
Enriching the Lives of Zebra Finches
Utilizing enrichment to maintain wild-type behavioral traits
2023 National Meeting Recap
Housing and Maintenance of Woodchucks in the Laboratory Animal Setting
Improved Method for Safe and Effective Injections in Mice
January/February 2024


Looking Forward to the Future by Bob
People & Places
New hires, promotions, awards, memorials
PROfiles
Navigating Export Control Laws in Animal Research: Key Considerations
By Rani Muthukrishnan, PhD
Meet Shawn Kozlov Career & Training
The Power of Neural Rewriting: Enhancing Public Speaking through Brain Transformation
National
Enriching the Lives of Zebra Finches
By Megan Warner-Hough, MS, RLATG
Overcoming the Hurdles of Lab Animal Enrichment: Natural Behaviors vs. Scientific Need
Morgan Mantz, RVT, RALAT, and Anna Pugerud, BA, RVT, RLATG
Precision Cut Lung Slices –A Versatile 3Rs Method for Pulmonary Research
By Julia Herbert, DVM, PhD
TECH TIPS
How Much Wood Could a Woodchuck Chuck if a Woodchuck Could Chuck Wood? Not Much if They’re Provided with the Right Enrichment!
By James Brennan, MLAS, CMAR, LATG
Switching to Vendor Decapsulated Artemia Embryos to Feed Zebrafish: A Cost and Labor Analysis
By Rachel Lenkey, BS, RLATG, Stephanie Slater, BS, RLATG, and Dante D’India, MS, RLATG A Cost-Effective Restraint Method for Safer Subcutaneous Scruff Injections and Reducing Stress in Mice and Handlers
By Anna Fitzwater, DVM and Luwen Zhang, PhD
AALAS
Connections
2024 National Meeting Updates AALAS Foundations
AALAS Foundation Announces Results of 2023 National Meeting Events Ad List
2 Laboratory Animal Science Professional January 2024 FEATURES January/February 2024 Vol. 12 Issue 1 4 6 44 40 46 50 52 54 60 56 10 12 28 32 36 DEPARTMENTS AALAS
Meeting
Recap
2023
Leadership Corner
Quinn
EXECUTIVE COMMITTEE
PRESIDENT
Robert H. Quinn
VICE PRESIDENT
James D. Macy
VICE PRESIDENT-ELECT
Jori K. Leszczynski
PAST PRESIDENT
Pamela A. Straeter
SECRETARY/TREASURER
Timothy D. Mandrell
EXECUTIVE DIRECTOR
Thomas L. Joseph
TRUSTEES
District One
Jennifer L. Asher
District Two
Erin E. Vogelsong
District Three
Donna D. Tignor
District Four
Janet Lynn Steele
District Five
Stacy R. Cantrell
District Six
Stephen I. Levin
District Seven
Adrienne Ferguson
District Eight
Katherine M. Marshall
AT-LARGE TRUSTEE
Debra L. Hickman
Kenneth B. Shapiro
Gordon Yee
Jason S. Villano
Thomas L. Joseph Executive Director Publisher
STAFF
Chris Lyons Associative Executive Director Associate Publisher
Ashlee Vaughn Associate Editor Degan Mesler Production Zara Garza Design
Michael T. Fallon Editor-in-Chief
Heather Lampi Ad Sales
EDITORIAL ADVISORY BOARD
Leslie Birke Louisiana State Univ
Andrew Burich Benaroya Research Institute
Bob Dauchy Tulane Univ School of Medicine
David DeOrnellis Champions Oncology
Penny Devlin Pennsylvania State Univ College of Medicine
Sonia Doss Duke Univ Medical Center
Kelly Ethun Emory University
Glenn Jackson Cornell University
Richard Marble Alpha Genesis Inc.
Elizabeth Nunamaker Charles River Laboratories
MISSION STATEMENT
Sara Oglesby Abbvie
Jane Olin Edwards Life Sciences
Karuna Patil Seattle Children's Research Institute
Amy Pierce Tulane Univ School of Medicine
Stacy Pritt The Texas A&M University System
Laboratory Animal Science Professional (LAS Pro) is the official magazine for American Association for Laboratory Animal Science members. LAS Pro provides a wide range of useful resources and knowledge to the association’s 15,000 laboratory animal science professionals who are involved in advancing responsible laboratory animal care and use to benefit people and animals. All signed articles, including, committee reports, news, and commentary, reflect the individual views of the authors and are not official views of AALAS.
Authorization to photocopy portions for personal or internal use is granted by the American Association for Laboratory Animal Science. Photocopying for purposes of resale or outside distribution is prohibited unless written approval is obtained from the AALAS Director of Communications.
Copyright 2024 by the American Association for Laboratory Animal Science.
Laboratory Animal Science Professional (USPS 010-730) is published bimonthly by the American Association for Laboratory Animal Science, 9190 Crestwyn Hills Drive, Memphis, TN 38125. Periodicals Postage paid at Memphis, TN 38101 and additional mailing offices. POSTMASTER: Send address changes to AALAS, 9190 Crestwyn Hills Drive, Memphis, TN 38125-8538.
HOW TO CONTACT AALAS
AALAS, 9190 Crestwyn Hills Drive, Memphis, TN 38125-8538
Phone: 901-754-8620 • Fax: 901-753-0046 • Email: info@aalas.org • Web: www.aalas.org

January 2024 Laboratory Animal Science Professional 3
THE LEADERSHIP CORNER
Looking Forward to the Future

I cannot believe I’m almost 2 months into my presidency already as I write this. The time is flying by quickly and there is just never enough time to complete all of the things I would like to during this single year. I’ve just returned from the FESAHANCCCAL/APCAL meeting in Panama City, Panama. I want to sincerely thank the meeting organizers for the opportunity to meet this amazingly warm and welcoming group of people attending from multiple central American countries. I made it challenging for them with no working knowledge of Spanish, but everyone was so understanding and helpful that I never felt like the outsider that I was.
In this issue we are focusing on environmental enrichment (EE). After many years of the general requirement for EE and many scientific studies looking at the effects of EE, it is clear that our animals benefit from having more than just a simple environment that meets their basic biological needs. In fact, many studies currently being conducted in neuroscience are revealing that animals appear to recover better from brain injuries or diseases when they are put into complex, extra-enriched environments. When we first started putting rodents into microisolator cages to protect them from disease and control exposure to variables that might affect research, these cages were often just bare environments with bedding, food and water. The thought back then was that controlling variables was the most important thing when it came to ensuring consistent research results. Nobody thought about how this very basic environment might be affecting the animal’s mental or emotional state. We now know that mental and emotional factors can have a huge influence over biology and response to disease. Although we are seeing increasing data on the benefits of an enriched environment, particularly in behavioral research, we still don’t have a really good understanding of how EE may affect different types of studies or how variations in the types and amounts of EE between cages may affect research results. This is an area that definitely needs more study, but in the mean time I believe we should always err on the side of creating a comfortable and stimulating environment for our animals as long as there is no clear evidence of it being detrimental to the animal or the research.
I hope to meet many of you in my upcoming travels to our many wonderful AALAS branch and regional meetings. Hearing all your ideas and thoughts about AALAS and our field makes my job as president so fulfilling and, as I said at the start, the time just flies by.
 Robert H. Quinn, DVM, DACLAM President American Association for Laboratory Animal Science
Robert H. Quinn, DVM, DACLAM President American Association for Laboratory Animal Science
4 Laboratory Animal Science Professional January 2024





Certification Showcase!

Anikka Smith, BS, RLATG
Madison Earnheart, RALAT
"Earning my certification has been something I have worked on for the last two years. This has been one of the things I wanted to accomplish in 2023 and seeing the word "pass" pop up on the screen was a feeling I couldn't describe. I proved to myself that I could do it and I accomplished something I thought was impossible for me to reach when I first started my career back in 2020. Thank you to all of those who pushed me to get where I am today, I couldn't have done it without you!"
This is a major accomplishment for me as I know the personal, physical-health struggles (Multiple Sclerosis) I was combatting during the first time I attempted to take the test. Failing the test was a major dig at my confidence, and I was very hard on myself. It took me some time to build up the confidence to take it again. Once I was in a better place physically and mentally, I was ready to finally conquer the beast of “failure”and take the LATG! Taking it the second time felt so much more natural and the answers were flying from my fingers as I checked the boxes! Obtaining my LATG is a testimony to myself that not only am I a knowledgeable professional in my field, but also that I can do hard things. It was a reminder to give myself grace and that even though we fall 9 times, we get up 10. ( A little MS humor, IYKYK. )


Stephanie Ventura, CMAR
Obtaining my CMAR certification allows me to offer well rounded knowledge in my management role in combination with my certification as a Veterinary Technologist. (CVT). I look forward to using this certification to advance in my career.
6 Laboratory Animal Science Professional January 2024 PEOPLE & PLACES - NEW HIRES, MEETING UPDATES, AND MEMORIALS
Chetna Patel, CMAR, LATG
My career in lab animal science began in 2018 as a supervisor over cage wash at UT MD Anderson Cancer Center, Houston, after moving countries and states and 20 years of managing in commercial animal production. I started my certification journey at the beginning, with ALAT with an end goal to attain CMAR as soon as I had 5 years of lab animal experience, knowing this would allow me to develop my knowledge and offer opportunities for growth in this vast, progressive field.


Yan Yuxi, BVM, RLATG, CMAR
The entire process of obtaining my CMAR (Certified Manager of Animal Resources) was very rewarding. Not only did I gain a deep understanding of animal facilities and resources, but I also learned valuable management knowledge. This has been of great help in my professional career.
Johnny R. Green, BS, CMAR, LATG
My CMAR journey was arduous but was fueled by my desire to achieve to my highest ability. With Phillip Massinger’s quote “A willing mind makes a hard journey easy,” resonating in my mind, I began studying after I had begun to feel stagnant in my career. I heavily relied on my years of experience in the field, the knowledge I had ascertained from mentors and peers, and the sheer desire to succeed in every facet of life that presents itself to me, to continue the road to completion. With each of the CM tests I passed, I had to realize that they were only stepping stones and the full CMAR designation was my terminal goal. I thank my family for their patience and support, allowing me time to focus on our bigger picture and while I’m ecstatic to have received the CMAR certification, I am always ready for the next path that opens for me so that we can share in the fruits of the teamwork we have established.

January 2024 Laboratory Animal Science Professional 7
New Faces at AALAS
 Degan Mesler Digital Media Marketing Strategist
Degan Mesler Digital Media Marketing Strategist
Degan Mesler is our new Digital Media Marketing Strategist at AALAS. She received her Master's in Sociology from The University of Memphis and is currently ABD towards her Ph.D in Communications at The UofM. She will be handling all of our communication and marketing efforts at AALAS and hopes to bring a modern and newly recognizable branding strategy to all of our digital media materials. When she's not busy raising her two year old, she likes to watch obscure movies with her husband or work on writing fiction.
Simon Tucker
Multimedia Design Coordinator
Hi, I’m Simon, and I am the Multimedia Design Coordinator for the AALAS Mar/Com team. I work on animated content, like the sort you’ll soon see on our various social media pages, as well as digital event production like our recent Demo Days webinar. I’m excited to continue working with my awesome team, and you might see me around if you’re at ILAM or National Meeting!
A fun fact about me is one of my siblings made it onto the Jimmy Kimmel show! We were in Hollywood on vacation and got pulled into the studio. My sibling got to audition and made it to the final round of a skit with Jimmy, and was ultimately picked to be on the show!

8 Laboratory Animal Science Professional January 2024 PEOPLE & PLACES - NEW HIRES, MEETING UPDATES, AND MEMORIALS


Getting Personal
What companion animals do you have?
Currently four dogs including a treeing walker coonhound, a bluetick coonhound, and two staffy mixes. The coonhounds came with my wife so please don't judge me.
Best binge-watching TV series?
Currently going back through Newsroom, Aaron Sorkin is wonderful.
What is the last book you read?
Leviathan Wakes by James S. A. Corey and what the TV show The Expanse was based on.
Where is your dream vacation spot?
The Colorado Mountains, luckily I live here now.
What is your favorite dessert?
That is a toss up between an Islay Scotch and a slice of pie, both would be great.
5
LAS PRO-files minutes with... Shawn Kozlov
Facility/Employer: Inotiv Co, Fort Collins, CO
Job Title: Laboratory Animal Research Veterinarian
How did you get in this field?
Coworkers of mine from an emergency veterinary clinic worked full-time at the NIH and recruited me to come work as an ICU veterinary technician. I was nervous at first because I knew very little about using animals in research but the hours were better and so was the pay. I quickly learned that my skills could be used to provide high quality care for animals helping to cure kids of cancer, save adults from coronary disease, and develop vaccines for diseases around the world; I never looked back.
Who were your mentors?
While there have been many mentors throughout my career, I can say that one in particular help me get to where I am today. Dr. Robert Hoyt was the AV with the National Heart, Lung, and Blood Institute and chief surgeon when I left the ICU and began working with NHLBI. Dr. Hoyt encouraged me to learn surgical skills, explore new anesthesia protocols, and used his educational budget to help me finish my undergraduate degree while working full time. Because of his encouragement and assistance, I completed my undergraduate degree and eventually went onto veterinary school.
What are your current interests in animal science? This may sound bad but I have little interest in doing my own research. I have learned that my passion for animal science comes from helping PIs and Sponsors to successfully complete their research goals. I love working in the surgical research field with an emphasis on medical device testing where I can tailor anesthetic protocols to each surgery for the best possible intra-op and post-operative outcomes. My heart is filled with joy every time I walk into our recovery room and see post-op animals up and moving around like nothing ever happened.
Where do you see yourself in 5 years?
I hope to still be working in the medical device field or perhaps another aspect of surgical research that will allow me to continue to apply my skills in anesthesia to obtain excellent research results. I am also interest in policy
and regulator medicine so could end up on the other side of the research review application for the FDA or finding my way into another regulatory agency such as PHS or USDA.
What is your favorite part of your job?
Tailoring anesthetic protocols to each individual research project instead of using a standard template is one of the most fulfilling parts of my job. Using a mix a local/regional blocks and multimodal approaches to anesthesia and analgesia management has led to less complications in the operating room and smoother recoveries for our animals.
What advice do you have for others just beginning their animal science career?
Ask questions. Scientist love to talk about their research and nothing helps you understand what is going on with the animals more than hearing them discuss their studies. Knowing why a particular mouse is needed can help you understand why certain husbandry is needed. Asking questions can lead to opportunities to learn new procedures, different lab techniques, and expose you to different aspect of science you may not have known about. Being curious in this field can lead to a number of doors opening for future career development.
What is the most rewarding aspect of your career?
I was a research technician for many years and had the privilege or learning many different surgical and technical skills that I now enjoy passing onto our research technicians. Seeing someone complete a challenging technical task for the fist time and know that they can now do it is a wonderful feeling.
If you’ve attended an AALAS National Meeting, share a memorable experience.
I have had the privilege to work as an instructor for the microsurgical workshops put on by Dr. Robert Hoyt at AALAS for many years. One year, while in Salt Lake City we found a yard sign for a local election that read “I’m for Bob!” and we couldn’t help but grab one for our beloved boss Dr. Hoyt. The instructors wrote messages on the sign that we planned to present to Dr. Hoyt at the workshop. Our live animal workshop was happening at the University of Utah and unfortunately, a group of strangers showing up with a “picket sign” caused a little bit of commotion that morning.
10 Laboratory Animal Science Professional January 2024


