



































MEANINGFUL CHANGE TAKES REAL EFFORT. With deep roots in immunology and a science-first approach, Incyte is tirelessly innovating and actively listening to you as change-makers to help you positively impact the lives of patients, one Dermatology solution at a time.





O p t i o n s f o r
m a n a g i n g
nThe darker the skin colour, the more potential for pigmentation changes following liquid nitrogen or laser therapy
by LOUISE GAGNON, Correspondent The Chronicle
The use of calcipotriol combined with 5-fluorouracil and the availability of tirbanibulin to treat actinic keratoses (AKs) on smaller and potentially larger surfaces of the skin are advances in the management of AKs. For locally advanced basal cell carcinomas (BCCs), the availability of another therapy offers an alternative to the hedgehog inhibitor vismodegib.
“It [calcipotriol] makes the 5-fluorouracil more effective,” explained Dr. Mark Lupin, a dermatologist in Victoria, B.C., Director and Founder of Cosmedica, and clinical instructor in the Department of Dermatology
and Skin Science in the Faculty of Medicine at the University of British Columbia in Victoria. “It has been proven with time that this is a reasonable regimen. Patients can comply with twice a day treatment for four days. It’s working quite well.”
The 5-fluorouracil monotherapy that lasts two or three weeks is the standard against which other treatments for AKs are compared with regards to efficacy, but it’s a challenge for patients to adhere to a regimen that lasts half a month or more, pointed out Dr. Lupin.
“It hard to do that standard treat-

Dr. Mark Lupin

Dr. Ilya Shoimer

Dr. Michael Stevens
ment of two or three weeks with 5fluorouracil [monotherapy],” said Dr. Lupin. “It seems quite effective to have only a four-day course of combination treatment, and it is more convenient for patients.”
Still another treatment option for AKs is the topical agent tirbanibulin, which initially received approval for field treatment up to 25 cm 2 in the U.S. and Canada. The field treatment was recently expanded to about 100 cm2 by the U.S. Food and Drug Administration, noted Dr. Ilya Shoimer, a dermatologist, Mohs micrographic surgeon, Clinical Assistant Professor, University of Calgary, Calgary, and VicePresident, Canadian Society for Dermatologic Surgery.
“I think the advantages [of tirbanibulin ointment] are the shorter duration and the less brisk inflammatory reaction,” said Dr. Shoimer, noting it is a therapy that would be expected to result in very good patient adherence. “It has a shorter course of treatment [than 5-fluorouracil]. You can treat a larger area, and there is less

2% salicylic acid + 10% glycolic acid + witch hazel


2% salicylic acid + witch hazel + zinc PCA


2% glycolic acid + 1% salicylic acid + zinc PCA + fragmented hyaluronic acid
Continued from page 4
inflammation caused by it.”
Specific populations and AK risk
Treatment choices need to take into account skin complexion, noted Dr. Michael Stevens, a dermatologist, Mohs micrographic surgeon, and Assistant Professor of Dermatology at Dalhousie University in Halifax.
“The darker the skin colour, the more potential for colour change after things like liquid nitrogen or laser therapy,” said Dr. Stevens. “It would just be something to discuss with the patient in terms of side effects. The biggest issue would be that some of the therapies have the potential to cause post-inflammatory hyper- or hypo-pigmentation.”
Transplant recipients are recognized as hav-
“
have undergone an organ transplant, noted Dr. Shoimer.
“Organ transplant recipients, if they have skin of colour, are going to be at higher risk for skin cancer and AKs, compared to individuals who have skin of colour who have not undergone an organ transplant,” said Dr. Shoimer.
Importance of field treatment for AKs
Clinicians agree that field treatment is an imperative in the management of AKs because of two important points: There is actinic damage that is not visible on the skin and because of the risk of AK recurrence.
“Actinic keratosis is a chronic disease,” said Dr. Lupin. “If you are thinking about actinic keratoses, there is a field effect.”
It is generally insufficient to treat AKs as solitary lesions in sun-damaged skin, agreed Dr.
T he darker the skin colour, the more potential for colour change after things like liquid nitrogen or laser therapy, Dr. Michael Stevens
ing an elevated risk of developing AKs because of their immunosuppressed status, which is why clinicians agree vigilance is required. “I would monitor [transplant recipients] more frequently,” said Dr. Stevens.
Even though patients with skin of colour are not regarded as high risk in terms of developing AKs, their risk for developing AKs increases if they
Shoimer.
“The idea is that for every AK that you can see, there are about five subclinical ones in the background that you may not see,” said Dr. Shoimer. “The importance of field treatment would be for patients who have extensive actinic damage or extensive sun damage. If there are really thick, crusted lesions, we have to curette
Continued from page 3
200 to 300%. Although not previously studied I am seeing significant increases in the rates of SCC as well as occurrence with more aggressive lesions. This article could not be more timely.
We also feature an article on the use of dermoscopy in challenging melanomas (see page 1). While I was attending the Spring Derm Update sessions in Vancouver, Drs. Joël Claveau and Max Sauder presented outstanding work when it comes to the management of melanoma and SCC. Joël has been a pioneer in dermoscopy and has trained many of us in that area. Our colleague Dr. Chris Sladden was pleased to hear I am a proud owner of a dermatoscope and actually use it.
On the pediatric front Dr. Jerome Coulombe educates us on the usefulness of ultrasound in the diagnosis of pediatric lesions (see page 1). We now see ultrasound being used in multiple
areas of dermatology including HS.
When it comes to the management of scalp disorders Dr. Renee A. Beach provides information on the management of scarring and nonscarring scalp inflammatory disorders, which is certainly a significant challenge in all of our practices. Thank you, Dr. Beach. Finally, I bring your attention to the second feature in our ongoing retrospective of 30 at 30, marking this publication’s 30th year of continuous publication. This instalment highlights the importance of devices including lasers, the ongoing issues of PRP as a treatment looking for a disease, the advent of genodermatology, all brought to you by the team THE CHRONICLE OF SKIN & ALLERGY.
As always, THE CHRONICLE team invites and welcomes your comments on this issue, or any other topic in dermatology, at www.derm.city
—Wayne P. Gulliver, MD, FRCPC, Medical Editor
those to help with the efficacy of the topical field therapies. Otherwise, the field therapy is not going to penetrate.”
Managing basal cell carcinomas
Surgery is the mainstay in the management of BCCs, but other treatment modalities may need to be selected depending on the location of a BCC, noted Dr. Lupin.
“Most primary surgeries are curative options,” said Dr. Lupin. “There is less chance of recurrence, but it may be tumour factors like location and size or patient factors like age that might dictate the direction [of treatment].”
One of the possible other modalities includes photodynamic therapy (PDT), noted Dr. Lupin.
“If we are using PDT to treat a large area like the face [for AKs], and there happens to be a small, superficial basal cell, you can use PDT [to treat it].”
The hedgehog inhibitor vismodegib has represented an advance in the treatment of BCCs that are locally advanced and/or in locations that make the lesion inoperable. There has been a limitation in the use of vismodegib, however, in that it can produce undesired side effects, making the availability of the checkpoint inhibitor cemiplimab beneficial, noted Dr. Shoimer
“It is nice to have another tool in your toolbox,” said Dr. Shoimer. “Vismodegib is often challenging to tolerate for some patients. They may experience side effects like hair loss or muscle cramps, and it is a medication that is hard to be on continuously. Usually, patients either have to take breaks [from vismodegib], or they need alternative dosing regimens of vismodegib. Cemiplimab would be an alternative to vismodegib, so it is a second-line option for locally advanced disease.”
Non-proprietary and brand names of therapies: calcipotriol combined with 5-fluorouracil (no branded products); tirbanibulin 1% ointment (ONAKTA, AVIR Pharma Inc.); vismodegib (ERIVEDGE, Roche); cemiplimab (Libtayo, Sanofi).

Ronald B. Vender, MD, FRCPC comments on recent findings and developments, and answers clinicians’ questions
This retrospective cohort study, conducted in Greece, analyzed real-world switching patterns and associated characteristics in patients with psoriasis treated with biologics (Archives of Dermatological Research 2025; 317:310 https://doi.org/10.1007/s00403-024-03746-y). The study utilized data from the Greek digital prescription database, including 6,772 biologic-naïve patients who initiated treatment between Jan. 1, 2016, and Dec. 31, 2020.
The results revealed several key findings regarding switching rates and patterns. The overall switching rate from first to second biologic treatment was 12.4% (Scenario 1) and 13.4% (Scenario 2). Anti-TNFα agents demonstrated higher switching frequencies compared to interleukin-targeting antibodies (20.9% vs. 9.8%). Notably, interclass switching from anti-TNFα to anti-interleukin agents was 3.3 times higher than the opposite transition. Among the specific drugs, brodalumab, ustekinumab, and secukinumab showed the lowest switching rates, while infliximab had the highest at 44%. Interestingly, ustekinumab and secukinumab had higher re-initiation rates than switches.
The study also examined the time to switch or re-initiation. The median time from first to second line was 43.3 months. Patients receiving brodalumab and certolizumab pegol did not reach median time to switch, while among those reaching median time, secukinumab and ustekinumab had the highest.
Several factors were associated with switching patterns. Brodalumab, secukinumab, and ustekinumab were associated with a lower risk of switching compared to adalimumab. Concomitant psoriatic arthritis (PsA) and female gender were associated with a higher risk of switching.
The study employed two scenarios in its methodology. Scenario 1 considered both treatment switches and re-initiations after a 90-day gap, while Scenario 2 focused solely on switches to different biologics. Statistical analyses included Kaplan-Meier curves, cumulative incidence functions, and Cox regression models.
These findings have several implications for clinical practice. The significantly reduced switching risk of interleukin-targeting antibodies compared to TNFα agents suggests better treatment persistence. The higher switching rates from anti-TNFα to interleukin-targeting treatments indicate a potential superiority of newer biologics in maintaining treatment response. The study also highlights the importance of considering patient characteristics, such as gender and presence of PsA, when selecting and managing biologic treatments for psoriasis. These insights provide valuable guidance for clinicians in selecting appropriate biologic agents and managing psoriasis treatment in real-world settings.
However, the study lacks clinical details on reasons for treatment switching or re-initiation, preventing conclusions on efficacy or safety as potential drivers of therapy modifications. The inability to assess the impact of evolving comorbidities or lifestyle changes on switching patterns is another limitation. Due to its retrospective nature, the study may have potential selection bias. Additionally, the study is limited to a specific time period, which may not capture long-term trends in biologic use.
This retrospective cohort study contributes valuable real-world data on biologic therapy persistence and switching patterns in psoriasis. The lower switching rates for IL-17 and IL-23 inhibitors compared to TNF-α inhibitors reinforce their superior long-term efficacy. The higher likelihood of switching from TNF-α to interleukin-targeting agents aligns with current clinical practice favouring newer biologics. The study is limited by the absence of clinical reasons for switching, preventing conclusions about efficacy versus safety concerns. However, the reason for switch is often primary or secondary loss of response. Despite these limitations, the findings offer practical insights into biologic treatment optimization and patient management in dermatology practice applicable to Canadian derm practices.
This paper describes the development of two measurement tools designed to assess Cumulative Life Course Impairment (CLCI) in patients with chronic skin diseases (J Eur Acad Dermatol Venereol wileyonlinelibrary.com/journal/jdv 2023;37:1626–1633). CLCI refers to the non-reversible damage caused by persistent disease burden over time, influencing patients' life trajectories through psychological, social, and emotional impairments. The study aimed to create instruments that measure persisting CLCI retrospectively and identify patients at risk for future CLCI.
The development process involved multiple stages, including a systematic literature review, analysis of patient registry data, open item selection, focus group interviews, item conception, cognitive debriefing, and finalization. The open item selection involved 162 patients with psoriasis, atopic dermatitis, or hidradenitis suppurativa, who identified key topics impacting their life course. Focus group interviews with 19 patients
and 13 experts further refined these topics and highlighted additional areas of concern.
Two measurement tools were developed: DermCLCI-r and DermCLCI-p. DermCLCI-r consists of 30 items assessing retrospective impairments due to skin disease over the disease life course, using 4-point and 5-point Likert scales. It also includes questions about ongoing burden and life-changing impact. DermCLCI-p, also comprising 30 items, evaluates current CLCI status and future risk within the last two weeks, primarily using a 4-point Likert scale.
The authors argue that these tools are more suitable and specific for dermatologic conditions compared to existing measures such as the Major Life Changing Decision Profile (MCLDP) and Life Change Index. They emphasize the importance of distinguishing between existing CLCI and the risk for future CLCI to provide appropriate support and interventions.
While the study presents a comprehensive approach to tool development, limitations include the focus on only three skin diseases and recruitment from a single centre in Germany. The authors acknowledge the need for further international studies and psychometric testing for validity and reliability. This project provides much-needed instruments to assess previous CLCI and contribute to the prevention of CLCI in patients with chronic skin diseases. The tools are expected to facilitate early identification of CLCI, enable adequate dermatological and psychosocial interventions, and potentially prevent CLCI. Future research plans include linguistic and cross-cultural validations for international use, as well as adaptations for additional skin diseases. These instruments represent an important step toward developing people-centered psychosocial interventions to prevent and reduce the risk of CLCI in patients' lives.
This study introduces DermCLCI-r and DermCLCI-p, two tools designed to assess Cumulative Life Course Impairment (CLCI) in chronic skin diseases. The strengths include a rigorous, multi-stage development process and diseasespecific focus, distinguishing it from broader psychosocial measures. The ability to assess both past and future CLCI enhances clinical relevance. However, limitations include its focus on only three diseases and single-centre recruitment, limiting generalizability. These tools provide a patientcentred approach to dermatologic care, enabling earlier psychosocial interventions. Future research should prioritize cross-cultural validation and adaptation for other skin conditions.
We invite your comments and questions about this feature at www.derm.city. Dr. Ron Vender is a certified dermatologist with more than 30 years of clinical practice experience and over 100 clinical trials in psoriasis. He is founder and director of Venderm Innovations in Psoriasis, a center of excellence for Psoriasis offering a comprehensive management solution for individuals with psoriasis. www.psoriasis.vip
H
ave a comment about the articles in this issue? Share your knowledge and opinions with peers worldwide.

Continued from page 1
Afro-textured hair, Dr. Renée A. Beach told delegates to the 10th annual Skin Spectrum Summit on Oct. 5 in Toronto. Treating folliculitis barbae involves a multi-pronged approach, while oral metformin has been shown to be effective in treating central centrifugal cicatricial alopecia.

Dr. Beach is a dermatologist practicing medical and cosmetic dermatology in Toronto. She is the founder of DermAtelier on Avenue in the Avenue-Lawrence area of the city. She is also the on-air dermatologist on the daytime talk show The Social.
Treating folliculitis barbae
Dr. Beach used the example of a male patient with Black skin who regularly developed papules, or razor bumps, from shaving his neck. The patient had fibrosis and dyspigmentation, and would likely end up with scarring. The condition is known as folliculitis barbae, and primarily affects men with Afro-textured hair. It can be caused by both exogenous factors, such as shaving which is often dictated by cultural norms or employer expectations, and endogenous factors, such as curvature of the hair follicle and kinky hair shafts. The patient develops inflammation of the hair follicle, which can progress to fibrosis, and may even lead to sycosis barbae, which is pustular and needs to be treated with antibiotics.
In Afro-textured hair, not only is the hair kinky, but the actual follicle is curved, said Dr. Beach. When hair grows from the follicle after it has been cut short, it may grow into the walls of the pilosebaceous unit.
Treatment of folliculitis barbae is multipronged, with the first step being addressing grooming, said Dr. Beach. She said she recommends that patients use a single-blade razor or a trimmer that is not set to the shortest level, and that they use short strokes in a single direction. She also recommended only shaving once per week, and, between shaving, washing with salicylic acid, or with alpha or beta hydroxy acid.
The next step is prescription therapy such as retinoids, said Dr. Beach. These help to shed keratinocytes and increase epidermal cell turnover. If patients are able to tolerate it, she said she recommends that patients initially use the therapy nightly. As the patients taper off, or in a maintenance phase, that may be reduced to three or four nights a week. Retinoids will also smooth papules and reduce hyperpigmentation. Dr. Beach said she prescribes tazarotene lotion for patients with public medication insurance or who are under 25 years old and use OHIP+.
For patients who require rescue therapy, Dr. Beach said she prescribes a combination of 5% benzoyl peroxide and 1% clindamycin two to three nights a week when needed. However, this treatment may cause irritation and may stain linens.
“ I n Afro-textured hair, not only is the hair kinky, but the actual follicle is curved.”
Procedural therapy, such as laser treatment, is the last prong of the treatment, said Dr. Beach. She said she uses an Nd:YAG 1,064 nm laser, and that treatments are every four to six weeks until clearance. Patients will require quarterly maintenance sessions, she cautioned.
Central centrifugal cicatricial alopecia
Dr. Beach said she has many female patients who say that their hair is breaking at the crown. This may present with thinning in the anterior or posterior vertex, or, in more advanced cases, with thinning in both the anterior and posterior vertices. These patients have central centrifugal cicatricial alopecia (CCCA), which occurs primarily in women with Afro-textured hair. Patients will ideally be treated early, before there is clinically significant hair loss which is difficult to conceal.
CCCA is a form of scarring hair loss which proceeds in a progressive, centrifugal pattern from a central point and may lead to fibrosis, said Dr. Beach. The condition may be asymptomatic, but patients may experience pain, burning, itch or tenderness. CCCA is not caused by styling, but glue-on extensions or wigs, or braids that are tight or kept in for too long may harm hair and affect hair retention.
The condition is associated with a protein defect, said Dr. Beach. Patients have an under-expression of the gene PRKAA2, which leads to a missense mutation, and downstream to a reduction in adenosine monophosphate kinase (AMPK).
There are multiple treatment options, said Dr. Beach. She said that for four or five years, she used 10% topical metformin once or twice a day to increase AMPK levels. But topical metformin did not produce outstanding clinical results. But she said that a recent study showed that oral metformin is a more effective and practical alternative. (Bao A et al. Low-Dose Metformin and Profibrotic Signature in Central Centrifugal Cicatricial Alopecia. JAMA Dermatol Nov. 1, 2024; 160(11):1211-1219. doi: 10.1001/jamadermatol.2024.3062. PMID: 39230880; PMCID: PMC11375521.)
The study showed that patients who took oral metformin 500 mg daily for at least six months, in addition to other treatments, showed a decrease in symptoms and one half of the patients showed signs of visible hair regrowth at six months, said Dr. Beach. While she said that patients may be reluctant to take an oral medication to treat a hair condition, she believes oral met-
Dr.
formin is the CCCA treatment that will be used going forward.
Dr. Beach said that she also provides secretosome therapy and microneedling in her office. Microneedling provides controlled injury to the scalp and boosts microcirculation, although it is not certain that microneedling treats fibrosis. Secretosomes increase growth factors. Microneedling alone has allowed some patients to improve their hairline. The microneedling then may be followed by secretosome therapy. Dr. Beach said that she uses secretosomes at a higher concentration as she believes that is more effective on the scalp. Patients are not allowed to rinse their scalp or to do anything that may sweat the secretosomes off the scalp. Patients receiving secretosome therapy have shown signs of clinically significant hair regrowth, she said.
Side-effects of secretosome therapy may include heaviness, headaches or soreness, said Dr. Beach. The procedure is costly, although the cost is coming down.
Bottom Line: Folliculitis barbae primarily affects men with Afro-textured hair, and can be caused by both exogenous factors, such as shaving, and endogenous factors, such as curvature of the hair follicle and kinky hair shafts. It is treated by first adjusting grooming practices such as shaving. The next step is prescribing topical retinoids, which help to shed keratinocytes and increase epidermal cell turnover, as well as smoothing papules and reducing hyperpigmentation. For rescue therapy, a combination of 5% benzoyl peroxide and 1% clindamycin can be used two to three nights a week when needed. The final step may be procedural therapy using a laser. Central centrifugal cicatricial alopecia (CCCA) occurs primarily in women with Afro-textured hair, and is a form of scarring hair loss which proceeds in a progressive, centrifugal pattern from a central point and may lead to fibrosis. CCCA is associated with a protein defect. Patients have an under-expression of the gene PRKAA2, which leads to a missense mutation, and downstream to a reduction in adenosine monophosphate kinase (AMPK). Oral metformin has been shown to be an effective treatment. Microneedling and secretosome therapy may also be used to treat CCCA.
Physicians are using tools such as confocal microscopy to delineate tumour boundaries more accurately before excision, reduce the unnecessary removal of healthy tissue while ensuring complete tumour clearance, and prioritize both oncologic control and patient quality of life (Indian Dermatol Online J Feb. 28, 2024; 15(2):278–292. doi: 10.4103/idoj.idoj_852_23, Cancers (Basel) Feb. 23, 2024; 16(5):895. doi: 10.3390/cancers16050895).
Based on tumour characteristics such as Breslow depth, updated guidelines on excision margins have improved oncologic outcomes and cosmetic results in wide surgical excision.
In some cases, such as advanced or anatomically challenging tumors, robotic systems are being explored to enhance precision and minimize morbidity, though this remains an emerging area (Cancer Surg 2024, 9:3).
Integrating reconstructive techniques, including adjacent tissue transfer, full-thickness and split-thickness grafts, into wide local excision procedures has become standard practice. Removing the delay between excision and reconstruction has improved functional and cosmetic outcomes for patients (Plast Reconstr Surg Glob Open Feb. 24, 2020;8(2):e2661. doi: 10.1097/GOX.0000000000002661).
Sentinel lymph node biopsy (SLNB) has revolutionized the management of melanoma and high-risk non-melanoma skin cancers by enabling targeted lymph node sampling. This technique improves staging accuracy and reduces the morbidity associated with more extensive lymph node dissections. The technique is associated with improved disease-free survival and is now a routine part of melanoma surgical management (Cancer Surg 2024, 9:3).
SLNB has also become a critical adjunct to surgical excision for staging melanoma. It allows precise identification of micrometastases in regional lymph nodes, guiding further treatment decisions. Advances in tracer technology, such as hybrid radioactive and fluorescent dyes, have further improved the accuracy of node detection during surgery. (Cancers (Basel) Feb. 23, 2024; 16(5):895.)

