
Embracing Technology to Ensure Quality in Organisations which both Sponsor and Host Research
James & Dr Nick Morley, Royal Cornwall Hospital Trust
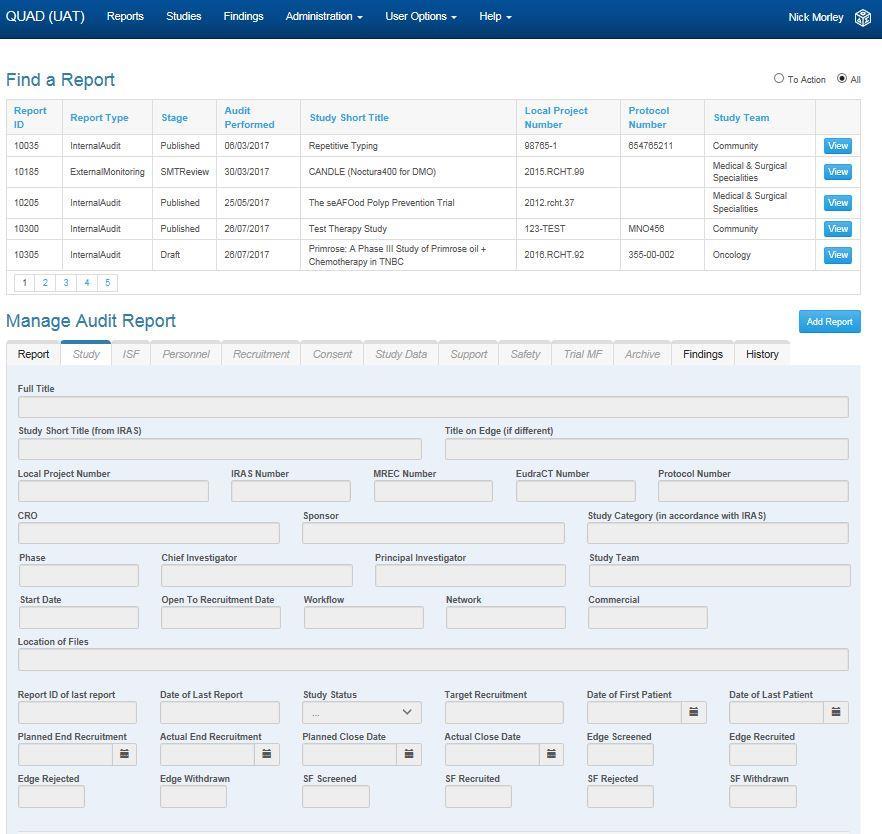
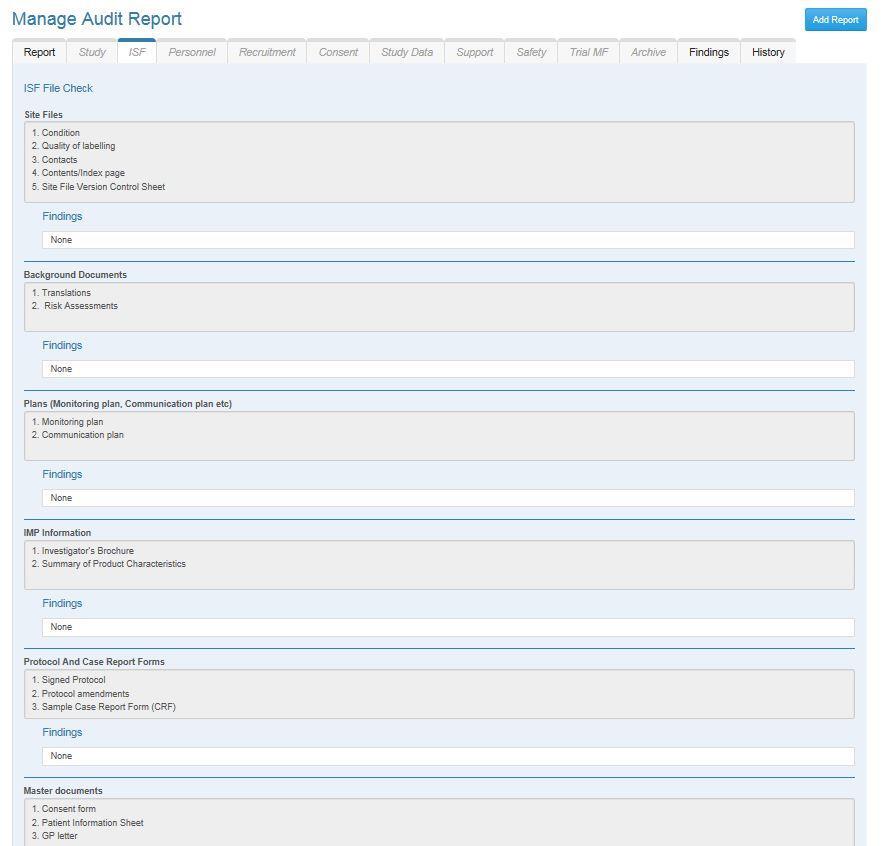
As an organisation which both hosts and sponsors research, we have developed electronic software to capture and collate findings from audit and monitoring reports to identify and respond to weakness in the systems and processes. The Quality Assurance Database (QuAD) is an innovative, purpose built, electronic system designed to facilitate audit and monitoring of research projects which are conducted in accordance with the principles of good clinical p ractice.
QuAD
The Quality Assurance Database


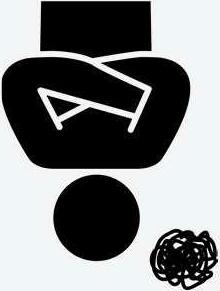
Sponsor performs monitoring visits
Report or just findings captured in Quad
Site or Sponsor develops audit plan to address weakness
Findings from multiple reports collated to identify weakness and system failures
Site and Monitor address the findings
Whilst eliminating the need for paper files the most significant benefit of the database is that it provides the sponsor and trial management team the opportunity to collate findings from audit and monitoring reports to give complete oversight.
QuAD can be used to determine how well all research staff, research teams and departments involved in research are working in accordance with the standards at single or multiple sites for single or multiple studies.
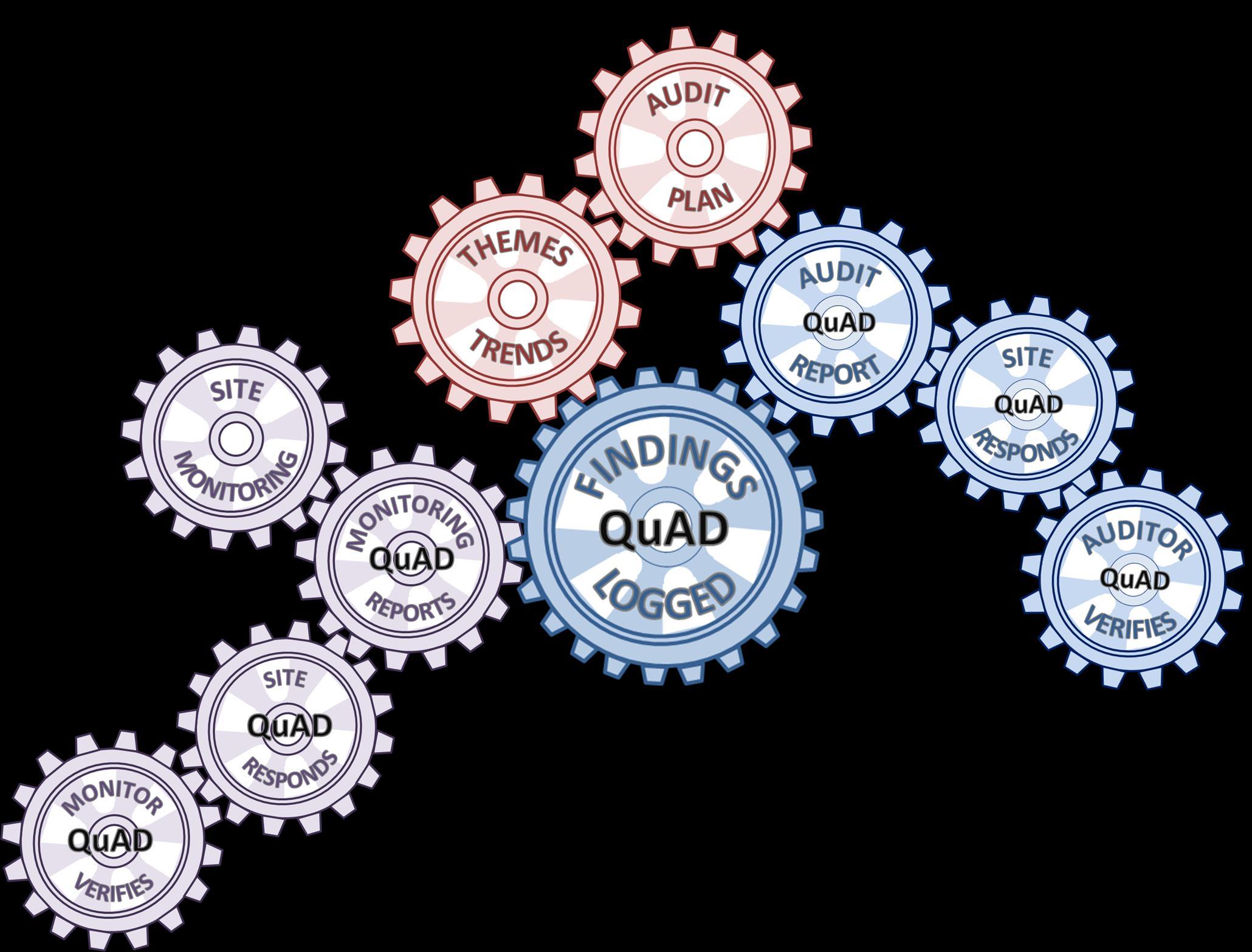
By combining data from other sources it is also possible to relate findings from a series of reports to a single individual which might otherwise be overlooked from a single report.
QuAD provides a rapid and effective means of improving the standards of research at site, ultimately to ensure the health and wellbeing of participants and the integrity of the study data. QuAD in an essential tool for sponsor oversight
Brendon













Enhancing Quality in Research at University Setting
S. Mata, J.O.
García, C.
Quality Research Service,
Quality
Key strategy Support activities Core facilities Research groups ISO 9001 certification ICH Q10 for GMP customers some GLP requirements in animal facilities for GLP customers. Knowledge transfer Non regulated framework GLP ISO 9001 ISO17025 GMPs Conclusion The strategy developed by the Quality Research Service together with the additional support activities offered contributes to enhance quality in research at the University of Barcelona. Control of Standard Operating Procedures (SOP) Custody of certified standards Training GLP Archive In apparatus calibration of common use in laboratories (balances, pipettes, thermometers, incubators and UV-Vis spectrometers Teachers and technical staff as internal auditors of ISO 9001 Staff in quality systems ( GLP, ISO 17025 and ISO 9001 )
R.M. Vilanova,
Bernad, M.
Navarro
Agency for
Assurance, University of Barcelona












Implementing Quality Systems in University Core Facilities: a Strategy to Spread Quality Culture in Research
Quality
Quality Assurance,
Equipment of high performance and the most current technology Accessible to a wide range of scientists Well trained and expert staff Verification and calibration of equipment Validated methods Data integrity Control of standards and reagents Core facilities are a good place to implement Quality Assurance best practices, spreading the quality culture to many researchers and contributing to the reliability of research data Core Facilities: centralized scientific research infrastructures that allow research scientists shared access to sophisticated and expensive technologies. Research groups: Obligation to release reliable and reproducible data to scientific community and society. Lack of experience using Quality Management tools Track from samples to results Training of students by expert staff Benefits QA System Core facilities values
R.M. Vilanova, C. Navarro
Research Service, Agency for
University of Barcelona




























in Semi-Virtual Clinical
Trials Using Direct to Patient (O -Site) Research Nurses –Practical Considerations for Sponsors and CROs

