NEONATOLOGY CATALOGUE
Vascular Access Devices





Vascular Access Devices
Pierre Simonet founded Vygon in France in 1962. He was one of the first to introduce the concept of disposable medical equipment. Shortly thereafter, the company established subsidiaries in Europe, the United States, India, South America, and more. Today it is present worldwide. Vygon also relies on many production units, starting with its first plant in France and continuing on to its latest, brand-new plant that opened in Portugal in 2015.
Belgium
Czech Republic
Denmark
France
Germany
Ireland
Italy
Netherlands
Norway
Poland
Portugal
Spain
Sweden
Switzerland
Turkey
United Kingdom




Vygon, committed to its customers worldwide
Vygon is a company that designs, manufactures and markets a wide range of medical devices for 4000 customers worldwide. Its success is due to its teams, who are available and committed. They are at the service of caregivers to offer them products and solutions that improve the safety and performance of care. The company that invented the single-use medical device, a true revolution in hospital care practices, has built its offer on the complementary skills that make up its business: industrial and technological know-how, clinical knowledge and understanding of hospital structures, and its own resources in research and development. Today, Vygon is an international company but it also remains a family business. The stability and commitment of its shareholders allow for a sustainable development that brings projects to fruition and its value system is a guide.
TYPES
Main insertion sites
Hand
Forearm
Antecubital fossa
Arm (under the axillary region)
Scalp (before 6 months)
Leg (before walking age)
Umbilical cord
Veins
Digital, metacarpal
Cephalic, basilic, median antebrachial
Medial basilic, medial cephalic, medial ulnar
Basilic, cephalic
Occipital, frontal, temporal
Large and small saphenous vein
Vena umbilicalis
Main insertion sites Arteries
Forearm
Leg
Foot
Umbilical cord
Radial
Posterior tibial
Dorsalis pedis
2 arteria umbilicalis
Frontal or metopic vein
Jugular vein
Subclavian vein
Cephalic vein
Median cubital vein
Basilic vein
Supra temporal vein
Occipital vein
Posterior auricular vein
Superior vena cava
Cephalic vein
POLYVINYL CHLORIDE (PVC)
Advantages
• DEHP ≤ 0.1%
• Acceptable mechanical resistance (traction, repeated flexing, clamping, pressure…)1
• Low-cost material
• Large variety of shore hardnesses
Disadvantages
• Risks of absorption and adsorption of some medications
• Release plasticizers
• More rigid in contact with some biological fluids
• Short term-use
POLYURETHANE (PUR)
Advantages
• Durable material
• Enhanced hemocompatibility, biocompatibility compared to PVC
• Good mechanical resistance (traction, repeated flexing, clamping, pressure, etc.)1, which allows thin-walled catheters with high flow rates
• Thermosensitive material2
• Versatility/processability:
- Can be extruded in extremely small diameters or multilumen configurations
- Can be coated or impregnated with specific polymers or additives to enhance their properties (lubricity, antimicrobial or antithrombogenic…)
• Large variety of shore hardness
• For short and medium term-use
Disadvantages
• Weakened by alcohol3
• Risk of absorption and adsorption of some medications
SILICONE
Advantages
• Excellent biocompatibility and hemocompatibility
• Possible prolonged use of the catheter4
• Soft, and flexible
• Atraumatic, best tolerated material, reduces the risk of phlebitis or vessels/cardiac wall erosion5
• Floating in the blood flow
• Good chemical compatibility with most drugs
Disadvantages
• More difficult placement due to softness4
• Thicker catheter wall than PUR / smaller inner diameter
• Tolerates less pressure than polyurethane
• Iodine-based solutions weaken silicone tubes
Vascular Access needs determined
Osmolality and PH of medications or solutions
Quality of vascular access Length of therapy Line decision
PIV = Peripheral Intravenous Catheter EPIV = Extended Dwell Peripheral Intravenous Catheter PICC = Peripheral Inserted Central Catheter
As umbilical catheters are used in emergencies, they are not included in the decision tree.
Indication of Umbilical catheter
UMBILICAL VEIN CATHETERIZATION
Allows infusion of parenteral nutrition, medication and other intravenous (IV) fluids
INDICATION
TYPE OF CATHETER
AVERAGE DWELL TIME
ADVANTAGES
Allows an access port for venous blood sampling
Provides access for an exchange transfusion
Single or multiple lumen
UMBILICAL ARTERY CATHETERIZATION
Monitoring arterial blood pressure
Sampling arterial blood for pH and gas analysis
Alternative IV access for fluids and medication, if none are available
Only single lumen
Up to 14 days (most of the time, removed before 8 days) Less than 5 days
Central infusion of drugs, blood products, hypertonic solutions or vasoactive products when the child is in severe respiratory distress6
Performing an exchange transfusion
Measurement of a central venous pressure
Used in an emergency approach to the resuscitation room at birth, for the cardiotonic products’ injection
Used as an approach for administering prolonged parenteral nutrition in the preemie up to 2 weeks7
Continuous monitoring of blood, for the umbilical arterial catheter8
Placed in the event of failure of peripheral venous access or the exhaustion of peripheral venous capital
Complete range of neonatal umbilical catheters
Umbilical catheter sizes / patient weights9-12
Common features
SINGLE-LUMEN PVC CATHETER
Blunt distal tip Preservation of the vessel
Partially transparent tube Visualization of blood return
Packed in a protective tube and over-packed in a peelable pouch
Radiopaque catheter Control of catheter positioning under X-ray
Numerical marking every cm from 4 to 25 cm Easy check of the length of catheter inserted
For arterial access For venous access
SINGLE-LUMEN PUR CATHETER
Partially transparent tube Visualization of blood return
Plugs
Red for arterial access
Blue for venous access
Radiopaque catheter
Control of catheter positioning under X-ray
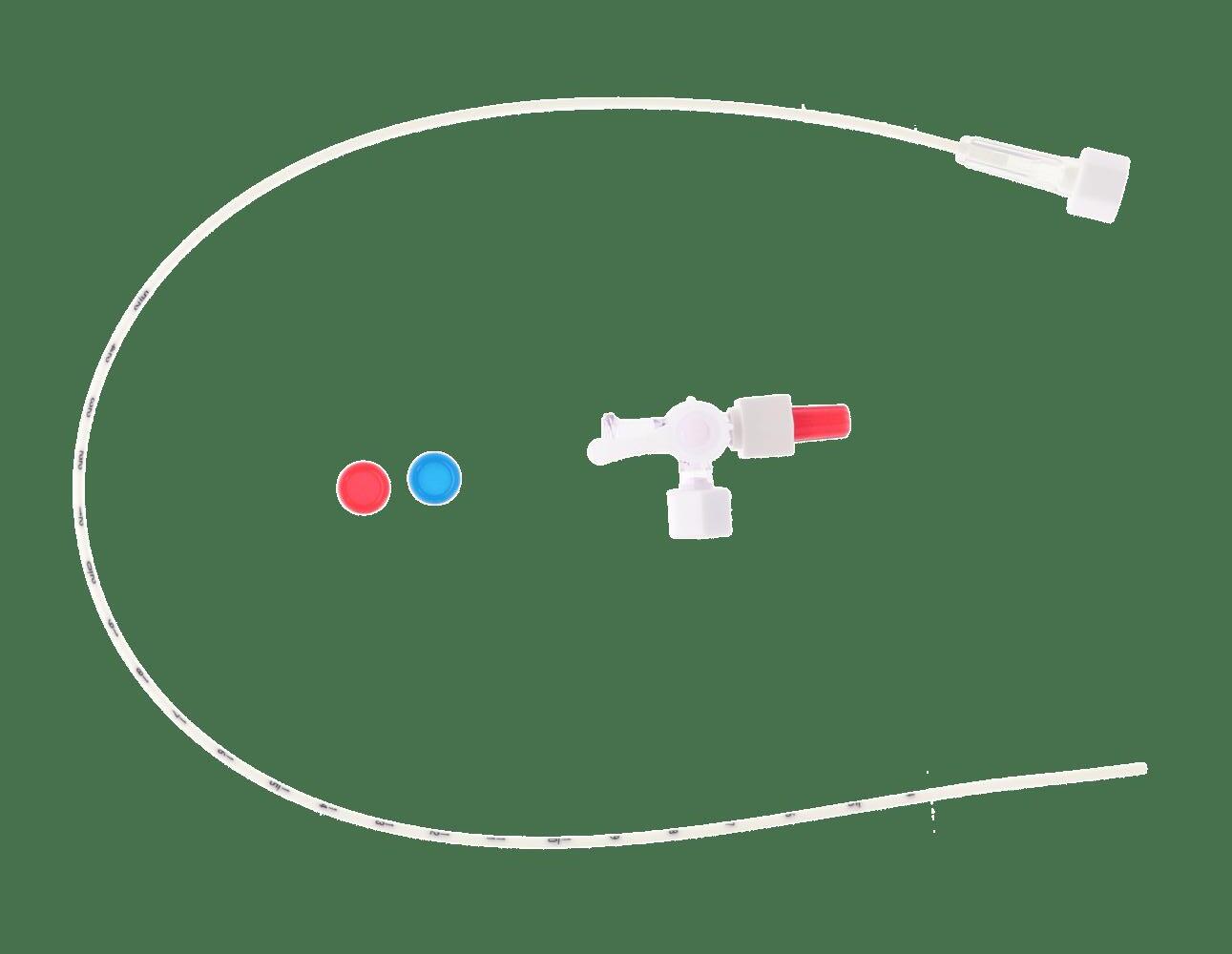
Female Luer connector
3-way stopcock

Numerical marking every cm from 4 to 25 cm
Easy check of the length of catheter inserted
Packed in a protective tube and over-packed with the stopcock in a peelable pouch
Blunt distal tip
Preservation of the vessel
For
For
The double-lumen PUR umbilical catheter can be used, in specific cases, which are :
• Infusion of incompatible drugs
• Infusion of medication and parenteral nutrition simultaneously
• Reduction of the number of peripheral routes required
Numerical marking every cm
Easy check of the length of catheter inserted
Preservation of the vessel
Double-lumen
To prevent incompatible drug mixing lines with small and rounded clamps
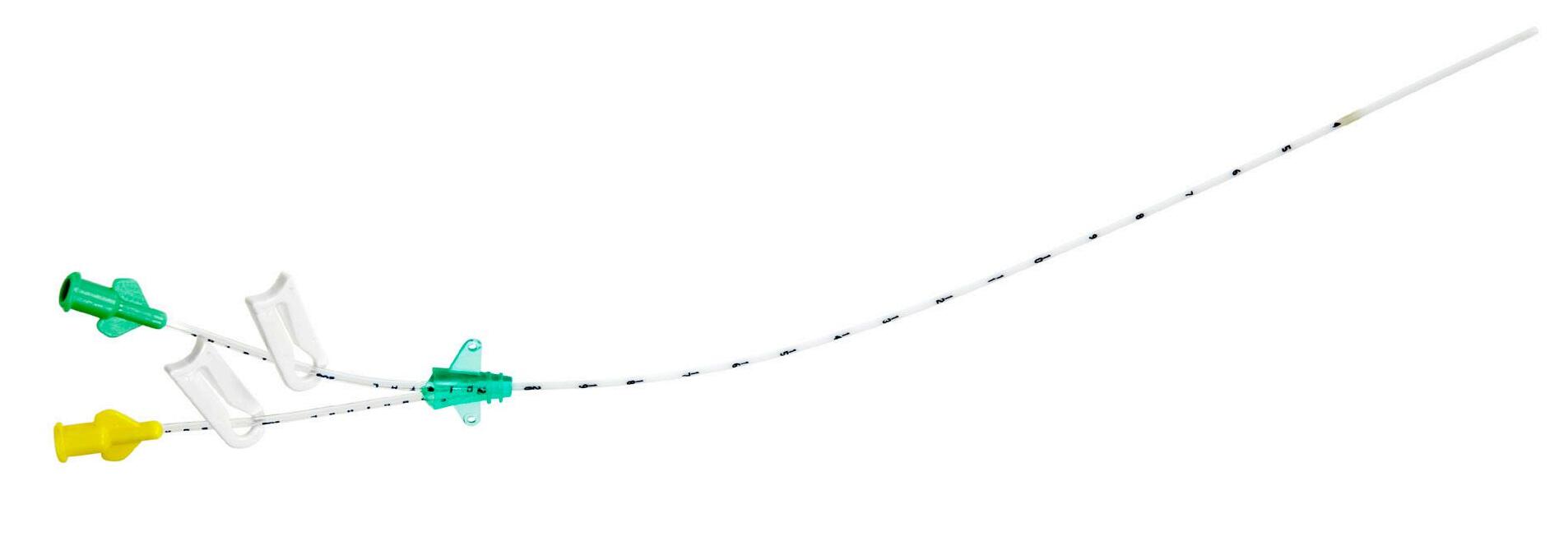
2 color-coded hubs marked “Distal” or “Proximal”
Radiopaque catheter
Control of catheter positioning under X-ray
Codes 1272: presented in a protective tube and over-packed in a rigid blister pack
Codes 1274: packed in a protective tube and over-packed in a peelable pouch
The triple-lumen PUR umbilical catheter can be used for :
• Extremely premature baby born around 24 weeks (to avoid multiple access and maintain continuous administration)
• Small-for-Gestational-Age (SGA) infant
• Term infant suffering from Persistent pulmonary hypertension (PPHN)
• New-born suffering from Hypoplastic Left Heart Syndrome (HLHS)
Numerical marking every cm Easy check of the length of catheter inserted
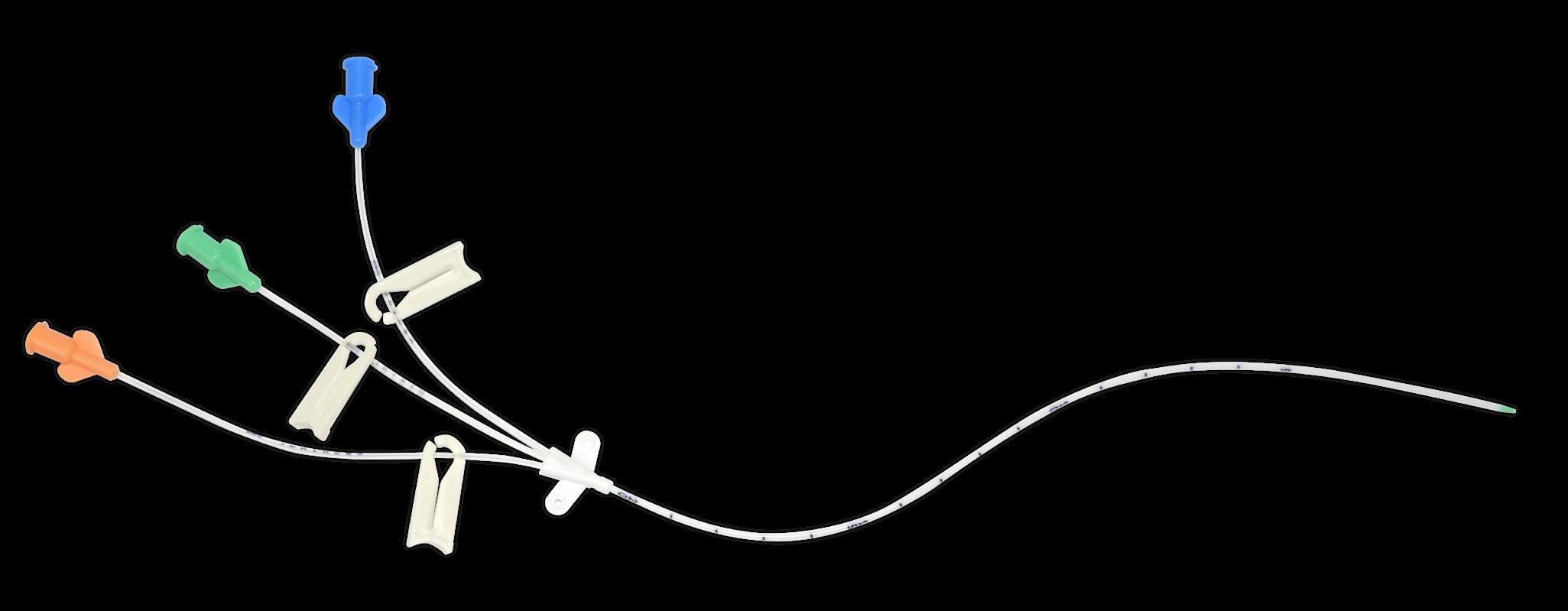
Triple-lumen
Reduced number of routes needed lines with small and rounded clamps
3 color-coded hubs marked “Distal”, “Medial” & “Proximal”
Packed in a protective tube and over-packed in a peelable pouch Indication Code
Radiopaque catheter
Separated exit points
Incompatible drugs mixing prevention
One distal & two lateral openings
Green distal tip
Flexible & smoothed
Preservation of the vessel
Control of catheter positioning under X-ray
The “Expert” umbilical catheters are the only umbilical catheters with an integrated antimicrobial technology to fight against Catheter-Related Bloodstream Infections (CRBSI) in NICUs.
This antimicrobial technology, called AgION™, is made of silver ions bounded into a zeolite, a bio-inert ceramic integrated within the wall of the catheter.
When the “Expert” catheter comes in contact with blood, the zeolite naturally releases the Ag+ ions and replace them by Na+ ions present in the blood. It is a pure ionic exchange. The silver ions are released from the internal and external surfaces of the catheter for both extraluminal and intraluminal protective action.
Numerical marking every cm Easy check of the length of catheter inserted
Ionic silver is a highly efficient antimicrobial technology with:
• A broad spectrum of action on Gram+, Gram - and fungi
• A low toxicity
• A tri-modal action
Because the silver ions are sensitive to light (photoreactive), the catheters are supplied within a green UV-protective tubing.
The AgIONTM technology
Ag+, ionic silver is released Zeolite
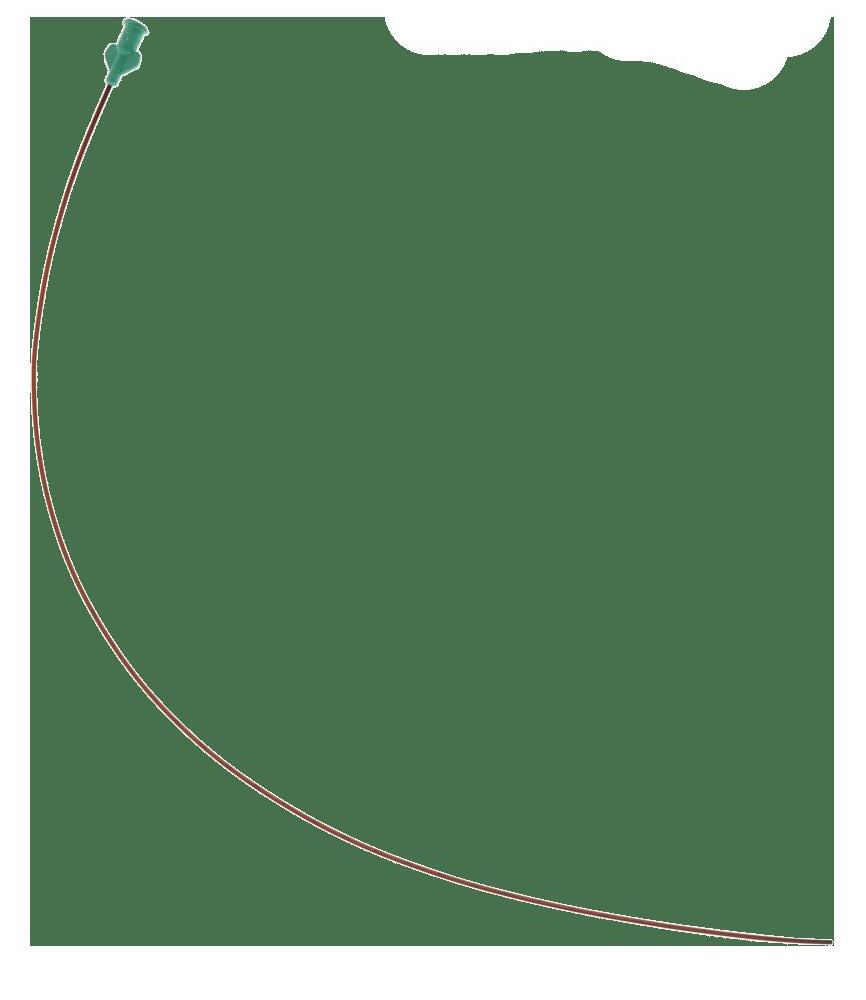
For arterial access For venous access
Brown opaque catheter tube due to the AglON™ antimicrobial agent made of silver ions
Blunt distal tip Atraumatic insertion Preservation of the vessel

Na+ sodium naturally present in the blood enters zeolite
Double-lumen
To prevent incompatible drug mixing Lines with small and rounded clamps 2 color-coded hubs marked “Distal”
SINGLE LUMEN: THE “HAUMONT KIT”
The Haumont kit allows to place safely and without pain a silicone catheter for long-term access in the umbilical vein with a specific introducer. The advantages of this technique compared to the percutaneous technique are:
• Longer term use of an umbilical catheter, as for the percutaneous catheter
• Chemical inertia of the silicone material
• Painless for the patient and easy access to the umbilical vein
• Preservation of the peripheral veins of the neonate
The catheter is inserted in the umbilical vein by using one of the 3 introducers (Ø 6 Fr, PVC) supplied in the kit. These introducers have 3 different lengths (8 cm, 9 cm and 11 cm) to suit the size of the baby and the distance of the cord section.
Procedure:
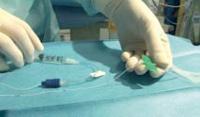
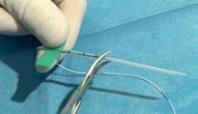
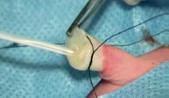
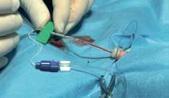
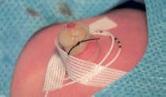
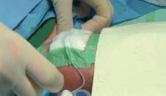
Contents of the kit:
Numerical marking every cm
Easy check of the length of catheter inserted
Radiopaque catheter
Control of catheter positioning under X-ray
Silicone No plasticizer release
Detachable Easy-lock connector Allow removal of the introducer
PUR extension line
Length >10 cm
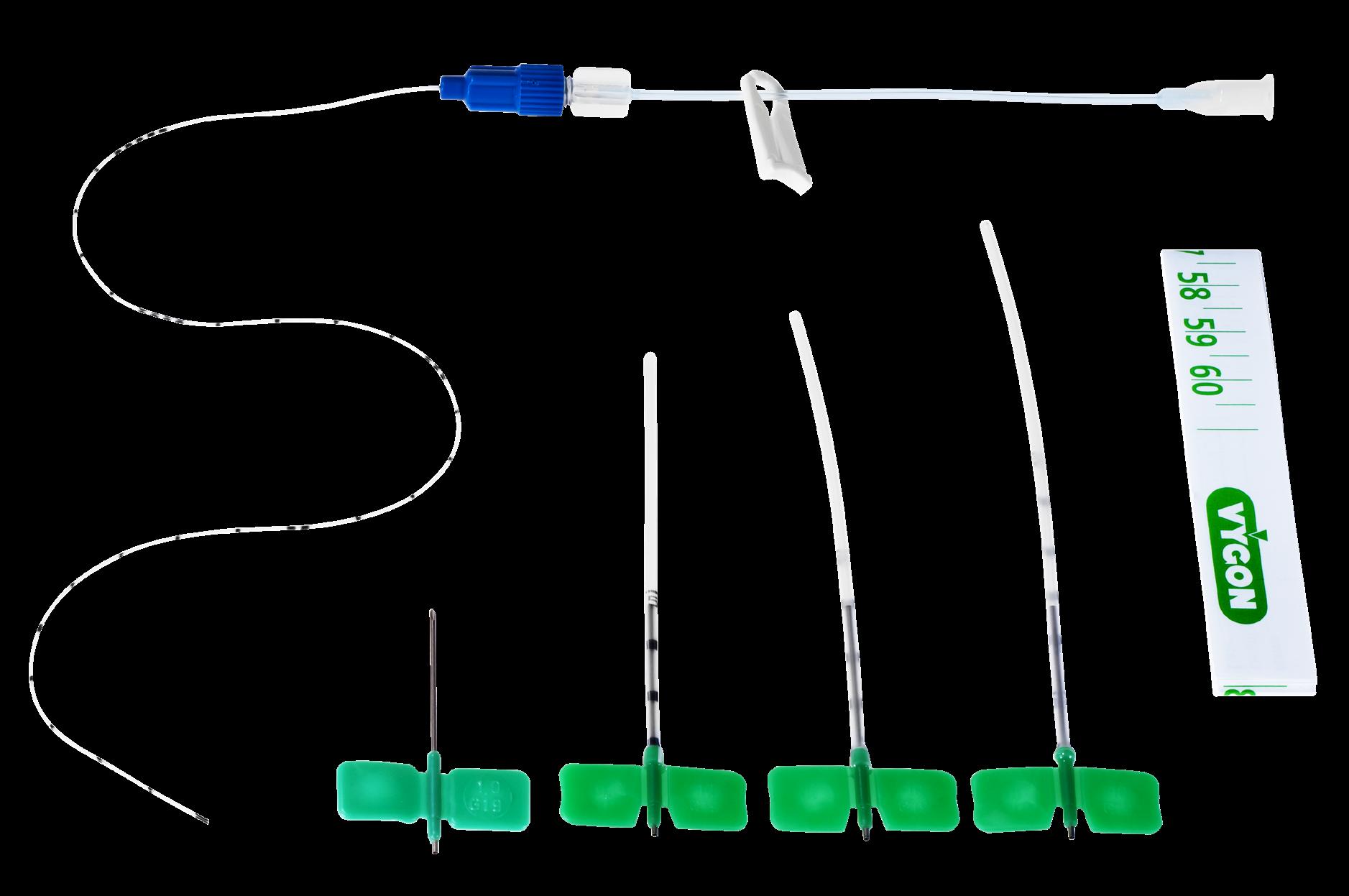
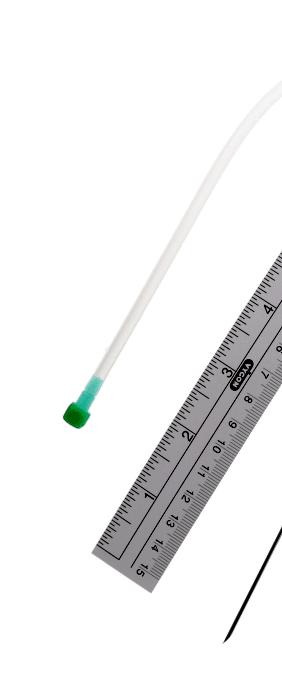
The kit is presented in a rigid blister containing:
• 1 epicutaneo cava silicone catheter (2Fr, 24G, length 30 cm)
• 3 introducers for inserting the catheter in the umbilical vein (6 Fr, length 8, 9 & 11 cm)
• 1 stainless steel winged needle (Ø 1.0 mm, 19 G, length 27 mm) if the user decides finally to insert the catheter in a peripheral vein instead of the umbilical vein
• 1 measuring tape
The exchange-transfusion tray is indicated for alloimmune hemolytic disease of the new-born, hyperbilirubinemia, severe anaemia, and polycythaemia. The technique consists of the replacement of most of the patient blood with donor blood.
This replacement is made by split and successive volumeto-volume exchanges, to remove abnormal blood components and circulating toxins, whilst maintaining adequate circulating blood volume.
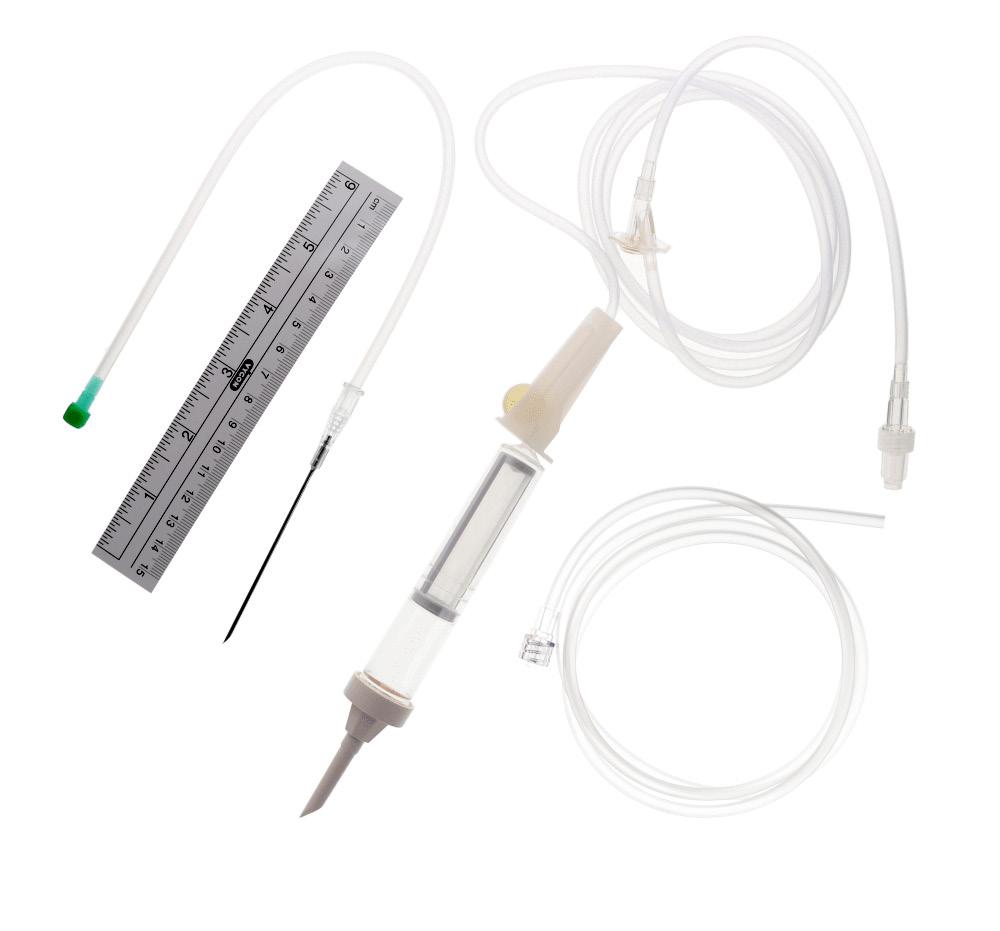
The tray contains:
• 1 fenestrated drape 50 x 60 cm
• 2 20 mL Luer-lock syringes
• 1 10 mL Luer-slip syringe
• 1 16 x 0.5 mm (25 G) Luer hypodermic needle
• 1 air-venting needle
• 3 8-ply swabs 50 x 50 mm
• 1 measuring tape 15 cm


• 1 four-way stopcock equipped with 1 injection site
• 1 transfusion set
• 1 graduated plastic container 2000 mL and their hooks
• 1 extension tube for the evacuation of the discarded blood
• 1 5 Fr exchange transfusion


catheter (PVC - XRO), 37 cm long, with 2 sides holes and centimetric markings
• 1 7 Fr exchange transfusion catheter (PVC - XRO), 37 cm long, with 2 sides holes and centimetric markings
• 1 pair of gloves (only for 275.00)
• 1 control sheet
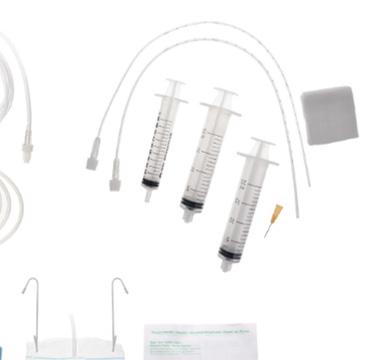
The pack is intended to be used for umbilical catheter placement. It contains all the instrumentation and accessories used during the insertion of an umbilical venous or arterial catheter and allows to carry out this procedure under optimal aseptic conditions
• Designed for neonates with chosen and adapted devices
• Saving time and money by reducing preparation time, improving stock management, and decreasing waste
• Improving patient safety by simplifying and standardizing the procedure, providing traceability label, and reducing the risk of nosocomial infections
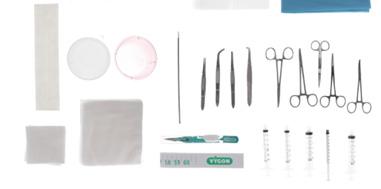

The kit is sterile, single use and presented in a supple blister containing :
• 1 tray 20 x 15 x 4 cm transparent
• 1 outer wrap 75 x 90 cm
• 2 hand towels 33 x 38 cm
• 1 peelable transparent drape 50 x 50 cm opening Ø 4 cm
• 2 drape towels 45 x 75 cm
• 1 gallipot 60 mL red
• 1 gallipot 60 mL crystal
• 1 syringe 1 mL Luer Slip
• 2 syringes 3 mL Luer-lock
• 2 syringes 5 mL Luer-lock
• 1 safety hypodermic needle 20 G, 38 mm, yellow
• 1 safety hypodermic needle 18 G, 38 mm, pink
• 10 swabs 5 x 5 cm, 4 ply white non-woven
• 10 swabs 10 x 10 cm, 4 ply white non-woven
• 1 paper measuring tape 60 cm
• 1 safety scalpel
• 1 umbilical cotton lace 2x30” 75 cm
• 1 dilator 14 cm Ø 2 mm
• 1 full curved Iris forceps 10 cm without teeth
• 1 straight Iris forceps 10 cm
• 1 Mayo Hegar needle holder 14 cm
• 1 straight haemostatic Halstead Mosquito forceps 13 cm
• 1 curved Reynold’s dissecting scissors 11 cm
• 2 curved Mosquito forceps 13 cm
• 1 straight Iris forceps 10 cm with teeth
• 1 half-curved Iris forceps 10 cm
• 1 silk suture 3-0 with curved
• needle
• 2 adhesive strips 148 x 12.5 mm
• 1 instruction for use
• 1 measuring guide
• 1 small zip lock bag
Indication of neonatal PICCs
INDICATION
TYPE OF CATHETER
AVERAGE DWELL TIME
ADVANTAGES
These Peripherally Inserted Central Catheters (PICCs) are indicated for premature babies, new-borns, and infants, They are intended for central intravenous (IV) administration of total parenteral nutrition (TPN), IV medications or fluids, specifically when the IV therapy requires:
• vesicant or irritant drugs,
• > 600 mOsm/kg solutions,
• drugs with pH <5 or >9,
• or more than 6 days of treatment
Single or double lumen
Short-term (≤ 30 days)
Only single lumen
Long term (> 30days)
Preserve peripheral vein integrity and avoid the trauma associated with repeated puncture
Can be implanted in a patient with coagulation disorders or ventilatory risk (no pneumothorax, no haemothorax…)
Elements to be taken in account when choosing the device: the painless puncture, the comfort of the patient and the ease of removal (particularly in patients who are potential candidates to return home during the treatment)
Complete range of neonatal PICCs
VYGON’s PICCs
Common features
FEATURES
Black mark at distal tip
Common centimetric marking for all Vygon’s PICC
X-ray opaque
Measuring tape
Adaptability
BENEFITS
To check that the entire catheter has been withdrawn
Facilitates accurate placement of catheter
(The actual cm is the middle of the marking. So, it is the middle black dash for the odd numbers or the space between the middle black dashes for the even numbers. See the illustration)
Generally, avoids use of iodine-based opacifiers
To measure distance from puncture site to vena cava
Can be used with different types of introducers
PUR PICCS
premicath – 1 FR PUR SINGLE-LUMEN PICC
Designed for the tiniest neonatal patients, premicath is intended to be used for short term (<30 days) venous access to deliver drugs and for parenteral nutrition.
Flushable stylet
For priming the catheter and facilitating its removal
Small removal round clamp Prevents from damaging the fragile skin
Pink hub
Allowing the fixation of the 24 G I.V. short cannula
premicath is presented in a rigid blister pack containing:
• 1 PUR catheter
• 1 introducer (except codes 1261.201/206/210/306/310)
• 1 measuring tape
Code 1261.21 only: 1 syringe adaptor to prime the introducer
Numerical marking every cm
Radiopaque
Pink hub to connect the non-peelable plastic cannula introducer (only for codes: 1261.152/153/206/207/302/306/307)
*See the introducers’ details page 29
nutriline – 2 FR / 3 FR / 4 FR PUR SINGLE-LUMEN PICC
A single-lumen PICC designed for premature babies, newborns and infants.
Flushable stylet
For priming the catheter and facilitating its removal
Small removal round clamp Prevents from damaging the fragile skin
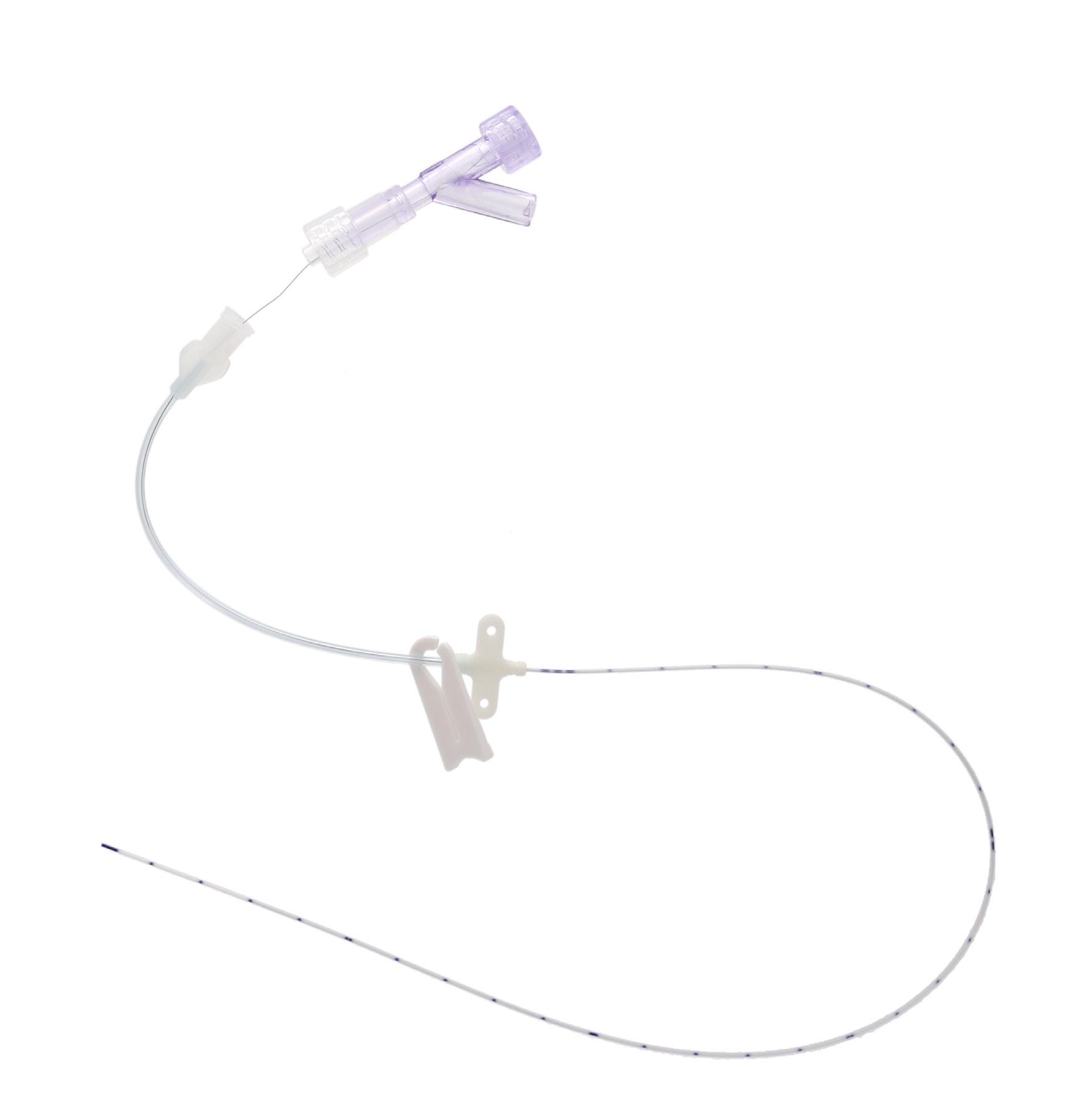
Small fixation wings Compatible with gripl

