

XIANG S. YIN MALT



Biochemical Changes During Malting
3.1 The Composition of Barley
Barley is unique in comparison with most other cereals in that the husk stays with the grain after harvest, except for the naked (hulless) varieties. The husk provides protection and contains chiefly nonviable empty cells consisting mostly of hemicellulose, cellulose, lignin, and low amounts of proteinaceous materials. As shown in the longitudinal section of the barley kernel (Fig. 3.1), the embryo is located toward the dorsal, or round, side of the kernel (Palmer, 1983). In mature barley kernels, the major tissue is the endosperm, which serves as the main nutrition storage organ of the kernel (Table 3.1). It is made up of large nonviable cells, each of which is packed with large and small starch granules (A- and B-granules, respectively) embedded in a protein matrix (Fig. 3.2). In the endosperm of mature barley kernels, all traces of the cell nuclei and other organelles have disappeared. The living cells of the barley grain are in the embryo and the aleurone layer, which is about 2–3 cells in cross section. The embryo and the aleurone layer are where the metabolic activities begin when the seeds are placed in humid and warm conditions. The scutellum between the embryo and the endosperm functions in transport of the hydrolytic enzymes to participate in degradation reactions within the endosperm to supply nutrients for the growth of rootlets and acrospires and to permit hormone release to the aleurone layer (Palmer, 1974; Palmer and Bathgate, 1976). In malted barley kernels, the cell walls and proteins become partially degraded and many of the small starch granules are hydrolyzed by enzymes (Fig. 3.3).

FIG. 3.1. Structure of a barley kernel. (Adapted from Palmer, 1983. Courtesy X. S. Yin— © ASBC.)
TABLE 3.1. Proportions of parts of barley grain (in approx. % dry basis)a,b
a Courtesy X. S. Yin—© ASBC.
b Data from Briggs, 1998; Morrison, 1993.
c n.a., not available.

FIG. 3.2. Scanning electron microscopy image of the endosperm of raw barley. (Courtesy A. W. MacGregor— © ASBC)

FIG. 3.3. Scanning electron microscopy image of the endosperm of malted barley. (Reproduced, by permission, from Yin, 2014— © ASBC)
3.2 Starch and Its Degradation
3.2.1
Starch Components
Most cereal starches consist of two main structurally distinct components, amylose and amylopectin. Starch from grain normally contains 20–30% amylose. Examples of exceptions are some waxy cereals (maize, barley, and sorghum), which can have <1% amylose, and certain genetically selected varieties of cereal, like amylomaize, may have amylose contents around 80%. Table 3.2 shows some characteristics of the two starch components.
TABLE 3.2. Comparison of the two components of barley starcha,b
Property
Ratio in starch of conventional barley
Structurec

(Essentially unbranched)
Linkage type
Average chain length (number of glucose residues)
α-(1,4) linkages (and a very low proportion of α-(1,6) linkage at rare branching points)

α-(1,4) linkage and α-(1,6) linkage at branching points
X-ray diffraction
Iodine coloration (λmax)
Enzymes to which starch component is susceptible
Intense blue (~660 nm)
α-Amylase
β-Amylase
α-Glucosidase/amyloglucosidase
Phosphorylase
Purple (530–550 nm)
α-Amylase
β-Amylase (limited by branching points)
Limit dextrinase
α-Glucosidase/amyloglucosidase
Phosphorylase
Tendency toward retrogradation in solution
Conversion into maltose by β-amylase
Conversion into maltose by debranching enzymes, then by β-amylase
a Courtesy X. S. Yin—© ASBC.
b Data from MacGregor and Fincher, 1993; Yin, 1986.
c Drawings courtesy A. W. MacGregor—© ASBC.
Amylose is generally regarded as a linear polymer with extremely few branching points. It is therefore very susceptible to intensive degradation, but the linear nature of the molecule makes it more prone to annealing and retrograding in solution when subjected to declining temperature, making it hard to remain soluble for further degradation. Amylopectin, in comparison, possesses a well-defined branched organization known as the cluster structure. It is relatively stable in solution even with a much higher molecular weight than amylose. Due to the differences in the dimensional structure from amylose, amylopectin has restrictions to amylase access at the branching points and therefore reacts differently to an enzyme attack, hence leading to differences in the beer quality and sensory characteristics, e.g., branched dextrin content that affects the mouthfeel and calories of the beer.
3.2.2 Gelatinization Temperature
It is generally recognized that both wheat starch and barley starch possess two types of granules, the A-granules (large) and the B-granules (small) (Fig. 3.4). The ratio of small to large starch granules varies according to the phylogenetic origins of the barley starch genes. It is generally accepted that 90% of the total starch granules (by number) in barley are the small granules, which account for about 10% of the total weight of the barley starch (Stark and Yin, 1986). The small granules are embedded in the protein matrix and are therefore more difficult to dissolve; however, during malting, they are preferentially degraded by enzymes. Amylose and amylopectin exist in a well-defined crystalline arrangement within the starch granule, making them difficult to dissolve in water without heating. Gelatinization of starch refers to the disassociation of the crystalline structure of the starch components, leading to the collapse of the granule. This is an essential step in order for starch to liquefy for enzymatic conversion in the

