CARDIOVASCULAR CATALOGUE
Sterile





Pierre Simonet founded Vygon in France in 1962. He was one of the first to introduce the concept of disposable medical equipment. Shortly thereafter, the company established subsidiaries in Europe, the United States, India, South America, and more. Today it is present worldwide. Vygon also relies on many production units, starting with its first plant in France and continuing on to its latest, brand-new plant that opened in Portugal in 2015.



Today the company is present in 100 countries and works for 4,000 clients worldwide. We owe this rapid expansion to all of our clients, both old and new, and to our employees’ capacity for innovation. Thanks to their commitment, we are able to offer the products and devices that healthcare professionals expect, and that provide their patients with relief. We are also grateful for all of our employees' positive attitudes, whether they work in our laboratories, in our plants, or in our subsidiaries. Everyone is devoted to their jobs in the service of healthcare. Today, our development and our results have led us to prioritise an even more efficient approach for project management and to structure our activity in five business units. Since our product range is managed in a decentralised fashion, our staff members are able to concentrate on more specific projects, shorten the innovation cycles, and be more responsive in managing their relations with you. Retaining the values, vocation, and professional approach that are unique to Vygon, and intensifying our inventive spirit, are the very objectives of this new organisational structure.
Stéphane Regnault CEOThe inflation device matching the majority of balloon catheter
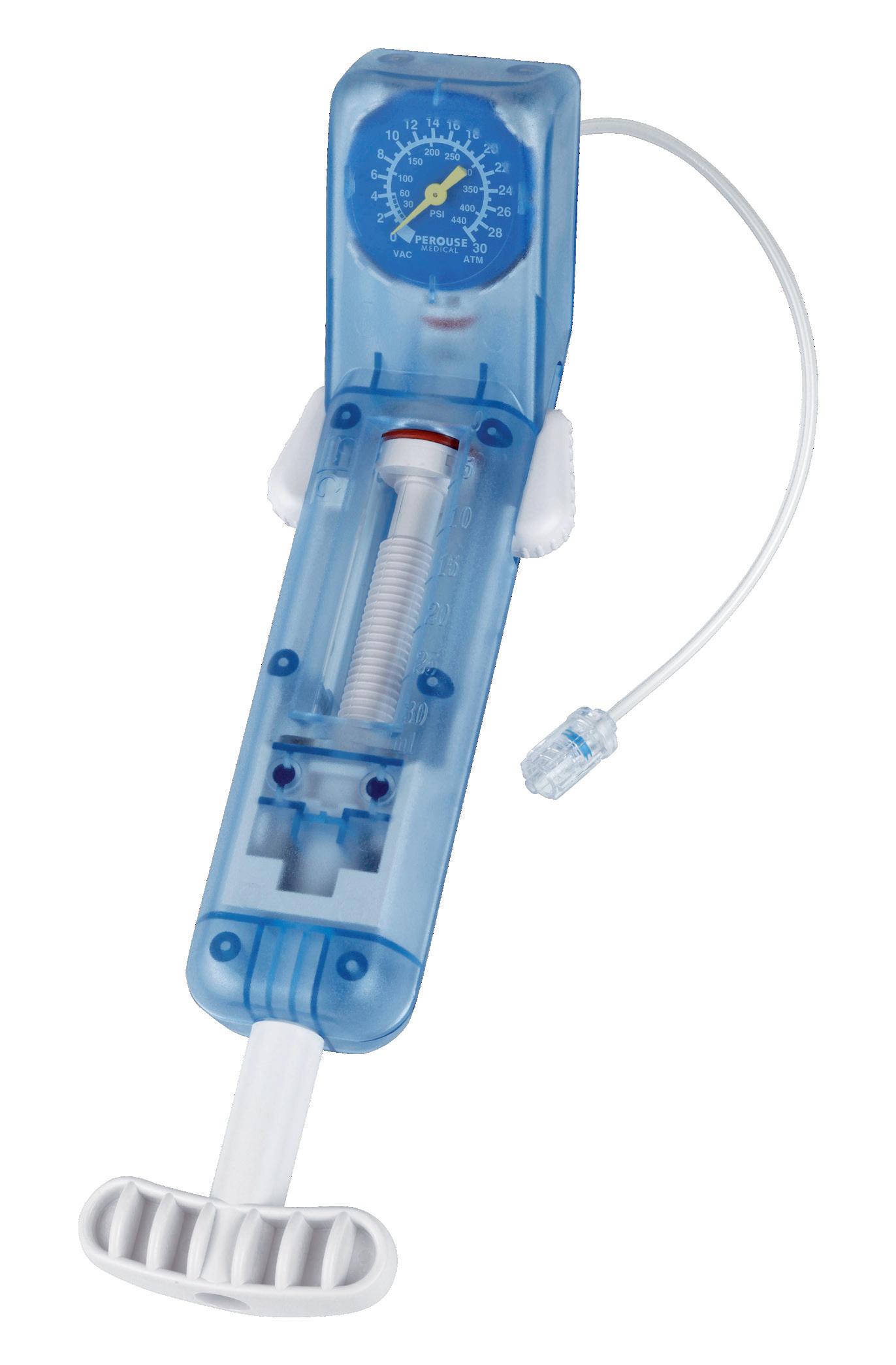
• 30 atm / 3040 kPa pressure gauge
• 30 cm3 syringe
• Ergonomic handle
BODY
HANDLE
PRESSURE GAUGE
BUTTONS
PRESSURE GAUGE
• 30 atm / 3040 kPa
- suitables for most of the procedures
BODY AND BUTTON
• 30 cm3 syringe
- small to big balloons (PTA & PTCA)
• Square shaped
- stable
• Automatic cam locking system
- easy handling
• Clear material
- easy air bubbles detection and removal
HANDLE
• T-shape handle
- high torque
- easy deflation
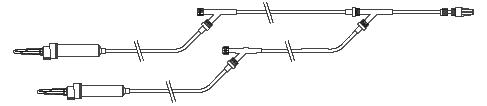
Y connector push-pull Metallic guidewire introducer Alligatork torque device Trefoil 3-way stopcock 0185QL 30 atm / 3040 kPa inflation device + PTCA set 3 items
Trefoil 3-way stopcock myshell lite Y connector 9F / 3mm, 20cm PVC connection line - 3-way stopcock
Metallic guidewire introducer Alligatork torque device
Please contact VYGON for non sterile and OEM products. Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
A compact and ergonomic inflation device
• 30 atm / 3040 kPa pressure gauge
• 20 cm3 syringe

