Tx for PsO persisted during Covid-19
n Canadian study analyses ixekizumab use before and during pandemic
by JOHN EVANS, Senior Editor, The Chronicle
n Canadian study analyses ixekizumab use before and during pandemic
by JOHN EVANS, Senior Editor, The Chroniclesee page 4
n The key role of histopathology is expanding to aid in diagnosis
by JOHN EVANS, Senior Editor, The Chronicle


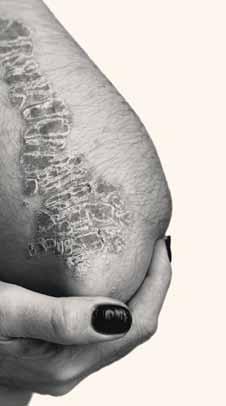
Pathology plays an important role in the diagnosis of dermatology conditions with many similarly appearing conditions, including vitiligo, cutaneous amyloid disorders, and sclerosing disorders. Recent research suggests histopathology can also aid in diagnosing IgG-4related conditions and chronic actinic dermatitis
This was the focus of a presentation by dermatologist and pathol-
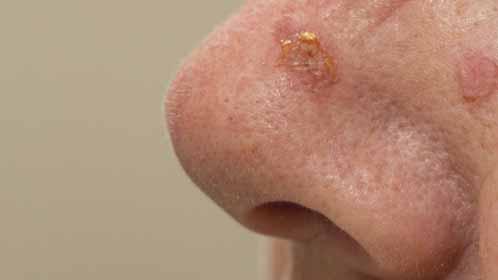
Dr. Sidiropoulos practices at the Odette Cancer Centre and Sunnybrook Health Sciences Centre in Toronto.
Pigmenting disorders
Other depigmenting disorders can mimic vitiligo, said Dr. Sidiropoulos. Using an S100 protein and Melan-A stain will highlight melanocytes in a biopsy. “[Vitiligo] mimickers can have a significant decrease in melanin as well as melanocytes,”
n Higher prevalence among Indigenous populations in central and midwestern Canada
by KRISHNA RAU, Correspondent, The ChronicleAsystematic review of literature on photodermatoses in North American Indigenous populations showed that the most common pathologies are polymorphous light eruption and actinic prurigo, Dr. Gregory Kost said in a presentation at the second annual Indigenous Skin Spectrum Summit.
Dr. Kost is a
page 8→
he said. “However, an S100 and Melan-A stain can reveal a complete loss of melanocytes and confirm a diagnosis of vitiligo. So we can do immuno-stains and we can look for complete loss of the melanocytes in vitiligo confirming that diagnosis.”
Plasma cell infiltrates
“Plasma cells are a key cell type to identify when we want to make a
Findings from a recent study show that Canadian patients with moderate-to-severe psoriasis persisted in biologic treatment through the early period of the Covid-19 pandemic, and many were also initiated on biologics during this time frame.
Published in JAAD (Sept. 2022; 87(3):AB91.), the study was initiated to assess persistence and adherence to ixekizumab in moderate-to-severe psoriasis in the Canadian Patient Support Program (PSP) before and during the Covid-19 pandemic.

The study included data on 2,450 patients aged 18 years or older who initiated ixekizumab treatment for psoriasis and had been enrolled in the PSP for at least six months. The data was collected between May 2016 and Jan. 1, 2021.
The researchers broke the data down into four study periods (SPs). These included:
• SP1: Initiated ixekizumab treatment more than two years before the start of the lockdown
n
◼ New therapies for patients who are resistant or intolerant to hedgehog inhibitorsDr. Wayne Gulliver

Photodermatoses in North American Indigenous peoples
During his presentation at the 2nd Annual Indigenous Skin Spectrum Summit, Dr. Gregory Kost described the higher prevalence of photodermatoses in North American Indigenous peoples, with the most common pathologies being polymorphous light eruption and actinic prurigo 1
Advancing Tx of BCC and AKs
Canadian dermatologists discuss new treatment options for BCCs and AKs.
Perspectives 2023: Rebuilding the confidence of Skin of Colour patients by Dr. Saima Ali
In partnership with DiTiDE, THE CHRONICLE OF SKIN & ALLERGY sponsored a short essay competition for Canadian dermatology residents, encouraging them to reflect on matters relating to inclusion, diversity, and equity in skin health 25
Chronicle Postgraduate Educational Supplement
In this issue’s educational supplement, a team of researchers from China identified the potential relationship between atopic dermatitis and Celastrol. Noting that atopic dermatitis was reversed following treatment with MRGPRX2OE indicating that Celastrol might affect atopic dermatitis through MRGPRX2 21
From the News Resources of The Chronicle
Researchers have identified an immune and lipid profile on the surface of the skin of infants that can predict the development of atopic dermatitis (AD) months before the onset of clinical illness. The findings were published in the Journal of Allergy and Clinical Immunology
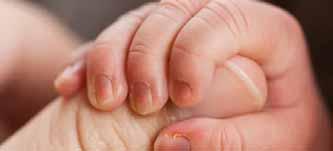
The researchers partnered with hospitals in the Republic of Korea, where physicians used non-invasive skin tapes to collect samples from the forearms of babies who were two months old, before signs of clinical AD typically present.
Study participants included those with and without a family history of atopic diseases. The babies were clinically monitored from birth until they reached age two years. Their skin cells were collected beginning at two months of age and were analyzed by mass spectrometry in the laboratory of Evgeny
Berdyshev, PhD. Dr. Berdyshev is the lead author of the study and the head of the mass spectrometry laboratory at National Jewish Health who pioneered this analytical assay.
Overall, 22 of 74 (29.7%) infants in the risk group and five of 37 (13.5%) in the control group developed AD.
The researchers found decreased levels of protein-bound ceramides in the stratum corneum of infants who went on to develop AD. Unsaturated sphingomyelin species, “short-chain” NS- and AS-ceramides were elevated in the skin of these children compared to healthy children.
Thymic stromal lymphopoietin (TSLP) and interleukin-13 (IL-13) levels were also increased in the stratum corneum of future AD subjects
Statistical analysis revealed that a combination of family history, type 2 cytokines and dysregulated lipids had a strong power for predicting AD.
copies: $10.00 per issue (plus 13% HST).
In this issue of THE CHRONICLE OF SKIN & ALLERGY, our editorial team continues to bring readers important clinical and scientific data covering diverse aspects of dermatology. Disorders that are common to our Indigenous patients including PMLE and actinic prurigo are presented on page 1. Another significant article relates to the dramatic impact of skin disorders in our patients with skin of colour (see page 1).
In an article that appears on page 1, we’re pleased to highlight and appreciate the important role of the dermatopathologist (friend of the dermatologist) in helping us diagnose and better manage many complex skin disorders. And what better way to approach spring than to provide an update on the management of AKs and BCCs, an update that should be appreciated by all.
Finally, a shout out to all dermatologists in Canada who did an outstanding job managing our dermatology patient population during the ongoing Covid-19 pandemic. This outstanding effort is con -
Please turn to Message page 13→
Published eight times per year by the proprietor, Chronicle Information Resources Ltd., with offices at 555 Burnhamthorpe Road, Ste 306, Toronto, Ont.
M9C 2Y3 Canada. Telephone: (416) 916-2476; Facs. (416) 3526199.
E-mail: health@chronicle.org
ISSN No. 1209-0581

Contents © Chronicle Information Resources Ltd., 2023 except where noted. All rights reserved worldwide.
The Publisher prohibits reproduction in any form, including print, broadcast, and electronic, without written permissions.
Printed in Canada. The Chronicle of Skin & Allergy is a Canadian publication. The Publisher certifies that advertising placed in this publication meets Revenue Canada requirements for tax deductibility.
Subscriptions: $85.60 per year in Canada, $129.95 per year in all other countries. Single
Canada Post Canadian Publications Mail Sales Product Agreement Number 40016917. Please forward all correspondence on circulation matters to: The Chronicle of Skin & Allergy, 555 Burnhamthorpe Road, Ste 306, Toronto, Ont. M9C 2Y3 Ideas in the Service of Medicinesm
Affiliated journals of the Chronicle Companies include The Chronicle of Cosmetic Medicine + Surgery, The Chronicle of Neurology + Psychiatry, Pediatric Chronicle, and Linacre’s Books/Les Editions Linacre

Medical Editor
Wayne Gulliver, MD, FRCPC
John P. Arlette, MD, FRCPC

Benjamin Barankin, MD, FRCPC
Marc Bourcier, MD, FRCPC
Eric Goldstein, MD, FRCPC
Peter Hull, MD, FRCPC
Richard Langley, MD, FRCPC
Danielle Marcoux, MD, FRCPC
R.A.W. Miller, MD, FRCPC
Editor, Cosmetic Dermatology Sheldon V. Pollack, MD, FRCPC
H. Eileen Murray, MD, FRCPC
Kim Papp, MD, FRCPC
Yves Poulin, MD, FRCPC
Melanie D. Pratt, MD, FRCPC
Denis Sasseville, MD, FRCPC
Jerry Tan, MD, FRCPC
Ronald B. Vender, MD, FRCPC
Founding Editor Colin A. Ramsay, MD, FRCPC (1936-2003)
Publisher Mitchell Shannon
Editorial Director R. Allan Ryan
Senior Editor John Evans
Lead Coordinator, Journals
Assistant Editor Jeremy Visser Kylie Rebernik
Manager,Operations Cathy Dusome
Comptroller Rose Arciero
n READER SERVICE: To change your address, or for questions about your receipt of the journal, send an e-mail to health@chronicle.org with subject line “Circulation,” or call during business hours at 416.916.CHROn (2476), or toll-free at 866.63.CHRON (24766).

n LETTERS: We welcome your correspondence by mail, fax (416.352.6199), or e-mail. Kindly use the co-ordinates listed above.
n ADVERTISING: For current rates and data, please contact the publisher.
(see page 4)
n REPRINTS: The content of this journal is copyrighted. Please contact Mitchell Shannon for reprint information.
“These [hedgehog inhibitors] are good options for very large or multiple BCCs, to try to reduce them prior to surgery or for more palliative care of very bothersome BCCs”
Dr. Alia Bosworth, dermatologist in Halifax

Diagnostic and therapeutic developments could expand choices for Canadian dermatologists who manage actinic keratoses (AKs), precursors to squamous cell carcinomas (SCCs), and basal cell carcinomas (BCCs).


Emerging diagnostic technology for AKs and BCCs
One of the cutting-edge diagnostic tools in the diagnosis of AKs and BCCs is vibrational optical coherence tomography (VOCT), which is used to determine the morphology and stiffness of benign and cancerous lesions such as AKs and BCCs.
“This is a non-invasive technology,” said Dr. Ilya Shoimer, a dermatologist in Calgary, who is familiar with the technology based on his clinical experience in Australia, but who noted VOCT is not yet available in Canada. “It would be akin to an ul-
trasound of the skin to try and detect what is going on in the skin. One of the advantages of VOCT is that you can make a diagnosis without doing a biopsy, although biopsy still remains the gold standard. The limitation of this technology is the [high] expense.”
An additional potential disadvantage is the steep learning curve associated with using VOCT and its utility being operator-dependent, said Dr. Shoimer. “If people are trained on this technology, then it may be something useful in the future,” he said, noting cost of the technology would go down as use of the technology increases.
Dr. Mark Lupin, a dermatologist
in Victoria, Founder of Cosmedica Laser Centre, and a Fellow of the American Society of Laser Medicine and Surgery, noted the technology is an exciting proposition but is in nascent stages for the moment.
“My view is this is still in its infancy as far as being of great clinical utility, and more work and time will help determine if this is of value or not,” said Dr. Lupin. “Certainly, there is a need for more noninvasive diagnostic tools related to managing the growing burden of skin cancer.”
Dr. Michelle Pratt, Clinical Assistant Professor of Dermatology at the Memorial University of Newfoundland in St. John’s, NL, noted this emerging technology sounds promising, particularly if the accuracy can be improved through the integration of artificial intelligence and machine learning.

“I think that VOCT would be a really interesting modality to use as a

The Soluver products for wart treatment are formulated in a unique acrylic delivery system which eliminates the need for bandage covering while keeping occlusion.
Soluver Original minimizes irritation to the healthy skin surrounding the wart, locks in moisture with a durable, watertight covering and allows for superior penetration of salicylic acid.
Soluver Plus, with its higher percentage of salicylic acid, is ideally suited for resistant common or plantar warts.

Soluver Original is available in a 14 mL size while Soluver Plus is available in a 10 mL size; both include a brush applicator.

AVAILABLE AT SHOPPERS DRUG MART AND MOST DRUG STORES.

Continued from page 4
complement to clinical inspection and dermoscopy, potentially increasing diagnostic accuracy, and minimizing the need for invasive biopsies.”
Cemiplimab for BCCs represents more tolerable treatment
An emerging therapy for BCCs is the PD-1 antibody cemiplimab, which was approved by the U.S. Food and Drug Administration in 2021 to treat BCCs, following treatment with a hedgehog pathway inhibitor (HHI) or for patients in whom these therapies are not appropriate.
Dr. Shoimer noted that hedgehog inhibitors such as vismodegib and sonidegib can present a challenge in terms of tolerability, so potentially having access to a non-hedgehog inhibitor would be beneficial. “Hedgehog inhibitors are tough medications to tolerate,” he said. “There are a lot of side effects.”
Dr. Alia Bosworth, a dermatologist in Halifax and lecturer at Dalhousie University, Division of Cutaneous Science and Dermatology, agreed that the disadvantage of hedgehog inhibitors is that they may be difficult to tolerate.
“These [hedgehog inhibitors] are good options for very large or multiple BCCs, to try to reduce them prior to surgery or for more palliative care of very bothersome BCCs,” said Dr. Bosworth. “They can be quite effective, but their use is often limited by their side effect profile.”
Dr. Pratt echoed the challenges of patient tolerability associated with hedgehog inhibitors.
“There have been some patients who have been able to achieve a sustained, complete response, but [hedgehog inhibitors] are very difficult to tolerate,” said Dr. Pratt. “There’s a high rate of side effects. The most common side effect is muscle spasms, but patients can also experience abnormal taste, hair loss, tiredness, nausea, and diarrhea. There is a high rate of discontinuation due to the side effects.”
Newer topical choices for AKs involve combining established treatment such as 5-fluorouracil with 0.005% calcipotriol ointment as an off-label treatment. “This [calcipotriol] releases Vitamin D,” said Dr. Shoimer. “This [combination] means a shorter course of treatment. You can administer it twice a day for four days.”
Other topical treatments include imiquimod 5% and imiquimod 3.75%, with the less strong concentration permitting treatment of a larger surface area.
Dr. Lupin selects 5-fluorouracil to treat AKs because of its efficacy and affordability and, like Dr. Shoimer, combines it with other therapies to bolster its efficacy.
“For patients with hypertrophic AKs, especially of the scalp, a combination of 0.5% 5-fluorouracil with 10% salicylic acid can be an option,” said Dr. Lupin. “I will often combine calcipotriol 0.005% with 5fluorouracil as a twice daily, four-day regimen when patients want a short course of treatment.”
An emerging option is tirbanibulin
1% ointment, a microtubule inhibitor administered once daily for five days. It received U.S. FDA approval in December 2020. “The advantage is that it is a relatively painless treatment with good results, and there is high compliance from patients as it is only once a day for five days,” said Dr. Lupin.
Dr. Shoimer added that the shorter treatment course and decreased inflammation associated with tirbanibulin would be appealing for patients.
The abbreviated treatment period associated with tirbanibulin is also desirable for patients, agreed Dr. Pratt. “To cut the [treatment] application period down to five days would be a big draw for a lot of people.”
Preventive measures with AKs
Dr. Pratt takes a proactive approach to managing AKs in her high-risk patients, which includes prescribing chemopreventive medications. “There is evidence to support the use of oral retinoids and oral niacinamide in reducing the incidence of AKs and squamous cell skin cancers in patients at higher risk, many of whom have a history of skin cancers,” she said.
Similarly, Dr. Bosworth employs a preventive approach to ward off the development of future AKs and SCCs.
“We do use oral niacinamide 500 mg twice daily for chemoprophylaxis for patients with numerous AKs and a history of non-melanoma skin cancers to reduce transformation of AKs to SCCs,” said Dr. Bosworth.
Non-proprietary and brand names of therapies: cemiplimab (Libtayo, Sanofi); vismodegib (Erivedge, Roche); sonidegib (ODOMZO, Sun Pharma); 5-fluorouracil with 0.005% calcipotriol ointment (Enstilar, LEO Pharma); imiquimod 5% (Aldara, Bausch Health); imiquimod 3.75% (Zyclara, Bausch Health); 0.5% 5-fluorouracil with 10% salicylic acid (Actikerall, Cipher Pharma); tirbanibulin 1% ointment (not approved in Canada).
DUOBRII is indicated for improving the signs and symptoms of plaque psoriasis in adult patients with moderate to severe plaque psoriasis.
DUOBRII is not indicated for patients under the age of 18 years.
Clinical trials with DUOBRII did not include sufficient patients aged 65 and older to establish efficacy and safety in geriatric patients.
CONTRAINDICATIONS:
• Hypersensitivity to the drug, any medicinal or non-medicinal ingredient in the formulation, any component of the container, or other corticosteroids or retinoic compounds.
• Viral lesions of the skin, bacterial or fungal skin infections, parasitic infections, skin manifestations relating to tuberculosis or syphilis, or eruptions following vaccinations.
Seborrheic dermatitis.
• Women who are pregnant or may become pregnant.
RELEVANT WARNINGS AND PRECAUTIONS:
Patients with skin diseases with impaired circulation
• Patients with chronic leg ulcers
• HPA axis suppression
Patients with hepatic impairment
• Patients with impaired immune system function
• Patients with concomitant skin infection
Patients with renal impairment
• Allergic contact dermatitis
• Patients with glaucoma
Striae, telangiectasias, folliculitis, or skin atrophy
Conditions where the skin barrier may be impaired
• Wind or cold weather
• Exposure to excessive sunlight or sunlamps, or to photosensitizing drugs
• Breastfeeding women
DUOBRII should be used with caution as topical corticosteroid use may lead to rebound relapses, development of tolerance, risk of generalized pustular psoriasis and development of local or systemic toxicity
• Conditions that augment systemic absorption
FOR MORE INFORMATION:
Please see the Product Monograph at https://healthproducts.canada.ca/dpd-bdpp/index-eng.jsp for important information on adverse reactions, drug interactions, and dosing not discussed in this piece. The Product Monograph is also available by calling 1-800-361-4261.
† Based on a prospective, multicentre, randomized, double-blind, phase III clinical trial, comparing DUOBRII lotion to the vehicle lotion, in 215 patients 18 years and older with moderate to severe plaque psoriasis.
REFERENCE:
1. Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: Results of 2 phase 3 randomized controlled trials. Journal of the American Academy of Dermatology. 2018;79(2):287–93.













• At Week 8 of treatment, 45.3% of DUOBRII patients (n=141) achieved treatment success‡ vs vehicle (12.5% (n=74); 1° endpoint; p<0.001).1
• 4 weeks after stopping treatment, at Week 12, 33.4% of DUOBRII patients (n=141) maintained treatment success rates vs vehicle (8.8% (n=74); 2° endpoint; p<0.001).1
Demonstrated sustained efficacy.
Indicated for improving the signs and symptoms of plaque psoriasis in adult patients with moderate to severe plaque psoriasis.

dermatology resident at the University of Saskatchewan in Saskatoon. The review, which Dr. Kost was involved in, looked at case reports and case series with a primary Indigenous focus, particularly on rural populations in Canada and the US. A finding from the review is that there is a higher prevalence of photodermatoses among Indigenous populations in central and midwestern Canada, he said.
Photodermatosis is when chromophores in the skin react with the ultraviolet radiation in sunshine to cause a reaction in the skin, he said.

In his presentation, he focused on immune-based causes of photodermatosis but pointed out the condition can also be caused by DNA repair disorders, chemicals, or medications, or can be photoaggravated dermatosis.
Polymorphous light eruption (PMLE) is a common form of photodermatosis, he said. PMLE affects more women, is mostly seen in people in their 20s or 30s, has no predilection for race or ethnicity and is often inversely related to latitude. PMLE is caused by exposure to UVB and UVA rather than visible light. Parents do not necessarily pass susceptibility to the condition on to children. The pathogenesis is a delayed hypersensitivity reaction or an abnormal immune response, said Dr. Kost.
When taking a clinical history, the key factors are onset and timing, he said. PMLE usually appears in spring or summer, or after a trip to a sunny locale. Within minutes or hours of exposure to the sun, the skin will react and become quite itchy, and it can take days or weeks to resolve. During winter, patients can be asymptomatic, Dr. Kost said.
Over a few months, a patient's skin may harden and become more resistant to PMLE, he said. This is thought to be due to an immunogenic reaction and the pa-
tient can be exposed to sun with little reaction.
PMLE can present with different morphologies, but most common signs include erythematous papules and plaques and papulovesicles, said Dr. Kost. PMLE affects areas exposed to sunlight, but in adults the face is usually not affected, perhaps due to hardening over time. A form of PMLE called juvenile spring eruption affects younger males, particularly on the skin of the ears.
Dr. Kost suggested that in making a differential diagnosis, physicians should be aware of lupus erythematosus.
The best way to prevent PMLE is by using a broad-spectrum, high SPF sunscreen that is reapplied frequently, he said. For treatment, he recommended topical corticosteroids or topical calcineurin inhibitors. Using narrowband UVB treatments for a few weeks in early spring may accelerate skin hardening and leave the patient asymptomatic for the summer. For severe flares, Dr. Kost suggested oral prednisone for short course treatment and hydroxychloroquine for longer-term maintenance therapy.
Evaluating actinic prurigo
Actinic prurigo is more common in North American Indigenous populations, he said. It is commonly seen at higher altitudes and in warm climates and may be passed on genetically. There is a specific HLA-DR4 antigen associated with patients.
The pathogenesis is thought to be activation through TNF alpha synthesis and upregulation, as well as interferon gamma-producing CD3 cells, which cause an inflammatory response, he said.
Dr. Kost said actinic prurigo is more common in childhood but can affect patients of any age. While it often resolves in adolescence, it can progress throughout a patient's life. Similar to PMLE, it is triggered by UV radiation and presents during spring or summer.
Actinic prurigo is intensely pruritic, which can be a clear distinguishing feature, said Dr. Kost. It presents with erythematous

Please turn to Photodermatoses page 18→
ILUMYA™ coverage now available
Enrol your patients in the Sun Patient Support Program for ILUMYA™ – designed to help you and your patients every step of the way
PrILUMYA™ (tildrakizumab injection) is indicated for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.



For more information:
Please consult the Product Monograph at: info.ilumya.ca/Product_Monographfor important information relating to contraindications, warnings, precautions, adverse reactions, interactions, dosing and conditions of clinical use.
The Product Monograph is also available by calling our medical information department at: 1-844-924-0656.
REFERENCE:
Current ILUMYA™
For patients with moderate-to-severe plaque psoriasis





This study in The Journal of Dermatology (DOI: 10.1111/1346-8138.16710) explored bone mineral density (BMD), the 10-year fracture risk, the number of prevalent fractures, and the frequency of falls in relation to psoriatic arthritis (PsA) disease characteristics in a cohort with PsA.
A total of 61 PsA patients and 69 consecutive, age-matched controls underwent a physical examination, bone mineral density (BMD, and T-score) assessments, and calculation of a 10-year fracture risk. The odds ratio (OR) for low BMD and the 10year risk of hip fracture was higher (p=0.0029; 0.0002, p< 0.0001, OR=21,9, p=0.014) in the PsA group. According to the investigators, the PsA patients were more predisposed to prevalent fractures, including peripheral and vertebral fractures, and falls (OR 3.42; 2.26; 13.33; 3.95, respectively), compared to the control group.
Scalp psoriasis and late-onset psoriasis were significantly associated with a greater number of prevalent fractures (p=0.0049; 0.029), and the number of
falls per year correlated with late-onset psoriasis and flexural psoriasis (p=0.007; 0.023).
The authors conclude that PsA is an independent risk factor for reduced bone density and falls, resulting in related bone fractures. Patients with late-onset psoriasis are more likely to experience falls and related fractures, particularly if their disease involves the scalp and body folds.
This study looked at another comorbidity that may be associated with psoriasis and psoriatic arthritis. A bone mineral density test was performed, and the 10year fracture risk was examined. A very high odds ratio for low bone mineral density and 10-year risk of hip fracture was seen in the psoriatic arthritis group. This study confirmed that psoriatic arthritis patients were more predisposed to fracture and reduction in bone mineral density. In addition, scalp psoriasis and late
onset psoriasis was associated with more fractures as well. The authors concluded that psoriatic arthritis was an independent risk factor for reduced bone density and potentially fractures.
This tells us that a new comorbidity such as osteoporosis that was not previously recognized in psoriatic disease may be important to examine, especially in late onset psoriasis or those who have psoriatic arthritis. This may prevent additional morbidities such as hip fractures, or other factors that can lead to further complications. Certainly further studies are required, however, primary care practitioners or other specialists associated with osteoporosis may consider testing bone mineral density in patients with psoriatic arthritis.
FOR THE NINTH ANNUAL
REGISTER NOW OCTOBER 21, 2023
Ronald B. Vender, MD, FRCPC comments on recent findings and developments, and answers clinicians’ questionsWe invite your comments and questions about this feature at www.derm.city. Dr. Ron Vender is a certified dermatologist with more than 30 years of clinical practice experience and over 100 clinical trials in psoriasis. He is founder and director of Venderm Innovations in Psoriasis, a center of excellence for Psoriasis offering a comprehensive management solution for individuals with psoriasis. www.psoriasis.vip

Chronicle's digital audio, e-newsletters, and web portal keep physicians updated reliably 24/7. Each is worthy of your time. And they're available without charge. Find them all at https://linktr.ee/dermcity


Since 2015, derm.city has been a leading hub and repository for dermatology information and interactive knowledge-exchange in video, text, images, and audio. It's the official site of The Chronicle of Skin & Allergy, now newly enhanced with added useful features. Thrice weekly updates on the latest findings in dermatology, summarized for quick learning. Visit derm.city at https://derm.city
A dermatology newsletter focused on ethnodermatology care from top physicians in the field, delivered to your inbox every Monday.
Supported by Bausch Health Canada
A biweekly newsletter on clinical and social issues affecting female patients and physicians, delivered straight to your inbox.
Supported by Galderma Canada
Dr. Geeta Yadav and expert dermatologists discuss clinical developments, news, trends and myths in acne treatment and research. First season of three episodes now available for listening and downloading.

Supported by Cipher Pharmaceuticals
Dr. Neil Shear, dermatologist and Professor Emeritus at the University of Toronto, speaks with many guests from the world of dermatology, including Dr. Boluwaji Ogunyemi, Dr. Marc Bourcier, and Dr. Rachel Asiniwasis, to hear their stories and insights. First season of eight episodes now available for listening and downloading.

Supported by Amgen Canada
Dr. Ron Vender delves into recent psoriasis studies and comments articles about the efficacy of PASI scores, intermittent fasting for psoriasis, and biologic fatigue. First season of six episodes now available for listening and downloading.
Supported by Sun Pharma Canada

Continued from page
(defined as Mar. 15, 2020).
• SP2: Initiated treatment one to two years before lockdown.
• SP3: Initiated treatment one year or less before
lockdown.
• SP4: Initiated treatment during the pandemic.
“If you look at the patient demographics, there are pretty good numbers here, overall,” said the study’s lead author Dr.
Wayne Gulliver in an interview with THE CHRONICLE OF SKIN & ALLERGY “There were 2,450 total patients, more than 900 initiated more than two years before, roughly 600 one to two years, another 600 zero to one years

At Arcutis, that means focusing on unmet needs of immune-mediated skin diseases. With deep medical dermatology development expertise and a unique development platform, we’re committed to meaningful advances—innovation that solves the most persistent challenges of patients and their families.
Learn more at arcutis.com
before, and another 200 during the pandemic.”
Dr. Gulliver is a Professor of Medicine at Memorial University in St. John's, NL, and has been in practice as a dermatologist for more than 30 years. He is medical
editor of this journal. Sample was typical of psoriasis patients The sample was typical psoriasis patients, said Dr. Gulliver. “Between 55 and 60 per cent were male, [and the patients] had extensive psoriasis with anywhere from 16 to 19 per cent body surface area involved.”
Patients had an average PASI score of 13.8, with some having scores as high as 14.4, and they were experiencing a severe impact on their quality of life.
“We didn’t have a lot of data on [psoriasis in] special sites, but almost 37 per cent [of the patients] had psoriasis in special sites such as the face or genital area,” Dr. Gulliver said.
“So this [sample] is a very much snapshot of psoriasis in Canada,” he said. “It shows who is being treated with biologics and that people are being appropriately treated with biologics.”
The researchers found that treatment persistence was high in all of the subgroups. It was highest in those with two or more years of treatment before lockdown (95.2%) and in those who initiated treatment during the pandemic (94.4%). While it was lowest in patients who initiated ixekizumab zero to one year before lockdown, persistence was still high (89.7%). The researchers also noted the risk of non-persistence was not significantly different between SP4 and other SPs.
“We had data over at least six months for each patient, and [ixekizumab treatment] was persistent for at least six months,” Dr. Gulliver said. “The patients were getting their medication and were receiving their monthly injections for the most part.”
Concerns about severe Covid in these patients not realized Dr. Gulliver noted that when the first guidelines for safely
We champion meaningful innovation within medical dermatology.
Continued from page 13
conducting dermatology practice during the pandemic were being released, there was some concern that patients treated with immune-modulating medications would be at risk of more severe Covid-19.
“There were a number of groups that suggested holding the biologic,” he said. “There were some programs that set up registries to track patients who had Covid-19 and psoriasis.”
As data from those programs began to come in, it seemed that patients on treatments such as methotrexate and cyclosporine had poorer outcomes while patients on biologics did not fare any worse than the general population, he said.
“So it took us a little bit of time to figure out what to do [regarding psoriasis patient treatment],” Dr. Gulliver said. “I think in Canada, most of the dermatologists sat back and took a very scientific approach. Some patients may have skipped a dose or two. If they got Covid-19 we held their treatment for a couple of weeks to a month.”
Some patients opted to stop immune-modulating therapy due to their concerns, but “the majority of patients continued on their medication,” he said.
It was reassuring to see how many patients with severe psoriasis were initiating biologic treatment during the pandemic, Dr. Gulliver said. “It meant that dermatologists were still working, we
were still seeing patients and were still treating severe psoriasis, even through the pandemic.”
Physicians cannot treat a patient’s psoriasis with a biologic through a telemedicine consult, he said.
“A physician cannot do a virtual visit and start someone on a biologic. Patients need a chest X-ray, they need a TB [tuberculosis] test, they need bloodwork and they need assessments. So obviously dermatologists are gowning up and taking the appropriate public health precautions, but still seeing patients and still bringing care to patients with psoriasis in Canada, coast to coast to coast.”
Dr. Gulliver said that it would be good to look at additional data to see how Canadian dermatologists and their patients have fared as the pandemic extends into more years.
“With the knowledge we have gained, we can treat our patients if they get Covid-19 and we know it is appropriate to hold [ixekizumab] for two to four weeks, and when the [Covid] symptoms have abated to initiate therapy again, at the appropriate time,” he said. “We know that among our patients, if they get Covid-19, some of them may experience a psoriasis flare. But if we start treatment again at the appropriate time the majority will clear again.”
“We are not seeing increased negative outcomes for psoriasis patients on biologics when it comes to Covid-19,” said Dr. Gulliver.
“I believe that if we did, we would not see a 94
to 97 per cent persistence in terms of patients maintained during the pandemic. Overall, this study has very reassuring data.”
Non-proprietary and brand names of therapy: ixekizumab (Taltz, Lilly).
Continued from page 3
firmed by the data on the management of severe psoriasis patients treated with ixekizumab (full disclosure, your editor is an author on this publication) and from what I have observed this high quality care likely applies to all aspects of our dermatology practice. Although our services are sometimes underappreciated, we all should be very proud of the important work we do dayto-day, especially under the trying circumstances of the pandemic and the ongoing challenges in our various healthcare systems across the country.
As always, THE CHRONICLE team invites and welcomes your comments on this issue, or any other topic in dermatology, at www.derm.city.
—Wayne P. Gulliver, MD, FRCPC, Medical EditorClinical use
Can be used with or without medicated topical therapies for atopic dermatitis.
Limitations of use: use in combination with other JAK inhibitors, biologic immunomodulators, or potent immunosuppressants, such as methotrexate and cyclosporine, has not been studied and is not recommended.
Most serious warnings and precautions
Serious infections: patients may be at increased risk for developing serious bacterial, fungal, viral and opportunistic infections that may lead to hospitalization or death; more frequently reported serious infections were predominately viral. If a serious infection develops, interrupt treatment until the infection is controlled. Risks and benefits of treatment should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Monitor for signs and symptoms of infection during and after treatment, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Malignancies: lymphoma and other malignancies were observed in patients taking JAK inhibitors to treat inflammatory conditions and were more frequently observed in patients with rheumatoid arthritis (RA) during a clinical trial with another JAK inhibitor versus TNF inhibitors. Thrombosis: including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients taking JAK inhibitors to treat inflammatory conditions. Many of these events were serious; some resulted in death. Consider risks and benefits prior to treating
patients who may be at increased risk. In a clinical trial in patients ≥50 years of age with RA, a higher rate of all-cause mortality and thrombosis occurred in patients treated with another JAK inhibitor versus TNF inhibitors. Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately.
Major adverse cardiovascular events (MACE): including non-fatal myocardial infarction, were observed more frequently in patients ≥50 years of age with RA during a clinical trial comparing another JAK inhibitor versus TNF inhibitors.
Other relevant warnings and precautions
• Driving or operating machinery
• Dose-dependent increase in blood lipid parameters, lipid monitoring and management
• Hematological abnormalities
• Use with potent immunosuppressants
• Vaccination
• Monitoring and laboratory tests
• Fertility
• Women of childbearing potential
• Pregnancy and breastfeeding
• Geriatrics
For more information
Consult the Product Monograph at http://pfizer.ca/pm/en/CIBINQO.pdf for important information regarding adverse reactions, drug interactions and dosing, which have not been discussed in this piece. The Product Monograph is also available by calling 1-800-463-6001.
References: 1. CIBINQO Product Monograph, Pfizer Canada ULC. 2. Bieber T, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med 2021;384:1101–12. † Results from a phase 3 randomized, double-blind, placebo-controlled, double-dummy, parallel group, multicentre study of CIBINQO in combination with background medicated topical therapies in patients aged ≥18 years with moderate-to-severe atopic dermatitis who had an inadequate response to topical therapy or had received systemic therapy, excluding dupilumab. 2:2:2:1 randomization to CIBINQO 200 mg (n=226), CIBINQO 100 mg (n=238), dupilumab (n=243) or placebo (n=131) for 12 weeks. CIBINQO dose: 200 mg or 100 mg taken orally once daily. Dupilumab dose: 300 mg administered subcutaneously every other week after a loading dose of 600 mg at baseline. Matching placebo was dosed accordingly
CIBINQO is indicated for the treatment of patients 12 years and older with refractory moderate-to-severe atopic dermatitis, including the relief of pruritus, who have had an inadequate response to other systemic drugs (e.g., steroid or biologic), or for whom these treatments are not advisable.

A new, highly selective oral JAK1 inhibitor for moderate-to-severe AD*
Once-daily dosing
Flexibility to start patients 12–64 years of age with a 100 or 200 mg dose based on individual goal of therapy and potential risk for adverse reactions. Exceeding 200 mg per day is not recommended.
Contact the P昀zerFlex™ Support Program for dermatology for enrolment forms and information about program services
1-855-935-FLEX (3539)
Monday to Friday, 8 am–8 pm EST
Refer to the page in the bottom-right for additional safety information and for a web link to the Product Monograph discussing:
• The most serious warnings and precautions regarding: serious infections; malignancies; thrombosis; major adverse cardiovascular events
• Other relevant warnings and precautions regarding: driving and operating machinery; dose-dependent increase in blood lipid parameters, lipid monitoring and management; hematological abnormalities; use with other potent
AD=atopic dermatitis; JAK1=Janus kinase 1.
* Clinical significance unknown.
CIBINQO@p昀zer昀ex.com
immunosuppressants; vaccination; monitoring and laboratory tests; fertility; special populations, including women of childbearing potential, pregnancy, breastfeeding, and geriatrics

• Conditions of clinical use, adverse reactions, drug interactions and dosing instructions
In addition, the page contains the reference list relating to this advertisement.
Significantly more CIBINQO patients achieved ≥75% improvement in the EASI score from baseline (defined as EASI-75) at Week 12 vs. placebo1,2†
Rapid and significant itch relief was seen as early as Week 2 vs. placebo as measured by PP-NRS4 (2º endpoint)
Patients achieving a PP-NRS response with ≥4-point improvement from baseline
31.8% § CIBINQO 100 mg
49.1%¶ ||
200 mg
vs.vs.
26.4% dupilumab 300 mg
13.8% placebo
Significantly more CIBINQO 200 mg patients achieved PP-NRS4 vs. dupilumab as early as Day 4 and remained higher through Week 2
Proportion of PP-NRS4 responders with CIBINQO 100 mg was similar to dupilumab at Week 2 and over time.
§ p<0.001 vs. placebo (multiplicity-controlled).
¶ p<0.0001 vs. placebo (multiplicity-controlled).
|| p<0.0001 vs. dupilumab (multiplicity-controlled); statistical comparison between either CIBINQO dose and dupilumab was only performed on the proportion of patients achieving PP-NRS4 at Week 2.
CIBINQO is only indicated in patients who have had an inadequate response to other systemic drugs or for whom these treatments are not advisable. Over 50% of patients in these studies did not have prior exposure to systemic therapy.
Adapted from Product Monograph and Bieber, et al.diagnosis [using histology],” said Dr. Sidiropoulos. “They shouldn't typically be in normal skin, so when we see them we know there is an abnormality. They present in a wide range of dermatoses as well as tumours.”
Plasma cells have a distinct appearance, with the nucleus pushed to one side, he said. When they are found in the skin they are associated with a range of potential diagnoses, including cutaneous amyloidosis, IgG 4-related diseases, infections, lymphadenopathic Kaposi's sarcoma, and lymphoma.
Plasma cell infiltration is also seen with nodular cutaneous amyloidosis, in band-like plaques, Dr. Sidiropoulos said.
The lack of plasma cells can make biopsies of macular and lichen amyloidosis appear normal, said Dr. Sidiropoulos.
“But we can do stains to help us tell if [a biopsy] is cutaneous amyloid and whether it is macular or lichen.” One option is a Congo Red stain, though there is a higher level of expertise involved in preparing a sample for that stain, he said. Another option are keratin antibodies, which will highlight the keratin within keratinocytes.
Immunohistochemical studies have shown a decrease or absence of CD 34-positive cells in many sclerosing disorders of the skin, Dr. Sidiropoulos said. Testing for CD 34 depletion is a good test to confirm sclerosing disorders. “We see [CD 34 depletion] in morphea. We see it in scleroderma, we see it with necrobiosis lipoidica, and we also see it in lichen sclerosis and scars.”
CD 34 “is a transmembrane glycoprotein. It is expressed in many cells—we are still learning exactly which cells it is expressed in and how many. But It is seen in dermal dendritic cells, embryonic fibroblasts and other key cells in the dermis,” he said.
Dermal dendritic cells that are CD 34 positive are important in maintaining the architecture of the dermis as well as modulating the immune response in the skin, he said. “Some people believe that it is this cell that is the primary target in sclerosing disorders such as morphea.”
Dr. Sidiropoulos noted that the sclerosing disorder nephrogenic systemic fibrosis (NSF) can be difficult to distinguish clinically from morphea. “What we can do is stain for CD 34 and the positivity is retained in NSF. A pathologist can see the significant contrast with morphea just with a simple stain.”
Identifying spirochete / Lyme organisms related to eosinophilic fasciitis
Dr. Sidiropoulos also discussed eosinophilic fasciitis, noting they are on the same spectrum of sclerosing disorders such as morphea. He noted there is research that has revealed that some cases of eosinophilic fasciitis are associated with spirochete organisms or stain positively for Borel-
lia DNA or antibodies. “Does that change management [of eosinophilic fasciitis]? Possibly not, but it shows that maybe the causative factor in a subset of these cases is Lyme or another spirochetal organism.”
IgG-4-related diseases
“IgG-4-related diseases are a somewhat newly recognized fibro-inflammatory conditions,” said Dr. Sidiropoulos. The diagnostic criteria for them are still something of a work in progress, he added.
“They can mimic malignancies, they can mimic infections, they can mimic inflammatory disorders. Histopathology at this time is currently key for diagnosis.”
For diagnosis, pathologists can look for infiltration of IgG-4-positive plasma cells. “We see a dense inflammatory infiltrate: lots of lymphocytes, lots of plasma cells. We see storiform fibrosis—a type of scar-like reaction which looks like a cartwheel—that takes over the organ.” While many IgG-4-related diseases are often associated with obliterative phlebitis, which is seen mainly in internal organs and is less often found in the skin, he said.
“The key with these entities is the mass effect,” said Dr. Sidiropoulos. Enlarging scar tissue takes over an organ and presses on other tissues and organs, causing further problems. IgG-4-related diseases “Often present with an infiltrated tumefactive swelling—puffy lesions—at multiple sites. In the skin, they are often found in the head and neck areas.”
These lesions are commonly clinically and pathologically misdiagnosed as pseudo lymphomas, he said.
The current consensus is that a confident diagnosis of an IgG-4-related disease requires positive IgG-4 staining as well as two of:
• Dense lymphocytic plasmocytic infiltrate;
• Fibrosis in a cartwheel-like pattern; and
• Obliterative phlebitis (in organs other than the skin).
Chronic actinic dermatitis is an eczematous hypersensitivity reaction to ultraviolet rays, Dr. Sidiropoulos noted. When it is more severe, it is also known as actinic reticuloid and it can mimic
several lymphoid infiltrate conditions such as cutaneous T-cell lymphoma (CTCL), and mycosis fungoides, he said.
“The key when we look at lymphoid infiltration in the skin is we want to make sure whether it is a reactive or a neoplastic process,” he said.
“When we work up lymphomas, those workups are quite extensive both clinically and pathologically,” said Dr. Sidiropoulos. “All this work up is done to make sure we are not calling something a lymphoma when it is actually reactive. And one of the things that we do not want to call neoplastic is chronic actinic dermatitis.”
The differential diagnosis is based on clinical, pathologic and, most importantly, photo biologic features, Dr. Sidiropoulos said.
“A low minimal erythema dose and pathology showing prominent dermal dendrocytes with multi-nucleation, and a high ratio between CD8-positive to CD4-positive cells in the infiltrate could help differentiate chronic actinic dermatitis from CTCL.”
“
lasma cells are a key cell type to identify when we want to make a diagnosis [using histology]. They shouldn't typically be in normal skin, so when we see them we know there is an abnormality”
Dr. Michael Sidiropoulos


Continued from page 8
papules and plaques and can also present with severe cheilitis. It affects areas exposed to sunlight, which can include the face. Conjunc-
tivitis is associated with actinic pruritis, and it may also produce scarring, he said.
The differential diagnosis for actinic pruritis can include more serious
conditions such as porphyria cutanea tarda or lupus erythematosus, he said.


Camp Liberté was created by a group of dermatologists dedicated to offering children with moderate to severe skin conditions an opportunity to enjoy a summer camp experience. With locations in eastern and western Canada, Camp Liberté hosts more than 40 children per summer at no cost to parents, thanks to the support of generous donors.
Our camps are fully equipped with volunteer dermatologists, residents and nurses to care for children with a wide range of skin conditions, including atopic dermatitis, epidermolysis bullosa, and alopecia areata.


HOW CAN YOU HELP?













plied frequently is the best way to reduce flares and control symptoms of actinic prurigo, said Dr. Kost. Topical steroids and calcineurin inhibitors can be used for ”
Le Camp Liberté a été créé par un groupe de dermatologues déterminés à offrir à des enfants qui ont des problèmes de peau variant de modérés à graves la possibilité de vivre l’expérience d’un camp d’été. Avec ses emplacements dans l’est et l’ouest du Canada, le Camp Liberté accueille plus de 40 enfants par été sans qu’il en coûte quoi que ce soit aux parents, grâce à l’appui de généreux donateurs. Nos camps bénéficient des services complets de dermatologues, de médecins résidents et d’infirmières bénévoles qui s’occupent d’enfants aux prises avec un vaste éventail de problèmes de peau, y compris la dermatite atopique, l’épidermolyse bulleuse et la pelade.

COMMENT POUVEZ-VOUS AIDER?
Un don au Camp Liberté permet à des enfants canadiens qui ont des problèmes de peau d’accroître leur confiance en soi et leur estime de soi en vivant une expérience multiculturelle de camping en plein air offerte dans un environnement bilingue, sécuritaire et amusant.
treatment, as can light therapy. Oral prednisone can be used for short course treatments, but azathioprine and cyclosporine can also be utilized. For resistant actinic pruritis, thalidomide has proven very effective in the later stages of treatment.
Bottom Line: North American Indigenous populations have a high prevalence of polymorphous light eruption and actinic prurigo. Both are caused by exposure to the ultraviolet radiation in sunlight and usually present in spring or summer. Sunscreen and topical treatments are the usual initial treatments, with more powerful medications used as needed.
Chronicle Companies is pleased to support Camp Liberté with monetary and inkind donations through Sandi’s Fund, established to honour our late friend and colleague, Sandra Gail Leckie, RN. Sandi was a nurse, pharmaceutical industry executive and health educator who had a life long affinity for children and children’s charities.

2021-Camp L berte Journal Ad-Ful Page-10 375x14 9-F na ndd 1 2021-02-10 6:41 PM

Have a comment about the articles in this issue?
Share your knowledge and opinions with peers worldwide.
where dermatology lives
Skin Spectrum Quarterly™ is a new worldwide dermatology journal that focuses on the emerging subspecialty of "Ethnodermatology." The journal will b e distributed in print and digitally and will champion "Skin Health for All," with a speci al focus on understudied groups of patients with richly pigmented skin (Fitzpatrick type s IV-IV.)

Skin Spectrum Quarterly™ will publish high-quality original res earch, review articles, correspondence, clinical news with commentary, recent book and publication reviews, editorials, and first-person opinion articles. The journal's in terests lie at the intersection of dermatology, race, and culture.

We invite authors to submit articles reflecting the journal's f ocus and interests. All submitted articles will undergo an independent, rigorous peer-r eview process prior to acceptance. To request more information on submitting an articl e, please email hello@skinspectrum.org. We are excited to hear from you.
The journal will launch separate editions in the USA and Canada in 2023, and begin distribution in other areas of the world the following year.
The submission deadline for Inaugural Issue is May 15, 2023.

Ciyu Yao,1 Wenzhen Ye,1 and Mengxue Chen1
1Department of Dermatology, Fuzhou Hospital of Traditional Chinese Medicine Affiliated to Fujian University of Traditional Chinese Medicine, No. 102, Gudong Road, Fuzhou, 350000 Fujian Province, China
ABSTRACT
Background: Atopic dermatitis is a common dermatological disease, and mast cell degranulation is believed to be related with the progression of atopic dermatitis. Mas-related G protein-coupled receptor-X2 (MRGPRX2), and calcium release-activated calcium channel protein 1-2 (ORAI-1, ORAI-2) are involved in mast cell degranulation. Celastrol is an active monomer of Tripterygium wilfordii, and it presents an antiatopic role.
Methods: 2,4-Dinitrofluorobenzene (DNFB) and compound 48/80 (C 48/80) were used to establish a slow and acute scratching animal model, respectively. Hematoxylin-eosin and toluidine blue staining was used to investigate tissue injury. Inflammatory factor concentration was measured with ELISA. The expression of MRGPRX2, ORAI-1, and ORAI-2 was detected with immunohistochemistry (IHC) staining. Gene expression profiling and microRNA array were performed to investigate gene differential expression. Results. Celastrol greatly inhibited atopic dermatitis-related tissues injury, mast cell production, histamine release, scratching level, inflammatory factor expression, and activation of MRGPRX2/ORAI axis in the DNFB-induced atopic dermatitis model. The influence of Celastrol on atopic dermatitis was remarkably reversed by overexpression of MRGPRX2. Conclusion: We found that the improvements of atopic dermatitis caused by Celastrol were reversed by treatment with MRGPRX2OE, indicating that Celastrol might affect atopic dermatitis through MRGPRX2. This study might provide a novel thought for the prevention and treatment of atopic dermatitis by regulating MRGPRX2.
Atopic dermatitis is a common dermatological disease. The repeated inflammation, stubborn itching, and skin barrier damage caused by atopic dermatitis can seriously affect the quality of life of patients.1,2 The pathogenesis of atopic dermatitis is not very clear. It may be caused by autoimmunity, genetic factors, environmental factors, skin barrier dysfunction, bacterial infection, and other pathogenic factors.3,4 At present, the treatment of atopic dermatitis is mainly through local medication or oral administration to reduce inflammation and itching.5 Topical glucocorticoid is a common local treatment for atopic dermatitis, but it may cause adverse reactions such as skin at-
rophy, telangiectasia, pigmentation, and systemic absorption, which limits its application.6
Mast cells activated in atopic dermatitis release a large number of inflammatory cytokines, histamine, and protease through degranulation7,8 and play an important role in regulating inflammatory response and itching.9,10 Mas-related G protein-coupled receptor-X2 (MRGPRX2) is specifically expressed by skin mast cells and is considered a new marker for regulating mast cell degranulation, chronic inflammation, and anaphylaxis.11 MRGPRX2 may utilize calcium release-activated calcium channel protein 1 (ORAI-1) and calcium release-activated calcium channel protein 2 (ORAI-2) to promote mast cell degranulation.12 The regulation of calcium influx by ORAI-1 is related to RhoA/Rock/MAPK signaling, which is
Reprinted with permission ©2023 Ciyu Yao, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Content has been edited to conform with the Canadian Press Publication Style Guide Chronicle.Academy develops bespoke structured learning programs for clinicians providing in-depth education to professionals in the healthcare industry.
closely related to mast cell degranulation.13 However, the mechanism by which ORAI-1 and ORAI-2 interact with MRGPRX2 to regulate mast cell degranulation is unclear.
Celastrol, as an active monomer of Tripterygium wilfordii, can play an antiatopic role by reducing calcium ion influx, β-hexosaminidase, histamine, Th2 cytokines, and cell adhesion molecules.14,15 However, whether Celastrol regulates atopic dermatitis through MRGPRX2 has not been reported. The purpose of this study is to explore whether the molecular mechanism of Celastrol intervention on mast cell degranulation in atopic dermatitis is related to the interaction of MRGPRX2/ORAI.
In the present study, a 2,4-dinitrofluorobenzene- (DNFB-) induced atopic dermatitis animal model was establish, and compound 48/80 (C 48/80) was used to establish an acute scratching animal model. We demonstrated that Celastrol could inhibit atopic dermatitis through suppressing MRGPRX2/ORAI axis. This research might provide a new insight for the prevention and treatment of atopic dermatitis.
All the experiments were approved by the Ethical Committee of Fujian University of Traditional Chinese Medicine. BALB/c mice (18-25 g, Charles River, Beijing, China) were used in this study. In total, four groups were set in this research with 10 mice in each group. The slow atopic dermatitis animal model was established with DNFB (#D1529, Sigma). 0.8% Na2S solution was applied to 2.5 cm x 2.5 cm back skin of mice for hair preparation, and 100 μL of 0.5% DNFB solution was applied on the back of the mice the next day. 100 μL of 0.5% DNFB solution was used to daub the back of the mice once a day for 14 days. The mice in the control group were treated with PBS with the same way. The animals in the DNFB+Celastrol and DNFB+Celastrol+MRGPRX2OE group were treated with 0.5% DNFB solution as described above. Meanwhile, the mice in the DNFB+Celastrol group were treated with Celastrol (3 mg/kg, #C0869, Sigma) gavage once a day. The mice in the DNFB+Celastrol+MRGPRX2OE group were treated with Celastrol (3 mg/kg) gavage once a day and injected with 50 μL MRGPRX2OE vectors (1x108 transfection units/mL) through
the tail vein once a day. At the 14th day, the mice were sacrificed, and the skin tissues were collected for the following experiments. The scratch bouts were recorded at the 0, 3, 6, 9, and 12 days. Each recording time lasts for 30 min, and scratch bout times were recorded.
Establishment of an acute atopic dermatitis animal model with C 48/80
The mice in the C 48/80 (#C2313, Sigma) and control group were treated with PBS (3 mg/kg) gavage once a day for seven days. The mice in the C 48/80+Celastrol group were treated with Celastrol (3 mg/kg) gavage once a day for seven days. The mice in the C 48/80+Celastrol+MRGPRX2OE group were treated with Celastrol (3 mg/kg) gavage once a day and injected with 50 μL MRGPRX2OE vectors (1x108 transfection units/mL) through the tail vein once a day for seven days. Then, an acute atopic dermatitis animal model was established through intradermal injection of C 48/80 (50 μg, 25 μL). After the intradermal injection of C 48/80, scratch bout times were recorded immediately for 30 min. The animals in the control group were injected with the same amount of PBS.
The serum level of histamine was measured with related commercial kit purchased from Nanjing Jiancheng Bioengineering Institute (#H171, Nanjing, China) according to instructions.
Immunohistochemistry (IHC) staining Paraffin sections were dewaxed firstly. Tissue sections were placed in EDTA antigen repair buffer and placed in a microwave oven for antigen repair (5 min). PBS (#ABA212278, Hyclone) was washed three times (5 min/time). The sections were put into 3% hydrogen peroxide solution and incubated for 25 min at room temperature in the dark to block endogenous peroxidase. The slides were washed three times in PBS (5 min/time). BSA was used and blocked for 30 min. The primary antibody was added to the sections, and the sections were incubated overnight at 4°C in a wet box. After washing three times in PBS, secondary antibody was added to incubate sections for 50 min at room temperature. Sections were incubated with DAPI dye for 5 min. Sections were observed under a fluorescence microscope, and images were collected. The antibodies used in this study were listed as follows: rabbit anti-MRGPRX2 anti-
Figure 1: Inhibition of DNFB-induced atopic dermatitis by Celastrol was reversed by overexpression of MRGPRX2. (a) HE staining was performed to unfold the tissue damage. (b) The skin thickness was analyzed. (c) Toluidine blue staining was used to observe mast cells. (d) The number of mast cells was counted. Yellow arrows represent hair follicles, and red arrows represent mast cells. * suggests p<0.005.
body (ab237047, Abcam), mouse antiOrai1 antibody (#SAB3500126, Sigma), and rabbit anti-Orai2 antibody (#HPA055137, Sigma).
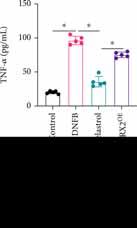
Detection of TNF-�� , IL-6, IL-1β, and IL-31
The serum levels of TNF-�� (#PT513), IL-6 (#PI326), and IL-1β (#PI301) were measured with related commercial kits purchased from Beyotime (Shanghai, China) according to instructions. The concentration of IL-31 (#ab243681) was detected with related commercial kit purchased from Abcam (UK).
Paraffin sections were dewaxed firstly, and the sections were successively put into xylene I for 20 min, xylene II for 20 min, absolute ethanol for 5 min, and 75% alcohol for 5 min. The sections were stained with hematoxylin staining solution (#G1004, Servicebio) for 3 min and washed with tap water. The sections were dehydrated with 85% and 95% gradient alcohol for 5 min and stained with eosin staining solution (#G1001, Servicebio) for 5 min. The sections were put into anhydrous ethanol for 5 min, xylene I for 5 min, and xylene II for 5 min.
The neutral gum was sealed. The slides were observed with a microscope (Carl Zeiss, Germany).
Total RNA from each sample was quantified using the NanoDrop ND-1000, and the RNA integrity was assessed using standard denaturing agarose gel electrophoresis. Agilent Array platform was
Figure 2: Celastrol greatly inhibited histamine and scratch bouts. (a) The level of histamine was analyzed. (b) An acute scratching animal model was established with C 48/80. (c) A slow scratching animal model was established with DNFB. * suggests p<0.05.
employed. The sample preparation and microarray hybridization were performed based on the manufacturer’s standard protocols. Briefly, total RNA from each sample was amplified and transcribed into fluorescent cRNA with the use of the manufacturer’s Agilent’s Quick Amp Labeling protocol. The labeled cRNAs were hybridized onto the Whole Mouse Genome Oligo Microarray (4x44K, Agilent Technologies). After washing the slides, the arrays were scanned by the Agilent Scanner G2505C. Agilent Feature Extraction software (version 11.0.1.1) was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v11.5.1 software package (Agilent Technologies). After quantile normalization of the raw data, genes were chosen for further data analysis. Differentially expressed genes were identified through fold change filtering.
Total RNA was harvested using TRIzol (Invitrogen) and miRNeasy mini kit (QIAGEN) according to the manufacturer’s instructions. After having passed RNA quantity measurement using the NanoDrop 1000, the samples were labeled using the miRCURY Hy3/Hy5 Power Labeling Kit and hybridized on the miRCURY LNA Array (v.18.0). Following the washing steps, the slides were scanned using the Axon GenePix 4000B microarray scanner. Scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. Replicated miRNAs were averaged, and miRNAs that have intensities =>30 in all samples were chosen for calculating normalization factor. Expressed
Figure 3: The inflammatory factor levels were suppressed by Celastrol. (a) The level of TNF-�� was measured. (b) The level of IL-31 was measured. (c) The level of IL-6 was measured. (d) The level of IL-1β was measured. * suggests p<0.05.
data were normalized using the median normalization. After normalization, differentially expressed miRNAs were identified through fold change filtering. Finally, hierarchical clustering was performed to show distinguishable miRNA expression profiling among samples.

The tissues were lysed firstly, and protein samples were separated with 10% SDS-PAGE. The proteins were transferred to nitrocellulose membrane (#Z358657, Millipore, USA). After blocking with 5% skim milk for 1 h, the related primary antibodies (1:1000) were used to culture membrane. Then, the membranes were incubated with secondary antibody (1:3000) for 2 h at room temperature. Finally, the protein grey was analyzed with ImageJ software. The antibodies used in this study were listed as follows: rabbit anti-MRGPRX2 antibody (ab237047, Abcam), mouse anti-Orai1 antibody (#SAB3500126, Sigma), and rabbit anti-Orai2 antibody (#ab180146, Sigma).
Statistical difference was analyzed with at least 3 repeated experiments. The results were shown as the mean ± standard deviation. Data among different groups were analyzed with ANOVA; p<0.05 was considered to be statistically different.
Inhibition of DNFB-induced atopic dermatitis by
To unfold the influence of Celastrol on atopic dermatitis, DNFB was used to induce an atopic dermatitis mice model. Compared with the control group, in the DNFB model group, the

Figure 4: The activation of the MRGPRX2/ORAI axis in the atopic dermatitis animal model was suppressed by Celastrol. (a) The expression of MRGPRX2 in the skin tissue was measured with IHC staining. (b) The expression of MRGPRX2 was analyzed. (c) The levels of ORAI-1 and ORAI-2 in the skin tissue were detected with IHC staining. (d) The levels of ORAI-1 and ORAI2 were analyzed. (e) The expression levels of the MRGPRX2/ORAI axis were measured with western blot; * indicates p<0.05.
inflammatory cell infiltration (Figure 1(a)) and skin thickness (Figure 1(b)) in the model site were significantly increased. After Celastrol treatment, the infiltration of inflammatory cells was significantly alleviated, and skin thickness was decreased. However, overexpression of MRGPRX2 greatly inhibited the influence of Celastrol (Figures 1(a) and 1(b)). Meanwhile, after DNFB induction, the mast cell number was significantly increased, but increased mast cells were suppressed by Celastrol (Figures 1(c) and 1(d)). In addition, the decreased mast cells by Celastrol were promoted by MRGPRX2 OE (Figures 1(c) and 1(d)).
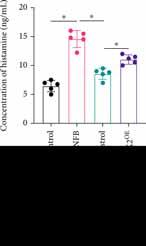
The level of histamine is closely related with itching degree. We found that histamine release was markedly promoted in the DNFB animal model (Figure 2(a)) compared with the control or DNFB+Celastrol group. However, after treatment with MRGPRX2OE, the level of histamine was significantly increased compared with the DNFB+Celastrol group (Figure 2(a)). An acute scratching animal model and slow scratching animal model were established with C 48/80 (Figure 2(b)) and DNFB (Figure 2(c)), respectively. The scratching bouts were greatly increased after C 48/80 or DNFB induction compared with the control group. However, administration with Celastrol remarkably suppressed the scratching levels, but simultaneous treatment with MRGPRX2OE increased the
times of scratching (Figures 2(b) and 2(c)).
The inflammatory factor levels were suppressed by Celastrol
The expression levels of inflammatory factors were measured. We found that the concentrations of TNF-�� (Figure 3(a)), IL-31 (Figure 3(b)), IL-6 (Figure 3(c)), and IL-1β (Figure 3(d)) were markedly promoted in the DNFB group compared to the control or DNFB+Celastrol group.
After treatment with Celastrol, the expression levels of inflammatory factors were greatly inhibited, and the influence of Celastrol on inflammatory factors was reversed by overexpression of MRGPRX2 (Figures 3(a)–3(d)).
The activation of the MRGPRX2/ORAI axis in the atopic dermatitis animal model was suppressed by Celastrol
The expression of the MRGPRX2/ORAI axis was evaluated by measuring the levels of MRGPRX2, ORAI-1, and ORAI2 in skin tissue (Figures 4(a)–4(d)).
After DNFB induction, the protein expression of MRGPRX2, ORAI-1, and ORAI-2 in skin tissue was remarkably promoted compared with the control group (Figures 4(a)–4(d)). However, the activation of the MRGPRX2/ORAI axis by DNFB was inhibited greatly by Celastrol. In addition, the suppression of the MRGPRX2/ORAI axis by Celastrol was greatly reversed by overexpression of MRGPRX2 (Figures 4(a)–4(d)).
Similar influence of Celastrol and overexpression of MRGPRX2 on expression of the MRGPRX2/ORAI axis were observed and validated with western blot (Figure 4(e))
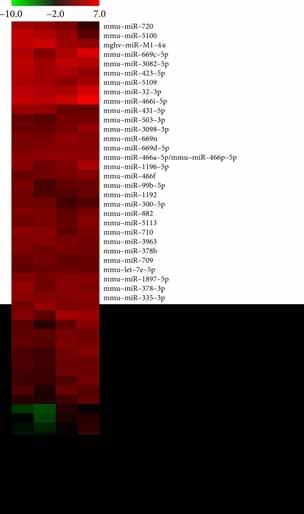
Gene expression profiling and microRNA array were performed to investigate the gene differential expression. The default fold change value of the three green broken lines is 2 (Figure 5(a)). The point above and below the green line is the gene fold change => 2.0 of the two comparative samples. If the expression difference between the two comparative samples is greater, the dispersion is greater. There was little difference in gene expression between the control and DNFB+Celastrol group (Figure 5(a)). However, significant gene expression differences were observed between the control, DNFB, and DNFB+Celastrol groups (Figure 5(a)). The gene expression heat map is presented in Figure 5(b). In addition, microRNA array was performed to investigate the expression differences of microRNAs in different groups (Figure 5(c)).
Atopic dermatitis is a common chronic, recurrent, and itchy skin disease.2,16
Studies have shown that in atopic der-
matitis, mast cells are recruited by chemokines and activated by IgE and non-IgE factors to produce a series of inflammatory factors;17 interact with nerve cells, T cells, eosinophils, keratinocytes, etc.; and jointly participate in the process of skin itching and inflammation in atopic dermatitis.16,18
Skin pruritus is one of the main symptoms of atopic dermatitis, which reflects the severity of atopic dermatitis to a certain extent.19 Mast cells are mononuclear immune cells that exist in skin and mucosal tissues.20 They can be activated by allergens such as allogeneic proteins and chemical small molecule drugs to cause allergic reactions.21 Its allergic mechanism is that the allergen binds to the immunoglobulin IgE receptor in mast cells, induces cells to release bioactive substances, produces degranulation, and causes physiological and pathological changes such as smooth muscle contraction, increased vascular permeability, and increased serous secretion.22 In this study, we found that both acute scratching and slow scratching caused by C 48/80 and DNFB, respectively, could be greatly alleviated by Celastrol. However, the relief of scratching by Celastrol was significantly reversed by overexpression of MRGPRX2, indicating that Celastrol might regulate scratching through inhibiting MRGPRX2.
Mast cells can act as both effector cells and immune regulatory cells during the occurrence and development of atopic dermatitis.23,24 Mast cells regulate and recruit the chemotaxis of other inflammatory cells to further promote the process of atopic dermatitis.25,26 Histamine is a receptor that is released when mast cells activate sensory nerve endings and release neuropeptides. Mast cell activation leads to degranulation, vasodilation, plasma extravasation, and recruitment of immune cells.27,28 Mast cell activation is involved in the pathogenesis of pain and many chronic inflammatory diseases, including atopic dermatitis.29,30
A previous study indicated that MRGPRX2-mediated mast cell activation contributed to local inflammation and sensory neuron excitation in atopic dermatitis.31 In addition, ORAI channels contributed to MRGPRX2-mediated mast cell activation and the inactivation of mast cells might provide a novel treatment for neuropeptide substance P-induced atopic dermatitis.13 However, these two studies lack in vivo experiment validation. Other publications related with the roles of MRGPRX2-mediated mast cells in atopic dermatitis are mainly reviews.10,32 In this study, we first found that Celastrol remarkably inhibited the number of mast cells and histamine release, which might be the regulatory mechanism of Celastrol to modulate scratching. However, the treatment with MRGPRX2OE greatly reversed the influence of Celastrol, suggesting that Celastrol might regulate mast cell
production and histamine release through targeting MRGPRX2.
It was reported that ORAI channel was involved in MRGPRX2-regulated mast cell activation, and inhibition of it might be the potential treatment method for MRGPRX2-mediated disorders.13 Meanwhile, Tribuli Fructus extract could suppress skin inflammation in atopic dermatitis mice through regulating calcium channels and mast cell activation by targeting ORAI-1.13,33 In this study, we found that the protein expression levels of MRGPRX2, ORAI-1, and ORAI-2 in the skin tissues were greatly suppressed by Celastrol, but the decrease of MRGPRX2/ORAI was promoted by MRGPRX2OE.
There are several limitations in this research. First, the influence of Celastrol on atopic dermatitis through the MRGPRX2/ORAI axis needs to be validated with human atopic dermatitis tissues. Second, further targeting signaling pathway needs to be further explored.
Third, the specific functioning microRNA needs to be studied.
In the present study, we demonstrated that Celastrol could greatly inhibit atopic dermatitis-related tissue injury, mast cell production, histamine release, scratching level, inflammatory factor expression, and activation of the MRGPRX2/ORAI axis in the DNFB-induced atopic dermatitis model. However, the regulatory role of Celastrol was remarkably reversed by overexpression of MRGPRX2, indicating that Celastrol might regulate atopic dermatitis through targeting MRGPRX2.
The datasets used in the current study are available from the corresponding author on reasonable request.
The study has been approved by the Ethics Committee of Fujian University of
 Figure 5: Gene differential expression analysis was performed. (a) Gene expression profiling was performed. (b) Gene expression heat map was analyzed. (c) MicroRNA array was performed.
Figure 5: Gene differential expression analysis was performed. (a) Gene expression profiling was performed. (b) Gene expression heat map was analyzed. (c) MicroRNA array was performed.
Traditional Chinese Medicine.

The authors declare that they have no competing interests.
CY and MC conceived and designed the experiments; CY, WY, and MC performed the experiments; MC wrote the paper. All authors read and approved the final manuscript. All authors agree with the publication of this manuscript.
This study was supported by the Fuzhou Science and Technology Project (2020-WS-53), the Scientific Foundation of Fujian Provincial Health Commission (2020QNA076), the Natural Science Foundation of Fujian Province (2022J011327), and the Fuzhou Traditional Chinese Medicine Hospital InHouse Project (2020-Ynky-01).
1. Oh JS, Seong GS, Kim YD, Choung SY: Deacetylasperulosidic acid ameliorates pruritus, immune imbalance, and skin barrier dysfunction in 2,4dinitrochlorobenzene-induced atopic dermatitis NC/Nga mice. International Journal of Molecular Sciences 2021; 23(1):226.
2. Yang TH, Chen PC, Lin YC, et al: Adolescents with atopic dermatitis have lower peak exercise load capacity and exercise volume compared with unaffected peers. International Journal of Environmental Research and Public Health 2022; 19(16).
3. Han RT, Kim HY, Ryu H, et al: Glyoxal-induced exacerbation of pruritus and dermatitis is associated with staphylococcus aureus colonization in the skin of a rat model of atopic dermatitis. Journal of Dermatological Science 2018; 90(3):276–283.
4. Elosua-Gonzalez M, Rosell-Diaz A, Alfageme-Roldan F, Siguenza-Sanz M, Roustan-Gullon G: Clinical remission of disseminated molluscum contagiosum infection in a patient with atopic dermatitis treated with dupilumab. Anais Brasileiros de Dermatologia
2022; 97(3):358–361.
5. Kahremany S, Hofmann L, Harari M, Gruzman A, Cohen G: Pruritus in psoriasis and atopic dermatitis: current treatments and new perspectives. Pharmacological Reports 2021; 73(2):443–453.
6. Fujii M: Current understanding of pathophysiological mechanisms of atopic dermatitis: interactions among skin barrier dysfunction, immune abnormalities and pruritus. Biological & Pharmaceutical Bulletin 2020; 43(1):12–19.
7. Lv WJ, Huang JY, Li SP, et al: Portulaca oleracea L. extracts alleviate 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. Frontiers in Nutrition 2022; 9:article 986943.
8. Shin N, Jung N, Lee SE, et al: Pimecrolimus interferes the therapeutic efficacy of human mesenchymal stem cells in atopic dermatitis by regulating NFAT-COX2 signaling. Stem Cell Research & Therapy 2021; 12(1):482.
9. Poto R, Quinti I, Marone G, et al: IgG autoantibodies against IgE from atopic dermatitis can induce the release of cytokines and proinflammatory mediators from basophils and mast cells. Frontiers in Immunology 2022; 13:article 880412.
10. Wang Z, Babina M: MRGPRX2 signals its importance in cutaneous mast cell biology: does MRGPRX2 connect mast cells and atopic dermatitis? Experimental Dermatology 2020; 29(11):1104–1111.
11. Zhang F, Hong F, Wang L, Fu R, Qi J, Yu B: MrgprX2 regulates mast cell degranulation through PI3K/AKT and PLCγ signaling in pseudo-allergic reactions. International Immunopharmacology 2022; 102:article 108389.
12. Fernandopulle NA, Zhang SS, Soeding PF, Mackay GA: MRGPRX2 activation in mast cells by neuromuscular blocking agents and other agonists: modulation by sugammadex. Clinical and Experimental Allergy 2021; 51(5):685–695.
13. Chaki S, Alkanfari I, Roy S, et al: Inhibition of Orai channel function regulates mas-related G protein-coupled receptor-mediated responses in mast cells. Frontiers in Immunology 2021; 12: article 803335.
14. Lee JK, Seok JK, Cho I, et al: Topical application of celastrol alleviates atopic dermatitis symptoms mediated
through the regulation of thymic stromal lymphopoietin and group 2 innate lymphoid cells. Journal of Toxicology and Environmental Health, Part A 2021; 84(22):922–931.
15. Kim Y, Kim K, Lee H, et al: Celastrol binds to ERK and inhibits FcεRI signaling to exert an anti-allergic effect. European Journal of Pharmacology 2009; 612(1-3):131–142.
16. Tominaga M, Takamori K: Peripheral itch sensitization in atopic dermatitis. Allergology International 2022; 71(3):265–277.
17. Chen WY, Chen SC, Hsu SY, et al: Annoying psoriasis and atopic dermatitis: a narrative review. International Journal of Molecular Sciences 2022; 23(9).
18. Yao TC, Wang IJ, Sun HL, et al: Taiwan guidelines for the diagnosis and management of pediatric atopic dermatitis: consensus statement of the Taiwan Academy of Pediatric Allergy, Asthma and Immunology. Journal of Microbiology, Immunology, and Infection 2022; 55(4):561–572.
19. Jeong NH, Lee S, Choi YA, Song KS, Kim SH: Inhibitory effects of euscaphic acid in the atopic dermatitis model by reducing skin inflammation and intense pruritus. Inflammation 2022; 45(4):1680–1691.
20. Ham B, Kim M, Son YJ, et al: Inhibitory effects of Pterocarpus santalinus extract against IgE/antigen-sensitized mast cells and atopic dermatitis-like skin lesions. Planta Medica 2019; 85(7):599–607.
21. Jeong NH, Lee S, Choi JK, et al: Polyozellin alleviates atopic dermatitislike inflammatory and pruritic responses in activated keratinocytes and mast cells. Biomedicine & Pharmacotherapy 2020; 122:article 109743.
22. Li G, Wu H, Sun L, et al: (-)-α-Bisabolol alleviates atopic dermatitis by inhibiting MAPK and NF-κB signaling in mast cell. Molecules 2022; 27(13).
23. Yang H, Chen JS, Zou WJ, et al: Vitamin A deficiency exacerbates extrinsic atopic dermatitis development by potentiating type 2 helper T celltype inflammation and mast cell activation. Clinical and Experimental Allergy 2020; 50(8):942–953.
24. Zeng HR, Zhao B, Rui X, et al: A TCM formula VYAC ameliorates DNCB-induced atopic dermatitis via blocking mast cell degranulation and suppressing NF- κB pathway. Journal of
Ethnopharmacology 2021; 280:article 114454.
25. Cho BO, Che DN, Yin HH, Shin JY, Jang SI: Diospyros lotus leaf and grapefruit stem extract synergistically ameliorate atopic dermatitis-like skin lesion in mice by suppressing infiltration of mast cells in skin lesions. Biomedicine & Pharmacotherapy 2017; 89:819–826.
26. Schumann J, Basiouni S, Guck T, Fuhrmann H: Treating canine atopic dermatitis with unsaturated fatty acids: the role of mast cells and potential mechanisms of action. Journal of Animal Physiology and Animal Nutrition 2014; 98(6):1013–1020.
27. Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M: Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. The Journal of Allergy and Clinical Immunology 2015; 136(2):351–359.e1.
28. Kang JA, Song HY, Byun EH, et al: Gamma-irradiated black ginseng extract inhibits mast cell degranulation and suppresses atopic dermatitislike skin lesions in mice. Food and Chemical Toxicology 2018; 111:133–143.
29. Kang H, Kim S, Lee KH, et al: 5 nm silver nanoparticles amplify clinical features of atopic dermatitis in mice by activating mast cells. Small 2017; 13(9).
30. Luo X, Chen J, Yang H, et al: Dendritic cell immunoreceptor drives atopic dermatitis by modulating oxidized CaMKII-involved mast cell activation. JCI Insight, 2022; 7(5).
31. Butuci ZBM, Wong A, Schanin J, Xu A, Kamboj A, Youngblood B: Mast cells are locally activated and respond to MRGPRX2 stimulation in atopic dermatitis ex vivo skin biopsies. The Journal of Allergy and Clinical Immunology 2022; 149(2):AB5.
32. Azimi E, Reddy VB, Lerner EA: Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch, 2017; 2(1):article e5.
33. Kang SY, Jung HW, Nam JH, et al: Effects of the fruit extract of Tribulus terrestris on skin inflammation in mice with oxazolone-induced atopic dermatitis through regulation of calcium channels, Orai-1 and TRPV3, and mast cell activation. Evidencebased Complementary and Alternative Medicine 2017; 2017:Article ID 8312946, 12 pages.
The Chronicle of Skin & Allergy, in partnership with the Dermatology Industry Taskforce on Inclusion, Diversity and Equity (DiTiDE), sponsored a short essay competition for Canadian dermatology residents in 2022.

This contest was open to any resident enrolled in a dermatology training program at a Canadian medical school, with enrolment confirmed by their Program Director. Entrants submitted a 350- to 500-word composition reflecting on matters relating to inclusion, diversity, and equity. Eight winners were announced.
The selected essays will be published in each issue of The Chronicle during 2023.
Dr. Saima Ali of The Univeristy of British Columbia was recognized for her essay “Rebuilding the confidence of Skin of Colour patients.”
DiTiDE is a volunteer working group composed of Canadian life sciences managers and executives, dermatologists, patient association leaders, and allied parties committed to improving the patient experience and outcomes of under-represented skin types and underserved ethnicities through developing physician education and resources.
The 2nd annual short-essay competition for Canadian dermatology residents is now open for entries. Details are available at http://derm.city/for-residents.
THE PARADOX OF A SKIN DISORDER lies in how its clarity of presentation rarely represents its complex influence on a patient’s sense of self. Every day, I see people of colour who not only suffer from physical skin symptoms, but also the emotional burden of feeling ashamed of their skin. Being able to provide a patient with a clear diagnosis and management plan has a powerful impact on their self-esteem, confidence, and overall quality of life. However, when patients are fearful of misdiagnosis, we, as physicians, are failing our patients and the trust they have in us.
I took a non-traditional path to dermatology, initially practicing as a family physician for two years before re-entering into UBC Dermatology residency in 2021. This experience allowed me to learn from a diverse population of patients and gain an understanding of the barriers patients face throughout their lives. Throughout my medical career, I have trained and worked in cities in British Columbia with large populations of immigrants. My community family clinic was unique in that the majority of my practice was composed of patients who were South Asian, Black, and Hispanic. Many of these individuals presented with fears and frustrations, tired of discussing their skin concerns because they felt that they were not heard or represented. These patients motivated me to re-enter residency in dermatology, and work on bridging the gap in their care.
Being a woman of South Asian descent, I grew up hearing my family and friends sharing their struggles with their skin, from undiagnosed eczematous dermatitis to post-inflammatory hyperpigmentation from widely available procedures. My culture also emphasized the use of lightening products with unsafe ingredients and spread misinformation regarding the need for sunscreen. I believe that stories of unsuccessful treatments and a lack of understanding of cultural practices also perpetuated the fear of seeking help.
It is time that the field of dermatology exhibit true inclusion and diversity. This goal can be pursued in two broad manners. First, skin of colour training from medical school through to residency should be emphasized with didactic teaching and clinical skills equally focusing on signs and symptoms in skin of colour. Just as important is ensuring that future physicians appreciate and understand differences in ethnic practices and are trained in cultural competency. I envision clinics where patients feel safe sharing their concerns with the knowledge that their symptoms will not be dismissed and the treatment approach will be individualized to their practices.
Secondly, education is an important tool not only for patient care, but also for coloured communities as a whole to dismantle misinformation regarding skin practices. Ideally, this type of patient space intertwined with community interventions would manifest as a Skin of Colour Clinic in every major city across Canada. As part of these clinics, research dedicated to disease entities affecting those with skin of colour could also be pursued.
My hope is that with these directed initiatives, we can rebuild the confidence our skin of colour patients has in the dermatology community.
Dr. Saima Ali is a fourth-year dermatology resident at the Unviersity of British Columbia (UBC). She is the Co-Chief of the UBC Dermatology Residency Program and the Co-Chair of the Canadian Dermatology Association Residents and Fellows Society .

PSORIASIS THERAPY SWITCHING PATTERNS ASSESSED
This retrospective cohort study used IBM (now Merative) MarketScan Commercial and Medicare claims (Jan. 1, 2006-Dec. 31, 2019) to evaluate patterns of switching, discontinuation, and nonswitching in two cohorts (oral, n=11,993; biologic, n=9,753) of psoriasis patients initiating systemic therapy. Total pre-switch and post-switch costs were reported per-patient per-month (PPPM).
Among the oral and biologic cohorts, 32% and 15% discontinued index and any systemic treatment within one year of initiation; 40% and 62% remained on index therapy; and 28% and 23% switched treatment, respectively. In the oral and biologic cohorts, total PPPM costs within one year of initiation for nonswitchers, discontinuers, and switchers were US$2,594, $1,402, and $3,956, respectively, and $5,035, $3,112, and $5,833, respectively.
Overall, there was lower persistence with oral treatment, higher costs associated with switching, and a need for safe and effective oral options to delay the switch to biologic therapy.
S Thai, J Zhuo, Y Zhong, Q Xia, X Chen, Y Bao, D Dhanda, L Priya, JJ Wu: Real-world treatment patterns and healthcare costs in patients with psoriasis taking systemic oral or biologic therapies, in The Journal of Dermatological Treatment (Feb. 16, 2023;1-37).
STUDY LOOKS AT IL -2 FOR CSU, AFTER FAILURE OF ANTIHISTAMINES
This retrospective study included 15 chronic spontaneous urticaria patients who received treatment with at least one cycle of IL-2, injected intramuscularly at a dose of 1.0 million IU daily for seven consecutive days, after failing treatment with H1-antihistamines. Patients were followed up for ≥ 12 weeks
Of the participants, seven (46.7%) and 11 (73.3%) achieved complete response at Week 2 and Week 12, respectively. The mean change of urticaria control test (UCT) and weekly urticaria activity score (UAS7) from baseline was 6.6 (95% CI, 4.2 to 8.9) and -16.9 (95% CI, -24.0 to -9.8), respectively, at Week 12. Local injection-site reactions were the most common adverse events. No serious adverse events were reported.
J Wang, L He, W Yi, Q Liang, L Jiang, Y Tan, G Zhang, Y Su, R Xiao, Q Lu, H Long: Consecutive injections of low-dose interleukin-2 improve symptoms and disease control in patients with chronic spontaneous urticaria, in Clinical Immunology (Feb. 2023; 247:109247).
Throughout the years, humans have smeared all sorts of things on their skin in the name of beauty: scorpion venom, snail mucin, mud, etc. Lately, a new trend has emerged on TikTok. According to an article published by the HuffPost (Jan. 10, 2023), period blood face masks, also known as menstrual masking, have become popular among some TikTok users who say that the practice has improved their skin and even treated their acne. Many dermatologists, such as Beverly Hills, Calif. dermatologist Dr. Ava Shamban, are not convinced of the mask’s reported benefits. “I think it is popularized by those who would dare to do anything in the name of beauty for social media— seeking glam for the ’gram regardless of the dermal disaster,” she said.
An article published in Insider (Feb. 23, 2023) listed seven dermatologistapproved products that can help soothe rosacea symptoms and improve the appearance of the skin. Dr. Jeremy Brauer, a dermatologic surgeon, recommends La Roche-Posay Toleriane Ultra-Sensitive Skin Face Moisturizer because it has ceramides, which strengthen the skin barrier and improve hydration. Dr. Anna Chacon, recommends Vanicream Moisturizing Cream because it contains hyaluronic acid and squalane, which help skin cells retain moisture. Other recommended products are Cetaphil Redness Relieving Moisturizer, Bioderma’s Sensibio AR Cream, Skinbetter’s Trio Rebalancing Moisture Treatment and the Avène Tolerance Control Soothing Skin Recovery Cream. The dermatologists also advise rosacea patients to avoid products that contain alcohol, parabens, retinoids, urea, fragrances, menthol, camphor, sodium lauryl sulfate, witch hazel, peppermint or eucalyptus oil, and salicylic, glycolic and lactic acids.
A . Seborrheic keratosis
B. Bowen's disease
C. Actinic keratosis
D. Basal cell carcinoma
THE EDITORS invite your participation in this regular feature of the journal. Please send all images and correspondence to: Medical Editor, The Chronicle of Skin & Allergy 555 Burnhamthorpe Road, Suite 306, Toronto, Ont. M9C 2Y3.
Telephone: (416) 916-2476
E-mail: health@chronicle.org
According to an article published on Allure (Feb. 23, 2023), ferulic acid products are underrated. The authors write that the antioxidant has been demonstrated to make other common ingredients work better, faster, and more efficiently. The publications consulted Dr. Hadley King, a New York City-based dermatologist, about the benefits of ferulic acid. Dr. King said that one of the main benefits of ferulic acid is its ability to stabilize certain ingredients, such as vitamins C and E, making them last longer without losing potency. She added that vitamin C, for example, is not stable on its own, so both ingredients have a synergistic effect for top performance. Dr. King also said all skin types can use ferulic acid and that its effects might be especially great for users dealing with dullness, dark circles, and hyperpigmentation.
An article published in Marie Claire (Feb. 25, 2023) discusses minocycline and how it can be used to treat acne. Minocycline, an antibiotic, is sometimes prescribed to control P. acnes, a bacteria that contributes to acne. While an effective medication, many dermatologists say minocycline should not be a firstline treatment. New York-based dermatologist Dr. Shari Marchbein even calls it one of their least favourite oral antibiotics and says she never prescribes it. She adds that it can contribute to development of antibiotic resistance, as well as unpleasant side effects. Minocycline is less favoured among dermatologists than other treatments such as isotretinoin or AviClear laser therapy
Photo courtesy of Wikimedia CommonsWhat THE LAY PRESS is saying about . . . Dermatology news your patients may be reading
FOR THE NINTH ANNUAL
The Canadian Conference on Ethnodermatology
OCTOBER 21, 2023
VISIT WWW.SKINSPECTRUM.CA TO REGISTER
USE THE CODE S+A2023 FOR 50% OFF THE REGISTRATION FEE





2% salicylic acid + 10% glycolic acid + witch hazel
2% salicylic acid + witch hazel
+ zinc PCA
2% glycolic acid
+ 1% salicylic acid
+ zinc PCA
+ fragmented hyaluronic acid