NUTRITIONAL ENRICHMENT MEDICATEDSPECIAL NEEDS Advancing Science. Enriching Animals. ISO 9001:2015 Certified Quality System 800-996-9908 U.S. & CANADA | 908-284-2155 INTERNATIONAL www.bio-serv.com

A A L A S 2023

74TH NATIONAL MEETING
RECAP NATIONAL MEETING
OCTOBER 22-26 SALT LAKE CITY, UT
The 2023 AALAS National Meeting was held in Salt Lake City, Utah from October 22 - 26, 2023. Total registration for the meeting was 3,488. We had 232 exhibitors participate this year, including 30 first time exhibitors! This year we again partnered with VRL Laboratories to bring you Zelda the zebrafish, our AI Chat Bot.
Facilitator Winners
The free registration to next year's meeting in Nashville was awarded to Sarah Choi, RLAT
$50 gift certificate to AALAS Bookstore were awarded to Michelle Adams, CMAR, RLATG and Juan Slaughter, RLAT
Poster Winners
Animal Welfare, Training, & the 3R’s
• Third Place:
P106-Cisterna Magna Ports in Rhesus Monkeys
Then and Now
First author: Brad E Smith
BE Smith*, DB Gilberto, HA Corcoran, GR Sitko, JM Beech, JA Werner, JL Hughes, WE Smallridge, LK Handt, TL Gray, SL Motzel
• Second Place:
P147-Assessing Stress Impact and Subcutaneous Dosing Effectiveness of a Scruffing Restraint Device in C57BL/6J Female Mice
First author: Kaitlyn E Benjamin KE Benjamin*, J Dunbar, H Kim
• First Place:
P124-Click It To Trick It: Sheep Training and its Positive Impact on Long-Term Residency
First author: Breanna L Morones BL Morones*
Clinical Winners
• Third Place:
P246- Secure Intravenous Ear Catherization Method for Pigs
First author: Adrianne Kaiser-Vry A Kaiser-Vry*, AP Kress, G Dell’Anna, MB Sauer
• Second Place:
P223- Enhanced Surgical Site Infection Prevention Protocols Improve Outcomes in Swine (sus scrofa domestica)
First author: Claire Brown C Brown*, K Patil, T Garcia, R Uthamanthil
• First Place:
P203- A novel method of health monitoring in laboratory zebrafish
First author: Amanda K Darbyshire AK Darbyshire*, V Johnson, A Horvath, A Leon

Husbandry and Management Winners
• Third Place:
P324- Utilizing Cage-Side Scales for Efficient Weight Collection in Non-Human Primates (NHPs): A Comparative Analysis
First author: Andrea Calvillo A Calvillo*
• Second Place:
P323- Mouse Allergen Exposure on Single-Use Versus Multi-Use Disposable Gowns: A Pilot Study
First author: Ayush Kumar A Kumar*, WO Williams, E Moore, B Singh
• First Place:
P312- Creation of a Premature Piglet PICU (Piglet Intensive Care Unit) Using Common Vivarium
Supplies
First author: Shawn Kozlov
S Kozlov*, K Stockton, B Wood
Lab Investigations Winners
• Third Place:
P423- Comparison of novel and traditional bleeding techniques in neonatal and juvenile mice
First author: Rebecca Prentiss
R Prentiss*, K Lamont, B Bollinger, K Gaston, C Fletcher, IA Galex
• Second Place:
P425- Interaction of juvenile stocking density and larval diet on adult size and sex differentiation in the laboratory zebrafish (Danio rerio).
First author: Candace Sparkman
C Sparkman*, J Darling, D Kennedy, L Schur, H Engle, E Liechty
• First Place:
P440- Validation of a fecal corticosterone metabolite immunoassay to assess changes in basal stress in the laboratory zebra finch (Taeniopygia guttata)
First author: Alanna G Backx
AG Backx*, A Wu, MS Fee, N Fabian
12 Laboratory Animal Science Professional January 2024




January 2024 Laboratory Animal Science Professional 13






14 Laboratory Animal Science Professional January 2024







January 2024 Laboratory Animal Science Professional 15





16 Laboratory Animal Science Professional January 2024






January 2024 Laboratory Animal Science Professional 17







18 Laboratory Animal Science Professional January 2024








January 2024 Laboratory Animal Science Professional 19



20 Laboratory Animal Science Professional January 2024


January 2024 Laboratory Animal Science Professional 21





22 Laboratory Animal Science Professional January 2024




January 2024 Laboratory Animal Science Professional 23






24 Laboratory Animal Science Professional January 2024






Small Animal Research Solutions for:
Low-Flow Anesthesia

Physiological Monitoring



























Noninvasive Blood Pressure
Ventilators
Animal Warming













Surgical Platforms






























































































kentscientifi c.com
2023 AALAS AWARD WINNERS
Marcel I. Perret-Gentil, DVM, MS


University Veterinarian & Director
The University of Texas at San Antonio
Nathan R. Brewer Lifetime Achievement Award
Dr. Perret-Gentil is University Veterinarian and Director at the University of Texas at San Antonio as well as Attending Veterinarian for four other institutions. He is President of Perret-Gentil Lab Animal Veterinary Services. He has contributed to 67 papers, articles, abstracts and book chapters and has delivered 494 special presentations. He has held 35 consulting positions including governmental, biotechnology, and institutions of higher education in the U.S. and abroad. He serves as ad hoc Specialist for AAALAC International. His passion is in helping programs worldwide with limited resources develop into robust animal care and use programs.
Victoria K. Baxter, DVM, PhD, DACLAM

Assistant Professor
Texas Biomedical Research Institute
Pravin N. Bhatt Scientific Investigator Award
Dr. Baxter received her BS degree in genetics and her DVM from Texas A&M University. She then completed a postdoctoral fellowship in laboratory animal medicine and a PhD in viral immunology from Johns Hopkins University School of Medicine. After serving as a faculty member and the biocontainment veterinarian at the University of North Carolina at Chapel Hill, Dr. Baxter joined the faculty at Texas Biomedical Research Institute in 2022. Her research primarily focuses on studying the pathogenesis of emerging viruses and understanding how genetic and environmental factors affect animal research studies in order to improve the translatability of animal models.
Craig L. Franklin, DVM, PhD, DACLAM

Professor of Veterinary Pathobiology University of Missouri
Charles River Prize
Dr. Craig Franklin received his DVM and PhD from the University of Missouri, where he is currently a Professor of Veterinary Pathobiology and Co-Director of the Mutant Mouse Resource and Research Center. His research, which has resulted in approximately 140 manuscripts and 250 posters or seminar presentations, centers on refinement of rodent models for optimal reproducibility and translatability, most recently focusing on the role of complex microbiota in model phenotype modulation. As Director of the Comparative Medicine Program and Veterinary Research Scholars Program, he has advised over 90 post-DVM trainees and 700 veterinary students exploring and pursuing biomedical research careers.
Coralie Zegre Cannon, DVM, DACLAM

Corporate Director and Attending Veterinarian
Becton Dickinson
Charles A. Griffin Award
Dr. Coralie Zegre Cannon earned her veterinary degree from North Carolina State College of Veterinary Medicine, completed post-doctoral training at National Institute of Environmental Health Sciences and University of North Carolina at Chapel Hill, and earned Diplomate of the American College of Laboratory Animal Medicine. Dr. Cannon is Corporate Director and Attending Veterinarian at Becton Dickinson medical device and biotech company with global responsibility for all laboratory animal programs. She is an AAALAC Ad Hoc Consultant and past president of North Carolina Academy of Laboratory Animal Medicine. Her passions include developing laboratory animal professionals, practicing clinical medicine and teaching as adjunct professor at NC State College of Veterinary Medicine.
Kiirsa Pokryfke, MS, CMAR

Managing Director of the Training Core and Technical Services University of Michigan
George R. Collins Education and Training Award
Kiirsa Pokryfke is the Managing Director of the Unit for Laboratory Animal Medicine (ULAM) Training Core and Technical Services Teams at the University of Michigan. In her 12+ years in this role, Kiirsa has developed and managed the training program that services approximately 3800 research and ULAM personnel. Previously, Kiirsa Pokryfke was employed at a contract research organization and developed their first training program. She earned her B.S. in Biology from Western Michigan University and completed a graduate degree in Strategy, Management and Leadership from Michigan State University.
Shandell Solbach, BS, RLATG, CMAR, ILAM

Laboratory Animal Manager Genetech
Joseph J. Garvey Management
Award
Ms. Solbach received her degree in Wildlife, Fisheries & Conservation Biology from the University of California, Davis in 1996. She immediately entered the laboratory animal field and worked as an Animal Resources Supervisor at the University for 14 years. She joined Genentech in 2010, supporting gRED Animal Resources as a Laboratory Animal Manager in Animal Care. She enjoys serving on the Genentech IACUC and Community Emergency Response Team. She is a Sacramento Valley (SV-AALAS) branch member, serving 5 years in various board positions, and honored with the 2007 Technician of the Year award. A dedicated National AALAS member since 1999, proudly achieved RLATG (2004), CMAR (2014), and graduated ILAM (2017).
Jennifer Crosby, BS, RLAT


Breeding Colony Specialist University of Michigan
Technician of the
Year Award
Jennifer Crosby is a Breeding Colony Specialist at the University of Michigan ULAM. She earned her Bachelor of Science in Animal Science at Michigan State University. Jennifer manages several colonies of immunocompromised mice for investigators across campus and recently completed a platform project on how to improve weanling survival in immunocompromised mouse strains. Jennifer is passionate about improving animal welfare practices by attending workshops and webinars. She plans to continue to grow in her professional role as a technical leader and advocate for both animals and humans alike. Outside of work, Jennifer enjoys listening to music, gardening, cooking, and fishing.
Think Stainless
Think quality.
Think durability.
Think custom.
Make it easy.
Think Ancare.










Better Products. Better Science. www.ancare.com
Enriching the Lives of Zebra Finches
By Megan Warner-Hough, MS, RLATG
Zebra finches are an uncommon animal model in the biomedical industry but are widely used in language development, memory, and learning studies. Native to Australia, they are desert birds that like warm days and 40%+ relative humidity. As a social species, they should be kept in aviaries of small or large groups with enrichment that helps them maintain and exhibit wild-type behavioral traits. The Guide for the Care and Use of Laboratory Animals emphasizes that enrichment can enhance animal well-being.4 As with all laboratory animals, efforts need to be made to provide enrichment and stimulation.
Perch swings
Zebra finches enjoy having areas to perch at different heights.8,9 More perches allow for a variety of options as well as increased availability for exercise in a limited space area.3 One or two perch swings are provided, depending on the number of birds per cage. The swinging nature of the perch mimics the wild behavior of perching on a twig or grass stalk (Figure 1). Caution is taken to place the swinging perches slightly off to the side of the cage so as not to inhibit the free-flying area.
Millet Spray
In the wild, zebra finches primarily consume seeds. This includes foraging for them on plant stalks and on the ground.11 Millet spray, a source of minerals and protein, has long tendrils of densely packed clusters of tiny seeds along the stalk.10 The stalks of millet are hung in plastic feeders that allow the birds access from all sides (Figure 2). The feeders are hung in the cage close enough to the side and perch that they can rest easily while eating but are not stationary. This mimics a more natural method of feeding. Providing millet spray can also help reduce the incidence of obesity and provide the opportunity for mental stimulation.2
Baths
Regular bathing time has been shown to be essential to the quality of life in zebra finches as they can have elevated stress levels when no access to bathing water is regularly granted.5 Water also helps the males clean their feathers, which can help facilitate their mating rituals as they rely primarily on their brightly colored feathers for mate attraction. The colony cages are provided access to baths weekly, based on recommendations.9 A small plastic tub is placed in the cage for 24 hours, allowing the birds enough time to drink from it and take a bath (Figure 3). The risk of drowning must be considered when providing a bath for birds that have fledglings.


28 Laboratory Animal Science Professional January 2024
FEATURE – LAB ANIMAL ENRICHMENT
Figure 1. Zebra finch swinging on a 3” Living World wooden perch swing.
Figure 2. Piece of millet hanging in a JW Pet Insight Millet Spray Holder.


Nesting Material
Male zebra finches collect nesting material while the female uses that material to shape the nest. They will use multiple types of nesting material in the wild, including wild grasses or feathers.7 Due to the nature of the colony room and space constraints, the zebra finches are kept in non-standard temperature and humidity conditions that can stimulate breeding (standard conditions are 12h:12h light cycle, 20-25°C, 40%+ RH8, with breeding conditions being set at 14h:10h, 23-25°C, 50%+ RH and regular greens provided). This means that the males, and sometimes the females, are exhibiting nest-building behaviors. Because of this, individuals will search for nesting material if none is provided, which can include feathers from other birds.

A mix of nesting material is provided to all cages consisting of jute fibers, cotton, sisal fibers, and coconut fibers. The mix allows the birds to pick the type of material they prefer.6 The nesting material is placed in a plastic holder off the ground to prevent contamination, waste, and underuse (Figure 4). If the nesting material was placed on the ground, it would be removed three times a week when the papers that line the cages are removed. The nesting holder prevents the nesting material from being consistently removed and needing to be replaced. Placing it on the floor also allows it to be pushed into the edges of the cage, where it can fall out of the cage. A consistent supply of nesting material reduces the amount of feather plucking, which can also be attributed to aggression or other factors and each cage is monitored to ensure the birds are not being bullied.9 A bird that is being bullied is removed and held in an isolation cage for 24 hours, which is placed directly in front of a new cage. Because they have time to interact through the cage bars, through both visual and audio means, this substantially reduces or eliminates the amount of aggression the new bird receives when entering a new pecking order. The nests, often made in a perch cup, are left until they are changed out on the standard cleaning schedule (Figure 5).
January 2024 Laboratory Animal Science Professional 29
Figure 3. Zebra finch bathing in Hagen plastic bird bath dish filled with lukewarm tap water.
Figure 4. A mixture of nesting material (jute fibers, cotton, sisal fibers, and coconut fibers) stored in a S.T.A Nesting Material Holder.
Figure 5. A nest built inside of a muffin liner, nested in a stainless steel seed cup that is used as a perch cup.


