1 NOVEMBER DECEMBER 2023 BLIZZARD SEASON How Technology Can Ease the Chaos BIOLOGIC COORDINATION An Illuminating Light in Dermatology BCOD CONFERENCE Highlights from the Third Annual Event CO-PAY SURPRISE Navigating Accumulator and Maximizer Plans CELEBRATING ONE YEAR OF CONTENT THAT HELPS MEMBERS NAVIGATE THE COMPLEX HEALTHCARE JOURNEY Anniv s y Issue































Introducing LITFULO (ritlecitinib)


The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12 1,2



TO LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM

One pill. Once a day.1
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details.
INDICATION


LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:





• with chronic or recurrent infection




Patient portrayal.

• who have been exposed to tuberculosis (TB)
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.





Confidence in Patient Support
Helping patients unlock access and reimbursement support for LITFULO™
Coverage Assistance
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Financial Assistance
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
Pharmacy Coordination
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
Live, Personal Support
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.

A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages. For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
TM
IMPORTANT SAFETY INFORMATION (cont’d)
SERIOUS INFECTIONS (cont’d)
In patients with a negative latent TB test, consider anti-TB therapy before initiating treatment with LITFULO in those at high risk and consider screening patients at high risk for TB during treatment with LITFULO.
Viral Reactivation
Viral reactivation, including cases of herpes virus reactivation (eg, herpes zoster), was reported in clinical trials. If a patient develops herpes zoster, consider interrupting treatment until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy with LITFULO. Patients with evidence of HIV infection or hepatitis B or C infection were excluded from clinical trials.
MORTALITY
In a large, randomized, postmarketing safety study of another Janus kinase (JAK) inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in RA patients treated with the JAK inhibitor compared with tumor necrosis factor (TNF) blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO. LITFULO is not approved for use in RA patients.
MALIGNANCIES
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical trials of LITFULO.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding NMSC) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
The risks and benefits of ritlecitinib treatment should be considered prior to initiating or continuing therapy in patients with a known malignancy other than successfully treated NMSC or cervical cancer.
Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of MACE (defined as cardiovascular death, non-fatal myocardial infarction [MI], and non-fatal stroke) was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LITFULO in patients that have experienced an MI or stroke.
THROMBOEMBOLIC EVENTS
Thrombosis has occurred in patients treated with LITFULO. An event of pulmonary embolism (PE) was reported in a patient receiving LITFULO. In a ritlecitinib higher dosing group, 1 patient reported an event of retinal artery occlusion.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, deep vein thrombosis, arterial thrombosis and PE were observed with the JAK inhibitor compared to those treated with TNF blockers.
Avoid LITFULO in patients who may be at increased risk of thrombosis. If symptoms of thrombosis or embolism occur, patients should interrupt LITFULO and be evaluated promptly and treated appropriately.
CONTRAINDICATION
LITFULO is contraindicated in patients with known hypersensitivity to ritlecitinib or any of its excipients.
HYPERSENSITIVITY
Serious reactions, including anaphylactic reactions, urticaria, and rash have been observed in patients receiving LITFULO in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue LITFULO and institute appropriate therapy.
LABORATORY ABNORMALITIES
Treatment with LITFULO was associated with decreases in lymphocytes and platelets. Prior to LITFULO initiation, perform absolute lymphocyte count (ALC) and platelet count. After initiating treatment with LITFULO, treatment interruption or discontinuation is recommended based on ALC and platelet count abnormalities.
Liver Enzyme Elevations: Treatment with LITFULO was associated with increased incidence of liver enzyme elevation compared to placebo. Increases of alanine transaminase (ALT) and aspartate aminotransferase (AST) ≥5 times the upper limit of normal were observed in patients in LITFULO clinical trials. Evaluate at baseline and thereafter according to routine patient management. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt LITFULO until this diagnosis is excluded.
Creatine Phosphokinase (CPK) Elevations: Treatment with LITFULO was associated with increased incidence of CPK elevation compared to placebo.
VACCINATIONS
No data are available on the response to vaccination in patients receiving LITFULO. Use of live attenuated vaccines should be avoided during or shortly prior to initiating treatment. Prior to initiating LITFULO, it is recommended that patients be brought up to date with all immunizations, including prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
HEPATIC IMPAIRMENT
LITFULO is not recommended in patients with severe hepatic impairment.
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥1%) are headache, diarrhea, acne, rash, urticaria, folliculitis, pyrexia, atopic dermatitis, dizziness, blood creatine phosphokinase increased, herpes zoster, red blood cell count decreased, and stomatitis.
DRUG INTERACTIONS
LITFULO can increase plasma concentrations of CYP3A and CYP1A2 substrates. Consider additional monitoring and dose adjustment of CYP3A and CYP1A2 substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
Coadministration with strong inducers of CYP3A is not recommended.
USE IN PREGNANCY
Available clinical trial data on LITFULO use in pregnant women are insufficient to identify a drug-associated risk from major birth defects, miscarriage or other adverse maternal or fetal outcomes. Advise pregnant females and females of reproductive potential to inform their healthcare providers if they are pregnant or intend to become pregnant during treatment with LITFULO.
If a patient becomes pregnant while receiving LITFULO, healthcare providers should report LITFULO exposure by calling 1-877390-2940.
LACTATION
Advise women not to breastfeed during treatment with LITFULO and for 14 hours after the last dose.
Please see Brief Summary of Prescribing Information, including BOXED WARNING, at the end of this advertisement.
References: 1. LITFULO. Prescribing information. Pfizer; 2023. 2. King B, Zhang X, Harcha WG, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b–3 trial. Lancet 2023;401(10387):1518-1529. doi:10.1016/S0140-6736(23)00222-2
© 2023 Pfizer Inc. All rights reserved. July 2023. PP-RIL-USA-0594
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
• Increased risk of serious bacterial, fungal, viral, and opportunistic infections leading to hospitalization or death, including tuberculosis (TB). Interrupt treatment if serious infection occurs until the infection is controlled. LITFULO should not be given to patients with active TB. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test
• Higher rate of all-cause mortality, including sudden cardiovascular death with another Janus kinase (JAK) inhibitor vs TNF blockers in rheumatoid arthritis (RA) patients. LITFULO is not approved for use in RA patients
• Malignancies have occurred in patients treated with LITFULO. Higher rate of lymphomas and lung cancers with another JAK inhibitor vs TNF blockers in RA patients
• Higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) with another JAK inhibitor vs TNF blockers in RA patients
• Thrombosis has occurred in patients treated with LITFULO. Increased incidence of pulmonary embolism, venous and arterial thrombosis with another JAK inhibitor vs TNF blockers
INDICATIONS AND USAGE
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment With LITFULO
• TB infection evaluation: LITFULO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of LITFULO
• Viral hepatitis screening in accordance with clinical guidelines: LITFULO initiation is not recommended in patients with hepatitis B or hepatitis C
• Treatment with LITFULO should not be initiated in patients with absolute lymphocyte count (ALC) <500/mm3 or a platelet count <100,000/mm3
• Update immunizations according to current immunization guidelines
Recommended Dosage
The recommended dosage of LITFULO is 50 mg orally once daily with or without food.
LITFULO capsules should be swallowed whole; not crushed, split, or chewed.
If a dose is missed, the dose should be taken as soon as possible unless it is less than 8 hours before the next dose; in which case, skip the missed dose and resume dosing at the regular scheduled time.
Patients With Severe Hepatic Impairment
LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
Treatment Interruption or Discontinuation
If treatment interruption is indicated, a temporary treatment interruption for less than 6 weeks is not expected to result in significant loss of regrown scalp hair.
Hematologic Abnormalities
• Treatment with LITFULO should be discontinued if platelet count is <50,000/mm3
• Treatment with LITFULO should be interrupted if ALC is <500/mm3 and may be restarted once ALC returns above this value
ALC and platelet counts are recommended before treatment initiation and at 4 weeks after treatment initiation, and thereafter according to routine patient management.
DOSAGE FORMS AND STRENGTHS
Capsules: 50 mg of ritlecitinib, size 3, opaque capsules with yellow body and blue cap. The body is printed with “RCB 50” and the cap is printed with “Pfizer” in black.
CONTRAINDICATIONS
LITFULO is contraindicated in patients with known hypersensitivity to ritlecitinib or any of its excipients.
WARNINGS AND PRECAUTIONS
Serious infections have been reported in patients receiving LITFULO. The most frequent serious infections have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO. Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
• with chronic or recurrent infection
• who have been exposed to TB
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt LITFULO if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
Screen patients for TB before starting therapy. LITFULO should not be given to patients with active TB. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB. In patients with a negative latent TB test, consider anti-TB therapy before initiating treatment with LITFULO in those at high risk and consider screening patients at high risk for TB during treatment with LITFULO.
Viral Reactivation
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), was reported in clinical trials. If a patient develops herpes zoster, consider interrupting treatment until the episode resolves.
Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy with LITFULO. Patients with evidence of HIV infection or hepatitis B or C infection were excluded from clinical trials.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO.
Malignancy and lymphoproliferative disorders, including nonmelanoma skin cancer (NMSC), were observed in clinical trials of LITFULO.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding NMSC) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
The risks and benefits of LITFULO treatment should be considered prior to initiating or continuing therapy in patients with a known malignancy other than a successfully treated NMSC or cervical cancer.
Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of MACE defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LITFULO in patients that have experienced an MI or stroke.
Thromboembolic Events
An event of pulmonary embolism (PE) was reported in a patient receiving LITFULO. In a ritlecitinib higher dosing group, 1 patient reported an event of retinal artery occlusion. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, deep vein thrombosis, and PE were observed compared to those treated with TNF blockers.
Avoid LITFULO in patients who may be at increased risk of thrombosis. If symptoms of thrombosis or embolism occur, patients should interrupt LITFULO and be evaluated promptly and treated appropriately.
Hypersensitivity
Serious reactions including anaphylactic reactions, urticaria, and rash have been observed in patients receiving LITFULO in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue LITFULO and institute appropriate therapy.
Laboratory Abnormalities
Treatment with LITFULO was associated with decreases in lymphocytes and platelets.
Prior to LITFULO initiation, perform ALC and platelet counts. After initiating treatment with LITFULO, treatment interruption or discontinuation are recommended based on ALC and platelet count abnormalities.
Liver Enzyme Elevations: treatment with LITFULO was associated with increased incidence of liver enzyme elevation compared to placebo. Increases of ALT ≥5 times the upper limit of normal (ULN) and increases of AST ≥5 times the ULN were observed in patients in LITFULO clinical trials. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt LITFULO until this diagnosis is excluded.
Creatine Phosphokinase (CPK) Elevations: treatment with LITFULO was associated with increased incidence of CPK elevation compared to placebo.
Vaccinations
No data are available on the response to vaccination in patients receiving LITFULO. Use of live attenuated vaccines should be avoided during or shortly prior to initiating treatment. Prior to initiating LITFULO, it is recommended that patients be brought up to date with all immunizations, including prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
ADVERSE REACTIONS
Clinical Trials Experience
The safety of LITFULO was evaluated in three randomized, placebo-controlled clinical trials and one long-term trial in patients with alopecia areata, including alopecia totalis and alopecia universalis, who were 12 years of age and older. A total of 1628 patients were treated with LITFULO representing 2085 patient-years of exposure. There were 1011 patients with at least 1 year of exposure to LITFULO. In the placebo-controlled period of clinical trials in alopecia areata, a total of 668 patients were exposed to LITFULO with 130 receiving 50 mg once daily for up to 24 weeks. The median age of patients was 33 years, 105 (11.9%) patients were 12 to <18 years old and 22 (2.5%) patients were 65 years of age or older. The majority of patients were White (70.7%) and female (63.6%).
Adverse reactions occurring at ≥1% in the treated groups and at a higher rate than placebo are presented in the following table. A total of 2 (1.5%) patients treated with LITFULO 50 mg were discontinued from the trials due to adverse reactions.
LITFULO™ (ritlecitinib) capsules, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
Adverse Reactions in Clinical Trials of LITFULO for the Treatment of Alopecia Areata
LITFULO 50 mg N=130 n (%)
Placebo N=213 n (%)
Headache 14 (10.8)18 (8.5)
Diarrhea 13 (10.0)8 (3.8)
Acne
8 (6.2)10 (4.7)
Rash 7 (5.4)2 (0.9)
Urticaria
6 (4.6)3 (1.4)
Folliculitis 4 (3.1)4 (1.9)
Pyrexia 4 (3.1)0
Dermatitis atopic
3 (2.3)1 (0.5)
Dizziness 3 (2.3)3 (1.4)
Blood CPK increased 2 (1.5)0
Herpes zoster 2 (1.5)0
Red blood cell count decreased 2 (1.5)0
Stomatitis 2 (1.5)0
Specific Adverse Reactions
Exposure adjusted incidence rates were adjusted by clinical trial size for all adverse reactions reported in this section.
Overall Infections
In the placebo-controlled trials, for up to 24 weeks, overall infections were reported in 66 patients (80.35 per 100 patient-years) treated with placebo and 43 patients (74.53 per 100 patient-years) treated with LITFULO 50 mg. Across clinical trials, including the long-term trial, overall infections were reported in 645 patients (50.71 per 100 patient-years) treated with LITFULO 50 mg or higher.
Serious Infections
In the placebo-controlled trials, for up to 24 weeks, 3 patients reported serious infections across all ritlecitinib doses studied. Across clinical trials, including the long-term trial, serious infections were reported in 12 patients (0.66 per 100 patient-years) treated with LITFULO 50 mg or higher. The most common serious infections were related to appendicitis, COVID-19 infection (including pneumonia), and sepsis.
Herpes Zoster
In the placebo-controlled trials, for up to 24 weeks, herpes zoster was reported in 4 patients across all ritlecitinib doses studied and 0 patients treated with placebo. Across clinical trials, including the long-term trial, herpes zoster was reported in 21 patients (1.17 per 100 patient-years) treated with LITFULO 50 mg or higher. Opportunistic infections of multi-dermatomal herpes zoster were reported in 1 patient (0.50 per 100 patient-years) treated with the ritlecitinib higher dose in the placebo-controlled trials and 2 patients (0.1 per 100 patient-years) treated with LITFULO 50 mg or higher in all clinical trials.
Malignancy
In the placebo-controlled trials, for up to 24 weeks, 1 malignancy (breast cancer) was reported in 1 patient (1.33 per 100 patient-years) treated with ritlecitinib higher dose and no malignancy was reported in patients treated with placebo. Across clinical trials, including the long-term trial, malignancies excluding NMSC were reported in 7 patients (0.37 per 100 patient-years) treated with LITFULO 50 mg or higher.
Thromboembolic Events
Across clinical trials, including the long-term trial, PE was reported in 1 patient (0.06 per 100 patient-years) treated with LITFULO. There was 1 report of retinal artery occlusion and 1 report of acute MI.
Urticaria
In the placebo-controlled trials, for up to 24 weeks, urticaria was reported in 28 patients treated in all ritlecitinib doses studied and 3 patients treated with placebo. The rate of urticaria was 8.23 per 100 patient-years in patients treated with LITFULO 50 mg and 4.03 per 100 patient-years in patients treated with placebo. Across clinical trials, including the long-term trial, urticaria was reported in 76 patients treated with LITFULO 50 mg or higher. Among all patients treated with LITFULO 50 mg or higher in the integrated safety analysis, the rate of urticaria was 4.10 per 100 patient-years. The median time to onset of an initial event was 8 weeks; median duration of urticaria was 7 days. Most of the cases were mild to moderate in severity.
Decreased Lymphocyte Counts
Across clinical trials, including the long-term trial, confirmed ALC <500/mm3 occurred in 1 patient (<0.1%) treated with LITFULO 50 mg. Age appeared to be a risk factor for lower ALC in patients ≥65 years of age.
Decreased Platelet Count
In the placebo-controlled trials, for up to 24 weeks, treatment with LITFULO was associated with a decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which platelet count remained stable at a lower level with continued therapy. Across clinical trials, including the long-term trial, 1 patient (<0.1%) had a confirmed platelet count <100,000/mm3. No patient had a confirmed platelet count <75,000/mm3
CPK Elevations
In the placebo-controlled trials, for up to 24 weeks, events of blood CPK increased were reported in 2 (1.5%) patients treated with LITFULO 50 mg and 0 patients treated with placebo.
Liver Enzyme Elevations
In the placebo-controlled trials, for up to 24 weeks, events of increases in liver enzymes ≥3 times the ULN were observed in patients treated with LITFULO.
DRUG INTERACTIONS
Effects of LITFULO on Other Drugs
CYP3A Substrates
Ritlecitinib is a CYP3A inhibitor. Concomitant use of ritlecitinib increases area under the curve (AUC) and Cmax of CYP3A substrates, which may increase the risk of adverse reactions of these substrates.
Consider additional monitoring and dosage adjustment in accordance with approved product labeling of CYP3A substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
CYP1A2 Substrates
Ritlecitinib is a CYP1A2 inhibitor. Concomitant use of ritlecitinib increases AUC and Cmax of CYP1A2 substrates, which may increase the risk of adverse reactions of these substrates. Consider additional monitoring and dosage adjustment in accordance with the approved product labeling of CYP1A2 substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
Effects of Other Drugs on LITFULO
CYP3A Inducers
Concomitant use of strong CYP3A inducer (e.g., rifampin) may decrease AUC and Cmax of ritlecitinib, which may result in loss of or reduced clinical response. Coadministration with strong inducers of CYP3A is not recommended.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry
If a patient becomes pregnant while receiving LITFULO, healthcare providers should report LITFULO exposure by calling 1-877-390-2940.
Risk Summary
Available data from clinical trials with LITFULO use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ritlecitinib to pregnant rats and rabbits during organogenesis caused fetotoxicity and fetal malformations at exposures equal to 49 and 55 times the maximum recommended human dose (MRHD) based on an AUC comparison, respectively.
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The estimated background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats, oral administration of ritlecitinib from gestation days 6 to 17 decreased fetal body weights and caused fetal skeletal malformations (malformed vertebrae and ribs) and variations (delayed ossification) at doses ≥175 mg/kg/day (49 times the MRHD based on AUC comparison). Maternal toxicity (lower body weights) was noted at 325 mg/kg/day (102 times the MRHD based on AUC comparison). There was no developmental toxicity at 75 mg/kg/day (16 times the MRHD based on AUC comparison).
In an embryo-fetal development study in pregnant rabbits, oral administration of ritlecitinib from gestation days 7 to 19 decreased mean fetal body weights and increased visceral malformations (malpositioned kidneys), skeletal malformations (supernumerary sternebrae, absent thoracic
arch, and/or fused thoracic centra), and skeletal variations (delayed ossification) at 75 mg/kg/day (55 times the MRHD based on AUC comparison). There was no developmental toxicity at doses up to 25 mg/kg/day (12 times the MRHD based on AUC comparison).
In a pre- and postnatal development study in rats, oral administration of ritlecitinib from gestation day 6 through lactation day 20 had no effects on pre- and postnatal development at doses up to 75 mg/kg/day (14 times the MRHD based on AUC comparison). At 175 mg/kg/day (41 times the MRHD based on AUC comparison), ritlecitinib caused adverse lower postnatal survival and lower offspring body weights, which correlated with delayed sexual maturation in both sexes. Bred females in the F1 generation also exhibited lower mean numbers of corpora lutea at 175 mg/kg/day.
Lactation
Risk Summary
There are no data on the presence of ritlecitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Ritlecitinib is present in the milk of lactating rats. When a drug is present in animal milk, it is likely that it will be present in human milk. Because of the serious adverse effects in adults, including risks of serious infection and malignancy, advise women not to breastfeed during treatment with LITFULO and for approximately 14 hours after the last dose (approximately 6 elimination half-lives).
Data
After a single oral 30 mg/kg dose of ritlecitinib to lactating rats, ritlecitinib concentrations in milk over time were higher than those in plasma. The mean milk to plasma AUC ratio was determined to be 2.2.
Pediatric Use
The safety and effectiveness of LITFULO for alopecia areata have been established in pediatric patients ages 12 years and older. A total of 181 pediatric patients ages 12 to <18 years were enrolled in alopecia areata clinical trials, with 105 pediatric patients ages 12 to <18 years with alopecia areata randomized in a pivotal, double-blind, placebo-controlled trial (Trial AA-I). Efficacy was consistent between the pediatric patients and adults. The adverse reaction profile in the pediatric patients was similar to adults.
The safety and efficacy of LITFULO have not been established in pediatric patients under 12 years of age.
Geriatric Use
No dose adjustment is required for patients ≥65 years of age.
A total of 28 patients enrolled in alopecia areata trials were 65 years of age and older, and none were 75 years of age and older. Clinical trials of LITFULO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
As there is a higher incidence of infections in the elderly population in general, caution should be used when treating the elderly.
Hepatic Impairment
No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
OVERDOSAGE
LITFULO was administered in clinical trials up to a single oral dose of 800 mg. Adverse reactions were comparable to those seen at lower doses and no specific toxicities were identified. Pharmacokinetics data up to and including a single oral dose of 800 mg in healthy adult volunteers indicate that more than 90% of the administered dose is expected to be eliminated within 48 hours.
There is no specific antidote for overdose with LITFULO. Treatment should be symptomatic and supportive, and monitor patients for signs and symptoms of adverse reactions.
In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
This brief summary is based on LITFULOTM (ritlecitinib) Prescribing Information LAB-1469-0.5.
Issued: June 2023.
The product’s label may have been updated. For full Prescribing Information, visit LITFULOHCP.com.
© 2023 Pfizer Inc. All rights reserved.
PP-RIL-USA-0414 June 2023
LITFULO™ (ritlecitinib) capsules, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
ACCESS DERMATOLOGY NOVEMBER/DECEMBER 2023 6 contents ACCESS DERMATOLOGY ISSUE 6
8 Founder's Letter Happy anniversary to us all! 40 Humira® Biosimilar Clinical Reference Guide Resource pages courtesy of Walgreens Clinical teams 48 Third Annual BCOD National Conference Keep advancing patient access 58 Employee Spotlight Recognizing outstanding patient service
69 Gifts for a Getaway Find the perfect present for the jet-setter on your list 72 Last Word An inspiring quote from author Leo Buscaglia
10 Biologic Coordination An illuminating light in dermatology 24 Prior Authorizations What they are and the best way to handle them 34 Avoiding Co-Pay Surprise How to navigate accumulator and maximizer plans 62 Surviving the Blizzard How technology can ease re-enrollment season chaos
COMMUNITY
LIFESTYLE
FEATURES


























































































































































bcofdermatology.com 7 24 34 10 Co-Pay 62 THOUGHTFUL PLAN INNOVATIVE TECHNOLOGIES A ROBUST ECOSYSTEM CONSISTENT CARE WITH NO LAPSE IN MEDICATION ACCESS WELL-EQUIPPED STAFF
letter from the founder / Craig Schuette
Exp ding the BC Community
It feels like yesterday when BCoD brought biologic coordinators together for the first time in Charleston to share best practices, learn from industry experts, and meet peers who share in the fight for the patient. Since then, BCoD has grown to become a community that welcomes more than one new member per day. As a result, we have a deeper drive and capability than ever before to enhance education and training for the BC.
We share our passion with you: our members. In our 2023 survey, you showed your love for the field of dermatology and the battle for the script! Those fights translate into remarkable prior authorization approval levels. Furthermore, our coaching programs demonstrate a significant percentage increase in appeal and approval times and even a quicker transfer out of bridge.
And we're not letting up. Some of the developments that we are excited to share with our members include:
• CERTIFICATION: BCoD has collaborated with healthcare organizations to offer the most significant training program for individuals seeking BC certification.
• FIELD REIMBURSEMENT FINDER:
Effective communication between BCs and FRMs/FRAs is crucial, and our locator tool has helped to improve it.
• CREATIVE PARTNERSHIPS:
We're making connections and building relationships to bring more innovation to our members' workflows.
• BUILDING COMMUNITY:
Our education managers guide tough cases through group and one-on-one
coaching sessions to help you and your patients with drug use.
Our gratitude comes from those who believe in us and trust us to assist with workflow and patients. I want to personally thank the BCoD team and our many contributing educational managers and volunteers for creating a space of nourishment and community. And I want to thank our partners for equally trusting us with your programs and the patients they serve. The patient suffers if there are cracks in the process and misunderstanding of programs. Our members are armed with the information to enhance access and change lives through collaboration!
We are excited to see you in San Antonio. We were almost at total capacity when writing this (early September), but we'll reserve space for you! New to the agenda this year, we're holding a Partner Pavilion and a Tough Case Blitz to help you through those bottlenecked cases.
If you can't make it this year, rest assured that we'll bring you recaps of the information presented. If you still need to join BCoD, we hope you take the time to reach out to us online at bcofdermatology.com or speak directly with our education team to learn more.
We look forward to welcoming you to our community, and we honor the work you do for patients every day.
With appreciation,
CRAIG SCHUETTE


ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 8





STILL GOLDEN. EHR | PM | PATIENT ENGAGEMENT | TELEHEALTH | ASC | INTEGRATED PAYMENTS | CONSULTING Don’t fall for anything less. Nextech is still the only EHR to receive the American Academy of Dermatology’s DataDerm™ Gold recognition. Start anew with the #1 solution for Dermatology
 By Ginger McWilliams DERMATOLOGY/IMMUNOLOGY , BRISTOL MYERS SQUIBB
By Ginger McWilliams DERMATOLOGY/IMMUNOLOGY , BRISTOL MYERS SQUIBB
BIOLOGIC COORDINATION
An Illuminating Light in Dermatology
It is often said that “Change is the only constant in life.” While this may be disarming and a bit uncomfortable, knowing that change is a constant force allows us to prepare for evolution and equips us for the ever-changing dermatology landscape. There is no one better equipped and ready than the biologic coordinator (BC).
Biologic coordinators are accustomed to challenges and can prevail through adversity for patients. For those of us who have worked in the dermatology space for a long time, the view today is quite different from previous years. Our ever-changing jobs are dynamic and constantly evolving. Even in this year’s membership survey with BCOD, 86% of BCs manage patients outside of biologics, which further complicates the role.1 Now more than ever, you are called to “illuminate” in ways you may not have before.
FDFDSFDSFDSFSDFSDDSFDSDS ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 10

“THERE IS ALWAYS LIGHT. IF ONLY WE’RE BRAVE ENOUGH TO SEE IT. IF ONLY WE ARE BRAVE ENOUGH TO BE IT.”
—AMANDA GORMAN
bcofdermatology.com 11
Over the past 25 years, we have seen an influx of responsibilities shift to the BCs in a highly complex terrain. The dermatology market is growing due to the rising geriatric population, an increase and awareness of dermatological diseases, and more personal spending. The positive news is this trend creates great job assurance. New treatments are constantly being approved with revolutionary mechanisms of action that often expand into multiples disease states, adding to the complexity you manage.
While the three largest PBMs—Express Scripts, CVS/Caremark, and Optum—have historically managed prescription drugs in vertical integration with the three largest health insurers, shifts are beginning to occur. New companies have launched, such as Mark Cuban’s CostPlus, with reduced costs for patients at its center, and
“Over the past 25 years, we have seen an influx of responsibilities shift to the BCs in a highly complex terrain.”
ClearNet by Express Scripts, which mimics the cost-plus model for prescription care. ClearNet is set to launch in early 2024.2
There are old faces from the industry and dozens of fresh new reimbursement staff to educate you and aid in patient onboarding. Additionally, you have a plethora of specialty pharmacy reps calling on you too—all with the intent to help you support your patients. In addition to the external changes, the office landscape has changed drastically over the past few decades.
Many of you may have started your career with one or two providers, and now you may be working for a private equity firm, hospital network, SP, or you might even work remotely. In dermatology, we have seen an explosion of private equity firms with a 275% growth and 50% decline of private practices.
2018 2019-2022
ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 12
DERMATOLOGY PRACTICES ARE UNDERGOING DRAMATIC TRANSFORMATION REGARDING PRACTICE TYPE, OWNERSHIP AND SIZE
4% 4% 5% 8% 79% Hospitals and IDNs have remained largely stagnant. Small MD Large MD IDN Hospital PE Solo groups dropped from 75% to 40% 25% large group growth PE grew to 15%+ IN JUST 2 YEARS, WE HAVE SEEN A 50% DECLINE IN SOLO PRACTICES AND A 275% INCREASE IN PE PRESENCE.
NURSE PRACTITIONERS
355,000 in the U.S.
36,000 new NPs completed their academic programs 2020-2021
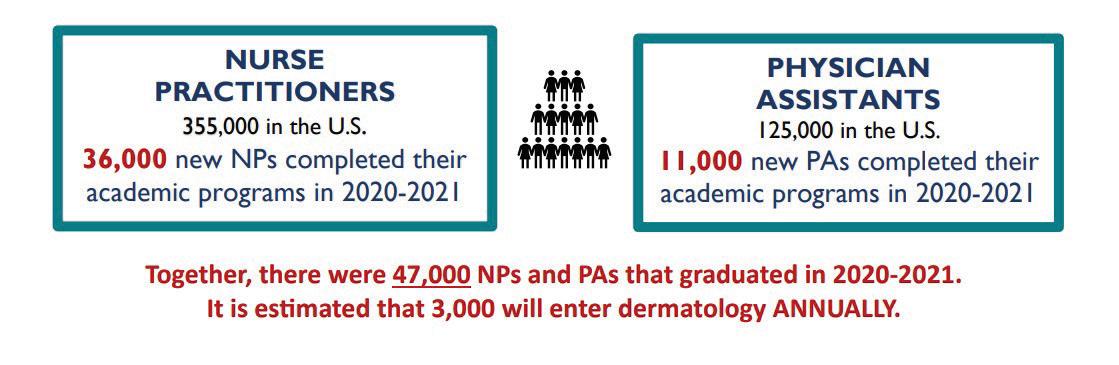
PHYSICIAN ASSISTANT
355,000 in the U.S.
36,000 new NPs completed their academic programs 2020-2021
Together, there were 47,000 NPs and PAs that graduated in 2020-2021. It is estimated that 3,000 will enter dermatology annually.
Your care team is evolving, too. To manage the current healthcare patient influx, more and more PAs and NPs are coming into your practices to manage patients.3 The current trends predict over 3,000 new NP or PAs will enter dermatology annually, often with little dermatology training in their curriculum, which may further complicate your job.
As the terrain changes and responsibilities grow, it is important that you as biologic coordinators focus on your “light” in managing patients through access advocacy. Employing the assistance of industry and specialty pharmacy partners is a mainstay. Staying abreast of the legislative changes in your state and nationally is key with the increase of PAs, LMNs and peer-to-peers. Recently, step therapy legislative changes have occurred in Tennessee, Pennsylvania, and Massachusetts, which AAD summarizes in their legislative updates.4 Additional
“In dermatology, we have seen an explosion of private equity firms with a 275% growth and 50% decline of private practices.”
efforts have been made to abolish co-pay accumulators and maximizers. To date, 19 states have passed legislation banning co-pay accumulators as of June 20235 (see map image).
The BC community, along with national and local advocacy organizations, shine a light on the needs of dermatology patients for a unified voice through their efforts and through organizations like the National Psoriasis Association or National Eczema Association. These local advocacy events provide opportunities to gain insights on newly developed patient resources, pertinent legislation, and various networking opportunities to support patients in your community.
As you forge ahead in dermatology, I encourage you to be your best advocate through education, BCOD certification, and networking opportunities for fruitful partnerships. Invest in mentorships with BCOD members, webinars, industry
13
bcofdermatology.com
education and offerings. Become an active reader or podcast attendee to familiarize yourself with the trends that are here today or coming tomorrow. Create a LinkedIn profile, follow BCOD members, patients, and healthcare industry experts and advocates. Make sure you invest in your own growth and document your skills and achievements on your resume. Remember: Your resume is your window to the community.
For over 25 years, I have been inspired by biologic coordinators as I have marketed infusibles, injectables and orals in immunology. Many of you have welcomed me into your world, often teaching me more than I can ever teach you. I have seen you grimace with frustration, dig in your heels, and shed tears of joy when a patient starts to heal from a medication you helped to secure. You walk this healthcare journey alongside
“Now more than ever, you are called to illuminate in ways that you may not have ever before.”
REFERENCES
1. Access Dermatology 2023 Survey Results
2. Drug Prices: Cigna Follows Mark Cuban’s Lead to Simplify Pricing - Bloomberg
each of your patients. You have taught me and so many others what it means to be beacons of light for patients. It is because of each of you that the BCOD was born, assembled and is thriving today. You have and continue to make a difference.
May you always illuminate—as I know you will!
3. AANP National Nurse Practitioner Database, 2022. American Association of Colleges of Nursing (AACN). (2022). 2021-2022 Enrollment and Graduations in Baccalaureate and Graduate Programs in Nursing. Washington, DC: AACN. Am J Manag Care. 2021;27(11):498504 provided by Margaret Bobonich, DNP.
4.Keeping you in the driver’s seat with step therapy and prior authorization reform (aad.org)
5. avalere-state-copayaccumulator-bans-impact-11-ofus-commercial-lives.pdf

ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 14
Figure 1. States with Laws Banning Copay Accumulator Use Note: State-level activity current as of June 21, 2023. Puerto Rico has also enacted a copay accumulator ban.
Copay Accumulator Bans (19)

Bringing collections that balance technical innovation and iconic design to fit the active lifestyle of today’s sophisticated and conscious woman.
A first-in-class,* selective TYK2 inhibitor for moderate-to-severe plaque psoriasis1,2


INDICATION


Clearer skin



SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Please see additional Important Safety Information and Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
*TYK2 is a member of the Janus kinase (JAK) family.

