INCREASE doxycycline dosage weekly* as follows:
WEEK 1
(50mg tablet): give 3/4 tablet in AM (7.65mg/kg) and 1/2 tablet (5.1mg/kg) in PM with food
WEEK 2
Give 3/4 tablet in AM and PM (7.65mg/kg) with food
WEEK 3
Give 1 tablet in AM (10.2mg/kg) and 3/4 tablet in PM (7.65mg/kg) with food
WEEK 4 onwards
Give 1 tablet in AM and 1 tablet in PM (10.2mg/kg) with food

*Aiming to increase dose to a level she will tolerate but will not cause inappetence or gastro-intestinal upset.
Continue marbofloxacin 25mg once daily (5mg/kg q 24 hours) with food.
24 July 2023 (7.5 weeks later)
The cat was tolerating the doxycycline dose changes and all draining tracts had resolved. There was a < 1cm firm subcutaneous nodule present on the left ventral abdomen. Her appetite had increased and she had regained some of her lost weight.
Management plan (patient weight 5.1 kg)
INCREASE doxycycline dosage weekly* as follows:
(Note: Now administering 100mg tablets, previously this was a 50mg tablet):
WEEK 1
Give 3/4 tablet of a tablet (14.70mg/kg) in AM and 1/2 of a tablet (9.80mg/kg) in PM with food
WEEK 2
Give 3/4 tablet (14.70mg/kg) in AM and PM with food
WEEK 3
Give 1 tablet (19.60mg/kg) in AM and 3/4 tablet (14.70mg/kg) in PM with food
WEEK 4 onwards
Give 1 tablet in AM and 1 tablet (19.60mg/kg) in PM with food
*Aiming to increase dose to a level she will tolerate but will not cause inappetence or gastro-intestinal upset.
Figure 3. Fine needle aspirates were collected under sedation
Continue marbofloxacin 25mg once daily (5mg/kg q 24 hours) with food.
18 September 2023 (8 weeks later)
Cat was tolerating the dosage changes and all lesions had resolved. Owner had clipped hair in area so closer observation of the skin was possible.
Management plan (patient weight 5 kg)
Continue doxycycline 100 mg twice daily (20 mg/kg q 12 hours) with food.
Continue marbofloxacin 25 mg once daily (5 mg/kg q 24 hours) with food.
Future plan
Aim to continue both antibiotics to 3-months beyond clinical resolution (from this day).
Consider switching from marbofloxacin to pradofloxacin if lesions recur.
Follow up planned in 12-weeks.
Discussion
Mycobacterium goodii is a non-tuberculous mycobacterium rarely reported in cats, but is an emerging nosocomial infection in humans, being reported in prosthetic device contaminations and less commonly identified in osteomyelitis, endophthalmitis, bursitis and respiratory tract infections.
The organism has been found in the environment and in water systems; and in cats, it is thought to be introduced by wounds contaminated by soil or punctures and foreign bodies.
Infections commonly occur in areas with more fatty tissue (e.g. ventral abdomen) which is the rationale behind encouraging weight loss in affected overweight cats.
In cats, the resulting lesions are typically ulcerated wounds and nodules often with draining tracts.
Diagnosis can be challenging as the organisms are often not identified on cytology or histopathology, requiring culture or PCR assays.
In this case we were able to obtain a suitable sample by FNA aspirate alone, not requiring a general anaesthetic.
Ideally, antibiotic treatment should be dictated by culture and sensitivity results; however, because there is a delay in achieving a diagnosis, empiric therapy is begun prior to results.

Current recommendation for treatment is a fluoroquinolone combined with doxycycline or a macrolide to reduce the chance of resistance developing. Treatment duration is for between 4-12 months depending on the clinical response.
Whilst surgical debulking of the infection has been recommended, this case challenges that assumption, with the cat’s lesions eventually resolving with medical treatment and weight loss, without the previously planned second surgical de-bulking.
If surgery is performed, it is recommended to use a rapidly absorbing suture material as it is thought that a biofilm development on slowly absorbing suture material can harbour the bacteria and delay resolution of the infection.
References
Wu C-Y, Diaz S, Ellis A, Jones R, Pucheu-Haston C. Cutaneous Mycobacterium goodii infection in an immunocompetent cat in Louisiana: clinical presentation, molecular identification, antimicrobial susceptibility and management. Journal of Feline Medicine and Surgery Open Reports 2022;8(1). doi:10.1177/20551169221090442
Ruth Waldron, Dympna Waldron, Eileen McMahon et al Mycobacterium goodii pneumonia: An unusual presentation of nontuberculous mycobacterial infection requiring a novel multidisciplinary approach to management, Respiratory Medicine Case Reports,Volume 26,2019,Pages 307-309
Salas NM, Klein N. Mycobacterium goodii: An Emerging Nosocomial Pathogen: A Case Report and Review of the Literature. Infect Dis Clin Pract (Baltim Md). 2017 Mar;25(2):62-65. doi:
Figure 4. 7-weeks post diagnosis, the cat had no draining tracts and hair was regrowing



FIGHTING FIP, A FIGHTING FIP, A GLOBAL EFFORT. GLOBAL EFFORT.
Feline Infectious Peritonitis (FIP) is a devastating disease that primarily affects cats, especially young or immunocompromised ones It is caused by a mutated strain of the feline coronavirus Historically, FIP was considered almost always fatal due to the absence of effective treatments, leaving veterinarians and caregivers desperate for solutions. However, the availability of legally approved treatments has revolutionised the outlook for FIP, thanks largely to companies like Bova, which have been instrumental in developing and providing quality-assured treatments on a global scale
As a global leader in veterinary compounding, Bova has taken significant strides in the fight against FIP Their legally produced and quality-assured antiviral medications include remdesivir, GS-441524 and EIDD-1931 Unlike unregulated alternatives, Bova’s products meet stringent quality and safety standards, allowing veterinarians to prescribe them with confidence Bova’s contribution extends beyond treatment it has strengthened the veterinary community’s understanding of FIP. By adhering to regulated frameworks, Bova has built trust among practitioners, encouraged further research, and promoted transparency in a field long burdened by misinformation

"As Australia’s leading veterinary compounder, Bova Aus is proud to set the benchmark for quality and innovation in animal healthcare. Our Quality Assured (QA) Program reflects our unwavering commitment to delivering safe, effective, and reliable solutions for veterinarians and their patients. We strive to empower veterinary professionals with the tools and confidence they need to achieve the best possible outcomes."
– Nick Bova, CEO, Bova Group
Additionally, Bova’s commitment to ethical and legal drug development has set a new standard in the veterinary sector
Their collaboration with researchers demonstrates the importance of integrity-driven innovation. This dedication has not only saved lives but also showcased the potential of pharmaceuticals to address previously untreatable conditions
The global reach of Bova’s treatments has opened a new chapter in FIP care, providing hope where little existed before By focusing on quality, legality, and accessibility, Bova has empowered veterinarians and caregivers to face FIP with renewed confidence As understanding of the disease continues to grow, Bova’s leadership remains a symbol of progress and a testament to the life-changing power of science and compassion
The proof is in the results.
A retrospective study of 307 cats showed that 84 4% of cats remained alive after the treatment period (12 weeks)
Bova's support tools for vets and caregivers.
Bova's support tools for vets and caregivers.
Bova is dedicated to empowering veterinarians and caregivers in the fight against FIP by providing quality treatment options and support tools. Key offerings include:
A dedicated FIP webpage with comprehensive resources on FIP and treatment
A home monitoring chart for tracking health indicators like appetite and weight
Tailored documents for veterinary teams and cat owners to ensure coordinated care
Webinars with experts on the latest FIP advancements. An FIP advice line for direct professional support
These resources emphasise Bova’s commitment to partnering with vets and caregivers to save feline lives.




Visit our website or speak to your Account Manager for more information on available support: bova.vet/fip-resource-page/


Q2: What three key insights have you gained from your FIP experience and studies you have undertaken or participated in?
1.
“Don’t give up on severe cases - One of the most striking lessons from our prospective study in Australia is that even moribund cats can make remarkable recoveries with treatment. For example, 3 out of 5 critically ill cats treated with IV remdesivir made full recoveries, sitting up and eating within days. These cases remind us that perseverance can yield extraordinary results
2.
Accurate diagnosis is critical - FIP can mimic a range of other diseases, making a thorough diagnostic workup essential. While it's reasonable to initiate antivirals in highly suspected cases (e.g., a pyrexic, icteric, hyperglobulinemic kitten with classic yellow high protein effusion), we must avoid rushing into treatment trials for less clear-cut cases. Too often, I see cats treated for months for "FIP" only to later be diagnosed with lymphoma, eosinophilic sclerosing fibroplasia, or other conditions Judicious use of antivirals ensures the best outcomes for our patients, their owners and protects the long-term efficacy of these drugs (antivirals are antimicrobials too!)
3.
What did the experts say?
What did the experts say?
We asked global feline specialists key questions about FIP, covering advancements in treatments, early diagnosis, and collaborative care. Their insights provide invaluable guidance for veterinarians and caregivers, helping to improve outcomes and offer hope for cats affected by this challenging disease
Q1: What would you say to a veterinary colleague who is feeling overwhelmed by FIP cases in their practice?
"I get it - this is all very new and represents a huge paradigm shift. The good news is that there is also an explosion of resources and guidance and research happening in this field And these new drugs are remarkably effective - treating FIP with antivirals is not rocket science. In most cases, this disease is so much more manageable than diabetes, for example You can do it! And there are lots of DVMs and reputable organizations here to help."

Dr Samantha Evans
DVM, PhD, DACVP
Assistant Professor
of Veterinary Clinical Pathology, Colarado State University, USA
There’s still so much to learn - FIP is anything but straightforward While antiviral therapy has revolutionised outcomes, many questions remain unanswered. The variability in clinical presentations and responses to treatment suggests we’re only scratching the surface. Over the next decade, I anticipate significant advancements that may completely reshape how we understand and treat this disease.”

Dr Sally Coggins
BVSc (Hons I) MANZCVS (Feline Medicine), PhD Candidate Postdoctoral research fellow (Diseases and Treatment of Cats) Sydney Infectious Diseases Institute.
Q3: What is the main advice you would offer to a vet with a possible FIP case presented to them?
“To think of the diagnosis like a puzzle, and 'pieces' such as signalment, history, clinical examination findings and blood results are important to allow you to see the picture of an FIP diagnosis. One of the most important 'pieces' is finding an effusion, even in a cat presenting with neurological or ocular signs, so use point-of-care ultrasound to look for the fluid!”

