

MASHING

Evan Evans
CHAPTER 5
Mash Substrates and Enzymes: Cell Walls
5.1 Know Thy Substrate: Cell Wall Non-Starch Polysaccharides
Cell walls are made up of from around 5–6 very high molecular weight nonstarch polysaccharides (NSP). Although NSP’s can be polymers of glucose like starch (e.g., β-glucan), the polymeric linkages of NSP’s are β-glucosidic and that their polymeric or substituting units can be based on sugars other than glucose, such as xylose, arabinose, xylose, galactose, etc. (Fig. 5.1). Also, in contrast to starch whose role is for energy storage, the primary role for NSP’s is structural. The composition of cereal grain cell walls is typically dominated by β-glucan and arabinoxylan (aka pentosan or hemicellulose). Typical barley cell wall compositions of importance to brewing are shown in Tables 5.1 and 5.2. The relative proportions are dependent on which cell walls are being analyzed and if the barley is 2-row (more plump, extra starch), or 6-row (less plump, less starch), or hull-less. The fact that hull-less barley has been developed indicates that a substantial amount of cell wall NSP is bound in the husk, which is cellulose-rich, but has minimal potential to be solubilized in the mash or during malting.
Most grain cell walls are composed of around 2–3X as much arabinoxylan as β-glucan. The exception is the barley endosperm (see Fig. 3.2) where β-glucan is 3X as prevalent as arabinoxylan. In barley aleurone and coleoptile cell walls (Fig. 3.2) the abundance pattern is reversed and similar to the cell wall composition of other grains such as wheat in which arabinoxylan dominates. Other cell wall NSP, generally in reduced proportions, include cellulose, heteromannan, xyloglucan and pectin. An exception of potential importance to brewing is rice endosperm, regularly used as an unmalted starch adjunct, where the proportion of cellulose is 36.3% and arabinoxylan is 32% (Table 5.2). Traditionally, brewers have been particularly interested in β-glucan and less so in the other cell wall NSPs, which was driven by their perceived impact on brewing efficiency. β- Glucan can, if not sufficiently modified or degraded, can reduce extract recovery, the lautering rate, and the filtration efficiency during the later phases of the brewing process, and potentially be responsible for undesirable hazes in the beer.
The molecular structure of the of cell wall NSPs is key to how they behave during the mashing and how they are hydrolyzed during both malting and mashing. β- Glucan (Fig. 5.1A) which is soluble in water, contains approximately
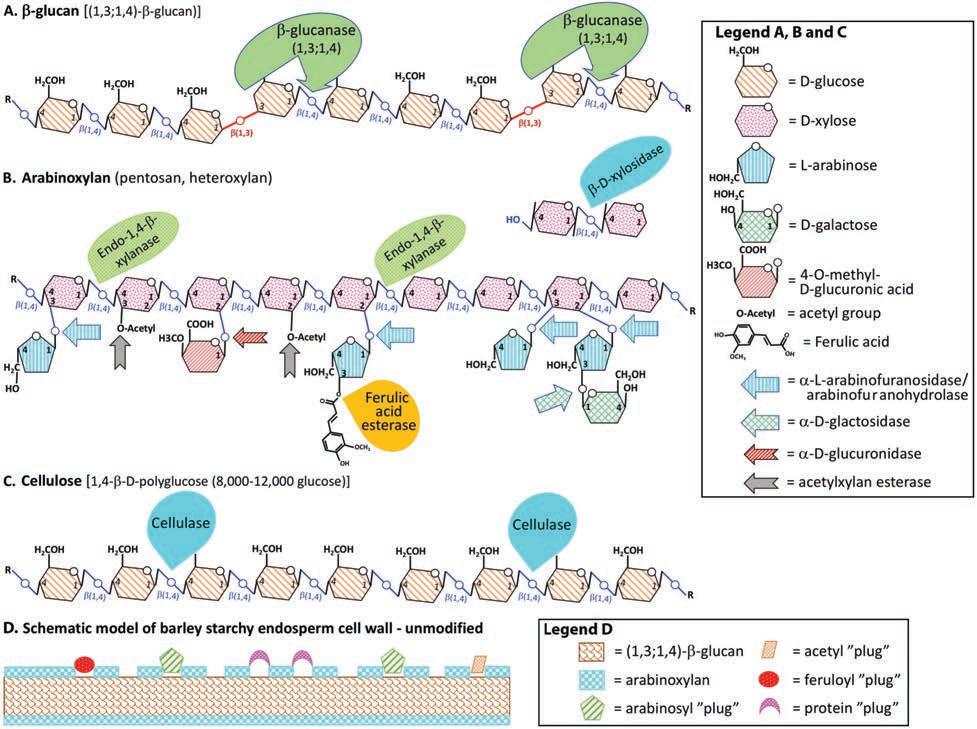
Fig. 5.1. Schematic representation of the major cell wall non-starch polysaccharides and their hydrolysis by enzymes during malting and mashing (based on Fincher and Stone, 1985). A, β- Glucan; B, arabinoxylan; C, cellulose; and D, a simple model proposed for the starchy endosperm cell wall by Bamforth and Kanauchi (2001). (Courtesy E. Evans—© ASBC and MBAA)
70% (1,4)-β-glycosyl and 30% (1,3)-β-glycosyl linkages or “kinks,” typically with a (1,3)-linkage interrupting sequences of 2–3 but up to 14 (1,4)-linkages (Fincher & Stone, 1985). The molecular weight for barley β-glucan has been reported to range from 31 × 103 to 2,340 × 103 (Lazaridou & Biliaderis, 2007), with typical average molecular weights for β-glucan ranging from 200,000 to 300,000 Da, which suggests degrees of polymerization of between 1,200–1,850 (Fincher & Stone, 1985).
Arabinoxylan, otherwise known as heteroxylan, pentosan, or hemicellulose (Fig. 5.1B), is based on a (1,4)-β-xylopyranosyl backbone with a high proportion of substitution through the C(O)2 and C(O)3 xylose atoms on that backbone. The typical substituents of the xylan backbone include α-arabinosyl, α-galactosyl, glucoronosyl, methyl, acetyl and ferulic acid.
The final major cell wall NSP to be discussed is cellulose. Cellulose is a polymer of (1,4)-linked β-glucopyranosyl residues containing thousands of residues per chain (Fincher & Stone, 1985). While a small amount of cellulose has been
Table 5.1. Estimates of the proportion of barley whole grain weight that can be attributed to β-glucan, arabinoxylan, cellulose and total non-starch polysaccharides (NSP)a
Proportion barley weight (%)
Sample number Reference
2.4–8.3 7.4–15.78.5–17.1 22.6–41.1 39d Holtekjolen et al., 2006
3.6–4.3 nr nr nr 5 Manzanares & Sendra, 1996
3.5–10. 6 nr nr nr
Unknown Bamforth, 1994
3.4–5.7 4.4–7.8 nr nr 17 Henry, 1986
2.4–3.6 nr nr nr 11 Forest & Wainwright, 1977 nr 3.7–4.3 nr nr Hull-less Egi et al., 2004 nr 4.4–9.8 nr nr 2-row Egi et al., 2004 nr 5.2–11.0 nr nr 6-row Egi et al., 2004
a Courtesy E. Evans—© ASBC and MBAA.
b nr = not reported.
c Cellulose primarily localized to the husk and effectively unextractable.
d Includes hull-less varieties.
Table 5.2. Comparison of cell wall non-starch polysaccharides in the walls of grains and seed (% w/w)a,b
a Courtesy E. Evans—© ASBC and MBAA.
b Based on Burton & Fincher, 2014.
c Fincher, 1975.
d nd = not detected.
e 4 days after germination (Gibeaut et al., 2005).
f nr = not reported.
g Bacic & Stone, 1981.
h Pellny et al., 2012.
i Shibuya & Iwasaki, 1978.
j Zhang et al., 2014.