brewhouse. The small granules have higher and broader gelatinization temperatures than large granules in general (Briggs et al., 2004).
The gelatinization temperature of starch is very dependent on the cereal source, possibly due to the makeup and texture of the starch granule. For starches from barley and oats, gelatinization occurs in the temperature range of 51–60°C, significantly lower than that for starches from other commonly used cereals such as maize (62–72°C), wheat (58–64°C), sorghum (68–78°C), and rice (68–78°C) (Lineback, 1984).
Differential scanning calorimetry (DSC) is a conventional technique that demonstrates the temperatures and heat flows associated with transitions in ingredients as a function of temperature and time in a controlled setting. The measurements present quantitative and qualitative data about the physical and chemical transitions associated with endothermic or exothermic processes and modifications in heat capacity. Figure 3.5 exhibits the DSC curves of two pairs of raw and malted wheat and barley samples (Yin, 2011). The results indicated that the malting process slightly increases the onset and peak temperatures of the barley and wheat samples but significantly reduces the energy required to gelatinize the starch granules in both sets of cereal samples. The wheat sample set showed lower gelatinization temperatures but higher energy demand than their barley counterparts. Rapid visco analysis (RVA) is currently utilized to provide more rapid information on the pasting behaviors of cereals and their malts (Yin et al., 2019), and more discussion is provided later in this chapter.

FIG. 3.5. Comparison of gelatinization curves of barley, barley malt, wheat, and wheat malt showing the varying onset and peak temperatures by sample type. Barley and malted barley (blue lines) appear to require less energy to gelatinize but higher onset peak temperatures than their wheat and malted wheat counterparts. The broken lines represent malted samples, and the solid lines represent the unmalted grain. (Reproduced from Yin, 2011. Courtesy X. S. Yin—© ASBC.)
3.2.3 Starch-Degrading Enzymes
Four major enzymes are involved in the degradation of barley starch (Table 3.3). The most basic one is α-amylase, an endoenzyme that randomly attacks α-(1,4) linkages in amylose and amylopectin. Studies have shown that α-amylase from germinated barley has multiple forms. β-Amylase is an exoenzyme that cleaves maltose units from the nonreducing ends of chains by the selective stepwise splitting of α-(1,4) glucosidic linkages. Due to its lower thermostability than that of α-amylase, about 55% of its enzyme activity survived during the drying and curing process at 80°C for 5 hours (Narziss and Rusitka, 1977). Multiple forms of β-amylase are present in the endosperm. The debranching enzyme, limit dextrinase, slowly hydrolyses α-(1,6) glucosidic linkages in
TABLE 3.3. Key starch-degrading enzymes in malta Enzymes
Stage of formation
Upon germination
Cleavage linkage Randomly on α-(1,4) linkages
Mode of cleavageb

Reaction product
and smaller dextrin, sugars
Bound form in grain but released during germination
Every other α-(1,4) linkage from the nonreducing end, stopping at the branching points forming limit dextrins
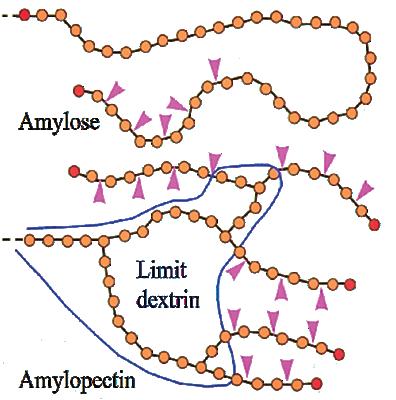
Upon germination During kernel formation before maturation
α-(1,6) linkage, i.e., branching points
Every α-(1,4) linkage from the nonreducing end