Dr Samantha Taylor
BVetMed(Hons) CertSAM DipECVIM‐CA MANZCVS FRCVS Internal Medicine Specialist Clinician, Lumbry Park Veterinary Specialists, UK

Hyperactive Syndrome in a Dog
Professor Emeritus Rick Atwell
e. r.atwell@uq.edu.au
m. 0409 065 255
C&T No. 6059

Image resource: freepik.com
A middle aged, desexed, female (very healthy looking) Dachshund was fostered (from Brisbane) in a small western town. The dog had access to inside and outside the house, in the spring/summer part of the year. It had boarded there before and had its usual array of toys and a den. There were 3 cats in the house and 2 adult people (both retired), moving in and out of the house and yard during the day.
The dog was not known to be allergic and was up to date with routine prophylaxis. The carers were fastidious re the general care, feeding, exercise etc and were demonstrative of their love of their various animals, which included a geriatric caged bird.
I found the whole experience incredulous being asked to intervene (as a retired vet.) prior to the critical history of the owner. It’s always in the history, seen so clearly in human medicine, where about 90% of primary organ issues can be gleaned by good history acquisition.
A dinner was being prepared (roast mutton/upright oven/ standard times and temperature etc)—a routine event once a week in this home. At some stage the dog became agitated, appearing anxious, hyperactive, running quickly in and out of the house, around the yard, yelping and barking. It could not be caught (older people?), nor verbally calmed. Instead, it continued (as above) until it was finally quartered and held. Even then it could not be calmed and continued in a hyperactive, excitable state, wriggling, barking etc.
At the time there were no bees, wasps etc evident in the garden. It was not known to be a plant-chewing-dog, nor were there any toads, snakes etc seen while attempting capture. There was no access to garden or household poisons.
The owner (their son) was contacted re previous veterinary care—the condition was explained and the first
question he asked (of the carers): ‘Are you cooking lamb or mutton in the oven?! ’
Historically, the dog was known to always react (with this excitable behaviour) whenever the owner cooked such food. The dog remained affected until the cooking was over and then resumed its usually quiet demeanor. Is this a specific syndrome? Fear-based? Past experiences? Does it have an Animal behaviour name?
The diagnosis seems sound—that this was the cause, as it was clearly associated before and ‘diagnosed,’ blindly, without any prompting, at distance over the phone. Does the dog ‘smell or hear’ the cooking—linking it to a hot fat, meat or a cooking apparatus burn of some sort? (This was not known to have happened, but the early puppyhood history was unknown).
Was it due to some fear/pain-based history triggered by exposure, that is ‘recalled’ producing a ‘run away’ attempt, (well-fenced property—no escape), and ‘verbalised’ fear?
I’m not aware of such animal behaviour, but perhaps other clinical experiences exist or expertise can correct and/or add to the above.
Comment courtesy of Dr
Isabelle Resch BVSc (Hons) MVS
MANZCVS (Small Animal Medicine Veterinary Behaviour) Resident – ANZCVS and American College of Veterinary Behavior
Co-Tutor of the Behavioural Medicine Distance Education course e. isabelle@cabvets.com.au
From the history and presenting signs in this case, it is difficult to make a behavioural diagnosis. However, they indicate that this dog may have an anxiety disorder.
Anxiety issues are very common in our companion animals, with up to 72.5% of dogs having problematic behaviours in a recent study.1 Noise sensitivity was the most common anxiety trait with 32% of dogs being highly fearful of at least one noise.1 Other common causes for behavioural issues are fear, stress, conflict, panic or frustration.2
This dog may have an anxiety disorder, but we really need more behavioural history to make a behavioural diagnosis. The signs of anxiety can be varied and individual and can vary within an individual in different situations. We often refer to the 4F’s of anxiety: Fight, flight, freeze and fiddle behaviours.
Flight and avoidance are common strategies. Fight behaviours include agitation, arousal, excessive movement, through to growling, and biting. They are distance-increasing behaviours, to gain distance from a perceived or real threat. Freeze behaviours occur when the animal is stiff and tense, at times freezing and not moving. Fiddle behaviours (also known as displacement behaviours) are more subtle signs of anxiety and can be normal behaviours displayed out of context. Common examples include lip licking, yawning, and shake off behaviours when not wet. Owners are often not well educated about the more subtle signs of anxiety, such as the freeze and fiddle behaviours, and may miss earlier signs of emotional discomfort and anxiety. As Prof Atwell has mentioned, ‘it is always in the history’.
This is especially true for veterinary behavioural medicine. This is why we use detailed behavioural questionnaires and lengthy consultations, as the history, context, duration, frequency and intensity of behaviours are vital in making a behavioural medicine diagnosis. We need to consider if this behaviour is a problem behaviour for the owners? Was the dog frustrated, anxious, anticipating, excited? There are many questions we still need to answer to arrive at a definitive diagnosis. Again, I agree with Professor Atwell – history is everything.
In any older dog with behavioural changes, we need to consider medical issues.
In humans, there is a strong relationship between painful conditions and the development of fear-related avoidance responses. Fagundes et al 20183 explored the connection of musculoskeletal pain and noise sensitivities, finding that cases with noise sensitivity associated with pain often had an older age of onset, and displayed a ubiquitous fearfulness to loud noises and extensive generalisation of the problem to the wider environment. The age of onset of noise sensitivity in dogs with pain was often older.3 Any pain issues, such as musculoskeletal, gastro-intestinal, itchy skin, or dental pain can lead to or contribute to behavioural changes.4 Given the breed and middle age, this dog may also have some comorbid pain issues, such as arthritic pain, intervertebral disc disease, and/or dental pain that may be leading to an exacerbation of the problem behaviour.
We need to be mindful that a dog’s hearing is far better than humans.
They can hear very soft sounds, from a range of -5 to -15 dB. The softest sound people can hear is 0 dB. They can also hear higher pitched sounds, up to 45 000 Hz, whereas people can only hear up to 20 000 Hz. This sensitivity to sounds and dogs ability to hear higher frequency sounds may contribute to storm and noise issues, such as this potential reaction to sizzling meat or oven noises.2 A dog’s sense of smell (olfaction) is 10 000
to 100 000 times better than humans at recognising scent in the environment. Dogs have between 220 million and 2 billion neurons in the olfactory epithelium (humans have 2 to 5 million). The olfactory system is closely associated with the limbic system, and this partially explains the strong emotional reaction that dogs have to scents.2
The terminology used in veterinary behavioural medicine is evolving.
There are differences in diagnostic terms used in different countries, by different veterinary behavioralists. This is similar to the field of veterinary medicine and human psychiatric medicine. Terminology changes and evolves, as we learn more about the emotional motivation for behaviours, associated medical conditions and have more published data and research. In this case, the anxiety response may be associated with auditory, olfactory and/or location-related triggers.
Past experiences, previous learning, genetics, epigenetic effects and medical disease also play a role. Animals may also generalise anxiety responses to similar sounds and smells after a traumatic incident. Noise issues or sensitivities are common issues in dogs, with very varied presentation and causes. This could also be a learnt behaviour. Perhaps the dog was fed lamb regularly, so that now, when he hears the sounds and smells the odours of cooking lamb, he gets excited and runs around in anticipation and excitement.
A possible diagnosis in this case is an anxiety disorder, likely a noise sensitivity.
The ‘run-away’ attempts and barking are common manifestations of anxiety and fear. The hyperactivity seen is a manifestation of the underlying emotional state of the dog—fear and anxiety based, possible panic, but excitement cannot be ruled out without more detailed information. There may be medical and behavioural co-morbidities that need assessing, and the history is an essential tool in gaining more information. The history, but also the medical and behavioural examination is vital to making a behavioural diagnosis. However, without a comprehensive behavioural history, it can be difficult to make a definitive diagnosis.
References
1. Salonen, M., et al.2020, Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Scientific Reports ,10(1): 2962.
2. Landsberg, G., L. Radosta, and L. Ackerman, 2023, Behavior Problems of the Dog and Cat -E-Book: Elsevier Health Sciences.
3. Lopes Fagundes, A.L., et al ., 2018 Noise Sensitivities in Dogs: An Exploration of Signs in Dogs with and without Musculoskeletal Pain Using Qualitative Content Analysis. Frontiers in Veterinary Science , 5.
4. Mills, D.S., et al. 2020, Pain and problem behavior in cats and dogs. Animals ,. 10(2): 318.
Systemic Amyloidosis in a Cat Presenting with Acute
Haemoabdomen & Hepatic Rupture
Alexander Teh
Resident in Anatomic Pathology
University of Sydney B14
e. alexander.teh@sydney.edu.au
C&T No. 6060
Signalment, History & Clinical Presentation
A 2-year-old male neutered Domestic Medium Hair cat presented to the University Veterinary Teaching Hospital Sydney for a 48-hour onset of lethargy, and 24-hour history of hyporexia. On examination, free abdominal fluid was observed on AFAST which had a PCV of 0.25 L/L (patient PCV was 13 L/L and total protein was 52 g/L) confirming haemoabdomen. Serum biochemistry showed hyperglycaemia (13.7), hyperamylasaemia (420), and elevated ALT activity (2799). Haematology showed a neutrophilia (0.82), eosinopenia, low red blood cell count (2.8), low haemoglobin, increased MCHC, and thrombocytopaenia (17). aPTT was prolonged (146). The cat was treated with an autotransfusion of peritoneal blood, subcutaneous phytomenadione, intravenous tranexamic acid, and maropitant. A second haemorrhagic event was then suspected to have occurred and the cat was euthanised and submitted for a post-mortem examination.
Necropsy Examination
The mucous and scleral membranes were pale. Haemoabdomen was present, and the abdomen contained 165 mL of sanguineous fluid (protein 59 g/L, PCV 0.26 L/L) ( Figure 1). The liver was moderately enlarged and friable with severe parenchymal haemorrhage ( Figure 2). Blood clots and a few fibrin strands were adhered to the capsular surface of the liver (Figure 2).
Histopathological Examination
Extracellular, eosinophilic, hyaline, dense amorphous material expanded the thyroid and renal cortical and medullary interstitial tissues, and the hepatic sinusoids and spaces of Disse ( Figures 3-4). This material stained