Numerical marking every cm
The following products will be made to order:
*See the introducers’ details page 29 For venous access
Designed for neonates and infants,
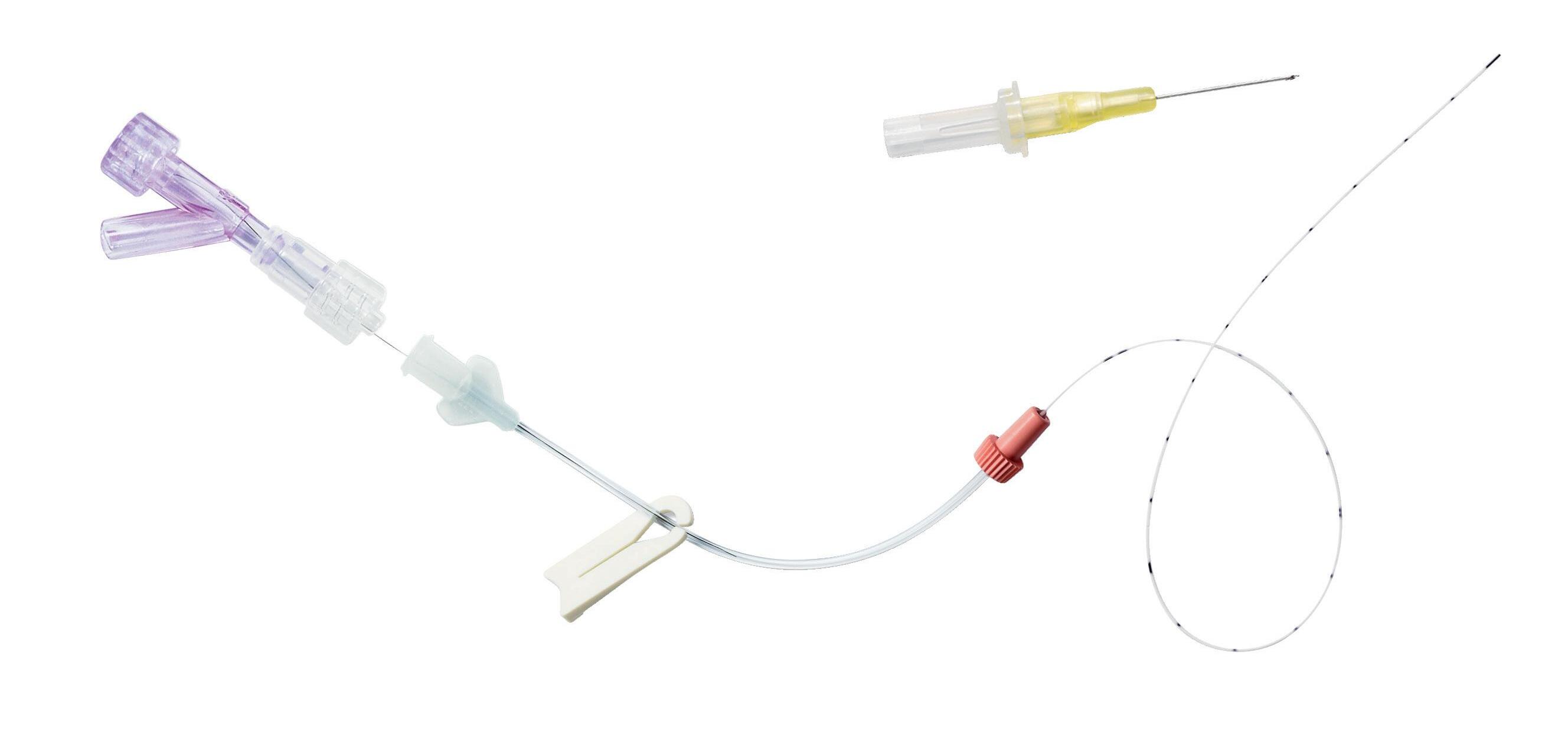
double-lumen catheter intended to be used for short term (< 30 days) venous access. This catheter is particularly recommended for simultaneous administration of incompatible drugs, or simultaneous infusion of medication and parenteral solutions. It also prevents trauma associated with repeated punctures and preserves venous access of the new-born.

Unique catheter taper
Allows high flow rates
Prevents the risk of kinking
Colour-codes connectors
For line’s identification
nutriline twinflo is presented in a rigid blister pack containing:
• 1 double-lumen 2 Fr PUR catheter
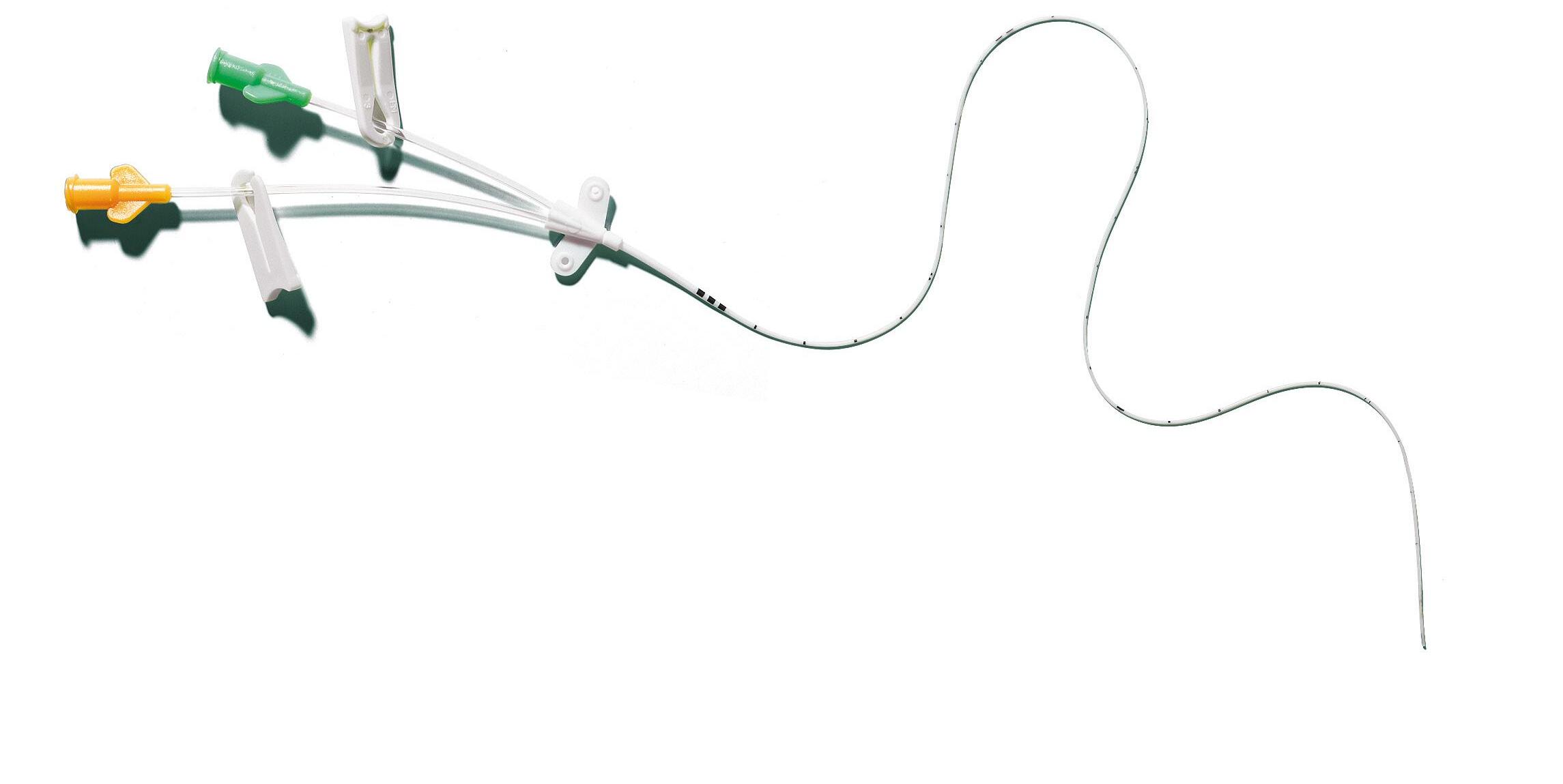
• 1 introducer (except for 1252.232/233)
• 1 neonatal PICC griplokTM
• 1 measuring tape
Numerical marking every cm
Radiopaque

nutriline twinflo is supplied with griplokTM securement device, which features an hypoallergenic adhesive specially designed for neonates.
*See the introducers’ details page 29
For venous access
premistar – Antimicrobial 1 Fr PUR single-lumen PICC
premistar is the only antimicrobial impregnated 1 Fr / 28 G PICC , especially developed to fight against catheterrelated bloodstream infections (CRBSI) in NICU. The Star Technology, used in this product, is the innovative combination of two active substances, Rifampicin and Miconazole, chosen for their synergic properties.
Rifampicin is a competitive inhibitor of bacterial RNA polymerase with potent activity against Grampositive microorganisms and has a high physico-chemical compatibility with polyurethane.
Miconazole is a synthetic antifungal with a wide spectrum of antimicrobial activity and a low toxicity and has been used for years in the topical and systemic treatment of mycotic infections.
Rifampicin and Miconazole are molecularly integrated into the polyurethane catheter material by using a patented
multistep diffusion-controlled incorporation process. The active ingredients are entirely “dissolved’ in the polymer like in an alloy.
When having contact to blood, body fluids or infusion solutions, the catheter releases Rifampicin and Miconazole to the catheter’s inner and outer surfaces at a slow and steady rate.
Suppression of microbial growth in the catheter’s biofilm reduces the incidence of catheter associated infections.
Due to the wide reservoir of this antimicrobial substance inside the catheter’s entire wall, the release mechanism provides release of Rifampicin and Miconazole for the entire life of the product.
Combined antimicrobial effect
• Synthetic antifungal with low toxicity
• Mechanical action: damages cell wall and inhibits peroxide degradation
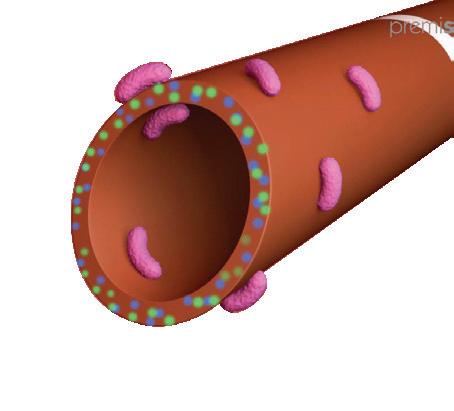
• High efficacy against fast growing and resting bacteria in biofilm
• Mechanical action: inhibits RNA synthesis
Designed for the tiniest neonatal patients (<1 kg), premistar catheter is intended to be used for short term (<30 days) venous access to deliver drugs and parenteral nutrition.
Large white mark
To ensure that the entire catheter has been withdrawn

Thermosensitive polyurethane
To provide comfort and safety to the patient
Brown opaque catheter tube
Due to antimicrobial agents’ impregnation
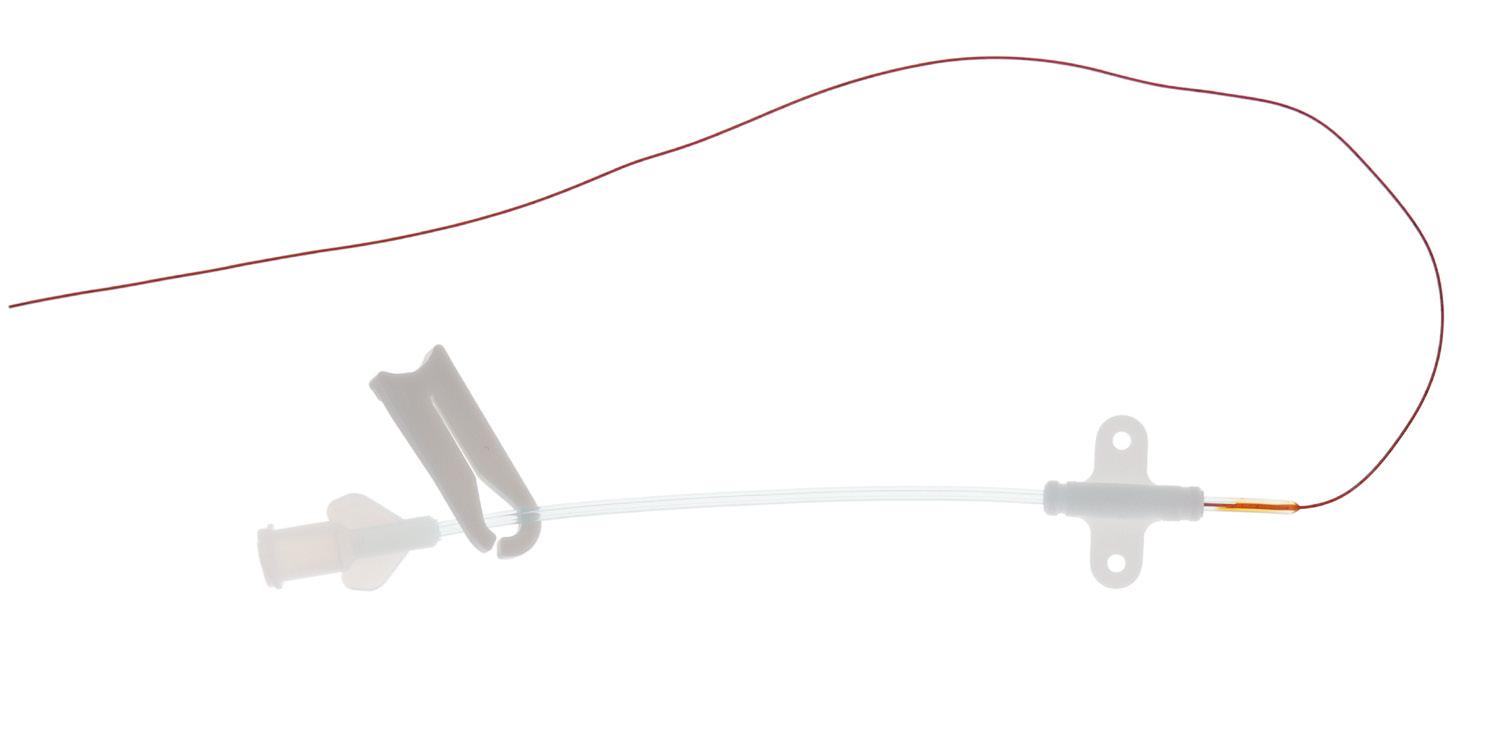
White markings every cm
Integral PUR extension tube
Measuring tape Radiopaque
Length > 8 cm, with small and rounded clamp
Integral extension with wings
To ensure the stabilization and the securement of the catheter, reducing the risks of mechanical phebitis
To connect the non-peelable plastic cannula introducer (only for the codes: 6261.206/306) Or
Pink hub
premistar is presented in a rigid blister pack containing:
• 1 PUR catheter
• 1 introducer (except codes 6261.206/306)
• 1 measuring tape


