

Under the Surface: A Clear Look Into the Cornea

2023 ASCRS 2nd Place Winner, Slit Lamp Photography
Inflammatory Pupillary Membrane in Patient with Endophthalmitis
Kingston Rodolfo Ureña-Wong
Asociación para evitar la ceguera en México, Mexico City, Mexico

Subscription Information
The Journal of Ophthalmic Photography (ISSN 0198-6155) is published biannually by the Ophthalmic Photographers’ Society, Inc. Membership in the Ophthalmic Photographers’ Society includes an annual subscription to the The Journal of Ophthalmic Photography. Membership requests, subscription inquiries, past issue requests and changes of address should be sent to Barbara McCalley, Executive Director, 1621 East Jody Circle, Republic, MO 65738; Phone: 417.725.0181; Email: ops@opsweb.org.
Instructions to Authors
Instructions to Authors can be found at http://www.opsweb.org/?page=journal
The Ophthalmic Photographers’ Society
The Ophthalmic Photographers’ Society (OPS) is a nonprofit international organization dedicated to the advancement of photography as applied to ophthalmology and visual science. Our membership, numbering over 1000, includes a broad spectrum of professionals in eye health care, including ophthalmologists, optometrists, photographers, nurses, ophthalmic medical personnel, and basic researchers. The Society’s educational programs, designed to supplement the professional expertise of our members, have earned the OPS recognition as being an organization dedicated to the elevation of standards in the craft. The Society’s major publication, The Journal of Ophthalmic Photography, presents tutorials and articles on photographic techniques, instrumentation, and related topics in ophthalmology. Visit us online at www.opsweb.org.
Copyright
The Ophthalmic Photographers’ Society. All rights reserved. Printed in the U.S.A. None of the contents may be reproduced by mechanical or electronic processes, stored in a retrieval system or transmitted by any means without prior written permission from the Editor.




Volume 46, Issue 1, 2024

Clearly the Cornea
Monica Motta
Original Article: What’s Your Diagnosis? Morning Rounds with Sharon F. Freedman, MD, and Melissa B. Daluvoy, MD
Esteban A. Peralta Chacon, MD; Melissa B. Daluvoy, MD; Sharon F. Freedman, MD; James Tian, MD; Edited By Ahmad A. Aref, MD, MBA
Case Report: Slit Lamp Photographs Comparing Healthy and Diseased Corneas
Monica Ardon-Vasallo, BS; Karolina Paulina Roszak, DVM
Original Article: An Update on Advances in Diagnosis and Treatment of Acanthamoeba Keratitis
Esteban A. Peralta Chacon, MD; Kourtney Houser ; James Tian, MD
Original Article: Eyes on a Miracle
Michael Penn; Photography by Chris Hildroth
Case Report: An Afternoon with Dr. Brooklynn Lafoon
Brooklynn LaFoon, DVM, DACVO
Gustavo Moreira Madeira, IOCM
Nicole Mantel
Anahli Vazquez-Galvan,
Sean Grout, OCT-C

in 1977


Editor in Chief
Kathleen Warren Duke University Eye Center Department of Ophthalmology 2351 Erwin Road Durham, NC 27710 kathleen.warren@duke.edu
Art Director
Jennifer Manning 5682 Dunnigan Road Lockport, NY 14094 jenmanningJOP@gmail.com








Editorial Review Board
Case Report / Technical Tactics Editor
Michael P. Kelly, FOPS Duke University Eye Center 2351 Erwin Road, Suite 209 Durham, NC 27710 michaelpkellydukeeye@gmail.com
Elizabeth Affel, MT, MS, OCT-C, FOPS Jefferson University Hospital Philadelphia, PA president@opsweb.org
Lisa Dennehy, BS Mass Eye and Ear Boston, MA lisa_dennehy@meei.Harvard.edu
Alan Frohlichstein, BFA, BS, CRA - ret., FOPS
Retinal Angiography Services Morton Grove, IL alanfroh@gmail.com
Christiaan Lopez-Miro Duke University Eye Center Durham, NC christiaan.lopezmiro@duke.edu
Paula Morris, BS, CRA, FOPS
John A. Moran Eye Center Salt Lake City, UT paula.morris@hsc.utah.edu
Assistant Editor
Monica Motta, SRA III, Supervisor I, BS, RVT, LATG
UC Davis School of Veterinary Medicine Comparative Ophthalmology
Vision Science Lab Davis, CA 95616
Ocular Services on Demand Madison, WI mjmotta@ucdavis.edu
Advertising Editor
Barbara McCalley 1621 East Jody Circle, Republic, MO 65738 Phone: 417.725.0181 Fax: 417.724.8450 ops@opsweb.org






Medical Advisor
Akbar Shakoor, MD
John A. Moran Eye Center University of Utah School of Medicine Salt Lake City, UT Akbar.Shakoor@hsc.utah.edu
Nicholas Patterson, CPT Duke University Eye Center Durham, NC nicholas.patterson@duke.edu
Kasi Sandhanam Singapore National Eye Centre Singapore kasi.sandhanam@snec.com.sg
Robert G. Shutt, CRA, OCT-C Connecticut Eye Consultants Danbury, CT shutteye@optimum.net
Jennifer Struck Duke University Eye Center Durham, NC jennifer.struck@duke.edu
Paola Torres, COT, CPT, CRA, OCT-C Duke University Eye Center Durham, NC paola.belleau-torres@duke.edu
Founded
by the Ophthalmic Photographers’ Society, Inc.; Don Wong, RBP, FOPS, Founding Editor




Quality Without Question
SPECTRALIS® multimodal imaging platform, the answer for unparalleled image quality, is uniquely designed to empower you to see more and do more for patients.
■ Pinpoint pathology through precise visualization and segmentation of the retinal layers
■ Reveal real change from visit-to-visit automatically with AutoRescan
■ Take patient comfort to the next level with TruTrack Active Eye Tracking
■ Preserve your investment as a customizable and upgradeable imaging platform
And so much more… Experience the SPECTRALIS difference.

Monica Motta, BS RVT, LATG UC Davis School of Veterinary Medicine
Comparative Ophthalmology Vision Science Lab Davis, CA 95616
mjmotta@ucdavis.edu
The cornea is probably the most underappreciated aspect of the eye… OK, maybe not the most underappreciated, but perhaps the most unexpected and surprising aspect of the eye. There is no other organ in the body that has a clear and functioning window. If you sit back and really understand what the cornea does, it’s absolutely magnificent. The fact that the cornea is made up of several cellular layers and nerves and still maintains transparency, is by far its most unique feature. The cornea is much more than the window into the eye; its functions and responsibilities
Clearly the Cornea

are just as fascinating. From the outside in, the cornea first acts as a protective layer and serves as the initial refraction of light into the lens, directing light to the retina. The cellular layers throughout the cornea maintain the transparency and shape of the cornea and as you approach the back of the cornea, you have a single layer of endothelial cells which pump water to the stroma and anterior chamber. That’s a lot of responsibility for a single layer of cells. The cornea is complex, yet deceptively simple… like a fine wine, one might say. I hope you enjoy our celebration of the cornea in this Journal issue.




Figure 2: NZW rabbit images. (a) Cornea. (b) Epithelium. (c) Stroma/neuro plex. (d) Endothelium.
Figure 1: NZW rabbit image.
Original Article

James Tian, MD
Esteban A. Peralta Chacon, MD
Melissa B. Daluvoy, MD
Sharon F. Freedman, MD
Edited By Ahmad A. Aref, MD, MBA
Corresponding Author: Sharon F. Freedman, MD
Duke University Eye Center Department of Ophthalmology 2351 Erwin Road Durham, NC 27710 919.684.4584 sharon.freedman@duke.edu
What’s Your Diagnosis? Morning Rounds with Sharon F. Freedman, MD, and Melissa B. Daluvoy, MD
IThe Discovery That Saved a Baby’s Life n December of 2016, Cornelius Crystal* was 6 weeks old, and both of his eyes were red and tearing. His parents took him to a local pediatric ophthalmologist, who diagnosed him with adenoviral conjunctivitis and prescribed a short course of TobraDex. At this time, the ophthalmologist noted that Cornelius also had a membrane under both upper and lower eyelids, and a membrane peeling procedure was performed. However, the membranes recurred despite repeated attempts at peeling over several months. After the fourth membrane peel, Cornelius’ parents brought him to us at the Duke Eye Center.
We Get a Look
When Cornelius presented to our eye clinic at 6 months of age, his mother reported that he had no apparent vision issues but noted that he was experiencing significant tearing and eye irritation. He had no other past medical or ocular history and had been born full-term at 39 weeks. During this consultation, Cornelius’ parents expressed concern that the membranes recurred each time they were peeled. Based on the history of chronicity and recurrence, we were concerned about a chronic toxic or foreign body reaction, sarcoidosis, amyloidosis, herpes simplex, or severe vernal or atopic keratoconjunctivitis.
The Exam
Cornelius’ vision was fix-and-follow in both eyes, as expected for his age. His IOP readings were normal, and he had no relative afferent pupillary defect. His visual fields and extraocular movements were full to clinic testing. The cycloplegic refraction showed a mild myopic astigmatism in both eyes. The exam showed bilateral (left larger than right) large lesions under the upper and lower eyelids, with conjunctival injection and increased tear meniscus (Figure 1a-b). There were no corneal or eyelid lesions, and the rest of the exam was unremarkable. We
* Patient name is fictitious.
decided to remove the membranes again in the operating room and send a specimen to pathology for evaluation. We everted both the right and left upper and lower eyelids and slowly peeled away the membranes with occasional use of a Tooke blade and micro-Westcott scissors. At the site of each excision, amniotic membrane attached with fibrin glue was placed to facilitate healing. Because of the constant tearing, we also took this opportunity to evaluate the nasolacrimal ducts. Fluorescein-dyed saline

Figure 1: (a-b) The patient experienced multiple recurrences of chronic conjunctivitis and membrane.
was instilled into each cannula and retrieved in the nose via nasal suction, ruling out a concurrent obstruction.
The Pathology Report
The pathology report revealed brightly eosinophilic amorphous and fibrillar material that stained brown with the fibrin immunohistochemical stain. With Masson trichrome, the specimen stained mostly blue and light red, typical of fresh and aging fibrin. There was no sign of amyloid using Congo red stain. The pathologist’s impression: these findings were consistent with ligneous conjunctivitis.
A Rare Condition
Ligneous conjunctivitis is a rare, chronic conjunctivitis characterized by fibrin-rich membranes or pseudomembranes. It is typically associated with plasminogen deficiency, which can result from a mutation of the PLG gene on chromosome 6.1 This gene is responsible for the production of plasminogen, the precursor protein of plasmin, which degrades fibrin and other matrix proteins into their byproducts.2
When microtrauma of the ocular surface induces the wound healing cascade, the lack of plasmin can cause extensive fibrin matrix deposition. As this fibrin matrix cannot be replaced with granulation tissue and is unable to be remodeled, wound healing is impaired.
Typical Presentation
Patients typically present with fibrinous pseudomembranes or true membranes on the palpebral conjunctiva.1 These membranes, often described as thick and “woody,” can be sessile or pedunculated, and range in color from white to yellow or even red. They can lead to chronic tearing, pain, corneal abrasions, and photosensitivity. Longterm complications include corneal scarring, corneal neovascularization, and symblepharon. A viral infection can trigger the development of these membranes, causing possible confusion with the pseudomembranes that may occur with viral conjunctivitis. Ligneous conjunctivitis is a very rare condition, estimated at about 1.6 patients per million (though this condition is likely underdiagnosed).1 In addition to ocular findings, patients can have systemic associations, including ligneous lesions in the respiratory tract, oral cavity, brain, and genital tract.3 It is important to coordinate with other specialties, particularly hematology, when managing patients.
Diagnosis
The differential diagnosis for a chronic recurrent membrane in the pediatric population includes chronic toxic or foreign body reaction, sarcoidosis, amyloidosis, herpes simplex, or severe vernal or atopic keratoconjunctivitis. Definitive diagnosis is made with analysis of a biopsy by pathology. The patient’s systemic level of plasminogen activity can be tested with an assay. In our patient, the plasminogen activity level was 14% of expected (normal range is 85%-120% of expected). Patients are considered
to have Type 1 plasminogen deficiency, also known as hypoplasminogenemia, when they have both decreased levels of plasminogen and decreased plasminogen activity.3 Patients with normal levels of plasminogen but reduced levels of activity are described as having Type 2 plasminogen deficiency, also known as dysplasminogenemia, which is usually asymptomatic. Genetic testing also can be performed for mutations in the PLG gene in the patient and family. A thorough multidisciplinary evaluation of the full extent of symptoms from plasminogen deficiency should also be performed. It is important not to stop at control of ocular symptoms when a diagnosis of plasminogen deficiency is made after presenting with ligneous conjunctivitis.
Management/Treatment
The current literature includes many reports of successfully treating the lesions with topical hyaluronidase or alpha chymotrypsin, both of which dissolve the fibrin matrix. After dissolution, anti-inflammatory treatment with topical steroids, topical cyclosporine, or systemic azathioprine has been shown to decrease the rate of recurrence.1 More recently, reports have trended toward supplementation of natural plasminogen with fresh frozen plasma to directly treat the underlying deficiency.4 It is important not to surgically remove these lesions without adjunctive therapy, as that would simply restart the wound healing cascade and cause recurrence of these membranes. In a series of 17 cases, surgical excision followed by aggressive topical heparin and steroids led to success in 76.4% (13/17) of patients.5 The mechanism of treatment with heparin is to inhibit thrombin formation, which then inhibits the production of fibrin—and, thus, fibrin matrix formation. Treatment of this condition should involve a multidisciplinary team, including a hematologist to coordinate laboratory and genetic workup. Referrals to other specialists should be made based on internal findings, due to possible lesions in the respiratory tract, oral cavity, brain, and genital tract.
Our Patient
We referred Cornelius to a clinical trial in which he received topical plasminogen drops.6 Shortly after therapy was initiated, his membranous lesions resolved. However, 3.5 years later, he developed voice hoarseness. A bronchoscopy was performed; this showed an irregular mass in his airway near his vocal cords (Figure 2). Had the earlier diagnosis not been made, the new mass might have been excised and then recurred, possibly growing larger. Instead, Cornelius was switched to weekly IV plasminogen and the mass disappeared. He is now 6 years old. In his most recent follow-up, his BCVA was 20/20 in both eyes, and there were no signs of membranes in any part of his body. His case is a reminder that if plasminogen deficiency is caught early enough, its detection can potentially save a life.

Case Report

Monica Ardon-Vasallo, BS
Karolina Paulina Roszak, DVM
University of California Davis School of Veterinary Medicine
References
1. Schuster V, Seregard S. Surv Ophthalmol. 2003; 48(4):369-388.
2. Castellino FJ, Ploplis VA. Thromb Haemost. 2005; 93(4):647-654.
3. Schuster V et al. J Thromb Haemost. 2007;5(12): 2315-2322.
4. Shapiro AD et al. Haematologica. 2020;105(3): 554-561.
5. De Cock R et al. Ophthalmology. 1995;102(11): 1654-1659.
6. Caputo R et al. Ophthalmology. 2022;129(8): 955-957.
About the Authors
Dr. Tian is currently in his Corneal fellowship; Dr. Peralta Chacon is an ophthalmology resident; Dr. Daluvoy is Associate Professor of Ophthalmology and Chief of the Cornea, External Disease, and Refractive Surgery Division; Dr. Freedman is Professor of Ophthalmology and Pediatrics, and Chief of the Pediatric Ophthalmology and Strabismus Division. All are affiliated with the Duke Eye Center in Durham, N.C. Relevant financial disclosures: None. For full disclosures, see this article at aao.org/eyenet.2
Department of Surgical and Radiological Sciences 1275 Med Science Drive Davis, CA 95616 mardon@ucdavis.edu
Slit Lamp Photographs Comparing Healthy and Diseased Corneas
The purpose of this study was to destroy endothelial cells by performing corneal cryoinjury. Corneal endothelial cells (CECs) maintain hydration and opacity within the cornea. However, if CECs cannot maintain fluidity, excess fluid in the cornea accumulates, causing the cornea to become cloudy (Figure 2) best known as corneal edema.
Based on previous research, we anticipated mild-to-moderate central corneal edema. When comparing both figures, the iris and pupil can no longer be seen as clearly as in Figure 1. Additionally, the lack of CECs, caused by cryo-injury, lead to corneal edema since CECs could no longer maintain the cornea in a state of dehydration (Figure 2). A slit lamp biomicroscope (Imaging Module IM 900; Haag Streit, Koeniz, Switzerland) was used to capture an oblique cross-section of the cornea, anterior chamber, iris, and lens (Figures 1 and 2).
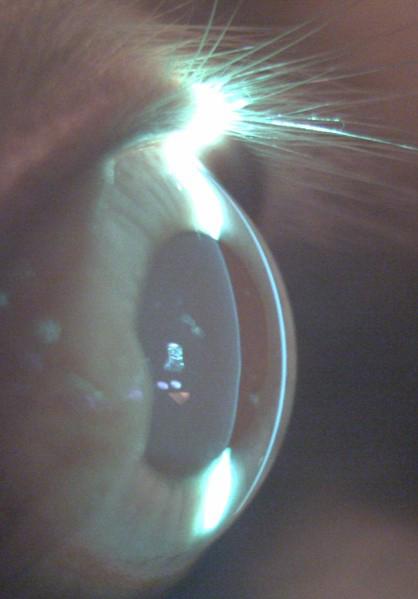

Figure 2: Bronchoscopy image of an irregular mass (arrow) in his airway near his vocal cords.
Figure 2: Three days following central corneal cryo-injury.
Figure 1: A healthy eye.

Esteban A. Peralta Chacon, MD
Kourtney Houser
James Tian, MD
Corresponding Author: Kourtney Houser
Duke University Eye Center Department of Ophthalmology 2351 Erwin Road Durham, NC 27710 kourtney.houser@duke.edu
An Update on Advances in Diagnosis and Treatment of Acanthamoeba Keratitis
Keywords
Acanthamoeba, Acanthamoeba keratitis, corneal ulcer, therapeutic penetrating keratoplasty, confocal microscopy
Introduction
Acanthamoeba keratitis (AK) is a potentially devastating infection of the ocular surface caused by amoebas of the genus Acanthamoeba. Although the organism is classically known for being difficult to detect and treat, recent advances in the field have greatly improved diagnostic accuracy and treatment efficacy. In this update, we review the current body of knowledge about AK epidemiology and pathogenesis, discuss the advances in diagnosis with confocal microscopy and polymerase chain reaction, and explore potential novel treatments such as voriconazole, miltefosine, topical steroids, phototherapeutic keratectomy, cross-linking and photodynamic therapy.
Pathogen and Pathophysiology
Acanthamoeba is an opportunistic, unicellular, free-living protist with at least 25 species and 23 genotypes so far identified (on 18S rRNA sequencing).1 Organisms have a two-stage life cycle: metabolically active trophozoites and quiescent double-walled cysts. Both trophozoites and cysts are small, usually 15–50 µm in length for trophozoites and 5–30 µm for cysts.2 Exposure to stress results in rapid encystment of Acanthamoeba, which are resistant to harsh environmental conditions and can lay dormant for years. Trophozoites are typically well treated with most antimicrobial therapies; however, treatment of the cysts requires specific cysticidal medications over a long period of time.
Acanthamoeba causes corneal infections through a multistep process beginning with epithelial breakdown, often through microtrauma from contact lens wear. This microtrauma and increased expression of epithelial glycoproteins allows Acanthamoeba to adhere
and release various enzymes and toxins that degrade the stroma.3 In particular, the binding is mediated by mannose-binding protein, along with two lamininbinding proteins, to the epithelial glycoproteins.4 Acanthamoeba then releases ecto-adenosine triphosphatases involved in caspase-3 activation, neuraminidases,5 phospholipase, elastase, glycosidase and metalloproteases, which work in concert to have a cytotoxic effect.4 Although Acanthamoeba trophozoites can penetrate the Descemet membrane, intraocular infection rarely occurs because of the intense neutrophil response in the anterior chamber.6 Trophozoites have been shown to demonstrate a chemotactic response to cells of neural crest origin, leading to infiltration of the corneal nerves and painful keratoneuritis.7
Epidemiology and Risk Factors
Acanthamoeba is found ubiquitously in water, air and soil, including swimming pools, hot tubs and ponds. It is also found frequently on contact lenses and contact lens cases, especially when not correctly sanitized.8 Common human exposures to these environments, especially in lower socioeconomic settings, trauma and recent increases in contact lens use have contributed to an increasing number of cases of Acanthamoeba keratitis (AK).1 The infection rate is estimated to range from 1–2 per million adults or 2–10 per thousand contact lens wearers.9 These estimated rates are highly variable due to the different prevalence of contact lens use, the amoebicidal efficacy of contact lens cleaning systems and the use of diagnostic tests for AK.
Clinical Features
AK should be considered in any presentation of keratitis, especially those associated with contact lens use or exposure to soil or water. Patients typically report subacute eye pain, photophobia and blurred vision. The pain is often out of proportion to examination


findings due to the presence of keratoneuritis. Tu et al. described five levels of AK severity: epitheliitis (with grey/ dirty epithelium), epitheliitis with radial neuritis, anterior stromal disease, deep stromal keratitis (Figure 1), or ring infiltrate (Figure 2), which is typically only seen in late stages of the disease.10 Rarely, Acanthamoeba can also cause scleritis, chorioretinitis, endophthalmitis, adnexal disease or disseminated encephalitis.11 As a result of the wide array of clinical presentations, the early epitheliitis stages can easily be confused with herpes simplex keratitis, whereas in the advanced stage, the deeper infiltrates can resemble bacterial or fungal keratitis.12 As late diagnosis is associated with worse outcomes, a high index of suspicion, recognition of both typical and atypical findings, lack of improvement with alternative treatments (especially herpetic), and ancillary testing are recommended to provide timely and accurate diagnosis.2
Diagnosis
The gold standard of diagnosis for AK is culture of corneal scrapings. This is typically done in a media with inactivated Escherichia coli, and examination for Acanthamoeba trails is then performed under light microscopy. Acanthamoeba will move away from the inoculation point to ‘graze’ on the E. coli.13 Co-infection with Acanthamoeba is common, possibly due to the presence of other bacteria, fungi, algae and viruses that Acanthamoeba feed on or have an endosymbiotic relationship with.13 Various stains such as acridine orange and calcofluor white can assist with the diagnosis on culture or smears. However, cultures are only positive in 52–67% of cases, possibly due to inadequate sample material, previous antimicrobial treatments and deeper stromal infection, and even lower rates have been reported; furthermore, calcofluor white works only for cysts.14 Even positive culture results are often delayed due to the slowgrowing nature of Acanthamoeba. Contact lenses may be cultured when there is a high index of suspicion, but they
should not be routinely cultured, as Acanthamoeba colonization has been found in an estimated 7–8% of contact lens storage cases used by asymptomatic individuals.15 Confocal microscopy is another valuable diagnostic tool that allows microscopic examination of individual corneal layers at a 2–4 µm resolution. Confocal microscopy is also noninvasive and rapid, and can be standardized for longitudinal examinations in the same patient to assess for progression. Studies have shown variable sensitivity (~60–90%) for confocal microscopy, which is likely to be due to the operator-dependent yield.16 The specificity is typically higher (~80–93%) due to the characteristic findings such as signet ring-shaped bodies, bright cyst with a dark background, hyperreflective bodies with spindle-like projections, and others.2 However, caution must be taken due to the similar appearance between cysts and inflammatory cells.17 Additionally, as confocal microscopy requires the patient to be imaged directly, it is not available at most institutions. Polymerase chain reaction of scrapings or biopsies can also identify the presence of amoebic DNA in a tissue sample after amplification of amoeba RNA in the laboratory.3 This process shows excellent specificity (99% or greater), similar to that for cultures, but also a higher sensitivity (73–87%); however, it is significantly more technical and expensive. 18
Treatment
Medical Treatment
Medical treatment of AK requires treatment of both the active trophozoite form and the stress-resistant cyst form.3 The trophozoite form is sensitive to a variety of antibiotics, antifungals, antiprotozoals and even antineoplastic therapies. However, the cystic form classically requires prolonged antiseptics such as polyhexamethylene biguanide (PHMB) and chlorhexidine (biguanides) or propamidine (also known as Brolene; Thornton & Ross, Linthwaite, UK) and hexamidine (diamidines).3 These positively charged molecules bind
Figure 1: External photograph of deep stromal Acanthamoeba keratitis. Source: Kourtney Houser, MD, and Joe Mastellone. Slit lamp photograph taken with patient consent per institutional protocol for de-identified images.
Figure 2: External photograph of a ring infiltrate in late-stage Acanthamoeba keratitis. Source: Kourtney Houser, MD, and Joe Mastellone. Slit lamp photograph taken with patient consent per institutional protocol for de-identified images.
to the phospholipid layer of the Acanthamoeba, disrupting the cell membrane and denaturing cytoplasmic proteins.19 Patients should be counselled that treatment often lasts several months and possibly up to a year (Figure 3).
PHMB and chlorhexidine have been shown to have relatively low minimal cysticidal concentrations, allow-



ing treatment of AK at low doses with relatively minimal damage to the corneal epithelium.20 However, prolonged use can still lead to epithelial toxicity. PHMB is typically dosed at 0.02% to 0.06%, chlorhexidine at 0.02% to 0.2%, propamidine at 0.1% and hexamidine at 0.1%, concentrations that are nearly 100-fold lower than when used as antiseptics.20
Bacterial co-infection is reported to be as high as 40%, and it is therefore common for initial treatment to include antibacterial drops as well as anti-AK drops.21 Many antibacterial treatments also have activity against the trophozoite form of Acanthamoeba
For refractory cases, success has been reported with the addition of voriconazole. Topical 1% voriconazole has been shown to have good outcomes when added as an adjunctive treatment,22 and studies have also shown success with topical or oral voriconazole monotherapy.23,24 Patients starting voriconazole should be counselled on the possibility of transient visual phenomena or hepatic toxicity.
Miltefosine is an alkylphosphocholine originally used for leishmaniasis that also selectively disrupts Acanthamoeba cell membranes. A few case series have shown success for refractory AK; however, there appears to be a high rate of initially worsened inflammation.25 Topical or oral steroids are often needed to treat the inflammatory response. Patients should also be counselled on gastrointestinal side effects such as nausea, vomiting, abdominal pain and diarrhea, as well as the possibility of liver and renal toxicity.
The use of topical steroids is controversial in AK. Although it is commonly accepted that the use of topical steroids before the diagnosis and treatment of AK is associated with worse outcomes (due to increased trophozoite expression),26,27 there may be a role for delayed topical steroids as an adjunct therapy to control inflammation. McClellan demonstrated in vivo that steroids increase the excystment of Acanthamoeba into the active trophozoite form, which allows the infection to be more sensitive to treatment but also increases the pathogenicity.28 Various case series have shown similar or noninferior outcomes in patients who received delayed topical corticosteroids, and both topical steroids or systemic immunosuppression therapy may especially play a role in patients who present with scleritis or a hypopyon.27,29,30 If topical steroids are used, it is recommended that patients receive anti-AK therapy for a minimum of 2 weeks before steroid treatment and continue for at least 4 weeks after cessation of steroids.
Procedural and Surgical Treatments
Although first-line treatment of AK remains medical therapy, procedural or surgical treatment can be added to facilitate treatment or as a last resort for refractory cases. For example, thorough epithelial debridement for early AK can help reduce pathogen load and also assist with penetration of topical therapy.3
Figure 3: External photograph of a patient with polymerase chain reaction-confirmed Acanthamoeba keratitis. (a) Ring infiltrate prior to treatment. (b) After 8 weeks of polyhexamethylene biguanide and Brolene treatment. (c) 6 months after diagnosis with resolution of infection and a stromal scar. Source: Kourtney Houser, MD, and Joe Mastellone. Slit lamp photograph taken with patient consent per institutional protocol for de-identified images.
In a case series by Kandori et al., four cases of superficial stromal AK that were resistant to medical therapy for a week were successfully treated with laser phototherapeutic keratectomy, with excellent visual outcomes.31 Patients underwent ablation to an average depth of 254 µm until infectious tissue was no longer seen.31
A few case series in refractory cases have reported success with riboflavin cross-linking with ultraviolet A light32 or rose bengal photodynamic antimicrobial therapy (RB-PDAT) with green light.33 Both processes are theorized to generate free radicals that have significant antimicrobial properties without significant corneal toxicity.34 In a study by Naranjo et al., 10 patients with AK unresponsive to standard medical therapy for at least 2 weeks were treated with 1–3 rounds of RB-PDAT, with success in seven patients.33 In a meta-analysis by Papaioannou et al., 11 patients were treated with ultraviolet A cross-linking, with success in 10, although five required repeat treatment.35 Although reports of these therapies are promising, there are currently insufficient data to recommend their incorporation into standard practice.
Therapeutic penetrating keratoplasty (PK) has been performed to help debulk infectious load but outcomes were poor and complicated by infection recurrence, graft rejection or graft failure from glaucoma.36 In a series of 32 therapeutic PK cases, Kashiwabuchi et al. reported a 40% rate of glaucoma, 56% rate of graft failure and 38% rate of a second PK.37 As medical therapy is generally successful, therapeutic PK is now typically reserved for severe refractory cases or for severe thinning or perforation. Anti-AK therapy is typically continued pre- and postoperatively, adopting a regimen that minimizes epithelial toxicity on the new graft. PK or deep anterior lamellar keratoplasty have also shown good outcomes in treating scarring and irregular astigmatism after quiescent AK.38
Prognosis
Prognosis is highly dependent on the severity of disease at presentation and treatment initiation. In a review of 349 patients, 90% of patients had a final vision of 20/40 or better, although ~5% lost all useful vision.20
Conclusions
AK is a potentially devastating infection of the ocular surface that is increasing in frequency due to contact lens use and ubiquitous environmental contamination. The clinical features of AK and imperfect confirmatory testing present a unique diagnostic challenge where progression has a significant impact on prognosis. We recommend a high index of suspicion for Acanthamoeba in contact lens wearers, especially those who have been diagnosed with herpetic keratitis. We also recommend recognition of typical and atypical symptoms, along with early and multimodal testing with culture and polymerase chain reaction or confocal microscopy if possible. Medical therapy with biguanides
and diamidines are often effective, although involve subjecting the patient to a long and painful treatment course. Exciting new strategies to manage refractory AK have been reported with voriconazole, miltefosine or topical steroids, which have been shown to be valuable adjuncts. Procedural interventions such as phototherapeutic keratectomy, cross-linking or PDAT have also shown promise, but the small-sample case series are insufficient to make recommendations for clinical practice. Ultimately, earlier awareness and evaluation for AK in patients with corneal infections can lead to better outcomes and a more tolerable clinical course.
References
1. Labbé A, Khammari C, Dupas B, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf. 2009;7:41–52.
2. Kaufman AR, Tu EY. Advances in the management of Acanthamoeba keratitis: A review of the literature and synthesized algorithmic approach. Ocul Surf. 2022;25:26–36.
3. Maycock NJ, Jayaswal R. Update on Acanthamoeba keratitis: Diagnosis, treatment, and outcomes. Cornea. 2016;35:713–20.
4. Rayamajhee B, Willcox MDP, Henriquez FL, et al. Acanthamoeba, an environmental phagocyte enhancing survival and transmission of human pathogens. Trends Parasitol. 2022;38:975–90.
5. Siddiqui R, Khan NA. Biology and pathogenesis of Acanthamoeba Parasit Vectors. 2012;5:6.
6. Illingworth CD, Cook SD, Karabatsas CH, Easty DL. Acanthamoeba keratitis: Risk factors and outcome. Br J Ophthalmol. 1995;79:1078–82.
7. Niederkorn JY, Alizadeh H, Leher HF, McCulley JP. The immunobiology of Acanthamoeba keratitis. Springer Semin Immunopathol 1999;21:147–60.
8. de Lacerda AG, Lira M. Acanthamoeba keratitis: A review of biology, pathophysiology and epidemiology. Ophthalmic Physiol Opt 2021;41:116–35.
9. Ibrahim YW, Boase DL, Cree IA. Factors affecting the epidemiology of Acanthamoeba keratitis. Ophthalmic Epidemiol. 2007;14:53–60.
10. Tu EY, Joslin CE, Sugar J, et al. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology 2008;115:1998–2003.
11. Bataillie S, Van Ginderdeuren R, Van Calster J, et al. How a devastating case of Acanthamoeba sclerokeratitis ended up with serious systemic sequelae. Case Rep Ophthalmol. 2020;11:348–55.
12. Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite. 2015;22:10.
13. Rayamajhee B, Subedi D, Peguda HK, et al. A systematic review of intracellular microorganisms within Acanthamoeba to understand potential impact for infection. Acanthamoeba. 2021;10:225.
14. Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–16.
15. Larkin DF, Kilvington S, Easty DL. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol 1990;74:133–5.
16. Kheirkhah A, Satitpitakul V, Syed ZA, et al. Factors influencing the diagnostic accuracy of laser-scanning in vivo confocal microscopy for Acanthamoeba keratitis. Cornea. 2018;37:818– 23.
17. Tu EY, Joslin CE, Sugar J, et al. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea. 2008;27:764–72.
18. Yera H, Ok V, Lee Koy Kuet F, et al. PCR and culture for diagnosis of Acanthamoeba keratitis. Br J Ophthalmol. 2021;105:1302–6.
19. Lim N, Goh D, Bunce C, et al. Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am J Ophthalmol. 2008;145:130–5.
20. Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: Diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487– 99.
21. Singh A, Sahu SK, Sharma S, Das S. Acanthamoeba keratitis versus mixed Acanthamoeba and bacterial keratitis: Comparison of clinical and microbiological profiles. Cornea. 2020;39:1112–16.
22. Musayeva A, Riedl JC, Schuster AK, et al. Topical voriconazole as supplemental treatment for Acanthamoeba keratitis. Cornea. 2020;39:986–90.
23. Bagga B, Sharma S, Gour RPS, et al. A randomized masked pilot clinical trial to compare the efficacy of topical 1% voriconazole ophthalmic solution as monotherapy with combination therapy of topical 0.02% polyhexamethylene biguanide and 0.02% chlorhexidine in the treatment of Acanthamoeba keratitis. Eye (Lond) 2021;35:1326–33.
24. Tu EY, Joslin CE, Shoff ME. Successful treatment of chronic stromal Acanthamoeba keratitis with oral voriconazole monotherapy. Cornea. 2010;29:1066–8.
25. Thulasi P, Saeed HN, Rapuano CJ, et al. Oral miltefosine as salvage therapy for refractory Acanthamoeba keratitis. Am J Ophthalmol 2021;223:75–82.
26. Robaei D, Carnt N, Minassian DC, Dart JK. The impact of topical corticosteroid use before diagnosis on the outcome of Acanthamoeba keratitis. Ophthalmology. 2014;121:1383–8.
27. Carnt N, Robaei D, Watson SL, et al. The impact of topical corticosteroids used in conjunction with antiamoebic therapy on the outcome of Acanthamoeba keratitis. Ophthalmology. 2016;123:984–90.
28. McClellan K, Howard K, Niederkorn JY, Alizadeh H. Effect of steroids on Acanthamoeba cysts and trophozoites. Invest Ophthalmol Vis Sci. 2001;42:2885–93.
29. Park DH, Palay DA, Daya SM, et al. The role of topical corticosteroids in the management of Acanthamoeba keratitis. Cornea 1997;16:277–83.
30. Iovieno A, Gore DM, Carnt N, Dart JK. Acanthamoeba sclerokeratitis: Epidemiology, clinical features, and treatment outcomes. Ophthalmology. 2014;121:2340–7.
31. Kandori M, Inoue T, Shimabukuro M, et al. Four cases of Acanthamoeba keratitis treated with phototherapeutic keratectomy. Cornea. 2010;29:1199–202.
32. Watson SH, Shekhawat NS, Daoud YJ. Treatment of recalcitrant Acanthamoeba keratitis with photoactivated chromophore for infectious keratitis corneal collagen cross-linking (PACK-CXL). Am J Ophthalmol Case Rep. 2022;25:101330.
33. Naranjo A, Arboleda A, Martinez JD, et al. Rose bengal photodynamic antimicrobial therapy for patients with progressive infectious keratitis: A pilot clinical study. Am J Ophthalmol. 2019;208:387–96.
34. Martinez JD, Arrieta E, Naranjo A, et al. Rose bengal photodynamic antimicrobial therapy: A pilot safety study. Cornea. 2021;40:1036–43.
35. Papaioannou L, Miligkos M, Papathanassiou M. Corneal collagen cross-linking for infectious keratitis: A systematic review and metaanalysis. Cornea. 2016;35:62–71.
36. Sony P, Sharma N, Vajpayee RB, Ray M. Therapeutic keratoplasty for infectious keratitis: A review of the literature. CLAO J 2002;28:111–18.
37. Kashiwabuchi RT, de Freitas D, Alvarenga LS, et al. Corneal graft survival after therapeutic keratoplasty for Acanthamoeba keratitis. Acta Ophthalmol. 2008;86:666–9.
38. Awwad ST, Parmar DN, Heilman M, et al. Results of penetrating keratoplasty for visual rehabilitation after Acanthamoeba keratitis. Am J Ophthalmol. 2005;140:1080–4

Coming Next Issue
Human and Animal-Based Research Clinical Trials
The Comparative Ophthalmology & Vision Sciences Laboratory at UC Davis School of Veterinary Medicine (COVSL), run by Drs. Sara Thomasy and Brian Leonard, strives to be a world leading center in translational comparative ophthalmology research. Our mission is to develop novel strategies for the diagnosis and treatment of ocular diseases in animal and human patients. We employ scientists, technicians, postdoctoral fellows, graduate students, and undergraduates to complete this mission
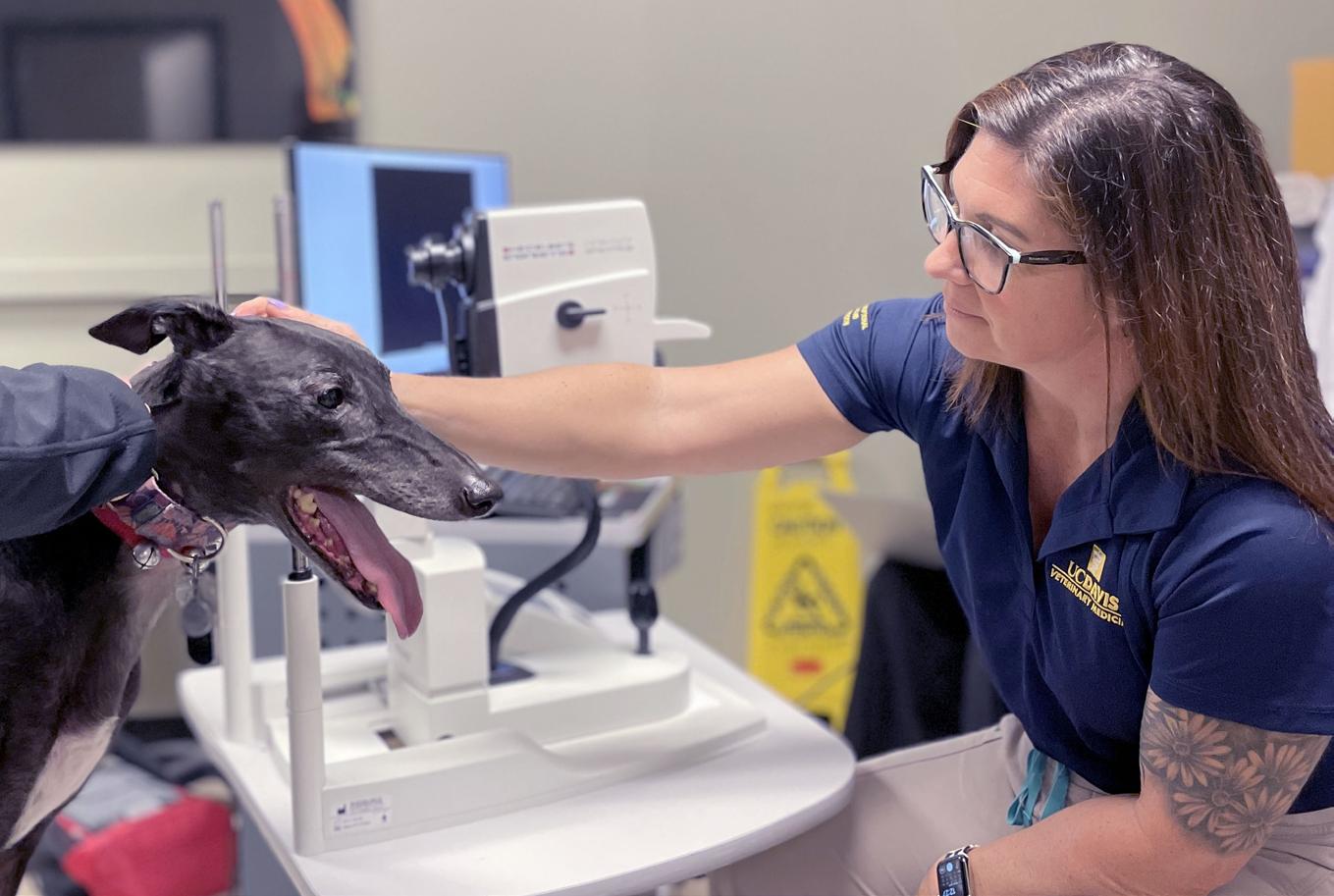

Members of the COVSL examine a Greyhound with an advanced imaging tool called optical coherence tomography that provides detailed images of the retina, the neural layer of the eye that transforms light into an electrical signal and transmits it to the brain.

Original Article

by Michael Penn Director of Duke Communications, Marketing, & Alumni Relations
310 Trent Drive Durham, NC, 27710
919.681.7760
m.penn@duke.edu

Eyes on a Miracle
Balu Sesay had been blind for 29 years when Lloyd Williams first met her. It was a sweltering day in July 2021, in a small hospital exam room in Freetown, the bustling port capital of Sierra Leone. The Duke eye surgeon saw immediately that both of Sesay’s eyes were badly damaged, the right one completely ruined and a milky haze covering the left, both the result of injuries she sustained as a teenager. Now 46 and the mother of five, Sesay had never seen her husband or her children.
What brought her to Freetown, nearly three hours from her home, was a promise she still couldn’t quite believe: that her left eye could be fixed, that her years in the dark might end.
The next day, Williams worked with a team of Sierra Leonean ophthalmologists to replace the opaque cornea in her left eye with one from a donor. It was one of eight corneal transplants that week, the first ever performed in the West African country. Twenty-four hours later, when doctors removed the patch protecting her repaired eye, she walked into the waiting room and toward a teenage child whose eyes were flooding with tears. “Girl, why are you crying?” she asked in Krio, the local language.
“Mom, it’s me,” the girl cried, reaching out to embrace her mother. “I am your daughter.”
Even for Williams, who has performed hundreds of vision-restoring surgeries in the United States and other countries,
by Chris Hildroth Rooster Media Productions Durham, NC roostermedia.com
those moments are soul-stirring. It’s why he’s back in Freetown nine months later, having traveled 7,000 miles, taking three planes and a boat, clutching a Styrofoam cooler packed with human eyeballs.
“It’s the closest we can come to performing miracles on Earth,” he says.
“Miracle” is a word that Williams, a devout Christian and the son of Lutheran missionaries, invokes with a good deal of reverence. He notes that healing the blind is among the most frequently described miracles attributed to Jesus. But as a scientist, he knows what he and others are doing to restore sight in places like Sierra Leone is not divine intervention. It’s actually pretty straightforward ophthalmology.
Consider that cataracts — the most common cause of blindness globally, leaving more than 17 million people with no functional vision — can usually be fixed with a simple, one-time surgery that costs as little as $12 to perform. Millions more, like Balu Sesay, could have their vision restored with a corneal transplant. In fact, according to the International Agency for the Prevention of Blindness, more than three-quarters of the estimated 43 million people around the world who are blind could regain sight through medical intervention.

That so many remain in darkness is primarily a function of the massive inequities in global health care. Ninety percent of people with preventable blindness live
In West Africa, Duke eye surgeon Lloyd Williams is helping local doctors restore sight to the blind, a mission that is both easier and harder than you’d think.
Photography
in low-income countries, which often lack the resources to offer anything like comprehensive eye care. In subSaharan Africa, there are just 2.5 trained eye specialists for every million people, akin to a city the size of Durham having only one eye doctor. Problems like cataracts or glaucoma, which are typically painless and progress slowly, often go untreated for years, eroding vision until a person is left completely blind.
“There are no campaigns in West Africa to tell people to go get their eyes checked,” says Leon Herndon, a Duke glaucoma specialist who has done clinical research and training in Ghana and Nigeria. “There’s very little education about eye diseases, and many people believe that once you lose vision, it’s just hopeless.”
And that makes restoring vision — and with it, hope — a powerfully addictive feat. Williams first felt its pull as a medical student at Tufts University, when he spent a summer break in 2001 volunteering at a hospital in the remote high plains of Zambia, in south central Africa. One day, an American doctor asked him to check on a blind woman who had undergone cataract surgery the previous day. Williams and the doctor removed a patch covering her eye and huddled over her bed, asking what she could see. “White people,” she responded in a local dialect.
“I thought, this is the most amazing thing I’ve ever seen,” Williams says. “For the most part [in medicine], you don’t get many wins. You’re just managing something. But, I thought, I could do this for the rest of my life and never wonder whether what I did was worthwhile.”
After finishing medical school and joining a private practice in Utah, Williams threw himself into that mission with characteristic, high-motor zeal. A competitive cyclist who builds guitars to unwind, he crammed his calendar with trips abroad, usually funded by himself or with small donations. He estimates he has done some 700 cataract surgeries in countries including Nepal, Guatemala and South Sudan. His most recent trip to Sierra Leone was his 16th to Africa since unbandaging his first patient in Zambia.
But such drop-in visits are a Band-Aid solution at best. Williams came to Duke in 2019 to work on something more sustainable. He now leads Duke’s Global Ophthalmology Program, adroitly rebranded as Duke GO, which is aiming to provide more structure to the international efforts of several Duke eye doctors across Africa, Asia, and Central America. The point is not just to help eye doctors in those places, but to empower them, he notes.
“No one person is going to end preventable blindness alone,” he says. “We need an army of people.”
In Sierra Leone, that army’s field general is Jalika Mustapha, a dynamic young ophthalmologist who leads the country’s national eye program. A native Sierra Leonean, Mustapha graduated from medical school in Kenya in 2016 with the highest board scores in all of Africa, and she has won international praise for expanding access to cataract surgery and preventive eye care in the country’s rural interior.


Since Mustapha’s team is already doing cataract surgeries, Williams is focusing on teaching more advanced procedures such as corneal transplants, which have only been done in a handful of places in Africa. Mustapha has now taken the scalpel for three transplants, becoming the first Sierra Leonean doctor to perform an organ transplant in her country.
That first earned Mustapha and Williams a spot on the national news and a meeting with the health minister, a big deal in a country still battling COVID-19 and other public health crises. With annual per capita spending on health care at just $46 — about 1 percent of what wealthy countries spend — eye care doesn’t often get such attention on the national agenda. But when the minister asked Mustapha to dream big, she didn’t miss a beat.
“We need a new eye center,” she said. “We need to be able to do more surgeries, and we need a place to train more people in our country.”
She came away with a promise of land to build the country’s first dedicated eye hospital. Williams hopes it will eventually be a site where Duke ophthalmology fellows can train. “Sierra Leone, I think, is at a tipping point,” he says. “The doctors there are very good, and with just a little bit more money and support, they have
Lloyd Williams M.D., Ph.D. arrives at the Connaught Hospital in Sierra Leone carrying 20 corneas from donors in the United States.
Located in Freetown, the Connaught Hospital is the main referral hospital in the country.


an opportunity to make a massive difference, not just for their country, but for all of West Africa.”
Of course, a tipping point can still be a precarious place to stand, especially when you’re holding a scalpel.
While she works toward construction of a new eye center, Mustapha runs Sierra Leone’s national eye program from two small rooms in Connaught Hospital, which occupies a gated complex just off the Atlantic Ocean in central Freetown. On his April 2022 trip, Williams has ferried 20 donated corneas from North Carolina, and all week, patients and families filter into a bright waiting room near the entrance, their anxious banter mixing with the sounds of trucks and motorcycles whizzing by on the city’s busy streets.
On one afternoon, the doctors are in the middle of surgery when the power goes out. Nurses grab their mobile phones, ready to shine light so the doctors can complete the procedure. The hospital’s backup generator kicks in within a minute, but it’s a reminder that medicine in a developing country can test one’s resourcefulness.
In Sierra Leone, for instance, there’s no general anesthesia for eye surgeries, only a local numbing shot, and so doctors must anticipate a patient twitching or squirming. When it’s time to make the delicate sutures to knit the donor cornea in place, everyone holds their breath. One patient, overcome by nerves, stands up right before the first cut and leaves. She returns a few days later and this time makes it through the procedure.
“It’s really remarkable what the doctors are able to do with the resources they have,” Williams says.
But any obstacles the doctors face pale compared to what their patients have overcome. Most African countries have virtually no accessibility services or legal protections for the disabled, making it significantly more difficult for people who are blind to support themselves and live independently. Many who lose their sight find they can no longer work or even navigate to do simple errands. Children or grandchildren may be held out of school to care for blind elders, perpetuating a cycle of poverty that can cripple a family for generations. In places like Sierra Leone, where most people support themselves through manual labor and farming, a blind person is sometimes described as “a mouth with no hands.”
For Williams, understanding the shame and scorn steeped into such labels makes those moments when the bandages come off all the more profound. He reflects for a moment on the notion that he is giving someone their life back, through his surgery.

“Honestly, in Africa, I think you are. When you think about the social and spiritual effects of believing you are worthless, believing you are a drain on your family … I think it is giving them their life back.”
You can sense the nervous anticipation as patients and families wait for preand post-operative appointments. On the
Day 1: The Need. Doctors were met with a packed waiting room of patients eager for their chance to be seen.
Day 2: Light and Response. Williams uses his cell phone and a flashlight to assess reaction. Sierra Leone physician Dr. Lloyd H. Williams joins Duke surgeon Dr. Lloyd B. Williams, to check on Junisa Sillah the day after surgery.
Day 3: Surgery. Williams observes as Dr. Jalika Mustapha performs a cornea transplant. Mustapha, seated, is the only female ophthalmologist in Sierra Leone and heads the national eye care program.


days following surgery, when they first realize they can see, everyone cries with a mix of joy and relief, thanking God and the doctors in equal measure.
But the sense of rebirth is never more palpable than when the people who received transplants during the first round of surgeries in 2021 return for follow-up appointments. Balu Sesay, the mother of five, has a job as a housekeeper, and she brags that she now can see when her children aren’t doing their chores. Another woman whose deteriorating vision nearly caused her to lose her position in the military shows up in combat fatigues, her career back on track.
One day, a 20-year-old woman strides into the exam room wearing a bright red top and a broad smile. There had been no smile when Williams first saw her nine months earlier. She had been blind in both eyes for 10 years and described herself as “hopeless” and “just crying
over and over about my sight.” When Williams asked her what she wanted to do with her life, she stared blankly at the floor and mumbled, “Nothing.”
Now, with a clear cornea in her left eye, she brims with newfound purpose. “It was not easy to lose [my] sight,” she says in a video interview after her follow-up appointment. “Before, someone walked with me, while now I am walking for myself. I can do everything for myself. I can read. I can write. I can even cross the streets. Before I was not doing any of this.” She tells the doctors she now aspires to be a politician.
A few weeks later, Williams is back in his Duke Health clinic, reminiscing about the trip. He watches the video again and smiles. “That’s a win,” he says. “That’s as big a win as you can get.” Because it’s not just that she’s regained her ability to see the world. It’s that now she can see herself in it.

Day 4: A Critical Moment. Williams stitches a live cornea in place. Corneal tissue awaiting transplant.
Day 5: First Sight. Firdaus Jalloh clasps Williams’ hand with joy, knowing she can see again.
2023 Ophthalmic Photography Exhibit
First Place Winners
These are the winning images of the 2023 American Society of Cataract and Refractive Surgery (ASCRS) ophthalmic photography exhibit. The OPS Scientific Exhibit Committee partners with ASCRS each year to make this competition a reality. We look forward to seeing your entries for the 2024 exhibit in Boston, MA. For further information, see https:// www.opsweb.org/page/SEC_OPSASCRS or contact Stuart Alfred at ops@ascrs.org.

BEST OF SHOW
Slit Lamp Photography
Pseudoexfoliation of Lens Capsule
Gary Miller CRA, OCT-C, FOPS
Geissinger Medical Center
Danville, Pennsylvania

Fundus Autofluorescence
Coxsackievirus-Associated Unilateral Acute Idiopathic Maculopathy, ON Drusen Sean Grout, OCT-C CPMC Department of Ophthalmology San Francisco, California

External Photography
Spontaneous Cataract Extrusion in a Patient with Corneal Melting
Gustavo Moreira Madeira, IOCM
Instituto de Olhos Ciencias Médicas
Belo Horizonte, Brazil
2023 Ophthalmic Photography Exhibit First Place Winners

Fundus Photography High Magnification 20°
The Leaning Optic Nerve Head - Fuchs Coloboma
Prasanna Ramesh, MS, DNB; Shruthy Ramesh, DNB, MS
Mahathma Eye Hospital Private Limited Trichy, India

Fundus Photography Wide Angle 45°+ PDR with Retinal Detachment
Noelle Pensec Rovensky, BSc, CRA
Columbia University Medical Center New York, New York

Fundus Photography Normal 30°- 40° Staphyloma of the Left Eye
Anahli Vazquez-Galvan, OCT-C Kresge Eye Institute Detroit, Michigan

Ultra-Widefield Imaging Sickle Cell Disease, Proliferative Retinopathy
Judith Gulian, BSc, OCT-C
University of Michigan Kellogg Eye Center Ann Arbor, Michigan
2023 Ophthalmic Photography Exhibit First Place Winners

Gonio Photography
Gonio
Veronica Jones, BA
Duke Eye Center
Durham, North Carolina

The Eye as Art
Santos Klimt
Veronica Jones, BA
Duke Eye Center
Durham, North Carolina

Monochromatic Photography
Oculocutaneous Albinism with Iris Transillumination Monochromatic Nicole Mantel Inselspital
Bern, Switzerland

Composite Image
Retinal Detachment
Sousa Jefferson, BEng, COMT Instituto Suel Abujamra (ISA)
São Paulo, Brazil
2023 Ophthalmic Photography Exhibit First Place Winners

Fluorescein Angiography
Choroiditis
Sean Grout, OCT-C
CPMC Department of Ophthalmology San Francisco, California

Corneal Endothelial Photography
Moon Craters (Corneal Guttata)
Anugraha Balamurugan, MS; Prasanna Ramesh, MS, DNB
Mahathma Eye Hospital Private Limited Trichy, India

Indocyanine Green Angiography
Punctate Inner Choroidopathy
Jessica Stephens, BA
New England Eye Center Boston, Massachusetts

Cross Categories
X-Linked Juvenile Retinoschisis
Sousa Jefferson, BEng, COMT
Instituto Suel Abujamra (ISA) São Paulo, Brazil
2023 Ophthalmic Photography Exhibit First Place Winners

Optical Coherence Tomography
The Whale Riding the Wave: Subluxated LENS with Vitreous in Anterior Chamber
Kumar Doctor, MD; Shivani Pattnaik, MS
Doctor Eye Institute Mumbai, India

OCT Angiography
Diabetic Retinopathy with Neovasularization
Kasi Sandhanam
Singapore National Eye Centre
Singapore

Gross Specimen Photography
Medusa’s Head: Ciliary Processes and Posterior Zonule Insertion
Kumar Doctor, MD; Shivani Pattnaik, MS
Doctor Eye Institute Mumbai, India
2023 Ophthalmic Photography Exhibit Second Place Winners

Fluorescein Angiography
Branch Retinal Artery Occlusion
Ben Serar, MA, CRA Serar Photography Prescott, Arizona

Composite Image Racemose Angiomatosis
Olivia Rainey, BA, COA, OCT-C
Retina Specialists of Michigan
Grand Rapids, Michigan

OCT Angiography
Choroidal Neovascularization
Kasi Sandhanam
Singapore National Eye Centre
Singapore

Fundus Photography High Mag. 20° Optic Atrophy
Tracey Troszak, CRA, OCT-C
Henry Ford Health System
Sterling Heights, Michigan

Fundus Photography Wide Angle 45°+
Stargardt’s Disease
Gary Miller CRA, OCT-C, FOPS Geissinger Medical Center Danville, Pennsylvania

Indocyanine Green Angiography
Smoke Stack ICG
Brian Lutman, CRA
Duke Eye Center
Durham, North Carolina

Karla
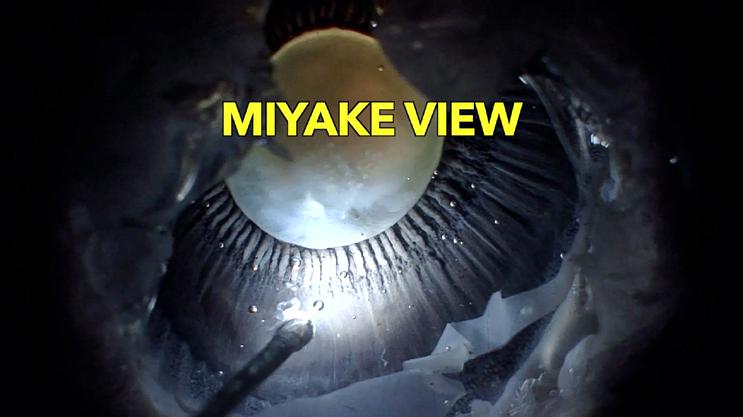
Gross Specimen Photography
95548 The Full Moon: Miyake-Apple VIEW
Kumar Doctor, MD; Shivani Pattnaik, MS
Doctor Eye Institute Mumbai, India
Fundus Photography Normal 30°- 40° Neuroretinitis with Macular Star
Woods, COA Geisinger Medical Center Danville, Pennsylvania
2023 Ophthalmic Photography Exhibit Second Place Winners

Gonio Photography
Iris Melanoma
Sarah Skiles, MA, BA, CRA
University of Iowa Hospitals and Clinics Iowa City, Iowa

Fundus Autofluorescence
GEO Atrophy
Tracey Troszak, CRA, OCT-C
Henry Ford Health System Sterling Heights, Michigan

Corneal Endothelial Photography
King’s Ransom – Polymegathism
Prasanna Ramesh, MS, DNB
Shruthy Ramesh, DNB, MS
Mahathma Eye Hospital Private Limited Trichy, India

Optical Coherence Tomography
Vogt Koyanagi Harada SyndromeAnterior Segment Manifestation with Anterior Ciliary Body Dislocation and Angle Closure Acute Glaucoma
Luca Gualdi, MD, COA
Ace Vision Group Boston, Massachusetts

Monochromatic Photography
Congenital Retinal Macrovessel
Bridgette Barnes, OSC
University of North Carolina Ophthalmology Chapel Hill, North Carolina

The Eye as Art
The Flow of Molten Gold (Fluorescein Angiography of Normal Disc)
Prasanna Ramesh, MS, DNB
Mahathma Eye Hospital Private Limited Trichy, India

Cross Categories
Central Retinal Artery Occlusion
Gary Miller CRA, OCT-C, FOPS
Geissinger Medical Center
Danville, Pennsylvania

Ultra-Widefield Imaging Capillary Dropout
Bradley Stern, BA, CRA, OCT-C
Henry Ford Health System Detroit, Michigan

