





Editor
Editorial Coordinators:


Dr. Sunita Tandulwadkar President, FOGSI (2025)
Dear Readers,
The field of general Gynaecology continues to advance rapidly, driven by innovations in precision medicine, imaging technologies, and a growing focus on individualized patient care. Worldwide, there is a clear shift from reactive treatment to proactive health strategies that emphasize prevention, early detection, and long-term wellbeing. Modern Gynaecological practice now integrates reproductive health, pregnancy care, and menopausal management across the entire female lifespan, with an increasing emphasis on accessibility, equity, and evidence-based decisionmaking. Digital health tools, multidisciplinary collaboration, and global research networks are further redefining how women’s health is delivered and studied.
Together, these contributions underscore the dynamic nature of modern Gynaecology and its ongoing commitment to integrating the best available evidence into clinical practice. As the field continues to evolve, the challenge remains to translate emerging knowledge into globally equitable care—ensuring that every woman, regardless of setting or circumstance, benefits from the progress of contemporary reproductive medicine.
CORTICOSTEROIDS: EVIDENCE-BASED UPDATE 2025 - Dr. Poonam Goyal
EVALUATION OF THE INDICATIONS OF MENOPAUSE HORMONE THERAPY - Dr. Jyoti Bhaskar
FIRST TRIMESTER FETAL EVALUATION: REDEFINING EARLY PREGNANCY CARE - Dr. Jaggina Lavanya, Dr. Chinmayee Ratha


EXECUTIVE EDITOR & PUBLISHER
Dom Daniel
22, Shreeji Bhavan, 275-279, Samuel Street, Masjid Bunder (W), Mumbai-4000 03, INDIA.
EMAIL: paradigminfocomobgyn@gmail.com
Website: ytpfuturechronicles.com
Published at 22 Shreeji Bhavan, 275/279, Samuel Street, Masjid Bunder (West), Mumbai - 400003. India.
“YTP FUTURE CHRONICLES” takes no responsibility for unsolicited photographs or material
ALL PHOTOGRAPHS, UNLESS OTHERWISE INDICATED, ARE USED FOR ILLUSTRATIVE PURPOSE ONLY.
Views expressed in this Journal are those of the contributors and not of the publisher. Reproduction in whole or in parts of texts or photography is prohibited. Manuscripts, Photographs and art are selected at the discretion of the publisher free of charge (advertising excluded). Whether published or not, no material will be returned and remains the property of the publishing house, which may make use of it as seen fit. This may include the withdrawal of publication rights to other publishing houses.
All rights reserved. Reproducing in any manner without prior written permission prohibited.
Published for the period of November -2025




Dr. Poonam Goyal
MD, FICOG, FICMCH, CIMP
Director & HOD OBG
Panchsheel Hospital, East Delhi
HOD IVF & Infertility
MAX Super Speciality Hospital, Vaishali
Dr. Jyoti Bhaskar
MD, FRCOG, FICOG, FICMCH
Additional Director Cloudnine, Delhi and Noida
Secretary Delhi Menopause Society
Dr. Chinmayee Ratha
MBBS, MS - Obstetrics & Gynaecology, MRCOG (UK), FIMSA, FICOG
Fetal Medicine Specialist
Director & Lead Consultant of Resolution Centre of Fetal Medicine, Hyderabad
KIMS Sunshine Hospital, Hyderabad
Dr. Jaggina Lavanya
Fellow- ICOG certification course in Fetal Medicine
Fetal Resolution Clinic, Hyderabad

Dr. Rohan Palshetkar President, YTP (2024 - 2026)
Dear Readers,
In this issue of YTP Future Chronicles, we bring together carefully curated, evidence-based articles that reflect the dynamic evolution of women’s healthcare. Our feature article, “Antenatal Corticosteroids: EvidenceBased Update 2025,” provides a comprehensive review of current guidelines and clinical implications for optimizing fetal outcomes. The second article, “Critical Evaluation of the Indications of Menopause Hormone Therapy,” offers a balanced analysis of evolving therapeutic perspectives, emphasizing patient safety and individualized care. Rounding off the issue, “First Trimester Fetal Evaluation: Redefining Early Pregnancy Care,” underscores the transformative potential of early diagnostic advancements in improving maternal-fetal health.
Through these contributions, we aim to empower clinicians with knowledge that supports informed decision-making and enhances patient care outcomes. As YTP continues its journey of fostering academic excellence and clinical leadership, we extend our gratitude to our contributors and readers for being an integral part of this mission.
Wishing you an insightful and enriching reading experience.

MBBS, DNB (Obstetrics & Gynecology)
Consultant Obstetrician & Gynaecologist
IVF Specialist at Angel’s Hope (a unit of Sawan Neelu Angels Hospital, Saket, New Delhi)
IVF consultant at Bloom IVF at Fortis La Femme, GK

MBBS, DGO, DFP, Fellowship in Reproductive Medicine.
Infertility Specialist, Laparoscopic Surgeon
Consultant IVF Specialist and Gynaecologist at Bloom IVF, Mumbai
Consultant Obstetrician, Gynaecologist, and IVF Specialist at APS Hospital & ICCU, Mumbai
Dear Readers,
It gives me great pleasure to present to you the this November edition of YTP Future Chronicles — a platform born from our collective vision to inspire, educate and evolve the field of Obstetrics and Gynaecology in India.
As members of FOGSI, we have always strived to stay ahead of the curve — continuously enhancing our knowledge, honing our clinical expertise and adapting to the everchanging landscape of women’s healthcare. With this publication, we take that mission a step further. YTP Future Chronicles aims to capture the pulse of modern gynaecology — the innovations, the challenges and the inspiring stories that define our practice today.
Women’s health is not a static concept; it evolves with time, culture and context. Whether it’s the young woman balancing career ambitions with reproductive choices or the homemaker navigating menopausal transitions, every woman’s journey is unique. Through this edition, we aim to highlight the diversity of these journeys — exploring solutions that are holistic, compassionate and evidence-based.
This issue delves into emerging dimensions of our field — from regenerative and functional gynaecology to discussions around sexual health, premature menopause and the psychosocial aspects of care. It challenges us to pause and reflect — what does true empowerment in women’s health really mean? And how can we, as clinicians, help shape that narrative?
I am deeply grateful to Dr. Rohan Palshetkar for his constant encouragement and guidance in shaping this initiative. His vision for the Young Talent Promotion Committee continues to inspire each of us to push boundaries and think beyond conventional practice.
I hope YTP Future Chronicles becomes not just a publication, but a dialogue — one that keeps evolving with your insights, contributions, and experiences. Here’s to learning, growing, and redefining the future of women’s health together.


Dr. Poonam Goyal
MD, FICOG, FICMCH, CIMP Director & HOD OBG
Panchsheel Hospital, East Delhi
HOD IVF & Infertility
MAX Super Speciality Hospital, Vaishali
Preterm birth continues to be the leading cause of neonatal morbidity and
INTRODUCTION mortality globally. Antenatal corticosteroids (ACS) remain one of the most effective interventions to reduce neonatal respiratory distress and mortality.1 Over the years, multiple global organizations — including WHO, FIGO, ACOG, NICE and FOGSI — have refined recommendations for optimal use, timing and safety, especially in low- and middleincome countries (LMICs).
Corticosteroids accelerate
fetal lung maturation by promoting surfactant synthesis, improving lung compliance and reducing alveolar surface tension.2 They also confer protection against intraventricular hemorrhage (IVH) and necrotizing enterocolitis (NEC).3
ACS are recommended for women at risk of preterm birth between 24 and 34 completed weeks of gestation 4-7
• ACOG (2023) and NICE (2024) recommend up to 34+6 weeks.5, 6
• WHO (2023) and FIGO (2024) extend
recommendations to 36+6 weeks in facilities with adequate newborn care.4,7
• FOGSI (2024) follows a 24–34 week range, advising selective use beyond 34 weeks.8
INDICATIONS INCLUDE:
• Threatened preterm labor.
• Preterm prelabor rupture of membranes (PPROM).
• Indicated preterm birth for maternal or fetal conditions (e.g., preeclampsia, IUGR).
CONTRAINDICATIONS
Absolute contraindications include chorioamnionitis, fetal demise, or conditions necessitating immediate delivery.4, 6
Multiple or routine repeat courses beyond one “rescue” dose are discouraged due to possible adverse fetal effects.5,7
Two corticosteroids are widely endorsed:
• Betamethasone: 12 mg intramuscularly, 2 doses 24 hours apart.
• Dexamethasone: 6 mg intramuscularly, 4 doses 12 hours apart.
WHO and FIGO recommend either, noting dexamethasone’s accessibility and cost advantage in LMICs.4, 7
REPEAT COURSES
A single repeat (“rescue”) course may be considered if:
• The first course was administered >14 days earlier, and
• Birth is again imminent within 7 days.5, 6
WHO (2023) and FIGO (2024) advise only one rescue course to minimize potential growth or neurodevelopmental impact.4, 7
The WHO ACTION-I Trial (2020) in seven LMICs demonstrated a 31% reduction in neonatal mortality and a 42% reduction in severe respiratory distress when ACS were used appropriately.9
However, the ACTION-II Trial (2021) found no mortality benefit in late preterm infants and raised concern for increased neonatal sepsis when neonatal care capacity was limited.10
A 2023 Cochrane Review reaffirmed ACS as a cornerstone of preterm management, reducing neonatal death by ~30% and IVH by ~20%.3
• ACOG (2023) recommends selective use in women likely to deliver within 7 days and who have not previously received ACS.5
• NICE (2024) supports similar use after shared decisionmaking.6
• WHO (2023) and FIGO (2024) restrict use to facilities with neonatal intensive care.4, 7
Appropriate ACS use in LMICs requires:
• Accurate gestational age determination,
• Availability of neonatal resuscitation, and
• Avoidance of use when gestation is uncertain or neonatal care is absent.4, 9, 11
The G-I-N LMIC Working Group (2024–26) emphasizes context-specific protocols to prevent overuse and ensure safety.12
Transient hyperglycemia is common post-ACS; hence, diabetic mothers require close glucose monitoring.6 ACS are generally safe, with no increase in maternal infection risk when used in aseptic settings.4, 7
When correctly used, ACS significantly reduce:
• Respiratory Distress Syndrome (RDS).
• Intraventricular Hemorrhage (IVH).
• Neonatal Mortality.3, 9
Long-term neurodevelopmental outcomes appear neutral or beneficial, according to systematic follow-up studies.11
Organization Recommended Gestation
WHO (2023) 24–34 wks
NICE (2024) 24–34+6 wks
ACOG (2023) 24–33+6 wks
Preferred Drug Repeat Course Late Preterm Use
Dexamethasone 1 rescue after 14 days Only with NICU
Betamethasone 1 repeat Considered
Betamethasone 1 rescue Selective
FIGO (2024) 24–34 wks Either 1 rescue Context-based
FOGSI (2024) 24–34 wks
Dexamethasone 1 rescue Limited use
Antenatal corticosteroids remain one of obstetrics’ most
1. Blencowe H, et al. Lancet Global Health. 2023;11(3):e423–e435.
2. Roberts D, Dalziel S. Cochrane Database Syst Rev. 2023;CD004454.
powerful interventions, saving thousands of preterm neonates annually. The 2025 global consensus reinforces judicious, guideline-based use, emphasizing the need for infrastructure readiness, proper timing, and equity of access. The ongoing goal is “right woman, right time, right dose” — ensuring every eligible baby benefits from this simple yet lifesaving intervention.
3. Cochrane Pregnancy and Childbirth Group. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Library; 2023.
4. World Health Organization. Recommendations on Antenatal Corticosteroid Therapy for Improving Preterm Birth Outcomes. Geneva: WHO; 2023.
5. American College of Obstetricians and Gynecologists (ACOG). Practice Bulletin No. 236: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2023;142(4):1053–1064.
6. National Institute for Health and Care Excellence (NICE). Preterm Labour and Birth (NG25). Updated March 2024.
7. International Federation of Gynecology and Obstetrics (FIGO). FIGO Good Practice Recommendations: Use of Antenatal Corticosteroids. FIGO; 2024.
8. Federation of Obstetric and Gynaecological Societies of India (FOGSI). FOGSI Clinical Practice Recommendations: Antenatal Corticosteroids in Preterm Birth. 2024.
9. WHO ACTION-I Trial Collaborators. Lancet. 2020;395(10260):1412–1423.
10. WHO ACTION-II Trial Collaborators. Lancet Global Health. 2021;9(9):e1235–e1246.
11. McGoldrick E, Stewart LA, et al. BMJ. 2023;381:e072846.
12. Guidelines International Network (G-I-N). LMIC Working Group Report 2024–26. GIN Secretariat; 2024.

TDr. Jyoti Bhaskar
MD, FRCOG, FICOG, FICMCH
Additional Director Cloudnine, Delhi and Noida
Secretary Delhi Menopause Society
he use of hormone therapy (MHT) in menopausal women
INTRODUCTION has, in last 3 decades, been one of the most debated topics in women’s health. This pendulum of MHT use appears to be driven not just by the sociocultural trends as by the emerging evidence from clinical trials.
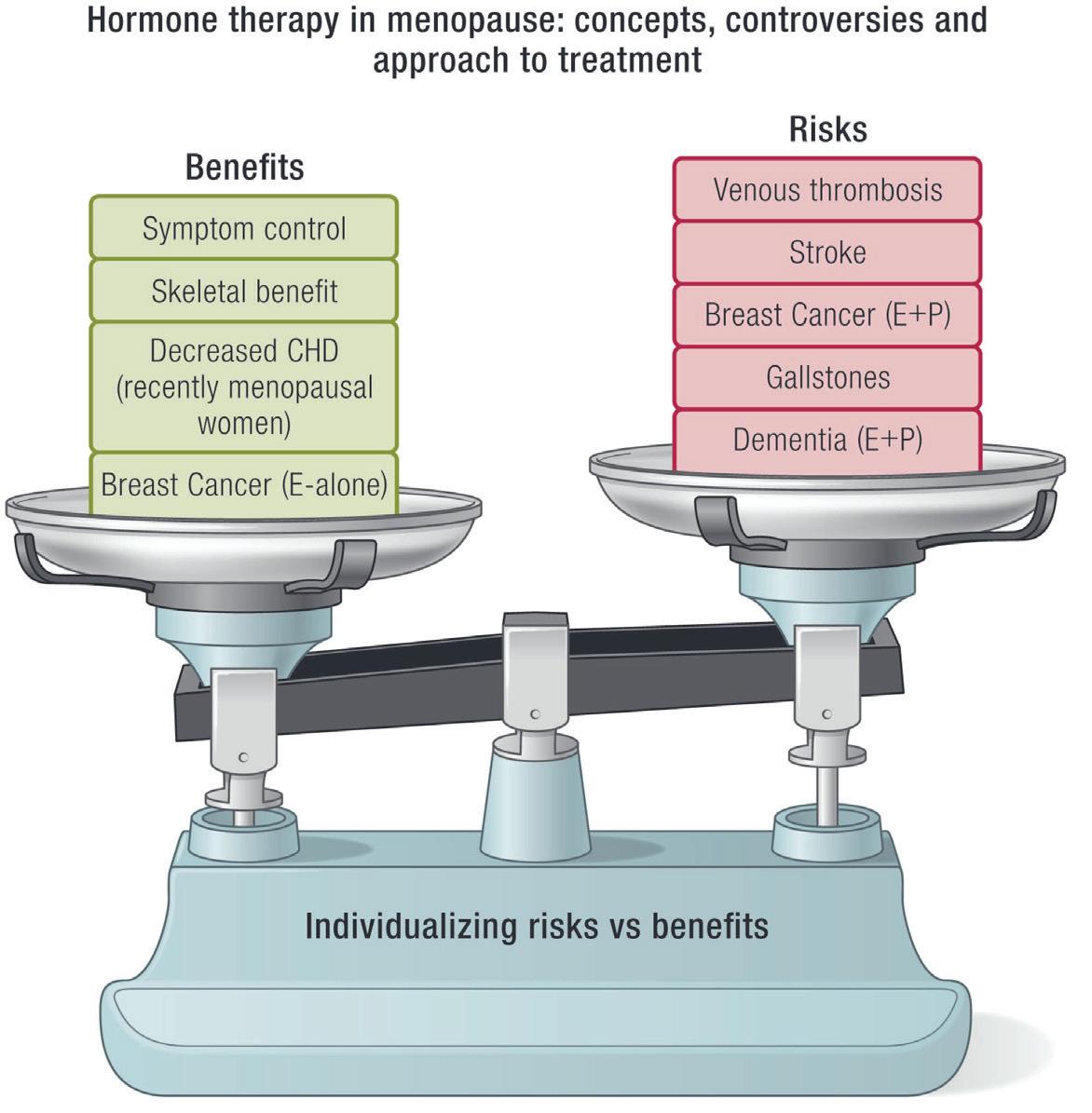
The abundance of observational data has shown that HT is not only effective against common menopausal symptoms such as hot flushes and night sweats, vaginal dryness and atrophy but also offers benefit against chronic disorders such as osteoporosis, coronary artery disease, dementia, and even all-cause mortality.
In this article today, we
aim to critically evaluate the indications for the Menopause Replacement Therapy based on evidence.
• Menopausal symptoms (Vasomotor symptoms)
• Genito urinary symptoms of Menopause (GSM).
• Premature ovarian insufficiency (premature menopause, oophorectomy).
• Postmenopausal osteoporosis (Accepted indication by few societies only).
• Coronary heart disease
• Colorectal cancer
• Diabetes mellitus
• Metabolic syndrome, Alzheimer’s disease, various atrophicdegenerative diseases (skin, mucous membranes, connective tissue), rheumatic diseases, certain forms of schizophrenic psychoses, Breast cancer (E alone).
1. Menopausal Symptoms

The cardinal symptoms causally associated with menopause are vasomotor symptoms (VMS), menstrual changes, disrupted sleep, and genitourinary symptoms. For Asian women, physical symptoms such as body aches and joint pains as well as psychological symptoms are recognized to be more prevalent than VMS. A recent systematic review and meta-analysis of prevalence data globally found that joint and muscular discomfort were the most prevalent menopause related symptoms at 65.43%.1
In addition, many women describe new onset mood and
cognitive symptoms, fatigue, palpitations, changes in body habitus and sexual dysfunction.
• Hot flashes are the most common reason for postmenopausal estrogen therapy, affecting 85% of women during late menopause and early post menopause, yet only 25% seek treatment despite impacts on sleep, quality of life, and daily function.
• MHT is considered the most effective currently available treatment for menopause symptoms with strong international consensus.
• Coexistence of other menopausal symptoms, such as depression, sleep disturbances, in these women often require treatment with both MHT and antidepressants (usually selective serotonin reuptake inhibitors [SSRIs].
• Women with mild hot flashes: Women with mild flashes usually do not need pharmacotherapy. Instead, simple behavioural measures, such as lowering room temperature, using fans, dressing in layers of clothing that can be easily shed, and avoiding triggers (such as spicy foods and stressful situations), can help reduce the number of hot flashes.
• Women with moderate to severe hot flashes — Hormonal or other pharmacotherapy is usually needed for women with more bothersome hot flashes. Women with an intact uterus need both estrogen and a progestin, while those who have undergone hysterectomy can receive estrogen only.
• Consider the initiation of MHT to be a safe option for healthy, symptomatic women
who are within 10 years of menopause or younger than age 60 years and who do not have contraindications to MHT.3
• For women who choose estrogen or combined estrogenprogestin therapies, shortterm use has been suggested (generally for not more than five years) based on the original interpretation of the Women's Health Initiative (WHI) data.
• However, several expert societies, including the Endocrine Society, the American College of Obstetrics and Gynecology, the North American Menopause Society, and the International Menopause Society no longer recommend a specific duration of treatment in symptomatic women, based upon revised safety data and the observation that hot flashes persist well beyond five years in many women.
2. Genito-Urinary Symptoms

The primary indication for treatment of GSM is to relieve symptoms that cause distress in a patient who has diminished ovarian estrogen production due to menopause or other etiologies. Vulvovaginal symptoms include vaginal dryness, burning, pruritus, dyspareunia, vaginal discharge, bleeding, or spotting. Urinary
tract symptoms include dysuria, urinary frequency, urethral discomfort or infrequently, haematuria.
Management is guided by the patient's symptoms, overall medical health and goals.
• Initial therapy – For most patients with GSM, nonhormonal vaginal moisturizers and lubricants are the preferred approach to initial therapy. Pelvic floor muscle (Kegel) exercises should be encouraged.
• Subsequent therapy3Vaginal ET is most effective in the treatment of urogenital atrophy. Low-dose vaginal preparations are as effective as systemic therapy.
Treatment should be started early to prevent irreversible atrophic changes and may need long-term treatment to maintain benefits. Regular sexual activity, including vaginal coitus, should be encouraged to maintain vaginal health.
• Limited data are available on the use of vaginal ET in women with breast cancer and EC.
• Recurrent urinary tract in this age after ruling out other causes may benefit from the local application of ET.
• Progesterone supplement for endometrial protection is not needed along with the use of vaginal estrogen.
• Endometrial surveillance is not necessary in low-risk asymptomatic woman.
• Low-dose vaginal estrogen formulations reported no incidence of increase in CHD, stroke, and VTE.
• There are no contraindications for use
in non-estrogen-dependent cancers.
3. Premature Ovarian Insufficiency or Early Menopause


Menopause occurring at an age less than 2 SD below the mean estimated age for the reference population is called as POI. It is a spectrum ranging from occult to overt POI.
The NFHS of 2015-16 collected information from a sample of more than 90,000 married women aged between 15 and 49 years and covering 99% of the India’s population living in 26 states; 3.7% of the women are already in menopause by the age of 30–34 years; and the incidence rises to 8% for the age bracket of 35–39 years.3
Women with untreated premature menopause are at increased risk of developing osteoporosis, CVD, dementia,
cognitive decline, and Parkinson’s and all-cause mortality.
• Appropriate counselling, lifestyle modification, and HT form the mainstay of treatment.
HT should be started as early as possible in women with POI and continued till the age of natural menopause.
• Emerging evidence suggests that physiological dose of HT is superior to hormone contraceptives. HT has bone protection effect.
• Androgen replacement may be considered for women with persistent fatigue and loss of libido, in spite of estrogen replacement.
• Counsel women that HT is not a contraceptive, and erratic ovulation and pregnancy may occur in POI.
• MHT should be considered in women aged <50 years who have undergone surgical menopause.
• Younger women may require higher doses for symptom relief or protection against bone loss.
• There is no evidence that HT increases risk of breast cancer, CVD, or dementia, over and above that found in menstruating women with a normally timed menopause.
4. Osteoporosis

A meta-analysis of RCTs found that oral and
transdermal estrogen (with or without progestogen) reduces hip, vertebral, and total fracture risk by 20%–37%.7 ET/ EPT prevents all osteoporotic fractures even in low-risk population; it increases lumbar spine BMD up to 7.6% and femoral neck BMD up to 4.5% over 3 years.3
• All preparations including low-dose, non-oral routes of estrogen are effective in preserving bone mass.
• Estrogen–progesterone therapy (EPT) or ET may be used for the prevention and treatment of osteoporosis in the early post menopause in symptomatic women unless there is a contraindication.
• MHT is included in postmenopausal osteoporosis guidelines, with greater fracture risk reduction seen in women starting before age 60.
• MHT should not be started solely for bone protection after 10 years of menopause. Extended use of MHT in women with reduced bone mass is an option after considering the risk–benefit analysis compared to the other available therapies for osteoporosis.
• While protection decreases after stopping MHT, there is no rebound fracture risk.
Although not universally approved for bone protection, MHT should be considered as a first option for bone protection in women with early or natural menopause over antiresorptive or osteoanabolic therapies, unless there are overriding reasons for bonespecific therapy use such as glucocorticoid associated bone
loss.8 Furthermore, MHT may be continued long-term if the benefits for bone health and menopause symptoms continue to outweigh the side effects and risks.
1. Cognitive function and Alzheimer’s disease
Midlife women commonly experience changes in their cognitive function as they transition through menopause and are often concerned if these symptoms may be initial stages of a more serious cognitive disorder.
Facts
• Menopause brain fog refers to the constellation of cognitive symptoms experienced by women around menopause, frequently manifesting in memory and attention difficulties like forgetting names, numbers and unable to multitask.
• Cognitive difficulties at midlife are linked to changes in E2, VMS, sleep and mood.
• Treating these symptoms may benefit cognition, although clinical trial data are not yet available to definitively recommend that approach.
MHT role in Cognition function2
• Based on current guidelines, MHT is not recommended at any age to treat cognitive concerns at menopause or prevent cognitive decline or dementia.
• The magnitude of the effect of MHT on dementia, whether beneficial or adverse, in the
literature is small.
• There is no reliable finding in the literature to guide treatment decisions about MHT formulation or duration of use on dementia risk.
• Clinical counselling should focus on a multi-pronged approach to reducing dementia through such modifiable risk factors as obesity, hypertension, diabetes, physical activity, smoking, cognitive activity, social interaction, hearing impairment and depression.
2. Primary prevention of CVD risk and all-cause mortality4 FACTS
• Incidence of CHD in women lags behind men by 10 years and incidence of MI and sudden death in women lags behind men by 20 years. This delay in onset of CVD appears due to the cardioprotective effects of endogenous estrogen.
• While premenopausal, women are protected from clinical manifestations of CVD relative to men, after menopause, CVD complications exceed those of men.
• Although there is an ageassociated increase in CVD incidence for women as there is for men, age-specific CVD incidence is two- to six-fold greater for postmenopausal than premenopausal women across the age range <40–54 years.
A Cochrane review in 2015 of RCTs of HT showed an overall reduction in risk of CHD and all-cause mortality in women who started HT within 10 years of menopause.
The safety of HT used early in menopause is further supported by the ELITE and KEEPS study.6
MHT has favourable or neutral effects on CHD risk when started in women younger than 60 years old or within 10 years of menopause, in the absence of contraindications.
HRT is a sex-specific and time-dependent primary CVD preventive therapy that concomitantly reduces allcause mortality as well as a diversity of other aging-related diseases with an excellent risk profile.
Despite this, no society guidelines have as yet recommended MHT for primary prevention of CVD.
It is time to recognize that HRT reduces all-cause mortality and CVD, and that it is all about timing.
Should women without valid indications be prescribed MHT?1
• Although MHT is primarily indicated for the relief of distressing menopause symptoms, it is often incorrectly promoted to women as an ‘elixir of youth’. It should not be given beyond prescribed core indications.
• It is important to counsel women from the outset that menopause symptoms such as VMS and sleep disturbances, mood swings and brain fog will usually improve with time and may not require treatment.
The ultimate goal is to
empower women with evidence-based information to make an individualized choice that is right for them.
• In healthy women less than 10 years since menopause onset, or younger than 60 years, hormone therapy is a safe, effective treatment option for menopausal symptoms; the benefits extend beyond the control of vasomotor symptoms and genitourinary syndrome of menopause to include reductions in risk of fracture and type 2 diabetes.
• MHT is the First line treatment for symptoms causally linked with menopause: VMS & related fatigue, sleep & mood changes and Urogenital atrophy related symptoms.
• For those experiencing loss of ovarian function at an earlier age than the average population norms, consideration for initiation of hormone therapy is advisable not only to mitigate the symptoms resulting from hypoestrogenism, but also to prevent the long-term health consequences on CVS and bone, associated with premature onset of estrogen insufficiency.
• There is good evidence that MHT reduces the incidence of osteoporosis and risk of osteoporosis-related fractures, and in some countries – for example, in the USA and Australia – this is also a primary indication for MHT.
• There are also good data supporting its use for reducing the risk of cardiovascular disease, thereby having a positive impact on life expectancy, but MHT is not currently licensed anywhere
globally for these indications.
• The research findings regarding the impact of MHT on cognition and dementia are considerably less reliable and require further research.
• Prior to prescribing, the predominant symptoms should be identified and realistic goals set as to the degree of
improvement expected and also over what timeline a response to treatment is expected.
1. Nick Panay, Seng Bin Ang, Rebecca Cheshire, Steven R. Goldstein, Pauline Maki, Rossella E. Nappi & on behalf of the International Menopause Society Board (2024) Menopause and MHT in 2024: addressing the key controversies – an International Menopause Society White Paper, Climacteric, 27:5, 441-457, DOI: 10.1080/13697137.2024.2394950
2. Maki, P. M., & Jaff, N. G. (2022). Brain fog in menopause: a health-care professional’s guide for decision-making and counseling on cognition. Climacteric, 25(6), 570–578. https://doi.org/10.1080/13697137.2022.2122792
3. Meeta M, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical practice guidelines on menopause: *An executive summary and recommendations: Indian menopause society 2019–2020. J Mid-life Health 2020; 11:55-95.
4. Hodis HN, Mack WJ. Menopausal Hormone Replacement Therapy and Reduction of All-Cause Mortality and Cardiovascular Disease: It Is About Time and Timing. Cancer J. 2022 May-Jun 01;28(3):208-223. doi: 10.1097/PPO.0000000000000591. PMID: 35594469; PMCID: PMC9178928
5. Flores VA, Pal L, Manson JE. Hormone Therapy in Menopause: Concepts, Controversies, and Approach to Treatment. Endocr Rev. 2021 Nov 16;42(6):720-752. doi: 10.1210/endrev/bnab011. PMID: 33858012
6. Mehta J, Kling JM and Manson JE (2021) Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front. Endocrinol. 12:564781. doi: 10.3389/fendo.2021.564781
7. L. Zhu, X. Jiang, Y. Sun, and W. Shu, “Effect of Hormone Therapy on the Risk of Bone Fractures: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials,” Menopause 23, no. 4 (2016): 461–470, https://doi.org/10.1097/gme.0000000000000519
8. Mukherjee, A. and Davis, S.R. (2025), Update on Menopause Hormone Therapy; Current Indications and Unanswered Questions. Clinical Endocrinology. https://doi.org/10.1111/cen.15211
Arecent study has demonstrated a direct link between the maternal gut microbiota,

specifically the commensal bacterium Bifidobacterium breve, and the regulation of placental function during pregnancy. Published in the Journal of Translational Medicine, the research provides the first definitive evidence that maternal microbial composition influences placental hormone production with downstream effects on fetal development. Using a controlled murine model, researchers observed that colonization with Bifidobacterium breve significantly altered over 150 biological processes in the placenta involving more than 400 proteins. These changes included enhanced nutrient transport, particularly of amino acids and lactate, and increased production of pregnancy-supporting hormones such as prolactins and glycoproteins. The presence of Bifidobacterium breve was associated with reduced fetal loss, improved placental efficiency, and enhanced fetal growth. These findings are particularly relevant given the prevalence of pregnancy complications such as fetal growth restriction, gestational diabetes, preeclampsia, and miscarriage. Approximately 10 % of first-time pregnancies result in low birth weight or fetal growth restriction, which are associated with adverse neonatal outcomes and increased long-term risk of neurodevelopmental disorders. The study highlights the potential of the maternal gut microbiome as both a diagnostic and therapeutic target in prenatal care. Bifidobacterium breve, a naturally occurring member of the human gut microbiota and widely available as a probiotic supplement, may contribute to improved maternal and fetal outcomes. Further research in human populations is required to validate these findings and assess the safety and efficacy of microbiota-based interventions. This growing body of evidence supports microbiome modulation as a promising nonpharmacological approach to enhancing pregnancy health.

Anewly published investigation Nature delineates a novel methodology for elucidating mechanisms
of treatment resistance in high-grade serous ovarian cancer (HGSOC), the predominant and most lethal histological subtype of ovarian carcinoma. HGSOC presents significant therapeutic challenges due to its early micrometastatic dissemination within the peritoneal cavity and frequent relapse following initial cytoreductive surgery, chemotherapy, and maintenance regimens. The study introduces CloneSeq SV, an integrative approach combining single-cell whole genome sequencing with targeted sequencing of structural variants, enabling high-
resolution tracking of clonal dynamics in treatment-resistant tumor cell populations via longitudinal circulating tumor DNA analysis. Structural variants serve as molecular barcodes facilitating discrimination of tumor subclones from diagnosis through recurrence. Analysis of serial blood specimens revealed pre-existing resistant subclones that expand post-therapy as sensitive populations are depleted. These resistant clones exhibit characteristic genomic alterations including oncogene amplification, chromothripsis, and whole genome doubling, which may represent exploitable therapeutic vulnerabilities. Notably, selective pressure from frontline treatment led to enrichment of ERBB2-amplified clones in one case, which responded favourably to targeted ERBB2 inhibition, resulting in durable clinical remission. This study underscores the utility of comprehensive longitudinal molecular profiling to elucidate intratumoral heterogeneity and resistance evolution. CloneSeq SV offers potential to refine precision oncology approaches in HGSOC and may be extended to other malignancies characterized by genomic instability. Future investigations will expand patient cohorts, incorporate additional tumor sampling, and explore broader oncological applications.


Dr. Jaggina Lavanya
Fellow- ICOG certification course in Fetal Medicine
Fetal Resolution Clinic, Hyderabad

Dr. Chinmayee Ratha
MBBS, MS - Obstetrics & Gynaecology, MRCOG (UK), FIMSA, FICOG
Fetal Medicine Specialist
Director & Lead Consultant of Resolution Centre of Fetal Medicine, Hyderabad
KIMS Sunshine Hospital, Hyderabad
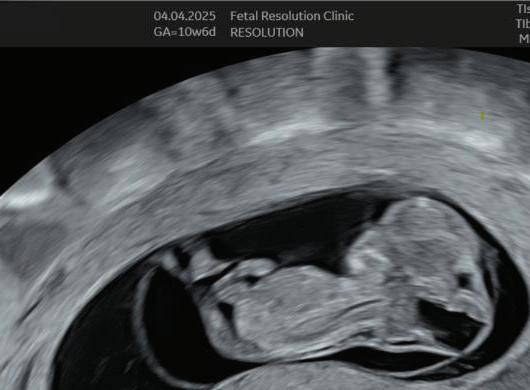
The first trimester— spanning from conception to 13+6 weeks—is no longer considered a “blind phase” of pregnancy. With the evolution of highresolution ultrasound, color Doppler and advanced biochemical screening, this period has transformed into a window of opportunity for early fetal evaluation, risk prediction and preventive strategies.
First-trimester fetal evaluation is not limited to dating the pregnancy or confirming viability—it is the foundation of predictive, preventive, and personalized obstetric care.
➢ Proposed by Nicolaides et al. (2011).
➢ It shifts the focus of antenatal care from the later stages of pregnancy to early pregnancy, emphasizing first-trimester screening.
➢ The traditional pyramid (more care in later pregnancy) is inverted, meaning most assessments and interventions are concentrated early, around 11–13 weeks gestation.
➢ Early identification of women at high risk for complications such as preeclampsia, fetal growth restriction, preterm birth and chromosomal abnormalities.
➢ Allows early prevention and management rather than late detection.


1. Establish viability and Confirmation of intrauterine pregnancy/ uterine integrity.
2. Accurate dating of pregnancy.
3. Early assessment of fetal anatomy.
4. Assessment of risk of obstetric complications.
5. Assessing risk for common forms of aneuploidies (trisomies 21, 18 and 13)
1. EARLY PREGNANCY SCAN (6–10 WEEKS)
Indications:
1. Confirmation of pregnancy.
2. location of pregnancy.
3. Number of fetus.
4. Cardiac activity.
5. Initial dating.
6. Assessment of uterine contour to ruleout any uterine anamolies.
7. Examnation of adenexa.
Findings:
• Gestational sac at ~4.5–5 weeks-Appears as anechoic fluid filled structure surrounded by a decidual reaction (Double Decidual sac sign).
• Measure Mean sac diameter .
• Yolk sac appear around ~5–5.5 weeks.
• Embryo with cardiac activity around 6 weeks.
2. NUCHAL TRANSLUCENCY (NT) SCAN
• Done @11–13+6 weeks.
• CRL 45–84 mm.
• This is the cornerstone of first trimester screening.
MEASUREMENTS THAT CAN BE OBTAINED ON
First Trimester Fetal Evaluation:
FETAL ULTRASOUND EXAMINATION AT 11 TO 14 + 0 WEEKS: BASIC BIOMETRY INCLUDES:


Nuchal Translucency

Biperital Diameter
(a) Crown Rump Length(CRL)
(b) Biperital diameter(BPD)
(c) Nuchal Translucency(NT)
DETAILED BIOMETRY INCLUDES:
Along with basic biometry
(a) Abdominal circumference
(b) Head circumference
(c) Femur length
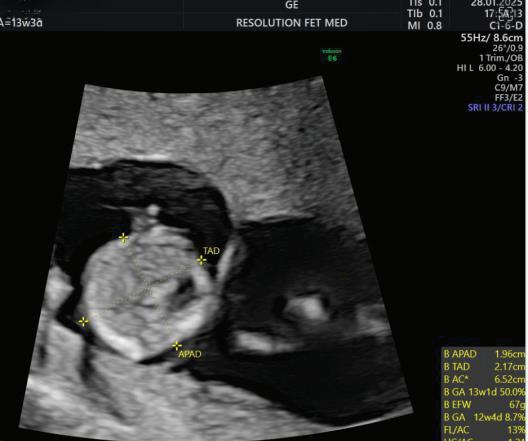
Abdominal Circumference

1. NT is the sonographic appearance of subcutaneous accumulation of fluid behind the fetal neck, below the skin in the first trimester of pregnancy.
2. Measured in mid-saggital section only.
3. Measured between 11-14 weeks.
4. CRL between 45-84mm.
5. Magnified to include only head and upper thorax.
6. Fetus should be in neutral position.
7. Demostrate fetus separate from amnion.
8. Measurement should be ON to ON (cross bar of the callipers should be such that it’s hardly visible and merges with white line, not nuchal fluid).
During the scan more than one measurement should be taken and maximum value should be considered.
INCREASED NT:
1. Aneuploidies
2. Structural Anamolies
3. Genetic Syndromes
4. Fetal infections
5. Metabolic disorders


NASAL BONE:
Hypoplasia defined as length <2.5mm.
Absent METHOD OF MEASUREMENT
• Mid saggital plane.
• Good face profile.
• At 45 degree angle with imaginary line from forehead to chin.
• 3 echogenic lines.
• Absent nasal bone is more common if The gestation is 11 than 13 weeks. The fetal nuchal translucency is high. The mother is Black.
ADDITIONAL SONOGRAPHIC MARKERS:



A Wave Reversal
MARKER
Ductus Venosus flow
Tricuspid Regurgitation
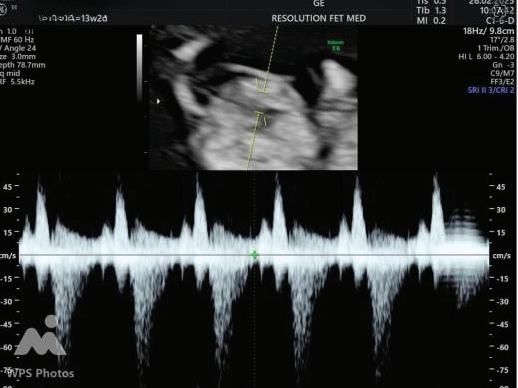
Tricuspid Regurtitation
SIGNIFICANCE
Reversed a-wave in aneuploidy or cardiac dysfunction
Suggests cardiac abnormality or aneuploidy
COMMONLY DETECTABLE MAJOR ANOMALIES:
ORGAN STRUCTURE POSSIBLE ANAMOLIES
Head and brain
Skull Midline falx
Choroid plexus
Posterior fossa

Acrania, cephalocele Holoprosenchephaly Ventriculomegaly
Chiari malformation, PF cystic anamolies

ORGAN STRUCTURE POSSIBLE ANAMOLIES
Spine
Vertebrae Open spina bidifa
Dorsal skin Kyphoscoliosis myelomeningocele
First Trimester Fetal Evaluation: Redefining
Early Pregnancy Care



ORGAN
Face Profile
Orbits
Thorax
Heart
GIT/Abdominal Wall
STRUCTURE
Anterior Palate
Lung Fields
Heart Activity
Cardiac Situs
Size And Position
Urinary Tract And Genitalia
Four Chambers
Three Vessels/Arches
Stomach
Cord Insertion
Bladder
Umblical Arteries
Kidneys
Limbs Active Movements
Three Segments
POSSIBLE ANAMOLIES
Micrognathia, Flat Facies
Microphthalmia
Hypotelorism
Cleft Palate
Pleural Effusion
CDH
CHAOS
Bradycardia/Arryt Hmias
Isomerism
CDH
Hypoplastic RT/LT Heart
Ectopia Cordis
Vascular Stenosis/Atresia, AV Septal Defect
Conotruncal Abnormalities, Valvular Stenosis/Atresia
Esophageal/Duodenal Atresia
Exomphalos
Gastrochisis
Body Stalk Anamoly
Bladder Exstrophy
B/L Renal Agenesis
Megacystitis
Single Umblical Artery
Renal Agenesis
Pelvic/Horseshoe Kidney
Cystic/Hyperechoic Kidneys
Hydronephrosis
Neuromuscular Anamolies
FADS
Limb Reduction Defects
Skeletal Dysplasia





ANEUPLOIDY SCREENING:
✔ Every pregnant women carries a risk of having fetus with chromosomal abnormalities –“Background risk or apriori risk”.
✔ Risk can be assessed based on screening teststo “high or low risk”
Ideally every pregnant women should be offered screening tests for aneuploidies.
SCREENING TESTS FOR FETAL ANEUPLOIDIES:
■ First trimester combined screening test.
■ Second trimester quadruple test
■ Cell free fetal DNA
■ Second trimester genetic sonogram
Second Trimester
(triple test)
Inhibin A (Quadruple test)
Second Trimester Sonogram
INTERPRETATION:
• Low PAPP-A → increased risk for aneuploidy, preeclampsia, and FGR.
• High β-hCG → often associated with trisomy 21.
FIRST TRIMESTER PREDICTION OF OBSTETRIC COMPLICATIONS
Preeclampsia screening:
Predictive markers:
• Mean Arterial Pressure (MAP)
• Uterine artery Doppler PI (11–13+6 weeks)
• Serum PAPP-A and PlGF levels
Early identification allows prophylactic use of low-dose aspirin (150 mg nightly till 36 weeks).
• PLACENTAL ABNORMALITIES
1. Placenta previa should not be reported at this stage.
2. Any abnormal findings such as masses, single or multiple cystic spaces or sub chorionic fluid > 5 cm should be noted and followed.
3. PAS disorders with prior cesarean delivery should be noticed carefully.
• SCAR PREGNANCY
Low anterior implantation of gestational sac next to scar niche.
MULTIPLE PREGNANCY EVALUATION
• Determine chorionicity and amnionicity (best done in first trimester).
• Lambda sign (twin peak) → dichorionic.
• T-sign → monochorionic.
• Abnormal NT or markers → Offer genetic counseling.
• Diagnostic tests:
• Chorionic villus sampling (CVS) between 11–13 weeks.
INTERPRETATION AND FOLLOW UP STRATEGIES:
added to standard screening time. The authors conclude that advanced multiplanar neurosonography enables reliable early detection of central nervous system abnormalities in the first trimester.3
• Poor acoustic window (maternal habitus, bowel gas) may reduce image quality.
• Fetal position may hinder measurement (especially NT or CRL).
COMMUNICATION WITH PATIENT:
• Reassure if normal:emphasise limitations
• If anomalies/markers found:schedule counselling, offer further testing.
➢ In fetal cardiology, a large retrospective screening of 77,396 fetuses at 11–13+6 weeks demonstrated a detection rate of ~70.5 % for congenital heart disease (CHD) using a standardised firsttrimester cardiac scanning strategy.2
➢ The prospective study “Fetal Advanced Neurosonography in the First Trimester of Pregnancy” evaluated 92 fetuses (CRL 60–82 mm) and found that an extended first-trimester CNS ultrasound protocol was feasible in 93.5% of cases, with excellent intra- and interobserver concordance and only 3 extra minutes
• Mistiming of gestational age (uncertain LMP, irregular cycles) may lead to misinterpretation.
• Not all anomalies are detectable in the first trimester; some evolve later (e.g., mild ventriculomegaly, placenta accreta changes) so normal early scan is reassuring but not definitive.
• Inter ‐ observer variability in NT measurement and other markers remains a challenge; ensure credentials and training.
1. https://www.isuog.org/static/f465db45-655c-42eb-96a196bcd2d34547/ISUOG-Practice-Guidelines-Updated-performance-of-1114-week-ultrasound-scan.pdf
2. https://pubmed.ncbi.nlm.nih.gov/40019943/?utm_source=chatgpt.com
3. https://doi.org/10.1159/000546460


Opening address by Mr. Dom Daniel (drscourseslive.com)

The welcoming address was delivered virtually by Prof. Dr. S. Sampathkumari.
The Fungal Master Class, Chennai, 6th September 2025 on “Overview of Effective Management Strategies for Vulvar and Perianal Infections” was successfully conducted, featuring approximately two hours of in-depth clinical discussion and expert engagement. The event was held under the auspices of the Young Talent Promotion (YTP) Committee of FOGSI (The Federation of Obstetric and Gynaecological Societies of India), in collaboration with the OGSSI (Obstetric and Gynaecological Society of Southern India) and drscourseslive.com. Marking its fifth edition, the Fungal Master Class, Chennai continues to serve as a trusted academic platform advancing knowledge on challenging infections that significantly impact women’s health. The session commenced with the Presidential address by Prof. Dr. S. Sampathkumari, President of OGSSI and Former Vice President of FOGSI (2022). The keynote speaker was Dr. Meenakshi Sundaram, Treasurer of OGSSI. The event was moderated by Dr. Meena Mahalingam, Secretary of OGSSI and Co-Chair of the Urogynae Committee. The Panel featured an esteemed group of experts: Dr. Josephine Rosy, OGSSI Executive Committee Member; Dr. Karpagambal Sairam, Consultant Gynaecologist; Dr. Jayashree Sharma, Consultant Gynaecologist; Dr. G. Shantha, Senior Consultant Gynaecologist; and Dr. Ramani Rao, Senior Consultant Gynaecologist. Together, the Panelists contributed valuable clinical perspectives on vulvar, perianal, and vulvovaginal fungal infections, enriching the session with practical strategies and evidence-based insights to improve outcomes in women’s health.


Speaker Dr. Meenakshi Sundaram shared her insightful presentation.
The Chennai masterclass featured a detailed presentation by Dr. Meenakshi Sundaram, who provided valuable insights into diagnosing and managing vulvar and perianal fungal infections, emphasizing pH testing, microscopy and targeted treatments. Recurrent cases were
addressed with species identification, extended therapy, probiotics and special consideration treatments. The presentation comprehensively covered vulvar and perianal infections along with vulvovaginal candidiasis.


Moderator Dr. Meena Mahalingam delivered an insightful presentation on urinary tract infections
The presentation session continued with a detailed overview of urinary tract infections, delivered by Dr. Meena Mahalingam. It covered the classification and various types of infections, as well as treatment strategies for both bacterial and fungal cases, providing valuable clinical insights for effective management.

Sharma, Dr. Ramani Rao, Dr. G. Shantha
The interactive panel discussion, moderated by Dr. Meena Mahalingam, featured expert contributions from Dr. Josephine Rosy, Dr. Karpagambal Sairam, Dr. Jayashree Sharma, Dr. Ramani Rao and Dr. G. Shantha. The session addressed key clinical challenges in diagnosing and managing vulvar and perianal fungal infections, including differential diagnosis, treatment-resistant
cases, advanced diagnostics, and care for special populations. This engaging discussion highlighted the need for personalized, multidisciplinary strategies to improve outcomes in patients with chronic or recurrent vulvoperineal infections.



This Fungal Master Class, Chennai was supported by Glenmark and concluded with a heartfelt note of appreciation from the Glenmark team to the esteemed faculty — Dr. Ramani Rao, Dr. Karpagambal Sairam, Dr. Meena Mahalingam, Dr. Meenakshi Sundaram, Dr. Josephine Rosy, Dr. Jayashree Sharma, Dr. G. Shantha — along with all other contributors, for their invaluable support in making the event insightful, impactful, and a resounding success.
Dr. K. Poongothai - The session effectively addressed common clinical concerns in a clear and well-structured manner. It was highly relevant to everyday practice, offering practical and actionable insights. As expected, the presentation was exceptional — informative, impactful, and enriched with valuable takeaways for practitioners.
Dr. M Prasanna Kumari: A superb session—wellorganized and highly informative. The content was valuable and directly applicable to clinical practice.
Dr. Senthamarai Samuel J.P: A highly practical session with valuable insights directly applicable to everyday clinical practice.
This Event was conceptualized and executed by Paradigm Infocom Pvt. Ltd, Publisher of this Newsletter. For more information, Contact: +919820507771
Email id: - paradigminfocomobgyn@gmail.com
Astudy published in JAMA Network demonstrates a significant association between premenstrual disorders (PMDs) and
reduced health-related quality of life in women of reproductive age. PMDs, encompassing both premenstrual syndrome (PMS) and its more severe form, premenstrual dysphoric disorder (PMDD), were found to negatively impact physical and psychological well-being. Using a combination of self-reported symptoms and diagnostic information from national health registers, researchers assessed quality of life through the EQ-5D-3L instrument, which evaluates dimensions such as mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. The findings indicated that individuals with PMDs report substantially lower quality of life

compared to those without such conditions, with PMDD linked to the most severe impairments. These associations remained evident even after adjusting for other health conditions and sociodemographic variables. Symptoms such as heightened anxiety, depressive mood, and physical discomfort were particularly prominent among those with PMDD, contributing to a significant burden on daily functioning. The study highlights the importance of recognizing PMDs as a public health concern and calls for integrated medical, psychological, and social interventions to improve the overall well-being of affected individuals.

Anew set of global guidelines published in The Lancet Global Health calls for a comprehensive
from the largest study on PPH to date and establish objective diagnostic criteria to improve timely clinical response. Previously, PPH was diagnosed after blood loss exceeded 500 milliliters; the new recommendations advise initiating treatment at 300 milliliters of blood loss if abnormal vital signs are present. Use of calibrated drapes is recommended for accurate blood loss measurement. Upon diagnosis, a standardized intervention bundle called MOTIVE should be applied, including uterine massage, administration of uterotonic drugs, tranexamic acid, intravenous fluids, genital tract examination, and escalation of care if bleeding continues. For severe cases, surgical procedures or blood transfusions may be necessary to stabilize the patient. The guidelines also highlight the importance of antenatal and postnatal care to address risk factors such as maternal anemia, recommending oral iron and folate supplementation or intravenous iron for rapid correction. Preventive strategies include avoiding routine episiotomy and promoting perineal massage late in pregnancy to reduce birth-related trauma. During the third stage of labor, administration of a prophylactic uterotonic preferably oxytocin or heat-stable carbetocin is advised, with misoprostol as an alternative in settings with limited resources. Outdated practices are discouraged in favour of standardized, evidencebased interventions suitable for a variety of healthcare contexts. Supported by training resources, policy guidance, and simulation-based education, these guidelines were launched at the 2025 FIGO World Congress. This initiative aims to enhance maternal care quality and significantly reduce preventable deaths related to postpartum haemorrhage worldwide. revision of postpartum haemorrhage (PPH) management, emphasizing earlier detection, rapid diagnosis, and prompt treatment to reduce maternal mortality and morbidity. PPH, defined as excessive bleeding after childbirth, is a leading cause of maternal death globally and can cause serious physical and psychological complications even when not fatal. Developed by the World Health Organization (WHO), the International Federation of Gynecology and Obstetrics (FIGO), and the International Confederation of Midwives (ICM), these guidelines incorporate data



