

MEMBER SURVEY
Shop talk and key findings
EVOLVING NEEDS
Increasing importance of BC role
STREAMLINE THE PROCESS
Discover new workflow systems
TOPICAL THERAPY
Psoriasis, PAs and treatment
OCTOBER
SEPTEMBER
2023
BCOD CONFERENCE 2023
ready for San Antonio
Get
Painting by Andrew Wilkie, 2023. Image provided by Incyte Corporation.
Please scan the code above to visit EUCRISAhcp.com and see the full Prescribing Information and Medication Guide.




























Please scan the code above to visit CIBINQOhcp.com and see Brief Summary of Prescribing Information, including BOXED WARNING, at the end of this advertisement.
Please scan the code above to visit LITFULOhcp.com and see Brief Summary of Prescribing Information, including BOXED WARNING, at the end of this advertisement.
Explore Innovation in Dermatology





Confidence in Patient Support
Helping patients unlock access and reimbursement support for Pfizer dermatology medication
Coverage Assistance
Pfizer Dermatology Patient AccessTM (PDPA) can provide assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Financial Assistance
No matter what type of insurance your patients have, PDPA can help identify financial support options.
Pharmacy Coordination
PDPA strives to make prescription fulfillment through the pharmacy as smooth as possible.
Live, Personal Support
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.

A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
TM © 2023 Pfizer Inc. All rights reserved. September 2023. PP-CIB-USA-0916
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
• Increased risk of serious bacterial, fungal, viral, and opportunistic infections leading to hospitalization or death, including tuberculosis (TB). Interrupt treatment if serious infection occurs until the infection is controlled. LITFULO should not be given to patients with active TB. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test
• Higher rate of all-cause mortality, including sudden cardiovascular death with another Janus kinase (JAK) inhibitor vs TNF blockers in rheumatoid arthritis (RA) patients. LITFULO is not approved for use in RA patients
• Malignancies have occurred in patients treated with LITFULO. Higher rate of lymphomas and lung cancers with another JAK inhibitor vs TNF blockers in RA patients
• Higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) with another JAK inhibitor vs TNF blockers in RA patients
• Thrombosis has occurred in patients treated with LITFULO. Increased incidence of pulmonary embolism, venous and arterial thrombosis with another JAK inhibitor vs TNF blockers
INDICATIONS AND USAGE
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment With LITFULO
• TB infection evaluation: LITFULO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of LITFULO
• Viral hepatitis screening in accordance with clinical guidelines: LITFULO initiation is not recommended in patients with hepatitis B or hepatitis C
• Treatment with LITFULO should not be initiated in patients with absolute lymphocyte count (ALC) <500/mm3 or a platelet count <100,000/mm3
• Update immunizations according to current immunization guidelines
Recommended Dosage
The recommended dosage of LITFULO is 50 mg orally once daily with or without food.
LITFULO capsules should be swallowed whole; not crushed, split, or chewed.
If a dose is missed, the dose should be taken as soon as possible unless it is less than 8 hours before the next dose; in which case, skip the missed dose and resume dosing at the regular scheduled time.
Patients With Severe Hepatic Impairment
LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
Treatment Interruption or Discontinuation
If treatment interruption is indicated, a temporary treatment interruption for less than 6 weeks is not expected to result in significant loss of regrown scalp hair.
Hematologic Abnormalities
• Treatment with LITFULO should be discontinued if platelet count is <50,000/mm3
• Treatment with LITFULO should be interrupted if ALC is <500/mm3 and may be restarted once ALC returns above this value
ALC and platelet counts are recommended before treatment initiation and at 4 weeks after treatment initiation, and thereafter according to routine patient management.
DOSAGE FORMS AND STRENGTHS
Capsules: 50 mg of ritlecitinib, size 3, opaque capsules with yellow body and blue cap. The body is printed with “RCB 50” and the cap is printed with “Pfizer” in black.
CONTRAINDICATIONS
LITFULO is contraindicated in patients with known hypersensitivity to ritlecitinib or any of its excipients.
WARNINGS AND PRECAUTIONS
Serious infections have been reported in patients receiving LITFULO. The most frequent serious infections have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO. Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
• with chronic or recurrent infection
• who have been exposed to TB
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt LITFULO if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
Screen patients for TB before starting therapy. LITFULO should not be given to patients with active TB. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB. In patients with a negative latent TB test, consider anti-TB therapy before initiating treatment with LITFULO in those at high risk and consider screening patients at high risk for TB during treatment with LITFULO.
Viral Reactivation
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), was reported in clinical trials. If a patient develops herpes zoster, consider interrupting treatment until the episode resolves.
Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy with LITFULO. Patients with evidence of HIV infection or hepatitis B or C infection were excluded from clinical trials.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO.
Malignancy and lymphoproliferative disorders, including nonmelanoma skin cancer (NMSC), were observed in clinical trials of LITFULO.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding NMSC) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
The risks and benefits of LITFULO treatment should be considered prior to initiating or continuing therapy in patients with a known malignancy other than a successfully treated NMSC or cervical cancer.
Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of MACE defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LITFULO in patients that have experienced an MI or stroke.
Thromboembolic Events
An event of pulmonary embolism (PE) was reported in a patient receiving LITFULO. In a ritlecitinib higher dosing group, 1 patient reported an event of retinal artery occlusion. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, deep vein thrombosis, and PE were observed compared to those treated with TNF blockers.
Avoid LITFULO in patients who may be at increased risk of thrombosis. If symptoms of thrombosis or embolism occur, patients should interrupt LITFULO and be evaluated promptly and treated appropriately.
Hypersensitivity
Serious reactions including anaphylactic reactions, urticaria, and rash have been observed in patients receiving LITFULO in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue LITFULO and institute appropriate therapy.
Laboratory Abnormalities
Treatment with LITFULO was associated with decreases in lymphocytes and platelets.
Prior to LITFULO initiation, perform ALC and platelet counts. After initiating treatment with LITFULO, treatment interruption or discontinuation are recommended based on ALC and platelet count abnormalities.
Liver Enzyme Elevations: treatment with LITFULO was associated with increased incidence of liver enzyme elevation compared to placebo. Increases of ALT ≥5 times the upper limit of normal (ULN) and increases of AST ≥5 times the ULN were observed in patients in LITFULO clinical trials. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt LITFULO until this diagnosis is excluded.
Creatine Phosphokinase (CPK) Elevations: treatment with LITFULO was associated with increased incidence of CPK elevation compared to placebo.
Vaccinations
No data are available on the response to vaccination in patients receiving LITFULO. Use of live attenuated vaccines should be avoided during or shortly prior to initiating treatment. Prior to initiating LITFULO, it is recommended that patients be brought up to date with all immunizations, including prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
ADVERSE REACTIONS
Clinical Trials Experience
The safety of LITFULO was evaluated in three randomized, placebo-controlled clinical trials and one long-term trial in patients with alopecia areata, including alopecia totalis and alopecia universalis, who were 12 years of age and older. A total of 1628 patients were treated with LITFULO representing 2085 patient-years of exposure. There were 1011 patients with at least 1 year of exposure to LITFULO. In the placebo-controlled period of clinical trials in alopecia areata, a total of 668 patients were exposed to LITFULO with 130 receiving 50 mg once daily for up to 24 weeks. The median age of patients was 33 years, 105 (11.9%) patients were 12 to <18 years old and 22 (2.5%) patients were 65 years of age or older. The majority of patients were White (70.7%) and female (63.6%).
Adverse reactions occurring at ≥1% in the treated groups and at a higher rate than placebo are presented in the following table. A total of 2 (1.5%) patients treated with LITFULO 50 mg were discontinued from the trials due to adverse reactions.
LITFULO™ (ritlecitinib) capsules, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
Adverse Reactions in Clinical Trials of LITFULO for the Treatment of Alopecia Areata
LITFULO
50 mg N=130 n (%)
Placebo N=213 n (%)
Headache 14 (10.8)18 (8.5)
Diarrhea 13 (10.0)8 (3.8)
Acne 8 (6.2)10 (4.7)
Rash 7 (5.4)2 (0.9)
Urticaria
6 (4.6)3 (1.4)
Folliculitis 4 (3.1)4 (1.9)
Pyrexia 4 (3.1)0
Dermatitis atopic 3 (2.3)1
Dizziness
Blood
Herpes
Red
Specific Adverse Reactions
Exposure adjusted incidence rates were adjusted by clinical trial size for all adverse reactions reported in this section.
Overall Infections
In the placebo-controlled trials, for up to 24 weeks, overall infections were reported in 66 patients (80.35 per 100 patient-years) treated with placebo and 43 patients (74.53 per 100 patient-years) treated with LITFULO 50 mg. Across clinical trials, including the long-term trial, overall infections were reported in 645 patients (50.71 per 100 patient-years) treated with LITFULO 50 mg or higher.
Serious Infections
In the placebo-controlled trials, for up to 24 weeks, 3 patients reported serious infections across all ritlecitinib doses studied. Across clinical trials, including the long-term trial, serious infections were reported in 12 patients (0.66 per 100 patient-years) treated with LITFULO 50 mg or higher. The most common serious infections were related to appendicitis, COVID-19 infection (including pneumonia), and sepsis.
Herpes Zoster
In the placebo-controlled trials, for up to 24 weeks, herpes zoster was reported in 4 patients across all ritlecitinib doses studied and 0 patients treated with placebo. Across clinical trials, including the long-term trial, herpes zoster was reported in 21 patients (1.17 per 100 patient-years) treated with LITFULO 50 mg or higher. Opportunistic infections of multi-dermatomal herpes zoster were reported in 1 patient (0.50 per 100 patient-years) treated with the ritlecitinib higher dose in the placebo-controlled trials and 2 patients (0.1 per 100 patient-years) treated with LITFULO 50 mg or higher in all clinical trials.
Malignancy
In the placebo-controlled trials, for up to 24 weeks, 1 malignancy (breast cancer) was reported in 1 patient (1.33 per 100 patient-years) treated with ritlecitinib higher dose and no malignancy was reported in patients treated with placebo. Across clinical trials, including the long-term trial, malignancies excluding NMSC were reported in 7 patients (0.37 per 100 patient-years) treated with LITFULO 50 mg or higher.
Thromboembolic Events
Across clinical trials, including the long-term trial, PE was reported in 1 patient (0.06 per 100 patient-years) treated with LITFULO. There was 1 report of retinal artery occlusion and 1 report of acute MI.
Urticaria
In the placebo-controlled trials, for up to 24 weeks, urticaria was reported in 28 patients treated in all ritlecitinib doses studied and 3 patients treated with placebo. The rate of urticaria was 8.23 per 100 patient-years in patients treated with LITFULO 50 mg and 4.03 per 100 patient-years in patients treated with placebo. Across clinical trials, including the long-term trial, urticaria was reported in 76 patients treated with LITFULO 50 mg or higher. Among all patients treated with LITFULO 50 mg or higher in the integrated safety analysis, the rate of urticaria was 4.10 per 100 patient-years. The median time to onset of an initial event was 8 weeks; median duration of urticaria was 7 days. Most of the cases were mild to moderate in severity.
Decreased Lymphocyte Counts
Across clinical trials, including the long-term trial, confirmed ALC <500/mm3 occurred in 1 patient (<0.1%) treated with LITFULO 50 mg. Age appeared to be a risk factor for lower ALC in patients ≥65 years of age.
Decreased Platelet Count
In the placebo-controlled trials, for up to 24 weeks, treatment with LITFULO was associated with a decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which platelet count remained stable at a lower level with continued therapy. Across clinical trials, including the long-term trial, 1 patient (<0.1%) had a confirmed platelet count <100,000/mm3. No patient had a confirmed platelet count <75,000/mm3
CPK Elevations
In the placebo-controlled trials, for up to 24 weeks, events of blood CPK increased were reported in 2 (1.5%) patients treated with LITFULO 50 mg and 0 patients treated with placebo.
Liver Enzyme Elevations
In the placebo-controlled trials, for up to 24 weeks, events of increases in liver enzymes ≥3 times the ULN were observed in patients treated with LITFULO.
DRUG INTERACTIONS
Effects of LITFULO on Other Drugs
CYP3A Substrates
Ritlecitinib is a CYP3A inhibitor. Concomitant use of ritlecitinib increases area under the curve (AUC) and Cmax of CYP3A substrates, which may increase the risk of adverse reactions of these substrates.
Consider additional monitoring and dosage adjustment in accordance with approved product labeling of CYP3A substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
CYP1A2 Substrates
Ritlecitinib is a CYP1A2 inhibitor. Concomitant use of ritlecitinib increases AUC and Cmax of CYP1A2 substrates, which may increase the risk of adverse reactions of these substrates. Consider additional monitoring and dosage adjustment in accordance with the approved product labeling of CYP1A2 substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
Effects of Other Drugs on LITFULO
CYP3A Inducers
Concomitant use of strong CYP3A inducer (e.g., rifampin) may decrease AUC and Cmax of ritlecitinib, which may result in loss of or reduced clinical response. Coadministration with strong inducers of CYP3A is not recommended.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry
If a patient becomes pregnant while receiving LITFULO, healthcare providers should report LITFULO exposure by calling 1-877-390-2940.
Risk Summary
Available data from clinical trials with LITFULO use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ritlecitinib to pregnant rats and rabbits during organogenesis caused fetotoxicity and fetal malformations at exposures equal to 49 and 55 times the maximum recommended human dose (MRHD) based on an AUC comparison, respectively.
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The estimated background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats, oral administration of ritlecitinib from gestation days 6 to 17 decreased fetal body weights and caused fetal skeletal malformations (malformed vertebrae and ribs) and variations (delayed ossification) at doses ≥175 mg/kg/day (49 times the MRHD based on AUC comparison). Maternal toxicity (lower body weights) was noted at 325 mg/kg/day (102 times the MRHD based on AUC comparison). There was no developmental toxicity at 75 mg/kg/day (16 times the MRHD based on AUC comparison).
In an embryo-fetal development study in pregnant rabbits, oral administration of ritlecitinib from gestation days 7 to 19 decreased mean fetal body weights and increased visceral malformations (malpositioned kidneys), skeletal malformations (supernumerary sternebrae, absent thoracic
arch, and/or fused thoracic centra), and skeletal variations (delayed ossification) at 75 mg/kg/day (55 times the MRHD based on AUC comparison). There was no developmental toxicity at doses up to 25 mg/kg/day (12 times the MRHD based on AUC comparison).
In a pre- and postnatal development study in rats, oral administration of ritlecitinib from gestation day 6 through lactation day 20 had no effects on pre- and postnatal development at doses up to 75 mg/kg/day (14 times the MRHD based on AUC comparison). At 175 mg/kg/day (41 times the MRHD based on AUC comparison), ritlecitinib caused adverse lower postnatal survival and lower offspring body weights, which correlated with delayed sexual maturation in both sexes. Bred females in the F1 generation also exhibited lower mean numbers of corpora lutea at 175 mg/kg/day.
Lactation
Risk Summary
There are no data on the presence of ritlecitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Ritlecitinib is present in the milk of lactating rats. When a drug is present in animal milk, it is likely that it will be present in human milk. Because of the serious adverse effects in adults, including risks of serious infection and malignancy, advise women not to breastfeed during treatment with LITFULO and for approximately 14 hours after the last dose (approximately 6 elimination half-lives).
Data
After a single oral 30 mg/kg dose of ritlecitinib to lactating rats, ritlecitinib concentrations in milk over time were higher than those in plasma. The mean milk to plasma AUC ratio was determined to be 2.2.
Pediatric Use
The safety and effectiveness of LITFULO for alopecia areata have been established in pediatric patients ages 12 years and older. A total of 181 pediatric patients ages 12 to <18 years were enrolled in alopecia areata clinical trials, with 105 pediatric patients ages 12 to <18 years with alopecia areata randomized in a pivotal, double-blind, placebo-controlled trial (Trial AA-I). Efficacy was consistent between the pediatric patients and adults. The adverse reaction profile in the pediatric patients was similar to adults.
The safety and efficacy of LITFULO have not been established in pediatric patients under 12 years of age.
Geriatric Use
No dose adjustment is required for patients ≥65 years of age.
A total of 28 patients enrolled in alopecia areata trials were 65 years of age and older, and none were 75 years of age and older. Clinical trials of LITFULO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients. As there is a higher incidence of infections in the elderly population in general, caution should be used when treating the elderly.
Hepatic Impairment
No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
OVERDOSAGE
LITFULO was administered in clinical trials up to a single oral dose of 800 mg. Adverse reactions were comparable to those seen at lower doses and no specific toxicities were identified. Pharmacokinetics data up to and including a single oral dose of 800 mg in healthy adult volunteers indicate that more than 90% of the administered dose is expected to be eliminated within 48 hours.
There is no specific antidote for overdose with LITFULO. Treatment should be symptomatic and supportive, and monitor patients for signs and symptoms of adverse reactions.
In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
This brief summary is based on LITFULOTM (ritlecitinib) Prescribing Information LAB-1469-0.5.
Issued: June 2023.
The product’s label may have been updated. For full Prescribing Information, visit LITFULOHCP.com.
LITFULO™ (ritlecitinib) capsules, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
(0.5)
3 (2.3)3 (1.4)
CPK increased 2 (1.5)0
zoster 2 (1.5)0
blood cell count decreased 2 (1.5)0
2 (1.5)0
Stomatitis
© 2023 Pfizer Inc. All rights reserved. PP-RIL-USA-0414 June 2023
CIBINQO™ (abrocitinib) tablets, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death; The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia. If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection. Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens. Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately.
INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Testing, Evaluations, and Procedures
Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
• Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO
• Viral hepatitis screening in accordance with clinical guidelines – CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C
• A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation.
Recommended Dosage
The recommended dosage of CIBINQO is 100 mg orally once daily. If an adequate response is not achieved with CIBINQO 100 mg orally daily after 12 weeks, consider increasing dosage to 200 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 200 mg once daily.
CIBINQO can be used with or without topical corticosteroids. If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
Recommended Dosage in Patients with Renal Impairment
Renal Impairment
CIBINQO dosage recommendation in patients with mild renal impairment (60-89 mL/minute) is 100 mg once daily. For patients with moderate renal impairment (30-59 mL/ minute), the recommended dosage is 50 mg once daily. CIBINQO is not recommended for patients with severe or End-Stage Renal Disease (ESRD). Severe renal impairment and End-Stage Renal Disease include patients on renal replacement therapy.
In subjects with mild and moderate renal impairment, if an adequate response is not achieved after 12 weeks, dose of CIBINQO can be doubled.
CIBINQO is not recommended for patients with severe renal impairment or ESRD.
Recommended Dosage in CYP2C19
Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Dosage Modifications due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19 reduce the dosage to 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Treatment Discontinuation due to Serious Infections or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Hematologic Abnormalities
• Discontinue CIBINQO if platelet count <50,000/mm3 and
follow with CBC until >100,000/mm3
• Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value
• Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value
• Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosing increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities.
DOSAGE FORMS AND STRENGTHS
• 50 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 50” on the other.
• 100 mg: Pink, round, film-coated tablet debossed with “PFE” on one side and “ABR 100” on the other.
• 200 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 200” on the other.
CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment.
WARNINGS AND PRECAUTIONS
Serious Infections
The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and benefits of treatment prior to initiating CIBINQO in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO. Tuberculosis Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation
Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information; Initial Approval: January 2022
CIBINQO™ (abrocitinib) tablets, for oral use
Mortality (continued)
treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with CIBINQO for atopic dermatitis.
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical studies of CIBINQO for atopic dermatitis.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in patients receiving CIBINQO in the clinical studies for atopic dermatitis.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
Laboratory Abnormalities
Hematologic Abnormalities Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a CBC. CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations
Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO.
Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter
elevations on cardiovascular morbidity and mortality has not been determined.
Immunizations
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Serious Infections
• Mortality
• Malignancy and Lymphoproliferative Disorders
Clinical Trials Experience
• Major Adverse Cardiovascular Events
• Thrombosis
• Laboratory Abnormalities
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO.
In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks.
The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%).
Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in the table below. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety profile of CIBINQO in the monotherapy and the combination trial(s) were similar.
Adverse Reactions from Placebo-Controlled Trials
Reported in ≥1% of CIBINQO Treated Subjects with Moderate to Severe Atopic Dermatitis and at Higher Rate than Placebo for up to 16 Weeks Weeks
Overall Infections In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events In the placebocontrolled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia, no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia In the placebo-controlled trials, for up to 16 weeks, confirmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the first 4 weeks of exposure.
Dermatitis
Abdominal
Abdominal
Herpes
Thrombocytopenia
Specific Adverse Reactions Exposure adjusted incidence rates were adjusted by trial size for all the adverse reactions reported in this section.
Lipid Elevations In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the final visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Retinal Detachment In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
0-16 CIBINQO 200 mg N=590 n (%a) CIBINQO 100 mg N=608 n (%a) PLACEBO N=342 n (%a) Nasopharyngitis 51 (8.7)75 (12.4)27 (7.9) Nausea 86 (14.5)37 (6.0)7 (2.1) Headache 46 (7.8)36 (6.0)12 (3.5) Herpes simplexb 25 (4.2)20 (3.3)6 (1.8) Increased blood creatine phosphokinase 17 (2.9)14 (2.3)5 (1.5) Dizziness 17 (2.9)11 (1.8)3 (0.9) Urinary tract infection 13 (2.2)10 (1.7)4 (1.2) Fatigue 8 (1.3)10 (1.6)2 (0.5) Acne 28 (4.7)10 (1.6)0 (0.0) Vomiting 19 (3.2)9 (1.5)3 (0.9) Impetigo 3 (0.5)9 (1.5)1 (0.3) Oropharyngeal pain 6 (1.0)8 (1.4)2 (0.6)
5 (0.8)7 (1.2)2 (0.7) Influenza 6 (1.1)7
8
Hypertension
(1.2)0 (0.0) Gastroenteritis
(1.3)7 (1.1)2 (0.6)
contact 3
(0.5)6 (1.1)1 (0.3)
pain upper 11 (1.9)4
(0.6)0 (0.0)
discomfort 7 (1.2)3
(0.5)1 (0.3)
zoster 7
(1.2)2 (0.3)0 (0.0)
9
Study size adjusted percentages b Herpes simplex also includes oral herpes, ophthalmic herpes, herpes dermatitis, genital herpes.
(1.5)0 (0.0)0 (0.0) a
CIBINQO™ (abrocitinib) tablets, for oral use
Specific Adverse Reactions (continued)
Creatine Phosphokinase Elevations (CPK) In the placebocontrolled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
Adolescent Subjects (12 to less than 18 years of age) The safety of CIBINQO was assessed in a trial of 284 subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis (Trial-AD-4). The safety profile of CIBINQO in these subjects, assessed through the initial treatment period of 12 weeks and the long-term period (213 with at least 52 weeks of abrocitinib exposure), was similar to the safety profile from trials in adults with atopic dermatitis.
DRUG INTERACTIONS
Effects of Other Drugs on CIBINQO
The table below includes drugs with clinically significant drug interactions affecting CIBINQO.
Clinically Significant Drug Interactions Affecting CIBINQO
Strong CYP2C19 Inhibitors
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors.
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9
Clinical Impact Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9.
Strong CYP2C19 or CYP2C9 Inducers
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response.
Intervention Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers.
Effects of CIBINQO on Other Drugs
The table below includes clinically significant drug interactions affecting other drugs.
Clinically Significant Interactions Affecting Other Drugs
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities
Clinical Impact
Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin).
Intervention Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO.
Antiplatelet Therapy Drugs
Clinical Impact Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia.
Intervention Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment are contraindicated with CIBINQO.
Pregnancy
Pregnancy Exposure Registry There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
Risk Summary Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 11 or 4 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Animal Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Animal Data In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossified metatarsals) were noted at 60 mg/kg/day (17 times the MRHD based on AUC comparison). In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (4 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (17 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (2.4 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/ day (11 times the MRHD based on AUC comparison).
Lactation
Risk Summary There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Animal Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5-6 elimination half-lives).
Animal Data Lactating female rats were orally administered a single dose of 10 mg/kg abrocitinib on lactation day 12. Abrocitinib AUC was approximately 5 times greater in milk than in plasma.
Females and Males of Reproductive Potential
Infertility Females Based on the findings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration.
Pediatric Use
The safety and effectiveness of CIBINQO in pediatric patients 12 years of age and older weighing 25 kg or more with atopic dermatitis has been established. In trials Trial-AD-1 and Trial-AD-2, 124 adolescent subjects 12 to less than 18 years old with moderate-to-severe atopic dermatitis were enrolled and randomized to receive either CIBINQO 100 mg (N=51), 200 mg (N=48), or matching placebo (N=25) in monotherapy. Additional 284 adolescent subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis, were enrolled and randomized to receive either CIBINQO 100 mg (N=95) or 200 mg (N=94) or matching placebo (N=95) in combination with topical corticosteroids in Trial-AD-4. Efficacy and adverse reaction profile were consistent between the pediatric patients and adults.
The safety and effectiveness of CIBINQO have not been established in pediatric patients below 12 years of age.
Juvenile Animal Toxicity Data In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day beginning on postnatal day 10 (approximately equivalent to a human infant) and continuing through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose-related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur. Abrocitinib produced adverse effects on bone development at all dose levels. Abrocitinib caused irreversible dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (0.8 times the MRHD based on AUC comparison).
Abrocitinib also irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (7.2 times the MRHD based on AUC comparison). At 75 mg/kg/ day (27 times the MRHD based on AUC comparison), paw fractures generally corresponded to limb impairment, a fractured tibia was noted in a single female. Irreversible bone findings have not been observed in older animals.
Geriatric Use
A total of 145 (4.6%) patients 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
A higher proportion of patients 65 years of age and older discontinued from clinical trials compared to younger patients. Among all patients exposed to CIBINQO, including the long-term extension trial, confirmed ALC <500/mm3 occurred only in patients 65 years of age and older. A higher proportion of patients 65 years of age and older had platelet counts <75,000/ mm3. The incidence rate of herpes zoster in patients 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of patients 18 to less than 65 years of age (3.44 per 100 patient-years).
Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30-59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min). This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement therapy. A dosage reduction in patient with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60-89 mL/min).
CIBINQO has not been studied in patients on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function. In clinical trials, CIBINQO was not evaluated in patients with severe (Child Pugh C) hepatic impairment.
CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates.
OVERDOSAGE
There is no experience regarding human overdosage with CIBINQO. There is no specific antidote for overdose with CIBINQO. In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
Rx only
This brief summary is based on CIBINQO™ (abrocitinib) Prescribing Information LAB-1424-2.0.
Issued: February 2023.
The product’s label may have been updated. For full Prescribing Information, visit CIBINQOPI.com.
See CIBINQO full Prescribing Information at CIBINQOPI.com.
© 2023 Pfizer Inc. All rights reserved. February 2023. PP-CIB-USA-0485
USE IN SPECIFIC POPULATIONS
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
MISSION STATEMENT: Access Dermatology aims to educate and empower the biologic coordinator by keeping them informed of the complex and everchanging drug and patient access landscape. Readers are engaged with editorial and lifestyle content equally suitable for dermatologic patients, so they too may gain a better sense of therapies and the patient services programs that can assist in their therapeutic journey.
EXECUTIVE DIRECTOR
Craig Schuette
EDITOR
Elizabeth Hole
CREATIVE DIRECTOR
Venera Alexandrova
ASSISTANT ART DIRECTOR
Lisa Servidio
PRODUCTION DIRECTOR
Communications regarding original articles as well as editorial suggestions for future issues should be addressed to Craig Schuette at cs@bcofdermatology.com. Any content forwarded to the publisher assumes no liability for the safety or return of unsolicited art, photographs, or manuscripts.
DISPLAY ADVERTISING: Contact Craig Schuette at cs@bcofdermatology.com.
7
content
ISSUE 6
September/October 2023 PRESIDENT Jonathan W. Moffly VICE PRESIDENT/BUSINESS Elena V. Moffly MOFFLY CUSTOM MEDIA 205 Main Street, Westport, CT 06880 telephone: 203-222-0600 email: mail@MofflyCustomMedia.com Follow the Biologic Coordinators of Dermatology FACEBOOK LINKEDIN
bcofdermatology.com
A magazine dedicated to supporting patient access with practical information and lifestyle
ACCESS DERMATOLOGY
|
© 2023 Access Dermatology and Biologic Coordinators of Dermatology. ALL RIGHTS RESERVED. The material in this publication is published by Moffly Custom Media and may not be reproduced or transmitted in any manner, in whole or in part, without the express written permission of Access Dermatology and Moffly Custom Media. NOTICE: The information contained within articles of this magazine represent the views and opinions of the original authors and do not necessarily represent the views and opinions of Access Dermatology or its affiliates. The mere appearance of content in the magazine does not constitute an endorsement by Access Dermatology or its affiliates. The content has been made available for informational and educational purposes only. Editorial advice is not specific, and readers are advised to seek medical, professional, or reimbursement help for individual circumstances. Access Dermatology hereby disclaims all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the content. CIRCULATION: To be added to the circulation, visit www.bcofdermatolgy.com/ magazine. REPRINTS: For educational, commercial, or promotional reprints, including author off-prints, please email contact@bcofdermatology.com.
CORRESPONDENCE:
Tim Carr Cover Art Painting by Andrew Wilkie, 2023. Image provided by Incyte Corporation.
FEATURES
12 Evolving Needs
The increasing importance of biologic coordinators in dermatology
22 Streamline the Process
Simplifying the job with technology-enabled workplace systems
66 Building a Note
Keys to success for getting biologic therapies approved
98 Topical Therapy
Psoriasis, Prior Authorizations and ZORYVE (ROFLUMILAST) CREAM 0.3%
COMMUNITY
32 Member Survey
Shop talk and key findings about the job
46 Dermatology Specialty Drug Guide
Resource page courtesy of OPTUM
49 BCoD Conference 2023
Thank you to our sponsors
70 BC Spotlight
Recognizing five committed biologic coordinators
LIFESTYLE
80 The Great Pumpkin
Get crafty and carve up some spooky fun
84 Mental Wellness
Six tips for maintaining your well-being this fall
90 A Little He Time
Discover the latest spa trends for men to feel youthful and healthy
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 8
contents
ACCESS DERMATOLOGY ISSUE 6


bcofdermatology.com 9 98 22 32 12
letter from the founder / Craig Schuette
Exp ding the BC Community
It feels like yesterday when BCoD brought biologic coordinators together for the first time in Charleston to share best practices, learn from industry experts, and meet peers who share in the fight for the patient. Since then, BCoD has grown to become a community that welcomes more than one new member per day. As a result, we have a deeper drive and capability than ever before to enhance education and training for the BC.
We share our passion with you: our members. In our 2023 survey, you showed your love for the field of dermatology and the battle for the script! Those fights translate into remarkable prior authorization approval levels. Furthermore, our coaching programs demonstrate a significant percentage increase in appeal and approval times and even a quicker transfer out of bridge.
And we're not letting up. Some of the developments that we are excited to share with our members include:
• CERTIFICATION: BCoD has collaborated with healthcare organizations to offer the most significant training program for individuals seeking BC certification.
• FIELD REIMBURSEMENT FINDER: Effective communication between BCs and FRMs/FRAs is crucial, and our locator tool has helped to improve it.
• CREATIVE PARTNERSHIPS: We're making connections and building relationships to bring more innovation to our members' workflows.
• BUILDING COMMUNITY:
Our education managers guide tough cases through group and one-on-one
coaching sessions to help you and your patients with drug use.
Our gratitude comes from those who believe in us and trust us to assist with workflow and patients. I want to personally thank the BCoD team and our many contributing educational managers and volunteers for creating a space of nourishment and community. And I want to thank our partners for equally trusting us with your programs and the patients they serve. The patient suffers if there are cracks in the process and misunderstanding of programs. Our members are armed with the information to enhance access and change lives through collaboration!
We are excited to see you in San Antonio. We were almost at total capacity when writing this (early September), but we'll reserve space for you! New to the agenda this year, we're holding a Partner Pavilion and a Tough Case Blitz to help you through those bottlenecked cases.
If you can't make it this year, rest assured that we'll bring you recaps of the information presented. If you still need to join BCoD, we hope you take the time to reach out to us online at bcofdermatology.com or speak directly with our education team to learn more.
We look forward to welcoming you to our community, and we honor the work you do for patients every day.
With appreciation,
CRAIG SCHUETTE



ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 10

Bringing collections that balance technical innovation and iconic design to fit the active lifestyle of today’s sophisticated and conscious woman.

EVOLV NG NEEDS
THE INCREASING IMPORTANCE OF BIOLOGIC COORDINATORS IN DERMATOLOGY

FRESHIDEASTOCK.ADOBE.COM
By Andrew Baker PA-C, MBA, WEST OHIO DERMATOLOGY
& Samantha Smith RN, WEST OHIO DERMATOLOGY

ABSTRACT
Biologic coordinators work in dermatology offices to increase access to innovative treatment options. The burdensome process to get a biologic medication approved and ready for patient use continues to become more complex. With the number of biologics medications expected to increase, the role of biologic coordinators will likely also increase. Healthcare providers recognize the utility of biologic coordinators and their ability to streamline many processes to enhance patient care. This article highlights the integral part biologic coordinators play within a dermatology office now and in the future.
bcofdermatology.com 13
“BCs play a central role in OVERCOMING THESE CHALLENGES TO PROVIDE ACCESS TO THESE AGENTS BY NAVIGATING BENEFITS, COMMUNICATING WITH INSURANCE COMPANIES and advocating for individual patients."

LITERATURE REVIEW
Over the last several years, there has been an increasing number of innovative therapy options and technologies available to dermatology health care providers (HCPs). The mechanism of action (MOA) of these novel biologic medications are readily applied toward individual patient care. For some diseases, there are numerous biologic medications available with varying routes of administration and MOA.
Along with the expanded use of biologics, the amount of time and labor for patients to successfully get access to these treatments is also increasing. The role of a biologic coordinator (BC) within a dermatology practice has been acknowledged and becoming recognized as a crucial staff member. The number of BCs are growing nationwide due to their versatility within a dermatology practice, along with the
need for improved patient care and patient satisfaction. These individuals have many roles within a dermatology office, all of which increase the flow of patients and enhance access to medication. With physician assistants (PAs) and nurse practitioners (NPs) prescribing increasingly more biologics, the need for BCs is paramount. In 2023, PAs and NPs accounted for 54% of prescribed biologics 1 (Chart on opposite page). Additionally, the amount of current phase 2 and phase 3 clinical trials in the dermatology space suggests the number of FDA approved biologics is only going to escalate 2.
BCs specialize in supporting dermatology offices by streamlining the process to get specialty medications approved and offer continued support tailored to the officespecific patient needs. BCs have been shown to modernize the process of getting patient therapy initiated by identifying potential issues to access and adherence.
Allowing both the office, patient, pharmacy and pharmaceutical company to have a single point of contact enhances patient satisfaction and improves patient outcomes. BCs face several challenges; however, as they work to ensure that
patients receive timely access to therapy. For example, patients do not always inform their providers when there have been changes in benefits or insurance carriers, which can result in denial or delayed access.
Currently, the two most common conditions associated with an increased number of biologics are psoriasis and atopic dermatitis. Understanding the immunological process of forming psoriasis or atopic dermatitis is integral to the prescriber. A comprehensive understanding of the disease process will certainly help dermatology HCPs better care for their patients. For example, for psoriasis, different classes of biologics are now available, including tumor necrosis factor (TNF)-α interleukin (IL) – 4, IL – 13, IL-12/23, IL-17 and IL-23 inhibitors. TNF-α inhibitors were the first biologics introduced for psoriasis treatment and include etanercept, infliximab, adalimumab and certolizumab.
The availability of biosimilars at a lower cost compared to originators is dramatically changing the landscape of patient access to treatment. In the coming years, studies will be ongoing to better identify subgroups of patients based on biomarkers for a
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 14
ANDREAS BERHEIDESTOCK.ADOBE.COM
more personalized treatment approach. The biosimilar medications will likely continue to flood the biologic market, further necessitating the need for BCs. The challenge of dealing with insurance companies who want to take charge of therapeutic decision-making for patients will be an ongoing issue, resulting in more denials and more time to get approvals.
HCPs have certainly noticed the approval process for biologics becoming increasingly cumbersome. The amount of insurance denials and excluded specialty medications can interfere with their ability to treat patients. HCPs are now being required to initiate prior authorizations, appeals and patient assistance programs. To get a biologic approved for the most affordable price, the patient, specialty pharmacy, insurance and dermatology office need to effectively communicate.
Prior to prescribing biologic medications, it is imperative the patient understands the process and anticipated timeline to
REFERENCES
ensure they will adhere to treatment plan. BCs are the point of contact for all the moving parts during this process. Insurance changes, lack of insurance coverage, and high co-pays can make it increasingly difficult for patients to begin and continue therapy. Without ongoing assistance, patients may put their treatment on hold or completely stop. Without an experienced BC in the dermatology office, it threatens HCPs ability to adequately treat patients due to the amount of time required to receive approval and shipment of these medications. BCs
play a central role in overcoming these challenges to provide access to these agents by navigating benefits, communicating with insurance companies and advocating for individual patients. BCs effectively communicate with pharmaceutical teams, such as reimbursement specialists and sales representatives, ensuring the appropriate resources required to treat patients with biologics is met.
Dermatology is a fast-paced area of medicine. HCPs, collectively, need congruency when prescribing biologics as well as consistent resource availability. Frequent checks on patients to confirm the receipt of their medication and reinforce the importance of medication adherence are imperative to successful treatment.
Streamlining the process of patient care has many positive outcomes for patients and the dermatology practice. Look for BCs to increase in number and become even more of an integral part of dermatology, both in and out of the office.
• IQVIA Institute for Human Data Science. Medicine use and spending in the U.S.: a review of 2018 and outlook to 2023. May 2019. www.iqvia.com/-/media/iqvia/ pdfs/institute-reports/medicine-use-and-spending-in-the-us---a-review-of-2018-outlook-to-2023.pdf. Accessed April 20, 2020.
• Drug Topics. (2023). The 2023 Dermatology Drug Pipeline. Retrieved from https://www.drugtopics.com/view/the-2023-dermatology-drug-pipeline

PA/NP 100% % of Biologic Prescriptions 80% 60% 40% 20% 0% Dermatologist % of treatment NNDANKOSTOCK.ADOBE.COM

CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.






IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death. The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia.
If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection.
Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral (including herpes zoster), and other infections due to opportunistic pathogens.





Avoid use of CIBINQO in patients with an active, serious infection, including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections or those who have resided or traveled in areas of endemic tuberculosis or endemic mycoses.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO.
Viral reactivation, including herpes virus reactivation (eg, herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves. Hepatitis B virus reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C.
To learn more about CIBINQO efficacy, visit CIBINQOhcp.com or scan QR code.
JAK=Janus kinase; AD=atopic dermatitis.
Committed to Patient Support
Helping patients unlock access and reimbursement support for CIBINQO™ (abrocitinib)