Mash thermostabilityc
a Courtesy X. S. Yin—© ASBC.
b Drawings courtesy A. W. MacGregor—© ASBC.
c Evans, 2020: Enzyme thermostability temperature is approximated by estimating the temperature at which 50% of activity remains after 30 minutes of heating.
the amylopectin molecules but rapidly hydrolyses starch-derived dextrins, hence the name “limit dextrinase.” Meanwhile, α-glucosidase catalyzes the conversion of the end products of the action by dextrinase and amylases on starch to glucose but has limited action on larger molecules. Its activity in germinated barley is stable around 50°C but declines at higher temperatures during the later stage of kilning. Limit dextrinase, α-(1,4) glucosidase, and β-amylase are inactivated more readily, but α-amylase remains relatively active even at low moisture of finished malt. However, if the diastatic activities are to be preserved for distillers, the condition of kilning should be controlled at a mild level. During mashing, as the temperature rises toward 70°C, most malt enzymes denature rapidly.
Additionally, starch phosphorylase, also known as α-glucan phosphorylase, catalyzes the degradation of amylose to glucose-1-phosphate and completes similar reactions on amylopectin together with limit dextrinase. However, the main function of this enzyme is for biosynthesis of α-(1,4) glucans using glucose-1-phosphate, since some starch phosphorylase activities have been identified and characterized in barley grains and shown to be present during starch synthesis. The exact role of the enzyme in starch synthesis still remains to be elucidated (Cuesta-Seijo et al., 2017).
In mature barley grain, β-amylase is present at high concentrations, although some of it is in a bound and ineffective form, while α-amylase and limit dextrinase are only present at very low levels. The activity of α-glucosidase is present but in limited amount in the grain. During germination, these enzymes are synthesized or activated to high levels. Acting in concert with other hydrolytic enzymes (β-glucanases, arabinoxylanases, and proteases), α-amylase passes into the endosperm cells and initiates starch degradation on the granules. Other starch-degrading enzymes play cooperative roles in further degrading the primary products of α-amylolysis of the native starch granules, resulting in pitted surfaces of the granules in affected parts of the endosperm (Fig. 3.6). The α- and β-amylases are the most important diastatic enzymes in malting and brewing. However, they can only degrade starch to yield products with about 70% fermentable sugars without the presence of limit dextrinase and α-glucosidase.
Similar to wheat starch, the size distribution of the barley starch granules exhibits a bimodal pattern. During malting, the small starch granules are preferentially degraded; however, in the mashing process, intact small granules tend to survive the brewhouse operations and remain in the spent grain.
3.3 Proteins and Proteolysis
Proteins are a critical quality aspect of barley. Proteins provide the base material for beer foam-active protein, enzymes and free amino nitrogen (FAN) for yeast nutrition, and they contribute to flavor and haze characteristics within the final product.