Background
Number of trials involving “home care”, “direct to patient” or “O -Site” component has been steadily increasing over the past 20 years. The use of o -site Research Nurses has further increased with the move towards virtual (or site-less) trials. Although technology has driven the change to this more decentralised trial model, the use of o -site Research Nurses is a key component of the more patient centric model.Following the ICH E6 (R2) update, European Medicines Agency (EMA) Inspectorate Working Group (WG) issued two new posts on the Q&A website in Dec 2018 to further clarify how compliance with ICH E6 (R2) can be achieved and what steps must be taken when including an external O -Site Nursing provider (“Provider”) in any trial model.
Objectives
The intention is to identify di erent categories of actions and responsibilities from the Sponsor/ Contract Research Organisation (CRO), Investigator Site (“Site”) and Provider, to comply with ICH GCP E6 (R2).
Methods
The available guidance was reviewed and interpreted by the members of the Illingworth Quality Assurance department, with further input and clarification from the Medicines and Healthcare products Regulatory Agency (MHRA), EMA and Health Research Authority (HRA) was obtained. The results were categorised, and responsibilities assigned to each stakeholder (Sponsor/CRO, Site and Provider).
Conclusions
Sponsors/CROs looking to implement virtual and semi-virtual trial models to achieve patient centricity should ensure early engagement of both the Site and the Provider.
This will allow for open communication and appropriate, documented division of responsibilities across all parties, in turn ensuring regulatory compliance.
Sponsor/CRO
Essential Documents Approvals & Permissions Contracts & Agreements Patient Safety Data Integrity Oversight
Study design, patient condition and drug indication support and justify o -site services. Protocol and other key documents (e.g. Lab Manual) identify procedures to be carried out o -site. Informed Consent Documentation reflective of o -site process including data sharing (with provider and partners e.g. courier).
IRB/IEC and Competent Authority approval of Protocol and ICD containing O -Site procedures.
Sponsor/CRO and Site agreement (e.g. mCTA) refers to the Provider and how delegation of Investigator duties will be documented.
Financial agreement between Sponsor and Provider.
Essential Documents Approvals & Permissions Contracts & Agreements
Risk Management Plan to include risks associated with o -site procedures.
Review of source data worksheet template.
Ensuring Provider has procedures for quality control and quality assurance (vendor qualification). Monitoring of data.
Regular communication with the -Site and Provider.
Site
Patient Safety Data Integrity Oversight
PI review of Provider Nurse’s CV and acceptance of proposed resource.
Written agreement confirming delegation of Investigator duties, as well as assurance of pre-engagement checks (training and experience) between the Site and Provider.
Other Institution requirements (e.g. insurance or indemnification arrangements).
Essential Documents Approvals & Permissions Contracts & Agreements
Pre-visit handover between Site and O -Site nurse to ensure all relevant safety information is completed.
Pre-agreed timelines for return of completed worksheets.
Regular contact between O -Site Nurseand Site.
Provider
Patient Safety Data Integrity Oversight
Develop instructions for the O -Site Nurses based on the protocol and relevant manuals.
Due diligence checks on the Protocol, ICD and other documentation to ensure OSite procedures are adequately described.
Strict pre-engagement checks for each potential O -Site Nurse.
Financial agreement with the Sponsor, delegation agreement with the Investigator (may be per trial or a master agreement with individual scope of work).
OR Tri-partite agreement including all stakeholders.
Strict process to ensure protection of Subject Identifiable Information. Detailed country-specific due diligence to ensure compliance with data protection regulations.
Ensuring Life Support training of O -Site Nurses.
Study specific risk assessment for o -site visits. Health and safety risk assessments for conducting osite visits.
“Meet the Patient” visits at Site prior to the first o -Site visit. StaySafe® platform for emergency notifications.
Procedure for direct reporting of SAEs and other AEs of interest to the Site.
Quality control processes for data collected o -site.
Timelines for data provision to sites.
Secure storage of worksheets before return to Site.
O -Site Nurse attending Site Initiation Visit and periodically visiting the site. Regular communication between Site and Provider to schedule visits and exchange feedback.
Ensuring ICH GCP Compliance
Website: www.illingworthresearch.com Email: info@illingworthresearch.com
about research, compassionate about patients
Natalia Janik and Nikki Cooper, Quality Assurance Managers at Illingworth Research Group Ltd.
Passionate
Consideration of appropriate management of the GLP computerized system for data integrity -Analysis of data integrity issues from FDA warning letters -