With the Copay Savings Card,
pay as little as $ 0 per month*
*Eligibility required. No membership fees. This is not health insurance. The maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. See full terms and conditions below.
Copay Savings Card: TERMS AND CONDITIONS
By using the Pfizer Dermatology Patient AccessTM Copay Savings Card, you acknowledge that you currently meet the eligibility criteria and will comply with the terms and conditions described below:
• You are not eligible to use this card if you are enrolled in a state or federally funded prescription insurance program, including but not limited to Medicare, Medicaid, TRICARE, Veterans Affairs health care, a state prescription drug assistance program, or the Government Health Insurance Plan available in Puerto Rico (formerly known as “La Reforma de Salud”).
• You must have commercial insurance. Offer is not valid for cash-paying patients.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for CIBINQO™ (abrocitinib) may pay as little as $0 per month. Eligible patients with commercial prescription drug coverage may receive a maximum benefit of $15,000 per calendar year, which is defined by the date of enrollment through December 31st of the enrollment year. After a maximum of $15,000, you will be responsible for paying the remaining monthly out-of-pocket costs.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for EUCRISA® (crisaborole) may pay as little as $10 per tube. Eligible patients with commercial prescription drug insurance coverage that does not cover EUCRISA may pay as little as $100 per tube. Individual savings are limited to $970 per tube. Individual patient savings are limited to $3,880 in maximum total savings per calendar year.
• This copay card is not valid when the entire cost of your prescription drug is eligible to be reimbursed by your commercial insurance plan or any other health or pharmacy benefit program.
• You must deduct the value of this copay card from any reimbursement request submitted to your commercial insurance plan, either directly by you or on your behalf.
• You are responsible for reporting use of the copay card to any commercial insurer, health plan, or other third party that pays for or reimburses any part of the prescription filled using the copay card, as may be required. You should not use the copay card if your insurer or health plan prohibits use of manufacturer copay cards.
• This copay card is not valid where prohibited by law.
• Copay card cannot be combined with any other savings, free trial, or similar offer for the specified prescription.
• Copay card will be accepted only at participating pharmacies.
• If your pharmacy does not participate, you may be able to submit a request for a rebate in connection with this offer.
• This copay card is not health insurance.
• Offer good only in the United States and Puerto Rico
• Copay card is limited to 1 per person during this offering period and is not transferable.
• A copay card may not be redeemed more than once per 30 days per patient.
• No other purchase is necessary.
• Data related to your redemption of the copay card may be collected, analyzed, and shared with Pfizer, for market research and other purposes related to assessing Pfizer’s programs. Data shared with Pfizer will be aggregated and de-identified; it will be combined with data related to other copay card redemptions and will not identify you.
• Pfizer reserves the right to rescind, revoke, or amend this offer at any time without notice.
• Offer expires 12/31/2025.
For questions or additional support, call 1-833-956-3376, write to Pfizer Inc. at PO Box 29387, Mission, KS 66201, or visit the CIBINQO website at www.CIBINQO.com or the EUCRISA website at www.EUCRISA.com.
Please see additional Important Safety Information and Brief Summary of full Prescribing Information on the following pages. For full Prescribing Information, including BOXED WARNING and Medication Guide, visit CIBINQOhcp.com.
TM
eligible commercially insured patients may Visit PDPACopayCard.com
& INDICATION
MORTALITY
In a large, randomized postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality (including sudden cardiovascular death) was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
MALIGNANCIES
Malignancies, including non-melanoma skin cancer (NMSC), were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
In a large, randomized postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA patients. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur.
THROMBOSIS
Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of overall thrombosis, DVT, and PE were observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and treat patients appropriately.
CONTRAINDICATION
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤ 81 mg daily), during the first 3 months of treatment.
LABORATORY ABNORMALITIES
Hematologic Abnormalities: Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia.
Prior to CIBINQO initiation, perform a complete blood count (CBC). CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations: Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy, and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
IMMUNIZATIONS
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines, including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
RENAL IMPAIRMENT
Avoid use in patients with severe renal impairment or end stage renal disease, including those on renal replacement therapy.
HEPATIC IMPAIRMENT
Avoid use in patients with severe hepatic impairment.
ADVERSE REACTIONS
Most common adverse reactions (≥ 1%) in subjects receiving 100 mg and 200 mg include: nasopharyngitis, nausea, headache, herpes simplex, increased blood creatine phosphokinase, dizziness, urinary tract infection, fatigue, acne, vomiting, oropharyngeal pain, influenza, gastroenteritis.
Most common adverse reactions (≥ 1%) in subjects receiving either 100 mg or 200 mg also include: impetigo, hypertension, contact dermatitis, upper abdominal pain, abdominal discomfort, herpes zoster, and thrombocytopenia.
Inform patients that retinal detachment has been reported in CIBINQO clinical trials. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision.
DRUG INTERACTIONS
Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO. See Prescribing Information for clinically relevant drug interactions.
USE IN PREGNANCY
Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Advise females of reproductive potential that CIBINQO may impair fertility.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
LACTATION
Advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose.
INDICATION
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable. Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
Please see full Important Safety Information throughout and full Prescribing Information, including BOXED WARNING, and Medication Guide.
IMPORTANT SAFETY INFORMATION
PP-CIB-USA-0665
© 2023 Pfizer Inc. All rights reserved. May 2023.
CIBINQO™ (abrocitinib) tablets, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death; The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia. If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection. Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens. Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately.
INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Testing, Evaluations, and Procedures
Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
• Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO
• Viral hepatitis screening in accordance with clinical guidelines – CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C
• A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation.
Recommended Dosage
The recommended dosage of CIBINQO is 100 mg orally once daily. If an adequate response is not achieved with CIBINQO 100 mg orally daily after 12 weeks, consider increasing dosage to 200 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 200 mg once daily.
CIBINQO can be used with or without topical corticosteroids. If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
Recommended Dosage in Patients with Renal Impairment
Renal Impairment
CIBINQO dosage recommendation in patients with mild renal impairment (60-89 mL/minute) is 100 mg once daily. For patients with moderate renal impairment (30-59 mL/ minute), the recommended dosage is 50 mg once daily. CIBINQO is not recommended for patients with severe or End-Stage Renal Disease (ESRD). Severe renal impairment and End-Stage Renal Disease include patients on renal replacement therapy.
In subjects with mild and moderate renal impairment, if an adequate response is not achieved after 12 weeks, dose of CIBINQO can be doubled.
CIBINQO is not recommended for patients with severe renal impairment or ESRD.
Recommended Dosage in CYP2C19
Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Dosage Modifications due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19 reduce the dosage to 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Treatment Discontinuation due to Serious Infections or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Hematologic Abnormalities
• Discontinue CIBINQO if platelet count <50,000/mm3 and
follow with CBC until >100,000/mm3
• Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value
• Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value
• Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosing increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities.
DOSAGE FORMS AND STRENGTHS
• 50 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 50” on the other.
• 100 mg: Pink, round, film-coated tablet debossed with “PFE” on one side and “ABR 100” on the other.
• 200 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 200” on the other.
CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment.
WARNINGS AND PRECAUTIONS
Serious Infections
The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and benefits of treatment prior to initiating CIBINQO in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO. Tuberculosis Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation
Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information; Initial Approval: January 2022
CIBINQO™ (abrocitinib) tablets, for oral use
Mortality (continued)
treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with CIBINQO for atopic dermatitis.
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical studies of CIBINQO for atopic dermatitis.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in patients receiving CIBINQO in the clinical studies for atopic dermatitis.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
Laboratory Abnormalities
Hematologic Abnormalities Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a CBC. CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations
Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO.
Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter
elevations on cardiovascular morbidity and mortality has not been determined.
Immunizations
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Serious Infections
• Mortality
• Malignancy and Lymphoproliferative Disorders
Clinical Trials Experience
• Major Adverse Cardiovascular Events
• Thrombosis
• Laboratory Abnormalities
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO. In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks.
The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%).
Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in the table below. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety profile of CIBINQO in the monotherapy and the combination trial(s) were similar.
Adverse Reactions from Placebo-Controlled Trials
Reported in ≥1% of CIBINQO Treated Subjects with Moderate to Severe Atopic Dermatitis and at Higher Rate than Placebo for up to 16 Weeks Weeks 0-16
Overall Infections In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events In the placebocontrolled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia, no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia In the placebo-controlled trials, for up to 16 weeks, confirmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the first 4 weeks of exposure.
Lipid Elevations In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the final visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Specific Adverse Reactions Exposure adjusted incidence rates were adjusted by trial size for all the adverse reactions reported in this section.
Retinal Detachment In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
CIBINQO 200 mg N=590 n (%a) CIBINQO 100 mg N=608 n (%a) PLACEBO N=342 n (%a) Nasopharyngitis 51 (8.7)75 (12.4)27 (7.9) Nausea 86 (14.5)37 (6.0)7 (2.1) Headache 46 (7.8)36 (6.0)12 (3.5) Herpes simplexb 25 (4.2)20 (3.3)6 (1.8) Increased blood creatine phosphokinase 17 (2.9)14 (2.3)5 (1.5) Dizziness 17 (2.9)11 (1.8)3 (0.9) Urinary tract infection 13 (2.2)10 (1.7)4 (1.2) Fatigue 8 (1.3)10 (1.6)2 (0.5) Acne 28 (4.7)10 (1.6)0 (0.0) Vomiting 19 (3.2)9 (1.5)3 (0.9) Impetigo 3 (0.5)9 (1.5)1 (0.3) Oropharyngeal pain 6 (1.0)8 (1.4)2 (0.6) Hypertension 5 (0.8)7 (1.2)2 (0.7) Influenza 6 (1.1)7 (1.2)0 (0.0)
8 (1.3)7 (1.1)2 (0.6)
contact 3 (0.5)6
pain upper 11 (1.9)4 (0.6)0 (0.0)
discomfort 7 (1.2)3 (0.5)1 (0.3)
zoster 7 (1.2)2 (0.3)0 (0.0)
9 (1.5)0 (0.0)0 (0.0)
Study size adjusted percentages b Herpes simplex also includes oral herpes, ophthalmic herpes, herpes dermatitis, genital herpes.
Gastroenteritis
Dermatitis
(1.1)1 (0.3) Abdominal
Abdominal
Herpes
Thrombocytopenia
a
CIBINQO™ (abrocitinib) tablets, for oral use
Specific Adverse Reactions (continued)
Creatine Phosphokinase Elevations (CPK) In the placebocontrolled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
Adolescent Subjects (12 to less than 18 years of age) The safety of CIBINQO was assessed in a trial of 284 subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis (Trial-AD-4). The safety profile of CIBINQO in these subjects, assessed through the initial treatment period of 12 weeks and the long-term period (213 with at least 52 weeks of abrocitinib exposure), was similar to the safety profile from trials in adults with atopic dermatitis.
DRUG INTERACTIONS
Effects of Other Drugs on CIBINQO
The table below includes drugs with clinically significant drug interactions affecting CIBINQO.
Clinically Significant Drug Interactions Affecting CIBINQO
Strong CYP2C19 Inhibitors
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors.
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9
Clinical Impact Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9.
Strong CYP2C19 or CYP2C9 Inducers
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response.
Intervention Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers.
Effects of CIBINQO on Other Drugs
The table below includes clinically significant drug interactions affecting other drugs.
Clinically Significant Interactions Affecting Other Drugs
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities
Clinical Impact
Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin).
Intervention Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO.
Antiplatelet Therapy Drugs
Clinical Impact Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia.
Intervention Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment are contraindicated with CIBINQO.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
Risk Summary Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 11 or 4 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Animal Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Animal Data In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossified metatarsals) were noted at 60 mg/kg/day (17 times the MRHD based on AUC comparison). In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (4 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (17 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (2.4 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/ day (11 times the MRHD based on AUC comparison).
Lactation
Risk Summary There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Animal Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5-6 elimination half-lives).
Animal Data Lactating female rats were orally administered a single dose of 10 mg/kg abrocitinib on lactation day 12. Abrocitinib AUC was approximately 5 times greater in milk than in plasma.
Females and Males of Reproductive Potential
Infertility Females
Based on the findings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration.
Pediatric Use
The safety and effectiveness of CIBINQO in pediatric patients 12 years of age and older weighing 25 kg or more with atopic dermatitis has been established. In trials Trial-AD-1 and Trial-AD-2, 124 adolescent subjects 12 to less than 18 years old with moderate-to-severe atopic dermatitis were enrolled and randomized to receive either CIBINQO 100 mg (N=51), 200 mg (N=48), or matching placebo (N=25) in monotherapy. Additional 284 adolescent subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis, were enrolled and randomized to receive either CIBINQO 100 mg (N=95) or 200 mg (N=94) or matching placebo (N=95) in combination with topical corticosteroids in Trial-AD-4. Efficacy and adverse reaction profile were consistent between the pediatric patients and adults.
The safety and effectiveness of CIBINQO have not been established in pediatric patients below 12 years of age.
Juvenile Animal Toxicity Data In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day beginning on postnatal day 10 (approximately equivalent to a human infant) and continuing through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose-related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur. Abrocitinib produced adverse effects on bone development at all dose levels. Abrocitinib caused irreversible dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (0.8 times the MRHD based on AUC comparison).
Abrocitinib also irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (7.2 times the MRHD based on AUC comparison). At 75 mg/kg/ day (27 times the MRHD based on AUC comparison), paw fractures generally corresponded to limb impairment, a fractured tibia was noted in a single female. Irreversible bone findings have not been observed in older animals.
Geriatric Use
A total of 145 (4.6%) patients 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
A higher proportion of patients 65 years of age and older discontinued from clinical trials compared to younger patients. Among all patients exposed to CIBINQO, including the long-term extension trial, confirmed ALC <500/mm3 occurred only in patients 65 years of age and older. A higher proportion of patients 65 years of age and older had platelet counts <75,000/ mm3. The incidence rate of herpes zoster in patients 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of patients 18 to less than 65 years of age (3.44 per 100 patient-years).
Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30-59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min). This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement therapy. A dosage reduction in patient with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60-89 mL/min).
CIBINQO has not been studied in patients on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function. In clinical trials, CIBINQO was not evaluated in patients with severe (Child Pugh C) hepatic impairment.
CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates.
OVERDOSAGE
There is no experience regarding human overdosage with CIBINQO. There is no specific antidote for overdose with CIBINQO. In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
Rx only
This brief summary is based on CIBINQO™ (abrocitinib) Prescribing Information LAB-1424-2.0.
Issued: February 2023.
The product’s label may have been updated. For full Prescribing Information, visit CIBINQOPI.com.
See CIBINQO full Prescribing Information at CIBINQOPI.com.
© 2023 Pfizer Inc. All rights reserved. February 2023. PP-CIB-USA-0485
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
© 2023 Pfizer Inc. All rights reserved. May 2023. PP-CIB-USA-0485
SIMPLIFYING DRUG APPROVALS AND PATIENT COMMUNICATION WITH TECHNOLOGY ENABLED WORKFLOW SYSTEMS
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 22 T R E A M L I N
T E S R E S
C
E H
P O
S
IBy Jay Mayers VICE PRESIDENT BUSINESS DEVELOPMENT - RXNEXUS™










n an era marked by groundbreaking advancements in health sciences, the field of medicine stands at the forefront of innovation. Novel specialty drugs, tailored to address specific medical conditions, offer hope to patients who were once left grappling with limited treatment options. However, this progress is often diminished by the overwhelming challenges that ensue when attempting to secure access to these specialized medications. The coordination of healthcare access for specialty drugs has emerged as a burdensome ordeal for clinics, exacerbated by patient tracking issues, and leaving patients in a state of perplexity. The maze of paperwork, phone calls and manual data entry has not only resulted in administrative inefficiencies but also led to delays in patients receiving the medications crucial for their well-being. The integration of technologybased practice management solutions, however, has paved the way for a more coordinated and patient-centric approach.
bcofdermatology.com 23
“Using
technology to streamline the medication approval process allows staff to spend less time on paperwork and more time with patients. Not only does this make the staff happier, but it also leads to more satisfied patients.”
–COLLIN M. BLATTNER, DO, FAAD
Clinics, traditionally seen as sanctuaries of healing, now find themselves entangled in a web of administrative complexities. The task of coordinating access to specialty drugs involves a convoluted dance between healthcare providers, pharmaceutical companies, insurance entities and regulatory bodies. Each step in this intricate process demands meticulous paperwork, hours of communication, and an unyielding persistence to ensure the seamless delivery of the required medication. The strain placed upon clinic resources by this bureaucratic battle diverts attention and resources away from the core mission of healthcare: to heal and alleviate suffering.
In this ever-evolving coverage environment, where precision and promptness can be the difference between a patient receiving treatment or ongoing suffering, the role of the biologic coordinator stands as a cornerstone to patient access and care. A crucial yet often overlooked aspect of this role’s success is the development of an access coordination strategy that leverages end-to-end workflow systems. Streamlined access coordination processes leveraging technological platforms with ongoing support can hold the potential to revolutionize the way BCs operate, save valuable resources, and ensure patients are connected to the process while facilitating improved quality of care for patients.
At its core, a technology-enabled workflow system for access coordination streamlines the intricate web of tasks, processes and
“The burden of medication approval and patient communication for specialty therapy is significant, even for our most experienced access coordinators and nursing staff. We are always looking for ways to improve the overall workflow for our staff and the patient experience. We have tried other technology solutions in the past without success. WHAT IS REQUIRED IS A TRULY END-TO-END WORKFLOW SUPPORT SYSTEM FOR OUR STAFF AND PATIENTS SUCH AS RxN EXUS to set expectations and manage their entire specialty therapy journey.”
–STEPHANIE BURKHOLDER, BSN, RN, DNC REGIONAL DIRECTOR, HEARTLAND DERMATOLOGY AND SKIN CANCER CENTER, PA
communications that biologic coordinators navigate on a daily basis. Beyond the enrollment process, staff can be supported in assisting therapy selection based on coverage, prior authorisations, appeals, renewals and connecting clinic tasks to patient engagement. Technology-enabled workflow systems help practices make access coordination less burdensome by synchronizing diverse elements to create seamless reimbursement operations. But the benefits extend far beyond operational fluidity.
A carefully optimized process leveraging a technology enabled workflow system enables BCs to allocate more time to patient-focused
activities. With administrative tasks efficiently managed, coordinators can engage in meaningful patient interactions, offering compassion, education and resources. Ongoing automated digital communications to patients foster patient engagement and reinforce educational information about the patient’s disease and prescribed therapy. This personal touch, reinforced by technology, not only nurtures patient-clinic relationships but also fosters an environment where patients feel heard, cared for and engaged, leading to increased patient satisfaction and loyalty.
Leveraging end-to-end workflow platforms, such as RxNexus™, to optimize clinic access
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 24
processes empower biologic coordinators to allocate resources with pinpoint accuracy. From patient tracking and communications to therapy initiation and renewal, every aspect is finely calibrated based on real-time needs. This precision prevents wastage, ensures equitable resource distribution, and it enhances the overall cost-efficiency of healthcare operations.
In a world driven by data and technology, optimized workflows provide BCs with accurate and up-to-date information at their fingertips. This information-driven approach enables coordinators to make informed decisions swiftly, adapting to changing circumstances while maintaining an unwavering focus on patient well-being. Data insights also pave the way for continuous improvement, allowing administrators to fine-tune processes for better patient outcomes over time.
The administrative burden associated with specialty medication access coordination on healthcare professionals is well-documented, often leading to burnout and decreased job satisfaction. An optimized access coordination workflow can significantly alleviate this burden by automating repetitive tasks, minimizing manual data entry, and providing intelligent notifications and reminders. Such technological support frees up valuable time and mental energy for coordinators to engage in more intellectually stimulating and impactful activities, which in turn reduce the clinic administrative costs.
Healthcare is a collaborative effort, requiring seamless communication and coordination among diverse teams and departments. Optimized access workflows provide a common platform for streamlined communication, ensuring that all stakeholders are on the same page. This enhanced collaboration fosters a sense of unity, reduces misunderstandings, and ultimately leads to a more synchronized and effective healthcare ecosystem. It further reduces the impact of
“At Clear Choice Dermatology we want to offer the best possible patient experience. This is often challenging with biologic and specialty medications due to challenges in medication approval. By incorporating software like RxNexus into our clinic workflow, we have been able to offer better care to our patients while making our processes more efficient.”
–NADIA VIZZA, MS, RHIA PRACTICE ADMINISTRATOR, CLEAR CHOICE DERMATOLOGY
administration on supporting prescribers and frees up their time to see more patients. The potential benefits of a technology enabled workflow system to optimize processes for biologic coordinators and patient communications are vast and transformative. From enriching patient experiences through enhanced communications and fostering data-driven decision-making to reducing administrative burdens and promoting seamless collaboration, an optimized workflow holds the power to elevate healthcare operations to new heights of efficiency and excellence. As the healthcare access landscape continues to evolve, embracing and harnessing the potential of technology optimized workflows will undoubtedly be a defining factor in delivering superior access to specialty medications and overall patient care.
“A technology and service to reduce the burden of coordinating patient, PBM, mandated specialty pharmacy, copay assistant and manufacturer-free drug programs with the dermatology office is something that all practices need today. THE PROCESS NEEDS TO BE TRACKED AND CLEAR TO THE PHYSICIAN, STAFF AND PATIENT ALIKE. We must evolve past the era of ‘you didn’t complete the right form’ or ‘guess which pharmacy can fill it.’ This solution is long overdue.”
–ANTHONY NUARA, MD, PHD






More than75% of commercially insured patient lives have coverage for OPZELURA®1
Covered at any tier. PA and other restrictions may apply. Coverage is plan specific and subject to change.







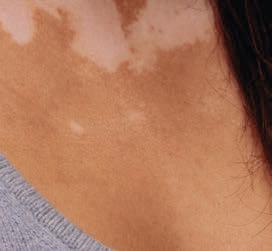



Not an OPZELURA® patient.
















For patients 12 years of age and older







The First and Only FDA-Approved Treatment for Repigmentation in Nonsegmental Vitiligo 2*



Proven to Promote Skin Repigmentation in Phase III Clinical Trials2


F-VASI75 Results at Weeks 24 and 52
Data From Week 24 And an Open-Label Extension
Nearly patients 1 in 3

~51%



Nearly1 in 3 patients achieved F-VASI75† at 24 weeks (primary endpoint; 29.9% vs 7.5% [P<0.0001] and 29.9% vs 12.9% [P<0.01]).2,3

About half the patients remaining in the studies who applied OPZELURA® from day 1 achieved F-VASI75 at 52 weeks: ~51% for OPZELURA® and ~28% for vehicle-to-OPZELURA® (Week 24 to Week 52).



Data were reported as observed. No conclusions of safety or efficacy should be made based on these results.2,4
Limitations of an open-label extension: In an open-label extension, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
OPZELURA® was studied in 2 double-blind, randomized, vehicle-controlled trials of identical design that enrolled 674 adult and adolescent patients with nonsegmental vitiligo ≥12 years of age. Patients had depigmented areas affecting ≥0.5% F-BSA, ≥3% nonfacial BSA, and total body vitiligo area (facial and nonfacial) of up to 10% BSA. Phototherapy was not permitted during the trials. In both trials, patients were randomized 2:1 to treatment with OPZELURA® or vehicle cream BID for 24 weeks followed by a 28-week open-label extension, wherein patients originally assigned to vehicle could switch to OPZELURA®. 2
*In patients 12 years of age and older.


BID, twice daily; BSA, body surface area; F-BSA, facial body surface area; FDA, Food and Drug Administration: F-VASI, Facial Vitiligo Scoring Index; F-VASI75, ≥75% improvement from baseline in Facial Vitiligo Area Scoring Index.
† F-VASI is a composite measurement of the overall area of facial vitiligo patches and degree of depigmentation within patches. As assessed, the face did not include surface area of the lips, scalp, eyelids, ears, or neck. 5





®2
CONSIDER OPZELURA





Patient Age
Indication

Tried and Failed Alternatives
Body Surface Area (BSA)
Combination Use

INDICATION
Common Prior Authorization Requirements
The patient must be 12 years of age or older.
OPZELURA® is indicated for nonsegmental vitiligo




This may include a topical corticosteroid (TCS) and/or topical calcineurin inhibitor (TCI). Be sure to include your patient’s most recent chart notes!
Indicate the BSA that will be treated:
• Nonsegmental vitiligo is up to 10%
Confirm OPZELURA® will not be used with:
• Therapeutic biologics
• Other JAK inhibitors
• Potent immunosuppressants




OPZELURA® was studied as a monotherapy treatment. Many plans do not approve combination use.
Turn the page to see the results with OPZELURA® at 52 weeks
OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.

Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death. Reported infections include:


• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.


Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled. Carefully consider the benefits and risks of treatment prior to initiating OPZELURA

in patients with chronic or recurrent infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA.


Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib.
No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.


Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.



Please see Important Safety Information continued on next page and Brief Summary of Full Prescribing Information, including Boxed Warning, on the following pages.












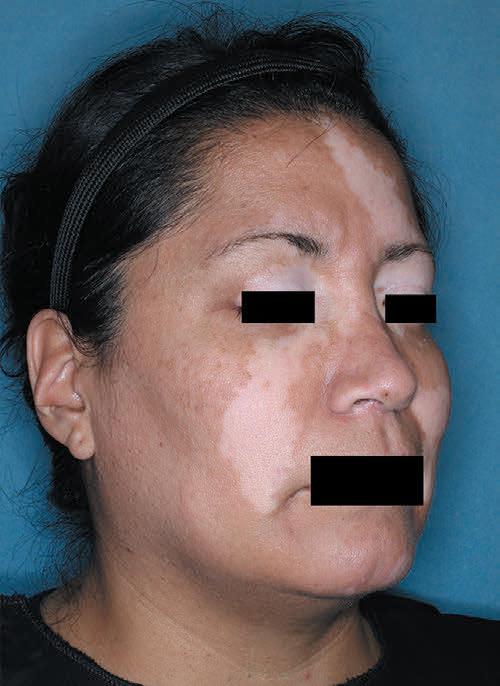

52-Week Results for a Clinical Trial Participant Whose Repigmentation Met the Primary Endpoint of F-VASI75 at 24 Weeks6‡





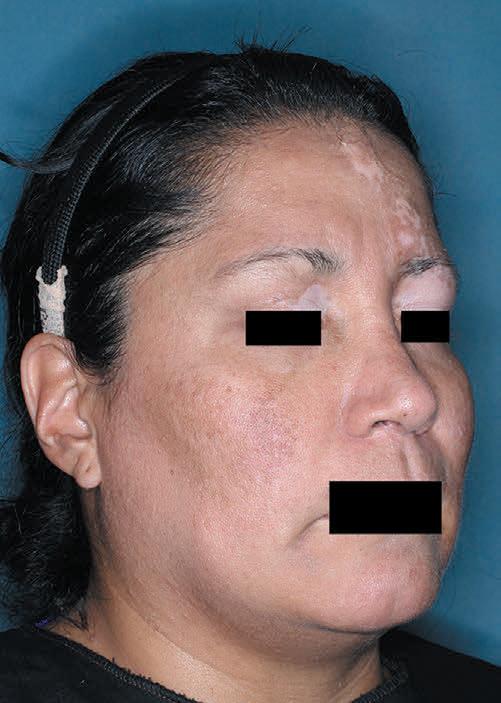



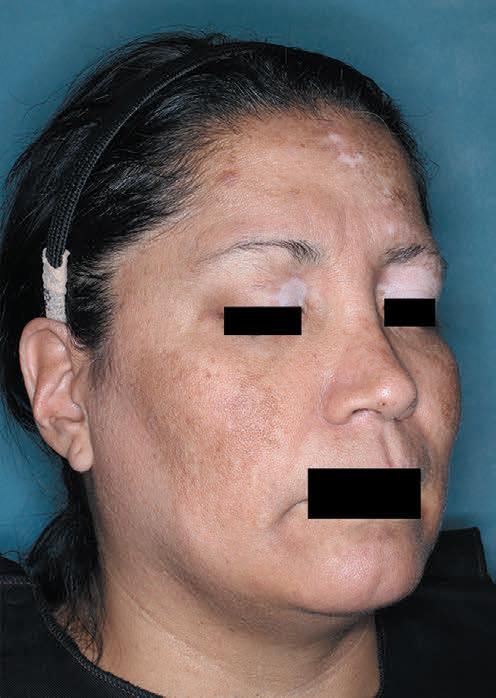


‡Results not typical. Actual patient treated with OPZELURA® in a clinical trial. Individual results may vary. Not for ophthalmic, oral, or intravaginal use.





See the Possibilities for Your Members With OPZELURA®
IMPORTANT SAFETY INFORMATION (continued)
MORTALITY



In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
MALIGNANCIES



Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin

cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers. Non-melanoma skin cancers, including basal cell and squamous cell carcinoma, have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.





W eek 0 F -VASI SCORE 1.5 W eek 24 F -VASI SCORE 0.3 80% Improvement From Baseline
ENDPOINT W eek 52 F -VASI SCORE 0.3 80% Improvement From Baseline BASELINE WEEK 24 WEEK 52
PRIMARY




IMPORTANT SAFETY INFORMATION (continued)
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)



In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
THROMBOSIS



Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately.

Thrombocytopenia, Anemia, and Neutropenia
Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations


Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Adverse Reactions
In nonsegmental vitiligo, the most common adverse reactions (incidence ≥1%) are application site acne (6%), application site pruritus (5%), nasopharyngitis (4%), headache (4%), urinary tract infection (2%), application site erythema (2%), and pyrexia (1%).



References:


Pregnancy
There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Lactation


Advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Please see Brief Summary of Full Prescribing Information, including Boxed Warning, on the following pages.

1. VANTAGE Fingertip Formulary, April 2023. 2. OPZELURA® (ruxolitinib) cream. Prescribing Information. Incyte Corporation. 3. Rosmarin D, Pandya AG, Grimes P, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo: 24-week results from 2 randomized, double-blind phase 3 studies. Abstract presented at: 30th European Academy of Dermatology and Venereology (EADV) Congress; September 29-October 2, 2021; virtual. 4. Rosmarin D, Passeron T, Pandya AG, et al. Efficacy and safety of ruxolitinib cream monotherapy for the treatment of vitiligo: results from two 52-week phase 3 studies. Presented at: American Academy of Dermatology Annual Meeting; March 25-29, 2022; Oral Presentation. 5. Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(suppl):1-121. 6. Data on File. Incyte Corporation.



OPZELURA, the OPZELURA logo, Incyte, and the Incyte logo are registered trademarks of Incyte.
© 2023, Incyte Corporation.
MAT-OPZ-01787 07/23














OPZELURA® (ruxolitinib) cream, for topical use
OPZELURA® (ruxolitinib) cream, for topical use
Brief Summary of FULL PRESCRIBING INFORMATION
Brief Summary of FULL PRESCRIBING INFORMATION
INDICATIONS AND USAGE: OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
INDICATIONS AND USAGE: OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
Limitations of Use: Use OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Reported infections include:
Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials. Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
The risks and benefits of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials. Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
The risks and benefits of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
MORTALITY
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
MALIGNANCIES
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat inflammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat inflammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
WARNINGS AND PRECAUTIONS
WARNINGS AND PRECAUTIONS
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to
Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke. Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total low-density lipoprotein (LDL) cholesterol, and triglycerides.
ADVERSE REACTIONS
ADVERSE REACTIONS
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%).
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%).
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, influenza-like illness, insomnia, nasal congestion, and vomiting.
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Pediatric Use: Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established.
Pediatric Use: Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These findings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, influenza-like illness, insomnia, nasal congestion, and vomiting.
DRUG INTERACTIONS
DRUG INTERACTIONS
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4) Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These findings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Geriatric Use: Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
Geriatric Use: Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
PATIENT COUNSELING INFORMATION
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4) Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
PATIENT COUNSELING INFORMATION
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions].
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Lactation
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions].
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions].
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Lactation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat inflammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions].
Thrombocytopenia, Anemia, and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Thrombocytopenia, Anemia, and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Specific Populations]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Specific Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Specific Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Specific Populations]
Manufactured for: Incyte Corporation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Manufactured for: Incyte Corporation
1801 Augustine Cut-off Wilmington, DE 19803
1801 Augustine Cut-off Wilmington, DE 19803
OPZELURA is a registered trademark of Incyte. All rights reserved.
U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870; 11219624
© 2022-2023 Incyte Corporation. All rights reserved.
Issued: July 2022 PLR-OPZ-00018-JUN23
OPZELURA is a registered trademark of Incyte. All rights reserved. U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870; 11219624 © 2022-2023 Incyte Corporation. All rights reserved. Issued: July 2022 PLR-OPZ-00018-JUN23

SURVEY Biologic Coordinator 2023



Our latest career report focuses on this important role, along with some key findings about the job and how the BC community helps patients get the care they need.

ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 32


WHO YOU ARE


bcofdermatology.com 33
ADVANCED DEGREE 93% FEMALE 7% MALE gender age ≤ 24 3% 25-29 9% 30-34 17% 35-39 17% 40-44 13% 45-49 14% ≥ 50 26% HIGH SCHOOL 11% 36% TWO-YEAR COLLEGE/ VOCATIONAL SCHOOL CERTIFICATE PROGRAM (MA) 30% FOUR-YEAR COLLEGE 3% 19% education
WHAT INSPIRES YOU?
Motivating factors to carry out the BC role
28% PATIENT OUTCOMES
19% OFFICE RELATIONSHIPS
13%
THE SCIENCE BEHIND MEDICINE
33% BEATING PAYORS TO GAIN APPROVALS
7%
COLLABORATING WITH SPECIALTY PHARMACIES AND DRUG MANUFACTURERS
“Being a resource for patients to provide a better quality of life.”
“Helping patients get the medication they need and seeing how it changes their lives!”
“Patient access and relationships—Iʼm here for the patient.”
“Beating payors to gain approvals and the patients, especially when they come back and show you the biologic that you worked on paid off because their condition has cleared.”
“Helping patients with severe disease navigate the system and gain access to a life-changing drug. Particularly the elderly medicare patients, as the system is (in my opinion) designed to make them give up on getting the medications due to cost.”
“Helping patients navigate a complex process of insurance, pharmacies and manufacturers.”
Do you consult dermatologists when it comes to the patient access landscape?
time spent working in access role
number of managedpatients per month
26%
38%
26%

10%
70% YES
30% NO
6% 18% 21% 35% ≤ 1 year 2-5 6-10 ≥ 10 ≤ 20 PATIENTS
≥ 100 PATIENTS
21-50 PATIENTS
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 34
51-100 PATIENTS
Do you manage patient access outside of biologics?
86% NO How many prior approvals do you have per month?
PATIENT ACCESS
Biggest challenges getting medication into patients’ hands...
“Doctors not working with staff to address or work around insurance company requirements but rather rely on the appeal of “I’M A DOCTOR...THEY ARE NOT.”
“Insurance step therapy requirements and specialty pharmacy communication.”
“My Specialty Pharmacy Accredo’s 5-8 business day script processing time.”
35%
INSURANCE REQUIREMENTS documentation and notes; step therapy; PA process; appeal process
31%
COST: HIGH COPAYS accumulator/maximizer plans
12%
SPECIALTY PHARMACIES communication; prescription clarifications; ease of use
12%
PATIENT ENGAGEMENT not answering phones; not completing forms
<1%
MANUFACTURERS access to resources; benefit verifications’ bridge programs and process
What factors negatively affect your job satisfaction?
bcofdermatology.com 35
14% Working conditions: hours and office staff 6% Career advancement 22% Compensation 15% Challenges with patient communication 16% Specialty pharmacy interaction 17% Payor interaction
YES
50 – 100 25 – 50 ≥ 100 ≥ 25 24% 27% 31% 18%
14%

Any additional programs represented made up less than 1%.
Do you have OTC-product discussions with patients?
Do you patientsoversee and medications in more than one location?



How many access staff professionals are in your office?
OFFICE DYNAMICS SPECIAL NOTE:
MODERNIZING MEDICINE (EMA) 63 ALLSCRIPTS 3 NEXTECH 12 ATHENA 2 What Electronic Medical Record program are you utilizing in your practice? 56% 1 (Me) 21% ≥ 2 45% 2 26% YES 74% NO 54% YES 46% NO EPIC EZDERM 3 ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 36


Most visited media platforms

























Where do you look most to gain additional job-related information/learnings?
PEER-TO-PEER PROGRAMS CONFERENCES
MANUFACTURER REPRESENTATIVES
JOURNAL ARTICLES














































SELFRESEARCH



























Do dermatology magazine advertisements increase brand recall and awareness?
61% NO
39% YES
Do advertisements make you consider utilizing its drug access program or service?
50% NEARLY
Are you as interested in (and do you look for) advertisements in dermatology journals as much as you are focused on the articles?
33% YES








YouTube 4% 50% Facebook 9% 13% 11% 11% Instagram None TikTok LinkedIn 1% X
67% NO SAY YES
bcofdermatology.com 37 1 2 3 4 5
A first-in-class,* selective TYK2 inhibitor for moderate-to-severe plaque psoriasis1,2


INDICATION

Clearer skin



SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Please see additional Important Safety Information and Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
*TYK2 is a member of the Janus kinase (JAK) family.

