Ellansé® An Expert's Guide
Seven years ago, we at Sinclair had the pleasure of meeting the great Pierre Nicolau. Already recognised by his peers as a plastic surgeon, a mentor and a teacher, Pierre had been involved with Ellansé and Silhouette Soft since their conception, working with the creators on development and clinical studies.

Today, more than 10 years since their launch, Ellansé and Silhouette Soft are still recognised as the most inspirational dermal fillers and threads on the market, and Pierre clearly contributed to this success. Pierre was dedicated to science, and dedicated to his patients. He was also an inspiration to many of his peers. He always wanted to learn more and to dive deeper. He was engaged and engaging, convinced and convincing, and sometimes unwilling to compromise. Most of our Ellansé and Silhouette Soft experts worldwide were initially trained by him, and today, we – as a family – all share the loss.
We are convinced that this book, one of his last projects with us, will be a game changer in convincing aesthetic practitioners to start using Ellansé in the most effective and safe way to achieve the best outcomes for their patients. For that reason and because “Spoken words fly away, written words remain” – "Verba volant, scripta manent” (Horace) - we would like to dedicate this Experts’ Guide to Pierre and to his family. May his passion for Ellansé keep inspiring the world through this book!
Dr Pierre Nicolau 1949 - 2022
Ellansé® An Expert's Guide
EXPERT ADVISERS
Dr Francisco de Melo, UAE
Dr Shang-Li Lin, Taiwan
Dr Ingrid López-Gehrke, Mexico
Dr Pierre Nicolau, Spain
Dr Amanda Ong, Australia
Foreword
In the past 20 years, our understanding of one of the most complex areas of the human body – the face – has improved dramatically, with several new anatomical structures having been identified.
At the same time, a plethora of non-surgical procedures have become available for treating the signs of ageing and restoring the youthful appearance of the face. Ellansé® is the first, and currently the only, collagen stimulator that is made of polycaprolactone microspheres, which contribute to its durable aesthetic enhancements. Ellansé’s unique properties mean it is a desirable option for a range of soft-tissue procedures.

In addition to independent and authoritative contributions from leading physicians in the field of aesthetics, this book incorporates the latest technology to deliver augmented reality assets that bring the Ellansé injection techniques to life. Facial anatomy and the consequences of ageing are covered, before recognised experts share best practice on patient preparation, injection technique and avoidance and management of complications and adverse events.
This book aims to support clinicians who have completed Ellansé injection training in their ongoing efforts to provide an optimal patient experience to those seeking to preserve their youthful appearance, balance or enhance facial features, or offset the effects of ageing.
Having read the book, you will be confident in the optimal use and benefits of Ellansé, becoming increasingly motivated to employ more advanced techniques and offer safer treatment to patients.
We at Sinclair hope you find this book rewarding and informative as it accompanies you on your Ellansé journey.
Testimonials
“This book is the result of collective clinical experience. We intend to share our expertise and knowledge in when and how to incorporate Ellansé in clinical practice. I hope it will benefit the reader the same way it has worked for me for the past 10 years: offering safer treatments with a better outcome and long-lasting results. Ellansé is a fundamental tool in my practice and has made me a better injector!”
Dr Francisco de Melo Plastic Surgeon, UAE

“Ellansé has been my favourite dermal filler for 7 years. This book will help you to master the use of Ellansé and you will fall in love with it.”
Dr Shang-Li Lin Dermatologist, Taiwan

ELLANS É ® | AN EXPERT'S GUIDE
“In daily clinical practice, we frequently need practical management guidelines to hand. This new book represents a simple and effective guide to best practice in the use of Ellansé. Since I learned about Ellansé several years ago, my clinical practice and the aesthetic results for my patients have developed considerably. The improvement in structure and skin quality resulting from Ellansé’s unique neocollagenesis is unmatched. Undoubtedly one of the best tools for clinics that want maximum efficacy and safety in an injectable product. Ellansé has the capacity to provide long-lasting lifting and enhanced facial structure with just a single session.”
Dr Ingrid López-Gehrke Dermatologist, Mexico

“I find great pleasure in using Ellansé due to its incredible volumising effect. This allows less product to be used, and through the real production of collagen type I, has a true capacity for skin regeneration. Many patients tell me: 'It is the first time I have something that lasts’, or 'Look at the quality of my skin’. Definitely my favourite filler.”
Dr Pierre Nicolau Plastic Surgeon, Spain

“Ellansé has become the principal filler treatment in my practice because it is a both a superior volumiser and a skin rejuvenator. The results are not only natural looking and long lasting, but also predictable. The Ellansé Expert’s Guide provides a detailed outline on everything you need to know about the product, including detailed protocols of all facial indications. This book will become your loyal best friend if you choose to embrace Ellansé as I do.”
Dr Amanda Ong Aesthetic Physician, Australia

01 – Introducing Ellansé® 01 02 – Anatomy of the Face 19 03 – The Ageing Process 35 04 – Patient Selection and Preparation 53 05 – Injection Techniques 69 06 – Case Studies 111 07 – Combined Treatments 129 08 – Adverse Event Management 147 Contents
Introducing Ellansé®
1
01
Sinclair vision: Leading future change
Sinclair’s vision is to use collagen-stimulation technology to harness the body’s ability to regenerate itself, delivering natural-looking and age-appropriate enhancements that will lead the way in the aesthetics industry.
Sinclair’s product portfolio is built around the belief that the shape of the face is the key to aesthetic beauty (Figure 1.1).
Ellansé resonates perfectly with Sinclair’s vision: improving defects in facial contours, and restoring and treating signs of ageing – including the appearance of wrinkles and folds, and loss of facial volume.

2 ELLANS É ® | AN EXPERT'S GUIDE Figure 1.1 Actual patient, results may vary.
Ellansé major milestones
Following extensive research and development, and clinical testing, Ellansé gained ISO 13485 Quality Management System certification in 20081 (Figure 1.2). In 2009, the European Conformity (CE) mark approval was granted, leading
to the highly successful launch of the product in the UK, Germany and Spain. Other launches followed, with Ellansé being registered in more than 69 countries by 2018. By 2019, the 10-year anniversary of Ellansé, more
2013
than 1 million syringes had been sold worldwide. But the success story didn’t stop there, with a new manufacturing site in the Netherlands starting production in 2020 and Ellansé launched in China in 2021.
2018
2007
2008 QMS ISO 13485 certification 2009 CE mark approval CE 0344 2010
R&D, product testing, production, intellectual property, QA/Reg set-up, literature research and preclinical testing
Launch in Spain, the UK, Germany
KFDA registration Launch in Korea 2014 Acquisition by Sinclair
2015 Launch in Taiwan
Registered in over 69 countries
2019
2021 Launched in China 2018 ANVISA registration Launch in Brazil
2021
1.8 million syringes sold worldwide
INTRODUCING ELLANSÉ 3
Figure 1.2 Ellansé major milestones ANVISA, Brazilian Health Regulatory Agency; CE, European Conformity; KFDA, Korea Food and Drug Administration (now called Ministry of Food and Drug Safety [MFDS]); QA/Reg, quality assurance and regulation; R&D, research and development.
10-year anniversary 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021
Sinclair Global Presence
Headquartered in London, UK, Sinclair is a global aesthetics company2 (Figure 1.3) that manufactures a range of nonsurgical aesthetic products, with a focus on stimulation of collagen.
Sinclair’s portfolio of differentiated, complementary aesthetics technologies are increasingly in demand as they strive to satisfy the growing need for effective, highquality, longer duration, naturallooking and minimally invasive aesthetic treatments.
Sinclair Presence (direct and indirect) COMPANY OPERATIONS
USA/CANADA
Key distribution agreement with Suneva
MEXICO CITY
Mexican directly-owned affiliate and direct sales
SÃO PAULO
Brazilian directly-owned affiliate and direct sales
4 ELLANS É ® | AN EXPERT'S GUIDE
Figure 1.3
Sinclair global presence
LONDON
Corporate head office, UK directly-owned affiliate and UK direct sales
CHESTER
Global technical operations
AMSTERDAM
Direct sales
ALMERE
Manufacturing site
LYON
Manufacturing site
MOSCOW
Directly - owned affiliate and direct sales
MADRID
Spanish directly-owned affiliate and direct sales
MANNHEIM
DACH directly-owned affiliate and direct sales (Germany, Austria and Switzerland)
DUBAI
UAE directly-owned affiliate and direct sales
SEOUL
South-Korean directly-owned affiliate and direct sales
HANGZHOU
Directly-owned affiliate and direct sales
SINGAPORE
Asian operations
PARIS
Global marketing, French directly-owned affiliate and direct sales
INTRODUCING ELLANSÉ 5
Unique composition
Ellansé is composed of a unique, patented blend of:
● 70% carboxymethyl cellulose (CMC)based gel carrier
● 30% polycaprolactone (PCL) microspheres (Figure 1.4)3,4,5
The PCL microspheres are held in homogeneous suspension in the CMC-based gel carrier. PCL and CMC both have an excellent and proven biocompatibility profile.

PCL
synthetic polycaprolactone microspheres


70% 30%
CMC
aqueous carboxymethyl cellulose gel carrier
6 ELLANS É ® | AN EXPERT'S GUIDE
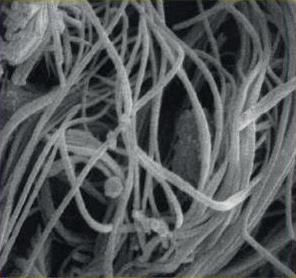
Figure 1.5
Scanning electron microscope and light microscope images of polycaprolactone spheres5
Figure 1.4
Unique composition of microspheres
Image courtesy of Dr Kim Jong-Seo, South Korea
PCL MICROSPHERES
PCL is a non-toxic medical polyester, first synthesised in the early 1930s4, that is attractive for use in dermal fillers because of its ease of bioresorption; it is naturally hydrolysed into carbon dioxide and water within the body5
The PCL microspheres used in Ellansé are designed to offer optimal biocompatibility6. They have a smooth surface, a spherical shape and a size of approximately 25–50 μm (Figure 1.5)2,3,4
PCL 30%
PROPERTIES OF CMC
CMC is a natural material derived from cellulose; it is not cross-linked, and is nontoxic. Its other properties include (Figure 1.7)4:
● It is a recognised pharmaceutical excipient
● It is hygroscopic
● It has been designated by the FDA as generally recognised as safe (GRAS)
● Resorption occurs in 2–3 months
Natural well-known cellulose derivative
PCL has an excellent safety profile3 and has been used in the biomedical field for more than 70 years for a range of applications, from sutures to tissue and organ replacements by 3D printing (Figure 1.6)4. It is also used in CE-marked and US Food and Drug Administration (FDA)-approved products.
Absorbable sutures
Adhesive barriers
Wound dressing
Haemostatics
Facial cosmetics
Orthopaedic and arthroscopic devices
Dental implant devices
Organ replacement
Natural material derivative
CMC gel carrier
Pharmaceutical excipient
GRAS – substance in food additive by FDA
Hygroscopic –forming a gel with water
INTRODUCING ELLANSÉ 7
Figure 1.6 Polycaprolactone applications4 PCL, polycaprolactone.
Figure 1.7 Properties of carboxymethyl cellulose GRAS, generally recognised as safe.
70%
Ellansé and inflammation
in three key ways:
1 Size and volume of particles: Ellansé PCL microspheres technology was designed to maintain the volume of the particles for a longer time, delaying the triggering of phagocytosis and intensity of the inflammatory reaction
2 Particle morphology: As the PCL microspheres can maintain their shape and smooth surface during the hydrolysis process, there is reduced risk, and decreased intensity, of secondary inflammation
3 Surface area: By maintaining the integrity of the PCL microspheres, the inflammatory reaction responsible for collagen production is sustained at a low intensity, promoting the synthesis of a more mature and organised collagen layer

The physical properties of Ellansé differ from those of other dermal fillers on the market (Figure 1.8)5,6,7,8
DURATION OF INFLAMMATION
The human body is capable of differentially responding to the total contact surface area of foreign material within the tissues. A gradual increase in the surface area will reach a tipping point where an acute inflammatory reaction is initiated by the body in an attempt to eradicate what it considers to be a tumoural process8. This explains the majority of late inflammatory reactions seen in daily practice following dermal filler use5
Ellansé maintains its integrity over time, with the PCL microspheres triggering a low-intensity inflammatory reaction that induces neocollagenesis, which favours the more lasting type I collagen4. The end result is the long-lasting aesthetic improvement seen with Ellansé of up to 24 months4,9,10



INTRODUCING ELLANSÉ 9
Figure 1.8
Physical composition of different fillers1
Sculptra® (50μm)
Radiesse® (30μm)
Ellansé (100μm)
Ellansé mechanism of action
Ellansé has two distinct phases of activity (Figure 1.9)1,4:
● Step 1: Immediately after injection, the CMC component provides temporary volume, which gradually decreases over 2–3 months
● Step 2: The PCL microspheres induce neocollagenesis of types I and III collagen, with more persistent type I collagen structure gradually increasing over 1–3 months and the PCL microspheres becoming embedded in the type I collagen scaffold. The resulting collagen volume replaces the initial volume increase caused by the CMC gel

The collagen scaffold stimulated by the PCL microspheres persists after they have been resorbed, leading to the durable volume increase seen with Ellansé (Figure 1.10)3

10 ELLANS É ® | AN EXPERT'S GUIDE
D1, day 1.
Figure 1.10
Ellansé dual mode of action1,4
12 months D1 M 24 months 4 weeks 12 weeks
S
1. Immediate restoration of volume
2. PCL effect
Clinical effect CMC effect PCL effect 1 2
3. Long-lasting volumisation
Figure 1.9 Ellansé dual mode of action3
Collagen stimulation by Ellansé: Scientific evidence
Ellansé has been tested in an animal model where rabbits were injected with either Ellansé S (PCL-1) or Ellansé M (PCL-2) to investigate neocollagenesis5
Nine months after injection of PCL-1, neocollagenesis had occurred and the PCL microspheres of PCL-1 had been completely resorbed (Figure 1.11)5 Meanwhile, with PCL-2 at 9 months, there was evidence of formation of type I and type III collagen around PCL microspheres. At 21 months postinjection, PCL-2 microspheres were still present in the injected tissue5

In a pilot study of Ellansé in humans, patients were enrolled to receive Ellansé injected intradermally into the temple region9. Histological analysis of tissue obtained from the biopsies revealed collagen formation around the injected PCL particles (Figure 1.12)9, supporting similar findings previously shown in rabbit tissue5





INTRODUCING ELLANSÉ 11
Figure 1.11
Collagen stimulation by Ellansé: Animal model5
Collagen
Microscopic images (13 months postinjection) show PCL microspheres surrounded with collagen deposition and a mild fibroblastic and histiocytic tissue response. Stainings were haematoxylin and eosin (A and B) and Martin's trichrome (C and D)9 40x C A 40x
Figure 1.12
stimulation
by Ellansé: Human model
B D 200x 200x
Resorption of Ellansé
CMC is resorbed 2–3 months after injection4. PCL-filler degradation occurs via hydrolysis (Figure 1.13)4 and is characterised by bulk degradation brought about by water penetrating the PCL microspheres, causing progressive internal hydrolysis of the ester bonds throughout the entire polymer matrix.
The length and the molecular weight of the polymer chains decrease over time, while the mass, volume and shape of the implant remain unchanged. Then, when hydrolysis has produced low-molecularweight polymer chains, diffusion of the small polymer fragments occurs4
Longevity of the microspheres ultimately depends on the hydrolytic breakdown of PCL crystalline regions. The PCL chains of the M version of Ellansé are longer than those of the S version, and consequently take longer to degrade4 The difference in duration of the two Ellansé products (S and M) is due to the differing chain length (molecular weight) of the PCL chains within the microspheres4

12 ELLANS É ® | AN EXPERT'S GUIDE
I Oδ+H2O Hydrolosis Ester bond Microsphere Oδ- Cδ+ II O OH C +HO +H2O III O CO2 + H2O HO Hydroxycaproic acid C H H C H H C H H C H H C H H C H H OH
Figure 1.13
Hydrolysis and bulk degradation of polycaprolactone microspheres4
Clinical/aesthetic effect
Ellansé gel’s elasticity is greater than that of volumising hyaluronic acid1,11 (HA), meaning that projection and volumisation of the injected area are instantly visible. Because the collagen-stimulating PCL microspheres are maintained in the injection area, aesthetic improvement is also more long term than that seen with HAs12. Ellansé also has excellent rheological properties and combines high elasticity4 with specifically designed viscosity that is suitable for subdermal injection3
In a randomised, prospective, blinded, split-face, single-centre study comparing Ellansé with HA in 40 patients, nasolabial folds (NLFs) treated with Ellansé showed statistically significant improvements on the Wrinkle Severity Rating Scale and greater improvements on the Global Aesthetic Improvement Scale (GAIS) compared with NLFs treated with an HAbased dermal filler12
Ellansé also improves skin quality, enhancing density, firmness, tonicity and texture from within.
A clinical trial was carried out to assess the effect of Ellansé on skin quality parameters in 24 patients up to 24 months post-treatment11 Although Ellansé was injected in the subdermis* of the midface, patient echographs clearly showed a substantial increase in tissue density in the dermis following treatment with Ellansé, compared with baseline11. This improvement was statistically significant at all time points during the 24-month study. Skin firmness, tonicity and smoothness were also statistically significantly improved with Ellansé, compared with baseline11

is not an actual patient. INTRODUCING ELLANSÉ 13 *Ellansé should not be placed intradermally. Please refer to Ellansé Instructions for Use for further Information.3
Model
Packaging and available options
Ellansé is available in two options that differ only in their duration of action. Packaging includes two ready-to-use syringes containing 1 ml of Ellansé and four 27G ¾” needles (Figure 1.14)1. The clinical and aesthetic improvements with Ellansé are immediate, long lasting and stable4,9


Ellansé L and E versions are no longer available.
● CE mark 2009
● Injectable implant
● Medical device: class III
● Ready-to-use syringes
● 2 x 1 ml syringe
14 ELLANS É ® | AN EXPERT'S GUIDE
As an injectable implant, Ellansé is considered a class III medical device.
1.14 Ellansé packaging and available options
Figure
ELLANSÉ S 18 months' longevity
ELLANSÉ M 24 months' longevity
Summary
The composition of Ellansé, 70% aqueous CMC-based gel carrier and 30% PCL composition, allows for an immediate filling effect caused by CMC, followed by stimulation of the body’s own collagen (neocollagenesis).
CMC is resorbed 2 to 3 months post-injection and is progressively replaced by the patient’s own collagen (predominantly type I) stimulated by PCL microspheres. The microspheres of PCL are also bioresorbable.
Ellansé has a number of attributes that make it an attractive option as a dermal filler:
1 Encapsulation of polymer microspheres, within approximately 1 month, and the associated collagen scaffold prevent further inflammatory reactions from occurring13
2 The enduring collagen type in the injected site is predominantly the ‘mature’ collagen scaffold of collagen type I5
a) The reduction of collagen type III means no further stimulation of the inflammatory response
3 The degradation of Ellansé constituents is completed by hydrolysis, leaving just water and carbon dioxide
4 Because the final volume within the treated area is greater than the volume of Ellansé injected, there is no requirement to ‘touch up’ the treatment
a) The final volume is greater than the volume injected by 20–30% due to formation of collagen type I fibres11
5 The availability of two versions of Ellansé with different durations of action means that the length of treatment effect can be tailored to a patient’s requirements
a) This is achieved by varying the length of the PCL chains, allowing for predictable, controlled and adjustable bioresorption
6 The treatment technique is the same regardless of the Ellansé product selected
a) Same:
● Rheological properties
● Technique
● Syringe
● Needle/cannula
INTRODUCING ELLANSÉ 15
Sinclair Training
Sinclair delivers scientifically advanced, aesthetic products and services made exclusively for the most highly skilled clinicians worldwide. Our educational arm, Sinclair College, exists to harness the power of science with excellence as standard through collaboration and insight with worldwide experts. We provide a forward-thinking approach to linked-up education across the Sinclair portfolio for ongoing engagement and education.
Our Sinclair College educational platform provides you with a whole host of informative webinars, courses, filmed video demonstrations and programmes to help support your professional
development in aesthetics. Additionally, our education team works to create a personalised learning journey that helps our trainers and key opinion leaders take clinicians through the portfolio, giving the best possible patient outcomes to those who use our products.
The Sinclair College website and My e-College App can be used anywhere, giving you free and flexible access to product and anatomy modules, and practice development insight.

You can access the Sinclair College e-learning website and app by scanning the below QR codes:


16 ELLANS É ® | AN EXPERT'S GUIDE
Sinclair College e-learning website My e-College App
References
1. Sinclair. Ellansé training materials.
2. Sinclair. Ellansé 10-year anniversary brochure.
3. Sinclair. Ellansé instructions for use.
4. Christen MO, Vercesi F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin, cosmet investig dermatol. 2020;13:31–48.
5. Nicolau PJ, and Marijnissen-Hofsté J. Neocollagenesis after injection of a polycaprolactone based dermal filler in a rabbit. Eur J Aesth Med Dermatol. 2013;3:19–26.
6. Laeschke K. Biocompatibility of microparticles into soft tissue fillers. Semin Cutan Medic Surg. 2004;23:214–7.
7. Anderson JM. Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993;2:33S–41S.
8. Nicolau JP. Long-lasting and permanent fillers: biomaterial influence over host tissue response. Plast Reconstr Surg. 2007;119: 2271–86.
9. Kim JA, Van Abel D. Neocollagenesis in human tissue injected with a polycaprolactonebased dermal filler. J Cosmet Laser Ther. 2015;17:99–101.
10. Moers-Capri MM, Sherwood S. Polycaprolactone for the correction of nasolabial folds: A 24-month, prospective, randomized, controlled clinical trial. Dermatol Surg. 2013;39:457–63.
11. Sinclair. Data on file.
12. Galadari H, van Abel D, Al Naumi K, Al Faresi F, Galadari I. A randomized, prospective, blinded, split-face, single-center study comparing polycaprolactone to hyaluronic acid for treatment of nasolabial folds. J Cosmet Dermatol. 2015;14:27–32.
13. Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg. 2004;113:1380–90.
INTRODUCING ELLANSÉ 17
Anatomy of the Face 02
19
The first rule of anatomy is variation. All faces are unique, and all have features that may be modifiable by aesthetic procedures. When assessing the face of an individual who has elected to have Ellansé or any other aesthetic enhancement procedure, there are three things to consider (Figure 2.1):

1 Height/width
2 Symmetry of the different segments and features
3 Proportion
Together, these factors add up to ‘attractiveness’, which has been described as ‘the visual properties of a face that are pleasing to the visual sense of an observer’1
Familiarity with these distinct facial layers will inform not only treatment intentions (volumisation alone or volumisation and biostimulation of novel collagen), but also injection depth and placement patterns.
Importantly, familiarity with facial anatomy will also allow clinicians to prevent and avoid complications arising from misplacement of product, or vascular and neurological complications. Good injection technique and detailed knowledge of the anatomy are paramount to achieving sustained improvements with minimal adverse effects or complications2
Facial anatomy is best examined by looking at the distinct facial layers as separate entities, starting with the skin and continuing through to the bone of the skull.
1/3 1/3 1/3 ½ ½ ⅔ ⅓ ⅕ ⅕ ⅕ ⅖ W=⅔H Hair line Nasion Subnasale Menton ⅕ ⅕
Introduction
Figure 2.1
Components of attractiveness Reproduced with kind permission of Dr Francisco de Melo
20 ELLANS É ® | AN EXPERT'S GUIDE
Before undertaking any assessment of a patient for aesthetic improvements with Ellansé, it is crucial to have a thorough understanding of the anatomy, from the skin to the underlying tissues, to the facial skeleton.
Anatomical layers of the face
The layers of the face run from superficial to deep. It is generally agreed that there are six discrete layers (Figure 2.2)3,4:
1 Skin
2 Subcutaneous (superficial fat)
3 Superficial musculoaponeurotic system (SMAS)
4 Retaining ligaments, spaces and deep fat compartments
5 Periosteum and deep fascia
6 Subperiosteal plane/bone
Figure 2.2
The layers of the face run from superficial to deep
6 ANATOMY OF THE FACE 21
1. SKIN
Skin is the envelope or canvas of the face, revealing the decrease in density and volume of the underlying bone and soft-tissue compartments over time5. The skin has different characteristics in different areas of the face in terms of pigmentation, thickness and subcutaneous adherence. In the infraorbital region, the so-called tear trough area, the skin is thin, transparent and firmly attached to the underlying orbicularis oculi muscle. In the buccal and parotideomasseteric region, the skin lies on a thick layer of superficial fat and has loose and variable connections to the underlying muscles of facial expression. In the perioral region, the skin is directly connected to the muscles of facial expression without a distinct superficial fat layer in between and without a macroscopically identifiable aponeurotic structure present.
The epidermis is a cell-rich stratified epithelium with a reduced extracellular component; whereas, the dermis is essentially made of extracellular fibres, namely collagen (Figure 2.3)6. Collagen provides structure and support to the skin as well as being involved in cellular communication7. The importance of collagen in skin and its role in the ageing process are covered in the next chapter.

2. SUBCUTANEOUS LAYER Superficial fat
Beneath the dermis is the subcutaneous layer, which consists of the superficial fat compartments and the fibrous retinacula cutis (skin ligaments). Both of these components vary in density, volume, proportion and arrangement in the different areas of the face.
There are a number of superficial fat compartments separated by delicate fascial tissue and septa that converge to form retaining ligaments. According to studies on the subcutaneous fat of the face using staining techniques8, superficial fat can be separated into the following discrete anatomical compartments (Figure 2.4)9,10:
● Central
● Middle forehead
● Lateral temporal-cheek (forehead)
● Superior orbital
● Lateral orbital
● Inferior orbital
● Medial
● Nasolabial
● Middle
● Lateral temporal-cheek
● Jowl
● Pre-platysma
These compartments also influence the appearance of the face following injection of dermal fillers: injection into the superficial nasolabial, middle cheek and jowl compartments leads to inferior displacement, whereas injection into the medial cheek, lateral cheek and superficial temporal compartments leads to an increase in volume without inferior displacement (i.e. an increase in local projection)9.
Skin ligaments
The fibrous retinacula cutis (skin ligaments) are numerous, small, fibrous bands that extend from the deep true ligaments originating in the periosteum and bone (level 6). As the ligaments become superficial, they branch to give support to the superficial structures (Figure 2.5)11,12. These skin ligaments are present extensively in the subcutaneous region of the face12. In a study of eight embalmed cadavers, macroscopic and microscopic examination demonstrated the widespread presence of fibrous strands linking the base of the dermis and the superficial fibres of the underlying deep fascia12. The investigators concluded that skin ligaments provide an anchorage of skin to deep fascia that is flexible and yet resistant to mechanical loading from multi-directional forces12
Figure 2.3
Reproduced with permission of MINERVA Research Labs Ltd
Collagen in the dermis6
Reproduced with permission of MINERVA Research Labs Ltd - London. 22 ELLANS É ® | AN EXPERT'S GUIDE
Middle forehead
Lateral temporalcheek (forehead)
Lateral orbital
Medial
Middle
Lateral temporal-cheek
Pre-platysma fat
Adipose tissue
Space
Central
Superior orbital
Inferior orbital
Nasolabial
Jowl
1 Dermis
2 Retinacula cutis
3 Superficial musculoaponeurotic system (SMAS)
4 Retaining ligament
5 Periosteum
Figure 2.4
Superficial fat compartments of the subcutaneous layer10
Figure 2.5
Fibrous retinacula cutis structures extend from the dermis to the deep fascia11
ANATOMY OF THE FACE 23
3. SUPERFICIAL MUSCULOAPONEUROTIC SYSTEM (SMAS)
The aponeurotic system is a continuous layer from the occipitofrontal area to the neck and includes all muscles of facial expression, which are predominantly placed over or around the orbital or oral cavities. The aponeurotic system consists of:
● Galea - scalp
● Temporoparietal or superficial
temporal fascia
● Orbicularis fascia - periorbital area
● SMAS - mid and lower face
● Platysma - neck
Within the SMAS, there are two layers of intrinsic muscles10,3:
1 Superficial sphincteric muscles (broad and flat muscles with minimal bone insertions [stabilised mainly by retaining ligaments]): these are the frontalis, orbicularis oculi, orbicularis oris, risorius muscle and platysma
2 Deep muscles with functional control over the sphincter muscles: these are the corrugator supercilii, procerus, zygomaticus major, zygomaticus minor, nasalis, depressor septi, levator labii superioris, levator labii superioris alaeque nasi, levator anguli oris, buccinator, mentalis, depressor labii inferioris, depressor anguli oris and auricular muscles
The muscles of the SMAS include the mimetic muscles of the face (see Figure 2.6)10. Developing from the first pharyngeal arch, the muscles of mastication are innervated by a branch of the trigeminal nerve called the mandibular nerve. They consist of10:
● Temporalis
● Masseter
● Lateral pterygoid
● Medial pterygoid
The mimetic muscles, or muscles of facial expression, are thin, flat muscles that act either as sphincters of facial orifices, as dilators or as elevators and depressors of the eyebrows and mouth10. Mimetic muscles are attached to the skin by the retinacula cutis fibres within the subcutaneous layer and much less strongly attached to the underlying facial skeleton3
The mimetic muscles include (but are not limited to):
Upper face:
● (Periorbital muscles) frontalis, corrugator supercilii, depressor supercilii procerus and orbicularis oculi
Midface and lower face:
● (Perioral muscles) levator muscles, zygomaticus major and minor, risorius, orbicularis oris, depressor anguli oris, depressor labii and mentalis
● (Nasal muscles) compressor naris, dilator naris and depressor septi
As the name suggests, this group of muscles is responsible for the facial movements that allow facial communication.
24 ELLANS É ® | AN EXPERT'S GUIDE
Occipitofrontails (frontal portion)
Orbicularis oculi Procerus
Orbicularis oculi (palpebral portion)
Zygomaticus minor
Zygomaticus major
Risorius
Levator anguli oris
Depressor anguli oris
Depressor labii inferioris
Sternohyoid muscle
Omohyoid
Corrugator supercilii
Levator labii
superioris alaeque nasi
Temporalis
Levator labii superioris alaeque nasi
Levator labii superioris
Compressor naris
Dilator naris
Depressor septi
Masseter

Buccinator
Orbicularis oris
Mentalis
Sternocleidomastoid muscle
Figure 2.6
ANATOMY OF THE FACE 25
The mimetic facial muscles
4. RETAINING LIGAMENTS AND DEEP FAT COMPARTMENTS
Retaining ligaments
There are two types of ligaments: true retaining ligaments, which are easily identifiable structures that connect the dermis to the underlying periosteum, and false retaining ligaments, which are more diffuse condensations of fibrous tissue that connect superficial and deep facial fascia.
The true retaining ligaments can be aligned into a single line located immediately lateral to the lateral orbital rim extending from the temporal crest to the mandible, creating the line of ligaments indicated in blue (Figure 2.7)14
Galea
Deep fat
While the superficial fat compartments lie above the muscles, the deep fat deposits provide volume and shape as well as acting as gliding planes that allow mimetic muscles to move smoothly10
Deep fat structures include (Figure 2.8)10:
● Suborbicularis oculi fat (SOOF), which is divided into:
● The medial component extending along the inferior orbital rim from the medial limbus (sclerocorneal junction) to the lateral canthus
● The lateral component from the lateral canthus to the temporal fat pad
The sublevator fat pad is an extension of the buccal fat pad and lies medial to the medial SOOF compartment. It represents the most medial of the deep infraorbital fat pads.
The buccal fat pad is an aesthetically important structure that sits on the posterolateral part of the maxilla superficial to the buccinator muscle and deep to the anterior part of masseter. It facilitates smooth movement of the surrounding muscles of mastication.
The galea fat pad lies deep to the frontalis in the forehead and extends superiorly for about 3 cm. It envelops the corrugator and procerus muscles and aids their movement.
● The retro-orbicularis oculi fat (ROOF) is part of the galea fat pad over the superolateral orbital rim from the middle of the rim to beyond the lateral part. It lies deep to the superolateral fibres of preseptal and orbital orbicularis oculi and contributes to the fullness (in youth) or heaviness (in senescence) of the lateral brow and lid
Mandibular ligament
Figure 2.7
The true retaining ligaments form a single line
Temporal ligamentous adhesion
Zygomatic ligament
This layer (retaining ligaments, spaces and deep fat compartments) 'is the battleground in which the fight between mobility and stability is played out'13
26 ELLANS É ® | AN EXPERT'S GUIDE
Retro-orbicularis oculi fat (ROOF)
Suborbicularis oculi fat (SOOF)
Lateral
Deep medial cheek fat
Medial
Medial Lateral
Buccal fat pad
The periosteum is a membranous tissue that covers the surface of the facial skeleton, except for where there are cartilage ligament attachments15 The periosteum is composed of two distinct layers and is involved in growth and repair of bones16. The deep fascia includes temporalis fascia, parotid–masseteric fascia, periosteum, perichondrium and orbital septum15 These fascia overlie the facial skeleton.
The facial skeleton forms the hard tissue of the face, providing important structural support and projection for the overlying soft tissues, as well as supplying attachment points for the SMAS.
Facial appearance is, to a large extent, determined by the convexities and concavities of the underlying facial bones10. The high cheekbones
and strong chin associated with attractiveness are attributable to the convexities and projection provided by the zygomatic bone and mental protuberance of the mandible. In a young face, there is a smooth transition between these compartments.
Age-related changes to the facial skeleton and their consequences for other facial tissues are covered in Chapter 3.
5. PERIOSTEUM AND DEEP FASCIA
6. SUBPERIOSTEAL PLANE/BONE
Figure 2.8
Deep fat compartments
ANATOMY OF THE FACE 27
Blood vessels
The skin and soft tissue of the face mostly receive their blood supply from the external carotid artery – the exceptions being the central forehead, eyelids and upper part of the nose, which are supplied through the internal carotid system by the ophthalmic arteries. The internal carotid system is of most concern when carrying out dermal filler injections because it transverses all facial layers from 6 to 1.
The facial artery in the midface and lower face is of concern because it runs at different distances from the skin and subcutaneous fat due to the different tissue thickness, and there is considerable variation in the course of the artery between different patients. A cadaver study demonstrated three terminal branch types with obvious implications for dermal filler injections





(Figure 2.9)17
Overall, the extensive nature of the blood supply to the face and the considerable variation in the course of the facial blood vessels, which frequently run superficial to the skin, mean that there are danger areas for the injection of dermal fillers. The veins and arteries of the face generally run through the SMAS, which means the most suitable layers for injection are layers 2 and 4.
Angular type Nasal type Alar type Nasal type 60% Angular type 22% Labial type 4% Hypoplastic type 2% Alar type 12% Labial type Hypoplastic type
Variation
arteries17 28 ELLANS É ® | AN EXPERT'S GUIDE
Figure 2.9
in facial

29
Model is not an actual patient.
Danger zones for Ellansé injection
UPPER FACE (FIGURE 2.10A):
● The supraorbital, supratrochlear, dorsal nasal and angular arteries anastomose in the nasoglabellar region to form a vascular arcade. This rich network means that intravascular injection can create retrograde propagation of a foreign body to the ophthalmic artery, so dermal fillers should never be applied here. The arteries quickly become more superficial after exiting the orbit and closely abut rhytides, especially the supratrochlear artery and the glabellar frown lines. Ellanse is contraindicated in the periorbital region (eyelids, under-eye dark circles, crow's feet) and glabella region.18
● The forehead region should be approached with caution and only by experienced physicians due to the presence of the supratrochlear and supraorbital arteries
● The frontal branch of the facial nerve crosses the zygomatic arch at the supraperiosteal level (mid third of the zygomatic arch) and runs
1.5–2 cm above the orbital rim. Special precautions should be taken when approaching the temporal area from below or when treating the zygomatic area
● In the temporal region, the superficial temporal artery resides in the temporoparietal fascia. However, as the vessel approaches the lateral border of the frontalis, just above the brow peak, it becomes subcutaneous2
MIDFACE (FIGURE 2.10B):
● Inadvertent intravascular injection of fillers around the eye can lead to occlusion of the central retinal vessels and potentially blindness7,18
● To avoid complications in the infraorbital area, Ellansé should be placed at the supraperiosteal level thus avoiding contour irregularities or potential vascular complications19
● Care should also be taken when treating nasolabial folds in the upper third, close to the nose, due to proximity of the facial artery17 (see Chapter 5 for more information)
LOWER FACE (FIGURE 2.10C):
● The facial artery is more exposed when crossing from the submaxillary fossa to the face, and it is right at the anterior border of the masseter. To help avoid the facial artery when treating the pre-jowl sulcus and jawline, it is advisable to locate its pulse at the anterior border of the masseter. Asking the patient to clench their jaw can assist with this
● Injections directly into the mental foramen should be avoided, as pressure can affect the mental nerve
Like all procedures of this type, there is a possibility of adverse events, although not everybody experiences them. These adverse events include, but are not limited to: hypersensitivity, allergic reactions, inflammation (redness, swelling, oedema, nodules/granuloma, etc.), infection, pain (which may be temporary or persistent in nature), haematoma or bruising. For a full list of adverse events, consult the Ellansé Instructions for Use 18
For further information on planning Ellansé treatment, obtaining better and consistent results, and preventing and avoiding complications and unwanted outcomes, see Chapters 4 and 8.
Additional information is available from Sinclair College. Please scan the QR code below for access

30 ELLANS É ® | AN EXPERT'S GUIDE
Scan the QR code to investigate the layers of the face using augmented reality and test your knowledge of facial anatomy, or use the following URL to explore the content:
www.ellanseexpertguide.com/2
 Figure 2.10A Danger zones for upper face
Figure 2.10B Danger zones for midface
Figure 2.10A Danger zones for upper face
Figure 2.10B Danger zones for midface
ANATOMY OF THE FACE 31
Figure 2.10C Danger zones for lower face
References
1. Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757–74.
2. de Melo F, Nicolau P, Piovano L, Lin SL, Baptista-Fernandes T, King MI, Camporese A, Hong K, Khattar MM, Christen MO. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. 2017;10:431–40.
3. Mendelson BC, Jacobson SR. Surgical anatomy of the midcheek: facial layers, spaces, and the midcheek segments. Clin Plastic Surg. 2008;35:395–404.
4. Cotofana S, Fratila AAM, Schenck TL, Redka-Swoboda W, Zilinsky I, Pavicic T. The anatomy of the aging face: A review. Facial Plast Surg. 2016;32:253–60.
5. Farkas JP, Pessa JE, Hubbard B, Rohrich RJ. The science and theory behind facial aging. PRS GO 2013;1:e8. DOI:10.1097/ GOX.0b013e31828ed1da.
6. Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesthet Res. 2021;8:2. DOI 10.20517/2347-9264.2020.153.
7. Christen MO, Vercesi F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31–48.
8. Rohrich RJ, Pessa JE. The fat compartments of the face: Anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219–27.
9. Schenck TL, Koban KC, Schlattau A, Frank K, Sykes JM, Targosinski S, Erlbacher K, Cotofana S. The functional anatomy of the superficial fat compartments of the face: a detailed imaging study. Plast Reconstr Surg. 2018;141:1351–9.
10. Prendergast PM. Anatomy of the face and neck. In: Shiffman M, Di Giuseppe A (eds) Cosmetic Surgery. Springer, Berlin, Heidelberg. DOI: org/10.1007/978-3-642-21837-8_2.
11. Mendelson B, Wong CH. Facial anatomy and ageing. In: Farhadieh RS, Bulstrode NW, Cugno S (eds). Plastic, reconstructive surgery: Approaches and techniques. John Wiley & Sons; 2014. p. 79–92.
12. Nash LG, Phillips MN, Nicholson H, Barnett R, Zhang M. Skin ligaments: regional distribution and variation in morphology. Clin Anat. 2004;17: 287–93.
13. Mendelson B. Facelift anatomy, SMAS, retaining ligaments and facial spaces. Available from: https:// plasticsurgerykey.com/faceliftanatomy-smas-retaining-ligamentsand-facial-spaces/. Accessed February 2022.
14. Casabona G, Bernardini FP, Skippen B, Rosamilia G, Hamade H, Frank K, Freytag DL, Sykes J, Onishi EC. How to best utilize the line of ligaments and the surface volume coefficient in facial soft tissue filler injections. J Cosmet Dermatol. 2019;00:1–9.
15. Dzubow LM. The fasciae of the face: an anatomic and histologic analysis. J Am Acad Dermatol. 1986;14:502–7.
16. Dwek JR. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010;39:319–23.
17. Pinar YA, Bilge O, Govsa F. Anatomic study of the blood supply of perioral region. Clin Anat. 2005;18:330–9.
18. Sinclair. Ellansé instructions for use.
19. Sinclair. Ellansé best practices and guidelines advisory board. IMCAS. Paris 2020.
32 ELLANS É ® | AN EXPERT'S GUIDE

