
Titanium for reliable stability and space maintenance


Titanium for reliable stability and space maintenance
Precise patient-specific
3-D printing
Fully microstructured surface protects from tissue ingrowth
Titanium for reliable stability and space maintenance
Less surgery time due to perfect fit without shape adaptation
Precise patient-specific
3-D printing
Less surgery time due to perfect fit without shape adaptation
Calculation of augmentation volume (optional)

”I have been using the new Yxoss with a dense structure for the last 2 years and I can state that they are very effective and predictable devices for horizontal and vertical ridge augmentation. When associated with 70% of autogenous bone chips and 30% of DBBM their efficacy is comparable withthe one of traditional PTFE non-resorbable membranes, but much easier and faster to be installed.“
Prof. Massimo Simion

”I have been using the new Yxoss with a dense structure for the last 2 years and I can state that they are very effective and predictable devices for horizontal and vertical ridge augmentation. When associated with 70% of autogenous bone chips and 30% of DBBM their efficacy is comparable withthe one of traditional PTFE non-resorbable membranes, but much easier and faster to be installed.
Integrated implant positioning (optional)
Calculation of augmentation volume (optional)
Integrated implant positioning (optional)
Head-to-head comparison as assessed by Dr. Seiler and Dr. Ronda
Head-to-head comparison as assessed by Dr. Seiler and Dr. Ronda
Prof. Massimo Simion



Head-to-head comparison as assessed by Dr. Seiler and Dr. Ronda

The Australasian
Journal of Oral and Maxillofacial Surgery is the official scientific journal of the Australian and New Zealand Association of Oral and Maxillofacial Surgeons
Aim and Scope
The Australasian Journal of Oral and Maxillofacial Surgery is the premier forum for the exchange of information for new and significant research in oral and maxillofacial surgery, promoting the surgical discipline in the Oceanic region.
Oceania comprises 19 countries, spread over one-sixth of the globe, but with Australia and New Zealand being the dominant developed countries.
The Journal comprises peer-reviewed scientific reports, reviews, case reports of rare and unusual conditions, and perspectives; all of value for continuing professional development.
Information for prospective authors, including author guidelines, publication ethics, malpractice statements and patient consent forms are available for download from the Australian and New Zealand Association of Oral and Maxillofacial Surgeons homepage. All correspondence with the Editor is via editorajoms@anzaoms.org
Advertising Information
The Australasian Journal of Oral and Maxillofacial Surgery accepts paid advertisements from companies involved with the surgical discipline. For information on advertising guidelines and rates contact Ms Belinda Mellowes, Executive Officer, Australian and New Zealand Association of Oral and Maxillofacial Surgeons: eo@anzaoms.org Submit advertisements to ajoms@anzaoms.org
Disclaimer
The Australasian Journal of Oral and Maxillofacial Surgery Editors and Editorial Board cannot be held responsible for error or consequence arising from the information contained in the Journal. The views and opinions expressed herein do not necessarily reflect those of the Australian and New Zealand Association of Oral and Maxillofacial Surgeons, AJOMS Editor or Editorial Board. Neither does publication of advertisements constitute any endorsement of the products advertised. © 2025 ANZAOMS

Alastair Goss
• Emeritus Professor of Oral and Maxillofacial Surgery, The University of Adelaide
• Emeritus Consultant Surgeon, The Royal Adelaide Hospital Adelaide, SA, Australia
Andrew Heggie, AM
• Clinical Professor, Department of Paediatrics, The University of Melbourne
• Senior Consultant Oral and Maxillofacial Surgeon, Royal Children’s Hospital of Melbourne Melbourne, VIC, Australia
Alexander Bobinskas
• Unit Director, Oral and Maxillofacial Surgery, The Canberra Hospital
• Associate Professor, School of Medicine, Australian National University Canberra, ACT, Australia
Website and Distribution
Belinda Mellowes
• Executive Officer, Australian and New Zealand Association of Oral and Maxillofacial Surgeons Sydney, NSW, Australia
AMPCo Production Editors
Laura Teruel
Kate Steyn
AMPCo Graphic Design
Peter Humphries
Catherine Offler
• Administrative Assistant to the Editor Adelaide, SA, Australia
Business Manager
Dieter Gebauer
• Senior Consultant in Oral and Maxillofacial Surgery, Royal Perth Hospital
• Clinical Associate Professor in Oral and Maxillofacial Surgery, The University of Western Australia
Perth, WA, Australia
Members
Arun Chandu
• Senior Consultant in Oral and Maxillofacial Surgery, Royal Dental Hospital of Melbourne
• Clinical Associate Professor of Oral and Maxillofacial Surgery, The University of Melbourne
Melbourne, VIC, Australia
Nigel Johnson
• Consultant Oral and Maxillofacial Surgeon, Princess Alexandra Hospital
• Senior Lecturer in Oral and Maxillofacial Surgery, The University of Queensland
Brisbane, QLD, Australia
Paul Sambrook, AM
• Director Oral and Maxillofacial Surgery, The Royal Adelaide Hospital
• Senior Lecturer in Oral and Maxillofacial Surgery, The University of Adelaide
Adelaide, SA, Australia
Darryl Tong
• Professor of Oral and Maxillofacial Surgery, The University of Otago
• Senior Consultant in Oral and Maxillofacial Surgery, Dunedin Public Hospital
Dunedin, New Zealand
Andrew Watkins
• Supervisor of Training, John Hunter Hospital
• Senior Lecturer, Charles Sturt University Newcastle, NSW, Australia
Ex Officio Members
Jasvir Singh
• President, Australian and New Zealand Association of Oral and Maxillofacial Surgeons
• Consultant in Oral and Maxillofacial Surgery, Prince of Wales Hospital
Sydney, NSW, Australia
Patrishia Bordbar
• Immediate Past President, Australian and New Zealand Association of Oral and Maxillofacial Surgeons
• Consultant in Oral and Maxillofacial Surgery, The Royal Children’s Hospital of Melbourne
• IAOMS Executive Representative – Oceania Region
Melbourne, VIC, Australia
John Harrison
• Regional Councillor for Oceania, International Association of Oral and Maxillofacial Surgeons
• Senior Consultant in Oral and Maxillofacial Surgery, Auckland City and Middlemore Hospital
Auckland, New Zealand
Jocelyn Shand
• Clinical Associate Professor, Department of Paediatrics, The University of Melbourne
• Chair, Australian and New Zealand Association of Oral and Maxillofacial Surgeons Research and Education Foundation
• Head of Section of Oral and Maxillofacial Surgery, The Royal Children’s Hospital of Melbourne
• Vice President Elect, International Association of Oral and Maxillofacial Surgeons
Melbourne, VIC, Australia
56
EDITORIAL
November 2025
Volume 2 | Issue 2
Personal reflections on the progress of team management of oral and oropharyngeal cancer
Wiesenfeld D
60 SCIENTIFIC ARTICLE
Retrospective analysis of surgically treated orbital fractures in a Western Australian hospital with a focus on Aboriginal and Torres Strait Islander outcomes
Hong L, Cooper T, Vujcich N, Ricciardo P and Bobinskas A
66
SCIENTIFIC ARTICLE
Does the degree of volume change after isolated orbital trauma have a relationship with rates and severity of ocular injury or demographic characteristics?
Narsinh P, Taneja K, Satheakeerthy S and Erasmus J
73 SCIENTIFIC ARTICLE
Loss of fat associated with haematoma in the traumatised, reconstructed orbit
Spencer S, Gebauer D, Day R, Collier R and Khadembaschi D
78
SCIENTIFIC ARTICLE
Hyperbaric oxygen for therapeutic treatment of osteoradionecrosis
Gamage SN, Jensen ED, Cheng A, Goss AN and Sambrook P
84 PERSPECTIVE
Disparities in orthognathic surgery between Australia and New Zealand
Roberts SL
88 SCIENTIFIC ARTICLE
What indication criteria are applied to patients receiving total temporomandibular joint replacement? A systematic review
Mian M, Woliansky M, Sklavos A, Sreedharan S and Kumar R
94 REVIEW ARTICLE
The use of autologous fat grafting in temporomandibular total joint replacement surgery: a systematic review
Liu A , Vu LC, Zhao DF and Dimitroulis G
101 CASE REPORT
Case report of a life-threatening bleed from oral hereditary haemorrhagic telangiectasia
van Kuijk M, Singleton C, Ke L and Singh T

Maxillo Facial Imaging (MFI) is Australia's leading dental radiology providersolely focusing on CBCT, OPG and Lat Ceph
The highest quality imaging & workups
Oral & Maxillofacial Radiologists
State-of-the-art imaging technology




Exceptional patient care
Convenient locations
24 hour turn around reporting service*




Oral and oropharyngeal cancer, predominately mucosal squamous cell carcinoma, provide significant challenges to health care worldwide. There is a wide variation in the incidence and prevalence of these cancers, particularly between developed and developing countries. The aetiologies of these cancers include tobacco and alcohol use, and the chewing of betel nut with various additives. Human papilloma virus (HPV) infection is recognised as a sexually transmitted cause of oropharyngeal cancers; it is identified by the presence of P16 protein as a surrogate marker in the formalin-fixed histological specimen.
The management of these cancers requires a highly skilled multidisciplinary team including medical, nursing and allied health participation. Treatment can often be disfiguring and interfere with essential functions including speech and swallowing. There are significant psychological impacts of treatment, which must be considered as part of care. There have been many improvements in patient care and disease management during my professional career over nearly fifty years. A key element of this improvement has been the concept of the multidisciplinary team. Team members all have expertise and novel experiences to enhance care. Developed from their wide-ranging education and life experience, the team must consider and prioritise the needs of the patient, their families and carers.
Common to all the treating specialties are the concepts of risk stratification, the development and acceptance of treatment protocols, striving for early diagnosis and tailoring care for the individual patient based on their unique circumstances and needs.
Dedicated nurses have always been indispensable in the operating theatres; assisting and facilitating surgeons to complete their procedures. In the wards, we have a cohort of expert nurses to provide physical and emotional care to patients to manage their recovery, wound care and initiate the plans for discharge. A new type of nurse has been introduced as the backbone of the team: the cancer nurse coordinator (CNC), who helps the patients and their families through an often-bewildering course of intensive treatment, and medical clinical attendances. The CNC works full-time rather than the three-monthly rotations that junior doctors experience and is therefore more available to assist in the patient’s whole cancer journey.
Major advances have been made in the understanding of radiation biology and the technical aspects of accurately planning and delivering the required doses to tumours while sparing uninvolved
ANNUAL SCIENTIFIC MEETING
6-8 AUGUST 2026 CHRISTCHURCH/OTAUTAHI NEW ZEALAND
The ANZAOMS 2026 Annual Scientific Meeting will be held in the beautiful Otautahi/ Christchurch, New Zealand. The 2026 ASM promises to deliver an engaging and thought-provoking scientific program alongside a dynamic exhibition and social program.


CALL FOR ABSTRACTS OPEN 7 December 2025
OPEN 9 February 2026 CALL FOR ABSTRACTS CLOSE 23 March 2026
PROGRAM RELEASED 11 May 2026
BIRD CLOSES 8 June 2026 ANZAOMS 2026 ASM 6 - 8 August 2026
tissues, thus reducing treatment-related morbidity and improving the chances of control. Included in these advances are intensity modulated radiation therapy, volumetric modulated arc therapy, and concurrent treatment with chemotherapy. The indications and protocols for adjuvant treatment, either radiation alone or concurrent chemoradiation are well documented and accepted internationally. Palliative radiotherapy is a versatile low morbidity treatment option for patients with advanced and incurable disease, either through disease extent or patient frailty. The dose and frequency provided can be modified to suit the Eastern Cooperative Oncology Group (ECOG) Performance Status scale.
Concepts of medical oncology in the management of oral and oropharyngeal cancers have changed dramatically with the inception of immunotherapy. Diligent controlled multicentre prospective studies and clinical trials are showing promising results in both the neoadjuvant and locally advanced inoperable cohorts with mucosal head and neck squamous cell carcinoma. The role of concurrent chemoradiation protocols using platinum-based chemotherapy is well established and accepted in the adjuvant setting with standardised case selection and protocols.

Standardised pathology reporting for head and neck cancer has led to improved risk stratification, prognostication, guidance for the need of elective neck dissection and selection of adjuvant therapy. Factors to be considered are disease clearance margins, depth of invasion, perineural and lymphovascular invasion within the primary tumour and evidence of extranodal extension from the neck dissection specimen. The evidence of HPV association of oropharyngeal cancers using the surrogate P16 protein has a significant bearing on treatment de-escalation and prognosis for these patients.
Computer tomography (CT) imaging, preferably with contrast, is essential for both pre-operative assessment of the neck and chest and ongoing surveillance. Magnetic resonance imaging (MRI), especially with enhancement, aids significantly in soft tissue and bone marrow assessment and is very useful for surveillance of the neck. Ultrasound can be used to guide fine needle or core biopsy of nodal disease and is helpful for assessing the tumour thickness of tongue and other soft tissue lesions.
Positron emission tomography (PET), often combined with CT scans, has revolutionised the assessment of metastatic, especially nodal, disease in head and neck cancer care and can be used for surveillance as well as pre-treatment staging.
“Early diagnosis saves lives” is more than a slogan. All involved in oral cancer care know that small tumours are easier to manage than advanced stage tumours. Education for general medical and dental practitioners in oral cancer diagnosis is essential to improve patient outcomes, equally community education is vital so that patients can recognise that a persistent ulcer or sore in your mouth needs attention. Community awareness has been of great assistance with skin and bowel cancer diagnosis as well as smoking reduction campaigns. Acknowledging that skin and bowel cancer are more common than oral cancers, we still need to increase awareness at the national and local levels; including photographs on cigarette packets is a start but not nearly enough.
The role of HPV is widely accepted in the development of oropharyngeal cancer, as well as genital and anal cancers. Support of HPV vaccination for young people before commencing sexual activity
will help reduce the incidence of HPV-related cancers and possibly eradicate this cancer. It is predicted that HPV-related cancers in all sites will disappear in the next 30 years.
Advances in diagnostic imaging and staging have led to improvements in our understanding of the anatomical requirements for complete resection of the tumour with clear margins. The availability of skilled reconstructive surgeons with advanced techniques for both reconstruction and rehabilitation have allowed for more extensive excisions when required, and for better functional and aesthetic rehabilitation. Modern surgical techniques combined with virtual surgical planning allow for increased collaboration between ablative and reconstructive surgeons to achieve functional results using free tissue transfer that were not previously considered possible, including dental rehabilitation. Despite current advances, patients still face the challenges of interference with speech, swallowing and deformity when they present with advanced disease. Neoadjuvant immunotherapy may be a future opportunity for us to reduce the morbidity and improve aesthetic outcomes of surgically excising advanced tumours.
We have active allied health teams that support our endeavours to manage patients. Speech pathologists and dietitians collaborate to manage feeding, especially when long term difficulty with swallowing is anticipated. Percutaneous endoscopic gastrostomy (PEG) feeding is appropriate for medium and long term feeding challenges when safe swallowing is affected by the tumour, its removal or radiotherapy. Pre-operative PEG feeding can also be used to assist patients who present as malnourished to improve their recovery after treatment. Clinical psychologists and social workers play a major role in assessing patient suitability for proposed treatment and assisting them to deal with the challenges that major surgery and prolonged hospitalisation present. The clinical psychologist can also assist
with long term survivorship issues. Patient support groups have been established by hospitals and community organisations to assist patients through their lived cancer experience and provide for self-help opportunities through sharing their experiences with other cancer survivors who face similar problems.
The complexity and availability of treatment for patients with oral cancer have changed and expanded over the course of my clinical career. Treatment options and success rates have improved. Patterns of disease and aetiology have changed with a reduction of smoking in the community, the adoption of habits from other countries through migration and the recognition of a growing cohort of non-smoking, non-alcohol drinking females who develop non-HPV-related oral tongue cancer.
June 2025
David Wiesenfeld AM
Deputy
Editor AJOMS
Professor David Wiesenfeld AM played a key role as Deputy Editor in the foundation of the Australasian Journal of Oral and Maxillofacial Surgery (AJOMS). His incisive reviews of submitted papers helped establish the high scientific level of AJOMS. His contributions will be missed, and we wish him all the best in his retirement. We are pleased to announce that Dr Alexander Bobinskas has joined Professor Andrew Heggie AM as a second Deputy Editor of AJOMS
Alastair Goss
Editor-in-Chief AJOMS
Author contributions
David Wiesenfeld AM: Conceptualization, investigation, original draft, reviewing and editing.
Conflicts of interest
The author declares that they have no conflicts of interest.
doi: 10.63717/2025.MS0053

