OAKWOOD HOSPITAL & MEDICAL CENTER-DEA
Summary by Body System, Sex, Class, Status and Best CS/AJCC Stage Report
Filter(s):Quick Filter: Year:1ST CONTACT YEAR 2017-2017

OAKWOOD HOSPITAL & MEDICAL CENTER-DEA
Summary by Body System, Sex, Class, Status and Best CS/AJCC Stage Report
Filter(s):Quick Filter: Year:1ST CONTACT YEAR 2017-2017
Oral Cavity & Pharynx - 31 (4%)
Lung & Bronchus - 138 (20%)
Pancreas - 24 (3%)
Kidney & Renal Pelvis - 38 (5%)
Urinary Bladder - 51 (7%)
Colon & Rectum - 73 (10%)
Prostate - 135 (19%)

Non-Hodgkin Lymphoma - 33 (5%)
Melanoma of the Skin - 2 (0%)
Leukemia - 20 (3%)
All Other Sites - 158 (22%)
Thyroid - 42 (4%)
Lung & Bronchus - 168 (17%)
Breast - 344 (35%)

Kidney & Renal Pelvis - 20 (2%)
Ovary - 33 (3%)
Uterine Corpus - 76 (8%)
Colon & Rectum - 62 (6%)
Non-Hodgkin Lymphoma - 30 (3%)
Melanoma of the Skin - 3 (0%)
Leukemia - 14 (1%)
All Other Sites - 182 (19%)
Images reprinted by the permission of the American Cancer Society, Inc. from www.cancer.org. All rights reserved.
BACKGROUND
• Chemotherapy is one of the mainstays of treatment for many oncologic disorders
• Chemotherapy use is often associated with myelosuppression, including neutropenia
• Febrile neutropenia (FN) is an oncologic emergency and is defined as:
o Absolute neutrophil count (ANC) of < 500 cells/mm3 or expected to decrease to < 500 cells/mm3 in the next 48 hours AND
o Temperature of ≥ 38.3°C (101°F) or ≥ 38.0°C (100.4°F) sustained over a 1-hour period
• Management of FN
o Prompt evaluation
o Initiation of an anti-pseudomonal antimicrobial
o Additional antimicrobial therapy is determined by risk factors for gram-positive, fungal, and mold infections
o Delay in antimicrobial administration or improper selection is associated with increased mortality
STUDY AIM
• The purpose of the study was to evaluate the appropriate use of antimicrobial agents for the management of FN at Beaumont Hospital - Dearborn.
STUDY DESIGN
• Single-center, retrospective cohort of patients with documented ICD-10 codes for FN between October 2016 to October 2017
• Descriptive statistics
PRIMARY OUTCOMES
• Evaluate the appropriate selection of antimicrobial agents in patients with documented FN
• Assess the appropriate duration of antimicrobial therapy in FN
SECONDARY OUTCOMES
• Document the use of granulocyte colony-stimulating factor (G-CSF) agents in patients with FN
INCLUSION CRITERIA
• Age ≥ 18 years of age
• Receipt of chemotherapy within 30 days of diagnosis of FN
• Fulfilled criteria for FN (or clear evidence of neutropenia episode with fever, if not explicitly stated)
EXCLUSION CRITERIA
• Received chemotherapy greater than 30 days of FN diagnosis or not on active chemotherapy
• No cancer diagnosis
• No documented evidence of fevers
Ciprofloxacin 750 mg PO Q12 hours + amoxicillinclavulanate 875/125 mg PO Q12 hours
Penicillin allergy (hives or bronchospasm): Ciprofloxacin
750 mg PO Q12 hours + clindamycin 300 mg PO Q6 hours
Ciprofloxacin or levofloxacin monotherapy
Indications for empiric gram-negative coverage (High risk)
Cefepime 2 g IV Q8 hours
If mucositis present: Consider piperacillin-tazobactam 4.5g IV Q6 hours (or adding metronidazole to cefepime)
Penicillin allergy: aztreonam 2 g IV Q8 hours
Extended-spectrum beta-lactamase (ESBL) risk: meropenem 1 g IV Q8 hours
Indications for empiric gram-positive coverage (Mainly MRSA)
Hemodynamic instability
Pneumonia documented radiographically
Suspected line of catheter-related infection
Colonization or infection with MRSA, VRE, or penicillin-resistant Streptococcus pneumoniae
Persistently febrile on anti-pseudomonal therapy for ≥48 hours
Additional considerations:
Evidence of severe sepsis
Blood culture for gram-positive bacteria
Skin or soft-tissue infection at any site
Severe mucositis (if ceftazidime used and fluoroquinolone prophylaxis had been given)
Linezolid and daptomycin for severe vancomycin allergy, history, or risk of VRE
• Appropriate antipseudomonal coverage was evaluated based on the National Comprehensive Cancer Network “Prevention and Treatment of Cancer-Related Infections” guideline and from an antimicrobial stewardship standpoint. The need for additional coverage such as anaerobes or ESBL resistant pathogens was assessed.
• Duration of therapy is determined by the organism and site of infection but should continue for at least the duration of neutropenia (ANC ≥ 500 cells/mm3 x 48 hours)
o For unexplained fevers the treatment should be continued until there are clear signs of marrow recovery (ANC ≥ 500 cells/mm3 x 48 hours)
o If a treatment course has been completed and all signs and symptoms have resolved a neutropenic patient may resume oral fluoroquinolone prophylaxis until marrow recovery (ANC ≥ 500 cells/mm3 x 48 hours)
• 131 patients screened
• 39 patients included for analysis and 92 patients excluded

