

NITINOL INNOVATION AND ITERATION.
AT THE SPEED OF CONFIDENCE.
Nitinol’s unique properties make it indispensable in advanced MedTech applications. From raw material to finished components, Resonetics offers unmatched expertise and in-house capabilities, ensuring top-quality nitinol for your devices. Accelerate your innovation with our comprehensive solutions, at the speed of confidence.


Drug delivery’s double threat, enabled by collaboration
Drug delivery technology advances are improving and preserving the health of millions of people around the world.
This issue of Medical Design & Outsourcing dives deep into drug delivery, with features focused on drug delivery’s dual abilities of disease prevention and health restoration, coverage of two key materials — adhesives and silicone — plus drug delivery design tips and regulatory expertise.
Our cover story explores nextgeneration inhalable vaccines and the nebulizer technology used to deliver them deep into the lungs. These drugs and delivery technologies show promising potential for more effective, more affordable and more accessible vaccinations for deadly respiratory diseases like influenza and COVID-19 — but they won’t make it to to patients without collaboration, warns Aerogen Director of R&D, Science and Emerging Technologies Ronan MacLoughlin.
We examine drug delivery’s restorative impact in our feature on type 2 diabetes technology, where automation, miniaturization, real-time information and device developer partnerships are making it easier for patients to treat their chronic condition.
This issue also includes coverage of technologies beyond drug delivery, including a feature on Medtronic’s development of adaptive deep brain stimulation therapy and lessons from the project shared by Senior Distinguished Engineer Scott Stanslaski.
In our Nitinol department, we take a first look at Boston Scientific’s investigational Faraflex pulsed field ablation (PFA) and mapping catheter with Dr. Brad Sutton, the chief medical officer of Boston Scientific’s Atrial Solutions Business.
In our Tubing department, we interview Shockwave Medical Chief Medical Officer Dr. Nick West to learn more about how the new Javelin intravascular lithotripsy catheter — recently launched by parent company Johnson & Johnson MedTech —
breaks up calcium in some of the most challenging clogged blood vessels
And ahead of their presentation at DeviceTalks Boston April 30-May 1, two lawyers offer advice for medical device startups on M&A and intellectual property in today’s tumultuous market.
I’d also like to thank the entire Medical Design & Outsourcing team for their work, which has once again been honored as a national finalist for overall excellence in business-to-business media by the American Society of Business Publication Editors (ASBPE).
This recognition wouldn’t be possible without the support of our readers, our advertisers, and the medtech insiders who graciously lend us their time and expertise to advance the medtech mission. We’re fortunate to have the opportunity to enable collaboration and innovation in the medical device industry, and we strive to consistently exceed your expectations in new and surprising ways.
As always, I hope you enjoy this edition of Medical Design & Outsourcing — and thanks for reading.













EDITORIAL
Editor in Chief
Chris Newmarker cnewmarker@wtwhmedia.com
Managing Editor Jim Hammerand jhammerand@wtwhmedia.com
Senior Editor Danielle Kirsh dkirsh@wtwhmedia.com
Associate Editor Sean Whooley swhooley@wtwhmedia.com
Editorial DirectorDeviceTalks Tom Salemi tsalemi@wtwhmedia.com
Managing EditorDeviceTalks Kayleen Brown kbrown@wtwhmedia.com
Editor in ChiefR&D World
Brian Buntz bbuntz@wtwhmedia.com

CREATIVE SERVICES
VP, Creative Director Matthew Claney mclaney@wtwhmedia.com
AUDIENCE DEVELOPMENT
Director, Audience Growth Rick Ellis rellis@wtwhmedia.com
Audience Growth Manager Angela Tanner atanner@wtwhmedia.com
CEO Matt Logan mlogan@wtwhmedia.com
Senior Vice President Courtney Nagle cseel@wtwhmedia.com 440.523.1685


DIGITAL MARKETING
VP, Operations Virginia Goulding vgoulding@wtwhmedia.com
Digital Marketing Manager Taylor Meade tmeade@wtwhmedia.com
Digital Marketing Coordinator Matthew Kulkin mkulkin@wtwhmedia.com
PRODUCTION SERVICES
Customer Service Manager Stephanie Hulett shulett@wtwhmedia.com
Customer Service Rep Tracy Powers tpowers@wtwhmedia.com
Customer Service Rep JoAnn Martin jmartin@wtwhmedia.com
Customer Service Rep Renee Massey-Linston renee@wtwhmedia.com
jbrownlee@wtwhmedia.com
mcooke@wtwhmedia.com
mfrancesconi@wtwhmedia.com
jpowers@wtwhmedia.com
btoole@wtwhmedia.com


















LEADERSHIP TEAM

DEPARTMENTS
HERE’S WHAT WE SEE:
Drug delivery’s double threat, enabled by collaboration
ADHESIVES:
Avoiding adhesive pitfalls in the CGM device design process
DRUG DELIVERY:
Nasal vaccine device design and regulatory need-to-knows
INTELLECTUAL PROPERTY:
Positioning medtech IP for funding and exits
MATERIALS:
How silicone is meeting healthcare’s toughest device challenges
NITINOL:
OR DIE:’ KEYS TO NEXT-GEN INHALABLE VACCINE DEVICE DESIGN FROM AEROGEN’S R&D DIRECTOR
“We are just at the beginning of a very exciting era,” says Aerogen Director of R&D, Science and Emerging Technologies Ronan MacLoughlin.


First Look: Boston Scientific’s next-gen Faraflex PFA and mapping catheter
SUSTAINABILITY:
Six sustainable design lessons from the development of a reusable autoinjector
TUBING:
How Shockwave’s Javelin IVL catheter breaks up bloodblocking calcium where balloons can’t reach



new options for hundreds of millions of diabetes patients.
37 A MEDTRONIC ENGINEER SHARES LESSONS FROM THE ADAPTIVE DEEP BRAIN STIMULATION PROJECT
The biggest technical challenge for aDBS was how small brain signals are.




April 30 - May 1st





Testing Laboratory Production & Material Processing Engineering Services


COMPREHENSIVE EXPERTISE: From early-stage product development to biocompatibility testing, we provide a full spectrum of services under one roof, ensuring a seamless process and faster time-to-market.
CUTTING-EDGE CAPABILITIES: With advanced equipment and industry know-how, we offer specialized testing in drug stability, device verification, and biocompatibility.
TAILORED SOLUTIONS: Whether you're a startup or a Fortune 500 company, we offer customized support, guiding your product through its entire life cycle with precision and care.

