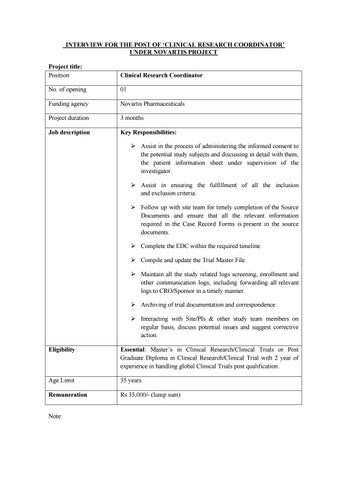
INTERVIEW FOR THE POST OF ‘CLINICAL RESEARCH COORDINATOR’ UNDER NOVARTIS PROJECT Project title: Position
Clinical Research Coordinator
No. of opening
01
Funding agency
Novartis Pharmaceuticals
Project duration
3 months
Job description
Key Responsibilities: ➢ Assist in the process of administering the informed consent to the potential study subjects and discussing in detail with them, the patient information sheet under supervision of the investigator. ➢ Assist in ensuring the fulfillment of all the inclusion and exclusion criteria. ➢ Follow up with site team for timely completion of the Source Documents and ensure that all the relevant information required in the Case Record Forms is present in the source documents. ➢ Complete the EDC within the required timeline. ➢ Compile and update the Trial Master File. ➢ Maintain all the study related logs screening, enrollment and other communication logs, including forwarding all relevant logs to CRO/Sponsor in a timely manner. ➢ Archiving of trial documentation and correspondence. ➢ Interacting with Site/PIs & other study team members on regular basis, discuss potential issues and suggest corrective action.
Eligibility
Essential: Master’s in Clinical Research/Clinical Trials or Post Graduate Diploma in Clinical Research/Clinical Trial with 2 year of experience in handling global Clinical Trials post qualification.
Age Limit
35 years
Remuneration
Rs 35,000/- (lump sum)
Note: