Exploring the Care and Use of Zebrafish in Research
The research studies with zebrafish are not only fascinating, but also vital to the world of laboratory animal science
2022 National Meeting in Review
Getting to Know Thomas Joseph, AALAS’s Executive Director
Zebrafish Nutrition: Everything Known and to be Known

January/February 2023
We back each ot her up for you r emergency nee d s .
We are an alliance of proven suppliers to the industry We are spread out across the country and globe to serve our local markets with LabDiet® and other supplies; and in times of troubles...service yours!
• We are positioned to provide feed, bedding, and more like no other supply-chain in the industry


• We have clean, organized, and safe facility environments.
• We are skilled professionals, trained and focused on your research standards.

A N I MA L S P ECI A LT I E S & P R OV I SIO N S ST E WA R T S FEE D L A B S U PP LY L A B S U PP LY L A B S U PP LY L A B S U PP LY A N I MA L S P ECI A LT I E S & P R OV ISIO N S A N I MA L S P ECI A LT I E S IN C . N E WCO N E WCO T R L A S T CIN CI N N AT I L A B SU P P LY G AT E WAY L A B SU PP LY T U S CU L U M FEE D FR O N T I E R D I ST R I B U T I O N WA L D S CH MI DT & S O N S S COT TP HA R M A S O L U T I O N S W. F. FI S H E R & S O N I N C . ©2 02 2 L A B D I E T A LL R I G HT S R E S E RV E D


Protect you and your animals with fast, effective and safer disinfectants. ALWAYS ON GUARD Peroxigard com Peroxigard™ is a member of the Virox family of brands. * Applicable to Peroxigard Ready-to-Use and Wipes only (except C.bovis claim with a 2-minute contact time). You must read and follow the instructions on the label for our product and use the product for the particular applications specified on the product label. Product shots have been digitally altered and are not accurate as shown. Refer to product label or reference sheet for a complete list of disinfection claims and additional features. Accelerated Hydrogen Peroxide® and Design are trademarks of Diversey, Inc. B-010923-8292-02F © 2023 Virox Technologies Inc. 1 Minute Disinfect in * Creators of
January/February 2023 Vol. 11 Issue 1
That’s A Wrap!
Reflecting on our 2022 National Meeting in Louisville, and setting our sights on Salt Lake City

20 22 26
28 30
Q & A
Learn more about Tom Joseph and his plans for AALAS

A Comprehensive Overview
The significance of zebrafish in biomedical research

The Pros of Hiring the Pros
The benefits of hiring specialized aquatic staff
Two Are Better than One
Why we need technology and technology needs us
Nutrition 101
What we’ve learned about zebrafish nutrition
On the cover: Joshua Barber, MA, LATG, is the Senior Manager of Aquatic and Reptilian Life at Columbia University in New York City, NY. Working alongside Morgan McCloud, he curated and guest edited all zebrafish columns. We cannot thank him enough for his meaningful contribution to this special topic edition of LAS Pro.

2 Laboratory Animal Science Professional January 2023 January/February 2023 2022 National Meeting in Review Getting to Know Thomas Joseph, AALAS’s Executive Director Zebrafish Nutrition: Everything Known and to be Known Exploring the Care and Use of Zebrafish in Research The research studies with zebrafish are not only fascinating, but also vital to the world of laboratory animal science 10
INSIDE THIS ISSUE...
18 20 28
Rodent dosing, refined.

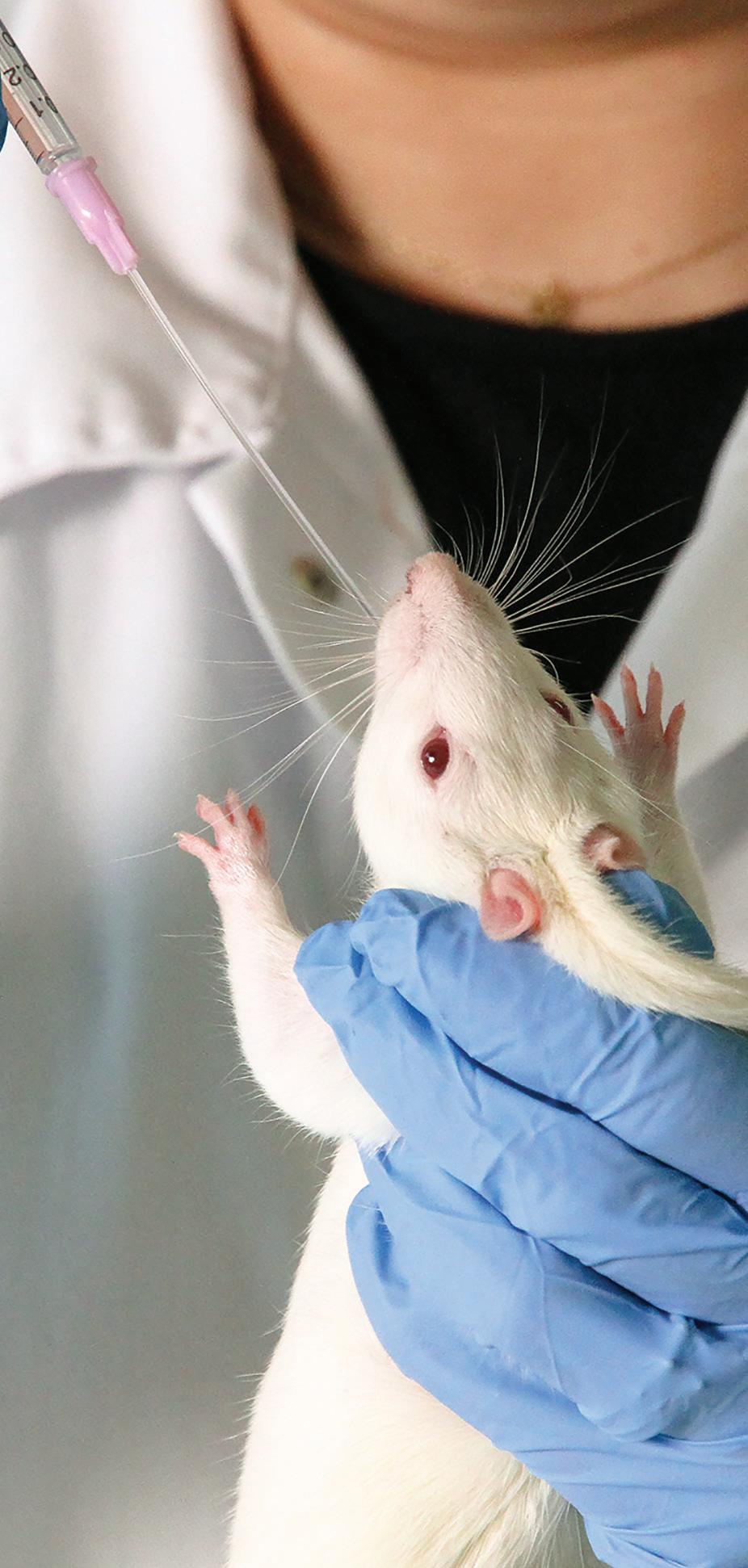
Our flexible gavage tubes have a soft tip to minimize trauma, and they are disposable to eliminate cross contamination and the hassle of cleaning. Available in a range of sizes for mice, rats and other rodents.









*Shown actual size.
Instech Laboratories, Inc. Plymouth Meeting, PA USA REQUEST FREE SAMPLES www.instechlabs.com/sample-request



4 Laboratory Animal Science Professional January 2023 into a polyculture nursery tank. (B) Rinsing the fish into the polyculture nursery tank DEPARTMENTS 56 54 5 Publisher’s Note Looking Forward to the Future 6 People & Places New hires, promotions, awards, memorials 8 PROfiles Meet Joshua Barber 34 Inside the IACUC IACUC Concerns and General Incubator Management 38 Career & Training Facing Difficulties in Virtual Learning 42 Tech Tips Insights on techniques and tactics 51 Crossword – Zebrafish 52 AALAS Connection 2023 AALAS Election 54 AALAS Foundation POE Winner for 2022 55 Ad Index 56 Pet Talk Gravy Boat 44
PUBLISHER’S NOTE

Looking Forward to the Future
First and foremost, I want to express how honored I am to join the AALAS staff as the new Executive Director. Ann Turner has been crucial in making my transition smooth and successful, and I look forward to building the next floor of this organization from Ann’s solid foundation. Along with thanking Ann, I’d like to thank the AALAS staff for the warm welcome. It’s evident that this field is filled with committed and passionate individuals, and I take pride in working alongside you all. I plan to honor the legacy of those who came before me and lead us into the
As we continue evolving and growing, staying informed with our peers in the laboratory animal science world remains vital. This recurring column in the magazine presents an opportunity for us to elevate the voices of leaders in our field. Advancements in our line of work come from one of the greatest attributes of our community: colleague interactions. Working and growing alongside each other
Staff
Publisher Thomas L. Joseph
Associate Publisher Chris Lyons

Managing Editor John Farrar
Associate Editor Morgan McCloud
Ad Sales John Farrar
Design/Production Zara Garza
Editorial Advisory Board
Leslie Birke Louisiana State Univ
Andrew Burich Benaroya Research Institute
Bob Dauchy Tulane Univ School of Medicine
David DeOrnellis Champions Oncology
Penny Devlin Pennsylvania State Univ College of Med
Sonia Doss Duke Univ Medical Center
Kelly Ethun Emory University
Glenn Jackson Cornell University
Richard Marble Alpha Genesis Inc
Elizabeth Nunamaker Charles River Laboratories
Sara Oglesby Abbvie
Karuna Patil Seattle Children's Research Institute
Amy Pierce Tulane Univ School of Medicine
Stacy Pritt UT Southwestern Medical Center
Robin Tucker Georgetown Univ
Mission Statement
of you, and it’s important to me to ensure that AALAS is an organization that
I wish you nothing but success for 2023 and want to remind you, that I along
Laboratory Animal Science Professional (LAS Pro) is the official magazine for American Association for Laboratory Animal Science members. LAS Pro provides a wide range of useful resources and knowledge to the association’s 14,000 laboratory animal science professionals who are involved in advancing responsible laboratory animal care and use to benefit people and animals. All signed articles, including, committee reports, news, and commentary, reflect the individual views of the authors and are not official views of AALAS.
Authorization to photocopy portions for personal or internal use is granted by the American Association for Laboratory Animal Science. Photocopying for purposes of resale or outside distribution is prohibited unless written approval is obtained from the AALAS Director of Communications.

Copyright 2023 by the American Association for Laboratory Animal Science.
Laboratory Animal Science Professional (USPS 010-730) is published bimonthly by the American Association for Laboratory Animal Science, 9190 Crestwyn Hills Drive, Memphis, TN 38125. Periodicals Postage paid at Memphis, TN 38101 and additional mailing offices.

POSTMASTER: Send address changes to AALAS, 9190 Crestwyn Hills Drive, Memphis, TN 38125-8538.
American Association for Laboratory Animal Science 9190 Crestwyn Hills Drive Memphis, TN 38125-8538
Phone: 901-754-8620
Fax: 901-753-0046
E-mail: info@aalas.org
Web: www.aalas.org
January 2023 Laboratory Animal Science Professional 5
-
Kelly S. Patterson Completes AAALAC Fellowship

!

After COVID postponed her trip, Kelly Patterson traveled to the UK to complete her AAALAC Fellowship. Here is what she had to say about her trip.

“I would like to say no words can come close to expressing my sincere gratitude for being awarded this fellowship. One US fellow I spoke to shortly before leaving for my trip mentioned it was “life-changing.” I wholeheartedly agree. I would do it again if I could, but instead, I am paying it forward and using my experience to engage colleagues and encourage them to apply for this once-in-a-lifetime opportunity. I would also like to sincerely thank AAALAC International, Datesand Group, MRC, NIH, and Priority One Services for sponsoring this amazing fellowship, all my new friends in the UK that served as a host and tour guides, and the selection committee and my nominating colleagues and friends for believing in me!”
Certifications


Autumn Ruiz successfully passed her AR exam on October 5, 2022. “I can’t wait to be able to say I passed the entire CMAR exams and earned my certification,” says Ruiz, “but these two steps are amazing.”
Gilberto Collazo successfully passed his CMAR exam in December of 2022.


Matthew Hope successfully passed his ALAT/RALAT exam in November of 2022. He says that his LAT is up next.
Emily Lambert successfully passed her ALAT/RALAT exam in November of 2022.
6 Laboratory Animal Science Professional January 2023
!
TOMIMIST.COM | 800.525.1698 UPGRADING YOUR DECON? WE’VE GOT YOUR SOLUTION. Need to decontaminate a vivarium, BSL 3&4, cabinet, or an entire facility? SteraMist products and service eliminate human error with superior compatibility. cGMP COMPLIANCE. NON-REACTIVE APPLICATION. FASTER RESULTS.
Gilberto Collazo
Matthew Hope
PEOPLE & PLACES - NEW HIRES, MEETING UPDATES, AND MEMORIALS
Emily Lambert
Features:
• Compliant with AVMA Guidelines


• Cycle starts automatically when cage is docked
• Choose between Adult and Neonates
• LED indicator lights show when cycle is running and when complete

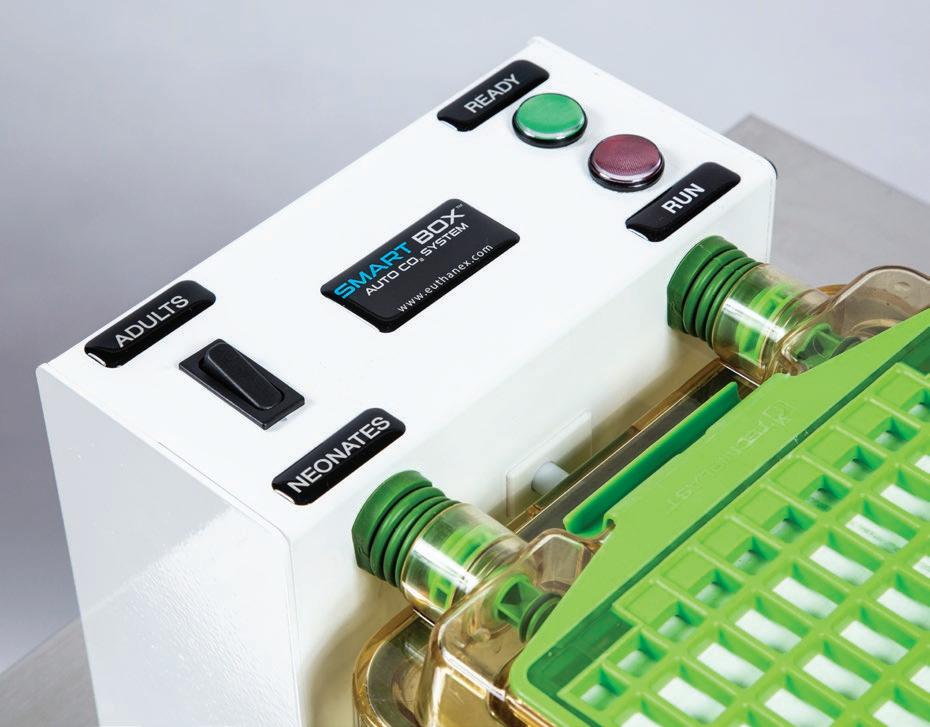
• Automatically shuts off if cage is removed for safety
• Uses house or bottle CO2 supply at 15-100 psi
• Super E-Z

EZ-DOCK SMARTBOX® Euthanasia Station When Simplicity and Home Surroundings Matter... Toll-Free: 1.877.559.0159 • Phone: 610.882.3800 www.ezsystemsinc.com
with
Other
Available. Scan to watch video ®
Shown
Tecniplast GM500 Cage.
Cage Types
Getting Personal
What companion animals do you have?
A tank full of fish and 3 snakes named Fluffy, Fancy, and Freddie.
Best binge-watching TV series? Superstore or Scrubs.
What is the last book you read?
The Quarterback by Allen Ginsberg.
Where is your dream vacation spot?
I don't think I'd be able to dream up a better vacation than my trip to South Africa.
What is your favorite dessert?
Death by Chocolate. Is there a better way to die?
minutes with... Joshua Barber, MA, LATG


LAS PRO-files
Facility/Employer: Columbia University
Current Job Title: Senior Manager of Aquatic & Reptilian Life
How did you get in this field? I was the sort of nerdy kid who memorized the Dewey Decimal system so that I could bee-line for the fish and reptile encyclopedias in the library. I majored in biology but I had never heard of laboratory animal science and a random interview to do zebrafish husbandry at the NIH turned into a career in aquatics. I now focus on management of facilities for a number of aquatic animals used in medical research.
Who were your mentors? My primary mentor was a research scientist named Chao Yang at HHMI Janelia. Although my focus was animal husbandry, he took a special interest in teaching me genetics, gene manipulation, screening techniques and microinjection. These skills helped to propel my career and I owe him a lot.
What are your current interests in animal science? I’m captivated by our ability to manipulate the genome. Almost every scientist in the lab does some aspect of this and we can edit DNA to code for anything we want. In particular, we’ve developed this protein synthesized within the animal called GCamp which lights up during brain activity and I think it’s extremely cool.
Where do you see yourself in 5 years? I love managing as much as I love fish and reptiles. I want to stay in this field but continue to grow our aquatics program to the point where it’s known nationally as a great place to do research. I also want to be a top dog in the zebrafish community!
What is your favorite part of your job? I think my favorite part of my job is working with zebrafish embryos. It’s so familiar and relaxing and takes me back to my technician days. I also love seeing them develop into little fish.
What advice do you have for others just beginning their animal science career? More than anything else, I found that a positive attitude and treating others with kindness goes so much farther than you’d expect. I’ve been in the field for 12 years and every single job has come from someone who recommended me. Obviously there’s some hard work in there but you’d be surprised by how much people remember a smile or a positive interaction.
What is the most rewarding aspect of your career? Plenty of us have imposter syndrome where we just don’t feel we measure up to our peers or even to our own job expectations. Once in a while I get this light-bulb moment when I’m helping out a researcher and I realize that I’m actually expert in my field. That sort of validation makes me proud to do what I do.
What is something unexpectedly interesting about your career? My job varies a lot in the day to day. To give an example, this week I implanted RFIDs in Xenopus frogs, gave a talk on rotifer culturing and assisted with design of a new aquatics facility. I also get to do necropsies like a pseudo-veterinarian!
8 Laboratory Animal Science Professional January 2023
5
Braintree Scientific has been providing research equipment to the Life Sciences Industry for over 40 years.
Our unique, hand-picked selection of products for mice and rats is extensive and has evolved with researchers’ needs and changing technology.
Scan to request our 2023 Braintree Scientic catalog
and ip through our pages!

