15 minute read
grown on titanium chips before and after washing
from silk
Sample Absorbance (nm) Absorbance (nm) after before wash wash
B.subtilis 0.56 0.558 E.coli+pUC19 0.585 0.58 E.coli+pBluescript II KS(-) 0.558 0.553
Advertisement
Table 3. Absorbance (595 nm) readings of LB broth in which biofilms were grown on titanium chips before and after washing
As it can be observed, the absorbance values for each sample do not present a significant difference before and after the wash.
Natural silk from a common house spider and synthetic silk were dissolved then analysed by the ATR technique, recording the reflectance of the crystals in the silk. The produced spectra were compared with IR spectra library from the apparatus in order to determine the composition, by a matching percentage. Figure 12 illustrates spectra of natural silk, whilst Figure 12 shows spectra of synthetic silk.
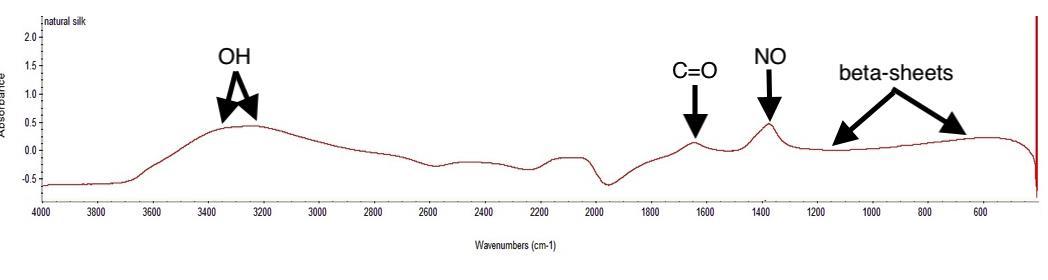
Figure 12. IR spectra of natural silk
As it can be observed, natural silk presents a resolved peak at 3300 cm -1 , however the absorption bands between 2300 and 1400 cm -1 are less easily interpretable. The IR library found that Manganese(II) nitrate hydrate presents a composition matching percentage of 50.26% with the sample.
Figure 13. IR spectra of synthetic silk

Figure 13 illustrates an IR spectra of dyed synthetic silk and presents a relative similarity with the analysed natural silk spectra. The synthetic silk peaks at 3300, 1600 between 1300 and 900 cm -1 . The IR library a composition matching rate of 58.53% with cellophane.
Table 4 represents the chemical functional group corresponding in the IR spectra region, which helps to identify the structure of the compounds. The functional class was chosen for stretching vibrations.
Wave number (cm -1 ) Functional groups
722 (CH 2 ) n 750-870 C-H in aromatics 1400 N-O asymmetric 1100 n (C-O) 1200 C-H (Amide III) 1650 C=O (Amide II) 2860 CH 2 2960 CH 3 3200 N-H (Amide I) 3400 OH
Table 4. Functional groups and their corresponding wavenumbers (Rani et al. 2017; Ciolacu, Ciolacu and Popa 2010).
Natural and synthetic silk did not reflect properly, resulting in poor IR spectra. The natural silk presented a peak at 1400 cm -1 , representing the nitro (NO) functional group and the absorption region between 3400 and 3200 cm -1 the hydroxyl group (OH) was a result of the ethanol used in the dissolving process. The absorbance band situated at around 1000 cm -1 represents the -sheets constructing the silk fibroin. The IR library found similarities in silk’s composition with Manganese(II) nitrate hydrate and Trimethylboroxine. On the other hand, the dyed synthetic silk spectra showed unsteady changes in the 3600-2400 cm -1 , comparing to the natural silk. The n(C-O) groups appeared around 1100 cm -1 and the hydroxyl class at 3400 cm -1 , and moreover, the larger peak around 3400 cm -1 can represent amounts of bound water, which contribute to the cellulose IR spectrum. The IR library found a relatively higher similarity of the synthetic silk composition with cellophane, which is a transparent sheet made of cellulose.
Discussion
Competent E.coliand B.subtilis cells were prepared for transformation. E.coli cells presented moderated efficiency transformation, whereas B.subtilis strain suffered total inefficiency in transformation, due to the competent cells preparation. One technique was chosen for both bacterial hosts, however B.subtilis cells led to failure in the blue/white colonies selection step due to the incompatibility of the used competent cells preparation technique. Competent B.subtilis cells preparation is more complex and it requires expression of B.subtilis competence transcription factor ComKfrom an IPTG-inducible promoter carried by an unstable plasmid to generate the genes required for the DNA uptake and further recombination. The ComKfactor will be lost after transformation due to its intrinsic instability and further genetic manipulation can be performed without any interferences (Nijland et al. 2010).
The blue/white colonies screening was performed in order to select the successful recombinant bacterial hosts, which have taken up the silk producing gene. The agar
plates containing diluted and undiluted E.coli with both inserts presented 190 white colonies, representing the recombinant cells and one blue colony, indicating the - galactosidase presence detected by X-gal. The host E.colistrain contains the lacZ deletion mutant, scientifically termed lacZM15, caring a mutant -galactosidase mutant with deleted 11 -41 N-terminal residues, termed the -peptide. The vectors pUC19 and pBluescript II SK(-) carry the lacZsequence which encodes the first 59 residues of -galactosidase, producing the -peptide. The white colonies show no functional -galactosidase enzyme, whilst the blue colonies indicate hydrolyzed Xgal and no interruption of lacZ(Aitken 2012).
On the other hand, the agar plates containing transformed B.subtilis cells did not show any colonies. Regardless whether both vectors contain the lacZ, B.subtilis does not carry the lacZoperon, leading to an unsuccessful blue/white colonies selection. Glycolic genes cggR, gapA, pgk, tpi, pgm and eno are transcribed as a hexacistronic operon, named gapA, repressed by the CggR regulator (Ludwig et al. 2001). A scientifically proved effective way to induce -galactosidase enzyme activity in B.subtillis is the use of the lacZgene from E.coli as a reporter gene, where the lacZsequence is attached to B.subtillis and use the blue/white colonies selection (Daniel et al. 1997). Another way to allow selection in presence of antibiotic in B.subtilis cells is the use of antibiotic-resistance cassettes containing genes encoding resistance to the antibiotics erythromycin, kanamycin, tetracycline and spectinomycin, which were cloned in the polylinker of E.coliplasmid vectors (Guérout-Fleury et al. 1995).
The performed dilutions were 10 -1 , 10 -2 and 10 -3 and the resulted colonies could be countable, however, the 10 -3 dilution did not show any colony on the plates. More dilutions could have been performed for more detailed investigation and accuracy. The recombinant percentage was calculated to be 99.47%, showing the efficiency of the technique applied on E.colicells. However, the viable competent cells were calculated to be 6.60 x 10 2 , 1.118 x 10 3 and 6 x 10 2 cells/ml. Table 4 presents the viable competent cells expressed in cfu (colony forming units)/g and a categorization of the competent cells efficiency according to Thermofisher standards (Thermofisher.com 2019).
Bacterial host Viable Viable High Medium Subcloning and vector competent competent (gold) (silver) (bronze) cells cells efficiency efficiency efficiency (cells/ml) (cfu/g) >10 9 10 8 -10 9 10 6 -10 7 cfu/g cfu/g cfu/g or less Undiluted 6.60 x 10 2 6.60 x 10 5 ✓ E.coli+pBluescript II SK (-) Undiluted 1.118 x 1.118 x ✓ E.coli+pUC19 10 3 10 6 E.coli+pUC19 6.00 x 10 2 6.00 x 10 3 ✓ diluted 1:10
Table 5. Competent cells efficiency compared to standard efficiency values (Thermofisher.com 2019)
Agar plates containing E.colicells and the insert showed bronze efficiency or even less viable competent cells to be categorized, whilst plates containing B.subtils cells showed total inefficiency of the competent cells.
Biofilms were grown in 96-well and 24-well microtiter plates in different volumes. As it can be observed in Figure 6, B.subtilis biofilms were formed on a 24-well plate well in 2ml volume grew on the bottom of the well, as well as on the side of it. E.coli biofilms were created on the bottom of the well. The 96-well plates indicated presence of E.coliand B.subtilis biofilms on the bottom of the wells, however this can represent a false positive due to the possibility of accumulation of the cells on the bottom of the well (Coventry University 2019).
Serial dilutions of non-recombinant E.coli and B.subtilis incubated at different times were used in creating the efficient conditions for biofilm formation. According to Figures 7 and 8, the biofilms showed a greater absorbance while diluted 1:10 with LB broth, indicating that the cells tend to grow more as bacterial communities in presence of LB broth rather than dry cells. The most beneficial incubation time for E.colibiofilms was determined to be 96h, whilst the best incubation time for B.subtilis biofilms was 4h. Conclusively, the 4h incubation time showed good absorbance values for both E.coliand B.subtilis biofilms. LB broth was used as negative control, however the absorbance readings indicated high values, showing contamination in the wells.
Recombinant E.colishowed a greater potential to create biofilms with the pUC19 insert for both diluted and undiluted samples, whilst the plasmid vector pBluescript II SK (-) inserted within the bacterial host demonstrated a tendency to remain planktonic cells more than E.coli with no insert.
Both pUC and pBluescript variations present a high-copy number, ampicillin resistance and a wide range of restriction endonucleases can be used to cut them for further cloning procedures. However, one difference between the two cloning vectors is that pBluescript vectors carry flanking promoters in the MSC, which allow the insert DNA transcription on either strand. Being phagemids, pBluescript vectors
contain a single-stranded filamentous bacteriophage origin of replication, termed M13 phage and therefore single-stranded DNA applications, such as DNA sequencing or site mutagenesis present a higher efficiency rather than replicating conventional double-stranded plasmids (Casali and Preston 2003). Thus E.coli with pBluescript II SK(-) did not produced effective biofilms.
Silk was expected to be produced by the biofilms due to the silk producing gene insert of both E.coli and B.subtilis hosts. The silk gene was first introduced into two different cloning vectors, pUC19 and pBluescript II SK(-) and inserted into the bacterial host to replicate. Cloning vectors are small segments of DNA, used to insert genes into host cells with the purpose of obtaining numerous copies of the gene insert, however they are not able to express the functions of the of the genes of interest. Expression vectors are DNA plasmids, which transcript and translate specific gene inserts into the host cells, forcing the mechanisms of the cell to produce the relevant gene. An expression vector is able to comprise enhancers, strong promoter region, termination codon, transcription and translation initiation sequences, origin of replication, restriction sites and a selectable marker, allowing the silk gene to successfully produce silk fibroins in the biofilms (Lakna 2017).
The microtiter plate biofilm assay was performed to determine whether biofilms where formed or not on the microtiter plates. This technique is based on staining the plate wells with crystal violet solution, then washing the dye with acetic acid solution for a proper preparation for absorbance reading. The results showed that E.coli biofilms indicate greater absorbance values than the B.subtlis biofilms. E.coli is Gram-negative bacteria and B.subtilis is a Gram-positive bacteria, the difference between these two groups is the colour change after staining with crystal violet solution due to the composition of the cell wall. This issue can lead to false positives in the microtiter plate assay. Gram-positive bacteria remain dark violet after staining due to the multi-layered peptidoglycan with embedded proteins cell wall which retain the purple colour of the stain. On the other hand, the cell wall of Gram-negative bacteria contains an extracellular membrane with a periplasmic space layer between the membrane and the peptidoglycan layer and does not allow the stain to be retained, resulting a fade red-pink colour after Gram staining (Thairu et al 2014).
An attempt of biofilm creation on titanium chips led to failure as no sight of bacterial community could be observed on titanium after 4h incubation. A possible cause of the failure could be the inability of the bacteria to adhere to the surface and form a biofilm further on. During bacterial adhesion, planktonic cells swim using the flagella or are attracted to the surface by Brownian motion, van der Walls attraction, gravitational forces, surface electrostatic charge and hydrophobic interaction, leading to irreversible adhesion. Bacterium’s capsule, flagella and pili help the organism to adhere to the surface. Hydrophobicity, chemical composition, roughness and topography of the surface can influence the adhesive behaviour, however surface coatings can bring an important contribution to solving this issue. Polymer coatings can promote cell adhesion, stimulating the exo-polysaccharide matrix formation if chosen correctly. Titanium surface can be coated with polymers by plasm polymerisation or electro-polymerisation. For instance, hexamethyldisiloxane coating could be fasten the attachment of the extracellular matrix, whilst polymer layers functionalised by N-acetyl-GCRGYGRDGSPG peptide can be beneficial for specific protein matrixes. Moreover, pre-treated titanium by grit blasting, acid etching or
plasma/laser treatment generate a stronger substrate-coating bonding and thus, firmer biofilm adhesion (Wang 2011).
The natural and synthetic silk were analysed using ATR, providing IR spectra. The composition of both types of silk was meant to be used as standards and compared to the composition of silk produced by the biofilms. However, regardless the failure of silk production, the experiment was carried out with the purpose of helping further investigations.
The presented IR spectra can be categorized as poor analysis due to the shape of the absorbance bands, the interpretation being relatively difficult, yet the IR library provided profiles of compounds with similar composition to identify the samples. An attempt of interpretation was done in order to justify the similarities with the compounds found by the IR library. As the results show, the natural silk’s crystalline domains reflected poorly, providing an IR spectra with wide bands. The intense bands resulted from the amide vibrations, representing the coupling of the peptide bonds in the cell. The spectral regions associated with the amide vibrations are 3200, 1600 and 1200 cm -1 (Rani et al. 2017).
The synthetic silk composition was determined to be cellulose. The broad band situated between 3600 and 3000 cm -1 spectral region resulted from the OH stretching vibration caused by the hydrogen bonds. The 2900 cm -1 band correspond to the C-H stretching vibration, determining the composition of cellulose (Ciolacu, Ciolacu and Popa 2010). A better characterization of silk could be given by scanning electron microscopy analysis, where elucidation of the complete sequence of silk proteins is possible, as well as a 3D structure (dos Santos-Pinto et al 2018).
Conclusion
The aim of the experiment was to create and investigate strong E.coliand B.subtilis biofilms, which can produce silk due to the silk producing gene inserted into the bacterial hosts. Unfortunately, this aim was not reached due to the wrong choice of the vectors in which the silk gene was ordered, wrong competent B.subtilis cells production and inability of the cells to attach to the titanium chips in order to form a biofilm. The information provided by this experiment could be used for further investigations.
Word count:
• Abstract: 336 words • Introduction: 998 words • Methods: 943 words • Results: 1343 words • Discussion: 1697 words • Conclusion: 85 words • TOTAL: 5402 words
List of references
Addgene.org. (2019). Addgene: Vector Database - pBluescriptII SK (-). [online] available from https://www.addgene.org/vector-database/1947/ [ 24 Mar. 2019].
Addgene.org. (2019). Addgene: Vector Database - pBluescript II SK (-). [online] available from https://www.addgene.org/vector-database/1947/ [24 Mar. 2019].
Casali N. and Preston A. (2003). E. Coli plasmid vectors: Methods and applications, Vol-235. Totowa: Humana Press.
Ciolacu, D., Ciolacu, F. and Popa, V. (2010). Amorphous cellulose- structure and characterization. Cellulose chemistry and technology, 45, pp.13-21.
Coventry University (2017). 226BMS Biomolcular Analysis LABORATORY CLASSES 2017-2018 DNA cloning Labs 1-3 Coventry: Coventry University
Coventry University (2017). 226BMS-Biomolecular Analysis Coventry: Coventry University
Daniel, R., Haiech, J., Denizot, F. and Errington, J. (1997). Isolation and characterization of the lacA gene encoding beta-galactosidase in Bacillus subtilis and a regulator gene, lacR. Journal of Bacteriology, 179(17), pp.5636-5638.
dos Santos-Pinto, J., Arcuri, H., Esteves, F., Palma, M. and Lubec, G. (2018). Spider silk proteome provides insight into the structural characterization of Nephila clavipes flagelliform spidroin. Scientific Reports, 8(1).
Garrett, T., Bhakoo, M. and Zhang, Z. (2008). Bacterial adhesion and biofilms on surfaces. Progress in Natural Science, 18(9), pp.1049-1056.
Guérout-Fleury, A., Shazand, K., Frandsen, N. and Stragier, P. (1995). Antibioticresistance cassettes for Bacillus subtilis. Gene, 167(1 -2), pp.335-336.
Kasoju, N. and Bora, U. (2012). Silk Fibroin in Tissue Engineering. Advanced Healthcare Materials, 1(4), pp.393-412.
Lakna (2017). Difference Between Cloning Vector and Expression Vector | Definition, Types, Uses, Similarities, Differences. [online] Pediaa.Com. available from http://pediaa.com/difference-between-cloning-vector-and-expression-vector/ [20 Mar. 2019].
Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F., Hecker, M. and Stülke, J. (2001). Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Molecular Microbiology, 41(2), pp.409-422.
McKeen, L. (2012). Film properties of plastics and elastomers. [Place of publication not identified]: William Andrew.
Mittmann, B. and Wolff, C. (2012). Embryonic development and staging of the cobweb spider Parasteatoda tepidariorum C. L. Koch, 1841 (syn.: Achaearanea tepidariorum; Araneomorphae; Theridiidae). Development Genes and Evolution, 222(4), pp.189-216.
Nhm.ac.uk. (201 9). [online] available from http://www.nhm.ac.uk/content/dam/nhmwww/our-science/dpts-facilitiesstaff/Coreresearchlabs/blue-white-colony-screening_aug12-118543.pdf [ 24 Mar. 2019].
Nijland, R., Burgess, J., Errington, J. and Veening, J. (2010). Transformation of Environmental Bacillus subtilis Isolates by Transiently Inducing Genetic Competence. PLoS ONE, 5(3), p.e9724.
O'Toole, G. (2011). Microtiter Dish Biofilm Formation Assay. Journal of Visualized Experiments, (47).
Openwetware.org. (2019). Wiese Lab:Competent Cell Prep - OpenWetWare. [online] available from https://openwetware.org/wiki/Wiese_Lab:Competent_Cell_Prep [ 21 Mar. 2019].
Percival, S., Yates, M., Williams, D., Chalmers, R. and Gray, N. (2014). Microbiology of waterborne diseases. Elsevier.
Pumure, I., Ford, S., Shannon, J., Kohen, C., Mulcahy, A., Frank, K., Sisco, S. and Chaukura, N. (2015). Analysis of ATR-FTIR Absorption-Reflection Data from 13 Polymeric Fabric Materials Using Chemometrics. American Journal of Analytical Chemistry, 06(04), pp.305-312.
Rani, K., Chandwani, N., Kikani, P., Nema, S., Sarma, A. and Sarma, B. (2017). Optimization and surface modification of silk fabric using DBD air plasma for improving wicking properties. The Journal of The Textile Institute, 109(3), pp.368- 375.
Sangunamani, D., Raghu, K. and Naik, S. (2019). ATR-FTIR Spectroscopy analysis of Silk. [online] available from https://www.chemarc.com/content/article/atrftirspectroscopy-analysis-of-silk/5c4a9afa6b17990f29c82d6d [ 21 Mar. 2019].
Siesler, H. (1980). Fourier transform infrared (ftir) spectroscopy in polymer research. Journal of Molecular Structure, 59, pp.15-37.
Thairu, Y., Usman, Y. and Nasir, I. (2014). Laboratory perspective of gram staining and its significance in investigations of infectious diseases. Sub-Saharan African Journal of Medicine, 1(4), p.168.
Thermofisher.com. (2019). Chemically Competent Cells | Thermo Fisher Scientific - UK. [online] available from https://www.thermofisher.com/uk/en/home/lifescience/cloning/competent-cells-for-transformation/chemicallycompetent.html#medium [ 19 Mar. 2019].
Van Die, I., Bergmans, H. and Hoekstra, W. (1983). Transformation In Escherichia coli: Studies On The Role Of The Heat Shock In Induction Of Competence. Microbiology, 129(3), pp.663-670.
Wang, A. (2011 ). Bacterial Biofilms and Biomineralisation on Titanium., Birmingham: University of Birmingham
Acknowledgements
I would like to express my appreciation to Dr. Sharon Williams for her guidance and help during this project. Without her valuable assistance, as well as the encouragement at various stages of the project period, I would not have achieved my best work.
I am also very thankful to the laboratory technicians during the data collection period for all their hard work to satisfy any need for my experiment.
Finally, I would like to thank the Faculty of Health and Life Sciences for offering me all the opportunities to reach my aims with cutting-edge technology and friendly staff that were constantly willing to help.






