

Diseases of Alfalfa
Dean K. Malvick and Deborah A. Samac
Introduction
Many different factors influence the establishment and productivity of alfalfa stands, and diseases are often very important factors. Diseases of alfalfa (Medicago sativa) result from the interactions among susceptible alfalfa varieties, pathogens, a conducive environment, and improper crop management practices. Alfalfa diseases can reduce yields, reduce forage quality, thin or destroy stands, decrease stand persistence, and increase susceptibility to winterkill, other diseases, and environmental stresses. The first step in managing alfalfa diseases is prevention through proper site selection and field management practices, including optimization of soil pH, fertility, drainage, and cutting management. Selection and planting of diseaseresistant alfalfa varieties adapted for the area where they w ill be planted is an important second step in managing many diseases. Selected seed treatments are important for managing some seedling diseases. Fungicides for controlling foliar diseases are available but must be applied before symptoms are apparent. Harvesting forage early can reduce yield losses caused by foliar diseases and reduce pathogen inoculum. Rotation to a nonlegume crop also removes residue and inoculum. This chapter summarizes symptoms, occurrence, and management options for important alfalfa diseases in the United States.
Common Alfalfa Diseases Known to Exist in the United States
Seedling Diseases
Phytophthora Root Rot
The causal pathogen of Phytophthora root rot is the oomycete Phytophthora medicaginis. Phytophthora root rot can affect plants throughout the growing season. It can kill seedlings and may damage or kill established plants in poorly drained soils. Seedlings are often infected as they emerge, then they wilt and collapse (“dampingoff ”). Pythium species cause similar symptoms, and this disease can also be confused with Aphanomyces root rot. Established plants wilt, become stunted, and often develop a yellow to reddishbrown color on their
leaves. Taproots infected with P. medicaginis develop a reddishbrown to black rotting on the surface, and lesions may enlarge until the root is mostly rotted or girdled into a pencilpoint symptom. Plants pull easily from the ground because they have a short taproot and few lateral roots. Infected lateral roots usually have few or no nodules.
This pathogen may survive for years in the soil and may attack alfalfa after long rotations to other crops. The disease is often seen during or after wet periods and can occur anytime during the growing season. It is most common in lowly ing fields, fields with slow drainage, and in soils with high clay content. Phytophthora root rot can also be damaging in welldrained areas during periods of heavy or persistent rainfall.
Several practices can assist with the management of Phytophthora root rot. Foremost is the use of alfalfa varieties with resistance to Phytophthora root rot. Treating seed with appropriate fungicides can help protect seedlings from Phytophthora dampingoff. Disease problems can also be decreased by selection of welldrained fields and improvement of water drainage where possible. Maintenance of adequate soil fertility to promote lateral root growth and harvesting to minimize plant stress may also reduce disease.
Pythium Seed and Root Rot
Pythium seed and root rot is caused by a group of soilborne oomycete organisms in the genus Pythium in the broad sense. Seed and root rot and dampingoff caused by Pythium species are common problems affecting alfalfa under wet and cool soil conditions worldwide. Pythium species cause the greatest damage to seedlings, but roots of older plants can also be injured. The result can be poor seedling establishment and reduced stands and growth of alfalfa (Fig. 2.1). Alfalfa seedlings appear to become less susceptible to the dampingoff phase about 5 days after germination, but infection of feeder roots can occur later. The disease can be devastating when seeds are sown in heavily infested soil and when environmental conditions favor the disease. High soil moisture and, in most cases, cool temperatures (near 61–68°F [16–20°C] or cooler) favor growth and infection by Pythium
Alfalfa seeds may be infected before or during germination, with their contents being reduced to a soft brown mass.

