National Tuberculosis Institute Epidemiology & Research Division Date: 27th May 2019
Recruitment of contractual project staff for Vaccine Trial (TB)
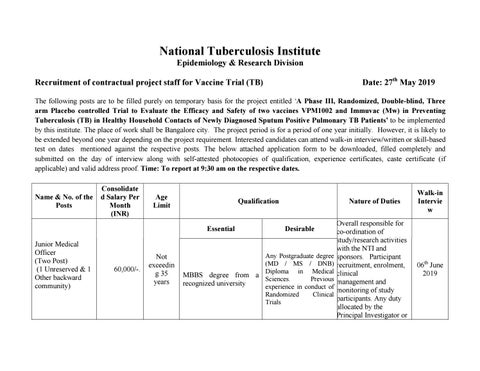
The following posts are to be filled purely on temporary basis for the project entitled ‘A Phase III, Randomized, Double-blind, Three arm Placebo controlled Trial to Evaluate the Efficacy and Safety of two vaccines VPM1002 and Immuvac (Mw) in Preventing Tuberculosis (TB) in Healthy Household Contacts of Newly Diagnosed Sputum Positive Pulmonary TB Patients’ to be implemented by this institute. The place of work shall be Bangalore city. The project period is for a period of one year initially. However, it is likely to be extended beyond one year depending on the project requirement. Interested candidates can attend walk-in interview/written or skill-based test on dates mentioned against the respective posts. The below attached application form to be downloaded, filled completely and submitted on the day of interview along with self-attested photocopies of qualification, experience certificates, caste certificate (if applicable) and valid address proof. Time: To report at 9:30 am on the respective dates.
Name & No. of the Posts
Consolidate d Salary Per Month (INR)
Age Limit
Qualification
Essential Junior Medical Officer (Two Post) (1 Unreserved & 1 Other backward community)
60,000/-.
Not exceedin g 35 years
Nature of Duties
Desirable
Any Postgraduate degree (MD / MS / DNB) MBBS degree from a Diploma in Medical Sciences. Previous recognized university experience in conduct of Randomized Clinical Trials
Overall responsible for co-ordination of study/research activities with the NTI and sponsors. Participant recruitment, enrolment, clinical management and monitoring of study participants. Any duty allocated by the Principal Investigator or
Walk-in Intervie w
06th June 2019