STUDY DESIGNS




Clearer choice
AT LAST...SOTYKTU
Superior skin clearance vs Otezla® (apremilast)†‡ in a once-daily, oral pill1
†POETYK PSO-1 (N=664) and POETYK PSO-2 (N=1020) were two, 52-week, multicenter, randomized, double-blind, placebo- and active (apremilast 30 mg twice daily)-controlled, Phase 3 studies to evaluate the safety and efficacy of SOTYKTU (6 mg once daily) in adult patients with moderate-to-severe plaque psoriasis who were eligible for systemic therapy or phototherapy. Patients had a body surface area (BSA) involvement of ≥10%, a Psoriasis Area and Severity Index (PASI) score ≥12, and a static Physician’s Global Assessment (sPGA) ≥3 (moderate or severe).
Both studies assessed the responses at Week 16 compared with placebo for the two co-primary endpoints:
• The proportion of patients who achieved at least a 75% improvement in PASI scores from baseline (PASI 75)
• The proportion of patients who achieved an sPGA score of 0 (clear) or 1 (almost clear)
There were multiple ranked secondary endpoints, including:
• The proportion of patients who achieved PASI 75 at Week 16 vs apremilast
STUDY RESULTS 1,3,4
‡Comparison between SOTYKTU and apremilast was a secondary endpoint.
Co-primary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs placebo: PSO-1: 58% (193/330) vs 13% (21/166), P<0.0001; PSO-2: 53% (271/511) vs 9% (24/255), P<0.0001
• sPGA 0/1 at Week 16 for SOTYKTU vs placebo: PSO-1: 54% (178/330) vs 7% (12/166), P<0.0001; PSO-2: 50% (253/511) vs 9% (22/255), P<0.0001
Select secondary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs apremilast: PSO-1: 58% (193/330) vs 35% (59/168); P<0.0001; PSO-2: 53% (271/511) vs 40% (101/254); P=0.0004.
SELECT IMPORTANT SAFETY INFORMATION
In the PSO-1 and PSO-2 trials, through Week 16, the most common adverse reactions (≥1% and higher than placebo) in patients taking SOTYKTU (n=840) were upper respiratory infections (19.2%), blood creatine phosphokinase increase (2.7%), herpes simplex (2.0%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%).
BSA=body surface area; PASI=psoriasis area and severity index; PASI 75=75% reduction from baseline in PASI; sPGA=static Physician’s Global Assessment; TYK2=tyrosine kinase 2.
Otezla® is a registered trademark of Amgen, Inc. SOTYKTU tablet is not actual size. Shown for illustrative purposes only.
Scan to learn more or visit SOTYKTUHCP.com
INDICATION
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Infections: SOTYKTU may increase the risk of infections. Serious infections have been reported in patients with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19. Avoid use of SOTYKTU in patients with an active or serious infection. Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing, have appropriate antimicrobial therapy initiated and be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex) was reported in clinical trials with SOTYKTU. Through Week 16, herpes simplex infections were reported in 17 patients (6.8 per 100 patient-years) treated with SOTYKTU, and 1 patient (0.8 per 100 patientyears) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent patient. During PSO-1, PSO-2, and the open-label extension trial, the majority of patients who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age. The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis (TB): In clinical trials, of 4 patients with latent TB who were treated with SOTYKTU and received appropriate TB prophylaxis, no patients developed active TB (during the mean follow-up of 34 weeks). One patient, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU. Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas: Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.
SOTYKTU (deucravacitinib) INDICATION AND IMPORTANT SAFETY INFORMATION
Rhabdomyolysis and Elevated CPK: Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
Laboratory Abnormalities: Treatment with SOTYKTU was associated with increases in triglyceride levels. Periodically evaluate serum triglycerides according to clinical guidelines during treatment. SOTYKTU treatment was associated with an increase in the incidence of liver enzyme elevation compared to placebo. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded.
Immunizations: Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition: It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
Most common adverse reactions (≥1% of patients on SOTYKTU and more frequently than with placebo) include upper respiratory infections, blood creatine phosphokinase increased, herpes simplex, mouth ulcers, folliculitis and acne.
SPECIFIC POPULATIONS
Pregnancy: Available data from case reports on SOTYKTU use during pregnancy are insufficient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Report pregnancies to the BristolMyers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Lactation: There are no data on the presence of SOTYKTU in human milk, the effects on the breastfed infant, or the effects on milk production. SOTYKTU is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Hepatic Impairment: SOTYKTU is not recommended for use in patients with severe hepatic impairment.
SOTYKTU is available in 6 mg tablets.
Please see Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
References: 1. SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 2. Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11(5):1763-1776.
3. Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 4. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51.
SOTYKTU and the related logo are trademarks of Bristol-Myers Squibb Company.
© 2023 Bristol-Myers Squibb Company. 1787-US-2300018 02/23
for oral use
Brief Summary of Prescribing Information. For complete prescribing information consult official package insert.
INDICATIONS AND USAGE
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use:
SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment Initiation
Evaluate patients for active and latent tuberculosis (TB) infection prior to initiating treatment with SOTYKTU. If positive, start treatment for TB prior to SOTYKTU use [see Warnings and Precautions]
Update immunizations according to current immunization guidelines [see Warnings and Precautions]
Recommended Dosage
The recommended dosage of SOTYKTU is 6 mg taken orally once daily, with or without food. Do not crush, cut, or chew the tablets.
Recommended Dosage in Patients with Hepatic Impairment
SOTYKTU is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations and Clinical Pharmacology (12.3) in full Prescribing Information] No dosage adjustment is needed for patients with mild to moderate hepatic impairment.
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU [see Warnings and Precautions]
WARNINGS AND PRECAUTIONS
Hypersensitivity
Hypersensitivity reactions such as angioedema have been reported in subjects receiving SOTYKTU. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU [see Adverse Reactions]
Infections
SOTYKTU may increase the risk of infections.
Serious infections have been reported in subjects with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19 [see Adverse Reactions]
Avoid use of SOTYKTU in patients with an active or serious infection.
Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with SOTYKTU. A patient who develops a new infection during treatment with SOTYKTU should undergo prompt and complete diagnostic testing; appropriate antimicrobial therapy should be initiated; and the patient should be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical trials with SOTYKTU. In the 16-week placebo-controlled period, herpes simplex infections were reported in 17 subjects (6.8 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent subject who received SOTYKTU. During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, the majority of subjects who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age.
The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Subjects with positive screening tests for hepatitis B or C, or chronic hepatitis B, or untreated hepatitis C were excluded from clinical trials. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis
In clinical trials, of 4 subjects with latent tuberculosis (TB) who were treated with SOTYKTU and received appropriate TB prophylaxis, no subjects developed active TB (during the mean follow-up of 34 weeks). One subject, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU.
Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients receiving SOTYKTU for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas
Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU (deucravacitinib) [see Adverse Reactions]
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU. Rhabdomyolysis and Elevated CPK
Cases of rhabdomyolysis were reported in subjects treated with SOTYKTU resulting in interruption or discontinuation of SOTYKTU dosing.
Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to treatment with placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Adverse Reactions]
Laboratory Abnormalities
Triglyceride Elevations - Treatment with SOTYKTU was associated with increases in triglyceride levels. The effect of this elevated parameter on cardiovascular morbidity and mortality has not been determined. Periodically evaluate serum triglycerides according to the clinical guidelines for hyperlipidemia while patients are receiving treatment with SOTYKTU. Manage patients according to clinical guidelines for the management of hyperlipidemia [see Adverse Reactions]
Liver Enzyme Elevations - Treatment with SOTYKTU was associated with an increase in the incidence of liver enzyme elevation compared to treatment with placebo. Liver serum transaminase elevations ≥3 times the ULN were reported in subjects treated with SOTYKTU. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded [see Adverse Reactions]
Immunizations
Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition
It is not known whether TYK2 inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
• Infections [see Warnings and Precautions]
• Malignancy including lymphomas [see Warnings and Precautions]
• Laboratory Abnormalities [see Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SOTYKTU was evaluated in two placebo- and active-controlled trials (PSO-1 and PSO-2) and an open-label extension trial in which subjects who completed PSO-1 or PSO-2 could enroll [see Clinical Studies (14) in full Prescribing Information]. In these clinical trials, a total of 1,519 subjects with moderate-to-severe plaque psoriasis who were candidates for systemic therapy or phototherapy received SOTYKTU 6 mg orally once daily. Of these, 1,141 subjects were exposed to SOTYKTU for at least one year.
In trials PSO-1 and PSO-2, 1,681 subjects were randomized to receive SOTYKTU 6 mg (840 subjects), placebo (419 subjects), or apremilast 30 mg twice daily (422 subjects). All subjects randomized to placebo switched to SOTYKTU at Week 16. All other subjects remained in their original treatment group until Week 24, at which point subjects could have continued on the same treatment or be switched to SOTYKTU or placebo. The mean age of subjects was 47 years. The majority of subjects were White (87%) and male (67%).
In the 16-week placebo-controlled period of the pooled clinical trials (PSO-1 and PSO-2), discontinuation of therapy due to adverse reactions in subjects who received SOTYKTU was 2.4%, compared to 3.8% for placebo.
Table 1 summarizes the adverse reactions that occurred in at least 1% of subjects in the SOTYKTU group and at a higher rate than the placebo group during the 16-week controlled period.
SOTYKTU™ (deucravacitinib)
tablets,
Table 1: Adverse Reactions that Occurred in ≥1% of Subjects with Plaque Psoriasis in the SOTYKTU (deucravacitinib) Group and More Frequently than in the Placebo Group in Trials PSO-1 and PSO-2 through Week 16
Adverse Reaction
a Includes upper respiratory tract infection (viral, bacterial, and unspecified), nasopharyngitis, pharyngitis (including viral, streptococcal, and unspecified), sinusitis (includes acute, viral, bacterial), rhinitis, rhinotracheitis, tracheitis, laryngitis, and tonsillitis (including bacterial, streptococcal)
b Includes oral herpes, genital herpes, herpes simplex, and herpes virus infection
c Includes mouth ulceration, aphthous ulcer, tongue ulceration, and stomatitis
d Includes acne, acne cystic, and dermatitis acneiform
Adverse reactions that occurred in <1% of subjects in the SOTYKTU group were herpes zoster.
Specific Adverse Reactions
Exposure adjusted incidence rates are reported for all the adverse reactions presented below.
Infections
In the 16-week placebo-controlled period, infections occurred in 29% of the SOTYKTU group (116 events per 100 person-years) compared to 22% of the placebo group (83.7 events per 100 person-years). The majority of infections were non-serious and mild to moderate in severity and did not lead to discontinuation of SOTYKTU.
In the 16-week placebo-controlled period, serious infections were reported in 5 subjects (2.0 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo. The most common serious infections reported during the 52-week treatment period were pneumonia and COVID-19.
Malignancies
During the 0-to-52-week treatment period of the two clinical trials, PSO-1 and PSO-2 (total exposure of 986 patient-years with SOTYKTU), malignancies (excluding non-melanoma skin cancer) were reported in 3 subjects treated with SOTYKTU (0.3 per 100 patient-years), including single cases each of breast cancer, hepatocellular carcinoma, and lymphoma after 24, 32, and 25 weeks of treatment, respectively.
During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, a total of 3 subjects (0.1 per 100 patient-years), developed lymphoma while receiving SOTYKTU after 25, 77, and 98 weeks of treatment.
Laboratory Abnormalities
Creatine Phosphokinase (CPK)
In the 16-week placebo-controlled period, increased CPK (including Grade 4) was reported in 23 subjects (9.3 per 100 patient-years) treated with SOTYKTU, and 5 subjects (4.1 per 100 patient-years) treated with placebo.
Liver Enzyme Elevations
Events of increases in liver enzymes ≥3 times the ULN were observed in subjects treated with SOTYKTU [see Warnings and Precautions]. In the 16-week placebo-controlled period:
• ALT elevations ≥3 times the ULN was reported in 9 subjects (3.6 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
• AST elevations ≥3 times the ULN was reported in 13 subjects (5.2 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
Decreased Glomerular Filtration Rate (GFR)
In the 16-week placebo-controlled period in subjects who had moderate renal impairment (eGFR 30-59 mL/min) at baseline, decreased GFR was reported in 4 subjects (1.6 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Two of the deucravacitinib-treated subjects had worsening of baseline proteinuria.
Lipid Elevations
Mean triglycerides increased by 10.3 mg/dL during the 16-week treatment period in subjects treated with SOTYKTU and by 9.1 mg/dL during the 52-week treatment period.
Safety Through Week 52
In PSO-1 and PSO-2, the exposure adjusted incidence rate of adverse reactions in subjects treated with SOTYKTU from Week 0 through Week 52 without switching treatment did not increase compared to the rate observed during the first 16 weeks of treatment.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data from case reports on SOTYKTU use during pregnancy are insufficient to evaluate a drugassociated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, no effects on embryo-fetal development were observed with oral administration of deucravacitinib to rats and rabbits during organogenesis at doses that were at least 91 times the maximum recommended human dose (MRHD) of 6 mg once daily (see Data)
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report pregnancies to the Bristol-Myers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Data
Animal data
Deucravacitinib was administered orally during the period of organogenesis at doses of 5, 15, or 75 mg/kg/day in rats and 1, 3, or 10 mg/kg/day in rabbits. Deucravacitinib was not associated with embryo-fetal lethality or fetal malformations in either species. These doses resulted in maternal exposures (AUC) that were 266 times (rat) or 91 times (rabbit) the exposure at the MRHD.
In a pre- and post-natal development study in rats, deucravacitinib was administered orally from gestation day 6 through lactation day 20, at doses of 5, 15, or 50 mg/kg/day. At 50 mg/kg/day, F1 offspring had reduced body weight gains during the pre-weaning period. After weaning, body weights of affected F1 offspring gradually normalized to control levels. No maternal effects were observed at 50 mg/kg/day (110 times the MRHD based on AUC comparison). No deucravacitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring were noted at doses up to 15 mg/kg/day (19 times the MRHD based on AUC comparison).
Lactation
Risk Summary
There are no data on the presence of deucravacitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Deucravacitinib is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU (deucravacitinib) and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Data
Animal Data
A single oral dose of 5 mg/kg radiolabeled deucravacitinib was administered to lactating (post-partum days 8 to 12) rats. Deucravacitinib and/or its metabolites were present in the milk of lactating rats.
Pediatric Use
The safety and effectiveness of SOTYKTU in pediatric patients have not been established.
Geriatric Use
Of the 1,519 subjects with plaque psoriasis treated with SOTYKTU, 152 (10%) subjects were 65 years or older and 21 (1.4%) subjects were 75 years or older.
During the Week 0-16 period, for those subjects (80 subjects ≥65 years old, including 12 subjects ≥75 years old) who received SOTYKTU without switching treatment arms, there was a higher rate of overall serious adverse reactions, including serious infections, and discontinuations due to adverse reactions compared with younger adults.
No overall differences in effectiveness of SOTYKTU have been observed between patients 65 years of age and older and younger adult patients.
Renal Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild, moderate, or severe renal impairment or in patients with end stage renal disease (ESRD) on dialysis [see Clinical Pharmacology (12.3) in full Prescribing Information]
Hepatic Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. SOTYKTU is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) [see Adverse Reactions and Clinical Pharmacology (12.3) in full Prescribing Information]
OVERDOSAGE
There is no experience regarding human overdosage with SOTYKTU. In case of overdose, consider contacting the Poison Help line (1-800-222-1222) for additional overdosage management recommendations. The extent of deucravacitinib elimination by hemodialysis was small (5.4% of dose per dialysis treatment), and thus hemodialysis for treatment of overdose with SOTYKTU is limited.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of deucravacitinib was assessed in 2-year rat and 6-month rasH2 transgenic mouse studies. No evidence of tumorigenicity was observed in male or female rats that received deucravacitinib at oral doses up to 15 mg/kg/day (51 times the MRHD based on AUC comparison). No evidence of tumorigenicity was observed in male or female Tg.rasH2 mice that received deucravacitinib at oral doses up to 60 mg/kg/day.
Deucravacitinib was not mutagenic in a bacterial mutagenicity assay (Ames test) or clastogenic in an in vitro chromosomal aberration assay (cultured Chinese hamster ovary cells) or in an in vivo rat peripheral blood micronucleus assay.
In male rats, deucravacitinib had no effects on reproductive parameters (mating, fertility, and sperm morphology) or early embryonic development of their offspring at oral doses up to 50 mg/kg/day (224 times the MRHD based on AUC comparison).
SOTYKTU 6 mg once daily N=840 n (%) Placebo N=419 n (%) Upper respiratory infectionsa 161 (19.2) 62 (14.8) Blood creatine phosphokinase increased 23 (2.7) 5 (1.2) Herpes simplexb 17 (2.0) 1 (0.2) Mouth ulcersc 16 (1.9) 0 (0.0) Folliculitis 14 (1.7) 0 (0.0) Acned 12 (1.4) 1 (0.2)
In female rats, deucravacitinib had no effects on mating, fertility, or early embryonic parameters at oral doses up to 50 mg/kg/day (171 times the MRHD based on AUC comparison).
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) before starting SOTYKTU (deucravacitinib) therapy and each time the prescription is renewed, as there may be new information they need to know. Advise patients of the potential benefits and risks of SOTYKTU.
Hypersensitivity Reactions
Advise patients to discontinue SOTYKTU and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions]
Infections
Inform patients that SOTYKTU may lower the ability of their immune system to fight infections and to contact their healthcare provider immediately if they develop any signs or symptoms of infection [see Warnings and Precautions]
Inform patients that herpes infections, including serious, may occur with use of SOTYKTU [see Warnings and Precautions]
Malignancies including Lymphomas
Inform patients that SOTYKTU may increase their risk of developing malignancies including lymphomas. Instruct patients to inform their healthcare provider if they have ever had any type of cancer [see Warnings and Precautions]
Rhabdomyolysis
Inform patients that SOTYKTU may increase their risk of developing rhabdomyolysis. Instruct patients to immediately inform their healthcare provider if they develop unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Warnings and Precautions]
Laboratory Abnormalities
Inform patients that SOTYKTU (deucravacitinib) may affect certain lab tests, and that blood tests may be
Inform patients that SOTYKTU (deucravacitinib) may affect certain lab tests, and that blood tests may be required before and during SOTYKTU treatment [see Warnings and Precautions]
Immunizations
Advise patients that vaccination with live vaccines is not recommended during SOTYKTU treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform the healthcare practitioner that they are taking
Advise patients that vaccination with live vaccines is not recommended during SOTYKTU treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform the healthcare practitioner that they are taking SOTYKTU prior to a potential vaccination [see Warnings and Precautions].
Pregnancy
Advise patients to report their pregnancy to Bristol-Myers Squibb Company at 1-800-721-5072 [see Use in Specific Populations]
Manufactured by: Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
U.S. License No. 1713
© 2022 Bristol-Myers Squibb Company.
SOTYKTU and the Bristol Myers Squibb logo are trademarks of Bristol-Myers Squibb Company.
[see Use
Issued: September 2022
Issued: September 2022
1787-US-2200566 09/22
1787-US-2200566 09/22
EDUCATION & NETWORKING EMPOWERS MEMBERS THROUGH Join our network of biologic coordinators landscape of drug access together. Sign up today at bcofdermatology.com Connect with BCoD:

Prior Authorizations
WHAT THEY ARE AND THE BEST WAY TO HANDLE THEM
ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 24
MRPANYASTOCK.ADOBE.COM
By Casey Butrus
SENIOR PHARMACIST, CLINICAL PHARMACY STRATEGIES | HIGHMARK INC.
The main purpose of a prior authorization (PA) is to determine therapeutic need, clinical efficacy to FDA label, optimize outcomes using disease state measures and evidence-based medicine, enforce clinical practice guidelines, contain cost and ensure medication safety.
Drugs that need PAs may be high-cost or high-spend (specialty) drugs, have serious adverse effects, be new-to-market, or have the potential for waste or misuse. Other examples—such as cosmetic or hair growth medicine—may not be covered by certain groups or agencies. Additionally, brand-name drugs with generic alternatives or non-preferred/non-formulary drugs can also fall under this PA-required category.
To manage the utilization of such therapies, payors will put stipulations in their formularies. Utilization management may include PA, step therapy and quantity limitations (QL). Step therapy typically requires use of a clinically recognized first-line drug before approval of a more expensive medication or use of a planpreferred medication first. QLs may be used to prevent stockpiling, promote adherence to best prescribing practices, or to prevent medication misuse or abuse. Limitations are usually consistent with FDA-approved durations/dosing, or maximum dosing. There are broadly two types of formularies. An open formulary allows for most or all FDA-approved drugs to be covered by the payor, but patients may pay more out-ofpocket for non-preferred products. This
It is possible more than one utilization management edit or nonformulary (NF) request may apply to a drug and be the cause of a rejection.
type of formulary provides more options for patients, but places more of the cost on both the patient and the organization.
A closed formulary includes a specific list of covered products provided by the plan. If medically necessary, patients may request exceptions beyond the formulary, but may pay extra to try formulary alternatives first. Closed formularies are designed to cover an appropriate number of medications per therapeutic category, and they’re based on clinical practice to limit the need for exception requests. This provides more limited options for patients, but it reduces overall healthcare cost.
It is possible more than one utilization management edit or non-formulary (NF) request may apply to a drug and be the cause of a rejection.
bcofdermatology.com 25
PHARMD

BEST PRACTICES FOR AVOIDING DENIALS & APPEALS
1
PROVIDE THE CORRECT DIAGNOSIS. ICD-10 codes

2 Examples include body surface area, global assessment
3
USE DISEASE SEVERITY SCORES.
FOCUS ON SAFETY Infection monitoring, live vaccines, co-morbid conditions, etc.
4
STATE PREVIOUS MEDICATIONS TRIALED WITH DURATIONS or any contraindications to standard therapy or reason not to prescribe guidelinerecommended agents.
Many insurance companies will post pharmacy policies outlining PA requirements orhave surveys available for submission. Not all rejections are for prior authorizations, so understanding the reason for denial (e.g., NF, QL, pharmacy network restriction, refill too soon, group exclusions, plan exclusions) may prove successful for getting a drug approved.
ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 26
JO PANUWAT DSTOCK.ADOBE.COM

More Than Software – A Practice Management Solution for Biologics Coordination
Key Features
• Realtime updates for clinic team, patients and industry
• Single view dashboard of all patients for both coverage and follow up tasks throughout the entire journey
• Advanced patient engagement with alerts and notifications
• Improves patient outcomes
• Reduces time spent on paperwork and phone calls
• Prevents patients “lost to follow-up”
• Ensures that patient “follow through”
• And much more…
www.rxnexus.com
Streamline Medication Approval & Improve Patient Communication






More than75% of commercially insured patient lives have coverage for OPZELURA®1
Covered at any tier. PA and other restrictions may apply. Coverage is plan specific and subject to change.











Not an OPZELURA® patient.
















For patients 12 years of age and older







The First and Only FDA-Approved Treatment for Repigmentation in Nonsegmental Vitiligo 2*



Proven to Promote Skin Repigmentation in Phase III Clinical Trials2

F-VASI75 Results at Weeks 24 and 52

Nearly patients 1 in 3

Data From Week 24 And an Open-Label Extension
~51%



Nearly1 in 3 patients achieved F-VASI75† at 24 weeks (primary endpoint; 29.9% vs 7.5% [P<0.0001] and 29.9% vs 12.9% [P<0.01]).2,3

About half the patients remaining in the studies who applied OPZELURA® from day 1 achieved F-VASI75 at 52 weeks: 51% for OPZELURA® and 28% for vehicle-to-OPZELURA® (Week 24 to Week 52). Data were reported as observed. No conclusions of safety or efficacy should be made based on these results.2,4



Limitations of an open-label extension: In an open-label extension, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
OPZELURA® was studied in 2 double-blind, randomized, vehicle-controlled trials of identical design that enrolled 674 adult and adolescent patients with nonsegmental vitiligo ≥12 years of age. Patients had depigmented areas affecting ≥0.5% F-BSA, ≥3% nonfacial BSA, and total body vitiligo area (facial and nonfacial) of up to 10% BSA. Phototherapy was not permitted during the trials. In both trials, patients were randomized 2:1 to treatment with OPZELURA® or vehicle cream BID for 24 weeks followed by a 28-week open-label extension, wherein patients originally assigned to vehicle could switch to OPZELURA®. 2
*In patients 12 years of age and older.


BID, twice daily; BSA, body surface area; F-BSA, facial body surface area; FDA, Food and Drug Administration: F-VASI, Facial Vitiligo Scoring Index; F-VASI75, ≥75% improvement from baseline in Facial Vitiligo Area Scoring Index.
† F-VASI is a composite measurement of the overall area of facial vitiligo patches and degree of depigmentation within patches. As assessed, the face did not include surface area of the lips, scalp, eyelids, ears, or neck. 5





®2
CONSIDER OPZELURA




Patient Age

Indication

Tried and Failed Alternatives
Body Surface Area (BSA)
Combination Use

INDICATION
Common Prior Authorization Requirements
The patient must be 12 years of age or older.
OPZELURA® is indicated for nonsegmental vitiligo




This may include a topical corticosteroid (TCS) and/or topical calcineurin inhibitor (TCI). Be sure to include your patient’s most recent chart notes!
Indicate the BSA that will be treated:
• Nonsegmental vitiligo is up to 10%
Confirm OPZELURA® will not be used with:
• Therapeutic biologics
• Other JAK inhibitors
• Potent immunosuppressants




OPZELURA® was studied as a monotherapy treatment. Many plans do not approve combination use.
Turn the page to see the results with OPZELURA® at 52 weeks
OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.

Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death. Reported infections include:


• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.


Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled. Carefully consider the benefits and risks of treatment prior to initiating OPZELURA

in patients with chronic or recurrent infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA.


Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib.
No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.


Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.



Please see Important Safety Information continued on next page and Brief Summary of Full Prescribing Information, including Boxed Warning, on the following pages.












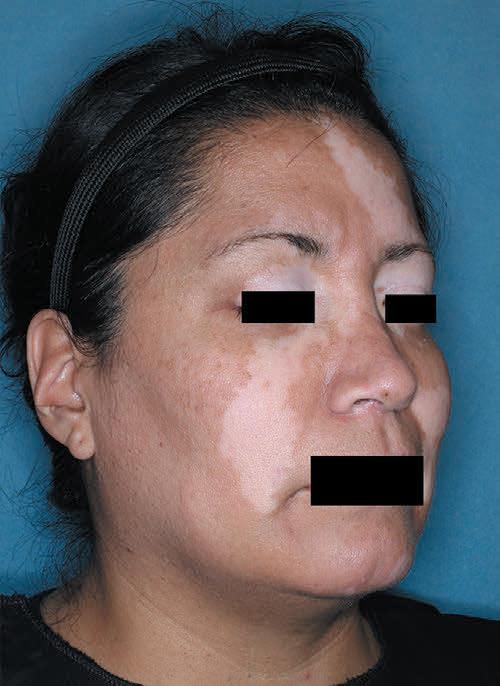

52-Week Results for a Clinical Trial Participant Whose Repigmentation Met the Primary Endpoint of F-VASI75 at 24 Weeks6‡





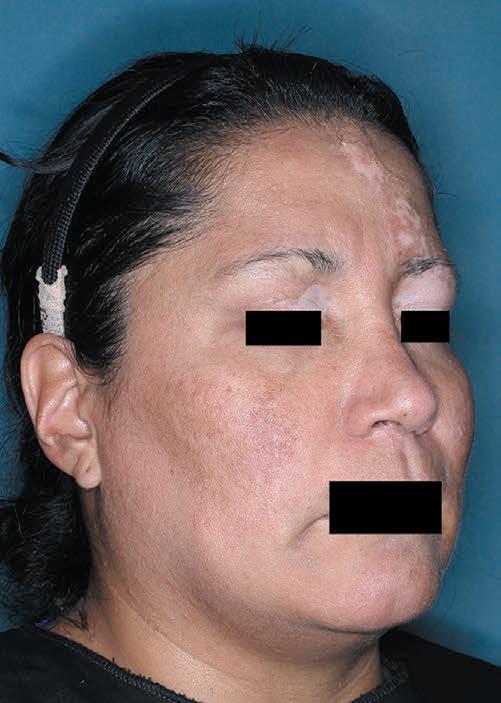



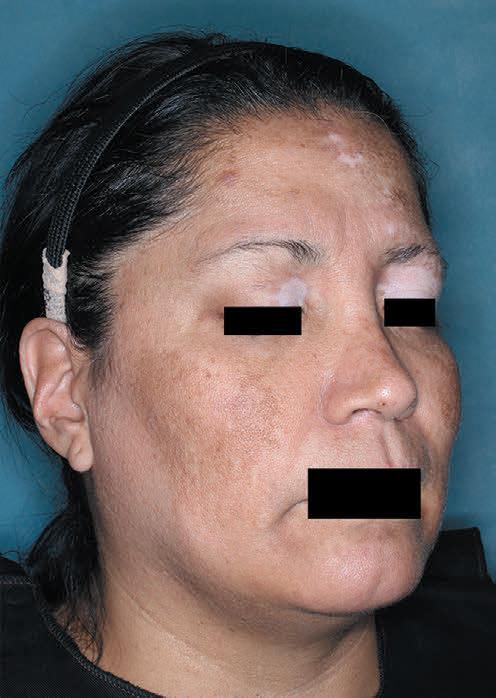


‡Results not typical. Actual patient treated with OPZELURA® in a clinical trial. Individual results may vary. Not for ophthalmic, oral, or intravaginal use.





See the Possibilities for Your Members With OPZELURA®
IMPORTANT SAFETY INFORMATION (continued)
MORTALITY



In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
MALIGNANCIES



Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin

cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers. Non-melanoma skin cancers, including basal cell and squamous cell carcinoma, have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.





W eek 0 F -VASI SCORE 1.5 W eek 24 F -VASI SCORE 0.3 80% Improvement From Baseline
ENDPOINT W eek 52 F -VASI SCORE 0.3 80% Improvement From Baseline BASELINE WEEK 24 WEEK 52
PRIMARY




IMPORTANT SAFETY INFORMATION (continued)
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke.





Thrombocytopenia, Anemia, and Neutropenia
Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations


Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
THROMBOSIS


Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately.
Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Adverse Reactions
In nonsegmental vitiligo, the most common adverse reactions (incidence ≥1%) are application site acne (6%), application site pruritus (5%), nasopharyngitis (4%), headache (4%), urinary tract infection (2%), application site erythema (2%), and pyrexia (1%).



References:


Pregnancy
There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Lactation


Advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Please see Brief Summary of Full Prescribing Information, including Boxed Warning, on the following pages.

1. VANTAGE Fingertip Formulary, April 2023. 2. OPZELURA® (ruxolitinib) cream. Prescribing Information. Incyte Corporation. 3. Rosmarin D, Pandya AG, Grimes P, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo: 24-week results from 2 randomized, double-blind phase 3 studies. Abstract presented at: 30th European Academy of Dermatology and Venereology (EADV) Congress; September 29-October 2, 2021; virtual. 4. Rosmarin D, Passeron T, Pandya AG, et al. Efficacy and safety of ruxolitinib cream monotherapy for the treatment of vitiligo: results from two 52-week phase 3 studies. Presented at: American Academy of Dermatology Annual Meeting; March 25-29, 2022; Oral Presentation. 5. Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet 2020;396(suppl):1-121. 6. Data on File. Incyte Corporation.



