
VOLUME 6/ISSUE 8 - JULY 2019 ™ 18183 UK VOLUX Aesthetics Journal Cover Advert JUN 2019.indd 1 18/06/2019 17:18 Re-treating Dissolved Filler Dr Saleena Zimri discusses HA treatment considerations after previous dissolution Using the Yellow Card Scheme Feza Haque and Mitul Jadeja explore the importance of Yellow Card reporting Special Feature: Chemical Peel Complications Practitioners advise on adverse events following chemical peels Mr Stagnell and Ms Berridge outline botulinum toxin concerns Complications of Toxin CPD AESTHETICSBOOKAWARDS NOW!



Mr Sami Stagnell and Ms Natasha Berridge present an overview of botulinum toxin complications by anatomical region and how to avoid them 29 Case Study: Vascular Compromise
Dr Zunaid Alli shares his management of a dermal filler vascular compromise 33 Treating the Male Periorbital Area

Miss Jennifer Doyle explores the gender differences around the eye 36 Re-treating After Dissolving Fillers
Dr Saleena Zimri discusses the treatment of patients with HA filler after previous dissolution and shares three successful case studies 41 Understanding Periocular Vascular Anatomy
Dr Zoya Diwan details the vascular anatomy in the orbital and ocular area to prevent blindness following fillers 44 Advertorial: Dealing with Complaints
Aesthetics speaks to senior claims technician Emma Bracchi about how Hamilton Fraser Cosmetic Insurance supports practitioners 47 Stress and Ageing
Dermatology nurse practitioner Emma Coleman explores links between stress and skin ageing 51 Considerations for HA Dissolution
Nurse prescriber Sharon Bennett highlights the importance of filler rheology when injecting hyaluronidase 55 Abstracts
A round-up and summary of useful clinical papers IN PRACTICE 57 Utilising Online Reviews
Business coach Alan Adams advises how clinic owners can utilise positive patient reviews 61 Understanding the Yellow Card Scheme
Medical device specialist Feza Haque and special projects manager Mitul Jadeja explore the importance of Yellow Card reporting 65 Mastering Local SEO

Digital marketing consultants Adam Hampson and Lottie Staples detail how to improve local SEO to target new patients
69 In Profile: Andrew Rankin
Nurse prescriber Andrew Rankin shares his career highlights and passion for creating a credible specialty
71 The Last Word
Nurse prescriber Jodie Nightingill argues the appropriateness of taking on dermal filler complications

MSc in Skin Ageing and Aesthetic Medicine at the University of Manchester.
Ms Natasha Berridge is an oral and maxillofacial surgeon with a specialist interest in reconstructive aesthetic surgery and aesthetic medicine. She is a fellow of the Royal College of Surgeons and member of the Faculty of Dental Surgery.


Dr Zunaid Alli is an aesthetic practitioner with a background in general medicine, acute medicine and oncology. Dr Alli is also lead medical trainer for Glow Aesthetic Training and a KOL for Vivacy UK.

Miss Jennifer Doyle has a Bachelor of Medicine, a Bachelor of Surgery and a Master’s in Medical Sciences. She currently works as an NHS specialist registrar in Ophthalmology, and owns Oxford Aesthetics.
Dr Saleena Zimri is the co-founder of the Skin Doctor Clinics and set up her first aesthetic skin and laser clinic 10 years ago in Yorkshire. She is an international speaker and key opinion leader.


Dr Zoya Diwan holds a first class degree in Anatomy and Human Sciences from King’s College London. She is the medical director of Trikwan Aesthetics, chair of the Academic Aesthetics Mastermind Group and also works as an aesthetic trainer.
Emma Coleman is a dermatology and advanced aesthetic nurse practitioner. She trained in aesthetics in London in 2015 and gained a distinction in her Clinical Dermatology Diploma with the University of South Wales in 2019.
Sharon Bennett is a cosmetic nurse prescriber and runs clinics in Harrogate and London. She is a cofounder of the British Association of Cosmetic Nurses (BACN) and has completed her MSc in Non-Surgical Cosmetic Interventions at Northumbria University.

Book your Aesthetics Awards tickets now! www.aestheticsawards.com Contents • July 2019 Subscribe Free to Aesthetics Subscribe to Aesthetics, the UK’s leading free-of-charge journal for medical aesthetic professionals. Visit aestheticsjournal.com or call 0203 096 1228
Feature: Managing Chemical Peel Complications Page 17
Special
Clinical Contributors
Mr Sami Stagnell is a specialist oral surgeon. He has practised in facial aesthetics for seven years and has completed an
06 News The
12
15
NEXT MONTH • IN FOCUS: Injectables • Filler Properties • Skin Disinfection • Celebrities and Aesthetic Treatments
latest product and industry news
On the Scene Out and about in aesthetics
News Special: Irish Regulation Aesthetics looks at regulation issues in Ireland
24
CLINICAL PRACTICE 17 Special Feature: Managing Chemical Peel Complications Four practitioners discuss adverse events following chemical peel treatments, showcasing two case studies
CPD: Avoiding Toxin Complications
The Benefits of Clinisept+
+ Optimum cleansing. Clinisept+ is a highly effective cleansing solution with antimicrobial properties.

+ Optimum skin compatibility. Clinisept+ is gentle. It has a skin neutral pH and has been proven non-toxic, non-mutagenic, nonirritant to skin, non-irritant to eyes and non-cytotoxic to re-growing skin cells.
Cleans, Calms and Cares.
+ Client benefits. Clinisept+ calms and soothes the skin. It reduces the appearance of redness and swelling, and provides the optimum conditions for skin to flourish.
+ Revolutionary. Clinisept + technology is revolutionary: it contains no alcohol petroleum, lanolin, or oils and is nonsensitising, even to sensitive skin.
Clinisept delivers significant benefits in client outcomes.
“Clinisept+ has been a great substitute for chlorhexidine for me in both my injectable and laser based procedures. I have noticed reduced skin irritation and erythema with its use versus chlorhexidine both during and after treatments”
Dr. Benji Dhillon
HIGHLY COMMENDED 2017
Winner Product Innovation of the Year Highly Commended Best UK Manufacturer
Meeting the needs of your business, delivering high satisfaction to your patients Call us on 01234 313130 | info@aestheticsource.com www.aestheticsource.com

THE BARRY KNAPP AWARD FOR PRODUCT INNOVATION OF THE YEAR, SUPPORTED
GROUP
WINNER 2017
BY MEDICAL AESTHETIC
Professional Use
Accredited Now
Patient Use
Chloé Gronow Editor & Communications Manager
Welcome to the July issue! As many of you will read on p.7, we are delighted to announce that Aesthetics Media has been acquired by Easyfairs, which organises CCR. We’re really looking forward to growing the Aesthetics brand thanks to the large-scale operations and event management team at Easyfairs, as well as, of course, our team’s expertise in producing this fantastic journal, our extensive digital presence, ACE and the Aesthetics Awards. While the sun is shining and many of you take summer holidays, we shouldn’t forget the serious side of aesthetics and how important it is to be prepared for all eventualities, especially when you are away from clinic. So this month, we talk preventing and managing complications. Nurse prescriber Sharon Bennett notes that the frequency of lip filler complication reports inspired her to investigate the breakdown of various hyaluronic acid fillers with hyaluronidase – she has summarised her findings in a fascinating article on p.51. Dr Saleena Zimri has also written an insightful piece on retreating patients
Clinical Advisory Board
after dissolving filler on p.36. But of course, it’s not just lip treatments that result in complications. Throughout this issue we explore various other types of concerns that could occur. Our Special Feature highlights the various methods that can be used to prevent and manage peel complications on p.17, Dr Natasha Berridge and Dr Sami Stagnell advise how to avoid adverse events from toxin use in their excellent CPD on p.24, Dr Zunaid Alli outlines successful management of a mid-face filler complication on p.29, Dr Zoya Diwan discusses the importance of understanding periocular vascular anatomy to avoid visual loss on p.41 and nurse prescriber Jodie Nightingill shares her thoughts on taking on someone else’s adverse events in this month’s Last Word on p.71
We are also delighted to feature an extremely valuable article from the MHRA on the importance of Yellow Card Reporting following a complication. Anecdotal reports suggest that this is not done enough, so Feza Haque and Mitul Jadeja from the MHRA detail exactly what you need to know and how simple it is to do on p.61. Thank you very much to all our contributors for sharing their experiences this month –they are all so valuable to practitioners’ continued learning.
Leading figures from the medical aesthetic community have joined the Aesthetics Advisory Board to help steer the direction of our educational, clinical and business content

WE WANT TO HEAR FROM YOU!
Sharon Bennett is chair of the British Association of Cosmetic Nurses (BACN) and the UK lead on the BSI committee for aesthetic nonsurgical medical standards. She is a registered university mentor in cosmetic medicine and has completed the Northumbria University Master’s course in non-surgical cosmetic interventions.

Mr Adrian Richards is a plastic and cosmetic surgeon with 18 years’ experience. He is the clinical director of the aesthetic training provider Cosmetic Courses and surgeon at The Private Clinic. He is also member of the British Association of Plastic and Reconstructive and Aesthetic Surgeons and the British Association of Aesthetic Plastic Surgeons.
Jackie Partridge is an aesthetic nurse prescriber with a BSc in Professional Practice (Dermatology). She has recently completed her Master’s in Aesthetic Medicine, for which she is also a course mentor. Partridge is a founding board member of the British Association of Cosmetic Nurses and has represented the association for Health Improvement Scotland.
Dr Christopher Rowland Payne is a consultant dermatologist and internationally recognised expert in cosmetic dermatology. As well as being a co-founder of the European Society for Cosmetic and Aesthetic Dermatology (ESCAD), he was also the founding editor of the Journal of Cosmetic Dermatology and has authored numerous scientific papers and studies.

Dr Raj Acquilla is a cosmetic dermatologist with more than 12 years’ experience in facial aesthetic medicine. In 2015 he won the Aesthetics Award for Aesthetic Medical Practitioner of the Year and in 2012 he was named Speaker of the Year. Dr Acquilla is a UK ambassador, global KOL and masterclass trainer for botulinum toxin and dermal fillers.
Dr Tapan Patel is the founder and medical director of PHI Clinic. He has more than 16 years’ clinical experience and has been performing aesthetic treatments for more than 14 years. Recently, he was listed in Tatler’s Top 30 AntiAgeing Experts. Dr Patel is passionate about standards in aesthetic medicine.

Mr Dalvi Humzah is a consultant plastic, reconstructive and aesthetic surgeon with more than 20 years’ experience and is director of P&D Surgery. He is an international presenter, as well as the medical director and lead tutor of the multi-award-winning Dalvi Humzah Aesthetic Training courses. Mr Humzah is founding member of the Academy of Clinical Educators at the Royal College of Physicians and Surgeons of Glasgow. ARTICLE


Do you have any techniques to share, case studies to showcase or knowledge to impart?
Email editorial@aestheticsjournal.com
Dr Stefanie Williams is a dermatologist with special interest in aesthetic medicine.



She is the founder and medical director of the multi-award winning EUDELO Dermatology & Skin Wellbeing in London. She lectures in the Division of Cosmetic Science and has published more than 100 scientific articles, book chapters and abstracts.
Dr Souphiyeh Samizadeh is a dental surgeon with a Master’s degree in Aesthetic Medicine and a PGCert in Clinical Education. She is the clinical director of Revivify London, an honorary clinical teacher at King’s College London and a visiting associate professor at Shanghai Jiao Tong University.

PDFs AND REPRO
contact@aestheticsjournal.com
T: 0207 148 1292 M: 07557 359 257
megan@aestheticsjournal.com
CUSTOMER LIAISON
Chloe Carville • Customer Liaison Executive
T: 0203 096 1228 | contact@aestheticsjournal.com
Lian Graham • Customer Liaison Executive T: 0203 096 1228 contact@aestheticsjournal.com
DESIGN
Peter Johnson • Senior Designer
T: 0203 096 1228 | peter@aestheticsjournal.com
Editor’s letter
as a limited company in
DISCLAIMER: The editor and the publishers do not necessarily agree with the views expressed by contributors and advertisers nor do they accept responsibility for any errors in the transmission of the subject matter in this publication. In all matters the editor’s decision is final. PUBLISHED BY PORTFOLIO MANAGEMENT Suzy Allinson • Brand Director T: 0207 148 1292 | M: 07500 007 013 suzy@aestheticsjournal.com Jenny Claridge • Commercial Director T: 0203 096 1228 | jenny@aestheticsjournal.com ADVERTISING & SPONSORSHIP Judith Nowell • Business Development Manager T: 0203 740 3886 | M: 07494 179535 judith@aestheticsjournal.com EDITORIAL & COMMUNICATIONS Chloé Gronow • Editor & Communications Manager T: 0207 148 1292 | M: 07788 712 615 chloe@aestheticsjournal.com Shannon Kilgariff • Deputy Editor T: 0207 148 1292 M: 07557 359 257 shannon@aestheticsjournal.com Megan Close • Journalist
Material may not be reproduced in any form without the publisher’s written permission. For PDF file support please contact Chloe Carville,
© Copyright 2019 Aesthetics. All rights reserved. Aesthetics is published by Aesthetics Media Ltd, which is registered
England; No 9887184
Aesthetics Journal @aestheticsgroup Aesthetics @aestheticsjournaluk
Mr Dalvi Humzah, Clinical Lead
ABC accredited publication
#DayOff
Talk #Aesthetics
Follow
#Training
Dr Raul Cetto @drcetto I love visiting #glasgow amazing delegates, great hospitality = one enjoyable experience. Thank you @victoria.teoxane, Karen and Michelle for a truly enjoyable day. #teoxane #medicaleducation #dermalfillers

Dr Beatriz Molina @Medikas1
I decided to wear my #gardening gloves instead of my #surgical ones! Very satisfying. @medikas anything is possible!

#BodyDysmorphicDisorder
Dr Fiona Durban
@drfiona_durban
Today’s filming with Mr Adrian Richards about Body Dysmorphic Disorder. A really important condition to recognise and manage appropriately.
#AestheticTraining #Filming
Skinade launches two new product lines

#Acquisition
Chloé Gronow
@chloe_aestheticseditor
Exciting news for our team! Global exhibition organiser Easyfairs, the owner of CCR, has acquired the Aesthetics Media portfolio!
#TransgenderPatients
Dr Greg Williams @Drgregwilliams
It was an honour to speak at this @idf_uk symposium on #HairTransplantSurgery in transgender patients.
@NHSEngland needs to recognise the importance to trans women of creating a female hairline and provide funding for the procedure.
#ScienceBehindTheBeauty
Dr Stefanie Williams

@DrStefanieW
It is a wrap for the ‘Meet the Expert’ @MaryKayGlobal symposium at @wcd2019milan, the olympics of derm, but I feel very energised as a dermatologist to continue increasing awareness on the harmful effects of pollution on the skin.

Two new lines have been added to the Skinade supplement portfolio called Skinade Targeted Solutions and Skinade|MD Pre+Post Care Programme. There are three products in the Skinade Targeted Solutions range, which are exclusively for professional use. Skinade Targeted Solutions Cellulite aims to deliver targeted nutraceuticals promoting high absorption and bioavailability to address cellulite-related concerns, while the Skinade Targeted Solutions Vitamin Boost A+D aims to stimulate natural skin cell renewal, elasticity and hydration. Finally, the Skinade Targeted Solutions Clear aims to allow the body to address causes of acne, including hormonal imbalance, inflammation and overactive sebaceous glands. The Skinade|MD Pre+Post Care Programme is a medical solution to help patients prepare and recover from surgical or invasive aesthetic procedures, such as deep chemical peels, CO2 lasers and surgery. It is exclusively designed for medical professionals to supply to their patients and it comes in a four-step protocol covering a 45-day period. The company explains that the programme integrates liquid and powder sachets, capsules and a sublingual spray, enabling the active ingredients to be consumed with optimal efficacy to improve results and mitigate procedure impact.
Skinade is distributed in the UK by Bottled Science Ltd.

Industry
Healthxchange recruits new members

Aesthetic supplier Healthxchange Group has expanded its team across multiple departments. Seema Hirani has been appointed as Obagi account executive for the North and greater London area which, the company states, will ensure that the existing team of account managers will spend more time with clients. As well as this, aesthetic nurse prescriber Jude Dunican is the newest recruit for the Obagi Consultation Prescribing Service for the London and South region. She will be joining nurse prescriber Jo Ward, pharmacist prescriber Mary Keltai and nurse prescriber Amanda Wilson, who leads the team. Karen Hill, managing director of Healthxchange Group said, “We are totally committed to serving our clients and meeting their needs. We never forget clients have a choice and work hard to make sure that the clients who put their trust in us for service and products get the best possible experience. This investment is just another visible demonstration of our commitment, but there is so much going on behind the scenes so watch this space!”

Supplements
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
us on Twitter @aestheticsgroup and Instagram @aestheticsjournaluk
Business update
Easyfairs acquires Aesthetics Media portfolio
Global exhibition organiser Easyfairs, the owner of CCR, has acquired the Aesthetics Media portfolio, which includes the Aesthetics Conference and Exhibition (ACE), the Aesthetics Awards and the Aesthetics journal print and digital platforms. According to Easyfairs, the acquisition will make the company the leading provider for aesthetic professionals in the UK.
Easyfairs notes that ACE sits perfectly with its own highly successful conference and exhibition, CCR, which is run in the autumn and, as well as being a leading event for medical aesthetic practitioners, also supports the surgical side of the market through its longstanding partnership with the British Association of Aesthetic Plastic Surgeons.
Matt Benyon, head of Easyfairs UK and Global, said, “We can now offer customers a phenomenal breadth of products and unparalleled opportunities to engage with aesthetic professionals – it’s a true 360 degree offering.”
He continued, “Suppliers in the sector now only need to make one phone call to one company to access the sector’s two major exhibitions, its most prestigious awards, and the promotional and advertising opportunities of the industry’s top journal and digital platform.” Jenny Claridge, commercial director of Aesthetics Media, said, “Culturally and strategically, there’s a real fit between businesses. The development opens up exciting new opportunities for the whole Aesthetics community; our advisors, the healthcare professionals and our customers. We are retaining the Aesthetics Media positioning, products and team, but gaining the considerable Easyfairs scale of operation and its expertise in areas such as event management, digital expertise and marketing.”
Pigmentation
mesoestetic updates dermamelan pack

Pharmaceutical and skincare manufacturer mesoestetic has updated the dermamelan pack, which the company explains is designed to reduce melanic spots. The pack features the existing oil-removing solution, dermamelan mask and dermamelan treatment, as well as two new solutions; the melan recovery and melan 130+ pigment control. According to the company, the melan recovery aims to calm and restore skin that has become sensitive and reddened due to the dermamelan exfoliation process, while the melan 130+ pigment control is incorporated for solar protection for hyperpigmented skin. mesoestetic explains that the first two products are used in clinic (the oil-removing solution and dermamelan mask) whilst the dermamelan treatment, melan recovery and melan 130+ pigment control are to be used by the patient at home following treatment. Adam Birtwistle, managing director of mesoestetic UK distributor Wellness Trading, commented, “The two new additions to the pack are designed to work alongside the treatment providing comfort and support for the consumer and containing active ingredients which continue the depigmenting action.”
Vital Statistics
Nearly 100% of social media users visit at least four platforms a day (GoodFirms, 2019)
14% of UK aesthetic practitioners aren’t sure if they are GDPR compliant (Hamilton Fraser Cosmetic Insurance, 2019)
A survey conducted in the UK demonstrated that 13% of dermal filler complications in 2018 were tear trough related (SaveFace, 2019)
63% of marketers currently use video as part of their marketing strategy (Wyzowl, 2019)
A UK survey demonstrated liposuction treatments rose by 9% in 2018 in comparison to the previous year (BAAPS, 2019)
(Alama, 2019)
82% of people aged between 21-35 believe injectable treatments are socially acceptable (Allergan, 2019)
40% of the population in the US are concerned with skin pigmentation
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Events Diary
20th September
International Association for Prevention of Complications in Aesthetic Medicine Symposium www.iapcam.co.uk
21st September
British College of Aesthetic Medicine Conference www.bcam.ac.uk
10th-11th October
CCR Expo & BAAPS Annual International Conference www.easyfairs.com/ccr-expo-2019 www.baaps.org.uk
7th-8th November
British Association of Cosmetic Nurses Conference www.bacn.org.uk
Botulinum toxin
Evolus announces US consumer campaign to promote Jeuveau
US-based medical aesthetic company Evolus Inc has begun a consumer campaign in the US called ‘#NEWTOX NOW’ in the hope to encourage patients to use its botulinum toxin. Jeuveau (prabotulinumtoxinA-xvfs), known as Nuceiva in the EU and Nabota in other markets, was introduced to the US market in May and is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines. The promotion of prescription-only medicines such as Jeuveau is not restricted in the US, unlike in the UK. Evolus chief marketing officer Michael Jafar, said, “#NEWTOX NOW is the next dimension of our launch strategy. It offers consumers $75 that can be used for Jeuveau treatment at participating practices. We believe this programme will further enable consumer conversion to Jeuveau, putting us on track to achieving the number two market position within 24 months of launch.” From a UK perspective, Dr Souphiyeh Samizadeh said, “For us in the UK, it is very unusual to see a POM being promoted to consumers. But those that have lived in the US or travelled there extensively will know this is not uncommon. It is great to have competition in the market and is a well researched product with good clinical studies surfacing, I assume they will promote it appropriately when they enter the UK market.”
Jeuveau (Nuceiva) is currently awaiting marketing authorisation by The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP).
Conference
7th December
The Aesthetics Awards 2019 www.aestheticsawards.com
Peels
AestheticSource launches second PEEL2GLOW
Aesthetic distributor AestheticSource has launched the second PEEL2GLOW formula, under the SkinTech brand, in a series of seven solutions scheduled to launch in the UK. Step one, the peel, remains the same as the existing formula (containing glycolic acid and mandelic acid) whereas step two, the glow, now contains tranexamic acid, which is designed to target photo damage and uneven pigmentation, improve fine lines and wrinkles and boost radiance, according to AestheticSource.
The PEEL2GLOW system is presented in two 1.5ml glass ampoules with an ampoule opener and a specially designed applicator, which is used to deliver the product during treatment in-clinic or at home.
IAPCAM Symposium confirmed for a third year
The director of the International Association for Prevention of Complications in Aesthetic Medicine (IAPCAM) Dr Beatriz Molina has announced that the organisation will be hosting its third symposium. Taking place on September 20 at the Church House Conference Centre in Westminster, the IAPCAM 3rd Symposium will feature sessions on specific complications, including inflammatory and non-inflammatory nodules, infections, granulomas and vascular complications. Speakers will present a description of each complication, followed by different case studies and their outcomes, Dr Molina said. Speakers already confirmed are plastic surgeons Mr Frank Rosengaus and Miss Francesca De Angelis, maxillofacial surgeon Mr Jeff Downie and ocular surgeon Mr Philippe Berros. Consultant radiologist Dr Carmen Soteras and aesthetic practitioners Dr Sherif Wakil, Dr Sophie Shotter and Dr Shirin Lakhani will also be joining the line-up, amongst others. Dr Molina said, “As medical practitioners, we must share our knowledge to avoid complications, carry out safer procedures and use the best tools available!”
Injection skills
MATA introduces Level 7 advanced masterclasses
Aesthetic training provider Medical Aesthetic Training Academy (MATA) has announced the launch of its new Level 7 advanced masterclasses in facial aesthetics. The company explains that the one to two-day courses, which are accredited by Ofqual, will be aimed at those with experience in injecting, looking to hone their skills with new techniques. The portfolio of masterclasses include: Super Masterclass – Full Facial Rejuvenation, Periorbital Masterclass, Nose Augmentation Masterclass, Jaw, Chin and Neck, Complications Masterclass, Lip and Perioral Rejuvenation and Toxins for the Lower Face and Neck. Mr Faz Zavahir, director of MATA said, “We are thrilled to offer these specialist masterclasses to those with experience in carrying out toxin and dermal filler treatments. These highly advanced workshops take even the most advanced practitioner to the next level and they can be offered as standalone classes, or alongside our Level 7 Postgraduate Diploma in Clinical Injectable Therapies.”
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Medik8 releases eye serum
Global skincare company Medik8 has added the r-Retinoate Day & Night Eye Serum to its product offering. The serum features the company’s patented formula, retinyl retinoate (a hybrid of traditional retinol and clinical strength retinoic acid) as well as caffeine, hyaluronic acid and hesperidin. It is designed to fade lines and brighten the eye area, Medik8 explains. The company states that the R-retinoate Day & Night Eye Serum can be applied in the morning as well as in the evening for accelerated use. It should be applied after cleansing, initially for twice a week for the first fortnight, then every other night for the following two weeks, before graduating to every evening use. Medik8 also advises practitioners to recommend everyday sunscreen use to patients.

BACN UPDATES
A roundup of the latest news and events from the British Association of Cosmetic Nurses BACN AUTUMN

AESTHETIC CONFERENCE
Injectables
Harley Academy introduces new foundation course
Aesthetic training provider Harley Academy has launched a new course called the Foundation Programme – Injectables. It is designed for medical professionals who are looking to take the first step in aesthetics using botulinum toxin and dermal fillers for facial rejuvenation, the company explains. The programme follows a threestep process that includes an eLearning platform designed to get practitioners ready for the course, an eight-hour foundation day led by aesthetic doctors, featuring case studies, live demonstrations, practical experience on silicone mannequins and details on how to manage complications. The last step is a 60-minute oneon-one session with a mentor that will focus on a practitioner’s first treatment. Harley Academy explains that practitioners will be guided through consultation and treatment of the patient and advised on how to carry out follow-up.
5 Squirrels expands portfolio
Private label cosmeceutical supplier, 5 Squirrels, has introduced the LIFT; a neck and décolletage firming cream. This is the seventh product in the Your Signature Skincare range to be launched without the inclusion of palm oil or palm derivatives to keep to the company’s commitment of being completely ‘palm free’ by 2021, co-founder Gary Conroy explained. The topical includes malus domestic, carnosine, resveratrol, heptapeptide, shea butter and lecithin to rejuvenate ageing skin, promote neocollagenesis, combat glycation, brighten, protect, hydrate and protect the skin, according to the company. As well this, to celebrate the launch and recent partnership with the Orangutan Foundation, the company is offering the first 24 customers who purchase 24 units an ‘OranguGary’ stuffed toy. The company has also employed two new team members; Soraya Alavi and Darren Nash. Alavi is joining the sales/ marketing department as commercial executive, while Nash has joined the design team to assist the company’s clients in establishing and perfecting their skincare brand’s bespoke designs.

Booking for the annual BACN Autumn Aesthetic Conference is open on the new-look BACN website – making it easier than ever for members to book on, check out exhibitors, and take a look at the programme for this year. The BACN is proud to announce a range of new speakers, who can all be found on the events page. BACN members once more have access to hotel packages and are encouraged to book earlier rather than later – last year the conference sold out with more than two weeks to go, and hotel packages with months left. The conference will have the same two-day format as last year – with a masterclass day on Thursday November 7 and the traditional conference with exhibitors on Friday 8. If you have any issues booking or want more information – contact Sarah Greenan, BACN operations manager at sgreenan@bacn.org.uk
BACN MEETINGS
Throughout June the BACN Board and staff members met to discuss a wide range of strategic objectives, with developments due to be put in place at the end of the year. We discussed how to deliver outcomes such as governance overhaul, along with outlining details for the BACN Aesthetic Nurse framework and how this will impact membership for 2020. After this meeting, BACN regional leaders got together with event manager Tara Glover to look at the Regional Meeting format and discuss new ideas for Autumn and the new year.
BACN TRAINING
There are still some places available for members to book the Facial Anatomy courses with a discount, which can be found on the events page of the BACN website. The BACN partners with training companies that offer an incredible opportunity for members to learn techniques and skills to really understand the facial structure with hands-on training.
This column is written and supported by the BACN
Eyecare
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Development
Lee Ison, Director of Springpharm
Who are Springpharm?

We are a family owned and run independent pharmacy chain based in the Midlands with more than 50 years’ experience. Our background stretches back through multiple generations and locations and, in recent years, we have taken a gradual step into the aesthetics sector, hoping to bring with us our cornerstones of operation, which is evidencebased practice and patient priority.
How will you become a more familiar name in medical aesthetics?
Springpharm is growing more rapidly than we ever expected. We have a strong emphasis on personable service and good customer support and when you match that with good prices and fast delivery we have the basic winning formula already. It’s important to note however, that we are striving to be more than just a ‘box it up and ship it out’ pharmacy.
You talk a lot about game-changing, what makes Springpharm so different?
Aesthetics is a very difficult field in the UK and we feel it’s only right that while medical professionals are fighting to raise standards we should also do our part in the pharmaceutical sector to help. Springpharm never loses sight of the end user, which ultimately is the patient whom we all have a duty of care towards, not just the practitioner. With this in mind, we are introducing support channels for our practitioners so they can offer their patients the best care and results. At present, we are developing a series of in-house aesthetic workshops for our clients, which will be held in our very own brand new clinical space, which is also available to use as a consultation or treatment room. We have made links with professional aesthetic association PIAPA who provide education and guidance for practitioners, and along with other pharmacy colleagues we hope to also provide informational materials for better prescribing and consultation.
Charity
Aesthetics Media to run 10K for the British Red Cross

The team from Aesthetics Media will participate in The Asics London 10K run on Sunday July 21 to support the British Red Cross. The British Red Cross, which helps millions of people around the world to get the support they need in the event of a crisis, is the 2019 charity partner for Aesthetics Media. The team will be running a route through Central London past some of the city’s renowned landmarks. They will start near Green Park Station, before running to Piccadilly Circus, down to St James’ Palace, over to Trafalgar Square, through to Charring Cross, then along the River Thames to Westminster, ending on Downing Street. Chloé Gronow, editor and communications manager at Aesthetics Media commented, “The British Red Cross is a wonderful cause and Aesthetics is delighted to support them by participating in the Asics London 10k run, as well as other initiatives throughout the course of the year. We will be running past many London aesthetic clinics so we encourage everyone to give us a wave and cheer us on! We would also appreciate any support through donations or social media encouragement.” To donate, visit the Aesthetics website or email contact@aestheticsjournal.com.

Laser
Cutera releases new device
Laser and light-based medical provider Cutera Medical Ltd has introduced an updated version of the Excel V laser, called the Excel V+ for vascular and pigmentary conditions. It features 1064 nm and 532 nm wavelengths and spot sizes of up to 16mm. According to Cutera, the device can address superficial and deep vascular lesions, pigment and skin revitalisation concerns with a high safety margin. Tim Tailor, UK country manager for Cutera Medical, said, “The new Excel V+ is a really exciting platform. The Excel V, in itself, was already a great vascular platform. What we have done is added more spot sizes for faster treatments and deeper penetrations of the lasers, as well as to offer longer lasting results and allow us to target hard-to-reach places such as around the nose and thread vessels. Treatment is also a lot faster when you are doing full face treatments for skin rejuvenation. The Excel V+ also features the new Green Genesis micro-pulsed 532 nm treatment, which is going to be even better for addressing redness and rosacea. You can also combine the Green Genesis with your standard Laser Genesis for a lovely facial.”

Industry
sk:n acquires Destination Skin
Medical skincare clinical group sk:n has announced the acquisition of Destination Skin, a chain of 16 aesthetic clinics. Darren Grassby, CEO at sk:n, said, “We’ve admired the Destination Skin brand for a long time. Together, we will be operationally and commercially stronger to innovate and shape the future of the aesthetic skincare market in the UK.” In a statement released by sk:n, it was explained that the acquisition and support from private equity firm TriSpan has enabled the company to now operate 68 clinics and has total revenues of approximately £40 million.
60
This column is written and supported by
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Ceremony
Booking open for the Awards
With entry for the Aesthetics Awards now closed and under review, booking is open for the most prestigious ceremony in the specialty’s calendar. The ceremony, taking place on December 7 at the Park Plaza Westminster Bridge Hotel, will celebrate Winners, Highly Commended and Commended finalists in 26 categories. Tickets can be bought individually or in tables of 10 and include entry to the dazzling ceremony, prosecco on arrival, a three-course meal with drinks, and entertainment throughout the night. AesthetiCare has also been announced as a new sponsor for the Award for Best Clinic North England, while Schuco will once again sponsor the Award for Outstanding Achievement. To find out more and to book your tickets visit aestheticsawards.com.
DNA REVIV introduces genetic analysis programme
Global wellness provider REVIV has launched a bespoke programme called REVIV Genetics that aims to offer treatments and products based on an individual’s genetic analysis. REVIV says it sequences the full genome to establish the gene variations in individuals that demonstrate how different aspects of the body work and behave. Practitioners have the option to offer two different analysing programmes to their patients; Beauty, which assesses collagen, moisture, oxidative stress and biological age and Beauty coupled with Weight & Nutrition, which covers genetic weight profile and genetic response to food groups. The REVIV clinics will also be offering three other options in their clinics; Health, Weight & Nutrition, Sports & Nutrition and Nutrition. CEO of REVIV, Sarah Lomas said, “With the advancement of technology, individuals are far more educated in terms of their own wellbeing; as a wellness sector we need to continue to provide the information that will allow people to take control and make lifestyle decisions based on advanced data.”

Training
Acquisition Aesthetics launches new course in Manchester
Training provider Acquisition Aesthetics will launch its Foundation Course in Botulinum Toxin and Dermal Fillers in Manchester this month.
According to Acquisition Aesthetics, which was Commended for Best Independent Training Provider at the Aesthetics Awards 2018, the CPD-registered course will provide delegates with the knowledge and skills necessary to perform foundation cosmetic procedures safely, effectively and with confidence.

The one-day course is complemented by theoretical e-learning modules, a video library of practical training, post-course mentoring and support, and hands-on injecting experience on live models. The trainers all have maxillofacial, dental and plastic surgery backgrounds. Leading the course will be dental surgeon Dr Yusra Al-Mukhtar and course directors Miss Priyanka Chadha and Miss Lara Watson. The first course will take place in Manchester on July 7, with more dates soon to be released. Delegates must be doctors, dentists or nurses with a valid GMC/GDC/NMC number or the international equivalent.
News in Brief
Survey highlights motivations for body procedures
A UK survey of 1,000 women conducted by laser developer and manufacturer Cynosure suggests that almost 50% are encouraged to refine their body shape and lose stubborn fat after seeing an unflattering photo of themselves. The survey also reported that other motivations were to get back into clothes that no longer fit (30.9%), as well as getting back into shape after having children (7.7%), after a divorce or breakup (4.6%), if their friends were also trying (4.4%) and seeing celebrities/role models’ bodies (2.6%).
Wigmore Medical appoints new KOL Aesthetic practitioner Dr Max Malik has been confirmed as a key opinion leader (KOL) for distribution company Wigmore Medical. Dr Malik has more than 20 years of medical experience and works within aesthetic medicine and hair restoration surgery. Product development and marketing director at Wigmore Medical, Raffi Eghiayan said, “Wigmore Medical is delighted that internationally renowned doctor, Max Malik has joined as a KOL. In conjunction with Wigmore, Dr Malik will also be running a series of educational seminars on the use of toxins.”
Lynton celebrates 25-year anniversary
Aesthetic technology manufacturer and skincare developer Lynton is celebrating its 25-year anniversary in the aesthetics specialty. To commemorate the milestone, the Manchester-based company has announced that it will be visiting 25 customers across the UK to hear about their individual success stories. The company will also be celebrating by working with students at The University of Manchester to design a ‘Lynton bee’ sculpture, the city’s best-known symbol that represents its reputation for hard work. When completed, the bee will be placed at the company’s recently refurbished office and laser training academy.
Image Skincare recruits new sales director
Clinical skincare company Image Skincare UK has hired Graham Clarke as its new sales director. Graham brings more than 20 years of beauty and wellness experience to Image Skincare, having worked with Space NK, AVEDA, Sothys and The Giving Brands Limited. As part of his new role, Graham will be leading the company’s growth strategies.
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
On the Scene
Out and about in the specialty
Aesthetic Exchange, London
The benefits of combining topical cosmeceutical formulations with lasers to maximise treatment success was highlighted at the Aesthetic Exchange event at the Bulgari Hotel on the evening of June 6. The event was organised by laser developer and manufacturer Hologic’s Cynosure UK division and cosmeceutical brand iS Clinical, which were celebrating their first collaboration. Guests were greeted by Cynosure UK country manager Ben Savigar-Jones. Alana Chalmers, founder and director of Harpar Grace International, the exclusive iS Clinical UK distributor, then spoke about the collaboration and how it aims to enhance patient results and provide business benefits to practitioners. Beauty journalist Alice Hart-Davis also spoke about what patients want from treatment, which she said was an overall fresher face. Delegates then heard from aesthetic practitioner Dr Tapan Patel and surgeon and aesthetic practitioner Miss Sherina Balaratnam, who presented the theory and benefits behind the approach of cosmeceuticals and laser science. The event ended with live treatment demonstrations, and networking opportunities. Chalmers said, “We had a full turnout and it was great to bring together a range of respected and interesting speakers, and the educational content has been a real highlight.” Savigar-Jones concluded, “We love energy-based technology but we do know that in isolation it only gets you so far, which is the same for cosmeceuticals, so to get truly happy patients, it’s about combining both of them, so we are pleased to partner with iS Clinical.”
BAS Conference, Windsor
On May 14 more than 80 surgeons, doctors and nurses attended the British Association of Sclerotherapists’ (BAS) conference at the Dorney Lake Conference Centre in Windsor. The day consisted of educational presentations and live demonstrations on topics including varicose vein treatments, microsclerotherapy, ultrasound-guided foam sclerotherapy as well as business talks on the importance of social media and blogs, newsletters and events, networking and cross referral partnerships. Consultant vascular physician and BAS chairman Dr Stephen Tristram commented on the day, “We are very happy that many of the delegates were attending the conference for the first time. The variety of speakers and information, the new ideas, the live demonstrations and the networking opportunities have all been commended, and the superb venue has been commented on more times than I can say.”

Cosmetic Courses Annual Conference, London
For a third year, practitioners were invited by training provider Cosmetic Courses to attend a full day of learning at the Royal Society of Medicine. Guests were welcomed by consultant plastic and reconstructive surgeon, and clinical director of Cosmetic Courses, Mr Adrian Richards and managing director Jim Savin. Among the topics covered were treatment planning and consultation by Cosmetic Courses’ clinical lead and nurse prescriber Mel Recchia, who also shared a presentation on facial contouring with aesthetic practitioner and clinical lead Dr Fiona Durban. Eddie Hooker, director of insurance provider Hamilton Fraser explored how to deal with complaints and claims handling, while business consultant Chris Gill and Allergan SPARK innovation marketing manager John Campbell introduced the new online SPARK initiative. Cosmetic Courses Nottingham clinical lead and dentist Dr Olha Vorodyukhina performed a lip augmentation demonstration and Professor David Sines, chair of the JCCP, provided an update on the role of regulation in the aesthetic sector. There was also an exhibition, which included companies such as AestheticSource, Allergan, Hamilton Fraser, Biotec Italia, 5 Squirrels, Celluma, T Chauhan Consultancy and Macom Medical. Aesthetics Media also took part, showcasing the Aesthetics journal, Aesthetics Awards and Aesthetics Conference and Exhibition.
Irish Excellence in Aesthetics Conference, Dublin
On May 20, aesthetic professionals were invited to attend the Irish Excellence in Aesthetics Conference hosted at the Marker Hotel in Dublin. The conference was organised by skincare developer AlumierMD, and was designed specifically for the aesthetic community in Ireland to discuss strategies for delivering excellence through multiple modality treatments, safe practice, topical skincare and effective business practice. Speakers at the event included aesthetic practitioners Dr Lee Walker and Dr Eithne Brenner, aesthetic nurse Anna Gunning, business coach Pam Underdown and AlumierMD brand spokesperson, Victoria Hiscock. Samantha Summerfield, AlumierMD marketing and events manager UK and Ireland said, “For AlumierMD, education and patient safety is at the core of our business and it was important to us to present speakers that were able to offer delegates valuable sessions that were easy to implement into their current business practices.” She concluded, “The event has been a remarkable success with our speaker panel, delegates and associated partners all giving us wonderful feedback. We are thrilled that we were able to offer the Irish market a conference that they found valuable.”

Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
new pack
dermamelan
depigmenting solution by mesoestetic
trust the leader*
®
the world’s leading professional depigmentation method



* Considering international distribution.
The new dermamelan® pack includes a patient’s home pack with two innovative complementary products specifically developed to achieve more efficient and lasting results.
 dermamelan® method efficacy has been demonstrated in numerous studies under medical supervision. More than 500,000 patients treated worldwide, in any skin phototype (I-VI) and in all ethnicities.
dermamelan® method efficacy has been demonstrated in numerous studies under medical supervision. More than 500,000 patients treated worldwide, in any skin phototype (I-VI) and in all ethnicities.
®
after
after before For more
visit
before
information
us at www.mesoestetic.com
Long term results obtained after 12 weeks demonstrates method’s efficacy on pigmentation reappearance control.
The World’s Only Anti-Aging System Powered by Umbilical Cord Lining Stem Cells

Cruelty free. Ethical.
I have a special interest in skin health and having worked in aesthetics for nearly 15 years running my own clinics I have found skincare a great challenge. There is limited scientific data regarding skincare products, so I was very impressed with the scientific data behind the CALECIM® range. I have personally used it for over 1 year and my skin has been transformed. Most importantly my patients love it too!



–
Dr Beatriz Molina, Medikas Founder of IAPCAM
CALECIM® has been a game changer in the management of my patients’ post plasma. It considerably reduces the downtime, improves healing and has been useful in the management of complications. It is now a mandatory component of my post plasma aftercare. In addition, we have introduced new protocols using plasma shower in conjunction with CALECIM® with outstanding results.
– Dr Shirin Lakhani, Elite Aesthetics
As seen on ITV’s This Morning
“ “ “ “
IT’S NO MIRACLE. IT’S SCIENCE®
distributed by London | Loughborough | Online Skincare@churchpharmacy.co.uk calecimprouk www.calecimprofessional.com 01509 357 300
Exclusively
Irish Regulation
Much like the UK, the subject of regulation is a hot topic in the Republic of Ireland.

On June 10, the Irish Minister for Health Simon Harris publically voiced concerns surrounding injectable treatments, with a particular focus on protecting young patients.
He has asked his department officials to explore the potential public health risks for those in Ireland undergoing botulinum toxin and dermal filler treatments. The Minister says, “There is a need to examine whether current regulations are sufficient or whether further regulation is required. This is not an issue solely confined to Ireland, but one that I believe we need to address as a matter of priority.”
The main reason behind this, the Minister states, is an increasing desire by young men and women in Ireland, as well as beyond, to look perfect, which is largely driven by social media. This, he claims, is increasing the popularity of treatments such as dermal fillers and botulinum toxin.
Aesthetic practitioner Dr Patrick Treacy, who owns Ailesbury clinic in Dublin and Cork, says, “The Irish Government focusing on aesthetic procedures has been a long time coming. We have been advocating for regulation change for at least 12 years with no response at all and there is no doubt that there are issues that need to be addressed.”
One of Minister Harris’ top concerns is the age at which the treatments are available to the public. He states, “This cannot be an area that goes unregulated or unsupervised. In the past, we have banned sunbeds for under-18s1 and I have asked my Department to assess whether we should impose a minimum age
for the use of these [injectable] products.”
Following the Minister’s announcements, The Royal Society of Public Health in the UK released a report titled Skins and Needles, which also called for the UK Government to make non-surgical cosmetic procedures illegal for patients under 18s.2
Concerns surrounding the minimum age for aesthetic treatments are not new. Last year Aesthetics explored the arguments for placing a ban on treatment under 18 in the UK, which included developmental issues, mental health, comprehension of treatment risks and to prevent non-medics treating the younger age group.3
Wider issues specific to Ireland
According to Dr Treacy and aesthetic nurse Anna Gunning, owner and director of The Laser and Skin Clinic in Dublin, Mullingar and Athlone, the government has missed the mark by putting such a strong focus on age. They believe age restrictions won’t help the wider issues. Dr Treacy explains, “There would be no ethical medical practitioner in Ireland who would be treating patients under the age of 18 for cosmetic purposes so I don’t think that the age group is really the problem. The fact that the light has been shone on the industry is good, but unfortunately in my mind, the light is being pointed in the wrong direction. There are many other concerns that need to be addressed.”
He believes, “The main issues in Ireland relate to dermal fillers because they do not need a prescription to administer. I believe there is a rising level of inadequately trained practitioners coming to practice in Ireland, many from abroad, who are not treating safely
and who are also using cheap dermal fillers, which are more enticing to younger people.” Gunning adds, “Like the UK, you don’t need to be a medical professional to administer dermal fillers, which I believe needs to stop as these procedures should be regulated. There are also issues in Ireland of who is providing training for non-medics to inject fillers and what responsibility they should take before signing off and certifying someone after only a few hours of training. The Department of Health should take notice of what is happening in the industry to protect patient safety.” In an unregulated field, Dr Treacy believes that practitioners working in accident and emergency need to be better educated in Ireland to deal with complications. “I don’t believe that hospitals in Ireland are well enough equipped and trained to deal with the increasing vascular occlusions that they are seeing. I have written to the Health Service Executive several times regarding this, it’s definitely something that needs to be addressed.” Gunning also feels that some insurance companies could be doing more. She says, “I think there is an issue where the insurance companies are insuring non-medical professionals for injectable treatments. So, the companies have a big responsibility to make sure they are not insuring people who have no medical background with limited or no proper training.”
Gunning also says that from a nursing perspective, there are misconceptions around Irish legislation and nurse prescribing of botulinum toxin, which need to be cleared up. “There is a little bit of confusion around nurses ordering botulinum toxin and there is some clarification needed, which will hopefully be resolved in the near future. This will make the public and our medical colleagues aware of our nursing scope of practice,” she explains.
Gunning and Dr Treacy both conclude that instead of an age restriction, the Government’s true focus should be on who is performing the treatments. Dr Treacy summarises, “The dangers occur when patients are treated in untrained and unchecked hands. What we need to do in Ireland, just like what I believe needs to be done in the UK, is pass legislation that only medically trained people should be performing these treatments.”
REFERENCES
1. Department of Health, Provisions relating to those under 18 commenced from 21 July 2014 <https://health.gov.ie/healthyireland/sunbeds/>
2. RSPH, Skins and Needles, June 2019. <https://www.rsph. org.uk/uploads/assets/uploaded/97c182fb-3d70-472c90ef36ded8da1b63.pdf>
3. Kilgariff, ‘Banning Under-18 Injectables’, Aesthetics journal, January 2018. <https://aestheticsjournal.com/feature/banningunder-18-injectables>
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Aesthetics looks at the regulation issues in Ireland and explores what concerns practitioners believe should be addressed
MEDICAL DERMATOLOGY


















CLINICAL PHOTOGRAPHY







@TSIS_London READY TO TRY? Telephone: +44 (0)781478805 Email: NMS@surfaceimaging.co.uk The Exclusive UK and Ireland distribution partner for The Worldwide leader, Medical Imaging Software, Photography Systems, Clinical Research Services Experience the most advanced clinical 3D Technology that enables your patients to see an accurate representation of themselves; thereafter to clearly express, visualise and document their aesthetic desires VECTRA®: Global market leader in medical 3D Imaging Solutions VISIA®: Most advanced clinical facial skin analysis system IntelliStudio®: Fully integrated medical studio photography system AESTHETIC SYSTEMS COMPLEXION ANALYSIS • VOLUME MEASUREMENTS • SIMULATIONS • BEFORE AND AFTER DOCUMENTATION See the full range of products and services at www.surfaceimaging.co.uk 2D / 3D IMAGING • BODY MAPPING • DERMOSCOPY •
Best in class 360° & 180° whole body imaging systems, designed specifically for dermatology. Single capture, macro quality resolution and fully integrated software to map and monitor lesions and distributed diseases of the skin. Other applications include documenting pigmented lesions, psoriasis and vitiligo. VECTRA® WB360: World class, whole body 3D Imaging • DermaGraphix®: Scalable 2D & 3D body mapping solution VEOS HD2/DS3: Compact Dermatoscope / Digital Dermoscopy • VISIOMED D200 evo: High-resolution dermoscopy imaging VISIOMED Luminis: Handheld high-end dermatoscope • VISIOMED Optima: Examination magnifier
Managing Chemical Peel Complications


Four practitioners discuss adverse events following chemical peel treatments and explore management, showcasing two successful case studies
Skin peeling dates back hundreds of thousands of years. Historically, it is thought that the ancient Egyptians used animal oils, salt, alabaster and sour milk (lactic acid), American Indians would use dried corncobs and Polynesians would use crushed shells to abrade the surface of their skin, all to improve its quality.1,2 Although today’s approaches are not as alternative, skin peeling is a significant part of most aesthetic practices, where it is often achieved through topical chemical formulations. Today’s patients are often over-worked, stressed and living in over-populated cities, which we know is having detrimental effects on the skin, so it’s no surprise that chemical peels are a popular solution. They can improve signs of dullness, reduce lines and even improve the appearance of mild scars, amongst many other common concerns.3-7
Medium Trichloroacetic acid (TCA) 10%+, high percentage glycolic acid, Jessner (contains salicylic acid, lactic acid and resorcinol)
Deep Phenol, Trichloroacetic acid (TCA) 35%+
Hyperpigmentation,

Wrinkles, sun damage, acne scarring, xanthelasma and hyperpigmentation
“We wear our skin every single day. It’s how we communicate with the outside world so it’s understandable that good skin quality is a top priority for so many patients,” says surgeon and aesthetic practitioner Miss Sherina Balaratnam. “Comments like ‘You look healthy’ or ‘You’re glowing’ don’t just come from having wrinkle-free skin or fuller lips, they come from the light reflections,” explains aesthetic practitioner Dr Max Malik. “I have absolutely seen a rise in patients mentioning skin quality in my practice and things that weren’t so bothersome before are now being highlighted, like freckles for example,” he adds. But it’s not to say that chemical peel treatments don’t come with their risks and complications, just like so many other aesthetic procedures do. Dr Malik says, “The industry is really focused on dermal filler complications at the moment, which is great, but I think what also needs to be highlighted is the fact that other treatments, such as chemical peels, can have significant negative outcomes and practitioners need to know how to diagnose and manage these.”
In this article, Miss Balaratnam, Dr Malik, aesthetic practitioner Dr Xavier Goodarzian and dermatologist Dr Harryono Judodihardjo explore the complications that can arise from chemical peels, discuss best practice for dealing with them and share two case studies of successful complication management.
Concerns around peels
“There’s no denying that we are dealing with a much more educated patient base now and many even use acid-based products, such as glycolic and salicylic acid, in their skincare regime. Patients may think they are well informed of what they are having because they know the name of the acid, but they should be informed about what ingredient or strength of peel they have had, why they are having it and what the true benefit and long-term effects are on their skin,” advises Miss
Erythema, swelling, sensitivity, skin peeling/ shedding
Increased skin sensitivity, activation of herpes virus, bacterial and fungal infections, allergic and irritant contact dermatitis, pigmentation
Increased skin sensitivity, activation of herpes virus, epidermolysis, bacterial and fungal infections, allergic and irritant contact dermatitis
Prolonged erythema, scarring, hypo- and post-inflammatory hyperpigmentation (PIH), epidermolysis
Prolonged erythema, scarring, hypo- and post-inflammatory hyperpigmentation (PIH)
Table 1: Overview of chemical peels and possible complications.8 Dr Judodihardjo assisted with the information included within this table. Note: this table does not provide an extensive list of possible complications – thorough training is always recommended.
Depth of peel Primary ingredients Examples of targeted concerns Expected skin response Moderate side effects/ complication Severe side effects/ complications Light/superficial Alpha hydroxy acids; glycolic acid, citric acid, lactic acid Trichloroacetic acid (TCA) -10% Skin tone, fine lines, acne scarring, pigmentation, acne Erythema, sensitivity, skin flaking Increased skin sensitivity, activation of herpes virus, fungal infections,
allergic and irritant contact dermatitis, prolonged erythema, pigmentation N/A
fine lines, acne scarring Erythema, sensitivity, skin peeling/shedding
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com

 REF: Efficacy test of a serum with gold nanocomplex combined with radiofrequency to reduce the cellulite effects. Endor Technologies S.L., Instituto de Fotomedicina, Dr. Joaquin Querol, Gabriel Buendía Bordera M. Sc., Dr. Jorge Gaviria, Dr. Juan Bascones.
REF: Efficacy test of a serum with gold nanocomplex combined with radiofrequency to reduce the cellulite effects. Endor Technologies S.L., Instituto de Fotomedicina, Dr. Joaquin Querol, Gabriel Buendía Bordera M. Sc., Dr. Jorge Gaviria, Dr. Juan Bascones.
Balaratnam. Dr Judodihardjo reiterates this point, explaining that due to peels being so available and performed by a wide variety of professionals, he worries that they may become trivialised. “We have to remember that essentially we are pouring acid onto the face so you must be well-informed on the type of acids used, its concentration, the pressure applied, and when they should be neutralised, as these will determine the depth that you want to go. In my opinion, deep chemical peels must only be performed by very experienced medical professionals as they come with the highest risks,”4 he explains.
Possible complications
Deciphering the difference between what is an expected skin response and what is a complication is key, emphasise all practitioners interviewed. This is further showcased in Table 1 Dr Malik highlights, “Some ‘complications’ that patients report, are, in fact, not complications at all, such as the peeling effect, erythema and sensitivity with a medium depth peel, for example. Therefore, when you are treating, it’s a good idea to provide patients with a clear timeline of what can happen and include every possible outcome on this timeline.” The best thing to do, recommends Dr Goodarzian, is to treat everything that is even slightly out of the ordinary. “Even if you think it’s probably nothing, pay attention to it and monitor it closely. You will save yourself later down the line by being extra cautious,” he says.
Although rare, scarring is one of the adverse events associated with deep chemical peels, which needs to be recognised early and must be well-managed for a good outcome, explains Dr Goodarzian.8 He says, “This complication could be due to excessive skin preparation with products such as tretinoin, injury to the stratum corneum prior to peeling (waxing, exfoliating, threading), excessive amount of peeling solution, excessive force of application of the acid, amongst many other reasons.8 Neglecting the signs could lead to permanent scarring, so that’s why it’s important to address any signs as early as possible.”
He continues, “Both products that I use in my clinic, the SkinTech Easy TCA Pain Control and Lip & Eye peels, are self-neutralising and scarring with these peels is extremely rare when the application methods of the manufacturer are strictly adhered to.10 Even after many years of using TCA and phenol peels, or combinations of them, I have never come across the issue of scarring. Early recognition is absolutely crucial to prevent actual scar formation.”
Transient hyper or hypopigmentation is also a common side effect from medium-depth and superficial peels,8 Dr Judodihardjo explains, saying, “This is generally more common in patients with darker skin types – that’s why it’s particularly important to highlight this prior to any treatment.” He continues, “Less common complications are infections. This is where we see the skin remain red for a prolonged time period. These are often caused by fungus overgrowth. Treatment for this may consist of oral and topical anti-fungal drugs. All peels can cause activation of herpes viral infection, so do warn the patients that this can happen and, if necessary, prescribe anti-herpes medications.”
Miss Balaratnam shares that patients who have had peels over a number of years can develop long-term skin damage if the treating practitioner has not been careful. “Some patients are having them too deep, too frequently and are getting the wrong peel for the wrong skin type. They are presenting with inflamed skin, stripped of its natural oils, with evidence of broken capillaries. The long-term damage needs to be managed early on,” she explains.
Patient management
Dr Judodihardjo explains that the key factor when dealing with skin complications, whether it’s one you have caused or not, is communication. “Patients want to feel that there are lots of options available to them and that there is a hope for a solution. Compromised skin quality can affect more than just the skin itself, it has huge physiological impacts too.”9 He continues, “Communicate with them throughout and keep them under a close watch. Alienating your patients is the worst thing that you can do, you need to hold their hand throughout.”
Dr Goodarzian also says that it is extremely important to know your limits of whether you can achieve the suitable outcome, “If you know that a complication, such as erythema, would respond better to a treatment you don’t offer, like a laser for example, refer them elsewhere and trust that they will respect you more for being open and honest,” he says.
Practitioners agree that all chemical peel complication management requires time and perseverance. Dr Malik says, “Repairing skin health is a long process and you aren’t going to see results overnight. Always make it very clear, whether you are treating a complication or maintaining successful results, that it will take time and that commitment is required from the patient too.”
Dr Judodihardjo highlights that it’s all about finding the right approach for your patient, which is usually a combination of treatment modalities. This is further emphasised in Dr Goodarzian’s case study.
Dr Judodihardjo reiterates, “Know that your first treatment approach may not work, so just keep persevering and be sure to explore all of your options.”
Continuing the journey
Miss Balaratnam recognises that patients who have experienced complications in the past may sometimes be reluctant to continue their aesthetic journey. “I think it is understandable to be a little weary of having more treatments in the future, however it comes down to working with the practitioner, the right team and the right topical ingredients. Show patients the results that you have achieved in the past in order to restore confidence, give them your time with an indepth consultation, and always a cooling-off period, given that it is not a medical emergency,” she explains.
With detailed consultations in place, knowledge on identifying a problem and communication throughout the journey, all practitioners agree that this will instil best practice at a time of potential distress.
Dr Judodihardjo concludes, “Probably nine times out of 10, patients’ confidence in you as a practitioner is increased because you have been the one to help them in a time of need. Patients will often respect you more when they can see that you can handle the complication and not run away from it.”
“Early recognition is absolutely crucial to prevent actual scar formation”
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Dr Xavier Goodarzian
“Gives the effect of a CO2 laser, but without the downtime... its safety profile is excellent. I cannot begin to say how Tixel has changed my practice and increased my patient footfall”

















 Sabrina
Sabrina
Shah-Desai Consultant ocular plastic surgeon, Perfect Eyes Ltd
















“I have been using Tixel for the past year and a half, one of the best devices I ever bought, particularly for peri-orbital rejuvenation, fantastic results for scarring and general skin sundamage”



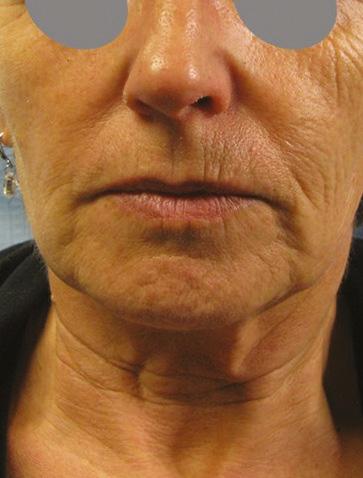 Dr
Dr



Tapan Patel – PHI Clinic London









EXCLUSIVELY to
AZTEC Services supply Tixel
doctors and nurses
For further information or to arrange a demo contact AZTEC Services at: az@aztecservices.uk.com | 01494 956644 | www.aztecservices.uk.com After 3 Tixel treatments
Before Tixel.indd 1 01/04/2019 14:33
Find out why medical professionals love
Mid and lower face case study shared
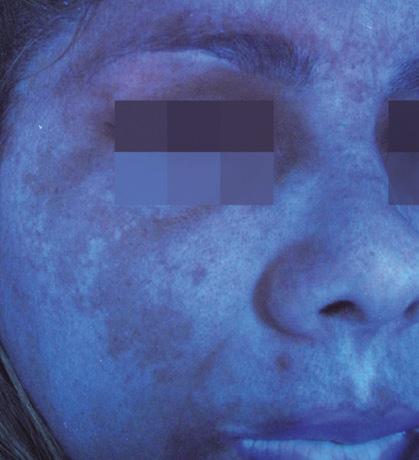
Before After
• Complication: first-degree chemical burn

• Treatment: debridement and skincare regime



Patient journey
This 44-year-old woman was a former patient of mine who had previously had several skincare treatments at my clinic. She called my practice after having a chemical peel from another clinic that resulted in burns to the face. I was away at a training course at the time, so I asked her to send images so I could assess the extent of the burn. When I saw them, I urged her to go straight to A&E as I was concerned that it was a medical emergency. In the meantime, I advised her to apply copious amounts of cool water and cover the burn in Vaseline to keep the wound moist and cool. She decided to book an appointment the following week upon my return, although I continued to express the urgency of seeking hospital treatment before coming in to see me.