Surgical Training
Advanced workshops, complex case discussions and cu ing-edge implant techniques.
Practical Perks Community
Priority access to AOMI events and exclusive local study clubs for surgeons only.
Professional Status & Leadership
Faculty tracks, leadership roles and the prestige of belonging to a surgeons only global network.
Member Value 20% member discount on AOMI education programs
Hong L (BSci, MD)1; Cooper T (BDSc, MBBS, MPhil, FRACDS(OMS))2,3; Vujcich N (BDSc, MBBS, FRACDS(OMS))2; Ricciardo P (BDSc, MBBS, FRACDS(OMS))2; Bobinskas A (BOH(DentSci), GradDipDent, MBBS, FRACDS(OMS), FRCS(OMFS))4
Orbital fractures are a frequently encountered type of maxillofacial injury, constituting up to 25% of isolated facial fractures.1 Epidemiological investigation across Asia, Europe and America consistently reveal that individuals sustaining orbital floor fractures are predominantly young males.2-6 From an Australian perspective, orbital fractures in Indigenous patients have only been studied as a subset of broader maxillofacial or ocular injury studies.7,8 Although these reports provide satisfactory case numbers, they lack comprehensive information about patient demographics and do not delve into detailed analyses of orbital fracture presentations.
It is estimated that there are over 980 000 Indigenous Australians in Australia, representing 3.8% of the total Australian population. Of these, approximately 120 000 live in Western Australia (WA), comprising 4.4% of the total WA population.9 Prior literature has highlighted a higher proportion of head injuries and mandibular fractures in the Indigenous Australian population.10-12 However, no datasets have focused on orbital fractures.
This study aims to review the epidemiology of orbital fractures and identify trends in patients undergoing orbital reconstruction in WA, with a specific emphasis on identifying potential differences between the Indigenous Australian and non-Indigenous populations.
A retrospective review was conducted on all cases of orbital reconstruction performed by the oral and maxillofacial unit at the
1Intensive Care Unit, Royal Perth Hospital, Perth, WA, Australia; 2Oral and Maxillofacial Surgery, Royal Perth Hospital, Perth, WA, Australia; 3Oral and Maxillofacial Surgery, Fiona Stanley Hospital, Perth, WA, Australia; 4Oral and Maxillofacial Surgery, Canberra Hospital, Canberra, ACT, Australia. Corresponding author: Lewis Hong ✉ lewish1807@gmail.com | doi: 10.63717/2025.MS0042
Purpose: Orbital fractures are a common type of maxillofacial injury, accounting for up to 25% of isolated facial fractures. This retrospective study examines orbital fractures requiring reconstruction in Western Australia (WA) over a five-year period, with a focus on differences between Indigenous Australians (Aboriginal and Torres Strait Islander peoples, hereafter referred to as Indigenous Australians) and non-Indigenous populations.
Methods: Data from 142 patients who had undergone orbital reconstruction at a tertiary trauma centre were reviewed, revealing that young males were predominantly impacted, with a median age of 33 years. Assault emerged as the leading cause of injury, especially among Indigenous Australians, who represented 17% of the study population, higher than their 4.4% representation in the WA population.
Results: Key findings include a significantly higher incidence of assault-related orbital fractures among Indigenous Australians (83% v 47%; P < 0.01) and higher rates of “lost to follow-up” in the Indigenous Australian group (46% v 24%; P = 0.04). No significant differences were observed in the timing of surgery, injury severity, or post-surgical outcomes between Indigenous Australians and non-Indigenous patients. The study underscores the need for targeted prevention strategies, such as reducing assault rates, and improving follow-up compliance through potential interventions that may include telehealth, enhanced health education for local medical officers and involvement of Aboriginal liaison officers.
Conclusions: These findings highlight the disparities in orbital fracture aetiology and follow-up care between Indigenous Australians and non-Indigenous populations. Further research is recommended to validate these findings and explore interventions.
Keywords: trauma | oral and maxillofacial surgery | orbital fixation | orbital fracture
* Indicates statistical significance for a P value less than 0.05.
Royal Perth Hospital (RPH), between January 2016 and December 2020. The study protocol was approved by the RPH Human Research Ethics Committee and the Aboriginal Health Council Ethics Committee (RGS0000005043).
Using the unique patient identifier, demographic attributes such as self-reported ethnicity, gender, age and residential address were obtained from the electronic patient management system. The paper files of these patients, including outpatient clinic notes, operation records, ward and emergency department notes, and discharge summaries were then reviewed.
The Modified Monash Model was used to determine if a patient came from an urban or rural area. As per the model, a patient was characterised as originating from a rural area if their residential address fell within territories categorised as Modified Monash 2 or higher, with each number above 2 signifying a higher degree of remoteness.13
Presenting signs and symptoms were identified as the latest documented before surgery. Total length of post-operative followup was calculated from day of surgery to the day of last review. A patient was deemed discharged if the outpatient clinic notes had formal documentation saying so, otherwise a patient was noted as ongoing with review. As per hospital policy, patients were categorised as “lost to follow-up” if they did not show up to at least two scheduled follow-up appointments.
Data were processed using Microsoft Excel and, where statistical analysis was performed, either the Fisher Exact Test or two-sample T-test was used with a two-tailed P value of less than 0.05 being deemed as statistically significant.
Table 2. Mechanism of injury and time from injury to surgery
Between January 2016 and December 2020, 142 patients underwent orbital reconstruction at RPH. Of these, 103 were male and 39 were female. Seventy-nine patients came from urban areas, while 54 came from rural areas, and 9 were international residents. In terms of ethnicity, 88 patients were of European descent, and there were 24 patients of Indigenous Australian heritage. The median age at the time of injury was 33 years, with 33.1% falling between the ages of 30 and 39 years, and 30.3% between 20 and 29 years (Table 1).
The most prevalent cause of orbital injuries was assault, accounting for 76 cases, followed by motor vehicle accidents and sport/ recreation-related incidents, each with 21 cases. There was no significant difference based on location. Notably, there was a significantly higher incidence of assault as the cause of injury within the Indigenous Australian patient population relative to non-Indigenous patients (83% v 47%; P < 0.01) (Table 2). When accounting for gender, the highest rate of assault as reported cause of injury was seen in Indigenous Australian males (88%) with the second highest seen in Indigenous Australian females (75%) (Table 3).
Most surgeries took place between 8 to 14 days after the injury, which was not significantly different when accounting for location or indigeneity (Table 2). Only 21 cases involved an isolated orbital fracture with no other facial injuries present. In other cases, ocular injuries were found in 75 cases, soft tissue injuries in 59 cases, and other facial fractures in 58 cases (Table 4). Among presenting issues, peri-orbital bruising was most frequently documented, affecting 100 patients, followed by diplopia in 89 patients. Acute vision loss was noted in 18 cases, and gaze restriction in 66 cases (Table 5). There was no significant difference for presenting issues or associated injuries when accounting for location or indigeneity.
Overall median follow-up duration was 49 days. Follow-up duration was significantly higher in the non-Indigenous population compared with the Indigenous Australians, being nearly three times longer (61 v 24 days; P = 0.03) (Table 5). A significantly higher proportion of Indigenous Australian relative to non-Indigenous patients were “lost to follow-up” (46% v 24%; P = 0.04). There were no significant differences in terms of post-operative follow-up duration or rate of “lost to follow-up” based on location. Thirteen patients required revision or removal of hardware after surgery. There was no significant difference for post-surgical outcome between the Indigenous Australian and non-Indigenous populations (Table 5).
This retrospective study aimed to identify the trends in orbital trauma requiring repair over a five-year period in WA, with a particular
emphasis on the Indigenous Australian population. The overall findings mirror previously published results, showing that the highest numbers of these traumatic injuries occur in young to middle-aged males of European descent.1-8 However, Indigenous Australian patients comprised 17% of the study population, which is higher than the overall Indigenous Australian population of 4.4% in WA, suggesting that there may be a potentially higher occurrence of orbital trauma in the Indigenous Australian population.9
Our results support assault being the leading cause of orbital bone fractures, followed by traffic accidents and sports activities. These findings are consistent with several studies, indicating universal trends or a strong association between the mechanism of assault and orbital fractures.2-7 Among the Indigenous Australian patients, there was a significantly higher incidence of assault compared with nonIndigenous individuals.
Oberdan and Finn observed similar trends on assault in Indigenous Australian individuals and identified a higher rate of assault on women in their study on mandibular fractures in Far North Queensland, suggesting a possible link to high rates of interpersonal/ domestic violence.11 Our study found no significant difference in incidence of assault for Indigenous Australian women when compared with other groups; however, there appears to be higher rates of violence in Indigenous Australian women versus nonIndigenous women in our study, which may not have been adequately captured due to low Indigenous Australian female patient numbers.
The data overall further support the notion that Indigenous Australian individuals are more vulnerable to assault.
No significant differences were noted in the timing of surgery between urban, rural, Indigenous Australian, and non-Indigenous comparisons, indicating adequate accessibility to maxillofacial services. The overall pre-operative findings and associated injuries did not significantly differ when comparing Indigenous Australian to non-Indigenous patients, suggesting that injuries sustained in the Indigenous Australian population are not inherently more severe.
In terms of post-operative follow-up, the rate of being “lost to followup” was significantly higher in the Indigenous Australian population. Location did not seem to be a contributing factor, as the rate of follow-up for rural and urban patients (both Indigenous Australians and non-Indigenous) did not significantly differ. This underscores a disparity in compliance with follow-up for the Indigenous Australian population, emphasising the need for services to address this issue.
In summary, our study illustrates some discrepancies between the Indigenous Australian and non-Indigenous populations concerning orbital floor fractures, in particular the need for improved prevention strategies to mitigate injury, such as reducing rates of assault, and improved follow-up services. One method of reducing rates of assault was described by Dorman and colleagues, where they demonstrated a remarkable reduction in the incidence of ocular trauma in Indigenous communities following the implementation of alcohol management plans in Far North Queensland, which prohibited the possession and consumption of alcohol in most Indigenous communities.14 However, they also rightfully acknowledged that implementing such measures was a potential infringement on civil liberties based on ethnicity.
Implementing health technology, such as telehealth, to enhance compliance with follow-up is a promising approach with well studied benefits both locally in Australia and globally.15-17 However, it is unclear if these benefits would be applicable to orbital trauma, partly due to the importance of, and difficulties in, performing an accurate clinical examination. Such health technologies still require patient compliance in attending appointments to be effective. While proven to be beneficial for rural areas, geographical isolation did not appear to be an issue for our cohort, as indicated by the non-significant differences in rural and urban patient follow-up rates, suggesting that other factors may be influencing follow-up for Indigenous Australian individuals.
Enhanced health education during hospitalisation and in clinic settings regarding the risks of patient-led discharge and the potential benefits of follow-up (including addressing ongoing diplopia with eye exercises or referral to an optometrist for corrective lenses and monitoring visual acuity) may also help with follow-up compliance. Involving relevant allied health staff such as the Aboriginal liaison
officer to aid in this task as well as helping address issues that hinder follow-up could be of benefit. Cheok and colleagues18 have shown routine involvement of an Aboriginal liaison officer within the orthopaedic multidisciplinary team to be effective in reducing the risk of self-discharge in Indigenous patients, with those who selfdischarged doing so only after critical aspects of their care were met.
Providing a guide of concerning symptoms to monitor for in a patient’s discharge summary and having the patient follow-up with their local general practitioner may also be a simple alternative as it leverages the strong patient–clinician relationships that exist at the local level. This is supported by the preference shown by some Indigenous Australian patients who opt for their local Aboriginal medical service over a hospital for assistance.19
Limitations of this study include a small sample size, partially attributed to focusing on operated orbital fractures rather than all fractures. The retrospective nature introduces potential bias due to non-standardised measurement of diplopia (clinician-dependent), possibly leading to intraoperative bias. As there was no standard proforma for assessment, some examination findings may not have been adequately documented. All assessments were conducted by a maxillofacial registrar or higher. A prospective study further investigating this issue would be valuable to validate findings and track trends in response to enhanced use of health technology.
In summary, this retrospective study highlights trends in orbital trauma, underscoring differences between the Indigenous Australian and non-Indigenous populations in WA. Notably, assault remains the predominant cause, with a heightened prevalence observed among Indigenous Australian individuals. Additionally, there is an apparent gap in compliance with post-operative follow-up, particularly among Indigenous Australian individuals. Further research and targeted interventions, particularly within the Indigenous Australian community, such as involving Aboriginal liaison officers or the local general practitioner for follow-up, would be of benefit to improve outcomes for individuals sustaining orbital fractures.
Hong L: Acquisition, analysis and interpretation of data, writing –original draft, writing – review and edit.
Cooper T: Conceptualization, writing – original draft, writing – review and edit.
Vujcich N: Writing – review and edit.
Ricciardo P: Writing – review and edit.
Bobinskas A: Writing – review and edit, supervision.
All authors declare that they have no conflicts of interest.
This study did not generate any original data that can be shared.
1 Alvi A, Doherty T, Lewen G. Facial fractures and concomitant injuries in trauma patients. Laryngoscope 2003; 113: 102-106.
2 Seifert LB, Mainka T, Herrera-Vizcaino C, et al. Orbital floor fractures: epidemiology and outcomes of 1594 reconstructions. Eur J Trauma Emerg Surg 2022; 48: 1427-1436.
3 Gosau M, Schöneich M, Draenert FG, et al. Retrospective analysis of orbital floor fractures--complications, outcome, and review of literature. Clin Oral Investig 2011; 15: 305-313.
4 Shere JL, Boole JR, Holtel MR, et al. An analysis of 3599 midfacial and 1141 orbital blowout fractures among 4426 United States Army Soldiers, 1980-2000. Otolaryngol Head Neck Surg 2004; 130: 164-170.
5 Chi MJ, Ku M, Shin KH, et al. An analysis of 733 surgically treated blowout fractures. Ophthalmologica 2010; 224: 167-175.
6 Chiang E, Saadat LV, Spitz JA, et al. Etiology of orbital fractures at a level I trauma center in a large metropolitan city. Taiwan J Ophthalmol 2016; 6: 26-31.
7 Cabalag MS, Wasiak J, Andrew NE, et al. Epidemiology and management of maxillofacial fractures in an Australian trauma centre. J Plast Reconstr Aesthet Surg 2014; 67: 183-189.
8 Ashraf G, Arslan J, Crock C, et al. Sports-related ocular injuries at a tertiary eye hospital in Australia: a 5-year retrospective descriptive study. Emerg Med Australas 2022; 34: 794-800.
9 Australian Bureau of Statistics. Estimates of Aboriginal and Torres Strait Islander Australians [website]. Canberra: ABS, 2021 . https://www.abs.gov.au/statistics/ people/aboriginal-and-torres-strait-islander-peoples/ estimates-aboriginal-and-torres-strait-islanderaustralians/30-june-2021 (viewed Oct 2023).
10 Australian Government Australian Institute of Health and Welfare. Hospitalised injury among Aboriginal and Torres Strait Islander people; 2011–12 to 2015–16. AIHW, 2019. https://www.aihw.gov.au/reports/injury/ hospitalised-injury-among-aboriginal-and-torres-st/ contents/table-of-contents (viewed Oct 2023).
11 Oberdan W, Finn B. Mandibular fractures in Far North Queensland: an ethnic comparison. ANZ J Surg 2007; 77: 73-79.
12 Diab J, Flapper WJ, Moore MH. Facial fractures in Indigenous and non-Indigenous populations of South Australia. J Craniofac Surg 2023; 34: 1207-1211
13 Australian Government Department of Health, Disability and Ageing. Modified Monash Model. Australian Government Department of Health, Disability and
Ageing. https://www.health.gov.au/topics/rural-healthworkforce/classifications/mmm (viewed Oct 2023).
14 Dorman A, O’Hagan S, Gole G. Epidemiology of severe ocular trauma following the implementation of alcohol restrictions in Far North Queensland. Clin Exp Ophthalmol 2020; 48: 879-888.
15 Mathew S, Fitts MS, Liddle Z, et al. Telehealth in remote Australia: a supplementary tool or an alternative model of care replacing face-to-face consultations? BMC Health Serv Res 2023; 23: 341.
16 Kichloo A, Albosta M, Dettloff K, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Health 2020; 8: e000530.
17 Gajarawala SN, Pelkowski JN. Telehealth benefits and barriers. J Nurse Pract 2021; 17: 218-221.
18 Cheok T, Berman M, Delaney-Bindahneem R, et al. Closing the health gap in Central Australia: reduction in Indigenous Australian inpatient self-discharge rates following routine collaboration with Aboriginal Health Workers. BMC Health Serv Res 2023; 23: 874.
19 Nolan-Isles D, Macniven R, Hunter K, et al. Enablers and barriers to accessing healthcare services for Aboriginal people in New South Wales, Australia. Int J Environ Res Public Health 2021; 18: 3014.



Narsinh P (MBBS, BDS, FRACDS (OMS))1; Taneja K (BMed, BDS)2; Satheakeerthy S (MBBS, MTrauma)3;
Orbital fractures that are commonly seen by oral and maxillofacial surgeons can be associated with a multitude of injuries to surrounding structures, including the globe, brain and cervical spine.1 Severe ocular injuries are estimated to occur in 2.7–13.7% of isolated orbital trauma cases.2 Jamal and colleagues reported a 10% incidence of major or blinding injuries and a 6% rate of traumatic optic neuropathy with zygomaticomaxillary fractures.3 Brown and colleagues found that 17.1% of patients had concurrent globe injuries associated with isolated orbital fractures.4
The burden of orbital trauma can be high, with pooled trauma registry data of 102 887 patients in Germany showing that 11.1% of patients sustained facial trauma.5 Orbital walls are estimated to be fractured in 30–40% of all facial fractures.6,7 Orbital fractures represent onefifth of all facial fractures in New Zealand.8-10 American data showed a 47% increase in orbital fracture presentation over an 11-year period leading to 2017, and that 40% of ocular admissions were due to orbital fractures.11
Clinical parameters as outlined by Burnstine, or visual assessment of fracture size guide the reconstructive surgeon on orbital fracture management.12,13 With access to high quality imaging techniques, three-dimensional (3D) orbital volume measurements are now being calculated to determine orbital volume changes that occur after trauma. Studies have indicated that orbital volume increases by more than 1.62 cm3 are an indication for orbital reconstruction to prevent enophthalmos.14 Studies also indicate that volume measurements have a repeatability of 95%, adding to their validity.15 However, there is no data assessing if there is an association between volume changes after isolated orbital trauma and ocular injuries or other demographic characteristics.
Purpose: This study aims to establish if there is a relationship between the degree of volume change after isolated orbital trauma and the rates and severity of ocular injury or demographic characteristics, which is a topic not previously investigated.
Methods: A retrospective analysis of 109 isolated orbital trauma cases presenting to the maxillofacial surgery department in Christchurch, New Zealand was completed. Ocular injuries and demographic data related to the fractures were analysed against orbital volume changes calculated using OsiriX MD software.
Results: Volume change versus ocular injury analysis was not significant (P = 0.55). The average volume increase in the orbital reconstruction group was 2.17 cm3 versus 1.07 cm3 in the nonoperative group. The total rate of ocular injury was 21.1% with 10.1% sustaining severe injuries.
Conclusions: No association between the degree of volume change after isolated orbital trauma and the rate or severity of ocular injury was found. In this retrospective analysis, 47.7% of the cohort were not formally assessed by ophthalmology, potentially leading to an under-reported incidence of ocular injuries. Findings therefore correlate with those formally assessed by ophthalmology. The severe ocular injury rate of 10.1% is consistent with international literature. Demographic analysis versus volume change showed no significant relationship.
Our study aims to determine if there is a relationship, linear or nonlinear, between the degree of volume change after isolated orbital trauma and the rate and severity of ocular injury. We hypothesise
1Oral and Maxillofacial Surgery Department, Te Whatu Ora Christchurch Hospital, Christchurch, New Zealand; 2Oral and Maxillofacial Surgery Department, Auckland Hospital, Auckland, New Zealand; 3Western Health, Melbourne, VIC, Australia.
Corresponding author: Pritesh Narsinh ✉ priteshnarsinh@hotmail.com | doi: 10.63717/2025.MS0045
computed
that after isolated orbital trauma, increased orbital volumes would have lower rates of ocular injury compared with non-displaced fractures or fractures with a volume decrease. If this hypothesis is true, it may aid in streamlining ophthalmology referrals based on imaging, as we may be able to determine fracture patterns in three dimensions associated with higher rates of ocular injury. This is a topic that has not been assessed in the literature. Our secondary aims are to assess if volume changes after isolated orbital trauma have any association to ethnicity, age, mechanism of injury and fracture patterns.
A retrospective analysis of all orbital fractures that presented within the catchment of the Canterbury District Health Board (CDHB), Christchurch, New Zealand from 5 January 2018 to 5 June 2021 was undertaken using the maxillofacial surgery department’s trauma registry.
Mild: no intervention required
Chemosis
Commotio retinae
Traumatic iritis
Traumatic mydriasis
Macular haemorrhage
Iridodialysis
Moderate: non-surgical intervention required or ophthalmic follow-up
Proptosis
Corneal abrasion
Raised intraocular pressure
Ptosis/moderate lid issues requiring non-surgical treatment
Exotropia
Intra-retinal haemorrhage
Iris sphincter tear
Conjunctival laceration
As per the New Zealand Health and Disability Ethics Committees’ requirements for ethical approval, local health board institutional ethical approval was obtained for patient consent, data release and analysis as carried out in the study (approval number: RO21172).
Two hundred and eighty-four patients were identified as having sustained fractures involving orbital walls during the study period. Patients who met the following inclusion criteria were included in the study: (i) patients with isolated orbital fractures either single or multi-walled, (ii) patients aged 10 years and over, and (iii) patients with complete computer tomography (CT) imaging of both orbits. Patients were excluded if they had bilateral orbital fractures or if orbital fractures (either single or multi-walled) were associated with additional facial fractures. Of the 284 patients, 109 patients met the inclusion criteria (38.38% of the initial cohort).
Orbital fractures were subsequently classified using the Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification system into medial wall, orbital floor, orbital roof, lateral wall or combined orbital fractures.
For the 109 patients identified, clinical notes were reviewed and demographic data collected pertaining to patient gender, age, ethnicity, mechanism of injury, side of injury and fracture pattern. The presence of diplopia and ocular injuries sustained was also collected. Ophthalmic examinations were initially carried out by emergency physicians, followed by maxillofacial junior medical officers. A normal ophthalmic examination was defined as normal or symmetrical visual acuity, normal pupillary responses, symmetrical pupillary size, and free range of extraocular muscles. Any presence of orbital trauma resulted in a phone consultation with the ophthalmology department. Abnormal examinations or any concerning features on examination led to a formal ophthalmology review. This pathway of referral was
Severe: vision threatening or requiring surgical management
Orbital compartment syndrome/ lateral canthotomy
Globe rupture
Traumatic optic neuropathy
Retinal detachment
Vitreous haemorrhage
Hyphaemia
Lens dislocation
Iridodialysis/cyclodialysis
Traumatic cataract
Choroid rupture
Entrapment
Table 1. Ocular injuries categorised into mild, moderate and severe
deemed safe and appropriate at our centre but has the potential to under-report the true incidence of ocular injuries, particularly those mild in nature, as not all the patients in the cohort were reviewed by ophthalmology. Those with ocular injuries as determined by ophthalmology had their ocular injuries graded as mild, moderate or severe based on grading systems from American studies by Zhong and colleagues2, and Rossin and colleagues.16 Mild injuries were those requiring no follow-up, moderate injuries those requiring non-surgical intervention or ophthalmology follow-up, and severe injuries being vision-threatening or ocular injuries requiring urgent surgical management. Examples of graded ocular injuries are outlined in Table 1. Patients were categorised based on their most severe injury. We excluded minor ocular signs such as periorbital oedema/ ecchymosis, subconjunctival haemorrhage and infraorbital nerve paraesthesia from the mild injury category. Periorbital lacerations were also excluded.
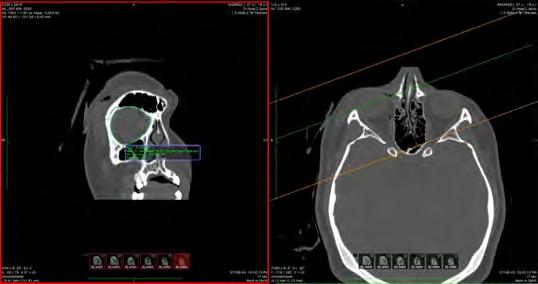
Once the retrospective analysis was completed, we measured the volumes of the left and right orbits for the 109 patients. We used the OsiriX MD software suite.17 Multi-planar reconstruction techniques were used to visualise and assess the orbital volumes. All CT scans were taken using Siemens Somatom force, drive or flash scanners. All fractures were imaged using a non-contrast scanning protocol with tube rotation time of one second, a pitch of 0.8 with 100 kV and 600 mAs. The Digital Imaging and Communications in Medicine data were procured for analysis. The axial, coronal and sagittal planes were meticulously aligned with consistent anatomical landmarks to facilitate a multi-planar reconstruction conducive to the precise anatomical segmentation of the orbit, as depicted in Figure 1
Selected landmarks for this alignment included the orbital apex, the superior aspect of the nasolacrimal duct, and the lateral wall of the orbital rim. The volumetric analysis was undertaken to calculate the volume in cubic centimetres (cm3) by multiplying the number of voxels within the region of interest by the volumetric size of each voxel.
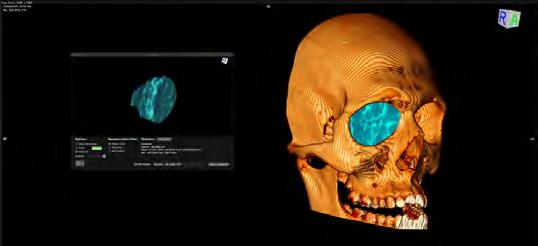
Subsequently, a three-dimensional model was generated to provide a visual representation of the volume within the osseous boundaries of the orbit, as depicted in Figure 2
To ensure uniformity in calibration and methodology, a single researcher (KT) was tasked with measuring the left and right orbital dimensions across the entire patient cohort.
Using the measured 3D volumes, orbital volume ratios (OVRs, %) were calculated using the volume of the traumatised orbit/volume of the unaffected orbit × 100.18 OVRs are proposed to allow for a more clinically accurate estimate of volume change after trauma by quantifying the percentage change in orbital volume.19 All 109 patients had volume measurements completed by orbital segmentation. All variables were analysed against volume change of the fractures to see if any association was present after isolated orbital trauma.
Analysis was completed with one-way ANOVA (with post hoc testing) with STATA 17.0 as well as sensitivity analysis using bootstrap resampling methods.
The average OVR for the 109 patients was 107.61%. As per Table 2, combined orbital floor and medial wall fractures had the largest OVR (115.45%), followed by isolated orbital floor fractures (107.3%) and isolated medial wall fractures (105.11%). The average orbital volume
Table 2. Orbital volume ratios (OVR) subclassified to fracture pattern and orbital volume increases in cm3
increase for the cohort was 1.60 cm3. The volume increase was 2.17 cm3 in the operative group versus 1.07 cm3 in the non-operative group. The OVR in the non-operative group was 105.99% and 108.3% in the operative group.
Of the cohort, 52.3% had a formal ophthalmology consultation and examination. The total rate of ocular injury was 21.1% (Table 3). Mild ocular injuries accounted for 6.4% of cases, moderate 4.6% and severe 10.1%. Volume change versus ocular injury analysis was not significant as determined by one-way ANOVA (P = 0.55). There was no appreciable relationship between volume change and ocular injury prevalence or severity. Table 3 outlines ocular injuries subcategorised into mild, moderate and severe. Of the severe isolated injuries listed in Table 3, the retrobulbar haemorrhage and muscle entrapment cases required urgent surgical intervention and the retinal detachment required urgent intraocular surgery.
Table 4 shows the relationship between ocular injuries and percentage orbital volume changes (classified into orbital volume decreases, 0–5% increases, 5–15% increases and greater than 15% increases). The 5–15% volume increase group, which included 45% of the cohort, had the highest ocular injury rates, with a 24.5% total ocular injury rate and a severe ocular injury rate of 14.3%. The lowest ocular injury rate total was in the volume decrease group at 14.3%, closely followed by the greater than 15% increase group at 16.7%. The lowest prevalence of severe ocular injury was in the 0–5% increase group at 3.6%, followed by the greater than 15% increase group at 5.5%.
As shown in Table 3, 56.9% of fractures were left-sided. Isolated orbital floor fractures made up 62.4% of cases, isolated medial wall fractures (23.9%), combined
and medial wall (10.1%), orbital
roof (2.8%) and isolated lateral wall fractures (0.9%). There was a statistically significant difference between groups as determined by one-way ANOVA (P = 0.01). A Tukey post hoc test revealed that volume change was statistically significantly higher in the combined floor and medial wall group compared with the floor alone group (8.14% ± 2.53%; one-way ANOVA, P = 0.015) or the medial wall alone group (10.3% ± 2.81%; one-way ANOVA, P = 0.00). However, there were no statistically significant differences with orbital roof and lateral wall fractures.
As per Table 3, males represented 68.8% of the cohort. The ethnic background was 67.0% New Zealand European, 19.3% Māori, 3.7% Pacific, with other groups making up the remaining 10%. The Indigenous Māori population is over-represented in the study population, as census data for the catchment area from 2018 indicated that Māori represent 9.9% of population, New Zealand Europeans 77.9%, and Pacific 3.8%.20 Volume change versus ethnicity was not significant (one-way ANOVA, P = 0.88). The mean cohort age was 41.4 years, with a range between 10 and 98 years.
The leading mechanism of injury was interpersonal violence (35.8%), followed by falls (24.8%) and sporting injuries (20.2%). Motor vehicle accidents accounted for only 4.6% of isolated orbital fractures. Volume change versus mechanism analysis was not significant (oneway ANOVA, P = 0.43).
Of the fractures, 47.7% underwent operative management with a mean time to surgery of 8.09 days. A one-way ANOVA showed a statistically significant difference in volume change between individuals who underwent surgery versus individuals who were not surgically treated. Post hoc testing revealed that individuals who had surgery had a 3.3% ± 1.5% difference in volume change compared with individuals who did not have surgery (one-way ANOVA, P < 0.03).
Diplopia on presentation occurred in 37.6% of patients, with 56.0% having no diplopia. There was no documentation for 6.4% of patients.
Volume change versus diplopia analysis was not significant (one-way ANOVA, P = 0.55). The rates of diplopia were higher in individuals requiring surgical management with 49.1% of individuals requiring orbital reconstruction having diplopia.
To account for anatomical variation between left and right orbits, a sensitivity analysis using bootstrap resampling methods was performed. This involved generating 1000 individual pseudo-datasets by resampling the original data and recalculating with models under different assumptions of variability between left and right orbits up 1%, 2.5% and 5% (equating to three bootstraps). The conclusions remained consistent across these scenarios, indicating that the observed differences in orbital volume are robust to changes in the assumed side-to-side variation.
The literature has shown that larger volume changes after orbital trauma can lead to sequalae such as enophthalmos.14,18,19 However, it is unknown if volume changes have a relationship with other factors such as ocular injuries or demographic characteristics such as ethnicity, age or mechanism of injury.
We found no statistically significant relationship between the degree of volume change after orbital trauma and the rate or severity of ocular injury. Of the cohort, 47.7% was not formally assessed by ophthalmology, potentially leading to an under-reported incidence of ocular injuries. The maxillofacial surgery department at Canterbury District Health Board has a good working relationship with the ophthalmology department, who, due to departmental constraints, are unable to see every orbital trauma patient for ocular assessment. Patients with a clinically normal eye examination are deemed at low risk of a clinically significant ocular injury and after phone consultation with ophthalmology are followed up by maxillofacial surgery alone. The standard follow-up for non-operative or operatively managed orbital fractures in the maxillofacial surgery department after initial assessment is at one, three and six weeks.
Our study acknowledges that with our retrospective analysis, the true incidence of ocular injury may be underestimated as every patient in the cohort was not assessed by ophthalmology. However, the ocular injuries potentially missed are likely to be mild in nature, as the cohort patients have multiple follow-ups with maxillofacial surgery where any concerning features would lead to an ophthalmologic referral for assessment. The moderate and severe ocular injury rates are less likely to be underestimated, but we acknowledge that the study’s findings relate to a cohort that had a formal ophthalmology examination. Any prospective analysis in this area would require all patients to undergo a formal ophthalmic examination to reduce the chance of an under-reported incidence of ocular injury.
The process of measuring orbital volumes, as well as anatomical variation between left and right orbits, can impact the accuracy of orbital volume measurements (OVMs). OVMs are often conducted by manual segmentation or planimetry, which involves outlining the orbital cavity based on known landmarks on serial slices on CT images.19 The accuracy of OVMs was assessed by comparing volume on CT images versus cadaveric analysis. The average difference between CT image and direct measurements was 0.40 ± 1.13 mL.15 The disadvantages of planimetry include the segmentation being
time intensive and that operator error or bias could occur.19 To ensure uniformity in calibration and methodology, a single researcher was tasked with measuring the left and right orbital dimensions across the entire patient cohort.
Normal anatomical variation also exists in individuals when volume is measured between the left and right orbits. Tandon and colleagues analysed 121 skulls and found an average orbital volume of 26.7 mL with an average difference of 0.8 mL or 2.9% between orbits.21 Statistical analysis using bootstrap resampling methods for our cohort indicated that the observed differences in orbital volume are robust to changes in the assumed side-to-side variation.
Our demographic analysis of gender, age and mechanism of injury had no significant association with volume change after orbital trauma. As anatomical segmentation for 3D volume measurement is time intensive, our cohort consisted of relatively small numbers. We acknowledge that this makes the study prone to Type II errors when analysing our cohorts’ lack of demographic trends. There were, however, consistent trends when comparing to local and international literature.
IPV was the leading cause of injury in our cohort while MVAs accounted for only 4.6% of isolated orbital trauma. These trends are similar to those found in the maxillofacial surgery department in Auckland, New Zealand.9 Over a five-year period of isolated orbital reconstructions, they found 83% of patients were male, 49.5% were aged between 18 and 30 years, IPV accounted for 45.6% of cases with MVAs only representing 2.9% of cases.9
In comparison to international data from New York, USA in those sustaining isolated orbital trauma, males made up 73.7% of the cohort, falls (44.1%) was the most common mechanism followed by IPV (21.5%) and MVA (19.9%).2 An epidemiological analysis of isolated orbital blowouts from Naples, Italy showed an average age of 48 years and MVA accounting for 30% of fractures versus 25% from IPV.6 In comparison to international literature, IPV accounts for a higher rate of isolated orbital trauma in New Zealand while MVA are a significantly lower cause.
Our fracture patterns followed a similar trend as Jung and colleagues who found 59.4% of fractures to be isolated orbital floor, 23.7%
medial wall and 15% had combined floor and medial wall defects in 2415 patients in South Korea.22
Our rates of ocular injury are consistent with previous literature. Severe ocular injuries are estimated to occur in 2.7–13.7% of isolated orbital trauma patients.2 The rate of severe ocular injury in our cohort was 10.1%. There was no appreciable relationship between volume change and ocular injury prevalence or severity. Zhong and colleagues had a 12.5% rate of severe ocular injuries when looking at 186 isolated orbital fracture patients in New York, USA.2 Terrill and colleagues, in Loma Linda, USA, found a 11.2% rate of major/severe ocular injury with a 2.9% rate of globe rupture.23 Our cohort had a 1.83% rate of globe rupture.
In conclusion, we found no association between the degree of volume change after isolated orbital trauma and the rate or severity of ocular injury, a topic not previously investigated. The total ocular injury rate of 21.1% and severe ocular injury rate of 10.1% are consistent with international literature. The main limitation of this retrospective analysis was that 47.7% of the cohort were not formally assessed by ophthalmology, potentially leading to an under-reported incidence of ocular injuries, particularly those mild in nature. Findings therefore correlate with those formally assessed by ophthalmology. Demographic analysis versus volume change showed no significant relationship.
Narsinh P: Lead author, literature review, data collection, data analysis, article write up.
Taneja K: Orbital volume analysis. Satheakeerthy S: Statistical analysis. Erasmus JH: Supervisor.
Abbish Kamalakkannan (BSc (Adv) (Hons I) PHD) for statistical support. This research did not receive funding from any sector; public, commercial, or not-for-profit sectors.
All authors declare that they have no conflicts of interest.
This study did not generate any original data which can be shared.
1 Chow J, Parthasarathi K, Mehanna P, et al. Primary assessment of the patient with orbital fractures should include pupillary response and visual acuity changes to detect occult major ocular injuries. J Oral Maxillofac Surg 2018; 76: 2370-2375.
2 Zhong E, Chou TY, Chaleff AJ, et al. Orbital fractures and risk factors for ocular injury. Clin Ophthalmol 2022; 16: 4153-4161.
3 Jamal B, Pfahler S, Lane K, et al. Ophthalmic injuries in patients with zygomaticomaxillary complex fractures requiring surgical repair. J Oral Maxillofac Surg 2009; 67: 986-989.
4 Brown MS, Ky W, Lisman RD. Concomitant ocular injuries with orbital fractures. J Craniomaxillofac Trauma 1999; 5: 41-48.
5 Seifert LB, Mainka T, Herrera-Vizcaino C, et al. Orbital floor fractures: epidemiology and outcomes of 1594 reconstructions. Eur J Trauma Emerg Surg 2022; 48: 1427-1436.
6 Troise S, Committeri U, Barone S, et al. Epidemiological analysis of patients with isolated blowout fractures of orbital floor: correlation between demographic characteristics and fracture area. J Craniomaxillofac Surg 2024; 52: 334-339.
7 Moore BK, Smit R, Colquhoun A, et al. Maxillofacial fractures at Waikato Hospital, New Zealand: 2004 to 2013. N Z Med J 2015; 128: 96-102.
8 Nguyen E, Lockyer J, Erasmus J, et al. Improved outcomes of orbital reconstruction with intraoperative imaging and rapid prototyping. J Oral Maxillofac Surg 2019; 77: 1211-1217.
9 Anand L, Sealey C. Orbital fractures treated in Auckland from 2010-2015: review of patient outcomes. N Z Med J 2017; 130: 21-26.
10 Buchanan J, Colquhoun A, Friedlander L, et al. Maxillofacial fractures at Waikato Hospital, New Zealand: 1989 to 2000. N Z Med J 2005; 118: U1529.
11 Iftikhar M, Canner JK, Hall L, et al. Characteristics of orbital floor fractures in the United States from 2006 to 2017. Ophthalmology 2021; 128: 463-470.
12 Burnstine M. Clinical recommendations for repair of isolated orbital floor fractures: an evidence-based analysis. Ophthalmology 2002; 109: 1207-1210.
13 Hwang K, You SH, Sohn IA. Analysis of orbital bone fractures: a 12-year study of 391 patients. J Craniofac Surg 2009; 20: 1218-1223.
14 Garcia Garcia B, Ferrer AD. Surgical indications of orbital fractures depending on the size of the fault area determined by computed tomography: a systematic review. Rev Esp Cir Oral Maxilofac 2016; 38: 42-48.
15 Diaconu SC, Dreizin D, Uluer M, et al. The validity and reliability of computed tomography orbital volume measurements. J Craniomaxillofac Surg 2017; 45: 1552-1557.
16 Rossin EJ, Szypko C, Giese I, et al. Factors associated with increased risk of serious ocular injury in the setting of orbital fracture. JAMA Ophthalmol 2021; 139: 77-83.
17 OsiriX MD [software]. Version 12.0. Pixmeo SARL. http:// www.osirix-viewer.com (viewed Dec 2023).
18 Choi SH, Kang DH, Gu JH. The correlation between the orbital volume ratio and enophthalmos in unoperated blowout fractures. Arch Plast Surg 2016; 43: 518-522.
19 Sentucq C, Schlund M, Bouet B, et al. Overview of tools for the measurement of the orbital volume and their applications to orbital surgery. J Plast Reconstr Aesthet Surg 2021; 74: 581-591.
20 Health New Zealand Te Whatu Ora. Canterbury Wellbeing Index. https://www.canterburywellbeing.org. nz/our-population/ (viewed Mar 2024).
21 Tandon R, Aljadeff L, Ji S, et al. Anatomic variability of the human orbit. J Oral Maxillofac Surg 2020; 78: 782-796.
22 Jung EH, Lee MJ, Cho BJ. The incidence and risk factors of medial and inferior orbital wall fractures in Korea: a nationwide cohort study. J Clin Med 2022; 11: 2306.
23 Terrill SB, You H, Eiseman H, et al. Review of ocular injuries in patients with orbital wall fractures: a 5-year retrospective analysis. Clin Opthalmol 2020; 14: 28372842.








