




*Guided by the World Health Organization’s National Assessment Tool on integrated antimicrobial stewardship programs

Nepal October 2022
In Collaboration With:

Pillar 1.
Pillar 2.
Pillar 3.
Pillar 4.
Pillar 5.
Conclusions
Establish and develop national coordination mechanisms for antimicrobial stewardship and develop guidelines
Ensure access to and regulation of antimicrobials
Improve awareness, education and training
Strengthen water, sanitation and hygiene, and infection prevention and control Surveillance, monitoring and evaluation
Pillar 1. Establish and develop national coordination mechanisms for antimicrobial stewardship and develop guidelines
Domain 1. Establish and maintain a national coordinating mechanism for AMS that is functional at national, subnational and district levels
Item 1.1. Is there a national policy on integrated AMS?
Item 1.2. Is there a central national coordination unit at the ministry of health or designated agency or institution focused on AMS?
Item 1.3. Has the central national coordination unit established a national coordination mechanism for integrated AMS activities with diverse membership, including civil society and the private sector?
Item 1.4. Have similar coordination mechanisms with diverse members, including from civil society and the private sector, been established at subnational and district levels?
Item 1.5. Are there clear reporting lines and feedback mechanisms from subnational mechanisms to the national coordination mechanism on integrated AMS activities?
Item 1.6. Is there funding allocated for the national coordinating unit and the national, subnational and district coordination mechanisms?
Item 1.7. Does the national coordination unit have clear terms of reference (TOR)?
Item 1.8. Is there a linkage to other relevant stakeholders e.g. from tuberculosis (TB), infection protection and control (IPC), water, sanitation and hygiene (WASH) or universal health coverage (UHC)?
Item 1.9. Is there a monitoring and evaluation (M&E) framework and have national targets been set for AMS activities based on nationally and internationally agreed indicators?
Item 1.10. Have other programmes, such as IPC, WASH, TB, malaria, HIV, UHC and primary health care (PHC), integrated AMS activities within their action plans?
Domain 2. Develop national treatment and stewardship guidelines, standards and implementation tools
Item 2.1. Have the national treatment guidelines for the management of infections been updated within the last 3–5 years?
Item 2.2. Do the national treatment guidelines include AMS principles?
Item 2.3. Is there monitoring of implementation and compliance to treatment guidelines?
Item 2.4. Is there coordinated guidance and interventions to improve availability and appropriate use of diagnostics to guide therapeutic decisions?
Item 2.5. Are there specific standard operating procedures for AMS activities in health-care facilities and in community settings?
Item 2.6. Are there mechanisms and activities for the dissemination of guidelines, standards and implementation tools on AMS activities?
Pillar 2. Ensure access to and regulation of antimicrobials
Domain 3. Improve access to essential, quality-assured, safe, effective and affordable antimicrobials
Item 3.1. Has the WHO Model List of Essential Medicines (EML) and Access, Watch, Reserve (AWaRe) system been incorporated into the national EML formulary and health-care facility treatment guidelines?
Item 3.2. Is there a system in place to monitor access to essential, quality-assured, safe, effective and affordable antimicrobials?
Item 3.3. Is there a system to periodically identify availability of affordable antibiotics at health-care facilities?
Item3.4.Isthereamechanisminplacetoreportshortagesandstock-outsofantibiotics inthecountry?
Item3.5.Isthereamechanismtoreporttheantibioticsusedbypatients?
Item3.6.IsthereaprocesstoreporttheantibioticsusedintheAWaResystem?
Domain4.Regulatesocialtriggersandremunerationpoliciesthatpromote responsibleantimicrobialprescriptionanddispensingbehaviors
Item4.1.Arehealthworkerbehavioralchangeprinciplesincorporatedintopolicies addressingdiagnosis,prescription,dispensingandadministrationofantimicrobials?
Domain5.Legislateandregulateresponsibleandappropriateuseanddisposalofantimicrobials
Item5.1.Istherearegulationonprescription-onlysale/dispensingofantibiotics?
Item5.2.Areregulationsondispensingantibioticsbyprescriptiononlybeingenforced (whereaccessisnotanissue)?
Item5.3.Isthereanenforceableregulatorymechanismtoprohibitsaleofsubstandardandfalsifieddrugs?
Item5.4.Aretherestandardsandcriteriaforresponsiblemanufacturinganddisposalof antimicrobialagents?
Pillar 3. Improve awareness, education and training
Domain6.Improveawarenessandengagementtosupportbehavioralchange ofantimicrobialsuse
Item6.1.Havestudiesondeterminantsofbehaviorinhealthprofessionalsandotherstakeholders, includingthegeneralpublic,beencompletedtosupportdesignofawarenesscampaigns?
Item6.2.DoesthecountryholdWorldAntimicrobialAwarenessWeek(WAAW)activitiesannually?
Item6.3.Doesthecountryhaveregularpublicawarenesscampaignsontheresponsibleand appropriateuseofantibiotics?
Item6.4.AretailoredAMSmessagesintegratedintobroaderhealthpromotion,prevention,treatment andrehabilitationservicesandinitiativessuchasWorldWaterDay,WorldToiletDay,World Children’sDay,immunizationcampaigns,WorldAIDSDay,WorldTBDay,WorldMalariaDay andWorldNutritionWeekforsustainedaction?
Item 6.5. Is regular assessment/evaluation of the impact of education and awareness campaigns on knowledge attitudes and behaviors of health workers and the public conducted?
Domain 7. Strengthen health worker capacity through the provision of tailored education and training packages according to health worker roles and functions
Item 7.1. Are AMS principles and strategies included in the educational curriculum of pre-service healthcare professionals?
Item 7.2. Is there access to in-service training, including continuous professional development (CPD) on antimicrobial prescribing and AMS for all healthcare professional groups in the country?
Item 7.3. Are AMS concepts and principles incorporated within the curriculum of other complementary disciplines e.g. the curriculum for IPC professionals
Pillar 4. Strengthen water, sanitation and hygiene and infection prevention and control
Domain 8. Enhance WASH in health facilities and communities
Item 8.1. Is there representation of the AMS coordinating unit on the WASH programmes and vice versa?
Item 8.2. Is the WHO WASH Facility Improvement Tool (FIT) being promoted to assess WASH in healthcare facilities?
Domain 9. Implement IPC core components in health facilities
Item 9.1. Is there representation of the AMS coordinating unit on the IPC programmes and vice versa?
Item 9.2. Are there systems linking the monitoring and reporting of healthcare-associated infections (HAIs), antimicrobial use, AMR, patient outcomes and quality of care?
Pillar 5. Surveillance, monitoring and evaluation
Domain 10. Surveillance of antimicrobial use and consumption
Item 10.1. Is there a national surveillance programme for antimicrobial resistance, use and consumption with defined structures, governance and work objectives (i.e. data collection, validation, analysis, reporting and data sharing with all stakeholders)?
Item 10.2. Are there guidelines/standard operating procedures for the use of (a) antimicrobial medicines use (AMU), (b) antimicrobial medicines consumption (AMC), and c) AMR data to inform action for national and institutional decision-making to inform action for national and institutional decision-making?
Domain 11. Surveillance of AMR
Item 11.1. Is there a plan to strengthen laboratory capacity to support accurate diagnosis for decision-making?
Domain 12. Monitoring and evaluation of AMS activities
Item 12.1. Is there a national M&E framework, including internationally and locally agreed key performance indicators for integrated AMS interventions and activities in human health?
Item 12.2. Is there an accountability mechanism put in place at the local level which includes positive feedback and recognition of local leadership?
Assessment report of AMS implementation at the national level

Antimicrobial stewardship (AMS) is one of the key strategies in the global and national action plans to control antimicrobial resistance (AMR). Nepal has taken significant strides in this area, establishing a national coordinating mechanism for AMS and drafting a comprehensive CostedNational Action Plan (Costed NAP). These initiatives demonstrate the country's commitment to addressing the growing burden of AMR. Despite commendable progress, the endorsement of the NAP is still pending, resulting in fragmented AMS activities carried out by different stakeholders. However, with the NAP's endorsement, Nepal aims to integrateandcoordinateeffortsacrossvarioussectorstostrengthenAMS and combat AMR more effectively. Nepal's proactive approach and dedication underscore its determination to promote responsible practices in healthcare and tackle AMR challenges head-on. By implementing the NAP, Nepal seeks to further enhance its efforts in antimicrobial stewardship,contributingtotheglobalfightagainstAMRandensuringthe sustainabilityofhealthcarepracticesinthecountry.
In this analysis, we used the World Health Organization (WHO)’s national assessment tool from the WHO policy guidance on integrated antimicrobial stewardship activities to evaluate AMS implementation at the national level with five pillars, 12 domains and 44 items (Figure 1). The12domainsrepresent12packagesofinterventionsandactivitiesthat need to be considered and implemented to create a strong backbone for an integrated AMS approach to preserve antimicrobials. The policy guidance also seeks synergy and efficiency for the implementation of comprehensiveinterventionsacrosskeysectorsandatalllevels.
The project aims to identify successes and achievements, areas for improvement, driving factors, and barriers to program implementation to inform further actions to improve the current status at the national level in Nepal. Below we summarize the overall state of national-level AMS implementation and the key findings from this assessment under each pillar.

AMR
AMS
AMU
AWaRe
DDA
DOHS
EDCD
ELMIS
EML
ETP
GAP
GLASS
GoN
HCW
IPC
LMS
MoHP
MSS
NAP
NHEICC
NHTC
NLEM
NPHL
NSSD
PPS
PMS
QSRD
SOP
STP
WASH
WHO
: Antimicrobial Resistance
: Antimicrobial Stewardship
: Antimicrobial Use
: Access, Watch, Reserve
: Department of Drug Administration
: Department of Health Services
: Epidemiology and Disease Control Division
: Electronic Logistics Management Information System
: Essential Medicine List
: Effluent Treatment Plant
: Global Action Plan
: Global Antimicrobial Resistance and Use Surveillance System
: Government of Nepal
: Healthcare Workers
: Infection Prevention and Control
: Logistic Management Section
: Ministry of Health and Population
: Minimum Service Standards
: National Action Plan
: National Health Education Information and Communication Center
: National Health Training Center
: National List of Essential Medicines
: National Public Health Laboratory
: Nursing and Social Security Division
: Point Prevalence Survey
: Post Marketing Software
: Quality Standard and Regulation Division
: Standard Operating Procedure
: Standard Treatment Protocol
: Water, Sanitation, and Hygiene
: World Health Organization
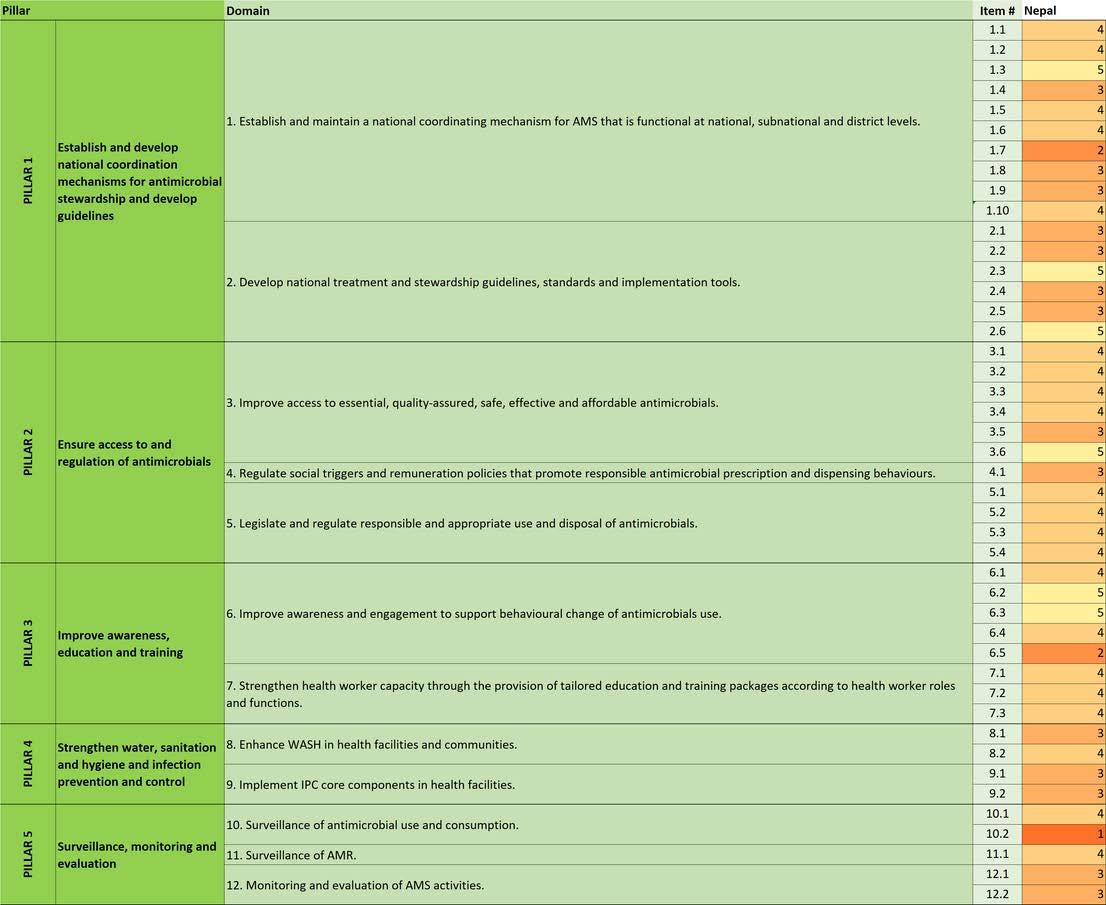

Establish and develop national coordination mechanisms for antimicrobial
Nepal has taken significant steps towards establishing a national coordinating mechanism for antimicrobial stewardship (AMS). The country has drafted a Costed-National Action Plan (Costed NAP) encompassing AMR, AMU, and AMS initiatives, aiming to integrate and coordinate all relevant stakeholders and activities. However, the endorsement of the Costed-NAP is pending, leading to fragmented AMS activities carried out by different stakeholders at individual institution levels due to the absence of a specific national policy. At the national level, the Antimicrobial Resistance Multisectoral Coordination Committee (AMR MSC), led by the secretary, oversees aspects related to AMR containment, including surveillance, stewardship, and antimicrobial use. While the national coordinating mechanism for AMS is operational and inclusive (includes diverse membership), coordination mechanisms at the provincial and local levels have been planned but not yet implemented. Funding for AMS activities is currently limited, relying on support from various partners such as the Fleming Fund. However, with the endorsement of the Costed-National Action Plan, a dedicatedbudgetallocationforAMSactivitieswillensuresustainedfunding.
The main challenges for and gaps in the implementation of the intervention underthisdomainincludes:
Lack of full endorsement and implementation of the Costed-National ActionPlan(CostedNAP)forantimicrobialstewardship(AMS)activities.
Insufficient dedicated funding from the government for AMS initiatives, relyingheavilyoninternationalsupport.
Absence of specific terms of reference (TOR) for the national coordinating unit,hinderingitsfocusedfunctioning.
DelayinestablishingcoordinationmechanismsforintegratedAMSactivities atthesubnational(provincialanddistrict)levels.
Poor linkage and integration of AMS principles in existing interconnected programmes such as tuberculosis, infection protection and control, water, sanitation,andhygiene,oruniversalhealthcoverageprograms.
Lack of developed standard operating procedures (SOPs) for AMS activities,resultinginunclearguidanceandinconsistentpractices.
Delay in integrating the AMS principles into updated national treatment guideline.
Delay in initiating and implementing monitoring and evaluation (M&E) frameworksforAMSactivitiestoassesstheirimpactandprogress.
Addressing these gaps is crucial to strengthen AMS efforts and combat antimicrobialresistanceeffectivelyinNepal.


Nepal has made notable progress in improving access to essential antimicrobials by partially incorporating the WHO Model List of Essential Medicines (EML) and the Access, Watch, Reserve (AWaRe) system into the national EML formulary and health-care facility treatment guidelines. The Drug Administration of Nepal (DDA) has been proactive in updating the national list of essential medicines (NLEM) based on WHO recommendations, tailored to the local context through AWaRe classification. While there are still pending implementations at the Ministry of Health and Population (MoHP) level, the country has established an electronic Logistics Management Information System (e-LMIS) to monitor distribution,stock,andconsumptionofmedicinesatthelocallevel.Effortsarealsoongoing to identify the availability of affordable antibiotics and address reporting challenges from district- and provincial-level hospitals. Nepal is actively working on a patient-level reporting system and future plans include post-marketing software to better track antimicrobial sales. To promote responsible antimicrobial prescription and dispensing behaviors, health worker behavioral change principles are being incorporated into policies, and antibiotic treatment guidelines, including AWaRe classification, are being updated. Additionally, Nepal is enforcing prescription audits and conducting national prescription surveys to ensure responsible use. Efforts to detect and recall substandard drugs through risk-basedpost-marketingsurveillance(PMS)areinplace,andregulationsonprescriptiononly sale of antibiotics have been established. Although there is room for improvement in enforcement at the retail level and specific disposal criteria, Nepal maintains standards for responsible manufacturing of antimicrobial agents, ensuring adherence to Good Manufacturing Practices (GMP) by manufacturers. With continuous progress, Nepal is activelystrivingtostrengthenantimicrobialstewardshipandpromoteresponsiblepractices tocombatantimicrobialresistanceeffectively.
Themaingapsremaininthisareainclude:
Partial implementation of the WHO Model List of Essential Medicines (EML) and AWaResystematthenationallevelduetochallengesinimplementationbytheMinistry ofHealthandPopulation(MoHP).
Limited data availability on the distribution and use of antimicrobials at district- and provincial-levelhospitalswheremaximumconsumptionofantimicrobialsoccurs.
Lack of systematic mechanism in place to periodically identify the availability of antibioticsatthehealthcarefacilities,resultinginsupplychainissue.
Theprocessofreportingantibioticsusedbypatientsisplannedbutnotstarted,andthe DDA currently relies on information from importers and wholesalers for a snapshot of antimicrobialsales.
The implementation of regulations on prescription-only sale/dispensing of antibiotics is onlypartiallyenforced,withantibioticsoccasionallybeingsoldwithoutprescriptions.
Lack of national-level standards and guidelines for pharmaceutical waste disposal and guidelines for the national-level disposal of expired medications have not been established.
Nepal has taken steps to implement studies on the determinants of behavior in health professionalsandthegeneralpublictosupportthedesignofawarenesscampaigns. Although these studies are only partially implemented. The government actively involves experts and professionals to prepare awareness materials. Nepal actively participates in World Antimicrobial Awareness Week (WAAW) activities, conducting various awareness initiatives and disseminating relevant information at national, provincial,andpubliclevels.
The National Health Education Information and Communication Center (NHEICC) of Nepal is fully engaged in conducting regular public awareness campaigns on the responsible use of antibiotics. While there are some challenges in integrating tailored AMS messages into broader health promotion campaigns, ongoing initiatives are beingundertakenacrossdifferenthealthsectors.
AMS principles and strategies are partially included in the educational curriculum for pre-service healthcare professionals, with an emphasis on general AMR principles covered in medical, nursing, para-medical, and pharmacy courses. In-service training on AMR, including antimicrobial prescribing and AMS, is available through the National Health Training Center (NHTC), but it is not yet mandatory and not fully accessibleinallprovinces.
While AMR concepts are integrated into complementary disciplines like infection prevention and control (IPC) and water, sanitation, and hygiene (WASH), dedicated AMS components remain limited. With the implementation of the National Action Plan (NAP on AMR), Nepal has the opportunity to further strengthen, and coordinate activities related to AMR, AMS, and appropriate antimicrobial use. These positive efforts demonstrate Nepal's dedication to combating antimicrobial resistance effectively.
Themaingapsidentifiedinthisareaincludes:
Limited studies conducted to understand the determinants of behaviour in health professionals and the general public to support the design of awareness campaignsandtailorAMSmessages.
Incomplete integration of AMS principles and strategies into the educational curriculumofpre-servicehealthcareprofessionals.
Limited accessibility and mandatory implementation of in-service training on antimicrobial prescribing and AMS for healthcare professionals, due to capacity andresourcechallenges.
Insufficient incorporation of AMS concepts and principles within the curriculum of complementary disciplines like infection prevention and control (IPC) programs
 Oxford
Oxford
Nepal is making positive strides towards enhancing Water, Sanitation, and Hygiene (WASH) inhealthfacilitiesandcommunitiesaswellasimplementingInfectionPreventionandControl (IPC) measures. Although the integration of the AMS coordinating unit with WASH and IPC programs has not started yet, there are clear intentions to establish collaborative efforts. The management division's work on Healthcare Facility WASH policies and standards is a step forward, even though implementation is conducted at individual hospitals and local levels. The introduction of the WHO WASH Facility Improvement Tool (FIT) and training in seven provinces indicate progress, despite limited nationwide coverage due to budgetary and infrastructure challenges. Similarly, while IPC programs exist and formal guidelines are awaiting approval, efforts to train human resources in IPC and consider animal health in zoonotic diseases show promising developments. Plans to link surveillance activities related to healthcare-associated infections (HAIs), antimicrobial use, AMR, patient outcomes, and quality of care demonstrate the commitment to comprehensive monitoring. Although full implementationandcoordinationremaininprogress,theseplannedinitiativesreflectNepal's determination to improve WASH and IPC practices in the country's healthcare facilities and communities.
The main gaps identified in the implementation of AMS in relation to WASH and IPC programsare:
Representation of the AMS coordinating unit on the WASH and IPC programs is planned but not yet started, indicating a need for better integration and collaboration between theseimportantcomponents.
WASH in healthcare facilities Improvement Tool (FIT) lacks a nationwide coverage due tobudgetaryconstraintsandinfrastructureneeds.
Even though IPC program exists in a healthcare facility, the formal guidelines are pendingapproval.
Systems linking the monitoring and reporting of HAIs, antimicrobial use, AMR, patient outcomes, and quality of care have not yet been fully established, with some surveillanceactivitiesstartedbutnotsystematicallyconnected.
Budgetary limitations and human resource constrain can impede the implementation of bothWASHandIPCinitiatives.
Although there are plans to link surveillance of healthcare-associated infections (HAIs), antimicrobial use, AMR, patient outcomes, and quality of care, actual implementation is pending.
Limited training and considerations for animal health, especially in the context of zoonotic diseases, may not be fully addressed leading to challenges in controlling and preventingzoonoticinfections.
Overall, these gaps represent significant challenges in the effective implementation of WASHandIPCinitiativesinhealthfacilitiesandcommunities.Addressingthesegapsrequires collaborative efforts, adequate resources, and strong commitment from relevant stakeholdersandauthorities.

Nepal has made significant progress in combating antimicrobial resistance (AMR) through its well-established national surveillance program, supported by 21 participating laboratories under the Fleming Fund and non-Fleming fund grants. The program has been in place since 2005 and focuses on collecting, validating, analyzing, and reporting data on 10 priority pathogens recommended by WHO GLASS (Global Antimicrobial Resistance and Use Surveillance System). This valuable information is shared on the GLASS portal and disseminated through newsletters.
To further enhance their efforts, Nepal has ambitious plans to strengthen laboratory capacity, including advanced molecular testing, to ensure accurate diagnoses for effective decisionmaking. The National Public Health Laboratory (NPHL) is actively involved in equipping itself with high-tech technologies and helping district hospitals in establishing microbiology laboratories to improve antimicrobial prescription practices based on microbiology culture and sensitivity reports. There are plans to expand sentinel sites and establish more microbiology labs inotherdistricthospitals.
Currently, Nepal lacks a national monitoring and evaluation (M&E) framework for integrated AMS interventions in human health. However, a National Action Plan (NAP) on AMR and AMS, yet to be endorsed, will set national targets and guidelines for evaluating progress. The drafted NAPincludesprovisionsforM&Esystemsandstakeholderroles.
There is no accountability mechanism at the local level that recognizes and provides positive feedback to local leadership for AMS activities. The implementation of NAP is expected to address this issue and specify roles, responsibilities, and scopes of different stakeholders, aligning efforts towards the common national goal of AMR containment, strengthening AMS, and optimizing AMU in a one-health approach. Amendments to policy documents, SOPs, and STPsmayoccurbasedoninitialevaluationreportsfollowingtheimplementationofNAP.
Importantgapsandareasforimprovementinthispillarare:
There is currently no national surveillance program for antimicrobial use and consumption (AMUandAMC)inNepal,withlimiteddataavailableonAMUatthepatientlevel. Guidelines or standard operating procedures (SOPs) for the use of AMU and AMC data to inform national and institutional decision-making are lacking, indicating a need for a structuredapproachtoutilizingthisdataforeffectiveaction.
There is no accountability mechanism in place at the local level that recognizes and rewards localleadershipforeffectiveAMSactivities.
The pending Endorsement of the National Action Plan (NAP) may hinder the coordinated effortsofvariousstakeholdersintacklingAMRandimplementingAMSinterventions. Nepal currently lacks a national M&E framework for integrated AMS interventions and activitiesinhumanhealth.
There may be challenges in resource allocation, training personnel, and maintaining quality standardsacrossallthemicrobiologylabsevenifitisplanned.
In summary, Nepal has made significant progress in tackling AMR and putting AMS measures into practice. However, there are ongoing efforts and areas where implementation is incomplete, emphasizing the need for sustained attention and action. Nepal's strategic approach, including its commitment to global initiatives and the development of a costed national action plan, highlights its determination to combat AMR. The establishment of a coordinated national system, adherence to WHO guidelines, and a fully operational national AMR surveillance program showcase Nepal's dedication to making informed decisions and improving access to essential antimicrobial treatments. Regular public awareness campaigns further underscore its proactiveengagementinpromotingresponsibleuseofantimicrobials.

However, certain specific areas demand more focus and advancement, such as refining guidelines, strengthening data collection and reporting, implementing AMS practices in healthcarefacilities,andenhancingthecapabilitiesofhealthcareworkers.
By making significant progress in these targeted areas, Nepal can continue its effective efforts against AMR, contributing significantly to the global fight against antimicrobial resistancewhilesafeguardingthehealthoffuturegenerations.
The global spread of AMR and its effects has been widely documented [1], with an estimation of about 10 million deaths per year due to AMR by 2050 [2]. Controlling AMR is an important societal priority as it is a cross-cutting and transdisciplinary issue that can negatively impact the achievements in human health, food and environmental security, as well as our collective targets in achieving the Sustainable Development Goals (SDGs) [3]. The use of antibiotics is directly contributing to the development and spread of AMR [4]. AMS is one of the key strategies in the global AMR Action Plan and has been shown to be effective in reducing the inappropriate use of antimicrobials and therefore helping to reduce AMR[5-8].
AMR stands as a global health and developmental threat demanding immediate crosssectoral measures to fulfill the SDGs, with WHO identifying it as a foremost global public health concern [13]. In 2011, health ministers from the South-East Asia Region, including Nepal, proactively acknowledged, the early threat by endorsing the Jaipur Declaration on AMR [12].The endorsement of the Global Action Plan (GAP) on AMR by the World Health Assembly in May 2015 and the recognition of AMR as a worldwide health concern in the Political Declaration of the High-Level Meeting of the General Assembly on AMR in September 2017 both highlight the worldwide concern about AMR. These policy actions acknowledge the overuse and misuse of antimicrobial as a primary factor contributing to the emergence of resistance as well as a need to optimize the use of antimicrobials [14]. The GAP-AMR adopted a "One Health" approach that recognizes the interconnectedness of human health, animal health, and the environment. This strategy advocates for a multifaceted,national-levelapproachtoeffectivelycombatAMR[12].
The 2019 National Health Policy of Nepal underscores the critical importance of addressing AMRasamajorpublichealthconcern.Ithighlightsthenecessitytoestablishanationalaction plan (NAP) and promote collaborative efforts across sectors for effective antibiotic management [9]. Alongside this, the Nepal Health Sector Strategy 2015–2020 acknowledges the significance of antibiotic resistance as a substantial public health challenge andincorporatesastrategicinitiativetocombatAMR,therebyenhancinghealthcaredelivery quality[11].
In 2021, the WHO developed a toolkit to evaluate the implementation of the AMS program at the national level [15]. In this project, we used the WHO Toolkit to identify areas of successes and achievements, areas for improvement, driving factors, and barriers to program implementation in order to assess and inform further actions to improve the currentstatusatthenationallevel.

We used the WHO toolkit to review current AMS implementation at the national level [15] using the specific questions categorized in below five key domains to guide our data collection,andanalysisandsynthesisoftheresults:
A.EstablishanddevelopnationalcoordinationmechanismsforAMSand developguidelines.
B.Ensureaccesstoandregulationofantimicrobials.
C.Improveawareness,education,andtraining.
D.Strengthenwater,sanitation,hygiene,andinfectionpreventionandcontrol.
E.Surveillance,monitoring,andevaluation
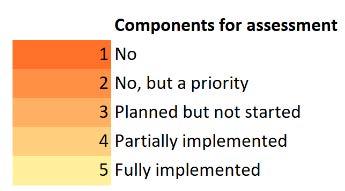
For each question, a rating of the current state of AMS implementation items is chosen together with justifications given for the response as well as an elaboration on the contextual factors that has contributed to the achievements and challenges. Following arethe5optionsofresponseforeachquestion,theresponderswereinstructedtoselect one of these options as the final response in each question during the data collection process:
1=No(orNotimplementing);
2=No,butapriority;
3=Plannedbutnotstarted;
4=Partiallyimplemented;
5=Fullyimplemented.
Thereviewwasbasedonthefollowingsourcesofinformation:
Review of policy documents of Nepal such as the Drafted NAP for AMR, National AMR Containment Action Plan-2016, Drafted Infection Prevention and Control Guideline, Standard Treatment Protocol for Basic, Health Service Package 2021/22 (2078 BS), Infectious Disease Control Guideline 2016, Nepal Antimicrobial treatment guideline 2014, National List of Essential Medicine in Nepal 2021 –sixth edition, the 98 Essential Medicine List (free), Drug Category Rule 1986/87 (2043BS), National Healthcare Waste Management SOP -2020, National Standard for WASH in HCF of Nepal, The Water and Sanitation for Health Facility Improvement Tool(WASHFIT)etc.

In-depth interviews of six key informants who are key members of Ministry of HealthandPopulation(MoHP)Nepal:
Former director of National Public Health Laboratory (NPHL): Distinguished and visionary leader who played a pivotal role in shaping and overseeing the strategic direction of the laboratory's initiatives, particularly in the field of AMRsurveillance.
Chief of Quality Standard and Regulation Division (QSRD): Highly experienced and skilled professional, dedicated to ensuring the highest standards of healthcare. Instrumental in developing and implementing quality assurance protocols and playing an important role in drafting AMS policyinNepal.
Senior Drug Administrator: Dedicated professional at the Department of Drug Administration (DDA), responsible for overseeing the safe and effective use of medications through pharmaceutical regulation and drug administration.

SeniorHospitalNursingAdministrator:Compassionateandskilledhealthcare professional with vast nursing administration experience. Notably, she drafted the first IPC guidelines in Nepal and demonstrates a strong commitmenttopatientcareandnursingexcellence.
Public Health Administrator: Proficient in healthcare management, with a background in public health and administration. Plays a key role in overseeing various healthcare programs and operations within the ManagementDivision.
Procurement Officer: Detail-oriented and resourceful expert in procurement processes and supply chain management within the Logistic Management Division. Ensures timely and efficient acquisition of essential medicalsuppliesandequipmentforsmoothhealthcareservicedelivery.

Establish and develop national coordination mechanisms for antimicrobial stewardship and develop guidelines
Current status:
Partially implemented
Establish and maintain a national coordinating mechanism for AMS that is functional at national, subnational and district levels
After formulation and approval of GAP by WHO in 68th World Health Assembly in 2015, Nepal being a member state signed a commitment for AMR containment. Under this, then honorary official of MoPH, along with National Public Health Laboratory (NPHL) in association with Government of Nepal members of ‘National alliance for prevention and control of Antimicrobial resistance’ prepared a NAP for combating AMR. (“a committee was formed with then Director General, NPHL and Chikitsa Mahasakha, which prepared a guidelinedocumentforAMRcontainmentaround10yearsbefore”).Thisdocumentoutlined different AMR containment action plans such as conduct AMR surveillance, promote capacity building by providing relevant trainings (which is routinely ongoing via National HealthTrainingCenterunderMoHPthatregularlyprovidesrelevanthealthtrainingsonAMR to doctors and nurses) strengthen infrastructure of laboratories working on AMR surveillanceofhumanandanimalsectors(whichiscurrentlyongoingtoo).
Similarly, regarding aspects on AMU (Antimicrobial use), Department of Drug Administration (DDA) has been working on this, for which DDA is a focal point too. (NPHL is afocalpointforAMRsurveillance).
Similarly, for AMS too, the status of AMS has been assessed in few hospitals utilizing the WHO AMS assessment toolkit for healthcare facilities by few medical colleges, private/community hospitals and institutions, rather than by government itself. Thus, so far, the pioneering baseline effort to integrate and bind all these AMR-AMS activities was initiated by the National AMR Containment Plan. Then after, after the approval and signed commitment of GAP, NAP was drafted. While drafting NAP, several stakeholders contributed. Each stakeholder had been undertaking several specific AMR-AMS related activities in their own scattered individual ways per various institutional draft documents. NAP policy document envisions to undertake a coordinated and cohesive action that will integrate all these stakeholders and the existing institution specific draft guidelines for addressing AMR and AMS. The drafted NAP has envisioned to form a specific Steering Committee with a chair, a Technical Working Group which will be chaired by QSRD Chair, and several Technical Working Committees specifically for AMS (to be led by QSRD), AMU (to be led by DDA) and AMR surveillance (to be led by NPHL). All these individual working groups are currently working independently. Very soon with the endorsement and functionalization of NAP, all these individually scattered groups and their activities will be integrated together in a coordinated fashion. Hence, at the moment though Nepal lacks a single specific national policy on AMS (which is NAP that takes all AMR, AMU and AMS together),individualactivitieshavebeenongoingscatteredatindividualinstitutionlevels.
Nepal has drafted the Costed-National Action Plan (Costed NAP) for 5 years, which is bit different compared to majority of other countries; only 1-2 countries have prepared the Costed NAP as Nepal. This document is currently at Ministry of Finance for the review process. After approval from Ministry of Finance, this policy document will be directed to thecabinetforapprovalandfinalendorsement.
Item 1.2.
Current status: Partially implemented
Item 1.3.
Current status: Fully implemented
Item 1.4.
Current status: Planned but not started
Is there a central national coordination unit at the Ministry of HealthordesignatedagencyorinstitutionfocusedonAMS?
Not just specifically focused on AMS, the National Coordination Committee which is of Secretary Level looks for overall AMS, AMR surveillance, AMU, andallaspectsunderall5pillarsofAMRcontainment.
Under this committee, is One Health focused Steering Committee led by Secretary of MoHP. There are joint Secretaries from different departments of MoHP, along with other ministries such as Ministry of Finance, Ministry of Home Affairs, and Ministry of Education. Under this committee is a Technical WorkingCommitteewhichisalsofocusedonOneHealthApproachandledby QSRD. Under this, there are different types of specific Technical Working Groups (TWGs). All these coordination units and committees have also been included in the NAP draft. What specific type of Technical Committee will be requiredtoaddresseachactivityundereachpillarsuchasthatspecificallyfor each AMS, AMR, AMU etc. have been included and envisioned in the drafted Costed-NAP policy. Till the real endorsement of the NAP, for now, National Technical Working Committees (NTWC) for AMR-AMS is being led (facilitation and regulation) by Secretary. As of now, this has been active and ongoing, including AMS. MoHP started and implemented the WHO healthcare facility specific AMS toolkit in few hospitals, of which it is running in 5 different hospitals. Apart from this, different hospitals have implemented this attheirowneffort,thedataofwhicharealsosharedwithMoHP.Interviewee (002-002) believe that when NAP for AMR will be materialized and endorsed, the AMS activities will be undertaken in institutions in more strengthened fashion. Till then, AMS related works are at least happening to someextent.
Has the central national coordination unit established a national coordination mechanism for integrated AMS activities with diverse membership, including civil society and the private sector?
Yes, national coordination mechanism for AMR-AMS under MoHP has diverse membership that includes civil society, private hospitals, public institutionsandtheirmedicalunits.
Have similar coordination mechanisms with diverse members, including including from civil society and the private sector, beenestablishedatsubnationalanddistrictlevels?
At Provincial (sub-national) level, this has not yet initiated. Till date, all AMR related activities at provincial level are being centrally governed from the national level itself. That sort of provincial and district level coordination units for AMR-AMS has not yet been envisioned either. However, to advocate the AMR related containment activities, and to gather feedback on the AMR-AMS-AMU action plans that were drafted in NAP,lastyear,MoHPlaunchedaprograminall7provinces.
Item 1.5.
Current status:
Partially implemented
ThiswasprimarilytoletallprovincesgetacquaintedabouttheimminentNAP,andto let know their specific roles in implementation of various pillars and activities under NAP at provincial levels. Thus, in the drafted NAP policy, such coordination mechanism with diverse membership has been envisioned at provincial and local levels. But, as of now, no such dedicated coordination mechanism or committee or body as such exists currently at provincial or local level. So, this is planned but not yet started. Despite the absence of such body, activities though have been ongoing atprovinciallevel.
Are there clear reporting lines and feedback mechanisms from subnational mechanisms to the national coordination mechanism on integratedAMSactivities?
Federal Government of Nepal (GoN) has a three-tier governance mechanism with central (national), provincial, and local levels. Under this governance mechanism, currently there is no exact reporting lines and feedback mechanisms from subnational to national level. However, as of earlier system, when needed, the subnational level must report to the central level. This mechanism of subnational to central level reporting has just been initiated/envisioned in NAP but due to the lack of subnational-specific coordination units, such reporting line does not literally exist as such. For example, for celebrating World antimicrobial awareness week (WAAW), national level communicated with the provincial level to conduct specified AMR awareness activities and convey the message accordingly to their local levels too. Later, when NAP is endorsed, WAAW activities will be initiated and conducted independently by each province themselves. But currently, this mechanism has not been established so far. Currently, all AMR-AMS activities at provincial and local levels are being centrally conducted, coordinated and bridged. NAP though has envisioned the reporting and communication mechanisms, and scope of activities at national,provincialandlocallevelsinalignmentwiththefederalgovernmentsystem of Nepal. Apart from this, under one health approach NAP has also envisioned the coordination mechanism for each division and sectors, such as that of human health, animalhealth,foodandagriculture.Thechainofcommunicationfromsubnationalto centrallevelexists,butthiswillbefurtherstrengthenedafterendorsementofNAP.
Item 1.6.
Current status:
Partially implemented
Is there funding allocated for the national coordinating unit and the national,subnationalanddistrictcoordinationmechanisms?
Currently, there is not much dedicated funding allocated from the government side to support the AMR-AMS coordination units and mechanisms at national and subnational levels. However, various partners such as the Fleming Fund and other agency who conduct such activities have been financially supporting such activities. Where the problem mainly lies is that the AMS in Nepal has only been in its infancy, and it is yet to be strengthened. GoN has neither yet been able to make AMS activities compulsory in institutions, nor been able to establish and bring program specific AMS activities. Thus, there are not specific government funds to addressthis.
Item 1.7.
Current status: No, but a priority
However, having said that, currently few institutions and agencies which think AMS is important and need to be prioritized are undertaking AMS related activities at their own initiatives and resources, either utilizing their own funds or in partnership with other agencies. Of these, the strongest partner has been the Fleming Fund, its regional and country grants have greatly supported AMS activities in Nepal. Of some private agencies working specifically in AMS in Nepal, for example is Group for Technical Assistance (GTA), which has been supported by Henry Ford Health International to conduct the AMS training activities. WHO has similarly helped conduct the AMSactivitiesthatGoNaretryingtoruninallprovinces.
Soon, when NAP will be endorsed, the AMR activities including AMS will definitely be rolled out in a sustainable way. Because in the costed-NAP, MoHP has envisioned all action plans and activities with specific budget required for five years for each of the activities to be implemented across all 3 levels of government. We are assured that with the endorsement of NAP, the AMS and AMR activities will be sustained with dedicated budget from governmentlevelbeingallocated.
Does the national coordination unit have clear terms of reference(TOR)?
As of now, the national coordination unit does not have a dedicated terms of reference specifically to address AMS. It is definitely a priority for GoN, but forming a separate unit and TOR just for AMS will create a redundancy as this hasalreadybeenenvisionedinthedraftedNAP.
Item 1.8.
Current status: Planned but not started
Item 1.9.
Current status: Planned but not started
Is there a linkage to other relevant stakeholders e.g. from tuberculosis (TB), infection protection and control (IPC), water, sanitation and hygiene (WASH) or universal health coverage(UHC)?
Not exactly that AMS activities currently have linkage to all existing relevant stakeholder. However, there is some linkage in stakeholder like WASH and IPC. The recent guideline on liquid waste management that WASH has developed does have a component of AMR. The 5-year road map that has been drafting under WASH program has incorporated AMR under liquid waste management guideline. The IPC protocol that MoHP is drafting also has an AMR component. Along with AMR, the AMS comes simultaneously in all of these activities. As both of these are in process, it is still in pipeline (yet tobeexecuted).
Is there a monitoring and evaluation (M&E) framework and have national targets been set for AMS activities based on nationally andinternationallyagreedindicators?
The M&E framework currently is not implemented. However, in the drafted NAP, the national targets have been set for evaluating the progress on each of AMR-AMS-AMU domains directed as per the action plans against each of thepillarsalongthe5-yearsofroadmap.
After the endorsement and implementation of NAP, MoHP will evaluate the progress accordingly. Also, it will also figure out what else and which further steps need monitoring, what needs to be evaluated and what feedback needs to be given to ensure strengthening of the plans. For now, in the absence of NAP which would be a single binding policy to unite all currently fragmented departments/units and their activities, currently there are no designated M&E frameworks to evaluate the progress of the each individually led programs. Once NAP will be endorsed it will specify roles, responsibility and scope of each different stakeholders (such as different divisions and departments under MoHP like QSRD, National Planning commission,NationalCoordinationDivision,NPHL,EpidemiologyandDiseaseControl Division (EDCD), Department of Curative Services, DDA, Department of Livestock (DLS), Department of Agriculture, Department of Food Technology and Quality Control (DFTQC); Ministry of Education, Ministry of Finance), and how the activities of each of those units will align and synergize to fulfill a common national goal to ensureAMRcontainment,strengthenAMS,andenhanceoptimalAMUinthecountry in a one health approach. After the implementation of NAP, and based on initial evaluation reports, then it might be necessary to amend specific policy documents, Standard Operating Procedures (SOPs), and Standard Treatment Protocols (STPs) accordingly.
Item 1.10. Have other programmes, such as IPC, WASH, TB, malaria, HIV, UHC and primary health care (PHC), integrated AMS activities within theiractionplans?
Current status:
Partially implemented
So far, there are no programs that have visualized the integration of dedicated AMS activities with their action plans. All these concerned programs are aware of AMS principles though and have some AMS component in their activities. For e.g., under TB program, we can say that a strong AMS activity is engrained in it, because a specific treatment protocol exists for TB that defines specific dose, duration and drug along with guidelines for dealing if any complication arises, which altogether in fact is the AMS principle (if the prescribed molecule is beneficial to the patient? And if the benefit of prescribed molecule outweighs the health risks and consequences to patients due to the given disease is basically what AMS is). The Directly Observed Therapy Short course (DOTS) program for TB has proven highly effective for TB treatment. For the treatment being directly observed,AMSisautomaticallyengrainedintheTBprogram.
Similarly, Nepal has Standard Treatment Protocol (STP) for malaria, HIV-AIDS (Anti-retroviral therapy too is directly observed, thus AMS can be said to be strongly implemented in HIV program too). Under IPC program, though it basically looks into the ways to mitigate hospital infections, we still need to work in this sector to strengthen AMS component, which is currently going on. Apart from this, other vertical programs that MoHP is running have AMS activities to some extent under their action plans, though a separate dedicated AMS committee lacks in them. Also in hospitals, the concerned authorities sit together to find the best antibiotics for the treatment of an infection that is found resistant to standard treatment, but there may not be dedicated and formal AMS committee whichwouldotherwisesitandreviewregularly.
Item 2.1.
Current status: Planned but not started
Have the national treatment guidelines for the management of infectionsbeenupdatedwithinthelast3–5years?
(They are updating the STP for infectious diseases under the name of National antibiotic and antimicrobials treatment guideline) MoHP has standard treatment protocol (STP) for different diseases and for different levels of health setups. For example, that specifically for emergency medicine, Basic health service package, musculo-skeletal conditions, gynecological conditions, and that for ongoing vertical programs. An antimicrobial treatment guideline was published by MoHP in 2014, which is currently being updated and completed consultation now, and the draft is in its final stage for being published. This new version of national treatment guideline for management of infection will soon be implemented and endorsed.
Item 2.2.
Current status: Planned but not started
Item 2.3.
Current status: Fully implemented
DothenationaltreatmentguidelinesincludeAMSprinciples?
In the updated national treatment guideline, they have followed international practices and also followed WHO guidelines on antimicrobial categories as Access, Watch and Reserve groups. It has also specified consultation directions to go if any complication arise. More or less, Principles of AMS are tried to be engrained in this upcoming updated version of national treatment guideline. This guideline is yet to be disseminated and endorsed at different levels of health system. MoHP also has a plan of digitizing the national treatmentguidelineusingappbasedinterface.
Is there monitoring of implementation and compliance to treatmentguidelines?
Yes, Department of curative health services under MoHP undertakes the monitoring of implementation and compliance to national treatment guideline. This is the sole primary responsibility of this department. The process is like, for e.g., after a specific STP is drafted and finalized, next process is to successfully roll out that STP under different levels of health systems. For this, first thing is to take and introduce this guideline to these levels (provincial, district and local levels) and train relevant healthcare workers there. MoHP allocates specific budget for this dissemination and training programs so that these healthcare levels can disseminate this guideline to all the health institutions under them. Once at implementation phase, MoHP undertakes monitoring activities to evaluate the degree of implementation and compliance to the given STP. Under this, one of evaluation metrics for the institution is to evaluate its Minimal Service Standard (MSS), which is an index to access the Readiness of the institution to operate, under 3 different domains: governance, clinical management and support system. During this M&E process, which happens in every six months, and which includes a self-evaluation and an external evaluation process, the monitoring team also evaluates if the Standard national treatmentguidelineshavealsobeenimplementedandtowhatextent.
Assessment report of AMS implementation at the national level
MoHP has the MSS mechanism that is functional from health post level till tertiary level. Utilizing this mechanism, the implementation status and any feedback regarding given treatment guideline is collected during this M&E process. However, via this MSS evaluation process, it may be challenging to exactly map the extent of implementation of given STP, as this will be quantitative evaluation with yes/no questions only. So MoHP has not been able to assess the real impact on the disease outcome after the implementation of given treatment guideline, though the MSS system can provide data on the level of implementation. To address this issue on assessing the impact of the treatment guideline, MoHP is preparing another framework called National Quality Assurance Framework, that is also medico-legally bound. And we have also finalized the Implementation Guideline for this framework, which has so far been disseminated to few provinces so far along with gathering feedback on this from Lumbini and Karnali province. Respondents believe that with this framework, the above-mentioned gaps will be addressed. With this, for it being a medico-legally approved framework, Respondent believe that compliance to this will also protect healthcare professionals against violence from agitated patient party in the instances of any misfortunate patient outcome, as they will have proof that they have followed the national treatment guideline. Till the full implementation of this Quality Assurance (QA) framework, MoHP is still in a primitive stage of assessingtheimpactofthenationaltreatmentguidelinesonpatientoutcome.
Current status: Planned but not started
Is there coordinated guidance and interventions to improve availability and appropriate use of diagnostics to guide therapeutic decisions?
The national STP itself specifies the types of diagnostic tools along with type of drugs to be used for the managements of specific syndrome. Next is the reports on different levels of medical/clinical audits (such as doctors audit, nursing audit) conducted by hospitals as required by government in at least large tertiary hospitals, which can also assess the impact/usefulness of treatment protocols (diagnostic tools and medications). In medical audit report, the morbidity patterns such as percentage of different patients’ outcome e.g. number of patients relieved and discharge, referred,thosewhohadunfavorableoutcomesarelookedupon.
Next MoHP has been doing in ~25 different hospitals (tertiary level government and private level) are a Point Prevalence Survey (PPS), where following things are assessed: if the treatment is as per STP or not, if that drug has worked as expected or not, if the prescription is as per the laboratory report or not, number and variants of resistance reported in the pathogens. The PPS has been ongoing in key tertiary hospitals, but not yet been able to roll out in all hospitals. Nursing and Social Security Division (NSSD) looks into nursing audits, surgical/medical audits are reviewed by Curative Service Division; PPS is reviewed by Quality Standard and Regulation Division (QSRD). Though these activities are happening, it has not been able to be replicatedinallhospitals.
Item 2.5.
Current status: Planned but not started
Are there specific standard operating procedures for AMS activitiesinhealth-carefacilitiesandincommunitysettings?
If we consider STPs (treatment protocols of various infections and that of different vertical programs) and PPS (that collects data on types and frequency of molecules being used in hospitals, AMR patterns) as AMS tools, then it can be said that we have the SOPs for guiding AMS activities. But MoHP has not developed any separate SOP specifically dedicated for AMS activities.
In terms of community setting, for example, for pharmacy, DDA has an audit system to monitor the types and quantity of molecules being sold, list of drug manufacturers and distributors in the country etc. DDA also needs to report this data regularly to the MoHP. However, utilizing this data from DDA for informing MoHP at policy level has not happened and is challenging. Also, MoHP has recently made decision of differently labelling the packaging of Antimicrobials being sold with RED marking (inflicting a sense of Danger sign), to induce an alert that this category of drug is different from others, and must be taken with caution under close consultation from prescribers among the end consumers. However, it is taking sometime to be endorsed as many manufacturing companies already have a logistical stock of previously producedpackagingmaterials,whichotherwisewouldbewasted.
Item 2.6.
Current status: Fully implemented
Are there mechanisms and activities for the dissemination of guidelines,standards&implementationtoolsonAMSactivities?
Yes, MoHP regularly disseminates the national guidelines and protocols at the provincial level. Along with dissemination, MoHP also does regular training activities on those guidelines and standards after being dissemination. Any updates in the guidelines or protocol are also disseminated from the central leveltotheprovinciallevelsaccordingly.

Item 3.1.
Has the WHO Model List of Essential Medicines (EML) and Access, Watch, Reserve (AWaRe) system been incorporated into the national EML formulary and health-care facility treatment guidelines?
Current status:
Partially implemented
Item 3.2.
Current status:
Partially implemented
With the involvement of healthcare professional and infectious disease specialist, DDA updated the national list of essential medicine (NLEM 2021) in accordance with WHO suggestions and added the AWaRe classification which was then further tailored to the local context. In addition to keeping NLEM as a book DDA has intended to implement it such as Reserved group antimicrobials must be available to only certain location with certain restriction for its prudent use. In recent time, as the antimicrobials are not used rationally, DDA has started the advocacy to implement it. Antimicrobials has already been classifiedaccordingtoAwareclassificationandisavailableonwebsite.
Regarding implementation, although DDA has been advocating, the MoHP has the primary responsibility for implementation. The DDA has discussed the methods of implementing the AWaRe classification with the MoHP. They have also addressed matters related to the accessibility and monitoring of antimicrobials in the watch and reserve groups, aiming to safeguard against improperusage.DiscussiononStewardshipisalsoongoingonMoHP,butasfar as interviewee (002-003) is aware, things have not yet reached the implementationstage.
Is there a system in place to monitor access to essential, qualityassured,safe,effectiveandaffordableantimicrobials?
Before federalization, all purchase and distribution of EML medicines including antimicrobials used to be undertaken centrally from central government to local levels. Till that time there used to be a complete data with Nepal government on distribution, use and shortage of these basic medicines. However, with federalization, these activities are undertaken by respective provinces, and thus a centralized data is no longer available at Logistics Management Division of DoHS Nepal. However, there is online information system known as eLMIS through which the distribution, stock, and consumptionrecordsofmedicinescanberetrieved.
Currently, the reporting of consumption and usage at the local level is reported in that system, allowing to view those statistics in eLMIS. The online reporting system has commenced at the local level overall. The main issue is that there may not always be complete reporting. But the system that Logistic ManagementSection(LMS)nowhaveiseLMIS.
The most recent instance involved procuring a large quantity of antibiotics, specifically ciprofloxacin and amoxicillin. This occurred before the COVID-19 lockdown. After that, no bulk supply has been done by LMS. Programmatically theantimicrobialsforchildrenaredistributedandistrackedbyeLMIS.
Assessment report of AMS implementation at the national level
Many medications are now bought at the local and provincial levels since the system has changed. Nonetheless, LMS does offer support when necessary. But now, they (local and provincial levels) currently, have three to four years of experience, thus they don't have any issues. If issue arises, it can be resolved. One of LMS work is, they supply basic medicine during Integrated Management of Neonatal and ChildhoodIllness(IMNCI)programifnecessary.
Challenge:
The barriers are gaps regarding linkage and communication with provincial bodies (as they more prefer to act independently now and may sometimes be hesitant in reporting or communicating with central level) and technical IT issues. Further, limitation of skilled human resources such as lack of pharmacists at dispensing sites and store management. Due to lack of people with pharmacy specific knowledge across Nepal, especially in rural, there are issues on quality of data reported and variations between actual scenario compared to reports on use and shortage of antimicrobials.
Current status:
Partially implemented
Though there is a national eLMIS system for reporting distribution and use of free EML antimicrobials across Nepal, it has not yet been able to fully integrate or input data from district- and provincial-level hospitals where maximum consumption of antimicrobials occur. The hospital pharmacies do not use national eLMIS system for their overall recording but use it for recording only basic “98 EML medicines”. For rest of large number of medicines and antimicrobials that are beyond national EML list, there is no mechanism to centrally record such data on their use or distribution. Soperiodically,reviewingmeetingsissometimesdonewithHospitalPharmacy.
Current status:
Partially implemented
In Nepal, there are more than 250 essential medicines, within NLEM however only 98 are enlisted as ‘free’ and are provided by the government for free of cost so the data of free medicine can be received. eLMIS system indicates a shortage and stock out of free medicines and also displays information about monthly stock, order interval, maximum storage, and minimum emergency order; hence, LMS also provides training on all of these topics. Following training, provincial and local levels will be able to order, distribute, and will also make them aware about stock outsandshortages.
National representative information can be obtained from eLMIS. The data from the eLMIS system can be shared if it is completely utilized. Despite the fact that eLMIS is online, updates are only made once a month, making it somewhat difficult to utilize. Hence, where eLMIS reporting is done, stock outs and shortagesofEML(freemedication)canbeknown.
Item 3.5.
Current status:
Planned but not started
If considered as part of regular work, the data obtained from eLMIS is sufficient; nevertheless, for AMR, central level hospitals' antimicrobials consumption data cannot be accessed there, despite the fact that it appears to be necessary. The major problem here is beyond of eLMIS' purview. The majority of patients are receiving treatment and are getting treated as well, but because the data is not centralized, the policy cannot be carried out, and the relevanceisalsoreduced,henceonlypartialinformationisreceived.
There is also insurance system, they might also keep those data, but there is a gap about connection to the insurance system. Interviewee (002-006) is also unaware of the gap. Insurance may not have consumption information but may be of treatment patient received. There are several medications on the insurance list. If insurance provides that data, then the majority of the EML mustbecovered.Butnotallplacesofferinsurance.
Isthereamechanismtoreporttheantibioticsusedbypatients?
Future plans call for us to go beyond patient level to retail. For the time being, DDA relies on first-hand information from importer regarding their import and sale activities. It could be either wholesale or retail data. Although it is not an actual data, it can be substituted for it and used as a snapshot. Regarding manufacturers, DDA only has that data that includes, how much raw material has been imported, how much final product has been manufactured in a fiscal year, which gives snapshot. DDA is still working to determine how much antimicrobials are sold at the retail level, currently they have a post marketing software (PMS), which is undergoing testing. Procurement provided and actual sales data can be obtained at the retail level after PMS. Even though it is retail level data, it can be assumed as patient level data (Sell volume = patient usage). Sometime even though patient purchase they might not take the full dose, but in some extent that it is patient level use. Hence, there is no information obtained or mechanism too share from hospital level about the antimicrobial usage.
Current status:
Fully implemented
Manufacture classifies the medicine according to molecule, DDA then reclassifies it as AWaRe classification. For instance, some imported may import 5 molecules, some manufacturers may make 10 molecules, while others may only make 2, so only those data are reported in accordance with consumption, molecule wise. After the DDA receives the data, further classification is done using the AWaRe classification; for example, they classified the data from 2016-2018inthismanner.
If those data are looked upon, it becomes clear that Nepal does not meet the WHO recommendation that access groups be used 60% of the time. The watch group and even the reserve group are being used excessively, whereas access antibiotic is being used sparingly, this also provide the snapshot. Even though thesearejustpreliminarydata,Nepalisrepresentedbythesedata.
Item 4.1.
Are health worker behavioral change principles incorporated into policies addressing diagnosis, prescription, dispensing and administrationofantimicrobials?
Current status:
Planned but not started
It’sinprocess.
Antibiotic treatment guidelines were previously established, and it is now being updated as part of ministry initiatives. AWaRe classification, first- and second-line antibiotics, Laboratory tests, and everything else will fall under it after its endorsement. Therefore, it is considerably better than the previous one and will besoonfinalized.Additionally,servingasanadvocacy.

Item 5.1.
Current status: Partially implemented
Is there a regulation on prescription-only sale/ dispensing of antibiotics?
Yes, it is clearly written in legal form.Medicines are divided into "ka(A) and kha(B)" categories (should have been kha(B) and gha(C)), neither of which should be sold without a prescription. Antibiotics, hormonal medications are collectively referred to as "kha(B)"; as such, they must be sold with a prescription and fall under the purview of the law. Additionally, it has the label"soldwithmedicalprescription".
In terms of implementation, usage may differ. Although it hasn’t been monitored yet, occasionally different research from interested parties reveals that antibiotics are also sold without a prescription. However, accordingtothelaw,itisaprescription-onlydrug.
In order to enforce, DDA has done public awareness programs. In addition, antibiotics will have red lines denoting that they should only be consumed with a prescription on their primary and secondary packaging, per a recommendationbythesallaharkarsamitee(advisorycouncil).
DDA also carried out other tasks over antimicrobial awareness week. Similarly, they also made jingles and promoted them on the radio. Also, time and time again, they discuss frequently regarding prudent use at provincial level with stakeholders. However, DDA is considering ways to connect with customers at the retail level so that only prescribed antibiotics are sold. For now, they are unable to do so at the moment, thus advocacy efforts are still ongoing.
Item 5.2. Are regulations on dispensing antibiotics by prescription only beingenforced(whereaccessisnotanissue)?
Current status: Partially implemented
For now, legally, antimicrobials are prescription only and DDA is advocating the same thing with stakeholders in the same ways everywhere. GoN lacks a mechanism for enforcing, prescription audits (when it is actually given only with a prescription). Future efforts will be made to address it, as well as the issue of stewardship and the use of antibiotics. National prescription surveycanalsobeconducted.Theyareadvocatingalltheseactivities.
Activities that have been conducted till now do not provide national level data but only consumption pattern. Some organizations have conduct point prevalencesurvey.
Current status:
Partially implemented
Current status:
Partially implemented
Assessment report of AMS implementation at the national level
Additionally, Interviewee (002-003) did a NAP survey as a sample at a certain hospital in a country. The results were really alarming; one level of advocacy for the use of antimicrobials is required, so designated person, such as a pharmacist, must be in place to have an overlook. So DDA will go to retail and advocate there. Legally, they will keep track of whether it has been adhered to, but there is currently no such mechanism. For Strictly enforcement, DDA can file a lawsuit, if it is sold without prescription; but it has not reached at that level yet. It is actually multi-sectorial involvement. For example, healthcare professional must be aware and similarly the retailandpublic.
When people have mild to moderate illnesses, they often visit the pharmacy instead of theadjacenthospital,sayingthingslike,"eventhoughitisnothing,givemesomething, Iwillseefor1,2daysandthenifitdoesnothelp,Iwillgotohospital".Forthisreason, DDAhavesomeplansforregularmonitoringandstrictenforcement.
Is there an enforceable regulatory mechanism to prohibit sale of substandardandfalsifieddrugs?
DDA does sample based, risk based post marketing surveillance. In a risk-based strategy, samples are gathered from various locations across the nation. These samples are then examined at a national medicine laboratory. If the results show that the samples are substandard or do not satisfy the specified criteria, it is recalled. Notices are posted for this purpose on DDA’s website, and some are even published in newspapers. DDA does have the mechanism but, it hasn't been used at the retail level yet. DDA do have the capability to evaluate the drug quality and do have laboratoryfacilities.
Are there standards and criteria for responsible manufacturing and disposalofantimicrobialagents?
Regarding manufactures. it is clear regarding criteria and GMP (Good Manufacturing Practices). License is issued only after the specified criteria/level are met. There is no problemwithmanufacturingcompany.DDAisalsofollowinginternationalpractice.
Regarding disposal, national level pharmaceutical waste disposal is still in draft only. However, manufacturer individually, must have their own effluent treatment plan. Principally, the amount of medicine expired must be received by them and then disposed through ETP, which promote environmental protection. However, for the time being, DDA does not have any measures in place to collect expired medications from homes. These medications end up as household waste, and if they do return, theyhaven'tyetmonitoredit.
Therefore, there are no policies, mechanisms, or guidelines at the national level. However, DDA have imposed individual responsibility. If they have an ETP, they must adhere to its rules, thus the water discharged from it should be at appropriate level andsuitableforuseingardeningandotherpurpose. It is mandatory to have ETP at manufacture level. DDA doesn’t test the water targeting Antimicrobial but they (the environment division) test for certain criteria such as suspending particle, BOD/COD (Biological oxygen level/ Chemical oxygen level). Water should be having the capacity for Survival of aquatic animal, and at some places fish are also farmed. Annually the water is tested; several environmental factorsareexamined.Buttheantibiotictargettestisnotperformed.

Item 6.1.
Have studies on determinants of behavior in health professionals and other stakeholders, including the general public, been completed to supportdesignofawarenesscampaigns?
Current status:
Partially implemented
Item 6.2.
Current status:
Fully implemented
There are no studies conducted on the behavioral determinants of the end users of antimicrobials to support design of awareness campaigns from the government side itself. However, all the information and advocacy document such as in the form of audio, video, printed versions, etc., that are prepare with the help of National Information Commission Nepal, we take inputs from the experts of specific field. Thus, though the government itself does not conduct such studies, the experts engage in preparation of such health-related information materials do have data/information on specific topics based on their expertise, experience or pilot studies. For example during WAAW, annually, MoHP provides relevant information from national to provincial and general public level on various aspects of AMR, AMS and AMU by conducting various awareness activities throughout a week, which includes various expert interview sessions aired via TV programs, display of information via standees, organizing competition programs on paintings, elocution on relevant topics, organizing workshops for prescribers and pharmacists, organizing seminars, consultation meetings at national and subnational levels etc. Thus, relevant information has been disseminated at different layers such as government, private, public, academia, engaging different stakeholders (prescribers, pharmacists, chemists, respective councils, academia, civil society) across one health sector. However, these awareness programs have not come as a result of recommendations drawnbyconductinganystudiesbygovernmentlevel.
Does the country hold World Antimicrobial Awareness Week (WAAW) activitiesannually?
Yes, Nepal has been regularly conducting annual WAAW activities since Nepal participatedinGlobalActionPlan(GAP)onAMR.OnthefirstdayofWAAW,message from the prime minister focusing on AMR awareness is printed on a national daily. Similarly, ministers of the 3 ministries representing one health components publish their commitment and wishes on the occasion of WAAW in national newspaper. GoN also publishes press brief regarding this. Throughout WAAW week, GoN conducts series of activities such as panel discussion, various expert interview sessions aired via TV programs, display of information via standees, organizing competition programs on paintings, elocution on relevant topics, organizing workshops for prescribers and pharmacists, organizing seminars, consultation meetings at national and subnational levels etc. Thus, relevant information has been disseminated at different layers such as government, private, public, academia, engaging different stakeholders (prescribers, pharmacists, chemists, respective councils, affils, academia,civilsociety)acrossonehealthsector.Andasasymbolicawarenessand
Item 6.3.
Current status: Fully implemented
Item 6.4.
Current status: Partially implemented
Item 6.5.
Current status: No, but a priority
commitmentagainstAMR,sincelastyear,wehavebeenlightingtheDharahara tower in blue shade (following “Go Blue” theme) projecting different awareness information. On the last day of WAAW, GoN organizes a walkathon involving different one health stakeholders starting from Department of Health Services (DoHS) and ending to MoHP. MoHP also sends out information tools like standees to provinces to encourage them to celebrate WAAW organizingdifferentactivitiesattheirownlevels.
Does the country have regular public awareness campaigns on the responsibleandappropriateuseofantibiotics?
National Information Commission Nepal under GoN regularly conducts various public awareness campaigns on different health topics including appropriate use of antibiotics utilizing various awareness methods. Via various radio and TV programs and press printed materials (such as information on printed calendars, books), Nepal information commission aims to raise public awarenessbyoccasionallycirculatinginformationonAMR.
Are tailored AMS messages integrated into broader health promotion, prevention, treatment and rehabilitation services and initiatives such as World Water Day, World Toilet Day, World Children’sDay,immunizationcampaigns,WorldAIDSDay,WorldTB Day, World Malaria Day and World Nutrition Week for sustained action?
This has not been done fully in all of these activities but been done partially on some of these awareness programs. For example, on some health campaigns such as World TB Day, World Malaria Day, World AIDS day, the main aim of these programs is to reduce the burden of specific diseases and so, on these campaigns, the main goals focus on strengthening the specific curative or supportive care specified for that disease or condition. Thus, the tailored AMS messages are also simultaneously and strongly advocated, because one of the main messages given during these campaigns focus around strictly following the drug regime (using antimicrobials as prescribed by the physicians, strictly completing full course of antimicrobials, discouraging incomplete antimicrobial courses, keep on regular medical visits). However, this may not be true on all otherhealthinitiatives.
Is regular assessment/evaluation of the impact of education and awareness campaigns on knowledge attitudes and behaviors of healthworkersandthepublicconducted?
No. This has not been done so far from the government level thought it might have happened at private levels. There are no immediate plans on conducting assessments/evaluation of impact of such awareness campaigns on end users. Butitisimportanttoconductanassessmentorevaluationoftheseprograms.
Item 7.1.
Current status:
Partially implemented
AreAMSprinciplesandstrategiesincludedintheeducationalcurriculum ofpre-servicehealthcareprofessionals?
If it is about 5 AMR principles and strategies as set by WHO, then these are not as such included in the current curriculum of healthcare workers. But general principles of AMR are certainly there in the medical curriculum, so all those studying medicine, nursing, para-medicals, and pharmacy have to study these components in their academiccourses.
Apart from these, National Health Training Center develops training package to conduct specific AMR related in-service trainings to the prescribers, nurses and paramedics. These training courses have been ongoing regularly since last 7-8 years whichareaccessibletoallprofessionalworkinginprivateandpublicinstitutions.
SeveralbatchesofprofessionalsspecificallytrainedonAMRcourseshavepassesout so far. This training is not yet sufficient. We provide specific trainings throughout the year in number of batches, with ~20 trainees/batch. The current challenge is thatGoNhasbeendeliveringthetrainingprogramsonlyfromthecentrallevel.Ithas not yet been able to conduct these specialized training courses across all 7 provincesasnotalltrainingcentersatprovinciallevelareyetfullycapacitated.
Item 7.2.
Current status:
Partially implemented
Is there access to in-service training, including continuous professional development (CPD) on antimicrobial prescribing and AMS for all healthcareprofessionalgroupsinthecountry?
Yes, as mentioned above, there is a provision of in-service training as continuous professional development on AMR, which includes antimicrobial prescribing and AMS to healthcare professionals. The training programs are under regular activity conducted by Nepal Health Training Center, which publish call for specific trainings. Interested professionals who apply to the training calls and who fit their criteria will get a chance to take these specialized in-service trainings. So, the trainings are on requestdependingontheactiveinitiativeoftheprofessionalhimself;ithasnotbeen made mandatory so far. It should be made mandatory. But the challenge is that not all healthcare professionals can be brought to the capital for the purpose of training. Instead, we need to build the capacity of the training centers at the provincial levels sothatthesecanbemoreaccessibleandcanbemademandatory.
Additionally, making the AMR specific in-service trainings mandatory would require these two aspects, first, the training resources (availability of specific trainers, training cost, site, and other logistics) need to be available and adequate; secondly, the intended person (trainee) should have that capacity and relevance. Thus, it wouldbegoodtomakethesetrainingmoreaccessibleandcompulsory.Sofar,these havebeenoptional.
Item 7.3. Are AMS concepts and principles incorporated within the curriculumofothercomplementarydisciplinese.g.thecurriculum forIPCprofessionals?
Current status: Partially implemented
AMR concepts and principles are embedded in other programs such as IPC, WASH. But if we look dedicated AMS components only in these disciplines, then it may not be there as such. We don’t have anything as just dedicated AMS as such, but we have activities/plans for AMR. When we talk about AMR, the AMS is an integral component of it, so AMS will come automatically. Only when AMS get strengthened, AMR will automatically be addressed and mitigated. So, when AMR related activities conducted and informationaredisseminated,thatforAMSwillbedeliveredsimultaneously.
We do have areas to be improved and opportunities to strengthen AMS. If we have had been discussing all these todays talks after the endorsement of NAP, we should have answers to several of these questions, metrics to measure their progresses, and analyses the strength of the strategies. Once the NAP is endorsed, these 3 topics: AMR, AMS, and AMU will come as separate domains with dedicated objectives and action plans. NAP has stipulated that there should be stewardship committee, IPC committee, and quality assurance division at institution level, with somewhat shared membership (for example the same physician, department chiefs, matron being a member of multiple of these committees). NAP has envisioned that these committees be supported and budgeted to ensure their sustained functionality, their activities be defined to strengthen their role in AMRAMS-AMU related monitoring, evaluation, protocol compliance audits). IPC guidelines are also prerequisite of the NAP. So, GoN is very hopeful that with NAP endorsement, activities on AMR-AMS-AMU will be coordinated and strengthened.
Currently, we assess IPC status of hospitals via minimal service standard (MSS)evaluationdocument.

Item 8.1.
Current status: Planned but not started
Is there representation of the AMS coordinating unit on the WASHprogrammesandviceversa?
Washprogramisof2level:CommunityWASHandHCFWASH.Management division has worked on HCF WASH. They provide document and framework and actually do policy level activity, implementation is actually done by respectivehospitals,locallevel.That’swhyforHCF,theyalsohavestandard, Standard for WASH for HCF for different level which include hand hygiene, menstrual hygiene. There is a standard passed by government. They also have SOP for Healthcare waste management. The work is ongoing on basis ofthosetwodocuments.Standardismadebyfederalgovernment,sothatis whattheydo.
The interviewee specifically is unaware of the AMS coordinating unit, but QSRD is in charge of AMR. They do have a technical working committee, which includes IPC, WHO, environmental, and healthcare representatives. There is no formal AMS coordinating unit for the WASH program, although there are on and off involvements of people there. Different people from theTWGplatformareinvolvedintheWASHprogram.
Community WASH has different focal point, The Department of Water and Sanitation, however, the management division handles the advocacy work, which focuses on community issues like mothers and children rather than infrastructure and other factors. Campaign such as open defecation free are donebythem.
Item 8.2.
Current status: Partially implemented
Is the WHO WASH Facility Improvement Tool (FIT) being promoted toassessWASHinhealthcarefacilities?
In 2017/18, WASH FIT was launched in Nepal. It was also translated into Nepali. Additionally, NHTC offers a 6-day integrated training program for healthcareprofessionalsthatcoversenvironmentalhealth,WASH,andwaste management. WASH FIT is therefore covered in this section of WASH. They also provided training about WASH FIT in 7 provinces. In Banke and Bardiya and the outcome was also good. Primary health service is chosen to launch the tool, as the WASH FIT tool is actually for lower level but it can be started in bigger hospitals by making MODULE WARDS. Management division have alsopresentedacasestudyofNepalinaregionalworkshop.
Although Budgeting is done, there are competing interests. Even though training is given, it is insufficient because planning requires gap identification, hazard and risk analysis, and then a budget is needed to carry out the implementation. Additionally, Infrastructure is required, which necessitate high capital. Though they can't start everything at once, they have for the time being started in 7 provinces. There is currently a second editionofWashFitthatisalsoavailableinNepalilanguage.
Additionally, they are trying to create a unique training program for WASH FIT, possibly lasting two days. they have chosen a municipality for the next year as well to launch the tool. They also have requested WASH-related institutions,touseFITtool,doKAPanalysisaccordingtoitandintervene. The USAID-supported program Swachhata is working in the province of Karnali.
WASH FIT is being promoted in leadership of government, but it costs a lot of money to train health personnel and fill the gap. The resource is constant, foreign country have provided aid but training for seven provinces is funded separately by the government. government do have WASH ownership. They are creating documentation and laying the way for a possible scaled-up deployment,butthereisnoyetnationwidecoverage.
WASHFITisactuallyamethodtoensureWASHincludinghealthcarewaste.It is also in SDG as joint monitoring programme, UNICEF and WHO combine have made indicator, national indicator is also there, health facility survey was also conducted from there also we can analyze where are we. We are also making national roadmap for WASH FOR 2030, we are setting the milestone.
Item 9.1.
Current status: Planned but not started
While the IPC paperwork procedure and approval process have just begun, the IPC program is still in place at HCFs. Regarding AMS, the program hasn't been launched in all government hospitals, but there is some concept of AMS present in private hospitals. Considering government policy, AMS and IPC are connected. IPC falls under stewardship. IPC guidelines have also been discussed in relation to stewardship and resistance. IPC guidelines are in approval process as it was their last year project, but it is impossible to predict when it will be approved. It could take a few days or longer, but the interviewee (002-004) believes it won't take more than a month at this point. However, for implementation it might take some time, possibly a few years, as stewardship should also go hand in hand. Implementation might begin at a tertiary level hospital. The guidelines can be used at community hospitals as well as tertiary level hospitals and basic level centers. It is designed for everyone, not just governmenthospitals.
The main challenge is budgetary issue. Setting up the IPC program at hospitals might not be challenge as everyone is aware of how important it is during COVID. IPC related program, IPC committee has been formed, even if the documentationisnothere.ThereisawastecommitteeandaQIcommitteethat go by different names at different HCF. Therefore, there may be less of a challenge. However, there might be limitation of HR or focal person. IPC is supposed to have a dedicated team and committee, but in our setting, the same people work in the ICU and the OPD, so they have many responsibilities. This is whythebiggestdifficultyisthattherecouldnotbeafocalpersonorHR.
Next thing is hospital leadership. There is a challenge at the individual level, if theytakeitseriouslyandareaccountable;otherwise,theremaybeachallenge. We can state that the government lacks in this area of monitoring and feedback, or auditing, thus this document had tried to incorporate it. At that time, IPC is integrated with MSS. The next is clinical audit, which is carried out by QSRD. Internal auditing is done in hospitals, so they have linked it to clinical audit and MSS. Therefore, if the external team visits, there is a significant IPC component in MSS that will also be monitored. Consequently, IPC have ties to MSS and Clinical audit. The IPC national committee has also been established; as a result, that body will also monitor, and once more, there is MSS and clinical audit, so NSSD have that provision. Document implementation, its status, and itschallengescanallbeseenfromit.
Every component in MSS has been addressed, including the IPC component, but as of right now, there are different health facility assessments. Therefore, many tasks are no longer necessary to be done. An IPC committee, a stewardship program, incident reporting, a surveillance system, and reporting are key indicators that will be monitored. Therefore, altogether it is about the quality of service. The same MSS team will perform an extended review; internally, HCF must also undertake an internal evaluation, as NSSD have stated twiceinayear.Buttheyhavelinkedthatexternallyaswell.
Is there representation of the AMS coordinating unit on the IPC programmes and vice versa?Current status: Planned but not started
NSSD has discussed about it. In terms of stakeholders, CSD, QRSD, and EDCD are all present.TheyalreadyhaveanEarlyWarning,AlertandResponseSystem(EWARS)for surveillance.
Although it is a crucial part of IPC, surveillance has not yet begun at government hospitals. While Gangalal, Medicity, Teaching hospitals, and other facilities have started, the government hospital has not. They have slowly begun to realize this, and havealsostarteditbyhandhygienemonitoringatsomeHCF.
IPC guideline has also linked HCF surveillance with the EDCD Surveillance section. After making the key indicator, they intended to link it to HIMS. The next point regarding patient safety is that QSRD is working on it right now. They have created a road map for patient safety that encompasses both patient safety and person safety, so healthcare workers' safety is also covered. As a result, they are currently creating an online system for that, which includes patient information and records all events, and the IPC department has planned to connect to it as well. Key indicators will undoubtedly be visible there as an outcome after that system. Overall, quality of care is what matters most, and the groundwork is already in place for this to happen. Therefore,ifnecessary,wecanlinkwithHIMS,EDCD,andCSD.
Therefore, although the base is prepared, it has not yet been started. As a result, connecting the various components together to form a system still needs to be done. NAP AMR and the Patient Safety Road Map will therefore address all of these. We all work well together across all departments, giving us a plan of action and the ability to mergeasnecessary.IbelievewecannowoperateeffectivelyinIPC.
Although there is no mechanism or data to track the use of antimicrobials, the health record has information about the treatments delivered, making it possible to use that data. The majority of healthcare facilities have electronic health records, but the data about antimicrobial usage are not organized in a systematic way. However, the basic details can be discovered, but an integrated system is not there. After the stewardship program, this system might exist. Although data is there, the system is notyetsetup.
There is nothing new about IPC. Globally, the IPC nurse serves as the organization's focal point, but in Nepal’s context, it would be preferable if there were a designated IPC representative; a course might be necessary for that. We now have a 3-month IPC specialized training course. They subsequently receive IPC nursing certification. They will gain knowledge about IPC, AMR and how surveillance is carried out because itisnotasimpletask.
Therefore, NSSD has trained HR, so if they form a dedicated team, it'll go smoothly. The animal health is somehow under looked. So now they are attempting to include it as well as every zoonotic disease originates from an animal. They have realized the importanceofanimalhealth.

Item 10.1.
Current status:
For AMR:
Fully implemented
For AMC and AMU:
Planned but not started
Is there a national surveillance programme for antimicrobial resistance, use and consumption with defined structures, governance and work objectives (i.e. data collection, validation, analysis, reporting and data sharing with all stakeholders)?
Yes, national surveillance program for AMR fully implemented since 2005, and still going on for 10 priority pathogens recommended by WHO GLASS. This is happening across Nepal via 21 participating laboratories under support of Fleming fund, as well as non-Fleming fund support grant. From these 21 laboratories of different hospitals, microbiology data on 10 GLASS priority pathogens are submitted to NPHL on a monthly basis. At NPHL, this data is cleaned, and is uploaded in WHO GLASS portal. Since 2017, NPHL is reporting data to GLASS, which thus reflects the scenarioofAMRinNepal.Timeandagain,thisdataisalsopublishedasnewletterfor disseminationpurpose.
Based on the microbiological data received from the participating laboratories, NPHL conducts on-site assessments of the lab, and provides needful trainings to the staffs, both by going to the sites as well as at NPHL. NPHL has also provided infrastructural development support to few sites. NPHL also visits the sites to interact with the site hospital clinicians. Recently, NPHL has also been helping participating sites such as different hospitals to develop antibiogram. Currently, there are 26 stakeholders under national AMR surveillance program, including participatinglaboratories,Flemingfund,andCentralveterinarylaboratory(CVL).
Challenges: data not submitted by participating labs in timely manner, submission of uncleandata,
Forconsumptionanduse:
Currently national surveillance programs on antimicrobial use and consumption as such is lacking in Nepal. DDA does have some information based on the volume of medicines including antimicrobials being imported and distributed by pharmaceutical companies, which can be used as proxy data of antimicrobial use and consumption. Theactualdatafromthepatientlevelisentirelylacking.MoHPLogisticManagement Division has a provision of using eLIMS system to record medicines, but it is only limited and mandatory to a free group of essential medicines list of Nepal and this system entirely excludes large antimicrobial consumers such as zonal and tertiary level hospitals of both government and private sectors (as the latter have their own onsitepharmacieswhichusetheirinventorymanagementsystems).
Item 10.2. Are there guidelines/standard operating procedures for the use of (a) antimicrobial medicines use (AMU), (b) antimicrobial medicines consumption (AMC), and c) AMR data to inform action for national and institutional decision-making to inform action for national and institutional decision-making?
Current status: No
(Forc):ThereisnospecificdesignatedguidelineorSOPspecifyinghowwill the collected AMR data be used to inform action at national or institutional decision making. Though there is no specific guideline on this, other MoHP departments use this data to make clinical protocols. Main objective and responsibility of NPHL is just to collect and generate AMR data across country. Deciding on how that data will be utilized is the role of other relevantdepartments,asthisneedsmulti-sectorialaction.
(For a & b) : Currently there are no guidelines or SOPs for use of AMU and AMCdatatoinformactionfornationalandinstitutionaldecisionmaking.
Item 11.1. Is there a plan to strengthen laboratory capacity to support accurate diagnosis for decision-making?
Current status: Partially implemented
Yes,ithasbeenlongwayforNPHLandlabcapacitystrengtheninghasbeen an ongoing process. Recently NPHL has also been equipped with high-tech technologies. It has also plan to incorporate fungal culture. Molecular lab is already established at NPHL. It will soon start routine molecular testing as well.
NPHL has also helped district hospital to establish microbiology lab. Ultimate aim of NPHL is not just involving laboratories as participating sites for AMR surveillance, but also to help build a good microbiology laboratory so that antimicrobials are prescribed based on microbiology culture and sensitivity reports. NPHL has plan to establish microbiology lab in rest of the district hospitals too. As relevant, NPHL has plans to increase sentinel sites. NPHL has also added in its laboratory guidelines that there must be a microbiology lab also in all private hospitals which have ICU units in them. Under minimal service standards, NPHL stipulates that there should be microbiologylabindistrictlevelhospitals.
Item 12.1.
Current status:
Planned, but not started
IsthereanationalM&Eframework,includinginternationallyandlocally agreedkeyperformanceindicatorsforintegratedAMSinterventions andactivitiesinhumanhealth?
002-004: We can state that the government lacks in this area of monitoring and feedback
002-002: The M&E framework currently is not implemented. However, in the drafted NAP, the national targets have been set for evaluating the progress on each of AMR-AMS-AMU domains directed as per the action plans against each of the pillars along the 5-years of roadmap. After the endorsement and implementation of NAP, MoHP will evaluate the progress accordingly. currently there are no designated M&E frameworks to evaluate the progress of the each individuallyledprograms.
Item 12.2. Isthereanaccountabilitymechanismputinplaceatthelocallevel whichincludespositivefeedbackandrecognitionoflocalleadership?
Current status:
Planned, but not started
Currently, in the absence of endorsement of NAP on AMR and AMS, the AMS activities are entirely lacking in hospitals of Nepal. Only few big private hospitals have functional AMS implementation activities ongoing based on their own guidelines, interest and resources. Thus, no monitoring and evaluation framework for AMS activities are available in Nepal. However, in drafted NAP guideline, there is a clear M&E system incorporated. As for now, Government of Nepal have identified some stakeholders but there is no accountability mechanism put in place at the local level which includes positive feedback and recognition of local leadership.
002-002: regarding aspects on AMU, Department of Drug Administration (DDA) has been working on this, for which DDA is a focal point too. (NPHL is a focal point forAMRsurveillance).
Once NAP will be endorsed it will specify roles, responsibility and scope of each different stakeholders (such as different divisions and departments under MoHP like QSRD, National Planning commission, National Coordination Division, NPHL, EDCD, Department of Curative Services, DDA, Department of Livestock (DLS), Department of Agriculture, Department of Food Technology and Quality Control (DFTQC); Ministry of Education, Ministry of Finance), and how the activities of each of those units will align and synergize to fulfill a common national goal to ensure AMR containment, strengthen AMS, and enhance optimal AMU in the country in a one health approach. After the implementation of NAP, and based on initial evaluation reports, then we may have to amend specific policy documents, SOPs,STPsaccordingly
TheWHOpolicyguidanceforanintegratedapproachto AMS promotes the principles of synergy and efficiency for the implementation of AMS programs at all levels. Following this guidance, national and local context and the structure of the health system need to be considered when carrying out AMS activities while focus our efforts on activities that are likely to provide the greatest benefits based on needs assessment. We also need to strengthen and use existing national and subnationalplatformsandcoordinatingmechanismsand resources to implement integrated AMS activities. It is important to build strong and effective linkages and synergies between the relevant areas and disciplines relatedtoAMR.
Nepal's key strength lies in its proactive and comprehensive approach, evident by the MoHP-led national coordination mechanisms with diverse participation, development of the Costed-National Action Plan, integration of WHO guidelines like AWaRe system into national EML formulary , incorporation of antimicrobial stewardship principles into healthcare policies, a strong national surveillance program for AMR, consistent public awareness campaigns, and active global collaborations, all underscoring a resolute dedication to combating antimicrobial resistance and fosteringresponsiblepractices.
However, several challenges, including delayed endorsement of the NAP and limited AMS coordination, need to be addressed for effective AMR containment. To strengthen Nepal's response to AMR and promote AMS, the following areas need to be further developed and strengthened for an effective integrated AMS programimplementation:
Expedite the endorsement and implementation of the NAP on AMR to enable coordinated and comprehensiveAMSactivities.
Develop specific guidelines and SOPs for the use of AMR and AMU data in decision-making at national andinstitutionallevels.
Establish surveillance systems and systematic documentation and reporting of antimicrobial use and consumption in healthcare facilities and communities to monitor the impact of AMS related decisionsandfollow-upinterventions.
Allocate dedicated financial resources from the government to support AMS initiatives to reduce dependence on external support, ensuring sustainable funding for the implementation of AMS programs
Increase effective coordination mechanisms to scale up the implementation of integrated AMS activities, with sustainable and adequate funding and with technically competent human resources and accountabilitymechanisms.
Strengthen WASH and IPC in healthcare facilities and communities, ensuring the interconnectedness between AMS and the other complementary programs.
Continue to strengthen and expand the national AMR surveillance network to inform the planning andimplementationofintegratedAMSactivities. Continue to implement laboratory strengthening efforts to ensure that there is adequate infrastructure for conducting quality microbiological practices, including isolation, identification and susceptibilitytesting.
Enhance pre-service and in-service training programs for healthcare professionals, integrating comprehensiveAMSmodules.
Strengthentheenforcementofregulationson prescription-onlysale/dispensingofantibiotics, andconductregularprescriptionauditstoensure complianceattheretaillevel.
Ensurethathealthcareprofessionalsatalllevels receivecomprehensivetrainingontheproper implementationofAMSprinciplesandguidelines. Enhancethefunctionalityandcoverageofthe eLMISsystemtocapturecompleteandaccurate dataonthedistribution,stock,andconsumption ofessentialmedicines,includingantimicrobials.
Establishmechanismsatthelocallevelto acknowledgeandrewardeffectiveAMS practicestoencouragehealthcareleadershipto activelyparticipateandcontributetothe broadergoalofAMRcontainment.
Recognizetheimportanceofanimalhealthinthe contextofzoonoticdiseasesandintegrate animalhealthsurveillanceintoIPCandAMR programs.Collaboratewithrelevant stakeholders,includingveterinarydepartments, todevelopcomprehensivestrategiesforthe preventionandcontrolofzoonoticdiseasesand thecontainmentofAMR
Enhancethedisseminationmechanismsfor guidelines,standards,andimplementationtools onAMSactivities.
Continuepublicawarenesscampaignsonthe responsibleuseofantibioticsandthethreatof AMR,andconductstudiesondeterminantsof behaviourtotailorawarenesscampaigns effectively.
Enhancecollaborationandcommunication betweencentral,provincial,andlocallevelsto ensurethesmoothimplementationof antimicrobialaccessandregulationstrategies. Fosterpartnershipsandcollaborationamong governmentagencies,healthcareinstitutions, educationalbodies,andinternational organizations DevelopacomprehensiveM&Eframeworkfor integratedAMSinterventionsandactivitiesat bothnationalandinstitutionallevels.
Inconclusion,Nepalhasshownastrongcommitmentto tacklingtheissueofantimicrobialresistance(AMR) throughitsproactiveapproach.Byfocusingonthefive keyareasmentionedearlier,Nepalhasmade commendablestridesindealingwiththemultifaceted challengesofAMR.Thiscommitmentreflectsits dedicationtoprotectingpublichealthandpromoting responsiblepractices.AsNepalcontinuestorefineand expanditsstrategies,ithasthepotentialtomakea lastingimpactontheglobalfightagainstAMR.The ongoingeffortsarepoisedtosignificantlymitigatethe risksassociatedwithresistantinfections.This determinedeffortholdstheprospectofgenerating lastingcontributionstotheworldwideinitiative,ensuring thehealthandprosperityofbothcurrentandfuture generationsbyeffectivelycounteringthethreatsposed byresistantinfections.
MurrayCJL,IkutaKS,ShararaF,etal.Globalburdenofbacterialantimicrobialresistancein2019:asystematic analysis.TheLancet2022;399(10325):629-55.doi:10.1016/S0140-6736(21)02724-0
1. deKrakerMEA,StewardsonAJ,HarbarthS.Will10MillionPeopleDieaYearduetoAntimicrobialResistanceby 2050?PLoSMed2016;13(11):e1002184.doi:10.1371/journal.pmed.1002184
2. WorldHealthOrganization.RegionalOfficeforEurope.Antimicrobialresistance:factsheetonSustainable DevelopmentGoals(SDGs):healthtargets,2017.
3. CostelloeC,MetcalfeC,LoveringA,etal.Effectofantibioticprescribinginprimarycareonantimicrobial resistanceinindividualpatients:systematicreviewandmeta-analysis.BMJ2010;340
4. DaveyP,MarwickCA,ScottCL,etal.Interventionstoimproveantibioticprescribingpracticesforhospitals inpatents.CochraneDatabaseSystRev2017;2:CD003543doi:10.1002/14651858.CD003543.pub4.
5. SchutsEC,HulscherMEJL,MoutonJW,etal.Currentevidenceonhospitalantimicrobialstewardshipobjectives: asystematicreviewandmeta-analysis.LancetInfectDis2016;16(7):847-56.doi:10.1016/S14733099(16)00065-7
7.
6. LeeCF,AsoH,FukudaK,etal.ImpactofantibioticstewardshipprogrammesinAsia:asystematicreviewand meta-analysis.JAntimicrobChemother2018;73(4):844-51.doi:10.1093/jac/dkx492
8.
HondaH,OhmagariN,TokudaY,etal.AntimicrobialStewardshipinInpatientSettingsintheAsiaPacificRegion: ASystematicReviewandMeta-analysis.ClinInfectDis2017;64(suppl_2):S119-S26.doi: 10.1093/cid/cix017
9.NationalHealthPolicy2076.(2019).NationalHealthPolicy2076.Kathmandu.GovernmentofNepal.
11.
10.MOHPNepal,KafleK,etal.(2014).NationalAntibiotictreatmentguidelines2014.doi:10.13140/RG.2.1.34. MinistryofHealthandPopulation(MoHP).2015.NepalHealthSectorStrategy2015-2020.
http://www.nationalplanningcycles.org/sites/default/files/planning_cycle_repository/nepal/nhss-englishbook-final-4-21-2016.pdf
12. WorldHealthOrganization.(2021,November17).Antimicrobialresistance.Retrievedfrom
MinistryofHealthandPopulation.(2021).NationalActionPlanforAntimicrobialResistance2021-2026. Retrievedfrom
https://www.MoHP.gov.np/uploads/AMR/National%20action%20plan%20for%20anti%20microbial%20resista nce.pdf
13.
https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
14.
WorldHealthOrganization.(2019).Antimicrobialstewardshipprogrammesinhealth-carefacilitiesinlow-and middle-incomecountries:AWHOpracticaltoolkit.https://www.who.int/publications/i/item/9789241515481
15.
WorldHealthOrganization.WHOpolicyguidanceonintegratedantimicrobialstewardshipactivities.Geneva: WorldHealthOrganization,2021.




TheOxfordUniversityClinicalResearchUnit(OUCRU)isalarge-scaleclinical andpublichealthresearchunitwithsiteofficesinVietNam,Indonesia,and Nepal.
PartoftheCentreforTropicalMedicineandGlobalHealthattheUniversityof Oxford(UK),OUCRUwasfirstestablishedinHoChiMinhCityin1991,hosted bytheHospitalforTropicalDiseases(HTD),VietNam.
In2003,OUCRUNepalwasestablishedinKathmandu,Nepal,hostedbyPatan HospitalandthePatanAcademyofHealthSciences.
OUCRUHaNoiwasestablishedin2006inpartnershipwiththeNational HospitalofTropicalDiseases(NHTD),VietNam.
In2008,OUCRUIndonesiawasestablishedinJakarta,Indonesia,inpartnership withtheFacultyofMedicineUniversityofIndonesia.
Ourvisionistohavealocal,regionalandglobalimpactonhealthbyleadinga locally-drivenresearchprogrammeoninfectiousdiseasesinSoutheastAsia.
Ourresearchprogrammecoversclinicalandlaboratoryresearchwithhospital andcommunity-basedpatientpopulations,includingepidemiology, immunology,hostandpathogengenetics,molecularbiology,microbiologyand virology,mathematicalmodelling,bioinformatics,biostatistics,andsocial science.Thisworkissupportedbyanextensiveclinicaltrialsunitanddata managementcentrecompliantwithnationalandinternationalregulationsand comprehensivemanagement,finance,publicengagement,andadministrative supportoffices.
OUCRUreceivesconsiderablesupportfromWellcomeaspartoftheAfricaand AsiaProgrammes.Togetherwithourpartners,wehaveledahighlysuccessful effortinenhancingtheinfrastructureandcapacitytoperformclinicaltrialsand basicscientificresearchinVietNam,Indonesia,andNepal.
Website:www.oucru.org
OUCRU-NP
Kathmandu, Nepal
OUCRU Indonesia
Jakarta, Indonesia
Assessment
OUCRU Hanoi
Viet Nam
OUCRU Ho Chi Minh City
Oxford University Clinical Research Unit
Website: oucru.org
For more information, contact us at: communications@oucru.org

