



TOGETHER FOR ACCESS NOT JUST SKIN DEEP Finding the Courage to Fight for Patients EVOLVING TREATMENTS The Impact of Biologic Medications FREE MARKET HEALTH Behind the Specialty Pharmacy Program
ISSUE 8 / 2024
Pati ts
Empow
WORKING

CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.






IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS

Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death. The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia.
If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection.
Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral (including herpes zoster), and other infections due to opportunistic pathogens.





Avoid use of CIBINQO in patients with an active, serious infection, including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections or those who have resided or traveled in areas of endemic tuberculosis or endemic mycoses.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO.
Viral reactivation, including herpes virus reactivation (eg, herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves. Hepatitis B virus reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C.
To learn more about CIBINQO efficacy, visit CIBINQOhcp.com or scan QR code.
JAK=Janus kinase; AD=atopic dermatitis.
Committed to Patient Support
Helping patients unlock access and reimbursement support for CIBINQO™ (abrocitinib)

With the Copay Savings Card,
pay as little as $ 0 per month*
*Eligibility required. No membership fees. This is not health insurance. The maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. See full terms and conditions below.
Copay Savings Card: TERMS AND CONDITIONS
By using the Pfizer Dermatology Patient AccessTM Copay Savings Card, you acknowledge that you currently meet the eligibility criteria and will comply with the terms and conditions described below:
• You are not eligible to use this card if you are enrolled in a state or federally funded prescription insurance program, including but not limited to Medicare, Medicaid, TRICARE, Veterans Affairs health care, a state prescription drug assistance program, or the Government Health Insurance Plan available in Puerto Rico (formerly known as “La Reforma de Salud”).
• You must have commercial insurance. Offer is not valid for cash-paying patients.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for CIBINQO™ (abrocitinib) may pay as little as $0 per month. Eligible patients with commercial prescription drug coverage may receive a maximum benefit of $15,000 per calendar year, which is defined by the date of enrollment through December 31st of the enrollment year. After a maximum of $15,000, you will be responsible for paying the remaining monthly out-of-pocket costs.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for EUCRISA® (crisaborole) may pay as little as $10 per tube. Eligible patients with commercial prescription drug insurance coverage that does not cover EUCRISA may pay as little as $100 per tube. Individual savings are limited to $970 per tube. Individual patient savings are limited to $3,880 in maximum total savings per calendar year.
• This copay card is not valid when the entire cost of your prescription drug is eligible to be reimbursed by your commercial insurance plan or any other health or pharmacy benefit program.
• You must deduct the value of this copay card from any reimbursement request submitted to your commercial insurance plan, either directly by you or on your behalf.
• You are responsible for reporting use of the copay card to any commercial insurer, health plan, or other third party that pays for or reimburses any part of the prescription filled using the copay card, as may be required. You should not use the copay card if your insurer or health plan prohibits use of manufacturer copay cards.
• This copay card is not valid where prohibited by law.
• Copay card cannot be combined with any other savings, free trial, or similar offer for the specified prescription.
• Copay card will be accepted only at participating pharmacies.
• If your pharmacy does not participate, you may be able to submit a request for a rebate in connection with this offer.
• This copay card is not health insurance.
• Offer good only in the United States and Puerto Rico
• Copay card is limited to 1 per person during this offering period and is not transferable.
• A copay card may not be redeemed more than once per 30 days per patient.
• No other purchase is necessary.
• Data related to your redemption of the copay card may be collected, analyzed, and shared with Pfizer, for market research and other purposes related to assessing Pfizer’s programs. Data shared with Pfizer will be aggregated and de-identified; it will be combined with data related to other copay card redemptions and will not identify you.
• Pfizer reserves the right to rescind, revoke, or amend this offer at any time without notice.
• Offer expires 12/31/2025.
For questions or additional support, call 1-833-956-3376, write to Pfizer Inc. at PO Box 29387, Mission, KS 66201, or visit the CIBINQO website at www.CIBINQO.com or the EUCRISA website at www.EUCRISA.com.
Please see additional Important Safety Information and Brief Summary of full Prescribing Information on the following pages. For full Prescribing Information, including BOXED WARNING and Medication Guide, visit CIBINQOhcp.com.
TM
eligible commercially insured patients may Visit PDPACopayCard.com
MORTALITY
In a large, randomized postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality (including sudden cardiovascular death) was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
MALIGNANCIES
Malignancies, including non-melanoma skin cancer (NMSC), were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
In a large, randomized postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA patients. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur.
THROMBOSIS
Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of overall thrombosis, DVT, and PE were observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and treat patients appropriately.
CONTRAINDICATION
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤ 81 mg daily), during the first 3 months of treatment.
LABORATORY ABNORMALITIES
Hematologic Abnormalities: Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia.
Prior to CIBINQO initiation, perform a complete blood count (CBC). CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations: Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy, and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
IMMUNIZATIONS
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines, including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
RENAL IMPAIRMENT
Avoid use in patients with severe renal impairment or end stage renal disease, including those on renal replacement therapy.
HEPATIC IMPAIRMENT
Avoid use in patients with severe hepatic impairment.
ADVERSE REACTIONS
Most common adverse reactions (≥ 1%) in subjects receiving 100 mg and 200 mg include: nasopharyngitis, nausea, headache, herpes simplex, increased blood creatine phosphokinase, dizziness, urinary tract infection, fatigue, acne, vomiting, oropharyngeal pain, influenza, gastroenteritis.
Most common adverse reactions (≥ 1%) in subjects receiving either 100 mg or 200 mg also include: impetigo, hypertension, contact dermatitis, upper abdominal pain, abdominal discomfort, herpes zoster, and thrombocytopenia.
Inform patients that retinal detachment has been reported in CIBINQO clinical trials. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision.
DRUG INTERACTIONS
Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO. See Prescribing Information for clinically relevant drug interactions.
USE IN PREGNANCY
Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Advise females of reproductive potential that CIBINQO may impair fertility.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
LACTATION
Advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose.
INDICATION
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable. Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
Please see full Important Safety Information throughout and full Prescribing Information, including BOXED WARNING, and Medication Guide.
rights reserved.
SAFETY INFORMATION
IMPORTANT
& INDICATION
2023 Pfizer Inc. All
May
PP-CIB-USA-0665
©
2023.
CIBINQO™ (abrocitinib) tablets, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death; The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia. If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection. Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens. Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately.
INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Testing, Evaluations, and Procedures
Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
• Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO
• Viral hepatitis screening in accordance with clinical guidelines – CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C
• A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation.
Recommended Dosage
The recommended dosage of CIBINQO is 100 mg orally once daily. If an adequate response is not achieved with CIBINQO 100 mg orally daily after 12 weeks, consider increasing dosage to 200 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 200 mg once daily. CIBINQO can be used with or without topical corticosteroids. If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
Recommended Dosage in Patients with Renal Impairment
Renal Impairment
CIBINQO dosage recommendation in patients with mild renal impairment (60-89 mL/minute) is 100 mg once daily. For patients with moderate renal impairment (30-59 mL/ minute), the recommended dosage is 50 mg once daily. CIBINQO is not recommended for patients with severe or End-Stage Renal Disease (ESRD). Severe renal impairment and End-Stage Renal Disease include patients on renal replacement therapy.
In subjects with mild and moderate renal impairment, if an adequate response is not achieved after 12 weeks, dose of CIBINQO can be doubled.
CIBINQO is not recommended for patients with severe renal impairment or ESRD.
Recommended Dosage in CYP2C19
Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Dosage Modifications due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19 reduce the dosage to 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Treatment Discontinuation due to Serious Infections or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Hematologic Abnormalities
• Discontinue CIBINQO if platelet count <50,000/mm3 and
follow with CBC until >100,000/mm3
• Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value
• Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value
• Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosing increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities.
DOSAGE FORMS AND STRENGTHS
• 50 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 50” on the other.
• 100 mg: Pink, round, film-coated tablet debossed with “PFE” on one side and “ABR 100” on the other.
• 200 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 200” on the other.
CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment.
WARNINGS AND PRECAUTIONS
Serious Infections The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and benefits of treatment prior to initiating CIBINQO in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO. Tuberculosis Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy. Viral Reactivation Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information; Initial Approval: January 2022
CIBINQO™ (abrocitinib) tablets, for oral use
Mortality (continued)
treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with CIBINQO for atopic dermatitis.
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical studies of CIBINQO for atopic dermatitis.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in patients receiving CIBINQO in the clinical studies for atopic dermatitis.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
Laboratory Abnormalities