Spencer S (MBBS, BDS)1; Gebauer D (BDSc, MBBS(Hons), FRACDS(OMS), Mast Clin Dent)1; Day R (MBiomedEng, BEng)2; Collier
Khadembaschi D (BDSc, MD, MPhil)3
Orbital reconstruction following facial trauma is a challenge for oral and maxillofacial surgeons. Accurate reconstruction of the bony orbital vault is predictable with careful technique assisted by fine slice computed tomography (CT) imaging and bioengineering adjuncts. The cosmetic and functional outcome is also dependent on the behaviour of the orbital soft tissues, which include fat, ligaments and the extraocular muscles. Symmetrical globe position is the desirable outcome of orbital reconstruction but may not be achieved despite adequate anatomical reconstruction of the bony orbital cavity.
It has been four decades since Bite and colleagues1 described various aetiologies for enophthalmos, each contributing to an imbalance between soft and hard tissue volumes. These aetiologies included displacement of orbital tissue from the bony orbit, tethering of the globe posteriorly by entrapped tissue, fat necrosis with decreased soft tissue volume, and posterior soft tissue fibrosis.
In the era of patient specific implants (PSIs), appreciation of the status of the orbital soft tissues is highly significant. It has been observed that the accuracy of reconstruction of orbital fractures was not significantly associated with the degree of post-operative enophthalmos.2 A review comparing PSIs to conventional implants for the reconstruction of orbital fractures3 failed to show a benefit from using PSIs when assessing post-operative diplopia, enophthalmos and orbital volume. A prospective study by Zimmerer and colleagues4 found that 4.2% of patients with adequate post-traumatic reconstruction of orbital blowout fractures had unfavourable globe positions > 2 mm, and 3.5% had both diplopia and unfavourable globe positions > 2 mm. There appears to be a process that may involve necrosis, fibrosis or atrophy, with or without ligamentous disruption, to account for cases where poorer than expected outcomes are observed.
The aims of this retrospective cohort study were to:
use automated segmental volumetric analysis software to analyse the orbital soft tissue compartments of injured and reconstructed orbits, with intact orbits as a control;
evaluate if there is a fat volume reduction in traumatised, reconstructed orbits compared with intact orbits; and
evaluate if the presence and volume of a haematoma correlates with fat volume change in traumatised, reconstructed orbits.
R (BEng(Hons))2;
Purpose: The purpose of this study was to measure orbital fat volume loss in traumatised reconstructed orbits with haematoma.
Methods: A retrospective cohort study identified subjects with orbital fractures and associated orbital haematoma managed by the oral and maxillofacial surgery service in Western Australia between January 2013 and 2023. Subjects required orbital reconstruction and post-operative computed tomography to proceed to segmental volumetric analysis. Volume change in orbital tissue compartments was analysed using a two-tailed paired t-test. A Pearson correlation test was used to assess whether a linear relationship between haematoma volume and fat volume change existed.
Results: Twenty-two subjects had orbital reconstructions with associated haematoma and underwent segmental volumetric analysis. A statistically significant orbital fat volume reduction of 1.84 mL (95% confidence interval [CI], 1.01–2.67 mL, P < 0.001) in reconstructed orbits was observed. Correspondingly, 3.07 mL (95% CI, 2.48–3.66 mL, P < 0.001) less orbital fat volume was observed in reconstructed orbits than their respective contralateral orbits on post-operative scans. No statistically significant correlation between orbital haematoma volume and orbital fat volume change was observed (P = 0.24).
Conclusions: Fractured orbits with associated orbital haematoma demonstrated a statistically significant reduction in orbital fat volume following reconstruction. This reduction in orbital fat volume did not correlate in a statistically significant manner with haematoma volume.
The primary null hypothesis is orbital fat compartment volumes of traumatised, reconstructed orbits with haematoma remain stable and comparable to the contralateral intact orbit. The secondary null hypothesis is there is no correlation between haematoma volume at the time of injury and fat volume change over time.
1Department of Oral and Maxillofacial Surgery, Royal Perth Hospital, Perth, WA, Australia; 2East Metropolitan Health Service, Perth, WA, Australia; 3Department of Oral and Maxillofacial Surgery, Fiona Stanley Hospital, Perth, WA, Australia. Corresponding author: Samuel Spencer ✉ sam.r.spencer@gmail.com | doi: 10.63717/2025.MS0054
Keywords: atrophy | fat haematoma | orbital reconstruction
Ethical approval for this retrospective cohort study was granted by the Royal Perth Hospital Human Research Ethics Committee (protocol 10.04.23, PRN RGS0000005554) and this research was carried out in accordance with the Helsinki Declaration. Operative orbital fractures at the Fiona Stanley Hospital, Fremantle Hospital and Royal Perth Hospitals were identified retrospectively by reviewing the theatre management systems for orbital reconstruction over a 10-year period from January 2013 to January 2023. Medicare item codes and operative logs maintained by the oral and maxillofacial surgery departments at each site for governance were interrogated. Subjects were excluded if they were under the age of 18 years at the time of operation. CT scans were reviewed for the presence of orbital haematoma. Subjects with operative orbital fractures with orbital haematoma and both diagnostic and post-operative CT scans proceeded to volumetric analysis.
CT data was imported into Materialise Mimics Innovation Suite (Materialise NV, Leuvin, Belgium). A sphere was manually placed to approximate the globe volume by marking three points on the globe and adjusting the sphere for size and position in three planes. The orbital rim anatomy was demarcated with the spline function. The manual identification of these landmarks was found to have good intra-observer and inter-observer reproducibility when tested on a sample of cases. Custom python scripts were used to isolate the orbital volume. The bony vault was delineated using an automated wrapping function, with parameters to capture herniated soft tissue and close small defects, including fissures and foramina, in the bony orbit. The anterior orbit was closed off with a linear wrap across the orbital rim. The volume within the wrapped bony vault and orbital rim after subtracting the globe was deemed the orbital volume (Figure 1). Using the contralateral intact orbit as a control allowed for the titration of orbital volumes over serial scans, ensuring any variation associated with CT protocol was minimised.
The various orbital tissue components of fat, muscle, blood and air were intersected by Mimics software using selected Hounsfield

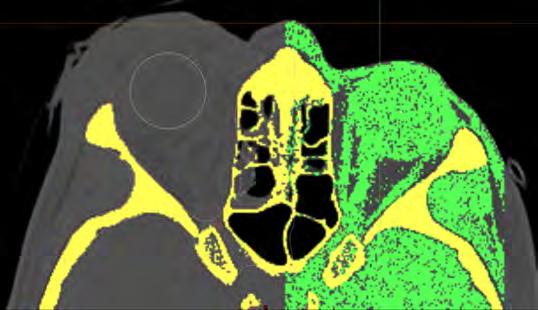
Unit thresholds (“masks”) to allow automated segmental volumetric analysis of each component per orbit (Figures 2 and 3).
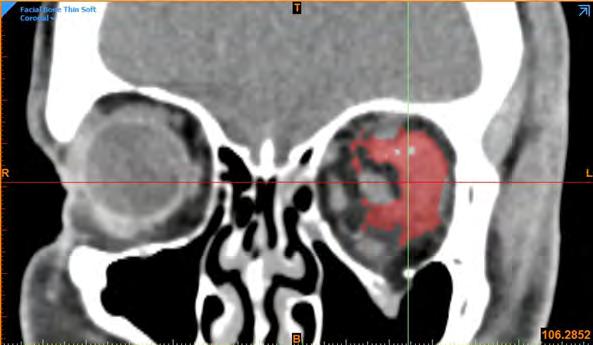
As it was not possible to reliably distinguish between haematoma and muscle on CT scan by threshold alone, the haematoma and muscle volumes were combined as a single segment for the automated volumetric analysis. Assuming no haematoma of the contralateral non-injured orbit and equal extraocular muscle volumes between orbits, orbital haematoma volume was calculated by subtracting the muscle volume of the non-injured (contralateral) orbit from the combined muscle–haematoma volume of the injured orbit. To ensure the integrity of this automated method, a random sample of haematomas was manually segmented by bioengineer RC, with a Pearson correlation coefficient finding of 0.91, representing good correlation between both methods (Figure 4).
The evolution of orbital fat volume was assessed using two methods:
The injured orbital fat volume was compared over time using serial scans, up to a period of six months post-operative. This method was limited by the possible effect of fat compartment oedema in the acute injury and post-operative phases.
The injured orbital fat volume was compared with contralateral intact orbital fat volume in a post-operative scan, up to six months post-operative.
The Statistical Package for Social Sciences version 30 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Volume change in various tissue compartments over time was analysed using a twotailed paired t-test. A Pearson correlation test was used to determine

subjects had their initial scan within two weeks (median 2 days) of injury.
Fourteen subjects had two CT scans available for serial volumetric analysis, with a mean duration between scans of 26 days. The remaining eight subjects had more than two CT scans available for serial analysis, with a mean duration between scans of 170 days. Nineteen subjects had isolated orbital fractures, whereas three were associated with non- or minimally displaced non-operative ZMC fractures. Of the pure orbital fractures, 12 involved multiple orbital walls (floor and medial wall) whereas seven were isolated orbital floor fractures. Twenty of the orbital haematomas were intraconal, whereas two subjects had both intraconal and extraconal haematomas. A summary of subject demographics and injuries is reported in Table 1
Following reconstruction, the mean difference between intact orbit and injured orbit volumes was 0.02 mL, suggesting a satisfactory reconstruction of bony vault anatomy. Mean orbital haematoma volume was 6.93 mL. As anticipated, there was a marked regression of haematoma volume within the first month.
whether a linear relationship between haematoma and fat volume change existed.
Over the study period (from 2013 to 2023), 488 subjects were identified as having had an orbital reconstruction. Of these, 25 (5.1%) subjects were found to have orbital haematoma and at least one diagnostic and post-operative scan allowing for serial orbital volumetric analysis. Two subjects had such poor quality CT scans (including thick slices > 2 mm, significant motion artifact and scatter) that accurate soft tissue segmentation was not possible and these subjects were excluded. Additionally, one subject had an associated grossly displaced zygomaticomaxillary complex (ZMC) fracture that had been previously operated with a post-operative deformity and thus was also excluded. Of the 22 remaining subjects, there were 47 CT scans available for volumetric analysis. Males accounted for 18 of the 22 subjects and the average age was 38 years. The majority of orbital fractures were caused by assaults, followed by falls and workplace injuries. At least 8 subjects underwent emergency canthotomy with cantholysis in the emergency department for signs of orbital compartment syndrome.
Eighteen of the 22 subjects had their initial CT scan on the day of injury, while the remaining four
Table 1. Subject demographics, mechanism of injury, fracture pattern and haematoma location
Orbital fat volume of the non-injured contralateral orbit did not change in a statistically significant manner across serial CT scans (P = 0.85), with a mean volume difference of only 0.04 mL (95% CI, -0.47 to 0.39 mL). Conversely, a statistically significant mean orbital fat volume reduction of 1.84 mL in the injured orbit was observed (95% CI, 1.01–2.67 mL, P < 0.001).
Twenty of the 22 injured and reconstructed orbits demonstrated a reduction in fat compartment volume on serial scans (Figure 5). An increase in fat volume, as evident in two cases, may be attributable to pronounced iatrogenic inflammation of the orbital soft tissues post-operatively, resulting in transient fat compartment oedema.
Comparison of the injured and intact orbital fat volumes in the post-operative scan revealed a mean orbital fat volume loss of 3.07 mL (95% CI, 2.48–3.66 mL, P < 0.001). Importantly, this statistically significant change was consistently demonstrated to be a relative deficit in fat volume in the reconstructed orbit, as demonstrated in Figure 6
The Pearson correlation coefficient between haematoma volume and change in orbital fat volume was 0.26 (P = 0.24), indicating no statistically significant correlation between haematoma and fat volume loss (Figure 7).
It is quite possible that what has been observed amounts to intraconal orbital fat necrosis and fibrosis in the setting of orbital haematoma. It remains unclear from this study if the orbital fat volume depletion was due to fat atrophy, necrosis, fibrosis or inadvertent fat removal during surgery.
Enophthalmos is multifactorial and varies depending on the severity and combination of the bony and soft tissue injuries. In an anatomical study,5 the interconnectedness of fat, bone, muscle and the ligamental system in the support of the globe and its position was highlighted. It was speculated that fat atrophy was not a predominant feature in most patients, but this anatomical study did describe the importance of intraconal fat in globe projection. With significant enophthalmos, there was a reduction of intraconal fat despite intact ligamentous support.
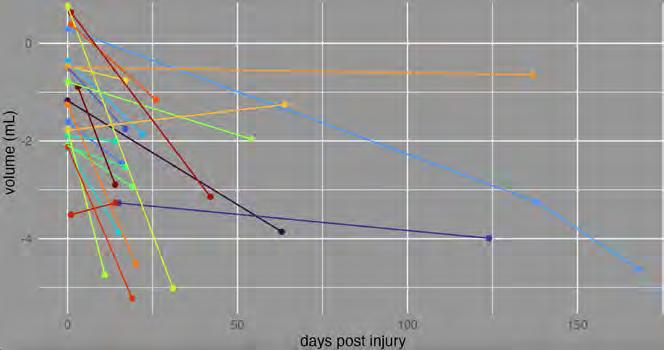