localized to the endosperm and aleurone layer cell walls, the overwhelming majority of cellulose is associated with the coleoptile cell walls (see Fig. 3.2) and most notably the husk (Tables 5.1 and 5.2) (Henry, 1988). The association of similar proportions of cellulose, arabinoxylan and lignin in the husk (Olkku et al., 2005) ensures that little of this NSP is available for extraction during mashing. Although the husk typically comprises 9–13% of barley weight (Evers & Miller, 2002; Whitmore, 1960), it is largely disposed of with the spent grains after mashing. The husk does serve a purpose in enabling efficient lautering and even with the finely ground grists used for mash filtration (Evans et al., 1999). The husk’s lack of potential extract has encouraged brewers, breeders and maltsters to consider hull-less or naked barley varieties for improving brewing efficiency, in
combination with mash filters (Evans et al., 1999). Although small scale results with hull-less barley malt have been very encouraging, commercial scale trials do not provide the expected extract yields due to ‘case hardening’ of the barley corns during kilning (Thomas 1986; Stewart et al., 2004; McCaig et al., 2006).
From the perspective of mashing there are two properties of cell wall NSP that are important. Firstly, there is their solubility or their propensity to be released into solution by enzyme action. Of course, the second important characteristics is their size. Although, cell wall NSPs (e.g., β-glucan) can ultimately be hydrolyzed down into sugars that can potentially be utilized by yeast, hydrolysis typically does not extend to this extent as a result of the malting and mashing processes. Hence, if the cell wall NSP is solubilized and is large enough it can cause β-glucan gel formation, and reductions in the efficiency of the lautering, extract recovery, gels and beer filtration processes. It is however clear that actual size trumps total amount because beers containing relatively high levels of β-glucan can filter efficiently if the molecular weight of that β-glucan is less than 120 kDa (Stewart et al., 1998).
5.2 The Non-Starch Degrading Enzymes
5.2.1 Cell Wall NSP Degrading Enzyme: β- Glucanase
β- Glucanase or rather (1,3;1,4)-β-D-glucan endohydrolase is considered to be primarily responsible for the degradation of cell walls (1,3;1,4)-β-glucan in the endosperm (Fincher 1989) and presumably the β-glucan component of the aleurone layer as well. Like LD, there is a portion of β-glucanase activity present in the mature barley grain, but the majority of activity is expressed after germination (Kunz and Bamforth, 2007; Cooper et al., 2016) There are two isoenzymes of β-glucanase: GI which is expressed by the scutellum and GII which is expressed by the aleurone layer (see Fig. 3.2) that are induced by GA and 10 mM Ca 2+, (Stuart et al., 1986). Both isoenzymes are secreted from the aleurone layer into the rapidly modifying endosperm after germination. The G1 iso- enzyme is substantially less thermostable than the GII enzyme, to the extent that all the activity in kilned malt can be attributed to the GII β-glucanase form (Loi et al., 1987). As the (1,3;1,4)-β-D-glucan endohydrolase name suggests, β-glucanases substrate binding and cleavage is dependent on the (1,3)-β-linkage being adjacent to at least one (1,4)-β-linkage (Fig. 5.1A) (Hrmova & Fincher, 2001).
The pH and thermostability characteristics of β-glucanase suggest it is more active during malting than mashing. The pH optimum of the β-glucanases can be narrowed to the range pH 3.7–5.0 (Chen et al., 1995; Stewart et al., 2001), which is broadly aligned with the pH ~5.0 measured for germinated barley endosperm (Mikola & Mikola, 1980; Simpson 2001) but somewhat lower than the typical mash pH range of 5.0–6.0 for typical mashes (Briggs et al., 2004). Finally, the consensus for the mash thermostability of β-glucanase (GII) during mashing is <50°C (Loi et al., 1987; Stewart et al., 2001; Chen et al., 1995), making the 45°C rest of the Congress mash (Fig. 2.1A) is truly a β-glucanase, not a prote-
ase rest (Jones, 2005). Consistent with this conclusion, Muller (1995) observed that the half-life of β-glucanase was approximately 40 min at 45°C. In addition, Kanauchi and Bamforth (2001) found that increased extraction of β-glucan from barley was progressively achieved using water heated to 45, 65, or even 80°C. Overall, it is probably safe to conclude that the majority of β-glucan hydrolysis by β-glucanase occurs during malting, although some further modification may occur during mashing, particularly when a β-glucanase rest at 45°C is included in the mashing temperature program.
