AMT® - RegeneraActiva protocol in Dermatology & Aesthetic Medicine


 Editors: Graziano A., D’Aquino R., Galvez R., Casanova J.M., Perego F
Editors: Graziano A., D’Aquino R., Galvez R., Casanova J.M., Perego F




 Editors: Graziano A., D’Aquino R., Galvez R., Casanova J.M., Perego F
Editors: Graziano A., D’Aquino R., Galvez R., Casanova J.M., Perego F

RegeneraActiva uses AMT® Autologous Micrografting Technology® by Rigenera© Technology: a simple and effective protocol with enormous therapeutic power to revolutionaze healthcare.
Autologous Micrografting Technology ® is an innovative, simple and effective regenerative method, capable of obtaining Autologous Micro-grafts using Rigenera technology. AMT ® protocols are based on stimulating self-regeneration by activating native progenitor cells located in the homologous tissues, through a simple process, without risks to the patient and with great therapeutic potential. In a single session, the patient is a donor and recipient of autologous micrografts, allowing the recipient area to benefit from the regenerative activity of the progenitor cells and growth factors extracted from the donor site.
The technique is based on clinical studies that show there is a high concentration of the progenitor cells obtained in solid tissues. Through a calibrated mechanical cutting process and filtering, cells and other precursor elements are concentrated. The Rigeneracon is a device designed and approved to mechanically disintegrate any biological tissue obtaining autologous micro-grafts in a minimally invasive way. Rigeneracon is a class II sterile and disposable medical device, consisting of a rotation and pressure propeller, a grid with 100 hexagonal holes 80 microns in diameter and 600 micro blades designed for optimal and effective cutting of different types of tissue. Each device is equipped with an RFID microchip that contains product information for security and traceability reasons.
The tissue to be treated is obtained through a biopsy that is introduced into the Rigeneracon device and is gently pressed against the microblades at a constant speed of 80 rpm, without damaging the cellular structure of the disaggregated tissue. The sample is processed between 2 and 4 minutes depending on the type of tissue. The calibrated holes act as a filter, selecting only particles and cells less than 80 microns. The complete procedure occurs in a single surgical time and in a sterile manner. AMT® obtains injectable micro grafts composed of: cells, extracellular matrix and growth factors derived from the patient’s own cells, with no other manipulation than mechanical disaggregation.

We care about the health of our patients, that is why we offer the best quality, personalized and safe treatments with cutting-edge technology:
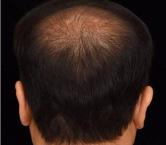
Androgenic alopecia is the most common baldness in our society, it affects both men and women. Analyzing the problem of hair loss with Handyscope (FotoFinder), a professional micro camera with polarized light, associated with a server where expert trichologists analyze the photos taken, is the best way to know the origin of the hair loss, essential for succeed in treatment.
AMT ® is an effective technique using injectable micro capillary graft, which slows down the evolution of hair loss by immunomodulation, as well as promoting hair growth. The micrograft solution obtained with AMT ® has the required regenerative properties, such as chemotaxis, cell proliferation, and high angiogenic potential.

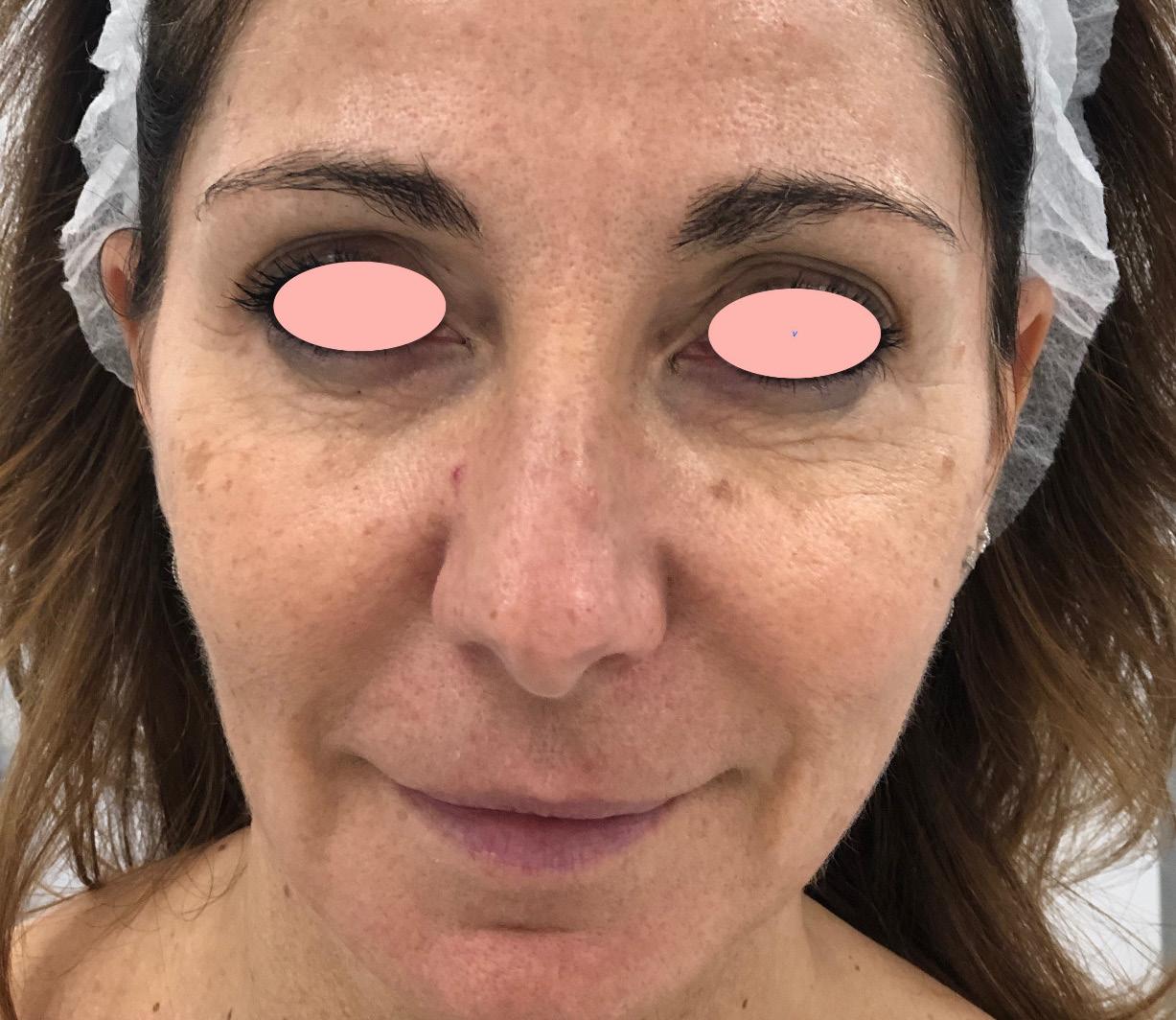
Vitiligo is an immune disorder of the skin, characterized by the presence of depigmented areas due to the lack of melanocytes. Vitiligo patients are more sensitive to sunburn. The contrast with the pigmented areas of the skin creates a major aesthetic problem for the patient.

AMT ® is a novel therapeutic tool for the field of dermatology. The melanocyte micro graft solution obtained with AMT ®, favors the re-pigmentation of the treated area.
A youthful appearance and care of our skin is essential as a business card in a society in which the image is increasingly important.
AMT ® is a novel therapeutic tool for the field of dermatology. The micrograft solution obtained with AMT ® has multiple regenerative properties, such as chemotaxis, cell proliferation, and high angiogenic potential. Our technology complements, enhances and makes the evolution of any aesthetic treatment aimed at skin rejuvenation shorter.


Our technology is based on two fundamental pillars of regenerative biology:
• Principle 1: The side population. Numerous scientific papers have demonstrated that progenitor cells (PC) reside within a population with determined morphological features. The main features are: (1) the size and (2) stem cell marker expression.
• Principle 2: The niche concept. Preserving the extracellular matrix (ECM) allows cells to maintain their physiological niche. This specific environment not only improves cell viability, but also gives the progenitor cells the appropriate growth factors that support their role in regenerative processes.
ANDROGENETIC ALOPECIA (AGA): VITILIGO SKIN REJUVENATION

This procedure is shown to be effective in delaying the biological evolution of Androgenetic Alopecia, as described in the scientific literature reported in the Bibliography section.
Specific considerations for patients undergoing “DERMIS MICROGRAFTS WITH RIGENERACONS FOR ANDROGENETIC ALOPECIA” protocol:
Patient suffering androgenetic alopecia must have enough hair follicles (hypotrophy) and a small bald pate area.
• The scalp should look healthy.
• Absence of local inflammatory pathology must be assured.
• Normal blood analysis (TSH below 2).
• A healthy food intake is ideal.
• Graft must be procured from a standard/healthy hair follicle area.
• Anesthesia without adrenaline and in a surrounding fashion (resembling a peripheral blockage and avoiding embibing the donor dermis).
Clinical record is mandatory in accordance to local laws and requirements, although this is a minimally invasive medical procedure. Signed consent is required, like in any other aesthetic medicine procedure. Its characteristics will be subjected to the local laws. Thus, it is important that the physician is acquainted with them.
Pictures must follow a previous stablished guideline. Light, distance to focus, zoom, and camera from the pre-treatment photo must be the same for all the follow-up photo(s), so they can be compared.
A. Prepare the patient:
I) Protect the surgical field. Although this is an ambulatory procedure and, could be performed outside an operating room, sterile handling of the sample must be guaranteed at all times.
II) Micrograft will be extracted from the mastoid hairy area, containing the Hair Follicle complex.
B. Prepare the equipment:
I) Rigenera® Machine N4SA: The machine must be plugged-in to the electrical power source. Turn it on. Be sure that the engine lever is up.
II) Rigeneracons® device: Peel the protective shield and discard it. Only the Rigeneracons® device, inside the second cover, is sterile.
III) Disposable material: Leave it on an accessory table. The whole set must be ready-to-use and kept sterile.
C. Prepare yourself:
I) Good light.
II) Goggles (optional).
III) Sterile gloves.
D. Biopsy retrieval:
I) Mark retrieval area, using a standard dermis marker.
II) Disinfect the retrieval area with the antiseptic solution of your choice (e.g.: alcohol 70% or Chlorhexidine ).
III) Anesthesia application in the periphery area without embedding the donor tissue (e.g.: 2% lidocaine without vasoconstrictor).
IV) Shave gently to remove the stratum corneum. Extract the samples with a dermal punch. Normally, the procedure will require 3 samples with a 2.5mm punches minimum.
NOTE: Using a 3- or 4-mm diameter dermal punches might require stitches.
NOW YOU ARE USING STERILE GLOVES! STERILE CONDITIONS AND SURGICAL BEHAVIOR FROM NOW ON!
E. Sample procurement:
The procedure is standard and extremely simple. However some complications, related with the small size of the sample, are relatively frequent:
• The tissue might stay inside the incision covered with blood. The tissue might stay inside the dermatome (dermal punch). It could be extracted with a needle.
• The tissue might get washed away by the blood.
• Mechanical compression is enough as hemostatic technique. Exceptionally, a stitch might be required.
F. Sample processing and micrograft solution generation:
I) Place the biopsies inside the Rigeneracons® device, over the grid under the helix rotor.
II) Add 1.2 ml sterile injectable physiologic solution through the specific connector of the Rigeneracons® with a non-Luer Lock® syringe. NOTE: The Grid has to be wet and the sample must slightly embedded, not submerged in it
NOTE: If more sterile saline solution has been used and the sample float into it we suggest to aspirat and discard the excess of saline solution.
III) Close the Rigeneracon® and place it in the Sicurlid, that could be sterilized if needed, couple the device with the Sicurstik.
IV) Place it in the Rigenera® N4SA machine rail into the main body and couple it with the machine rotor.
V) Press the central button located in the machine front. For this specific protocol the sample should be disaggregated for 2 minutes, each pulse lasts 1 minute (1minute/pulse).