Biocompatibility & Toxicology


5368 Kuhl Road, Erie PA, 16510
814-464-0790 | info@psnlabs.com www.psnlabs.com
































Enabling The Future of Diabetic Innovation


M icro Molding & M icro Automated Assembly
Miniaturization Exper ts
Micro features including 3-micron uidic channels, tight tolerances, micro holes, thin walls, and/or complex geometries
All In-House
Prototyping, ultra-precision tooling, micro molding, automated assembly, and CT scanning
Experienced
35 years as a trusted partner, 7,000+ molds
R isk Mitigation
Quick turn protot yping, D fM, D fA, D fX
World- Class Automation
Achieving <1.5 micron positional accurac y
Materials Exper tise
All thermoplastic, PEEK , long-term implantable, bioresorbable, silicone, and uoropolymer resins
Nissha Medical Technologies' expertise in endoscopic device design and Isometric’s micro-molding capabilities are transforming minimally invasive surgical instruments and robotics. Together, we drive innovation in miniaturization and precision, enhancing medical device e ciency and performance.
Avoiding adhesive pitfalls in the CGM device design process
There’s no one-size-fits-all adhesive for CGM wearable devices, but considering materials and user needs will lead to an optimal solution.
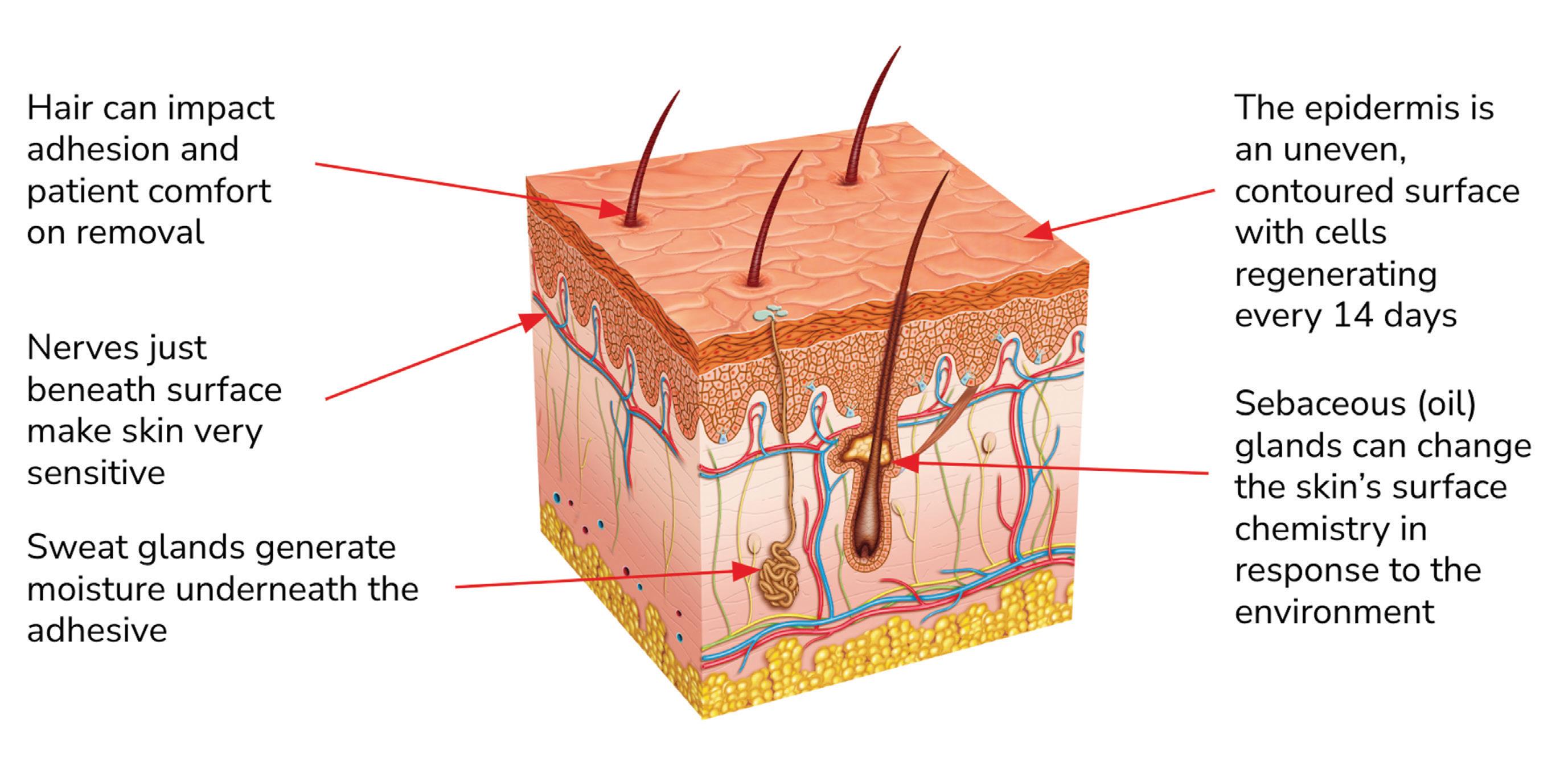
Skin’s anatomical properties present a variety of CGM adhesive challenges. Image courtesy of Avery Dennison

Wearable electronic device technology is revolutionizing diabetes care, allowing patients to get on with their daily lives while enjoying less invasive glucose monitoring and drug delivery.
And it’s no wonder why so many manufacturers want in on this revolution: The continuous glucose monitoring (CGM) device segment is estimated to be worth $11 billion globally and is forecast to grow at a CAGR of 10% between 2024 and 2029.
the device could lead to a device failure.
Nevertheless, engineers may be tempted to de-prioritize the selection of an adhesive. After spending significant time and resources to develop elements such as the processor, transmitter, and power source, they simply specify the most economical or easy-to-access off-the-shelf adhesive solution. This is a missed opportunity at best, and a pitfall in the design process at worst.
By Henry Milliman Avery Dennison Performance Tapes
Adhesive selection is a critical element of the device design process One of the keys to success is reliability. Providers and patients expect durable, effective devices that allow the wearer to experience daily life without discomfort. Any failure of any element of
Adhesives have a significant effect on device reliability. Modern medical adhesives are increasingly sophisticated and diverse, building on decades of innovation. Different adhesive chemical formulations can make a device better suited to different applications.
Taking the time to get the adhesive selection right can truly differentiate a device in a competitive marketplace. >>
ADHESIVES
Device engineers owe it to themselves to treat the adhesive as an investment and approach adhesive selection as an integrated part of the overall device design process.
Key challenges for CGM device adhesives
Accounting for the human element can be a daunting technical challenge. This is certainly true when selecting an adhesive for a wearable CGM device. Here are some key challenges device engineers should consider:
1. Bonding to various device substrates
Adhesives used in device construction (for bonding parts together and bonding the entire device to the skin adhesive) must be compatible with the types of substrate materials used in the device.
Most CGM devices are made using polyethylene, silicone or polycarbonate. Each of these substrates presents different challenges to an adhesive, and certain adhesives are more effective with certain substrates. Low-surface-energy silicone, for example, can be more difficult to bond to than a high-surfaceenergy material such as polycarbonate. However, adhesives optimized for various surface energies are readily available.
2. Skin compatibility
A device adhesive must minimize the risk of skin irritation while ensuring sufficient adhesive strength throughout the device’s wear time. There’s much variation in skin quality and adhesive compatibility from person to person, often based on factors such as age, health, activity level, and the general condition of their skin.
It’s crucial to balance the adhesive’s strength and its compatibility with skin types, necessitating a careful selection process to align with standards such as ISO 10993 and wearer well-being. An adhesive should work appropriately for the majority of the user group.
3.
Wear time
CGM devices are typically worn for at least seven to 14 days, so a device must withstand up to two weeks of real-world stress including bodily movement, exposure to moisture, temperature variance, and rubbing against clothing. A device adhesive should offer a secure bond under such conditions while being breathable, flexible and hypoallergenic to minimize skin irritation or damage.
Wear-time testing is essential for evaluating adhesive performance
in terms of wearability, adhesive strength and user comfort. Such testing is optimal when conducted with scientific rigor, but even casual testing — perhaps involving a handful of company volunteers willing to wear a device and record their experiences — can provide useful insights during the development process.
4. Quality of life
One of the great advantages of CGM wearables is they allow patients to go about their daily lives without being preoccupied with glucose monitoring. It may be a stretch to think of a device as comfortable to wear, but device engineers should work to create an overall positive experience for the wearer. This means (among other things) selecting an adhesive that securely attaches the device without excessive pressure or tension, accommodates natural movement, and minimizes discomfort or pain upon removal.
Silicone-based adhesive chemistries are renowned for their gentle release properties but typically do not have the bonding power of more traditional acrylic-based chemistries. Adhesive selection may involve striking a balance between performance and comfort.

Image courtesy of Avery Dennison

Determining the right adhesive for your needs
While there’s no one-size-fits-all adhesive for CGM wearable devices, there likely is an optimal adhesive based on a device’s materials and target wearer’s needs. Determining that optimal adhesive is the tricky part, but it can pay handsome dividends once a device is in-market.
I’ve provided a framework to help you understand the challenges of choosing an adhesive. But thoroughly understanding your options will likely mean working closely with an adhesive manufacturer, preferably one that can provide design support such as samples, prototyping and wear-test guidance.
As stated earlier, adhesive selection should be an integral part of the overall device design process. That may mean additional investment. But considering the effect of adhesive on device reliability, it’s an investment worth making.
Henry Milliman is a senior research and development manager at Avery Dennison Performance Tapes. He’s the author of six scientific journal publications and holder of more than 200 citations and 10 patent applications, and has extensive experience in adhesive synthesis, formulation and processing across a wide variety of pressure-sensitive adhesive and tape applications.
illustration depicts
many layers
a



Medical Vacuum Heat Treating Services
Your high-value medical parts need special treatment. Solar’s leading-edge vacuum heat treating technology produces clean, bright, consistent results. From annealing to age hardening, rest assured knowing your life-critical parts were vacuum heat treated to your exact specs.
For your prosthetics, guide wires, stents, surgical tools, device and battery cases, hypodermics and hypodermic tubing, brazements for analytical devices...and more, trust Solar Atmospheres to provide you with uncompromising quality.