OPZELURA, the OPZELURA logo, Incyte, and the Incyte logo are registered trademarks of Incyte.
© 2023, Incyte Corporation.
MAT-OPZ-01787 07/23














OPZELURA® (ruxolitinib) cream, for topical use
OPZELURA® (ruxolitinib) cream, for topical use
Brief Summary of FULL PRESCRIBING INFORMATION
Brief Summary of FULL PRESCRIBING INFORMATION
INDICATIONS AND USAGE: OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
INDICATIONS AND USAGE: OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
Limitations of Use: Use OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Reported infections include:
Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials. Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
The risks and benefits of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials. Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
The risks and benefits of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
MORTALITY
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
MALIGNANCIES
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat inflammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat inflammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
WARNINGS AND PRECAUTIONS
WARNINGS AND PRECAUTIONS
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke. Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia , and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia , and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total low-density lipoprotein (LDL) cholesterol, and triglycerides.
ADVERSE REACTIONS
ADVERSE REACTIONS
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%).
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%).
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, influenza-like illness, insomnia, nasal congestion, and vomiting.
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Pediatric Use: Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established.
Pediatric Use: Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These findings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, influenza-like illness, insomnia, nasal congestion, and vomiting.
DRUG INTERACTIONS
DRUG INTERACTIONS
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4) Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These findings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Geriatric Use: Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
Geriatric Use: Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
PATIENT COUNSELING INFORMATION
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4) Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
PATIENT COUNSELING INFORMATION
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions]
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Lactation
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions]
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions]
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Lactation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions]
Thrombocytopenia, Anemia , and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Thrombocytopenia, Anemia , and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Specific Populations]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Specific Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Specific Populations].
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Specific Populations].
Manufactured for: Incyte Corporation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
1801 Augustine Cut-off Wilmington, DE 19803
Manufactured for: Incyte Corporation 1801 Augustine Cut-off Wilmington, DE 19803
OPZELURA is a registered trademark of Incyte. All rights reserved.
U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870; 11219624
© 2022-2023 Incyte Corporation. All rights reserved.
Issued: July 2022 PLR-OPZ-00018-JUN23
OPZELURA is a registered trademark of Incyte. All rights reserved. U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870; 11219624 © 2022-2023 Incyte Corporation. All rights reserved. Issued: July 2022 PLR-OPZ-00018-JUN23





































NOVEMBER/DECEMBER MRPANYASTOCK.ADOBE.COM
DUBNYTSKAYA PHOTOSTOCK.ADOBE.COM
By Mallory Schmoll, PharmD, AAHIVP
THERAPY LEAD, CLINICAL PHARMACY STRATEGY WALGREEN CO.
Accumulator and maximizer plans have become particularly difficult to navigate, causing confusion, and in some instances leaving patients with difficult financial decisions that could negatively impact health outcomes. There are steps, however, that patients and providers can take to stay informed and be proactive with planning for medical needs when faced with one of these plans.
Before accumulator and maximizer plans existed, co-pay assistance for expensive medications was fairly straightforward. For example, if insurance approved a medication but left a patient with a co-pay of $500, it is likely that the drug manufacturer offered a co-pay assistance program that would cover 100 percent of that co-pay or reduce the patient’s responsibility to a low flat rate like $5 or $10. Even though the $500 co-pay was covered through a manufacturer assistance program, that payment still applied toward the patient’s deductible and/or the out-of-pocket maximum. This was great because if the patient happened to exhaust the co-pay assistance benefits, the deductible had likely been met through that program, and subsequently would leave the patient with a lower insurance co-pay.
When accumulator benefit designs were introduced in 2018, it meant that the funds offered through a manufacturer co-pay assistance program would no longer apply toward the patient’s deductible or
out-of-pocket maximum responsibility. In addition, it seemed that many patients were not aware that their insurance plan adopted an accumulator benefit design, leaving those with high deductible plans at risk for a co-pay surprise. This means that a patient may have been utilizing manufacturer co-pay assistance with no hiccups, but once those benefits were exhausted, the patient was suddenly faced with a high insurance co-pay all over again, as they had made no progress toward meeting the deductible.
In my experience as a pharmacist, when these situations occurred, it was often the start of a cascade of tough decisions. If the patient could not afford the medication and had exhausted all financial assistance benefits, they may choose to discontinue their medication. When faced with having to choose between food, medication or rent, most people prioritized food and shelter.

I have also seen patients get creative and space out doses so their medication would last longer, reducing the cost. Discontinuing medication or self-adjusting the dose without speaking with a provider or pharmacist is risky and can result in worsened symptoms, as well as poor quality of life and health outcomes.
Maximizer plans are similar to accumulator plans in that both restrict co-pay assistance funds from contributing to deductibles and out-of-pocket amounts. The difference is that maximizer plans take a distributed approach where the maximum value of manufacturer co-pay assistance is spread evenly throughout the benefit year. The patient’s cost sharing amount is equal to the total annual value of co-pay assistance, so while the patient should not experience “co-pay surprise” for that specific medication, they are still responsible for their full deductible and out-of-pocket maximums. Both accumulator and maximizer plans are strategies for payors, or insurance companies, to increase their profits.
Pharmacists are a valuable resource to support patients and prescribers by conducting thorough benefit investigations, navigating insurance nuances, and providing information on alternative or more cost-effective therapy options. A common challenge is that many specialty medications are brand only, with no available generic alternative. In fact, 99.6 percent of co-pay
“Accumulator and maximizer plans have become particularly difficult to navigate…leaving patients with difficult financial decisions that could negatively impact health outcomes.”
JO PANUWAT DSTOCK.ADOBE.COM
ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 36 KULNIZSTOCK.ADOBE.COM
CONSUMER EXPERIENCES WITH HEALTH INSURANCE
Over 1/3 of consumers do not understand the meaning of “health plan deductible.”
Almost 2/3 of consumers do not understand the meaning of “out-of-pocket maximum.”
58% of adults say they have experienced a problem using health insurance in the past year, and nearly half of these patients were unable to satisfactorily resolve the problem.
4 in 10 insured adults report skipping or delaying care in the past year due to cost.
27% of consumers report insurance paying less than expected 18% of consumers report insurance not paying anything when they thought services/ products were covered.

assistance is used for branded drugs without a generic alternative1. While the specialty pharmacy pipeline is robust and more generics and biosimilars are entering the market, specialty therapy options can be limited, and costs continue to be a barrier.
While these subjects are not popular choices for light weekend reading and may be viable options for falling asleep, they are important to understand. Even the most seasoned professionals have difficulty navigating accumulator and maximizer plans, so imagine patients with no medical background trying to grasp these concepts while facing a difficult diagnosis. According to a KFF Survey2, most patients have faced health insurance issues over the last year.
Activities that seem preferable to navigating health insurance include doing taxes, setting up a new internet or
TV connection, or re-setting a forgotten password only to be told “you may not use a previous password.” Healthcare professionals have significant opportunity to collaborate in this space and to lighten the load for patients by equipping them with knowledge, tools and resources to navigate their specialty therapy benefits and financial options. Preparation is key, and steps can be taken proactively to predict the financial impact of specialty treatments and prevent gaps in care.
As many are anticipating potential health plan changes in the new calendar year, patients should be on the lookout for communications from their plan.
Language like “changes for 2024,” “adjustment programs” or “accumulator adjustment” may indicate an accumulator and/or maximizer plan for the upcoming
37
bcofdermatology.com
EXUBERATIONSTOCK.ADOBE.COM
“For patients, providers and pharmacies, knowledge is power, and preparation is key when it comes to starting a new medication and navigating health insurance.”
year. Plan member handbooks, employer HR departments and insurance member services lines are all resources that can identify if a specific plan is an accumulator or maximizer benefit design.
The patient, provider, and pharmacy should all have a thorough understanding of health and prescription insurance benefits prior to starting a specialty medication. A proactive and thorough benefit investigation along with detailed communication between all parties will help spot any co-pay surprises that may appear down the road. This approach allows for shared decision making between the patient and provider to select the best treatment plan, and financial assistance options can be explored ahead of time to prevent gaps in treatment.
It is no surprise that accumulator and maximizer plans have created a lot of noise in the political and advocacy worlds. Nineteen states plus Puerto Rico have banned co-pay accumulator plans, and on September 29, 2023, the U.S. District Court for the District of Columbia reinstated a ban on co-pay accumulators for medications that do not have generic alternatives in both federally and stateregulated insurance plans 1. The Help

Ensure Lower Patient (HELP) Co-Pays Act would ban accumulators at the federal level; it was introduced in 2021 and reintroduced in February 2023 but has not yet been passed.
For patients, providers and pharmacies, knowledge is power, and preparation is key when it comes to starting a new medication and navigating health insurance. Every member of a patient care team has an important role to play, and as an African Proverb states, “If you want to go fast, go alone. If you want to go far, go together.” Through collaboration, communication and education, patients will be empowered on their own healthcare journey and equipped with the tools and resources needed to stay on treatment, achieve the optimal health outcomes and maintain the best quality of life.
REFERENCES
1. Immune Deficiency Foundation. Addressing copay accumulators. Updated 2023. Accessed November 15, 2023. https://primaryimmune. org/get-involved/advocate/ addressing-copay-accumulators
2. Pollitz KP, Montero AM, Lopes LL, et al. Kaiser Family Foundation (KFF). KFF survey of consumer experiences with health insurance. Published June 15, 2023. Accessed November 15, 2023. https://www.kff.org/mental-health/ poll-finding/kff-survey-of-consumerexperiences-with-health-insurance/
3. Drug Channels. Copay accumulator and maximizer update: adoption plateaus as insurers battle patients over copay support. Published February 22, 2023. Accessed November 15, 2023. https://www.drugchannels. net/2023/02/copay-accumulatorand-maximizer-update.html
4. Drug Channels. The economics of copay accumulators, maximizers, and alternative funding programs.
Published August 1, 2023.
Accessed November 15, 2023. https://www.drugchannels. net/2023/08/the-economics-ofcopay-accumulators.html
ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 38
RETHEA BOER/PEOPLEIMAGES.COMSTOCK.ADOBE.COM

Multiple Humira biosimilars entered the market in July 2023, and the biosimilar landscape continues to expand. On the following pages, you’ll find a guide to help clarify the available biosimilar therapeutic options. This document will continue to be updated by Walgreens Clinical teams.
bcofdermatology.com 39 HUMIRA® BIOSIMILAR CLINICAL
REFERENCE GUIDE
LIVE AREA 8" x 5" 3213877-3919 | ©2023 Walgreen Co. All rights reserved. This isn’t just about healthy skin. It’s helping people feel good in their own skin. This is one of many moments made possible by the inspirational work of Biologic Coordinators of Dermatology. Your tireless efforts make us glow with pride.
Product studying/seeking studying/seeking Y studying/seeking studying/seeking
Link to copay card website
https://www.humira.com/humi ra-complete/cost-and-copay
https://www.pfizerflex. ca/
https://www.amjevita. com/supportplus/finan cial-support
https://patient.boehrin geringelheim.com/us/pro ducts/cyltezo/savingon-cyltezo
https://organonpro.co m/enus/product/hadlima/pa tient-support/
https://www.hulio.com /support
ACCESS DERMATOLOGY NOVEMBER/DECEMBER 2023 40 Trade Name Humira® (Reference/Innovator Product) Abrilada ™ Amjevita ™ Cyltezo® Hadlima ™ Hulio ™ Generic Name adalimumab adalimumab-afzb adalimumab-atto adalimumab-adbm adalimumab-bwwd adalimumab Manufacturer AbbVie Pfizer Amgen Boehringer Ingelheim Samsung Bioepis/ Organon Mylan Interchangeability Interchangeable
N/A Reference/Innovator
Starter Kits (SK) 40 mg/0 8 mL #6 Pens Humira Pen-CD/UC/HS starter N/A N/A 4 40 mg/0 8 mL SK CD/UC #6 Pens N/A N/A 80 mg/0 8 mL #3 Pens Humira Pen-CD/UC/HS SK N/A N/A N/A N/A N/A 80 mg/0.8 mL #8 Pens Humira Pen-Ped UC SK N/A N/A N/A N/A N/A 80 mg/0 8 mL and 40 mg/0 4 mL #2 prefilled syringe (PFS) Humira PFS Ped CD SK N/A N/A N/A N/A N/A 80 mg/0 8 mL and 40 mg/0 4 mL #3 Pens Humira Pen-Psor/Uveit SK N/A N/A N/A N/A N/A 80 mg/0 8 mL #3 PFS Humira PFS Ped CD SK N/A N/A N/A N/A N/A Low-Concentration Dosage Forms 10 mg/0 2 mL PFS N/A N/A 10 mg/0.2 mL #1 PFS 1 10 mg/0 2 mL #2 PFS N/A N/A 20 mg/0 4 mL PFS N/A N/A 20 mg/0.4 mL #1 PFS 2 20 mg/0 4 mL #2 PFS N/A 20mg/0.4mL 40 mg/0 8 mL PFS Humira 40 mg/0.8 ml #2 PFS N/A 40 mg/0.8 mL #1 PFS N/A N/A N/A N/A N/A 4 40 mg/0 8 mL #2 PFS 40 mg/0.8 mL #2 PFS 40mg/0.8mL 40 mg/0 8 mL Pens Humira 40 mg/0.8 mL #2b Pens N/A 40 mg/0.8 mL #1 Pen N/A N/A N/A 40 mg/0.8 mL #2 Pens 4 40 mg/0 8 mL #2 Pens 40 mg/0.8 mL #2 Pens 40mg/0.8mL High-Concentration Dosage Forms 10 mg/0 1 mL #2 PFS Humira CF 10 mg/0.1 mL PFS N/A N/A N/A N/A N/A 20 mg/0 2 mL #2 PFS Humira CF 20 mg/0.2 mL PFS N/A N/A N/A N/A N/A 40 mg/0 4 mL #2 PFS Humira CF 40 mg/0.4 mL PFS N/A N/A N/A 40 mg/0.4 mL #2 PFS N/A 40 mg/0 4 mL #2 Pens Humira CF 40 mg/0.4 mL Pen N/A N/A N/A 40 mg/0.4 mL #2 Pens N/A 80 mg/0 8 mL #2 Pens Humira CF 80 mg/0.8 mL Pen N/A N/A N/A N/A N/A Copay Assistance for Eligible Commercially Insured Patients Patient cost after savings $5 copay Savings card processed like credit card Assistance levels vary $0 copay $0 copay $0 copay $0 copay
(Y/N)
For full prescribing
CD = Crohn’s disease, CF = Citrate-free, HS = hidradenitis suppurativa, UC = ulcerative colitis
80mg/0.8mL SK Ped CD #3 PFS N/A
80mg/0.8mL & 40mg/0.4mL SK plaque psoriasis ( PsO ) #3 Pens N/A
80mg/0.8mL & 40mg/0.4mL SK Ped CD #2 PFS N/A N/A N/A N/A
Boehringer Ingelheim Samsung Bioepis / Organon Mylan Sandoz Fresenius Celltrion Coherus Alvotech / Teva studying/seeking studying/seeking TBD TBD studying/seeking TBD studying/ seeking
https://www.hulio.com /support
https://portal.trialcard. com/Sandoz/hyrimoz
2 adalimumabadbm adalimumabbwwd adalimumabfkjp adalimumabadaz adalimumabaacf adalimumabaaty adalimumambaqvh Biologics License Application (BLA) currently under FDA Review
Biologics License Application (BLA) currently under FDA Review
T
https://portaltrialcard. com/Freseniuskabi/idacio TBD TBD TBD
For full prescribing information including boxed warnings for all drugs listed above visit: https://dailymed.nlm.nih.gov/dailymed/
bcofdermatology.com 41 a ™ Hulio ™ Hyrimoz ™ Idacio® Yuflyma® Yusimry ™ AVT02 adalimumab-bwwd adalimumab-fkjp adalimumab-adaz adalimumab-aacf adalimumab-aaty
adalimumamb-aqvh
Sandoz Fresenius
Coherus
studying/seeking studying/seeking TBD TBD studying/seeking TBD studying/ seeking N/A N/A 40 mg/0.8 mL SK CD/UC #6 Pens N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A 80mg/0.8mL SK CD/UC/HS #3 Pens N/A N/A N/A N/A N/A 80mg/0.8mL & 40mg/0.4mL SK Ped CD #2 PFS N/A N/A N/A N/A N/A 80mg/0.8mL & 40mg/0.4mL SK plaque psoriasis
Pens N/A N/A N/A N/A N/A 80mg/0.8mL SK Ped CD #3 PFS N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A 20mg/0.4mL #2 PFS N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A mg/0.8 mL #2 PFS 40mg/0.8mL #2 PFS N/A 40 mg/0.8 mL #2 PFS N/A N/A N/A N/A N/A N/A N/A N/A N/A mg/0.8 mL #2 Pens 40mg/0.8mL #2 Pens N/A 40 mg/0.8 mL #2 Pens N/A 40 mg/0.8 mL #2 Pens N/A N/A 10 mg/0.1 mL #2 PFS N/A N/A N/A N/A N/A 20 mg/0.2 mL #2 PFS N/A N/A N/A N/A mg/0.4 mL #2 PFS N/A 40 mg/0.4 mL #2 PFS N/A 40 mg/0.4 mL #1 PFS N/A N/A mg/0.4 mL #2 Pens N/A 40 mg/0.4 mL #2 Pens N/A 40 mg/0.4 mL #2 Pens N/A N/A N/A 80 mg/0.8 mL #2 Pens N/A N/A N/A N/A $0 copay $0 copay $0 copay TBD TBD TBD
Bioepis/ Mylan
Celltrion
Alvotech/ Teva
(PsO) #3
organonpro.co us/product/hadlima/pa support/
y l t e z o ®
a
m a
m
o
m
V
H
d l i
™ H u l i o ™ H y r i
o z ™ I d a c i
® Y u f l y m a ® Y u s i
r y ™ A
0
m g / 0 . 8 m L S K D / U C # 6 P e n s N/A N/A
N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A
N/A N/A
N/A N/A
N/A 40 mg/0.8 mL SK CD/UC #6 Pens
80mg/0.8mL SK CD/UC/HS #3 Pens
N/A N/A
N/A
N/A
N/A N/A
N/A
N/A N/A N/A
N/A N/A
N/A N/A
m g / 0 . 2 m L # 2 P F S N/A N/A N/A N/A N/A N/A N/A m g / 0 4 m L # 2 P F S N/A 20mg/0.4mL #2 PFS N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A m g / 0 . 8 m L # 2 P F S 40 mg/0.8 mL #2 PFS 40mg/0.8mL #2 PFS N/A 40 mg/0.8 mL #2 PFS N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A m g / 0 8 m L # 2 P e n s 40 mg/0.8 mL #2 Pens 40mg/0.8mL #2 Pens N/A 40 mg/0.8 mL #2 Pens N/A 40 mg/0.8 mL #2 Pens N/A N/A N/A N/A 10 mg/0.1 mL #2 PFS N/A N/A N/A N/A N/A N/A N/A 20 mg/0.2 mL #2 PFS N/A N/A N/A N/A N/A 40 mg/0.4 mL #2 PFS N/A 40 mg/0.4 mL #2 PFS N/A 40 mg/0.4 mL #1 PFS N/A N/A T r a d e N a m e H u m i r a ® ( R e f e r e n c e / I n n o v a t o r P r o d u c t ) A b r i l a d a ™ A m j e v i t a ™ C y l t e z o ® H a d l i m a ™ H u l i o ™ H y r i m o z ™ I d a c i o ® Y u f l y m a ® G e n e r i c N a m e adalimumab adalimumabafzb adalimumabatto adalimumabadbm adalimumabbwwd adalimumabfkjp adalimumabadaz adalimumabaacf adalimumab M a n u f a c t u r e r AbbVie Pfizer Amgen Boehringer Ingelheim Samsung Bioepis / Organon Mylan Sandoz Fresenius Celltrion I n t e r c h a n g e a b i l i t y I n t e r c h a n g e a b e ( Y / N ) N/A Reference/Innovator Product studying/seeking studying/seeking Y studying/seeking studying/seeking TBD TBD studying/seeking S t a r t e r K i t s ( S K ) 4 0 m g / 0 8 m L # 6 P e n s Humira PenCD/UC/HS starter N/A N/A 4 0 m g / 0 8 m L S K C D / U C # 6 P e n s N/A N/A N/A 40 mg/0.8 mL SK CD/UC #6 Pens N/A 8 0 m g / 0 8 m L # 3 P e n s Humira PenCD/UC/HS SK N/A N/A N/A N/A N/A N/A N/A N/A 8 0 m g / 0 8 m L # 8 P e n s Humira PenPed UC SK N/A N/A N/A N/A N/A 80mg/0.8mL SK CD/UC/HS #3 Pens N/A N/A 8 0 m g / 0 8 m L a n d 4 0 m g / 0 4 m L # 2 p r ef i l l e d s y r i n g e ( P F S ) Humira PFS Ped CD SK N/A N/A N/A N/A N/A 80mg/0.8mL & 40mg/0.4mL SK Ped CD #2 PFS N/A N/A 8 0 m g / 0 8 m L a n d 4 0 m g / 0 4 m L # 3 P e n s Humira PenPsor / Uveit SK N/A N/A N/A N/A N/A 80mg/0.8mL & 40mg/0.4mL SK plaque psoriasis ( PsO ) #3 Pens N/A N/A 8 0 m g / 0 8 m L # 3 P F S Humira PFS Ped CD SK N/A N/A N/A N/A N/A 80mg/0.8mL SK Ped CD #3 PFS N/A N/A L o wC o n c e n t r a t i o n D o s a g e F o r m s 1 0 m g / 0 2 m L P F S N/A N/A 10 mg/0.2 mL #1 PFS 1 0 m g / 0 2 m L # 2 P F S N/A N/A N/A N/A N/A 2 0 m g / 0 4 m L P F S N/A N/A 20 mg/0.4 mL #1 PFS 2 0 m g / 0 4 m L # 2 P F S N/A 20mg/0.4mL #2 PFS N/A N/A N/A 4 0 m g / 0 . 8 m L P F S Humira 40 mg/0.8 ml #2 PFS N/A 40 mg/0.8 mL #1 PFS N/A N/A N/A N/A N/A N/A N/A N/A 4 0 m g / 0 8 m L # 2 P F S 40 mg/0.8 mL #2 PFS 40mg/0.8mL #2 PFS N/A 40 mg/0.8 mL #2 PFS N/A 4 0 m g / 0 8 m L P e n s Humira 40 mg/0.8 mL #2b Pens N/A 40 mg/0.8 mL #1 Pen N/A N/A N/A N/A N/A N/A 40 mg/0.8 mL #2 Pens 4 0 m g / 0 8 m L # 2 P e n s 40 mg/0.8 mL #2 Pens 40mg/0.8mL #2 Pens N/A 40 mg/0.8 mL #2 Pens N/A H i g hC o n c e n t r a t i o n D o s a g e F o r m s 1 0 m g / 0 . 1 m L # 2 P F S Humira CF 10 mg/0.1 mL PFS N/A N/A N/A N/A N/A 10 mg/0.1 mL #2 PFS N/A N/A 2 0 m g / 0 2 m L # 2 P F S Humira CF 20 mg/0.2 mL PFS N/A N/A N/A N/A N/A 20 mg/0.2 mL #2 PFS N/A N/A 4 0 m g / 0 4 m L # 2 P F S Humira CF 40 mg/0.4 mL PFS N/A N/A N/A 40 mg/0.4 mL #2 PFS N/A 40 mg/0.4 mL #2 PFS N/A 40 mg/0.4 mL 4 0 m g / 0 . 4 m L # 2 P e n s Humira CF 40 mg/0.4 mL Pen N/A N/A N/A 40 mg/0.4 mL #2 Pens N/A 40 mg/0.4 mL #2 Pens N/A 40 mg/0.4 mL 8 0 m g / 0 8 m L # 2 P e n s Humira CF 80 mg/0.8 mL Pen N/A N/A N/A N/A N/A 80 mg/0.8 mL #2 Pens N/A N/A C o p a y A s s i s t a n c e f o r E l i g i b l e C o m m e r c i a l l y I n s u r e d P a t i e n t s P a t i e n t c o s t a f t e r s a v i n g s $5 copay Savings card processed like credit card Assistance levels vary $0 copay $0 copay $0 copay $0 copay $0 copay $0 copay TBD https:// patient.boehrin
N/A

CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.





IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death. The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia.
If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection.
Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral (including herpes zoster), and other infections due to opportunistic pathogens.





Avoid use of CIBINQO in patients with an active, serious infection, including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections or those who have resided or traveled in areas of endemic tuberculosis or endemic mycoses.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO.
Viral reactivation, including herpes virus reactivation (eg, herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves. Hepatitis B virus reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C.
To learn more about CIBINQO efficacy, visit CIBINQOhcp.com or scan QR code.
JAK=Janus kinase; AD=atopic dermatitis.
Committed to Patient Support
Helping patients unlock access and reimbursement support for CIBINQO™ (abrocitinib)

pay as little as $ 0 per month*
With the Copay Savings Card, eligible commercially insured patients may Visit PDPACopayCard.com
*Eligibility required. No membership fees. This is not health insurance. The maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. See full terms and conditions below.
Copay Savings Card: TERMS AND CONDITIONS
By using the Pfizer Dermatology Patient AccessTM Copay Savings Card, you acknowledge that you currently meet the eligibility criteria and will comply with the terms and conditions described below:
• You are not eligible to use this card if you are enrolled in a state or federally funded prescription insurance program, including but not limited to Medicare, Medicaid, TRICARE, Veterans Affairs health care, a state prescription drug assistance program, or the Government Health Insurance Plan available in Puerto Rico (formerly known as “La Reforma de Salud”).
• You must have commercial insurance. Offer is not valid for cash-paying patients.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for CIBINQO™ (abrocitinib) may pay as little as $0 per month. Eligible patients with commercial prescription drug coverage may receive a maximum benefit of $15,000 per calendar year, which is defined by the date of enrollment through December 31st of the enrollment year. After a maximum of $15,000, you will be responsible for paying the remaining monthly out-of-pocket costs.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for EUCRISA® (crisaborole) may pay as little as $10 per tube. Eligible patients with commercial prescription drug insurance coverage that does not cover EUCRISA may pay as little as $100 per tube. Individual savings are limited to $970 per tube. Individual patient savings are limited to $3,880 in maximum total savings per calendar year.
• This copay card is not valid when the entire cost of your prescription drug is eligible to be reimbursed by your commercial insurance plan or any other health or pharmacy benefit program.
• You must deduct the value of this copay card from any reimbursement request submitted to your commercial insurance plan, either directly by you or on your behalf.
• You are responsible for reporting use of the copay card to any commercial insurer, health plan, or other third party that pays for or reimburses any part of the prescription filled using the copay card, as may be required. You should not use the copay card if your insurer or health plan prohibits use of manufacturer copay cards.
• This copay card is not valid where prohibited by law.
• Copay card cannot be combined with any other savings, free trial, or similar offer for the specified prescription.
• Copay card will be accepted only at participating pharmacies.
• If your pharmacy does not participate, you may be able to submit a request for a rebate in connection with this offer.
• This copay card is not health insurance.
• Offer good only in the United States and Puerto Rico
• Copay card is limited to 1 per person during this offering period and is not transferable.
• A copay card may not be redeemed more than once per 30 days per patient.
• No other purchase is necessary.
• Data related to your redemption of the copay card may be collected, analyzed, and shared with Pfizer, for market research and other purposes related to assessing Pfizer’s programs. Data shared with Pfizer will be aggregated and de-identified; it will be combined with data related to other copay card redemptions and will not identify you.
• Pfizer reserves the right to rescind, revoke, or amend this offer at any time without notice.
• Offer expires 12/31/2025.
For questions or additional support, call 1-833-956-3376, write to Pfizer Inc. at PO Box 29387, Mission, KS 66201, or visit the CIBINQO website at www.CIBINQO.com or the EUCRISA website at www.EUCRISA.com.
Please see additional Important Safety Information and Brief Summary of full Prescribing Information on the following pages. For full Prescribing Information, including BOXED WARNING and Medication Guide, visit CIBINQOhcp.com.
TM
MORTALITY
In a large, randomized postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality (including sudden cardiovascular death) was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
MALIGNANCIES
Malignancies, including non-melanoma skin cancer (NMSC), were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
In a large, randomized postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA patients. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur.
THROMBOSIS
Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of overall thrombosis, DVT, and PE were observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and treat patients appropriately.
CONTRAINDICATION
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤ 81 mg daily), during the first 3 months of treatment.
LABORATORY ABNORMALITIES
Hematologic Abnormalities: Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia.
Prior to CIBINQO initiation, perform a complete blood count (CBC). CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations: Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy, and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
IMMUNIZATIONS
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines, including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
RENAL IMPAIRMENT
Avoid use in patients with severe renal impairment or end stage renal disease, including those on renal replacement therapy.
HEPATIC IMPAIRMENT
Avoid use in patients with severe hepatic impairment.
ADVERSE REACTIONS
Most common adverse reactions (≥ 1%) in subjects receiving 100 mg and 200 mg include: nasopharyngitis, nausea, headache, herpes simplex, increased blood creatine phosphokinase, dizziness, urinary tract infection, fatigue, acne, vomiting, oropharyngeal pain, influenza, gastroenteritis.
Most common adverse reactions (≥ 1%) in subjects receiving either 100 mg or 200 mg also include: impetigo, hypertension, contact dermatitis, upper abdominal pain, abdominal discomfort, herpes zoster, and thrombocytopenia.
Inform patients that retinal detachment has been reported in CIBINQO clinical trials. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision.
DRUG INTERACTIONS
Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO. See Prescribing Information for clinically relevant drug interactions.
USE IN PREGNANCY
Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Advise females of reproductive potential that CIBINQO may impair fertility.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
LACTATION
Advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose.
INDICATION
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable. Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
Please see full Important Safety Information throughout and full Prescribing Information, including BOXED WARNING, and Medication Guide. ©
SAFETY INFORMATION
INDICATION
IMPORTANT
&
All
May
PP-CIB-USA-0665
2023 Pfizer Inc.
rights reserved.
2023.
CIBINQO™ (abrocitinib) tablets, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death; The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia. If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection. Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens. Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately.
INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Testing, Evaluations, and Procedures
Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
• Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO
• Viral hepatitis screening in accordance with clinical guidelines – CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C
• A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation.
Recommended Dosage
The recommended dosage of CIBINQO is 100 mg orally once daily. If an adequate response is not achieved with CIBINQO 100 mg orally daily after 12 weeks, consider increasing dosage to 200 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 200 mg once daily.
CIBINQO can be used with or without topical corticosteroids. If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
Recommended Dosage in Patients with Renal Impairment
Renal Impairment
CIBINQO dosage recommendation in patients with mild renal impairment (60-89 mL/minute) is 100 mg once daily. For patients with moderate renal impairment (30-59 mL/ minute), the recommended dosage is 50 mg once daily. CIBINQO is not recommended for patients with severe or End-Stage Renal Disease (ESRD). Severe renal impairment and End-Stage Renal Disease include patients on renal replacement therapy.
In subjects with mild and moderate renal impairment, if an adequate response is not achieved after 12 weeks, dose of CIBINQO can be doubled.
CIBINQO is not recommended for patients with severe renal impairment or ESRD.
Recommended Dosage in CYP2C19
Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Dosage Modifications due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19 reduce the dosage to 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Treatment Discontinuation due to Serious Infections or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Hematologic Abnormalities
• Discontinue CIBINQO if platelet count <50,000/mm3 and
follow with CBC until >100,000/mm3
• Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value
• Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value
• Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosing increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities.
DOSAGE FORMS AND STRENGTHS
• 50 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 50” on the other.
• 100 mg: Pink, round, film-coated tablet debossed with “PFE” on one side and “ABR 100” on the other.
• 200 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 200” on the other.
CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment.
WARNINGS AND PRECAUTIONS
Serious Infections
The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and benefits of treatment prior to initiating CIBINQO in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO. Tuberculosis Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation
Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information; Initial Approval: January 2022
CIBINQO™ (abrocitinib) tablets, for oral use
Mortality (continued)
treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with CIBINQO for atopic dermatitis.
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical studies of CIBINQO for atopic dermatitis.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in patients receiving CIBINQO in the clinical studies for atopic dermatitis.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
Laboratory Abnormalities
Hematologic Abnormalities Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a CBC. CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations
Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter
elevations on cardiovascular morbidity and mortality has not been determined.
Immunizations
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Serious Infections
• Mortality
• Malignancy and Lymphoproliferative Disorders
Clinical Trials Experience
• Major Adverse Cardiovascular Events
• Thrombosis
• Laboratory Abnormalities
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO.
In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks.
The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%).
Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in the table below. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety profile of CIBINQO in the monotherapy and the combination trial(s) were similar.
Adverse Reactions from Placebo-Controlled Trials
Reported in ≥1% of CIBINQO Treated Subjects with Moderate to Severe Atopic Dermatitis and at Higher Rate than Placebo for up to 16 Weeks Weeks 0-16
Overall Infections In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events In the placebocontrolled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia, no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia In the placebo-controlled trials, for up to 16 weeks, confirmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the first 4 weeks of exposure.
Lipid Elevations In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the final visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Specific Adverse Reactions Exposure adjusted incidence rates were adjusted by trial size for all the adverse reactions reported in this section.
Retinal Detachment In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
CIBINQO 200 mg N=590 n (%a) CIBINQO 100 mg N=608 n (%a) PLACEBO N=342 n (%a) Nasopharyngitis 51 (8.7)75 (12.4)27 (7.9) Nausea 86 (14.5)37 (6.0)7 (2.1) Headache 46 (7.8)36 (6.0)12 (3.5) Herpes simplexb 25 (4.2)20 (3.3)6 (1.8) Increased blood creatine phosphokinase 17 (2.9)14 (2.3)5 (1.5) Dizziness 17 (2.9)11 (1.8)3 (0.9) Urinary tract infection 13 (2.2)10 (1.7)4 (1.2) Fatigue 8 (1.3)10 (1.6)2 (0.5) Acne 28 (4.7)10 (1.6)0 (0.0) Vomiting 19 (3.2)9 (1.5)3 (0.9) Impetigo 3 (0.5)9 (1.5)1 (0.3) Oropharyngeal pain 6 (1.0)8 (1.4)2 (0.6) Hypertension 5 (0.8)7 (1.2)2 (0.7) Influenza 6 (1.1)7 (1.2)0 (0.0)
8 (1.3)7 (1.1)2 (0.6)
contact 3 (0.5)6
pain upper 11 (1.9)4 (0.6)0 (0.0)
discomfort 7 (1.2)3 (0.5)1 (0.3)
zoster 7 (1.2)2 (0.3)0 (0.0)
9 (1.5)0 (0.0)0 (0.0)
Study size adjusted percentages b Herpes simplex also includes oral herpes, ophthalmic herpes, herpes dermatitis, genital herpes.
Gastroenteritis
Dermatitis
(1.1)1 (0.3) Abdominal
Abdominal
Herpes
Thrombocytopenia
a
CIBINQO™ (abrocitinib) tablets, for oral use
Specific Adverse Reactions (continued)
Creatine Phosphokinase Elevations (CPK) In the placebocontrolled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
Adolescent Subjects (12 to less than 18 years of age) The safety of CIBINQO was assessed in a trial of 284 subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis (Trial-AD-4). The safety profile of CIBINQO in these subjects, assessed through the initial treatment period of 12 weeks and the long-term period (213 with at least 52 weeks of abrocitinib exposure), was similar to the safety profile from trials in adults with atopic dermatitis.
DRUG INTERACTIONS
Effects of Other Drugs on CIBINQO
The table below includes drugs with clinically significant drug interactions affecting CIBINQO.
Clinically Significant Drug Interactions Affecting CIBINQO
Strong CYP2C19 Inhibitors
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors.
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9
Clinical Impact Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9.
Strong CYP2C19 or CYP2C9 Inducers
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response.
Intervention Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers.
Effects of CIBINQO on Other Drugs
The table below includes clinically significant drug interactions affecting other drugs.
Clinically Significant Interactions Affecting Other Drugs
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities
Clinical Impact
Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin).
Intervention Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO.
Antiplatelet Therapy Drugs
Clinical Impact Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia.
Intervention Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment are contraindicated with CIBINQO.
Pregnancy
Pregnancy Exposure Registry There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
Risk Summary Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 11 or 4 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Animal Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Animal Data In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossified metatarsals) were noted at 60 mg/kg/day (17 times the MRHD based on AUC comparison). In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (4 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (17 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (2.4 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/ day (11 times the MRHD based on AUC comparison).
Lactation
Risk Summary There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Animal Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5-6 elimination half-lives).
Animal Data Lactating female rats were orally administered a single dose of 10 mg/kg abrocitinib on lactation day 12. Abrocitinib AUC was approximately 5 times greater in milk than in plasma.
Females and Males of Reproductive Potential
Infertility Females
Based on the findings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration.
Pediatric Use
The safety and effectiveness of CIBINQO in pediatric patients 12 years of age and older weighing 25 kg or more with atopic dermatitis has been established. In trials Trial-AD-1 and Trial-AD-2, 124 adolescent subjects 12 to less than 18 years old with moderate-to-severe atopic dermatitis were enrolled and randomized to receive either CIBINQO 100 mg (N=51), 200 mg (N=48), or matching placebo (N=25) in monotherapy. Additional 284 adolescent subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis, were enrolled and randomized to receive either CIBINQO 100 mg (N=95) or 200 mg (N=94) or matching placebo (N=95) in combination with topical corticosteroids in Trial-AD-4. Efficacy and adverse reaction profile were consistent between the pediatric patients and adults.
The safety and effectiveness of CIBINQO have not been established in pediatric patients below 12 years of age.
Juvenile Animal Toxicity Data In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day beginning on postnatal day 10 (approximately equivalent to a human infant) and continuing through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose-related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur. Abrocitinib produced adverse effects on bone development at all dose levels. Abrocitinib caused irreversible dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (0.8 times the MRHD based on AUC comparison).
Abrocitinib also irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (7.2 times the MRHD based on AUC comparison). At 75 mg/kg/ day (27 times the MRHD based on AUC comparison), paw fractures generally corresponded to limb impairment, a fractured tibia was noted in a single female. Irreversible bone findings have not been observed in older animals.
Geriatric Use
A total of 145 (4.6%) patients 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
A higher proportion of patients 65 years of age and older discontinued from clinical trials compared to younger patients. Among all patients exposed to CIBINQO, including the long-term extension trial, confirmed ALC <500/mm3 occurred only in patients 65 years of age and older. A higher proportion of patients 65 years of age and older had platelet counts <75,000/ mm3. The incidence rate of herpes zoster in patients 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of patients 18 to less than 65 years of age (3.44 per 100 patient-years).
Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30-59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min). This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement therapy. A dosage reduction in patient with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60-89 mL/min).
CIBINQO has not been studied in patients on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function. In clinical trials, CIBINQO was not evaluated in patients with severe (Child Pugh C) hepatic impairment.
CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates.
OVERDOSAGE
There is no experience regarding human overdosage with CIBINQO. There is no specific antidote for overdose with CIBINQO. In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
Rx only
This brief summary is based on CIBINQO™ (abrocitinib) Prescribing Information LAB-1424-2.0.
Issued: February 2023.
The product’s label may have been updated. For full Prescribing Information, visit CIBINQOPI.com.
See CIBINQO full Prescribing Information at CIBINQOPI.com.
© 2023 Pfizer Inc. All rights reserved. February 2023. PP-CIB-USA-0485
USE IN SPECIFIC POPULATIONS
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
© 2023 Pfizer Inc. All rights reserved. May 2023. PP-CIB-USA-0485