NOTE: A green led will indicate that the process has started. It is automatic and it lasts 1 minute.
VI) Collect approx. 1.2 ml of micrograft solution through the specific connector of the Rigeneracons® (PRE-VIAL 1, more concentrated). Avoid using non-Luer Lock® syringe.
VII) Repeat steps from “ii” to “vi” with the same Rigeneracon® and sample to obtain (PRE-VIAL 2, more diluted).
VIII) Pull together and mix PRE-VIAL 1 and PRE-VIAL 2 in a 3 ml Luer Lock® syringe, the approx. micrograft solution volume is 3 ml.
XI) Split the volume into another 3 ml Luer Lock® syringe with a transfer (1.5 ml in each)
X) Increase the volume up to 3 ml with sterile injectable physiologic solution in both syringes, this syringe is now ready to use.
NOTE: 6ml of micrograft solution is enough for the Androgenetic Alopecia treatment of the damaged scalp area.
G. Application through mesotherapy:
I Injection will be performed with a 30G of 4 mm needle.
II The micrograft injection is performed both perpendicularly than with a moderate inclination to the scalp, depending on skin thickness. The solution should be injected in a superficial subcutaneous plane/deep dermis and not strictly intra-dermal .
III The injections shall be approximatively 0.05/0.1 ml* cm2
NOTE: 6ml of micrograft solution is enough for the Androgenetic Alopecia treatment of the damaged scalp area.
• Hemostasis is achieved by mechanical compression. Normally, stitches are not needed. If necessary, put one stich
• Vicryl rapid 4/o for each incision.
• After the procedure, pictures must be taken under the same conditions from the preoperatory
• Avoid activities that increase blood hydrostatic pressure he first 48 hours: sport, yoga, sauna, etc.
• Wound hygiene should be controlled.
• Sun protection is mandatory.
This protocol supports Dermatologist, Plastic surgeon and Aesthetic Doctors in using Dermis Micrografts to improve the skin texture by modulating the inflammatory and regenerative processes.
• For the first noticeable signs of skin aging / to prevent skin aging of the face and neck.
• Indicated for the relaxation / elastosis in “difficult” regions such as the lower third of the face, neck and décolleté.
• In association with fractional or ablative laser treatments, in order to enhance the induced regenerative effect, also modulating the inflammatory response of the laser and reducing its invasiveness.
• In association with Micro-needling treatments, in order to enhance the induced regenerative effect.
• To treat post-acne and post-surgical scars.
• For the preparation and maintenance of cervico-facial face-lifting operations, especially in the areas more predisposed to the relapse with hypo-elasticity of the skin (such as the neck and the sub-mental region).
• In association with lipofilling of the face, with the aim of enriching the adipose tissue.
Clinical record is mandatory in accordance to local laws and requirements, although this is a minimally invasive medical procedure. Signed consent is required, like in any other aesthetic medicine procedure. Its characteristics will be subjected to the local laws. Thus, it is important that the physician is acquainted with them.
Pictures must follow a previous stablished guideline. Light, distance to focus, zoom, and camera from the pre-treatment photo must be the same for all the follow-up photo(s), so they can be compared.
• Rigenera Machine N4SA
• RIGENERACONS
• Surgical drapes and sterile gloves
• Chlorhexidine solution
• Sterile dressings
• Biopsy punch (2,5/3 mm diameter)
A. Prepare the patient:
• Scalpel n° 15
• Lidocaine 2% without vasoconstrictor
• Luer lock Syringe of 1.0 ml for mesotherapy
• Needle 30/32 G
• NaCl solution
• Anatomical clamp and scissors
I) Protect the surgical field. Although this is an ambulatory procedure and, could be performed outside an operating room, sterile handling of the sample must be guaranteed at all times.
II) Micrograft can be extracted from the mastoid hairless area (close to the pre-hairline), behind the earlobe, or using the excess skin removed during a facial surgical procedure.
B. Prepare the equipment
I) Machine Rigenera®: The machine must be plugged-in to the electrical power source. Turn it on. Be sure that the engine lever is up.
II) Rigeneracons® device: Peel the protective shield and discard it. Only the Rigeneracons® device, inside the second cover, is sterile.
III) Disposable material: Leave it on an accessory table. The whole set must be ready-to-use and kept sterile.
C. Prepare yourself:
I) Good light.
II) Goggles (optional).
III) Sterile gloves (personal choice).
D. Biopsy retrieval:
I) Mark retrieval area, using a standard dermis marker.
II) Skin samples are performed in the area without hair follicles, back-auricular, mastoid or in other concealable regions (folds, furrows or areas of ongoing cutaneous removals during surgical procedures on the face).
III) Disinfect the retrieval area with the antiseptic solution of your choice (e.g.: Chlorhexidine).
IV) Anesthesia application in the periphery area without embedding the donor tissue (e.g.: 2% lidocaine without vasoconstrictor).
V) Shave gently to remove the stratum corneum with a scalpel n.15. -
VI) with a 2.5 / 3.0 mm punch, collect the full thickness skin samples.
Normally, the procedure will require:
• 3 Samples for face
• 4/6 samples for Face + Neck
• 6/7 samples for Face + Neck + Hands