6261.20
*See the introducers’ details page 29
For venous access
SILICONE PICCS
epicutaneo cava – 2 Fr SILICONE single-lumen PICC
Designed for neonatal patients, epicutaneo cava is intended to be used for long term (>30 days) venous access to deliver drug and parenteral nutrition
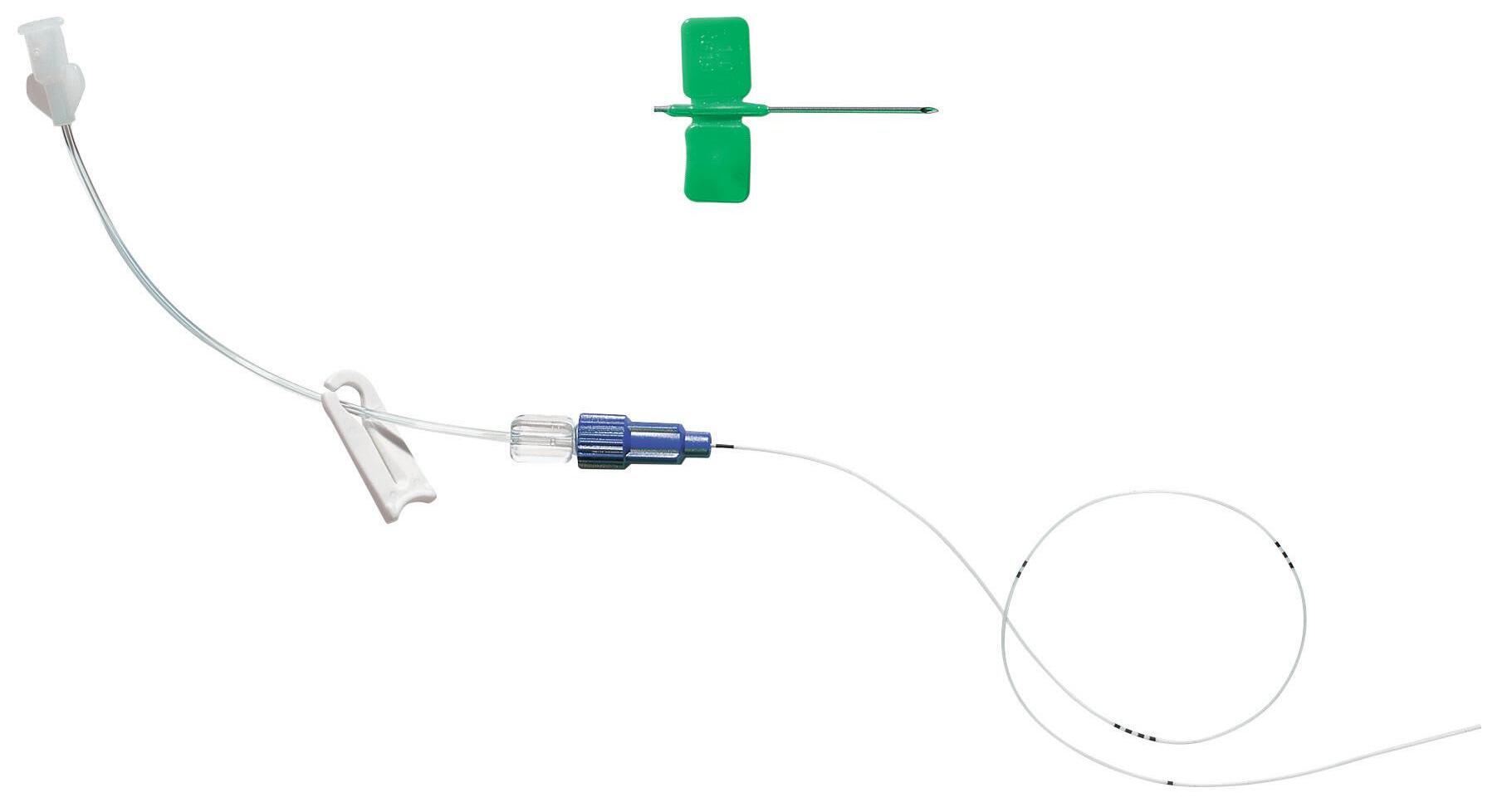
Integral PUR extension tube
Length = 10 cm, with small and rounded clamp
Detachable easylock type connector
Numerical marking every cm
Large black mark At the distal tip to ensure that the entire catheter has been withdrawn
Radiopaque
epicutaneo cava is presented in a rigid blister pack containing:
• 1 silicone catheter
• 1 introducer
• Integral PUR extension tube (length 10 cm) with a detachable easylock type connector
• 1 measuring tape
*See the introducers’ details page 29
For venous access
Soft silicone
To enhance patient safety against cardiac tamponade
epicutaneo 2 – 1.9 Fr SILICONE single-lumen PICC
Designed for neonatal patients, epicutaneo 2 catheter is intended to be used for long term (>30 days) venous access to deliver drug and parenteral nutrition
Integrated silicone extension tube Length = 4 cm, without clamp (to avoid damaging the silicone tube)
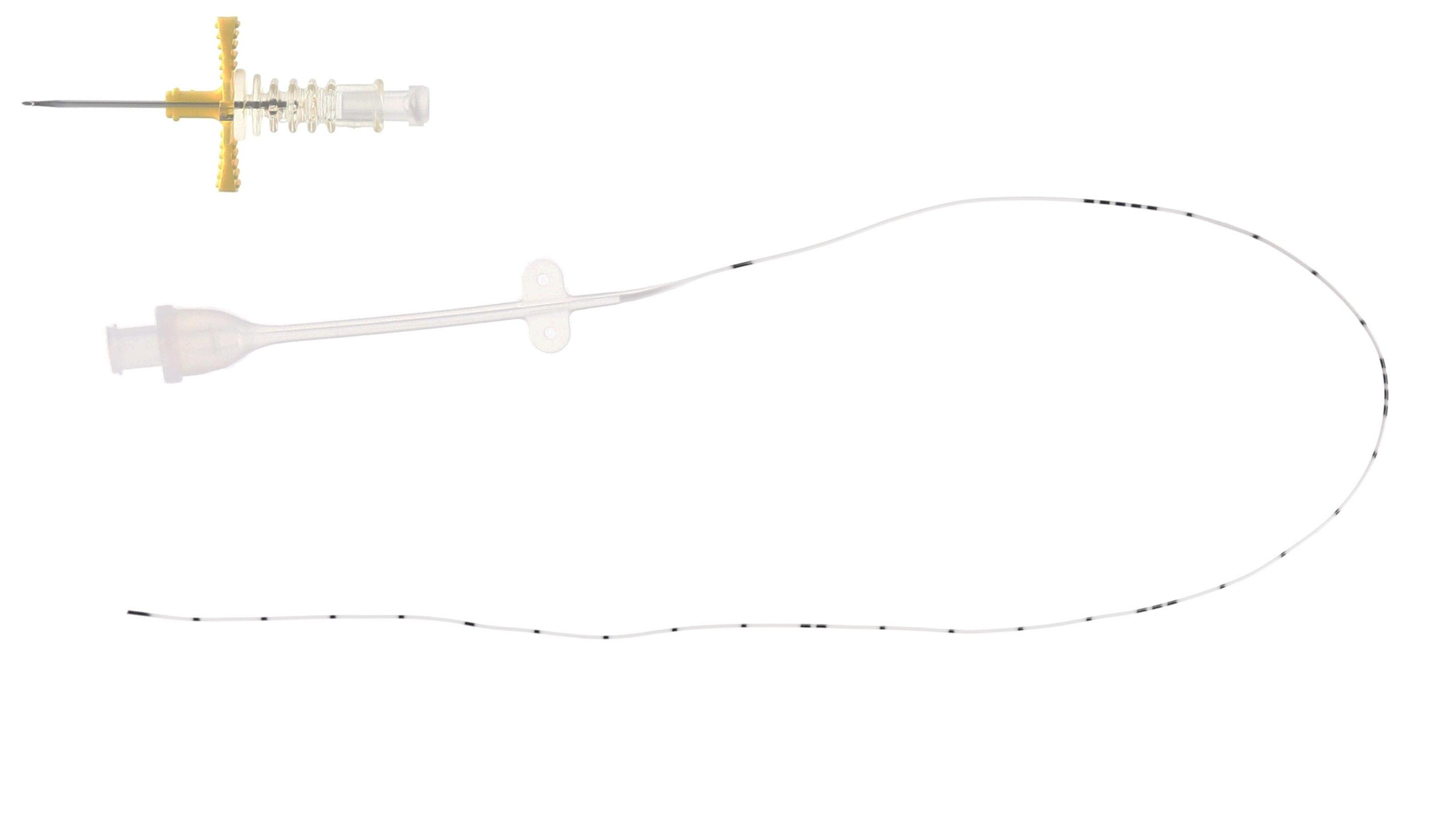
Large black mark
At the distal tip to ensure that the entire catheter has been withdrawn
Soft silicone fixation wings To enhance the stabilization and securement
Radiopaque
Soft silicone
To enhance patient safety against cardiac tamponade
Numerical marking every cm
epicutaneo 2 is presented in a rigid blister pack containing:
• 1 silicone catheter
• 1 introducer
• 1 measuring tape Indication
*See the introducers’ details page 29
For venous access




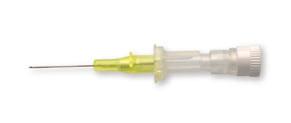

Splitting needle
The splitting needle introducer is intended to be used for 1 Fr or 2 Fr PICC placement

Stainless steel needle Siliconized to ease its insertion in the vein Double wings
Compatible with premicath, premistar, epicutaneo 2, nutriline, nutriline twinflo
Supplied in a rigid blister pack
microflash
microflash is a peelable cannula introducer, intended to be used for 1 Fr or 2 Fr PICC placement.
Radiopaque stripe
Clear cannula

Ribbed wings Preventing finger slippage while splitting the cannula
Lateral eye For early detection of blood flashback Can be totally withdrawn after catheter placement
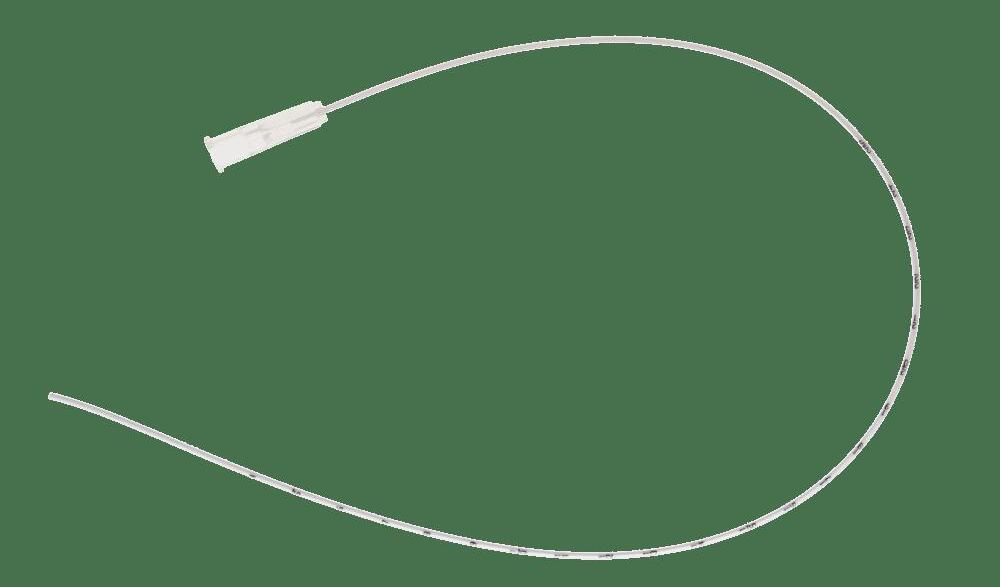
microsite® – Modified Seldinger Technique introducer
Designed for premature babies, microsite is an insertion kit for 1 Fr or 2 Fr central venous catheters introduced with the Modified Seldinger Technique. The microsite introducer is intended to be used for small veins difficult to catheterize.
Guidewire insertion aid

Guidewire
• Offers increased puncture accuracy with a smaller gauge introducer
• Facilitates poor peripheral venous access
• Reduces the risk of multiple punctures and venous trauma
• Enhances the rate of successful placement.