Hematologic Abnormalities Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a CBC. CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations
Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter
elevations on cardiovascular morbidity and mortality has not been determined.
Immunizations
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Serious Infections
• Mortality
• Malignancy and Lymphoproliferative Disorders
Clinical Trials Experience
• Major Adverse Cardiovascular Events
• Thrombosis
• Laboratory Abnormalities
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO. In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks. The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%).
Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in the table below. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety profile of CIBINQO in the monotherapy and the combination trial(s) were similar.
Adverse Reactions from Placebo-Controlled Trials Reported in ≥1% of CIBINQO Treated Subjects with Moderate to Severe Atopic Dermatitis and at Higher Rate than Placebo for up to 16 Weeks
Overall Infections In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events In the placebocontrolled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia, no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia In the placebo-controlled trials, for up to 16 weeks, confirmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the first 4 weeks of exposure.
Lipid Elevations In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the final visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Specific Adverse Reactions Exposure adjusted incidence rates were adjusted by trial size for all the adverse reactions reported in this section.
Retinal Detachment In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
0-16 CIBINQO 200 mg N=590 n (%a) CIBINQO 100 mg N=608 n (%a) PLACEBO N=342 n (%a) Nasopharyngitis 51 (8.7)75 (12.4)27 (7.9) Nausea 86 (14.5)37 (6.0)7 (2.1) Headache 46 (7.8)36 (6.0)12 (3.5) Herpes simplexb 25 (4.2)20 (3.3)6 (1.8) Increased blood creatine phosphokinase 17 (2.9)14 (2.3)5 (1.5) Dizziness 17 (2.9)11 (1.8)3 (0.9) Urinary tract infection 13 (2.2)10 (1.7)4 (1.2) Fatigue 8 (1.3)10 (1.6)2
28 (4.7)10 (1.6)0
19 (3.2)9
3 (0.5)9
(0.3) Oropharyngeal pain 6 (1.0)8 (1.4)2 (0.6) Hypertension 5 (0.8)7 (1.2)2 (0.7) Influenza 6 (1.1)7 (1.2)0 (0.0) Gastroenteritis 8 (1.3)7 (1.1)2 (0.6)
contact 3 (0.5)6 (1.1)1 (0.3)
pain upper 11 (1.9)4
discomfort 7 (1.2)3 (0.5)1 (0.3)
zoster 7 (1.2)2 (0.3)0 (0.0)
9 (1.5)0
Study size adjusted percentages b Herpes simplex also includes oral herpes, ophthalmic herpes, herpes dermatitis, genital herpes.
Weeks
(0.5) Acne
(0.0) Vomiting
(1.5)3 (0.9) Impetigo
(1.5)1
Dermatitis
Abdominal
(0.6)0 (0.0) Abdominal
Herpes
Thrombocytopenia
(0.0)0 (0.0) a
Specific Adverse Reactions (continued)
Creatine Phosphokinase Elevations (CPK) In the placebocontrolled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
Adolescent Subjects (12 to less than 18 years of age) The safety of CIBINQO was assessed in a trial of 284 subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis (Trial-AD-4). The safety profile of CIBINQO in these subjects, assessed through the initial treatment period of 12 weeks and the long-term period (213 with at least 52 weeks of abrocitinib exposure), was similar to the safety profile from trials in adults with atopic dermatitis.
DRUG INTERACTIONS
Effects of Other Drugs on CIBINQO
The table below includes drugs with clinically significant drug interactions affecting CIBINQO.
Clinically Significant Drug Interactions Affecting CIBINQO
Strong CYP2C19 Inhibitors
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors.
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9
Clinical Impact Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9.
Strong CYP2C19 or CYP2C9 Inducers
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response.
Intervention Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers.
Effects of CIBINQO on Other Drugs
The table below includes clinically significant drug interactions affecting other drugs.
Clinically Significant Interactions Affecting Other Drugs
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities
Clinical Impact Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin).
Pregnancy
Pregnancy Exposure Registry There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
Risk Summary Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 11 or 4 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Animal Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively. Animal Data In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossified metatarsals) were noted at 60 mg/kg/day (17 times the MRHD based on AUC comparison). In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (4 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (17 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (2.4 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/ day (11 times the MRHD based on AUC comparison).
Lactation
Risk Summary There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Animal Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5-6 elimination half-lives).
Animal Data Lactating female rats were orally administered a single dose of 10 mg/kg abrocitinib on lactation day 12. Abrocitinib AUC was approximately 5 times greater in milk than in plasma.
Females and Males of Reproductive Potential
Infertility Females
Based on the findings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration.
Pediatric Use
Intervention Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO.
Antiplatelet Therapy Drugs
Clinical Impact
Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia.
Intervention Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment are contraindicated with CIBINQO.
The safety and effectiveness of CIBINQO in pediatric patients 12 years of age and older weighing 25 kg or more with atopic dermatitis has been established. In trials Trial-AD-1 and Trial-AD-2, 124 adolescent subjects 12 to less than 18 years old with moderate-to-severe atopic dermatitis were enrolled and randomized to receive either CIBINQO 100 mg (N=51), 200 mg (N=48), or matching placebo (N=25) in monotherapy. Additional 284 adolescent subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis, were enrolled and randomized to receive either CIBINQO 100 mg (N=95) or 200 mg (N=94) or matching placebo (N=95) in combination with topical corticosteroids in Trial-AD-4. Efficacy and adverse reaction profile were consistent between the pediatric patients and adults.
The safety and effectiveness of CIBINQO have not been established in pediatric patients below 12 years of age.
Juvenile Animal Toxicity Data In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day beginning on postnatal day 10 (approximately equivalent to a human infant) and continuing through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose-related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur. Abrocitinib produced adverse effects on bone development at all dose levels. Abrocitinib caused irreversible dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (0.8 times the MRHD based on AUC comparison).
Abrocitinib also irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (7.2 times the MRHD based on AUC comparison). At 75 mg/kg/ day (27 times the MRHD based on AUC comparison), paw fractures generally corresponded to limb impairment, a fractured tibia was noted in a single female. Irreversible bone findings have not been observed in older animals.
Geriatric Use
A total of 145 (4.6%) patients 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
A higher proportion of patients 65 years of age and older discontinued from clinical trials compared to younger patients. Among all patients exposed to CIBINQO, including the long-term extension trial, confirmed ALC <500/mm3 occurred only in patients 65 years of age and older. A higher proportion of patients 65 years of age and older had platelet counts <75,000/ mm3. The incidence rate of herpes zoster in patients 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of patients 18 to less than 65 years of age (3.44 per 100 patient-years).
Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30-59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min). This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement therapy. A dosage reduction in patient with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60-89 mL/min).
CIBINQO has not been studied in patients on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function. In clinical trials, CIBINQO was not evaluated in patients with severe (Child Pugh C) hepatic impairment.
CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates.
OVERDOSAGE
There is no experience regarding human overdosage with CIBINQO. There is no specific antidote for overdose with CIBINQO. In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
Rx only
This brief summary is based on CIBINQO™ (abrocitinib) Prescribing Information LAB-1424-2.0.
Issued: February 2023.
The product’s label may have been updated. For full Prescribing Information, visit CIBINQOPI.com.
See CIBINQO full Prescribing Information at CIBINQOPI.com.
© 2023 Pfizer Inc. All rights reserved. February 2023. PP-CIB-USA-0485
USE IN SPECIFIC POPULATIONS
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
CIBINQO™ (abrocitinib) tablets, for oral use
© 2023 Pfizer Inc. All rights reserved. May 2023. PP-CIB-USA-0485
MISSION STATEMENT: Access Dermatology aims to educate and empower the biologic coordinator by keeping them informed of the complex and everchanging drug and patient access landscape. Readers are engaged with editorial and lifestyle content equally suitable for dermatologic patients, so they too may gain a better sense of therapies and the patient services programs that can assist in their therapeutic journey.
EXECUTIVE DIRECTOR
Craig Schuette EDITOR
Elizabeth Hole
CREATIVE DIRECTOR
Venera Alexandrova
ASSISTANT ART DIRECTOR
Lisa Servidio
PRODUCTION DIRECTOR
Tim Carr
CORRESPONDENCE: Communications regarding original articles as well as editorial suggestions for future issues should be addressed to Craig Schuette at cs@bcofdermatology.com. Any content forwarded to the publisher assumes no liability for the safety or return of unsolicited art, photographs, or manuscripts.
DISPLAY ADVERTISING:
Contact Craig Schuette at cs@bcofdermatology.com.
ACCESS DERMATOLOGY / ISSUE 8 / 2024 6
dedicated to supporting patient access with practical information and lifestyle content
ISSUE 8 | 2024 PRESIDENT Jonathan W. Moffly VICE PRESIDENT/BUSINESS Elena V. Moffly MOFFLY CUSTOM MEDIA 205 Main Street, Westport, CT 06880 telephone: 203-222-0600 email: mail@MofflyCustomMedia.com Follow the Biologic Coordinators of Dermatology FACEBOOK LINKEDIN
A magazine
ACCESS DERMATOLOGY
© 2024 Access Dermatology and Biologic Coordinators of Dermatology. ALL RIGHTS RESERVED. The material in this publication is published by Moffly Custom Media and may not be reproduced or transmitted in any manner, in whole or in part, without the express written permission of Access Dermatology and Moffly Custom Media. NOTICE: The information contained within articles of this magazine represent the views and opinions of the original authors and do not necessarily represent the views and opinions of Access Dermatology or its affiliates. The mere appearance of content in the magazine does not constitute an endorsement by Access Dermatology or its affiliates. The content has been made available for informational and educational purposes only. Editorial advice is not specific, and readers are advised to seek medical, professional, or reimbursement help for individual circumstances. Access Dermatology hereby disclaims all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the content. CIRCULATION: To be added to the circulation, visit www.bcofdermatolgy.com/ magazine. REPRINTS: For educational, commercial, or promotional reprints, including author off-prints, please email contact@bcofdermatology.com.




