Dated: 7th August 2021
MCC/3118/2020-CON-IRB
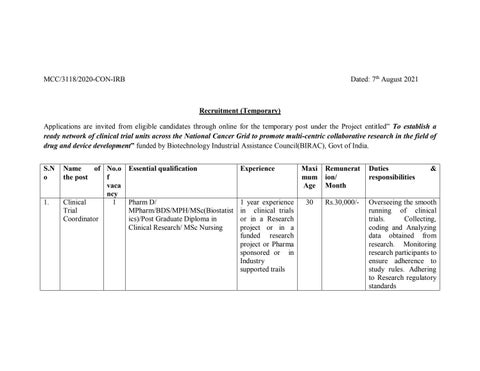
Recruitment (Temporary) Applications are invited from eligible candidates through online for the temporary post under the Project entitled” To establish a ready network of clinical trial units across the National Cancer Grid to promote multi-centric collaborative research in the field of drug and device development” funded by Biotechnology Industrial Assistance Council(BIRAC), Govt of India. S.N o
1.
Name of No.o the post f vaca ncy Clinical 1 Trial Coordinator
Essential qualification
Experience
Pharm D/ MPharm/BDS/MPH/MSc(Biostatist ics)/Post Graduate Diploma in Clinical Research/ MSc Nursing
1 year experience in clinical trials or in a Research project or in a funded research project or Pharma sponsored or in Industry supported trails
Maxi mum Age
Remunerat ion/ Month
Duties responsibilities
&
30
Rs.30,000/-
Overseeing the smooth running of clinical trials. Collecting, coding and Analyzing data obtained from research. Monitoring research participants to ensure adherence to study rules. Adhering to Research regulatory standards