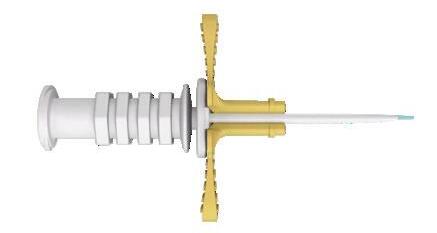
Peelable sheath and dilator Puncture needle
The kit is presented in a rigid blister pack containing:
• 1 24 G (Ø 0,55 mm) puncture needle, with an immediate visualization of the blood flashback
• 1 Nitinol guidewire, with symmetrical ball-protective ends
• Guidewire insertion aid
• 1 peelable sheath and dilator, for 1 Fr or 2 Fr PICC introduction.
24 G Short I.V. cannula
24 G short I.V. cannula is an introducer intended to be used for 1 Fr catheter that features (premicath 1261.xx or premistar 6261.20) a pink connector only. The cannula should be connected to the pink connector.
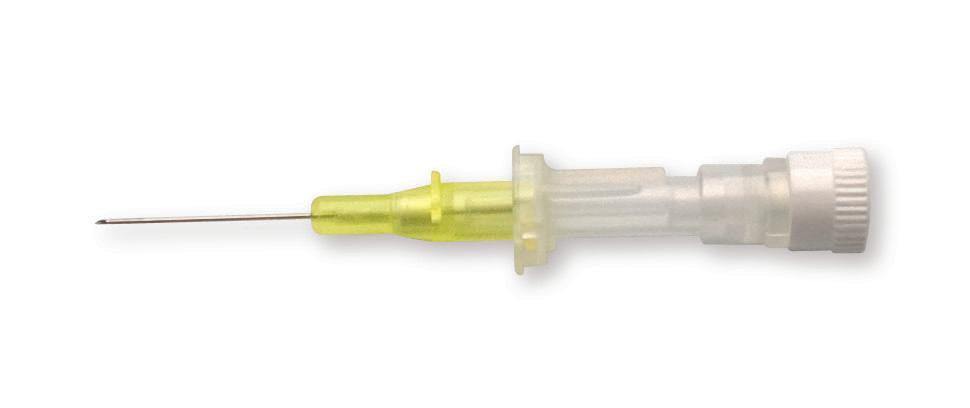
Transparent cannula
To see blood return
Not available separately
Supplied with premicath catheter codes 1261.102/152/153/207/302/307 (see page 20)
Winged needle
The winged needle introducer is intended to be used with the epicutaneo cava catheter (2184.xx and 1284.xx)
Double wings for handling
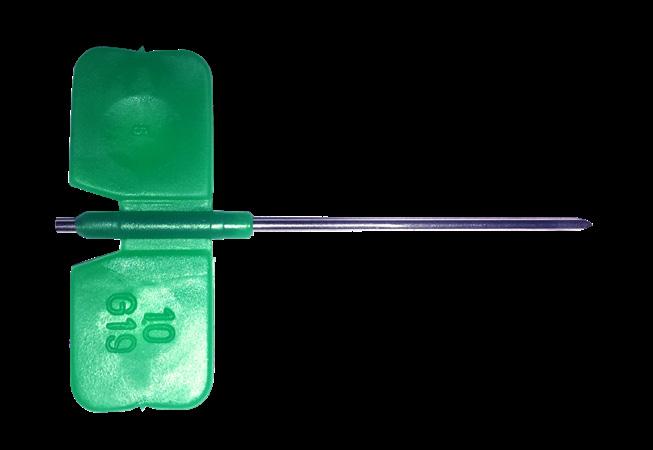
Siliconized stainless steel needle
The kit is intended to be used for the placement of a neonatal PICC , containing all the instrumentation and accessories that are necessary and allowing to carry out the procedure under optimal aseptic conditions.
Product benefits:
• Designed for neonates with chosen and adapted instruments
• Saves time and money by reducing preparation time, improving stock management and decreasing waste
• Improve patient safety, by simplifying the procedure, providing traceability label and reducing the risk of nosocomial infections
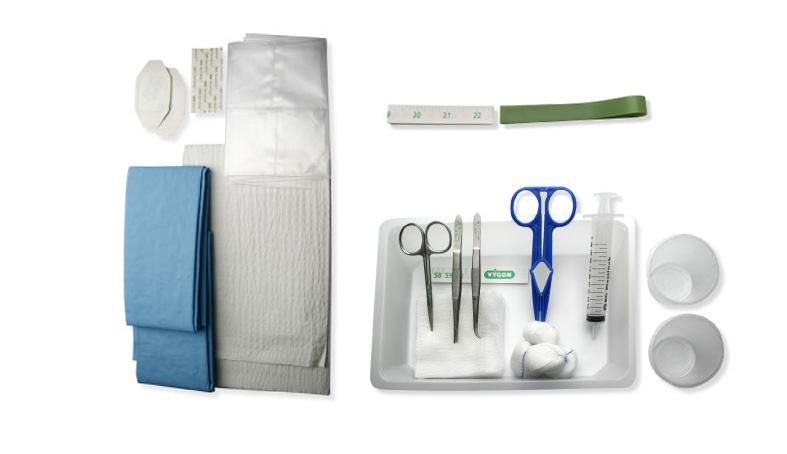
• 1 tray 20 x 15 x 4 cm transparent
• 1 outer wrap 75 x 90 cm
• 2 hand towels 33 x 38cm
• 1 peelable fenestrated transparent drape with “EasyPeel” 50 x 50 cm (opening Ø 4 cm)
• 2 drape towels 45 x 75 cm (absorbent/impermeable)
• 1 red gallipot 60 mL
• 1 transparent gallipot 60 mL
• 1 syringe 10 ml (Luer Lock)
• 1 blue prep forceps
• 4 ball swabs XRO
• 5 swabs 7.5 x 7.5 cm, non-woven
• 1 silicone neonatal tourniquet 9x180 mm
• 2 measuring tapes 60 cm
• 1 straight Reynolds scissors 9 cm
• 1 straight Iris forceps 10 cm
• 1 curved Iris forceps 10cm
• 6 adhesives strips 6 x 38 mm per 6
• 2 Tegaderm® dressings 4 x 4 cm
• 1 instruction for use
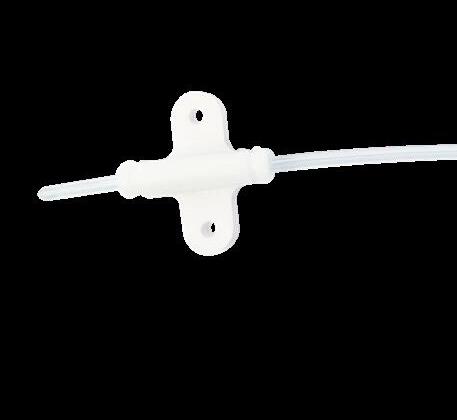
Fixation device for pediatric PICC catheters. griplok™ ensures the catheter fixation to prevent accidental removal of catheter migration.
Product benefits:
• Safe and secure for the patient: low risk of irritation and allergic reaction, efficient skin fixation, efficient catheter fixation to prevent migration and reduction of injection risk
• Easy and safe for the user: griplok™ sutureless system and sterile
• Efficient fixation for all Vygon neonatal and pediatric catheters
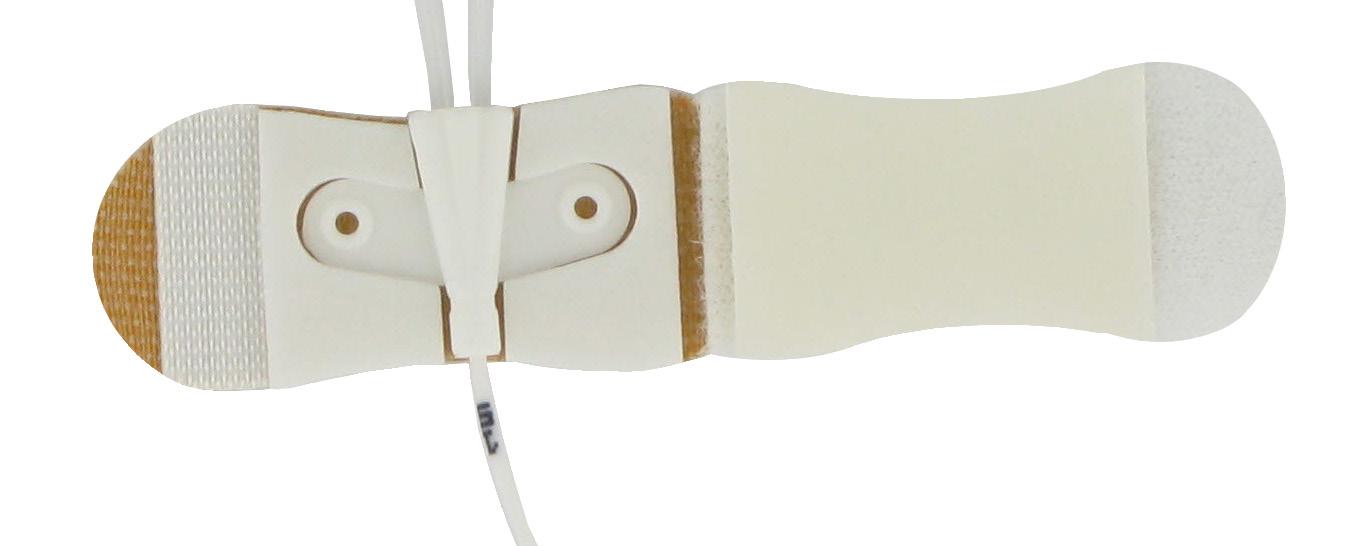
For arterial access For venous access
Short IV cannulas are used in neonates for short time IV access, and infusion of non-irritant fluids hub or lateral port with ISO standard colour coding
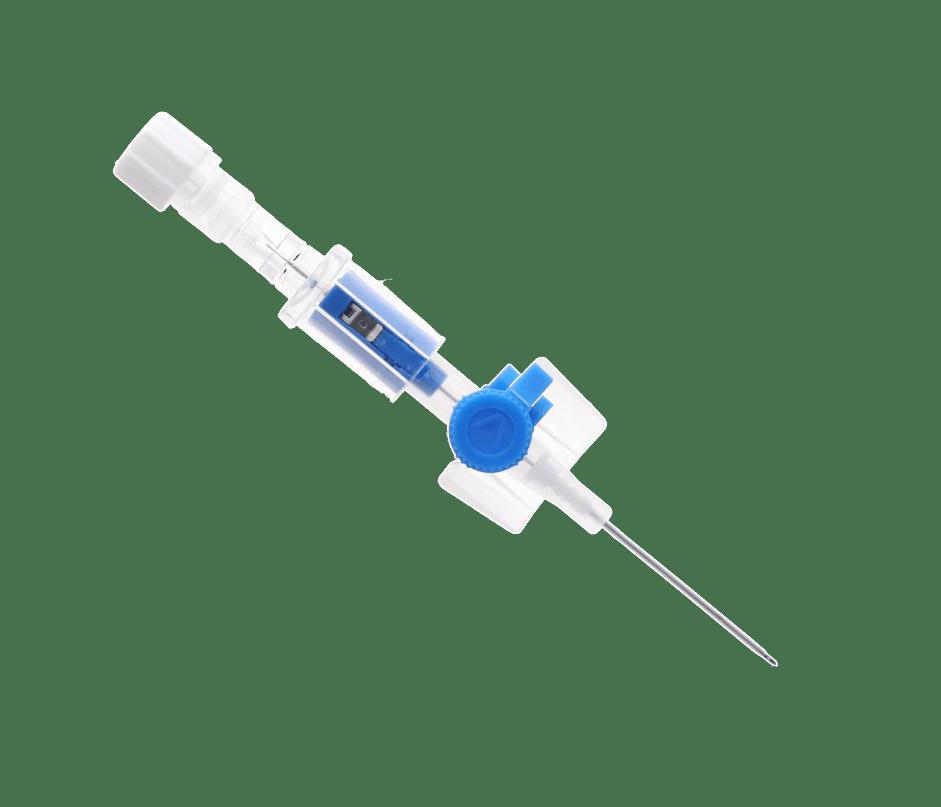
engineered passive safety system to protect the health-care workers from accidental infections due to needlestick injury or blood splatter.
vygonüle V – STANDARD SHORT I.V. CANNULA WITH LATERAL INJECTION PORT
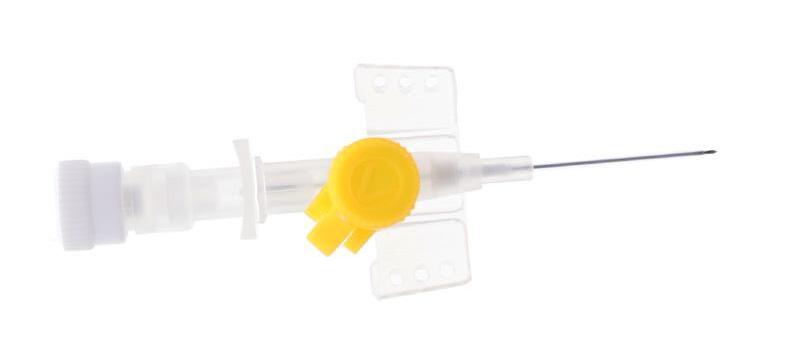
Internal needle guide With a transparent hub to visualise the blood reflux when puncturing
vygonüle V is a ported peripheral IV cannula, intended to be used for I.V. therapy
Supplied in a rigid blister pack
intraflon 2 – PTFE I.V. CANNULA
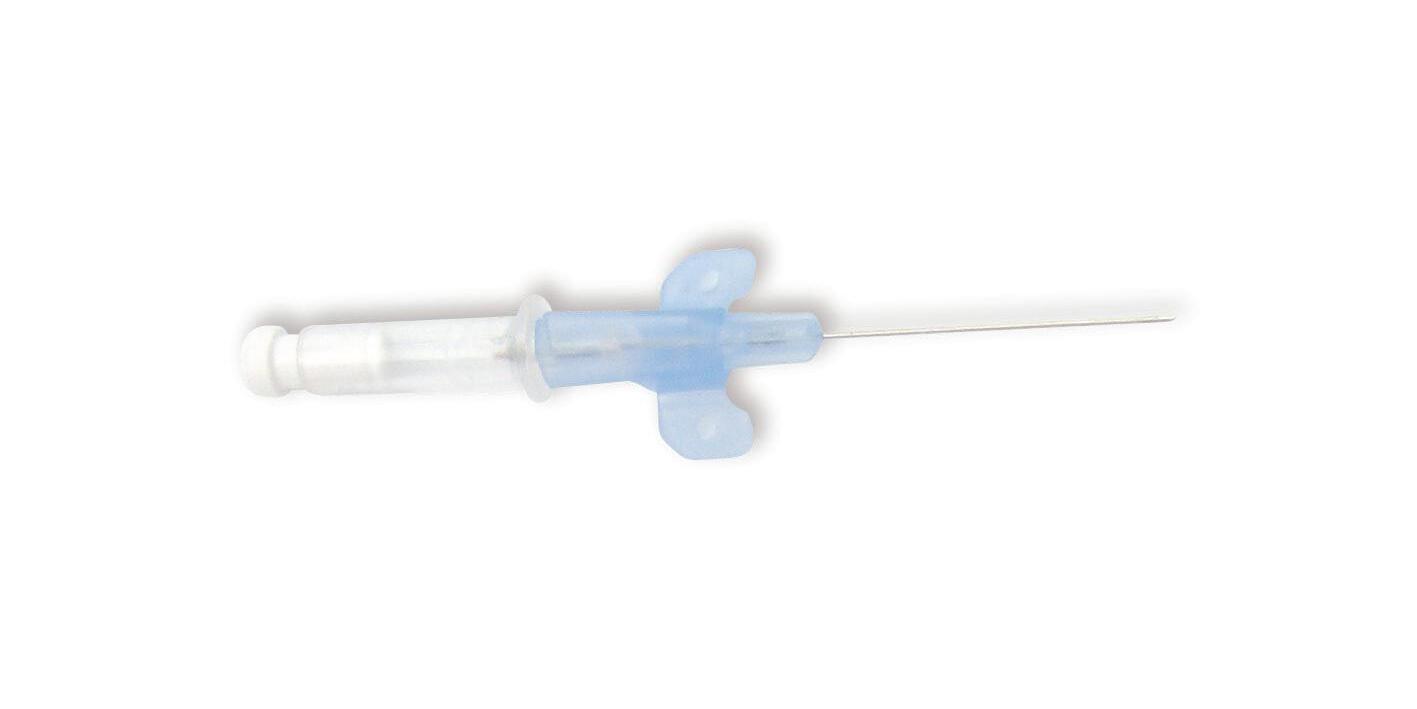
colour coding
Sharp punctured needle
Transparent PTFE cannula With winged hub and internal needle
intraflon 2 is intended to be used for short IV therapy.
Supplied in a rigid blister pack
Indications
For venous access
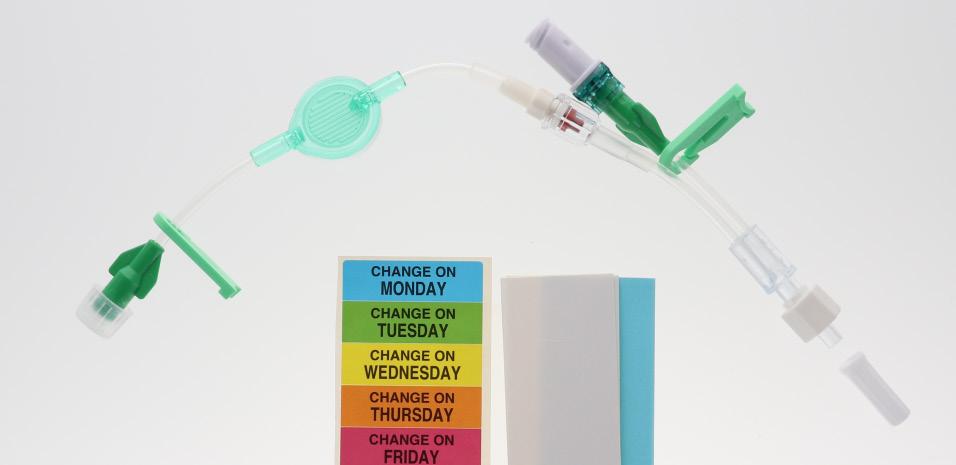
Vygon offers a complete range of modular I.V. accessories specially designed for neonates and paediatrics. It is intended to be used for multiple simultaneous infusion of drugs in neonates via a single vascular access device . It allows the practitioner to select the most suitable I.V. administration system, removing the need for additional 3-way taps, ramps, and extension lines, therefore reducing the risks associated with multiple connections.
The range incorporates
• Filters
• Needless connectors
• Anti-reflux valves
• Miniature slide clamps
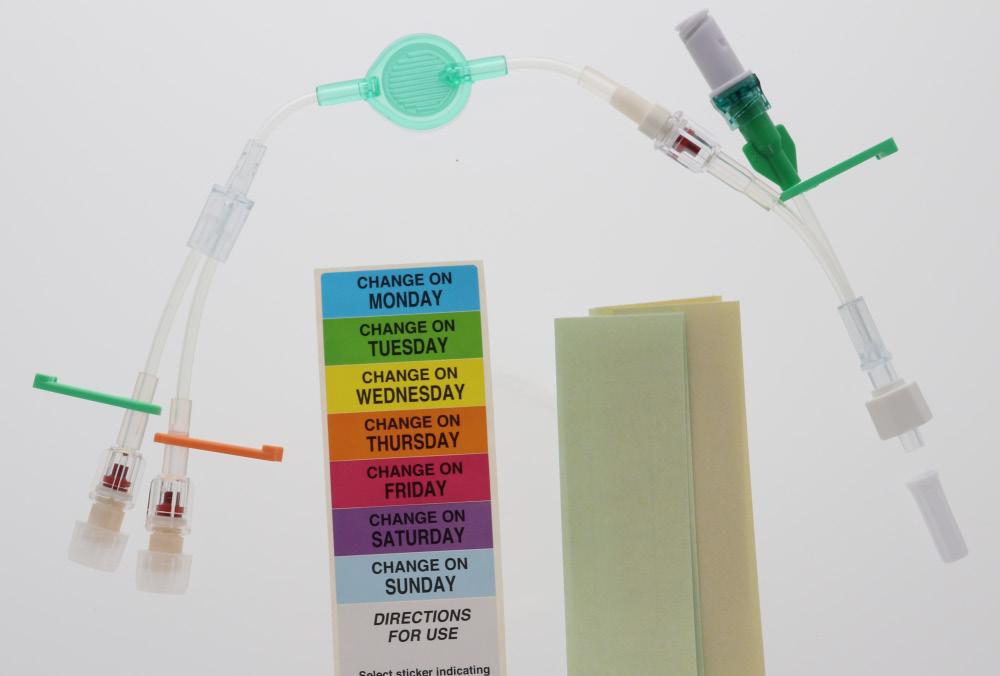
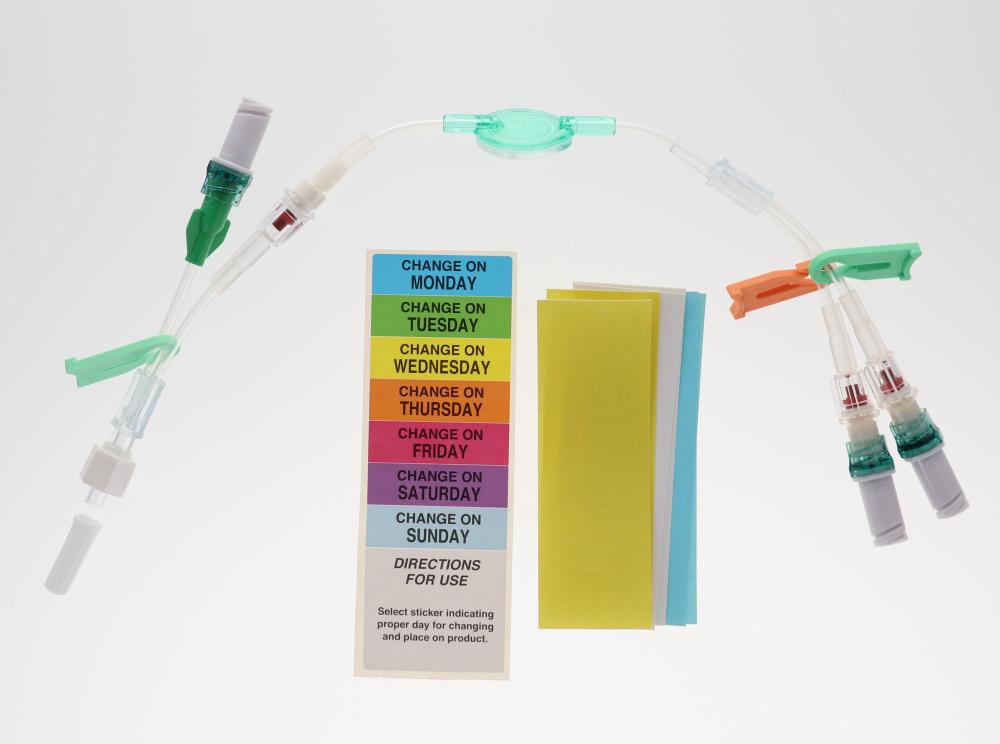
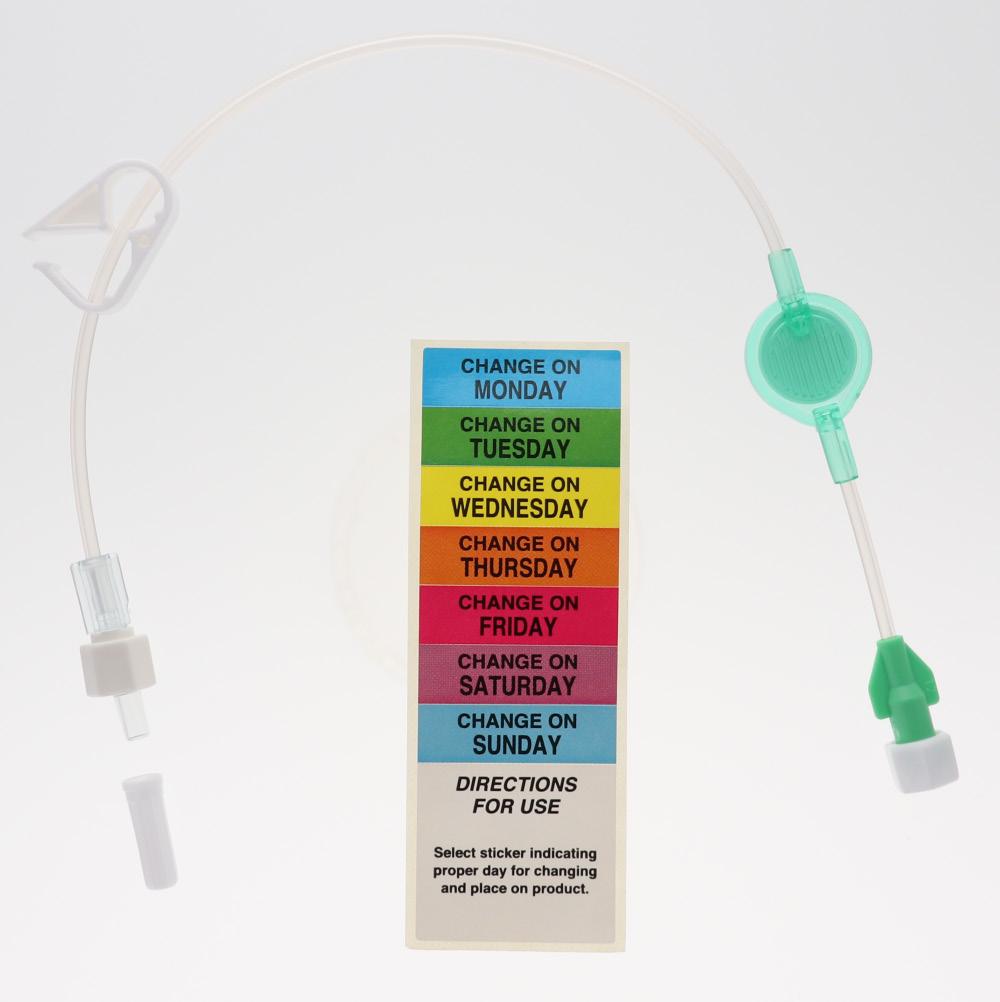
These extension lines (Code: 836.201, 836.202, 836.203, 836.204, 836.205, 836.210, 807.204, 807.205, 807.504, 807.506) are supplied with labels indicating the day they must be replaced.
Features/benefits
• Eliminates need of 3-way taps, ramps and extension lines
• Reduced dead space: small volume bolus dose medication very close to the VAD.
• Lightweight: reducing the drag on babies’ limbs.
• Ensures the elimination of backtracking and easy line management. (anti-reflux valves)
• Protect against needle stick injury (bionector)
• Minimum number of connections reduces contamination via manipulation, reduces the risk of accidental disconnection.
• Protect against contamination: Ensures maximum protection for your babies, from bacteria, particulate matter, air embolism and endotoxins. Ensuring safe, reliable infusions.
• Simply identify each infusion and filter dwell time thanks to colour coding: - by clamps - by (day change) labels
• Comfort for the nurses
• Ensures, safe identification of all administration sets
• Additionally highlights when the set requires changing
• Labels can be used to identify the lines: Easy to recognise - by size of filters colour
• Bacterial 0,22 µm green
• Lipidic 1,2 µm blue
The filter aims to remove inadvertent microbial contaminants, associated endotoxins, particulates, and air.
The neonatal filters are equipped with low-blinding protein membrane.