The patient presented to clinic four days later and upon initial assessment, it was very clear that she had gone against my strong recommendations to seek urgent medical care. She had a clear first-degree chemical burn and the skin presented with cracks and crust. When I asked her why she didn’t go to A&E, she explained that it was due to being too embarrassed. I was very disappointed by this as the chances of infection and post-inflammatory hyperpigmentation had increased. I once again advised her to seek specialist help at a burns unit; however, the patient resisted and said that she only
wanted me to help her due to my previous experience in burns reconstruction. The patient wasn’t aware of what peel was used and based on my examination, it appeared that the peel was not neutralised due to lack of product training in a new product which had been introduced to the clinic.
Treatment plan
During the first treatment, I cleaned the area and debrided the excess dead skin and cleaned the rest of the area using isopropyl alcohol. I gave her hypochlorous acid (Clinisept+) to use on a daily basis to keep the wound as clean as possible. I also gave her an emollient repair balm (iS Clinical Sheald) to keep the area moist and she was advised to use this daily, both morning and night. All steps were to disinfect the skin and encourage re-epithelialisation. I also advised that daily use of an SPF – this was especially important as she has olive skin, so her risk of developing PIH was higher.
She came back every two days, so that I could clean the area and debride until eventually there was a small scab, which lifted off after three weeks.
Following this, we moved onto weekly appointments. When I was happy with her progress, and the skin was no longer broken, I advised her to use a mild glycolic acid cleanser (iS Clinical Cream Cleanser) both morning and night and a 15% topical vitamin C with additional anti-inflammatory ingredients to manage post burn erythema.
Results
Considering the injury she initially presented with, her results have turned out very well; the patient is very pleased and so am I. She has a mild erythema, consistent with the healing phase, and I have discussed laser treatment to help manage this after the summer. I have advised the patient that medical grade skincare will work well to keep the healing of her skin optimised in the meantime and to manage any discolouration, and I will continue her treatment plan in the autumn.
Figure 1: Patient on day four when initial assessment took place and six weeks after a treatment plan, consisting of debridement and a topical regime. Images courtesy of S-Thetics, taken through the VISIA Complexion Analysis system.
The patient has mild erythema, consistent with the healing phase, I have discussed laser treatment to help manage this after the summer
by Miss Sherina Balaratnam
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com 14:33



HELP COSMETIC SURGERY SCARS FADE WITH KELO-COTE ® Find out more: www.kelo-cote.co.uk AL/3833/06.19/0.001 Date of Preparation: June 2019 CLINI CALLY PROVEN
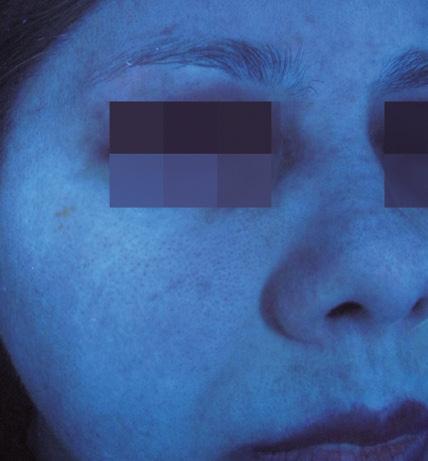
Periorbital case study
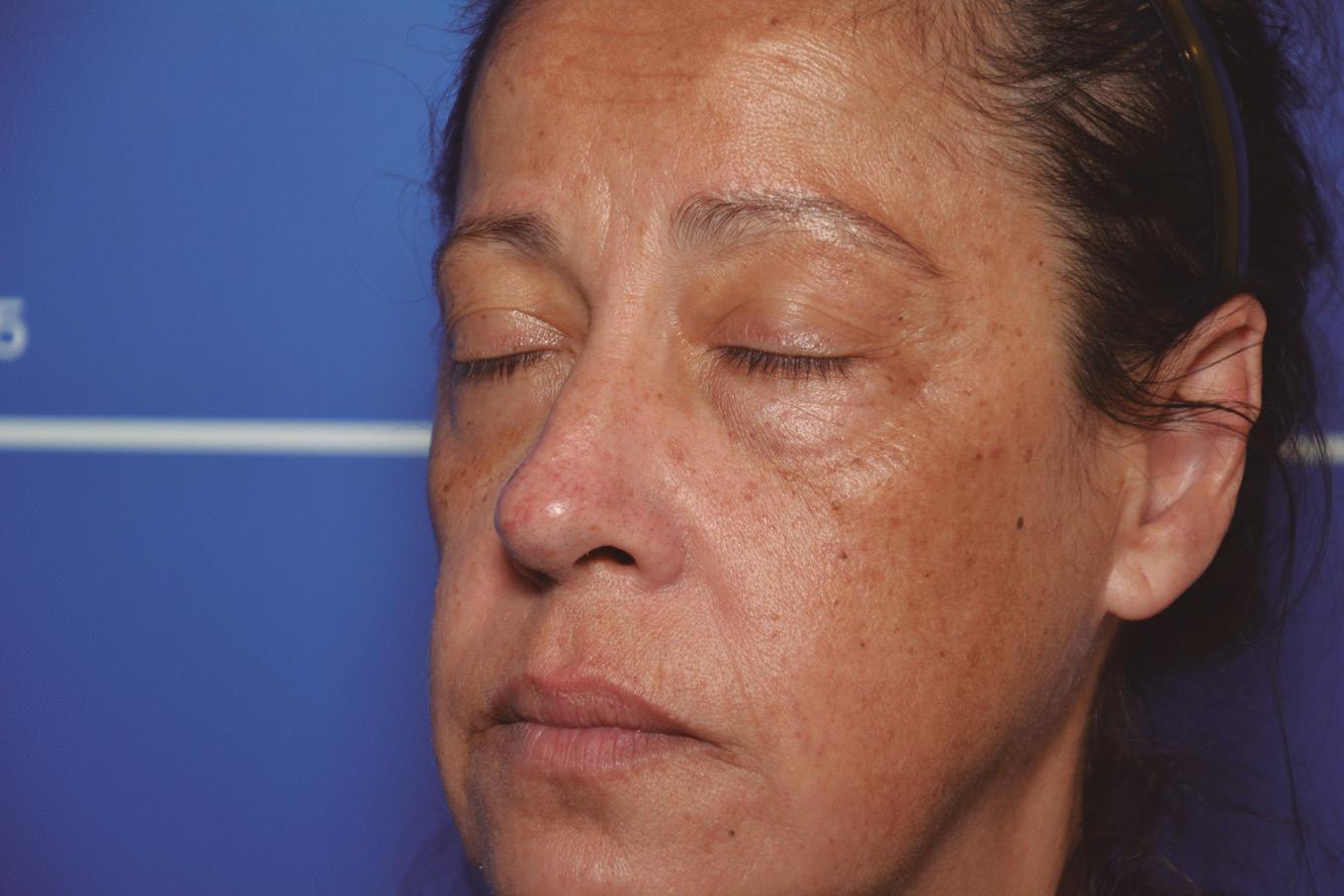
• Complication: hypertrophic reaction of the periorbital region
• Treatment: topical and injectable steroids, LED therapy, microneedling and silicone topical gel
Patient journey
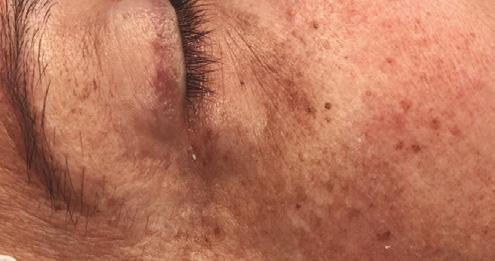
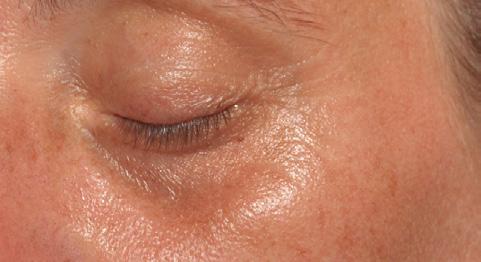
A 52-year-old patient came into my clinic with overall concerns of skin laxity, specifically on the upper and lower eyelids. I decided to perform a deep peel as I believe it to be one of the best treatments for eyelid skin laxity. The skin was cleansed and disinfected with alcohol and was degreased with acetone. I treated her with a mosaic chemical peel, consisting of a phenol peel around the eye area and a medium-depth TCA peel on the rest of the face, all performed according to the manufacturer’s protocol. At day three the patient was reviewed and all appeared normal. There was overall oedema of the face and periorbital region, darkening of the facial skin and drying of the periorbital scabs, with slight erythema as expected. The patient was reviewed on day eight with no signs of concern. I advised her to then immediately begin sunscreen use; recommending the SkinTech Melablock factor 50. At day 11 the patient was reviewed again. The scabbing had come off the periorbital area, however I noticed delayed healing and epidermalisation in the medial sections of the eyelids, which was not expected. Fusidic acid ointment was prescribed for those areas to aid with healing and avoid infection. Four days later the skin had fully epidermalised, which was a positive result, and the patient was advised to use IPLase post-peel treatment cream to reduce erythema and continue using sunscreen. However, during a scheduled review on day 20, the patient pointed out some red patches that had developed around the eye area. I noted three distinct areas of excessive erythema with induration and slight hypertrophy. The affected areas were the medial aspects of the right lower eyelid and upper and lower left eyelid (Figure 2)



Treatment plan
I prescribed a topical corticosteroid (Betnovate) to be used twice daily on the affected areas which is designed to reduce inflammation and reduce risk of scarring. Four days later, the erythema had improved significantly and the area of induration seemed less visible. However, I made the decision to administer 0.12ml of intralesional triamcinolone (Kenalog) as this would simply increase the anti-inflammatory effect. I had been taught about this two-pronged attack at a medical conference a few years ago and felt that this was the most suitable option for the patient given her circumstances. The patient also underwent Dermalux LED red light therapy daily for around two weeks and continued with the topical
REFERENCES
1. Loftus C, The History of Exfoliation, Cinta Aveda Institute, November 2014 <https://blog.cintaaveda. edu/2014/11/the-history-of-exfoliation/>
2. Brody H, Monheit G et al., A History of Chemical Peeling, Dermatologic Surgery, December 2001
3. NHS.uk, Chemical peels <https://www.nhs.uk/conditions/cosmetic-procedures/chemical-peels/>
4. WebMD, Chemical Peels and Your Skin <https://www.webmd.com/beauty/cosmetic-procedureschemical-peel-treatments#1>
5. Verma A, Dull skin: reasons which make the skin dull, Lifealth, May 2018 <https://www.lifealth.com/ lifestyle/beauty/dull-skin-reasons-which-make-the-skin-dull-av/76457/>
steroid twice daily for three weeks. In addition, silicone topical gel Kelo-cote was also given for twice-daily application. One week later, good progress was noticed and the hypertrophic areas had at least halved in size (Figure 2). Another 0.12ml dose of Kenalog was injected and topicals were continued as above. A week later the hypertrophic areas had improved again and my advice was to continue with the topicals (both topical corticosteroid and silicone topical gel). Three weeks later, another 0.17ml dose of Kenalog was administered and results continued to improve. In another two weeks, the area had further improved and only 0.05ml of Kenalog was administered. Four weeks after this, I decided to perform microneedling with 1mm depth into the entire periorbital area. This was repeated one and three months later to improve the skin texture further.
Results
After more than three months of a consistent and continued treatment plan, the patient and I are extremely happy with the results and there is no further evidence of erythema or scarring. The skin texture continues to improve and I have recommended that she continues the use of SPF for daily use.
6. Forthwithlife.co.uk, Great Britain and Stress – How bad is it and why is it happening? <https://www. forthwithlife.co.uk/blog/great-britain-and-stress/>
7. Harvey F, More than 95% of world’d population breathe dangerous air, major study finds, The Guardian, April 2018
8. Nikalji N, Godse K et al., Complications of Medium Depth and Deep Chemical Peels, Journal of Cutaneous and Aesthetic Surgery, October-December 2012 9. Clay R, The link between skin and psychology, American Psychological Association, February 2015 10. SkinTech Pharma Group, Peelings <https://www.skintech.info/index.php/products/peelings>
Figure 2: Patient on day 20 when complication was noted, one month after initial treatment and then a following three months after a combination treatment plan. Images courtesy of Dr Xavier G clinic, taken through the VISIA Complexion Analysis system.
Before 1 month after treatment
Before 3 months after treatment
shared by Dr Xavier Goodarzian
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Avoiding Botulinum Toxin Complications
Oral surgeon Mr Sami Stagnell and maxillofacial surgeon Ms Natasha Berridge present an overview of the common complications of botulinum toxin by anatomical region and how to avoid them
In recent years, practitioners’ awareness regarding the potential complications of injectable treatments has vastly improved. The growth of groups such as the International Association for Prevention of Complications in Aesthetic Medicine (IAPCAM) and the Aesthetics Complications Expert (ACE) Group, as well as the rise of selfregulation, are a snowballing form of justice within the industry that strives to establish its credibility.
Following the parliamentary debate led by conservative MP Alberto Costo earlier this year, the Government has reiterated that it will continue to explore options to strengthen the regulation of nonsurgical cosmetic procedures and enhance patient safety through more streamlined training of practitioners.1 In fact, in May 2019 the Department of Health and Social Care launched a patient safety consumer campaign to help spread the word of the dangers behind surgical and non-surgical aesthetic treatments.2
Undeniably, there are now greater numbers of patients seeking cosmetic treatments to enhance their natural visage, fuelled by reality TV personalities and social media icons alike.3,4 Patients are often unaware of the associated serious risks that could occur if treatments are not performed correctly. Fundamentally, it is crucial that medical aesthetic practitioners maintain a diligent, standardised approach to patient selection and assessment. Furthermore, with the majority of practitioners engaging with the continuous learning required to develop as a trustworthy provider, it is imperative that the profession remains abreast of managing and dealing with complications. This article is not an instructional guide for complication prevention and management, but aims to raise awareness of how to minimise the incidence of botulinum toxin type A (BoNT-A) complications based upon the following facial aesthetic zones: upper face, mid-face and lower face and neck.
Consultation
Contraindications to BoNT-A treatment should be identified in the patient assessment: this includes infection at injection site, known hypersensitivity, as well as patients taking aminoglycosides and skeletal muscle relaxants as it may potentiate the effects of these drugs. However, in our experience, the injector is unlikely to come across these medications in routine practice. BoNT-A should be used with caution in patients with a history of neuromuscular disease e.g. myasthenia gravis.5,6
As part of the comprehensive patient assessment, careful evaluation of the underlying musculature, blood vessels and associated nerves are vital to optimising a successful aesthetic outcome. Furthermore,
the treating clinician must have an appreciation of the individual patient’s own muscle activity and determine if any pre-existing asymmetry exists and its cause;7 it is not unusual for BoNT-A to be used in the management of facial asymmetry following medical issues such as facial palsy.8
General risks of BoNT-A and avoidance
As with all injectables, there are risks associated with treatment, which might present simply as a side effect of treatment itself without being attributed to a specific causative factor; this includes an inability to meet patient expectations or undesired over/undertreatment of muscle groups.7,9 A retrospective review of a database endorsed by the American Society of Plastic Surgeons between 2008-2013 reported that despite a greater uptake of aesthetic facial procedures, when compared to younger patients, older patients (over 65) had a complication rate that was statistically insignificant despite confounding factors such as raised BMI, diabetes mellitus or health risks in the older age group.10
Fortunately, adverse events secondary to BoNT-A have been reported to be relatively uncommon and the majority tend to be mild and temporary; these include ecchymosis, flu-like illness, local injection site pain and erythema. Mild adverse events tend to occur in approximately 3% of patients.11,12 Most occur if the toxin is inadvertently injected into unintended muscle groups or erroneously diffuses into muscles in the surrounding region.13
More commonly occurring risks include pain, bleeding/bruising and headache.14 We recommend the use of pre-treatment application of ice and a local anaesthetic cream such as EMLA, avoid the repeated use of a blunted needle (risk when injecting with insulin syringes) and reconstituting BoNT-A with preserved normal saline to minimise the perception of pain.14,15 Good lighting and a proper injection technique will allow the practitioner to identify blood vessels before they are inadvertently damaged. Should bleeding occur, then sustained local pressure and/or ice usually suffices and the patient can be advised that bruising can be reduced with local application of arnica.16 Should post-injection headache occur then simple and effective analgesia is recommended.17,18
Infections are usually self-limiting and can be treated with antibiotics as required.19 These may be the result of inappropriate management of skin preparation prior to injection,20 or the presence of local infection at the time of injection.19
Serious complications associated with the injection of BoNT-A tend to be linked to pre-existing medical conditions such as
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
neuromuscular disease and severe hypersensitivity reactions. Botulinum toxin has the potential to exacerbate conditions such as myasthenia gravis, Eaton-Lambert syndrome or amytrophic lateral sclerosis secondary to the temporary blockage of acetylcholine release at the neuromuscular junction.21
1. Frontalis muscle
Always inject at the mid-forehead or above when treating the frontalis muscle. This should be at least 2cm above the brow in all patients and for older patients, ensure injections are at least 4cm above the brow.
2. Injection depth When treating the forehead, by injecting intradermally rather than intramuscularly, one study reported a lower incidence of ptosis with no reduction in results.26
3. Glabella muscle Always inject the glabellar at the same time as the forehead and never treat the frontalis muscle in isolation, particularly in patients over 50 years of age.2
Table 1: Recommendations for safe and successful BoNT-A treatments in the upper face. Table adapted from King 2016.25
Hypersensitivity reactions, including urticaria and life-threatening anaphylaxis, are often due to components that the toxin may have been prepared with, namely human albumin, lactose and sodium succinate.20
These can be managed with antihistamines, or in more serious cases of imminent airway compromise, practitioners should escalate care to the emergency services, whilst ensuring that the patient’s airway, breathing and circulation is intact. Deployment of standard medical emergency equipment is expected of practitioners; supplemental oxygen should be administered and, if available, an epinephrine autoinjector administered.22
There is one paper reported in the literature in 2005 regarding anaphylaxis and subsequent death from botulinum toxin administration; note that the woman was being treated for chronic neck and back pain rather than a cosmetic intervention.23 Death as a result of BoNT-A administration is incredibly unlikely and those reported to the FDA are typically as a result of therapeutic injections with significantly higher doses rather than following cosmetic interventions.24 However, this study by Cote et al. on reporting to the FDA highlights only data prior to 2005 in the US and does not take into account reporting elsewhere in the world, where reporting mechanisms are likely to vary.24 It should be noted that practitioners should be extra cautious treating patients who have had previous facial surgery due to distorted local anatomy and tissue fibrosis secondary to scarring.25
The upper face
In this region, most complications are isolated to injections into the frontalis and associated depressor muscles (orbicularis oculi, corrugators and depressor supercilii) and include eyelid ptosis, brow ptosis and ‘spock’ eyebrow.25 This area is where most clinicians new to injectables begin to practice and a region that is considered by many to be ‘safe’, although this is not necessarily the case if simple rules are not adhered to (Table 1).25
It must be noted that with experience, and as injectors become accustomed to variations in anatomy and clinical outcomes of administration of BoNT-A, it is likely that injectors will move away from conventional ‘prescribed’ injection patterns.27,28
There are two crucial elements to assess when considering the brow:
1. The anatomy of the frontalis muscle
2. Any pre-existing brow ptosis. It is important to note that there is a difference between brow ptosis as a result of inadequate elevator activity (latent myogenic ptosis) and a true blepharoptosis, which are often confused.29
Be aware that patients may present to treat the forehead
as a result of rhytid formation in this area, that is in fact ‘compensated’ brow ptosis.30 This means that the patient is subconsciously elevating/contracting the frontalis in an effort to avoid brow droop.30 If the underlying cause of brow/upper lid ptosis (general ageing, levator dehiscence or dermatochalasia) remains unrecognised and untreated, then treating the frontalis with BoNT-A will cause the patient to complain of a ‘heaviness’ in the area as their compensatory mechanism has been temporarily eliminated by the toxin.31,32 King (2016) suggested that in those aged over 50, the glabella region should be treated first to observe the clinical reaction before injecting the forehead.25,33
In 2007, Olson highlighted that inappropriate central placement of product within the forehead can result in an unbalanced brow, widely referred to as the ‘Spock brow’.31 This may also result following inappropriate assessment of frontalis anatomy. It is well documented that by simply injecting further smaller volumes of BoNT-A in the lateral frontalis, 2cm above the brow will correct the ‘Spock brow’.34,35
In 2012, Braz and Sakuma did a retrospective analysis of pictures taken from 83 patients and identified three different anatomical variations in the frontalis muscle. These were: total pattern in 50.6% of cases, medial pattern in 25.3% of cases, and lateral pattern in 24% of cases. This observation prompted their suggestion that different injection patterns might be required to treat the frontalis based upon this anatomical variation.36
Mid-face
The most common complications in the middle third of the face tend to be those affecting the periocular region. The reported incidence of upper eyelid ptosis following treatment of the upper third of the face (glabella and forehead region) with BoNT-A is up to 5.4%.37 This occurs as a result of injection or diffusion of the toxin into the
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Serious complications associated with the injection of BoNT-A tend to be linked to pre-existing medical conditions such as neuromuscular disease and severe hypersensitivity reactions
Anatomical region Complication
Upper face and mid-face
• Asymmetry
Ptosis of eyebrow or eyelid
• Unmasking of pre-existing, compensated eyelid ptosis (weakening of frontalis)
• Impairment of eyelid function/ocular physiology (weakening of orbicularis oculi)
• Lower lid retraction/scleral show (weakening of orbicularis oculi)
• Lip ptosis (weakening of lip elevators when addressing nasal indications)
• Atrophy
Lower face
• Asymmetry
• Oral motor insufficiency e.g. impaired ability to raise or lower the lip
• Impairment of dental show in animation (smiling)
• Impaired muscular support of lower face
• Dysphagia (when targeting platysma)
• Neck weakness (when targeting platysma)
• Dry mouth (when targeting platysma)
orbital septum, affecting the levator palpebrae superioris muscle.38 Total reversal of upper lid ptosis caused by BoNT-A can take up to three months. Therefore, in cases where ptosis affects vision or concerns the patient enough to warrant treatment, the use of 0.5% apraclonidine ophthalmic drops is recommended. Apraclonidine is an adrenergic agonist, which selectively increases eyelid elevation in the absence of levator function by stimulating Muller’s muscle to raise the lid by 1-2mm.39
It is important to appreciate the nearby associated musculature when treating the periocular region for lateral canthal lines (crow’s feet). BoNT-A injected into or diffusing into the inferior aspects of orbicularis oculi (OO) and/or the lower lid may result in increased scleral show.40 If severe, a lower lid ectropion may develop, ultimately leading to exposure keratitis41 or corneal damage.42 Should the aforementioned happen, treatment with lubricating eye drops and/or the application of an eyepatch at night may be indicated. Rarely, diplopia may occur if BoNT-A diffuses into the ocular muscles. Although temporary (usually four to six weeks) it can be severely debilitating and, whilst present, may require the patient to wear prism glasses to correct the oculomotor imbalance.43
The zygomaticus major (ZMa) and minor (ZMi) muscles have bony insertions (origins) at the lateral and medial aspects of the zygomatic buttress.44 Care must be taken when injecting the lateral or inferior aspects of the OO and control must be exercised on the depth of
injection to ensure ZMa or ZMi are not treated, resulting in ptosis of the lip on the ipsilateral side.45
This is more evident when the patient is asked to smile (asymmetric smile). Complications associated with BoNT-A injected into the periocular region can be avoided by injecting at least 1cm away from the lateral orbital margin.25
Lower face and neck
Over the years, BoNT-A has been used to effectively treat perioral rhytids.46 However, given the complex arrangement of musculature that exists in this region, the risk of diffusion and over-dosage is highly likely.35 Hence, the perioral region is a treatment area considered suitable for experienced injectors only. Treatment in or around the depressors (depressor labii inferioris, depressor anguli oris) is usually used to help lift corners of the mouth or contour the chin,47,48 but improper treatment may result in difficulty smiling and controlling the lower lip with eating and even drooling. 49,50 Notably, small doses with subsequent review and re-treatment are advocated: this was highlighted in the study by Carruthers (2010) that showed small doses of only BoNT-A in treating perioral ageing resulted in the least improvement, and so retreatment or combination with dermal fillers was recommended.51
Other areas in the lower face that may be indicated for treatment with BoNT-A, include the masseters.52 This is typically the case for patients who request jaw sculpting as a consequence of masseteric hypertrophy.53 Masseteric hypertrophy is commonly due to an underlying parafunctional habit, such as clenching or grinding.23 Despite the paucity of evidence towards the efficacy of treatment in this manner and one systematic review has found promising outcomes with this type of treatment.54 However, despite its so-called aesthetic effects, a 2018 study by Peng found that complications in 680 patients treated in the masseter muscles included:55
• Temporary mastication force decrease: 30%
• Bruising: 2.5%
• Headaches after 12 hours: 0.58%
• Smile limitation: 0.15%
• Paradoxical bulging: 0.49%
• Sunken cheeks (sub-zygomatic volume loss): 0.44%
• Sagging around the jawline: 0.20%
The ‘Nefertiti’ neck lift, as it is often marketed as, is a popular BoNT-A treatment for those requesting a non-surgical lift and/or improvement in the appearance of unsightly platysmal bands.56 BoNT-A is injected superficially intradermally, yet despite relatively low dosage (50-70u – Allergan cosmetic dosing units), it has been observed that patients may struggle with ‘sit-up’ type movements57 amongst other potential complications based on systematic reviews58 (Table 3).
Summary
When administered by a medical professional with adequate training, the administration of BoNT-A for cosmetic purposes is largely a predictive and relatively safe exercise in the vast majority of
Table 2: Potential adverse events from aesthetic use of botulinum toxin type A according to anatomical region. Table from Sundaram et al 10
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
The evidence around longterm serious adverse events for the use of BoNT-A for cosmetic procedures is poorly reported
patients with most complications avoidable or transient in nature. The evidence around long-term serious adverse events for the use of BoNT-A for cosmetic procedures is poorly reported. Despite a general agreement on the common complications anticipated with BoNT-A treatment, consensus groups have highlighted the need for further clinical studies to better understand the impact in different regions of the face and have also suggested a need for panfacial assessment and appropriate treatment planning to improve outcomes.59 It is believed that most incidents typically occur in therapeutic cases where doses administered are significantly higher than recommended and/or where patient health may contribute as a confounding factor. Nevertheless, it is important that the aesthetic practitioner is vigilant in their approach to managing patients. Ultimately, this will allow for appropriate treatment and consent, as well as having a strategy in place should they be faced with complications, thereby ensuring the best outcomes for their patients.
Mr Sami Stagnell is a specialist oral surgeon with a clinical interest in facial aesthetics having actively practised in the field for seven years. He has completed an MSc in Skin Ageing and Aesthetic Medicine with the University of Manchester. He has contributed to the JCCP stakeholder groups representing dental interests, as well as publishing in the dental literature on facial aesthetics.

Ms Natasha Berridge is an oral and maxillofacial surgeon with a specialist interest in reconstructive aesthetic surgery and aesthetic medicine. A fellow of the Royal College of Surgeons and member of the Faculty of Dental Surgery, Ms Berridge has nine years of experience in treating facial hyperhidrosis and other maxillofacial conditions with botulinum toxin.
REFERENCES
1. Kilgariff, S, News Special: Westminster Regulation Debate, Aesthetics, March 2019. <https:// aestheticsjournal.com/feature/news-special-westminster-regulation-debate>
2. Aesthetics, UK Government to launch patient safety campaign, Aesthetics journal, April 2019. <https:// aestheticsjournal.com/news/uk-government-to-launch-patient-safety-campaign>
3. ISAPS, ISAPS internationals survey on aesthetic/cosmetic procedures performed in 2017. https://www. isaps.org/wp-content/uploads/2018/10/ISAPS_2017_International_Study_Cosmetic_Procedures.pdf, 2017.
4. Newswire, P., Medical Aesthetics Market worth $17.07 Billion by 2023. https://www.prnewswire.com/ news-releases/medical-aesthetics-market-worth-17-07-billion-by-2023-877401471.html, 2018.
5. Klein AW, ‘Contraindications and complications with the use of botulinum toxin’, Clin Dermatol, 22 (2004), pp.66-75.
6. BOTOX (onabotulinumtoxinA) Label, FDA, Highlights Of Prescribing Information <https://www. accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf>
7. COX SE, ADIGUN CG. Complications of injectable fillers and neurotoxins. Dermatologic Therapy. 2011;24:524-536
8. Kim J. Contralateral botulinum toxin injection to improve facial asymmetry after acute facial paralysis. Otol Neurotol.2013 Feb;34(2):319-24.
9. Vartanian AJ,Dayan SH. Complications of botulinum toxin A use in facial rejuvenation. Facial Plast Surg Clin North Am.2005 Feb;13(1):1-10.
10. Sundaram H, Signorini M et al. Global Aesthetics Consensus: Botulinum Toxin Type A—Evidence-Based Review, Emerging Concepts, and Consensus Recommendations for Aesthetic Use, Including Updates on Complications. 2016. Plast Recons Surg 137 (3). 518-529
11. Monheit G, Carruthers A, Brandt F, Rand R. A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose.Dermatol Surg.2007;33:51–59
12. Kane MA, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Huber MB Reloxin Investigational G. Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg.2009;124:1619–1629.
13. BK Satriyasa. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: a literature review of clinical use and pharmacological aspect. Clin Cosmet Investig Dermatol. 2019; 12: 223–228
14. Bakheit AM. The possible adverse effects of intramuscular botulinum toxin injections and their management. Curr Drug Saf.2006 Aug;1(3):271-9.
15. Allen SB,Goldenberg NA. Pain difference associated with injection of abobotulinumtoxinA reconstituted with preserved saline and preservative-free saline: a prospective, randomized, side-by-side, doubleblind study. Dermatol Surg.2012 Jun;38(6):867-70.
16. Hamman MS, Goldman MP. Minimizing bruising following fillers and other cosmetic injectables.J Clin Aesthet Dermatol.2013;6(8):16–18
17. MacKay D, Miller AL. Nutritional support for wound healing.Altern Med Rev.2003;8(4):359–377
18. Leu S, Havey J, White LE, et al. Accelerated resolution of laser-induced bruising with topical 20% arnica: a rater-blinded randomized controlled trial.Br J Dermatol.2010;163(3):557–563
19. Management of Acute Skin Infections E Davies, M King, J Clin Aesthet Dermatol. 2017 Feb; 10(2): E5–E7.
20. Immunogenicity of botulinum toxins. M Naumann,LM Boo AH. Ackerman CJ. Gallagher. J Neural Transm. 2013 Feb; 120(2): 275–290.
21. Janis JE. Essentials of plastic surgery. 2nd Edition. 2014. Quality Medical Publishing Inc
22. Emma Davies, Anaphylaxis, ACE Group, 2018. <http://acegroup.online/guidelines/>
23. Li M, Goldberger BA, Hopkins C, Fatal case of BOTOX-related anaphylaxis? J Forensic Sci. 2005 Jan;50(1):169-72.
24. Cote TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases.J Am Acad Dermatol.2005;53:407–415
25. King M. Management of Ptosis.J Clin Aesthet Dermatol. 2016;9(12):E1-E4.
26. Redaelli A, Forte R. How to avoid brow ptosis after forehead treatment with botulinum toxin. J Cosmet Laser Ther. 2003 Dec; 5(3-4):220-2.
27. Olson JJ. Balanced botox chemodenervation of the upper face: symmetry in motion. Semin Plast Surg.2007 Feb;21(1):47-53.
28. J Anido, D Arenas, C Arruabarrena,et al., Tailored botulinum toxin type A injections in aesthetic medicine: consensus panel recommendations for treating the forehead based on individual facial anatomy and muscle tone. Clin Cosmet Investig Dermatol. 2017; 10: 413–421
29. Ali F, Khan MS, Sharjeel M, Din ZU, Murtaza B, Khan A. Efficacy of brow suspension with autogenous fascia lata in simple congenital ptosis.Pak J Med Sci. 2017;33(2):439-442.
30. Wu-Fienberg Y,Bafna KR,Guyuron B. Horizontal Forehead Lines: A Reflection of Eyelid Ptosis or Blepharodermachalasia. Aesthetic Plast Surg.2018 Dec;42(6):1551-1555. Epub 2018 Jul 20.
31. Olson JJ. Balanced botox chemodenervation of the upper face: symmetry in motion.Semin Plast Surg. 2007;21(1):47-53.
32. Jun JY, Park JH, Youn CS, Lee JH. Intradermal Injection of Botulinum Toxin: A Safer Treatment Modality for Forehead Wrinkles.Ann Dermatol. 2018;30(4):458–461. doi:10.5021/ad.2018.30.4.458
33. Prado AC, Andrades PR. Caution in using botox in patients with previous frontal surgery. Plast Reconstr Surg. 2002 Apr 1; 109(4):1472-3
34. Cho ES, Hwang JY, Kim ST. A proposal to prevent the “Mephisto sign” side effect of botulinum toxin type A injection in chronic migraine.Yonsei Med J. 2013;54(6):1542-4.
35. Levy LL, Emer JJ. Complications of minimally invasive cosmetic procedures: prevention and management.J Cutan Aesthet Surg. 2012;5(2):121–132. doi:10.4103/0974-2077.99451
36. Braz AV, Sakuma TH. Patterns of contraction of the frontalis muscle: a pilot study. Surg Cosmet Dermatol. 2010;2(3):191-4.
37. Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, Walker P, Eadie N, BOTOX Glabellar Lines I Study Group. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002 Jun; 46(6):840-9
38. Başar E, Arıcı C. Use of Botulinum Neurotoxin in Ophthalmology.Turk J Ophthalmol. 2016;46(6):282-290.
39. Scheinfeld N The use of apraclonidine eyedrops to treat ptosis after the administration of botulinum toxin to the upper face. Dermatol Online J. 2005 Mar 1;11(1):9.
40. Kaynak-Hekimhan P. Noncosmetic periocular therapeutic applications of botulinum toxin.Middle East Afr J Ophthalmol. 2010;17(2):113-20.
41. Ricci LH, Navajas SV, Carneiro PR, Söderberg SA, Ferraz CA. Ocular adverse effects after facial cosmetic procedures: a review of case reports.J Cosmet Dermatol. 2015 Jun; 14(2):145-51

42. Vartanian AJ, Dayan SH. Complications of botulinum toxin A use in facial rejuvenation. Facial Plast Surg Clin North Am. 2005 Feb; 13(1):1-10
43. Rutstein RP,Cogen MS. Elimination of paradoxical diplopia following treatment with botulinum toxin and prism. Binocul Vis Strabismus Q.2004;19(1):35-8.
44. Beer JI, Sieber DA, Scheuer JF, Greco TM. Three-dimensional Facial Anatomy: Structure and Function as It Relates to Injectable Neuromodulators and Soft Tissue Fillers.Plast Reconstr Surg Glob Open. 2016;4(12 Suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):e1175.
45. Carruthers, J. and Carruthers, A. (2003), Aesthetic Botulinum A Toxin in the Mid and Lower Face and Neck. Dermatologic Surgery, 29: 468-476. doi:10.1046/j.1524-4725.2003.29115.x
46. Semchyshyn N, Sengelmann RD. Botulinum toxin A treatment of perioral rhytides. Dermatol Surg. 2003 May; 29(5):490-5; discussion 495.
47. Goldman A, Wollina U. Elevation of the Corner of the Mouth Using Botulinum Toxin Type A. J Cutan Aesthet Surg., (2010) 3(3), pp.145- Dermatol Surg. (2010) 36 Suppl 4, pp.2121-34.
48. Carruthers J, Carruthers A. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin.Dermatol Surg.1988;24:1189–94
49. Sarrabayrouse MA. Indications and limitations for the use of botulinum toxin for the treatment of facial wrinkles. Aesthetic Plast Surg. 2002 Jul-Aug; 26(4):233-8
50. Goldman A, Wollina U. Elevation of the corner of the mouth using botulinum toxin type a.J Cutan Aesthet Surg. 2010;3(3):145–150. doi:10.4103/0974-2077.74490
51. Carruthers A, Carruthers J, Monheit GD, Davis PG, Tardie G. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxinA and hyaluronic acid dermal fillers (24mg/ml smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010 Dec;36 Suppl 4:2121-34.
52. Al-Muharraqi MA1, Fedorowicz Z, Al Bareeq J, Al Bareeq R, Nasser M. Botulinum toxin for masseter hypertrophy. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007510.
53. Zohaib Ullah, Lower Face Aesthetics, 2018 <https://aestheticsjournal.com/feature/lower-faceaesthetics>
54. De la Torre Canales G, Câmara-Souza MB, do Amaral CF, Garcia RC, Manfredini D. Is there enough evidence to use botulinum toxin injections for bruxism management? A systematic literature review. Clin Oral Investig. 2017 Apr;21(3):727-734.
55. Peng HP, Peng JH. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J Cosmet Dermatol. 2018 Feb;17(1):33-38.
56. Brandt FS Boker A. Botulinum toxinfor the treatment of neck lines and neck bands. Dermatol Clin.2004 Apr;22(2):159-66.
57. Tamura BM The Effect of Botulinum Toxin on the Platysma Muscle. Current Dermatology Reports 2012; 1(2),89–95
58. Conor M Sugrue, Jack L Kelly, Niall McInerney; Botulinum Toxin Treatment for Mild to Moderate Platysma Bands: A Systematic Review of Efficacy, Safety, and Injection Technique,Aesthetic Surgery Journal, Volume 39, Issue 2, 17 January 2019, Pages 201–206
59. Sundaram H, Signorini M, Liew S, et al. Global Aesthetics Consensus: Botulinum Toxin Type A-Evidence-Based Review, Emerging Concepts, and Consensus Recommendations for Aesthetic Use, Including Updates on Complications.Plast Reconstr Surg. 2016;137(3):518e–529e.
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com






















BEAUTY BOOSTING REDENSIFICATION HYDRATION FIRMNESS RADIANCE
Case Study: Vascular Compromise
This article will describe a vascular complication that arose during injection of hyaluronic acid filler to the anterior cheek. Many practitioners may not be faced with an acute presentation of a complication such as this, but it is important to be prepared for all eventualities. As such, the article will stress the importance of having the emergency kit on site so as to treat immediately, thereby re-establishing blood flow, preventing tissue anoxia, ischaemia and necrosis.1
The consultation and treatment plan
A 35-year-old female patient presented for dermal filler injection to the anterior cheek following consultation. She had the medial and lateral cheek treated three months prior, with an appropriate volumising hyaluronic acid filler administered by another practitioner. She was happy with the medial and lateral projection of her cheeks from that procedure, but felt that she needed some projection in the anterior cheek as it appeared flat when viewed from the side. The anterior cheek area was not treated during the previous session. On history, the patient was otherwise healthy and a non-smoker and had consumed no alcohol in the days prior to the procedure. There was a known allergy to penicillin and previous gastric sensitivity to nonsteroidal anti-inflammatory drugs (NSAID). On aesthetic history, the patient previously had upper face toxin and lip filler, with minor lumps removed with hyaluronidase. In preparation for the procedure, the area to be treated was disinfected with a chlorhexidine solution and marked. The plan was to inject volumising filler (0.3% lidocaine) indicated for the cheek in a direct injection to the periosteum with micro bolus placement in a small dose of around 0.1ml0.3ml per cheek using the product provided with 27 gauge needles.
The treatment and complication
The right cheek was treated first. The needle was placed onto the periosteum and aspiration for 10 seconds was performed with no flashback in the needle hub. A total of 0.2ml was injected. There was minimal bleeding at the injection site, which subsided with mild pressure. No bruising was noted. The area was massaged to facilitate optimal placement of the filler. A good resultant projection of the anterior cheek on the right was achieved and this was shown to the patient, which she was happy with. The needle on the syringe was changed to a new 27 gauge needle as supplied and the left side was prepared for treatment. The needle was placed on the periosteum and aspirated again for 10 seconds, with no flashback in the needle hub and the injection of filler commenced. Around 0.1ml of filler was injected when acute skin changes, loss of colour and blanching was noted. The anterior and lower cheek on the left side turned pale and the patient reported pain of 5/10 at the injection site. The location included the area medial to the nasolabial fold and the upper lip. The injection was immediately stopped, needle and syringe removed and the patient was notified that a complication had occurred. Injection into the vasculature or vascular territory of the infraorbital artery was identified as the most likely cause. On examination, capillary refill time was noted at six seconds over the affected area; as we know, normal capillary refill time is usually 1-2 seconds.2 The patient was reassured that emergency treatment would commence and that the remainder of the procedure would be abandoned. I had my emergency kit close to hand, which needs to include hyaluronidase, water for reconstitution, and a heat pack, among other items.3 The patient became anxious, but was reassured that the complication could be handled successfully. A warm gauze pack was applied to the area to encourage vasodilatation while the hyaluronidase was reconstituted. In accordance with the ACE Group consensus paper,4 the hyaluronidase 1,500 IU was reconstituted with 2.5ml of normal saline to a concentration of 60 IU/0.1ml to facilitate adequate coverage of the affected area. The solution of hyaluronidase was injected in four points in and around the area of filler injection using a 30 gauge needle. The remainder of the solution was injected along the course of
the vasculature in the territory of the mid and lower cheek, as well as the upper lip in 0.1ml aliquots approximately 5-10mm apart. The area was firmly massaged and after around four minutes the area started to blanch, at which point a hyperaemic response was noted. Massages to the area alternating with the warm gauze were done for a total of 75 minutes at five to eight-minute intervals. The second vial of hyaluronidase was reconstituted and kept to the side for further infiltration, but was not required. The patient was not keen to take any aspirin due to NSAID gastric sensitivity. The patient experienced no visual disturbances or ocular or periocular pain after hyaluronidase injection. The character of the pain in the affected area had changed to a burning-type pain and the patient also reported feeling that the nasolabial and the upper lip area were a little numb. Overall, pain was tolerable and was reported as 6/10. Capillary refill time was improved following the infiltration of hyaluronidase and had improved from three seconds at 20 minutes after hyaluronidase to two seconds at 75 minutes.
Following treatment
The patient was reviewed the following day and prescribed clarithromycin 500mg twice a day for 14 days as a precaution for potential infection as well as prednisolone 20mg daily for four days to reduce inflammation. Pain was managed using co-codamol (30/500) two tablets six hourly, or as required. On examination, there was marked bruising to the affected area as well as a subconjunctival haemorrhage on the affected side. Capillary refill time was two seconds and some livedo reticularis was present over the area. Visual acuity was tested and found to be normal with no ocular or periocular pain. Neurosensory examination was done bilaterally and found to be intact. The patient was reassured and also given a vitamin K oxide cream for topical application to reduce bruising. When I reviewed and examined the patient on day three, the swelling around the area was steadily decreasing and pain was 2/10 with some numbness over the cheek. This was most likely due to the soft tissue inflammation following hyaluronidase injections. I told the patient to continue prednisolone for a further six days. On the day five examination, the swelling and bruising was <50% compared to day one and the subconjunctival haemorrhage was almost completely resolved. The patient could feel her lip and the bruising was confined to the infraorbital region. The lower region
Dr Zunaid Alli shares a filler complication case study and his steps for management
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
A guiding light in laser aesthetics

Cynosure has consistently set the market standard since 1991 and continues to do so.
Significant body of clinical evidence behind our technology so you can be confident you are making a safe choice.
Significant investment in research and development so you can be confident you are buying from the right long term partner.
Outstanding clinical training so you can be confident in getting the most out of your laser.
Consistent first class after sales service and support so you can be confident your lasers are always at peak performance.
There has never been a better time to invest with Cynosure.
Body Contouring • Cellulite Treatment •
Hair Removal • Skin Pigmentation & Revitalisation Tattoo Removal • Vascular Treatment • Acne Scars
Gynaecology
To learn more visit www.cynosureuk.com or call us on 01628 5222 52
of the cheek, which appeared pale, had a capillary refill time that was normal, and the inflammation in and around this area in particular was improving. The patient continued to be reviewed at two to three day intervals with examination and reassured every day throughout the treatment process. By day 14 the inflammation and bruising had resolved with no further symptoms. The patient continues to be actively monitored.

Discussion
A report of two case studies describes early hyaluronidase use in preventing skin necrosis after treatment with dermal fillers.5 The study further describes skin necrosis is the most significant complication after dermal filler injection in areas with terminal vascular circulation.5 As a recommendation, aspiration during infiltration is suggested. This was conducted prior to injection bilaterally and the needle was changed after treatment on the right so as to ‘dry aspirate’. Despite a negative result in both aspirations, the complication occurred. Further studies show marked differences in obtaining true positive results following aspiration tests, with one study reporting reliability of 53%6 and a larger study citing 33%;7 however, the limitation in both studies are confined to physiological mimicked tests. Withdrawal of the syringe plunger with no visible blood in the syringe does not eliminate the possibility of intravascular placement of the syringe needle.8 Further difficulties with aspiration tests are movement of the needle as the injector changes hand position to apply pressure onto the plunger to commence injection.7 The two primary diagnostic symptoms of vascular occlusion are pain and changes in skin colour,9 which were present in this case. This risk is higher for vascular events when large bolus injections are sent deeper into tissues for volume enhancement and when smaller needles are used.10
Learning points
Complications can happen to even the most experienced practitioners, and every time there is a complication important factors are highlighted. The main learning points from this case are as follows:
• Identify immediate complications, especially vascular ones and act accordingly. Remain calm.
• New injectors must ensure that they attend courses where complications are covered in detail and experienced injectors should ensure that they adhere to the latest guidelines in managing complications. All practitioners need to refresh their knowledge and remain up-to-date with local guidelines and current best evidence.
• Inform the patient that a complication has occurred and to be open, honest and provide reassurance. This includes detailed explanations about what the root cause of the complication is and how it is to be managed, thereby providing the best care possible.
• Provide accessible contact information including out of hours.
• Ensure that early appropriate referrals are made, for example, if there is ocular issues the patient must be referred to an ophthalmologist.
• Provide and maintain clearly documented communication with the patient throughout their treatment journey. In this case, the patient was gracious enough to recognise that complications do occur, which was because they were explained to her in detail in the consultation. However, she was still, nonetheless, very anxious about any permanent damage or delayed consequences.
• Provide that any steroids, antibiotics, vasodilators, analgesia and any other adjunctive treatment are promptly prescribed and dispensed.
• Ensure that the injector’s emergency kit is readily available with all products in date and all consumables are present to prevent
unnecessary delays.9
• Where possible, attempt to use dermal cannulas when injecting filler so to minimise risk, especially in the danger areas, for example the anterior cheek, the glabella or the nasolabial folds.

• Do not rely on aspiration alone. This case is a clear example that a negative aspiration does not rule out possible vascular involvement.
Summary
Vascular complications may rarely arise when injecting dermal fillers so it is important that the practitioner knows the anatomy of the vasculature, the vascular territory and has a dermal filler emergency kit to hand. Swift recognition of complications, especially vascular ones need to be treated timely to avoid further consequences such as necrosis and tissue loss.
Dr Zunaid Alli is an aesthetic practitioner with a background in general and acute medicine, and oncology. Dr Alli is the lead clinical trainer for Glow Aesthetic Training and a KOL for Vivacy UK.


REFERENCES
1. ACE Group, Guidelines. ACE Group Emergency kit v2.2 <http:// acegroup.online/guidelines>
2. Emma Davies & Martin King, The ACE Group Emergency Kit. <file:///Users/shannon.kilgariff/Downloads/ACE-GroupEmergency-Kit-v2.2.pdf>
3. StevenMcGeeMD,‘Peripheral Vascular Disease’, Evidence-Based Physical Diagnosis (Fourth Edition), 2018.
4. Martyn King, Cormac Convery & Emma Davies, The use of hyaluronidase in Aesthetic Practice (v2.4), Journal of clinical and aesthetic dermatology, 11(2018), E61-E66
5. Francesco Ciancio, Maria Stella Tarico, Giuseppe Guidice & Rosario Emanuele Perrotta, Early Hyaluronidase use in preventing skin necrosis after treatment with dermal fillers: Report of two cases
6. Gabriela Casabona, Blood aspiration test for cosmetic fillers to prevent accidental intravascular injection in the face, Deramtologic Surgery. 2015 July. 41(7): 841-847

7. Jani AJ Van Loghem, James J Fouche & Job Thuis, Sensitivity of aspiration as a safety test before injection of soft tissue fillers, J Cosmet Dermatol. 2018;17:39-46.
8. Carey W and Weinkle S, Retraction of the plunger on a syringe of hyaluronic acid before injection: Are we safe?, Dermatol Surg 2015 December; 41 Suppl 1:S340-6

9. Urdiales-Gálvez F, et al.,Treatment of Soft Tissue Filler
Complications: Expert Consensus Recommendations, Aesthetic Plast Surg. 2018 Apr;42(2):498-510.
10. Claudio De Lorenzi, complications of injectable fillers, Part 2: Vascular complications, Aesthetic Surgery Journal, 2014, vol. 34(4) 584-600
Day 1 Day 3 Day 5 Week 2
Figure 1: Patient complication journey from day one to two weeks following the complication.
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com







High Intensity Focused Ultrasound (HIFU) works by focusing ultrasound so that the defocused beam passes through the epidermis painlessly and at the focal point reaches temperature to break down collagen or fat. Why the Cambridge Stratum SkinTyte II?
• An evolution from the proven SkinTyte - faster, more comfortable, easier to use 3-year warranty
• 3D multi-line scanning - twice as fast and more comfortable than single line machines
• 3mm, 4.5mm and 13mm standard heads. Head options: 1.5mm to 16mm available
• Long life consumables (guaranteed up to 10K lines with expected use of up to 20K)
• Smart mode to simplify operation and Professional mode for full flexibility • Robust, compact, portable clam-shell design • Full marketing support
SkinTyte II An effective treatment for skin tightening and body contouring DEFINE SCULPT CONTOUR
• Training included • Finance available
To find out more or to arrange a demonstration, please contact us Tel: 01223 846881 Email: info@cambridgestratum.com www.cambridgestratum.com High Intensity Focused Ultrasound (HIFU) works by focusing ultrasound so that the defocused beam passes through the epidermis painlessly and at the focal point reaches temperature to break down collagen or fat. Why the Cambridge Stratum SkinTyte II?
• An evolution from the proven SkinTyte - faster, more comfortable, easier to use 3 -year warranty
scanning - twice as fast and more comfortable than single line machines • 3mm, 4.5mm and 13mm standard heads. Head options: 1.5mm to 16mm available • Long life consumables (guaranteed up to 10K lines with expected use of up to 20K) • Smart mode to simplify operation and Professional mode for full flexibility • Robust, compact, portable clam-shell design • Full marketing support • Training included • Finance available SkinTyte II An effective treatment for skin tightening and body contouring DEFINE SCULPT CONTOUR To find out more or to arrange a demonstration, please contact us Tel: 01223 846881 Email: info@cambridgestratum.com www.cambridgestratum.com High Intensity Focused Ultrasound through the epidermis painlessly and Why the Cambridge Stratum SkinTyte • An evolution from the proven SkinTyte • 3D multi- line scanning - twice as • 3mm, 4.5mm and 13mm standard • Long life consumables (guaranteed • Smart mode to simplify operation • Robust, compact, portable clam • Full marketing support • Training included • Finance available SkinTyte An effective treatment DEFINE To find out more or Tel: 01223
• 3D multi-line
Treating the Male Periorbital Area
With facial aesthetic treatments becoming more popular amongst males,1 practitioners need to facilitate a gender-adjusted approach for rejuvenation treatments and familiarise themselves with how they may need to amend their techniques.

The Men’s Maximum Difference study in 2015 by Allergan explored 600 men’s attitudes towards age-related facial changes. It found that the periorbital area was the greatest area of concern for respondents, with lateral canthal lines and tear troughs being the top features that 80% of respondents were most likely to get treated first.2
A different survey carried out by the American Society of Plastic Surgeons between 20082011 found that the number of men in the US seeking botulinum toxin injections rose by 268%.1 A trend that has likely continued as aesthetic treatments in general have become more popular. Despite the fact that more men are jumping on the aesthetic ‘bandwagon’, the majority of most practitioners’ clientele remain female.1,2 There is therefore a lack of experience as well as a lack of supporting evidence when we think about facial aesthetic treatments in males. This can lead to suboptimal outcomes, unless one recognises the anatomical differences, and aesthetic goals between the sexes. This article will explore the differences in the periorbital area between men and women to help practitioners provide a gender-adjusted approach for their male patients.
Anatomical differences between the sexes
The differences in facial anatomy between the sexes start at the skeletal level. Forensic science has taught us that the skull is the second most reliable indicator of sex assessment of a skeleton, coming second only to the pelvis.3-6 The female skull tends to have a flat supraorbital ridge with a smooth convexity of the forehead, whereas the male skeleton has a prominent supraorbital ridge. In the periorbital area, the glabellar and frontonasal suture are more pronounced in males and the eyebrows are flatter, whereas they tend to be subtler and more curved in females.7,8 These underlying skeletal differences give rise to differences within the soft tissues above. When we think about what is considered desirable and aesthetically pleasing in males, strong contours such as a sharply-defined brow and chiselled cheek-bones often come to mind.9-12 These masculine facial features develop with testosterone exposure during puberty and many studies have shown that they are found to be attractive.9-12 Due to the prominent supraorbital ridge, males also tend to have more deep-set eyes. The brow in males is more horizontal and normally sits at the level of the supraorbital rim, rather than arching above it like in females. Females also tend to have an upward tilt of the lateral canthus (corner of the eye) whereas in males this landmark tends to be level.13
Ageing changes to the periorbita
The ageing process affects all layers of the face from the skin down to the skull. The bony orbital aperture increases in size and width in both males and females. It has been found that the inferior orbital rim recedes most significantly laterally in females, whereas in males the entire rim recedes.14 Males have bulkier facial muscles and the loss of subcutaneous fat and connective tissue that occurs with age means that men tend to develop wrinkles earlier and more severely than in women – notably in the periorbital, glabellar and forehead.15
Treatment considerations
Research has shown that there tends to be a lower adoption rate for aesthetic procedures amongst men than women.2 Of the 600 men surveyed in The Men’s Maximum Difference study, the trial rate for all procedures was low (2-6%).2 When asked about this in the survey, 47% of men said that they felt that they did not yet need the treatment.2 So, whilst women strive for the appearance of youth, why are men more content to let the ageing process take its natural course? A large study looked at online users of a free dating site across four large US cities (186,935 subjects) and found that whilst women were deemed on average to reach their peak attractiveness at 18, men peak at age 50.16 The trend for finding women more attractive in youth and males more attractive in older age has also been observed in other studies.17,18 There are many theories as to why this is the case. Many opinions suggest these findings align with evolutionary theories of mating where youth suggests fertility, encouraging males to find young and potentially more fertile females more attractive. Females on the other hand are thought to be attracted to males who are
Ophthalmologist Miss Jennifer Doyle explores the gender differences in the periorbital area and how to treat men using injectables
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
The glabellar and frontonasal suture are more pronounced in males and the eyebrows are flatter, whereas they tend to be subtler and more curved in females











likely to be able to provide for offspring, and age may indicate a position of power that can facilitate this.19 Therefore, when treating the male patient, it’s important to keep the face masculinised, not just aiming for youthfulness. Social structural theorists argue that the differences in age between partners may be fuelled by sociallydetermined gender roles.20
Botulinum toxin
With lateral canthal lines being one of the top concerns amongst aesthetically-minded males,2 treatment with botulinum toxin to the area may be considered. Whilst I recommend starting with the standardised dosing, it is important to be aware that males are thought to respond less to toxin products than females.21 This is generally suggested to be due to the increased muscle bulk compared to females. Practitioners should be aware that an increased proportion of males are likely to require further units at a two-week review than females. It is important to also remember the underlying anatomical differences between the sexes, and not to feminise the male face, unless this is requested. For example, when using toxin to treat the glabella and frontalis, one must be careful not to arch or lift the brow, as this would not be in keeping with the masculine brow which tends to be low and flat.7,8 Ensuring that one treats the lateral aspect of the frontalis will avoid any lateral ‘spocking’ which again, whilst in some female patients can give an attractive outcome with an enhanced arch of the brow, would only feminise the male face. Unfortunately, there is a paucity of clinical data with regards to botulinum toxin use in males, and many have called for further studies regarding the dosing and efficacy.22 There are only two studies to date that account for sex in their study design or subgroup analysis, generally finding that toxin is less effective in males and they often require higher doses than females.22
Acknowledging the theory that males become more attractive with age, it would make sense for practitioners not to overdo the toxin for the male lateral canthal lines, as some lines could still be deemed attractive in men. However, I cannot find any studies supporting this.
Dermal filler
When treating the periorbital area with filler, we need to remember to respect and preserve the masculine features. Tear trough deformity is, alongside lateral canthal lines, one of the most likely areas to be treated first amongst males.2 The orbital aperture increases in size in both genders, contributing towards a tear trough deformity which can be addressed with dermal filler. CT scans of the skull have shown us that the orbital rim recedes unevenly, and there are important differences between males and females. Whilst in females the inferolateral orbital rim recedes more significantly,14 in males the inferomedial orbital rim also has a tendency to recede.23 This can affect the morphology of the tear trough deformity, and one should consider this when placing your filler to the tear trough area.
There are some features in the periorbital area that we would wish to keep, such as a prominent supraorbital ridge. Whilst in women this feature may lend itself to filler treatment to restore the smooth convexity of the forehead, obliterating this masculine feature in male patients risks feminising the face.24,25
Summary
When we are treating our male patients, it is important to remember the underlying anatomical differences between the sexes, and to adapt our technique accordingly. Whilst men make up a small percentage of aesthetic patients, they are on the increase and yet
the evidence and experience amongst practitioners of treating males is often lacking. When treating the periorbital area in males, strive to retain masculine features, unless the patient’s aim is to feminise their face, and be aware of the underlying anatomical differences that can affect the response to treatment, and adapt your practice accordingly.
Miss Jennifer Doyle has a Bachelor in Medicine and a Bachelor of Surgery with distinction, as well as a Master’s in Medical Sciences from the University of Oxford. She has completed the Level 7 in Injectables and is a lead trainer at Harley Academy. Miss Doyle currently works as an NHS registrar in ophthalmology, as well as leading her clinic Oxford Aesthetics.

REFERENCES
1. American Society for Plastic Surgery 2011 statistics. <from:http://www.plasticsurgery.org/News-andResources/2011-Statistics-.html>
2. Allergan, Allergan Presents Men’s Maximum Difference Data at American Society of Dermatologic Surgery Meeting, 2015. <https://www.allergan.com/news/news/thomson-reuters/allergan-presentsmen-s-maximum-difference-data-at.aspx>
3. Ubelaker DH, Volk CG. A test of the Phenice method for the estimation of sex.Journal of Forensic Sciences.2002;47(1):19–24.
4. France DL. Observation and metric analysis of sex in the skeleton. In: Reichs KJ, editor.Forensic Osteology: Advances in the Identification of Human Remains.Springfield, Ill, USA: Charles C. Thomas; 1998. pp. 163–18
5. Pickering RB, Bachman DC.The Use of Forensic Anthropology.Boca Raton, Fla, USA: CRC Press; 1997
6. Bass WM.Human Osteology: A Laboratory and Field Manual.5th edition. Missouri Archaeological Society Columbia; 2005
7. Marquardt SR. Dr Stephen R Marquardt on the golden decagon and human facial beauty. Interview by Gottlieb.J Clin Orthod2002;36(6):339-47.
8. Toledo Avelar LE, Cardoso MA, Santos Bordoni L, de Miranda Avelar L, de Miranda Avelar JV. Aging and Sexual Differences of the Human Skull.Plast Reconstr Surg Glob Open. 2017;5(4):e1297. Published 2017 Apr 27.
9. Fink B, Neave N, Seydel H. Male facial appearance signals physical strength to women. American Journal of Human Biology. Volume 19. Issue 1. December 2006.
10. Rennels, J. L., Bronstad, P. M., & Langlois, J. H. (2008). Are attractive men’s faces masculine or feminine? The importance of type of facial stimuli.Journal of Experimental Psychology: Human Perception and Performance, 34(4), 884-893.
11. Thornhill R, Gangestad. Facial attractiveness. Trends in Cognitive Sciences. Volume 3, Issue 12, 1 December 1999 Pages 452-460
12. Fink B, Penton-Voak I. Evolutionary Psychology of Facial Attractiveness. Current Directions in Psychological Science. 1 October 2002
13. Mark A. Codner, MD,Clinton D. McCord, Jr., MD Eyelid and Periorbital Surgery, Second Edition CRC Press,22 Jun 2016
14. David M. Kahn, Robert B. Shaw; Aging of the Bony Orbit: A Three-Dimensional Computed Tomographic Study,Aesthetic Surgery Journal, Volume 28, Issue 3, 1 May 2008, Pages 258–264,<https://doi.org/10.1016/j.asj.2008.02.007>
15. Luebberding S. Krueger N. Kerscher M. Quantification of Age-Related Facial Wrinkles in Men and Women Using a Three-Dimensional Fringe Projection Method and Validated Assessment Scales. Dermatology Surgery Volume 40 Issue 1. 25 November 2013.
16. Bruch E, Newman M.E.J. Aspirational pursuit of mates in online dating markets. 5 Sci Adv 4 (8), eaap9815.
17. C. Rudder,Dataclysm: Love, Sex, Race, and Identity—What Our Online Lives Tell Us about Our Offline Selves(Crown Books, 2015)
18. P.England,E. A.McClintock,The gendered double standard of aging in US marriage markets.Popul. Dev. Rev.35,797–816(2009).
19. Buss, D. M. (1989). “Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures”.Behavioral and Brain Sciences.12(1): 1–14.
20. Luke, N (2005). “Confronting the ‘Sugar Daddy’ Stereotype: Age and Economic Asymmetries and Risky Sexual Behavior in Urban Kenya”.International Family Planning Perspectives.31: 6–14.
21. Derek H. Jones, MD; Martina Kerscher, MD; Thorin Geister, MD; Michael A. Hast, MD; Petra Weissenberger, MD. “Efficacy of Xeomin for the Treatment of Glabellar Frown Lines in Male Subjects: Post-Hoc Analyses from Randomized, Double-Blind Pivotal Studies,” ASDS annual meeting, October 2017.
22. Keaney TC, Alster TS. Botulinum toxin in men: review of relevant anatomy and clinical trial data. Dermatol Surg. 2013 Oct;39(10):1434-43.
23. Pessa JE, Chen Y (2002) Curve analysis of the aging orbital aperture. Plast Reconstr Surg 109:751–755.
24. Mutaz B. Habal. Aesthetics of feminizing the male face by craniofacial contouring of the facial bones. .Aesthetic Plastic Surgery. December 1990, Volume 14, Issue 1, pp 143-150
25. De Maio, Mauricio. Ethnic and Gender Considerations in the Use of Facial Injectables: Male Patients. Plastic and Reconstructive Surgery, Volume 136, Supplement 1, November 2015.
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Re-treating after Dissolving Fillers
Dr Saleena Zimri discusses the treatment of patients with hyaluronic acid after previous dissolution and shares three successful case studies
With the increasing use of hyaluronic acid (HA) dermal fillers in the non-surgical aesthetic industry,1 we inevitably will come across more and more patients that require filler removal. This is either due to incorrect placement, poor results, lumps and bumps or simply through changes in aesthetic trends. Removal due to trends and more complications in general are certainly what I am seeing in my practice. In this article, I will discuss the use of hyaluronidase, what happens physically and anatomically when HA is dissolved, considerations around when to re-treat and best practice guidance. It’s important to note that emergency reversal is not covered and is outside the scope of this article.

What is hyaluronidase?
Hyaluronidases are a family of enzymes widely used as off-label drugs by aesthetic practitioners to prevent complications such as necrosis, treat nodules and remove unwanted or overcorrected HA. They are also naturally occurring enzymes found in the human body. 2 Hyaluronidases have been used widely in medicine for a number of years as a diffusing agent.2 Some examples of its use have been in treatment of vitreous haemorrhage, prevention of tissue damage in extravasation of substances and in fertility treatments to remove the cumulus-corona-oocyte complex formed during intracytoplasmic sperm injection.2 Hyaluronidase is derived from ovine/bovine testicles, recombinant human hyaluronidase, leech/hook worm or microbial.2 In the UK, we have access to Hyalase, a 1500 unit ampoule of freezedried powder for reconstitution which is of ovine origin.3 It is reported that those with an allergy to bee stings (or other stinging insects) would also be highly likely to be allergic to this form of hyaluronidase due to some cross allergenicity with the hyaluronidase in bee venom.4,5,6 Other brands of hyaluronidase include Vitrase and Hylenex however, for the purpose of this article, I will focus on Hyalase due to having most experience using this particular product.
Recombinant human hyaluronidase (Hylenex for example) has a purity 100 times higher than other formulations and is a non-foreign protein, so is proven through a number of multiple clinical studies to have a lower incidence of allergic reactions.4,7
Dosing
How does it work?
Hyaluronidase acts by hydrolysing HA in tissues, increasing cell membrane permeability, reducing viscosity and leaving tissues more pervious to injected fluids.8
The effects of hyaluronidase on skin tissue is very poorly studied at present with very little literature on it.
Often patients ask me if using hyaluronidase can impact their natural collagen. According to a study by Cavallini et al., which tested human fibroblast and human skin cultures in a lab setting, hyaluronidases did not affect fibroblast proliferation or human skin viability at low dosages of around 14 units.9
Buhren et al., also suggest in their in vitro experimental data that any degradation of the human body’s own HA by injection of hyaluronidase will be immediately replaced by de novosynthesis of HA in fibroblasts.10
Large dose studies are yet to be conducted.
A consensus opinion in the literature review conducted by King et al.,4 on behalf of the Aesthetic Complications Expert (ACE) Group, states five units of Hyalase is needed to break down 0.1ml of 20mg/ml HA. The group has also broken this down by providing guidelines on hyaluronidase units per region (Figure 1) which in my opinion isn’t clear given that each practitioner and every face is unique, therefore require different quantities of filler. We should note that there aren’t any recommendations for the lips specifically.4 However, the ACE Group states that in the event of a suspected vascular obstruction, a high-dose protocol, between 450-1,500 units, should be adopted.4 In my practice, I often dissolve and correct poor lip filler treatment and superficially-placed tear trough filler.
Figure 1: Aesthetic Complications (ACE) Group UK guidelines on hyaluronidase units per region4
While I am not aware of recommendations for dosages in the lips, I personally find that there is a need for higher doses than the recommended amounts for other areas for a number of reasons:
• Most patients have had numerous previous treatments in this area • Practitioners in my opinion tend to select a higher viscosity and cross-linked product and so it can be more difficult to breakdown11
• I personally find intramuscular deposits of filler are harder to remove due to spreadability from muscle movement
• To avoid the need to repeat hyaluronidase treatment and reduce allergen potential
Hyaluronidase units Nasal and perioral skin 15-30 Periorbital 3-4.5 Infraorbital 10-15 Lower eyelid 1.5
Region
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Case Study 1
This 29-year-old patient had HA dermal filler injected in her lips by another practitioner. She felt that after a few months the filler appeared to have been placed incorrectly giving her a ‘duck lip’, had migrated and that the upper lip was too projected and stiff. The patient wanted to dissolve the old filler and start fresh with my own technique.





The first stage was to dissolve. I used 350 units of Hyalase 1,500 ampoule which brought the lips back to their near original state. Some filler remained in her lower lip but it

wasn’t unsightly and so I decided not to dissolve any further. The patient returned two weeks later and I re-filled with 1ml of HA dermal filler in both her upper and lower lip using a combination of needle and cannula. I generally try to leave it two weeks (a week longer than necessary) to allow for any side effects such as bruising and swelling to have completely resolved before re-treating. I usually advise patients to take an antihistamine for any swelling and avoid heavy exercise for one to two days, by which time most of the swelling has resolved.
Typically if a patient has had more than 1ml (which is more than likely for the lips), I use around 200-500 units, sometimes more, to remove an entire lip in one or two sittings. I make this decision based on the above listed reasons but after carrying out a full, indepth consultation. It’s important to note that one size does not fit all and dosage is patient dependent. In tear troughs, it is usually the complete opposite. I find very low doses are more than adequate to remove filler and largely due to the opposite reasons of the first three listed. In my experience, I inject 10-20 units on average. I like to use small fine 0.3ml micro-syringes with pre-attached needles to allow for better precision in the tear trough.
Re-treating a patient
When injected, hyaluronidase has an immediate working effect on HA in tissue. It is known to have a half-life of around two minutes, however, its duration of action is longer at around 48 hours.4,7
It is thought the reasons behind this are:
1. Low number of units required to have a clinically significant effect and so its action continues despite degradation
2. Initial breakdown of HA crosslinking leaves free HA in the skin, which is known to have a half-life of 24-48 hours4
Case Study 2
This 34-year-old patient had undergone numerous treatments prior to presenting in my clinic; however, the original anatomy wasn’t respected by the treating practitioner. Upon clinical examination, I observed that too much filler had been placed in the border of the lip. After repeated treatment, this had migrated into the orbicularis muscle. Overall, the lip was highly volumous and, in my opinion, looked distorted. She reached out to me to try and improve the size and overall shape. I dissolved the filler using 1,000 units of hyaluronidase after knowing the patient’s previous history and products used. Following treatment there was still some filler remaining in the lower lip. As it was still in the correct tissue plane and the patient preferred volume in the lips, I decided to leave it there. We refilled after two weeks with the aim of providing good volume, however respecting the natural anatomy and keeping in line with her lip borders. In total, 1.5ml of dermal filler was used to create this.
I personally only re-treat after two weeks to allow for any bruising and swelling to have completely resolved
Poorly placed filler Immediately after dissolution
Re-treatment after 2 weeks
Poorly placed filler
Immediately after dissolution
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Re-treatment after 2 weeks
Case Study 3
This 40-yearold patient had her tear troughs injected some months prior by an inexperienced practitioner who unfortunately placed most of the filler superficially, creating pooling and a Tyndall effect (blue appearance). She asked for her existing filler to be dissolved and for a re-treatment to help improve the hollows under her eyes. After the usual patch testing



I placed 15 units per trough superficially using a 0.3ml micro-syringe with a pre-attached needle and massaged for 60 seconds to help with spread. This helped to dissolve the filler, restoring the area to the original state. I re-treated two weeks later to allow for any side effects to settle with a low-density HA filler, placing it deeper to help avoid the problem repeating again.
Considerations
It is important to be aware of the consideration associated with not only dissolving filler but re-treating too. If re-treated too soon after dissolution, you may also dissolve the new filler placed, which may require further treatment and dissatisfaction, as well as increased cost, for the patient.
As well as this, one of the key considerations is that sometimes we are unaware of what filler was used or how much for that matter, making it difficult to determine effective dosing. In this case I would advise to always over-dose, for the reasons explained above.
Conclusion
As our industry expands it is just as important for practitioners to maintain good knowledge about how to reverse and correct treatments as well as performing them in the first place. Although literature around this topic is limited, it seems promising that there is no indication so far of long-term damage to skin or fibroblast activity. Of course more research surrounding this is necessary. I would recommend that practitioners always patch test and aren’t afraid to help patients achieve their desired aesthetic outcome through dissolution of HA dermal filler and re-treatment. As always, ensure that a full medical history is taken and you are competent and experienced in the specific indication.
Dr Saleena Zimri is the co-founder of the Skin Doctor Clinics and set up her first aesthetic skin and laser clinic 10 years ago in Yorkshire. The company has three branches now with the recent opening of a clinic in Manchester to add to previous Leeds and York locations. She is an international speaker and KOL for Sinclair Pharma and has had numerous publications in the press.

REFERENCES
A small study on murine conducted by Kim HJ et al., concluded that six hours after reinjection of a monophasic filler following hyaluronidase, the filler materials restored almost its original volume and there were no significant differences from the positive control.12 Considering the above and evidence of free HA in the skin post hyaluronidase, I would support re-treatment no earlier than 48 hours to avoid breakdown of any consecutive re-treatment with HA filler. I personally only re-treat after two weeks to allow for any bruising and swelling to have completely resolved. There may also be an argument for patch testing for hyaluronidase before a patient has filler for the first time. This is particularly important, in my opinion, due to the lack of available human hyaluronidase in the UK market at present and the rise in patients having filler removed.13
Patch testing
Before proceeding to treat with hyaluronidase it is safe and expected practice that a patch test is performed, unless in an emergency situation, according to the ACE Group.14 This is usually with eight to 20 units of hyaluronidase injected intradermally on the patient’s forearm and observe.14 A positive reaction consists of a wheal with pseudopods (arm-like projections) appearing within five minutes and persisting for 20 to 30 minutes, accompanied by localised itching. Transient vasodilation (i.e. erythema) at the site of the test is not generally regarded as a positive reaction.4 Note also that false negative patch tests do occur so I would advise that you make sure your clinic is fully equipped with anaphylaxis kits.
1. LaingBuisson, The UK cosmetic surgery market, October 2018 <https://www.laingbuissonevents. com/wp-content/uploads/2018/10/Liz-Heath.pdf>
2. Cavallini M, Gazzola R et al., The Role of Hyaluronidase in the Treatment of Complications From Hyaluronic Acid Dermal Fillers, Aesthetic surgery journal, 2013
3. Medsafe.govt.nz, Product Summary, Hyalase <https://medsafe.govt.nz/Profs/Datasheet/h/ Hyalaseinj.pdf>
4. King M, Convery C, Davies E, This month’s guideline: The use of Hyaluronidase in Aesthetic Practice, Journal of Clinical and Aesthetic Dermatology, June 2018 < https://europepmc.org/ articles/pmc6011868>
5. Ewan P, Venom allergy, British Medical Journal, May 1998 <https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC1113072/>
6. Bailey S, Cohen J et al., Etiology, Prevention and Treatment of Dermal Filler Complications, Aesthetic Surgery Journal, January 2011 < https://academic.oup.com/asj/article/31/1/110/273423>
7. ScienceDirect, Recombinant Hyaluronidase < https://www.sciencedirect.com/topics/ neuroscience/recombinant-hyaluronidase>
8. Girish K, The magic glue hyaluronan and eraser hyaluronidase: A biological overview, Life Sciences, May 2007 < https://www.sciencedirect.com/science/article/pii/ S002432050700210X#aep-section-id29>
9. Cavallini M, Antonioli B et al., Hyaluronidases for treating complications by hyaluronic acid dermal fillers: evaluation of the effects on cell cultures and human skin, European journal of plastic surgery, August 2013
10. Buhren B, Schrumpf et al.,Hyaluronidase: from clinic application to molecular and cellular mechanisms, European Journal of Medical Research, February 2016
11. Funt D, Pavicic T, Dermal fillers in aesthetics: an overview of adverse events and treatment approaches, Clinical, Cosmetic and Investigational Dermatology, December 2013 <https://www. ncbi.nlm.nih.gov/pmc/articles/PMC3865975/>
12. Kim H, Kwon S et al., The duration of hyaluronidase and optimal timing of hyaluronic acid (HA) filler reinjection after hyaluronidase injection, Journal of Cosmetic Laser Therapy, February 2018
13. Cherrington R, Lip Filler Removal Guide: From How Hyaluronidase Works to Picking A Safe Practitioner, Huffington Post, January 2017
14. ACE Group, The Use of Hyaluronidase in Aesthetic Practice, February 2014 <http://acegroup. online/wp-content/uploads/2016/01/ACE-Group-Hyaluronidase-v1.2.pdf>
Poorly placed filler
Re-treatment after 2 weeks
Immediately after dissolution
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com










PARK PLAZA WESTMINSTER BRIDGE HOTEL SECURE YOUR TICKETS TODAY INDIVIDUAL TICKET: £305 +VAT TABLE OF 10: £2,900 +VAT WWW.AESTHETICSAWARDS.COM JOIN THE CELEBRATIONS! UNMISSABLE NETWORKING OPPORTUNITIES A GLAMOROUS DRINKS RECEPTION WITH 800+ INDUSTRY PEERS FIRST-CLASS ENTERTAINMENT HOSTED BY A TOP COMEDIAN WITH LIVE MUSIC FROM AN UNMISSABLE BAND DELICIOUS THREE-COURSE DINNER COMPLEMENTARY GLASS OF BUBBLY, TABLE WINE AND PETIT FOURS INCLUDED RENOWNED AWARDS CEREMONY PRACTITIONERS, CLINICS AND COMPANIES ALL RECOGNISED FOR STELLAR ACHIEVEMENTS Group CATEGORY SPONSORS

Understanding Periocular Vascular Anatomy

off an emboli that can backflow into any of the arteries directly supplying the optic nerve or retina.8 The key consideration with understanding this anatomy is that there is a huge network of anastomoses between all of these arteries. This is clearly demonstrated in Figure 2 where you can see all of the branches of the ophthalmic artery and its anastomoses with other branches, as well as with arterial branches of the ECA.9 This includes branches of the ECA anastomosing
LOV
following
this complication is rare, understanding how to avoid it and how to treat it if it did happen, is a vital part of being an aesthetic practitioner. On the rare occasion that LOV does occur, it will usually be within seconds after an appropriate artery has been affected. The central retinal artery, being one of the most important, once occluded leaves only a 60 to 90-minute window before LOV is irreversible. This is demonstrated in a study conducted by CJ Hwang et al. in 2019 where New Zealand red rabbits
injectable fillers Whilst
vascular anatomy
orbital and ocular
Supraorbital Central retinal Dorsal nasal Long & short posterior & anterior ciliary Lacrimal Muscular Posterior & anterior ethmoidal Internal palpebral Frontal Table 1: Arterial branches of the ophthalmic artery supplying the orbital and ocular regions of
Figure 1: Key arteries surrounding and supplying the orbit2,3 Middle meningeal artery Long and short posterior and anterior ciliary Zygomaticofacial artery Facial artery Infraorbital artery Superior labial artery Angular artery Dorsal nasal artery Nasociliary artery Supratrochlear artery Supraorbital artery Central retinal artery Lacrimal artery Ophthalmic artery Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Dr Zoya Diwan details the
in the
area to prevent blindness as a potential complication following filler treatment
the eye2,5
Artery Supply
Supraorbital
Dorsal nasal
Central retinal
Long and short posterior and anterior ciliary
Infraorbital
Middle meningeal
This artery supplies the forehead and eyebrows as well as the superior rectus and levator palpebrae superioris muscle.2
As a terminal branch of the OA, it supplies the superior region of the nose.2
This is the very first branch of the OA and supplies the retina of the eye.2
Together these arteries generally supply the iris, ciliary bodies, sclera and rectus muscles, and are the main supply of the optic nerve head.6
This is a branch of the ECA that comes off of the maxillary artery and exits the skull via the infraorbital foramen. It supplies the lacrimal sac and duct, and partly to some of the extraocular muscles of the eye, as well as the orbital floor.3
This gives off an orbital branch through the superior orbital fissure and does not usually supply the orbit itself.3
Table 2: Branches of the ophthalmic artery and the regions they supply
were used to stimulate hyaluronic acid gel filler-associated vascular occlusive blindness.10 Fundus photography and electroretinogram changes were recorded at 30, 60, 90, and 120 minutes. Out of the eight eyes experimented on, two had recorded partial occlusion and six eyes had fully occluded ophthalmic arteries. One of the partially occluded eyes demonstrated some improvement 60 minutes after injection of retrobulbar hyaluronidase; however, electroretinogram readings remained flat over the 120-minute period of observation.10 A literature review conducted by S Tobalem et al. in 2018, found this time to be much shorter however. They reported the retinal ganglion cells, supplied by the central retinal artery, are part of the central nervous system which undergoes infarction after non-perfusion of 12-15 minutes or less.11 The most common signs and symptoms of impending blindness include pain, sudden LOV or blurred vision, ptosis and weakness/ paralysis of ocular muscles (ophthalmoplegia).8 This is due to the disturbed flow to multiple arteries surrounding the eye including, for example, the supraorbital and infraorbital arteries. A meta-analysis conducted by Dr K Beleznay in 2015 concluded that there had been 98 cases of LOV after filler injections (including autologous fat, hyaluronic acid, poly-L-lactic acid and calcium hydroxyapatite) reported.6 After injection into the glabella region, causing 38 out of the 98 cases of LOV, the nasal, nasolabial fold and forehead were the most common regions that resulted in LOV after filler injections.6,8,12 Almost 50% of these were due to autologous fat injections, followed by 23.5% caused by hyaluronic acid fillers and the rest due to poly-L-lactic acid, paraffin,
collagen polymethyl methacrylate, silicone oil and calcium hydroxyapatite.6,12 The periocular region, including the tear trough region and cheeks, poses a moderate risk, accounting for a total of 11 out of the 98 injected regions that resulted in blindness, according to Dr Beleznay’s 2015 paper.6 It was reported that most cases of vision loss did not recover. Central nervous system complications were seen in 23.5% of the cases.6 The same authors then carried out a second review to update this information in 2019. It was concluded that 48 new published cases of partial or complete vision loss after filler injections were identified from January 2015 to September 2018.13 The most common and highest risk regions that results in LOV were the nasal region, glabella, forehead and nasolabial fold. Hyaluronic acid filler was the cause of these complications in 81.3% of cases.13 Ten cases experienced complete recovery of vision, whereas eight reported only partial recovery.13 In 2018, there was also a paper published by Thanasarnaksorn et al., which highlighted a case series of six patients that developed sudden loss of vision secondary to hyaluronic acid dermal fillers.14 Treatment areas included the nose (4), temples (1) and forehead (1). The researchers found that fast and early intervention including hyaluronidase injections and ocular massage were effective at reversing or at least restoring some of the ophthalmic circulation in some patients.14
Walker and King created a list for the emergency management:8
1. If a patient complains of eye pain or sudden visual loss, then stop treatment immediately 2. Place patient in supine position 3. Call emergency services and prepare to transfer patient to the nearest specialist eye hospital via blue light with detailed notes and contact the ophthalmologist at the receiving hospital 4. Whilst waiting, reduce intraocular pressure with timolol 0.5% 1-2 drops, use a re-breathe paper bag and give 300mg of aspirin 5. Attempt to dislodge the embolus by using ocular massage 6. Use emergency dose hyaluronidase and inject it into the treated area (retrobulbar injections should only be performed by an ophthalmic specialist within a 90-minute window)
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Internal
Central retinal artery Posterior ciliary arteries Muscular arteries Lacrimal artery Supraorbital artery Lateral palpebral artery Medial palpebral artery (or could brand from dorsonasal) Dorsonasal artery Supratrochlear artery Inner retina Short ciliary arteries (10-20) Long ciliary arteries (2) Lateral (superior) Medial (inferior) Anterior ethmoid artery Posterior ethmoid artery Ethmoid and frontal sinuses Ethmoid and sphenoid sinuses SR, SO, levator Lacrimal sac (through suprachoroid) Choroid Optic nerve head Ciliary body and iris Anterior ciliary arteries (from rectus) m. vessels LR, SR, SO, levator MR, IR, IO Episclera and conjunctiva Nasal cavity Eyelids Lacrimal gland Zygomatic arteries Recurrent meningeal artery Circle of Zinn Middle meningeal artery Orbicularis Zygomatic artery Superficial temporal artery Anterior temporal artery Transverse facial artery External carotid artery Angular artery Facial artery Maxillary artery IR, IO Infraorbital artery Major circle of the iris Palpebral arcades Anastomosis Anastomosis supplying lower lid, nose and cheek Anastomosis supplying forehead
Anastomoses between the
the
different regions and muscles that are supplied by different arteries.
carotid artery Ophthalmic artery
Figure 2:
ICA and branches of
ECA. This figure also shows the
Adapted from L Remington9
There is also the Aesthetics Interventional Induced Visual Loss (AIIVL) Consensus Group, which launched a few years ago, which aims to provide guidelines for managing such complications.15,16 In addition to the list provided by Walker and King, the group advises giving glyceryl trinitrate spray sublingually and providing guidance for specialists in a secondary care setting, when the patient is not in clinic for example. The AIIVL Consensus Group also provides guidance on how best to support the patient and report the complication to authorities.16

Conclusion
The vascular anatomy of the eye is complex with a multitude of anastomoses between the ECA branches and the OA and its branches. This explains why certain arterial areas of the face, including the nasolabial fold and glabella region, are high risk for LOV complications. Of course, there are factors that you could adopt to make injection technique safer, which is outside the scope of this article, but sound understanding of the anatomy of the vascular system surrounding the eye is of key importance for any aesthetic practitioner practising dermal fillers.
Dr Zoya Diwan graduated from St Bartholomew’s Hospital and the London Medical School and holds a first class degree in Anatomy and Human Sciences from King’s College London. She is currently the medical director of Trikwan Aesthetics, chair of the Academic Aesthetics Mastermind Group and also works as an aesthetic trainer.

REFERENCES

1. Meyer F. Zur Anatomie der Orbitalarteien. Morphol Jahr 1887; 12: 414–458.
2. Hayreh SS. Orbital vascular anatomy.Eye (Lond). 2006 Oct. 20(10):1130-44.[Medline]
3. (https://emedicine.medscape.com/article/1189696-overview) Oct 20, 2017Author: Hon-Vu Q Duong, MD; Chief Editor: Thomas R Gest, PhD


4. Forrester, JV. The Eye (Fourth Edition), Basic Sciences in Practice, 2016.
5. Morrison, J.C., van Buskirk, E.M., 1984. Am. J. Ophthalmol. 97, 372–383 in Figure11.25f in Bron, A.J., Tripathi, R.C., Tripathi, B.J., 1997. The choroid and uveal vessels. In: Wolff’s Anatomy of the Eye and Orbit, eighth ed. Chapman and Hall Medical, London (Chapter 11)
6. Beleznay, K.,Carruthers, J.D. A.,Humphrey, S., Jones, D., (2015)Avoiding and Treating Blindness From Fillers: A Review of World Literature.Dermatologic Surgery, October 2015 <https://www.ncbi.nlm.nih. gov/pubmed/26356847>
7. Hayreh, SS. Posterior Ciliary Artery Circulation in Health and Disease The Weisenfeld Lecture. Investigative Ophthalmology & Visual ScienceMarch 2004, Vol.45, 749-757. doi:10.1167/iovs.03-0469
8. Walker, Lee, and Martyn King. “This month’s guideline: Visual Loss Secondary to Cosmetic Filler Injection.”The Journal of clinical and aesthetic dermatology vol. 11,5 (2018): E53-E55.

9. Lee Ann Remington, The Clinical Anatomy and Physiology of the Visual System, Third Edition, 2012.
10. Hwang CJ, Mustak H et al., Role of Retrobulbar Hyaluronidase in filler-associated blindness: evaluation of fundus perfusion and electroretinogram Reading in an Animal Model, Ophthalmic Plastic & Reconstructive Surgery, 2019 <https://www.ncbi.nlm.nih.gov/pubmed/29877958>
11. Tobalem S, Schutz JS et al., Central retinal artery occlusion – rethinking survival time, BMC Ophthalmology, April 2018 <https://www.ncbi.nlm.nih.gov/pubmed/29669523>
12. Townshend, A. Blindness after facial injection. Journal of Clinical and Aesthetic Dermatology.Published online December 2016.
13. Belezany K, Carruthers JDA, Humphrey S, Jones DJ, Update on Avoiding and Treating Blindness From Fillers: A Review of the World Literature. Dermatologic Surgery, May 2019 <https://www.ncbi.nlm. nih.gov/pubmed/30805636>
14. Thanasarnaksorn W,Cotofana S Rudolph C Kraisak P Chanasumon N Suwanchinda A. Severe vision loss caused by cosmetic filler augmentation: Case series with review of cause and therapy. J Cosmet Dermatol.2018 Oct;17(5):712-718. doi: 10.1111/jocd.12705. Epub 2018 Jul 13.
15. Gronow C, News Special: The AIIVL Consensus Group, July 2017. <https://aestheticsjournal.com/ feature/news-special-the-aiivl-consensus-group>
16. Humzah MD,Ataullah S,Chiang C,Malhotra R,Goldberg R, The treatment of hyaluronic acid aesthetic interventional induced visual loss (AIIVL): A consensus on practical guidance. J Cosmet Dermatol.2019 Feb;18(1):71-76. doi: 10.1111/jocd.12672. Epub 2018. https://www.ncbi.nlm.nih.gov/ pubmed/29885087
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Dealing with Complaints
Aesthetics asks senior claims technician
Emma Bracchi how Hamilton Fraser Cosmetic Insurance supports practitioners faced with complaints and complications

What are the main reasons aesthetic practitioners contact you?
Practitioners usually get in touch with Hamilton Fraser Cosmetic Insurance for advice and reassurance. If they’ve had a situation where a patient is unhappy, which can be days, weeks or even months after a treatment, practitioners value our help in managing it successfully. We’re here every step of the way to advise and guide them on what they can do to avoid a complaint escalating.
What are the most common reasons patients may not be happy?
Aesthetic patients can be unhappy either because the results of treatment do not meet their expectations or they may have experienced a side effect or complication following treatment. We find the most common reason is that they’re simply unhappy with the results. Some patients have the impression that dermal filler or botulinum toxin treatment will change their face completely, so it is important practitioners manage expectations and make clear to patients that results should be judged on an individual basis.
What would Hamilton Fraser do to support a practitioner in a situation where a patient is not happy?
Our in-house claims team will go through a step-by-step process with the practitioner to identify the type of complaint that has been raised. They will need to find out exactly what treatment the patient came in for, what products were used, whether anything went wrong and clarify the main reason the patient is unhappy. We will assess the communication the practitioner had with the patient and work out the best course of action. This will result in advising whether the complaint should be dealt with as ‘dissatisfaction’ or a ‘formal claim’. Accurate identification of the type of complaint is crucial as it will determine whether it triggers a practitioner’s insurance policy. In the case of a formal claim, using the insurance policy will be necessary, whereas if we deem the issue as dissatisfaction, we will advise the practitioner how best to manage it without making a claim against their insurance.
Dissatisfaction
• Unhappy with results/outcome of the treatment
• Treatment not successful
• Not happy with aftercare
• Request for refund or free treatment
If a regular patient, who has had no issues in the past, complains that they are unhappy with the result of a treatment and it hasn’t caused a complication, we generally manage the complaint as dissatisfaction. This means the complaint will be dealt with swiftly and the practitioner will hopefully retain the patient. Sometimes, the evidence provided by the practitioner indicates that no mistakes have been made and expectations were clearly outlined to the patient before treatment. In these cases, we provide helpful advice on how best to communicate this to the patient and not impact retention. Of course, if mistakes are recognised, or it appears that expectations weren’t managed effectively, then we would discuss and advise whether a refund or future free treatment would be appropriate for this patient. It is important that practitioners understand that offering refunds or free treatments is a commercial decision, so wouldn’t be something that their insurance would cover. It will, however, hopefully resolve a complaint with limited impact to a practitioner’s reputation and practice.
Formal claim
• Bodily injury
• A solicitor’s letter of suggestion of legal intervention
• Request for compensation
If it appears that the complaint relates to a complication so significant that it would be
Tops tips to avoid a complaint
• Review policy regularly: ensure that you are covered for all the treatments you perform and that the products you use are specified. If you are a large company or organisation, you will need to ensure all your practitioners are also noted on your policy
• Patient selection: if there are any indications during the consultation and consent process that suggest the patient is not suitable for treatment, it may be worth advising them that you do not think you are the right practitioner for them as you cannot meet their expectations
• Consent form: ensure it details all eventualities and the patient thoroughly understands the risks of treatment before signing
• Expectations: be open and honest as to whether you feel like their expectations are achievable – ensure patients understand the limitations, expected results, side effects and potential complications that could occur following aesthetic treatment
• Treatment time: do not treat patients on the same day as consultation – it’s best practice to give the patient time to consider their options and the associated risks
• Before and after photographs: take high-resolution images immediately before and after treatment and ensure these are time-stamped
•
Before and after care: give patients leaflets to take home with your recommendations and follow up with an email copy of this advice to create a documented paper trail
• Contactable post treatment: if you know you are not going to be available, make sure you provide contact details for a peer or colleague who your patient can contact instead
• Complaints procedure: this will provide you with a clear and professional process in the event that you do receive a complaint, which can be dealt with timely and efficiently
Aesthetics | July 2019 44 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com Advertorial Hamilton Fraser
necessary to claim against insurance, we class it as a formal claim. In these cases, we will ask the practitioner for the full patient file. This should include before and after pictures, consultation notes, copies of the patient’s signed consent form and the completed medical questionnaire. We will also need the solicitor’s letter or request for compensation, a training certificate for the practitioner who performed the treatment, correspondence with the patient and a full summary of events, detailing how the practitioner feels the treatment went and whether they agree with the allegations. We really encourage practitioners to be honest straightaway. If they know something went wrong during the procedure, it’s so much better that we know from the start so we can deal with the complaint most efficiently.
It’s really important that practitioners read their policy wording when they take out insurance, because there are certain criteria that have to be met in order for a claim to be dealt with under their policy. For example, with any injectable treatment claim, the practitioner must be able to supply photographs taken immediately before the treatment and we strongly recommend having the ‘after’ photographs too. These will be your biggest line of defence if a patient claims a treatment didn’t work. Hamilton Fraser is proud of the fact that between May 2018 and May 2019, we dealt with 316 dissatisfactions and 213 reported claims. A greater proportion of these would have become formal claims without our intervention in resolving the matter.
In a scenario where a practitioner has contacted you because the patient has been injured, what would you do?
First of all, we would find out what the injury is and whether the risk of it occurring has been outlined in the consent form. While we don’t write consent forms ourselves, we advise that practitioners consult manufacturer guidelines for the products they use, which usually have consenting advice within them. Anything that could happen should be noted on the consent form, regardless how insignificant it may seem. If it’s not and a patient raises a complaint about, for instance, bruising following an injection – which as we know is to be expected – we would still have to class it as a formal claim.
Hamilton
Annual Complications Statistics
A survey of Hamilton Fraser Cosmetic Insurance customers found that:
While a practitioner’s medical malpractice policy relates to issues with treatment, we offer a Cosmetic Redress Scheme (CRS) to manage any concerns with service. These could be things like cancelled appointments, issues with payments or general bad customer service. For a one-off payment to join (which will differ depending on the size of the clinic) any service complaints will be efficiently managed by our handlers on a practitioner’s behalf. Each complaint is subject to a fee and practitioners can sign up to the CRS by visiting our website or giving us a call.
Hamilton Fraser is here to guide practitioners through any complications or complaints that may arise and to help minimise the chances of things going wrong in the first place. We provide lots of advice in the form of guides and articles on our website, as well as in our monthly newsletters. We’d also encourage practitioners to attend our Aesthetic Business Conference (ABC) on September 26, which will be packed with educational sessions from leading industry experts in a variety of fields.
Email: info@hamiltonfraser.co.uk Phone: 0345 310 6300 Website: hamiltonfraser.co.uk/cosmetic-insurance
Aesthetics | July 2019 45 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Advertorial
Fraser
What would happen if a practitioner contacted you about a complaint relating to their service?
How can practitioners seek further advice from you and the Hamilton Fraser team?
63%
of
63 % 71 % reassured
81 % 93 % managed complications independently needed
33 %
complications were minor and self limiting
the patient without fur ther inter vention had no complications in 5 years
fur ther inter vention by a medical professional


work and health worries.1 Stress varies based on an individual’s perception of what is stressful and their perceived abilities to cope. Human sympathetic nervous system stress responses are designed for short term situations and vary widely depending on individual perception,
suggesting that one person may cope better than another when placed under the same stress and whether the stress is acute or chronic. If it exceeds the body’s ability to
A study that compared hair cortisol concentrations (HCC) in both identical and non-identical twins demonstrated that stress perception
The study shows that in 72% of subjects, HCC was an inherited trait. The research also provides evidence of a link between perceived stress, neuroticism and depressive symptoms with genetic factors. Although more large scale, long term studies are required, these findings highlight that stress perception is part of our genetic makeup and will likely vary widely
There is much evidence to suggest that both acute and chronic stress directly impacts
This is caused by hypothalamic-pituitary-adrenal (HPA) axis disruption, leading to neurogenic and inflammatory response triggers at skin Additionally, skin actively participates
The main clinical symptoms of skin ageing are wrinkle formation, hyperpigmentation and loss of Skin and its appendages are highly sensitive to environmental changes and act as stress and anxiety response

ageing from natural, physiological changes over time, usually genetically preinfluenced by UVA and UVB, repetitive muscle movements, diet, sleep
The classification of stress is also two-fold: where inner thoughts and feelings crowd the mind, leading to feelings of sadness and anxiety, unrealistic
caused by situations arising around us, often beyond our control such as employment or family
One controlled murine study demonstrated that a stressed mouse group developed UV-induced neoplasia at a much faster rate compared with the non-stressed control group.18 With this in mind, when assessing patients for stress levels, it is important to
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019
Topicals for stressed skin
Patients should be given access to appropriate skincare alongside any advice and treatment to help combat external and internal stress effects on the ageing processes. Some studies have highlighted that application of topical lipids such as ceramides and free fatty acids will restore stratum corneum homeostasis, limiting skin’s stress response.38,39 Antioxidant-rich skincare serves to protect skin from long-term effects of external, internal and physical stressors, particularly vitamin C, lycopene, vitamin E and niacinamide.40 I would advise patients with inflamed, sensitive skin to avoid products containing synthetic Q10 which has a high contact dermatitis rate.40 For dry and flaky skin, emollients such as octyl octanoate or octyl stearate fill in the rough spaces of corneocytes leading to improved skin smoothness.41 Sunscreen is also absolutely fundamental for all patients, but particularly those who are experiencing stress, as research has indicated that physical and emotional stressors profoundly heighten skin’s inflammatory response.18,19,21
explore their exposure to physical stressors including UVA and UVB light and air pollutants including carbon monoxide, cigarette smoke and sulphur oxide, which in combination with stress may further negatively impact skin’s ageing process.19-21
Hyperpigmentation and melasma
Cortisol is the primary human stress hormone, and when its levels are heightened, epidermal and hair follicle keratinocytes, melanocytes, sebocytes, and mast cells respond via the HPA axis by secreting corticotropin-releasing hormone (CRH).22
CRH is pro-inflammatory, acting to prevent keratin cell synthesis whilst promoting mast cell degranulation.23 Under these circumstances, melanocytes and dermal fibroblasts increase production of adrenocorticotropic hormone (ACTH) and corticosterone, creating a chain where interleukin-18 production in keratinocytes, alongside melanocyte cell production, is stimulated.22
Melanocyte production is further accelerated by raised levels of alphamelanocyte stimulating hormone (a-MSH).14 Although minimal, there is evidence to suggest a link between sudden onset of human melasma and acute emotional stress.24 Adding to this is heightened TYR gene activity, coded to promote further melanocytic proliferation through production of tyrosinase, leading directly to hyperpigmentation.25
Wrinkle formation
Rhytids form where there is reduction in dermal and epidermal thickness.26 The rhytids may be static (visible without expression)27 or dynamic (observed at all ages and appearing with expression).28 There is evidence that suggests links between stress-induced inflammation and mitochondrial defects, directly contributing to wrinkle formation.9
Mitochondria can be described as the ‘power house’ of cells as they manufacture energy to drive all cellular functions, so it follows that mitochondrial disturbances disrupt protein production, leading to decline in autophagic degradation and an increase in reactive oxygen species (ROS), cellular organelle deterioration and ultimately, mitochondrial DNA (mtDNA) damage.29
One study provided evidence that depleted mtDNA function produced in mice through induction of dominant-negative mutation in the polymerase domain of POLG1, alongside the introduction of doxycycline to further impair mitochondrial function, directly resulted in formation of wrinkles and inflammatory infiltrate at histological level; interestingly, with the removal of doxycycline the murine mtDNA disfunction was reversed over a one-month period.9
Another study exerted sustained overcrowding onto mice to create
a stressful environment, and found that mild wrinkle formation, dryness and impaired barrier function were a result.30 Furthermore, mtDNA disfunction and epidermal stem cell function are thought to be co-dependent, directly causing skin ageing.31 In another trial, elevated epinephrine (also known as adrenaline), norepinephrine and cortisol levels in acutely stressed mice led to increased cellular DNA damage five-fold.12
The stress hormones also disrupted DNA repair and altered the cell cycle, highlighting the importance of cortisol homeostasis in wrinkle prevention.12
Loss of elasticity and dryness
Human and murine studies have provided evidence that stress impacts skin barrier function, causing it to heal poorly and appear flaky, dry, dull and more noticeably wrinkled.32,33 The exact mechanism is unknown, although lipid production is an essential part of restoring and maintaining stratum corneum health and integrity, and there is evidence to support that stress-induced corticosteroids inhibit lipid synthesis by reducing lamellar body number.34 Elastin and collagen are structural proteins produced in the dermis by fibroblasts, which help to maintain skin firmness.35 Reduction in these fibres results in skin laxity, giving an overall impression of ageing. Raised calcium levels caused by heightened epinephrine disturbs fibroblast function and thereby collagen and elastin synthesis.36
More recently, various studies have highlighted links to telomere shortening with specific causes of psychological stress, including relative caregiving, childhood adversity and domestic violence.14,37
Telomeres act as nucleoprotein ‘caps’, helping to maintain chromosomal integrity, and shortening can lead to the disruption of mitochondrial biogenesis, stem cell activity and ROS production.37
Adapting your in-practice technique
Many patients lead a busy and stressful life and some of my patients often arrive breathless, barely able to speak having just served dinner and put the kids to bed, or having rushed from the station after a twelve-hour working day, for example. Some have even arranged an appointment with me at 6.30am to accommodate family and work commitments. Assessing patients’ behaviours and state-of-mind clues, as well as what they are saying, must be an integral part of the consultation process; patients may appear well on the surface but may be experiencing mental, emotional or behavioural stress symptoms in their every day lives.42 Looking for signs of extreme stress will help to maintain best practice, including patient safety.43
For this reason, when assessing patients I have switched to a holistic consultation process, encompassing the usual relevant medical history but also questions about their lifestyle factors such as diet and sleep patterns, emotional intelligence, and coping mechanisms. This allows me to create an appropriate treatment and advice package as, in my experience, the patient’s mental state can very much determine how they react to a treatment. For example, a highly-stressed patient may be hyper-reactive to a chemical peel due to presence of a compromised skin barrier.32,33 Furthermore, Carter Singh, Hankins et al., explored in their human study in 2006 the psychosocial effects of cosmetic botulinum
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
toxin treatment and observed distress, worry, expectation and dependence in the study group.43 This study aimed to pave the way for practitioners to better understand the pressures of their patients during and after cosmetic injectables, and create a heightened sense of accountability for these potentially vulnerable patients. As observed in several studies, one extra, perceived pressure on top of an already heightening mountain of stress can have huge consequences for health and wellbeing.6,7 For a patient screened and identified as highly stressed with recognised symptoms,42 I would recommend an initial exploratory and educational chat to identify potential triggers and ways which they can unwind at home first – using lavender essential oil at bedtime for example, or maybe getting their skin conditioned before considering injectables with a course of pampering facials. One study found that patients exposed to lavender essential oil during botulinum toxin injections had reduced heart rate post treatment, compared to the control group.44 For this reason, I always burn lavender essential oil in clinic. Another important factor to consider is the effect of chronic stress on the body’s immune responses. According to a study conducted by Maddock and Pariante in 2001, there is evidence to suggest that biofilm formation following dermal filler treatment is more likely to occur in patients with low immunity.45,46 Furthermore, their skin barrier may be impaired, leading to delayed healing times.32,33 For this reason, I do not advocate use of dermal filler to treat a patient exhibiting signs of profound stress and anxiety.
Conclusion
Many of the physiological studies I have drawn upon here are murine-based, highlighting a need for more human studies. Research has demonstrated that physical, combined with emotional stressors, profoundly heighten skin’s inflammatory response, so it is imperative to remind patients to wear daily sunscreen throughout the year. We can’t see stress, but successful treatment outcomes for patients must involve more than treating the evident facial ageing; a comprehensive and holistic initial consultation looking at lifestyle factors with stress and anxiety assessment is a must.
Emma Coleman is a dermatology and advanced aesthetic nurse practitioner. She trained in aesthetics in London in 2015 and gained a distinction in Clinical Dermatology Diploma with the University of South Wales in 2019. She is a member of the British Dermatological Nursing Group (BDNG) and clinical director at the four Emma Coleman Skin clinics across Kent and London.

REFERENCES
1. forthwithlife.co.uk, Great Britain and Stress: How Bad Is It and Why is it Happening?, 2018
2. mentalhealth.org.uk Stressed Nation: 74% of UK Overwhelmed or Unable to Cope At Some Point in the Past Year 2018
3. Walter Cannon, ‘Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Research Into the Function of Emotional Excitement,’ 2nd ed, 1929, Appleton-Century-Crofts
4. Alicia Garside ‘4 Signs of Stress and How to Manage It’ 2019, < https://www.copemanhealthcare. com/resources/4-signs-stress-manage>
5. mentalhealth.org.uk, Stress: Are We Coping? 2019
6. Richard Lazarus and others, Stress Appraisal and Coping. Cited by: Gillian Butler, ‘Definitions of Stress’, 1993, Royal College of General Practitioners <https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC2560943/?page=561>
7. Hans Selye, The Stress of Life, Cited by: Gillian Butler, ‘Definitions of Stress’, 1993, Royal College of General Practitioners <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2560943/?page=561>
8. Liz Reitschel and others, Hair Cortisol in Twins: Heritability and Genetic Overlap with Psychological Variables and Stress-System Genes, Scientific Reports 15351, 2017 < https://www. nature.com/articles/s41598-017-11852-3>
9. Bhupendra Singh et al., Reversing Wrinkled Skin and Hair Loss in Mice by Restoring Mitochondrial Function,’ Cell Death & Disease, 2018
10. Akihiro Aioi et al., Effect of High Population Density Environment on Skin Barrier Function in Mice, Journal of Dermatological Science, 2001
11. Massimo Bonora et al., Mitochondrial DNA Keeps You Young, Cell Death & Disease, 2018 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6155168/>
12. Melanie Flint et al., Induction of DNA Damage, Alteration of DNA Repair and Transcriptional Activation by Stress Hormones. Psychoneuroendocrinology, 2007
13. Andrea Evers et al., How Stress Gets Under the Skin: Cortisol and Stress Reactivity in Psoriasis Br Journ Dermatol, 2010
14. Ying Chen et al., Brain-Skin Connection: Stress, Inflammation and Skin Aging, Inflamm Allergy Drug Targets 2014 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4082169/>
15. Mina Yaar, ‘Chapter 2: Clinical and Histological Features of Intrinsic versus Extrinsic Skin Aging,’ In: Skin Aging edited by Barbara Gilchrest and Jean Kruttman, 2006
16. Wilma Bergfeld, ‘The Aging Skin,’ Int. J. Fertil. Womens Med, 1997 <https://www.ncbi.nlm.nih.gov/ pubmed/9160214 >
17. anxiety.org.uk Stress <https://www.anxietyuk.org.uk/anxiety-type/stress/>
18. Jason Parker et al., Chronic Stress Accelerates Ultraviolet-Induced Cutaneous Carcinogenesis, J Am Acad Dermatol, 2004
19. Barbara Gilchrest, Photoaging, J Invest Dermatol 2013 < https://www.jidonline.org/article/S0022202X(15)41893-7/fulltext>
20. Andrea Vierktter et al., Airborne Particle Exposure and Extrinsic Skin Ageing, Society for Investigative Dermatology, 2010
21. Miaozhu Li et al., Epidemiological Evidence That Indoor Air Pollution from Cooking with Solid Fuels Accelerates Skin Ageing in Chinese Women, J Dermatol Sci, 2015
22. Andrzej Slominski et al., Steroidogenesis in the Skin: Implications for Local Immune Functions, J Steroid Biochem Mol Biol, 2013
23. Theoharis.C. Theoharides et al., Corticotropin-Releasing Hormone Induces Skin Mast Cell Degranulation and Increased Vascular Permeability, A Possible Explanation for its Proinflammatory Effects,’ Endocrinology, 1998
24. Ronni Wolf et al., Melasma: A Mask of Stress, British Journal of Dermatology, 1991
25. Gertrude Costin et al., ‘Human Skin Pigmentation: Melanocytes Modulate Skin Color in Response to Stress,’ FASEB <http://citeseerx.ist.psu.edu/viewdoc/ download?doi=10.1.1.333.8267&rep=rep1&type=pdf>
26. Gottfried Lemperle et al., A Classification of Facial Wrinkles,Plastic and Reconstructive Surgery, 2001
27. Moetaz El Domyati, Forehead Wrinkles: A Histological and Immunohistochemical Evaluation,Journal of Cosmetic Dermatology, 2014 <http://mc.minia.edu.eg/research/admin/ Publications/21102018Forehead%20Wrinkles%20JCD%202014.pdf>
28. Tsutomu Fujimura et al., ‘The Preliminary Study of the Relationship Between Facial Movements and Wrinkle Formation,’ Skin Res Technol, 2012 <https://onlinelibrary.wiley.com/doi/abs/10.1111/ j.1600-0846.2011.00557.x 18>
29. Antero Salminem et al., ‘Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process’ Int. J. Mol. Sci. 2013,<https://www.mdpi.com/1422-0067/14/2/3834/htm>
30. Akihiro Aioi et al., ‘Effect of High Population Density Environment on Skin Barrier Function in Mice.’ J Dermatol Sci. 25, 2001, <https://www.jdsjournal.com/article/S0923-1811(00)00133-X/ fulltext>
31. Massimo Bonora et al., ‘Mitochondrial DNA Keeps You Young,’ Cell Death and Disease, 2018 <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6155168/>
32. Amit Garg et al., Psychological Stress Perturbs Epidermal Permeability Barrier Homeostasis: Implications for the Pathogensis of Stress-Associated Skin Disorders, Arch Dermatol, 2001 (p:53-59)
33. Peter Elias et al., Barrier Function in K-10 Heterozygote Knockout Mice.J Invest Dermatol, 2000
34. Ying-Ho Choi and others, Mechanisms by which Psychologic Stress Alters Cutaneous Permeability Barrier Homeostasis and Stratum Corneum Integrity, J Invest Dermatol, 2005 <https://core.ac.uk/download/pdf/82583204.pdf>
35. Amanda Oakley, Terminology in Dermatology, 1997 <www.dermnetnz.org>
36. Bruna Romana-Souza and others, Stress-Induced Epinephrine Levels Compromise Murine Dermal Fibroblast Activity Through β-Adrenoceptors,’.Exp Dermatol. 2011
37. Eguin Sahin E et al., Axis of Ageing: Telomeres, p53 and Mitochondria’, Nat Rev Mol Cell Biol, 2012 <http://info-centre.jenage.de/assets/pdfs/library/sahin_et_al-NATURE-03_2010.pdf>
38. Patricia Oyetkin-White et al., Does Poor Sleep Quality Affect Skin Ageing? Clinical and Experimental Dermatology, 2014 39. Eung-Ho Choi and others, Mechanisms by Which Psychologic Stress Alters Cutaneous Permeability Barrier Homeostasis and Stratum Corneum Integrity, J Invest Dermatol, 2005 40. Inja Bogdan et al., Antioxidants Used in Skin Care Formulations, Skin Therapy Letter, 2008 41. Zoe Draelos, The Science Behind Skin Care: Moisturizers. Cosmet Dermatol, 2018 <https:// onlinelibrary.wiley.com/doi/abs/10.1111/jocd.12490>
42. Alicia Garside ‘4 Signs of Stress and How to Manage It’,2019 <https://www.copemanhealthcare. com/resources/4-signs-stress-manage> 43. Carter Singh et al., Psychosocial Aspects of Botox in Aesthetic Surgery, Aesthetic Plastic Surgery, 2006 44. Lisa Grunebaum et al., Effects of Lavender Olfactory Input on Cosmetic Procedures, Journ Cos Dermatol, 2011 45. Clementine Maddock et al., How Does Stress Affect You? An Overview of Stress, Immunity, Depression and Disease, Epidemology and Psychiatric Sciences, 2001 46. Wojciech Marusa et al., Probable Biofilm Formation in the Cheek as a Complication of Soft Tissue Filler Resulting from Improper Endodontic Treatment of Tooth 16, Int J Nanomedicine 7, 2012
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com



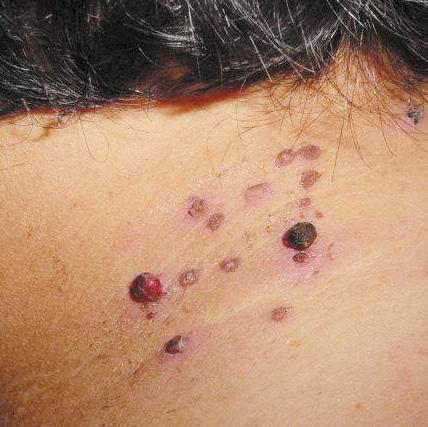


EXCLUSIVE TO the pursuit of skin perfection 0333 358 3904 info@naturastudios.co.uk naturastudios.co.uk For the removal of skin imperfections Get rid of unsightly warts, skin tags and benign lesions with CryoPen® Want to learn more about introducing CryoPen® into your clinic? Book an in clinic demonstration with our experienced sales rep today! Call us on 0333 358 3904 or email us on info@naturastudios.co.uk Ideal to treat: Skin tags, pigmentation, warts, age spots, sun spots, liver spots and verrucas Age spot treatment Hemangioma treatment MANY GPS IN THE UK WILL NO LONGER PROVIDE CRYOTHERAPY THROUGH THE NHS FOR BENIGN LESIONS. COMMENDED 2017 TREATMENT OF THE YEAR HIGHLY COMMENDED 2017 DISTRIBUTOR OF THE YEAR TREATMENT OF THE YEAR EQUIPMENT SUPPLIER OF THE YEAR DERMAPEN DIFFERENT CARTRIDGE SIZES OF 8G AND 16G WHICH DELIVER CONSTANT FLOW OF 120-240 SECONDS QUICK TREATMENT TIMES AND LOW ONGOING RUNNING COSTS MEANS QUICK RETURN ON INVESTMENT PINPOINT ACCURACY THAT CAN TREAT LESIONS FROM 1MM-10MM IN SIZE SO CAN SAFELY TREAT LESIONS CLOSE TO EYE AREA PRODUCES ULTRA-COLD N2O AT -89 C THAT RUPTURES THE CELL MEMBRANE DESTROYING THE CELLS QUICK RESULTS OF ONLY ONE TREATMENT ON COMMON WARTS WITH OTHER DEEPER WARTS REQUIRING MORE THAN ONE SMALL, PORTABLE PEN LIKE DEVICE MAKES TREATMENTS EASY TO PERFORM
Considerations for
there is no official guidance available that specifically focuses on the lip. For this area, I am noticing that instead of injecting 15-30 units as per guidance for the perioral skin, injectors are suggesting the use of much higher doses from 150 units upwards. This is also acknowledged in Dr Saleena Zimri’s article on p.36 8
With this in mind, I wanted to see how common fillers actually behave when they are injected with hyaluronidase and determine whether or not they differ.
Current research
There is some, but not a lot, of research available that compares the dissolution of some of the different HA fillers available.9-14
One recent study published in 2018 analysed the interactions of three HA fillers and hyaluronidase (Hylase). The authors concluded that hyaluronidase showed a significant degradation of two of the fillers and no effect of hyaluronidase was observed for the third. In their analysis, they found that the filler with the highest content of HA was most resistant to degradation as compared to fillers with lower concentrations. They also observed that the monophasic gel was comparably sensitive to degradation as the biphasic gel.9 In line with other studies, they also

As aesthetic practitioners are aware, hyaluronidase is an important drug that is commonly used off-label to dissolve hyaluronic acid (HA) dermal filler.1-3 Whether it be for the correction of an unaesthetic result, or, in more serious and time-sensitive situations, the resolution of a major complication such as a vascular occlusion, hyaluronidase is the only way we are able to remove a hyaluronic acid (HA) dermal filler without causing damage to the skin’s surface.1,4 When it comes to hyaluronidase dosing, there are guidelines available for practitioners to refer to that outline its use for different anatomical areas,1 and dosing is often outlined in published papers.5-7
The Aesthetics Complications Expert (ACE)
Group guidelines which, to my knowledge, are what most practitioners in the UK use, state that hyaluronidase dosage depends on several factors according to the individual filler’s characteristics; for example, whether it is particulate or non-particulate (monophasic or biphasic), the amount of cross-linking it has and the concentration of hyaluronic acid. How exactly the different properties impact a filler’s breakdown with hyaluronidsase is not explained. The Group therefore recommends to inject as much hyaluronidase as required to obtain the desired effect rather than following an absolute dosage.1,6
Through my own experiences of using hyaluronidase, training others, my involvement with the BACN and observing posts on social media groups, I have noticed that practitioners are commonly reporting that they need to inject far more hyaluronidase to get the desired result than is described in guidance (Table 1).
The situations where I am seeing this are more evident for removal of lip fillers, where particularly larger amounts seem to be used in the event of an emergency procedure. Note that although there is guidance for the nasal and perioral skin,
HA Dissolution Nurse prescriber Sharon Bennett highlights the need for practitioners to understand the rheology of different fillers when injecting hyaluronidase Region Hyaluronidase (units) Nasal and perioral skin 15-30 Periorbital 3-4.5 Infraorbital 10-15 Lower eye lid 1.5 Table 1: An indication of how much hyaluronidase is required to obtain desired effect per anatomical area according to the ACE Group.1
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
There will very likely be a difference in how quickly a dermal filler complication can be managed, which can be particularly problematic in emergency situations
noted stronger resistance to degradation for the highly cross-linked filler.9,11,14 However, for me to fully understand the concept of how the fillers dissolve differently, I decided to see it in practice and find out for myself, so I performed a small experiment in my clinic.
Experimenting with hyaluronidase
Limitations
I would firstly like to emphasise that this was not an official study, and there are certainly limitations to the results. Namely, I have not analysed the property differences between the fillers and how exactly each property may impact the interaction with hyaluronidase. The time was limited to five minutes; which I felt would give a general understanding of the filler behaviour. It would also be interesting to repeat the experiment several times to determine consistency of results.
Method
I chose four different HA dermal filler brands that are commonly used in the lip. I focused on fillers for this area because in general, when you look at the complication rates, and therefore the need for hyaluronidase, lips seem to have the most reported number of complications. This has certainly been reported in latest
Filler number My assessment
ACE Group and Save Face statistics.15 The brands I chose were all specifically indicated for injection into the body of the lip. The chosen fillers do have variations in particle size, cohesivity and different cross-linking methods, which as discussed above may explain different reactions. I placed 0.2ml of each dermal filler onto a flat mirror surface to obtain the best photographs possible. I reconstituted 1,500 of hyaluronidase (Hyalase) with 5ml of saline to give me 300 units per ml, equalling 30 units per 0.1ml. I then injected 30 units of hyaluronidase into each 0.2ml dermal filler. Upon injection, I observed the behaviour of the gels over a five-minute time-period (Table 2 and Figure 1).
I observed that out of the four HA gels, two of them (A and D) broke down very quickly, simply, and, in my opinion, satisfactorily. After five minutes, they resembled a liquid. The two others did have a reaction to the hyaluronidase (B and C), but less so in comparison to the others and I noticed that C reacted the slowest. Instead of liquefying, B and C separated into larger particles, or ‘clumps’, which suggests that more hyaluronidase would be required for these two fillers to breakdown, become less particulate and fully liquefy.


Discussion
My small experiment supports the findings of the aforementioned studies in that there are marked differences between how different HA products react to hyaluronidase.9,10 I believe this indicates that there will very likely be a difference in how quickly a dermal filler complication can be managed, which can be particularly problematic in emergency situations. Fillers that separate into larger particles or ‘clumps’, are likely to need to be treated with more units of hyaluronidase compared to those that don’t, or practitioners will need to do more repeated treatments to
A Broke down quickly, simply and satisfactorily
B Broke down into clumps or larger particles. More hyaluronidase needed to complete breakdown
C Broke down into three distinct separate clumps or larger particles. More hyaluronidase needed to complete breakdown
D Broke down quickly, simply and satisfactorily
Table 2: My assessment of the fillers’ ability to react to hyaluronidase
get to the same point. The issue with repeated injections of hyaluronidase, as with other injectable treatments, is that there are more chances of inflammation, bruising and trauma to the area of injection.16 The concern with increasing the dose of hyaluronidase is that it carries some risks, such as allergic response.17-19 In my opinion, we should try to avoid injecting large doses of hyaluronidase units unnecessarily, and the least amount of hyaluronidase we can give someone to resolve an issue the better. One author acknowledges that it is necessary to over-inject hyaluronidase in the event of a clinical emergency,4 and this is because higher doses often result in more rapid resolution.20 In my experience, high doses are essential in an emergency. A protocol has been published for emergency vascular adverse events called the High-Dose Pulse Hyaluronidase Protocol (HDPH) and this is also being adopted and included within the ACE guidelines.1 This guidance involves repeated administration of relatively high doses of hyaluronidase (450-1,500 units) into the ischaemic tissue hourly until resolution.6 As discussed, dosing guidance for hyaluronidase does not currently specify exactly how practitioners may need to tailor the dosing according to the type and the brand of HA or their properties, and there is nothing specific to the lip. This is particularly challenging to novice injectors, who may strictly adhere to the guidelines and be inexperienced in identifying how exactly to amend their dosing.
Conclusion
Although I acknowledge that we don’t always know what filler has been injected, my small experiment, as well as the few studies in this area, highlight the need for practitioners to understand and consider the fact that hyaluronidase interacts differently with various filler brands and properties. This is of particular importance due to the number of fillers available and of new fillers coming into the market. This knowledge can influence how we approach emergency complications; if you are trying to maintain vascularisation and the skin is deteriorating, then the faster the product is dissolved the better the outcome is for the patient. If a certain filler takes longer to breakdown, then practitioners should have an understanding of why this is and recognise that higher and more concentrated hyaluronidase doses may be
Figure 1: Four different types of hyaluronic acid (0.2ml) that were dissolved with 30 units of hyaluronidase. The after image is five minutes following hyaluronidase injection.
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
required to ensure patient outcomes are not affected. It’s particularly important for new practitioners to consider that the required dosing of hyaluronidase may be much higher than current guidelines recommend, and not assume that one HA filler is the same as another. By researching our chosen fillers, using peer group discussions, reading the research that is available and drawing from our medical understanding and clinical judgement, we can determine if, and how much, more hyaluronidase is needed. I certainly think there is scope for large studies to be performed that really examine the interaction of different hyaluronic acid fillers with hyaluronidase. It would be beneficial for practitioners to have more clarity on hyaluronidase units and time required to break down each filler for different indications. Not only would it benefit us as practitioners but our patients will be managed appropriately.
Sharon Bennett is a cosmetic nurse prescriber and runs clinics in Harrogate and London. She is a cofounder and current chair of the British Association of Cosmetic Nurses (BACN) and introduced the first hyaluronic acid filler (Restylane) into the UK in 1996. She has undertaken an MSc in Non-Surgical Cosmetic Interventions at Northumbria University and is a trainer and KOL for Galderma.

REFERENCES
1. Martyn King, Cormac Convery, Emma Davies, This month’s guideline: The Use of Hyaluronidase in Aesthetic Practice (v2.4), J Clin Aesthet Dermatol. 2018 Jun; 11(6): E61–E68.


2. Deok-Woo Kim, Eul-Sik Yoon, Yi-Hwa Ji, Seung-Ha Park, Byung-Il Lee, Eun-Sang Dhong, Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management, Journal of Plastic, Reconstructive & Aesthetic Surgery (2011) 64, 1590-1595
3. Claudio DeLorenzi, Complications of Injectable Fillers, Part I, Aesthetic Surgery Journal 2013 33: 561
4. Glynis Ablon, Understanding How to Prevent and Treat Adverse Events of Fillers and Neuromodulator, Plast Reconstr Surg Glob Open. 2016 Dec; 4(12 Suppl): e1154.
5. Brandon E. Cohen, Sameer Bashey, Ashley Wysong, The Use of Hyaluronidase in Cosmetic Dermatology: A Review of the Literature, J Clin Investigat Dermatol, December 2015 Volume 3, Issue 2.
6. DeLorenzi C, ‘New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events,’ Aesthet Surg J



7. The Kinetics of Reversible Hyaluronic Acid Filler Injection Treated With Hyaluronidase. Dermatol Surg. 2017 Jun;43(6):841-847.
8. Saleena Zimri, ‘Re-treating After Dissolving Fillers’,
9. Bettina Alexandra Buhren, Holger Schrumpf, et al., ‘Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase’, Eur J Med Res. 2018; 23: 37.

10. Rao V, Chi S, Woodward J. Reversing facial fillers: interactions between hyaluronidase and commercially available hyaluronic-acid based fillers. J Drugs Dermatol. 2014 Sep;13(9):1053-6.
11. Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010;36:804–809.
12. Juhasz MLW, Levin MK, Marmur ES. The kinetics of reversible hyaluronic acid filler injection treated with hyaluronidase. Dermatol Surg. 2017;43:841–847.
13. Hwang E, Song YS. Quantitative correlation between hyaluronic acid filler and hyaluronidase. J Craniofac Surg. 2017;28:838–841.
14. Sall I, Ferard G. Comparison of the sensitivity of 11 crosslinked hyaluronic acid gels to bovine testis hyaluronidase. Polym Degrad Stabil. 2007;92:915–919.
15. Megan Close, News Special: Lip Filler Complications, aestheticsjournal.com/feature/news-special-lip-filler-complications
16. El-Shafey elSI.Complications from repeated injection or puncture of old polyacrylamide gel implant sites: case reports.Aesthetic Plast Surg.
17. A Reum Park, Woong Mo Kim, and Bong Ha Heo, Delayed Allergic Reaction to Secondary Administrated Epidural Hyaluronidase, The Korean Journal of Pain, Korean J Pain 2015 April; Vol. 28, No. 2: 153-155.
18. Wu L, et al, Delayed allergic hypersensitivity to hyaluronidase during the treatment of granulomatous hyaluronic acid reactions.J Cosmet Dermatol. 2018 Dec;17(6):991-995. doi: 10.1111/jocd.12461. Epub 2017 Nov 21.




19. Medicines.org, Hyalase 1500 I.U. Powder for Solution for Injection/Infusion or Hyaluronidase 1500 I.U. Powder for Solution for Injection/Infusion.
20. Alam M, et al., Effectiveness of Low Doses of Hyaluronidase to Remove Hyaluronic Acid Filler Nodules: A Randomized Clinical Trial. JAMA Dermatol. 2018 Jul 1;154(7):765-772.
The UK’s medical aesthetic event



10 + 11 OCTOBER 2019 | OLYMPIA LONDON, UK
CCR_Ad2019_RegisterHalfPagevertical_.indd 1 17/06/2019 17:21:15
SCHOOL OF HEALTH SCIENCES
MSC NONSURGICAL AESTHETIC MEDICINE
The MSc in Non-surgical Aesthetic Medicine prepares U.K. registered doctors, nurses, dentists and pharmacists to deliver safe, personcentred non-surgical aesthetic practice.
The programme is delivered by an expert faculty, internationally recognised for delivering outstanding teaching and research, and with expertise in non-surgical aesthetic medicine. The programme lead, Stephen Prydderch, has extensive experience within nonsurgical aesthetic practice and has been actively involved in the development of various U.K. practice standards for non-surgical aesthetic practice.
If you would like further information regarding this programme, please contact: Stephen Prydderch 01248 383619 s.prydderch@bangor.ac.uk www.bangor.ac.uk/health-sciences @shsbangor
• Part Time & Distance taught.
• This course includes practice based components.
• You may have the option to complete a Non-Medical Prescribing module.
• The programme is open to Doctors, Nurses, Dentists & Pharmacists.
• You can learn via live online lectures.
• The taught components of the programme are delivered at our Wrexham campus by an expert faculty.


A summary of the latest clinical studies
Title: The Role of Hyaluronidase for the Skin Necrosis Caused by Hyaluronic Acid Injection-Induced Embolism
Authors: Li J, Xu Y, Wang Y et al.
Published: Aesthetic Plastic Surgery, May 2019
Keywords: Complication, Hyaluronidase, Skin necrosis
Abstract: This paper investigates the effects of hyaluronidase injection timing on the treatment of skin necrosis. In an in vitro experiment, the carbazole method was used to determine the degradation time of hyaluronic acid gels in a large volume of hyaluronidase. In vivo experimental rabbit ear models were developed to simulate the skin necrosis caused by hyaluronic acid and the test animals distributed into five groups. Except one control group, the other four groups were injected with a large volume of hyaluronidase as treatment at 2h, 4h, 8h and 16h, respectively, after models were built. The necrosis degree of models was analyzed with necrotic area and histologic examination on the postoperative 7th day. Besides, temperatures of rabbit ears were observed to demonstrate the healing process of flap models. The average necrotic area of flaps in the 2-h and 4-h injection groups showed a significant difference compared with that of the control group (p < 0.05; p < 0.05). The histologic examination showed that there were HA embolisms, vascular thrombolytic recanalization and arteriovenous thromboses in the survival area. In addition, the mean temperatures of the rabbit ear flaps fluctuated over time and showed clear differences between distal and proximal parts. The area of flap necrosis positively correlates with injection timing of the large volume of hyaluronidase. More importantly, when injection timing is within 4h, treatment effectiveness will be significantly improved.
Title: Amniotic fluid-derived mesenchymal stem cell products combined withmicroneedlingfor acne scars
Authors: El-Domyati M, Moftah NH, Nasif GA et al.
Published: Journal of Cosmetic Dermatology, June 2019
Keywords: Acne scars, Microneedling, Growth factors
Abstract: Postacne scars are still a challenge in its management. Microneedling is a popular minimally invasive technique in treatment of such scars. However, the addition of topical stem cell products after microneedling is considered a new treatment regimen for these scars. To compare efficacy of amniotic fluidderived mesenchymal stem cell-conditioned media (AF-MSCCM) and microneedling vs microneedling alone in management of atrophic acne scars. Ten cases with atrophic postacne scars received five sessions of microneedling, with 2-week interval on both sides of the face. Then, AF-MSC-CM was topically applied to right side of the face after microneedling. Clinical examination with histopathological and computerized histometric analysis was done 1month after the sessions. There was significant increase in the improvement percentage of acne scars on right side (dermaroller and AF-MSC-CM) vs left side of face (dermaroller; P<0.001). Histologically, improvement of character of collagen and elastic fibers was noticed, especially on right side. Meanwhile, significant increase in epidermal thickness on both sides of face was detected.
Title: A Cross-cultural exploration on the psychological aspects of skin color aesthetics: implications for sun-related behavior
Authors: Chen HY, Robinson JK, Jablonski NG


Published: Translational Behavioral Medicine, May 2019
Keywords: Body image, Skin colour, Cross-cultural psychology
Abstract: This study explores how sociocultural contexts shape attitudes toward skin color and sun-related behaviors in three groups of genetically Chinese women, located on a spectrum from predominantly Chinese culture to predominantly Euro-American culture. Using ethnographic and qualitative comparative approaches, interviews were conducted with (a) 15 Chinese women (Mage = 25; SD = 2.73) who grew up in mainland China until at least age 18 years and then moved to the United States, (b) 15 second-generation Chinese Americans (Mage = 20; SD = 1.16) raised in the United States by Chinese parents, and (c) 18 Chinese adoptees (Mage = 21; SD = 1.13) raised in the United States by Euro-American parents. Overall, Chinese women leaned toward Chinese culture, preferred lighter skin, and engaged in more sun-protection practices. Chinese adoptees leaned toward Euro-American culture, preferred tanned skin and sun-seeking behaviors, and experienced more sunburns. Chinese Americans had mixed results, exemplifying a double-bind in adherence to either Euro-American or Chinese cultural values. Findings elucidate the connections between sun-related behaviors and sociocultural backgrounds, especially how embracing Euro-American culture might increase sun exposure and sunburn tendency. Since sun exposure contributes to health outcomes (e.g., skin cancer, vitamin D status, and bone density), these findings have significant implications for public health prevention efforts.
Title: Topical skin therapies in subjects undergoing full facial rejuvenation
Authors: Dayan SH, Bacos JT, Ho TT et al.
Published: Journal of Cosmetic Dermatology, June 2019
Keywords: L-ascorbic acid, Trichloroacetic acid, Growth factor Abstract: The aim of this study was to characterize the effect of skincare products in subjects undergoing full facial rejuvenation with abobotulinumtoxinA and hyaluronic acid in improving facial skin appearance, patient satisfaction, and projected first impressions. Twenty subjects were recruited and divided into two treatment groups. Subjects in Group A followed a standard skin care regimen, whereas subjects in Group B received a more robust skin care regimen including chemical peels and antioxidant formulations. All subjects underwent facial rejuvenation treatments with hyaluronic acid at Visit 2 and with abobotulinumtoxinA at Visit 7. Patient aesthetic improvement, satisfaction, self-esteem, and first impression were evaluated via the Fitzpatrick Wrinkle Assessment Scale, Global Aesthetic Improvement Scale, Skin Quality Assessment, Heatherton & Polivy State Self-Esteem Scale, Subject Satisfaction Scale, and the First Impressions Questionnaire. Both treatment groups experienced significant improvements in the Skin Quality Assessment, Fitzpatrick Wrinkle Assessment Scale, Heatherton and Polivy State Self-Esteem Scale, and First Impressions Questionnaire. Larger studies are needed to determine the most efficacious combination of topical skin therapies with facial rejuvenation therapy.
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com


MATA Level 7 Post Graduate Diploma in Facial Aesthetics (Incorporating OTHM Level 7 Certificate/Diploma In Clinical Aesthetic Injectable Therapies) Our Diploma Provides – 150 Hours of Online Learning – 2 Days of Foundation Training – 2 Days of Advanced Training – (Including Hyperhydrosis) – Mentorship Days Up to date with the latest JCCP guidelines Free Half Day Complications Training Learn Facial Aesthetics to a Higher Standard Call: 020 3126 4870 Discounted from £9000+VAT to £5250+VAT Includes Free Half Day Complications Training using code. AJ5250 1 Harley Street, London, W1G 9QD www.matacourses.com Special price available for delegates who want to upgrade to the new 60 Credit MATA Diploma from the existing 28 Credit Level 7 Certificate Subject to RPL
Utilising Online Reviews
Running a clinic can be challenging, demanding and tiring at times, but when you’ve been able to deliver brilliant results for your patients and turn them into flag flyers for your business, the hard work and long hours you’ve put in instantly become worthwhile.
There are many ways in which you can leverage successes to attract and retain patients so they continue their journey with you. In this article, I will discuss the importance of clinic reviews that are published on thirdparty websites; detailing how to obtain them and how you can best utilise reviews as a powerful marketing tool. Before we discuss this further, I think it is important to decipher the difference between testimonials and reviews. Testimonials are generated and managed by the business offering that particular service, whereas reviews, generally speaking, are managed by a third party.1
Why secure reviews?
With the increasing popularity of review sites, including Facebook pages, Google My Business, TripAdvisor and Manta,2 securing positive reviews and ensuring they are kept up-to-date can make a huge difference to the lead conversion rate of potential patients seeking services that you offer. In BrightLocal’s Local Consumer
Review Survey 2018, it was reported that 86% of consumers read reviews for local businesses. The survey found that an average of 10 online reviews are read before a consumer feels able to trust a local business, and 43% of consumers said they read reviews for the medical/healthcare sectors.3 When reading reviews of your clinic, searchers may come across mentions of friendliness of staff, attentiveness, price, quality of service, treatment results and location – all of which are wants and needs that many of your prospective patients might be searching for. This may well be the decider that will lead them to book an appointment, or at the other end of the scale, may put them off from finding out any more.
In my opinion, reviews are an absolute must if you’re committed to growing your clinic and turnover. Star rating is commonly used as a rating scale and, according to digital software company Vendesta, it is the number one factor used by consumers to judge a business.4 It has been reported that 57% of consumers will only use a business if it has four or more stars, and this rises to 61% of consumers over 55 years old.5 Reviews can be displayed as quotes and/ or star ratings. They also can be collated to create statistics.
Obtaining reviews
There are several ways that you can encourage a customer to leave you a review, but it will depend on how you communicate with your patients the most. You could ask them face-to-face whilst in clinic whether they’d like to write a review or leave you a star rating on a third party website of your choice. You could also make a request within your general marketing emails or via a personalised follow-up email to check they were happy with your services.
For example, ‘We hope you loved your treatment. If you have a few spare minutes, could you rate your experience out of 5?’. You should then include a hyperlink straight to your Google My Business or Facebook page to make it easier for the review to be posted. If you’re looking to boost your volume of positive reviews online, you could also set yourself (and your team) a target to work towards, such as to have 10 new reviews on Google, Facebook or Yelp in the next three months. As a bit of friendly competition, and to encourage your team to seek reviews from their loyal patients, you could also enter your team members into an internal prize draw with a reward of your choosing.
The power of reciprocity
According to marketing and psychology university professor Dr Robert Cialdini’s Six Principles of Persuasion, reciprocity is when a person responds to a positive action with another positive action.6 In this case for example, offering loyal patient’s a gift or reward that will hopefully make them feel more inclined to do something for you in return. This small gesture aims to encourage patients to spare the time to write and leave you a review, rather than influencing the content of it. Dr Cialdini’s contrast theory is another highly effective technique and works by asking someone for something which requires more effort, for example a two-page case study (or anything that you think they will turn down). Rather than retreating and accepting the no (which he calls the ‘rejection then retreat’ technique7), he recommends responding with another, smaller request – one which you were really interested in all along – such as a quote for an online review.
According to Dr Cialdini, this works because the person you’ve asked feels like they’ve successfully influenced you to do less.
Using reviews in marketing
Star ratings and quotes can be used within your wider marketing and sales funnels,
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Business coach and author Alan Adams discusses how clinic owners can utilise positive patient reviews and maximise them as part of a continued growth strategy








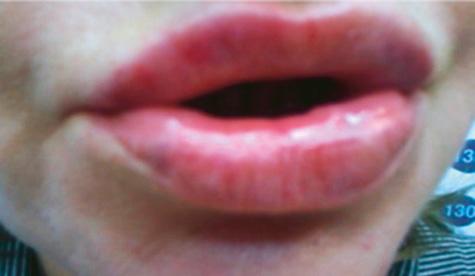
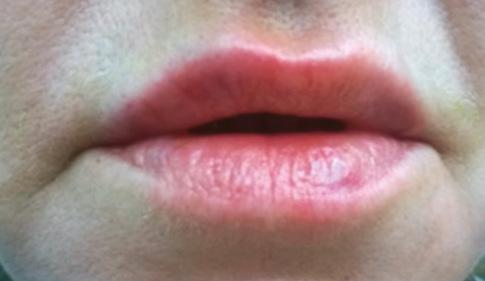


L.C.E. Balm Cebeline ® Blackcurrant POST PROCEDURE REPAIR CARE FRAGRANCE FREE PRESERVATIVE FREE REPAIR Laminin 5 active fragment Restores the mechanical and biological properties of the dermoepidermal junction ACCELERATES HEALING Increases capillary strength, improves microcirculation REDUCES BRUISING www.4tmedical.com | info@4tmedical.com | 01223 440285 Preserves capillary elasticity and permeability Prevents venous insufficiency REDUCES OE DEMA Escin extract (Horse Chestnut) Results Blepharoplasty: DAY 7 Lip Filler: IMMEDIATELY AFTER Rhinoplasty: IMMEDIATELY AFTER DAY 14 AFTER 2 HOURS DAY 7
such as within brochures, leaflets, on your website, and within newsletters and followup communications. For example, on your website, you could say ‘10 out of 10 people recommend [Clinic Name] to others’. Whilst you are legally able to lift reviews from third party websites, it’s important to note that you don’t ‘own’ these reviews, even though they are related to your business.8 As they are published on an external website, they are technically not your content.8 You should, in this case, always credit where the reviews came from. Adding reviews from such websites onto your own platform can be great for your conversion rates, however they will not impact your search engine optimisation. In order to do this you would need to ‘embed’ reviews that you have obtained, which is a topic to be explored outside of this article.8
According to the Advertising Standards Authority (ASA), marketers must hold documentary evidence that a testimonial or endorsement used in a marketing communication is genuine and hold contact details for the person who, or organisation that, gives it. So, if you have asked your patient if you can reuse a quote they’ve left you on Facebook, for example, you should also ask if they’re happy for their name to be used. Each case may be different – some may not want their name included, and some may not want you to reuse it at all. You should always check their preference beforehand. Again, you should ensure you have this question and answer in written form. If concerned about approvals, I’d recommend reading the ASA guidelines.9
Converting your website visitors
So, a potential patient has read a positive review on your Facebook business page about treatments that you offer with a mention of friendly and well-educated staff, and they have now clicked onto your website, what next? They are more than likely exploring your site and forming their opinions about you, while seeking materials to help them decide whether to get in touch or not. You can’t control the reviews other people leave online, but on your website you can use your case studies as another type of review to fully leverage opportunities.
It is vital that you obtain written consent from the patient for publication of case studies, and any testimonials.10 The most important part of the process is making sure that they’re happy with anything you’ve put together before it gets issued or used, and that they have the
opportunity to make any tweaks if needed. This is more likely to be relevant for a case study, which you’ll be responsible for drafting or having a third-party agency put together. Using something that’s not been fully approved, whether it be photos of a patient without their consent or a full name associated with a written testimonial, means you run the risk of damaging the relationship you have, potentially losing them as loyal patients altogether, or breaching confidentiality.10
Acting on negative reviews
I find that many practitioners are slightly nervous about promoting their presence online in case they are opening themselves up to negative reviews and comments. In such a socially connected world, negative reviews and comments cannot always be prevented and they can appear whether you’re present online or not.

They can be left on any of the aforementioned websites such as Google My Business or Facebook, and, in the case of the latter, if you don’t have a Facebook page but patients are regularly using your business’s name to review it or tag your name, a page will be automatically generated for it.11 If you’re actively choosing not to be online, you might not even know what reviews are being left for you and could potentially be losing out on potential patients because of it.
Surprisingly, some brands still choose to avoid social media due to the risk of the unknown and receiving negative reviews, but simply staying clear doesn’t mean that these won’t appear at all. In fact, in my opinion, negative reviews can improve a brand’s reputation if the response to such criticism is managed well.
Take one of the best loved clothes retailers, H&M, as an example. One of its customers shared a post about their inaccurate and disheartening clothing sizes, which later went viral and rallied consumer support across the UK and beyond. So much so that the brand actively pledged to make improvements to its sizing.12
Dealing with a negative review effectively can actually help to improve your brand’s image, services and offerings. If you’ve received a bad review, it’s always good to respond to it in a timely and professional manner.
For example, ‘Thank you for your feedback and bringing this to our attention. We’d love the opportunity to put this right — could you email us on customerservice@yourclinic.com with further details so that we can help?’
Always remember that other people will be
able to see your response, so you shouldn’t go into specific details on the post about anything personal, or defend your services and/or actions. Any issues you’re trying to resolve or respond to should be taken off the review site itself and done privately and in a professional manner.
Conclusion
It may sound like a lot of work while you’re in a full-time business, but quite often search results and the accompanying reviews are the first thing a prospective patient will come across or seek out about your brand. So why not take full advantage of these tools, promote what your patients already love about you, and enhance your online reputation amongst new patients?
Alan Adams is an awardwinning business coach and bestselling author. He is also a member of the Chartered Management Institute and the Professional Speaking Association. Adams has been recognised by Enterprise Nation as one of the Top 50 Advisors in the UK. The publication of his third book, The Beautiful Business: Secrets to Sculpting Your Ultimate Clinic, focuses specifically on the medical, cosmetic and aesthetic clinic sector.
REFERENCES
1. Vanguard86, What’s the difference? Testimonials vs review, February 2017 <https://www.vanguard86.com/blog/why-reviewsare-better-than-testimonials>
2. Search Engine Land, Giigle’s growth in online local reviews continues to dominate but…, February 2018 <https:// searchengineland.com/googles-growth-in-online-local-reviewscontinues-to-dominate-but-292571>
3. Bright Local, Local Customer Survey 2018
4. Vendesta, 50 Important Stats You Need to Know About Online Reviews, 2016 <https://www.vendasta.com/blog/50-stats-youneed-to-know-about-online-reviews>
5. Bryan Caplan, Online Statistics, 2019 <https://www.bryancaplan. com/blog-posts/top-25-online-review-statistics-2019>
6. Influence at Work, Robert Cialdini’s Six Principles of Persuasion < https://www.influenceatwork.com/principles-of-persuasion/>
7. Einstein Marketer, The Rejection then Retreat Technique, February 2019 < https://www.einsteinmarketer.com/rejectionthen-retreat/>
8. Review Trackers, How to incorporate online reviews on your business website the right way, December 2018 <https://www. reviewtrackers.com/incorporate-online-reviews-website/>
9. Advertising Standards Authority, Testimonials and endorsements, January 2019 <https://www.asa.org.uk/adviceonline/testimonials-and-endorsements.html>
10. BMJ, Gaining consent for publication in difficuly cases involving children <https://www.bmj.com/bmj/section-pdf/185996?path=/ bmj/337/7670/Analysis.full.pdf>
11. Facebook, How do I claim an unmanaged page? <https://www. facebook.com/help/168172433243582?helpref=uf_permalink>
12. Allure, H&M’s sizing gets slammed in viral Facebook post, May 2017 <https://www.allure.com/story/hm-sizing-criticism-viralfacebook-post>
FURTHER READING:
• Simoes T, Damage control, Aesthetics journal, February 2014 <https://aestheticsjournal.com/feature/damage-control>
• Ross E, Why you shouldn’t ignore online clinic reviews, Aesthetics journal, July 2015, <https://aestheticsjournal.com/ feature/why-you-shouldn-t-ignore-online-clinic-reviews>
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Featuring state-of-the-art patented tip technology and the largest spot size in the industry, the NanoFractional RF™ applicator is the only skin resurfacing system with SmartScan™ technology for increased patient comfort.




APPLICATORS for anti-ageing, body contouring, skin tightening




The DiamondPolar™ applicator is used for the face and neck, and the OctiPolar™ applicator is used for the body. Both are powered by our patented (MP)2 technology, which combines Multi-Polar Radio Frequency and Pulsed Electro-Magnetic Fields.

DISCOVER THE MOST VERSATILE SYSTEM. FOR A HIGHER RETURN ON INVESTMENT. 0208 748 2221 info.uk@venusconcept.com www.venusconcept.com WINNER FINALIST THREE MODALITIES. ONE ADVANCED WORKSTATION. DIAMONDPOLAR™ & OCTIPOLAR™ NANOFRACTIONAL™ RADIO FREQUENCY IPL APPLICATORS
APPLICATOR for skin resurfacing
APPLICATORS for photorejuvenation, acne reduction and hair removal 7 available
Intense Pulsed Light (IPL) with real-time cooling and SmartPulse™ technology, which ensures precise and consistent energy delivery to the targeted treatment area.
DELIVERING THE RESULTS 1 SR IPL, 1 DiamondPolar™, & 1 NanoFractional RF™ treatment Courtesy of venus Concept 4 NanoFractional RF™ treatment Courtesy of Matt Mahlberg, MD BEFORE AFTER BEFORE AFTER SEE US ON STAND D36 20-21 October 2019 The RDS, Dublin SEE US ON STAND K38 EventCity Manchester 13-14 October 2019 Untitled-33 1 10/08/2018 09:59
Understanding the Yellow Card Scheme
Senior medical device specialist Feza Haque and special projects manager Mitul Jadeja explore the importance of Yellow Card reporting
As a medical aesthetic practitioner, you will be familiar with the use of medicines and equipment that is classed as a medical device. You will be aware of occasions where you may be presented with an issue or adverse event associated with the product that you are using. In these cases, reporting to the UK’s regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), is vital as it helps to identify and solve problems to prevent future issues occurring. This article will explore the Yellow Card Scheme, explaining why it’s your duty as a medical professional to report, when you should do so, as well as the steps required.
What is the Yellow Card Scheme?
The Yellow Card Scheme is the reporting route used by the MHRA to monitor the safety of all medical devices and medicines in the UK to ensure they are acceptably safe for patients and those that use them.1 It is the route for healthcare professionals and members of the public to report actual or suspected:2
• Adverse drug reactions (ADRs), which are commonly referred to as side effects
• Medical device adverse incidents – injury or risk of injury from a medical device
• Defective medicines (drug or packaging quality problems)
• Suspected counterfeit/fake medicines or medical devices
• Unclear instructions for use or markings and labelling problems that may affect the safety of a medicine or device
The Yellow Card Scheme can be accessed online. It acts primarily as an early warning system, alongside other sources of information, for the identification of safety issues. It also aims to gain further information about the occurrence of incidents in clinical practice to strengthen the safety profile of medicines and devices to protect public health.
What’s the difference between a medicine and a medical device?
A medicinal product is defined as either:3
(a) Any substance or combination of substances presented as having properties for treating or preventing disease in human beings
(b) Any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis
In the aesthetic field, medicines that you are likely to be familiar with include botulinum toxin and lidocaine and, in both cases, these would be being used for medical purposes.
Medical devices are products which have an intended medical purpose. The regulatory requirements covering medical devices are set out in the Medical Devices Directive 93/42/ EEC, which also includes the definition of a medical device in Article 1.4 Intended purpose means the use for which the device is intended by the manufacturer and specified on the labelling and in the instructions and/or in promotional materials.4 Breast implants and, depending on their intended purpose, dermal fillers, laser devices for hair or tattoo removal and liposuction devices, would be regarded as medical devices under current legislation. Currently, dermal fillers, laser devices for hair or tattoo removal and liposuction devices would only be regulated as medical devices if they have an intended medical purpose (with or without an accompanying cosmetic purpose). However, under the new Medical Device Regulation 2017/745, which fully comes into force in May 2020, these products will be regulated as medical devices whether or not they have an intended medical purpose.5
Why should you report to the MHRA?
No drug or device is without risk. Reporting incidents to the MHRA provides information that may be directly responsible for preventing similar problems from happening again. Manufacturers must, by law, report problems with their medicine or medical device to the MHRA, but for healthcare professionals it is not mandatory to report, although it is considered best practice.6 It is strongly encouraged by the MHRA to report actual or suspected problems because it helps to spot patterns or emerging problems more quickly. This will lead to safer and more effective products available for you and your patients. Reporting is also considered best clinical practice by a medical practitioners’ regulatory body. The General Medical Council, for example, highlights the importance of reporting in its Guidance for Doctors Who Offer Cosmetic Interventions 7
What to report and when?
Problems with any medicine or medical device that a medical aesthetic professional uses should be reported, however minor. Each individual incident may not seem significant, or require specific feedback, but each case is key to giving the MHRA a national perspective. Practitioners are urged to report as soon as possible after the event. If you haven’t got all the information to hand, you can send the MHRA the basics, discussed in the sections below, and then follow up with more details later.
Side effects to medicines (ADR)
There are four minimum pieces of information required to submit a suspected adverse drug reaction (ADR) report:
1) Suspected drug(s)
2) Suspected reaction(s)
3) Basic information about the patient
4) Your contact details so the MHRA can get in touch if more information is needed and to acknowledge the receipt of your report
Other useful information such as concomitant medicine(s), patient history and details of the outcome and treatments given are not compulsory to have when completing a Yellow Card but can provide useful additional context to help the MHRA assess your report. Information such as brands and batch numbers are very important, especially for biological products or where it is suspected there may be a batch-related problem to help the MHRA quickly identify potential safety
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
issues. It is particularly important to notify the MHRA if the patient is pregnant, and details about their pregnancy.
You should report all suspected adverse drug reactions that are:
• Serious, medically significant or result in harm. Serious events are: fatal, life-threatening, a congenital abnormality, disabling or incapacitating, or result in hospitalisation.
• Associated with newer drugs and vaccines (medicines marked with a Black Triangle ▼ which are under additional monitoring).8 The most up-to-date list of black triangle medicines is available online.9
Essentially, if you are ever in doubt whether you should report a suspected adverse drug reaction, it’s always best to just complete a Yellow Card. You can access interactive Drug Analysis Profiles (iDAPs) on the Yellow Card website or through the Yellow Card app.10-12 Each iDAP contains complete data for all spontaneous suspected adverse drug reactions which have been reported on that drug substance to the MHRA, via the Yellow Card Scheme.
Adverse incidents involving a medical device
An adverse incident is an event that produces, or has the potential to produce, unwanted effects involving the safety of patients, users or other persons.13 You should report these through the Yellow Card Scheme and your local incident reporting system, if appropriate. The reporting differs if you are a practitioner in Northern Ireland, whereby you should report device problems to the Northern Ireland Adverse Incident Centre (NIAIC).15 In Scotland, report to Health Facilities Scotland,15 unless you are a private facility providing care to private patients, in which case you should report to the MHRA (Yellow Card) and the Care Inspectorate.16
The information required includes:
• The reporter’s name
• Reporting organisation
• Address of organisation
• Type of device
• Manufacturer name
• Type of injury
• Details of incident/nature of device defect
There is the option to give more details such as:
• Device model and serial numbers
• Dates of the initial procedure
• Location of use on the face or body
• Volume of product used
It’s encouraged to provide as much information and context as possible; however, all reports are still welcomed, even if there is very little device information available. It all helps the MHRA get a better idea of the national or international picture.
Examples of medical device problems reported to the MHRA include:
• Dermal fillers: syringes with dermal filler breaking or leaking, unclear packaging and labelling resulting in wrong product type being injected into the wrong place.
• Liposuction: problems with the vacuum container maintaining vacuum, leakage of tubing, packaging issues and problems with sterility.
• Lasers and IPLs: over-treatment (using for too long) or insufficient personal protective equipment can result in eye or skin damage to the patient/client or the practitioner.
Fake medicines or medical devices
Counterfeit or fake medicines and medical devices may lead to the patient being exposed to negative health consequences, as well as credit card or identity fraud. There is currently a #FakeMeds campaign run by the MHRA that provides tools to avoid fake medical products when shopping online.17 If you think a website is selling medicines illegally or you suspect a counterfeit, you should tell the MHRA by reporting a Yellow Card. The MHRA also has a register to check if a website is legally allowed to sell medicines. Look out for the Distance Selling Logo (Figure 1) that must be displayed on every web page that offers to sell human medicines.18

How to report?
The Yellow Card Scheme is an online system with several options, depending on the problem you want to report.19
The databases for reports on suspected adverse drug reactions to medicines is separate from the database for handling adverse incidents for medical devices. For ADRs you can create an account which retains the reporter’s account details for subsequent reports. For medical devices, reporters will have to re-enter their details each time they submit a new report. The Yellow Card app is also for reporting incidents involving medicines only, but you can use it to view safety data about both medicines and devices, including access to MHRA’s Drug Safety Update, iDAPs, and medicines alerts.2,22 For reporting suspected adverse drug reactions, there are also other ways to report, one example is calling the Yellow Card reporting line on 0808 100 3352,21,22 however the most common reporting method is online.

What happens to your report?
Once you submit the report, you will get an acknowledgement email with a MHRA reference number. You should quote this reference number if you contact the MHRA about your report, or wish to add information at a later date. All reports are evaluated, together with additional sources of information such as clinical trial data, medical literature or data from international regulators, to identify previously unknown safety issues, and to gain a better picture of emerging safety trends.
Figure 1: Benefits of reporting suspected side effects with medicines
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Figure 2: Distance Selling Logo19
Medicines
When a report of a suspected adverse drug reaction to a medicine is received by the MHRA, it is assessed and added to its internal pharmacovigilance database to make it available for ‘signal detection’. Signal detection is the continual review of all ADR reports to identify previously unrecognised side effects or a change in frequency of an ADR, including potential unidentified drug interactions. When a potential signal is identified, it is evaluated along with all other available evidence, including from medical literature and other international medicines regulators.24
A multi-disciplinary team at the MHRA including scientists, pharmacists and physicians oversee the signal detection process and investigate further to see if regulatory action is required. Additional safety information can also be requested from the pharmaceutical company such as in-depth studies. The MHRA can also seek advice from the Commission on Human Medicine and its advisory groups, which advises ministers on the safety, efficacy and quality of medicinal products.25 Regulatory action is communicated when necessary by the MHRA and may include changes or warnings in the product information, changing or restricting how a medicine should be used (including indications), change of legal status of medicines (such as from over-the-counter to prescription only) and, in rare cases, removal from the market if risks are found to outweigh the benefits. The recent safety issues to which Yellow Cards have contributed to can be viewed online.22
Medical devices
All reports are risk assessed by a team of medical device specialists to determine the level of investigation required, and sent to the manufacturer for them to investigate, who then report findings back the MHRA along with an analysis on why the event occurred and what action they are taking as a result. Reports submitted by members of the public have the option to anonymise their report, so the manufacturer is not able to identify or contact them when the report is sent to them for investigation. Some of the actions the manufacturer might take to address a problem identified from reports of adverse incidents include: design improvements, technical or software upgrades, recommendations for training users (including patients and healthcare professionals), and changes to the instructions for use. Investigations will be used for wider signal detection activities. If a serious safety issue is identified, the MHRA expects the manufacturer to inform its customers by issuing a notice called a ‘Field Safety Notice’.26 In some cases, the MHRA may also publish their own safety communication to users called Medical Device Alerts 27
Conclusion
It is important for everyone to report suspected and actual problems with medicines and medical devices to the MHRA via the Yellow Card Scheme. Under-reporting is a known issue with spontaneous reporting systems worldwide, so report as soon as possible after the event, and then follow up with more details later. Reporting is considered good clinical practice and part of a healthcare professional’s duty. Reporting incidents to the MHRA provides information that may be directly responsible for preventing similar problems from happening again, improving information and education about the safety of products and helps make sure the medicines and medical devices available to patients in the UK work and are acceptably safe.
Feza Haque is responsible for investigating adverse incident reports of suspected device failures at the Medicines and Healthcare products Regulatory Agency (MHRA). She has spent more than 14 years at the MHRA, and she has also contributed in audits and counterfeit cases.

Mitul Jadeja works at the Medicines and Healthcare products Regulatory Agency (MHRA) in pharmacovigilance and medicines safety. Having managed teams responsible for adverse drug reactions, Jadeja now leads on Yellow Card Strategy.

Acknowledgement
The authors are very grateful to their colleagues involved in the safety and surveillance of medical devices, vigilance and risk management of medicines and the Yellow Card Scheme at the MHRA for their contribution to this article.
REFERENCES
1. MHRA, About Us, 2019. <https://www.gov.uk/government/organisations/medicines-and-healthcareproducts-regulatory-agency/about>
2. About Yellow Card, MHRA. <https://yellowcard.mhra.gov.uk/the-yellow-card-scheme/>
3. Directive 2001/83/Ec Of The European Parliament And Of The Council of 6 November 2001 on the Community code relating to medicinal products for human use. <https://ec.europa.eu/health/sites/ health/files/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf>
4. B COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning medical devices (OJ L 169, 12.7.1993, p. 1) <https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:EN:PDF>
5. Medical devices: EU regulations for MDR and IVDR, Guidance for Annex XVI manufacturers <https:// www.gov.uk/guidance/medical-devices-eu-regulations-for-mdr-and-ivdr>
6. Gov.uk, Medical devices: guidance for manufacturers on vigilance <https://www.gov.uk/government/ collections/medical-devices-guidance-for-manufacturers-on-vigilance>
7. GMC Guidance for doctors who offer cosmetic interventions, 2016. <https://www.gmc-uk.org/-/media/ documents/Guidance_for_doctors_who_offer_cosmetic_interventions_210316.pdf_65254111.pdf>
8. The Yellow Card Scheme: guidance for healthcare professionals, patients and the public <www.mhra. gov.uk/blacktriangle>
9. European Medicines Agency, List of medicines under additional monitoring. <https://www.ema.europa. eu/en/human-regulatory/post-authorisation/pharmacovigilance/medicines-under-additional-monitoring/ list-medicines-under-additional-monitoring>
10. Yellow Card, Interactive Drug Analysis Profiles. <https://yellowcard.mhra.gov.uk/idap>
11. Yellow Card, MHRA, iTunes. <https://itunes.apple.com/us/app/apple-store/id990237487?mt=8>
12. Yellow Card Scheme, Google Play. <https://play.google.com/store/apps/details?id=uk.org.mhra. yellowcard&referrer=utm_source%3DEYC%26utm_medium%3Dcpc%26anid%3Dadmob>
13. Guidelines on Medical Devices MEDDEV 2.7/3 revision 3 May 2015. <https://ec.europa.eu/docsroom/ documents/16477/attachments/1/translations/en/renditions/native>
14. Department of Health, Northern Ireland Adverse Incident Centre (NIAIC). <https://www.health-ni.gov.uk/ topics/safety-and-quality-standards/northern-ireland-adverse-incident-centre-niaic>
15. NHS, Health Facilities Scotland, How to report an Adverse Incident. <http://www.hfs.scot.nhs.uk/ services/incident-reporting-and-investigation-centre-iric/how-to-report-an-adverse-incident/>
16. Care Inspectorate, <http://www.careinspectorate.com/>
17. HM Government, Helping you protect your health when buying medical products online. <https:// fakemeds.campaign.gov.uk/>
18. Gov.UK, Check if a website can legally sell medicines online. <https://www.gov.uk/checkmedicines-online>
19. Yellow Card, Welcome to the reporting site for the Yellow Card Scheme. <www.mhra.gov.uk/yellowcard>
20. Gov.uk, Drug Safety Update. <https://www.gov.uk/drug-safety-update>
21. MHRA, Contribution of Yellow Cards to identifying safety issues. <https://assets.publishing.service.gov. uk/government/uploads/system/uploads/attachment_data/file/731851/Contribution_of_Yellow_Cards_ to_identifying_safety_issues.pdf>
22. Gov. UK, The Yellow Card Scheme: guidance for healthcare professionals, patients and the public. <https://www.gov.uk/guidance/the-yellow-card-scheme-guidance-for-healthcare-professionals#howto-report>
23. Yellow Card, What will happen to a Yellow Card report that I complete? <https://yellowcard.mhra.gov.uk/ faqs/?page=1>
24. Gov.uk, Latest from the Commission on Human Medicines. <https://www.gov.uk/government/ organisations/commission-on-human-medicines>
25. Alerts and recalls for drugs and medical devices, Field Safety Notices. <https://www.gov.uk/ drug-device-alerts?parent=&keywords=&alert_type%5B%5D=field-safety-notices&issued_ date%5Bfrom%5D=&issued_date%5Bto%5D=>
26. Alerts and recalls for drugs and medical devices, Medical Device Alerts. <https://www.gov.uk/ drug-device-alerts?keywords=&alert_type%5B%5D=devices&issued_date%5Bfrom%5D=&issued_ date%5Bto%5D=>
FURTHER READING
• Gov.uk, Patient information leaflets and summaries of product characteristics, <https://www.gov.uk/ pil-spc>
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com

Mastering Local SEO
detailed by the device they’re using, and presents them with the most relevant results. The results shown are websites that Google has deemed the most likely to be valuable to the searcher’s experience; they’ll contain informative content, mobile-optimised design, keywords and locations, and contact details.3
Local SEO also heightens your chances of becoming a featured GMB profile, which means your business listing could appear before the many others in your area. This is why it is so important and can be so lucrative, because it can be used to target those seeking your services in your area; in fact, 76% of people who look for a nearby business on their devices visit within a day’s time,4 turning searchers into real-time patients. These are more likely to turn into return patients just through their proximity to your clinic.5
How to optimise your local SEO
Local search engine optimisation (SEO) is an important digital marketing tool that helps young, independent, and smaller businesses stand out in their local area.1 It’s been documented that Google is changing its approach to favour local SEO in a bid to improve the average searcher’s experience of their services.2
In a new focus on its ease of usability and how useful it can be to its traffic, Google is now placing more of an emphasis on the quality of the results it shows to its searchers, and other search engines are likely to follow.
Understanding local SEO
Local SEO for small to medium clinics, and even individual practitioners, can make the difference between numerous new patient bookings and a comparatively quieter clinic. It involves a series of tasks that help your clinic’s website and Google presence climb the search engine rankings to become one of the most highly-rated search results for people in your area. The combination of high-quality content, and by this we mean that it is informative and well-written, a Google My Business (GMB) profile and a website that is optimised by location and keywords can be formidable.
A GMB profile is a free tool Google allows you to set up for your business. When all the fields are completed, it shows search traffic your business name, location, contact information, and opening hours amongst other useful information. If you don’t have a GMB profile already, this is something that comes highly recommended and will only complement your other local SEO marketing activities.
How local SEO makes a difference
When someone searches for ‘dermal fillers near me’, Google doesn’t literally trawl websites for this exact phrase. Instead, they take the valuable keywords of ‘dermal fillers’ and use the searcher’s location, as
Optimising your website and web presence for local SEO consists of many different factors and tasks that can be largely categorised as: website content, website engagement and GMB profiles. Improving your local SEO will involve segmenting some time to focus on your website’s SEO, and another healthy portion on your GMB profile. Your GMB and organic local SEO both use similar ranking factors, though both also need attention in equal measure. This complex cocktail of different SEO tasks is why you need a good mix of them in your SEO strategy, to ensure your website and your GMB are at their optimum performance.
Optimising website content
Optimising your website content for local SEO is simple, yet very effective. Google’s users search for product or service and location, for example ‘dermal fillers in Nottingham’. Therefore, placing each web page’s relevant keyword and your clinic’s location in a text header and in your written content will help Google flag your website as a provider of these treatments. The affiliation you create between your website, the service searched for, and your location will contribute to your website featuring higher in the search engine ranks and therefore the potential for new patients to click-through. Even your images and videos, be they professional stock imagery or your own content, can
Digital marketing consultant Adam Hampson and digital marketing consultant and content executive Lottie Staples details how you can improve your local SEO to target new patients in your area Local SEO for small to medium clinics can make the difference between numerous new patient bookings and a quieter clinic Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com









WIN A HOLIDAY IN ANTIGUA or £5000 CASH PRIZE! Follow the evidence to win the game Here’s how you can play • Register for an online account at www.springpharm.co.uk check out our FAQs for more info how to do this •Receive a clue every time you order with us • Log into your account and enter your clue code • Make an accusation of Room, Character and Weapon every time you receive a clue • The more clues you get, the more accusations you can make, the more chances you have of winning Enter Promo Code: AJx3 on your first order to triple your clues The Game-Changing Pharmacy 0345 319 4000 info@springpharm.co.uk www.springpharm.co.uk
‘Google Local Pack and Local Finder’
A Google Local Pack presents itself when you search for a service and location. It typically appears at the top or very high on the first search engine results’ page. It shows a map of your city with three location pointers to local businesses associated with this service, and beneath this map are the three business’ Google My Business Profiles. These profiles will include useful action buttons such as directions, call, and website to encourage an enquiry. Naturally, these are high-performing places and highly sought after in local SEO. A Google Local Finder is presented when you click on ‘More Places’ and see the other businesses ranking after this top three; their location pins also appear on the map.10,11
be optimised through keyword and location alt tagging, which is a HTML attribute added to an image and provides search engines with another term to crawl.6 Any content, whether it is a blog post, photo with alt tagging, or simply a treatment page, can benefit from the addition of your location mentioned somewhere in the page.
Website engagement
Google still uses engagement as a ranking factor in organic local SEO, and it takes into account your website’s bounce rate (the frequency of searchers leaving your website without interacting with it), session duration (how long users spend on your site), and your user interaction (link clicks and contact form submissions, for example).7 These are known as ‘behavioural signals’,8 so the more positive behaviour conducted on your website, the higher value and therefore search engine results’ page (known as SERP) position Google will reward you with. If people in your area are searching for ‘dermal fillers’, find your website, but quickly bounce off it again without converting or spending upwards of a few seconds on it, then your website isn’t going to climb the slippery search engine ladder. This is why you not only need to optimise your content and ensure its informative, but make sure your website functions well and presents plenty of opportunities for interaction.
Google My Business profiles
To compound the efforts you’re making across your website, you should also create a GMB profile for your clinic or spend a little more time optimising it if you already have one. Google’s focus on creating the best and most useful experience for its searchers has led it to value all the information ‘checkpoints’ of users’ search journey, one of those being your GMB and name, address, phone number, and website (NAPW). Making sure your GMB is fully optimised with your NAPW will lead Google to recognising its value, because it can see that your clinic is providing accurate and useful contact
information for your potential patients. Not only this, but your GMB can now also be linked to your social media profiles such as Facebook, Instagram, Twitter, and YouTube. It’s beneficial to do this because the more cross-platform and ‘useful’ information you provide to Google’s searchers, the more it will value your business profile.
Google My Business reviews
Google values user engagement and interaction possibly even more so than your own SEO efforts, because it ranks websites and content on what other searchers have seemingly deemed useful and trustworthy. This is why websites with lower bounce rates and longer session duration encounter higher Google search rankings, because they’re recognised by both web traffic and search engines as being ‘valuable’. This is also why GMB profiles with multiple reviews are more likely to appear higher up in the Local Pack and Local Finder (see boxed information). A GMB profile that has multiple reviews shows Google that your clinic has been highly rated and trusted by other people similar to your web traffic. Around 86% of people looking for a service read the reviews of local businesses, and 57% will only use a business if it has four or more stars,9 demonstrating the influence positive reviews have on new patients’ interaction with you. Therefore, Google will rank your GMB higher because it wants to provide its traffic with reliable information and services.
Summary
Local SEO is just as competitive as more widespread efforts because of the need to conquer your location and lay claim to the potential patients. Combining your efforts to optimise not only your website, but your Google My Business profile, in keeping with a comprehensive SEO strategy will undoubtedly gain your clinic great online visibility and the potential for new patients.
Adam Hampson is the founder and director of Cosmetic Digital, a web design and digital marketing agency in Nottingham that works with clients in the cosmetic medical sector. He has recently launched 13 Digital Training and offers digital marketing training courses for dentists, doctors and nurses of all abilities.

Lottie Staples is a content executive and copywriter at Cosmetic Digital, specialising in writing engaging content for websites, blogs, and marketing materials. Lottie has worked both as in-house marketing support for aesthetic clinics and within a specialist digital marketing agency.

REFERENCES
1. Moz, Local Search Ranking Factors 2018: Local Today, Key Takeaways, and the Future, < https://moz. com/blog/local-search-ranking-factors-today-takeaways-future>
2. Think It With Google, The consumer behaviors shaping the next generation of mobile experiences, <https://www.thinkwithgoogle.com/advertising-channels/mobile-marketing/consumer-behavior-mobile-digital-experiences/>
3. Google Policies, Why does Google use location information? <https://policies.google.com/technologies/location-data>
4. Search Engine Journal, How to Use Local Keywords to Rank Higher on Google, <https://www.searchenginejournal.com/rank-higher-local-keywords/262899/#close>
5. Social Media Today, How Proximity/Geo-Marketing Can Grow Your Business, <https://www.socialmediatoday.com/social-networks/how-proximitygeo-marketing-can-grow-your-business>
6. Moz, Alt Text, <https://moz.com/learn/seo/alt-text>
7. Google Support, Bounce rate, <https://support.google.com/analytics/answer/1009409?hl=en>
8. Moz, 2018 Local Search Ranking Factors, <https://moz.com/local-search-ranking-factors>
9. BrightLocal, Local Consumer Review Survey 2018, <https://www.brightlocal.com/research/local-consumer-review-survey/>
10. Moz, 2018 Local Search Ranking Factors, <https://moz.com/local-search-ranking-factors>
11. Local SEO & Google My Business Pages, Cosmetic Digital, 2019. <https://www.cosmeticdigital.co.uk/ mastering-local-seo-need-know/>
people looking
a service read
businesses
Around 86% of
for
the reviews of local
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com








FROM THE NATURAL ESSENCE OF SALVIA HAENKEI TO THE FORMULA THAT NOURISHES THE SKIN Exclusive UK & Ireland distributor / training provider of IBSA’s aesthetic products GENTLY POWERFUL Enhancing & maintaining skin cellular vitality Improves skin elasticity & firmness Advanced complex combining high & low molecular weight of hyaluronic acid & Haenkenium® MULTI-ACTION ANTIOXIDANT FORMULA
“I will keep pushing for a safer specialty”
From a nursing assistant in a psychiatric hospital to an aesthetic clinic owner and strong advocator for high industry standards, nurse prescriber Andrew Rankin has had an interesting career. He has been practising nursing for almost 25 years, and began his journey into aesthetics in 2006. He reminisces, “I grew up in a small spa town called Malvern and, when I was 18, I began working as a nursing assistant in a small psychiatric hospital. I thoroughly enjoyed this – a combination of caring for others and the inspiring people I worked with that sparked my interest in nursing.” So, Rankin left home and moved to London for nursing training. Throughout his career, Rankin mainly spent his time in intensive care, which he found very challenging and rewarding. However, after 20 years of working in the NHS it was time for a change. He explains, “I had a conversation with my wife, and now clinic manager, and she suggested looking into aesthetics, which I was initially uncertain about. But, after I did some research, the possibilities and the opportunity to work for myself was quite appealing.” Rankin underwent injectable training in April 2006. He adds, “I then spent a few months gaining experience and confidence. When I was confident I could practice carefully, safely and achieve adequate results, I opened my clinic Regenix in September.” When Rankin entered the aesthetic specialty, he never thought his career would lead him on a path so involved in broader industry issues, such as standards of practice, regulation and training. He explains, “I’ve always felt morally obligated to do the best for my patients and felt quite strongly about upholding safety standards in the industry – I want to work in an industry that’s credible. It is upsetting when you hear some of the horror stories and people doing things that are questionable.”
Something that was very ‘questionable’, Rankin remembers, was remote prescribing of botulinum toxin. He explains, “I was extremely against remote prescribing and strongly believe in a face-to-face consultation as it otherwise starts to lose sight of our moral and statutory guidelines. I voiced this opinion and Emma Davies, the then chair of the BACN, supported this and invited me to join the BACN board in 2010, which was a great opportunity. The GMC later banned remote prescribing in 2012.”

Rankin has fond memories of his time at the BACN. “It was fantastic because I’ve met a lot of very intelligent peers who are like-minded and all very keen to make a difference,” he says. His role as a board member led Rankin to co-author the BACN prescribing guidelines, work with the Department of Health on the HEE Expert Reference Group to help develop the qualification requirements for delivery of cosmetic procedures, and become BACN vice-chair. He is also very
proud to have been heavily involved in the formation of the JCCP and recently became a trustee and co-chair of its Practitioner Register Committee, which is why he gave up his post as BACN vice-chair in 2018. He says, “It’s easy to get frustrated with the state of the industry at present as there are a lot of problems, but you have to take it one step at a time. With the Joint Council, we have accomplished so much in terms of the agreements with the regulatory bodies, and updated competency and training frameworks. In my mind, it’s already been successful and I will keep pushing for a safer specialty.” Complications, Rankin reiterates, are a large safety focus at present. “Undoubtedly, I think there are issues in that many practitioners aren’t competent enough in identifying complications,” he adds. Interestingly, Rankin has seen a shift in dermal filler complications over his career. He explains, “We are presently seeing a lot of lip complications, such as vascular occlusions, but when I originally trained, these were virtually unheard of! Previously, glabella vascular occlusions were much more common and we don’t see much of that anymore, probably because of our progressive understanding of the and changing of fashions.” Rankin recommends that if practitioners want to see industry change, they need to do their best to become involved in not just the voluntary registers and associations, but also the overall aesthetics community. “Going to events, meeting colleagues, not isolating yourself, and being involved is so important. Working in this field can be quite stressful, and continuously trying to push for change can be tiring and involves commitment, but you must be committed and you have to keep going,” he concludes.

What’s your ethos or motto?
I have lived by the motto ‘If in doubt, then don’t’. This has served me well in this specialty and is something that I think all practitioners should bear in mind.
What’s your top advice for success?
Truly care about what you do. Take your time with your patients and make sure that they and their welfare are the focus of all decisions that you make.
What are your hobbies?
At the moment, my hobby is regulation! But something that I’ve not done for years and was my absolute favorite hobby was scuba diving – I have dived all over the world.
1985-1990 Nursing training & Coronary Care Unit, Hammersmith Hospital, London 1990-1993 Cardiothoracic Intensive Care, Royal Brompton Hospital, London 1993-2006 General Intensive Care, Worcester Royal Infirmary 1997-1999 Cardiothoracic intensive care, Prince Sultan Cardiac Centre, Saudi Arabia 2006 Aesthetic training and opening of Regenix clinic 2009 Independent prescriber 2012 BACN board member 2013 BACN vice-chair 2014 HEE Expert Reference Group 2018 JCCP Trustee and co-chair of Practitioner Register Committee Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Aesthetics gets to know nurse prescriber Andrew Rankin and explores his passion for creating a credible specialty





AESTHETICSJOURNAL.COM Keep up-to-date with the latest aesthetic developments and best practice guidance on your desktop, tablet or phone AESTHETICSJOURNAL.COM @aestheticsjournaluk Aesthetics @aestheticsgroup
The Last Word
There is no denying that the popularity of non-surgical procedures, or ‘tweakments’ as they are sometimes referred to, is booming.1 With many one-day courses promising the title of ‘aesthetic practitioner’ and pop-up clinics in every town, non-surgical cosmetic procedures are now more accessible than ever before. Unfortunately, as the number of aesthetic procedures carried out each day rises, the possibility of being presented with a complication also increases.2,3 But when should we, as responsible medically qualified and competent practitioners, step in and treat someone else’s complication?
The problem
In my opinion, the problem really is multifactorial. The first being that in an ideal world, all aesthetic practitioners would be able to manage and resolve their own complications or, when unable to do so, should take responsibility to appropriately refer their patient to another professional who can help.4 However, with the lack of regulation and professional standards in the field of aesthetic practice, this is not the reality we are faced with. Aside from the lack of regulation however, practitioners may be unavailable at the time the complication presents –they may be away on holiday for example. I believe that practitioners should always make arrangements for periods where they
are inaccessible to their patients and linking with another local practitioner is a great management plan for holiday cover. Even when the practitioner is available, the problem might actually be that the patient simply feels uncomfortable returning to their original practitioner or perhaps they had a negative experience and don’t wish to relive it. It’s important to note that practice guidelines and policies are usually a fundamental ‘go to’ for healthcare professionals. Unfortunately, as there are no standardised guidelines on how to deal with such a scenario, the decision you make will need to be based solely on your experience and clinical judgement. Another issue is that many in the specialty don’t always understand a complication’s urgency. Before taking on a complication, I believe we should be asking ourselves, ‘Is this an emergency or can the intervention wait?’.
Understanding the urgency


Immediate intervention
If the complication requires immediate intervention, for example you suspect vascular occlusion following dermal filler treatment, you will be faced with the challenge of making a very quick decision. In this instance, treating someone else’s complication, where you are competent to do so, could be considered to be in the best interest of the patient, in my opinion. However many problems also start here, with unqualified practitioners treating outside of their remit.5
Solution
An intervention is not without its own potential complications and undesirable results. We know that hyaluronidase, as used in cases of vascular occlusions, is related to oedema and even severe allergic reaction.6 For that reason, unless you are experienced, prepared and equipped to deal with any eventuality, in my opinion, you should not get involved in treating the complication.
If you’ve already concluded that you’re unable to address the complication directly, you can still support the patient until help is arranged. The first port of call should be to the primary treating practitioner; have the patient call their practitioner and narrate the problem to them. You should then give them your professional assessment and recommendation as indicated. By doing this it demolishes the idea of competing between practitioners and shows a more seamless and professional relationship. Also, I would argue the importance of not being afraid to ask the practitioner if they are suitably qualified, experienced and competent and have the appropriate protocol for dealing with such an event. You can then be assured that the patient’s urgent complication will be dealt with in a proper and timely manner.
Nurse prescriber Jodie Nightingill argues when and why it may be deemed appropriate to take on another practitioner’s dermal filler complication
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
An intervention is not without its own potential complications and undesirable results
Black & White offers a clear-cut, forward-thinking approach to communications. No grey-areas, no false promises. Simply your brand, meaningfully amplified.
If you are interested in building strategic connections with media & influencers across Print, Digital & Social connect with us www.blackandwhitecomms.com blackandwhitecomms
Delayed intervention
On the other hand, can intervention wait? Is the complication related to patient satisfaction? Perhaps there is asymmetry for example? In this scenario, you will have more time to consider your next step or may even choose not to get involved at all.
Solution
In an intervention that you believe doesn’t require immediate treatment, you might like to explore the complication history of the patient. However, before you do so, you need to be confident that you have enough training and experience to safely decide that the complication couldn’t be any more serious. For example, many misdiagnose vascular occlusions as bruising.7 Once you are comfortable with your decision, you can ask what effort the patient has made to contact their original practitioner, if any, and what has been the outcome of this?8
I believe that promoting referral back to the original practitioner is key for continuity of care and, in my opinion, it develops improved relationships with patients and ultimately leads to improved clinical outcomes.
I would advise that practitioners contact the original practitioner personally and discuss the case at hand, perhaps offer your assessment and advice and mediate a transition of the patient back to their care. If a patient has been unsatisfied with the cosmetic outcome from an aesthetic treatment performed elsewhere, consider if their expectations are realistic; ask yourself if you are confident you can manage these expectations before getting involved. Where there is no risk of harm to a patient my preference is to avoid involvement and encourage referral back to the original practitioner. Complications can arise for even the most experienced and skilled injectors and dealing with them can be complex, unpredictable and time-consuming.
Complication history
It is important to note that if you do decide to treat the complication, in an emergency situation or otherwise, it is crucial to think about the possibility of any future implications.
If the patient chooses to make a formal complaint about the original complication, by intervening you are now directly involved in the history of that complaint and you should check with your insurance company about the appropriate cover.9 This is particularly important with the compensation culture that is becoming commonplace in the UK.9
It is good practice to keep accurate records of your involvement, no matter how limited, detailing communication with the patient, assessment of the complication, communication with the original practitioner and any advice you have given. I think that the value of accurate documentation (including written notes and before and after pictures) should never be underestimated. Good record keeping takes a short amount of time but will be extremely valuable if you’re ever called to discuss a case.
Seek support
Until the time comes when there are more robust regulations and restrictions for people accessing training courses and emerging as aesthetic practitioners, I don’t anticipate this complex area of care will come to an end any time soon.
For now, and until such a time occurs, I believe practitioners must continue to educate themselves on managing complications and consider joining a forum or association such as the ACE Group,10 the International Association for Prevention in Complications in Aesthetic Medicine (IAPCAM),11 the British Association of Aesthetic Nurses (BACN)12 or the British College of Aesthetic Medicine (BCAM).13 All of which enable you to access a wide range of support from an expert group of aesthetic professionals. This will not only give you credibility and peace of mind in your aesthetic
practice, but will ensure your patients receive the best and safest possible care. Whether you chose to take on treating someone else’s complication or not will depend on a number of factors relating not only to the complication itself, but patient and practitioner circumstances too. Evaluating complication urgency, patient expectations and practitioner skill and competence are all key considerations; however, I believe that statutory guidelines around this would eliminate fear and help to implement best practice regarding when you should be taking on someone else’s complication.
Jodie Nightingill is an aesthetic nurse prescriber, BACN member and ACE Group registered practitioner. She completed her aesthetic training with Cosmetic Courses and The Allergan Medical Institute. Nightingill is currently working as an NHS clinical nurse specialist for malignant haematology as well as running her clinic, The Dolls House Essex.
REFERENCES
1. The American Society for Aesthetic Plastic Surgery, Statistics 2018 <https://www.surgery.org/sites/default/files/ASAPSStats2018-Trends.pdf>
2. Saner E, The rise of non-surgical beauty: My mum said my lip looked liked a rubber dinghy, Guardian, April 2017 <https:// www.theguardian.com/lifeandstyle/2017/apr/03/non-surgicalcosmetic-procedures-botox-lip-filler-beauty>

3. Save Face. Consumer Complaints Audit Report 201718. Available at: https://docs.google.com/viewerng/ viewer?url=https://www.saveface.co.uk/wp-content/ uploads/2018/11/Save-Face-Consumer-Complaints-Report2017-18-FINAL.pdf&hl=en
4. Cosmetic Standards, JCCP and CPSA Guidance for Practitioners Who Provide Cosmetic Interventions <http:// www.cosmeticstandards.org.uk/uploads/1/0/6/2/106271141/ jccp_cpsa_code_of_practice.pdf>
5. NMC (2018) The Code, <https://www.nmc.org.uk/ globalassets/sitedocuments/nmc-publications/nmc-code.pdf>
6. Summary of Product Characteristics (2015) Hyalase 1500 I.U. Powder for Solution for Injection/Infusion or Hyaluronidase <https://www.medicines.org.uk/emc/product/1505/smpc>
7. Grunebaum L.D. · Funt D.K. Goldberg DJ (ed): Dermal Fillers. Aesthet Dermatol. Basel, Karger, 2018, vol 4, pp 128-140
8. Sudhakar-Krishnan, Vidya, and Mary C J Rudolf, ‘How important is continuity of care?’Archives of disease in childhoodvol. 92,5, 2007
9. Data on file.
10. ACE Group <http://acegroup.online/welcome-ace-groupwebsite/>
11. International Association for Prevention of Complications in Aesthetics Medicine <https://iapcam.co.uk/>
12. British Association of Cosmetic Nurses <https://www.bacn. org.uk/>
13. British College of Aesthetic Medicine <https://bcam.ac.uk/>
Reproduced from Aesthetics | Volume 6/Issue 8 - July 2019 @aestheticsgroup @aestheticsjournaluk Aesthetics aestheticsjournal.com
Keep accurate records of your involvement, no matter how limited





Highly Commended




Trilogy advanced laser hair removal
restylane.co.uk

Demand a higher standard.

#jawlineenvy
RES19-03-0116 DOP March 2019
























































 dermamelan® method efficacy has been demonstrated in numerous studies under medical supervision. More than 500,000 patients treated worldwide, in any skin phototype (I-VI) and in all ethnicities.
dermamelan® method efficacy has been demonstrated in numerous studies under medical supervision. More than 500,000 patients treated worldwide, in any skin phototype (I-VI) and in all ethnicities.


















































 Sabrina
Sabrina













 Dr
Dr




































































































































































































