



The first described species in Magnaporthales was the rice stem rot fungus. It was named Sclerotium oryzae on the basis of the sclerotial morph (Cattaneo, 1877) (Fig. 1.1). Its conidial morphs were subsequently named Nakataea sigmoidea (Hara, 1939). The sexual morph of this fungus was not discovered until almost a century later. Krause and Webster (1972) established a new genus, Magnaporthe in Diaporthales, to accommodate the sexual morph of this species as Magnaporthe salvinii Four more species were added to the genus on the basis of their sexual morphology: M. grisea, M. oryzae, M. poae, and M. rhizophila (Fig. 1.2).
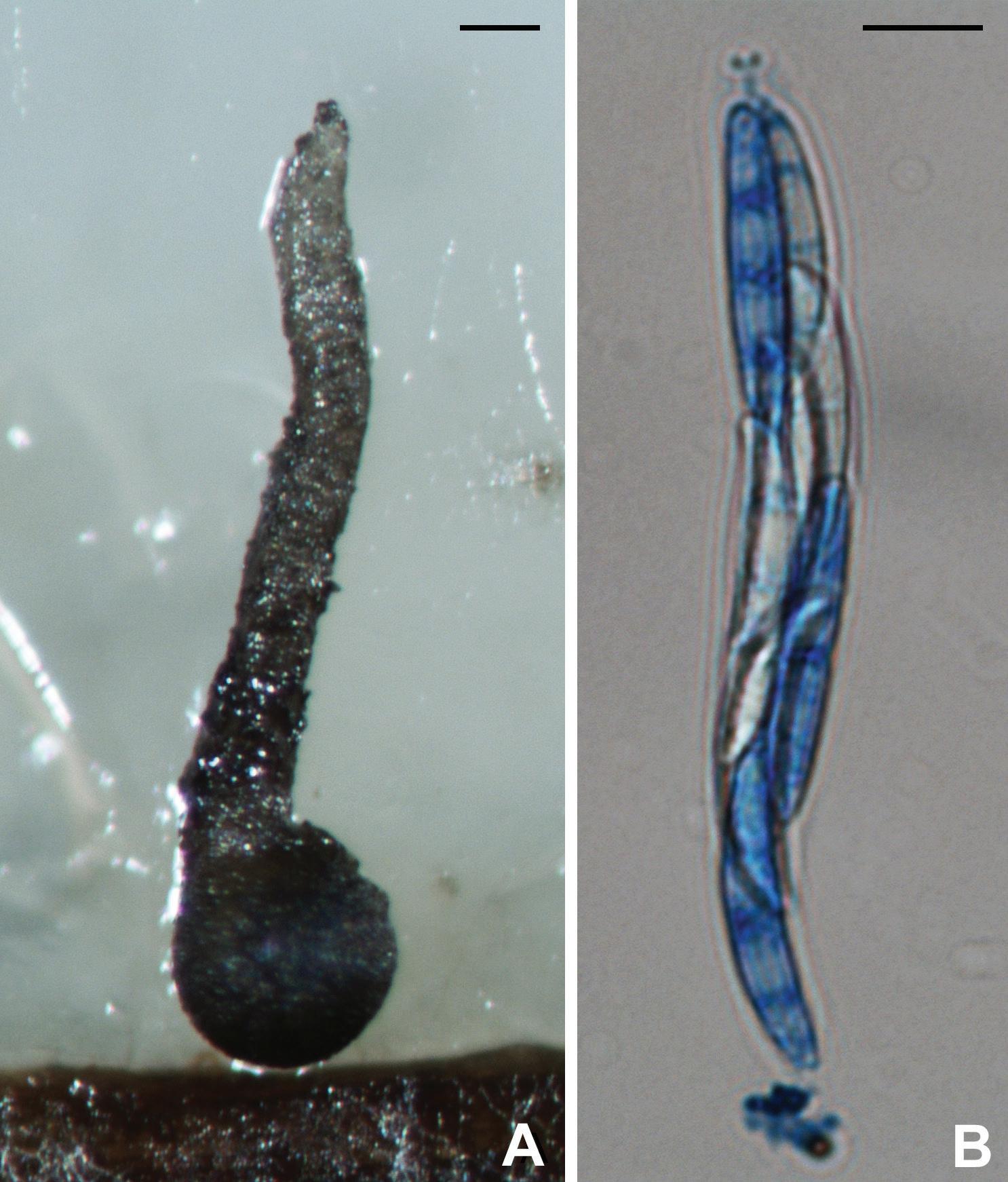
In 1880, Saccardo established the genus Pyricularia (pyri: pear-shaped conidia) (Fig. 1.3) based on P. grisea on crabgrass (Saccardo, 1880) and including primarily asexual species. The rice isolates were designated P. oryzae by Cavara (1892), which now is known as the rice blast fungus. Since then, over 70 names have been placed in Pyricularia, many of which cause blast diseases of monocotyledonous plants. However, only a fraction of Pyricularia species have been included in modern phylogenetic analyses (Tosa and Chuma, 2014).
The sexual morph of P. grisea was first developed from crossing experiments in culture (Hebert, 1971), and it was placed in the genus Ceratosphaeria. Barr (1977) later moved it to Magnaporthe because its ascospore morphology was similar to that of the
type species, M. salvinii. The sexual morph of the rice blast fungus also was obtained by laboratory crossing experiments and was named M. oryzae (Couch and Kohn, 2002). However, phylogenetic and phylogenomic analyses demonstrate that the rice blast fungus does not belong in Magnaporthe (Luo

and Zhang, 2013; Luo et al., 2015a; Murata et al., 2014; Zhang et al., 2011) (Fig. 1.4). Rather the type species of Magnaporthe, M. salvinii, belongs in Magnaporthaceae and is not congeneric with P. grisea and P. oryzae placed in Pyriculariaceae. Placement of the sexual morph of the rice blast fungus, P. oryzae, in Magnaporthe was based on incomplete data.
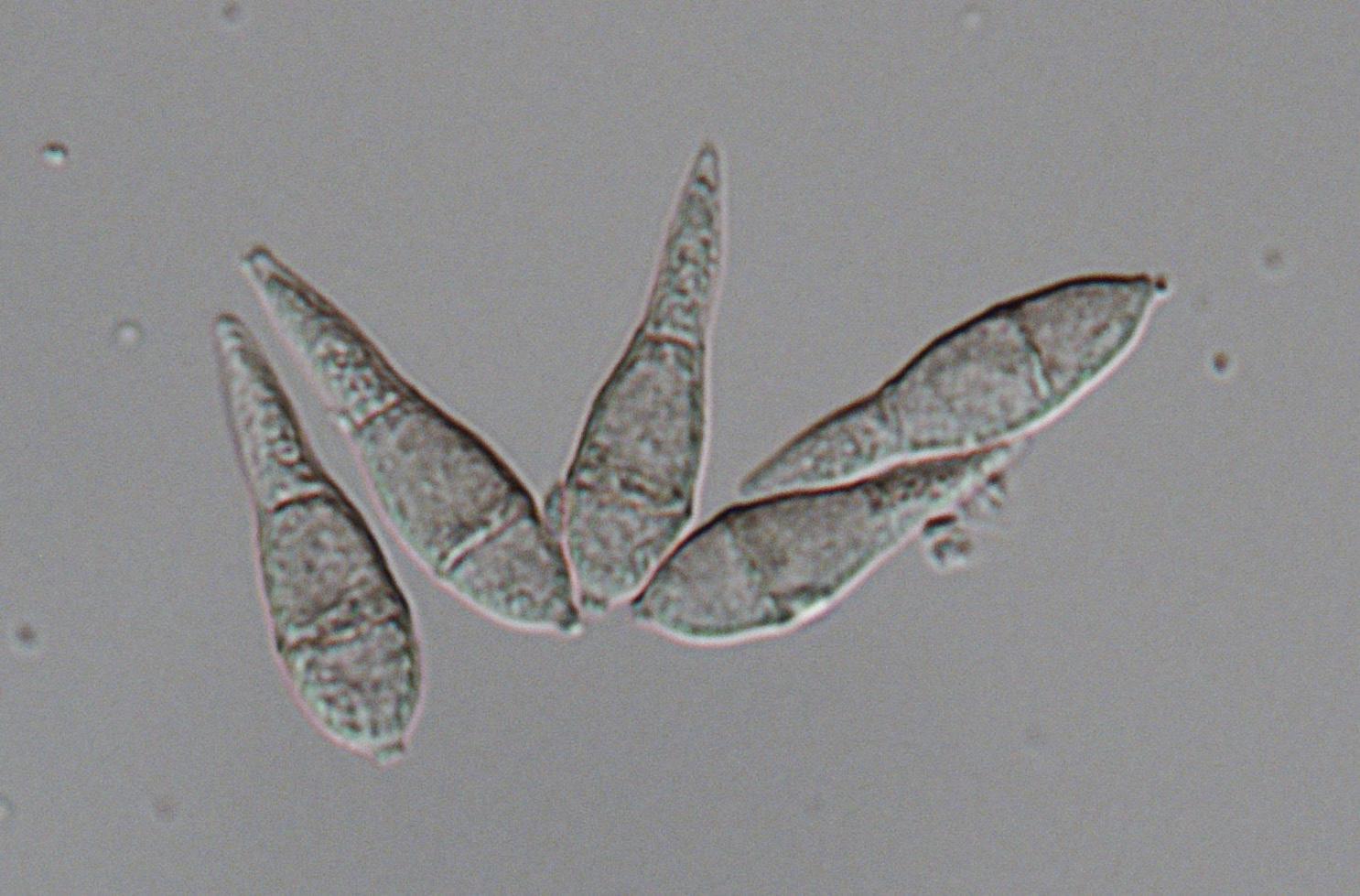
Traditionally, mycologists have given considerable weight to ascospore morphology, such as shape, size, pigmentation, and wall ornamentation, in delimiting genera in ascomycetes. With the development of polymerase chain reaction and DNA sequencing technology in the early 1990s, molecular phylogeny has become a powerful approach for understanding fungal systematics. Molecular phylogenetic studies suggest that taxonomy based on traditional morphological characteristics does not always reflect evolutionary history. A six-gene phylogeny, specifically the largest subunit of the RNA polymerase II gene (RPB1), translation elongation factor 1-alpha gene (TEF1), a DNA replication licensing factor gene (MCM7 ), the internal transcribed spacer (ITS) region of the nuclear rRNA genes, 18S rRNA gene (18S), and 28S rRNA gene (28S), has been applied to genera in Magnaporthales. Zhang et al. (2011) pointed out that both Gaeumannomyces and Magnaporthe, two important genera in Magnaporthales, are polyphyletic. Taxonomic revisions of these taxa subsequently have been made by Luo and Zhang (2013), Klaubauf et al. (2014), Hernández-Restrepo et al. (2016), and Zhang et al. (2016). Pathology and characteristics of the asexual morph, rather than ascospore morphology, correspond better with phylogenetic relationships of these fungi. The six-gene phylogeny also recognizes two major clades in these fungi; one clade includes root-infecting species that produce phialophora-like conidia, while the other clade includes fungi causing blast diseases that produce pyricularia-like conidia (Zhang et al., 2011).
The family Magnaporthaceae was proposed for a group of cereal- and grass-associated fungi centered on species causing rice stem rot and blast symptoms, along with species of Buergenerula, Clasterosphaeria, Gaeumannomyces, Herbampulla, and Omnidemptus, most of which are necrotrophic or hemibiotrophic pathogens (Cannon, 1994). Early taxonomic studies placed the rice blast fungus and allied species in different orders in Ascomycota, such as Diaporthales, Phyllachorales, and Xylariales (Barr, 1977; Krause and Webster, 1972). Later molecular phylogenetic analyses supported a monophyletic Magnaporthaceae, as defined by Cannon (1994), placing it in the subclass Sordariomycetidae (Sordariomycetes, Ascomycota). However, subsequent analyses supported variable phylogenetic affinities for this family. For example, in Zhang and Blackwell’s (2001) 18S rDNA tree, Magnaporthaceae formed a sister clade with Ophiostomatales. The 28S rDNA tree generated by Huhndorf et al. (2008) proposed a close relationship between Magnaporthaceae and Sordariales,
Chaetosphaeriales, and Boliniales. In the 18S tree of Thongkantha et al. (2009), Magnaporthaceae formed a clade sister to Diaporthales and Ophiostomatales; however, in the same paper, the 28S rDNA tree grouped them with Chaetosphaeriales and Sordariaceae (Thongkantha et al., 2009). Zhang et al. (2006) generated a four-gene phylogeny (TEF1; the second largest subunit of RNA polymerase II, RPB2; 18S; and 28S) and suggested that the rice blast fungus was most closely related to Diaporthales. However, no other taxa in Magnaporthaceae were included in the analysis (Zhang et al., 2006). Poor taxon sampling resulting
from a lack of sequence data from nonmodel species in Magnaporthaceae and phylogenetic heterogeneity among gene loci (Ebersberger et al., 2012; Fitzpatrick et al., 2006) likely explain the conflicting tree topologies in these studies.
In 2009, a new order, Magnaporthales, with a single family was established (Thongkantha et al., 2009), characterized by nonstromatic black perithecia usually with long, hairy necks, persistent asci, and fusiform (spindle-shaped) or filiform (needle-shaped) ascospores. The asexual morphs are hyphomycetous and variable but can be categorized into two types:
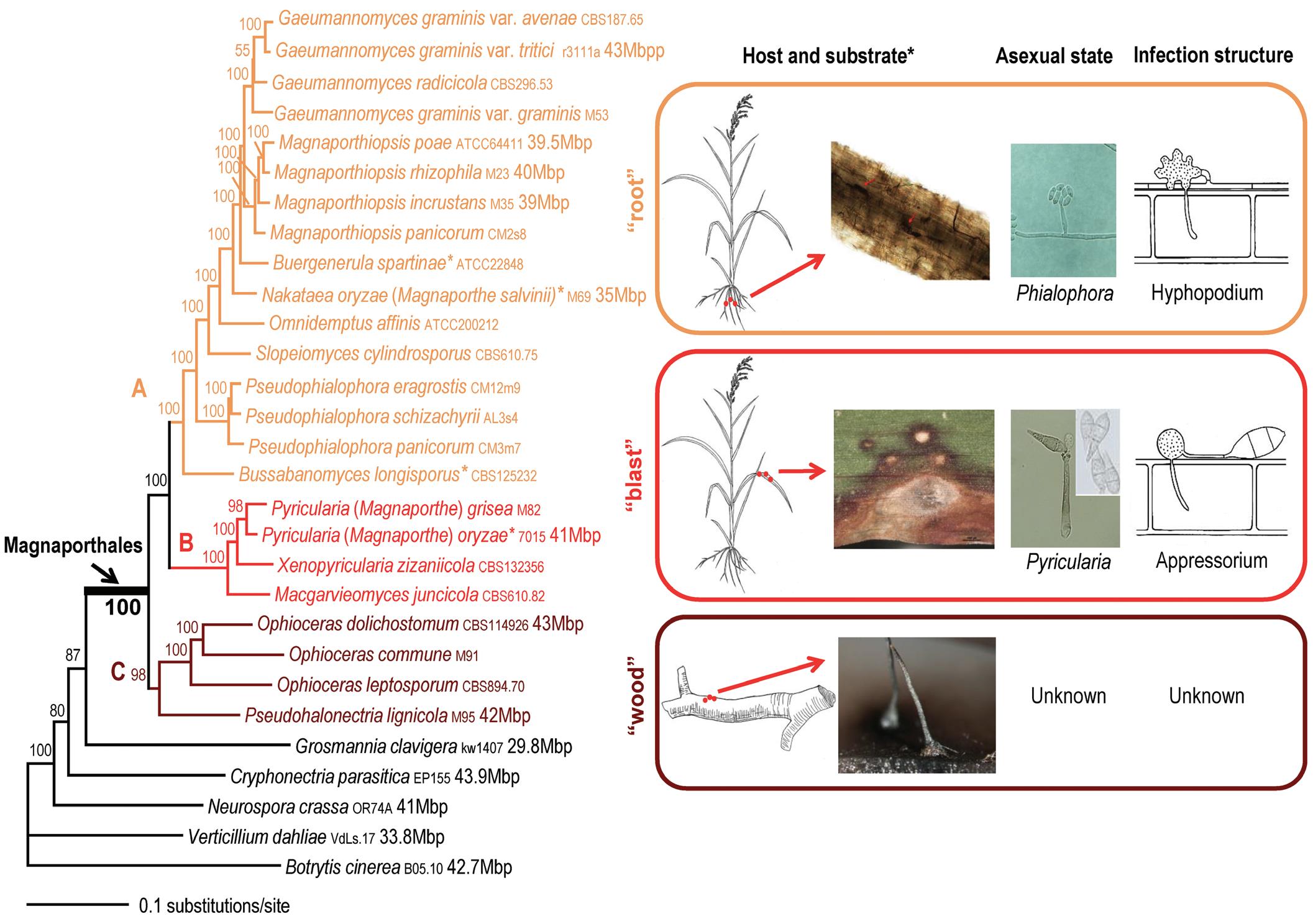
FIG. 1.4. Maximum likelihood tree of 24 Magnaporthales species and five Sordariomycetes species used as outgroup species based on 82,715 amino acid positions derived from 226 genes. The infection sites and the morphology of sexual and asexual states for the three major Magnaporthales clades are illustrated (right panel). The supporting values for each node were estimated using 1,000 bootstrap replicates. The strain number and genome size (if available) for each species are provided (Luo et al., 2015a). Note that Buergenerula spartinae, Bussabanomyces longisporus, Nakataea oryzae, and Omnidemptus affinis are associated with aboveground parts of host plants, which is an exception in clade A. Pyricularia oryzae is associated with both the leaf and root of the host plant, which is an exception in clade B. (Reproduced according to terms of Creative Commons Attribution 4.0 International License from Luo, J., Qiu, H., Cai, G., et al. Phylogenomic analysis uncovers the evolutionary history of nutrition and infection mode in rice blast fungus and other Magnaporthales. Sci. Rep. 5, 9448 (2015). doi.org/10.1038/srep09448)
pyricularia-like and phialophora-like (Fig. 1.4). Several asexual genera have been linked to Magnaporthales, including Clasterosporium, Didymobotryum, Harpophora, Mycoleptodiscus, Nakataea, Phialophora, Pseudotracylla, Pyricularia, Sclerotium, and Trichocladium (Cannon, 1994; Cannon and Alcorn, 1994; Gams, 2000; Huhndorf et al., 2008; Kohlmeyer and VolkmannKohlmeyer, 1995; Thongkantha et al., 2009). Currently, Clasterosporium, Nakataea, Pseudotracylla, and Pyricularia are maintained in Magnaporthales (Luo and Zhang, 2013; Zhang et al., 2016). Harpophora is treated as a synonym of Gaeumannomyces (Luo et al., 2015b), while Didymobotryum, Mycoleptodiscus, Phialophora, Sclerotium, and Trichocladium are excluded from Magnaporthales (Klaubauf et al., 2014). Some
pathogenic species produce specialized infection structures (appressoria and hyphopodia) when colonizing their host plants. The hyphal pressing organs can generate strong turgor pressure to penetrate the host cells (Dean et al., 2005). Magnaporthales currently contains over 200 species (Table 1.1). In addition, an increasing number of environmental sequences of uncultured members of Magnaporthales have been deposited in GenBank, suggesting cryptic diversity of the order.
To generate a robust phylogeny for Magnaporthales, Luo et al. (2015a) performed a phylogenomic analysis of more than 200 genes. The results indicated that Magnaporthales is a monophyletic order, sister to Ophiostomatales (Fig. 1.5) (Luo et al., 2015a).
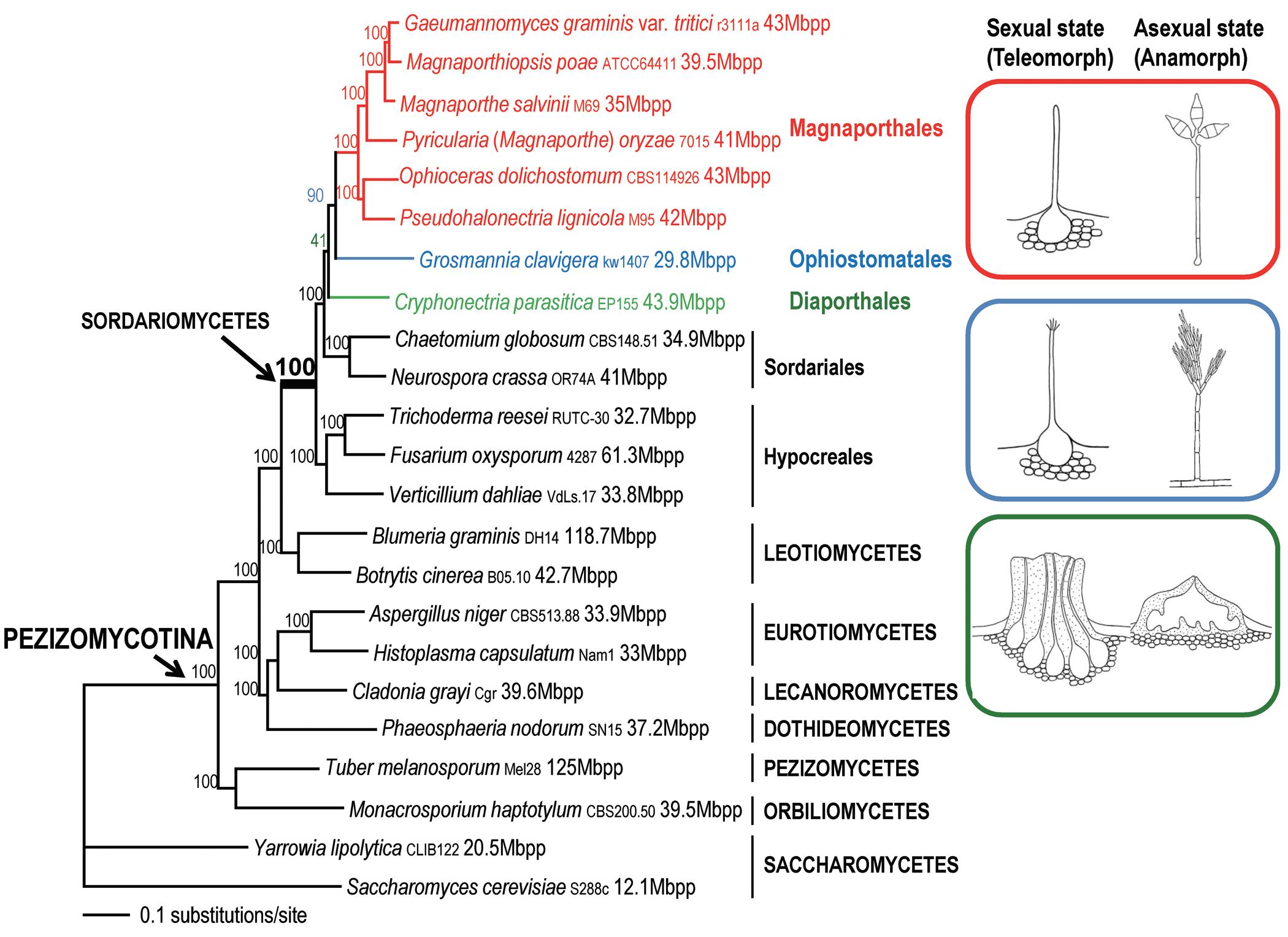
FIG. 1.5. Maximum likelihood tree of 21 Pezizomycotina spp. and two Saccharomycetes spp. used as outgroup species based on 83,616 amino acid positions derived from 226 genes. The morphology of the sexual and asexual states for Magnaporthales and closely related species is shown (right panel). The supporting values for each node were estimated using 1,000 bootstrap replicates. The strain number and genome size for each species are provided (Luo et al., 2015a). (Reproduced according to terms of Creative Commons Attribution 4.0 International License from Luo, J., Qiu, H., Cai, G., et al. Phylogenomic analysis uncovers the evolutionary history of nutrition and infection mode in rice blast fungus and other Magnaporthales. Sci. Rep. 5, 9448 (2015). doi.org/10.1038/srep09448)
TABLE 1.1. Species currently included in Magnaporthalesa
Taxa
Magnaporthaceae
Aquafiliformis lignicola Submerged wood Saprotroph China Luo et al., 2019
Bifusisporella sorghi Sorghum bicolor Endophyte Brazil Silva et al., 2019
Buergenerula biseptata Carex sp.