STUDY DESIGNS1




Clearer choice
AT LAST...SOTYKTU
Superior skin clearance vs Otezla® (apremilast)†‡ in a once-daily, oral pill1
†POETYK PSO-1 (N=664) and POETYK PSO-2 (N=1020) were two, 52-week, multicenter, randomized, double-blind, placebo- and active (apremilast 30 mg twice daily)-controlled, Phase 3 studies to evaluate the safety and efficacy of SOTYKTU (6 mg once daily) in adult patients with moderate-to-severe plaque psoriasis who were eligible for systemic therapy or phototherapy. Patients had a body surface area (BSA) involvement of ≥10%, a Psoriasis Area and Severity Index (PASI) score ≥12, and a static Physician’s Global Assessment (sPGA) ≥3 (moderate or severe).
Both studies assessed the responses at Week 16 compared with placebo for the two co-primary endpoints:
• The proportion of patients who achieved at least a 75% improvement in PASI scores from baseline (PASI 75)
• The proportion of patients who achieved an sPGA score of 0 (clear) or 1 (almost clear)
There were multiple ranked secondary endpoints, including:
• The proportion of patients who achieved PASI 75 at Week 16 vs apremilast
STUDY RESULTS 1,3,4
‡Comparison between SOTYKTU and apremilast was a secondary endpoint.
Co-primary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs placebo: PSO-1: 58% (193/330) vs 13% (21/166), P<0.0001; PSO-2: 53% (271/511) vs 9% (24/255), P<0.0001
• sPGA 0/1 at Week 16 for SOTYKTU vs placebo: PSO-1: 54% (178/330) vs 7% (12/166), P<0.0001; PSO-2: 50% (253/511) vs 9% (22/255), P<0.0001
Select secondary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs apremilast: PSO-1: 58% (193/330) vs 35% (59/168); P<0.0001; PSO-2: 53% (271/511) vs 40% (101/254); P=0.0004.
SELECT IMPORTANT SAFETY INFORMATION
In the PSO-1 and PSO-2 trials, through Week 16, the most common adverse reactions (≥1% and higher than placebo) in patients taking SOTYKTU (n=840) were upper respiratory infections (19.2%), blood creatine phosphokinase increase (2.7%), herpes simplex (2.0%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%).
BSA=body surface area; PASI=psoriasis area and severity index; PASI 75=75% reduction from baseline in PASI; sPGA=static Physician’s Global Assessment; TYK2=tyrosine kinase 2.
Otezla® is a registered trademark of Amgen, Inc.
SOTYKTU tablet is not actual size.
Shown for illustrative purposes only.
Scan to learn more or visit SOTYKTUHCP.com
INDICATION
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Infections: SOTYKTU may increase the risk of infections. Serious infections have been reported in patients with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19. Avoid use of SOTYKTU in patients with an active or serious infection. Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing, have appropriate antimicrobial therapy initiated and be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex) was reported in clinical trials with SOTYKTU. Through Week 16, herpes simplex infections were reported in 17 patients (6.8 per 100 patient-years) treated with SOTYKTU, and 1 patient (0.8 per 100 patientyears) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent patient. During PSO-1, PSO-2, and the open-label extension trial, the majority of patients who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age. The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis (TB): In clinical trials, of 4 patients with latent TB who were treated with SOTYKTU and received appropriate TB prophylaxis, no patients developed active TB (during the mean follow-up of 34 weeks). One patient, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU. Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas: Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.
SOTYKTU (deucravacitinib) INDICATION AND IMPORTANT SAFETY INFORMATION
Rhabdomyolysis and Elevated CPK: Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
Laboratory Abnormalities: Treatment with SOTYKTU was associated with increases in triglyceride levels. Periodically evaluate serum triglycerides according to clinical guidelines during treatment. SOTYKTU treatment was associated with an increase in the incidence of liver enzyme elevation compared to placebo. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded.
Immunizations: Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition: It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
Most common adverse reactions (≥1% of patients on SOTYKTU and more frequently than with placebo) include upper respiratory infections, blood creatine phosphokinase increased, herpes simplex, mouth ulcers, folliculitis and acne.
SPECIFIC POPULATIONS
Pregnancy: Available data from case reports on SOTYKTU use during pregnancy are insufficient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Report pregnancies to the BristolMyers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Lactation: There are no data on the presence of SOTYKTU in human milk, the effects on the breastfed infant, or the effects on milk production. SOTYKTU is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Hepatic Impairment: SOTYKTU is not recommended for use in patients with severe hepatic impairment.
SOTYKTU is available in 6 mg tablets.
Please see Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
References: 1. SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 2. Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11(5):1763-1776.
3. Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 4. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51.
SOTYKTU and the related logo are trademarks of Bristol-Myers Squibb Company.
© 2023 Bristol-Myers Squibb Company. 1787-US-2300018 02/23
for oral use
Brief Summary of Prescribing Information. For complete prescribing information consult official package insert.
INDICATIONS AND USAGE
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use:
SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment Initiation
Evaluate patients for active and latent tuberculosis (TB) infection prior to initiating treatment with SOTYKTU. If positive, start treatment for TB prior to SOTYKTU use [see Warnings and Precautions]
Update immunizations according to current immunization guidelines [see Warnings and Precautions].
Recommended Dosage
The recommended dosage of SOTYKTU is 6 mg taken orally once daily, with or without food. Do not crush, cut, or chew the tablets.
Recommended Dosage in Patients with Hepatic Impairment
SOTYKTU is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations and Clinical Pharmacology (12.3) in full Prescribing Information] No dosage adjustment is needed for patients with mild to moderate hepatic impairment.
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU [see Warnings and Precautions]
WARNINGS AND PRECAUTIONS
Hypersensitivity
Hypersensitivity reactions such as angioedema have been reported in subjects receiving SOTYKTU. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU [see Adverse Reactions]
Infections
SOTYKTU may increase the risk of infections.
Serious infections have been reported in subjects with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19 [see Adverse Reactions]
Avoid use of SOTYKTU in patients with an active or serious infection.
Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with SOTYKTU. A patient who develops a new infection during treatment with SOTYKTU should undergo prompt and complete diagnostic testing; appropriate antimicrobial therapy should be initiated; and the patient should be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical trials with SOTYKTU. In the 16-week placebo-controlled period, herpes simplex infections were reported in 17 subjects (6.8 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent subject who received SOTYKTU. During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, the majority of subjects who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age.
The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Subjects with positive screening tests for hepatitis B or C, or chronic hepatitis B, or untreated hepatitis C were excluded from clinical trials. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis
In clinical trials, of 4 subjects with latent tuberculosis (TB) who were treated with SOTYKTU and received appropriate TB prophylaxis, no subjects developed active TB (during the mean follow-up of 34 weeks). One subject, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU.
Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients receiving SOTYKTU for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas
Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU (deucravacitinib) [see Adverse Reactions]
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.
Rhabdomyolysis and Elevated CPK
Cases of rhabdomyolysis were reported in subjects treated with SOTYKTU resulting in interruption or discontinuation of SOTYKTU dosing.
Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to treatment with placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Adverse Reactions].
Laboratory Abnormalities
Triglyceride Elevations - Treatment with SOTYKTU was associated with increases in triglyceride levels. The effect of this elevated parameter on cardiovascular morbidity and mortality has not been determined. Periodically evaluate serum triglycerides according to the clinical guidelines for hyperlipidemia while patients are receiving treatment with SOTYKTU. Manage patients according to clinical guidelines for the management of hyperlipidemia [see Adverse Reactions]
Liver Enzyme Elevations - Treatment with SOTYKTU was associated with an increase in the incidence of liver enzyme elevation compared to treatment with placebo. Liver serum transaminase elevations ≥3 times the ULN were reported in subjects treated with SOTYKTU. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded [see Adverse Reactions]
Immunizations
Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition
It is not known whether TYK2 inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
• Infections [see Warnings and Precautions]
• Malignancy including lymphomas [see Warnings and Precautions]
• Laboratory Abnormalities [see Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SOTYKTU was evaluated in two placebo- and active-controlled trials (PSO-1 and PSO-2) and an open-label extension trial in which subjects who completed PSO-1 or PSO-2 could enroll [see Clinical Studies (14) in full Prescribing Information]. In these clinical trials, a total of 1,519 subjects with moderate-to-severe plaque psoriasis who were candidates for systemic therapy or phototherapy received SOTYKTU 6 mg orally once daily. Of these, 1,141 subjects were exposed to SOTYKTU for at least one year.
In trials PSO-1 and PSO-2, 1,681 subjects were randomized to receive SOTYKTU 6 mg (840 subjects), placebo (419 subjects), or apremilast 30 mg twice daily (422 subjects). All subjects randomized to placebo switched to SOTYKTU at Week 16. All other subjects remained in their original treatment group until Week 24, at which point subjects could have continued on the same treatment or be switched to SOTYKTU or placebo. The mean age of subjects was 47 years. The majority of subjects were White (87%) and male (67%).
In the 16-week placebo-controlled period of the pooled clinical trials (PSO-1 and PSO-2), discontinuation of therapy due to adverse reactions in subjects who received SOTYKTU was 2.4%, compared to 3.8% for placebo.
Table 1 summarizes the adverse reactions that occurred in at least 1% of subjects in the SOTYKTU group and at a higher rate than the placebo group during the 16-week controlled period.
SOTYKTU™ (deucravacitinib)
tablets,
Table 1: Adverse Reactions that Occurred in ≥1% of Subjects with Plaque Psoriasis in the SOTYKTU (deucravacitinib) Group and More Frequently than in the Placebo Group in Trials PSO-1 and PSO-2 through Week 16
Adverse Reaction
a Includes upper respiratory tract infection (viral, bacterial, and unspecified), nasopharyngitis, pharyngitis (including viral, streptococcal, and unspecified), sinusitis (includes acute, viral, bacterial), rhinitis, rhinotracheitis, tracheitis, laryngitis, and tonsillitis (including bacterial, streptococcal)
b Includes oral herpes, genital herpes, herpes simplex, and herpes virus infection
c Includes mouth ulceration, aphthous ulcer, tongue ulceration, and stomatitis
d Includes acne, acne cystic, and dermatitis acneiform
Adverse reactions that occurred in <1% of subjects in the SOTYKTU group were herpes zoster.
Specific Adverse Reactions
Exposure adjusted incidence rates are reported for all the adverse reactions presented below.
Infections
In the 16-week placebo-controlled period, infections occurred in 29% of the SOTYKTU group (116 events per 100 person-years) compared to 22% of the placebo group (83.7 events per 100 person-years). The majority of infections were non-serious and mild to moderate in severity and did not lead to discontinuation of SOTYKTU.
In the 16-week placebo-controlled period, serious infections were reported in 5 subjects (2.0 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo. The most common serious infections reported during the 52-week treatment period were pneumonia and COVID-19.
Malignancies
During the 0-to-52-week treatment period of the two clinical trials, PSO-1 and PSO-2 (total exposure of 986 patient-years with SOTYKTU), malignancies (excluding non-melanoma skin cancer) were reported in 3 subjects treated with SOTYKTU (0.3 per 100 patient-years), including single cases each of breast cancer, hepatocellular carcinoma, and lymphoma after 24, 32, and 25 weeks of treatment, respectively.
During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, a total of 3 subjects (0.1 per 100 patient-years), developed lymphoma while receiving SOTYKTU after 25, 77, and 98 weeks of treatment.
Laboratory Abnormalities
Creatine Phosphokinase (CPK)
In the 16-week placebo-controlled period, increased CPK (including Grade 4) was reported in 23 subjects (9.3 per 100 patient-years) treated with SOTYKTU, and 5 subjects (4.1 per 100 patient-years) treated with placebo.
Liver Enzyme Elevations
Events of increases in liver enzymes ≥3 times the ULN were observed in subjects treated with SOTYKTU [see Warnings and Precautions]. In the 16-week placebo-controlled period:
• ALT elevations ≥3 times the ULN was reported in 9 subjects (3.6 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
• AST elevations ≥3 times the ULN was reported in 13 subjects (5.2 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
Decreased Glomerular Filtration Rate (GFR)
In the 16-week placebo-controlled period in subjects who had moderate renal impairment (eGFR 30-59 mL/min) at baseline, decreased GFR was reported in 4 subjects (1.6 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Two of the deucravacitinib-treated subjects had worsening of baseline proteinuria.
Lipid Elevations
Mean triglycerides increased by 10.3 mg/dL during the 16-week treatment period in subjects treated with SOTYKTU and by 9.1 mg/dL during the 52-week treatment period.
Safety Through Week 52
In PSO-1 and PSO-2, the exposure adjusted incidence rate of adverse reactions in subjects treated with SOTYKTU from Week 0 through Week 52 without switching treatment did not increase compared to the rate observed during the first 16 weeks of treatment.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data from case reports on SOTYKTU use during pregnancy are insufficient to evaluate a drugassociated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, no effects on embryo-fetal development were observed with oral administration of deucravacitinib to rats and rabbits during organogenesis at doses that were at least 91 times the maximum recommended human dose (MRHD) of 6 mg once daily (see Data)
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report pregnancies to the Bristol-Myers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Data
Animal data
Deucravacitinib was administered orally during the period of organogenesis at doses of 5, 15, or 75 mg/kg/day in rats and 1, 3, or 10 mg/kg/day in rabbits. Deucravacitinib was not associated with embryo-fetal lethality or fetal malformations in either species. These doses resulted in maternal exposures (AUC) that were 266 times (rat) or 91 times (rabbit) the exposure at the MRHD.
In a pre- and post-natal development study in rats, deucravacitinib was administered orally from gestation day 6 through lactation day 20, at doses of 5, 15, or 50 mg/kg/day. At 50 mg/kg/day, F1 offspring had reduced body weight gains during the pre-weaning period. After weaning, body weights of affected F1 offspring gradually normalized to control levels. No maternal effects were observed at 50 mg/kg/day (110 times the MRHD based on AUC comparison). No deucravacitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring were noted at doses up to 15 mg/kg/day (19 times the MRHD based on AUC comparison).
Lactation
Risk Summary
There are no data on the presence of deucravacitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Deucravacitinib is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU (deucravacitinib) and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Data
Animal Data
A single oral dose of 5 mg/kg radiolabeled deucravacitinib was administered to lactating (post-partum days 8 to 12) rats. Deucravacitinib and/or its metabolites were present in the milk of lactating rats.
Pediatric Use
The safety and effectiveness of SOTYKTU in pediatric patients have not been established.
Geriatric Use
Of the 1,519 subjects with plaque psoriasis treated with SOTYKTU, 152 (10%) subjects were 65 years or older and 21 (1.4%) subjects were 75 years or older.
During the Week 0-16 period, for those subjects (80 subjects ≥65 years old, including 12 subjects ≥75 years old) who received SOTYKTU without switching treatment arms, there was a higher rate of overall serious adverse reactions, including serious infections, and discontinuations due to adverse reactions compared with younger adults.
No overall differences in effectiveness of SOTYKTU have been observed between patients 65 years of age and older and younger adult patients.
Renal Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild, moderate, or severe renal impairment or in patients with end stage renal disease (ESRD) on dialysis [see Clinical Pharmacology (12.3) in full Prescribing Information]
Hepatic Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. SOTYKTU is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) [see Adverse Reactions and Clinical Pharmacology (12.3) in full Prescribing Information]
OVERDOSAGE
There is no experience regarding human overdosage with SOTYKTU. In case of overdose, consider contacting the Poison Help line (1-800-222-1222) for additional overdosage management recommendations. The extent of deucravacitinib elimination by hemodialysis was small (5.4% of dose per dialysis treatment), and thus hemodialysis for treatment of overdose with SOTYKTU is limited.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of deucravacitinib was assessed in 2-year rat and 6-month rasH2 transgenic mouse studies. No evidence of tumorigenicity was observed in male or female rats that received deucravacitinib at oral doses up to 15 mg/kg/day (51 times the MRHD based on AUC comparison). No evidence of tumorigenicity was observed in male or female Tg.rasH2 mice that received deucravacitinib at oral doses up to 60 mg/kg/day.
Deucravacitinib was not mutagenic in a bacterial mutagenicity assay (Ames test) or clastogenic in an in vitro chromosomal aberration assay (cultured Chinese hamster ovary cells) or in an in vivo rat peripheral blood micronucleus assay.
In male rats, deucravacitinib had no effects on reproductive parameters (mating, fertility, and sperm morphology) or early embryonic development of their offspring at oral doses up to 50 mg/kg/day (224 times the MRHD based on AUC comparison).
SOTYKTU 6 mg once daily N=840 n (%) Placebo N=419 n (%) Upper respiratory infectionsa 161 (19.2) 62 (14.8) Blood creatine phosphokinase increased 23 (2.7) 5 (1.2) Herpes simplexb 17 (2.0) 1 (0.2) Mouth ulcersc 16 (1.9) 0 (0.0) Folliculitis 14 (1.7) 0 (0.0) Acned 12 (1.4) 1 (0.2)
In female rats, deucravacitinib had no effects on mating, fertility, or early embryonic parameters at oral doses up to 50 mg/kg/day (171 times the MRHD based on AUC comparison).
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) before starting SOTYKTU (deucravacitinib) therapy and each time the prescription is renewed, as there may be new information they need to know. Advise patients of the potential benefits and risks of SOTYKTU.
Hypersensitivity Reactions
Advise patients to discontinue SOTYKTU and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions]
Infections
Inform patients that SOTYKTU may lower the ability of their immune system to fight infections and to contact their healthcare provider immediately if they develop any signs or symptoms of infection [see Warnings and Precautions]
Inform patients that herpes infections, including serious, may occur with use of SOTYKTU [see Warnings and Precautions]
Malignancies including Lymphomas
Inform patients that SOTYKTU may increase their risk of developing malignancies including lymphomas. Instruct patients to inform their healthcare provider if they have ever had any type of cancer [see Warnings and Precautions]
Rhabdomyolysis
Inform patients that SOTYKTU may increase their risk of developing rhabdomyolysis. Instruct patients to immediately inform their healthcare provider if they develop unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Warnings and Precautions]
Laboratory Abnormalities
Inform patients that SOTYKTU (deucravacitinib) may affect certain lab tests, and that blood tests may be required before and during SOTYKTU treatment [see Warnings and Precautions]
Immunizations
Advise patients that vaccination with live vaccines is not recommended during SOTYKTU treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform the healthcare practitioner that they are taking SOTYKTU prior to a potential vaccination [see Warnings and Precautions]
Pregnancy
Advise patients to report their pregnancy to Bristol-Myers Squibb Company at 1-800-721-5072 [see Use in Specific Populations]
Manufactured by: Bristol-Myers Squibb Company Princeton, NJ 08543 USA
U.S. License No. 1713
© 2022 Bristol-Myers Squibb Company.
SOTYKTU and the Bristol Myers Squibb logo are trademarks of Bristol-Myers Squibb Company.
Issued: September 2022
1787-US-2200566 09/22
EDUCATION & NETWORKING EMPOWERS MEMBERS THROUGH Join our network of biologic coordinators landscape of drug access together. Sign up today at bcofdermatology.com Connect with BCoD:
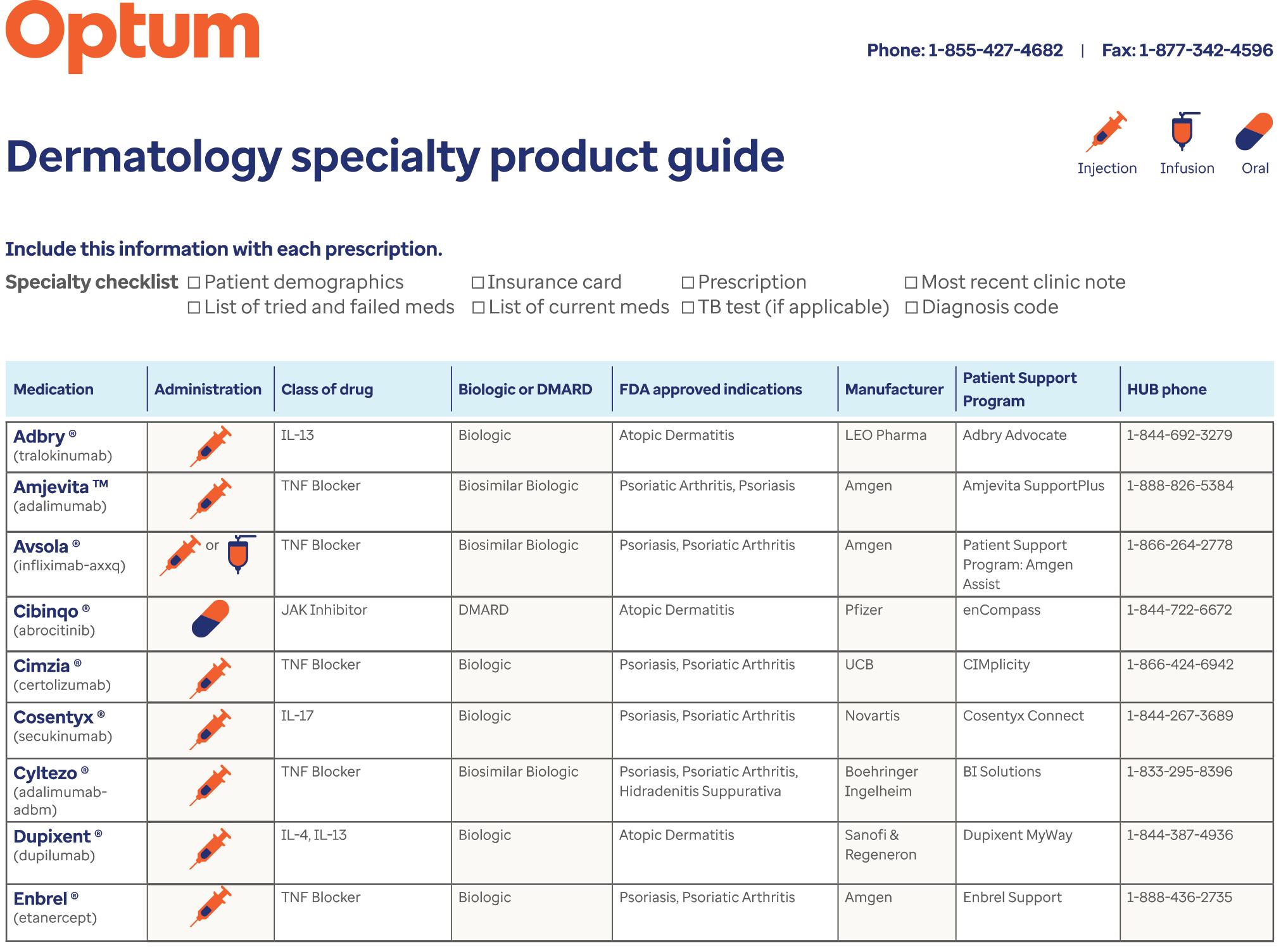
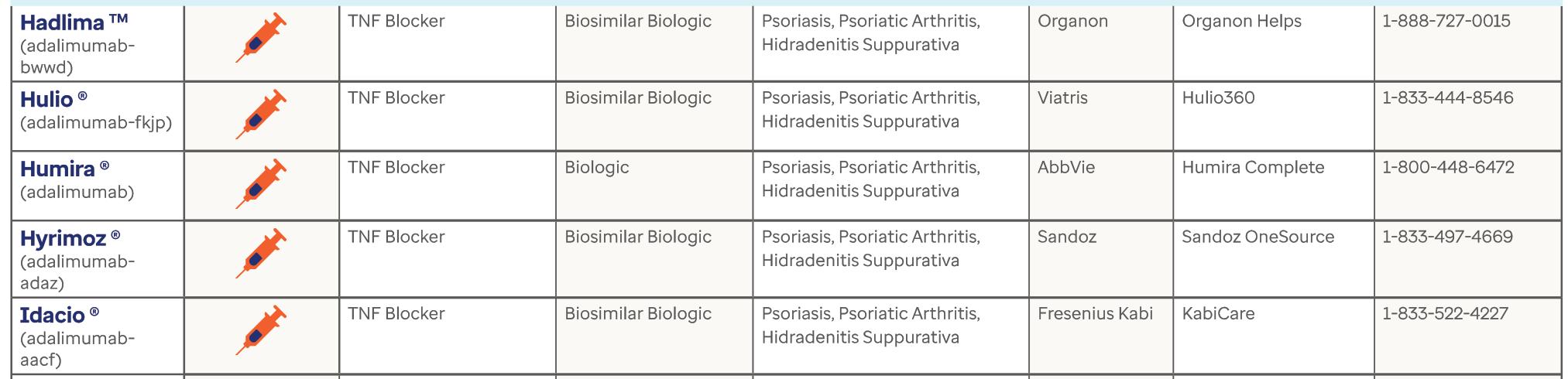
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 46
bcofdermatology.com 47

More Than Software – A Practice Management Solution for Biologics Coordination
Key Features
• Realtime updates for clinic team, patients and industry
• Single view dashboard of all patients for both coverage and follow up tasks throughout the entire journey
• Advanced patient engagement with alerts and noritications
• Improves patient outcomes
• Reduces time spent on paperwork and phone calls
• Prevents patients “lost to follow-up”
• Ensures that patient “follow through”
• And much more…
www.rxnexus.com
Streamline Medication Approval & Improve Patient Communication


Antonio, Texas • November 5 – 7, 2023 Make a Connection Make a Difference JW Marriott San Antonio Hill Country Resort CONFERENCE 2023
BC OD RAWPIXEL.COMSTOCK.ADOBE.COM; CONTRIBUTED
San
BCOD ANNUAL
Diagnosed with a Passion to Serve
Dear BCoD Members,
There's a saying that you should walk in someone's shoes to understand their experiences, challenges, and thinking. That advice reminds us to be empathetic to those around us. While you don't have to be a dermatology patient suffering from a skin disease, you can say it fuels my passion daily. BCoD is filled with incredible BCs who aim to improve the patient experience, as a community. I was in those shoes at a young age, and I remain a biologic patient to this day. So, it is personal for me. And it is truly our privilege to bring forth information to members aimed at educating, simplifying, and enhancing processes.
November is approaching, and I couldn't be more excited. The air is brisk, and my son is playing post-season football. Moreover, there is the BCoD Conference! In its third year, BCoD has seen tremendous growth and transformation, and our mission to educate and empower our members has never been stronger. Those in attendance last year can attest to the energy and engagement of BCs and the saturation of insightful content. I can't wait to see everyone in San Antonio. We say, "Together We Advance," and we mean it.
And to our members and industry partners, we thank you for taking the journey with us to advance patient access. You are vital to our mission, and we truly appreciate your partnership.
HEATHER SAWREY
President BCoD, Biologic Coordinator George Washington University, Dermatology
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 50
PRESIDENT'S LETTER
WHAT ARE YOU LOOKING FORWARD TO AT THE 2023 BCOD NATIONAL CONFERENCE?
“I'm looking forward to expanding my knowledge of the biologic industry and learning more helpful tips that I can bring back not only to my providers and staff, but also to my patients. We strive to give our patients top quality care and I believe that continuing to learn new techniques and staying informed on new information is key. Also looking forward to networking and meeting new BCs. Last year was my first year, and I've kept in touch and reached out when I needed it. Happy to be a part of such a great group of people that strive for the same goal.”
Stephanie Poznanski HIGHTOWER DERMATOLOGY SUN CITY CENTER, FL
“I’m very eager to learn and gather new information and apply it to help me work more efficiently in the field of patient access.
I look forward to learning the utilization of insurance, specialty pharmacy and manufacturer portals that can help my workflow and save me from spending countless hours on the phone. With manufacturer companies making changes to their bridge programs and insurance companies often updating their formularies, there are many changes to step edits and other requirements, and attending this conference opens up a great opportunity to learn from the BC community as to what is working well for our patients.
This will also create bonding, and learning their thoughts and sharing tips on documentation, insurance intricacies and streamlining the process of the workflow.”
Lisa
Morris Biologic Coordinator COASTAL SKIN CARE AND WELLNESS CENTER VICTORIA, TX
“I am looking forward to meeting and networking with other biologic coordinators to learn from them and see what their processes are for best practice to help increase efficiency and productivity to best help and serve the patients. I recognize the importance of these medications and the difficulties that face the patients and practices.
I think the more we can learn from each other and the nuances that we face, the more productive and efficient we can be.”
Leea Rink, Practice Manager DERMATOLOGY AND SKIN SURGERY ERIE, PA




51
bcofdermatology.com
HALFPOINTSTOCK.ADOBE.COM
We are grateful to these PARTNERS WHO SUPPORT the mission of advancing PATIENT ACCESS.
THANK YOU! Your information, resources, and technologies empower our members every day.
BC OD


































AS OF SEPTEMBER 1


ABBVIE abbvie.com
ABBVIE abbvie.com
AAbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. Follow @abbvie on Twitter, Facebook or LinkedIn.
bbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. Follow @abbvie on Twitter, Facebook or LinkedIn.
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 54 SPECIAL CONFERENCE PARTNER SECTION
Simply committed to patient support
We’re working to support eligible patients to access their medication by offering affordability and individualized assistance.

