
11 minute read
The key role of catalysts



Dr Meritxell Vila, MERYT Catalysts & Innovation, Spain, explains the importance of catalysts in processing CO2 to chemicals and fuels, and takes a look at the latest research from experts in the field.


In the context of the energy transition, experts are currently advocating for technologies based on the circularity of resources: recycling, reusing, recovering and reducing. This applies not only to plastics and residues, but also to the main environmental concern: CO2 emissions. It is important to point out that in August 2021, the Intergovernmental Panel on
Climate Change (IPCC) determined that the increase in CO2 emissions is causing defi nitive changes to our climate.1 There are many different ways to approach this issue, and efforts from all sides are needed to solve the problem. However, among all of the possible options to reduce and manage CO2 emissions, it is important to remember what the most recognised experts in chemistry advised many years ago. The concept of the ‘Green Carbon Science’ and its importance was introduced by Prof. Dr Avelino Corma and
Prof. Dr Mingyuan. They established a new future scenario where required chemicals could be produced based on the hydrogen from water, and renewable energy and carbon from biomass and CO2. 2,3 The Nobel Laureate Prof. Dr George A. Olah published his book ‘Beyond Oil and Gas: The Methanol Economy’ in 1994, where he advanced that the solution to replace fossil fuels would come through CO2 conversion to methanol and other chemicals by reaction with green hydrogen.4
This article will corroborate again how catalysts play a crucial role in the energy transition, specifi cally in the conversion of CO2 to chemicals and fuels, and will review the current status of some major industrial projects.
The electrifi cation of cars is one important element of the energy transition, but there are some considerations to be made. On one side, the renewal of the car park and the gas station network is very expensive. Batteries also have a high cost, heavy weight, and short life, as well as short autonomy and long charging times. On the other side, electrifi cation cannot be applied to aviation or heavy vehicles or ships. For all these reasons, technologies that are based on a circular economy of carbon are becoming more relevant. In this way, keeping the same infrastructure and engines, and combining them with the use of biofuels, would make reaching the carbon neutrality goals set for 2050 more possible.
The CO2 molecule is extremely stable due to the two double bonds carbon to oxygen. This high stability means that the energy needed to convert this chemical to other substances is extremely high. Consequently, the role of catalysts is more important than ever, because they are required to lower the activation energy to obtain the desired reactions. The CO2 molecule is also extremely versatile. Thanks to catalytic reactions, 17 different chemical compounds can be obtained


Figure 1. A new catalyst developed by the MIT: a layer of ionic liquid (hexagonal lattices) is added between the catalyst layer of gold or platinum in the bottom (grey spheres) and the material to be catalysed at the top (red spheres). On the left, a detail of how oxygen (red) and hydrogen (green) can combine to form water at an enhanced rate through this process.
Figure 2. A new catalyst developed by Caltech and the UCLA to convert CO2 to ethylene, based on copper nanowires: first, the smooth nanowire is synthesised, and then activated by applying a voltage to get the rough stepped surface that is very selective for CO2 to ethylene.


from CO2: methanol, ethanol, ethylene, acetaldehyde, dimethyl ether, methane, carbon monoxide, acetone, acetate, formate, alkylalcohol, 1-propanol, glycolaldehyde, propionaldehyde, ethylenglycol, hydroxyacetone and glyoxal.5
It is well known that CO2 is a chemical already widely used in the industry, including several industrial processes that use CO2 as a raw material to convert it into value-added chemicals. Currently, the annual volume of CO2 used by the industry is approximately 120 million t, which is less than 0.5% of the total annual anthropogenic emissions of CO2 (34 billion t).6 For this reason, the search for CO2 applications where this chemical is a precursor for chemical processes is a very urgent task.
Current industrial plants of carbon capture and utilisation (CCU)
Currently, there are several industrial plants around the world that convert captured CO2 to fuels or chemicals. Some of these are detailed here:
In Iceland, Carbon Recycling International started producing renewable methanol from CO2 at industrial scale in 2011, producing up to 4000 tpy of methanol. This company is also responsible for the Fresme Project (which obtains methanol as a marine fuel from CO2 captured from a steel manufacturing plant in Sweden), as well as the MEFCO2 and Shunli CO2 to methanol projects (which capture 150 000 tpy of CO2 to produce methanol).7
In Saudi Arabia, the United Jubail Petrochemical Co., an affi liate of SABIC, started the operation of a CCU plant in 2015, capturing 500 000 tpy of CO2/yr from ethylene glycol production plants. This CCU plant purifi es and converts this CO2 to produce urea and methanol.8
The Canadian company Carbon Engineering has been producing fuels from CO2 captured directly from the air (by direct air capture [DAC]) since 2017, with the technology AIR TO FUELSTM and several other ongoing projects. 9
In Germany, Covestro uses captured CO2 to react with propylene oxide to produce polyols that are destined to manufacture polyurethane foams. The plant started its production in 2016 with 5000 tpy of polyols.10
In Spain, Repsol has a project to build a plant to produce synthetic fuels from captured CO2 and green hydrogen from a 10 mW plant. The production will be 3.6 million liters/yr of fuels for cars and planes. This technology has been developed by Repsol TechLab in collaboration with Saudi Aramco.11
Research trends in catalysts for conversion of CO2 to chemicals and fuels
Researchers around the world who specialise in catalysis are intensively studying many different catalysts that can convert CO2 to chemicals and fuels. Some of the most important research centres of catalysis that have important projects in this fi eld include the following:
In Saudi Arabia, the King Abdullah University of Science and Technology (KAUST) is working on the conversion of CO2 to methane thanks to a catalyst built from nickel nanoparticles on a layer of barium titanate under the action of sunlight (photohermal catalysis). The catalyst shows good yield and excellent selectivity at ambient temperature.12 Other lines of research include the conversion of CO2 to ethanol over Rh/TiO2 catalyst, among other investigations.13
In the US, at the Massachusetts Institute of Technology (MIT), researchers have developed a way to improve electrocatalytic processes by adding a catalyst layer of ionic liquid, improving the effi ciency of the reaction by fi vefold. The catalyst layer is made of gold or platinum. This discovery can be used in the conversion of CO2 to chemicals and fuels, as seen in Figure 1.14
Also in the US, researchers at the University of Delaware are working on several catalytic projects to convert the CO2 into chemicals and fuels. One project consists of the electrochemical production of formic acid from CO2 without a purifi cation step, on a reactor formed by membranes and layers electrically charged. Another interesting project is the design of a two-step electrochemical process to produce ethylene and acetic acid from the CO2 emitted from coal-fi red power plants.15
In California, US, there are several important groups of researchers on catalysis. One is the group formed by the Joint Center for Artifi cial Photosynthesis, run by the California Institute of Technology, in partnership with the Lawrence Berkeley National Laboratory, together with the Max Planck Institute for Chemical Energy Conversion in Mülheim, Germany, who together form the ‘Sunrise Consortium’. This team is working on photocatalysts, which require high energy ultraviolet light to generate the electrons to convert CO2 to methanol, formaldehyde, and formic acid. The main objective is to
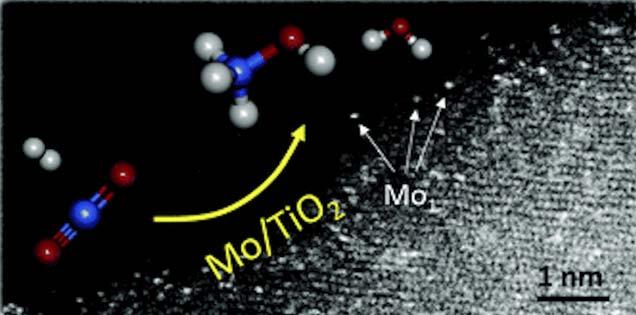
Figure 3. A new catalyst developed by the CNRS in collaboration with other research centres, based on Mo/TiO2, to convert CO2 to methanol, with atomic dispersion of the molybdenum atoms.
Figure 4. A new catalyst developed by the Hokkaido University to convert CO2 to methanol, based on Re supported on TiO2, that works under mild operation conditions (T= 150ºC, pCO2= 1 MPa, pH2 = 5 Mpa).