Fig. 2.1. Symptoms of Pythium seed and root rot and damping- off on left compared to healthy seedlings on right. (Courtesy D. A. Samac)
After germination, pre and postemergence dampingoff can occur. The radicle and cotyledons may become brown and soft, and infected areas of the hypocotyl and root become water soaked, flaccid, and finally collapse and wither. The seedlings fall over (dampoff ) or may become stunted, with small darkgreen cotyledons, and die in a few days. With moderate temperatures and sufficient soil moisture, some seedlings with infected primary roots may survive by producing secondary roots above the lesion. Seedlings with sublethal infections of Pythium may have reduced root system size, length, branching, complexity, and growth rates.
Many different Pythium species can infect and damage alfalfa seedlings. The species of Pythium that infect alfalfa can vary regionally and seasonally among and within fields, and they can have different optimal soil temperatures for infection. Pythium seedling blight and root rot often can be reduced via several tactics. Increasing soil drainage where possible in problem areas of fields can be beneficial. Prepare a firm seedbed and adjust soil pH and fertility to optimal levels for growth of alfalfa. Plant when soil and weather conditions favor rapid emergence and early growth of seedlings. Fungicides, such as metalaxyl and mefenoxam, applied to seed can reduce infection and stand loss.
Aphanomyces Root Rot
Aphanomyces root rot is caused by the soilborne oomycete
Aphanomyces euteiches. Two races of A. euteiches are known to infect alfalfa, which interact differently with different sources of resistance in alfalfa. This disease is most commonly noted when it stunts and kills alfalfa seedlings, but it may also cause root disease of established plants. Infected seedlings are typically stunted and develop chlorotic, purpletinted cotyledons prior to chlorosis of leaf tissues (Fig. 2.2). Infected roots and hypocotyls of seedlings are initially tan and become darker brown over time. Seedlings infected by Aphanomyces usually remain rigid and upright for some time after infection, whereas seedling affected by other diseases that occur under similar

Fig. 2.2. Symptoms of Aphanomyces root rot on seedlings. Diseased seedlings are stunted and show purple discoloration. (Courtesy D. Malvick— © APS)
wet soil conditions—especially Pythium dampingoff and Phytophthora root rot—usually collapse soon after infection. Symptoms on established plants are often not clear, but cortical tissues in lateral roots develop a brown decay, have reduced root mass, and frequently either lack or have decayed nitrogenfi xing nodules. Brown lesions on the surface of the taproot often develop where lateral roots were destroyed. Fo l iage of infected plants becomes stunted, chlorotic, and may develop symptoms that resemble nitrogen deficiency (Fig. 2.3). Infected plants may have increased susceptibility to winter injury and may exhibit slow growth following winter dormancy or harvest.
Oospores can often be observed with a microscope in the hypocotyls and roots of infected plants. When infected seedlings are floated in water for 1 to 2 days, sporangia with grapelike clusters of spherical primary zoospores are formed. A. euteiches likely survives for years as oospores in tissues of infected plants or in soil. Watersaturated soils at warm temperatures, 70–82°F (22–28°C), are optimal for infection and disease development. Many different legume species can be infected by A. euteiches.
Aphanomyces root rot is managed by planting resistant alfalfa cultivars and improving field drainage. Alfalfa cultivars are available with resistance to Aphanomyces root rot caused

Stunting and thinning of plant stand caused by Aphanomyces root rot. (Courtesy D. Malvick— © APS)
by races 1 and 2. Cultivars with the highest level of resistance available should be chosen and planted. Resistance to Aphanomyces root rot is most effective for improving alfalfa establishment and stand life when combined with resistance to Phytophthora root rot. Practices that improve soil drainage can reduce the risk of this disease. New seed treatments are under development that may have the potential to reduce Aphanomyces root rot. Resistant varieties or alternative crops that are not hosts of A. euteiches should be considered for crop rotation practices
Root and Crown Diseases
Crown Rot Complex
Crown rot is caused by a complex of pathogenic organisms, including species of the fungal pathogens Fusarium, Phoma, and Rhizoctonia. Bacteria and nematodes may also play a role. Crown rot is a common problem in alfalfa and generally increases as stand age increases. Brown to black areas develop in the crown tissue or root cortex and may initially be visible only when the crown is split (Fig. 2.4). The central core of the crown and root may also be rotted or hollow. As crown rot becomes more severe, plants become stunted, wilting may occur, and considerable stand thinning and shortened stand life may result.
Conditions that favor crown rot include poor fertility (low potassium in par ticular), low soil pH, insect damage (such as

from the clover root curculio), and other diseases. Crown rot may also be increased by damage to crowns from harvesting machinery and grazing animals; crop stress caused by improper harvesting (too frequently or too late in the fall) also increases damage. Management of crown rot is a challenge, but this problem may be decreased by minimizing stresses and damage to alfalfa and by choosing adapted cultivars for the area of production.
Bacterial Wilt
Bacterial wilt is caused by Clavibacter insidiosus. Symptoms typically first appear in 2 to 3yearold stands, and the disease can be difficult to identify definitively in the field. Severely infected plants appear yellowgreen and stunted; they also have spindly stems and small, deformed leaflets (Fig. 2.5A, B). In early stages of disease development, infected plants are often scattered throughout a field. Symptoms include mild cupping, curling, and mottling of leaves. Infected plants are often most easily noticed in regrowth after the first forage harvest. Crosssections of the upper portion of infected roots have a yellowishbrown discoloration in the vascular system, and the discoloration may spread across the entire root as the disease becomes more severe (Fig. 2.6). Where resistant varieties are grown, the disease does not cause large production losses.
The pathogen grows slowly in culture and is difficult to isolate from plant material, because other organisms readily
Fig. 2.3.
Fig. 2.4. Split crown showing brown or black areas caused by crown rot. (Courtesy D. A. Samac)


Fig. 2.5. A , Stunting and B, foliar discoloration of plant infected with bacterial wilt. (Courtesy D. Malvick— © APS)
overgrow it. Colonies are shiny, mucoid, and slightly raised on sucrose peptone agar. They are initially white, then turn pale yellow, and may develop swirls of purpleblue pigment after 10 to 14 days. The bacterial cells are short, grampositive rods that are nonmotile and do not form spores. Polymerase chain reaction (PCR)based tests are available for conclusive identification.
This pathogen survives in plant residue in the soil. The bacterium infects plants through wounds in the roots and crown or through the cut ends of newly mowed stems. The pathogen can be transmitted by infested seeds, although reported levels of seed transmission are extremely low, and C. insidiosus levels decline rapidly in stored seed.
Bacterial wilt occurs throughout most alfalfagrowing regions worldwide. Disease incidence and severity are increased by the interaction of the bacterial wilt pathogen with the northern rootknot nematode and the stem nematode. Resistant varieties are the primary method used to manage bacterial wilt. Where susceptible varieties are grown, rotate fields out of alfalfa for 3 or more years. Avoid mowing plants when leaves and stems are wet to reduce transmission of the pathogen from infected to uninfected plants. Young stands should be harvested before older stands if the same harvesting equipment is used.
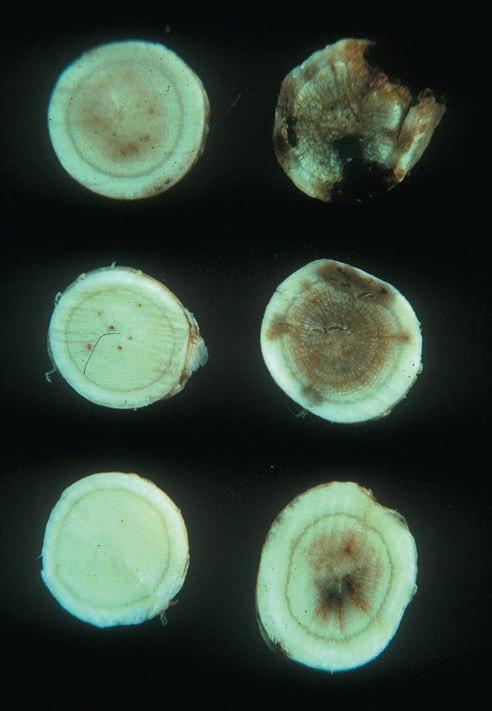
Fig. 2.6. Internal root discoloration caused by bacterial wilt. (Courtesy D. A. Samac)
Brown Root Rot
Leptosphaeria sclerotioides (syn. Phoma sclerotioides) is the causal agent of brown root rot. This fungus occurs on alfalfa in the northern United States, including Alaska, and in the high mountain valleys of Colorado and New Mexico. Brown, roughly circular lesions often outlined by a dark margin are found on taproots and lateral roots (Fig. 2.7). Lesions can girdle roots, resulting in plant death. Pycnidia are often found on crowns, bases of stems, and lower petioles of infected plants. Because of the compromised root system, infected plants are slow to break dormancy and regrow after forage harvest. The disease contributes to winterkill in older stands, because rotting of the root and crown reduces nutrient acquisition and storage. The disease is difficult to diagnose by symptoms alone, and the pathogen grows slowly in culture. A PCRbased assay is available for detecting the pathogen in roots and soil.
Seven infraspecific varieties of the fungal pathogen have been identified based on morphology and gene sequences. Optimal growth of the fungus occurs at 50–64°F (10–18°C).
The fungus overwinters as a saprophyte on soil organic matter. Roots are penetrated directly. Disease develops primarily during host dormancy from late fall to early spring, and disease severity is highly dependent on weather conditions and plant stress levels. Symptoms may take three or more winters

Fig. 2.7. Symptoms (small, round lesions that expand to irregularly s haped necrotic areas) of brown root rot on taproot. (Courtesy C. Hollings worth— © APS)
to become apparent. In addition to alfalfa, L. sclerotioides causes disease on winter wheat and other perennial legume forage crops.
A few cultivars are known to be tolerant. Rotating 2 to 3 years with a springsown annual cereal crop w ill decrease inoculum in soil. Crop management strategies to reduce plant stress w ill mitigate the effects of brown root rot.
Fusarium Wilt
The causal agent of Fusarium wilt of alfalfa is the fungal pathogen Fusarium oxysporum f. sp. medicaginis. Fusarium wilt is a common disease in many areas where alfalfa is grown. When Fusarium wilt is beginning to develop, wilting may occur, but plants often recover their stature overnight when plant stress decreases. Infected plants usually occur in scattered patches in a field. Stems and leaves of only one side of a plant may initially discolor or wilt, and leaves and stems develop chlorosis. As the disease progresses, the entire plant may wilt, develop a yellow or reddish tint, and die after several months (Fig. 2.8A). A key diagnostic feature is to cut crosssections of the upper part of the root and look for dark brown discoloration near the center as a complete or partial circle (Fig. 2.8B). This soilborne fungus infects through wounds in roots and survives in soil and in infested plant debris. This disease can occur throughout the growing season and is most common in stands that are 2 or more years old. Warm soil


Fig. 2.8. A , Plant with symptoms of Fusarium wilt next to healthy plant. B, Cross- section of root showing discoloration caused by Fusarium wilt. (A, Courtesy D. Malvick— © APS. B, Courtesy V. Marble— © APS)
temperatures favor this disease. The severity of Fusarium wilt can be increased by rootknot nematode injury. The keys to disease management are using alfalfa cultivars resistant to Fusarium wilt and to the rootknot nematode.
Phymatotrichopsis Root Rot
The causal fungal pathogen, Phymatotrichopsis omnivora, also infects many other plants during hot weather, including cotton. This destructive disease of alfalfa (also named “cotton root rot” and “Texas root rot”) is known to occur primarily in the southcentral and southwestern areas of the United States into central Mexico. This disease occurs in circular patches


common); the Columbia rootknot nematode, M. chitwoodi; the southern rootknot nematode, M. incognita; the Japanese rootknot nematode, M. javanica; and the peanut rootknot nematode, M. arenaria These nematodes are widely distributed and have broad host ranges.
(Fig. 2.9), where the fungus spreads out radially from a central infection site. Leaves and stems become yellow and brown, and whole plants can wilt and die. Roots develop root rot and become covered with light brown fungal growth (Fig. 2.10). Management of this disease is difficult, although some fungicides have shown some efficacy.
Root-Knot Nematodes
Five species of rootknot nematode are of concern on alfalfa: the northern rootknot nematode, Meloidogyne hapla (most
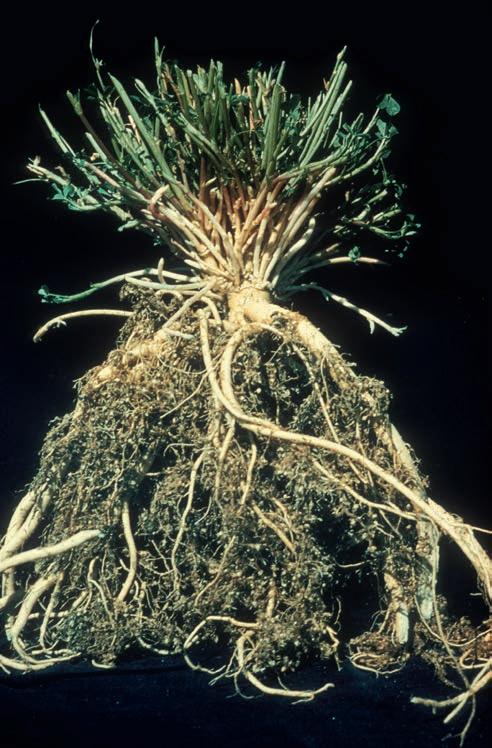
Plants infected with rootknot nematodes are often stunted and have shallow roots that branch profusely and develop many small galls (Fig. 2.11). The galls can be similar to or smaller in size than nodules formed by nitrogenfi xing bacteria. Infected plants may be localized or found throughout the field. Infection with rootknot nematodes increases severity of other alfalfa diseases, including bacterial wilt, Fusarium wilt, and Phytophthora root rot. A major economic impact of rootknot nematodes can be the damage to highvalue crops in rotation with alfalfa.
The rootknot nematode is found in most soils, but it prefers sandy loam soils. Optimal temperatures for hatching, mobility, invasion, and growth are 59–77°F (15–25°C). The life cycle can be completed within 4 to 6 weeks, with multiple generations per year.
Diagnosis of the nematode problem is impor tant for selecting proper management practices. Planting resistant cultivars is the most practical control method. Rotation with corn
Fig. 2.10. Brown growth of Phymatotrichopsis omnivora on alfalfa roots. (Courtesy V. Marble— © APS)
Fig. 2.9. Phymatotrichopsis root rot: field symptoms in circular patch. (Courtesy V. Marble— © APS)
Fig. 2.11. Severe root proliferation and root galls caused by the root- k not nematode. (Courtesy D. A. Samac)
or cereal grains may reduce nematode populations if broadleaf weeds are controlled.
Root-Lesion Nematodes
Pratylenchus penetrans is the most important rootlesion nematode species associated with alfalfa, although some other Pratylenchus species also cause economic damage to alfalfa. Rootlesion nematodes have broad host ranges.
Plants infected with rootlesion nematodes do not typically have clear diagnostic symptoms. High populations of rootlesion nematodes may kill seedlings and impair stand establishment. Older infected plants may be stunted and appear in scattered patches in a field. Areas with disease may be weedy and have reduced stand density and yield. Infected plants may have dark, brownblack lesions on the surface of roots (Fig. 2.12). Fine, fibrous roots and nodules may be absent because of nematode feeding, resulting in impaired nutrient uptake. Rootlesion nematode infections may increase susceptibility to other root diseases, such as bacterial wilt and Fusarium wilt, and may increase the severity of winter or drought damage.
Damage to alfalfa is most common in fields with sandy soils that can enhance movement of nematodes. All motile

stages can invade roots. Generation time is 4–8 weeks with multiple generations produced in a year.
Commercial varieties with resistance to Pratylenchus species are available. However, because of variations in nematode pathogenicity, cultivars reported as resistant to rootlesion nematodes in one location may not have the same level of resistance elsewhere. Crop rotation is of limited value as a control measure, and the cost of chemical control is prohibitive. Infested fields w ill generally remain infested indefinitely because the rootlesion nematode has many crop and weed hosts. Fallowing with weed control can reduce nematode populations. Plant and harvest infested fields last, and clean equipment between fields to avoid spreading nematodes from infested soils to new fields.
Stem Nematode
The causal agent is the nematode Ditylenchus dipsaci. This nematode is a serious pest of alfalfa in the western United States. It can be a problem all season in irrigated areas, although the stem nematode is often most problematic in the first cutting. Stems, stem nodes, and crown buds of plants infected with stem nematodes are swollen (Fig. 2.13). Infected stems are shorter than healthy stems because of decreased internode

Fig. 2.12. Lesions on root surface from feeding by the root- lesion nematode. (Courtesy J. A. Theis)
Fig. 2.13. Swollen stems and nodes caused by feeding of the stem nematode. (Courtesy V. Marble— © APS)

White flagging caused by the stem nematode. (Courtesy J. R. Gray— © APS)
length. Heavily infected plants may have curled and distorted leaves, stems may become white (Fig. 2.14) or blackened and die, and the entire plant may be killed. Severe infections can reduce stem numbers and predispose plants to winterkill and other diseases, such as bacterial wilt and Fusarium wilt.
Moderate temperatures favor infection and reproduction of the nematode, although infection and reproduction can occur at temperatures in the range 41–86°F (5–30°C). Stem nematode is common in parts of the western United States and is most serious in fields with heavy soils and heavy rainfall or irrigation. The stem nematode is spread by irrigation water, rain, alfalfa debris, and harvesting equipment.
Plant damage can be reduced by planting alfalfa varieties resistant to stem nematode. Two or 3year rotations out of alfalfa to a nonhost crop, such as corn, small grains, or beans, can reduce nematode populations to low levels, but fields can be quickly infested. Avoid contamination of fields with irrigation water, soil, contaminated seed, and plant debris.
Verticillium Wilt
Verticillium wilt is caused by Verticillium alfalfae (previously named V. albo-atrum). Infected plants become yellowed and stunted, and leaves may fall off the lower portion of the infected stem. In the early stages of disease, the yellowing often first appears as a Vshaped area on tips of individual leaves and is often associated with the upward rolling of leaflets along their length (Fig. 2.15). As the disease progresses, the leaves may become bleached (white to pink in color) and die. Dead leaves frequently remain attached to green stems. Infected stems have a bunchy appearance. The disease slowly invades the crown and kills the plant over a period of months. An orangebrown ring may develop inside the root in the vascular system.
Verticillium wilt occurs over much of the northern half of the United States. This disease is rarely found in firstyear stands, and the most severe symptoms often occur in the spring of the third production year. Infection can occur in

stems or roots. Affected plants usually are scattered in a field. Verticillium spores are produced on cut stubble and are spread by harvesting equipment, insects, and foraging animals. Spores can also be spread by contaminated seed.
Planting alfalfa varieties with resistance to Verticillium wilt is the most effective way to control this disease. Plant certified, clean seed. Rotate to another crop for 2–3 years, harvest young fields before old fields, and clean equipment between fields. Control weeds, even during periods of nonalfalfa rotations, because some weeds, such as black medic, are hosts and can sustain the pathogen.
Lower Stem and Crown Diseases
Anthracnose
Anthracnose is primarily caused by the fungus Colletotrichum trifolii, although C. destructivum, C. dematium f. sp. truncatum, and C. gloeosporioides have also been shown to cause anthracnose on alfalfa. Two major pathotypes (races) of C. trifolii are known to occur in the United States. This disease affects stems and the crown. It is characterized by strawcolored dead shoots scattered in a field, with many having a shepherd’s crook (Fig. 2.16). Large, sunken, diamond shaped, strawcolored lesions with dark brown borders develop on the stems (Fig. 2.17) and may girdle them, resulting in wilting or drooping. Fungal acervuli with dark setae and pinkishorange spore masses often form in lesions. Crown infections have a bluishblack color in the upper crown tissue, although color may vary if other pathogens are present. Crown infection often w ill cause dead bleached stems without apparent stem lesions. Crown infection may occur, and plant death may result without evidence of stem lesions.
The pathogen can be seedborne when conidia contaminate seed during harvest, and it can survive on infected crop debris and crowns for several months. Moisture is impor tant for spore dissemination and infection. Anthracnose is favored by warm, humid conditions and becomes more severe within a year after the first forage harvest. Maximum severity occurs between harvests in the late summer. This disease is often most
Fig. 2.14.
Fig. 2.15. V- s haped chlorosis on leaves caused by Verticillium wilt. (Courtesy D. Malvick— © APS)

Fig. 2.16. Shepherd’s crook on stem, symptomatic of anthracnose. (Courtesy L. H. Rhodes— © APS)

Fig. 2.17. Straw- colored lesions on stems, characteristic of anthracnose. (Courtesy L. H. Rhodes— © APS)
destructive in low, shaded, or protected areas of a field, where plants retain moisture on the surfaces of leaves and stems. The most effective method for disease management is to select and plant alfalfa cultivars with resistance to anthracnose. Clean debris from harvest equipment before the first harvest in the spring and again during the growing season. Harvest young stands before older stands. Rotate crops out of alfalfa for 1 year or more.