5.2.2 Cell Wall NSP Degrading Enzyme: Cellulase and β- Glucan Solubilase
Cellulose is degraded by cellulase, also known as (1,4)-β-glucanase (Fig. 5.1C).
One of the earliest reports observing a cellulase in malt was from Sandegren and Enebo (1952), although later studies showed most malt cellulase activity is contributed by the microflora inhabiting the external layers (husk) of the grain (Hoy et al., 1981). Fincher (1989) concluded that the level of barley cellulase in germinating barley is low and is mostly contributed by the grains microflora during the wet phases of malting. Certainly, the grain microbes have been shown to contribute a number of enzymes that may be useful during mashing such as cellulase, β-glucanase and proteases (Laitila, 2008; van Nierop et al., 2006), but to date, the level of the contribution does not always appear to be measurable or predictable.
Perhaps the most enduring ‘enzyme’ still requiring complete characterization is ‘β-glucan solubilase.’ Bamforth and Martin (1981) identified a relatively thermostable enzyme whose activity that could survive mashing to increase the level of β-glucan in the wort. β- Glucan solubilase was initially postulated to increase the release of hemicellulose into solution (Bamforth & Martin, 1981) but was never been purified to homogeneity. Subsequently, Yin et al. (1989) provided robust evidence that β-glucan solubilase activity originated from cellulases that are contributed by fungi. Over time, various enzyme activities have been linked with β-glucan solubilase which include (1-3)-β-glucanase, carboxypeptidase, phospholipases, (1- 4)- endo-β-glucanase, feruloyl esterase, xyloacetyl esterase and arabinofuranosidase (Jin et al., 2004; Kunz & Bamforth, 2007; Bamforth, 2009; Bamforth, 2020). Still, an activity that releases relatively high molecular weight β-glucan and arabinoxylan during mashing was observed, be it derived from fungi or “β-glucan solubilase” or from a carboxy-peptidase or a ferulic acid esterase (Moore et al., 1996). The point is that if these enzyme/s reduce wort viscosity, whatever the source, then they are of practical value for brewing. A simple model of the endosperm cell wall was proposed by Bamforth and Kanauchi (2001) which suggested that ferulic acid esterase, or even carboxy-peptidase activity, could improve the release of arabinoxylan and by extension, β-glucan, to hasten the solubilization of the cell wall (Fig. 5.1D). Further work is still required to understand this activity and to better understand the importance of ferulic acid esterase to mashing and malt quality, and how it might be measured and manipulated to improve malt quality for mashing.
5.2.3
Cell Wall NSP Degrading Enzyme: Xylanase
Although arabinoxylan or heteroxylan is based on a (1,4)-β-xylopyranosyl backbone, the high proportion of substitutions, mainly through the C(O)2 and C(O)3 xylose atoms, ensures that several enzymes are required for its full hydrolysis (Fig. 5.1B). Each of the substitutions to the xylose backbone requires the appropriate enzyme to hydrolyzing the substituted group:
1. α-L-arabinofuranosidase/arabinofuranohyrolase (L-arabinose)
2. α-D-galactosidase (D-galactose)
3. α-D-glucuronidase (4- O-methyl-D-glucuronic acid)
4. Ferulic acid esterase (ferulic acid)
5. Acetylxylan esterase (O-acetyl groups)
The final two enzymes hydrolyze the xylose back bone, the exo-β-D-xylosidase and finally the endo-(1,4)-β-xylanase or endo-xylanase. A recent report observed that including purified arabinofuranohyrolase into a Congress mash of a problematic Chinese variety (Dan’er) improved mash separation and resultant wort quality (Li et al., 2015).