LAB RESEARCH PRODUCTS 781.917.9526 | info@braintreesci.com | braintreesci.com
All the laboratory products you need right at your ngertips.
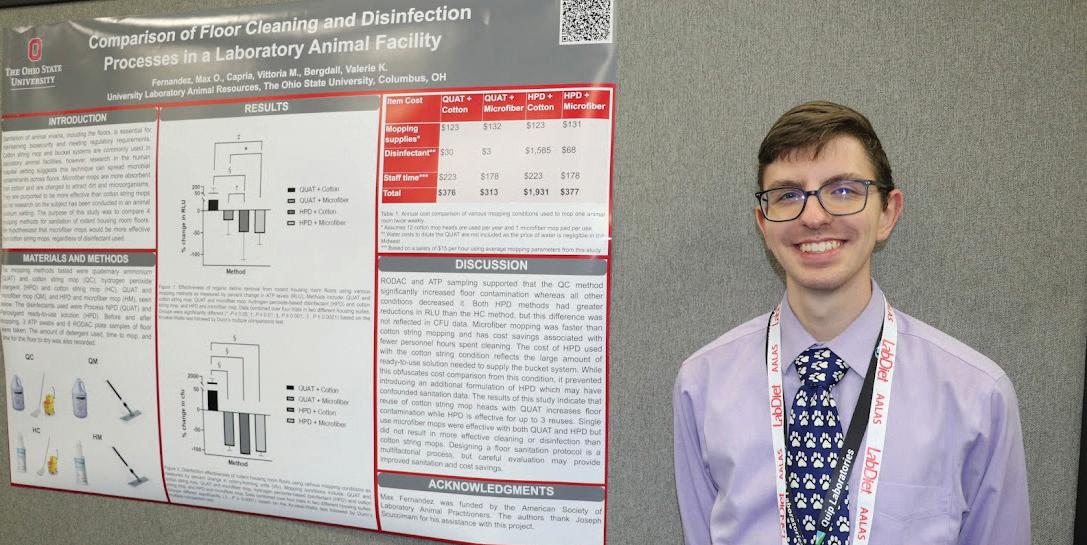
The 2022 AALAS National Meeting was held in Louisville, KY, from October 23-27. Total registration for the meeting was 3118. We had 211 exhibitors participated this year. The event app was downloaded 2,299 times. This year, we partnered with VRL Laboratories to bring you Zelda the Zebrafish, our AI chat bot.
Facilitator Winners
The free registration to next year’s meeting was awarded to Mr. Joshua Frost, RLATG.
$50 gift certificates to the AALAS Bookstore were awarded to Dr. Vittoria M Capria and Mr. Juan E Sanchez-Lopez, RLATG.
Poster Winners
Animal Welfare, Training, and 3Rs Winners
Third Place: P36 Adopting an Extended-release Buprenorphine Postoperative Analgesic Regimen in Transgenic Mice; Melissa Reding
Second Place: P25 Design and Evaluation of an Artificial Intelligence Model for Automated Tail Vein Administration in Rodent Models, Jonguk Kim
First Place: P27 Precision Cut Lung Slices: A Versatile 3R Method for Pulmonary Research, Julia Herbert
Clinical Winners
Third Place: P114 Effects of Capromorelin in Buprenorphine-induced Poor Appetite in New Zealand
White Rabbits (Oryctolagus cuniculus), Hironori Kawano
Second Place: P124 Idiopathic Systemic Amyloidosis in Laboratory Society Finches (Lonchura striata domestica)
First Place: P109 Continuous Dosing of Buprenorphine Hydrochloride with A Subcutaneously Implanted Osmotic Minipump for Postlaparotomy Analgesia in Ferrets, Cara Goodrum
Husbandry/Management Winners
Third Place: P221 Who's that Spiny Mouse?
Identification Methods for Acomys cahirinus, Joette Crews
Second Place: P236 How I Learned to Quit Worrying and Love Cold Water, Samantha Crow
First Place: P200, Semiautomated Aquatic Live Feed Culture: A System Designed to Support Limited or Varying Production Needs, Adedeji Afolalu
Laboratory Investigations Winners
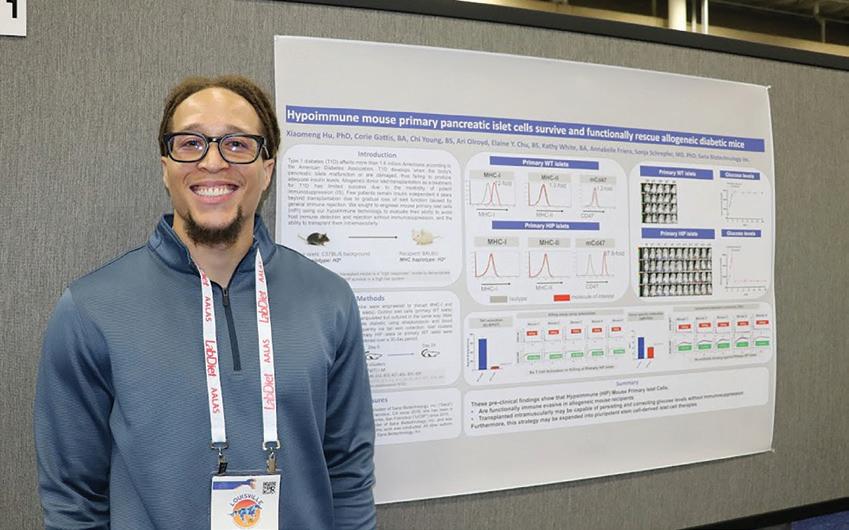
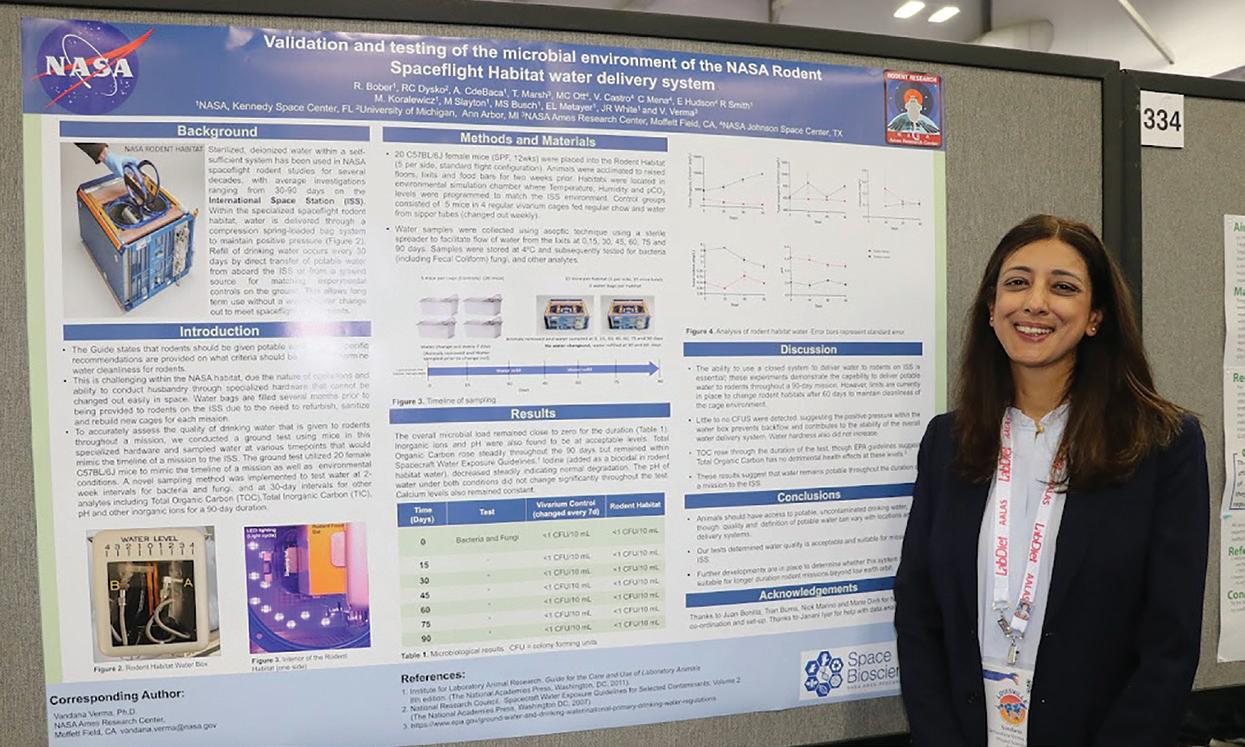
Third Place: P342 Testing and Validation of the Microbial Environment of the NASA Rodent Spaceflight Habitat Water Delivery System, Vandana Verma
Second Place: P313 Ear Slicing of PD 7 C57BL/6 Mice as a Method for Permanent Identification and Genotyping, Diane Chen
First Place: P344 Analysis of Water-based Foam as a Depopulation Method for Adult Cattle, Vittoria Capria
73 RD AALAS NATIONAL MEETING RECAP


10 Laboratory Animal Science Professional January 2023 FEATURE – NATIONAL MEETING RECAP
AALAS Foundation National Meeting News



Thanks to everyone who made this year's AALAS Foundation Appreciation Reception & Live Auction a HUGE successespecially all our generous Live Auction donors and bidders. The AALAS Foundation is excited to announce the Texas Branch as the winner of the 2022 Branch Public Outreach & Education (POE) Award! Texas Branch was awarded a $100 gift card and certificate during the AALAS Foundation’s Appreciation Reception at the 2022 AALAS National Meeting in Louisville, Kentucky.
Congrats to all the “Racing for Research” contest winners!
Individual Category Winner: #001- Dianna Laurent Branch Category Winner: #084 - Research Triangle BranchArtists: RTB Executive Committee
Corporate Category Winner: 029 - BMS RWC- Vet Sciences
- Artists:,Deborah Calantropioi-Covington, Lindsay Bates, Angelica Cabrera, James Champion, Michael-Ann Sowick, Kelly Walton and Holli Zampano
Institution Category Winner: #010 - Emory National Primate Research Center; Artist: Elyse McClosky
Fan Favorite Winner: #003 - Central Ohio Branch; Artist: Carla
Waddell
Best of Show Winner: #003 - Central Ohio Branch; Artist: Carla
Waddell
Thanks to everyone who participated and supported this contest to benefit the AALAS Foundation and its mission!
LOUI SVILLE
January 2023 Laboratory Animal Science Professional 11









12 Laboratory Animal Science Professional January 2023





January 2023 Laboratory Animal Science Professional 13








14 Laboratory Animal Science Professional January 2023






January 2023 Laboratory Animal Science Professional 15




16 Laboratory Animal Science Professional January 2023





CLEARH2O.COM 1-888-493-7645 AquaPak® HydroGel DietGel MediGel® LabGel FiberBites® AquaFeed® Z The Clearly Better Solution • Ready-to-Use Pouches • No Capital Investment • Reduced Labor Pre-filled Pure Water AquaPak® INTRODUCING OUR LATEST INNOVATION
2022 AALAS AWARD WINNERS
Michael J. Huerkamp, DVM, DACLAM
Executive Director and Attending Veterinarian
Emory University
Nathan R. Brewer Lifetime Achievement Award

Dr. Huerkamp earned his veterinary degree in 1984 from The Ohio State University, completed his residency in laboratory animal medicine under the tutelage of Drs. Ben Cohen and Dan Ringler at the University of Michigan in 1987, and earned Diplomate status with ACLAM in 1988. He has been employed by Emory University since 1987 where he currently serves as Executive Director of the Division of Animal Resources and Attending Veterinarian with appointment as Professor in the Department of Pathology and Laboratory Medicine.
Jean A. Nemzek–Hamlin, DVM, MS, DACVS

Clinical Professor and Assistant Director of Research Unit for Laboratory Animal Medicine, University of Michigan
Pravin N. Bhatt Scientific Investiagtor Award
Dr. Nemzek is a Clinical Professor, Program Director for the Animal Surgery Operating Rooms, and Assistant Director of Research in the Unit for Laboratory Animal Medicine at University of Michigan. She is a graduate of University of Minnesota College of Veterinary Medicine and board certified in small animal surgery. Research in the Nemzek Lab explores novel immunomodulatory therapies for sepsis and acute lung injury, with emphasis on clinical relevance and welfare refinement of the animal models. Through these studies, Dr. Nemzek has mentored the research training of numerous students and graduate veterinarians at all stages of career development.
Mangala Gunatilake, BVSc, PhD, FSLCVS, MLAS

Professor in Physiology
Faculty of Medicine, University of Colombo
Charles River Prize
Dr. Gunatilake is a professor in the Department of Physiology, Faculty of Medicine, University of Colombo. She played a principal role in developing ethical guidelines for the use of laboratory animals in research in Sri Lanka. Dr. Gunatilake is the Founding President of the LAS Association and the Founding Director of the 3Rs Centre for LAS in Sri Lanka. She also organized the first postgraduate certificate and diploma courses in laboratory animal science in the Asian region. She is a recipient of National Honours and several national and international awards in recognition of her pioneering work and dedication to the field.
Jessica M. Stukes, BS, LATG

Education & Training Manager
Division of Laboratory Animal Resources
Duke University Medical Center
George R. Collins Education and Training Award
Ms. Stukes is a native of Charlotte, NC and a proud graduate of North Carolina A&T State University. After completing her bachelor of science in laboratory animal science, Jessica began an intentional journey of discovery ranging from animal husbandry, NHP anesthesia, PET imaging, transgenic colony management, and behavioral study investigation. She is currently the Education and Training Manager at Duke University Medical Center and continues to advance the lab animal community by serving on the IACUC of her alma mater and as the membership chair of LAWTE. Her training philosophy is “all great achievements require time” by Maya Angelou.
Diana Baumann, BSc (Hons), RLATG, CMAR

Head, Reptile and Aquatics Stowers Institute for Medical Research
Joseph J. Garvey Management Award
Ms. Baumann received her undergraduate science degree in the United Kingdom, followed by postgraduate work in education. She worked as a science teacher before moving into the laboratory animal field. She graduated from Institute of Laboratory Animal Management (ILAM) in 2008, serving as class president, and obtained CMAR certification in 2010. She has served on three AALAS committees and is a member of the LAMA Education committee. She is President of the Australia/New Zealand Association of Aquarium Professionals and is an ad hoc specialist for AAALAC International. She has published and presented on a diversity of topics and is recipient of multiple awards including the AALAS George R. Collins Education and Training award.
Kayla A. Vore, LVT, RLAT

Animal Husbandry Technician Senior University of Michigan Technician of the Year Award
Ms. Vore is a Senior Animal Husbandry Technician with the University of Michigan and has been for the past 4 years. She works mainly with the nonhuman primates, providing specialized enrichment and training opportunities to the long-term macaque colony. Kayla graduated from Stautzenberger College with a degree in veterinary technology and earned her veterinary technician license in 2016. She enjoys spending time with her border collie, Harley, and is actively involved with the cow breeding program on her family’s farm, Vore Farms LLC.
18 Laboratory Animal Science Professional January 2023
SPECIAL NEEDS

NUTRITIONAL
MEDICATED
ENRICHMENT
Getting to Know AALAS’s New Executive Director: Thomas “Tom” L. Joseph, MPS, CAE

research to help advance the care and health of humans and animals around the world.




How has your previous experience helped you prepare for this role?


I have been tremendously fortunate to work at many state, national, international, professional membership, and trade associations. Also, I have worked within these associations at almost every different level and area, from accounting to strategic planning, so I am very familiar with how a successful membership association runs and the important role it plays within the professional community and society.

Which leaders do you draw inspiration from, and how do you incorporate their values into the work that you do?
When you first heard of this opportunity, what piqued your interest?





It combines my entire association experience, my love for animals, and my love for Tennessee, so I couldn’t pass it up.
What preconceived notions did you have about laboratory animal research before learning more about our field?








I have seen firsthand the benefits of, and the need for, animal

Individuals that lead by example and listen are the leaders that I most admire. Abraham Lincoln (that’s why I’m currently reading a book about Lincoln), who led a nation with a cabinet of former political foes, and my mother, who recently passed at the age of 90, who, along with my father, raised eight children by setting the tone within our house - treat everyone equally and always include them in whatever you are doing. As the new Executive Director at AALAS, I intend to follow this game plan.
What is your vision for your first year here at AALAS?


I have a plan for my first 30/60/90 days and beyond, it calls for me to meet and listen to all AALAS’ staff, volunteer leaders, key opinion leaders, and corporate partners, gaining knowledge and building relationships for now and the future.

20 Laboratory Animal Science Professional January 2023
FEATURE – A NEW FACE AT
Somno Low-flow electronic vaporizers kentscientifi c.com/somnofl o Sept/Oct 22 LasPro
AALAS
What is the best advice you were given regarding this role?