bcofdermatology.com 7 contents ACCESS DERMATOLOGY | ISSUE 8 13 Founder's Letter The Year of the Member 16 Sculpting Skin Wellness The Vital Role of a Dermatology Biologic Coordinator 22 What is Free Market Health? Behind the Specialty Pharmacy Program from Blue Cross Blue Shield 30 Not Just Skin Deep Finding the Courage to Fight for Patients 34 Empower Patients Working Together for Specialty Medication Access 39 Specialty Pharmacy What to Look for and How to Choose One 40 Evolving Treatments The Impact of Biologic Medications 44 Biologic Passion Why I Love My Job
INDICATIONS
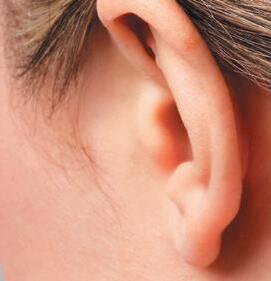
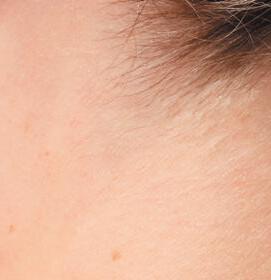
OPZELURA is indicated for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adult and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
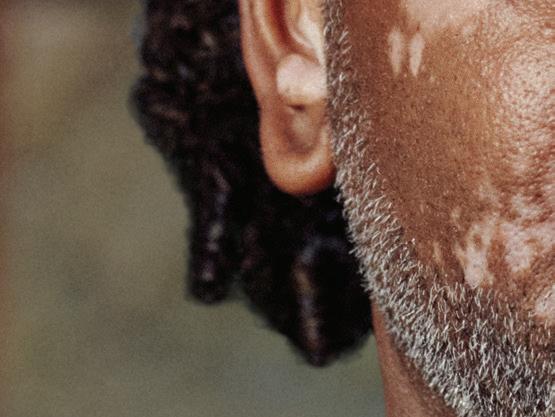
OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.





TREATMENT REIMAGINED TREATMENT REIMAGINED
FIRST-EVER FDA-APPROVED TOPICAL JAK INHIBITOR1
TARGETS THE SIGNALING OF KEY CYTOKINES IN AD
(IL-4, IL-13, IL-31, and TSLP)1-4*





FIRST + ONLY FDA-APPROVED TREATMENT FOR NONSEGMENTAL VITILIGO REPIGMENTATION1*








BROAD COVERAGE for commercially insured patients


*The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.1 For topical use only. Not for ophthalmic, oral, or intravaginal use.1
AD, atopic dermatitis; IFN-y, interferon gamma; IL, interleukin; JAK, Janus kinase; TSLP, thymic stromal lymphopoietin.
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death. Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled. Carefully consider the benefits and risks of treatment prior to initiating OPZELURA in patients with chronic or recurrent infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA.
Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.

















































IMPORTANT SAFETY INFORMATION (CONTINUED)










Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.


#1 PRESCRIBED JAK INHIBITOR IN DERMATOLOGY #1 PRESCRIBED JAK INHIBITOR IN DERMATOLOGY OVER 200,000 PATIENTS TREATED AND COUNTING6 5 SEE
































Please see the Brief Summary of the Full Prescribing Information, including Boxed Warning, and Medication Guide on the last page.








THE RESULTS
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma skin cancers, including basal cell and squamous cell carcinoma, have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately.
Thrombocytopenia, Anemia, and Neutropenia
Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations
Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Adverse Reactions
In atopic dermatitis, the most common adverse reactions (≥1%) are nasopharyngitis (3%), diarrhea (1%), bronchitis (1%), ear infection (1%), eosinophil count increased (1%), urticaria (1%), folliculitis (1%), tonsillitis (1%), and rhinorrhea (1%).
In nonsegmental vitiligo, the most common adverse reactions (incidence ≥1%) are application site acne (6%), application site pruritus (5%), nasopharyngitis (4%), headache (4%), urinary tract infection (2%), application site erythema (2%), and pyrexia (1%).
Pregnancy
There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Lactation
Advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Please see the Brief Summary of the Full Prescribing Information, including Boxed Warning, and Medication Guide on the next page.
REFERENCES: 1. OPZELURA [Prescribing information]. Wilmington, DE: Incyte Corporation. 2. Kim BS, Howell MD, Sun K, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145(2):572582. doi:10.1016/j.jaci.2019.08.042 3. Smith P, Yao W, Shepard S, et al. Developing a JAK inhibitor for targeted local delivery: ruxolitinib cream. Pharmaceutics. 2021;13(7):1044. doi:10.3390/ pharmaceutics13071044 4. Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi:10.3389/fimmu.2019.02342 5. Data on File. This information is an estimate derived from the use of information under license from the following IQVIA information service: NPA Market Dynamics for the period from July 2022 through June 2023. IQVIA expressly reserves all rights, including rights of copying, distribution and republication. 6. Data on File. This information is an estimate derived from the use of information under license from the following IQVIA information service: NPA Market Dynamics for the period from October 2021 through June 2023. IQVIA expressly reserves all rights including rights of copying, distribution and republication.
OPZELURA, the OPZELURA logo, Incyte, and the Incyte logo are registered trademarks of Incyte. © 2023, Incyte Corporation. 09/23
MAT-OPZ-01402
IMPORTANT SAFETY INFORMATION FOR OPZELURA® (RUXOLITINIB) CREAM 1.5% (CONTINUED)
OPZELURA® (ruxolitinib) cream, for topical use
Brief Summary of FULL PRESCRIBING INFORMATION
INDICATIONS AND USAGE: OPZELURA is indicated for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adult and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable and for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
The risks and benefits of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions]
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
WARNINGS AND PRECAUTIONS
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who
have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials.
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat inflammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia , and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
ADVERSE REACTIONS
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Atopic Dermatitis: In two double-blind, vehicle-controlled clinical trials
or with patients for treatment with opportunistic trials with kinase patients for OPZELURA reactivation (e.g., used to herpes inflammatory unknown. clinical trials. elevations reported in initiation is not inhibitor least one sudden inhibitor patient lymphomas, conditions. Malignancies, to treat an oral non-melanoma skin those treated with the cancers to those additional individual patients cancers), current or cell and Perform treatment as protective postmarketing least one (MACE) non-fatal stroke Patients benefits and OPZELURA, with other serious patients OPZELURA. (PE), and used to serious and an oral cardiovascular compared to increased OPZELURA and anemia , and benefits prior to indicated. If anemia, and increases lipoprotein (LDL) varying directly the rates clinical trials
(TRuE-AD1 and TRuE-AD2), 499 adult and pediatric subjects 12 years of age and older with atopic dermatitis were treated with OPZELURA twice daily for 8 weeks. In the OPZELURA group, 62% of subjects were females, and 71% of subjects were White, 23% were Black, and 4% were Asian. The adverse reactions reported by ≥ 1% of OPZELURA treated subjects and at a greater incidence than in the vehicle arm through week 8 are as follows for OPZELURA (N=499) vs Vehicle (N=250), respectively: Subjects with any treatment emergent adverse event (TEAE) 132 (27%) vs 83 (33%), Nasopharyngitis 13 (3%) vs 2 (1%), Bronchitis 4 (1%) vs 0 (0%), Ear infection 4 (1%) vs 0 (0%), Eosinophil count increased 4 (1%) vs 0 (0%), Urticaria 4 (1%) vs 0 (0%), Diarrhea 3 (1%) vs 1 (<1%), Folliculitis 3 (1%) vs 0 (0%), Tonsillitis 3 (1%) vs 0 (0%), and Rhinorrhea 3 (1%) vs 1 (<1%).
Adverse reactions that occurred in TRuE-AD1 and TRuE-AD2 in < 1% of subjects in the OPZELURA group and none in the vehicle group were: neutropenia, allergic conjunctivitis, pyrexia, seasonal allergy, herpes zoster, otitis externa, Staphylococcal infection, and acneiform dermatitis.
Nonsegmental Vitiligo: In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%)
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, influenza-like illness, insomnia, nasal congestion, and vomiting.
DRUG INTERACTIONS
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4) Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure). Lactation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Pediatric Use: Atopic Dermatitis: The safety and effectiveness of OPZELURA for the topical treatment of mild-to-moderate atopic dermatitis have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-AD1 and TRuE-AD2, which included 92 pediatric subjects aged 12 to 17 years with mild-to-moderate atopic dermatitis. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with atopic dermatitis have not been established. Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established. Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These findings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Geriatric Use: Of the 1249 total subjects with atopic dermatitis in clinical trials with OPZELURA, 115 (9%) were 65 years of age and older. No clinically meaningful differences in safety or effectiveness were observed between subjects less than 65 years and subjects 65 years and older.
Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
PATIENT COUNSELING INFORMATION
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide). Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions]
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions]
Thrombocytopenia, Anemia , and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Specific Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Specific Populations]
Manufactured for: Incyte Corporation
1801 Augustine Cut-off Wilmington, DE 19803 OPZELURA
is a registered trademark of Incyte. All rights reserved. U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870; 11219624 © 2022-2023 Incyte Corporation. All rights reserved. Issued: July
PLR-OPZ-00022-JUN23
2022
 letter from the founder
Craig Schuette
letter from the founder
Craig Schuette
The Year of the Member
With 2024 underway, I am excited for all that lies ahead for the Biologic Coordinators of Dermatology (BCoD). It’s a pivotal time for the association, as we declare 2024 The Year of the Member. This year, we are doubling down on our commitment to providing exceptional education and support to our members, the lifeblood of our community.
There has been remarkable growth in our membership over the past several years. We’re thrilled BCoD members find the resources we provide so valuable, and your involvement is a true reflection of that success.
In the spirit of pharmaceutical brand planning, we have delved deep into understanding the challenges that you, our valued members, face in navigating the multifaceted healthcare landscape. We recognize the fragmentation, turnover and evolving access environment that plague our industry.
We remain committed to providing meaningful solutions to these challenges. By listening to your concerns and aspirations, we strive to create a vibrant community where BCs and office access staff can thrive. We're proud to have assembled a remarkable group of professionals dedicated to advancing patient access to innovative treatments.
Coming soon: the relaunch of our website. This upgraded platform will feature an expanded offering of educational resources, keeping you up-to-date on the latest industry developments and best practices. Stay tuned for more details.
In addition, we are delighted to introduce the first-of-its-kind certificate program, developed in partnership with esteemed healthcare institutions. This comprehensive program provides unparalleled professional development opportunities for members.
Together, we are advancing patient access and transforming the healthcare landscape. In this issue of Access Dermatology, you will discover the unwavering commitment of BCs to putting patients first. Their insights and guidance offer valuable direction in optimizing patient care.
Thank you for reading and being such a valuable part of BCoD!
CRAIG SCHUETTE

bcofdermatology.com 13



Effectively control seborrheic dermatitis and simplify treatment with a steroid-free foam.1