• 90° rotary pressure gauge BUTTON BODY
PRESSURE GAUGE HANDLE
PRESSURE GAUGE
• 30 atm / 3040 kPa
- one product for all procedures
• 90° rotating manometer
- “custom” handling
• Colored pressure scale
- easy reading
BODY AND BUTTON
• Unique mechanism for rapid pressure increase
- efficiency - fast procedures
• 20 cm3 graduated syringe
- compact system
• Grip design
- good handling
• Clear material
- easy air bubbles detection and removal
HANDLE
• Ergonomic design
- easy handling
• Progressive resistance while inflating
- pressure feedback
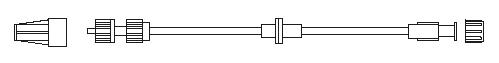
myshell lite Y connector 9F / 3mm 20 cm PVC connection line - 3-way stopcock metallic guidewire introducer alligatork torque device
myshell lite Y connector 7F / 2,33 mm 20 cm PVC connection line - 3-way stopcock
metallic guidewire introducer twisting torque device
Y connector push-pull 7 F / 2,33mm
20 cm PVC connection line with 3 way stopcock
metallic guidewire introducer
3 way stopcock
atm / 3040 kPa
twisting torque device 0218KR
set 3 items
Y connector push-pull 7 F / 2,33mm
20 cm PVC connection line with 3 way stopcock
metallic guidewire introducer
Twisting torque device
Please contact VYGON for non sterile and OEM products. Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
Radial Compression Device
• High visibility of the puncture site
• Targeting the radial artery
• Precise adjustment and control of compression level
• Secured and precise decompression
• Simplified handling: no syringe required for compression & decompression
• Easy stock management: only one reference
• Adapter pad for small wrists (circumference ≤ 16 cm) supplied with seal one®

HIGH VISIBILITY OF THE PUNCTURE SITE
• Transparent compression pad
• Central marker on compression pad
TARGETING THE RADIAL ARTERY
• Specific design of the compression pad
> Enabled venous return (1)
SECURED WRIST STRAP BY A TAB
PRECISE ADJUSTMENT AND CONTROL OF COMPRESSION LEVEL
• Compression / Decompression knob with scale
SECURED & PRECISE DECOMPRESSION
• Secured button
> Limited risk of unintended decompression
• Decompression / compression knob with scale
> Step by step decompression
EASIER MONITORING OF DECOMPRESSION PROTOCOL
• Display of seal one® positioning time
SIMPLIFIED HANDLING: NO SYRINGE REQUIRED FOR COMPRESSION & DECOMPRESSION
EASY STOCK MANAGEMENT: ONLY ONE REFERENCE:
• Suitable for right or left wrist
• Adjustable wristband
• Adapter pad for small wrists (circumference ≤ 16 cm) supplied with seal one®
Reference Description Packaging
0259NA Radial compression device 30/box
Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459)
(1) Jirouš S et al_Patent haemostasis and comparison of two compression devices after transradial coronary catheterization and intervention. Cor et Vasa. 2018; Volume 60, Issue 2: Pages e122-e126.
Aspiration catheter

