
BRIDGE PROGRAMS Road to Access AFFORDABLE HEALTHCARE Financial Assistance Tools ROOMS WITH A VIEW Great Summer Getaways
SOCIAL MEDIA
The New Etiquette A
AUGUST 2023
JULY
STORY
PIRITUAL JOURNEY
OF TRANSFORMATION S




























Introducing LITFULO (ritlecitinib)
Introducing LITFULO (ritlecitinib)
Introducing LITFULO (ritlecitinib)


Introducing LITFULO (ritlecitinib)
The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12. 1,2
The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12 1,2
The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12. 1,2
The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12 1,2









One pill. Once a day.1
One pill. Once a day.1
One pill. Once a day.1








TO LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details. TO LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details.
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details. TO
TO LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details.
INDICATION
INDICATION
INDICATION
INDICATION













LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
SERIOUS INFECTIONS
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
SERIOUS INFECTIONS
SERIOUS INFECTIONS
SERIOUS INFECTIONS
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:











One pill. Once a day.1 Patient portrayal.
Patient portrayal.
Patient portrayal.
Patient portrayal.
• with chronic or recurrent infection
• with chronic or recurrent infection
• with chronic or recurrent infection

• who have been exposed to tuberculosis (TB)
• who have been exposed to tuberculosis (TB)
• with chronic or recurrent infection
• who have been exposed to tuberculosis (TB)



• with a history of serious infection or an opportunistic infection
• with a history of serious infection or an opportunistic infection
• who have been exposed to tuberculosis (TB)
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• who have resided or traveled in areas of endemic TB or mycoses, or
• who have resided or traveled in areas of endemic TB or mycoses, or
• with underlying conditions that may predispose them to infection
• with underlying conditions that may predispose them to infection
• with underlying conditions that may predispose them to infection
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
Tuberculosis
Tuberculosis
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.
LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM



Confidence in Patient Support
Confidence in Patient Support
Confidence in Patient Support
Confidence in Patient Support
Helping patients unlock access and reimbursement support for LITFULO™
Helping patients unlock access and reimbursement support for LITFULO™
Helping patients unlock access and reimbursement support for LITFULO™
Helping patients unlock access and reimbursement support for LITFULO™
Coverage Assistance
Coverage Assistance
Coverage Assistance
Coverage Assistance
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Financial Assistance
Financial Assistance
Financial Assistance
Financial Assistance
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
Pharmacy Coordination
Pharmacy Coordination
Pharmacy Coordination
Pharmacy Coordination
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
Live, Personal Support
Live, Personal Support
Live, Personal Support
Live, Personal Support
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.

A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages. For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages. For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages.
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages. For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
TM
TM
TM
TM
IMPORTANT SAFETY INFORMATION (cont’d)
SERIOUS INFECTIONS (cont’d)
In patients with a negative latent TB test, consider anti-TB therapy before initiating treatment with LITFULO in those at high risk and consider screening patients at high risk for TB during treatment with LITFULO.
Viral Reactivation
MORTALITY
Introducing LITFULO (ritlecitinib)
MALIGNANCIES
Introducing LITFULO (ritlecitinib)
Viral reactivation, including cases of herpes virus reactivation (eg, herpes zoster), was reported in clinical trials. If a patient develops herpes zoster, consider interrupting treatment until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy with LITFULO. Patients with evidence of HIV infection or hepatitis B or C infection were excluded from clinical trials.
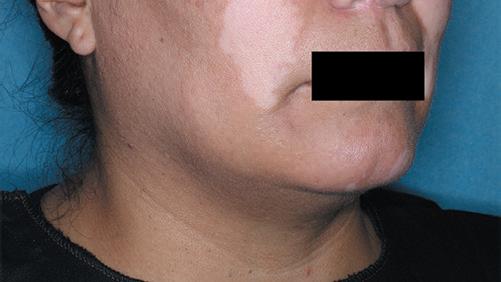
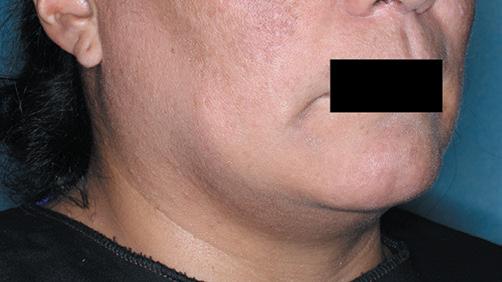
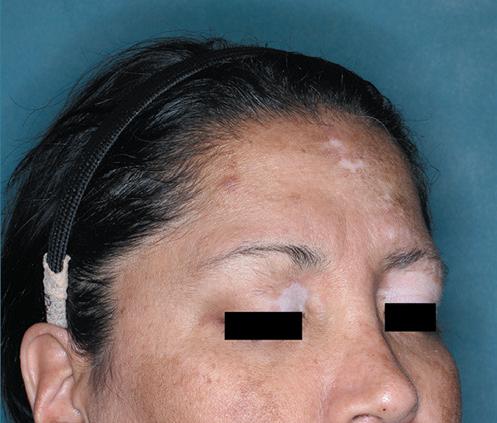
The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12. 1,2
The first and only FDA-approved treatment for severe alopecia areata in both adults and adolescents as young as 12. 1,2





In a large, randomized, postmarketing safety study of another Janus kinase (JAK) inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in RA patients treated with the JAK inhibitor compared with tumor necrosis factor (TNF) blockers. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO. LITFULO is not approved for use in RA patients.
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical trials of LITFULO.

TO LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding NMSC) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
TO LEARN MORE ABOUT LITFULO VISIT LITFULOHCP.COM
Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
One pill. Once a day.1
One pill. Once a day.1







The risks and benefits of ritlecitinib treatment should be considered prior to initiating or continuing therapy in patients with a known malignancy other than successfully treated NMSC or cervical cancer.




In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of MACE (defined as cardiovascular death, non-fatal myocardial infarction [MI], and non-fatal stroke) was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details.
Perform testing, evaluations, and procedures prior to LITFULO initiation. See Brief Summary for details.
Patient portrayal.
Patient portrayal.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with LITFULO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LITFULO in patients that have experienced an MI or stroke.
INDICATION
• with chronic or recurrent infection
THROMBOEMBOLIC EVENTS
INDICATION
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
• who have been exposed to tuberculosis (TB)
• with chronic or recurrent infection
• with a history of serious infection or an opportunistic infection
• who have been exposed to tuberculosis (TB)
Thrombosis has occurred in patients treated with LITFULO. An event of pulmonary embolism (PE) was reported in a patient receiving LITFULO. In a ritlecitinib higher dosing group, 1 patient reported an event of retinal artery occlusion.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
• who have resided or traveled in areas of endemic TB or mycoses, or
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, deep vein thrombosis, arterial thrombosis and PE were observed with the JAK inhibitor compared to those treated with TNF blockers.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
• with underlying conditions that may predispose them to infection
• with underlying conditions that may predispose them to infection
IMPORTANT SAFETY INFORMATION
Avoid LITFULO in patients who may be at increased risk of thrombosis. If symptoms of thrombosis or embolism occur, patients should interrupt LITFULO and be evaluated promptly and treated appropriately.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
LITFULO is contraindicated in patients with known hypersensitivity to ritlecitinib or any of its excipients.
HYPERSENSITIVITY
SERIOUS INFECTIONS
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
SERIOUS INFECTIONS
Serious reactions, including anaphylactic reactions, urticaria, and rash have been observed in patients receiving LITFULO in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue LITFULO and institute appropriate therapy.
LABORATORY ABNORMALITIES
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Patients treated with LITFULO are at increased risk of serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death, including tuberculosis (TB). The most frequent serious infections reported with LITFULO have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Treatment with LITFULO was associated with decreases in lymphocytes and platelets. Prior to LITFULO initiation, perform absolute lymphocyte count (ALC) and platelet count. After initiating treatment with LITFULO, treatment interruption or discontinuation is recommended based on ALC and platelet count abnormalities.
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefits of treatment prior to initiating LITFULO in patients:
Tuberculosis
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt treatment if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.
LITFULO should not be given to patients with active TB. Screen patients for TB before starting and monitor during therapy. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB.
Liver Enzyme Elevations: Treatment with LITFULO was associated with increased incidence of liver enzyme elevation compared to placebo. Increases of alanine transaminase (ALT) and aspartate aminotransferase (AST) ≥5 times the upper limit of normal were observed in patients in LITFULO clinical trials. Evaluate at baseline and thereafter according to routine patient management. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt LITFULO until this diagnosis is excluded.
Creatine Phosphokinase (CPK) Elevations: Treatment with LITFULO was associated with increased incidence of CPK elevation compared to placebo.
VACCINATIONS
No data are available on the response to vaccination in patients receiving LITFULO. Use of live attenuated vaccines should be avoided during or shortly prior to initiating treatment. Prior to initiating LITFULO, it is recommended that patients be brought up to date with all immunizations, including prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
Confidence in Patient Support
HEPATIC IMPAIRMENT
Confidence in Patient Support
Helping patients unlock access and reimbursement support for LITFULO™
LITFULO is not recommended in patients with severe hepatic impairment.
Helping patients unlock access and reimbursement support for LITFULO™
ADVERSE REACTIONS
Coverage Assistance
Most common adverse reactions (incidence ≥1%) are headache, diarrhea, acne, rash, urticaria, folliculitis, pyrexia, atopic dermatitis, dizziness, blood creatine phosphokinase increased, herpes zoster, red blood cell count decreased, and stomatitis.
DRUG INTERACTIONS
Coverage Assistance
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
Pfizer Dermatology Patient AccessTM (PDPA) provides assistance throughout the coverage process, including benefits investigation, prior authorization, and the appeals process.
LITFULO can increase plasma concentrations of CYP3A and CYP1A2 substrates. Consider additional monitoring and dose adjustment of CYP3A and CYP1A2 substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
Coadministration with strong inducers of CYP3A is not recommended.
USE IN PREGNANCY
Financial Assistance
Financial Assistance
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
No matter what type of insurance your patients have, financial support may be available. Eligible, commercially insured patients may save with the Copay Savings Card.*
Available clinical trial data on LITFULO use in pregnant women are insufficient to identify a drug-associated risk from major birth defects, miscarriage or other adverse maternal or fetal outcomes. Advise pregnant females and females of reproductive potential to inform their healthcare providers if they are pregnant or intend to become pregnant during treatment with LITFULO.
Pharmacy Coordination
If a patient becomes pregnant while receiving LITFULO, healthcare providers should report LITFULO exposure by calling 1-877390-2940.
LACTATION
Pharmacy Coordination
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
Advise women not to breastfeed during treatment with LITFULO and for 14 hours after the last dose.
PDPA strives to make LITFULO prescription fulfillment through the pharmacy as smooth as possible.
Please see Brief Summary of Prescribing Information, including BOXED WARNING, at the end of this advertisement.
Live, Personal Support
Live, Personal Support
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.
You and your patients can connect with a Patient Support Representative by calling 1-833-956-DERM (1-833-956-3376), Monday-Friday, 8AM-8PM ET.
A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
A Pfizer Field Reimbursement Manager (FRM) can support your patients enrolled in Pfizer Dermatology Patient Access by providing your o ce with access and reimbursement requirements. Visit PfizerDermFRM.com or scan the QR code to the right to find your local FRM.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
*Eligibility required. No membership fees. This is not health insurance. Maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. Terms and conditions apply.
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages. For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
References: 1. LITFULO. Prescribing information. Pfizer; 2023. 2. King B, Zhang X, Harcha WG, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b–3 trial. Lancet 2023;401(10387):1518-1529. doi:10.1016/S0140-6736(23)00222-2
© 2023 Pfizer Inc. All rights reserved. July 2023. PP-RIL-USA-0594
Please see additional Important Safety Information and Brief Summary of Prescribing Information on the following pages. For Prescribing Information, including BOXED WARNING and Medication Guide, visit LITFULOhcp.com.
TM
TM
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE), AND THROMBOSIS
• Increased risk of serious bacterial, fungal, viral, and opportunistic infections leading to hospitalization or death, including tuberculosis (TB). Interrupt treatment if serious infection occurs until the infection is controlled. LITFULO should not be given to patients with active TB. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test
• Higher rate of all-cause mortality, including sudden cardiovascular death with another Janus kinase (JAK) inhibitor vs TNF blockers in rheumatoid arthritis (RA) patients. LITFULO is not approved for use in RA patients
• Malignancies have occurred in patients treated with LITFULO. Higher rate of lymphomas and lung cancers with another JAK inhibitor vs TNF blockers in RA patients
• Higher rate of MACE (defned as cardiovascular death, myocardial infarction, and stroke) with another JAK inhibitor vs TNF blockers in RA patients
• Thrombosis has occurred in patients treated with LITFULO. Increased incidence of pulmonary embolism, venous and arterial thrombosis with another JAK inhibitor vs TNF blockers
INDICATIONS AND USAGE
LITFULO is a kinase inhibitor indicated for the treatment of severe alopecia areata in adults and adolescents 12 years and older.
Limitations of Use: Not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, cyclosporine or other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment With LITFULO
• TB infection evaluation: LITFULO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of LITFULO
• Viral hepatitis screening in accordance with clinical guidelines: LITFULO initiation is not recommended in patients with hepatitis B or hepatitis C
• Treatment with LITFULO should not be initiated in patients with absolute lymphocyte count (ALC) <500/mm3 or a platelet count <100,000/mm3
• Update immunizations according to current immunization guidelines
Recommended Dosage
The recommended dosage of LITFULO is 50 mg orally once daily with or without food.
LITFULO capsules should be swallowed whole; not crushed, split, or chewed.
If a dose is missed, the dose should be taken as soon as possible unless it is less than 8 hours before the next dose; in which case, skip the missed dose and resume dosing at the regular scheduled time.
Patients With Severe Hepatic Impairment
LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
Treatment Interruption or Discontinuation
If treatment interruption is indicated, a temporary treatment interruption for less than 6 weeks is not expected to result in signifcant loss of regrown scalp hair.
Hematologic Abnormalities
• Treatment with LITFULO should be discontinued if platelet count is <50,000/mm3
• Treatment with LITFULO should be interrupted if ALC is <500/mm3 and may be restarted once ALC returns above this value
ALC and platelet counts are recommended before treatment initiation and at 4 weeks after treatment initiation, and thereafter according to routine patient management.
DOSAGE FORMS AND STRENGTHS
Capsules: 50 mg of ritlecitinib, size 3, opaque capsules with yellow body and blue cap. The body is printed with “RCB 50” and the cap is printed with “Pfzer” in black.
CONTRAINDICATIONS
LITFULO is contraindicated in patients with known hypersensitivity to ritlecitinib or any of its excipients.
WARNINGS AND PRECAUTIONS
Serious infections have been reported in patients receiving LITFULO. The most frequent serious infections have been appendicitis, COVID-19 infection (including pneumonia), and sepsis. Among opportunistic infections, multi-dermatomal herpes zoster was reported with LITFULO. Avoid use of LITFULO in patients with an active, serious infection. Consider the risks and benefts of treatment prior to initiating LITFULO in patients:
• with chronic or recurrent infection
• who have been exposed to TB
• with a history of serious infection or an opportunistic infection
• who have resided or traveled in areas of endemic TB or mycoses, or
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with LITFULO. Interrupt LITFULO if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with LITFULO should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored. LITFULO may be resumed once the infection is controlled.
Tuberculosis
Screen patients for TB before starting therapy. LITFULO should not be given to patients with active TB. Anti-TB therapy should be started prior to initiating therapy with LITFULO in patients with a new diagnosis of latent TB or previously untreated latent TB. In patients with a negative latent TB test, consider anti-TB therapy before initiating treatment with LITFULO in those at high risk and consider screening patients at high risk for TB during treatment with LITFULO.
Viral Reactivation
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), was reported in clinical trials. If a patient develops herpes zoster, consider interrupting treatment until the episode resolves.
Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy with LITFULO. Patients with evidence of HIV infection or hepatitis B or C infection were excluded from clinical trials.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the benefts and risks for the individual patient prior to initiating or continuing therapy with LITFULO.
Malignancy and lymphoproliferative disorders, including nonmelanoma skin cancer (NMSC), were observed in clinical trials of LITFULO.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding NMSC) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
The risks and benefts of LITFULO treatment should be considered prior to initiating or continuing therapy in patients with a known malignancy other than a successfully treated NMSC or cervical cancer.
Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of MACE defned as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefts and risks for the individual patient prior to initiating or continuing therapy with LITFULO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue LITFULO in patients that have experienced an MI or stroke.
Thromboembolic Events
An event of pulmonary embolism (PE) was reported in a patient receiving LITFULO. In a ritlecitinib higher dosing group, 1 patient reported an event of retinal artery occlusion. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, deep vein thrombosis, and PE were observed compared to those treated with TNF blockers. Avoid LITFULO in patients who may be at increased risk of thrombosis. If symptoms of thrombosis or embolism occur, patients should interrupt LITFULO and be evaluated promptly and treated appropriately.
Hypersensitivity
Serious reactions including anaphylactic reactions, urticaria, and rash have been observed in patients receiving LITFULO in clinical trials. If a clinically signifcant hypersensitivity reaction occurs, discontinue LITFULO and institute appropriate therapy.
Laboratory Abnormalities
Treatment with LITFULO was associated with decreases in lymphocytes and platelets.
Prior to LITFULO initiation, perform ALC and platelet counts. After initiating treatment with LITFULO, treatment interruption or discontinuation are recommended based on ALC and platelet count abnormalities.
Liver Enzyme Elevations: treatment with LITFULO was associated with increased incidence of liver enzyme elevation compared to placebo. Increases of ALT ≥5 times the upper limit of normal (ULN) and increases of AST ≥5 times the ULN were observed in patients in LITFULO clinical trials. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in ALT or AST are observed and drug-induced liver injury is suspected, interrupt LITFULO until this diagnosis is excluded.
Creatine Phosphokinase (CPK) Elevations: treatment with LITFULO was associated with increased incidence of CPK elevation compared to placebo.
Vaccinations
No data are available on the response to vaccination in patients receiving LITFULO. Use of live attenuated vaccines should be avoided during or shortly prior to initiating treatment. Prior to initiating LITFULO, it is recommended that patients be brought up to date with all immunizations, including prophylactic herpes zoster vaccinations, in agreement with current immunization guidelines.
ADVERSE REACTIONS
Clinical Trials Experience
The safety of LITFULO was evaluated in three randomized, placebo-controlled clinical trials and one long-term trial in patients with alopecia areata, including alopecia totalis and alopecia universalis, who were 12 years of age and older. A total of 1628 patients were treated with LITFULO representing 2085 patient-years of exposure. There were 1011 patients with at least 1 year of exposure to LITFULO. In the placebo-controlled period of clinical trials in alopecia areata, a total of 668 patients were exposed to LITFULO with 130 receiving 50 mg once daily for up to 24 weeks. The median age of patients was 33 years, 105 (11.9%) patients were 12 to <18 years old and 22 (2.5%) patients were 65 years of age or older. The majority of patients were White (70.7%) and female (63.6%).
Adverse reactions occurring at ≥1% in the treated groups and at a higher rate than placebo are presented in the following table. A total of 2 (1.5%) patients treated with LITFULO 50 mg were discontinued from the trials due to adverse reactions.
LITFULO™ (ritlecitinib) capsules, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
Adverse Reactions in Clinical Trials of LITFULO for the Treatment of Alopecia Areata
LITFULO 50 mg N=130 n (%)
Placebo N=213 n (%)
Headache 14 (10.8)18 (8.5)
Diarrhea 13 (10.0)8 (3.8)
Acne 8 (6.2)10 (4.7)
Rash 7 (5.4)2 (0.9)
Urticaria 6 (4.6)3 (1.4)
Folliculitis 4 (3.1)4 (1.9)
Pyrexia 4 (3.1)0
Dermatitis atopic
Dizziness
Blood CPK increased
Herpes zoster
Red
Stomatitis
Specifc Adverse eactions
xposure adjusted incidence rates were adjusted by clinical trial size for all adverse reactions reported in this section.
Overall Infections
In the placebo-controlled trials, for up to 2 weeks, overall infections were reported in 66 patients (80.35 per 100 patient-years) treated with placebo and patients ( .5 per 100 patient-years) treated with LITFULO 50 mg. Across clinical trials, including the long-term trial, overall infections were reported in 6 5 patients (50. 1 per 100 patient-years) treated with LITFULO 50 mg or higher.
Serious Infections
In the placebo-controlled trials, for up to 2 weeks, patients reported serious infections across all ritlecitinib doses studied. Across clinical trials, including the long-term trial, serious infections were reported in 12 patients (0.66 per 100 patient-years) treated with LITFULO 50 mg or higher. The most common serious infections were related to appendicitis, COVID-19 infection (including pneumonia), and sepsis.
Herpes Zoster
In the placebo-controlled trials, for up to 2 weeks, herpes zoster was reported in 4 patients across all ritlecitinib doses studied and 0 patients treated with placebo. Across clinical trials, including the long-term trial, herpes zoster was reported in 21 patients (1.1 per 100 patient-years) treated with LITFULO 50 mg or higher. Opportunistic infections of multi-dermatomal herpes zoster were reported in 1 patient (0.50 per 100 patient-years) treated with the ritlecitinib higher dose in the placebo-controlled trials and 2 patients (0.1 per 100 patient-years) treated with LITFULO 50 mg or higher in all clinical trials.
Malignancy
In the placebo-controlled trials, for up to 2 weeks, 1 malignancy (breast cancer) was reported in 1 patient (1. per 100 patient-years) treated with ritlecitinib higher dose and no malignancy was reported in patients treated with placebo. Across clinical trials, including the long-term trial, malignancies excluding NMSC were reported in patients (0. per 100 patient-years) treated with LITFULO 50 mg or higher.
Thromboembolic Events
Across clinical trials, including the long-term trial, P was reported in 1 patient (0.06 per 100 patient-years) treated with LITFULO. There was 1 report of retinal artery occlusion and 1 report of acute MI.
Urticaria
In the placebo-controlled trials, for up to 2 weeks, urticaria was reported in 28 patients treated in all ritlecitinib doses studied and 3 patients treated with placebo. The rate of urticaria was 8.2 per 100 patient-years in patients treated with LITFULO 50 mg and .0 per 100 patient-years in patients treated with placebo. Across clinical trials, including the long-term trial, urticaria was reported in 6 patients treated with LITFULO 50 mg or higher. Among all patients treated with LITFULO 50 mg or higher in the integrated safety analysis, the rate of urticaria was .10 per 100 patient-years. The median time to onset of an initial event was 8 weeks; median duration of urticaria was 7 days. Most of the cases were mild to moderate in severity.
Decreased Lymphocyte Counts
Across clinical trials, including the long-term trial, confrmed ALC <500/mm3 occurred in 1 patient (<0.1%) treated with LITFULO 50 mg. Age appeared to be a risk factor for lower ALC in patients ≥65 years of age.
Decreased Platelet Count
In the placebo-controlled trials, for up to 2 weeks, treatment with LITFULO was associated with a decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which platelet count remained stable at a lower level with continued therapy. Across clinical trials, including the long-term trial, 1 patient ( 0.1%) had a confrmed platelet count <100,000/mm3. No patient had a confrmed platelet count <75,000/mm3
CPK Elevations
In the placebo-controlled trials, for up to 2 weeks, events of blood CPK increased were reported in 2 (1.5%) patients treated with LITFULO 50 mg and 0 patients treated with placebo.
Liver Enzyme Elevations
In the placebo-controlled trials, for up to 2 weeks, events of increases in liver enzymes ≥ times the ULN were observed in patients treated with LITFULO.
DRUG INTERACTIONS
Effects of LITFULO on Other Drugs
CYP3A Substrates
Ritlecitinib is a CYP3A inhibitor. Concomitant use of ritlecitinib increases area under the curve (AUC) and Cmax of CYP3A substrates, which may increase the risk of adverse reactions of these substrates.
Consider additional monitoring and dosage adjustment in accordance with approved product labeling of CYP3A substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
CYP1A2 Substrates
Ritlecitinib is a CYP1A2 inhibitor. Concomitant use of ritlecitinib increases AUC and Cmax of CYP1A2 substrates, which may increase the risk of adverse reactions of these substrates. Consider additional monitoring and dosage adjustment in accordance with the approved product labeling of CYP1A2 substrates where small concentration changes may lead to serious adverse reactions when used with LITFULO.
Effects of Other Drugs on LITFULO
CYP3A Inducers
Concomitant use of strong CYP3A inducer (e.g., rifampin) may decrease AUC and Cmax of ritlecitinib, which may result in loss of or reduced clinical response. Coadministration with strong inducers of CYP3A is not recommended.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry
If a patient becomes pregnant while receiving LITFULO, healthcare providers should report LITFULO exposure by calling 1-8 - 90-29 0.
Risk Summary
Available data from clinical trials with LITFULO use in pregnant women are insuffcient to identify a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ritlecitinib to pregnant rats and rabbits during organogenesis caused fetotoxicity and fetal malformations at exposures equal to 49 and 55 times the maximum recommended human dose (MRHD) based on an AUC comparison, respectively.
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The estimated background risks in the U.S. general population of major birth defects and miscarriages are 2- % and 15-20% of clinically recognized pregnancies, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats, oral administration of ritlecitinib from gestation days 6 to 17 decreased fetal body weights and caused fetal skeletal malformations (malformed vertebrae and ribs) and variations (delayed ossifcation) at doses ≥1 5 mg kg day ( 9 times the MRHD based on AUC comparison). Maternal toxicity (lower body weights) was noted at 325 mg/kg/day (102 times the MRHD based on AUC comparison). There was no developmental toxicity at 75 mg/kg/day (16 times the MRHD based on AUC comparison).
In an embryo-fetal development study in pregnant rabbits, oral administration of ritlecitinib from gestation days 7 to 19 decreased mean fetal body weights and increased visceral malformations (malpositioned kidneys), skeletal malformations (supernumerary sternebrae, absent thoracic
arch, and/or fused thoracic centra), and skeletal variations (delayed ossifcation) at 5 mg kg day (55 times the M D based on AUC comparison). There was no developmental toxicity at doses up to 25 mg/kg/day (12 times the MRHD based on AUC comparison).
In a pre- and postnatal development study in rats, oral administration of ritlecitinib from gestation day 6 through lactation day 20 had no effects on pre- and postnatal development at doses up to 75 mg/kg/day (14 times the MRHD based on AUC comparison). At 175 mg/kg/day (41 times the MRHD based on AUC comparison), ritlecitinib caused adverse lower postnatal survival and lower offspring body weights, which correlated with delayed sexual maturation in both sexes. Bred females in the F1 generation also exhibited lower mean numbers of corpora lutea at 175 mg/kg/day.
Lactation
Risk Summary
There are no data on the presence of ritlecitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Ritlecitinib is present in the milk of lactating rats. When a drug is present in animal milk, it is likely that it will be present in human milk. Because of the serious adverse effects in adults, including risks of serious infection and malignancy, advise women not to breastfeed during treatment with LITFULO and for approximately 14 hours after the last dose (approximately 6 elimination half-lives).
Data
After a single oral 30 mg/kg dose of ritlecitinib to lactating rats, ritlecitinib concentrations in milk over time were higher than those in plasma. The mean milk to plasma AUC ratio was determined to be 2.2.
Pediatric Use
The safety and effectiveness of LITFULO for alopecia areata have been established in pediatric patients ages 12 years and older. A total of 181 pediatric patients ages 12 to <18 years were enrolled in alopecia areata clinical trials, with 105 pediatric patients ages 12 to <18 years with alopecia areata randomized in a pivotal, double-blind, placebo-controlled trial (Trial AA-I). ffcacy was consistent between the pediatric patients and adults. The adverse reaction profle in the pediatric patients was similar to adults.
The safety and effcacy of LITFULO have not been established in pediatric patients under 12 years of age.
Geriatric Use
No dose adjustment is re uired for patients ≥65 years of age.
A total of 28 patients enrolled in alopecia areata trials were 65 years of age and older, and none were 75 years of age and older. Clinical trials of LITFULO did not include suffcient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients. As there is a higher incidence of infections in the elderly population in general, caution should be used when treating the elderly.
Hepatic Impairment
No dose adjustment is re uired in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. LITFULO is not recommended in patients with severe (Child Pugh C) hepatic impairment.
OVERDOSAGE
LITFULO was administered in clinical trials up to a single oral dose of 800 mg. Adverse reactions were comparable to those seen at lower doses and no specifc toxicities were identifed. Pharmacokinetics data up to and including a single oral dose of 800 mg in healthy adult volunteers indicate that more than 90% of the administered dose is expected to be eliminated within 48 hours.
There is no specifc antidote for overdose with LITFULO. Treatment should be symptomatic and supportive, and monitor patients for signs and symptoms of adverse reactions.
In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
This brief summary is based on LITFULOTM (ritlecitinib) Prescribing Information LAB-1 69-0.5.
Issued: June 2023.
The product’s label may have been updated. For full Prescribing Information, visit LITFULOHCP.com.
LITFULO™ (ritlecitinib) capsules, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information
3 (2.3)1 (0.5)
3
(1.4)
(2.3)3
2
(1.5)0
2
(1.5)0
2
blood cell count decreased
(1.5)0
2
(1.5)0
202 Pfzer Inc. All rights reserved. PP- IL-USA-0 1 une 202
MISSION STATEMENT: Access Dermatology aims to educate and empower the biologic coordinator by keeping them informed of the complex and everchanging drug and patient access landscape. Readers are engaged with editorial and lifestyle content equally suitable for dermatologic patients, so they too may gain a better sense of therapies and the patient services programs that can assist in their therapeutic journey.
DISPLAY ADVERTISING:
Contact Craig Schuette at cs@bcofdermatology.com.
EXECUTIVE DIRECTOR
Craig Schuette
EDITOR
Elizabeth Hole
CREATIVE DIRECTOR
Venera Alexandrova
ASSISTANT ART DIRECTOR
Lisa Servidio
ART INTERN
Sammi Fern
PRODUCTION DIRECTOR
Tim Carr
CORRESPONDENCE:
Communications regarding original articles as well as editorial suggestions for future issues should be addressed to Craig Schuette at cs@bcofdermatology.com. Any content forwarded to the publisher assumes no liability for the safety or return of unsolicited art, photographs, or manuscripts.