Trial 203 and STRATUM studies evaluated ZORYVE (n=458) vs vehicle (n=225) once daily for 8 weeks in patients with seborrheic dermatitis. The primary endpoint was IGA Success at Week 8, defined as a score of Clear (0) or Almost Clear (1) and a ≥2-grade improvement from baseline.
ZORYVE is for topical use only and not for ophthalmic, oral, or intravaginal use.1
IGA = Investigator Global Assessment

INDICATION
ZORYVE foam, 0.3%, is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients 9 years of age and older.
IMPORTANT SAFETY INFORMATION
ZORYVE is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C).
Flammability: The propellants in ZORYVE are flammable. Avoid fire, flame, and smoking during and immediately following application.
The most common adverse reactions (≥1%) include nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
Please see brief summary of full Prescribing Information for ZORYVE foam on the following page.
References:
1. ZORYVE® foam. Prescribing information. Arcutis Biotherapeutics, Inc; 2023.
2. Data on File. Arcutis Biotherapeutics, Inc.
See the results at zoryvehcp.com/foam
NOW AVAILABLE! ®
DOWN TO AGE 9
one.
One foam. Once a day. Anywhere.1 SebD
DRAMATIC 77% IGA SUCCESS AT WEEK 81,2
A 2023 Arcutis survey of 93 adults diagnosed with seborrheic dermatitis found that an average of 15 products (including over-the-counter, alternative, and prescription treatments) were reportedly used on a yearly basis. 2
Actor portrayal
US-COM-154-00125 01/24
© 2024 Arcutis Biotherapeutics, Inc. All rights reserved.



Brief Summary of Prescribing Information for ZORYVE® (roflumilast) foam, 0.3%, for topical use. See package insert for full Prescribing Information.
INDICATIONS AND USAGE
ZORYVE foam, 0.3%, is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients 9 years of age and older.
DOSAGE AND ADMINISTRATION
Shake can prior to each use. Apply a thin layer of ZORYVE foam, 0.3%, once daily to affected areas on skin and/or scalp when they are not wet. Rub in completely.
Wash hands after application.
Avoid fire, flame, and smoking during and immediately following application.
ZORYVE foam, 0.3%, is for topical use only and not for ophthalmic, oral, or intravaginal use.
CONTRAINDICATIONS
ZORYVE foam, 0.3%, is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C).
WARNINGS AND PRECAUTIONS
Flammability
The propellants in ZORYVE foam, 0.3%, are flammable. Avoid fire, flame, and smoking during and immediately following application.
ADVERSE REACTIONS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two multicenter, randomized, double-blind, vehicle-controlled trials (Trial 203 and STRATUM), 683 adult and pediatric subjects 9 years of age or older with seborrheic dermatitis were treated with ZORYVE foam, 0.3%, or vehicle foam once daily for 8 weeks. The combined trial population was 79% White, 11% Black, and 5% Asian; for ethnicity, 79% identified as non-Hispanic/Latino and 21% identified as Hispanic/Latino. Fifty percent (50%) were male and 50% were female. The median age was 41 years (range 9 to 87 years). The median body surface area (BSA) affected was 2.5%.
Table 1 presents adverse reactions that occurred in at least 1% of subjects treated with ZORYVE foam, 0.3%.
Table 1: Adverse Reactions Reported in ≥1% of Subjects with Seborrheic Dermatitis Treated with ZORYVE Foam, 0.3%, for 8 Weeks in Trial 203 and Trial STRATUM
Adverse Reaction
Nasopharyngitis
Nausea
Headache
ZORYVE foam, 0.3% (N=458) n (%)
7 (1.5)
6 (1.3)
5 (1.1)
Vehicle foam (N=225)
n (%)
1 (0.4)
0 (0)
0 (0)
The following additional adverse reactions were reported in fewer than 1% of subjects treated with ZORYVE foam, 0.3%: diarrhea and insomnia. In 408 subjects who continued treatment with ZORYVE foam, 0.3%, for up to 24 to 52 weeks in an open-label, long-term trial, the adverse reaction profile was consistent with that observed in vehicle-controlled trials.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are insufficient data available on the use of ZORYVE foam, 0.3%, in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, roflumilast administered orally to pregnant rats and rabbits during the period of organogenesis produced no fetal structural abnormalities at doses up to 30 and 26 times the maximum recommended human dose (MRHD), respectively. Roflumilast induced post-implantation loss in rats at oral doses greater than or equal to 10 times the MRHD. Roflumilast induced stillbirth and decreased pup viability in mice at oral doses 16 and 49 times the MRHD, respectively. Roflumilast has been shown to adversely affect pup post-natal development when dams were treated with an oral dose 49 times the MRHD during pregnancy and lactation periods in mice.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor and delivery
Avoid using ZORYVE foam, 0.3%, during labor and delivery. There are no human studies that have investigated effects of ZORYVE foam, 0.3%, on preterm labor or labor at term; however, animal studies showed that oral roflumilast disrupted the labor and delivery process in mice.
Data
Animal data
In an embryo-fetal development study, pregnant rats were dosed orally during the period of organogenesis with up to 1.8 mg/kg/day roflumilast (30 times the MRHD on a mg/m2 basis). No evidence of structural abnormalities or effects on survival rates were observed. Roflumilast did not affect embryo-fetal development at a maternal oral dose of 0.2 mg/kg/day (3 times the MRHD on a mg/m2 basis).
In a fertility and embryo-fetal development study, male rats were dosed orally with up to 1.8 mg/kg/day roflumilast for 10 weeks and females for 2 weeks prior to pairing and throughout the organogenesis period. Roflumilast induced pre- and post-implantation loss at maternal oral doses greater than or equal to 0.6 mg/kg/day (10 times the MRHD on a mg/m2 basis). Roflumilast did not cause fetal structural abnormalities at maternal oral doses up to 1.8 mg/kg/day (29 times the MRHD on a mg/m2 basis).
In an embryo-fetal development study in rabbits, pregnant does were dosed orally with 0.8 mg/kg/day roflumilast during the period of organogenesis. Roflumilast did not cause fetal structural abnormalities at the maternal oral doses of 0.8 mg/kg/day (26 times the MRHD on a mg/m2 basis).
In pre- and post-natal developmental studies in mice, dams were dosed orally with up to 12 mg/kg/day roflumilast during the period of organogenesis and lactation. Roflumilast induced stillbirth and decreased pup viability at maternal oral doses greater than 2 mg/kg/day and 6 mg/kg/day, respectively (16 and 49 times the MRHD on a mg/m2 basis, respectively). Roflumilast induced delivery retardation in pregnant mice at maternal oral doses greater than 2 mg/kg/day (16 times the MRHD on a mg/m2 basis). Roflumilast decreased pup rearing frequencies at a maternal oral dose of 6 mg/kg/day during pregnancy and lactation (49 times the MRHD on a mg/m2 basis). Roflumilast also decreased survival and forelimb grip reflex and delayed pinna detachment in mouse pups at a maternal oral dose of 12 mg/kg/day (97 times the MRHD on a mg/m2 basis).
Lactation
Risk Summary
There are no data on the presence of roflumilast or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production.
Roflumilast and/or its metabolites are excreted into the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZORYVE foam, 0.3%, and any potential adverse effects on the breastfed infant from ZORYVE foam, 0.3%, or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use ZORYVE foam, 0.3%, on the smallest area of skin and for the shortest duration possible while breastfeeding. To avoid direct infant exposure, advise breastfeeding women not to apply ZORYVE foam, 0.3%, directly to the nipple or areola. If applied to the patient’s chest, avoid exposure via direct contact with the infant’s skin.
Data
Animal data
Roflumilast and/or its metabolite concentrations measured 8 hours after an oral dose of 1 mg/kg given to lactating rats were 0.32 and 0.02 mcg/g in the milk and pup liver, respectively.
Pediatric Use
The safety and effectiveness of ZORYVE foam, 0.3%, for the treatment of seborrheic dermatitis have been established in pediatric patients 9 years of age and older. Use of ZORYVE foam, 0.3%, in this age group is supported by data from two 8-week, vehiclecontrolled trials which included 32 pediatric subjects 9 to 17 years of age, of whom 17 received ZORYVE foam, 0.3%, and from open-label trials of up to 52 weeks which included 23 pediatric subjects treated with ZORYVE foam, 0.3%. The adverse reaction profile was consistent with that observed in adults.
The safety and effectiveness of ZORYVE foam, 0.3%, in pediatric patients below the age of 9 years have not been established.
Geriatric Use
Of the 683 subjects with seborrheic dermatitis exposed to ZORYVE foam, 0.3%, or vehicle for up to 8 weeks in the controlled clinical trials, 98 (14%) were 65 years of age or older, and 33 (5%) were 75 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Hepatic Impairment
Oral roflumilast 250 mcg once daily for 14 days was studied in subjects with hepatic impairment. The systemic exposure of roflumilast and roflumilast N-oxide were increased in subjects with moderate (Child-Pugh B) hepatic impairment. ZORYVE foam, 0.3%, is contraindicated in patients with moderate to severe liver impairment (ChildPugh B or C). No dosage adjustment is needed in patients with mild (Child-Pugh A) hepatic impairment.
PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information).
Flammability
Because the propellants in ZORYVE foam, 0.3%, are flammable, instruct the patient to avoid fire, flame, and smoking during and immediately following application.
Lactation
Advise patients to use ZORYVE foam, 0.3%, on the smallest area of skin and for the shortest duration possible while breastfeeding. Instruct patients who are breastfeeding not to apply ZORYVE foam, 0.3%, directly to the nipple or areola to avoid direct infant exposure. Instruct patients to avoid inadvertent contact of treated areas with infant skin.
© 2024 Arcutis Biotherapeutics, Inc. All rights reserved.




