

Accuracy of a New Noninvasive Automatic Ocular Surface Analyzer for the Diagnosis of Dry Eye Disease-Two-Gate Design Using Healthy Controls
Janosch Rinert , Giacomo Branger , Lucas M Bachmann , Oliver Pfaeffli , Katja Iselin , Claude Kaufmann , Michael A Thiel , Philipp B Baenninger
Affiliations
PMID: 35543570 DOI: 10.1097/ICO.0000000000003052
Abstract
Purpose: The purpose of this study was to assess the diagnostic performance of measurements from a new noninvasive, automated ocular surface analyzer (IDRA) in the diagnosis of dry eye disease (DED).
Methods: We prospectively identified patients with and without DED using best practice methods. Subsequently, all participants underwent IDRA analysis, consisting of 5 components: noninvasive tear film break-up time, tear meniscus height, lipid layer interferometry, eye blink quality, and infrared meibography. The manufacturer provides cutoff values for a pathologic result for each of these components. Using a stepwise augmentation multivariate logistic regression model, we identified the components with the strongest association for the presence of DED. For the 3 components with the strongest association (interferometry, tear meniscus, and infrared meibography), we calculated the probability of DED.
Results: We enrolled 40 patients (80 eyes) with DED (mean age 60.5 years; women 78.3%) and 35 healthy subjects (70 eyes, mean age 31.1 years; women 21.7%). The IDRA had an area under the curve of 0.868 (95% confidence interval: 0.809-0.927) to detect DED. A normal (≥80) interferometry combined with a normal (>0.22) tear meniscus and a normal (≤40) infrared meibography was associated with an estimated probability of 18% for the presence of DED, whereas the estimated probability of DED was as high as 96% when all 3 findings were pathologic.
Conclusions: The results of IDRA showed a positive concordance with routine clinical diagnostic tests. The new analyzer is an easyto-access diagnostic tool to rule out the presence of DED in the extramural setting and to guide a timely DED treatment.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
PubMed Disclaimer
References
Clegg JP, Guest JF, Lehman A, et al. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13:263–274.
Verjee MA, Brissette AR, Starr CE. Dry eye disease: early recognition with guidance on management and treatment for primary care family physicians. Ophthalmol Ther. 2020;9:877–888.
Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–387.
Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–365. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283.
Ozulken K, Aksoy Aydemir G, Tekin K, et al. Correlation of non-invasive tear break-up time with tear osmolarity and other invasive tear function tests. Semin Ophthalmol. 2020;35:78–85.
Gumus K, Crockett CH, Rao K, et al. Noninvasive assessment of tear stability with the tear stability analysis system in tear dysfunction patients. Invest Ophthalmol Vis Sci. 2011;52:456–461.
Eom Y, Lee JS, Kang SY, et al. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155:1104–1110.e2.
Jung JW, Park SY, Kim JS, et al. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2016;57:4076–4083.

Review
PromisingHigh-TechDevicesinDryEyeDiseaseDiagnosis
AndreaDeLuca,AlessandroFerraro,ChiaraDeGregorio,MariateresaLaborante,MarcoCoassin , RobertoSgrullettaandAntonioDiZazzo*
OphthalmologyComplexOperativeUnit,UniversityCampusBio-Medico,00128Rome,Italy
* Correspondence:a.dizazzo@policlinicocampus.it
Abstract: Background:Dryeyedisease(DED)isacommonanddebilitatingconditionthataffects millionsofpeopleworldwide.Despiteitsprevalence,thediagnosisandmanagementofDED canbechallenging,astheconditionismultifactorialandsymptomscanbenonspecific.Inrecent years,therehavebeensignificantadvancementsindiagnostictechnologyforDED,includingthe developmentofseveralnewdevices.Methods:Aliteraturereviewofarticlesonthedryeye syndromeandinnovativediagnosticdeviceswascarriedouttoprovideanoverviewofsomeofthe currenthigh-techdiagnostictoolsforDED,specificallyfocusingontheTearLabOsmolaritySystem, DEviceHygrometer,IDRA,Tearcheck,Keratograph5M,CorneaDomeLensImagingSystem,I-PEN OsmolaritySystem,LipiViewIIinterferometer,LacryDiagOcularSurfaceAnalyzer,Tearscope-Plus, andCobraHDCamera.Conclusions:Despitethefactthatconsistentuseofthesetoolsinclinical settingscouldfacilitatediagnosis,nodiagnosticdevicecanreplacetheTFOSalgorithm.
Keywords: dryeyedisease;diagnosticdevice;ocularsurface
1.Introduction
Citation: DeLuca,A.;Ferraro,A.;De Gregorio,C.;Laborante,M.;Coassin, M.;Sgrulletta,R.;DiZazzo,A. PromisingHigh-TechDevicesinDry EyeDiseaseDiagnosis. Life 2023, 13, 1425. https://doi.org/10.3390/ life13071425
AcademicEditors:José-María Sánchez-González,Carlos Rocha-de-Lossadaand AlejandroCerviño
Received:8May2023
Revised:15June2023
Accepted:19June2023
Published:21June2023

Copyright: ©2023bytheauthors. LicenseeMDPI,Basel,Switzerland. Thisarticleisanopenaccessarticle distributedunderthetermsand conditionsoftheCreativeCommons Attribution(CCBY)license(https:// creativecommons.org/licenses/by/ 4.0/).
TheTFOSDEWSII(TearFilmandOcularSurfaceSocietyInternationalDryEye WorkshopII,2017)definesdryeyedisease(DED)as“amultifactorialdiseaseoftheocular surfacecharacterizedbyalossofhomeostasisofthetearfilmandaccompaniedbyocular symptoms,inwhichtearfilminstabilityandhyperosmolarity,ocularsurfaceinflammation anddamage,andneurosensoryabnormalitiesplayetiologicalroles”[1].Theprevalenceof DEDexhibitsapositivecorrelationwithadvancingageandvariesbetweenfivepercentand fiftypercentacrosstheoverallpopulation[2].DEDischaracterizedbyarangeofsymptoms suchasocularpain,burning,stinging,discomfort,aforeignbodysensation,poorvisual acuity,photophobia,andirritation[1,2].Thesymptomsofdryeyediseasecanrangefrom minordiscomforttosubstantialgrievancesthatinterferewithdailyfunctioning,decrease qualityoflife,andcarrynotableconsequencesforthesocioeconomicstructure[1,2].
Thefirstphaseintheprocessofdiagnosingdryeyediseaseinvolvestheutilizationof triagequestions,whichcouldestablishtheneedforadditionalDEDevaluationandexclude disorderssuchasconjunctivitis,blepharitis,Sjögrensyndrome,infection,andlid-related disease.
BasedontheTFOSDEWSII,adryeyediagnosisrequiresthepatienttoscorepositivelyononeoftwospecificsymptomquestionnaires(DEQ-5,DryEyeQuestionnaire-5 score ≥ 6orOSDI,OcularSurfaceDiseaseIndexscore ≥ 13).Thismustbefollowedbythe presenceofaminimumofonepositiveclinicalsign,suchasdecreasedtearfilmstability (NIBUT,non-invasivetearbreak-uptime,<10s),elevatedtearosmolarity(>308mOsm/L), significantinter-eyedisparityinosmolarity(>8mOsm/L),orocularsurfacedamageindicatedbyfluoresceinandlissaminegreen(>5cornealspots,>9conjunctivalspots,orlid margin ≥ 2mmlengthand ≥ 25%width).
OSDIreferstoaverifiedquestionnairethatiscommonlyemployedinclinicaltrials duetoitsabilitytoprovidearapidassessmentofdryeyedisease(DED)anditsimpacton thequalityoflife(QoL)ofpatients[3].TheOSDIconsistsof12itemsthataimtoevaluate
thesymptomsexperiencedbypatientsduringtheprecedingweek.Itisorganizedinto threesections:theoccurrenceofsymptoms,theimpactonvision-relatedqualityoflife,and theidentificationofanyenvironmentaltriggers[3].Eachitemisevaluatedusingascale rangingfromzerotofour.Thescaleusedtomeasurefrequencyisasfollows:noneofthe time(0),someofthetime(1),halfofthetime(2),themajorityofthetime(3),andtheentire time(4).Theoverallnumericalvalueiscalculatedutilizingarangethatextendsfromzero toonehundred,wherebyelevatedscoresdenoteheightenedlevelsofimpairment.Ascore of0to12isregardedasnormal;ascoreof13to22suggestsmilddisease;ascoreof23to 32denotesmoderateDED;andascoreof33to100indicatessevereDED[3].Furthermore, OSDIdoesnotdiscriminatebetweenevaporativeandaqueousdeficiencies[4].
ItispossiblethattheTFOSDEWSIIdiagnosticalgorithmisnotthemosteffective methodwhenusedinaclinicalenvironment,despitethefactthatitprovidesbotha comprehensiveandfullapproachtodetectingdryeyedisease.Althoughitoffersacomplete evaluation,thediagnosticproceduretypicallyconsistsofanumberofstepsthatcanbe challengingtocarryoutinfast-pacedclinicalsettingswithlimitedtime.
Therefore,inthispaper,weexaminenewhigh-techimagingsystemsforocularsurface evaluation.Thesesystemsclaimtohaveseveralbenefitsovertraditionalmethodsofdiagnosis,suchasbeingnon-invasive,providingstandardizedandobjectiveresults,enabling themonitoringofdiseaseprogressionandtreatmenteffectiveness,beinguser-friendly,and enablingrapidtaskexecution.However,regardlessofthestudiesreviewed,thereliability ofthesedevicesislow.
2.MaterialsandMethods
Aliteraturereviewofarticlesondryeyesyndromeandinnovativediagnosticdevicespublishedinthelast15yearsandavailableontheNationalLibraryofMedicine wascarriedoutwithoutanyrestrictionoflanguage,especiallyfocusingontheTearLab OsmolaritySystem,IDRA,Tearcheck,Keratograph5M,I-PenOsmolaritySystem,Lipiview IIInterferometer,LacryDiagOcularSurfaceAnalyzer,CorneaDomeLensImagingSystem, DEviceHygrometer,Tearscope-Plus,andCobraHDCamera.Allpublishedpeer-reviewed randomizedclinicaltrials,meta-analyses,systematicreviews,andobservationalstudies aboutthesetopicswereevaluated.Table 1 showsallthedevicesanalyzedandtheexams theyperform.
TearLabOsmolaritySystem® (TearLabCorporation,SanDiego,CA,USA)isanoninvasivediagnosticdevicethatanalyzestheosmolarityofapatient’stears.Osmolarity referstothetotalconcentrationofdissolvedsubstancespresentinasolutionwithout regardtotheirdensity,size,molecularweight,orelectricalcharges.Theprocessoftears evaporating,adecreaseintheproductionoftears,andthedysfunctionofthemeibomian glandareallfactorsthatcontributetoanelevationintearosmolarity.Theevaluationof tearosmolarityisconsideredahighlyeffectivediagnostictoolforeverytypeofdryeye syndrome[5].Thetearfilmoftheexposedocularsurfaceareaexhibitsalowerosmolarity. Variousenvironmentalfactorssuchaswind,cigarettesmoke,indoorairconditioning,and heat,aswellasprolongedcomputeruseleadingtoreducedblinkingfrequency,havebeen identifiedaspotentialimpedimentstoevaporation,therebyaffectingtearosmolarity[6]. Tearosmolaritywasalsofoundtocorrelatewithincreasedconcentrationsofinflammatory cytokinesandmatrixmetalloproteinases(MMPs),aswellasHLA-DR(HumanLeukocyte Antigen-DR)overexpression,suggestingthattearosmolaritycouldpotentiallyserveasa predictivemeasureforocularsurfacediseasesthatarelinkedtohighlevelsofinflammatory mediators[6].
TearLabOsmolaritySystem®
Tearcheck®
Keratograph5M®
I-PENOsmolaritySystem®
LipiView® IIinterferometer
LacryDiagOcularSurfaceAnalyzerx
CorneaDomeLensImagingSystem® x
Tearscope-PlusTM x
CobraHDCamera
NIBUT,non-invasivetearbreak-uptime;LLT,lipidlayerthickness;TMH,tearmeniscusheight;RH,relativehumidity.
TheTearLabOsmolaritySystemiscomposedofasetofinstruments,includinga readerdevice,pens,andtestcards.Thereaderisasmallcountertopdevicethatcalculates andshowstheosmolaritytestresultsonaliquidcrystaldisplay.TheTearLabosmolarity deviceisequippedwithapairofpensthatareusedtoholdthetestcardandtransmitthe datatothereader.Thetestcardisattachedtothepensandtakesasampleof50nLin milliosmolesperliter(mOsm/L)units.Thecontactbetweenthetearfilmandtheeyelid occursatthetemporalmargin.Afterhearingthebeepconfirmingsuccessfultearcollection, thepenisdockedintothereader[7].
Thisosmometerhasseveraladvantages,includingbeingasmall,portabledevicethat canbeusedinadoctor’sofficeandrequiringlessthan100nLoftearfluid[5].Furthermore, itusesatemperature-correctedtearfluidimpedancemeasurement,enablinganindirect evaluationoftearosmolarityandprovidingpreciseresultswithinabrieftimeframe[5,8].It mayalsobeusedincombinationwithotherdiagnostictools,suchastheLipiViewsystem, toprovideamoredetailedimageofapatient’sdryeyecondition.AccordingtoSzczesnaIskander,itisnecessarytotakeaminimumofthreeconsecutivemeasurementstoobtain clinicallytrustworthytearosmolarityvaluesusingtheTearLabOsmolaritySystem.TheutilizationofthehighestosmolarityvaluetoidentifyDEDrequirescarefulconsiderationdue tothefrequentoccurrenceofanomalousreadingsoftearosmolarity[9].Nevertheless,Szalai etal.foundsignificantoverlapintearosmolarityvaluesmeasuredwiththeTearLabsystem inthecontrol(22healthyindividuals)anddryeyegroups(21patientswithnon-Sjögren syndromedryeye(NSSDE)and20patientswithSjögrensyndromedryeye(SSDE)),implyingthatmeasurementoftearosmolarityutilizingtheTearLabosmometerishighlyvariable anddoesnotdistinguishindividualsdiagnosedwithdryeyediseasefromhealthycontrols (meantearosmolaritywas 296.77 ± 16.48mOsm/L inNSSDE, 303.36 ± 17.22mOsm/L in SSDE,and303.52 ± 12.92mOsm/Linthecontrolgroup; p =0.018)[10].
Asaresult,TearLabisaquicktoolinclinicalpractice,butitsusefulnessislimitedby theliterature-reportedlowreliabilityinrecognizingDEDanditsprimaryuseinevaporative dryeye.
IDRA® OcularSurfaceAnalyzer(SBMSISTEMI,Inc.,Torino,Italy)isadiagnostictool thatusesinfraredandultravioletlighttoevaluatethehealthoftheocularsurface.Changes inthetearfilmandmeibomianglands,whichcanbeindicativeofDED,canbedetected bytheinstrument.TheinstrumentisabletoidentifyMGaswellasallthreelayersofthe tearfilm(lipid,aqueous,andmucin).Thisallowsphysicianstodeterminewhichpartsof thetearfilmrequiretreatmentbasedonthetypeofinsufficiency.IDRAmustbeplaced betweenaslitlampandabiomicroscope.Itspinshavebeendesignedtofitperfectlyinto theholeleftbyremovingtheplateusedforthetonometer,anditconductsa5-minute non-invasivetest[11].Theinstrumentproducesabeamofwhitelightontothecorneal surface,andtheresultantreflectionoflightfromthetearfilmpresentsawhite,fan-shaped regionthatcoverstheinferiorthirdofthecornea[12].ThefiveparametersincludeNIBUT, TMH,lipidlayerinterferometry,ocularblinkquality,andinfraredmeibography.The non-invasivebreak-uptime(NIBUT)isdeterminedthroughtheutilizationofPlacido’s disctoprojectringpatternsonthecornea,followedbythemeasurementoftheduration insecondsbetweenthecompleteblinkandthefirstdisturbanceofthereflectedimage onthecornea[11].Theutilizationofinfraredilluminationinnon-invasivemeibography hasthecapabilitytoidentifymorphologicalalterationsinthemeibomiangland.Onthe otherhand,tearinterferometrycanbeemployedtoassessthelipidlayerofthetearfilm. Theevaluationofmeibomianglandmorphologyoffersvaluableclinicalevidenceforthe diagnosisofevaporativeDED,whileassessmentsofthelipidlayerofthetearfilmfacilitate themonitoringofmeibomianglandfunction[10].Thephotographshowstheidentification oftheupperandlowertearmeniscusaswellastheevaluationoftearmeniscusheight alongthelowerlidmargin[11].Aprospectivestudywith75patients(40withDEDand 35healthysubjects)foundgoodconcordancebetweentheIDRAocularsurfaceanalyzer andstandarddiagnosticproceduresindifferentiatingbetweenindividualswithnormal
ocularfunctionandthosewithdryeyedisease.Ithadanareaunderthecurveof0.868 (95%confidenceinterval:0.809–0.927)todetectDED[13].
Thelipidlayerisimportantforregulatingtheevaporationofthetearfilm.Atestfor lipidlayerpattern(LLP)evaluationisbasedoninterferencephenomena,butitisinfluenced bysubjectiveinterpretationofthepatterns[14].Thelipidlayerthicknessesaredetermined throughtheutilizationofDr.Guillon’sinternationalgradingsystem,whichenablesthe calculationoftheaverage,maximum,andminimumthicknessesofthelipidlayerpattern grades[15].Thegradesareconvertedtonanometerunitsandclassifiedintoarangeof 15to100nmaccordingtotheobservedpatterns.ThemaximumcutoffwavelengthofIDRA is100nm[16].
DespitethefactthatIDRAhasthebenefitofassessingmultipleocularsurfaceparameterswithasingledevice,thefindingsarecontradictory.Inaretrospectivecross-sectional studywith47non-Sjögrendryeyepatients,Jeonetal.demonstratedasignificantcorrelationbetweendryeyesymptomsandthepartialblinkrateaswellasmeibomiangland dropoutratesmeasuredusingIDRA.Ontheotherhand,Leeetal.demonstratedthat IDRAexhibitedaconsiderablylowerpercentageofmeibomianglanddropoutandagreater partialblinkratethananotherdevice,LipiView® II,inacross-sectional,single-visitobservationalstudywith47participants[11,12].Thesefindingssuggestthatthesedevices shouldnotbeusedinterchangeablywhenassessingmeibomianglanddropoutsandpartial blinkrates[11].Rinertetal.demonstratedafavorablecorrelationbetweeneveryday clinicaldiagnosticexaminations.Theresearchersfoundthattheutilizationofpathologic meibography,interferometry,andtearmeniscusmeasurementswiththeanalyzerproduced a96%estimatedprobabilityofdryeyedisease.Simultaneously,thepercentageofeyesexhibitingpathologicalobservationsinthethreesetsofexaminationswasrelativelyminimal, suggestingthatdryeyedisease(DED)maymanifestindiverseclinicalpresentationsand necessitateacomprehensiveassessment[13].
Thus,IDRAappearstobeaninterestingdevicefordiagnosingDEDbecauseitevaluatesallthreecomponentsofthetearfilm;however,theliteraturepresentsconflictingand limitedfindings.
Tearcheck® (E-Swin,Houdan,France)isastand-alonedevicewithanintegrated screenthatallowstheusertoviewallacquisitionsandexamsinrealtime.Thedevice facilitatesquickevaluationsthatincludenineexaminations:non-invasivebreaktime,tear filmstability,ocularsurfaceinflammatoryassessment,meibographyIR,Demodex,eye redness,abortiveblinking,tearmeniscusheight,andtheOSDIquestionnaire.Thisresults inasimpledryeyeanalysis.
UsingtheDemodexexam,anenlargedimageofthebaseoftheeyelashescanbe obtained,allowingforthetracingandvisualizationofsignsindicatingthepresenceof Demodexmites.Asaresult,thedevicetakeshigh-resolutionimagesoftheocularsurface, enablingthedetectionofchangesinthecornea,conjunctiva,andtearfilm,suchasthe existenceofinflammationorocularsurfacedamage(dryspotsorerosion).
Althoughthedeviceisnon-invasiveandsimpletouse,thereisnoevidenceinthe literaturetosupportitsuseinthediagnosisandmanagementofDED.
Keratograph5M® (Oculus,Wetzlar,Germany)isadiagnosticdevicethatusesnoninvasiveimagingtechnologytoevaluatethehealthofthecornea.Thedevicecanidentify changesinthesurfaceandshapeofthecornea,whichcanbeindicativeofDED.Itisa cornealtopographerthatisequippedwitharealkeratometerandacolorcamera.Its purposeistocaptureexternalimagesbyprojectingaringpatternfromaplacidodisconthe tearfilmsurfaceusinganinfraredlightsource.Itmayevaluatenon-invasivebreak-uptime (NIBUT),meibography,bulbarredness,tearmeniscusheight,lipidlayer(interferometry), andtearfilmdynamics(monitoringoftearfilmparticleflow,fromwhichinferencesabout tearfilmviscositycanbeinferred).
Thefindingsofthistoolintheliteraturearealsocontradictory.Ontheonehand, thekeratographhassignificantexaminerbias[17],butontheotherhand,ithasbeen reportedtohavestronginter-examinerreproducibility(meandifferencebetweenexaminers
of0.08 ± 0.55and0.13 ± 0.50gradeunitsintwoseparatesessions,respectively)with lowwithin-subjectvariability(95%limitsofagreementfortwodifferentexaminersof 1.02to+1.10and 1.27to+1.09gradeunits,respectively)[18].Inaprospectivestudy with42patientswithDEDand42healthysubjects,Tianetal.foundthatutilizingthe non-invasiveKeratographtearbreak-uptime(NIKBUT)andtearmeniscusheight(TMH) measurementsthroughtheK5Mdevicecouldserveasastraightforwardandnon-invasive methodforscreeningdryeyewhilealsoexhibitingsatisfactorylevelsofrepeatabilityand reproducibility(coefficientofvariation(CV%) ≤ 26.1%andintraclasscorrelationcoefficient (ICC) ≥ 0.75forallmeasurements).Nevertheless,itwasobservedthatNIKBUTsexhibited greaterreliabilityinindividualswithdryeyedisease(DED)ascomparedtoTMH[19].
Sutphinetal.concludedthatkeratographicmeasurescannotbeconsideredinterchangeablealternativesforcommonlyusedclinicalmeasures.Furthermore,theyfound thatthereisnospecifictestthatcanprovideobjectivesupportforthediagnosis[20].Indeed, accordingtoPérez-Bartolomé etal.,theKeratograph5Mwasobservedtooverestimate ocularrednessscoresincomparisontosubjectivegradingscaleswhenutilizedforthe purposeofassessingthedegreeofocularredness[21].Furthermore,inanobservational cross-sectionalstudyof47subjectswithDEDand41normalcontrolsubjects,Chenetal. showedalimitedassociationbetweenthekeratographtearmeniscusheight(TMH)and Schirmerscoresamongindividualswithdryeyedisease(DED).Theyalsodemonstrated thatinthecomparisonofFourier-domainopticalcoherencetomography(FD-OCT)andthe Keratograph5M,bothinstrumentsexhibitednotablediagnosticprecisionindistinguishingbetweennormalpatientsandthosewithdryeyedisease.However,itwasobserved thattheFD-OCTmeasurementsoftearmeniscusheight(TMH)weremoredependable thanthekeratographdataintheDEDgroup[22].Specifically,whilethekeratographand FD-OCTmeasurementsofTMHwerecloselycorrelated,theformertendedtoyieldlower measurementsthanthelatter[23].
DespitethefactthattheKeratograph5Misanon-invasivediagnostictopographerfor DED,itisnotyetasubstituteformultipleclinicaltestssuchastheSchirmertestandFBUT becauseitsreliabilityisveryweak.
I-PENOsmolaritySystem® (I-MEDPharmaInc.,Dollard-des-Ormeaux,QC,Canada) isaportableelectricaltoolthatassessestheosmolarityoftearsbymeasuringtheelectrical impedanceoftheeyetissuesonthepalpebralconjunctivalmembrane.Theoccurrenceofan inflammatorycascadeattheocularsurfaceisinitiatedbytearfilmhyperosmolarity,which ultimatelyleadstothelossofgobletcells,epithelialinjury,andtheproductionofcytokines. Thisconditionisresponsibleforcausingoculardistressandvisionimpairmentinpatients withDED.TheI-PEN’susefulnesshasbeenquestionedintheliterature,thoughnumerous studieshaveconsideredtheI-Penappropriateandreliableforclinicaluse[24–26].Parketal. concludedthattheI-Penosmometerdemonstratesfavorableperformanceindiagnosing DEDinclinicalsettings;however,itshouldnotbesolelyrelieduponforevaluatingDED. Nonetheless,theI-Penosmometercanserveasavaluabletoolforscreeningandidentifying dryeyedisease[24].Incontrast,someresearchersfailedtoestablishanycorrelations betweentearfilmosmolarityvaluesacquiredthroughtheI-PENsystemandvarious subjectiveorobjectiveparametersofdryeyedisease(DED).Furthermore,theyshowed thattheI-PENsystemwaslesseffectivethantheTearLabOsmolaritySystemindelineating subjectswithandwithoutdryeyedisease[7,27,28].Shimazakietal.foundnostatistically significantdifferenceinmeantearfilmosmolaritybetweentheDED(871eyes)andnonDED(51eyes)groupsusingtheI-PENsystem(294.76 ± 16.39vs.297.76 ± 16.72mOsms/L, respectively, p =0.32).Furthermore,motionmayaffectosmolarityreadingsacquired throughtheI-Pensystem;however,theinfluenceofthisfactorcanbeminimizedifthe measurementsarecarriedoutbyahighlytrainedclinician[29].Alanazietal.evaluatedthe relationshipbetweenosmolarityresultsacquiredbytheTearLabTMandI-Pen® systemsin individualswithahighbodymassindex(BMI).TheI-Pen® results(294–336mOsm/Linthe studygroupof30malesubjectswithahighBMIand278–317mOsm/Linthecontrolgroup of30healthymales)weresignificantlyhigherthantheTearLabTM scores(278–309mOsm/L
inthestudygroupand263–304mOsm/Linthecontrolgroup).Furthermore,theoutcomes obtainedfromtheI-Pen® measurementsexhibitedsignificantvariationsinosmolarity valuesanddemonstratedaconsiderablelackofaccuracyindistinguishingnormaleyesas comparedtotheTearLabTM system[29].
Asaresult,theI-PENisaportable,easy-to-use,autocalibrateddevicethatisnot affectedbyvariationsintearfilmvolumeandrequiresonlyasimpletouchofthepalpebral conjunctiva.Nevertheless,itcanonlydetecttearosmolarity,andfurtherinvestigationsare requiredtodetermineitsutility.
LipiView® IIinterferometer(TearScienceInc.,Morrisville,NC,USA)isadevicefor ocularimagingthatexaminestheinterferometricpatternofthetearfilm.Itachievesthisby measuringthelipidlayerthickness(LLT)ofthetearfilmwithnanometeraccuracy;however, ithasanuppercut-offtoassessLLTvaluesof100nm[11].Inadditiontothat,itrecords thedynamicsofblinkingandimagesthestructureofthemeibomiangland.Comparedto IDRA,inacross-sectionalsingle-visitobservationalstudywith47participants,MinLee etal.foundnosignificantdifferenceinLLT.However,IDRAhadaconsiderablylowerrate ofMGdropoutandahigherPBR(IDRAMGdropout 45.36 ± 21.87 andPBR 0.23 ± 0.27; LipiView® IIinterferometerMGdropout36.51 ± 17.53andPBR0.51 ± 0.37)[11].In comparisontoKeratograph5M(K5M),Wongetal.showedthatLVIIexhibitedastatistically significantreductioninmeiboscoresandalowerpercentageofMGdropoutin20subjects (1.43 ± 0.78vs.1.90 ± 0.81, p =0.001)[30].Theseresultssuggestthatthereispoor interchangeabilitybetweenthemethodsusedtoevaluateDEDfeatures,particularlyMG dropoutsandPBR.
Inconclusion,thedataobtainedfromtheLVIILLTshouldbecomparedtoother instruments.However,additionalstudieswithlargersamplesizesarenecessary.
LacryDiagOcularSurfaceAnalyzer(QuantelMedical,Cournon-d’Auvergne,France) isanophthalmicdeviceusedtodiagnoseandmonitorthetearfilmandmeibomianglands. Ittakesnon-invasivephotographswithwhiteorinfraredlighttoassesstheheightof thelowertearmeniscus,thedistancebetweentheupperandlowereyelids,tearfilm interferometry,andnon-invasivetearfilmbreak-uptime.Tothetal.demonstratedthatit isanon-invasive,simple-to-usedeviceabletoanalyzethetearfilmandsavephotosfor lateruse[31].Despitethisresult,thereisagreatdealofvariabilitybetweenmeasurements performedbythisinstrumentandthoseperformedbyanotherinnovativedevice,such astheOCULUSKeratograph5M,possiblyreflectingdifferencesinimageprocessingor theneedforsubjectiveevaluationbytheobserverforaconsiderablenumberofthese measurements[32,33].Wardetal.,forinstance,evaluatedtherepeatabilityoftheLacryDiag OcularSurfaceAnalyzerforbothintra-andinter-observermeasurementsandcompared ittotheOCULUSKeratograph5Min30healthysubjects.Theirfindingsrevealedagood relationshipbetweenthedevices(nodifferencesinmeanvaluesfortearmeniscusheight, NIBUT,ortearfilminterferometry,exceptforlipidlayerinterferometry),butlowagreement foranyindividualobserver(intra-observervariabilityforNIBUTwassignificantlyhigher fortheKeratograph, p =0.0003forobserverAand p <0.0001forobserverB).Accordingto theauthors,theobservedinconsistencycouldbeattributedtotheutilizationofrepeated testingandtheinclusionofsubjectswithoutdryeyeconditions.Therefore,theauthors concludedthatintheidentificationandfollow-upofpatientswithdryeyedisease(DED), itisessentialtoconsiderthereproducibilityofthetestinginstrumentandtheutilizationof differentoutcomemeasures[33].
Insummary,LacryDiagisapromisinginstrumentforassessingtheocularsurface,but thereisalackofresearchaboutitinthemedicalliterature.
CorneaDomeLensImagingSystem® (Occyo,Innsbruck,Austria)isanimagingsystem thatattemptstoprovideuniformocularsurfacecolorphotographsrespectingposition, illumination,focus,andoperatorindependence.Thisisachievedbyovercomingthe limitationsofobjectivemethodsthatarebasedondigitalocularsurfaceimages.Thedevice iscomposedofanovelimaginglensthatconformsaccuratelytothecurvatureoftheeye, enablinghigh-resolutionimagingofthevisibleocularsurface.Inaddition,thedevice
incorporatesafixationtargetthatguaranteesacentralizedviewintothelens,thereby minimizingeyemovements.Furthermore,thesetiscomposedofsoftwaredesignedfor eyetrackingaswellasanilluminationunit.Tomaintainthestabilityofthepatient’shead, achinrestandaforeheadbandareutilized.Theaimofthesystemistoobtainphotographs inastandardizedmannerwithouttheneedforhumanintervention.Therefore,according toLins,thistoolpossessestheabilitytoevaluatetheextentofbulbareyerednessinan objectiveandreplicablemanner,utilizingtheimagesithascaptured[34].
Thistechnologyhasthepotentialtoprovideanobjectivetechniquebasedonthedigital ocularsurfaceforassessingbulbareyeredness,overcomingthelimitationsofsubjective photographicscalesthatsufferfrominter-ratervariability.However,itsroleintheDED diagnosticprocessshouldbeinvestigatedinfuturestudies.
DEviceHygrometer© (AI,Rome,Italy)isacomplete,low-costdiagnostic-therapeutic toolforocularsurfacemanagementthathasthecapabilitytoquicklyidentifytheentities ofproduction,clearance,andstability,alongwiththeseverityoftearfilmevaporation, anddrivesthesubsequenttherapythroughtheutilizationofsimplealgorithms.The deviceoperatesbydetectingchangesintherelativehumidity(RH)levelswithinaconfined environmentsurroundingtheocularsurface(Figure 1).
Thediagnosticcomponentofthedeviceworksbymeasuringtheevaporationofthe tearfilmfromtheocularsurfaceatavariablespeedthatmaybemodifiedbytheuser. Atacertaintemperature,thedevicemeasuresthebaselineandpost-stimulusrelative humidityvalues.Thesensorisplacedinacupontheorbitaledgesbytheoperator.The measurementsarecarriedoutinaclosedenvironmentmadeupofthecupandtheocular surfacesystem.Usingtheacquireddata,itispossibletoconstructprogressioncurvesfor relativehumidity(RH)thathavebeencorrectedfortemperature.Thecollecteddataare “basal”valuesthatarecombinedwithmeasurementstakeninreactiontodiversekinds ofstimuli,suchasairblows,alterationsintemperaturewithinthemicroenvironment surroundingtheocularsurface,lightstimuli,andsoon.Additionally,anon-contact samplemechanismbuiltintothedeviceallowsforthecollectionofacertainamountof tearevaporation.Despitethefactthatincompleteblinkingandtearclearancemayhavean impactontheaccuracyofmeasurementsobtainedfromtheDEvice© ,itrepresentsalowcost,efficient,accurate,rapid,andsafer(asitisnon-contact)instrumentformeasurementof tearfilms.Indeed,apreliminaryobservationalpilotstudywith8patients(2withDEDand 6healthysubjects)hasshownthatindividualswithdryeyedisease(DED)showedhigher relativehumidityvaluescomparedtohealthyindividuals.However,additionalstudies involvingalargersamplesizearenecessarytoconfirmthesefindings.Thediagnostic deviceexhibitspotentialforlocaldrugnebulization,therebypresentinganoptionfor alternativetherapeuticapplicationsinthefuture[35].
Thus,eventhoughitisapromisingdiagnostictool,itsuseinclinicalpracticeisnow limitedtoevaporativedryeye.
Tearscope-PlusTM (Keeler,Windsor,UK)isarelativelynewportabledevicethatmay beconnectedtoaslitlampforconductingnon-invasiveassessmentsofthetearfilm.It allowstheexaminationoftheinterferencepatternsofthelipidlayeracrossthewhole corneawithouttheneedforfluorescein,therebyenablingtheevaluationofnon-invasive break-uptime(NIBUT),tearmeniscalheight(TMH),andlipidlayerthicknessofthetear film(LLT).Guillon’sclassificationisemployedforthepurposeofdeterminingthethickness ofthelipidlayer(LLP,lipidlayerpattern).Thesystemincludesfivedifferenttypesof lipidlayerpatterns:openmeshwork(OM),closedmeshwork(CM),wave(W),amorphous (AM),andcolorfringe(CO).InadditiontonormalLLPsandevents,atypicaloneswere described.Thismethodiseffectiveforinvestigatingthequalityandstructureofthetear film;nevertheless,itisdependentontheobserver’sjudgment,whichcanbeaffectedby thesortofpatternthatisviewed[36].Visualizingthickerlipidlayerscanbechallenging duetothelackofdistinguishingmorphologicaltraitsandcolorfringes.Additionally, thesubjectiveperceptionoftheobservercaninfluencethefindings.García-Resúaetal. showedthat,althoughtherewasasignificantcorrelationbetweenclassificationsmadeby
experiencedobserversbasedonGuillon’sschema,misinterpretationsofthepatternsmight stilloccur,evenwithinthesameobserver[14].
findings. The diagnostic device exhibits potential for local drug nebulization, thereby presenting an option for alternative therapeutic applications in the future [35].

The figure shows a side and rear
schematic representation of a monosensor diagnostic prototype with: an eyepiece cup (10); the sensor (20) placed inside it; a processing board (30) equipped with processor (31), memory (34), and wireless connection device (32); the optional connection cable (33); a digital screen (40) with buons (41); and a rechargeable power supply baery (50) placed in the handle [35].
Figure1. Aschematicdesignofthediagnostictoolsystem.Thefigureshowsasideandrearviewof theschematicrepresentationofamonosensordiagnosticprototypewith:aneyepiececup(10);the sensor(20)placedinsideit;aprocessingboard(30)equippedwithprocessor(31),memory(34),and wirelessconnectiondevice(32);theoptionalconnectioncable(33);adigitalscreen(40)withbuttons (41);andarechargeablepowersupplybattery(50)placedinthehandle[35].
Thus, even though it is a promising diagnostic tool, its use in clinical practice is now limited to evaporative dry eye.
DespitethefactthatFodoretal.demonstratedthatlowertearmeniscusheightmeasurementsweremorerepeatablewithTearscopethanslit-lampbiomicroscopywithout stainingin31healthyindividuals,thesubjectivityinherentinitsuselimitsbothitsrepeatabilityanditsutilityincomparisontootherinstrumentsthataremoreobjective(Oculus® Keratograph5MandLipiView® )[37,38].
Finally,Tearscopepresentsareliableandconsistentmethodofincreasingclinical observationandidentificationofocularphysiologicalalterations;yet,thisautomated procedureissusceptibletohumanerror.
Tearscope-PlusTM (Keeler, Windsor, UK) is a relatively new portable device that may be connected to a slit lamp for conducting non-invasive assessments of the tear film. It allows the examination of the interference paerns of the lipid layer across the whole cornea without the need for fluorescein, thereby enabling the evaluation of non-invasive break-up time (NIBUT), tear meniscal height (TMH), and lipid layer thickness of the tear film (LLT). Guillon’s classification is employed for the purpose of determining the thickness of the lipid layer (LLP, lipid layer paern). The system includes five different
CobraHDCamera(CSO,Florence,Italy)isanon-mydriaticdigitalfundusdevice withmodulesdesignedforretinalscreeninganalysis.Additionally,itincludesadedicated moduleformeibography[17].
References
InastudyconductedbyPult,theassociationbetweenage,sex,anddryeyesymptoms, aswellasthequantificationofmeibomiangland(MG)loss,wasinvestigatedusingaCobra funduscamera.Thestudyinvolved112participantsandrevealedsubstantialstandard deviationsinthemeanMGlossbetweenparticipantswithandwithoutdryeyesymptoms (30 ± 17%and45 ± 18%,respectively)[39].
IphraandGantzinvestigatedtheinter-sessionrepeatability(ISR),inter-examiner reproducibility(IER),andwithin-subjectvariability(WSV)oftheCobraHDfunduscamerameibographer.ThisstudyutilizedPhoenixsoftware.Participantswereclassifiedas eithersymptomaticorasymptomaticfordryeyebasedontheirOcularSurfaceDisease Index(OSDI)questionnairescores.TodeterminetheIER,seventy-fourparticipantswere evaluatedonthesamedaybytwoexaminers,referredtoasExaminer1andExaminer 2.Subsequently,sixty-sixoftheseparticipantswerere-examinedbyExaminer1ona differentdatetocalculatetheISR.TheresultsshowedthattheCobraHDfunduscamera meibographerhadgoodrepeatabilityandreproducibility,andclinicallysimilarfindings shouldbeobtainedwhenusedbydifferentexaminersondifferentoccasions[40].
Inconclusion,althoughtheCobraHDcameracanonlydetectmeibomianglandloss, itisusefulforthemeibographicassessmentandfollow-upofDEDprogression.
3.Discussion
Easyandrapiddryeyediseasediagnosisisstillachallengingunmetneedinopthalmology.Thealgorithmsproposedbyvariousinternationalsocietiesandcommitteesare frequentlytime-consumingandcostly,andtheiruseinthecontextofabusymedicalsetting islimited.Although20%ofourpatientssufferfromDEDandoculardiscomfortimpact almost40%ofsurgeonspractice,aproperDEDdiagnosticmethodisstillmissed[41–43]. Therefore,severalnewdiagnostictoolsaimtofillthisgap,makingthediagnosiswitha single“click”,althoughatahighercost.
Theconsistentuseoftheseinstrumentsinclinicalsettingsmayfacilitatethediagnosis, tracking,andpreventionofocularsurfacediseasessuchasdryeyesyndrome.However, theresultsintheliteraturearefewandinconsistent.Mostlikely,thereisnoclearway forpractitionerstousetheseautomatedtoolstodiagnosedryeyesinastandardizedway. Furthermore,thelackofintra-andinterobserverrepeatabilityincertainmeasurement instrumentslimitsneutralityandincreasesbias,influencingtheiruseanddistribution. Apotentialdrawbackoftheseimagingsystemsistheirhighcost,whichcanlimittheir accessibilityinmanyhealthcarecenters.
4.Conclusions
NodiagnosticinstrumentcanreplacethecomplexTFOSalgorithm,andthereis nosingletoolforaspecificdiagnosis,butresearchinthisfieldisveryactive,andsuch primordialdevicesmaybeapromisingrealityintheverynearfuture.
Funding: Thisresearchreceivednoexternalfunding.
InstitutionalReviewBoardStatement: Notapplicable.
InformedConsentStatement: Notapplicable.
DataAvailabilityStatement: Datasharingnotapplicable.
ConflictsofInterest: Theauthorsdeclarenoconflictofinterest.
1. Craig,J.P.;Nichols,K.K.;Akpek,E.K.;Caffery,B.;Dua,H.S.;Joo,C.-K.;Liu,Z.;Nelson,J.D.;Nichols,J.J.;Tsubota,K.;etal.TFOS DEWSIIDefinitionandClassificationReport. Ocul.Surf. 2017, 15,276–283.[CrossRef][PubMed]
2. Stapleton,F.;Alves,M.;Bunya,V.Y.;Jalbert,I.;Lekhanont,K.;Malet,F.;Na,K.-S.;Schaumberg,D.;Uchino,M.;Vehof,J.;etal. TFOSDEWSIIEpidemiologyReport. Ocul.Surf. 2017, 15,334–365.[CrossRef][PubMed]
3. Hashmani,N.;Munaf,U.;Saleem,A.;Javed,S.O.;Hashmani,S.ComparingSPEEDandOSDIQuestionnairesinaNon-Clinical Sample. Clin.Ophthalmol. 2021, 15,4169–4173.[CrossRef][PubMed]
4. Machali´nska,A.;Zakrzewska,A.;Safranow,K.;Wiszniewska,B.;Machali´nski,B.RiskFactorsandSymptomsofMeibomian GlandLossinaHealthyPopulation. J.Ophthalmol. 2016, 2016,7526120.[CrossRef]
5. Srinivasan,S.;Nichols,K.K.Editorial:Collectingtearosmolaritymeasurementsinthediagnosisofdryeye. ExpertRev.Ophthalmol. 2009, 4,451–453.[CrossRef]
6. Versura,P.;Campos,E.C.TearLab® OsmolaritySystemfordiagnosingdryeye. ExpertRev.Mol.Diagn. 2013, 13,119–129. [CrossRef]
7. Tavakoli,A.;Markoulli,M.;Flanagan,J.;Papas,E.Thevalidityofpointofcaretearfilmosmometersinthediagnosisofdryeye. OphthalmicPhysiol.Opt. 2022, 42,140–148.[CrossRef]
8. Benelli,U.;Nardi,M.;Posarelli,C.;Albert,T.G.TearosmolaritymeasurementusingtheTearLabOsmolaritySysteminthe assessmentofdryeyetreatmenteffectiveness. ContactLensAnteriorEye 2010, 33,61–67.[CrossRef]
9. Szczesna-Iskander,D.H.MeasurementvariabilityoftheTearLabOsmolaritySystem. ContactLensAnteriorEye 2016, 39,353–358. [CrossRef]
10. Szalai,E.;Berta,A.;Szekanecz,Z.;Szûcs,G.;Módis,L.Evaluationoftearosmolarityinnon-SjögrenandSjögrensyndromedry eyepatientswiththeTearLabsystem. Cornea 2012, 31,867–871.[CrossRef]
11. Lee,J.M.;Jeon,Y.J.;Kim,K.Y.;Hwang,K.-Y.;Kwon,Y.-A.;Koh,K.Ocularsurfaceanalysis:AcomparisonbetweentheLipiView® IIandIDRA® Eur.J.Ophthalmol. 2021, 31,2300–2306.[CrossRef]
12. Jeon,Y.J.;Song,M.Y.;Kim,K.Y.;Hwang,K.-Y.;Kwon,Y.-A.;Koh,K.Relationshipbetweenthepartialblinkrateandocularsurface parameters. Int.Ophthalmol. 2021, 41,2601–2608.[CrossRef]
13. Rinert,J.M.;Branger,G.;Bachmann,L.M.M.;Pfaeffli,O.;Iselin,K.;Kaufmann,C.;Thiel,M.A.M.;Baenninger,P.B.Accuracyofa NewNoninvasiveAutomaticOcularSurfaceAnalyzerfortheDiagnosisofDryEyeDisease-Two-GateDesignUsingHealthy Controls. Cornea 2023, 42,416–422.[CrossRef]
14. García-Resúa,C.;Pena-Verdeal,H.;Miñones,M.;Giráldez,M.J.;Yebra-Pimentel,E.Interobserverandintraobserverrepeatability oflipidlayerpatternevaluationbytwoexperiencedobservers. ContactLensAnteriorEye 2014, 37,431–437.[CrossRef]
15. Guillon,J.P.Non-invasivetearscopeplusroutineforcontactlensfitting. ContactLensAnteriorEye 1998, 21 (Suppl.S1),S31–S40. [CrossRef]
16. Zhao,Y.;Tan,C.L.S.;Tong,L.Intra-observerandinter-observerrepeatabilityofocularsurfaceinterferometerinmeasuringlipid layerthickness. BMCOphthalmol. 2015, 15,53.[CrossRef]
17. Garduño,F.;Salinas,A.;Contreras,K.;Rios,Y.;García,N.;Quintanilla,P.;Mendoza,C.;Leon,M.G.Comparativestudyoftwo infraredmeibographersinevaporativedryeyeversusnondryeyepatients. EyeContactLens 2021, 47,335–340.[CrossRef]
18. Ngo,W.;Srinivasan,S.;Schulze,M.;Jones,L.Repeatabilityofgradingmeibomianglanddropoutusingtwoinfraredsystems. Optom.Vis.Sci. 2014, 91,658–667.[CrossRef]
19. Tian,L.;Qu,J.-H.;Zhang,X.-Y.;Sun,X.-G.RepeatabilityandReproducibilityofNoninvasiveKeratograph5MMeasurementsin PatientswithDryEyeDisease. J.Ophthalmol. 2016, 2016,8013621.[CrossRef]
20. Sutphin,J.E.;Ying,G.-S.;Bunya,V.Y.;Yu,Y.;Lin,M.C.;McWilliams,K.;Schmucker,E.;Kuklinski,E.J.;Asbell,P.A.;Maguire, M.G.;etal.CorrelationofMeasuresfromtheOCULUSKeratographandClinicalAssessmentsofDryEyeDiseaseintheDryEye AssessmentandManagementStudy. Cornea 2022, 41,845–851.[CrossRef]
21. Pérez-Bartolomé,F.;Sanz-Pozo,C.;Martinez-De-La-Casa,J.M.;Arriola-Villalobos,P.;Fernández-Pérez,C.;García-Feijoó,J. Assessmentofocularrednessmeasurementsobtainedwithkeratograph5Mandcorrelationwithsubjectivegradingscales. J.Fr. Ophtalmol. 2018, 41,836–846.[CrossRef][PubMed]
22. Chen,M.;Wei,A.;Xu,J.;Zhou,X.;Hong,J.ApplicationofKeratographandFourier-DomainOpticalCoherenceTomographyin MeasurementsofTearMeniscusHeight. J.Clin.Med. 2022, 11,1343.[CrossRef]
23. Baek,J.;Doh,S.H.;Chung,S.K.ComparisonofTearMeniscusHeightMeasurementsObtainedwiththeKeratographandFourier DomainOpticalCoherenceTomographyinDryEye. Cornea 2015, 34,1209–1213.[CrossRef][PubMed]
24. Park,J.;Choi,Y.;Han,G.;Shin,E.;Han,J.;Chung,T.-Y.;Lim,D.H.EvaluationoftearosmolaritymeasuredbyI-Penosmolarity systeminpatientswithdryeye. Sci.Rep. 2021, 11,7726.[CrossRef][PubMed]
25. Fagehi,R.;Al-Bishry,A.;Alanazi,M.;Abusharha,A.;El-Hiti,G.;Masmali,A.Investigationoftherepeatabilityoftearosmolarity usinganI-PENosmolaritydevice. TaiwanJ.Ophthalmol. 2020, 11,168–174.[CrossRef][PubMed]
26. Chan,C.C.;Borovik,A.;Hofmann,I.;Gulliver,E.;Rocha,G.ValidityandReliabilityofaNovelHandheldOsmolaritySystemfor MeasurementofaNationalInstituteofStandardsTraceableSolution. Cornea 2018, 37,1169–1174.[CrossRef]
27. Shimazaki,J.;Sakata,M.;Den,S.;Iwasaki,M.;Toda,I.TearFilmOsmolarityMeasurementinJapaneseDryEyePatientsUsinga HandheldOsmolaritySystem. Diagnostics 2020, 10,789.[CrossRef]
28. Nolfi,J.;Caffery,B.Randomizedcomparisonof invivo performanceoftwopoint-of-caretearfilmosmometers. Clin.Ophthalmol. 2017, 11,945–950.[CrossRef]
29. Alanazi,M.;El-Hiti,G.;Alhafy,N.;Almutleb,E.;Fagehi,R.;Alanazi,S.;Masmali,A.CorrelationbetweenosmolaritymeasurementsusingtheTearLabTM andI-Pen® systemsinsubjectswithahighbodymassindex. Adv.Clin.Exp.Med. 2022, 31,1413–1418. [CrossRef]
30. Wong,S.;Srinivasan,S.;Murphy,P.J.;Jones,L.Comparisonofmeibomianglanddropoutusingtwoinfraredimagingdevices. ContactLensAnteriorEye 2019, 42,311–317.[CrossRef]
31. Tóth,N.;Szalai,E.;Rák,T.;Lillik,V.;Nagy,A.;Csutak,A.Reliabilityandclinicalapplicabilityofanoveltearfilmimagingtool. GraefesArch.Clin.Exp.Ophthalmol. 2021, 259,1935–1943.[CrossRef]
32. Garcia-Terraza,A.L.;Jimenez-Collado,D.;Sanchez-Sanoja,F.;Arteaga-Rivera,J.Y.;Flores,N.M.;Pérez-Solórzano,S.;Garfias,Y.; Graue-Hernández,E.O.;Navas,A.Reliability,repeatability,andaccordancebetweenthreedifferentcornealdiagnosticimaging devicesforevaluatingtheocularsurface. Front.Med. 2022, 9,893688.[CrossRef]
33. Ward,C.D.;Murchison,C.E.;Petroll,W.M.;Robertson,D.M.EvaluationoftheRepeatabilityoftheLacryDiagOcularSurface AnalyzerforAssessmentoftheMeibomianGlandsandTearFilm. Transl.Vis.Sci.Technol. 2021, 10,1.[CrossRef]
34. Lins,A.Image-basedEyeRednessUsingStandardizedOcularSurfacePhotography.InProceedingsofthe2ndMCIMedical TechnologiesMaster’sConference,Innsbruck,Austria,27–29September2021.
35. Gaudenzi,D.;Mori,T.;Crugliano,S.;Grasso,A.;Frontini,C.;Carducci,A.;Yadav,S.;Sgrulletta,R.;Schena,E.;Coassin,M.;etal. AS-OCTandOcularHygrometerasInnovativeToolsinDryEyeDiseaseDiagnosis. Appl.Sci. 2022, 12,1647.[CrossRef]
36. Guillon,J.P.Abnormallipidlayers.Observation,differentialdiagnosis,andclassification. Adv.Exp.Med.Biol. 1998, 438,309–313.
37. Fodor,E.;Hagyó,K.;Resch,M.;Somodi,D.;Németh,J.ComparisonofTearscope-plusversusslitlampmeasurementsofinferior tearmeniscusheightinnormalindividuals. Eur.J.Ophthalmol. 2010, 20,819–824.[CrossRef]
38. Markoulli,M.;Duong,T.B.;Lin,M.;Papas,E.ImagingtheTearFilm:AComparisonBetweentheSubjectiveKeelerTearscopePlusTM andtheObjectiveOculus® Keratograph5MandLipiView® Interferometer. Curr.EyeRes. 2018, 43,155–162.[CrossRef]
39. Pult,H.Relationshipsbetweenmeibomianglandlossandage,sex,anddryeye. EyeContactLens 2018, 44,S318–S324.[CrossRef]
40. Ifrah,R.;Quevedo,L.;Gantz,L.RepeatabilityandreproducibilityofCobraHDfunduscamerameibographyinyoungadults withandwithoutsymptomsofdryeye. OphthalmicPhysiol.Opt. 2023, 43,183–194.[CrossRef]
41. Coassin,M.;Mori,T.;DiZazzo,A.;Poddi,M.;Sgrulletta,R.;Napolitano,P.;Bonini,S.;Orfeo,V.;Kohnen,T.Effectof minimonovisioninbilateralimplantationofanovelnon-diffractiveextendeddepth-of-focusintraocularlens:Defocuscurves, visualoutcomes,andqualityoflife. Eur.J.Ophthalmol. 2022, 32,2942–2948.[CrossRef]
42. Bandello,F.;Coassin,M.;DiZazzo,A.;Rizzo,S.;Biagini,I.;Pozdeyeva,N.;Sinitsyn,M.;Verzin,A.;DeRosa,P.;Calabrò,F.;etal. Oneweekoflevofloxacinplusdexamethasoneeyedropsforcataractsurgery:Aninnovativeandrationaltherapeuticstrategy. Eye 2020, 34,2112–2122.[CrossRef][PubMed]
43. Antonini,M.;Gaudenzi,D.;Spelta,S.;Sborgia,G.;Poddi,M.;Micera,A.;Sgrulletta,R.;Coassin,M.;DiZazzo,A.OcularSurface FailureinUrbanSyndrome. J.Clin.Med. 2021, 10,3048.[CrossRef][PubMed]
Disclaimer/Publisher’sNote: Thestatements,opinionsanddatacontainedinallpublicationsaresolelythoseoftheindividual author(s)andcontributor(s)andnotofMDPIand/ortheeditor(s).MDPIand/ortheeditor(s)disclaimresponsibilityforanyinjuryto peopleorpropertyresultingfromanyideas,methods,instructionsorproductsreferredtointhecontent.
OPENACCESS
EDITEDBY
AlejandroNavas, InstitutodeOftalmologíaFundación deAsistenciaPrivadaConde deValenciana,I.A.P,Mexico
REVIEWEDBY
MelisPalamar, EgeUniversity,Turkey PabloDeGracia, MidwesternUniversity,UnitedStates QihuaLe, Eye,Ear,Nose,andThroatHospital ofFudanUniversity,China
*CORRESPONDENCE
José-MaríaSánchez-González jsanchez80@us.es
SPECIALTYSECTION
Thisarticlewassubmittedto Ophthalmology, asectionofthejournal FrontiersinMedicine
RECEIVED 07May2022
ACCEPTED 21July2022
PUBLISHED 10August2022
CITATION
Sánchez-GonzálezMC, Capote-PuenteR, García-RomeraM-C, De-Hita-CantalejoC, Bautista-LlamasM-J,Silva-VigueraC andSánchez-GonzálezJ-M(2022)Dry eyediseaseandtearfilmassessment throughanovelnon-invasiveocular surfaceanalyzer:TheOSAprotocol. Front.Med. 9:938484.
doi:10.3389/fmed.2022.938484
COPYRIGHT
©2022Sánchez-González, Capote-Puente,García-Romera, De-Hita-Cantalejo,Bautista-Llamas, Silva-VigueraandSánchez-González. Thisisanopen-accessarticle distributedunderthetermsofthe CreativeCommonsAttributionLicense (CCBY).Theuse,distributionor reproductioninotherforumsis permitted,providedtheoriginal author(s)andthecopyrightowner(s) arecreditedandthattheoriginal publicationinthisjournaliscited,in accordancewithacceptedacademic practice.Nouse,distributionor reproductionispermittedwhichdoes notcomplywiththeseterms.
TYPE Methods
PUBLISHED 10August2022
DOI 10.3389/fmed.2022.938484
Dryeyediseaseandtearfilm assessmentthroughanovel non-invasiveocularsurface analyzer:TheOSAprotocol
MaríaCarmenSánchez-González,RaúlCapote-Puente, Marta-CGarcía-Romera,ConcepciónDe-Hita-Cantalejo, María-JoséBautista-Llamas,CarmenSilva-Vigueraand José-MaríaSánchez-González*
VisionScienceResearchGroup,VisionSciencesoftheUniversityofSeville(CIVIUS),Department ofPhysicsofCondensedMatter,OpticsArea,UniversityofSeville,Seville,Spain
WedescribetheroleofOSAasanewinstrumentinthestudyofdry eye,andwerecommendaprotocolforconductingthetestsaswellas describetheadvantagesanddisadvantagescomparedwithotherinstruments. Acomparisonwithotherocularsurfacedevices(TearscopePlus,Keratograph 5M,anterior-segmentocularcoherencetomography,EasyTearView-Plus, LipiView,IDRA,andLacryDiag)werepresentedduetomanualorautomatic procedureandobjectiveorsubjectivemeasurements.Thepurposeofthis studywastodescribetheOSAasnewnon-invasivedryeyediseasediagnostic device.TheOSAisadevicethatcanprovideaccurate,non-invasiveand easy-to-useparameterstospecificallyinterpretdistinctfunctionsofthetear film.ThisOSAprotocolproposedalessertohighernon-invasiveocular surfacedryeyediseasetearfilmdiagnosticmethodology.Acompleteand exhaustiveOSAandOSAPlusexaminationprotocolwaspresentedwithin thesubjectivequestionnaire(DryEyeQuestionnaire5,DEQ5),limbaland bulbarrednessclassification(withintheEfrongradeScale,interferometry lipidlayerthickness(LLT)(accordingtoGuillonpattern),tearmeniscusheight (manuallyorautomatic),firstandmeannon-invasivebreakuptime(objective andautomatic)andmeibomiangland(MG)dysfunctiongradeandpercentage (objectiveandautomatic).TheOSAandOSAPlusdevicesarenoveland relevantdryeyediseasediagnostictools;however,theautomatizationand objectivityofthemeasurementscanbeincreasedinfuturesoftwareordevice updates.Thenewnon-invasivedevicessupposedrepresentarenewalinthe dryeyediseasediagnosisandintroduceatendencytoreplacetheclassic invasivetechniquesthatsupposedlessreliabilityandreproducibility.
KEYWORDS
ocularsurfaceanalyzer,dryeyedisease(DED),dryeyesyndromediagnostic,tearfilm, non-invasiveoculardevices
Introduction
Ocularsurfacepathologyisageneraltermthatincludes dryeye,withinvolvementofthecornea,conjunctiva,eyelids, andmeibomianglands(MGs).Dryeyeisagroupofdisorders characterizedbylossoftearfilmhomeostasis,duetoeither lipidlayeralterationowingtotheMGs(evaporativedryeye) orinsufficientaqueoustearproduction(hyposecretorydryeye) leadingtotissuedamageandinflammation(1).
Therearevarioustechniquesformeasuringanddiagnosing dryeye.Themostcommontestsforthisdiagnosisare invasiveandcanyieldresultsthatdifferfromthenatural propertiesofthetear,sonon-invasivemethodswould bemoreappropriate(2).Ocularsurfacediagnostictests fordryeyediseaseshouldcombinehighprecision,good sensitivityandreproducibility.Amongthemostcommonly useddiagnosticdevices,Placidomethodringshavebeen usedindifferentstudiesasanalternativetobreak-uptime (BUT)toavoidtheuseoffluorescein,althoughtheyhave aweakcorrelationwithotherdryeyediseasediagnostic measurements(3).
Ithasbeenrecommendedthatocularsurfacemeasurements beperformedfromlessinvasivetomoreinvasive(4).Such measurementsincludetheuseofaquestionnairetocollect symptoms(5),evaluationoflimbalandbulbarconjunctival hyperemia(6),assessmentoftearmeniscus(7),studyoflipid layerthickness(LLT)andpattern(8),non-invasivetearbreakuptime(NIBUT)(9)andinfraredmeibography(10).However, someofthemeasuresusedtoevaluatedryeyecanbeinfluenced bythesubjectivityoftheexaminer.
Amongthenon-invasivedevicesfordryeyemeasurement areTearscopePlus R (Keeler,Windsor,UnitedKingdom), Polaris(bonOptic,Lübeck,Germany),EasyTearViewplus R (EasyTear,Rovereto,Italy),OculusKeratograph5M R (Oculus,Arlington,WA,UnitedStates)(K5M),LipiView R interferometer(TearScienceInc.,Morrisville,NC, UnitedStates),IDRA R OcularSurfaceAnalyzerfromSBM System R (Orbassano,Torino,Italy),LacryDiag R Ocular SurfaceAnalyzer(QuantelMedical,Cournon-d’Auvergne, France)andOcularSurfaceAnalyzer(OSA)fromSBM System R (Orbassano,Torino,Italy)(11–13).Asummaryof thefunctionalitiesoftheocularsurfacedevicesispresented in Table1.RegardingTearscopePlus,thedeviceisattached totheslitlamp,andthemeasurementisachievedthrough imageanalysissoftware(14).PolarisusesLEDlighttoimprove thevisibilityofboththelipidlayerofthetearfilmandthe tearmeniscus(15).Ontheotherhand,OculusKeratograph introducestearanalysissoftwarewithanintegratedcaliper thatallowscapturingimagesforabettermeasurementofthe heightofthetearmeniscus(16).Anteriorsegmentoptical coherencetomography(AS-OCT)alsoallowsthemeasurement oftheheightofthetearmeniscusthroughintegratedsoftware, producingaveryhigh-qualityresolutioninmicrometers.
AS-OCTandKeratographaretwocomparablemethods(17). EasyTearViewplus R isalsoattachedtotheslitlamp,and throughwhiteLEDlights,itachievesanalysisofthelipidlayer, NIBUTandtearmeniscus;withinfraredLEDs,itperforms meibography,andthesoftwarequantifiestheimagestructures (18).LipiView R allowsautomatedmeasurementsofthelipid layerwithnanometerprecision.Thelimitationisthatonly valuesgreaterthan100nmaredisplayed(19).IDRA R is attachedtotheslitlamptoperformthemeasurementquickly andinafullyautomatedmanner(20).LacryDiag R useswhite lightinitssystemtocaptureimagesandinfraredlightfor theanalysisoftheMGs(13).Finally,OSA R isdesignedto performdryeyeassessmentbasedonthefollowingdiagnostic measurements:DryEyeQuestionnaire(DEQ-5),limbaland bulbarconjunctivalrednessclassification,tearmeniscus height,LLTinterferometry,NIBUT,andmeibographygland dysfunctionlosspercentage.
Inthepresentstudy,wedescribetheroleofOSA asanewinstrumentinthestudyofdryeye,andwe recommendaprotocolforconductingthetestsaswellas describetheadvantagesanddisadvantagescomparedwith otherinstruments.
Materialsandequipment Questionnaire
Manyquestionnairestoanalyzeandclassifysymptoms areenteredintothesoftwareoftheinstrumentsfordry eyeassessment:OcularSurfaceDiseaseIndex(OSDI) inKeratograph5M(21),StandardPatientEvaluation ofEyeDrynessQuestionnaire(SPEED)inIDRA(20) andDryEyeQuestionnaire(DEQ-5)inOSA(5).Onthe contrary,LD(3, 22),LipiView(19, 20),EasyTearViewplus, PolarisandTearscopePlus(23, 24)havenoquestionnaires intheirsoftware.
Thesensibilityandspecificityareinfluencednotonlyby thenumberofitemsineachquestionnaire,orthetimestudied butalsobythecapacitytoclassifysymptoms.TheOSDI isa12-itemquestionnairefocusingondryeyesymptoms andtheireffectsinthepreviousweek.Insubjectswith andwithoutdryeyedisease,theOSDIhasshowngood specificity(0.83)andmoderatesensitivity(0.60)(25).The SPEEDhaseightitemstoevaluatethefrequencyandseverity ofsymptomsinthelast3months.Sensibilityandspecificity valuesare0.90and0.80,respectively(26, 27).IntheDEQ5,thesymptomsinthepastweekareanalyzedthroughfive questions.Thissurveyhasbeenvalidatedincomparisonto theOSDI(Spearmancorrelationcoefficients, r =0.76)(28) and(r =0.65, p < 0.0001).Thesensitivityis0.71,andthe specificityis0.83(29).Thus,anyofthesethreequestionnaires couldbeagoodoptiontoanalyzedryeyesymptoms,although
theDEQ-5mightbequickertouse,giventhenumberof items.TheadvantagethatOSApresentswithrespecttoother dryeyeanalyzersisthatthequestionnairehasfewitems andiscompletedquickly.However,asdisadvantages,wefind thatquestionnaireswithagreaternumberofitemshave greaterrepeatability.
Limbalandbulbarredness classification
Regardingthelimbalandbulbarrednessclassifications (LBRC),Keratograph5Mhassoftware(RScan)tosaveimages andobjectivelyclassifythemintofourdegreesrangingfrom0 to3(30).IDRA,LacryDiagandOSAusesubjectiveprocedures, giventhatthesoftwareonlyshowstheimagetakenandthe analysismustbecarriedoutbyanobserverusingascale(31).
Efronissoftwarewidelyusedtosubjectivelyclassify rednessineyes(enteredinOSA,IDRAandLacryDiag). TheEfronscalehasachievedexcellentreproducibility(32, 33)andisoneofthemoreaccuratescalesbasedonfractal dimension(34).Comparingobjectiveandsubjectiveredness classifications,thehighestreproducibilityisobservedwhen hyperemiaisassessedandscoredautomatically(6, 30).
Amongtherestoftheocularsurfacedevices,TearscopePlus, Polaris,EasyTearViewplusandLipiViewinterferometerdo notofferarednessanalyzer.Therefore,theidealdevicehas toimplementandautomatic,objective,non-invasiveLBRC assessmentintegratedintoaplatformandsoftwarewithin therestoftheocularsurfaceparameters.Theadvantage thatOSApresentswithrespecttootherdryeyeanalyzersis thattheLBRCiscarriedoutaccordingtotheinternational scaleestablishedbyEfron.However,asdisadvantages,we findthattheanalysisofrednessissubjectivewhilethe Keratograph5Mpresentsasoftwarethatperformsitobjectively andautomatically.
Lipidlayerthickness
Therearedifferentdevicestomeasurethethicknessofthe lipidlayer,mostofwhicharebasedonopticalinterferometry, suchasOSA.ThesedevicesareTearscopePlus,EasyTear Viewplus,Polaris,Keratograph5M,andLipiView.Thebasic technologyinthemisthesame;themeasurementisperformed non-invasivelybyobservingthephenomenonofinterference fringes,whichallowsthethicknessofthelipidlayersecretedby theMGstobeanalyzed.
WithTearscopePlus,EasyTearViewplusandPolaris,the resultobtainedhasasubjectiveandqualitativecomponent, astheobservercomparestheimageheseeswiththesame classificationthatexistsforthethicknessofthelipidlayer infivedifferentcategoriesasdescribedbyGuillon(35) (amorphousstructure,marbledappearance,wavyappearance, yellow,brown,blueorreddishinterferencefringes).This sameclassificationallowsaquantitativeequivalent(from thinnertothicker: < 15nm–notpresent, ∼15nm–open meshwork, ∼30nm–closedmeshwork, ∼30/80nm–wave, ∼80nm–amorphous, ∼80/120nm–colorfringes, ∼120/160nm–abnormalcolor)usedbyOSAandIDRA. Keratograph5Musesfourinterferometricpatternsinsteadof five1=openmesh(13–15nm);2=closedmesh(30–50nm); 3=wave(50–80nm);and4=colorfringe(90–140nm).Inboth devices,thesubjectivityoftheobserverisinfluentialduring classification;thistypeofmeasurementisconsideredtobe morereliableandrepeatable,withlessdeviationintheresults (36–38).
OnlyLipiViewiscapableofmeasuringwithnanometer precision(39).Itisanon-invasiveinstrumentthattakeslive digitalimagesofthetearfilm,measuresitslipidcomponent,and assessesLLTusinganinterferencecolorunit(ICU)score(usual average ≥ 75scorepoints).Illuminationisprojectedoverthe lowerthirdofthecorneafromacolorinterferencepatternasa resultofthespecularreflectionatthelipidaqueousborder.The

detectedcolorisrelatedtothedeviceandisshownasanICU, whichisequivalenttonanometers.
DifferentpublicationssupportthereliabilityoftheLLT measurementwithLipiView,bothinitsvalueasadiagnostic elementcomparedtootherdevicesinwhichtheobserver intervenesandinitsintra-andinterobserverrepeatability(19, 20, 40, 41).TheadvantagethatOSApresentswithrespect totherestofdryeyeanalyzersisthattheclassificationof thelipidpatternofthetearfilmiscarriedoutinaccordance withtheinternationalscaleestablishedbyGuillon.However,as disadvantages,wefindthattheanalysisofthelipidthicknessis ofaqualitativenature,whileLipiViewpresentsasoftwarethat measuresthethicknessofthelipidlayerquantitatively.
Tearmeniscusheight
Severalocularsurfacedevices(EasyTearViewplus,ASOCT,Keratograph5M,LipiView,OSAandIDRA)present thepossibilityofmeasuringtearmeniscusheight,andthe
acquisitionofmultipleimagesisperformednon-invasively,as thewatercontentcanbeaccuratelyevaluatedwithanintegrated caliperalongtheedgeofthelowerorsuperioreyelid.OSA PlusandIDRAareuniquedevicesthatautomaticallyand objectivelymeasurethetearmeniscusheightofthelowerlid. Scientificevidenceisneededtoestablishtherepeatabilityand reproducibilityofthesedevices.
Theworkspresentedontearmeniscusheightarescarce,but theysupportitsrepeatability,inboththeonecarriedoutina slitlamp(42)andtheonecompletedwithKeratograph5M, whichhasasignificantcorrelationwithtraditionaldiagnostic testsfordryeyedisease(43, 44).Futurelinesofresearchshould measurethetearmeniscusvolumeinsteadoftheheightto estimatetheaqueouslayerofthetear.TheadvantagethatOSA presentswithrespecttootherdryeyeanalyzersisthattheheight ofthetearmeniscusismeasuredmanually(withOSA)and automatically(withOSAPlus),makingitanobjectivetest.In thissense,therestofthedryeyeanalyzerdevicesperforma manualmeasurementoftheheightofthetearmeniscus.
Non-invasivebreak-uptime
NIBUTisobjectivelymeasuredbyKeratograph5M,OSA, IDRAandLacryDiag.Thesedevicesrecordthefirstalterationof thetearfilm(FNIBUT)aswellastheaverageBUTforallpoints ofmeasurement(MNIBUT).Keratograph5M(45–48)performs themeasurementautomaticallyfor24s,butusingOSA(49), IDRA(12, 50, 51)andLacryDiag(13, 52),theclinicianmanually activatesandstopsvideorecording.Keratograph5Mhas showngoodrepeatabilityandreproducibilityinpatientswith dryeyeandhealthycontrols(43).Itisthemostcommonly utilizedinstrumentinocularsurfacestudiesandisusedfor thevalidationoftheotherdevices(11, 13, 36, 53).OSAand LacryDiagmeasurementsofNIBUTareobtainedthroughthe detectionofdistortionsincircularringsthatarereflectedinthe tearfilmusingthePlacidoringsaccessory(13).EmployingOSA PlusandIDRA,gridscanbeinsertedintotheinternalcylinder ofthedevicetoprojectstructuredimagesontothesurfaceof thetearfilm,andtheexaminercanchoosebetweenmanualor automaticanalysis.Inavalidationstudy,IDRAshowedgood sensitivityandspecificityvaluesforNIBUT(12).
NIBUTcanbesubjectivelymeasuredbyTearscopePlus, PolarisandEasyTearViewplus.Theseinstrumentsprojectagrid ofequidistantcirclesoflightontothesurfaceoftheeyethat areblurredbythetearfilmrupture.TheNIBUTistakenasthe timeelapseduntiltheblurofthelinescanbeobserved.Polaris (54),EasyTearViewplus(55),TS(56–58)andKeratograph 5MproducedsimilaraverageresultsrelatingtoNIBUTinthe studycarriedoutbyBandlitzetal.(11).BecauseKeratograph 5MistheonlydevicethatperformstheNIBUTmeasurement fullyautomatically,itistherecommendedinstrumentfor themeasurementofthisparameter.TheadvantagethatOSA

presentswithrespecttotherestofdryeyeanalyzersisthat themeasurementoftheFNIBUTandMNIBUTiscarriedout automaticallyandobjectively.Therefore,itisonaparwith otherdryeyeanalyzerdevicessuchastheKeratograph5M andtheLacryDiag.
Meibomianglanddysfunction
Non-contactinfraredmeibographyisatechniqueused tostudyMGdysfunctionbyevaluatingMGdropout.The qualificationofthedegreeofMGdropoutcanbedetermined subjectivelybymeansofascaleorobjectivelythroughsoftware thatautomaticallycalculatestherelationshipbetweentheareaof lossofMGandthetotalareaoftheeyelid(valuerangingfrom 0to100%)(59).Automaticobjectivemeasuresmaybemore usefulfordetectingearlyglandloss(60).
Thenon-invasiveinstrumentsthatcanperformthestudy ofMGdysfunctionareKeratograph5M,OSA,IDRA,EasyTear Viewplus,LacryDiagandLipiView.Theanalysisofmeibography withEasyTearViewplusandLipiView(20, 61, 62)iscarried
outsubjectivelybycomparingitwithascale.InLacryDiag, theanalysisissemiautomatic.Theexaminermanuallydelimits theexamarea,andthesoftwareprovidesthepercentageof MGloss(13).OSA(49)andIDRA(12, 20, 50, 51)have automatic,semiautomaticormanualproceduresforanalyzing thepresentandabsentglandareaandshowMGlossina classificationoffourdegrees:0–25,26–50,51–75,and76–100%.Inthemanualprocedure,theexaminerselectsthearea inwhichtheMGsarelocated.Inaddition,OSAPlusand IDRAperformautomatic3Dmeibography.UsingKeratograph 5M,theanalysiscanbesubjectivebycomparingtheimage obtainedwithareferencescalewithfourdegrees(ranging from0to3)(13, 45, 46)orsemiautomaticthroughthe ImageJsoftwarethatprovidesthetotalareaanalyzedandthe areacoveredbyMGs(47, 60, 63, 64).Theadvantagethat OSApresentswithrespecttotherestofdryeyeanalyzers isthatthemeasurementoftheMGDpercentageiscarried outautomaticallyandobjectively.Therefore,itrepresentsan improvementoverotherdryeyeanalyzerdevicessuchasthe Keratograph5MandtheLacryDiagthatperformmanualor semi-automaticmeasurementusingsoftware.

FIGURE3
Lipidlayerthicknessassessmentwithintheopticinterferometer. (A) Nolipidpresent(<15nmoflipidthickness). (B) Openmeshworkpattern (∼15nmoflipidthickness). (C) Closemeshworkpattern(∼30nmoflipidthickness). (D) Wavepattern(∼30/80nmoflipidthickness). (E) Amorphouspattern(∼80nmoflipidthickness). (F) Colorfringespattern(∼80/120nmoflipidthickness)andnopatientachievedabnormal color( 120/160nmoflipidthickness).

FIGURE4
Tearmeniscusheight(TMH)measuredwiththecaliper.Thecentralgreencirclerepresentsastandardmeasureofreferencetocalculatethe TMH. (A,B) Imagesrepresenttherightandlefteye,respectively.Aresult ≤0.20mmimpliesanabnormalTMHand >0.20mmsupposeawithin thenormTMH.
Theocularsurfaceanalyzer protocol:Methodsandanticipated
results
Non-invasivetearfilmanalysisisperformedwiththe IntegratedClinicalPlatform(ICP)withintheOSA.TheOSA includesafullassessmentoftheocularsurfacethrougha combinationofdryeyediseasediagnostictests.Thetestallows thequickassessmentofthedetailsofthetearfilmcomposition, includingthelipid,aqueousandmucinlayers,inadditionto conjunctivalrednessclassificationandMGassessment.The instrumentisfitintheslitlamptonometerhall.Regarding thetechnicaldata,theimageresolutionissixmegapixels,the acquisitionmodeismultishotandmovieacquisition,thefocus canbemanualorautomatic,andPlacidodiscandNIBUTgrids areavailable.Furthermore,thecolorandsensitivitytoinfrared camerasareaccessible,andthelightsourceisaninfraredorblue light-emittingdiode(LED).AnOSAdeviceimagewaspresented in Figure1
TheOSAprotocolexaminationincludesallavailablenoninvasivedryeyediseasetestsinthedevice.Temperature andhumidityroomexaminationconditionsmustbestable duringallmeasurements.Illuminationoftheroomshould beperformedundermesopicconditions.Thepatientmust notwearsoftorrigidcontactlensesatleast48hprior totheexamination.Inaddition,nolubricants,eyedropsor make-upshouldbeusedbeforethemeasurements.Ocular surfacetestsaretakeninalternatingfashionbetweenbotheyes. Furthermore,betweenOSAmeasurementsteps,thesubjects blinknormallywithin1min.Priortothenextmeasurement, thesubjectblinksdeliberatelythreefulltimes.Theorderofthe measurementsisfromminortomajortearfilmfluctuationsin thefollowingorder.
Subjectivequestionnaire
ThequestionnaireincludedintheOSAplatformisthe DEQ-5(5, 65–67).Ithasfivequestionsdividedintothreeblocks: (I)Questionsabouteyediscomfort:(a)Duringatypicalday inthepastmonth,howoftendidyoufeeldiscomfort(from nevertoconstantly)and(b)Whenyoureyesfeeldiscomfort, howintensewasthefeelingofdiscomfortattheendofthe day,within2hofgoingtobed?(fromneverhaveittovery intense).(II)Questionsabouteyedryness:(a)Duringatypical dayinthepastmonth,howoftendidyoureyesfeeldry?(from nevertoconstantly)and(b)Whenyoufeltdry,howintense wasthefeelingofdrynessattheendoftheday,within2hof goingtobed?(fromneverhaveittoveryintense).(III)Question aboutwateryeyes:(a)Duringatypicaldayinthepastmonth, howoftendidyoureyeslookorfeelexcessivelywatery?(from nevertoconstantly).
Attheendofthequestionnaire,theOSAplatform summarizestheresults,withscoresrangingfrom0to4for questionsI-a,II-aandIIIandscoresrangingfrom0to5 forquestionsI-bandII-b.Thetotalpossiblescoreinthis questionnaireis22points.Chalmersetal.(5)describedmean healthypopulationresultsof2.7 ± 3.2pointswithinaclinical differencetodetectsixpoints(68)(basedonthevariation betweenseverityclassification)(5).
Limbalandbulbarredness classification
TheLBRCwasdetectedwithinthebloodvesselfluidityof theconjunctivatoevaluatetherednessdegreewiththeEfron (69)Scale(0=normal,1=trace,2=mild,3=moderate and4=severe).Forthismeasurement,noconewasplaced onthedevice.Acentralpicturemustbetakentoassesslimbal conjunctivalredness(Figure2).Therefore,anasalandtemporal picturemustbetakentoassessbulbarconjunctivalredness (Figure1).Efron(69)andWuetal.(30)didnotreportmean healthypopulationvalues,althoughtheyestablishedclinically normalasgrade0–1.Theclinicaldifferencetodetectis0.5 grading(68).
Lipidlayerthickness
Atthispoint,thequalityofthetearfilmlipidwasassessed. TheLLTevaluationwasperformedwithopticinterferometry. Furthermore,theevaluationofthequantityofthelipidlayer wasclassifiedintosevendifferentpatterncategoriesdefined byGuillon(35).Forthismeasurement,aplainconeis placedonthedevice.Thepatientmustblinknormallyduring anapproximately10-svideorecording.Later,thevideois comparedwiththesevenvideostomatchtheexactlipidlayer pattern(Figure3).
Tearmeniscusheight
TheTMHtestevaluatestheaqueouslayerquantitywithin amillimetercaliper(≤ 0.20mm–abnormaland > 0.20mm–normal).Forthismeasurement,theplainconeisplacedon thedevice.Thepictureconsistsofacentralcaptureofthe tearmeniscusfocalizedinthecenterofthegreensquare (Figure4).Later,themillimetercaliperisplacedatthestart andendofthetearmeniscus,andtheheightisobtained. Multiplemeasurementscanbeperformedaswellasnasal ortemporalTMH.Meanhealthypopulationresultswere presentedbyseveralauthors.Nicholsetal.(42)reported 0.29 ± 0.13mm(measuredwithaslitlamp),Weietal.(44) reported0.29 ± 0.04mm(measuredwithKeratograph4),

(A) Placiddiskringsreflectedontearfilmjustafterinitialdoubledeliberateblinks. (B) FirstPlacidorings deformation(difficulttoseevisuallybyahuman)thismomentautomatedestablishesthefirstnon-invasivebreak-uptime(FNIBUT). (C) Mean andgeneralPlacidoringsdeformation(difficulttoseevisuallybyahuman)thismomentautomatedestablishesthemeannon-invasivebreak-up time(MNIBUT).

ormanualestablishesglandspresence. (A) Righteyeuppereyelidrealmeibomianglandpattern. (B) Lefteyeuppereyelidrealmeibomiangland pattern. (C) Righteyeloweyelidrealmeibomianglandpattern. (D) Lefteyeloweyelidrealmeibomianglandpattern.

Analyzer(OSA)fromSBMSystem R (Orbassano,Torino,Italy). (A) Simulated3Drighteyeuppereyelidrealmeibomianglandpattern. (B) Simulated3Dlefteyeuppereyelidrealmeibomianglandpattern. (C) Simulated3Drighteyeloweyelidrealmeibomianglandpattern. (D) Simulated3Dlefteyeloweyelidrealmeibomianglandpattern.
Tianetal.(43)reported0.27 ± 0.12mm(measuredwith Keratograph5M),Lietal.(70)reported0.19 ± 0.02mm (measuredwithocularcoherencetomography,OCT)and Wangetal.(71)reported0.34 ± 0.15mm(measuredwith OCT).Theminimalclinicaldifferencetodetectwassetat 0.1mm(68).
Non-invasivebreak-uptime
Regardingthismeasurement,thetearfilmmucinlayer quantityisassessed.TheFNIBUTandMNIBUTareevaluated withaspecialgridcone,whichevaluatesthetearfilmbreakin seconds.ThePlacidoconeissetforthistest.Thepatientmust deliberatelyblinktwotimes;afterthis,thevideorecordingstarts andstopsatthefirstinvoluntaryblink.Thedeviceautoanalyzes themeasurementandreportsthefirstpointoftheblurgridas theFNIBUTandthegeneralizedtearfilmBUTastheMNIBUT (Figure5).Meanhealthypopulationresultswereestablishedby Nicholsetal.(58)11.2 ± 6.8s(measuredwithTearscopePlus) andTianetal.(43)10.4 ± 4.2s(measuredwithKeratograph 5M).Theminimalclinicaldifferencetodetectwassetat5s(68).
Meibomianglandsdysfunction
TheMGdysfunctionpercentageusmeasuredwithan infrarednon-contactcamerathatevaluatestheupperandlower lidafterevertingitwithaswab.Forthismeasurement,no coneisplacedonthedevice.MGpicturesoftheupperand lowereyelidsmustbecapturedinsidethegreensquare.After thecatch,MGassessmentcanbeperformedautomatically ormanually(Figure6).Inaddition,acombinationofboth methodscanbeperformedwiththesemiautomatedmethodthat allowstheadditionorremovalofnon-detectedMGsmanually. TheMGdysfunctionpercentagecanbeclassifiedintofour degrees: ∼0%–Grade0, < 25%–Grade1,26–50%–Grade2, 51–75%–Grade3and > 75%–Grade4(72, 73).Thedevice permittoperformasimulatedorreal(withOSAPlus)3DMG pattern(Figure7).
Futureresearchlinesand limitations
Newemerginglinesofresearcharefocusedonthesearchfor identifiersthatallowustorecognizebiomarkersoftheeffectsof theocularsurfaceinamoreobjective,automatedandminimally invasiveway.Toenhancethefield,thedevelopmentofnew algorithmiccalculationsandtheincorporationofsoftware fordataanalysis,suchbigdataandmachinelearning,will allowustorecognize,detectandclassifymoreaccuratelythe differentvalues,includingtheinterrelationsbetweenthem,inan
automatedwaywithdifferentparameters(74).Independentand dissociatedobservationofthetearfilm,inclusionofpalpebral parametersandanalysisofproinflammatoryfactorswithoutthe needforinvasive,expensive,rapidorinvitedtestsarepotential futuredirectionsthatshouldbeanalyzed(75, 76).
Futureresearchersshouldconsiderthattheintensity ofilluminationproducedbytheseinstrumentsintheir measurementscancauseanincreaseintheblinkrateand reflextearing(77).Therefore,themainlimitationsfound arethelackofobjectivityandautomationinthemeasures conducted,absenceofcorrelationsbetweenexistingtestsand lackofextrapolationtoothersimilarsystems.However,the lackofintra-andinterobserverrepeatabilityinsomeofthe measurementtoolsduetotheinteractionofanobserver limitsneutralityandincreasesbiases,whichimpactthevalidity oftheresults.Withinthelimitationsofthisstudy,an accuracyandrepeatabilityresearchisneededtovalidatethis ocularsurfacedevice.
Conclusion
TheOSAisadevicethatcanprovideaccurate,noninvasiveandeasy-to-useparameterstospecificallyinterpret distinctfunctionsofthetearfilm.Theuseofvariablesand subsequentanalysisofresultscangeneraterelevantinformation forthemanagementofclinicaldiagnoses.TheOSAand OSAPlusdevicesarenovelandrelevantdryeyedisease diagnostictools;however,theautomatizationandobjectivity ofthemeasurementscanbeincreasedinfuturesoftware ordeviceupdates.
Dataavailabilitystatement
Theoriginalcontributionspresentedinthisstudyare includedinthearticle/supplementarymaterial,furtherinquiries canbedirectedtothecorrespondingauthor.
Authorcontributions
MS-G,RC-P,M-CG-R,CD-H-C,M-JB-L,CS-V,and J-MS-G:conceptualization,methodology,writing—original draftpreparation,writing—reviewandeditingandsupervision. Allauthorsreadandagreedtothepublishedversion ofthemanuscript.
Funding
ThisstudyreceivedfundingfromESTEVEPharmaceuticals S.A(EnglishEditingServicesandArticleProcessingCharges).
Thefunderwasnotinvolvedinthestudydesign,collection, analysis,interpretationofdata,thewritingofthisarticleorthe decisiontosubmititforpublication.
Acknowledgments
Weappreciatethesupportofferedbythemembersof theDepartmentofPhysicsofCondensedMatter,Faculty ofPhysics,UniversityofSeville,withspecialthanks toJavierRomero-LandaandClaraConde-Amiano.In addition,wealsoappreciatethetechnicalsupportoffered bythemembersandfacilitiesoftheFacultyofPharmacy, UniversityofSeville,withspecialthankstoMaríaÁlvarez-deSotomayor.
References
1.JonesL,DownieLE,KorbD,Benitez-del-CastilloJM,DanaR,DengSX,etal. TFOSDEWSIIManagementandTherapyReport. OculSurf. (2017)15:575–628. doi:10.1016/j.jtos.2017.05.006
2.KottaiyanR,YoonG,WangQ,YadavR,ZavislanJM,AquavellaJV.Integrated multimodalmetrologyforobjectiveandnoninvasivetearevaluation. OculSurf. (2012)10:43–50.doi:10.1016/J.JTOS.2011.12.001
3.RemonginPE,RousseauA,BestAL,BenHadjSalahW,LegrandM,Benichou J,etal.[MultimodalevaluationoftheocularsurfaceusingathenewLacrydiag device]. JFrOphtalmol. (2021)44:313–20.doi:10.1016/J.JFO.2020.06.045
4.FoulksGN.Challengesandpitfallsinclinicaltrialsoftreatmentsfordryeye. OculSurf. (2003)1:20–30.doi:10.1016/S1542-0124(12)70004-6
5.ChalmersRL,BegleyCG,CafferyB.Validationofthe5-ItemDryEye Questionnaire(DEQ-5):discriminationacrossself-assessedseverityandaqueous teardeficientdryeyediagnoses. ContactLensAnteriorEye. (2010)33:55–60.doi: 10.1016/j.clae.2009.12.010
6.PetersonRC,WolffsohnJS.Sensitivityandreliabilityofobjectiveimage analysiscomparedtosubjectivegradingofbulbarhyperaemia. BrJOphthalmol. (2007)91:1464–6.doi:10.1136/BJO.2006.112680
7.NiedernolteB,TrunkL,WolffsohnJS,PultH,BandlitzS.Evaluationof tearmeniscusheightusingdifferentclinicalmethods. ClinExpOptom. (2021) 104:583–8.doi:10.1080/08164622.2021.1878854
8.AritaR,FukuokaS,MorishigeN.Functionalmorphologyofthelipidlayerof thetearfilm. Cornea. (2017)36:S60–6.doi:10.1097/ICO.0000000000001367
9.LanW,LinL,YangX,YuM.Automaticnoninvasivetearbreakuptime (TBUT)andconventionalfluorescentTBUT. OptomVisSci. (2014)91:1412–8. doi:10.1097/OPX.0000000000000418
10.AritaR,SuehiroJ,HaraguchiT,ShirakawaR,TokoroH,AmanoS.Objective imageanalysisofthemeibomianglandarea. BrJOphthalmol. (2014)98:746–55. doi:10.1136/BJOPHTHALMOL-2012-303014
11.BandlitzS,PeterB,PflugiT,JaegerK,AnwarA,BikhuP,etal.Agreementand repeatabilityoffourdifferentdevicestomeasurenon-invasivetearbreakuptime (NIBUT). ContLensAnteriorEye. (2020)43:507–11.doi:10.1016/J.CLAE.2020.02. 018
12.VigoL,PellegriniM,BernabeiF,CaronesF,ScorciaV,GiannaccareG. Diagnosticperformanceofanovelnoninvasiveworkupinthesettingofdryeye disease. JOphthalmol. (2020)2020:5804123.doi:10.1155/2020/5804123
13.WardCD,MurchisonCE,PetrollWM,RobertsonDM.Evaluationof therepeatabilityofthelacrydiagocularsurfaceanalyzerforassessmentofthe meibomianglandsandtearfilm. TranslVisSciTechnol. (2021)10:1.doi:10.1167/ TVST.10.9.1
14.UchidaA,UchinoM,GotoE,HosakaE,KasuyaY,FukagawaK,etal. NoninvasiveinterferencetearmeniscometryindryeyepatientswithSjögren syndrome. AmJOphthalmol. (2007)144:6.doi:10.1016/J.AJO.2007.04.006
Conflictofinterest
Theauthorsdeclarethattheresearchwasconductedinthe absenceofanycommercialorfinancialrelationshipsthatcould beconstruedasapotentialconflictofinterest.
Publisher’snote
Allclaimsexpressedinthisarticlearesolelythoseofthe authorsanddonotnecessarilyrepresentthoseoftheiraffiliated organizations,orthoseofthepublisher,theeditorsandthe reviewers.Anyproductthatmaybeevaluatedinthisarticle,or claimthatmaybemadebyitsmanufacturer,isnotguaranteed orendorsedbythepublisher.
15.AbdelfattahNS,DastiridouA,SaddaSVR,LeeOL.Noninvasiveimagingof tearfilmdynamicsineyeswithocularsurfacedisease. Cornea. (2015)34:S48–52. doi:10.1097/ICO.0000000000000570
16.BaekJ,DohSH,ChungSK.Comparisonoftearmeniscusheight measurementsobtainedwiththekeratographandfourierdomainoptical coherencetomographyindryeye. Cornea. (2015)34:1209–13.doi:10.1097/ICO. 0000000000000575
17.Arriola-VillalobosP,Fernández-VigoJI,Díaz-ValleD,Peraza-NievesJE, Fernández-PérezC,Benítez-Del-CastilloJM.Assessmentoflowertearmeniscus measurementsobtainedwithKeratographandagreementwithFourier-domain optical-coherencetomography. BrJOphthalmol. (2015)99:1120–5.doi:10.1136/ BJOPHTHALMOL-2014-306453
18.ZhouN,EdwardsK,ColoradoLH,SchmidKL.Developmentoffeasible methodstoimagetheeyelidmarginusinginvivoconfocalmicroscopy. Cornea. (2020)39:1325–33.doi:10.1097/ICO.0000000000002347
19.LeeY,HyonJY,JeonHS.Characteristicsofdryeyepatientswith thicktearfilmlipidlayersevaluatedbyaLipiViewIIinterferometer. Graefes ArchClinExpOphthalmol. (2021)259:1235–41.doi:10.1007/S00417-020-05 044-5
20.LeeJM,JeonYJ,KimKY,HwangK-Y,KwonY-A,KohK.Ocularsurface analysis:acomparisonbetweentheLipiView R IIandIDRA R EurJOphthalmol. (2021)31:2300–6.doi:10.1177/1120672120969035
21.GuarnieriA,CarneroE,BleauAM,Alfonso-BartolozziB,Moreno-Montañés J.RelationshipbetweenOSDIquestionnaireandocularsurfacechangesin glaucomatouspatients. IntOphthalmol. (2020)40:741–51.doi:10.1007/s10792019-01236-z
22.VerrecchiaS,ChiambarettaF,KodjikianL,NakouriY,ElChehabH,Mathis T,etal.Aprospectivemulticentrestudyofintravitrealinjectionsandocularsurface in219patients:IVISstudy. ActaOphthalmol. (2021)99:877–84.doi:10.1111/aos. 14797
23.LawrensonJG,BirhahR,MurphyPJ.Tear-filmlipidlayermorphologyand cornealsensationinthedevelopmentofblinkinginneonatesandinfants. JAnat. (2005)206:265–70.doi:10.1111/j.1469-7580.2005.00386.x
24.PrabhasawatP,TesavibulN,KasetsuwanN.Performanceprofileofsodium hyaluronateinpatientswithlipidteardeficiency:randomised,double-blind, controlled,exploratorystudy. BrJOphthalmol. (2007)91:47–50.doi:10.1136/bjo. 2006.097691
25.SchiffmanRM,ChristiansonMD,JacobsenG,HirschJD,ReisBL.Reliability andvalidityoftheocularsurfacediseaseindex. ArchOphthalmol. (2000)118:615–21.doi:10.1001/archopht.118.5.615
26.NgoW,SituP,KeirN,KorbD,BlackieC,SimpsonT.Psychometric propertiesandvalidationofthestandardpatientevaluationofeyedryness questionnaire. Cornea. (2013)32:1204–10.doi:10.1097/ICO.0b013e318294 b0c0
27.HashmaniN,MunafU,SaleemA,JavedSO,HashmaniS.Comparingspeed andosdiquestionnairesinanon-clinicalsample. ClinOphthalmol. (2021)15:4169–73.doi:10.2147/OPTH.S332565
28.SimpsonTL,SituP,JonesLW,FonnD.Dryeyesymptomsassessedby fourquestionnaires. OptomVisSci. (2008)85:b013e318181ae36.doi:10.1097/OPX. 0b013e318181ae36
29.AkowuahPK,Adjei-AnangJ,NkansahEK,FummeyJ,Osei-PokuK,Boadi P,etal.Comparisonoftheperformanceofthedryeyequestionnaire(DEQ-5)to theocularsurfacediseaseindexinanon-clinicalpopulation. ContactLensAnterior Eye. (2021)2021:101441.doi:10.1016/j.clae.2021.101441
30.WuS,HongJ,TianL,CuiX,SunX,XuJ.Assessmentofbulbarrednesswith anewlydevelopedkeratograph. OptomVisSci. (2015)92:892–9.doi:10.1097/OPX. 0000000000000643
31.BaudouinC,BartonK,CucheratM,TraversoC.Themeasurementofbulbar hyperemia:challengesandpitfalls. EurJOphthalmol. (2015)25:273–9.doi:10.5301/ ejo.5000626
32.Pérez-BartoloméF,Sanz-PozoC,Martínez-delaCasaJM,Arriola-Villalobos P,Fernández-PérezC,García-FeijoóJ.Assessmentofocularrednessmeasurements obtainedwithkeratograph5Mandcorrelationwithsubjectivegradingscales. JFr Ophtalmol. (2018)41:836–46.doi:10.1016/j.jfo.2018.03.007
33.AmparoF,WangH,Emami-NaeiniP,KarimianP,DanaR.Theocular rednessindex:anovelautomatedmethodformeasuringocularinjection. Invest OphthalmolVisSci. (2013)54:4821–6.doi:10.1167/IOVS.13-12217
34.SchulzeMM,HutchingsN,SimpsonTL.Theuseoffractalanalysisand photometrytoestimatetheaccuracyofbulbarrednessgradingscales. Investig OphthalmolVisSci. (2008)49:1398–406.doi:10.1167/iovs.07-1306
35.GuillonJP.Non-invasivetearscopeplusroutineforcontactlensfitting. ContactLensAnteriorEye. (1998)21:S31–40.doi:10.1016/S1367-0484(98)80035-0
36.MarkoulliM,DuongTB,LinM,PapasE.Imagingthetearfilm:a comparisonbetweenthesubjectivekeelertearscope-plusTM andtheobjective oculus R keratograph5mandlipiview R interferometer. CurrEyeRes. (2018) 43:155–62.doi:10.1080/02713683.2017.1393092
37.TongL,TengLS.Reviewofliteratureonmeasurementsofnon-invasivebreak uptimes,lipidmorphologyandtearmeniscalheightusingcommerciallyavailable hand-heldinstruments. CurrEyeRes. (2018)43:567–75.doi:10.1080/02713683. 2018.1437454
38.García-MarquésJV,Talens-EstarellesC,García-LázaroS,CerviñoA. Validationofanewobjectivemethodtoassesslipidlayerthicknesswithoutthe needofaninterferometer. GraefesArchClinExpOphthalmol. (2022)260:655–76. doi:10.1007/S00417-021-05378-8
39.FinisD,PischelN,SchraderS,GeerlingG.Evaluationoflipidlayerthickness measurementofthetearfilmasadiagnostictoolforMeibomianglanddysfunction. Cornea. (2013)32:1549–53.doi:10.1097/ICO.0B013E3182A7F3E1
40.ChouYB,FanNW,LinPY.Valueoflipidlayerthicknessandblinking patterninapproachingpatientswithdryeyesymptoms. CanJOphthalmol. (2019) 54:735–40.doi:10.1016/J.JCJO.2019.03.005
41.ZhaoY,TanCLS,TongL.Intra-observerandinter-observerrepeatabilityof ocularsurfaceinterferometerinmeasuringlipidlayerthickness. BMCOphthalmol. (2015)15:53.doi:10.1186/S12886-015-0036-9
42.NicholsKK,MitchellGL,ZadnikK.Therepeatabilityofclinical measurementsofdryeye. Cornea. (2004)23:272–85.doi:10.1097/00003226200404000-00010
43.TianL,QuJH,ZhangXY,SunXG.Repeatabilityandreproducibilityof noninvasivekeratograph5mmeasurementsinpatientswithdryeyedisease. J Ophthalmol. (2016)2016:8013621.doi:10.1155/2016/8013621
44.WeiA,LeQ,HongJ,WangW,WangF,XuJ.Assessmentoflowertear meniscus. OptomVisSci. (2016)93:1420–5.doi:10.1097/OPX.0000000000000986
45.YuT,ShiW-Y,SongA-P,GaoY,DangG-F,DingG.Changesofmeibomian glandsinpatientswithtype2diabetesmellitus. IntJOphthalmol. (2016)9:1740. doi:10.18240/IJO.2016.12.06
46.WangX,LiJ,ZhangR,LiN,PangY,ZhangY,etal.Theinfluenceofovernight orthokeratologyonocularsurfaceandmeibomianglanddysfunctioninteenagers withmyopia. JOphthalmol. (2019)2019:5142628.doi:10.1155/2019/5142628
47.LiuS,LiS,LiM,ZengS,ChenB,ZhangL.Evaluationoftheocularsurface andmeibomianglandinobstructivesleepapneahypopneasyndrome. FrontMed. (2022)9:832954.doi:10.3389/FMED.2022.832954
48.WangX,LuX,YangJ,WeiR,YangL,ZhaoS,etal.Evaluationofdryeye andmeibomianglanddysfunctioninteenagerswithmyopiathroughnoninvasive keratograph. JOphthalmol. (2016)2016:6761206.doi:10.1155/2016/6761206
49.TotukÖMG,KabadayıK,ÖzkapıC,AykanÜ.Efficacyofintensepulsed lighttreatmentformoderatetosevereacuteblepharitisorblepharoconjunctivitis:
aretrospectivecaseseries. TurkishJOphthalmol. (2021)51:89–94.doi:10.4274/tjo. galenos.2020.28924
50.JeonYJ,SongMY,KimKY,HwangKY,KwonYA,KohK.Relationship betweenthepartialblinkrateandocularsurfaceparameters. IntOphthalmol. (2021)41:2601.doi:10.1007/S10792-021-01819-9
51.MartaA,BaptistaPM,MarquesJH,AlmeidaD,JoséD,SousaP,etal. Intensepulsedpluslow-levellighttherapyinmeibomianglanddysfunction. Clin Ophthalmol. (2021)15:2803.doi:10.2147/OPTH.S318885
52.TóthN,SzalaiE,RákT,LillikV,NagyA,CsutakA.Reliabilityandclinical applicabilityofanoveltearfilmimagingtool. Graefe’sArchClinExpOphthalmol. (2021)259:1935.doi:10.1007/S00417-021-05162-8
53.BestN,DruryL,WolffsohnJS.ClinicalevaluationoftheOculusKeratograph. ContLensAnteriorEye. (2012)35:171–4.doi:10.1016/J.CLAE.2012.04.002
54.SandraJohannaGP,AntonioLA,AndrésGS.Correlationbetweentype2 diabetes,dryeyeandMeibomianglandsdysfunction. JOptom. (2019)12:256. doi:10.1016/J.OPTOM.2019.02.003
55.FagehiR,Al-BishryA,AlanaziM,AbusharhaA,El-HitiG,MasmaliA. InvestigationoftherepeatabilityoftearosmolarityusinganI-PENosmolarity device. TaiwanJOphthalmol. (2021)11:168.doi:10.4103/TJO.TJO_65_20
56.PrabhasawatP,TesavibulN,MahawongW.Arandomizeddoublemaskedstudyof0.05%cyclosporineophthalmicemulsioninthetreatmentof meibomianglanddysfunction. Cornea. (2012)31:1386–93.doi:10.1097/ICO. 0B013E31823CC098
57.GuillonM,TheodoratosP,PatelK,GuptaR,PatelT.Pre-contactlensand pre-cornealtearfilmkinetics. ContactLensAnteriorEye. (2019)42:246–52.doi: 10.1016/j.clae.2019.02.001
58.NicholsJJ,NicholsKK,PuentB,SaracinoM,MitchellGL.Evaluationoftear filminterferencepatternsandmeasuresoftearbreak-uptime. OptomVisSci. (2002) 79:363–9.doi:10.1097/00006324-200206000-00009
59.HanSB,LiuYC,Mohamed-NoriegaK,TongL,MehtaJS.ObjectiveImaging DiagnosticsforDryEyeDisease. JOphthalmol. (2020)2020:3509064.doi:10.1155/ 2020/3509064
60.PultH,Riede-PultB.Comparisonofsubjectivegradingandobjective assessmentinmeibography. ContLensAnteriorEye. (2013)36:22–7.doi:10.1016/ J.CLAE.2012.10.074
61.ZhangJ,WuZ,SunL,LiuXH,LiuYC.Functionandmorphologyofthe meibomianglandsusingalipiviewinterferometerinrotatingshiftmedicalstaff. J Ophthalmol. (2020)2020:3275143.doi:10.1155/2020/3275143
62.WangCY,HoRW,FangPC,YuHJ,ChienCC,HsiaoCC,etal.Thefunction andmorphologyofMeibomianglandsinpatientswiththyroideyedisease:a preliminarystudy. BMCOphthalmol. (2018)18:9.doi:10.1186/S12886-018-0763-9
63.SatitpitakulV,RattanaphongT,PruksakornV.Meibomianglandsdropout inpatientswithinactivethyroidrelatedorbitopathy. PLoSOne. (2021)16:250617. doi:10.1371/JOURNAL.PONE.0250617
64.LiJ,MaJ,HuM,YuJ,ZhaoY.Assessmentoftearfilmlipidlayerthickness inpatientswithMeibomianglanddysfunctionatdifferentages. BMCOphthalmol. (2020)20:8.doi:10.1186/S12886-020-01667-8
65.FernandezCA,GalorA,ArheartKL,MusselmanDL,VenincasaVD,Florez HJ,etal.Dryeyesyndrome,posttraumaticstressdisorder,anddepressionin anoldermaleveteranpopulation. InvestOphthalmolVisSci. (2013)54:3666–72. doi:10.1167/iovs.13-11635
66.GalorA,FelixER,FeuerW,ShalabiN,MartinER,MargolisTP,etal.Dryeye symptomsalignmorecloselytonon-ocularconditionsthantotearfilmparameters. BrJOphthalmol. (2015)99:1126–9.doi:10.1136/bjophthalmol-2014-306481
67.CampA,WellikSR,TzuJH,FeuerW,ArheartKL,SastryA,etal.Dryeye specificqualityoflifeinveteransusingglaucomadrops. ContactLensAnteriorEye. (2015)38:220–5.doi:10.1016/j.clae.2015.02.001
68.WolffsohnJS,AritaR,ChalmersR,DjalilianA,DogruM,DumbletonK, etal.TFOSDEWSIIDiagnosticMethodologyreport. OculSurf. (2017)15:539–74. doi:10.1016/j.jtos.2017.05.001
69.EfronN.Gradingscalesforcontactlenscomplications. OphthalmicPhysiol Opt. (1998)18:182–6.doi:10.1016/S0275-5408(97)00066-5
70.LiJ,ShenM,WangJ,MaH,TaoA,XuS,etal.Clinicalsignificanceof tearmenisciindryeye. EyeContactLens. (2012)38:183–7.doi:10.1097/ICL. 0b013e318252ce0c
71.WangJ,PalakuruJR,AquavellaJV.Correlationsamongupperandlowertear menisci,noninvasivetearbreak-uptime,andtheschirmertest. AmJOphthalmol. (2008)145:795–800.doi:10.1016/j.ajo.2007.12.035
72.AritaR,ItohK,InoueK,AmanoS.Noncontactinfraredmeibographyto documentage-relatedchangesofthemeibomianglandsinanormalpopulation. Ophthalmology. (2008)115:911–5.doi:10.1016/j.ophtha.2007.06.031
73.AritaR,ItohK,InoueK,KuchibaA,YamaguchiT,Amano S.Contactlenswearisassociatedwithdecreaseofmeibomian glands. Ophthalmology. (2009)116:379–84.doi:10.1016/j.ophtha.2008. 10.012
74.AshwiniDL,VeRS,NoschD,WilmotN.Efficacyofblinksoftwarein improvingtheblinkrateanddryeyesymptomsinvisualdisplayterminalusersAsingle-blindedrandomizedcontroltrial. IndianJOphthalmol. (2021)69:2643–8. doi:10.4103/ijo.IJO_3405_20
75.McMonniesCW.Diagnosisandremediationofblinkinefficiency. ContLens AnteriorEye. (2021)44:101331.doi:10.1016/j.clae.2020.04.015
76.PetrilloF,PignataroD,LavanoMA,SantellaB,FollieroV,ZannellaC, etal.Currentevidenceontheocularsurfacemicrobiotaandrelateddiseases. Microorganisms. (2020)8:8071033.doi:10.3390/microorganisms8071033
77.AritaR,FukuokaS,MorishigeN.Newinsightsintothemorphologyand functionofmeibomianglands. ExpEyeRes. (2017)163:64–71.doi:10.1016/j.exer. 2017.06.010
applied sciences
Review

AdvancesintheNoninvasiveDiagnosisofDryEyeDisease
LucaDiCello 1,† ,MarcoPellegrini 2,3,4,† ,AldoVagge 1 ,MassimilianoBorselli 5 ,LorenzoFerroDesideri 1 , VincenzoScorcia 5 ,CarloE.Traverso 1 andGiuseppeGiannaccare 5, *
1 IRCCSOspedalePoliclinicoSanMartino,UniversityEyeClinicofGenoa,16132Genoa,Italy; luca.di.cello88@gmail.com(L.D.C.);aldo.vagge@gmail.com(A.V.);lorenzoferrodes@gmail.com(L.F.D.); mc8620@mclink.it(C.E.T.)
2 DepartmentofOphthalmology,OspedaliPrivatiForlì “VillaIgea”,47122Forlì,Italy; marco.pellegrini@hotmail.it
3 IstitutoInternazionaleperLaRicercaeFormazioneinOftalmologia(IRFO),47122Forlì,Italy
4 DepartmentofTranslationalMedicine,UniversityofFerrara,44121Ferrara,Italy
5 DepartmentofOphthalmology,UniversityMagnaGraeciaofCatanzaro,88100Catanzaro,Italy; mborselli93@gmail.com(M.B.);vscorcia@libero.it(V.S.)
* Correspondence:giuseppe.giannaccare@unicz.it;Tel./Fax:+39-096-1364-7041
†Theseauthorscontributedequallytothework.
Citation: DiCello,L.;Pellegrini,M.; Vagge,A.;Borselli,M.;FerroDesideri, L.;Scorcia,V.;Traverso,C.E.; Giannaccare,G.Advancesinthe NoninvasiveDiagnosisofDryEye Disease. Appl.Sci. 2021, 11,10384. https://doi.org/10.3390/app112110384
AcademicEditors:ItziarFernández MartínezandAlbertoLópez-Miguel
Received:3October2021
Accepted:29October2021
Published:5November2021
Publisher’sNote: MDPIstaysneutral withregardtojurisdictionalclaimsin publishedmapsandinstitutionalaffiliations.