OPTIMIZED TIP DESIGN
• Smooth tip designed to increase effortless deliverability
OPTIMAL TRACKABILITY
• Unique hydrophilic coating
EXCELLENT PUSHABILITY
• With an innovative shaft
KINK RESISTANCE
• Specifically designed for aspiration catheter
• cathfish 6F (2mm) = 0301NA
• cathfish 7F (2,33mm) = 0301ND
Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
The haemostasis valve designed for your hand
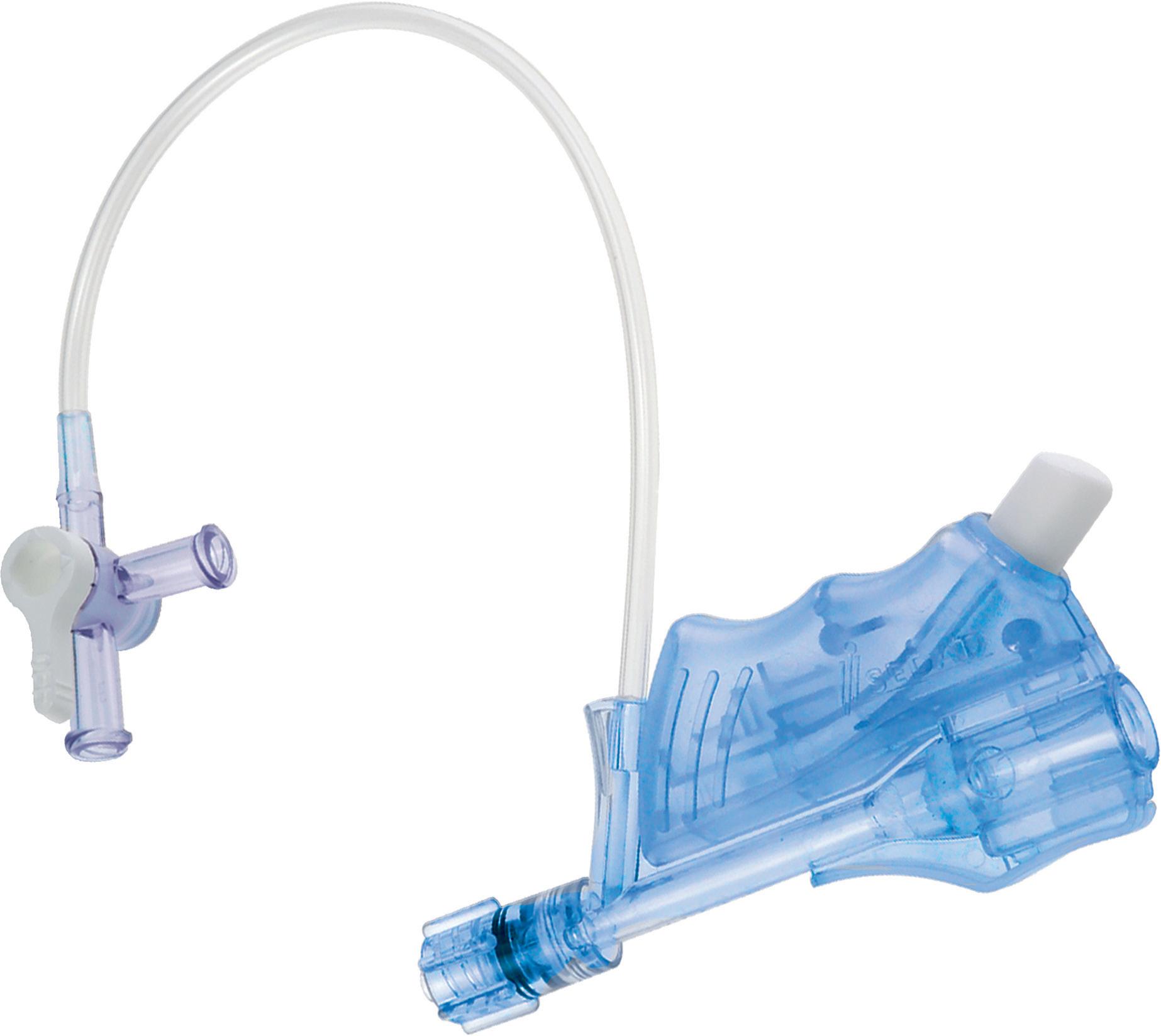
• Push-push closing system
• Double silicone valve
• Inner diameter of 7F / 2,33 mm or 9F / 3mm
CONNECTION LINE AND STOPCOCK
BUTTON
DOUBLE SILICONE VALVE
CONNECTION LINE AND STOPCOCK
• 20 cm connection line
• Stopcock
- Allows drug injection and blood aspiration
BODY AND BUTTON
• Push-push system
- single-handed use
• Compact system
- ergonomic handling
SILICONE DOUBLE VALVE
• Wire and catheter control through closed valve
- minimum handling
• Soft material
- keeps the integrity of the coated catheters
• 7F / 2,33 mm or 9F / 3 mm option
- 9F / 3mm suitable for kissing
Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
squyd
Push-pull haemostasis valve
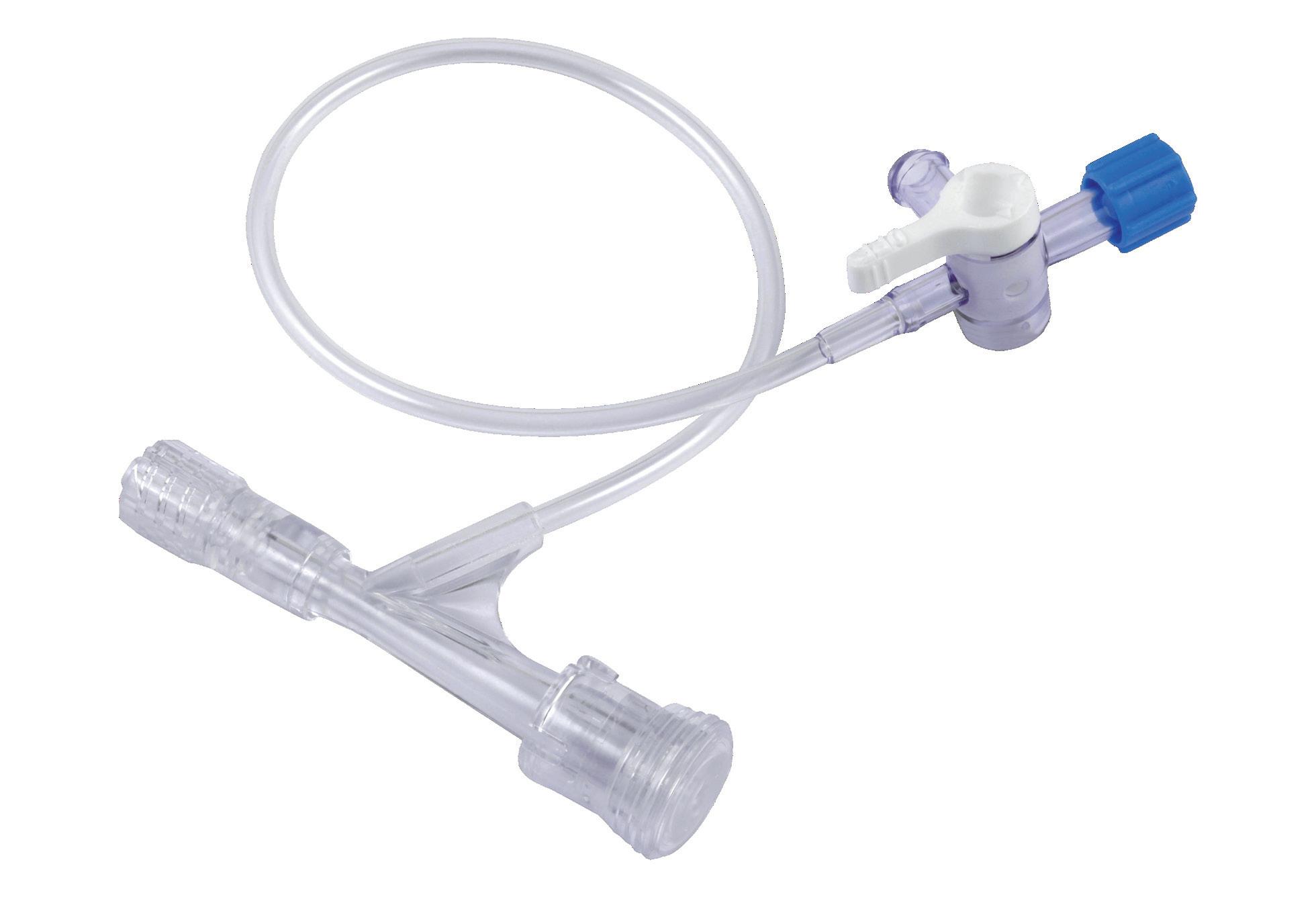
• Push-pull system
• Silicone valve
• 7F / 2,33 mm inner lumen
CONNECTION LINE AND STOPCOCK
PUSH-PULL SYSTEM
• Single-handed operation
• Minimized devices manipulation with sealed valve
• Limited blood loss
SILICONE VALVE
• Non-aggressive material
• Preserves catheter coatings
WITH OR WITHOUT CONNECTION LINE
• Numerous configurations adapted to your needs
(1) Haemostasis valve strength measured in vitro applying the injection pressure through the connection line.
valve with a 20 cm connection line
introducer
valve with a 20 cm connection line
introducer
Twisting torque device
valve with a 50 cm connection line
00270NC
00270ND
00270NE
introducer
valve without connection line
introducer
peelable insertion tool
valve with a 50 cm connection line Guidewire introducer
Twisting torque device
Syringes for contrast media injectors
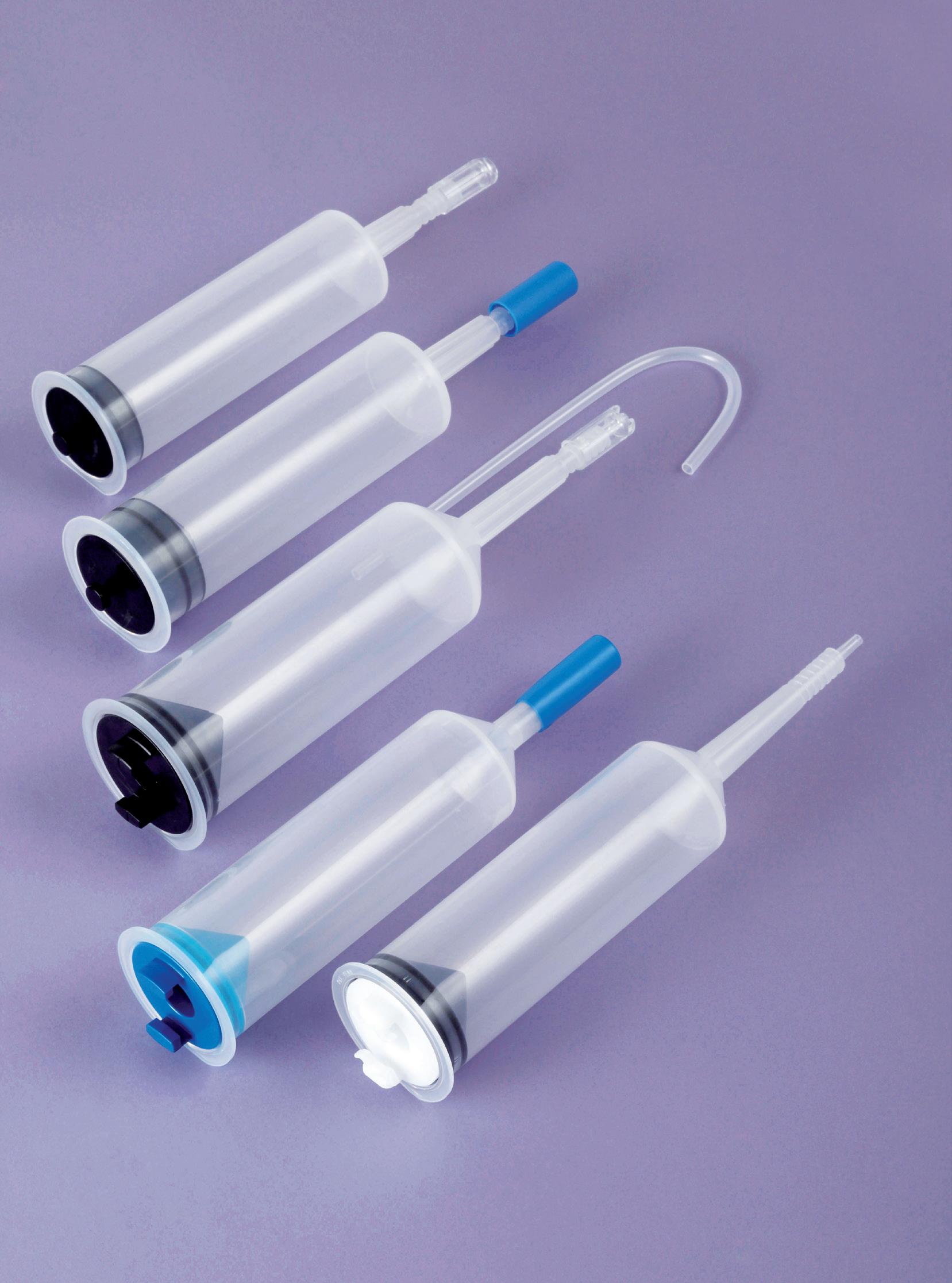
RESISTANT TO 300 PSI (21 BARS /2069 KPA) OR 1200 PSI (83 BARS / 8274 KPA)
• For low or high pressure injections
CAPACITY
• From 130ml to 200ml
Medrad injectors
Medtron injectors
Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
Connection lines
• Wide range of connection lines to meet all your needs

DESIGNED FOR 1200 PSI (83 BARS / 8274 KPA) OR 300 PSI (21 BARS / 2069 KPA)
• For low and high pressure injections
LENGTHS
• From 30 cm to 200 cm
NYLON BRAIDED, PU OR PVC
ROTATING, FIXED OR SPINLOCK ADAPTORS
High pressure lines (1200 psi / 83 bars / 8274 kPa)
References
0209NH 100
0209NK 120
0209NL 150
0209NM 180
Low pressure lines (300 psi / 21 bars / 2069 kPa)
0163PQ 50 0163PR 75 0163PM 100 0163PS 150
Polyvinyl chloride (PVC) lines + Spinlock male Luer-lock Inner diameter: 2.9mm
Polyurethane (PU) lines
Rotating male Luer lock + Inner diameter: 1.7mm
Low pressure coiled lines (300 psi / 21 bars / 2069 kPa)
References Length (cm)
0163QA 150
0163QD 150
Polyvinyl chloride (PVC) line
Fixed coiled male Luer-lock + Inner diameter: 1.5mm
Polyvinyl chloride (PVC) line with non-return valve
Fixed male Luer-lock + Inner diameter: 1.5mm
The CE conformity assessment has been conducted by GMED (0459). Please contact VYGON for non sterile products.
Procedures made easier with sets designed for injections

• Filling and injection sets
• Patient lines with non-return valves
Filling and injection set (maximum pressure 300 PSI / 21bars / 2069 kPa)
References Description
For double head injector:
• 4 non-return valves
Packaging
0170PS
- 1 contrast media line 100cm with a drip chamber + tubing of 10cm
- 1 saline line 100cm with a drip chamber + tubing of 40cm
- 1 patient line 10cm
25/box
0170NQ
For double head injector: - patient line 150 cm
- syringe to syringe line 50 cm
Patient line (maximum pressure 300PSI / 21bars / 2069 kPa)
References Description
0170NN
0170NT
• 1 non-return valve
- patient line 60cm
• 1 non-return valve
- patient line 120cm
Packaging
50/box
• manifold
• alligatork
Torque device

• Metallic Guide Wire introducer

manifold
0170TJ Three ports manifold RIGHT OFF: 600 PSI (41,3 bars / 4137 kPa)
0170SQ Three ports manifold RIGHT ON: 600 PSI (41,3 bars / 4137 kPa)
torque device
Metallic Guide Wire introducer
Please contact VYGON for non sterile products. The CE conformity assessment has been conducted by GMED (0459).
EN 13795:
• Two levels of performance:
• Standard performance : classification of medical devices used in invasive surgical interventions satisfying minimal performance requirements.
• High performance : classification of medical devices used in invasive surgical interventions satisfying maximal performance requirements.
• Two levels of requirements depending on the area of product usage:
• Less critical area: this area is less likely to be involved in a procedure where the transfer of infectious agents could be transferred to or from the wound.
• Critical area: this area has a higher risk of being involved in a procedure where the transfer of infectious agents could be transferred to or from the wound.
With its drapes range, Vygon drives to offer drapes meeting the requirements of “ High performancecritical area” in all areas of the products. Thus, the level of safety measured in any area of a Vygon drape is greater than the level of safety required for a “High performance –critical area” draping.
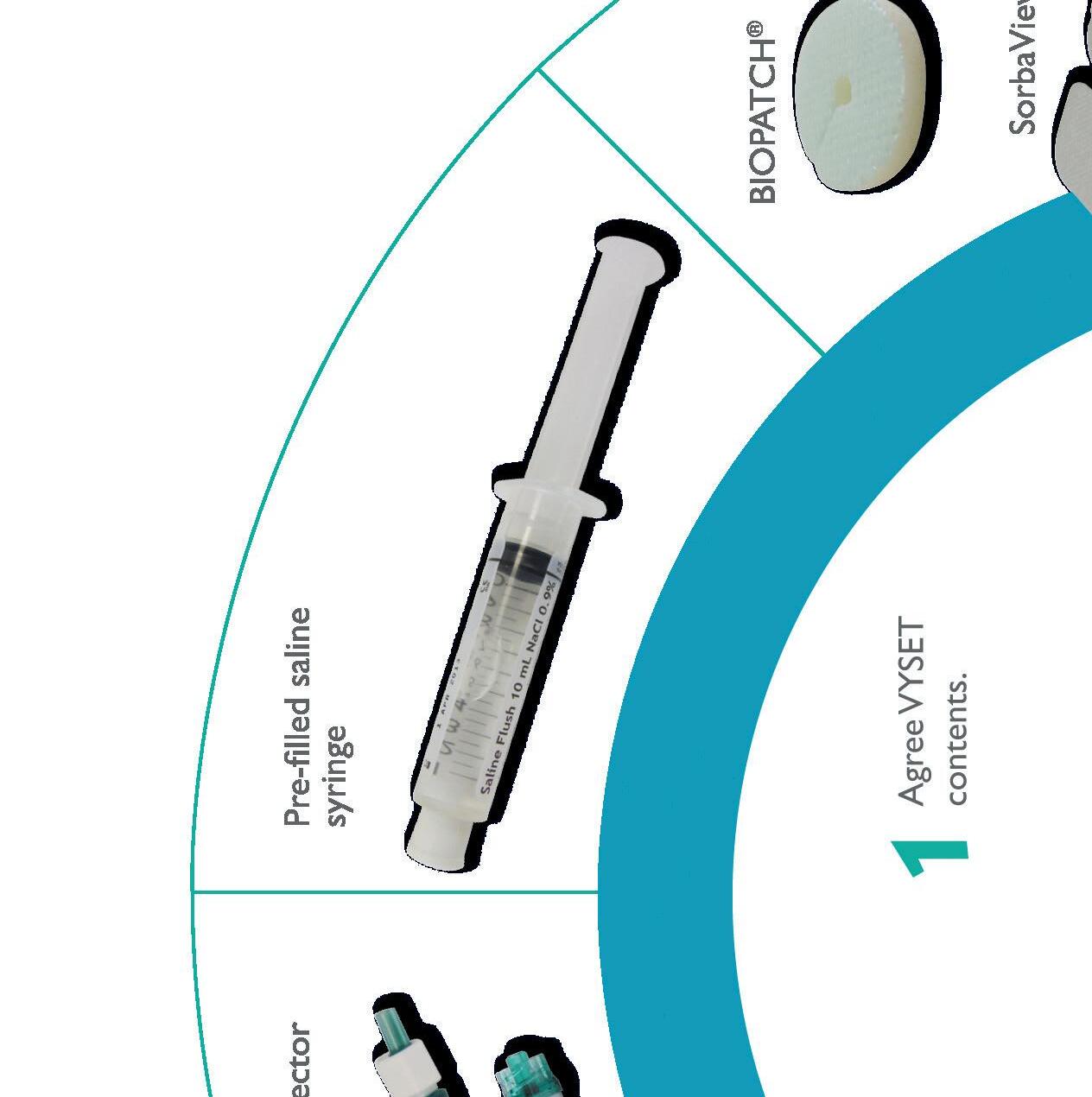
Find our drapes to add in Vyset custom sets page 64
smartpack Sets with most of the necessary components for angiography

smartpack Sets with most of the necessary components for angiography +
*The drape inside the 3 sets is the drape code 79507008 (see next page)

Drapes are impermeable on the entire surface and have a central band highly absorbent.





* Discover our Vyset customed sets concept page 64

ANGIODRY 1:
Large size to match the dimensions of new tables
79507020
240 cm x 400 cm
4 openings: - aligned
- ø 9 cm and 6 x 8 cm
ANGIODRY 2:
High level of absorption with a small footprint and an easy draping
79507030
240 cm x 360 cm
4 openings : - aligned
- ø 9 cm and 6 x 8 cm
ANGIODRY 3:
Enhanced absorption in the middle of the drape
79507040
220 cm x 360 cm
4 openings : - aligned
- ø 9 cm and 6 x 8 cm
79507021
240 cm x 400 cm
4 openings : - staggered
- ø 9 cm and 6 x 8 cm
79507031
240 cm x 360 cm
4 openings : staggered
- ø 9 cm and 6 x 8 cm
79507040
220 cm x 360 cm
4 openings : - staggered
- ø 9 cm and 6 x 8 cm
• poly patch ®
• polyarch ®






• poly branch ®
• poly these®
• poly maille® C




• poly maille® extra thin







Woven polyester vascular graft
polythese IC/ICT vascular prosthesis is indicated for replacement or bypass of arteries presenting aneurysms or obliterative arterial diseases. Its indication is restricted to thoracic (polythese® ICT), abdominal and peripheral surgery, not involving the crossing of the knee joint.

Straight or bifurcated
Woven polyester
Water permeability less than 10 mL / cm2 / min at 120 mm of mercury
Black guide lines
• Good suturability and handling;
• Dimensional stability: Good resistance to circumferential dilatation(1)
• Patented technology of impregnation: Collagen CXE, formaldehyde and glutaraldehyde free(2)
• External velvet-like surface: support for neighbour tissues attachment and periprosthetic encapsulation
• Smooth and texturized endoluminal wall: support for pseudo-intimal cells ingrowth
• Two black guide lines: single and double lines opposite each other over the entire length of the prosthesis to avoid « twistting » during the procedure
polythese® IC straight tube
polythese® ICT straight thoracic tube
* Only on request.
(1) Rapport RRD-0101-01 rev00 - 29/10/2012.
(2) LNE certificate n°F014288 (2005): analysis of Perouse Grafts: formaldehyde free and glutaraldehyde free. Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
Woven polyester vascular graft
polythese® vascular prostheses are indicated for replacement or bypass of arteries presenting aneurysms or obliterative arterial diseases. The polythese® IC-3GL prosthesis is especially indicated in aortic root and ascending thoracic aorta surgery


Woven polyester
Impregnated with type 1 bovine collagen
Water permeability less than 10 mL / cm2 / min at 120 mm of mercury
3 black guidelines at an equal angle of 120° over the circumference
• Good suturability and handling;
• Dimensional stability: Good resistance to circumferential dilatation(1)
• Patented technology of impregnation: Collagen CXE, formaldehyde and glutaraldehyde free(2)
• External velvet-like surface: support for neighbour tissues attachment and periprosthetic encapsulation
• Smooth and texturized endoluminal wall: support for pseudo-intimal cells ingrowth
polythese® IC-3GL
* Only on request.
(1) Rapport RRD-0101-01 rev00 - 29/10/2012.
(2) LNE certificate n°F014288 (2005): analysis of Perouse Grafts: formaldehyde free and glutaraldehyde free. Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
polythese® polybranch vascular prostheses are indicated for replacement or bypass of the thoracic aorta