To listen. To everyone and anyone, take it all in, and then begin to evaluate, learn, and understand the laboratory animal science field, knowing that it will be an ongoing process.

What are the top three things you are most looking forward to as Executive Director?

1) Expanding my knowledge about the laboratory animal research and science community and field; 2) Learn more about the needs within the community and 3) Develop new and continue to build upon existing relationships.









You are making a big move from Chicago to Memphis! What plans do you have for exploring the city?
My wife and I are taking it one step at a time, trying all the local (non-chain) restaurants, and visiting the various parks and downtown areas within the Memphis-Germantown-Collierville area. We enjoy walking and biking, so we’ll incorporate these activities whenever possible.

On the topic of traveling, where is your favorite vacation spot?
I have been very fortunate to travel to many beautiful places within the United States and internationally because of my previous work (and personal vacations), but one of my favorite places is Germany. I have been there several times, most recently with my wife, and we plan on returning soon with our children.
What do you like to do in your free time?
I enjoy spending time with my wife, two daughters, and son as much as possible, family vacations and just hanging out. We do family movie nights and enjoy other activities such as biking and hiking, and traveling.
What is a food/dessert you can’t say no to?
My one indulgence is Peanut M&Ms.
Name the last good book you read.
I just started reading Lincoln and the Irish by Niall O’Dowd. Before starting this book, I completed the Fatal Journey by Peter C. Mancall. I am an avid lover of non-fiction within the history or survival genre.
What are your favorite movies and television shows?
It’s a Wonderful Life is by far my favorite movie. Currently, I enjoy watching the Tennis Channel, World Cup, and many NBA and NFL games that are televised. Basically, everything and anything sports related.
Lastly, what would you like to say to the members of our organization?
AALAS is here for them now and moving forward. The staff’s servant leadership mindset always ensures that our members’ needs come first and that we are always available. I also look forward to meeting them in person at the various activities/ meetings we will be hosting in the future.
January 2023 Laboratory Animal Science Professional 21
Features & Benefits of Low-Flow: Flow rates as low as 50mL/min Saves money by using less than 1 mL/hr of isoflurane Built-in air compressor Uses ambient air or compressed gas No servicing or calibration needed Cost-e ective, reliable equipment • A8/22/22
River Dolphin. Amongst the hundreds of species of animals that can be found in this river, a small unassuming fish makes its home along the shallows and in its tributaries. It grows no longer than 5cm and possesses a striping pattern down its whole body. Zebrafish, Danio rerio, were first discovered by a Scottish physician named Francis Hamilton in the early 1800s. He wrote about them in his book, “An Account of the Fishes Found in the River Ganges and its branches” published in 1822.6 Over time they became a mainstay in the aquarium hobby due to how easily they reproduced and their beautiful striped pattern.
The rise to scientific prominence for zebrafish was facilitated by Dr. George Streisinger, a molecular biologist who most regard as the founding father of zebrafish research. In 1959, he was hired by the University of Oregon to serve as the Director of the Institute of Molecular Biology.9 There he carried out much of his research using zebrafish as a new animal model. In the late 70s, he perfected a technique by which he could use
embryo.8 The significance of this accomplishment cannot be overstated. Cloning provides an excellent way to create recessive mutations and, while easy to do in lesser species like bacteria, zebrafish have a much closer application to human medicine. His landmark paper on this experiment was published in Nature in 1981. Through Streisinger’s trailblazing work, zebrafish became a well-known model for genetics.
Zebrafish have made many inroads into biomedical research due to their useful attributes as model organisms. While mice remain the gold standard model, zebrafish maintain some key advantages. Unlike mice, they externally fertilize their eggs which makes it incredibly easy to track embryonic development in a dish. They develop most major organ systems before any pigment so they can be imaged easily and labeled with fluorescent markers. A single spawn can produce several hundred offspring as opposed to a litter of mice which usually doesn’t exceed 10. Not only do mice produce many fewer offspring, but the females also expend 3 weeks’ worth of energy

22 Laboratory Animal Science Professional January 2023
caring for their young. Zebrafish can develop and survive on their own without any parental care. Zebrafish can also be housed in much greater numbers due to their size and space requirements. Twenty fish can easily be housed in a footprint the size of a mouse cage limited to 5 mice. The zebrafish genome is fully sequenced and over 70% of the fish genome has a human orthologue which has direct application to clinical research. There are other fish that have similar attributes to zebrafish and might have been selected as the most prevalent fish model but this species has run away with the title.
The use of zebrafish in biomedical research spans a wide breadth of disciplines. They are used in studies that characterize hundreds of different diseases. We will touch on a few in this article.
Like all bony fish, zebrafish possess a two-chambered heart; one atrium and one ventricle. Recent genetic work has identified a mutation in genes nkx2.5 and nkx 2.7 which cause congenital heart disease in humans.1 The gene itself contains transcription factors that affect a number of expression pathways. On the cellular level, it becomes difficult to fully interrogate this mutation using human tissue so zebrafish can be an excellent intermediary. The Targoff Lab at Columbia University induced this same mutation in embryonic zebrafish and fixed the heart at different time points, labeling the atrial and ventricular heart cells (cardiomyocytes).5 They discovered that mutations in these genes led to dysregulation in cardiomyocyte differentiation patterns. The mutated hearts developed with many more atrial cells than ventricular ones, leading to a lop-sided and barely functional organ. Homozygous mutants were embryonic lethal. Understanding this mechanism can give important insight into the development of treatments for congenital diseases like this.
Zebrafish also possess a remarkable ability to regenerate. This has been demonstrated in several labs that study heart injury. Heart injuries are considered survival surgeries and consist of anesthetizing the fish, cutting open the chest to reveal the heart, snipping 20% of the ventricle or using a metal rod dipped in liquid nitrogen to damage via cryoinjury.4 Both intend to replicate the trauma and cellular death associated with a heart attack. Incredibly, the zebrafish can survive and rebuild its heart in approximately 60 days. The zebrafish heart is capable of clotting quickly and stopping potentially fatal blood loss, clearly an evolutionary adaptation from being a prey species in the wild. Cardiomyocytes near the trauma site spring into action, dividing quickly and repairing the heart to its original form. Amazingly, this mechanism leaves no scar tissue. A heart attack in a human patient leads to very permanent heart damage and if extreme enough it could require a new heart. If we could harness even a little of the regenerative

capabilities of the zebrafish heart, we wouldn’t need a heart transplant list anymore.
A single embryo requires very little water to develop properly so they have a major advantage in high throughput experiments. Each one can be placed into a 96-well plate and treated with an experimental chemical for either toxicity work or potential treatment dosages.7 Scaled up, these experiments can be duplicated hundreds or thousands of times over. This is impossible to do in any mammalian study. It allows scientists to screen tens of thousands of potential cures for various cancers, Alzheimer’s disease, and many more.
Brain imaging in larval zebrafish came about in a much different way than in mammalian studies and has proved to be useful in its own way. For most land creatures, a head post is fixed to the skull and then secured tightly to a microscope to image without fear of movement out of the field of view. This is not a reality in fish for a myriad of reasons including a liquid environment, the size of the animal, and the thickness of the skull. Neuroscientists found a way around these obstacles. They discovered that they could imbed the whole larva into a low boiling point agarose gel, securing them gently and allowing for high resolution images and videos to be taken while conscious. Behavior studies were run using this method, during which a larva was shown video of a moving substrate giving it the illusion of drifting and triggering neurons implicated in
January 2023 Laboratory Animal Science Professional 23
FEATURE – ZEBRAFISH IN RESEARCH
locomotion.3 Larval zebrafish have also been commonly used in optogenetic studies. Optogenetics is a technology used in neuroscience for the purposes of behavior research. Zebrafish DNA can be altered such that channels within their neurons can be made light sensitive. Using focused light, scientists can activate or inhibit specific groups of neurons, a rudimentary form of brain control, leading to altered decision making in test animals.2 Once again, low boiling point agarose is used to imbed the fish to ensure that focused light is hitting the correct neurons. Experimental designs like this have unlocked new ways to approach neuroscience.
Throughout this article, it remains clear that within the sphere of biomedical research, zebrafish are here to stay. They continue to be a tool to answer incredibly complex questions about behavior, development, and disease. The next time you visit a pet store, be on the lookout for a small, greenish-striped fish that just might change the world for the better.
Joshua Barber, MA, LATG, is the Senior Manager of Aquatic and Reptilian Life at Columbia University in New York City, NY.
REFERENCES:
1. Benson, D. W., Silberbach, G. M., Kavanaugh-McHugh, A., Cottrill, C., Zhang, Y., Riggs, S., Smalls, O., Johnson, M. C., Watson, M. S., Seidman, J. G., Seidman, C. E., Plowden, J., & Kugler, J. D. (1999). Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. The Journal of clinical investigation, 104(11), 1567–1573.
2. Del Bene, F., & Wyart, C. (2012). Optogenetics: a new enlightenment age for zebrafish neurobiology. Developmental neurobiology, 72(3), 404–414.
3. Dunn, T. W., Mu, Y., Narayan, S., Randlett, O., Naumann, E. A., Yang, C. T., Schier, A. F., Freeman, J., Engert, F., & Ahrens, M. B. (2016). Brain-wide mapping of neural activity controlling zebrafish exploratory locomotion. eLife, 5, e12741.
4. Ellman, D. G., Slaiman, I. M., Mathiesen, S. B., Andersen, K. S., Hofmeister, W., Ober, E. A., & Andersen, D. C. (2021). Apex Resection in Zebrafish (Danio rerio) as a Model of Heart Regeneration: A Video-Assisted Guide. International journal of molecular sciences, 22(11), 5865.


5. George, V., Colombo, S., & Targoff, K. L. (2015). An early requirement for nkx2.5 ensures the first and second heart field ventricular identity and cardiac function into adulthood. Developmental biology, 400(1), 10–22.
6. Hamilton, F. 1822. An account of the fishes found in the river Ganges and its branches.
7. Kithcart, A., & MacRae, C. A. (2017). Using Zebrafish for High-Throughput Screening of Novel Cardiovascular Drugs. JACC. Basic to translational science, 2(1), 1–12.
8. Streisinger, G., Walker, C., Dower, N., Knauber, D., & Singer, F. (1981). Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature, 291(5813), 293–296.
9. Varga M. (2018). The Doctor of Delayed Publications: The Remarkable Life of George Streisinger (1927-1984). Zebrafish, 15(3), 314–319.
24 Laboratory Animal Science Professional January 2023
C M Y CM MY CY CMY K



Fishing for Help: Considerations for Staffing in Aquatics Facilities
By Haley Engle, BS, & Emma Liechty, DVM, DACLAM
Like many businesses in the United States today, research aquatics facilities are experiencing significant labor shortages. Recruitment and retention of specialized technical staff are challenging in the best of circumstances. Yet according to the U.S. Bureau of Labor Statistics, the number of unemployed persons per job opening is at a ten-year low.1 Meanwhile in 2022, labor costs increased by 4.5%, the largest annual increase since 2001.2 Staff are therefore both increasingly difficult to recruit and more expensive than ever to retain. Concurrently, a growing trend towards increased regulatory oversight of aquatic research species has compelled many organizations to take a greater role in veterinary care and management of aquatics operations. This has necessitated greater investment in aquatics facilities and raised questions about how best to staff these facilities. Here we review lessons learned from the development of a large, centrally managed zebrafish facility at the Northwestern University Center for Comparative Medicine and make a case for aquatics-specific approaches to hiring and supporting staff.
Job Roles and Responsibilities
With this overarching goal in mind, we propose that skills necessary for aquatics operations can be divided into four categories: basic, intermediate, and advanced life support functions, and veterinary care. These diverse program models have different constraints on staffing, but all have the same common need for an aquatic life support system that functions appropriately and protects critical aspects of animal welfare. Therefore, the primary goal of each facility must be the development of staff who have a detailed understanding of system operations and who can both identify and correct problems.
With this overarching goal in mind, we propose that skills necessary for aquatics operations can be divided into four categories: basic, intermediate, advanced life support functions, and veterinary care (Figure 1). This framework can aid in the creation of a job matrix, in which compensation reflects the staff’s technical ability. Alternatively, it can be used to identify basic system support tasks and to equally divide effort in each category across individuals in a team. In our facility, we’ve created two hourly positions (Aquatics Technician I and II) that perform tasks in the basic through advanced categories, depending on years of experience. An aquatics supervisor and clinical veterinarian share responsibilities for staff oversight, advanced technical support, and veterinary care.
Staff Recruitment
Recruitment of staff with previous aquatics experience is highly desirable. Not only does this ensure continued staff engagement, but it improves trust between researchers and aquatics caretakers. Although zebrafish are the most prevalent aquatic animal model in the research environment, many programs house aquatic invertebrates, amphibians, or other teleost species in smaller numbers. Personnel with experience in aqua-
Category Examples of skills
Basic
• Life support system operation & maintenance
• Basic water quality assessment
• Identification of sick or dead animals
• Feeding and sanitation
Intermediate
• Life support system diagnostics
• Advanced water quality assessment & diagnostics
• Larviculture
• Live food preparation
Advanced
• Cryopreservation
• Embryo microinjection
• Genotyping
• Animal import, export, and quarantine
Veterinary Care
• Health surveillance & biosecurity
• Disease diagnostics
• Anesthesia, analgesia, & treatment protocols
culture, zoos, or the aquarium hobby trade have basic training in the maintenance and operation of aquatic life support systems and often bring diverse perspectives on improving workflow efficiency, troubleshooting life support system issues, and problem solving. It is worthwhile to create a job title and description that emphasizes aquatics experience. This improves targeted recruitment on large hiring platforms. Utilization of career sites with the capability to reach recent graduates from natural resources, aquaculture, laboratory animal, and agricultural colleges is also recommended. The development of aquat-
26 Laboratory Animal Science Professional January 2023
Figure 1
FEATURE – ZEBRAFISH IN RESEARCH
ics intern or externship positions can be used to incentivize applicants from aquaculture or other undergraduate programs. In our experience, the development of faculty contacts within ecology, biology, or natural sciences departments has proven useful in the identification of qualified candidates.
Retention
Aquatic animal husbandry tasks can be repetitive. Unless care is taken to maintain engagement, staff attrition rates may be high. As with other technical labor forces, a combined approach emphasizing staff recognition programs, continuing education, team building, and community engagement is beneficial. Aquatics staff recognition can be incorporated into pre-existing institutional programs. We also find that less formal, team-based merit programs are useful for incentivizing employee engagement and problem-solving. Supporting attendance at continuing education webinars, local conferences, or short courses is extremely valuable. The Zebrafish Husbandry Association, Aquaneering Inc., University of Alabama-Birmingham, and MDI Biological Laboratory offer regular webinars and short courses developed for the laboratory zebrafish community. Possibilities for targeted team building activities supporting continuing education for aquatics staff include behind the scenes aquarium or zoo visits, guest lectures or lab space tours from research stakeholders, and visits to other local research aquatics facilities. At the Center for Comparative Medicine, we also develop individualized projects for each team member. These may be as simple as assessing feed delivery devices or be targeted to more complex questions, such as quantifying fertility rates within a given diet regimen. Accountability for the success of these projects vests each staff
member with the ability to implement change and serves as a powerful tool for enhancing engagement.

Conclusion
As the use of zebrafish as an animal model in biomedical research becomes more widespread, both the research community and the animal husbandry community are advancing towards improved standardization and replicability of zebrafish husbandry protocols. This progression has the potential to increase the impact and significance of studies that use zebrafish as an animal model, and the practice of hiring specialized aquatics staff is an important component of this effort. Although there is no “one size fits all” staffing solution for the wide variety of zebrafish facility management models which exist around the world, it is critical to recognize both the unique skillset required for those working in a zebrafish facility, as well as the considerable source of personnel available from the aquarium, zoo, and commercial aquaculture industries.
Haley Engle, BS, is an Aquatics Supervisor at Northwestern University in Chicago, IL.
Emma Liechty, DVM, DACLAM, is a Senior Clinical Veterinarian at Northwestern University in Chicago, IL.
REFERENCES
1. U.S. Bureau of Labor Statistics. [Internet]. 2022. Job Openings and Labor Turnover Summary. [Cited 7 September 2022] Available at: https://www.bls.gov/charts/job-openings-and-laborturnover/unemp-per-job-opening.htm#
2. U.S. Bureau of Labor Statistics. [Internet]. 2022. Employment Cost Index. [Cited 7 September 2022]. Available at: https:// www.bls.gov/news.release/pdf/eci.pdf
January 2023 Laboratory Animal Science Professional 27
People Need Technology and Technology Needs People
By Gennifer Caesar, BS, ALAT
Lines wrapped around buildings for miles, social media posts buzzing, and thousands of pre-orders to reserve your spot whenever the newest tech is announced. Let’s face it: our society goes into a frenzy awaiting the launch date when the newest tech item is set to be available. We are sold on all the great new capabilities and how this new tech will assuredly improve our lives. Now while that may be true to a small degree, often, users will not be able to enjoy all the tech has to offer without a learning curve. Simply possessing tech does not make an individual more efficient, more organized, or data driven. Owning a smartwatch or the latest mobile phone does not automatically change your life without significant knowledge of the functionality of the tech and how you intend to use it. These attributes are gained and built upon either trial and error or investing time into learning the tech. So, when we think about adopting new technology or digital solutions, a very important piece required for success is often overlooked: the human component. A tool is only as powerful as the individual that wields it. Investing in people is the only way to ensure the successful adoption of any digital initiative.
In the laboratory animal research industry, technological advancements have changed the way vivarium facilities operate as well as how research is conducted. From large digital cage-washing systems that heat up to the exact temperature for efficient decontamination to digital booking platforms that allow researchers to effectively plan out space for their experiments, technology has affected research operations for the better. However, with the adoption of these new technologies, their success weighed heavily on how well the staff and operators were prepared, trained, and involved with the new process. Strategic planning and implementation should always include a business culture and process initiative to be ready for any new technology adoption. Starting out, an organization should be aware of its “why.” Why is the new technology needed, followed by, “What problem will it help solve?” When the questions of what and why are clearly defined, it gives a foundation to then develop a strategy for the end-users. Key components of a business culture & process initiative should include:
1. Evaluation of current processes: To change how a process is done, it should be fully understood how it is currently com-
pleted. For instance, if you want to change the animal order process from a paper form to a digital process, you must know all the stages to complete the form as well as who is involved with the approval.
2. Established team to lead initiative: Depending on your program size and available resources, developing a team of staff to lead the initiative may be the most beneficial. In the previous animal order example, the individual responsible for overseeing and approving animal orders regularly would be the ideal candidate for leading the digital initiative. Teaming them up with the project manager would be beneficial because they know all the nuances of the process and would be able to provide the best feedback for training and piloting the new process.
3. Timeline for piloting processes: A clearly defined timeline for training and piloting processes will help keep initiatives on track. Creating benchmarks with a time component helps to evaluate what changes are needed faster and reach the launch date on time. For example, if the animal ordering initiative had a goal of 6 months to implement, the team could properly schedule time for each stage to test, assess, and re-evaluate as needed. If no timeline is defined, a project that should only take 3 months could end up taking 6 months or more, eating up time and resources.
4. Development of SOPs: Once a process has been tested and evaluated, creating documentation as guidelines is crucial. SOPs define a process step-by-step to ensure successful completion and serves as a resource to users, new and existing. They also serve to eliminate “single-super users,” which is the individual who happens to be the only one who knows how to complete or teach a specific task or process.
Remember these key components when starting the adoption of a digital solution, and prioritizing the readiness of staff, as well as involving them in the adoption of the new technology, will help to ensure a successful implementation.
 Gennifer Caesar, BS, ALAT, is the founder and Chief Digital Transformation Consultant for Vivalytics Consulting in Las Vegas, NV.
Gennifer Caesar, BS, ALAT, is the founder and Chief Digital Transformation Consultant for Vivalytics Consulting in Las Vegas, NV.
FEATURE – TECHNOLOGY IN LAS
Although common misconceptions about technology adoption in the laboratory animal research community can typically boil down to resistance to change in organizational culture or financial constraints, these aren’t the only ones. It is common, even as individuals, to get excited about technology without fully realizing that it will only improve something for you if you know how to use it to your advantage.
Think Stainless





Think quality. Think durability. Think custom. Make it easy. Think Ancare.





Better Products. Better Science. www.ancare.com
Zebrafish Nutrition: Everything Known and to be Known
 By Ehsan Ramezani-Fard, PhD
By Ehsan Ramezani-Fard, PhD
In a successful aquaculture system, good and balanced nutrition is the key that leads to the production of healthy and high-quality animals. Furthermore, feed is one of the most significant costs of aquaculture and represents 40–50% of fish production costs. Therefore, it is essential to formulate an optimized diet that meets the nutrition requirements of fish before starting commercial culture of any aquatic animals. Zebrafish aquaculture started with the ornamental tropical fish industry, where the fish were often held in large outdoor ponds and fed on the natural production of zooplanktons, stimulated by fertilization, and possibly supplemented with a variety of low to average quality formulated diets. Such food combinations met most of the fish’s nutritional requirements, and its culture was profitable. Once zebrafish research industry hit its stride in the 1980s and several laboratories started using zebrafish as a research animal, feeding strategies were adopted from the ornamental fish industry whereas edible fish aquaculture industry benefitted from balanced species-specific diets formulated after conducting well-designed scientific research by aquaculture nutritionists. Therefore, zebrafish laboratories started culturing live feed such as paramecium, rotifer, and Artemia, and further studies demonstrated that zebrafish cultured on a live diet from the first feeding until the adult stage (almost 3 months old) had higher growth performance and fecundity rates compared to those on the available formulated diets.5,6 However, the dry feed used in those studies may not be the best formulated diet to meet zebrafish nutritional requirements. Live fish culture is very labor intensive and has variable nutritional values. Live feed also has the potential to act as a pathogen career. Artemia and rotifer cultures are quite literally bacterial soups that support diverse microbial communities, including numerous Vibrio species.11 Therefore, the elimination of their cultures and raising zebrafish on a formulated diet that meets all its nutritional requirements is highly desirable.


Diet Standardization
Despite the widespread use of zebrafish (Danio rerio) as a research animal in many studies including toxicologic and biomedical research, the nutritional requirements of this species have yet to be determined. 12 It is still a common practice in most zebrafish laboratories to use live feed as the first feed given to the fish larvae; meanwhile, live feed, formulated feed, or a combination of them are given to the adult fish. While the contribution of specific nutrients in any of those diets is not clear, numerous studies have shown the effect of feed quality on growth and breeding performance, body composition, metabolism, gene expression, and overall health status of fish. 1,7
Due to this, consideration of both quantity and quality of specific nutrients is essential. Because this can be more easily achieved in standard diets, this highlights the importance of feeding them to animals used as biomedical research models for human disease.

30 Laboratory Animal Science Professional January 2023
FEATURE – ZEBRAFISH IN RESEARCH
Several aquafeed companies currently produce zebrafish diets that, with limited knowledge of zebrafish nutrition, are mostly based on perceived or empirical general fish nutrition knowledge. Their ingredients are also the intellectual property of the companies and not available to the scientists that might be using the feed. Furthermore, most of the formulated diets contain ingredients with antinutritional factors such as soybean meal and additives such as pigments and preservatives that can impact fish genetics, genomics, transcriptomics, proteomics, metabolomics, or epigenetics and may compromise the results of research.10 These concerns lead to the conclusion that there is a need for formulated diet of known ingredients, defined nutrients, and consistent and reproducible quality. A standard reference diet formulation could be the next step in the evolution of zebrafish research. 12

Nutrition Requirements
Information about 3 macronutrient classes of protein, lipids, and carbohydrates is required to formulate a high-quality balanced diet for zebrafish. With the current limited knowledge of zebrafish nutrient requirements, feed formulators have no choice but to either wait until more information is available or largely rely on the dietary information available for similar omnivorous aquaculture species such as some tropical cyprinids and tilapia.
Protein, the major component of fish body (65-75% of dry weight) and its diet, is the main concern and most expensive component of fish feed. The dietary protein is digested or
hydrolyzed and releases free amino acids, which will then be distributed through the blood to other organs or tissues and used to synthesize new proteins. Inadequate protein in the diet reduces fish growth and has adverse physiological impacts. Excessive protein in the diet cannot be used to make new proteins, and the remainder will be converted to energy. There is currently a dearth of information on the dietary protein requirements of zebrafish and their impact on different physiological characteristics. Fernandes recommended a level of dietary protein of 37.6% for maximum weight gain and 44.8% for maximum protein retention for zebrafish.2
Lipids act as the main energy source in the diet, supplying energy about two times more than protein and carbohydrates. In fish, lipids can be utilized to spare dietary protein for growth purposes. Consumption of protein as an energy source, resulting from inadequate amounts of dietary lipid, may lead to protein deficiency for growth. In addition, lipids are an important source of essential fatty acids (EFAs), phospholipids, and fat-soluble vitamins. Fish are unable to synthesize essential fatty acids so they must be provided for optimum growth. Although fish generally have a higher lipid demand compared to cattle and poultry, the exact amount of crude fat and essential fatty acid required for each species is different. Fowler showed that zebrafish female spawning success decreased as dietary total lipid and omega-6 to omega-3 ratio increased from 8 to 14% and 1.4:1 to 9.5:1, respectively.3 There is no more published information about the dietary lipid and omega-3 to omega-6 ratio requirements of zebrafish at this time.
January 2023 Laboratory Animal Science Professional 31
Most fish are primarily either carnivorous or omnivorous, and carbohydrates are not a major components of their diets. Therefore, they have a limited ability to digest and utilize carbohydrates. However, carbohydrates are the cheapest aquafeed ingredients, and it would be economically beneficial to use them as an energy source to spare protein and lipids for growth and metabolic process. Such a sparing effect has been previously shown in Tilapia, another omnivorous tropical fish.9 Robisona showed that adult zebrafish should not be fed a carbohydrate free diet as it would reduce their growth rate.8 They suggested a minimum of 5% dietary carbohydrate is required for zebrafish and showed that its manipulation impacts physiological response and hepatic gene expression in the fish.
Other than having a nutritional value to meet the fish’s dietary requirements, the feed should be relatively water stable, palatable, made of digestible ingredients, and pelleted in the appropriate size for the extremely small zebrafish mouth.
Guerrera showed that zebrafish mouths are functional at 3 days post fertilization (dpf) and exocrine pancreas differentiation ends at 7 dpf when a wide range of present digestive enzymes are already active, and the fish has an effective digesting capability.4 Therefore, fish should be able to effectively digest a well formulated diet, made with high quality and easily digestible ingredients of appropriate size, palatable, and relatively water stable at their first feeding at 7 days post fertilization. However, it should be noted that a progressive increase in the surface of the intestinal mucosa and an increase in the quantity of some digestive enzymes like trypsin and lipase were observed until 47 days post fertilization. 4 This may show the importance of ingredients used in the diet that must be readily digestible for fish.
Feeding Ratio and Frequency
The next challenge in zebrafish feeding is to determine the daily ration. In edible fish aquaculture, it is common practice to calculate the ration based on body weight (e.g., 1-4% of total biomass in the rearing unit). This is very labor intensive for zebrafish facilities and could also be stressful to the small experimental zebrafish that has a fast daily growth rate. Its weight would need to be monitored regularly so that a set percentage of the average body weight could be calculated. Because of this, the common practice in many zebrafish facilities is “x minute” rule in which the fish are given any amount of feed that can be consumed in X minutes (e.g., 5-10 minutes). This non-standard subjective feeding strategy needs a lot of attention and observational skill or may adversely affect water quality, increases the cost of feeding, and even adversely affect animal health, compromising research outcomes.
Conclusion
Though the zebrafish research industry is growing rapidly, information about their dietary requirement is limited and the
aquafeed industry is still in the early stages of a zebrafish-specific diet development. Physiological responses and metabolic changes associated with an inconstant or unbalanced diet formulation would be a major source of experimental variability which must be minimized within and among research studies in order to produce repeatable and reliable results. Zebrafish research and aquaculture nutrition communities should work closely together to standardize and optimize zebrafish nutrition in the near future.
Ehsan Ramezani-Fard (aka. Ethan Ramsey) is an aquaculture nutritionist and lecturer currently working as Aquatic Research Facility Manager at the Animal Care and Veterinary Service (ACVS), University of Ottawa, ON, Canada.
REFERENCES
1. Fang L, Liang XF, Zhou Y, Guo XZ, He Y, Yi TL, Liu LW. Yuan XC, Tao YX. 2014. Programming effects of high-carbohydrate feeding of larvae on adult glucose metabolism in zebrafish, Danio rerio. Br J Nutr 111: 808-818.
2. Fernandes H, Peres H, Carvalho AP. 2016. Dietary protein requirement during juvenile growth of zebrafish (Danio rerio). Zebrafish 13: 548-555.
3. Fowler LA, Dennis-Cornelius LN, Dawson JA, Barry RJ, Davis JL, Powell ML, Yuan Y, Williams MB, Makowsky R, D’Abramo LR, Watts SA. 2021. Both dietary ratio of n-6 to n-3 fatty acids and total dietary lipid are positively associated with adiposity and reproductive health in zebrafish. Curr Dev Nutr 19
4. Guerrera MC, Pasquale FD, Muglia U, Caruso G. 2016. Digestive enzymatic activity during ontogenetic development in zebrafish (Danio rerio). J Exp Zool 324B: 699-706.
5. Karga J, Mandal SC. 2017. Effect of different feeds on the growth, survival and reproductive performance of zebrafish, Danio rerio (Hamilton, 1822). Aquac Nutr 23: 406-413.
6. Markovich ML, Rizzuto NV, Brown PB. 2007. Diet affects spawning in zebrafish. Zebrafish 4: 69-74.
7. Panserat S, Kaushik SJ. 2010. Regulation of gene expression by nutritional factors in fish. Aquac Res 41: 751-762.
8. Robisona BD, Drewa RE, Murdochc GK, Powellb M, Rodnicke, KJ, Settlesd M, Stoneb D, Churchilla E, Hillc RA, Papasanic MR, Lewisc SS, Hardyb RW. 2008. Sexual dimorphism in hepatic gene expression and the response to dietary carbohydrate manipulation in the zebrafish (Danio rerio). Comp Biochem Physiol D 3: 141-154
9. Shiau SY. 1997. Utilization of carbohydrates in warmwater fish - with particular reference to tilapia, Oreochromis niloticus × O. aureus. Aquaculture 151: 79-96
10. Watts SA, Powell M, D’Abramo LR. 2012. Fundamental approaches to the study of zebrafish nutrition. ILAR J 53: 144160.
11. Watts SA, Lawrence C, Powell M, D’Abramo LR. 2016. The vital relationship between nutrition and health in zebrafish. Zebrafish 13: S72-S76.
12. Watts SA, D’Abramo LR. 2021. Standardized reference diets for zebrafish: addressing nutritional control in experimental methodology. Annu Rev Nutr 41: 511-527.
32 Laboratory Animal Science Professional January 2023
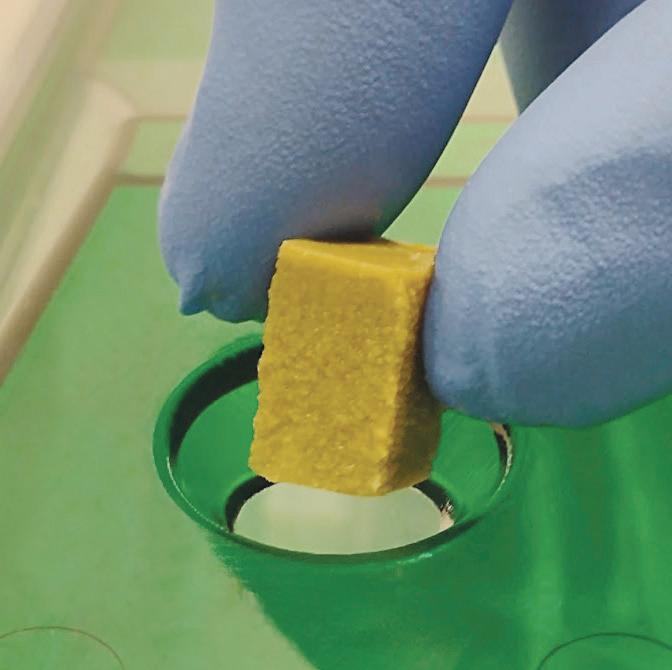
AquaFeed® Z
The First Gel Diet Specifically Formulated for Zebrafish

Nutrient Specific
Formulation based on nutrient requirements that researchers have identified as specific for zebrafish growth, reproduction, and maintenance.
Quality Ingredients
ClearH2O’s gel technology incorporates purified ingredients, including sustainable protein with high solubility, for optimum nutrient utilization. No fillers, added starches, or binders.
Natural Feeding
Gel cube properties provide for a longer feeding period and more natural grazing behavior, reducing stress and competition for food, resulting in improved fish welfare.
Consistent Unit of Measure
Designed to be cut with the AquaFeed® Cutting Device into uniform 1 gram cubes for ease of clean and consistent feed administration.
To learn more about our full line of products, go to clearh2o.com or call today at 1-888-493-7645

REQUEST A SAMPLE TODAY!
INTRODUCING
AquaPak® HydroGel® DietGel® MediGel® LabGel® FiberBites® AquaFeed® Z
INSIDE the IACUC Incubator Management for Investigators and IACUCs
Zebrafish continue to be a very popular laboratory animal model in biomedical research, with more investigators studying immediate post-fertilization changes in embryos. Caring for these precious embryos has become a subspecialty in laboratory animal medicine, in and of itself. Incubator systems for fish, typically housed outside of traditional animal facilities, tend to be the preferred storage site.
This issue of Laboratory Animal Science Professional gave us the opportunity to highlight the experience of Stephen Frederickson, Wendy Pridgen, Irene Ginty, and Dr. Tannia Clark with IACUC oversight of incubator management for zebrafish. The article touches upon some important topics including incubator setup, care, husbandry, maintenance, and disaster planning. Requirements for IACUC oversight of incubators, and how the IACUC should accomplish its oversight, are also discussed.
The authors hoped to provide a detailed discussion of how to properly use and maintain incubators and explain how the IACUC can best support their use, and we think we did just that! The article contains valuable information that can assist any program with their zebrafish oversight and certainly adds to the body of literature of care for this important aquatic species.
We hope you enjoy this “deep dive” into zebrafish care!

Stacy Pritt, DVM, MS, MBA, CPIA, CHRC, DACAW is the Associate Vice President of Research Support and Regulatory Management and Assistant Professor in Psychiatry at the University of Texas Southwestern Medical Center in Dallas, TX.