Copyright: ©2021bytheauthors. LicenseeMDPI,Basel,Switzerland. Thisarticleisanopenaccessarticle distributedunderthetermsand conditionsoftheCreativeCommons Attribution(CCBY)license(https:// creativecommons.org/licenses/by/ 4.0/).
Abstract: Dryeyedisease(DED)isamultifactorialdiseasethatrepresentsoneofthemostcommon ophthalmologicconditionsencounteredineverydayclinicalpractice.Traditionaldiagnostictests forDED,suchassubjectivequestionnaires,tearfilmbreak-uptimeandtheSchirmertest,areoften associatedwithpoorreproducibilityandreliability,whichmakethediagnosis,follow-up,and managementofthediseasechallenging.Newadvancesinimagingtechnologiesenableobjectiveand reproduciblemeasurementsofDEDparameters,thusmakingthediagnosisamultimodalimagingbasedprocess.Theaimofthisreviewistosummarizeallthecurrentandemergingdiagnostic toolsavailableforthediagnosisandmonitoringofDED,suchasnon-invasivetearbreakuptime, thermography,anteriorsegmentopticalcoherencetomography,meibography,interferometry, invivo confocalmicroscopy,andopticalqualityassessment.Althoughthereisnotagoldstandardimaging technique,newmulti-imaging-integrateddevicesarepreciousinstrumentstohelpclinicianstobetter copewiththediagnosticcomplexityofDED.
Keywords: dryeye;diagnosis;noninvasivediagnosis;advancedimaging;NIBUT
1.Introduction
Dryeyedisease(DED)isoneofthemostcommonophthalmologicconditionsencounteredineverydayclinicalpractice[1].The2017reportbytheTearFilmandOcularSurface Society(TFOS)DryEyeWorkshop(DEWSII)publishedthereneweddefinitionofDED, whichwasdefinedasanocularsurfacedisorderinwhichmultiplepathologicalevents, includingtearfilminstability,hyperosmolarity,inflammationandneurosensoryabnormalities,leadtothelossofthehomeostasisoftheentiresystem[2].Commonsymptoms ofDEDinclude,amongothers,irritation,redness,foreignbodysensation,blurryvision, tearing,andsensitivitytolight.Itisachronicconditionthatalsorepresentsanimportant economicburdenforbothpatientsandsociety[3].
PerformingasuitableclinicaldiagnosisofDEDcanbechallenging,asitssignsand symptomsareoftenpoorlycorrelated.Combinationsofsubjectivesymptomsevaluatedby questionnaires,slitlampexaminationand(non-)invasivediagnostictestshavebeenused toobtainthediagnosis.TheTFOSDEWSIIDiagnosticMethodologyreportidentifieda diagnosticalgorithmforastepwiseanalysisthatincludes,aftertriagingquestions,theevaluationofsymptomsandhomeostasismarkers(noninvasiveorfluoresceinbreak-uptime, tearosmolarityandocularsurfacestaining)[4].Nonetheless,thereportacknowledged
N.I.B.U.T
Meibography
Interferometry
TearMeniscus
BulbarRedness
thatnosinglegoldstandardtesthasyetbeenestablished,andthatthereisaneedfornew, reliablediagnosticbiomarkers.
Inrecentyears,agreatnumberofimagingtechniquesanddevicesfortheexamination oftheocularsurfacehavebeendevelopedandplacedonthemarket(Table 1).These devicesoffertheadvantageofprovidingautomatedresultsoftheexaminedtests,thus avoidingobserverbias;moreover,sincemostoftheseexaminationsarenoninvasive,they donotaltertheresultsofsubsequenttests,representingusefulscreeningtoolsfordiscriminatinghealthysubjectsfrompatientsaffectedbyoratriskforDED.Finally,combining differenttechniquesinacomprehensiveocularsurfaceworkupmayincreasesensitivity andspecificitytodiagnosethediseaseandmonitoritscourseafterspecifictreatments[5–7].
Inthisreview,wesummarizethecurrentresearchavailableaboutthedevelopment anduseofnovelnoninvasivediagnostictechniquesforthediagnosisandfollow-upofDED.
IDRA KERATOGRAPH O.S.A. LACRYDIAGTEARCHECK
Automaticevaluation oftearfilmbreak-up time
Viewofthepresence ofabnormalgland structuresina high-resolution 3Dimage
Automaticevaluation ofthelipidlayer
Estimationofthetear filmquantityupto 5values
Comparisonwithall internationalgrading scales(efron, cclru,jenvis)
Automaticevaluationof tearfilmbreak-uptime withinfrared illumination
Morphologicalchangesin theglandtissuearemade visibleusingthe Meibo-Scanandcanbe classifiedwiththe JENVISMeibo GradingScales
Thethicknessofthelipid layerisautomatically assessedbasedonthe structureandcolor
Theheightofthetear meniscuscanbeprecisely measuredwithan integratedruler
TheR-Scanautomatically detectsthebloodvessels intheconjunctivaand evaluatesthedegree ofredness
Automaticevaluation oftearfilm break-uptime
Viewofthepresence ofabnormalgland structuresina high-resolution 3Dimage
Manualevaluationof thelipidlayer
Estimationofthetear filmquantityupto fivevalues
Comparisonwithall internationalgrading scales(efron,cclru, jenvis)
Automaticevaluation oftearfilm break-uptime
Automaticdetection ofmeibomianglands andautomatic calculationofthe percentageofloss
Qualitativeand quantitativeanalysis ofthelipidlayer Evaluationoflipid layerthicknessbased onagradingscale
Measurementoftear meniscus height(mm)
Automaticevaluation oftearfilm break-uptime
Viewoftherateof glandlossin%
Notavailable
Calculatemanually theheightofthetear meniscus
Notavailable Available
NIBUT=noninvasivebreakuptime,LACRYDIAG(QuantelMedical),IDRA(SbmSistemi,Inc.,Torino,Italy),OSA(SbmSistemi,Inc., Torino,Italy),KERATOGRAPH5M(OculusOptikgeräteGmbH,Wetzlar,Germany),TEARCECK(NewTechs.p.a.,MI,Italy).
2.NoninvasiveTearBreak-UpTime
Pre-cornealfilmstabilityplaysacriticalroleinthehomeostasisoftheocularsurface, anditisanimportantparametertobeconsideredforDEDdiagnosis[4,8].Infact,thetear filmisthefirstopticalinterfacebetweentheairandtheocularsurface,andsincethereis alargedifferenceinrefractiveindexbetweentheairandthetear,inhomogeneityinits structurehasamajorimpactontheopticalquality.InDED,thereisaquantitativeand/or qualitativeteardeficiencythatdeterminesirregularitiesand/ortheearlybreak-upofthe tearfilm[9,10].Inclinicalpractice,themostfrequentlyemployedtestforthemeasurement oftearfilmstabilityisthefluoresceintearfilmbreakuptime(BUT),whichistheinterval timebetweentheeyelidopeningafteracompleteblinkandthefirstbreakinthetear film.SinceBUTrequirestheuseoffluorescein,whosequantityandconcentrationcan alsoaffectthefinalmeasurement,noninvasiveBUT(NIBUT)hasbecomewidelyused inbothclinicalpracticeandresearchsettings.DEWSIIindicatedthatacutoffvalueof NIBUT ≤ 10sisanindicatorforthediagnosisofDED,withasensitivityof82–84%anda
specificityof76–94%[4].ThereareseveralcommerciallyavailableNIBUTsystems,based ontopographicorvideokeratographicmethods,whichanalyzetheinter-blinkchangesof reflectedplacidomires.Achangeintheedgesofthemiresreflectscompromisedtearfilm integrity.Bandlitzetal.demonstratedthatthereisagreementandrepeatabilityofmeasure betweensubjectiveandobjectivedevices[11],whileLeeetal.demonstratedthatthe agreementbetweentheresultsextrapolatedfromtwodifferentinstruments(Tomey RT-7000 AutoRefractor-KeratometerandOculusKeratograph)waspoor[12].TheKeratograph5M (OculusOptikgeräteGmbH,Wetzlar,Germany)allowsthemeasureofquantitativevalues, suchastheNIBUTf,whichistheinitialtearfilmbreakup,whereastheNIBUTavrepresents theaverageofalltearfilmbreakupsoccurringovertheentirecornea.Bothparameters havebeenreportedtobecorrelatedwithOcularSurfaceDiseaseIndex(OSDI)score[13,14].
3.LightScatter
Lightscatteringisaphysicalphenomenoninwhichlightwhenhitsasmallobject (aparticleoramolecule)changesitsdirection.Inhumaneyes,aberrationsandlight scatteringarethemainfactorsinthedegradationofopticalquality[15].InDED,theloss ofintegrityofthetearfilmandtheearlyexposureoftheroughepitheliumcanleadto anincreaseintheocularforwardlightscatteringdetectedbyC-Quantstraylightmeter (OculusGmbH,Wetzlar,Germany).InDEDeyeswithsuperficialpunctatekeratopathy, thereisalsoanincreaseincornealbackwardlightscatteringfromtheanteriorcornealpart, detectedbyaScheimpflugcamera(PentacamHR;OculusGmbH),comparedtonormal eyes[16].Tanetal.evaluatedopticalqualitychangesinDEDbyusingtheOpticalQuality AnalysisSystem(OQASII;VisiometricsS.L.,Tarrasa,Spain).Theyanalyzedaberrations andintraocularscatteringbyusingtheObjectiveScatterIndex(OSI)[17]andfoundthat thisparameterwasincreasedinpatientswithDED,anditsrateofchangewascorrelated withseverityofDED[18].Furthermore,Geetal.demonstratedthatseveralparameters (OSI,OSIstandarddeviation, ∆ OSI, ∆ OSI/time,blinkingchange,andblinkingfrequency) werecorrelatedwithBUTandstainingscore[19].
4.Aberrometer
Aberrometryuseswavefrontsensingtoanalyzedeviationsinthewavefrontexiting theeyefromareferencewavefront.Thistechniqueisusefulformeasuringthecomplete refractivestatus,includingirregularastigmatism,oranyotheropticalirregularity[20].
Kohetal.demonstratedthatBUTwasassociatedwithincreasedhigher-orderaberrations(HOAs)bothinphotopicandscotopicconditions[21].Sequentialmeasurementof HOAsdemonstratedthatnormaleyescanbeclassifiedintothreesub-groupsashaving stable,small-fluctuation,andsawtoothpatterns,respectively.Inthelattergroup,significant changeswerefoundinthesequentialpost-blinkchangesinthecoma-likeandsphericallikeaberrationsandtotalHOAs[22].IneyeswithDEDorirregularitiesintheirrefractive surfaces,dynamicwavefrontanalysispost-blinkcandetectanincreaseoftheHOAs,and thisiscorrelatedwiththeOSDIscoreandBUT[23].
InpatientswithDED,superficialpunctatekeratopathymayaggravatebothbaseline HOAsandsequentialpost-blinkchangesinHOAs[24].IneyeswithashortBUT,a prolongedblinkintervalleadstoincreasedHOAs,suggestingthatsuppressedblinking (e.g.,forworkerswhooperatevideodisplayterminals),canresultinreducedoptical quality[25].
Deschampsetal.demonstratedtheimpactoftearfilm-relatedaberrationchangeson activitiesofdailylivinginDED,specificallyduringadrivingsimulatortest[26].
Aninterventionalstudyinvestigatedtheeffectsoftopicaldrops(sodiumhyaluronate) ondynamicaberrometryinpatientswithDEDanddiscoverednosignificantchanges[27]. Ontheotherhand,anotherstudyexaminingtheeffectofrebamipide2%(amucinsecretagogue)demonstrateddecreasedHOAsafter4weeksoftreatmentinDEDpatientswith shortBUT[28].
5.AnteriorSegmentOpticalCoherenceTomography
Opticalcoherencetomography(OCT)isanoninvasivetechniquedevelopedtoobtain tomographicimagereconstructionofabiologicaltissuewithalongitudinalandlateral spatialresolutionofafewmicrometersbyusinglow-coherenceinterferometry[29,30]. AnteriorsegmentOCT(AS-OCT)canobtainimagesofimportantstructuresoftheocular surface[31]andisausefultoolinthediagnosisandfollow-upofseveraldiseasesof theanteriorsegmentoftheeye.InDED,themeasurementofparametersofthetear filmbymeansofAS-OCT,suchaspre-cornealtearfilmthickness(TFT),tearmeniscus height(TMH),curvature(TMR),cross-sectionalarea(TMA)andtearmeniscusdepth (TMD),arewidelyusedinroutineclinicalpracticethankstothenoncontactnatureof theseexaminationandtherapidimageacquisitionbytheinstrument[32,33].Theaverage valueofTFTmeasuredbyAS-OCTinhealthysubjectsisapproximately5 µm,whileit isreducedinDEDpatients.Thisreductioniscorrelatedwithobjectiveandsubjective assessments,suchasBUTandOSDI[34].ThevalueofTFTincreasesupto24hwiththe topicaladministrationofchitosan-N-acetylcysteine,perfluorohexyloctaneorlow-dose hydrocortisone[35,36],butislessinfluencedbylow-viscosityagents.TMHandTMAare twousefulbiomarkersofDED,assuggestedbytheDEWSIIReport.SpectralDomain AS-OCTdemonstratedasensitivityof80.5%and86.1%andaspecificityof89.3%and85.3% forTMHandTMA,respectively;however,thediagnosticabilityofevaporativeDEDwas low(<50%)[37].Inclinicalpractice,someconfoundingfactorsrelatedtotheenvironment ortheocularsurface’sanatomy(e.g.,conjunctivochalasis,lid-parallelconjunctivalfolds, disordersoflidmargincongruity)shouldbetakenintoaccount.AS-OCTisalsousefulfor the3Dhigh-resolutionofMeibomianglands(MG)[38].InpatientswithMeibomiangland dysfunction(MGD),AS-OCTdemonstratedadecreasedMGlengthandwidth,which correlatedwithoculardiscomfortsymptomsandsigns[39].
6.InVivoConfocalMicroscopy
Invivo confocalmicroscopy(IVCM)isausefulimagingtechniqueforthe invivo microscopicobservationofthecornealmicrostructurethatenablesclinicianstogain deepinsightintothepathophysiologyofocularsurfacediseases[40].Althoughitis anon/minimallyinvasivetest,thereareconcernsaboutitsroutineuseinclinicalpractice: (i)theexaminationrequiresdirectcontactwiththeocularsurface;(ii)asmallfieldof viewisobtained;(iii)onlyonez-planeisinvestigated;(iv)theeyetrackerfunctionisnot feasible[41].
IVCMofthecornealepitheliumdemonstratedsignificantalterationsinDEDpatients, presumablyduetoincreaseddesquamationofthesuperficialcelllayercausedbyhyperosmolarityofthetearfilmassociatedwithincreasedtearevaporationandelevated inflammatorymediators.Furthermore,IVCMallowstheevaluationofimmuneandinflammatorycells,cornealnerves,keratocytes,andMGstructuresonacellularlevel[42].
SeveralstudiesdemonstratedareduceddensityofsuperficialepithelialcellsinSjögren’sandnon-Sjögren’sDED(respectively,PSDEandNSDE)[43].Tuominenetal.reportedareductioninstromalthicknessinpatientswithPSDEaswellasabnormalkeratocytehyperreflectivity[44].BenitezdelCastilloetal.presentedanincreaseddensityin anteriorandposteriorstromalcellsinthePSDEgroupcomparedtotheNSDEgroup,but withoutstatisticalsignificance[45].
InaccordancewiththerecentevidenceofneuraldysregulationinDED,studies regardingcornealnerveparametersreportedanincreasedtortuosityandreflectivityofthe cornealsub-basalnerveplexusandanincreasednumberofbead-likeformationsinboth PSDEandNSDEcomparedtohealthycontrols[41,43].TheresultsobtainedbyLinetal. demonstratedthattherewasalsoimmunedysregulation:infact,whileinhealthysubjects dendriticandleukocytesepithelialcellsofthecorneadecreasefromtheperipherytowards tothecenter,inDEDtheyshowtheoppositetrend[46].
IVCMenablesthevisualizationoftheultrastructureofMG[47–49].Villanietal. demonstratedanincreasedacinardilatation,ahigherreflectivity(grades1–4)ofmeibum,
adecreaseddiameterofMGorificesandincreasedinhomogeneityoftheacinarwallin patientswithSSandMGDcomparedtocontrols[47].Ibrahimetal.demonstrateda reductioninthedensityanddiametersoftheacinarunitsinMGD[49].Randonetal., proposedanewclassificationofMGDbasedonIVCMfindings(type0fornormality,type1 formeibumobstruction,type2forinflammation,andtype3forfibrosis),anddemonstrated astrongcorrelationbetweentheIVCMscoreandthemeibographyscores[50].Inacrosssectionalstudy,ZhaofoundthatDEDsymptomswerenegativelycorrelatedwithIVCM parametersofMGandpositivelycorrelatedwithconjunctivalinflammatorycells[51].
7.Meibography
MeibographyallowstheobservationofthemorphologicalstructureoftheMGina two-dimensionalplane.Thetechniquewasintroducedforthefirsttimein1977byTapie, whousedtransilluminationfromthepalpebralskinwithanilluminatingprobetocapture imagesofMGs.Theapproachwasthenrefinedbyothermethodologiesthatallowthe visualizationofMGonblack-and-whitefilm,oninfraredfilmwithanear-infraredCCD (charge-coupleddevice)camera,orwithaninfraredCCDcamera[52].Non-contacttechniquesbasedonaninfraredfilterandinfraredCCDcamerathatmadepossiblerecording thetransilluminationimagefromtheconjunctivalsideoftheeyelidweredevelopedinthe lastdecade[53].Non-contactmeibographyisbasedontheautofluorescenceofahealthy meibumwhenilluminatedwithinfraredlight,whichcanbedetectedbyaninfraredchargecoupleddevicecamera;thus,theglandsappearaslightareasagainstadarkerbackground andanyalterationsinmeibumorlossofacinartissueappearasadarkarea[54].Recent technologyhasledtothedevelopmentofseveralmobile,handheld,pen-shapedandmultifunctionality(slitlamp-based,mobileandtopography-equipped)systemswithinfrared light-emittingdiodesfixedtoinfraredcamerasthatallowthecaptureofvideosandimages ofMGandincreasethefeasibilityofoutpatientcareassessment[55,56].
Inclinicalpractice,thegradingsystemsofMGstructurescanbeusedtodocument thepresence,progression,andtreatmentresponsetoMGD[57].Themostusefulsystems aretheMeiboscoreandtheMeibograde;bothfeatureascorebasedonthepercentageof lossoftheglandulararea,althoughthelatteroffersahighersensitivityandshouldbeable todetectminorchangesinMG[58].GulmezSevimetal.demonstratedthatOSDIscore, BUTandlissaminegreenstainingweresignificantlycorrelatedwithMGDgradeandMG arealoss[59].AlthoughfunctionalandmorphologicalchangesinMGareoftenthoughtto bewellcorrelated[60],meibographyalonedoesnotappeartobesufficientforreachingthe diagnosisofMGD,butitshouldbeconsideredinthecontextofotherclinicalparameters (e.g.,BUT,lidmarginexamination)orotherimagingtechniques(e.g.,IVCM).
8.Interferometry
Thetearfilmlipidlayerischieflycomposedofmeibomianlipid,whichisderived fromthemarginalreservoirsofthelids.Thelipidlayerplaysacriticalroleintearfilm stabilityforthemaintenanceofocularsurfacehealthbypreventingexcessiveevaporation oftheaqueouslayer.InobstructiveorhyposecretoryMGD,thinningofthelipidlayer leadstoexcessiveevaporation,causingevaporative-typeDED[61].Whenadequatelight hitsanoilylayer,theresultisthegenerationofaninterferometricfringe.Furthermore, interferencepatternsareproducedduetothephasedifferencebetweenthelightreflected fromthelipidlayerandthelightthatisreflectedfromthecornealepithelium[62,63]. Interferencesbythinfilmsdisplaydifferentcolors,dependingonthethicknessofthefilm, fromadarkcolor,causedbyathinnerfilmarea,toabrightercolor,causedbyathickerfilm area.Imaging-basedocularsurfaceinterferometryisanoninvasivetechniquethatallows themeasurementoflipidlayerthickness(LLT)onananometer(nm)scale.
Inhealthyeyes,LLThasbeenestimatedandreportedtobeapproximately70nm.Variousmethodsutilizinginterferencepatternshavebeenusedtocharacterizethisparameter. In1968,McDonaldusedagoosenecklight[64],in1980Hamanoetal.abiodifferential microscope[65],Polaroidfilters[66],monochromaticlight[67],spectraldiscrimination[68],
andasimpleinterferometermadeofpapertoolforlipidlayerevaluation[69].Thereare severalinterferenceimagingdevices:theDR-1tearinterferencecamera(KowaCo.,Nagoya, Japan)[70],theLipiViewIIinterferometer(TearScienceInc.,Morrisville,NC,USA)[71], theLipiscanner1.0(VisualOptics,Chuncheon,Korea),anadd-ontoanexistingslitlamp biomicroscope[72],theOculusKeratograph5M(Oculus,Arlington,WA,USA)[73],and theIDRA(SbmSistemi,Inc.,Torino,Italy)[74,75].
TheDR-1 α cameraallowsaqualitativeanalysisofthelipidlayer.Yokoietal.proposed agradingsystembasedonthefirststableframesfromtheDR-1 α camera[76].
Gotoetal.producedacomputer-synthesizedcolorchartofahumantearlipid interferenceimagefortheconversionfromtearinterferencecolorinformationtotearlipid layerfilmthicknessdata[77].ByusingtheDR-1 α camera,itispossibletoevaluatethe kineticspreadandstabilityofthelipidlayer.Gotoetal.evaluatedthespeedandpattern oflipidspreadaftereyeopeningandthestabilityofthelipidfilmafterspread,andfound thatinpatientswithlipidlayerdeficiency,thelipidspreadwasslowandresultedina verticallystreakingandnon-uniformpattern[78].Aritaetal.demonstratedthattheDR-1 α interferometercanmeasuretheTMHasreliablyasAS-OCTandthattheinterferometric TMHcorrelatedwithSchirmer’sscore.[79].DR-1 α interferometrydevicecanassistinthe differentialdiagnosisofdifferentsubtypesofDED;indeed,Aritaetal.,bycomparingthree interferometricpatterns(pearl-like,Jupiter-like,orcrystal-like),foundadirectcorrelation withBUT,NIBUTandSchirmertestvalue[80].
TheLipiViewII(LVII)interferometercanquantitativelymeasuretheaverageLLT byanalyzingtheinterferometricpatternofthetearfilm,apartialblinkrate,anduses aninfraredlightsourceforimagingtheMG.LeeY.etal.showedthattheaverageLLT valuehasasignificantlypositivecorrelationwithage,OSDI,andOcularStainingScore, andnocorrelationwiththeSchirmertesttypeI,BUT,ormeiboscore[81].ByusingLVII, WengdemonstratedthatyoungerpatientswithDEDexperiencedmoreseveresubjective symptoms,moreincompleteblinks,andathinnerLLT[82].
9.Thermography
Thestabilityofthetearfilmisstrictlylinkedtotheintegrityofthelipidlayerthat controlsevaporation.Thereiscurrentlynocommerciallyavailableinstrumentdedicatedto thedetectionoftearfilmevaporation,butthermographypermitsanindirectevaluationof theevaporationratebymeasuringthetemperatureoftheocularsurfaceinanoninvasive manner,usingathermographiccameraoperatingwithintheinfraredrange[83–85].In1995, Morganetal.,measuredocularsurfacetemperatureinpatientswithDEDandreported thatthesurfacetemperaturewassignificantlyhigherthaninnormaleyes,whilethe temperatureatthecenterofthecorneaofDEDeyesbecamelowerthanthatinnormaleyes aftersustainedeyeopening[86].Inthelastdecade,advancesintechnologyhaveallowed themeasurementoftheocularsurfacetemperaturewithincreasingaccuracy,resolution, andspeed[87].Suetal.,showedastrongspatialandtemporalcorrelationbetweenBUT andtearfilmevaporation[88].
Kamaoetal.reportedthatDEDwasassociatedwithagreaterdecreaseintheocular surfacetemperatureat10saftereyeopeningandsuggestedthatmeasurementsobtained over10shavesensitivityandspecificityvaluesof0.83and0.80,respectively,forscreening DEDeyes[87].
10.BulbarRednessAssessment
Bulbarredness(BR)isanon-specificocularconditioncausedbythevasodilationof theconjunctivaland/oranteriorscleralbloodvessels,whichisitselfcausedbyenhanced bloodflowtoandcapillarypermeabilityintheanterioroculartissuesinresponseto variousstimuli[89].Hyperemiaisafeatureofseveralocularconditions:inflammationof theanteriorsegmentoftheeye[90],theadverseeffectsofglaucomamedications[91,92], contactlenswearing[93],allergicorinfectiveconjunctivitis[94],andDED[95].
SincethefirstintroductionbyMcMonniesandChapman-Daviesin1987ofanimagebasedbulbarhyperemiagradingscale[89],severalsubjectiveratingscaleshavebeen introducedtoimproveinter-orintra-observervariability[96,97].Scientificstudiestriedto overcometheselimitationsbydevelopingnovelmodelsofcomputer-basedphotographanalysistechniques,whichhavenowprogressedtoautomatedimageanalysisthroughthe applicationofartificialintelligence(AI)[98].Villumesenetal.developedthefirstcomputerassistedconjunctivalhyperemiaquantificationsystembyusingthepixeledgedetection analysisofa3 × 3mmregionofbulbarconjunctiva[99].Willinghamanalyzedthemean relativeredness(RR)andthebloodvesselarearatio(VA)ofdigitalimagesoftheexternal eyebyusingaclinicalphotographicbiomicroscope,anMVCvideocameraandcomputer imagingsystem,andcustomsoftware[100].Rodriguezetal.developedanautomated computerrednessgradingsystem(Ora,Inc.,Andover,MA,USA,patentpending)forthe evaluationofthelocationandprominenceoffinehorizontalconjunctivalvesselsinDED anddemonstratedagreementwithagroupofinvestigatorsusingtheestablishedclinical scale[101].
Wuetal.evaluatedtheclinicalassessmentofbulbarrednessbyusinganOculusKeratograph5MTopographer(OculusOptikgerateGmbH,Wetzlar,Germany)equippedwith automatedscanningandscoringsoftware.Theyfoundastatisticallysignificantcorrelation betweentheOculusIndexscoreandthescoresdeterminedusingthreesubjectivescales (theInstituteforEyeResearchscale,theEfronscale,andtheValidatedBulbarRedness 10-picturegradingscale)[96,102].Moreover,theyfoundthattheKeratographyielded higherintra-andinter-observerreproducibility,suggestingthattheKeratographcouldbe atime-savingdevice[103].
Despitetheintroductionofnovelincorporatedinstrumentsthatmaketheclinical assessmentofBRinDEDquickerandeasier,themajorityofautomaticgradingsystemsare unabletodifferentiatebulbarconjunctivalhyperemiafromepiscleralandscleralhyperemia; thus,therearemanyconcernsovertheuseofBRasabiomarker[104].
11.ImageModalityBasedComputerizedDetectionTechniques
ThroughrecentadvancesinAIandtherapidprogressionofanalytictechniques, researchersaretryingtoovercomethemainpitfallsofclinicaltesting,suchasthelong timerequiredforacquisitionandtheneedforskillfulmaneuvers[105].Theaimofthe computerizationofclinicaltestsistohelpclinicianstoincreaseaccuracyandreducethe timetakentoformadiagnosis.
Inthelastdecade,severalcomputerizedDEDdetectiontechniqueshavebeenproposed,eachbasedondifferenttechnologies.ThefirstattempttoautomaticallydetectDED wasundertakenbyYedidyaetal.,whousedamulti-stepalgorithmtoevaluateBUTfrom aneyevideothatwascapturedusinganEyescandevice[106].Ramosetal.proposedan automaticmethodologyforcharacterizingtearfilmdynamicsovertheexposedcorneal surfacefromtheemergenceofthefirstbreak-upinthetearfilmuntilthesubsequentblink. Tothisend,theBUTmeasurementwascomputed,andthebreak-upareasweresegmented ineachvideoframetoanalyzeotherbreak-upparameters,suchasthesizeorspatialextensionoftheBUT[107].Suetal.proposedanautomaticmethodtodetecttheBUTareausing adeeplearningconvolutionalneuralnetwork(CNN)model,ahierarchicalmultilayered neuralnetworkthatcanlearnvisualpatternsdirectlyfromimagepixels[108,109].The proposedCNNmodeldetectedbreak-upwithanaccuracyrateof98%[110].
Twotechniquesbasedontheinterferencepatternofthelipidlayerhavebeenproposed. Thefirstisacomputer-aidedsystemtosupportDEDdiagnosisbasedontearfilmmaps (CASDES),andthesecondiscallediDEAS.Theformersystem,whichanalyzestheimages acquiredbyTearscope,allowstherecognitionofdryarearegionsinagreementwiththe regionannotatedmanuallybytheophthalmologist[111].Thelatterisaweb-basedsystem forDEDassessment,inwhich,oncethepatient’sinterferencepatternimagesareuploaded, asupportvectormachineclassifierbasedonstatisticallearningtheoryallocatestheimages intofivecategoriesbasedonGuillonclassification[112,113].
Acharyaetal.developedatechniquebasedonthermalinfraredimages.Theresult producedbythistechniqueisbinary;thatis,inputimagesofeithernormaleyeordry eyeareconvertedinto1Ddataandthenfedintodifferentclassifiers,suchask-Nearest Neighbor(KNN),decisiontree,ProbabilisticNeuralNetwork(PNN),SVMandNaive Bayes(NB).Theauthorsobtainedanaveragesensitivityof99.8%,specificityof99.8%and classificationaccuracyof99.8%usingPNNandKNNclassifier[114].
12.ProsandConsofNoninvasiveDEDImaging
Thenovelnon-invasivediagnostictechniquesforDEDoffernumerousadvantages overconventionaltests.Themainadvantageisrelatedtotheirautomatednature,which meansthattheydonotrequiretheclinician’sjudgmenttodetermineascore.Thisis clinicallyrelevantassubjectiveDEDmarkers,suchasfluoresceinBUTandcornealstaining, featurelowinter-observerrepeatabilityduetotheirlackofstandardization[4].Moreover, mostofthesenoveldiagnostictechniquesdonotrequiredirectcontactwiththeeye,and thereforehavelittle/noeffectonthevolumeandpropertiesofthetearfilm.Thus,they canbeusedasscreeningtools(e.g.,priortoocularsurgery)bytrainedmedicalpersonnel (notnecessarilyanophthalmologist),beforeproceedingwithmoreinvasiveocularsurface examinationsincaseofthedetectionofabnormalvalues.Furthermore,theyprovideclear anddetailedreportssummarizingalltheresultsofthetestsforeachpatientexamination, whichcanbeusedforfuturereferenceandcomparison(Figures 1 and 2).

Thedailyreportisusefulforeducatingpatientsandencouragingtreatmentcompliance:“Apictureistrulyworthathousandwords”.All-in-onedevices(onemachinefor moretasks)makeitpossibletoreachthediagnosisofDEDinlesstimecomparedtoa traditionalworkup(e.g.,3minareenoughtoperformacompleteocularsurfaceworkup withIdra),andrepresentaguideforimaging-basedtreatment:thedeficientlayer(s)ofthe tearfilmis/areidentifiedandatargetedtherapycanbeprescribed.Similarly,monitoring patient’scourseovertimeisalsomadeeasierbyusingthesedevicesandthetrendof asingleparameter(e.g.,NIBUT)afteragiventherapycanbegraphicallyreportedand analyzed(Figure 3).

DryEyereportobtainedwiththeuseofIDRA(SbmSistemi,Inc.,Torino,Italy),includingnoninvasivebreak-uptime,eyeblink,lipidlayer,tearmeniscusheight,andlossareaofmeibomianglands.

TrendinggraphobtainedwiththeuseofIDRA(SbmSistemi,Inc.,Torino,Italy)relatedto noninvasivebreak-uptimevaluescollectedatdifferenttimepoints.
Nevertheless,therearesomedrawbackstothesenewdiagnostictechniquesthat needtobementioned.Inparticular,thecostmayrepresentamajorlimitationfortheir widespreadacceptance,andcost-effectivenessevaluationsarestillrequiredtosupporttheir adoptiononalargescale.Moreover,ophthalmologicalconditionsotherthanDEDmay causesomephenomenathatreducethediagnosticaccuracy,particularlyiftheimageinterpretationsarenotperformedbyanophthalmologistand/oriftheslitlampexaminationis notperformed.Forinstance,conjunctivochalasismayhampertheaccuratemeasurement ofTMH,leadingtooverestimationofthevalue(Figure 4),whileconjunctivallesions,such aspterygium,maysignificantlyalterbulbarrednessbyincreasingitsvalueintheaffected area(Figure 5).Itshouldalsobenotedthatnoveltestscannotyetreplaceallthetraditional DEDmetricsandslitlampexaminationisstillrequiredtocompletethediagnosticworkup.


Finally,wewouldliketoprovidesomerecommendationsfortheimproveduse ofnoninvasivediagnostictools.Firstly,asintraditionalDEDtests,inthenoninvasive workupattentionshouldbepaidtotheorderinwhichdiagnosticexamsareperformed. Infact,althoughthesetestsusuallydonotrequirecontactwiththeocularsurface,lightinducedtearingshouldbeconsidered,sinceitcouldinfluencetheresultsofsubsequent examinations.Secondly,inthecaseofsignificantdifferencesbetweenthetwoeyesofthe samepatient,resultsshouldbeinterpretedwithcaution,andmultipleevaluationsofboth eyesareadvisable.
13.Conclusions
DEDrepresentsaveryfrequentoculardiseaseworldwideanditsprevalenceiscontinuouslygrowing[115].Severaldiagnostictoolshaverecentlybeenintroducedinclinical practicetoassistcliniciansinthe(early)diagnosisandmonitoringofDED;furthermore, thesedevicesallowtheidentificationoftheaffectedlayer(s)ofthetearfilmandrepresenta guideforimaging-basedtargetedtreatment.Theinformationgeneratedprovidesobjective resultsandpicturesthatareusefulforhelpingpatients’awarenessaboutDED,thusimprovingtreatmentcompliance.Soon,theadventofAImayfurtherhelpcliniciansinthis complexdiagnosticpathway.Inthisdirection,further,larger-scale,clinicaltrialsshould providemoreevidenceontheroleoftheseemergingdiagnostictechniquesintheDED diagnosticbatterybyestablishinguniversalcut-offvaluesforeachexam.
AuthorContributions: Conceptualization,L.D.C.andG.G.;methodology,L.D.C.;formalanalysis, G.G.;datacuration,L.D.C.,M.P.,A.V.,M.B.,L.F.D.,V.S.,C.E.T.,G.G.;writing—originaldraftpreparation,L.D.C.,M.P.,M.B.;writing—reviewandediting,L.D.C.,M.P.,A.V.,G.G.;supervision,V.S., C.E.T.;projectadministration,V.S.,C.E.T.Allauthorshavereadandagreedtothepublishedversion ofthemanuscript.
Funding: Thisresearchreceivednoexternalfunding.
InstitutionalReviewBoardStatement: Notapplicable. InformedConsentStatement: Notapplicable.
ConflictsofInterest: Theauthorsdeclarenoconflictofinterest.
References
1. Lemp,M.A.;Foulks,G.N.TheEpidemiologyofDryEyeDisease:ReportoftheEpidemiologySubcommitteeoftheInternational DryEyeWorkShop(2007). Ocul.Surf. 2007, 5,93–107.[CrossRef]
2. Stapleton,F.;Alves,M.;Bunya,V.Y.;Jalbert,I.;Lekhanont,K.;Malet,F.;Na,K.-S.;Schaumberg,D.;Uchino,M.;Vehof,F.;etal. TFOSDEWSIIEpidemiologyReport. Ocul.Surf. 2017, 15,334–365.[CrossRef]
3. McDonald,M.;Patel,D.A.;Keith,M.S.;Snedecor,S.J.EconomicandHumanisticBurdenofDryEyeDiseaseinEurope,North America,andAsia:ASystematicLiteratureReview. Ocul.Surf. 2016, 14,144–167.[CrossRef][PubMed]
4. Wolffsohn,J.S.;Arita,R.;Chalmers,R.;Djalilian,A.;Dogru,M.;Dumbleton,K.;Gupta,P.K.;Karpecki,P.;Lazreg,S.;Pult,H.;etal. TFOSDEWSIIDiagnosticMethodologyreport. Ocul.Surf. 2017, 15,539–574.[CrossRef][PubMed]
5. Schmidl,D.;Schlatter,A.;Chua,J.;Tan,B.;Garhöfer,G.;Schmetterer,L.NovelApproachesforImaging-BasedDiagnosisof OcularSurfaceDisease. Diagnostics 2020, 10,589.[CrossRef]
6. Vigo,L.;Pellegrini,M.;Bernabei,F.;Carones,F.;Scorcia,V.;Giannaccare,G.DiagnosticPerformanceofaNovelNoninvasive WorkupintheSettingofDryEyeDisease. J.Ophthalmol. 2020, 2020,5804123.[CrossRef]
7. Aragona,P.;Giannaccare,G.;Mencucci,R.;Rubino,P.;Cantera,E.;Rolando,M.Modernapproachtothetreatmentofdryeye,a complexmultifactorialdisease:AP.I.C.A.S.S.O.boardreview. Br.J.Ophthalmol. 2021, 105,446–453.[CrossRef]
8. Willcox,M.D.P.;Argüeso,P.;Georgiev,G.A.;Holopainen,J.M.;Laurie,G.W.;Millar,T.J.;Papas,E.B.;Rolland,J.P.;Schmidt,T.A.; Stahl,U.;etal.TFOSDEWSIITearFilmReport. Ocul.Surf. 2017, 15,366–403.[CrossRef]
9. Herbaut,A.;Liang,H.;Denoyer,A.;Baudouin,C.;Labbé,A.Tearfilmanalysisandevaluationofopticalquality:Areviewofthe literature. J.Fr.Ophtalmol. 2019, 42,226–243.[CrossRef]
10. Giannaccare,G.;Scorcia,V.FalseMythsversusMedicalFacts:TenCommonMisconceptionsRelatedtoDryEyeDisease. Biomedicines 2020, 8,172.[CrossRef]
11. Bandlitz,S.;Peter,B.;Pflugi,T.;Jaeger,K.;Anwar,A.;Bikhu,P.;Nosch,D.S.;Wolffsohn,J.S.Agreementandrepeatabilityoffour differentdevicestomeasurenon-invasivetearbreakuptime(NIBUT). ContactLensAnteriorEye 2020, 43,507–511.[CrossRef]
12. Yeo,S.;Lee,R.;Aung,H.T.;Tong,L.Agreementofnon-invasivetearbreakuptimemeasurementbetweenTomeyRT-7000Auto Refractor-KeratometerandOculusKeratograph5M. Clin.Ophthalmol. 2016, 10,1785–1790.[CrossRef][PubMed]
13. Fuller,D.G.;Potts,K.;Kim,J.NoninvasiveTearBreakupTimesandOcularSurfaceDisease. Optom.Vis.Sci. 2013, 90,1086–1091. [CrossRef]
14. Hong,J.;Sun,X.;Wei,A.;Cui,X.;Li,Y.;Qian,T.;Wang,W.;Xu,J.AssessmentofTearFilmStabilityinDryEyeWithaNewly DevelopedKeratograph. Cornea 2013, 32,716–721.[CrossRef][PubMed]
15. Goto,E.;Yagi,Y.;Matsumoto,Y.;Tsubota,K.Impairedfunctionalvisualacuityofdryeyepatients. Am.J.Ophthalmol. 2002, 133, 181–186.[CrossRef]
16. Koh,S.;Maeda,N.;Ikeda,C.;Asonuma,S.;Mitamura,H.;Oie,Y.;Soma,T.;Tsujikawa,M.;Kawasaki,S.;Nishida,K.Ocular ForwardLightScatteringandCornealBackwardLightScatteringinPatientswithDryEye. Investig.Opthalmol.Vis.Sci. 2014, 55, 6601–6606.[CrossRef][PubMed]
17. Galliot,F.;Patel,S.R.;Cochener,B.ObjectiveScatterIndex:WorkingTowardaNewQuantificationofCataract? J.Refract.Surg. 2016, 32,96–102.[CrossRef]
18. Tan,C.;Labbé,A.;Liang,Q.;Qiao,L.;Baudouin,C.;Wan,X.;Wang,N.DynamicChangeofOpticalQualityinPatientsWithDry EyeDisease. Investig.Opthalmol.Vis.Sci. 2015, 56,2848–2854.[CrossRef]
19. Ye,F.;Jiang,F.;Lu,Y.;Xue,C.Y.;Zhu,X.M.;Wu,Y.;Huang,Z.P.Objectiveopticalassessmentoftear-filmqualitydynamicsin patientswithmeibomianglanddysfunctionandaqueous-deficientdryeyeopticalqualitychangesindifferentdryeyesubtypes. IndianJ.Ophthalmol. 2019, 67,599–603.[CrossRef]
20. Maeda,N.Clinicalapplicationsofwavefrontaberrometry-areview. Clin.Exp.Ophthalmol. 2009, 37,118–129.[CrossRef]
21. Koh,S.;Maeda,N.;Kuroda,T.;Hori,Y.;Watanabe,H.;Fujikado,T.;Tano,Y.;Hirohara,Y.;Mihashi,T.Effectoftearfilmbreak-up onhigher-orderaberrationsmeasuredwithwavefrontsensor. Am.J.Ophthalmol. 2002, 134,115–117.[CrossRef]
22. Koh,S.SerialMeasurementsofHigher-OrderAberrationsafterBlinkinginNormalSubjects. Investig.Opthalmol.Vis.Sci. 2006, 47,3318–3324.[CrossRef]
23. Denoyer,A.;Rabut,G.;Baudouin,C.TearFilmAberrationDynamicsandVision-RelatedQualityofLifeinPatientswithDryEye Disease. Ophthalmology 2012, 119,1811–1818.[CrossRef][PubMed]
24. Koh,S.;Maeda,N.;Hirohara,Y.;Mihashi,T.;Bessho,K.;Hori,Y.;Inoue,T.;Watanabe,H.;Fujikado,T.;Tano,Y.Serial MeasurementsofHigher-OrderAberrationsafterBlinkinginPatientswithDryEye. Investig.Opthalmol.Vis.Sci. 2008, 49, 133–138.[CrossRef]
25. Koh,S.;Maeda,N.;Hori,Y.;Inoue,T.;Watanabe,H.;Hirohara,Y.;Mihashi,T.;Fujikado,T.;Tano,Y.EffectsofSuppressionof BlinkingonQualityofVisioninBorderlineCasesofEvaporativeDryEye. Cornea 2008, 27,275–278.[CrossRef][PubMed]
26. Deschamps,N.;Ricaud,X.;Rabut,G.;Labbé,A.;Baudouin,C.;Denoyer,A.TheImpactofDryEyeDiseaseonVisualPerformance WhileDriving. Am.J.Ophthalmol. 2013, 156,184–189.e3.[CrossRef][PubMed]
27. Lekhanont,K.;Chuckpaiwong,V.;Vongthongsri,A.;Sangiampornpanit,T.EffectsofSodiumHyaluronateonWavefront AberrationsinDryEyePatients. Optom.Vis.Sci. 2014, 91,39–46.[CrossRef]
28. Koh,S.;Inoue,Y.;Sugmimoto,T.;Maeda,N.;Nishida,K.EffectofRebamipideOphthalmicSuspensiononOpticalQualityinthe ShortBreak-upTimeTypeofDryEye. Cornea 2013, 32,1219–1223.[CrossRef][PubMed]
29. Huang,D.;Swanson,E.A.;Lin,C.P.;Schuman,J.S.;Stinson,W.G.;Chang,W.;Hee,M.R.;Flotte,T.;Gregory,K.;Puliafito,C.A.; etal.OpticalCoherenceTomography. Science 1991, 254,1178–1181.[CrossRef]
30. Pellegrini,M.;Vagge,A.;Desideri,L.F.F.;Bernabei,F.;Triolo,G.;Mastropasqua,R.;DelDelNoce,C.;Borrelli,E.;Sacconi,R.; Iovino,C.;etal.OpticalCoherenceTomographyAngiographyinNeurodegenerativeDisorders. J.Clin.Med. 2020, 9,1706. [CrossRef]
31. Ang,M.;Baskaran,M.;Werkmeister,R.M.;Chua,J.;Schmidl,D.;dosSantos,V.A.;Garhöfer,G.;Mehta,J.S.;Schmetterer,L. Anteriorsegmentopticalcoherencetomography. Prog.Retin.EyeRes. 2018, 66,132–156.[CrossRef]
32. Cui,L.;Wang,J.;Perez,V.L.;Shen,M.;Yuan,Y.;Wang,M.R.VisualizationofthePrecornealTearFilmUsingUltrahighResolution OpticalCoherenceTomographyinDryEye. EyeContactLensSci.Clin.Pract. 2012, 38,240–244.[CrossRef]
33. Ramos,J.L.B.;Li,Y.;Huang,D.Clinicalandresearchapplicationsofanteriorsegmentopticalcoherencetomography-areview. Clin.Exp.Ophthalmol. 2009, 37,81–89.[CrossRef]
34. Schmidl,D.;Witkowska,K.J.;Kaya,S.;Baar,C.;Faatz,H.;Nepp,J.;Unterhuber,A.;Werkmeister,R.M.;Garhofer,G.;Schmetterer, L.TheAssociationbetweenSubjectiveandObjectiveParametersfortheAssessmentofDry-EyeSyndrome. Investig.Ophthalmol. Vis.Sci. 2015, 56,1467–1472.[CrossRef]
35. Kallab,M.;Szegedi,S.;Hommer,N.;Stegmann,H.;Kaya,S.;Werkmeister,R.M.;Schmidl,D.;Schmetterer,L.;Garhöfer,G.Topical LowDosePreservative-FreeHydrocortisoneReducesSignsandSymptomsinPatientswithChronicDryEye:ARandomized ClinicalTrial. Adv.Ther. 2020, 37,329–341.[CrossRef][PubMed]
36. Schmidl,D.;Bata,A.M.;Szegedi,S.;DosSantos,V.A.;Stegmann,H.;Fondi,K.;Krösser,S.;Werkmeister,R.M.;Schmetterer,L.; Garhöfer,G.InfluenceofPerfluorohexyloctaneEyeDropsonTearFilmThicknessinPatientswithMildtoModerateDryEye Disease:ARandomizedControlledClinicalTrial. J.Ocul.Pharmacol.Ther. 2020, 36,154–161.[CrossRef]
37. Binotti,W.W.;Bayraktutar,B.;Ozmen,M.C.;Cox,S.M.;Hamrah,P.AReviewofImagingBiomarkersoftheOcularSurface. Eye ContactLensSci.Clin.Pract. 2020, 46,S84–S105.[CrossRef][PubMed]
38. Hwang,H.S.;Shin,J.G.;Lee,B.H.;Eom,T.J.;Joo,C.-K.InVivo3DMeibographyoftheHumanEyelidUsingRealTimeImaging Fourier-DomainOCT. PLoSONE 2013, 8,e67143.[CrossRef][PubMed]
39. Liang,Q.;Pan,Z.;Zhou,M.;Zhang,Y.;Wang,N.;Li,B.;Baudouin,C.;Labbé,A.EvaluationofOpticalCoherenceTomography MeibographyinPatientsWithObstructiveMeibomianGlandDysfunction. Cornea 2015, 34,1193–1199.[CrossRef]
40. Wang,Y.E.;Tepelus,T.C.;Vickers,L.A.;Baghdasaryan,E.;Gui,W.;Huang,P.;Irvine,J.A.;Sadda,S.;Hsu,H.Y.;Lee,O.L.Roleof invivoconfocalmicroscopyinthediagnosisofinfectiouskeratitis. Int.Ophthalmol. 2019, 39,2865–2874.[CrossRef]
41. Niederer,R.;McGhee,C.Clinical invivo confocalmicroscopyofthehumancorneainhealthanddisease. Prog.Retin.EyeRes. 2010, 29,30–58.[CrossRef]
42. Giannaccare,G.;Pellegrini,M.;Sebastiani,S.;Moscardelli,F.;Versura,P.;Campos,E.C. Invivo confocalmicroscopymorphometric analysisofcornealsubbasalnerveplexusindryeyediseaseusingnewlydevelopedfullyautomatedsystem. Graefe’sArch.Clin. Exp.Ophthalmol. 2019, 257,583–589.[CrossRef]
43. Villani,E.;Galimberti,D.;Viola,F.;Mapelli,C.;Ratiglia,R.TheCorneainSjögren’sSyndrome:AnInVivoConfocalStudy. Investig.Opthalmol.Vis.Sci. 2007, 48,2017–2022.[CrossRef][PubMed]
44. Tuominen,I.S.J.;Konttinen,Y.T.;Vesaluoma,M.H.;Moilanen,J.A.O.;Helinto,M.;Tervo,T.M.T.CornealInnervationand MorphologyinPrimarySjögren’sSyndrome. Investig.Opthalmol.Vis.Sci. 2003, 44,2545–2549.[CrossRef]
45. DelCastillo,J.M.B.;Wasfy,M.A.S.;Fernandez,C.;Garcia-Sanchez,J.AnInVivoConfocalMaskedStudyonCornealEpithelium andSubbasalNervesinPatientswithDryEye. Investig.Opthalmol.Vis.Sci. 2004, 45,3030–3035.[CrossRef]
46. Lin,H.;Li,W.;Dong,N.;Chen,W.;Liu,J.;Chen,L.;Yuan,H.;Geng,Z.;Liu,Z.ChangesinCornealEpithelialLayerInflammatory CellsinAqueousTear–DeficientDryEye. Investig.Opthalmol.Vis.Sci. 2010, 51,122–128.[CrossRef]
47. Villani,E.;Ceresara,G.;Beretta,S.;Magnani,F.;Viola,F.;Ratiglia,R.InVivoConfocalMicroscopyofMeibomianGlandsin ContactLensWearers. Investig.Opthalmol.Vis.Sci. 2011, 52,5215–5219.[CrossRef]
48. Matsumoto,Y.;Sato,E.A.;Ibrahim,O.M.;Dogru,M.;Tsubota,K.Theapplicationof invivo laserconfocalmicroscopytothe diagnosisandevaluationofmeibomianglanddysfunction. Mol.Vis. 2008, 14,1263–1271.
49. Ibrahim,O.M.;Matsumoto,Y.;Dogru,M.;Adan,E.S.;Wakamatsu,T.H.;Goto,T.;Negishi,K.;Tsubota,K.TheEfficacy,Sensitivity, andSpecificityofInVivoLaserConfocalMicroscopyintheDiagnosisofMeibomianGlandDysfunction. Ophthalmology 2010, 117,665–672.[CrossRef][PubMed]
50. Randon,M.;Aragno,V.;Abbas,R.;Liang,H.;Labbé,A.;Baudouin,C.;Hong,L. Invivo confocalmicroscopyclassificationinthe diagnosisofmeibomianglanddysfunction. Eye 2018, 33,754–760.[CrossRef][PubMed]
51. Zhao,H.;Chen,J.-Y.;Wang,Y.-Q.;Lin,Z.-R.;Wang,S. Invivo ConfocalMicroscopyEvaluationofMeibomianGlandDysfunction inDryEyePatientswithDifferentSymptoms. Chin.Med.J.(Engl.) 2016, 129,2617–2622.[CrossRef]
52. Arita,R.Meibography:AJapanesePerspective. Investig.Opthalmol.Vis.Sci. 2018, 59,DES48–DES55.[CrossRef]
53. Robin,J.B.;Jester,J.V.;Nobe,J.;Nicolaides,N.;Smith,R.E.InVivoTransilluminationBiomicroscopyandPhotographyof MeibomianGlandDysfunction. Ophthalmology 1985, 92,1423–1426.[CrossRef]
54. Giannaccare,G.;Bonifazi,F.;Sebastiani,S.;Sessa,M.;Pellegrini,M.;Arpinati,M.;Moscardelli,F.;Versura,P.;Campos,E. MeibomianGlandDropoutinHematologicalPatientsBeforeHematopoieticStemCellTransplantation. Cornea 2018, 37,1264–1269.[CrossRef]
55. Arita,R.;Itoh,K.;Maeda,S.;Maeda,K.;Amano,S.ANewlyDevelopedNoninvasiveandMobilePen-ShapedMeibography System. Cornea 2013, 32,242–247.[CrossRef]
56. Pult,H.;Riede-Pult,B.Non-contactmeibography:Keepitsimplebuteffective. ContactLensAnteriorEye 2012, 35,77–80. [CrossRef][PubMed]
57. Bernabei,F.;Versura,P.;Pellegrini,M.;Moscardelli,F.;Bonifazi,F.;Sessa,M.;Arpinati,M.;Scorcia,V.;Giannaccare,G. LongitudinalAnalysisofInfraredMeibographyinPatientsUndergoingHematopoieticStemCellTransplantation. Cornea 2020, 39,812–817.[CrossRef][PubMed]
58. Wise,R.J.;Sobel,R.K.;Allen,R.C.Meibography:Areviewoftechniquesandtechnologies. SaudiJ.Ophthalmol. 2012, 26,349–356. [CrossRef]
59. Sevim,D.G.;Gumus,K.;Unlu,M.Reliable,NoncontactImagingToolfortheEvaluationofMeibomianGlandFunction:Sirius Meibography. EyeContactLensSci.Clin.Pract. 2020, 46,S135–S140.[CrossRef]
60. Kim,H.M.;Eom,Y.;Song,J.S.TheRelationshipbetweenMorphologyandFunctionoftheMeibomianGlands. EyeContactLens Sci.Clin.Pract. 2018, 44,1–5.[CrossRef]
61. Abusharha,A.A.;Pearce,E.I.TheEffectofLowHumidityontheHumanTearFilm. Cornea 2013, 32,429–434.[CrossRef] [PubMed]
62. Doane,M.G.AnInstrumentforInVivoTearFilmInterferometry. Optom.Vis.Sci. 1989, 66,383–388.[CrossRef]
63. Yokoi,N.;Yamada,H.;Mizukusa,Y.;Bron,A.J.;Tiffany,J.M.;Kato,T.;Kinoshita,S.RheologyofTearFilmLipidLayerSpreadin NormalandAqueousTear–DeficientDryEyes. Investig.Opthalmol.Vis.Sci. 2008, 49,5319–5324.[CrossRef][PubMed]
64. McDonald,J.E.Surfacephenomenaoftearfilms. Trans.Am.Ophthalmol.Soc. 1968, 66,905–939.[CrossRef]
65. Hamano,H.;Hori,M.;Kawabe,H.;Umeno,M.;Mitsunaga,S.;Ohnishi,Y.;Koma,I.ClinicalApplicationsOfBioDifferential InterferenceMicroscope. ContactIntraocularLensMed.J. 1980, 6,229–235.
66. Guillon,J.-P.Tearfilmphotographyandcontactlenswear. J.Br.ContactLensAssoc. 1982, 5,84–87.[CrossRef]
67. Doane,M.G.;Lee,M.E.TearFilmInterferometryasaDiagnosticToolforEvaluatingNormalandDry-EyeTearFilm. Adv.Exp. Med.Biol. 1998, 438,297–303.
68. Khamene,A.;Negahdaripour,S.;Tseng,S.Aspectral-discriminationmethodfortear-filmlipid-layerthicknessestimationfrom fringepatternimages. IEEETrans.Biomed.Eng. 2000, 47,249–258.[CrossRef]
69. Hwang,H.S.;Kim,E.C.;Kim,M.S.NovelTearInterferometerMadeofPaperforLipidLayerEvaluation. Cornea 2014, 33,826–831. [CrossRef]
70. Goto,E.;Tseng,S.C.G.Kineticanalysisoftearinterferenceimagesinaqueousteardeficiencydryeyebeforeandafterpunctal occlusion. Investig.Opthalmol.Vis.Sci. 2003, 44,1897–1905.[CrossRef]
71. Eom,Y.;Lee,J.-S.;Kang,S.-Y.;Kim,H.M.;Song,J.-S.CorrelationBetweenQuantitativeMeasurementsofTearFilmLipidLayer ThicknessandMeibomianGlandLossinPatientsWithObstructiveMeibomianGlandDysfunctionandNormalControls. Am.J. Ophthalmol. 2013, 155,1104–1110.e2.[CrossRef][PubMed]
72. Cho,B.-J.;Jee,D.H.;Kim,W.J.;Shin,M.C.;Kim,E.C.;Kim,M.S.;Hwang,H.S.DirectVisualizationofContinuousMeibum SecretionFromtheOrificesofMeibomianGlandstotheTearFilm. Cornea 2019, 38,1245–1252.[CrossRef]
73. Molina-Solana,P.;Domínguez-Serrano,F.D.B.;Garrido-Hermosilla,A.M.;Montero-Iruzubieta,J.;Fernández-Palacín,A.; Rodríguez-De-La-Rúa-Franch,E.;Caro-Magdaleno,M.ImprovedTearFilmStabilityinPatientswithDryEyeAfterHyaluronic AcidandGalactoxyloglucanUse. Clin.Ophthalmol. 2020, 14,1153–1159.[CrossRef][PubMed]
74. Lee,J.M.;Jeon,Y.J.;Kim,K.Y.;Hwang,K.-Y.;Kwon,Y.-A.;Koh,K.Ocularsurfaceanalysis:AcomparisonbetweentheLipiView® IIandIDRA® . Eur.J.Ophthalmol. 2020.[CrossRef][PubMed]
75. Giannaccare,G.;Vigo,L.;Pellegrini,M.;Sebastiani,S.;Carones,F.OcularSurfaceWorkupWithAutomatedNoninvasive MeasurementsfortheDiagnosisofMeibomianGlandDysfunction. Cornea 2018, 37,740–745.[CrossRef]
76. Yokoi,N.;Mossa,F.;Tiffany,J.M.;Bron,A.J.Assessmentofmeibomianglandfunctionindryeyeusingmeibometry. Arch. Ophthalmol. 1999, 117,723–729.[CrossRef][PubMed]
77. Goto,E.;Dogru,M.;Kojima,T.;Tsubota,K.Computer-synthesisofaninterferencecolorchartofhumantearlipidlayer,bya colorimetricapproach. Investig.Opthalmol.Vis.Sci. 2003, 44,4693–4697.[CrossRef]
78. Goto,E.DifferentiationofLipidTearDeficiencyDryEyebyKineticAnalysisofTearInterferenceImages. Arch.Ophthalmol. 2003, 121,173–180.[CrossRef]
79. Arita,R.;Yabusaki,K.;Hirono,T.;Yamauchi,T.;Ichihashi,T.;Fukuoka,S.;Morishige,N.AutomatedMeasurementofTear MeniscusHeightwiththeKowaDR-1α TearInterferometerinBothHealthySubjectsandDryEyePatients. Investig.Opthalmol. Vis.Sci. 2019, 60,2092–2101.[CrossRef]
80. Arita,R.;Morishige,N.;Fujii,T.;Fukuoka,S.;Chung,J.L.;Seo,K.Y.;Itoh,K.TearInterferometricPatternsReflectClinicalTear DynamicsinDryEyePatients. Investig.Opthalmol.Vis.Sci. 2016, 57,3928.[CrossRef]
81. Lee,Y.;Hyon,J.Y.;Jeon,H.S.CharacteristicsofdryeyepatientswiththicktearfilmlipidlayersevaluatedbyaLipiViewII interferometer. Graefe’sArch.Clin.Exp.Ophthalmol. 2021, 259,1235–1241.[CrossRef]
82. Weng,H.-Y.;Ho,W.-T.;Chiu,C.-Y.;Tsai,T.-Y.;Chang,S.-W.Characteristicsoftearfilmlipidlayerinyoungdryeyepatients. J.Formos.Med.Assoc. 2021, 120,1478–1484.[CrossRef][PubMed]
83. Purslow,C.;Wolffsohn,J.TheRelationbetweenPhysicalPropertiesoftheAnteriorEyeandOcularSurfaceTemperature. Optom. Vis.Sci. 2007, 84,197–201.[CrossRef]
84. Craig,J.P.;Singh,I.P.;Tomlinson,A.;Morgan,P.;Efron,N.Theroleoftearphysiologyinocularsurfacetemperature. Eye 2000, 14, 635–641.[CrossRef][PubMed]
85. Versura,P.;Giannaccare,G.;Fresina,M.;Campos,E.C.SubjectiveDiscomfortSymptomsAreRelatedtoLowCornealTemperature inPatientswithEvaporativeDryEye. Cornea 2015, 34,1079–1085.[CrossRef]
86. Morgan,P.;Tullo,A.B.;Efron,N.Infraredthermographyofthetearfilmindryeye. Eye 1995, 9,615–618.[CrossRef]
87. Kamao,T.;Yamaguchi,M.;Kawasaki,S.;Mizoue,S.;Shiraishi,A.;Ohashi,Y.ScreeningforDryEyeWithNewlyDeveloped OcularSurfaceThermographer. Am.J.Ophthalmol. 2011, 151,782–791.e1.[CrossRef][PubMed]
88. Su,T.-Y.;Chang,S.-W.;Yang,C.-J.;Chiang,H.K.Directobservationandvalidationoffluoresceintearfilmbreak-uppatternsby usingadualthermal-fluorescentimagingsystem. Biomed.Opt.Express 2014, 5,2614–2619.[CrossRef]
89. Baudouin,C.;Barton,K.;Cucherat,M.;Traverso,C.TheMeasurementofBulbarHyperemia:ChallengesandPitfalls. Eur.J. Ophthalmol. 2015, 25,273–279.[CrossRef]
90. Leibowitz,H.M.TheRedEye. N.Engl.J.Med. 2000, 343,345–351.[CrossRef]
91. Schuman,J.S.Short-andlong-termsafetyofglaucomadrugs. ExpertOpin.DrugSaf. 2002, 1,181–194.[CrossRef]
92. Giannaccare,G.;Pellegrini,M.;Bernabei,F.;Senni,C.;Aloi,M.;Scalzo,G.C.;Ceravolo,D.;Iovino,C.;Scorcia,V.Comparative analysisofocularrednessscoreevaluatedautomaticallyinglaucomapatientsunderdifferenttopicalmedications. Eur.J. Ophthalmol. 2020.[CrossRef]
93. Giannaccare,G.;Blalock,W.;Fresina,M.;Vagge,A.;Versura,P.Intolerantcontactlenswearersexhibitocularsurfaceimpairment despite3monthsweardiscontinuation. GraefesArch.Clin.Exp.Ophthalmol. 2016, 254,1825–1831.[CrossRef]
94. Vagge,A.;Senni,C.;Bernabei,F.;Pellegrini,M.;Scorcia,V.;ETraverso,C.;Giannaccare,G.TherapeuticEffectsofLactoferrinin OcularDiseases:FromDryEyeDiseasetoInfections. Int.J.Mol.Sci. 2020, 21,6668.[CrossRef][PubMed]
95. Roda,M.;Corazza,I.;Reggiani,M.L.B.;Pellegrini,M.;Taroni,L.;Giannaccare,G.;Versura,P.DryEyeDiseaseandTearCytokine Levels—AMeta-Analysis. Int.J.Mol.Sci. 2020, 21,3111.[CrossRef]
96. Schulze,M.;Jones,D.;Simpson,T.L.TheDevelopmentofValidatedBulbarRednessGradingScales. Optom.Vis.Sci. 2007, 84, 976–983.[CrossRef]
97. Park,I.K.;Chun,Y.S.;Kim,K.G.;Yang,H.K.;Hwang,J.-M.NewClinicalGradingScalesandObjectiveMeasurementfor ConjunctivalInjection. Investig.Opthalmol.Vis.Sci. 2013, 54,5249–5257.[CrossRef][PubMed]
98. Singh,R.B.;Liu,L.;Anchouche,S.;Yung,A.;Mittal,S.K.;Blanco,T.;Dohlman,T.H.;Yin,J.;Dana,R.Ocularredness—I:Etiology, pathogenesis,andassessmentofconjunctivalhyperemia. Ocul.Surf. 2021, 21,134–144.[CrossRef][PubMed]
99. Villumsen,J.;Ringquist,J.;Alm,A.Imageanalysisofconjunctivalhyperemia. ActaOphthalmol. 2009, 69,536–539.[CrossRef]
100. Willingham,F.F.;Cohen,K.L.;Coggins,J.M.;Tripoli,N.K.;Ogle,J.W.;Goldstein,G.M.Automaticquantitativemeasurementof ocularhyperemia. Curr.EyeRes. 1995, 14,1101–1108.[CrossRef]
101. Rodriguez,J.D.;Johnston,P.R.;Iii,G.W.O.;Abelson,M.B.;Smith,L.M.Automatedgradingsystemforevaluationofocular rednessassociatedwithdryeye. Clin.Ophthalmol. 2013, 7,1197–1204.[CrossRef]
102. Efron,N.;Morgan,P.B.;Katsara,S.S.Validationofgradingscalesforcontactlenscomplications. OphthalmicPhysiol.Opt. 2001, 21, 17–29.
103. Wu,S.;Hong,J.;Tian,L.;Cui,X.;Sun,X.;Xu,J.AssessmentofBulbarRednesswithaNewlyDevelopedKeratograph. Optom.Vis. Sci. 2015, 92,892–899.[CrossRef]
104. Roy,N.S.;Wei,Y.;Kuklinski,E.;Asbell,P.A.TheGrowingNeedforValidatedBiomarkersandEndpointsforDryEyeClinical Research. Investig.Opthalmol.Vis.Sci. 2017, 58,BIO1–BIO19.[CrossRef]
105. Rajalakshmi,R.;Dutt,S.;Sivaraman,A.;Savoy,F.Insightsintothegrowingpopularityofartificialintelligenceinophthalmology. IndianJ.Ophthalmol. 2020, 68,1339–1346.[CrossRef]
106. Yedidya,T.;Hartley,R.;Guillon,J.-P.;Kanagasingam,Y.AutomaticDryEyeDetection.In MedicalImageComputingandComputerAssistedIntervention–MICCAI2007;Springer:Brisbane,Australia,2007;pp.792–799.
107. Ramos,L.;Barreira,N.;Mosquera,A.;Currás,M.;Pena-Verdeal,H.;Giráldez,M.J.;Penedo,M.G.ComputationalApproachfor MeasuringtheTearFilmBreak-UpTimeinanUnsupervisedManner. Commun.Netw. 2013,254–267.
108. Krizhevsky,A.;Sutskever,I.;Hinton,G.E.ImageNetclassificationwithdeepconvolutionalneuralnetworks. Adv.NeuralInf. Process.Syst. 2012, 25,1097–1105.Availableonline: https://proceedings.neurips.cc/paper/2012/file/c399862d3b9d6b76c8436e9 24a68c45b-Paper.pdf (accessedon14July2021).
109. Litjens,G.;Kooi,T.;Bejnordi,B.E.;Setio,A.A.A.;Ciompi,F.;Ghafoorian,M.;vanderLaak,J.A.;vanGinneken,B.;Sánchez,C.I.A surveyondeeplearninginmedicalimageanalysis. Med.ImageAnal. 2017, 42,60–88.[CrossRef][PubMed]
110. Su,T.-Y.;Liu,Z.-Y.;Chen,D.-Y.TearFilmBreak-UpTimeMeasurementUsingDeepConvolutionalNeuralNetworksforScreening DryEyeDisease. IEEESens.J. 2018, 18,6857–6862.[CrossRef]
111. Remeseiro,B.;Mosquera,A.;Penedo,M.G.;García-Resúa,C.TearFilmMapsbasedontheLipidInterferencePatterns.In ProceedingsoftheInternationalConferenceonAgentsandArtificialIntelligence,Angers,France,6–8March2014;SCITEPRESS: Setúbal,Portugal,2014;Volume1,pp.732–739.
112. Guillon,J.-P.Non-invasivetearscopeplusroutineforcontactlensfitting. ContactLensAnteriorEye 1998, 21,S31–S40.[CrossRef]
113. Remeseiro,B.;Barreira,N.;García-Resúa,C.;Lira,M.;Giráldez,M.J.;Yebra-Pimentel,E.;Penedo,M.G.iDEAS:Aweb-based systemfordryeyeassessment. Comput.MethodsProgramsBiomed. 2016, 130,186–197.[CrossRef][PubMed]
114. Acharya,U.R.;Tan,J.H.;Koh,J.E.;Sudarshan,V.K.;Yeo,S.;Too,C.L.;Chua,C.K.;Ng,E.;Tong,L.Automateddiagnosisofdry eyeusinginfraredthermographyimages. InfraredPhys.Technol. 2015, 71,263–271.[CrossRef]
115. Giannaccare,G.;Vaccaro,S.;Mancini,A.;Scorcia,V.DryeyeintheCOVID-19era:Howthemeasuresforcontrollingpandemic mightharmocularsurface. Graefe’sArch.Clin.Exp.Ophthalmol. 2020, 258,2567–2568.[CrossRef][PubMed]
IntOphthalmol(2021)41:2601–2608
Relationshipbetweenthepartialblinkrateandocular surfaceparameters
YoungJoonJeon . MiYeonSong . KookYoungKim . Kyu-YeonHwang . Young-A.Kwon . KyungminKoh
Received:12September2020/Accepted:16March2021/Publishedonline:25March2021
TheAuthor(s),underexclusivelicencetoSpringerNatureB.V.2021
Abstract
Purpose Toelucidatetherelationshipbetweenpartialblinkrate(PBR)andocularsurfaceparameters.
Methods Weconductedaretrospectivecross-sectionalstudyofthepatientswhovisitedtheKim’sEye HospitalbetweenMarch2020andJuly2020.Blinking dynamics,tearfilmlipidlayerthickness(LLT),noninvasivetearfilmbreak-uptime(NITBUT),tear meniscusheight(TMH),andmeibomiangland(MG) dropoutratewereassessedusingtheIDRA Ocular surfaceanalyzer(SBMSISTEMI,Inc.,Torino,Italy) (IDRA).Dryeyesymptomswerequantifiedbythe StandardPatientEvaluationofEyeDryness(SPEED) questionnairescores.
Results Atotalof47non-Sjogrendryeyepatients (47righteyes,meanage=56.8 ± 14.5[20–75] years,66%female)wereenrolled.Amongtheocular surfaceparameters,PBRhadstrongcorrelationswith MGdropoutrate(r =0.811; p \ 0.01)andmoderate correlationswithSPEEDscores(r =0.596; p \ 0.01).MGdropoutrateandagehadmoderate correlationswithSPEEDscores(r =0.416; p =0.04 and r =0.322; p =0.03,respectively).Comparisons
Y.J.Jeon M.Y.Song K.Y.Kim K.-Y.Hwang Y.-A.Kwon K.Koh(&) DepartmentofOphthalmology,Kim’sEyeHospital, KonyangUniversityCollegeofMedicine,136 Youngshinro,Youngdeungpo-gu,Seoul07301,Republic ofKorea
e-mail:kmkoh@kimeye.com
bysexrevealednosignificantdifferenceincharacteristics,exceptfortheTMHvalue.TheTMHoffemale patients(0.17 ± 0.07)wassignificantlyhigherthan thatofmales(0.14 ± 0.04; p =0.04).LLTshowedno significantcorrelationwiththeothervariables.
Conclusion PBRandMGdropoutratesmeasured withIDRAweresignificantlycorrelatedtodryeye symptoms.
Keyword Blink IDRA Interferometry Lipid layer Meibomianglanddysfunction
Introduction
Dryeyedisease(DED)isamultifactorialsyndrome relatedtoimpairedtearfilmhomeostasisandocular surfaceinflammation[1].Becauseofthelarge discrepancybetweenapatient’ssymptoms,the observedsigns,andthedryeyesymptomsoverlapping withthoseofotherocularsurfacediseases,diagnosing DEDisdifficultinpractice[2].Therefore,anew diagnosticdeviceusingtechnologiessuchasinterferometryandmeibographyisdesirable.
Thetearfilmconsistsoftwophases,alipidlayer overlyingamuco-aqueouslayer[3].Tearlipids secretedfromthemeibomianglands(MG)aidsthe tearfilmmaintainingitssolidity[4].Meibomiangland dysfunction(MGD),themaincauseofevaporativedry
eye,ismorefrequentthanaqueous-deficientdryeye [5].TheevaluationofboththefunctionandmorphologyofMGisessentialforthediagnosisofMGD[6]. Meibographycandetectthemorphologicalalterations ofMG,whereastearinterferometrypermitsassessmentsofthelipidlayerofthetearfilm.Such assessmentsofMGmorphologyprovideclinical evidencecontributingtothediagnosisofevaporative dryeye,whereasthatofthelipidlayerofthetearfilm allowsthemonitoringofMGfunction[7].Blinking canretainadynamicbalanceofocularsurfacetear capacityandisimportantforthedevelopmentand distributionofthelipidlayer[8, 9].
VariousinstrumentsevaluatingDEDarecurrently beingused[10].Themostuseddeviceisthe LipiView IIocularsurfaceinterferometer(TearScienceInc.,Morrisville,NC,USA)(LVII),launched in2011.ItprovidesanabsoluteLLT,apartialblink rate(PBR),andanimagingoftheMG.Similartothe LVII,IDRA Ocularsurfaceanalyzer(SBMSISTEMI,Inc.,Torino,Italy)(IDRA),whichwasreleased in2018,providesLLT,MGdropouts,non-invasive tearbreak-uptime(NITBUT),tearmeniscusheight (TMH),andblinkingpattern[11].
TherewasnosignificantdifferencebetweenLVII andIDRAinLLTmeasurements,PBRwasmeasured higherinIDRAthanLVII[11].Apaperhasbeen reportedusingLVIItoinvestigatetherelationship betweenpartialblinkingandocularsurfaceparametersrelatedwithDED[12],butnoresearchhas identifiedthecorrelationofPBRtootherdryeye parametersmeasuredusingIDRA.
Thisstudyaimedtoelucidatetherelationship betweenblinkingpatternandocularsurfaceparametersbyIDRAinDEDpatients.
Materialsandmethods
Weconductedaretrospectivecross-sectionalstudyof thepatientswhovisitedtheKim’sEyeHospital betweenMarch2020andJuly2020whenthecoronavirusdisease2019(COVID-19)pandemicwas raging.ByMay2020,thereweremorethan4million confirmedCOVID-19casesworldwide,withnearly 300,000deaths[13].ThereisahighriskoftransmissionofCOVID-19becauseophthalmologicalpractice duringbothslitlampexaminationanddirectophthalmoscopyareconductedclosetothepatient’sface.
Therefore,ourhospitalmadethorougheffortsto preventinfection.Allpatientsfilledoutquestionnaires onfever,respiratorysymptoms,andwhetherthey visitedothercountrieswithin14days.Inaddition,a mandatorynon-contacttemperaturecheckwasconductedatthehospitalentrance.Allpatientsand hospitalstaffwererequiredtowearmasksthroughout theirstayinthehospital.Hospitalstaffincluding doctors,nurses,andexaminerswashedtheirhands usingwaterlesshandsanitizerbeforeandaftercontact withpatients.Participantsinthestudy,whovisitedthe hospitalasanoutpatient,didnottaketheCOVID-19 testbecauseonlyhospitalizedpatientsweretested.
Thisstudyreceivedethicalapprovalfromthe InstitutionalReviewBoardatKim’sEyeHospital, Seoul,RepublicofKorea(2020–08-001)andadhered tothetenetsoftheDeclarationofHelsinki.Consideringtheretrospectivenatureofthestudyandtheuse ofdeidentifiedpatientdata,thewritteninformed consentwaswaivedbytheInstitutionalReviewBoard ofKim’sEyeHospital,Seoul,RepublicofKorea.Data ofblinkingdynamics,tearfilmLLT,NITBUT,and MGdropoutrateswereassessedusingIDRA.
Inclusioncriteriawereadultpatientsfrom20to 80yearsoldwhometthefollowingtwocriteria: StandardPatientEvaluationofEyeDryness(SPEED) C 6pointsandNITBUT \ 10s.Patientswere excludedfromthestudyiftheywerediagnosedwith supra-nuclearmotorweaknessinfluencingmovements oftheeyelid,ptosis,pre-existingocularpathology, historyofocularsurgeryortrauma,historyofuseof anysystemicmedications,withsystemicautoimmune orconnectivetissuediseaseincludingSjo ¨ grensyndrome,hadahistoryofusingapunctualplug,previous eyeinfection,andcontactlensusage.Asingle investigator(JYJ)handledtheIDRAthroughoutthe study.Testvaluesofonlytherighteyewereevaluated.
Dryeyequestionnaire
DEDsymptomswereevaluatedusingtheSPEED validatedquestionnaire(0–28)[14, 15].Asingle ophthalmologist(KK)diagnosedDEDbasedonthe TearFilmandOcularSurfaceSocietyDryEye WorkshopIIcriteria,withSPEED C 6points[16]. ApreviousstudyshowedthattheSPEEDquestionnairewascompatiblewiththeOcularSurfaceDisease Index(OSDI)[17].

oldmalepatienttakenwiththeIDRA . b Comparedtothe imageofLipiView II,therearesmallerareasthatcanbe
identifiedbyinterferometry.Inthispatient,theLLTvalueswere measuredbyIDRA asfollows:average68nm,maximum 85nm,andminimum58nm

imageshowsmeibomiangland(MG)dropout of40yearsoldmalepatient.Thelowereyelidswereturnedover andMGswereobservedusinganinfraredtransmittingfilter, meibomianglandsareapparentasareasofhighreflectivity. IDRA automaticallycalculatesthedropoutrateofMGs,and thispatientcameoutat45%.AsIDRA calculatesthe
Interferometricassessmentoflipidlayerthickness
TheIDRA,releasedin2018,isanoveldeviceforthe specificevaluationoftearfilmpermittingarapid detailedstructuralanalysisofthetearcomponents. Thedevicecananalyzealllayersofthetearfilmand MG,allowingclinicianstodeterminethecomponents tobetreated,accordingtothetypeofinsufficiency.
IDRAperformsanon-invasivetestforapproximately 5min,anditshouldbeincorporatedbetweenaslit lampandbiomicroscope.Itspinshavebeenbuilttofit completelyintothehole,foundwhentheplateusedfor thetonometeriseliminated.Theparticipantssat comfortablyusingachinholderandwereaskedto lookatthecamerawithnaturalblinkingoftheireyes. Videoscanberecordedforafewseconds,withthe adjustmentoftherecordingtimeaccordingtothe convenienceoftheclinician.Inthisstudy,itwas recordedfor20stoproceed.Thedeviceprojects whitelightoverthecornea,andthelightreflectedfrom thetearfilmcanbeobservedasawhitefan-shaped
remainingpartofmeibomianglands,sotosubtractthedropout ratio,itwaswrittenasthevaluesubtractedfrom100. b IDRA imageaboutblinkingpatternof72-year-oldfemalepatient.It showsapartialblink2timesper20s,totalblink7timesper 20s,andpartial/totalblinkratio0.29
areacoveringthelowerthirdofthecornea.The automaticinterferometryIDRAtestdetectedthe interferenceofcolorsfromthelipidlayeronthetear film.Itdeterminedtheaverage,maximum,and minimumLLTusingtheinternationalgradescale withthethicknessesrelatedtoeachgradeofthelipid layerpattern(Fig. 1)[18, 19].Dependingonthe patterns,thegradeswereconvertedtonanometersand couldbeclassifiedbetween15and100nm.Like LVII,IDRAhasanuppercutoffof100nm[16].
MGdropoutmeasurement
TheLVIIonlyproducesMGdropoutresultsonthe image.Incontrast,IDRAcalculatedtheMGdropout rateoflowerlidintheresultingitem(Fig. 2a).The dropoutratewascalculatedasapercentageby dividingthenon-glandularzonebythetotalvisible areaofthelowerlid[20].
Fig.1a Imageaboutlipidlayerthickness(LLT)of58years Fig.2a IDRASPEED,standardpatientevaluationofeyedrynessvalidated questionnaire(0–28); NITBUT, Non-invasivetearbreak-up time; TMH,Tearmeniscusheight; nm,nanometer; Numberof partialblinks,numberofpartialblinksper20s; Numberof completeblinks,numberoftotalblinksminuspartialblinks; Numberoftotalblinks, numberoftotalblinksper20s; MGD meibomianglanddysfunction
Dataarepresentedasthemean ± SD
Blinkingpatternmeasurement
Participantswereaskedtoblinkfreely.IDRAautomaticallydetectsandanalyzesblinkrateandquality usingrecordedvideo(Fig. 2b).Itisnumerically displayedthenumberofcompleteandpartialblinks andblinkfrequencies.Eachstageintheblinkingcycle wasmeasuredandrecordedduringtheexamination (Fig. 2B),andthepartialblinksweredefinedasblinks withouttouchingtheupperandlowereyelids[12].
NITBUTandTMH
IDRAcandetermineNITBUTbyusingtheprojected ringpatternsfromaPlacido’sdiskontothecornea. NITBUTevaluatesthestabilityofthetearfilmby measuringthetimefromthefullblinktothepresence
c Fig.3a Pearsoncorrelationscatterplotforpartialblinkingrate andmeibomianglanddropoutrate.Itshowedsignificant correlations(Spearman’s r =0.811, p \ 0.01). b PearsoncorrelationscatterplotforpartialblinkingrateandStandardPatient EvaluationofEyeDryness(SPEED)questionnairescores.It showedsignificantcorrelations(Spearman’s r =0.596, p \ 0.01). c PearsoncorrelationscatterplotforSPEEDscores andmeibomianglanddropoutrate.Itshowedsignificant correlations(Spearman’s r =0.416, p =0.04)
ofthefirstdisruptionofthereflectedimageonthe corneainseconds.Participantswererequiredtoblink twiceandthenkeeptheireyesopenaslongas possible.IDRAcanalsomeasureTMH,whichcanbe recordednon-non-contactinvasivelyinaflashby takingaphotograph.Thedevicecandetecttheupper andlowertearmeniscusandevaluatetheTMHalong thelowerlidmargininthephotograph.
Statisticalanalysis
DatawereanalyzedwiththeIBMStatisticalPackage fortheSocialSciences(SPSS)version22(Chicago, USA),andapvalueof \ 0.05wasconsidered statisticallysignificant.Continuousvariableswere presentedasmeans ± standarddeviation.Statistical analyseswereperformedusinglinearregression analysis,Pearsoncorrelationanalysis,andStudent’s ttests.Pearsoncorrelationanalysisandlinearregressionanalysiswereusedtoevaluatethecorrelation betweentheSPEEDscoresandocularsurfacedata obtainedbyIDRA.Correlationsofeachvaluewere analyzedwiththePearsoncorrelationcoefficient(r). Student’sttestswereusedtocompareocularsurface databetweenmalesandfemales.
Results
Fifty-sevenpatientswithdryeyewererecruited. Amongthem,10patientswithLLT C 100nmwere excluded.Thiscouldnotbepreciselycalculatedas IDRAhasanuppercutoffof100nm.Wefinally enrolled16menand31women(meanage=56.8 ± 14.5[21–79]years)inourstudy.Meanage,spherical equivalent,non-contacttonometrymeasurements,and centralcornealthicknessarelistedinTable 1.Comparisonsbysexrevealednosignificantdifferencein characteristics,exceptfortheTMHvalue.TheTMH

offemalepatients(0.17 ± 0.07)wassignificantly higherthanthatofmales(0.14 ± 0.04)(p =0.041). Lookingattheclinicaldryeyeparametersofthe patients,theaverageSPEEDscorewas12.53 ± 5.33 points,averageNITBUTmeasuredbyIDRAwas 8.22 ± 1.23s,andTMHwas0.16 ± 0.06nm.The meanvalueofaverageLLTmeasuredwas 75.45 ± 12.19nm,andmeanvalueofmaximum LLTwas85.53 ± 13.33nm,andmeanvalueof minimumLLTwas66.11 ± 13.99nm.Themean valuesofblinkdynamicsareshowninTable 1.
TherewasnosignificantrelationshipbetweenLLT andSPEEDortheMGdropoutrate.Amongtheocular surfaceparameters,PBRhadstrongcorrelationswith theMGdropoutrate(r =0.811; p \ 0.01)and moderatecorrelationswithSPEEDscores (r =0.596; p \ 0.01)(Fig. 3aand3b).TheMG dropoutratehadmoderatecorrelationswithSPEED scores(r =0.416; p =0.04)(Fig. 3c).Theresultsof correlationsbetweenocularsurfaceparameters, SPEEDquestionnaires,andblinkingparametersare summarizedinTable 2
Discussion
Inourstudy,PBRwascorrelatedwiththeMGdropout rateandSPEEDscore.TheMGdropoutratecorrelatedwiththeSPEEDscore.Itisthefirststudyto assesstherelationshipbetweenblinkingpatternsand ocularsurfaceparametersmeasuredbyIDRA.Many aspects,suchasocularsurfacedisorders,psychologicalstatus,andsystemicdiseases,mayaffectthe blinkingrate[21].Blinkingservesavitalfunctionin preservingmoistureandunityofthelipidlayer’s ocularsurfaceandextendingthetearlipids[22]. Inadequatelipiddistributionmayoccur,whichmay increaseevaporationwithariseinpartialblinking [23].ThisisassociatedwithTBUTandMGD, possiblythroughitscontributiontoMGobstruction andsubsequentlossoftearfilmhomeostasis[12]. Rightblinkingandstabilityoftearfilmarecritical elementsintheprotectionoftheocularsurface[23].
Previousstudieshaveshownthatthevolumeofthe lipidlayeriscorrelatedtothenumberandfunctionof MGs[24].LLTandPBRaresignificantlyrelatedto DEDsymptoms[23].WhencomparingforLLT,the fieldofthewhitelightprojectedtocauseinterference ofcolorsisdirectedatthelowerthirdofthecornea,
Table2 CorrelationbetweenSPEEDquestionnairescoresand ocularsurfaceparametersmeasuredbyIDRA Ocularsurface analyzer(SBMSISTEMI,Inc.,Torino,Italy)
ParametersVersusSPEEDVersusMGDR rprp
Age 0.3220.031*0.1210.438
TMH 0.2540.0940.1290.418
NITBUT0.0920.559-0.0130.958
AverageLLT0.2230.1440.0720.659
MaximumLLT0.1340.3780.0560.737
MinimumLLT0.1210.4360.0510.762
Partialblinks0.542 \ 0.01*0.627 \ 0.01*
Completeblinks-0.1920.192 - 0.442 \ 0.01*
Totalblinks0.2040.1690.0930.546
Partialblinkrate0.596 \ 0.01*0.811 \ 0.01*
MGDR 0.4160.04*
NITBUT, Non-invasivetearbreak-uptime; TMH, Tear meniscusheight; nm, nanometer; Partialblinks, numberof partialblinksper20s; Numberofcompleteblinks,numberof totalblinksminuspartialblinks; Numberoftotalblinks, numberoftotalblinksper20s; LLT,lipidlayerthickness; SPEED,standardpatientevaluationofeyedrynessvalidated questionnaire(0–28)
r,Pearsoncorrelationcoefficient, - 1 B r B 1.The correlationwasstatisticallyanalyzedbylinearregression *Indicatestatisticallysignificantassociation(p \ 0.05). MGDR meibomianglanddropoutrate
approximately1mmabovetheinferiortearmeniscus, inLVII[16],whileitisdirectedslightlyhigher, approximately2mmabovetheinferiortearmeniscus, inIDRA(Fig. 1).
AstudyinDEDpatientsshowedthatincreasedage waspositivelyassociatedwithLLT[25].Inaretrospectiveanalysisof153patients,SPEEDvalues showedasignificantcorrelationwithageandLLT [15].Likethisstudy,SPEEDscoreswerecorrelated withage.However,therewasnostatisticallysignificantcorrelationbetweenSPEEDvaluesandLLTin ourstudy.Thismightbebecauseofthesmallnumber ofparticipantsinourstudyorbecauseofthecharacteristicsofIDRA.
Figure 2 showstheMGdropoutdisplayofanMGD patient.ThelowereyelidswereturnedoverandMGs wereobservedusinganinfraredtransmittingfilter, whileMGswereapparentasareasofhighreflectivity. IDRAautomaticallycalculatesthedropoutrateof MGs.AsIDRAcalculatestheremainingpartofthe
MGstosubtractthedropoutratio,itwaswrittenasthe valuesubtractedfrom100.IDRArecognizesalterationsofthelightintensityacrossthesurfaceand compensatesforthevariationsinlidthickness betweenparticipantsthroughtheilluminationattached tothelideverter,knownastheadaptivetransillumination.Thistransilluminationalsoformsshadowson areaswhereMGsarelocated;thus,anyglandslocated belowthevisibleexteriororatthesuboptimal positionsmaybeseenwiththeillumination.
IDRAhastheadvantageofbeingabletoconduct testsforvariousocularsurfaceparameterswithone equipment.ThemeasurementofLLTthroughautointerferometryalongwiththatofTMH,auto-NIBUT, blinkingquality,meibography,andbulbarredness classificationisfeasible.Inaddition,white-to-white measurementandpupillometryarepossiblewiththis device.
Thereareseverallimitationsininterpretingthe resultsofourstudy.First,therelativelysmallnumber ofparticipantsincludedinthestudymayhave influencedtheresults.Second,thestudysubjectswere homogenous,consistingonlyoftheKoreanpopulation.Futurestudiesneededtoverifywhetherother raceshavethesameresultsinalargernumberof participants.Despitethelimitations,webelievethat ourresultsshowedaclearcorrelationbetweenocular surfaceparametersoftheIDRA.
Inconclusion,PBRwascorrelatedwiththeMG dropoutrateandSPEEDscore.TheMGdropoutrate correlatedwiththeSPEEDscore.IDRAhasthe advantageofbeingabletoconducttestsforvarious ocularsurfaceparameterswithoneequipment.
Acknowledgements ThisstudywassupportedbytheKim’s EyeHospitalResearchCenter.
Authors’contribution Allauthorsattestthattheymeetthe currentICMJEcriteriaorAuthorship.JYJandKKplannedthe clinicalstudy,contributedtotheconceptionanddesignofthe study,andtheacquisition,analysis,andinterpretationofthe data.MYSandYAKcontributedtotheanalysisand interpretationofthedata.KYK,KYH,andKKcontributedto theconceptionanddesignofthestudy,analysisofdata,the draftingofthemanuscriptanditscriticalrevisionforimportant intellectualcontent.Allauthorsreadandapprovedthefinal versionofthemanuscriptandagreedtobeaccountableforall aspectsofthestudy.
Funding Theauthorshavenofundingreceivedforthiswork todisclose.
Dataavailabilityandmaterials Moredataifnecessaryare availablefromthecorrespondingauthoronreasonablerequest.
Declarations
Conflictofinterest Nopotentialconflictofinterestrelevant tothisarticlewasreported.
Ethicalapproval Allproceduresperformedinstudies involvinghumanparticipantswereinaccordancewiththeethicalstandardsoftheinstitutionaland/ornationalresearch committeeandwiththe1964Helsinkideclarationanditslater amendmentsorcomparableethicalstandards.Thestudywas approvedbytheInstitutionalReviewBoardofKim’sEye Hospital,Seoul,RepublicofKorea(2020-08-001).
Informedconsent Consideringtheretrospectivenatureofthe studyandtheuseofdeidentifiedpatientdata,thewritten informedconsentwaswaivedbytheInstitutionalReviewBoard ofKim’sEyeHospital,Seoul,RepublicofKorea.
References
1.CraigJP,NicholsKK,AkpekEK,CafferyB,DuaHS,Joo CK,LiuZ,NelsonJD,NicholsJJ,TsubotaK,StapletonF (2017)TFOSDEWSIIdefinitionandclassificationreport. OculSurf15(3):276–283. https://doi.org/10.1016/j.jtos. 2017.05.008
2.HyonJY,KimHM,LeeD,ChungES,SongJS,ChoiCY, LeeJ(2014)Koreanguidelinesforthediagnosisand managementofdryeye:developmentandvalidationof clinicalefficacy.KoreanJOphthalmol:KJO 28(3):197–206. https://doi.org/10.3341/kjo.2014.28.3.197
3.CraigJP,NelsonJD,AzarDT,BelmonteC,BronAJ, ChauhanSK,dePaivaCS,GomesJAP,HammittKM,Jones L,NicholsJJ,NicholsKK,NovackGD,StapletonFJ, WillcoxMDP,WolffsohnJS,SullivanDA(2017)TFOS DEWSIIreportexecutivesummary.OculSurf 15(4):802–812. https://doi.org/10.1016/j.jtos.2017.08.003
4.LeeJH,KimCH,ChoeCM,ChoiTH(2020)Correlation analysisbetweenocularsurfaceparameterswithsubjective symptomseverityindryeyedisease.KoreanJOphthalmol: KJO34(3):203–209. https://doi.org/10.3341/kjo.2019.0133
5.NicholsKK,FoulksGN,BronAJ,GlasgowBJ,DogruM, TsubotaK,LempMA,SullivanDA(2011)Theinternationalworkshoponmeibomianglanddysfunction:executivesummary.InvestOphthalmolVisSci52(4):1922–1929. https://doi.org/10.1167/iovs.10-6997a
6.AritaR,FukuokaS,MorishigeN(2017)Newinsightsinto themorphologyandfunctionofmeibomianglands.ExpEye Res163:64–71. https://doi.org/10.1016/j.exer.2017.06.010
7.AritaR,FukuokaS,MorishigeN(2017)Functionalmorphologyofthelipidlayerofthetearfilm.Cornea36(Suppl 1):S60-s66. https://doi.org/10.1097/ico.0000000000001367
8.HuangJ,HindmanHB,RollandJP(2016)Invivothickness dynamicsmeasurementoftearfilmlipidandaqueouslayers withopticalcoherencetomographyandmaximum-likelihoodestimation.OptLett41(9):1981–1984. https://doi.org/ 10.1364/ol.41.001981
9.BandlitzS,PurslowC,MurphyPJ,PultH(2014)Time courseofchangesintearmeniscusradiusandblinkrate afterinstillationofartificialtears.InvestOphthalmolVis Sci55(9):5842–5847. https://doi.org/10.1167/iovs.1414844
10.AbdelfattahNS,DastiridouA,SaddaSR,LeeOL(2015) Noninvasiveimagingoftearfilmdynamicsineyeswith ocularsurfacedisease.Cornea34(Suppl10):S48-52. https:// doi.org/10.1097/ico.0000000000000570
11.LeeJM,JeonYJ,KimKY,HwangKY,KwonYA,KohK (2020)Ocularsurfaceanalysis:Acomparisonbetweenthe LipiView( )IIandIDRA( ).EurJOphthalmol. https:// doi.org/10.1177/1120672120969035
12.JieY,SellaR,FengJ,GomezML,AfshariNA(2019) Evaluationofincompleteblinkingasameasurementofdry eyedisease.OculSurf17(3):440–446. https://doi.org/10. 1016/j.jtos.2019.05.007
13.NguyJ,HitchenSA,HortAL,HuynhC,RawlinsMDM (2020)TheroleofaCoronavirusdisease2019pharmacist: anAustralianperspective.IntJClinPharm 42(5):1379–1384.
https://doi.org/10.1007/s11096-02001067-4
14.AmparoF,SchaumbergDA,DanaR(2015)Comparisonof twoquestionnairesfordryeyesymptomassessment:the ocularsurfacediseaseindexandthesymptomassessmentin dryeye.Ophthalmology122(7):1498–1503. https://doi.org/ 10.1016/j.ophtha.2015.02.037
15.FinisD,PischelN,KonigC,HayajnehJ,BorrelliM, SchraderS,GeerlingG(2014)ComparisonoftheOSDIand SPEEDquestionnairesfortheevaluationofdryeyedisease inclinicalroutine.DerOphthalmologe:Zeitschriftder DeutschenOphthalmologischenGesellschaft 111(11):1050–1056.
https://doi.org/10.1007/s00347-0143042-z
16.ZhaoY,TanCL,TongL(2015)Intra-observerandinterobserverrepeatabilityofocularsurfaceinterferometerin measuringlipidlayerthickness.BMCOphthalmol15:53. https://doi.org/10.1186/s12886-015-0036-9
17.AsieduK,KyeiS,MensahSN,OcanseyS,AbuLS,Kyere EA(2016)Ocularsurfacediseaseindex(OSDI)versusthe standardpatientevaluationofeyedryness(SPEED):astudy
ofanonclinicalsample.Cornea35(2):175–180. https://doi. org/10.1097/ico.0000000000000712
18.GuillonJP(1998)Abnormallipidlayers.Observation,differentialdiagnosis,andclassification.AdvExpMedBiol 438:309–313
19.GuillonJP(1998)Non-invasivetearscopeplusroutinefor contactlensfitting.ContactLensAntEye:JBrContact LensAssoc21(Suppl1):S31-40. https://doi.org/10.1016/ s1367-0484(98)80035-0
20.EomY,NaKS,ChoKJ,HwangHS,KimSW,ChungTY, JunRM,SongJS,KimHS(2019)Distributionandcharacteristicsofmeibomianglanddysfunctionsubtypes:a multicenterstudyinSouthKorea.KoreanJOphthalmol: KJO33(3):205–213. https://doi.org/10.3341/kjo.2018.0104
21.McGovernJE,MasucciMD,LeTP,CohenAS(2020)The (b)linkbetweenamotivationandpsychosis:Insights throughphasiceyeblinkrate.PsychiatrRes294:113490. https://doi.org/10.1016/j.psychres.2020.113490
22.FinisD,PischelN,SchraderS,GeerlingG(2013)Evaluationoflipidlayerthicknessmeasurementofthetearfilmasa diagnostictoolforMeibomianglanddysfunction.Cornea 32(12):1549–1553. https://doi.org/10.1097/ICO. 0b013e3182a7f3e1
23.ChouYB,FanNW,LinPY(2019)Valueoflipidlayer thicknessandblinkingpatterninapproachingpatientswith dryeyesymptoms.CanJOphthalmolJCanD’ophtalmol 54(6):735–740. https://doi.org/10.1016/j.jcjo.2019.03.005
24.LinX,XuB,ZhengY,CourseyTG,ZhaoY,LiJ,FuY, ChenX,ZhaoYE(2017)Meibomianglanddysfunctionin type2diabeticpatients.JOphthalmol2017:3047867. https://doi.org/10.1155/2017/3047867
25.JungJW,ParkSY,KimJS,KimEK,SeoKY,KimTI (2016)Analysisoffactorsassociatedwiththetearfilmlipid layerthicknessinnormaleyesandpatientswithdryeye syndrome.InvestOphthalmolVisSci57(10):4076–4083. https://doi.org/10.1167/iovs.16-19251
Publisher’sNote SpringerNatureremainsneutralwith regardtojurisdictionalclaimsinpublishedmapsand institutionalaffiliations.



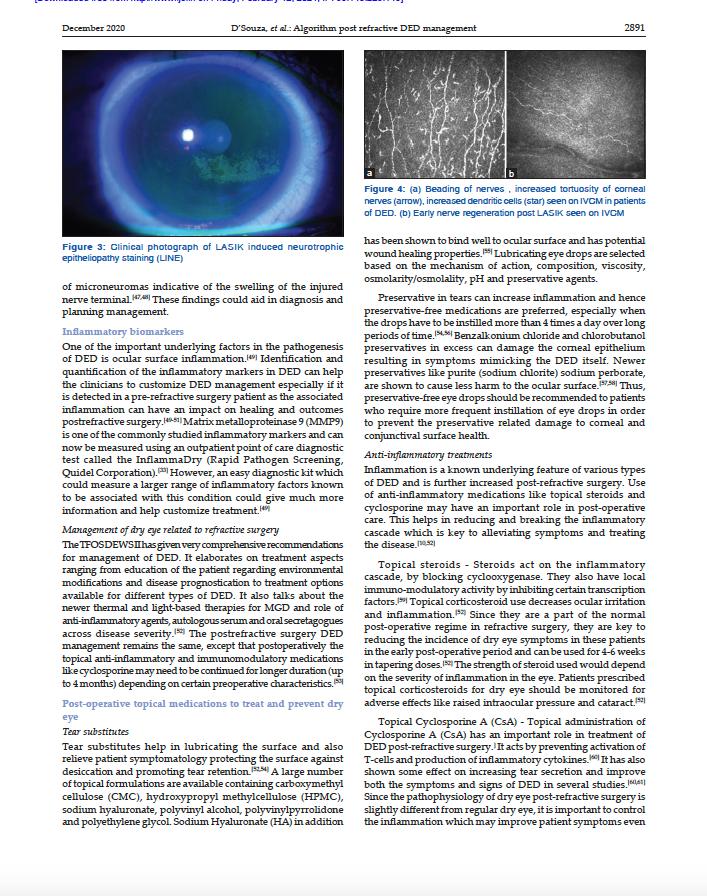




Hindawi
Journal of Ophthalmology
Volume 2020, Article ID 5804123, 6 pages https://doi.org/10.1155/2020/5804123
DiagnosticPerformanceofaNovelNoninvasiveWorkupinthe SettingofDryEyeDisease
LucaVigo,1 MarcoPellegrini,2 FedericoBernabei,2 FrancescoCarones,1 VincenzoScorcia , 3 andGiuseppeGiannaccare 3
1CaronesOphthalmologyCenter,Milan20122,Italy
2OphthalmologyUnit,S.Orsola-MalpighiUniversityHospital,UniversityofBologna,Bologna40138,Italy
3DepartmentofOphthalmology,UniversityMagnaGræciaofCatanzaro,Catanzaro88100,Italy
CorrespondenceshouldbeaddressedtoGiuseppeGiannaccare;giuseppe.giannaccare@gmail.com
Received 2 August 2020; Revised 15 November 2020; Accepted 1 December 2020; Published 11 December 2020
Academic Editor:AntonioBenito
Copyright © 2020LucaVigoetal.ThisisanopenaccessarticledistributedundertheCreativeCommonsAttributionLicense, whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
Purpose.Toevaluatethediagnosticperformanceofanovelnoninvasiveautomatedworkupemployedforthediagnosisofdryeye disease(DED). Methods.OnehundredpatientswithmildtomoderateDEDand100matchedcontrolsubjectswereenrolledin thiscross-sectionalstudy.OcularsurfaceexaminationswerecarriedoutbymeansofIDRAPlus(SBMSistemi,Turin,Italy),which allowstheautomatedevaluationofnoninvasivebreakuptime(NIBUT),lipidlayerthickness(LLT),tearmeniscusheight(TMH), infraredmeibographyforthemeasurementofmeibomianglandloss(MGL),andblinkinganalysis.Continuousvariableswere comparedbetweenpatientswithDEDandcontrolsbyusingtheMann–Whitney U test.Theareaunderthecurve(AUC)of receiveroperatingcharacteristiccurveswascalculated.Thecorrelationsbetweenocularsurfaceparameterswereevaluatedwith Pearsoncorrelationanalysis. Results.PatientswithDEDshowedsignificantlylowervaluesofNIBUT,LLT,andTMHcomparedto controls(6.9 ± 2.5vs10.4 ± 2.4s, P < 0.001;64.6 ± 20.3vs73.4 ± 21.9nm, P � 0.003;0.231 ± 0.115vs0.289 ± 0.164, P � 0.012, respectively).Conversely,nosignificantdifferenceswereobservedforMGLandblinkinganalysis(both P > 0.05).NIBUThadthe highestdiagnosticpower(AUC � 0.841,sensitivity � 0.89,andspecificity � 0.69),followedbyLLT(AUC � 0.621,sensitivity � 0.89, andspecificity � 0.55),TMH(AUC � 0.606,sensitivity � 0.57,andspecificity � 0.63),blinkanalysis(AUC � 0.533, sensitivity � 0.48,andspecificity � 0.59),andMGL(AUC � 0.531,sensitivity � 0.54,andspecificity � 0.48).InpatientswithDED, NIBUTshowedasignificantcorrelationwithTMH(R � 0.347, P � 0.002)andblinkinganalysis(R � 0.356, P < 0.001),while blinkinganalysiswasnegativelycorrelatedwithMGL(R �−0.315, P � 0.008). Conclusions.Theautomatednoninvasiveworkup validatedinthisstudymaybeausefultoolforreachinganoninvasivediagnosisofDEDwithagoodperformance,especially forNIBUT.
1.Introduction
Dryeyedisease(DED)isamultifactorialdiseaseoftearsand ocularsurfacethatrepresentsoneofthemostfrequent ophthalmologicalcomplaints,affectinghundredsofmillions ofpeopleworldwide[1].BasedonthedefinitionbyTearFilm andOcularSurfaceSocietyDryEyeWorkshop(TFOS DEWS)II,multiplefactorsincludingtearfilminstability, tearhyperosmolarity,inflammation,andneurosensoryabnormalitiesplayakeyroleinthepathogenesisofDED[2].
Nosinglegold-standarddiagnosticmarkerhasyetbeen established,mainlyduetodifferentaspectsofthedisease includingthemultifactorialandcomplexpathogenesis,the poorcorrelationbetweensymptomsandsigns,andthe significantfluctuationovertimeandseasonofcurrently availablemetrics[3–6].Assuch,nowadaysthediagnosisof DEDisreachedifoculardiscomfortsymptomsarepresent inassociationwithatleastonemarkerofdisruptedhomeostasisoftheocularsurfaceamongcornealstaining,tear filminstability,andincreasedtearosmolarity[7].
Recently,novelmetrics,includingamongotherstear filminterferometry,infraredmeibography,tearmeniscus height,andevaluationofblinkingcharacteristics[8–14], havebeendevelopedtocomplementthediagnosisofDED traditionallyreachedwithslit-lampexaminationandvital dyestaining.Theadvantagesofthesetestsincludethe noninvasivenatureandtheautomatedcalculationofthe resultsthatcanprovidereliablebiomarkersofthedisease, avoidingobserverbias[15].However,thecurrentlackof validatedcutoffvaluesforreachingthediagnosisofDED hamperedtheirwideadoptionintheclinicalpractice.
Thepurposeofthisstudywastoevaluatetheperformanceofanovelnoninvasiveautomatedworkupfor reachingthediagnosisofDED.
2.MaterialsandMethods
2.1.StudyDesign. Thiscross-sectionalstudywasconducted attheDepartmentofOphthalmologyoftheUniversity MagnaGræciaofCatanzaro(Italy)betweenDecember2019 andFebruary2020.Thestudywasperformedinaccordance withtheprinciplesoftheDeclarationofHelsinkiandwas approvedbythelocalethicscommittee(ComitatoRegione CalabriaSezioneAreaCentro-Protocoln.280/2019).Consecutivepatientsover18yearsofagewithaconfirmeddiagnosisofDEDwhoattendedtheocularsurfaceofficefor controlvisitswerescreenedforenrolment.Thediagnosisof DEDwasreachedaccordingtoTFOSDEWSIIcriteria, whichrequireanocularsurfacediseaseindexscore ≥ 13plus onebetweentearbreakuptime(TBUT) < 10secondsor > 5 spotsofcornealstaining[7].Healthysubjectsattendingour centerforroutineophthalmicvisits,whowerematchedby ageandgender,wereselectedasthecontrolgroup.Exclusioncriteriaforbothgroupswerecontactlenswearing, previouscornealsurgery,andactiveoculardiseasesincludingallergyaswellasuncontrolledsystemicdiseases.
2.2.OcularSurfaceExamination.
AllocularsurfaceexaminationswereperformedusingthenewlydevelopedIDRA Plus(SBMSistemi,Turin,Italy),anall-in-onedevicewhich allowstheautomatedmeasurementof(i)noninvasive breakuptime(NIBUT)(Figures1(a)and1(b));(ii)lipid layerthickness(Figures1(c)and1(d));(iii)tearmeniscus height(Figures1(e)and1(f));(iv)infraredmeibography (Figures1(g)and1(h));and(v)blinkinganalysis (Figures1(i)and1(j)).Indetail,NIBUTwasmeasured withouttheneedforfluoresceindyeafteraskingthepatient toblink3consecutivetimesandthenholdtheeyesopen.The measurementwasrepeated3times,andthemeanvaluewas recorded.Lipidlayerthicknesswasestimatedbyobserving theinterferencepatternandcoloursofthemovinglipidtear film.Tearmeniscusheightwasmeasuredalongthelowerlid marginimmediatelybelowthepupil.Infraredmeibography wasperformedafterevertingthesuperioreyelid,and meibomianglandlosswasdefinedasthepercentageofgland lossinrelationtothetotaltarsalareaofthelid.Theblinking analysiswasperformedbyrecordinga30-secondvideo whilethepatientwasaskedtoblinknaturallybyavoiding
forcedblinking,andthepercentageclosureofmaximal palpebralfissureopeningwasnoted.Thetestswereperformedinthefollowingchronologyinordertoavoid/ minimizepotentialconfoundingeffectsonthereadingsof subsequentmeasurements[16]:blinkanalysis,tearmeniscus height,lipidlayerthickness,NIBUT,andinfrared meibography.
2.3.StatisticalAnalysis. ThestatisticalanalysiswasconductedwithR(version4.0.0)andRStudio(version1.2.5042) software.Examinationswereperformedinbotheyesof patients,andvaluesfromtheworsteyeaccordingtoTFOS DEWSIIcriteriawereusedforthestatisticalanalysis. Continuousvariableswerecomparedbetweenpatientswith DEDandcontrolsbyusingtheMann–Whitney U test. Receiveroperatingcharacteristic(ROC)curvesweredrawn toassessthediagnosticsignificanceofocularsurfaceparametersbyusingthepROCpackage[17].Theaccuracyof eachocularsurfaceparameterfordiscriminatingpatients withDEDfromcontrolswasevaluatedbycalculatingthe areaunderthecurve(AUC).Theoptimalcutoffvalueofeach parameterwasdeterminedasthepointontheROCcurve thatwasnearesttothecoordinate(1,1).Thecorrelations betweenocularsurfaceparameterswereevaluatedwith Pearsoncorrelationanalysis.ABonferronicorrectionfor multiplecomparisonswasapplied.A P value < 0.05was consideredstatisticallysignificant.
3.Results
Overall,100eyesofDEDpatientsand100eyesofcontrol subjectswereincluded.Nosignificantdifferencesbetween thetwogroupswereobservedforgenderdistribution(74% femalesintheDEDgroupvs70%femalesinthecontrol group, P � 0.637)andage(50.5 ± 31.1yearsvs54.0 ± 14.7, P � 0.075).
Theresultsoftheocularsurfaceexaminationinthetwo groupsarereportedinTable1.Comparedtocontrolsubjects,patientswithDEDshowedasignificantlylower NIBUT(P < 0.001),lipidlayerthickness(P � 0.003),andtear meniscusheight(P � 0.012).Conversely,nosignificant differencesinmeibomianglandlossandblinkinganalysis wereobserved(both P > 0.05).
TheAUCofROCcurvesalongwithoptimalcutoffvalues withcorrespondingsensitivityandspecificityoftheocular surfaceparametersanalyzedisreportedinTable2:NIBUThad thehighestdiagnosticpower(AUC � 0.841,sensitivity � 0.89, andspecificity � 0.69),followedbylipidlayerthickness (AUC � 0.621,sensitivity � 0.89,andspecificity � 0.55),tear meniscusheight(AUC � 0.606,sensitivity � 0.57,andspecificity � 0.63),blinkinganalysis(AUC � 0.533,sensitivity � 0.48, andspecificity � 0.59),andmeibomianglandloss (AUC � 0.531,sensitivity � 0.54,andspecificity � 0.48).Figure2showstheROCcurvesofNIBUT,lipidlayerthickness, andtearmeniscusheight.
InpatientswithDED,NIBUTshowedasignificant correlationwithtearmeniscusheight(R � 0.347, P � 0.002) andblinkinganalysis(R � 0.356, P < 0.001);moreover,









1:Ocularsurfaceworkupinarepresentativecontrolsubject(a,c,e,g,i)andinapatientwithdryeyedisease(b,d,f,h,j).(a,b) Measurementofnoninvasivebreakuptime.(c,d)Tearfilminterferometryforthemeasurementoflipidlayerthickness.(e,f)Measurement oftearmeniscusheight.(g,h)Infraredmeibography.(i,j)Blinkanalysis.
Table 1:Ocularsurfaceparametersinpatientswithdryeyediseaseandcontrolsubjects.
Table 2:Areaunderthecurve(AUC)with95%confidenceintervals(CIs),optimalcutoffvalues,andcorrespondingsensitivityand specificityfortheanalyzedocularsurfaceparameters.
blinkinganalysiswasnegativelycorrelatedwithmeibomian glandloss(R �−0.315, P � 0.008).Noothersignificant correlationswereobserved.
4.Discussion
TheprevalenceofDEDvariesconsistentlyacrossdifferent population,andthisispartiallyduetotheheterogeneityof diagnosticcriteriausedindifferentstudies[1].Toaddress thisissue,theTFOSDEWSIIguidelinesdevelopeda consensusdiagnosticbatteryoftestsforDEDincluding breakuptime,tearosmolarity,ocularsurfacestaining,and symptomatology[7].Nevertheless,theDEWSIIDiagnostic MethodologySubcommitteeacknowledgedthelackofa gold-standardtesttodiagnoseDEDandtheneedofidentifyingnewreliablebiomarkers[7].Inthesamereport,ithas beenhighlightedthatstudiesevaluatingnoveldiagnostic testsarefrequentlyaffectedbyselectionandspectrumbiases. Theformeroccurswhenanoveltestiscomparedto establishedonesthatwereusedasinclusioncriteria, resultinginapparentlypoorperformance.Thelatterrefersto theexclusionfromclinicaltrialsofpatientswithmilddisease,withoverestimationofthediagnosticperformance. Conversely,toavoidboththesebiasesandobtainreliable estimatesofthediagnosticperformance,noveltestsshould bedevelopedandvalidatedusingdatafromthepopulation
inwhichtheyareintendedtobeused[18].Therefore,inthe presentstudy,weincludedconsecutivepatientswitha confirmeddiagnosisofDEDpresentingtoourcenterfor routinecontrolvisits.SinceDEDdiagnosishadbeenalready reachedpreviously,wedidnotuseconventionalteststo selectandgradepatients.Thisresultedintheinclusionofa broadpopulationofmildtomoderateDEDpatients,producingresultsthataregeneralizabletoreal-lifeclinical practice.
PatientswithDEDshowedsignificantlylowervaluesof NIBUT,lipidlayerthickness,andtearmeniscusheight comparedtocontrols,whilenodifferencesinmeibomian glandlossandblinkinganalysiswereobserved.TheROC analysisshowedthatNIBUTwastheparameterwiththe highestsensitivityandspecificitytodiagnoseDED.Lipid layerthicknessandtearmeniscusheighthadmoderate diagnosticutility,whiletheperformancesofmeibomian glandlossandblinkinganalysiswerepoor.
Inagreementwiththeseresults,alsopreviousworks focusedonbothhyposecretory[19]andevaporativeDED [10]foundthatNIBUTwasthebestsinglediagnostictestfor reachingthediagnosis.Ourresultsfurtherconfirmtherole oftearfilminstabilitymeasurementasareliableindicatorof DEDdiagnosis.Comparedtoconventionalbreakuptime, NIBUThastheadvantageofavoidingcontactwiththe ocularsurfaceaswellasdisruptionofthetearfilminduced byfluoresceininstillation[20].Interestingly,theoptimal NIBUTcutoffvalueinthisstudywas7.75seconds,whichis lowerthanthecutoffof10secondsproposedinprevious works[7,21].
Tearmeniscusheightandlipidlayerthicknessshowed moderatediagnosticperformancetodifferentiateDEDfrom controls.Singhandcolleaguesrecentlyreportedhigher accuracyoftearmeniscusheight(sensitivity0.98and specificity0.96)inpatientswithmoderatetosevereDED [13].Notsurprisingly,theperformanceofthisparameterin patientswithmilderdiseases,likethoseincludedinour study,wasfoundtobelower.Conversely,thepreviously reportedaccuracyoflipidlayerthicknessforthediagnosisof meibomianglanddysfunction(sensitivity0.65andspecificity0.63)isconsistentwiththeresultsofourstudy[22].
NodifferencesinmeibographybetweenDEDand controlswereobservedinthisstudy.Althoughwedidnot classifypatientsaccordingtothesubtypeofDED(aqueous, evaporative,ormixed),thisfindingcouldbeexplainedby thelimitednumberofpatientswithevaporativeDEDincludedinthestudy.Infact,ithasbeenshownthatmeibomianglandchangesareusuallymorepronouncedin meibomianglanddysfunctioncomparedtoDEDofother types[23].
Althoughblinkinganalysisshowedlimiteddiagnostic utility,thisparametershowedasignificantbutrelatively weakcorrelationwithbothNIBUTandmeibomiangland loss.Jieandcoauthorsreportedsimilarassociationsand speculatedthatincompleteblinkingcouldleadtoinadequate meibomianglandexpressionandsubsequenttearfilminstability[14].Itshouldbenotedthatastandardized methodologytoevaluateincompleteblinkinghasnotyet beendeveloped.Wemeasuredthepercentageofeyeclosure
whilepatientsblinkingspontaneously,butalsoother methodssuchastheincompleteblinkratemightprovide additionalinformationforthecharacterizationofeyelid dynamics[24,25].
Thisstudysuffersfromsomelimitationsthatdeserve mentioning.Inparticular,inordertobestreflecteveryday practice,weincludedpatientswithaconfirmeddiagnosisof DEDregardlessofdiseaseseverityand/orDEDsubtype. Furtherstudieswithmorerigorousinclusioncriteriaare neededtoevaluatethepossiblechangesofdiagnosticperformanceindifferentDEDscenarios.Furthermore,future studiesarewarrantedtoinvestigatethecorrelationbetween theresultsobtainedwiththisnewnoninvasivediagnostic deviceandDEDclinicalandmolecularfindings.
5.Conclusions
Theautomatednoninvasiveworkuppresentedandvalidated inthisstudymaybeausefultooltodiagnoseDEDwithgood valuesofsensitivityandspecificity,especiallyforNIBUT. Furthermore,sincetheeffectsofthisworkuponvolumeor propertiesofthetearfilmarenegligible,itcanbeusedasan effectivescreeningtoolfordiscriminatinghealthysubjects frompatientsaffectedoratriskforDED,beforeproceeding withinvasiveocularsurfaceexaminationsrequiredfora bettercharacterizationofthedisease.
DataAvailability
Thedatathatsupportthefindingsofthisstudyareavailable fromthecorrespondingauthoruponreasonablerequest.
ConflictsofInterest
Theauthorsdeclarethattheyhavenoconflictsofinterest.
Authors’Contributions
LucaVigoandMarcoPellegriniequallycontributedtothis workandshareprimaryauthorship.
References
[1]F.Stapleton,M.Alves,V.Y.Bunyaetal.,“TFOSDEWSII epidemiologyreport,” TheOcularSurface,vol.15,no.3, pp.334–365,2017.
[2]J.P.Craig,K.K.Nichols,E.K.Akpeketal.,“TFOSDEWSII definitionandclassificationreport,” TheOcularSurface, vol.15,no.3,pp.276–283,2017.
[3]J.E.Moore,J.E.Graham,E.A.Goodalletal.,“Concordance betweencommondryeyediagnostictests,” BritishJournalof Ophthalmology,vol.93,p.66,2009.
[4]G.Fuentes-Paez,J.M.Herreras,Y.Cordero,A.Almaraz, M.J.Gonzalez,andM.Calonge,“Lackofconcordancebetweendryeyesyndromequestionnairesanddiagnostictests,” ArchivosdelaSociedadEspanoladeOftalmologia,vol.86, p.37,2011.
[5]B.D.Sullivan,L.A.Crews,E.M.Messmeretal.,“Correlations betweencommonlyusedobjectivesignsandsymptomsfor thediagnosisofdryeyedisease:clinicalimplications,” Acta Ophthalmologica,vol.92,p.16,2014.
[6]P.Aragona,G.Giannaccare,R.Mencucci,P.Rubino, E.Cantera,andM.Rolando,“Modernapproachtothe treatmentofdryeye,acomplexmultifactorialdisease:a P.I.C.A.S.S.O.boardreview,” BritishJournalofOphthalmology,vol.23,2020.
[7]J.S.Wolffsohn,R.Arita,R.Chalmersetal.,“TFOSDEWSII diagnosticmethodologyreport,” TheOcularSurface,vol.15, no.3,pp.539–574,2017.
[8]J.J.Nichols,K.K.Nichols,B.Puent,M.Saracino,and G.L.Mitchell,“Evaluationoftearfilminterferencepatterns andmeasuresoftearbreak-uptime,” OptometryandVision Science,vol.79,no.6,pp.363–369,2002.
[9]Y.Ban,S.Shimazaki-Den,K.Tsubota,andJ.Shimazaki, “Morphologicalevaluationofmeibomianglandsusing noncontactinfraredmeibography,” TheOcularSurface, vol.11,no.1,pp.47–53,2013.
[10]G.Giannaccare,L.Vigo,M.Pellegrini,S.Sebastiani,and F.Carones,“Ocularsurfaceworkupwithautomatednoninvasivemeasurementsforthediagnosisofmeibomiangland dysfunction,” Cornea,vol.37,no.6,pp.740–745,2018.
[11]G.Giannaccare,F.Bonifazi,S.Sebastianietal.,“Meibomian glanddropoutinhematologicalpatientsbeforehematopoietic stemcelltransplantation,” Cornea,vol.37,no.10, pp.1264–1269,2018.
[12]L.Vigo,L.Taroni,F.Bernabeietal.,“Ocularsurfaceworkup inpatientswithmeibomianglanddysfunctiontreatedwith intenseregulatedpulsedlight,” Diagnostics,vol.9,no.4, p.147,2019.
[13]A.Singh,M.Vanathi,A.Kishore,N.Gupta,andR.Tandon, “Evaluationofstripmeniscometry,tearmeniscusheightand depthinthediagnosisofdryeyediseaseinAsianIndianeyes,” TheOcularSurface,vol.17,no.4,pp.747–752,2019.
[14]Y.Jie,R.Sella,J.Feng,M.L.Gomez,andN.A.Afshari, “Evaluationofincompleteblinkingasameasurementofdry eyedisease,” TheOcularSurface,vol.17,no.3,pp.440–446, 2019.
[15]N.S.Roy,Y.Wei,E.Kuklinski,andP.A.Asbell,“Thegrowing needforvalidatedbiomarkersandendpointsfordryeye clinicalresearch,” InvestigativeOpthalmology&VisualScience,vol.58,no.6,pp.BIO1–BIO19,2017.
[16]S.Koh,C.Ikeda,S.Watanabeetal.,“Effectofnon-invasive tearstabilityassessmentontearmeniscusheight,” Acta Ophthalmologica,vol.93,pp.135–139,2015.
[17]X.Robin,N.Turck,A.Hainardetal.,“pROC:anopen-source packageforRandS+toanalyzeandcompareROCcurves,” BMCBioinformatics,vol.12,p.77,2011.
[18]J.A.Usher-Smith,S.J.Sharp,andS.J.Griffin,“Thespectrum effectintestsforriskprediction,screening,anddiagnosis,” BMJ,vol.353,2016.
[19]H.Pult,C.Purslow,andP.J.Murphy,“Therelationship betweenclinicalsignsanddryeyesymptoms,” Eye,vol.25, no.4,pp.502–510,2011.
[20]L.E.Downie,“Automatedtearfilmsurfacequalitybreakup timeasanovelclinicalmarkerfortearhyperosmolarityindry eyedisease,” InvestigativeOphthalmology&VisualScience, vol.56,p.7260e8,2015.
[21]L.S.Mengher,A.J.Bron,S.R.Tonge,andD.J.Gilbert,“A non-invasiveinstrumentforclinicalassessmentoftheprecornealtearfilmstability,” CurrentEyeResearch,vol.4,1985.
[22]D.Finis,N.Pischel,S.Schrader,andG.Geerling,“Evaluation oflipidlayerthicknessmeasurementofthetearfilmasa diagnostictoolforMeibomianglanddysfunction,” Cornea, vol.32,no.12,pp.1549–1553,2013.
[23]R.Arita,K.Itoh,S.Maeda,K.Maeda,A.Tomidokoro,and S.Amano,“Efficacyofdiagnosticcriteriaforthedifferential diagnosisbetweenobstructivemeibomianglanddysfunction andaqueousdeficiencydryeye,” JapaneseJournalofOphthalmology,vol.54,2010.
[24]T.Wan,X.Jin,L.Lin,Y.Xu,andY.Zhao,“Incomplete blinkingmayattributetothedevelopmentofmeibomian glanddysfunction,” CurrentEyeResearch,vol.41,no.2, pp.179–185,2016.
[25]J.D.Rodriguez,K.J.Lane,G.W.Ousler,E.Angjeli, L.M.Smith,andM.B.Abelson,“Blink:characteristics, controls,andrelationtodryeyes,” CurrentEyeResearch, vol.43,no.1,pp.52–66,2018.
Graefe's
Tear film layers and meibomian gland assessment in patients with type 1 diabetes mellitus using a noninvasive ocular surface analyzer: a cross‑sectional case–control study
María‑Carmen Silva‑Viguera1,2 · Alicia Pérez‑Barea1 · María‑José Bautista‑Llamas1,2
Received: 21 September 2022 / Revised: 24 November 2022 / Accepted: 27 November 2022 / Published online: 13 December 2022
© The Author(s) 2022
Abstract
Purpose To assess the tear film layers and Meibomian glands by a noninvasive ocular surface analyzer in patients with and without type 1 diabetes mellitus (T1DM).
Methods Eighty-eight participants were enrolled in this study: 44 patients with T1DM without diabetic retinopathy, and 44 patients as a control group, between 18 and 49 years old. Limbal and bulbar redness classification, lipid layer thickness (LLT), tear meniscus height (TMH), first and mean noninvasive tear break-up time (FNIBUT and MNIBUT, respectively), and Meibomian glands loss (MGL) were assessment through the ICP Ocular Surface Analyzer (OSA). Schirmer’s I test (SIT), the fluorescein tear break-up time test (TFBUT), OSDI and SPEED questionnaires, and percentage of glycosylated hemoglobin (HbA1c) were also tested.
Results The T1DM group showed higher limbal and bulbar redness (p = 0.010) and lower LLT (p < 0.001), TMH (p < 0.001), FNIBUT (p < 0.001), MNIBUT (p < 0.001), SIT (p = 0.001), and TFBUT (p < 0.001) than the control group. A higher percentage of MGL was found in the T1DM group in the upper (p = 0.097) and lower (p < 0.001) eyelids. No significant differences were found in dry eye symptoms across the OSDI and SPEED questionnaires between the two groups.
Conclusion Patients with T1DM without signs of retinopathy showed involvement of the mucoaqueous and lipid layers of the tear film, as well as a higher percentage of MGL, using a noninvasive analyzer. Dry eye disease in people with T1DM cannot be ruled out by anamnesis and subjective symptom questionnaires alone; therefore, these patients should undergo regular anterior pole examinations.
Key messages
Diabetes mellitus has been associated with dry eye disease, there are few studies in adults with type 1 diabetes mellitus without retinopathy or neuropathy and none using a noninvasive device that anal yses all layers of the tear film and Meibomian glands.
There are different studies on the relationship between type 1 diabetes mellitus and dry eye disease, as well as its relationship with the state of the Meibomian glands, and none that use a non-invasive device that analyzes all the layers of the tear film.
Patients with type 1 diabetes mellitus showed involvement of the mucoaqueous and lipid layers of the tear film, as well as a higher percentage of the Meibomian glands loss, using a noninvasive analyzer.
Keywords Dry eye disease · Meibomian glands dysfunction · Ocular surface · Tear film · Type 1 diabetes mellitus
Introduction
* María-José Bautista-Llamas mbautista5@us.es
Extended author information available on the last page of the article
Diabetes mellitus (DM) is a systemic and chronic degenerative disease characterized by chronic hyperglycemia due to deficiency in the production or action of insulin, affecting
the metabolism of carbohydrates, proteins, and fats. In type 1 diabetes mellitus (T1DM), the β cells of the pancreas are destroyed, which leads to an absolute insufficiency of insulin in the blood [1]. It can affect practically all ocular structures, with corneal dysfunction being the main complication in the anterior segment, with the appearance of abnormal sensitivity, delayed corneal re-epithelialization, diabetic keratopathy, progressive decrease in density and/or alteration of the corneal nerve, and opacity of the lens at a younger age [2].
We can also find alterations of the tear film, the function of which is to lubricate and maintain a smooth and refractive surface for optimal visual performance. Dry eye disease (DED) is defined as a disorder of the tear film and the ocular surface caused by insufficient tears or excessive evaporation of the same, which produces damage to the interpalpebral ocular surface and symptoms of discomfort [3].
The stability of the tear film can be assessed with different tests, such as the Schirmer test, the invasive tear break-up time, noninvasive tear break-up time (NIBUT), lipid layer thickness (LLT), or the tear meniscus height (TMH). The measurement of these parameters is important to diagnose DED. Normally, an observer takes measurements using the slit lamp and the use of fluorescein to assess the tear break-up time (TFBUT), which implies that the measurement is not entirely reliable. These tests evaluate different aspects of the tear, such as its production or quality, to classify DED into tear deficiency or excessive evaporation. In the latter, the Meibomian glands (MG) play an important role; Meibomian glands dysfunction (MGD) is commonly characterized by terminal duct obstruction and/ or qualitative/quantitative changes in glandular secretion and is the leading cause of evaporative DED [2].
Eyes with an abnormally low tear film break-up time and the presence of subjective symptoms are considered to have DED; however, the Dry Eye Workshop II (DEWS II) of the Tear Film and Ocular Surface Society (TFOS) has recently published dry eye definition as follows: “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” [4]. Stapleton F et al. in a meta-analysis concluded that the prevalence of dry eye can range from 5 to 50% depending on age, sex, race, or geographic region analyzed [5]. The study by Millán et al., on the 11-year incidence of dry eye and risk factors in a cohort of 209 adults in Spain, obtained an incidence of 25.4% and 31.6% in signs and symptoms of dry eye, respectively, significantly associated with age (p < 0.05). In addition, they also concluded that some factors may increase the risk of dry eye signs and others of dry eye symptoms [6].
Therefore, it is necessary to make evaluations of the tear film as objective as possible. Newer diagnostic tests, such as tear film osmolality, refractive meniscometry for
measurement of TMH, infrared meibography, interferometry to analyze the lipid layer of the tear film, the stability of the tear film (video keratography), and ocular surface thermography, are some of the instruments used to objectify these examinations.
There are different studies on the relationship between diabetes and DED. Although most of these studies have been carried out in patients with type 2 diabetes mellitus (T2DM) [7–9] or in patients with T1DM in childhood [10, 11], none of them presents objective data of patients with T1DM as well as their relationship with the state of the MG.
Although DM has been associated with DED, there are few studies in adults with T1DM without retinopathy or neuropathy and none using a noninvasive device that analyzes all layers of the tear film and Meibomian glands. The diagnosis of DED aims not only to detect this pathology to treat it but also to reduce the factors that lead to serious corneal complications that can compromise vision, such as corneal inflammation, corneal abrasions, or ulcers [3].
The aim of this research was to assess the tear film layers and meibomian glands by a noninvasive ocular surface analyzer in patients with and without T1DM.
Material and methods
Study design and ethics
A cross-sectional, case–control study was carried out in the Optometry Facilities of the Faculty of Pharmacy of the University of Seville (Seville, Spain) between January and May 2022. The study was conducted according to the ethical principles for medical research set out in the Declaration of Helsinki and was approved by the Ethical Committee of Andalusia (code 1474-N-19).
Selection of participants
For the selection of participants with T1DM, a proposal for participation in the study was sent by email to the association of diabetics of Seville (ANADIS). In addition, an age- and sex-matched control group was selected among volunteers of the university community of the Faculty of Pharmacy of Seville. All participants signed an informed consent form after an explanation of the nature and consequences of the study.
The inclusion criteria were as follows: (1) age between 18 and 50 years; (2) absence of systemic or ocular disease; (3) absence of pharmacological prescription except for T1DM; and (4) absence of a previous history of ocular surgery.
The exclusion criteria were as follows: (1) ocular infection or inflammation; (2) taking any ophthalmic or systemic
medications with tear film or ocular surface effects; (3) pregnant or breastfeeding patients; (4) a recent history of contact lens wearing; and (5) use of any ocular lubricant 1 week before.
Subjects with T1DM were controlling their DM with insulin injections, had to be diagnosed by a diabetes physician specialist with the disease at least 3 years earlier, and had no signs of retinopathy on fundus imaging according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) [12]. Controls should have a percentage of capillary glycosylated hemoglobin (HbA1c) of no more than 5.6%.
Material and measurements
The symptomatology of dry eye was evaluated using two subjective questionnaires: the Ocular Surface Disease Index (OSDI) [13] and the Standard Patient Evaluation of Eye Dryness (SPEED) test [14]. A nonmydriatic retinography (CSO nonmydriatic fundus camera Cobra HD, Italy) examination was performed to rule out the presence of signs of diabetic retinopathy. HbA1c was also tested in all subjects using the Cobas b-101 analyzer (Roche Diagnostic) to determine glycemic control. HbA1c defines the average blood glucose level of the previous 2–3 months, and in patients with DM, it reflects the success of diabetes management [15].
The noninvasive analysis of the tear film was performed through the Integrated Clinical Platform (ICP) Ocular Surface Analyzer (OSA) from SBM System® (Orbassano, Torino, Italy) [ 16 –18 ]. OSA provides a complete noninvasive assessment of the ocular surface by combining several tests for the diagnosis of DED. This device was placed in the tonometer hall of the slit lamp. The measurements performed using this instrument were the limbal and bulbar redness classification, lipid layer thickness (LLT), tear meniscus height (TMH), first and mean noninvasive tear break-up time (FNIBUT and MNIBUT, respectively) (objective and automatic), and Meibomian gland loss (MGL) percentage and grade (objective and automatic) [19].
Tear volume was measured by Schirmer’s I test (SIT) [20], and the invasive analysis of tear film stability was carried out through the fluorescein tear break-up time test (TFBUT) using cobalt blue illumination of a slit lamp (Topcon SL-6E, Japan). TFBUT was the average of the three measures of each eye [21].
Examination procedure
After verification of compliance with the previously established criteria, subjects were included in the study.
This was followed by noninvasive examination of the tear film by OSA in the following order [19]: (I) limbal and bulbar redness. The degree of redness was assessed by
comparing the image taken by the device with the Efron scale [22] (0 = normal, 1 = trace, 2 = mild, 3 = moderate, 4 = severe). Reddening values above grade 2 were considered abnormal. (II) Interferometric LLT. The interferometric patterns were classified into the 7 categories defined by Guillon [23] and their quantitative equivalence in LLT (from thinner to thicker: 0 < 15 nm – not present,
1 ~ 15 nm – open meshwork, 2 ~ 30 nm – close meshwork,
3 ~ 30/80 nm – wave, 4 ~ 80 nm – amorphous, 5 ~ 80/120 nm – color fringes, 6 ~ 120/160 nm – abnormal color). Categories below the wave pattern were considered abnormal and corresponded to reduced lipid layer thickness. (III) TMH. This test assessed the mucoaqueous layer of the tear. TMH was measured in millimeters along the lower lid margin at the center of the cornea by the magnification tool. TMH values ≤ 0.20 mm were considered abnormal [24]. (IV) Noninvasive tear break-up time. With this measurement, the tear film stability was evaluated. The device automatically provides the FNIBUT and MNIBUT in seconds (s). Values of MNIBUT < 10 s were considered abnormal and indicative of eye [25]. (V) Meibomian gland assessment. The percentage and degree of MGL were determined by noncontact infrared meibography and subsequent automatic analysis, or semiautomatic when this was not possible, on the lower and upper eyelids separately. The percentage of MGL was represented as the percentage of the area of missing glands in the region of the lower and upper tarsal plates and graded according to a 4-stage scale (Meiboscale) [26] (0 ~ 0%, 1 < 25%, 2 = 26% – 50%, 3 = 51% – 75%, 4 > 75%). An MGL value above 25% (grade 2) was considered abnormal.
Finally, invasive tear film analysis was performed using SIT and TFBUT. ST < 10 mm and TFBUT < 10 s were considered abnormal [25].
All examinations were performed at the same location, under the same temperature and humidity conditions, by an experienced examiner using the same instrumentation.
Statistical analysis
Because there were no significant differences observed in measurements between the right and left eyes, data from one eye per patient were considered for further analysis, choosing the right eye or left eye by simple and computergenerated random numbers.
Data were analyzed using IBM SPSS® Statistics 26 for Windows version (IBM Corporation, Armonk, NY). The normality of the variables was checked using the Shapiro–Wilk test (p > 0.05). Quantitative variables that conformed to normal were described by the mean ± standard deviation (SD), and those that were not described, by the median (interquartile range). Comparisons between the two groups were analyzed using the independent T test (Mann–Whitney U test for nonparametric). The chi-square
test was used for qualitative variables. The relationship between the variables considered was assessed using the correlation Pearson test (Spearman for nonparametric). For all comparisons, a p value < 0.05 was considered statistically significant.
The sample size was determined in the GRAMMO® calculator (Institut Municipal d’Investigació Mèdica, Barcelona, Spain, Version 8.0) based on the TMH as the main study variable. The common SD in the TMH is assumed to be 0.07 for the group with type 1 diabetes based on the results obtained by Han et al. [27]. Accepting an alpha risk of 0.05 and beta risk of 0.2 in a two-sided test, a minimum of 31 subjects are necessary in each group to recognize as statistically significant a difference greater than or equal to 0.05 mm in the TMH.
Results
Demographics and clinical characteristics
Eighty-eight subjects (36 males and 52 females) were enrolled in this study, including 44 patients with T1DM and 44 controls between 18 and 49 years old. All subjects were Spanish Caucasians. The demographics and clinical characteristics of the participants are shown in Table 1.
The T1DM and control groups were similar with respect to age, sex, correct distance visual acuity, and subjective dry eye symptom questionnaires score points OSDI and SPEED; however, there was a statistically significant difference in the percentage of HbA1c between the two groups (p < 0.001).
Scores on the OSDI and SPEED questionnaires were directly correlated (ρ = 0.697, p < 0.001).
Noninvasive tear film test
Comparisons of the values obtained in the OSA tests between the two groups of participants, the statistical significance (p value), and subjects with abnormal values in each variable are summarized in Table 2
The T1DM group showed higher limbal and bulbar redness than the control group (p = 0.010). Fifty-four percent of the participants had conjunctival redness equal to or greater than grade 2 (mild) on the Efron scale [22], and 35% were in patients with T1DM compared to 19% without T1DM. An example of conjunctival redness examination is presented in Fig. 1.
In the LLT assessment, lower lipid pattern values were obtained in the T1DM group than in the control group (p < 0.001). Seventy-seven percent of the participants (45% in the T1DM group and 32% in the control group) had a lipid pattern category below the wave pattern (~ 30/80 nm), thus reducing LLT. Examples of examinations of different lipid patterns found are presented in Fig. 2
The TMH in the T1DM group was lower than that in the control group, with a statistically significant median difference of 0.09 mm (p < 0.001). Twenty-eight percent of the participants (26% with T1DM and 2% without T1DM) had a reduced meniscus height, indicating a decreased mucoaqueous layer.
In the assessment of tear film stability, patients with T1DM had a lower FNIBUT and MNIBUT than controls ( p < 0.001). Sixty-six percent of participants (43% with
CDVA corrected distance visual acuity, DMT1 type 1 diabetes mellitus, IQR interquartile range, OSDI Ocular Surface Disease Index, SD standard deviation, SPEED Standard Patient Evaluation of Eye Dryness Values are presented as mean ± SD (range) or median (IQR) (range) in quantitative variables and as frequency (percentage) in qualitative variables
a Statistically significant differences through independent T test
Variables a
group (
value Subjects with abnormal values,
T1DM type 1 diabetes mellitus, MGL Meibomian glands loss, FNIBUT first noninvasive break-up time, MNIBUT mean noninvasive break-up time, TFBUT fluorescein break-up time test
a Values are presented as mean ± SD or median (IQR)
b Statistically significant differences by Mann–Whitney U test
c Statistically significant differences by independent T test

T1DM and 28% without T1DM) presented tear film instability and therefore the presence of dry eye signs by showing an MNIBUT of less than 10 s [25].
Meibomian gland assessment
A higher percentage of MGL was found in the T1DM group in the upper and lower eyelids, although the difference between the two groups was only statistically significant in the case of the lower eyelid (p < 0.001) (Table 2). Regarding the lower eyelid MGL percentage, in 64% of participants (46% with T1DM and 16 without T1DM), it was higher than 25% (grade 2). In contrast, the upper eyelid MGL percentage was higher than 25% (grade 2) in only 32% of the participants (20% with T1DM and 18% without T1DM). Some examples of upper and lower eyelid MGLs through OSA are shown in Fig. 3.
Invasive tear film analysis
In the SIT, lower values were obtained in the T1DM group than in the control group ( p = 0.001) (Table 2). Twentyseven percent of the participants (20% with T1DM and 7% without T1DM) had a reduced tear volume according to this test. Regarding the TFBUT, the T1DM group also had a lower value than the control group (p < 0.001) (Table 2).

sent (0 < 15 nm). B Open meshwork (~ 15 nm). C Close meshwork (~ 30 nm). D Wave (~ 30/80 nm). E Amorphous (~ 80 nm). F Color fringes (80/120 nm)
Seventy-two percent of participants (48% with T1DM and 31% without T1DM) had values of less than 10 s, indicating tear film instability.
Correlation analysis of HbA1c and diabetes duration with the tests
The percentage of HbA1c was directly correlated with limbal and bulbar redness (ρ = 0.226, p = 0.034) and lower
eyelid MGD (r = 0.396, p < 0.001). In addition, HbA1c was inversely correlated with TMH (ρ = 0.584, p < 0.001), LLT (ρ = 0.361, p = 0.001), FNIBUT (ρ = 0.525, p < 0.001), MNIBUT ( ρ = 0.399, p < 0.001), SIT ( ρ = 0.317, p = 0.003), and TFBUT (ρ = 0.566, p < 0.001).
In the T1DM group, an inverse correlation of diabetes duration with LLT (ρ = 0.400, p = 0.007) and a direct correlation of diabetes duration with upper eyelid MGD (ρ = 0.413, p = 0.005) were obtained.
= 31% (grade 2);
the upper eyelid MGL = 8% (grade 1); C
= 58% (grade 3); D
= 33% (grade 2)

Discussion
In this study, tear film layers and MGs were assessed in adult patients with and without T1DM using a noninvasive ocular surface analyzer. A statistically significant impairment of the mucoaqueous and lipid layers, as well as a greater loss of lower eyelid MGs, was found in patients with T1DM. However, no significant differences were found in dry eye symptoms across the OSDI and SPEED questionnaires between the T1DM and control groups. This could be because some of the T1DM patients may have a lower corneal sensitivity caused by peripheral neuropathy [28]. Diabetic neuropathy is a serious complication of diabetes and the main cause of nerve damage [29]. In some cases, because of this, patients with T1DM may be asymptomatic even with severe damage to the ocular surface, indicating progression of peripheral neuropathy [30].
Noninvasive tear film test
The tear film was quantified noninvasively, obtaining LLT and bulbar network measurements by optical interferometry, TMH, FNIBUT, and MNIBUT. In all of them, lower results were obtained in the T1DM group compared to the control group, except for bulbar redness, which was greater in the T1DM group, and the differences were also statistically significant in all of them. Several authors describe the differences in NIBUT between people with and without DM, although the methods of measurement are very different, and many measure it invasively (TFBUT) and in T2DM [31] or in children [10, 32], always describing lower values for patients with DM.
We cannot make an exhaustive comparison of the FNIBUT or the MNIBUT, two values of recent appearance with the use of noninvasive devices, which are usually compared with the reference values in subjective methods.
Few studies perform a complete noninvasive examination of the tear film, such as that of Zeng et al. [33], which found no differences between patients with DM and the control group in the TMH and NIBUTM measured with Keratograph, nor in the LLT, measured with the Tearscope, although in this case they are again T2DM; or Garzon P et al. [34] that quantifies NIBUT, LLT with interferometry, and TMH with OCT in T2DM, also obtaining lower values, but without statistically significant differences. Additionally, Han et al. [27] used a dry eye analyzer similar to the one used in the present study, describing worse results in the analyzed group, although they were again T2DM patients; similarly, Nadeem et al. [28] described a lower TMH and no relationship between this loss and the duration of the disease and who also suffered from diabetic retinopathy.
Only Misra et al. [35] noninvasively analyzed NIBUT and LLT in patients with T1DM, obtaining lower values. Only the former was significant, but these patients were diagnosed with corneal neuropathy.
Meibomian gland assessment
Patients with T1DM showed a higher percentage of MGL than patients without T1DM, with the highest value found in the lower eyelid of patients with T1DM, grade 2 according to the Meiboscale [26]. Eom et al. [36] reported a statistically significant higher lower eyelid and upper eyelid MGL in patients with obstructive MGD, but no studies have been found in people with T1DM. Because of MGD, a reduction in the lipid layer was also found in this study [37]. The relationship of MGD with T2DM has been studied by several authors [9, 20, 33, 34, 38–40], mostly indicating a higher MGD in these patients; however, studies in the population with T1DM are more limited, and this is the first one in an adult population. There are recent publications in children and adolescents with T1DM [41, 42]. In the study by Koca et al. [41], children with T1DM had a higher percentage of GML than controls, although this did not correlate with HbA1c or duration of diabetes; however, the study by Gunay et al. [42] reported certain altered ocular surface parameters but no differences with controls with respect to MGL.
Invasive tear film tests
Participants with T1DM had a reduced tear volume compared to controls in the SIT. These results are consistent with other research in adults with T1DM [43, 44], children and adolescents [42, 45], and subjects with T2DM [33, 46–49] but disagree with Ferdousi et al. [50]. Shujaat et al. [45] suggested that diabetes may cause damage to the microvasculature and innervation of the main lacrimal gland, affecting tearing.
Regarding TFBUT, patients with T1DM had lower TFBUT values than controls, corroborating the results of other authors [45, 50] and supporting the results obtained in the NIBUT, although there are also some authors who disagree with this result.
It is logical to suppose that if SIT values are lower in patients suffering from DM, as has been described by some authors, and with which we agree, and the MG are more affected, we would find lower values of LLC and TMH. This would be related to the fact that the mean value of bulbar reddening is higher in T1DM, also considering that the mucoaqueous layer of the tear is reduced in this group.
Correlation analysis of HbA1c and diabetes duration with the tests
The percentage of HbA1c correlated significantly with all tests performed except the upper eyelid MGL, indicating a relationship between high HbA1c values and deterioration of the tear film layers and the lower eyelid MGL. Tavakoli et al. [51] indicated a significant inverse correlation between
HbA1c and corneal nerve fiber density, which would indicate the protective factor of good glycemic control against corneal complications.
A statistically significant moderate relationship of diabetes duration with lower LLT (p = 0.007) and higher upper eyelid MGL (p = 0.005) was also found, showing a direct relationship with the presence of evaporative-type dry eye. Other studies have also indicated that tear film instability increases with diabetes duration, finding a relationship with TFBUT in patients with T1DM [10, 45].
Limitations
Given the cross-sectional design, this study only reports a relationship between the factors and the results obtained, but we cannot draw conclusions about the cause. Future longitudinal studies are needed to confirm these results. Moreover, an examiner masked to the type of patient would reduce the risk of bias. In addition, a larger sample size would be recommended to be able to group patients according to HbA1c percentage, duration of diabetes, age, or sex.
Conclusion
Patients with T1DM without signs of retinopathy showed involvement of the mucoaqueous and lipid layers of the tear film, as well as a higher percentage of MGL, using a noninvasive analyzer. DED in people with T1DM cannot be ruled out by anamnesis and subjective symptom questionnaires alone; therefore, these patients should undergo regular anterior pole examinations. Noninvasive instruments, such as the OSA, are useful to make a rapid diagnosis of dry eye signs, causing less discomfort to patients than invasive methods.
Acknowledgements The authors would like to thank all the participants in the project for their collaboration. In addition, the authors also appreciate the support offered by the members of the Faculty of Pharmacy of the University of Seville, and the facilities of the Degree in Optics and Optometry.
Funding Funding for open access publishing: Universidad de Sevilla/ CBUA
Data availability The data showed in this research are available on request from the corresponding author. The data are non-publicly available due to their containing information that could compromise the privacy of research participants.
Declarations
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of Andalusia (code 1474-N-19) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest The authors declare no competing interests.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/
References
1. American Diabetes Association (2020) Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S14–S31. https://doi.org/10.2337/ dc20-S002
2. Ha M, Oh SE, Whang WJ et al (2022) Relationship between meibomian gland loss in infrared meibography and meibum quality in dry eye patients. BMC Ophthalmol 22(1):292. https:// doi.org/10.1186/s12886-022-02509-5
3. Shimazaki J (2018) Definition and diagnostic criteria of dry eye disease: historical overview and future directions. Investig Ophthalmol Vis Sci 59(14 Special Issue):DES7–DES12. https:// doi.org/10.1167/iovs.17-23475
4. Craig JP, Nichols KK, Akpek EK et al (2017) TFOS DEWS II. Definition and classification report. Ocul Surf 15:276–283. https://doi.org/10.1016/j.jtos.2017.05.008
5. Stapleton F, Alves M, Bunya VY et al (2017) TFOS DEWS II Epidemiology Report. Ocul Surf 15(3):334–365. https://doi.org/ 10.1016/j.jtos.2017.05.003
6. Millán A, Viso E, Gude F et al (2018) Incidence and risk factors of dry eye in a Spanish adult population: 11-year follow-up from the Salnés eye study. Cornea 37(12):1527–1534. https://doi.org/ 10.1097/ICO.0000000000001713
7. Manchikanti V, Kasturi N, Rajappa M, Gochhait D (2021) Ocular surface disorder among adult patients with type II diabetes mellitus and its correlation with tear film markers: a pilot study. Taiwan J Ophthalmol 11(2):156. https://doi.org/10.4103/tjo.tjo_56_20
8. Naik K, Magdum R, Ahuja A et al (2022) Ocular surface diseases in patients with diabetes. Cureus 14(3):e23401. https://doi.org/10. 7759/cureus.23401
9. Zou X, Lu L, Xu Y et al (2018) Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: The Beixinjing eye study. BMC Ophthalmol 18(1). https://doi.org/10.1186/s12886-018-0781-7
10. Inanc M, Kiziltoprak H, Hekimoglu R et al (2020) Alterations of tear film and ocular surface in children with type 1 diabetes mellitus. Ocul Immunol Inflamm 28(3):362–369. https://doi.org/10. 1080/09273948.2019.1571212
11. Wei A, Le Q, Hong J et al (2016) Assessment of lower tear meniscus. Optom Vis Sci 93(11):1420–1425. https://doi.org/10.1097/ OPX.0000000000000986
12. The Diabetic Retinopathy Research Group (1991) Fundus photographic risk factors for progression of diabetic retinopathy.
3
Ophthalmology 98(5):823–833. https://doi.org/10.1016/S01616420(13)38014-2
13. Schiffman RM, Christianson MD, Jacobsen G et al (2000) Reliability and validity of the ocular surface disease index. Arch Ophthalmol 118(5):615–621. https://doi.org/10.1001/archopht.118.5. 615
14. Ngo W, Situ P, Keir N et al (2013) Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea 32(9):1204–1210. https://doi.org/10.1097/ICO. 0b013e318294b0c0
15. Akil H, Buluş A, Andiran N, Alp M (2016) Ocular manifestations of type 1 diabetes mellitus in pediatric population. Indian J Ophthalmol 64(9):654–658. https://doi.org/10.4103/0301-4738. 194336
16. Sánchez-González JM, De-Hita-cantalejo C, Martínez-Lara C, Sánchez-González MC (2022) Lipid, aqueous and mucin tear film layer stability and permanence within 0.15% liposome crosslinked hyaluronic acid versus 0.15% non-crosslinked hyaluronic acid measured with a novel non-invasive ocular surface analyzer. J Clin Med 11(13):3719. https://doi.org/10.3390/jcm11133719
17. Sánchez-González MC, De-Hita-Cantalejo C, Martínez-Lara C, Sánchez-González JM (2022) Oral isotretinoin for acne vulgaris side effects on the ocular surface: hyaluronic acid and galactoxyloglucan as treatment for dry eye disease signs and symptoms. Front Med 9:2192. https://doi.org/10.3389/fmed.2022.959165
18. Sánchez-González JM, De-Hita-Cantalejo C, Sánchez-González MC (2022) Hyaluronic acid and galacto-xyloglucan eyedrop efficacy in young-adult oral contraceptive users of childbearing age. J Clin Med 11(15):4458. https://doi.org/10.3390/jcm11154458
19. Sánchez-González MC, Capote-Puente R, García-Romera MC et al (2022) Dry eye disease and tear film assessment through a novel non-invasive ocular surface analyzer: The OSA protocol. Front Med 9. https://doi.org/10.3389/fmed.2022.938484
20. Fan F, Li X, Li K, Jia Z (2021) To find out the relationship between levels of glycosylated hemoglobin with meibomian gland dysfunction in patients with type 2 diabetes. Ther Clin Risk Manag 17:797–807. https://doi.org/10.2147/TCRM.S324423
21. Sánchez-González MC, Madroñero M, García-Romera MC et al (2021) Effect of blue light filters on tear and contrast sensitivity in individuals using electronic devices. Eye Contact Lens 47(12):642–646. https://doi.org/10.1097/ICL.0000000000000843
22. Efron N (1998) Grading scales for contact lens complications. Ophthalmic Physiol Opt 18:182–186. https://doi.org/10.1016/ S0275-5408(97)00066-5
23. Guillon JP (1998) Non-invasive tearscope plus routine for contact lens fitting. Contact Lens Anterior Eye 21(SUPPL. 1):S31-40. https://doi.org/10.1016/S1367-0484(98)80035-0
24. García-Resúa C, Santodomingo-Rubido J, Lira M et al (2009) Clinical assessment of the lower tear meniscus height. Ophthalmic Physiol Opt 29(5):526–534. https://doi.org/10.1111/j.1475-1313. 2009.00634.x
25. Wolffsohn JS, Arita R, Chalmers R et al (2017) TFOS DEWS II Diagnostic Methodology report. Ocul Surf 15(3):539–574. https:// doi.org/10.1016/j.jtos.2017.05.001
26. Pult H, Riede-Pult B (2013) Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye 36(1):22–27. https://doi.org/10.1016/j.clae.2012.10.074
27. Han JX, Wang H, Liang HH, Guo JX (2021) Correlation of the retinopathy degree with the change of ocular surface and corneal nerve in patients with type 2 diabetes mellitus. Int J Ophthalmol 14(5):750–758. https://doi.org/10.18240/ijo.2021.05.17
28. Nadeem H, Malik TG, Mazhar A, Ali A (2020) Association of dry eye disease with diabetic retinopathy. J Coll Physicians Surg Pak 30(5):493–497. https://doi.org/10.29271/JCPSP.2020.05.493
29. Pathak R, Sachan N, Chandra P (2022) Mechanistic approach towards diabetic neuropathy screening techniques and future challenges: a review. Biomed Pharmacother 150:113025. https://doi. org/10.1016/j.biopha.2022.113025
30. Han SB, Yang HK, Hyon JY (2019) Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging 14:53–63. https://doi.org/10.2147/CIA.S190713
31. Yu L, Chen X, Qin G et al (2008) Tear film function in type 2 diabetic patients with retinopathy. Ophthalmologica 222(4):284–291. https://doi.org/10.1159/000140256
32. Chen Z, Xiao Y, Qian Y et al (2021) Incidence and risk factors of dry eye in children and adolescents with diabetes mellitus: a 3-year follow-up study. Front Med 8. https://doi.org/10.3389/ fmed.2021.760006
33. Zeng X, Lv Y, Gu Z et al (2019) The effects of diabetic duration on lacrimal functional unit in patients with Type II diabetes. J Ophthalmol 2019:1–11. https://doi.org/10.1155/2019/8127515
34. Garzón PSJ, López-Alemany A, Gené-Sampedro A (2019) Correlation between type 2 diabetes, dry eye and meibomian glands dysfunction. J Optom 12(4):256–262. https://doi.org/10.1016/j. optom.2019.02.003
35. Misra SL, Patel DV, McGhee CNJ et al (2014) Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res 2014:848659. https://doi.org/10.1155/2014/848659
36. Eom Y, Choi KE, Kang SY et al (2014) Comparison of meibomian gland loss and expressed meibum grade between the upper and lower eyelids in patients with obstructive meibomian gland dysfunction. Cornea 33(5):448–452. https://doi.org/10.1097/ICO. 0000000000000092
37. Viso E, Rodríguez-Ares MT, Abelenda D et al (2012) Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci 53(6):2601–2606. https://doi.org/10.1167/IOVS.11-9228
38. Yu T, Shi WY, Song AP et al (2016) Changes of meibomian glands in patients with type 2 diabetes mellitus. Int J Ophthalmol 9(12):1740–1744. https://doi.org/10.18240/ijo.2016.12.06
39. Yu T, Han XG, Gao Y et al (2019) Morphological and cytological changes of meibomian glands in patients with type 2 diabetes mellitus. Int J Ophthalmol 12(9):1415–1419. https://doi.org/10. 18240/ijo.2019.09.07
40. Wu H, Fang X, Luo S et al (2022) Meibomian glands and tear film findings in type 2 diabetic patients: a cross-sectional study. Front Med 9:762493. https://doi.org/10.3389/fmed.2022.762493
41. Koca S, Koca SB, İnan S (2022) Ocular surface alterations and changes of meibomian glands with meibography in type 1 diabetic children. Int Ophthalmol 42(5):1613–1621. https://doi.org/ 10.1007/s10792-021-02155-8
42. Gunay M, Celik G, Yildiz E et al (2016) Ocular surface characteristics in diabetic children. Curr Eye Res 41(12):1526–1531. https://doi.org/10.3109/02713683.2015.1136421
43. Saito J, Enoki M, Hara M et al (2003) Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea 22(1):15–18. https://doi.org/10. 1097/00003226-200301000-00004
44. Goebbels M (2000) Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol 84(1):19–21. https://doi. org/10.1136/bjo.84.1.19
45. Shujaat S, Jawed M, Memon S, Talpur KI (2017) Determination of risk factors and treatment of dry eye disease in type 1 diabetes before corneal complications at Sindh Institute of Ophthalmology And Visual Sciences. Open Ophthalmol J 11(1):355–361. https:// doi.org/10.2174/1874364101711010355
46. Liu R, Ma B, Gao Y et al (2019) Tear inflammatory cytokines analysis and clinical correlations in diabetes and nondiabetes with
dry eye. Am J Ophthalmol 200:10–15. https://doi.org/10.1016/j. ajo.2018.12.001
47. Lyu Y, Zeng X, Li F, Zhao S (2019) The effect of the duration of diabetes on dry eye and corneal nerves. Contact Lens Anterior Eye 42(4):380–385. https:// doi. org/ 10. 1016/j. clae. 2019. 02.011
48. Achtsidis V, Eleftheriadou I, Kozanidou E et al (2014) Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care 37(10):e210–e211. https://doi.org/10.2337/ dc14-0860
49. Beckman KA (2014) Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea 33(8):851–854. https://doi.org/10.1097/ICO.0000000000000163
Authors and Affiliations
50. Ferdousi M, Petropoulos IN, Kalteniece A et al (2018) No relation between the severity of corneal nerve, epithelial, and keratocyte cell morphology with measures of dry eye disease in type 1 diabetes. Invest Ophthalmol Vis Sci 59(13):5525–5530. https://doi. org/10.1167/iovs.18-25321
51. Tavakoli M, Kallinikos P, Iqbal A et al (2011) Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med 28(10):1261–1267. https://doi.org/10.1111/j.1464-5491. 2011.03372.x
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
María‑Carmen Silva‑Viguera1,2 · Alicia Pérez‑Barea1 · María‑José Bautista‑Llamas1,2
1 Department of Physics of Condensed Matter, Optics Area, Physics Faculty, University of Seville, Reina Mercedes St, Seville, Spain
2 Vision Research Group (CIVIUS), University of Seville, Seville, Spain
3
Tear film lipid layer thickness measurement from Ocular Surface Analyzer as a marker to monitor treatment of meibomian gland dysfunction in a study comparing physiological detergent-free eyelid wipes with conventional therapy: A randomized trial
Neelam Runda1,2, Souvik Manna1, Murugesan Vanathi1, Radhika Tandon1, Noopur Gupta1
Purpose: To compare the efficacy of physiological, non‑detergent eyelid wipes with conventional lid hygiene in patients with meibomian gland dysfunction (MGD). Methods: Fifty participants with MGD were recruited and randomized into two groups. Participants in group I used Evolve Pure Eyewipes twice a day to clean the eyelid debris along with standard therapy (antibiotic and lubricants) and participants in group II followed lid hygiene with warm compresses along with standard therapy. Symptoms, ocular surface assessment (lipid layer thickness, tear meniscus height, non‑invasive tear film breakup time, and meibography), slit‑lamp biomicroscopy (eyelash contamination, meibomian gland blockage, meibomian gland secretion, and meibomian gland telangiectasia) and tear film osmolarity were noted at baseline and 90 days after therapy. Results: Significant improvement in symptoms and signs of MGD was observed in both groups after treatment (P < 0.001); however, the clinical improvement was better with the use of eyelid wipes. Lipid layer thickness increased significantly in group I (P = 0.0006) and group II (P = 0.0002), which was maintained even after adjusting for sociodemographic variables such as age, sex, and severity score of symptoms and signs. Conclusion: Lipid layer thickness of the tear film is a sensitive marker in monitoring response to treatment in patients with MGD. The use of physiological detergent‑free eyelid wipes is non‑inferior to lid hygiene and warm compresses, which remains the mainstay for treatment of MGD; the clinical improvement with eyelid wipes was noted to be better.
Key words: Eyelid wipes, lid hygiene, lipid layer thickness, meibomian gland blockage, meibomian gland dysfunction, ocular surface analyzer
Meibomian gland dysfunction (MGD), as described by the International Tear Film and Ocular Surface Society (TFOS) workshop, is a “chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.”[1] MGD is a leading cause of evaporative dry eye, and its global prevalence has been reported to be 35.8%.[2]
The management of MGD, as recommended by the TFOS subcommittee, includes lid hygiene as the mainstay therapy for MGD, which usually consists of two components, including the application of heat and mechanical massage of the eyelids.[3] Overall, maintenance of eyelid hygiene not only plays a pivotal role in the management of MGD but also contributes to the health of the lacrimal functional unit.[4] Several premedicated eyelid wipes are commercially available. Of note, wet eyelid wipes, namely Oculeaf (tea tree oil, glycerine, sodium
1Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, 2Department of Ophthalmology, CGHS, Prayagraj, Uttar Pradesh, India
Correspondence to: Dr. Noopur Gupta, Additional Professor of Ophthalmology, Cornea, Cataract and Refractive Surgery Services, Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi ‑ 110 029, India. E‑mail: noopurgupta@ hotmail.com
Received: 15 Nov 2021 Revision: 15 Jan 2022
Accepted: 17 Feb 2022 Published: 31 May 2022
Access this article online
Website: www.ijo.in
DOI: 10.4103/ijo.IJO_2885_21
PMID:
*****
Quick Response Code:

hyaluronate, and panthenol), Cliradex® (4‑ terpineol), Blephapad Combo (terpinen‑4‑ol and hyaluronic acid), and Blephaclean (capryloyl glycine, iris florentina, and sodium hyaluronate) have been used with proven efficacy in anterior and posterior blepharitis.[5‑7] However, there is a lack of robust evidence to support their routine recommendation as has also been documented in a systematic review, apart from the fact that they induce ocular inflammation due to the presence of surfactants and detergents.[8] The eyelid wipes used in the current study (Evolve Pure Eyewipes) are physiological, non‑detergent, single‑use wipes impregnated with sodium chloride, potassium chloride, calcium chloride dehydrate, magnesium chloride hexahydrate, sodium acetate, sodium hydroxide, and purified water, similar to the human tear film. Therefore, the current study was planned to assess the efficacy of these physiological eyelid wipes in patients with MGD and document the change in tear film parameters and
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
For reprints contact: WKHLRPMedknow_reprints@wolterskluwer.com
Cite this article as: Runda N, Manna S, Vanathi M, Tandon R, Gupta N. Tear film lipid layer thickness measurement from Ocular Surface Analyzer as a marker to monitor treatment of meibomian gland dysfunction in a study comparing physiological detergent-free eyelid wipes with conventional therapy: A randomized trial. Indian J Ophthalmol 2022;70:1963-70.
meibomian gland morphology by objective monitoring using the non‑invasive ocular surface analyzer (OSA).[9]
Methods
Study setting and study participants
A prospective, randomized double‑arm controlled interventional study was undertaken to evaluate the efficacy of physiological eyelid wipes in subjects with MGD. Ethical approval for the study was obtained from the institute’s ethics committee (IEC‑377/06.07.2018, RP‑5/2018). The study was conducted from March 2019 to March 2020 in compliance with the Declaration of Helsinki, registered at Clinical Trials Registry‑India [CTRI/2018/08/015482]. Participants aged 18 years and above, who presented with a clinical diagnosis of MGD, defined as a minimum combined score of 5 for the signs 1) eyelash contamination (flaky material on the eyelash and lid margin comprising desquamated material/debris/glandular secretion), 2) meibomian gland blockage, 3) meibomian gland secretion, and 4) meibomian gland telangiectasia and/or symptoms of 1) dryness, 2) foreign body sensation, 3) burning, 4) redness, 5) crusting, or 6) sticky lids as per MGD symptom questionnaire developed by Schein et al. [10] were voluntarily recruited from the ophthalmology outpatient department of a tertiary eye care hospital after obtaining informed consent. Patients with acute ocular infection or inflammation other than MGD, with chronic ocular disease, using topical medications or contact lens, who underwent ocular surgery within the last 6 months, pregnant or lactating females, and/or physically or mentally challenged individuals were excluded.
Clinical assessment
A symptom questionnaire was administered to all participants to assess the dry eye symptomatology focusing on key ocular manifestations of MGD, such as a feeling of dryness, foreign body sensation, burning sensation, and feeling of stickiness. These four symptoms were graded on a scale of 0–4 from none to very severe disabling manifestation [Appendix 1]. This was administered by a separate ophthalmologist who did not perform the clinical examination.
The clinical assessment included measurement of ocular surface parameters and slit‑lamp biomicroscopy for evaluation of MGD. Ocular surface analysis was conducted by an optometrist by using the ICP Tearscope (SBM Sistemi, Turin, Italy) in the following order: lipid layer thickness (LLT), tear meniscus height (TMH), non‑invasive tear film breakup time (NIBUT), and meibography. The least invasive test was performed first to minimize perturbation of tear film physiology for the subsequent tests; tear film osmolarity, slit‑lamp examination, and meibomian gland expression were performed sequentially in that order. Analysis of LLT was performed using interference patterns by capturing the video for 5–10 s, allowing for blinking of eyes 3–5 times, then comparing the patient’s lipid layer to the standard image of the lipid layer, and grading was done as per modified Guillon’s classification.[11] The lower TMH was assessed using digital imaging under high magnification, captured by the ICP Tearscope, taking a picture with a green cross focused on the pupil and then focusing on the tear meniscus seen along the lower eyelid margin due to internal reflection. The TMH was calculated using an inbuilt software, and three measurements near the center of the lower meniscus were averaged and graded [Appendix 2]. NIBUT was recorded as the time taken following a blink for the grid reflection to first show distortion,
and tear break‑up time was calculated using an automated inbuilt software. Three measurements were averaged for each eye and graded.[11] Meibography was performed by capturing infrared images with the BG‑4M noncontact meibography system (SBM Sistemi, Turin, Italy). Images were digitally analyzed using ImageJ software (freely available in the public domain). From the captured image, meibomian gland dropout areas were graded on the five‑point meiboscale [Appendix 2].
Slit‑lamp examination included assessment of eyelash contamination, lid margin thickening, telangiectasia, foaming, eyelash crusting, the number of blocked meibomian gland orifice, and meibum viscosity and expression graded on a five‑point Likert scale [Appendix 1].[11] Tear film osmolarity was evaluated for each patient at baseline and at 90‑day follow‑up visit by using a lab‑on‑a‑chip technique (TearLab®; TearLab Corporation, San Diego, CA). A test card was used to collect and analyze 50 nanoliters of tear sample from the lower tear meniscus near the lateral canthus by capillary action. Quality control was performed before each study day as recommended by the manufacturer. An osmolarity result of ≥308 mOsmol/L was taken as abnormal. To avoid diurnal fluctuation of tear osmolarity, all measurements were taken in the morning, between 8.00 and 11.00 AM.[12,13]
Treatment
Subjects were randomly assigned in 1:1 ratio to one of the two groups of standard therapy for MGD (antibiotics and lubricants) along with eyelid wipes (group I) and those prescribed standard therapy for MGD along with lid hygiene and warm compresses (group II) by an independent clinical research coordinator according to a computer‑generated randomization list. Eyelid hygiene measures employed were eyelid scrubs and eyelid massage. Lid hygiene was done by warm compression with a hot wet towel for 10 min followed by mechanical lid massage with fingertips and cleaning of the expressed sebum with cotton buds. The upper eyelid followed by the lower eyelid was first massaged by around 15 firm downward strokes, which were then scrubbed with cotton bud along the lid margin. At the end of the scrub, the ointment was applied along the lid margin at the base of the eyelashes. The randomization sequence was created between 1 and 2 by using the RAND function in Excel 2013 (Microsoft Inc., Redmond, WA) and was stratified with a 1:1 allocation. Thus, the ophthalmologist, who did the clinical examination, was masked to the treatment received by the patient. Standard therapy for MGD included warm compression with mechanical lid massage along with cleaning of the expressed sebum from lid margin and antibiotic eye drop (gatifloxacin 0.3%) instillation four times a day and gatifloxacin 0.3% ointment application at night for 2 weeks along with lubricating eyedrop (polyethylene glycol 0.4% and propylene glycol 0.3%) four times a day for 3 months. Participants with severe MGD were prescribed systemic antibiotics (doxycycline 50 mg twice a day for 2 weeks). Lifestyle changes and advice on digital screen use were imparted in certain patients, whenever the same was contributory to MGD. The participants were followed up on days 15, 30, 60, and 90; however, study parameters were noted and analyzed between the baseline and final visit at completion of 3 months.
Statistical methods
The study was designed and powered to demonstrate the non‑inferiority of the eyelid wipes relative to lid hygiene with respect to the symptoms and signs of MGD. The sample size of 20 subjects (40 eyes) was calculated to test for non‑inferiority based on a minimum power of 90%, one‑sided type I error of
0.025, non‑inferiority limit of 85%, and a true mean difference of 25% (as a percentage of the non‑inferiority limit).[14]
Data analyses were performed to determine the main effects of intervention, testing occasion, and the interaction effects of group and time. To determine whether the patients in either group experienced changes in their ocular surface analyzer and slit‑lamp parameters, a paired t test was used. The primary dependent variables were ocular surface
analyzer parameters (NIBUT, LLT, and TMH), and the independent variable was the testing occasion (baseline vs. 90‑day follow‑up visit). For the comparison of intergroup ocular surface analyzer parameters, a between‑subjects ANOVA design (two‑way analysis of variance) was performed on the dependent variables (NIBUT, LLT, and TMH), while the independent variable was the intervention in each group (eyelid wipes versus lid hygiene). To detect interaction effects between the groups and testing


*Statistically significant difference between groups
*statistically significant difference (<0.05)
occasion, a 2 × 2 mixed‑model ANOVA design was used with dependent measures of NIBUT, LLT, and TMH as the main outcomes; the group (eyelid wipes versus lid hygiene) as the between‑subjects independent variable; and the time (baseline vs. 90‑day follow up visit) as the repeated
measures within‑subjects independent variable. The data were analyzed using the Stata 14.2 statistical package for Windows by using the intention‑to‑treat principle. Differences between the groups in the clinical and demographical variables were analyzed using the Chi‑squared test. The outcome variables
were normally distributed and analyzed using parametric tests. A stepwise linear regression model was used to assess the differences within and between groups and to determine the predictors of change in the outcome variables. The level of significance was taken as 0.05.
Results
A total of 71 participants with MGD were screened and assessed for eligibility. Out of these, 25 patients who met the eligibility criteria were recruited in each group by using random number tables. These 50 participants with MGD were included in the study [Fig. 1]. A total of 37 patients with MGD completed the follow‑up of 90 days. Out of these, 40 eyes of 20 patients (14 men and 6 women; mean age: 33.4 ± 10.53 years) were treated with eyelid wipes along with standard therapy, and 34 eyes of 17 patients (11 men and 6 women; mean age: 42.7 ± 11.25 years) were prescribed standard therapy along with lid hygiene. The two groups had similar demographic and clinical profiles [Table 1]. Moreover, the outcome measures, including the grade of disease, were comparable at baseline between the two groups (P > 0.05) [Table 2].
Dry eye symptoms
There was a significant improvement in all the subjective dry eye symptoms from baseline to the 90‑day follow‑up visit (P < 0.001) [Table 2]. The symptom of dryness improved in 70.0% of the patients using eyelid wipes and standard therapy (group I) compared to 64.7% of patients using lid hygiene along with standard therapy (group II). Foreign body sensation ameliorated in 45.0% patients in group I in comparison to 41.2% of patients in group II. Half (50%) of the patients in group I reported relief in burning sensation compared to 41.2% in Group II. At the last follow‑up visit, stickiness of eyelids was present in only 15.0% of the patients in group I, and improvement in this symptom was noted in 35% and 29.4% in groups I and II, respectively. Both groups demonstrated marked improvement after 90 days of therapy in all the above symptoms (P < 0.001). However, symptomatic improvement was better in participants using the physiological eyelid wipes.
Slit-lamp parameters
All the slit‑lamp parameters showed significant improvement in both groups after 90 days of therapy (P < 0.05). No eyelash contamination was observed at the 90‑day follow‑up visit in 25.0% of the patients in group I versus 61.8% of the patients in group II, whereas slight contamination was seen in 45.0% versus 64.7% in the aforementioned groups, respectively. None of the patients in either group had discharge in the eyelashes at the 90‑day follow‑up visit. Statistically significant improvement in meibomian gland telangiectasias was observed in both study groups (P = 0.005 in group I and <0.001 in group II). No signs of telangiectasia were present at the 90‑day follow‑up visit in 27.5% and 23.5% of patients in groups I and II, respectively. None of the patients in either study group had more than grade 3 telangiectasia, but the majority (62.5% and 70.6% in groups I and II, respectively) had less than one‑fourth involvement (grade I telangiectasia) at the 90‑day follow‑up visit. Meibomian gland blockage demonstrated a statistically significant response with therapy (P = 0.001 in both groups). At the last follow‑up visit, most patients (67.5% and 70.6% in groups I and II, respectively) demonstrated less than one‑fourth meibomian gland blockage (grade 1). The quality of meibomian gland secretions also improved significantly. Clear meibum was expressed in 47.5% and 38.2% of the patients in groups I
and II, respectively (grade 0). Thick, white particulate meibum or non‑expressibility was observed in neither of the group participants at the last follow‑up visit (grades 3 and 4) [Table 2].
Ocular surface parameters
The integrated platform for analysis of the ocular surface helped in recording the change in LLT, TMH, NIBUT, and MGL along with meibography images before and after treatment [Fig. 2].
LLT increased markedly after using eyelid wipes and standard therapy ( P = 0.0006), and a similar trend was noted among patients using lid hygiene and standard therapy (P = 0.0002). TMH also improved significantly among eyelid wipes users ( P = 0.012), whereas NIBUT showed a favorable response after lid hygiene (P = 0.020). In both groups, meibomian gland loss did not show any appreciable change after 90 days of treatment. When testing the baseline and 90‑day intervention effects with the ocular surface analyzer parameters as the dependent variables in a two‑way ANOVA for repeated measures, the interaction effect was non‑significant, demonstrating that changes in the ocular surface analyzer parameters were the same in the two groups. The multiple linear regression was done to determine the change in ocular surface analyzer parameters at the 90‑day follow‑up visit, adjusting for the effect of the same parameter at baseline and other independent variables [Table 3]. It indicated that LLT at the 90‑day follow‑up visit was significantly altered from its baseline values, even after adjusting for the use of eyelid wipes and sociodemographic variables such as age, sex, and symptom or sign score (P < 0.001). On the contrary, NIBUT and TMH at the 90‑day follow‑up visit were not significantly changed from baseline, after adjusting for these parameters.
Tear film osmolarity showed no significant improvement at the 90‑day follow‑up visit (311.12 ± 13.02 vs. 315.54 ± 26.80 mOsmol/L in groups I and II, respectively) nor any difference was noted between the groups [Table 2].
Discussion
The primary objective of the current study was to evaluate the efficacy of the eyelid wipes as compared to lid hygiene and warm compresses in the treatment of MGD. Although eyelid wipes are shown to be non‑inferior to the mainstay therapy with regard to an efficacy endpoint and both groups demonstrated improvement in both symptoms and signs of MGD with treatment, the clinical improvement with eyelid wipes was noted to be better. To the best of our knowledge, this is the first study on the use of physiological, non‑detergent eyelid wipes in MGD patients where objective monitoring was performed using an ocular surface analyzer in patients with MGD.
Remarkable symptomatic relief in dryness, foreign body sensation, burning, and stickiness of lids was observed in both groups, corroborating with the results of a study performed in patients with MGD and anterior blepharitis by using Blephaclean eyelid wipes.[7] This study, however, did not have a control arm receiving treatment as per standard of care. Moreover, the use of a non‑invasive ocular surface analyzer and meibography makes the current study more comprehensive and holistic. As depicted in previous trials, the ocular surface analyzer is a non‑invasive, reproducible method for ocular surface workup, characterized by the standardized recording of meibomian gland function eliminating interobserver bias.[15]
The symptomatic relief in the study patients corroborated with significant amelioration of eyelid margin health as observed by a d ecrease i n e yelash c ontamination a nd meibomian gland blockage with improvement in quality of meibomian gland secretion as has been reported previously.[16]
The study participants demonstrated betterment in meibomian gland telangiectasia, which was not noted with the use of eyelid wipes in available published literature.
Both the therapeutic arms in the current study facilitated an increase in the LLT of the patients with MGD, as has been reported by previously published studies.[17‑19] On the contrary, other parameters such as TMH and NIBUT did not show an adequate trend of improvement. This result was in contrast to earlier studies.[19,20] In the current study, tear osmolarity demonstrated no significant change in either group as observed in a previous study where automated thermodynamic treatment [Lipiflow®, Johnson & Johnson Inc., New Brunswick, NJ] was compared with standard lid hygiene procedure.[18] Thus, it may be inferred that LLT is a sensitive ocular surface parameter that corroborated the symptoms and signs of MGD and can serve as a useful test in monitoring the amelioration of disease with intervention.
Nonetheless, our study reiterates that lid hygiene with warm compresses combined with standard therapy remains the mainstay for treatment of MGD.[3] Conventional therapy of hot fomentation and massage addresses the pathophysiology of MGD. The melting point of the expressed meibum is raised to 32.2°C–35.3°C, higher than the normal meibum due to the altered composition consisting of desquamated epithelial cells. Thus, raised temperature facilitates the secretion and delivery of meibum to the ocular surface.[21]
Our study has a few limitations. The sample size in each group was small, resulting in an insignificant change in breakup time and tear osmolarity after intervention for MGD. The minimum sample size was taken based on feasibility and resource availability. The follow‑up of all patients could not be completed due to the onset of the COVID‑19 pandemic, which restricted travel and transport due to the nationwide lockdown in the country. A large‑scale study with longer follow‑up may be undertaken in the future.
Conclusion
The current study demonstrates that lipid layer thickness (LLT) of the tear film, as determined by the Ocular Surface Analyzer (OSA) is a sensitive parameter to monitor the therapeutic response in patients with MGD. Although lid hygiene and warm c ompresses a long w ith a ntibiotics i s th e m ainstay for tr eatment o f M GD, th e a dditive u se o f p hysiological detergent‑free eyelid wipes can enhance clinical improvement in these patients.
Acknowledgements
Mr. Vikrant is ac knowledged fo r his c ontribution to d ata acquisition. M r. Deepak Kum ar and M s. Subhi Jain are acknowledged for their contribution to the management and analysis of the data.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in public, commercial, or not‑for‑profit sectors.
Conflicts of interest
There are no conflicts of interest.
References
1.Nelson JD, Shimazaki J, Benitez‑del‑Castillo, Jose M, Craig JP, McCulley JP, et al The international workshop on meibomian gland dy sfunction: Report of t he definit ion andclassificat ion subcommittee. Invest Ophthalmol Vis Sci 2011;52:1930–7.
2.Hassanzadeh S, V armaghani M, Zarei‑Ghanav ati S, Heravian Shandiz J, Azimi Khorasani A. Global prevalence of meibomian gland dysfunction: A systematic review and meta‑analysis. Ocul Immunol Inflamm 2021;29:66‑75.
3.Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’Brien T, et al The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of m eibomian gl and d ysfunction. I nvest Oph thalmol Vis S ci 2011;52:2050–64.
4.Benitez‑Del‑Castillo JM How to promote and preserve eyelid health. Clin Ophthalmol 2012;6:1689–98.
5.Qiu TY, Yeo S, Tong L. Satisfaction and convenience of using terpenoid‑impregnated eyelid wipes and teaching method in people without blepharitis. Clin Ophthalmol 2018;12:91–8.
6.De Luca V, Carnev ali A, Carnov ale Scalzo G, Piccoli G, Bruzzichessi D, S corcia V E fficacy and s afety o f wet wipes containing Hy ‑Ter ® solut ion compared wit h st andard care for bilateral posterior blepharitis: A preliminary randomized controlled study. Ophthalmol Ther 2019;8:313‑21.
7. Guillon M, Maissa C, Wong S. Symptomatic relief associated with eyelid hygiene in anterior blepharitis and MGD. Eye Contact Lens 2012;38:306–12.
8. Gostimir M, Allen LH. Is there enough evidence for the routine recommendation of eyelid wipes? A systematic review of the role of eyelid wipes in the management of blepharitis. Can J Ophthalmol 2020;55:424–36.
9. Gilbard JP. Human tear film electrolyte concentrations in health and dry‑eye disease. Int Ophthalmol Clin 1994;34:27–36.
10.Schein OD, T ielsch JM, M unõz B, Band een‑Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population‑based perspective. Ophthalmology 1997;104:1395‑401.
11. Pult H, Riede‑Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye 2013;36:22–7.
12. Versura P, Campos EC. TearLab® osmolarity system for diagnosing dry eye. Expert Rev Mol Diagn 2013;13:119–29.
13. Szalai E, Berta A, Szekanecz Z, Szûcs G, Módis L Jr. Evaluation of tear osmolarity in non‑Sjögren and Sjögren syndrome dry eye patients with the TearLab system. Cornea 2012;31:867‑71.
14. Flight L, Julious SA. Practical guide to sample size calculations: Non‑inferiority and equivalence trials. Pharm Stat 2016;15:80–9.
15.Giannaccare G, Vigo L, Pellegrini M, Sebastiani S, Carones F. Ocular surface w orkup w ith a utomated n oninvasive m easurements for the diagnosis of meibomian gland dysfunction. Cornea 2018;37:740‑5.
16.Guillon M, Maissa C, Wong S. Ey elid margin modification associated with ey elid hygiene in anterior blepharitis and meibomian gland dysfunction. Eye Contact Lens 2012;38:319–25.
17. Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens 2003;29:96–9.
18. Finis D, Hayajneh J, König C, Borrelli M, Schrader S, Geerling G. Evaluation of an automated thermodynamic treatment (LipiFlow®) system f or m eibomian gl and d ysfunction: A pr ospective, randomized, observer‑masked trial. Ocul Surf 2014;12:146‑54.
19.Li B, Fu H, Liu T, Xu M. Comparison of the therapeutic effect of Meibomian Thermal Pulsation LipiFlow® on obstructive and hyposecretory meibomian gland dysfunction patients. Int Ophthalmol 2020;40:3469‑79.
20.Wei S, Ren X, Wang Y, Chou Y, Li X. Therapeutic effect of intense pulsed light (IPL) combined with meibomian gland expression (MGX) on meibomian gland dysfunction (MGD). J Ophthalmol 2020;2020:3684963.
21. Terada O, Chiba K, Senoo T, Obara Y Ocular surface temperature of meibomian gland dysfunction patients and the melting point of meibomian gland secretions. Nippon Ganka Gakkai Zasshi 2004;108:690‑3.
Parameters
Symptomology (discomfort, severity & frequency)
Dryness
Foreign body sensation
Burning sensation
Lid stickiness
Slit‑lamp signs
Eyelash contamination
Meibomian gland blockage
Meibomian gland secretion
Meibomian gland telangiectasia
Parameter
Tear Osmolarity (mOsmol/L)
Lipid layer thickness (nm)
Tear meniscus height (mm)
Noninvasive tear breakup time (s)
Meibomian gland loss (%)
None Mild and/or episodic; occurs under environmental stress
Clear
None
Clear liquid
None
Slight contamination <25% block Yellowish liquid <25% glands
Grading scale
Moderate episodic or chronic, stress or no stress
Mild 25% <50% Opaque and toothpaste‑like consistency 25% <50%
Severe frequent or constant without stress
Severe and/or disabling and constant
Moderate
50% <75%
Thickened white material
50% <75%
Severe 75% or more
Not possible 75% or more
Grading Categories
295‑<308 (hypersecretory MGD)
≥308‑370 (hyposecretory MGD)
Grade 0=absence of lipids
Grade 1=13‑30 (open meshwork)
Grade 2=31‑50 (closed meshwork)
Grade 3=51‑80 (wave)
Grade 4=81‑90 (amorphous)
Grade 5=91‑140 (color fringes)
Grade 0 >0.25 (normal)
Grade 1 ≤0.25‑>0.17 (mild DED)
Grade 2 0.17 to 0.11 (moderate to severe DED)
Grade 0=instantaneous
Grade 1 = <6
Grade 2 = ≥6‑<12
Grade 3 = ≥12‑24
Grade 0: 0%
Grade 1: >0% to≤25%
Grade 2: 26%‑50%
Grade 3: 51%‑75%
Grade 4: >75%
MGD=Meibomian gland dysfunction; DED=dry eye disease
Appendix 1: Grading scales showing the severity of symptoms and signs of evaporative dry eye disease and MGD