Introduction
Globally, data integrity findings have been issued


Method Result



Data integrity issues according to data lifecycle phase Regulatory
What should be the focus point to improve data integrity at the GLP facilityinJapan?
Step 1



















In facilities where sufficient training on data integrity is not provided to staff, there is a concern that a lack of understanding will cause data integrity issues. Also, many facilities use legacy systems (insufficient functionality) and effective controls are required to ensure data integrity in such systems. Therefore, we adopted the methods described herein to consider appropriate management of the GLP computerized system focused on data integrity in Japan.
Purpose
To identify the focus point to improve data integrity based on categorizing according to the data lifecycle.

Discussion and Conclusion

The extracted findings from the FDA warning letters were categorized into the 5 phases of data lifecycle. About 85% of data integrity issues extracted from FDA warning letters were categorized in the early phases of data lifecycle (generation and processing). Data integrity issues are likely to occur in the early phases of data lifecycle and may be the inspector's focus point. As a result, it is considered that the priority for GLP facilities to improve its response to data integrity should be focusing on the generation and processing phases of the data lifecycle. We plan to conduct activities to propose to Japan GLP facilities, measures for data integrity focusing on the generation and processing phases of the data lifecycle and to establish procedures for that purpose.
Focus point of the data lifecycle to improve data integrity at the GLP facilityfrom this approach
IsaoWatanabe: Shin Nippon Biomedical Laboratories, Ltd. Drug Safety Research Laboratories (SNBL DSR), Kagoshima, Japan Co-author: Japan Society of Quality Assurance (JSQA), GLP Division, Study Group 3, Subgroup 2, Team A
staff training sufficient ? Are effective controls in place for legacy systems ?
on FDA
Warning letters
to August 2019 *One issue may be classified into multiple phases 33warning letters
in
62 issues were categorized
the 5 phases Warning Letter No. DataIntegrity Issue Data lifecycle phases* GenerationProcessingReview/ Reporting/Use Archive/ Retrieval Destruction 320-16XX The trial injection data files were stored on separate drives from the reported test result data. 320-16XX Multiple integrations were conducted without justification. ・・・・・・ Collected the FDA warning letters of data integrity issues related to computerized systems Step 2 Categorized the data integrity issues of the FDA warning letters to the 5 phases of the data lifecycle Generation Processing Review/Reporting/Use Archive/Retrieval Destruction FDAwarning letters Focus point Inadequate control of computer clock or audit trail Generation Processing Generation Processing Review/ Reporting/Use Archive/ Retrieval Sharing a username and password Multiple integrations or data deletion without just ification System administrator rights are assigned to staff with a direct interest in the data Only the passing result was reported in the officia l record. Certificates of Analysis can be manipulated or deleted Unable to retrieve the data Storing on separate storage from the reported data Data lifecycle PhaseMain finding (There were no applicable issues in this phase.)
inspection Is
Search
website
from April 2016
from 9 countries
cGMP
into
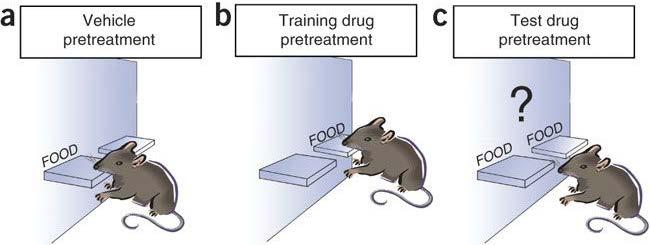
Challenges of abuse liability studies conducted in compliance with GLP
1. Introduction
The preclinical abuse liability assessment is a critical component in the development of new molecular entities (NMEs) with targeted or indirect effects on the central nervous system (CNS) supporting the safety evaluation as required by the Regulatory Agencies (FDA, 2017; ICH M3 [R2], 2009; EMA, 2006).
Drug abuse is defined as the intentional, non-therapeutic use of a drug product or substance to achieve a desired psychological or physiological effect.
Specific preclinical abuse-related studies are usually conducted in rats once the therapeutic dose range is determined after completion of Phase 2 clinical trials in order to evaluate:
whether a drug has rewarding or reinforcing properties (self-administration study and conditioned place preference study)
whether a drug has subjective and interoceptive effects that are similar to known drugs of abuse (drug discrimination study)
the ability of a drug to produce physical dependence after chronic administration (spontaneous withdrawal symptoms)
http://www.med-associates.com/medlines/Summer2012/InYourLab-Summer2012.html

2. Regulatory Requirements
Recent FDA guidance recommends that preclinical abuse liability evaluation must be conducted in compliance with GLP.

However, the requirement for GLP compliance in this specific field is difficult due to: the nature of the studies, where experimental designs are complex and phase progression is based on individual animals’ behaviour, thus not predictable in advance
the validation of computerised systems that have been developed to be used in a research environment
a complex data flow from the acquisition to the statistical elaboration and final reporting
Figure 1: Schematic representation of an operant chamber used for a self-administration experiment (upper figure) or drug discrimination experiment (lower figure). Figure 2: Data flow from acquisition to archiving
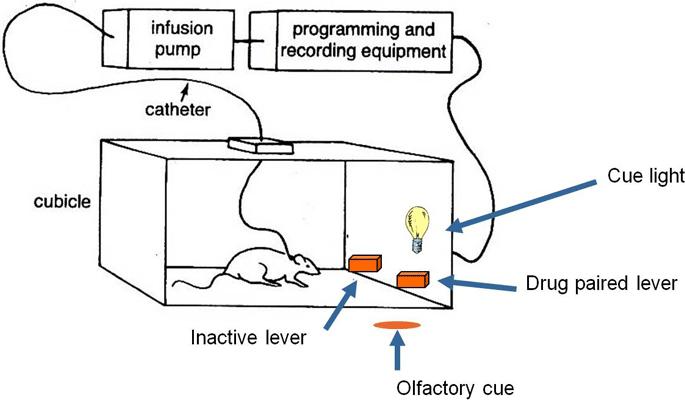
Risk assessment to identify the critical steps and put in place the controls and check points to mitigate the risk on data integrity in these steps:
1. Data acquisition
2. At the end of acquisition data are saved on the lab computer as a text file that is automatically transferred in a secure network area
3. Data are loaded into a non-editable MS Access database through MED2Access tool; validated queries are then executed to assess the data
4. If further data analyses are required, the data are imported in SAS for statistical elaboration and 100% quality checked
5. Data are moved from the secure area to an electronic archive
3. GLP implementation approach
QUALITY ASSURANCE PROGRAMME
Figure 3: GAMP 5: a Risk-Based Approach to Compliant Computerized Systems.
Following the GAMP guideline a risk based approach has been used to commensurate the validation effort of the systems used for data acquisition, transfer, elaboration and archive:
MED-PC for operant chamber control and data acquisition
MED to Access tool is a bespoke tool for data transfer
SAS for data elaboration
NuGenesis for electronic data archiving
The Quality Assurance Programme is based on a documented risk based approach.
The following inspections are performed:
Protocol/Amendment
Study-based inspections on critical phases identified with support of Study Directors/Scientist
Process-based inspections are conducted to monitor procedures or processes of a routine and repetitive nature
Facility inspection that covers the laboratory
Report and data review
Periodic compliance review of computerized systems
Corporate Headquarters: Evotec SE, Manfred Eigen Campus, Essener Bogen 7,
info@evotec.com Web: www.evotec.com
22419 Hamburg, Germany Email:
From Solinas et al., Nature, 2006
Barbara Vaccarini1, Michela Tessari2 Massimo Breda3 and Gabriele Pesavento1
Aptuit (Verona) Srl, an Evotec Company, Via Alessandro Fleming, 4-37135 Verona Italy, Quality Assurance, QU; 2Abuse Liability/Safety Assessment, PCD; 3Preclinical Sciences & GLP/GCP Compliance