In the overall collection of arabinoxylan degrading enzymes, it appears that endo-xylanase has the most important activity for mashing, and, for the subsequent lautering and beer filtration stages of the brewing process. Xylanase activity is present in the mature barley grain and the level of activity approximately doubles as a result of malting (Kuntz & Bamforth, 2007). The endo-xylanase thermostability of approximately 55–60°C and the optimal pH range of 4.5–7.0 suggests that the enzyme may be active both during malting and mashing (Kanauchi et al., 2013; Li et al., 2005; Slade et al., 1989). In support of this contention, Li et al. (2005) showed that during a stepped mash (49°C/62°C/71°C), wort arabinoxylan content decreased from 1,510 to 1,170 mg/L during the course of mashing. At the very least, recent analysis shows that malt xylanase activity is highly correlated with Congress wort viscosity (n = 34), r = –0.84, where the correlation with βglucanase was r = –0.580 and cellulase was r = 0.454 (P < 0.01) (Cornaggia et al., 2019). Within the same investigation, malt xylanase activity was also shown to be an important parameter (along with viscosity and KI) for predicting the results for the SWIFT prediction of eventual beer filterability (Stewart et al., 2000).
5.3 Summary of Cell Wall NSPs and Hydrolyzing Enzymes
A brewer’s primary concern with cell wall NSP’s are their potential impact on process filtration events (lautering and beer filtration), where they reduce process efficiency, and as a potential undesirable factor with respect to beer hazes. The two-main cell wall NSP’s are (1,3;1,4)-β-glucan and arabinoxylan whose schematic structures are shown in Figure 5.1. The level of β-glucan is approximately 2–3X the level of arabinoxylan in barley endosperm cell walls, but the proportions are reversed for all other cell walls. The key impact of these two
NSPs is their size and solubility. The size of NSPs is important in terms of viscosity and also blocking the pores of filters. (1,3;1,4)-β- Glucanase is the primary enzyme for hydrolyzing β-glucan. Its relatively low pH optima range 3.7–5.0, and relatively low thermostability (<50°C) suggests that most of β-glucanase action occurs mostly during malting unless a 45°C β-glucanase rest is included during mashing. It should be noted that β-glucan size is most important with respect to detrimental brewing effects with a molecular weight of 120kDa seemingly being the key cut- off threshold (Stewart et al., 2000). Endo-xylanase is the other key NSP enzyme and has been less rigorously studied in the brewing context compared to β-glucan. The pH 4.5–7.0 optima range for endo-xylanase and thermostability range of 55–60°C suggests that endo-xylanase may operate both during malting and mashing.
References
Bacic, A., and Stone, B. A. (1981). Chemistry and organization of aleurone cell wall components from wheat and barley. Funct. Plant Biol. 8:475- 495.
Bamforth, C. W. (1994). β-glucan and β-glucanases in malting and brewing: Practical aspects. Brew. Dig. 69:12-21.
Bamforth, C. W. (2009). Current perspectives on the role of enzymes in brewing. J. Cereal Sci. 50:353-357.
Bamforth, C. W. (2020). The Horace Brown Medal. Forever in focus: Researches in malting and brewing sciences. J. Inst. Brew. 126:4-13.
Bamforth, C. W., and Kanauchi, M. (2001). A simple model for the cell wall of starchy endosperm in barley. J. Inst. Brew. 107:235-240.
Bamforth, C. W., and Martin, H .L. (1981). β-glucan and β-glucan solubilase in malting and mashing. J. Inst. Brew. 87:365-371.
Briggs, D. E., Boulton, C. A., Brookes, P. A. and Stevens, R. (2004). Brewing: Science and practice. CRC Press, New York.
Burton, R. A., and Fincher, G. B. (2014). Evolution and development of cell walls in cereal grains. Fron. Plant Sci. 11:456- 471.
Chen, L., Garrett, T. P. J., Fincher, G. B., and Hoj, P. B. (1995). A tetrad of ionizable amino acids Is important for catalysis in barley β-glucanases J. Biol. Chem. 270:8093- 8101.
Cooper, C., Evans, D. E., Yousif, A., Metz, N., and Koutoulis, A. (2016). Comparison of the impact on performance of small-scale mashing with different proportions of unmalted barley, Ondea Pro®, malt and rice. J. Inst. Brew. 122:218-227.
Cornaggia, C., Evans, D. E., Draga, A., Mangan, D., and McCleary, B. V. (2019). Prediction of potential malt extract and beer filterability from conventional and novel malt quality assessment parameters. J. Inst. Brew. 125:294-309.
Egi, A., Speers, R. A., and Schwartz, P. B. (2004). Arabinoxylans and their behaviour during malting and brewing. MBAA Tech. Q. 41:248-267.