Buergenerula caricis Carex aquatilis
Unknown Canada, U.K. Cannon et al., 1985
Unknown Canada Arx, 1977
Buergenerula spartinae Spartina sp. Saprotroph Argentina, Canada, U.K., U.S.A Kohlmeyer and Gessner, 1976
Buergenerula zelandica Carex sinclairii, Carex virgata
Unknown New Zealand McKenzie, 1991
Bussabanomyces longisporus Amomum siamense Endophyte Thailand Bussaban et al., 2003; Klaubauf et al., 2014
Clasterosporium caricinum Carex appendiculata, Carex aquatilis, Carex spicata, Scirpus acutus
Clavatisporella musicola Musa sp.
Pathogen Canada, Poland, Russia, U.K., U.S.A. Simmons, 1995
Unknown Indonesia Hyde, 1995
Falciphoriella solaniterrestris Soil Saprotroph Netherlands Hernández-Restrepo et al., 2016
Falciphora oryzae Oryza granulata Endophyte China Luo et al., 2015b; Yuan et al., 2010
Gaeumannomycella caricis Carex rostrata
Gaeumannomycella caricicola Carex remota
Gaeumannomyces amomi Amomum siamense, Alpinia malaccensis
Gaeumannomyces arxii Pennisetum clandestinum, Stenotaphrum secundatum
Gaeumannomyces australiensis
Triticum aestivum
Unknown U.K. Hernández-Restrepo et al., 2016
Unknown Germany Crous et al., 2019
Endophyte Thailand Bussaban et al., 2001
Unknown Australia, U.S.A. Hernández-Restrepo et al., 2016
Unknown Australia Hernández-Restrepo et al., 2016
Gaeumannomyces avenae Agrostis sp., Avena sp. Pathogen Australia, Canada, France, Ireland, Japan, Netherlands, South Africa, U.K., U.S.A.
Gaeumannomyces californicus Stenotaphrum secundatum
Hernández-Restrepo et al., 2016; Walker, 1973;
Unknown U.S.A. Hernández-Restrepo et al., 2016
Gaeumannomyces ellisiorum Deschampsia caespitosa Unknown U.K. Hernández-Restrepo et al., 2016
Gaeumannomyces floridanus Stenotaphrum secundatum
a Courtesy J. Luo and N. Zhang—© APS.
Unknown U.S.A. Hernández-Restrepo et al., 2016
b The following website is used in addition to the listed references: Farr, D. F., and Rossman, A. Y. n.d. Fungal Databases, Systematic Mycology and Microbiology Laboratory. Agricultural Research Service, U.S. Department of Agriculture. http://nt.ars-grin.gov/fungaldatabases/ (continued )
TABLE 1.1. Species currently included in Magnaporthalesa
Magnaporthaceae
(continued )
Gaeumannomyces fusiformis Oryza sativa
Gaeumannomyces glycinicola Glycine max
Gaeumannomyces graminicola
Ctenanthe sp., Stenotaphrum secundatum, Eremochloa ophiuroides
Gaeumannomyces graminis Cynodon sp., Agropyron sp.
Gaeumannomyces hyphopodioides
Avena sp., Pennisetum clandestinum, Triticum aestivum, Zea mays
Unknown U.S.A. Hernández-Restrepo et al., 2016
Unknown U.S.A. Hernández-Restrepo et al., 2016
Unknown Netherlands, U.S.A.
Hernández-Restrepo et al., 2016
Pathogen Italy, U.S.A. Hernández-Restrepo et al., 2016
Pathogen Australia, Germany, Poland, U.K. Hernández-Restrepo et al., 2016
Gaeumannomyces licualae Licuala sp. Saprotroph Brunei Fröhlich and Hyde, 2000
Gaeumannomyces mirabilis Sasa veitchii
Gaeumannomyces nanograminis Cynodon sp.