h
Amino-acid and endotoxin filters can stay in place up to 96 hours and are colour coded green. The filters are not suitable for blood, blood products or lipids. The products are available in a supple blister
•
Ready-Set “Feed” is an extension tube with additional access port. It is intended to be used for infusion via the 96 hours filter.
Lumen 2
Close-system needless bionector) and can be used for either for intermittent injections of medication or for continuous
Lumen 1
0,22 micrometer endotoxin bacterial, particulate, and air filter, 96 hours with low protein adsorption membrane
Luer Female Connector
Connector
For venous access Protector
• READY-SET «COMPLETE»
Ready-Set “Complete” is an extension tube with 2 access ports. It enables the user to run multiple infusions via the 96 hours filter without the need for additional 3 ways taps and connections.
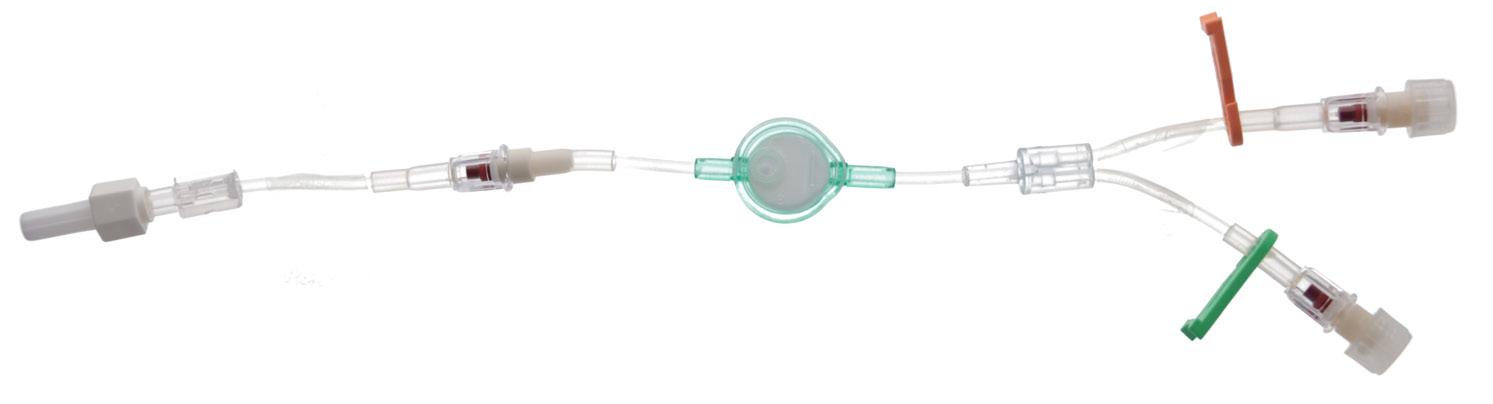
Non-return valve
Polyurethane extension tube with 2 access incorporing nonreturn valves and clamps
• SAFE-T-PIECE
Safe-T-Piece is a Luer-locking «T» piece extension set. It is intended to be used for infusion via 96 hours filter.