Please scan QR code to learn more about SOTYKTU 360 SUPPORT and to view the terms and conditions. 1-888-SOTYKTU (1-888-768-9588)


SOTYKTU and the related logo are trademarks of Bristol-Myers Squibb Company. © 2023 Bristol-Myers Squibb Company. 1787-US-2300608 08/23
bcofdermatology.com 55 SPECIAL CONFERENCE PARTNER SECTION


DERMAVANT dermavant.com

D@DERMAVANT
ermavant Sciences, a subsidiary of Roivant Sciences, is a biopharmaceutical company dedicated to developing and commercializing innovative therapeutics in immuno-dermatology. Dermavant’s focus is to develop therapies that have the potential to address high unmet medical needs while driving greater efficiency in research and clinical development. The company’s medical dermatology pipeline includes commercialized, late-stage and earlier-development

product candidates that target specific unmet needs in two of the largest growing immunodermatology markets, plaque psoriasis and atopic dermatitis, as well as other immunological and inflammatory diseases. Dermavant is marketing VTAMA® (tapinarof) cream, 1%, for the topical treatment of plaque psoriasis in adults. The FDA approved VTAMA cream for the topical treatment of mild, moderate, and severe plaque psoriasis in May 2022. For more information, please visit dermavant.com
SCIENCES
DERMAVANT
SPECIAL CONFERENCE PARTNER SECTION ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 56



GALDERMA galderma.com






Galderma is a pure-play dermatology category leader, present in approximately 90 countries. We deliver a science-based portfolio of premium brands and services that span the full spectrum of dermatology through Injectable Aesthetics, Dermatological Skincare and Therapeutic Dermatology. Through our purpose to advance dermatology for every skin story, we are dedicated to meeting individual consumer and patient needs with superior outcomes in partnership with healthcare professionals.
SPECIAL CONFERENCE PARTNER SECTION bcofdermatology.com 57



GENEFIC SPECIALTY PHARMACY: Redefining Personalized Healthcare

Genefic Specialty Pharmacy stands at the forefront of transforming personalized healthcare. With a clear and unwavering mission to redefine the Specialty Pharmacy experience by providing every patient with high-touch care, Genefic sets the standard in patientcentricity, process efficiency, and improvement of outcomes. This mission serves as a guiding light, ultimately propelling the pharmacy forward in its pursuit of excellence. Driven by a vision to become the epitome of a best-in-class Specialty Pharmacy solution, Genefic draws from more than 30 years
of knowledge and experience in the field. Genefic is well-prepared to lead the charge in reshaping healthcare delivery and holds a 50-state license while upholding all necessary URAC and NABP accreditations. The commitment to reduce workloads and ensure a hassle-free experience for all stakeholders underscores Genefic's dedication to setting new standards of quality.
Genefic's philosophy revolves around harnessing the power of technology to transform and improve processes and outcomes. By creating innovative solutions that enhance the lives of patients
and streamline operations for healthcare professionals, the company epitomizes the synergy of healthcare expertise and technological innovation.
Genefic Specialty Pharmacy is poised to revolutionize the specialty biologics space, promising a future where personalized healthcare is accessible, seamless, and patientfocused. With its unwavering mission, visionary goals, and innovative philosophy, Genefic Specialty Pharmacy stands as a beacon of progress in the healthcare industry.
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 58 SPECIAL CONFERENCE PARTNER SECTION


bcofdermatology.com 59 SPECIAL CONFERENCE PARTNER SECTION ACCESS DERMATOLOGY S EPTEMBER / O C T O BE R 202 3 Get the help you need.
may be treating patients for atopic dermatitis or nonsegmental vitiligo with a treatment from Incyte Dermatology. As you know,
have
–
authorizations – that
make patient access
of your workday. NAVIGATING PATIENT ACCESS Meet your Field Pharmacy Manager (FPM). Incyte FPMs are experts on insurance and reimbursement requirements. It’s easy to connect with your FPM.
Log into the BCoD
portal
Incyte and the Incyte logo are registered trademarks of Incyte. © 2023, Incyte Corporation. MAT-DRM-01041 08/23
You
Payers often
specific requirements
like prior
can
part
•
members
• Search “Incyte Field Pharmacy Manager Support” • Reach out to your FPM for the information and assistance you need to help your patients access their prescribed medication

Janssen Supports and Celebrates Biologic Coordinators Like You
Biologic coordinators are some of the most important advocates for patients in health care–yet too often, their work goes unrecognized. A biologic coordinator is an essential member of a medical practice’s office staff–a doctor, nurse, physician’s assistant, medical assistant and/or an office/practice manager–that helps patients navigate the sometimes complex process of obtaining access to prescribed biologic medications.
As a connecting force between the patient, prescribing healthcare provider, specialty pharmacy, manufacturer
and insurance company, biologic coordinators work hard to ensure patients are able to start and stay on their prescribed biologic treatments.
November 1, 2023, marks the third annual National Biologic Coordinators Day–a day established by The Janssen Pharmaceutical Companies of Johnson & Johnson to recognize the daily commitment of biologic coordinators who go above and beyond for the patients they serve. We are proud to shine a light on these unsung heroes of patient care.
© Janssen Biotech, Inc. 2023 08/23 cp-334516v2
BIOLOGIC
DAY NOVEMBER 1 SPECIAL CONFERENCE PARTNER SECTION ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 60
NATIONAL
COORDINATORS


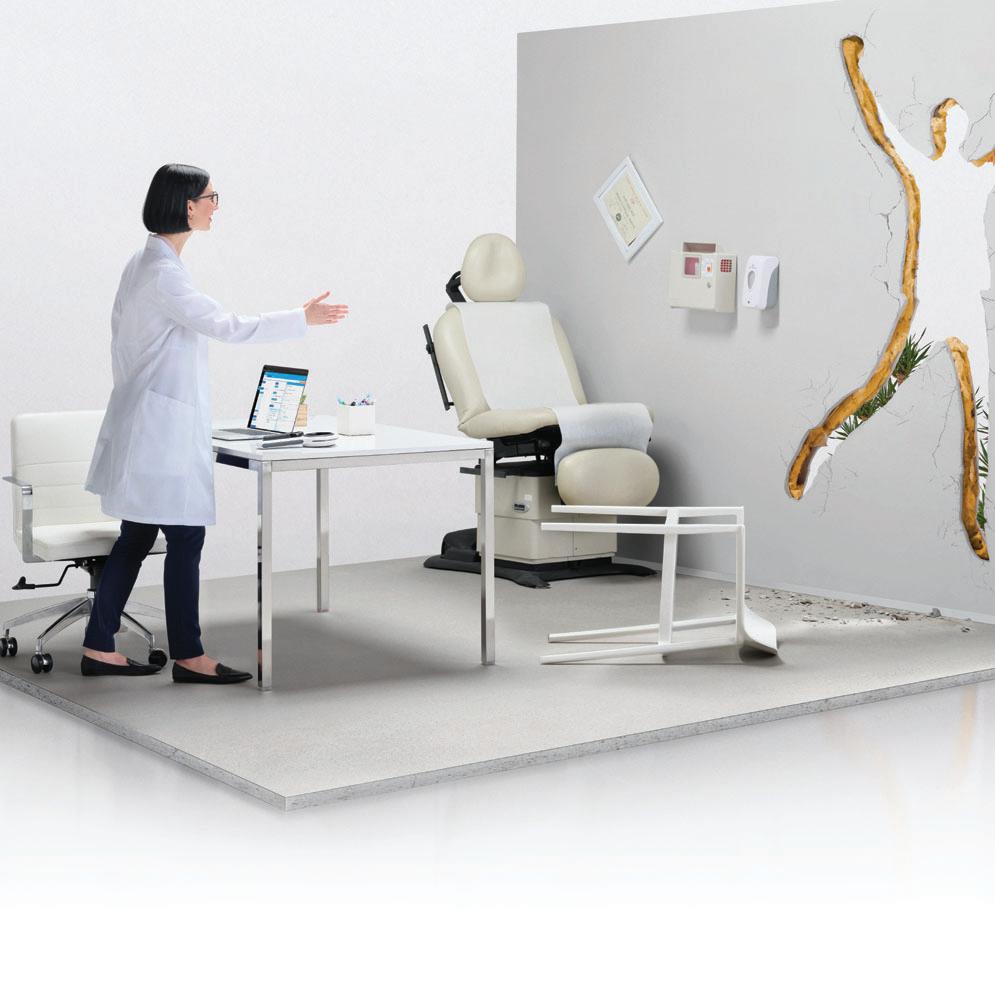





LILLY
1-800-545-5979 (1-800-LillyRx) lilly.com
olumiant.com
Lilly is a global healthcare leader that unites caring with discovery to create medicines that make life better for people around the world. Across the globe, Lilly employees work to discover and bring life-changing medicines to those who need them, improve the understanding and management of disease, and give back to communities through philanthropy and volunteerism. To learn more about Lilly, please visit lilly.com and newsroom.lilly.com/social-channels.

DISCOVER THE DIFFERENCE AT TALTZ.COM/HCP FDA Approved ELI LILLY AND COMPANY ELI LILLY AND COMPANY @ELILILLYCO SPECIAL CONFERENCE PARTNER SECTION bcofdermatology.com 61


NEXTECH
landing.nextech.com/bcod-demo

Nextech understands each physician has specific needs that must be fulfilled by their Practice Management and EMR vendor in order to meet and exceed the demands of their patients and the general healthcare industry. That is why Nextech continues to evolve its product with the thoughtful and strategic input of specialty physicians. Nextech’s Electronic Medical Record and Practice Management software is fully integrated to boost workflow efficiencies, manage practice revenue trends, enhance patient engagement and increase productivity through its innovative mobile platform.
SPECIAL CONFERENCE PARTNER SECTION ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 62


PFIZER DERMATOLOGY PATIENT ACCESS

The Pfizer Dermatology Patient Access™ program is designed to provide savings and support resources for patients, and to help them access the Pfizer dermatology medication that’s been prescribed for them. We can help patients along their dermatology treatment access journey by supporting them every step of the way.
SPECIAL CONFERENCE PARTNER SECTION bcofdermatology.com 63
Help eligible patients start and stay on track with their therapy




















The DUPIXENT MyWay® patient support program can help facilitate patient access to DUPIXENT® (dupilumab) throughout the treatment journey.
Coverage support
DUPIXENT MyWay provides assistance
navigating the insurance process with benefits investigations, prior authorization support, and education about the appeals process.
Patient access support
DUPIXENT MyWay may have support for eligible patients who need help with their out-of-pocket costs.
Education and support
Every enrolled patient is assigned a dedicated DUPIXENT MyWay Case Manager, who takes a patient-centric approach to providing tools, support, resources, and education throughout the treatment journey.
Support for your patients begins with a complete DUPIXENT MyWay Enrollment Form
You can help patients enroll in DUPIXENT MyWay by:
• Faxing the form to DUPIXENT MyWay at 844-387-9370
• Submitting the form through the DUPIXENT MyWay Document Drop at www.patientsupportnow.org
For more information:
Visit us on the exhibit floor
Call DUPIXENT MyWay at 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am – 9 pm ET
Learn more at DUPIXENThcp.com
For any questions or concerns, or to report side effects with DUPIXENT® (dupilumab), please contact 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am – 9 pm ET
DUPIXENT® and DUPIXENT MyWay® are registered trademarks of Sanofi Biotechnology.
© 2023 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved.
DUP.23.08.0466 09/2023
D UPIXEN ry (dupilumab) Injection
DUPIXENT myway) sanofi |



UCB ucb-usa.com
At UCB, we come together every day to work, laserfocused, on a simple question: How will this create value for people living with severe diseases? Patient value is not just what we say, but how we live. It is our culture of care, embodied by our patient value strategy. That’s because how we do business— from discovery to development to delivery—has been transformed and redesigned around the patient and
their individual experience. Patients are at the heart of everything we do, inspiring us, driving our scientific discovery, and leading us to rethink the patient experience. By fulfilling our commitment, driving innovation, and providing patients a meaningful experience, more impactful solutions are on the horizon.
With a team of approximately 8,300 employees and operations in nearly 40 countries, we are a global biopharmaceutical company
investing more than a quarter of our revenue in cutting-edge scientific research to meet unmet patient needs. Global headquarters are in Brussels, Belgium, with U.S. headquarters in Atlanta, Georgia. Additional U.S. UCB sites include R&D in Raleigh and Durham, North Carolina, Bedford and Cambridge, Massachusetts and Seattle, Washington, and a policy office in Washington, D.C.
SPECIAL CONFERENCE PARTNER SECTION bcofdermatology.com 65
A NOTE BUILDING
KEYS TO SUCCESS FOR GETTING BIOLOGIC THERAPIES APPROVED

MONKEY BUSINESSSTOCK.ADOBE.COM
By Kereen Crider BS, PACS, BIOLOGICAL COORDINATOR, PORTER PREMIERE DERMATOLOGY AND SURGERY CENTER


In a conversation with Dr. Anthony Porter of Porter Premiere Dermatology and Surgery Center in Melbourne, Florida, Biologic Coordinator Kereen Crider discusses why a stellar note is critical for obtaining approved biologic therapies.
“It is so important we aren’t just producing words on a page. The note should have substance and specificity. THE END GOAL IS TO GET THE PATIENT THE MEDICATION THEY NEED.”
—DR.
ANTHONY PORTER, PORTER PREMIERE DERMATOLOGY AND SURGERY CENTER
What is HPI?
HPI stands for “history of present illness.” This is important so that a complete narrative portrays what is going on with the patient. HPI is usually recorded by the medical assistant, and it’s a good idea to put everything in a patient’s own words to relay what they are currently experiencing. Plan to make this the well-rounded bulk of the note. The medical assistant should ask pertinent information related to the condition including disease symptoms, severity and length of time the patient has present symptoms.
67
bcofdermatology.com
Diagnosis Section
The next key part of the note a BC should look for is the diagnosis section. This is where the prescriber diagnoses the actual condition. It may also include a biopsy to confirm or substantiate the diagnosis. (Obviously, HS is a clinical diagnosis only.) When I speak with a patient, this is where good scribing comes in to play. I ask the patient to reiterate some of the questions they may have already been asked. For this portion of the note, use the acronym “OLD CARTS.”
OLD CARTS:
O NSET
L OCATION
D URATION
C HARACTERISTICS
A LLEVIATING AND AGGRAVATING FACTORS
(What triggers it?)
R ADIATING OR RELIEVING FACTORS
(What helps, what calms it down?)
T IMING
(How long have they had it?)
S EVERITY
(Constant or gradual?)

Disease-Specific Key Points Within the Note
Most insurances have standard information necessary to approve certain medications, so I make sure to address this within the note. BCs should keep an eye out for these and draw attention them. Examples of things to highlight, include:
20 NODULAR LESIONS FOR PN
SALT SCORE OF 50 OR HIGHER FOR ALOPECIA AREATA
PASI SCORE FOR PSORIASIS
BODY SURFACE AREA AND ITCH INTENSITY SCALE
IGA SCORE FOR ANY INFLAMMATORY CONDITION
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 68 MASTER1305STOCK.ADOBE.COM
BC PEARLS
Look for the provider cues that relay how the disease affects sleep patterns. (This is a big one. If your sleep patterns are off, it affects the patient’s quality of life immensely.)

Family history of the same condition.
Length and intensity of itching; itch scale. (10 being the worst)
What else affects the condition? (Hopefully this is documented, i.e. stress.)
Look for the documentation of significant discomfort and pain.
What Helps Aid Approvals?
A good HPI with a quality physical exam that documents the intensity and severity of the disease.
Clear documentation of what has been tried and failed for their disease specifically.
Quality of life. (Stress this point and highlight.)
If the provider can dictate a letter of medical necessity within the note or separately, this helps bolster the case.
Good communication between the provider and biologic coordinator. (I set aside time weekly to discuss with my BC any issues, denials or pharmacy faux pas, to come up with solutions that best fit a patient’s specific needs. This can include starting patient on samples, addressing reactions and signing appeal letters.)
Paint the best picture of your patient’s condition so that the insurance companies have no other choice but to approve the requested therapy.
bcofdermatology.com 69
Dr. Anthony Porter jumps for joy knowing biologics were approved for his patients.










RECOGNIZING
ECOGNIZING
BIOLOGIC COORDINATORS

Biologic Coordinators of Dermatology (BCoD) applauds our members and access managers who ensure a positive patient experience. These dedicated professionals are committed to their patients, helping them secure the types of therapy needed. For our fall issue, we would like to recognize four stand-out biologic coordinators through the words of their dermatology offices. BCoD salutes these individuals for their valiant effort in advancing patient access.
their























































DTammynison
BIOLOGIC COORDINATOR
DermSpecialists
A FOREFRONT DERMATOLOGY PRACTICE
Elizabethtown, KY
“I would like to nominate our team lead and biologic coordinator Tammy Dennison. She has worked in our office for over 15 years, and for the last 8-10 years, she has managed biologic access for patients. She is tireless in her efforts to gain access for patients and help them receive their needed medication.
I can think of many instances where she found ways to help get medication approved or get financial assistance for patients when it seemed that they were out of luck. She finds creative ways to help them and personally communicates progress with them. I often hear compliments from patients about how much she has helped them. I also hear accolades from drug company representatives that she is one of the best coordinators in their region or the country.”
—CHAD A BROWN, MD
bcofdermatology.com 71
LETTER of R ECOGNITION BC OD
LETTER of R ECOGNITION







































Patrice Kane
BIOLOGIC COORDINATOR
PENNSYLVANIA DERMATOLOGY GROUP, P.C.
Huntingdon Valley, PA
“The biologic coordinator position is integral to any successful dermatology practice. It’s also one of the hardest jobs in the dermatology practice. Our biologic coordinator, Patrice Kane, works tirelessly day after day spending hours on the phone with insurance companies to make sure our patients get the medication they need as quickly as possible. I know there are days that she is extremely frustrated, yet she continues to push forward, knowing that the job is bigger than her and the patients are the ones who suffer with any delayed access to medicines. This job is sometimes thankless, as the expectations of the patients are different than the reality of the timeline it takes to coordinate these medications and get approvals from insurance.
We are so appreciative of everything she does for our practice and everything that she does to fight for our patients. Our practice would not be the same without her. Although she knows this, we would love to highlight her as someone who has worked so hard and will continue to work hard for the betterment of our patients. Other biologic coordinators could learn a lesson from Patrice, as she has all the tricks from supplementing patients with samples, to writing stellar letters of appeal, to checking on status daily until she gets approvals.”
— LYNNE JASTRZEBSKI, OFFICE MANAGER
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 72
BC
OD







































BIOLOGIC COORDINATOR
NOMS DERMATOLOGY Sandusky, OH
“Verna goes above and beyond to make sure our patients continue to get their medications, whether that means spending hours on the phone with a pharmacy fighting for the patient, combing through the literature to find new and creative arguments for insurance companies to get patients coverage, or mastering the numerous patient assistance programs out there to make sure medications stay covered and affordable. Our patients and office staff enjoy working with her, and we are so lucky to have her on staff.”
— EMILY PETITTI, MD
bcofdermatology.com 73
Verna Maltese,
BC OD
LETTER of R ECOGNITION







































Ysabel Capuzi
BIOLOGIC COORDINATOR
DUNDEE DERMATOLOGY
West Dundee, IL
“It is delightfully satisfying to have the opportunity to nominate our biologic coordinator, Ysabel Capuzi, for recognition of her many years of contribution in her increasingly crucial role at Dundee Dermatology.
Medical staff and patients alike continually share their high esteem and confidence in Ysabel’s expert handling of all aspects of specialty medication coordination. What makes Ysabel stand out is her ability to connect with patients of all backgrounds, especially our large population of native Spanish-speakers.
As a Spanish speaker of Mexican heritage, Ysabel understands the cultural needs and challenges our non-English speaking patients face. She can explain complex treatment plans in a patient’s native tongue, ensuring full understanding. Patients immediately feel at ease with Ysabel and trust her guidance and her confident demeanor.
Beyond invaluable bilingual skills, Ysabel is unwaveringly dedicated to each patient. She helps them navigate a challenging, confusing and often adversarial system. In the past year, Ysabel confirmed insurance benefits, assisted with copay applications, coordinated injection training and handled all medication logistics for well over 100 patients, ensuring access to essential care.
Ysabel’s grace under pressure, reassuring and confident demeanor, and problem-solving skills are invaluable to our patients. Even when dealing with insurance denials or pharmacy delays, Ysabel handles every obstacle with professionalism and care, and she advocates fiercely for patients. Also, her commitment as a bicultural and bilingual biologics coordinator has increased health equity and the quality of care we provide.
Ysabel Capuzi deserves recognition and acknowledgement for her tireless advocacy for the medical needs of dermatology patients in a very diverse patient population.”
— PAUL GETZ, MD FAAD
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 74
BC OD
LETTER of R ECOGNITION
LETTER of R ECOGNITION







































Zue Dejesus
BIOLOGIC COORDINATOR GLICK SKIN INSTITUTE
Margate, FL
“I am Dr. Brad Glick, and I've been in practice for 28 years. We provide comprehensive care for our patients with immune-mediated diseases, and we utilize a lot of systemic therapies. I'd like to highly recommend a biologic/systemic therapy coordinator, Zue Dejesus, who is a dedicated staff member in my dermatology practice.
While Zue has been with our practice for less than a year, what she has accomplished is remarkable. Her turnaround time for most stomach therapies with a definitive answer is about 48 to 72 hours, sometimes even sooner. Moreover, she cares about patients, converses with them regularly and keeps them in the loop about the sometimes-challenging activities we face with drug approvals these days.
Additionally, in simplistic terms, as part of the mantra of our practice, she 'fights the fight' to get the systemic therapies for our patients.
I'd like to recognize Zue for her efforts, going far above and beyond for our practice and patients.”
— BRAD P. GLICK, DO, MPH, FAAD
bcofdermatology.com 75
BC OD
BIOLOGIC COORDINATORS OF DERMATOLOGY 2023
NATIONAL CONFERENCE

Join the largest community of biologic coordinators and office staff supporting the patient access journey. The BCoD conference is the premier experience for members and industry partners to come together for education, training, networking, and thoughtful insight exchanges.
CONFERENCE HIGHLIGHTS!
• First-of-its-kind Certification
• Industry Networking Pavilion
• 25+ Educational Talks
• Touch Cases Blitz
NOV 5–7
JW MARRIOTT COUNTRY HILL RESORT
SAN ANTONIO, TX
NO REGISTRATION FEE
Meet peers and market expects while making lasting industry connections! Register here today!








Redefining
Personalized Healthcare

A biologic-focused pharmacy experience like no other.



The Choice of Biologic Coordinators and a Proud Benefactor Sponsor of BCoD!


WHY GENEFIC SPECIALTY PHARMACY?
• LICENSED IN 50 STATES
•Optimal management across ALL patient populations
•Rigorous care management, patient engagement, and critical data reporting
•Cutting-edge technology for streamlined operations and improved efficiency
• Removes burden from office staff, effectively freeing up employees
Care for your complex patients, improve clinical quality and safety, harness clinical data, and manage associated costs more effectively.
NATIONALLY-LICENSED AND ACCREDITED

Office: (833) 928-7660
Fax: (833) 928-7661
www.GeneficRx.com
ELEVATED PATIENT CARE
UNMATCHED EXPERTISE
30+ years of providing specialty pharmacy services with proven results.
HASSLE-FREE EXPERIENCE
Streamlined processes and improved efficiency for patients and providers.
REGULAR COMMUNICATION
Constant communication and updates throughout the treatment journey.
PATIENT-CENTERED EXCELLENCE












Tailored, high-quality experience that enhances patient healthcare outcomes.
Genefic Specialty Pharmacy in redefining personalized healthcare! Rev.GSP_MM1016_09/11/23
Join
BIOLOGIC COORDINATOR NOMINATE A FOR THEIR SERVICE
Biologic Coordinators of Dermatology (BCoD) understands the commitment to patient care. Our members are at the forefront of access, helping achieve patients’ confidence within their skin.
BCoD would like to acknowledge biologic coordinators across the country who are advocates for their patients, work tirelessly to find solutions and resources to assist in drug access, and create an atmosphere focused on celebrating the patient.
To all the biologic coordinators working for the dermatology patient, we applaud you!
Please submit nominations to contact@bcofdermatology.com with a description and example of how the biologic coordinator demonstrates a dedication and work ethic for patient care.
Individuals will be highlighted in future publications.
Any content will be reviewed with offices for compliance and privacy purposes.





Guiding You Every Step of the Way
We offer personalized care for patients living with complex and chronic conditions, including:
> Limited distribution medications and biosimilars access


> Home infusion and support from our pharmacists and nurses
> Educational resources to help patients manage their medication


> Benefits investigation and financial assistance support





AcariaHealth.com
© 2023 AcariaHealth. All rights reserved. A2018_230404
more by
today
Learn
contacting us
Sales@AcariaHealth.com | 800.511.5144
the great
KNIVES OUT! ’TIS THE SEASON TO GET CRAFTY AND CARVE UP SOME SPOOKY FUN
pumpkin


KRIINA2000STOCK.ADOBE.COM
By Eileen Bartels
Bring on the pumpkin-spiced lattes, scary movies and pet costumes. Symbolic of every Halloween is the humble pumpkin. (More than 150 million Americans buy one every October.) The tradition of carving pumpkins dates back to ancient Celtic times, with the Irish bringing the practice to America. This year, join Linus in the pumpkin patch and get ready to create your very own great pumpkin.

PICK OF THE CROP
Selecting a fresh healthy pumpkin ensures longer lasting decor. The ideal pumpkin is firm to the touch with a deep orange color and hard rind. The greener the stem, the fresher the pumpkin. A stem that’s too dry or brown is an older pumpkin. Thump the side and listen for a hallow sound, indicating health. Look for a flat bottom to prevent rolling. And, obviously, avoid those with soft spots or visible decay. Also think outside of the round-pumpkin box. Many farmers grow heirloom pumpkins and gourds in a variety of colors and with rinds featuring bumpy skin—ideal for Halloween.

TOOLS OF THE TRADE
You’ll need a good pumpkin carving kit. Drugstores sell cheap plastic kits for under $10, but this year consider an upgrade. Amazon offers a number of higher-quality carving sets. The better the tool, the less chance the handle breaks mid-spooky smile cut. I purchased a kit from Williams Sonoma over a decade ago, and every October I roll out the orange canvas filled with every carving tool I need. A good kit will last you a lifetime of Halloweens.
81
bcofdermatology.com
PUMPKINS BY MICHAEL FLIPPOSTOCK.ADOBE.COM

HAVE A VISION
Once you have the tools, it’s time to look for inspiration. Are you going for a spooky haunted house vibe, social commentary or way to make first-time trick or treaters smile? Search the internet for ideas. You’ll find templates to carve or paint everything from Baby Yoda to Halloween classics like witches, cats or bats. Print your favorite template, tape it to the pumpkin and carve. Patterns can be found for both carving or painting.
GLITTER AND GLOW
Have a fear of combining sharp objects with small children? Painting a pumpkin is a great option—plus, the pumpkin will last a little longer. Depending on temperatures, a carved pumpkin will only last three to five days on the counter before drawing fruit flies or on the porch before becoming a squirrel motel.
The key to a lasting coat of paint on pumpkins is to mix tempura or kid-friendly washable paint with school glue in a 50/50 ratio, this allows the paint to stick to the pumpkin skin and prevents peeling and flaking.
Consider adding a little dazzle to your pumpkin with glitter. Let kids apply school glue and top with glitter.
Simple ideas can yield big designs. Paint a pumpkin white, and use a black marker to create a Hello Kitty face; stack a trio of white painted pumpkins and make Frozen’s Olaf; let the kids paint a pumpkin green, and you are just a few black Sharpie marks from Frankenstein.
For added oomph, Crayola makes neon paints that will stand out. And Glow Magic offers glow-in-the-dark paint. Swap a standard front porch light bulb with a black light bulb available at drug and hardware stores, then pair with a glow-in-the-dark painted pumpkin and you’re Halloween ready.

WHAT ’ S ON THE INSIDE COUNTS
If you’re carving your pumpkin, you’ll find a tasty treat inside. Once you gut and clean out the interior, don’t toss that treasure trove of goodness. Send the kids to the sink with a strainer to separate the seeds from the pulp. Rinse and dry the seeds. Toss the seeds in bowl with a little olive oil and seasoning. Spread on a cookie sheet and bake at 350 degrees for twenty minutes, shaking the pan occasionally to move seeds. Roasted pumpkin seeds are a great snack or salad topper.






ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 82 GOURDS © PIXARNOSTOCK.ADOBE.COM ; CHILD PAINTING PUMPKINS © KRIINA2000STOCK.ADOBE.COM




















































TAKE A NEW LOOK Incyte and the Incyte logo are registered trademarks of Incyte. © 2022, Incyte Corporation. MAT-DRM-00755 09/22 VISIT INCYTE AT THE 2023 BC o D ANNUAL CONFERENCE

IRISSCASTOCK.ADOBE.COM
By Dr. Derek Berberian NORTH JERSEY HEALTH AND WELLNESS
M tal

Wellness
6 TIPS FOR MAINTAINING YOUR WELL-BEING THIS FALL
Now that fall is here, the new season brings a variety of changes to our everyday routines. But we’re not just talking about swapping flips-flops for boots and iced coffee...

DON’T LET COOLER TEMPERATURES STOP YOU FROM GETTING OUTSIDE
Even if it’s a quick walk around the block, spending time outside to get some fresh air can do wonders for your mental wellness. Some of our favorite ways to enjoy the outdoors during the fall include picnics with hot apple cider, hiking on new trails, and going apple picking. The colors will be at their peak this time of year, so it should be easy to find something beautiful wherever you go!
1
IRISSCASTOCK.ADOBE.COM

SET “NO PHONE ZONE” TIMES FOR YOURSELF
Do you often feel like your phone is constantly occupying your time, whether that means scrolling social media platforms or checking work emails for hours at a time? It’s common for this to happen to many individuals, especially young adults who have shown an increase in mental health issues due to digital media over the last decade.
While a little technology is okay and occasionally necessary, the goal should be to moderate the amount of time you spend in front of the screen. Family dinners, road trips with friends, and date nights are just a few examples of when our

phone should stay out of sight, out of mind. By doing so, you will not only be more engaged in the present, but you will also be less preoccupied by things that don’t require your attention at that moment.
Fall is a time of change. But it is also a reminder that our mental health needs to be taken care of properly. These tips can help you maintain mental wellness at this turning point in the year, and we encourage you to share them with anyone who may need guidance when it comes to maintaining positive mental wellness in the fall season.
GET IN THOSE ZZZ’S!
Because it helps regulate hormones in the brain that affect our emotions, energy levels, cognitive abilities, appetite, and more, sleep plays a role in mental wellness. Sleep deprivation can also cause you to have trouble thinking clearly or remembering things, which makes it hard for your work performance at school or on the job. When this happens, it can cause a lack of confidence and lower self-esteem, which can take a toll on our mental health.
Therefore, creating a sleep schedule and sticking to it is so crucial for staying on top of our day-to-day. 3 2
TIM-FOSTER/UNSPLASH.COM; NEW AFRICASTOCK.ADOBE.COM

MOVE YOUR BODY!
Exercise is a key part of our physical, mental, and emotional wellness. Getting in 30 minutes of light, moderate, or vigorous activity can help improve your mood, reduce stress and anxiety, alleviate symptoms of depression, and strengthen the immune system. When you exercise your heart pumps blood throughout the body, which releases endorphins that make us feel good or “high.” Exercise also provides a sense of accomplishment for many people when they set personal goals like finishing a 5K or completing their first marathon. Yoga is another terrific way to get in a more relaxed workout, reset your inner well-being, and keep up your mental wellness in the fall.
4
STUDIO ROMANTICSTOCK.ADOBE.COM

MAINTAIN A WELL-BALANCED, NUTRITIOUS DIET
We all know that food can have a significant impact on our physical health. But did you know what we eat can also influence our mental health? For example, eating too much sugar causes mood swings, while fatty foods can slow down blood flow in your body. In contrast, eating a balanced diet that is rich in fruits and vegetables, whole grains, lean protein, and omega-3s may offer a mood boost and help you feel more grounded and less stressed. It can also give you the fuel you need to function at your best, making for more energized, productive days!
5 ELLA OLSSON/UNSPLASH.COM

SET BOUNDARIES FOR YOURSELF AND STICK TO THEM
6
It is important to set boundaries for your time so you can focus on giving your energy to what is most important and avoid feeling overwhelmed. Sometimes, it can be extremely difficult to say no to friends, social functions, or big plans that require a lot of your time. But the key is to remember times in the past when overstretching yourself caused you stress, anxiety, or frustration afterward. By creating a schedule that is healthy and maintainable, you’ll show others that you respect your time, which will teach them how to also respect your time.
To learn more about North Jersey Health and Wellness, visit njhwllc.com or contact (201) 588-3491.

helping people feel good in their own skin. This is one of many moments made possible by the inspirational work of Biologic Coordinators of Dermatology. Your tireless efforts make us glow with pride.
bcofdermatology.com 89
SNAPVAULTSTOCK.ADOBE.COM
LIVE AREA 8" x 5" 3213877-3919 | ©2023 Walgreen Co. All rights reserved.
It’s
This isn’t just about healthy skin.


COMING OFF-SCREEN AND BACK TO IN-PERSON MEETINGS AND DATING, GUYS WANT TO LOOK AND FEEL NOT LIKE THEIR OLD SELVES, BUT LIKE THEIR NEW, POST-PANDEMIC SELVES— YOUTHFUL, HEALTHY AND READY TO RE-ENGAGE WITH THE WORLD





BODY, Y MIND NDSPIRIT HE TIME















OUR WRITER TOM CONNOR HAD THE ROUGH JOB OF VISITING SOME SOUTHERN CONNECTICUT SPAS TO FIND OUT JUST WHAT THE GUYS ARE UP TO.









RUSLAN GRUMBLESTOCK.ADOBE.COM ; OPPOSITE PAGE BY VALUA VITALYSTOCK.ADOBE.COM


We’ve been looking at ourselves on-screen for more than three years, and it's not always pretty. The pandemic has left many men with more wrinkles and weight, less hair and energy, and a general disapproval of the way we look.
“Studies show that the increase of webinars and virtual meetings have led to an increase in facial dissatisfaction overall,” notes Kim Nichols, M.D., the celebrity dermatologist and owner of NicholsMD of Greenwich, CT.
So what? We’re guys! Who cares how we look? Well, it’s finally time to admit the obvious: We do! Women, of course, have long known both the benefits and the sublime pleasures of spa treatments. And for almost as long, they’ve been trying to get the men in their lives—husbands, boyfriends, fathers, brothers and sons—to experience them, too. »
Massage is just one of the popular options for men.
Making changes to one's face takes time— and a strategy.

Now, as guys are back to in-person meetings and dates in real time, we’re having to do so without Zoom’s “Touch up my appearance” feature or the filter that smooths wrinkled skin. In fact, we’re steadily leaving virtual rooms and showing up in lightfilled, calm and soothing spas in record numbers. According to the International SPA Association, men’s presence in spas has shot up from 31 percent ten years ago to 47 percent today.
In the past, guys had to travel to the grand spas of Europe for aesthetic treatments or to only a handful of iconic American spas— The Golden Door in Southern California during Men’s Week, for example, or the Homestead in Hot Springs, Virginia (full, obnoxious disclosure: I’ve been to both). But the intersection of Covid, prolonged screen time and men’s growing concern for their health and wellness has given rise not only to new aesthetic centers, but also to the opening of half a dozen or more medical spas across Fairfield County in the past two years alone.
Men can work on skin perfection with deep-cleansing facials.
SO WHAT? WE’RE GUYS! WHO CARES HOW WE LOOK?
WELL, IT’S FINALLY TIME TO ADMIT THE OBVIOUS: WE DO!
ANDREY KISELEVSTOCK.ADOBE.COM
THE ZOOM EFFECT
Launched in 2013, Zoom reported 200 million— both free and paying— daily meeting participants in March 2020, the first official month of the Covid pandemic. According to Business Insider, by the following month another 100 million users were in daily Zoom meetings. But enough about them! Seeing myself on Zoom one night earlier this year, I realized I looked like Ted Kaczynski, the Unabomber, the day the FBI pulled him from his remote cabin in rural Montana after more than two decades of living in isolation. I felt like turning myself in.
“The Zoom Effect is a real phenomenon,” says Merry Thornton, who opened Element Medical Aesthetics in New Canaan, CT, in March of this year. “The pandemic has increased sensitivity to looking old and tired.”
Bags under the eyes and flabby skin on the neck—these, she and others say, are the result both of the stress from pandemic isolation and from the virus itself.


GIVEN THE PROMINENCE OF THE FACE, AN IDEAL STARTING POINT FOR POST-ZOOM CARE IS A THIRTY-MINUTE HYDRAFACIAL, A DEEP-CLEANSING SPA TREATMENT THAT CLEANS, EXTRACTS AND HYDRATES THE SKIN AND NECK.

NICHOLSMD OF FAIRFIELD Fairfield, CT
NICHOLSMD OF GREENWICH Greenwich, CT
FACING OURSELVES
Given the prominence of the face, an ideal starting point is a thirty-minute Hydrafacial, a deep-cleansing spa treatment that cleans, extracts and hydrates the skin and neck. And an ideal spa for the treatment is NicholsMD of Fairfield, CT, the third office of celebrity dermatologist Kim Nichols, M.D., who, with perfect skin, has appeared on Today and The Dr. Oz Show. The new office opened in May of this year in the Brick Walk near Fairfield’s downtown. Alyson, a youthful-looking registered nurse, has me lie back on a comfortable white lounge chair. After she washes my face— something I clearly should be able to do myself—she uses a laser device with HydroPeel Tips and Vortex-Fusion technology on the skin. The device, I learn, creates a vortex-like effect to vacuum up dead skin and extract “debris,” as she calls it, from the pores.(A superficial detail that, nonetheless, preoccupies me: The “debris” from the vacuumed pores gets captured and collected in a trap. Trust me, you don’t want to know anything more about this.)
The final step in the treatment has her infusing the pores with nourishing serums and intense moisturizers and saturating the surface with antioxidants and peptides. I leave looking fabulous.

PAINTED-WALL SYNDROME
Faces are multifaceted, so medical spas like NicholsMD divide treatment sessions into facial units that require different solutions and that offset the potential danger of too much treatment in one area. The problem with these treatments, however, is similar to that of painting one wall of a room: By comparison, the other walls immediately cry out for repainting.
I make an appointment to see Merry Thornton at Element Medical Aesthetics.
Located in a second-floor, 2,000-square-foot space in New Canaan, CT, Element is super-clean, white, bright and uncluttered. After reviewing my medical history and concerns, Merry washes and applies numbing cream to my face and neck. It's a good thing, too, because she now runs the tip of an Ultra laser gun up, down and across the surface of my skin, a procedure that feels like a regiment of mildly agitated yellow jackets stinging me. No matter. The treatment leaves my face feeling warm and tingly and looking slightly sunburned, and far easier for me to face myself onscreen.
For more serious skin issues, Merry suggests the Genius, a system for administering radiofrequency microneedling— forty-nine tiny needles that penetrate the skin and emit radio frequency—to get rid of dead skin and encourage collagen, a structural protein that tightens it.
“It’s a bit uncomfortable,” she tells me. Actually, the Ultra treatment was “a bit uncomfortable,” so I think I’m good for now, I tell her. »
bcofdermatology.com 93
ELEMENT MEDICAL AESTHETICS New Canaan, CT
MIRROR,MONTEGO6STOCK.ADOBE.COM; ALL OTHERS CONTRIBUTED
below: The calming minimalism of Element Medical Aesthetics
BODY,MIND ANDSPIRIT
HAIR THERE, BUT NOT EVERYWHERE
While many men want hair added to thinning areas, others are interested in removing it from unwanted places.
Unless we’ve been wearing baseball caps in Zoom meetings, another noticeable fallout from the pandemic has been the fallout of our hair. Spa owners report an uptick in male clients inquiring about medical treatments to encourage hair growth in bald or thinning areas of the scalp.
One of the more popular treatments for hair restoration is PRP (platelet-rich plasma). For the procedure, a patient’s blood is drawn, spun in a centrifuge, then injected into the scalp with microneedling, which opens channels in the follicles to encourage hair growth. (Think of this as a bag of liquid Scott’s Turf Builder dumped onto the bald areas of the scalp then worked in with a sharpened spade.)
For unwanted body hair, medical spa personnel suggest laser treatments, in which the emission of light and heat damages hair follicles. The drawbacks are that it’s uncomfortable (some have likened it to a rubber band snapping against the skin), and it can be expensive. As many as six treatments may be required, to the tune of roughly $250 each treatment, and then permanent removal isn’t guaranteed.
A faster and far less expensive method of hair deforestation is traditional waxing. Here, strips of cloth dipped in hot wax are laid on a victim’s skin, then ripped off one strip at a time. (On second thought, I may opt for the snapped rubber bands!)
One new treatment at Dream Spa & Salon in Westport, CT, has owner Lori Dodd mincing words. “I don’t know if you’re ready for this,” she tells me, “but we’ll soon be offering manscaping in the form of manzillians”—in other words, Brazilians for men. (Note: I should have stopped Lori at “I don’t know if you’re ready for this.” I wasn’t.)

“I DON’T KNOW IF YOU’RE READY FOR THIS,” SHE TELLS ME, “BUT WE’LL SOON BE OFFERING MANSCAPING IN THE FORM OF MANZILLIANS— IN OTHER WORDS, BRAZILIANS FOR MEN.”

THE MASSAGE IS THE MESSAGE
Even before the pandemic, Stephanie Torres, the manager of the Delamar Greenwich Harbor Spa in Greenwich, CT, saw a significant number of men making appointments for a range of spa treatments, but especially massages—Swedish, Sports, Deep Tissue. “The kind of massage,” she says, “depends on whether clients want to relax or work on specific muscles and areas of the body that need to be stretched and massaged.”
What may be helpful for some guys to know is that spa treatments aren’t only for the high-powered Greenwich male. For years, one of Torres’s male clients let the gift certificates from his wife pile up before manning up and giving it a try. “He finally came in, and he was blown away,” she says. “Now he’s booking appointments every two weeks.” The gentleman’s occupation? Greenwich police officer, which makes a lot of sense: Standing in the middle of Greenwich
Avenue directing Range Rovers and well-heeled pedestrians is reason enough for regular, stress-reducing massages.
The problem with scheduling an appointment with Stephanie Torres? She’s so in demand that she’s booked out a month or even more.
Standing appointments for weekly or monthly massages is the norm among male clients at Artistex Salon & Spa in Westport, CT, which merged with Born of Earth Spa earlier this year. “A massage helps male clients de-stress,” says Anna, a masseuse at the spa for the past nine years. “Sitting in a chair all day hunched over a laptop can cause a lot of stress on the neck muscles and back muscles, even leg muscles. But beyond physical stress, there is mental and emotional stress, and I think men see the spa as somewhere they can get away from the world.”
Just off the busy Post Road near downtown, stepping into one of the massage rooms at Artistex feels like a full retreat from life outside. The room is narrow, the dark walls a relief from the stark white of medical spa treatment rooms.
I strip to shorts and lie under warm sheets on the massage table. Anna, who is Polish and has large, strong hands,
WAX STICK BY PIXEL-SHOTSTOCK.ADOBE.COM; MASSAGE BY HBSSTOCK.ADOBE.COM ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 94

“DEPENDS ON WHETHER CLIENTS WANT TO RELAX OR WORK ON SPECIFIC MUSCLES AND AREAS OF THE BODY THAT NEED TO BE STRETCHED AND MASSAGED.”
Not all massages are just about relaxation. Some work on athletic recovery.
RENEWABLE ENERGY
Athletically minded men are trying I.V. vitamin and hydration drip treatments at Elivate Med Spa.

ARTISTEX SALON & SPA Westport, CT DELAMAR GREENWICH HARBOR SPA Greenwich, CT
attended university to become a teacher but found her calling in this country when her son was diagnosed at age four with rheumatoid arthritis.
“I always hid my big hands,” she says, “until I realized why I was given them.” After seven years of massaging her son’s limbs and joints to relieve his pain, she accepted her calling to become a massage therapist.
Employing a mix of soft Swedish massage and harder deep-tissue massage, she uses long sweeping strokes to massage the back and leg muscles, and her fingers find and relieve tension knots in the shoulders and neck. “When we are stressed, we lower our heads and raise our shoulders,” she says.
The massage lasts an hour, though time is blurred by the background ambient music— wind, storms and waves mixed with flutes, strings and horns played, it seems to me, by Himalayan spirit sherpas in yurts blowing into yak antlers. Or something.
There’s a term spa workers use to describe the effect of multiple treatments on men and women: “Spa brain.” I think it may be kicking in, because all I want to do is lie on this massage table, talking to Anna and listening to this music.
Younger men are skipping hair treatments, and for good reason: All their hair is on their heads! Instead, they’re making appointments for I.V. vitamin and hydration drip bags.
“I.V. therapy was a hit, especially in the beginning of the pandemic, because everyone wanted to be as strong as possible in case they did get sick from Covid,” says Melissa PulciniButtine, the founder of Elivate in Old Greenwich, CT.
As at other spas across the county, men are coming on their own these days as opposed to being dragged in by the women in their lives.
Clients at Elivate lounge in comfortable leather recliners in one of two I.V. rooms and watch movies on a large flatscreen TV as the vitamins—zinc, vitamin C, vitamin D, vitamin B-12 and immune booster cocktails— course through their veins.
The vitamins are custom mixed based on a consultation with Pulcini-Buttine or on blood work done in the on-site lab. Treatments, which take about an hour, provide multiple benefits—improved mood, energy, weight loss, immune system strengthening, enhanced athletic performance—with the intravenous vitamins absorbed far faster and more effectively than if taken orally. »


KEVIN MAYERSTOCK.ADOBE.COM; CONTRIBUTED
BODY,MIND ANDSPIRIT
GETTING GROUNDED
With the ordeal of medical spa treatments behind us, it’s time for treatments that both feel good and are good for us. One of the first steps for guys seeking a new lease on life is a pedicure. That’s right: A pedicure!
This is because men have slowly been discovering the benefits of well-tended feet that have spent most of their lives imprisoned in boots or shoes, where some of the nastiest conditions on Earth prevail.
Fortunately, spas are as much centers of health information as they are palaces of pleasure, and a little information can go a long way to getting men in the door for this muchneeded treatment. As I learn from Jeannie, who has been rejuvenating feet at Dream Spa & Salon in Westport, CT, for sixteen years, the feet contain more sensory nerve endings per square centimeter than any other part of the body, continually supplying information about the surfaces we’re trodding for better balance, stability and shock absorption. With more than 250,000 sweat glands there, each foot can produce four to six or more ounces of perspiration a day. Enough said.
As with other spa treatments, gift certificates for pedicures are what usually drag men in the door for the first time. “They’ll come in for a pedicure, because they have a gift certificate from a wife or daughter or girlfriend,” Jeannie says. “A lot of the guys will say that when they cut their own toenails, they usually end up bleeding.”
I change my socks and go see her.
The pedicure room at Dream is a small, clean, warm room set off from the rest of the spa so that men don’t feel self-conscious in a sea of chatting women, says Jeannie. She is Greek and has a natural Mediterranean warmth that itself is soothing.
The procedure begins with slipping the bare feet in a warm, shallow bath of powdered milk

water mixed with lemongrass essential oil. After drying them on a towel on her lap, Jeannie clips the nails without blood or digit loss, then applies cuticle eliminator ointment to soften the dead skin on the nails that is pushed back and scraped off. Next, she files the calluses with soft and gritty sandpaper-like files, and finally washes and massages the feet and calves with a washcloth and vanillaorange and brown sugar scrub, the sugar giving a mild grit to the wash. I feel special.
Jeannie recommends that men come back for a pedicure every four to six weeks, which is when toenails have grown long enough to be in need of clipping. I leave feeling not only grounded, but being able to see my reflection in my polished toenails.
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 96
DREAM SPA & SALON Westport, CT
FEET: SANDOR KACSOSTOCK.ADOBE.COM; CONTRIBUTED

BODY, MIND AND SPIRIT
One of the best ways to wrap up a program of spa treatments, both medical and aesthetic, might be with a visit to Jane Kohler, a Westport, CT-based structural integration therapist and masseuse, who is much sought after for her holistic approach to bodywork. In addition to feeling really good after one of her long sessions, clients receive an education across a wide, free-ranging spectrum of information—from yoga and meditation to Rolfing, reflexology and qigong, a traditional Chinese medicine that uses movement to optimize energy and maintain healthy mind, body and spirit.
Kohler’s Westport studio is a spacious, sun-dappled space in an old house furnished with antique Oriental carpets and a simple, cushioned massage table.
“Before clients get on the table, I look at their structure— how they’re standing, how they’re breathing and how they’re moving,” she says. “If the ankles and shoulders and ears aren’t aligned, there are issues that will continue until they’re resolved.”
Kohler focuses on the fascia— the connective tissue of the body—to realign posture and to open tissue that’s tightened due to stress, injury or other factors that cause chronic pain and limited mobility. Some issues involve ingrained habits of sitting, walking and breathing that inhibit daily functioning. Other issues are emotional. Kohler uses a mix of slow, deep, stretching movements and applied pressure to balance the fascial. The release of emotions, memories and/or traumas that have been stored in the tissue can make clients aware of the opportunity for change.
She stretches the limbs, realigns the frame, gently massages sore spots and applies pressure to tension knots, one area at a time. I leave feeling better than I have in years.

“IF
THE ANKLES AND SHOULDERS AND EARS AREN’T ALIGNED, THERE ARE ISSUES THAT WILL CONTINUE UNTIL THEY’RE RESOLVED.”
CAVEAT
One warning, of sorts: Spa services for men aren’t for the faint of wallet. Aside from prescriptions, which PAs can write, most spa treatments are for those with disposable dead skin, tension and income.
“What we do is purely discretionary, so men have to be able to afford it,” says Dream Spa & Salon’s Lori Dodd.
Laser treatments and Hydrafacials can get pricey, while basic pedicures and massages are often more reasonable. As with most treatments, packages of three to six lower the cost per treatment considerably. (A second, more obnoxious disclosure: All of the above services were free for me. Someone's gotta do it.)
Spa treatments can also be addictive. The Greenwich police officer, who disregarded gift certificates from his wife the way scofflaws disregard parking tickets, now schedules monthly appointments at the Delamar Spa for stresseliminating massages.
Finally, because of that spa brain, you may want to avoid treatments on days when you need to use your brain— like if you've got to give a big presentation. Then again, who cares? Explain that you’ve just come from a massage and pedicure and aren’t wearing shoes so that you can look at yourself in your polished toenails. Spa men will understand.
JANE KOHLER Westport, CT
GUDENKOASTOCK.ADOBE.COM
ANDSPIRIT
BODY,MIND



PSORIASIS, PRIOR AUTHORIZATIONS AND ZORYVE


Topical Therapy
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 98
CREAM 0.3% CREAM BY IMAGEHUBSTOCK.ADOBE.COM
(ROFLUMILAST)
By Neomia “Neo” Cuellar, CCMS, PACS

“If there was ever a time or place to be diagnosed with psoriasis, IT’S NOW .”
This is a phrase I tell every patient I meet who is diagnosed with psoriasis.
Honestly, it’s true. The advances in therapies have come a very long way, and it’s not only the injectable/oral therapies that are evolving, but now topical therapies, such as ZORYVE, that are an option for some of our younger patients. ZORYVE is a topical treatment for plaque psoriasis, including intertriginous areas in patients 12 years old and older. With the burdens of burnout and turnover rates growing the last few years, coupled with increasing insurance denials across all drugs from coast to coast, I’m here to help by pursuing a ZORYVE approval for your patients to relieve some of the pressures faced by your office.
As medical professionals in dermatology, we have seen different levels of patients struggling with psoriasis. This creates a negative impact not only on a physical level,
bcofdermatology.com 99





but on a mental one as well. We must decide which therapy will be effective while keeping in mind location and possible side effects of therapy options. With the release of ZORYVE, there is a non-steroidal option that can be used even in sensitive intertriginous areas, avoiding the risk of atrophy. Additionally, it is an alternative for patients who may have a needle phobia, seen especially in our adolescent patients, those who have no at-home injection help or those who struggle with swallowing pills.

While the options for psoriasis treatment have grown and produced progressive therapies, such as ZORYVE, there has also been an increase in prior authorization denials in the medical landscape for all drugs. This has caused a burden for our offices, which can lead to burnout and turnover. Some offices are under the impression that an attempt at approval is futile.
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 100
KBISCUITSTOCK.ADOBE.COM


WHY PURSUE APPROVAL?
This seems to be the 2023 question of the year. There’s a two-part answer to this. First and foremost, let’s look at the medication, ZORYVE, and explain the benefits of the therapy for our patient to the insurance company. The average psoriasis patient struggles with intense burning, itching and plaques, not just to the exposed skin, but to areas we can’t see. ZORYVE is non-steroidal and has convenient dosing, only needing to be applied once daily, and is not associated with side effects of folliculitis or atrophy. ZORYVE does not contain potentially irritating ingredients such as isopropyl alcohol, propylene glycol, or ethanol, which are concerns we may
have when choosing a topical therapy for our patients. For patients who have several topical drug allergies, this gives them an option where there may not have been one before. I had a call from a patient with a high-gluten sensitivity, not just internally, but to topical items that contained gluten which made her breakout. I was extremely happy to deliver the news that ZORYVE does not have gluten in the ingredients, and we were able to start ZORYVE therapy for her. As healthcare professionals, we want to minimize making a patient who is already suffering from experiencing any more discomfort.
As a provider, you should also have the fundamental right to choose what you feel the appropriate therapy should be for each patient. If you don’t tend to write

for biologics or believe the patient “isn’t there yet,” for injectable/oral therapies for psoriasis, then ZORYVE provides you with an option and/or an intervention pre-biologic initiation. Furthermore, providers may choose to use ZORYVE complementary to a biologic therapy. Even with biologic/oral therapy, there can be breakthroughs and flares of psoriasis. I am a witness to this, living and working in Arizona, where there is pretty much 0% humidity all year. With numerous patients who do get flares, especially in the winter months and need adjunct therapy, I have successfully gotten ZORYVE approved for patients to use alongside their injectable or oral therapies for those breakthrough flares. Additionally, ZORYVE can be used for long-term management for our patients’ psoriasis, where we may have struggled to find a long-term topical therapy before. The physical manifestation of psoriasis can cause great mental anguish. This leads me to the second part of “Why pursue ZORYVE approval?” Many psoriasis patients are embarrassed about their condition and omit locations such as sensitive skin folds. I have received calls from countless patients over my last almost
“As medical professionals in dermatology, we have seen different levels of patients struggling with psoriasis. THIS CREATES A NEGATIVE IMPACT NOT ONLY ON A PHYSICAL LEVEL, BUT ON A MENTAL ONE AS WELL."
OLGA TERNAVSKAYASTOCK.ADOBE.COM bcofdermatology.com 101






15 years in dermatology who applied a topical steroid to an intertriginous area, because they did not want to tell the provider about flaring under the breasts or buttocks. Since we prescribed a steroid for the locations they did tell us about during the visit, they thought it would work the same, regardless of what area they were applying the topical steroid to, causing atrophy. This humiliation that kept them from telling their healthcare professional about a flare in the groin area also drives how they live on a daily basis, causing social discord and has led to the development of anxiety and depression.
This can cause psychosocial embarrassment—whether the patient is a 14-year-old in class who attends middle school or an adult who simply goes out to buy groceries. If you believe a therapy like ZORYVE could help improve a patient’s psoriasis, in turn, reducing embarrassment in their life, it’s worth fighting for treatment.

In my own practice, we also faced a high burnout rate that led to turnover and ultimately deterred people from doing prior authorizations (PAs) for topical medications, let alone an appeal. We felt as if many of the staff were too new and not educated on the prior authorization/appeal process… or we just simply didn’t have the time and resources to pursue many drugs that were seen as similar. The decision was made from not having a strong topical therapy option. This changed with the release of ZORYVE. I sat with the providers and explained not only the benefits of ZORYVE, but I laid out the facts about the PA and appeal process itself.
Insurance companies have more procedures in place that make it hard to navigate coverage, which can disincentivize us from pursuing an appeal. The fact of the matter is, according to an AMA survey, “84% of physicians reported seeing an increase of PA requests within
the last five years.”* Ultimately, those who actually sought an appeal had “seen over 80% of those appeals were overturned as approved,” according to a Kaiser analysis of their Medicare patients.
With this knowledge, let’s review what an office can do to pursue a ZORYVE approval. First and foremost, ensure you are adding any tried and failed therapies into the note/ supporting documentation. This may sound simple, but it is often the simplest tasks that are overlooked. This is one of the most common reasons for a coverage denial by the insurance company. In addition to missing tried and failed medications, we may forget to add the location of the psoriasis. With how fast-paced the visit can be, it is easy to counsel on a condition but forget to click on a body part in our EHR systems, thus leading to a denial.
Adding the clinical information is a great start, but please don’t forget to include anything that speaks to how the condition
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 102
KBISCUITSTOCK.ADOBE.COM
“The advances in therapies have come a very long way, and it’s not only the injectable/oral therapies that are evolving, but NOW TOPICAL THERAPIES, SUCH AS ZORYVE, THAT ARE AN OPTION FOR SOME OF OUR YOUNGER PATIENTS. "
affects the patient, not just physically, but mentally. My passion is fighting for medication approvals, such as ZORYVE. Over the years, one of the most important things I have learned through insurance prior authorizations is that a well-worded statement about how the condition affects a patient’s quality of life can also be a factor in a successful approval.
Psoriasis can cause mental harm when we are forced to go through step therapies, creating frustration in the patient since they are not seeing an improvement. This experience may inadvertently reflect on their view of the practice/provider. If there is a reason the provider feels the alternative therapy would be ineffective or has a concern about an adverse effect, also add this to your PA or Appeal.
Some of my own tips to get a ZORYVE approval (besides listing locations that are affected and what they have tried and failed) include why this medicine is preferred, along with the provider’s reasons about how it will improve the condition. For example, if a patient does have psoriasis in their intertriginous areas, I want to avoid topical steroids because of the risk of atrophy. If my patient has had problems with rashes before or they
have a large body surface area that would burn with most topical therapies, I explain we need ZORYVE approved since it is an effective treatment option and does not have alcohol, ethanol, or propylene glycol, which would cause irritation to the already open, burning plaques.
Not just compiled from literature, but from real-life experience, I continuously try to remember the impact psoriasis can have on patients and include this in the notes. For example, I’ve had patients explain they don’t feel like seeking intimacy or a partner because of the sensitive areas affected, such as the intertriginous areas. Alongside these experiences, I have seen some of the same patients who experienced atrophy and feel that “nothing can be done.”
Some of my patients cannot perform job responsibilities because their hands
are affected or they are broken out on an exposed area, causing embarrassment. Explain in a note how the condition affects the patient both professionally and personally, which can also be used in a PA or appeal.
When I am short on time to do prior authorizations and appeals for medications like ZORYVE, I enlist the help of specialty pharmacies. Working with an experienced specialty pharmacy can be helpful. I work with several that have a tremendous amount of knowledge on payer processes, so our office doesn’t have to navigate it on our own. Since they’re seeing these things multiple times a day, they can help you figure out a complicated process. I understand the strain on an office when we are short-staffed. Specialty pharmacies can assist with the PA or appeal processes for medications such as ZORYVE.
With advancements in therapies like ZORYVE, it is important to remember the benefits for patients and the justification of attempting to pursue an approval for it, even with the landscape of burnout and office turnover. Simply listing what a patient has failed helps; however, be sure to remember the greater cause and why we practice medicine. For these patients
bcofdermatology.com 103
TRIOCEANSTOCK.ADOBE.COM


who may not have had many options before, or who fear alternatives therapies, ZORYVE may be the treatment that fits them best. We are also taking back the fundamental right to practice medication and prescribing from the hands of the insurance companies, since they may try to dictate therapies that are against our wishes for the patient. If we need assistance pursuing an appeal but don’t want to take too much time from our own staff, specialty pharmacies are out there waiting to help with this.
Lastly, when we do get an approval for a medication like ZORYVE and the patient can adhere to the therapy and sees


IMPORTANT SAFETY INFORMATION
The use of ZORYVE is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C). The most common adverse reactions (≥1%) include diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), application site pain (1%), upper respiratory tract infection (1%), and urinary tract infection (1%). Please see full prescribing information at zoryvehcp.com.
improvement, we are not only improving their physical condition, but also improving their mental quality of life. Preventing embarrassing moments and empowering the patient is critical.
For example, a 16-year-old patient got the courage to try out and successfully made the varsity swim team only after getting clear skin with her ZORYVE prescription. I worked with a 28-year-old autism patient who struggled with alternative therapies, but he was able to switch to ZORYVE and now has the ability to control his disease. What we prescribe and how we pursue these medications can mean the world to our patients.
REFERENCES
*https://www.medicarerights.org/medicare-watch/2023/02/09/prior-authorization-data-and-proposed-rule-reflect-need-for-stronger-medicare-advantage-rules
**https://www.medicarerights.org/medicare-watch/2023/02/09/prior-authorization-data-and-proposed-rule-reflect-need-for-stronger-medicare-advantage-rules
“Over the years, one of the MOST IMPORTANT THINGS I HAVE LEARNED THROUGH INSURANCE PRIOR AUTHORIZATIONS IS THAT A WELL-WORDED STATEMENT ABOUT HOW THE CONDITION AFFECTS A PATIENT’S QUALITY OF LIFE can also be a factor in a successful approval. "
ACCESS DERMATOLOGY SEPTEMBER/OCTOBER 2023 104 KBISCUITSTOCK.ADOBE.COM





STILL GOLDEN. EHR | PM | PATIENT ENGAGEMENT | TELEHEALTH | ASC | INTEGRATED PAYMENTS | CONSULTING Don’t fall for anything less. Nextech is still the only EHR to receive the American Academy of Dermatology’s DataDerm™ Gold recognition. Start anew with the #1 solution for Dermatology
Help eligible patients start and stay on track with their therapy



















The DUPIXENT MyWay® patient support program can help facilitate patient access to DUPIXENT® (dupilumab) throughout the treatment journey.
Coverage support
DUPIXENT MyWay provides assistance navigating the insurance process with benefits investigations, prior authorization support, and education about the appeals process.
Patient access support
DUPIXENT MyWay may have support for eligible patients who need help with their out-of-pocket costs.
Education and support
Every enrolled patient is assigned a dedicated DUPIXENT MyWay Case Manager, who takes a patient-centric approach to providing tools, support, resources, and education throughout the treatment journey.
Support for your patients begins with a complete DUPIXENT MyWay Enrollment Form
You can help patients enroll in DUPIXENT MyWay by:
• Faxing the form to DUPIXENT MyWay at 844-387-9370
• Submitting the form through the DUPIXENT MyWay Document Drop at www.patientsupportnow.org
For more information:
Visit us on the exhibit floor OR
Call DUPIXENT MyWay at 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am–9 pm Eastern time
For any questions or concerns, or to report side effects with DUPIXENT® (dupilumab), please contact 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 am–9 pm Eastern time.
and DUPIXENT MyWay® are registered trademarks of Sanofi Biotechnology. © 2023 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved. 07/2023 DUP.23.06.0234
DUPIXENT®
D (dupilumab) Injection
DUPIXENT myway) sanofi