Platelet-rich plasma (PRP) has evolved from a niche therapeutic option to a versatile tool with diverse applications.
Through the 1990s, PRP emerged as a breakthrough for chronic wounds, leveraging growth factors such as platelet-derived and vascular endothelial growth factors to accelerate healing. Researchers have studied PRP in several kinds of hard-to-heal wounds, including diabetic ulcers, venous ulcers, and pressure ulcers. In some cases, time to healing has been reduced by up to 50% (J Am Acad Dermatol Sept. 2019; 81(3):834-846. doi: 10.1016/j.jaad.2019.04.037, Indian Dermatol. Online J. Sept.-Oct. 2020; 11(5):685-692 doi:10.4103/idoj.IDOJ_369_19).
Emerging applications of PRP
In the 2010s, PRP also began being used for hair restoration, skin rejuvenation and anti-aging treatments, scar and striae treatment, and supporting fat grafting—all addressing cosmetic factors that can significantly impact patient quality of life. (Cosmoderma June
2021; 1:12 doi: 10.25259/CSDM_7_2021). PRP has been shown to significantly improve skin texture, firmness, elasticity, and reduce wrinkles and pigmentation. Objective measurements demonstrate increased collagen density, dermal and epidermal thickness, and decreased transepidermal water loss after PRP therapy (Cureus Aug. 12, 2024; 16(8):e66734, Skin Res Technol April 22, 2024; 30(4):e13714, Exp Ther Med 2020; 19(4):3024-3030).
PRP injections have been shown to stimulate hair regrowth in androgenetic alopecia and other hair loss conditions. Studies report mean increases of 18 to 27.7 hairs/cm² in treated areas, with improved hair density and thickness, especially when PRP is used prior to or after hair transplantation (Cureus Aug. 12, 2024, J Cosmet Dermatol July 14, 2021; 20(9):2712–2714).
Most recently, researchers have explored combining non-cultured epidermal cell suspensions with PRP for repigmenting in vitiligo, achieving 70 to 80% repigmentation in stable cases (Int J Dermatol April 2019; 58(4):472-476).
However, significant heterogeneity remains in PRP preparation methods and treatment protocols, highlighting a need for standardization and large-scale trials (Cureus Aug. 12, 2024, EMJ Dermatol Nov. 17, 2023).
Gene-based dermatology has undergone transformative advancements over the past three decades, shifting from experimental concepts to clinically validated therapies that address the genetic roots of skin diseases. These innovations underscore a shift from palliative care to curative strategies, particularly for previously untreatable genetic disorders.
CRISPR-based gene editing has shown promise in correcting mutations causing epidermolysis bullosa (EB) and ichthyosis.
Gene-altered skin grafts are promising in EB, with studies finding grafts have persisted for years via stem cell repopulation with no genotoxicity (Hum Gene Ther Sept. 2023; 34(17-18):763-775. doi: 10.1089/hum.2023.152. Mol Ther June 26, 2023; 31(7):1860–1861. doi: 10.1016/j.ymthe.2023.06.002).For junctional EB, a 2017 study that described the transplantation of epidermal sheets with corrected LAMB3 genes—which code for the beta subunit of laminin 332, a protein vital for cell adhesion—to replace 80% of a patient’s skin. The researchers were able to achieve full wound closure and sustained collagen VII expression for more than five years (Nature Nov. 8, 2017; 551(7680):327–332).
In 2023, the U.S. FDA approved the first topical gene therapy for EB, beremagene geperpavec gel (B-Vec). A study of B-Vec showed 67% of wounds treated with B-Vec had complete healing at six months compared with 22% of those not treated with the therapy. (N Engl J Med Dec. 14, 2022; 387(24):1–9. doi:10.1056/NEJMoa2206663.) Since the gel is stable at room temperature, it represents an accessible treatment for clinics that may have fewer resources.
Systemic therapies are still in preclinical stages of research, but models have shown the use of adeno-associated viruses to deliver COL7A1—a gene that provides instructions for making a protein called pro-α1(VII) chain, which is part of type VII collagen—systemically have led to expression of type VII collagen in skin, liver, and lungs (Int J Mol Sci Feb. 13, 2024; 25(4):2243, Ther Clin Risk Manag June 14, 2023; 19:455-473).
Continued from page 1
Nodules or bumps or lumps that present in a pediatric patient can be a diagnostic challenge and generally fall into one of four categories, said Dr. Coulombe. They can be cysts, vascular lesions, inflammatory dermatologic presentations, or tumours that are benign or malignant. “The majority of them [lumps or bumps] will be benign,” said Dr. Coulombe.
Clinical clues for lumps and bumps, imaging, and excision
There are various characteristics that help clinicians determine if lumps or bumps that present in pediatric patients are benign or malignant, according to Dr. Coulombe.
Benign bumps and lumps tend to have a ruby colour like a hemangioma, may have a Darier sign that suggests a mast cell tumour, may have a “tent” appearance that signals a growth such as a dermatofibroma, may be lipomas, may be vascular malformations, may be boils, may be abscesses, or may be infections, noted Dr. Coulombe.
In contrast, symptoms and signs of borderline or malignant bumps or lumps include neonatal onset, rapid growth, pain, ulceration, and bleeding, size that exceeds more than three cm, and onset in an immunosuppressed patient, added Dr. Coulombe.
Following clinical evaluation, clinicians can categorize a bump or a lump as a benign entity,
should either have classic features that denote benign status, or as having concerning findings. If there are concerning findings, employing Doppler ultrasound is useful to characterize the bump or lump and determine if it is benign or possibly malignant, explained Dr. Coulombe, citing a published reference (Pediatric Dermatology 2022; 39:679-688).
If a bump or a lump cannot be characterized after using Doppler ultrasound, additional imaging such as magnetic resonance imaging or computed tomography may be necessary or a biopsy may be necessary. “It may have to be referred for surgery [excision],” said Dr. Coulombe.
Lumps and bumps on the neck and pilomatrixoma
The midline anterior neck is a location where lumps and bumps can occur in young pediatric patients, noted Dr. Coulombe. One novel lesion that was recently described was the midline anterior neck inclusion cyst, a congenital anomaly (Pediatr Dermatology 2018 Jan; 35(1):55-58).
“These are completely benign,” he said.
A type of calcified cyst, pilomatrixoma, which is hard as a rock to the touch, normally develops before puberty and its clinical appearance is dependent upon its depth in the skin, pointed out Dr. Coulombe.
While these cysts are benign, they may be surgically removed if they become ulcerated or for cosmetic improvement as they may appear in
prominent locations such as on the nose and on other parts of the face.
Granulomas (IFAG) and mastocytomas
Idiopathic facial aseptic granulomas are benign inflammatory nodules that can resolve on their own in several months (Annales de Dermatologie et Vénérologie 2023; 150:173-179).
“They can occur in preschoolers and in prepubescent children,” said Dr. Coulombe. “Parents may want to accelerate the management even though idiopathic facial aseptic granulomas will disappear over time.”
One of the management options are oral antibiotics for six to eight weeks, and surgery should be avoided, noted Dr. Coulombe.
Mastocytomas usually present early in childhood, have a yellowish appearance and they will resolve on their own and do not need treatment, pointed out Dr. Coulombe. “Ultrasound is not necessary as [mastocytomas] can be diagnosed clinically.”
Value of dermoscopy
Juvenile xanthogranuloma (JXG) is a rare skin presentation, appearing as a red-yellow papule that resolves on its own and can occur in patients up to 30 years of age (An Bras Dermatol 2018; 93(1):138-140).
“Dermoscopy is valuable in obtaining the diagnosis,” said Dr. Coulombe, noting dermoscopy is also useful in detecting JXG in patients with darker skin tones revelling the sunset sign.

Recent studies conducted by researchers at the Hospital for Special Surgery (HSS) in New York contribute to understanding the biological mechanisms underlying systemic sclerosis, or scleroderma. Their findings may help to explain why women are four times more likely to be diagnosed with scleroderma than men.
The research, published in the March issue of the Journal of Experimental Medicine, identifies genetic receptors TLR7 and TLR8 on the X chromosome as key drivers of the disease. These receptors activate plasmacytoid dendritic cells (pDCs), which contribute to chronic fibrosis. In healthy individuals, one X chromosome is typically inactivated, but in scleroderma patients, TLR7 and TLR8 evade this deactivation process in pDCs, leading to their overactivation.
“The magnitude of this escape was striking,” noted lead researcher Dr. Franck Barrat in a press release. In healthy individuals, about 10 to 15% of cells can evade X chromosome deactivation, but in scleroderma patients, this occurs in more than 35% of pDCs. Dr. Barrat noted that “The expression of two copies of the TLR7 and TLR8 in such a large number of cells can very well explain the chronic activation of these immune cells and why this disease is so prevalent in female patients.”
In a separate study, Dr. Barrat and colleagues investigated why inflammation fails to resolve in scleroderma patients. They identified the cytokine CXCL4 as a primary culprit, which prevents immune suppression and keeps pDCs in a state of chronic activation, promoting fibrosis. “We show that CXCL4 prevents the normal termination of the immune response in the skin,” Dr. Barrat said. “Basically, the pDCs are attracted by the fibrosis, but instead of being suppressed as they should be, CXCL4 keeps them active, in turn contributing to the cycle of fibrosis in these patients.”
These findings may also highlight potential therapeutic strategies. Dr. Barrat said several therapies targeting pDCs in clinical trials have shown promise in blocking pDCs and preventing skin lesions in patients with lupus.
“This body of research makes a very strong case for exploring drugs that target and interfere with pDCs. There are already drugs in development that we can try,” Dr. Barrat said.
Both studies were collaborative efforts involving researchers from HSS and international institutions.

S u r vey i n g
t h e c u r r e n t
Dermatologic Dermatologic
l i t e r a t u r e
RRecently come across something from the peer-review literature that you consider to be interesting or impactful? Share it with your colleagues. E-mail your clippings, along with your comments, to: health@chronicle.org
ESEARCHERS FOUND NO INEQUALITY IN ACCESS TO ADVANCED PSORIASIS THERAPIES OR CARE IN CANADA BASED ON PATIENTS’ drug insurance coverage. This finding came from a study published in the Journal of Health Economics and Outcomes Research (Feb. 7, 2025; 12(1):51-57).
The authors of this paper note that the specifics of a patient’s public or private insurance drug coverage may have a negative impact on access to treatment for patients with psoriasis covered by public plans compared with private plans.
To clarify these impacts, researchers compared (1) psoriasis severity scores when an advanced therapy was prescribed; (2) psoriasis severity scores at follow-up; (3) treatment response; and (4) delay between prescription and first dose of advanced therapy in patients with psoriasis treated with advanced therapy in public vs. private drug insurance groups.
Researchers included patients with psoriasis treated by advanced therapy prescribed by a dermatologist between Sept. 2015 and Aug. 2019, in a tertiary academic care center in Québec City, Canada. They collected data from medical records. They found patients treated with an advanced therapy for psoriasis covered under the provincial public drug insurance plan (n=78) and under a private drug plan (n=93) did not differ regarding the studied outcomes. Patients' characteristics differed between groups. Patients in the public group were older (p<0.0001), more socioeconomically deprived (p<0.05), and more likely to benefit from compassion from the industry to access a prescribed medication free of charge (p<0.0001) compared with patients from the privately insured group.
The investigators note the high prevalence of the use of compassionate programs from the industry in the public insurance group (42% vs. 14%), and the high prevalence of psoriasis on difficult-to-treat areas (face, genitalia, and/or palmoplantar areas) in the cohort (85.4%) may mask differences in access to advanced therapy between the two groups.
—For more information visit https://tinyurl.com/4wv6t4fx
THE FIRST STUDY TO ASSESS THE PUSH-D, PHQ-9 AND GAD-7 PATIENT-REPORTED OUTCOME SCORES IN PATIENTS WITH generalized pustular psoriasis (GPP) has identified a significant clinical burden and negative impact on their daily lives. Published in Orphanet Journal of Rare Diseases (Feb. 8, 2025; 20:61), this multicentre study assessed the demographics and impact on quality of life (QoL) of patients with GPP in France.
A questionnaire asking questions on demographics and QoL was sent to patients followed for GPP in 10 different dermatology departments in France. QoL was assessed using the Dermatology Life Quality Index (DLQI), Patient Unique Stigmatization Holistic tool in Dermatology (PUSH-D), EuroQol-5 Dimensions (EQ-5D) utilities, Patient Health Questionnaire (PHQ-9), and Generalized Anxiety Disorder 7 (GAD-7), as well as a Visual Analog Scale (VAS) for joint pain, skin pain, fatigue, and perceived stress.
Investigators recruited 41 patients, mostly women (n=25). The mean (standard deviation) age was 52.3 (15.8) years for women and 49.2 (10.4) for men, and 34% of patients were obese. The diagnosis of GPP was made at the hospital (by an emergency physician or dermatologist) in 87% of cases, in outpatient setting for the remaining 13%.
Survey data showed that GPP was more common in women, and the proportion of patients who were obese was twice the national average in France.
Patients with GPP experienced a significant clinical burden that negatively impacted their daily lives. The DLQI score showed that more than half of patients faced an important or very important impact on their QoL, reflecting a greater impact than plaque psoriasis. More than one-third of patients stated they had taken sick leaves with almost a month per leave.
“GPP and plaque psoriasis require distinct approaches to their management and treatment,” the authors write. “With the advent of new treatments for GPP, it would be interesting to evaluate the effect that new biologics may have on these elements of clinical burden.”
—For more information visit https://tinyurl.com/3rh83f9p
GIVEN THE CHRONIC NATURE OF VITILIGO AND ITS SLOW TREATMENT RESPONSE, TAILORED HEALTH EDUCATION STRATEGIES focusing on improving knowledge and attitudes are essential for standardizing treatment and enhancing patient adherence. This is the conclusion of a study published in Scientific Reports (Feb. 8, 2025; 15(1):4741).
The authors of the paper write that knowing the provoking factors of vitiligo progression and how to avoid them is essential to patients successfully self-managing vitiligo. Cultivating a positive attitude is also essential to avoid focusing on the negative aspects of the disease and to fuel proactive practice.
In this study, the investigators evaluate the knowledge, attitudes, and practices (KAP) of vitiligo patients and identify related factors.
They recruited 517 (54.55% male) adult vitiligo patients in Beijing and Nanjing. Participants’ mean knowledge, attitude, and practice scores were 5.45±2.74 (possible range: 0-10), 23.15±6.78 (possible range: 8-40), and 37.13±6.78 (possible range: 10-50), respectively. Mediation analysis indicated that knowledge (β=0.24, p=0.023), attitude (β=-0.28, p<0.001), disease duration (β=-1.49, p<0.001), and negative emotions (β=-1.54, p=0.008) had direct effects on practice. Income (β=-0.29, p=0.003), disease duration (β=0.16, p=0.033), lesion duration (β=0.25, p=0.003), education (β=0.11, p=0.024), and age (β=0.14, p=0.036) had indirect effects on practice. Vitiligo patients exhibited significant gaps in knowledge and attitudes toward their condition despite active management practices.
—For more information visit https://tinyurl.com/yc6hwhpc

Could residents adequately assess the severity of skin lesions in mycosis fungoides/Sézary syndrome? Evaluation of
Hanna Cisoń,1,* Alina Jankowska-Konsur,1 and Rafał Białynicki-Birula2
1University Centre of General Dermatology and Oncodermatology, Wroclaw Medical University, 50-556 Wroclaw, Poland
2Faculty of Medicine, Wroclaw University of Science and Technology, 50-370 Wroclaw, Poland
* Corresponding Author
Keywords: Mycosis fungoides/diagnostic imaging; Sezary syndrome/diagnostic imaging; ultrasonography
ABSTRACT
Background/Objectives: Cutaneous T-cell lymphoma (CTCL), including mycosis fungoides (MF) and Sézary syndrome (SS), is a challenging-to-diagnose lymphoproliferative malignancy characterized by T-cell dysfunction and progressive cutaneous and extra cutaneous involvement. Disease severity assessment in CTCL is crucial for guiding treatment. This study aims to evaluate the interrater agreement and interrater reliability of mSWAT among dermatology residents and identify lesion types most prone to scoring variability.
Methods: Sixteen dermatology residents with varied experience levels assessed 14 patients with confirmed MF/SS diagnoses. Using mSWAT, residents independently scored lesions severity on a standardized set of patient’s photos. The results were compared with reference mSWAT scores provided by an experienced clinician. Descriptive statistics and the Shapiro–Wilk test were applied to evaluate data distributions, while Student’s t-test assessed score deviations from reference values. Furthemore, we conducted a pilot of the high frequency ultrasound (HFUS) study on a single patient, whose mSWAT score and photographs are also presented in the manuscript.
Results: Significant discrepancies were observed in 64.29% of cases (nine/14), with tumours and infiltrative lesions in erythrodermic SS patients posing particular scoring challenges. Misclassification of tumours as patches or plaques was a frequent issue, leading to underestimations in mSWAT scores. Residents’ assessments of infiltrative lesions were also notably inconsistent.
Conclusions: This study highlights significant interobserver variability in mSWAT scoring among less experienced dermatology residents, particularly with tumour and erythrodermic lesions. Findings underscore the need for enhanced training and standardized scoring protocols to improve mSWAT reliability. Similar to other comparable indices such as PASI, the mSWAT should be employed consistently by the same physician during each assessment to systematically monitor and evaluate the skin condition of a patient under observation. However, broader application requires the acquisition of sufficient experience. The study suggests the use of the HFUS as an objective method of assessment of the skin lesion infiltration in MF/SS patients.
INTRODUCTION
Cutaneous T-cell lymphoma (CTCL) represents a heterogeneous group of malignancies predominantly involving skin-tropic Tlymphocytes. In contrast to nodal non-Hodgkin lymphoma, which mainly originates from B-cells, approximately 75% of primary cutaneous lymphomas are of T-cell origin. Among these T-cell lymphomas, two-thirds are classified as mycosis fungoides (MF) or as Sézary syndrome (SS).1 Therefore, we
will focus only on MF and SS. MF generally manifests as slowly progressing cutaneous lesions. In advanced stages, MF may exhibit extracutaneous involvement, affecting lymph nodes, the bloodstream, and, less frequently, other organs.2 On the other hand, SS is a leukemic variant of CTCL. It is characterized by erythroderma, palmoplantar keratoderma, pruritus, and peripheral blood involvement.3 CTCL is marked by the progressive dysfunction of various components of the immune system. SS is thought to derive from thymic memory T cells whereas MF is hypothesized to originate from the malignant transformation of cutaneous-resident effector memory T cells. This differentiation implies that SS is a distinct entity from MF.4 CTCL is a debilitating condition, strongly influencing patients’ quality of life. Initial management protocols for patients diagnosed with MF or SS typically include a comprehensive physical examination
using the modified Severity Weighted Assessment Tool (mSWAT), histological exam, blood morphology analysis, and relevant imaging tools such as Computed Tomography (CT) and Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) scans. Peripheral blood flow cytometry is generally advocated at all stages of MF whereas for intermediate or aggressive cutaneous lymphomas, it is recommended to perform a bone marrow biopsy and aspiration.5 The immunophenotypic profile of MF and SS cells typically includes the expression of CD2, CD3, CD5, CD4, CCR4, TCR-beta, and CD45RO, with a lack of CD7 and CD26 markers.6 Additionally, adverse prognostic factors in MF, independent of clinical stage, include older age (>60 years), large cell transformation, elevated lactate dehydrogenase (LDH) levels, and low CD30+ expression (<10%).7,8 The staging of MF and SS is based on the tumour-node-
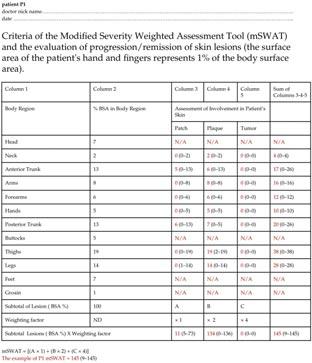
Figure 1: The mSWAT questionnaire, originally available in English, was utilized in its Polish version in our study to accommodate Polish-speaking residents. In the questionnaire is shown data of our patients (P1), (the English version) indicated in red colour, and mSWAT=145. The values in parentheses represent the range of assessments provided by the resident physicians. For legal reasons some body regions were not assessed: head, buttocks, groin. Feet were free of lesions in all patients. These cases were stated: N/A.
Reprinted with permission © 2024 by the authors. Licensee MDPI, Basel, Switzerland. This is an open access article distributed under the terms of the Creative Commons Attribution Licence CC-BY 4.0., which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Content has been edited to conform with the Canadian Press Publication Style Guide Chronicle.Academy develops bespoke structured learning programs for clinicians providing in-depth education to professionals in the healthcare industry.




guish them from random differences for the measurement.
A precise classification of skin lesion severity relies heavily on the subjective assessment of clinical manifestations by a physician, making the doctor’s experience crucial.15 Assessing disease severity can be particularly challenging for young, inexperienced dermatologists. The choice of assessment method depends on whether it is intended for research purposes or routine clinical practice. Proper classification of the patient according to disease severity is essential for selecting an appropriate treatment modality. MF presents distinctively compared to other malignant skin tumours. In its early stages, MF often resembles inflammatory skin conditions, such as psoriasis or eczema, which frequently leads to misdiagnosis.16 This clinical overlap makes it challenging to classify MF accurately within the traditional TNMB staging system. A key limitation of applying TNMB staging to MF lies in the objective assessment of body surface area (BSA) involvement. In early-stage MF, the classification system only distinguishes between lesions covering less than 10% (T1) and greater than 10% (T2) of the total BSA, failing to account for substantial differences in disease burden, such as between patients with 20% versus 50–70% BSA involvement. These limitations highlight the need for the development of new tools to assess disease severity more accurately in patients with MF/SS. The mSWAT is particularly advantageous for evaluating MF/SS, where various lesion types can co-exist within a single patient. It quantifies disease severity by recording the percentage of body surface area (BSA) affected by patch, papule/plaque, and tumour lesions. Tumours are defined as solid or nodular lesions exhibiting depth or vertical growth with a diameter of ≥1 cm. Each lesion type is assigned a relative weight—1 for patches, 2 for plaques, and 4 for tumors. These weighted values are then summed to produce a total score. BSA involvement is determined using the palmar surface of all five fingers (approximately 1% of BSA) (Figure 1). However, as indicated by both the literature and findings from our study, mSWAT has limitations as an assessment tool for MF/SS severity. It remains, to a significant extent, subjective and is influenced by the experience level of the evaluating physician. Furthermore, the weighting assigned to tumours in the mSWAT score has been debated due to the high prognostic value of these lesions. Tumours exhibit a much denser dermal infiltrate and greater neoplastic cell content than patches. Nonetheless, mSWAT may under-represent tumor burden relative to patches and plaques, complicating potential adjustments.12 The high variability in classifying lesions

as plaque or tumor complicates increasing the tumour weighting factor and highlights the need for a consistent assessor in clinical trials. The findings of this study align with our results, indicating statistically significant deviations from reference (normal) values in nine of 14 patients (64.29%). Among the enrolled patients, only three presented with tumors (Figure 3 and Table 1 and Table 2). However, statistically significant discrepancies in assessments were observed in two of these three cases (P2 and P4), where tumours were misinterpreted as patches and plaques, leading to an underestimation of the mSWAT scores. The third patient with tumours (P3) also showed lower mSWAT scores, but these differences did not reach statistical significance. Assessment challenges were also evident in patients with erythroderma and infiltrative lesions. Specifically, for erythroderma in SS patients, P8 demonstrated statistically significant deviations in resident evaluations, while P7 showed lower mSWAT scores that, although not statistically significant, were noticeably distinct from random variations. This suggests resident physicians encountered difficulty in accurately evaluating mSWAT scores in erythrodermic presentations. Infiltrative skin lesions also posed additional challenges. All patients with infiltrative lesions (P6, P10, P13, and P14) demonstrated statistically significant underestimations in mSWAT scores by resident physicians. Interestingly, the highest-rated changes were observed in infiltrative and erythematous lesions of a single patient (P1), where statistically significant differences in mSWAT assessments were noted. For P5 and P12, mSWAT scores were lower but not statistically significant, whereas P11’s mSWAT scores were assessed accurately (Figure 3 and Table 1 and Table 2). These findings underscore the challenges resident physicians face in accurately evaluating mSWAT scores, particularly in complex presentations such as tumors, erythroderma, and infiltrative lesions. Although the currently available literature includes articles on mSWAT as a tool for assessing the severity of skin lesions in MF, highlighting its usefulness in monitoring disease progression and response to treatment,17 and publications providing information on the application of mSWAT for assessing skin tumour burden in patients with MF/SS,18 only one direct study comparing mSWAT assessments conducted by different physicians has been identified.19 In 2007, Scarisbrick, et al19 conducted a study to evaluate the variability in skin scoring among healthcare professionals. Physicians and nurses attended a 2-h lecture on skin scoring methods, combining the Rule of Nines and the Palmar method. Following the lecture, six patients with different stages of MF were assessed by both the lecturer and the
attendees. A total of 22 attendees provided 122 scores, which were compared to the lecturer’s scores. For patient 1, the lecturer’s score was 73.6 compared to the attendees’ median of 50 (range: 26.4–99). For patient 2, the scores were 125.6 vs. 76.2 (range: 63–164), for patient 3, 50.7 vs. 57 (range: 17–91), for patient 4, 3.5 vs. 9.3 (range: 1.5–15.4), for patient 5, 4.7 vs. 15.9 (range: 4.2–25), and for patient 6, 17.3 vs. 17.2 (range: 14–28). Individual attendees showed consistent tendencies to score either above or below the lecturer’s assessments. The widest range of scores was observed for hypopigmented MF, while the least variability was noted in scoring erythroderma. Despite standardized training, significant interobserver variability in skin scoring was evident. This variability was partly attributed to differences in defining the palmar surface area (palm, fingers, and thumb) and its allocation of percentage surface area in different scoring systems.
Our study appears to be the second to address the topic of interobserver agreement in the use of mSWAT. Moreover, both our study and the similar study conducted by Scarisbrick, et al19 demonstrated that mSWAT assessment is highly subjective and significantly influenced by the experience of the evaluating physician. However, the authors of the first study primarily focused on differences in the assessment of Body Surface Area (BSA) among the participating physicians, rather than on distinguishing the types of skin lesions in patients with CTCL.
The recent literature highlights that HFUS provides precise evaluation of infiltration depth and morphological characteristics of MF/SS lesions, offering valuable insights for the staging of CTCL. Moreover, the unique ultrasonographic features identified in MF and SS have the potential to support their diagnosis.20-24 In a study by Wang, et al20 26 patients underwent HFUS examination. Among 16 patients with patches or plaques (early-stage group), a subepidermal low-echogenic band was identified, with only three plaque-stage lesions showing partial extension into the superficial dermis. Conversely, seven patients with tumours (advanced-stage group) exhibited lesions infiltrating the deep dermis or subcutaneous tissue. Significant differences were observed between early and advanced stages in terms of infiltration depth, boundary definition, and echo homogeneity. Furthermore, two folliculotropic MF lesions and one SS lesion displayed distinctive features, including a well-defined subepidermal lowechogenic band and patchy hypoechoic regions surrounding hair follicles within the dermis. Furthermore, in the study by Polańska, et al21 the relationship between histopathological infiltrates containing clonal T cells and ultrasonographic findings was analyzed.
HFUS performed on 10 patients with MF revealed the presence of a subepidermal low-echogenic band (SLEB). Notably, a strong correlation was identified between SLEB thickness and the thickness of subepidermal infiltration (r=0.994, p<0.05). The authors highlighted that SLEB is a characteristic feature of the infiltrative stage of MF, with its thickness potentially varying based on the type of skin lesion. The study suggests that HFUS may serve as a reliable noninvasive method for the quantitative assessment of MF, reflecting the extent of T-cell infiltration observed in histopathology. In another study by Polańska, et al22 HFUS was utilized to objectively monitor the treatment response in three patients with MF over a five-year period. The findings demonstrated a reduction in the thickness of the SLEB during phototherapy. The authors suggested that regular SLEB thickness measurements could provide a valuable supplementary method for monitoring therapeutic outcomes. Furthermore, the incomplete resolution of SLEB was associated with a lack of complete remission as assessed by mSWAT. Next, in a study conducted by Wohlmuth-Wieser, et al23 epidermal thickness, SLEB thickness, and dermal thickness were measured and compared among patients with CTCL, atopic dermatitis (AD), and psoriasis. The mean epidermal thickness in CTCL patch lesions was 271 ± 124 μm. A subepidermal low-echogenic band (SLEB) was present in all CTCL patients, with grade 2 observed in 16.7% and grade 3 in 83.3% of cases. The mean SLEB thickness was 193 ± 78 μm, and the mean dermal thickness was 1847 ± 460 μm. Patients with CTCL and psoriasis showed a trend toward increased epidermal thickness compared to those with AD, suggesting a notable difference in epidermal characteristics between these conditions.
We suggest that, alongside mSWAT scoring, the HFUS examination might be performed to objectively monitor disease progression and treatment effectiveness in patients with MF/SS.24 Thanks to this method the real infiltration could be estimated and counted in mm (Figure 4).
Moreover, other diagnostic tools, such as dermoscopy and reflectance confocal microscopy (RCM), may also be useful for diagnosis, assessment of disease severity, and monitoring remission of patients with CTCL. In the study by Soliman, et al25 88 MF patients were analyzed using H&E-stained sections, showing CD3, CD4, and CD8 positivity with CD7 negativity. Dermoscopy identified common features: non-homogeneous pink to erythematous background, orange discolouration, whitish scales, dotted and short linear vessels, and spermatozoa-like vessels, with variations across MF subtypes. In another study by Melhoranse Gouveia,
et al26 38 MF lesions were analyzed by RCM, with 19 re-assessed by RCM after six months. In total, 50 biopsies were performed (38 at baseline, 12 at followup). An RCM checklist combining four features—Pautrier’s microabscess, epidermal and junctional lymphocytes, and interface dermatitis—predicted disease severity with statistical significance.
This study has several limitations. First, the small sample size of both patients and resident examiners may limit the generalizability of the findings. Second, patient photographs were utilized to standardize the assessment results while ensuring anonymity and respecting patient dignity. For legal and ethical reasons, facial and genital areas were not photographed, as shown in Figure 2. These constraints, although necessary for ethical and legal compliance, may have limited the comprehensiveness of visual assessments.
This study underscores the challenges and variability in the assessment of MF/SS using the mSWAT, particularly among less experienced resident physicians. Significant deviations from reference values were observed in 64.29% of patients, revealing the subjective nature of mSWAT scores and their sensitivity to the assessor’s expertise (Figure 3 and Table 1 and Table 2). Tumours and infiltrative lesions were identified as particularly challenging for resident physicians to evaluate accurately. Misclassification of tumours as patches or plaques in patients with advanced lesions led to substantial underestimation of mSWAT scores, demonstrating the difficulty in distinguishing between lesion types and the limitations of the current weighting system.
The findings highlight that erythrodermic and infiltrative lesions, especially in SS patients, also pose significant assessment challenges, with notable discrepancies in mSWAT scores among resident physicians for these cases. The interobserver variability evident in this study suggests a need for enhanced training and standardized assessment protocols to improve consistency and accuracy in mSWAT scoring, particularly for less experienced assessors.
Despite mSWAT’s utility in monitoring disease progression and treatment response, its limitations—such as the under-representation of tumour burden—indicate that it may not fully capture the complexity of MF/SS disease manifestations. This study is the second to address interobserver agreement in mSWAT use, pointing to the necessity of a more objective assessment tool as HFUS to monitor treatment in MF/SS patients (Figure 1, Figure 3, and Figure 4).
AUTHOR CONTRIBUTIONS
Conceptualization, H.C. and R.B.-B.;
methodology, H.C. and R.B.-B.; software, H.C.; validation, H.C. and R.B.-B.; formal analysis, H.C. and R.B.-B.; investigation, H.C.; resources, H.C.; data curation, H.C.; writing—original draft preparation, H.C.; writing—review and editing, R.B.-B.; visualization, H.C. and R.B.-B.; supervision, R.B.-B. and A.J.-K.; project administration, R.B.-B.; funding acquisition, H.C. and R.B.-B. All authors have read and agreed to the published version of the manuscript.
This research was funded by grant number SIMPLE: SUBK.C260.24.059 and the APC was funded by grant number SIMPLE: SUBK.C260.24.059. The Wroclaw Medical University of Silesian Piasts in Wrocław, Ludwik Pasteur Str. 1, 50-367 Wrocław.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The Bioethics Committee at the Medical University in Wrocław (protocol code KB-851/2021, date of approval 28 October 2021).
The data presented in this study are available on request from the corresponding author.
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
1.Campo E, Jaffe ES, Cook JR, et al: The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the clinical advisory committee. Blood 2022; 140:1229–1253.
2. Vergier B, de Muret A, Beylot-Barry M, et al: Transformation of mycosis fungoides: Clinicopathological and prognostic features of 45 cases. French Study Group of Cutaneous Lymphomas. Blood 2000; 95:2212–2218.
3. Olsen EA, Rook AH, Zic J, et al: Sézary syndrome: Immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol 2011; 64:352–404.
4. Campbell JJ, Clark RA, Watanabe R, Kupper TS: Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: A biologic rationale for their distinct clinical behaviors. Blood 2010; 116:767–771.
5. Hristov AC, Tejasvi T, Wilcox RA: Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol 2023; 98:193–209.
6. Agar NS, Wedgeworth E, Crichton S, et al: Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging Proposal. J Clin Oncol 2010; 28:4730–4739.
7. Scarisbrick JJ, Prince HM, Vermeer MH, et al: Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol 2015; 33:3766–3773.
8. Sokołowska-Wojdyło M, Olszewska B, Chmielowska E, et al: Primary cutaneous lymphomas: Diagnostic and therapeutic guidelines of the Polish Dermatological Society (PTD) and Polish Lymphoma Research Group (PLRG). Dermatol Rev 2023; 110:647–674.
9. Bunn PA Jr., Lamberg SI: Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treat Rep 1979; 63:725–728.
10. Olsen E, Vonderheid E, Pimpinelli N, et al: Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A Proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110:1713–1722, Erratum in Blood 2008; 111:4830.
11. Stevens SR, Ke MS, Parry EJ, et al: Quantifying skin disease burden in mycosis fungoides–type cutaneous T-cell lymphomas: The Severity-Weighted Assessment Tool (SWAT). Arch Dermatol 2002; 138:42–48.
12. Olsen EA, Whittaker S, Kim YH, et al: Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A Consensus Statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol 2011; 29:2598–2607.
13. Trautinger F, Eder J, Assaf C, et al: European Organisation for Research and Treatment of Cancer Consensus Recommendations for the Treatment of Mycosis Fungoides/Sézary Syndrome—Up-
date 2017. Eur J Cancer 2017; 77:57–74.
14. Hair JF Jr., Hult GTM, Ringle CM, et al: Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R: A Workbook; Springer: Cham, Switzerland, 2021.
15. Sokołowska-Wojdyło M, Maj J, Robak E, et al: Primary cutaneous lymphomas—Diagnostic and Therapeutic Guidelines of the Polish Dermatological Society. Dermatol Rev 2017; 104:243–268
16. Hodak E, Geskin L, Guenova E, et al: Real-life barriers to diagnosis of early mycosis fungoides: An International Expert Panel Discussion. Am J Clin Dermatol 2023; 24:5–14.
17. Combalia A, Estrach T: The Modified Severity-Weighted Assessment Tool: A PASI/EASI system for mycosis fungoides. Actas Dermosifiliogr 2017; 108:936–937.
18. Scarisbrick JJ: Skin Scoring for Mycosis Fungoides and Sézary Syndrome. In: Measuring the Skin; Humbert, P., Maibach, H., Fanian, F., Agache, P., Eds.; Springer: Cham, Switzerland, 2015.
19. Scarisbrick JJ, Morris S: How big is your hand and should you use it to score skin in cutaneous T-cell lymphoma? Br J Dermatol 2013; 169:260–265.
20. Wang Y, Niu Z, Liu J, et al: Value of high-frequency ultrasound in accurate staging of mycosis fungoides/Sézary syndrome. J Ultrasound Med 2020; 39:1927–1937.
21. Polańska A, Bowszyc-Dmochowska

M, Olek-Hrab K, et al: High-frequency ultrasonography: A new quantitative method in evaluation of skin lymphomas—first comparative study in relation to histopathology. Skin Res Technol 2019; 25:720–724.
22. Polańska A, Dańczak-Pazdrowska A, Olek-Hrab K, et al: High-frequency ultrasonography—New non-invasive method in assessment of skin lymphomas. Skin Res Technol 2018; 24:517–521.
23. Wohlmuth-Wieser I, Ramjist JM, Shear N, Alhusayen R: Morphologic features of cutaneous T-cell lymphomas using dermoscopy and high-frequency ultrasound. J Clin Med 2020; 10:17.
24. Cisoń H, Cisoń W, Białynicki-Birula B, et al: Mycosis fungoides unveiled following dupilumab treatment in a patient with a history of atopic dermatitis: Usefulness of HFUS in monitoring skin features. A review with a case report. Adv Dermatol Allergol 2024; 41.
25. Soliman SH, Ramadan WM, Elashmawy AA, et al: Dermoscopy in the diagnosis of mycosis fungoides: Can it help? Dermatol Pract Concept 2023; 13:e2023284.
26. Melhoranse Gouveia B, Wells J, Kim J, et al: Reflectance confocal microscopy role in mycosis fungoides follow-up. Skin Res Technol 2020; 27:414–421.

RACIAL DIFFERENCES IN PEDIATRIC HS CLARIFIED
The authors of this paper note understanding the potential racial differences in presentation, severity, and outcomes of pediatric hidradenitis suppurativa (HS) can help physicians deliver equitable and individualized care. To assess potential differences, researchers conducted a single-centre retrospective analysis of 70 pediatric patients diagnosed with HS who were seen at University Hospitals Cleveland Medical Center in Cleveland. There was no statistically significant difference in biologic treatments between non-Hispanic Black (NHB) patients when compared to non-Hispanic White (NHW) patients, although NHW patients overall received biologic therapy more frequently (17.4%) than NHB patients (12.8%). The authors conclude the observed racial differences in pediatric HS highlight the need for further investigation of possible underlying factors contributing to disease heterogeneity and treatment response to optimize the care provided and patient outcomes.
T Onamusi, J Murphy, and SD Shah: Racial differences in disease characteristics of pediatric hidradenitis suppurativa, in Pediatric Dermatology (Feb. 9, 2025, online ahead of print).
LONG -TERM TX WITH BIMEKIZUMAB S AFE, EFFECTIVE
Investigators evaluated the long-term efficacy and safety of the IL-17A/IL-17 F inhibitor bimekizumab in the treatment of moderate to severe plaque psoriasis in 56 patients across two dermatology clinics in Italy.Researchers recruited adults with either a baseline Psoriasis Area and Severity Indes (PASI) score >10 or PASI <10 with sensitive area involvement and followed them for 16 to 52 weeks. They measured clinical outcomes by PASI 75, 90, and 100 responses and Dermatology Life Quality Index (DLQI) scores at 4, 16, 36, and 52 weeks. At week 16, 97.5% of patients achieved PASI 75, 76.7% reached PASI 90, and 66% attained PASI 100. By week 52, 91.5% achieved PASI 90 and 85.1% reached PASI 100, with 95.7% reporting a Dermatology Life Quality Index (DLQI) score of 0 or 1, indicating minimal impact on daily life. Researchers found similar efficacy across bio-naïve and bio-experienced groups, and between normal-weight and obese patients, without statistically significant differences.
E Mortato, F Artosi, C Borselli, et al: Bimekizumab for the treatment of moderate to severe psoriasis: a real-world experience over 52 weeks from two Italian dermatology clinics, in The Journal of Dermatological Treatment (2025; 36(1):2432932)
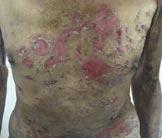
What are these lesions?
A . Dermatitis herpetiformis
B. Pemphigus vulgaris
C. Erythema multiforme
D. Linear IgA dermatosis
THE EDITORS invite your participation in this regular feature of the journal. Please send all images and correspondence to: Medical Editor, The Chronicle of Skin & Allergy 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4 Telephone: (416) 916-2476
E-mail: health@chronicle.org
Dermatology news your patients may be reading
Woman&Home reports (Feb. 20, 2025) that the slow aging trend is gaining popularity across generations, emphasizing long-term skin health over quick fixes. Unlike traditional anti-aging approaches, slow aging promotes preventive care, holistic wellness, and sciencebacked skincare. Experts attribute its rise to increased awareness of longevity science and social media’s role in amplifying wellness trends. Cosmetic physician Dr. Emmaline Ashley describes slow aging as a shift from reversing signs of aging to preserving skin function. Liverpoolbased dermatologist Dr. Sonia Khorana highlights consistency, hydration, sun protection, and stress management as key elements of the trend. Aesthetic physician Dr. Sindhu Siddiqi encourages a “pro-aging” mindset, embracing subtle treatments instead of drastic procedures. Recommended skincare includes antioxidants such as vitamin C, collagen-boosting peptides, retinoids, daily SPF, holistic health practices and investing in tools like LED masks and silk pillowcases.
An MSN article (Feb. 18, 2025) explores dermatologist-recommended treatments for hair thinning and shedding. New York-based dermatologist Dr. Blair Murphy-Rose highlights common causes, including hormonal thinning, telogen effluvium, and nutritional deficiencies. She advises addressing underlying issues before using treatments. Dr. Brendan Camp, a dermatologist also based in New York, emphasizes that hair loss products require at least three to four months to show results. Recommended treatments include minoxidil (Rogaine), which helps extend the hair growth phase, and ketoconazole shampoo (Nizoral), which may reduce inflammation and hormonal effects on hair loss. The experts also recommend rosemary oil for its potential to stimulate growth and DHTblocking shampoos containing saw palmetto and pumpkin seed oil. While these products may aid in regrowth, dermatologists stress the importance of professional evaluation for effective treatment.
A RollingOut 25 article (Feb. 20, 2025) detailed dermatologist insights on fever blisters caused by the herpes simplex virus type 1 (HSV-1), which affects over 3.7 billion people. Dr. Michael Harris explained that the virus remains dormant but can be triggered by stress, UV exposure, and immune changes. According to the report, research linked stress-related cortisol spikes to increased outbreaks, emphasizing the importance of stress management. Toronto-based immunologist Dr. Sarah Thompson highlighted immune support as a key factor in reducing recurrence. Diet and environmental factors also played roles in outbreak frequency. Sun exposure heightened viral activation, while nutrient-rich diets improved healing. Sleep quality significantly influenced immune response and recovery time. Experts stressed early intervention with cold therapy and topical treatments for better results.
An article in HealthCentral (Jan. 13, 2025) explores how skin damage manifests differently in people of colour and emphasizes the importance of recognizing signs of skin stress to maintain overall health. Dermatologists note that medical literature often focuses on white skin, leading to underdiagnosis and latestage detection of conditions such as melanoma in BIPOC individuals. Differences in melanin content, skin thickness, and barrier function make conditions such as dyspigmentation, inflammation, and dryness more prevalent and distinct in darker skin tones. Inflammatory issues such as acne, eczema, and psoriasis are often underdiagnosed due to subtler redness. Additionally, BIPOC individuals may experience heightened skin sensitivity, dryness, and keloid scarring due to structural differences. Hyperpigmentation and hypopigmentation are also more common in darker skin. Experts recommend regular skin checks, diligent sun protection, and using hydrating, hypoallergenic skincare to maintain a healthy skin barrier.