 By Stephen C. Frederickson, B.S; Wendy Pridgen, B.S.; Irene Ginty, B.S. & Tannia Clark, DVM, MS, DACLAM
By Stephen C. Frederickson, B.S; Wendy Pridgen, B.S.; Irene Ginty, B.S. & Tannia Clark, DVM, MS, DACLAM
Introduction
Incubators are a great way to maintain a consistent micro-environment for laboratory animals, embryos, and larvae, such as zebrafish, medaka, Xenopus tadpoles, or even chicken eggs. In addition, incubators can be located outside animal facilities and closer to benchtop work, can be under the exclusive care of research staff, and the same model of incubator used for bacterial plates, cell cultures can alternatively house thousands of fish larvae! This great asset for the investigators presents a unique challenge to an Institutional Animal Care and Use Committee (IACUC), as incubators housing fish larvae, such as zebrafish, may meet the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals (Policy) definition of satellite facility (SF).1 This standard piece of laboratory equipment easily blends into the research setting, and benign oversight of investigators could lead to unexpected surprises during facility inspections. Sharing methods of SF documentation, oversight, and inspection can only benefit the animal research community as we work towards a shared goal of maintaining animal welfare. This article will focus particularly on the use of incubators housing fish used for biomedical research.
Satellite Facility (SF) Incubators:
The PHS Policy applies to “Any live, vertebrate animal used or intended for use in research, research training, experimentation, or biological testing or for related purposes,” therefore, the PHS Policy applies to fish. It also defines SF, as any containment outside of an animal facility in which animals are housed for more than 24 hours.4 The Office of Laboratory Animals and Welfare (OLAW) further clarifies that egg-laying vertebrates, like zebrafish, offspring are considered to live animals immediately upon hatching.2 In zebrafish reared under standard husbandry practices, hatching occurs approximately 3 days post fertilization (dpf).5 Therefore, under the PHS Policy, all incubators outside of a core facility that house zebrafish at 3+ dpf for more than 24 hours are to be considered as SF. These incubator sites should be approved by the IACUC and include documentation of animal care.
Incubator Setup:
Since laboratory incubators may have multiple uses for purposes other than animal housing (i.e., bacteria growth, cell culture, etc.), there should
F.
Claire Hankenson, DVM, MS, DACLAM, is the Associ-
ate Vice Provost for Research and Attending Veterinarian and Executive Director, University Laboratory Animal Resources, at the University of Pennsylvania in Philadelphia, PA.
34 Laboratory Animal Science Professional January 2023
Figure 1. Zebrafish being socially housed and interacting within their environment. Photo credit: Stephen C. Frederickson.
be clear signage delineating which incubator(s) have been designated for animal use. We recommend using only dedicated satellite facility incubators for animals.
Larval fish development is often temperature-dependent1 so a method for monitoring the temperature within the incubator is recommended, as extreme heat (or cold) could harm the animals. Most incubators on the market utilize a digital display of the internal temperature sensor value. Alternatively, an analog display or a small, independent thermometer could be placed in the incubator to indicate the status. Additionally, we recommend there should be a nearby sign or resource that notes the acceptable temperature range as well as emergency contacts, such as: the lead investigator, veterinarian and/or facility manager for the incubator.
Incubators can be a good solution for off-system housing of zebrafish used for chemical screens and other research that would not be possible on a system with shared, recirculating water. There are special considerations that need to be given when animals are being exposed to drugs, chemicals or other agents while also being housed in an incubator. The appropriate Animal Biosafety Level and precautions for the specific hazard(s) should be determined by consultation with an institution’s safety department/official, and cautions should be clearly posted on the incubator. Designated supplies (trays, embryo disposal cups, etc.) should be used for chemical exposure and not shared for any other purpose or area.
Regular Incubator Care and Husbandry:
Maintaining documentation of equipment and animal care within the incubators is required and should be standardized via standard operating procedures (SOPs) across an institution. As stated in the Guide for the Care and Use of Laboratory Animals (Guide): Whenever possible, routine procedures for maintaining animals should be documented to ensure consistency of management and care (p.52).3 The frequency of the health check, husbandry care, and the resulting documentation of such will vary with species, age, research study endpoints, animal care, and use program, as well as veterinary care requirements.
We recommend researchers conduct both incubator (equipment) checks and animal health checks (Figure 2). When incubators house zebrafish, daily checks are common. At a minimum, OLAW expects animals to be observed daily per the Guide. Therefore, daily incubator checks are required, and an alternative plan with less than daily monitoring would only be acceptable for disaster-related situations. Alarms should be encouraged with the ability of staff to promptly intervene when established parameters are exceeded. Performance standards which eliminate daily health checks are usually not acceptable.
Incubator Animal
Welfare N/A
Labelling/ Signage Emergency contact(s), temperature range
Physical Environment Powered, properly functioning (no odd sounds)
Incubator Maintenance:
Incubators require minimal maintenance; however, periodic (annual/ semi-annual) temperature verification of each incubator is important as temperature is directly linked to development1 and temperatures can vary during times when not observed by staff. We recommend a temperature logging probe, such as the Hobo MX100, to trend temperature over time, and if a light cycle is used, we recommend a logger with a light sensor, such as the Hobo UX100-003. Temperature verification for 24+ hours should catch the typical temperature swings and variation that comes from normal use, as well as the variance in the environmental conditions created by the unit working to maintain a stable temperature (Figure 3). Alarms and remote monitoring should be encouraged, with the ability of staff to intervene when established parameters are exceeded promptly.
Disaster Planning:
Appropriate emergency response plans should be developed to address major system failures (Guide, p. 87) 3. The authors recommend that incubators housing animals are included within an institution’s disaster planning. While a typical incubator is not overly complex, there are a few circumstances that could arise and require a response that should be addressed in your disaster plan. When available, the incubator should be placed on the emergency power circuit to prevent issues from potential power outages. In the event that an incubator loses power (or fails) and function cannot be restored in a timely manner, do not open the door. The insulation built into the unit will allow maintenance of a suitable temperature for the longest time possible. A slightly lower or higher temperature may change the rate of development but is not an immediate animal welfare concern. If the function cannot be restored and relocation to other incubators is not feasible, alternate methods of heating or cooling may be considered. Supplemental temperature assistance, like hand warmers or similar heating pads (often used in the shipment of fish) or cold packs, may be used to help maintain temperatures within an acceptable range. However, it is important to note that all disaster planning must include euthanasia and researchers should know how to appropriately euthanize based on species and development.
Suggestions for Review and Oversight:
As indicated, the Guide provides requirements, guidelines and language regarding the environment, housing, and management of fish in a laboratory setting.3 While incubator satellite facility management is not expressly discussed, OLAW clarifies that they are to be checked daily since these house animals (as defined upon immediate egg-hatching).
Documentation
Temperature, semiannual temperature verification and light cycle verification (if applicable)
Alive, no illness
Age of embryos/ animals, owners of containers
Housing containers are intact
Frequency of health checks, feeding/water changes (if applicable)
Health & Equipment Checks: Using performance standards, the IACUC with veterinary input, should ensure the frequency of the health check, husbandry care, and the resulting documentation of animals cared for within incubators (Figure 2) are appropriate based on the species, age, and research study endpoints. As with terrestrial species, aquatic animals should receive daily care from qualified personnel (Guide, p 87).3 Our IACUC has outlined that zebrafish satellite incubators (with animals present) receive both equipment and health checks a minimum of every three days, with daily checks being optimal.
Feeding: Incubators and typical larval containers (e.g., petri dishes) are not designed to allow for easy access with feed devices, though feeding and care can be provided during temporary removal. Researchers and veterinarian staff should establish an SOP when first
January 2023 Laboratory Animal Science Professional 35
Figure 2: Recommended checks and documentation for an incubator Satellite Facility
feedings and frequency are expected for animals housed within an incubator. Per OLAW: if feeding is withheld for research purposes, then it must be approved following IACUC review by DMR or FCR and provided in the semiannual report to the IO as a departure unless established performance standards exist.

Semi-Annual Facility Inspection: IACUCs are required to inspect satellite facilities. During semi-annual walk-throughs, members should verify that their established requirements are being met, such as documentation and signage being correct, that any animals in containers appear healthy, containers are clean, and that the temperature is in the desired range.
Animal Numbers: The PHS Policy requires that investigators an approximate number of animals to be used, and as such, institutions must have a mechanism to track animals, including zebrafish in incubators, that are acquired and use including animals euthanized when not needed.22021</edition><dates></dates><urls><related-urls><url>https://olaw.nih.gov/faqs/#/guidance/faqs?anchor=question50289</url></related-urls></urls><access-date>10/20/22</access-date></record></Cite></EndNote> In many institutions, much of the research is done on fish, embryos, and larvae that are housed in an incubator. For example, zebrafish are to be immediately counted upon hatching (which could occur anytime before or after 3+ dpf).
Reporting Animal Welfare Concerns: Institutions should always have a method for promptly reporting any animal welfare concerns. As incubators are considered satellite facilities, we recommend posting the same animal welfare requirements established for other animal holding areas and regularly reviewing or training to ensure that the policy is known to the staff so that they would feel comfortable using it, if needed.
Conclusion:
Incubators are a great research tool that provide proper environmental conditions for animals. It allows investigators to bring the subjects of the research closer to the lab space where most of the work is done. An IACUC can easily embrace this solution while still ensuring animal welfare and regulatory requirements are met.
Stephen Frederickson, BS, LATG, Contractor- Charles River, is the NHGRI Zebrafish Research Specialist at Charles River in Bethesda, MD.
Wendy Pridgen, BS, LATG, ILAM, is the NHGRI Animal Resources Program Coordinator at Charles River in Bethesda, MD.
Irene Ginty BS, RLATg, CMAR, Contractor- Priority One Services, Inc., NHGRI Animal Operations Specialist at Charles River in Bethesda, MD.
Tannia Clark, DVM, MS, DACLAM, is the NHGRI Animal Program Director at Charles River in Bethesda, MD.
The editors would like to thank Dr. Neera V. Gopee for her time and expertise as a contributing OLAW reviewer for this article.
REFERENCES:
1. Bartlett DH, Silk SB. Office of Laboratory Animal Welfare Comments. Zebrafish 2016;13:563-564.
2. Frequently Asked Questions (FAQs) PHS Policy on Humane Care and Use of Laboratory Animals. [Cited January 17,2023]. Available at: https://olaw.nih.gov/faqs#/guidance/faqs?anchor=50289
3. ILAR Guide for the Care and Use of Laboratory Animals. 8th Edition, 2011
4. PHS Policy on Humane Care and Use of Laboratory Animals 2015.
5. Urushibata H, Sasaki K, Takahashi E, et al. Control of Developmental Speed in Zebrafish Embryos Using Different Incubation Temperatures. Zebrafish 2021;18:316-325.
36 Laboratory Animal Science Professional January 2023
Figure 3. Recorded temperature and relative humidity inside an incubator over several days. Each sudden temperature drop (and corresponding humidity increase) is associated with the incubator door being opened. Graph created using HOBOware.
GEMMA Micro - The complete nutrition for all lifestages of zebrafish

GEMMA Micro is a unique, patented diet that offers complete nutrition for every lifestage of zebrafish. Integration of GEMMA Micro to the zebrafish feeding regime requires NO Artemia, and can reduce feed frequency to only one feeding event per day for adult fish Smaller sizes of GEMMA Micro allow for earlier transition to microdiet from live feed.
Fe e ding G EMMA Mic r o for t h e entire life cycle of zebrafish leads to health y, robust, an d hi g hly fecund zebrafish