Perch cups
Empty seed cups are provided as an area for the birds to make nests, perch, and sleep in (Figure 6). The perch cups are lined with muffin liners. This provides a comfortable place for the birds to sit while allowing for them
to be removed when dirty, which reduces the frequency of the cups needing to be changed. They have been seen sitting, laying eggs, or sleeping with another bird in the empty perch cups as well (Figure 7).
Lettuce
Zebra finches are known to consume fresh, leafy greens in large quantities.1 This can be provided to zebra finches as an extra food supplement item, providing both novel enrichment (if not supplied daily) and essential nutrients. As well, regular provision of greens will stimulate breeding.9 The zebra finch breeding season typically starts when the rainy season begins, which brings about an abundance of green sprouts that they love to consume.
Megan Warner-Hough, MS, RLATG is a Vivarium Manager at Wellesley College in Wellesley, MA.
Figure Images. All photographic images were taken by Megan Warner-Hough, MS, RLATG REFERENCES
1. Allen LR, Hume ID. 1997. The importance of green seed in the nitrogen nutrition of the Zebra Finch Taeniopygia guttata. Austral Eco. 22:412–418.
2. Gray G. 2016. Enhancing the welfare of zebra finches through the use of environmental enrichment. Anim Tech and Welfare, 15:147–150.
3. Hawkins P, editor. 2001. Laboratory birds: refinements in husbandry and procedures. Lab Anim. 35: Supp 1.
4. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
5. Krause ET, Ruploh T. 2016. Captive domesticated zebra finches (Taeniopygia guttata) have increased plasma corticosterone concentrations in the absence of bathing water. App Anim Behavior Sci. 182:80–85.
6. Lehtonen TK, Helanterä H, Solvi C, Wong BM, Loukola OJ. 2023. The role of cognition in nesting. Philos Trans R Soc Lond B Biol Sci. 378.
7. Muth F, Healy SD. 2011. The role of adult experience in nest building in the zebra finch, Taeniopygia guttata. Anim Behavior. 82:185–189.
8. Olson CR, Wirthlin M, Lovell PV, Mello CV. 2014. Proper care, husbandry, and breeding guidelines for the zebra finch, Taeniopygia guttata. Cold Spring Harb Protoc. 12:1243-1248.
9. RSPCA, Research Animals Department. [Internet]. 2011. Zebra finches: Good practice for housing and care, 4th edition. Available at: https://www. rspca.org.uk/documents/1494935/9042554/Zebra+finches+%282011%29+%28PDF+410KB%29.pdf/2609d4bff960-77cf-33c5-c29287a92b9d?t=155766508271
10. Sachdev N, Goomer S, Singh LR, Pathak VM, Aggarwal D, Chowhan RK. 2023. Current status of millet seed proteins and its applications: A comprehensive review. App Food Research. 3.
11. Vriends M. 2004. Feeding finches the conventional way. Am Fed of Aviculture. 31:16–20.
30 Laboratory Animal Science Professional January 2024
Figure 7. Eggs were laid inside of the muffin liner placed in a perch cup.
Figure 6. A female bird sitting on her eggs in a perch cup.

Overcoming the Hurdles of Lab Animal Enrichment: Natural Behaviors vs. Scientific Need
Morgan Mantz, RVT, RALAT and Anna Pugerud, BA, RVT, RLATG
Introduction
The Animal Resources Core (ARC) at the Abigail Wexner Research Institute (AWRI) at Nationwide Children’s Hospital (NCH) faces many of the same challenges other institutions manage in terms of enrichment for laboratory animals. A Behavior Assessment Team (BAT) was developed to mitigate some of these challenges in the ARC. The BAT assesses, manages, and treats behavioral concerns for all species by providing adequate enrichment without impeding research outcomes. As previously reviewed1, the term “environmental enrichment” for research animals was first used in 1962.2 While the opportunities for developing and improving enrichment for laboratory animals are abundant, it is a complicated subject that can include opinions on both data driven and anecdotal driven benefits. Environmental enrichment should be used to promote expression of species-specific behavior, enhance animals’ psychological and physiological well-being, and aid in preventing abnormal behaviors.5
Aquatics
Providing enrichment for zebrafish has proven more challenging than other more traditional laboratory animal species due to the limitations and novelty when verifying the efficacy of enrichment.3 The ARC’s primary sources of enrichment for zebrafish are social housing and live feeds. Alternative enrichment includes plastic plants and gravel stickers placed under the tanks, but these reduce visibility of the fish, must be regularly cleaned, and require additional staff time to maintain them. The ARC utilizes live plants when zebrafish are singly housed or when tank housing density is low.
Rodents
Rodent enrichment is a widely known area of laboratory animal care and most facilities are familiar with standard products available such as huts, LifeSpans™ (Lab Products, LLC), plastic tubes, and social housing. Rodents are nocturnal, agile, and active creatures that spend time exploring, foraging, and nesting, among other behaviors.4 Mimicking the natural environment while still accommodating the research needs remains a challenge for rodent enrichment. Strain constraints and study specific
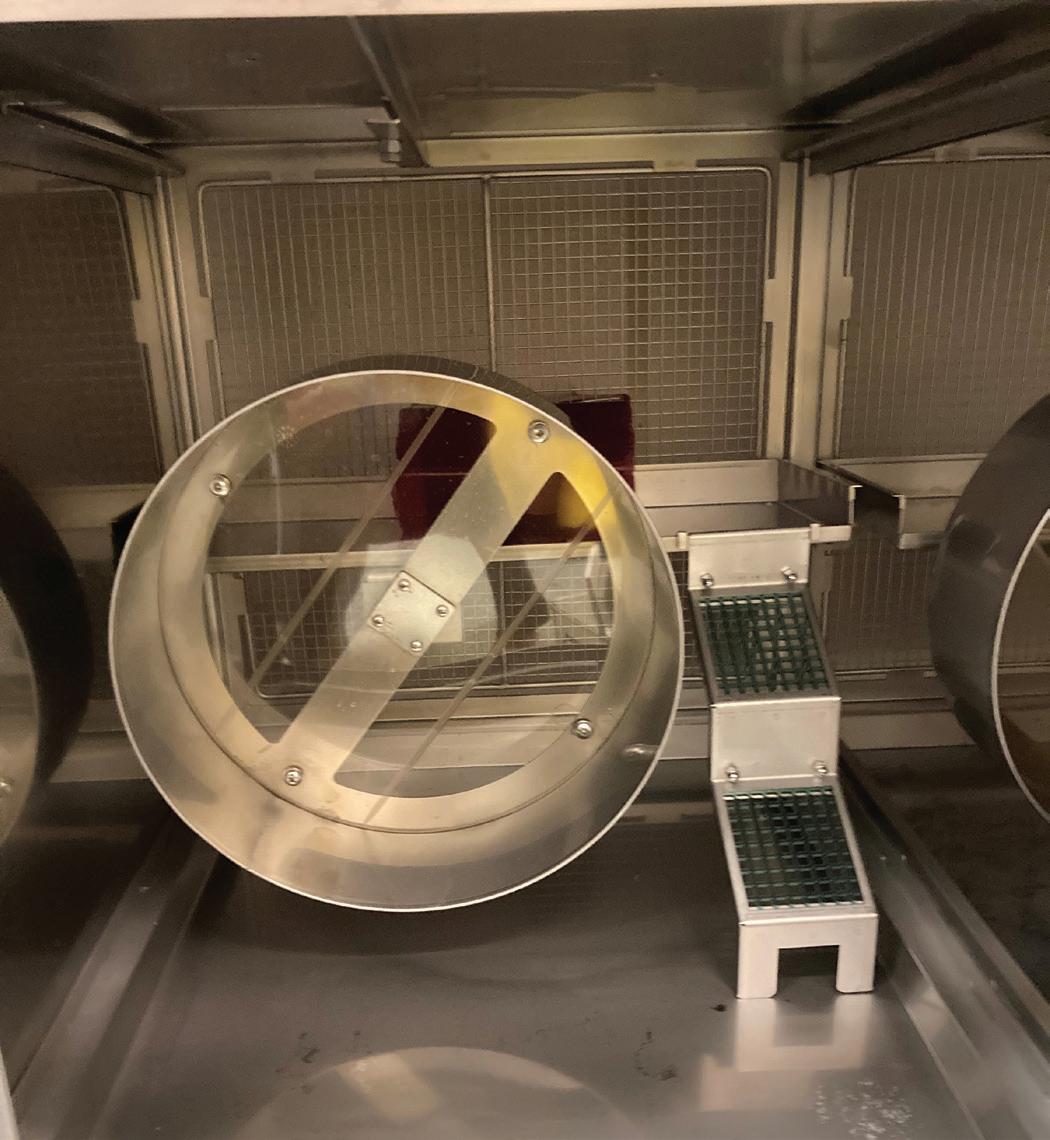
issues can restrict the type and amount of enrichment. In some cases, rodents may develop abnormal or detrimental behaviors due to their surroundings and enrichment may be used to try and alleviate these symptoms.6
Chinchillas
Chinchillas are social, nocturnal animals that normally live in large colonies. They are known for burrowing, jumping, and perching and need access to items for chewing to wear down teeth. Group housing is often limited due to research requirements, so several alternative methods of enrichment have been tested. The ARC purchased new caging which includes areas for perching, climbing, wheels that allow exercise, and options for group housing (Figure 1). Foraging boxes made from cardboard boxes and stuffed with hay and chewing blocks have also been successful in providing enrichment. Dust baths are also provided frequently to help maintain cleanliness and mimic natural behaviors.
32 Laboratory Animal Science Professional January 2024
FEATURE – LAB ANIMAL ENRICHMENT
Figure 1. Chinchilla cage.

Rabbits
Rabbits are social animals that will often engage in social grooming and enjoy digging, jumping, gnawing, and foraging. Our current studies utilizing rabbits require individual housing due to study restrictions. Since the Guide for Care and Use of Laboratory Animals5 states that social animals should be housed in stable pairs or groups of compatible individuals unless they must be housed alone for experimental reasons or because of social incompatibility, single housing was approved by the Institutional Animal Care and Use Committee (IACUC) and rabbits are required to have extra enrichment. ARC rabbits are provided foraging boxes, dig boxes, huts, lofts, gnawing devices, floor exercise, and positive human interaction (Figure 2).

Pigs
Pigs are inquisitive and social animals known for their highly developed olfactory signals that allow them to interact with their environment through exploration and
sniffing. The ARC prefers to socially house our swine, but they do occasionally require IACUC approved individual housing due to study related concerns. In those cases, we have noticed the pigs benefit the most from daily human socializations. Novel enrichment is provided by offering foraging boxes, smoothies, ice blocks, TV, mirrors, music, and opportunities to paint, run in the hallway, and swim in a baby pool (Figure 3).

Ruminants
Sheep and goats are herd animals with a social hierarchy that can lead to incompatibility among groups. All herds in the ARC are grouped based on personality, weight, and age to decrease aggressive behaviors. Ruminants typically forage, which the ARC promotes with foraging boxes (Figure 4), ice blocks, or smearing fruit and vegetable mixtures on the walls and toys. Ruminants are very inquisitive and use their lips and mouths to touch and sense their surroundings. Depending on the size of the herd, ruminants are provided 5-15 hanging toys for inquisitory play and 1-3 floor toys for hiding treats. All ruminants are on a rotating schedule that allows them to explore and exercise in designated hallway space.
Conclusion
Although scientific data has not been collected regarding improvements, since the BAT was established in 2019 the ARC has seen anecdotal evidence of improvements in rodents showing abnormal behaviors receiving wheels and alternative foraging materials. Our large animal colonies appear more relaxed and compliant due to the intensive socialization program. In addition, animals that require intensive care (ICU) after surgery are acclimated to various ICU activities 1-2 weeks prior to surgery. We have observed noticeable improvements in stress levels after surgery and animal recovery outcomes. The chinchilla colonies have shown increased activity levels and
January 2024 Laboratory Animal Science Professional 33
Figure 2. Rabbits with various enrichment.
Figure 3. Pigs in ball pit.
Figure 4. Sheep using foraging box.
conspecific behaviors with their new caging. The ARC’s future goals include collecting scientific data to see if it is comparable to the anecdotal evidence that providing enrichment has shown decreased anxiety and abnormal behaviors in many of the species housed in the ARC.
Acknowledgements
We would like to thank Dr. Carmen Arsuaga for editing assistance. Additionally, we would like to thank everyone in the ARC, past and present, for remaining dedicated to the enrichment program for our animals.
Morgan Mantz, RVT, RALAT is a Senior Veterinary Technical Specialist at Abigail Wexner Research Institute, Animal Resources Core in Columbus, OH.
Anna Pugerud, BA, RVT, RLATG is a Preclinical Behavior and Imaging Technologist at the Abigail Wexner Research Institute, Animal Resource Core in Columbus, OH.
REFERENCES
1. Benefiel AC, Dong WK, Greenough WT. 2005. Mandatory “enriched” housing of laboratory animals: the need for evidence-based evaluation. ILAR Journal 46:95–105. Available at: https://doi.org/10.1093/ilar.46.2.95
2. Krech D, Rosenzweig MR, Bennett EL. 1962. Relations between chemistry and problem-solving among rats raised in enriched and impoverished environments. J Comp Physiol Psychol 55:801–807.
3. Krueger LD, Thurston SE, Kirk J, Elsaeidi F, Freeman ZT, Goldman D, Lofgren JL, Keller JM. 2020. Enrichment preferences of singly housed zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 59:148–155. Available at: https:// doi.org/10.30802/AALAS-JAALAS-19-000078
4. Liss C. 2015. Comfortable quarters for laboratory animals. Washington, D.C.: Animal Welfare Institute.
5. National Research Council. 2011. Guide for the care and use of laboratory animals. Washington, D.C.: National Academies Press.
6. Toth LA, Kregel K, Leon L, Musch TI. 2011. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 61:314–321.



THE VRL ADVANTAGE

Laboratory Animal Science Professional January 2024
For a complete range of products and services, request our catalog or view it online at www.vrl.net ANIMAL HEALTH DIAGNOS TICS Animal Health Monitoring • Virology • Chemistry • Bacteriology • Aquatics • Parasitology • Environmental Testing Visit our new improved website for your testing needs www.vrl.net
Reliable Results • Fast Turnaround Times Knowledgeable Scientific Staff Customer Focused Independent Laboratory Client & Technical Service Diagnostic Testing Fast Turnaround Times Quality Diagnostics For more than 30 years Our priority has been to ensure our clients accessibility to the highest level of expertise and customer service in the industry. We are dedicated to providing you non-biased high-quality results with fast turnaround times with unmatched customer service.
All the laboratory products you need right at your ngertips.