positive (congophilic) with a Congo Red stain ( Figures 5-6) and exhibited apple-green birefringence with polarised light ( Figure 7 ) confirming this material as amyloid. Furthermore, congophilic staining and applegreen birefringence was preserved with Wright’s potassium permanganate reaction (suggestive of amyloid AL). Additional deposits of amyloid with similar special staining features were also found in the gallbladder and spleen. Altogether, the findings were consistent with systemic amyloidosis.
Further findings included severe hepatic haemorrhage with associated necrosis and inflammation, moderately severe lymphoplasmacytic interstitial nephritis, and reactive lymphoid hyperplasia of the spleen and the sternal, mandibular, and mesenteric lymph nodes.
Figure 1. 165 mL of sanguineous fluid was present in the abdomen consistent with a haemoabdomen
Figure 2. Liver after removal at necropsy showing severe parenchymal haemorrhage and rupture
Systemic Amyloidosis
Systemic amyloidosis is the most common presentation of amyloidosis in domestic animals, and involves the extracellular deposition of misfolded, insoluble, and aggregated proteins with a β-pleated sheet conformation in multiple organs. Systemic amyloidosis can be due to a primary process involving the deposition of amyloid AL which is produced from immunoglobulin light chains due to a monoclonal B-cell proliferation (such as plasma cell disorders). Alternatively, it can be due to a secondary process involving the deposition of amyloid AA as a consequence of chronic inflammation and the sustained overproduction of serum amyloid A, a positive acutephase protein and the precursor of amyloid AA. A familial form of systemic AA amyloidosis is also recognised in Abyssinian, Siamese, and Oriental cats but the mode of inheritance has not been fully elucidated; however, an autosomal dominant with incomplete penetrance is suspected in Abyssinian breeds. Amyloid infiltration and deposition within the liver predisposes the organ to rupture and haemorrhage, and this was considered the presumed cause of the hepatic rupture and consequential haemoabdomen in this case.
Wright’s potassium permanganate method was also trialled which is a process to distinguish between the different amyloid fibrils. It is thought that the removal of congophilic staining following pre-treatment with potassium permanganate is indicative of amyloid AA (secondary amyloidosis), whereas retention of congophilic staining is indicative of amyloid AL (primary amyloidosis). In this case, congophilic staining was retained after pre-treatment with potassium permanganate. However, whilst this feature may be suggestive of amyloid AL/primary amyloidosis, many exceptions to the potassium permanganate reaction have been reported and this method is currently considered obsolete in human medicine diagnostics to differentiate between AA and AL amyloidosis. Chronic inflammation was also identified in the kidney and to a lesser extent in the liver, in addition to reactive lymphoid hyperplasia in the spleen and sternal, mandibular, and mesenteric lymph nodes. Therefore, secondary/AA amyloidosis due to chronic antigenic stimulation also remains a possibility in this case.

3

4 5 6


Figure 3. Histology of the thyroid gland showing expansion of the interstitial tissue with eosinophilic, dense, amorphous, hyaline material (amyloid)
Figure 4. Histology of the liver. Black arrows show expansion of the sinusoids and space of Disse with eosinophilic, dense, amorphous, hyaline material (amyloid)
Figure 5. Histology of the thyroid gland with Congo Red staining demonstrating positive/congophilic staining of the amyloid expanding the interstitial tissues
Figure 6. Histology of the kidney with Congo Red staining demonstrating positive/congophilic staining of the amyloid expanding the interstitial tissues

Questions
1. Why is the aPTT prolonged?
2. Why is the platelet count low?
3. What additional measures might have been used to try to save this cat?
4. What is the treatment for hepatic amyloidosis?
Email your answers to cve.marketing@sydney.edu.au
The winner of the best and most complete answer wins a CVE$300 voucher.


Congratulations!
To the members/readers who eagerly replied to the December 2024 ‘What’s YOUR Diagnosis?’ on 24, 25 & 26 December. All three answers were excellent but the best was contributed by Robert Bird (NZ) who wins the CVE$300.
In the spirit of our first 60th anniversary issue, we are awarding a CVE$100 voucher each to the runners up: Kayla Hamilton (TAS) and Tomas Gilead (NSW).
See the answer on page 34.
A harmless-looking papilloma on Monday morning, a fitting Bulldog on Wednesday afternoon, a pyometra last thing on Friday—The Veterinary General Practice Casebook echoes life at the coalface of companion animal practice. Organised into ‘clinics’, each chapter feels like a day in the life of a busy general practitioner, where emergencies are juggled with routine consultations and every so often something unexpected pops up.
This unique and exciting textbook makes a persuasive argument for general practice as a speciality in its own right and celebrates the role of general practice veterinarians and their vital contribution to animal health and welfare in the community.
Ideal for senior veterinary students, new graduates, those returning to practice, and those seeking a general practice workout, this book presents cases as they appear in real life, often messier and less complete than textbook descriptions. The clinical content is complemented by topics on wellbeing and professional practice, as well as recipes for simple and nutritious meals to feed veterinary body and soul. cve.edu.au/GP-casebook
Figure 7. In the thyroid gland, the congophilic material exhibits apple-green birefringence when viewed with polarised light confirming this material as amyloid
iCatCare Veterinary Society, formerly the International Society of Feline Medicine (ISFM), partner with the CVE to deliver the Feline Medicine Distance Education course. Due to its comprehensive content, it is spread over 2 years to allow the busy practitioner to fully engage with the course whilst maintaining life and work balance.
Feline Ageing and Age-Related Disease What Test and When?
Nathalie Dowgray
BVSc MRCVS MANZCVS (feline) PGDip IAWEL PhD
C&T No. 6061
The pattern of cats attending veterinary clinics has a trend for more frequent visits as a kitten, reducing from 1–2-years-of-age and then increasing again when the cat is ‘old’. In running a feline specific senior health clinic, the goal is to encourage cat owners to bring their cats into the clinic before the cat becomes old. This provides us with the opportunity to diagnose and manage agerelated diseases before they start to have a significant impact on the cat’s health and welfare.
Download the poster icatcare. org/resources/how-old-isyour-cat-handout.pdf
When do cats become at risk of age-related diseases?
Ageing categories in cats have traditionally called cats ‘senior’ from 11 years-of-age and ‘geriatric’ or ‘super senior’ from 15 years.

Cats aged 7–10 years are considered ‘mature’. However, if we consider the literature on age-related disease (summarised in Table 1), these diseases do start to occur in cats of this age range and the pathophysiological changes that lead to the development of these diseases will be occurring for some time before clinical disease becomes apparent. Additionally, in a study of 206 cats aged 7–10 years-of-age, of the 176 that were completely examined, 45% were overweight or obese, 29% had a heart murmur, 5% had elevated systolic blood pressure, 54% had dental disease, 58% had abnormalities on orthopaedic examination, 10% were azotaemic, 3% were hyperthyroid and only 12% were ‘healthy’.27 So, ideally, a feline ageing cat clinic should be inviting cats aged 7 years and above.
How often should cats be attending an ageing clinic?
The individual cat’s disease status should always be taken into account as this is often the main factor in determining the frequency of visits. It is important to note that we do not know how frequently healthy cats should be visiting the clinic.
Guidelines produced are based on the best evidence available and that evidence is poor. Super seniors, or cats with a known age-related disease, should probably be visiting the veterinarian every 3 months. With a healthy super senior, a longer visit every 6 months and a shorter weight check every 3 months could be considered. For senior cats, every 6 months is likely to be the most appropriate, and for mature cats, an annual veterinarian visit with a short 6-month weight check should be sufficient ( Figure 2).
Key points for clinical examination
Weigh the cat at every appointment and record the body condition score (BCS).
Record the muscle condition. This may be a formal muscle condition score system or just a descriptive note of what you are seeing.
Always look in the cat’s mouth; dental disease in cats is very common.
Palpate for a goitre.
Listen to the heart on 2–3 different occasions during the examination as the presence of a murmur in cats will vary with the heart rate; listen when they first arrive and then later when they are settled.
Have a good look at the coat and note any areas of over or under-grooming.
During your history and examination, try to observe the cat walking around the room and perform an orthopaedic examination if indicated.
Diagnostics
Blood pressure
Ideally, this should be part of an annual examination from 7-years-of-age and at every appointment for cats who are hyperthyroid or have chronic kidney disease (CKD). Blood pressure will increase with age, so starting from 7 years-of-age will get cats used to the procedure and hopefully reduce the ‘white coat effect’. Cats with a reading over 140 mmHg (if they have CKD) or over 160 mmHg should have their retinas examined for signs of hypertensive retinopathy. Record the site the blood pressure was measured from, the cuff size used and the temperament of the cat during the measurement.
Urine sample
Ideally, collect a urine sample annually in cats over 7-years-of-age and more frequently in cats over 10-years-of-age. Test urine specific gravity, carry out a dipstick for blood and bilirubin, and a sediment examination for crystals and inflammatory cells.
Blood sample
Blood sampling could be included as part of a standard senior health check package on an annual or twice-yearly basis. Alternatively, you may choose to perform bloods when there is an indication to do so. Tables 2 and 3 summarise clinical indications for blood tests and what should be included in a minimum database.
Conclusions
Weigh the cat at every appointment; always try to record the ‘normal’ for the individual cat. This will make it easier to identify changes when they occur. Develop a general structure for your clinic but always be prepared to modify it for the individual.

The ICatCare Veterinary Society is the veterinary division of the pioneering cat welfare charity International Cat Care. Trusted by vets and nurses, it provides a worldwide resource on feline health and wellbeing, via the Journal of Feline Medicine of Surgery, by fostering an international community of veterinary professionals with a shared vision of feline welfare, and supporting professional development with practical CPD. Additionally, International Cat Care’s website provides a valuable resource of accurate information delivering what both vets and cats would want owners to know.
Figure 2. Recommended examinations at different life stages.
Table 1. Age-related diseases
Disease
Chronic kidney disease (CKD)
Hyperthyroidism
Hypertension
Diabetes mellitus
Osteoarthritis (OA )/ Degenerative joint disease (DJD)
Effect of age on prevalence of disease
Increases with age in cats over the age of 8–10¹
Prevalence of 37.4% in cats aged 0–4.9 years, 40.9% in cats aged 5–9.9 years, 42.1% in cats aged • 10–14.9 years and 80.9% in cats aged 15–20 yrs²
Between 18–30.5% of a geriatric cohort (aged 9+ years) developed azotaemia within a year3,4
UK prevalence of 2.5% rising to 8.7% in cats aged 10 years and above⁵ 6% in cats aged over 10 years also in the UK⁶
3.89% in cats aged over 10 years in Hong Kong⁷ 8.9% in cats aged over 9 years in Japan⁸
Prevalence of hypertension is higher in cats aged over 10 years9,10
Secondary hypertension is commonly associated with CKD and hyperthyroidism11,12 Cats show a significant increase in systolic blood pressure with age over a 12-year period13
An age of 6 years and over was determined as a risk factor for disease14
Overall prevalence of 92% with at least one joint affected by OA, with each year of additional age being associated with a 13.6% increase in a cat’s arthritis score (number of joints affected and severity of lesions)15
Overall prevalence of 61% of at least one joint with OA and 48% with more in one joint in cats aged 6 years and older16
33.9% prevalence in a group of cats with an average age of 6.5 years17
90% prevalence in a group of cats with an average age of 15.2 years18
Heart murmurs and hypertrophic cardiomyopathy (HCM)
Weight and body condition
Prevalence of heart murmurs: 24.1% in cats aged 6–12 months, 37.5% in cats aged 1–3 years, 44.1% in cats aged 3–9 years and 59.8% in cats aged over 9 years
Prevalence of HCM: 14.7% across a population of 780 cats rising 29.4% in cats over 9 years. Presence of a heart murmur and increasing age are significant risk factors for HCM19
Cachexia (loss of lean body mass) is associated with a number of age-related diseases in cats20,21
Sarcopenia (loss of lean body mass in the absence of disease) is associated with ageing in cats22
Body condition tends towards obesity between 7 and 13 years of age23
Dental disease
Cancer
A higher prevalence of tartar and periodontal disease is reported in cats over 3 years of age than cats under 3 years of age with an overall prevalence of 68%24
12.3% of cats over 5 years of age die due to neoplasia25
The frequency of tumour development increases with age in all types and locations26
Table 2. Clinical indications for blood tests and what should be included in a minimum database
Clinical indication Routine blood samples that should be run
Owner observed polyuria/polydipsia
Reduction in weight/BCS/muscle condition/development of entropion
Dental disease
Hypertension
Minimum database ± T4
Minimum database ± T4
Minimum database pre general anaesthesia
Minimum date base ± T4
New heart murmur/increasing grade of murmur T4 and PCV
Palpation of a goitre T4
Abnormal renal palpation
Arthritis diagnosed and NSAID are prescribed
Urine specific gravity under 1.035 without dietary explanation
Minimum database
Minimum database
Minimum database ± T4
BCS = body condition score; NSAIDs = non-steroidal anti-inflammatory drugs; PCV = packed cell volume; T4 = thyroxine
Table 3. Components to be tested
Test Components
Haematology
Biochemistry
Packed cell volume, total protein, smear evaluation or full CBC if clinically indicated
Albumin, total protein, urea, creatinine, phosphate, ALT, ALP, glucose and electrolytes if clinically indicated
ALT = alanine aminotransferase; ALP = alkaline phosphatase; CBC = complete blood count
Article references can be downloaded here: cve.edu.au/references-cnt-317
iCat Care Research Roundup
1. JFMS Clinical Spotlight article: Overweight and obesity in domestic cats: epidemiological risk factors and associated pathologies
2. Evaluation of laboratory findings indicating pancreatitis in healthy lean, obese and diabetic cats
3. Clinical findings, treatment, and outcomes in cats with naturally occurring hypoadrenocorticism: 41 cases
4. Clinical and prognostic relevance of Mycoplasma felis PCR detection in feline lower respiratory tract disease
5. Intraoperative nociceptive and clinical comparisons between ventral midline and flank ovariectomy in feral and stray cats

Read here: cve.edu.au/rr-march-25

What’s Your Diagnosis?
C&T No. 6045 (Issue 317 Dec 2024)
cve.edu.au/wyd-317-dec
Dr Ester Quilez
Specialist in Veterinary Clinical Pathology
University Veterinary Teaching Hospital Camden Veterinary Pathology Diagnostic Services
Sydney School of Veterinary Science
e: ester.quilez@sydney.edu.au
C&T No. 6062


Questions
What are the main diagnostic possibilities based on the gross appearance of the cutaneous preputial mass?
What is your diagnosis of the inguinal mass based on the cytology?
Which further diagnostics would you recommend?
Answer
High grade mast cell tumour
Main differentials for the cutaneous preputial mass
The most common preputial tumours in dogs include squamous cell carcinoma (SCC), transmissible venereal tumour and mast cell tumour (MCT).1 Any skin tumour common to haired skin may occur within the prepuce, and papilloma, haemangioma, fibroma, sebaceous adenoma, fibrosarcoma, and haemangiosarcoma have been reported within the prepuce.1
Cytological findings of the inguinal mass
The smears were highly cellular with excellent preservation of the cells. The nucleated cells consisted of a population of round cells seen individualized, in sheets and in small aggregates occasionally intermixed with a pink fibrillar material and at times interspersed with small capillaries. Amidst this population there were also moderate numbers of small lymphocytes and eosinophils, mild numbers of plasma cells, occasional medium sized lymphocytes and occasional macrophages/histiocytes. The background was clear and contains frequent variably sized pale blue cytoplasmic fragments.
The population of round cells showed a moderate N:C ratio and a round to rarely slightly polygonal to spindle (Figure 6) cell outline. Nuclei are round, central to paracentral with fine to coarsely granular to coarsely clumped chromatin and indistinct to distinct; one to several small to medium sized round to bizarrely shaped nucleoli. Anisonucleoliosis was identified. Cytoplasm was mild to moderate in volume, moderately basophilic and occasionally showed small amounts of dusty to granular magenta material. Anisocytosis was moderate. Anisokaryosis was moderate to marked. Frequent bi- and fewer multi-nucleated (up to 4 nuclei) forms were seen along with frequent mitotic figures including atypical forms.
Interpretation
Consistent with round cell tumour. Probable high grade mast cell tumour.
Diagnosis
The cytological findings, in light of the reported location for the inguinal mass, supported aspiration of an effaced lymph node with a metastatic high grade mast cell tumor.
Figure 2. Image of cutaneous preputial mass and large subcutaneous left inguinal mass
Figure 1. FNA from large subcutaneous left inguinal mass (Rapid Diff, X500 magnification)
Further testing
The preputial mass was surgically excised with no margins and submitted for histopathology ( Figure 3 ): the dermis was expanded by a large, nodular, non-encapsulated mass composed of round cells with moderate amounts of amphophilic cytoplasm arranged in sheets within the pre-existing stroma. Rare cells were faintly granulated with toluidine blue ( Figure 4). Anisocytosis and anisokaryosis were moderate with common binucleation. Mitotic figures were present at a rate of 18 in 2.37mm.2
Large numbers of eosinophils were scattered throughout the mass. (Histopathology description and interpretation provided by Dr Cheryl Rae Sangster).
AFAST (abdominal focused assessment) scan performed post-operatively revealed multiple hepatic and splenic lesions suggestive of metastasis. FNA of these lesions was not performed.
The patient was discharged with palliative care.
Discussion
Canine MCTs can be divided into cutaneous mast cell tumours, subcutaneous mast cell tumours, mucosal mast cell tumours, extracutaneous/extramucosal mast cell tumours without skin involvement, and mast cell leukaemia.2 In most cases, MCTs can be cured by surgical excision; however, a subset of MCTs is locally invasive and progress to fatal metastatic disease.3 Subungual, oral, and other mucous membrane sites, and preputial and scrotal tumours are associated with high grade tumours and worse prognosis.2,4
Cutaneous MCTs are often referred to as the ‘great pretender’ due to their ability to mimic a variety of clinical appearances and are sometimes inadvertently mistaken for nonneoplastic lesions. Well-differentiated MCTs tend to be solitary, small, slow-growing, nonulcerated tumours while undifferentiated MCTs tend to be rapidly growing, ulcerated lesions that cause considerable irritation and attain a large size.4 Metastatic rates for undifferentiated MCTs range from 55% to 96%, and most dogs with these tumours die of their disease within a year. The majority disseminate first to local lymph nodes, then to spleen and liver.2,4
Prognostic factors for mast cell tumours in dogs include histologic grade, clinical stage, location, cell proliferation rate (mitotic index, Ki67, argyrophilic nucleolus organizer regions-AgNORs), growth rate, microvessel density, recurrence, systemic signs, breed, tumour size, c-kit mutation, DNA copy number variation, and sex.4 Aberrant expression of KIT protein as detected with IHC has been shown to be a negative prognostic indicator for canine cutaneous MCTs.6,7 Duplication mutations activating exon 11 of c-kit are present in about 20% of canine cutaneous mast cell tumours and are more prevalent in
high-grade MCTs while mutations in exon 8 of c-kit are not associated with aggressive behaviour.6,8 Combining histologic grading with analysis of AgNORs, Ki67, KIT expression and detection of mutations in exons 8 and 11 provides the most detailed prognostic assessment.6
Histologic grade is considered the most consistent and reliable prognostic factor available for dogs with MCTs, although it will not predict the behaviour of every tumour.2,3,4,5,6 Although 3 histologic grading schemes exist, 2 are utilized commonly: the Patnaik system and the newer 2-tier system (Kiupel system). While no grading system is associated with 100% accuracy in predicting biological behaviour, the Kiupel system decreases interobserver variation and provides strong correlations with overall survival, MCT-associated mortality and risk of metastasis.3,6 This system divides canine cutaneous MCTs into high and low grade MCTs with the first group showing shorter survival times.3,5,6


Figure 4. Histopathology of cutaneous preputial mass (Toluidine blue, X400 magnification). Arrows indicate rare granulated mast cells.
Figure 3. Histopathology of cutaneous preputial mass (H&E, X400 magnification).
Poorly Granular
At Least Two of:
Mitotic Figures
>50% Anisokaryosis High Grade
Nuclear Pleomorphism
Binucleation or Mutlinucleation
Cytological mast cell tumour grading, while not yet validated, offers an easy, low-invasive and fast screening tool to provide treatment guidance and prognostic information in the initial stages of tumour grading. Efforts have been made to establish a standardized cytologic grading system5,9,10 for canine cutaneous mast cell tumours, yet none has gained widespread acceptance within the veterinary pathology community. A unique cytology grading system has been suggested by Camus et. al (2016) based on the 2-tier grading criteria ( Figure 6). In the proposed grading, MCT are considered high grade if poor granulation is identified, or if there are 2 of the following 4 cytologic features: presence of any mitotic figures, anisokaryosis >50%, binucleation or multinucleation, or nuclear pleomorphism.5 This scheme was found to be predictive of survival and correlated well with the 2-tier histologic grading system (sensitivity 88.2% and specificity 94.8%) although showed a higher false positive rate compared to histology. Although it’s not ideal to have a higher false positive rate in cytology compared to histology, it’s preferable for a screening test to minimize false negatives. This ensures that high-grade tumours, which require more aggressive treatment, are less likely to be overlooked.5,6

Summary
Figure 5. Algorithm for application of cytologic grading scheme for canine cutaneous mast cell tumours⁵
Encouraging cytological evaluation of MCTs can offer significant prognostic insights, particularly when identifying high-grade characteristics. This information prompts further assessment and helps tailor treatment strategies accordingly. High-grade MCTs often signify a poorer prognosis, necessitating more aggressive therapeutic interventions such as wider surgical margins or additional therapies like radiation or chemotherapy. However, it’s important to acknowledge that cytology serves as a screening tool and may not always accurately reflect histological grading, requiring confirmation through histopathological examination for definitive treatment planning.
References
1. Henry CJ, Higginbotham ML. Male reproductive tumors. In: Cancer management in small animal practice. 1st ed. Elsevier Saunders; 2010: 282-286.
2. Willmann M, Yuzbasiyan-Gurkan V, Marconato L, et al Proposed diagnostic criteria and classification of canine mast cell neoplasms: A consensus proposal. Front Vet Sci. 2021; 8
3. Sledge DG, Webster J, Kiupel M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet J . 2016; 215: 43-54.
4. Withrow SJ, Rodney LP, Vail DM. Mast cell tumors. In: Withrow and MacEwen’s Small animal clinical oncology . 5th ed. Elsevier Saunders; 2013: 335-355.
5. Camus MS, Priest HL, Hoehler JW, et al Cytologic criteria for mast cell tumor grading in dogs with evaluation of clinical outcome. Vet Clin Pathol. 2016; 53 (6): 1117-1123.
6. Kiupel M, Camus M. Diagnosis and prognosis of canine cutaneous mast cell tumors. Vet Clin Small Anim . 2019; 49: 819836.
7. Kiupel M, Webster JD, Kaneene JB, et al The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol . 2004; 41: 371-377.
8. Webster JD, Yuzbasiyan-Gurkan V, Kaneene JB, et al The role of c-KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia . 2006; 8: 104-11.
9. Scarpa F, Sabattini S, Bettini G. ‘Cytological grading of canine cutaneous mast cell tumours’. Vet Comp Oncol . 2016; 14 (3): 245-251.
10. Hergt F, Bomhard W, Kent MS, et al Use of a 2-tier histologic grading system for canine cutaneous mast cell tumors on cytology specimens. Vet Clin Pathol . 2016; 45 (3): 477-483.
Thank you to Natalie Courtman and Cheryl Sangster for their assistance with this case.
Figure 6. Arrow indicates a spindle-shaped mast cell
Winner - Best Visuals
Entitled to a a CVE$300 voucher
Possum Box Design
Niko Clemente
e. Niko.clemente@outlook.com
C&T No. 6063
After the 2019 bushfire season hit our area of Wombeyan Caves, seeing the direct impact on both the flora and fauna of the region was quite confronting.
One highly impacted habitat was the old growth gums and dead trees. Their hollows are crucial for the shelter and reproduction of many local species: microbats, sugar gliders, possums, cockatoos and Peregrine Falcons to name a few. With larger hollows taking over 100 years to form, human intervention becomes essential for rehabilitation. It is also a form of intervention that is accessible and financially viable to the individual.
My partner Daniel and I decided to design our own box, which accounted for our altitude and seasonal conditions, namely opting for hard wood with 25mm thick walls to better insulate in deep winter and temperature regulate in the heat of summer that we are prone to in our region. There are great resources about the basic principles that are required for housing many native species online (see links below). Once you have an understanding of them, you can be as creative or minimalistic as you desire in designing and building your own boxes.
https://www.environment.nsw.gov.au/researchand-publications/publications-search/ guide-to-making-a-possum-house
https://www.bct.nsw.gov.au/resources/managing-yourland/habitat-enhancement
https://www.abc.net.au/gardening/how-to/building-abat-box/9432924
https://www.lls.nsw.gov.au/__data/assets/pdf_ file/0006/656610/GS-LLS-Wildlife-Nest-Box-10-2017Accessible.pdf



large animal
Lumpy Skin Disease Exclusion Diagnosing
Severe
Dermatophilus Infection in a Weaner
Heifer
Dr Meg Parsons BVBiol BVSc
District Veterinarian, Local Land Services, Northern
Tablelands
e. mparsons@live.com.au
C&T No. 6064
Summary
Dermatophilosis is a bacterial skin infection caused by Dermatophilus congolensis. For this bacteria to cause disease, it is widely accepted that in addition to the carrier animal, two elements need to be present: skin damage and moisture. This case was unique as it did not have these aspects present. Furthermore, it was unusually severe in its presentation.
History
In April 2024, near Inverell in Northern New South Wales, a single heifer out of a mob of 60 head of Shorthorn cattle presented to their private veterinary practice for severe skin changes. Upon discussion with the client, Local Land Services was contacted and subsequently performed a property visit for the purposes of excluding Lumpy Skin Disease (LSD).
Two weeks prior to the property visit, the producer noticed skin lumps on the body of the affected heifer, which continued to spread and significantly worsen. The heifer had been weaned one week before the consultation, and while still eating and drinking, had lost a significant amount of weight in comparison to her herdmates. The producer did not believe she had been itchy, she was defecating normally, and was bright and alert. The heifer had not received any treatments.
No other cattle on the property had developed skin lesions. There were no adverse weather events, heavy rainfall or long wet periods in the month prior. The weaners had no access to any supplements, minerals or hay prior to weaning. The cattle were grazed on primarily native pasture with a small amount of sub-tropical plant species sewn down previously. There were no known
toxic plants or forage crops, including lucerne, vetch and cowpeas. The dams were up to date with clostridial and leptospirosis vaccinations and had received Multimin® injections. The property owner had not initiated a Pestivirus control or vaccination program, raising questions about the possibility of the heifer being a persistently infected calf.
Clinical Examination
Upon examination the heifer was bright and alert. Up to 80% of her skin was covered with grey, crusted, raised, coalescing lesions. When removed, the superficial dermal layer and hair were detached to leave raw, red skin underneath. There was no evidence of the characteristic purulent discharge under the scabs, often associated with Dermatophilus spp. Several areas appeared slightly necrotic where scabs had been previously removed. The heifer was mildly pruritic and did not seem painful.


Figure 2. Distribution of lesions along the backline
Figure 1. Wet hair allowing for the distribution of skin masses over the hindquarter to be appreciated


A B C

There was no erythema, heat or discharge observed; clinical signs that are commonly associated with an active infection. There were no lesions on the coronary bands or the mucous membranes, including the eyes, nose, tongue, gingiva, and vulva. The heifer had a fever of 40.7oC and had a body condition score of 2/5; the rest of her physical examination was within normal limits.
Lithium heparin, EDTA and plain blood samples, along with fresh and fixed scabs and biopsies were sent to Elizabeth Macarthur Agricultural Institute (EMAI).
Diagnosis
LSD testing was negative. Histopathology showed diffuse, marked, chronic exudative, neutrophilic dermatitis and folliculitis, characterised with ulceration, hyperkeratosis, haemorrhage and filaments of Gram-positive cocci bacteria (Chamings & Jordan, 2024). The histological changes and morphology of the bacteria was consistent with infection with the actinomycete Dermatophilus congolensis. A bacterial culture was then undertaken with profuse mixed growth including Dermatophilus congolensis (Chamings & Jordan, 2024).
The heifer was then tested for Pestivirus, to determine if she was a persistently infected animal. Testing was negative.
Treatment
The heifer was treated with topical chlorhexidine washes and a course of procaine penicillin at 15mg/kg as an intramuscular injection, once daily, for 5 days. Over a period of a month, the crusting and scabs fell off leaving hairless skin underneath. By the end of June 2024, most of her hair had regrown.
Discussion
Dermatophilus congolensis is a filamentous, branching, facultatively anaerobic, gram-positive actinomycete bacteria (Parkinson, Vermunt & Malmo, 2010; Lee, Rogers and Hillbrich, 2019). Infection causes a superficial, exudative dermatitis which is commonly known as ‘rain scald,’ ‘lumpy wool’ or ‘strawberry foot rot’ (Moriello, 2019). It can affect many domestic species including cattle, sheep, goats and horses. The bacteria may persist in healthy carrier animals and are not highly invasive (Read, 2011). Parkinson et. al. indicated that it is ‘virtually impossible’ to establish infection and lesions on skin that is intact and undamaged without moisture and skin abrasions (Smith, 2009; Parkinson, Vermunt and Malmo, 2010).
This severe presentation of dermatophilosis seems more commonplace overseas, although there have been reports of severe dermatitis cases in Australian cattle. (Read, 2011; Lee, Rogers and Hillbrich, 2019). Research in Australia has been predominantly focused around Dermatophilus spp. infection in sheep, with ‘lumpy wool’ or ‘fleece rot’ being significantly more common in Australia and having a production impact on wool enterprises (Parkinson, Vermunt and Malmo, 2010; Tellam et. al., 2021).
Factors which impact its ability to colonise and form clinical disease include weather conditions which result in prolonged wetting of the animal, and high temperature and humidity leading to further skin maceration (Moriello, 2019). Disruption of the integument by ectoparasites, particularly ticks in cattle, also influences the prevalence of disease (Ndhlovu and Masika, 2016; Tellam et. al., 2021). Other sources of skin damage can include prickly vegetation or biting flies. Our case had no known external factors precipitating the infection.
Figure 3. Skin lesions on the perineum, periorbital area and ear pinna
Dermatophilosis may be seen in animals of all ages but is most prevalent in young animals; particularly if they are chronically exposed to moisture or immunocompromised (Moriello, 2019). It is for this reason that Pestivirus testing was undertaken on the affected heifer. Other causes of immunocompromise, such as mineral deficiencies, were not investigated due to the excellent health of the remaining mob of cattle.
While it is likely the stress of weaning exacerbated the heifer’s disease, skin lesions were present and worsening prior to this. It is uncommon to see Dermatophilus spp. infections this severe in the Northern Tablelands; they are predominantly seen in tropical or subtropical regions (Parkinson, Vermunt and Malmo, 2010). It is even more uncommon to see them in a relatively dry season with low pasture density and without prolonged periods of wetting. Cases like this are a prime opportunity to rule out potentially serious exotic diseases, such as LSD.
This case report first appeared in the Flock and Herd publication.

References
Chamings, A. & Jordan, A. (2024). Laboratory Report; Final Report [PDF]. Department of Primary Industries, Elizabeth Macarthur Agricultural Institute.
Lee, E., Rogers, J. & Hillbrich, E. (2019). Dermatophilus and lice causing severe dermatitis in a heifer. Flock & Herd. https://www.flockandherd. net.au/cattle/ireader/dermatophilus.html
Moriello, K. A. (2019). Dermatophilosis in Animals. MSD Manual Veterinary Manual. https://www.msdvetmanual.com/integumentary-system/ dermatophilosis/dermatophilosis-in-animals
Ndhlovu, D. N. & Masika, P. J. (2016). Bovine dermatophilosis: Awareness, perceptions and attitudes in the small-holder sector of north-west Zimbabwe. Onderstepoort Journal of Veterinary Research, 83(1). 10.4102/ ojvr.v83i1.1004
Parkinson, T. J., Vermunt, J. J., & Malmo, J. (2010). Diseases of Cattle in Australasia. Vetlearn, Wellington.
Read, L. (2011). Dermatophilosis in weaner cattle. Flock & Herd. https:// www.flockandherd.net.au/cattle/reader/dermatophilosis.html
Tellam, R. L., Vuocolo, T., Denman, S., Ingham, A., Wijffels, James, P. J., Colditz, I. G. (2021). Dermatophilosis (lumpy wool) in sheep: a review of pathogenesis, aetiology, resistance and vaccines. Animal Production Science, 62(2), 101-113. https://doi.org/10.1071/AN21119
Get FREE Literature Updates to Your Inbox
C&T No. 6065
VetLit is an online resource set up by Simon Cook, Senior Lecturer in Emergency and Critical Care at the Royal Veterinary College, to help vets stay up to date with current veterinary literature.
FREE and users can subscribe to monthly updates and specialty emails
Can be used to look for specific topics, or article types including case reports, retrospective studies, review articles and clinical guidelines
"Articles are automatically added to the site as soon as they are published, ensuring users have immediate access to the latest research and findings. Many of the articles are open access.
VetLit is an excellent resource for busy veterinarians. In this attention economy, we need to actively decide where we get our information and what we give our attention to. The collation of abstracts provided by VetLit allows you to see the overall trends emerging in veterinary publications as well as allowing you to hone in on the articles which pique your interest. With veterinary science evolving and improving at an exponential rate, remaining up to date with veterinary medicine has never been more important. VetLit allows veterinarians to do this efficiently. We should never stop learning and we should always remain curious.
_ Elizabeth Thrift BVSc (Hons) FANZCVS Small animal medicine specialist
2024 Leptospirosis Update for NSW
With recommendations for dogs travelling to Queensland or the Northern Territory
Dr Christine Griebsch Dr med vet DipECVIM-CA (Small Animal)
EBVS® European Veterinary Specialist in Small Animal Internal Medicine
Senior Lecturer in Small Animal Medicine at SSVS
e. christine.griebsch@sydney.edu.au
t. +61 2 9351 3437
m. 0405 969008
C&T No. 6066
In 2024 there were 11 cases of canine leptospirosis in NSW: 1 in January (Jewells, Newcastle), 1 in February (Albury, Victorian border), 2 in March (Wollamia and Browns Mountain, South Coast), 2 in April (East Kangaloon, Southern Highlands; Bellevue Hill, Eastern suburbs), 3 in June (Vincentia, South Coast; Kangaroo Valley, Local Government Area (LGA) Shoalhaven; Weston, near Canberra), 1 in August (Matraville, Sydney Eastern suburbs) and 1 in September (Bondi Junction, Sydney Eastern suburbs).
Diagnosis was based on the presence of typical clinicopathological findings and positive PCR in urine (n=4), blood (n=2), blood and urine (n=5) and seroconversion (n=8). This brings the total number of canine leptospirosis cases to 90 since we started recording in 2017.
Case fatality was 45% (5/11), 4 dogs were euthanased due to severe anuric renal failure and one dog was euthanased due to financial constraints. Four dogs had been vaccinated with Leptospira interrogans serovar Copenhageni (Protech® C2i, Boehringer Ingelheim). One dog had completed a primary vaccination course seven months prior and recovered. One dog had completed a primary vaccination course 4½ years prior, but no booster vaccination had been given since then and this dog recovered. One dog had completed a primary vaccination course 2 months prior and recovered. One dog had received one vaccination 3 years prior; however, had not completed a primary vaccination course and this dog recovered. All of these previously vaccinated dogs tested positive for serovar Australis on MAT which was assumed to be the infecting serovar.
Microscopic agglutination testing (MAT) was conducted in 10/11 dogs and the results of the highest titres are summarized in Table 1.
We recommend vaccination against leptospirosis in dogs living at the South Coast and Newcastle area, in Inner Sydney, Sydney’s Eastern suburbs and Inner West or in any area if the dog is in contact with rats or other rodents
Vaccination in other geographic locations with confirmed leptospirosis cases should be considered and discussed with clients; however if there is any doubt about the risk of exposure we would recommend vaccination. The South Coast has been a major leptospirosis hotspot in 2022 with 27 cases reported to date and serovar Australia was the causative serovar in all cases in which an MAT was performed (Griebsch et al., 2024). While serovar Copenhageni is still the predominant serovar in the Greater Sydney area, serovar Australis has also been found in this region. Our research data has contributed to development of a new bivalent vaccine containing serogroup Australis serovar Bratislava and serogroup Icterohaemorrhagiae serovar Copenhageni (Nobivac Lepto 2, INTERVET AUSTRALIA PTY LIMITED) which is now available in Australia. This vaccine is thought to induce cross protection against serovar Australis. We recommend using this vaccine in all dogs residing in or travelling to the South Coast and in all dogs travelling to Queensland or the Northern Territory in which serovar Australis is the predominant infecting serovar. The new bivalent Nobivac Lepto 2 vaccine should also be considered and offered to all dog owners in the Greater Sydney area and for dogs that have never been vaccinated against leptospirosis and puppies we recommend vaccinating with this vaccine in Greater Sydney. Of note, if switching to the new bivalent vaccine a primary vaccination course (two vaccines given 2-4 weeks apart) is necessary in all dogs even if they have previously been vaccinated against leptospirosis Therefore, some clients in the Greater Sydney area might prefer to continue with a yearly booster with Protech® C2i from Boehringer Ingelheim containing serovar Copenhageni which has previously been the only registered leptospirosis vaccine for dogs in NSW.
Interestingly, we did not only find regional differences of infecting serovars but also some differences in clinical presentation. Dogs infected with serovar Copenhageni were significantly more likely to have hepatic involvement with significantly higher liver enzyme activities, bilirubin concentration and icterus whereas dogs with serovar Australis were significantly more likely to have glucosuria (Griebsch et al., 2024).
While there currently seems to be suffcient vaccination supply we have had some vaccination shortages in past years. In the event of a vaccination shortage, for concerned owners unable to complete a primary vaccination course or the annual booster vaccination, we recommend providing the following advice to clients:
Risk mitigation methods are the most important measures to prevent leptospirosis. Contact with sources of infection should be limited. This includes limiting swimming or drinking of stagnant water and avoiding contact with possible reservoir hosts such as rodents and farm animals, which can be achieved by fencing and rodent control. Similarly, contact with dogs with leptospirosis should be avoided. In endemic areas—especially during leptospirosis outbreaks—close dog-to-dog contact like doggy day care and boarding in kennels should be reconsidered.
Please contact the author for more information or if you would like to participate in any ongoing leptospirosis studies.
The University of Sydney is continuing to investigate leptospirosis cases to determine the causative serovars and if there is any specific source of infection and risk factors that can be identified. We are also testing dogs who have been in contact with dogs with clinical leptospirosis to assess their risk of infection. Other current ongoing studies include investigating the role of leptospirosis in dogs with PU/PD or CKD and the duration of shedding after commencing antibiotic treatment.
Leptospirosis may be suspected in any dog with
Nonspecific clinical signs like lethargy, vomiting and diarrhoea, which can precede more obvious clinical signs like icterus
Azotaemia +/- hyperbilirubinaemia, elevated liver enzymes +/- glucosuria
Important information to ask
Is there any contact with rats?
Is there any contact with stagnant water (e.g. ponds)?
Which area is the animal from?
Has there been any travel into areas in which there have been reported cases (Albion Park, Albury, Annandale, Ashfield, Balmain, Bardia, Bayswood, Bellevue Hill, Bobs Farm, Bondi Junction, Browns Mountain, Burradoo, Cambewarra, Cardiff, Cardiff Heights, Cheltenham, Clovelly, Cooks Hill, Crows Nest, Darlinghurst, East Kangaloon, Elanora Heights, Erskineville, Falls Creek, Figtree, Firefly, Glebe, Gresford, Horsley Park, Huskisson, Ingleside, Jewells, Jervis Bay, Kangaroo Valley, Kembla Grange, Lurnea, Marrickville, Matraville, Medowie, Newcastle, Newtown, Old Erowal Bay, Paddington, Potts Point, Randwick, Rangari, Redfern, Robertson, Sanctuary Point, Speers Point, South Coast, St Georges Basin, Sanctuary Point, Surry Hills, Sussex Inlet, Tomerong, Tuggerah, Trunkey Creek, Vincentia, Wallsend,
Waterloo, Weston, Woollamia, Worrowong Heights)?
Of particular importance are the movements of the dog in the 30 days prior to developing clinical signs.
In suspicious cases, we recommend the following
Collect urine and EDTA blood samples BEFORE giving antibiotics and send to IDEXX or Vetnostics for PCR—if you obtain a positive result, please inform us about the case and request the laboratory ships leftover samples to us after obtaining client consent—these will be useful for further research
Collect a serum sample—send to IDEXX or Vetnostics for antibody testing (this will help to identify the infecting serovar). If there is a high index of suspicion of leptospirosis but the PCR is negative it is important to perform another titre 2 weeks later to determine whether there has been seroconversion. Similarly, in confirmed cases of leptospirosis, a follow up titre will be helpful to determine the causative serovar. Ensure appropriate PPE (gloves and gowns) are worn when handling the animal, as leptospirosis is a zoonotic disease.
Start treatment with IV fluids and antibiotics immediately after collecting diagnostic samples (do not wait for results). Intravenous penicillin derivatives such as ampicillin or amoxicillin are recommended initially; however, these will not clear the organisms from the kidneys. To clear the infection, oral doxycycline (5mg/kg BID or 10mg/kg SID) should be given for 14 days once the patient can tolerate oral medication.
The animal should be isolated from other animals and only be handled with appropriate PPE. We currently recommend isolation for 72 hours following the commencement of antibiotics. Ideally a urinary catheter should be placed to monitor urine output and avoid contamination of the environment with urine.
If you have a suspicious case and would like to participate in the ‘duration of shedding after antibiotic treatment’ study, please contact us promptly for further advice.
The owner/s should be advised to seek medical advice.
We request your help, please!
Report any suspicious cases to the author
Obtain and store serum, EDTA and urine.
Separate serum, use small urine tubes if possible and freeze samples if stored for >1 week if storage time is less, we can collect the samples or organise a courier. If you have a high index of suspicion for leptospirosis
The client is financially constrained, please contact christine.griebsch@sydney.edu.au and send us the history and blood results for the patient. We have a small amount of research funds available to cover costs for leptospirosis testing in those cases.
In-contact dogs should also be treated with a 14-day course of doxycycline.
If possible, and after obtaining client consent, collect whole (EDTA) blood, urine and serum from these in contact dogs before starting doxycycline This will help us to assess if in-contact dogs are infected without having clinical signs (silent shedders) or have been exposed to leptospirosis without being infected.
We will provide you with an appropriate submission form and cover the costs for testing in contact dogs and will inform you of the results.
Questions or you’d like to discuss a case in person?
Contact Dr Christine Griebsch as per the contact details at the beginning of this article.
References
Griebsch, C., Kirkwood, N., Ward, M. P., So, W., Weerakoon, L., Donahoe, S., & Norris, J. M. (2022). Emerging leptospirosis in urban Sydney dogs: a case series (2017-2020). Aust Vet J, 100(5), 190-200. doi:10.1111/avj.13148
Griebsch, C., Kirkwood, N., Ward, M., & Norris, J. (2024). Serovar Australis replaces serovar Copenhageni as the most common cause of canine leptospirosis in New South Wales, Australia. Australian Veterinary Journal – accepted 14 November 2024
hop://doi.org/10.1111/avj.13401 hops://onlinelibrary.wiley.com/share/ author/547TWSP2DCP57HGGKEA6?target=10.1111/avj.13401
AUS, Australis, ARB, Arborea; BAT, Batavia; BRA, Bratislava; BUL, Bulgarica; CAN, Canicola; COP, Copenhageni; CYN, Cynopteri; DJA, Djasiman; HAR, Hardjo; ICT, Icterohaemorrhagiae; KRE, Kremastos; PAN, Panama; POM, Pomona; ZAN, Zanoni
Table 1: Cross reaction may occur with MAT. Titres in bold and highlighted in dark blue indicate the serovar we think is responsible based on other cases in the region but confirmation of this is not possible until a serovar specific PCR test is validated. Titres highlighted in light blue demonstrate cross reaction with other serovars versus exposure to multiple different serovars.
The Busyness Epidemic in Veterinary Medicine: Reality
or Self-Fulfilling Prophecy?
Dr Jessica Moore-Jones
Unleashed Coaching & Consulting Is first and foremost about people. We believe whole-heartedly (and have the data to back it up) that focusing on people first IS the answer to profit and process, not the other way around.
Keeping Veterinary Professionals in the Veterinary Profession through resilient teams, supported managers, and sustainable cultures.
e. jessica@unleashedconsulting.com.au w. unleashedconsulting.com.au
C&T No. 6067
How often in a day do you tell people you’re busy? In a week?
How often is it the standard, default response to ‘How’s your day going? ’ or ‘Hi, how are you? ’.
Somehow, ‘busy’ has become an expected state of being. A default response for how we are, irrespective of what’s happening in our lives. In a small social study in 2019, a researcher found that nearly 8 out of 10 people responded to ‘How are you? ’ with some variation of the word ‘Busy’.
Of course, sometimes we are genuinely busy. Sometimes the workload is extreme, and out of the ordinary. Some days really are manic, and we’re chasing our tails and running between consults and wondering when it’s going to end.
But the problem with ‘busy’ is that it’s infectious
In the clinic, as soon as someone mentions their busyness, others feel the need to be / act / feel busy. If a couple of people are pulling their hair out with chaos, the rest of the room feels a sort of peer pressure to be doing the same.
If someone asks me how I am and I DON’T say busy, does that make me lazy? Or am I not being helpful enough? Am I a poor team player, or will I be asked to do more? I
can’t exactly respond to their exasperated schedule with ‘ I’m pretty chill, thanks for asking.’ And so I agree about how busy we are, maybe even see your ‘busy’ and raise you a ‘hectic’. And next time we spiral upwards towards ‘insane’ or ‘slammed’.
And where can we go from there?
Ever upwards in a self-fulfilling prophecy of feeling obliged to one-up each other’s (or our own) state of busyness, that has little room for us to ground ourselves lest we suddenly admit to having a quiet day and feel like something must be wrong with us. Or worse, that we might be redundant.
We somehow have become a culture that congratulates being busy, competing for the ultimate confirmation of our martyrdom when our schedule out-busies that of our colleagues. We seek validation that our consult list is longer, our surgeries more hectic, our personal lives more packed. And God forbid that someone feels comfortable enough to say out loud that they had a full 9 hours of sleep last night! To quote the 1987 movie Wall Street: ‘Lunch is for wimps’
What’s wrong with being busy?
Our brains are wired towards confirmation bias.
When we focus on the frazzled feeling of always rushing between things, each new addition to a task list confirms for us this constant and inescapable state of being. Each consult that runs late, every blown catheter, that stupid broken tooth root, or that colleague who isn’t keeping up with their share of the list, further solidifies to us that we can’t possibly keep up. That this workload is untenable. That our busy has become ‘slammed’ and that becomes ‘impossible’ and then suddenly, where can we go from there? Even if we do get a couple of hours where everyone avoids saying the dreaded Q-word, as soon as the pace picks back up again we flit right back to the confirmation that we are too busy to cope.
There’s nowhere to downgrade, nowhere to find pause
And certainly no room for people around you to feel like they’re allowed to have a cruisey day. If the heroes are those who stay late, skip lunch breaks and repeatedly get UTIs from not having time to pee, how is a new graduate or freshly hired nurse supposed to calmly and dramafree announce that she’s heading to sit in the sunshine for her whole allocated lunch break?
Worse, research has shown that when the task-positive network of your brain, the one where you’re focused on attention-demanding tasks, is overly active, we are less good at using our default network—the one which thinks
beyond the present. The one which helps us be creative, to experience meaning and fulfilment, to enjoy social interactions. In other words, excessive busyness limits your capacity to thrive.
While that might seem obvious, acknowledging it is a vital step in choosing to create a culture where we stop rewarding the tail-chasing, stop revering the timemartyrs, and definitely catch our own brains before we let ourselves fall into a trap that might be stopping us from getting what we want out of our day.
What can we do about it?
While it’s not easy to go against the tide of a global societal trend towards busyness, we can (and absolutely should) set a culture in our clinics that makes it absolutely clear that we value meaningful achievement rather than constant activity.
Establish and reinforce the culture
Leaders need to set an example. Take breaks. Go home on time, or at least appear to. Announce loudly when you’re leaving early to watch the kid’s soccer game. Respond to requests for your time with considered words that make clear you are valuing your priorities appropriately. If you are working outside of business hours, use your technology to ensure emails don’t send until 8am.
Experienced staff role model what they want younger or newer staff to experience. Leave the building for lunch. Sit in the sunshine instead of at a computer. Do one task at a time.
For every competition your workplace runs that might invertedly add busyness (e.g. who can book the most dentals in, who can see the most consults), ensure there is a separate competition that makes clear what your organisation really values. Have a competition for who took the MOST lunch breaks that month. Who did the LEAST overtime. Who said no, or delegated a task, or asked for help. Find ways of validating and publicly recognising that the heroes of this organisation are not the people who look the busiest, but the people who successfully do their job in a calm, considered, sustainable manner.
Set yourself up for success
Avoid multi-tasking. It makes us feel active and therefore can convince us that we’re being productive, but research is clear that it doesn’t work. Multi-tasking simply makes us FEEL busy, while being less productive at either task.
Don’t let technology hijack your priorities. Turn off notifications on your emails, and only check them at specified times (before lunch and at the end of the day are highly recommended so that they don’t derail all
your bigger priorities at the start of the day). Set autoresponses to make this clear, and letting people know to call you if they need a more urgent response. Leave your phone in your locker. Yes, even managers should do this. There are rarely emergencies so great that they aren’t as easily fixed at lunch time as now (in fact, you may find a bunch of those ‘emergencies’ sorted themselves out before you got to it, when people are forced to manage a situation themselves). Do one thing at a time.
The words we choose matter
When someone asks you how you are, find a different adjective. It’s as simple as that! You are very rarely only feeling one emotion at a time; pick a different one. Or simply find a synonym that brings down the drama associated with busy. ‘ Steady ’ is a good one, or ‘ productive’ is much more fulfilling.
If you must say busy, challenge yourself to add additional information that reframes the cycle. You can be ‘ busy, and productive’, or ‘busy, and excited by business picking up’ or ‘ busy, I learned a new surgery which slowed me down but it was worth it ’ . Give an explanation for your busyness that finds the positive and avoids adding to the culture of chaos.
If you cannot find a different, calmer adjective, and you cannot find a way to explain why your activity has been productive and fulfilling, then the final option is to add a ‘BUT’ on the end of your sentence. You can be busy, IF you are doing something about it. So you could be ‘ busy, but I’ve scheduled myself a break in half an hour ’ , or ‘ busy, but I’ve got a holiday booked next week ’ , or ‘ busy, but I’ve spoken to my manager about how we can adjust my schedule to allow me to fit more meaningful tasks into the morning’. Again, this is simply about acknowledging the truth of the matter but without letting it become a measure of success. Being clear to yourself, and to anyone else who might be listening, that busyness is NOT something you consider a badge of honour, but a problem that you are committed to fixing.
Veterinary practice often is busy. There are genuinely hectic days, and it’s ok to acknowledge that. But if we use it as our default state of being, then how do we not become the boy who cried wolf? Where do we go from there if each day must be busier, harder, more hectic than the last in order for us to maintain our sense of value and usefulness? So allow yourself permission to NOT be busy. To find a new word, to reframe your productivity, to carve out calm. And importantly, to make it clear to others around you that the heroes around here are those who can do veterinary medicine sustainably, not the ones who skip the most lunch breaks.
Being busy is not a virtue—being productive is.
PersPective Nasal Disease in Cats
Rachel Korman BVSc MANZCVS (Internal Medicine)
FANZCVS (Feline Medicine) Specialist Feline Medicine
Alison Jukes BVSc (Hons) FANZCVS
Specialist Feline Medicine
Cat Specialist Services
1-15 Lexington Road, Underwood QLD
t. +61 7 3841 7011
e. drkorman@catspecialists.com.au
Rachel and Alison are Co-Tutors in the Feline Medicine Distance Education course cve.edu.au/feline-medicine
Perspective No. 165
Chronic nasal disease in cats may be caused by various aetiologies including infection, inflammatory and neoplastic disease. Definitive diagnosis can require advanced imaging and biopsy, yet despite utilisation of these modalities and depending on the disease process, treatment ultimately is about long term management rather than cure in many cases.
Aetiology
Infectious
Inflammatory
Viral e.g. Feline Herpesvirus and chronic rhinosinusitis
Fungal e.g. Cryptococcus,
Aspergillus,
Mycobacteria
Tooth root abscess
Oronasal fistula
Nasopharyngeal polyp Chronic lymphocytic plasmocytic rhinitis
“nasal IBD”
Foreign body
Neoplasia
Lymphoma
Adenocarcinoma
Squamous cell carcinoma
Congenital Choanal atresia
Nasopharyngeal stenosis
A recent study evaluated 400 nasal biopsy samples from cats in the United Kingdom. The most common nasal disease was rhinitis, followed by neoplasia and polyps. Nasal lymphoma was the most common neoplasia, followed by adenocarcinoma and undifferentiated carcinomas, with benign tumours being very uncommon. There was no association identified between skull
conformation and the type of nasal disease. Polyps were more likely to have been identified in younger male cats (median age 8 years) with a meocephalic skull and no nasal discharge. A previous older study of 77 cases identified neoplasia most commonly (lymphoma) followed by chronic rhinitis. Unsurprisingly, neoplasia was more likely in older cats. These cats were also more likely to be dyspnoeic and have a haemorrhagic or unilateral nasal discharge.
Clinical signs associated with sinonasal disease are typically fairly obvious and include a combination of nasal discharge, noisy breathing and sneezing. Epistaxis and facial distortion may be seen. Appetite can be variably affected. Acute, intractable sneezing would be suggestive of a foreign body, whereas chronic intermittent sneezing would be suggestive of chronic rhinosinusitis or neoplasia.
Alterations in swallowing, paroxysmal reverse sneezing, stertor and an obstructive respiratory pattern (inspiratory dyspnoea) suggest involvement of the caudal nasal cavity or nasopharynx. Stertor is often most audible during inspiration. Upper airway sounds that are audible during both inspiration and expiration suggest a fixed obstruction (e.g. neoplasia or nasopharyngeal stenosis). Owners may also report that their cat has recently started snoring.
Ocular discharge may occur due to obstruction of the nasolacrimal duct. Peripheral vestibular signs, head shaking or scratching can be seen with nasopharyngeal polyps. Intracranial neurological signs can be seen with neoplasia or fungal infection with extension of disease through the cribiform plate.
Signalment is a major consideration in cats with chronic nasal disease. Young cats are more likely affected with upper respiratory infections, foreign bodies, nasopharyngeal polyps and nasopharyngeal stenosis. Older cats are more likely affected with neoplasia or chronic rhinosinusitis.
Physical examination yields many useful diagnostic clues. A systematic review of all body systems is mandatory in cats presenting with signs of upper respiratory disease as involvement of other organs may yield important information (e.g. lymphoma). Examination of the nasal cavity includes visual inspection and palpation of the head, eyes and muzzle for conformation, symmetry and defects. The external nares are inspected for colour, presence and characteristics of nasal discharge. Evaluation of patency and airflow can be accomplished


with a clean compact disc or glass slide observing for condensation during expiration.
The hard palate can be evaluated for conformation change suggestive of a space occupying lesion causing ventral deviation of the palate. The upper dental arcade is examined for dental disease (e.g. tooth root abscess). Careful palpation of the submandibular lymph nodes and an otoscopic examination are also important.
Serology can be performed prior to investigations that require sedation or anaesthesia. A latex cryptococcal antigen agglutination test (LCAT) detects cryptococcal antigen in serum. A positive titre confirms active infection. A bedside IMMY test is also available.
Haemorrhagic nasal discharge needs a separate consideration. In addition to nasal cavity disease (e.g. foreign body, neoplasia, fungal disease), systemic diseases also need to be given consideration (e.g. hypertension, polycythemia, coagulopathy, hyperviscosity syndrome, immune mediated thrombocytopenia). If the nasal discharge is primarily haemorrhagic, then investigations such as assessment of a manual platelet count, total protein, systolic blood pressure and assessment of clotting times must be performed prior to any invasive testing.
If enlarged local lymph nodes or facial deformity is present, then aspiration for cytology and culture can be performed prior to more invasive testing. Fine needle aspirates through the soft palate can also be obtained if there is obvious ventral deviation.
Bacterial culture of superficial nasal swabs is rarely helpful. The nasal cavity of healthy cats can be colonised with Cryptococcus sp. So definitive diagnosis of Cryptococcus as the causative organism requires a positive LCAT or cytological evidence of a large organism burden and inflammation.
Diagnostic imaging plays an important role in the investigation of nasal disease and ultimately requires general anaesthesia. Imaging is performed prior to invasive procedures so that haemorrhage does not affected findings.
Nasal and sinus radiography rarely yield a definitive diagnosis but are able to detect asymmetry, bone destruction or the presence of a soft tissue opacity. Typical views include dorsoventral, open-mouth ventrodorsal and lateral views. Oblique lateral views and skyline views are often required for assessment of the frontal sinus and bulla. Patient positioning is important.
Computed tomography (CT) provides extensive detail of the nasal cavity and sinuses and is the gold standard for evaluation of nasal disease, particularly early lesions. It is also superior for evaluating the extent of invasive nasal disease e.g. involving the cribiform plate or orbital involvement and can facilitate guidance for biopsy.
Magnetic resonance imaging (MRI) also provides a sensitive modality for assessment of soft tissue structures and can readily distinguish between soft tissue structures and fluid or mucous accumulations. However, it may be less sensitive in identification of early bony or cartilaginous changes and slice thickness may affect visualisation of changes to the cribiform plate. If intracranial involvement is suspected however, MRI is superior. Limitations of both CT and MRI include cost, technical skill and availability of equipment.
Rhinoscopy facilitates direct visualisation of the nasal cavity and nasopharynx. This can be limited in cats due to their size, availability of appropriately sized scoping equipment and technical skill. When combined with CT or MRI, rhinoscopy allows complete evaluation of the nasal cavity. Rhinoscopy alone can identify large focal lesions or diffuse inflammatory or infectious disease, but does not allow complete evaluation of the entire nasal cavity (often access to the dorsal meatus is limited) and lesions may be missed.
The nasopharynx is examined first to avoid iatrogenic haemorrhage obscuring visualisation as blood will pool in the nasopharynx. The nasopharynx, choanae and caudal nares are evaluated by retroflexing a flexible endoscopy (2-5 mm outer diameter) through the oral cavity and dorsally over the soft palate. Lesions identified in this location include polyps, foreign bodies, nasopharyngeal stenosis, fungal granulomas and neoplasia. Biopsies
Figure 1. Nasal discharge in a cat with nasal lymphoma
Figure 2. Retropharyngeal grass foreign body in a cat with chronic rhinitis
can be obtained using a flexible endoscopy or a spay hook can be used to retract the soft palate and curved biopsy forceps used to blindly biopsy the nasopharyngeal mucosa. Be ready for bleeding from these locations.
Prior to anterior rhinoscopy biopsy or flushing, it is important to protect the airway by packing the nasopharynx with gauze swabs (throat pack) to prevent aspiration of fluid or material. These swabs must be removed prior to recovery and evaluated for evidence of tissue or foreign body material that maybe important for sampling purposes.
The left and right nasal passages are then examined by directing a rigid endoscope through the nares. Soft tissue masses, nasal foreign bodies and turbinate loss suggestive of fungal rhinitis can be identified and appropriate biopsies obtained.
Nasal biopsy is vital for a definitive diagnosis and is essential to differentiate chronic rhinitis and fungal rhinitis from neoplasia. Rhinoscopic assessment of nasal disease does not always correlate with the severity of inflammation detected histologically. Biopsies can be obtained via blinded or guided techniques. It is vital to ensure that the cribiform plate is avoided. This is done by measuring the distance from the nares to the medial canthus of the eye and marking this point (e.g. using tape) on the biopsy instrument. Nasal biopsies often result in a large amount of bleeding. It is good standard practice to ensure assessment of a platelet count and ideally coagulation parameters (e.g. APTT, PT or activated clotting time) prior to sampling.
Blind biopsy techniques include the use of cup or pinch biopsy forceps, straight haemostatic forceps. Measuring to the level of a lesion identified on imaging can help guide the location. The forceps are inserted closed, then opened slightly towards the level of the lesion, advanced another few millimetres, closed again to “grasp” the sample and then removed quickly.