www.skrettingusa.com
GEMMA
Facing Difficulties in Virtual Learning in a Zebrafish Facility
Written by Karen Dunford and Joseph Upstone
Introduction
Since March 2016, the University College London
Zebrafish Facility has run the modular course for those wishing to use this model legally, which is required by the UK Home Office. The course had a successful format, with high pass rates. In 2020, we faced two changes that resulted in declining exam scores: the addition of 178 standardized questions provided by UK accrediting bodies, including the Royal Society of Biology, and the advent of an unexpected global pandemic. When the world went into lockdown, our original training paradigm was no longer possible, and face-to-face training (F2F) had to be quickly modified to fit the mold of a virtual course. The transition was difficult, and it may have also been detrimental to the performance of the students who now found that their living spaces had become their classroom. This was reflected in the overall decline of exam scores (ES). While the 2019 classes saw a passing rate of 82%, a sharp decline was seen in 2020 with only 59% of students passing the exam on the first attempt in the virtual course (Figure 1).
The results indicate that the F2F format was not as successful in a virtual environment. The alarming change required a re-examination of virtual methods. This led to a retrospective review of the new exam questions and the virtual transition. We hypothesized that increasing the quantity of virtual self-led interactive study material would raise exam performance.
Exam Analysis
Initially, the exams were analyzed to determine if the new questions were too difficult. An item difficulty index (IDI) is frequently used in education to evaluate multiple choice exams by calculating the proportion of questions correctly answered in past exams1. This index, created retrospectively, results in a value, p. Questions with a high p-value are considered easy, and those with a low p-value are considered difficult. The IDI was created for each individual question, then the average p-value of each class is taken to create an overall value for the year (Table 1).
38 Laboratory Animal Science Professional January 2023 Management/Career & Training
83.9%81.8% 59.3% 0% 50% 100% F2F2020Virtual Number of Exams Passed % CourseType Pass/FailofExams
Module Learning Objective Question 2016 2017 2018 2019 F2F 2020 Virtual 2020 2021 2022 Avg p-value Rating E1 1 MC1 1.00 0.78 0.53 0.65 1.00 0.85 0.73 0.79 2 TF1 0.93 0.73 1.00 0.89 1 2 MC1 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1 MC2 0.75 0.86 0.93 0.90 1.00 1.00 0.91 1 TF1 1.00 1.00 1 MW1 0.88 0.73 0.83 0.82 1
Figure 1: The percentage of exams passed over the changes in the licence course: face to face, changes to exam questions, and virtual course due to the pandemic.
Table 1: A selection of the item difficulty index (IDI) used to determine each question's p-value for the ethics module.
Face-to-Face
Course Format
Our study then turned to the new virtual format. We used a local version of the education platform Moodle and attempted to provide as similar an experience as possible to the F2F; one main exception was the group activities, which were made individual (Table 2).
However, with the decline in exam performance, changes in the format were required, although the learning outcomes had to remain the same. These occurred over 5 phases, which were implemented slowly over 2021 (Table 3). These included the re-introduction of the group-based activities and the creation of self-led study materials such as flash cards, and quizzes; the study materials were not mandatory. The course books also became interactive and therefore more virtually appealing.
Review: Item Difficulty Index
Three groups were created for analysis (Figure 2).
• Group 1: All exams March 2016 – 2019
• Group 2: All exams January - March 2020
• Group 3: All exams July 2020 - December 2021
The IDI is determined by taking the total number of correct answers divided by total number of people.
p = t / n
p = p-value; t = total number of correct answers; n = total number of
A p-value was determined for each exam question and the average of these over time was applied to the overall difficulty of the question. Each question was then categorized as easy (1 point), medium (2 points), or hard (3 points). This index then allowed for scoring each course’s exam to determine its overall difficulty (Table 4).
January 2023 Laboratory Animal Science Professional 39
people
Virtual Coursebooks Hardcopies of textbook and study guide/exercise book Digital copies of textbook and study guides/exercise book Group activities Culture of Care Culture of Care Sexing Fish Recognition of Pain Practicals Handling Sexing Fish (individually assessed) Embryo Identification Recognition of Pain Water Testing Embryo Identification Handling (in-person) Summative Assessment Exam Exam Course Assessment Feedback form in course Feedback form online
Phase Additions Average ES 1 Course creation: includes all material from F2F as pdfs 76.8% All group activities are done individually 2 Study material added: ethics vocab flash cards 81.3% 3 Study material added: biology vocab flash cards 80.0% Activities are
4 Study material added: ethics quiz,
quiz,
quiz 81.5% Course books changed to interactive 5 Study material added: learning objectives
as separate files 82.5%
Table 2: The differences between the first virtual
course
and the established F2F course.
returned to group activities Addition of a networking virtual room for use during breaks
legislation
welfare
created
Difficulty Range Full Course (total points) Partial Course (total points) Hard 71-105 51-75 Medium 36-70 26-50 Easy 0-35 0-25 Table 4:
ranges that determine if an exam is difficult, medium, or easy.
Table 3: The five phases of the virtual course, with the average exam score for each phase.
The
Figure 2: The three groups for analysis, showing the overlapping factors (delivery and question set).
A Kruskal-Wallis test showed that the average ES from Group 1 and Group 3 are significantly different (Figure 3). Group 2 is not significantly different from either group. However, another Kruskal-Wallis test showed that the difficulty score of all exams were not significantly different between any of the three groups
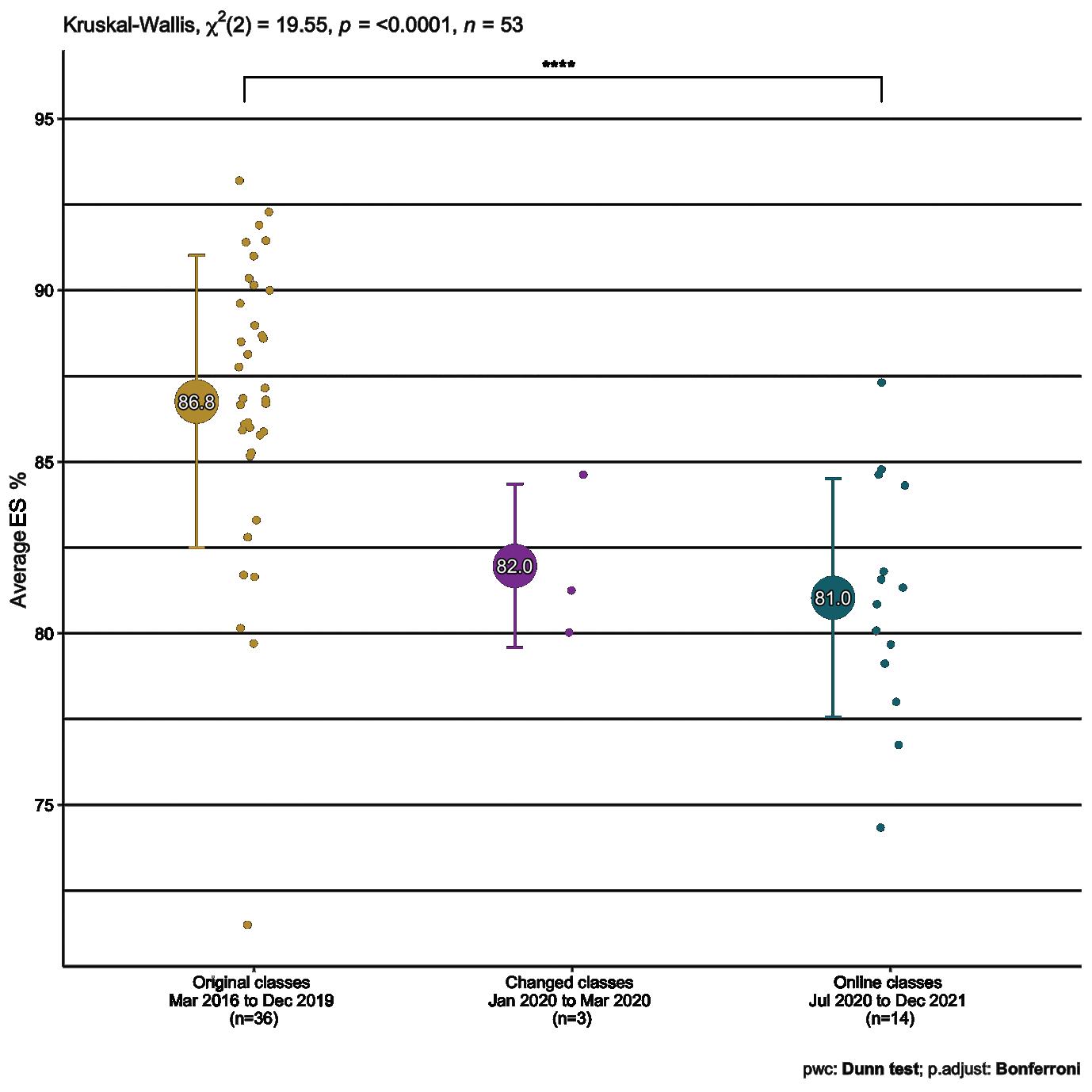
(Figure 4), and all exam difficulty scores fell within the medium category.
This determined that the virtual exams fell into an acceptable level of difficulty while still expecting a passing mark.
Virtual Format
The second review only looked at Group 3, the virtual group, and the changes made. Analysis of the efficacy of these changes was based on the average frequency with which students accessed the material available (data provided by the website). These data were then measured against exam scores.
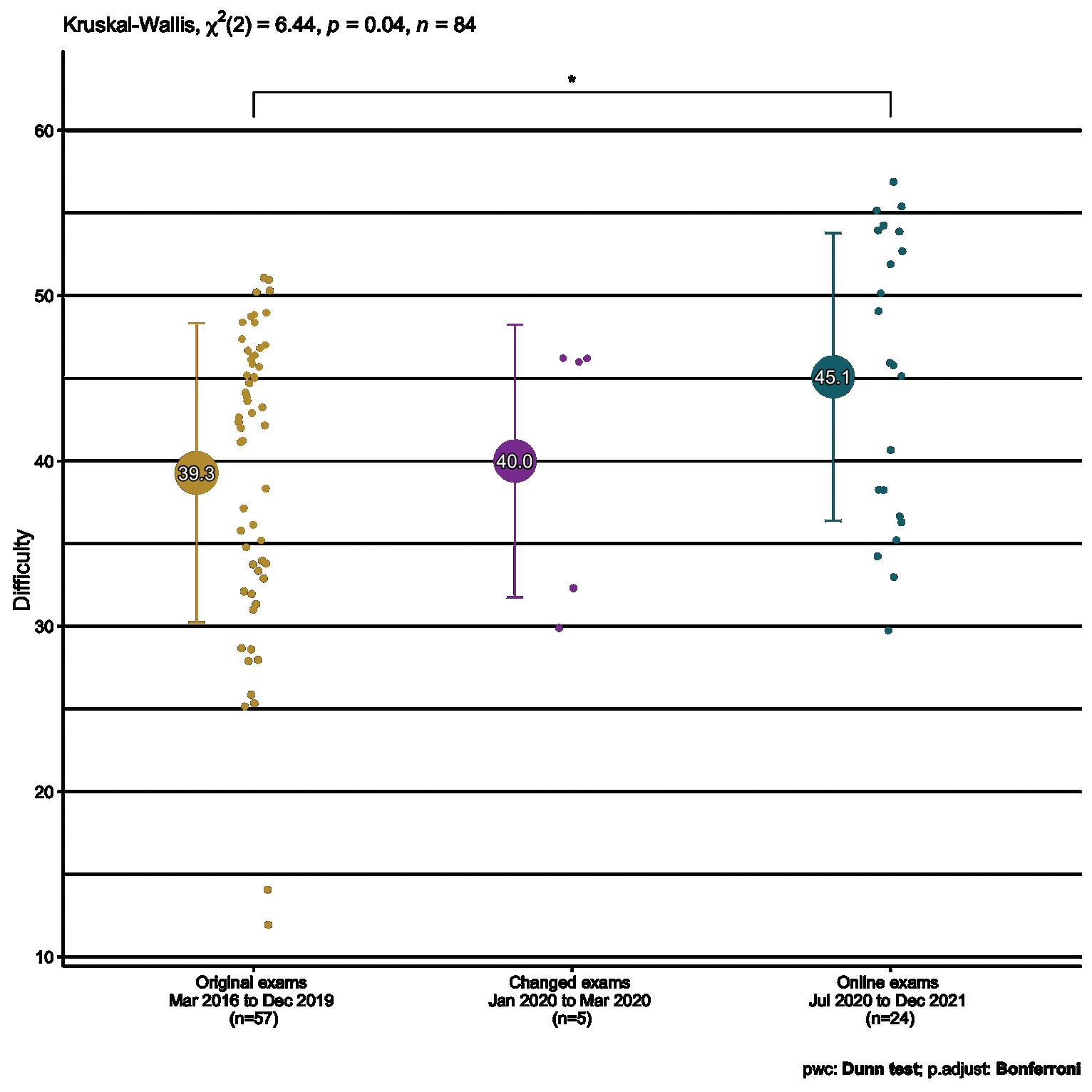
Overall, exams scores increased from 76.8% in Phase 1 to 82.5% in Phase 5 (Table 3). Surprisingly, the results show a declining trend of ES across the entirety of the virtual course for those who accessed the material more frequently.
When individual phases are examined, however, the difference is all in Phase 4, which saw the highest creation of material; it has the highest average of frequency of access by students at 4.15, but only in those who failed. The other phases are approximately equal between those who pass and fail; those who passed averaged 1.9 frequency, and those who failed ranged between 1.7 and 2.1.
Discussion
The addition of new questions can be eliminated as the reason for the decrease in exam scores, as they all were scored for medium difficulty. Therefore, the issue lay with the virtual format.
The results for the review of the online material were surprising, as the hypothesis was that more material would increase ES. The changes made to adapt to online learning were helpful, as there was an overall increase in ES, but not to pre-COVID levels.
Phase 1 has a lower exam average than phases 2-5, indicating a positive impact from the inclusion of more material. But Phase 4, the greatest volume of material added, shows that a higher frequency of access resulted in failures. The danger is overloading the learner with too much or complex information. Improvements for a virtual format could be brevity and simplicity in material available, such as the simplistic vocabulary flash cards added in Phases 2 and 3 (Figure 5), instead of the more intensive quizzes added in Phase 4.
Returning to group activities in Phase 3, as in the original delivery, saw an improvement in ES compared to Phase 1, but was lower than the other phases (table
40 Laboratory Animal Science Professional January 2023 Management/Career & Training
Figure 3: The average ES from Groups 1 (87.3) and 3 (81.9) are statistically different. There is no statistical difference between Group 1 and 2 (82.9) and 3 and 2.
Figure 4: A Kruskal-Wallis test showed no significant difference between the average difficulty for all exams within each group (i.e. partial and full course exams).
3), which may indicate that human interaction is more impactful when in person. This is a current issue in the education field2,3. Despite this, it is crucial to include group activities in virtual learning as it is overall beneficial to students’ performance.
It should be noted, however, the above analyses cannot account for study habits, individual ability, and especially personal situations due to the pandemic. Further analysis of continued virtual learning performance, or the re-introduction of F2F learning, would help determine the latter’s role.
The results of translating a F2F course to virtual in a hurry implies that the intangibles of in-person learning were lost in transition and is therefore not effective. Both formats are advantageous, but virtual learning has to be carefully planned. Key points for improving a virtual transition are including human interaction and addition
of simplistic and brief material. Training is not static, but dynamic and needs to change according to context. COVID has forced people to adapt and rethink ‘how we’ve always done it’. Just as we strive to constantly improve the ethical use of animals in research, we must apply that same ambition to how we approach training.
References
1. DiBattista D, Kurzawa L. 2011. Examination of the Quality of Multiple-choice Items on Classroom Tests. The Canadian Journal for the Scholarship of Teaching and Learning. 2(2).
2. Mahmood S. 2021. Instructional Strategies for Online Teaching in COVID-19 Pandemic. Human Behavior and Emerging Technologies. 3(1):199-203.
3. Nguyen MH, Gruber J, Marler W, Hunsaker A, Fuchs J, Hargittai E. 2022. Staying connected while physically apart: Digital communication when face-to-face interactions are limited. New Media & Society. 24(9):2046-2067.
January 2023 Laboratory Animal Science Professional 41
Figure 5: The vocabulary flash cards provided are direct and simplified in design.
Navigating an Aquatic Research Facility with Multiple Species
By Nuno Pereira, DVM, Inês Santos, MsC, and Ana Borges, PhD
Intro
The Aquatic Facility of the Instituto Gulbenkian de Ciência (IGC) provides services on zebrafish (Danio rerio), turquoise killifish (Nothobranchius furzeri), and the African clawed frog (Xenopus laevis). Over the last two decades, the facility has grown to progressively incorporate these three species, adapting to the needs of research programs. The co-existence of multiple aquatic species in a vivarium poses challenges at many levels, namely the training of dedicated human resources, facility design, biosecurity, and health control.9 In this article, we will describe some of the strategies that we have been developing over the years to overcome these challenges.
Team
There are multiple aspects that are unique to aquatic units, such as water quality control, maintenance of filtration/life support systems, production of live feeds, nursery care, and health management. Therefore, the aquatic facility team should master the previously listed aspects, as well as the biology and husbandry of all species involved. Even though each individual may not possess all the listed skills, overall, the team must have them through the combination of its members. Furthermore, in a multi-species facility, all staff members should be aware of the many biosecurity challenges, be trained to keep effective biosecurity barriers, and know about the zoonotic risks of each species.
To fulfill these requirements, the IGC dedicated aquatic team has formal training in aquatic species, according to the specific function within the facility, following the European guidelines on training.4 In addition, each person undergoes in-house practical training, and specific training through webinars, seminars, and courses.
Regarding health management, the designated veterinarian of an aquatic animal multi-species facility should be proficient in the particularities of aquatic animal medicine (e.g. fish, amphibians, and cephalopods), which are very different from mammal veterinary medicine. These competencies are slowly starting to be included in the veterinary faculties’ curricula, so veterinarians working in this area should seek postgraduate training, preferably a specialty status whenever available. In our facility, there is a veterinarian dedicated to aquatic animals.
Biosecurity
The IGC campus was built in the late 1960s, at the time with a vivarium dedicated to mammalian species. It was only a few decades later, in 2005, that a small zebrafish (Danio rerio) facility was installed (capacity for 1500 fish). This initial facility was progressively expanded to increase the housing capacity
for zebrafish up to 20,000 fish, and later to include the african clawed frog Xenopus laevis, and the turquoise killifish Nothobranchius furzeri (Fig. 1). The aquatic vivarium was not initially designed as an integrated structure to house several species, so, as it grew, the multiple expansions were designed and built on existing laboratories that were adapted (Fig. 2A). Zebrafish and Xenopus are still used today at the IGC, but the turquoise killifish colony, was kept for a 2-year project (2017-2019; Fig.1).
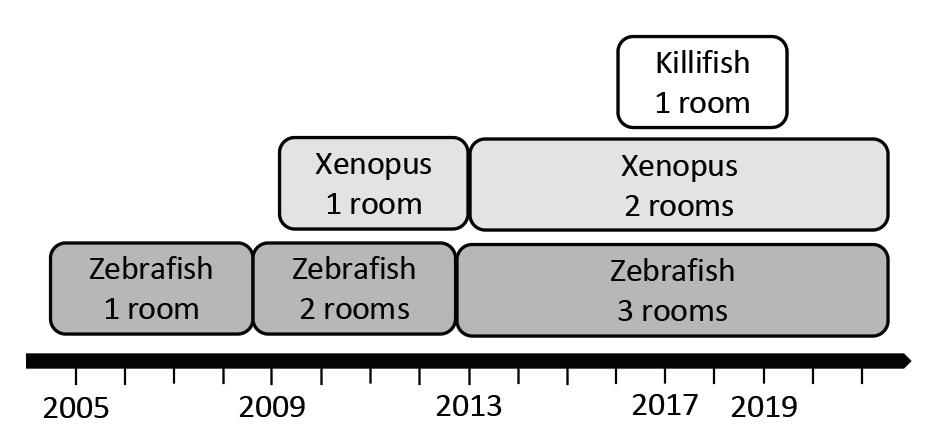
Animals are housed in state-of-the-art recirculation systems (which include mechanical filtration, activated carbon, and UV irradiation), except for the Xenopus quarantine (E5), where there is a flow-through system, developed in-house.
The implementation of a multi-species facility was done by having a reinforced biosecurity program as a guide to mitigate the main concern, the prevention of infectious diseases and cross-contamination among the different species.
“A biosecurity program is the set of procedures and barriers designed to protect a facility, against biological and chemical
42 Laboratory Animal Science Professional January 2023 TECH TIPS - HUSBANDRY, ENRICHMENT, NEW TECHNIQUES AND TACTICS
Figure 1. Timeline of the expansion of the IGC Aquatic Facility.
Figure 2A. Spatial distribution of animal rooms throughout the IGC campus.
Flavobacterium columnare
Sessile protozoa YES
Microsporidia Pseudoloma neurophilia Glugea sp. ND
Probably Probably Yes
of Epistylis sp in X. laevis (2018)
Table 1. Common pathogens to zebrafish, turquoise killifish, and Xenopus. This table is a simplified version of the one from Mocho el al 2022a, (supplementary material Table S3). It illustrates the high cross-contamination risk between the various species and their UEs. This risk would decrease substantially, but not to zero, if comparing species with a higher taxonomic gap such as fish versus mammals. Aeromonas hydrophila, more aggressive mycobacteria species, Piscinoodinium spp, sessile protozoa such as Epistylis sp and even ranavirus represent a health risk if cross contamination occurs. ND- not determined.
racks or systems likely to share the same water or for which cross-contamination is unavoidable.”9 Within an EU, all animals are susceptible to contamination with the same pathogen or hazard. Importantly, EU barriers are not only physical barriers but also the workflow and the possibility of cross-contamination between rooms and systems.
At the aquatic facility of the IGC, 5 EUs were defined (Fig.2B) based on two main criteria. The first, was to create barriers between the different aquatic species, and the second was to separate the units based on their health status (e.g., holding rooms vs quarantine, existing pathogens, and prevalence).
To keep barriers between the different EUs, each unit has dedicated equipment, to avoid the circulation of objects among rooms. This includes reagents, consumables, kits (e.g., water test kits), devices (e.g., water probes), and equipment (e.g., fridges, incubators, stereoscopes). In addition, we implemented a “one way traffic flow” from cleaner areas to dirtier ones, according to the defined biosecurity risk (Fig. 2C). This rule applies to the circulation of personnel, fish, material/equipment, and feeds. If objects must return to a cleaner area, they must be properly disinfected before re-entering the cleaner area.
Water
hazards”, as defined in (10). These hazards can be originated from incoming animals, water, quarantine, visitors, pests, and food sources.
Therefore, one important role of the multi-species facility manager and designated veterinarian is to assess the risks and to implement appropriate physical barriers and workflows to reduce the possibility of cross-contamination between species, and epidemiological units.
When establishing the biosecurity program, one must define the epidemiological units (EU) that compose the multi-species facility. Mocho defined the EU as “a set of tanks,

The IGC aquatic facility is supplied by a municipal water source which, at the IGC, undergoes purification through mechanical filtration, activated carbon, and UV treatment upstream of the housing systems.
There are dedicated water purification systems in three rooms (zebrafish main room, Xenopus main room, and Xenopus quarantine). However, the other three share the same water source, which, at first, could elicit biosecurity concerns. However, cross-contamination is very unlikely to occur through the water supply because this reverse osmosis (RO) water production system is located upstream of all three units, and is not readily accessible, nor handled routinely by the facility personnel.
January 2023 Laboratory Animal Science Professional 43
Pathogens Zebrafish Killifish Xenopus
YES YES YES Common opportunistic bacteria
YES Probably ND
Comments Aeromonas hydrophila
Edwardsiella ictaluri
YES Probably ND Mycobacterium
YES YES YES Episode
YES YES
YES YES Episode
sp.
of M. haemophilum in X. laevis (2011) Piscinoodinium sp. YES
Ranavirus
Figure 2B. Definition of Epidemiological Units EU1 to EU5. Each room corresponds to an unit, with exception of ZF main Room and ZF Behavior Room which are considered the same unit despite being located in different buildings.
Figure 2C. Biosecurity risk assessment of the epidemiological units.
Legend: ZF-zebrafish; Q –Quarantine; KF-Killifish; EU-Epidemiological Unit
Zebrafish (EU1 and EU2)
Sample Periodicity Tests
Food
Prefiltration
sentinel fish Biannually (soon to be quarterly)
(c. 50/year in EU1 *)
Necropsy; Bacterial culture #; Histopathology $; PCR “
System water Biannually Bacterial culture
Colony fish (sick or retired) Through the year (c. 80/year in EU1 *) Necropsy; Histopathology
Live feed Annually (soon to be at least biannually) PCR (Mycobacteria)
Sump biofilm Biannually (soon to be quarterly) PCR (Mycobacteria)
Ziehl-Neelsen and Luna stains; “ extended profile annually and mycobacteria+Pseudoloma neurophilia biannually
Killifish (EU3)
Sample Periodicity Tests
Routine testing on randomly picked fish
Annually Necropsy; Bacterial culture #; Histopathology $; PCR (Mycobacteria)
Colony fish (sick or retired) Through the year Necropsy; Bacterial culture #; Histopathology; PCR (If suspicion of mycobacteria infection)
System water Annually Bacterial culture
Feed » Annually PCR (Mycobacteria)
Sump biofilm Annually PCR (Mycobacteria)
Table 2B. Health Program – Brief description of the screening protocol for the turquoise killifish. # coelom or suspected tissue; $ hematoxylin and eosin, Ziehl-Neelsen and Luna stains; » artemia and blood worms
African clawed frog (EU4 and EU5)
Sample Periodicity Tests
New arrivals at quarantine (EU5)
Colony animals (sick or retired) (EU4)
Al least one animal at the beginning of quarantine and if possible, another before quarantine end
Biannually – at least 1 animal tested
Commercial processed diets for fish and frogs are stored centrally, outside the EUs, and distributed directly to each unit. Each housing room has refrigerated storage for holding any additional dedicated diet.
Live feeds produced in-house for zebrafish include rotifers and Artemia salina and are produced in the zebrafish main room, EU1, and then distributed through EU1 and EU2, daily. Containers used to transport food to the zebrafish quarantine (EU2) travel back to the EU1 only after sanitation to remove potential contaminants.
For turquoise killifish, Artemia salina was produced specifically in the EU3, to preserve the biosecurity barrier. This colony was also fed on frozen bloodworms (red mosquito larvae), which were stored in EU3.
Animals
We favor an “eggs only” policy, where incoming embryos from external sources (preferably surface disinfected with sodium hypochlorite) are housed in the quarantine. In addition, the process of rederivation (from quarantine to the main room) and colony maintenance are done through surface disinfected eggs. Adult and embryo experiments are usually performed within the respective EU, for which each EU has dedicated equipment. Behavior experiments are performed in a dedicated room, contained within the EU1. For this, adult animals are transferred from the zebrafish main room to the experimental room and are not allowed to return. If zebrafish (adult or embryo) experiments are performed outside the EU1 (e.g., confocal microscope), the animals must not return to the original room. Usually, these experiments are terminal, or exceptionally, the animals may be housed in quarantine.
For the turquoise killifish, only one room existed (E3), thus there was no circulation between units and all actions were confined to a single room. The colony was established from a single batch of embryos. Hence, there was no need for quarantine. If the turquoise killifish project had been prolonged in time, we would probably need to install a quarantine unit.
Necropsy; Bacterial culture #; Histopathology $; PCR “
Adult frogs are procured from authorized sources and imported into a quarantine room (EU5), where they are housed for 6-8 weeks to acclimate and perform health tests (Tables 1 and 2). Upon favorable health screening results, the animals are moved into the Xenopus Main Room (EU4). Once in this room, they are not allowed to leave, only their products can (eggs, embryos, or other tissues).
Personnel
Sump biofilm Annually PCR (Mycobacteria)
Table 2C. Health Program – Brief description of the screening protocol for the African clawed frog. # coelom or suspected tissue; $ hematoxylin and eosin, Ziehl-Neelsen and Gram stains; “ ranavirus, Chlamydophila sp., Batrachochytrium dendrobatidis and if paraffin block positive to Ziehl-Neelsen– attempt species ID.
The daily workflow of the animal facility staff generally moves from the room with the lowest to the room with the highest biosecurity risk (Fig. 2C). However, research staff is dedicated to each EU based on the species with which individuals work. All rooms are equipped with a footbath with disinfectant at the entrance.
Facility personnel wear gloves, laboratory coats, and overshoes/clogs in all rooms. For research staff working in a single EU, gloves are mandatory but labcoats are optional.
44 Laboratory Animal Science Professional January 2023
Table 2A. Health Program – Brief description of the screening protocol for zebrafish. * Borges et al 2016; # coelom or suspected tissue; $ hematoxylin and eosin,
Sanitation
All tanks and their accessories, equipment, food, and supplies for each EU are dedicated and cleaned separately, with the exception of EU2 and EU3. In this case, tanks and accessories are washed and disinfected in a common external washing room. To minimize cross-contamination between these units, the respective materials are washed and disinfected on alternate days, following appropriate sanitation of the washing facility.
Health control
Health control programs for fish research facilities are well described in the literature.2,6,8,10,11 For other aquatic animals like amphibians, literature on laboratory amphibian medicine is still scarce, so additional information should be adapted from scientific publications on amphibian medicine.3,12,13,14,16
We started by implementing a health control program for zebrafish, based on the limited available literature at the time.1,5,7,15 With the introduction of additional species in the facility, we had to address new issues, namely strengthening the biosecurity measures, due to the increased number of EUs and the risk of cross-contamination between them because some pathogens can impact different species. A literature search was done to determine which relevant pathogens can affect a single species vs multiple species (Table 1) which led us to design a pathogen screening protocol specific to each EU (Table 2).
Sampling protocols were tailored to our facility, since the usage of a statistically significant number of sampled animals, puts up some obstacles, as exemplified in Mocho.9 Therefore, we try to compensate for the reduced sample size by adding environmental and feed testing (Table 2).
The diagnostic techniques employed differ between species. In zebrafish, due to their size, antemortem tests are not widely used. In contrast, in African clawed frogs, clinical exams with manual restraint or under sedation are more frequent. In bigger fish (e.g., seabass, tilapias, salmonids, elasmobranchs), tests like gill biopsies, blood collection, and imaging under sedation or anesthesia, would be feasible.
An evident difference between the screening protocol of zebrafish and African clawed frog is that in the previous, we
mainly test pre-filter sentinels, while in the latter, we import a surplus of animals for lethal screening during quarantine and throughout their stay at the EU4. This and other protocols adapted to different species are thoroughly described in recent literature (9, 10).
Over the years following this program, we diagnosed two cases of relevant infectious disease in the African clawed frog, Mycobacterium haemophilum and Epistylis spp., which were managed without cross-contamination in the other two fish species (Figure 3), even if they may infect other fish and amphibians. One important component of health programs is reporting. Since the only species that we currently export is zebrafish, it is the only one for which we produce regular health reports. Along with the pathogen and diseases historical report we also make available a husbandry report.2
Currently, we are in a transition period, adapting our whole health and biosecurity program to the recent FELASA/ AALAS recommendations.9,10 We will move from biannual routine sampling to a quarterly pattern and rearrange some of our diagnostic techniques while still maintaining a strong histopathology component, screening a large percentage of the mortalities and of the retired animals.
Conclusion
The multi-species facility poses additional challenges when compared to those of single species. In this more complex context, personnel with appropriate expertise, biosecurity, and health monitoring become major concerns. Consequently, we dedicate important resources to these areas. Here, we described the biosecurity and health programs of the IGC aquatic facility, which were developed to minimize the possibility of cross-contamination of pathogens originating in different host species, by incorporating strong preventive measures. Even though we observed a few outbreaks over the years, these were confined, and not spread to other species, or EUs. This suggests that the biosecurity barriers put into practice were effective. Furthermore, the health control program has been important not only to validate the effectiveness of the barriers, but also to characterize the health status of each EU, to protect research projects, and to promote animal welfare. We hope that this information is useful to others attempting to establish multi-species aquatic facilities.
January 2023 Laboratory Animal Science Professional 45
Fig 3. Two relevant infectious disease cases that were contained in the affected epidemiological unit. A . Abundant acid-fast bacilli in the spleen of an african clawed frog (Ziehl-Neelsen stain, 200x). B. Wet mount of Epistylis sp. found in african clawed frog (200x).
Nuno Pereira, DVM, is the Designated Veterinarian at the Aquatic Facility of Instituto Gulbenkian de Ciência, Oeiras, Portugal, Veterinarian at Oceanário de Lisboa, Portugal, and Lecturer at the Veterinary Faculty, Universidade Lusófona, Lisboa, Portugal.
Inês Santos, MsC, is an Animal Care Technician at the Aquatic facility of the Instituto Gulbenkian de Ciência, Oeiras, Portugal.
Ana Borges, PhD, is Manager of the Aquatic Facility of the Instituto Gulbenkian de Ciência, Oeiras, Portugal.
REFERENCES
1. Borges AC, Pereira N, Franco M, Vale L, Pereira M, Cunha MV, Amaro A, Albuquerque T, Rebelo M. 2016. Implementation of a zebrafish health program in a research facility: a 4-year retrospective study. Zebrafish, 13(S1), 115–126.
2. Collymore C, Crim M, Lieggi C. 2016. Recommendations for health monitoring and reporting for zebrafish research facilities. Zebrafish 13.S1: 138–148.
3. Divers SJ, Stahl SJ, editors. 2018. Mader’s reptile and amphibian medicine and surgery-e-book. Elsevier Health Sciences.
4. European Commission. 2014. Caring for animals – aiming for better science. Education and Training Framework.
5. Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, MacLaw J, Sánchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. 2009. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 149.2: 240–248.
6. Lieggi C. 2019. Health Surveillance Programs, p 415–429. In: Cartner S, Eisen JS, Farmer SF, Guillemin KJ, Kent ML, Sanders GE, editors. The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications. Academic Press, Elsevier.
7. Matthews JL. 2004. Common diseases of laboratory zebrafish. Methods in cell biology 77: 617–643.
8. Mocho JP, Pereira N. 2022. Health monitoring, disease, and
clinical pathology, p 81–100. In: D’Angelo L, Girolamo P. Laboratory Fish in Biomedical Research. Academic Press, Elsevier.
9. Mocho JP, Collymoore C, Farmer SC, Leguay E, Murray KN, Pereira N. 2022. FELASA-AALAS Recommendations for Monitoring and Reporting of Laboratory Fish Diseases and Health Status, With an Emphasis on Zebrafish (Danio rerio). Comparative Medicine 74(3): 127–148.
10. Mocho JP, Collymoore C, Farmer SC, Leguay E, Murray KN, Pereira N. 2022. FELASA-AALAS recommendations for biosecurity in an aquatic facility, including prevention of zoonosis, introduction of new fish colonies, and quarantine. Comparative Medicine 74(3): 149–168.
11. Murray KN, Varga ZM, Kent ML. 2016. Biosecurity and health monitoring at the Zebrafish International Resource Center. Zebrafish 13(S1): 30–38.
12. Mylniczenko N. 2009. Amphibians, p. 73-111. In: Mitchell MA, Tully TN, editors. Manual of Exotic Pet Practice. Saint Louis: W.B. Saunders.
13. RSPCA – Royal Society for the Prevention of Cruelty to Animals. [Internet]. 2005. Reed BT. Guidance on the housing and care of the African clawed frog Xenopus laevis. [Cited 30 November 2022].Available at: https://www.rspca.org.uk/webContent/ staticImages/Downloads/GuidanceXenopusLaevisReport.pdf?_ gl=1*yp4o68*_ga*MTA0NDQxNDgxNy4xNjY5ODkwMTUw*_ga_ FQYR2JQR29*MTY2OTg5MDE1MC4xLjEuMTY2OTg5MDMxMC4wLjAuMA
14. Green SL, editor. 2010. The Laboratory Xenopus sp.. Boca Raton, London, New York: CRC Press. Taylor and Francis Group, LLC.
15. Westerfield M, editor. 2007. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th Edition. Eugene (OR): University of Oregon Press.
16. Wright KM, Whitaker BR, editors. 2001. Amphibian Medicine and Captive Husbandry. Malabar (Fl): Krieger Publishing Company.
46 Laboratory Animal Science Professional January 2023
Is There Something Fishy Going On in Your Lab?
Find out by using our:
Zebrafish Health Monitoring Services

• Histopathology services
• Infectious disease PCR
• NSG sequencing
• Flexible and custom program options
• NEW: Expanded infectious disease PCR panel
Creating a Rotifer Polyculture Tank for the Use of Rearing Larval Zebrafish

 By Logan A Fehrenbach MS and Raphael A Malbrue DVM, MS, CertAqV, DACLAM
By Logan A Fehrenbach MS and Raphael A Malbrue DVM, MS, CertAqV, DACLAM
Introduction
The use of zebrafish as a research model and the number of research labs using zebrafish has been rapidly increasing since the early 1990s.5 Currently, most facilities rear larval and juvenile zebrafish to maturity in-house. There are multiple methods of rearing larval zebrafish used within the field, with different ratios of live and prepared feeds being added to nursery tanks.2 In this article, we present the technique and setup of a rotifer polyculture first introduced in Zebrafish. 1

To raise larval zebrafish using the rotifer polyculture method, most zebrafish facilities currently use one of two marine rotifer species, Brachionus plicatilis or Brachionus roundiformis. These two rotifer species are small enough to fit the larval mouth gape of larval zebrafish that start exogenous feeding at 5 days post fertilization (dpf), as well as being easily digestible and an attractive prey item.1,4
Tank Set Up
The Abigail Wexner Research Institute at Nationwide Children’s Hospital uses a 1.8-L Aquaneering tank for creating a nursery polyculture. The 1.8-L size tanks allow for sufficient room for the polyculture mix of rotifers in solution (feed out) and fish system water. It also allows more tanks to fit in the nursery due to the 1.8-L tanks’ narrow width. Larger tank sizes can be used if needed, but the volume of rotifer feed out and system water may need to be adjusted to allow sufficient room for larval fish to feed. Ensure polyculture tanks are fitted with a baffle micron fry screen no larger than 400 microns situated in the rear of the tank to prevent larval fish from escaping
(Figure #1). A back baffle should also be added to the tank to allow normal water flow when a drip is started into the tank. A lid should be fitted on the tank after the polyculture tank is created.
Addition of Rotifer Feed Out and System Water
Rotifer feed out to be added to the polyculture tank should be highly dense (e.g. 1500 rotifers/ml) and at a salinity range between 5-10 part per thousand (ppt) which is low enough to allow high survival rates for larval zebrafish but high enough to allow for survival and reproduction of rotifers to continue. The ratio of system water to rotifers in the tank should maintain a salinity of 2-7 ppt. A salinity below 2 ppt will result in lower rotifer survivability and cease rotifer reproduction, while a salinity above 7 ppt will result in higher mortality rates in larval zebrafish.4 Our facility uses a ratio of 150 ml of rotifer feed out culture kept at a density of 1500 rotifers per milliliter with a salinity of 5 ppt to 200 ml of fish system water kept at ~0 ppt (600 μS/cm), resulting in a final volume of 350 ml and salinity of ~2.15 ppt.
Addition of Larval Fish
After the tank has been set up and the rotifer feed out and system water has been added, the larval zebrafish are ready to be added to the tank. Larval fish should be added at 5 dpf when they start exogenous feeding and their yolk sac is depleted.3,4 If necessary, larval fish can be added at 6 dpf or 7 dpf, but this will result in lower survival rates in the tank. Larval fish can be added in densities up to 50 fish/L, but we suggest keeping fish
B A
48 Laboratory Animal Science Professional January 2023
TECH TIPS - HUSBANDRY, ENRICHMENT, NEW TECHNIQUES AND TACTICS
Figure 1. (A) Tank supplies needed to create a polyculture nursery tank including housing tank, 400 micron fry screen, back baffle, and lid. (B) Proper placement of a fry micron screen and back baffle inside the rear a polyculture nursery tank.
Figure 1. (A) Tank supplies needed to create a polyculture nursery tank including housing tank, 400 micron fry screen, back baffle, and lid. (B) Proper placement of a fry micron screen and back baffle inside the rear a polyculture nursery tank.
Figure 1. (A) Tank supplies needed to create a polyculture nursery tank including housing tank, 400 micron fry screen, back baffle, and lid. (B) Proper placement of a fry micron screen and back baffle inside the rear a polyculture nursery tank.
A B
B
fish into the polyculture tank, slowly decant the petri dish or container containing fish into the polyculture tank (Figure #2).




Once the polyculture tank has been created, it will be placed into the nursery in the static phase with no extra rotifer feed or system water added into the tank. The static phase can last anywhere from 3-7 days (8 dpf to 12 dpf). We suggest placing a piece of test tube tape on the lid over the feeding hole with -
BACompleted polyculture nursery tank with rotifer feed out, system water, and larval fish (B) Piece of tape over the feeding hole on the lid with the date that the static phase of the polyculture is completed.

enly feeding or adding system water during the static phase (Figure #3). Once the static phase is complete, a slow drip of 1 drip every 2-3 seconds will be started into the tank and additional food will be added on a daily basis.
Conclusion
The rotifer polyculture method for rearing larval zebrafish is widely used in the field and highly successful. Rotifer cultures are easy to maintain and harvest, which allows facilities to keep a plentiful source of feed in-house for larval and juvenile zebrafish.1 Using marine rotifers to raise larval zebrafish has multiple benefits when compared to using other methods such as a dry feed.
tank with rotifer feed out, system water, and larval fish. (B) with the date that the static phase of the polyculture is completed.
densities around 15-20 fish/L so that there is no need to separate them at a later stage of life. Separating high-density larval

In the nursery tank, the marine rotifers used in zebrafish facilities are able to stay in the water column and reproduce, which gives larval and juvenile zebrafish access to copious amounts of food during the early stages of their development. The size of rotifers used in zebrafish facilities fit the larval mouth gape of zebrafish once they first start exogenous feeding around 5 dpf and are also highly attractive digestible prey.1,4 In addition, rotifers can be gut loaded which allows facilities to target nutrients beneficial for larval zebrafish growth. Rotifers serve as a vessel, holding these nutrients in their guts

January 2023 Laboratory Animal Science Professional 49
Figure 2. (A) Decanting larval zebrafish from a petri dish into a polyculture nursery tank. (B) Rinsing the petri dish with embryo media to wash out any left-over larval fish into the polyculture nursery tank
Figure 2. (A) Decanting larval zebrafish from a petri dish into a polyculture nursery tank (B) Rinsing the petri dish with embryo media to wash out any left-over larval fish into the polyculture nursery tank.
Figure 2. (A) Decanting larval zebrafish from a petri dish into a polyculture nursery tank. (B) Rinsing the petri dish with embryo media to wash out any left-over larval fish into the polyculture nursery tank.
Figure 3. (A) Completed polyculture nursery tank with rotifer feed out, system water, and larval fish. (B) Piece of tape over the feeding hole on the lid with the date that the static phase of the polyculture is completed.
until they are consumed by larval zebrafish. Using rotifers in a nursery tank slows the build-up of ammonia in the water when compared to using a dry feed. Any uneaten dry feed in the tank will fall to the bottom of the tank and start to decompose, resulting in a raised ammonia content in the tank. Any dead and uneaten rotifers will also result in ammonia build up in a tank. However, because rotifers are able to survive in nursery tanks and stay in the water column, this results in more of the food being eaten by the fish in the tank and less buildup of decomposing detritus at the bottom of the tank.
Logan Fehrenbach, MS, is an Aquatic Tech Specialist at The Abigail Wexner Research Institute at Nationwide Children’s Hospital in Columbus, Ohio.
Raphael Malbrue, DVM, MS, CertAqV, DACLAM, is the Associate Director at The Abigail Wexner Research Institute at Nationwide Children’s Hospital in Columbus, Ohio.


REFERENCES:
1. Best J, Adatto I, Cockinton J, James A, Lawrence C. 2010. A novel method for rearing first-feeding larval zebrafish: polyculture with type L saltwater rotifers (Brachions plicatilis). Zebrafish 7: 289-295.
2. Carvalho AP, Araujo L, Santos MM. 2006. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aquac Res 37: 1107-1111.
3. Harper C, Lawrence C. 2010. The Laboratory Zebrafish (Laboratory Animal Pocket Reference). CRC Press.
4. Lawrence C, Best J, Cockington J, Henry EC, Hurley S, James A, Lapointe C, Maloney K. 2016. The Complete and Updated “Rotifer Polyculture Method” for Rearing Fist Feeding Zebrafish. Jove-J Vis Exp 107:e53629.
5. Teame T, Zhang Z, Ran C, Zhang H, Yang Y, Ding Q, Xie M, Gao C, Ye Y, Duan M, Zhou Z. 2019. The use of zebrafish (Danio rerio) as biomedical models. Anim Front 9:68-77.
We’re proud to announce the grand opening of our facility located in Quebec, Canada! With this facility, access to more services and added shipping conveniences back VRL’s continued efforts –strengthening relationships by way of exceptional customer service.
50 Laboratory Animal Science Professional January 2023
ANIMAL HEALTH DIAGNOS TICS Animal Health Monitoring • Virology • Chemistry • Bacteriology • Aquatics • Parasitology • Environmental Testing THE VRL ADVANTAGE Knowledgeable Scientific Staff Fast Turnaround Times Customer Focused Reliable Results
www.vrl.net GRAND OPENING
AALAS Crossword
By Kari Buchanan, RLATG
Zebrafish #2
ACROSS
2 To examine mucus scraped from the skin of an aquatic species, what substance is used to prepare the slide?
5 At what time of day do zebrafish spawn?
7 Producing eggs that develop and hatch outside the maternal body is called:
9 What fins are associated with a musculoskeletal girdle connected to the skull?
11 What can be used to maintain moisture on the skin of zebrafish during technical procedures such as in-vitro fertilization or surgery?
13 How many barbels do zebrafish have?
16 How many hours post fertilization do zebrafish embryos acquire a fish-like appearance, rudimentary neural tube and eye twitches?
17 What is the most important factor in the microenvironment for Zebrafish?
21 What carrier molecule can be used to administer compounds orally in zebrafish?
22 What common practice is performed in order to isolate genetic material from zebrafish for the purpose of genotyping?
23 Approximately % of the total water in a recirculating system should be drained off and replaced daily
1 For gastric gavage of fish, the volume administered should not exceed % of body weight.
2 How many unpaired fins do zebrafish have?
3 Zebrafish embryos are , which means you can watch development as it happens
4 From which location can serial blood samples be obtained from zebrafish?
6 What injection route requires zebrafish to be fasted for 24 hours prior to avoid perforating the intestine?
8 What laboratory bred strain of zebrafish lacks skin pigmentation?
10 What abbreviation is used for the phase “days post fertilization”?
12 Zebrafish are meaning they can tolerate a diverse range of temperatures
14 What pigment pattern do wild type zebrafish have?
15 How many heart chambers do fish have?
18 Zebrafish don't have a stomach, which means they also lack a(n) bladder.
19 Embryos transferred from quarantine to the main system are surface disinfected in what solution?
20 How many fin types do zebrafish have?
January 2023 Laboratory Animal Science Professional 51
DOWN
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
2023 National Meeting Update
Submit a Topic or Abstract for 2023
We hope you will consider submitting a topic or abstract for the 2023 National Meeting.
The Program Committee and AALAS President Pam Straeter invite you to submit proposals for abstracts, technical trade presentations, panel discussions, seminars, special topic lectures, and workshops for the 2023 National Meeting.
Go to Abstract Central to access the portal. Those who have before will have a password and username. New users must create an account. The deadline for topic submissions is March 15, and the deadline for abstracts is June 1.
Exhibit Your Products and Services in Salt Lake City
Exhibitor registration is open. Please remember that the exhibitor priority point deadline is February 5, 2022. As an exhibitor, you have an opportunity to interact with more than 4,500 AALAS attendees from the academic community, research institutions, government organizations, and commercial companies.

Interested in partnering with AALAS and supporting our mission? Consider becoming a National Meeting sponsor. Review our 2023 Media Kit on our website and consider the opportunities to promote your products to our AALAS decision-makers. For more information, contact John Farrar at john.farrar@aalas.org.
Recognize a Deserving Colleague with an AALAS Award
Six professional and technical categories are recognized at the AALAS National Meeting for excellence in the laboratory animal science field. The six awards which will require nominations are the Bhatt, Brewer, Collins, Garvey, Griffin, and Technician of the Year. Nominees and nominators for all awards must be current national members of AALAS as of January 1 of the nominating year. Nominations are due by April 1, 2023. Over $13,000 in prize money will be awarded!
The 2023 AALAS Election: Is it Time for You to Lead?
The 2023 Nominations Committee, chaired by John Park, is looking for our next AALAS leaders. We need your time, energy, and ideas to advance and improve our association and the many programs and benefits we offer to members.
Are you ready for a leadership role?
• Do you want to influence current and future AALAS programs?
• Do you want to give back to your professional association?
• Do you want to get a sense of satisfaction from helping AALAS members reach their goals?
• Do you want to interact with people who will enrich your life?
• Do you want to gain experience, learn new skills, network, and demonstrate your work ethic?
52 Laboratory Animal Science Professional January 2023 AALAS CONNECTION
• Do you want to gain more leadership skills?
• Do you want to work with and learn from the best association staff anywhere?
If you answered yes to any of these questions, you should consider running for one of our open positions!
The ballot for the 2023 AALAS Election has the following open positions:
• Vice President-Elect
• At-Large Trustee Seat 4
• District 1 Trustee
• District 2 Trustee
• District 2 Alternate
• District 3 Alternate
• District 4 Trustee
• District 7 Trustee
The qualifications for running for a leadership position can be found on the AALAS website’s nominations page (https:// www.aalas.org/leadership/leadership-nomination) along with details about the application process. Candidates are required to submit an application, candidate agreement, nominating petition (Trustees/Alternates), and CV (Vice President-Elect).
If you are need more information about the time commitment or other required duties, please reach out to former or current Executive Committee Members, Trustees, or the AALAS office.
Apply Now for AALAS’ Grants for Laboratory Animal Science
By Ashlee Vaughn, PhD
Application forms and instructions are now available online for the AALAS Grants for Laboratory Animal Science (GLAS) program. The GLAS program provides two types of research grants: the standard grant, of up to $50,000 for studies with a sound hypothesis and preliminary data; and the small grant, for up to $7,500 for innovative or pilot studies. Applications are due on February 1, 2023.
The GLAS mission is to enhance scientific knowledge in laboratory animal health and welfare through research and to promote collaborative efforts by the AALAS membership within the broader scientific community. The GLAS program contributes to the AALAS mission to advance responsible laboratory animal care and use to benefit people and animals in such areas as environmental conditions, housing and enrichment, pain and distress, health and welfare, euthanasia, and advancements in animal care and use. Since the first grants were awarded in 2007, the GLAS program has awarded 92 grants totaling $1,743,310. As a result of the GLAS program, nearly 400 cited presentations, publications, and posters stemming from GLAS-sponsored projects have been produced and are listed on the AALAS website. Through these outcomes, AALAS has had a visible role in advancing research and knowledge in laboratory animal science.
Canada, Spain, and Sweden. The GLAS application form and details about the GLAS program, application instructions, and an application tutorial may be viewed at www.aalas.org/glas.
This website also has recordings of the AALAS Scientific Advisory Committee’s perspectives and suggestions to prospective GLAS candidates on how to craft a successful GLAS application. The recordings are the panel discussion and Q&A session from last year’s National Meeting.
If you have a proposal to improve laboratory animal health and welfare in research, we hope you will submit your idea in a GLAS application.
The GLAS program requires that the principal investigator be an AALAS member at any level, but it is not restricted geographically. GLAS awards have been made to recipients in
January 2023 Laboratory Animal Science Professional 53
Ashlee Vaughn, PhD, is an Educational Resource Editor at AALAS in Memphis, TN.
Since 2007 the GLAS program has awarded 92 grants totaling $1,743,310.
AALAS Foundation – Branch Public Outreach & Education (POE) Award
The AALAS Foundation is excited to announce the Texas Branch as the winner of the 2022 Branch Public Outreach & Education (POE) Award! Texas Branch was awarded a $100 gift card and certificate during the AALAS Foundation’s Appreciation Reception at the 2022 AALAS National Meeting in Louisville, Kentucky.
The Branch POE Award recognizes branches for their members’ public outreach efforts conducted each year during the time frame of September 1 through August 31.

Members of the Texas Branch sent submission forms to the AALAS Foundation Branch Committee representing a total of nine (9) public outreach activities conducted by various members of the Texas Branch in 2022.
Submission forms are currently being accepted for consideration for the 2023 Branch POE Award. Any Branch member conducting a public outreach activity is eligible to submit a POE Award submission form on behalf of their branch – the outreach activity does not have to be organized by the branch.
The AALAS Foundation offers free “What’s Happening in Research?” speaker-ready PowerPoint presentations to help with your public outreach efforts. These presentations are fully scripted with speaker notes and help explain how animals are helping advance medical progress in seven different areas of disease research:

What’s Happening in Breast Cancer Research?
What’s Happening in Heart Disease Research?
What’s Happening in Alzheimer’s Disease Research?
What’s Happening in Diabetes Research?
What’s Happening in Arthritis Research?
What’s Happening in Transplant Research?
What’s Happening in COVID-19 Research?
The presentations are suitable for presenting in person or via an online platform, such as Zoom, to adults, college students, and/or high school students.
Additionally, the AALAS Foundation offers its “Celebrate Animal Research & Education” (CARE) YouTube channel to assist with your outreach efforts. The CARE YouTube channel contains a growing collection of short videos highlighting
how animals have helped advance medical discoveries. https:// tinyurl.com/CARE-ANIMALS
The AALAS Foundation also continues to offer its free handout materials, such as the highly popular “Animal Roles in Medical Discoveries” poster and the “Careers in Biomedical Research” brochure. These materials, and others, may be ordered directly from the AALAS online Book Store where shipping of these items are free, too!
Email foundation@aalas.org for more details on all the free resources available to you and to learn more about how your public outreach efforts might help your branch be the next winner of our Branch POE Award!
54 Laboratory Animal Science Professional January 2023
AALAS Crossword Answers
Zebrafish #2
January 2023 Laboratory Animal Science Professional 55
1O N 2T A N K W A 3T E R H R R A 4D E 5D A W N 6 I 7O V I P A R O U S 8C E S N R A 9P E C T O R A L F I N S S A R A P 10D R A L 11W E T S P O N G 12E E P A R F U N E 13T W O P A I R 14S R T R 15T R T Y I 16T W E N T Y F O U R 17W A T E R Q 18U A L I T Y O A I H R O 19B 20F P 21G L U T E N I 22F I N C L I P P I N G 23T E N R N E E V S M A A A E A R L C L Y H —Advertising List— Contact us for more information. Visit us at aalas.org or call John Farrar at (901) 754-8620.
in LAS Pro COMPANY WEBSITE PAGE Allentown LLC www.allentowninc.com back cover Ancare Corp www.ancare.com 28 Bio-Serv www.bio-serv.com 19 Braintree Scientific Inc. www.braintreesci.com 9 Charles River Laboratories www.criver.com 47 Clear H2O Inc www.clearh2o.com 17,33 E-Z Systems Inc. www.criver.com 7 Instech Laboratories Inc. www.instechlabs.com 3 IWAKI Aquatic www.iwakiaquatic.com 25 Kent Scientific Corporation www.kentscientific.com 20/21 Lomir Biomedical Inc www.lomir.com IBC PlasLabs Inc. www.plas-labs.com 24 PMI LabDiet www.labdiet.com inside frontcover Skretting www.skretting.com 37 SteraMist by TOMI www.tomimist.com 6 Virox Technologies www.peroxigard.com 1 VRL Laboratories-USA www.vrlsat.com 50
Advertise
Gravy Boat
 By Annika Helle
By Annika Helle
Gravy is a wonderful large 9-year-old tuxedo cat who came into my life after a friend had to give her up. “Watch your groceries, she’s an absolute fiend for food” was the parting statement from her past owner. From then on, I’ve never had to eat a meal alone as Gravy is always just a whiskers length away carefully supervising my food intake. I learned pretty quickly that the crock pot must be taped closed and no butter can be left to soften on the counter unless I wished to come home to a pork roast juice and butter covered cat (and a lost opportunity for baking those cookies). Gravy’s impressive appetite is just one of her many shining qualities. She is actually a very talented singer. Her 3 am renditions of Motzart’s Der Hölle Rache would have even the most seasoned of opera fans rousing from their slumber and weeping with emotion. As a woman of fierce conviction and even fiercer determination, Gravy has mastered the art of wordless demands. One steely-eyed gaze at her little brother has him instantly backing away from his bowl of kibble, yielding it to Gravy herself so she may use it to grow her body and fuel her reign (or at least that is what she believes). She can convince even the most apathetic of contractors and house guests to provide her with a snack by dutifully leading everyone who enters the home straight to the bin where the kibbles are kept and giving them those adoring big eyes. While not focused on her necessity to vacuum up food crumbs like Kirby, Gravy fills her time with marathon naps.

Years of consistency and persistent training have granted Gravy
the ability to remain unconscious for borderline concerning amounts of time. Her favorite locations for her nap sessions are on my chest giving my lungs some serious resistance training and on that pile of important papers I currently need to access. Through all this, Gravy is a remarkably affectionate cat who accepts all who wish to greet her, even when it’s some stranger’s dog. Her thrumming reliable purr can mend any wound and bring at least a statistically significant amount of peace to any soul that needs it. A novel wouldn’t cover the impact of Gravy on my life and her natural talent for being an icon, but I will keep this abridged. Harnessing strength, facing fears with courage, embracing and nurturing yourself, offering gentle support, investing yourself in hobbies you enjoy, taking change in stride, and finding joy in the small things are just a handful of the lessons that Gravy has enriched my life with. I continue to look forward to each day I have with her and her hungry lovable self.

56 Laboratory Animal Science Professional January 2023 Be a part of LAS Pro's column this year! Email us a photo of you with your pet and we will put you in the running for a spot in one of our upcoming issues. LAS PRO 's PET
TALK
FollowGravyBoatonInstagram:ohgravyboat


















































































































































































































Learn more at www.AllentownInc.com Visit our online store at Store.AllentownInc.com 01-2023 Solutions for multiple research program areas, from a partner you can trust. Your Global Solutions Provider ◼ ANIMAL HOUSING ◼ WORKSTATIONS ◼ WASHING ◼ GLASSWARE WASHING ◼ STERILIZATION ◼ CONTAMINATION CONTROL ◼ ENRICHMENT ◼ RESEARCH ANESTHESIA ◼ HOME CAGE MONITORING ◼ COLONY HEALTH MONITORING ◼ VIVARIUM SOLUTIONS






































































































































 Gennifer Caesar, BS, ALAT, is the founder and Chief Digital Transformation Consultant for Vivalytics Consulting in Las Vegas, NV.
Gennifer Caesar, BS, ALAT, is the founder and Chief Digital Transformation Consultant for Vivalytics Consulting in Las Vegas, NV.











 By Ehsan Ramezani-Fard, PhD
By Ehsan Ramezani-Fard, PhD










 By Stephen C. Frederickson, B.S; Wendy Pridgen, B.S.; Irene Ginty, B.S. & Tannia Clark, DVM, MS, DACLAM
By Stephen C. Frederickson, B.S; Wendy Pridgen, B.S.; Irene Ginty, B.S. & Tannia Clark, DVM, MS, DACLAM
























 By Annika Helle
By Annika Helle


































































































