THIRD ANNUAL BCOD NATIONAL CONFERENCE 2023
convened in San Antonio, TX, November 5-7 to bring together biologic coordinators and office staff from across the country for education and networking.



ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 48
Photography by Brighthill Company

Keep PatientAdvancing Access

This year, BCoD provided select attendees with the first- ofits-kind BC Certificate Program. This newly offered program provides the most relevant and meaningful training ever made available to BCs.
We had over 220 BCs register for the event and more than 35 exhibiting partners in attendance.
LOOKING AHEAD TO 2024
Our membership is growing exponentially, and we are extremely excited to continue bringing innovative programs to our members and strengthening our collaboration with our partners to advance patient access.


EVENTS THIRD ANNUAL BCOD CONFERENCE bcofdermatology.com 49


“The relevant content, speakers, and engaging design for industry partners was sensational. My deepest congratulations to all for being phenomenal architects at a meeting that transforms the market.”
– Industry Partner





THIRD ANNUAL BCOD CONFERENCE ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 50
EVENTS




“I can gladly say that there was not one program that I did not learn something from!”
- Member







EVENTS THIRD ANNUAL BCOD CONFERENCE bcofdermatology.com 51





“Thank you! I love my job and helping people feel better! This conference gave me a wealth of information to do that.”
- Member



THIRD ANNUAL BCOD CONFERENCE ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 52
EVENTS








EVENTS THIRD ANNUAL BCOD CONFERENCE bcofdermatology.com 53
EVENTS








ACCESS DERMATOLOGY / NOVEMBER/DECEMBER 2023 54
THIRD ANNUAL BCOD CONFERENCE











EVENTS THIRD ANNUAL BCOD CONFERENCE bcofdermatology.com 55
THANK YOU! BC OD
I
want to extend my gratitude to our members, whose relentless pursuit of patient care is the cornerstone of BCoD's mission. We convened at our third annual conference for you, propelled by a single ambition: to enrich your capabilities, to bridge information chasms, and to ensure your indispensable role is empowered and acknowledged.
The unveiling of our certificate program and our strong partnerships in creating its content demonstrate our commitment to providing a deep network of resources. The certificate isn't just a badge— it's a testament to our collective expertise and commitment to
advancing patient access.
To our partners, your presence is a pillar of strength. Your shared vision with BCoD helps sculpt a pathway toward better patientcare outcomes. It's a symbiotic endeavor that not only amplifies the impact, but also paves the way for a promising patient journey.
Thank you to everyone who joined us in San Antonio. Our future is full of endless possibilities, and we are destined to make an unforgettable impact together, thanks to those individuals who epitomize BCoD.
CRAIG SCHUETTE, CO-FOUNDER & EXECUTIVE DIRECTOR, BCOD FOUNDER, ACCESS DERMATOLOGY







































EMPLOYEE SPOTLIGHT

ARECOGNIZING OUTSTANDING PATIENT SERVICE
t Biologic Coordinators of Dermatology (BCoD), we celebrate our members and access managers who ensure a positive patient experience. These dedicated professionals are committed to their patients, helping them secure the types of therapy needed. We would like to recognize two stand-out employees through the words of their dermatology offices. BCoD salutes these individuals for their remarkable efforts in advancing patient access.


















LETTER of R ECOGNITION



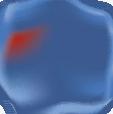



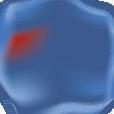













BC OD


















Tracy Vidrich
RN and BIOLOGIC COORDINATOR
ASSOCIATED DERMATOLOGY & SKIN CANCER CLINIC
Helena, Montana
“Associated Dermatology would like to nominate Tracy Vidrich, RN, our long-term employee for over 13 years. Tracy took on the responsibility of Associated Dermatology Biologic Coordinator two years ago. During this time, her passion for patient care helped to save the life of a two-year-old girl, who was suffering. Her mom had been searching for an answer for at least a year and had seen multiple doctors for a rash, and no one could find a diagnosis. Diagnosed with atopic dermatitis as a young child with no access to a child dose of Dupixent, Tracy worked with Dupixent on treatment.
Since this family had no transportation to our office, Tracy took the time to go to their home and give her the much-needed injection and care. If not for Tracy doing this, who knows what outcome the child would have had. Now she is a vibrant three-year-old with mostly clear skin and is thriving.
This is just one example of what Tracy does to go the extra mile as a biologic coordinator. Tracy has a comprehensive knowledge of the various medications, diagnosis, processes and programs each manufacturer offers. She provides valuable input and assistance to the prescriber and patient during the process. Tracy tirelessly advocates and navigates the complicated ever-changing process to get patients access to medication so they can enjoy the quality of life they deserve.”
— DEBI SIMON, OFFICE MANAGER
“I would like to nominate Tracy Vidrich for outstanding nurse, but I do not have a computer. My daughter is now a healthy three-year-old. She was suffering from a severe skin disease when we turned to dermatology. Starting last year, Tracy would regularly come to our home to deliver the medication for my daughter when we had no transportation. She would take care of us even when no one else would take an interest. She arranged Christmas gifts for our entire family. My daughter is so much better, and Tracy's actions saved her life. My daughter had previous hospitalizations for widespread bacterial infections. Her clothing would stick to her skin with all the disease and infection. My daughter was in constant pain and was not developing well; I was worried we would lose her. Thanks to Tracy, she is growing and healthy today!”
—PATIENT’S MOTHER
bcofdermatology.com 59
LETTER of R ECOGNITION





















BC OD


















Talisa Soto
BIOLOGIC COORDINATOR
CHICAGO SKIN CLINIC
Chicago, IL
“I am reaching out to enthusiastically nominate Ms. Talisa Soto, our dedicated biologic coordinator at Chicago Skin Clinic, for the recognition she wholeheartedly deserves.
Chicago Skin Clinic stands proudly as a haven for a diverse and often underserved patient base in the heart of Chicago. A significant number of our patients either lack insurance or face the daunting challenge of losing it, especially after commencing biologic treatments. Amidst such challenges, Talisa emerges as a lifeline for our patients, ensuring they don't miss a beat in their journey toward better skin health.
Talisa's ingenuity is evident in her creation of a customized spreadsheet used within our clinic. This invaluable tool allows our team to seamlessly monitor status updates on patient progress, ensuring timely interventions and adjustments to their care. Her ability to leverage this tool, combined with her proactive communication skills, means patients and pharmacies are always kept in the loop, and potential hurdles are addressed before they escalate.
Her knack for effortlessly handling medication requests, especially for our uninsured patients, epitomizes her commitment to patient care. It's no exaggeration to state that she acts as both an advocate and guardian for our patients, always putting their needs at the forefront of her actions.
The spirit and ethos of BCoD resonate deeply with the work Talisa does daily. Her dedication to ensuring every patient feels celebrated, valued, and heard makes her an embodiment of the principles you champion.
It's professionals like her who truly uphold the dignity and purpose of our vocation.”
— DR. DANILO C. DEL CAMPO, MD FAAD




ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 60

Guiding You Every Step of the Way
We offer personalized care for patients living with complex and chronic conditions, including:
> Limited distribution medications and biosimilars access


> Home infusion and support from our pharmacists and nurses
> Educational resources to help patients manage their medication


> Benefits investigation and financial assistance support





AcariaHealth.com
© 2023 AcariaHealth. All rights reserved. A2018_230404
more
Learn
by contacting us today Sales@AcariaHealth.com | 800.511.5144















































HOW TECHNOLOGY CAN EASE RE-ENROLLMENT SEASON CHAOS


SURVIVING the BLIZZARD

































ACCESS DERMATOLOGY
NOVEMBER/DECEMBER 2023 LUMOS SPSTOCK.ADOBE.COM





 By Adam Rosenberg Senior Director, RxLightning RxLightning
By Adam Rosenberg Senior Director, RxLightning RxLightning


































known as .

bcofdermatology.com



ne of the most challenging times of the year for biologics coordinators, providers, medication access staff, and other team members who support specialty medication onboarding is re-verification and re-enrollment season—aptly Blizzard Season



While insurance selections are finalized and patients are counting down the days until the new year, coverage modifications on the back-end can cause significant delays in medication access. The most common adjustments include: updates to a coverage policy and a new prior authorization requirement; reset deductibles; the end of co-pay assistance and patient assistance programs (PAP); or the selection of an entirely new insurance provider. Some of these changes can wholly prevent the continuation of medication fulfillment, while others—like deductible and financial assistance resets—may surprise patients enough to forego the unexpected costs and adherence to their meds.
Patient support teams, manufacturers and hubs often hire short-term staff to meet the influx of benefits verification (BV) demands, but this model is inefficient and expensive. One manufacturer even resorted to hiring 400 temporary workers to complete 50,000 BVs over the course of a six-week period (Pharmacy Times, 2019). While much of the work cannot be started until updated forms and insurance details are finalized, a robust Blizzard Season plan and digital technology are necessary to alleviate undue burdens during this frenzied time.



