
EMPOWERING PATIENTS with THEIR OWN VOICE

CIBINQO is indicated for the treatment of adults with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.





JAK=Janus kinase; AD=atopic dermatitis.
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death. The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia.
If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection.
Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral (including herpes zoster), and other infections due to opportunistic pathogens.






Avoid use of CIBINQO in patients with an active, serious infection, including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections or those who have resided or traveled in areas of endemic tuberculosis or endemic mycoses.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO.
Viral reactivation, including herpes virus reactivation (eg, herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves. Hepatitis B virus reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C.
To learn more about CIBINQO efficacy, visit CIBINQOhcp.com or scan QR code.
Confidence in Patient Support
Helping patients with access and reimbursement support for CIBINQO™
Coverage Assistance
Pfizer Dermatology Patient Access™ (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Financial Assistance
Eligible, commercially insured patients may save with the Copay Savings Card.* No matter what type of insurance your patients have, financial support may be available.
Pharmacy Coordination
PDPA strives to make CIBINQO prescription fulfillment as smooth as possible through the preferred pharmacy.
Live, Personal Support
A Patient Support Representative is available by phone, Monday – Friday, 8 am – 8 pm ET, for your patients, as well as for you and your office staff. Call 1- 833-956-DERM (1-833-956-3376).

To learn more about PDPA, your Field Reimbursement Manager (FRM), and how they can help support your prescribed patients, contact PDPA at 1-844-496-8707, Monday – Friday, 8 am – 8 pm ET.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
Please see additional Important Safety Information and Brief Summary of full Prescribing Information on the following pages. For full Prescribing Information, including BOXED WARNING and Medication Guide, visit CIBINQOhcp.com. TM
& INDICATION
MORTALITY
In a large, randomized postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality (including sudden cardiovascular death) was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
MALIGNANCIES
Malignancies, including non-melanoma skin cancer (NMSC), were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
In a large, randomized postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding nonmelanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA patients. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur.
THROMBOSIS
Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of overall thrombosis, DVT, and PE were observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and treat patients appropriately.
CONTRAINDICATION
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤ 81 mg daily), during the first 3 months of treatment.
LABORATORY ABNORMALITIES
Hematologic Abnormalities: Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a complete blood count (CBC). CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations: Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy, and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
IMMUNIZATIONS
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines, including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
RENAL IMPAIRMENT
Avoid use in patients with severe renal impairment or end stage renal disease, including those on renal replacement therapy.
HEPATIC IMPAIRMENT
Avoid use in patients with severe hepatic impairment.
ADVERSE REACTIONS
Most common adverse reactions (≥ 1%) in subjects receiving 100 mg and 200 mg include: nasopharyngitis, nausea, headache, herpes simplex, increased blood creatine phosphokinase, dizziness, urinary tract infection, fatigue, acne, vomiting, oropharyngeal pain, influenza, gastroenteritis. Most common adverse reactions (≥ 1%) in subjects receiving either 100 mg or 200 mg also include: impetigo, hypertension, contact dermatitis, upper abdominal pain, abdominal discomfort, herpes zoster, and thrombocytopenia.
Inform patients that retinal detachment has been reported in CIBINQO clinical trials. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision.
DRUG INTERACTIONS
Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO. See Prescribing Information for clinically relevant drug interactions.
USE IN PREGNANCY
Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Advise females of reproductive potential that CIBINQO may impair fertility.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770.
LACTATION
Advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose.
INDICATION
CIBINQO is indicated for the treatment of adults with refractory, moderateto-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
Please see full Important Safety Information throughout and full Prescribing Information, including BOXED WARNING, and Medication Guide.
© 2023 Pfizer Inc. All rights reserved. January 2023. PP-CIB-USA-0430
SAFETY INFORMATION
IMPORTANT
CIBINQO™ (abrocitinib) tablets, for oral use
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death; The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia. If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection. Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens. Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately.
INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Testing, Evaluations, and Procedures
Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
• Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO
• Viral hepatitis screening in accordance with clinical guidelines CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C
• A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/ mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation.
Recommended Dosage
The recommended dosage of CIBINQO is 100 mg orally once daily.
If an adequate response is not achieved with CIBINQO 100 mg orally daily after 12 weeks, consider increasing dosage to 200 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 200 mg once daily.
CIBINQO can be used with or without topical corticosteroids. If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
Recommended Dosage in Patients with Renal Impairment
Renal Impairment
CIBINQO dosage recommendation in patients with mild renal impairment (60-89 mL/minute) is 100 mg once daily. For patients with moderate renal impairment (30-59 mL/ minute), the recommended dosage is 50 mg once daily. CIBINQO is not recommended for patients with severe or End Stage Renal Disease (ESRD). Severe renal impairment and End-Stage Renal Disease include patients on renal replacement therapy.
In subjects with mild and moderate renal impairment, if an adequate response is not achieved after 12 weeks, dose of CIBINQO can be doubled.
CIBINQO is not recommended for patients with severe renal impairment or ESRD.
Recommended Dosage in CYP2C19
Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Dosage Modifications due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19 reduce the dosage to 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Treatment Discontinuation due to Serious Infections
or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Hematologic Abnormalities
• Discontinue CIBINQO if platelet count <50,000/mm3 and
follow with CBC until >100,000/mm3
• Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value
• Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value
• Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosing increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities.
DOSAGE FORMS AND STRENGTHS
• 50 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 50” on the other.
• 100 mg: Pink, round, film-coated tablet debossed with “PFE” on one side and “ABR 100” on the other.
• 200 mg: Pink, oval, film-coated tablet debossed with “PFE” on one side and “ABR 200” on the other.
CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment.
WARNINGS AND PRECAUTIONS
Serious Infections
The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and benefits of treatment prior to initiating CIBINQO in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and benefits of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO. Tuberculosis Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information; Initial Approval: January 2022
CIBINQO™ (abrocitinib) tablets, for oral use
Mortality (continued)
treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with CIBINQO for atopic dermatitis.
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical studies of CIBINQO for atopic dermatitis.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in patients receiving CIBINQO in the clinical studies for atopic dermatitis.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
Laboratory Abnormalities
Hematologic Abnormalities Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a CBC. CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations
Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter
elevations on cardiovascular morbidity and mortality has not been determined.
Immunizations
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Serious Infections
• Mortality
• Malignancy and Lymphoproliferative Disorders
Clinical Trials Experience
• Major Adverse Cardiovascular Events
• Thrombosis
• Laboratory Abnormalities
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO.
In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks.
The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%). While subjects aged 12 to 17 years were included in these trials, CIBINQO is not approved for use in pediatric subjects. Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in the table below. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety profile of CIBINQO in the monotherapy and the combination trial(s) were similar.
Adverse Reactions from Placebo-Controlled Trials
Reported in ≥1% of CIBINQO Treated Subjects with Moderate to Severe Atopic Dermatitis and at Higher Rate than Placebo for up to 16 Weeks
size for all the adverse reactions reported in this section.
Overall Infections In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events In the placebocontrolled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia, no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia In the placebo-controlled trials, for up to 16 weeks, confirmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the first 4 weeks of exposure.
Lipid Elevations In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the final visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Specific Adverse Reactions
Exposure adjusted incidence rates were adjusted by trial
Retinal Detachment In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
Weeks
CIBINQO 200 mg N=590 n (%a) CIBINQO 100 mg N=608 n (%a) PLACEBO N=342 n (%a) Nasopharyngitis 51 (8.7)75 (12.4)27 (7.9) Nausea 86 (14.5)37 (6.0)7 (2.1) Headache 46 (7.8)36 (6.0)12 (3.5) Herpes simplexb 25 (4.2)20 (3.3)6 (1.8) Increased blood creatinine phosphokinase 17 (2.9)14 (2.3)5 (1.5) Dizziness 17 (2.9)11 (1.8)3 (0.9) Urinary tract infection 13 (2.2)10 (1.7)4 (1.2) Fatigue 8 (1.3)10 (1.6)2 (0.5) Acne 28 (4.7)10 (1.6)0 (0.0) Vomiting 19 (3.2)9 (1.5)3 (0.9) Impetigo 3 (0.5)9 (1.5)1 (0.3) Oropharyngeal pain 6 (1.0)8 (1.4)2 (0.6) Hypertension 5 (0.8)7 (1.2)2 (0.7) Influenza 6 (1.1)7 (1.2)0 (0.0) Gastroenteritis 8 (1.3)7 (1.1)2 (0.6) Dermatitis contact 3 (0.5)6 (1.1)1 (0.3)
pain upper 11 (1.9)4 (0.6)0 (0.0)
discomfort 7 (1.2)3 (0.5)1 (0.3)
zoster 7 (1.2)2 (0.3)0 (0.0)
9 (1.5)0 (0.0)0 (0.0) a Study size adjusted percentages b Herpes simplex also includes oral herpes, ophthalmic herpes, herpes dermatitis, genital herpes.
0-16
Abdominal
Abdominal
Herpes
Thrombocytopenia
CIBINQO™ (abrocitinib) tablets, for oral use
Specific Adverse Reactions (continued)
Creatine Phosphokinase Elevations (CPK) In the placebo-controlled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
DRUG INTERACTIONS
Effects of Other Drugs on CIBINQO
The table below includes drugs with clinically significant drug interactions affecting CIBINQO.
Clinically Significant Drug Interactions Affecting CIBINQO
Strong CYP2C19 Inhibitors
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors.
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9
Clinical Impact
Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9.
Strong CYP2C19 or CYP2C9 Inducers
Clinical Impact
Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response.
Intervention Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers.
Effects of CIBINQO on Other Drugs
The table below includes clinically significant drug interactions affecting other drugs.
Clinically Significant Interactions Affecting Other Drugs
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities
Clinical Impact
Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or lifethreatening toxicities (e.g., digoxin).
Intervention Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or lifethreatening toxicities when coadministered with CIBINQO.
Antiplatelet Therapy Drugs
Clinical Impact
Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia.
Intervention Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the first 3 months of treatment are contraindicated with CIBINQO.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant
women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770.
Risk Summary Available data from pregnancies reported in clinical trials with CIBINQO are not sufficient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 14 or 5 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Animal Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively.
Animal Data In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/day (14 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossified metatarsals) were noted at 60 mg/kg/day (22 times the MRHD based on AUC comparison).
In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (5 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/day (14 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (22 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (3 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/day (14 times the MRHD based on AUC comparison).
Lactation
Risk Summary There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Animal Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse findings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5-6 elimination half-lives).
Animal Data Lactating female rats were orally administered a single dose of 10 mg/kg abrocitinib on lactation day 12. Abrocitinib AUC was approximately 5 times greater in milk than in plasma.
Females and Males of Reproductive Potential
Infertility Females Based on the findings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration.
Pediatric Use
The safety and effectiveness of CIBINQO have not been established in pediatric patients.
Juvenile Animal Toxicity Data In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day beginning on postnatal day 10 (approximately equivalent to a human infant) and continuing through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose-related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur. Abrocitinib produced adverse effects on bone development at all dose levels. Abrocitinib caused irreversible
dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (1.1 times the MRHD based on AUC comparison). Abrocitinib also irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (10 times the MRHD based on AUC comparison). At 75 mg/kg/day (36 times the MRHD based on AUC comparison), paw fractures generally corresponded to limb impairment, a fractured tibia was noted in a single female, and effects noted at lower doses were increased in frequency and severity. Irreversible bone findings have not been observed in older animals.
Geriatric Use
A total of 145 (4.6%) patients 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
A higher proportion of patients 65 years of age and older discontinued from clinical trials compared to younger patients. Among all patients exposed to CIBINQO, including the long-term extension trial, confirmed ALC <500/mm3 occurred only in patients 65 years of age and older. A higher proportion of patients 65 years of age and older had platelet counts <75,000/mm3. The incidence rate of herpes zoster in patients 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of patients 18 to less than 65 years of age (3.44 per 100 patient-years).
Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30-59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min). This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement. A dosage reduction in patient with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60-89 mL/min). CIBINQO has not been studied in patients on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function. In clinical trials, CIBINQO was not evaluated in patients with severe (Child Pugh C) hepatic impairment.
CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates.
OVERDOSAGE
There is no experience regarding human overdosage with CIBINQO. There is no specific antidote for overdose with CIBINQO. In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
Rx only
This brief summary is based on CIBINQO™ (abrocitinib) Prescribing Information LAB-1423-1.0.
Issued: January 2022.
The product's label may have been updated. For full Prescribing Information, visit CIBINQOPI.com.
See CIBINQO full Prescribing Information at CIBINQOPI.com.
© 2022 Pfizer Inc. All rights reserved. February 2022. PP-ABR-USA-0329
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
MISSION STATEMENT: Access Dermatology aims to educate and empower the biologic coordinator by keeping them informed of the complex and everchanging drug and patient access landscape. Readers are engaged with editorial and lifestyle content equally suitable for dermatologic patients, so they too may gain a better sense of therapies and the patient services programs that can assist in their therapeutic journey.
DISPLAY ADVERTISING: Contact Craig Schuette at cs@bcofdermatology.com.
EXECUTIVE DIRECTOR
Craig Schuette
EDITOR
Elizabeth Hole
CREATIVE DIRECTOR
Venera Alexandrova
ASSISTANT ART DIRECTOR
Lisa Servidio
PRODUCTION DIRECTOR
Tim Carr
CORRESPONDENCE:
Communications regarding original articles as well as editorial suggestions for future issues should be addressed to Craig Schuette at cs@bcofdermatology.com. Any content forwarded to the publisher assumes no liability for the safety or return of unsolicited art, photographs, or manuscripts.
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 6
practical information and lifestyle content
Jonathan W.
V. Moffly
Amill MOFFLY CUSTOM MEDIA 205 Main Street, Westport, CT 06880 telephone: 203-222-0600 fax: 203-222-0937 email: mail@MofflyCustomMedia.com Follow the Biologic Coordinators of Dermatology FACEBOOK LINKEDIN
A magazine dedicated to supporting patient access with
ACCESS DERMATOLOGY ISSUE 2 | January/February 2023 PRESIDENT
Moffly VICE PRESIDENT/BUSINESS Elena
CHIEF REVENUE OFFICER Andrew
© 2023 Access Dermatology and Biologic Coordinators of Dermatology. ALL RIGHTS RESERVED. The material in this publication is published by Moffly Custom Media and may not be reproduced or transmitted in any manner, in whole or in part, without the express written permission of Access Dermatology and Moffly Custom Media. NOTICE: The information contained within articles of this magazine represent the views and opinions of the original authors and do not necessarily represent the views and opinions of Access Dermatology or its affiliates. The mere appearance of content in the magazine does not constitute an endorsement by Access Dermatology or its affiliates. The content has been made available for informational and educational purposes only. Editorial advice is not specific, and readers are advised to seek medical, professional, or reimbursement help for individual circumstances. Access Dermatology hereby disclaims all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the content. CIRCULATION: To be added to the circulation, visit www.bcofdermatolgy.com/ magazine. REPRINTS:
educational, commercial,
promotional reprints, including author off-prints,
For
or
please email contact@bcofdermatology.com.
COMING SOON
Patient-driven digital enrollment for faster first fill rates, increased patient persistency and reduced adminstrative work
Visit BCofDermatology.com/partnership to request more information!

What Matters Most
Welcome to the latest edition of Access Dermatology.
There is so much pressure with the advent of a new year—to be something more, to experience some revelatory moment.
For us at BCoD, we didn't declare any New Year's resolutions. Instead, we're focused on broader, more long-term goals that enrich the daily lives of the access coordinator community.
Our first edition of the year includes a wealth of material focused on wellness for the body and mind. We also feature content that empowers patients to become an advocate for themselves. As this year unfolds, I encourage our readers to focus on what matters most for you and your loved ones. My collegiate coach would say to “pay attention to the rocks before the sand.” If you first fill your vessel with sand,
you won't have room for the rocks. Rather, focus on the more significant elements—and then add in the sand to fill the extra space.
As for us, we'll continue to bring you more meaningful and vibrant content that innovates and challenges therapeutic magazine standards, giving our readers sound advice to lead patients through therapy and toward confidence.
With that, read on, and I hope when you put this copy down, you'll feel newly inspired in your personal and professional endeavors.
CRAIG SCHUETTE

ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 8
letter from the founder /
Craig Schuette



LIFESTYLE


SHOP TALK
bcofdermatology.com 9 contents
45 BC Spotlight: Q&A with two office professionals 50 BCoD Conference Highlights from 2nd annual event
ACCESS DERMATOLOGY ISSUE 2 COMMUNITY
56 New Year. New You. Experts share wellness tips 68 Fitness Gadgets Tech that helps you achievehealth and wellness goals
74 Patient Access Program Survey 81 Path to Affordability VTAMA's Copay Program
14 Accumulator Plans Tips for keeping patients on therapy 18 Patient Advocacy Help and empower your patient 24 FRM Assistance Our co-pilots in dermatology 30 Patient Access Best practices for gathering information 24 50 68 81 30 56
FEATURES























VTAMA cream can give plaque psoriasis patients significantly clearer skin. So they’re free to think about…literally anything.

VTAMA cream is a once-daily, non-steroidal topical for adults with plaque psoriasis.
36% and 40% Physician Global Assessment (PGA) success rate* in two 12-week pivotal studies vs 6% and 6% for vehicle 2






Safe and well-tolerated even on the face, neck, intertriginous areas, inframammary areas, axillae, genitalia, and anal crux.1,3,4


See the data: VTAMA.com
A median ~4 month off-treatment effect seen in long-term, open-label extension study of patients (n=73)2

*PGA success rate defined as PGA=0 or 1 and ≥2-grade improvement from baseline at week 12.




References:









Important Safety Information: Indication: VTAMA® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults. Adverse Events: The most common adverse reactions (incidence ≥ 1%) in subjects treated with VTAMA cream were folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu). You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088
1. Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219–2229. 2. VTAMA (tapinarof) cream, 1%. Prescribing Information. Dermavant; 2022. 3. Strober B, Stein Gold L, Bissonnette R, et al. Tapinarof cream 1% once daily for plaque psoriasis: long-term extension trial of a novel therapeutic aryl hydrocarbon receptor modulating agent. Oral presentation at European Academy of Dermatology and Venereology; September 30, 2021. 4. Stein Gold L, Kircik L, Lebwohl M, et al. Tapinarof cream 1% once daily for plaque psoriasis: favorable local tolerability in two pivotal phase 3 trials. Poster presentation at Innovations in Dermatology 2021; March 16–20, 2021.


Please see the Brief Summary of VTAMA cream on the following page.




© 2023 Dermavant Sciences, Inc. All Rights Reserved. All trademarks are the property of Dermavant Sciences, GmbH. US-VTAMA-2200072-01









Now FDA Approved


BRIEF SUMMARY
IMPORTANT INFORMATION ABOUT
VTAMA® (Vee-TAM-uh) (tapinarof) cream, 1%
This summary contains important information about VTAMA cream. It is not meant to take the place of your doctor’s instructions. Read this information carefully before you start using VTAMA cream. Ask your doctor or pharmacist if you do not understand any of this information or if you want to know more about VTAMA cream. For full Prescribing Information and Patient Information, please see the package insert.
WHAT IS VTAMA cream?
VTAMA cream is a prescription medicine used on the skin (topical) to treat plaque psoriasis in adults. It is not known if VTAMA cream is safe and effective in children under 18 years of age.
Do not use VTAMA cream for a condition for which it was not prescribed. Do not give VTAMA cream to other people, even if they have the same symptoms you have. It may harm them.
Important: VTAMA cream is for use on the skin (topical use) only. Do not use VTAMA cream in your eyes, mouth, or vagina.
WHAT SHOULD I TELL MY DOCTOR BEFORE USING VTAMA cream?
Before you use VTAMA cream tell your doctor about all of your medical conditions, including if you:
•Are pregnant or plan to become pregnant. It is not known if VTAMA cream will harm your unborn baby; and/or



•Are breastfeeding or plan to breastfeed. It is not known if VTAMA cream passes into your breast milk. Talk to your doctor about the best way to feed your baby during treatment with VTAMA cream.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
HOW SHOULD I USE VTAMA cream?
•Use VTAMA cream exactly as your doctor tells you to use it.
•Apply a thin layer of VTAMA cream only to your psoriasis skin lesions one (1) time a day. Avoid applying VTAMA cream to unaffected areas of your skin.
•Wash your hands after application, unless VTAMA cream is for treatment of your hands.
•If someone else applies VTAMA cream for you, they should wash their hands after application.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF VTAMA cream?
The most common side effects include: folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu).
These are not all the possible side effects of VTAMA cream. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA at www.fda.gov/ medwatch or call 1-800-FDA-1088.
HOW SHOULD I STORE VTAMA cream?
•Store VTAMA cream at room temperature, between 68℉ to 77℉ (20℃ to 25℃).
•Do not freeze VTAMA cream.
•Protect VTAMA cream from exposure to excessive heat.
•Keep VTAMA cream and all medicines out of the reach of children.
WHAT ARE THE INGREDIENTS IN VTAMA cream?
Active ingredient: tapinarof
Inactive ingredients: benzoic acid, butylated hydroxytoluene, citric acid monohydrate, diethylene glycol monoethyl ether, edetate disodium, emulsifying wax, medium-chain triglycerides, polyoxyl 2 stearyl ether, polyoxyl 20 stearyl ether, polysorbate 80, propylene glycol, purified water, and sodium citrate dihydrate.
VTAMA is a trademark of Dermavant Sciences, GmbH or its affiliates.
Dermavant Sciences Inc., Long Beach, CA 90806 USA


S:10" T:10.875" B:11.25"
Accumulator Plans
TIPS FOR KEEPING PATIENTS ON THERAPY
What is the biggest challenge you face when getting your patients to stay on biologics? For me, it’s making biologics affordable for the entire year. You can get your patients on therapy, but keeping them on it seems to be a whole new challenge. Yes, I'm talking about accumulator plans. Let's begin with the basic definition of each phrase.
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 12 CAGKANSTOCK.ADOBE.COM
ACCUMULATOR PLANS
These plans exclude copay assistance dollars from applying to a patient’s deductible or out-of-pocket maximums. Once the patient uses the available assistance for that year, they are responsible for any deductible or out-ofpocket per the benefit, which can lead to unexpected, substantial mid-year expenses for members. The result is that the employer’s plan spend is reduced, while the total patient out-of-pocket likely increases in the long run.
By Christine Ramig

MAXIMIZERS
Sometimes called variable copay programs, maximizers reclassify specialty medications as “non-essential,” removing the ACA requirements related to maximum out-ofpocket. These medications are assigned a percentage of coinsurance within the benefit, but patients are never charged for these drugs. Pharmacy Benefit Managers or (PBMs) identify the available copay assistance and spread that across a patient’s benefit year to maximize the use of those available funds, essentially maxing out the copay card over the 12-month period. While this sounds better for the patient, remember, the insurance is still exhausting all funds of the copay card just stretched out over the year.
PBM
This is a third-party administrator of a prescription drug program that is primarily responsible for processing and paying prescription drug claims. In addition, they typically negotiate discounts and rebates with drug manufacturers, contract with pharmacies and develop and maintain the drug formulary. Think of PBMs as middlemen. They are hired by the patient's insurance company to arrange the delivery and sale of prescriptions to the pharmacies. The pharmacy delivers the drugs to the patients, and the PBM reimburses the pharmacy for dispensing the prescription.
bcofdermatology.com 13
Accumulator and maximizer plans are becoming more common, so we need to educate ourselves now to help as many patients as possible. In 2022, these accumulator plans took about 23% of the specialty products, particularly focusing on marketplace plans. Experts are predicting that percentage will likely double by 2023. Today, one in five patients have accumulator plans and that is only going to get worse.
Did you know that 15 states (as of November 2022) have banned the use of accumulator plans? That's the good news; the bad news is that 35 states still haven’t. Even if the state you live in has a ban in place, the insurance company might not have to follow that ban if your main employer is in a state that doesn’t ban it.
OUR GOAL AS BIOLOGIC COORDINATORS: “How do we keep our patients on therapy while maintaining our sanity?!”
Our goal is to figure out how we can help. What can one person do to help change the way these programs are run?
Write a letter to congress, pay attention and vote in your cities. You can also
educate patients to call their insurance and find out how their benefits work. We know that pharmaceutical companies are looking at ways to be able to help our patients, but our patients need to help in this fight too!
The goal from the insurance company is how to save money, while the goal from the pharmaceutical company is how to keep patients on their therapies. Our goal as biologic coordinators: “How do we keep our patients on therapy while maintaining our sanity?!” We want to make sure we are working with our field reimbursement managers to know what options are available. Review the tips below for keeping patients on therapy, and know that people are in your corner trying to make things easier for you.
4
WE
5
ENCOURAGE PATIENTS WHO HAD COPAY PROBLEMS FIGURE OUT HOW TO SOLVE THIS FOR 2023.
6
GIVE PATIENTS THE PHONE NUMBERS TO COPAY CARDS AND EXPLAIN HOW THEIR HSA BENEFITS WORK.
7
YOU CAN ALSO SUGGEST PATIENTS LOG INTO THEIR INSURANCE PORTALS AFTER FILLING THEIR MEDICINE TO SEE IF THE DEDUCTIBLE HAS CHANGED.

ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 14
HOW CAN WE MAKE SURE OUR PATIENTS CONTINUE THEIR MEDICATIONS?
TALK TO YOUR FRMS, ARMS OR FAS.
FIND OUT WHAT THEIR COMPANIES HAVE IN PLACE FOR THESE SITUATIONS.
JUST BECAUSE A PATIENT MAXED OUT THE COPAY CARD.
1
2
3 DON’T CHANGE MEDICINES
NEED TO FIND SOLUTIONS WHEN A CARD IS MAXED OUT. THE SAME ISSUE WILL HAPPEN WITH A NEW MEDICINE.



50% of Rxs are processed without extra paperwork or phone calls

Thanks to EHR connectivity, CVS Specialty® can securely access essential data to help cut down on paperwork and simplify onboarding.




EHR (Electronic health record)
*CVS Health® Enterprise Analytics, April 15, 2020. Actual results may vary depending on bene�t plan design, member demographics, programs implemented by the plan and other factors.
Specialty Expedite is available exclusively for providers who use compatible EHR systems that participate in the Carequality Interoperability Framework or have an eligible EHR with CVS Health-enabled data connections and a fully executed connectivity agreement. All data sharing and usage complies with applicable privacy laws. Some payors have speci�c utilization management requirements related to prior authorization submissions (e.g., review, submission and/or physical signature by prescribers). Specialty Expedite is only used where permissible by payor requirements and applicable law. Patient privacy is important to us. Our employees are trained regarding the appropriate way to handle patients’ private health information.
©2022 CVS Specialty. All rights reserved.
75-1144729A 092622
your
to
Specialty®.
the
Send
prescription referrals
CVS
Scan
QR code to learn what else CVS Specialty o�ers. Can’t scan? Go to cvs.co/Derm
*
 By Neomia “Neo” Cuellar CCMS, PACS, ALLIANCE DERMATOLOGY
By Neomia “Neo” Cuellar CCMS, PACS, ALLIANCE DERMATOLOGY
Patient Advocacy
HOW TO HELP AND EMPOWER YOUR PATIENTS
As biologic coordinators, it is incumbent upon us to not only be an advocate for our patients, but also to teach them how to be their own advocates. I realized how critical this process was not as a BC, but as the parent of a patient.
Before I learned the lesson of advocacy from personal experience, I was accustomed to the day-to-day clinic flow. A patient comes in, the provider decides therapy, prior authorization begins, insurance determines access, and we usually conform to the directives of the insurance company. This is followed by a call made to the patient explaining the insurer requires step therapies first, which don’t always reach the therapeutic effectiveness needed by the patient.
Then life happened. My oldest stepson was diagnosed with bone cancer on the Monday after Thanksgiving. One day we were sitting around the table giving thanks for what we have, then within a few days’ time…pause…blank. Unknown.
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 16
CONTRIBUTED

“To help educate a patient, WE MUST STEP BACK AND REMEMBER THEY ARE NOT FAMILIAR WITH THE PROCESS. “
PRESSMASTERSTOCK.ADOBE.COM

“As biologic coordinators, it is incumbent upon us to not only be an advocate for our patients, BUT ALSO TO TEACH THEM HOW TO BE THEIR OWN ADVOCATES. ”
PRESSMASTERSTOCK.ADOBE.COM
PERSONAL JOURNEY
Everything happened so fast. After the doctors poked, prodded and diagnosed, we were told by the nurse of a children’s hospital that the chemotherapy and radiation would drop his white blood cell count, putting him at risk for infection. This could be prevented with an injection, but there were choices. On one hand, he could inject himself every day to keep the count up, or there was a therapy that would be only two injections a month. Side note—he’s nine years old. It was a no brainer. Let’s do the one that would be better for his quality of life—only two injections a month so he would not have to have the constant reminder of the diagnosis. The chemotherapy would do that enough as it was.
The office coordinator asked my husband, “What medical insurance do you have?” We had not provided the card yet. My husband responds, “Blue Cross Blue Shield” and her face drops. My mind flutters and a light bulb in my brain goes off, understanding why the look on her face, and I announce, “Of Tennessee!” See, I figured her fight against insurances and their formulary coverages must be just as bad or maybe worse than my own to get an insurance to approve a medication or procedure for a patient. We know not all formularies are the same. They can differ from state to state and plan to plan. Once she had the card she left and returned not too long later with an approval for the twice-a-month medication that would do the exact same job as the generic, which would have been every day. However, the branded medication she got approved would give my stepson one less thing that would cause him more pain in his life while on chemotherapy.
She acted as his patient advocate. She expressed that the patients acting as their own advocate can make or break the medication being approved. Only the patient can truly tell the story of how the condition affects them and their quality of life. This was the catalyst that changed the way I perform my job—no longer accepting the insurer’s decision.

ENCOURAGE ADVOCACY AND FIGHT FOR QUALITY OF LIFE
The conditions we see in dermatology such as psoriasis, atopic dermatitis, hidradentitis supportiva, alopecia and countless others affect a patient’s quality of life. These conditions dictate a patient’s day-to-day functioning: How they do their hair; Will they go to school or not because they are embarrassed; What clothes to wear; Should they be intimate with their partner; Do they have to sleep in plastic wrap and be uncomfortable. In addition, these conditions have such a negative impact that the patient can develop anxiety, depression and/or suicidal tendencies. There are countless studies correlating these diagnoses and suicide. A patient advocate fights to get the therapy needed to help that patient, which improves their quality of life, and relieves them of psychosocial embarrassment.
“Only the patient can truly tell the story of how the condition affects them and their quality of life. THIS WAS THE CATALYST THAT CHANGED THE WAY I PERFORM MY JOB... ”
Strive to educate your patients on how to be their own patient advocate in life. Patients are usually under the assumption they are prisoners of the decisions insurance companies make. Empower them with the knowledge that they have the right to call the insurance company and inquire about the status of prior authorizations, and, if denied, also initiate their own patient appeal. These steps can be used in all specialties for any medication and procedure.
To help educate a patient, we must step back and remember they are not familiar with the process. If prior authorizations and appeals are confusing to us in the field, it must seem even more confusing to the patients who have no experience with them. This is where we lose compassion sometimes in clinic, forgetting that we didn’t know the process at one point either. When we take a second to remember that it allows the dialog to open and creates a bridge to help explain the process in an understandable way. This first step empowers your patient with the knowledge and confidence of knowing the process. When they develop these two traits, they
bcofdermatology.com 19
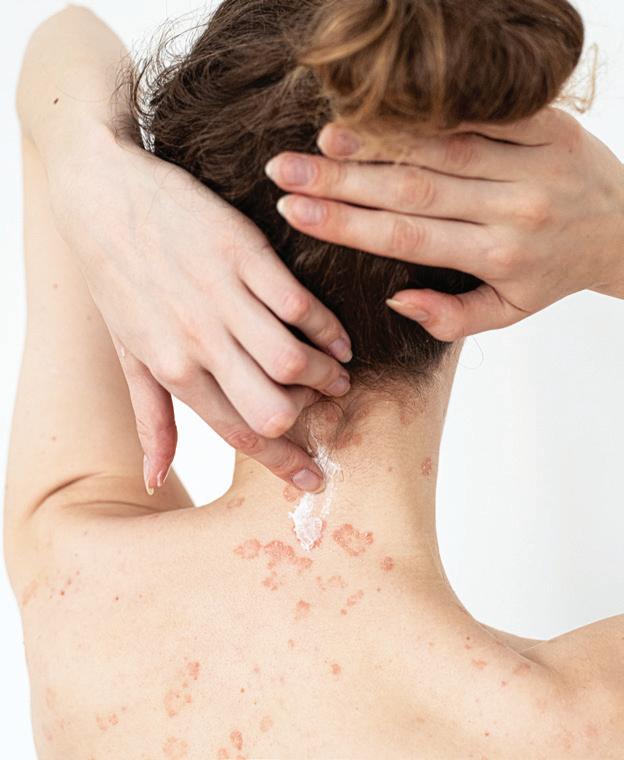
When Cuellar's stepson (above) was diagnosed with bone cancer at age 9, she became his patient advocate.

feel more inclined to take the role as their own patient advocate.
When a patient calls the insurance company, invite them to question who they are speaking with and to ask the questions that could impact decisions such as, “How long have you been authorized to process these kinds of cases?” and “What are your medical credentials?” These are important questions, because anyone who handles a prior authorization or appeal must have medical credentials of some kind that are backed by HIPAA certification. If the person on the other end of the phone does not have credentials or refuses to give them, patients should get their name and supervisor’s name or be transferred. If there are no credentials, the patient has the right to report this to the Insurance Board and this should be verbalized to the supervisor.
“Patients who reach out show the insurance company THEY ARE PART OF THE PROCESS AND WANT ANSWERS.”
Patients who reach out show the insurance company they are part of the process and want answers. Insurance companies are not used to the patient being an advocate in the PA and appeal processes. This is an advantage for the patient to raise the success rate of getting the medication approved. The insurance company cannot “sit” on the prior authorization since the patient is checking in, forcing them to move forward with a decision. In addition to the empowerment the patient receives from knowing the process, this kind of patient advocacy allows them to develop understanding and empathy for the office staff. My personal experiences include having to handle upset patients who call the office and demand, “Why isn’t it approved yet?” or “Why can’t you get the approval faster?” This opens the door of communication to explain the
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 20
Patient advocates Stan and Alice with Neo Cuellar
waiting game the insurance may put us through, and it allows me to help them know the next steps to move the process along.
OFFICE RESOURCES
An office resource with expectations of the patient’s role can empower them and make the process more rewarding. Patients should understand that no one can fight for them as hard as they can, because no one is better at explaining how their conditions affect their quality of life. They need to know the insurance companies aren’t motivated to approve a specialty drug right away. To that effect, even generic medications are rejected daily. Written instructions give a reference of who to call and a timeline to follow. Your handout can explain this process and be a reminder for a patient to answer their phone, which can be one of the hurdles to getting patients onboard. The material can also explain that patients should call the insurance company (not the office) if they want status of a prior authorization. This not only starts the patient serving as their own advocate, but it also offsets a lot of phone calls we get in the office, which simply result in the insurance stating they have not made a decision yet. I am not surprised by the burnout or turnover rate within offices. I relate with the struggles of the day-to-day in a clinic being short-staffed. There are more patients than staff. Open the line of communication with the patient, getting them onboard and empowering them with the knowledge that they can act as their own patient advocate. This can lead to success in approvals for the medications they need to improve their quality of life. While this takes some of the burden off the office staff, it also gives the patient a feeling of self-worth, commitment to therapy (since they also fought for it) and more patience with the office now that they understand we are all at the mercy of the insurance companies. Ultimately, a patient has the power to fight the insurance company, because they are not just a patient, but a patient advocate.

bcofdermatology.com 21
FIZKESSTOCK.ADOBE.COM

ALLIANCESTOCK.ADOBE.COM
By Heather Sawrey
GEORGE WASHINGTON UNIVERSITY, DEPT OF DERMATOLOGY, BIOLOGIC COORDINATOR

FRM Assistance
HOW TO UTILIZE FIELD REIMBURSEMENT MANAGERS
FRM. FAS. FRAS. ARM. Regardless of their title, the job of a field reimbursement manager (FRM) is to help offices (in our case, biologic coordinators) obtain the necessary information to get prior authorizations approved. Or, in the event of a denial, help put the patient on a bridge program until an approval is granted. Being our only HIPPA-certified entity, these FRMs truly are our co-pilots.
I have been in dermatology for 15 years, and I admit that when I first started as a BC—and probably for the next five or six years—I refused to work with a FRM. Like a lot of nurses and BCs, I had my own process, and I did not need anyone’s
help, regardless of who they were. Fast forward to when Dupixent was launched. This was for a new indication of biologics that had not been approved yet, and there were different guidelines to learn. In addition, we had to understand the
process and all the rules for the bridge program. At that point, I realized I would be hurting myself and our patients if I didn’t utilize the help and knowledge FRMs brought to the table. Since opening my eyes to FRMs at that point in my
23
bcofdermatology.com
career, the help I’ve received has been nothing short of amazing.
Field reimbursement managers assist us in so many areas of the patient spectrum. From help with prior authorizations, appeals support and bridge program push throughs, to specialty pharmacy phone calls, they are our heroes in dermatology.
For anyone who is not familiar with a FRM, they are different from the sales specialist (or drug rep) that comes to see the providers about their biologic. The sales specialists are there to help sell the drug, and while they do touch on access and affordability, it is not their biggest touch point when speaking to the HCPs. They offer valuable information, but sales specialists focus mainly on promotional information. The FRM concentrates on supporting the clinics, offering services that help both the clinic and the patient. These services include, but are not limited to, tracking payers, assisting with all our touch points, advocating for patients, monitoring the prescription journey from start to fulfillment, pushing through obstacles, assisting with copay issues and reviewing copay card enrollment. Essentially, they can help with anything that we need for our patients. One of the most important aspects of the field reimbursement manager is to be HIPAA certified. FRMs who are HIPAA certified can discuss specific patient information with the clinic and make phone calls on our behalf.
Let’s talk about the importance of FRMs in the daily process of biologic patients. I realize there are many different processes that biologic coordinators have, and maybe the current ones do not include contacting the FRM. Some BCs use specialty pharmacies to do the prior authorizations, while others do their own authorizations and then send it to the mandated specialty pharmacy upon approval. Think about all the steps that come before the prescription is sent to the pharmacy.
PREPARING PRESCRIPTIONS
This is not to say that our pharmacies are not good at what they do and how they handle our patients. We all need someone who is concentrated in the respected drug who knows the insurance layout for our own geography. Sometimes they can make things happen that SPs cannot. One of the main issues is having a patient who has exhausted their copay card. Finding someone within the pharmacy to help the patient and to help us often leads to a dead end. This is where the FRM can step in and tell us within a matter
“From help with PRIOR AUTHORIZATIONS, APPEALS SUPPORT AND BRIDGE PROGRAM PUSH THROUGHS, TO SPECIALTY PHARMACY PHONE CALLS, they (FRMs) are our heroes in dermatology.”
of minutes what we need to communicate to the patient to fix their issue. Once the patient does their part, the FRM can keep an eye on it and make sure that it goes through the proper channels.
For several years now, I have utilized the FRM for any biologic or oral medication that our office prescribes. Even if I do not utilize the support center (also known as the HUB), I always fax the drug enrollment form to make sure the FRMs have the HIPAA acknowledgement on file they need to assist patients. Without that acknowledgement, they cannot speak with us.
Field reimbursement managers are the ones who back us up and lend support, speaking with us about patients and any special issues happening. It is good practice to have the contact names and numbers handy in the event that you need them, regardless if they are a support option used in the everyday process. Reach out to the sales team to find out who the FRM is for the territory where your clinic is located. You will not be sorry that you did, because we all need a good co-pilot!
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 24
1
DOES THE PRIOR AUTHORIZATION? 2
HELPS WITH THE COPAY CARD? 3
SETS UP THE BRIDGE PROGRAM IF NEEDED? 4 WHERE IS THE PRESCRIPTION AT IN THE PROCESS? 5 HAS THE SPECIALTY PHARMACY DROPPED THE BALL? 6 IS THE AUTHORIZATION IN PLACE, BUT THE CLAIM IS NOT GOING THROUGH? 7 HAS THE PATIENT EXHAUSTED THE COPAY CARD AND IT IS ONLY APRIL? 8
WHO
WHO
WHO
WE CALL
A PATIENT
EXHAUSTED
THE
WHO DO
WHEN WE HAVE
WHO HAS AN
COPAY CARD AND IS NOT ABLE TO AFFORD
DRUG?
ENHANCE AND EXPEDITE PATIENT-FOCUSED COLLABORATION
Biologic Coordinators of Dermatology has partnered with Quincy to bring technological innovation to the FRM and BC workstreams.
Speed time to therapy and increase the efficiency of Field Reimbursement Managers and Biologic Coordinator collaboration with HIPAA-compliant secure messaging.
• Reduce patient onboarding time
• Increase employee productivity
• Increase the number new patients processed
• Increase staff and patient satisfaction
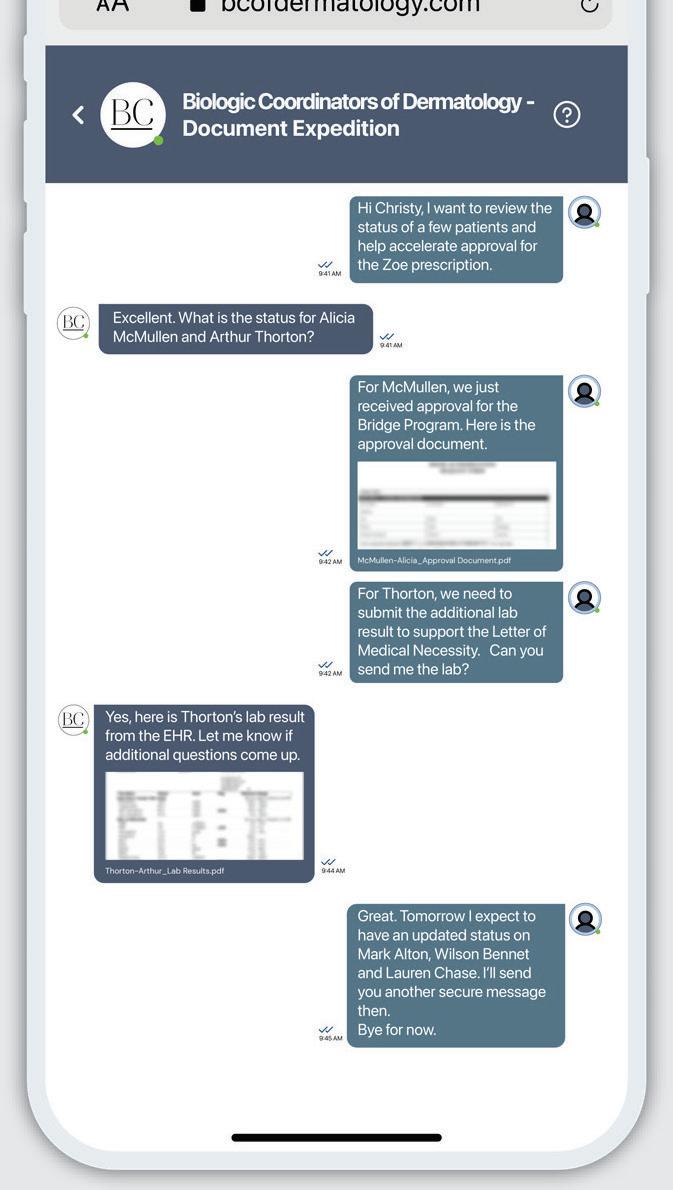

FEATURES:
• Initiate secure conversations between Field Reimbursement Managers and Biologic Coordinators
• Create Quick Messages for commonly used communications
• Personalized url to increase trust in the identity of the communicator
• Optional: Add Face to Face Video virtual visits for a personal touch

To learn more, see a demo, and take advantage of partnership discounts, please reach out to contact@bcofdermatology.com
SECOND ANNUAL BCOD CONFERENCE EVENTS



PatientAdvancing Access
The Second Annual BCoD Conference convened in Orlando, FL, November 3-5, 2022, to bring together biologic coordinators from around the country for education, networking and partnership. Over 170 biologic coordinators registered for the conference, with 102 of them receiving certification training. The largest academic institutions and supergroups in the country were in attendance with over 30 industry partners joining to share in the latest therapeutic and patient access solutions.
2 3 1
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 26
1. Heather Sawrey, Shayli Nagelkerk, Janelle Ball, Craig Schuette, Tommie Major, Christine Ramig, Marc Del Bono, Amy Brennan 2. Kareen Crider, Laura Cohoon, Christina Jordan, Ingrid Mollick 3. Megan Peebles, Kalie Carlton, Catherine O’Brien, Bayleigh Buckley





“It’s all about getting patients on therapy and letting them have a better quality of life.”


6
4. Heather Sawrey
5. Reeli Reina, PA-C; Carla Voorhees, Janssen
6. Kim Johnson, Sherice Money, Desiree Parker
7. Maria Maile, Eli Lilly; Beth Finnerty, Eli Lilly; Kim DesRosiers, Eli Lilly; Heather Hickey, Eli Lilly
8. Jessie Ehrman, Chloe Adams, Kalyn Khalaf, PxTechnology 9. Rose Myers, Abbvie; Courtney Mayfield, Sage Wodarz, Abbvie
10. Jeremy Osten, Leo Pharma 10
What is the conference about?
8 4 5 7 9 bcofdermatology.com 27
— Sherice Money






13
11. Margaret Bobonich, CNP, DNP 12. Erickson Brown, Janssen; Tara Haltom Biggers; Lisette Marchant 13. Brandie Shepherd, Christy Roof, Amy May 14. Shanna Settlemire, Amgen; Angela Lee
14 11 SECOND ANNUAL BCOD CONFERENCE 12 15 16 ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 28
15. Craig Schuette, BCoD; Scott Drew, DO, FAOCD; Celine LaFratte, Dermavant Sciences; Fred Hockenjos, Dermavant Sciences 16. Amanda Pulver, Jaime Boyer, Brandi Eikenberry
EVENTS



17. Kirk Gautier, PA-C 18. Rachel Stubbs, Celina Barrios 19. Marc Del Bono; Brittney Hubbard, Ortho Dermatologics; Janelle Ball; Kirsten Kidd, Ortho Dermatologics
20. Orlando Symphony


LOOKING AHEAD TO 2023
F or the first time, BCoD will open its conference attendance to prescribers and anyone in the practice office that oversees any part of the patient access journey, including medical assistants and office managers.
The 2023 conference will deliver an unprecedented experience— bringing together the most biologic coordinators and dermatologists under one roof seeking to ensure access for their patients.
VISIT
BCOFDERMATOLOGY.COM to learn more about this highly anticipated event that is guaranteed to be sold out.
2022 BCOD CONFERENCE PARTNERS
17 18
Abbvie • Acaria Health • ACMA • Amgen • Arcutis Biotherapeutics • AssistRx • BioplusRx• Blue Sky Pharmacy • BMS CareMetx • CVS Health • Dermavant Sciences • Eli Lilly • Humana Centerwell Specialty Pharmacy • Impiricus • Incyte Janssen • Kroger Health • Leo Pharma • MinderaHealth • MLinkRx • Novartis • Ortho Dermatologics • Pfizer • PxTechnology Rep Reach • RxVantage • Sanofi | Regeneron • SenderraRx • Sun Pharma • Trial Card | Policy Reporter • UCB
19
20
bcofdermatology.com 29
New Year NEW
EXPERTS SHARE WELLNESS TIPS
After the fanfare of the holidays, the new year is the perfect time for reflection and a chance to focus on personal goals and aspirations. If health is your main priority for 2023, you are not alone. Over 50% of the population has a health-related goal as their top resolution. To set our sights on an even better year, we’ve consulted experts on the best tips, practices and products for a happier and heathlier 2023.
ACCESS DERMATOLOGY JANUARY/FEBRUARY 2023 30





YOU BE WELL IN 2023!

 BY JACKIE FIGUEIREDO Founder of Fig Nutrition & Wellness Certified Nutritionist & Holistic Health Practitioner
BY JACKIE FIGUEIREDO Founder of Fig Nutrition & Wellness Certified Nutritionist & Holistic Health Practitioner
Healthy Eating
During this time of year, people often wonder how they can do better or simply “reset.” A lot of my clients are still feeling pressure from overindulging at the holidays and honestly, who isn’t? This is the first year in a long time that many people have been able to spend the holiday season with their extended friends and family. This contributed to many of us throwing caution to the wind when it comes to nutrition, self-care and sleep—what I like to call, “Being whole body healthy.” Follow my five tips for becoming a more healthful self, and you'll be back on track.

FORGET THE IDEA THAT WORKOUTS AND NEW WAYS OF EATING NEED TO START ON MONDAYS. MONDAY IS AN ARBITRARY DAY OF THE WEEK THAT GIVES NEGATIVE ENERGY AND CAN BE OVERWHELMING.
Sabotaging your entire weekend gives a person more to “clean up” on Monday, instead of taking things day by day or even meal by meal. I coach clients to think of each meal as a chance to do better. If a person wants to indulge over an amazing brunch, I say do it—knowing that your mid-afternoon snack and dinner are going to be loaded with more nutritious foods. Life is about enjoyment, not denying yourself. It’s a matter of balance each day. Use the 80/20 rule as a guide—80% of the time consume food that is nutritious and healthy, and 20% of the time, enjoy whatever you want.
HOW TO GET “ WHOLE BODY HEALTHY ”
KNOW THAT DIETS AND CLEANSES DON’T WORK. ALTHOUGH A SHORT-TERM FIX IN SOME CASES, THE LONG-TERM EFFECTS ARE NOT THE OUTCOME THAT ANYONE SEEKS.
What does work, is retraining your brain to healthier habits and lifestyle choices and arming yourself with the knowledge of anything you put into your body MUST have a benefit to your health. This should be non-negotiable. If something on your plate or on a menu doesn’t have a health benefit, it’s not welcome in your body. And if you are reading a label on packaged food and the words do not resonate as words you know as real food, it’s also not welcome.

QUICK TIP: Shop the perimeter of the grocery store for fresh and healthy foods—many of the items in center aisles are sitting on shelves with added chemicals and preservatives.

CHECK IN WITH YOUR SLEEP QUALITY. I’M A FIRM BELIEVER IN GETTING GREAT SLEEP EACH NIGHT TO HELP SUPPORT A PERSON’S GOALS AND HEALTH.
I can’t say how much sleep, because each person differs on the amount they need for optimal performance. Getting at least seven hours of solid, sound sleep each night helps with overall health. When someone doesn’t sleep well, the body over produces the hormone gherlin (also known as “the hunger hormone”) and the “satisfaction hormone” called leptin, lowers— which in turn leads to people overeating when they really are not that hungry. Often, they are just tired. This turns into a vicious cycle of cravings and overindulging.

MOVE FOR AT LEAST 30 MINUTES A DAY.
This doesn’t mean you have to go to a gym or run miles and miles. It means that any kind of movement that gets your heart rate going for at least 30 minutes per day will do your body well. This can be chasing your kids around the house or going for an easy walk. Studies show that people who move their bodies with low to moderate impact each day have a lower chance of developing heart-related illness later in life.

MOST IMPORTANT IS HYDRATION!
Water, water, water. I can’t say this enough because it really is the fuel to the fire of your overall health. Hydrating with real water is simple, but I get that a lot of people don’t love plain water. Like I said before, READ labels. There are some great drinks out there to substitute with, but make sure they are not loaded with sugar or even worse, chemicals. If you don’t like plain water, add some lemon juice or grapefruit but get at least 90 ounces of water each day. Remember: dehydration can mask itself as hunger, so the more hydrated someone is, the less likely they are to overeat.
bcofdermatology.com 33

 BY ARTUR KIRSH Artur Kirsh Salon in Philadelphia
BY ARTUR KIRSH Artur Kirsh Salon in Philadelphia
Best Tressed
When it comes to healthy hair, the most important thing you can do is to see your hairdresser routinely, just like you would for a doctor checkup. It is recommended to go every six to 12 weeks whether it is for a consultation, hair color, or a simple healthy trim. In between visits, the easiest thing you can do at home to maintain healthy hair is to follow six basic tips.
HAIRCARE TOOLS & TIPS

1. SHAMPOO ONLY THE ROOTS TO AVOID EXCESSIVE DRYING OF THE ENDS.
2. CONDITION ONLY THE ENDS TO AVOID EXCESSIVE BUILDUP AT THE SCALP, WHICH RESULTS IN FLAT HAIR.
3. USE A NATURAL BOAR BRISTLE BRUSH, AS IT DISTRIBUTES YOUR SCALP OIL MORE EVENLY ACROSS YOUR HAIR.
4. WHEN BLOW DRYING YOUR HAIR, USE PRODUCTS TO PROTECT THE HAIR BEING DAMAGED BY EXCESSIVE HEAT. HEAT PROTECTANTS ADD PROTECTION AGAINST BREAKAGE AND ADD MOISTURE TO YOUR HAIR.

HAIR RESCUE BY A.S.K.
This leave-in hydrating hair therapy contains provitamin D5 to repair damaged hair by sealing split and broken ends. Smoothing proteins and conditioners prevent hair damage from heat and frizz.

Not only does this brush cause less damage and breakage, but it also circulates natural hair oils more evenly.
bcofdermatology.com
5. WHEN YOU SHAMPOO YOUR HAIR, MASSAGE YOUR HAIR GENTLY FOR BLOOD CIRCULATION TO STIMULATE THE FOLLICLES AND ENCOURAGE HAIR GROWTH.
PRODUCT RECOMMENDATIONS
6. TRY NOT TO WASH YOUR HAIR EVERY DAY SO YOU DON’T STRIP THE NATURAL OILS, OR SEBUM, THAT IS PRODUCED INSIDE THE SKIN ALONGSIDE YOUR HAIR FOLLICLES. THIS NATURAL OIL HYDRATES YOUR SCALP AND CONDITIONS AND PROTECTS YOUR HAIR NATURALLY.

ARTUR FULL BODY SPRAY
Add incredible volume to the hair without a stiff or tacky feel. This alcohol-free styling spray gives you shine and volume without drying the hair.
35

 BY DR. GARY GOLDENBERG and DR. KRISTINA GOLDBERG Goldenberg Dermatology
BY DR. GARY GOLDENBERG and DR. KRISTINA GOLDBERG Goldenberg Dermatology
Skincare
Starting the new year with healthy habits is no different than focusing on the health of your skin. Good skincare and an anti-aging regimen start with four steps, and we highly recommend this simple process.

FOLLOW DAILY REGIMEN
Use a gentle cleanser and sunscreen with a moisturizer in the morning, followed by a cleanser and retinol with a moisturizer at night.
This basic habit will get a patient to 90% of their goal. You can always increase product utility after that, but we often see patients who have too many products end up not using any because of confusion or they use all the wrong ones. These extremes can be avoided by staying true to the basics.

CUSTOMIZE YOUR APPROACH

Each person is different and may require a customized approach to skincare. Ingredients to look for depend on the goals of the patient. If you have sensitive skin, use a moisturizer that is hypoallergenic and a cleanser that is hydrating. For those with acne prone or oily skin, it is recommended to use salicylic acid or glycolic acid to help take oil away, shrink pores, and maintain even-toned skin.
TOP PRODUCTS FOLLOW DAILY REGIMEN
Hydration is very important to skin health. Just because your face cream has a moisturizer doesn’t mean your skin is hydrated. If you’re not hydrated, you skin will suffer.

GO LOW AND SLOW WITH PROCEDURES
For many people, their new year’s resolution to improve the appearance of their skin goes beyond lotions and ingredients, and instead relies on cosmetic procedures. We encourage supplementing procedures with a skincare regimen, but with this important philosophy: achieving a natural look is more important long-term than trying to gain a quick fix with an excess of fillers or procedures. Patients look best when they are refreshed and naturally enhanced rather than achieving unnatural proportions or sizes of features. Find a doctor that you trust who does not push the most procedures you can get.

LA ROCHEPOSAY TONER
An exfoliating acne toner with salicylic acid and glycolic acid, this product helps remove pore-clogging debris and smooths the skin texture. laroche-posay.us

VANICREAM
Formulated with hyaluronic acid and ceramides, Vanicream hydrates and retains the skin’s moisture. vanicream.com
bcofdermatology.com

CETAPHIL
Cetaphil is hypoallergenic, with a non-comedogenic formula for sensitive skin that is free of fragrances, parabens and sulfates. cetaphil.com

AQUAFOR
This skin protectant ointment contains Panthenol and Glycerin to nourish and protect skin. aquaphorus.com
37

 BY BEN HUNTER, MD Medical Director of Professional Services, Skyland Trail
BY BEN HUNTER, MD Medical Director of Professional Services, Skyland Trail
State of Mind

Mental health is complex and multifactorial, and this means there are many levers we can pull to improve our mental wellbeing. Mindfulness, psychotherapy ("talk therapy"), gratitude practices, healthy dietary choices, and social engagement are only some of the important items on an extensive list of practices that have received scientific validation in randomized controlled trials. However, perhaps the most effective and accessible driver of mental wellness is exercise.
Exercise promotes healthy hormone and neurotransmitter balance, reduces body and brain inflammation, and promotes neuroplasticity— the healthy growth and remodeling of the brain. It improves connections between functional regions of the brain and improves blood supply to all areas of the body, including brain tissue. Perhaps equally important, engaging in regular exercise has a profound psychological effect, promoting self-efficacy, self-regulation, self-esteem, and healthy reward response. Moreover, regular exercise tends to lead to other health-promoting behaviors such as healthy eating and improved sleep patterns. Numerous studies have demonstrated a significant role for exercise in both treating and preventing symptoms of depression, anxiety, and other mental illnesses. (See Sources on opposite page.)

Those new to exercise may find the prospect of regular physical activity daunting. However, research suggests that workouts or other forms of physical movement do not need to be arduous or time-consuming. While the greatest effect seems to come from a combination of strength training and cardiovascular exercise—a combination found in almost all exercise classes—even low-intensity training such as walking or light calisthenics seems to significantly benefit mental wellness.



1. CHOOSE A FORM OF EXERCISE YOU ENJOY. THE BEST EXERCISE REGIMEN IS THE ONE YOU WILL COMPLETE!
Start slowly. Choose a routine that is easy to accomplish within the constraints of your schedule, and don't overdo it in terms of intensity. Nothing is more discouraging than doing a few workouts and getting derailed by muscle soreness or injuries. Consult with a licensed professional trainer to maximize your chances of success.

2. CREATE ACHIEVABLE, MOTIVATING FITNESS GOALS AND TRACK YOUR PROGRESS. THIS WILL HELP YOU REMEMBER WHY YOU’RE PUTTING FORTH THE EFFORT.
• Find an accountability partner. Exercise is often more fun with others, and a workout buddy can help motivate you to exercise on those days you'd rather skip the gym!
• Couple exercise with mindfulness practices, healthy eating, breath work, or psychotherapy for additive benefit.
• Always consult with a healthcare provider before beginning any new exercise regimen.

3. MOST IMPORTANTLY, DON'T BE DISCOURAGED IF THINGS DON'T GO EXACTLY ACCORDING TO PLAN.
Flexibility and resilience in the face of challenge are hallmarks of mental health, so use your exercise routine as a chance to practice these key wellness tenets!
SOURCES
1. Smith PJ, Merwin RM. The Role of Exercise in Management of Mental Health Disorders: An Integrative Review. Annu Rev Med. 2021 Jan 27;72:45-62. doi: 10.1146/annurev-med-060619-022943. Epub 2020 Nov 30. PMID: 33256493; PMCID: PMC8020774.
2. Merom D, Phongsavan P, Wagner R, et al. Promoting walking as an adjunct intervention to group cognitive behavioral therapy for anxiety disorders--a pilot group randomized trial. J Anxiety Disord 2008; 22:959. 3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am J Psychiatry 1998; 155:603.
4. Saeed SA, Antonacci DJ, Bloch RM. Exercise, yoga, and meditation for depressive and anxiety disorders. Am Fam Physician 2010; 81:981. 5. Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. META-ANALYSIS OF ACUTE EXERCISE EFFECTS ON STATE ANXIETY: AN UPDATE OF RANDOMIZED CONTROLLED TRIALS OVER THE PAST 25 YEARS. Depress Anxiety 2015; 32:624.
6. Herring MP, Hallgren M, Campbell MJ. Acute exercise effects on worry, state anxiety, and feelings of energy and fatigue among young women with probable Generalized Anxiety Disorder: A pilot study. Psychol Sport Exerc 2017; 33:31.
bcofdermatology.com 39
FIT BODY & MIND
Once a prescribing decision has been made to prescribe TREMFYA® (guselkumab), enroll patients in
A dedicated support program for TREMFYA® patients
TREMFYA withMe provides a range of dedicated support to help make it easier for patients as they begin, and continue, their TREMFYA® treatment journey.
If a patient has been prescribed TREMFYA® for approved on-label use and is 18 or older, they are eligible for this program.
Visit tremfyawithme.com to learn more about all that TREMFYA withMe offers patients.

Scan this QR CODE with your phone’s camera
Here’s how you can sign up patients.
Scan or Visit:
Visit tremfyawithme.com/ healthcare-professionals to sign up patients and for more information.

Portrayal
Actor

The TREMFYA withMe Guide
At the core of the program is a TREMFYA withMe Guide, a dedicated and qualified healthcare professional* who can work one-on-one with patients to:



Answer questions about prescription fulfillment and cost support
Patients will receive education on specialty medication fulfillment and options that could make their treatment more affordable.
Connect patients to injection support
You are the best person to review the injection process with your patients and help them understand what to expect. The TREMFYA withMe Guide is also available, after you have talked with your patients, if they have questions about injections.
Help with treatment expectations
Patients will get information on what to expect with their TREMFYA® treatment.
Provide regular updates and reminders at the patient’s request
Patients may receive support to help them stay on track with their treatment, plus updates on their TREMFYA® treatment journey.
Our goal is to provide a live person who can compassionately deliver the precise information that patients may want to help them with their treatment.
The TREMFYA withMe Guide can also help patients access other channels of support.
*Guides do not provide medical advice.






Actor Portrayal
© Janssen Biotech, Inc. 2022 09/22 cp-333177v1

NOVEMBER 5-7, 2023
BCoD 2023 Annual Conference
JW Marriott San Antonio Hill Country Resort & Spa
The BCoD Annual Conference is the premier event for uniting biologic coordinators, office staff, and expert resources for candid discussion and timely best practices that reduce patient burden and advance patient access, in the heart of beautiful Texas hill country.
Join us and tap into expert acumen delivered through immersive workshops, training sequences, certification offerings, and exciting networking opportunities.

”The ability to learn from and rely on my peers has helped me stay up to date with the market and changes in patient programs, but it has also allowed me to see real change in our processes. These changes have ultimately led to getting drugs into the hands of patients more efficiently and effectively. I highly recommend this community to anyone in the industry looking to grow and improve their skills.”
2022 MEETING REACHED CAPACITY FOR MEMBERS AND INDUSTRY.
LEARN MORE AT BCOFDERMATOLOGY.COM
WITH TWO OFFICE PROFESSIONALS
BiologicCoordinator Spotlight Q&A
Biologic Coordinators of Dermatology celebrates our members and everyone who ensures a positive patient access journey. In order to bring prominence to the biologic coordinator role and to build a stronger BC network, we are highlighting two well-respected professionals who have shown extraordinary commitment and teamwork and enriched the lives of their patients.
Tina Brooke
TRIAGE COORDINATOR, BIOLOGIC COORDINATOR, RESEARCH ASSISTANT
DERMATOLOGY ASSOCIATES OF PLYMOUTH MEETING PLYMOUTH MEETING, PA
Q: WHAT IS ONE PROGRAM OR PLATFORM YOU CANNOT LIVE WITHOUT?
A: Abbvie Complete Portal
WHAT KIND OF CASES INSPIRE YOU TO GAIN PATIENT APPROVAL?
Minors who are extremely uncomfortable because of the severity of their skin condition. I recall a three-year-old who was referred to our office from an allergist. Her atopic dermatitis relapsed after discontinuing medication because of Covid. The little girl’s condition was so bad that she came in with her hands in a sock. Despite her having previously been on every topical therapy imaginable, her insurer denied the prescribed biologic because they wanted her to try MTX or three other biologics first.
WHAT WAS YOUR JOURNEY LIKE BECOMING A BIOLOGIC COORDINATOR?
I worked in a primary care office for 25 years as a medical assistant. Transitioning to dermatology was natural for me, but it required learning all new medications. I love my job and the patients I work with, and I’m happy I made the change.
HOW HAVE YOU SEEN THE BIOLOGIC COORDINATOR ROLE EVOLVE,

AND DO YOU BELIEVE IT WILL CONTINUE TO DO SO?
Before, everything was done on paper and by faxing to the insurance companies. Now it has evolved on the platform of ePA on CoverMyMeds, and it’s done in one place.
WHAT KEEPS YOU BUSY OUTSIDE OF THE OFFICE?
Bike riding, motorcycle riding with my husband and son, swimming, keeping up with my three dogs.
ANY TIPS FOR OFFICE EFFICIENCY?
Use Excel to track your patients. I have a spreadsheet for seven physicians, which lists every biologic, and under each of them is the patient’s name, date of birth, account number, lab date for TB Gold, last office visit, expiration date of prior authorization, insurance company and specialty pharmacy. I also have spreadsheets that contain the list of specialty pharmacies, all the biologics with directions, manufacturer names and programs, hub phone numbers, fax numbers, drug representatives, and patient assistance phone numbers.
bcofdermatology.com 45
Melanie Leister, office manager; Tina Brooke, biologic coordinator
Rebecca Cullen
MA/CDT MEDICAL PATIENT LIAISON
PIEDMONT HEALTHCARE/ PAVIOL DERMATOLOGY
CHARLOTTE, NC
Q: WHAT IS ONE PROGRAM OR PLATFORM YOU CANNOT LIVE WITHOUT?
A: CoverMyMeds
DO YOU HAVE ANY PATIENTS THAT KEEP YOU UP AT NIGHT?
A female with alopecia who has complete hair loss, including her eyebrows, presented psoriasis. Despite continued prior authorizations, appeal letters and peer to peer efforts, the insurer would not approve the preferred medication. They recommended another drug but since she was of childbearing age, we didn’t want her taking it. Her PsO was treated with a biologic through a manufacturer drug program. She is clear now, but the program has run out and she needs to be placed on a new one. Through it all, her spirit was commendable, and that further drives me to do everything I can for my patients.
WHAT WAS YOUR JOURNEY LIKE BECOMING A BIOLOGIC COORDINATOR?

I worked with a previous physician for 13 years as an MA prior to starting with Paviol Dermatology. My role took me from the front desk to transitioning to a triage and biologic coordinator.
DOES THE TITLE BIOLOGIC COORDINATOR ENCOMPASS
WHAT YOU DO?
There is more than the title may insinuate. I also take on triage and assist with nurse visits, including blood draws, suture removals and injections.
WHAT KEEPS YOU BUSY OUTSIDE OF THE OFFICE?
My four children and four grandchildren.
ANY TIPS FOR PATIENT COMMUNICATION?
Be constant and consistent. I always try to keep my patients well-informed. This helps to build a relationship with them. It is important that they trust you and know you’ll follow through on patient responsibility.
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 46
Q&A WITH TWO OFFICE PROFESSIONALS
Pamela Myers, Rebecca Cullen, Scott Paviol, MD
 By Staci Piner BIOLOGIC COORDINATOR, DERMATOLOGY AND SKIN CANCER SURGERY
By Staci Piner BIOLOGIC COORDINATOR, DERMATOLOGY AND SKIN CANCER SURGERY
Path to Afford ility
While not a biologic, VTAMA® (tapinarof) 1% cream is the first topical, non-steroidal medication in its class for the treatment of adult patients with plaque psoriasis. VTAMA has proven to be a competitive treatment option for psoriasis and has continued to show convincing results among our psoriasis population. It is steroid-free and safe to use in all affected, external areas as demonstrated in clinical studies over 52 weeks.
My experience with a MyVTAMA patient savings card has been positive, and I’d like to share my knowledge of moving through the prior authorization (PA) process as effortlessly as possible.
 VTAMA'S COPAY PROGRAM
VTAMA'S COPAY PROGRAM
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 47
COST MATTERS
As a patient myself, I want the cheapest copay possible for my medications, and I take on that same mindset when trying to get my patients on therapy. The MyVTAMA patient savings card provides commercially insured patients with a $0 copay if approved, or a $75 copay when a prior authorization is not completed, denied, or VTAMA is excluded from a patient’s insurance plan. We owe it to our patients to take advantage of this $0 dollar offering. While the path of least resistance will be to have the pharmacy use the copay card as a secondary insurance and have the patient pay $75, this drug is often readily obtained at $0. In my experience, the PA process for VTAMA carries little friction, and when using a specialty or preferred pharmacy to assist with completing the PA, I ensure they follow through on completing the PA to attain the best possible price.
MINDFUL APPROACHES
TO ACCESSING VTAMA
Appropriate chart documentation is of utmost importance. Take a minute to review the patient’s chart before submitting a prior authorization to ensure all necessary information has been documented. Step therapy can be required before VTAMA is approved. Step therapy requirements may consist of a trial or failure of a strong


“In my experience, THE PA PROCESS FOR VTAMA CARRIES LITTLE FRICTION, and when using a specialty or preferred pharmacy to assist with completing the PA, I ENSURE THEY FOLLOW THROUGH ON COMPLETING THE PA TO ATTAIN THE BEST POSSIBLE PRICE.”
to mid-potency topical corticosteroid, and a topical vitamin D analog such as calcipotriene. We often see psoriatic patients having struggled with their disease for several years prior to being treated at our office, and in most cases, they have tried and failed numerous topical steroids and non-steroidal agents. Be investigative about your patients’ prior tried and failed therapies. While their response may be, “I have been on so many different creams, but I cannot recall specific names,” take a few extra minutes to run medication names by them (i.e., clobetasol, triamcinolone, betamethasone, protopic (tacrolimus), calcipotriene). This often allows them to recognize the familiarity of a name, and this recall will strengthen your position in the prior authorization process. The biggest obstacle with the PA is the trial/failure of a topical vitamin D analog such as calcipotriene, Enstilar and Såorilux, among others. If you come across a patient who does not have a true failure to a topical vitamin D analog, have your physician send a prescription to the pharmacy during their visit.
Proactively submit a prior authorization for VTAMA before a prescription is sent to the pharmacy. I provide my patient with a few samples as well as a VTAMA brochure. Then I’ll have the patient scan the QR code on the back of the brochure so they can register for the MyVTAMA patient savings
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 48
“When submitting an appeal, I like to use key phrases like, ‘PSORIASIS NEGATIVELY IMPACTS PATIENTS’ QUALITY OF LIFE’ OR ‘PLEASE APPROVE MEDICATION FOR MY PATIENT WHO REQUIRES AND DESERVES APPROPRIATE TREATMENT OF THIS DEVASTATING DISEASE.’”
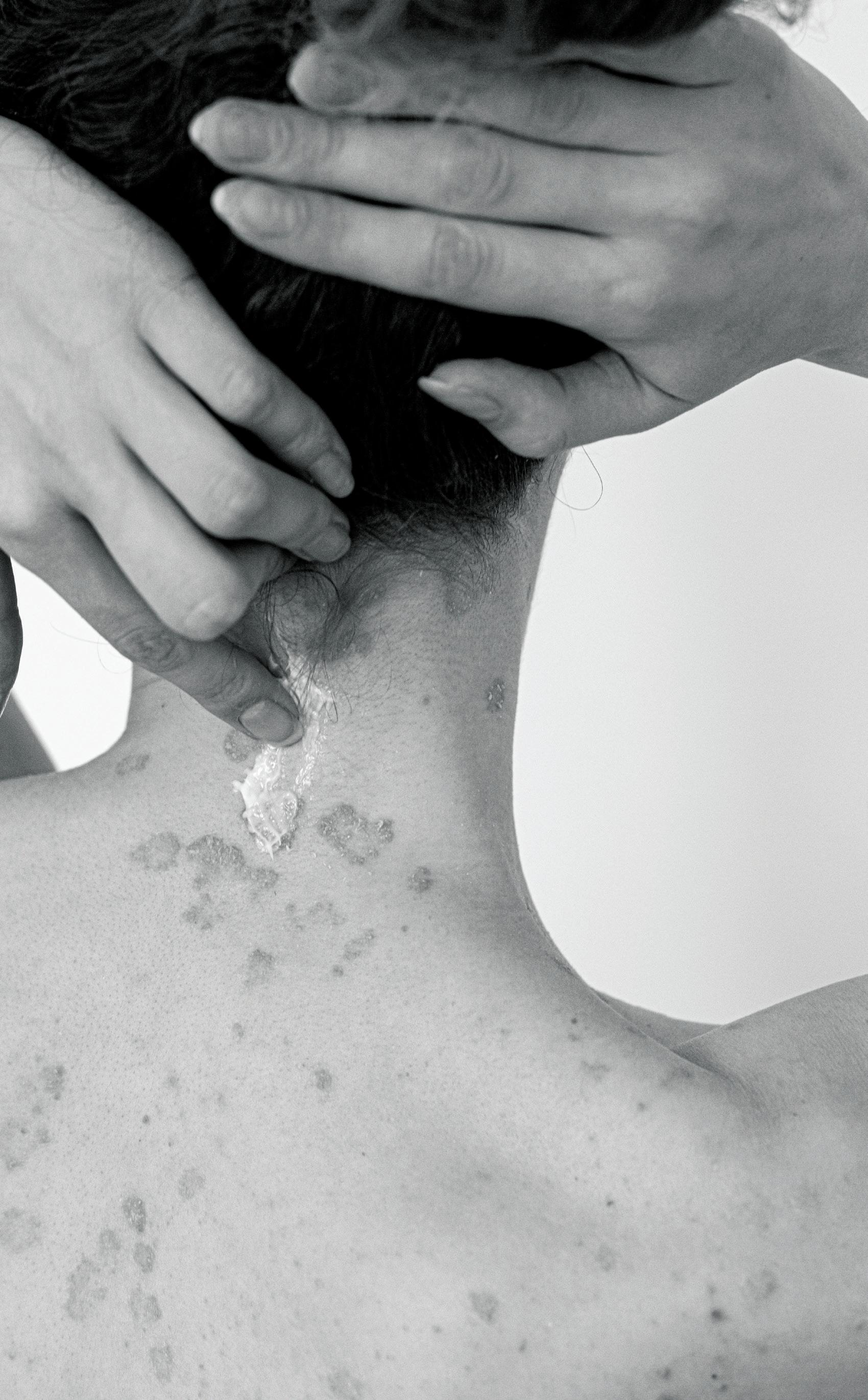
card. The registration process should not take the patient more than a couple of minutes to complete. Pharmacies should also have this readily available if the patient does not register.
Once I receive the prior authorization outcome, I will send the VTAMA prescription to the preferred pharmacy along with the PA approval or denial letter. I have learned that by refraining from sending the prescription during the office visit, it helps to prevent duplicating prior authorization requests, which can further delay on the prescription process.
If I do receive a prior authorization denial, I will submit an appeal that day. I personally recommend creating a fill-in-the-blank style appeal template that can be used for all patients. Taking a few minutes to do this will keep the process simple and efficient, requiring minimal effort on your end moving forward. I have had a high volume of prior authorization denials overturned with an appeal. When submitting an appeal, I like to use key phrases like, “Psoriasis negatively impacts patients’ quality of life” or “Please approve medication for my patient who requires and deserves appropriate treatment of this devastating disease.” My goal with an appeal letter is to paint a clear picture for the insurance company to illustrate why my patient requires VTAMA.
CONCLUSION
The MyVTAMA patient savings card provides an often predictably positive experience, allowing patients to obtain VTAMA at an affordable price. When appropriate effort is made, I find that affordable price to be $0, which also increases patient adherence and persistency. Even if the prior authorization is not successful, I love that my patients can still obtain their VTAMA medication for $75.
I currently manage over 200 VTAMA prescriptions and our patients have consistently achieved meaningful improvement and even clearance. It's rewarding for me to see my patients report a zero-itch rating.
bcofdermatology.com 49







VTAMA cream can give plaque psoriasis patients significantly clearer skin. So they’re free to think about…literally anything.


VTAMA cream is a once-daily, non-steroidal topical for adults with plaque psoriasis. 36% and 40% Physician Global Assessment (PGA) success rate* in two 12-week pivotal studies vs 6% and 6% for vehicle 2
Safe and well-tolerated even on the face, neck, intertriginous areas, inframammary areas, axillae, genitalia, and anal crux.1,3,4

See the data: VTAMA.com

A median ~4 month off-treatment effect seen in long-term, open-label extension study of patients (n=73)2
*PGA success rate defined as PGA=0 or 1 and ≥2-grade improvement from baseline at week 12.
Important Safety Information:
References:
1. Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219–2229.

Indication: VTAMA® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults. Adverse Events: The most common adverse reactions (incidence ≥1%) in subjects treated with VTAMA cream were folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu). You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
2. VTAMA (tapinarof) cream, 1%. Prescribing Information. Dermavant; 2022. 3. Strober B, Stein Gold L, Bissonnette R, et al. Tapinarof cream 1% once daily for plaque psoriasis: long-term extension trial of a novel therapeutic aryl hydrocarbon receptor modulating agent. Oral presentation at European Academy of Dermatology and Venereology; September 30, 2021. 4. Stein Gold L, Kircik L, Lebwohl M, et al. Tapinarof cream 1% once daily for plaque psoriasis: favorable local tolerability in two pivotal phase 3 trials. Poster presentation at Innovations in Dermatology 2021; March 16–20, 2021. Please see the Brief Summary of VTAMA cream on the following page.


Now FDA Approved © 2022 Dermavant Sciences, Inc. All Rights Reserved. All trademarks are the property of Dermavant Sciences, GmbH. US-VTAMA-2200072-03




BRIEF SUMMARY
IMPORTANT INFORMATION ABOUT
VTAMA® (Vee-TAM-uh) (tapinarof) cream, 1%
This summary contains important information about VTAMA cream. It is not meant to take the place of your doctor’s instructions. Read this information carefully before you start using VTAMA cream. Ask your doctor or pharmacist if you do not understand any of this information or if you want to know more about VTAMA cream. For full Prescribing Information and Patient Information, please see the package insert.
WHAT IS VTAMA cream?
VTAMA cream is a prescription medicine used on the skin (topical) to treat plaque psoriasis in adults. It is not known if VTAMA cream is safe and effective in children under 18 years of age.
Do not use VTAMA cream for a condition for which it was not prescribed. Do not give VTAMA cream to other people, even if they have the same symptoms you have. It may harm them.
Important: VTAMA cream is for use on the skin (topical use) only. Do not use VTAMA cream in your eyes, mouth, or vagina.
WHAT SHOULD I TELL MY DOCTOR BEFORE USING VTAMA cream?
Before you use VTAMA cream tell your doctor about all of your medical conditions, including if you:
•Are pregnant or plan to become pregnant. It is not known if VTAMA cream will harm your unborn baby; and/or
•Are breastfeeding or plan to breastfeed. It is not known if VTAMA cream passes into your breast milk. Talk to your doctor about the best way to feed your baby during treatment with VTAMA cream. Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
HOW SHOULD I USE VTAMA cream?
•Use VTAMA cream exactly as your doctor tells you to use it.
•Apply a thin layer of VTAMA cream only to your psoriasis skin lesions one (1) time a day. Avoid applying VTAMA cream to unaffected areas of your skin.
•Wash your hands after application, unless VTAMA cream is for treatment of your hands.
•If someone else applies VTAMA cream for you, they should wash their hands after application.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF VTAMA cream?
The most common side effects include: folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu).
These are not all the possible side effects of VTAMA cream. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA at www.fda.gov/ medwatch or call 1-800-FDA-1088.
HOW SHOULD I STORE VTAMA cream?
•Store VTAMA cream at room temperature, between 68℉ to 77℉ (20℃ to 25℃).
•Do not freeze VTAMA cream.
•Protect VTAMA cream from exposure to excessive heat.
•Keep VTAMA cream and all medicines out of the reach of children.
WHAT ARE THE INGREDIENTS IN VTAMA cream?
Active ingredient: tapinarof
Inactive ingredients: benzoic acid, butylated hydroxytoluene, citric acid monohydrate, diethylene glycol monoethyl ether, edetate disodium, emulsifying wax, medium-chain triglycerides, polyoxyl 2 stearyl ether, polyoxyl 20 stearyl ether, polysorbate 80, propylene glycol, purified water, and sodium citrate dihydrate.
VTAMA is a trademark of Dermavant Sciences, GmbH or its affiliates.
Dermavant Sciences Inc., Long Beach, CA 90806 USA


PATIENT ACCESS PROGRAM SURVEY
Manufacturer patient assistance programs are in place to augment existing prescription drug coverage. Our biologic coordinators routinely lean on these types of programs to help patients access treatment .
We surveyed 139 biologic coordinators to gain insight into their experience with current manufacturer patient access programs, touching on overall satisfaction, quality of nursing programs, efficacy of HUBs, and their interest to learn more.
GEORGIISTOCK.ADOBE.COM bcofdermatology.com 53
Programs with the Easiest Path to Access Copay Support

SKYRIZI COMPLETEABBVIE | SKYRIZI
DUPIXENT MY WAYREGENERON | DUPIXENT
HUMIRA COMPLETEABBVIE | HUMIRA
TALTZ TOGETHERLILLY | TALTZ
COSENTYX CONNECTNOVARTIS | COSENTYX
TREMFYA WITH MEJANSSEN | TREMFYA
OTEZLA SUPPORT PLUSAMGEN | OTEZLA
RINVOQ COMPLETEABBVIE | RINVOQ
ADBRY RAPID RESPONSE PROGRAMLEO PHARMAADBRY
OLUMIANT TOGETHERLILLY | OLUMIANT
STELERA WITH MEJANSSEN | STELERA
INCYTE CARESINCYTE | OPZELURA MY VTAMADERMAVANT | VTAMA PFIZER DERMATOLOGY PATIENT ACCESSPFIZER | CIBINQO ZORYVE DIRECTARCUTIS BIOTHERAPEUTICS | ZORYVE
ILLUMYA
ILLUMYA
SUPPORTSUN PHARMA |
SOTYKTU 360 SUPPORTBMS | SOTYKTU CIMPLICITYUCB | CIMZIA
SILIQ SOLUTIONS
DERMATOLOGICS | SILIQ 1 105 82 74 71 62 59 41 41 28 24 23 20 14 10 4 4 3 3 120 100 80 60 40 20 0 *RESPONDENTS SELECTED UP TO 5. ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 54
ORTHO
84 SKYRIZI COMPLETEABBVIE | SKYRIZI 72 DUPIXENT MY WAYREGENERON | DUPIXENT 68 TALTZ TOGETHERLILLY | TALTZ HUMIRA COMPLETEABBVIE | HUMIRA 64 COSENTYX CONNECTNOVARTIS | COSENTYX 49 TREMFYA WITH MEJANSSEN | TREMFYA 41 OTEZLA SUPPORT PLUSAMGEN | OTEZLA 37 RINVOQ COMPLETEABBVIE | RINVOQ 34 ADBRY RAPID RESPONSE PROGRAMLEO PHARMAADBRY 31 SOTYKTU 360 SUPPORTBMS | SOTYKTU 26 ILLUMYA SUPPORTSUN PHARMA | ILLUMYA 17 STELERA WITH MEJANSSEN | STELERA 17 OLUMIANT TOGETHERLILLY | OLUMIANT 15 CIMPLICITYUCB | CIMZIA 12 PFIZER DERMATOLOGY PATIENT ACCESSPFIZER | CIBINQO 1 SILIQ SOLUTIONSORTHO DERMATOLOGICS | SILIQ 90 80 70 60 50 40 30 20 10 0 0 *RESPONDENTS SELECTED UP TO 5. bcofdermatology.com 55
Fastest HUBs for Starting Patients on Therapy
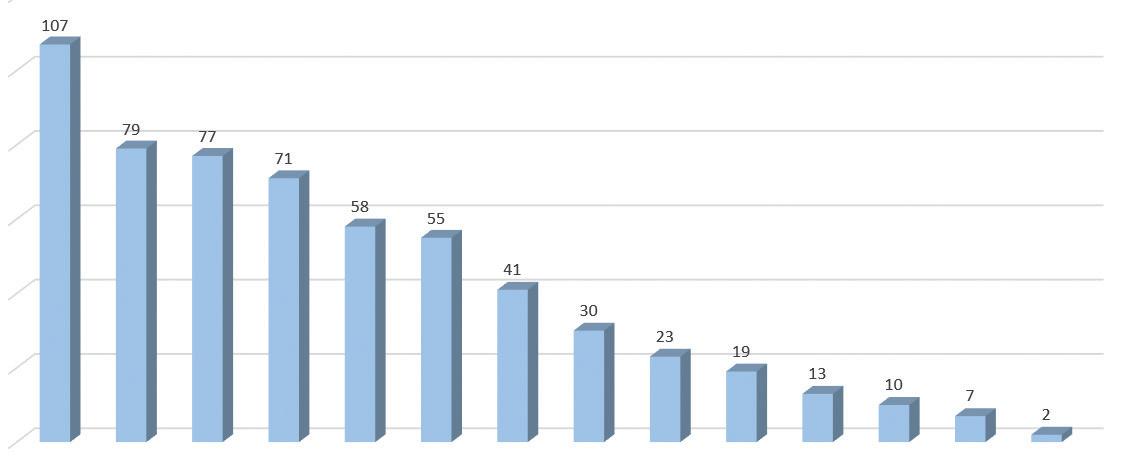
SKYRIZI DUPIXENT
DUPIXENT
COMPLETE
ABBVIE | HUMIRA
TOGETHERLILLY | TALTZ
NOVARTIS | COSENTYX
JANSSEN | TREMFYA RINVOQ COMPLETEABBVIE | RINVOQ STELERA WITH MEJANSSEN | STELERA
RAPID RESPONSE PROGRAMLEO PHARMAADBRY OLUMIANT TOGETHERLILLY | OLUMIANT SOTYKTU 360 SUPPORTBMS | SOTYKTU ILLUMYA SUPPORTSUN PHARMA | ILLUMYA CIMPLICITYUCB | CIMZIA PFIZER DERMATOLOGY PATIENT ACCESSPFIZER | CIBINQO 2 107 79 77 71 58 55 41 30 23 19 10 13 7 120 100 80 60 40 20 0 *RESPONDENTS SELECTED UP TO 5. ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 56
Most Satisfying Nursing Programs SKYRIZI COMPLETEABBVIE |
MY WAYREGENERON |
HUMIRA
-
TALTZ
COSENTYX CONNECT
TREMFYA WITH ME
ADBRY
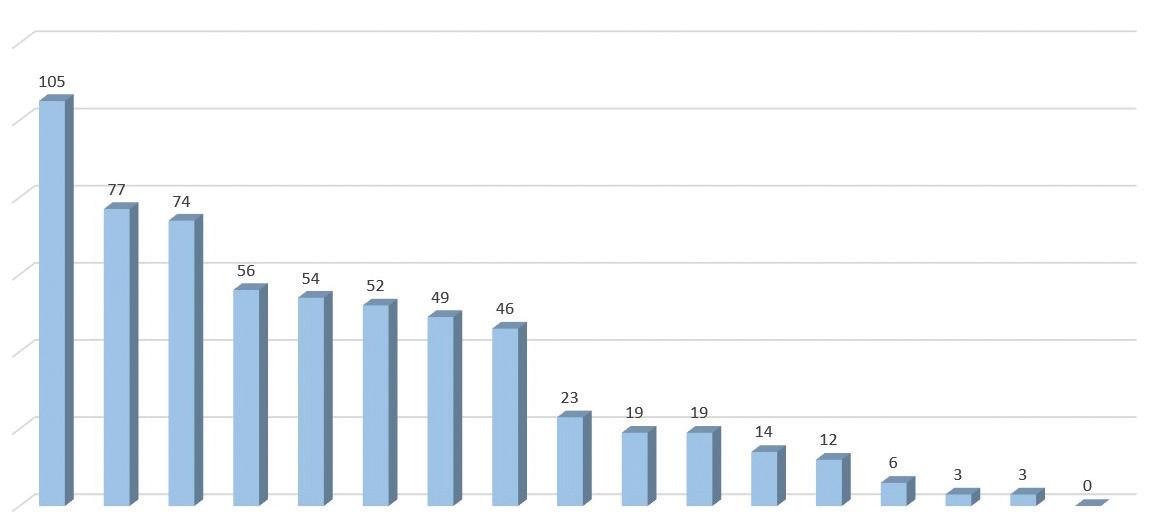
SKYRIZI COMPLETEABBVIE | SKYRIZI DUPIXENT MY WAYREGENERON | DUPIXENT HUMIRA COMPLETEABBVIE | HUMIRA TALTZ TOGETHERLILLY | TALTZ OTEZLA SUPPORT PLUSAMGEN | OTEZLA TREMFYA WITH MEJANSSEN | TREMFYA COSENTYX CONNECTNOVARTIS | COSENTYX RINVOQ COMPLETEABBVIE | RINVOQ OLUMIANT TOGETHERLILLY | OLUMIANT ADBRY RAPID RESPONSE PROGRAMLEO PHARMAADBRY STELERA WITH MEJANSSEN | STELERA SOTYKTU 360 SUPPORTBMS | SOTYKTU ILLUMYA SUPPORTSUN PHARMA | ILLUMYA CIMPLICITYUCB | CIMZIA MY VTAMADERMAVANT | VTAMA PFIZER DERMATOLOGY PATIENT ACCESSPFIZER | CIBINQO SILIQ SOLUTIONSORTHO DERMATOLOGICS | SILIQ 0 105 77 74 56 54 52 49 46 23 19 19 14 12 6 3 3 120 100 80 60 40 20 0 *RESPONDENTS SELECTED UP TO 5. bcofdermatology.com 57
Most Simplified Start Form or Enrollment Process
Programs Carrying the Most Interest to Learn More

INCYTE CARESINCYTE | OPZELURA
ZORYVE DIRECTARCUTIS BIOTHERAPEUTICS | ZORYVE
ADBRY RAPID RESPONSE PROGRAMLEO PHARMAADBRY MY VTAMADERMAVANT | VTAMA
SOTYKTU 360 SUPPORTBMS | SOTYKTU
OLUMIANT TOGETHERLILLY | OLUMIANT
TREMFYA WITH MEJANSSEN | TREMFYA
STELERA WITH MEJANSSEN | STELERA
ILLUMYA SUPPORTSUN PHARMA | ILLUMYA
OTEZLA SUPPORT PLUSAMGEN | OTEZLA
PFIZER DERMATOLOGY PATIENT ACCESSPFIZER | CIBINQO RINVOQ COMPLETEABBVIE | RINVOQ
TALTZ TOGETHERLILLY | TALTZZZ CIMPLICITYUCB | CIMZIA
COSENTYX CONNECTNOVARTIS | COSENTYX
*RESPONDENTS ASKED TO SELECT AS MANY PROGRAMS THAT APPLY.
ABBVIE
SKYRIZI 39 39 38 35 33 30 28 27 26 26 25 23 21 19 18 18 18 17 16 30 25 20 15 10 5 0 40 35
DUPIXENT MY WAYREGENERON | DUPIXENT SILIQ SOLUTIONSORTHO DERMATOLOGICS | SILIQ HUMIRA COMPLETEABBVIE | HUMIRA SKYRIZI COMPLETE -
|
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 58
Addressing the toughest challenges in atopic dermatitis takes all of us.
We fight the toughest health challenges with advanced science, putting our passion to work where the need is greatest. Because our purpose as a global biopharmaceutical company is to make a remarkable impact on people’s lives.
It takes all of us to turn possibilities into medicine that reaches millions. So we partner with governments, academic institutions, scientific and advocacy groups to make it happen. Together, we’re inventing the future of medicine. Learn

more at
abbvie.com
 By Shayli Nagelkerk
By Shayli Nagelkerk
PATIENT ACCESS
Best practices in patient information gathering
A methodical approach to gathering and communicating patient information paves the way for a successful journey to access. In any medical office, it is important to encourage staff to adhere to these practices and convey the value they serve the patient. By following these six steps when taking patient information, you can help your patient get approval for the treatment they need.

ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 60
PIEDMONT PLASTIC SURGERY AND DERMATOLOGY


START THE INFORMATION GATHERING PROCESS BY ENSURING THE FRONT OFFICE IS DILIGENT IN GETTING THE CORRECT INFORMATION FROM PATIENTS.
The importance of collecting insurance information and the pharmacy benefit card sets the tone for what potential hurdles BCs and providers may face while the prescribing process is taking place. While many companies offer a bridge program, you may still find yourself asking, “What drug is covered?” If the insurance information is not in the chart, we as coordinators are faced with the unknown. Patients often forget what insurance they have, what specialty pharmacy they are mandated to, and if they have a specific carve-out in coverage. It is extremely important for patients to provide this information for a seamless process from start to finish. The most repeated request with my front office? “Please get updated insurance information.” It is forgotten more times than not. This is critical.
bcofdermatology.com 61 1
PHOTO CREDIT: SMOLAW11STOCK.ADOBE.COM

SMAKE SURE REGISTRATION PAPERWORK IS COMPLETED, ESPECIALLY PATIENT HISTORY.
omeone coming in as a new patient who has never seen a dermatologist before will have a completely different prior authorization outcome versus someone who has been seen at a different dermatology office and has tried multiple medications for their specific disease. Communicating this to the patient and front office staff is also very important. Some patients get frustrated with certain forms of check-in material, whether it be a paper process or simply having them fill out information on an iPad. They may feel it takes too long, the information is not pertinent, or they cannot navigate the technology well enough to interpret their history. A rule of thumb: Make sure you exhaust their history when they are pulled into an exam room. Sometimes patients get frustrated with having to repeat this information, but simply remind them that you are trying to update the provider and best manage any ensuing steps required by insurance.
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 62 2
PHOTO CREDIT: LENETS_TANSTOCK.ADOBE.COM
INCLUDE QUALITY OF LIFE IN CHART NOTES AND HAVE AN EXTENSIVE HISTORY OF PATIENT ILLNESS (HPI).
When rooming patients and doing patient intake, ask enough questions to build up the patient’s HPI. It may be the case that multiple staff members are part of a single office visit; however, some may only be handed information later in the day or revert back after hours have passed. If your nursing staff is rooming patients and providing information within an HPI to be used later, advise them to
make that information as useful as possible. If a patient tells you how their job performance is suffering, make sure to put that in your notes. FOR EXAMPLE: “My job is suffering, because I am required to type, and I can’t use my hands. My fingertips are cracking and bleeding and it’s so painful when they hit the keys of the keyboard.” By adding these details to your notes, the BC can plead the patient’s case when obtaining approval
for a biologic or medication. This is a direct issue with a patient’s quality of life. When you give examples of how someone’s disease is affecting daily workflow, insurance often shows leniency in providing an approval. Also, list any tried and failed therapies including light therapy, DMARDS or topical steroids. These factors all help with the approval process.
bcofdermatology.com 63 3
PHOTO CREDIT: CHRISTIN LOLASTOCK.ADOBE.COM

HUSE THE ENROLLMENT FORM AS A STARTING POINT AND GET SIGNATURES.
aving the staff trained to obtain signatures on every enrollment form is a great process to implement within your practice. Whether you send every form in or not, having one on file just in case is key. Some BCs may send every form in, regardless, and this is a fruitful practice to follow. It allows the FRM to have eyes on each patient sent through the HUB, and it enables the manufacturer to access it should any hiccups occur along the way. In fact, I send every form into the HUB. I’ve had patients lose insurance a few months into therapy or experience changes in coverage along the way. These issues alike require additional manufacturer programs, but at the mercy of having forms signed and submitted. Personally, I like my office to simply capture signatures and allow me to fill the form out in its entirety. This gives the autonomy to review the form with a fine-tooth comb before submission.
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 64
4
PHOTO CREDIT: TEROVESALAINENSTOCK.ADOBE.COM
HAVE A SINGLE POINT OF CONTACT, INCLUDING THE PHARMACIES AND HUBS AND BE THE SINGLE POINT OF CONTACT FOR YOUR PATIENTS.

Having a direct contact within all areas of medicine when dealing with biologics is a game changer. Don’t be afraid to take on the role of being the main point of contact at your office when it comes to patient access. All patients are told about the biologic coordinator and what roles they oversee within the office. A business card for the BC is handed to each patient. By providing a direct person for these patients to contact, you avoid multiple derailments in their journey and fires behind
the scenes. It holds one person responsible. A single point of contact for the BC at the pharmacy is important as well. The direct contact then becomes aware of specific wants and needs within an office and can apply them accordingly. It also helps avoid getting someone on the phone who has no idea what is going on with a specific patient. Imagine using a pharmacy who does not have a point of contact. This opens the door for anyone and everyone to work on your cases and creates too much room for error.

“By providing a direct person for these patients to contact, YOU AVOID MULTIPLE DERAILMENTS IN THEIR JOURNEY and fires behind the scenes.”





bcofdermatology.com 65 5
PHOTO CREDIT: ALEXKICHSTOCK.ADOBE.COM
MAKE SURE YOUR PATIENT IS EDUCATED ABOUT THE COPAY CARD AND PATIENT ASSISTANCE.
Informing patients about what they should expect to pay for a medication is important, because they often think they cannot afford the medication being prescribed. Educating them on coverage helps them understand options and what is needed for them to obtain their medication. For example, PAP may be an alternative, but a conversation around its application, activation phone calls, and proof of insurance, brings the patient along in the journey and may even provide comfort for some patients who are worried about being presented with scams. Make the patient fully aware of the 1-800 numbers that may be calling. If a message is left, it is important to return these phone calls to avoid delayed starts in therapy.

ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 66 6
PHOTO CREDIT: MICROGENSTOCK.ADOBE.COM
BIOLOGIC COORDINATOR NOMINATE A FOR THEIR SERVICE
Biologic Coordinators of Dermatology (BCoD) understands the commitment to patient care. Our members are at the forefront of access, helping achieve patients’ confidence within their skin.
BCoD would like to acknowledge biologic coordinators across the country who are advocates for their patients, work tirelessly to find solutions and resources to assist in drug access, and create an atmosphere focused on celebrating the patient.
To all the biologic coordinators working for the dermatology patient, we applaud you!
Please submit nominations to contact@bcofdermatology.com with a description and example of how the biologic coordinator demonstrates a dedication and work ethic for patient care.
Individuals will be highlighted in future publications.
Any content will be reviewed with offices for compliance and privacy purposes.
A first-in-class,* selective TYK2 inhibitor for moderate-to-severe plaque psoriasis1,2

INDICATION
Clearer skin
SOTYKTU (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Please see additional Important Safety Information and Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
*TYK2 is a member of the Janus kinase (JAK) family.


Clearer choice
STUDY DESIGNS
*POETYK PSO-1 (N=664) and POETYK PSO-2 (N=1020) were two, 52-week, multicenter, randomized, double-blind, placebo- and active (apremilast 30 mg twice daily)-controlled, Phase 3 studies to evaluate the safety and efficacy of SOTYKTU (6 mg once daily) in adult patients with moderate-to-severe plaque psoriasis who were eligible for systemic therapy or phototherapy. Patients had a body surface area (BSA) involvement of ≥10%, a Psoriasis Area and Severity Index (PASI) score ≥12, and a static Physician’s Global Assessment (sPGA) ≥3 (moderate or severe).
Both studies assessed the responses at Week 16 compared with placebo for the two co-primary endpoints:
• The proportion of patients who achieved at least a 75% improvement in PASI scores from baseline (PASI 75)
• The proportion of patients who achieved an sPGA score of 0 (clear) or 1 (almost clear)
There were multiple ranked secondary endpoints, including:
• The proportion of patients who achieved PASI 75 at Week 16 vs apremilast
STUDY RESULTS1,3,4
†Comparison between SOTYKTU and apremilast was a secondary endpoint.
Co-primary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs placebo: PSO-1: 58% (193/330) vs 13% (21/166), P<0.0001; PSO-2: 53% (271/511) vs 9% (24/255), P<0.0001
• sPGA 0/1 at Week 16 for SOTYKTU vs placebo: PSO-1: 54% (178/330) vs 7% (12/166), P<0.0001; PSO-2: 50% (253/511) vs 9% (22/255), P<0.0001
Select secondary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs apremilast: PSO-1: 58% (193/330) vs 35% (59/168); P<0.0001. PSO-2: 53% (271/511) vs 40% (101/254); P=0.0004
SELECT IMPORTANT SAFETY INFORMATION
In the PSO-1 and PSO-2 trials, through Week 16, the most common adverse reactions (≥1% and higher than placebo) in patients taking SOTYKTU (n=840) were upper respiratory infections (19.2%), blood creatine phosphokinase increase (2.7%), herpes simplex (2.0%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%).
BSA=body surface area; PASI=psoriasis area and severity index; PASI 75=75% reduction from baseline in PASI; PASI 90=90% reduction from baseline in PASI; PSSD=Psoriasis Symptoms and Signs Diary; sPGA=static Physician’s Global Assessment; ss-PGA=scalp-specific Physician’s Global Assessment; TYK2=tyrosine kinase 2. Otezla® is a registered trademark of Amgen, Inc. SOTYKTU tablet is not actual size. Shown for illustrative purposes only.
AT LAST... SOTYKTU Superior skin clearance vs Otezla® (apremilast)* † in
once-daily, oral pill1 Scan to learn more or visit SOTYKTUHCP.com
a
INDICATION
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Infections: SOTYKTU may increase the risk of infections. Serious infections have been reported in patients with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19. Avoid use of SOTYKTU in patients with an active or serious infection. Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing, have appropriate antimicrobial therapy initiated and be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex) was reported in clinical trials with SOTYKTU. Through Week 16, herpes simplex infections were reported in 17 patients (6.8 per 100 patient-years) treated with SOTYKTU, and 1 patient (0.8 per 100 patient-years) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent patient. During PSO-1, PSO-2, and the open-label extension trial, the majority of patients who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age. The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis (TB): In clinical trials, of 4 patients with latent TB who were treated with SOTYKTU and received appropriate TB prophylaxis, no patients developed active TB (during the mean follow-up of 34 weeks). One patient, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU. Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas: Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.
SOTYKTU (deucravacitinib) INDICATION AND IMPORTANT SAFETY INFORMATION
Rhabdomyolysis and Elevated CPK: Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
Laboratory Abnormalities: Treatment with SOTYKTU was associated with increases in triglyceride levels. Periodically evaluate serum triglycerides according to clinical guidelines during treatment. SOTYKTU treatment was associated with an increase in the incidence of liver enzyme elevation compared to placebo. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded.
Immunizations: Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition: It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
Most common adverse reactions (≥1% of patients on SOTYKTU and more frequently than with placebo) include upper respiratory infections, blood creatine phosphokinase increased, herpes simplex, mouth ulcers, folliculitis and acne.
SPECIFIC POPULATIONS
Pregnancy: Available data from case reports on SOTYKTU use during pregnancy are insu icient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Report pregnancies to the Bristol-Myers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Lactation: There are no data on the presence of SOTYKTU in human milk, the e ects on the breastfed infant, or the e ects on milk production. SOTYKTU is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU and any potential adverse e ects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Hepatic Impairment: SOTYKTU is not recommended for use in patients with severe hepatic impairment.
SOTYKTU is available in 6 mg tablets.
Please see Brief Summary of U.S. Full Prescribing Information, including Medication Guide, for SOTYKTU on the following pages.
References: 1. SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 2. Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11(5):1763-1776.
3. Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 4. Data on file. BMS-REF-DEU-0021. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
SOTYKTU and the related logo are trademarks of Bristol-Myers Squibb Company. ©2022 Bristol-Myers Squibb Company. 1787-US-2200564 09/22
tablets, for oral use
Brief Summary of Prescribing Information. For complete prescribing information consult of cial package insert.
INDICATIONS AND USAGE
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use:
SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment Initiation
Evaluate patients for active and latent tuberculosis (TB) infection prior to initiating treatment with SOTYKTU. If positive, start treatment for TB prior to SOTYKTU use [see Warnings and Precautions]
Update immunizations according to current immunization guidelines [see Warnings and Precautions]
Recommended Dosage
The recommended dosage of SOTYKTU is 6 mg taken orally once daily, with or without food. Do not crush, cut, or chew the tablets.
Recommended Dosage in Patients with Hepatic Impairment
SOTYKTU is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Use in Speci c Populations and Clinical Pharmacology (12.3) in full Prescribing Information] No dosage adjustment is needed for patients with mild to moderate hepatic impairment.
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU [see Warnings and Precautions]
WARNINGS AND PRECAUTIONS
Hypersensitivity
Hypersensitivity reactions such as angioedema have been reported in subjects receiving SOTYKTU. If a clinically signi cant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU [see Adverse Reactions]
Infections
SOTYKTU may increase the risk of infections.
Serious infections have been reported in subjects with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19 [see Adverse Reactions]
Avoid use of SOTYKTU in patients with an active or serious infection. Consider the risks and bene ts of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with SOTYKTU. A patient who develops a new infection during treatment with SOTYKTU should undergo prompt and complete diagnostic testing; appropriate antimicrobial therapy should be initiated; and the patient should be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical trials with SOTYKTU. In the 16-week placebo-controlled period, herpes simplex infections were reported in 17 subjects (6.8 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent subject who received SOTYKTU. During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, the majority of subjects who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age.
The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Subjects with positive screening tests for hepatitis B or C, or chronic hepatitis B, or untreated hepatitis C were excluded from clinical trials. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis
In clinical trials, of 4 subjects with latent tuberculosis (TB) who were treated with SOTYKTU and received appropriate TB prophylaxis, no subjects developed active TB (during the mean follow-up of 34 weeks). One subject, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU.
Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be con rmed. Monitor patients receiving SOTYKTU for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas
Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU (deucravacitinib) [see Adverse Reactions]
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU. Rhabdomyolysis and Elevated CPK
Cases of rhabdomyolysis were reported in subjects treated with SOTYKTU resulting in interruption or discontinuation of SOTYKTU dosing.
Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to treatment with placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Adverse Reactions]
Laboratory Abnormalities
Triglyceride Elevations - Treatment with SOTYKTU was associated with increases in triglyceride levels. The effect of this elevated parameter on cardiovascular morbidity and mortality has not been determined. Periodically evaluate serum triglycerides according to the clinical guidelines for hyperlipidemia while patients are receiving treatment with SOTYKTU. Manage patients according to clinical guidelines for the management of hyperlipidemia [see Adverse Reactions]
Liver Enzyme Elevations - Treatment with SOTYKTU was associated with an increase in the incidence of liver enzyme elevation compared to treatment with placebo. Liver serum transaminase elevations ≥3 times the ULN were reported in subjects treated with SOTYKTU. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded [see Adverse Reactions]
Immunizations
Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition
It is not known whether TYK2 inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
• Infections [see Warnings and Precautions]
• Malignancy including lymphomas [see Warnings and Precautions]
• Laboratory Abnormalities [see Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not re ect the rates observed in practice.
The safety of SOTYKTU was evaluated in two placebo- and active-controlled trials (PSO-1 and PSO-2) and an open-label extension trial in which subjects who completed PSO-1 or PSO-2 could enroll [see Clinical Studies (14) in full Prescribing Information]. In these clinical trials, a total of 1,519 subjects with moderate-to-severe plaque psoriasis who were candidates for systemic therapy or phototherapy received SOTYKTU 6 mg orally once daily. Of these, 1,141 subjects were exposed to SOTYKTU for at least one year.
In trials PSO-1 and PSO-2, 1,681 subjects were randomized to receive SOTYKTU 6 mg (840 subjects), placebo (419 subjects), or apremilast 30 mg twice daily (422 subjects). All subjects randomized to placebo switched to SOTYKTU at Week 16. All other subjects remained in their original treatment group until Week 24, at which point subjects could have continued on the same treatment or be switched to SOTYKTU or placebo. The mean age of subjects was 47 years. The majority of subjects were White (87%) and male (67%).
In the 16-week placebo-controlled period of the pooled clinical trials (PSO-1 and PSO-2), discontinuation of therapy due to adverse reactions in subjects who received SOTYKTU was 2.4%, compared to 3.8% for placebo.
Table 1 summarizes the adverse reactions that occurred in at least 1% of subjects in the SOTYKTU group and at a higher rate than the placebo group during the 16-week controlled period.
SOTYKTU™ (deucravacitinib)
Adverse Reaction
a Includes upper respiratory tract infection (viral, bacterial, and unspecified), nasopharyngitis, pharyngitis (including viral, streptococcal, and unspecified), sinusitis (includes acute, viral, bacterial), rhinitis, rhinotracheitis, tracheitis, laryngitis, and tonsillitis (including bacterial, streptococcal)
b Includes oral herpes, genital herpes, herpes simplex, and herpes virus infection
c Includes mouth ulceration, aphthous ulcer, tongue ulceration, and stomatitis
d Includes acne, acne cystic, and dermatitis acneiform
Adverse reactions that occurred in <1% of subjects in the SOTYKTU group were herpes zoster.
Speci c Adverse Reactions
Exposure adjusted incidence rates are reported for all the adverse reactions presented below.
Infections
In the 16-week placebo-controlled period, infections occurred in 29% of the SOTYKTU group (116 events per 100 person-years) compared to 22% of the placebo group (83.7 events per 100 person-years). The majority of infections were non-serious and mild to moderate in severity and did not lead to discontinuation of SOTYKTU.
In the 16-week placebo-controlled period, serious infections were reported in 5 subjects (2.0 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo. The most common serious infections reported during the 52-week treatment period were pneumonia and COVID-19.
Malignancies
During the 0-to-52-week treatment period of the two clinical trials, PSO-1 and PSO-2 (total exposure of 986 patient-years with SOTYKTU), malignancies (excluding non-melanoma skin cancer) were reported in 3 subjects treated with SOTYKTU (0.3 per 100 patient-years), including single cases each of breast cancer, hepatocellular carcinoma, and lymphoma after 24, 32, and 25 weeks of treatment, respectively.
During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, a total of 3 subjects (0.1 per 100 patient-years), developed lymphoma while receiving SOTYKTU after 25, 77, and 98 weeks of treatment.
Laboratory Abnormalities
Creatine Phosphokinase (CPK)
In the 16-week placebo-controlled period, increased CPK (including Grade 4) was reported in 23 subjects (9.3 per 100 patient-years) treated with SOTYKTU, and 5 subjects (4.1 per 100 patient-years) treated with placebo.
Liver Enzyme Elevations
Events of increases in liver enzymes ≥3 times the ULN were observed in subjects treated with SOTYKTU [see Warnings and Precautions]. In the 16-week placebo-controlled period:
• ALT elevations ≥3 times the ULN was reported in 9 subjects (3.6 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
• AST elevations ≥3 times the ULN was reported in 13 subjects (5.2 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
Decreased Glomerular Filtration Rate (GFR)
In the 16-week placebo-controlled period in subjects who had moderate renal impairment (eGFR 30-59 mL/min) at baseline, decreased GFR was reported in 4 subjects (1.6 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Two of the deucravacitinib-treated subjects had worsening of baseline proteinuria.
Lipid Elevations
Mean triglycerides increased by 10.3 mg/dL during the 16-week treatment period in subjects treated with SOTYKTU and by 9.1 mg/dL during the 52-week treatment period.
Safety Through Week 52
In PSO-1 and PSO-2, the exposure adjusted incidence rate of adverse reactions in subjects treated with SOTYKTU from Week 0 through Week 52 without switching treatment did not increase compared to the rate observed during the rst 16 weeks of treatment.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data from case reports on SOTYKTU use during pregnancy are insuf cient to evaluate a drugassociated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, no effects on embryo-fetal development were observed with oral administration of deucravacitinib to rats and rabbits during organogenesis at doses that were at least 91 times the maximum recommended human dose (MRHD) of 6 mg once daily (see Data )
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report pregnancies to the Bristol-Myers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Data
Animal data
Deucravacitinib was administered orally during the period of organogenesis at doses of 5, 15, or 75 mg/kg/day in rats and 1, 3, or 10 mg/kg/day in rabbits. Deucravacitinib was not associated with embryo-fetal lethality or fetal malformations in either species. These doses resulted in maternal exposures (AUC) that were 266 times (rat) or 91 times (rabbit) the exposure at the MRHD.
In a pre- and post-natal development study in rats, deucravacitinib was administered orally from gestation day 6 through lactation day 20, at doses of 5, 15, or 50 mg/kg/day. At 50 mg/kg/day, F1 offspring had reduced body weight gains during the pre-weaning period. After weaning, body weights of affected F1 offspring gradually normalized to control levels. No maternal effects were observed at 50 mg/kg/day (110 times the MRHD based on AUC comparison). No deucravacitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring were noted at doses up to 15 mg/kg/day (19 times the MRHD based on AUC comparison).
Lactation
Risk Summary
There are no data on the presence of deucravacitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Deucravacitinib is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data ). The developmental and health bene ts of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU (deucravacitinib) and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Data
Animal Data
A single oral dose of 5 mg/kg radiolabeled deucravacitinib was administered to lactating (post-partum days 8 to 12) rats. Deucravacitinib and/or its metabolites were present in the milk of lactating rats.
Pediatric Use
The safety and effectiveness of SOTYKTU in pediatric patients have not been established.
Geriatric Use
Of the 1,519 subjects with plaque psoriasis treated with SOTYKTU, 152 (10%) subjects were 65 years or older and 21 (1.4%) subjects were 75 years or older.
During the Week 0-16 period, for those subjects (80 subjects ≥65 years old, including 12 subjects ≥75 years old) who received SOTYKTU without switching treatment arms, there was a higher rate of overall serious adverse reactions, including serious infections, and discontinuations due to adverse reactions compared with younger adults.
No overall differences in effectiveness of SOTYKTU have been observed between patients 65 years of age and older and younger adult patients.
Renal Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild, moderate, or severe renal impairment or in patients with end stage renal disease (ESRD) on dialysis [see Clinical Pharmacology (12.3) in full Prescribing Information]
Hepatic Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. SOTYKTU is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) [see Adverse Reactions and Clinical Pharmacology (12.3) in full Prescribing Information]
OVERDOSAGE
There is no experience regarding human overdosage with SOTYKTU. In case of overdose, consider contacting the Poison Help line (1-800-222-1222) for additional overdosage management recommendations. The extent of deucravacitinib elimination by hemodialysis was small (5.4% of dose per dialysis treatment), and thus hemodialysis for treatment of overdose with SOTYKTU is limited.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of deucravacitinib was assessed in 2-year rat and 6-month rasH2 transgenic mouse studies. No evidence of tumorigenicity was observed in male or female rats that received deucravacitinib at oral doses up to 15 mg/kg/day (51 times the MRHD based on AUC comparison). No evidence of tumorigenicity was observed in male or female Tg.rasH2 mice that received deucravacitinib at oral doses up to 60 mg/kg/day.
Deucravacitinib was not mutagenic in a bacterial mutagenicity assay (Ames test) or clastogenic in an in vitro chromosomal aberration assay (cultured Chinese hamster ovary cells) or in an in vivo rat peripheral blood micronucleus assay.
In male rats, deucravacitinib had no effects on reproductive parameters (mating, fertility, and sperm morphology) or early embryonic development of their offspring at oral doses up to 50 mg/kg/day (224 times the MRHD based on AUC comparison).
S:10.375" T:10.875"
Table 1: Adverse Reactions that Occurred in ≥1% of Subjects with Plaque Psoriasis in the SOTYKTU (deucravacitinib) Group and More Frequently than in the Placebo Group in Trials PSO-1 and PSO-2 through Week 16
SOTYKTU 6 mg once daily N=840 n (%) Placebo N=419 n (%) Upper respiratory infectionsa 161 (19.2) 62 (14.8) Blood creatine phosphokinase increased 23 (2.7) 5 (1.2) Herpes simplexb 17 (2.0) 1 (0.2) Mouth ulcersc 16 (1.9) 0 (0.0) Folliculitis 14 (1.7) 0 (0.0) Acned 12 (1.4) 1 (0.2)
In female rats, deucravacitinib had no effects on mating, fertility, or early embryonic parameters at oral doses up to 50 mg/kg/day (171 times the MRHD based on AUC comparison).
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) before starting SOTYKTU (deucravacitinib) therapy and each time the prescription is renewed, as there may be new information they need to know. Advise patients of the potential bene ts and risks of SOTYKTU.
Hypersensitivity Reactions
Advise patients to discontinue SOTYKTU and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions]
Infections
Inform patients that SOTYKTU may lower the ability of their immune system to ght infections and to contact their healthcare provider immediately if they develop any signs or symptoms of infection [see Warnings and Precautions]
Inform patients that herpes infections, including serious, may occur with use of SOTYKTU [see Warnings and Precautions]
Malignancies including Lymphomas
Inform patients that SOTYKTU may increase their risk of developing malignancies including lymphomas. Instruct patients to inform their healthcare provider if they have ever had any type of cancer [see Warnings and Precautions]
Rhabdomyolysis
Inform patients that SOTYKTU may increase their risk of developing rhabdomyolysis. Instruct patients to immediately inform their healthcare provider if they develop unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Warnings and Precautions]
Laboratory Abnormalities
Inform patients that SOTYKTU (deucravacitinib) may affect certain lab tests, and that blood tests may be required before and during SOTYKTU treatment [see Warnings and Precautions]
Immunizations
Advise patients that vaccination with live vaccines is not recommended during SOTYKTU treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform the healthcare practitioner that they are taking SOTYKTU prior to a potential vaccination [see Warnings and Precautions]
Pregnancy
Advise patients to report their pregnancy to Bristol-Myers Squibb Company at 1-800-721-5072 [see Use in Speci c Populations]
Manufactured by: Bristol-Myers Squibb Company Princeton, NJ 08543 USA
U.S. License No. 1713
© 2022 Bristol-Myers Squibb Company.
SOTYKTU and the Bristol Myers Squibb logo are trademarks of Bristol-Myers Squibb Company.
Issued: September 2022
1787-US-2200566 09/22



GADGET GIVEAWAY
Calling all BCoD members!
As a thank you to serving the dermatology patients, we are holding a special drawing. Enter for your chance to win one of the fitness devices shown on page 69.
TO ENTER, VISIT bcofdermatology.com/giveaway
The drawing is open to members of BCoD only.
FITNESS GADGETS
Tech that helps you achieve health and wellness goals
We see the impact of technology on healthcare every day. Whether it is machinery for in-office procedures, sending and storing patient information, or the bestin-class medicine, patient outcomes rely on innovation. We’ve rounded up our favorite technology devices and accessories that you can personally leverage to help boost health and wellness.
ACCESS DERMATOLOGY / OCTOBER 2022 76 VIDI STUDIOSTOCK.ADOBE.COM
ENTER TOWIN
GALAXY BUDS PRO
Wireless earbuds seamlessly switch between noise canceling and fully adjustable ambient sound. samsung.com

WHOOP
This product monitors your sleep, recovery and daily effort around the clock to deliver actionable insights on how you can optimize your performance. whoop.com





GARMIN VENU® 2S
Fitness tracking watch offers phone-free music listening. garmin.com

OURA RING
Unlock personalized sleep, health and activity insights. ouraring.com

WITHINGS SLEEP TRACKING MAT
This mat offers sleep cycles analysis (deep, light and REM), heart rate tracking and snore detection. withings.com
77
bcofdermatology.com
NOOM APP
Noom is a food tracking, weight loss app based on cognitive behavioral therapy to learn what drives your eating habits and how to make positive, sustainable changes. noom.com

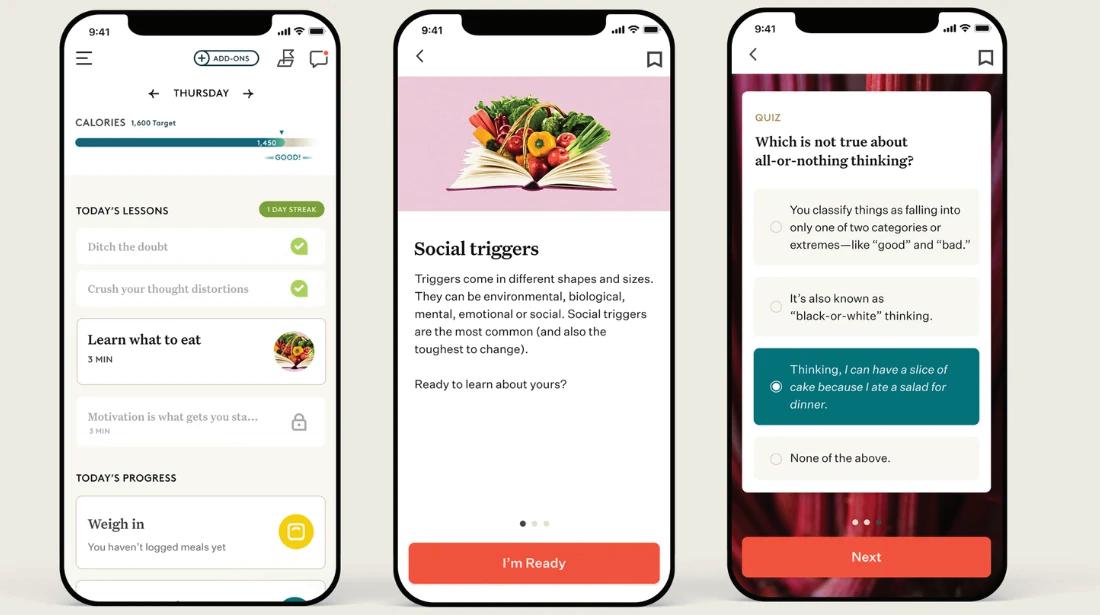
SENSATE
Sensate is designed to calm your fight-flight-freeze response, to relax you in the moment and improve your stress resilience. getsensate.com
FITBIT ARIA AIR SCALE
Smart scale displays your weight and syncs with the Fitbit app to view BMI and track trends over time. fitbit.com



CALM APP
Find your calm with an app designed to improve your health and happiness. calm.com
ACCESS DERMATOLOGY / JANUARY/FEBRUARY 2023 78

MARTON SZALAIUNSPLASH

KOVOP58STOCK.ADOBE.COM

MIRROR | LULULEMON STUDIO
Smart mirror delivers fitness classes at home. mirror.com



QUARDIOARM BLOOD PRESSURE MONITOR
Track blood pressure regularly with this smart, wireless device. qardio.com
HYDROW ROWER
Stream live and on-demand rowing workouts from stunning waterways around the world. hydrow.com
GADGET GIVEAWAY Calling all BCoD members!
As a thank you to serving the dermatology patients, we are holding a special drawing. Enter for your chance to win one of the fitness devices shown on page 69.
TO ENTER, VISIT bcofdermatology.com/giveaway
The drawing is open to members of BCoD only.
bcofdermatology.com
81






BCoD JOB

BCoD understands the critical role of biologic coordinators and office staff in advocating for patient access and therapeutic coordination. Members of BCoD can now view job listings for open positions across the United States.
This service is free for dermatologists.




JOB BOARD


If you are a dermatologist or office seeking staff to manage patient access, BCoD will post your open position at no cost. We’ll connect you with our engaged and experienced community of knowledgeable BCs.


To submit a job, reach out to contact@bcofdermatology.com.

bcofdermatology.com













VTAMA cream can give plaque psoriasis patients significantly clearer skin. So they’re free to think about…literally anything.
VTAMA cream is a once-daily, non-steroidal topical for adults with plaque psoriasis. 36% and 40% Physician Global Assessment (PGA) success rate* in two 12-week pivotal studies vs 6% and 6% for vehicle 2
Safe and well-tolerated even on the face, neck, intertriginous areas, inframammary areas, axillae, genitalia, and anal crux.1,3,4

See the data: VTAMA.com

A median ~4 month off-treatment effect seen in long-term, open-label extension study of patients (n=73)2
Important Safety Information:
References:
*PGA success rate defined as PGA=0 or 1 and ≥2-grade improvement from baseline at week 12.
Indication: VTAMA® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for the topical treatment of plaque psoriasis in adults. Adverse Events: The most common adverse reactions (incidence ≥1%) in subjects treated with VTAMA cream were folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu). You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088
1. Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219–2229.
Please see the Brief Summary of VTAMA cream on the following page.



2. VTAMA (tapinarof) cream, 1%. Prescribing Information. Dermavant; 2022. 3. Strober B, Stein Gold L, Bissonnette R, et al. Tapinarof cream 1% once daily for plaque psoriasis: long-term extension trial of a novel therapeutic aryl hydrocarbon receptor modulating agent. Oral presentation at European Academy of Dermatology and Venereology; September 30, 2021. 4. Stein Gold L, Kircik L, Lebwohl M, et al. Tapinarof cream 1% once daily for plaque psoriasis: favorable local tolerability in two pivotal phase 3 trials. Poster presentation at Innovations in Dermatology 2021; March 16–20, 2021.

Now FDA Approved © 2022 Dermavant Sciences, Inc. All Rights Reserved. All trademarks are the property of Dermavant Sciences, GmbH. US-VTAMA-2200072-02




BRIEF SUMMARY
IMPORTANT INFORMATION ABOUT
VTAMA® (Vee-TAM-uh) (tapinarof) cream, 1%
This summary contains important information about VTAMA cream. It is not meant to take the place of your doctor’s instructions. Read this information carefully before you start using VTAMA cream. Ask your doctor or pharmacist if you do not understand any of this information or if you want to know more about VTAMA cream. For full Prescribing Information and Patient Information, please see the package insert.
WHAT IS VTAMA cream?
VTAMA cream is a prescription medicine used on the skin (topical) to treat plaque psoriasis in adults. It is not known if VTAMA cream is safe and effective in children under 18 years of age.
Do not use VTAMA cream for a condition for which it was not prescribed. Do not give VTAMA cream to other people, even if they have the same symptoms you have. It may harm them.
Important: VTAMA cream is for use on the skin (topical use) only. Do not use VTAMA cream in your eyes, mouth, or vagina.
WHAT SHOULD I TELL MY DOCTOR BEFORE USING VTAMA cream?
Before you use VTAMA cream tell your doctor about all of your medical conditions, including if you:
•Are pregnant or plan to become pregnant. It is not known if VTAMA cream will harm your unborn baby; and/or
•Are breastfeeding or plan to breastfeed. It is not known if VTAMA cream passes into your breast milk. Talk to your doctor about the best way to feed your baby during treatment with VTAMA cream.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
HOW SHOULD I USE VTAMA cream?
•Use VTAMA cream exactly as your doctor tells you to use it.
•Apply a thin layer of VTAMA cream only to your psoriasis skin lesions one (1) time a day. Avoid applying VTAMA cream to unaffected areas of your skin.
•Wash your hands after application, unless VTAMA cream is for treatment of your hands.
•If someone else applies VTAMA cream for you, they should wash their hands after application.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF VTAMA cream?
The most common side effects include: folliculitis (red raised bumps around the hair pores), nasopharyngitis (pain or swelling in the nose and throat), contact dermatitis (skin rash or irritation, including itching and redness, peeling, burning, or stinging), headache, pruritus (itching), and influenza (flu).
These are not all the possible side effects of VTAMA cream. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA at www.fda.gov/ medwatch or call 1-800-FDA-1088.
HOW SHOULD I STORE VTAMA cream?
•Store VTAMA cream at room temperature, between 68℉ to 77℉ (20℃ to 25℃).
•Do not freeze VTAMA cream.
•Protect VTAMA cream from exposure to excessive heat.
•Keep VTAMA cream and all medicines out of the reach of children.
WHAT ARE THE INGREDIENTS IN VTAMA cream?
Active ingredient: tapinarof
Inactive ingredients: benzoic acid, butylated hydroxytoluene, citric acid monohydrate, diethylene glycol monoethyl ether, edetate disodium, emulsifying wax, medium-chain triglycerides, polyoxyl 2 stearyl ether, polyoxyl 20 stearyl ether, polysorbate 80, propylene glycol, purified water, and sodium citrate dihydrate.
VTAMA is a trademark of Dermavant Sciences, GmbH or its affiliates.
Dermavant Sciences Inc., Long Beach, CA 90806 USA


Join our network of biologic coordinators and navigate the fluid and complex landscape of drug access together. Sign up today at bcofdermatology.com Connect with BCoD: EDUCATION & NETWORKING EMPOWERS MEMBERS THROUGH































































































 By Neomia “Neo” Cuellar CCMS, PACS, ALLIANCE DERMATOLOGY
By Neomia “Neo” Cuellar CCMS, PACS, ALLIANCE DERMATOLOGY







































 BY JACKIE FIGUEIREDO Founder of Fig Nutrition & Wellness Certified Nutritionist & Holistic Health Practitioner
BY JACKIE FIGUEIREDO Founder of Fig Nutrition & Wellness Certified Nutritionist & Holistic Health Practitioner






 BY ARTUR KIRSH Artur Kirsh Salon in Philadelphia
BY ARTUR KIRSH Artur Kirsh Salon in Philadelphia





 BY DR. GARY GOLDENBERG and DR. KRISTINA GOLDBERG Goldenberg Dermatology
BY DR. GARY GOLDENBERG and DR. KRISTINA GOLDBERG Goldenberg Dermatology









 BY BEN HUNTER, MD Medical Director of Professional Services, Skyland Trail
BY BEN HUNTER, MD Medical Director of Professional Services, Skyland Trail













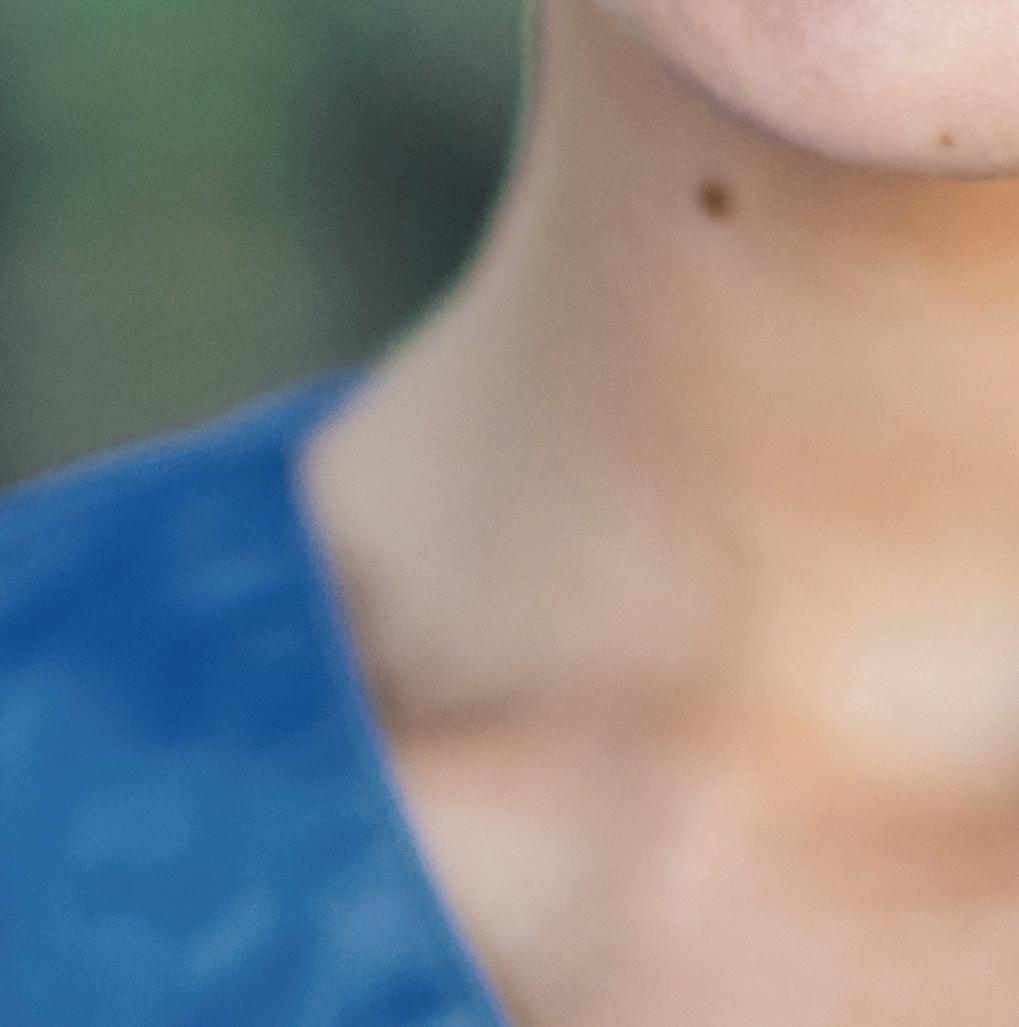









 By Staci Piner BIOLOGIC COORDINATOR, DERMATOLOGY AND SKIN CANCER SURGERY
By Staci Piner BIOLOGIC COORDINATOR, DERMATOLOGY AND SKIN CANCER SURGERY
 VTAMA'S COPAY PROGRAM
VTAMA'S COPAY PROGRAM
























 By Shayli Nagelkerk
By Shayli Nagelkerk












































