This
wearable medical device that sticks to a patient’s skin. Image courtesy of Avery Dennison




of Scientific Affairs
Julie Suman
Nasal vaccine device design and regulatory need-to-knows
Nasal vaccine devices have the potential to offer more convenient and more effective protection against a variety of viruses.
n 2024, the FDA approved a nasal spray as the first at-home influenza vaccination, allowing adults to vaccinate themselves or someone in their care without a needle jab or a trip
Intranasal vaccines are in development for RSV, HPV, norovirus, hepatitis B and avian flu. A nasal drop vaccination for COVID-19 is already approved and in use in India, and researchers are evaluating whether a nasal spray might be a more effective method of preventing COVID infections than those drops or the intramuscular injections that are approved in the U.S.
There have also been promising developments with antibodies or peptides delivered via nasal sprays to protect people from infections or to treat them.
To learn more about nasal vaccine devices and how they’re designed, we spoke with Aptar Pharma VP of Scientific Affairs Julie Suman, who’s been working in the nasal space for more than 20 years.
The COVID-19 pandemic “jumpstarted the need to consider multiple routes of vaccinations [for] multiple
shots on goal,” Suman said. “We are excited about the opportunities for intranasal vaccination.”
Why is there so much more attention now on nasal vaccine devices?
Suman: “One of the reasons why nasal became so attractive is the concept of mucosal immunity that would potentially limit the spread of the virus. So not only could you develop the ability to fight the infection yourself, you would not necessarily be shedding the virus and infecting others. In addition, with COVID it’s generally thought that the virus deposits in the nose and interacts with those ACE receptors and then will translocate to the lungs, so if we can stop it in the nasal cavity the person would have a better outcome, preventing that disease from evolving into a full-blown infection.”
What exactly is mucosal immunity?
Suman: “There’s two mechanisms of immunity. Systemic is when you get your shots, it’s circulating in the bloodstream, and different type of antibodies will fight the virus. >>
Nasal vaccine devices could one day be used to prevent COVID-19, avian flu, RSV, HPV, norovirus and hepatitis B.
Photo courtesy of Aptar
DRUG DELIVERY
The nose will generate a different type of antibody that’s sitting around waiting to interact: an IgA (immunoglobulin A) antibody, versus IgG (immunoglobulin G) when you get a systemic immunization. Different types of antibodies get generated from those routes of administration, and it’s thought that IgA — mucosal — is key for preventing the spread of infection. … There is significant immune tissue in the nasal cavity that can induce that immune response, so it has the opportunity to be used for for more than than respiratory viruses.”
How do sprays activate that response?
Suman: “Generally, the spray will deposit in the nasal cavity and interact with the lymphoid tissue in the back of the nasal cavity called the NALT (nasal-associated
Does droplet size matter, and can you control that?
Suman: “The size of the droplets will determine whether they would deposit in the nose or the lung. If you want to deposit droplets in the lung, they have to be smaller than 5 microns. In the nose, the droplets are generally larger than that. … In the orifice of the device, there are mechanisms for generating the droplet size so the device itself will work with the fluid to produce the different sizes of droplets. That’s a very important part of our design process and control process to make sure that we’re delivering appropriate and reproducible droplet sizes.”
“The nose will generate a different type of antibody that’s sitting around waiting to interact. ... Different types of antibodies get generated from those routes of administration, and it’s thought that [mucosal] is key for preventing the spread of infection.”
lymphoid tissue). There are also dendritic cells in the nasal epithelium that allow the vaccine to interact. The dendritic cells are throughout the nasal cavity and the NALTs in the back. When we administer our sprays, we want to target those regions in the nasal cavity.”
Do you need to design nasal vaccine devices specifically to hit those targets?
Suman: “We want to make sure we’re controlling the droplet size and the shape of the spray, if you will. How the person uses it can also play a role, depending on how the device operates. But generally, you want to aim the device in that target region. What we don’t want to do is aim the vaccine toward the roof of the nasal cavity. There was a case maybe 15 years ago where a component of a nasal vaccine entered the brain through the olfactory region and caused unwanted side effects, so the general school of thought is we don’t want to really hit that region of the nose with a vaccine.”
Are there pediatric design considerations?
Suman: “We want to make sure that it’s comfortable for a child to use, because their noses are smaller, and some of the devices you’ll see are indicated for children and even neonates. … It depends on the shape of the nozzle itself. And an adult is going to be upright when getting a vaccine, but that might not be the case with a child. You might be cradling them. So we’ll look at things like how the orientation of your head or the child’s head may affect where the product would distribute in the nose.”
What’s the advantage of sprays versus droppers for nasal vaccines?
Suman: “I’m not sure that a dropper will work with Western regulations, because it’s a little more challenging to monitor the dose or control the dose with a dropper, compared to sprays that are metered so we know we’re giving a very specific volume — which equates to the dose — when we spray it. Droppers are certainly a more
cost-positive solution for developing countries, but I don’t know that we’d see droppers in the Western world.”
Besides safety and efficacy, what sort of regulatory considerations are there for nasal drug delivery devices?
Suman: “There are guidelines on human factors. Can the person or the medical practitioner understand how to use the device? Can they use it correctly? We have a company in our portfolio called Noble that does human factors studies and helps produce instructions for use that our pharma companies can incorporate into their packaging and labeling. There are requirements around the materials and biocompatibility. These devices are injection molded, so there’s criterion around quality. … There are also FDA guidelines for quality control tests they want to see. We look at the shape of the spray, the size of the droplets. Certainly, dose reproducibility is No. 1. Compatibility of the formulation with the device itself is also important.”
How are nasal delivery devices sterilized?
Suman: “Everybody has a different process to sterilize a device. Some people use steam, some use gamma radiation. When we throw in a biologic or vaccine, that becomes trickier because the sterilization process can damage the molecule itself and maybe render it ineffective. The interesting thing about nasal is that from a regulatory standpoint, there is no FDA guidance that says a nasal spray must be sterile. So there’s not an obligation. However, customers may want for various reasons to have a sterile product. … Anybody who’s in this space is probably having that conversation with their pharma [customer].”














By Jim Hammerand Managing Editor
Positioning medtech IP for funding and exits
Despite global volatility, there’s still opportunity for medtech startups with strong intellectual property protection.
Greenberg Traurig attorneys David Dykeman and Roman Fayerberg are scheduled to present at DeviceTalks Boston on April 30.
Their panel, “Positioning IP for Funding & Exits,” will include Aerin Medical Chief Legal Officer Irina Ridley and Bluwave Capital Partner Jonathan Black, who is also CEO of Wellsense and was previously CEO of DuraMedic, Liberty Medical and Omnis Health.
Ahead of DeviceTalks Boston, Dykeman and Fayerberg jointly answered questions from Medical Design & Outsourcing about medtech M&A and intellectual property (IP) considerations for medical device startups.
How would you characterize the market for medtech M&A and IPOs right now, and do you anticipate a shift in the near future?
“The U.S. economy and the stock market are volatile with uncertainty right now, which makes IPOs more difficult. While these uncertainties can also impact M&A deals, the medtech giants have large piles of cash and are willing to pay a premium for new technology that has regulatory approval, strong intellectual property protection, and growing markets. The billion-dollar-plus M&A deals of 2024 and 2025 meet these criteria.
Many analysts are predicting a medtech M&A boom in the second half of 2025. Larger medtech companies continue to acquire smaller companies with innovative technologies, both to grow revenue and to streamline product development. Robotic surgery remains a hot space as well as interventional cardiology, orthopedics and pain management.
To position themselves as attractive targets, medtech companies should de-risk their commercial, regulatory and intellectual property strategies. Buyers
are conducting deeper due diligence, and companies that understand their competitive landscape and highlight potential synergies will be best positioned to attract premium valuations.”
What are the biggest IP pitfalls that could hurt a company when it’s time to exit?
“One of the most damaging IP pitfalls is failing to secure IP protection for the company’s technology. Most startups file one or more patent applications early, but then do not dedicate the necessary resources to protect the changes in their products, including filing additional patent applications covering incremental improvements. Public disclosure of an invention before filing a patent application — whether through a publication, presentation, website, or conversation with a customer or investor — can jeopardize patent rights, especially outside the U.S., where absolute novelty is required. Companies must be vigilant about confidentiality to preserve their IP value.
Another major risk is trade secret misappropriation. The financial losses from IP theft are staggering in medtech, often hundreds of billions of dollars annually.
A company that fails to protect its trade secrets could face enormous legal costs and lose its competitive edge, major red flags for potential acquirers or investors.”
What sort of protection should startups have when talking with potential buyers?
“Startups should never disclose confidential or proprietary information without a signed nondisclosure agreement (NDA). Even after execution of an NDA, startups should avoid revealing trade secrets or critical technical details until later in the due diligence process. Protecting IP through patent filings before any discussions also ensures that the company retains its rights regardless of how talks progress. Thus, it is important to update the patent portfolio before starting the due diligence process.
How much access to give a potential buyer in the ‘kicking-the-tires’ phase often depends on how much leverage the buyer has, but generally startups should start by revealing public information such as published articles, patents and patent applications and reveal additional information as talks progress and the buyer becomes more serious.”


Visit wtwh.me/IPtips to read more from David Dykeman and Roman Fayerberg.
Carefully controlled manufacturing practices are required to form unvulcanized silicone into sheeting without contamination or flaw. Machines do heavy lifting, but the final stage of calendering is all about the human touch, with technicians teaming up to make sure each sheet is perfect before it moves on.
How silicone is meeting healthcare’s toughest device challenges
Breaking down why medical-grade silicone is at the heart of CGMs and other healthcare innovations.



By Jay Rutherford Lubrizol
Jay Rutherford is a new product development engineer at Lubrizol’s specialty silicone manufacturing plant in Montana. He has over 12 years of experience in R&D and is responsible for developing quality manufacturing of silicone medical devices and components.
Precise, high-quality silicone is increasingly essential for devices like catheters, surgical tools, prosthetics, long-term implants and wearable devices like continuous glucose monitors.
Biocompatible and breathable, medical-grade silicone is ideal for sealing, bonding and creating barriers of elastomeric components. Indeed, it can be a versatile, effective tool in a medical designer’s tool kit. But medical-grade silicone must meet the strictest purity standards to be trusted for life-critical devices.
Let’s explore some of the standards that high-quality silicone should meet, how its properties can provide advantages in some critical applications, and how medical device engineers can apply the material to meet today’s demands.
Getting to know silicone
The raw materials for silicone medical devices are produced by a number of suppliers. NuSil, DuPont, Wacker and Elkem are just a few examples of leading
raw material providers, many of which have FDA master files and/or ISO 10993 testing to support their use in medical device applications.
From these raw materials, a huge variety of medical devices can be produced, many of which make use of silicone film and sheeting. Medical-grade sheeting from Lubrizol is manufactured per ISO 13485 (QMS requirements) in ISO Class 7 cleanrooms. The resulting material can be cut and shaped to fit just about any need. Precision cutting is handled by die cutting and laser cutting. There are also multilayer options having (typically) two or three silicone layers that add another dimension of versatility.
Device developers also have plenty of options when it comes to thicknesses and configurations. With calendering, you get thicknesses from 0.005 to 0.125 in., while extrusion and knife coating can create ultrathin films as slim as 0.0005 in. The material is available in durometers from 1 to 80 Shore A, meaning it can be as soft as
Photo courtesy of Lubrizol

chewing gum or as firm as the sole of a dress shoe, depending on the requirement. It can also be reinforced with mesh for extra strength or foamed for extra softness. Add to that a choice of matte or glossy finishes, as well as a variety of colors, and engineers have a highly customizable material to deploy depending on their specific needs.
Silicone also has unique functional properties that make it a go-to material for improving patient outcomes, especially in challenging applications.
Silicone films particularly shine in wound care because they are both gas-permeable and liquid-proof, which protects against bacteria while allowing breathability. Silicone gel sheets used for scar healing, for example, are often worn for a week or more and keep the skin hydrated while allowing water vapor to escape to prevent problems like overly soft skin. And because it is chemically inert, medical-grade silicone won’t cause irritation or allergies.
Silicone is frequently used as a primary wound dressing placed in direct contact with wounds. The perforated silicone film dressing stays in place for extended periods of time, so secondary wound dressings — used to absorb wound exudate — can be changed without disturbing the delicate healing skin. (Mepitel One manufactured by Mölnlycke Health Care, and Cuticell Contact manufactured by Essity are two examples of this type of product.) Plus, the transparency of the film means you can inspect the wound without removing the dressing, a huge benefit for undisturbed healing and cutting down on the number of changes.
How silicone is overcoming design challenges in critical applications
Reinforced sheeting takes silicone to the next level with embedded materials like polyester or nylon mesh. Depending on the application, meshes of varying thickness and aperture size (open area percentage) can be incorporated. Reinforced sheeting is ideal for devices and components that need to stay flexible but not stretch too far or tear.
A common use of reinforced sheeting is at suture locations. Without reinforcement, sutures will cut through silicone, causing it to

These fine-tipped forceps are gently lifting an ultra-thin medicalgrade silicone film — possibly as slim as 0.0005 in. — demonstrating the delicate handling required for this thin and flexible material in both manufacturing and surgical settings.
Photo courtesy of Lubrizol
tear. Proper reinforcement makes suture sites stronger than the sutures themselves. Tissue expanders used in breast reconstruction or skin repair, for example, use suture tabs made from reinforced sheeting to ensure they remain in place.
Devices that are implanted in the brain, such as cerebral shunts and electrodes, can also rely on reinforced sheeting to handle physical stresses while being safe for nearby tissues. The
silicone encapsulation prevents tissue from growing into the mesh and minimizes trauma to the surrounding tissue if the device moves or must be removed.
The bottom line is that silicone is a trusted material for OEMs because it’s tough when needed but soft enough for delicate uses.
And its versatility gets even better when you bring vulcanized and unvulcanized silicone together. In part two of this series, we explore how this duo works in harmony to create innovative, practical solutions for device assembly. We’ll also dive deeper into how customizations like pigments, radiopaque additives and medicalgrade inks are helping meet other safety and functionality needs for devices that rely on high-quality silicones.
Go to wtwh.me/ LubrizolPart2 to read part two of this series.





First Look: Boston Scientific’s next-gen Faraflex PFA and mapping catheter
Boston Scientific’s Faraflex pulsed field ablation (PFA) and mapping catheter has 19 sensing electrodes on the selfexpanding, hexaspline nitinol structure.
Image courtesy of Boston Scientific


oston Scientific recently announced the first-in-human procedures using its nextgeneration Faraflex pulsed field ablation (PFA) and mapping catheter.
The investigational catheter was built specifically for PFA and mapping, said Dr. Brad Sutton, the chief medical officer of Boston Scientific’s Atrial Solutions Business, which includes PFA systems for treating atrial fibrillation (AFib).
In an interview with Medical Design , Sutton described Faraflex as “a pretty nice blend” of Boston Scientific’s high-density mapping catheter technology — he noted the Intellamap Orion mapping catheter in particular — with “what we think the market needs and patients need moving forward in the PFA world.”
At 8 Fr, Faraflex is smaller than Boston Scientific’s 12 Fr Farawave PFA catheter, which the FDA approved with the Farapulse PFA system for treating AFib in January 2024, shortly after Medtronic won the first approval for PFA.
Farawave is a single-shot catheter, while Faraflex is a large focal catheter. Boston Scientific said Faraflex is “designed to optimize mapping and ablation in a single catheter with a wide-area tip and high-fidelity sensing electrodes.”
Faraflex is a navigation-enabled catheter and “will be integrated into the
OPAL HDx Mapping System enabling precise diagnostic mapping to understand arrhythmia mechanism and target ablations accordingly,” the company said. (Boston Scientific added magnetic navigation capabilities to its Farawave catheter and won FDA approval for that upgrade in October 2024).
Sutton described the expandable catheter as a flexible lattice with a pre-specified, nonadjustable shape, 19 sensing electrodes and a next-gen handling system.
“We expect it to be atraumatic and easy to navigate to all the nooks and crannies you might expect in the chambers of the heart,” he said. “There was a prototype, sort of first-generation Faraflex that the Farapulse company had developed. They toyed around with it a little bit for atrial flutter on the right side of the heart. It was a catheter that really wasn’t commercially viable, so this is using the same concept but a much more sophisticated design.”
Pulmonary vein isolation and posterior wall isolation can handle most de novo paroxysmal and persistent AFib cases.
“That’s a huge chunk of the market,” Sutton said. “It’s a very easy workflow. It’s not the most intellectually sophisticated, stimulating thing to do because it’s all anatomical, and
electrophysiologists love to do more complex ablations. Frankly, there’s a clinical need to do more complex ablations, and single-shot tools aren’t the best set of tools for that.”

“The unmet need [for] Faraflex is what if you have a redo patient with a complex atrial arrhythmia and you really need to understand both the electrical substrate, potentially the mechanism of the arrhythmia, where is it coming from, what is the electrical circuit — how do I tackle it?” Sutton continued.
“And anything outside the atrium — the ventricle is its own complex animal, and [with] ventricular tachycardia ablation, we’re very much on the front end of that journey with PFA, but it’s really promising,” he said. “What Faraflex represents is a bit more precision and a much higher fidelity mapping capability to understand the heart tissue itself, how much is scar, what is healthy,
Asahi_2023-MDO_printer.pdf 1 7/12/2023 3:39:01 PM
and what is the electrical framework and structure and functionality of that chamber of the heart.”
Dr. Ante Anic performed the first-inhuman procedures in Croatia, observed by Cleveland Clinic Drs. Oussama Wazni and Walid Saliba.
Those first cases “went as well as we could have possibly hoped for,” Sutton said, characterizing their feedback on the catheter as an “easy-to-use, utilitarian sort of tool for anything complex [that] stacks up very favorably to anything they’ve used from the competition.”
Sutton said he hopes to launch an investigational device exemption (IDE) trial in the second half of 2025 or early 2026.
“Right now, we’re having conversations around the IDE design, leadership of the global study, what that looks like, and looking to understand exactly what the regulatory path is going to be,” he said.

Boston Scientific Atrial Solutions Chief Medical Officer Dr. Brad Sutton
Best in Class, High Performing Components for Your Medical Devices








CDMO / CMO Services

Minimally Invasive Device Solutions
Co-development Opportunities
Access, Delivery, & Retrieval Systems
Wire & Catheter Based Devices
Contract Manufacturing
Vascular Access Devices
Guidewires, Therapeutic & Diagnostic


Braided & Coiled Catheter Shafts
Class 8 Clean Room


Precision Gears and Superior Mechanical Components
Our Certifications
ISO 9001 + AS9100
ITAR Compliant-DDTC Registered
DFARS Compliant
RoHS & R.E.A.C.H. Compliant
NIST SP 800-171 Compliant
Turning Ideas into Reality since 1950
Engineering development, design, and manufacturing expertise combined with mechanical and electromechanical component options.
Streamline Your Supply Chain
We deliver parts and subassemblies complete, eliminating your need for additional sourcing, time, and cost.
Customized Solutions
Custom Gears & Mechanical Components
Custom Gearheads & Gearboxes
Complex Gear Assemblies
Mechanical Assembly
Electromechanical Assembly





Robotic Arms & Endowrists
Six sustainable design lessons from the development of a reusable autoinjector
Jabil experts offer design for sustainability advice to reduce the environmental footprint of drug delivery devices and other high-volume medtech products.
Sustainability plays a critical role during the early design phase of a medical device. About 80% of a product’s environmental impact is determined in its design phase.


By Oliver Eden Jabil
If sustainability is a key requirement for the product, designers should leverage design for sustainability (DFS) principles to deliver a solution that will account for the reduction of resources used and maximize conservation across the manufacturing value chain while also lowering the total cost of goods over the lifecycle of the product.
This experience was leveraged to maximize the sustainability of the Qfinity autoinjector, giving it an 80% lower carbon footprint than market-leading mechanical disposable autoinjectors.
Here are six lessons from the Qfinity autoinjector’s design and development that medtech engineers can use to develop more sustainable devices.
1. Clearly understand the device’s intended use, function and lifecycle. The type of device you are building will impact all of the sustainability considerations we’ll discuss below. Engagement with patients and healthcare professionals during the design process is very important.
Understanding their needs and behaviors helped guide the Qfinity autoinjector’s design to deliver medication as simply as possible, improving patient adherence and compliance while reducing the risk of errors and misuse scenarios, which maximizes the device’s sustainability.
Devices also need to be appropriately designed to be robust and reliable for their expected lifecycle. Engineering safety factors need to
be sufficiently robust to meet the requirements but not over-engineered, which can add cost and complexity when not needed.
For the Qfinity reusable autoinjector, we leveraged cross-industry experience designing products for sustainability and focused on getting the product requirements document (PRD) correctly defined. As a device moves through the design process, these disciplines must be maintained or you run the risk of reducing the product’s overall sustainability performance.
2. Optimize your material selection. Optimized material selection is one of the most crucial elements of sustainable design. At Jabil, we’ve developed a database to compare various materials for medical devices on a range of criteria for sustainability, including the recycling method, environmental impact of the material’s production, carbon footprint of the material’s supply chain, the supplier’s sustainability credentials, and the material’s expected outcome post-use.
It’s important to consider the environmental impact of the materials used, especially plastic resins and other common medtech materials. The use of recycled plastics is a potential frontier, but there are challenges due to scale (with reliable sources of recycled materials lacking) and the risks of contamination and material traceability. However, another option is a biobased resin that is not sourced from fossil fuels. For example, Celanese has developed Hostaform MT POM ECO-B, a sustainable medical grade material which is almost identical to their existing POM product, but is made up of around 97% bio-content. >>
Jabil
This biogas-derived material has less than 50% of the carbon footprint of the original material produced from fossil fuels but meets the same performance criteria.
3. Modularity matters. When possible, device design should leverage interchangeable parts and allow for easy repair or replacement without discarding the device.
The Qfinity autoinjector’s platform design covers both 1 mL and 2.25 mL, connected and mechanical, with mechanisms that have 80% common parts, assemblies, and manufacturing processes. This modular platform approach allows the manufacturer to industrialize a single manufacturing cell to serve all variants, reducing the manufacturing footprint and energy consumption.
4. Get circular.
Reusability was a critical area of focus for our Qfinity autoinjector design team. The European Federation of Pharmaceutical Industries and Associations (EFPIA) released a whitepaper calling on the pharma industry to increase adoption of circular economy principles to better preserve resources and maximize product lifecycles. A reusable autoinjector is a compelling solution for the opportunities described within EFPIA’s analysis and bears out advantages that single-use injector products just cannot match.

5. Consider sustainable manufacturing methods during design. Energy use during production adds up for high-volume devices like autoinjectors. Injection molding is highly energy efficient when producing large quantities of identical parts. Meanwhile, engineered thermoplastics ensure durability and compatibility with medical standards but also support recycling potential, further enhancing sustainability.
6. Stay sharp on industry regulations. Designers need to ensure sustainability initiatives align with regulations and standards such as the Waste Electrical and Electronic Equipment (WEEE), Restriction on Hazardous Substances (RoHS), Registration, Evaluation, and Authorization of Chemicals (REACH), and the Energy Using Products (EuP), all of which have already positively impacted the sustainability of medical devices containing electronics.
Designers must also stay aware of national and international laws — like the European Union’s Corporate Sustainability Reporting Directive (CSRD) — that may have a trickledown effect on their design choices.
There are now programs to reduce medical device waste by returning used devices for recycling, repurposing, refurbishing and reuse. Jabil operates three medical device reprocessing plants in Maple Grove, Minnesota, that process and repackage thousands of medical devices every year.
But these programs present additional regulatory hurdles, such as establishing a safe means of returning the devices with a low burden on the user and developing infrastructure to introduce the returned devices safely and efficiently into the circular economy. Designers should consider how or if medical reprocessing will play a role in their design for sustainability and understand the associated regulatory challenges.
Conor Mulcahy is the senior director of research and development in Jabil‘s healthcare division, responsible for the development of strategic capabilities to support pharmaceutical solutions, and was the Qfinity autoinjector development engineer responsible for the technical development of the solution to meet the product requirements. Mulcahy holds a master’s degree in engineering design from University College Dublin and a bachelor’s degree in mechanical engineering from Ulster University.
Oliver Eden is a senior business unit director for Jabil‘s healthcare division, focused on pharmaceutical solutions, and was the product manager responsible for strategy to deliver the Qfinity autoinjector and meet customer and market requirements. Eden holds a Ph.D. in biomaterials engineering and master’s degree in mechanical engineering, both from the University of Exeter.

THE PERFECT PARTNERSHIP
The experts in design, development, and large-scale manufacturing of interventional catheter-based devices and implants.





PARTNERING WITH YOU EVERY STEP OF THE WAY
Learn more about our offerings at www.confluentmedical.com or email sales@confluentmedical.com for a custom quote. NITINOL
TUBING
COMPONENTS BALLON CATHETERS BIOMEDICAL TEXTILES

Shockwave Medical designed the Javelin peripheral intravascular lithotripsy (IVL) catheter to break up calcium in blood vessels through which previous IVL devices could not pass.
Illustration courtesy of Johnson & Johnson

Sean Whooley Associate Editor

How Shockwave’s Javelin IVL catheter breaks up blood-blocking calcium where balloons can’t reach
Shockwave’s novel Javelin intravascular lithotripsy catheter focuses acoustic energy forward to clear a path through calcium-clogged blood vessels.
Johnson & Johnson MedTech’s
Shockwave Medical aims to deliver intravascular lithotripsy (IVL) to a different type of blood vessel with a first-of-its-kind catheter platform.
The Javelin Peripheral IVL catheter features new technology for modifying calcium and crossing extremely narrowed vessels in patients with peripheral artery disease (PAD).

Shockwave says the novel, nonballoon IVL platform delivers a similar safety and efficacy profile as its legacy catheters, bolstering the device developer’s market-leading IVL portfolio.
“We believe this technology has the ability to transform the way that physicians are able to treat patients with peripheral vascular disease,” Shockwave Chief Medical Officer Dr. Nick West said in a Medical Design & Outsourcing interview.
“We hope that we’re answering a clear unmet need not just from physicians, but from the patients they also serve.”
Shockwave’s IVL technology
Shockwave’s IVL technology uses acoustic energy to treat calcified narrowings in blood vessels. It’s an adaptation of extracorporeal shockwave lithotripsy used to treat kidney stones, but miniaturized and tuned for use inside blood vessels.
Shockwave IVL sparks an electrical current between two electrodes, traditionally encased in a balloon placed in the blood vessel. The spark enables the formation of a vapor bubble in the mixture of contrast and saline within the balloon. As the bubble collapses, it emits an acoustic pressure delivered to the tissues.
The Javelin system doesn’t have a balloon, however. It has a single emitter behind the tip of the catheter, designed to create acoustic pressure waves in the same way the balloonbased catheter does, according to West. That 120-pulse lithotripsy emitter helps enable use in sub-total occlusions or extremely narrowed
MedTech
Shockwave Medical Chief Medical Officer
Dr. Nick West
vessels through which a wire can cross but devices can not.
With this new platform, the sonic pressure waves expand spherically from the forward-shifted emitter beyond the tip of the catheter, which has a working length of 150 cm. This approach allows modification of the obstructive calcification that facilitates device crossing.
What difference does Javelin make? In track and field, the javelin event can sometimes be viewed “as a kind of brute force event,” West said, but javelin throwers often aren’t “the biggest and most muscular guys.”
Similarly, Shockwave’s Javelin is “not all about brute force to get this through the lesion.”
“It’s partly about the technique,” he said, “but also we hope that it encapsulates that ability to cross those
very, very difficult lesions and also modify them on the way.”
In an FDA investigational device exemption (IDE) study, Javelin’s results “closely mimic those of our balloon-based platforms, showing excellent safety and efficacy in modifying calcium and allowing the crossing of these lesions,” West said.
The big difference without a balloon is that the catheter is more deliverable, he said. And physicians are already familiar with microcatheters and catheterbased technologies for treating complex lesions, so Javelin’s design is immediately familiar to them. Additionally, the Javelin emitter is similar to those in the Shockwave balloon platform.
Effectively, the system can deliver lithotripsy capable of breaking up calcium a physician couldn’t reach with the balloon-based system.
“We transitioned that emitter
technology from the balloon-based platform to this new forward IVL platform, but we’ve replicated the same efficacy and safety as that prior parent platform,” West said.
While estimates vary, West said as many as 12 million people in the U.S. have peripheral vascular disease, with many more cases globally, leaving many at risk of losing limbs and possibly their lives through heart attack or stroke. The most severe form, chronic limb-threatening ischemia (CLTI), brings even more risk.
“There is a real need to be able to treat these patients,” West says. “Endovascular treatments are not the only option available to these patients. But, should an endovascular approach be used, given the prevalence of calcification, we think this tool could really be a difference-maker in those patients that are very difficult to treat.”
Innovative Interconnect solutions from BAYCABLE can transform the way our customers deliver care. Turn design challenges into next-generation, market-leading medical devices with our extensive manufacturing capabilities and engineering expertise.
MEDICAL MOLDED CABLE ASSEMBLIES
Industry expertise:
• Robotic surgery system cable assemblies
• Video cable assemblies
Turn your design challenges into next-generation, marketleading medical devices with our extensive manufacturing capabilities and engineering expertise. We have facilities in Fremont, CA and Santa Ana Sonora Mexico.
• Patient monitoring cable assemblies
• Single use cable assemblies
• Sensor probe cable assemblies

Highlights:
• ISO 13485:2016 and ISO 9001:2015 certification in our Fremont, CA and Mexico facilities
• Highly trained technicians and trainers certified to IPC/WHMA-A-620D
• Custom molded cable assemblies and strain reliefs
• 100% final product electrical testing
• Fine wire termination capabilities
• Laser stripping, laser marking, and ultrasonic welding capabilities


Nebulizers can create drug particles of varying sizes to target different parts of the respiratory tract, offering potential for next-generation vaccines and other inhalable drugs. Illustration for Medical Design & Outsourcing by Artery Studios (arterystudios.com/healthcarevisuals)



N“WE ARE JUST AT THE BEGINNING OF A VERY EXCITING ERA,” SAYS AEROGEN DIRECTOR OF R&D, SCIENCE AND EMERGING TECHNOLOGIES RONAN MACLOUGHLIN.
ot too long from now, you might be able to skip the pharmacy or your doctor’s office for certain vaccinations and instead visit an automated dispensing station for immediate self-administration.
Instead of sticking yourself with a syringe, it’d be as easy as sipping from a cup. It might be less expensive than an intramuscular injection and even offer you better protection with fewer side effects.
“We’ve tried to make it as easy as possible in the sense that our vaccination station has a user interface and it gives you on-screen instructions,” Aerogen Director of R&D, Science and Emerging Technologies Ronan MacLoughlin said in an interview. “… Like you’re filling a cup at McDonald’s, it fills up with air, you take that, you exhale, you inhale one long, slow, deep breath, hold for four seconds and you’re done.”
“The only limit is the potential for cross contamination between patients,” he
continued. “Through our fixturing and our vaccination station — for which we got our patents granted [in March], actually — we removed that risk.”
Aerogen is the nebulizer manufacturer that developed the world’s first approved inhalable COVID-19 vaccine with biopharmaceutical company CanSinoBIO, which has been used on more than 30 million patients since 2022. Delivering that vaccine via inhalation needs just one-fifth of the dose used for an intramuscular injection, which means pharmaceutical manufacturers can inoculate five times as many people.
“It’s going to open the floodgates for us, because we know that inhaled vaccines work,” MacLoughlin said.
There’s much left to learn about inhalable vaccines, but MacLoughlin said his team has invested years and more than $150 million to get a head start on the devices that will deliver these next-gen drugs. >>
BY JIM HAMMERAND MANAGING EDITOR
KEYS TO NEXT-GEN INHALABLE VACCINE DEVICE DESIGN FROM AEROGEN’S R&D DIRECTOR
“Given the school of hard knocks and the lessons we learned in our trials that didn’t do particularly well, as well as all the lessons that we’ve taken from device development at scale, drugdevice-combination development and learning everything there is to know about aerosols and aerosol generation — we have 600 patents, and we have hundreds of clinical papers and bench studies that underpin our technology — we can now apply that to the vaccine space,” he said. “We’re unparalleled in that regard.”
Designing drug-delivery devices for next-gen vaccines
Inhalable vaccines have been studied for more than half a century, with research showing the potential for more efficient and effective inoculation. Until recently it’s just been easier and less expensive to administer vaccines orally or with an injection.
possible. Instead of developing a less expensive single-use nebulizer to deliver the new vaccine, Aerogen built a system to reuse the same nebulizer for hundreds or thousands of patients by dispensing the inhalable vaccine into a disposable plastic cup with a custom-designed lid.
“The reason we went with a cup is because it was globally available. We didn’t need to make that and we don’t want to make that. … We will sell you our delivery system, but the cups — here’s the instructions. Make them yourself,” MacLoughlin said. “… It’s not just any old sippy cup. There’s a lot of engineering, a lot of IP around this. We did a huge amount of work and generated a lot of data very quickly. I could make subtle changes to it and make it a terrible device.”
The smaller dose required for inhalable vaccines is another factor
But fear of needles is estimated to cause a significant share of people to avoid intramuscular injections, and in the early days of the COVID-19 pandemic, manufacturing capacity and cold chain distribution limited the supply of vaccines approved in the U.S.
Taking the inhalable route, CanSinoBIO asked Aerogen for billions of its nebulizers at a price that wasn’t
vaccine companies working on inhaled clinical studies and development and moving toward inhaled vaccine studies, and they can now spend money on doing the necessary validations to get to that first-in-human as opposed to trying to make loads of it beforehand.”
Vibrating mesh nebulizers can nebulize and fully deliver doses as small as 5 microliters with specific droplet sizes for diseases where droplet size matters.
“Influenza binds to sialic acid receptors — α2-6, α23, and α2-8 receptors — in your airways: α2-6 is primarly
“Diabetes, cancer, the number of diseases that are vaccine-preventable or -treatable is ginormous. The inhaled route may not be suitable for them all. However, they all have potential.”
in your upper airways and some in the alveolar region, and α2-3 is really only in the lower airways, the alveolar region. So if you have an influenza that preferentially binds to α2-3, there’s no point in delivering to the upper airways. You have to get it into lower areas to bind and elicit that immune response,” he said. “That’s if it’s receptor-bound. For something like tuberculosis, you’re looking to see T cell response and need to get it to where those T cells are. Depending on a huge number of different factors, that could be upper, lower or just everywhere.”
that lowers vaccination costs, not only for R&D but for scaled manufacturing and distribution.
“When you’re getting five times as much out of a single facility because you’re getting that protection when you inhale it at one-fifth the dose, that opens up a whole new route,” MacLoughlin said. “I’m heavily involved now with a number of
There are pediatric design considerations as well. Researchers are studying the best route for RSV vaccinations in babies, which primarily breathe through their nose.
“No matter what droplet size you put in there, their nose is going to filter out the bigger droplets, and all that’s going to get into their lungs is the small droplets,” MacLoughlin said. “So if you can start small, you bypass the losses in the upper airways and get down into the lungs.”
(continued on page 32)
McMaster University researchers are testing a new inhalable COVID-19 vaccine delivered with Aerogen nebulizer technology.
Image courtesy of McMaster University
The Aerogen Solo nebulizer features palladium vibrating mesh technology with 1,000 precisionformed holes in the 5 mm central aperature plate. This mesh vibrates at 128,000 times per second to create a fine mist of uniformly sized droplets between 1 and 5 microns in diameter, which is ideal for deep lung penetration. Image courtesy of Aerogen

(continued from page 30)
Inhalable vaccines have potential applications beyond respiratory diseases. Vaccines inhaled and delivered to respiratory mucus can confer immunity to mucosal surfaces elsewhere in the body, like the GI tract.
“Diabetes, cancer, the number of diseases that are vaccine-preventable or -treatable is ginormous,” MacLoughlin said. “The inhaled route may not be suitable for them all. However, they all have potential. And the beauty of it is you can get away with a very small dose. … We know that you can get infected with influenza with as few as 10 individual viral particles being inhaled. So in theory, you could get away with as few as 10 vaccine particles to give you that protection.”
“We are just at the beginning of a very exciting era,” he continued. “We now know what can be done, we know it can be done at scale, we know the technology exists where if we come up with this wonder vaccine it can actually be delivered.”
‘Collaborate or die’ MacLoughlin recounted helping a team working on a pneumococcal vaccine after their trials told them an inhalable drug wouldn’t work — or so they thought. He asked what device they used and if they had one handy, and when he turned it on he almost immediately felt the heat it generated.
easily assembled out of low-cost materials like cardboard, or automation that minimizes the risk of a doctor or patient incorrectly administering the vaccine.
“You need to maintain flexibility because not everywhere is in the First World, not everywhere has power, not everybody can read,” he said. “Your usability, your user and human factors come into it in a huge way [to ensure] your product is ultimately going to be used.”
Five years after the COVID-19 pandemic started, MacLoughlin recalls device development insights from unusual places, like a wind turbine manufacturer that wanted to manufacture a basic ventilator when they were in short supply.
“Those guys had a completely different view on things and some of the assumptions and thinking that they brought to their project, we were able to
“You don’t need to be a genius, you don’t need to be any more than 5 years old. You just need to be able to sit down and logically think through something and be willing to accept feedback. Collaborate or die.”
“What they’d used in the clinical trial cooked the vaccine. … There’s so many learnings that that vaccine developers haven’t had to think about. I think now we’re having these conversations more and more that we’ll certainly be in a position where we’ll see a lot more momentum in the next couple of years. … Maybe you’re looking for disease mitigation or injury mitigation or maybe just looking for transmission reduction. There’s so many levers that we can pull, and we’re still trying to figure out what the levers do.”
More than ever, device and drug developers will need to consider human factors. MacLoughlin pointed to Mexico’s inhalable measles vaccine campaign in the 1980s.
“It was a thundering success, an amazing success,” he said. “All these kids got full protection. The device, however, was not fit for purpose [because] this particular device needed an air compressor that was really only ever found at car mechanics. … What you’re looking for is reliable, reproducible delivery of whatever it is in the real-world setting.”
That might mean systems powered by solar or hand cranks for areas without reliable electricity, equipment that can be
apply to other projects,” he said.
Even his own son, Eoin, contributed to inhalable vaccine research when he was just 5 years old by designing a simple mask for testing inhalable drugs on pigs.
“Pig models are very, very good for humans because we share a very similar immune response and airway geometries — way better than mice and ferrets and rats and golden hamsters and all that, which are valuable but not as good,” MacLoughlin said. “Eoin designed a face mask that’s been used in hundreds of trials now for pigs, teasing out the understanding of how inhaled vaccines work or don’t work.”
“The lesson from that is you don’t need to be a genius, you don’t need to be any more than 5 years old,” he continued. “You just need to be able to sit down and logically think through something and be willing to accept feedback. Collaborate or die.”
THEIR SIGHTS TO DEVELOPERS TURN DIABETES DEVICE

The Insulet Omnipod 5 was the first automated insulin delivery (AID) system cleared by the FDA for type 2 diabetes patients.
BY SEAN WHOOLEY ASSOCIATE EDITOR
ABBOTT, DEXCOM, INSULET, MEDTRONIC AND MORE ARE DEVELOPING NEXT-GEN SENSORS, PUMPS AND ALGORITHMS TO OFFER NEW OPTIONS FOR HUNDREDS OF MILLIONS OF DIABETES PATIENTS.
More than 90% of people with diabetes have type 2 diabetes, but most of the diabetes technology on the market is for type 1 patients.
That’s something diabetes device developers are hoping to change as they adapt miniaturized, automated type 1 technology for the much larger type 2 patient population. The International Diabetes Federation projects that approximately 783 million people will be living with diabetes by 2045.
“The people who need insulin therapy in type 2 also deserve to have a much easier, reduced burden,” former Insulet CEO Jim
Hollingshead said in an interview shortly before his company’s Omnipod 5 became the first automated insulin delivery (AID) system cleared by the FDA for type 2 last year.
More AID systems are in development, while continuous glucose monitor (CGM) makers are also bringing more options to type 2 diabetes patients.
“We have much work to do as an industry to really bring to the type 2 population the technologies and brands that are pretty well covered and supplied into that smaller type 1 population,” Abbott EVP of Diabetes Care Chris Scoggins said as his company pushed forward with type 2 tech last year. >>
Photo courtesy of Insulet





What is different about developing tech for the type 2 population?
Diabetes device companies had already developed much of the technology needed for the type 2 population. In fact, many type 2 patients were using type 1 systems off label before regulatory authorization.
Insulin pumps, for example, don’t need hardware changes to expand to type 2, but the algorithms that ensure the safe and effective delivery of insulin need adjustments. Insulin can cause serious complications if under- or over-delivered.
Some type 2 patients don’t even need insulin, unlike type 1, which always requires dosing to make up for a patient’s inability to naturally produce enough insulin. Type 2 patients may need more insulin over time as the chronic disease progresses.
Tandem Diabetes Care President and CEO John Sheridan said it’s “a natural evolution” to bring AID systems beyond type 1 and into the type 2 population. His company’s Control-IQ+ AID algorithm won the second FDA clearance for type 2 patients in February.
Insulet and Tandem both recruited more than 300 subjects for clinical trials to win their type 2 clearances.
Insulet also targeted health equity in its trial, Hollingshead said, with the type 2 population often featuring significant ethnic and socioeconomic diversity. The system, which needed to
keep A1c in range and avoid an increase in hypoglycemia in the study, delivered “very efficacious, very safe” results with no adverse effects and “terrific clinical benefit,” including reduction of diabetes distress, Hollingshead said.
Medtronic plans to follow Insulet and Tandem with an FDA submission for type 2 this year.
Beta Bionics, which developed the iLet bionic pancreas, has FDA clearance for type 1 diabetes. Expanding a type 1 diabetes system’s indication to type 2 is “exceedingly important [but] tough,” co-founder Ed Damiano said on a panel in March.
Beta Bionics has a couple thousand users managing their type 2 diabetes with the iLet off-label, Damiano said, and his company may seek an indication for that population.
Another notable AID developer that hasn’t yet moved into type 2 is Sequel Med Tech. The company is beginning commercial efforts for its Twiist system after integrating the pump with Abbott CGMs. While Twiist’s current indication covers type 1, Sequel CEO and cofounder Alan Lotvin said the company is kicking off a clinical trial for type 2.
The window may be closing for potential AID competitors.
Embecta received FDA clearance for a disposable, open-loop type 2 patch pump last year, but killed the product within months instead of
Kevin Sayer
President and CEO Jim Hollingshead
The T:slim X2 insulin pump is Tandem Diabetes Care’s flagship AID system.
Photo courtesy of Tandem Diabetes Care
pushing toward its goal of launching an automated system for type 2.
“The pump market has continued to evolve, and we anticipate that competition in closed-loop type 2 indicated products may continue to intensify and our offering would require incremental investments to be market competitive,” Embecta CEO Dev Kurdikar explained at the time. “… Upon FDA clearance of our open-loop pump … we performed a market check to identify potential opportunities that would allow us to monetize the asset. Since that did not surface any viable options, we acted promptly to discontinue the program.”
Meanwhile, Modular Medical is commercializing its MODD1 product, a 90-day patch pump that features new microfluidics technology for low-cost pumping of insulin for type 1 and type 2. Users can monitor the pump activity with their cell phone and do not need an external controller. The pump uses a
single-use, disposable battery.
Modular Medical’s aim is to bring a reliable, smaller form factor at a low cost to serve those “neglected by the industry,” CEO Jeb Besser said in an interview.
“Our target is not the ultra-sophisticated, superhigh-functioning user,” he said. “There are lots of products on the market today that do a good job of serving that user.”
“The pump market has continued to evolve, and we anticipate that competition in closed-loop type 2 indicated products may continue to intensify.”
Type 2 tech beyond AID systems
The developers of CGMs that integrate with those insulin pump technologies also have their eyes on the type 2 opportunity. Dexcom and Abbott CGMs have been available for the type 2 population for years. They have now launched over-the-counter (OTC) CGMs for type 2 patients who don’t use insulin.
Dexcom won the first such FDA clearance with its Stelo glucose biosensor. The company estimates approximately 25 million type 2 patients in the U.S. don’t use insulin but could benefit from CGM use. Someone who currently has insulin dependence has to worry about controlling glucose in the present, Dexcom Chair and CEO Kevin Sayer said after winning that clearance, while a person who doesn’t use insulin might not have complications today, but could face blindness, heart attacks, kidney failure and amputations in the future. >>








































































