MODULES AND OP TIONS
| INNOVATION | SERVICE
Recognized by the FDA for use in all phases of Clinical and PMA Trials


QUALITY
RETURN TO TABLE OF CONTENTS



RETURN TO TABLE OF CONTENTS



Curve
Sensitivity
Utilizing
Vision

. .
. . . .
(ACSFS) . . . . . . . . . .


. . . . . . . . . . . . . . .
.
. . . . . . . . . .
. .
TABLE O F CONTENTS MODULES: Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 EVA Electronic Visual Acuity – e-ETDRS / ATS Algorithms . . . . . . . . . . . 5 DVA-5000 Standardized Automated ETDRS Testing . . . . . . . . . . . . . 6 Automated ETDRS – Testing at Distance, Intermediate, and Near . . . . . . . . 7 5.5" Ultra-High Resolution Display . . . . . . . . . . . . . . . . . . . . 8 Distance OneTM Ultrasonic Distance Measurement Device 9 Contrast Acuity Testing . . . . . . . . . . . . . . . . . . . . . . . . 10 Contrast Threshold Testing . . . . . . . . . . . . . . . . . . . . . . . 11 Linear Sine Wave Gratings . . . . . . . . . . . . . . . .
. . . 12 Automated Contrast
Function System
. 13 Defocus
–
Automated ETDRS
. 14 Web-based
Testing .
. . . . . .
.
. . 15 eLVTTM – electronic low vision testing 16 OPTIONS: Luminance Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . 17 Glare Testing System (GTS) . . . . . . . . . . . . . . .
. . . . . . 18







FEATURES •Configured to use e-ETDRS or ATS Protocols • Monitor calibrations set to ANSI, ISO specifications and FDA recommendations of 85 cd/m2 for visual acuity testing • Utilizes an infinite number of chart randomizations • Eliminates manual recording, technician bias and data reporting errors • Reliable repeatable results • Test results can be automatically exported in an XML or CSV format to any EDC or Reading Center providing immediate access for statistical analysis • Significantly reduces data collection expense • Saves space! No big bulky cabinet to wheel in and out • Multiple hardware configurations available • Conforms to all ANSI, ISO and FDA guidelines for vision testing • CE marked for distribution to the European Union EVA Electronic Visual Acuity e-ETDRS ATS Algorithms Tablet Controller Test Results M&S | Clinical Trial Suite CTS Similarly, for Contrast Sensitivity testing, the above symbol sets can be displayed at varying levels of contrast 0.4% to 100% (black) Any symbol set can be selected and inserted into the EVA protocol to accommodate international investigator sites. •Sloan Letters •British Standard Institution Letters •Euro-Wide Letter Set •Landolt Rings •Numbers •Tumbling E’s •LEA Symbols® • HOTV Symbols Sets Available mstech-eyes.com 1-877-225-6101 1-847-763-0500 Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS RETURN TO TABLE OF CONTENTS VISUAL ACUITY Recognized by the FDA for use in all phases of Clinical and PMA Trials ®
DVA-5000 Standardized Automated ETDRS
Recognized by the FDA for use in all phases of Clinical and PMA Trials
FEATURES
• Clinical Trial testing with consistent, repeatable results from site-to-site and visit-to-visi
• Quickly and accurately guides technicians through computer-automated, algorithm-based AutomatedETDRS testing eliminating technician bias, calculation errors, and erroneous test results
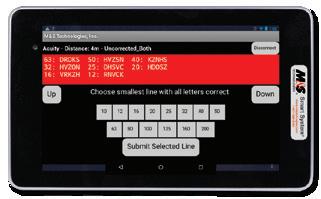
• Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers

• Memorization Effect is eliminated with an infinite number of computer-randomized optotype, letter, and shape presentations ensuring consistent, repeatable and unbiased test results
• Can be configured to “stop” testing if environmental luminance levels exceed Sponsor requirements

• Pre-programmed testing protocols by visit - Customized per sponsor specifications
• Product of choice by major Pharmaceutical Companies and Research Institutions
• Optotypes are precisely calibrated for both distance-to-subject and pixels-per-inch, strictly following ANSI Z80.21-2010 (R2015), ISO 8596:2017, and ISO 10938 guidelines regarding size, spacing between optotypes, and spacing between lines
• Standardized, background luminance is set to 85 cd/m2 as recommended by the FDA (ANSI/ISO) and can be customized from 80 to 320 cd/m2 to fit any luminance levels and distance required for a study
• Letter-contrast luminance as well as ambient environmental room conditions are automatically calibrated and measured with the Luminance Sensor

• Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter
• Export test results automatically in an XML or CSV format to any EDC or Reading Center for immediate access to statistical analysis - Results indicate ETDRS numerical score, visual acuity level, and logMAR value



•
to all
Union
•
& validated
College of
-
Visual Performance
®
Tablet Controller Sample Test Result
Conforms
ANSI, ISO and FDA guidelines for vision testing
CE marked for distribution to the European
Published
by Southern
Optometry, Published Optometry and
Volume 6, April 2018 Test Results Letter Score: 81 Equivalent Acuity: 20/25 Log: 0.12
mstech-eyes.com 1-877-225-6101 1-847-763-0500 Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS VISUAL ACUITY
•
• Quickly and accurately guides technicians through computer-automated, algorithm-based Automated-ETDRS testing eliminating technician bias, calculation errors, and erroneous test results
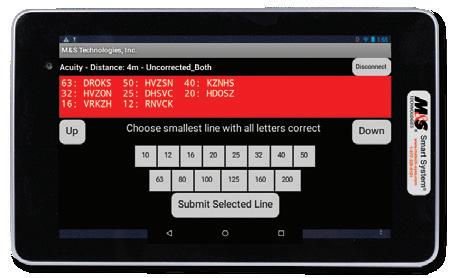
• Calibration set for 40 cm for intermediate and 66 cm for near testing and is easily configurable to any distance
• Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers

• Memorization Effect is eliminated with an infinite number of computer-randomized optotype, letter, and shape presentations ensuring consistent, repeatable and unbiased test results
• Can be configured to “stop” testing if environmental luminance levels exceed Sponsor requirements
• Optotypes are precisely calibrated for both distance-to-subject and pixels-per-inch, strictly following ANSI Z80.21-2010 (R2015), ISO 8596:2017, and ISO 10938 guidelines regarding size, spacing between optotypes, and spacing between lines
• Standardized, background luminance is set to 85 cd/m2 as recommended by the FDA (ANSI/ISO) and can be customized from 80 to 320 cd/m2 to fit any luminance levels and distance required for a study
• Letter-contrast luminance as well as ambient environmental room conditions are automatically calibrated and measured with the Luminance Sensor

• Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter
• Export test results automatically in an XML or CSV format to any EDC or Reading Center for immediate access to statistical analysis - Results indicate ETDRS numerical score, visual acuity level, and logMAR value

•

Conforms to all ANSI, ISO and FDA guidelines for vision testing - CE marked for distribution to the European Union
Published & validated by Southern College of Optometry, Published Optometry and Visual Performance Volume 6, April 2018
High-Resolution Laptop Required
® Automated E Testing at Distance, Intermediate and N Tablet Controller Sample test result RETURN TO TABLE OF CONTENTS VISUAL ACUITY M&S | Clinical Trial Suite CTS Recognized by the FDA for use in all phases of Clinical and PMA Trials FEATURES 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
5.5" Ultra-High Resolution


Tablet Controller




®
Display
•Precision display of letters/symbols at near and intermediate test distances • Allows testing down to 28 cm with clear optotypes • Customized test distances from 28 cm to 66 cm • Fully automated testing algorithm for both ETDRS and Contrast Acuity • Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter • Utilizes an infinite number of chart randomizations • Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers • Export test results automatically in an XML or CSV format to any EDC or Reading Center for immediate access to statistical analysis • Small Footprint compared against ETDRS near charts • Add the Distance One TM measurement device to maintain precise display-to-subject distance • Seamless integration into Clinical Trial Suite • USB powered / HDMI connected • Conforms to all ANSI, ISO and FDA guidelines for vision testing - CE marked for distribution to the European Union • Published & validated by Illinois College of Optometry, Published Clinical and Experimental Optometry. Volume 103 2020
Ultra high-resolution display for ultra-sharp optotypes
Testing down to 28cm RETURN TO TABLE OF CONTENTS VISUAL ACUITY M&S | Clinical Trial Suite CTS Recognized by the FDA for use in all phases of Clinical and PMA Trials FEATURES 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS





•Ultrasonic distance detection accurately measures and maintains specified calibration distance from the LED display to subject •Ultrasonic sensors send and receive data every 0.025 seconds, verifying the distance to the patient •Sponsor determines the minimum and maximum limits to ensure accuracy when testing at near and intermediate distances •Auto-Motion Detection will pause the test while notifying the technician based on participant deviation in distance to LED display •Easily calibrated without requiring trial frames or any correction •Seamless integration with all CTS systems for both near and intermediate testing •Accurate to within 1 cm Conforms to all ANSI, ISO, and FDA guidelines for vision testing CE marked for distribution to the European Union Distance OneTM ULTRASONIC DISTANCE MEASUREMENT DEVICE mstech-eyes.com 1-877-225-6101 1-847-763-0500 Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS DISTANCE ONETM Recognized by the FDA for use in all phases of Clinical and PMA Trials FEATURES ®
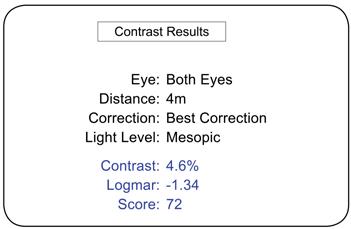
Contrast Acuity Testing

• Quickly and accurately guides technicians through computer-automated, algorithm-measured Contrast Acuity Testing eliminating technician bias, calculation errors, and erroneous test results


• Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers

• Memorization Effect is eliminated with an infinite number of computer-randomized optotype, letter, and shape presentations ensuring consistent, repeatable and unbiased test results
• System calibration is set in meters at virtually any distance and is adjustable and precise to within 1 cm
• Optotypes are precisely calibrated for both distance-to-subject and pixels-per-inch, strictly following ANSI Z80.21-2010 (R2015), ISO 8596:2017, and ISO 10938 guidelines regarding size, spacing between optotypes, and spacing between lines
• Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter
• Standardized, background luminance is set to 85 cd/m2 as recommended by the FDA (ANSI/ISO) and can be customized from 80 to 320 cd/m2 to fit any luminance levels and distance required for a study
• Letter-contrast luminance as well as ambient environmental room conditions are automatically calibrated and measured with the Luminance Sensor

• Export test results automatically in an XML or CSV format to any EDC or Reading Center for immediate access to statistical analysis - Results indicate ETDRS numerical score, contrast acuity level, and logMAR value

• Conforms to all

Tablet Controller Test Results
ANSI, ISO and FDA guidelines for vision testing - CE marked for distribution to the European Union • Published & validated by Southern College of Optometry, Optometry Vision Science. Volume 97, May 2020 RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS CONTRAST SENSITIVITY Recognized by the FDA for use in all phases of Clinical and PMA Trials FEATURES ® 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
Trial
Contrast Threshold Testing
by the
for use
and PMA Trials
• Quickly and accurately guides technicians through computer-automated, algorithm-measured Contrast Threshold Testing eliminating technician bias, calculation errors, and erroneous test results

• FIXED SIZE: Letters are displayed at 20/100 size and begin at 10% contrast (-1.0 log) and will decrease -0.1 log steps (contrast threshold testing)

• Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers
• Memorization Effect is eliminated with an infinite number of computer-randomized optotype, letter, and shape presentations ensuring consistent, repeatable and unbiased test results
• System calibration is set in meters at virtually any distance and is adjustable and precise to within 1 cm
• Optotypes are precisely calibrated for both distance-to-subject and pixels-per-inch, strictly following ANSI Z80.21-2010 (R2015), ISO 8596:2017, and ISO 10938 guidelines regarding size, spacing between optotypes, and spacing between lines

• Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter
• Standardized, background luminance is set to 85 cd/m2 as recommended by the FDA (ANSI/ISO) and can be customized from 80 to 320 cd/m2 to fit any luminance levels and distance required for a study
• Letter-contrast luminance as well as ambient environmental room conditions are automatically calibrated and measured with the Luminance Sensor

• Export test results automatically in an XML or CSV format to any EDC or Reading Center for immediate access to statistical analysis - Results indicate ETDRS numerical score, contrast threshhold level, and logMAR value
• Conforms to all ANSI, ISO and FDA

Union

• Published & validated by
2021
for vision testing - CE marked for distribution to the


College of Optometry, Optometry and Physiological
. Volume
guidelines
European
Southern
Optics
41, July
Tablet Controller Test Results RETURN TO TABLE OF CONTENTS M&S | Clinical
Suite CTS CONTRAST SENSITIVITY Recognized
FDA
in all phases of Clinical
FEATURES ® 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
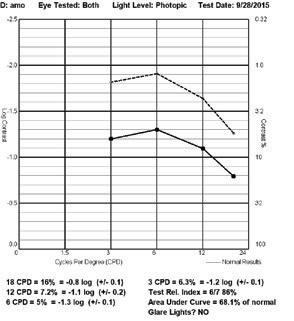
Linear Sine Wave Gratings FEATURES

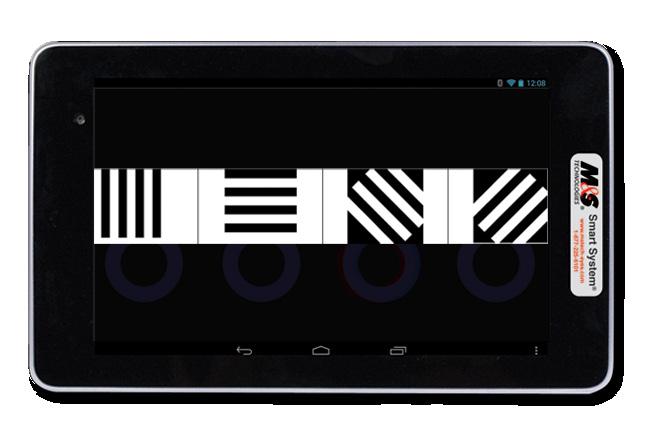
• Conduct Contrast Sensitivity Function testing at 1.5, 3, 6, 12 and 18 cycles-per-degree at either photopic or mesopic luminance levels
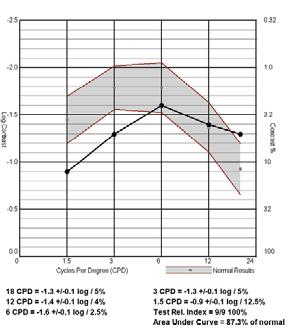
• Contrast levels start at 0.6 log units above normative data allowing for expedited test time

• Average test time is less than 4 minutes per eye
• Complies with FDA Task Force recommendations of target and background luminance
• Background luminance is standardized and calibrated to 85 cd/m2 to comply with ANSI and ISO testing, with certification on brightness, contrast and color temperature
to accommodate a specific
• Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter

• Randomized presentations are precisely calibrated for both distance-to-subject and pixelsper-inch - Utilizes an infinite number of chart
• Test results can be automatically exported in an XML or CSV format to any EDC or Reading Center providing immediate

• System
fully
Tablet Controller Test Results
levels
Customizable
randomizations
access for statistical analysis
is
customizable to accommodate any study requirements • Conforms to all ANSI, ISO and FDA guidelines for vision testing • CE marked for distribution to the European Union RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS CONTRAST SENSITIVITY
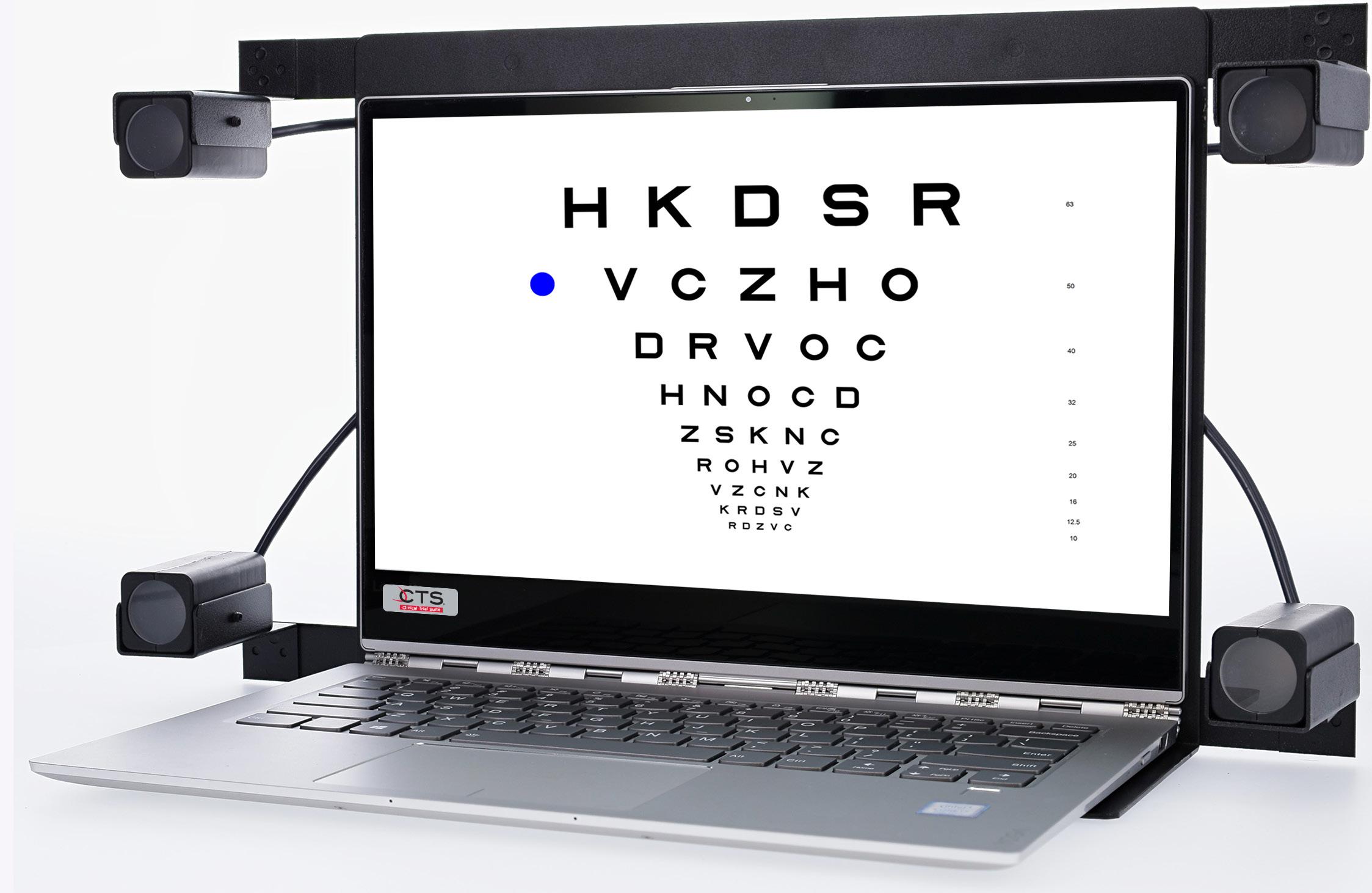
Laptop
shown
with optional Glare
Testing Lights Recognized by the FDA for use in all phases of Clinical and PMA Trials ® 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
FEATURES
• Conduct Contrast Sensitivity Function testing at 1.5, 3, 6, 12 and 18 cycles-per-degree at either photopic or mesopic luminance levels

• Contrast levels start at 0.3 log units above normative data, allowing for expedited test time

• Average test time is less than 5 minutes per eye, followed by 2-3 minutes for existing subjects by starting at 0.3 log units above previously stored values
• Eliminate patient bias with randomly presented optotypes and non-rotational symmetric optical aberrations such as residual astigmatism, coma, trefoil, etc.
• Replaces biased testing methods for with-the-rule aberrations such as vertical astigmatism, vertical coma, and biased against aberrations such as against-the-rule and horizontal coma
• Complies with FDA Task Force recommendations of target and background luminance levels
• Background luminance is standardized and calibrated to 85 cd/m2 to comply with ANSI and ISO testing, with certification on brightness, contrast and color temperature

• Mesopic testing at 3 cd/m2 is available by utilizing a neutral density filter

• Designed with the maximum response time to eliminate the focusing effect and predetermined screen blanking time, and to allow the retina to refresh between displays
• Randomized presentations are precisely calibrated for both distance-to-subject and pixels-per-inchUtilizes an infinite number of chart randomizations
• Test results can be automatically exported

•
format to any EDC
in an XML or CSV
or Reading Center providing immediate access for statistical analysis
Conforms to all ANSI, ISO and FDA guidelines for vision testing - CE marked for distribution to the European Union • Published & validated by Southern College of Optometry, Optometry and Visual Performance. Volume 7, January 2019
Laptop shown with Sinusoidal Bullseye and optional Glare Testing Lights
Tablet Controller Automated Contrast Sensitivity Function System (ACSFS) Test Results RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS CONTRAST SENSITIVITY Recognized by the FDA for use in all phases of Clinical and PMA Trials ® 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
Defocus Curve

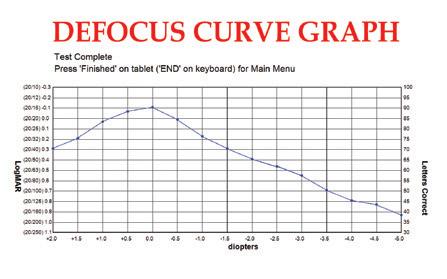
Utilizing Automated ETDRS

by
FEATURES
and PMA Trials
• Defocus Curve automates ETDRS testing from the standard +2.0 diopters through -5.0 and also customizable depending on specific study
• Diopters from +0.5 to -0.5 decrease in .25 increments
• Quickly and accurately guides technicians through computer-automated, algorithm-based visual acuity testing eliminating technician bias, calculation errors, and erroneous test results


• Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers
• Memorization Effect is eliminated with an infinite number of computer-randomized optotype, letter, and shape presentations ensuring consistent, repeatable and unbiased test results
• System calibration is set in meters at virtually any distance and is adjustable and precise to within 1 cm
• Optotypes are precisely calibrated for both distance-to-subject and pixels-per-inch, strictly following ANSI Z80.21-2010 (R2015), ISO 8596:2017, and ISO 10938 guidelines regarding size, spacing between optotypes, and spacing between lines
• Standardized, background luminance is set to 85 cd/m2 as recommended by the FDA (ANSI/ ISO) and can be customized from 80 to 320 cd/m2 to fit any luminance levels and distance required for a study
• Results indicate ETDRS numerical score, visual acuity level, and logMAR value
• Export test results automatically

•
XML or CSV format to any EDC or Reading Center for
® Tablet Controller Test Results
in an
immediate access to statistical analysis
Conforms to all ANSI, ISO and FDA guidelines for vision testing - CE marked for distribution to the European Union • Published & validated by Southern College of Optometry, Published Optometry and Visual Performance. Volume 6, April 2018 RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS VISUAL ACUITY Recognized
the FDA for use in all phases of Clinical
1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
FEATURES




• Maintain regular status of patients health • Increased patient engagement • Results are securely transmitted and stored on HIPAA compliant server • Keep clinics safe and sanitary • Convenient scheduling for both doctor & patient • Increased patient volume • Doctor is able to view results immediately • Convenient web-based testing on any computer or tablet • Simple user friendly interface • Easy to calibrate • Fast, accurate results transmitted to the doctor • Tests include: e -ETDRS, ATS/HOTV and Screening protocols ® Web-based Vision Testing •••••••••••• Straightforward and Secure RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS VISUAL ACUITY 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
•
•
hand motion
manual recording, technician
• Memorization Effect
letter,
including Sloan letters,


white
and data reporting errors

number of computer-randomized


repeatable

® • Can accurately determine VA levels greater than 20/10,000 and is repeatable • Proprietary technology allows testing at 3 meters, 1 meter and 25 centimeters *** • Testing algorithm includes a combination of test targets
Tumbling E, black with white gratings and black and
discrimination
Eliminates
or finger detection
Eliminates
bias
is eliminated with an infinite
optotype,
and shape presentations ensuring consistent,
and unbiased test results • Test results can be automatically exported in an XML or CSV format to any EDC or Reading Center providing immediate access for statistical analysis • Conforms to all ANSI, ISO and FDA guidelines for vision testing - CE marked for distribution to the European Union Sample test results e LVT TM electronic low vision test Make your test complete Another industry first from M&S, the eLVT™ system eliminates the need to count fingers (CF) or detect hand motion (HM) for vision assessment and allows the clinician to determine the visual thresholds of low vision patients with accuracy and repeatability. RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS VISUAL ACUITY Recognized by the FDA for use in all phases of Clinical and PMA Trials FEATURES 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
Luminance Sensor



by
FEATURES
and

Trials
technician
® OPTIONS RETURN TO TABLE OF CONTENTS
• Complies with FDA Task Force recommendations of target and background luminance levels • System can be customized to reflect any luminance level for both the display device as well as environmental light levels • Automated protocol for setting luminance levels removes all variability and
involvement thereby providing consistent levels from investigator site-to-investigator site • Typical photopic range is 85 cd/m2 +/- 5%, well within guidelines • Can be configured to “stop” testing if environmental luminance levels exceed Sponsor requirements • Conforms to all ANSI, ISO and FDA guidelines for vision testing • CE marked for distribution to the European Union Recognized
the FDA for use in all phases of Clinical
PMA
1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
Glare Testing System (GTS)
Recognized by
GTS from M&S is the most accurate and innovative Glare Testing System on the market today. It delivers consistent, repeatable results to effectively evaluate the effect of glare on contrast sensitivity and visual acuity. Glare Testing System complies with the FDA task force recommendations on reducing the contrast function without washing out the subjects vision.
The proprietary lenses minimize the “light spray”, starburst and halo effects typically associated with conventional glare testing methods. Patented technology allows for an equal amount of light to be dispersed over the surface of the eye for balanced luminance distribution. Peer review and publication(s) available for review.

•Consistent luminance at required FDA testing distances
• Test with Contrast Sensitivity and Visual Acuity
•Can be used at both Photopic and Mesopic luminance levels
• Choose Sloan letters (10), British Standard Institution letters (12), Euro-wide letters (12), Tumbling E, Landolt C Rings, LEA SymbolsTM, and numbers
• Utilizes an infinite number of chart randomizations
• Easy-to-install and utilizes long-life, high-intensity LEDs


• Conforms to all ANSI, ISO and FDA guidelines for vision testing CE marked for distribution to the European Union

• Published & validated by Southern College of Optometry, Optometry and Visual Performance. Volume 7, January 2019

FEATURES Close-up of single LED light RETURN TO TABLE OF CONTENTS M&S | Clinical Trial Suite CTS
the FDA for use in all phases of Clinical and PMA Trials
Laptop shown with optional Glare Testing Lights
OPTIONS® 1-877-225-6101 1-847-763-0500 mstech-eyes.com Software development in USA The First Choice in Vision Testing Systems ©2022 M&S and Smart System are registered trademarks of the Hilsinger Company Parent LLC. All rights reserved. The Hilsinger Company Parent LLC holds US Patents 7,354,155; 7,926,948; 8,425,040; 8,167,429; 8,419,184; 8,550,631; 8,992,022; 9,433,347; 9,820,644; 10,244,938 and 10,182,713. Other Patents Pending. YEARS
FOR MORE INFORMATION


QUALITY | INNOVATION | SERVICE
Please call: 1-847-763-0500 Visit us at: www.mstech-eyes.com RETURN TO TABLE OF CONTENTS