Cover photography by Cat Davis



ACCESS DERMATOLOGY JULY/AUGUST 2023 6
with practical information and lifestyle content
ISSUE 5
July/August 2023 PRESIDENT Jonathan W. Moffly
V. Moffly
Andrew Amill MOFFLY CUSTOM MEDIA 205 Main Street, Westport, CT 06880 telephone: 203-222-0600 fax: 203-222-0937 email: mail@MofflyCustomMedia.com Follow the Biologic Coordinators of Dermatology FACEBOOK LINKEDIN
A magazine dedicated to supporting patient access
ACCESS DERMATOLOGY
|
VICE PRESIDENT/BUSINESS Elena
CHIEF REVENUE OFFICER
© 2023 Access Dermatology and Biologic Coordinators of Dermatology. ALL RIGHTS RESERVED. The material in this publication is published by Moffly Custom Media and may not be reproduced or transmitted in any manner, in whole or in part, without the express written permission of Access Dermatology and Moffly Custom Media. NOTICE: The information contained within articles of this magazine represent the views and opinions of the original authors and do not necessarily represent the views and opinions of Access Dermatology or its affiliates. The mere appearance of content in the magazine does not constitute an endorsement by Access Dermatology or its affiliates. The content has been made available for informational and educational purposes only. Editorial advice is not specific, and readers are advised to seek medical, professional, or reimbursement help for individual circumstances. Access Dermatology hereby disclaims all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the content. CIRCULATION: To be added to the circulation, visit www.bcofdermatolgy.com/ magazine. REPRINTS:
promotional reprints,
For educational, commercial, or
including author off-prints, please email contact@bcofdermatology.com.

Guiding You Every Step of the Way
We offer personalized care for patients living with complex and chronic conditions, including:
> Limited distribution medications and biosimilars access


> Home infusion and support from our pharmacists and nurses
> Educational resources to help patients manage their medication


> Benefits investigation and financial assistance support





AcariaHealth.com
© 2023 AcariaHealth. All rights reserved. A2018_230404
more by
us today
Learn
contacting
Sales@AcariaHealth.com | 800.511.5144
nt ts
FEATURES
63, 70 News from OptumRX
COMMUNITY
66 BC Spotlight: Q&A with Amy Tarkington of Wilmington Health Dermatology
LIFESTYLE
50 Rooms with a View Distinctive summer getaways with a twist
64 Book No Further
fascinating summer reads
72 Last Word
ACCESS DERMATOLOGY JULY/AUGUST 2023 8
co
ACCESS DERMATOLOGY ISSUE 5
Five
The road to access 22 Affordable Healthcare Financial assistance tools to share with patients
Spiritual Journey A story of recovery and transformation
Social
Etiquette Guidelines for your dermatology practice
18 Bridge Programs
26
36
Media
Improving affordability for patients








bcofdermatology.com 9 18 26 50 BANK: STOCK.ADOBE.COM ; SUSY: TWIN FLAME PHOTOGRAPHY; PILL BOTTLE: KUPREVICHSTOCK.ADOBE.COM 22
letter from the founder / Craig Schuette

New Advances in the Field
The year 2023 is shaping up to be significant for dermatology, highlighting the impressive abilities of biologic coordinators to adapt and remain skillful.
We are witnessing the introduction of new, innovative medications that offer newfound relief to patients. Additionally, biosimilars, drugs that have demonstrated biosimilarity, are making lifechanging treatments more affordable.
Thanks to these new therapies and the patient programs accompanying them, access staff demonstrate their flexibility and dedication in navigating this emerging market.
Highlighted in this issue is a patient that reminds us: no matter how the market evolves, our mission is to achieve successful patient outcomes.
Susy shares a touching story that brings humanity to her ongoing struggle with alopecia areata. The personal experiences of patients like her underscore the importance of the
healthcare system and remind us that patients are much more than the happy faces we see in TV commercials for drugs. Despite challenges, Susy’s passionate spirit remains unbroken, and we are thrilled to invite her to speak at the BCoD National Conference this November.
As the season nears its end, I wish you a continued pleasant summer. If you have yet to make a summer escape, you might enjoy our “Rooms with a View” piece. In it, you’ll discover unique lodgings that deliver the rejuvenation we all need as we embark upon the second part of the year.
CRAIG SCHUETTE


ACCESS DERMATOLOGY JULY/AUGUST 2023 10

Bringing collections that balance technical innovation and iconic design to fit the active lifestyle of today’s sophisticated and conscious woman.



More th a n 75% of commerci a lly insured patient lives have coverage for OPZELURA®1






















Not an OPZELURA® patient.


For your members 12 years of age and older



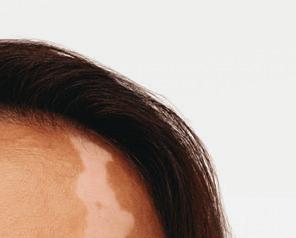
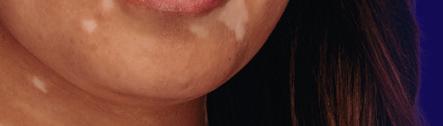
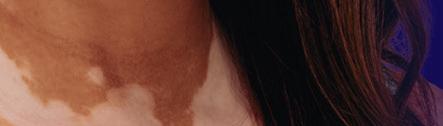
The First a nd Only

FDA-Approved Treatment for Repigmentation in Nonsegmental Vitiligo 2*
Proven to Promote Skin Repigmentation in Phase III Clinical Trials2


F-VASI75 Results at Week 24 and 52


Data From Week 24 And an Open-Label Extension
Nearly patients 1 in 3


Nearly1 in 3 patients achieved F-VASI75† at 24 weeks
(primary endpoint; 29.9% vs 7.5% [P<0.0001] and 29.9% vs 12.9% [P<0.01]).2,3


~51%

About half the patients remaining in the studies who applied OPZELURA® from day 1 achieved F-VASI75 at 52 weeks: ~51% for OPZELURA® and 28% for vehicle-to-OPZELURA® (Week 24 to Week 52).
Data were reported as observed. No conclusions of safety or efficacy should be made based on these results.2,4
Limitations of an open-label extension: In an open-label extension, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out.
*In patients 12 years of age and older.



OPZELURA® was studied in 2 double-blind, randomized, vehicle-controlled trials of identical design that enrolled 674 adult and adolescent patients with nonsegmental vitiligo ≥12 years of age. Patients had depigmented areas affecting ≥0.5% F-BSA, ≥ 3% nonfacial BSA, and total body vitiligo area (facial and nonfacial) of up to 10% BSA. Phototherapy was not permitted during the trials. In both trials, patients were randomized 2:1 to treatment with OPZELURA® or vehicle cream BID for 24 weeks followed by a 28-week open-label extension, wherein patients originally assigned to vehicle could switch to OPZELURA®. 2

BID, twice daily; BSA, body surface area; F-BSA, facial body surface area; FDA, Food and Drug Administration: F-VASI, Facial Vitiligo Scoring Index; F-VASI75, ≥75% improvement from baseline in Facial Vitiligo Area Scoring Index.
† F-VASI is a composite measurement of the overall area of facial vitiligo patches and degree of depigmentation within patches. As assessed, the face did not include surface area of the lips, scalp, eyelids, ears, or neck. 5


CONSIDER OPZELURA®2







See the Results With OPZELURA® at 52 Weeks




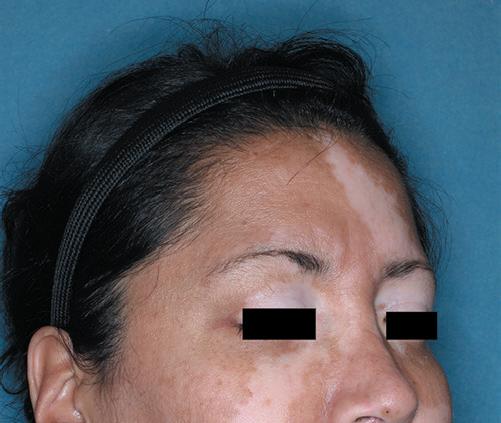
52-Week Results for a Clinical Trial Participant Whose Repigmentation Met the Primary Endpoint of F-VASI75 at 24 Weeks6‡
PRIMARY ENDPOINT

WEEK 24


WEEK 52









W eek 52

INDICATION
F
‡Results not typical. Actual patient treated with OPZELURA® in a clinical trial. Individual results may vary. Not for ophthalmic, oral, or intravaginal use.


OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.








IMPORTANT SAFETY INFORMATION
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for inflammatory conditions are at risk for developing serious infections that may lead to hospitalization or death. Reported infections include:


• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.


Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled. Carefully consider the benefits and risks of treatment prior to initiating OPZELURA in patients with chronic or recurrent infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA.
Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib.
No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat inflammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.



Please see Brief Summary of Full Prescribing Information, including Boxed Warning, on the following pages.





eek 0 F -VASI SCORE 1.5
W
eek 24 F -VASI SCORE 0.3 80% Improvement From Baseline
BASELINE W
-VASI
0.3 80%
Baseline
SCORE
Improvement From




Vitiligo Is an Autoimmune Condition That Impacts People From All Different Backgrounds


Vitiligo is a chronic, autoimmune skin condition affecting people of all ethnicities, skin types, and sexes.7


15.3%




In a retrospective study, 15.3% of patients with vitiligo had other autoimmune conditions.8*

See the Possibilities for Your Members With OPZELURA®
* Study Design: Retrospective chart review of 1487 US patients with a diagnosis of vitiligo between February 2005 and February 2015. Use of OPZELURA® in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.2

IMPORTANT SAFETY INFORMATION (continued)
SERIOUS INFECTIONS (continued)


Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat inflammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.


MALIGNANCIES




Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
MORTALITY



In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.




Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers. Non-melanoma skin cancers, including basal cell and squamous cell carcinoma, have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.












MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)


In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke.


Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.

THROMBOSIS



Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately.

References:






Thrombocytopenia, Anemia, and Neutropenia


Thrombocytopenia, anemia, and neutropenia were reported in the clinical trials with OPZELURA. Consider the benefits and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA.

Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically significant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations
Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.


Adverse Reactions
In nonsegmental vitiligo, the most common adverse reactions (incidence ≥1%) are application site acne (6%), application site pruritus (5%), nasopharyngitis (4%), headache (4%), urinary tract infection (2%), application site erythema (2%), and pyrexia (1%).
Pregnancy


There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Lactation
Advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).


Please see Brief Summary of Full Prescribing Information, including Boxed Warning, on the following pages.
1. VANTAGE Fingertip Formulary, April 2023. 2. OPZELURA® (ruxolitinib) cream. Prescribing Information. Incyte Corporation. 3. Rosmarin D, Pandya AG, Grimes P, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo: 24-week results from 2 randomized, double-blind phase 3 studies. Abstract presented at: 30th European Academy of Dermatology and Venereology (EADV) Congress; September 29-October 2, 2021; virtual. 4. Rosmarin D, Passeron T, Pandya AG, et al. Efficacy and safety of ruxolitinib cream monotherapy for the treatment of vitiligo: results from two 52-week phase 3 studies. Presented at: American Academy of Dermatology Annual Meeting; March 25-29, 2022; Oral Presentation. 5. Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet 2020;396(suppl):1-121. 6. Data on File. Incyte Corporation. 2023. 7. Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatol. 2020;236(6):571-592. 8. Hadi A, Wang JF, Uppal P, Penn LA, Elbuluk N. Comorbid diseases of vitiligo: a 10-year cross-sectional retrospective study of an urban US population. J Am Acad Dermatol. 2020;82(3):628-633.



OPZELURA, the OPZELURA logo, Incyte, and the Incyte logo are registered trademarks of Incyte.


© 2023, Incyte Corporation. 05/23 MAT-OPZ-01666








OPZELURA™ (ruxolitinib) cream, for topical use
OPZELURA™ (ruxolitinib) cream, for topical use
Brief Summary of FULL PRESCRIBING INFORMATION
Brief Summary of FULL PRESCRIBING INFORMATION
INDICATIONS AND USAGE: OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
INDICATIONS AND USAGE: OPZELURA is indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
Limitations of Use: Use of OPZELURA in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
SERIOUS INFECTIONS
Patients treated with oral Janus kinase inhibitors for in ammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
initiating OPZELURA in patients: with chronic or recurrent infection; with a history of a serious or an opportunistic infection; who have been exposed to tuberculosis; who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or with underlying conditions that may predispose them to infection. Closely monitor patients for the development of signs and symptoms of infection during and after treatment with OPZELURA. Interrupt OPZELURA if a patient develops a serious infection, an opportunistic infection, or sepsis. Do not resume OPZELURA until the infection is controlled.
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat in ammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Tuberculosis: No cases of active tuberculosis (TB) were reported in clinical trials with OPZELURA. Cases of active TB were reported in clinical trials of oral Janus kinase inhibitors used to treat in ammatory conditions. Consider evaluating patients for latent and active TB infection prior to administration of OPZELURA. During OPZELURA use, monitor patients for the development of signs and symptoms of TB.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat in ammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Patients treated with oral Janus kinase inhibitors for in ammatory conditions are at risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions and Adverse Reactions].
Reported infections include:
Reported infections include:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Invasive fungal infections, including cryptococcosis, and pneumocystosis.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Viral Reactivation: Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical trials with Janus kinase inhibitors used to treat in ammatory conditions including OPZELURA. If a patient develops herpes zoster, consider interrupting OPZELURA treatment until the episode resolves.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat in ammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials. Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. If a serious infection develops, interrupt OPZELURA until the infection is controlled.
The risks and bene ts of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Hepatitis B and C: The impact of Janus kinase inhibitors used to treat in ammatory conditions including OPZELURA on chronic viral hepatitis reactivation is unknown. Patients with a history of hepatitis B or C infection were excluded from clinical trials. Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking oral ruxolitinib. OPZELURA initiation is not recommended in patients with active hepatitis B or hepatitis C.
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
The risks and bene ts of treatment with OPZELURA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with OPZELURA [see Warnings and Precautions].
MORTALITY
MORTALITY
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing an oral JAK inhibitor to tumor necrosis factor (TNF) blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor [see Warnings and Precautions].
MALIGNANCIES
MALIGNANCIES
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat in ammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
Malignancies were reported in patients treated with OPZELURA. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat in ammatory conditions. In RA patients treated with an oral JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk [see Warnings and Precautions].
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (de ned as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
Mortality: In a large, randomized, postmarketing safety study of an oral JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA.
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat in ammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat in ammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (de ned as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue OPZELURA in patients who have experienced a myocardial infarction or stroke [see Warnings and Precautions].
THROMBOSIS
THROMBOSIS
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat in ammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
Malignancy and Lymphoproliferative Disorders: Malignancies, including lymphomas, were observed in clinical trials of oral JAK inhibitors used to treat in ammatory conditions. Patients who are current or past smokers are at additional increased risk. Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat in ammatory conditions. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA, particularly in patients with a known malignancy (other than successfully treated non-melanoma skin cancers), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Non-melanoma Skin Cancers: Non-melanoma skin cancers including basal cell and squamous cell carcinoma have occurred in patients treated with OPZELURA. Perform periodic skin examinations during OPZELURA treatment and following treatment as appropriate. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) de ned as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
Thromboembolic events were observed in trials with OPZELURA. Thrombosis, including pulmonary embolism (PE), deep venous thrombosis (DVT), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat in ammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with an oral JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid OPZELURA in patients at risk. If symptoms of thrombosis occur, discontinue OPZELURA and treat appropriately [see Warnings and Precautions].
WARNINGS AND PRECAUTIONS
WARNINGS AND PRECAUTIONS
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and bene ts of treatment prior to
Serious Infections: Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving oral Janus kinase inhibitors. Serious lower respiratory tract infections were reported in the clinical development program with topical ruxolitinib. Avoid use of OPZELURA in patients with an active, serious infection, including localized infections. Consider the risks and bene ts of treatment prior to
Major Adverse Cardiovascular Events (MACE): In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) de ned as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with OPZELURA , particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue OPZELURA in patients that have experienced a myocardial infarction or stroke.
Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat in ammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Thrombosis: Thromboembolic events were observed in clinical trials with OPZELURA. Thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat in ammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of an oral JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. Avoid OPZELURA in patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue OPZELURA and evaluate and treat patients appropriately.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia , and neutropenia were reported in the clinical trials with OPZELURA. Consider the bene ts and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically signi cant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Thrombocytopenia, Anemia, and Neutropenia: Thrombocytopenia, anemia , and neutropenia were reported in the clinical trials with OPZELURA. Consider the bene ts and risks for individual patients who have a known history of these events prior to initiating therapy with OPZELURA. Perform CBC monitoring as clinically indicated. If signs and/or symptoms of clinically signi cant thrombocytopenia, anemia, and neutropenia occur, patients should discontinue OPZELURA.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Lipid Elevations: Treatment with oral ruxolitinib has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides.
ADVERSE REACTIONS
ADVERSE REACTIONS
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not re ect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%).
Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not re ect the rates observed in practice. In two double-blind, vehicle-controlled clinical trials (TRuE-V1 and TRuE-V2), 449 adult and pediatric subjects 12 years of age and older with nonsegmental vitiligo were treated with OPZELURA twice daily for 24 weeks. In the OPZELURA group, 55% of subjects were females, and 81% of subjects were White, 5% were Black, and 4% were Asian. The adverse reactions reported by OPZELURA treated subjects with an incidence of ≥ 1% and at least 1% greater incidence than in the vehicle arm in the 24-week double-blind period are as follows for OPZELURA (N=449) vs Vehicle (N=224), respectively: Subjects with any treatment emergent adverse event (TEAE) 214 (48%) vs 79 (35%), Application site acne 26 (6%) vs 2 (1%), Application site pruritus 23 (5%) vs 6 (3%), Nasopharyngitis 19 (4%) vs 5 (2%), Headache 17 (4%) vs 6 (3%), Urinary tract infection 7 (2%) vs 1 (<1%), Application site erythema 7 (2%) vs 1 (<1%), and Pyrexia 6 (1%) vs 0 (0%).
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, in uenza-like illness, insomnia, nasal congestion, and vomiting.
Data: Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13 times the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
Pediatric Use: Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established.
Pediatric Use: Nonsegmental Vitiligo: The safety and effectiveness of OPZELURA for the topical treatment of nonsegmental vitiligo have been established in pediatric patients aged 12 to 17 years of age. Use of OPZELURA in this age group is supported by evidence from TRuE-V1 and TRuE-V2, which included 55 pediatric subjects aged 12 to 17 years with nonsegmental vitiligo. No clinically meaningful differences in safety or effectiveness were observed between adult and pediatric subjects. The safety and effectiveness of OPZELURA in pediatric patients younger than 12 years of age with nonsegmental vitiligo have not been established.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These ndings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Adverse reactions that occurred in TRuE-V1 and TRuE-V2 in ≥ 0.5% to < 1% of subjects in the OPZELURA group and none in the vehicle group were: application site dermatitis, hypertension, anxiety, application site discoloration, application site folliculitis, contusion, dermatitis contact, diarrhea, ear infection, gastritis, gastroenteritis, hordeolum, in uenza-like illness, insomnia, nasal congestion, and vomiting.
DRUG INTERACTIONS
DRUG INTERACTIONS
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4). Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Juvenile Animal Toxicity Data: Oral administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These ndings were observed at systemic exposures that are at least 40% the MRHD clinical systemic exposure.
Geriatric Use: Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include suf cient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
Geriatric Use: Of the 831 total subjects enrolled with nonsegmental vitiligo in clinical trials with OPZELURA, 65 (8%) were 65 years of age and older. Clinical trials of OPZELURA in subjects with nonsegmental vitiligo did not include suf cient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
PATIENT COUNSELING INFORMATION
Drug interaction studies with OPZELURA have not been conducted. Ruxolitinib is known to be a substrate for cytochrome P450 3A4 (CYP3A4). Inhibitors of CYP3A4 may increase ruxolitinib systemic concentrations whereas inducers of CYP3A4 may decrease ruxolitinib systemic concentrations.
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
PATIENT COUNSELING INFORMATION
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Strong Inhibitors of CYP3A4: Avoid concomitant use of OPZELURA with strong inhibitors of CYP3A4 as there is a potential to increase the systemic exposure of ruxolitinib and could increase the risk of OPZELURA adverse reactions.
USE IN SPECIFIC POPULATIONS
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Advise the patient or caregivers to read the FDA-approved patient labeling (Medication Guide).
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Pregnancy Exposure Registry: There is a pregnancy registry that monitors pregnancy outcomes in pregnant persons exposed to OPZELURA during pregnancy. Pregnant persons exposed to OPZELURA and healthcare providers should report OPZELURA exposure by calling 1-855-463-3463.
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not suf cient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Infections: Inform patients that they may be at increased risk for developing infections, including serious infections, when taking Janus kinase inhibitors. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of an infection. Advise patients that Janus kinase inhibitors increase the risk of herpes zoster, and some cases can be serious [see Warnings and Precautions]
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions].
Risk Summary: Available data from pregnancies reported in clinical trials with OPZELURA are not suf cient to evaluate a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of ruxolitinib to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes at doses associated with maternal toxicity. The background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risk in the U.S. general population of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
Data
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Malignancies and Lymphoproliferative Disorders: Inform patients that Janus kinase inhibitors may increase the risk for developing lymphomas and other malignancies including skin cancer. Instruct patients to inform their health care provider if they have ever had any type of cancer. Inform patients that periodic skin examinations should be performed while using OPZELURA. Advise patients that exposure to sunlight, and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen [see Warnings and Precautions].
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat in ammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Major Adverse Cardiovascular Events: Advise patients that events of major adverse cardiovascular events (MACE) including non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, have been reported in clinical studies with Janus kinase inhibitors used to treat in ammatory conditions. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events [see Warnings and Precautions]
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat in ammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions].
Animal Data: Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30, or 60 mg/kg/day in rats and 10, 30, or 60 mg/kg/day in rabbits. There were no treatment-related malformations at any dose. A decrease in fetal weight of approximately 9% was noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 22 times the clinical systemic exposure at the maximum recommended human dose (MRHD; the clinical systemic exposure from ruxolitinib cream, 1.5% applied twice daily to 25-40% atopic dermatitisaffected body surface area is used for calculation of multiples of human exposure). In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose resulted in systemic exposure approximately 70% the MRHD clinical systemic exposure. In a pre-and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse effects on embryofetal survival, postnatal growth, development parameters or offspring reproductive function at the highest dose evaluated (3.1 times the MRHD clinical systemic exposure).
Lactation
Lactation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse ndings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
Thrombosis: Advise patients that events of DVT and PE have been reported in clinical studies with Janus kinase inhibitors used to treat in ammatory conditions. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions].
Thrombocytopenia, Anemia , and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Thrombocytopenia, Anemia , and Neutropenia: Advise patients of the risk of thrombocytopenia, anemia, and neutropenia with OPZELURA. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of thrombocytopenia, anemia , or neutropenia [see Warnings and Precautions]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Administration Instructions: Advise patients or caregivers that OPZELURA is for topical use only [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Advise patients to limit treatment to one 60 gram tube per week or one 100 gram tube per 2 weeks [see Dosage and Administration]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Speci c Populations]
Pregnancy: Inform patients to report their pregnancy to Incyte Corporation at 1-855-463-3463 [see Use in Speci c Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Speci c Populations]
Lactation: Advise a patient not to breastfeed during treatment with OPZELURA and for about four weeks after the last dose [see Use in Speci c Populations]
Manufactured for: Incyte Corporation
Risk Summary: There are no data on the presence of ruxolitinib in human milk, the effects on the breastfed child, or the effects on milk production. Ruxolitinib was present in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse ndings in adults, including risks of serious infections, thrombocytopenia, anemia, and neutropenia, advise women not to breastfeed during treatment with OPZELURA and for approximately four weeks after the last dose (approximately 5-6 elimination half-lives).
1801 Augustine Cut-off Wilmington, DE 19803
OPZELURA is a trademark of Incyte. All rights reserved.
U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870 ; 11219624
© 2022 Incyte Corporation. All rights reserved.
Incyte Corporation. All rights reserved.
Issued: July 2022 PLR-OPZ-00018
Issued: July 2022 PLR-OPZ-00018
for: Incyte Corporation 1801 Augustine Cut-off Wilmington, DE 19803 OPZELURA is a trademark of Incyte. All rights reserved. U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 9079912; 9974790; 10639310; 10610530; 10758543; 10869870 ; 11219624 © 2022
Manufactured
BRIDGE
ACCESS DERMATOLOGY JULY/AUGUST 2023 18
By Christine Ramig

While most of us are familiar with bridge programs, it’s helpful to do a quick overview. These programs are for our commercial-insured patien ts who have a prior authorization denial on file. When bridge programs first came out, they were used for new-to-market biologics that didn’t have the commercial insurance coverage in place yet. This enabled us to get the drug in patients’ hands quickly, while the manufacturers fought to get that biologic on formularies. Their purpose was to give the power back to the provider on which biologic they wanted to use. This “bridge” between the delay of the insurance approval served as a fill-in until approval was finalized.
THE ROAD TO ACCESS PROGRAMS
bcofdermatology.com 19
Although that process is still in use, we have come to use bridge programs a lot more freely for our patients. It has become our way to establish care. We must ask ourselves how we begin to transition our patients from bridge programs to actual insurance approvals. In addition, we need to educate office staff, medical assistants, registered nurses and providers on what is needed in office notes. We need to elevate these notes: What is documented, tried and failed, additional medications the patient is on, and other co-morbidities the patient has. Other things to consider include the severity of the patient’s disease and location of plaques. What job does the patient hold? Is the patient in school and the plaques are visible? All these questions should be in the medical notes.
WHILE STATES ARE SLOWLY STARTING TO MAKE LAWS REGARDING STEP EDITS, THEY ARE STILL IN THE PICTURE. ASK YOURSELF THE FOLLOWING QUESTIONS:

What is the number one reason for a denial?
Did the patient need to try and fail topical use? Is there a reason we are not using topicals?
Can they be prescribed with the biologic you are trying to get approved, so when you write that appeal letter you can now add that topical use?
Was the denial due to not trying and failing a systemic?
In our last issue of Access Dermatology, we gave some great tips on Methotrexate avoidance. Is there a contraindication on why the patient can’t try Methotrexate? What other medications is the patient on that could be considered a contraindication?

“ WE WANT OUR PATIENTS TO SEE THOSE APPROVALS, and we want them to utilize their insurance they have paid for! "
The goal for each of our bridge patients is to get them insurance approval. The bridge is temporary, and we have seen our patients get pulled off these bridge programs. For example, we had a patient who was approved for a bridge program, and we completed each step that was required. We did a PA, appeal and second-level appeal once they were denied. Our next step was to have the patient apply for patient assistance. The patient was over the income for patient assistance, so he was pulled off the biologic that was working for him. We had to explain to the patient that he needed to come off his medicine and do what the step edit asked us to do, so we could get the approval through the insurance. It is an exhausting, vicious circle that can be avoided for the most part, with one extra step. Now I’m not saying this happens all the time, but if we educate our providers and sometimes explain that just a simple topical could be prescribed to get a biologic approved, isn’t that the route we should go? We want our patients to see those approvals, and we want them to utilize their
insurance they have paid for!
Think back to when you did a peer to peer. Do you remember some of the questions they asked about why we wanted a specific biologic? We had a strong answer, we laid out our whys and got them overturned quite often. Do you know what the physician at the insurance company is saying on the other end of the phone call now? They are telling us the medicine is going to get denied, but that is okay because the patient can just go on the bridge program. The insurance companies want us to have the patients go on these programs because that means they don’t have to approve it; they don’t have to pay out for that patient’s prescription since they know the patient is going to get it anyway. That is not the way it should be. The goal is for the provider to be the decision maker and have power to write what they want to write. It should not be the insurance company saying they are not approving it, but it is “okay because the patient will get it anyway.” Let’s fight together to get more approvals for patients!
bcofdermatology.com 21

Afford le
FINANCIAL ASSISTANCE TOOLS TO SHARE WITH PATIENTS


ACCESS DERMATOLOGY JULY/AUGUST 2023 22
By Kereen Crider BS, PACS, BIOLOGICAL COORDINATOR, PORTER PREMIERE DERMATOLOGY AND SURGERY CENTER

Healthc e
When it comes to helping people, Mr. Rogers said it best:
LOOK FOR THE HELPERS. You will always find people who are helping. To this day, especially in times of disaster,' I remember my mother's words, AND I AM ALWAYS COMFORTED BY REALIZING THAT THERE ARE STILL SO MANY HELPERS so many caring people in this world."
– FRED ROGERS OF MISTER ROGERS NEIGHBORHOOD TELEVISION FAME
The PAN foundation (Patient Access Network) is exactly that.
A helper to those who need additional assistance with the cost of their disease, whether it is for medications, transportation, mental or physical support.
bcofdermatology.com 23
WHAT IS PAN?
The credo is taken directly from panfoundation.org:
“ WE HELP UNDERINSURED PEOPLE WITH LIFE -THREATENING, CHRONIC, AND RARE DISEASES GET THE MEDICATIONS AND TREATMENTS THEY NEED by assisting with their out-of-pocket costs and advocating for improved access and affordability.”
PAN is a privately funded organization that receives funds by way of private or corporate donations. Patients receive funding for a full year.
IDENTIFYING PATIENTS
Typically, patients who do not have insurance or have previously been screened for patient assistance foundations through the manufacturers would be ideal candidates. We would already know they require some form of assistance, and likely will need additional grants to ease the burden of their disease. If you are able, I recommend having candid conversations with patients to see what their individual needs and unique situations are.
FUND FINDER
This is a unique tool on the website that allows patients to search their rare or chronic condition to see if funds are available.
Atopic dermatitis and plaque psoriasis are two of many diseases the PAN foundation supports; however, currently their enrollment is closed. I still encourage my patients to check back or join the waitlist, in the event registration reopens.
Patients can apply for funding, and, if accepted, they are then provided PAN portal entrance and guided instruction on how to manage their grant. If the funds are closed, patients have the option to be added to a waitlist and are encouraged to check back often, as the status can change.
WHY IS THIS IMPORTANT TO BCS AND OUR PATIENTS?
Simply put, this leads to improved health outcomes. Patients may abandon therapy prematurely if they think they have run out of resources or funding. This organization helps BCs provide valuable information and possible funds to our patients, as most of them likely do not know about this option or know to research it on their own. Several of our patients have used PAN foundation, and they understand the onus is on them. While it is their responsibility, they always appreciate any additional guidance I can provide.
The goal is to add another useful resource to our BC toolbelt.
If you get a chance, visit panfoundation.org. I find it heartwarming to look at the pictogram of the United States, demonstrating the number of patients assisted within each state. Since its inception, across all states, the PAN foundation has assisted more than one million patients.
Keep calm and BC on.
*Source: panfoundation.org
ACCESS DERMATOLOGY JULY/AUGUST 2023 24
BIOLOGIC COORDINATOR ST ART PROGRAM
COLLABORATE WITH BIOLOGIC COORDINATORS OF DERMATOLOGY (BCOD) TO SET YOU AND YOUR BIOLOGIC OFFICE TEAM UP FOR SUCCESS.
Whether you are new to the patient access role, or have several years of experience, BCoD ofers hands-on support to enhance and refne your access workfow by providing education, guidance, and materials to thoroughly cover the approval process and broad patient access management.
Receive your own Specialty Drug Resource Binder which documents a clear layout of every step most specialty drugs need to go through to avoid pitfalls and achieve approval.
Our team of experts average over 15 years of biologic coordinator experience, and our Specialty Binder is resource that has evolved over 10 years – being tried and tested against all insurance plans for maximum approval efciencies.
• Reduce burnout and staf turnover with a customized ofce workstream Do you use EMA for your EHR? Our trained EMA “Super-users” will coach and upskill on how to implement data integration tools and or organize your practice to make the prior authorization process easier.
Our seasoned Biologic Coordinator experts take pride in patient access. We are here to help streamline your approval procedures, reduce burnout, and foster patient communication with patient-education handouts. Live and virtual assistance available.
Reach us at contact@bcofdermatology.com to learn how we can help you advance patient access.

ACCESS DERMATOLOGY JULY/AUGUST 2023 26 CAT DAVIS

Susy Markoe Schieffelin was everything a Greenwich girl is supposed to be—smart, ambitious, thoughtful, raised to believe anything is possible if you work hard, study hard, go to the right schools and belong to the right clubs. She and her three equally talented younger sisters were known around town as the four blond Schieffelin girls. “We wore matching outfits and always had bows in our hair,” she says. But Susy was hiding a secret beneath her golden locks, a secret that led her down a shame-based path of addiction. She started losing her hair when she was seven. “As a young girl, my hair was such a huge part of my identity. I just remember thinking, ‘what’s wrong with me?’”
Susy was diagnosed with an autoimmune disease called alopecia areata. “I was worried people would think I was a freak and ugly and I wouldn’t fit in. I developed a lot of social anxiety as a result,” she says. In middle school she discovered alcohol. “It was like this magical elixir that quieted my mind. I could finally relax and have fun.” For
years she hid her condition from all but a few trusted confidants. She maintained the façade of perfection through high school, college and, eventually, into a full-time job working for an upscale restaurant group in New York City. As her addiction to drugs and alcohol worsened, Susy started to feel hopeless. One night, she wrote a note to
her family and prepared to take her own life. Miraculously, she woke up the next morning in her bed. She called her mother, younger sister Louisa (“my angel,” as Susy refers to her) and a friend, and they came to pack her up and bring her home to Greenwich. Susy left for treatment a few days later.
“I knew that something had to change for
ACCESS DERMATOLOGY JULY/AUGUST 2023 28
MADRIGAL
RACHEL
"It shifted my frequency FROM ADDICTION AND A SCARCITY MINDSET to a newfrequency OF HEALING AND ABUNDANCE and unconditional love"
her,” says Louisa, about that morning. “I said, ‘Enough is enough. You need to come home with us and get help.’”
That was in September 2015. Since then, the thirty-four-year-old has been on a powerful journey of recovery. She moved to California. Ditched her all-black wardrobe. Stopped taking any substances. Got off her anxiety medications. Developed a strong sober support network. Began going to sound baths. Began leading sound baths. Got certified as a reiki master and kundalini yoga instructor. Developed a big following on Instagram and YouTube. Launched a business. Shaved her head and threw away her wig. Met and married her husband, Mark Doody, who is the vice president of a technology company. Had a baby.
Talking to Susy now, it’s hard to square the hopeless, self-loathing addict she used to be with the warm, confident, effusive woman she has become. Her energy is palpable, her gratitude inspirational. Around her, it’s impossible not to feel just a little bit lighter in mind and spirit. “I’ve been given so much,” she says. “I feel this great need to pay it forward.”
YOU CAN GO HOME AGAIN
In January, Susy and her nine-month-old son, Jack, traveled from Santa Monica to her hometown, where she led a “healing through harmony” breakfast sound bath at a local restaurant for forty people. The next day, Susy led a longer sound bath at a

church, her first since 2019. Longtime friend Gabriella Gentil was among the sixty or so attendees. “There’s a science behind it. She uses her voice to guide you to a very safe place. The sound makes you feel like you’re wrapped in a warm blanket,” she says. There is a science behind sound baths. Participants lie down on a mat and close their eyes. The sound healer then begins a guided mediation while playing a collection of crystal bowls. The healing frequencies activate and align the chakras, stimulate alpha, theta and delta brain-wave states, and promote deep rest and relaxation. Put simply, “a sound bath is like an energetic deep-tissue massage for your body, mind and spirit,” Susy says. “After just one session, most people feel very calm and relaxed.”
GROWING PAINS
Susy will be the first to admit that part
of her shame was fostered by growing up in Greenwich. “You’re surrounded by people who are smart and successful and beautiful. There can be a lot of pressure to keep up with that.”
When she first started losing her hair, the teachers thought she might be pulling it out, recalls her mother, Susan. “I told them there was no way she was doing that.” Together they made the rounds of traditional doctors and dermatologists. They prescribed lotions, pills and cortisone injections. She says it was heartbreaking to watch her daughter struggle with her affliction. “When she was in high school, she loved to dance. But she always stayed in the junior dance corps, because the movements were less extreme, and she could be more controlled. She was so afraid of a bald spot showing.” Her mother was determined to find the cause and began taking her daughter to energy healers, ayurvedic healers and acupuncturists.
“At that time, in the late nineties, it wasn’t really common to explore alternative healing in the way she did for me,” Susy adds. “I think that really does tie back to where I am today with the work I do.”
From the outset, Susy didn’t drink the way her friends did. “I went from this lovely girl who did what she was supposed to do and had it all together and got straight A’s and dressed appropriately to being the girl who let it all out—saying whatever was on my mind and having no inhibitions. I would
bcofdermatology.com 29
CLOSE UP OF HAND BY ALO MOVES

wake up the next morning and feel really ashamed.”
Susy excelled as a student at Greenwich Academy, where she learned Chinese and spent a semester abroad in LeShan in China’s Sichuan province. She graduated cum laude and was accepted into the University of Virginia early decision, where she majored in East Asian and Religious studies.
She dreamed about getting married, buying a house in Greenwich with a white picket fence and having a baby—all by the time she was twenty-four. The universe had other plans. She moved to New York, did a six-month program at culinary school, then started working in the hospitality industry.
Plagued by constant anxiety, Susy turned to prescription medications. “It was a glorious but also dangerous cocktail when mixed with booze,” she says. “I’d end up at the emergency room and not know how I got there. My parents and my
friends were like, you might want to think about controlling your drinks.” But she was powerless over her addiction.
Though she could go days, weeks, even months without drinking, once she started, she couldn’t stop. For Susy, this was the beginning of the end.
“I feel like there were angels around me even as I was going down this dark path. I could see I needed help.”
She started an outpatient program at the Freedom Institute in New York. That experience planted a seed, but it would be another few years before she was ready to leave substance abuse behind. That moment brings us back to the night in August of 2015 when she almost took her life but managed to make it through with the help of her family and friends, and get herself into an in-patient rehab.
Once there, Susy began a daily gratitude practice, something she remembers Oprah
talking about during a speech at Greenwich Academy. “The concept of gratitude was something I didn’t understand. I felt like the victim. Why is my hair falling out, why didn’t my relationship work out? Why can’t I stop drinking.
Nothing was going according to my plan, and I wasn’t grateful.”
With the help of her counselor, she began to turn those thoughts around. After thirty days, something shifted. She started to read spiritual books, had reiki healings, began meditating daily, did music therapy—“all things that connected me back to my essence,” she says. “I started paying attention to the breadcrumbs.”
Newly sober and living at home with her parents, she followed the breadcrumbs to L.A. to support her younger sister, who was presenting research at a psychology conference. Like many diehard East Coasters, Susy had always made fun of the laid-back California lifestyle. No one was more surprised than she was when she got there and felt at home. “I realized I needed to live there.”
Every day in her meditation she began visualizing her life in California. “I could see and feel the details, even though I didn’t know how or when they would happen,” she says. In May she saw a Facebook post from a friend who was moving out of her apartment in Santa Monica. “It was the apartment I’d been seeing in my meditation,” Susy says. “Right down to the beachfront location.”
She signed the lease sight unseen and moved in July 2016.
The only people Susy knew in L.A. were her mother’s sister and a sober friend, who connected her to a twelve-step group. When her aunt invited her to a sound bath, she said yes, without a moment’s hesitation.
For Susy, the experience was lifechanging. “It’s something that my soul had been searching for all those years of reaching for pills and alcohol,” she says.
“I finally felt calm and at peace, and that everything was okay.” She started attending
ACCESS DERMATOLOGY JULY/AUGUST 2023 30
RACHEL MADRIGAL
“THE CONCEPT OF GRATITUDE WAS SOMETHING I DIDN’T UNDERSTAND. I felt like the victim. Why is my hair falling out, why didn’t my relationship work out? ”
sound baths on a regular basis, began training to be a reiki master, got certified as a yoga instructor. She lost weight. The light started to come back into her eyes. She says the cumulative effect of the different healing modalities was transformational. “It shifted my frequency from addiction and a scarcity mindset to a new frequency of healing and abundance and unconditional love.”
Another pivotal moment came in October 2016, when she attended her first breath-work sound bath. “This message came to me—this has helped you so much, you need to learn this and share it with other people.” The instructor offered her a mentorship. “He would pay me $100 a night, and I was like, ‘this is amazing. I’m getting paid for doing something I love, and I would have done it for free.’”
After nine months as his assistant, she started to feel like it was time to leave her full-time job. “I sensed the work I was doing wasn’t in alignment with my spirit anymore. But the job was too cushy to leave,” she says. In April 2017, she read the book The Surrender Experiment by Michael Singer, author of the Untethered Soul. “I finished it and said out loud, ‘I surrender. Put me on my path.’” The next day she received a phone call from her boss, offering her a position at a corporate office in downtown L.A. Susy declined. “I knew if I went back to my former lifestyle, I would drink,” she says.

She called her mom and said, “I just quit my job. I’m a healer now. I’m going to start my own business.”
THERE ARE NO COINCIDENCES
On her last day of work, Susy went down to the beach, smoked a cigarette and threw away the rest of the pack. “I’m done holding myself back,” she remembers saying. “I’m a healer now.” She turned around, packed a bag and flew to New York, where she had been invited to speak about her recovery at the Freedom Institute’s annual fundraising gala at the Mandarin Oriental. She still has the off-the-shoulder black dress she wore that night—the only item of black clothing in her wardrobe.
Starting a successful business didn’t happen overnight. “I made it up as I went along,” she says. She used her savings to hire a team of professionals—coaches, lawyers and accountants—to help. She gave herself a deadline. She already had a name for her new venture—The Copper Vessel.
“I woke up one night thinking about a bunch of names; and as I reached for a glass of water from the copper vessel I kept on my nightstand, I thought, hmm, the copper vessel.” She pauses at the memory. “Copper represents the divine feminine. It’s a healing metal and a great conductor of energy.”
When a friend asked why copper? Why not platinum or gold? Susy said, “It’s not gold or platinum. It’s not the Susy show. It’s a humble metal. It’s here to serve.”
She launched the Copper Vessel in May 2017 and offered her first public sound bath at the beach on July 22. She texted all her friends, and twenty-six people showed up and donated a total of $500. Within a month she led her first class at a local meditation studio, which led to a gig doing sound baths at the sober living house at USC. She started sharing sound baths at a twelve-step meeting she attended. From there, the concept caught on and Susy was able to expand her audience and help more people.
Over the years, Susy’s sound baths have been viewed by millions of people worldwide and can be experienced on
bcofdermatology.com 31
CAT DAVIS
WORK WITH SUSY
VIRTUAL SOUND BATHS
Susy holds live virtual sound baths twice a month for the new and full moons. Relax and heal in the comfort of your own home for $33. For upcoming events, visit thecoppervessel.com/calendar.
SOUND HEALING MEMBERSHIP
For relaxation on demand, join Susy's online sound healing membership
The Copper Vessel Collective for $44 per month or $444 per year. Members get access to an extensive library of on-demand sound baths, free access to Susy's live virtual events, and VIP access and discounts to her in-person events and healing programs. To learn more about Susy's healing membership, visit thecoppervessel.com/tcvc.
THE SOUND HEALER'S ACADEMY
For those who have been touched by sound healing and would like to learn how to play crystal singing bowls, share sound healing professionally, and build a successful business as a healer, Susy offers a three-month professionally accredited sound healing certification training program called The Sound Healer's Academy. The training is held virtually twice per year and tuition is $4,444. For details to become a certified sound healer, visit thecoppervessel.com/tsha.
PRIVATE INSTRUCTION
Susy also offers a limited number of three month one-on-one deep healing containers (virtually and in person) as well as private business mentorships for healers and spiritual entrepreneurs. To learn more and inquire about working one-on-one with Susy please email info@thecoppervessel.com.

every United flight. She has collaborated with notable artists such as Leann Rimes and played her crystal bowls with the Los Angeles Philharmonic. She has brought sound healing to corporate groups at Google and Pandora, among others, and has established a loyal following.
THE FUTURE
Susy envisions a time when her Sound Healer’s Academy will be the international standard for training, she will be a published author, and she will have elevated sound healing into a mainstream activity,
performing at venues like Madison Square Garden and the Sydney Opera House. Most of all, she will continue to bring hope and healing to people struggling with anxiety, addiction and other mental health issues.
Given her track record, it’s easy to believe she’ll accomplish all of this and more. As her friend Gabriella says, “I believe in Susy and her light. Her honesty is disarming. She makes herself vulnerable so others can make themselves vulnerable. I think some people are real life fairies. She walks out of your house, and she leaves a trail of fairy dust in her wake.”
ACCESS DERMATOLOGY JULY/AUGUST 2023 32
ALO MOVES
BIOLOGIC COORDINATOR NOMINATE A FOR THEIR SERVICE
Biologic Coordinators of Dermatology (BCoD) understands the commitment to patient care. Our members are at the forefront of access, helping achieve patients’ confdence within their skin.
BCoD would like to acknowledge biologic coordinators across the country who are advocates for their patients, work tirelessly to fnd solutions and resources to assist in drug access, and create an atmosphere focused on celebrating the patient.
To all the biologic coordinators working for the dermatology patient, we applaud you!
Please submit nominations to contact@bcofdermatology.com with a description and example of how the biologic coordinator demonstrates a dedication and work ethic for patient care.
Individuals will be highlighted in future publications.
Any content will be reviewed with offces for compliance and privacy purposes.






NOVEMBER 5-7, 2023
BCoD 2023 Annual Conference
JW Marriott San Antonio Hill Country Resort & Spa
The BCoD National Conference brings together the largest community of biologic coordinators and o ce sta supporting the patient access journey. This conference is the premier experience for biologic coordinators, o ce access sta , and industry partners to come together for education, training, networking, and thoughtful insight exchanges.
Join us to tap into expert acumen delivered through immersive workshops, training sequences, and presentations from industry wide organizations.
NEW THIS YEAR:
•Never-before-offered BC certification. BCoD has partnered with medical institutions to develop the most relevant and meaningful certification course for biologic coordinators and access office staff in Dermatology
“The ability to learn from and rely on my peers has helped me stay up to date with the market and changes in patient programs, but it has also allowed me to see real change in our processes. These changes have ultimately led to getting drugs into the hands of patients more e ciently and e ectively. I highly recommend this community to anyone in the industry looking to grow and improve their skills.”
- BCoD Member
“I’ve been going to Dermatology conferences for over 10 years and I haven’t seen such spirit and enthusiasm in the attendees as I did at BCoD’s national meeting.”
-Industry Exhibitor
SCAN TO LEARN MORE!




LEARN MORE AT BCOFDERMATOLOGY.COM






























ACCESS DERMATOLOGY JULY/AUGUST 2023 36
By Sarah Pinowski Jansen

Social Media Etiquette
GUIDELINES FOR YOUR DERMATOLOGY PRACTICE
In today’s digital age, social media has become central to our lives, influencing how we communicate, compile information and make decisions. It has the power to enhance patient communications and engagement. Still, social media practices must take certain precautions to craft a responsible online presence—and to avoid ethical and legal snags along the way.
bcofdermatology.com 37
MARKETING & COMMUNICATIONS CONSULTANT
HIPAA COMPLIANCE IN THE AGE OF SOCIAL MEDIA
Oversharing on social media can be detrimental for anyone, but the result could become downright devastating when HIPAA enters the chat. It's important to mention that social media rules about HIPAA do not exist, because HIPAA was enacted years before social networks became part of our lives. That said, biologic coordinators and their teams still need to avoid HIPAA violations due to social media misuse. With the advent of smartphones and
other digital devices, sensitive patient or practice information and gossip can be sent to any number of recipients anywhere in the world. Ensuring that new employees receive adequate training, as well as ongoing refresher courses, is crucial. Offices can also integrate social media instruction into their initial HIPAA training and annual HIPAA retraining initiatives to keep everyone up to date. At best, operational and employment policies should detail consistent information concerning your office's social media guidelines.

TYPICAL SOCIAL MEDIA HIPAA VIOLATIONS







•


Information that personally identifies patients


Images or videos of patients shared without their consent


• frame



Images or videos of a practice, including patients or Protected Health Information (PHI) in the


“A study by the American Management Association found that cyberloafing could cost larger employers up to $600,000 per year. But that's not the only loss.
ENGAGING IN CYBERLOAFING CAN LEAD TO DIRE CONSEQUENCES IF AN
EMPLOYEE
BREACHES HIPAA
REGULATIONS OR SLANDERS A PATIENT. This can result in much bigger issues than just a dip in productivity."




EMPLOYEE RIGHTS
Employees have the right to communicate with colleagues, whether or not they’re union members. Federal law protects their ability to engage in union-related and “protected concerted” activities, even in cyberspace. According to the National Labor Relations Board (NLRB), "protected concerted" activities dictate that employees can address work-related gripes related to pay, benefits and working conditions with their coworkers. However, protected activity does not extend to egregiously scathing or knowingly false remarks about the employer's products and services without relating the comments to any actual labor controversy.

But the vast audience of the internet blurs the lines on cases of employee-onemployer rants.
In a legal case known as the Facebook Firing, the NLRB ruled in favor of the employee, citing that the employer's general policies restricted employees from participating in concerted activities, including discussing the terms and conditions of their employment with coworkers and others. Peers and coworkers supported her post and shared their feedback, making the exchange a laboradjacent discussion.
CYBERLOAFING
Taking time out of the day for social media opens the door to potential cyberloafing. While it can serve as a moment of rejuvenation for employees, the cost of scrolling time adds up fast. A study by the American Management Association found that cyberloafing could cost larger employers up to $600,000 per year. But that's not the only loss. Engaging in cyberloafing can lead to dire consequences if an employee breaches HIPAA regulations or slanders a patient. This can result in much bigger issues than just a dip in productivity.
The NLRA cases tend to favor the employer when it comes to how employees spend their business hours.
Back in 2011, a respiratory therapist made negative comments about a coworker on Facebook while on the way to an emergency call in an ambulance. Although she claimed mistreatment by a colleague, the NLRB ruled that her behavior did not qualify as protected concerted activity as it was a personal issue, not a collective concern. The therapist was expected to focus on the emergency instead of coworker challenges.
bcofdermatology.com 39
“It is crucial for the policy to CLEARLY STATE WHETHER EMPLOYEES CAN EXPECT PRIVACY WHEN USING SOCIAL MEDIA ON WORK DEVICES AND NETWORKS IN THE MEDICAL PRACTICE SETTING , as well as the extent to which the employer may monitor their activities."

MONITORING YOUR TEAM
It isn’t uncommon for a manager to scope out a potential new hire, but now, more healthcare employers mull regular social media monitoring.
Your office should have a documented social media policy regarding their activity and employee monitoring.
It is crucial for the policy to clearly state whether employees can expect privacy when using social media on work devices and networks in the medical practice setting, as well as the extent to which the employer may monitor their activities. This policy should be regularly reviewed and updated. When considering the privacy expectations of an employee, it’s crucial to weigh them against the potential risks posed to an employer’s reputation and confidential information. If there’s any uncertainty, seeking legal counsel for advice is highly recommended.




HOW CRITICAL IS YOUR PRESENCE ONLINE?
Various social media platforms—Facebook, Instagram, LinkedIn and TikTok, to name a few—empower dermatology offices to engage with their current and future patients. Through these channels, practices can toot their own horn, provide helpful resources, and deliver evidence-based content to combat misinformation pumped out by nonprescribers across the web. Your office can also capture vital marketing and branding exposure, essentially free advertising for existing and building a community with followers.
ACCESS DERMATOLOGY JULY/AUGUST 2023 40
EDUCATION & NETWORKING EMPOWERS MEMBERS THROUGH Join our network of biologic coordinators landscape of drug access together. Sign up today at bcofdermatology.com Connect with BCoD:

CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.


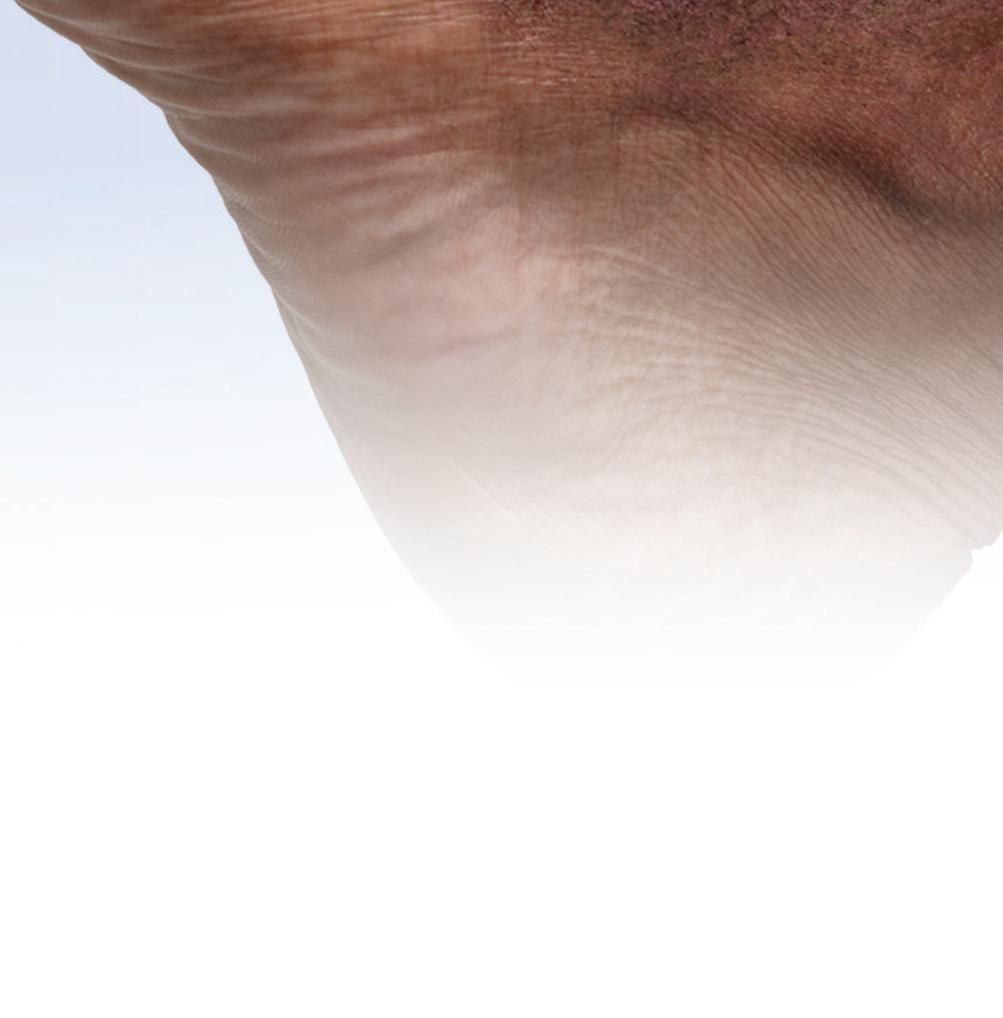



IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS

Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death. The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia.
If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection.
Reported infections from Janus kinase (JAK) inhibitors used to treat inflammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral (including herpes zoster), and other infections due to opportunistic pathogens.

To learn more about CIBINQO e cacy, visit CIBINQOhcp.com or scan QR code.




Avoid use of CIBINQO in patients with an active, serious infection, including localized infections. The risks and benefits of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections or those who have resided or traveled in areas of endemic tuberculosis or endemic mycoses.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO.
Viral reactivation, including herpes virus reactivation (eg, herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves. Hepatitis B virus reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C.
JAK=Janus kinase; AD=atopic dermatitis.
Committed to Patient Support
Helping patients unlock access and reimbursement support for CIBINQO™ (abrocitinib)

pay as little as $ 0 per month*
With the Copay Savings Card, eligible commercially insured patients may Visit PDPACopayCard.com
*Eligibility required. No membership fees. This is not health insurance. The maximum benefit per patient is $15,000 per calendar year. Only for use with commercial insurance. If you are enrolled in a state or federally funded prescription insurance program, you may not use the copay card. See full terms and conditions below.
Copay Savings Card: TERMS AND CONDITIONS
By using the Pfizer Dermatology Patient AccessTM Copay Savings Card, you acknowledge that you currently meet the eligibility criteria and will comply with the terms and conditions described below:
• You are not eligible to use this card if you are enrolled in a state or federally funded prescription insurance program, including but not limited to Medicare, Medicaid, TRICARE, Veterans A airs health care, a state prescription drug assistance program, or the Government Health Insurance Plan available in Puerto Rico (formerly known as “La Reforma de Salud”).
• You must have commercial insurance. O er is not valid for cash-paying patients.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for CIBINQO™ (abrocitinib) may pay as little as $0 per month. Eligible patients with commercial prescription drug coverage may receive a maximum benefit of $15,000 per calendar year, which is defined by the date of enrollment through December 31st of the enrollment year. After a maximum of $15,000, you will be responsible for paying the remaining monthly out-of-pocket costs.
• By using this copay card at participating pharmacies, eligible patients with commercial prescription drug insurance coverage for EUCRISA® (crisaborole) may pay as little as $10 per tube. Eligible patients with commercial prescription drug insurance coverage that does not cover EUCRISA may pay as little as $100 per tube. Individual savings are limited to $970 per tube. Individual patient savings are limited to $3,880 in maximum total savings per calendar year.
• This copay card is not valid when the entire cost of your prescription drug is eligible to be reimbursed by your commercial insurance plan or any other health or pharmacy benefit program.
• You must deduct the value of this copay card from any reimbursement request submitted to your commercial insurance plan, either directly by you or on your behalf.
• You are responsible for reporting use of the copay card to any commercial insurer, health plan, or other third party that pays for or reimburses any part of the prescription filled using the copay card, as may be required. You should not use the copay card if your insurer or health plan prohibits use of manufacturer copay cards.
• This copay card is not valid where prohibited by law.
• Copay card cannot be combined with any other savings, free trial, or similar o er for the specified prescription.
• Copay card will be accepted only at participating pharmacies.
• If your pharmacy does not participate, you may be able to submit a request for a rebate in connection with this o er.
• This copay card is not health insurance.
• O er good only in the United States and Puerto Rico.
• Copay card is limited to 1 per person during this o ering period and is not transferable.
• A copay card may not be redeemed more than once per 30 days per patient.
• No other purchase is necessary.
• Data related to your redemption of the copay card may be collected, analyzed, and shared with Pfizer, for market research and other purposes related to assessing Pfizer’s programs. Data shared with Pfizer will be aggregated and de-identified; it will be combined with data related to other copay card redemptions and will not identify you.
• Pfizer reserves the right to rescind, revoke, or amend this o er at any time without notice.
• O er expires 12/31/2025.
For questions or additional support, call 1-833-956-3376, write to Pfizer Inc. at PO Box 29387, Mission, KS 66201, or visit the CIBINQO website at www.CIBINQO.com or the EUCRISA website at www.EUCRISA.com.
Please see additional Important Safety Information and Brief Summary of full Prescribing Information on the following pages. For full Prescribing Information, including BOXED WARNING and Medication Guide, visit CIBINQOhcp.com.
TM
MORTALITY
In a large, randomized postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality (including sudden cardiovascular death) was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
MALIGNANCIES
Malignancies, including non-melanoma skin cancer (NMSC), were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat inflammatory conditions. Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
In a large, randomized postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA patients. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur.
THROMBOSIS
Deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat inflammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of overall thrombosis, DVT, and PE were observed when compared with TNF blockers. CIBINQO is not approved for use in RA patients.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and treat patients appropriately.
CONTRAINDICATION
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤ 81 mg daily), during the first 3 months of treatment.
LABORATORY ABNORMALITIES
Hematologic Abnormalities: Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia.
Prior to CIBINQO initiation, perform a complete blood count (CBC). CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations: Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy, and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The e ect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
IMMUNIZATIONS
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines, including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
RENAL IMPAIRMENT
Avoid use in patients with severe renal impairment or end stage renal disease, including those on renal replacement therapy.
HEPATIC IMPAIRMENT
Avoid use in patients with severe hepatic impairment.
ADVERSE REACTIONS
Most common adverse reactions (≥ 1%) in subjects receiving 100 mg and 200 mg include: nasopharyngitis, nausea, headache, herpes simplex, increased blood creatine phosphokinase, dizziness, urinary tract infection, fatigue, acne, vomiting, oropharyngeal pain, influenza, gastroenteritis.
Most common adverse reactions (≥ 1%) in subjects receiving either 100 mg or 200 mg also include: impetigo, hypertension, contact dermatitis, upper abdominal pain, abdominal discomfort, herpes zoster, and thrombocytopenia.
Inform patients that retinal detachment has been reported in CIBINQO clinical trials. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision.
DRUG INTERACTIONS
Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO. See Prescribing Information for clinically relevant drug interactions.
USE IN PREGNANCY
Available data from pregnancies reported in clinical trials with CIBINQO are not su cient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Advise females of reproductive potential that CIBINQO may impair fertility.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
LACTATION
Advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose.
INDICATION
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable. Limitations of Use: CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
Please see full Important Safety Information throughout and full Prescribing Information, including BOXED WARNING, and Medication Guide.
SAFETY INFORMATION
IMPORTANT
& INDICATION
© 2023 Pfizer Inc. All rights reserved. May 2023. PP-CIB-USA-0665
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, and THROMBOSIS
Serious Infections
Patients treated with CIBINQO may be at increased risk for developing serious infections that may lead to hospitalization or death; The most frequent serious infections reported with CIBINQO were herpes simplex, herpes zoster, and pneumonia. If a serious or opportunistic infection develops, discontinue CIBINQO and control the infection. Reported infections from Janus kinase (JAK) inhibitors used to treat in ammatory conditions:
• Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Test for latent TB before and during therapy; treat latent TB prior to use. Monitor all patients for active TB during treatment, even patients with initial negative, latent TB test.
• Invasive fungal infections, including cryptococcosis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
• Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens. Avoid use of CIBINQO in patients with an active, serious infection including localized infections. The risks and bene ts of treatment with CIBINQO should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIBINQO, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Mortality
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another JAK inhibitor to TNF blocker treatment, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor. CIBINQO is not approved for use in RA patients.
Malignancies
Malignancies were reported in patients treated with CIBINQO. Lymphoma and other malignancies have been observed in patients receiving JAK inhibitors used to treat in ammatory conditions. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in patients treated with CIBINQO. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (de ned as cardiovascular death, myocardial infarction, and stroke), was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients treated with CIBINQO. Thrombosis, including PE, DVT, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat in ammatory conditions. Many of these adverse reactions were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid CIBINQO in patients at risk. If symptoms of thrombosis occur, discontinue CIBINQO and treat appropriately.
INDICATIONS AND USAGE
CIBINQO is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable.
Limitations of Use CIBINQO is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Testing, Evaluations, and Procedures
Prior to Treatment Initiation
Perform the following tests and evaluations prior to CIBINQO initiation:
• Tuberculosis (TB) infection evaluation – CIBINQO initiation is not recommended in patients with active TB. For patients with latent TB or those with a negative latent TB test who are at high risk for TB, start preventive therapy for latent TB prior to initiation of CIBINQO
• Viral hepatitis screening in accordance with clinical guidelines – CIBINQO initiation is not recommended in patients with active hepatitis B or hepatitis C
• A complete blood count (CBC) – CIBINQO initiation is not recommended in patients with a platelet count <150,000/mm3, an absolute lymphocyte count <500/mm3, an absolute neutrophil count <1,000/mm3, or a hemoglobin value <8 g/dL
Complete any necessary immunizations, including herpes zoster vaccinations, in agreement with current immunization guidelines prior to CIBINQO initiation.
Recommended Dosage
The recommended dosage of CIBINQO is 100 mg orally once daily. If an adequate response is not achieved with CIBINQO 100 mg orally daily after 12 weeks, consider increasing dosage to 200 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 200 mg once daily. CIBINQO can be used with or without topical corticosteroids. If a dose is missed, administer the dose as soon as possible unless it is less than 12 hours before the next dose, in which case skip the missed dose. Thereafter, resume dosing at the regular scheduled time.
Recommended Dosage in Patients with Renal Impairment
Renal Impairment
CIBINQO dosage recommendation in patients with mild renal impairment (60-89 mL/minute) is 100 mg once daily. For patients with moderate renal impairment (30-59 mL/ minute), the recommended dosage is 50 mg once daily. CIBINQO is not recommended for patients with severe or End-Stage Renal Disease (ESRD). Severe renal impairment and End-Stage Renal Disease include patients on renal replacement therapy.
In subjects with mild and moderate renal impairment, if an adequate response is not achieved after 12 weeks, dose of CIBINQO can be doubled.
CIBINQO is not recommended for patients with severe renal impairment or ESRD.
Recommended Dosage in CYP2C19
Poor Metabolizers
In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Dosage Modi cations due to Strong Inhibitors
In patients taking strong inhibitors of cytochrome P450 (CYP) 2C19 reduce the dosage to 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily.
Treatment Discontinuation due to Serious Infections or Hematologic Adverse Reactions
Serious or Opportunistic Infections
If a patient develops a serious or opportunistic infection, discontinue CIBINQO and control the infection. The risks and bene ts of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO.
Hematologic Abnormalities
• Discontinue CIBINQO if platelet count <50,000/mm3 and
follow with CBC until >100,000/mm3
• Treatment should be temporarily discontinued if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value
• Treatment should be temporarily discontinued if ANC is less than 1,000 cells/mm3 and may be restarted once ANC return above this value
• Treatment should be temporarily discontinued if Hb is less than 8 g/dL and may be restarted once Hb return above this value
CBC evaluations are recommended at baseline, 4 weeks after treatment initiation and 4 weeks after dosing increase of CIBINQO. Laboratory evaluations may be extended for patients on chronic CIBINQO therapy who develop hematologic abnormalities.
DOSAGE FORMS AND STRENGTHS
• 50 mg: Pink, oval, lm-coated tablet debossed with “PFE” on one side and “ABR 50” on the other.
• 100 mg: Pink, round, lm-coated tablet debossed with “PFE” on one side and “ABR 100” on the other.
• 200 mg: Pink, oval, lm-coated tablet debossed with “PFE” on one side and “ABR 200” on the other.
CONTRAINDICATIONS
CIBINQO is contraindicated in patients taking antiplatelet therapies, except for low-dose aspirin (≤81 mg daily), during the rst 3 months of treatment.
WARNINGS AND PRECAUTIONS
Serious Infections
The most frequent serious infections reported in clinical studies with CIBINQO for atopic dermatitis were herpes simplex, herpes zoster, and pneumonia. Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving JAK inhibitors used to treat in ammatory conditions.
Avoid use of CIBINQO in patients with active, serious infection including localized infections.
Consider the risks and bene ts of treatment prior to initiating CIBINQO in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses
• with underlying conditions that may predispose them to infection
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with CIBINQO. If a patient develops a serious or opportunistic infection, discontinue CIBINQO. Initiate complete diagnostic testing and appropriate antimicrobial therapy. The risks and bene ts of treatment with CIBINQO should be carefully considered prior to reinitiating therapy with CIBINQO. Tuberculosis Evaluate and test patients for TB before starting CIBINQO therapy and consider yearly screening for patients in highly endemic areas for TB. CIBINQO is not recommended for use in patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, or for patients with a negative test for latent TB but who are at high risk for TB infection, start preventive therapy for latent TB prior to initiation of CIBINQO. Monitor patients for the development of signs and symptoms of TB, including patients who were tested negative for latent TB infection prior to initiating therapy.
Viral Reactivation Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies with CIBINQO. If a patient develops herpes zoster, consider interrupting CIBINQO until the episode resolves.
Hepatitis B virus (HBV) reactivation has been reported in patients receiving JAK inhibitors. Perform viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with CIBINQO. CIBINQO is not recommended for use in patients with active hepatitis B or hepatitis C. Monitor patients with inactive HBV for expression of HBV DNA during therapy with CIBINQO. If HBV DNA is detected during therapy with CIBINQO, consult a liver specialist.
Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients
tablets, for oral use SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION Brief Summary of full Prescribing Information; Initial Approval: January 2022
CIBINQO™ (abrocitinib)
CIBINQO™ (abrocitinib) tablets, for oral use
Mortality (continued)
treated with the JAK inhibitor compared with TNF blockers. CIBINQO is not approved for use in RA.
Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with CIBINQO.
Malignancy and Lymphoproliferative Disorders
Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical studies with CIBINQO for atopic dermatitis.
Perform periodic skin examination for patients who are at increased risk for skin cancer. Exposure to sunlight and UV light should be limited by wearing protective clothing and using broad-spectrum sunscreen.
Malignancies, including lymphomas, have occurred in patients receiving JAK inhibitors used to treat in ammatory conditions. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer (NMSC)) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Major Adverse Cardiovascular Events
Major adverse cardiovascular events were reported in clinical studies of CIBINQO for atopic dermatitis.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) de ned as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. CIBINQO is not approved for use in RA. Patients who are current or past smokers are at additional increased risk. Consider the bene ts and risks for the individual patient prior to initiating or continuing therapy with CIBINQO, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue CIBINQO in patients that have experienced a myocardial infarction or stroke.
Thrombosis
Deep venous thrombosis (DVT) and pulmonary embolism (PE) were observed in patients receiving CIBINQO in the clinical studies for atopic dermatitis.
Thrombosis, including DVT, PE, and arterial thrombosis have been reported in patients receiving JAK inhibitors used to treat in ammatory conditions. Many of these adverse reactions were serious and some resulted in death. In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers. CIBINQO is not approved for use in RA.
Avoid CIBINQO in patients that may be at increased risk of thrombosis. If symptoms of thrombosis occur, discontinue CIBINQO and evaluate and treat patients appropriately.
Laboratory Abnormalities
Hematologic Abnormalities
Treatment with CIBINQO was associated with an increased incidence of thrombocytopenia and lymphopenia. Prior to CIBINQO initiation, perform a CBC.
CBC evaluations are recommended at 4 weeks after initiation and 4 weeks after dose increase of CIBINQO. Discontinuation of CIBINQO therapy is required for certain laboratory abnormalities.
Lipid Elevations Dose-dependent increase in blood lipid parameters were reported in patients treated with CIBINQO. Lipid parameters should be assessed approximately 4 weeks following initiation of CIBINQO therapy and thereafter patients should be managed according to clinical guidelines for hyperlipidemia. The effect of these lipid parameter
elevations on cardiovascular morbidity and mortality has not been determined.
Immunizations
Prior to initiating CIBINQO, complete all age-appropriate vaccinations as recommended by current immunization guidelines including prophylactic herpes zoster vaccinations. Avoid vaccination with live vaccines immediately prior to, during, and immediately after CIBINQO therapy.
ADVERSE REACTIONS
The following clinically signi cant adverse reactions are described elsewhere in the labeling:
• Serious Infections
• Mortality
• Malignancy and Lymphoproliferative Disorders
Clinical Trials Experience
• Major Adverse Cardiovascular Events
• Thrombosis
• Laboratory Abnormalities
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not re ect the rates observed in practice.
The safety of CIBINQO was evaluated in four randomized, placebo-controlled clinical trials (2 monotherapy, 1 combination therapy with topical corticosteroid, and 1 dose-ranging) and one long-term extension trial in subjects with moderate to severe atopic dermatitis (AD). A total of 1623 subjects with moderate to severe atopic dermatitis were treated with CIBINQO in these clinical trials representing 1428 patient-years of exposure. There were 634 subjects with at least 1 year of exposure to CIBINQO. In the placebo-controlled clinical trials, a total of 1198 subjects were exposed to CIBINQO with 608 subjects receiving CIBINQO 100 mg once daily and 590 subjects receiving CIBINQO 200 mg once daily for up to 16 weeks.
The median age of subjects was 33.0 years, 124 subjects (8.1%) were 12 to less than 18 years old and 94 subjects (6.1%) were 65 years of age or older. The majority of subjects were White (68.7%) and male (53.9%).
Adverse reactions occurring at ≥1% in any of the treated groups and at a higher rate than in the placebo group are presented in the table below. A total of 61 (5.1%) subjects treated with CIBINQO were discontinued from the trials due to adverse reactions. The safety pro le of CIBINQO in the monotherapy and the combination trial(s) were similar.
Adverse Reactions from Placebo-Controlled Trials Reported in ≥1% of CIBINQO Treated Subjects with Moderate to Severe Atopic Dermatitis and at Higher Rate than Placebo for up to 16 Weeks
Weeks 0-16
Overall Infections In the placebo-controlled trials, for up to 16 weeks, overall infections were reported in 90 subjects (126.8 per 100 patient-years) treated with placebo, 211 subjects (168.8 per 100 patient-years) treated with CIBINQO 100 mg and 204 subjects (159.5 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, overall infections were reported in 427 subjects (91.8 per 100 patient-years) treated with CIBINQO 100 mg and 394 subjects (103.2 per 100 patient-years) treated with CIBINQO 200 mg.
Serious Infections In the placebo-controlled trials, for up to 16 weeks, serious infections were reported in 2 subjects (2.6 per 100 patient-years) treated with placebo, 6 subjects (3.9 per 100 patient-years) treated with CIBINQO 100 mg, and 2 subjects (1.3 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, serious infections were reported in 18 subjects (2.3 per 100 patient-years) treated with CIBINQO 100 mg and 16 subjects (2.3 per 100 patient-years) treated with CIBINQO 200 mg. The most commonly reported serious infections were herpes simplex, herpes zoster, and pneumonia.
Herpes Zoster In the placebo-controlled trials, for up to 16 weeks, opportunistic infections were generally cases of multidermatomal cutaneous herpes zoster. Herpes zoster was reported in 0 subjects treated with placebo, 3 subjects (1.9 per 100 patient-years) treated with CIBINQO 100 mg and 8 subjects (5.1 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, herpes zoster was reported in 16 subjects (2.0 per 100 patient-years) treated with CIBINQO 100 mg and 35 subjects (5.2 per 100 patient-years) treated with CIBINQO 200 mg.
Malignancy In the placebo-controlled trials, for up to 16 weeks, no malignancy was reported in subjects treated with placebo or CIBINQO 100 mg and in 1 patient (0.65 per 100 patient-years) treated with CIBINQO 200 mg. In all 5 clinical trials, including the long-term extension trial, malignancy was reported in 4 subjects (0.5 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombosis In all clinical trials, including the long-term extension trial, pulmonary embolism was reported in 3 subjects (0.4 per 100 patient-years), who were treated with CIBINQO 200 mg. Deep vein thrombosis was reported in 2 subjects (0.3 per 100 patient-years) who were treated with CIBINQO 200 mg. No thrombosis occurred in subjects treated with CIBINQO 100 mg.
Major Adverse Cardiovascular Events In the placebocontrolled trials, for up to 16 weeks, major adverse cardiovascular event (MACE) was reported in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, MACE was reported in 1 patient (0.1 per 100 patient-years) treated with CIBINQO 100 mg and 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 200 mg.
Thrombocytopenia In the placebo-controlled trials, for up to 16 weeks, treatment with CIBINQO was associated with a dose-related decrease in platelet count. Maximum effects on platelets were observed within 4 weeks, after which the platelet count returned towards baseline despite continued therapy. In all 5 clinical trials, including the long-term extension trial 6 subjects (0.9 per 100 patient-years) treated with CIBINQO 200 mg had adverse reactions of thrombocytopenia, no subjects treated with CIBINQO 100 mg had an adverse reaction of thrombocytopenia.
Lymphopenia In the placebo-controlled trials, for up to 16 weeks, con rmed ALC <500/mm3 occurred in 2 subjects (1.2 per 100 patient-years) treated with CIBINQO 200 mg and 0 subjects treated with CIBINQO 100 mg or placebo. Both cases occurred in the rst 4 weeks of exposure.
Lipid Elevations In the placebo-controlled trials, for up to 16 weeks, there was a dose-related percent increase in low-density lipoprotein cholesterol (LDL-c), total cholesterol, and high-density lipoprotein cholesterol (HDL-c) relative to placebo at Week 4 which remained elevated through the nal visit in the treatment period. Adverse reactions related to hyperlipidemia occurred in 1 subject (0.6 per 100 patient-years) exposed to CIBINQO 100 mg, 3 subjects (2.0 per 100 patient-years) exposed to CIBINQO 200 mg.
Speci c Adverse Reactions
Exposure adjusted incidence rates were adjusted by trial size for all the adverse reactions reported in this section.
Retinal Detachment In the placebo-controlled trials, for up to 16 weeks, retinal detachment occurred in 1 subject (0.6 per 100 patient-years) treated with CIBINQO 100 mg. In all 5 clinical trials, including the long-term extension trial, retinal detachment occurred in 2 subjects (0.3 per 100 patient-years) treated with CIBINQO 100 mg.
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
CIBINQO 200 mg N=590 n (%a) CIBINQO 100 mg N=608 n (%a) PLACEBO N=342 n (%a)
51 (8.7)75 (12.4)27 (7.9) Nausea 86 (14.5)37 (6.0)7 (2.1) Headache 46 (7.8)36 (6.0)12 (3.5) Herpes simplexb 25 (4.2)20 (3.3)6 (1.8) Increased blood creatine phosphokinase 17 (2.9)14 (2.3)5 (1.5) Dizziness 17 (2.9)11 (1.8)3 (0.9) Urinary tract infection 13 (2.2)10 (1.7)4 (1.2) Fatigue 8 (1.3)10 (1.6)2 (0.5) Acne 28 (4.7)10 (1.6)0 (0.0) Vomiting 19 (3.2)9 (1.5)3 (0.9) Impetigo 3 (0.5)9 (1.5)1 (0.3) Oropharyngeal pain 6 (1.0)8 (1.4)2 (0.6) Hypertension 5 (0.8)7 (1.2)2 (0.7) In uenza 6 (1.1)7 (1.2)0 (0.0) Gastroenteritis 8 (1.3)7 (1.1)2 (0.6) Dermatitis contact 3 (0.5)6 (1.1)1 (0.3) Abdominal pain upper 11 (1.9)4 (0.6)0 (0.0) Abdominal discomfort 7 (1.2)3 (0.5)1 (0.3) Herpes zoster 7 (1.2)2 (0.3)0 (0.0) Thrombocytopenia 9 (1.5)0 (0.0)0 (0.0) a Study size adjusted percentages b Herpes simplex also includes oral herpes, ophthalmic herpes, herpes dermatitis, genital herpes.
Nasopharyngitis
CIBINQO™ (abrocitinib) tablets, for oral use
Speci c Adverse Reactions (continued)
Creatine Phosphokinase Elevations (CPK) In the placebocontrolled trials, for up to 16 weeks, events of blood CPK increased were reported in 6 subjects (7.5 per 100 patient-years) treated with placebo, 11 subjects (6.9 per 100 patient-years) treated with 100 mg of CIBINQO and 19 subjects (12.3 per 100 patient-years) treated with 200 mg of CIBINQO. Most elevations were transient, there were no reported adverse reactions of rhabdomyolysis.
Adolescent Subjects (12 to less than 18 years of age) The safety of CIBINQO was assessed in a trial of 284 subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis (Trial-AD-4). The safety pro le of CIBINQO in these subjects, assessed through the initial treatment period of 12 weeks and the long-term period (213 with at least 52 weeks of abrocitinib exposure), was similar to the safety pro le from trials in adults with atopic dermatitis.
DRUG INTERACTIONS
Effects of Other Drugs on CIBINQO
The table below includes drugs with clinically signi cant drug interactions affecting CIBINQO.
Clinically Signi cant Drug Interactions Affecting CIBINQO
Strong CYP2C19 Inhibitors
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 inhibitors increases the combined exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Dosage reduction of CIBINQO is recommended when coadministered with strong CYP2C19 inhibitors.
Moderate to Strong Inhibitors of both CYP2C19 and CYP2C9
Clinical Impact Coadministration of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9 increases the exposure of abrocitinib and its two active metabolites, M1 and M2 which may increase the adverse reactions of CIBINQO.
Intervention Avoid concomitant use of CIBINQO with drugs that are moderate to strong inhibitors of both CYP2C19 and CYP2C9.
Strong CYP2C19 or CYP2C9 Inducers
Clinical Impact Coadministration of CIBINQO with strong CYP2C19 or CYP2C9 inducers decreases the combined exposure of abrocitinib and its two active metabolites, M1 and M2, which may result in loss of or reduced clinical response.
Intervention Avoid concomitant use of CIBINQO with strong CYP2C19 or CYP2C9 inducers.
Effects of CIBINQO on Other Drugs
The table below includes clinically signi cant drug interactions affecting other drugs.
Clinically Signi cant Interactions Affecting Other Drugs
P-gp Substrate Where Small Concentration Changes May Lead to Serious or Life-threatening Toxicities
Clinical Impact Coadministration of CIBINQO with P-gp substrate increases plasma concentrations of P-gp substrates and may result in potential adverse reactions of the P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities (e.g., digoxin).
Pregnancy
Pregnancy Exposure Registry There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to CIBINQO during pregnancy. Pregnant women exposed to CIBINQO and health care providers are encouraged to call 1-877-311-3770 or visit CIBINQOPregnancyRegistry.com.
Risk Summary Available data from pregnancies reported in clinical trials with CIBINQO are not suf cient to establish a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of abrocitinib to pregnant rats and rabbits during organogenesis at exposure 11 or 4 times the maximum recommended human dose (MRHD) based on AUC comparison, respectively, resulted in maternal dystocia and skeletal variations in rats and no adverse effects in rabbits (see Animal Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies carry some risk of birth defects, loss, or other adverse outcomes. The background risks in the U.S. general population of major birth defects and miscarriages are 2-4% and 15-20% of clinically recognized pregnancies, respectively. Animal Data In an embryofetal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, or 60 mg/kg/day during the period of organogenesis. No fetal malformations were observed. Abrocitinib increased the incidence of skeletal variations of short 13th ribs at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Increased embryofetal lethality and additional skeletal variations (cervical arches with reduced ventral processes, thickened ribs, and unossi ed metatarsals) were noted at 60 mg/kg/day (17 times the MRHD based on AUC comparison). In an embryofetal development study, abrocitinib was administered orally to pregnant rabbits at doses of 10, 30, or 75 mg/kg/day during the period of organogenesis. No abrocitinib-related maternal or developmental toxicity was noted at doses up to 75 mg/kg/day (4 times the MRHD based on AUC comparison).
In a prenatal and postnatal development study, abrocitinib was administered orally to pregnant rats at doses of 10, 30, and 60 mg/kg/day beginning on gestation day 6 and continuing through lactation day 20. Dystocia with prolonged parturition and reduced offspring body weights were noted at 30 mg/kg/ day (11 times the MRHD based on AUC comparison). Postnatal survival was markedly decreased at 60 mg/kg/day (17 times the MRHD based on AUC comparison). No maternal toxicity was observed at 10 mg/kg/day (2.4 times the MRHD based on AUC comparison). No abrocitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring was noted at doses up to 30 mg/kg/ day (11 times the MRHD based on AUC comparison).
Lactation
Risk Summary There are no data on the presence of abrocitinib in human milk, the effects on the breast-fed infant, or the effects on milk production. Abrocitinib was secreted in milk of lactating rats (see Animal Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the serious adverse ndings in adults, including risks of serious infections, malignancy, and thrombosis, advise women not to breastfeed during treatment with CIBINQO and for one day after the last dose (approximately 5-6 elimination half-lives).
Animal Data Lactating female rats were orally administered a single dose of 10 mg/kg abrocitinib on lactation day 12. Abrocitinib AUC was approximately 5 times greater in milk than in plasma.
Females and Males of Reproductive Potential
Infertility Females Based on the ndings in rats, oral administration of CIBINQO may impair female fertility. Impaired fertility in female rats was reversible 1 month after cessation of abrocitinib oral administration.
Pediatric Use
Intervention Monitor appropriately or dose titrate P-gp substrate where small concentration changes may lead to serious or life-threatening toxicities when coadministered with CIBINQO.
Antiplatelet Therapy Drugs
Clinical Impact
Coadministration of CIBINQO with antiplatelet therapy drugs may increase the risk of bleeding with thrombocytopenia.
Intervention Antiplatelet drugs, except for low-dose aspirin (≤81 mg daily), during the rst 3 months of treatment are contraindicated with CIBINQO.
The safety and effectiveness of CIBINQO in pediatric patients 12 years of age and older weighing 25 kg or more with atopic dermatitis has been established. In trials Trial-AD-1 and Trial-AD-2, 124 adolescent subjects 12 to less than 18 years old with moderate-to-severe atopic dermatitis were enrolled and randomized to receive either CIBINQO 100 mg (N=51), 200 mg (N=48), or matching placebo (N=25) in monotherapy. Additional 284 adolescent subjects 12 to less than 18 years of age with moderate-to-severe atopic dermatitis, were enrolled and randomized to receive either CIBINQO 100 mg (N=95) or 200 mg (N=94) or matching placebo (N=95) in combination with topical corticosteroids in Trial-AD-4. Ef cacy and adverse reaction pro le were consistent between the pediatric patients and adults.
The safety and effectiveness of CIBINQO have not been established in pediatric patients below 12 years of age.
Juvenile Animal Toxicity Data In a juvenile animal toxicity study, abrocitinib was administered orally to juvenile rats at doses of 5, 25, and 75 mg/kg/day beginning on postnatal day 10 (approximately equivalent to a human infant) and continuing through postnatal day 63 (approximately equivalent to an adolescent). Abrocitinib caused a reversible, dose-related decrease in the primary spongiosa in the metaphysis of the proximal tibia and distal femur. Abrocitinib produced adverse effects on bone development at all dose levels. Abrocitinib caused irreversible dose-related small or misshapen femoral heads at doses ≥5 mg/kg/day (0.8 times the MRHD based on AUC comparison).
Abrocitinib also irreversibly decreased femur size and caused paw malrotation and limb impairment at doses ≥25 mg/kg/day (7.2 times the MRHD based on AUC comparison). At 75 mg/kg/ day (27 times the MRHD based on AUC comparison), paw fractures generally corresponded to limb impairment, a fractured tibia was noted in a single female. Irreversible bone ndings have not been observed in older animals.
Geriatric Use
A total of 145 (4.6%) patients 65 years of age and older, while 25 (0.8%) were 75 years of age and older, were enrolled in CIBINQO clinical trials. Clinical trials of CIBINQO did not include suf cient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
A higher proportion of patients 65 years of age and older discontinued from clinical trials compared to younger patients. Among all patients exposed to CIBINQO, including the long-term extension trial, con rmed ALC <500/mm3 occurred only in patients 65 years of age and older. A higher proportion of patients 65 years of age and older had platelet counts <75,000/ mm3. The incidence rate of herpes zoster in patients 65 years of age and older treated with CIBINQO (7.40 per 100 patient-years) was higher than that of patients 18 to less than 65 years of age (3.44 per 100 patient-years).
Renal Impairment
In patients with severe (eGFR <30 mL/min) and moderate (eGFR 30-59 mL/min) renal impairment, the combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2, is increased compared to patients with normal renal function (eGFR ≥90 mL/min). This may increase the risk of adverse reactions such as infections.
CIBINQO is not recommended for use in patients with severe renal impairment and ESRD including those on renal replacement therapy. A dosage reduction in patient with moderate renal impairment is recommended. No dosage adjustment is required in patients with mild renal impairment (eGFR 60-89 mL/min).
CIBINQO has not been studied in patients on renal replacement therapy. In Phase 3 clinical trials, CIBINQO was not evaluated in patients with atopic dermatitis with baseline creatinine clearance values less than 40 mL/min.
Hepatic Impairment
Avoid use of CIBINQO in patients with severe (Child Pugh C) hepatic impairment.
Dosage adjustment is not required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment based on similar combined exposure (AUCinf,u) of abrocitinib and its two active metabolites, M1 and M2 compared to patients with normal hepatic function. In clinical trials, CIBINQO was not evaluated in patients with severe (Child Pugh C) hepatic impairment.
CYP2C19 Poor Metabolizers
In patients who are CYP2C19 poor metabolizers, the AUC of abrocitinib is increased compared to CYP2C19 normal metabolizers due to reduced metabolic clearance. Dosage reduction of CIBINQO is recommended in patients who are known or suspected to be CYP2C19 poor metabolizers based on genotype or previous history/experience with other CYP2C19 substrates.
OVERDOSAGE
There is no experience regarding human overdosage with CIBINQO. There is no speci c antidote for overdose with CIBINQO. In case of an overdose, call Poison Control Center at 1-800-222-1222 for latest recommendations.
Rx only
This brief summary is based on CIBINQO™ (abrocitinib) Prescribing Information LAB-1424-2.0.
Issued: February 2023.
The product’s label may have been updated. For full Prescribing Information, visit CIBINQOPI.com.
See CIBINQO full Prescribing Information at CIBINQOPI.com.
© 2023 P zer Inc. All rights reserved. February 2023. PP-CIB-USA-0485
USE IN SPECIFIC POPULATIONS
SEE PACKAGE INSERT FOR FULL PRESCRIBING INFORMATION
© 2023 Pfizer Inc. All rights reserved. May 2023. PP-CIB-USA-0485






BCoD JOB

BCoD understands the critical role of biologic coordinators and offce staff in advocating for patient access and therapeutic coordination. embers of BCoD can now view ob listings for open positions across the nited tates.
This service is free for dermatologists.





JOB BOARD


If you are a dermatologist or offce seeking staff to manage patient access, BCoD will post your open position at no cost. e’ll connect you with our engaged and experienced community of knowledgeable BCs.


o submit a ob, reach out to contact bcofdermatology.com.

bcofdermatology.com

 By Sarah Pinkowski Jansen MARKETING & COMMUNICATIONS CONSULTANT
By Sarah Pinkowski Jansen MARKETING & COMMUNICATIONS CONSULTANT
ROOMS WITH A VIEW
DISTINCTIVE SUMMER GETAWAYS WITH A TWIST
An anticipated 85% of Americans plan to enjoy a summer getaway, according to a 2023 trend report from The Vacationer. This summer's travel surge shows no signs of slowing down anytime soon. So, in the spirit of summertime pleasure, we've listed some of the best destinations that don't require a passport, deliver awe-inspiring views, and leave you feeling like you're staying at a retreat that's anything but ordinary.
ACCESS DERMATOLOGY JULY/AUGUST 2023 50





THE GREEN O
THE LAKE HOUSE
PRIMLAND MOUNTAIN RESORT



Twin Farms
BLACKBERRY MOUNTAIN THE OASIS AT DEATH VALLEY POST RANCH INN
TWIN FARMS TROUTBECK
Blackberry Farm Troutbeck
The Lake House on Canandaigua
Post Ranch Inn Primland Mountain Resort Death Valley National
DEATH VALLEY NATIONAL PARK BY BILL PERRYSTOCK.ADOBE.COM
the green o
Park
WALLAND, TENNESSEE
Blackberry Mountain
Ditch the hustle and bustle of everyday life and connect with nature at Blackberry Mountain. Wrapped in Tennessee's natural beauty, this retreat offers adventure, wellness and sophistication. From rock climbing and mountain biking to mountaintop yoga sessions and a world-class spa, there's something for everyone. Learn how to forage or participate in hands-on workshops with expert artisans to tap into your creativity. And with farm-totable cuisine showcasing local flavors, your taste buds will surely be delighted. Experience the perfect getaway at Blackberry Mountain.

Set on 5,200 acres, Blackberry Mountain offers “one mountain and many paths” for wellness, dining and adventure.

GREENOUGH, MONTANA
the
green o
Step under the sprawl of towering pines in western Montana at the green o, a hidden gem that beckons to adventurous souls in pursuit of an unrivaled blend of excitement and tranquility. Here, off the beaten path, adult guests breathe in the freshly scented pine air while partaking in unforgettable experiences like expert-led fly fishing, exhilarating mountain bike riding, archery and shooting sports, and horseback riding. Whether you seek peace and serenity, a lulled sleep under the stars, or a high-stepping adventure with the resort's unique equestrian program, the green o delivers an abundance of attractions and activities.
ACCESS DERMATOLOGY JULY/AUGUST 2023 52
Billed as a “sophisticated woodland hideaway,” the green o serves award-winning cuisine at Social Haus.


DEATH VALLEY, CALIFORNIA
The Oasis at Death Valley
Discover the Oasis at Death Valley, a luxurious and peaceful haven nestled within the magnificent landscapes of California's Death Valley. Enjoy warm and hospitable service while immersing yourself in the unique wonders of this breathtaking desert. Embark on Jeep excursions through rugged canyons, invigorating hikes, or tee off at the world's lowest-elevation golf course— all while taking in the majestic surroundings. Enjoy pampering spa treatments or take a refreshing dip in natural spring-fed pools. Savor exceptional cuisine while gazing out at towering mountains. The Oasis at Death Valley is the ultimate destination for a rejuvenating desert retreat that will invigorate your mind and body.

Post Ranch
Inn
BIG SUR, CALIFORNIA

Escape the ordinary and enjoy pure relaxation at the Post Ranch Inn in Big Sur, California. Nestled on majestic cliffs, this luxurious sanctuary offers a harmonious blend of natural beauty and eco-friendly architecture. Immerse yourself in breathtaking coastal vistas, rejuvenating spa treatments among the redwood trees, stargazing from infinity-edge pools, exquisite dining with Pacific Ocean views, and guided nature walks through enchanting landscapes. Experience unparalleled service and indulgence at the renowned Post Ranch Inn.
Channel Old Hollywood at this desert getaway, which once played host to stars like Marlon Brando and Clark Gable.
DEATH VALLEY NATIONAL PARK BY GARYTOGSTOCK.ADOBE.COM
This romantic cliffside inn sits 1,200 feet above the Pacific Ocean and is known for breathtaking views.

BARNARD, VERMONT
Twin Farms
This renowned retreat offers a personalized experience tailored to guests' every whim. Immerse yourself in outdoor adventures, from exhilarating hikes to thrilling skiing. Unwind with revitalizing spa treatments amidst tranquil surroundings. From the enchantment of individually designed accommodations, to the exquisite farm-to-table creations crafted from the finest local ingredients and complemented by an outstanding selection of wines, you won't forget Twin Farms. This retreat beckons you to embrace an active escape where relaxation, luxury and heartfelt hospitality converge.

AMENIA, NEW YORK
Troutbeck
Discover the enchanting Troutbeck, where history, natural beauty and contemporary comfort converge. Embracing its storied past, Troutbeck embodies the spirit of a summerhouse haven on the eastern edge of the Hudson Valley. Here, guests blaze new trails hiking and biking through picturesque landscapes, unleash creativity via immersive classes and workshops, and find solace alongside a mesmerizing trout-filled stream. Troutbeck offers a memorable and timeless escape with activities steeped in relaxation and inspiration.

ACCESS DERMATOLOGY JULY/AUGUST 2023 54
Guests of this estate hotel can be described as “locavores, creative, curious and adventurous.”
At this four-seasons haven, guests ages 14 and up are welcome to experience a stay here.


The Lake House
CANANDAIGUA, NEW YORK
Enjoy lakefront activities, relax at the spa or tour area wineries at this elegant Finger Lakes destination.
Uncover enchantment at the Lake House on Canandaigua, where a casual yet upscale New England summer atmosphere welcomes you to this beautiful resort in Upstate New York's Finger Lakes region. Spend your days swimming or boating in the serene lake waters, indulge in the local wineries' flavors, or relax at the Willowbrook Spa. With several atmospheric dining options against the pristine waterfront, this sustainable luxury retreat offers something for everyone and will captivate you with its natural beauty.
Primland Mountain Resort
MEADOWS OF DAN, VIRGINIA

Located in the magnificent Blue Ridge Mountains, Primland Resort merges luxury with the natural beauty of Virginia. For the adventurer, many thrilling activities await, including a championship mountaintop golf course, exhilarating off-road escapades, and hunting and fishing expeditions. Guests can gaze at the observatory's starry spectacle, where the vastness of space reveals its celestial wonders. Then, indulge in revitalizing spa treatments inspired by indigenous traditions or relax in the peaceful atmosphere of an idyllic sanctuary.

55
bcofdermatology.com
This Auberge resort offers mountain views, whether you stay in the lodge, cottages or treehouses.
A first-in-class,* selective TYK2 inhibitor for moderate-to-severe plaque psoriasis1,2


INDICATION

Clearer skin



SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Please see additional Important Safety Information and Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
*TYK2 is a member of the Janus kinase (JAK) family.

STUDY DESIGNS




Clearer choice
AT LAST...SOTYKTU
Superior skin clearance vs Otezla® (apremilast)†‡ in a once-daily, oral pill1
†POETYK PSO-1 (N=664) and POETYK PSO-2 (N=1020) were two, 52-week, multicenter, randomized, double-blind, placebo- and active (apremilast 30 mg twice daily)-controlled, Phase 3 studies to evaluate the safety and efficacy of SOTYKTU (6 mg once daily) in adult patients with moderate-to-severe plaque psoriasis who were eligible for systemic therapy or phototherapy. Patients had a body surface area (BSA) involvement of ≥10%, a Psoriasis Area and Severity Index (PASI) score ≥12, and a static Physician’s Global Assessment (sPGA) ≥3 (moderate or severe).
Both studies assessed the responses at Week 16 compared with placebo for the two co-primary endpoints:
• The proportion of patients who achieved at least a 75% improvement in PASI scores from baseline (PASI 75)
• The proportion of patients who achieved an sPGA score of 0 (clear) or 1 (almost clear)
There were multiple ranked secondary endpoints, including:
• The proportion of patients who achieved PASI 75 at Week 16 vs apremilast
STUDY RESULTS 1,3,4
‡Comparison between SOTYKTU and apremilast was a secondary endpoint.
Co-primary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs placebo: PSO-1: 58% (193/330) vs 13% (21/166), P<0.0001; PSO-2: 53% (271/511) vs 9% (24/255), P<0.0001
• sPGA 0/1 at Week 16 for SOTYKTU vs placebo: PSO-1: 54% (178/330) vs 7% (12/166), P<0.0001; PSO-2: 50% (253/511) vs 9% (22/255), P<0.0001
Select secondary endpoints:
• PASI 75 at Week 16 for SOTYKTU vs apremilast: PSO-1: 58% (193/330) vs 35% (59/168); P<0.0001; PSO-2: 53% (271/511) vs 40% (101/254); P=0.0004.
SELECT IMPORTANT SAFETY INFORMATION
In the PSO-1 and PSO-2 trials, through Week 16, the most common adverse reactions (≥1% and higher than placebo) in patients taking SOTYKTU (n=840) were upper respiratory infections (19.2%), blood creatine phosphokinase increase (2.7%), herpes simplex (2.0%), mouth ulcers (1.9%), folliculitis (1.7%), and acne (1.4%).
BSA=body surface area; PASI=psoriasis area and severity index; PASI 75=75% reduction from baseline in PASI; sPGA=static Physician’s Global Assessment; TYK2=tyrosine kinase 2.
Otezla® is a registered trademark of Amgen, Inc.
SOTYKTU tablet is not actual size.
Shown for illustrative purposes only.
Scan to learn more or visit SOTYKTUHCP.com
(deucravacitinib) INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use: SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions such as angioedema have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU.
Infections: SOTYKTU may increase the risk of infections. Serious infections have been reported in patients with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19. Avoid use of SOTYKTU in patients with an active or serious infection. Consider the risks and benefits of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing, have appropriate antimicrobial therapy initiated and be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex) was reported in clinical trials with SOTYKTU. Through Week 16, herpes simplex infections were reported in 17 patients (6.8 per 100 patient-years) treated with SOTYKTU, and 1 patient (0.8 per 100 patientyears) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent patient. During PSO-1, PSO-2, and the open-label extension trial, the majority of patients who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age. The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis (TB): In clinical trials, of 4 patients with latent TB who were treated with SOTYKTU and received appropriate TB prophylaxis, no patients developed active TB (during the mean follow-up of 34 weeks). One patient, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU. Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas: Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.
SOTYKTU
Rhabdomyolysis and Elevated CPK: Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
Laboratory Abnormalities: Treatment with SOTYKTU was associated with increases in triglyceride levels. Periodically evaluate serum triglycerides according to clinical guidelines during treatment. SOTYKTU treatment was associated with an increase in the incidence of liver enzyme elevation compared to placebo. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded.
Immunizations: Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition: It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
Most common adverse reactions (≥1% of patients on SOTYKTU and more frequently than with placebo) include upper respiratory infections, blood creatine phosphokinase increased, herpes simplex, mouth ulcers, folliculitis and acne.
SPECIFIC POPULATIONS
Pregnancy: Available data from case reports on SOTYKTU use during pregnancy are insu icient to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Report pregnancies to the BristolMyers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Lactation: There are no data on the presence of SOTYKTU in human milk, the e ects on the breastfed infant, or the e ects on milk production. SOTYKTU is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU and any potential adverse e ects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Hepatic Impairment: SOTYKTU is not recommended for use in patients with severe hepatic impairment.
SOTYKTU is available in 6 mg tablets.
Please see Brief Summary of U.S. Full Prescribing Information for SOTYKTU on the following pages.
References: 1. SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 2. Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11(5):1763-1776.
3. Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022. 4. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: e icacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51.
SOTYKTU and the related logo are trademarks of Bristol-Myers Squibb Company.
© 2023 Bristol-Myers Squibb Company. 1787-US-2300018 02/23
tablets, for oral use
Brief Summary of Prescribing Information. For complete prescribing information consult of cial package insert.
INDICATIONS AND USAGE
SOTYKTU™ (deucravacitinib) is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use:
SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
DOSAGE AND ADMINISTRATION
Recommended Evaluations and Immunizations Prior to Treatment Initiation
Evaluate patients for active and latent tuberculosis (TB) infection prior to initiating treatment with SOTYKTU. If positive, start treatment for TB prior to SOTYKTU use [see Warnings and Precautions]
Update immunizations according to current immunization guidelines [see Warnings and Precautions]
Recommended Dosage
The recommended dosage of SOTYKTU is 6 mg taken orally once daily, with or without food. Do not crush, cut, or chew the tablets.
Recommended Dosage in Patients with Hepatic Impairment
SOTYKTU is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Use in Speci c Populations and Clinical Pharmacology (12.3) in full Prescribing Information] No dosage adjustment is needed for patients with mild to moderate hepatic impairment.
CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU [see Warnings and Precautions]
WARNINGS AND PRECAUTIONS
Hypersensitivity
Hypersensitivity reactions such as angioedema have been reported in subjects receiving SOTYKTU. If a clinically signi cant hypersensitivity reaction occurs, institute appropriate therapy and discontinue SOTYKTU [see Adverse Reactions].
Infections
SOTYKTU may increase the risk of infections.
Serious infections have been reported in subjects with psoriasis who received SOTYKTU. The most common serious infections reported with SOTYKTU included pneumonia and COVID-19 [see Adverse Reactions]
Avoid use of SOTYKTU in patients with an active or serious infection.
Consider the risks and bene ts of treatment prior to initiating SOTYKTU in patients:
• with chronic or recurrent infection
• who have been exposed to tuberculosis
• with a history of a serious or an opportunistic infection
• with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with SOTYKTU. A patient who develops a new infection during treatment with SOTYKTU should undergo prompt and complete diagnostic testing; appropriate antimicrobial therapy should be initiated; and the patient should be closely monitored. Interrupt SOTYKTU if a patient develops a serious infection. Do not resume SOTYKTU until the infection resolves or is adequately treated.
Viral Reactivation
Herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical trials with SOTYKTU. In the 16-week placebo-controlled period, herpes simplex infections were reported in 17 subjects (6.8 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Multidermatomal herpes zoster was reported in an immunocompetent subject who received SOTYKTU. During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, the majority of subjects who reported events of herpes zoster while receiving SOTYKTU were under 50 years of age.
The impact of SOTYKTU on chronic viral hepatitis reactivation is unknown. Subjects with positive screening tests for hepatitis B or C, or chronic hepatitis B, or untreated hepatitis C were excluded from clinical trials. Consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with SOTYKTU. If signs of reactivation occur, consult a hepatitis specialist. SOTYKTU is not recommended for use in patients with active hepatitis B or hepatitis C.
Tuberculosis
In clinical trials, of 4 subjects with latent tuberculosis (TB) who were treated with SOTYKTU and received appropriate TB prophylaxis, no subjects developed active TB (during the mean follow-up of 34 weeks). One subject, who did not have latent TB, developed active TB after receiving 54 weeks of SOTYKTU. Evaluate patients for latent and active TB infection prior to initiating treatment with SOTYKTU. Do not administer SOTYKTU to patients with active TB. Initiate treatment of latent TB prior to administering SOTYKTU.
Consider anti-TB therapy prior to initiation of SOTYKTU in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be con rmed. Monitor patients receiving SOTYKTU for signs and symptoms of active TB during treatment.
Malignancy including Lymphomas
Malignancies, including lymphomas, were observed in clinical trials with SOTYKTU (deucravacitinib) [see Adverse Reactions]
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with SOTYKTU, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer) and patients who develop a malignancy when on treatment with SOTYKTU.
Rhabdomyolysis and Elevated CPK
Cases of rhabdomyolysis were reported in subjects treated with SOTYKTU resulting in interruption or discontinuation of SOTYKTU dosing.
Treatment with SOTYKTU was associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis compared to treatment with placebo. Discontinue SOTYKTU if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Adverse Reactions]
Laboratory Abnormalities
Triglyceride Elevations - Treatment with SOTYKTU was associated with increases in triglyceride levels. The effect of this elevated parameter on cardiovascular morbidity and mortality has not been determined. Periodically evaluate serum triglycerides according to the clinical guidelines for hyperlipidemia while patients are receiving treatment with SOTYKTU. Manage patients according to clinical guidelines for the management of hyperlipidemia [see Adverse Reactions]
Liver Enzyme Elevations - Treatment with SOTYKTU was associated with an increase in the incidence of liver enzyme elevation compared to treatment with placebo. Liver serum transaminase elevations ≥3 times the ULN were reported in subjects treated with SOTYKTU. Evaluate liver enzymes at baseline and thereafter in patients with known or suspected liver disease according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt SOTYKTU until a diagnosis of liver injury is excluded [see Adverse Reactions]
Immunizations
Prior to initiating therapy with SOTYKTU, consider completion of all age-appropriate immunizations according to current immunization guidelines including prophylactic herpes zoster vaccination. Avoid use of live vaccines in patients treated with SOTYKTU. The response to live or non-live vaccines has not been evaluated.
Potential Risks Related to JAK Inhibition
It is not known whether TYK2 inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
• Infections [see Warnings and Precautions]
• Malignancy including lymphomas [see Warnings and Precautions]
• Laboratory Abnormalities [see Warnings and Precautions]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not re ect the rates observed in practice.
The safety of SOTYKTU was evaluated in two placebo- and active-controlled trials (PSO-1 and PSO-2) and an open-label extension trial in which subjects who completed PSO-1 or PSO-2 could enroll [see Clinical Studies (14) in full Prescribing Information]. In these clinical trials, a total of 1,519 subjects with moderate-to-severe plaque psoriasis who were candidates for systemic therapy or phototherapy received SOTYKTU 6 mg orally once daily. Of these, 1,141 subjects were exposed to SOTYKTU for at least one year.
In trials PSO-1 and PSO-2, 1,681 subjects were randomized to receive SOTYKTU 6 mg (840 subjects), placebo (419 subjects), or apremilast 30 mg twice daily (422 subjects). All subjects randomized to placebo switched to SOTYKTU at Week 16. All other subjects remained in their original treatment group until Week 24, at which point subjects could have continued on the same treatment or be switched to SOTYKTU or placebo. The mean age of subjects was 47 years. The majority of subjects were White (87%) and male (67%).
In the 16-week placebo-controlled period of the pooled clinical trials (PSO-1 and PSO-2), discontinuation of therapy due to adverse reactions in subjects who received SOTYKTU was 2.4%, compared to 3.8% for placebo.
Table 1 summarizes the adverse reactions that occurred in at least 1% of subjects in the SOTYKTU group and at a higher rate than the placebo group during the 16-week controlled period.
SOTYKTU™ (deucravacitinib)
Table 1: Adverse Reactions that Occurred in ≥1% of Subjects with Plaque Psoriasis in the SOTYKTU (deucravacitinib) Group and More Frequently than in the Placebo Group in Trials PSO-1 and PSO-2 through Week 16
Adverse Reaction
a Includes upper respiratory tract infection (viral, bacterial, and unspecified), nasopharyngitis, pharyngitis (including viral, streptococcal, and unspecified), sinusitis (includes acute, viral, bacterial), rhinitis, rhinotracheitis, tracheitis, laryngitis, and tonsillitis (including bacterial, streptococcal)
b Includes oral herpes, genital herpes, herpes simplex, and herpes virus infection
c Includes mouth ulceration, aphthous ulcer, tongue ulceration, and stomatitis
d Includes acne, acne cystic, and dermatitis acneiform
Adverse reactions that occurred in <1% of subjects in the SOTYKTU group were herpes zoster.
Speci c Adverse Reactions
Exposure adjusted incidence rates are reported for all the adverse reactions presented below.
Infections
In the 16-week placebo-controlled period, infections occurred in 29% of the SOTYKTU group (116 events per 100 person-years) compared to 22% of the placebo group (83.7 events per 100 person-years). The majority of infections were non-serious and mild to moderate in severity and did not lead to discontinuation of SOTYKTU.
In the 16-week placebo-controlled period, serious infections were reported in 5 subjects (2.0 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo. The most common serious infections reported during the 52-week treatment period were pneumonia and COVID-19.
Malignancies
During the 0-to-52-week treatment period of the two clinical trials, PSO-1 and PSO-2 (total exposure of 986 patient-years with SOTYKTU), malignancies (excluding non-melanoma skin cancer) were reported in 3 subjects treated with SOTYKTU (0.3 per 100 patient-years), including single cases each of breast cancer, hepatocellular carcinoma, and lymphoma after 24, 32, and 25 weeks of treatment, respectively.
During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, a total of 3 subjects (0.1 per 100 patient-years), developed lymphoma while receiving SOTYKTU after 25, 77, and 98 weeks of treatment.
Laboratory Abnormalities
Creatine Phosphokinase (CPK)
In the 16-week placebo-controlled period, increased CPK (including Grade 4) was reported in 23 subjects (9.3 per 100 patient-years) treated with SOTYKTU, and 5 subjects (4.1 per 100 patient-years) treated with placebo.
Liver Enzyme Elevations
Events of increases in liver enzymes ≥3 times the ULN were observed in subjects treated with SOTYKTU [see Warnings and Precautions]. In the 16-week placebo-controlled period:
• ALT elevations ≥3 times the ULN was reported in 9 subjects (3.6 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
• AST elevations ≥3 times the ULN was reported in 13 subjects (5.2 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
Decreased Glomerular Filtration Rate (GFR)
In the 16-week placebo-controlled period in subjects who had moderate renal impairment (eGFR 30-59 mL/min) at baseline, decreased GFR was reported in 4 subjects (1.6 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Two of the deucravacitinib-treated subjects had worsening of baseline proteinuria.
Lipid Elevations
Mean triglycerides increased by 10.3 mg/dL during the 16-week treatment period in subjects treated with SOTYKTU and by 9.1 mg/dL during the 52-week treatment period.
Safety Through Week 52
In PSO-1 and PSO-2, the exposure adjusted incidence rate of adverse reactions in subjects treated with SOTYKTU from Week 0 through Week 52 without switching treatment did not increase compared to the rate observed during the rst 16 weeks of treatment.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data from case reports on SOTYKTU use during pregnancy are insuf cient to evaluate a drugassociated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal reproduction studies, no effects on embryo-fetal development were observed with oral administration of deucravacitinib to rats and rabbits during organogenesis at doses that were at least 91 times the maximum recommended human dose (MRHD) of 6 mg once daily (see Data)
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Report pregnancies to the Bristol-Myers Squibb Company’s Adverse Event reporting line at 1-800-721-5072.
Data
Animal data
Deucravacitinib was administered orally during the period of organogenesis at doses of 5, 15, or 75 mg/kg/day in rats and 1, 3, or 10 mg/kg/day in rabbits. Deucravacitinib was not associated with embryo-fetal lethality or fetal malformations in either species. These doses resulted in maternal exposures (AUC) that were 266 times (rat) or 91 times (rabbit) the exposure at the MRHD.
In a pre- and post-natal development study in rats, deucravacitinib was administered orally from gestation day 6 through lactation day 20, at doses of 5, 15, or 50 mg/kg/day. At 50 mg/kg/day, F1 offspring had reduced body weight gains during the pre-weaning period. After weaning, body weights of affected F1 offspring gradually normalized to control levels. No maternal effects were observed at 50 mg/kg/day (110 times the MRHD based on AUC comparison). No deucravacitinib-related effects on postnatal developmental, neurobehavioral, or reproductive performance of offspring were noted at doses up to 15 mg/kg/day (19 times the MRHD based on AUC comparison).
Lactation
Risk Summary
There are no data on the presence of deucravacitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Deucravacitinib is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). The developmental and health bene ts of breastfeeding should be considered along with the mother’s clinical need for SOTYKTU (deucravacitinib) and any potential adverse effects on the breastfed infant from SOTYKTU or from the underlying maternal condition.
Data
Animal Data
A single oral dose of 5 mg/kg radiolabeled deucravacitinib was administered to lactating (post-partum days 8 to 12) rats. Deucravacitinib and/or its metabolites were present in the milk of lactating rats.
Pediatric Use
The safety and effectiveness of SOTYKTU in pediatric patients have not been established.
Geriatric Use
Of the 1,519 subjects with plaque psoriasis treated with SOTYKTU, 152 (10%) subjects were 65 years or older and 21 (1.4%) subjects were 75 years or older.
During the Week 0-16 period, for those subjects (80 subjects ≥65 years old, including 12 subjects ≥75 years old) who received SOTYKTU without switching treatment arms, there was a higher rate of overall serious adverse reactions, including serious infections, and discontinuations due to adverse reactions compared with younger adults.
No overall differences in effectiveness of SOTYKTU have been observed between patients 65 years of age and older and younger adult patients.
Renal Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild, moderate, or severe renal impairment or in patients with end stage renal disease (ESRD) on dialysis [see Clinical Pharmacology (12.3) in full Prescribing Information]
Hepatic Impairment
No dose adjustment of SOTYKTU is recommended in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. SOTYKTU is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) [see Adverse Reactions and Clinical Pharmacology (12.3) in full Prescribing Information]
OVERDOSAGE
There is no experience regarding human overdosage with SOTYKTU. In case of overdose, consider contacting the Poison Help line (1-800-222-1222) for additional overdosage management recommendations. The extent of deucravacitinib elimination by hemodialysis was small (5.4% of dose per dialysis treatment), and thus hemodialysis for treatment of overdose with SOTYKTU is limited.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of deucravacitinib was assessed in 2-year rat and 6-month rasH2 transgenic mouse studies. No evidence of tumorigenicity was observed in male or female rats that received deucravacitinib at oral doses up to 15 mg/kg/day (51 times the MRHD based on AUC comparison). No evidence of tumorigenicity was observed in male or female Tg.rasH2 mice that received deucravacitinib at oral doses up to 60 mg/kg/day.
Deucravacitinib was not mutagenic in a bacterial mutagenicity assay (Ames test) or clastogenic in an in vitro chromosomal aberration assay (cultured Chinese hamster ovary cells) or in an in vivo rat peripheral blood micronucleus assay.
In male rats, deucravacitinib had no effects on reproductive parameters (mating, fertility, and sperm morphology) or early embryonic development of their offspring at oral doses up to 50 mg/kg/day (224 times the MRHD based on AUC comparison).
SOTYKTU 6 mg once daily N=840 n (%) Placebo N=419 n (%) Upper respiratory infectionsa 161 (19.2) 62 (14.8) Blood creatine phosphokinase increased 23 (2.7) 5 (1.2) Herpes simplexb 17 (2.0) 1 (0.2) Mouth ulcersc 16 (1.9) 0 (0.0) Folliculitis 14 (1.7) 0 (0.0) Acned 12 (1.4) 1 (0.2)
In female rats, deucravacitinib had no effects on mating, fertility, or early embryonic parameters at oral doses up to 50 mg/kg/day (171 times the MRHD based on AUC comparison).
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) before starting SOTYKTU (deucravacitinib) therapy and each time the prescription is renewed, as there may be new information they need to know. Advise patients of the potential bene ts and risks of SOTYKTU.
Hypersensitivity Reactions
Advise patients to discontinue SOTYKTU and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions].
Infections
Inform patients that SOTYKTU may lower the ability of their immune system to ght infections and to contact their healthcare provider immediately if they develop any signs or symptoms of infection [see Warnings and Precautions]
Inform patients that herpes infections, including serious, may occur with use of SOTYKTU [see Warnings and Precautions]
Malignancies including Lymphomas
Inform patients that SOTYKTU may increase their risk of developing malignancies including lymphomas. Instruct patients to inform their healthcare provider if they have ever had any type of cancer [see Warnings and Precautions]
Rhabdomyolysis
Inform patients that SOTYKTU may increase their risk of developing rhabdomyolysis. Instruct patients to immediately inform their healthcare provider if they develop unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever [see Warnings and Precautions]
Laboratory Abnormalities
Inform patients that SOTYKTU (deucravacitinib) may affect certain lab tests, and that blood tests may be required before and during SOTYKTU treatment [see Warnings and Precautions]
Immunizations
Advise patients that vaccination with live vaccines is not recommended during SOTYKTU treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform the healthcare practitioner that they are taking SOTYKTU prior to a potential vaccination [see Warnings and Precautions]
Pregnancy
Advise patients to report their pregnancy to Bristol-Myers Squibb Company at 1-800-721-5072 [see Use in Speci c Populations]
Manufactured by: Bristol-Myers Squibb Company Princeton, NJ 08543 USA
U.S. License No. 1713
© 2022 Bristol-Myers Squibb Company.
SOTYKTU and the Bristol Myers Squibb logo are trademarks of Bristol-Myers Squibb Company.
Issued: September 2022
1787-US-2200566 09/22

Optum Rx Launches Price Edge to Automatically Provide Members the Best Available Prescription Drug Price
• NEW SOLUTION SCANS AND COMPARES DIRECT-TO-CONSUMER PRICING WITH INSURANCE PRICING TO GIVE MEMBERS THE BEST AVAILABLE PRICE
• PRESCRIPTION DRUG PURCHASES AUTOMATICALLY INCLUDED IN MEMBERS DEDUCTIBLES TO MAXIMIZE THEIR BENEFITS
Optum Rx, UnitedHealth Group’s (NYSE: UNH) pharmacy services company, has launched Price Edge, a tool that seamlessly compares available directto-consumer pricing for traditional generic drugs with insurance pricing to ensure members always get the lowest prescription drug price. Price Edge is being offered to all Optum Rx clients.
Compared to most direct-to-consumer prescription drug prices, Optum Rx already offers a lower price nearly 90% of the time and Price Edge ensures a competitive consumer price on generic drugs with every transaction. Price Edge scans available prices and automatically provides the lowest available pricing to the member. If there is a lower cost to the member outside of their insurance benefit, Price Edge automatically applies that price.
Unlike other direct-to-consumer pharmacy solutions or cash market pricing, transactions initiated through Price Edge count toward the member’s deductible and out-of-pocket maximum. Plan sponsors also do not incur additional administrative fees for implementing Price Edge and their members automatically access the tool
within their plan at no cost. Additionally, by capturing all transactions within the member benefit, Price Edge maintains continuity of safety protocols and safeguards against contraindications between medications.
“Providing people affordable access to prescription drugs is at the heart of everything we do, and Price Edge ensures our members get the best price and peace of mind that their medications count toward out-of-pocket spending,” said Jon Mahrt, chief operating officer and pharmacy benefits manager president, Optum Rx. “We will continue to empower clients and consumers with innovative solutions that simplify pharmacy benefits, make pricing more transparent and ensure cost savings are easily accessible.”
It is the latest in a suite of products and tools designed to improve the pharmacy experience for consumers and healthcare professionals. For example, PreCheck MyScript offers healthcare providers real-time information on a patient’s pharmacy benefits and formulary to help identify the right drug to prescribe, obtain any prior authorizations and see the drug
costs. Solutions including MyScript Finder and Proactive Savings Alerts allow consumers, clients and providers to get the information they want and need to make informed decisions about their care—including real-time prior authorization, point-of-sale rebates, and notifications on savings opportunities.
ABOUT OPTUM RX Optum Rx is a pharmacy care business providing people with more affordable access to prescription medications and therapies. This strategy has proven to lower costs for clients and members, improve quality of care and enhance the member experience. Powered by deep clinical expertise and integrated data and analytics, our full-spectrum pharmacy services simplify how consumers, clients and partners navigate the pharmacy space to deliver improved experiences, better health outcomes and a lower total cost of care. Optum Rx is part of Optum, a leading information and technology-enabled health services business dedicated to helping make the health system work better for everyone. For more information, visit optum.com/ optumrx or follow @OptumRx on Twitter.
bcofdermatology.com 63
 By Emily Liebert
By Emily Liebert
Book No Furth




Summer is hotter with these five fascinating reads, including career and life strategies from a powerhouse CEO-meetsreality-TV-star, a novel inspired by the legendary actress Audrey Hepburn, two electrifying thrillers, and nostalgic fiction about former college sweethearts.




























THE AUDREY HEPBURN ESTATE
Brenda Janowitz


































The majestic Long Island estate where Emma Jansen grew up is going to be demolished. Well, she didn’t exactly live there. Her mother and father worked for the owners and they occupied the space over the garage, in the same way Audrey Hepburn’s character did in the film Sabrina. Still, Emma experienced a lot of firsts at the estate. Thus, she’s compelled to return, though she only felt truly accepted by the family’s grandson, Henry and by their driver’s son, Leo. As plans for the property unfold, Emma, Henry, and Leo come together after ten years and Emma is caught between two worlds and two loves. When a shocking secret about her own family is revealed, she’ll need to figure out what kind of life she currently desires and whom she wants to spend it with.
You probably know him as Kyle Richards’ husband on The Real Housewives of Beverly Hills or as the star of his new Netflix series Buying Beverly Hills, but Mauricio Umansky’s greatest professional achievement is building his internationally renowned real estate brand, The Agency. From the challenges of his childhood to outgrowing his father’s textile business and leaving his brother-in-law Rick Hilton’s company, while also raising a family and maintaining a strong marriage for over twenty-seven years, Mauricio shares plenty of advice and lessons for aspiring entrepreneurs and self-starters on how to find success at work and at home. His journey has been anything but boring. So, get ready to join his exclusive and exciting world.





ACCESS DERMATOLOGY 2023 64 / JULY/AUGUST
Mauricio Umansky 2 THE REAL DEAL 1







What happens when two former college sweethearts—who broke up before graduation and vowed to never speak to each other again—wake up a decade later in the same bed with wedding bands on their fingers and no idea how they got there? That’s the predicament Frankie and Ezra find themselves in on New Year’s Eve of 1999, while back on their snowy, picturesque New England campus together, at the turn of the century, for the wedding of mutual friends. Frankie has a successful career as a music manager and Ezra is ready to propose to his girlfriend. Everything should have gone smoothly, if they’d only been able to avoid each other. But now, with Ezra’s grandmother’s diamond on Frankie’s finger, they have to put aside old grievances and deal with their dilemma.

The New York Times bestselling author, Sally Hepworth, has done it again with this gripping and addictive novel about complex relationships, family, infidelity, mental illness, and the deceitfulness that nudges us to our breaking point. Gabe and Pippa live in a beautiful cottage, in a quiet coastal town, set on a dangerous cliff—a spot where people attempt to end their lives and often succeed. Unless Gabe intervenes. Remarkably, he’s managed to, quite literally, talk multiple jumpers off the edge. Until one day when his valiant plan fails and a woman falls to her death…leaving a number of unanswered questions. As Pippa begins to unravel the truth, the perfect facade of her marriage becomes fractured and she’s forced to ask herself if Gabe is really her soulmate after all.





In this fiendish thriller from USA Today bestselling author Hank Phillippi Ryan, the question is, which character is the cat, and which is the mouse? When Alyssa Macallan is dumped by her rich and powerful husband, and a toxic divorce is inevitable, she’s horrified by what’s in store for her. Will she be left alone and broke as a result of his manipulative behavior? What she needs is a good friend. Someone who can support her through the tumultuous times to come. That’s when Bree Lorrance enters the picture. Bree is running from a dangerous relationship of her own and Alyssa offers up her guest house as a safe haven. Very quickly, the two become trusted confidantes. When Bree makes a surprising, but tempting offer, Alyssa wonders if they can help solve each other’s problems.
bcofdermatology.com 65
THE HOUSE
Sally
THE SOULMATE
Hank5RyanPhillippi Ryan
GUEST Allison WinnScotch THE REWIND
Hepworth





































BBC SP TLIGHT SP












iologic Coordinators of Dermatology (BCoD) applauds our members and access managers for their commitment to patients and for securing the types of therapy needed. We salute these professionals across the country who ensure a positive patient experience. This month, we spotlight Amy Tarkington, CMA, Biologics Coordinator.



















JULY/AUGUST







Amy Tarkington
CMA, BIOLOGICS COORDINATOR WILMINGTON HEALTH DERMATOLOGY
WHERE DO YOU WORK AND WHAT IS THE NAME OF THE OFFICE?
I work at Wilmington Health Dermatology— Midtown in Wilmington, North Carolina, and I have been there for 13 years.
WHAT IS YOUR TITLE? PLEASE TELL US ABOUT YOUR JOURNEY GETTING THERE.
I am a CMA and biologic coordinator. Initially, as a CMA, I would see patients for their routine appointments. They would be upset if their insurance denied their biologic medication, or when they couldn't afford the medication, or if they had trouble getting through with the specialty pharmacy. Patients were often confused or upset they were going to be late for their injection, or off-schedule. I thought to myself, “That must be an awful feeling.”
As people, we can relate to patients who get upset when they don't have their day-to-day medication, because the pharmacy needs to order them, and it may take a few days. What would it be like if we had to wait every month to get something that we really need like daily meds? Not only that, but it is a whole different process—everything is done over the phone and not faceto-face at the local pharmacy. It seemed so hectic for patients, and I finally
realized one day that we need to step up and help these patients figure out a better process. So, when we had an 80-year-old patient with dementia pay over $3,000 for medication in one month, I knew we had to find additional help and didn’t settle until we did. I found a resource to get the patient medication at an affordable price, and it was so rewarding to see the relief and gratitude from the family. I knew right away I wanted to continue having that feeling for patients and myself. I had another patient who was denied for everything. This patient could not take TNF drugs due to history of colon cancer, but the payors continued to put up roadblocks. After speaking with pharmaceutical representatives, I was able to leverage additional information and resources to get that patient on an oral medication, and she has been doing well ever since. In fact, every Christmas she brings me a beautiful card and gift thanking me for going above and beyond for her!
CAN YOU DESCRIBE A PARTICULAR PATIENT YOU CAN’T HELP BUT WANT TO FIGHT FOR, AND WHY?
There are three patients who come to mind that I can't help but fight for, which
is another reason that led me in this direction. The first one is Jaiden. He was 10 at the time (now 16) and in middle school. He had atopic dermatitis, and his insurance would never cover Dupixent due to his age at the time.
This kid missed his field day/fun day at school because his skin was so bad, and he quit playing football because of his condition. Not to mention, he also had asthma on top of that. I fought his insurance company to the very end; they continued denying him because of his age. However, in the end, we got him approved, saw him six months after starting Dupixent; he was happier than ever and was back out on the football field. That made me so happy for him and his parents!
The second patient that comes to mind is Molly. She has had psoriasis for years, and she’s been on every injection possible. The only thing that worked for her was Cosentyx. She is in her 80s and has dementia. She and her husband were paying over $3,000 a month. I stepped in and asked if I could try and help them. Her husband was like, “Yes please! That's our mortgage.” I was able to get them assistance, and they have been more than appreciative!
The third patient I think about is
67
bcofdermatology.com












BC SP TLIGHT




Kathy. She had colon cancer and was previously with another dermatologist. She came to us in the hope that we could get her on a therapy she could afford, and one that was safe given her prior cancer history. We were able to get her on Otezla, and she has been wonderful ever since!
HAVE YOU HAD A PATIENT WHO EXPERIENCED EXTREME DIFFICULTY IN GETTING DRUG APPROVAL?
I had a tough time getting a patient approved for one of our biologics, but I did not give up! I kept trying and finally was able to get patient approval. I ended up providing a sample for the patient and re-did the authorization for maintenance only, and then I was able to get her approved.






 Amy Tarkington, CMA, Biologics Coordinator
Amy Tarkington, CMA, Biologics Coordinator
IS THERE A PROCESS WITHIN THE OFFICE YOU HAVE ADOPTED FOR EFFICIENCY PURPOSES? HOW DO YOU COLLABORATE WITH OTHER STAFF MEMBERS?
The process that I use first is to do the PA on Cover My Meds. If that is not possible, then I send it to one of the specialty pharmacies for further help if needed. I have learned and tried helping the staff learn the PBM of patient's plans and navigate them to Cover My Meds, instead of just sending it to a designated pharmacy of their choice. However, I am pretty OCD, therefore I normally have the staff send it to me so I can monitor and track the progress.
DO YOU HAVE ANY OTHER TIPS THAT MAKE YOUR JOB EASIER?






My personal tip would be to make sure you know the patient's PBM. There are certain plans you cannot do on Cover my Meds, as well as some you cannot send to My Preferred Specialty Pharmacy due to contract purposes. I am not one to send something for them to just do the PA. If they can't fill the prescription, I am surely not going to send them the stuff just to complete the PA for me. That is very unprofessional, plus they would have to transfer it to the dispensing pharmacy and that would be considered a third party. You might as well figure it out and handle yourself.
WHY IS PATIENT COMMUNICATION IMPORTANT, AND WHAT IS THE GOAL?
I feel like communication is the key. The office and patient must communicate with one another. I encourage patients if they have an issue with their medication to reach out to their ambassador and specialty pharmacy. That way, they can get their questions answered if they can't reach our office.



LIVE AREA 8" x 5" 3213877-3919 | ©2023 Walgreen Co. All rights reserved.
isn’t
It’s
This
just about healthy skin.
helping people feel good in their own skin. This is one of many moments made possible by the inspirational work of Biologic Coordinators of Dermatology. Your tireless efforts make us glow with pride.



Optum Rx Expands Choice, Improves Affordability for Patients With Two Additional HUMIRA Biosimilars
DECISION MAINTAINS CLINICAL QUALITY WHILE GIVING PATIENTS AND PHYSICIANS MORE CHOICE AND ENCOURAGES MANUFACTURER COMPETITION TO LOWER PRICES
Article courtesy of United Health Group
As of July 1, clients of Optum Rx have access to three biosimilar alternatives for HUMIRA, a highly utilized biologic medication used in the treatment of autoimmune diseases. The Optum Rx standard formulary will include the following treatments at parity with HUMIRA:
• Hyrimoz and adalimumab-adaz (low list) (Sandoz), both high concentration treatments.
• Cyltezo (Boehringer Ingelheim), the first interchangeable biosimilar, and, when available in 2024, access to its low-list version.
• Amjevita (Amgen), including both list versions, which were added earlier this year.
“As an industry leader driving competition and biosimilar access, we are bringing unprecedented choice and value in this highly utilized but historically very expensive drug class,” said Heather Cianfrocco, CEO, Optum Rx. “PBMs have a responsibility to leverage competition to lower cost and create choice. Access to new biosimilars and their available list price options provides physicians, clients and patients with affordable treatment options.”
Optum Rx was the first pharmacy services company to add a HUMIRA biosimilar to its formulary at parity with the original product and the first to include both the high-list and low-list options. The company will continue evaluating the addition of new entrants to the adalimumab category this year. Members will experience the same
or lower out-of-pocket expenses and Optum Rx pharmacists are ready to support patients with questions about treatment options.
This year, Optum Rx launched Price Edge, a consumer tool that ensures members always get the lowest available prescription drug price, and introduced expanded client solutions that enable choice and drive transparency and affordability in pharmacy benefits for employer, union and health plan customers it serves. Optum Rx also recently announced community and independent pharmacy initiatives to reduce administrative burdens, improve patient health outcomes and ensure pharmacists are paid fairly.
ABOUT OPTUM RX Optum
Rx, UnitedHealth Group’s (NYSE: UNH)
pharmacy care business, is a pharmacy care business providing people with more affordable access to prescription medications and therapies. This strategy has proven to lower costs for clients and members, improve quality of care and enhance the member experience. Powered by deep clinical expertise and integrated data and analytics, our full-spectrum pharmacy services simplify how consumers, clients and partners navigate the pharmacy space to deliver improved experiences, better health outcomes and a lower total cost of care. Optum Rx is part of Optum, a leading information and technology-enabled health services business dedicated to helping make the health system work better for everyone. For more information, visit optum.com/ optumrx or follow @OptumRx on Twitter.
ACCESS DERMATOLOGY JULY/AUGUST 2023 70












last word

“We don't heal in isolation, but in community."
– S. KELLEY HARRELL
SVITLANASTOCK.ADOBE.COM ACCESS DERMATOLOGY JULY/AUGUST 2023 72
ENHANCE AND EXPEDITE
ATIENT FOC SED COLLABORATION
Biologic Coordinators of Dermatology has partnered with uincy to bring technological innovation to the FRM and BC workstreams.
Speed time to therapy and increase the efciency of Field Reimbursement Managers and Biologic Coordinator collaboration with HI AA-compliant secure messaging.
• Reduce patient onboarding time
• Increase employee productivity
• Increase the number new patients processed
• Increase sta and patient satisfaction


FEAT RES
• Initiate secure conversations between Field Reimbursement Managers and Biologic Coordinators
• Create Quick Messages for commonly used communications
• Personalized url to increase trust in the identity of the communicator
• Optional: Add Face to Face Video virtual visits for a personal touch

To learn more see a demo and take advantage of partnership discounts please reach out to contact bcofdermatology.com


from UV damage with 100% mineral sunscreens
sun damaged skin with patented DNA Repairsomes®













Ask your local dermatologist or visit isdin.com/us for more information.




















































































































































































































































































































































































 By Sarah Pinkowski Jansen MARKETING & COMMUNICATIONS CONSULTANT
By Sarah Pinkowski Jansen MARKETING & COMMUNICATIONS CONSULTANT

































 By Emily Liebert
By Emily Liebert


























































































































































 Amy Tarkington, CMA, Biologics Coordinator
Amy Tarkington, CMA, Biologics Coordinator











