THE VITAL ROLE OF A DERMATOLOGY BIOLOGIC COORDINATOR
By Barry Williams
SCULPTING SKIN WELLNESS
In the intricate realm of dermatology, a profession devoted to crafting healthy skin, one indispensable figure emerges—the dermatology biologic coordinator (BC). With a passion for assisting those grappling with chronic skin conditions, I, as a Certified Medical Assistant, have navigated the dermatology landscape since 2009. For the past four years, my role as a biologic coordinator has evolved into an unexpected but deeply rewarding venture, amplifying not only my commitment to dermatological care but also transforming the way we care for our patients. In this article, I share insights garnered from my experiences as a coordinator, offering recommendations to enhance the efficacy of this crucial role.
bcofdermatology.com

17
CERTIFIED MEDICAL ASSISTANT AND BIOLOGIC COORDINATOR
OLEKSIISTOCK.ADOBE.COM

MEEKO MEDIASTOCK.ADOBE.COM
The BC serves as the linchpin in the patient care journey, seamlessly connecting the dots from diagnosis to treatment. To ensure success in this multifaceted role, implementing a robust protocol is paramount. From the moment an enrollment form is received to the administration of the loading dose and scheduling of subsequent appointments, having a structured process in place is essential. Injecting new biologics in-office not only facilitates patient education on proper usage, but also allows for vigilant post-injection observation, ensuring no adverse reactions and providing documentation of the initial injection.
One often overlooked aspect is the utilization of enrollment form stations equipped with all necessary materials, including copay cards and patient information. This setup streamlines the process, providing quick access for medical assistants and healthcare providers. Leveraging the expertise of drug representatives and their access field specialists further enhances efficiency, ensuring that forms are up-to-date and samples are readily available. The enrollment process is made smoother with a judicious approach—sending enrollment forms only with approval or denial, directing patients appropriately based on their status.
In the digital age, embracing online pharmacy portals proves to be invaluable.

"The BC serves as the linchpin in the patient care journey."
Platforms such as Accredo, CVS Specialty Pharmacy, OptumRx and Availity offer the capability to track prescriptions and upload prior authorization documents, easing the administrative burden. Equally important is enrolling in manufacturer portals provided by companies like Janssen, Lilly and AbbVie. These portals empower coordinators by
facilitating patient enrollment, providing access to copay cards and streamlining various savings programs, while allowing tracking of the overall process.
Networking emerges as a vital component in the biologic coordinator’s arsenal. Attending drug representative dinners not only fosters connections, but also serves as a platform for shared knowledge. The collaborative atmosphere is conducive to the exchange of innovative ideas and strategies for navigating the complex landscape of insurance approvals. Harnessing this collective wisdom helps alleviate the arduous task of securing approvals, sparing both time and effort.
In the intricate dance of dermatological care, the biologic coordinator stands as a sculptor of skin wellness, shaping the patient journey with passion and precision. From the meticulous administration of biologics to the strategic coordination of resources, this role is pivotal in elevating the quality of life for those battling chronic skin conditions.
As we continue to share insights, tips and tricks, the collective effort of coordinators, providers, pharmacies, manufacturers and drug representatives ensures that our patients receive the best care possible. It is with unwavering joy and dedication that we forge ahead, creating a future where dermatological wellness is within reach for all.
bcofdermatology.com 19 SYDA PRODUCTIONSSTOCK.ADOBE.COM
JOIN US AT THE PREMIER CONFERENCE FOR BIOLOGIC COORDINATORS
OCTOBER 27-29, ORLANDO, FL | JW MARRIOTT BONNET CREEK
This year, we’re introducing an array of new learning tracks and dynamic workshops designed to empower biologic coordinators at every career stage.
With ample networking opportunities and a resource-packed experience, this event is tailored to inspire, educate, and connect our community like never before!
WHAT’S IN IT FOR YOU?
NEW LEARNING TRACKS
Tailored sessions for all career stages.
INTERACTIVE WORKSHOPS
Hands-on skill development with experts.
NETWORKING OPPORTUNITIES
Connect with peers and industry leaders.
Learn more at BCOFDERMATOLOGY.COM
PROFESSIONAL DEVELOPMENT
Advance your career with cutting-edge insights.
RESOURCE-PACKED EXPERIENCE
Leave with valuable tools and knowledge.
SAVE THE DATE




 By Tara Biggers
CCMA, CDT // CLINICAL ASSISTANT/BIOLOGIC COORDINATOR
By Tara Biggers
CCMA, CDT // CLINICAL ASSISTANT/BIOLOGIC COORDINATOR
JIM GIMPELSTOCK.ADOBE.COM





What is Free Market Health?
BEHIND THE SPECIALTY PHARMACY PROGRAM FROM BLUE CROSS BLUE SHIELD
When Free Market Health was introduced last fall, we were left with many questions. What is going on with our patients’ prescriptions? Who is and will be affected? How do we navigate this new process? People were talking, and I needed to find answers. First things first: What is Free Market Health?
Blue Cross Blue Shield (BCBS) of North Carolina’s Free Market Health (FMH) program launched October 1, 2023.
FMH is a marketplace where approved or covered prescriptions go through an automated bidding process to determine who will dispense certain medications and at what cost. BCBS NC states on their website that an “authorized referral will be matched by Free Market Health to a specialty pharmacy best suited to service the member.” This process reportedly only takes a matter of hours. In other words, the specialty pharmacy with the lowest bid obtains the ability to fill the prescription for the patient. The objective of BCBS, when creating this program, is to lower the cost of specialty medications, as the use and cost of covering them has significantly increased in recent years.
The program affects fully insured commercial members with prescription coverage who are prescribed and approved for specific specialty medications. Members with copayment plans will not see a lower copay; however, members with health savings accounts or coinsurance plans may see lower costs at an in-network pharmacy. BCBS members currently receiving their medications from previously mandated pharmacies were not supposed to experience
an impact from this change. Most of the injectable and oral specialty medications that are used to treat dermatologic conditions are included in this new program. Lists of the participating specialty pharmacies and medications are on the BCBS website.
I remember my first BCBS patient affected by FMH. (We’ll call them “Patient Zero.”)
Patient Zero is an established patient who has been on a specialty medication for two years. They had been receiving medication from the same specialty pharmacy for the entirety of their treatment journey. After trying to obtain medication, the patient contacted me to send a new prescription to the specialty pharmacy. Patient Zero was instructed that a new prescription was necessary, since the old one was canceled.
“That is odd,” I thought. Although I sent the prescription multiple times, my patient still couldn’t get the medication. The pharmacy finally alerted me they were no longer able to dispense the medication. I notified the patient, and they were very upset the change in pharmacy caused delays in receiving medication. Luckily, we always keep plenty of samples on hand for patients when problems
arise. At the time, I did not realize this would become a bigger issue.
Following the implementation of FMH, I found myself deeply concerned about the uncertainty surrounding our patients' prescriptions. The influx of calls from patients struggling to obtain medication, compounded with various specialty pharmacies claiming to be the new dispensers, left me confused. Some pharmacies even demanded clinical notes and labs before releasing medications. This chaotic scenario affected both new and established BCBS patients, causing significant delays in medication access.
I consulted several pharmaceutical reps to understand the issue, but clarity remained elusive. Speaking with biologic coordinators yielded similar frustrations, yet each provided valuable insights that contributed to unraveling the puzzle. Determined to uncover the root cause, I decided to reach out to a pharmacist for expertise. Given our partnership with Blue Sky, an enhanced specialty pharmacy, I contacted Jennifer, my trusted contact there. Known for her intelligence and helpfulness, I was confident
ACCESS DERMATOLOGY / ISSUE 8 / 2024 24