Symptoms of infection (brown lesions) caused by Rhizoctonia solani on alfalfa stems. (Courtesy L. H. Rhodes— © APS)
Rhizoctonia Stem, Bud, and Foliage Blight
Rhizoctonia diseases of alfalfa occur nearly everywhere alfalfa is grown. The fungal pathogen Rhizoctonia solani can damage alfalfa in many different ways: infecting seeds and seedlings and roots, damaging crowns and developing buds, and causing blight of stems and leaves. In all cases, damage from R. solani usually occurs under warm and moist conditions. This section is focused on the damage that this pathogen causes to stems and leaves.
Brown lesions can girdle stems and result in death of entire stems (Fig. 2.18). Stem and leaf infection most often develops in areas with long periods of warm and very humid weather, such as in much of the southeastern United States. Some seed treatment fungicides may reduce seed and seedling infection, but other damage caused by R. solani is difficult to manage. Early harvesting when foliar blight begins to develop can reduce losses. Alfalfa varieties do not have high levels of resistance, but some may be less susceptible to severe damage than others.
Sclerotinia Crown and Stem Rot
The primary causal agent of Sclerotinia crown and stem rot in most areas appears to be the fungus Sclerotinia trifoliorum; however, S. sclerotiorum may be involved in some locations. This disease is favored by wet and cool weather in the fall and winter and is most common in areas without long and cold winters. Circular spotty areas in fields or entire fields may be destroyed. Initial symptoms are small, tan spots on leaves and stems that develop in the fall in most areas where this disease occurs, although it can also be a problem in the spring. Infected leaf, stem, and crown tissues discolor and become soft, and stems wilt (Fig. 2.19). In some cases, entire plants may rot, leaving minimal trace of the plant or pathogen. Round to cylindrical black structures (sclerotia) about ⅛ inch to ¼ inch (3–6 mm) develop in or on the infected plant part. A whitish, cottony, weblike growth can often be seen during wet conditions on stems and crowns of infected plants (Fig. 2.20).
Fig. 2.18.



Disease incidence can often be reduced by planting in spring or early summer in most parts of the United States. Avoid planting in fall when this disease has been a problem. Late summer planting should be done as early as possible to enable plants to become well established prior to release of pathogen spores in late autumn. Alfalfa varieties with different levels of resistance are available. Plowing may reduce disease
by burying sclerotia, and using clean seed free of sclerotia can reduce the spread to new fields.
Leaf and Stem Diseases
Alfalfa Mosaic
Alfalfa mosaic is caused by alfalfa mosaic virus. Alfalfa mosaic is the most impor tant and widespread viral disease of alfalfa. Symptoms are highly variable, depending on virus strain, plant genotype, and environmental conditions. Generally, mosaic symptoms (Fig. 2.21) are more severe in spring and fall, with mild yellowing or inconspicuous symptoms during the summer. Infection may result in reduced plant vigor and stand decline.
Commercial ELISA assays are available to detect the pathogen. The virus is composed of one spherical and three bacilliform virions. The virus overwinters in alfalfa crowns. It is transmitted by at least 14 species of aphids, with the pea aphid (Acyrthosiphon pisum) the most impor tant vector in the United States. Alfalfa mosaic virus can also be transmitted mechanically and through pollen. The virus is highly seed transmissible and can persist in seeds for 10 years or more. Alfalfa mosaic virus has a very wide host range, naturally causing disease in more than 150 plant species.
Use of virusfree seeds and varieties with aphid resistance reduces the incidence of alfalfa mosaic. Genotypes with resistance to specific strains have been developed.
Common Leaf Spot
Common leaf spot is caused by the fungus Pseudopeziza medicaginis. This pathogen spreads and survives between crops on dead leaves, where it produces spores that are dispersed to new growth. This disease can occur throughout the growing season and is favored by prolonged periods of cool and wet weather.
Typical symptoms of common leaf spot are small brown spots that typically remain as separate lesions (Fig. 2.22). Lower leaves are generally attacked first. This disease can
Fig. 2.20. White cottony fungal growth on plant infected by Sclerotinia trifoliorum. (Courtesy V. Marble— © APS)
Fig. 2.19. Sclerotinia crown and stem rot infection of plant crown. (Courtesy D. Malvick— © APS)
Fig. 2.21. Alfalfa mosaic symptoms (chlorotic streaks) on leaves. (Courtesy L. H. Rhodes— © APS)