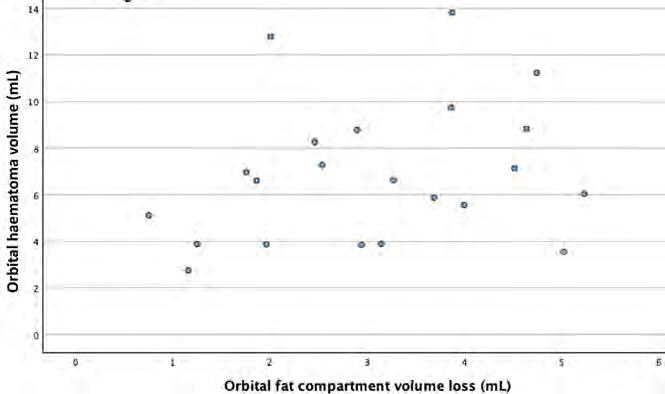
One can speculate that orbital haematomas, whether induced from the original trauma or iatrogenically created during reconstruction, will have some effect on the adjacent orbital soft tissues, including the orbital fat compartment and ligamentous system, although this is yet to be definitively demonstrated.
Retrobulbar haemorrhage is uncommon in the setting of facial trauma, with an incidence of 3.6%.6 The relationship between CT scan parameters of orbital fractures and clinical outcomes was examined in a systematic review in 2022,7 which found conflicting outcomes between studies, which were nearly all retrospective in
design and at risk of selection and/or observer bias. There was no mention of the influence of orbital haematoma on outcome.
Fat volume loss may be attributed to various aetiologies in the setting of trauma. Haematoma may cause tissue fibrosis, as shown histologically in the setting of autologous blood injections into the superior joint space and temporomandibular joint capsule in cases of recurrent temporomandibular joint dislocation.8 An alternative mechanism, referred to as traumatic fat necrosis and typically affecting the subcutaneous tissues of the shins, buttocks and thighs, occurs following sudden increases in pressure resulting in rupture of the fat microlobules and surrounding septae/blood vessels, leading to fat cell injury, hydrolysis and fibrosis.9 This process may apply to orbital fractures, whereby according to the “hydraulic theory” for blowout fractures,10 high orbital pressures are transmitted to orbital soft tissues following blunt force directed to the globe.
This study demonstrates the value of volumetric analysis software for research that will inform clinical practice. CT imagery has been used for volumetric analysis of orbital tissues for decades, with high levels of accuracy reported.11,12 The software program Mimics has been validated for calculating CT orbital soft tissue volumes, including segmentation into various soft tissue components, when a specific protocol is followed.13 Accommodation of post-traumatic orbital soft tissue changes within the reconstructive design may become the standard of care should a predictive soft tissue model be developed. Reliable soft tissue models may also stimulate research in novel targeted treatments such as orbital autologous fat injections, which have been used experimentally in rabbits14 and selectively in humans.15
As this is a retrospective study, there is some risk of bias associated with incomplete and heterogenous data. CT scan protocol and timing, for instance, was driven by clinical decisions and resource availability, and as such were not uniform. Similarly, the effects of surgery, including inflammation and bleeding, cannot be controlled when analysing volumes in operated orbits. The use of the contralateral
1 Bite U, Jackson IT, Forbes GS, et al. Orbital volume measurements in enophthalmos using three-dimensional CT imaging. Plast Reconstr Surg 1985; 75: 502-508.
2 Kim JI, Chang M. Factor influencing postoperative enophthalmos after reconstruction of orbital wall fracture. J Craniofac Surg 2022; 33: 1147-1149.
3 Maher DI, Hall AJ, Gwini S, et al. Patient-specific implants for orbital fractures: a systematic review. Ophthalmic Plast Reconstr Surg 2022; 38: 417-424.
4 Zimmerer RM, Gellrich NC, von Bulow S, et al. Is there more to the clinical outcome in posttraumatic reconstruction of the inferior and medial orbital walls than accuracy of implant placement and implant surface contouring? A prospective multicenter study to identify predictors of clinical outcome. J Craniomaxillofac Surg 2018; 46: 578-587.
5 Manson PN, Clifford CM, Su CT, et al. Mechanisms of global support and posttraumatic enophthalmos: I. The anatomy of the ligament sling and its relation to intramuscular cone orbital fat. Plast Reconstr Surg 1986; 77: 193-202.
6 Fattahi T, Brewer K, Retana A, et al. Incidence of retrobulbar hemorrhage in the emergency department. J Oral Maxillofac Surg 2014; 72: 2500-2502.
orbit as a control may introduce inaccuracy, given the known variation in volumes between left and right orbits.16,17 The statistical effect of this natural variation reduces with increasing number of subjects. Moreover, a more recent magnetic resonance imaging study involving 1453 subjects failed to find a statistically significant difference between left and right orbital volumes.18 Finally, the small sample included in the analysis reflects the rarity of operated orbital trauma in the presence of an orbital haematoma.
This retrospective cohort study used segmental volumetric analysis to demonstrate a statistically significant reduction in orbital fat volume in reconstructed, traumatised orbits in the presence of orbital haematomas. No correlation between orbital haematoma volume and orbital fat volume loss was observed. These findings support the need for a multicentre prospective study to further our understanding of the pathological changes in the traumatised orbital soft tissue compartments, the clinical significance of these changes, and assist in guiding clinical decisions.
Author contributions
Spencer S: Conceptualization, investigation, methodology, writing –original draft.
Gebauer D: Supervision, project administration, writing – review and editing.
Day R: Software, data curation, visualisation.
Collier R: Software, validation.
Khadembaschi D: Formal analysis.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Data sharing
The data underlying this article are available for sharing upon reasonable request to the corresponding author.
7 Wevers M, Strabbing EM, Engin O, et al. CT parameters in pure orbital wall fractures and their relevance in the choice of treatment and patient outcome: a systematic review. Int J Oral Maxillofac Surg 2022; 51: 782-789.
8 Gulses A, Bayar GR, Aydintug YS, et al. Histological evaluation of the changes in temporomandibular joint capsule and retrodiscal ligaments following autologous blood injection. J Craniomaxillofac Surg 2013; 41: 316320.
9 Braun-Falco OPG, Wolff HH, Burgdoft WHC. Dermatology. 2nd ed. Berlin Heidelberg New York: Springer Verlag; 2000. 867 p.
10 Pfeiffer RL. Traumatic enophthalmos. Trans Am Ophthalmol Soc 1943; 41: 293-306.
11 Deveci M, Ozturk S, Sengezer M, et al. Measurement of orbital volume by a 3-dimensional software program: an experimental study. J Oral Maxillofac Surg 2000; 58: 645-648.
12 Chepurnyi Y, Chernohorskyi D, Prykhodko D, et al. Reliability of orbital volume measurements based on computed tomography segmentation: validation of different algorithms in orbital trauma patients. J Craniomaxillofac Surg 2020; 48: 574-581.
13 Regensburg NI, Kok PH, Zonneveld FW, et al. A new and validated CT-based method for the calculation of orbital soft tissue volumes. Invest Ophthalmol Vis Sci 2008; 49: 1758-1762.
14 Cakir B, Aygit AC, Omur-Okten O, et al. Retro-orbital intraconal fat injection: an experimental study in rabbits. J Oral Maxillofac Surg 2012; 70: 242-250.
15 Sidhu N, Agrawal S, Pushker N, et al. Autologous fat transfer for orbital volume augmentation in sockets with small nonseeing eyes. J Plast Reconstr Aesthet Surg 2023; 82: 170-175.
16 Forbes G, Gehring DG, Gorman CA, et al. Volume measurements of normal orbital structures by computed tomographic analysis. AJR Am J Roentgenol 1985; 145: 149-154.
17 Parsons GS, Mathog RH. Orbital wall and volume relationships. Arch Otolaryngol Head Neck Surg 1988; 114: 743-747.
18 Erkoc MF, Oztoprak B, Gumus C, et al. Exploration of orbital and orbital soft-tissue volume changes with gender and body parameters using magnetic resonance imaging. Exp Ther Med 2015; 9: 1991-1997.
Gamage SN (MBBS, BDS, BscDent(Hons))1,2; Jensen ED (BDS, BSCDent(Hons), DClinDent(Paed))1,2; Cheng A (MBBS, BDS, FRACDS(OMS))2; Goss AN (DDSc, FRACDS(OMS))1,2; Sambrook P (MBBS, MDS, FRACDS(OMS))1,2
Osteoradionecrosis (ORN) is a condition in which there is exposed necrotic bone after radiation therapy that fails to heal within three months, in the absence of neoplastic disease and anti-resorptive therapy.1-3 Despite recent advancements in radiation therapy techniques, ORN remains among one of the most commonly encountered complications of radiotherapy to the head and neck.2 The incidence of ORN following dentoalveolar surgery ranges from 2% to 30%, and although ORN is considered a late complication of radiation therapy, the majority of cases occur within three years of treatment.4,5 The global incidence of head and neck malignancy is 5–6% and is increasing, along with the subsequent use of radiation therapy for its primary or adjuvant management.6 Therefore, the general population are at risk of an increasing incidence of ORN, despite the overall reduction in cases of ORN across the last few decades.7
Theoretically, any bone in the body can be affected by ORN, but it is predominantly diagnosed in the mandible and may very rarely occur in the maxilla and temporal bone.1,3 The oral presentation of ORN can vary from a small area of exposed bone intraorally, to a draining extraoral fistula with or without pathological fracture of the jaw. The risk of ORN is reported to be higher in the posterior mandible and if more than 60 Gy of radiation has been received.5 Pain, swelling, infection, difficulties with mouth opening and mastication are all possible sequelae, which can result in a significant impact on quality of life.4 Spontaneous ORN may occur but it more commonly follows insult to the underlying bone; such as during dentoalveolar surgery including dental extraction or implant placement, or secondary to denture irritation or periodontal disease.
Initially, ORN was primarily considered an infection. Irradiated bone was injured through traumatic events, which breached the overlying mucosa (through dentoalveolar procedures) and allowed the ingress of bacteria.8,9 Hence, treatment protocols followed the standard principles of infection management, including removal of the cause, debridement, drainage and antibiotics. Subsequent research challenged this concept and a suggestion was made that ORN was a hypocellular, hypovascular and hypoxic wound that results from radiation treatment.1 On this basis, within hypoxic
Purpose: The aim of this study was to evaluate a consecutive series of patients with osteoradionecrosis secondary to radiotherapy for head and neck cancer who were treated with hyperbaric oxygen therapy.
Methods: Patients who had osteoradionecrosis of the mandible treated with a full course of hyperbaric oxygen at the Royal Adelaide Hospital between 2008 and 2020 were identified. The patient demographics, treatment modalities and outcomes were documented.
Results: Thirty-eight individuals (67.9%) completed the full hyperbaric oxygen course. Of the individuals who completed therapy, resolution of osteoradionecrosis occurred in 68.4% at one year and 84.2% at two years after treatment.
Conclusions: This study suggests a potential benefit of hyperbaric oxygen therapy in management of osteoradionecrosis; however, the lack of a control group limits definitive conclusions. Further research is needed to establish the efficacy of hyperbaric oxygen in osteoradionecrosis treatment.
injured tissues, macrophages are not stimulated to re-organise the wound, fibroblasts fail to lay down new collagen and a chronic non-healing wound results.10 Microorganisms could be considered surface contaminants rather than the cause of the disease process.1,9 Prevention and treatment strategies, including the use of hyperbaric oxygen (HBO), were then considered.5,11 HBO was suggested to increase the oxygen tension in tissues and, as a result, reduce hypoxia, promote angiogenesis and therefore wound healing.1,5,11
The HBO treatment protocol includes 90 to 120 minutes of inhalation of 100% oxygen at pressures of 2–2.4 ATA usually daily, five to six times per week, within a hyperbaric chamber.9 The high pressure oxygen exposure leads to elevated oxygen levels in blood plasma and tissues, subsequently reducing hypoxia and stimulating angiogenesis in hypovascular tissue. Regular, periodic exposure of hypoxic tissues to high levels of oxygen have been shown to enhance leucocyte function, stimulate fibroblast growth and collagen formation, as well
1Adelaide Dental School, University of Adelaide, SA, Australia; 2Oral and Maxillofacial Surgery Unit, Royal Adelaide Hospital, SA, Australia. Corresponding author: Andrew Cheng ✉ ahacheng@hotmail.com | doi: 10.63717/2025.MS0007
Keywords: osteoradionecrosis | hyperbaric oxygen | therapeutics | mandible
Treatment phase
I. Initial treatment
II. Following phase I without adequate response
III. Following phase II without adequate response
Table
Suggested treatment
Chlorhexidine 0.2% mouth rinse
Sequestrectomy and/or debridement
Continue phase I
Systemic antibiotics
Continue phase II
Surgical intervention with or without hyperbaric oxygen therapy
for

Figure 1. Orthopantomographic images of individuals with osteoradionecrosis based on anatomical extent using the Notani classification system17: (a) stage I confined to the alveolar bone (black arrows), (b) stage II extending above (white arrows) the mandibular alveolar canal (dashed white lines) and (c) stage III diffuse involvement extending below (dashed black arrows) the level of the mandibular alveolar canal
as promote the growth of capillaries.12 These high levels of oxygen are also toxic to aerobic and anaerobic bacteria and therefore inhibit bacterial toxin formation.9 However, there are costs associated with this treatment, time requirements to complete therapy and the possibility of it leading to enhanced tumour growth or cancer recurrence.2,4
Treatment of ORN is largely based on the stage of ORN and often employs a multimodality approach using non-surgical measures for early disease with limited evidence for effectiveness (analgesia, antibiotic therapy, antiseptic mouth rinse, teriparatide, with or without local debridement and nutritional support) while reserving surgical resection and reconstruction for more advanced or refractory cases (Table 1).14-16 Staging of ORN may be based on the site-
specific response to treatment, clinical or radiographic progression of the disease or the extent of anatomical destruction. Notani and colleagues17 describe the anatomical extent of ORN and present the preferred staging classification of a number of centres due to the clear clinical and radiographic signs (Figure 1). The use of HBO therapy varies among centres, with some employing HBO for all stages of ORN, and some using it only as an adjunct in the advanced stages of ORN.
Since the early inception of HBO treatment protocols, there has been conflicting evidence in its efficacy in treatment of ORN. There is limited high quality evidence in the form of prospective, double-blinded, randomised, controlled trials,14 and prospective randomised trials,5 and ongoing concerns with poor study design
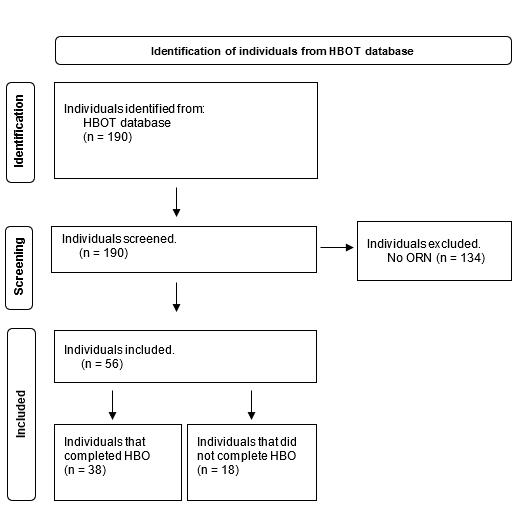
This cross-sectional audit was from January 2008 to December 2020 for individuals who received HBO therapy through the Royal Adelaide Hospital (Adelaide, South Australia) with the cohort being described previously.21 Ethical approval was received from the Central Adelaide Local Health Network HREC Ethics Committee (CALHN-HREC Ref Number 13787); however, the committee did not support a control group where HBO treatment was randomly withheld or incomplete.
The inclusion criterion was a diagnosis of ORN with a history of radiotherapy to the jaws following a diagnosis of cancer. The exclusion criterion was individuals with ongoing malignancy in the jaws. Individuals who were diagnosed with ORN received HBO treatment according to the Wolford Hall HBO therapy protocol.11 The protocol includes 30 treatments at 2.4 ATA for 90 to 120 minutes per treatment once a diagnosis of ORN was made, and 10 treatments following any surgical intervention (sequestrectomy or resection). Individuals were deemed to have not completed the HBO treatment if less than 30 sessions were completed.
(with selection, detection and performance biases), heterogeneity of classification and severity of ORN evaluated.6 Evidence indicates that there may be a slightly reduced risk of ORN with the use of prophylactic HBO use,5,9,18 but with a lack of high quality evidence and significant heterogeneity between studies. In 2012, the Royal College of Surgeons of England and the British Society for Disability and Oral Health published a clinical guideline on the prevention and management of ORN.19 This guideline recommends the use of HBO before and following dental extractions to prevent ORN. In addition, the guideline suggested that HBO may be considered for the management of advanced and severe cases of ORN.
There are currently no Australian guidelines for the prevention and management of ORN. A prospective cohort study in South Australia, Australia, evaluated the effect of HBO as prophylaxis for ORN and included the use of an HBO treatment protocol.9 Individuals who completed the prophylactic HBO treatment protocol had a 3.4% incidence of ORN whereas individuals who did not complete the protocol had a 14.2% incidence of ORN. The sample size was small but the results were in support of HBO therapy as a prophylaxis to individuals at risk of ORN. There is currently a single randomised controlled trial protocol registered in the clinical trial registry.20 There is a need for updated reporting of Australian centre protocols for HBO therapy for the management of ORN. Therefore, the aims of this study were to: (i) select individuals who had undergone HBO therapy following a diagnosis of ORN in an Australian centre, and (ii) describe each individual’s relevant lifestyle choices, ORN staging, previous treatments and treatment outcomes.
Medical records were sourced to obtain information regarding the individual’s age, gender, smoking history, alcohol use, site of malignancy, primary tumour treatment, radiation method, dentoalveolar surgery, pre-radiation dental check, severity staging of ORN (Notani’s classification),17 completion of HBO protocol and adjunctive treatments for ORN.
Statistical analysis was performed using SPSS (IBM; Version 28.0) produced by cross-tabulations. Due to the lack of a control group, comparative statistical analyses were not performed. Descriptive statistics were used to summarise the outcomes of patients who completed HBO therapy.
Independent/predictor variables were the individual’s details, including ability to complete the HBO treatment, age, gender, smoking history, alcohol history, cancer diagnosis, adjunctive surgical management and history of HBO prophylactic protocol. The dependent variable/outcome was the resolution of ORN at three months following cessation of HBO treatment based on clinical and radiographic signs as per Epstein’s clinical criteria.22
Fifty-six individuals satisfied the inclusion criteria with a diagnosis of ORN (Figure 2). The age of participants ranged from 44 to 100 years (mean 68.3 ± 10.8 years), with a greater proportion of males (44; 78.6%) (Table 2). One individual (1.8%) was taking a bone modifying medication (oral bisphosphonate). The recorded original tumour sites were 24 oral (42.9%), 20 oropharyngeal (35.7%) and 12 other (21.4%).
The management options of ORN included HBO only, sequestrectomy, surgical resection and surgical resection with free flap, with the outcomes measured at three months following HBO treatment (with or without surgical intervention) (Table 3). Thirty-eight (67.9%) individuals completed the full HBO treatment protocol. Among those who completed HBO treatment, ORN had resolved in 26 individuals (68.4%) at one year following HBO treatment (with or without surgical intervention). At two years following HBO treatment, ORN had resolved in 32 individuals (84.2%) who completed HBO treatment.
Postoperative symptoms, including pain, trismus and dysphagia, were reported in three (7.9%) individuals who completed HBO treatment. Clinical signs of infection, pathological fracture and orocutaneous fistula were reported for eight (21.1%) individuals. Primary tumour recurrence occurred in three (7.9%) individuals.
Smoking and alcohol were not significantly associated with the resolution of ORN at three months following HBO treatment (with or without surgical intervention). Binary logistic regression found age, smoking and alcohol consumption to have no significance in predicting the resolution of ORN (Table 4).
This study found that a substantial proportion of individuals who completed HBO therapy for ORN experienced resolution of the condition by two years after treatment. Our findings suggest a potential benefit of HBO therapy in ORN management, but these results should be interpreted cautiously. The absence of statistical significance testing due to study limitations means we cannot make strong claims about the superiority of HBO therapy over other treatment modalities.
Complication signs and symptoms associated with ORN were proportionally less for individuals who completed HBO in this study. Pathological fracture, infection, orocutaneous fistula, pain, trismus and dysphagia are serious complications of ORN that impact on quality of life (QOL). Although our study did not directly measure QOL, the reduction in associated symptoms of established ORN in some patients who completed HBO treatment is a notable observation. Prospective trials have found no improvement in the QOL for individuals who completed HBO prophylaxis but fewer reported acute symptoms.2 Reduction in associated symptoms of established ORN should not be overlooked as a positive outcome of HBO treatment. However, further prospective studies would benefit from direct evaluation of patient-reported QOL related to the use of HBO as a therapeutic option for ORN.
In this study, there was no significant difference between the Notani staging of ORN and the resolution of ORN at three months, one year or two years after HBO treatment. This observation suggests that HBO therapy may potentially be beneficial across different stages of ORN, but again, this finding is limited by the lack of a control group and should be interpreted with caution. Existing clinical guidelines have suggested that HBO treatment for ORN be reserved for advanced and severe cases of ORN and first-line conservative
Notani staging17
HBO = hyperbaric oxygen; ORN = osteoradionecrosis.
Table 2. Characteristics of individuals diagnosed with osteoradionecrosis
Table 3. Outcome of osteoradionecrosis after hyperbaric oxygen treatment protocol after 1 year
management and drug therapies have been recommended for less severe cases.19 A reconsideration of the aetiology of ORN to radiation-induced fibrosis from overactive fibroblasts has led to the exploration of antioxidant and antifibrosis medications.23 Significant resolution of ORN was observed using drug therapies and a protocol of pentoxifylline and tocopherol for both dental extraction prophylaxis pre-irradiation and for the treatment of ORN along with surgical debridement has been proposed.24 Clodronate (a bisphosphonate with anti-fibroblast activity) has also been added to protocols in cases of refractory ORN. These treatments were not widely available at the time of starting this study in 2008. Further studies are required to investigate the association between resolution of ORN with HBO therapy and drug therapies before robust clinical guidelines can be established. The limitations of this study, particularly the lack of a control group, highlight the need for further research in this area. Prospective, randomised, controlled trials would be ideal to establish the true efficacy of HBO therapy in ORN treatment. Such studies should also consider comparing HBO therapy to other treatment modalities, including conservative management and drug therapies such as pentoxifylline and tocopherol.
The advantages of this study were the large sample size, single HBO treatment protocol and inclusion of other adjunctive therapies including sequestrectomy, resection and free flap surgery. However, the retrospective nature of the study and the lack of a control group significantly limit the strength of our conclusions. Previous research
investigating the efficacy of HBO treatment relating to the Notani stage of ORN had smaller sample sizes of 17 and 27 individuals with ORN.25,26 Their studies provided evidence recommending HBO treatment to individuals with stage I and II ORN and when stage III had poor soft tissue peripheries. Another study used the Wilford Hall guidelines and a single protocol,11 allowing their work to be comparable to other hospital cohorts. Although the present study was retrospective and was without a control group, the information gathered from individual’s medical records was of a high standard without missing data for the independent/predictor variables. The relative contraindications for HBO treatment also include pneumothorax and chronic obstructive pulmonary disorder, claustrophobia, optic neuritis and active malignancy, which has been reported elsewhere. Although our study found a positive relationship between the completion of HBO treatment and the resolution of ORN at three months, one year and two years after treatment, we cannot definitively attribute this outcome to HBO therapy alone due to the study’s limitations. Improvement of signs and symptoms related to ORN were also observed in some patients who completed HBO treatment, but these findings should be interpreted cautiously.
In conclusion, although our study suggests potential benefits of HBO therapy in ORN management, the limitations of our methodology preclude definitive conclusions. HBO treatment for ORN may still have a place in contemporary management, particularly as prophylaxis before dentoalveolar surgery, but further well designed, prospective studies are needed to establish its true efficacy. Future research should focus on randomised controlled trials comparing HBO therapy to other treatment modalities and placebo groups, with careful consideration of patient selection, standardised treatment protocols, and long term follow up.
Author contributions
Gamage SN: Data curation, writing – original draft.
Jensen ED: Formal analysis, writing – review and editing.
Cheng A: Supervision, project administration. Goss AN: Supervision, project administration. Sambrook P: Supervision, project administration.
The authors acknowledge the patients whose clinical data were included in this study, and thank the clinical and hyperbaric unit staff at the Royal Adelaide Hospital for their role in patient care and support of this research.
Current 0.73 (0.77–0.48) 0.34
Former/unknown/no-alcohol 1.00
CI = confidence interval.
Table 4. Unadjusted association between predictor variables and osteoradionecrosis resolution
Author Alastair Goss is the Editor-in-Chief of The Australasian Journal of Oral and Maxillofacial Surgery and was not involved in any editorial decision making about this article. All other authors declare that they have no conflicts of interest.
sharing
The data underlying this article are available upon reasonable request to the corresponding author, subject to approval by the Central Adelaide Local Health Network Human Research Ethics Committee.
1 Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983; 41: 283-288.
2 Shaw RJ, Butterworth CJ, Silcocks P, et al. HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): a randomized controlled trial of hyperbaric oxygen to prevent osteoradionecrosis of the irradiated mandible after dentoalveolar surgery. Int J Radiat Oncol Biol Phys 2019; 104: 530-539.
3 McGowan K, Ivanovski S, Acton C. Osteonecrosis of the jaws: a 14‐year retrospective survey of hospital admissions. Aust Dent J 2018; 63: 202-207.
4 Kolokythas A, Rasmussen J, Reardon J, et al. Management of osteoradionecrosis of the jaws with pentoxifylline–tocopherol: a systematic review of the literature and meta-analysis. Int J Oral Maxillofac Surg 2019; 48: 173-180.
5 Marx RE, Johnson RP, Kline SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. J Am Dent Assoc 1985; 111: 49-54.
6 Forner LE, Dieleman FJ, Shaw RJ, et al. Hyperbaric oxygen treatment of mandibular osteoradionecrosis: combined data from the two randomized clinical trials DAHANCA-21 and NWHHT2009-1. Radiother Oncol 2022; 166: 137-144.
7 Lee IJ, Koom WS, Lee CG, et al. Risk factors and dose–effect relationship for mandibular osteoradionecrosis in oral and oropharyngeal cancer patients. Int J Radiat Oncol Biol Phys 2009; 75: 1084-1091.
8 Meyer I. Infectious diseases of the jaws. J Oral Surg 1970; 28: 17-26.
9 Vudiniabola S, Pirone C, Williamson J, et al. Hyperbaric oxygen in the prevention of osteoradionecrosis of the jaws. Aust Dent J 1999; 44: 243-247.
10 Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol 1987; 64: 379-390.
11 Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg 1983; 41: 351-357.
12 Knighton D, Silver I, Hunt T. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery 1981; 90: 262-270.
13 Sultan A, Hanna GJ, Margalit DN, et al. The use of hyperbaric oxygen for the prevention and management of osteoradionecrosis of the jaw: a Dana-Farber/Brigham and Women’s Cancer Center multidisciplinary guideline. Oncologist 2017; 22: 343-350.
14 Annane D, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J Clin Oncol 2004; 22: 4893-4900.
15 Camolesi GC, Ortega KL, Medina JB, et al. Therapeutic alternatives in the management of osteoradionecrosis of the jaws. Systematic review. Med Oral Patol Oral Cir Bucal 2021; 26: e195-e207.
16 Jacobson AS, Buchbinder D, Hu K, et al. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol 2010; 46: 795-801.
17 Notani Ki, Yamazaki Y, Kitada H, et al. Management of mandibular osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck 2003; 25: 181-186.
18 Sulaiman F, Huryn JM, Zlotolow IM. Dental extractions in the irradiated head and neck patient: a retrospective analysis of Memorial Sloan-Kettering Cancer Center protocols, criteria, and end results. J Oral Maxillofac Surg 2003; 61: 1123-1131.
19 Kumar N, Brooke A, Burke M, et al. The oral management of oncology patients requiring radiotherapy, chemotherapy and/or bone marrow transplantation. Faculty Dental Journal 2013; 4: 200-203.
20 Bulsara VM, Bulsara MK, Lewis E. Protocol for prospective randomised assessor-blinded pilot study comparing hyperbaric oxygen therapy with PENtoxifylline+TOcopherol±CLOdronate for the management of early osteoradionecrosis of the mandible. BMJ Open 2019; 9: e026662.
21 Dang B, Gamage S, Sethi S, et al. The role of hyperbaric oxygen in osteoradionecrosis—a prophylactic insight. Aust Dent J 2023; 68: 171-178.
22 Epstein J, Rea G, Wong FL, et al. Osteonecrosis: study of the relationship of dental extractions in patients receiving radiotherapy. Head Neck Surg 1987; 10: 48-54.
23 Delanian S, Lefaix J-L. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 2004; 73: 119-131.
24 Delanian S, Chatel C, Porcher R, et al. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherolclodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys 2011; 80: 832-839.
25 Vudiniabola S, Pirone C, Williamson J, et al. Hyperbaric oxygen in the therapeutic management of osteoradionecrosis of the facial bones. Int J Oral Maxillofac Surg 2000; 29: 435-438.
26 Dieleman F, Phan T, van den Hoogen F, et al. The efficacy of hyperbaric oxygen therapy related to the clinical stage of osteoradionecrosis of the mandible. Int J Oral Maxillofac Surg 2017; 46: 428-433.

Roberts SL (FRCS(OMFS))1,2
Orthognathic surgery, although elective, addresses profound functional and psychosocial impairments in patients with severe malocclusion and craniofacial anomalies. While not lifesaving, it is frequently life altering by improving airway function, mastication, speech, and self-perception. Yet in publicly funded health systems under persistent strain, access to this form of care is increasingly restricted, especially in New Zealand, where funding structures exacerbate inequities. Across Australasia, rising acute surgical demand — particularly for trauma, oncology, and airway-compromising infections — has intensified pressure on operating theatre availability and workforce resources. This burden has led to the recurrent cancellation or indefinite deferral of orthognathic procedures within public hospitals. Many orthognathic cases meet the threshold of “medically necessary” when defined as interventions required to restore essential function or mitigate clinically significant psychosocial distress. This distinction is central to access policies and funding eligibility in different jurisdictions. This article compares the accessibility of orthognathic surgery in Australia and New Zealand, with a focus on how structural funding models influence patient pathways. Drawing on utilisation data and ethical principles, we argue that the contrasting insurance frameworks underpin the disparity in access, affording Australian patients’ greater opportunity for
Orthognathic surgery provides substantial functional and psychosocial benefits for patients with severe dentofacial deformities. Despite its nonemergent nature, it often addresses conditions that significantly impair mastication, airway function, speech, and psychological wellbeing. This article compares access to orthognathic surgery in Australia versus New Zealand, highlighting the impact of differing health system structures and insurance frameworks on service delivery.
Australia’s hybrid public–private model allows for broader access, with private health insurance frequently covering orthognathic procedures deemed medically necessary. In contrast, New Zealand’s predominantly public system, coupled with restrictive insurance policies, results in limited availability, long delays, and reduced surgical volumes. These disparities disproportionately affect vulnerable populations, particularly Māori patients, contributing to inequities in care.
Drawing on workforce data, insurance policies and national service utilisation, we outline how funding models influence patient pathways and clinician decision making. Ethical concerns surrounding access, justice, and prioritisation frameworks are also examined. We propose targeted reforms, including insurance mandates, designated public surgical quotas, and hybrid funding initiatives to improve equitable access.
This comparative analysis underscores the need for structural reform to ensure timely, functionally justified orthognathic care across both nations.