could shed light on the situation.
Jennifer didnʼt disappoint; she provided a concise overview of FMH and shared her own research efforts to support colleagues at Blue Sky grappling with the changes.
When conversing with Jennifer, she revealed that the pharmacy had insufficient notice about the implementation of FMH. However, she received some modules to aid in navigating the new process. She faced difficulties logging into their portal, causing delays in assigning pharmacies that had secured the prescription bids. To obtain this crucial information, she had to email the help desk on the portal as notices were only faxed to offices.
“FMH is a marketplace where approved or covered prescriptions go through an automated bidding process to determine who will dispense certain medications and at what cost.” she
Initially, the response time was sluggish, but it gradually improved over the weeks ahead. Jennifer noted a pattern that resonated with observations made by other biologic coordinators as well as myself: Certain pharmacies were consistently winning more bids. Notably, hospitalassociated specialty pharmacies like Novant Health Specialty Pharmacy in my vicinity emerged as clear victors. This trend was consistent among biologic coordinators across different regions of North Carolina.
The challenge we face with hospitalassociated pharmacies lies in the delays encountered during medication dispensing upon prescription receipt. These pharmacies began requesting notes and labs upfront, diverging from the usual streamlined process, thereby increasing workload for both parties. Initially hesitant due to HIPAA compliance concerns, I was reluctant to provide the requested information.
However, after discussing with a supervisor at Novant Health Specialty Pharmacy, I learned that their accreditation necessitated this data for prescription fulfillment. Failure to comply could result in prescription rejection, restarting the bidding process, leading to prolonged delays— an outcome I aimed to avoid. Initially, this process took weeks, but improvements have been made, though some delays still persist, extending to almost a month.
bcofdermatology.com 25
GIMPELSTOCK.ADOBE.COM
JIM
SOLUTIONS THAT WORK
The initial rollout of FMH has been challenging, lacking warning or assistance from BCBS, which has not been ideal. Despite my reservations about the new process, I have found some solutions that work. Throughout recent months, I've identified several practices that help expedite medication access for my patients.
1.
Upon receiving a fax for prescription transfer from BCBS to a hospital specialty pharmacy, I promptly send the prescription along with relevant chart notes and labs, ensuring I notify the pharmacist of the sent items.

ACCESS DERMATOLOGY 26





STILL GOLDEN. EHR | PM | PATIENT ENGAGEMENT | TELEHEALTH | ASC | INTEGRATED PAYMENTS | CONSULTING Don’t fall for anything less. Nextech is still the only EHR to receive the American Academy of Dermatology’s DataDerm™ Gold recognition. Start anew with the #1 solution for Dermatology










UNLOCK YOUR POTENTIAL WITH BCOD

TOGETHER WE ADVANCE PATIENT ACCESS

Join the Biologic Coordinators of Dermatology (BCoD) and discover a world of member benefits designed to elevate your professional journey and enhance patient care.
MEMBER BENEFITS

EDUCATIONAL TRAINING
Exclusive invites to educational trainings, webcasts, and meetings.
RESOURCES
Office and patient resources for easier approvals and workflow management.


PROFESSIONAL DEVELOPMENT
Content designed for personal and professional growth. IMPROVE PATIENT OUTCOMES
Tools and insights that enhance patient outcomes.
COMMUNITY
A platform that connects you with peers nationwide.
Embrace the vibrant community of BCoD, where each member plays a crucial role in advancing patient access.
Learn more at BCOFDERMATOLOGY.COM

 By Sarah Bailey BIOLOGIC COORDINATOR/LPN CENTER FOR SKIN WELLNESS, SARASOTA, FL
By Sarah Bailey BIOLOGIC COORDINATOR/LPN CENTER FOR SKIN WELLNESS, SARASOTA, FL
Not Just
SKIN DEEP
FINDING THE COURAGE TO FIGHT FOR PATIENTS
Biologic coordinators are a unique and dedicated group. Serving as advocates and patient confidants, we lead teams to make objectives happen. Often, it is a challenge to get a patient the medication they need in a timely fashion. As biologic coordinators (BCs), we must fight for the patient using every skillset we have.
Patients can be uncomfortable in their own skin, literally suffering for months and sometimes years. This not only affects them physically, but can also change their emotional status, hobbies, ADLs, relationships, job performance and employment.
ACCESS DERMATOLOGY / ISSUE 8 / 2024 30

PHIMWILAISTOCK.ADOBE.COM
PATIENT EXPERIENCES
Unfortunately, it is common for conditions to go beyond the skin and external psychosocial issues. I recently had two patients in our practice diagnosed with generalized pustular psoriasis. This skin condition changes rapidly—at such an alarming pace that patients can be at risk for tachycardia, multisystem organ failure, sepsis or death. This dangerous disorder must be managed carefully to include the patient, prescriber, BC, sales representative, field reimbursement specialist and infusion specialist. Now more than ever, find your voice and speak out, because patients are fighting to survive.
Both of my cases were entirely different. Each patient was on biologics therapy prior to initiation of Spevigo. The first patient had plaque psoriasis and a flare, which was diagnosed as GPP. She had commercial insurance, and the enrollment forms for Spevigo were sent to the hub. I was assured the infusion center would handle the prior authorization (PA) and schedule the patient.
However, they dropped the ball. I quickly interjected and handled the PA, which was denied. Then I filed an appeal, including the prescribing information. It is critical for the office staff to document the GPP score in the note. I rerouted the patient to a different infusion center and was pleased she was


seen quickly and with great results.
The second patient was diagnosed with psoriatic arthritis, which was managed by her rheumatologist. She had a flare of a rash, presented to our office, and was diagnosed with GPP. Her condition was
more severe and advancing quickly. Adding fuel to the fire (pun intended), the patient did not have commercial insurance. While this seems easier in disguise, it was one of my most challenging cases. The patient had a medi-share plan. After the PA was
ACCESS DERMATOLOGY / ISSUE 8 / 2024 32 NUZZA11STOCK.ADOBE.COM
sent, the insurers delayed their decision. When implored to send a determination, they indicated that 72 hours was required even though it was a life-threatening illness. Instead, the insurance company offered to pray with me, which was clearly a stall tactic, not to mention humiliating for the patient.
GETTING TREATMENT
The known risk to my patient caused me to search for any way possible to assist her. As a consideration, I contacted the infusion center where my prior Spevigo patient was treated. They were willing to help by making her a self-pay patient—a true lifesaving measure.
Eventually, the treatment was approved; however, a medi-share plan doesn’t always pay claims. They take claims “into consideration after the service is rendered.” The cost of the medication is approximately $52,000 per infusion, and the patient is responsible for both the medication and infusion costs. Sometimes, patients are required to have two infusions within a week. This is where BCs need to get creative and advocate for patients. Due to the delay in a decision from medi-share, the Spevigo hub recommended sending a foundation application on behalf of the patient. The foundation request was approved. However, since the patient was “insured” they wanted a quote for the cost of the medication. After trying to get quotes without success, it dawned on me that since the patient is under the care of medi-share, she is truly self-insured. This changed the whole dynamic. Immediately, the patient was approved, and the medication was sent to the infusion center.
HELPFUL RESOURCE
To complicate things further, the medishare plan contracts with a third party. After the medication was scheduled with a tracking number, the plan wanted to determine if the practice was trying to get the patient on foundation assistance and to schedule with their pharmacy. Be watchful, as they can be sneaky! I checked with the foundation to confirm my delivery was legitimately scheduled.
An additional resource to utilize during the treatment period is Telehealth appointments. These appointments are valuable, due to the complexities of the disease process, recovery questions, and concerns raised by the patient and/or family members. Moreover, patients may be a considerable distance from the practice or unable to travel because of their health status. This allows the prescriber a one-on-one conversation to manage care, reassure the patient, provide education and expectations of the medication, and explain a skin care routine to help them recover after the infusion.
CONCLUSION
Biologic coordinators lead their teams to get the prescriptions their patients need to succeed. This process resonates with me, as I have psoriatic arthritis. Granted, my condition is not a life-threatening issue. It is important to remember how our job impacts people’s lives. Often, the formulary alternates are either not indicated for the disease process, antiquated, or have higher risk factors compared to safer medications in the realm of biologics. We must continue to have courage and seek what is in the best interest of our patients and help save lives. If not, who will?
SHERRY YOUNGSTOCK.ADOBE.COM
By Brynn Christensen CMA (AAMA), BIOLOGICS COORDINATOR // DERMATOLOGY ASSOCIATES OF LINCOLN
EMPOWER Patients
WORKING TOGETHER FOR SPECIALTY MEDICATION ACCESS
As biologic coordinators, we are driven and do our job with enthusiasm. Our goal has been and will always be to gain affordable access to biologic medications at the lowest cost possible for our patients. We spend countless hours educating ourselves on insurance policies, pharmacy benefit requirements, manufacturer copay programs and patient assistance programs.
ACCESS DERMATOLOGY / ISSUE 8 / 2024 34 ALEX S/PEOPLEIMAGES.COMSTOCK.ADOBE.COM