Proteins are the major ‘working molecules’ within cells, as proteins catalyze reactions, act as crucial building blocks for many cellular structures, and provide storage for the nitrogenous material in grain. Proteins are present in different types and have different primary locations in the kernel.
When barley kernels are subjected to warm and humid conditions, proteins readily break down to build new tissues such as rootlets and acrospires. The largemolecular-weight storage proteins are degraded to smaller, soluble fragments that travel from the endosperm to the growing tissues. Therefore, amino acids and peptides are intermediary metabolites in the germinating phase of the grain. At the point when germination is arrested during kilning, about 35–50% of the proteins become soluble, as indicated by the malt Kolbach index (KI). Some of the amino acids are transferred to the rootlets, which are then removed from the grain. Consequently, the nitrogen level in malt is normally about 0.2% less than that of barley depending on the intensity of respiratory loss.
Historically, barley proteins have been categorized by their solubility in water, a dilute salt solution, an alcohol solution, dilute alkali or acid solutions, or in the presence of surfactant (Table 3.4). While this basic and simple fractionation practice is still broadly used today, classification by functional properties has become increasingly more common. Storage proteins, i.e., hordeins and globulin, account for >50% of the protein content of barley grains and are therefore critical to the functionality of malt. The globulins are present in the embryo and aleurone layer, and hordeins (prolamins) are present in the endosperm. The hordeins are the most abundant storage proteins, accounting for 30–55% of the total proteins, and impact much of the malt quality.
Glutelin Soluble in weak alkali, acid, or surfactant
Hordein (prolamin) Soluble in 70–80% alcohol 35–55%
Globulin Soluble in dilute salt solution and mash 15–20%
Albumin Water soluble (also alkali soluble)
a Courtesy X. S. Yin—© ASBC.
b Data from Osborne, 1907; Steiner et al., 2011.
Located in aleurone; insignificant contribution to wort nitrogen with most left in spent grains
Endosperm prolamins; major contributor to wort nitrogen; rich in proline and glutamic acid but poor in lysine and methionine; degraded during germination, but some remains in spent grain
Located in embryo and aleurone; serves many functions like forming enzymes; boiling does not entirely precipitate; rich in lysine and methionine
Serves in enzymes; precipitated by boiling; rich in lysine and methionine; susceptible to enzyme attack
Figure 3.7 illustrates the distribution of proteins in the barley kernel, showing that the protein component is not uniformly distributed throughout the kernel. In reality, proteins tend to concentrate mostly toward the outer portion of the kernel. Therefore, partial modification of the grain will lead to the preferential release of proteins in the outer layers while the components in the inner regions of the endosperm, such as β-glucans, remain intact. In the hydrated areas of the kernel, proteolysis and cytolysis run parallel to each other, and it is difficult to control them separately.
Like the starch-degrading enzymes, protein-degrading enzymes follow either an endo or exo mode of reaction. An exopeptidase catalyzes the cleavage of the terminal peptide bond of the peptide chain, releasing a single amino acid (residue), not a dipeptide. An exopeptidase could be either aminopeptidase or a carboxypeptidase depending on whether the attack is from the amino or the carboxy terminal, respectively. Endopeptidases, referred to as proteinases or proteases, catalyze the hydrolysis of protein in no specific order on the peptide chain, releasing smaller peptides instead of amino acids (Fig. 3.8).
More detailed reports (Jones, 2005; Steiner et al., 2011) on the proteinases in barley and malt have indicated that there are more than 40 different enzymes based on their mechanisms of enzymatic reaction. The progression of the solubilization of protein through enzymatic degradation in malting and brewing is not well understood, but it is recognized that endoproteases initiate the breakdown of proteins to smaller fragments and then exopeptidases further degrade the peptides to individual amino acids. Activities of proteases reach the highest levels usually after 3 days into germination. As germination progresses, soluble protein increases, as shown in the study of germinating malt over a period of days in the malthouse (Yin et al., 2017). In Figure 3.9, the release of soluble protein was observed in samples of the barley varieties Pinnacle and CDC Copeland (both are two-rowed barleys) over a period of 4 days during germination. However, it should be noted that the release of soluble protein progressed much more slowly in the sample of Pinnacle barley, as the value doubled in the same period for the sample of Copeland from day 1 to day 4 of germination. Major classes of proteolytic enzymes include cysteine-, serine-, aspartic-, and metallo-class proteases (Jones, 2005; Steiner et al., 2011). The cysteine-class proteases and metalloproteases likely play more important roles in protein solubilization than the aspartic- and serine-class proteases. The cysteine-class proteases are active at acidic pH levels ranging from 3.8 to 5.0, and

FIG. 3.7. Protein distribution in barley endosperm observed under the light microscope. The eosin yellow staining (pink color) highlights that the protein is concentrated in the aleurone layer and the outer layers of the endosperm. Note the three layers of aleurone cells, characteristic of barley. (Courtesy A. W. MacGregor—© ASBC)

FIG. 3.8. Hydrolyzing mechanism of protein-degrading enzymes. (Courtesy X. S. Yin—© ASBC)

FIG. 3.9. Change in soluble protein level in barley during germination. Varieties: Pinn, Pinnacle; Cope, Copeland. D, Days of germination. (Data from Yin et al., 2017. Courtesy X. S. Yin— © ASBC.)
metallo-class proteases are optimally active between pH levels of 4.7 and 6.5. Carboxypeptidase is considered to be the main exopeptidase in the degradation of storage proteins and possibly in the liberation of bound β-amylase, with pH optima of 4.8–5.7. Aminopeptidase has a more neutral and alkalinic pH optima than carboxypeptidase, hence having a lesser role in releasing amino acids during the malting and brewing processes. The thermostability of these proteolytic enzymes is relatively low (around 55°C or 131°F). Therefore, the production of free amino acids, particularly in isothermal infusion mashes at 65°C, is quite minimal.
3.4 Cell Walls and Their Degradation
3.4.1
Structure of Cell Walls
The major nonstarchy polysaccharides in the cell walls of barley are arabinoxylans and mixed linkage (1,3)(1,4)-β-D-glucans, or simply referred to as pentosans and β-glucans, respectively. Their molecular structures are not as well defined as the components of starch. Therefore, their physical properties, like solubility and rheological behaviors, vary widely depending on their genotypic and cellular origins. Table 3.5 summarizes the basic biochemical properties of the two components in detail.