Public hospitals in major Australasian centres (including Auckland, Wellington, Sydney and Melbourne) frequently operate at or near their theatre occupancy rates, with emergency procedures taking precedence.1,2 In high pressure environments, elective maxillofacial operations — especially those requiring multidisciplinary teams and lengthy operating times — are often the first to be delayed or cancelled.3,4 Clinicians across both nations report increasing discomfort at initiating complex orthodontic preparations, only to face indefinite surgical deferral due to these constraints (unpublished source). This disruption of care continuity undermines the multidisciplinary planning that is central to achieving successful outcomes, particularly when preoperative orthodontic phases are prolonged without definitive surgical endpoints. Orthognathic surgery has proven quality of life benefits outside of the functional and physical benefits, including psychological and wellbeing, social functioning and relationship benefits.5 Although both Australian and New Zealand systems experience capacity pressures, outcomes for patients differ markedly due to divergent funding models.
Orthognathic surgery coverage varies significantly between Australia and New Zealand due to differing private health insurance policies and their integration into each nation’s health care system. In Australia, major insurers, such as Bupa, may provide coverage for orthognathic procedures when deemed medically necessary. Bupa’s hospital and extras policies, for instance, may include such surgical interventions under specific conditions, typically requiring clinical justification beyond aesthetic reasons, such as correction of functional impairment or significant malocclusion.6 In this context, “medically necessary” is interpreted to include dysfunctions such as obstructive sleep apnoea, impaired mastication, or speech anomalies. This contrasts sharply with New Zealand, where major health insurers often classify orthognathic surgery as a dental or elective procedure and therefore exclude it from standard policy coverage. Southern Cross Health Insurance (New Zealand) outlines specific exclusions for procedures deemed dental, and while coverage is subject to eligibility criteria, orthognathic surgery is frequently not included.7,8 Similarly, New Zealand’s AA Health Insurance explicitly lists orthognathic surgery among the services not covered under its private hospital and specialist plans.9 These exclusions reflect insurers’ discretionary authority in defining procedural categories, often in the absence of unified national regulation. This regulatory vacuum contributes to inconsistencies in access, placing a disproportionate burden on clinicians to justify and advocate for care on a case-by-case basis. Government policies in Australia, including rebates and tax incentives, encourage the uptake of private insurance, expanding its reach and sustaining its role in elective surgical care. As a result, patients in Australia can more readily access orthognathic surgery through dual avenues: public provision (albeit limited) and privately insured care. In contrast, New Zealand’s health care system is predominantly publicly funded, with minimal integration of private insurance into mainstream surgical access. Only about 25% of the New Zealand population holds private health insurance, and many
policies exclude orthognathic surgery unless strictly indicated for non-cosmetic, medically necessary reasons. Even then, coverage is often partial and unpredictable. As public hospitals remain the primary avenue for such care, patients face prioritisation tools that restrict access to only the most severe cases, typically those associated with major congenital deformities, while excluding chronic, non-urgent functional or psychological impairments from eligibility. In contrast to New Zealand’s restrictive eligibility criteria for publicly funded orthognathic surgery — typically limited to patients with cleft lip and palate, severe craniofacial deformities, or significant comorbidities10 — the United Kingdom’s National Health Service (NHS) employs the Index of Orthognathic Functional Treatment Need (IOFTN) to guide access based on functional and psychosocial indicators. Patients scoring in the higher categories (grades 4 and 5) are eligible for NHS-funded care, which encompasses those with severe malocclusion, masticatory dysfunction, obstructive sleep apnoea, or significant psychological distress.11 As a result, the NHS performs about 3000 orthognathic procedures annually,12 in stark contrast to New Zealand, where national volumes are markedly lower. A 2017–18 survey reported a median of five cases per surgeon per year.13 Furthermore, New Zealand has a markedly limited number of publicly employed orthodontists, with the majority working in private practice, unlike Australia, where public sector orthodontic services are more developed.14,15 Without strategic efforts to make public orthodontic roles more appealing, including competitive remuneration and clearer career pathways, the capacity to initiate multidisciplinary orthognathic treatment planning and expand publicly funded surgical access will remain severely constrained.14,16
A 2017–18 survey of the New Zealand oral and maxillofacial surgery (OMS) workforce found orthognathic surgery among the least commonly performed procedures, with a mean of 11 cases per surgeon annually and a median of 5, serving a population of over 5 million.13 A retrospective review from the University of Otago reported only 92 cases over nine years, with a trend towards older patient cohorts and a low representation of Māori patients (5.5%), indicating potential ethnic disparities in access.17 This underrepresentation may reflect both access barriers and reduced referral rates, suggesting a systemic failure to meet the needs of Indigenous populations with historically poorer oral health outcomes.
In Australia, by contrast, a 2011 national OMS survey reported that 67% of surgeons performed orthognathic surgery, with a median annual caseload of 25 and some exceeding 100 cases.17
A 2020 orthodontic survey found usage rates of orthognathic surgery in 5% of orthodontic cases overall, rising to 10% in some states or territories.18 Australia’s larger OMS workforce and greater concentration of private practice infrastructure partly explains this, but the decisive factor appears to be the financial architecture facilitating access.
The Australian Institute of Health and Welfare (AIHW) reported 5614 orthognathic operations performed nationally in 2023–24 across both public and private sectors.19,20,21 Procedures were coded by
type of osteotomy or ostectomy, and all maxillary, mandibular, and genioplasty cases were included in the analysis. Of these, about 1800 were undertaken in the public system, with the remaining twothirds funded privately. With an estimated 200 oral and maxillofacial surgeons actively performing orthognathic surgery, this equates to a median of approximately 28 cases per surgeon annually, consistent with prior workforce survey findings.18
In contrast, New Zealand’s data are collated and published by Te Whatu Ora/Health New Zealand, which also separates public and private sector activity using comparable osteotomy, ostectomy, and genioplasty coding systems. The most recent dataset, covering July 2021 to June 2022, recorded 190 procedures in the public system and 77 in the private sector, giving a national total of 267 orthognathic cases for that year.22
The data are summarised in Table 1, which further illustrates the number of procedures performed per surgeon in Australia and New Zealand. The disparity is striking: each oral and maxillofacial surgeon in Australia performs an average of 23.3 orthognathic cases annually, compared with 5.6 cases per surgeon in New Zealand. When
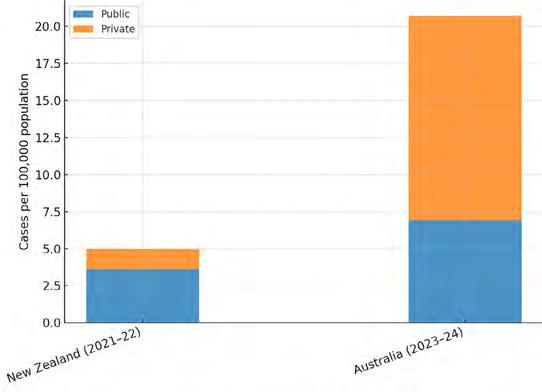
adjusted for population, the contrast remains marked, with Australia performing more than four times as many orthognathic cases per 100 000 population (Figure 1). However, these comparisons should be interpreted with caution, as the datasets are separated by two years. Given that orthognathic surgery is elective, volumes may have been influenced by temporal factors, particularly the closer proximity of the New Zealand reporting period to the COVID-19 pandemic.
Moreover, studies have demonstrated that orthognathic surgery yields substantial improvements in patient-reported outcome measures (PROMs), including enhanced quality of life, body image, and psychological wellbeing, underscoring its impact well beyond aesthetics or function.
The disparity in access between these two nations underscores critical ethical concerns. Justice in health care demands not only equal access but equitable responsiveness to suffering, particularly where chronic dysfunction and psychological harm are involved. When funding models systematically limit access to care for those unable to pay privately, and when triage systems fail to account for cumulative morbidity, an ethical deficit arises. The principle of equity is compromised, as is the dignity of patients whose conditions, although non-fatal, profoundly impair daily life. While resource allocation requires prioritisation, frameworks that overlook cumulative harm in favour of immediacy risk reinforcing cycles of neglect for chronically underserved populations. These concerns are especially acute for Indigenous communities such as Māori in New Zealand and Aboriginal and Torres Strait Islander peoples in Australia, who already face structural disadvantages in accessing specialist care. Both groups are disproportionately affected by social determinants of health and often experience barriers to referral, continuity of care, and culturally competent surgical services. In this context, the exclusion of orthognathic care further entrenches systemic inequities in health outcomes.
Mandate minimum insurance standards: in New Zealand, require private insurers to cover medically necessary orthognathic surgery, supported by clear eligibility criteria and incentives for compliance.
Designate public surgical quotas: allocate defined surgical capacity within public systems for non-urgent yet high impact procedures, including orthognathic interventions.
Incorporate ethical triage frameworks: embed long term functional and psychosocial burden into surgical prioritisation tools, ensuring equitable access beyond acute morbidity.
Implement hybrid funding models: develop co-payment vouchers or public–private partnerships that enable access to care regardless of income or insurance status.
Orthognathic surgery straddles a fault line in health system design: it is not lifesaving, but it is often life-transforming. Australia and New Zealand share comparable burdens of disease and clinical need but diverge sharply in who receives care and how. The difference lies in
1 Mackie B, Weber S, Mitchell M, et al. Chemical, biological, radiological, or nuclear response in Queensland Emergency Services: a multisite study. Health Secur 2022; 20: 222-229.
2 Wong D, Popham S, Wilson A, et al. Postoperative critical care and high‐acuity care provision in the United Kingdom, Australia, and New Zealand. Br J Anaesth 2019; 122: 460-469.
3 Chua I, Duff J, Munday J. Elective day of surgery cancellations: a retrospective observational study. Collegian 2023; 30: 721-726.
4 Zimmermann M, Nkenke E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J Craniomaxillofac Surg 2020; 48: 521-526.
5 Bär A, Meier A, Konzack O, et al. Quality of life in patients undergoing orthognathic surgery: a multidimensional survey. J Clin Med 2025; 14: 1923.
6 Bupa Australia. Packaged cover [website]. Bupa. https:// www.bupa.com.au/health-insurance/packages (viewed June 2025).
7 Southern Cross Health Society. Eligibility criteria [website]. Southern Cross. https://www.southerncross. co.nz/society/for-health-professionals/eligibility-criteria (viewed June 2025).
8 Southern Cross Health Society. Unapproved Healthcare Services. Southern Cross. https://www.southerncross. co.nz/society/for-members/making-a-claim/unapprovedhealthcare-services (viewed June 2025).
9 AA Health Insurance. Private Hospital and Specialist Cover Policy Document [Internet]. AA New Zealand; 2023 https://www.aa.co.nz/content/dam/nzaa/insurance/ health-insurance/6104871.PDF (viewed June 2025).
funding. Australia’s hybrid system facilitates access through public and private means; New Zealand’s predominantly public model too often leaves patients behind. Addressing this inequity requires not only theatre time or staffing, but structural reform rooted in ethical clarity. Whether acute or chronic, it is not the urgency of a condition alone that should guide access, but its impact. Systems that overlook this will continue to fail those most in need. Without meaningful reform, the disparity, like a shifting midline will continue to divide not just jaws, but the justice afforded to patients across the Tasman Sea.
Author contributions
Roberts SL: conception and design of the study, acquisition and interpretation of data, drafting and critical revision of the manuscript, and approval of the final version for publication.
The author declares no conflicts of interest.
The data underlying this article are available upon reasonable request to the corresponding author.
10 Goodwin-O’Neill A. Public funding criteria for orthognathic (jaw) surgery (official information act request). https://fyi.org.nz/request/3283-public-fundingcriteria-for-orthognathic-jaw-surgery (viewed Aug 2025).
11 Ireland AJ, Cunningham SJ, Petrie A, et al. An index of orthognathic functional treatment need (IOFTN). J Orthod 2014; 41: 77-83.
12 British Orthodontic Society. Statement on Orthognathic Treatment [website]. 16 Feb 2016. https://bos.org.uk/ statements/bos-statement-orthognathic-treatment/ (viewed June 2025).
13 Bridgman JB, Fulton G, Lou SM, et al. The New Zealand oral and maxillofacial surgeon workforce in 2017-18: characteristics, practice and prospects. N Z Med J 2020; 133: 11-22.
14 Dental Council of New Zealand. Workforce Analysis 2020–2022. Wellington: DCNZ, 2023. https://dcnz.org. nz/assets/Uploads/Publications/workforce-analysis/ Workforce-Analysis-2020-2022.pdf (viewed Aug 2025).
15 Australian Government, Australian Institute of Health and Welfare. Oral health and dental care in Australia: dental workforce. Canberra: AIHW, 2024. https://www. aihw.gov.au/reports/dental-oral-health/oral-healthand-dental-care-in-australia/contents/dental-workforce (viewed Aug 2025).
16 Mathew R, Sampson WJ, Spencer AJ. Orthodontic practices in Australasia: practice activity. Aust Orthod J 2005; 21: 1-10.
17 Parton AL, Tong DC, De Silva HL, et al. A nine-year review of orthognathic surgery at the University of Otago. N Z Dent J 2011; 107: 117-120.
18 Ricciardo P, Bobinskas A, Vujcich N, et al. Survey of Australasian oral and maxillofacial surgeons 2011— scope and workforce issues. Int J Oral Maxillofac Surg 2015; 44: 1569-1573.
19 Lam R, Mustac S, Goonewardene MS. A clinically based review of patient and treatment characteristics in West Australian private orthodontic practices. Australasian Orthodontic Journal 2020; 36: 9-19.
20 Australian Government, Australian Institute of Health and Welfare. Procedures and healthcare interventions (ACHI 12th edition), Australia, 2023-24. Canberra: AIHW, 2024. https://www.aihw.gov.au/reports/hospitals/proceduresdata-cubes/contents/summary (viewed Aug 2025).
21 Commonwealth of Australia, Department of Health and Aged Care. Medicare Benefits Schedule Book: Category 4 – Oral and Maxillofacial Services [Internet]. Canberra: Department of Health and Aged Care; updated 1 July 2024. https://www.mbsonline.gov. au/internet/mbsonline/publishing.nsf/Content/ B013E0789A07D708CA258B030013670A/$File/ PDF%20Version%20-%201%20July%202024%20 Category%204%20-%20Oral%20and%20 Maxillofacial%20Services.pdf (viewed Aug 2025).
22 Tūwhatu Ora – Health New Zealand. Hospital event web tool [website]. Wellington: Tūwhatu Ora – Health New Zealand, 2024.https://www.tewhatuora.govt.nz/forhealth-professionals/data-and-statistics/hospital-event/ web-tool (viewed June 2025).
23 Australian Health Practitioner Regulation Agency. Dental Board of Australia: Registrant data, 1 January 2025 to 31 March 2025. Melbourne: Ahpra, 2025. https://www. dentalboard.gov.au/About-the-Board/Statistics.aspx (viewed Aug 2025).
24 Dental Council of New Zealand. Annual report 2023/2024. Wellington (NZ): DCNZ, 2024. https://dcnz. org.nz/assets/Uploads/Publications/Annual-reports/ Dental-Council-Annual-Report-2024.pdf (viewed Aug 2025).
Mian M (BDS, MD, MPH)1; Woliansky M (BDS, MD)2; Sklavos A (MD, MPhil, BDS)1; Sreedharan S (MD)3; Kumar R (BHB, MBChB, BDS, FRACDS(OMS))1
Temporomandibular joint (TMJ) replacement (TMJR) is used to treat a wide range of pathological conditions, including trauma, inflammatory and degenerative arthritides, idiopathic condylar resorption and joint ankylosis.1 Long term outcomes for TMJR are generally favourable, with predictable improvements in diet, mouth opening and other functional limitations.2 However, TMJR incurs significant cost and is technique-sensitive. Experience with interpositional implants has demonstrated the potential harm when these surgeries are overused, and careful patient selection is paramount to successful surgical outcomes.3,4
The role of TMJ surgery, in particular joint replacement, is poorly defined and a range of surgical classifications have been proposed to assist in surgical decision making. The Research Diagnostic Criteria for Temporomandibular Disorders (RDC-TMD), widely used in epidemiological studies, emphasises the importance of psychosocial dysfunction (Axis II) alongside a physical diagnosis (Axis I).5 While helpful in the escalation of non-surgical management, the RDC-TMD provides limited clarification regarding what constitutes advanced or “end-stage” disease, and when surgical management is warranted.6
The American Academy of Orofacial Pain addresses many of the issues associated with the RDC-TMD; however, does not stratify disease severity, similarly limiting its use in guiding surgical treatment. The Wilkes classification system has been widely adopted by TMJ surgeons and escalates joint pathology in five stages to guide management.7 It focuses on internal derangement and osteoarthritic changes, with little attention to other joint pathologies. In response to the shortcomings associated with previous classification systems, Dimitroulis published a surgical classification system that divides TMJ disorders into five categories of escalating disease severity and attempts to specify the role of TMJ surgery across the spectrum of disease.6 A major shortcoming is that it makes no attempt to separate
Purpose: Temporomandibular joint (TMJ) replacement is an accepted treatment for end-stage joint disease. There are various definitions and guidelines available to identify and select patients suitable for TMJ replacement (TMJR) surgery. The aim of this systematic literature review was to assess which criteria are used to select patients undergoing TMJR surgery.
Methods: An electronic search was performed using the Preferred Reporting Items for Systematic Reviews and MetaAnalyses (PRISMA) guidelines and recommendations to identify all studies evaluating TMJR. Extracted data included bibliometric details, patient details, prostheses, primary pathology, and the criteria or justification for TMJR.
Results: A total of 10 329 patients with 13 237 TMJ prostheses were included in the study from 397 separate studies. There were 253 publications (63.7%) that included surgical criteria for TMJR. Five per cent of publications referenced existing guidelines or classification systems as part of their patient selection. There were 18 criteria identified as indications for TMJR. The most frequent criteria were post-ablative surgery (21.2%), joint ankylosis (21.2%), alterations to the fossa or condyle anatomy (10.3%), and failure of conservative management or initial surgery (6.3%).
Conclusions: The findings from this study suggest that existing publications on TMJR surgery do not use existing guidelines or classifications in their selection of cases for joint replacement. Identifying evidence-based criteria is difficult. Future studies should develop more universal criteria for TMJR case selection.
1Oral and Maxillofacial Unit, Royal Melbourne Hospital, Melbourne, VIC, Australia; 2Oral and Maxillofacial Unit, Barwon Health, Geelong, VIC, Australia; 3Department of Radiology, Royal Melbourne Hospital, Melbourne, VIC, Australia.
Corresponding author: Anton Sklavos ✉ antonsklavos@hotmail.com | doi: 10.63717/2025.MS0048
Keywords: temporomandibular joint
#1
(“replacement” OR “prosthesis” OR “alloplastic” OR “artificial joint” OR “custom” OR “personalized” OR “Concepts” OR “biomet” OR “christensen” OR “nexus” OR “omx”)
“TMJ” OR “Temporomandibular Joint” OR “TM joint”
Web of Science Core Collection
#1
(“replacement” OR “prosthesis” OR “alloplastic” OR “artificial joint” OR “custom” OR “personalized” OR “Concepts” OR “biomet” OR “christensen” OR “nexus” OR “omx”)
“TMJ” OR “Temporomandibular Joint” OR “TM joint”
Cochrane
#1 (“replacement” OR “prosthesis” OR “alloplastic” OR “artificial joint” OR “custom” OR “personalized” OR “Concepts” OR “biomet” OR “christensen” OR “nexus” OR “omx”)
#2 “TMJ” OR “Temporomandibular Joint” OR “TM joint”
#3 #1 AND #2
Embase
(“replacement” OR “prosthesis” OR “alloplastic” OR “artificial joint” OR “custom” OR “personalized” OR “Concepts” OR “biomet” OR “christensen” OR “nexus” OR “omx”)
2206 reviews, 62 protocols, 181 565 trials, 63 editorials, 48 clinical answers
Total: 183 944
13 reviews, 2197 trials
Total: 2210
1 review, 154 trials
Total: 155
(“replacement” OR “prosthesis” OR “alloplastic” OR “artificial joint” OR “custom” OR “personalized” OR “Concepts” OR “biomet” OR “christensen” OR “nexus” OR “omx”)
“TMJ” OR “Temporomandibular Joint” OR “TM joint”
TMJ disorders based on their underlying pathology, with the focus being instead on the components of the TMJ system, and which components are salvageable or non-salvageable.
Given the absence of a universally adopted surgical classification system that characterises “end-stage” disease, several guidelines have been published to provide recommendations about when total TMJR may be indicated. The British Association of Oral and Maxillofacial Surgeons has published a consensus statement on behalf of surgeons performing TMJR in the UK.4 The key components of this guideline included a prerequisite of failed conservative management, a diagnosis based on three-dimensional imaging, a disease process involving condylar bone loss and one of six indications, noting that pain alone was insufficient to justify joint replacement. These recommendations were subsequently adopted by the National Institute for Health and Care Excellence (NICE).8 Yoda and colleagues9 published similar guidelines emphasising extended resorption or defect in the condylar process of the mandible due to various pathological processes as the distinguishing findings and essential criteria for determining the indication for TMJR. A less prescriptive set of guidelines have been produced by the American College of Oral and Maxillofacial Surgeons regarding the role of surgery in the various TMJ pathologies;10 however, the role of joint replacement is not discussed.
As TMJR becomes an increasingly acceptable treatment option for end-stage disease, there is a growing imperative to define what “endstage” represents. Careful patient selection is paramount to good surgical outcomes. The aim of this systematic review was to identify the criteria applied in diagnosing patients with end-stage disease necessitating TMJR surgery.
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and recommendations.11 Electronic searches were conducted using MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, the Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science. A broad search strategy was applied to identify any article describing the implantation of an alloplastic TMJ prosthesis. Concepts were divided into “temporomandibular joint” and “replacement” linking related terms with “OR” (within each concept) and “AND” (across concepts). Filters were applied strategically to titles, abstracts and full-text fields. The search period ranged from 25 July 2023 to 20 August 2023. No time restriction was applied. Further information regarding the search strategy is provided in Table 1
All randomised controlled trials, prospective or retrospective cohort studies, case-control studies, case series and case reports published in English, reporting on total TMJR, for any indication, in one or more human subjects were included. Articles describing the use of TMJR as part of a broader treatment, for example, in combination with free
tissue transfer or extended craniofacial implants were included. Nonclinical studies, review articles, editorials, commentaries, technical notes, and published abstracts were excluded.
Two independent reviewers (MM and MW) screened titles and abstracts of all studies for eligibility using the Covidence platform. If a study was considered possibly relevant, the full text was retrieved for further evaluation. Full texts were compared against the inclusion criteria to determine eligibility. Extracted data included bibliometric information (title, authors, year, journal), patient details (number of patients, age, sex), joint details (number, stock versus custom, brand), primary pathology, and criteria (or justification) for joint replacement, whether implied or explicitly stated. Where no criteria (or justification) for joint replacement were stated, this was also recorded. In cases that were uncertain or ambiguous, a consensus decision was made in consultation with the senior author (RK). Two independent reviewers (MM and MW) individually appraised the quality of each study. The level of evidence was assessed according to the Oxford Centre for Evidence-Based Medicine System.12
Data was summarised using descriptive statistics. Where possible, logistic regression analysis was used to determine factors associated with publication of inclusion criteria. Statistical analysis was performed using R Statistical Software (version 2.14.0; R Foundation for Statistical Computing, Vienna, Austria).
A total of 6722 articles were retrieved from database searches. Thirty-four additional articles were identified through manual searches. After excluding duplicate references, 3070 titles and abstracts were reviewed. There were 891 studies identified for fulltext evaluation. Of these, 494 did not meet the inclusion criteria. In summary, 397 studies, consisting of 10 329 patients implanted with 13 237 prostheses were included. This information is summarised in Figure 1. There were 145 case reports, 109 case series, 145 cohort studies and eight cross-sectional studies included. The date of publication ranged from 1951 to 2023. The most common prostheses reported were the Biomet/Lorenz implant (n = 98; 23.9%), TMJ Concepts/Techmedica (n = 88; 21.5%) and the Christensen prosthesis (n = 17; 4.1%). Twenty-nine studies (7.1%) reported using a combination of devices. The implanted device was not specified in 33 studies (8.0%).
There were 253 full-text articles (63.7%) that included surgical criteria for TMJR, compared with 144 articles (36.3%) which did not. Case reports were the most likely to publish criteria (78.7%), followed by case series (68.0%), cohort studies (47.6%), and cross-sectional studies (37.5%). The lower rate of published criteria for cohort studies and cross-sectional studies, compared with case reports, was statistically significant (P < 0.05). Twenty articles (5.0%) referred to TMJR guidelines or disease classification systems in the selection of TMJR patients. The most common of these were the NICE guidelines8 (n = 8), followed by the Dimitroulis classification6 (n = 7), and Wilkes

classification7 (n = 5). This information is summarised in Table 2 and Table 3
The most common published criteria for TMJR were post-ablative (n = 84, 21.2%), and joint ankylosis (n = 84, 21.2%), followed by alterations in fossa or condyle anatomy (n = 41, 10.3%), failure of conservative management or other surgeries (n = 25, 6.3%), craniofacial syndromes or congenital anomalies (n = 23, 6.0%), and previous failed autogenous graft or alloplastic implant (n = 23, 6.0%). Pain was listed as a criterion in 22 cases (5.6%). Sixteen studies
Table 2. Proportion of articles specifying surgical criteria
(4.0%) required positive imaging findings. A full list of published criteria is provided in Table 4
In the current literature, only 64% of authors provide an indication for TMJR and only 5% refer to existing peerreviewed guidelines or classification systems. Case reports and case series were most likely to include surgical criteria for TMJR compared with cohort studies. Indications for TMJR were wide and varied. The most common indications for TMJR were post-ablative reconstruction (21.2%), joint ankylosis (21.2%), altered fossa or condyle anatomy (10.3%), failure of conservative management or other surgeries (6.3%), craniofacial syndrome or congenital anomaly (6.0%) and previous failed autogenous graft or alloplastic implant (6.0%). Pain was an indication in 5.6% of cases. Publications identified in this review represented Level 2b evidence or lower. Current evidence relating to the indications for TMJR is therefore lacking.
Most publications included in this study did not reference existing classifications or treatment guidelines in the decision for TMJR. Case reports were more likely to report criteria compared with cohort and cross-sectional studies. This may reflect that case series report on a smaller number of patients who may conform to specific inclusion criteria. Owing to the lack of published studies adhering to existing criteria or classifications of TMJ disease, end-stage TMJ disease as an indication for TMJR is loosely defined and there is a potential risk of overtreatment. The rise and failure of interpositional Proplast–Teflon implants in the 1980s is an important cautionary tale illustrating the dangers of overtreatment.13 In that case, hundreds of predominantly young female patients underwent discectomy and insertion of an interpositional implant for treatment of internal derangement of the TMJ.13 Most developed increasingly severe pain, condylar resorption, malocclusion and a proliferative foreign body reaction, resulting in the removal of the implant from the market in the early 1990s.13 TMJR is not without considerable risks and careful patient selection
Table 3. Study type versus inclusion of surgical criteria
is paramount to positive surgical outcomes. In this context, defining clear criteria for TMJR is an important future research goal.
This review found that post-ablative reconstruction was the most common indication for TMJR reported in the literature. In part, this is probably due to these articles being more likely to report the indication in the context of describing the primary pathology being treated. In large cohort studies where indications for TMJR are published (for example, the UK’s TMJR database) post-ablative reconstruction represented less than 1% of patients receiving alloplastic TMJR.14 In Australia, TMJR is commonly used in cases of advanced osteoarthritis. However, of the 64% of studies that reported criteria for TMJR, this was not the most common indication. Osteoarthritis may be captured by the synonymous and related terms “altered condyle and fossa anatomy” and “pain”. This may in part be due to researchers being more likely to report the indication when TMJR is used in oncology, pathology, or joint ankylosis procedures. This example serves to highlight the importance of improved reporting of TMJ criteria in the literature to reflect and inform everyday clinical practice. Existing evidence relating to TMJR for post-ablative reconstruction is limited. The largest case series of 141 patients followed up over a mean of 7.8 years was described by Marx and colleagues, reporting on a heterogenous group of patients treated for both benign and malignant pathology, involving the TMJ primarily or secondarily. They reported favourable results, with an overall complication rate of 10.6%, with six patients, all of whom were irradiated, experiencing plate exposure.15 If the TMJ is involved in the
primary pathology and preservation of the condyle is not possible, it appears that key considerations regarding reconstruction include (i) whether preservation of the articular disc is possible; (ii) previous or future radiotherapy to the area and; (iii) occlusal support and joint loading.16 In these cases, the role for alloplastic TMJR is made on a case-by-case basis. Higher risk patients may be better served with vascularised bone or composite free flap.16
Previous reviews investigating the surgical management of TMJ ankylosis have shown that alloplastic TMJR has several benefits compared with autologous reconstruction options (such as costochondral grafts), including lower pain scores, lower risk of recurrent ankylosis and lower risk of re-operation.17 Alloplastic TMJR for ankylosis in skeletally immature patients is more controversial, with understandable concerns about growth disturbance and the need for future additional surgeries.18 Emerging research in the paediatric cohort demonstrates that alloplastic TMJR is a viable treatment option, not only after failed conventional treatment, but potentially as a first-line surgical modality.19 Despite the lack of growth potential, most cases do not require secondary or revision surgery once the patient reaches skeletal maturity,19 although these conclusions are based largely on case reports and case series.
This review found that congenital craniofacial anomalies were an indication for TMJR in 6% of studies. There is a growing role for TMJR in the management of congenital craniofacial deformities, especially in combination with orthognathic surgery. Some advantages include the ability to design bespoke prostheses that meet complex anatomical requirements. With bespoke prostheses, the mandible can be advanced or vertically lengthened and the fossa component has a defined posterior stop improving surgical stability.20 Low level evidence, comparing alloplastic TMJR with autogenous grafts in patients with congenital craniofacial anomalies, suggests that alloplastic TMJR provides more stable and predictable improvements in function, aesthetics and pain reduction.20,21 However, higher level evidence is needed to inform clinical decision making, including patient selection.
Pain was listed as an indication in 5.6% of studies, always noted in addition to other indications. As noted elsewhere, pain is highly subjective and influenced by psychological and environmental factors.22 The role of pain assessment tools as criteria for TMJR remains controversial.23,24 Despite an overall mean reduction in pain scores, a high proportion of patients treated with alloplastic TMJR still have persisting moderate to severe pain post-operatively.23 Risk factors include severe pre-operative pain scores, regular opioid use and multiple previous open TMJ surgeries.24 Patients with osteoarthritis have been previously found to have higher preoperative pain scores compared with other groups. However, after controlling for pre-operative pain and other factors, indication for TMJR is not associated with post-operative pain outcomes.24
The lack of a universal classification or criteria for joint replacement described in this review is also present in the orthopaedic literature pertaining to total knee and hip replacements.25 There is a heterogeneity in the patients’ disease state at time of surgery. A consequence is that evidence-based indication criteria are lacking,
and there are limited guidelines to help in optimal timing and patient selection.25 Existing guidelines on indications for knee and hip replacement revolve around the three domains of: pain, function and radiological changes. Radiological changes are based on the Kellgren Lawrence system, as osteoarthritis is the main indication for total hip arthroplasty/total knee arthroplasty. This system enables grading of the severity of osteoarthritis, with a grade III or greater typically indicating replacement.26 Further research is required to examine thresholds for TMJ domain-specific criteria to guide timing of joint replacement.
This study includes data from over 10 000 TMJRs reported in the literature. A limitation of this study is that it does not attempt to identify which criteria or indications result in improved surgical outcomes. This was initially attempted, but given the low rate of reporting indications for TMJR in the literature, the highly heterogenous profile of publications and the absence of data within publications relating indication to outcome, this was not possible. This study utilised a broad search strategy to identify any publications that described a patient being implanted with an alloplastic TMJ implant. Studies that were investigating related but separate factors may reasonably not report the criteria or indication for TMJR, partially contributing to the low proportion of articles found to report this information. In addition, specific information regarding the jurisdiction of the studies was not included to avoid the risk of bias. Therefore, regional variations in practice were not considered. Finally, data pertaining to a single joint could potentially be counted more than once if that joint is included in multiple publications. In cases where
1 Elledge R, Mercuri LG, Attard A, et al. Review of emerging temporomandibular joint total joint replacement systems [comment]. Br J Oral Maxillofac Surg 2020; 58: 380.
2 Yaseen M, Abdulqader D, Audi K, et al. Temporomandibular total joint replacement implant devices: a systematic review of their outcomes. J Long Term Eff Med Implants 2021; 31: 91-98.
3 Kent JN, Block MS, Halpern J, et al. Update on the vitek partial and total temporomandibular joint systems. J Oral Maxillofac Surg 1993; 51: 408-415.
4 Sidebottom AJ. British Association of Oral and Maxillofacial Surgeons. Guidelines for the replacement of temporomandibular joints in the United Kingdom. Br J Oral Maxillofac Surg 2008; 46: 146-147.
5 Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014; 28: 6-27.
6 Dimitroulis G. A new surgical classification for temporomandibular joint disorders. Int J Oral Maxillofac Surg 2013; 42: 218-222.
7 Wilkes CH. Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg 1989; 115: 469-477.
8 National Institute for Health and Care Excellence. Total prosthetic replacement of the temporomandibular joint. NICE, 2014. https://www.nice.org.uk/guidance/ ipg500/resources/total-prosthetic-replacement-of-thetemporomandibular-joint-pdf-1899871678209733 (viewed Aug 2023).
9 Yoda T, Ogi N, Yoshitake H, et al. Clinical guidelines for total temporomandibular joint replacement. Jpn Dent Sci Rev 2020; 56: 77-83.
authors followed up a defined cohort, this was avoided, but in other cases, this was not possible.
There is significant heterogeneity in the criteria applied and indications for TMJR in the existing literature. A large proportion of studies do not provide any indication for TMJR and those that do often do not refer to any peer-reviewed classifications system or criteria. Future studies should look to develop a more universal list of criteria for end-stage disease to assist with decision making in TMJR surgery.
Mian M: Conceptualization, methodology, data collection, analysis, validation, draft writing, reviewing and editing.
Woliansky M: Conceptualization, methodology, data collection, analysis, validation, draft writing, reviewing and editing.
Sklavos A: Analysis, validation, draft writing, reviewing and editing.
Sreedharan S: Analysis, validation, draft writing.
Kumar R: Conceptualization, methodology, validation, supervision.
All authors declare that they have no conflicts of interest.
The data underlying this article are available for sharing upon reasonable request to the corresponding author.
10 Bouloux G, Koslin MG, Ness G, et al. Temporomandibular joint surgery. J Oral Maxillofac Surg 2017; 75(8S): e195-e223.
11 Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336-341.
12 OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence. Oxford Centre for EvidenceBased Medicine, 2011. http://www.cebm.net/index. aspx?o=5653 (viewed Aug 2023).
13 Spagnoli D, Kent JN. Multicenter evaluation of temporomandibular joint Proplast-Teflon disk implant. Oral Surg Oral Med Oral Pathol 1992; 74: 411-421.
14 Elledge R, Attard A, Green J, et al. UK temporomandibular joint replacement database: a report on one-year outcomes. Br J Oral Maxillofac Surg 2017; 55: 927-931.
15 Marx RE, Cillo JE Jr, Broumand V, et al. Outcome analysis of mandibular condylar replacements in tumor and trauma reconstruction: a prospective analysis of 131 cases with long-term follow-up. J Oral Maxillofac Surg 2008; 66: 2515-2523.
16 Bredell M, Grätz K, Obwegeser J, et al. Management of the temporomandibular joint after ablative surgery. Craniomaxillofac Trauma Reconstr 2014; 7: 271-279.
17 Al-Moraissi EA, El-Sharkawy TM, Mounair RM, et al. A systematic review and meta-analysis of the clinical outcomes for various surgical modalities in the management of temporomandibular joint ankylosis. Int J Oral Maxillofac Surg 2015; 44: 470-482.
18 Keyser BR, Banda AK, Mercuri LG, et al. Alloplastic total temporomandibular joint replacement in skeletally immature patients: a pilot survey. Int J Oral Maxillofac Surg 2020; 49: 1202-1209.
19 Sultan D, Pellecchia R, Mercuri LG. Alloplastic TMJ replacement in the skeletally immature patienta systematic review. J Craniomaxillofac Surg 2024: 52: 821-828.
20 Wolford L, Kesterke M, Harrison C. The efficacy of patientfitted total joint prostheses and orthognathic surgery to reconstruct patients with congenital craniofacial deformities and temporomandibular joint malformation: a systematic review. Frontiers Oral Maxillofac Med 2023; 5: 21.
21 Zimmerer RM, Sander AK, Schönfeld A, et al. Congenital mandibular hypoplasia: patient-specific total joint replacement as a line extension in the treatment of complex craniofacial anomalies. J Maxillofac Oral Surg 2023; 22: 410-418.
22 Xu X, Huang Y. Objective pain assessment: a key for the management of chronic pain. F1000Res 2020; 9: F1000 Faculty Rev-35.
23 Linsen SS, Teschke M, Heim N, et al. Is the risk of chronic pain after total temporomandibular joint replacement independent of its indications? A prospective cohort study. Br J Oral Maxillofac Surg 2023; 61: 337-343.
24 Gerber S, Saeed N. Predictive risk factors for persistent pain following total prosthetic temporomandibular joint replacement. Br J Oral Maxillofac Surg 2022; 60: 650-654.
25 Gademan MG, Hofstede SN, Vliet Vlieland TP, et al. Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord 2016; 17: 463.
26 Goh GS, Schwartz AM, Friend JK, et al. Patients who have Kellgren-Lawrence Grade 3 and 4 osteoarthritis benefit equally from total knee arthroplasty. J Arthroplasty 2023; 38: 1714-1717.
Liu A (BBiomed, DDS, MD, MSurg, FRACDS (GDP))1; Vu LC (BBiomed, MD)2,3; Zhao DF (BA, MD)2,4; Dimitroulis G (MDSc, PhD, FDSRCS(Eng), FFDRCS(Irel), FRACDS(OMS))5
Patients with severe temporomandibular joint (TMJ) disease often present with disabling pain, limitation in mouth opening and functional decline. This can be due to conditions such as degenerative joint disease, ankylosis or post-traumatic dysfunction. For patients with end-stage joint disease, alloplastic total temporomandibular joint replacement (TMJR) with either stock or custom prostheses is a well established and effective treatment modality.1 However, success rates and long term outcomes can be affected by complications such as heterotopic ossification (HO) and fibrosis. HO is the formation or presence of ectopic lamellar bone inside the joint apparatus where bone normally does not exist,2 which can occur following TMJ surgical procedures.3 The formation of a haematoma creates an environment that promotes the migration and differentiation of osteoblasts that deposit bone.4 This can lead to TMJ ankylosis, pain and limited mobility that may necessitate reoperation.4,5
One adjunctive technique to reduce complications in TMJR surgery is autologous fat grafting (AFG) around the prosthesis. Adipose tissue is readily accessible, abundant and cost-effective; intraoperatively, it is easy to handle and adapt to fit joint cavities of varying sizes.6 Being autologous, fat grafts are also biocompatible, with minimal graft site reactivity or immunological response that can cause rejection.7 AFG is a minimally invasive procedure that has widespread application, including aesthetic reconstruction or enhancement,8 and has been successfully used in other areas of joint reconstructive surgery.9 It has been adapted for use as an adjunct in TMJR surgery with similar benefits.4,10-18
In 1992, Thomas9 first proposed the use of AFG in hip prosthetic replacement surgery. Subsequently, Wolford and Karras4 developed the application of AFG around alloplastic TMJ prostheses. This key early study compared two small groups of
Patients with end-stage temporomandibular joint disease experience severe pain and functional limitations. Temporomandibular joint replacement is an effective treatment but may be complicated by postoperative issues such as heterotopic ossification, fibrosis and ankylosis. Autologous fat grafting serves as an adjunct to enhance outcomes by forming a biological barrier around the prosthesis.
This systematic review aims to evaluate the efficacy of autologous fat grafting in temporomandibular joint replacement surgery in improving joint function and mobility, reducing pain and preventing complications such as heterotopic ossification and fibrosis.
This review followed PRISMA guidelines, systematically searching multiple databases for English-language studies on temporomandibular joint replacement with autologous fat grafting. Data on study characteristics, patient demographics, surgical technique, type of prosthesis and outcomes measured were extracted and synthesised. Evidence quality was assessed using the GRADE framework.
The search identified ten studies involving 516 patients (753 joints). Autologous fat grafting was associated with significantly improved incisal opening, although pain reduction varied (up to 74%). Heterotopic ossification was significantly reduced or absent in most studies where autologous fat grafting was used. However, low to moderate study quality, small sample sizes and lack of control groups limited generalisability.
1Monash Health, Melbourne, VIC, Australia; 2NSW Health Pathology, Sydney, NSW, Australia; 3Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, Sydney, NSW, Australia; 4Douglas Hanly Moir Pathology, Sydney, NSW, Australia; 5MAXONIQ, Melbourne, VIC, Australia.
Corresponding author: Aimee Liu ✉ aimee.liu27@gmail.com | doi: 10.63717/2025.MS0046
Autologous fat grafting showed potential in improving functional outcomes; however, the evidence is limited by methodological weaknesses and variability in existing studies. More robust randomised control trials with larger sample sizes are required to establish the clinical value of autologous fat grafting in temporomandibular joint replacement surgery. Keywords: autologous
patients; one with AFG and the other without. The non-AFG group had a higher rate of reoperation. In 2004, Dimitroulis19 introduced the abdominal dermis-fat graft to TMJ surgery, demonstrating its safety and efficacy in treating TMJ ankylosis with a low re-ankylosis rate. In a subsequent study on 61 TMJ discectomies with AFG, Dimitroulis and colleagues20 reported improved quality of life, reduced pain and enhanced jaw function.
There are several mechanisms that contribute to the effectiveness of AFG around TMJ prostheses. Firstly, the graft acts as a barrier by filling dead space, preventing haematoma formation and reducing inflammation and fibrosis.4,19 Secondly, the graft undergoes neoadipogenesis by integrating with the local vasculature.19 In an animal study, Dimitroulis and colleagues21 demonstrated that neoadipogenesis disrupts condylar head regeneration, preventing new bone and cartilage growth, which may help to further inhibit HO. A follow-up magnetic resonance imaging study confirmed graft growth over time,22 supporting its long term integration. Finally, AFG provides a mechanical buffer, reducing shear stress and enhancing prosthesis durability under functional loads. Long term histological analysis of surgically retrieved fat grafts in TMJ discectomy patients by Dimitroulis23 demonstrated good joint cavity adaptation.
Although AFG does appear to mitigate HO and fibrosis, some patients remain susceptible, indicating that there may be other potential patient factors and surgical techniques influencing outcomes.24 Recent clinical discussions suggest variability in the use of AFG in TMJR surgery, with limited existing literature on its benefits and efficacy.24
This aim of this systematic review is to provide an overview and evaluate the current evidence on the use of AFG as an adjunct in TMJR surgery.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Eligibility criteria
The inclusion criteria encompassed retrospective case series, cohort studies, prospective studies and randomised control trials (RCTs) evaluating outcomes and complications of AFG in TMJR surgery with either custom or stock prostheses. Studies on other TMJ procedures (eg, arthroplasty, discectomy) and non-English publications were excluded.
A comprehensive literature search was conducted across PubMed, MEDLINE, Web of Science and the Cochrane Library, covering studies from database inception to October 2024. In addition, reference lists of relevant articles were screened to identify additional studies. The primary search terms included: “temporomandibular joint replacement”, “TMJ prosthesis”, “autologous fat grafting”, “fat graft”, “heterotopic ossification” and “joint function”.
Studies identified through database searches were initially screened for relevance by reviewing titles and abstracts, followed by a full-text assessment against the eligibility criteria. Two reviewers independently assessed each article for inclusion, with any discrepancies resolved through discussion with a third reviewer.
Information was collected across the following categories: study characteristics (design, sample size, patient demographics, follow-up duration), surgical techniques, prosthesis type, reported outcomes and complications.
Study results were categorised and synthesised, highlighting common themes and findings related to the use of AFG. Key outcomes analysed included improvements in maximal incisal opening (MIO), lateral excursion, pain levels, quality of life assessment, and complications including HO, nerve injuries and infection.
The included studies were evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, which evaluates evidence quality across four domains. Risk of bias was determined based on study design and randomisation. Inconsistency was evaluated by examining variability in outcomes across studies. Indirectness considered the applicability of study findings to the research question, while imprecision was measured based on sample size and follow-up duration. Each study was assigned an overall rating of high, moderate, low or very low quality, or a range between two categories.
A total of 275 studies were initially identified through electronic database searches and supplementary sources, including reference lists. After removing duplicates and irrelevant studies, 19 potentially relevant articles were retrieved for detailed screening. Ten studies, published between 1997 and 2023, met the inclusion criteria, and included 516 patients across Australia, the United States, India, the United Kingdom and China (Figure 1). These studies comprised retrospective cohort, prospective studies and case series. Sample sizes ranged from 3 to 151 patients, and mean follow-up periods ranged from 15.3 to 68 months. Most studies used custom devices (eg, TMJ Concepts, OMX), while stock prostheses (Biomet or Lorenz) were reported in three studies.13,14,18 Study characteristics, outcomes and patient demographics are summarised in Table 1. Notably, three studies by Wolford, and Wolford and colleagues contained overlapping data since 1992.4,11,12
The overall evidence quality of effectiveness of AFG in TMJR surgery was rated as low to moderate. Table 2 summarises the GRADE rating for each study. Most studies had a moderate to high risk of bias as they were often single-centre, single-surgeon, retrospective
cohort designs, and lacked control groups or randomisation. Only two studies used a prospective design, thereby reducing selection and detection biases.13,16
All studies showed consistent improvement in the objective measure of MIO, with variability in pain reduction and HO prevention.4,11,15 Most studies directly evaluated AFG within the context of TMJR, although some were specific to certain prosthetic systems (eg, TMJ Concepts, OMX, Biomet stock). Some studies examined prostheses that included AFG in the surgical protocol but not specifically isolated,16-18 contributing to indirectness. Finally, imprecision varied across studies, with small sample sizes and variable followup durations (12 to 73 months) further limiting the validity and generalisability of the findings.
Across the ten studies, a total of 516 patients and 753 joint prostheses were included. Most patients were female (84.4%), with males comprising 15.5%. Where specified, patient ages ranged from 11 to 76 years, with a weighted mean age of 42.6 years. The most common indications for TMJR surgery were degenerative joint disease and ankylosis. Osteoarthritis was the most common diagnosis, found in 80% of cases by Zhang and colleagues14 and 57.8% by Brierly and colleagues.17 Trauma was the leading cause of TMJ ankylosis,
identified in 73.2% of cases by Roychoudhury and colleagues13 and 53% by Wolford and colleagues.12 Other causes included infection (17.1%), idiopathic condylar resorption and osteoma (6.7% each), ankylosing spondylitis (2.4%) and unspecified factors (7.3%).13
Maximal incisal opening (MIO) and joint mobility
Preoperative MIO values for patients undergoing TMJR surgery ranged from 10 to 15 mm, with all studies reporting significant improvements in joint mobility. Roychoudhury and colleagues13 documented an increase from 12 mm to 35 mm in 41 patients with TMJ ankylosis, with no cases of re-ankylosis. In the only comparative retrospective cohort study, Wolford and Karras4 analysed 35 patients (baseline MIO, 26.9 mm) and found a significantly greater MIO improvement in the grafted group (38.7 mm) compared with the non-grafted group (33.1 mm) (P < 0.01). A followup study of 32 patients receiving AFG reported MIO improvement from 14.5 mm to 35 mm.12 Similarly, Dimitroulis and colleagues16 reported an MIO increase from 29.2 mm to 38.2 mm (P < 0.05) in 38 patients with custom prostheses, while a follow-up five-year retrospective study by Brierly and colleagues17 on 151 patients showed an increase from 30.8 mm to 39.1 mm (P < 0.05).
Significant reductions in TMJ pain scores, measured using visual analogue scales (VAS) were generally observed across studies. Wolford and colleagues12 reported a decrease in median TMJ pain scores from 8.0 before the surgery to 1.5 after the surgery; however, an earlier study6 found no statistically significant long term pain reduction. A prospective analysis by Mercuri and colleagues10 of 20 patients undergoing TMJR surgery with AFG reported a 52% reduction in mean pain scores. Dimitroulis and colleagues16 observed a 74.4% reduction in VAS scores (from 5.8 to 1.5), while Brierly and colleagues17 found a similar reduction from 6.14 to 0.87 (P < 0.001).
The prevalence of HO varied from 0%13 to 35% across studies. In a retrospective cohort study of 151 patients, Wolford and colleagues11 reported no cases of HO in patients that had received AFG compared with 35% in patients that had not received AFG. Another follow-up study of 115 patients by Wolford and colleagues12 found no radiographic or clinical evidence of HO at follow-up, even in patients requiring surgical prosthesis removal due to mechanical complications. Over a five-year period, Brierly and colleagues17 reported only one HO case (0.66%). In a retrospective analysis of 19 joints, Zhang and colleagues14 found no periprosthetic bone abnormalities in 17 joints (89.5%) with AFG, whereas HO was observed in the two joints (10.5%) that did not receive AFG.
et al.16
Brierly et al.17
joint disease MIO, pain (VAS), TMJ QoL *I = control group; ** = fat graft group. HO = heterotopic ossification; ICR = idiopathic condylar resorption; LE = lateral excursion; MIO = maximal incisal opening; NR = not recorded; OA = osteoarthritis; QoL = quality of life; TMJ = temporomandibular joint; TMJR = temporomandibular joint replacement; VAS = visual analogue scale.
cohort
Table 1. Study and patient characteristics
Wolford and Karras4
High
non-randomised control group
potential confounders
Mercuri et al.10
High
retrospective observational analysis
no control group
Wolford et al.11
High
large sample but retrospective design
no control group
Wolford et al.12
Roychoudhury et al.13
Moderate
retrospective design
Moderate
clear improvement in MIO
pain outcome not significantly different
Low consistent improvement in pain and MIO
Moderate
consistent improvement in MIO and jaw function, but not LE
pain not significantly different
no control group Low
Moderate
prospective study
no control group
limited controls for confounders
Zhang et al.14
High
retrospective design
no control group
Selbong et al.15
High
very small case series
Dimitroulis et al.16
Moderate
prospective design
Brierly et al.17
Carter et al.18
Low
direct evaluation of AFG in TMJR
specific to TMJ Concepts prostheses used
Low
direct evaluation of AFG as part of treatment of TMJ re-ankylosis
Low
High small sample size in both groups (35)
limited statistical power
High
small sample size (20)
limited statistical power
direct evaluation of AFG in TMJR Low
large sample size (115) adequate long term follow-up (mean 68 months) although (12–168)
consistent improvement in MIO and pain Low direct evaluation of AFG in TMJR
Low
consistent improvement in MIO
no cases of re-ankylosis or HO
Low
direct evaluation of AFG in TMJR
specific to stock prostheses
Moderate
consistent improvement in reduction in ossification in joints receiving AFG
direct evaluation of AFG in TMJR
lack of quantitative analysis
Moderate
consistent improvement in MIO
variable outcomes in pain
no control group Low
Moderate
retrospective design but large sample size
Moderate
retrospective design
potential selection and recall bias
Moderate
AFG part of protocol treating highly specific cases of recurrent HO
consistent improvements in MIO, pain and function Moderate
preliminary outcomes of custom TMJ prostheses
not direct evaluation of AFG
Low
consistent improvement MIO and pain
Low
consistent improvement in QoL, pain reduction and functional outcomes
Moderate
long term outcomes of custom TMJ prostheses
not direct evaluation of AFG
Moderate
comparison of QoL outcomes between stock custom TMJ prostheses
not direct evaluation of AFG
small sample size (32) limited statistical power
small sample size (41)
Moderate
small sample size (15)
limited statistical power
High
very small sample size (3)
no statistical analysis possible
lack of long term data Very
High
small sample size (38)
limited follow-up duration (12–24 months)
Low
large sample size (151)
adequate long term follow-up (mean 36 months)
Moderate
medium sample size (66)
minimum 24 months follow-up
Moderate
Moderate to high
Moderate
AFG = autologous fat grafting; GRADE = Grading of Recommendations Assessment, Development and Evaluation; HO = heterotopic ossification; LE = lateral excursion; MIO = maximal incisal opening; QoL = quality of life; TMJ = temporomandibular joint; TMJR = temporomandibular joint replacement.
Table 2. GRADE framework to rate overall evidence quality
Other surgical complications included nerve injury, infection, joint dislocation, mechanical failure, metal hypersensitivity and donor site morbidity. Brierly and colleagues17 reported transient temporal branch facial nerve injury in 14.6% of patients, while Roychoudhury and colleagues13 found facial nerve paresis in 9.8%, although over half of these patients had pre-existing deficits. Postoperative infections were rare, with one documented case successfully treated with surgical debridement and intravenous antibiotics.12 Condylar dislocations occurred in six patients, all within 24 hours of surgery, and were managed by reduction under general anaesthesia or intravenous sedation.13,16 Wolford and colleagues12 reported one additional case requiring light elastic traction for two weeks.
Mechanical complication rates varied significantly. Roychoudhury and colleagues13 reported no mechanical failures, whereas Wolford and colleagues12 documented high rates of device failure, including fractures and screw loosening, as well as metal hypersensitivity, necessitating the removal of 29 prostheses, although these were unrelated to AFG. Postoperative malocclusion was noted in eight cases by Roychoudhury and colleagues,13 which were managed conservatively with elastic guidance for 4 to 6 weeks. Brierly and colleagues17 reported three patients (2%) requiring revision surgery. Donor site complications included two patients (1.8%) who developed abdominal cysts requiring surgical removal, eight cases (6.9%) of seromas requiring aspiration, and two cases requiring temporary drain insertion.12
This systematic review analysed ten studies that evaluated outcomes using AFG in TMJR surgery for HO prevention and functional restoration. Findings suggest that AFG offers significant benefits, including enhanced joint mobility, pain relief and fewer postoperative complications, supporting its potential as an adjunct technique. However, despite these promising outcomes, the reliability of conclusions is limited by the quality of evidence and methodological constraints of the included studies.
Research on AFG remains limited, with no RCTs comparing its efficacy in major orthopaedic procedures such as hip or knee joint replacements. This review builds on findings of van Bogaert and colleagues25 incorporating four additional studies;13,16-18 however, issues with study design and heterogeneity persist. The available evidence is primarily based on retrospective observational studies and case series, and despite clear recommendations for RCTs, none have been conducted to date.
The extent of MIO limitation often reflects the severity of the underlying TMJ disorder. In the ten selected studies, functional recovery was evaluated through various metrics such as MIO and pain relief, with most showing significant improvements in joint mobility. However, variability may reflect differences in patient demographics (eg, age, comorbidities) and surgical techniques. Only one study (Roychoudhury and colleagues13) documented a comprehensive postoperative physiotherapy program, which plays a crucial role in optimising functional recovery. The impact
of postoperative care remains underexplored, and the absence of standardised rehabilitation protocols across studies may be a potential confounding factor.
Most patients experienced significant improvements in pain after surgery, although outcomes varied, reflecting the complex nature of pain in TMJ disorders. Factors such as preoperative pain levels, opioid use, prior TMJ procedures and surgical complications may contribute to this variability.25 In a two-year study of 50 TMJR surgeries, Dimitroulis and colleagues16 reported no device failures or infections, yet 23.7% of patients still had residual pain at rest. Similarly, in a larger follow-up study, Brierly and colleagues17 found that some patients with persistent postoperative joint pain required referral to chronic pain specialists. This variability highlights the importance of preoperative counselling to help patients develop realistic expectations regarding the potential benefits and limitations of TMJR surgery.
HO is a well recognised complication following TMJR surgery, potentially leading to ankylosis. Mercuri and colleagues10 treated 20 patients with re-ankylosis using custom TMJ replacement and AFG, demonstrating its safety and effectiveness as a management strategy. Similarly, Selbong and colleagues15 reported a case series of three patients with recurrent HO around TMJ prostheses, managed through heterotopic bone resection, prosthesis removal and re-implantation with abdominal periarticular AFG. At the 12 to 18 months follow-up, all patients had preserved mobility, further supporting the role of AFG in mitigating HO risk.
Various methods have been used to manage HO, including nonsteroidal anti-inflammatory drugs, bisphosphonates, low-dose radiation and surgical interventions.26 However, local TMJ radiation carries significant risks to adjacent structures such as the brain, middle ear and parotid gland. In orthopaedic surgery, indomethacin and etidronate have been trialled with mixed success.27,28 Although pharmacological therapy has been suggested after prosthetic TMJ reconstruction, there is no evidence to support its effectiveness. In contrast, AFG is a promising and minimally invasive option. However, to enhance reproducibility and validate its effectiveness, standardised protocols for graft harvesting and processing should be implemented.
AFG is most commonly harvested from the abdomen,4,11,12 particularly the lower region due to the abundance of fat tissue, but this method carries the risk of donor-site complications. Zhang and colleagues14 demonstrated a retro-mandibular approach, which provides a discreet incision site with minimal aesthetic concerns. Alternatively, Roychoudhury and colleagues13 used the buccal fat pad, which offers a readily available and well vascularised source of adipose tissue, potentially enhancing graft survival.
This review has several inherent limitations, including the retrospective nature of many studies and the variability in surgical techniques and prosthesis types. The high risk of bias and absence of comparison groups in most studies limits the ability to control for confounding factors and attribute outcomes solely to AFG, limiting the generalisability of findings. Small sample sizes (many with fewer than 50 patients) reduce statistical power and external validity. Publication
bias is another concern, as most studies were conducted by a small group of researchers, with limited negative data on AFG. The variable duration of follow-up, ranging from 12 to 73 months, limits the ability to assess long term outcomes such as HO recurrence and prosthesis longevity.
Future research should prioritise well designed RCTs with large sample sizes to evaluate the efficacy and safety of AFG in TMJR surgery. Ideally, multicentre studies involving multiple surgeons should be conducted to establish optimal fat grafting protocols, including graft quantity, placement, timing and integration with other surgical techniques.
This systematic review provides valuable insights into the effectiveness of AFG in TMJR surgery. Although MIO improvements were consistently observed, pain outcomes varied, and long term efficacy remains unclear. The small sample sizes in many studies, absence of RCTs and predominance of retrospective, single-centre studies increase the risk of bias, making it difficult to establish a causal link between AFG and improved outcomes. Despite its
1 Dimitroulis G. Management of temporomandibular joint disorders: a surgeon’s perspective. Aust Dent J 2018; 63: S79-90.
2 Bossche LV, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005; 37: 129-136.
3 Salman NJ, Trento GD, Carvalho PH, et al. Heterotopic ossification around temporomandibular joint prosthesis: case report and a scoping review. J Bone Res 2021; 9: 106.
4 Wolford LM, Karras SC. Autologous fat transplantation around temporomandibular joint total joint prostheses: preliminary treatment outcomes. J Oral Maxillofac Surg 1997; 55: 245-251.
5 Lindqvist C, Söderholm AL, Hallikainen D, et al. Erosion and heterotopic bone formation after alloplastic temporomandibular joint reconstruction. J Oral Maxillofac Surg 1992; 50: 942-949.
6 Dimitroulis G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int J Oral Maxillofac Surg 2011; 40: 561-568.
7 Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg 2001; 28: 111-119.
8 Guisantes E, Fontdevila J, Rodríguez G. Autologous fat grafting for correction of unaesthetic scars. Ann Plast Surg 2012; 69: 550-554.
9 Thomas BJ. Heterotopic bone formation after total hip arthroplasty. Orthop Clin North Am 1992; 23: 347 358.
10 Mercuri LG, Ali FA, Woolson R. Outcomes of total alloplastic replacement with periarticular autogenous fat grafting for management of reankylosis of the temporomandibular joint. J Oral Maxillofac Surg 2008; 66: 1794-1803.
11 Wolford LM, Morales-Ryan CA, Morales PG, Cassano DS. Autologous fat grafts placed around temporomandibular
potential as a minimally invasive adjunct, further high quality research with long term follow-up is required to confirm its effectiveness and support broader clinical implementation.
Liu AJ: Conceptualization (lead), writing – original draft (lead), formal analysis (equal), writing – review and editing (equal) – led the study design, drafted the manuscript, performed data analysis, and contributed equally to revisions.
Vu LC: Writing – review and editing (equal) – contributed to manuscript revisions and provided feedback on content.
Zhao DF: Methodology (lead), formal analysis (equal), writing – review and editing (equal) – led the methodological framework, performed data analysis, contributed equally to manuscript revisions.
Dimitroulis G: Writing – review and editing (equal) – provided critical feedback and revisions to the manuscript.
George Dimitroulis is the founder and director of MAXONIQ, which manufactures the TMJ Arthrojaw (formerly known as OMX). All other authors declare that they have no conflicts of interest.
joint total joint prostheses to prevent heterotopic bone formation. Proc (Bayl Univ Med Cent) 2008; 21:248-254.
12 Wolford L, Movahed R, Teschke M, et al. Temporomandibular joint ankylosis can be successfully treated with TMJ concepts patient-fitted total joint prosthesis and autogenous fat grafts. J Oral Maxillofac Surg 2016; 74: 1215-1227.
13 Roychoudhury A, Yadav P, Alagarsamy R, et al. Outcome of stock total joint replacement with fat grafting in adult temporomandibular joint ankylosis patients. J Oral Maxillofac Surg 2021; 79: 75-87.
14 Zhang S, Liu H, Yang C, et al. Modified surgical techniques for total alloplastic temporomandibular joint replacement: one institution’s experience. J Craniomaxillofac Surg 2015; 43: 934-939.
15 Selbong U, Rashidi R, Sidebottom A. Management of recurrent heterotopic ossification around total alloplastic temporomandibular joint replacement. Int J Oral Maxillofac Surg 2016; 45: 1234-1236.
16 Dimitroulis G, Austin S, Lee PV, et al. A new threedimensional, print-on-demand temporomandibular prosthetic total joint replacement system: preliminary outcomes. J Craniomaxillofac Surg 2018; 46: 11921198.
17 Brierly G, Thomas A, Dimitroulis G. A five-year review of the OMX temporomandibular prosthetic total joint replacement system. Oral Maxillofac Surg 2023; 27: 131-139.
18 Carter MJ, Ellis OG, Tocaciu S, et al. A 2-year comparison of quality of life outcomes between Biomet stock and OMX custom temporomandibular joint replacements. Advances Oral Maxillofac Surg 2022; 5: 100221.
19 Dimitroulis G. The interpositional dermis-fat graft in the management of temporomandibular joint ankylosis. Int J Oral Maxillofac Surg 2004; 33: 755-760.
20 Dimitroulis G, McCullough M, Morrison W. Quality-of-life survey comparing patients before and after discectomy of the temporomandibular joint. J Oral Maxillofac Surg 2010; 68: 101-106.
21 Dimitroulis G, Slavin J, Morrison W. Histological fate of abdominal dermis–fat grafts implanted in the temporomandibular joint of the rabbit following condylectomy. Int J Oral Maxillofac Surg 2011; 40: 177-183.
22 Dimitroulis G, Trost N, Morrison W. The radiological fate of dermis-fat grafts in the human temporomandibular joint using magnetic resonance imaging. Int J Oral Maxillofac Surg 2008; 37: 249-254.
23 Dimitroulis G. Macroscopic and histologic analysis of abdominal dermis-fat grafts retrieved from human temporomandibular joints. J Oral Maxillofac Surg 2011; 69: 2329-2333.
24 Gerber S, Saeed N. Predictive risk factors for persistent pain following total prosthetic temporomandibular joint replacement. Br J Oral Maxillofac Surg 2022; 60: 650-654.
25 Van Bogaert W, De Meurechy N, Mommaerts MY. Autologous fat grafting in total temporomandibular joint replacement surgery. Ann Maxillofac Surg 2018; 8: 299-302.
26 Joshi SS, Bagade SP, Gotmare PN, et al. Effectiveness of dermis fat graft versus temporalis myofascial flap for interposition in temporomandibular joint ankylosis: a systematic review. J Maxillofac Oral Surg 2023; 22: 321-328.
27 Ritter MA, Gioe TJ. The effect of indomethacin on para-articular ectopic ossification following total hip arthroplasty. Clin Orthop Relat Res 1982; 167: 113-117.
28 Francis MD, Graham R, Russell G, et al. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science 1969; 165: 1264-1266.
van Kuijk M (BDS(Hons) FRACDS(GDP))1; Singleton C (BSc, MBBS, BDS, FRACDS (OMS))1; Ke L (BDS(Hons))1; Singh T (BDS, MBChB, MPhil, FRACDS (OMS))1
A 76-year-old female patient presented to the Waikato Hospital emergency department with a three-day history of profuse intraoral bleeding. She reported approximately 1.5 L of blood loss over the preceding three days. At presentation, she was haemodynamically stable and denied having any syncopal episodes or bleeding from other sites.
The patient had a history of hereditary haemorrhagic telangiectasia (HHT), which was initially diagnosed in her 30s. She had ongoing epistaxis three to four times daily even after intranasal laser (yttrium aluminium garnet) cauterisation for nasal telangiectasias in 2010. A chest computed tomography (CT) scan, magnetic resonance imaging (MRI) of the brain and upper gastrointestinal endoscopy showed no evidence of arteriovenous malformations (AVMs). Genetic testing in 2020 confirmed a pathogenic variant in the ACVRL1 gene, which is associated with HHT. She has a strong family history with her mother, elder sister and younger brother diagnosed with HHT. She had multiple cutaneous telangiectasias (Figure 1A).
Other significant medical history included atrial fibrillation, osteoarthritis (right knee replacement), bariatric surgery, hypertension and anaemia. Her regular medications included dabigatran, digoxin, fluoxetine, frusemide, bisoprolol and iron supplementation, and she had had an adverse reaction to penicillin.
On examination there were multiple telangiectatic lesions over her anterior dorsum of the tongue that were non-pulsatile (Figure 1B). There was no blood in the nose or external auditory meatus. She was admitted under the care of the oral and maxillofacial surgery department. Initially she was managed with 1 g intravenous tranexamic acid every 6 hours as recommended by haematology, and her dabigatran was withheld. Otolaryngology performed flexible nasoendoscopy and found a small telangiectasia on the tip of the epiglottis but no active nasopharyngeal bleeding. On day two of her admission her haemoglobin level dropped from 99 to 84 g/L. She received one unit of red blood cells (235 mL) and 1 g of ferrinject (ferric carboxymaltose) in 250 mL NaCl.
Hereditary haemorrhagic telangiectasia (HHT) is a vascular disorder that results in the formation of multiple mucocutaneous arteriovenous malformations (AVMs). Clinically, it manifests as telangiectasias resulting in epistaxis, gastrointestinal bleeds and subsequent iron deficiency anaemia, along with visceral AVMs involving the lungs, liver and brain. HHT rarely presents in the oral cavity. It is inherited in an autosomal dominant pattern and commonly presents in the second decade of life with episodes of recurrent epistaxis. This case report outlines a rare presentation of a severe tongue bleed in an individual with HHT in the oral cavity and its management. With oral HHT being uncommon, treatment protocols have not been established.
A computed tomography angiogram (CTA), carotid angiogram and MRI scan of the neck found multiple enlarged abnormal appearing vessels bilaterally in the anterior third of the tongue (Figure 2A and Figure 2B). The imagery noted distention and prominent vessels of the facial veins, right parapharyngeal fat, bilateral soft palate and submucosa of the upper and lower lips.
Following initial conservative treatment with tranexamic acidsoaked gauze, applied with pressure, bleeding restarted. Discussion with interventional radiology concluded that embolisation of the vessels carried significant risk of ischaemic necrosis of the tongue. Further management options discussed with the patient included laser ablation to telangiectasias with intralesional bevacizumab injections, or formal surgical management. She opted for a more definitive solution and underwent a partial glossectomy, excising the telangiectasic lesions. This was performed by excising a wedge of 25 mm × 27 mm × 8 mm of tissue from the anterior aspect of the tongue with monopolar diathermy, and the underlying muscle was ablated with CO2 laser (Figure 3A). On the posterior aspect of the tongue, the deeper vessels were cauterised with laser followed by bipolar diathermy, then mattress-sutured with 4/0 Vicryl Rapide (Ethicon). Closure was with 4/0 Vicryl Rapide with layered mattress and interrupted sutures. Post-operatively, she was immediately extubated and transferred to the ward. She was commenced on a
1Department of Maxillofacial Surgery, Waikato Hospital, Hamilton, New Zealand. Corresponding author: Maria van Kuijk ✉ Maria.VanKuijk@healthpartners.com.au | doi: 10.63717/2025.MS0037
Keywords: hereditary haemorrhagic telangiectasia | tongue bleed
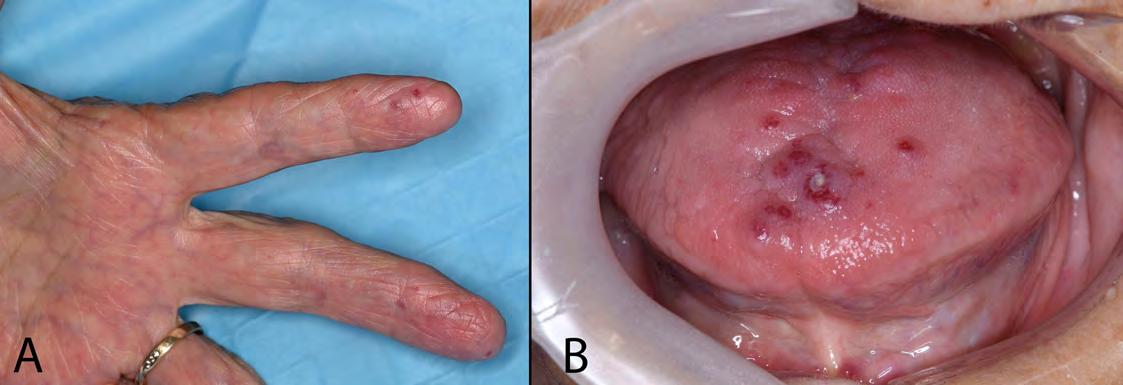
clear fluid diet, which was well tolerated and gradually upgraded as tolerated. The specimen was sent for histopathological examination, which showed large dilated vascular channels lined by flattened endothelial cells, features in keeping with a vascular malformation and the clinical diagnosis of HHT.
Post-operatively, she recovered well in hospital over the following five days. There were no further episodes of bleeding from her tongue and the surgical sites healed unremarkably. Her dabigatran was recommenced four days post-operatively. Unfortunately, her recovery was complicated by acquiring COVID-19. She was discharged on non-steroidal anti-inflammatory mouthwash and antibiotics.
The patient was reviewed in the outpatient clinic at one- and sixmonths post-operatively (Figure 3B). She had reported no further bleeding and had good tongue function, including speech and swallow with no dietary restrictions.
Hereditary haemorrhagic telangiectasia is a disorder of the vascular endothelium. It was first discovered by pathologist Henry Gawen Sutton in 1864; however, it would be another 32 years until it was distinguished from haemophilia by Henri Jules Louis Marie Rendu.1,2 It is an autosomal-dominant inherited disorder with a prevalence of 1 in 5000 to 8000.3
HHT1 has a mutation in the ENG gene (chromosome 9q33-34), HHT2 has a mutation in the ACVRL1 gene and HHT associated with juvenile polyposis has a mutation in the gene MADH4 4-6 The vessels in HHT have compromised cytoskeleton and dysfunctional remodelling of the endothelium. This results in chronically dilated vessels with poor elasticity that are susceptible to bleeding.4,7 Acute haemorrhage can be life-threatening if it occurs in a closed space (eg, cerebral AVMs), leading to organ dysfunction. Acute bleeds into open spaces such as the nasal cavity and gastrointestinal tract are better tolerated.

Anaemia occurs in approximately 50% of individuals with HHT, primarily due to chronic bleeding and depletion of iron stores.8
Telangiectasias in HHT normally develop between the ages of 20 and 30 years and increase in occurrence with age.9 Recurrent bleeding from AVMs and telangiectasias increase in severity and frequency with age.9,10 Over 93% of patients with HHT experience epistaxis with an average frequency of 18 times a month for 7.5 minutes.11 The mean age of onset is 12 years.11 The telangiectasias will blanch to pressure and promptly refill as they often present superficially on the mucosal surface. Intraoral trauma from mastication can result in bleeding for these patients.
Investigations include a carotid angiogram and MRI scan of the neck. Other investigations can include chest CT scan, brain MRI and upper gastrointestinal endoscopy to assess for other AVMs.
The Curacao diagnostic criteria for HHT were developed in 2000.12 An individual meeting three of the criteria of spontaneous epistaxis, cutaneous and mucosal telangiectasias, visceral lesions and positive family history is identified as a “definite case”. Visceral AVMs are abnormal capillary-free communications between the pulmonary and systemic circulations, which is associated with arterial hypoxaemia caused by right-to-left shunts.13
Treatment options for individuals with HHT are dependent on the location of vascular malformations and severity of bleeds and range from medical to surgical. The combination of oestrogen and progesterone helps stimulate metaplasia and increases thickness of nasal mucosa; however, this has restricted use due to hormonal therapy side effects. Tranexamic acid has been shown to reduce the duration of epistaxis.12,14 Bevacizumab is a recombinant humanised antibody that inhibits circulating vascular endothelial growth factor (VEGF), which down-regulates angiogenesis. In mouse models, increased VEGF was shown to drive telangiectasia and AVMs. Normalisation of these levels shows a decrease in formation of the AVMs in type 2 HHT.15 Thalidomide is an immunomodulatory drug
that down-regulates VEGF and improves the vascular wall; however, due to its side effects such as neuropathy it has lost popularity.16,17
A recent systematic review by Thiele and colleagues found that sclerotherapy reduced the frequency and severity of epistaxis.18
Niklasson and colleagues19 conducted a literature review of 103 articles related to oral manifestations of HHT. The overall conclusion was a clear need for higher quality research examining larger populations to establish a more robust evidence-based treatment guide for clinicians. Additionally, HHT patients with known pulmonary AVMs should be given antibiotic prophylaxis before dental procedures to minimise the risk of bacteraemia.20
Dentists and other health professionals who examine the oral cavity can aid in the early diagnosis of HHT as most telangiectasias will present in the mucosa, gingiva, palate, tongue and lips.19 Nasal telangiectasias will more often present in children; however, they may go on to develop oral telangiectasias. Oral bleeds, especially significant ones, are rare, and knowledge of treatment options is limited. Thus, documentation of the management of oral bleeds is important, particularly where the outcome has been successful.
Author contributions
van Kuijk M: Conceptualization, methodology, formal analysis, visualization, original draft.
Singleton C: Conceptualization, data curation, reviewing and editing, consultation.
Ke L: Methodology, formal analysis, original draft.
Singh T: Conceptualization, investigation, data curation, visualization, reviewing and editing, consultation.
Patient consent
The patient provided written consent for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.
1 Sutton HG. Epistaxis as an indication of impaired nutrition, and of degeneration of the vascular system. Med Mirror 1864; 1: 769-781.
2 Rendu M. Epistaxis repetes chez unsujet porteur de petits angiomies cutaneset muqueux. Bull Mem Soc Med Hop Paris 1886; 13: 731-733.
3 Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med 1999; 245: 31-39.
4 Fernández LA, Sanz-Rodriguez F, Blanco FJ, et al. Hereditary hemorrhagic telangiectasia, a vascular dysplasia affecting the TGF-beta signaling pathway. Clin Med Res 2006; 4: 66-78.
5 Jassim T, Sheng T, Zhang S, et al. Novel fusion KTN1PRKD1 in cribriform adenocarcinoma of salivary glands located in the parotid gland: case report including cytologic findings. Human Pathol: Case Reports 2021; 24: 200496.
6 Sadick H, Hage J, Goessler U, et al. Mutation analysis of “Endoglin” and “Activin receptor-like kinase” genes in German patients with hereditary hemorrhagic telangiectasia and the value of rapid genotyping using an allele-specific PCR-technique. BMC Med Genet 2009; 10: 53.
7 Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol 1990; 95: 422-427.
8 Kasthuri RS, Montifar M, Nelson J, et al. Prevalence and predictors of anemia in hereditary hemorrhagic telangiectasia. Am J Hematol 2017doi: 10.1002/ ajh.24832.
9 Plauchu H, De Chadarévian J-P, Bideau A, et al. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32: 291-297.
10 Ahamed SK, Al-Thobaiti Y. Life-threatening oral bleed—a rare presentation of hereditary hemorrhagic telangiectasia. J Oral Maxillofac Surg 2015; 73: 1465. e1-5.
11 Sami Aassar O, Friedman CM, White RI Jr. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 1991; 101: 977-980.
12 Geisthoff UW, Seyfert UT, Kübler M, et al. Treatment of epistaxis in hereditary hemorrhagic telangiectasia with tranexamic acid - a double-blind placebo-controlled cross-over phase IIIB study. Thromb Res 2014; 134: 565-571.
13 Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91: 66-67.
14 Gaillard S, Dupuis-Girod S, Boutitie F, et al. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: a European cross-over controlled trial in a rare disease. J Thromb Haemost 2014; 12: 1494-1502.
15 Han C, Choe S-W, Kim YH, et al. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014; 17: 823-830.
16 Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 2010; 16: 420-428.
17 Hosman A, Westermann CJ, Snijder R, et al. Follow-up of thalidomide treatment in patients with hereditary haemorrhagic telangiectasia. Rhinology 2015; 53: 340-344.
18 Thiele B, Abdel-Aty Y, Marks L, et al. Sclerotherapy for hereditary hemorrhagic telangiectasia-related epistaxis: a systematic review. Ann Otol Rhinol Laryngol 2023; 132: 82-90.
19 Niklasson J, Rönnblom A, Lidian A, et al. Oral manifestations and dental considerations of patients with hereditary hemorrhagic telangiectasia: a scoping review. Oral Surg Oral Med Oral Pathol Oral Radiol 2023; 136: 691-702.
20 Shovlin C, Bamford K, Sabbà C, et al. Prevention of serious infections in hereditary hemorrhagic telangiectasia: roles for prophylactic antibiotics, the pulmonary capillaries-but not vaccination. Haematologica 2019; 104: e85-e86.



Now with TiUltraTM and XealTM surfaces
‒ Designed for soft tissue health, and stability, to minimize complications.
Proven and immediate solution ‒
The graftless solution that allows immediate loading, backed by 81 studies.
‒ Excellent long-term survival rates at implant and prosthetic level, with up to 20 years’ follow up.
treatment option
‒Immediate improvement in quality of life for patients with severe maxillary bone resorption.


More than 25 years’ clinical experience1
81 Clinical studies published
2 ,700+ Patients
6,500+
Nobel Biocare implants placed
Designed for ease of placement and high primary stability, with a proven apex geometry.


