F. No. D.21013/03/2020-DC
Government of India
Directorate General of Health Services
Central Drugs Standard Control Organization (Administration Division)
FDA Bhavan, Kotla Road New Delhi
Dated: 30.01.2023
Vacancy Notice
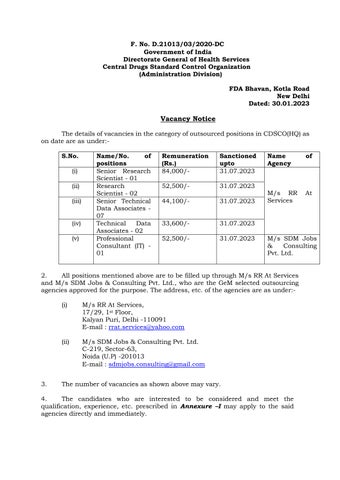
ThedetailsofvacanciesinthecategoryofoutsourcedpositionsinCDSCO(HQ)as ondate are asunder:-
2. All positions mentioned above are to be filledupthrough M/sRRAt Services and M/s SDM Jobs & Consulting Pvt. Ltd., who are the GeM selected outsourcing agenciesapprovedforthe purpose.The address,etc.ofthe agenciesare asunder:-
(i) M/sRRAtServices, 17/29,1st Floor, KalyanPuri,Delhi -110091
E-mail:rrat.services@yahoo.com
(ii) M/sSDMJobs&ConsultingPvt.Ltd. C-219,Sector-63, Noida(U.P)-201013
E-mail:sdmjobs.consulting@gmail.com
3. The numberofvacanciesasshownabove mayvary.
4. The candidates who are interested to be considered and meet the qualification, experience, etc. prescribed in Annexure –I may apply to the said agenciesdirectlyandimmediately.
Annexure to CDSCO’s Vacancy Notice dated 30.01.2023
(i) Sr. Research Scientist : Rs. 84,000/- p.m.
Qualification : B. Tech/B.E in Electrical/Instrumentation/Clinical/Biomedical Engineering/Biotechnology from recognized Institute/University; Post Graduation or Advanced Post Graduate Diploma in similar discipline would be desirable.In addition, M. Pharm, M.S in Medical DevicesTechnology.
Experience : Working in Medical Devices manufacturing/Medical Devices research/medical devices testing laboratory for minimum periodof05years.
(ii) Research Scientist : Rs. 52,500/- p.m.
Qualification : B.Tech/B.E in Electrical/Instrumentation/Clinical/Biomedical Engineering/Biotechnology from recognized Institute/University; Post Graduation or Advanced Post Graduate Diploma in similar discipline would be desirable. In addition, M.S in Medical Devices TechnologyandM.Pharm.
Experience : Working in Medical Devices manufacturing/Medical Devices research/medical devices testing laboratory for minimum periodof02years.
(iii) Sr. Technical Data Associates : Rs. 44,100/- p.m.
Sr. Technical Data Associates for Medical Devices:
Qualification : B. Tech/M. Tech in Clinical/Biomedical Engineering/Bio-technology, or M.Sc., Ph. D in Molecular Biology/Biochemistry/Bio-Technology, or BDS/M.D. in Clinical Pharmacology.Inaddition,M.SinMedicalDevicesTechnology.
Experience : Working in Medical Device manufacturing/Research/Testingforminimumperiodof twoyear.
For other Sr. Technical Data Associates :
Qualification : B. Tech/M. Tech in Clinical/Biomedical Engineering/Bio-technology, or M.Sc., Ph. D in Molecular Biology/Biochemistry/Bio-Technology, or BDS/M.D. in Clinical Pharmacology.In addition, M.S in Medical Devices Technology/ M. Pharmacy/Pharm. D with 03 year’s Industrial experience in R & D Manufacturing,TestingandRegulatoryaffairs.
(iv) Technical Data Associates (TDA) - Rs. 33,600/- p.m.
TDA for Medical Devices :
Qualification & Experience : The candidate should be B. Tech/M.Tech in Clinical/Biomedical Engineering/Bio-technology, M.S in Medical Devices Industry, preferably with 01 year Industrial experience in Medical Device Technology. The candidate should also havesoundknowledgeofcomputeroperation.Preferencewillbegiven to candidates having experience in manufacturing/testing of drugs/drugsregulatoryaffairs.
For Other TDAs :
Qualification & Experience : The candidate should be M.S in Pharmaceutical Sciences, B. Pharmacy or M.Sc. in Biochemistry, Pharmaceutical Chemistry/Microbiology/Biotechnology. The candidate should also have sound knowledge of computeroperation. Preference will be given to candidates having experience in manufacturing/testingofdrugs/drugsregulatoryaffairs.
(vi) Professional Consultant (IT) : Rs. 52,500/- p.m.
Qualification:-
1. B-Tech./B.E(computerscience)orMCA
2. 5 years’ experience in management/ development of software inareputedorganization.
Job description:-
1.Tofacilitate E-governance project.
2. To train the staff to implement E- Governance and File TrackingSystem.
3.Developmentofelectronic Archivingandretrievalsystem.
4.TomaintainDocumentation&NationalDataBank.
5.Upgradationofwebsite anditsupkeep.
6.InstallationofVideoconferencingfacilitieswithwifisystem.
7.Preparationofusermanualetc.
8. Any other duties assigned by Drugs Controller of India from time totime