Those patients “need a better tool now,” he said.
Abbott soon followed with FDA over-the-counter clearances for its Lingo biosensor and Libre Rio sensor, the latter of which is indicated for type 2 patients who aren’t on insulin and manage diabetes through lifestyle modifications.
Dexcom EVP and Chief Operating Officer Jake Leach said in an interview that his company is taking a twopronged approach toward type 2 diabetes: make products easier to use, and generate clinical evidence to support the expansion.
“You have to generate that evidence to show the benefits that CGM can provide,” he said. “There were questions in the beginning, like will someone with type 2 diabetes benefit from CGM like those with type 1? We were very confident they would, but we had to generate the evidence and that evidence generation continues. … The addressable market is very large. It’s our job to figure out how to get people the CGMs that they need.”
What to watch for in the type 2 market Insulet and Tandem have launched their type 2 AIDs, and Medtronic’s MiniMed 780G submission is expected to reach the FDA soon. Medtronic and Tandem are also developing patch pumps.
Senseonics has developed the long-term implantable Eversense E3 CGM for both type 1 and type 2 diabetes. The implant offers 365 days of continuous glucose monitoring and has an integrated CGM (iCGM) indication, meaning it can pair with AID systems in the future.
Dexcom is working on a next-gen CGM that will be 50% smaller, packing more powerful electronics into a thinner, lower-circumference form factor.
“The more powerful electronics means it can handle three different readings at the same time,” Sayer said, noting the device was in feasibility studies when we interviewed him in January. “It’ll be a while — like a couple years — before that is through all of our work process.”
And at Insulet, Hollingshead said the team is working on improved algorithms for more automation and enhanced form factors.
“People living with diabetes deserve to have better and simpler care,” he said. “That’s where we’re going.”
Beta Bionics received the first FDA clearance for its fully automated iLet bionic pancreas in 2023. Photo courtesy of Beta Bionics
A Medtronic engineer shares lessons from the adaptive
stimulation project deep brain
THE BIGGEST TECHNICAL CHALLENGE FOR ADBS WAS HOW SMALL BRAIN SIGNALS ARE.
Seventeen years before Medtronic’s recent FDA approval for the world’s first adaptive deep brain stimulation (DBS) system for Parkinson’s disease, Scott Stanslaski was just starting his work on the project.
Stanslaski — who’s now a senior distinguished engineer in Medtronic’s neuromodulation operating unit — was part of the team trying to learn more about the brain signals associated with Parkinson’s and how they changed with medication or sleep.
“Part of that initial phase was to design a device that we could put into research studies, and then both learn about the physiology effects but also learn about how to design our devices better,” Stanslaski said in an interview with Medical Design & Outsourcing ahead of the project’s latest milestone.
How Medtronic developed and designed devices for aDBS
Around 2007, Stanslaski and his team took the Activa neurostimulator (which a decade earlier became the first FDA-approved DBS therapy system for Parkinson’s) and added brain sensing.
“By doing that, we could make just an incremental design change and then try to start learning from it,” he said. “We went through three learning cycles to get to the device we have now.”
The first was the Medtronic Activa device, followed by an improved

BY JIM HAMMERAND MANAGING EDITOR
rechargeable research device, and then the technology that led to Percept and adaptive DBS (aDBS).
“Our first device was pretty limited in terms of the number of frequencies we could monitor as well as stimulate at and do both those at the same time,” Stanslaski said. “… Parkinson’s patients, depending on the patient type, can need different stimulation frequencies to appropriately treat their disease, so you need to build the electronics to be flexible, to stimulate at multiple different stimulation frequencies and still record the sense signal.” >>
This illustration depicts a Medtronic neurostimulator and SenSight leads implanted in a patient for deep brain stimulation. Image courtesy of Medtronic
“That was really where a lot of those revisions took place to try to accomplish that,” he continued. “We were also trying to become more efficient with how we did it so when you deploy this into a commercial product, the amount of energy that we were consuming to deliver the therapy was a manageable level.”
Medtronic’s deep brain stimulation portfolio includes the Percept PC and RC (rechargeable)
made on the rechargeable device in parallel with work on Medtronic cardiac devices — was digitizing the signal after amplification within an implant for digital signal processing (DSP).
Another challenge was working with four-electrode stimulating leads that weren’t designed for sensing.
“One of the things that would happen to them is you could get fluid ingress into the lead body, and that would degrade your ability to sense brain signals,” Stanslaski said. “You could still stimulate because those levels were high, but they were more difficult to sense with.”

The team had their initial human cases in 2013 and learned enough to demonstrate aDBS in that first modified Activa device in 2015.
“Once we saw that and thought we could do it, we had enough momentum to start that rechargeable product design,” Stanslaski said.
The biggest technical challenge over all those years was how small brain signals are. At just a couple of microvolts, those signals are easily drowned out by the volts (1 million times greater than a microvolt) of stimulation energy being delivered.
One big advance early on with the modified Activa device was building amplifiers with low energy consumption and the ability to perform at extremely low noise levels. A later advance —
Around 2015, they pushed ahead with a brand new lead built for brain sensing with eight electrodes. Those new SenSight leads were designed for directional sensing to minimize signal interference and featured new materials and a new manufacturing method.
Stanslaski declined to go into too much detail for competitive reasons, but discussed more generally how the team addressed fluid ingress.
“When you’re building a lead that’s going to go into your brain, it’s got to come out of the skull, go down the back of your neck and then into the chest,” he said. “The insulation materials have to be free of any pinhole defects, and that’s true for both the outer insulation as well as the inner insulation between the eight wires running back for each
lead. And for the metal electrodes inside the brain, how the sealing around those electrodes occurs is also critical. You have to prevent any kind of fluid from getting up into the lead body.”
Lessons learned from Medtronic’s aDBS project
One lesson the team learned the hard way was that testing a device before shipping it out can cause unexpected problems. When deploying those first Activa research devices, the team inserted a pin block to connect each one to test equipment and verify it functioned as intended.
“As we tested multiple devices, we ended up finding [the pin block] was cutting some of the inner seals of the implantable neurostimulator,” Stanslaski said. “While that didn’t impact the stimulation, it had a big impact on the sense performance. We started seeing that both in our animal studies as well as some of the initial human implants, so we had to go off and try to figure out how it was happening and make corrective actions for it.”
“It was a really good learning experience,” he continued. “You’ve really got to think through both the design of the device but also how you’re testing and manufacturing it.”
Asked for advice he’d offer to other device engineers, Stanslaski encouraged them to “have a tremendous amount of persistence to keep at it, both to solve the technical problems, but also to make sure you’re communicating where you’re having trouble, where you’re not having trouble. That’s really big.”


This Medtronic illustration of its SenSight directional leads shows 1) A “1-3-3-1 electrode configuration to more precisely direct the stimulation,” 2) “1.5 mm and 0.5 mm electrode spacing options to suit various targeting and patient needs,” and 3) “Completely insulated orientation markers to guide directional programming.”
Image courtesy of Medtronic
“And in engineering, the more you can be in the lab testing and learning, there’s no substitute for that because you can only learn so much from computer simulations and reading papers,” he continued. “Until you really have it in the lab — and for us, doing our animal experiments — there’s just no replacement for how much you can learn in those environments. And that persistence to keep learning as you’re going throughout your whole career is just so important.”
Stanslaski credited his team’s collaboration with other teams within Medtronic, as well as feedback from physicians treating patients, for the project’s success.
“It was definitely a back-and-forth with them both during the design phase, but also physicians had the willingness to let us be there for patient visits,” he said. “I personally think that was one of the more valuable things for me, because I learned so much about Parkinson’s disease just being there and seeing what the physicians went through with
the patients. And then you start to see, ‘Oh, wow, that’s a pain point right there, and we can pretty easily solve that.’ That collaboration is really important.”
The patients are another group of crucial collaborators. Stanslaski said he often traveled to research sites like Stanford University and the University of California San Francisco to meet with Parkinson’s patients offering their own assistance with the project.
“It gives them this new sense of purpose, almost like they’re coming to work,” he said. “You’re learning from them, and they’re getting to see their own brain signals. It’s a pretty enjoyable research environment.”
One interaction that stood out for Stanslaski was a January 2025 trip to Europe when Medtronic earned a CE mark for aDBS.
“He was a Dutch patient who couldn’t speak English, but he was so excited to have me there,” Stanslaski said. “He hugged me three or four times throughout the visit. That [felt] pretty good.”

“This just the start, because as we continue to learn more about the brain and how the signals are useful in either predicting or giving us feedback on how well the therapy is working, we think that there’s a lot more room here to make DBS therapy work better for patients.”
Medtronic VP and Brain Modulation GM Amaza Reitmeier





































































Superior North American & International Hospital-grade Cords without Tariffs or Delays.
Interpower® North American and international hospital-grade cord sets provide the correct amperages and voltages for medical devices such as portable CT scanners and X-ray machines, medical-grade treadmills, and ECMO machines worldwide. All Interpower hospital-grade conductors inside the plug are anchored by a stainless steel ring that ensures electrical continuity when you need AC power in critical medical settings. Critical medical cords with no tariffs—avoid the unpredictable.
North American and Japanese hospital-grade plugs and receptacles bear the green dot, signifying the plugs have passed the rigorous UL 817 Abrupt Removal Test (UL 817, 18.2.4.1) and C22.2 No. 21-14 requirements for hospital-grade cords. Other countries using hospital-grade cords such as Australia and Denmark have proprietary requirements.
Interpower cords and components are manufactured in accordance with its product quality plan: hipot testing, continuity testing, and ground testing with multiple inspections. Interpower offers value-added options such as lengths, colors, packaging and labeling!
• 1-week manufacturing lead times
• Same-day shipping on in-stock products
• Tariff free if shipped anywhere in the U.S.
