

Trial and Error: Diving Into Clinical Research

2023 OPS Scientific Exhibit 1st Place Winner
Photo/Electron Micrography
Tapioca Melanoma SEM
Ralph Eagle Jr., MD
Wills Eye Hospital, Philadelphia, Pennsylvania
In Memoriam: Johnny Justice Jr., CRA, FOPS

It is with great sorrow that we report the passing of our founder, mentor, colleague and friend, Johnny Justice Jr., CRA, FOPS, on December 15, 2024.
If not for Johnny, the OPS would not exist. Perhaps another organization may have eventually been formed, but the Ophthalmic Photographers’ Society was his vision and passion as he sought to bring recognition to our fledgling profession by organizing an independent professional society: an organization of and for photographers which would exist to educate imagers and physicians alike about all aspects of our field and our role in ophthalmic patient care.
Johnny was brilliant and motivated, friendly and gregarious, loyal and dedicated. His Southern charm was legendary. He was an inspiration to those who knew him, and he delighted in the company of ophthalmic imagers. Through the years many of us benefitted from his personal encouragement and his enthusiasm for the traditions and innovations in ophthalmic imaging. He never stopped promoting the science and art of our profession.
There have been a number of articles in The Journal of Ophthalmic Photography about Johnny and the early days of the OPS. In one of those pieces Tim Bennett invoked the phrase, “standing on the shoulders of giants”. That sentiment is especially applicable in reference to Johnny as our society has grown and evolved since its inception in 1969. He was the ultimate pioneer whose determination and foresight brought the OPS to fruition and all ophthalmic imagers, OPS members and non-OPS members alike, owe Johnny a huge debt of gratitude. Our society is his lasting legacy. We send our deepest sympathy to his wife Carol, his sons, the Justice family, and his close friends who loved him.
Photo courtesy of Alan Frohlichstein, CRA, FOPS. L to R: Marshall Tyler, Alan Frohlichstein, Terry Tomer, Ditte Hess, Johnny Justice, Jr. Thanks to Paula Morris for crafting this announcement.
Subscription Information
The Journal of Ophthalmic Photography (ISSN 0198-6155) is published biannually by the Ophthalmic Photographers’ Society, Inc. Membership in the Ophthalmic Photographers’ Society includes an annual subscription to the The Journal of Ophthalmic Photography. Membership requests, subscription inquiries, past issue requests and changes of address should be sent to Barbara McCalley, Executive Director, 1621 East Jody Circle, Republic, MO 65738; Phone: 417.725.0181; Email: ops@opsweb.org.
Instructions to Authors
Instructions to Authors can be found at http://www.opsweb.org/?page=journal
The Ophthalmic Photographers’ Society
The Ophthalmic Photographers’ Society (OPS) is a nonprofit international organization dedicated to the advancement of photography as applied to ophthalmology and visual science. Our membership, numbering over 1000, includes a broad spectrum of professionals in eye health care, including ophthalmologists, optometrists, photographers, nurses, ophthalmic medical personnel, and basic researchers. The Society’s educational programs, designed to supplement the professional expertise of our members, have earned the OPS recognition as being an organization dedicated to the elevation of standards in the craft. The Society’s major publication, The Journal of Ophthalmic Photography, presents tutorials and articles on photographic techniques, instrumentation, and related topics in ophthalmology. Visit us online at www.opsweb.org.
Copyright
The Ophthalmic Photographers’ Society. All rights reserved. Printed in the U.S.A. None of the contents may be reproduced by mechanical or electronic processes, stored in a retrieval system or transmitted by any means without prior written permission from the Editor.
Volume 46, Issue 2, 2024

Editorial: Veterinary Ophthalmology Clinical Trials
Monica Motta, BS RVT, LATG
Original Article: Trials and Future of Intervention of Achromatopsia
Monica Ardon-Vasallo, BS

Original Article: Non-Exudative Age-Related Macular DegenerationAdvances in Imaging, Genetic Insights, and Clinical Implications
Johnathan Hawkins, CRA, CDOS, OCT-C, FOPS
Original Article: Patient Centered Innovation in Clinical Trials
Leticia Tarilonte, MS
Case Report: Corneal Cross-Linking with Photo-Activated Riboflavin in a Dog with Stromal Keratitis
Michelle H Ferneding, BS, RVT; Bianca Martins, DVM, PhD, DACVO; Dr. Savannah Vig, DVM, DACVO ; Sara Thomasy, DVM, PhD, DACVO
Q & A: New Therapy for Glioma Receives FDA Approval
Carol Harbers, MA

Images by Michael P. Kelly, FOPS
Original Article: The Promise of Gene TherapyBringing Hope to Patients with Inherited Retinal Disease
Vicki D Frye
Presentation: PRGF – Plasma Rich in Growth Factors
Bradley Stern, CRA, OCT-C



Editor in Chief
Kathleen Warren Duke University Eye Center Department of Ophthalmology 2351 Erwin Road Durham, NC 27710 kathleen.warren@duke.edu
Art Director
Jennifer Manning 5682 Dunnigan Road Lockport, NY 14094 jenmanningJOP@gmail.com








Editorial Review Board
Case Report / Technical Tactics Editor
Michael P. Kelly, FOPS
Duke University Eye Center 2351 Erwin Road, Suite 209 Durham, NC 27710 michaelpkellydukeeye@gmail.com
Elizabeth Affel, MT, MS, OCT-C, FOPS Thomas Jefferson University Hospital Philadelphia, PA Elizabeth.affel@gmail.com
Lisa Dennehy, BS Mass Eye and Ear Boston, MA lisa_dennehy@meei.Harvard.edu
Alan Frohlichstein, BFA, BS, CRA - ret., FOPS
Retinal Angiography Services Morton Grove, IL alanfroh@gmail.com
Christiaan Lopez-Miro Duke University Eye Center Durham, NC christiaan.lopezmiro@duke.edu
Paula Morris, BS, CRA, FOPS
John A. Moran Eye Center Salt Lake City, UT paula.morris@hsc.utah.edu
Assistant Editor
Monica Motta, SRA III, Supervisor I, BS, RVT, LATG
UC Davis School of Veterinary Medicine Comparative Ophthalmology
Vision Science Lab Davis, CA 95616 Ocular Services on Demand Madison, WI mjmotta@ucdavis.edu
Advertising Editor
Barbara McCalley 1621 East Jody Circle, Republic, MO 65738 Phone: 417.725.0181 Fax: 417.724.8450 ops@opsweb.org






Medical Advisor
Akbar Shakoor, MD
John A. Moran Eye Center University of Utah School of Medicine Salt Lake City, UT Akbar.Shakoor@hsc.utah.edu
Nicholas Patterson, CPT Duke University Eye Center Durham, NC nicholas.patterson@duke.edu
Kasi Sandhanam Singapore National Eye Centre Singapore kasi.sandhanam@snec.com.sg
Robert G. Shutt, CRA, OCT-C Connecticut Eye Consultants Danbury, CT shutteye@optimum.net
Jennifer Struck Duke University Eye Center Durham, NC jennifer.struck@duke.edu
Paola Torres, COT, CPT, CRA, OCT-C Duke University Eye Center Durham, NC paola.belleau-torres@duke.edu
Founded in 1977 by the Ophthalmic Photographers’ Society, Inc.; Don Wong, RBP, FOPS, Founding Editor

Monica Motta, BS RVT, LATG
UC Davis School of Veterinary Medicine
Comparative Ophthalmology Vision Science Lab Davis, CA 95616
mjmotta@ucdavis.edu
Veterinary Ophthalmology Clinical Trials
LLet us take moment to recognize our furry family members who encounter many of the same ocular diseases and challenges as us humans. Dogs, in particular, commonly encounter dry eye disease, glaucoma and endothelial dystrophy, to name a few. Dogs also


have species-specific diseases such as SARDS, Sudden Acquired Retinal Degeneration Syndrome, which is a sudden onset of permanent blindness. UC Davis is a leading university in the field of research and amongst those is Veterinary Ophthalmology. Veterinary clinical trials serve as the foundation to advanced treatment in animal based ocular diseases.
Development, funding, implementation and follow through of clinical trials is a result of dedicated veterinarians, staff and clients over sometimes very long periods of time lasting two or more years, depending on the treatment and disease progression process. The success of clinical trials heavily depends on the support and cooperation of the client owners. Clients of pets are asked to attend time sensitive appointments, sometimes lasting hours or multiple days. These clients share a passion for their pets, the same which is shared amongst the clinical trials teams for the science behind the treatments. Together the two passions combine and create the opportunity to advance into a healthier future for our furry family members.
To inquire about UC Davis Veterinary Clinical Trials, please visit: https://clinicaltrials.vetmed.ucdavis.edu/.

Figure 1: Posterior indirect examination.
Figure 3: Anterior slit Lamp examination.
Figure 2: Posterior segment OCT.

Monica Ardon-Vasallo, BS Graduate Student Researcher
University of California, Davis School of Medicine Department of Ophthalmology
1275 Med Science Dr. Davis, CA 95616 mardon@ucdavis.edu
The Trials and Future of Intervention of Achromatopsia
Achromatopsia (ACHM) is an inherited autosomal recessive disorder that causes legal blindness, photophobia, and absent cone-mediated electroretinographic amplitudes.1,2 It affects 1 in 30,000 people in the United States and has no current treatment.1–3 There are currently six known genes that have been linked to ACHM: CNGA3, CNGB3, GNAT2, ATF6, PDE6C, and PDE6H. 4–6
Mutations in these genes have slightly different clinical presentations, especially in their fovea, but do have some similarities. In individuals with CNGA3, CNGB3, GNAT2, and PDE6C related ACHM, their mean foveal thickness and mean foveal outer nuclear layer (ONL) thickness are significantly lower than normal populations.2 In subjects with CNGA3 and CNGB3 related ACHM, they have an optical empty cavity that is visible in the cone cell layer. Subjects with ATF6-ACHM have a different phenotype where they do not have foveal ONL thickness reduction when compared to control, CNGA3, or CNGB3. 7 Furthermore, in patients with deletions of exons 2 and 3 in the ATF6 gene, OCT images revealed severe foveal hypoplasia and focal disruption in the ISe layer at the site of the presumed foveal pit.8
Attempts to rectify this issue include testing the safety and efficiency of using rAAV-based gene therapy. Gene therapy is used to deliver the functioning copy of the faulty gene to the cone photoreceptors in hopes of restoring cone photoreceptor function. There have been several studies where researchers have used rAAV-based gene therapy to treat
CNGA3, CNGB3, GNAT2, and PDE6C ACHM in multiple species who have either been engineered to contain the mutation or who have it naturally.9 Similarities in the studies include obtaining species who previously had no cone photoreceptor response present from birth, delivering the functioning gene to the retina via subretinal injection, and then seeing a partial rescue of the cone photoreceptor response.10–13 While there is no FDA approved gene therapy available as of now, continuing to research the safety and efficiency of using AAV-based gene therapy continues to prove itself as a strong candidate to treat ACHM.
In 2019, we have identified a non-human primate model of PDE6C-ACHM who has a homozygous R565Q missense mutation in the catalytic domain of PDE6C 14 These primates exhibit a clinical phenotype similar to human PDE6C-ACHM where both species have a completely absent cone function with a normal rod response by ERG. Since our animal model nearly mirrors human PDE6C-ACHM, then there is great potential to accelerate

Figure 1: One affected primate has progressive macular atrophy. Spectral domain-optical coherence tomography (SD-OCT) scans of the foveal center are shown from one six-year-old wildtype adult (a) and a homozygote adult at 5 (b) and 8 (c) years of age showing macular atrophy progression. Fundus autofluorescence shows normal macular autofluorescence of the same wildtype 6-year-old adult (d) but reveals obvious foveal hyperautofluorescence of the same 5-year-old homozygote adult (e) that progresses over time (f). Fundus photography shows normal macular appearance in wildtype adults (g) and prominent foveal pigmentation of homozygote primate that worsens with age (h-i).
translation of therapeutic approaches to human patients. When examining the natural progression of disease, we found that it may be important to intervene when our primates are less than a year old since there is some evidence of disease progression over time (Figure 1).15, 16
References
1. Pang, J.-J. et al. Achromatopsia as a Potential Candidate for Gene Therapy. in Retinal Degenerative Diseases (eds. Anderson, R. E., Hollyfield, J. G. & LaVail, M. M.) vol. 664 639–646 (Springer New York, New York, NY, 2010).
2. Sundaram, V. et al. Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology 121, 234–245 (2014).
3. Khan, N. W., Wissinger, B., Kohl, S. & Sieving, P. A. CNGB3 Achromatopsia with Progressive Loss of Residual Cone Function and Impaired Rod-Mediated Function. Investig. Opthalmology Vis. Sci. 48, 3864 (2007).
4. Georgiou, M. et al. Deep Phenotyping of PDE6C-Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 60, 5112–5123 (2019).
5. Weisschuh, N. et al. Mutations in the gene PDE6C encoding the catalytic subunit of the cone photoreceptor phosphodiesterase in patients with achromatopsia. Hum. Mutat. 39, 1366–1371 (2018).
6. Hirji, N., Aboshiha, J., Georgiou, M., Bainbridge, J. & Michaelides, M. Achromatopsia: clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 39, 149–157 (2018).
7. Mastey, R. R. et al. Characterization of Retinal Structure in ATF6 -Associated Achromatopsia. Investig. Opthalmology Vis. Sci. 60, 2631 (2019).
8. Lee, E.-J. et al. Multiexon deletion alleles of ATF6 linked to achromatopsia. JCI Insight 5, e136041 (2020).
9. Michalakis, S., Gerhardt, M., Rudolph, G., Priglinger, S. & Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 26, 51–59 (2022).
10. Michalakis, S. et al. Restoration of Cone Vision in the CNGA3−/− Mouse Model of Congenital Complete Lack of Cone Photoreceptor Function. Mol. Ther. 18, 2057–2063 (2010).
11. Pang, J. et al. AAV-Mediated Cone Rescue in a Naturally Occurring Mouse Model of CNGA3-Achromatopsia. PLoS ONE 7, e35250 (2012).
12. Dai, X. et al. Long-term retinal cone rescue using a capsid mutant AAV8 vector in a mouse model of CNGA3-achromatopsia. PLOS ONE 12, e0188032 (2017).
13. Moshiri, A. et al. Contributed Session III: AAV-mediated gene therapy for PDE6C achromatopsia: Progress and challenges. J. Vis. 23, 81 (2023).
14. Moshiri, A. et al. A nonhuman primate model of inherited retinal disease. J. Clin. Invest. 129, 863–874 (2019).
15. Ardon, M. et al. Onset and Progressino of disease in nonhuman primates with PDE6C cone disorder. Investig. Opthalmology Vis. Sci.
16. Ardon, M., Nguyen, L., Chen, R., Rogers, J., Stout, T., Thomasy, S., & Moshiri, A. (2024). Onset and Progression of Disease in Nonhuman Primates With PDE6C Cone Disorder. Investigative Ophthalmology & Visual Science, 65(14), 16. https://doi.org/10.1167/iovs.65.14.16


Johnathan Hawkins, CRA, CDOS, OCT-C, FOPS
Research Photographer
Retina Consultants of Texas
713.524.3434 x1855
jhawk27@icloud.com.
Non-Exudative Age-Related Macular Degeneration: Advances in Imaging, Genetic Insights, and Clinical Implications
Introduction
on-exudative age-related macular degeneration (AMD), commonly known as dry AMD, is a prevalent and progressive retinal condition affecting millions worldwide. With the aging global population, the burden of AMD continues to rise, underscoring the need for advanced diagnostic tools and therapeutic strategies. This article explores recent innovations in imaging techniques, genetic research, and clinical implications that are revolutionizing our understanding and management of non-exudative AMD.
Fundus Autofluorescence (FAF)
Fundus autofluorescence (FAF) imaging has emerged as a pivotal technique for visualizing metabolic changes associated with non-exudative AMD. FAF captures the natural fluorescence emitted by lipofuscin, a byproduct of photoreceptor outer segment degradation, within the retinal pigment epithelium (RPE). Increased FAF signal correlates with elevated lipofuscin accumulation, providing clinicians with insights into disease progression and areas of RPE dysfunction.1
Fluorescein Angiography (FA) and Geographic Atrophy
Fluorescein angiography (FA) remains a cornerstone in the evaluation of AMD, offering insights into retinal vascular changes and complications such as geographic atrophy (GA). GA is a hallmark feature of advanced non-exudative AMD, characterized by the progressive loss of retinal pigment epithelium (RPE) and photoreceptors in the macula, leading to irreversible central vision impairment.4
During FA, intravenous injection of fluorescein dye allows visualization of retinal vasculature dynamics and abnormalities. In non-exudative AMD, FA reveals characteristic findings such as window defects and hyperfluorescent areas corresponding to regions of RPE atrophy and outer retinal loss. These changes reflect the underlying
pathology of GA, where the gradual loss of RPE cells and photoreceptors results in decreased choroidal fluorescence due to tissue thinning and reduced metabolic activity.4
OCT Angiography (OCTA)
OCT angiography (OCTA) represents a non-invasive imaging modality that has revolutionized the visualization of retinal vasculature in AMD. Unlike traditional angiography techniques, OCTA generates high-resolution, depthresolved images of retinal and choroidal blood flow without the need for dye injection. In non-exudative AMD, OCTA allows for detailed assessment of vascular changes, including flow deficits and alterations in the choriocapillaris, contributing to a comprehensive understanding of disease pathophysiology.2
Pegcetacoplan and Its Role in AMD Research
Pegcetacoplan is a targeted C3 inhibitor that has shown promise in slowing the progression of geographic atrophy (GA) associated with non-exudative AMD. By inhibiting the complement cascade at the level of C3, pegcetacoplan reduces inflammation and immune-mediated damage to retinal cells. Clinical trials have demonstrated that pegcetacoplan can significantly slow the growth of GA lesions, offering a potential therapeutic option for patients with advanced non-exudative AMD.3
Genetic Insights and Disease Pathogenesis
Genetic studies have identified several susceptibility genes implicated in AMD pathogenesis, including CFH, ARMS2, and C3. These genes play critical roles in complement activation, lipid metabolism, and inflammatory responses within the retina, influencing disease susceptibility and progression. Variants in these genes contribute to the dysregulation of cellular pathways involved in RPE and photoreceptor function, ultimately leading to the development of GA and vision loss.5
Clinical Implications and Disease Management
Geographic atrophy represents a significant clinical challenge in AMD management, as current therapeutic options are limited. Early detection and monitoring of GA progression are crucial for optimizing patient outcomes and implementing timely interventions. Advanced imaging techniques such as spectral-domain optical coherence tomography (SD-OCT) and fundus autofluorescence (FAF) complement FA findings by providing detailed structural and functional assessments of retinal changes associated with GA.4
Future Directions and Research Opportunities
Despite significant advancements in understanding GA and its underlying mechanisms, challenges remain in developing effective treatments that can halt or reverse disease progression. Longitudinal studies integrating multimodal imaging and genetic analyses are essential for elucidating the natural history of GA and identifying novel therapeutic targets. Moreover, the standardization of imaging protocols and validation of biomarkers associated with GA will facilitate personalized medicine approaches aimed at preserving vision and improving quality of life for patients with non-exudative AMD.5
Conclusion
In conclusion, the integration of advanced imaging techniques such as FA, OCTA, and FAF, coupled with genetic insights, has transformed our approach to diagnosing and managing geographic atrophy in non-exudative AMD. These innovations provide clinicians with valuable tools for early detection, precise monitoring, and personalized treatment strategies, ultimately enhancing clinical outcomes and quality of life for patients affected by this debilitating condition.
References
1. Sparrow JR, et al. Lipofuscin and retinal pigment epithelial cell metabolism in the pathogenesis of age-related macular degeneration. Progress in Retinal and Eye Research. 2023; 85: 101994.
2. Wu Z, et al. Optical coherence tomography angiography in agerelated macular degeneration: a comprehensive review. Eye. 2022; 36(4): 1050-1065.
3. Guymer RH, et al. Pegcetacoplan for the treatment of geographic atrophy in age-related macular degeneration: results of a phase 2/3 clinical trial. Ophthalmology. 2023; 130(6): 789-797.
4. Holz FG, et al. Geographic atrophy in age-related macular degeneration: clinical features and potential therapeutic approaches. Ophthalmology. 2023; 130(4): 541-555.
5. Fritsche LG, et al. A large genome-wide association study of agerelated macular degeneration highlights contributions of rare and common variants. Nature Genetics. 2023; 55(4): 487-495.

Original Article

Leticia Tarilonte, MS Vice President of Global Clinical Operations Brainstorm Cell Therapeutics
1325 Avenue of Americas, 28th Floor
New York, NY 10019
617.293.5013
Leticia.Tarilonte@gmail.com
https://www.linkedin.com/in/leticiatarilonte/
Patient Centered Innovation in Clinical Trials
Throughout much of the 20th century, clinical trials operated in a fragmented manner across different regions worldwide. However, the advent of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) marked a pivotal shift towards a more unified approach. Nowadays, many clinical trials are geared towards facilitating the global approval of new drugs, resulting in the emergence of multinational trials.
Despite the potential efficiency gains associated with global trials, they come with their own set of challenges, especially for participants. Firstly, certain regions may insist on enrolling specific numbers of patients from their locale and healthcare setting. Secondly, regulatory bodies in different regions often have divergent criteria concerning endpoints, trial durations, comparators, and other trial parameters, complicating the development of universally accepted trial protocols. Lastly, while significant strides have been made towards harmonization, the intricate regulatory and ethical frameworks at the national level add layers of complexity to conducting trials across multiple regions.
Clinical trials are not at all easy – they are quite complex, lengthy, and are required to follow several rules and regulations to ensure compliance with different standards.
Key Challenges in Clinical Trials for Sponsors
Slow Recruitment
Patient recruiting is one of the most difficult challenges and determines if the clinical trial will begin successfully. The main reason for recruitment challenges is the trial’s specific inclusion and exclusion criteria. Finding the right patients that meet all the inclusion and exclusion criteria proves to be quite difficult. For Sponsors, time is money, the longer the recruitment period, the more expensive the trial will cost.
Patient Diversity
When trial participants are homogeneous (e.g., primarily of the same gender, race/ethnicity, or age group), findings may be skewed, resulting in a body of clinical knowledge that is not generalizable. Incorporating a diverse group of people
into clinical research could lead to more robust and full data, which further leads to a better understanding of variances in treatment responses and fewer discrepancies in outcomes.
Regulations
Compliance with the evolving rules and regulations of each country must be maintained throughout the trial, while coordination among sites, partners, vendors, and other stakeholders is unquestionably a recipe for a complex and tangled web of compliance regulations. Approval of protocols and materials by institutional review boards and ethics committees takes time, and each entity has its own requirements and its own pace at which things are approved.
Data Management
To achieve successful data analysis, dynamic data monitoring is essential. Institutions participating in the study face resource constraints, resulting in significant delays in entering data into the electronic data capture system (EDC). It is challenging to integrate lab results, imaging, and health records rapidly, especially when we continuously rely on manual spreadsheet methods.
Patient Retention
Patients frequently experience a lack of appreciation during their involvement in clinical trials, feeling as though they are merely treated as data points by both the attending physician and the sponsoring entity. The primary cause of patient disengagement stems from insufficient consideration of their time, coupled with an absence of communication, trials updates, and trial results. Inadequate interaction with patients often results in dissatisfaction and diminishes their willingness to participate in future trials. Consequently, patient dropout can result in a restricted dataset for the sponsor, leading to inconclusive endpoints.
Key Challenges in Clinical Trials for Patients
Long Waits to Enter a Trial
Patients may encounter prolonged waiting periods before being able to participate in a clinical study. Several factors contribute to these delays, including concomitant medication
wash-out, where patients are required to cease certain medications and wait for the complete elimination from the body before receiving the investigational drug. Additionally, patients typically undergo a 28-day screening period before being eligible to enter the clinical study and receive the investigational drug. Some patients may fail safety laboratory assessments during screening, necessitating re-screening and resulting in further delays before receiving the investigational drug. In Phase I clinical trials, patients are often placed on a waitlist while the sponsor clears a cohort and refines the next dose, which can extend the waiting period for weeks as patients eagerly anticipate entry into the clinical study.
Long and Multiple Visits
Numerous studies entail prolonged visits, requiring patients to spend entire days in the clinic and return the following day for additional blood samples and assessments. For many patients, social and cultural factors present significant challenges, as they may be unable to allocate extensive personal time away from their families or jobs to participate fully in clinical research.
Complex Studies
Sponsors frequently impose stringent eligibility criteria for clinical trials, encompassing restrictions on medications, procedures, and specific dosing requirements for investigational drugs. This complexity adds challenges to patients’ disease management. Moreover, patients are often required to provide substantial blood samples for pharmacokinetic assessments and exploratory biomarkers, as well as undergo invasive and painful procedures like fresh biopsies.
Insurance and Billing
Although federal law mandates most health insurance plans to cover routine patient care expenses in clinical trials, they are not obligated to cover research costs or outof-network doctors or hospital visits. In instances where a patient experiences a serious adverse event unrelated to the study and requires medical care outside of the trial, insurance may be billed. However, there are cases where insurance companies attempt to reject such claims citing the patient’s participation in clinical research.
Lack of Updates
Healthcare providers play a pivotal role in patient recruitment and retention. When patients perceive clinical trials support staff as unavailable or disinterested, or when they frequently interact with unfamiliar personnel, patient recruitment and retention may decline. Building patient trust in the clinical trial process fosters greater participation.
No Trial Results
Enhancing patient trust could be achieved by demonstrating that their perspectives have been integrated into the data sharing process. Analyses of shared data should employ scientifically valid methods and statistics to prevent biased conclusions. Patients desire involvement in the entire research process, which includes receiving study results, knowing their treatment assignment, and understanding their response compared to the overall study population.
Patient Recruitment Strategies in Clinical Trials
Sponsors initiating clinical research must take proactive steps to tackle patient recruitment challenges by crafting customized strategies to overcome potential hurdles at every stage of the study. Researchers should adopt the following crucial considerations to navigate typical pitfalls related to patient recruitment, ensuring timely completion of the study while reducing costs.
Nurse Navigators: Sponsors can hire nurse navigators to help identify suitable patients for the trial. These navigators can help patients understand their medical records and match their disease profile to the clinical trial inclusion and exclusion criteria.
Patient Transportation: Arranging for patient transport to the trial site for study visits can greatly impact patient recruitment and retention.
Patient Expenses: Providing coverage for patient expenses incurred during the trial, such as parking fees, meals, hotel accommodations, etc., that are necessary for study visits.
Recruitment Strategies: Designing a country and sitespecific recruitment strategy prior to study initiation.
Medical Community: Engaging with the local medical community can enhance recruitment efforts. Effective methods include distributing “Dear Doctor” letters and hosting “Lunch & Learn” sessions about the clinical trial at neighboring clinics and hospitals to disseminate information effectively.
Educating Patients: Educating patients on clinical trials during routine outpatient department (OPD) visits can help patients and their families consider participating in clinical studies.
Creating a Positive Awareness: Generating favorable perception about clinical trials among people through press, mass media and advocacy groups can greatly impact recruitment efforts.
Patient Engagement Strategies in Clinical Trials
After a patient has consented to participate in a clinical trial, implementing effective and beneficial strategies can significantly enhance patient retention, offer support, and cultivate a positive experience throughout their involvement in the study.
Provide Educational Materials: Offering visual aids to the trial participants: pamphlets, videos, patient newsletters, a study website, and social media.
Educate and Share Information: Ensuring that patients receive information about the trial before, during, and after its completion is essential. Educating patients on trial requirements, time commitments, and being transparent about reimbursement, payment, travel arrangements, and time constraints fosters trust and facilitates their engagement in the study.
Engage the Patient: Facilitating patient teleconferences to stay in touch with the patient and share study updates via email or text message.
Reducing Patient Burden
Implementing strategies to minimize patient burden through a patient-centered experience seamlessly integrated into everyday life can result in exceptional enrollment and retention rates. This approach not only enhances patient compliance but also accelerates study timelines and reduces costs.
Stress: Reduce anxiety and frustration by removing logistical barriers. Sharing trial information with the patient periodically can reduce the stress of the unknown benefits of the investigational drug.
Compliance: Boost patient compliance by fostering stronger connections between participants and study sites, thus reducing drop-out rates. Sites can leverage technology to maintain regular communication with patients.
Patient Satisfaction: Offer Patient Concierge Services (PCS) to address trial-related concerns and streamline the participant experience, while gathering feedback from patients and utilize it to enhance operational processes.
Administrative Burden: Centralized technology platforms can assist with enrollment, facilitate communication with patients, and track participant progress effectively.
Travel Expenses: Implementing Patient Travel Services (PTS) to help patients with travel logistics, meals, and hotel logistics.
Reimbursement: Implement a Patient Reimbursement Program (PRP) with flexible payout options that reduce patient and caregiver stress and lessen the burden on patients, their families and sites.
Technologies in Clinical Trials for the Benefit of the Patient
The increasing utilization of electronic health records, mobile apps, and wearable gadgets holds significant promise for revolutionizing clinical trials, enhancing their practicality and efficiency. While numerous hurdles must be addressed before these advancements can become standard practice in the operations of randomized, controlled trials, we have seen a major impact in clinical trials when new and advanced technology is implemented.
Data Capturing Improvements
Capturing patient data has become easier because of mobile and wearable devices. Patient data is at the forefront of decision-making as wearable technology is proven to be an excellent tool for automating patient monitoring. These improvements allow the Sponsor to keep a statistical eye on the data and provide virtual and real-time access to data across study locations, enhancing patient engagement to improve clinical data management.
Remote Monitoring
Remote site access and monitoring tools have become an important part of the clinical trial process, serving as a link between the sponsor, CROs, and participating sites.
Sponsors and CROs are satisfying the needs of sites by connecting to the site’s existing technology infrastructure (EMR) and/or by implementing an Electronic Investigator Site File (eISF) system that connects directly to the sponsor’s Electronic Trial Master File (eTMF).
Real Time Data
Sponsors can achieve real-time data access by partnering with sites to conduct hybrid clinical trials, combining on-site visits with decentralized approaches. This involves implementing remote patient visits and collecting data directly from patients in real time. However, uncontrolled risks, such as patient compliance at home and variations in technological experience among patient populations, must be carefully assessed, with mitigation plans in place. Decentralized clinical trials (DCTs) offer a unique opportunity by eliminating geographical barriers between a patient’s home and the clinical site, thereby revolutionizing the conduct of clinical trials.
Faster Digital Recruitment
Implementing mobile communications can overcome the challenge of distance. The use of globalized transportation apps like UBER which makes private transportation more accessible in isolated locations can greatly impact trial recruitment. Implementation of digital technologies can also help with recruitment efforts such as: e-consent, digital sources which can transfer data directly into EDC, electronic clinical outcome assessments, web-connected medical equipment, etc.
Improve Patient Experience
Patient experience can be enhanced by involving local sites, so patients do not have to travel long distances for routine labs. Advocacy Groups can help Sponsors learn about the patient journey and apply required changes to the clinical study to make decentralized trials possible. Allowing patients to submit some data from home directly into EDC can similarly impact the patient experience. Patients desire to be involved in many steps of the research process, Sponsor can obtain patient input into trial design by conducting a patient focus group before enrollment starts and include patient input into the design and informed consent language.
Digital Technology to Produce Patient-centric Solutions
Implementing digital technology to develop patient-centric solutions is crucial to encourage patient participation, leveraging mobile health, e-health records, and patientgenerated data. Technologies like wearables, mHealth, and real-world evidence (RWE) have enabled smaller trials, where everything, from patient enrollment to medication reminders and follow-ups, can be managed through a phone provided to trial participants.
The emergence of wearable technologies has generated considerable excitement for innovative approaches to healthcare data collection. Wearables have diverse applications across various therapeutic areas. To support ongoing assessment and integration of these technologies, it is essential to carefully consider analytical and clinical validation components, tailored to the specific context of use.
Device Type
Wrist Worn
Data Collected
Actigraphy, HR (Heart Rate), BP (Blood Pressure), EDA (Electrodermal Activity)
Skin Patch ECG (Electrocardiography), Actigraphy, Skin Temperature
Cuffs BP, HR
Finger Worn HR, SpO2
Clothing Embedded Sensors HR, HRV (Heart Rate Variability), ECG, Breathing Rate, Actigraphy
Headbands EEG (Electroencephalogram), EMG (Electromyography)
Table 1: Examples of wearable sensors to collect data in clinical trials.
Decentralization and Remote Elements of Clinical Trials
Decentralized clinical trials (DCTs) represent a departure from traditional trial settings, with some or all activities occurring outside clinical trial sites. These decentralized approaches utilize digital health technologies (DHTs) to collect healthcare data directly from participants. DHTs include portable devices like activity trackers and glucose monitors, as well as interactive mobile apps for assessing various aspects of patient well-being.
The increasing popularity of DCTs and DHTs is driven by advancements in technology, which simplify electronic data collection, transmission, and storage. Additionally, the growing familiarity with telemedicine among patients and healthcare providers enhances acceptance of remote trial participation.
The COVID-19 pandemic underscored the importance of remote trial facilitation, prompting sponsors to embrace remote elements like informed consent processes and patient monitoring via telemedicine. Many participants prefer receiving treatments at home or local clinics, easing recruitment and retention efforts by eliminating travel burdens. Furthermore, DHTs enable more frequent data collection, providing valuable insights into treatment effectiveness and safety in real-world settings.
Overall, DCTs offer long-term time and cost savings by reducing reliance on traditional sites. However, initial investments in technology adoption and training may be necessary.
Leveraging technology to decentralize clinical trials can ease participation for patients by reducing or eliminating the need to travel to specific study sites:
• Electronic Consent Form
• Home nursing visits
• Electronic patient reported outcomes
• Decentralized data collection
• Electronic self-reporting of adverse events
• Involvement of local doctors, laboratories, imaging
• Direct-to-patient drug delivery
• Remote monitoring of trial data
•Telemedicine: remote visits
• Activation of sites close to the patient’s geographical location
Patient Advocacy Groups
Establishing transparency and effective communication channels among patients, doctors, and researchers is crucial. Patient advocacy groups (PAGs) play a pivotal role in facilitating patient recruitment by providing a platform where patients, as experts on their condition, can connect with clinical trial sites offering potentially beneficial treatments. However, for certain rare conditions, patient availability for clinical trials may be limited. Collaborating with PAGs becomes particularly vital in such cases, specifically when doing research on rare diseases. These groups ensure that patients receive the support and understanding they need, fostering a sense of community and encouragement for participation in innovative trials. Working alongside advocacy groups also offers study sites assurance that they are engaging with motivated patients who are eager to contribute to research and provide valuable insights. PAGs actively disseminate information about clinical trials within the patient community, possessing deep insights into patient needs and preferences that can inform study design and enrollment strategies. Their involvement in protocol and consent review, study design, and patient recruitment can greatly benefit researchers. Furthermore, PAGs contribute to shaping research agendas, ensuring the feasibility and success of research protocols, and supporting training programs. This collaborative partnership with PAGs can significantly enhance the trial’s success.
Summary
The importance of the patient voice in clinical research is increasingly recognized and in many instances is considered mandatory by funding organizations (Sponsors). Incorporating the patient’s perspective into clinical research is crucial as it enhances the relevance and impact of the research for patients themselves. Patients possess unique expertise in their own illness, offering invaluable insights into their experiences, needs, and optimal research approaches. Involving patients in the research process often leads to improved outcomes, including:
1. Enhanced Understanding: Patients gain a deeper comprehension of their condition and treatment through active involvement in research.
2. Improved Adherence: Patients demonstrate higher adherence to the trial protocol when they play an active role in the research process.
3. Increased Diversity: By involving patients, clinical trials can represent a more diverse population with varied experiences, thereby enhancing the generalizability of research findings.
Researchers must develop expertise in effective methods to engage patient partners in the research process from the point of research prioritization through study design, execution, and dissemination of results. The patient must remain the central focus of healthcare and research practice.

2023 OPS Scientific Exhibit
First Place Winners: Print Division
The purpose of the annual OPS Scientific Exhibit is to encourage members to share their outstanding images with the ophthalmic community. The entire exhibit, featuring 42 categories in the print and stereo divisions, is displayed as part of the Scientific Exhibit at the Annual Meeting of the American Academy of Ophthalmology. For further information contact: Kathleen Warren, Scientific Exhibit Chair, kathleen.warren@duke.edu
BEST OF PRINT DIVISION
Slit Lamp Photography
Stitch Abscess
Angela Chappell, CRA, OCT-C Flinders Medical Centre Bedford Park, South Australia


Gonio Photography
Iris Mass
Mark Harrod, CRA, OCT-C Cleveland Clinic, Cole Eye Institute Cleveland, Ohio

2023 OPS Scientific Exhibit
First Place Winners: Print Division

Fundus Photography High Magnification 20° Papilledema
Meghan Menzel, CRA
University of Iowa Hospitals and Clinics Iowa City, Iowa

Fundus Photography Normal 30°- 40° Macular Hole
Shannon Howard, COA, CDOS, ROUB Mayo Clinic Rochester, Minnesota

Fundus Photography High Magnification 20° Untitled Chitaranjan Mishra Trilochan Netralaya Sambulpur, India

Fundus Photography Wide Angle 45°+ Morning Glory
Jody Troyer, CRA
University of Iowa Hospitals and Clinics Iowa City, Iowa

2023 OPS Scientific Exhibit
First Place Winners: Print Division

Fluorescein Angiography
Subretinal Fluid with Macular Oedema
Kasi Sandhanam
Singapore National Eye Centre
Singapore

Cross Categories
Best Disease
Jody Troyer, CRA
University of Iowa Hospitals and Clinics
Iowa City, Iowa

Indocyanine Green Angiography
Ocular Histoplasmosis
Mark Harrod, CRA, OCT-C
Cleveland Clinic, Cole Eye Institute Cleveland, Ohio

Monochromatic Photography
Macular Pucker
John Leo
Rajan Eye Care Hospital Pvt Ltd Chennai, India

2023 OPS Scientific Exhibit
First Place Winners: Print Division

External Photography Descemetocele
Sarah Skiles, CRA
University of Iowa Hospitals and Clinics Iowa City, Iowa

Henry Ford Health Detroit, Michigan

Gross Specimen Photography
Soemmerring Ring IOL
Ralph Eagle Jr., MD
Wills Eye Hospital Philadelphia, Pennsylvania

Composite Image
Circumferential Bone Spicule-Like Pigmentation in Retinitis Pigmentosa
Michael Edrington
University of Iowa Hospitals and Clinics Iowa City, Iowa
The Eye as Art Chicken-a-la-Rothko
Bradley Stern, CRA, OCT-C

2023 OPS Scientific Exhibit
First Place Winners: Print Division

Surgical Photography
Temporal Artery Biopsy
David Miller, CRA
Wake Forest University Eye Center
Winston-Salem, North Carolina

Fundus Autofluorescence
Progression of Ampiginous Choroiditis
Barbara Klemenc
University Eye Hospital Ljubljana
Ljubljana, Slovenia

Instrumentation Scientist at Work
Denice Barsness, CRA, FOPS
California Pacific Medical Center
Mill Valley, California

Clinical Setting
The COVID Years
Denice Barsness, CRA, FOPS
California Pacific Medical Center
Mill Valley, California

2023 OPS Scientific Exhibit
First Place Winners: Print Division

Optical Coherence Tomography
Epiretinal Membrane
Kasi Sandhanam
Singapore National Eye Centre
Singapore

OCT Angiography
NVD
Darrin Landry, CRA, OCT-C, FOPS
Bryson Taylor, Inc.
Saco, Maine

Ultra-Widefield Imaging
Asteroid Hyalosis in Vitreous Cells
Michael Edrington
University of Iowa Hospitals and Clinics
Iowa City, Iowa

Photo/Electron Micrography
Tapioca Melanoma SEM
Ralph Eagle Jr., MD
Wills Eye Hospital
Philadelphia, Pennsylvania

2023 OPS Scientific Exhibit
Second Place Winners: Print Division

Fluorescein Angiography
Sickle Cell Retinopathy
Kasi Sandhanam
Singapore National Eye Centre Singapore

Indocyanine Green Angiography
CNV Feeder Vessel
Darrin Landry, CRA, OCT-C, FOPS
Bryson Taylor Inc. Saco, Maine

Fundus Photography High Mag 20° NAION
Meghan Menzel, CRA
University of Iowa Hospitals and Clinics
Iowa City, Iowa

Fluorescein Angiography Unknown
Christiaan Lopez-Miro
Duke Eye Center Durham, North Carolina

Indocyanine Green Angiography Laser Scars
Dena Harris, CRA
University of Michigan Kellogg Eye Center Ann Arbor, Michigan

Fundus Photography Normal 30°- 40°
Angioid Streaks
Sarah Skiles, CRA
University of Iowa Hospitals and Clinics
Iowa City, Iowa

Fluorescein Angiography CRVO
Judith Gulian, OCT-C
University of Michigan Kellogg Eye Center Ann Arbor, Michigan

Fundus Photography High Mag 20° Optic Nerve Head Nevus
Ben Serar
Serar Photography Prescott, Arizona

Fundus Photography Wide Angle 45°+ Stargardt Disease
Michael Edrington
University of Iowa Hospitals and Clinics
Iowa City, Iowa

2023 OPS Scientific Exhibit Second Place Winners: Print Division

Fundus Photography Wide Angle 45°+ CRVO
Sean Grout, OCT-C
California Pacific Medical Center
San Francisco, California

Lamp Photography Iron Ring
Sean Grout, OCT-C
California Pacific Medical Center San Francisco, California

Gonio Photography Ciliary Body
David Miller, CRA
Wake Forest University Eye Center
Winston-Salem, North Carolina


Slit Lamp Photography Iris Growth
Huynh Van, CRA
Byers Eye Institute at Stanford San Jose, California
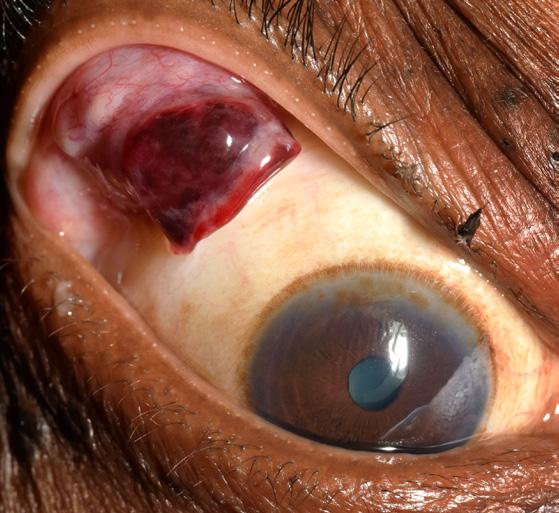
External Photography Hemorrhagic Lid Mass
David Miller, CRA
Wake Forest University Eye Center Winston-Salem, North Carolina

Slit Lamp Photography Irregular Iris
Huynh Van, CRA
Byers Eye Institute at Stanford San Jose, California

Gross Specimen Photography Melanoma Cells in Angle
Ralph Eagle Jr., MD Wills Eye Hospital Philadelphia, Pennsylvania

Ultra-Widefield Imaging
Slit
Fundus Autofluorescence
Choroidal Rupture
Tony Medina, CRA, FOPS
University of Michigan Kellogg Eye Center Ann Arbor, Michigan
Von Hippel Lindau Syndrome
Clint Downer, OCT-C
University of Michigan Kellogg Eye Center
Ann Arbor, Michigan

2023 OPS Scientific Exhibit
Second Place Winners: Print Division

Photo/Electron Micrography
Akreos IOL Calcification SEM
Ralph Eagle Jr., MD
Wills Eye Hospital Philadelphia, Pennsylvania

The Eye as Art The Beauty of Dystrophies
Barbara Klemenc
University Eye Hospital Ljubljana Ljubljana, Slovenia

Cross Categories
Iris Melanoma
Mark Harrod, CRA, OCT-C
Cleveland Clinic, Cole Eye Institute Cleveland, Ohio

Composite Image
Ischemic Diabetic Retinopathy
Kasi Sandhanam
Singapore National Eye Centre Singapore

The Eye as Art
Rising Planet Kepatoeidous & Moon
Huynh Van, CRA
Byers Eye Institute at Stanford San Jose, California

Optical Coherence Tomography
Anterior Chamber IOL with Angle Closure
Megan Walsh, CRA, OCT-C
Eyecare Medical Group
Portland, Maine

Composite Image Untitled Chiedozie Ukachukwu
Lurie’s Childrens Hospital Northwestern Univ, Chicago, Illinois

The Eye as Art Thinker
Pablo Gili, MD
Hospital Universitario Fundacion Alcorcon Madrid, Spain

OCT Angiography Angiogenesis
Darrin Landry, CRA, OCT-C, FOPS
Bryson Taylor, Inc.
Saco, Maine





2023 OPS Scientific Exhibit
Third Place/Honorable Mention: Print Division
Fluorescein Angiography
3rd: Sickle Cell Retinopathy, Christiaan Lopez-Miro
3rd: VKH, Govindarajan Jayaraman
3rd: Choroidal Rupture, Shannon Howard, COA, CDOS, ROUB
3rd: Capillary Dropout Diabetic Retinopathy, Judith Gulian, OCT-C
HM: Scleral Buckle with Silicone Oil, Kathleen Warren, OCT-C
Indocyanine Green Angiography
3rd: Polypoidal Choroidal Vasculopathy, Kasi Sandhanam
HM: AMD with PCV, Nipan Yodmanee
Fundus Photography High Magnification 20°
3rd: Cavernous Angioma, Sarah Skiles, CRA
HM: Nerve Neovascularization, Ben Serar
HM: RPE Hypertrophy, Mark Harrod, CRA, OCT-C
Fundus Photography Normal 30°-40°
3rd: ILM Hemorrhage Dehemoglobinizing, Meghan Menzel, CRA
HM: Anomalous Vessel, John Leo
Fundus Photography Wide Angle 45°+
3rd: MIDD with Outer Retinal and RPE Atrophy, Michael Edrington
3rd: Coloboma, Nicole Sclafani, OCT-C
HM: Myopic Degeneration, Ben Serar
HM: Retinal Dystrophy, Bradley Stern, CRA, OCT-C
HM: CRVO with Macular Edema, Michael Edrington
HM: APMPPE, Meghan Menzel, CRA
Slit Lamp Photography
3rd: ICE Syndrome, John Leo
3rd: Globe After Scleral Buckle Surgery, Kathleen Warren, OCT-C
HM: Open Globe, Govindarajan Jayaraman
HM: Uveitis, Nick Vigo
External Photography
3rd: By A Thread, Alexandra Copple
HM: Lid Lesion, Denice Barsness, CRA, FOPS
Gross Specimen Photography
3rd: Melanophages on Sectioned Iris, Ralph Eagle Jr., MD
Gonio Photography
3rd: Lesion, Jody Troyer, CRA
HM: Unknown, Christiaan Lopez-Miro
Monochromatic Photography
3rd: Macular Oedema, Kasi Sandhanam
HM: CRAO, Kasi Sandhanam
HM: Geographic Atrophy, Megan Walsh, CRA, OCT-C
Surgical Photography
3rd: Tubes for Vision, Denice Barsness, CRA, FOPS
HM: Healing Hands, Denice Barsness, CRA, FOPS
Photo/Electron Micrography
3rd: Fuchs Dystrophy DMEKK, Ralph Eagle Jr., MD
Composite
3rd: Retinal Detachment, Mark Harrod, CRA, OCT-C
The Eye as Art
3rd: Cerulean Cataract, Megan Walsh, CRA, OCT-C
3rd: Cue the Eye Ball, Kathleen Warren, OCT-C
HM: Paw Print, Sean Grout, OCT-C
Cross Categories
3rd: Macular Star, Barbara Klemenc
3rd: Central Serous Chorioretinopathy with Massive RPE Tear, Michael Edrington
HM: Eyelash in Anterior Chamber, Allison Koenke, CRA
Optical Coherence Tomography
3rd: Macular Hole, Kasi Sandhanam
HM: ERM, Bradley Stern, CRA, OCT-C
HM: Anterior Synechiae, Diogo Correla
OCT – Angiography
3rd: Choroidal Neovascularization, Kasi Sandhanam
3rd: Diabetic Retinopathy with Neovascularization, Kasi Sandhanam
HM: Choroideremia, Sarah Skiles, CRA
Fundus Autofluorescence
HM: Optic Disc Drusen, John Leo
Ultra-Widefield Imaging
3rd: Serous Choroidal Detachment, Michael Edrington
3rd: Ruptured Globe, Sarah Skiles, CRA
HM: Giant Retinal Tear, Alexandra Copple
HM: Choroidal Detachment with Retinal Tear, Christiaan Lopez-Miro
HM: Total Retinal Detachment, Nicole Radunzel, CRA

2023 OPS Scientific Exhibit
First Place Winners: Stereo Division


Gonio Photography ExPRESS and Xen Stents
Tim Costello, CRA
University of Michigan, Kellogg Eye Center, Ann Arbor, Michigan
Cross Categories CSC
Pablo Gili, MD
Hospital Universitario Fundacion Alcorcon
Madrid, Spain

Clinical Setting Photography 3D Surgery
Pablo Gili, MD
Hospital Universitario Fundacion Alcorcon
Madrid, Spain



Instrumentation Photography
B-Scan 20 MHz Oil Leak
Tim Costello, CRA
University of Michigan
Kellogg Eye Center Ann Arbor, Michigan


CSABA MARTONYI BEST OF SHOW AWARD and BEST OF STEREO DIVISION

2023 OPS Scientific Exhibit
First Place Winners: Stereo Division


Fundus Photography High Magnification 20°
Optic Disc Pit
Pablo Gili, MD
Hospital Universitario Fundación Alcorcón Madrid, Spain
Fundus Photography Normal 30°- 40°
Choroidal Tumor
Hoang Nguyen, CRA, OCT-C, FOPS University of Colorado Eye Center Aurora, Colorado

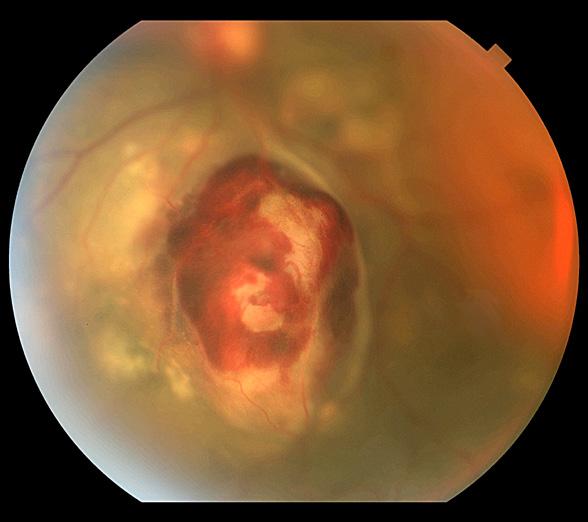


Fundus Photography Wide Angle 45°+
Asteroid Hyalosis
Sarah Skiles, CRA University of Iowa Hospitals & Clinics Iowa City, Iowa
Fundus Photography Wide Angle 45°+
Prepapillary Vascular Loop
Pablo Gili, MD
Hospital Universitario Fundación Alcorcón Madrid, Spain



2023 OPS Scientific Exhibit
First Place Winners: Stereo Division
Fluorescein Angiography
Subretinal Fluid with Macular Oedema
Kasi Sandhanam
Singapore National Eye Centre
Singapore




Fluorescein Angiography
Neovessels
Pablo Gili, MD
Hospital Universitario Fundacion
Alcorcon
Madrid, Spain
Indocyanine Green Angiography
Subretinal Fluid with Choroidal Neovascularization
Kasi Sandhanam
Singapore National Eye Centre
Singapore


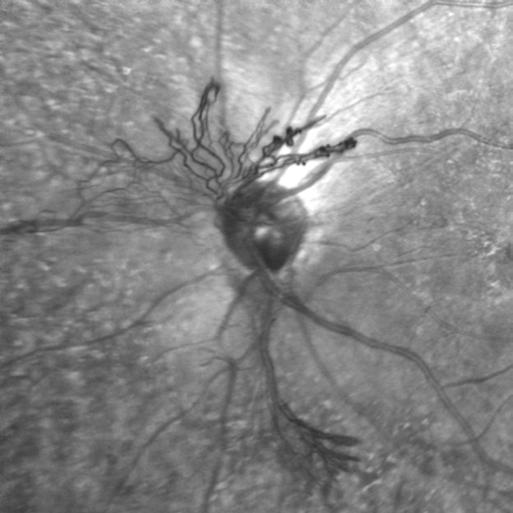

Monochromatic Photography
Optic Disc Neovascularization
Kasi Sandhanam
Singapore National Eye Centre
Singapore

2023 OPS Scientific Exhibit
First Place Winners: Stereo Division

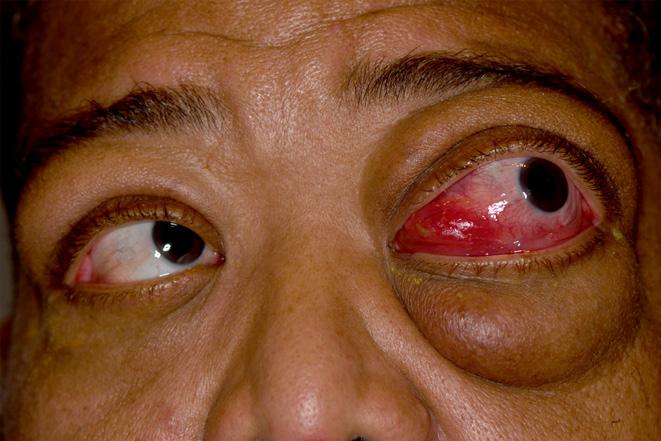
External Photography
Sarcoidosis
Mark Harrod, CRA, OCT-C
Cleveland Clinic
Cole Eye Institute Cleveland, Ohio
External Photography
Traumatic Cataract with Interior
Capsule Violation
Tony Medina, CRA. FOPS
University of Michigan
Kellogg Eye Center
Grand Blanc, Michigan




Surgical Photography
Extraction of Dislocated
Intraocular Lens
Pablo Gili, MD
Hospital Universitario Fundacion Alcorcon
Madrid, Spain
Gross Speciman
Merkel Cell Carcinoma
Dena Harris, CRA
University of Michigan
Kellogg Eye Center Ann Arbor, Michigan



2023 OPS Scientific Exhibit
First Place Winners: Stereo Division
Composite
Ocular Hamartoma
Jaime Tesmer, CRA, OCT-C
Mayo Clinic Rochester, Minnesota

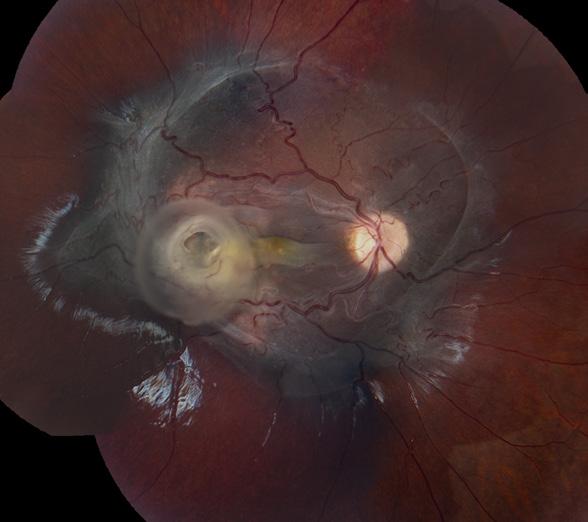


Ultra-Widefield Imaging
Retinal Detachment
Mark Harrod, CRA, OCT-C
Cleveland Clinic
Cole Eye Institute Cleveland, Ohio
Slit Lamp Photography
Inclusion Cyst
Jaime Tesmer, CRA, OCT-C Mayo Clinic Rochester, Minnesota




Autofluorescence
Choroidal Melanoma
Mark Harrod, CRA, OCT-C
Cleveland Clinic
Cole Eye Institute Cleveland, Ohio

2023 OPS Scientific Exhibit
Second Place Winners: Stereo Division


Fluorescein Angiography
Choroidal Hemangioma
Kasi Sandhanam
Singapore National Eye Centre, Singapore

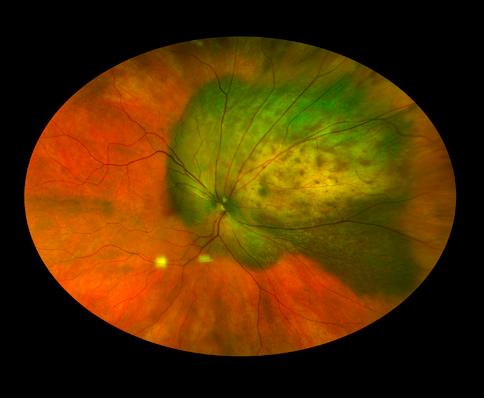
Ultra-Widefield Imaging NVM
Downer, OCT-C


Fundus Photography Normal 30°-40° Papilledema
Hoang Nguyen, CRA, OCT-C, FOPS
University of Colorado Eye Center, Aurora, Colorado

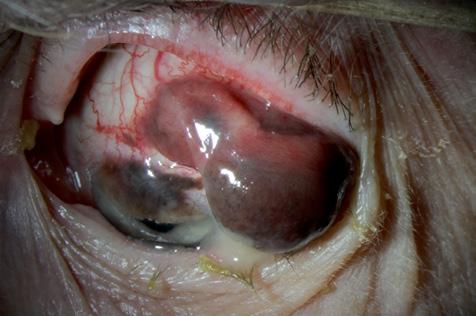


Indocyanine Green Angiography
Subretinal Fluid with Macular Oedema
Kasi Sandhanam
Singapore National Eye Centre, Singapore


Fundus Photography High Magnification 20° Papilledema


Fundus Photography Wide Angle 45°+
Severely Fibrosed Macula Due to AMD
Chris Keth, CRA, OCT-C
Duke Eye Center, Durham, North Carolina


Clint
University of Michigan, Kellogg Eye Center, Ann Arbor, Michigan
Bradley Stern CRA, OCT-C Henry Ford Health, Detroit, Michigan
Slit Lamp Photography Conjunctival Melanoma
Mark Harrod, CRA, OCT-C Cleveland Clinic, Cole Eye Institute, Cleveland, Ohio
Slit Lamp Photography S/P Enucleation
Tim Costello CRA University of Michigan, Kellogg Eye Center, Ann Arbor, Michigan



External Photography
Band Keratopathy with Pseudophakic Bullous Keratopathy
Tony Medina, CRA, FOPS
University of Michigan Kellogg Eye Center, Grand Blanc, Michigan


Monochromatic Photography
Optic Nerve Coloboma
Pablo Gili, MD
Hospital Universitario Fundación Alcorcón, Madrid, Spain


Gonio Photography
Artificial Iris s/p Iris Melanoma Removal
Jaime Tesmer, CRA, OCT-C Mayo Clinic, Rochester, Minnesota


Autofluorescence
Pachychoroid/CSCR
Tony Medina, CRA, FOPS
University of Michigan, Kellogg Eye Center, Grand Blanc, Michigan


Composite IPCV
John Leo


Surgical Photography
Intraocular Lens InJection
Pablo Gili, MD
Hospital Universitario Fundación Alcorcón, Madrid, Spain
Rajan Eye Care Hospital Pvt Ltd, Chennai, India

2023 OPS Scientific Exhibit
Third Place/Honorable Mention: Stereo Division
Fluorescein Angiography
3rd: Polypoidal Choroidal Vasculopathy, Kasi Sandhanam
3rd: Amelanotic Choroidal Lesion, Clint Downer, OCT-C
3rd: Hyperfluorescent Choroidal Lesion, Tim Costello, CRA
HM: Epiretinal Membrane, Pablo Gili, MD
HM: Stereo View of Non-proliferative Diabetic Retinopathy, Michael Edrington
HM: CRVO with CME, Tony Medina, CRA, FOPS
Indocyanine Green Angiography
3rd: Polypoidal Choroidal Vasculopathy, Kasi Sandhanam
3rd: Malignant Tumor, Tim Costello, CRA
Fundus Photography High Magnification 20°
3rd: Optic Disc Anomalie, Ben Serar
3rd: Optic Disc Drusen, Pablo Gili, MD
HM: Dense Epiretinal Membrane, Jaime Tesmer, CRA, OCT-C
Fundus Photography Normal 30°-40°
3rd: Untitled, John Leo
HM: Fibrotic Neovascularization, Darrin Landry, CRA, OCT-C, FOPS
Fundus Photography Wide Angle 45°+
3rd: IPCV, John Leo
HM: PDR with Diabetic Tractional Detachment, Aaron Howard, CRA, OCT-C
HM: Choroidal Melanoma, Jaime Tesmer, CRA, OCT-C
Slit Lamp Photography
3rd: Epithelial Growth, Tim Costello, CRA
HM: Untitled, Sean Grout, OCT-C
Gonio Photography
3rd: Iris Melanoma, Tim Costello, CRA
HM: Lesion in Angle, Sarah Skiles, CRA
Monochromatic Photography
3rd: Retinal Traction Detachment, Pablo Gili, MD
HM: Papilledema, Pablo Gili, MD
Surgical Photography
3rd: Capsulorhesis, Pablo Gili, MD
Composite
3rd: Choroidal Melanoma, Jaime Tesmer, CRA, OCT-C
HM: Epiretinal Membrane, Pablo Gili, MD
Fundus Autofluorescence
3rd: Leukemic Retinopathy,Tony Medina, CRA, FOPS
HM: Retinal Dystrophy, Mark Harrod, CRA, OCT-C
Ultra-Widefield Imaging
3rd: Congenital Retinal Fold, Tim Costello, CRA
HM: Retinal Detachment with Single Break, Mark Harrod, CRA, OCT-C

Case Report

Michelle H Ferneding, BS, RVT
Bianca Martins, DVM, PhD, DACVO
Dr. Savannah Vig, DVM, DACVO
Sara Thomasy, DVM, PhD, DACVO
University of California School of Veterinary Medicine
Comparative Ophthalmology and Vision Sciences Laboratory One Shields Ave. Davis, CA 95616
530.752.6967 mhferneding@ucdavis.edu
Corneal Cross-Linking with Photo-Activated Riboflavin in a Dog with Stromal Keratitis
T, a 9-year-old MC Chihuahua, was presented to the UC Davis Ophthalmology Service with a malacic ulcer as a possible candidate for the corneal cross-linking trial.
The right eye was previously enucleated. The left eye was blepharospastic with mild mucoid discharge. The direct pupillary light reflex of the left eye was reduced due to miosis. There was 10mm x 10mm diameter of epithelial loss with severely malacic and bullous stroma with cellular infiltrate in the axial cornea surrounded by stromal thinning (~40% depth). Moderate, diffuse corneal edema with 3 mm of superior perilimbal corneal fibrosis at the 11 o’clock position with infiltrating vessels was also

Figure 1: (a) Diffuse illumination with digital slit lamp photography using a Topcon D4 demonstrates a large malacic corneal ulcer with cellular infiltrate present inferiorly. (b) With the slit beam, the region of cellular infiltrate is more easily visible (arrow) and moderate corneal edema is also observed. (c) A digital image was taken with a Canon EOS 6D demonstrates axial stromal retention of fluorescein. A Schirmer tear test strip was used to measure ulcer diameter. Images courtesy of UC Davis Comparative Ophthalmology Service.

observed. The anterior chamber was well formed with hypopyon occupying the inferior aspect, the remaining intraocular could not be assessed due to corneal opacity. Due to the size of the ulcer, the patient was not enrolled in the clinical trial. However, corneal crosslinking was performed by exposing the eye to a photosensitizing 0.1% riboflavin solution for 20 minutes. The irradiation field of diameter of the Peschke cross-linking unit was set to the ulcer diameter and there is a distance of 50mm between the beam aperture and the eye. The patient was prescribed both topical and oral medications. Each of the topical medications were instructed to be given one drop in the left eye every two hours. The topical medications prescribed were Moxifloxacin, 0.5% ophthalmic solution (topical antibiotic); cefazolin, 40mg/ ml ophthalmic solution (topical antibiotic); Dorzolamide/ Timolol, ophthalmic solution (topical anti-glaucoma); Serum, ophthalmic solution (topical healing aid). Oral medications were also prescribed which included Clavamox 62.5mg tablets one tablet by mouth every 12 hours (antibiotic); Prednisolone 5mg tablets one half tablet by mouth every 24 hours (steroidal anti-inflammatory); Doxycycline 50mg tablets one half tablet by mouth every 12 hours (antibiotic and anticollagenase).
2: Spectral-domain optical coherence tomography demonstrates axial epithelial loss with markedly thickened, hyper-reflective corneal
QT was healed with full vision when examined at his three month recheck by the referring veterinarian.
Figure
stroma (OCT-IR, horizontal line scan). Images courtesy of UC Davis Comparative Ophthalmology Service.

Carol Harbers, MA
Senior
Director
Communications and Marketing
Duke Neurosurgery
919.699.3245
Carol.harbers@duke.edu
New Therapy for Glioma Receives FDA Approval
TDuke brain tumor researchers are part of earliest collaborations that led to the development of the drug, shown to more than double progression-free survival he FDA has approved a new targeted drug specifically for brain tumors called low-grade gliomas. The drug, vorasidenib, was shown in clinical trials to delay progression of low-grade gliomas that had mutations in the IDH1 or IDH2 genes.
“Although there have been other targeted therapies for the treatment of brain tumors with the IDH mutation, [this one] has been one of the most successful in survival prolongation of brain tumor patients,” said Darell Bigner, MD, PhD, the E. L. and Lucille F. Jones Cancer Distinguished Research Professor and founding director of the Preston Robert Tisch Brain Tumor Center at Duke.
In clinical trials, progression-free survival was estimated to be 27.7 months for people in the vorasidenib group versus 11.1 months for those in the placebo group.
Dr. Bigner, Katherine Peters, MD, PhD, professor of neurology and neurosurgery, and others at the Duke Brain Tumor Center played pivotal roles in the development and approval of the drug: Dr. Bigner in the early collaborations with Johns Hopkins University that led to the discovery of the IDH mutation, and Dr. Peters, more recently, as lead investigator in the clinical trials.
Patents developed from the early collaborations were licensed to industry through the Duke University Office for Translation and Commercialization, making this the seventh drug currently on the market with Duke intellectual property roots.
Drs. Bigner and Peters as well as Hai Yan, MD, PhD (formerly the Henry S. Friedman Distinguished Professor of Neuro-Oncology at Duke) answered questions about the work that led to vorasidenib.
Discovery
How did the discovery of the IDH gene mutation contribute to the overall understanding of brain cancer?
Bigner: The discovery of the mutant IDH gene is one of the most important discoveries in neuro-oncology. The IDH mutation has been incorporated by the World Health Organization into the rapid and accurate diagnosis and classification of astrocytic, oligodendroglial, and glioblastoma multiforme brain tumors. Never before has there been a single gene mutation that contributed so greatly to classification. Most importantly, it was immediately recognized that the IDH mutation could be targeted with drugs to treat the group of patients that had malignant brain tumors that expressed the IDH mutation.
Describe the collaboration between Johns Hopkins and Duke that led up to the development of this drug.

Figure 1: Duke researcher Darell Bigner, former Duke researcher Hai Yan, and Duke neuro-oncologist Katherine Peters were instrumental in the discovery and clinical research that led to a new drug for glioma, now approved by the Food and Drug Administration.
Bigner: Perhaps the most important collaboration between Johns Hopkins and Duke came in the work that led to the discovery of the IDH mutation. The National Cancer Institute had established a program in which genome sequencing of all the major cancers was to be done and decided that glioblastoma would be the first cancer that they investigated. The Johns Hopkins and Duke group decided to also perform complete genome sequencing of glioblastoma. The NCI did not do complete genome sequencing. Using the Duke material, the Johns Hopkins group sequenced the entire genome that could be done at that time, in 2008. The sequencing at that time was very laborious, rather than in the automated manner that can be done now. By doing almost complete genome sequencing, the Johns Hopkins and Duke group discovered the IDH mutation. The collaboration with Johns Hopkins was strengthened when [we] recruited Dr. Hai Yan [to Duke] in 2003. Dr. Yan had just completed a 5-year period as a post-doctoral research fellow at Johns Hopkins with Dr. Bert Vogelstein in Cancer Molecular Genetics.
Yan: Subsequent research from these teams produced numerous publications that further elucidated the pathological roles of IDH mutations, leading to the reclassification of gliomas in the WHO CNS classification. This body of work ultimately paved the way for the development of targeted therapies, culminating in the approval of [vorasidenib]. This collaboration … exemplifies the power of interdisciplinary and inter-institutional cooperation in driving scientific discovery and innovation in cancer treatment.
Can you briefly explain mechanism of action of vorasidenib?
Yan: Mutations in the IDH1 or IDH2 genes result in elevated levels of the oncometabolite D-2HG, disrupting normal cellular functions and contributing to tumorigenesis. Vorasidenib selectively binds to the mutated IDH1 and IDH2 enzymes, inhibiting their activity and thereby reducing the production of D-2HG. This inhibition helps to restore normal cellular processes, reduce tumor cell proliferation, and promote the differentiation of cancer cells.
How does the development and approval of vorasidenib affect the broader future of cancer research and treatment? Are there plans to study vorasidenib in combination with other treatments or in different types of brain cancers?
Yan: The development and approval of vorasidenib represent a significant milestone in the field of oncology, particularly in the treatment of brain cancers. It validates the approach of targeting specific genetic mutations with precision therapies and reinforces the importance of personalized medicine in oncology. This success is likely to inspire further research into targeting other genetic mutations and metabolic pathways in various cancers.
Bigner: There are indeed plans to explore the potential of vorasidenib beyond its current indications. Researchers are investigating its use in combination with other thera-
pies, such as immune checkpoint inhibitors, to enhance therapeutic efficacy. Additionally, studies are being planned or are already under way to assess the effectiveness of vorasidenib in treating other types of brain cancers, solid tumors and leukemia with IDH mutations. The ongoing research aims to expand the therapeutic applications of vorasidenib and optimize its use in various clinical settings, potentially benefiting a broader spectrum of cancer patients.
What the Clinical Research Showed
What were the outcomes of the clinical trial for vorasidenib?
Peters: The INDIGO clinical trial was a phase 3 trial of vorasidenib, an oral inhibitor of mutant IDH1/2 that can readily cross the blood-brain barrier, versus placebo in patients with mutant IDH1/2 glioma. Treatment with vorasidenib significantly improved progression-free survival (27.7 months vorasidenib vs. 11.1 for placebo).
The key secondary endpoint was time to next intervention, which means the time to needing chemotherapy, radiation therapy, or more surgery. For patients receiving placebo, the median time to next intervention was 17.8 months, but for patients receiving vorasidenib, the median time to next intervention has not yet been reached. Thus, patients on vorasidenib could significantly delay chemotherapy, radiation therapy, or more surgery. Most importantly, the vorasidenib was well tolerated with only 3.6% of patients needing to stop the drug because of an adverse event.
What about quality of life for patients on the trial?
Peters: Results showed that throughout the study, patients with IDH mutant low grade glioma had a good quality of life, and it was preserved throughout the study. Patients on vorasidenib were able to maintain their cognitive abilities and did not have any decline in their quality of life or cognition.
How might this drug influence future research and development in neuro-oncology?
Peters: At Duke, we are conducting studies of vorasidenib on patients with high-grade tumors and enhancing disease. Most of these studies look at combining vorasidenib with immunotherapy. It will be exciting to see what will happen with the INDIGO study’s long-term outcomes.
What does the approval of vorasidenib mean for the treatment landscape of low-grade gliomas?
Peters: It is exciting to have a drug specifically targeted for these patients by inhibiting the mutant IDH enzyme. With vorasidenib being orally available, well-tolerated, and does not impair quality of life or cognition, we can extend people’s lives and delay the use of treatments such as radiation therapy and chemotherapy. I am so thankful to all the patients who participated in the groundbreaking study and for paving the way for future patients.
2024 Ophthalmic Photography Exhibit
First Place Winners
These are the winning images of the 2024 American Society of Cataract and Refractive Surgery (ASCRS) ophthalmic photography exhibit. The OPS Scientific Exhibit Committee partners with ASCRS each year to make this competition a reality. We look forward to seeing your entries for the 2025 exhibit in Los Angeles, CA. For further information, see https://www. opsweb.org/page/SEC_OPSASCRS or contact Jonathan Hawkins at johnathan.hawkins@retinaconsultants-texas.com
BEST OF SHOW
Retinal Fluorescein Angiography
Wyburn-Mason Syndrome
Stephanie Burke, MS, CRA, OCT-C Cole Eye Institute, Cleveland Clinic Cleveland, Ohio


Fundus Autofluorescence Stargardt Disease
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center Ann Arbor, Michigan

Slit Lamp Photography
Ectropic Iris
Denice Barsness, BA, CRA, COMT, ROUB
EyeEducateU
Rhonert Park, California
2024 Ophthalmic Photography Exhibit
First Place Winners

Fundus Photography Normal 30°- 40°
Bilateral Papilledema
Christopher Keth, BA, CRA, OCT-C
Duke University Eye Center
Durham, North Carolina

Fundus Color Ultra-Widefield Photography > 60°
Retinal Hem Leukoma
Meghan Menzel, BA, CRA
University of Iowa Hospitals and Clinics
Iowa City, Iowa

Fundus Photography Wide Angle 45°+ CRAO
Tracey Troszak, CRA Henry Ford Health System Detroit, Michigan

Indocyanine Green Angiography Neovascularization
Kasi Sandhanam
Singapore National Eye Centre
Singapore
2024 Ophthalmic Photography Exhibit First Place Winners

Monochromatic Photography NVE
Megan Walsh, CRA
Eyecare Medical Group Portland, Maine

External Photography
Squamous Cell Carcinoma
Jason Calhoun, COA
Mayo Clinic Jacksonville, Florida

Composite Image
Retinal Artery Occlusion
Jason Calhoun, COA Mayo Clinic Jacksonville, Florida

Optical Coherence Tomography
Glaucoma Shunt Tube
Sean Grout, OCT-C
CPMC Department of Ophthalmology
San Francisco, California
2024 Ophthalmic Photography Exhibit
First Place Winners

The Eye as Art Vortex Veins
James Gilman, BSc, CRA
John A. Moran Eye Center
Salt Lake City, Utah

Gonio Photography Iris Melanoma
James Gilman, BSc, CRA
John A. Moran Eye Center
Salt Lake City, Utah

Cross Categories
Pigmented Paravenous Chorioretinal Atrophy
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center
Ann Arbor, Michigan
2024 Ophthalmic Photography Exhibit Second Place Winners

Fluorescein Angiography
Type 1 Diabetic Retinopathy
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center Ann Arbor, Michigan

Fundus Photography Normal 30°- 40° Melanocytoma
Stephanie Burke, MS, CRA, OCT-C
Cole Eye Institute, Cleveland Clinic Cleveland, Ohio

Slit Lamp Photography
Corneal Pigmentation
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center Ann Arbor, Michigan

OCT Angiography
Retinal Detachment with Macular Hole
Nipan Yodmanee, OD, COA
Mettapracharak (Wat Rai Khing) Hospital
Tha Talat, Thailand

Fundus Photography Wide Angle 45°-60° Drusen
Tracey Troszak, CRA
Henry Ford Health System Detroit, Michigan

Gonio Photography Iridectomy
James Gilman, BSc, CRA
John A. Moran Eye Center Salt Lake City, Utah

Fundus Autofluorescence
Chorioretinal Degeneration
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center
Ann Arbor, Michigan

Fundus Color Ultra-Widefield
Photography > 60°
Wyburn Mason
Meghan Menzel, BA, CRA
University of Iowa Hospitals and Clinics Iowa City, Iowa

The Eye as Art
Iris Watercolor
Denice Barsness, BA, CRA, COMT, ROUB
EyeEducateU Rhonert Park, California
2024 Ophthalmic Photography Exhibit
Second Place Winners

External Photography
Keratoprosthesis – Grafting the Curve
Prasanna Ramesh, MS, DNB
Mahathma Eye Hospital Private Limited Tiruchirappalli, India

Composite Image
Flecked Fundus
Shruthy Ramesh, FRCOphth, MS, DNB
Mahathma Eye Hospital Private Limited Tiruchirappalli, India

Cross Categories
Sub-Internal Limiting
Membrane Hemorrhage
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center Ann Arbor, Michigan

Indocyanine Green Angiography
Choroidal Mass with RD. Nipan Yodmanee, OD, COA
Mettapracharak (Wat Rai Khing) Hospital Tha Talat, Thailand

Monochromatic Photography
Axenfeld-Rieger Syndrome
Sean Grout, OCT-C
CPMC Department of Ophthalmology San Francisco, California
Los Angeles, California, April 25–28, 2025
2024 Ophthalmic Photography Exhibit
Third Place Winners and Honorable Mention
Fluorescein Angiography
3rd: Apmppe, Meghan Menzel, BA, CRA
HM: Serous Detachment, David Miller, BSc, CRA
HM: Vascular Occlusive Disease, James Gilman, BSc, CRA
HM: Unknown Unilateral Dx, Not Thought to be Retinitis Pigmentosa, Sara Ash, BA
Indocyanine Green Angiography
HM: Choroidal Lesion, Judith Gulian, BSc, OCT-C
HM: Retinal Capillary Hemangioma, Nipan Yodmanee, OD, COA
Fundus Photography High Magnification 20°
HM: Optic Nerve Cupping, Lacee Cooper, BA, COA, CRA
HM: Idiopathic Intracranial Hypertension, Nicole Radunzel, BA, CRA
Fundus Photography Normal 30°-40°
3rd: Cherry Red Spot, Shilpi Narnaware, DO; Dr. Prashant Bawankule, MS
HM: Macular Scarring, Bridgette Barnes
Fundus Photography Wide Angle 45°-60°
3rd: Macroaneurysm, Bradley Stern, BA, CRA, OCT-C
HM: Exudative Age-Related Macular Degeneration, Charlie Hain, BA
HM: Astrocytic Hamartoma, Cnvm, Sean Grout, OCT-C
Fundus Color Ultra-Widefield Photography > 60°
3rd: CRVO, Megan Walsh, CRA
HM: Peripheral Vessel Arborization with Macular Staphyloma, Judith Gulian, BSc, OCT-C
HM: Central Serous Chorioretinopathy, Nicole Radunzel, BA, CRA
Slit Lamp Photography
3rd: Persistent Pupillary Membrane: The Uncut Cord, Kumar Doctor, MD
HM: Choroidal Melanoma (ANTERIOR), Jason Calhoun, COA
HM: Pseudoexfoliaton Syndrome, Jason Calhoun, COA
HM: Granular Corneal Dystrophy... Stars in Your Eyes., Luisa Yañez Ortega, MD
HM: Fuchs Heterochromic Cyclitis with Subluxed IOL, Megan Walsh, CRA
HM: Cataracta Traumatica, Nicole Mantel
Gonio Photography
3rd: Iris Melanoma, Stephanie Burke, MS, CRA, OCT-C
HM: Unknown, Christiaan Lopez-Miro, BA
Monochromatic Photography
3rd: Crystal Clear Crystalline Lens in the Anterior Chamber, Shruthy Ramesh, FRCOphth, MS, DNB
HM: Best Disease, Bradley Stern, BA, CRA, OCT-C
HM: Drusen on Retro Mode, Shilpi Narnaware, DO; Dr. Prashant Bawankule, MS
External Photography
3rd: Basal Cell Carcinoma, David Miller, BSc, CRA
Gross Specimen Photography
HM: Wet Lab Enucleated Eye Dissection, Anuj Kodnani, MBBS, MS, DNB
HM: Nigrans, Rodrigo Tzec
HM: Phaco Failed, SICS to the Rescue, Rodrigo Tzec
Composite
3rd: Choroideremia, Christopher Keth, BA, CRA, OCT-C
HM: Geographic Atrophy, Jason Calhoun, COA
HM: Cscr, Hydrocephalus, Ventriculomegaly, Optic Nerve Edema, Sean Grout, OCT-C
The Eye as Art
3rd: The Wicked Forest, John Leo, BSc
HM: Water Eye, James Gilman, BSc, CRA
HM: Rough Waters Ahead, Sarah Skiles, MA, BA, CRA
Cross Categories
3rd: Idiopathic Choroidal Folds, Shilpi Narnaware, DO; Dr. Prashant Bawankule, MS
HM: Plaquenil Toxicity, Megan Walsh, CRA
HM: Leopard Skin Retinopathy, Sousa Jefferson, BEng, COMT
Optical Coherence Tomography
3rd: Anterior Synechia Following Ocular Trauma, Diogo Correia, BSc
HM: Dislocated Crystalline Lens in AC, Prasanna Ramesh, MS, DNB
HM: Posterior Polar Cataract Shaped like a Christmas Tree: Santa’s Present, Shivani Pattnaik, MS
OCT – Angiography
3rd: Vermicelli in the Optic Nerve Head, Anugraha Balamurugan, MS
HM: Choroidal Neovascularization, Kasi Sandhanam
Fundus Autofluorescence
3rd: Atypical Retinitis Pigmentosa with Rod-Cone Dysfunction, Judith Gulian, BSc, OCT-C
HM: Retinal Detachment, Nipan Yodmanee, OD, COA
HM: Bear Tracks, Sean Grout, OCT-C
Corneal Endothelial Photography
HM: Nor Big or Small – Asymmetrical Polymegathism Between the Eyes, Shruthy Ramesh, FRCOphth, MS, DNB
Intraoperative/Surgical Microscope/Refractive
Surgery Images
HM: Intra Operative Photo of Fundus with Multiple Fungal Seedings, Shilpi Narnaware, DO; Dr. Prashant Bawankule, MS
Eye Candy
images by Michael P. Kelly, FOPS

This is a choroidal rupture from blunt-force ocular trauma; the mechanical compression and hyperextension of the globe results in a crescent-shaped break in the choroid, Bruch’s membrane and RPE, along with subretinal hemorrhage in some cases. The optic nerve serves as an anchor of sorts during trauma to the eye. FAF imaging can nicely reveal the break(s) as hypo-autofluorescent. Later, choroidal neovascularization occurs in response. Fresh ruptures can be obscured by hemorrhage and may only become visible as the blood dissipates. The crescent moon-sign is almost always concentric following the shape of the proximal side of the optic nerve, is white or yellow in color, and pathognomonic for choroidal rupture, so the diagnosis can be made by the ophthalmic photographer during imaging. These curvilinear breaks can be of any size and there may be more than one. The repetitive linear reflections serve as another visual cue as to the extent of the three dimensional retinal damage inflicted, likely from puckering centrally. A word about magnification in all disorders: compose the image by choosing the magnification to best fit the area of interest, leaving one to two disc diameters of normal retina around it; ultra-wide field imaging is not the best choice for every disorder.
This is iris atrophy which is best imaged using a low-level fill light and strong retro-illumination in an eye just a minute or two after installing dilating drops, so the pupil is just in the beginning stages of dilation. Fixation should be slightly off-center to ‘place’ the optic nerve-head in the path of retro-illumination; this allows for more light as the optic nerve is more reflective than the surrounding retina. The retro-illumination light should be reduced in size to fit through the pupil, without touching the iris edge. The low-level fill light illuminates the conjunctival vessels and the intact portions of the iris, making the image even more dramatic and useful.


Vicki D Frye
vicki.frye@intersection19.com intersection19.com
https://www.linkedin.com/in/vickifrye/ 919.428.7736
The Promise of Gene Therapy: Bringing Hope to Patients with Inherited Retinal Disease
Inherited retinal degenerations are genetic diseases that cause irreversible blindness. Developing treatments for these conditions is a challenging task, particularly because they are caused by mutations in over 300 genes that lead to varying types and severity of vision loss.
Historically there has been no cure for inherited retinal degenerations. Recent innovations in research have provided a promising type of treatment called gene therapy. This approach aims to correct defective genes by replacing them with healthy ones.
Bench to Bedside
The introduction of gene therapy to practical ophthalmology is a remarkable example of how a complex human disease can be addressed by combined efforts of scientists and clinicians through a “bench-to-bedside” approach. This approach highlights the tremendous efforts of laboratory research teams behind any development of breakthrough medications.
One particularly severe type of inherited blindness is Leber congenital amaurosis, which impacts children at a young age. The first breakthrough in understanding the cause of blindness for those with Leber’s was in the 1990s, when scientists discovered that the disease arises from an error in the RPE65 gene. This set the stage to take action against this disease.
During the same time another group of scientists found that the same gene, RPE65, is affected in a group of dogs who are blind. This discovery led to an unprecedented opportunity to determine if supplying these dogs with a healthy RPE65 gene would enable them to regain vision.
A few years later, a dog named Lancelot made an unusual visit to the U.S. congress. Lancelot had been born blind but could now clearly see the members of the congress after receiving a revolutionary gene therapy treatment. A collaborative effort by teams of scientists and clinicians made history by showing that replacement
of the damaged RPE65 gene with a healthy one could restore vision in animals.
The path to curing blindness in human patients was now in sight. The treatment entered clinical trials, and for the first time, children treated with a healthy replacement gene could see the faces of their parents and siblings. Upon completion of the trial, the drug now known as Luxturna became the first gene therapy approved by the FDA to treat any genetic blinding condition. The landmark bench-to-bedside development of Luxturna emphasized just how much science can do to improve the wellbeing of the public; but it also emphasized how many resources are required at every step from scientific discovery to clinical application.
Luxturna Comes to Duke
Duke Eye Center is a premier destination for the treatment of vitreoretinal diseases, offering the latest in patient care. As a recently Certified Center of Excellence for Luxturna, Duke is the only Luxturna treatment center between Philadelphia and Miami for patients on the East Coast of the U.S. Patients eligible for Luxturna treatment are cared for by a team of expert clinicians, including specialists in the diagnosis and management of inherited retinal diseases and vitreoretinal surgeons with advanced expertise in delivering gene therapy medications to the retina. The clinical team is supported by a dedicated genetic counselor working with patients and their families to uncover their diagnoses through specialized genetic testing.
An important aspect of patient care is a comprehensive retinal electrophysiology facility, where patients undergo sophisticated testing, such as electroretinography (ERG), to measure the ability of the retina to detect light. There, patients who are potential candidates for Luxturna treatment undergo detailed testing in preparation for treatment and to monitor treatment response.


Beyond Current Therapy: The Quest for Universal Therapies
The development of Luxturna is a monumental achievement, but this is just a first step in helping patients suffering from inherited retinal degenerations. Patients eligible for Luxturna treatment represent less fthan one in a thousand of all people suffering from these conditions. This is because there are more than 300 other genes whose defects lead to various forms of this disease, with more still discovered every year and only a handful addressed in ongoing trials. A major challenge with developing new therapies, especially gene therapy, is that it takes a tremendous amount of time and money to bring each treatment to clinic.
To overcome this challenge, scientists at Duke Eye Center are working to develop universal therapies that can save sight regardless of which genes are mutated. The scientific foundation of this pursuit is their discovery that many mutations causing inherited retinal degenerations lead to a buildup of damaged proteins in the retina. As everywhere else in the body, the retina has a built-in system for garbage disposal called proteasomes, which eliminate damaged proteins to keep the retina healthy. In retinas affected by inherited degenerations, proteasomes may be overwhelmed and become unable to keep up with

2: The concept of proteasome enhancement, to assist in clearing of damaged proteins in the retina, was discovered in the laboratory of Vadim Arshavsky, PhD. His laboratory reported promising treatment results in animals with the same mutations as those found in human patients.
Inherited Retina Dystrophies
Clinical Team
Oleg Alekseev, MD, PhD (see honors and awards folder)
Ramiro Maldonado, MD (see honors and awards folder)
Surgical Team
Xi Chen, MD, PhD (see honors and awards folder)
Lejla Vajzovic, MD (see honors and awards folder)
Genetic Counselor
Anna Duemler, MS
Laboratory Research Team (5886)
Left to right, back row: Tylor Lewis, PhD; Penelope FerryLeeper, BS, GCTC, RAC; Oleg Alekseev, MD, PhD. Left to right front row: Natasha Klementeva, PhD; Lauren Cao, BS; Lin Yu, PhD; Stella Finkelstein, MS; Carson Castillo, MS; Margaux Kreitman, PhD; Vadim Arshavsky, PhD.

the disposal of damaged proteins. A sound solution would be to bring new proteasomes to the rescue, and this is exactly what Duke scientists strive to bring to degenerating retinas in order to clear proteins damaged by diseasecausing mutations.
The concept of proteasome enhancement was discovered in the laboratory of Vadim Arshavsky, PhD, a
Figure 1: Oleg Alekseev, MD, PhD joined Duke Eye Center faculty in 2022 help translate proteasome enhancement into an effective treatment.
Figure 3: Dr. Alekseev and Dr. Arshavsky.
Figure
world-renowned retina scientist and Vice Chair for Basic Science Research at Duke Eye Center. His laboratory reported promising treatment results in animals with the same mutations as those found in human patients.
Much more remains to be done to translate this scientific discovery into an effective treatment. These efforts are spearheaded by Oleg Alekseev, MD, PhD who joined Duke Eye Center faculty in 2022. Trained as both a scientist and a clinician, he is uniquely qualified to move this program forward. “Learning from my patients in the clinic inspires me to work tirelessly in the laboratory to better understand their conditions and to devise novel treatments to improve the quality of their lives”, said Alekseev.
Pioneering the Future of Vision at Duke
To integrate, facilitate and expand the audacious efforts of these and other researchers, Duke Eye Center has an exciting plan to establish the Center for Innovative Treatments of Retinal Degenerations. This will establish a
Presentation

DJanet Teng, COMT
Duke Eye Center janet.teng@duke.edu
robust translational research pipeline, whereby the ideas generated in laboratories and clinics will be converted into novel therapies. In this pipeline, new discoveries will be evaluated in blind animals first, followed by a streamlined process of developing and testing new therapeutics, and subsequently advancing the most promising candidates to clinical trials.
Other scientists at Duke Eye Center engaged in drug discovery and development to cure inherited blindness are Sidney Gospe, MD, PhD, who addresses rare but disastrous and currently incurable blinding conditions affecting the optic nerve, and Jeremy Kay, PhD, who is tackling a severe form of childhood blindness by using gene therapy.
Duke is fortunate to have a broad range of expertise on the same campus, which allows for enhanced collaboration. With the expertise in place, financial resources are needed to continue groundbreaking research that will hopefully one day cure blindness for people with inherited retinal degenerations.
PRGF –Plasma Rich in Growth Factors
uke Eye Center has been offering PRGF (Plasma Rich in Growth Factors) eye drops for more than six years. PRGF are autologous eye drops made from a patient’s own blood to treat ocular surface diseases. Because platelets are “activated” during its processing, PRGF contains a higher concentration of nutrients and growth factors compared to autologous serum tears. These drops are often used for advanced healing needs like wound repair and tissue regeneration but are also very effective for dry eye syndrome.
PRGF was initiated at Duke by former Foster Center for Ocular Immunology director Victor Perez, MD. Upon Perez’ departure from Duke, the program continued under the leadership of Amol Sura, MD, assistant professor of ophthalmology, cornea and ocular surface disease specialist
and Janet Teng, COMT. Teng became a certified phlebotomist and learned the laboratory work which requires tubes of a patient’s blood to undergo a specific centrifugation and incubation process to isolate and concentrate platelets.
After doctors identify candidates, Teng and her PRGF team will counsel patients, draw blood, create the product in an on-site sterile laboratory, and deliver the final product to the patient.
Today, we offer PRGF twice a week to our patients and appointments are always full. Many patients travel across the country to obtain PRGF treatment at Duke.
Duke is fortunate to offer this cutting-edge therapeutic technology. “PRGF is the premier option for autologous plasma tears, I am proud to be a part of this program and that we can offer it to patients at Duke Eye Center,” said Teng.