RE-ENROLLMENT READINESS
"In order to accelerate the benefits verification process and reduce the resources (and costs) required, teams must prepare well before Blizzard Season begins," says Julia Regan, Founder and CEO, RxLightning.
Stakeholders should understand which providers, plans, manufacturers, FRMs and hubs they may be working with and collect information about patient coverage and health changes, so that re-submitting paperwork can be more seamless. Additionally, teams should communicate with patients early to understand who may be changing plans, alert them of potential upcoming requests (signatures required, plan details, etc.) and support them.
While many programs officially transition on January 1, enrollment or PAP forms
are often available one or two months ahead, enabling teams to understand what information is required to get a head start on the re-enrollment process. With the right plan of attack, Blizzard Season can commence without too many unknowns.
ACCESS DERMATOLOGY NOVEMBER/DECEMBER 2023 64
SYDA PRODUCTIONSSTOCK.ADOBE.COM
HOW TECHNOLOGY CAN HELP
There will always be a need for real people to manage the more complex patient onboarding cases wherein new coverage restrictions, financial assistance or prior authorization is required to continue a patient’s care regimen. However, a significant portion of re-enrollments and re-verifications can be streamlined by using various types of technology.
In many cases, the first step for re-enrollment is finding the appropriate medication or PAP paperwork and completing the paper form correctly. While this may seem simple, finding and completing thousands of paper forms is a tedious and error-prone task. With new digital enrollment technologies, providers and care teams can be assured that they are completing the most up-to-date forms, with no missing information, greatly reducing the risk of a downstream issue in the application process.
Another important step of the process is reconfirming the patient’s insurance plan and completing an eligibility and
benefits check. Instead of manually scrolling through thousands of pages of documents on a plan’s website or calling a representative to confirm coverage, real-time connectivity to insurance databases can accurately display patient plan details while providing insight into cost and restrictions required. This automated process considerably reduces time spent confirming information, and it allows stakeholders to move on to more complex steps of the process.
Next, collecting consent. While patient and provider signatures are necessary to re-enroll for a specialty medication or PAP with the click of a button, email and SMS notifications can be sent to appropriate stakeholders to securely sign documents from wherever they might be. No waiting until the next in-person visit or stop-atthe-desk to collect a signature; instead, consent can be collected remotely in a matter of seconds.
As mentioned, with deductibles, copays and PAP all resetting, it is essential for technology to surface affordability options that can support patients—whether that is long-term PAP enrollment, ad hoc
“In order to accelerate the benefits verification process and reduce the resources (and costs) required, TEAMS MUST PREPARE WELL BEFORE BLIZZARD SEASON BEGINS. "
—JULIA REGAN, FOUNDER AND CEO, RXLIGHTNING
foundation or grant support, or short-term low-cost options a patient can receive in the interim (while the specialty medication enrollment is being finalized). With an interconnected network of coupon and discount providers, care teams can easily identify and communicate with patients about affordable options available to them.
One of the most time-consuming tasks of the re-enrollment process is filtering which patients are qualified for PAP or financial assistance programs. Major credit check companies now allow technology vendors to connect to their systems to provide real-time insight into income and credit score, enabling an initial verification of assistance eligibility. Combined with pre-seed questions, stakeholders using technology can immediately filter eligible patients and fasttrack certain patient cases over others.
Clearly there are many areas where technology can support stakeholders through the Blizzard Season slog. Luckily, RxLightning’s robust solution suite can support each and every stage of this journey, from completing digital enrollment paperwork to selecting patient affordability options. By leveraging innovative technologies, interconnected systems, and automation to alert and support teams through re-enrollment steps, Blizzard Season can be a more personalized, successful and error-free experience.
bcofdermatology.com 65



Brief Summary of Prescribing Information for ZORYVE® (roflumilast) cream, for topical use. See package insert for full Prescribing Information.
INDICATIONS AND USAGE
ZORYVE cream is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in patients 6 years of age and older.
DOSAGE AND ADMINISTRATION
Apply ZORYVE cream to affected areas once daily and rub in completely. Wash hands after application, unless ZORYVE cream is for treatment of the hands.
ZORYVE cream is for topical use only and not for ophthalmic, oral, or intravaginal use.
CONTRAINDICATIONS
ZORYVE cream is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C).
ADVERSE REACTIONS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two multicenter, randomized, double-blind, vehicle-controlled trials (DERMIS-1 and DERMIS-2), 881 adult and pediatric subjects 6 years of age or older with plaque psoriasis were treated with ZORYVE cream or vehicle topically once daily for 8 weeks.
The median age was 47 years (range 6 to 88). The majority of the subjects were male (64%) and White (82%). The median body surface area (BSA) affected was 5.5% (range 2% to 20%). The proportion of subjects who discontinued treatment due to an adverse reaction was 1.0% for subjects treated with ZORYVE cream and 1.3% for subjects treated with vehicle cream. The most common adverse reaction that led to discontinuation of ZORYVE cream was application site urticaria (0.3%).
Table 1 presents adverse reactions that occurred in at least 1% of subjects treated with ZORYVE cream, and for which the rate exceeded the rate for vehicle cream.
Table 1. Adverse Reactions Reported in ≥1% of Subjects with Plaque Psoriasis Treated with ZORYVE Cream (and More Frequently than Vehicle Cream) for 8 Weeks in Trials DERMIS-1 and DERMIS-2
In a fertility and embryo-fetal development study, male rats were dosed orally with up to 1.8 mg/kg/day roflumilast for 10 weeks and females for 2 weeks prior to pairing and throughout the organogenesis period. Roflumilast induced pre- and postimplantation loss at maternal oral doses greater than or equal to 0.6 mg/kg/day (3 times the MRHD on a mg/m2 basis). Roflumilast did not cause fetal structural abnormalities at maternal oral doses up to 1.8 mg/kg/day (9 times the MRHD on a mg/m2 basis).
In an embryo-fetal development study in rabbits, pregnant does were dosed orally with 0.8 mg/kg/day roflumilast during the period of organogenesis. Roflumilast did not cause fetal structural abnormalities at the maternal oral doses of 0.8 mg/kg/day (8 times the MRHD on a mg/m2 basis).
In pre- and post-natal developmental studies in mice, dams were dosed orally with up to 12 mg/kg/day roflumilast during the period of organogenesis and lactation. Roflumilast induced stillbirth and decreased pup viability at maternal oral doses greater than 2 mg/kg/day and 6 mg/kg/day, respectively (5 and 15 times the MRHD on a mg/m2 basis, respectively). Roflumilast induced delivery retardation in pregnant mice at maternal oral doses greater than 2 mg/kg/day (5 times the MRHD on a mg/m2 basis). Roflumilast decreased pup rearing frequencies at a maternal oral dose of 6 mg/kg/day during pregnancy and lactation (15 times the MRHD on a mg/m2 basis). Roflumilast also decreased survival and forelimb grip reflex and delayed pinna detachment in mouse pups at a maternal oral dose of 12 mg/kg/day (29 times the MRHD on a mg/m2 basis).
Lactation
Risk Summary
There are no data on the presence of roflumilast or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production.
Roflumilast and/or its metabolites are excreted into the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZORYVE cream and any potential adverse effects on the breastfed infant from ZORYVE cream or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use ZORYVE cream on the smallest area of skin and for the shortest duration possible while breastfeeding. To avoid direct infant exposure, advise breastfeeding women not to apply ZORYVE cream directly to the nipple or areola. If applied to the patient’s chest, avoid exposure via direct contact with the infant’s skin.
Data
Animal data
Roflumilast and/or its metabolite concentrations measured 8 hours after an oral dose of 1 mg/kg given to lactating rats were 0.32 and 0.02 mcg/g in the milk and pup liver, respectively.
Pediatric Use
In 594 subjects who continued treatment with ZORYVE cream for up to 64 weeks in open-label extension trials, the adverse reaction profile was consistent with that observed in vehicle-controlled trials.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are insufficient data available on the use of ZORYVE cream in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, roflumilast administered orally to pregnant rats and rabbits during the period of organogenesis produced no fetal structural abnormalities at doses up to 9 and 8 times the maximum recommended human dose (MRHD), respectively. Roflumilast induced post-implantation loss in rats at oral doses greater than or equal to 3 times the MRHD. Roflumilast induced stillbirth and decreased pup viability in mice at oral doses 5 and 15 times the MRHD, respectively. Roflumilast has been shown to adversely affect pup post-natal development when dams were treated with an oral dose 15 times the MRHD during pregnancy and lactation periods in mice.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor and delivery
Avoid using ZORYVE cream during labor and delivery. There are no human studies that have investigated effects of ZORYVE cream on preterm labor or labor at term; however, animal studies showed that oral roflumilast disrupted the labor and delivery process in mice.
Data
Animal data
In an embryo-fetal development study, pregnant rats were dosed orally during the period of organogenesis with up to 1.8 mg/kg/day roflumilast (9 times the MRHD on a mg/m2 basis). No evidence of structural abnormalities or effects on survival rates were observed. Roflumilast did not affect embryo-fetal development at a maternal oral dose of 0.2 mg/kg/day (equivalent to the MRHD on a mg/m2 basis).
The safety and effectiveness of ZORYVE cream for the treatment of plaque psoriasis have been established in pediatric patients 6 years of age and older. Use of ZORYVE cream in pediatric patients 6 to less than 18 years of age is supported by data from two 8-week, vehicle-controlled safety and efficacy trials which included 18 pediatric subjects 6 to 17 years of age, of whom 11 received ZORYVE cream. Use of ZORYVE cream in pediatric patients 12 to 17 years of age is also supported by data from open-label trials of 2 and 24 weeks duration which included 18 pediatric subjects 12 to 17 years of age treated with ZORYVE cream. Use of ZORYVE cream in pediatric patients 6 to less than 12 years of age is also supported by data from one 4-week, open-label, safety and pharmacokinetic (PK) study which included 20 pediatric subjects 6 to less than 12 years of age. The adverse reaction profile in subjects 6 to less than 18 years of age was consistent with that observed in adults.
The safety and effectiveness of ZORYVE cream in pediatric patients below the age of 6 years have not been established.
Geriatric Use
Of the 881 subjects with psoriasis exposed to ZORYVE cream or vehicle for up to 8 weeks in 2 controlled clinical trials, 106 were 65 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the geriatric and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Based on available data for roflumilast, no adjustment of dosage in geriatric patients is warranted.
Hepatic Impairment
Oral roflumilast 250 mcg once daily for 14 days was studied in subjects with hepatic impairment. The systemic exposure of roflumilast and roflumilast N-oxide was increased in subjects with moderate (Child-Pugh B) hepatic impairment. ZORYVE cream is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C). No dosage adjustment is needed in patients with mild (Child-Pugh A) hepatic impairment.
PATIENT COUNSELING INFORMATION

Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information).
Lactation
Advise patients to use ZORYVE cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Instruct patients who are breastfeeding not to apply ZORYVE cream directly to the nipple and areola to avoid direct infant exposure. Instruct patients to avoid inadvertent contact of treated areas with infant skin.
© 2023 Arcutis Biotherapeutics, Inc. All rights reserved. 10/2023
Adverse Reaction ZORYVE Cream (N=576) n (%) Vehicle Cream (N=305) n (%) Diarrhea 18 (3.1) 0 (0.0) Headache 14 (2.4) 3 (1.0) Insomnia 8 (1.4) 2 (0.7) Nausea 7 (1.2) 1 (0.3) Application site pain 6 (1.0) 1 (0.3) Upper respiratory tract infection 6 (1.0) 1 (0.3) Urinary tract infection 6 (1.0) 2 (0.7)

GOT A JET-SETTER ON YOUR LIST?
WE’RE ABOUT TO MAKE THEM VERY HAPPY
Gifts for a Getaway
This is your year, the year you finally thrill that picky traveler in your life with the perfect gift. There will be parades in the town square celebrating your shopping acumen. From the luxury PJs and lounge memberships to thoughtful and affordable stocking stuffers, we’ve got you covered.
bcofdermatology.com 69 HANNA AIBETOVASTOCK.ADOBE.COM
BE THEIR PRIORITY
Without a first-class ticket or elite status, getting into an airport lounge can be difficult, if not impossible. Gift your favorite traveler the peace, quiet and free alcohol of airport lounges around the globe. Priority Pass membership offers access to over 1,400 lounges and experiences.
At JFK , membership opens the door to Air France’s luxe lounge and the Virgin Atlantic Clubhouse (among others). In some airports and terminals chair massages at Be Relax are included. Prices start at $99 for the occasional traveler and up to $469 for a frequent traveler. prioritypass.com

BE SQUARE
Monogrammed packing cubes are soft-zippered clothing cases that make packing and unpacking easier. Whether you’re a roller or a folder, you can fit at least five to six pairs of undergarments into the small cubes, six to eight pairs of pants in the medium one, and nine to ten shirts (or two to three bulkier tops, like sweatshirts) in the big one. tourparavel.com
It’s easy to unpack when you arrive: simply toss the cubes into the hotel drawers and voilà, you’re unpacked.
NO STRINGS ATTACHED
You can fit more clothes in your carry on if you use them like compression bags (meaning, jam ‘em in tight).

Your organization is sure to impress any TSA agent who randomly searches your bag.

Your clothes stay wrinkle- free packed neatly in their own little pouches.

At first glance an AirFly bluetooth headphone connector could seem like a boring tech gift, but this little device is a game changer. It allows you to connect your wireless headphones (any brand) to the airline’s inflight entertainment system. No more cheap, stringy airline headphones. And if you’re traveling with a friend and want to watch the same program, simply connect both sets of headphones. It’s like a modern-day Lady and the Tramp. They range in price from $34.99 for the Duo to $64.95 for the pro. apple.com
Easily separate the clean from the dirty when you head home.





ACCESS DERMATOLOGY /NOVEMBER/DECEMBER 2023 70
HIRE THE PAPARAZZI



If you don’t have a photo in front of the Eiffel Tower, did you even go to Paris? Never hand your iPhone to a stranger to capture your travel memories again. Flytographer has professional photographers in over 350 cities around the world and makes the perfect gift for a loved one planning a special trip. You can gift a photo shoot for as little as $285 for a thirty-minute session. The price goes up as you add time, photos and multiple shoot locations. Unlike many other phographers, all of the digital images are included in the price. flytographer.com














ADAPT TO THE ROAD
A universal adapter is the perfect stocking stuffer. The ingenious little device gives Transformer vibes. For $23 you can charge not one, not two, not three, but four devices with just one adapter. The Epicka Universal Travel Adapter works in over 150 countries and was the No. 1 pick of The New York Times Wirecutter team and our travel editor. Editor’s note: make sure your hair styling appliances are dual voltage, or you run the risk of setting them on fire. amazon.com



everything except a private jet? Medjet. What does Medjet
do? If a member is hospitalized 150 miles or more from home, Medjet will arrange a medical transfer to the hospital in the home country of their choice. Note: This does not replace travel insurance. The emergency medical evacuation benefits within a travel insurance policy provides transportation to the nearest adequate facility. Medjet offers hospital-to-hospital transport, making sure that very ill people requiring continued hospitalization get moved to the hospital they’d rather be in at home. Memberships start at $99 for an eight-day trip. Annual MedjetAssist memberships, where you can travel as much as you want in a year, are $295 for an individual and $399 for a family (two adults and up to five kids). Read the fine print and various membership options at medjetassist.com

71
bcofdermatology.com

“Too often we underestimate the power of a touch, a smile, a kind word, a listening ear, an honest compliment, or the smallest act of caring, all of which have the potential to turn a life around."
—LEO BUSCAGLIA, AUTHOR
last word ACCESS DERMATOLOGY JULY/AUGUST 2023 72
ENHANCE AND EXPEDITE PATIENT-FOCUSED COLLABORATION
Biologic Coordinators of Dermatology has partnered with Quincy to bring technological innovation to the FRM and BC workstreams.
Speed time to therapy and increase the efficiency of Field Reimbursement Managers and Biologic Coordinator collaboration with HIPAA-compliant secure messaging.
• Reduce patient onboarding time
• Increase employee productivity
• Increase the number new patients processed
• Increase staff and patient satisfaction


FEATURES:
• Initiate secure conversations between Field Reimbursement Managers and Biologic Coordinators
• Create Quick Messages for commonly used communications
• Personalized url to increase trust in the identity of the communicator
• Optional: Add Face to Face Video virtual visits for a personal touch

To learn more, see a demo, and take advantage of partnership discounts, please reach out to contact@bcofdermatology.com
Help eligible patients start and stay on track with their therapy




















The DUPIXENT MyWay® patient support program can help facilitate patient access to DUPIXENT® (dupilumab) throughout the treatment journey.
Coverage support
DUPIXENT MyWay provides assistance navigating the insurance process with benefits investigations, prior authorization support, and education about the appeals process.
Patient access support
DUPIXENT MyWay may have support for eligible patients who need help with their out-of-pocket costs.
Education and support
Every enrolled patient is assigned a dedicated DUPIXENT MyWay Case Manager, who takes a patient-centric approach to providing tools, support, resources, and education throughout the treatment journey.
Support for your patients begins with a complete DUPIXENT MyWay Enrollment Form
You can help patients enroll in DUPIXENT MyWay by:
• Faxing the form to DUPIXENT MyWay at 844-387-9370
• Submitting the form through the DUPIXENT MyWay Document Drop at www.patientsupportnow.org
For more information:
Visit us on the exhibit floor OR
Call DUPIXENT MyWay at 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am–9 pm Eastern time
For any questions or concerns, or to report side effects with DUPIXENT® (dupilumab), please contact 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am–9 pm Eastern time.
and DUPIXENT MyWay® are registered trademarks of Sanofi Biotechnology. © 2023 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved. 07/2023 DUP.23.06.0234
DUPIXENT®
D (dupilumab) Injection
DUPIXENT myway) sanofi
























































































































































































































 By Ginger McWilliams DERMATOLOGY/IMMUNOLOGY , BRISTOL MYERS SQUIBB
By Ginger McWilliams DERMATOLOGY/IMMUNOLOGY , BRISTOL MYERS SQUIBB
































































































































































































































































































































































































































 By Adam Rosenberg Senior Director, RxLightning RxLightning
By Adam Rosenberg Senior Director, RxLightning RxLightning