• SOPs general and Unit specific • Internal Audit JACIE Clinical Indicators TCTO • CAPA, Trial SOPs, • Internal Audit Trial Indicators UOPI GLP TF • Internal Audit Methods Validation TCL GCLP methods SOPs • CAPA • General SOPs • Internal Audit GCP/ISO OSR (ISO 9001 based Quality System ) SCP • GLP methods SOPs • CAPA A Quality Manager for each system is appointed , responsible for the set up and maintenace of standards requirements and adequate communication between different systems SR-TIGET ATMP DEVELOPMENT MODEL Basic Research Pre-Clinical Research Clinical Research Phase I to III Clinical Proof of concept Registration phase Clinical Study Reports, MAA Market Access Achieved also with Pharma Industry Alliances IDEA! Pre-Clinical Proof of concept in vivo and in vitro model
Helene Clavien, Quality Manager Clinical Development Unit Nestlé Research, Lausanne Switzerland






































nutritional research, what
QA compliance?
Surveys in
matters for
3rd
November 2019 Nutritional surveys are not clinical trials but imply collection of health-related personal data. Compliance with ethical and scientific principles is essential, though adherence to full ICH GCP may not be applicable . Health, nutrition, and lifestyle surveys are agile research tools for understanding trends and emerging concerns in nutrition and health. Used effectively, risk-based approach is the best tool to support compliance decisions and ensure data integrity. • What might go wrong in survey research type? • What are the likelihood and consequences if something goes wrong? • How to control and mitigate the risks? Quality Management – QA and QC Medical Expertise - CRO / Vendor Trial Design - Trial Management Investigator selection - Allocation of responsibilities Compensation to subjects and investigators - Financing Notification/Submission/Review to regulatory authorities - IRB/IEC Record access - Monitoring – Audit – Non compliance Trial closure - Clinical trial study report Investigational Products Management Safety Adverse reaction reporting Appropriate informed consent process Data handling compliant as per local data protection and privacy laws Data integrity
European QA Conference in Dublin,
Introduction
Report ng results n a regu atory aboratory, has evolved rapidly through electron c systems becoming more complex and ga ning in functiona ity Whi e technology has moved on at a rap d rate, has our approach to audit ng the generated data kept pace?
Have the following questions been asked: Where is the risk?
Where should the effort of audit be focused?
Background
MHRA trend analysis of GLP findings from 2014/15 - 2017/18 show a 6.5 fold increase in major deficiencies categorised as Study Conduct. This can be partially explained by the increased use of e-systems, of increasing increasing complexity and the publication of the MHRA Data Integrity Guidance.
A key function of QA is to provide assurance to stakeholders by performing compliance assessments (audits). The conventional approach to these assessments is reactive; a greater benefit to the organisation is for them to be proactive
The Challenge
The convent ona audit checks the f na va ues against the raw data and verif es the work can be reconstructed The aud ts performed are e ther observational (observing the phase be ng performed) or retrospective (recreat ng the work us ng records/raw data, SOPs, work instructions and plans)
Key areas frequently overlooked; the steps to generate the final reported data the e-system controll ng the process or phases of the process interviewing personnel re-occurr ng ssues
Conventional
Innovative
Planning the audit programme
When planning an aud t programme the entire process from cradle to grave should be considered

Process map the entire sequence of events from observing the process and identify the steps wh ch are; managed by an e-system interaction between e-systems e.g instrument software to LIMS managed by human ntervention
If the process or steps are managed by the e-system t s not necessary to audit for every study The functionality of the e-system must be understood Is there functionality to nterrogate the records to identify potential issues? Such as aud t tra ls or runn ng queries The audit must prov de confidence of the functionality and continual va idation status of the e-system. Records must be available for any changes to the system by the use of change control
If the process or steps are managed manually the impact of an error in that step must be assessed for risk It s not necessary to aud t for every study f there s historical ev dence the process or steps are robust
QA identified values which had been omitted from the table of sample concentration data provided for audit. Values missing were from an entire ‘analytical run’. The findings were reported to the responsible scientist. QA reported that this was a common occurrence and yet had not been investigated.