Braintree Scientific has been providing research equipment to the Life Sciences Industry for over 43 years.
Our unique, hand-picked selection of products for mice and rats is extensive and has evolved with researchers’ needs and changing technology.
Scan to request our 2024 Braintree Scientic catalog and ip through our pages!
781.917.9526 | info@braintreesci.com | braintreesci.com
Precision Cut Lung Slices – A Versatile 3Rs Method for Pulmonary Research
By Julia Herbert, DVM, PhD
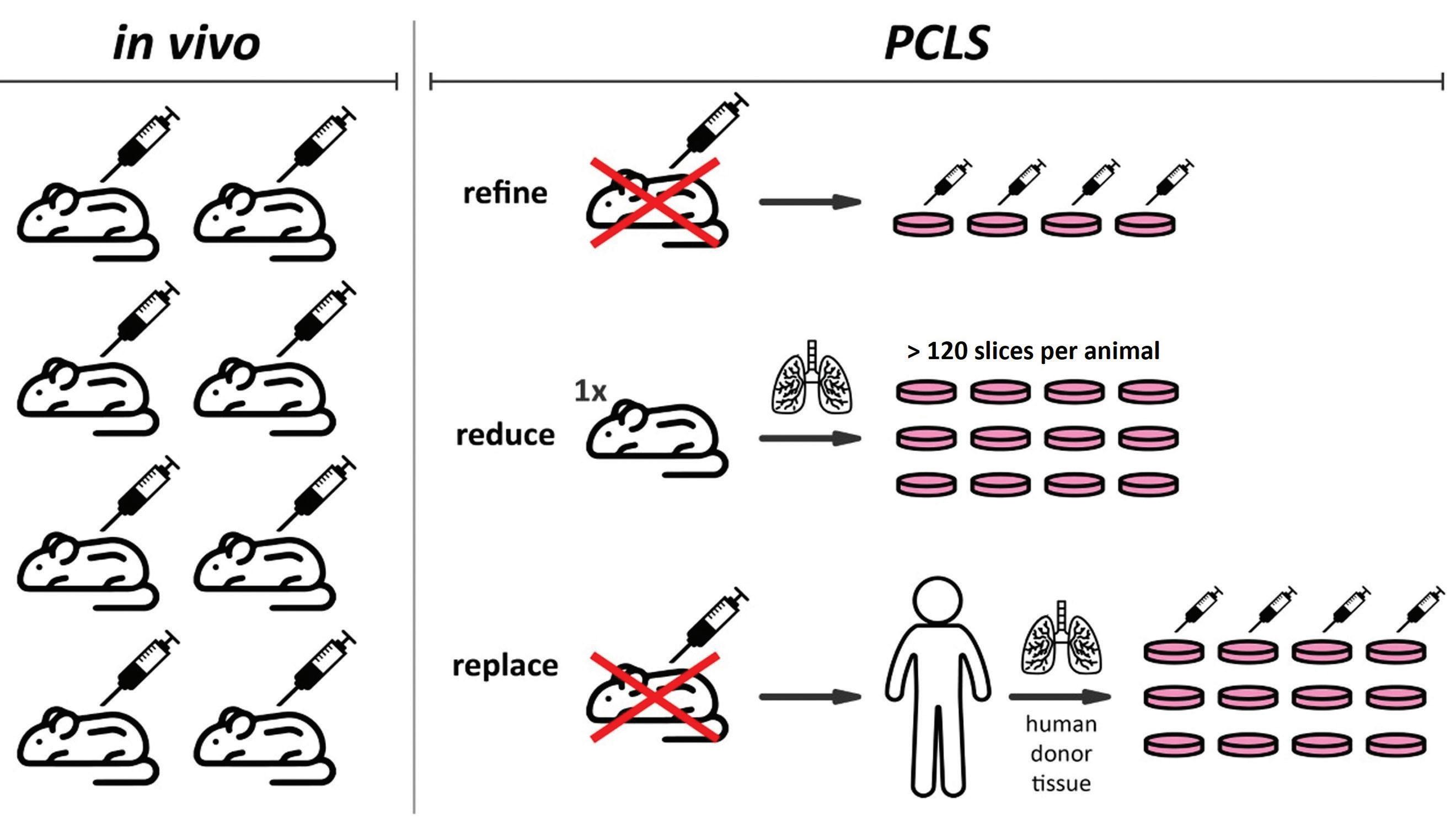
In recent years, alternative methods for translatable basic and clinical research have been developed that do not or only partially rely on the use of laboratory animals.5 These include methods like organ-on-a-chip, organoids, and complex in vitro cell models13 and have an important role in reducing animal use in research. For pulmonary disease modeling and pharmacology/toxicology studies, alternative models are available as well, but many lack the ability to mimic the complex structural make-up and functional mechanics of lung tissue.22,26 A more suitable model for genotoxicity, chemical compound toxicity testing, lung pathogenesis studies, and three-dimensional lung modeling involves the use of Precision Cut Lung Slices (PCLS).10,1,24,3,4,15 This ex vivo method features viable slices of lung tissue which retain the three-dimensional structure of the lung, comprising all resident cell types and maintaining parenchymal functionality.14 Additionally, this method complies with the ‘3Rs’ principles, which refers to the reduction, refinement, and replacement of animal use in research. By using PCLS, animal involvement is reduced to harvesting of lung tissue, because the slices serve as the experimental sample.
Total animal numbers are decreased due to a high yield of technical replicates that are generated from just one or two lung specimens.
Unique endpoints can be examined in PCLS that are challenging to analyze in vivo or in vitro, making the method an intermediary between live animal and cell culture experiments. Insights on immediate functional (airway responsiveness and ciliary beating), cellular (viability, metabolism, immune responses) and structural (histology, immunohistochemistry) changes connected to pulmonary disease or responses to pharmaceutical compounds/toxicants can be provided. Slices can be prepared from animal or human lung tissue,10 which contributes to the refinement and even replacement of animal experimentation, but also enhances the translatability of results. The 3Rs advantages of PCLS are depicted in Figure 1.
Current literature demonstrates the use of PCLS for investigation of immune responses in resident lung cells after challenge with immunoactive compounds, assessment of acid-induced injury and repair,2 investigation of genotoxicity,24 and evaluation of toxicity of a broad range of chemicals. Such
36 Laboratory Animal Science Professional January 2024
FEATURE – LAB ANIMAL ENRICHMENT
Figure 1. 3Rs (reduce, refine, replace) advantages of Precision Cut Lung Slices (PCLS). Picture credit: Dr. Benjamin Wong, US Army Combat Capabilities Command Army Research Laboratory (DEVCOM ARL).
chemicals include personal hygiene products and cleaning detergents,27 but also e-cigarettes6 or even chemical warfare agents.7-9 PCLS are also used to investigate the onset of fibrosis, pathogenesis of asthma/COPD, and other non-infectious or infectious pulmonary diseases, including studies on SARS-CoV-2.10,3,15,4
The general process of PCLS preparation is very similar among laboratories using this technique, and for the most part only requires standard equipment that can be found in most laboratories utilizing cell culture or animal models. Lung specimens can be collected from many different species. The tissue varies mainly in size and lobulation; for smaller species, entire lung lobes can be sliced, whereas lungs from larger species might need to be sectioned and tissue cores prepared. For human lung tissue, acquisition can be difficult and often requires a laboratory-hospital collaboration, patient-consent as well as regulatory oversight by an internal Institutional Review Board. Rejected transplants or tissues from lobectomies are a common source of human lung tissue for PCLS.1,19,15
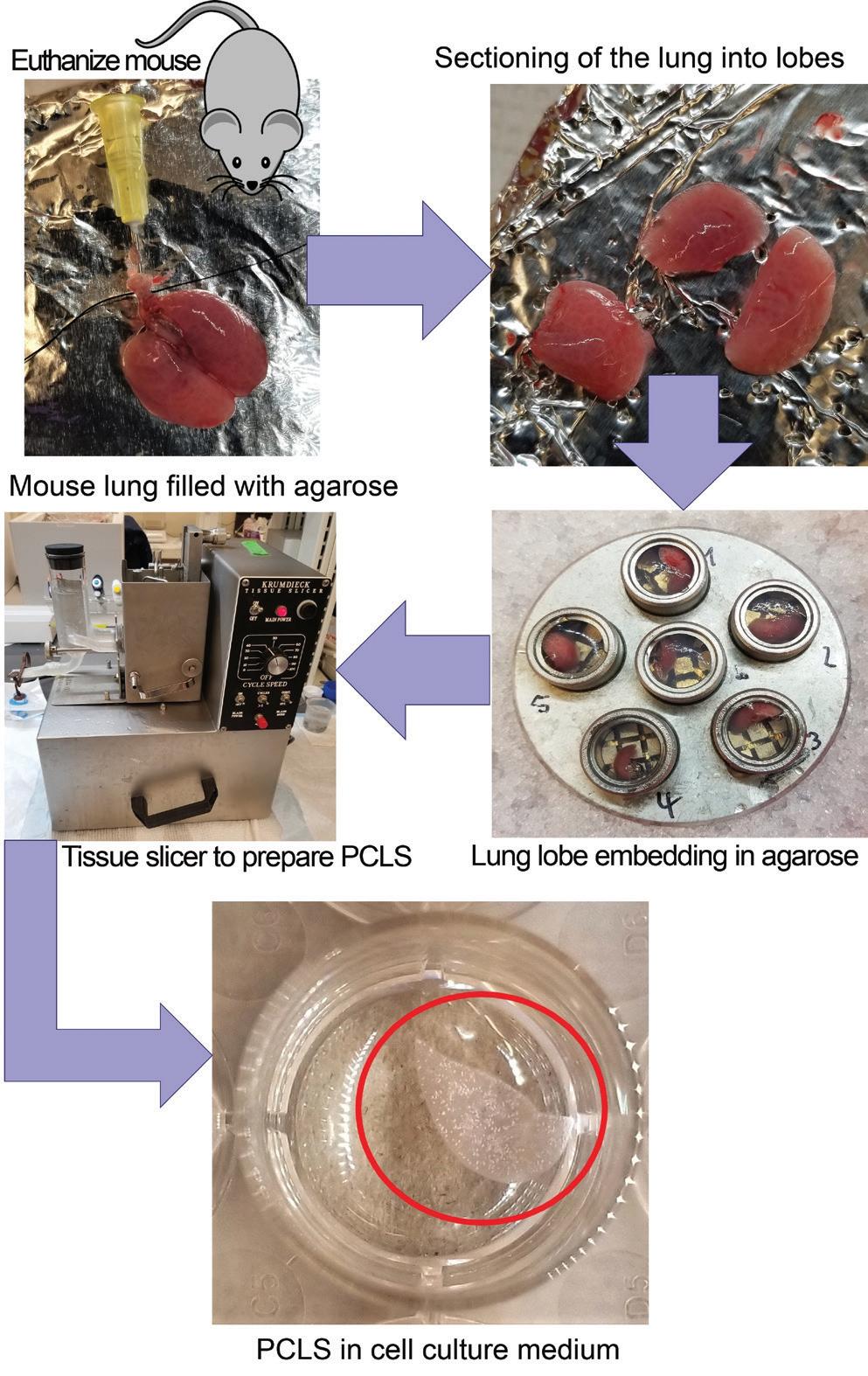
Popular laboratory animal species for PCLS preparation include mice, rats, and guinea pigs. To prepare PCLS from these species, the animal is euthanized, a tracheotomy is performed, and agarose is instilled into the lung via the airways. After congealment of the agarose, the lung is excised and separated into lobes and tissue cores. To prepare PCLS, a tissue-slicing device is needed such as the Alabama Research & Development Tissue Slicer (aka Krumdieck slicer) or the Precisionary Instruments Compresstome.18 Depending on the slicer and the source of the lung tissue, embedding of the lung specimen in agarose prior to slicing might be necessary. Slices are commonly cut to a thickness of 300-350 micrometers, which ensures sufficient diffusion of nutrients and oxygen. After slicing, PCLS are kept in microtiter plates containing warm culture media supplemented with antibiotics and/or antimycotics and placed in an incubator, preferably on an orbital shaker. The culture media is usually exchanged several times right after slicing and at least every 24 hours thereafter to replenish nutrients and eliminate debris. A schematic of PCLS preparation from mouse lung tissue can be seen in Figure 2.
PCLS have been shown to remain viable under cell culture conditions for up to 29 days.21 Commonly used assays to test slice viability include lactate-dehydrogenase (LDH) and water-soluble tetrazolium salt-1 (WST-1) assays.21 LDH is an enzyme in the cellular cytoplasm, which is released upon destruction of a cell. The LDH content in the supernatant of PCLS reflects the overall well-being of the slices and can, for example, be used to quantify the toxicity of an exposure compound. WST-1 is a water-soluble tetrazolium salt which is converted to formazan by mitochondrial dehydrogenases. The amount of formazan formed is an indicator of working mitochondria and the viability of cells in PCLS and can be measured photometrically. These viability assays in conjunction with other endpoints can be used to test pulmonary toxicity. For such testing, PCLS can be directly exposed to a dilution
January 2024 Laboratory Animal Science Professional 37
Figure 2. Precision Cut Lung Slice (PCLS) preparation.
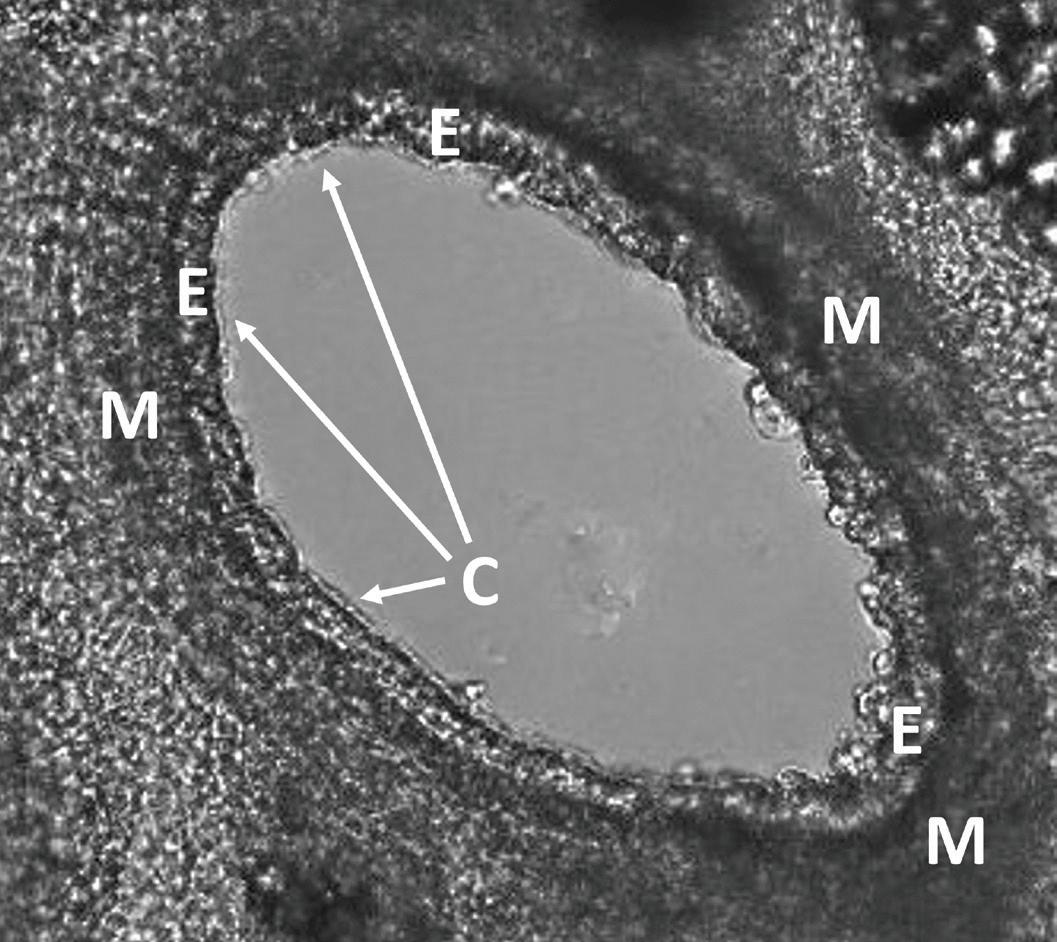
Figure 3. Airway in Precision Cut Lung Slice under the microscope. M = smooth muscle layer; E = epithelial cell layer; C = cilia seam
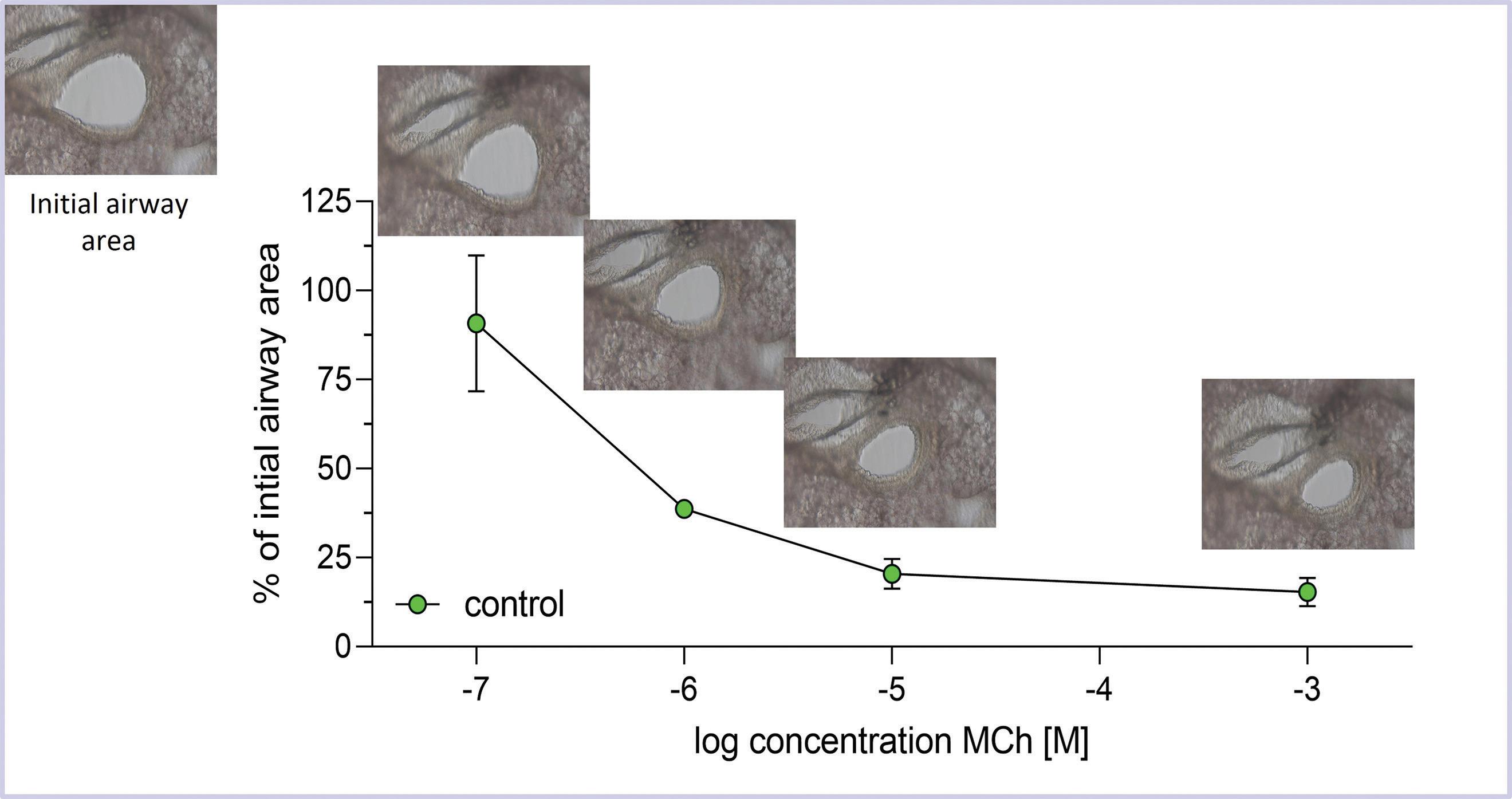
Figure 4. To measure airway responsiveness in Precision Cut Lung Slices, photographs of tissue slices are taken before and after treatment with a compound. In this case, the open airway area is calculated before and 10 minutes after treatment with increasing concentrations of methacholine (MCh) using ImageJ software (https://imagej.net/ij/). For each concentration, the data are plotted as the percentage of airway area after treatment compared to the airway area before treatment. Increasing concentrations of methacholine (from left to right) cause progressively greater loss of airway area. Using ImageJ, the airway area is measured, and values for each MCh dose are related to the initial airway area (set as 100%).
of a toxicant in a microtiter plate in a submerged setting or cultured at an air-liquid interface, where PCLS are placed on permeable membrane inserts with exposure to both air above and cell culture media beneath.25 This approach allows PCLS exposure to gaseous toxicants.
A unique advantage of utilizing PCLS is the ability to measure and image basic pulmonary functions like airway contractions12,17 and ciliary beating in real-time.23 Figure 3 shows an airway in a PCLS under the microscope. After administration of a constricting substance like methacholine, airway contraction can be observed, and the reduction of the airway area can be quantified (Figure 4). Similarly, the frequency of ciliary beating can be measured and quantified.23 Both assays allow conclusions about a toxicant's effects on these functional parameters, and how they might translate to the lung. For example, in a study investigating the toxicity of menthol flavoring in e-cigarette aerosols in PCLS, the contractility of airways and the ciliary beating frequency were significantly reduced, indicating that menthol flavoring causes functional impairment in the lungs.6 In addition to functional and cellular endpoints, PCLS can be used in conjunction with a variety of well-established in vitro or in vivo biomolecular experimental techniques. These include RNA extraction for real-time qPCR, Western Blot, immunohistochemistry and fluorescence, pathological histology, glycolysis and mitochondrial activity assays, oxidative stress analyses, live-cell-imaging, flow cytometry, and many more.20,28,16,11,4
Like all methods, PCLS have some limitations which should be kept in mind when interpreting results. The ability of the tissue to regenerate and function like the whole organ
inside the body is limited due to the slicing process and absent physiologic ventilation and perfusion cycles. Cell responses are confined to the resident population, but can also be useful in studies where isolation of tissue-specific signaling is needed, such as those determining the toxicity mechanism of a compound. Similarly, the absence of infiltrating immune cells allows the investigation of early-onset immune signals, which is a unique feature of PCLS. However, systemic immune responses cannot be investigated. And due to the slicing process, barrier functions of the tissue are compromised because cells and structures are exposed that normally would not be.
Despite these limitations, PCLS should be considered when conducting any type of pulmonary research. They are highly suitable for high-throughput screening studies as well as basic research approaches and offer unique functional endpoints that cannot be replicated using in vivo or in vitro experimentation. PCLS can serve all three of the 3Rs, with replacement possible if human lung tissue is available. Their use and preparation is also easy and does not require much specialized or costly equipment. It is a well-established methodology that can add a new layer of understanding to a great variety of pulmonary research projects.
Julia Herbert, DVM, PhD is an Assistant Research Professor in the Department of Pharmacology and Toxicology at Rutgers, The State University of New Jersey in Piscataway, NJ.
REFERENCES
1. Alsafadi HN, Uhl FE, Pineda RH, Bailey KE, Rojas M, Wagner DE, Konigshoff M. 2020. Applications and Approaches for Three-Dimensional Precision-Cut Lung Slices. Disease Modeling
38 Laboratory Animal Science Professional January 2024
and Drug Discovery. Am J Respir Cell Mol Biol 62:681-691.
2. Basoalto R, Damiani LF, Bachmann MC, Fonseca M, Barros M, Soto D, Araos J, Jalil Y, Dubo S, Retamal J, Bugedo G, Henriquez M, Bruhn A. 2021. Acute lung injury secondary to hydrochloric acid instillation induces small airway hyperresponsiveness. Am J Transl Res 13:12734-12741.
3. Gerckens M, Alsafadi HN, Wagner DE, Lindner M, Burgstaller G, Konigshoff M. 2019. Generation of Human 3D Lung Tissue Cultures (3D-LTCs) for Disease Modeling. J Vis Exp.
4. Gerhards NM, Cornelissen J, van Keulen LJM, Harders-Westerveen J, Vloet R, Smid B, Vastenhouw S, van Oort S, Hakze-van der Honing RW, Gonzales JL, Stockhofe-Zurwieden N, de Jong R, van der Poel WHM, Vreman S, Kortekaas J, Wichgers Schreur PJ, Oreshkova N. 2021. Predictive Value of Precision-Cut Lung Slices for the Susceptibility of Three Animal Species for SARS-CoV-2 and Validation in a Refined Hamster Model. Pathogens 10.
5. Gorzalczany SB, Rodriguez Basso AG. 2021. Strategies to apply 3Rs in preclinical testing. Pharmacol Res Perspect 9:e00863.
6. Herbert J, Kelty JS, Laskin JD, Laskin DL, Gow AJ. 2023. Menthol flavoring in e-cigarette condensate causes pulmonary dysfunction and cytotoxicity in precision cut lung slices. Am J Physiol Lung Cell Mol Physiol 324:L345-L357.
7. Herbert J, Laskin DL, Gow AJ, Laskin JD. 2020. Chemical warfare agent research in precision-cut tissue slices-a useful alternative approach. Ann N Y Acad Sci 1480:44-53.
8. Herbert J, Thiermann H, Worek F, Wille T. 2019. COPD and asthma therapeutics for supportive treatment in organophosphate poisoning. Clin Toxicol (Phila) 57:644-651.
9. Herbert J, Thiermann H, Worek F, Wille T. 2017. Precision cut lung slices as test system for candidate therapeutics in organophosphate poisoning. Toxicology 389:94-100.
10. Hess A, Wang-Lauenstein L, Braun A, Kolle SN, Landsiedel R, Liebsch M, Ma-Hock L, Pirow R, Schneider X, Steinfath M, Vogel S, Martin C, Sewald K. 2016. Prevalidation of the ex-vivo model PCLS for prediction of respiratory toxicity. Toxicol In Vitro 32:347-361.
11. Kortekaas RK, Geillinger-Kastle KE, Borghuis T, Belharch K, Webster M, Timens W, Burgess JK, Gosens R. 2023. Interleukin-11 disrupts alveolar epithelial progenitor function. ERJ Open Res 9.
12. Koziol-White C. 2022. Human Precision-Cut Lung Slices: Generation of and Measurement of Contractility and Relaxation of Small Airways. Methods Mol Biol 2506:111-117.
13. Lee SY, Lee DY, Kang JH, Jeong JW, Kim JH, Kim HW, Oh DH, Kim J-M, Rhim S-J, Kim G-D, Kim HS, Jang YD, Park Y, Hur SJ. 2022. Alternative experimental approaches to reduce animal use in biomedical studies. Journal of Drug Delivery Science and Technology 68:103131.
14. Liu G, Betts C, Cunoosamy DM, Aberg PM, Hornberg JJ, Sivars KB, Cohen TS. 2019. Use of precision cut lung slices as a translational model for the study of lung biology. Respir Res 20:162.
15. Liu Y, Wu P, Wang Y, Liu Y, Yang H, Zhou G, Wu X, Wen Q 2022. Application of Precision-Cut Lung Slices as an In Vitro Model for Research of Inflammatory Respiratory Diseases. Bioengineering (Basel) 9
16. Lyons-Cohen MR, Thomas SY, Cook DN, Nakano H. 2017. Precision-cut Mouse Lung Slices to Visualize Live Pulmonary Dendritic Cells. J Vis Exp.
17. Martin C, Uhlig S, Ullrich V. 1996. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur Respir J 9:2479-2487.
18. Morin JP, Baste JM, Gay A, Crochemore C, Corbiere C, Monteil C. 2013. Precision cut lung slices as an efficient tool for in vitro lung physio-pharmacotoxicology studies. Xenobiotica 43:63-72.
19. Neuhaus V, Schaudien D, Golovina T, Temann UA, Thompson C, Lippmann T, Bersch C, Pfennig O, Jonigk D, Braubach P, Fieguth HG, Warnecke G, Yusibov V, Sewald K, Braun A. 2017. Assessment of long-term cultivated human precision-cut lung slices as an ex vivo system for evaluation of chronic cytotoxicity and functionality. J Occup Med Toxicol 12:13.
20. Niehof M, Hildebrandt T, Danov O, Arndt K, Koschmann J, Dahlmann F, Hansen T, Sewald K. 2017. RNA isolation from precision-cut lung slices (PCLS) from different species. BMC Res Notes 10:121.
21. Nussbaum SM, Krabbe J, Boll S, Babendreyer A, Martin C 2022. Functional changes in long-term incubated rat precision-cut lung slices. Respir Res 23:261.
22. Primavessy D, Metz J, Schnur S, Schneider M, Lehr CM, Hittinger M. 2021. Pulmonary in vitro instruments for the replacement of animal experiments. Eur J Pharm Biopharm 168:62-75.
23. Sisson JH, Stoner JA, Ammons BA, Wyatt TA. 2003. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 211:103-111.
24. Switalla S, Knebel J, Ritter D, Dasenbrock C, Krug N, Braun A, Sewald K. 2013. Determination of genotoxicity by the Comet assay applied to murine precision-cut lung slices. Toxicol In Vitro 27:798-803.
25. Switalla S, Knebel J, Ritter D, Krug N, Braun A, Sewald K 2010. Effects of acute in vitro exposure of murine precision-cut lung slices to gaseous nitrogen dioxide and ozone in an air-liquid interface (ALI) culture. Toxicol Lett 196:117-124.
26. Upadhyay S, Palmberg L. 2018. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol Sci 164:21-30.
27. Watson CY, Damiani F, Ram-Mohan S, Rodrigues S, de Moura Queiroz P, Donaghey TC, Rosenblum Lichtenstein JH, Brain JD, Krishnan R, Molina RM. 2016. Screening for Chemical Toxicity Using Cryopreserved Precision Cut Lung Slices. Toxicol Sci 150:225-233.
28. Wijeweera JB, Gandolfi AJ, Parrish A, Lantz RC. 2001. Sodium arsenite enhances AP-1 and NFkappaB DNA binding and induces stress protein expression in precision-cut rat lung slices. Toxicol Sci 61:283-294.
January 2024 Laboratory Animal Science Professional 39
Navigating Export Control Laws in Animal Research: Key Considerations
By Rani Muthukrishnan, PhD
ABOUT THE EXPORT CONTROLS OFFICIAL
All academic institutions which perform fundamental or restricted research must demonstrate that they have a system in place to identify and mitigate export control compliance risk. The 2007 U.S. Sentencing Commission Guidelines at Section 8B2.1 require academic institutions to have an export controls program with the following elements:
• Standards and Procedures
• Governance, Organization, and Reporting
• Assignment of Substantial Authority
• Training and Education
• Monitoring and Auditing, Program Assessment, & Helpline
• Consistent Enforcement and Discipline
• Responses to Noncompliance and Program Modifications
Institutions often adopt a simple and effective way to manage export control compliance risk by identifying a single individual with overall responsibility for export control compliance on campus. The appointed official (Export Control Official or Export Control Officer) will be responsible for export control and serve as the point of contact for all export control questions or issues. The office or designee is granted “substantial authority” to make independent decisions void of organizational conflicts of interest. The official should have direct reporting lines to the university’s executive level. If the University is registered under ITAR, subject to 22 CFR 120-130, an Empowered Official with sufficient authority to verify accuracy of transactions is necessary.
Introduction
Animal research has a vital role in advancing scientific knowledge, medical breakthroughs, and innovations. The cutting-edge research with animal models often involves the use of controlled materials, advanced technologies, and technical information that may have potential dual use and thus falls under export control regulations. Items classified under dual use can be used both for civilian and military applications and often weaponized or worse, used for terrorism. Forge (2010)2 identifies dual use as having three distinct parts, or three related kinds of things that comprise the category of dual use: research, technologies, and artefact. Thus, it is possible that common or innocuous items used in laboratory or animal welfare can require export controls oversight. Navigating export control laws falls under biosecurity and use of biological materials in animal research requires a thorough understanding of the regulations, compliance procedures, and potential implications.
The export control regulations are designed to safeguard national security, protect sensitive technologies, and prevent the unauthorized sharing of controlled items with foreign entities. Export control laws are implemented by governments to regulate the transfer of certain items, technologies, and information to foreign individuals or entities. Many federal agencies are involved in implementing the regulations that are considered critical to national security and may cover a wide range of items, including equipment, biological materials, chemicals, software, and technologies used in animal research. Researchers involved in animal studies must be aware of potential export-controlled elements in their projects, as non-compliance can lead to severe legal and financial consequences. In many institutions, the researcher is responsible for initial determination of the applicability of export control regulations.
An Overview of Export Control
There are three U.S. government agencies that control the majority of exports: the Department of Commerce, the State Department, and the Treasury Department. By far the biggest regulatory body is the Department of Commerce - especially the Bureau of Industry and Security (BIS) which implements and enforces the Export Administration Regulations (EAR), which lists products under the Commerce Control List (CCL). Depending on the nature of research, Department of Defense Trade Controls (DDTC) which enforces International Traffic in Arms Regulations (ITAR) might be applicable. Oth-
er agencies that may have oversight over animal activities include USDA, U.S. Fish and Wildlife Service, USPS, and Customs and Border Protection. There is not a single agency or department that regulates all U.S. exports. Institutions must be aware of the various regulations and have documented processes that ensure that compliance is always maintained. In addition, institutions are responsible for safe-guarding select agents, which are bacteria, viruses, toxins, or other biological agents and substances that pose a severe threat to public health and safety if they are accidentally released. The U.S. government maintains a list of select agents and toxins that are subject to special restrictions and oversight when it comes to possessing, using, storing, or transferring. All select agents are subject to strict export controls and are regulated through the following agencies:
• The Centers for Disease Control and Prevention (CDC) regulates the possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public health and safety. The CDC maintains a list of select agents and toxins that fall under these oversight regulations.
• The Animal and Plant Health Inspection Service (APHIS) provides oversight for the export of certain plant pathogens and livestock/animal pathogens. They maintain a list of regulated plant pests and animal pathogens.
• The Department of Commerce regulates the export of dual-use microorganisms through the Commerce Control List. Dual-use items are those with both commercial and military applications.
In addition to the select agent lists, other microbes like those that cause severe human disease (even if not on the select agent lists) or that could be used to manufacture biological weapons may require export licenses from the CDC, APHIS, or Commerce Department. Strict permitting and registration processes must be followed for exportation. These controls aim to prevent restricted microbes from falling into the wrong hands and threatening public health or national security. Given the layers of oversight required and the large number of microbes, the U.S. Department of Commerce has issued an Export Control Classification Number (ECCN; a 5 character alpha-numeric designation) to identify dual-use items for export controls purposes. Your export controls officer will be able to provide you with the internal screening process at your institution.
40 Laboratory Animal Science Professional January 2024