NOTE: Using a 3- or 4-mm diameter dermal punches might require stitches.
E. Sample procurement:
The procedure is standard and extremely simple. A couple of setbacks, related with the small size of the sample, are relatively frequent:
• The tissue might stay inside the incision covered with blood.
• The tissue might stay inside the dermatome (dermal punch).
• It could be extracted with a needle.
• The tissue might get washed away by the blood.
• Mechanical compression is enough as hemostatic technique.
• Exceptionally, a stitch might be required.
F. Sample processing and micrograft solution generation:
I) Place the biopsies inside the Rigeneracons® device, over the hollowed disc under the rotor.
NOTE: Disaggregate maximum 3 skin samples at the time, since you have more add the remaining samples following the same procedure here described.
II) Add exactly 1.5 ml sterile injectable physiologic solution through the specific connector of the Rigeneracons® with a non-Luer Lock® syringe.
NOTE: Sample must slightly embedded, not submerged in it.
III) Close the Rigeneracon® and place it in the Sicurlid, that could be sterilized if needed, couple the device with the Sicurstik.
IV) Place it in the Rigenera® N4SA machine rail into the main body and couple it with the machine rotor.
V) Press the central button located in the machine front. For this specific protocol the sample should be disaggregated for 2 minutes, each pulse lasts 1 minute (1minute/pulse).
NOTE: A green led will indicate that the process has started. It is automatic and it lasts 2 minutes.
VI) After you have disaggregated all the samples needed, collect the micrografts through the specific hole of the Rigeneracons®. Avoid using non-Luer Lock® syringe.
VII) Dilute obtained Micrografts with sterile physiological solution, the dilution deepens on the area to treat:
• Face: 7.0 ml total solution = 140 injections
• Face + Neck: 15.0 ml total solution = 300 injections
• Face + Neck + Hands 22.0 ml total solution = 440 injections
The mesotherapy treatment is performed with a 30/32 G needle, in the deep intra-dermal and superficial sub-dermal (depending on the thickness of the treated regions), with the clinical observation of a series of papules on the skin.
• Useful for this purpose to maintain an inclination of the needle of about 30-45 °.
• For a correct execution of the technique, it is necessary to use 1.0 ml luer-lock syringes with 0.05 ml injections, spaced 1.5 cm apart.
Hemostasis is achieved by mechanical compression. Normally, stitches are not needed. After the procedure, pictures must be taken under the same conditions from the preoperatory. Recommendations:
• Avoid activities that increase blood hydrostatic pressure he first 48 hours: sport, yoga, sauna, etc.
• Wound hygiene should be controlled. Sun protection is mandatory.
NOW YOU ARE USING STERILE GLOVES! STERILE CONDITIONS AND SURGICAL BEHAVIOR FROM NOW ON!
Based on the combined use of the Dermis Micrografting with fractional / Ablative Laser treatments (Erbium-Yag or CO2).
The contextual action of these two methods in a single treatment allows the realization of a complete protocol for the prevention and treatment of facial skin, neck and décolleté aging.
The action exerted by the laser and the synergistic use of the Dermis Micrografts for the bio-revitalization also allows the use of the Laser in a particularly smoother way, with a clear series of advantages for the patient in terms of reducing side effects and complications (erythema, edema, pigmentation), as well as a clear reduction in recovery times.
A. Skin preparation:
Already during the first visit an adequate cutaneous preparation is suggested, generally carried out with the prescription of a specific topical exfoliating product and modulating melanogenesis, 15/30 days prior to the laser treatment.
B. Laser Treatment:
I) The skin conditions are classified with a particular scheme of diagnosis, based on the photo-type diagnosis (simplified Fitzpatrick’s classification) and on the level of the inesthetisms(simplified Rubin’s classification).
II) 30 minutes before starting the treatment a thorough skin cleansing is performed and a thin layer of topical anesthetic cream is applied (only if necessary).
III) At the end of the Laser treatment, while the preparation for the “Dermis Micrografting for Skin Biorevitalization protocol” is starting, it is possible to apply a specific serum / ointment or moisturizing and soothing mask on the cutaneous regions treated with the laser.
C. Skin regeneration treatment
Once the necessary sterile field is set up, proceed with the steps described in “Dermis Micrografting for Skin Bio-revitalization protocol”.
Based on the combined use of the “Dermis Micrografting for Skin Bio-revitalization protocol” with Micro- Needling treatment. Micro-needling is a mechanical anti-aging bio-remodeling method for rejuvenatin the epidermis and correcting various types of skin imperfections. The skin, by means of micro-needles (33G), is subjected to multiple micro-perforations, triggering natural tissue repair mechanisms that induce the production of collagen, hyaluronic acid.
The contextual action of these two methods in a single treatment allows the realization of a complete and synergistic protocol for the prevention and treatment of facial skin aging, neck and décolleté.
The optimal association of the two methods is suggested specifically for the treatment of “sagging skin” of the mandibular profile, of the under-chin region and of the neck.
Especially, it is well indicated in case the patient is considered less suitable for an excessive procedure (like the one implemented with the laser); in this context the patient is ready to receive a milder treatment, still able to create a superficial and deep dermal stimulus, without the longer sequences of erythema and edema typical of laser treatments in such regions of the cervico-facial district.
A. Perform the Micro needling procedure.
B. At the end of the treatment, while the preparation for the “Dermis Micrografting for Skin Bio-revitalization protocol” is starting, it is possible to apply a specific serum / ointment or moisturizing and soothing mask on the cutaneous regions treated
C. Once the necessary sterile field is set up, proceed with the steps described in “Dermis Micrografting for Skin Bio-revitalization protocol”.
Clinical record is mandatory in accordance to local laws and requirements, although this is a minimally invasive medical procedure. Signed consent is required, like in any other aesthetic medicine procedure. Its characteristics will be subjected to the local laws. Thus, it is important that the physician is acquainted with them.
Pictures must follow a previous stablished guideline. Light, distance to focus, zoom, and camera from the pre-treatment photo must be the same for all the follow-up photo(s), so they can be compared.
A. Prepare the patient:
I) Protect the surgical field. Although this is an ambulatory procedure and, could be performed outside an operating room, sterile handling of the sample must be guaranteed at all times.
II) Micrograft will be extracted from the mastoid hairy area, containing hair follicle melanocytes.
B. Prepare the equipment:
I) Machine Rigenera®: The machine must be plugged-in to the electrical power source. Turn it on. Be sure that the engine lever is up.
II) Rigeneracons® device: Peel the protective shield and discard it. Only the Rigeneracons® device, inside the second cover, is sterile.
III) Disposable material: Leave it on an accessory table. The whole set must be ready-to-use and kept in sterile conditions.
C. Prepare yourself:
I) Good light.
II) Goggles (optional).
III) Sterile gloves (personal choice).
D. Biopsy retrieval:
I) Mark retrieval area, using a standard dermis marker.
II) Disinfect the retrieval area with the antiseptic solution of your choice.
III) Anesthesia application in the periphery area without embedding the donor tissue (e.g.: 2% lidocaine without vasoconstrictor).
IV) Shave gently to remove the corneal epithelium.
V) Extract the samples with a 4 mm diameter dermal punch, the procedure will require 3 samples.
NOTE: 4 samples are required for an area of 100cm².
Using 3- or 4-mm diameter dermal punches might require stitches.
E. Sample procurement:
The procedure is standard and extremely simple. A couple of setbacks, related to the small size of the sample, are relatively frequent:
• The tissue might stay inside the incision covered with blood.
• The tissue might stay inside the dermatome (dermal punch).
• It could be extracted with a needle.
• The tissue might get washed away by the blood.
· Mechanical compression is enough as hemostatic technique.
· Exceptionally, a stitch might be required.
F. Sample processing and micrograft solution generation:
I) Place the biopsies inside the Rigeneracons® device, over the hollowed rack under the rotor
NOTE: avoid placing the sample over the rotor.
II) Add sterile injectable physiologic solution through the specific connector of the Rigeneracons® with a non-luer Lock® syringe.
NOTE: Sample must slightly embedded, not submerged in it.
III) Close the Rigeneracon® and place it in the machine adaptor, that could be sterilized if needed.
IV) Place it in the Rigenera® machine rail into the main body and couple it with the machine rotor. Use the machine lateral button to shift the rotor.
V) Press the central button located in the machine front. For this specific protocol the sample should be disaggregated for 2 minutes, each pulse lasts 1 minute (1minute/pulse).
NOTE: A green led will indicate that the process has started. It is automatic and it lasts 2 minutes.
VI) Collect the micrograft solution through the specific connector of the Rigeneracons®.
VII) Avoid using a non-Luer Lock® syringe.
VIII) Increase the volume with sterile injectable physiologic solution.
NOTE: From the extraction of the micrograft to its processing and application, it should not take more than 30 minutes to maintain a cell viability >92%.
G. Application
I) The injection will be made with 30G1/2 needle using the intradermal superficial mesotherapy technique, which creates papules throughout the hypo-pigmentated area.
II) Injections of 0,1ml each cm2.
III) Phototherapy 3 times/week for 6 months after the procedure with UVB.
Volume are to treat is 100cm²the injections should be 0,1ml per 1cm2.
Total number of injections will be: 100cm2*1cm2= 100 injection.
Total volume of the solution will be: 100 Injections * 0,1ml = 10ml.
Dilute the micrografts with sterile saline solution until you obtain 10ml.
Hemostasis is achieved by mechanical compression. Normally, stitches are not needed.
After the procedure, pictures must be taken under the same conditions from the preoperatory
Recommendations:
Avoid activities that increase blood hydrostatic pressure for the first 48 hours: sport, yoga, sauna, etc.


AFTER 4 MONTHS BEFORE

AFTER 12 MONTHS BEFORE


BEFORE


Dr. Casanova Spain
AFTER 9 MONTHS


BEFORE




Dra. Markova
Dr. Shadi
BEFORE



Spain
Spain
AFTER 30 DAYS
AFTER 30 DAYS BEFORE






BEFORE BEFORE
AFTER 6 MONTHS
AFTER 6 MONTHS
AFTER 6 MONTHS

AFTER BEFORE
AFTER BEFORE




AMT® (Autologous Micrografting Technology)

Balli M, Chui J, Athanasouli P et al. Activator protein-1 transcriptional activity drives soluble micrograft-mediated cell migration and promotes the matrix remodeling machinery. Stem Cells International Volume 2019 In press.
Riccio M, Marchesini A, Zingaretti N, Carella S et al. The use of micrografts in the reconstructing full-thickness post-traumatic skin defects of the limbs. a whole innovative concept in regenerative surgery. Stem Cells International Volume 2019 In press.
Balli M, Vitali F, Janiszewski A, Caluwé E, Cortés-Calabuig A, Carpentier S, Duelen R, Ronzoni F, Marcelis L, Bosisio FM, Bellazzi R, Luttun A, De Angelis MGC, Ceccarelli G, Lluis F, Sampaolesi M. Autologous micrograft accelerates endogenous wound healing response through ERK-induced cell migration. Cell Death Differ. 2019 Oct 25. doi: 10.1038/s41418-0190433-3.
Ruiz RG, Rosell JMC, Ceccarelli G, et al. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. J Cell Physiol. 2019;0–0. https://doi.org/10.1002/ jcp.29335
Trovato L, D’Aquino R, Graziano A. Techniques and Processing. In: Regenerative Medicine Procedures for Aesthetic Physicians. ISBN 978 3-030-15458-5 (eBook) https://doi.org/10.1007/978-3- 030-15458-5. Springer Nature Switzerland AG 2019.
Trovato L, Graziano A, D’Aquino R. Injection/Application of Micrografts. In: Regenerative Medicine Procedures for Aesthetic Physicians. ISBN 978-3-030-15458-5 (eBook) https://doi.org/10.1007/978- 3-030-15458-5. Springer Nature Switzerland AG 2019.
Andreone A, den Hollander D. A Retrospective Study on the Use of Dermis Micrografts in Platelet-Rich Fibrin for the Resurfacing of Massive and Chronic Full-Thickness Burns. Stem Cells International Volume 2019, https://doi.org/10.1155/2019/8636079.
Tresoldi MM, Graziano A, Malovini A, Faga A, Nicoletti G. The Role of Autologous Dermal Micrografts in Regenerative Surgery: A Clinical Experimental Study. Stem Cells International Volume 2019, https://doi.org/10.1155/2019/9843407
Uehara M and Shimizu F. Progress report for intractable ulcer and osteomyelitis cases using autologous micrografts. SAGE Open Medical Case Reports 2019; Volume 7: 1 –4
Kawakami S, Shiota M, Kon K, Shimogishi M, Kasugai S.The Effect of Dissociated Soft Tissue on Osteogenesis: A Preliminary In Vitro Study. Int J Oral Maxillofac Implants. 2019 May/June;34(3):651–657. doi: 10.11607/jomi.7021.
Lupi SM, Rodriguez Y Baena A, Todaro C, Ceccarelli G, Rodriguez Y Baena R Maxillary Sinus Lift Using Autologous Periosteal Micrografts: A New Regenerative Approach and a Case Report of a 3-Year Follow- Up.. Case Rep Dent. 2018 Jul 24;2018:3023096. doi: 10.1155/2018/3023096.
Viganò M, Tessaro I, Trovato L, Colombini A, Scala M, Magi A, Toto A, Peretti G, de Girolamo L. Rationale and pre-clinical evidences for the use of autologous cartilage micrografts in cartilage repair. J Orthop Surg Res. 2018 Nov 6;13(1):279. doi: 10.1186/s13018-018-0983-y.
Miranda R, Farina E, Farina MA. Micrografting chronic lower extremity ulcers with mechanically disaggregated skin using a micrograft preparation system. J Wound Care 2018 Feb 2;27(2):60-65.
Ferrarotti F, Romano F, Gamba MN, Quirico A, Giraudi M, Audagna M, Aimetti M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J Clin Periodontol. 2018 Jul;45(7):841850. doi: 10.1111/jcpe.12931.
Francesco De Francesco, Silvia Mannucci, Giamaica Conti, Elena Dai Prè, Andrea Sbarbati and Michele Riccio. A Non Enzymatic Method to Obtain a Fat Tissue Derivative Highly Enriched in Adipose Stem Cells (ASCs) from Human Lipoaspirates: Preliminary Results. Int. J. Mol. Sci. 2018, 19, 2061; doi:10.3390/ijms19072061.