Routine audits did not identify any issues with the analytical phase.
A non-routine audit comprising of the data transfer from the e-system to the final reported data format, a CSV file, was conducted.
The Documented Process (SOP)
A CSV file is set-up for each study
The CSV file was designed for multiple data transfers from the e-system
On each data transfer the CSV file is saved as a new document therefore no overwriting of files
The file name format was studynumber_date
The run data and CSV file is subject to QC check as each is completed. The request for QC is against a specific file name
The Observed Process
Analyst opened the latest CSV file as identified by the date in the file name with the notification 'File in Use the file is locked for editing' this message was ignored and the data was transferred
The CSV file was saved with the prefix ‘copy’
This generated a 'copy' of the CSV file - the analyst was unaware of what this meant The CSV file was QC'd as each run completed therefore no errors found Investigation
When the file is opened by another user the error message appears. A copy is created which does not include the data added by the other user
The folder containing the CSV files included duplicate files with the prefix ‘copy’ Analysts were not transferring data to the latest version of the CSV file.
Learnings from the case study
Process mapping of the steps from e-system to tab es detailed the documented procedure and d d not ref ect the actual process followed
The testing of the transfer from the e-system to the CSV file had not inc uded concurrent users
System error messages were not questioned or invest gated and there was no documented process for the handling of errors Version contro s of the CSV file were not appropriate Rout ne audits d d not cover the entire processes of the study
There was no verificat on that the QC process was appropr ate
There was no check of the complete f nal data set
QA findings were reported n iso ation without a method of dentifying recurr ng ssues
Advancing the audit programme
The deal purpose of an e-system is to generate records which minimises the evel of manual recording
To design an aud t program two elements must be considered; Human intervention Val dated functional ty
The audits must be ta lored according to the e-system, frequency of use, impact on the data and regu atory requirements.
Final thoughts
The
Plan /
Analysis Observat onal Audit; Instrument Setup Loading of samples Retrospect ve Audit; Instrument setup Sample Sequence Observational Audit; None Observational Aud t; Sample Rece pt Sample Storage Preparation of standards,samples Observat onal Audit; None Retrospect ve Audit; Review records for: sample rece pt standards samples Retrospect ve Audit; Regulatory requirements met? Retrospective Audit; Sample rev ew of reported numbers Retrospective Audit; Evidence of QC
Request Preparation
ht ps:/ assets pub ish ng serv ce gov uk/governmen /uploads/system/up oads/a tachmen da a f e 7 69279 GLP De iciency metr cs 2017 2018 pdf MHRA GXP Da a Integr ty Gu dance and De n t ons, March 2018 1 2
Observational Aud t or Retrospective Audit; Assignment of Respons ble Person Generation of plan / request
Set-up of electronic system (e-system) Qual ty Control
References Reporting Observational Audit; None Review Release Retrospective Audit; None Observational Audit; None Observational or Retrospective Audit; Final approval of data Transfer to stakeholder 1 2 Observat onal Audit; How the QC s performed What is checked; audit trails, manual entry Is the QC suitable for the e-systems in use? Observat onal Audit; How the values are transferred to the report Retrospective Audit; e-system nterrogated for anomalies audit tra ls changes to set-up Observational Aud t; Sample Rece pt, how automated i e barcodes Sample Storage Preparation of standards, samples e-Systems Process map Electronic versus human intervention Functionality validation status Risk assessment Contact us for more info www.tmqa.co.uk or visit TMQA on stand #21
Case Study
Authors Cate Ovington, Principal Associate Rita Silvestre, Associate
audit programme must be rev ewed but when? e-system changes new e-systems has risk been owered areas identified as an increased risk changes n the regu atory requirements changes n the fac lity Revisit the process map, verify if the sequence of events have evolved, this can be conducted during the audit program or as a separate exerc se
The
aud ts and aud
e
aud ts
must be
understanding
ent re
and
w th n QA
data s generated, managed and how to nterrogate to reconstruct the ana ys s to be ab e to des gn an integrated aud t program w th n the quality system
conventional approach to
t programmes may not a ways su tab
with e-systems In order for
to be a igned to current techno ogy there
an
of the
process; both e-system
human ntervention po nts
shou d fu ly understand the e-system; how









































































