Model is not an actual patient. ANATOMY OF THE FACE 33
The Ageing Process 03
35
Facial ageing: Our evolving understanding
Multiple theories have been presented over the past 100 years regarding facial ageing, with our understanding of the process constantly evolving2 In the past 20 years, several new anatomical structures have been identified, contributing to an improved understanding of one of the most complex areas of the human body (Figure 3.1)3
At the same time, a plethora of procedures, both invasive and noninvasive, have been recruited for the purpose of reducing the signs of ageing and restoring the youthful appearance of the face.
Optimal use of all of these different procedures requires a detailed understanding of the three-dimensional composition and layered nature of the underlying facial anatomy, as well as an appreciation of the changes that take place as we age.
36 ELLANS É ® | AN EXPERT'S GUIDE
DLCF, deep lateral cheek fat; DMCF, deep medial cheek fat; lig, ligament; LOT, lateral orbital thickening; ROOF, retro-orbicularis oculi fat; SMAS, superficial musculoaponeurotic system; SOOF, suborbicularis oculi fat.
Figure 3.1
Timeline of description of structures in the human face3
Facial ageing is an inevitability for us all, with the first signs becoming apparent between the ages of 20 and 30 years, depending on lifestyle and environment1.
1800s 1900s 1910s 1920s 1930s 1940s 1912 Modiolus P. Eisler
ROOF (Charpy's fat pad) M. Charpy
space
Kostrubala
organ
Chievitz
fat pad
Bichat
1909
1945 Buccal
J.
1885 Juxtaoral
J.
1801 Buccal
X.
2013
Premaxillary space
C.H. Wong
2008
DMCF Ristow space
R. Rohrich
2002
Prezygomatic space
B. Mendelson
1989 Mandibular lig. Platysma-cutaneous lig.
DW. Furnas
1973
Loré's fascia
J. Loré
1959
Zygomatic lig. (McGregor's patch)
M. McGregor
1951
Intra-orbital fat pads
S. Castañares
1976 SMAS
V. Mitz
2008
2012 DLCF
M. Gierloff
Premasseter space
B. Mendelson
2002
Orbicularis retaining lig.
AZ. Muzaffar
1992
Masseteric lig.
JM. Stuzin
2008
Mandibular septum jowl fat
E. Reece
2009
Deep chin fat
R. Rohrich
1995 SOOF
A. Aiache
2007
Subcutaneous fat compartments
R. Rohrich
2000
LOT
J. Moss
2012
Tear trough lig.
CH. Wong
THE AGEING PROCESS 37
1950s 1960s 1970s 1980s 1990s 2000s 2010s
Skin
Collagen is an important component of the extracellular matrix (ECM) (Figure 3.2), having an essential structural role as well as being a functional protein that interacts at different cellular levels5,6 There are 28 types of collagen in the body5, with 12 types7 found in the skin (of which, the most important are
type I [85%] and type III [10%])5,8. Type I collagen is the prototype collagen and also the most abundant type in the body. It consists of a long-chain triple-helix structure, comprising a heterotrimer of two identical α1 chains and one α2 chain (Figure 3.3)5
COLLAGEN
Provides infrastructure for elastin and hyaluronic acid
ELASTIN
Helps the skin retain its elasticity
HYALURONIC ACID
Water binds to hyaluronic acid, keeping the skin moist
Well-structured collagen network


DECREASED COLLAGEN
DECREASED ELASTIN

DECREASED HYALURONIC ACID
Disorganised collagen network
38 ELLANS É ® | AN EXPERT'S GUIDE
Collagen is the predominant protein type in the skin, accounting for 70% of total skin mass4.
Collagen from young human dermis
Collagen from aged human dermis
Figure 3.2
Collagen in young and ageing skin
Collagen fibres are responsible for the resilience and main mass of the dermis5,8. Fibrillar and non-fibrillar collagen structures exist, with specific functions5,8. The production of type III collagen is faster and more abundant during the primary processes of tissue repair, as type III is more concerned with protection and type I collagen is involved in longer-term regeneration (Table 3.1)9 As wound repair progresses, production of type III collagen decreases in tandem with the formation of larger and tougher type I collagen fibres9
Collagen fibres
With increasing age, the amount, quality and type of collagen changes. In both sexes, total skin collagen and skin thickness decrease over time. Aside from solar exposure causing wrinkling, there are a number of intrinsic and extrinsic ageing factors5,8,10. Intrinsic or natural ageing is inevitable, whereas extrinsic ageing is caused by a range of lifestyle factors (Table 3.2)5,8,10
Collagen fibrils
Collagen molecules (triple helices) α-chains
THE AGEING PROCESS 39
Figure 3.3
PRO PRO PRO
Type I collagen: A long-chain triple helix GLY, glycine; HYP, hydroxyproline; PRO, proline.
HYP HYP HYP GLY GLY GLY
Physical organisation
Type III: Protection
Individual thin fibres
0.5–1.5 μm
● Loose framework
● Thin
● Weakly birefringent
● Green fibres
Ultra-structures
Distribution
● In normal skin
● In protective, scar tissue
Differences between intrinsic and extrinsic ageing5,8,10
Intrinsically (naturally) aged skin
Causes: Natural ageing
Thin
Atrophic
Finely wrinkled
Loosely packed, thin fibrils, 45 nm, more uniform diameter
Takes 9 days to appear
4–5 weeks to complete
● 5–15%
● 80–85%
Type I: Regeneration
Bundles of thick fibres
2–10 μm
● Closely packed
● Thick
● Strongly birefringent
● Yellow or red fibres
Densely packed, thick fibrils, 75 nm, marked variations in diameter
Takes 5–6 weeks to appear
3–4 months to complete
● 85–95%
● 12–20%
Extrinsically aged skin
Causes: Smoking, excessive alcohol consumption, poor nutrition and sun exposure
Thickened
Mottled discolouration
Deep wrinkles
Dry Laxity
Variety of benign neoplasms
CHARACTERISTICS OF SKIN AGEING
Despite the wrinkles in the skin apparent in older people, ageing is actually more pronounced in the dermis than the epidermis (Figure 3.4), with collagen loss of 1.0–1.5% seen every year from early adulthood5. These changes should be considered to be a consequence of ageing, rather than the cause. One theory is that the ageing process is partly caused by the accumulation of remnants of collagen type I fibres from constant degradation and reconstruction
● Roughness
● Telangiectasia
● Pre-malignant lesions/skin cancers
of collagen over time5. Build-up of these type I fibre remnants eventually prevents mechano-transductional communication between type I fibres and the fibroblasts that produce collagen5. During the ageing process, the proliferative and metabolic activity of fibroblasts decreases and their functions are impaired, leading to reduction of the synthesis of collagen, elastin and hyaluronic acid11.
In aged skin, collagen is permanently modified into thickened fibrils, organised in rope-like bundles that appear to be in disarray in comparison with the pattern observed in younger skin8. The ratio of collagen type I to type III increases with increasing age; however, an earlier increase in type I collagen in the facial skin can cause premature ageing12
The progress of involutionary changes, deformation type of facial ageing and early development of ptosis is more rapid in patients with a reduced collagen type I/III ratio13
40 ELLANS É ® | AN EXPERT'S GUIDE
Table 3.2
Table 3.1
Types I and III collagen
Younger skin
Epidermis
Oxytalan
Elastin
Collagen
Older skin
Epidermis
Oxytalan
Elastin
Collagen
Collagen is an integral part of the biological interplay between skin cells, including communication between fibroblasts and keratocytes, as well as the ECM (elastin, hyaluronic acid, water molecules, etc.) (see Figure 3.2)5,8. With intrinsic ageing, elastin fibres become thickened and coiled in the papillary dermis, with fibres becoming less elastic8 and also decreasing in number. The metabolic turnover of elastin is slow, with a half-life comparable to the normal human life span14, meaning that, unlike collagen fibres, elastin is not constantly synthesised and degraded. Elastin is,
therefore, vulnerable to both intrinsic and extrinsic factors (see Table 3.2), with the process dependent on elastolytic enzymes, elastases, which are present in many tissues14
Melanocytes are also subject to ageing, with 10–20% of epidermal melanocytes lost every decade after 30 years of age15. Furthermore, vasculature decreases, particularly in the papillary dermis, leading to depleted nutrient exchange and inhibited thermoregulation8
Smoother skin
Hyaluronic acid and water
Fibroblast
Capillary vessel
Deep wrinkle
Hyaluronic acid and water
Fibroblast
Capillary vessel
THE AGEING PROCESS 41
Figure 3.4 Ageing is more pronounced in the dermis than the epidermis
Fat
As discussed in Chapter 2, the face has two types of fat: the deep fat compartments and the superficial fat of the hypodermis (Figure 3.5).
Previous debate about whether signs of facial ageing were partly due to selective hypertrophy of the upper portion of the cheek fat pad, as shown by magnetic resonance imaging (MRI), or whether the age-related increase in volume shown
by MRI was due to displacement of the deep fat from beneath the superficial musculoaponeurotic system (SMAS) into the superficial fat layers16 had led to a confused picture. However, the cadaver study using coloured dyes carried out
Suborbicularis oculi fat (SOOF)
Lateral
by Schenck et al16 has helped to provide a better understanding of the discrete compartments of the different fat layers of the face and their implication in facial ageing (Figure 3.6)16
Deep medial cheek fat
Medial
Medial Lateral
Buccal fat pad
Superficial facial fat
42 ELLANS É ® | AN EXPERT'S GUIDE
Figure 3.5
The face has two types of fat7
The superficial fat compartments are shown on the right in purple areas, while the deep fat compartments are shown on the left in yellow and pink areas, with labels
Cadaveric dissection illustrating superficial fat compartments16
Cadaveric dissection of the left side of a face after the superficial (subcutaneous) fat compartments have been injected with coloured dye: superficial nasolabial (1, red dye), medial cheek (2, blue dye), middle cheek (3, red dye), lateral cheek (4, violet dye), superficial superior temporal (5, blue dye), superficial inferior temporal (6, red dye) and the jowl fat compartment (7, blue dye). Asterisk marks the platysma


SUPERFICIAL FAT
This compartmentalised anatomy of the superficial subcutaneous fat has implications in the ageing process. Volume loss appears to occur at different rates in different compartments, leading to irregularities in facial contour and loss of the seamless, smooth transitions between the convexities and concavities of the face associated with youthfulness and beauty17. Superficial fat compartments, such as the superficial nasolabial fat compartment, undergo hypertrophy
with ageing3, with inferior displacement of the superficial nasolabial and jowl compartments16. These changes, in particular, have substantial influence on the appearance of the face as it ages.
Above the sulcus of the nasolabial fold, the subcutaneous fat loses its stability due to a number of ageing factors, including bone resorption3, and slides inferiorly, bulging over the sulcus (Figure 3.7).
DEEP FAT
In the midface, the suborbicularis oculi fat (SOOF) and deep cheek fat provide volume and shape to the face and act as gliding planes for the SMAS17. The retroorbicularis oculi fat (ROOF) compartment is part of the galea fat pad over the superolateral orbital rim from the middle of the rim to beyond the lateral part17
The ROOF lies deep to the superolateral fibres of preseptal and orbital orbicularis oculi and contributes to the fullness (in youth) and heaviness (in older age) of the lateral brow and eyelid17
With ageing, the retaining ligaments under the eye attenuate (lengthen). This, together with volume loss in both the superficial and deep fat compartments, results in visible folds and grooves in the cheeks and under the eyes17
Treatment should aim to reposition the displaced superficial fat and compensate for the reduced volume of the fat in the deep compartments, rather than fill hollows or grooves7
Deep fat
● Deep fat loses volume and slides
● Sliding is halted by strong retaining ligaments
● Deepening increased by muscle retraction
THE AGEING PROCESS 43
Figure 3.7
The ageing process in the area above the nasolabial sulcus3
Figure 3.6
Muscle
Facial muscles are generally cutaneous muscles. Most have at least one attachment to the skin and one to bone. Those with deeper bony insertions are responsible for protective movements, for example opening and closing of eyes and mouth, and have strong attachments so they can act at speed or generate power, such as for chewing.


Youthful
Deep fat
Long, curved thin muscle




Superficial fat
Bone
Ageing
Superficial fat
Transfer of deep fat towards superficial fat
Short, thick, straight muscle
Bone


44 ELLANS É ® | AN EXPERT'S GUIDE
3.8 Muscle ageing and its consequences 23-YEAR-OLD 55-YEAR-OLD
Figure
The physiological age-dependent process of losing muscle mass and proper function of muscles is known as sarcopenia3. Facial muscles change with age, increase in tone and have a shorter amplitude of movement, potentially entering into permanent
contracture (Figure 3.8)3. The clinical effect of these changes might be a general tightening of the muscles of the face, with the permanent contractures resulting in a potential shifting of fat and thus an accentuation of skin creases, and permanent skin wrinkling with a
transformation of dynamic facial lines to static facial lines3
Tension caused by tautening of ageing muscles also has a major effect on bone resorption at the point of attachment (Figure 3.9).
Arrows indicate the areas of the facial skeleton susceptible to resorption with ageing. The size of the arrow correlates with the amount of resorption
The darker areas are those of the greatest bone loss. The stigmata of ageing, manifested by the facial soft tissues, corresponds with the areas of weakened skeletal support


THE AGEING PROCESS 45
Figure 3.9
Facial skeleton resorption and increased orbital aperture18
Bone
Although traditionally less well appreciated than the soft-tissue changes to the face, changes to the facial skeleton contribute hugely to the appearance of ageing18.

While the facial skeleton tends to expand throughout life, selective resorption occurs in specific areas of the facial bones18. The orbital aperture increases with age, in both area and width (Figure 3.10)18. Resorption is, however, uneven and site specific. The superomedial and inferolateral aspects of the orbit have the greatest tendency to resorb.
This contributes to increased prominence of the medial fat pad, elevation of the medial brow and lengthening of the lid–cheek junction18. Meanwhile, the nasal tip droops due to changes in the bony foundation that supports the nose in youth, the paired nasal bone and the ascending processes of the maxillae18.
46
Model is not an actual patient.
CONSEQUENCES OF BONE AGEING18
General consequences of the resorption of the facial skeleton include retrusion of the periosteum, which alters the position of the outer surfaces of the bones and changes the attachment locations of facial ligaments and related muscles through the periosteum.


The areas most affected by reduced skeletal prominence correspond to those areas of the face that manifest the most prominent stigmata of ageing18, as detailed below.
Upper face:
The lateral brow assumes a drooped look, while the medial orbital fat pad also becomes more prominent with age, possibly associated with the recession of the superomedial orbital rim (see Figure 3.10).
Midface:
The midcheek manifests the most complex softtissue changes with ageing. The development of tear trough deformity, malar mounds and prominent nasolabial fold and groove are attributed to agerelated loss of the projection of the maxilla.
Lower face:
The changes over the lower face are less complex, with the jowl appearing more prominent in relation to the area of reduced skeletal support in the pre-jowl area of the mandible and at the mandibular angle – all of these being due to the strong muscular pull increased by their progressive contracture3
Figure 3.10
THE AGEING PROCESS 47
Ageing of the skeletal upper face18
AGEING PATTERNS IN MEN AND PREMENOPAUSAL WOMEN
There is a shared average facial ageing pattern in men and premenopausal women, with the average pace of ageing being twice as high in females as in males.
The pattern of facial ageing comprises local shape changes (Figure 3.11)1:











● Relatively smaller eyes
● Thinner lips
● Apparent widening of the lower face due to the global retraction of the face19





48 ELLANS É ® | AN EXPERT'S GUIDE
Figure 3.11
Male ageing pattern 35 years 50 years 70 years 90 years
Ageing patterns in men and premenopausal women Reproduced with permission of MINERVA Research Labs Ltd - London.
35 years 50 years 70 years 90 years Premenopausal female
ageing pattern
AGEING PATTERNS IN POSTMENOPAUSAL WOMEN
After menopause, women show a stronger reduction in the jaw area, particularly the chin, compared with men. Female faces change up to three times as fast as male faces, most noticeably between the ages of 50 and 60 years (Figure 3.12)1
This sex difference is likely to result from hormonal changes during menopause and andropause, which are dominated by oestrogen reduction in females and testosterone reduction in males1
THE AGEING PROCESS 49
Facial ageing rate (Procrustes distance per year) 0.004 0.003 0.002 0.001
Male 40 45 50 55 60 65 70 Mean age (years)
Figure 3.12
Rates
of male and female facial ageing1
Female
Summary and aims of treatment
Ageing is a multifactorial process involving all layers of the face:

● Skin
● Fat
● Muscle
● Bone
The aim of treatment with Ellansé is to:
Repair the consequences of ageing by:
● Compensating volume loss
● Repositioning fat
● NOT filling hollows and grooves
● Improving soft tissues and skin quality due to the neocollagenesis20
50 ELLANS É ® | AN EXPERT'S GUIDE
Model
is not an actual patient.
References
1. Windhager S, Mitteroecker P, Rupić I, Lauc T, Polašek O, Schaefer K. Facial aging trajectories: A common shape pattern in male and female faces is disrupted after menopause. Am J Phys Anthropol. 2019;169: 678–88.
2. Farkas JP, Pessa JE, Hubbard B, Rohrich RJ. The science and theory behind facial aging. Plast Reconstr Surg Glob Open. 2013;1:e8–15.
3. Cotofana S, Fratila AAM, Schenck TL, Redka-Swoboda W, Zilinsky I, Pavicic T. The anatomy of the aging face: A review. Facial Plast Surg. 2016;32:253–60.
4. Gniadecka M, Nielsen OF, Wessel S, Heidenheim M, Christensen DH, Wulf HC. Water and protein structure in photoaged and chronically aged skin. J Invest Dermatol. 1998;111: 1129–33.
5. Reilly DM, Lozano J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast Aesthet Res. 2021;8:2.
6. Christen MO, Vercesi F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31–48.
7. Sinclair. Ellansé trainer presentation.
8. Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–51.
9. Nicolau PJ, Marijnissen-Hofsté J. Neocollagenesis after injection of a polycaprolactone based dermal filler in a rabbit. Eur J Aesth Med Dermatol. 2013;3:19–26.
10. Yaar M, Eller MS, Gilchrist BA. Fifty years of skin aging. J Investig Dermatol Symp Proc. 2002; 7:51–8.
11. de Araújo R, Lobo M, Trindade K, Silva DF, Pereira N. Fibroblast growth factors: A controlling mechanism of skin aging. Skin Pharmacol Physiol. 2019;32:275–82.
12. Cheng W, Yan-hua R, Fang-gang N, Guo-an Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr J Biotechnol. 2011;10:2524–9.
13. Manturova NE, Smirnova GO, Stupin VA, Silina EV. The ratio of collagen types I/III as a marker of skin aging and prognosis of aesthetic facial surgery results. J Pharm Sci Res. 2018;10:2543–6.
14. Uitto J, Li Q, Urban Z. The complexity of elastic fibre biogenesis in the skin –a perspective to the clinical heterogeneity of cutis laxa. Exp Dermatol. 2013;22:88–92.
15. Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: biology and development. Postepy Dermatol Alergol. 2013;1:30–41.
16. Schenck TL, Koban KC, Schlattau A, Frank K, Sykes JM, Targosinski S, Erlbacher K, Cotofana S. The functional anatomy of the superficial fat compartments of the face: Plast Reconstr Surg. 2018;141:1351–9.
17. Prendergast PM. Anatomy of the face and neck. Cosmetic Surgery 2013. M.A. Shiffman and A. Di Giuseppe (eds.). DOI: 10.1007/9783-642-21837-8_2.
18. Mendelson B, Wong CH. Changes in the facial skeleton with aging: Implications and clinical applications in facial rejuvenation. Aesth Plast Surg. 2012;36:753–60.
19. Lambros V. Facial aging: A 54-year, three-dimensional population study. Plast Reconstr Surg. 2020;145:921–8.
20. Sinclair. Ellansé Instructions for use.
THE AGEING PROCESS 51
Patient Selection and Preparation 04
53
What is Ellansé used for?
Beautification
The treatment intention is to provide balance and symmetry to the face or correct areas lacking volume
Rejuvenation
Reversing the signs of ageing, which is achieved by the biostimulatory nature of Ellansé

Prevention
Preventing the signs of ageing from appearing through subtle touch-ups that will improve the quality of the skin
54
The adaptable and predictable nature of Ellansé means it is suitable for three different treatment intentions:
Model is not an actual patient.
Who is Ellansé for?
Ellansé should not be injected into the periorbital region (eyelids, under-eye dark circles, crow’s feet) or glabella region, as there is a risk of ocular ischaemic events leading to loss of vision1. It is also contraindicated for the lips. Like all procedures of this type, there is a possibility of adverse events, although not everybody experiences them.

These adverse events include, but are not limited to, infection, minimal acute inflammatory tissue reaction (redness, swelling, rash, oedema, erythema, lumps/nodules, etc.), pain (which may be temporary or persistent in nature), transient haematoma or bruising. For a full list, consult the Ellansé Instructions for Use1
Ellansé is suitable for men or women looking for lasting correction of wrinkles and facial ageing consequences or conditions2. Ellansé may be used in the following locations (Figure 4.1)1:
PATIENT SELECTION AND PREPARATION 55
Suitable
for
3 4 2 9 1 9 2 4 3 5 8 6 10 7 11 10 6 8 5 Upper face 1 Frontal 2 Eyebrow 3 Temporal 4 Zygomatic Midface 5 Preauricular 6 Malar/cheek 7 Nose Lower face 8 Mandibular 9 Nasolabial folds 10 Marionette lines 11 Chin (mental area)
Figure 4.1
treatment areas
Ellansé2,4
Who should not use Ellansé?
Patients presenting with any of the following should not be treated with Ellansé (Figure 4.2)2
Known hypersensitivity to any of the components1
Acute or chronic skin disease (infection or inflammation)1
Known susceptibility to keloid formation or hypertrophic scarring1
Active sepsis or infection
Current medication cortisone treatment, anticoagulants and medications that can prolong bleeding, e.g. aspirin or warfarin
Breastfeeding
Pregnancy
Coagulation/bleeding disorders
Severe allergies manifested by a history of anaphylaxis, or history or presence of multiple severe allergies1
Autoimmune disease
56 ELLANSÉ ® | AN EXPERT'S GUIDE
4.2 Who should not use Ellansé?2
Figure
Ellansé patient selection
Each patient should undergo a holistic and multilevel aesthetic evaluation to allow development of an adequate and personalised treatment plan. However, it is possible to define some characteristics that can help to identify candidate patients for Ellansé (Figure 4.3).
Patients with large facial volumes, integumentary heaviness or prominent fat pads are not good candidates for Ellansé.