Noda S, Sumita Y, Ohba S, Yamamoto H, Asahina I. Soft tissue engineering with micronized-gingival connective tissues. J Cell Physiol. 2018; 233:249–258.
Hernán Pinto, Rafael Gálvez2, José Casanova. Dermoscopy Is the Crucial Step for Proper Outcome.
Prospection When Treating Androgenetic Alopecia with the Regenera® Protocol: A Score Proposal. International Journal of Clinical and Developmental Anatomy 2018; 4(1):15-18. doi: 10.11648/j.ijcda.20180401.12
Álvarez X, Valenzuela M, Tuffet J. Clinical and Histological Evaluation of the Regenera® Method for the Treatment of Androgenetic Alopecia. International Educational Applied Scientific Research Journal 2018 Jan;3(1): 2456-5040.
Dorta Fernandez A, Baroni Luengo A. Biostimulation of Knee Cartilage Using Autologous Micro-Grafts: A Preliminary Study of the Rigenera Protocol in Osteochondral Lesions of the Knee. Rehabilitation Sciences 2018; 3(1): 8-12.
Pietro Gentile, Giovanna Maria Scioli, Alessandra Bielli, Augusto Orlandi & Valerio Cervelli. Comparing different nanofat procedures on scars: role of the stromal vascular fraction and its clinical implications. Regen Med. 2017 Dec;12(8):939952. doi: 10.2217/rme-2017-0076.
Álvarez X, Valenzuela M, Tuffet J. Microscopic and Histologic Evaluation of the Regenera® Method for the Treatment of Androgenetic Alopecia in a Small Number of Cases. International Journal of Research Studies in Medical and Health Sciences 2017;2(8):19-22.
Rodriguez Y Baena R, D’Aquino R, Graziano A, Trovato L, Aloise AC, Ceccarelli G, Cusella G, Pelegrine AA, Lupi SM. Autologous Periosteum-Derived Micrografts and PLGA/HA Enhance the Bone Formation in Sinus Lift Augmentation. Front Cell Dev Biol. 2017 Sep 27;5:87.doi: 10.3389/fcell.2017.00087. eCollection 2017.
Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig 2017;4:58.
Jimi Shiro, Kimura Masahiko, De Francesco Francesco, Riccio Michele, Hara Shuuji, Ohjimi Hiroyuki. Acceleration Mechanisms of Skin Wound Healing by Autologous Micrograft in Mice. Int. J. Mol. Sci. 2017, 18, 1675; doi:10.3390/ijms18081675.
Ceccarelli G, Gentile P, Marcarelli M, Balli M, Ronzoni FL, Benedetti L, Cusella De Angelis MG.
In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals (Basel). 2017 Jun 13;10(2). pii: E53. doi: 10.3390/ ph10020053.
Lampinen M, Nummi A, Nieminen T, Harjula A, Kankuri E. Intraoperative processing and epicardial transplantation of autologous atrial tissue for cardiac repair. Journal of Heart and Lung Transplantation 2017 Sep;36(9):1020-1022. doi: 10.1016/j.healun.2017.06.002.
Nummi A, Nieminen T, Pätilä T, Lampinen M, Lehtinen ML, Kivistö S, Holmström M, Wilkman E, Teitti-nen K, Laine M, Sinisalo J, Kupari M, Kankuri E, Juvonen T, Vento A, Suojaranta R, Harjula A; AADC consortium. Epicardial delivery of autologous atrial appendage micrografts during coronary artery bypass surgery-safety and feasibility study. Pilot Feasibility Stud. 2017 Dec 20;3:74. doi: 10.1186/ s40814-017-0217-9. eCollection 2017.
De Francesco F, Graziano A, Trovato L, Ceccarelli G, Romano M, Marcarelli M, Cusella De Angelis GM, Cillo U, Riccio M, Ferraro GA. A Regenerative Approach with Dermal Micrografts in the Treatment of Chronic Ulcers. Stem Cell Rev. 2017 Feb;13(1):149. doi: 10.1007/s12015-016-9698-9.
Bocchiotti MA, Bogetti P, Parisi A, Rivarossa F, Frenello A, Baglioni EA. Management of Fournier’s gangrene non-healing wounds by autologous skin micrograft biotechnology: a new technique. J Wound Care. 2017 Jun 2;26(6):314-317. doi: 10.12968/jowc.2017.26.6.314.
Monti M, Graziano A, Rizzo S, Perotti C, Del Fante C, d’Aquino R, Redi C, Rodriguez Y Baena R. In Vitro and In Vivo Differentiation of Progenitor Stem Cells Obtained after Mechanical Digestion of Human Dental Pulp. J Cell Physiol 232: 548–555, 2017 doi: 10.1002/jcp.25452.
Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Reconstruction of Alar Nasal Cartilage Defects Using a Tissue Engineering Technique Based on a Combined Use of Autologous Chondrocyte Micrografts and Platelet-rich Plasma: Preliminary Clinical and Instr umental Evaluation. Plast Reconstr Surg Glob Open. 2016 Oct 26;4(10):e1027.
Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V (2016) A combined use of Chondrocytes Micro Grafts (CMG) Mixed with Platelet Rich Plasma (PRP) in Patients Affected by Pinch Nose Deformity. J Regen Med 5:2. doi: 10.4172/2325-9620.1000129.
Trovato L, Failla G, Serantoni S, Palumbo FP. Regenerative Surgery in the Management of the Leg Ulcers. J Cell Sci Ther 2016; 7: 238. doi:10.4172/2157-7013.1000238.
Marcarelli M, Trovato L, Novarese E, Riccio M, Graziano A. Rigenera protocol in the treatment of surgical wound dehiscence. Int Wound J. 2017 Feb;14(1):277-281. doi: 10.1111/iwj.12601.
D’Aquino R, Trovato L, Graziano A, Ceccarelli G, Cusella de Angelis G, Marangini A, Nisio A, Galli M, Pasi M, Finotti M, Lupi SM, Rizzo S, Rodriguez Y Baena R. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. Journal of Translational Science 2016, 2(2) 125-29 doi: 10.15761/JTS.1000128.
Baglioni E, Trovato L, Marcarelli M, Frenello A, Bocchiotti MA. Treatment of Oncological Post-surgical Wound Dehiscence with Autologous Skin Micrografts. Anticancer Research 2016; 36(3): 975-980.
Purpura V, Bondioli E, Graziano A, Trovato L, Melandri D, Ghetti M, Marchesini A, Cusella de Angelis MG, Benedetti L, Ceccarelli G, Riccio M. Tissue Characterization After a New Disaggregation Method for Skin Micro-Grafts Generation. J Vis Exp. 2016 Mar 4;(109).e53579 doi: 10.3791/53579.
Svolacchia F, De Francesco F, Trovato L, Graziano A, Ferraro GA. An innovative regenerative treatment of scars with dermal micrografts. J Cosmet Dermatol. 2016 15(3):245-53. doi: 10.1111/ jocd.12212.
Trovato L, Monti M, Del Fante C, Cervio M, Lampinen M, Ambrosio L, Redi CA, Perotti C, Kankuri E, Ambrosio G, Rodriguez Y Baena R, Pirozzi G, Graziano A. A New Medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol, 2015;230:2299-303.
Giaccone M, Brunetti M, Camandona M, Trovato L, Graziano A. A New Medical Device, Based on Rigenera Protocol, in the Management of Complex Wounds. J Stem Cells Res, Rev & Rep. 2014;1(3):1013.
Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose Derived Stem Cells and Growth Factors Applied on Hair Transplantation. Follow-Up of Clinical Outcome. Journal of Cosmetics, Dermatological Sciences and Applications. 2014;24:268-274. DOI: 10.4236/jcdsa.2014.44036.
Aimetti M, Ferrarotti F, Cricenti L, Mariani GM, Romano F. Autologous Dental Pulp Stem in periodontal regeneration: A Case Report. Int. J Periodontics Restorative Dent 2014, 34(suppl): s27-s33; doi: 10.11607/prd.1991.
Brunelli G, Motroni A, Graziano A, D’Aquino R, Zollino I, Carinci F. Sinus lift tissue engineering using autologous pulp micrografts: A case report of bone density evaluation. J Indian Soc Periodontol. 2013;17(5):644-7. doi: 10.4103/0972-124X.119284.
Graziano A, Carinci F, Scolaro S, D’Aquino R. Periodontal tissue generation using autologous dental ligament micro-grafts: case report with 6 months follow-up. Annals of Oral & Maxillofacial Surgery. 2013;1(2):20.
Riccardo d’Aquino, Alfredo De Rosa, Vladimiro Lanza, Virginia Tirino, Luigi Laino, Antonio Graziano, Vincenzo Desiderio, Gregorio Laino and Gianpaolo Papaccio. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. European Cells and Materials 2009; 18:73-85
Aimetti M, Ferrarotti F, Mariani GM, Cricenti L, Romano F. Use of Dental Pulp Stem Cells/Collagen Sponge Biocomplex in the Treatment of Non-contained Intrabony Defects: A Case Series. Clinical Advances in Periodontics, 2013; doi 10.902/ cap.2013.130047

Worldwide Distributor
Legal headquarters and office: C. Sant Ferran, Local 10 - 12 08031, Barcelona
Contact information: Tel. +34 93 514 57 56
info@regeneraactiva.com

www.regeneraactiva.com


Legal headquarters: C.so Galileo Ferraris, 63 - 10128 Torino
Offices and Laboratories: Via Pinerolo, 101 - 10060 Candiolo (TO) Tel.: +39 011.993.45.08
www.rigenerahbw.com