Proximal end Closed-system needless connector (bionector)
Distal end is a “T” piece With a male Luer-lock hub on a side, a latex-free injection membrane on the other side
Ready-Set “Unite” is an extension tube intended to be used for multiple infusions via the 96 hours filter, featuring 2 access ports and additional access port.
Main line
2 access ports incorporating non-return valves and clamps
Polyurethane
extension tube with 3 access ports
Non-return valve
On the distal part of the line avoids the occlusion of the filter by reflux of lipidic solutions if the other line is used for that purpose
Closed-system needless connector (bionector) and clamp, can be used either for intermittent injections of medication or for continuous infusions
• READY-SET «UNITE» WITH GLUED bionector
Ready-Set “Unite” is a polyurethane extension tube with 2 access ports and additional access port. It is intended to be used for infusion via the 96 hours filter.
Lumen 2
Closed-system needleless connector (bionector) and clamp, can be used either for intermittent injections of medication or for continuous infusions
Non-return valve on the distal tip
Of the line avoids the occlusion of the filter by reflux of lipidic solutions if the other line is used for that purpose
Main line 2 access ports incorporating closed-system needleless connectors (bionector), non-return valves and clamps
• FILTER-A-LINE – PAEDIATRIC/NEONATAL ENDOTOXIN RETENTIVE I.V. FILTER
Paediatric/Neonatal endotoxin retentive I.V. filter is intended to be used for the removal of inadvertent microbial contaminants, associated endotoxins, particulates, and air.
Locking device
• FILTER-A-LINE – PAEDIATRIC ENDOTOXIN RETENTIVE I.V. FILTER WITH INTEGRAL EXTENSION LINE
Paediatric/Neonatal endotoxin retentive I.V. filter with integral extension line is intended to be used for the removal of inadvertent microbial contaminants, associated endotoxins, particulates, and air
Specially adapted for neonates and paediatric patients, Lipid-Filter is an add-on device for I.V. catheters intended to be used for lipidic solutions. Not compatible with blood. 24 h
Specially adapted for neonates and paediatric patients, Lipid-Filter is an add-on device for I.V. catheters intended to be used for lipidic solutions. Not compatible with blood.
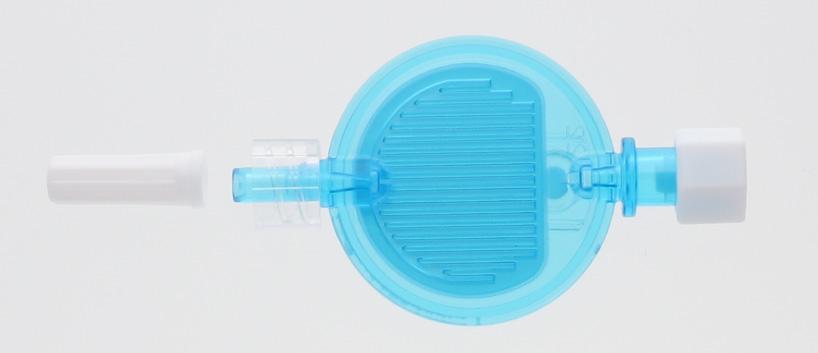
Filter-A-Line is intended to be used for the administration of lipids via a syringe pump. Not compatible with blood. It can be connected to the Bionector port of Ready-Set “Feed” and “Unite”.
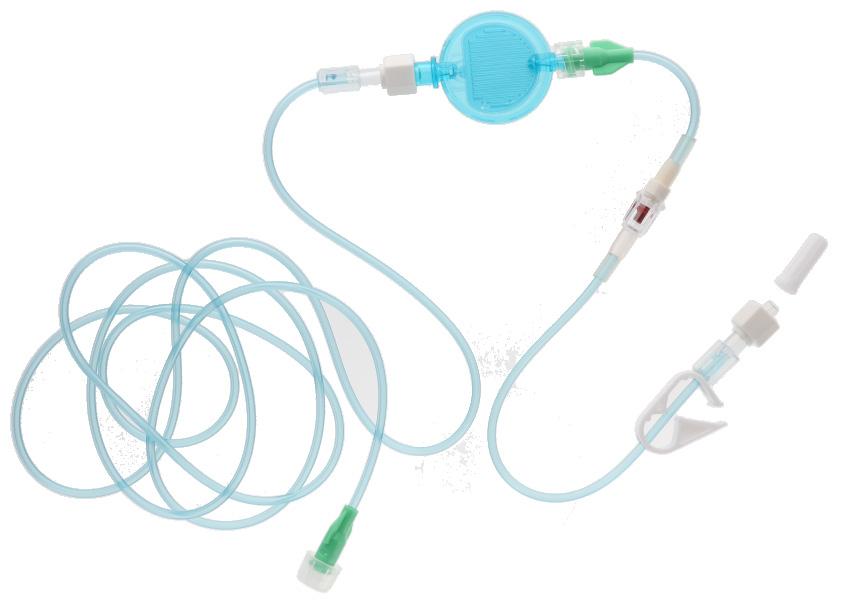
• readyset readyset is an extension tube intended to be used for multiple infusions via the 24 hours filter.
extension tube
2 access ports fitted With clamps, non-return valves and a 1.2 micrometre lipid, bacterial particulate and air filter, 24 hours for lipidic solutions Indication
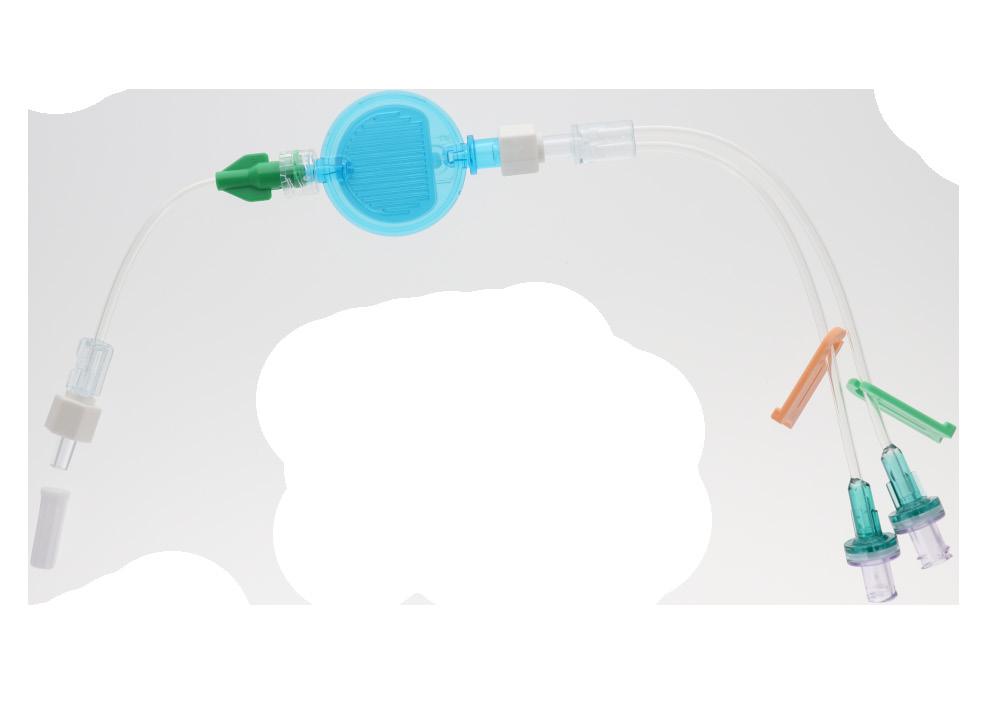
• readycheck 2, code 836.203
readycheck 2 is a double lumen extension tube. It has been designed to add versatility to the readyset range. It can be used in conjunction with readyset «Feed», «Complete» or «Unite», thus offering additional access.
Two identical extension lines 10 cm long, with non-return valves and clamps

Presented in a supple blister.
For venous access
Polyurethane double-lumen extension tube
neosafe
neosafe is a singled winged needle intended to be used for safe venous blood sampling in neonates and infants.


• No extra sharp end
Single wing
• Good handling
• Easy to identify in the cot
• Color-coded needle size
• Maximised needle stability (no wind recoil)


Sharp bevel
• Easy venous puncture
• Perfectly aligned with wing (bevel guide)
• Direct blood collection in container
Presented in a rigid blister pack
For venous access
Abbreviations
PVC: Polyvinyl chloride
PUR: Polyurethane
SIL: Silicone
PICC: Peripherally Inserted Central Catheter
CRBSI: Central-venous-catheter bloodstream infections
TPN: Total Parenteral Nutrition
G: Gauge
RNA: ribonucleic acid
IV: Intravenous
Glossaire
- Short term: <30 days
- Long term: > 30 days
References
1. Smirk C, Soosay Raj T, Smith AL, Morris S. Neonatal percutaneous central venous lines: fit to burst. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F298-300. doi:10.1136/adc.2008.147900
2. VYGON. Néonatologie - Cathéters en néonatolgie. https://www.vygon.fr/produits/?prd=00125430&doc=1554
3. Comes AM. Chemical Resistance and Stress Cracking in Polyurethanes. :8.
4. Chapter 4: Central Venous Catheters: Materials, Designs, and Selection. 2003rd ed. Thieme Verlag; 2003. doi:10.1055/b-0034-51397
5. Polyurethane vs. Silicone PICC Catheters - PDF Free Download. coek.info. Accessed February 21, 2022. https://coek.info/ pdf-polyurethane-vs-silicone-picc-catheters.htmL
6. Bouissou A, Rakza T, Storme L, et al. Le cathétérisme veineux ombilical et épicutanéocave chez le nouveau-né. Archives de Pédiatrie. 2008;15(9):1447-1453. doi:10.1016/j.arcped.2008.06.023
7. LAFTOUHI K. Catheter Ombilical En Néonatologie : Complications Immédiates, à Moyen et à Long Terme (A Propose de 55 Cas). Université Sidi Mohammed Ben Abdellah; 2013.
8. Anderson J, Leonard D, Braner DAV, Lai S, Tegtmeyer K. Umbilical Vascular Catheterization. N Engl J Med. 2008;359(15):e18. doi:10.1056/NEJMvcm0800666
9. Ainsworth S, Furness J, Fenton A. Randomized comparative trial between percutaneous longlines and peripheral cannulae in the delivery of neonatal parenteral nutrition. Acta Paediatrica. 2007;90(9):1016-1020. doi:10.1111/j.1651-2227.2001. tb01357.x
10. Dumpa V, Avulakunta ID. Umbilical Artery Catheterization. In: StatPearls. StatPearls Publishing; 2022. Accessed February 21, 2022. http://www.ncbi.nlm.nih.gov/books/NBK559111/
11. Gomella’s Neonatology: Management, Procedures, On-Call Problems, Diseases, and Drugs, 8e | AccessPediatrics | McGraw Hill Medical. Accessed February 21, 2022. https://accesspediatrics.mhmedical.com/book.aspx?bookID=2762
12. Ronco, Bellomo, Kellum, Ricci. Critical Care Nephrology. Elsevier; 2019. doi:10.1016/C2015-0-00412-9
13. Rodriguez OQ. Department of Pediatric Newborn Medicine. :10.
14. Scott-Warren V, Morley R. Paediatric vascular access. BJA Education. 2015;15(4):199-206. doi:10.1093/bjaceaccp/mku050