Water permeability less than 10 mL / cm2 / min at 120 mm of mercury
Pre-sewn multi branch intended for introduction of extracorporeal circulation
• Good resistance to circumferential dilatation1
• Makes surgical technique easier by increasing reliability and reducing operating time2, 3
• Help with ECC to create antegrade flow
• Fitted for ECC cannulas
• Low porosity: < 10 mL/min/cm²/120mmHg
• Patented technology of impregnation: Collagen CXE, formaldehyde and glutaraldehyde free
1. Rapport RRD-0101-01 rev00 – 29/10/2012
Also available, on request: - body diameter from 16 to 38mm, with ECC side branch of 8mm. - body diameter from 16 and 18mm, with ECC side branch of 10mm.
Please read carefully the Instructions for Use. The CE conformity assessment has been confucted by GMED (0459).
Woven polyester vascular graft
polythese® polyarch vascular prostheses are indicated for replacement of the ascending aorta and aortic arch in cases of aneurysms and dissections of the ascending aorta
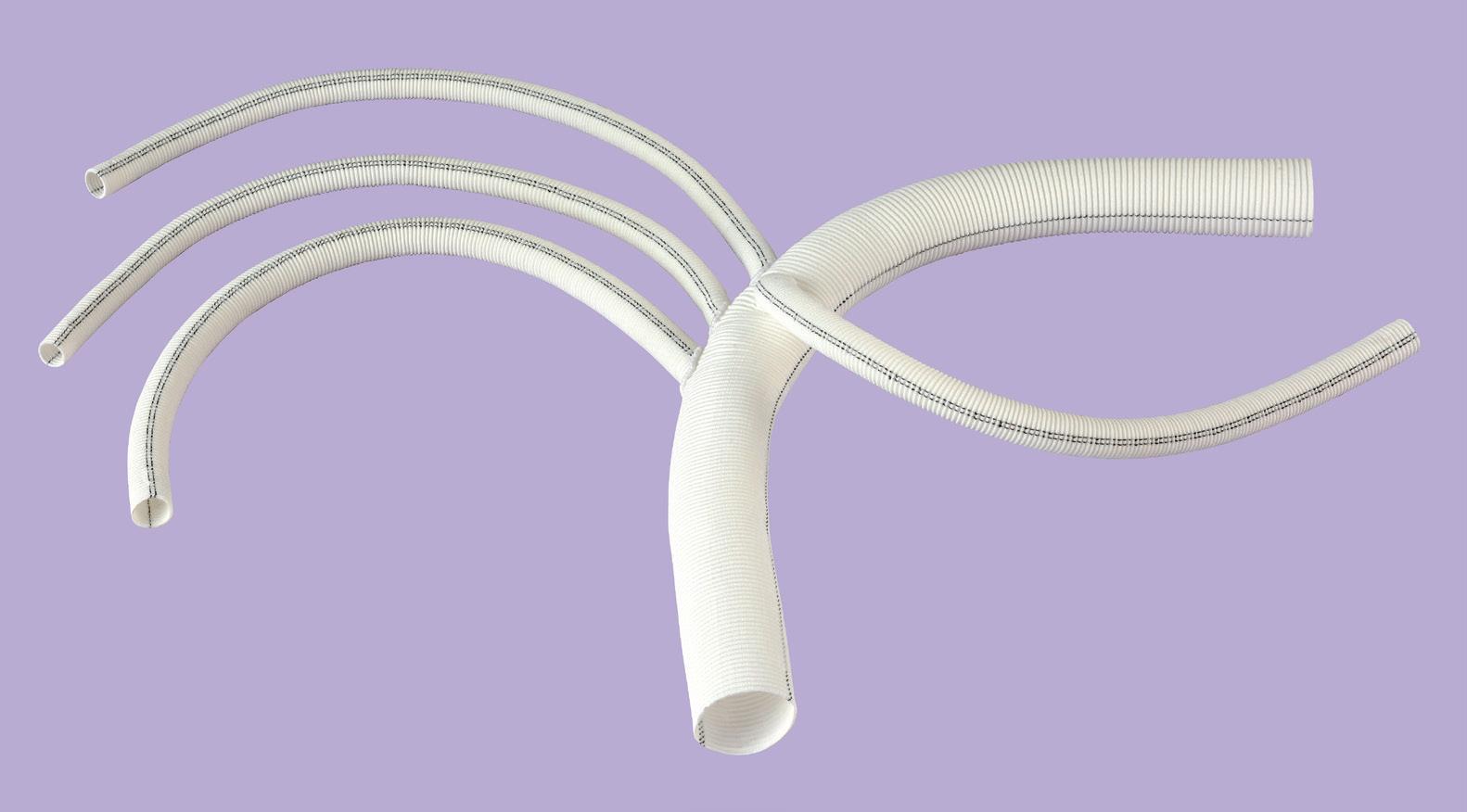
Pre-sewn branch intended for introduction of extracorporeal circulation
Water permeability less than 10 mL / cm2 / min at 120 mm of mercury
• Good resistance to circumferential dilatation1
• Reduced risk of embolic stroke2
• Potential for reduced operating time2
• Reduced risk of neurological deficits2
• Low porosity: < 10 mL/min/cm²/120mmHg
• Patented technology of impregnation: Collagen CXE, formaldehyde and glutaraldehyde free
PAR221088
PAR241088
PAR261088
* Only on request.
(3) Okita Y. et al. Total arch replacement using antegrade cerebral perfusion. The Journal of Thoracic and Cardiovascular Surgery Volume 145 N°35.
(4) Rapport HAS- Implants de pontage 2013/04.
Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
polymaille® C vascular prosthesis is indicated for replacement or bypass of arteries presenting aneurysm or obliterative arterial disease. Its indication is restricted to abdominal and peripheral surgery not crossing the knee flexion crease
• Good suturability and handling;
• Dimensional stability: Good resistance to cir
• Patented technology of impregnation: Collagen CXE, formaldehyde and glutaraldehyde free1
• Soft and flexible textile structure: enhance the graft handling and host vessel conformability
Bifurcated tube
Knitted polyester
Impregnated with type 1 Bovine collagen on both sides
Water permeability less than 5 mL / cm2 / min at 120 mm of mercury
polymaille® C bifurcated

polymaille® C straight
* Only on request.
(1) Rapport RRD-0394-01 rev00 - 27/04/2015.
(2) LNE certificate n°F014288 (2005): analysis of Perouse Grafts: formaldehyde free and glutaraldehyde free. Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).
Knitted polyester vascular patch
INDICATIONS
polymaille® extra thin vascular grafts are indicated for replacement or bypass of arteries damaged by aneurysm or arterial occlusive disease
The indication is limited to abdominal and peripheral surgery, not crossing the hollow of the knee
• SHR® technology;
• Thin wall ≤ 0.4mm.
SHR® reinforcement (Stretch Helicoidal Reinforcement)

Water permeability less than 5 mL / cm2 / min at 120 mm of mercury
Impregnated with type 1 bovine collagen on both sides
polymaille® extra thin straight
polymaille® extra thin straight with reinforcement


Please read carefully the Instructions For Use. The CE conformity assessment has been conducted by GMED (0459).

Knitted polyester vascular graft
polypatch vascular patches are indicated in vascular angioplasty and for carotid and femoral endarterectomy.
Knitted polyester
Anatomic shape
Impregnated with type 1 bovine collagen on both sides
Rectangular shape
Black alignment guideline
• Anatomical and rectangular shapes available: for carotid and femoral procedures


• Wall thickness ≤ 0,5 mm
• Patented technology of impregnation: Collagen CXE, formaldehyde and glutaraldehyde free(1)
• Suture retention strength ≥ 8N

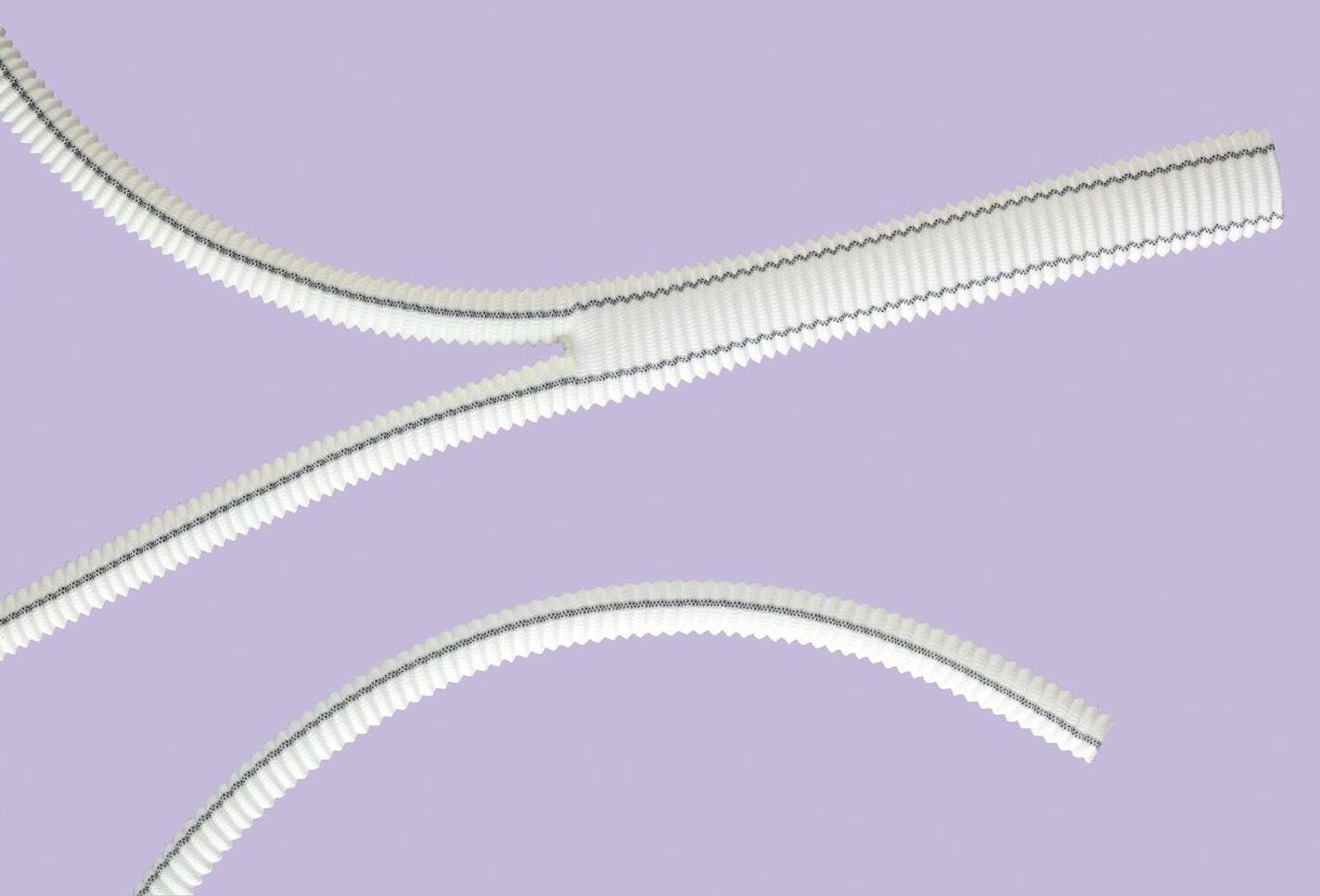
The vein stripper is developed for incompetent and dilated superficial veins stripping, especially for the great saphenous and small saphenous veins stripping.
1 handle allowing better gripping strength
1,2m long polyamide cable
• High tensile strength
• 2 straight tips pierced for possible recall wire placement
4 olives of different sizes for conventional method
• With or without olives: for endo or exo-stripping
• Handle: for better gripping strength during the removal of a superficial vein
• Single or double kit: for uni or bilateral saphenous vein stripping

THE INVAGINATED METHOD
• Resection by invagination
THE CONVENTIONAL METHOD

• Allows to catch possible invaginated and broken saphena sections, remaining in the vein after rupture
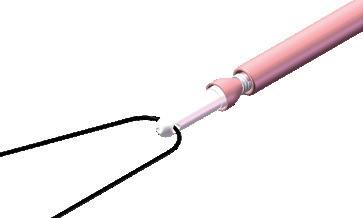

Vein ligature on the cable upstream the tip Olive fixation
Beginning of vein invagination onto recall wire
The CE conformity assessment has been conducted by GMED (0459).
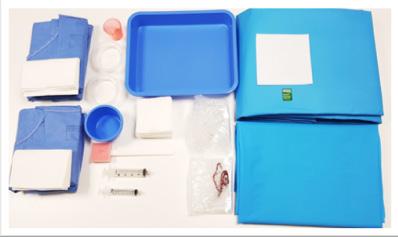
These pack are composed of necessary components to ensure the removal of varicose veins either on one leg or on both legs simultaneously.
• Absorbent and waterproof all over
• Double packaging
• Double-U-drape for easier patient set up
Composed by:
To support you in your varicose vein procedures, vein strippers are available for you, p.46 !

Also you can use derma+flex to close your surgical incision p.60 !

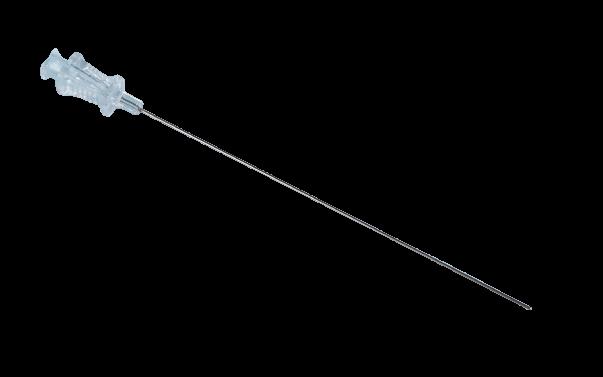
The arterial embolectomy balloon catheters (single lumen) are indicated for the removal of fresh, soft emboli and thrombi from vessels in the arterial system.
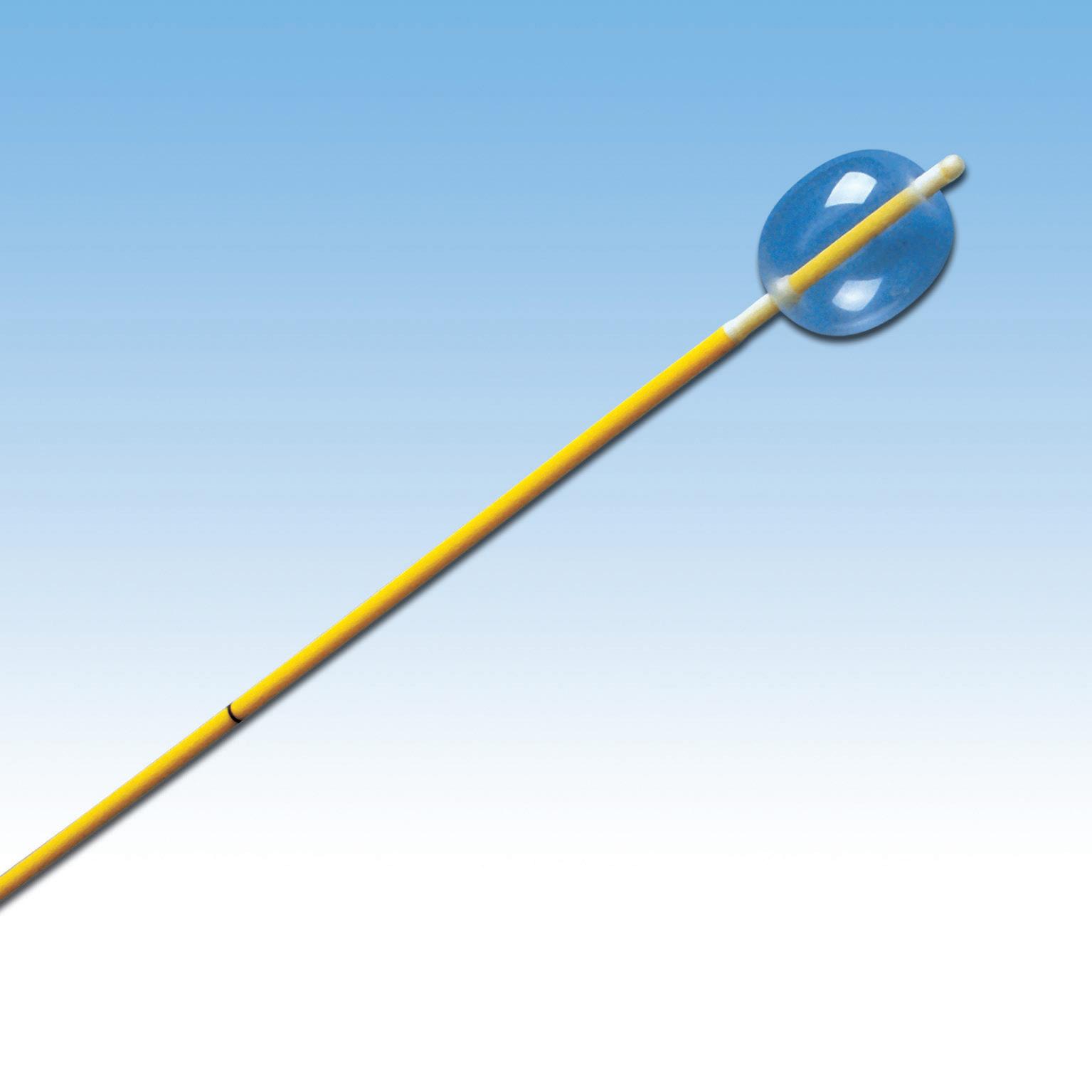
• Colour coded catheter: for easy identification of the French sizes
• Printed catheter: Maximum balloon inflation capacity printed on the proximal end of the catheter for safety!
• Graduation every 10 cm
• Short and flexible end: allowing easy introduction into the artery while reducing risks of plaque lesion.
The sternum guard is a surgical border drape, used in cardiac surgery with median sternotomy. This device is applied to the sternal edge and provides a mechanical protection of the sternal edge. The Sternum Guard range is intended for use in operating room.
• Haemostatic effect 1,2 through a natural reaction to compression and blood absorption
• Coverage of the entire incision angle and subcutaneous tissue
• Low particle release
• Specific mechanical protection
• Easy to use
• Maintaining a dry surgical site
sternum guard complies with EN 13795*, with a high performance level**
*EN 13795 aims to prevent the transmission of infectious agents between patients and surgical staff during surgery and other invasive procedures, leading directly to a fight against nosocomial infections. **High performance is defined as: “product intended to be used for surgeries to high exposure to body fluids or other, with many mechanical constraints or long surgeries”
The CE conformity assesment has been conducted by GMED SAS (0459).
1. Seiichi Ohta et al. Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent. Journal of Bioscience and Bioengineering. Volume 119, Issue 6, June 2015, Pages 718-723



2. K. M. Lewis, DVM et al. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur Surg. 2013; 45(4): 213–220

INDICATIONS
Standard pack of drapes for general surgery.
UNIVERSAL PACK
• Absorbant and waterproof all over
• Double packaging
References
REINFORCED UNIVERSAL PACK
• Absorbant and waterproof all over
• Double packaging
• Reinforced head and foot drapes
Gowns are intended for the protection of hospital staff and the patient. Several gowns are available.

asepskinTM is used to proceed skin preparation of the patient submitted to surgery. This device can be used for all types of surgical interventions!


Aborbents towels
Complete set (911.52)
Choice of antiseptic solutions to adapt to all patients
Compatible with povidone iodine and chlorexidine alcohol
Several sets availables to cover all steps of skin preparation
Handle to avoid patient’s contact
Avoid the risk of cross-contamination
(2nd and 3rd application
1 tray with 2 integral gallipot
1 surgical brush 2 washing gloves 3 absorbants towels 1 tray with integral gallipot
In single packaging : 1 tray with 2 integral gallipot
1 surgical brush
2 washing gloves
3 absorbants towels
1 tray with integral gallipot
1 sponge stick In double packaging :
1 tray with integral gallipot 1 sponge stick
derma+flex® is a topical skin adhesive, it can be used by medical professionals to close surgical incisions and minor traumatic wounds with easily opposable skin edges in areas of low skin tension. derma+flex® may be used in conjunction with, but not in place of, deep dermal sutures.

White connector provides optimal distance between the tube and the applicator tip for the enhanced visibility.
“Blend of 60% 2-octyl-cyanoacrylate and 40% n-butyl cyanoacrylate 0.7 mL tube”
Convenient storage
Multiple
Gel
No refrigeration required
use on a single patient
like viscosity will not run or seep into wounds
• Easy to apply
• Creates a microbial barrier
• Suture strength
• Highly flexible
• Suture & staple compatible
• Sets within 60 secs
• Duo tip allows for both precise and broad application
• Waterproof, shower friendly formula
• Painless application
• No suture removal
• Minimal exothermic effect (it won’t burn or sting)
• Cosmetically appealing
• Fast wound closure
• Lower cost
• A&E friendly
• No sharps risks
• Room temperature storage
• Safe and reliable

• Alternative to medical sedation
• Anxiety & pain reduction
• Before, during & after the procedure
• Clinically proven device
• For adults & children
• Interventional cardiology
• Interventional radiology
• Venous surgery
• Arterial surgery
• Carotid surgery
• Procedures in
- Orthopedic
- Urology
- Pediatrics
- Gynecology
- Oncology
- Emergency
ALTERNATIVE TO MEDICAL SEDATION
• Digital therapy combining clinical hypnotherapy and integrative therapeutic techniques through Virtual Reality


MORE COMFORT & SECURITY FOR THE PATIENT
• Reduction of anxiety & pain1 4 5
• Reduction of apnea during the procedure1
• No side effects or adverse events reported
• 100% of patients are satisfied1
MORE COMFORT FOR THE MEDICAL TEAM
• Reduction of involuntary movement during the procedure1
• 100% of anesthetists are satisfied1
SIGNIFICANT TIME SAVING
• Reduction in procedure duration1
SUITABLE FOR MOST OF THE PATIENTS
• 4 modules for children & 4 modules for adults
• 12 languages available
Version with smartphone controller
Customization with VYSET® allows you to create a package specific to your needs ensuring maximum barrier precautions, thus reducing the risk of associated infection.

IMPROVING SAFETY
• Reducing the risk of infection
• Reducing the risk of errors
• Improving working conditions
• Traceability (one label + barcode)

• Simplifying procedures which helps in emergency cases or when a practitioner works alone
SAVING TIME AND MONEY
• Reducing preparation time
• Improving stock management.
• Reducing waste
IMPROVING SAFETY
• Assembly and sterilization in our own facilities in Europe

• Selection of only high-quality medical devices


• Sales teams at your disposal to provide any additional training, help and advice needed to ensure your pack is a success