Rhinoscopy can also be used to guide nasal biopsies and obtain deep, targeted mucosal biopsies.
Nasal flushing can be performed, however nasal flush cytology may not be reliable for a definitive diagnosis. Samples obtained from nasal flush correlate with histopathological diagnoses in only 50% of dogs with nasal neoplasms. Identification of neoplastic epithelial cells or fungal hyphae or yeast may be useful, however. Certainly, nasal flushing can move inspissated secretions and provide relief to patients. Flush may also dislodge fragments of friable tumours or granulomas. A 10 mL syringe filled with room temperature, sterile saline is forcefully infused into the ventral nasal meatus with the contralateral naris blocked. This is repeated two to three times on each side. Ensure evaluation of the nasopharyngeal swabs for potential samples.
Haemorrhage can occur following nasal biopsy. Bleeding can be controlled utilising infusions of cold saline, ophthalmic phenylephrine (2.5%) instilled directly into the nasal cavity or the use of cotton tips soaked in adrenaline (diluted 1:100 000).
Biopsy samples are submitted in formalin for histopathology and plain samples in a sterile container for culture (e.g. fungal and bacterial) and PCR. Primary bacterial rhinitis is very rare and most bacterial infections are secondary colonizers. Bacterial culture is likely significant if there is a heavy growth of a single organism. Macerated tissue culture maybe of more diagnostic value than culture of nasal secretions or flush samples. Positive fungal cultures are likely accurate.


Figure 3. Nasal mass in a cat with carcinoma
Figure 4. Reactive lymphoid hyperplasia on rhinoscopy in a cat with chronic rhinitis
Figure 5. Destructive sinorbital rhinitis on CT in a cat

This course presented by Taronga will support veterinary professionals and veterinary nurses to develop knowledge and skills in native wildlife triage, including first aid, initial treatment and emergency care.
• Supported by the NSW Government Department of Planning and Environment
Email: tarongprofvet@zoo.nsw.gov.au
Visit: https://taronga.org.au/vet-professional-training
• AVA and VNCA certified
• Online Course (20 CPD points)
• Hands-on Workshop (12 CPD Points) –available in NSW, QLD and Vic (see website for details)
• Subsidised positions available to eligible applicants nationally Semester 2, 2025 Apply from Monday 12 May




Workshops available at:

