
NATIONAL BIOLOGIC COORDINATORS DAY NOVEMBER 1



Janssen Supports and Celebrates Biologic Coordinators Like You
Biologic coordinators are some of the most important advocates for patients in health care –yet too often, their work goes unrecognized. A biologic coordinator is an essential member of a medical practice’s office staff–a doctor, nurse, physician’s assistant, medical assistant and/or an office/practice manager–that helps patients navigate the sometimes complex process of obtaining access to prescribed biologic medications. As a connecting force between the patient, prescribing healthcare provider, specialty pharmacy, manufacturer and insurance company, biologic coordinators work hard to ensure patients are able to start and stay on their prescribed biologic treatments.
November 1, 2022, marks the second annual National Biologic Coordinators Day–a day established by The Janssen Pharmaceutical Companies of Johnson & Johnson to recognize the daily commitment of biologic coordinators who go above and beyond for the patients they serve. We are proud to shine a light on these unsung heroes of patient care.
© Janssen Biotech, Inc. 2022 09/22 cp-334516v1
Join our network of biologic coordinators landscape of drug access together. Sign up today at bcofdermatology.com Connect with BCoD: EDUCATION & NETWORKING EMPOWERS MEMBERS THROUGH
Access Dermatology aims to educate and empower the biologic coordinator by keeping them informed of the complex and everchanging drug and patient access landscape. Readers are engaged with editorial and lifestyle content equally suitable for dermatologic patients, so they too may gain a better sense of therapies and the patient services programs that can assist in their therapeutic journey.
CORRESPONDENCE: Communications regarding original articles as well as editorial suggestions for future issues should be addressed to Craig Schuette at cs@bcofdermatology. com. Any content forwarded to the publisher assumes no liability for the safety or return of unsolicited art, photographs, or manuscripts.
DISPLAY ADVERTISING: Contact Craig Schuette at cs@bcofdermatology.com.
EXECUTIVE DIRECTOR
Craig Schuette
EDITOR
Elizabeth Hole
CREATIVE DIRECTOR
Venera Alexandrova
ASSISTANT ART DIRECTOR
Lisa Servidio
PRODUCTION DIRECTOR
Tim Carr

ACCESS DERMATOLOGY / OCTOBER 2022 2
magazine dedicated to supporting patient access with practical information and lifestyle content ACCESS DERMATOLOGY Issue 1 | October 2022 PRESIDENT Jonathan W. Moffly
V. Moffly
Andrew Amill
MEDIA 205 Main Street, Westport, CT 06880 telephone: 203-222-0600 fax: 203-222-0937 email: mail@MofflyCustomMedia.com Follow the Biologic Coordinators of Dermatology FACEBOOK LINKEDIN ON THE COVER Design by Venera Alexandrova The skin tone-inspired elements represent the cooperative relationship between the patient, physician and biologic coordinator.
A
VICE PRESIDENT/BUSINESS Elena
CHIEF REVENUE OFFICER
MOFFLY CUSTOM
MISSION STATEMENT:
© 2022 Access Dermatology and Biologic Coordinators of Dermatology. ALL RIGHTS RESERVED. The material in this publication is published by Moffly Custom Media and may not be reproduced or transmitted in any manner, in whole or in part, without the express written permission of Access Dermatology and Moffly Custom Media. NOTICE: The information contained within articles of this magazine represent the views and opinions of the original authors and do not necessarily represent the views and opinions of Access Dermatology or its affiliates. The mere appearance of content in the magazine does not constitute an endorsement by Access Dermatology or its affiliates. The content has been made available for informational and educational purposes only. Editorial advice is not specific, and readers are advised to seek medical, professional, or reimbursement help for individual circumstances. Access Dermatology hereby disclaims all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the content. CIRCULATION: To be added to the circulation, visit www.bcofdermatolgy.com/ magazine. REPRINTS: For educational, commercial, or promotional reprints, including author off-prints, please email contact@bcofdermatology.com.



50% of Rxs are processed without extra paperwork or phone calls

Thanks to EHR connectivity, CVS Specialty® can securely access essential data to help cut down on paperwork and simplify onboarding.




EHR (Electronic health record)
*CVS Health® Enterprise Analytics, April 15, 2020. Actual results may vary depending on bene�t plan design, member demographics, programs implemented by the plan and other factors.
Specialty Expedite is available exclusively for providers who use compatible EHR systems that participate in the Carequality Interoperability Framework or have an eligible EHR with CVS Health-enabled data connections and a fully executed connectivity agreement. All data sharing and usage complies with applicable privacy laws. Some payors have speci�c utilization management requirements related to prior authorization submissions (e.g., review, submission and/or physical signature by prescribers). Specialty Expedite is only used where permissible by payor requirements and applicable law. Patient privacy is important to us. Our employees are trained regarding the appropriate way to handle patients’ private health information.
©2022 CVS Specialty. All rights reserved.
75-1144729A 092622
your
to
Specialty®.
the
Send
prescription referrals
CVS
Scan
QR code to learn what else CVS Specialty o�ers. Can’t scan? Go to cvs.co/Derm
*

A magazine dedicated to supporting patient access with practical information and lifestyle content
ACCESS DERMATOLOGY / OCTOBER 2022 4
ACCESS DERMATOLOGY Issue 1 contents
bcofdermatology.com 5 6 LETTER FROM THE FOUNDER 16 BIOLOGIC COORDINATORS: How somene in this role impacts my practice 18 BENEFITS VERIFICATION: Do they matter? 56 THE EVOLUTION OF HUBS: Providing solutions today's users want and need 60 UTILIZING YOUR EHR: With preparation comes success 62 YOGA IN THE OFFICE: How to become energized and peaceful at work 31 THE VOICE OF PSORIASIS: Tribute to Dr. Craig Leonardi 32 PATIENT TALK: Why are you asking me that? 28 TAKING THE LEAP: Starting buy-and-bill in your practice 66 DIET & PSORIASIS: Lifestyle changes for long-term improvement 70 CULINARY THERAPY: Get cooking to relax and relieve stress 54 EASY ON THE EYES: Find the perfect pair of blue light glasses 48 BIOLOGIC COORDINATOR CAREER SURVEY REPORT 2022 27 DRUG HOLIDAY: Consult your physician before taking a break from biologics 36 SCRUB STYLE: Functional options with a fashionable twist 42 PRIOR AUTHORIZATION DENIED! NOW WHAT? 34 INGREDIENTS MATTER: Experts share the best skin care products










Biologic

Coordinators
HOW SOMEONE IN THIS ROLE IMPACTS MY PRACTICE
By G. Scott Drew, DO, FAAD, FAOCD

Patients and office staff can be intimidated by the change of therapies from traditional topical and oral medications to injectable therapies. The dosing frequency is different, the notion of a shot is foreign, and the very concept of a biologic immune modulating medicine is an innovation provoking questions for many patients. Understandably, patients can be hesitant to embrace these therapies and may require additional consultation and time.
Once the decision to prescribe a biologic medication for patients suffering with atopic dermatitis, psoriasis, hidradenitis suppurativa or other dermatologic disease is made, many believe the patient is on their way to an improved quality of life. Sadly, for many patients, that struggle has only begun. For many reasons, including step edits, plan exclusions, costs of medications and the lack of nationwide continuity in getting biologics approved, patients can experience months-long delays in accessing medications. Fortunately, with the advent and increased availability of biologic coordinators, patients can have speedier and more reliable access to the medications.
Providers are usually busy tending to histories, examinations, procedures and patient counseling. Time-consuming biologic conversations can be daunting, but fortunately, a biologic coordinator can be a conduit between the physician, pharmacy, and payer to make the transition to a biologic therapy less stressful for everyone.
Biologic coordinators are relatively new in the dermatology space, and they are a welcome relief. My BCs—and so many across the country—communicate in patient-friendly language so that everyone understands the biologic journey they have
“A biologic coordinator can be a conduit between the physician, pharmacy, and payer that makes the transition to a biologic therapy less stressful for everyone.”
just begun. It can be a complicated process, so it’s important to have a trusted, informed friend on the ride with you. Our BCs provide educational brochures from specialty pharmacies and pharmaceutical companies, along with information from our office that includes contact details, drug information, and process protocols so the patient is comfortable with their new therapeutic regimen. Perhaps most importantly, our BC becomes the patient advocate and ombudsman. Patients have a bio-buddy who
can troubleshoot, reassure, or just listen. They set up and reinforce expectations on timing, dosing, initiation paperwork and signatures, delivery mechanisms, co-pays, bridge programs, patient assistance, prior autos and reauthorizations, lab testing and injection training. The essential glue that keeps this complicated machine running, BCs understand various disease processes and help patients have a better quality of life.
Satisfied patients are the best advertising. If they become better with your treatment, this makes for a very happy patient.
Not every office believes it can afford a BC. However, I challenge you to think of the efficiency created when a BC counsels the patient and provides support in other areas, including medical assistant (MA) or receptionist responsibilities. It is beneficial to have someone manage the parsimonious payers. Although pharma is there to help, they are often constrained by unduly restrictive compliance policies.
Like any profession, balance can be achieved with the correct tools, training, and environment. In a perfect world, biologics would be available as easily a tub of Triamcinolone. Until that day comes, the BC is an invaluable asset to a busy complex medical practice.
bcofdermatology.com 17
OPPOSITE PAGE: VEGEFOX.COMSTOCK.ADOBE.COM; THIS PAGE: CONTRIBUTED
by Amy Brennan

Benefit Verifications : Do they matter?
Each time a script goes out, a benefit investigation or verification is returned. For the purposes of consistency, I will refer to them as BVs throughout the article. Whether you work directly with a specialty pharmacy or manufacturer’s HUB, you are provided with a benefit verification for every single patient. The question is: How do we read and use them?
I am Amy Brennan of Skin Care Physicians in Boston, MA. Working with Dr. Jeffrey Sobell results in hundreds of patients, new and renewed, that pass my desk each month with thousands currently on biologics. Each time a script is sent in, or when it comes time for annual reverification, a BV is returned to me. In this article I am going to highlight the reasons why this is such an important document and how you may use it within your practice. Understanding that many of you already have a system, I will try to share some pearls for those of us that are newer to the Biologic Coordinator role or are handling the access process within your office.
When it comes to getting a BV, we have three options: call the payer ourselves,
rely on a specialty pharmacy or the manufacturer’s HUB. I tend to lean toward the HUB pathway. These HUBs work with the medications each day and understand the nuances of the individual program.
The process begins by faxing in the start, or enrollment form. If completed correctly, the BV is returned in 24-48 hours. Through Abbvie’s Complete Pro portal I can often receive a BV back within seconds, which is a huge plus.
For our newer Biologic Coordinators: The benefit verification is the road map on how to complete the prior authorization. Providing specifics about the patient’s insurance plan, including Group and ID numbers, it will also tell you if they have a mandated pharmacy. At times, payer policy will dictate where a script needs to be sent. While a HUB will most likely triage the script there for you, it is important to know if you need to send a follow up prescription and who to call to verify it was received. The BV is also instrumental in understanding the prior authorization processes for a patient’s individual plan.
Some key sections to look for prior to submitting:
ACCESS DERMATOLOGY / OCTOBER 2022 18 CONTRIBUTED
ARE THERE ANY STEP THERAPIES REQUIRED?
If you can document that the patient has tried and failed the necessary step therapies, it will be imperative for approval. If you are not able, you will need to provide rationale as to why the patient will not be trying those therapies first.
DO YOU HAVE THE RIGHT PA PAPERWORK, AND HOW IS THE PLAN REQUESTING YOU SUBMIT?
The BV will provide instruction on whether you are able to submit electronically, by phone, fax or using covermymeds.com. It is important to follow these instructions as described to reduce the amount of time the patient is waiting for approval.
1 2 3 4
DOES THE PATIENT HAVE A HIGH DEDUCTIBLE PLAN?
Work with your FRM to understand the max benefit on the copay savings program. You can determine if the patient will be able to receive the medication at a low or no cost amount and ensure they do not run out of benefits for the rest of the year.
5
ELIGIBLE FOR CO-PAY ASSISTANCE?
Typically, if a patient has commercial insurance, it will automatically enroll the patient in co-pay support and will let you know on the BV that this has occurred. It will also share the expected co-pay amount, and where they are within their yearly benefits.
WHO IS YOUR FRM?
The FRM is the expert on all things patient support. If you are using a medication within your office, it is a great idea to have the FRM on speed dial for questions related to your patients and the program specifics. They can also help keep you informed on where your patients stand in the approval process.
bcofdermatology.com 19
To stay organized, I take the fax or electronic version of the BV and upload it into the patient’s chart. This allows for quick referencing any time there is a question related to coverage. Although I am the main person in the office who will utilize this information, some offices do have multiple people who may work on this process. One centralized place for information is key!
This is a vital piece that jumpstarts the entire process for who covers it, how they cover it and how to obtain access for your patient.
When it comes to the HUBs we are using today, accuracy and timing are EVERYTHING. At times I have noticed that HUBs will rely on their experience with a particular PBM, without confirming the patient’s specific coverage. I had one BV returned that stated a patient would have no out of pocket expenses, but it turned out they had a 20% co-insurance. For a 14k medication, this is not a simple mistake. Abbvie now returns a lot of BVs within seconds. This is instrumental in moving a patient along and getting them approved!
A lot of HUBs will return the paper form of a prior authorization. While this used to be super helpful, we have moved to a more digital world. Unless there is a state sponsored plan that requires FAX submission, you can save the time sending us the paper form.
I wish all my fellow BCs luck, as we continue to navigate this ever changing, ever complex world of biologics.

VEGEFOX.COMSTOCK.ADOBE.COM
Hub services evolved to help patients and providers overcome barriers associated with accessing, affording, and adhering to specialty medicines.
The problem is, stakeholders often interact with these hubs through a portal- and today, each specialty therapy and manufacturer has a distinct portal that supports their brand.
For providers who see dozens of patients throughout a given day, this often means toggling between dozens of portals throughout the day. Put simply, this is not sustainable in the long term.
That’s where CareMetx can help.
CareMetx developed a portal that allows providers and their staff to access hub services without having to leave the native workflows they’re already comfortable using. With this ONE portal, users can:
• Receive the prescription from the electronic medical record (eliminating the need to initiate a prescription via fax or manually on a unique brand portal)
• Per form the benefit investigation
• Process the prior authorization
• Assist in the appeal process
• Locate the correct specialty pharmacy and transfer the prescription there
• Connect patients with copay programs and patient support services for all products
Let’s onboard patients faster, and help them receive critical treatment sooner.




Interested in a portal that providers actually want to use?




















THE ONE-OF-A-KIND TOPICAL JAK INHIBITOR
For uncontrolled, mild to moderate atopic dermatitis in non-immunocompromised patients aged ≥12 years1

> Clear or almost clear skin (IGA 0/1)* in >50% of patients at week 8 (53.8% vs 15.1% and 51.3% vs 7.6% vehicle†; P<0.0001)1,2


> Meaningful itch relief (Itch NRS4) in >50% of patients at week 8 (52.2% vs 15.4% and 50.7% vs 16.3% vehicle†; P<0.0001)1,2‡

*With a ≥2-grade improvement from baseline.1
†In TRuE-AD1 and TRuE-AD2, respectively.1,2
— Itch NRS4 response seen as early as day 3 (18.4% OPZELURA vs 4.2% vehicle and 13.2% OPZELURA vs 0% vehicle†)3
OPZELURA was studied in 2 identically designed, double-blind, randomized, vehicle-controlled trials (TRue-AD1 and TRuE-AD2). The 2 studies included 1249 adult and adolescent patients ≥12 years of age with an affected BSA of 3%-20% and an IGA score of 2 or 3 on a severity scale of 0-4. Patients were randomized to monotherapy with OPZELURA, ruxolitinib cream 0.75%, or vehicle twice daily for 8 weeks.1,2
‡≥4-point improvement in NRS among patients with a score of ≥4 at baseline.1



BSA=body surface area; IGA=lnvestigator's Global Assessment; JAK=Janus kinase; NRS=numeric rating scale.



Explore extension results through 52 weeks






















INDICATION
OPZELURA is indicated for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adult and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Limitation of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.

IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS

Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death. Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.

Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib.
No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.

• Invasive fungal infections, including cryptococcosis and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.



Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled. Carefully consider the benefits and risks of treatment prior to initiating OPZELURA in patients with chronic or recurrent infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA.
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
Please see additional Important Safety Information on following page.
Please see Brief Summary of Full Prescribing Information, including Boxed Warning, on following pages.





IMPORTANT SAFETY INFORMATION for OPZELURA™ (ruxolitinib) cream 1.5% (continued)
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma skin cancers, including basal cell and squamous cell carcinoma, have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately.
Thrombocytopenia, Anemia and Neutropenia
Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations
Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Adverse Reactions
In atopic dermatitis, the most common adverse reactions (≥1%) are nasopharyngitis (3%), diarrhea (1%), bronchitis (1%), ear infection (1%), eosinophil count increased (1%), urticaria (1%), folliculitis (1%), tonsillitis (1%), and rhinorrhea (1%).
Pregnancy
There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Lactation
Advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Please see Brief Summary of Full Prescribing Information, including Boxed Warning, on following pages.
References:
1. Opzelura. Prescribing Information. Incyte Corporation; 2022.
2. Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. Published online May 3, 2021. doi:10.1016/j.jaad.2021.04.085.
3. Data on file. Incyte Corporation. 2021.
OPZELURA is a trademark of Incyte. Incyte and the Incyte logo are registered trademarks of Incyte. © 2022, Incyte Corporation. MAT-OPZ-01070 09/22
OPZELURA™ (ruxolitinib) cream, for topical use
Brief Summary of FULL PRESCRIBING INFORMATION
INDICATIONS AND USAGE: OPZELURA is indicated for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adult and pediatric patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
The risks and benefits of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions]
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
WARNINGS AND PRECAUTIONS
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of
OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat in fl ammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat in fl ammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat in fl ammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials.
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the bene fi ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat inflammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat inflammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the bene fi ts and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically signi fi cant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid in lipid cholesterol, ADVERSE
Clinical conditions, directly rates (TRuE-AD1 with OPZELURA were treated follows treatment 13 (3%) count
Folliculitis
Adverse OPZELURA pyrexia, acneiform DRUG
Drug known may decrease
Strong inhibitors ruxolitinib
USE
Pregnancy
Pregnancy outcomes persons exposure
Risk are not or other administration resulted
The populations other birth Data
Animal period 60 mg/kg/day
A decrease maternally approximately human twice of multiples increased 60 mg/kg/day. clinical animals 30 mg/kg/day. postnatal highest
Lactation
Risk on the of lactating present serious breastfeed last Data (30 for up the several
infections. patients: with infection; who endemic them symptoms patient resume trials kinase patients for OPZELURA reactivation used herpes ammatory unknown. trials. elevations reported in is not inhibitor least one sudden inhibitor patient lymphomas, conditions. Malignancies, treat oral JAK cancer) with TNF inhibitor observed in with TNF overall initiating or (other malignancy cell and Perform treatment as protective postmarketing least (MACE) stroke Patients ts and OPZELURA , other serious OPZELURA in OPZELURA. arterial ammatory death. In a patients 50 overall Avoid symptoms of appropriately. , and bene ts prior to indicated. If and
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
ADVERSE REACTIONS
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not re ect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-AD1 and TRuE-AD2), 499 adult and pediatric subjects 12 years of age and older with atopic dermatitis were treated with OPZELURA twice daily for 8 weeks. In the OPZELURA group, 62% of subjects were females, and 71% of subjects were White, 23% were Black, and 4% were Asian. The adverse reactions reported by ≥ 1% of OPZELURA treated subjects and at a greater incidence than in the vehicle arm through week 8 are as follows for OPZELURA (N=499) vs Vehicle (N=250), respectively: Subjects with any treatment emergent adverse event (TEAE) 132 (27%) vs 83 (33%), Nasopharyngitis 13 (3%) vs 2 (1%), Bronchitis 4 (1%) vs 0 (0%), Ear infection 4 (1%) vs 0 (0%), Eosinophil count increased 4 (1%) vs 0 (0%), Urticaria 4 (1%) vs 0 (0%), Diarrhea 3 (1%) vs 1 (<1%), Folliculitis 3 (1%) vs 0 (0%), Tonsillitis 3 (1%) vs 0 (0%), and Rhinorrhea 3 (1%) vs 1 (<1%).
Adverse reactions that occurred in TRuE-AD1 and TRuE-AD2 in < 1% of subjects in the OPZELURA group and none in the vehicle group were: neutropenia, allergic conjunctivitis, pyrexia, seasonal allergy, herpes zoster, otitis externa, Staphylococcal infection, and acneiform dermatitis.
DRUG INTERACTIONS
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4) Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not suf cient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitis-affected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Lactation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse ndings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Pediatric Use: Atopic Dermatitis: The safety and effectiveness of OPZELURA for the topical treatment of mild-to-moderate atopic dermatitis have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-AD1 and TRuE-AD2, which included 92 pediatric subjects aged 12 to 17 years with mild-to-moderate atopic dermatitis. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with atopic dermatitis have not been established.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These ndings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Geriatric Use: Of the 1249 total subjects with atopic dermatitis in clinical trials with OPZELURA, 115 (9%) were 65 years of age and older. No clinically meaningful differences in safety or effectiveness were observed between subjects less than 65 years and subjects 65 years and older.
PATIENT COUNSELING INFORMATION
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions]
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat in ammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat in ammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions]
Thrombocytopenia, Anemia, and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions].
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Speci c Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Speci c Populations]
Manufactured for:
Incyte Corporation
1801 Augustine Cut-off Wilmington, DE 19803
OPZELURA is a trademark of Incyte. All rights reserved.
U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870; 11219624
© 2022 Incyte Corporation. All rights reserved. Issued: July 2022 PLR-OPZ-00020
By Alexander Fogel, MD, MBA
DRUG HOLIDAY

Consult Your Physician Before Taking a Break from Biologics

The idea of a drug holiday, or lapse in medication, is becoming a common topic in the era of biologic treatment. First, because the medicines work so well, patients—who can have clear or nearly clear skin for long stretches of time—wonder whether they need to continue taking the drug indefinitely. Secondly, the dosing regimens can be long intervals, ranging from several weeks to several months, so it is easy to miss an injection and be off of
treatment for a period of time. Finally, as these medicines may carry higher costs, changes in insurance can lead to lapses in coverage for the medication.
It is no surprise that the fall and winter seasons often impact a patient’s biologic injections. Patients may purposefully or mistakenly forego their scheduled dose when adding seasonal festivities or travel to their routines. And while the name “drug holiday” is not synonymous with turkey or balsam fir
wreaths, the two tend to go hand in hand.
For biologic coordinators, it is critical to know which patients are considering taking a drug holiday. Given the close relationship with the patient, BCs are often the patient’s most important and accessible office contact, and patients should be encouraged to discuss this scenario as well as any issues related to insurance coverage. Equally essential is the communication between a biologic coordinator and the treating physician. Consulting on potential eligibility of bridge products, such as topicals, phototherapy or orals, can help these patients while waiting for payer approval as lapses commonly require resubmission of paperwork.
For patients contemplating a drug holiday, I would strongly recommend a discussion with the treating dermatologist. That way, there are fewer surprises with respect to the potential for relapse, and additional treatments can be prescribed for the interim, such as topical therapy. Additionally, there is data that certain biologic medicines can be dosed at a longer interval frequency in some patients. The treating dermatologist will be aware of which medicines can have their dosing interval extended.
Overall, when thinking about a drug holiday, consultation between patient and provider is paramount to assess risk and ensure proper course for future treatment.
bcofdermatology.com 27
ADULT & PEDIATRIC DERMATOLOGY SPECIALISTS, WESTPORT, CT YALE UNIVERSITY DEPARTMENT OF DERMATOLOGY
NELLY KOVALCHUKSTOCK.ADOBE.COM; CONTRIBUTED
By Kristi Hawley, DO, FAAD OWNER AND DERMATOLOGIST OF THE DERM INSTITUTE OF WEST MICHIGAN

TAKING THE LEAP
Starting buy-and-bill in your practice
As reimbursement continues to plummet while the cost of running a practice rises, finding ancillary income is becoming vital to survive in the private practice setting. While biologics may often be the best therapeutic option for a patient, they are expensive to prescribe. Enter the role of the buy-and-bill biologic. This route of administration allows for those patients that may not always have access, and it helps offset the cost of overhead often required to prescribe these life-changing medications.
Due to the failed attempts to buy-and-bill in the past, along with a lot of money lost by those who participated, many dermatologists have avoided this as an option and are concerned about risk. However, when done correctly, an office can now profit off the work, time and risk that is already occurring when prescribing through pharmacy benefits. I have always felt frustrated that pharmacies
get to benefit from my personal and staff’s labor and time, and I believe dermatologists should be the ones who get paid for the work and cost that is being incurred. This method of prescribing also opens access for patients who would otherwise be limited in choices due to high deductibles, restrictive formularies, and high coinsurance premiums. Buy-and-bill medications can seem
overwhelming at first, but the companies have revamped their programs and have made the process risk-free. My biggest regret is that I did not implement it sooner!
There are several biologics that can be prescribed in this manner, which include Ilumya, Cimzia, Stelara, and Xolair. If it is your first time venturing into buy-and-bill, I recommend starting with Ilumya. Aside from
THIS PAGE: CONTRIBUTED; OPPOSITE PAGE: TATIANASTOCK.ADOBE.COM ACCESS DERMATOLOGY / OCTOBER 2022 28

bcofdermatology.com 29
"I typically choose buy-and-bill for my Medicare patients who otherwise cannot get biologics, those with a fear of injecting themselves, and patients that need to be monitored more closely."
its favorable injection schedule and safety profile, the company has done a fantastic job of making the process seamless and risk-free. I typically choose buy-and-bill for my Medicare patients who otherwise cannot get biologics, those with a fear of injecting themselves, and patients that need to be monitored more closely.
The first step in implementing buy-and-bill is to sit down with the company and have them walk you and your team through the process. It is important to utilize both a specialty pharmacy and the manufacturer’s HUB; the company will protect you and ensure you will be getting reimbursed prior to injection. The specialty pharmacy will initiate any prior authorization paperwork, and the HUB will provide a detailed benefit investigation. This makes it very clear on what to expect for reimbursement, along with determining eligibility for co-pay assistance and coverage outcomes. Submitting to the manufacturer’s HUB also alerts the field reimbursement manager (FRM) that we are considering a new patient and allows them to work together with my biologic coordinator (BC) to get the patient on the drug as soon as possible. In addition, I have my BC run the benefits themselves, as this will help reduce the risk of failed reimbursement.
Establishing a relationship with the FRM is vital, and they are instrumental in the following:
• Continuously updating the office on the Prior Authorization (PA) status
• Corresponding with the insurance company
• Understanding how to utilize and activate a co-pay card
• Acting as a secondary set of eyes for the busy biologic coordinator
• Providing a detailed outline of what is entailed when buying-and-billing for their medication
In addition, manufacturers continue to support our office and patients by providing money to cover high deductibles and out-ofpocket expenses. Be sure to discuss with
LESSONS I’VE LEARNED ALONG THE WAY TO HELP AVOID LOSS AS YOU IMPLEMENT THIS PROCESS:
1
Make sure the authorization states that the approval is a “medical approval” versus a “pharmacy approval.”
2
If you are utilizing a thirdparty billing company and pay them a percentage of collections, be sure to negotiate their fees regarding buy-and-bill reimbursements so they do not keep a percent prior to cost of product being backed out. My office has negotiated a flat fee for buy-and-bill patients.
3
DO NOT START ON SAMPLES!
Wait until the patient is approved prior to first injection. Most companies do not backdate authorization, no matter how severe a patient is.
the companies’ FRM about the co-pay program and how it is utilized.
In closing, if you are an office that writes biologics regularly, I encourage you to consider implementing this to maximize your profits and patients’ access to biologics. Find the perfect fit, take it slow, ask for help, learn the process, and ask the FRM to hold your hand during this exploration. Reach out to an office that has been doing it successfully. Your patients and practice will thank you.
ACCESS DERMATOLOGY / OCTOBER 2022 30
By Christine Ramig
The Voice of Psoriasis
TRIBUTE TO DR. CRAIG LEONARDI OF CENTRAL DERMATOLOGY IN ST. LOUIS, MO
Where do I even begin to honor a man who has given so much to not only his patients, but to the whole psoriasis community?
For me, let’s go back 21 years to when I first walked into his office at age 17 covered in psoriasis. My previous dermatologist told me there was nothing else he could do for me, but there was this doctor that was changing how psoriasis patients were being treated.
If you were to ask all his patients how they felt about him, you would get thousands of stories that mirror mine; we don't know where we would be without Dr. Leonardi. He has been the advocate for psoriasis patients his entire career. Some would even say he is the voice of psoriasis. Dr. Leonardi deserves this semi-retirement more than any other physician I know. He has always gone above and beyond for his patients. He truly never gives up on you and your skin.
Let's continue to celebrate a man that has devoted his life to helping people. My life and every single psoriasis patient's life has been changed by him. It's hard to put into words what he has not only done for me, but how he has helped psoriasis patients he deals with day in and out. He saw a need and he did everything in his power to fix it. His staff at Central Dermatology will miss him, but we know he is still out there caring for his patients the way they deserve.

ACCESS DERMATOLOGY / OCTOBER 2022 31
By Aaron Farberg, MD FAAD DOUBLE BOARD-CERTIFIED DERMATOLOGIST AND MOHS SURGEON
PATIENT TALK

Why Are You Asking Me That?

You might be one of 8 million people in the U.S. living with psoriasis. Still, you should feel confident that you’re getting a thoughtful consultation with your dermatologist—the person most intimately involved in caring for your skin condition. Unfortunately, we often hear stories, and even read yelp reviews, that doctors aren’t giving patients the personal attention or consideration that is expected. While I can’t speak for all dermatologists, I want to shed light on the consultative approach that I and other trained professionals take with patients to uncover the information necessary to tailor therapies. In other words, let’s answer the question, “Why is my dermatologist asking me these questions?”
Dermatologists consider many facets of a patient’s skin condition, medical and family history, and their lifestyle, to construct an effective therapy recommendation. Just like an airline pilot hits every checkpoint for their aircraft, physicians employ their own checklist to best assess you and your condition.
While it may seem rudimentary, impersonal (if a doctor is not looking at you but a tablet), or repetitive, and sometimes difficult to answer, my questions are a means to the most personalized medicine. You may have heard, or should expect to hear questions about prior medications, family history and other specifics.
I am not testing your memory or purposely making your experience more cumbersome by asking you to take photos of medications or even bring them in. We are in this together!
STUDIO ROMANTICSTOCK.ADOBE.COM; CONTRIBUTED ACCESS DERMATOLOGY / OCTOBER 2022 32
1
HOW LONG HAVE YOU HAD YOUR CONDITION?
2
HOW IS IT AFFECTING YOU AT SCHOOL, WORK OR AT HOME?
3
DO YOU HAVE DIFFICULTY SLEEPING?
4
DO YOU HAVE ANXIETY OR DEPRESSION?
5 HAVE YOU HAD PROBLEMS WITH YOUR GASTROINTESTINAL TRACK?
6
WHAT IS YOUR FAMILY HISTORY?
7
WHAT MEDICATIONS HAVE YOU TRIED IN THE PAST AND WHAT WAS YOUR RESPONSE, AND BE SPECIFIC?
“Just like an airline pilot hits every checkpoint for their aircraft, physicians employ their own checklist to best assess you and your condition."
professor grading a final exam, insurers are inspecting notes to determine if you’re granted the prescribed medication. These algorithms may even differ from state to state, so by simply crossing state lines, you’re now under a different microscope of an insurer who may have different step therapy requirements. Remember that question about what specific medications have you tried?
I encourage patients not to hold back their information. Research shows that half of patients cannot recall the medications they have been prescribed. And even a higher percentage are reported to have withheld important information that is relevant to their health and the therapy treating their condition.
Medicine is a collaborative decision-making exercise. While methodical in nature, it allows us to customize therapy based on the information you provide.
On top of that, dermatologists seek and identify information to increase the likelihood of obtaining that therapy, as insurers implement multiple stop-points, or barriers, that could jeopardize the approval of a medication based on insufficient or irrelevant evidence. Information is the key to unlocking these doors. We get there by our standardized checklist. Payers are not satisfied with histories of formulations or classes of drugs. Like a
In addition, my biologic coordinator may ask or repeat these questions to you because they are the sole touchpoint for you, and they need to dot the I’s and cross the T’s to ensure the right information is reaching your payer. In fact, they are the ones that will relay your story to the manufacturer drug programs, specialty pharmacies and the insurance company through prior authorizations, letters of medical necessity and appeals. While it may feel that your privacy is being infringed upon, an example like knowing where you have psoriasis and how it may impact your personal life can be the difference between approval and denial. The biologic coordinator is there to support you, and you should feel as comfortable sharing your story with them as you do with your doctor.
bcofdermatology.com 33

“My
Dermatologist
recommended Avene’s RetrinAL 0.1 because of my sensitive skin and history of not tolerating other retinol products.”
Biologic coordinator


Ingredients Matter
Dermatology, as the study of skin, is unmatched by other specialties in the sense that no other branch of medicine can visually capture the efficacy of its topical therapies. This visual satisfaction is why consumer spending in skin care continues to increase. According to the U.S Census Data and Simmons National Consumer Survey, Americans are spending up to $2,000 a year on skin care products. With biologic coordinators not only around the most innovative and promoted products on the market, but often discussing skin care with patients, we asked them which products are in their beauty arsenal.
TULASĀRA™ BRIGHT
CONCENTRATE BY AVEDA
RETRINAL 0.1 INTENSIVE
CREAM BY AVENE
This product also contains AVENE Thermal Spring Water, which has been shown to rebalance PH levels and calm the skin.
To appreciate RetrinAL, we must understand that it belongs to the family of retinoids. Retinoids are a group of vitamin A derivates. Their efficacy is demonstrated through skin cell turnover, collagen production, decreased acne, and soothing fine lines and wrinkles.
Vitamin A, a potent antioxidant, undergoes a conversion process before it becomes retinoic acid and influences your skin. Retinaldeyde converts to retinoic acid faster than some other OTC retinoids. For this reason, it is considered more potent than other retinal products.
WHEN TO USE:
Every day underneath or over your makeup
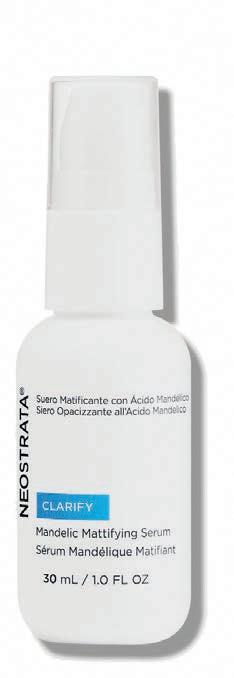

MANDELIC MATTIFYING SERUM BY NEOSTRATA
“I happen to be someone with a shiny T-zone which is why Neostrata is a staple in my skin routine.”
Biologic coordinator
Mandelic Mattifying Serum absorbs surface oil to control shine and minimize appearance of pores. It is formulated with a 8% exfoliating blend of mandelic acid and polyhydroxy acid (PHA). Mandelic acid, an alpha hydroxy acid (AHA) is lipophilic, meaning it has an affinity for oils, which reduces shine on your forehead and nose. Gluconolactone provides antiaging properties by way of exfoliation. Fragrance free and non-acnegenic.
“I prefer natural ingredients which is why I use Aveda and the Tulasāra serum.”
Biologic coordinator
Bright Concentrate is a 98% naturally derived serum, with including Licorice root extract, a traditional Ayurvedic ingredient, and molasses extract. Their effects are restoring radiance, evening skin tone, and reducing discoloration. Additionally, glucosamine supports cell turnover while vitamin C helps fade pigmentation.
ACCESS DERMATOLOGY / OCTOBER 2022 34
WHEN TO USE: a.m. and p.m. before moisturizing WHEN TO USE: Nightly to the face, neck and décolleté. Sunscreen is recommended during use of this or any retinoid product. LEAF BY MOUSTACHEGIRLSTOCK.ADOBE.COM; ORANGES BY OKEASTOCK.ADOBE.COM: ]OPPOSITE PAGE: BEAURY SERUM BY KAT KASTOCK.ADOBE.COM; COFFEE BEAN BY AMY LVSTOCK.ADOBE.COM



FACIAL FUEL DAILY ENERGIZING MOISTURE TREATMENT FOR MEN
BY KIEHL’S
ERYFOTONA ACTINICA DAILY MINERAL SPF 50+ SUNSCREEN
BY ISDIN PHOTO
“Working in dermatology has made me more cognizant of the sun and what I put on my face to protect it. I like ISDIN for its dual properties.”
Biologic coordinator
The most important product one can acquaint their skin with is sunscreen. This daily mineral SPF 50+ sunscreen from ISDIN is a silky, ultralight emulsion, guarding against sun damage while simultaneously repairing it. The formula contains Patented DNA Repairsomes®, photolyase enzymes clinically proven to restore existing sun damage.
Invigorating, non-greasy formulation

Caffeine is an alkaloid known for its stimulating properties. It may aid in making your skin noticeably less puffy by restricting the blood vessels and smoothing out the skin. It is a natural antioxidant that helps neutralize free radicals. (https://:doi: 10.1159/000343174) Vitamin Cg, or ascorbyl glucoside, is a vitamin C derivative that can boost radiance and smooth skin texture. Chestnut extract benefits users with its ability to improve blood circulation and improve the function of capillary walls.
“I just like the way it feels on my face. It really does wake you up!”
Biologic coordinator


WHEN TO USE: After cleaning, toning, and applying treatment products
WHEN TO USE:
After cleansing. The cooling menthol also makes it a great aftershave product.


TOLERIANE DOUBLE REPAIR FACE MOISTURIZER BY LA ROCHE-POSAY
“I’ve always been taught that ceramides are important. La Roche-Posay is my go-to ceramide product.”
Biologic coordinator
Ceramides are lipids in your skin with the ability to provide barrier function and hydration. Age and sun decrease the effectiveness of your natural skin composition.
Toleriane Double Repair Face Moisturizer is formulated with ceramide-3, niacinamide, and glycerin, to maintain skin integrity and prevent transepidermal water loss. Glycerin, a humectant, hydrates by boosting water absorption while niacinamide, a form of vitamin B3, promotes ceramide skin barrier growth.
bcofdermatology.com
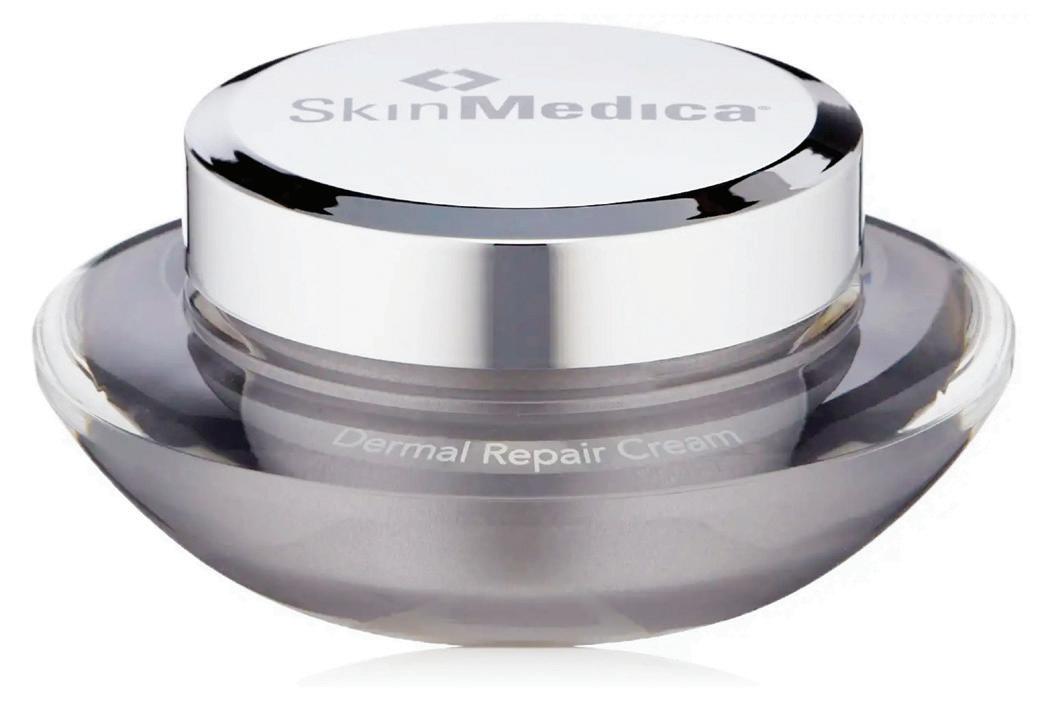
DERMAL REPAIR CREAM BY SKINMEDICA
Dermal Repair Cream delivers with its high concentration of vitamins C and E, antioxidants that also brighten skin. Vitamin C shields the skin from damage caused by free radicals as well as UV-induced inflammation by neutralizing them before there are harmful consequences to the skin. (https://:doi:10.3390/nu9080866)
In addition, the highly effective moisturizing ingredient sodium hyaluronate pulls and retains water in the skin for increased hydration. A derivative of hyaluronic acid, this ingredient can retain up to 40 times its weight in water on the surface of the skin.
“Deeply hydrating. I really feel I can see a difference when I use it.”
Biologic coordinator
WHEN TO USE: After cleaning, toning, and applying treatment products WHEN TO USE: Every day. Can also be used as a base for makeup.

ACCESS DERMATOLOGY / OCTOBER 2022 36

SCRUB STYLE
Functional Options with a Fashionable Twist
When it comes to how you present yourself, professionalism, behavior, attitude, and dress are all important factors. It was the 1970s that set in motion medical attire trends such as surgical greens that reduced eye fatigue by surgeons. While these greens remain in place, there has been vast evolution of office dress. Today, we can be more selective with how we represent our office and how we present ourselves to the patient. We can parsimoniously choose performance, comfort, and colors to suit our preferences. While needing functional wear, uniformity and standardization is important to operations and patient care.
We’ve highlighted several on-trend brands that deliver on both professionalism and style.
bcofdermatology.com 37 NASTYA PALEHINASTOCK.ADOBE.COM
NOEL ASMAR UNIFORMS
noelasmaruniforms.com
Noel Asmar Uniforms dare to be different with a heightened style to their premium scrubs. In fact, they are often worn by staff of iconic brands like The


 Ritz-Carlton and Four Seasons.
Women’s Faux Linen Maya Tunic $84.00
Jada with Matte Black Zipper $86.00
Ritz-Carlton and Four Seasons.
Women’s Faux Linen Maya Tunic $84.00
Jada with Matte Black Zipper $86.00
ACCESS DERMATOLOGY / OCTOBER 2022 38
Men’s Urban Fusion $82.00
FIGS wearfigs.com
FIGS, through Threads for Threads, donates scrubs and masks to healthcare professionals who work in resource-poor countries and lack proper uniforms to treat patients effectively and safely.



LIFETHREADS SCRUBS
Life-threads.com
Everyday scrubs for everyday shifts, this line also offers compression socks.

Off Shift Women’s Hoodie Sweatshirt $78.00 Inala Slim Scrub Top $44.00
The site offers matching sneakers.
bcofdermatology.com 39
Compression Socks $16.99

WONDERWINK SCRUBS wonderwinkscrubshop.com
This line offers wrinkle-free styles you can wear straight out of the dryer. It also partners with Carhartt for those with an outdoor spirit.
 Carhartt Men’s Solid Utility Ripstaop Top $28.99
Carhartt Men’s Solid Utility Ripstaop Top $28.99
ACCESS DERMATOLOGY / OCTOBER 2022 40
JAANUU Jaanuu.com
Jaanuu was created by a physician to address the needs of the profession. A bonus during your online search, this brand helps you select colors by explaining their meaning. For instance, gray is recommended as a practical choice. Gray: “A neutral color that’s easy on the eyes, and it’s also a darker shade than white, meaning it’s easier to keep clean. Gray is also less imposing than an authoritydriven black, making this particular hue a true middle-ground for scrub options.”

Women’s Relaxed 3-Pocket Top $36.00

Women’s Essential 5-Pocket Jogger Pant $46.00
bcofdermatology.com 41

NATALISTOCK.ADOBE.COM
Prior Authorization Denied! NOW WHAT?
By Marc Del Bono, CMA, PACS

If
doing a prior authorization in your office is a dreaded task, what do you do when the prior authorization is denied? Sure, it would be easy to throw up your hands and say we did what we could, but in today’s landscape, it
may not always be enough.
WHO IS THE BIOLOGIC COORDINATOR?
The term biologic coordinator (BC) is a recent term, and the position has grown in popularity within the last five years.
Traditionally, the role of a BC is organically grown. If you are knowledgeable, organized, and willing to fight for the patient, you might be the one in the office that gets designated as the BC. You will most likely be asked to wear multiple hats in the office and be great at juggling and prioritizing the daily workflow. Biologic coordinators are a key component in the bridge between the prescribers writing the therapy and the patient receiving the specialty medication.
“A medical assistant sees the problem; a Biologic Coordinator sees THROUGH the problem to get to the solution.”
—DR. NEAL BHATIA
NOVEMBER 1
IS NATIONAL BIOLOGIC COORDINATOR’S DAY
A special thanks to Janssen Biotech for all the hard work to make this possible.
With all the new and emerging drug therapies in development, more than half are biologics or specialty medications which will require an amount of paperwork to access. Meaning our ability to work closely with the manufacturers, submit clear and complete enrollment packages, as well as understanding the nuances of each support program will be an integral part of our job.
Why do prior authorizations get denied?
• Missing information
• Off-label use/wrong ICD.10 code
• Step therapy requirements
• Formulary exclusions (NDC block)
Once denied, you have a few options: leave it behind, switch to a formulary product, or appeal. Due to a lack of knowledge around the appeals process, staffing, and time allocation, about 65% of denied claims are never resubmitted, according to Healthcare Financial Management Association (HFMA). We can do better than this for our patients.
bcofdermatology.com 43
CONTRIBUTED
TIPS for the APPEAL PROCESS: 1 2 3 4 5
WORK WITH THE PRESCRIBER
Together you can develop a strategy based on their reasons for prescribing in the first place.
Prescribers should embrace this and foster educating their biologic coordinators as much as possible.
The prior authorization and appeal must be submitted by the prescriber, but once I receive the denial, we discuss the case and mutually come up with a plan that is best suited for the patient’s specific needs.
NOTIFY YOUR FRM AND MANUFACTURER HUB
FRMs are well versed on denials and tips and tricks to help gain access to the medication they represent.
The FRM can also direct you on requirements related to a manufacturer bridge program and help get your patient started as soon as possible.
The Field Reimbursement Manager (FRM) or Field Reimbursement Access Specialist (FRAS) is a unique position that is underutilized by medical offices and may be one of the most valuable assets for biologic coordinators when we get a difficult case.
UNDERSTAND THE DENIAL REASONS
SHARE THE DENIAL LETTER WITH THE HUB
Based on the benefit verification, your experience, and the denial letter, make sure you understand all the reasons for denial.
This can often jump-start the FRM’s ability to help and meet any requirements for continued bridge shipments.
**It is important to note that, in most cases, without documented appeals, the patient will not be able to maintain their accessibility to a bridge program and will need to discontinue the medication that they are currently taking. **
USE A
TEMPLATE
Most manufacturers will offer some sort of template. While they are not all created equal, it may be helpful to develop a template that you can pull up quickly and utilize.
ACCESS DERMATOLOGY / OCTOBER 2022 44
ADDRESS THE REASONS FOR DENIAL IN THE FIRST PARAGRAPH
Not all receiving the letter are going to take the time to scour through chart notes to find the information.
USE BULLET POINTS
PROVIDE RATIONALE
INCLUDE THE PATIENT’S OWN LANGUAGE
ATTACH CHART NOTES
By listing bulleted information, it makes for a quick read for the payer. If you are trying to gain approval over required step therapies, provide rationale why each one cannot be used, or if any were tried and failed. Include as much information as possible.
Have the patient write down in their own words a short description of how their disease state affects them personally and professionally.
Chart notes can help illustrate the patient’s journey to this medication.
Childbearing potential, hepatic toxicity, high risk of SCC to genitals, detriment to their employment are just a few examples of reasons why these formulary options will not work.
With the recent introduction of JAK inhibitors, it seems that insurance companies are afraid of their shadow with a black box warning, yet they continue to demand an off-label failure of drugs like methotrexate, cyclosporine, and acitretin that are not only off label but carry upwards of 13 black box warnings, yet the insurance companies still believe these are the best options for the patient. If the prescriber does not agree, we need to provide this rationale.
As another example, to get a drug approved the patient needs to try and fail two mid-potency topical corticosteroids, and a topical calcineurin inhibitor, provide rationale why light therapy is not appropriate for the patient—never mind the $40 copay, $6 gallon of gas, and time lost from work three days per week for a treatment that is not FDA-approved for eczema.
Poor quality of life, the consistent changing of bed sheets and the need to vacuum the house throughout the day because of shedding skin, hits home and is hard not to reverse the determination when you hear this from the patient directly.
As we focus on the new and emerging drug therapies coming to market, including the 2023 biosimilars, the challenges will continue, and drug approval processes will rely heavily on the knowledge, experience, and compassion of the biologic coordinator. It is up to us to “complete the circle,” seeing that our patients receive the much-needed therapy they deserve so that they can enjoy the quality of life that the rest of us, at times, may take for granted.
bcofdermatology.com 45
7 6 8 10
9
Once a prescribing decision has been made to prescribe TREMFYA® (guselkumab), enroll patients in
A dedicated support program for TREMFYA® patients
TREMFYA withMe provides a range of dedicated support to help make it easier for patients as they begin, and continue, their TREMFYA® treatment journey.
If a patient has been prescribed TREMFYA® for approved on-label use and is 18 or older, they are eligible for this program.
Visit tremfyawithme.com to learn more about all that TREMFYA withMe offers patients.

Scan this QR CODE with your phone’s camera
Here’s how you can sign up patients.
Scan or Visit:
Visit tremfyawithme.com/ healthcare-professionals to sign up patients and for more information.

Portrayal
Actor

TheTREMFYAwithMe Guide
At the core of the program is a TREMFYA withMe Guide, a dedicated and qualified healthcare professional* who can work one-on-one with patients to:



Answer questions about prescription fulfillment and cost support
Patients will receive education on specialty medication fulfillment and options that could make their treatment more affordable.
Connect patients to injection support
You are the best person to review the injection process with your patients and help them understand what to expect. The TREMFYA withMe Guide is also available, after you have talked with your patients, if they have questions about injections.
Help with treatment expectations
Patients will get information on what to expect with their TREMFYA® treatment.
Provide regular updates and reminders at the patient’s request
Patients may receive support to help them stay on track with their treatment, plus updates on their TREMFYA® treatment journey.
Our goal is to provide a live person who can compassionately deliver the precise information that patients may want to help them with their treatment.
The TREMFYA withMe Guide can also help patients access other channels of support.
*Guides do not provide medical advice.






Actor Portrayal
© Janssen Biotech, Inc. 2022 09/22 cp-333177v1
BIOLOGIC COORDINATOR
CAREER SURVEY REPORT
2022
The biologic coordinator’s (BC) position has and continues to grow organically. The BC wears multiple hats and oversees intricate daily workflows. The job description never truly captures the interestingly and equally diverse responsibilities. In our first career survey, we looked closely at the fundamental analysis of the biologic coordinator position, including education, working hours, salary, job satisfaction, workload, and office dynamics. Thank you for your participation in this survey. Future surveys will continue to dive into the activity and mindset of our biologic coordinators. We look forward to listening to you, supporting you, and building a stronger community together. Your value and impact are immense. The future is yours to shape.
ACCESS DERMATOLOGY / OCTOBER 2022 48
KEY FINDINGS
Many of you are not limited to only managing biologics, but rather procedures (15%) and all other therapies (51%) as well.
Not surprising as we entered the medical field to help people, 60% reported patient care and outcome as most important to job satisfaction.
With your experience and insight into the patient access programs, 81% of your dermatologists consult with you on suitable therapies based on patient coverage.
While Covid has led to more working from home, 85% are back to full-time office hours, with 72% in the office 5 days a week.
Many respondents are putting in long hours with 35% working 41+ hours per week.
BCoD collected 78 surveys from biologic coordinators in July 2022.
49
bcofdermatology.com
ARE YOU PAID HOURLY OR SALARY? Salary Hourly Less than 2 2 to 10 11 to 15 More than 15 7% HOW MANY YEARS HAVE YOU BEEN WORKING IN DERMATOLOGY AS A BIOLOGIC COORDINATOR? 13% 24% 56% 88% 12% ON AVERAGE, HOW MANY PATIENTS DO YOU MANAGE A MONTH? 26 to 100 More than 100 10 to 25 17% 32% 51%
2022 BIOLOGIC COORDINATOR 1 4 2 $14 - $19 $20 - $24 $30 - $35 $25 - $29 $36+ SALARY RATE* 38% 3% 3% 36% 9 respondents reporting a salary rate 2 respondents did not report actual rate 67 respondents reporting a hourly rate 2 respondents did not report actual rate
Salary respondents noted compensation structure based off multiple office titles HOURLY RATE $40,000-$49,000 $50,000-$69,000 $70,000+
is available to members on the BCoD website: bcofdermatology.com
is available to members on the BCoD website: bcofdermatology.com. ACCESS DERMATOLOGY / OCTOBER 2022 50
CAREER SURVEY REPORT
*
Data
Data
72% 4% 4% 20% WHAT IS MOST IMPORTANT TO YOU FOR JOB SATISFICATION? HOW MANY DAYS ARE YOU PHYSICALLY IN THE DERMATOLOGY OFFICE FOR WORK? I am fully remote 1 to 2 Every day 3 to 4 WHAT PERCENTAGE OF YOUR DAYS ARE FOCUSED ON BIOLOGIC COORDINATION? 0 to 25 25 to 75 75 to 100 High school Medical assistant LPN, RN Associate degree Bachelor's degree 42% 43% 15% 39% 23% 15% 9% 14% 0% 20% 40% 60% 60% 19% 14% 4% 3% Other Work-life balance Compensation Patient care and outcome Office collaboration and relationships WHAT IS YOUR HIGHEST DEGREE OF EDUCATION? bcofdermatology.com 51
I am the only one
DOES YOUR DERMATOLOGIST CONSULT WITH YOU ON WHICH MEDICATIONS TO PRESCRIBE PATIENTS DUE TO MANAGED CARE? No,
Yes No
Teams of 3 or more
DO YOU MANAGE ACCESS TO MEDICATIONS OUTSIDE OF BIOLOGICS?
All therapies
and biologics
only biologics
Procedures
Yes No 16% 33% 51% 19% 81% 87% 13% 69% 23% 8%
WILL YOU BE LOOKING FOR A DIFFERENT POSITION/EMPLOYER IN THE NEXT 12 MONTHS?
ARE YOU THE ONLY BIOLOGIC COORDINATOR IN THE OFFICE OR ARE YOU PART OF A TEAM OF BCS?
2022 BIOLOGIC COORDINATOR ACCESS DERMATOLOGY / OCTOBER 2022 52
Teams of 2 CAREER SURVEY REPORT
The physician spends more time in person, but I spend more time over the phone
I spend less time with the patient
I spend more time with the patient
We spend an equal amount of time with the patient
30
40
AVERAGE
WEEK DO YOU WORK?
AVERAGE, HOW MANY PATIENTS DO YOU
PER
10 to 25 26 to 100 more than 100
less than 30
to 40 more than
ON
HOW MANY HOURS A
ON
MANAGE
MONTH?
WHAT IS THE SINGLE BIGGEST FACTOR, IF AT ALL, THAT NEGATIVELY AFFECTS YOUR JOB SATISFACTION?
OF
INTERACTION VS. THE PRESCRIBER? Challenges with patient access Compensation Career Advancement Office relationships of Dermatologist Other* None Number of working hours 0% 10% 20% 30% 5% 15% 25% 35% 31% 22% 14% 10% 10% 10% 3% 32% 17% 51% 64% 35% 1% 0% 20% 40% 60% 10% 30% 50% 54% 28% 8% 10%
HOW WOULD YOU COMPARE YOUR AMOUNT
PATIENT
bcofdermatology.com 53

Easy on the Eyes
FIND THE PERFECT PAIR TO PROTECT YOU FROM BLUE LIGHT EXPOSURE
As biologic coordinators, we spend a lot of time in front of the computer. This screen, as is with other tablets and smartphones, emits highenergy blue light similar to the sun. Extended exposure may cause eye fatigue and discomfort, and has been connected to headaches, sore eyes and stiff necks. Blue light is also linked to disturbing sleep patterns because this artificial blue light tells our brain to stop producing melatonin. Without this sleep-inducing hormone we may take longer to fall asleep or have trouble staying asleep.
One way to combat this issue is to wear blue light glasses. For performance and style, we have rounded up some sleek options.
ACCESS DERMATOLOGY / OCTOBER 2022 54 OLEG GEKMANSTOCK.ADOBE.COM
SUITABLE FOR MEN AND WOMEN, THESE METAL CLASSIC AVIATOR FRAMES FEATURE SOFT NOSE PADS AND EASY ADJUSTMENT FOR LONG HOURS OF USE.
Richard by YesGlasses $63.60 yesglasses.com


THESE GLASSES TAKE ON A SPORTS-INSPIRED DESIGN WITH EVOLVE LIGHT RESPONSIVE LENSES THAT SHIFT FROM CLEAR TO GREY AS YOU TRANSITION BETWEEN INSIDE AND OUTSIDE.
Olympian I Deluxe Blue-Light Clear Evolve by Ray-Ban $204.00 ray-ban.com
Hunter by Roka $105.00 roka.com
THESE GLASSES ARE ULTRALIGHTWEIGHT AND COME IN SIZE REGULAR AND SMALL.

THESE COMBINATION SUNGLASSES ARE ANTI-BLUE LIGHT AND PHOTOCHROMIC, FOR ALL-DAY WEAR.
Blue Light Protection Squared Sunglasses with Havana-colored Acetate Frame Monte Blanc $395.00 montblanc.com


Percey by Warby Parker $95.00
THIS CLASSIC LOOK SHOWCASES ROUND LENSES AND A KEYHOLE BRIDGE, AND IT’S AVAILABLE IN DIFFERENT WIDTHS. warbyparker.com
Daisy by Warby Parker $95.00 warbyparker.com
SLENDER AND SCRATCHRESISTANT, THESE GLASSES HAVE ANTI-REFLECTIVE AND IMPACTRESISTANT MATERIAL.

bcofdermatology.com
55
The Evolution of Hubs
PROVIDING SOLUTIONS TODAY'S USERS WANT AND NEED
Article courtesy of CareMetx
Healthcare
is a $4 trillion industry experiencing a tremendous push for simple digital healthcare solutions. Unfortunately, as we wait for this digital shift to be fully leveraged and embraced, patients and healthcare providers alike are still frequently forced to navigate the cumbersome complexities of healthcare issues like figuring out insurance, determining out-of-pocket costs, and coordinating treatment plans—with little support.
Hub services have evolved to provide that support and help stakeholders overcome these barriers, especially the ones associated with patients accessing, affording, and adhering to specialty medicines.
SHIFT IN THE HUB INDUSTRY
The hub industry was originally built on technologies like phones, faxes, and paper forms, so managing those hubs required a large workforce of administrative experts and trained staff. These operations have always been a critical way for manufacturers to develop a personal connection with patients, helping to establish trust and confidence in their specialty therapy brand. On the downside, this people-based model was often impractical for those who needed to interact with it. Provider staff were spending hours on the phone coordinating
benefits verifications, and prior-authorization delays were holding up care.
To simplify, leading hubs began taking a fresh look at delivering the best possible patient experience via the digitally enabled, patient-focused model. The most comprehensive new hubs of this nature can leverage direct connectivity with payers and providers, multi-channel interfaces, process automation, and are typically supported by virtual call centers and staffed with welltrained individuals who can ensure these transactions run seamlessly throughout the patient journey.
These radically efficient hubs automate rote hub transactions, while directing more complex ones to skilled personnel who are both knowledgeable and empathetic to the patients they serve. Many also offer solutions with a specific focus on the

adherence portion of the patient's journey. These comprehensive solutions can provide stakeholders with the ability to oversee the entire treatment experience—including reimbursement, fulfillment, ongoing adherence, outcomes and data collection— from one single platform.
THE NEED TO FURTHER SIMPLIFY
The problem is, stakeholders often interact with these hubs via a portal, and today, each specialty therapy and manufacturer have a distinct portal and user experience that supports their brand. So, for providers who see dozens of patients prescribed different therapies throughout a given day, this often means organizing separate portal tabs bookmarked on their computers, remembering various usernames and entering numerous passwords throughout

the day as they toggle from portal to portal. Put simply, this administrative standard is not sustainable in the long term.
“In the specialty space, there are new products and indications coming to market every year,” says Chad Thompson, Vice President of Prescriber Networks at CareMetx.
“With the introduction of each new brand, providers and their staff members are required to familiarize themselves with a new interface, navigate a new hub service provider, create all new portal logins, and so on. As this dynamic currently stands, we are burdening individuals whose primary responsibility is treating patients, and whose time would be better spent with those patients.”
Prescribing specialty medications already takes a great deal of time and effort from a provider’s schedule, and they often need to designate one full-time employee or an entire team to manage the many tasks that are required to get these complex medications to their patients. These tasks typically include:

“The goal of a digital hub has always been to connect patients, providers, and other stakeholders to ultimately improve patient access and outcomes."
With portals from hubs, end users can:
Receive the prescription from the electronic medical record
(eliminating the need to initiate a prescription via fax or manually on a unique brand portal)
Perform the benefit investigation
Process the prior authorization
Assist in the appeal process
Locate the correct specialty pharmacy and transfer the prescription there
Investigating a patient’s insurance benefits for the prescribed medication
Submitting a prior authorization to the payer in order to confirm if said medication will be covered and approved
If an authorization is denied, navigating an appeal of the decision
Finding the correct specialty pharmacy for that patient based on their insurance
Sending the prescription to the appropriate pharmacy after it has been approved
Once those steps have been completed— which often can take weeks—the provider’s staff must also determine whether a co-pay program is offered in support of that medication, or they must try to connect the patient with other financial support programs that may be available. To date, many practices haven’t had a way to track all these fragmented processes in one place.
“ONE-STOP” PROCESS
To navigate this challenge, leading hubs have developed portals that allow providers and their staff to access hub services without having to leave the native workflows they're already comfortable using.
Solutions of this nature address all the above needs with one single process for all products. It enables providers to complete the tasks quickly and efficiently, while providing full visibility, in real time, to the entire prescription journey. This is the kind of efficiency that can reduce the time it takes for patients to access therapy from weeks to days.
Connect patients with co-pay programs and patient support services for all products
A NECESSARY EVOLUTION IN PATIENT SUPPORT
The goal of a digital hub has always been to connect patients, providers, and other stakeholders to ultimately improve patient access and outcomes. Of late, the frequent use of specialty pharmaceuticals, infused specialty medications and other novel treatment regimens is driving an urgent need for even more complex product access to journeys and delivery solutions. Therefore it’s critical to invest in a comprehensive and logical evolution in the patient support service industry. Simply put, these more practical solutions can revolutionize any brand’s patient access program—but most importantly, they can help get patients on therapy faster.
ACCESS DERMATOLOGY / OCTOBER 2022 58
HAVE A NICE DAYSTOCK.ADOBE.COM
BIOLOGIC COORDINATORS OF DERMATOLOGY
recognizes the following partners in their support of the 2022 BCoD national conference






















Thank you for your sharing in our mission to impact patients’ lives and advance access.

Utilizing Your EHR
WITH PREPARATION COMES SUCCESS
At some point the patients in your practice are going to need a medication or a new medication. Like an attorney in a trial, evidence will sway the jury into proving what you are saying is factual and lead to the guilty or not guilty verdict. Trying to convince a payer that your patients need a particular medication is not that different. You are trying to convince a non-medical professional to give you an approval for a medication their employer (payer or PBM) thinks the patient should not have. Your electronic heath record (EHR) program is your eyewitness, forensics and you are the attorney. Using chart notes to document everything from the patient's visit can come in handy during this process. The more detailed you can be, the more success you will have later when completing a prior authorization, letter of medical necessity or appeal.
ACCESS DERMATOLOGY / OCTOBER 2022 60
PIXELS HUNTERSTOCK.ADOBE.COM
As with many practices, a single patient may touch several healthcare providers during their treatment journey; therefore, organization and process is key. To help with this, there are several EHR systems specific to dermatology. Designing your EHR system based on its capabilities and necessities of your practice can help the access process run more smoothly for your office and patients.
First, there is communication. Through an intramail system, you can communicate within your practice by creating tasks without having to interrupt the flow of the day, and you can share “to-dos” with one another on the patient’s specific chart.
Another is documentation. Once documents are received, they can be scanned into the patient’s chart. This is your fact-finding tool to support the prescriber’s ask for a particular medication.
Items that should always be included or scanned in are:
BENEFITS INVESTIGATION OR VERIFICATION
2 CORRESPONDENCE FROM PAYER
• Prior authorization
• Formulary details
• Denial letter
• Step therapy requirements
• Co-pay card
• Insurance card
3 PRESCRIPTIONS If your office utilizes e-prescribing, you can quickly see what, when and where the medication was sent
DIAGNOSIS AND ICD.10 CODE
TRIED AND FAILED MEDICATIONS
CONTRAINDICATIONS
PGA AND IGA
“With preparation, you can quickly gain access to the therapy patients so desperately need.”
—MAVIS WALKER
EHR systems allow you to have pre-populated questions for certain diagnosis codes. This can help to organize notes for reference. Incorporate specific templates or questions that populate for atopic dermatitis and psoriasis. Collect patients’ height, weight, tried and failed medications, and any background as to why a medication may have failed. We also want to collect their BSA or EASI, PGA, and IGA scores specific for PSO or AD. What medication they are currently on and how have they been doing is also important. These items should be carried over to each new note. This helps to show the history of the patient, quickly, and concisely
By Mavis Walker
for the insurance company, compiling a story of their medical history. Building your EHR system to the things you will find most useful in your pull through efforts will help get the information from those within the patient’s room to your desk. Nurses, MAs, and the prescribers alike will be able to provide you with the details needed to pull the patient through to covered medication.
Keep in mind that a good EHR system will be built around the needs of your office. If you need help developing a template, look at previous prior authorizations and denial letters. Build your macros and automatic populations of questions to align with what is most frequently asked. Work alongside your provider(s) and ensure they can collect this information and that it will not slow down the visit and process of treating patients.
To technically implement, it can be helpful to pull in your EHR representative to help build this template for you. Do you know who your representative is?
With a series of chart notes you can demonstrate the trial and outcomes of certain medications and establish a baseline for the patient. It will help to develop a protocol for how chart notes should be written. By doing this you are creating an established process that will help you immensely when the time should come to complete a PA, letter of medical necessity or appeal. With preparation, you can quickly gain access to the therapy patients so desperately need.
bcofdermatology.com 61

Yoga in the Office
HOW TO BECOME ENERGIZED AND PEACEFUL AT WORK
By Caren Paskel

Is it possible to practice yoga in the office? Absolutely! It is not confined to just a yoga mat or a 90-degree yoga studio. Yoga is a practice of self-awareness, self-care, and self-love for anyone, anywhere, and at any time. It is not about posing; it’s about positioning your whole self as connected and centered. Yoga may be practiced at home, in the workplace, in relationships, and in all aspects of your life. While the poses and breathing techniques are wonderful and effective for physical health, there’s a larger mental component that shifts your state of mind, attitude, and the way you work. You can practice both in the office.
There’s a way to be energized and peaceful at work. Some people may have a ton of energy and be hurried, worried, and stressed out. Their minds may ramble a bit. Another group may be chilled out but not get as much done. They may find it hard to achieve their goals and get tasks completed.
A yogi is full of energy with a calm mind. Imagine feeling energized and peaceful while consulting with clients, being able to fully listen and have impactful conversations. Think about being a caregiver dedicated to individual patients and feeling stressed out, tired, and overworked. How does this affect your performance and productivity? How do you feel at the end of the day? Do you think this benefits you and your patients? There is a way to work peacefully rather than stressfully, managing your projects without feeling overworked. Yoga in the office is effective when your body is fueled, and your mind is present while being engaged.
How do you get your Zen on at work? Many yogis get their yoga fix at a studio class or by taking a virtual session. Shortly
afterwards, the Zen state disappears. Something happens, whether it’s traffic, relationship drama, work conflicts, schedule changes, or world issues… and now another yoga fix is needed! Yoga practice is applying what was learned to real life. The great news is you don’t need a yoga mat or even a structured class. When you develop selfawareness and instill self-care and self-love into your thoughts, actions, and interactions, you are practicing yoga.
Self-awareness is a focused awareness of yourself. Just because you’re in an office does not mean you need to disconnect or lose your sense of self. When you are selfaware, you will become more connected to your clients because you are more tuned in. The connection will foster a sense of joy. When you enjoy working you will be happy at your job and not feel like it's “work.” You will look forward to going and feel grateful for and within your job. This attitude shifts your entire week into an experience you feel you GET to rather than MUST do. You will feel lighter, not as heavy, and exhausted
by the end of your work week. Selfawareness means recognizing how you are doing. You are aware of your thoughts. Are they aligned with the conversation you are about to have? Adjust your thinking to one of being of service.
What are your feelings? What type of emotions are you encouraging and are they serving you properly? Being aware of your thoughts and feelings allows an opportunity to refine them if necessary to be at your best and to have optimal interactions.
What type of actions are you making? An awareness of your actions ensures they are the right actions. To-do lists may be endless until the day you die! Even after retirement, there will be things to do. What is a priority? What actions can be delegated? What are you doing that is ineffective or unnecessary? Awareness of action helps you establish what’s most important in aiding your patients. When you’re self-aware, you will be fully present, which makes a perfect action. You will have more energy because
bcofdermatology.com 63
OPPOSITE PAGE: KIEFERPIXSTOCK.ADOBE.COM; THIS PAGE: CONTRIBUTED
OFFICE YOGA ROUTINE
1
2
3
4
5
6
7
8
9
10
Close your eyes and take five deep breaths in and out of your nose if your airways are clear. If not, breathe through your mouth.
On an exhale allow your left ear to melt toward your left shoulder; you may also drape your left arm over your head for an added weight, stretching your neck. Inhale and raise your head back up to neutral. Change sides.
Take some shoulder rolls to loosen neck and shoulder tension.
Interlace your fingers and make a figure eight shape in both directions to stretch your wrists.
Inhale and reach the arms over your head. Exhale, grab your right wrist with your left hand, and lean to the left. Take a few breaths. Inhale and come up. Exhale and lean to the other side.
Inhale and raise your arms over the head once again. Exhale and twist your torso to the left. Gently hold on to your desk or chair on the left side as you rotate. Turn your gaze to your chest. Unwind and change sides.
Create space so you have room to place your left ankle over your right knee, toward your thigh in a figure four shape. Lean forward to intensify the sensation allowing opening of your left hip. Take 5-10 deep breaths. Change sides.
Straighten your legs and allow your upper body to fold forward over your lower body to stretch your hamstrings and release low back tension. You may sit in your chair to do this or stand up if you have the room and it’s appropriate.
Sitting midway on your chair, interlace your hands behind your head. Allow your head to lean back into your hands while arching your spine as you look toward the ceiling.
Bring your hands together in front of your heart, pressing the palms together. Acknowledge your self-awareness, self-care, and self-love.
ACCESS DERMATOLOGY / OCTOBER 2022 64
you will be focused on priorities rather than multitasking. When you complete one thing at a time, that one thing will be flawlessly done, and you can move to the next thing. There is no need to be hurried. The more self-aware you are the more productive you will be. Plus, it allows you to be yourself, fully engaged in each moment.
Self-awareness is a prerequisite for self-care. Without awareness, you won’t know how to care for yourself or meet your needs. By helping yourself, you are more available to help others. Self-care allows you to honor what you need to be at your best and thrive. When you need a bathroom break, you don’t ignore your body. You go to the bathroom! When you are hungry, you nourish yourself rather than starve. Being uncomfortable or hungry sets up agitation that may be projected onto your patients. Likewise, proper hydration is important, rather than depleting yourself with caffeine to set up a midday crash. Self-care may include taking a short walk outdoors, standing up to stretch your legs, or doing some yoga breathing and chair yoga poses.
Neglecting yourself is the opposite of self-care.
A client was having severe sciatic pain from working long hours sitting at his computer. I guided him through 30-minute yoga sessions three days a week, and he would tell me his pain level so we could address the issue. I told him to develop a habit of standing up every 20-30 minutes. He set an alarm as a reminder. He asked if I could create a 15-minute morning stretch routine to help prevent and reduce the pain. This was very effective in easing his pain! Self-care does not require hours.
Become self-aware to figure out how to take care of yourself!
Self-love is believing you are worthy of self-care. Self-love is how you maintain your self-care! When you love yourself, you emanate compassion. Patients will sense your loving vibrations through the phone.
Your words will come out as kind and loving. Self-love is the definition of yoga. When you love yourself, you feel love for everyone. This does not mean you like everyone, and that’s okay! Self-love is understanding there is a greater force inside you and every being that connects us all. This union of universal love is yoga. The root word of yoga is yuj, meaning to unite. When we love ourselves, we recognize that we are more alike than different. Love unites us rather than separates us.
Self-love will allow you to connect lovingly more deeply, creating winning conversations and happy patients. There are simple and efficient yoga breathing exercises and movements that can be performed in your chair! Yoga breathing is about being conscious of and using controlled breath. If you want to practice yoga breathing, all you need to do is be aware of your breath. You may notice your breath is short or shallow, or it may be rapid and labored. An easy way to calm your body is to slow your breathing and elongate the inhale and exhale. Think about stretching your breath to two to three times its natural length. That’s all. Voila! When you focus on your breath and count out 3-5 seconds for each inhale and exhale, you will notice a calm feeling. This is an easy tool for destressing and energizing. Practice
breathing for a minute or two. Go longer if you have the time. Go outside, close your eyes, and take some deep breaths. You will be refreshed. It’s better than a cup of coffee without later side effects.
Regarding yoga poses, think of keeping the spine happy by moving in six different directions: forward, backward, sideways, and twisting. These can be done while sitting. If you are at a keyboard for long periods, there are easy stretches for your wrists. If you are sitting all day, simple yoga poses for your neck and shoulders release tension. The hips and hamstrings get tight from sitting too, so let’s address some postures for those areas as well.
BIO
Caren Paskel's life’s purpose and fulfillment are to spiritually educate through master storytelling. She is the author of The Power of Self-Belief and the founder of Mental Training. Using yoga principles and philosophy, she evokes others to transform into fearless, independent, and radiant leaders. Caren has achieved abundant happiness and believes that it is every human’s birthright. Caren continues to work on her self-development to transform herself, others, and the world in a practical, playful, and tough-loving way. Her mission is to spread positivity globally.

bcofdermatology.com 65 SIRAPHOLSTOCK.ADOBE.COM
By Karan Lal, DO MS FAAD DOUBLE BOARD-CERTIFIED ADULT AND PEDIATRIC DERMATOLOGIST, FELLOWSHIP TRAINED COSMETIC SURGEON

DIET & PSORIASIS
Simple Lifestyle Changes Create Long-Term Improvement
Psoriasis as we know is a system-wide inflammatory disease. Studies have shown associations with inflammatory arthritis, obesity, heart disease, polycystic ovarian syndrome, smoking, and non-alcoholic fatty liver disease.
Many of these conditions have a metabolic component. Both obesity and psoriasis cause increased inflammation in your body by production of cytokines such as TNF-alpha. This is important to know as therapies must be adjusted according to comorbidities, inflammatory profiles, and weight. Some biologics such as infliximab and ustekinumab are weight-based, and being overweight may contribute to poor response to other medications that are better at treating psoriasis.
Psoriasis is also associated with obesity in children. This is important because fatty deposits, which contribute to heart disease, start to develop in the early second decade of life. Because I see both children and adults, I make sure to assess weight, height, and BMI starting in childhood. This is a great
indirect way of assessing metabolic health and allows us to intervene early. All my psoriasis patients, obese or not, get smoking cessation counseling, alcohol cessation counseling, and a referral to nutrition.
We know smoking is a risk factor for cancer, but it also allows neutrophils to be more aggressive, and these are one of the primary cells involved in psoriasis. Psoriasis is also associated with increased alcohol consumption. Alcohol not only contributes to poor metabolic health, but is high in carbohydrates, and consumption can lead to fatty liver disease. This is a contraindication to methotrexate therapy. Taking weight out of the equation, psoriasis can still be associated with an increased risk of heart disease. This is a reminder that all patients, not just obese patients, require education about better
eating habits (low fat and glycemic diet) with a focus on promoting the Mediterranean diet. Red meat is pro-inflammatory and can cause flares of disease in patients. Moreover, it adds to the risk of developing obesity and heart disease. A recent study on 97 patients with psoriatic arthritis were randomized to a low-calorie diet and fish oil, low-calorie diet and placebo supplement, or placebo supplement to assess if lifestyle changes impact skin and joint disease. After 12 weeks, they found improvement in multiple disease severity scores in the low-calorie and placebo group. They found low-calorie with fish oil resulted in weight loss, reduced body fat, and waist circumference. This shows that lifestyle modification with a low-calorie diet can improve disease severity and that fish
ACCESS DERMATOLOGY / OCTOBER 2022 66
CONTRIBUTED

CHACHEVASTOCK.ADOBE.COM
DARIA
oil supplementation may add additional improvement in weight loss (https://doi: 10.1186/s42358-022-00243-6).
Another study used a mouse model and showed a western diet increased skin and joint inflammation with increased Th-17 inflammatory mediators (https://doi: 10.1016/j. jid.2020.11.032). While only a mouse study, this certainly points to the inflammatory aspect of the traditional western diet and its effect on system-wide inflammation.
What can we do?
Restrictive diets are very hard to maintain. Referral to nutrition can help prevent restrictive and fad diets with short-term improvement. What we want is long-term improvement with simple, easy lifestyle changes. When I meet patients, I tell them the first place to start is cutting out unnecessary carbohydrates. This includes soda, sugary cereals, pastries and white bread. The high glycemic foods and beverages cause short-term energy, and over time, this contributes to insulin resistance. Instead I tell patients to switch to diet sodas, whole wheat bread, and whole grain low sugar (<6g per serving) cereal.
Next, I tell patients to reduce red meat consumption. Red meat is full of inflammatory mediators and low in healthy natural fats. I recommend patients focus on getting red and pink fish in their diet with sources such as salmon and tuna. Tuna is a great steak alternative. Turkey is underrated and is full of health antioxidants.
Lastly, I focus on improving vegetable intake. While vegetables are excellent, low-calorie, vitamin and antioxidant-rich, some vegetables are pro-inflammatory. Vegetables like eggplants, peppers and tomatoes contain solanine, which can promote inflammation. I recommend that patients limit intake of these vegetables and instead focus on the greens (Brussel sprouts, spinach, kale, broccoli, and lettuce). This is just an introduction to promoting a healthier lifestyle, which should not only improve skin and joint health, but promote overall heart and vascular health.

ACCESS DERMATOLOGY / OCTOBER 2022 68
OKKIJAN2010STOCK.ADOBE.COM

Confidently Prescribe the Right Biologic Drug Class for Your Psoriasis Patients

92% PPV

• Painless, minimally invasive test
• Patch placed on skin for 5 minutes



• Evaluates over 7,000 biomarkers per test sample















Please scan this code for more information minderahealth.com 5795 Kearny Villa Rd, San Diego, CA 92123 | Tel: (858) 810-6070


If you have questions, please contact a Mindera Health representative via email at mind.px@minderahealth.com. Mindera Laboratory is a CLIA-certifi ed, CAP-accredited, and HIPAA compliant laboratory (CLIA #05D2189599, CAP #8813410)


© Copyright 2022, Mindera Health
MAR01217.A
No More Trial & Error

Culinary Therapy
GET COOKING TO RELAX AND RELIEVE STRESS
During the colder months, there’s something soothing about baking and filling your home with wonderful smells of apples, chocolate and warm bread. Cooking is a relaxing way to unwind after work, but recent studies also show that spending time in the kitchen can help reduce stress and improve overall mental health. One study by the University of Toronto found that participants who baked “felt less stressed, had more patience, and were more creative than those who didn’t bake.”
We turned to Chef Michael Batt from Food Design, a catering and events company based in Greenwich, CT, for some decadent dessert recipes and a specialty cocktail to create this season. Carve out some time and serve up these treats for a cozy night at home.
RECIPES COURTESY OF CHEF MICHAEL BATT FROM FOOD DESIGN
A catering and events company based in Greenwich, CT
MATUCHA12STOCK.ADOBE.COM

CHOCOLATE BREAD PUDDING 8 to 10 servings
Ingredients
2 cups heavy cream
2 cups whole milk
1 vanilla bean, split, seeds scraped
1 pound bittersweet chocolate, finely chopped
8 large egg yolks
¼ cup sugar
¾ teaspoon kosher salt
1 pound brioche, cut into 1-inch pieces
1 ½ cups bittersweet chocolate chips (9 ounces)
Instructions
1. In a medium saucepan, combine the heavy cream with the milk and vanilla bean and bring just to a boil over moderately high heat. Remove from the heat and let stand for 5 minutes. Discard the vanilla bean and bring the cream mixture just to a boil. Remove from the heat and add the finely chopped chocolate. Let stand for 2 minutes, then whisk until smooth.

2. In a large bowl, beat the egg yolks with the sugar and salt. Very gradually whisk in the hot cream mixture until smooth. In another large bowl, toss the brioche with half of the chocolate cream and the chocolate chips. Spread the soaked bread in a 9-by-13-inch baking dish and then pour the remaining chocolate cream on top. Cover with plastic wrap and let stand at room temperature for 30 minutes.
3. Preheat the oven to 325°. Bake the bread pudding for 35 to 40 minutes, until the top is glossy, and the center is set. Let stand for 15 minutes. Serve the bread pudding warm with fresh whipped cream.

APPLE CIDER MULE
1 serving
Ingredients
2 ounces Apple Cider
2 ounces Vodka
.5 ounces Fresh Lime Juice
Ginger Beer
Ice Cinnamon stick
Apple Slice
Instructions
1. Fill copper mule mug with ice
2. Add 2 ounces of vodka
3. Add .5 ounces of fresh lime juice
4. Add 2 ounces of apple cider
5. Fill mug with ginger beer
6. Serve with cinnamon stick garnish and apple slice

bcofdermatology.com 71
CHOCOLATE
-
STOCK.ADOBE.COM
BY ALEKSS
STOCK.ADOBE.COM; CONNAMON STICKS BY TAN4IKK
Addressing the toughest challenges in atopic dermatitis takes all of us.
We fight the toughest health challenges with advanced science, putting our passion to work where the need is greatest. Because our purpose as a global biopharmaceutical company is to make a remarkable impact on people’s lives.
It takes all of us to turn possibilities into medicine that reaches millions. So we partner with governments, academic institutions, scientific and advocacy groups to make it happen. Together, we’re inventing the future of medicine.
Learn

more
at abbvie.com
INCREASE BRAND VISIBILITY AND SUPPORT THE EDUCATION OF BIOLOGIC COORDINATORS

Biologic coordinators are champions who liaise with patients, payors, manufacturers, and specialty pharmacies to manage disease and process. Reach us at: contact@bcofdermatology.com to discuss partnering with ACCESS DERMATOLOGY on customized content to increase brand visibility and advance patient access.



Help eligible patients start and stay on track with their therapy



















The DUPIXENT MyWay® patient support program can help facilitate patient access to DUPIXENT® (dupilumab) throughout the treatment journey.
Coverage support


DUPIXENT MyWay provides assistance navigating the insurance process with benefits investigations, PA support, and education about the appeals process.
Patient access support
DUPIXENT MyWay may have support for eligible patients who need help with their out-of-pocket costs.
Education and nursing support
Every enrolled patient is assigned a phone-based DUPIXENT MyWay Nurse Educator, who takes a patient-centric approach to providing tools, support, resources, and education throughout the treatment journey.
Support for your patients begins with a complete DUPIXENT MyWay Enrollment Form
You can help patients enroll in DUPIXENT MyWay by:
• Faxing the form to DUPIXENT MyWay at 844-387-9370
• Submitting the form through the DUPIXENT MyWay Document Drop at www.patientsupportnow.org
For more information: Visit us on the exhibit floor OR Call DUPIXENT MyWay at 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 AM–9 PM Eastern time
For any questions or concerns, or to report side effects with DUPIXENT® (dupilumab), please contact 1-844-DUPIXEN(T) (1-844-387-4936) Option 1, Monday–Friday, 8 AM–9 PM Eastern time.
DUPIXENT® and DUPIXENT MyWay® are registered trademarks of Sanofi Biotechnology. © 2022 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved. 08/2022 DUP.22.08.0202




















































































































 Ritz-Carlton and Four Seasons.
Women’s Faux Linen Maya Tunic $84.00
Jada with Matte Black Zipper $86.00
Ritz-Carlton and Four Seasons.
Women’s Faux Linen Maya Tunic $84.00
Jada with Matte Black Zipper $86.00





 Carhartt Men’s Solid Utility Ripstaop Top $28.99
Carhartt Men’s Solid Utility Ripstaop Top $28.99













































































