Evans, D. E., Vilpola, A., Stewart, D. C., Stenholm, K., Pöyri, S., Washington, J. M., Barr, A. R., and Home, S. (1999). Pilot scale investigation of the importance of the barley husk for mash filtration. MBAA Tech. Q. 36:375-382.
Evers, T., and Millar, S. (2002). Cereal grain structure and development: Some implications for quality. J. Cereal Sci. 36:261-284.
Fincher, G. B. (1975). Morphology and chemical composition of barley endosperm cell walls. J. Inst. Brew. 81:116-122.
Fincher, G. B. (1989). Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Ann. Rev. Plant Physiol. Plant Mol. Biol. 40:305-346.
Fincher, G. B., and Stone, B. A. (1985). Cell Walls and Their Components in Cereal Grain Technology. American Association of Cereal Chemists, St. Paul, MN.
Forrest, I. S., and Wainwright, T. (1977). Differentiation between desirable and troublesome β-glucans. Pages 401-413 in: Proc. 16th Eur. Brew. Conv. Cong., Amsterdam.
Gibeaut, D. M., Pauly, M., Bacic, A., and Fincher, G. B. (2005). Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 221:729-738
Henry, R. J. (1986). Genetic and environmental variation in the pentosan and β-glucan contents of barley and their relation to malting qualty. J. Cereal Sci. 4:269-277.
Henry, R. J. (1988). The carbohydrates of barley grains—A review. J. Inst. Brew. 94:71-78.
Holtekjolen, A. K., Uhlen, A. K., Brathen, E., and Knutsen, S. H. (2006). Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem. 94:348-358.
Hoy, J. L., MacCauley, B. J., and Fincher, G. B. (1981). Cellulases of plant and microbial origin in germinating barley. J. Inst. Brew. 87:77- 80.
Hrmova, M., and Fincher, G. B. (2001). Structure-function relationships of β-D-glucan endo- and exohyrolases from higher plants. Plant Mol. Biol. 47:73-91.
Jin, Y.-L., Speers, R. A., Paulson, A. T., and Stewart, R. J. (2004). Barley β-glucans and their degradation during malting and brewing. MBAA Tech. Q. 41:231-240.
Jones, B. L. (2005). Endo-proteinases of barley and malt. J. Cereal Sci. 42:139-156.
Kanauchi, M., and Bamforth, C. W. (2001). Release of beta-glucan from cell walls of starchy endosperm of barley. Cereal Chem. 78:121-124.
Kanauchi, M., Chijimi, A., Ohnishi-Kameyama, M., and Bamforth, C. W. (2013). An investigation of two xylan- degrading enzymes and a novel xylanase inhibitor in malted barley. J. Inst. Brew. 119:32- 40.
Kuntz, R. J., and Bamforth, C. W. (2007). Time course for the development of enzymes in barley. J. Inst. Brew. 113:196-205.
Laitila, A. (2008). More good than bad: Microbes in the maltings. Brew. Dist. Int. 4:52-54. Lazaridou, A., and Biliaderis, C. G. (2007). Molecular aspects of β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 46:101-118.
Li, X., Gao, F., Cai, G., Jin, X., Lu, J., Dong, J., Yin, H., Yu, J., and Yang, M. (2015). Purification and characterisation of arabinoxylan arabinofuranose hydrolase I responsibile for the filterability of malt. Food Chem. 174:286-290.
Li, Y., Lu, J., and Gu, G. (2005). Control of arabinoxylan solubilization and hydrolysis in mashing. Food Chem. 90:101-108.
Loi, L., Barton, P. A., and Fincher, G. B. (1987). Survival of barley (1-3, 1- 4)-β-glucanase isoenzymes during kilning and mashing. J. Cereal Sci. 5:45-50.
Manzanes, P., and Sendra, J. M. (1996). Determination of total (1-3),(1- 4)-β-D-glucan in barley and malt flour samples. J. Cereal Sci. 23:293-296.
McCaig, R., Sawatzky, K., Egi, A., and Li, Y. (2006). Brewing with Canadian hull-less barley varieties CDC Freedom, CDC McGwire, and CDC Gainer. J. Am. Soc. Brew. Chem. 64:118-123.