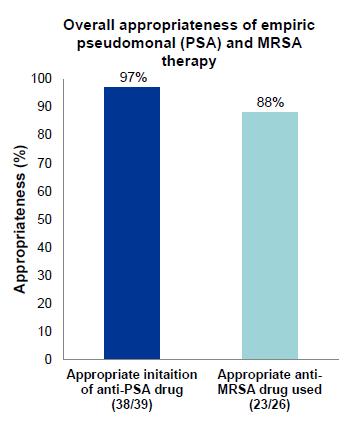
• Overall appropriate empiric anti-PSA and anti-MRSA therapy at FN onset

• Largely inappropriate selection of anti-pseudomonal agent, considering specific risk factors
• Duration of empiric antimicrobial therapy is consistent with resolution of neutropenia
• High use of G-CSF agents in FN patients considering that it is not routinely recommended in the treatment of febrile neutropenia
• Action plan:
o Present findings at next oncology section meeting
o Consider automatic consult to oncology and infectious diseases
o Education to oncology, infectious disease, and internal medicine staff
o Involve the antimicrobial stewardship program
o Investigate feasibility of febrile neutropenia order set
o Continue tracking G-CSF agent use and duration of therapy
1. Prevention and treatment of cancer-related infections. National Comprehensive Cancer Network. 2017.
2. Siegel RL, Miller KD, and Ahmedin J. Cancer Statistics 2017. CA Cancer J Clin. 2017; 67:7-30.
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2011; 52(4): 56-93.
4. Lyman GH and Rolston KV. How we treat febrile neutropenia in patients receiving cancer chemotherapy. J Oncol Pract. 2010; 6(3): 149-152.
5. Lyman GH, Michels SL, Reynolds MW, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010; 5555-5563.
6. Michels SL, Barron RL, Reynolds MW, et al. Costs associated with febrile neutropenia in the U.S. Pharmacoeconomics. 2012; 30(9): 809-823.
7. NCCN guidelines – Myeloid Growth Factors
8. Bennett CL, Djulbegovic B, Norris LB, et al. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013; 368(12): 1131-1139.
9. Raja AS. Stop treating all patients with febrile neutropenia similarly. N Engl J Med - Journal Watch. 2016