Export Controls for Biological Organisms
ECCN 1C353 applies to genetically modified organisms that contain, or have any genetic element that codes for any gene, genes, translated product, or translated products specific to any virus controlled by 1C351.a or .b5 or 1C354.c5; any gene or genes specific to any bacterium controlled by 1C351.c5 or 1C354.a5, or any fungus controlled by 1C351.e5 or 1C354.b5, and any toxins, or their subunits, controlled by 1C351.d5. If your institution creates or generates genetically modified organisms, including organisms in which the nucleic acid sequences have been created or altered by deliberate molecular manipulation, export controls may be required. ECCN 1C354 a, b, c, d, e covers a plant pathogen list that includes bacteria, fungi, and viruses.
If you have federally funded studies that include the creation of genetically modified organisms or working with select agents, a Biosecurity/Technology Control Plan (B/TCP) may be required to prevent unauthorized access by foreign nationals to technology or biologicals controlled for export under the BIS, International Traffic in Arms Regulations (ITAR), or the Export Administration Regulations (EAR). Under export control laws, access to some of these materials is considered as deemed export. The creation of a B/TCP sets forth the security measures that the department, principal investigator, and project personnel agree to implement in the performance of this project to prevent unauthorized foreign persons from gaining access to controlled technologies. For federally funded studies with international collaborations, it is best to consult with an export controls officer before sharing any item, technology, or information. The
institution must obtain necessary licenses or use appropriate communication channels to avoid violations.
Shipments
Shipping of all live animals, semen, ovules, and embryos to foreign countries is considered an export. The export control regulations apply to the final destination and sending animals to most destinations requires license from APHIS3 and other agencies. The regulatory framework for export controls takes into consideration a detailed look at the context by including questions about –
• Who’s exporting and who is receiving it?
• What’s being exported?
• When is the export taking place and how often?
• Where is the final destination of the animal?
• Why are you exporting the animal?
• How is this export being facilitated?
Because the list of items and countries are long and complex, the Export Administration Regulations (EAR) has created the Entity list6 of names of certain foreign persons – including businesses, research institutions, government and private organizations, individuals, and other types of legal persons – that are subject to specific license requirements for the export, re-export, and/or transfer (in-country) of specified items. Additionally, BIS maintains an Unverified List (UVL)7 of parties that are ineligible to receive items subject to the
January 2024 Laboratory Animal Science Professional 41
Figure 1. Depicts the generalized steps to follow for compliance with export controls regulations.
Export Administration Regulations (EAR). They may be allowed to receive live animals by means of a license exception. Exporters must file an Automated Export System record for all exports to parties listed on the UVL and obtain a statement from such parties prior to exporting, re-exporting, or transferring to such parties any item subject to the EAR. Countries subject to embargoes or sanctions have additional regulations, and even sharing non-controlled information might be considered a violation.
If you are shipping animals to international destinations or receiving animals from other countries, it is crucial to pay meticulous attention to detail to ensure compliance with export control laws (see Figure 1 for an outline). Researchers must be proactive in identifying export-controlled elements within their projects and take necessary steps to obtain licenses, authorizations, and approvals. When institutions fail to comply with export controls, it can result in legal penalties, damage to reputation, and potential harm to national security. By fostering a culture of awareness and adherence to export control regulations, researchers can contribute to both scientific advancements and the safeguarding of sensitive technologies. As the landscape of export controls evolves, researchers must remain vigilant and adapt their practices to ensure responsible and lawful animal research. Simple steps and consistency can go a long way to ensure that your animal research is compliant and protected.
If you are interested to know more about the enforcement aspects of export controls in animal research, here are some case studies pertaining to biosecurity.
1. Export of Human Pathogen4: A researcher violated regulations when he exported the human pathogen Yersinia pestis (also known as the Plague) to Tanzania without the required U.S. Department of Commerce license.
2. Stem Cell Research1 and indictment text9: Three researchers were charged in a two-count superseding indictment with knowingly and willfully conspiring to violate the Iranian Transactions and Sanctions Regulations (ITSR), in violation of 50 U.S.C. § 1705 and 31 C.F.R. §§ 560.203 and 560.204, and knowingly and willfully attempting to export biological items from the United States to Iran without first obtaining the required authorization from the United States Department of Treasury’s Office of Foreign Assets Control (OFAC), in violation of 50 U.S.C. § 1705, 31 C.F.R. §§ 560.203, 560.204, and 18 U.S.C. § 2.
3. Lying on NIH Grant8: A rheumatology professor with strong ties to China was sentenced to 37 months in prison for making false statements to federal authorities as part of an immunology research fraud scheme. The researcher was also ordered to pay more than $3.4 million in restitution to the National Institute of Health (NIH) and approximately $413,000 to the university.
Rani Muthukrishnan, PhD is the Director of Research Compliance at Texas A&M University in San Antonio, TX
REFERENCES
1. Catanzaro M. [Internet]. 2019. United States drops charges against two more scientists after Iran prisoner swap. Nature. Available at: https://www.nature.com/articles/d41586-01903856-y
2. Forge J. 2010. A note on the definition of “dual use”. Sci Eng Ethics 16:111–118.
3. U.S. Department of Agriculture, Animal and Plant Health Inspection Service. [Internet]. 2023. International regulations (IRegs) for animal exports home. Available at: https://www. aphis.usda.gov/aphis/ourfocus/animalhealth/export/iregs-for-animal-exports/ct_iregs_animal_exports_home
4. U.S. Department of Commerce, Bureau of Industry and Security. [Internet]. 2020. Electronic FOIA. Available at: https://efoia.bis.doc.gov/index.php/documents/export-violations/302-e1011/file
5. U.S. Department of Commerce, Bureau of Industry and Security. [Internet]. 2023. Commerce control list. Supplement No. 1 to Part 774. Category 1: Special materials and related equipment, chemicals, “microorganisms”, and “toxins”. Available at: https://www.bis.doc.gov/index.php/documents/ regulations-docs/2332-category-1-materials-chemicals-microorganisms-and-toxins-4/file
6. U.S. Department of Commerce, Bureau of Industry and Security. [Internet]. 2024. Entity list. Available at: https://www. bis.doc.gov/index.php/policy-guidance/lists-of-parties-of-concern/entity-list
7. U.S. Department of Commerce, Bureau of Industry and Security. [Internet]. 2024. Unverified list. Available at: https:// www.bis.doc.gov/index.php/policy-guidance/lists-of-parties-ofconcern/unverified-list
8. U.S. Department of Justice, Office of Public Affairs. [Internet]. 2021. University researcher sentenced to prison for lying on grant applications to develop scientific expertise for China. Available at: https://www.justice.gov/opa/pr/university-researcher-sentenced-prison-lying-grant-applications-develop-scientific-expertise
9. United States v. Soleimani. [Internet]. 2019. Opinion: Criminal case no 1:18-cr-00216-ELR-RGV. Case text, Thomson Reuters. Available at: https://casetext.com/case/united-states-v-soleimani-1
42 Laboratory Animal Science Professional January 2024
Need something to isten to?