improve current titanium dioxide catalysts that only act under ultraviolet light by doping it with nitrogen to make it more active even with only visible light.16
Also in California, researchers from the Lawrence Berkeley National Laboratory and from ExxonMobil have discovered a new Material Organic Framework (MOF) called tetraamine-functionalised MOF that can capture more than 90% of the CO2 emitted from industrial sources. This is up to six times more effective than conventional amine-based carbon capture technologies.17
In the same area, a research team from Caltech and the UCLA Samueli School of Engineering are working on an electrocatalyst that converts CO2 into ethylene. The design of the catalyst is based on copper nanowires with highly active ‘steps’ – similar to a set of stairs arranged at atomic scale, which achieve high selectivity to ethylene, avoiding the formation of undesired products, and with a conversion rate of greater than 70%, as seen in Figure 2.18
In Spain, the Instituto de Tecnología Química (ITQ) de Valencia is working on catalysts to promote CO2 photothermal catalytic hydrogenation to CO, ethylene, methanol, or formic acid with high selectivity. The ITQ is working together with other prestigious universities and research centres on the project FlowPhotoChem, which is a multi-national, EU-funded research project, with the objective of developing chemicals from CO2 and sunlight.19
In France, researchers at the CNRS of Lyon, in collaboration with other research centres, are developing Mo/TiO2 catalysts for CO2 hydrogenation to methanol, working at the atomic scale on the dispersion of the molybdenum atoms at the surface of the catalyst to obtain the highest activity and selectivity of the reaction, as shown in Figure 3.20
At the ETH in Zurich, Switzerland, researchers in collaboration with Total have developed a catalyst that improves the In2O3 catalyst to convert the CO2 to methanol, by replacing indium atoms in the active In3O5 with palladium atoms.21
In Russia, researchers from the Boreskov Institute of Catalysis are also working on CO2 use, in this case for the reaction with propylene oxide to form propylene carbonate using zeolitic imidasolate frameworks MAF-5 and MAF-6.22
In Croatia, researchers from the Croatian Ruđer Bošković Institute (RBI), in collaboration with colleagues from the Institute of Chemistry (KI) in Slovenia and McGill University in Canada, have developed a new bimetallic metal-organic crystalline CuZn framework (MOF-74) that presents moderate activity for the synthesis of methanol from CO2 comparable to the industrial CuZn Alumina catalysts.23
In the UK, researchers from the University of Cardiff are working on catalysts based on a combination of Cu/ZnO catalysts and ZSM-5 zeolites to produce methanol and dimethyl ether from CO2 and hydrogen.24
In South Africa, a team of researchers from the Catalysis Institute at the University of Cape Town, in collaboration with Sasol, have made important advancements in the use of commercial iron catalyst to convert captured CO2. The iron catalyst can achieve CO2 conversions greater than 40%, producing ethylene and light olefi ns, which can be used as chemical feedstocks, and signifi cant quantities of kerosene range hydrocarbons (jet fuel).25
In China, at the Dalian Institute, the project ‘Liquid Sunshine’ converts CO2 to methanol using a catalyst based on zinc oxide/ zirconium oxide. The selectivity to methanol reaches 98%, and the content of methanol is 99.5%. Additionally, the catalyst is resistant to poisoning and sintering.26
In Japan, at the Hokkaido University, researchers are working on a catalyst of Re supported on TiO2 (1%wt Re) that promotes the reaction of CO2 to methanol with high selectivity (82%) and under mild conditions (pCO2 = 1 MPa; pH2 = 5 MPa; T = 150 °C), shown in Figure 4.27
Therefore, the most brilliant brains in catalysis are intensively working on ways to improve catalysts and discover new ones to convert the captured CO2 to fuels and chemicals. It is extremely signifi cant that, very recently, the Nobel Prize for Chemistry has been awarded to Prof. Benjamin List (Max-Planck-Institute, Germany) and Prof. David W.C. MacMillan (Princeton University, US) for their research into new organocatalysts. This research will help develop new catalysts for the conversion of CO2 to chemicals. In conclusion, all of this immense effort carried out by so many extraordinary researchers will provide good and profi table results, and will help us to reach the CO2 neutrality objectives set for 2050.
Note
For the full list of references, please visit: https://www. hydrocarbonengineering.com/refining/10102021/the-key-role-ofcatalysts--references/