Regarding the product itself, selection of the appropriate Ellansé option is key. It is essential to explain to the patient the durability of the available variants. In the experience of experts contributing to this book, both the Ellansé S and Ellansé M options provide long-term results and are well accepted by patients*.
Ellansé is ideal for patients with the following clinical conditions:
1 Patients with signs of ageing due to volume loss, moderate skin laxity and elastosis. The patient will benefit from durable volume replacement and improved tissue quality

2 Patients with mild volume loss and good-quality soft tissues. Ellansé biostimulation will provide a durable and consistent result
For details of injection techniques see Chapter 6: Case studies
For details of injection techniques see Chapter 6:
3 Patients looking to balance their facial features, with or without signs of ageing. Ellansé can provide long-lasting results, with tissue consistency similar to the natural soft tissues due to neocollagenesis




4 Patients seeking long-lasting results that can be adapted to last more than 18 or 24 months according to the patient’s requirements*
*Treatment effects have variable duration dependent on the Ellansé variant used, practitioner injection technique, patient lifestyle and metabolic rate. Any opinions, views or advice provided are those of the physicians or patients. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed.
TOP TIPS
Personalised treatment
Each patient should undergo thorough evaluation to develop a personalised treatment plan.
Ellansé options
Selection of the appropriate Ellansé option is key. Ellansé S and Ellansé M options both provide long-term results and are well accepted by patients.
PATIENT SELECTION AND PREPARATION 57
Patient showing signs of ageing (before and after)
Younger patient looking to use Ellansé for beautification (before and after)
Patient looking to prevent signs of ageing
Figure 4.3
Personalised treatment and selection of Ellansé option Actual patients, results may vary.
Before Before Before Month 4 Month 3 Month 6
Case studies"
Preparation
The following steps should be taken as part of the pre-consultation process3:
Gather a complete medical history with close attention to any illnesses, medications, lifestyle habits (smoking, exercise, sun exposure, alcohol consumption, etc.)
Obtain all information about previous treatments (including satisfaction with results), i.e.:
● Previous facial cosmetic surgeries
● Injections (product, volume, areas, date, number of sessions, etc.)
● Resurfacings (peelings, dermabrasions, laser)
● Deeper treatments (radiofrequency, superficial or lasers)
Obtain patient expectations, reasons for seeking treatment and treatment priorities
Discuss the area(s) to be treated with the patient
Discuss future treatment options to develop a successful and progressive treatment plan
Combination treatments should generally be considered in order to provide optimal results
Highlight existing asymmetries
Assess the patient’s needs for pain management
Pre-treatment photographs are recommended
58 ELLANSÉ ® | AN EXPERT'S GUIDE
Patient consent
Details of the patient’s motivation and expectations should be recorded in order to identify the optimal treatment and any possible contraindications, and to prevent complications.

PATIENT SELECTION AND PREPARATION 59
A signed patient informed consent form should always be obtained prior to any preparation for procedures2,3,4.
Actual patient, results may vary.
Taking appropriate photographs
● Before and after photographs should always be taken
● Photographs should be taken in front of a plain, dark background, with the subject lit directly from the front to avoid shadows
● Patient should stand 1 m from the backdrop
● The patient should have their hair pulled back from the face, with ears fully visible and without jewellery
● In addition to two-dimensional photographs, it is recommended to take images with a three-dimensional imaging system, where possible
● Photographs should include five positions as a basis:
● Facing camera
● Correctly taken* 3/4 views (left and right)
● Both profiles
● Photographs should be taken:
● Before application
● Immediately at the end of the procedure
● 15 days after application
● 3 months after application
● Follow up at 1 year is recommended
*3/4 views are often not taken correctly:
Although many practitioners think the nose should reach the lateral line of the inner profile, this line should be totally visible, allowing for perfect appreciation of both inner (3/4) and outer profiles. Therefore, 3/4 photos should show the outer canthus.
Additional information is available from Sinclair College. Please scan the QR code below for access.

60 ELLANSÉ ® | AN EXPERT'S GUIDE
Managing patient expectations
The patient must be made aware of realistically achievable results. Treatment and post-treatment plans, as well as potential risks, must be discussed in light of the patient’s expectations2

PATIENT SELECTION AND PREPARATION 61
Actual patient, results may vary.
Preparing the patient
1 Preparation should start with removal of all make-up and full cleaning of the face with an antiseptic. The face should be free from any inflammation or infection
2 Use the anatomical landmarks of the face to mark the areas to be treated (Figure 4.4)

3 Mark the entry points, taking into consideration the anatomy to avoid complications or adverse events
4 Show the patient the treatment plan so it is clear which areas will be treated
5 Facial marking should always be carried out with the patient seated in a semi-supine position
62 ELLANSÉ ® | AN EXPERT'S GUIDE
There is no standard way to mark the areas to be treated. However, there are some standard procedures to follow:
Figure 4.4
in different ways Model is not an actual patient.
Marking of the face may be done
Preparing the workspace
● The procedure should be carried out in a clean, clinical environment

● Any instruments and devices should be kept in a sterile tray or drape (especially if using a cannula)
● Have an assistant present whenever possible
● It is recommended to have a swab impregnated with disinfectant close by at all times during the procedure. This swab should be used regularly to clean surrounding surfaces and objects that may not be sterile. It is also advisable to replace gloves or instruments if you think they may have
PATIENT SELECTION AND PREPARATION 63
Actual patient, results may vary.
Consider pain management
1 Assess the patient’s need for pain management to reduce patient anxiety and make treatment more comfortable3
2 Nerve blocks are a good option if there is a need for more prolonged and wider area of pain anaesthesia (the anaesthetic injection site will not be within the treatment area)3
3 Topical and/or local infiltration analgesia are also suitable; however, be aware of possible deformity to the treatment area3
4 The use of ice and vibration devices may also help to effectively reduce discomfort3

Model is not an actual patient. 64
Post-procedural care
For the first 24 hours after treatment, patients should be told to4:
Avoid sleeping face down
Avoid excessive sun exposure, ultraviolet lamp exposure and extreme cold weather, until any initial swelling and redness has resolved
Keep face clean
Use no make-up
Avoid swimming/bathing for the first 24 hours post-treatment
Avoid alcohol consumption for up to 72 hours post-treatment
Topically apply ice packs; this may help reduce oedema and bruising in the first 24–48 hours
Avoid laser treatment, chemical peeling or any other procedure based on active dermal response

PATIENT SELECTION AND PREPARATION 65
Figure 4.5
Post-procedure care for the first 24 hours
Summary
● Patient selection is key; Ellansé should not be offered to unsuitable candidates or those for whom it is contraindicated (see Figure 4.2)
● Informed consent should always be obtained before carrying out any procedure
● Before and after photographs are crucial to demonstrate aesthetic improvements, especially over time. Patient expectations should be managed and pain management considered

● Meticulous preparation of the patient and the workspace is advised to reduce chance of infection
● Post-procedural care is key to optimising patient outcomes
● Injection technique will be covered in the next chapter
66
Model is not an actual patient.
References
1. Sinclair. Ellansé instructions for use.
2. de Melo F, Nicolau P, Piovano L, Lin SL, BaptistaFernandes T, King MI, Camporese A, Hong K, Khattar MM, Christen MO. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. l 2017;10:431–40.
3. Sinclair. Ellansé injection technique guide.
4. Sinclair. Ellansé injection guidelines.
PATIENT SELECTION AND PREPARATION 67
Injection Techniques 05
69
Introduction
The information in this chapter is provided as a guide to injecting Ellansé® in the most common areas of the face. Please also refer to other chapters in this book for full details of selecting and managing patients who may be candidates for Ellansé treatment.
[NB: patient selection, work-up, contraindications and preparation are all covered in the previous chapter]
Ellansé should only be used by healthcare professionals trained in the field of soft-tissue augmentation after fully familiarising themselves with the product and its complete instruction leaflet. Training with a Sinclair-approved trainer is strongly recommended before using the product.
Before proceeding with Ellansé injections, please be sure that you are familiar with all the information in this book.
Ellansé may be used in several areas of the face. These areas are broadly separated into the following regions (Figure 5.1 and Figure 5.2)1:
● Upper face

● Midface
● Lower face
Model is not an actual patient. 70
Nose
Nasolabial folds


Malar and submalar
Zygomatic
Midface
Frontal area
Temporal area Brow area
Upper face
Mandibular area
Marionette lines
Chin (mental area)
Lower face
Upper face
Figure 5.2
Suitable treatment areas for Ellansé2,4
3 4 2 9 1 9 2 4 3 5 8 6 10 7 11 10 6 8 5
Figure 5.1 Regions of the face
1 Frontal 2 Eyebrow
3 Temporal
4 Zygomatic Midface 5 Preauricular 6 Malar/cheek 7 Nose Lower face 8 Mandibular 9 Nasolabial
folds
10
Marionette lines
11
Chin (mental area)
INJECTION TECHNIQUES 71
General guidelines for the use of Ellansé2
1. Depth of injection
● Subcutaneous fat (hypodermis) – level 2
● Supraperiosteal – level 4
2. Points of entry
● These should be planned according to the areas to be treated, taking the following aspects into consideration:
● Offer easy access to the plane of injection
● Avoid major vascular and nervous structures
● Limit the number of entry points, without compromising safety and result
● Local anaesthetic (LA) or topical anaesthetic (TA) infiltration in the entry points according to the device to be used. For a cannula, lidocaine 2% plus epinephrine (0.1–0.2 ml/point) should be used. For a needle, TA or LA should be used
3. Device
● Cannula: 25G (50 mm)
● Needle: 27G
4. Patterns of injection
● Retrograde threading or fanning:
● Assures an even distribution
● Increases the area of biostimulation
● More effective neocollagenesis
● Bolus:
● Should be limited to a maximum of 0.1–0.2 ml per bolus to avoid potential nodules
72 ELLANS É ® | AN EXPERT’S GUIDE
5. Low pressure injection
7. Massage
● Assures precise placement of the filler
● Reduces barotrauma
● Reduces swelling
● Reduces bruising
6. Volume
● Assures a better and even distribution of the filler
● Should be done by the treating physician immediately after the injection
● Recommend that the patient does not massage treated areas to avoid migration or displacement of the fillers
● Refers to the average volumes placed to obtain correction
● Varies depending on the area as well as individual needs and requirements
● Should be planned to obtain a full correction, except for a slight under-correction in areas with deficient soft-tissue coverage (including close to the orbital rim, nose and pre-jowl)
EXPERT TIP*
8. Mixing with lidocaine
● Ellansé can be used as presented and does not require mixing with lidocaine; the objective of mixing with lidocaine is purely to offer an extra layer of comfort to the patient (not a dilution)*
● Maximum volume is 0.2 ml/1.0 ml of Ellansé
● Lidocaine does not alter the carboxymethyl cellulose gel physical properties4
EXPERT TIP*
"In my clinic, I generally use a 22G cannula for facial augmentation work."
Dr Franciso de Melo
"In my practice, I wait for the results of neocollagenesis to be visible (~2 months) to avoid overcorrection with early top-ups."
Dr Franciso de Melo
*The information contained within this presentation has been provided by medical professionals in their medical practices independently of any involvement of Sinclair. Any opinions, information, views or advice provided or expressed are those of such medical professionals and not of Sinclair. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk INJECTION TECHNIQUES 73
Injection placement
For Ellansé in facial treatment areas, placement of the product in the subcutaneous and supraperiosteal plane is recommended (Figure 5.3)4.
For natural volume and enhancement of tissues, Ellansé is often injected in a deeper plane, such as the supraperiosteal layer1
For superficial volume, Ellansé is usually injected at the subdermal level (subcutaneous fat/hypodermis)5
EXPERT TIP*
EXPERT TIP*
The supraperiosteal technique is based on the deep application of the product in the superior temporal region. The objective of this technique is to apply the treatment in the supraperiosteal region, moving away from the areas of vascular risk. However, it has the disadvantage that larger amounts of product are required to achieve adequate volumetric make-up. Some experts have abandoned this technique and prefer only the use of cannula at the interfascial level.
The interfascial technique is currently the most used technique by most injector experts. The benefits of the application of polycaprolactone (PCL) in this region include:
● Reduced risks of intravascular application
● Need for smaller amounts of product
● No risk of migration of the product towards the middle third of the face This application should always be carried out with cannulas no smaller than 25G (ideally 22G* or 23G) and careful choice of site of access, depending on the ease provided by the anatomical characteristics of the patient:
● An approach site superior to the level of the rim of the frontal bone or
● A site inferior to the height of the zygomatic arch
In both cases, the initial insertion of
the cannula should be at 90o and the cannula should slide so that it freely penetrates the space between the temporal fasciae at the moment of contact with the bone. It is essential to feel that the cannula may slide smoothly and freely to ensure correct positioning.
A third site of approach by other expert injectors is in the area of hair insertion; however, some experts maintain that the interfascial approach is easier with the insertion points previously described.
Dr Ingrid López-Gehrke
"Adequate superficial fat injection with a cannula allows for improvement of skin textures. It is recommended that a 27G (¾–1¼-inch) needle or 25G or 27G (40–50 mm) cannula is used with this technique."*
Dr Shang-Li Lin
*Any opinions, views or advice provided are those of the healthcare professionals.
provide any warranty
not accept any liability for the opinions, views or advice expressed by expert contributors to this book.
Sinclair does not
and does
Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas3
74 ELLANS É ® | AN EXPERT’S GUIDE
Subcutaneous plane
Supraperiosteal plane
Injection technique
Linear threading, fanning, crosshatching in a retrograde manner
Bolus deposition and linear threading
Amount suggested
0.05–0.1 ml per linear thread or line in the the fanning technique, or per bolus
0.1–0.2 ml per linear thread or line in the the fanning technique, or per bolus'
Purpose of technique
Treatment intention
● Subdermal volume increase
● Biostimulation of collagen formation
To create a scaffold within the fat compartments and subsequently to restore the volume
To increase the surface area of the craniofacial platform
Epidermis



Dermis
Superficial fat
Ellansé
Muscle/superficial musculoaponeurotic system
Deep fat
Bone
Subdermal injection
Supraperiosteal injection
Treatment intention
● Deep volume increase
Figure 5.3
Injection placement for specific treatment intention
INJECTION TECHNIQUES 75
Injection techniques
EXPERT TIP*
There are three global techniques that may be employed in the administration of Ellansé:
2 3
This type of technique is based on the supraperiosteal application of the product in small boluses (less than 0.2 ml) in areas where it is recognised that support and bone and/or ligament structure are lost over time. The technique is based on maintaining the integrity of the line of facial anatomical ligaments.
Ellansé should be applied using lowcalibre needles, at 45o or 90o application angles, with gentle injection and in a single movement. Aspiration to determine if an intravascular puncture was performed can be justified as long as the practitioner ensures that the aspiration movement does not relocate the needle to a more superficial position, increasing the risk of complications. The use of a needle facilitates the supraperiosteal approach; however, in risk areas such as the frontal region, the use of a cannula achieves a similar effect.
This technique is currently one of the most commonly used with facial biostimulation treatments. It involves the subdermal application of PCL in small fan-shaped vectors, using a cannula with a retrograde injection to achieve homogeneous distribution of the product in the desired areas and trying to potentiate the lifting effect of the tissues.
It is always recommended to check the injection plane because if the product is applied deeply, facial volumisation only will be achieved, and if it is superficially applied, the product will be noticeable. Subcutaneous fans can be performed, applying amounts no greater than 0.1 ml per vector to avoid unwanted volumising effects. It is an ideal technique for thin skin with tegumentary flaccidity as well as for the presence of lateral wrinkles of the face, mainly in the middle third of the face or for the perioral region (not in the mucosa).
Depending on the individual needs of the patient, the described techniques can be combined, and cannulas can be used when facial volume replacement is needed.
A common way to achieve a harmonious combination is by applying the technique of structural points, for example in the zygomatic region, repositioning volumes with the cannula in the anterior malar portion. Combining this approach with lateral vectors on the face will reposition the lateral tissues and achieve a more subtle and natural transition between the middle third of the face and the subzygomatic portion of the cheek. The combination of techniques is feasible as long as care is taken to minimise the risk of complications.
Dr Ingrid López-Gehrke
9 ELLANS É ® | AN EXPERT’S GUIDE
1
Structural points technique (needle) Technique in subdermal facial vectors (cannula) Combined technique *Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. 76

INJECTION TECHNIQUES 10 77
Injection types
A comprehensive understanding of injection techniques is essential to obtain safe and efficient results. The most commonly used techniques in aesthetics are linear threading, serial puncture and fanning. However, cross-hatching or depot techniques are also possible options2,6.
An overview of the different possible techniques that may be used to administer Ellansé is provided below, and illustrated in Figure 5.42
Fanning technique2
● This method involves making puncture injections (point by point), known as a bolus
● This technique should only be used at the supraperiosteal, and not the subcutaneous, level
● The volume injected per bolus should not be more than 0.1–0.2 ml to reduce the chance of nodule formation
Linear threading/ retrograde2
● This technique is widely used, and is very comfortable for the patient
● It consists of inserting the needle/cannula along the length of the area to be treated, then depositing the product regularly while removing the needle/cannula
● Using a single injection point, this method makes it possible to change the direction of the needle or cannula and precisely inject the product into the whole zone
● The technique involves inserting the needle or cannula and depositing the product while slowly but not fully withdrawing the needle/ cannula, and then repositioning it again until the entire zone is filled
Cross-hatching2
● This technique involves multiple threading injections, in a grid pattern, in vertical and horizontal directions
Bolus/microbolus2
78 ELLANS É ® | AN EXPERT’S GUIDE
Bolus/microbolus
Static needle
Fanning
Linear threading/retrograde
Cross-hatching
Arrows indicate direction of needle when injecting
Injection techniques/patterns
Direction of needle when injecting
Injections are retrograde and overlapping
Figure 5.4
INJECTION TECHNIQUES 79
Levels of difficulty
Ellansé should only be used by healthcare professionals trained in the field of soft-tissue augmentation, and with expertise in the correction of volume defects after fully familiarising themselves with the product and its complete instruction leaflet7
The techniques described in this chapter are presented by difficulty level according to experience with Ellansé and biostimulators, beginning with the procedures that require the
least experience to perform (one-star procedures) to procedures that should only be performed by practitioners with considerable experience and expertise (three-star procedures).
Use of Ellansé should be reserved for practitioners with experience with filler injections, and all practitioners should begin with the areas marked with one star in this guide. Inexperienced practitioners should ensure that they are proficient with non-biostimulatory
products and have become very familiar with Ellansé and highly skillful in the injection technique in the one-star areas before progressing to the next level in order to avoid complications such as nodules, irregularities and vascular/nerve injuries.
It is imperative that all recommendations are followed to avoid complications due to technical errors with any type of filler.
Ellansé should only be used by trained practitioners in the field of soft tissue augmentation.
80 ELLANS É ® | AN EXPERT’S GUIDE

Actual patient, results may vary. 81
Treatment sequence
Work on the upper third, then the middle and then the lower third of the face. It is increasingly shown that an effect on one area can improve the adjacent lower region.
The above approach, which does not apply in all cases, is a general recommendation to help to structure and develop a treatment protocol for a patient with cosmetic needs.8,9
In general terms, the above sequence tends to be adopted when undertaking facial cosmetic procedures using injectable treatments, although this will depend on the areas that require treatment (Figure 5.5).
First inject the lateral portions of the face (bearing in mind the natural anatomical line formed by the facial ligaments). This will achieve the lifting of the tissues and will avoid the application of excessive materials.
Increasing volume in the deep midfacial fat compartments does not cause inferior displacement of the injected material. This underscores the role of deep soft-tissue filler injections (i.e. in contact with the bone) in providing support for overlying structures and resulting in anterior projection8,9
Please see Chapter 2 for further details on this subject.
The structural bases in facial treatments are the foundation for the work that is being carried out, meaning the supraperiosteal or deep fat pad regions are treated first.
A sequential treatment plan is recommended to decide the areas of the face that need to be worked on, in order to achieve effective results and with fewer risks.
From top to bottom 1
From the outside to the inside 2
82 ELLANS É ® | AN EXPERT’S GUIDE
From the deep to the superficial 3

1 2 2 3
Figure 5.5
INJECTION TECHNIQUES 83
Sequence of treatment areas
Indirect effects of area-specific treatments
When planning treatment, areas with more bone and ligament support are typically treated first, as adding volume to these compartments will create a lifting effect and reduce the need for larger volumes in the more superficial and medial compartments.
In order to reduce the need for greater injection volumes, a knowledge of anatomy may be used to improve cosmetic results by balancing volume restoration with projection and a lifting effect.
Well-planned treatments mean that the benefits will extend to adjacent areas, providing an improved and concordant cosmetic result, with significantly less risk10,11,12
The rest of this chapter describes the optimal injection techniques for the following facial areas:
● Zygomatic area
● Malar/cheek area
● Nasolabial folds
● Preauricular area
● Mandibular area
● Chin (mental) area
● Eyebrow area
● Marionette lines
● Temporal area
● Frontal area
● Nose area
84 ELLANS É ® | AN EXPERT’S GUIDE

Actual patient, results may vary. 85
Injection technique for the zygomatic area
Difficulty level6
Treatment intention: To provide a uniform contour as part of rejuvenation of the midface1
Injection depth: Level 4/supraperiosteal6
Needle/cannula: 22G* or 25G cannula6
Injection pattern: Linear retrograde injection6
Injection volume: 0.5–1.0 ml (per side)6
Concerns/considerations: Particular attention should be paid to the frontal branch of the facial nerve1
The cannula allows for better distribution of the filler than a needle, while encouraging a better distension of the zygomatic retaining ligament6.
EXPERT TIP* *
Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas3
86 ELLANS É ® | AN EXPERT’S GUIDE
InferiorSuperiorzygomaticarchlimit zygomaticarchlimit
Upperlimit15–20°
angletoparallelthejawline
Jawline

Upperlimit
Entry point
Delimitation of the area to treat Cannula
Limits and landmarks
Figure 5.6
Injection technique for the zygomatic area
INJECTION TECHNIQUES 87
Injection technique for the malar/cheek area
Difficulty level6
Treatment intention:
To restore volume and rejuvenate an area associated with atrophy of the deep fat compartment and bone loss1,2
Needle/cannula:
27G needle or 25G cannula6
Injection pattern:
Microbolus (needle)6
Linear retrograde injection (cannula)6
EXPERT TIP*
It is quite common to treat the zygomatic area and mid-malar area in the same treatment plan to provide a more uniform contour, as long as attention is paid to the frontal branch of the facial nerve1.
Sometimes, the upper third of the nasolabial fold (NLF) may also be included in the same treatment plan.
Injection depth:
Level 4/supraperiosteal (needle)6
Level 4/supraperiosteal and level 2/ subcutaneous (cannula)6
Injection volume: 0.5 ml (per side) (needle)6 0.5–1.0 ml (per side) (cannula)6
Concerns/considerations:
● Some experts prefer to use a cannula for this procedure because it allows better distribution of the filler and can reach posteriorly to the extended ligament6
● When using a cannula above the infraorbital foramen, Ellansé should be placed at the supraperiosteal level (level 4) to compensate for bone resorption and suborbicularis oculi fat volume loss. Below the infraorbital foramen, Ellansé is better placed at the subcutaneous level (level 2), as the supraperiosteal plane is difficult to reach and would require a large amount of product to obtain the desired volumetric enhancement6
● When using a needle, placement should be at the supraperiosteal level (level 4), and always above the infraorbital foramen
● Placement should always be below the orbital rim (upper limit), whether using cannula or needle
*Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. 88 ELLANS É ® | AN EXPERT’S GUIDE
Needle Cannula
Entry point
Delimitation of the area to treat Cannula


Bolus
Limits and landmarks
Infraorbital
Infraorbital
Nasolabialfatpad
Malar/
Malar/
foramen
foramen Nasolabialfatpad
Orbital rim Orbital rim
zygomatic projection
zygomatic projection Level 4 Level 4 Level 2
Figure 5.7
INJECTION TECHNIQUES 89
Injection technique for the malar/cheek area
Injection technique for the nasolabial folds
Difficulty level6
Treatment intention:
To add volume to reduce depth of sulcus in NLFs and provide a smoother transition from the midface to the upper lip1,2
Needle/cannula:
27G needle or 22G* to 25G cannula6
Injection depth:
Level 4/supraperiosteal and level 2/ subcutaneous (needle)6
Level 4/supraperiosteal (cannula)6
Injection volume: 0.5–1.0 ml (per side) (needle)6 0.5 ml (per side) (cannula)6
Injection pattern: Linear retrograde injection/microboluses (needle and cannula)6
Concerns/considerations:
A combined needle and cannula approach is recommended6
Injections should be supraperiosteal in the upper third (pyriform fossae) and subcutaneous in the lower two-thirds1.
The upper third of the NLF should be injected with a needle to the supraperiosteal level and a cannula should be used for the bottom twothirds in layer 2 (subcutaneous level)1,6.
EXPERT
TIP*
*Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas3 90 ELLANS É ® | AN EXPERT’S GUIDE
Upper third –Level 4
Lower twothirds – Level 2
Needle
Upper third –Level 4
Lower twothirds – Level 2
Cannula
Nasolabialfatpad
Nasolabialfatpad
Entry point
Delimitation of the area to treat Cannula


Needle
Bolus
Limits and landmarks
Figure 5.8
Injection technique for the nasolabial folds
INJECTION TECHNIQUES 91
Injection technique for the preauricular area
Difficulty level6
Treatment intention:
● Facial rejuvenation1,2
● Treatments to this area tend to add a lifting effect due to the very dense superficial reticulatum (see Chapter 2). As layers 1, 2, 3 and 4 are quite adherent to each other, a small volume will distend and 'lift' the area
Needle/cannula:
● 27G needle or 25G cannula6
Injection pattern:
● Linear retrograde injection (needle or cannula)6
Injection depth:
● Level 2/subcutaneous (needle and cannula)6
Injection volume:
● 0.3–0.5 ml (0.1 ml/3 vectors) (needle)6
● 0.3–0.5 ml (cannula)6
Concerns/considerations:
● Experts recommended injecting in the subcutaneous level when using either a needle or cannula6
It can be easier to inject some patients with a cannula if the patient has lax tissues6.
EXPERT TIP* *Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. 92 ELLANS É ® | AN EXPERT’S GUIDE
However, in the case of tight tissues, a needle may be easier6.
Entry point
Delimitation of the area to treat Cannula


Needle
Bolus
Limits and landmarks
Jowl
Jowl Chin Chin Inferiorzygomaticarchlimit Inferiorzygomaticarchlimit
Cannula Needle
Figure 5.9
INJECTION TECHNIQUES 93
Injection technique for the preauricular area
Injection technique for the mandibular area
Difficulty level6
Treatment intention:
● To improve jawline definition, mandible angle projection and to correct pre-jowl volume loss. Commonly requested as a beautification treatment, in combination with the chin1
Needle/cannula:
● 27G needle or 22G* to 25G cannula6
Injection pattern:
● Bolus (needle)6
● Linear retrograde injection (cannula)6
Injection depth:
● Level 2/subcutaneous (needle and cannula)6
Injection volume:
● 0.5–1.0 ml per side (needle and cannula)6
Concerns/considerations:
● The posterior part of the mandible should be injected at the subcutaneous level, and not supraperiosteal, which is difficult to access6
A combined approach using a needle (supraperiosteal) and cannula (linear retrograde injection) is recommended.
*Any
opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas3
TIP* 94 ELLANS É ® | AN EXPERT’S GUIDE
The needle should be used to attain the angle of the mandible and the pre-jowl area, while the cannula should be used for the subcutaneous level in the same areas6.
EXPERT

Mandibular angle Entry point Delimitation of the area to treat Cannula Bolus Limits and landmarks
5.10 Injection technique for the mandibular area 0.1 0.1 0.1 INJECTION TECHNIQUES 95
Jowl Chin Mandibularinferiorlimit
Needle and cannula combination Figure
Injection technique for the chin (mental area)
Difficulty level6
Treatment intention:
● To increase the volume, projection and length of the mental region (chin)
● Typically requested for beautification and to improve the overall shape of the face, or to balance signs of ageing
● It is common to treat the chin and jawline at the same stage
Needle/cannula:
● 27G needle or 25G cannula6
Injection pattern:
● Linear retrograde injection (needle and cannula)6
Injection depth:
● Level 4/supraperiosteal and level 2/ subcutaneous (needle and cannula)6
Injection volume:
● 0.8–1.5 ml (needle and cannula)6
Concerns/considerations:
● Injections directly into the mental foramen should be avoided as pressure can affect the mental nerve6
● It is worth considering extending the treatment to the pre-jowl area, along the same anatomical plane, as increasing the chin length and/or projection can create a more noticeable deformity in these areas1
Some experts prefer to use a needle over a cannula because it is easier to navigate under the mentalis in the supraperiosteal level6.
A bolus should be used when treating the chin with a needle6.
EXPERT TIP* *Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. 96 ELLANS É ® | AN EXPERT’S GUIDE
Each bolus should be limited to a maximum of 0.1–0.2 ml in all areas, to avoid the risk of nodules6.



0.1 Level 2 Level 4 0.1 0.1
point Delimitation of the area to treat Cannula Needle Bolus
landmarks
Needle Cannula Entry
Limits and
Mandible lower limit INJECTION TECHNIQUES 97
Figure
5.11 Injection technique for the chin (mental area)
Injection technique for the eyebrow area
Difficulty level6
Treatment intention:
● To give a subtle improvement in shape, position and elevation1,2
Needle/cannula:
● 27G needle or 25G cannula6
Injection pattern:
● Bolus (needle) or fanning (cannula)6
Injection depth:
● Level 4/supraperiosteal (needle and cannula)6
Injection volume:
● 0.1–0.2 ml per side6
Concerns/considerations:
● Owing to the presence of important vascular and nerve structures emerging from the supraorbital and supratrochlear foramen (terminal branches of the supraorbital artery), this area should be approached with caution and only by experienced physicians2
● Due to the zone of vascular risk, some experts recommend that a cannula should only be used in the eyebrow area6
*Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book.
The injection must be made above the orbital ligament6.
EXPERT TIP* 98 ELLANS É ® | AN EXPERT’S GUIDE
Injections that are too superficial are likely to result in a ‘lumpy’ appearance, and should therefore be placed in a supraperiosteal plane to avoid this risk1,2.

Orbitalrim 0.1 0.05 Delimitation of the area to treat Needle Bolus Limits and landmarks
INJECTION TECHNIQUES 99
Figure 5.12 Injection technique for the
eyebrow
area
Injection technique for marionette lines
Difficulty level7
Treatment intention:
● To reverse the depressed appearance of the oral commissures, highlighted by the formation of a fold (melomental fold or marionette lines), caused by the downward sliding of a small fat compartment, the anterior border of which follows the anterior border of the depressor anguli oris1,2
Needle/cannula:
● 27G needle or 25G cannula6
Injection pattern:
● Linear retrograde injection (needle and cannula)6
Injection depth:
● Level 2/subcutaneous (needle or cannula)6
Injection volume:
● 0.3–0.5 ml (0.1 ml/3 vectors) (needle)6
● 0.3–0.5 ml (cannula)6
Concerns/considerations:
● This is a higher risk area to treat due to the lack of structural support and the dynamic role played by the major muscles in the perioral area1
*Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book.
Points of entry can be the same for the cannula and needle because they are both being injected at the subcutaneous level6.
EXPERT TIP* 100 ELLANS É ® | AN EXPERT’S GUIDE
It is common to combine the pre-jowl areas and marionette lines areas in the same treatment to obtain a better result.
Needle Cannula
Entry point
Delimitation of the area to treat Cannula


Needle
Bolus
Figure 5.13
INJECTION TECHNIQUES 101
Injection technique for marionette lines
Injection technique for the temporal area
Difficulty level6
Treatment intention:
● To restore lost volume to the temporal area to enhance the overall appearance of the face1,2
Needle/cannula:
● 25–27G needle or 22G* cannula6
Injection pattern:
● Linear retrograde injection deep to superficial temporal fascia (cannula)6
Injection depth:
● Level 4/deep to the superficial temporal fascia6
Injection volume:
● 0.5–1.0 ml (per side)6
Concerns/considerations:
● Needle injection in the lower temple is not recommended due to the zone of vascular risk6
● The deep temporal fascia cannot be penetrated with a 22G* cannula
● When using a 25G cannula, care must be taken to ensure the correct plane is located, as it might move to the subcutaneous level with a risk of contour irregularities and increased risk of vascular issues (superficial temporal artery or veins)6
EXPERT
*Any
opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas3
When injecting, either try to stay superficially, or inject into the deep temporal fascia. It is difficult to stay in the same layer when injecting superficially with a needle6.
102 ELLANS É ® | AN EXPERT’S GUIDE
TIP*
Entry point
Delimitation of the area to treat Cannula
Limits and landmarks

0.5–1.0cmabovethesuperiorzygomaticarchlimit Orbitalrim
Temporal crest
Figure 5.14
INJECTION TECHNIQUES 103
Injection technique for the temporal area
Injection technique for the frontal area
Difficulty level6
Treatment intention:
● To improve anterior projection and convexity and contours of the frontal area (forehead)1,2
● Treatment in this area is typically used for beautification and to balance face proportions, and not to reverse the signs of ageing1,2
Needle/cannula:
● 22G* to 25G cannula6
Injection pattern:
● Linear retrograde injection6
● Fanning6
An extended period of oedema (1 week) may be expected. This is related to the procedure being carried out at the supraperiosteal level and not to Ellansé itself.
Injection depth:
● Level 4/supraperiosteal6
Injection volume:
● 1.0–3.0 ml6
Concerns/considerations:
● Owing to the presence of important vascular and nerve structures emerging from the supraorbital and supratrochlear foramen (terminal branches of the supraorbital artery), this area should be approached with caution and only by experienced physicians1
● Ellansé should be deposited above the orbital rim (1 cm)
To avoid any irregularities, the area should be massaged immediately after injection to ensure an even distribution of the product1,2
EXPERT TIP*
*Any
104 ELLANS É ® | AN EXPERT’S GUIDE
opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas3
Entry point
Delimitation of the area to treat Cannula
Limits and landmarks
 Temporal crest Orbital rim Orbital rim
Nasion
Temporal crest
Figure 5.15
Temporal crest Orbital rim Orbital rim
Nasion
Temporal crest
Figure 5.15
INJECTION TECHNIQUES 105
Injection technique for the frontal area
Injection technique for the nose area
Difficulty level6
Treatment intention:
● To improve the volume of the dorsum, to smooth contour irregularities (dorsal hump, for example), for mild lifting of the tip and to correct post-surgical minor contour irregularities1,2
Needle/cannula:
● 27G needle or 25G cannula6
Injection pattern:
● Linear retrograde injection (needle and cannula)6
Injection depth:
● Level 4/supraperiosteal (needle and cannula)6
Injection volume:
● 0.3–0.8 ml (needle and cannula)6
Concerns/considerations:
● The use of soft-tissue fillers for nose reshaping after rhinoplasty due to anatomical changes resulting from surgery and scartissue formation is more prone to additional complications2
● This treatment should only be performed by experienced physicians1,2
Scan the QR code to explore the interactive and augmented reality content for injection techniques and test your knowledge, or use the following URL to explore the content: www.ellanseexpertguide.com/5.

*Any
for the
advice
by expert contributors to this book.
opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability
opinions, views or
expressed
EXPERT
106 ELLANS É ® | AN EXPERT’S GUIDE
Experts prefer to use a cannula because it can better distribute the filler and can reach posteriorly to the extended ligament6.
TIP*
Needle step 1
Needle step 2
Cannula step 1
Cannula step 2
Entry point
Delimitation of the area to treat




Cannula
Needle
Bolus
Limits and landmarks
Nasion Interpupillary line Nasion 0.1 Dorsal hump 0.1
INJECTION TECHNIQUES 107
Figure 5.16 Injection technique for the nose area
Summary
Ellansé can be used to achieve specific outcomes across a number of different regions of the face. Appropriate techniques vary from region to region, and it is essential that the correct procedures are followed in order to achieve the desired outcomes.



A certain level of competence and experience is required before attempting to use Ellansé in the circumstances described, and a practitioner should ensure that they are familiar and comfortable with the less technically demanding areas (marked with one or two stars) before progressing to more challenging (three stars or more) areas. Not only will this approach help to obtain consistently better results, but it will also reduce the chances of complications and unwanted cosmetic results.
If the recommendations provided in this chapter are followed carefully by a capable and experienced practitioner, the patient should expect to see highly positive outcomes from their Ellansé treatment (Figure 5.17).
The following chapter provides case studies describing real-world examples of patient transformation following appropriate use of Ellansé.
Figure 5.17
may vary.
Examples of treatment outcome following Ellansé administrations (Caucasian case) Total volume of Ellansé injected: 5.0 ml
Actual patient, results
Month 12 Month 24 108 ELLANS É ® | AN EXPERT’S GUIDE
References
1. de Melo F, Nicolau P, Piovano L, Lin SL, Baptista-Fernandes T, King MI, Camporese A, Hong K, Khattar MM, Christen MO. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. 2017;10:431–40.
2. Sinclair. Ellansé injection guide.
3. Sinclair. Data on file. Ellansé extrusion force test report. 2019.
4. Christen MO, Vercesi F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31–48.
5. Rauso R. 5-year study of a polyacrylamide hydrogel-based filler for rehabilitation of HIV-related facial lipoatrophy. Aesthet Surg J. 2015;35:1021–9.
6. Sinclair Ellansé best practices and guidelines advisory board. IMCAS. Paris 2020.
7. Sinclair. Ellansé instructions for use.
8. Cotofana S, Gotkin RH, Frank K, Koban KC, Targosinski S, Sykes JM, Schlager M, Schlattau A, Schenck TL. The functional anatomy of the deep facial fat compartments: A detailed imaging-based investigation. Plast Reconstr Surg. 2019;143:53–63.
9. Casabona G, Bernardini FP, Skippen B, Rosamilia G, Hamade H, Frank K, Freytag DL, Sykes J, Onishi EC, Cotofana S. How to best utilize the line of ligaments and the surface volume coefficient in facial soft tissue filler injections. J Cosmet Dermatol. 2019;19:303–11.
10. Haddock NT, Saadeh PB, Boutros S, Thorne CH. The tear trough and lid/cheek junction: Anatomy and implications for surgical correction. Plast Reconstr Surg. 2009;123: 1332–40.
11. Rohrich RJ, Pessa JE, Ristow B. The youthful cheek and the deep medial fat compartment. Plast Reconstr Surg. 2008;121:2107–12.
12. de Melo F, Carrijo A, Hong K, Trumbic B, Vercesi F, Waldorf HA, Zenker S. Minimally invasive aesthetic treatment of the face and neck using combinations of a PCL-based collagen stimulator, PLLA/PLGA suspension sutures, and cross-linked hyaluronic acid. Clin Cosmet Investig Dermatol. 2020;13:333–44.
INJECTION TECHNIQUES 109
Case Studies
06 111
Aims of treatment
The three main aims of soft-tissue augmentation procedures are typically1:
Rejuvenation
Prevention Beautification
VOLUME RESTORATION
Ellansé achieves immediate volume restoration due to the optimal rheology of the carboxymethyl cellulose (CMC) gel2
In terms of the clinical and aesthetic effect, the elasticity of the gel in Ellansé is greater than that of volumising hyaluronic acid (HA)3,4 This provides an immediate projection and volumisation of the injected area and allows the microspheres to be maintained in the area of injection until the formation of the collagen scaffold, leading to low risks of migration5 In addition, it allows for a longer term volumising effect than for HAs6
Rejuvenation is typically achieved through a combination of volume restoration and biostimulation; prevention is typically achieved through biostimulation; and beautification is typically achieved through contouring1
This chapter provides details for each of these treatment objectives, using a case study in each instance to illustrate the techniques required.
BIOSTIMULATION
Over the course of a few months, a foreign body response to the implanted microspheres stimulates the body's natural type I collagen production7,8
The evidence for collagen stimulation by Ellansé is derived from both animal and human models. Use of Ellansé in a rabbit model resulted in neocollagenesis of primarily type I collagen from 9 months, and the effect was maintained until at least 21 months (see Chapter 1)7
Collagen production with Ellansé was also demonstrated in a human model, with histological analysis at 13 months after intra-dermal injection with Ellansé revealing collagen formation around the polycaprolactone (PCL) particles (see Chapter 1)8
CONTOURING
Ellansé dermal filler has two distinct phases of activity: an immediate effect and a longer, sustained effect2:
Immediately after injection, the CMC gel component provides temporary volume according to the filling capacity of the injected volume and the highly hygroscopic properties of CMC. The CMC gel is resorbed within 2–3 months2
This immediate effect is followed by gradual neocollagenesis with a more persistent type I collagen structure gradually increasing over 1–3 months, and the PCL microspheres becoming embedded in the type I collagen scaffold2
These attributes allow Ellansé to restore volume, redefine contours, reduce wrinkles and improve skin quality2
112 ELLANS É ® | AN EXPERT'S GUIDE
Case studies
The remainder of this chapter is devoted to the presentation of six case studies that demonstrate the possible outcomes from the use of Ellansé* in real-world clinical examples.

device is intended to be used by trained healthcare professionals for injection into the face to fill skin depressions, wrinkles and fine lines, and also for augmentation of tissue volume. The product is intended to be used in adult patients (aged 18 years or older) who are not pregnant or breastfeeding and are deemed appropriate for treatment by the healthcare professional.
*The
CASE STUDIES 113
Case study
1 (Courtesy of Dr Amanda Ong)
Dr Amanda Ong

"The patient was a 60-year-old Caucasian female who worked as a dog groomer, had a healthy lifestyle and no other medical conditions. She had previously used botulinum toxin and chemical peels."

PROCEDURE
The patient was treated with supraperiosteal and subdermal applications of Ellansé to the temples, cheeks, nasolabial folds (NLFs), prejowl sulcus, marionette lines and preauricular areas using a needle and cannula.
A bolus supraperiosteal and lines subdermal pattern of injection was adopted.
The patient was seated at an angle of approximately 60° during the procedure. Moulding was applied immediately after treatment only.
Ellansé was mixed with lidocaine (0.2 ml per 1 ml Ellansé).
Cheeks
1.2 ml/1 ml supraperiosteal
NLFs
0.15 ml/0.15 ml supraperiosteal
Pre-jowl
0.3 ml/0.3 ml supraperiosteal
Marionette lines
0.3 ml/0.3 ml subdermal
Total volume injected: 7.2 ml (6 ml of Ellansé with 1.2 ml of lidocaine)
FOLLOW UP
Preauricular 0.9 ml/0.6 ml subdermal
Temple
0.5 ml supraperiosteal, 0.5 ml layer 4
The patient was followed up 4 months after the procedure. Using the Global Aesthetic Improvement Scale (GAIS), the physician graded the outcome as ‘much improved’ and the patient graded the outcome as an ‘excellent corrective result’ (see Figure 6.1).
The patient will be followed in the future with 6- to 12-monthly reviews and possible touch-up retreatment.
114 ELLANS É ® | AN EXPERT'S GUIDE





Before After
Before
4 months after
[Images courtesy of Dr Amanda Ong] Actual patient, results may vary. Any opinions, views or advice provided are those of the physician. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed. CASE STUDIES 115
Figure
6.1 Case study 1:
Ellansé treatment (top) and
Ellansé treatment (bottom)
Case study 2
(Courtesy of Dr Shang-Li Lin)
Dr Shang-Li Lin

"The patient was a 42-year-old female, presenting with forehead flattening, concavity above the supraorbital ridge, drooping of the lateral third of the brows, infraorbital hollowing, medial cheek flattening, downshifts of midface fat pads and drooping of the corner of the mouth."

PROCEDURE
The patient was treated as follows:
Pre-zygomatic space (0.4 ml x2)
Pre-maxillary space (0.4 ml x2
● Drooping of corner of the mouth (0.1 ml x2)
● Perioral fat (0.15 ml x2)
Canine fossa (0.3 ml x2)
Total volume injected: 6.0 ml of Ellansé
Forehead (0.9 ml)
Drooping of lateral third of the brows (0.3 ml)
● Deep medial cheek fat (0.2 ml x2), retro-orbicularis oculi fat (0.2 ml x2)
● Zygomatic eminence (0.15 ml x2), zygomatic arch (0.3 ml x2 + 0.2 ml x2)
FOLLOW UP
The patient was followed up for 24 months after the procedure (Figure 6.3).
116 ELLANS É ® | AN EXPERT'S GUIDE






 Figure 6.3
Figure 6.3
[Images
Actual patient, results may vary.
Case study 2: 0–24
months after Ellansé treatment
courtesy of Dr Shang-Li Lin]
Figure 6.2
[Images
Actual patient,
Month 0 Month 2 Month 12 Month 6 Month 24 Any opinions, views or advice provided are those of the physician. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed. CASE STUDIES 117
Case study 2: Before Ellansé treatment
courtesy of Dr Shang-Li Lin]
results may vary.
Case
study
3 (Courtesy of Dr Shang-Li Lin)
Dr Shang-Li Lin

"The patient was a 42-year-old Caucasian female who worked as an English teacher."
PROCEDURE
The patient was treated with supraperiosteal and deep subcutaneous applications of Ellansé to the temples, cheeks and chin using a needle and cannula. A bolus and fanning pattern of injection was used as per the below voumes.
Forehead supraperiosteal fanning (2.2 ml)
Temples supraperiosteal bolus (1.0 ml left, 1.2 ml right)
Infraorbital supraperiosteal bolus (0.4 ml each side)†
Canine fossa supraperiosteal bolus (0.4 ml each side)
The patient was seated throughout the procedure, and moulding was applied immediately after injection.
Total volume injected: 10 ml (8.3 ml of Ellansé with 1.7 ml of lidocaine)
Cheeks supraperiosteal bolus (1.1 ml each side)
NLFs subcutaneous fanning (0.4 ml each side)
Mandibular angle subcutaneous fanning (0.5 ml each side)
FOLLOW UP
The patient was followed up 6 and 24 months after the procedure (Figure 6.5).
†Ellansé is Intended to be used by trained practitioners in the field of soft-tissue augmentation. Opinions and methods presented here are the physician’s personal experience. Refer to the Ellansé instructions for use for details of specific indications.
 Ellansé was mixed with lidocaine (0.2 ml per syringe).
Ellansé was mixed with lidocaine (0.2 ml per syringe).
118 ELLANS É ® | AN EXPERT'S GUIDE





 Figure 6.4
Case study 3: Before Ellansé treatment [Images courtesy of Dr Shang-Li Lin]
Figure 6.4
Case study 3: Before Ellansé treatment [Images courtesy of Dr Shang-Li Lin]
Actual
patient, results may vary.
Figure 6.5
6
24 Any opinions, views or advice provided are those of the physician. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed. CASE STUDIES 119
Case study 3: 6 and 24 months after Ellansé treatment
[Images
courtesy of Dr Shang-Li Lin]
Actual
patient, results may vary.
Month
Month
Case study 4
(Courtesy of Dr Shang-Li Lin)
Dr Shang-Li Lin
"The patient was a 38-year-old East Asian male who worked frequent night shifts in his role as a designer."

PROCEDURE
The patient was treated with supraperiosteal and deep subcutaneous applications of Ellansé to the temples and cheeks using a needle and cannula. A bolus and fanning pattern of injection was used, as per the below voumes.
Ellansé was mixed with lidocaine (0.2 ml per syringe).
The patient was treated as follows:
Temple supraperiosteal bolus (0.2 ml each side)
Infraorbital supraperiosteal bolus (0.8 ml each side)†
Canine fossa supraperiosteal bolus (0.4 ml each side)†
The patient was seated throughout the procedure, and moulding was applied immediately after the procedure.
Total volume injected: 6 ml (5 ml of Ellansé with 1 ml of lidocaine)
Cheeks supraperiosteal bolus (0.8 ml each side)
Eyebrow supraperiosteal bolus (0.4 ml each side)
Mandibular angle subcutaneous fanning (0.4 ml each side)
FOLLOW UP
The patient was followed up 18 months after the procedure (Figure 6.6).
†Ellansé is Intended to be used by trained practitioners in the field of soft-tissue augmentation. Opinions and methods presented here are the physician’s personal experience. Refer to the Ellansé instructions for use for details of specific indications.

120 ELLANS É ® | AN EXPERT'S GUIDE





 Figure 6.6
Case study 4: Before Ellansé treatment (top) and 18 months after Ellansé treatment (below)
Figure 6.6
Case study 4: Before Ellansé treatment (top) and 18 months after Ellansé treatment (below)
Actual patient, results may vary. Before After Any opinions, views or advice provided are those of the physician. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed. CASE STUDIES 121
[Images courtesy of Dr Shang-Li Lin]
Case study 5
(Courtesy of Dr Ingrid López-Gehrke)
Dr Ingrid López-Gehrke

"The patient was a 32-year-old Caucasian female who worked as a doctor. She had previously received HA treatment."
PROCEDURE
The patient was treated with supraperiosteal applications of Ellansé to the frontal region, malar and zygomatic structural points, piriformis fossa and mandibular structure points using a needle and cannula (to the frontal region). A structural points pattern of injection was adopted.
Ellansé was mixed with lidocaine (0.2% simple lidocaine mixture).
The patient was treated as follows:
Temporal (0.2 ml each side)
Malar (0.2 ml each side)
Pre-jowl (0.2 ml each side)
Mandibular (0.2 ml each side)
The patient was seated at an angle of approximately 45o during the procedure. No patient massage was applied.
Total volume injected: 2.4 ml (Ellansé with 0.2% lidocaine)
FOLLOW UP
Zygomatic (0.2 ml each side)
Piriformis fossa (0.2 ml each side)
The patient was followed up 3 months after the procedure. The physician graded the outcome as Glogau type 1, and the patient scored the outcome as 95 on the FACE-Q scale (scores are from 0 to 100 where a higher score indicates a better outcome) (Figure 6.7).

122 ELLANS É ® | AN EXPERT'S GUIDE

 Figure 6.7
Case study 5: Before Ellansé treatment (left) and at 3 months after Ellansé treatment (right)
Figure 6.7
Case study 5: Before Ellansé treatment (left) and at 3 months after Ellansé treatment (right)
Before After Any opinions, views or advice provided are those of the physician. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed. CASE STUDIES 123
[Images courtesy of Dr Ingrid López-Gehrke] Actual patient, results may vary.
Case study 6 (Courtesy of
Dr Shang-Li Lin)
Dr Shang-Li Lin

"The patient was a 38-year-old East Asian female. She presented with dark circles and a tired appearance due to infraorbital hollowing, saggy and heavy lower cheeks due to medial cheek flattening and downshifting, and drooping of the lateral third of the brows, as well as temporal hollowing, concavities of the forehead and a flat nasal bridge."

PROCEDURE
The patient was treated with supraperiosteal and deep subcutaneous applications of Ellansé to the areas indicated below using a needle and cannula. A bolus and fanning pattern of injection was used, with a total volume of 8 ml.
Ellansé was mixed with lidocaine (0.2 ml per syringe).
The patient was treated as follows:
Infraorbital supraperiosteal bolus (1.2 ml total)†
Forehead supraperiosteal bolus (1.2 ml total)
Eyebrow supraperiosteal bolus (0.8 ml total) Temple supraperiosteal bolus and subcutaneous fanning (2 ml total)
Cheeks supraperiosteal bolus and subcutaneous fanning (1.2 ml total)
Canine fossa
supraperiosteal bolus (0.8 ml total)
The patient was seated throughout the procedure, and moulding was applied immediately after the procedure.
Nose supraperiosteal bolus (0.8 ml total)
Total volume injected: 8 ml (6.7 ml of Ellansé with 1.3 ml of lidocaine)
FOLLOW UP
The patient was followed up 4 months after the procedure; with a period of 1 month between each 2 ml administration (Figure 6.8).
No adverse events requiring medical intervention were reported.
†Ellansé is Intended to be used by trained practitioners in the field of soft-tissue augmentation. Opinions and methods presented here are the physician’s personal experience. Refer to the Ellansé Instructions for Use for details of specific indications.
124 ELLANS É ® | AN EXPERT'S GUIDE
Actual patient, results may vary.
Before After



 Figure 6.8
Case study 6: Before Ellansé treatment (top) and 4 months after Ellansé treatment (bottom) with 1 month between each 2 ml administration, giving a total injection volume of 8 ml
[Images courtesy of Dr Shang-Li Lin]
Figure 6.8
Case study 6: Before Ellansé treatment (top) and 4 months after Ellansé treatment (bottom) with 1 month between each 2 ml administration, giving a total injection volume of 8 ml
[Images courtesy of Dr Shang-Li Lin]
Any opinions, views or advice provided are those of the physician. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed. CASE STUDIES 125
Ellansé can effectively be used for rejuvenation, prevention and beautification by exploiting its ability to achieve volume restoration, biostimulation and contouring1.
With experience and careful adherence to treatment guidelines and product information, highly satisfactory results can be achieved, as demonstrated by the case studies presented in this chapter.

126
Summary
References
1. de Melo F, Nicolau P, Piovano L, Lin SL, Baptista-Fernandes T, King MI, Camporese A, Hong K, Khattar MM, Christen MO. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. 2017;10:431–40.
2. Christen MO, Vercesi F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31–48.
3. Sinclair. Ellansé trainer slide presentation.
4. Sinclair. Data on file.
5. Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg. 2004;113:1380–90.
6. Galadari H, van Abel D, Al Nuami K, Al Faresi F, Galadari I. A randomized, prospective, blinded, split-face, single-center study comparing polycaprolactone to hyaluronic acid for treatment of nasolabial folds. J Cosmet Dermatol. 2015;14:27–32.
7. Nicolau PJ, Marijnissen-Hofsté J. Neocollagenesis after injection of a polycaprolactone based dermal filler in a rabbit. Eur J Aesthetic Med Dermatol. 2013;3:19–26.
8. Kim JA, Van Abel D. Neocollagenesis in human tissue injected with a polycaprolactonebased dermal filler. J Cosmet Laser Ther. 2015;17:99–101.
CASE STUDIES 127
Combined Treatments 07
129
These guidelines provide only general support, not an individualised treatment plan. The optimal treatment plan always results from a physician’s individual knowledge (anatomy, ageing physiology, product characteristics), training (injection or insertion techniques), general clinical competence, responsibility and wisdom, combined with the patient’s values and preferences.
The information contained in this chapter is guided by input from medical professionals, based on their medical practice. Sinclair offers these guidelines as expert guidance, rather than advice from its team*.
Use of Sinclair’s products in combination with any other product or accessory that is not listed within the Instructions for Use shall be undertaken entirely at the medical professional’s risk.
*Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk. Model is not an actual patient.

The information in this chapter is intended to provide an overview of the different approaches that may be adopted when treatments are combined to achieve optimal results.
Introduction
130
Use of combination treatment
USING COMBINED TREATMENTS: THE NEW STANDARD OF CARE
Minimally invasive procedures (MIPs) are increasingly used in combination protocols to improve outcomes in aesthetic procedures1. Indeed, MIPs now represent ~90% of aesthetic interventions2. Furthermore, in 2020, 60% of all patients undergoing aesthetic procedures in the United States who requested MIPs received multiple procedures3
The modern concept of natural and harmonious rejuvenation is based on a comprehensive, three-dimensional, multi-layered approach, combining multiple agents and techniques to attain multiple goals, such as relaxation, volumisation, volume repositioning, reshaping, resurfacing or tightening, depending on specific patient needs (Figure 7.1).
 Figure 7.1
Figure 7.1
COMBINED TREATMENTS 131
Combined treatments in patients undergoing aesthetic procedures
BENEFITS OF COMBINING TREATMENTS
Facial ageing is a multifactorial process involving structural changes in all anatomical layers of the face including the bone, muscles, ligaments, adipose tissue and skin, as well as dynamic interactions among these tissues (Figure 7.2). The importance of each component in terms of age-related structural changes is different. Bone and the deep fat compartments are most important, followed by muscle and skin, while ligaments are considered to be the most stable structures. While the deep fat compartment is affected by age,
the superficial fat (hypodermis) of the adipose tissue is predominantly affected by bodyweight, rather than ageing.
Adopting a combined treatment approach to aesthetic interventions allows the different and individual aspects of the multifactorial process of facial ageing to be addressed, providing optimal outcomes1. A number of studies have demonstrated that, compared with single-agent or single technique approaches, the use of combination treatments offers an additive or
Bone
synergistic benefit. The outcomes of combination treatments tend to be more favourable, longer lasting and not associated with any increase in the severity or rate of any related adverse events4,5,6,7,8. A combined approach also improves patient satisfaction by reducing the time required to achieve improvements and limiting the number of office visits required8
These findings have informed clinical practice such that combined treatments are now considered the standard of care1
Skin
Muscles Ligaments
Figure 7.2
Multifactorial process of facial ageing
Adipose tissue
132 ELLANS É ® | AN EXPERT'S GUIDE
OVERVIEW OF MIPS
The term, MIP, encompasses a wide range of injectable agents, devices and techniques (Figure 7.3)1
Botulinum neurotoxins
Hyaluronic acid (HA)-based fillers
INJECTABLES
Biostimulatory fillers Ellansé®
Threads Silhouette Soft®
Others Mesotherapy cocktails (skin rejuvenation)
Platelet-rich plasma and other peptides
Chemical-induced lipolysis
Energy-based devices
Radiofrequency high-intensity focused ultrasound
Low-intensity focused ultrasound, infrared light
OTHERS
Mechanical (suction, vibration)
Ablative and non-ablative lasers
Broadband lights and intense pulsed lights
Cryolipolysis
Figure 7.3
Mechanism of action of different minimally invasive procedures
COMBINED TREATMENTS 133
Sinclair clinical experts' recommendations on treatments that can be combined with Ellansé
Silhouette Soft® threads
EXPERT TIP*
Treatment with Ellansé and Silhouette Soft should be carried out at different times. Priority should always be given to volume replacement (Ellansé) over volume displacement (Silhouette Soft).
EXPERT TIP*
In clinical practice, Silhouette Soft threads (Figure 7.4) work synergistically with Ellansé. Patients seeking a thread lift will typically present with sagging of the superficial fat pads, jowls and/or nasolabial folds. On facial assessment, the patient is likely to have deep volume deficiency due to the normal ageing processes of bone resorption and deep fat pad loss, leading to the sliding down of the superficial tissues.
Silhouette Soft threads target the repositioning of soft tissue, but do not address deep volume loss. It is important to replace volume as a priority to target the ageing process changes (the bone and deep fat loss).
Ellansé is an ideal choice to provide the deep volume and structural support required for a clinically successful thread lift. This is especially the case in the pre-jowl/marionette areas, the malar groove and the pyriform aperture where it can assist an improved outcome with Silhouette Soft threads in the jowl and nasolabial areas, respectively.
Hyaluronic acid (HA) fillers are shorter lasting and will not sustain this deep supporting role over a long period.
For younger patients with good skin quality, Ellansé can be injected in the pyriform aperture and pre-jowl/ marionette line locations to provide
deep structural support, with Silhouette Soft threads being placed at a later date (allowing sufficient time for healing)9
For older patients with poorer skin laxity, as well as replacing deep volume, it is also beneficial to improve subdermal tissue quality in the subzygoma cheeks and preauricular areas with Ellansé in the subdermal plane before embarking on the placement of threads. Ellansé stimulates type I collagen and, therefore, will improve tissue thickness and firmness, subsequently making patients better candidates for Silhouette Soft threads.

*Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book.
Figure 7.4 Silhouette Soft
Dr Francisco de Melo
134 ELLANS É ® | AN EXPERT'S GUIDE
Dr Amanda Ong
Hyaluronic acid (HA)
EXPERT TIP*
For certain applications, such as deep volume replacement, Ellansé may be preferable to HA fillers, delivering a longlasting, predictable and natural-looking result. For the intention of improving skin laxity and skin quality, the collagenstimulating benefits of Ellansé injected in a fanning technique with a cannula
into the subdermal layer deliver a more favourable outcome than HA fillers. However, Ellansé is contraindicated in the periorbital region (eyelids, under-eye dark circles, crow’s feet) and glabella region (for full list of contraindications, consult Instructions for Use).10 Therefore, HA fillers can be used for lip
Botulinum toxin injections
EXPERT TIP*
Botulinum toxin is ideal for relaxing the muscles involved in causing dynamic creases and wrinkles. The most common areas for treatment are the glabella, forehead and periorbital areas (Figure 7.6).

Botulinum neurotoxin is frequently used in combination with Silhouette Soft to relax the orbicularis oculi, for eyebrow lifts and the depressor angulis orbis and masseters for lower face treatments.

Botulinum toxin also works well when injected prior to Ellansé. For example, in the upper face, Ellansé in the lateral brow and/or temple, following botulinum toxin injection in the superior lateral orbicularis oculi muscle, can achieve a successful brow lift. For the lower face, relaxing the depressor anguli oris and mentalis muscles with prior botulinum toxin can improve clinical outcomes when addressing the pre-jowl/marionette lines and chin augmentation with Ellansé.
enhancement, for tear trough correction2 and for plumping up superficial lines and wrinkles in the dermis (Figure 7.5).
 Figure 7.5
Figure 7.5
*Any opinions, views, advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk.
Perfectha® and MaiLi
Figure 7.6
Botulinum toxin injections –Botulinum toxin treatment areas include the glabella, forehead and periorbital areas (information provided by Dr Francisco de Melo) Model not actual patient.
Dr Amanda Ong and Dr Francisco de Melo
COMBINED TREATMENTS 135
Dr Amanda Ong
Energy-based devices (EBDs)
Patients often require EBDs for skin tightening to address sagging and thinning skin. The benefits of such treatments include minimal pain, no ‘downtime’ and a relatively short procedure duration (Figure 7.7).
EBDs, such as micro-focused ultrasound, lasers and radiofrequency (RF), stimulate collagen in the skin and deeper layers through thermal injury. Ablative laser resurfacing devices, such as the CO2 laser and the 2,940 nm erbium-doped yttrium aluminium garnet (Er:YAG) laser vaporise the entire dermis and part of the epidermis, leading to collagen shrinkage and increased collagen production11
Non-ablative fractional lasers (NAFLS) are also successful at decreasing wrinkles and improving skin texture, particularly skin laxity; however, multiple treatments may be required to provide long-term results11. Intense pulsed light may treat dyschromia and vascular lesions but offers no significant improvement in periorbital rhytides11 Meanwhile, RF devices use rapidly alternating electric current and generate

heat deep in the dermis and hypodermis, with limited effects on the epidermis11 Finally, microfocused ultrasound causes points of microthermocoagulation at specific depths into the dermis, resulting in discrete foci of skin contraction, with an overall effect of skin tightening and improvement in skin laxity11
The limitations of these devices are:
● Stimulation of type I and III collagen by thermal injury
● Positive results may not be apparent for several months
● Specific areas of deep volume loss and lack of structural support cannot be precisely targeted
Use of Ellansé injections may enhance any skin-tightening programme to achieve improved outcomes. Areas of volume loss and structural support can be targeted more precisely, allowing for an immediate filling effect along with the long-term benefit of collagen stimulation.
GUIDELINES FOR COMBINING TREATMENTS:
Guidelines on rejuvenation treatment of the face and the neck using combinations of MIPs were developed by a multidisciplinary, multinational board of plastic surgeons and dermatologists, representing a worldwide perspective1
All options for the best combined rejuvenation treatment on predefined target areas were submitted to plenary votes to identify formal consensual statements. As a result of this process, a series of key recommendations to manage combination treatments were consensually agreed1
Figure 7.7
Energy-based devices Model not actual patient.
TIP*
EXPERT
136 ELLANS É ® | AN EXPERT'S GUIDE
Dr Amanda Ong
Combining treatments: Key principles
KEY PRINCIPLE 1
The first key principle to arise from the consensus document on the use of combination approaches in the aesthetic treatment of the face relates to procedures that should not be performed simultaneously. In particular, the guidelines recommend that performing multiple procedures on the same area during the same session is inadvisable, because data are lacking on the possible interactions, making it difficult to accurately determine the responsible agent or procedure in case of adverse events (Figure 7.8)1
KEY PRINCIPLE 2
The second key principle arising from the consensus document states that the rejuvenation protocol should successively aim for two main objectives (Figure 7.9)1:
1. Volume adjustment (reduction, replenishment/augmentation or creation), followed by
2. Tissue repositioning
A final optional step that is frequently needed to improve the overall result is ‘touch-up’ and skin quality improvement procedures. As with all procedures, the techniques used must follow the product-recommended guidelines. It is also essential that sufficient time is allowed between procedures for adequate healing1


Replacement, replenishment, creation with Ellansé or MaiLi
Reduction with the physician's preferred techniques
Touch up with with Ellansé or MaiLi
Reposition with Silhouette
Volume adjustment Step 1 Tissue repositioning Step 2 Touch up (optional) Step 3
Figure 7.8
Key principle 1 for combining treatments
Figure 7.9
COMBINED TREATMENTS 137
Key principle 2 for combining treatments
Botulinum toxin1,12
Botulinum toxin injections are typically used for eyebrows and neck rejuvenation (Figure 7.10). The injection should be administered approximately 7–10 days before Silhouette Soft insertion, as complete muscular relaxation allows better cone encapsulation, which leads to a more stable outcome. The use of botulinum toxin injection in other areas is optional1
EXPERT TIP*
Botulinum toxin injections can also be administered at the same time as fillers (including HA and Ellansé), provided they are administered in a different area13. A 2-week interval between administration of botulinum toxin and a filler is recommended when the same area is concerned.

EBD procedures for tightening
EXPERT TIP*
It is recommended that EBD procedures for tightening should be performed in separate sessions (Figure 7.11)1
An interval of around 6–8 weeks is recommended before the Silhouette insertion to allow the collagen to begin remodelling, resulting in better support for the cones1

Similarly, when an EBD procedure is performed before the administration of fillers (HA and Ellansé), a 6- to 8-week interval is also recommended.
If EBD procedures for tightening are performed after suture insertion or filler injection, the interval should be longer (8–12 weeks) to avoid the risk of suture breakage or filler meltdown1
Figure 7.11
EBD procedures for tightening Model is not actual patient.
Figure 7.10
Botulinum toxin use in combination treatment
Dr Amanda Ong
138 ELLANS É ® | AN EXPERT'S GUIDE
Dr Amanda Ong
EBD procedures for resurfacing
EBD procedures for resurfacing should ideally be performed 8–12 weeks after the Silhouette Soft® insertion or filler injection (HA or Ellansé) (Figure 7.12)1
If the resurfacing procedure is performed first, it is important that the skin is properly re-epithelialised and free of any resurfacing-associated complications or adverse events (bacterial or viral infection, delayed healing areas) before suture insertion or filler injection (HA and Ellansé)1


Optional touch-up treatment
Optional treatment should be injected 4–6 weeks after initial volume replenishment/augmentation when using Ellansé, or 2 weeks after initial filler treatment when using MaiLi (Figure 7.13)1
Figure 7.12
EBD procedures for resurfacing Model is not actual patient.
Figure 7.13
*Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk. COMBINED TREATMENTS 139
Optional touch-up treatment Model is not actual patient.
Area-specific algorithms for combined treatment
A number of area-specific treatment algorithms were developed by the consensus process described previously. These algorithms are intended to provide guidance for healthcare professionals administering combinations of treatments for aesthetic facial procedures (Figure 7.14)1
Specifications are presented according to area-specific rejuvenation priorities1:
● Upper face
● Midface
● Lower face
Upper face (MaiLi or Ellansé injections for eyebrow elevation with volume replacement if required)
The sequence and timing of treatment of the upper face (MaiLi or Ellansé injections for eyebrow elevation) are shown in the algorithm (Figure 7.15).
Treatment should begin with botulinum toxin injections, associated with volume replacement1 The intention of treatment is to achieve relaxation of the eyebrow depressors and for volume replacement when needed.

The final touch-up/refinement step is optional1
Algorithm for upper face rejuvenation when using cross-linked hyaluronic acid or polycaprolactone collagen stimulator injections for eyebrow elevation BoNT, botulinum toxin; HA, hyaluronic acid; PCL, polycaprolactone.
Figure 7.15
Figure 7.14
BoNT 1b. TEMPLE and/or FOREHEAD VOLUME REPLACEMENT 1c. EYEBROW ELEVATION 2.
Cross-linked HA Cross-linked HA (or) PCL collagen stimulator PCL collagen stimulator 2 weeks 6/8 weeks Same session
Area-specific algorithms Model is not actual patient. 1a. RELAXATION ORBICULARIS OCULI (systematic)
TOUCH UP (optional)
140
Upper face (Silhouette Soft® for eyebrow elevation)

The sequence and timing of treatment of the upper face (Silhouette Soft threads for eyebrow elevation) are shown in the algorithm (Figure 7.16).
Treatment should begin with botulinum toxin injections, followed by simultaneous Silhouette Soft threads and volume replacement9. The final touch-up/ refinement session is optional1
When using Silhouette Soft for a brow elevation, it is crucial to first relax the lateral eyebrow depressors in order to obtain better suture stabilisation and an effective eyebrow elevation. The treatments should be carried out 10–15 days apart.
Cross-linked HA
BoNT PCL collagen stimulator
2 weeks
PLLA/PLGA sutures – 8 cones
Algorithm
BoNT, botulinum toxin; HA, hyaluronic acid; PCL, polycaprolactone; PLGA, poly(lactide-co-glycolide); PLLA, poly-(L)-lactic acid.
*Spacing between steps 2 and 3: 4 to 6 weeks if step 1 treatment was Ellansé or 2 weeks if step 1 treatment was MaiLi
Spacing according to step 1 treatment*
PCL collagen stimulator
Cross-linked HA
1a. RELAXATION ORBICULARIS OCULI (systematic)
1b. TEMPLE and/or FOREHEAD VOLUME REPLACEMENT
2. EYEBROW ELEVATION
3. TOUCH UP (optional)
Figure 7.16
for upper face rejuvenation when using PLLA/PLGA sutures for eyebrow elevation
COMBINED TREATMENTS 141
Midface
The sequence and timing of treatment of the midface are shown in the algorithm (Figure 7.17).
Midface rejuvenation should begin with volume replacement, followed by tissue repositioning. The third ‘touch-up’ step is optional and should not be performed before the results of the previous steps have stabilised1

EXPERT TIP*
Over the past 5 years, for any patient aged 45–50 years or older treated with Silhouette, I have added a session of biostimulation with 0.5 ml of Ellansé per side at week 5 (i.e. once the thread is
fully encapsulated). This has contributed greatly to improved skin quality and has ensured the longevity of the results.
PCL collagen stimulator
(or)
Cross-linked HA
2 weeks
PLLA/PLGA
Cross-linked HA
Collagen stimulator
Spacing according to step 1 treatment*
*Spacing between steps 2 and 3: 4 to 6 weeks if step 1 treatment was Ellansé or 2 weeks if step 1 treatment was MaiLi
Figure 7.17 Algorithm for midface rejuvenation HA, hyaluronic acid; PCL, polycaprolactone; PLGA, poly(lactide-co-glycolide); PLLA, poly-(L)-lactic acid.
1. VOLUME REPLACEMENT
3. TOUCH UP (optional)
2. TISSUE REPOSITION
sutures
142 ELLANS É ® | AN EXPERT'S GUIDE
Dr Pierre Nicolau
*
Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk.
Lower face
The sequence and timing of treatment of the lower face are shown in the algorithm (Figure 7.18).
The overall lower face rejuvenation procedure should begin with volume adjustment (reduction or replacement/ augmentation). Application of botulinum toxin to the lower face is optional; if carried out, it should be separated by a 2-week interval from the next step (tissue repositioning and suture insertion). The final ‘touch-up’ step is also optional1

1a. VOLUME REPLACEMENT/ AUGMENTATION
1b. VOLUME REDUCTION
Cross-linked HA PCL collagen stimulator Liposuction and cryolipolysis
BoNT
Cross-linked HA PCL collagen stimulator
PLLA/PLGA
Algorithm for lower face rejuvenation BoNT, botulinum toxin; DAO, depressor anguli oris; HA, hyaluronic acid; PCL, polycaprolactone; PLGA, poly(lactideco-glycolide); PLLA, poly-(L)-lactic acid.
*Spacing between steps 2 and 3: 4 to 6 weeks if step 1 treatment was Ellansé or 2 weeks if step 1 treatment was MaiLi
1. VOLUME ADJUSTMENT
2. RELAXATION (optional) Masseter, DAO, mentalis, platysma
4. TOUCH UP (optional)
3. TISSUE REPOSIT
sutures
Injection lipolysis Same session 2 weeks* 6 to 8 weeks 2 to 6 weeks 12 weeks (or) (or)
Figure 7.18
COMBINED TREATMENTS 143
The practice guidelines described in this chapter have been written to help practitioners achieve optimal patient outcomes when combining treatments for the rejuvenation of different facial areas.
As always, individual treatment plans should be adapted according to the physician’s individual competence and the patient’s preferences and needs1 . Practitioners should always be mindful of which treatments may be performed simultaneously, and perform procedures separately if in doubt (see Figure 7.9).

144
Summary
Model is not an actual patient
References
1. de Melo F, Carrijo A, Hong K, Trumbic B, Vercesi F, Waldorf HA, Zenker S. Minimally invasive aesthetic treatment of the face and neck using combinations of a PCL-based collagen stimulator, PLLA/PLGA suspension sutures, and cross-linked hyaluronic acid. Clin Cosmet Investig Dermatol. 2020;13:333–44.
2. Matarasso A, Nikfarjam J, Abramowitz L. Incorporating minimally invasive procedures into an aesthetic surgery practice. Clin Plast Surg. 2016;43:449–57.
3. American Society of Plastic Surgeons. Plastic surgery statistics report. 2020. Available from: https:// www.plasticsurgery.org/documents/ News/Statistics/2020/plasticsurgery-statistics-full-report-2020. pdf (Accessed November 2021).
4. Carruthers J, Burgess C, Day D, Fabi SG, Goldie K, Kerscher M, Nikolis A, Pavicic T, Rho NK, Rzany B, Sattler G, Sattler S, Seo K, Werschler WP, Carruthers A. Consensus recommendations for combined aesthetic interventions in the face using botulinum toxin, fillers, and energy-based devices. Dermatol Surg. 2016;42:586–97.
5. Werschler WP, Calkin JM, Laub DA, Mauricio T, Narurkar VA, Rich P. Aesthetic dermatologic treatments: Consensus from the experts. J Clin Aesthet Dermatol. 2015;8(10 Suppl):S2–S7.
6. Lorenc ZP, Daro-Kaftan E. Optimizing facial rejuvenation outcomes by combining poly-Llactic acid, hyaluronic acid, calcium hydroxylapatite, and neurotoxins: two case studies. J Drugs Dermatol. 2014;13:191–5.
7. Alam M, Kakar R, Nodzenski M, Ibrahim O, Disphanurat W, Bolotin D, Borovicka JH, Pace N, Alster TS, Arndt KA, Beer KR, Berlin JM, Bernstein LJ, Brightman LA, Butterwick K, Cox SE, Chotzen V, Fabi SG, Fitzpatrick RE, Geronemus RG, Goldman MP, Groff WF, Kaminer MS, Kilmer S, Rohrer TE, Tanzi EL, Silva SK, Yoo SS, Weinkle SH, Strasswimmer J, Poon E, Dover JS. Multicenter prospective cohort study of the incidence of adverse events associated with cosmetic dermatologic procedures: Lasers, energy devices, and injectable neurotoxins and fillers. JAMA Dermatol. 2015;151:271–7.
8. Langelier N, Beleznay K, Woodward J. Rejuvenation of the upper face and periocular region: Combining neuromodulator, facial filler, laser, light, and energy-based therapies for optimal results. Dermatol Surg. 2016;42(Suppl 2):S77–S82.
9. Sinclair Silhouette Soft Physician Guide.
10. Sinclair. Ellansé instructions for use.
11. Heitmiller K, Ring C, Saedi N, Biesman B. Nonsurgical light and energy–based devices: Utility in eyelid and periorbital surgery. Facial Plast Surg Clin North Am. 2021;29:323–34.
12. Môle B. Griffose faciale: Traitement des rides dynamiques du visage par injections dermiques simultanées de toxine botulique A et d'acide hyaluronique [Scratched faces: Treatment of dynamic facial wrinkles through the simultaneous combined use of botulinium toxin A and hyaluronic acid]. Ann Chir Plast Esthet. 2012;57:194–201.
13. Sinclair. Data on file. Understanding publication on combined treatment. 2021.
COMBINED TREATMENTS 145
Adverse Event Management 08
147
Ellansé safety record
The biocompatibility, biodegradation and bioresorption of Ellansé have been extensively demonstrated3,4, while its good safety profile has been confirmed in numerous clinical efficacy and safety studies5,6,7,8. In addition, the safety profile of Ellansé has been continuously monitored since its launch in vigilance surveys as well as by clinical experts in daily practice1,9,10
In a review of adverse events (AEs) from launch to October 2021, the AE rate with Ellansé was 0.0619%, which equates to one event for every 1,616 syringes used11 The majority of the AEs reported were considered minor, and tended to be events such as nodules and swelling that are related to the injection procedure itself (Table 8.1). No trends towards specific AEs were noted.
The information in this chapter is designed to help avoid any complications, including those due to the injection procedure. Detailed information on the contraindications, warnings, precautions and directions for use is provided in the Instructions for Use of the product, which should be reviewed before any procedures are undertaken.
● Ellansé is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies
● Ellansé is not to be used in patients with known hypersensitivity to any of the components
● Ellansé should not be used in the case of acute or chronic skin disease (infection or inflammation) or autoimmune disease
● Ellansé should not be used in patients with known susceptibility to keloid formation or hypertrophic scarring
● Ellansé should not be administered in case of current cortisone treatment as the growth of connective tissue fibres might be inhibited
● Ellansé should not be used in or close to sites where previous skin augmentation procedures have been applied, especially with permanent implants
● Ellansé should not be injected into the periorbital region (eyelids, under-eye dark circles, crow's feet) or glabella region as there is a risk of ocular ischaemic events leading to loss of vision
The safety of Ellansé has been evaluated throughout its development by studying its individual components and the finished ready-to-use product1,2.
Table 8.1 Reported adverse event rate for Ellansé by type of event for the 3 years to 15 October 202111
8.1 Ellansé contraindications12 Nodules/lumps 0.0277% Swelling 0.0181% Inflammation 0.0059% Bruising/haematoma 0.0015% Infection 0.0012% Hardness induration 0.0075%
Figure
148 ELLANS É ® | AN EXPERT'S GUIDE
Ellansé contraindications
● Severe allergies manifested by a history of anaphylaxis, or history or presence of multiple severe allergies
● Known hypersensitivity to any of the components
● Acute or chronic skin disease (infection or inflammation)
● Active sepsis or infection, active (or history of) autoimmune disease
● Known susceptibility to keloid formation or hypertrophic scarring
● Current cortisone treatment (reduces neocollagenesis)
For full list of contraindications and warnings, consult the Instructions for Use (IFU).
Ellansé should never be injected into the eyelids, under-eye dark circles, crow's feet, lips or glabella region13. In addition, Ellansé should not be injected into the muscles13
If Ellansé is to be used in combination with any other treatment, the principles outlined in this chapter should be followed closely14. In particular, adequate time must be allowed to elapse between different procedures, depending on the treatment type14

Periorbital region Lips
To reduce the risk of complications, Ellansé should never be injected in patients with the following contraindications (Figure 8.1)13:
to
ADVERSE EVENT MANAGEMENT 149
Figure 8.2 Treatment areas
avoid12 Glabella Eyelids Crow's feet
Advice for safe use of Ellansé
EXPERT TIP*
EXPERT TIP*
Ellansé can be mixed with a maximum of 0.2 ml of lidocaine (with or without adrenaline), according to published guidelines2, to offer comfort to the patient during treatment.
The recommended volume does not change the characteristics of the gel. However, Ellansé should not be diluted or mixed with other pharmaceuticals, water or normal saline, as this will alter the gel composition and rheology, and may lead to an increased risk of AEs and complications.
Risk of AEs can be reduced by always adhering to the proper injection technique (please refer to Chapter 5 for a full discussion of the correct injection technique) (Table 8.2 and Figure 8.3). In particular, it is important to mark the entry points and inject with a low pressure, remaining mindful of the potential to cause pain12. Small volumes in each passage or bolus should always be injected (i.e. 0.1–0.2 ml boluses and 0.05–0.1 ml lines)2,7
For Ellansé treatment in facial areas, placement of the product in the subcutaneous or supraperiosteal plane is recommended (Figure 8.4)2. It is recommended to use a 27G, ¾–1¼-inch needle or a 22G* to 25G cannula.
For subdermal injections, always consider using a cannula. If using a needle, however, make the path as short as possible12. Be aware of the different anatomical levels during injection.
Dr Francisco de Melo
GOOD INJECTION TECHNIQUE
*Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk. Ellansé extrusion force testing has only been carried out for 25G and 27G cannulas11
Adherence to the injection guidelines and good clinical judgement are the most important requirements to avoid complications and AEs in the use of Ellansé.
Dr Francisco de Melo
Level of depth Subdermal plane Supraperiosteal plane Injection technique Linear threading, fanning, cross-hatching in a retrograde manner Bolus deposition, linear threading and fanning Amount suggested 0.05–0.1 ml/cm 0.1–0.2 ml/cm2
of technique To create a scaffold within the fat compartments and subsequently restore the volume
improve volume in the deep compartments
8.2
depths and associated techniques
150 ELLANS É ® | AN EXPERT'S GUIDE
Purpose
To
Table
Injection
EXPERT TIP* Dr Shang-Li Lin
Bolus/microbolus
Linear
Static needle
Fanning
Arrows indicate direction of needle when injecting
Treatment intention
● Subdermal volume increase
● Biostimulation of collagen formation



Cross-hatching
Injections are retrograde and
Treatment intention
● Deep volume increase
Superficial fat
threading/retrograde
Figure 8.3
Injection patterns for Ellansé
Figure 8.4
ADVERSE EVENT MANAGEMENT 151
Placement of product in the subdermal and supraperiosteal plane2 SMAS, superficial musculoaponeurotic system.
The pyramid technique, which involves injecting perpendicular to the surface of the skin down towards the deep planes and injecting the product in a retrograde manner, should be avoided (Figure 8.5). The pyramid technique can be dangerous because if a vessel is inadvertently punctured on the way down, the product will be infused intravascularly on the way back.
Dr Francisco de Melo
TOP TIPS TO ACHIEVE OPTIMUM RESULTS:
Always inject with a low pressure
Gradually bring the treatment area to the required level of correction
The pyramid technique, which involves injecting perpendicular to the surface of the skin down towards the deep planes and injecting the product in a retrograde manner, should be avoided
Gently mould the injected area (if needed) after treatment to obtain the desired result
There is no need to overcorrect, as the volume will slowly increase over time (10–12 weeks)
Figure 8.5 Avoid the pyramid technique
EXPERT TIP* 152 ELLANS É ® | AN EXPERT'S GUIDE
Potential complications with fillers
There are a number of incorrect procedures and poor practices that may occasionally lead to complications with the use of fillers. Such complications are rare, but are occasionally seen with all types of filler.
Generally, the AEs associated with dermal fillers are classified by the time

of onset. Those that occur up to several days post-treatment, including injectionsite reactions, infection, hypersensitivity, irregularities, skin discoloration and vascular events, are considered early events. Filler complications that occur from weeks to years post-treatment, such as atypical infection, foreign body granuloma, migration, immune reactions, nodules, persistent discoloration, persistent scarring and malar oedema,

are defined as delayed events (Figure 8.6; Table 8.3)15
The most common issues associated with the use of all types of filler include overcorrection, leading to accumulation of the product, and improper superficial placement, leading to issues such as oedema12
 Dr Francisco de Melo
Figure 8.6
Dr Francisco de Melo
Figure 8.6
[Images courtesy of Dr
Actual patient, results may vary. None of these cases were related to use of Ellansé
Complications with fillers. A, B vascular complications; C nodules; D persistent oedema.
Shang-Li Lin]
*Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book.
Improper choice of long-lasting, slowly resorbable products leads to an increased risk of granuloma, while too much product in one focal area increases the risk of nodules. Finally, potentially the most severe complication is injection into blood vessels causing vascular impairment12
Methods for avoiding these issues and subsequent complications are discussed in further detail later in this chapter.
EXPERT TIP*
A B C D ADVERSE EVENT MANAGEMENT 153
BRUISING
Bruising is a common complication of filler injections, with patients who take anticoagulants, antiplatelet agents, nonsteroidal anti-inflammatory drugs, herbal medications or alcohol at an increased risk16. Bruising is more common when fanning or threading techniques are used in the dermal and immediate subdermal planes16. In general, the risk of bruising can be reduced with the use of blunt tip cannulas, low-pressure injections with smaller volumes of product, and use of the recommended needle gauge16. Most post-procedure bruising is benign and will resolve spontaneously. If bruising is extensive, or distressing to the patient, it can be treated with a pulsed dye laser or intense pulsed light to aid recovery16
DISCOLORATION
A slight blue-grey discoloration at the site of filler injection is often a sign that the product was placed too superficially16. This phenomenon, known as the Tyndall effect, can be avoided by injecting filler products at the proper depth within the tissue16
SWELLING AND OEDEMA
Swelling after treatment with any type of injectable, particularly in the periorbital area, is frequent and usually benign –generally resolving over 1 to 2 weeks16
However, if the swelling is significant or persistent, it may be due to lymphatic obstruction, overcorrection or hypersensitivity reaction16. Lymphatic obstruction is not typically associated with erythema or pruritus and may present as malar oedema after tear trough correction16. Lymphatic obstruction is probably multifactorial and related to the patient’s degree of preprocedural lymphatic obstruction, the volume injected and the viscosity of the filler used16
Table 8.3
Dermal filler complications and adverse events by time of onset15
Early events (occurring up to several days posttreatment)
Injection-site reactions
● Erythema
● Oedema
● Pain/tenderness
● Bruising
● Itching
Infection
● Erythema
● Oedema
● Pain/tenderness
● Acne papule formation
● Nodule/abscess
Hypersensitivity
● Erythema
● Oedema
● Pain/tenderness
● Nonfluctuant nodules
Lumps, asymmetries, contour irregularities caused by technique and placement errors
Skin discoloration
● Redness
● Whiteness
● Hyperpigmentation
Local tissue necrosis caused by vascular occlusion
Delayed events (occurring from weeks to years post-treatment)
Infection (atypical; e.g. mycobacterial)
● Erythema
● Oedema
● Pain/tenderness
● Nodule/abscess
● Systemic responses to infection
● Biofilm
Foreign body granuloma
● Varying from subclinical histological changes to disfiguring nodules
Migration of implant material
Immune reactions
● Local, site injection and generalised
Persistent discoloration
Persistent scarring
Malar oedema
Dr Shang-Li Lin
Risk of discoloration can be minimised by placing the product supraperiosteally or at the subdermal level in small amounts.
TIP* 154 ELLANS É ® | AN EXPERT'S GUIDE
EXPERT
NODULES
A nodule is a small collection of tissue that is palpable at any level of the skin (epidermis, dermis or subcutis) or in another tissue of the body. Nodules characteristically range in size from 1 to 2 cm in diameter (Figure 8.7)17

GRANULOMA
A granuloma is a chronic inflammatory reaction, with a large involvement of immune cells19. Granulomas are caused by granulomatous inflammation after the aggregation of macrophages in response to large foreign bodies19
The histopathology of granulomas shows an overabundance of host tissue reaction to a relatively small amount of product, with large numbers of foreign body giant cells (although the presence of a granuloma is not dependent on the volume of foreign material)7
A granuloma has a clinical definition, and typically appears suddenly, 6–24 months post-injection19, and is located at all injected sites at the same time19,20 (Figure 8.8). Unlike nodules, granulomas become larger than the volume of filler that was injected and can be associated with skin discoloration and oedema19,21
Granulomas have been reported with all currently available commercial devices including collagen, hyaluronic acid (HA), poly(l-lactic acid), silicone, calcium hydroxylapatite, polymethyl methacrylate and polyacrylamide gel22. True inflammatory granulomas are unpredictable and rare (the reported rate of clinically detectable granuloma formation varies between 0.01% and 1.0%)21,22
DISTINCTION BETWEEN NODULES AND GRANULOMAS
As nodules have often been described incorrectly as granulomas in the medical literature, practitioners should clearly know the differences with regard to their causes, features and management.
Diagnosis of a nodule or granuloma is made clinically, with distinctions becoming clear under examination with a microscope22. Clinical characteristics of granulomas and nodules are listed in Table 8.4.
Diagnosis may be confirmed histopathologically. A nodule reveals an overabundance of product, while the histopathology of a true granuloma shows an overabundance of host reaction to a small amount of product, indicating the latter is an immune response21,22
EXPERT TIP*
The areas susceptible to nodules tend to be where there is no fat and intramuscular placement in sphincteric muscles, leading to increased nodule formation.
Nodules typically appear 1–2 months after an injection and occur when too much material accumulates in an area as a result of poor technique (overcorrection or use of a filler for an incorrect indication such as intramuscular placement in a sphincteric muscle)7,15,16,18
Nodules are more frequent when large volumes are injected in any single location7,18. Nodule size depends on the volume injected and remains stable over time15
Dr Amanda Ong
A small amount of filler placed in a scar tissue area, which is a dense tissue by nature, will be sufficient to create the necessary tension to trigger a physiological response and generate a nodule. The same small amount of filler in non-scar tissue will not have the same effect, as it will not be able to generate the pressure gradient necessary to trigger the response.
Nodules are seen with all fillers, including HAs12

Distinction between a nodule and a granuloma [Lemperle 2006]
Characteristic Nodule
Appearance One to 2 months after injection, after swelling subsides; noninflammatory
Location Single nodules, close to facial muscles, particularly in the lips

Size Remain the same size (similar to a lentil or pea), hard
Granuloma
Sudden, 6 to 24 month after injection, often inflammatory, telangectasia
At all injected sites at the same time
Grow to the size of a bean, accompanied by skin discolouration, oedema; rather soft Borders
Well confined by fibrous capsule
Until absorption (or permanent)
Persistence
Histology Foreign-body reaction; particles or microspheres are packed
Treatment Corticosteroids have little effect; must wait for absorption or excision
Cause Often improper technique or intramuscular injection
Grow fingerlike into surrounding tissue
If untreated, they disappear after 1 to 5 years
Foreign-body granuloma; droplets, particles, or microspheres are scattered
Respond well to intralesional or systemic corticosteroids
Still unknown
Table 8.4
Figure 8.7
Examples of nodules20 Actual patient, results may vary. © Georg Thieme Verlag KG
Figure 8.8
*
accept any liability for the opinions,
is not listed within the IFU shall be undertaken entirely at the
Simultaneous appearance of granuloma. [Image courtesy of Dr Shang-Li Lin] Actual patient, results may vary.
Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not
views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that
medical professional's risk.
Dr Francisco de Melo
EXPERT TIP*
ADVERSE EVENT MANAGEMENT 155
Prevention of complications
RISK REDUCTION BEFORE USE
There are several considerations that should be followed prior to use of Ellansé to reduce the risk of complications12. First, it is important to acquire a detailed anatomical awareness of the likely position and depth of named vessels in the treatment areas12 Appropriate markings should be made on the skin to show the areas to be treated and those where injections should be avoided (Figure 8.9)2
Low-pressure techniques should be adopted12, and a small bolus or line for each applied injection point2,7 should be used. Placement of the product in the subdermal and supraperiosteal plane should be adhered to12
It is important to check the depth of the injection. Local anaesthesia with adrenaline may be used before filler treatment12
PREVENTION OF NODULES
There are several steps that should be followed in order to reduce the risk of nodules following a filler procedure1,12

The injection should always be carried out with a low-pressure gradient1,12 Large volumes (i.e. boluses or lines of more than 0.2 ml per line) should not be used, as they will generate unnecessary tension1
No injection should be made within the muscles, lips or eyelids1,12
PREVENTION OF GRANULOMAS

To prevent the formation of granulomas, it is essential to adhere to the proposed injection techniques and recommended volume per area, as described in Chapter 5.
Granulomas are the result of nonspecific immunity and can happen with any product or filler24 Do not inject in the same zone where a permanent filler has previously been injected12
Figure 8.9
Appropriate markings prior to treatment help prevent injecting areas that should be avoided
156 ELLANS É ® | AN EXPERT'S GUIDE
Scan the QR code to learn more about preventing complications at Sinclair College.
Management of complications
This section is based on the 2018 World Expert Meeting roundtable on AE management attended by Drs Pierre Nicolau, Francisco de Melo and Shang-Li Lin25
OVERCORRECTION
It is recommended to undercorrect rather than overcorrect when using Ellansé, as the volume injected will slowly increase over time. However, if overcorrection does occur at an early stage, it is due to the volume provided by the carboxymethyl cellulose gel, and it can be dispersed by local infiltration with normal saline with or without lidocaine, and massage1
If diagnosed between the 10th day and the 5th week, it is still possible to disperse Ellansé using saline, but it is usually better to add methylprednisolone1 Oral corticosteroids may also be useful.
SWELLING
Treatment should be conservative as the adverse event is of limited duration and size. General measures should be used initially, such as head elevation, procedures to improve lymphatic drainage, and reduction of smoking. Pharmacological measures, including oral non-steroidal anti-inflammatory drugs, short courses of oral steroids or intralesional steroid injection, should be reserved for more persistent cases.
NODULES (FIGURE 8.10)
● In the early stages (first 2 weeks) nodules are essentially an overcorrection, and should be treated as such

● After 2 weeks, because encapsulation will have begun, adding a steroid to the local injection will help to reduce the intensity of the foreign body reaction
● Beyond 2 months, this is now a true nodule, and repeated intralesional steroid injections will be necessary
● Surgical excision is an option, but should be reserved for selected cases, depending on the nodule location and resulting scar visibility
 Figure 8.10
Figure 8.10
ADVERSE EVENT MANAGEMENT 157
Management of nodules [Images courtesy of Dr Shang-Li Lin] Actual patient, results may vary.
GRANULOMA

● The treatment of granulomas is nonspecific and focused on the reduction of the immune response (Figure 8.11)
● Treatment with oral steroids (prednisolone 1 mg/kg) is usually the first line of action
● Intralesional treatment with steroids (methylprednisolone, triamcinolone) or 5-fluorouracil (5-FU) is recommended as a complement to the oral treatment
● In the case of cystic granulomas, drainage and local infiltration with steroids has also been recommended21





REMOVAL OF PRODUCT
In certain rare situations, it may be necessary to remove the product because of AEs.

VASCULAR COMPLICATIONS (FIGURE 8.12)
Vascular complications are considered a medical emergency and, as a consequence, all measures are permissible. In this situation, the following steps should be followed:
1. Hyaluronidase injection: increases vascular permeability and offers a vasodilator effect
2. Chewable aspirin (81 mg) or an initial loading dose (325 mg) followed by 100–125 mg/day: offers an antiaggregant effect
3. Pentoxifylline 400 mg/day: improves red blood cell deformability (known as a haemorrheological effect), reduces blood viscosity and decreases the potential for platelet aggregation and blood clot formation
4. Sildenafil (50 mg/day) or tadalafil (5 mg/day): alleviates vasospasm and reduces severe ischaemia
5. Hyperbaric oxygen
INVESTIGATION OF AEs
All AEs should be thoroughly reviewed and investigated (Figure 8.13).
Please report all AEs to Sinclair’s Vigilance and Compliance Department:
Email: quality@sinclair.com
● Sinclair website
● By notifying any Sinclair employee or distributor
Resolution
complications [Images
Actual patient, results may vary.
Figure 8.12
of vascular
courtesy of Dr Francisco de Melo]
Management
[Images
Actual patient, results may vary.
Figure 8.11
of granuloma
courtesy of Dr Pierre Nicolau]
Methotrexate has also been recommended in a weekly dose of 5 mg supplemented with folic acid 10 mg/day.
EXPERT TIP* 158 ELLANS É ® | AN EXPERT'S GUIDE
Dr Pierre Nicolau
Collagenase (Xiapex® in Denmark and Spain; Xiaflex® in the US) should be injected at a dose of 0.05 ml per point, to a maximum injection per site of 0.20–0.25 ml every 4–6 weeks12
Positive findings in the patient history:
● Associated pathology (mainly autoimmune)
● Any trigger event before the appearance of the lesion: infection, surgery, medication, etc.
● Patient smoking habits
● Any previous filler treatment in the same area (permanent fillers?)
Initial treatment:
● Ellanse S or M?
● Mixing or dilution
● Volume injected
● Technique: cannula/needle, fanning/bolus
● Top-ups?
Adverse event diagnosis:
● Interval between initial treatment and the onset of symptoms
● Single or multiple lesions, simultaneous appearance, hard or soft, associated inflammatory signs
● Investigations: ultrasound scan or other radiology exams; biopsy, etc.
Treatment:
● What was done
● Result
● Recurrence
Vascular complication:
● Which area was affected?
● Immediate or delayed?
● What injection technique was used?
● What was the initial treatment?
● How did it evolve?
Considerations for investigation of adverse events
Figure 8.13
[Figure courtesy of Dr Francisco de Melo]
*Any opinions, views or advice provided are those of the healthcare professionals. Sinclair does not provide any warranty and does not accept any liability for the opinions, views or advice expressed by expert contributors to this book. Use of Sinclair’s products in combination with any other product or accessory that is not listed within the IFU shall be undertaken entirely at the medical professional's risk.
In addition, ancillary measures, such as topical antibiotic ointment and oral steroids should be offered to reduce oedema and inflammation associated with ischaemia. Oral antibiotics and wound care treatment should also be considered as needed.
Dr Francisco de Melo
Hyaluronidase can be used to help diffuse the corticosteroids, but has no direct effect on polycaprolactone.
EXPERT TIP* EXPERT TIP* ADVERSE EVENT MANAGEMENT 159
Dr Pierre Nicolau
Summary
All injectable soft-tissue fillers contain particles and induce a foreign body inflammatory reaction. The intensity and duration of the effect are directly related to the substance’s physical (rheological) properties, size, shape and longevity of the particles.
Ellansé has a unique, patented technology that was specifically designed to stimulate mature collagen, producing a prolonged and predictable effect by inducing a limited low-grade foreign body reaction that allows Ellansé to stimulate type I collagen1

All injectable products have AEs. Most of the complications/AEs associated with injectable fillers are associated with injection technique and the physician’s experience; Ellansé is no exception11
Ellansé has an excellent safety profile5,6,7,8. The AEs associated with Ellansé can be minimised by avoiding use in areas recently treated with injectable biostimulators or permanent fillers14
Being familiar with the mechanism of action of Ellansé, understanding the biostimulation process, adopting a good injection technique and a proper therapeutic plan are the most important steps to avoid complications and AEs. Continued medical education, training and clinical experience are necessary to ensure optimal and safe results with Ellansé.
Scan the QR code to test your knowledge of managing adverse events, or use the following URL to explore the content:
www.ellanseexpertguide.com/8

160
Model is not an actual patient.
References
1. Christen MO, Vercesi F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020;13:31–48.
2. de Melo F, Nicolau P, Piovano L, Lin SL, Baptista-Fernandes T, King MI, Camporese A, Hong K, Khattar MM, Christen MO. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. 2017;10:431–40.
3. Laeschke K. Biocompatibility of microparticles into soft tissue fillers. Semin Cutan Med Surg. 2004;23:214–7.
4. Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002;28:484–90.
5. Figueiredo VM. A five-patient prospective pilot study of a polycaprolactone based dermal filler for hand rejuvenation. J Cosmet Dermatol. 2013;12:73–7.
6. Galadari H, van Abel D, Al Nuami K, Al Faresi F, Galadari I. A randomized, prospective, blinded, split-face, single-center study comparing polycaprolactone to hyaluronic acid for treatment of nasolabial folds. J Cosmet Dermatol. 2015;14:27–32.
7. Lin SL, Christen MO. Polycaprolactone-based dermal filler complications: A retrospective study of 1111 treatments. J Cosmet Dermatol. 2020;19:1907–14.
8. Moers-Carpi MM, Sherwood S. Polycaprolactone for the correction of nasolabial folds: A 24-month, prospective, randomized, controlled clinical trial. Dermatol Surg. 2013;39:457–63.
9. Sinclair. Ellansé brochure.
10. Sinclair. Ellansé post-market surveillance report. 2020.
11. Sinclair data on file. AE rate –Ellansé data. 19 October 2021.
12. Sinclair. Ellansé adverse event management.
13. Sinclair. Ellansé instructions for use.
14. de Melo F, Carrijo A, Hong K, Trumbic B, Vercesi F, Waldorf HA, Zenker S. Minimally invasive aesthetic treatment of the face and neck using combinations of a PCL-based collagen stimulator, PLLA/PLGA suspension sutures, and cross-linked hyaluronic acid. Clin Cosmet Investig Dermatol. 2020;13:333–44.
15. Funt D, Pavicic T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.
16. Vanaman M, Fabi SG, Carruthers J. Complications in the cosmetic dermatology patient: A review and our experience (part 1). Dermatol Surg. 2016;42:1–11.
17. MedTerms Medical Dictionary A-Z list. Available from: https://www. medicinenet.com/nodule/definition. htm (Accessed February 2022).
18. Nicolau PJ. Skin fillers complications. IMCAS. Paris 2016.
19. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: Pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;52:232–9.
20. Fitzgerald R, Bertucci V, Sykes JM, Duplechain JK. Adverse reactions to injectable fillers. Facial Plast Surg. 2016;32:532–55.
21. Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006;26:356–64.
22. Fitzgerald R, Vleggaar D. Facial volume restoration of the aging face with poly-l-lactic acid. Dermatol Ther. 2011;24:2–27.
23. Phumyoo T, Tansatit T, Rachkeaw N. The soft tissue landmarks to avoid injury to the facial artery during filler and neurotoxin injection at the nasolabial region. J Craniofac Surg. 2014;25:1885–9.
24. Schwartz LR, Thompson C, Campanelli C. Calcium hydroxylapatite filler foreign body granulomas: A case report of a rare occurrence and review of the literature. Cos Derm. 2014:E1–4? Or change url to: https://cdn.mdedge. com/files/s3fs-public/Document/ September-2017/CD000010001_e. pdf.
25. Sinclair. World Expert meeting 2018. Roundtable meeting.
ADVERSE EVENT MANAGEMENT 161
Sinclair
1st Floor Whitfield Court 30-32 Whitfield Street London W1T 2RQ United Kingdom.
E. ellanse@sinclair.com ellanse.com
Date of Preparation: March 2022
UKBR-00665954-001-000
Copyright © 2022 Sinclair
All rights reserved. This book is protected by copyright.
No part of this book may be reproduced, stored or transmitted in any form or by any means, including photocopying, or utilised by any information storage without the prior written permission of the copyright holder.
The editor, authors and publisher make no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein.





































 Figure 2.10A Danger zones for upper face
Figure 2.10B Danger zones for midface
Figure 2.10A Danger zones for upper face
Figure 2.10B Danger zones for midface







































































 Temporal crest Orbital rim Orbital rim
Nasion
Temporal crest
Figure 5.15
Temporal crest Orbital rim Orbital rim
Nasion
Temporal crest
Figure 5.15






















 Figure 6.3
Figure 6.3





 Figure 6.4
Case study 3: Before Ellansé treatment [Images courtesy of Dr Shang-Li Lin]
Figure 6.4
Case study 3: Before Ellansé treatment [Images courtesy of Dr Shang-Li Lin]





 Figure 6.6
Case study 4: Before Ellansé treatment (top) and 18 months after Ellansé treatment (below)
Figure 6.6
Case study 4: Before Ellansé treatment (top) and 18 months after Ellansé treatment (below)


 Figure 6.7
Case study 5: Before Ellansé treatment (left) and at 3 months after Ellansé treatment (right)
Figure 6.7
Case study 5: Before Ellansé treatment (left) and at 3 months after Ellansé treatment (right)



 Figure 6.8
Case study 6: Before Ellansé treatment (top) and 4 months after Ellansé treatment (bottom) with 1 month between each 2 ml administration, giving a total injection volume of 8 ml
[Images courtesy of Dr Shang-Li Lin]
Figure 6.8
Case study 6: Before Ellansé treatment (top) and 4 months after Ellansé treatment (bottom) with 1 month between each 2 ml administration, giving a total injection volume of 8 ml
[Images courtesy of Dr Shang-Li Lin]


 Figure 7.1
Figure 7.1



 Figure 7.5
Figure 7.5

















 Dr Francisco de Melo
Figure 8.6
Dr Francisco de Melo
Figure 8.6






 Figure 8.10
Figure 8.10