Gaeumannomyces oryzicola Oryza sativa
Gaeumannomyces radicicola Zea mays
Gaeumannomyces setariicola Setaria italica
Unknown Japan Hino and Katumoto, 1955; Vasilyeva, 1998
Pathogen U.S.A. Vines et al., 2021
Unknown U.S.A. Hernández-Restrepo et al., 2016
Pathogen Canada, China, South Africa Hernández-Restrepo et al., 2016; Luo et al., 2015b
Unknown South Africa Hernández-Restrepo et al., 2016
Gaeumannomyces tritici Triticum sp., Hordeum sp., Elymus repens, Agropyron sp. Pathogen Australia, U.S.A. Hernández-Restrepo et al., 2016
Gaeumannomyces walkeri Stenotaphrum secundatum Unknown U.S.A. Hernández-Restrepo et al., 2016
Gaeumannomyces wongoonoo Stenotaphrum secundatum Pathogen Australia Wong, 2002
Herbampulla crassirostris Carex firma, Sesleria varia Unknown Australia, Switzerland Scheuer and Nograsek, 1993
Kohlmeyeriopsis medullaris Juncus roemerianus Unknown U.S.A. Klaubauf et al., 2014
Magnaporthiopsis agrostidis Agrostis stolonifera Pathogen Australia Wong et al., 2015
Magnaporthiopsis cynodontis Agrostis sp., Cynodon sp., Festuca sp., Poa sp. Pathogen U.S.A. Vines et al., 2020
Magnaporthiopsis incrustans Poa pratensis, Poa annua, Festuca sp., Cynodon sp., Zoysia sp.
Magnaporthiopsis maydis Zea mays
Pathogen U.S.A. Landschoot and Jackson, 1989a; Luo and Zhang, 2013
Pathogen Egypt, Hungary, India, Israel, Portugal, Spain Klaubauf et al., 2014; Samra et al., 1963
continued )
TABLE 1.1. Species currently included in Magnaporthalesa
Taxa Hosts
Magnaporthaceae
Magnaporthiopsis meyeri-festucae
Agrostis sp., Poa annua, Festuca sp.
Magnaporthiopsis panicorum Panicum sp.
Magnaporthiopsis poae Agrostis sp., Festuca sp., Lolium perenne, Poa annua, Poa pratensis
Magnaporthiopsis rhizophila Cynodon dactylon, Panicum virgatum, Poa pratensis, Triticum aestivum
Nutritional modes Geographic locations Referencesb
Pathogen U.S.A. Luo et al., 2017
Unknown U.S.A. Luo et al., 2014
Pathogen China, U.S.A. Landschoot and Jackson, 1989b; Luo and Zhang 2013
Pathogen Australia, South Africa, U.S.A.
Muraeriata africana Bark or wood Saprotroph Kenya
Muraeriata collapsa Bark or wood Saprotroph U.S.A.
Luo and Zhang 2013; Scott and Deacon, 1983
Huhndorf et al., 2008
Huhndorf et al., 2008
Nakataea curvularioides Bromelia pinguin Unknown Cuba Arnold and Castañeda-Ruiz, 1987
Nakataea multiseptata Unidentified tree branches Unknown China Ma et al., 2018
Nakataea oryzae Oryza sp., Zizania sp., Echinochloa sp.
Pathogen Worldwide Luo and Zhang, 2013
Nakataea rarissima Byrsonima crassifolia Unknown Cuba Castañeda-Ruiz and Kendrick, 1990
Nakataea serpens Plant debris Unknown U.S.A. Shearer and Crane, 1979
Nakataea setulosa Unidentified tree branches Unknown China Ma et al., 2014
Neogaeumannomyces bambusicola
Unidentified bamboo Saprotroph Thailand Liu et al., 2015
Omnidemptus affinis Panicum effusum Pathogen Australia Cannon and Alcorn, 1994; HernándezRestrepo et al., 2019
Omnidemptus graminis Unidentified grass Unknown Spain Hernández-Restrepo et al., 2019
Omnidemptus lunatus Carpobrotus glaucescens Unknown Australia Hernández-Restrepo et al., 2019
Pseudophialophora angusta Dichanthelium acuminatum Unknown U.S.A. Luo et al., 2015c
Pseudophialophora dichanthii Dichanthelium acuminatum Unknown U.S.A. Luo et al., 2015c
Pseudophialophora eragrostis Eragrostis sp., Poaceae sp. Unknown U.S.A. Luo et al., 2014
Pseudophialophora magnispora Dichanthelium acuminatum Unknown U.S.A. Luo et al., 2015c
Pseudophialophora panicorum Panicum sp., Poaceae sp. Unknown U.S.A. Luo et al., 2014
Pseudophialophora schizachyrii Schizachyrium sp., Poaceae sp. Unknown U.S.A. Luo et al., 2014
(continued ) (continued )