Affordable access has changed over the years, and we reminisce how easy it was to enroll a patient in manufacturer copay programs, bridge programs or a patient assistance program. The biologic access landscape has changed, and we are faced with the continued presence of accumulator and maximizer plans, pharmacy benefit third-party administrators and ineligibility for copay programs, bridge programs and patient assistance.
PHARMACY BENEFIT THIRD-PARTY ADMINISTRATORS
With the rapid increase of pharmacy benefit third-party administrators, there is often a lack of coverage for specialty medications. This has made patient access more difficult, forcing patients to decide if they should empty their bank account to pay the cash price of their prescribed medication or go without and suffer from the painful symptoms and comorbidities associated with their untreated condition.
So, what does a pharmacy benefit thirdparty administrator do? A person in this role has been hired by the insurance pharmacy benefit to assist and enforce coverage decisions and to lower prescription costs for the employer and employee. The pharmacy benefit manager (PBM) has been chosen to provide prescription drug benefit management, where the third-party administrator (TPA) ensures the policies of the health plan are being followed.
In layman’s terms, the chosen insurance pharmacy benefit plan with third-party administration is marketed as “cost saving” and “Everybody wins!” The reality is that employees enrolled in these types of
plans are suffering from the lack of or no coverage of specialty medications. This is not only affecting dermatology patients, but impacting any patient who is prescribed a specialty medication of any kind.
ACCESS TO MEDICATION
To best identify these patients, begin with completing a prior authorization and benefits verification through the manufacturer hub. Third-party administrators who are not covering specialty medications may be oblivious to the verbiage of the response received. Often it will state, “No specialty medications covered” or “Specialty medication is a policy exclusion.”
Instead of a traditional denial letter, you may receive notice that the medication is not covered, and prior authorization is not accepted. The goal of the pharmacy benefit third-party administrator is to keep costs down. This is done by not covering any prescribed specialty medications, as they expect manufacturer programs to provide free medication to all patients. This has led to a standoff between manufacturers
and insurance companies, leaving patients without medication unless they choose to pay out-of-pocket.
Patients paying for a commercial insurance policy are unable to use the benefit for their specialty medications, and they’re ineligible to receive manufacturer copay card assistance. Having a commercial insurance policy with a pharmacy benefit that does not cover specialty medication does not automatically qualify a patient as underinsured or uninsured, and they will be viewed as ineligible for patient assistance programs.
READ THE FINE PRINT
It is important to know the contractual agreements with manufacturers’ copay assistance programs as well as eligibility criteria. Reading the fine print on a patient application is critical, as some manufacturer programs have stated they will no longer work with thirdparty administrators, deeming a patient ineligible for copay cards even when they are commercially insured.
Many patient assistance programs are no longer working with pharmacy benefit
ACCESS DERMATOLOGY / ISSUE 8 / 2024 36
SYDA PRODUCTIONSSTOCK.ADOBE.COM

“It is important to
know the contractual agreements with manufacturers’ copay assistance programs as well as eligibility criteria.”
bcofdermatology.com 37
“Patients who are empowered can take charge of their health and medication access alongside you, becoming one of your best allies.”
third-party administrators, because they are also fighting for the patient’s rights. Patients who have obtained a commercial policy are paying a monthly premium; therefore, they should be entitled to receive prescribed medication through the pharmacy benefit.
UTILIZE FIELD REIMBURSEMENT AND ACCESS MANAGERS
To gain access to biologic medication, it is imperative you keep in contact with your field reimbursement and access managers for extra guidance. Some patients may be grandfathered into their current copay program even if they have a PBM with a TPA, which does not provide specialty medication coverage. Seek help to identify these patients. Commercially insured patients with pharmacy benefits are ineligible for most patient assistance programs.
On the rare occasion, you can still enroll your patient in a patient assistance program after several attempts of being unable to gain insurance coverage—if your

FIGHT THE GOOD FIGHT
You and your patient need to work together to “fight the good fight” and persevere in accessing medication. Set expectations for the process, as it is sure to be long and frustrating with numerous communications between manufacturer copay programs, patient assistance programs, the pharmacy benefit administrator and the patient.
patient meets the income guidelines of the chosen program. This is not common, so educate yourself and fully explain to your patient the exact plan; our goal is to gain medication access, not give a false sense of hope. Patients are capable of understanding and assisting in access to their medications.
As biologic coordinators, we need to empower our patients to help obtain their specialty medications. Educate your patient so they know exactly what their policy states, as well as the steps you are taking to assist them with securing their biologic.
Encourage your patient to speak to their HR department and share denial documentation. Unfortunately, many employers were misguided, thinking their insurance PBM/ TPA change was a positive one. They are now learning specialty medications are not covered within their chosen policy.
Patients who are empowered can take charge of their health and medication access alongside you, becoming one of your best allies. They can make changes to their workplace pharmacy benefit policy by bringing awareness to their employer and sharing access hurdles. Not only will they be helping themselves, but also assisting coworkers and their families in obtaining prescribed specialty medications by being a voice in their workplace.
ACCESS DERMATOLOGY / ISSUE 8 / 2024 38
VINNSTOCKSTOCK.ADOBE.COM

SPECIALTY PHARMACY
What to look for and how to choose one
The term “specialty pharmacy” is something we as biologic coordinators hear all day—every day. There are so many choices when it comes to pharmacies and what will best cater to our clinics’ needs. I’m sure you’ve seen a pharmacy whose workload is too big for their capacity, and they sell out. It is not a one-size-fits-all process, so how do we know what pharmacy is the best choice?
When searching for a specialty pharmacy (SP), many factors come into play. Sometimes you can shop around and use multiple pharmacies, instead of sticking to one specifically. Most SPs have a rep that will try and persuade you to use them. You should always ensure what the rep promises is provided. Getting to know all the services a pharmacy can offer your clinic is a must. Some provide services such as PA support, appeal support, filling the script, and even helping patients with copay assistance. The PA support is usually what BCs utilize most, and it should be a quick and correct PA submission.
Finding a point-of-contact comparable to a biologic coordinator (BC) is also very important. You should be able to contact that person for whatever might arise when you need fast and clear communication about your patient. A portal is an effective way to communicate and keep your eye on the scripts. Rather than wonder what process your script is in, it is much better to have over-communication.
On the flip side, there are many things a specialty pharmacy should not do. It is frustrating when a pharmacy over promises on services and then under delivers. First of all, SPs should never let your patients fall through the cracks. The PAs should be completed in a timely manner and the pharmacy should have superior communication with you, whether it is via fax or portal. Constantly having to call or email your point of contacts is not something you should need to do. Be sure to express your concerns too; if they don’t know about it, they can’t fix it. It is not a good sign if you need to keep scheduling meetings with the pharmacy to remind them of your expectations. Remember, if you are using a pharmacy and are not happy, you are not tied to them. You are able to transfer your scripts at any time if they are not meeting the needs of your clinic.
In the BC role, you can choose what to let the pharmacy do from your workload. It could be all-in or just what you think will benefit you best. For example, if you feel comfortable renewing PAs but think starting a patient is tricky, try utilizing them for new starts. Some clinics may need to use them for all of their scripts, depending on their circumstances.
Utilizing a specialty pharmacy is all about what fits your needs best. There are so many options out there, along with many pros and cons. Make sure your needs and wants are heard at the pharmacy, as they should customize to suit you and your clinic.
By Trinity Hoke
NOKHOOGSTOCK.ADOBE.COM
PIEDMONT PLASTIC SURGERY & DERMATOLOGY // BIOLOGIC COORDINATOR
39
bcofdermatology.com
 By Lacie Chennault
By Lacie Chennault
BAY DERMATOLOGY AND COSMETIC SURGERY // OFFICE MANAGER, MEDICAL ASSISTANT, BIOLOGICS COORDINATOR AND PRIOR AUTHORIZATION CERTIFIED SPECIALIST
ALLME3DSTOCK.ADOBE.COM

Evolving Treatments
THE IMPACT OF BIOLOGIC MEDICATIONS
In my six years of experience at Bay Dermatology and Cosmetic Surgery, working as a medical assistant and biologics coordinator (BC), I have witnessed a remarkable transformation in the field of dermatology. The influx of new technologies and medications has not only made my work rewarding, but has also provided patients with groundbreaking solutions for conditions like psoriasis, hair loss, prurigo nodularis and atopic dermatitis.
CHANGING LANDSCAPE
In recent years, the dermatology world has evolved significantly with biologic medications emerging as a beacon of hope for patients seeking relief. We have seen hundreds of individuals walk through our doors, each with unique struggles. The advancements in biologics have played a pivotal role in addressing their concerns.
PERSONAL JOURNEY
My role as a BC has been a continuation of learning, as I navigate the dynamic world of biologic medications. Witnessing the mostly positive impact on patients' lives has been a wonderful journey. The joy from knowing our providers and staff are on the front lines, offering relief, is immeasurable. When I first started doing biologic medications as a medical assistant, there was so much back and forth with insurance companies, specialty pharmacies and medication assistance programs.
Recently, there has been a much better turnaround to get my patients the help they need and the quality of life they deserve. There are hiccups here and there, but overall people who deal with these lifealtering conditions can get treatment in a timely matter. Having designated BCs has helped tremendously for providers and patients to execute the care plan.
PATIENT ACCESS AND QUALITY OF LIFE
Biologic medications have opened new doors for patients, providing access to treatments that significantly enhance their quality of life. Despite the challenges of dealing with insurance companies, the satisfaction gained from helping patients overcome obstacles and receive the care they need outweighs the struggles.
On the technological side of dermatology, the integration of cutting-edge technology has revolutionized our patient care. With innovative medications to support programs offered by drug companies, patients now have unprecedented opportunities to seek relief and improve their overall well-being. My favorite resources are the portals we can use to access the patient’s road map on their treatment plan. It’s a quick and easy way to see where we are on the patient’s healing journey. It is my hope that soon all drug companies and specialty pharmacies will have this technology.
ORGANIZATIONAL SUCCESS
Staying organized and gathering accurate documentation, including past medical history, has been key to achieving positive outcomes for patients. The meticulous approach ensures that patients receive the best possible coverage, facilitating a smoother journey toward recovery. One
struggle I have identified within the field is the documentation on BSA, itch scale, etc. Personally, I have found that speaking with our expert providers and medical assistants really helps improve this challenge. Another trick that has helped is having a biologics bible in the MA lab, so medical assistants and providers can reference the process and inform the patients about this process if I am not available.
CONCLUSION
In the ever-evolving field of dermatology, the impact of biologic medications for psoriasis, hair loss, prurigo nodularis and atopic dermatitis is undeniable. As a BC, the joy derived from witnessing patients' happiness and improved quality of life is a testament to the transformative power of these advancements.
Despite the challenges, the commitment to helping patients access the relief they deserve remains unwavering. The future looks promising, with ongoing support programs and technological innovations paving the way for even better outcomes in dermatological care.
ACCESS DERMATOLOGY / ISSUE 8 / 2024 42

Biologic
medications have opened new doors for patients, providing access to treatments that significantly enhance their quality of life."
ALLME3DSTOCK.ADOBE.COM bcofdermatology.com 43











ACCESS DERMATOLOGY / ISSUE 8 / 2024 44
BACKGROUND: MR.ILKINSTOCK.ADOBE.COM; HAND/HEART: RICHARDSTOCK.ADOBE.COM
By Brynn Christensen, CMA (AAMA) DERMATOLOGY ASSOCIATES OF LINCOLN // BIOLOGIC COORDINATOR

BIOLOGIC PASSION
WHY I LOVE MY JOB
When I say that I love biologics and my job, that is no exaggeration.
I LOVE my job! Being a biologic coordinator feeds my soul, and I hope it does for you too. This career allows us to feel all the human emotions: empathy, sympathy and competitiveness, while experiencing highs and lows and—let’s be honest—pure frustration at times. However, the pure joy that comes from patients receiving their medication and watching their quality of life improve is why I show up every day.
PIXELS HUNTERSTOCK.ADOBE.COM
So why do you do the job?
We all have our own stories of how we became biologic coordinators. For some, you may have started out on the clinic floor and then slowly eased into biologics. Before you knew it, you were buried in a mountain of paperwork for the hub. For others, maybe you knew someone who worked in the clinic and heard of the open position that needed to be filled, or perhaps you were part of the front desk staff who also handles prior authorizations for the office. No matter how you became a biologic coordinator, the question is and always has been, “What is your why?”
For me, I live for the wins! My Type A organizational spirit gets giddy filling out my Excel sheet and adding one more patient. Will this be the week I hit 600 active patients on biologics? I feel the rush of how fast I can get the prior authorization approved, the prescription sent to the specialty pharmacy and filled, and then sent to the patient. I always try to beat my best record, but admittedly, I think five days is the fastest I can get. If you haven’t noticed yet, I have a competitive spirit.
In all seriousness, I live for this job. It is my passion, it is my calling, and I am eager to jump out of bed each morning ready to tackle the day. There is no better feeling than
I will never forget the way their lives have changed for the better, and honestly, it has forever changed me."

a patient receiving their prescribed biologic, approved by insurance and with a minimal copay improving their skin and quality of life. And yes, I have cried tears of joy when I hear patient stories on how the biologic I helped them gain access to has cleared their skin, improved their joints, and made them confident to go out into the world. There are certain patients I will never forget the way their lives have changed for the better, and honestly, it has forever changed me.
Now I understand this is not always the case, as there are frustrations, roadblocks and mountains to climb. But I believe if you wake up each day excited, ready to fight and learn, you too can have a rewarding career.
FUEL YOUR PASSION
Biologic access does not need to be scary or intimidating. It truly is one of the best careers, and you are lucky to be a part of it. This is one of the most rewarding jobs, and my hope is you find your excitement and passion. Remember your why. Get that fire in your belly that excites you and fuels your passion. You are amazing and your patients and providers admire all you do to help gain affordable access for medications. Your efforts not only help treat inflammatory conditions, but also give your patients back their lives.
" ACCESS DERMATOLOGY / ISSUE 8 / 2024 46


















bcofdermatology.com 47

















HELPFUL METHODS
ORGANIZATION
Know who is established on drug, who is starting, dates of bloodwork, appointments and prior authorization status. I use the Excel sheet provided by the BCOD, which can be found on bcofdermatology.com . You can modify the cells to include the information you desire at your fingertips.
SUBMIT ALL PRIOR AUTHORIZATIONS
I recommend using Cover My Meds, insurance forms, or an insurance plan dedicated website. Submit the forms electronically, as this provides the fastest turnaround.
DRAFT AND SUBMIT ALL APPEAL LETTERS IN-OFFICE INJECTION TRAINING
I understand this isn’t an ideal situation for every office, but I enjoy getting to spend a 30-minute sit-down with a patient explaining how to properly inject, review their injection schedule, appointment schedule, required bloodwork, and ensure they understand how to reorder their medication from their pharmacy.
UTILIZE THE HUB
This step is one of the most important. If you are not submitting enrollment forms to
the manufacturer HUBs by fax, portal, or Dropbox, your Field Reimbursement Manager (FRM) cannot correctly assist you. When you submit the enrollment form to the HUB, your FRM can have “eyes on the case” and help navigate tricky situations and walk you through any documentation or necessary steps that are missing.
Also, by completing the enrollment form and submitting it to the HUB, you will receive a benefits verification which will tell you everything you need to know, such as insurance coverage, deductibles, copays and specialty pharmacy.
The HUB can also flag patients
who may have accumulator/ maximizer plans and assist with access options.
UNDERSTAND COPAY CARDS/ PATIENT ASSISTANCE
Consistently educate yourself on manufacturer programs such as copay assistance and patient assistance programs. These plans have different variables, and each manufacturer has slightly different conditions than the next, so be sure you understand them. If something is ever in question, reach out to your FRM.
ACCESS DERMATOLOGY / ISSUE 8 / 2024 48
L TO R: RETHEA BOER/PEOPLEIMAGES.COMSTOCK. ADOBE.COM; DXFOTO.COMSTOCK.ADOBE.COM; C DAVIDS/PEOPLEIMAGES.COMSTOCK.ADOBE.COM ; KASTOSTOCK.ADOBE.COM


Help eligible patients start and stay on track with their therapy





















The DUPIXENT MyWay® patient support program can help facilitate patient access to DUPIXENT® (dupilumab) throughout the treatment journey.
Coverage support
DUPIXENT MyWay provides assistance navigating the insurance process with benefits investigations, prior authorization support, and education about the appeals process.
Patient access support
DUPIXENT MyWay may have support for eligible patients who need help with their out-of-pocket costs.
Education and support
Every enrolled patient is assigned a dedicated DUPIXENT MyWay Case Manager, who takes a patient-centric approach to providing tools, support, resources, and education throughout the treatment journey.
Support for your patients begins with a complete DUPIXENT MyWay Enrollment Form
You can help patients enroll in DUPIXENT MyWay by:
• Faxing the form to DUPIXENT MyWay at 844-387-9370
• Submitting the form through the DUPIXENT MyWay Document Drop at www.patientsupportnow.org
For more information:
Visit us on the exhibit floor OR
Call DUPIXENT MyWay at 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am–9 pm Eastern time
For any questions or concerns, or to report side effects with DUPIXENT® (dupilumab), please contact 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am–9 pm Eastern time.
DUP.23.06.0234
DUPIXENT® and DUPIXENT MyWay® are registered trademarks of Sanofi Biotechnology. © 2023 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved. 07/2023
D (dupilumab) Injection
DUPIXENT myway) sanofi

ePrescribe
We Are AcariaHealth

Submitting Prescriptions
ePrescribe
> Find AcariaHealth in your electronic medical record (EMR) system
Phone Call
800.511.5144 Ext 608.0033 to directly to our pharmacists
Fax

> Dial 800.511.5144 Ext 608.0033 to speak directly to our pharmacists
Fax
> Send completed referral form via fax to 877.541.1503

As a national comprehensive specialty pharmacy, we are transforming lives with compassionate care by serving patients living with complex medical conditions. We put patients first and improve health outcomes.
> Find AcariaHealth in your electronic medical record (EMR) system
Provider Services

> Referrals via phone, fax, and e-prescription
> Prior authorization completion
> Single point of contact to guide your team
> Support for your patients, including copay assistance
> Appeals support and guidance

> Send completed referral form via fax to 877.541.1503
PHARMACY ADDRESS
Accessible in Most EMR Systems
45K+

AcariaHealth Family of Pharmacies
Download our referral forms: Prescriptions ADDRESS
Address ahrx.com/AH-referrals
Send Us a Fax 877.541.1503
Send Us a Fax 877.541.1503
Download our drug list:
ahrx.com/AH-drug-list
Most EMR Systems referral forms:Download our drug list: ahrx.co/AH-referrals ahrx.co/AH-drug-list

98% 35K+
patients served each month of patients report high satisfaction based on proprietary patient data collection
patients engaged with our digital solutions, including text, psychographics, etc.
















































90%+
of those patients achieve optimal therapy adherence based on proprietary patient data collection
Contact us today to learn more about our DERMATOLOGY services sales@acariahealth.com | Phone: 800.511.5144 | Fax: 877.541.1503 or visit us at AcariaHealth.com
Download our referral forms:Download our drug list: ahrx.co/AH-referrals ahrx.co/AH-drug-list

© 2024 AcariaHealth. All rights reserved.































































































































 letter from the founder
Craig Schuette
letter from the founder
Craig Schuette





















 By Tara Biggers
CCMA, CDT // CLINICAL ASSISTANT/BIOLOGIC COORDINATOR
By Tara Biggers
CCMA, CDT // CLINICAL ASSISTANT/BIOLOGIC COORDINATOR



























 By Sarah Bailey BIOLOGIC COORDINATOR/LPN CENTER FOR SKIN WELLNESS, SARASOTA, FL
By Sarah Bailey BIOLOGIC COORDINATOR/LPN CENTER FOR SKIN WELLNESS, SARASOTA, FL








 By Lacie Chennault
By Lacie Chennault





































































