The LASt Word
an AALAS podcast
AALAS is proud to present a rand-new podcast that eatures some o our communit 's rightest minds discussing their research.

Listen here:



The Power of Neural Rewriting: Enhancing Public Speaking through Brain Transformation
By Sarah E. Jones, MLAS, CMAR
The mere thought of delivering a presentation has consistently triggered anxiety for me, creating a persistent and apprehensive reaction even before the actual act of presenting. For many of us, public speaking skills are crucial for career advancement, especially in the management realm of lab animal science. Success often hinges on the ability to effectively exchange knowledge, present research data, and provide comprehensive training. Whether communicating findings to colleagues, delivering training sessions, or disseminating crucial information, mastering public speaking is integral to thriving in various roles within biomedical research.
Although public speaking is a skill that many aspire to master, it often remains a source of anxiety and discomfort for many people. Traditional public speaking courses typically emphasize aspects such as crafting presentation slides, knowing your subject matter well, maintaining a non-distracting speaking style, and recommending repetitive practice, and suggest methods like mirror rehearsals and self-recording to address them. However, these conventional classes tend to overlook crucial aspects of managing anxiety and fear of public speaking.
The key to overcoming these challenges might lie in the fascinating concept of ‘rewriting’ your brain by utilizing neuroplasticity, which refers to the brain’s ability to reorganize itself and form new neural connections. This process offers a promising approach for individuals seeking to enhance their public speaking abilities.
Understanding Neural Rewriting
Neural rewriting involves deliberately reshaping thought patterns and behaviors by forging new neural pathways. This process is rooted in the idea that the brain is adaptable and can be molded through conscious effort and repetition. When applied to public speaking, this technique aims to reframe perceptions, reduce anxiety, and foster confidence.
Identifying Negative Thought Patterns
Many individuals harbor negative thought patterns associated with public speaking which include fear of judgment, self-doubt, or a sense of inadequacy. Recognizing and acknowledging these patterns is the first step towards rewriting the brain. Mindfulness, the practice of cultivating a heightened awareness of the present moment with a
44 Laboratory Animal Science Professional January 2024 Management/Career & Training
non-judgmental mindset, plays a pivotal role in this process. Mindfulness techniques can be instrumental, enabling individuals to observe their thoughts without judgment and identify areas for improvement. This integrated approach, combining the recognition of negative thought patterns with mindfulness practices, forms a powerful strategy for fostering personal growth and enhancing public speaking abilities.
Imagine a researcher named Jack. Jack consistently experiences high levels of anxiety and nervousness when presenting data at conferences or during internal meetings. This anxiety hinders effective communication and may impact his confidence and overall professional growth. Acknowledging the challenge, Jack decides to adopt a mindful approach to presentation preparation. This approach, characterized by cultivating heightened awareness and a non-judgmental mindset, empowers Jack to intentionally shift his focus. Instead of dwelling solely on potential negative outcomes, the emphasis transforms to the value of sharing knowledge and contributing to the scientific community. This mindful approach becomes a cornerstone in Jack’s journey to overcome presentation anxiety and intentionally foster a more positive and constructive mindset.
Positive Affirmations and Visualization
Once negative thought patterns are identified, replacing them with positive affirmations becomes crucial. Affirmations are powerful tools that can reshape beliefs and attitudes. By consistently reinforcing positive statements about one’s ability to speak in public, individuals can begin to rewrite their brain’s default responses.
Jack incorporates positive affirmations into his routine. Affirmations focus on reinforcing confidence and competence, such as “I am well-prepared, and my expertise adds value to this presentation”.
Visualization is another effective technique. Picturing successful public speaking scenarios in vivid detail helps create a positive mental framework. The brain is incapable of differentiating between actual and mentally rehearsed experiences, so it reacts to these cognitive rehearsals as if they were genuine events, progressively reducing anxiety and bolstering confidence. Mentally rehearsing and visualizing an event aids in conditioning the brain to respond in more positive ways, especially in the face of potentially stressful situations such as public speaking.
Prior to presentations, Jack engages in positive visualization. This involves imagining a successful and well-received presentation, envisioning an engaged and interested audience, and feeling a sense of accomplishment.
Gradual Exposure and Desensitization
Fear of public speaking often stems from a fear of judgment or failure. Neural rewriting involves gradual exposure to these fears in a controlled environment. Start with small, friendly audiences or even practice in front of a mirror. As the brain becomes accustomed to the experience without triggering a stress response, it begins to rewrite its associations with public speaking, transforming fear into familiarity. Jack engages in gradual exposure to public speaking. This could involve starting with smaller, less formal settings before progressing to larger conferences. Gradual exposure helps build confidence over time.

Repetition and Consistency
Neural rewriting requires consistent effort. Repetition is key to strengthening new neural connections. Regular practice, whether through mock presentations, speaking clubs, or solo rehearsals, reinforces positive associations with public speaking. Over time, the brain adopts these new patterns, making confidence and composure the default responses to speaking engagements.
Instead of viewing feedback as solely evaluative, Jack reframes it as an opportunity for growth. Constructive criticism is seen as valuable input for refining presentation skills rather than a reflection of personal failure. Through consistent application of these neural rewriting strategies, Jack experiences a shift in his response to public speaking situations. Over time, the anxiety associated with presentations diminishes, and Jack develops a more confident and composed demeanor during research talks. This not only enhances his effectiveness as a communicator but also contributes to a more positive and fulfilling professional experience.
In this example, neural rewriting is applied to reshape Jack’s cognitive and emotional responses to public speaking, fostering a more positive and adaptive mindset.
Seeking Professional Guidance
For those seeking a more structured approach, professional guidance can be invaluable. Cognitive-behavioral therapy (CBT) and public speaking courses specifically designed to address anxiety and build confidence can provide tailored strategies for neural rewriting.
Conclusion
Rewriting your brain for improved public speaking is a transformative journey that requires dedication and patience. By identifying negative thought patterns, incorporating positive affirmations and visualization, gradually exposing oneself to speaking situations, and maintaining consistency through repetition, individuals can reshape their neural pathways and develop a newfound confidence in public speaking. Embracing the concept of neuroplasticity opens up exciting possibilities for personal growth and enhanced communication skills.
Sarah E. Jones, MLAS, CMAR is an Associate Scientist in San Diego, CA.
REFERENCES:
1. Dispenza J. 2007. Evolve your brain: the science of changing your mind. Deerfield Beach (FL): Health Communications, Inc.
2. Arden JB. 2010. Rewire your brain: think your way to a better life. Hoboken (NJ): Wiley.
January 2024 Laboratory Animal Science Professional 45
How Much Wood Could a Woodchuck Chuck if a Woodchuck Could Chuck Wood? Not Much if They’re Provided with the Right Enrichment!
By James Brennan, MLAS, CMAR, LATG
Introduction
The Eastern woodchuck (Marmota monax) is a large, burrowing rodent with native territories throughout parts of the US and Canada. Frequently known by its more common names (Woodchuck, Groundhog, Land Beaver, or Whistle Pig), it is a valuable laboratory animal species used as a model for hepatitis B virus (HBV), food intake, obesity, hibernation, and annual cycles. Woodchucks are susceptible to infection with woodchuck hepatitis virus (WHV), a hepadnavirus that is endemic in certain wild woodchuck populations. WHV demonstrates numerous similarities with another member of the hepadnaviridae family, human hepatitis B virus (HBV). This fact has led to the emergence of WHV-infected woodchucks as a valuable and highly translatable animal model of viral hepatitis and oncogenesis. Despite the clear utility of this species for a variety of research applications, the housing and maintenance of woodchucks in a laboratory setting is relatively uncommon and information regarding their care requirements is limited. In this article, I will discuss our facility’s approach to woodchuck husbandry, particularly environmental enrichment.
Materials & Methods
All work was performed under an approved Institutional Animal Care and Use Committee (IACUC) protocol at an AAALAC International accredited facility. Animals were obtained from both captive-bred and wild-caught sources. They were maintained on a commercially available diet (Mazuri Herbivore) and reverse-osmosis (RO) drinking water via automatic water lixits. Fresh produce was given daily, along with a variety of edible enrichment items. Before starting any study work, animals were acclimated for several weeks. They were first acclimated to the basic husbandry practices (feeding/ watering, daily health checks, cage/pan changes, etc.), environmental conditions (temperature/humidity, lighting, etc.), and then study conditions. Like many lab animals, woodchucks may have the tendency to bite when handled. A Kevlar glove on one hand was utilized for restraint, along with a custom made “woodchuck restrainer”. Animals were slowly acclimated to this procedure, with very positive results. Typically, 30-45 animals were maintained at the facility at any one time. Before starting our woodchuck enrichment program, we considered their species-specific behaviors and tailored our program around those parameters. Woodchucks are ground dwelling, herbivores, and true hibernators. They have a body

built for burrowing with short powerful legs, sharp curved claws, a rounded head, and fur-covered ears designed to keep out dirt. In addition, they have long sharp teeth that will grow too long if they do not wear them down by chewing on things. Woodchucks are diurnal, foraging for food in the morning and afternoon. They will retreat to their burrow when they are threatened and spend a large portion of their time in their burrow.
Next, we broke down our program into 3 enrichment types or groups: tactile enrichment (anything the animals can physically make contact with), food enrichment, and social enrichment
First to address tactile enrichment. Since the burrow is such an important aspect of the woodchuck’s environment, the first item we added was a hut in the cage. Working with Allentown LLC, we were able to design and fabricate one from stainless steel (see Figure 1). The dimensions were approximately 14” (length) × 8” (width) × 8” (height), allowing for an adult woodchuck to fit comfortably in the hut. We chose stainless steel for durability and its ability to be sanitized. It was also one of the only materials the woodchucks could not chew through! When added to the cage, the woodchucks utilized the huts almost immediately, and spent most of their time in them. To reduce animal stress from trying to frequently “catch” the
46 Laboratory Animal Science Professional January 2024 TECH TIPS - HUSBANDRY, ENRICHMENT, NEW TECHNIQUES AND TACTICS
Figure 1. Woodchuck hut.





animals and remove them from their cage for study activities, adding a track (for a door) on the front, along with a carrying handle on the top, allowed us to turn the hut into a transport container which they very much preferred over hand catching. The next items we added were objects to chew, such as wood sticks, Wood Gnawing Blocks, Timothy Hay, Bio-Serv Nylon Bones Certified, Dental Stars Certified, and Dumbbells Certified, all purchased from Bio-Serv (Figure 2). Most of the woodchucks enjoyed these items, with wood sticks being their favorite (most likely since it was the most similar to what they would find in their natural habitat). We also added hanging toys and mirrors to each cage but noticed little interest in these items.
For food enrichment, we tried just about every type of produce you can think of, including carrots, cucumbers, lettuce, tomatoes, celery, broccoli, cabbage, green beans, apples, pears, banana, kiwi, mango, and sweet potato, just to name a few. Overall, we found there were individual preferences between animals, with cantaloupe and dried mango being the top picks (Figure 3). We also found some of the woodchucks could be trained to cooperate during study activities if given the right

treat. One animal would sit still for routine blood collection and/or vascular access port (VAP) maintenance as long as she was given a bag of mini carrots to munch on during the activity. Once the carrots were gone though, so was her patience and so was she!
To address the social enrichment, we thought it would be important that they have visual, auditory, and some tactile contact with the other woodchucks in the room. Working with Allentown LLC we developed “woodchuck” caging, similar to 6-cage rabbit racks (Figure 4), which were equipped with dividers that were solid on the bottom half and had small holes on the top half to allow for contact between cage mates. The dividers were removable for when we had more social individuals who preferred to be paired (Figure 5). This was determined by in-person and remote monitoring (via mobile cameras) of animals for signs of potential compatibility (spending much of their time next to the divider, sniffing, eye contact, etc.). Only same-sex animals were paired to avoid potential breeding. We also had to acclimate the animals to humans, which we did shortly after their arrival at the facility and continued to do frequently throughout their time in the facility. To do this, we implemented a “Woodchuck Acclimation Team” which would gradually habituate each animal to routine husbandry and study activities, including daily cage cleaning and water checks, cage changes, body weights, and oral dosing (Figure 6). The team also habituated the animals to be comfortable coming out for study activities such as dos-
January 2024 Laboratory Animal Science Professional 47
Figure 2. Tactile enrichment items.
Figure 3. Suzie enjoying some delicious cantaloupe.
Figure 4. Woodchuck caging.


ing, blood collection, and VAP maintenance. Similar to many acclimation programs, the schedule and pace of the acclimation was completely dependent on the individual animal’s comfort level, to avoid any unnecessary stress. Some animals habituated quickly (1-2 wk) and others took rather long (4-6 wk) to get used to these procedures. In general, the less human contact and the more treats a woodchuck was given, the better!
Conclusion
Overall, we found that developing a new enrichment program for woodchucks was challenging at times, but very rewarding overall. Watching an individual woodchuck progress from the time they arrived at the facility, to the time they left, was incredibly rewarding. They were far more relaxed, (as evidenced by the decrease in behaviors such as over-chewing, “barking”, and startling easily), and they quickly became accustomed to “life in a lab”.
In summary, the woodchuck is a very unique and useful animal model with valuable research applications. The procedures outlined here have been developed to create a highly effective program for the care and use of this non-traditional species.
Acknowledgments
We thank Gary Marks, Shirley Walker, Kasey Fitch, Jade Arnold, and the rest of the BMS Team for all their hard work and compassion towards developing an innovative Woodchuck Enrichment Program!
James Brennan, MLAS, CMAR, LATG is a Program Manager & Training Coordinator at the University of Connecticut, Storrs, CT.
48 Laboratory Animal Science Professional January 2024
Figure 6. Oral dosing.
Figure 5. Woodchuck social housing.
Essential online training for investigators, technicians, veterinarians, managers, and IACUC members in the laboratory animal science field.

Animal Biosafety Training Program Certificate – 16 courses
260+ courses on animal research and compliance – courses customizable
Translatable instantly to 100+ languages – website and courses
Training documentation
American Association for Laboratory Animal Science | 9190 Crestwyn Hills Drive, Memphis, TN 38125-8538 (901) 754-8620 | (901) 753-0046 | www.aalas.org | info@aalas.org
https://aalaslearninglibrary.org
Switching to Vendor Decapsulated Artemia Embryos to Feed Zebrafish: A Cost and Labor Analysis
By Rachel Lenkey, BS, RLATG, Stephanie Slater, BS, RLATG, and Dante D’India, MS, RLATG
Artemia salina nauplii (brine shrimp) are a favorite and commonly fed food of zebrafish. Artemia eggs are sold as a thick-shelled cyst which can remain dormant for years until they are exposed to saltwater. To remove this outer cyst, which is not digestible and provides no nutritional benefit to zebrafish, a multistep process called decapsulation is performed in-house. Decapsulation calls for hydrating the cysts in water, soaking them in a combination of chilled saltwater, lye, and sodium hypochlorite, followed by rinsing them, then soaking them in a neutralization solution, rinsing again, and finally portioning out the embryos into glass bottles topped off with saltwater. When a new culture of Artemia is needed, one bottle of embryos is added to 12 L of aerated saltwater, and 48 h later the hatched Artemia are collected and dispersed to feed bottles. This is a labor-intensive process. However, multiple vendors are now offering Artemia embryos that have been decapsulated and can be fed directly to fish without culturing them. They have become widely accepted in the zebrafish community after it was shown that switching to these vendor decapsulated embryos provided no negative effects on growth, fecundity, and mortality rate and often improved

these metrics.1,2 We assessed the labor and potential cost savings of switching to vendor decapsulated Artemia embryos, including a long-term labor comparison of two Artemia sources.
Materials and Methods
Grade A Brine Shrimp Eggs Breeders Special and Hatching Shell-Free Brine Shrimp Eggs E-Z Egg 1 KG, (Brine Shrimp Direct, Ogden, UT) were used to compare in-house and vendor decapsulated Artemia cost and labor needs (Figure 1).
All activities were approved by Seattle Children’s Institutional Animal Care and Use Committee.
To calculate the total cost of in-house decapsulated Artemia we added the costs of the following: 1) supplies, including the cans of cysts, salt, and decapsulation chemicals (sodium hypochlorite, sodium hydroxide, and sodium thiosulfate); and 2) animal technician labor.
To calculate the total cost of vendor decapsulated Artemia we added the following: 1) supplies, including the bottle of decapsulated embryos; and 2) animal technician labor.


A B C D

50 Laboratory Animal Science Professional January 2024 TECH TIPS - HUSBANDRY, ENRICHMENT, NEW TECHNIQUES AND TACTICS
Figure 1. A) Starting product of encapsulated eggs for in-house process. B) Bottle with end product of decapsulated eggs via the in-house process. C) Container of E-Z Egg containing vendor-prepared decapsulated eggs in solution. D) Bottle with solution of in-house prepared decapsulated eggs from E-Z Egg bottle.
To calculate the total labor cost for the animal technician we tracked the following time spent: 1) for in-house decapsulated Artemia, we tracked the labor involved with preparation of solutions (salt, lye, saturated brine, sodium thiosulfate, and buffered salt), cyst hydration, decapsulation of cysts, neutralization and rinsing of cysts, preparation of refrigerated storage bottles with ‘ready to hatch’ Artemia embryos; and the set-up of hatchers, harvesting, and cleaning processes; and 2) for vendor decapsulated Artemia, we tracked the labor involved in the preparation and cleaning of feed bottles. A minimum of 15 min was charged for each step. The animal technician’s hourly wage plus 30% to account for benefits was used to calculate labor costs.
Discussion
Based on the results of this cost and labor analysis, we made the decision to switch to vendor decapsulated Artemia fully in September 2023. Another consideration was the size of our zebrafish program. Our facility currently averages around 2,500 fish and 250 tanks. If the census were to double in the future the in-house decapsulation process would have to occur more frequently, increasing the cost of labor and supplies. By feeding vendor decapsulated Artemia embryos, even if supplies doubled in cost, the labor cost would be unaffected because making additional or larger feed bottles adds a negligible amount of time to the process and our program would still see an overall cost savings.
At the time we performed our cost and labor study, we also collected stock performance data on growth, fecundity, and mortality and found that results were consistent with the referenced literature.
Disclaimer
The mention of trade names or commercial products in this study is solely for supplying specific information and does not imply recommendations or endorsement by Seattle Children’s Research Institute.
Results
The total monthly cost of decapsulating and feeding in-house decapsulated Artemia was US $429.49 and the total monthly cost for feeding vendor decapsulated Artemia embryos was US $262.25 (Table 1).
The in-house decapsulation and bottling process required 4 h and was performed every 7 wk. Set-up, harvesting and cleaning of the Artemia hatchers required 30 min. The monthly in-house decapsulation labor cost was calculated at US $380.92 (Table 1). The time required was estimated as 106 h annually which is 5.10% of the total hours worked in an 8 h workday. Preparation and cleaning of vendor decapsulated Artemia bottles required 15 min, 3 times a week. The monthly vendor decapsulated labor cost was calculated at US $137.93 (Table 1). The time required was estimated at 39 h annually, which is 1.88% of the total hours worked in an 8 h workday.
Switching to vendor decapsulated Artemia embryos led to a monthly cost savings of US $167.24 and labor needs were reduced by 63.21%. Annually, the cost of vendor decapsulated Artemia embryos cost US $3,147.00 versus US $5,153.88 for in-house decapsulated Artemia, accounting for a 39% cost savings of US $2,006.88 annually.
Acknowledgments
We thank the incredible members of Seattle Children’s Research Institute for improving the lives of children every day and the Office of Animal Care staff who take such excellent care of the animals.
Rachel Lenkey, BS, RLATG is a Quality Assurance Specialist with the Office of Animal Care at Seattle Children’s Research Institute, Seattle, WA.
Stephanie Slater, BS, RLATG is an Animal Technician III with the Office of Animal Care at Seattle Children’s Research Institute, Seattle, WA.
Dante D’India, BS, MS, RLATG is the Manager of the Office of Animal Care at Seattle Children’s Research Institute, Seattle, WA.
REFERENCES
1. Lim LC, Dhert P, Sorgeloos P. 2003. Recent developments in the application of live feeds in the freshwater ornamental fish culture. Aquaculture. 227:319–331.
2. Tye M, Rider D, Duffy EA, Seubert A, Lothert B, Schimmenti LA. 2015. Nonhatching decapsulated Artemia cysts as a replacement to Artemia nauplii in juvenile and adult zebrafish culture. Zebrafish. 6:457–461.
January 2024 Laboratory Animal Science Professional 51
Monthly cost (US$) Type of cost IHD VD Supplies: 48.57 124.32 Labor: 380.92 137.93 Total monthly costs: 429.49 262.25 Total annual costs: 5,153.88 3,147.00
Table 1. The monthly and annual cost of feeding in-house decapsulated (IHD) and vendor decapsulated (VD) Artemia
A Cost-Effective Restraint Method for Safer Subcutaneous Scruff Injections and Reducing Stress in Mice and Handlers
By Anna Fitzwater, DVM and Luwen Zhang, PhD
Introduction
Mice share over 95% sequence homology with humans, enabling valuable insights into human gene function and disease. Manipulating the mouse genome has generated numerous strains to examine research questions. Mice provide a practical, cost-effective model compared to larger mammals, with fewer ethical concerns. The combination of genetic relevance, physiological similarity, and practicality has made the mouse a fundamental biomedical research tool.
Subcutaneous injection is a vital technique in mousebased biomedical research.2 Compared to other injections, the subcutaneous method poses fewer technical challenges and reduces acute toxicity risks due to slower absorption.1 The subcutaneous bleb formation also can confirm proper delivery. Given the frequent need for injections in mouse studies, subcutaneous proficiency is a core research skill.2
Several suitable subcutaneous injection sites exist in mice, including the scruff/neck, flank, abdomen, and chest. The scruff provides the most convenient site for easy access to loose skin for tenting and injections. However, this site allows mice to see the incoming needle and potentially bite or scratch. Resulting movements increase stress in both the mouse and handler. To resolve these issues, we have developed a method for safe, effective scruff injections that mitigate associated risks to mouse and handler.
All animal procedures were performed in accordance with all relevant institutional and governmental ethics guidelines and regulations. This protocol was approved by the Institutional Animal Care and Use Committee of the University of Nebraska-Lincoln.
Materials
and Methods
Materials unique to this method are 50 mL centrifuge tubes or waste plastic bottles, colored paper/tape or non-transparent duct tape, a ruler, and scissors (Figure 1A). Materials needed for routine subcutaneous injection are the injectable substance, sterile syringes with needles, alcohol swabs, and gauze pads.
1. Wrap a 50 mL centrifuge tube or cut waste plastic bottle with colored tape or non-transparent duct tape to make it light proof (Figure 1B). If an opaque tube is used, this step may be omitted. If a waste bottle is used, leave at least 2 inches of uncut material at the closed end to provide enough space for the mouse head.
Caution: Sterile tubes are strongly recommended. A Fisherbrand conical polypropylene centrifuge tube (catalogue #: 05-539-13; 3-millimeter diameter) was used in Figure 1. Opaque black 50 mL centrifuge tubes are better options available from Light Labs (catalogue # HD4429-1; https://www.lightlabsusa.com/Opaque-Black50ml-Centrifuge-Tubes.html). If emptied plastic water bottles are used, they should be decontaminated according to the SOP and BSL requirements governing the procedure. For repeated use of the device, you can choose either opaque centrifuge tubes or wrap translucent tubes twice with autoclave indicator tape, such as 3M Comply Steam Indicator Tape 3/4” (ITEM#1322-18MM). Both options allow repeated autoclaving as needed.
2. Cut a gap in the taped tube at the open end (Figure 1C). The width should be 0.4-0.7 cm and the depth should be 2-3 cm.
3. Secure the taped tube to a lab bench with additional tape, keeping the open gap at the top (Figure 1D).
The lab bench should have a smooth surface so the animal cannot gain traction and move forward or backward suddenly.
4. Gently but firmly grasp the mouse by the neck scruff. 5. Insert the mouse’s head into the taped tube, positioning the scruff inside the gap. Gently pull the mouse’s neck against the wall of the tube to restrain it and block its vision (Figure 1E).
The diameter of the device should be appropriate for the rodent size (Figure 1F). This method can used for injecting rats with the use of an appropriately sized tube diameter and gap dimensions. Vision blocking is tempo-
52 Laboratory Animal Science Professional January 2024 TECH TIPS - HUSBANDRY, ENRICHMENT, NEW TECHNIQUES AND TACTICS
rary and does not cause undue distress.
6. Select the desired subcutaneous injection site(s).
7. Follow standard procedures using sterile equipment, disinfectant, and proper injection technique to administer the substance. After injection, monitor the mouse as usual post-injection.
Discussion
These devices use routine labware or plastic bottles, so the cost is minimal. The key is to block the vision of the mouse using a simple restraint. Several major benefits are associated with this procedure.
• Reduced bite risk; because of the device structure, the mouse cannot bite or scratch the handler, improving safety, especially for injections of potentially hazardous substances.
• Easier handling; removing visual stimuli helps minimize the mouse’s instinct to struggle or suddenly move during injection. The mouse is less likely to resist. This makes injections easier to perform.
• Reduced stress; not seeing the injection reduces anxiety and fear responses in the mouse, helping keep the
Figure 1. How to make the subcutaneous injection device.
A) Necessary materials needed for making a device: 1) A 50 mL centrifuge tube (with a diameter of 3 centimeters) or a waste plastic bottle with a suitable diameter, 2) Opaque colored tape or duct tape, 3) Scissors, and 4) A ruler.
B) Cut lengthwise, ensuring that you leave at least 2 inches of uncut material at the closed end. Wrap the tube with colored paper or autoclavable tape.
C) Create a gap in the wrapped tube using scissors. The width should be 0.4-0.7 cm, and the depth should be 2-3 cm. Make sure the edges of the cut surface are not sharp.
D) Attach the tube with tape to a flat, smooth surface such as a bench or table top.
E) Insert the mouse’s head into the taped tube. Gently pull the mouse's neck (scruff) toward the gap and against the tube. The mouse is now ready for injection.
F) Ensure that the diameter of the device is appropriate. A smaller mouse (Panel E) might require a 50 ml conical tube whereas a large mouse (Panel F) will require a larger tube.
animal calmer. In addition, the safer injection procedure will also reduce handler stress.
In summary, we describe the use of a simple device for safe and effective subcutaneous injections of mice and possibly other rodents, which can reduce stress for both animals and handlers.
Anna Fitzwater, DVM, is the Senior Clinical Veterinarian at University of Nebraska-Lincoln in Lincoln, NE.
Luwen Zhang, PhD, is Professor of Biological Sciences at the Nebraska Center for Virology at the University of Nebraska in Lincoln, NE.
REFERENCES
1. Levin-Arama M, Abraham L, Waner T, Harmelin A, Steinberg DM, Lahav T, Harlev M. 2016. Subcutaneous compared with intraperitoneal ketamine-xylazine for anesthesia of mice. J Am Assoc Lab Anim Sci 55:794-800.
2. Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613.
January 2024 Laboratory Animal Science Professional 53
CONNECTION

A A L A S 2024

2024 National Meeting Update
Submit a Topic or Abstract for 2024
We hope you will consider submitting a topic or abstract for the 2024 National Meeting in Nashville, TN.
The Program Committee and AALAS President Robert Quinn invite you to submit proposals for abstracts, technical trade presentations, panel discussions, seminars, special topic lectures, and workshops for the 2024 National Meeting.
Go to Abstract Central to access the portal. Those who have submitted before will have an existing username and password. New users must create an account. The deadline for topic submissions is March 15, 2024 and the deadline for abstract submissions is June 1, 2024.
Exhibit Your Products and Services in Nashville!
Exhibitor registration is now open! As an exhibitor you have an opportunity to interact with more than 4,500 AALAS attendees from the academic community, research institutions, government organizations and commercial companies.
Interested in partnering with AALAS and supporting our mission? Consider becoming a National Meeting sponsor! Review our 2024 Media Kit on our website and consider the opportunities to promote your products to our AALAS decision-makers. For more information contact Heather Lampi at hlampi@aalas.org.
Recognize a Deserving Colleague with an AALAS Award
Six professional categories are recognized at the AALAS National Meeting for excellence in the laboratory animal science field. The six awards which will require nominations are the Bhatt, Brewer, Collins, Garvey, Griffin, and Technician of the Year. Nominees and nominators for all awards must be current national members of AALAS as of January 1 of the nominating year. Nominations are due by April 1, 2024. Over $13,000 in prize money will be awarded!
54 Laboratory Animal Science Professional January 2024 AALAS
NASHVILLE,
75TH NATIONAL MEETING NOVEMBER 3 - 7
TN
Join us in achieving our 25th Anniversary goal by contributing a $25 donation on the 25th of each month. Don't worry - if you miss the 25th, your donation on any other date in the month still counts! Consider the convenience of signing up for automatic monthly donations to effortlessly support our cause. Together, let's make a meaningful impact!
With your help, we look forward to another successful twenty-five years of advocacy and providing free resources to assist AALAS members with their public outreach activities.
1950 2025 th ANNIV E RSARY
25 25 ON AALASFOUNDATIONCAMPAIGN 25CAMPAIGN 25 25 ON
25 25 ON AALAS FOUNDATION CAMPAIGN
AALASFOUNDATIONCAMPAIGN
AALAS Foundation Announces Results of 2023 National Meeting Events
The AALAS Foundation thanks everyone who participated in all of its events at the 2023 National Meeting in Salt Lake City, Utah.
A special thanks to the Foundation’s National Meeting Chair, Jessica Hunt and National Meeting Vice Chair, Sarah Gilliam, and the committee members who helped organize and plan this year’s events – along with all of the many volunteers who helped out at the AALAS Foundation’s booth!
The following is a breakdown of the results for each event:







“Boot Up for Research” Contest











This year’s fundraising contest required entrants to creatively decorate an 11” wood cowboy boot. There were seventy-three contestants in this year’s contest. The contest raised a total of $9,650 to benefit the AALAS Foundation and its mission.
Congratulations to the following contest winners!



Branch Category Winner
Central Ohio Branch; Artist: Carla Waddell
Corporate/Company Winner
AbbVie Animal Care Staff; Artists: Cassy Weinberger, Brandon Cuevas, Kaitlyn Kuzmic, Carly Stoneman & Andrea Kachellek
Individual Category Winner
Jessica Hunt; Artist: Jessica Hunt
Institution/Organization Category Winner
University of Maryland; Artist: Denise Castellon
56 Laboratory Animal Science Professional January 2024
Brian Ebert - AbbVie - Corporate Contest Winner
Larry Shelton - University of Maryland - Inst. Winner of Contest
Jessica Hunt - Individual Winner - Contest

The “Fan Favorite” Winner Central Ohio Branch; Decorator: Carla Waddell
The “Best of Show” Award went to University of Texas Health Science Center at Houston; Artists: Kelly Williams & Jeanette Pearson
Silent Auction
The AALAS Foundation’s virtual Silent Auction was a great success – thanks to all of the generous auction donors and bidders! Everyone’s support of the virtual Silent Auction helped raise a total of over $9,000 to benefit the AALAS Foundation and its mission.



January 2024 Laboratory Animal Science Professional 57
Molly, Lucy, Lisa, and Vicki at reception
Auction Display Cases
Aaron and Sarah at booth
Jessica Hunt and Lynne at booth



Appreciation Reception & Live Auction
The 2023 Appreciation Reception and Live Auction was a fabulous success! Thanks to our amazing auctioneer, Brian Ebert, and to all who donated so many incredible auction items, along with everyone who generously submitted bids! Your efforts helped us raise over $25,000!
We are especially thankful to all of our Appreciation Reception & Live Auction sponsors who made this important event possible.
$10,000 Level Sponsor
Gruenberg Dry Heat Sterilizers
$5,000 Level Sponsors
Allentown
Lomir Biomedical, Inc.
Priority One Services, Inc.
Systems Engineering - SE Lab Group, Inc.
$1,000 Level Sponsors
American Protective Products
Animal Care Systsems
Bio-Serv
Innovive
Inotiv
Pharmacal
The AALAS Foundation was also happy to recognize the following companies for being a first time Platinum+ level donor:
American Protective Products
Gruenberg-Dry Heat Sterilizers
Process Control Solutions
Systems Engineering - SE Lab Group, Inc.
The AALAS Foundation was honored to recognize the following individuals who were recently added to the virtual Memorial Wall of Honor – each of whom made lasting contributions and impact to the laboratory animal science community during their lifetime:
U. Kristina Stephens
Dr. Richard Fish
Dr. John Young
Dr. Jerald Silverman
Camellia “Cammie” Symonowicz
Dr. Nicole Duffee
Branch POE Award Winner
The Texas Branch was recognized during the Appreciation Reception as the 2023 Branch POE Award winner. This award is based on public outreach efforts conducted by branch members during the time period of September 1st through August 31st. The Texas Branch received a $100 gift card and certificate of appreciation for their outstanding public outreach efforts in 2023.
Thanks again to all who supported the AALAS Foundation at the 2023 National Meeting! We look forward to seeing everyone at the 2024 National Meeting in Nashville, Tennessee!
58 Laboratory Animal Science Professional January 2024
Gruenberg plaque presentation
Systems Engineering plaque presentation
Lisa Kelly - Outgoing Board Chair Presentation







January 2024 Laboratory Animal Science Professional 59
Branch Poe Award Winner: Texas Branch
Bruce Kennedy - John Young Award Presentation
Gail Thompson - U. Kristina Stephens Award Presentation
Peg Hogan - Richard Fish Award Presentation Mike Fallon - Nicole Duffee Award Presentation Lisa Secrest - Cammie Symonowicz Award Presentation
Lisa Barber and Mark Sharpless - Jerald Silverman Award Presentation
60 Laboratory Animal Science Professional January 2024 —Advertising List— Contact us for more information. Visit us at aalas.org or call Heather Lampi at (901) 754-8620. Advertise in LAS Pro COMPANY WEBSITE PAGE Allentown LLC www.allentowninc.com back cover Ancare Corp www.ancare.com 27 Bio-Serv www.bio-serv.com 11 Braintree Scientific Inc. www.braintreesci.com 35 Clear H2O Inc www.clearh2o.com 1 E-Z Systems Inc. www.ezsystemsinc.com 5 Hilltop Lab Animals Inc www.hilltoplabs.com 60 Kent Scientific Corporation www.kentscientific.com 25 PMI LabDiet www.labdiet.com inside frontcover VRL Laboratories-USA www.vrlsat.com 34

WE’ M T YA THERE!
75TH AALAS NATIONAL MEETING NOVEMBER 3 - 7, 2024
Join us for the 75th AALAS National Meeting in Nashville, Tennessee. Each fall since 1950, the American Association for Laboratory Animal Science has held its annual National Meeting. During the five days of the meeting, members and nonmembers come together to enoy the workshops, lectures, poster sessions, and exhibits. The program is designed to have topics relevant to the entire membership. Exhibitors have an opportunity to interact with AALAS members from the academic community, research institutions, government organizations, and commerical companies.
The AALAS National Meeting is the largest gathering in the world of professionals concerned with the production, care, and use of laboratory animals.
1950 2025 th ANNIV E RSARY


01-2024 Learn more: www.AllentownInc.com www.ClorDiSys.com
dioxide gas
residue-free, safe sterilization
research
worry-free decontamination
most
AIRFLOW TECHNOLOGY WASHING/ AUTOMATION STERILIZATION DECONTAMINATION COLONY HEALTH MONITORING
Expanding our global solutions so we can serve you better.
■ Chlorine dioxide gas and UV-C decontamination and disinfection equipment and services to enhance your research requirements ■ Safety first: Setting the benchmark for unparalleled decontamination, especially in sensitive research environments – your safety is our priority ■ Experience peace of mind: Chlorine
ensures
for
equipment, providing
in your
critical environments HOUSING
VIVARIUM DESIGN SOLUTIONS



















 Degan Mesler Digital Media Marketing Strategist
Degan Mesler Digital Media Marketing Strategist



















































































































































































































































































