Mikola, L., and Mikola, J. (1980). Mobilization of proline in the starchy endosperm of germinating barley grain. Planta 149:149-154.
Moore, J., Bamforth, C. W., Kroon, P. A., Bartolome, B., and Williamson, G. (1996). Ferulic acid esterase ccatalyses the solubilization of β-glucans and pentosans from the starchy endosperm cell walls of barley. Biotechnol. Lett. 18:1423-1426.
Muller, R. (1995). Factors influencing the stability of barley malt β-glucanase during mashing. J. Am. Soc. Brew. Chem. 53:136-140.
Olkku, J., Kotoviita, E., Salmenkallio-Marttila, M., Sweins, H., and Home, S. (2005). Connection between structure and quality of barley husk. J. Am. Soc. Brew. Chem. 63:17-22.
Pellny, T. K., Lovegrove, A., Freeman, J., Tosi, P., Love, C. G., Knox, J. P., Shewry, P. R., and Mitchell, R. A. C. (2012). Cell walls of developing wheat starchy endosperm: Comparison of composition and RNA-Seq transcriptome. Plant Physiol. 158:612- 627.
Sandegren, E., and Enebo, L. (1952). Cell wall decomposing enzymes of barley and malt. I. Determination and stability investigations. J. Inst. Brew. 58:198-203.
Shibuya N., and Iwasaki, T. (1978). Polysaccharides and glycoproteins in rice endosperm cell-wall. Agric. Biol. Chem. 42:2259-2266
Simpson, D. J. (2001). Proteolytic degradation of cereal prolamins—The problem with proline. Plant Sci. 161:825- 838.
Slade, A. M., Hoj, P. B., Morrice, N. A., and Fincher, G. B. (1989). Purification and characterization of three (1- 4)- β-D-xylan endohydrolases from germinating barley. Eur. J. Biochem. 185:533-539.
Stewart, D. C., Freeman, G. and Evans, D. E. (2000). Development and assessment of a small-scale wort filtration test for the prediction of beer filtration efficiency. J. Inst. Brew. 106:361-366.
Stewart, D. C., Hawthorne, D., and Evans, D. E. (1998). Cold sterile filtration: A small scale filtration test and investigation of membrane plugging. J. Inst. Brew. 104:321-326.
Stewart, D. C., Sterenberg, N., Washington, J., and Hughes, G. (2004). Hull-less barley— A commercial malting and brewing evaluation. Pages 13-15 in: Proc. 28th Inst. Guild Brew., Asia Pacific, Hanoi.
Stewart, R. J., Varghese, J. N., Garrett, T. P. J., Hoj, P. B., and Fincher, G. B. (2001). Mutant barley (1-3,1- 4)-β-glucan endohydrolases with enhanced thermostability. Protein Eng. 14:245-253.
Stuart, I. M., Loi, L., and Fincher, G. B. (1986). Development of (1,3;1,4)-β-D-glucan endohydrolase isoenzymes in isolated scutella and aleurone layers of barley (Hordeum vulgare). Plant Physiol. 80:310-314.
Thomas, D. A. (1986). A novel result of malt fiabilimeter analysis: Case-hardened malt. J. Inst. Brew. 92:65- 68.
van Nierop, S. N. E., Rautenbach, M., Axcell, B. C., and Cantrel, I. C. (2006). The impact of microorganisms on barley and malt quality - a review. J. Am. Soc. Brew. Chem. 64:69-78.
Whitmore, E. T. (1960). Rapid method for determination of the husk content of barley and oats. J. Inst. Brew. 66:407- 408.
Yin, X. S., MacGregor, A. W., and Clear, R .M. (1989). Field fungi and β-glucanase solubilase in barley kernals. J. Inst. Brew. 95:195-198.
Zhang, Q., Cheetamun, R., Dhugga, K. S., Rafalski, J. A., Tingey, S. V., Shirley, N. J., Taylor, J., Hayes, K., Beatty, M., Bacic, A., Burton, R. A., and Fincher, G. B. (2014). Spatial gradients in cell wall composition and transcriptional profiles along elongating maize internodes. BMC Plant Biology 14:27- 40.