Slit Lamp Photography Inflammatory Pupillary Membrane in Patient with Endophthalmitis
Kingston Rodolfo Ureña-Wong
Asociación para evitar la ceguera en México Mexico City, Mexico
2023 Ophthalmic Photography Exhibit
Third Place Winners and Honorable Mention
Composite
3rd: Artery Occlusion, Jason Calhoun, COA
HM: Neuroretinitis with Macular Star, Sean Grout, OCT-C
HM: Albinism (White Retina), Jason Calhoun, COA
HM: Retinal Vasculitis, Jason Calhoun, COA
Corneal Endothelial Photography
HM: Asteroid Showers - Extracellular Matrix in Descemet’s Membrane, Prasanna Ramesh, MS, DNB
HM: Lunar Craters in Corneal Endothelium, Prasanna Ramesh, MS, DNB; Shruthy Ramesh, DNB, MS
Cross Categories
3rd: Oculocutaneous Albinism with Iris Transillumination 4x, Nicole Mantel
HM: Choroidal Neovascular Membrane, Megan Walsh, BSc, CRA
HM: Pterygium Cyst, Sean Grout, OCT-C
HM: Choroidal Folds Secondary to Grave’s Disease, Bryan Carmichael, BSc, OSA, CST, COT
HM: Focal Choroidal Excavation, Hailey Litzer, OCT-C, CPSS
External Photography
HM: When the Eyeball Is Replaced By a Dermolipoma, Sara Ettouri, PhD
HM: Conjunctival Papilloma, Kathleen Warren, OCT-C
HM: Anridia, Sarah Skiles, MA, BA, CRA
Fluorescein Angiography
3rd: Telengectasia, John Leo, BSc
HM: Proliferative Diabetic Retinopathy, Darrin Landry, CRA
HM: Sickle Cell Retinopathy, Olivia Rainey, BA, COA, OCT-C
HM: Central Retinal Artery Occlusion, Noelle Pensec Rovensky, BSc, CRA
HM: Sickle Cell, Christiaan Lopez-Miro, BA
HM: Atypical Macular Telangiectasia, Megan Walsh, BSc, CRA
Fundus Autofluorescence
3rd: Rhodopsin Dominant RP , Darrin Landry, CRA
HM: Benign Flecked Retina, John Leo, BSc
HM: Faf RD Optos , Jack Rodriguez
Fundus Photography High Magnification 20°
3rd: Abducens Nerve Palsy, Nicole Radunzel, BA, CRA
Fundus Photography Normal 30°-40°
3rd: Vitreoretinal Tuft, Sarah Skiles, MA, BA, CRA
HM: Macular STAR, John Leo, BSc
HM: Wet ARMD, Megan Walsh, CRA
HM: Weiss Ring, Jason Calhoun, COA
Fundus Photography Wide Angle 45°+
3rd: Doyne’s Honeycomb Retinal Dystrophy, Noelle Pensec Rovensky, BSc, CRA
HM: Malattia Leveninese, Sarah Skiles, CRA
HM: Stargardt’s Disease, Jason Calhoun, COA
Gonio Photography
3rd: Istent, Jason Calhoun, COA
HM: Iris Nevus, Sarah Hugar, CRA
Indocyanine Green Angiography
3rd: Number 3, Sarah Schmid-Ainsworth, BSc
HM: Posterior Uveitis, Christopher Keth, BA, CRA
Instrumentation Photography
HM: LASIK on a Contact Lens, David Miller, BSc, CRA
Monochromatic Photography
HM: Macular Star, Robert Mays, Karla Woods, COA
HM: Vitreous Heam, John Leo, BSc
Optical Coherence Tomography
3rd: Macular Hole with Operculum, Bradley Stern, BA, CRA
HM: Idiopathic Intracranial Hypertension, Sean Grout, OCT-C
HM: Choroidal Metastasis, Sean Grout, OCT-C
HM: A Weaver’s Hitch of Fibrin Membrane - the Frequent Complication, Prasanna Ramesh, MS, DNB; Shruthy Ramesh, DNB, MS
HM: The Leaky Barrier of Corneal Endothelial Dystrophy, Anugraha Balamurugan, MS; Prasanna Ramesh, MS, DNB
OCT – Angiography
3rd: Myopic Choroidal Neovascularization, Kasi Sandhanam
Slit Lamp Photography
3rd: Dislocated Lens, Kasi Sandhanam
HM: Netarsudil-Associated Honeycomb Edema, Sean Grout
HM: Neovascularisation of IRIS, John Leo
HM: Ciliary Body Melanoma, Noelle Pensec Rovensky, CRA
HM: Veil over the Window - Occlusio Pupillae, Shruthy Vaishali Ramesh
HM: Pseudoexfoliation, Jason Calhoun
The Eye as Art
HM: Special Effects Visual Flower - Iris Patterned Petals, Prasanna Ramesh, MS, DNB
HM: Fish Food “Pisciform Lesion”, Bryan Carmichael, BSc, OSA, CST, COT
HM: The Arctic Circle: Capsular Phimosis, Kumar Doctor, MD; Shivani Pattnaik, MS
Ultra-Widefield Imaging
3rd: Pigmented Retinochoroidal Atrophy, Jason Calhoun, COA
HM: Bietti’s Crystalline Retinopathy, Lisa Spraragen, MA, COA
HM: Angiod Streaks, Tracey Troszak, CRA
HM: Dislocated Lens, Teesha McClam, BSc, OCT-C, COT
Q & A with VisionGift

What is VisionGift?
VisionGift is a non-profit organization dedicated to improving lives through eye donation, transplantation, and research. Our mission is to Honor Donors by Advancing Sight for all Humankind, while providing hope and healing to individuals in need of corneal transplants. Our vision that Everyone has a Fundamental Right to See is supported through our education of the public and key partners about the importance of eye donation. Since our founding in 1975, we have helped facilitate over 70,000 corneal transplants and much more through advancements in ocular research, giving recipients of the gift of vision a renewed sense of hope for their future. At VisionGift, we are committed to honoring the gift of donation and making a difference in the lives of those we serve.
Where do donations come from?
VisionGift is proud of the many different functions we perform on the path to sight restoration in our communities. Through collaboration with our donation partners throughout the region, we are able to screen and recover donated tissues throughout Oregon, southwest Washington, and western Idaho from generous individuals who have registered as organ, eye and tissue donors through the online registry or by designating their wishes on their driver’s license. Finally, we strive to continue our strong tradition of involvement in ocular research initiatives, both within our own Vision Research Laboratory and by providing tissues to outside research facilities. Through programs like these, we can continue to increase the safety and efficacy of the tissues we provide.

Evelyne Tschibelu
27-43 Wormwood Street
Suite 100
Boston, MA 02210
evelyne.tschibelu@visiongift.org



KeraNatural® arc (long-term preserved corneal tissue) transplanted in lower half of patient eye, barely visible.
KeraNatural® arc (long-term preserved corneal tissue), held by forceps.


How do you collect the tissue?
CMS CoPs (Centers for Medicare Services, conditions of participation) requires that any death that takes place in a hospital that receives Medicare funds (pretty much any hospital) has to refer that death to the local procurement agency (that’s us in our region) and when medically suitable donation wishes need to be honored or legal next-of-kin needs to be given the opportunity to donate if wishes are not documented. Once we receive a referral we screen known medical history through either EMR (electronic medical records) or with the referring organization to determine initial suitability for donation. If yes, we check the registry to see if the individual is a registered donor and then contact the appropriate nextof-kin and either notify them of their loved ones wishes, or give them the opportunity to donate on their loved one’s behalf. An extensive questionnaire is completed with a knowledgeable historian to determine the potential donors risk assessment (DRAI) for transplant. Once authorization and DRAI is obtained we send a technician out to the hospital, funeral home, ME office, etc. to recover the gift along with obtaining a blood sample from the donor for serologic testing. It’s then brought back to the eye bank where another group goes into a more extensive review of past medical history, obtains serology testing results for potential infectious diseases, and then is ultimately either cleared for transplant or designated for important research or medical education. f the gift will go to transplant, the next group looks at the tissue itself and determines the type of transplant it’s suitable for and then moves the tissue for additional processing to prepare it for whatever transplant will be performed. Once processed and ready to be sent to the surgeon, it’s packed up in a temperature-controlled package on wet ice and is transported to the surgery center where it will finally restore sight to an individual in need.
What is the success rate of a transplant of tissue?
Corneal transplants are very successful, having a greater than 90% success rate when cases are not complicated. Graft survival does drop at 5-10 years, but most of the publications on graft survival are older and tend to focus on penetrating keratoplasty (full thickness cornea) rather than the more common lamellar keratoplasty (partial thickness) that we do now. 1
1. DMEK (Descemet Membrane Endothelial Keratoplasty https:// www.healio.com/news/ophthalmology/20220222/concernsremain-about-longterm-graft-survival-in-dmek#:~:text=Graft%20 survival%20rate%20was%20estimated,graft%20survival%20 at%2010%20years.
Cornea in plastic chamber with storage media, set in chamber holder.
Patient Ready DMEK® is processed and loaded into a glass injector and set in chamber, ready for use in the OR.
Challenging/Interesting Cases

Chris
c.keth@duke.edu
Slit lamp work has become one of my favorite aspects of the job, whether it’s working with a child in pain (next page, bottom right), cancer patients (below, right), or strange accidents such as a run in with a pressure washer (next page, top group). I value our ability to help people in need while being enriched by the constant flow of interesting situations and beautiful visuals.



Mooren’s Ulcer (in stereo)
Pigmentary Growths in Metastatic Melanoma







Iris Pigment Lesion
Corneal Blood Staining
Dislocated Lens w Vit Prolapse


