
ANNUAL REPORT | 2020-2021
February 2021
Prepared by the Consortium for Medical Marijuana Clinical Outcomes Research
For more information about the Consortium visit: www.mmjoutcomes.org
For questions or comments, contact:
Almut G Winterstein, PhD Director, Consortium for Medical Marijuana Clinical Outcomes Research
Email: mmj.outcomes@cop.ufl.edu
Phone: 352-273-6984
TABLE OF CONTENTS Executive Summary ....................................................................................................... 5 Consortium Research Program ....................................................................................5 Consortium Research Plan 2021-2022 8 Introduction 10 Consortium Leadership and Administrative Structure 12 The Consortium for Medical Marijuana Clinical Outcomes Leadership 12 Consortium Administrative Structure 12 Board Meetings ............................................................................................................ 13 Consortium Efforts in FY 2021 .................................................................................... 15 Grants Program ............................................................................................................15 2019 Grants Program 16 Executive Summary of Final Reports of the 16 2019 Awarded Research Projects 2020 Grants Program 21 Summary of 2020 Awarded Research Projects 21 2021 Grants Program 24 Highlights of Grant Awardee Progress Reports 24 MMJ Clinical Outcomes Research Data Repository (MEMORY) 26 MEMORY Data Dictionary ...................................................................................... 27 Clinical Research Core ................................................................................................ 28 MMJ Contact Registry .......................................................................................... 28 MMJ Patient Cohort 29 Outreach 30 Cannabis Clinical Outcomes Research Conference 31 Provider Survey 33 Website 34 Newsletter .............................................................................................................. 35 Evidence Core ............................................................................................................. 35 Research Priorities for 2021-2022....................................................................... 36 Scientific Journal of the Consortium 37 Consortium Bibliography 38 Future Plans for the Consortium 40 Summary of Consortium Research Priorities 40 Research Plan 2021-2022 40 Grants Program ..................................................................................................... 40 MEMORY ................................................................................................................. 41 Clinical Core ........................................................................................................... 41 Outreach 41 Evidence Core 41 Appendix A 42 The Consortium for Medical Marijuana Clinical Outcomes Research Board Appendix B ................................................................................................................... 47 Leadership and Staff of the Consortium for Medical Marijuana Clinical Outcomes Research Appendix C .................................................................................................................. 51 List of Reviewers of the 2020 Research Grants Program

4 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
EXECUTIVE SUMMARY
Beginning with the Compassionate Use Act passed in 2014 and followed by several amendments, Florida law allows the use of marijuana for the treatment of certain debilitating conditions. Persons seeking Medical Marijuana (MMJ) may suffer from serious health conditions and symptoms, many of which are not responsive to approved medications. While MMJ could improve health outcomes, there are also significant safety concerns related to cognitive effects, risk for accidents, interactions with other medications, psychosis, and addiction. Moreover, MMJ varies significantly in terms of its specific components and mode of administration (including smoking and vaping), but little is known about how and which types, doses and delivery methods provide the optimal risk-benefit profile. There is a substantial need to understand how MMJ impacts health and safety outcomes. But due to the complex federal and state legal restrictions for both MMJ use and MMJ research, the development of evidence on the safety and effectiveness of medical marijuana is lagging far behind its rapid uptake. As of January 2021, more than 450,000 Floridians are licensed to use medical marijuana.
To address the need for more evidence, the state legislature established the Consortium of Medical Marijuana Clinical Outcomes Research (Consortium) to conduct, disseminate and support rigorous scientific research on the clinical effects of medical marijuana. In July 2019, the Florida State University System Board of Governors designated the University of Florida (UF) the lead university of the Consortium. Eight additional universities have joined the Consortium to-date, each with one designee to form the Consortium Research Board. The endorsed charter defines the Consortium purpose as “creating an effective medical marijuana clinical outcomes research program which would mobilize the scientific and medical resources that presently exist in the state to determine the appropriate and best use of marijuana to treat illness.” Consortium responsibilities as defined in the charter and consistent with statute include:
• Conduct rigorous scientific research
• Disseminate research
• Guide statewide policy on ordering and dosing practices for the medical use of marijuana.
The following report details activities of the Consortium completed during the second year of its existence (July 2020 – Feb 1, 2021). The first annual report specified by statute was submitted on February 15, 2020 and is available at https://mmjoutcomes.org/ourconsortium/annualreport/
CONSORTIUM RESEARCH PROGRAM
The Consortium research program rests on five pillars aimed at supporting the Consortium mission to foster medical marijuana clinical outcomes research including: A Grants Program, a unique research data repository known as the MEdical Marijuana Clinical Outcomes RepositorY (MEMORY), a Clinical Research Core, an Evidence Core and an Outreach Program. The Consortium has made great strides across all five pillars and intends to
2021 ANNUAL REPORT | 5
build on this strong foundation further as proposed in the new 2021-2022 research plan. Following is a brief overview of the purpose and accomplishments for each pillar.
The Consortium Grants Program, launched in September 2019, awarded 10 proposals to Faculty from 5 Consortium member institutions. Executive summaries of the final reports by the awardees in January 2021 are presented in this report. The Consortium launched its 2nd year grants cycle in February 2020 and received 20 proposals. Nine awards were made to faculty belonging to 5 of the Consortium member institutions. Research outcomes and health conditions in these proposals include glioblastoma, anorexia nervosa, chemotherapy induced peripheral neuropathy, anxiety, olfactory function, food-predicting sensory cues, drug interactions, chronic pain, and pattern and motivation for MMJ use. Progress reports were submitted by these awardees in Jan 2021 and a succinct summary is included in this report.
The Consortium launched its third grants cycle in December 2020 with release of its Request for Proposals and updated research priorities that were derived in consultation with scientific and subject matter experts, evidence review, input from stakeholder engagement and NIH areas of programmatic interest. The early start of the cycle allows completion of application reviews by the end of the fiscal year and expeditious funding of prioritized proposals, once the Consortium budget for FY 2022 is approved.
Highlights of Early Outputs of the Consortium Research Program
Though early in the research process with only 1 year after the first grants were awarded and despite the COVID-19 pandemic, our awardees and other Consortium researchers have made great strides.
6 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Research findings are presented at scientific meetings and in peer-reviewed publications, and the new data has already served as the basis for 1 patent and 6 new extramural grant applications. Noteworthy, 41 trainees have been involved in the funded research grants and one new course has been approved, supporting the development of MMJ research capacity in the state.
The Consortium Outreach Program has continued is quarterly newsletter, expanded information on its website at https://mmjoutcomes.org/, and has completed a state-wide MMJ provider survey to gather input on research and training needs. As key activity for this year, the Consortium is holding its inaugural annual Cannabis Clinical Outcomes Research Conference (CCORC) on April 8th and 9th, 2021 (http://ccorc.mmjoutcomes. org/). This will be a research-centric meeting, open to researchers, patients and providers, to share research findings and stimulate research collaborations throughout the state and nationally. A scientific program committee with participation from consortium member institutions advises the organizing committee on conference themes and scope of scientific content. The planning and progress milestones thus far are presented in this report.
The Clinical Research Core was established to complement MEMORY, and provide infrastructure support for prospective studies (including randomized controlled trials) involving collection of new data, which may include detail on patient reported outcomes or other experiences related to MMJ use. To facilitate such studies, the Clinical Core is working with patients, providers, researchers and industry partners. A MMJ patient contact registry to support recruitment into research studies has been rolled out statewide, with a registry portal on the consortium website. To date, 204 individuals who are using or are planning to use MMJ have registered. Providers and industry partners are now listed in the online “Connect and Advance Research for Medical Marijuana Analysis” (CARMMA) database. The clinical core has also launched planning for the first Florida MMJ user cohort, governed by an 11-member scientific planning committee. The core has also successfully aided an investigator to obtain a FDA Investigational New Drug (IND) authorization for his clinical research.
MEMORY is designed to establish the infrastructure for real-world MMJ clinical outcomes evaluations similar to those employed by the US Food and Drug Administration (FDA). Specifically, the Consortium aims to link the Office of Medical Marijuana Use (OMMU) MMJ registry data with other clinical databases commonly used for outcomes research to create a robust research-ready repository. Linkage of detailed MMJ dispensing information with clinical outcomes data allows controlled studies on MMJ effectiveness and safety and establishes an active surveillance platform that can detect emerging safety

2021 ANNUAL REPORT | 7
signals. The data use agreements are currently under review by the Department of Health for use of OMMU registry data and the Agency for Health Care Administration (AHCA) for use of Medicaid data. Use of vital data has been granted. The MEMORY team has developed the repository architecture and data dictionaries that will guide MEMORY development upon final data acquisition.
Finally, the Consortium Evidence Core has continued its synthesis of evidence to inform the 2021 Consortium research agenda and to disseminate such evidence as resource for providers, patients and researchers. Several publications, scientific and educational activities produced by consortium members are listed in this report. The Evidence Core developed research priorities for FY20 based on a comprehensive literature review, national expert panel prioritization, and review of statutory mandates. In the current year, we built further on these research priorities from the latest inputs from our state-wide physician survey and review of NIH areas of programmatic interest to refine research priorities for FY22. These research priorities are applied in the Consortium’s 2021 grants program and guide all Consortium activities.
2021-22 RESEARCH PRIORITIES
1. Clinical Outcomes of Medical Marijuana use
• Emphasis on chronic pain, anxiety/PTSD, cancer symptoms, sleep disorders, opioid/ substance use
• Effects on the developing brain, pregnancy and breastfeeding, other serious side effects
2. Route of Administration
Effect of dosing and routes (especially vaping) on efficacy and safety
3. Interactions of Medical Marijuana with other drugs/medications
Emphasis on common co-medications and polysubstance use
4. Epidemiology Research
Cannabis use and cannabis use disorder patterns, including the impact of COVID-19
5. Research on the human endocannabinoid system
6. Measurement and effects of components of medical marijuana/cannabis
• Safety and efficacy of different components and potency levels
• Standards for measuring cannabis dose, intoxication, and impairment
Consortium Scientific Journal
As new outlet for the Consortium’s scientific output, the Consortium has signed a collaborative agreement with the journal “Medical Cannabis and Cannabinoids,” to serve as the consortium’s official journal. Consortium leadership has joined the journal’s editorial board and launched a new Consortium-led series “Evidence in Context.” This series will feature appraisals of new published evidence that is relevant to clinical practice.
CONSORTIUM RESEARCH PLAN 2021-2022
Since its inception in July 2019, the Consortium has made great strides towards facilitating and conducting research that informs clinical care and policy about the medical use of marijuana to improve public health. To build on these initial efforts, the Consortium board agreed to continue development of the five Consortium research program pillars: The Grants program, MEMORY, the Clinical Core, Outreach and Evidence Core Activities. The specific goals and plans for each pillar for FY22 are described below.
8 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
• In light of the impressive response to the Consortium’s Grants program over two cycles, the board will continue this effort. With awards anticipated to be made in July 2021, awardees will have a 12-month funding period to complete their research. The most noteworthy modification of the grants cycle is the focus on updated specific research priorities.
• Plans for MEMORY development remain unchanged for fiscal year 2022 with the key focus on establishing data sharing agreement and procedures with OMMU. As envisioned, MEMORY can then support controlled studies on MMJ effectiveness and safety and active surveillance for emerging safety issues among MMJ users.
• Goals for the Clinical Core will include continuation of recruitment for the first prospective cohort of MMJ users, allowing detailed ascertainment of data on MMJ use and outcomes. The Clinical Core will also continue guidance for investigators on regulatory issues involving use of MMJ in research studies and provide other research support.

• The Consortium will increase its Outreach through its inaugural annual conference Cannabis Clinical Outcomes Research Conference (CCORC) in April 2021. For the coming fiscal year, we envision conversion of the virtual meeting platform to a live event in Orlando. The Consortium will continue all other outreach efforts including its newsletter, website and attendance of key scientific meetings.
• The Evidence Core will build on its emerging evidence reviews series in collaboration with the Consortium journal, Medical Cannabis and Cannabinoids, and continue its development of patient/provider info sheets. The purpose of these info sheets is to provide unbiased, evidence-based and up-to-date information for important topics on MMJ clinical outcomes in a format that is clear and concise for all members of the public. The evidence core will also continue its update of evidence to support the Consortium research agenda and coordinate its scientific output.
The Consortium board and staff look forward to continuing this critical work in fiscal year 2021-2022 to support the Florida Medical Marijuana program’s primary intent to improve the health of Florida citizens.
2021 ANNUAL REPORT | 9
INTRODUCTION
Beginning with the Compassionate Use Act passed in 2014 and followed by several amendments, Florida law allows the use of marijuana for the treatment of certain debilitating conditions. Persons seeking Medical Marijuana (MMJ) may suffer from serious health conditions and symptoms, many of which are not responsive to approved medications. While MMJ could potentially improve health outcomes, there are also significant safety concerns related to cognitive effects, risk for accidents, interactions with other medications, psychosis, and addiction. Moreover, MMJ varies significantly in terms of its specific components and mode of administration (including smoking and vaping), but little is known about how MMJ improves clinical outcomes and what components, doses and delivery methods provide the optimal risk-benefit. There is a substantial and urgent need to understand how MMJ impacts health and safety outcomes to guide both policy and clinical decision-making. But due to the complex federal and state legal restrictions for both MMJ use and MMJ research, the development of evidence on the safety and effectiveness of MMJ is lagging far behind its rapid uptake.
The number of Floridians certified for use of MMJ has increased steadily with more than 450,000 persons actively registered with the Florida Office for Medical Marijuana Use (OMMU) in January 2021. While the COVID-19 pandemic has slowed many economies, the number of MMJ patients and the overall amount of marijuana dispensed have increased in the second half of 2020. Over 146 kg of tetrahydrocannabinol (THC) was dispensed in just one week ending December 31, 2020.
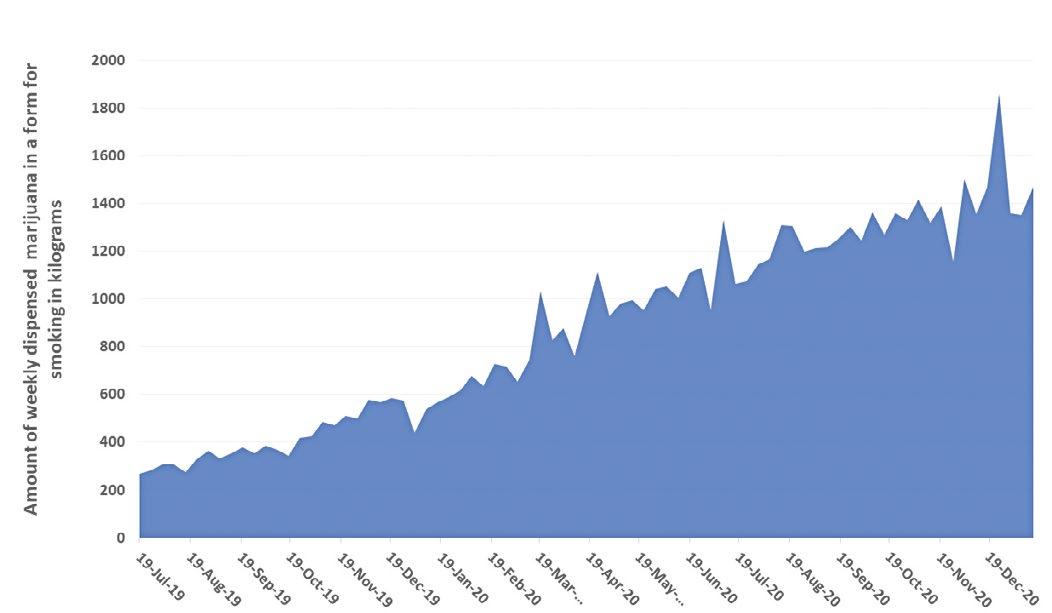
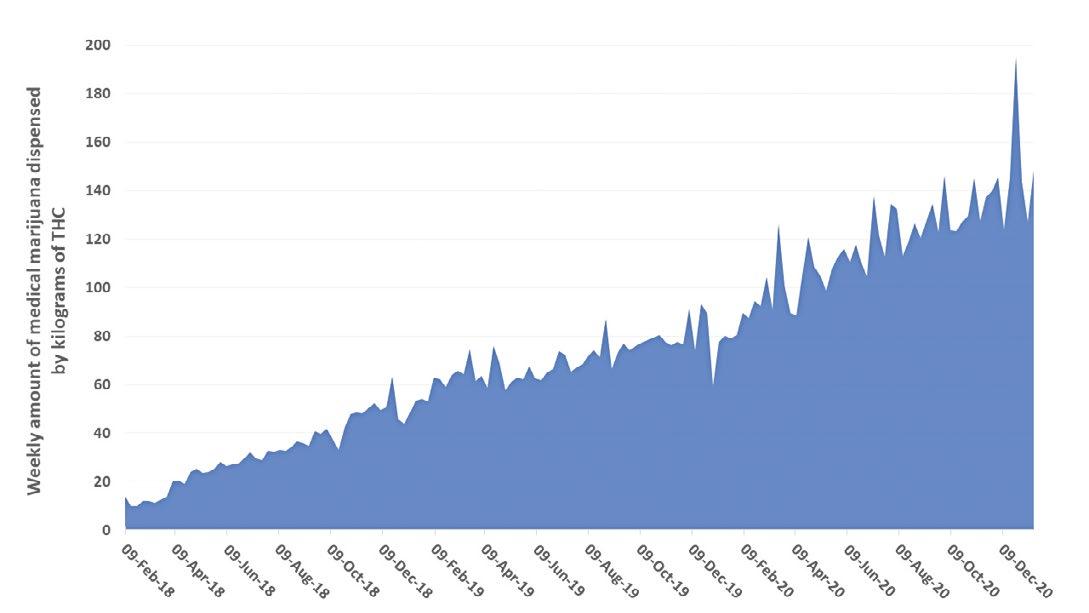

KILOGRAMS THC FEB. 2018 – JAN. 2021
KILOGRAMS OF FORMS FOR SMOKING JUL. 2019– JAN. 2021
10 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
MONTHLY NUMBER OF MEDICAL MARIJUANA QUALIFIED PATIENTS (ID CARD) IN FLORIDA (JAN. 2018 - JAN. 2021)
WEEKLY AMOUNT OF DISPENSED MEDICAL MARIJUANA IN FLORIDA
To address the need for rigorous evidence on the safety and effectiveness of MMJ for the various patient populations who are seeking certification for use, the state legislature introduced Section 1004.4351, Florida Statutes, to establish the Consortium of Medical Marijuana Clinical Outcomes Research to conduct, disseminate and support rigorous scientific research on the clinical outcomes of medical marijuana use. In July 2019, the Florida State University System Board of Governors, following a competitive request for proposals, designated the University of Florida as the lead university of the Consortium. Eight additional universities have joined the Consortium to-date.

The charter defines the Consortium purpose as “creating an effective medical marijuana clinical outcomes research program which would mobilize the scientific and medical resources that presently exist in the state to determine the appropriate and best use of marijuana to treat illness.” Consortium responsibilities as defined in the charter and consistent with statute include:

• Conduct rigorous scientific research
• Disseminate research
• Guide statewide policy on ordering and dosing practices for the medical use of marijuana.
The Consortium of Medical Marijuana Clinical Outcomes Research presents its second Annual Report on its accomplishments in research projects, research findings, community outreach initiatives, and future plans for the consortium.
2021 ANNUAL REPORT | 11
FLORIDA STATE UNIVERSITY
CONSORTIUM LEADERSHIP AND ADMINISTRATIVE STRUCTURE
THE CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES LEADERSHIP
The Consortium of Medical Marijuana Clinical Outcomes Research is open to all public and private universities in Florida. The Consortium is directed by the Medical Marijuana Research Board, which is composed of representatives from each participating university. Board members represent a variety of scientific and medical fields as required per statute. Profiles of the Consortium Board members are available in Appendix A
CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH BOARD
BOARD MEMBER INSTITUTION
Roger B. Fillingim, PhD (Chair)
Daniel C. Flynn, PhD (Vice Chair)
William Anderson, PhD
W. Dalton Dietrich, PhD
Timothy Gilbertson, PhD
Eric H. Holmes, PhD
Cynthia Hughes Harris, PhD, OTR, FAOTA, FASAHP
Max. C. E. Orezzoli, PhD
Martha S. Rosenthal, PhD
University of Florida
Florida Atlantic University
Florida International University
University of Miami
University of Central Florida
Florida State University
Florida A&M University
Florida Memorial University
Florida Gulf Coast University
CONSORTIUM ADMINISTRATIVE STRUCTURE
Pursuant to statute, the board appointed Almut G Winterstein, RPh, PhD, FISPE, Professor and Dr. Robert and Barbara Crisafi Chair in Pharmaceutical Outcomes and Policy and Director of the Center for Drug Evaluations and Safety (CoDES) at UF as its director. Leveraging her 20year experience in directing research on drug outcomes in real-world populations and her 6-year tenure as chair of the FDA Drug Safety and Risk Management Advisory Committee, Dr. Winterstein leads the development of MEMORY, the Consortium research data repository, administers the grants program, and has assumed responsibility for Consortium administration and support of the Board as defined by statute. She is supported by Dr. Robert Cook, MD, MPH, professor of Epidemiology and Internal Medicine and director of the Southern HIV and Alcohol Research Consortium (SHARC) Center for Translational HIV Research. Dr. Cook has assumed responsibility for developing the Clinical Research Core, leads the statewide MMJ Provider Partners Group, and oversees Consortium outreach activities.
12 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
They are supported by a faculty lead for the data science team charged with the development of MEMORY, a faculty lead for development of the clinical research core and a faculty lead who directs development of the Consortium evidence core, which is supported by a national scientific expert group. Program staff include a program coordinator to support day-to-day operations, and a communication expert who leads outreach activities, in addition to a data science team. Because of its academic base, Consortium leadership involves several trainees in Consortium activities. Profiles of the Consortium leadership and staff are available in Appendix B.
BOARD MEETINGS
To expedite Consortium progress, the board has met eight times between August 2019 and Feb 1, 2021. Key accomplishments and decisions at the board meetings are summarized in the following section and complete meeting minutes are available on the Consortium website at https://mmjoutcomes.org/our-consortium/board-members/ The following are the highlights of the board meetings that were held since the First Annual Report was submitted.
JUNE 4, 2020
• The board convened via a web-based meeting to finalize the selection of research proposals for funding.
• The Consortium Board agreed in moving forward with the agreement with the Journal Medical Cannabis and Cannabinoids, making it the official journal of the consortium.
JULY 23, 2020
• Budget updates and an overview of the FY21 research plan were shared with the Consortium board. Dr. Joshua Brown was introduced as the new Faculty Lead for MEMORY
• The board was updated on plans for the patient contact registry and development of a patient cohort, and a new platform to foster collaborations amongst researchers, providers, and the community.
• The FY21 budget was presented and approved by the Consortium board.
• Board members approved the plans for the inaugural conference of the consortium as a virtual event.
DECEMBER 4, 2020
• The board members were updated on progress toward FY21 goals.
• The board reviewed objectives and the preliminary agenda of the consortium’s inaugural conference, Cannabis Clinical Outcomes Research Conference (CCORC).
• A summary of results from the state-wide physician survey was shared with the board.
• The board approved the proposed plans for the 2021 research grants program, including an updated list of research priorities.
JANUARY 29, 2021
• The Consortium board approved the 2021-22 research plan and the outline of the second annual report.
2021 ANNUAL REPORT | 13
TIMELINE OF KEY CONSORTIUM ACTIVITIES
SINCE ITS INCEPTION (JULY 2019 – FEB 2021)
14 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
CONSORTIUM EFFORTS IN FY 2021
Central to the Consortium is its mission to foster clinical outcomes research on MMJ across the state. Five pillars constitute the Consortium Research Program: A Grants Program, a new and unique data repository known as the MEdical Marijuana Clinical Outcomes RepositorY (MEMORY), a Clinical Research Core, Outreach activities and an Evidence Core Consistent with its charter, the Consortium has engaged scientists with relevant research programs to participate in the Consortium and foster research collaborations to accelerate the development of evidence on MMJ clinical outcomes. The following sections provide a brief description of each of the Consortium functions and a detailed progress report.
CONSORTIUM OF MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH: ORGANIZATIONAL STRUCTURE AND ACTIVITIES 2021.
GRANTS PROGRAM
Each year, the Consortium intends to offer the Consortium MMJ Clinical Outcomes Research Grants Program, open to all members of the Consortium and teaching nursing homes. The research focus of the grants program is prioritized based on statutory guidance and the annual Consortium research agenda to ensure optimal fund utilization. Applications are reviewed by external reviewers, recruited from out-of-state, using NIH review criteria. Final grant awards are made by the Medical Marijuana Research board based on study quality, impact and relevance to the Consortium research priorities. Calls for proposals are disseminated by each board member within their university systems and through the Consortium website and newsletter.
We have completed the 2019 grants program cycle, are mid-way through the 2020 grants program cycle and have launched the 2021 grants cycle.
2021 ANNUAL REPORT | 15
2019 Grants Program
Ten awards were made by the Consortium board in the 2019 Grant Program cycle from amongst 41 proposals, based on scientific merit, innovation, potential impact, relevance to the Consortium research charge, qualifications of the PI, potential for future extramural funding, and immediate clinical and/or demonstrable translational relevance to inform medical use of marijuana.
Executive Summary of Final Reports of the 2019 Awarded Research Projects
The ten proposals, selected for funding from five of the Consortium member institutions, included 4 clinical and 5 translational studies, reflect research areas prioritized by statute and the 2020-2021 research priorities adopted by the Consortium board.
The 2019 awardees were provided an extension until December 2020, to compensate for delays caused by the COVID-19 pandemic. The 2019 awardees, their affiliations, and executive summaries of their final reports, are as follows.
PI: PAUL BORSA, PH.D.

University of Florida
Research proposal titled “Efficacy of a controlled short-term trial of CBD ingestion on reducing symptomatic response and facilitating recovery after induced muscle injury”
EXECUTIVE SUMMARY OF FINAL REPORT: Many physically active Americans have reported pain-relieving effects of cannabidiol (CBD) that can reduce or eliminate use of nonsteroidal anti-inflammatory drugs (NSAIDs). Currently its biological and therapeutic effects have not been explained, and clinical research in humans regarding its effectiveness is urgently needed. We sought to investigate if a controlled short-term trial of CBD ingestion was effective in reducing a symptomatic response (e.g., musculoskeletal pain and pain-related anxiety) and facilitating functional recovery (strength
loss) following induced muscle injury. Secondarily, we wanted to investigate if the therapeutic effects are dose dependent.
We have secured Investigational New Drug (IND) status for our investigational product from the US Food & Drug Administration (FDA) Center for Drug Evaluation and Research (CDER). In addition, we were able to receive IRB approval from UF. Our IND approved investigational product has been manufactured and processed through a Florida-based CBD company (SunFlora, Inc, St. Petersburg, FL), which has agreed to source our hemp-derived CBD for the project as well as for future studies. Patient recruitment has commenced after initial delays due to the pandemic. Data from this study will help shape future grant applications on identifying an efficacious dose range of CBD, as well as determining the cellular and molecular mechanisms that contribute to symptom resolution and recovery.
16 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
THE GRANTS PROGRAM CONTINUES TO SUPPORT INVESTIGATOR-INITIATED RESEARCH AT CONSORTIUM INSTITUTIONS WITH A PURPOSEFUL FOCUS ON RESEARCH PRIORITIES REPRESENTING THE GREATEST NEED FOR EVIDENCE.
PI: HELEN BRAMLETT, PH.D.

University of Miami Research proposal titled “Therapeutic dosing of a cannabinoid (CBD) after mild and moderate brain injury for translation to the clinic”
EXECUTIVE SUMMARY OF FINAL REPORT: Cannabidiol (CBD) has been shown to have anti-inflammatory, neuroprotective effects and its administration may be a therapeutic strategy in the treatment of traumatic brain injury (TBI). The objective of the present study was to assess two therapeutic doses (3 or 5mg/kg) of CBD using a clinically-relevant oral administration regimen in two pre-clinical models of brain injury: a pressure wave-induced blast injury and a fluid percussioninduced (FPI) moderate TBI. We anticipated conducting and analyzing several neurocognitive, sensorimotor, hearing, and vestibular behavioral outcome measures, as well as histological analyses evaluating neuro- and cytoprotection and inflammatory responses. All FPI and Blast TBI groups have been completed, undergone their respective behavioral testing paradigms, and tissue processed for histological analyses.
We observed several interesting trends with CBD treatment after TBI. In the moderate FPI model, there was a slight preservation of sensorimotor skills as indicated by reduced asymmetrical usage of forelimbs, albeit this was not significant. Cognitive evaluation showed no CBD effect on spatial memory acquisition and retention after injury, however short-term working memory skills trended towards sham uninjured levels. Histological assessment revealed reduced cortical atrophy and contusion volumes in animals treated with CBD. Furthermore, we qualitatively observed decreased microglia activation, a hallmark indicator of TBI-induced neuroinflammation. In the Blast experiments, there were no improved hearing outcomes at the time of assessment, which contrasted our earlier findings in similar experiments with intraperitoneal CBD administration.
In summary, in our studies, we found that oral consumption of CBD may have reduced inflammation, protected vulnerable brain regions, and reversed certain memory and sensorimotor deficits that are observed after brain injury. The appearance of reduced microglia reactivity, led us to believe that a higher oral dose may be more efficacious in reversing neuropathological sequelae. Further investigation, possibly coupled with higher sensitivity testing paradigms, is needed to fully evaluate CBD as a therapeutic avenue in TBI. Our plan is to continue to evaluate the use of CBD after TBI for a future grant submission to an appropriate funding agency.
PI: JOSHUA BROWN, PHARM.D., PH.D., M.S University of Florida Research proposal titled “Characterizing community and physician-level factors associated with medical marijuana prescriber registration and patient access”


EXECUTIVE SUMMARY OF FINAL REPORT: This project created a dynamic data visualization tool and linkable database to cross-reference cannabis-licensed physician practices, cannabis dispensary locations,
and community-level and physician-level indicators of access and health.
We first compared physicians authorized to submit patient orders for medical cannabis in Florida versus those without authorization by specialty. Further, we compared physician specialties by authorization status for measures that assess past prescribing behaviors for opioids, benzodiazepines, and brand name drugs. The general trend observed was that authorized physicians, overall and for select specialties, prescribed more opioids and more benzodiazepines than non-authorized physicians of the same specialty. Prescribing for brand name drugs was more comparable for authorized versus non-authorized physicians. Physician prescribing, practice location, dispensary locations, and other community-level measures were overlayed using Tableau Server mapping software to visually assess patterns of geographic disparities. These community-level measures were adjusted for population and the findings revealed that, during the study period, there was lack of medical cannabis access in rural areas as compared with more densely populated areas in Florida. Additionally, we documented a strong correlation between cannabis access and utilization of opioids.
Data curation and linkage will continue with the aim of hosting the server and interactive maps on the Consortium’s website permanently for use by the public and other researchers. Findings will be used for future research evaluating disparities in access as well as for investigating important potential confounding variables once the MEMORY database is established. The investigators will pursue funding from the NIH and Robert Wood Johnson Foundation with a focus on health policy as well as pharmacoepidemiological and health outcomes studies using these curated and linked data.
PI: ANDREA CIPPITELLI, PH.D.
Florida Atlantic University Research proposal titled “Cannabidiol: A potential treatment for migrainelike pain, negative emotion and photophobia”
EXECUTIVE SUMMARY OF FINAL REPORT: Migraine is a complex condition characterized by the tendency to have headache with sensory disturbances and comorbid anxiety and depression. Based on the wide range of cannabidiol (CBD)’s pharmacological actions, including anxiolytic and antidepressant effects, modulatory effects on neuronal transmission, and pain relief, we aimed to investigate if CBD has a therapeutic role in migraine pathology.
We used a calcitonin gene-related peptide (CGRP) migraine model since peripherally administered CGRP (0.1 mg/kg) reliably provided measures of cephalic allodynia, spontaneous pain as assessed by facial signs of discomfort, altered light sensitivity (photophobia) and conditioned place aversion (CPA) in mice.
The most important finding so far is that CGRPinduced cephalic allodynia is successfully blocked by CBD treatment (30 mg/kg, ip) both in male and female C57BL/6J mice. We determined that the painful responses manifested as a facial grimace were attenuated 60 min post-CGRP injection by systemic administration of 30 mg/kg CBD in female mice, whereas signs of facial discomfort in male mice
2021 ANNUAL REPORT | 17
were prominent at 15 min post-injection and were not reversed by similar CBD dose. We also observed reliable photosensitivity both in male and female mice, however CBD pretreatment was not effective in blocking CGRP-induced photophobia. CGRP produced anxiogenic-like activity only in male mice, an effect reversed by CBD. Lastly, we determined that avoidance for the compartment previously associated with CGRP injection was not reversed by CBD. This experiment was conducted only in male mice.
Collectively, our results suggest that CBD is effective in relieving migraine-like pain and anxiety comorbid to headache pain, but fails in providing protection from other symptoms experienced by migraineurs such as photophobia. Additional preclinical research is needed to demonstrate the suitability of CBD as a treatment for migraine and to identify the mechanisms of protective effects. Results from our studies aid in the progression of MMJ research, as they inform on pharmacological and behavioral effects of CBD.
PI: GREGORY McMANUS, PH.D. Florida Gulf Coast University Research proposal titled “Rapid identification and quantification of heavy metals and microplastics in CBD oil.”

EXECUTIVE SUMMARY OF FINAL REPORT: Cannabis has shown great promise for the treatment of many medical conditions. There are, however, substantial uncertainties surrounding the nature and content of contaminants in cannabis plants. The goal of this project was to develop reliable, rapid, inexpensive techniques for the determination of key contaminants within the cannabis plant and to accelerate research in this promising industry to ensure consumer/patient safety.
The specific aims of our projects were to: develop a methodology to rapidly quantify the heavy metal contaminants, including arsenic, cadmium, cobalt, copper, lead and mercury in commercially available samples of CBD oil via Wavelength Dispersive X-ray Fluorescence (WDXRF); and identify microplastic polymer contaminants in CBD oil using coupled Thermal Gravimetric Analysis – Differential Scanning Calorimetry (TGA–DSC). For the purposes of this study we collected 25 different CBD oil samples from 15 different vendors across the country. All CBD oil samples are in the form of tinctures with CBD oil concentrations ranging between 250 mg to 3000 mg per 30 mL bottle.
Analysis showed that heavy metals were not present in the samples within the detection limits of the WDXRF instrument. However, analysis did identify the presence of trace amounts of silicon in at least 12 of the 25 samples measured. Presumably, due to the low concentrations of microplastics in the samples, no characteristic endothermic phase transition temperatures were observed for the samples.
Our conclusions are that these contaminants are not present in the over two dozen commercially available CBD oil samples we analyzed within the detection limits of our instruments. These findings should help reassure consumers and policy makers that CBD oil products are inherently safe. For future direction, we
propose using coupled Thermal Gravimetric analysis and Mass Spectroscopy (TGA-MS) techniques to determine contaminates present in the marijuana vaping products. For this purpose, we will seek funding through the NIH R03 program.
PI: MANDIP SINGH SACHDEVA, PH.D.

Florida Agricultural & Mechanical University.
Research proposal titled “Hyaluronic acid functionalized, Cannabidiol-loaded Mesenchymal Stem Cells (MSC)-Derived Exosomes for Drug Resistant Cancers”
EXECUTIVE SUMMARY OF FINAL REPORT: About 1014 % of all breast cancers are triple negative (TNBC), which represents an important clinical challenge, as these tumors often develop resistance to conventional chemotherapeutics. While cannabidiol (CBD) may have favorable effects in various cancers, poor solubility and increased metabolism by cytochromes P450 (CYP) enzymes limit the bioavailability of CBD.
In our laboratory, we observed that CBD besides having anticancer activity against MDA-MB-231, MDA-MB-468 and DOX-resistant MDA-MB-231 cells was also a sensitizer for anticancer drugs like doxorubicin and docetaxel. Exosomes derived from human mesenchymal stem cells (hUCMSCs-EX) have shown their potential clinical applications in various diseases including cancer. Exosomes derived from hUCMSCs were fully characterized and were formulated with CBD. CBD Exosomes significantly decreased the proliferation of MDA-MB-231 and MDA-MB-468 cells. We further functionalized these exosomes by using Hyaluronic acid (HA) and it was observed that these exosomes significantly decreased the proliferation of MDA-MB-468 cells (i.e., IC50 value: 1.76 µM) with significant increase in G1 phase cell cycle arrest when compared to CBD alone at similar doses.
Our results demonstrate significant benefits of CBD in TNBC. Further, hUCMSCs-EX were found to be a suitable delivery system for CBD to make it a clinically feasible formulation. Further studies using tumor models in animals will determine the benefits of the HA coated CBD-EX, which are in progress.
PI: JACQUELINE SAGEN, PH.D., M.B.A.

University of Miami Research proposal titled “Evaluation of medical marijuana for the treatment of chronic spinal cord injury pain using a rat central neuropathic pain model”
EXECUTIVE SUMMARY OF FINAL REPORT: Although the most frequently reported use of MMJ is for pain relief, there has been a paucity of preclinical studies evaluating the effects of cannabis in chronic pain models. Chronic pain following spinal cord injury (SCI) occurs in a majority of patients and can be so severe that it is their top quality of life concern. The goal of this study was to rigorously evaluate the effects of the two major but mechanistically distinct cannabis components, CBD
18 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
and THC, and their potential synergistic pain-relieving combination, using a preclinical SCI rodent model. However, the work was hampered by the inability to obtain THC due to delays resulting from the Covid-19 pandemic. Therefore, another prominent component of Cannabis, the sesquiterpene β-caryophyllene (BCP) was substituted for these initial studies. BCP is a major component (up to 35%) in the essential oils of Cannabis sativa. It acts as a CB2-receptor-selective agonist and has shown pain-reducing effects in rodent models. Thus, its potential pain-reducing mechanism is distinct from CBD and THC and may be a valuable addition in SCI pain management.
The chronic SCI pain-reducing effects of CBD and BCP were evaluated using a battery of behavioral tests for neuropathic SCI pain in both male and female rats with clip compression spinal injury. Dose-response analyses were done for both drugs and the A50s (doses producing half-maximal effect) were calculated. Since CBD and BCP act via distinct receptors to reduce pain, their combination in appropriate ratios may provide further beneficial effects at lower doses of each. To determine this, dose-response evaluations of the combination based on the fixed ratio of the A50s were done, and potential synergism assessed using isobolographic analysis. Results showed that the CBD/BCP combination synergistically reduced cold allodynia in both male and female rats with chronic SCI neuropathic pain. The combination produced additive effects in reducing tactile allodynia.
Thus, the combination of two major Cannabis components, CBD and BCP, show promise in furthering a pain management treatment for SCI neuropathic pain. These results and further development will be pursued via the National Center for Complementary and Integrative Health (NCCIH) special interest program in Analgesic Properties of Minor Cannabinoids and Terpenes. In addition, once the THC is obtained, it will be tested in combination with CBD and BCP, in order to select the most potent SCI pain-reducing ratios and targeted for future NIDA/NINDS pain funding programs.
PI: KRISHNA VADDIPARTI, PH.D., M.P.E., M.S.W. University of Florida Research proposal titled “A feasibility study of real-time monitoring of Posttraumatic Stress Disorder related sleep disturbances and other symptoms among patients on medical marijuana”

EXECUTIVE SUMMARY OF FINAL REPORT: In the US several states permit the medical use of marijuana by individuals with Posttraumatic Stress Disorder (PTSD) but, at this point, we lack evidence on the appropriateness of marijuana as a therapy for PTSD. The goal of this pilot grant is to demonstrate the feasibility of recruiting and retaining patients with PTSD on MMJ in a prospective study and examine in real-time how MMJ affects PTSD related sleep disturbances and recovery from PTSD symptoms and distress, using Ecological Momentary Assessment (EMA) delivered via smartphone and surveys.
Covid-19 restrictions have caused a slight delay in launching the study as we had to amend the study methods and procedures that involve face-to-face interactions with the participants to virtual methods. Also, the travel of research assistants to MMJ clinics was restricted to physically station at the clinic and screen referrals. To test our hypothesis, we recruited 15 patients with confirmed PTSD seeking to start MMJ for their PTSD symptoms from cannabis clinics in North-Central Florida and assessed them at different phases of MMJ treatment.
Our preliminary analysis suggests that there were significant improvements in sleep and mental health well-being, and decreases in PTSD symptoms and nightmares, with effects lasting at least 70 days after initiation of MMJ. This pilot study also confirms that patients with PTSD on MMJ could be recruited and retained in longitudinal real-time monitoring research.
PI: JENNY L. WILKERSON, PH.D. University of Florida Research proposal titled “Marijuanaderived terpenes for the treatment of chemotherapy-induced pain”

EXECUTIVE SUMMARY OF FINAL REPORT: Paclitaxel, commonly used to treat breast, lung and other cancers, can also produce persistent and debilitating side effects such as chemotherapy induced peripheral neuropathy (CIPN). Anecdotal reports suggest marijuana may be an effective analgesic. The primary constituent of marijuana, Δ-9 tetrahydrocannabinol, produces most of its physiological actions via cannabinoid type 1 receptors (CB1R), predominately distributed on neurons, and cannabinoid type 2 receptors (CB2R), predominately distributed on immune cells. Marijuana also contains a multitude of other compounds (i.e., terpenes) that have not been well studied and may hold therapeutic promise as pain therapeutics. We examined the ability of a subset of terpenes found in marijuana: γ-terpinene, α-terpineol, β-caryophyllene to reduce a common behavior associated with CIPN pain in mice. Each terpene reversed this pain-related behavior in paclitaxel-treated mice. We found differential cannabinoid receptor involvement underlying each terpene’s ability to produce antipain effects in paclitaxel-treated mice. Further, each terpene relied on downstream inflammatory factors to produce analgesia. Cannabis-based terpenes possess a pharmacological profile that may yield new efficacious analgesics.
These data open exciting research questions on numerous fronts. The first new question may be that additional chemical modifications may be made to these terpenes to enhance the therapeutic dosing window of these compounds. Another exciting question involves the examination of the pharmacokinetic profile of these terpenes alone and in combination. Both questions have initiated collaborations using pilot data generated from this award, with the intention of submitting an R01 to NIDA. An additional collaboration with the UF Cancer Center is underway to examine the potential of these terpenes to co-treat both cancer-induced pain as well as cancer-induced cellular senescence; a R01 to NCI is expected as well.
2021 ANNUAL REPORT | 19
PI: ALI M. YURASEK, PH.D.
University of Florida
Research proposal titled “The Relationship between State Medical Marijuana Laws, Substance Use and Mental Health Disorder Diagnoses, and Associated Health Care Costs”

EXECUTIVE SUMMARY OF FINAL REPORT: Despite the potential of MMJ to assist with mental health conditions, marijuana use is also associated with increased participation in substance use treatment and risk for the development of psychosis and mood-related disorders. Yet, whether the passage of MMJ laws is associated with changes in substance use, mental health diagnoses or healthcare costs remains unclear. The purpose of this study was investigating the trends of substance use diagnosis, mental health diagnosis, and treatment utilization in states with and without MMJ laws between 2012-2018.
As of 2020, there are 38 states that have passed MMJ laws. Our preliminary analyses examined the treatment
costs associated with 8 different mental health disorder diagnosis in 2012, including Opioid Use Disorder (OUD), Cannabis Use Disorder (CUD), Alcohol Use Disorder (AUD), Post-Traumatic Stress (PTSD) related disorders, Anxiety Disorders (AD), Depressive Disorders (DD), Psychosis related disorders (PD), and Sleep Disorders (SD). In 2012, 19 states had passed MMJ laws. In 2012, states that passed MMJ laws had higher rates of OUD, CUD, AUD, PTSD, DD, and PD (ps <.001) than those that did not yet pass MMJ laws. Similarly, the healthcare costs were significantly higher across all disorders examined in states with MMJ laws compared to those without MMJ laws. Additional analysis will be conducted to examine within-state changes in these costs across the 5-year timespan, as well as changes in the prevalence of these different diagnoses over time and in states with and without MMJ laws. Findings will provide policy-relevant information about the influence of MMJ laws on health care utilization for substance use and mental health diagnoses.
20 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
2020 Grants Program
After the tremendous response to its inaugural grants program the Consortium released its second Request for proposals on February 3, 2020. The new RFP listed the following research priorities for funding.
1. Clinical Outcomes: with particular emphasis on the following conditions – chronic pain, anxiety, and symptomatic treatment of cancer
2. Route of Administration: effect of dosing and routes on efficacy and safety; of particular interest are studies that evaluate effects of smoking and vaping
3. Interactions of Medical Marijuana with other drugs/medications: with particular focus on medications that are commonly used by patients who seek medical marijuana treatment
Summary of 2020 Awarded Research Projects
The 2020 Grants Program received 21 letters of intent (LOIs) from faculty from consortium member universities, submitted through a new online submission platform. A total of 20 proposals were reviewed by 37 ad-hoc outof-state reviewers with no conflicts and demonstrated expertise in the respective research area. The Consortium Board met in June 2020 and based on reviewer scores and responsiveness of the proposals to the research mission of the consortium, ranked the proposals in order of funding priority. The final decision on the 2020 grants awards was made after the FY21 budget was approved by the Governor and adopted by the Board.
Nine grant awards for FY21 were announced in July 2020, including faculty from 5 of the Consortium member institutions (Florida A&M University, Florida Atlantic University, Florida State University, University of Central Florida, and University of Florida). Funded research proposals include 6 clinical and 3 translational studies.
2021 ANNUAL REPORT | 21
The 2020 awardees, their affiliations, along with the project narratives and anticipated impact of their proposed research, are presented below.
PI: HASSAN AZARI, PH.D. University of Florida Research proposal titled “Hemp derived extracellular vesicles (EVs) for the treatment of glioblastoma”

PROJECT NARRATIVE: Glioblastoma (GBM) is an aggressive brain tumor with a poor prognosis despite surgery and chemoradiotherapy. We have isolated and characterized extracellular vesicles (EVs) like nanoparticles (NPs) with strong anti-glioma effects from the hemp plant. These NPs contain cannabinoids in their acidic form and can be delivered into the brain via a non-invasive intranasal route. This project will examine anti-glioma therapeutic efficacy of the hemp EVs on mouse models of glioma and also their impact on standard of care temozolomide chemotherapy.
ANTICIPATED IMPACT: Hemp EVs with their natural payload of non-psychoactive acidic cannabinoids represent a novel natural nanodrug source for brain tumor therapy. While we are initially testing this therapy in a model of primary brain cancer it could be applied to a broad range of other cancers metastasizing to the brain, and inflammatory neurodegenerative diseases. Given the scalability of hemp EVs and the simplicity of its application, positive results from this study would provide a strong rationale to test the approach clinically in a wide range of neurological disorders.
PI: LISA ECKEL, PH.D.


Florida State University
Research proposal titled “Cannabinoid medication for treatment of a preclinical model of anorexia nervosa”
PROJECT NARRATIVE: Anorexia nervosa (AN) is a serious psychiatric illness with poor treatment outcome. Our work examines the endocannabinoid system (ECS) as a novel therapeutic target for AN, based on the critical role it plays in regulating food intake, energy expenditure, and reward processing, all of which are dysregulated in AN. The goal of this study is to investigate whether treatment with THC and/or CBD attenuates weight loss and normalizes ECS function in a pre-clinical animal model of AN.
Anticipated Impact: This pre-clinical study investigating the therapeutic potential of THC and CBD in alleviating AN symptoms in rodents, offers a translational model for the development of new cannabinoid-based pharmacotherapies, including the use of MMJ, that would help to ease the high personal and societal costs of AN.
PI: DEBRA FADOOL, PH.D.
Florida State University
Research proposal titled “Mechanisms of Action for Cannabidiol (CBD) in a Mouse Model of Anxiety”
PROJECT NARRATIVE: CBD is a nonpsychoactive ingredient of cannabis that has demonstrated changes in anxiety, chronic pain, sleep, and prevention of substance abuse in mouse and human
subjects. We are using a newly found mouse model that exhibits anxiety and attention deficit to examine behavioral intervention of chronic CBD therapy across three stages of development (fetal, young adult, aged). We are also pairing our behavior studies with energy expenditure and eating behavior.
ANTICIPATED IMPACT: Investigators can either induce anxiety by adding a stressor (“state” anxiety), or use a mouse model whereby the “trait” anxiety does not vary over time. Because CBD is not addictive, it has high therapeutic potential for chronic treatments and the use of the latter model affords an advantageous tool to explore reduction or elimination of anxiety- or ADHD-associated behaviors. This study will develop a preclinical tool for probing the molecular and electrical changes that mitigate anxiety and attention deficit behaviors.
PI: JASON FORD, PH.D.

University of Central Florida
Research proposal titled “Patterns, Motives, and Risks Associated with Marijuana Use: A Comparison of Medical Marijuana Patients and Non-Patient Marijuana Users in Florida”
PROJECT NARRATIVE: Little is known about medical cannabis among young adults, even though they are the age group with the highest prevalence of substance use. The main goal is to compare medical cannabis patients to non-patient cannabis users with regard to patterns and motives of use, psychosocial correlates of use, associations with substance use and other risky behaviors, and the effects of the COVID-19 pandemic on cannabis use. Regarding medical cannabis patients, we can assess the various health-related conditions they use cannabis to treat, their experiences at medical cannabis dispensaries, any side effects experienced due to cannabis use, and how patterns of cannabis use change after becoming a patient.
ANTICIPATED IMPACT: The current research will help us understand patterns and characteristics associated with cannabis use among young adults in the state of Florida, particularly how use is associated with physical and mental health. An important outcome is identifying how medical cannabis users are different from non-patient users. We will be able to determine how the COVID-19 pandemic impacted patterns of cannabis use and learn about medical cannabis patients’ experiences with licensed dispensaries.
PI: JOHN MARKOWITZ, PH.D.

University of Florida Research proposal titled “An Assessment of the Drug Interaction Potential Between Oral Cannabidiol (Epidiolex®) and the CES1 Substrate Methylphenidate in Healthy Volunteers”
PROJECT NARRATIVE: Drug-drug interactions (DDIs) are a significant cause of emergency department visits, hospital admissions and increased morbidity and mortality, yet most are preventable. The DDI potential
22 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
of conventional prescription medications is evaluated as part of the FDA approval process, a process not applied to medical cannabis which is frequently used in combination with prescription medications. The present DDI assessment is a rigorous clinical study in healthy subjects (n=12) which will determine if the major medical cannabis constituent, cannabidiol (CBD) can inhibit a major drug metabolizing enzyme (CES1) in a clinically significant manner.
ANTICIPATED IMPACT: The results of the project will be impactful in several ways. First, it will inform the scientific and medical community as to whether in vitro DDI study methodology (more rapid and relatively inexpensive) can accurately predict clinical DDIs in healthy volunteers. Secondly, as numerous therapeutics agents are CES1 substrates and reliant on the enzyme for both detoxification and clearance as well as activation of certain prodrugs, the study results will have implications for co-prescribing of prescription medications with medical cannabis. Numerous drugs are potentially affected including many ACE Inhibitors, several antivirals, immunosuppressive drugs, some antiplatelet drugs and others. Project results can be rapidly disseminated to the scientific and clinical community.
PI: DAVID NEWMAN, PH.D.
Florida Atlantic University
Research proposal titled “Assessing and Supporting Effective and Safe Use of Medical Marijuana for Older Adults with Chronic Pain”
PROJECT NARRATIVE: The proportion of Florida’s older population is growing rapidly. Among this older group musculoskeletal disorders with associated chronic pain are common, but symptom management including chronic pain management can be challenging. Medications to relieve these symptoms, especially opioids, can increase the risk of confusion, constipation, falls and injury. This purpose of this mixed methods study is to identify what older adults need, to effectively and safely use MMJ, and to identify what age appropriate, evidence-based education is required.
ANTICIPATED IMPACT: Develop evidence-based education for the safe and effective use of MMJ for older adults as well as provide guidance for policy for clinical practice.
PI: MANDIP SACHDEVA, PH.D.

Florida Agricultural & Mechanical University Research proposal titled “Preclinical evaluation of exosomal cannabinoid formulations in chemotherapy induced peripheral Neuropathy”
PROJECT NARRATIVE: Docetaxel (DTX) is widely used for Triple negative breast cancer (TNBC), but DTXinduced peripheral neuropathy (DIPN) is a major clinical concern. Cannabidiol (CBD) alleviates mechanical allodynia and anxiety in peripheral neuropathy models by acting on (TRPV1)/CB2/5-HT1A/α3-Glycine receptors. Exosomes derived from human umbilical cord mesenchymal stem cells (hUCMSCs) have potential clinical applications especially in peripheral nerve injury
induced neuropathy. Hence a synergistic effect can be expected if we constitute an exosomal CBD formulation. We hypothesize that oral administration of Enteric Coated Mini Capsules encapsulating CBD exosomes will serve as an ideal delivery platform for significant alleviation of DIPN in non-tumor and MDA-MB-231 tumor bearing mice.
ANTICIPATED IMPACT: This project will allow us to evaluate the role of CBD exosomes formulation to treat peripheral neuropathy induced by chemotherapy, which impacts majority of the breast cancer patients. Further this approach can also be extended for other ailments like Diabetes induced neuropathy.
PI: DOUGLAS STORACE, PH.D. Florida State University Research proposal titled “The influence of cannabinoid receptors on olfactory function”
PROJECT NARRATIVE: Cannabinoids play an important therapeutic role in the stimulation of appetite and promotion of food seeking behavior. One potential underlying mechanism is the modulation of the olfactory system by cannabinoids. The current study uses a combination of functional imaging and behavior assays to determine how cannabinoid receptor modulation alters olfactory sensory processing and perception.
ANTICIPATED IMPACT: Although our hunger level is determined by physiological signals that reflect our nutritional status, external sensory cues such as olfactory stimuli can enhance our appetite. Changes in the perception of a food-related odor could impact appetite and food-seeking behavior. The goal of these studies is to define how cannabinoids alter the representation of olfactory stimuli in the brain, and to link these changes to perception.
PI: ROBERTO VINCIS, PH.D. Florida State University Research proposal titled “Endocannabinoid mechanism in the neural processing of food-predicting sensory cues.”


PROJECT NARRATIVE: In this proposal we aim to investigate the role of cannabinoid modulation of the mouse insular cortex (IC), a cortical area known to process motivationally salient stimuli associated with food reward cues and drive food-oriented behaviors
ANTICIPATED IMPACT: Investigating the neuromodulatory effects of endocannabinoids on neural processing of food-predicting cues within the IC will increase our understanding of the neurobiological mechanisms of cannabinoid actions and provide crucial information for the development of cannabinoid-based pharmacotherapies.

2021 ANNUAL REPORT | 23
2021 Grants Program
The Consortium launched its third grants cycle in December 2020, releasing its 2021 Request for Proposals and updated research priorities that were derived in consultation with scientific and subject matter experts, evidence review, input from stakeholder engagement and NIH areas of programmatic interest.
25 LOIs were received on Feb 1, 2021, which will be administratively reviewed to invite full proposals. Each of these proposals will be reviewed by two ad-hoc out of state subject matter experts. Based on these reviews and recommendations of the consortium board, and pending state funding of the Consortium for FY22, awards will be announced in July 2021.
Highlights of Grant Awardee Progress Reports
Though early in the research process with only 1 year after the first grants were awarded and despite the COVID-19 pandemic, our awardees have made significant progress. Research findings are presented at scientific meetings and in peer-reviewed publications, and the new data generated has already served as the basis for 1 patent and 3 new extramural grant applications. Noteworthy, 37 trainees have been involved in the funded research grants and one new college course has been approved, supporting the development of MMJ research capacity in the state. The following are the highlights of the Grants program thus far, based on the research findings of the 2019 and 2020 awarded research studies.
• Provisional patent application filed: by Dr. Hassan Azari (UF/2020 awardee), titled “Cannabinoid containing plant derived extracellular vesicles and therapeutic methods using the same.” Hassan Azari, Nasser Nassiri Koopaei, Thomas D Schmittgen, Brent A Reynolds. Sept, 2020
• Media interview: of Dr. Jenny Wilkerson (UF/2020 awardee), titled “How CannabisBased Therapeutics Could Help Fight COVID Inflammation” (Medscape, 2020) https:// www.medscape.com/viewarticle/940265
• Investigational New Drug (IND#147985) authorization: to Dr. Paul Borsa (UF/2019 awardee) for their investigational product from the US Food & Drug Administration (FDA) Center for Drug Evaluation and Research (CDER).
• Dynamic data visualization tool and linkable database created: by Dr. Joshua Brown (UF/2019 awardee) to cross-reference cannabis-licensed physician practices, cannabis dispensary locations, and community-level and physician-level indicators of access and health. The dashboard will be available soon for public use through the Consortium’s website.
• Courses:
» A new course “Chemistry of Medicinal Plants” (CHM 2282C) developed by Dr. Gregory McManus (FGCU/2019 awardee) and Prof. Martha Rosenthal has been approved by the State University System of Florida.
» Training through a course “Physiological Pharmacology” by Dr. Debra Fadool (FSU/2020 awardee), includes appraisal of published literature on cannabinoid action and effects on anxiety/attention to train undergraduates on research project background, methods details and statistical analyses.
• Research and training opportunity for 37 undergraduate scholars, postdoctoral fellows and graduate students, three of whom are from groups that are underrepresented in science.
24 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Extramural grants submitted by grant awardees (based on findings emerging from the consortium awarded research; awardee names in bold)
• Jenny Wilkerson (PI), Lance McMahon (Co-I), Abhisheak Sharma (Co-I), Francisco Leon (Co-I) submitted a grant to NCCIH Titled “Terpenes and minor cannabinoids as novel analgesics.”
• Hassan Azari and Brent Reynolds submitted a grant to NIH Titled “Acidic cannabinoids for the treatment of high-grade glioma.”
• Hassan Azari and Brent Reynolds submitted a grant to DOD Titled “Cannabinoids Acidic for the treatment of Glioblastoma.”
Publications/conference abstracts from grant awardees (names in bold)
1. Sachdeva, M. Role of Cannabinoids for Breast and Lung Cancer Cell lines. FAMU Pharmacy Clinical Symposium Mar 6, 2020
2. Wilkerson, J. Axially-Chiral Cannabinols as Novel Therapeutics for Neuropathic Pain. UF SPAR (Pain and Addiction Research) Conference, Gainesville, FL. December 2019
3. May, C., C. Silver, C. Huffstetler, B. Cochran, N. Maykut, and D.A. Fadool. Does CBD (Cannabidiol), the Non-Psychoactive Ingredient of Cannabis, Mitigate Anxiety in Mice? Florida Undergraduate Research Conference (FURC), Florida State University, Feb. 26-27, 2021.
4. Hassan Azari, Nasser Nassiri Koopaei, Mohammad-Zaman Nouri, Jesse D. Hall, Nancy D. Denslow, Thomas D. Schmittgen, Brent A. Reynolds. Hemp derived extracellular vesicles (evs): a potential anti-glioma therapy. Society for neurooncology (SNO) annual meeting. Virtual meeting, November 2020
5. Wilkerson, J. Cannabinols and Terpenes as Novel Therapeutics for Neuropathic Pain. International Cannabinoid Research Society Annual Conference, July 2020
6. Vaddiparti K., Crump C., Wang Y., Williamson J., Cook R. Does Medical Marijuana Help Improve Sleep and Symptoms of Posttraumatic Stress Disorder? Evidence from a pilot study. (under review for presentation at the College on Problems of Drug Dependence, June 2021).
7. Sachdeva, M. Anti-cancer and Chemosensitization effects of Cannabidiol in TNBC cells: Role of GADD45A, integrins and autophagy (manuscript under review).
8. Bilbrey, J.A., Ortiz, Y., Felix, J.S., McMahon, L.R., Wilkerson, J.L. Evaluation of the terpenes β-caryophyllene, α-terpineol, and γ-terpinene in the mouse chronic constriction injury model of neuropathic pain: possible cannabinoid receptor involvement. (manuscript under review in Behavioral Brain Research)
9. Wilkerson, J.L., Bilbrey, J.A., Felix, J.S., Makriyannis, A., McMahon, L.R., Untapped endocannabinoid pharmacological targets: pipe dream or worthy of the therapeutic buzz? (manuscript under review in Behavioral Brain Research)
10. Blanton, H.R., Barnes R.C., McHann M.C., Bilbrey, J.A., Wilkerson, J.L., Guindon, J. Sex Differences and the Endocannabinoid System in Pain. (manuscript under review in Pharmacology, Biochemistry and Behavior)
11. Huffstetler, C., B. Cochran, C. May, C. Silver, N. Maykut, and D.A. Fadool. Therapeutic potential of cannabidiol (CBD) and WIN 55, 212-2-mesylate in a mouse model of anxiety and ADHD. (under review for presentation at the Experimental Biology Conference, hosted Virtually by American Physiological Society, April 27-30, 2021).
2021 ANNUAL REPORT | 25
MMJ CLINICAL OUTCOMES RESEARCH DATA REPOSITORY (MEMORY)
Unlike medications that have undergone rigorous testing, only a small number of controlled studies are available for MMJ. For prescription drugs, in addition to a rigorous approval process, the Food and Drug Administration (FDA) can require the drug manufacturer to conduct additional studies after drug approval. Such studies use oftentimes controlled observational designs, where experiences of real-world populations are evaluated. MEMORY will establish the infrastructure for real-world MMJ clinical outcomes evaluations similar to those employed by the FDA. Specifically, the Consortium aims to link the Office of Medical Marijuana Use (OMMU) MMJ dispensing data with other clinical databases commonly used for outcomes research to create a robust research-ready repository. The planned linkages will optimize detail on MMJ use (type, dose, route, originating plant from the OMMU registry) and detail on patient health history, other treatments and outcomes (from linked clinical encounter data), and facilitate controlled longitudinal studies on safety and effectiveness outcomes. Pending relevant approvals, the Consortium plans to make a de-identified version of the repository available to Consortium researchers, thus providing state-wide infrastructure for real-world clinical outcomes research.
Core tasks to develop MEMORY include:
a) data acquisition, curation and linkage, resulting in a well-documented longitudinal database of patients who initiated MMJ and adequate control groups who have not (yet) initiated MMJ,
b) provision of adequate study cohort data for researchers along with analytical support, and
c) the development and implementation of policies and procedures to access and use the data.
After several delays, most recently involving the COVID-19 pandemic, the legal departments at the Department of Health (DoH) and the University of Florida (UF) have agreed on interpretations of the statutory requirements for DoH to share OMMU registry data with the Consortium. The data use agreement (DUA) is currently under review by DoH. The DUA with the Agency for Health Care Administration (AHCA) for use of Medicaid data has been submitted and is pending AHCA approval. The DUA agreement with DoH for obtaining vital data (birth and death certificates) has been approved and the related request to the DoH Institutional Review Board (IRB) has been submitted.
26 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
MEMORY Data Dictionary
The MEMORY data science team has developed the data architecture and the data dictionary that will define variables after final data acquisition from the various stakeholders. The data dictionary contains specifications for 91 unique variable types that are organized in the following three data tables. This dictionary will guide all analytical work once data have been received.
MEMORY Table 1-DD (Data Dictionary) – Patient-Certification Record
Table 1-DD includes information at the patient-certification level (i.e., each record is a unique combination of an individual patient identifier and an individual certification period). The figure “longitudinal structure” shows the overall timeline of data anchored to a certification period as summarized in table 1-DD.
Data in MEMORY table 1-DD are always anchored on the start date of the patient certification. The look-back and look-forward periods for the ascertainment of baseline variables and clinical outcomes might overlap with periods for another certification record for the same patient. Baseline characteristics such as disease history and clinical outcomes are ascertained from linked Medicaid data.
1 YEAR
LONGITUDINAL STRUCTURE FOR PATIENT CERTIFICATION (TABLE 1-DD)
MEMORY Table 2-DD – Marijuana Utilization
Table 2-DD contains information obtained from OMMU on each individual order within each certification period and the corresponding dispensing of MMJ reported by the dispensary.
MEMORY Table 3-DD. Medication Utilization
Data in the medication utilization table is ascertained throughout the time period of Table 1-DD using the prescription claims data from Medicaid. Prescription drug utilization will be coded, similar to MMJ use, for specific use periods to allow longitudinal assessment of MMJ safety and effectiveness in the context of other medications.
2021 ANNUAL REPORT | 27
CLINICAL RESEARCH CORE
To complement MEMORY, which facilitates retrospective studies of routinely collected data, thus maximizing sample size and the ability to examine long-term outcomes or rare safety events, the clinical research core provides infrastructure support for prospective studies (including randomized controlled trials) involving collection of new data such as patientreported outcomes. Support services in collaboration with the Clinical and Translational Science Institutes (CTSIs) at UF, the University of Miami and Florida State University, include assistance with patient recruitment, data collection or analysis, storage of specimen (biorepositories), and access to laboratory experts for product analysis.
To facilitate patient recruitment, the Consortium has assembled a group of Provider Partners. Provider partners are physicians throughout the state who are willing to recruit patients for Consortium research studies or provide input on pressing clinical outcomes research needs. Collaborating providers and dispensaries are available in the Connecting and Advancing Medical Marijuana research (CARMMA) database established by the Consortium in spring 2020 at https://mmjoutcomes.org/collaborate
The Clinical Core complements MEMORY by providing support for prospective studies on MMJ outcomes, through its patient contact registry, planned patient cohort and research collaborations (CARMMA).
MMJ Contact Registry
The clinical core has built a statewide MMJ contact registry to facilitate patient recruitment for future research on MMJ clinical outcomes. The registry was approved by the University of Florida IRB in November 2020. As of January 2021, 204 current or prospective MMJ users across the state have agreed to be listed in the contact registry. Initial analyses suggest that participants are representative of the state’s MMJ users. The mean age of the group is 45 years (standard deviation = 14.5), with approximately equal distribution between genders (53.4% female, 46.1% male, 0.5% non-binary). The race/ethnicity distribution of the patients is 82.1% White, 10.4% Black, 4.5% Multiracial, 1.5% American Indian or Alaska Native, 0.5% Asian, and 12.9% Hispanic/Latino.
28 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
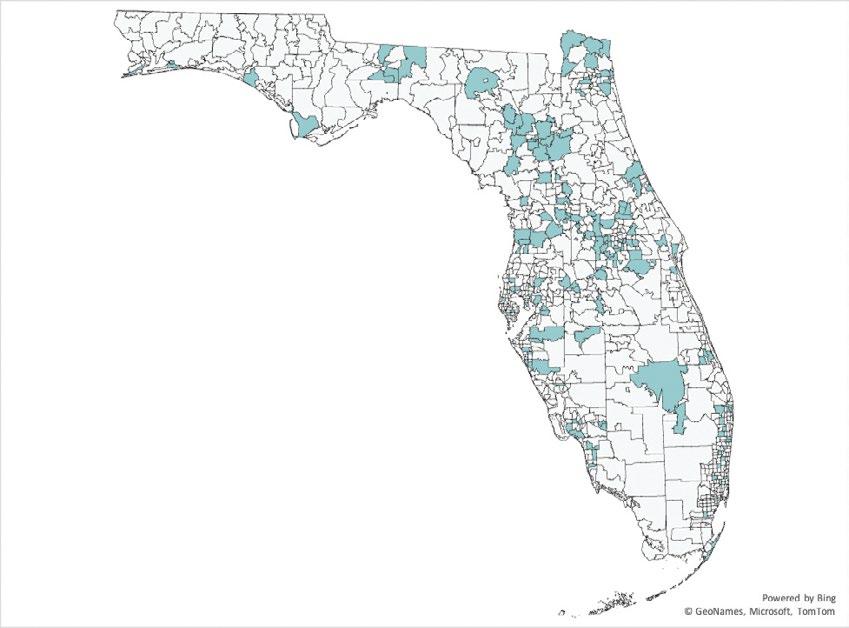
Medical Marijuana Patient Contract Registry Recruitment: (A) Clinics assisting in patient recruitment
(B) Geographic representation of MMJ registry participants
MMJ Patient Cohort

New for this fiscal year was the launch of a prospective patient cohort of MMJ users to study detail on MMJ dosing, administration routes, health effects, and side effects of MMJ. As first step toward launch of this cohort, the Consortium has established a scientific planning committee consisting of 11 members, including six researchers at consortium universities, four MMJ physicians, and one MMJ patient representative. The planning committee guides and oversees patient recruitment and assessments.
SCIENTIFIC PLANNING COMMITTEE MEMBERS - MMJ PATIENT COHORT PARTICIPANT INSTITUTION
George Burgess University of Florida*
John Crump, MD Releafe Now
Justin Davis, MD Florida Marijuana Doctors
Jason Ford, PhD University of Central Florida*
Raul Gonzalez, PhD Florida International University*
Patricia Green-Powell, PhD Florida A&M University*
Dushyantha Jayaweera, MD University of Miami*
Jonathon Quinonez, DO
CannaMD
Martha Rosenthal, PhD Florida Gulf Coast University*
Denise Vidot, PhD University of Miami*
Michelle Weiner, DO, MPH Spine Wellness America
*Consortium member institution
ROLE ON PLANNING GROUP
MMJ Patient Liaison
Certified MMJ Provider
Certified MMJ Provider
MMJ Researcher
MMJ Researcher
MMJ Researcher
MMJ Researcher
Certified MMJ Provider
MMJ Researcher
MMJ Researcher
Certified MMJ Provider
2021 ANNUAL REPORT | 29
(A) (B)
OUTREACH
The Consortium’s outreach activities are directed towards patients, providers, researchers and industry, to maximize participation in research and keep these stakeholders abreast of the latest research findings.
In addition to its website and newsletter, the Consortium enhanced its outreach activities by planning its inaugural annual Cannabis Clinical Outcomes Research Conference (CCORC) and a state-wide provider survey.

30 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Cannabis Clinical Outcomes Research Conference (CCORC)
The consortium is planning its inaugural annual Cannabis Clinical Outcomes Research Conference (CCORC) to be held on April 8th and 9th, 2021 (http://ccorc.mmjoutcomes.org/ ).


This will be a research-centric meeting, open to patients and providers, to share research findings, disseminate the latest evidence of the health effects of marijuana, and stimulate research collaborations throughout the state and nationally.
The CCORC save-the-date announcements and call for abstracts have been widely disseminated and we expect a large turnout to this conference, which is organized as a virtual event to facilitate interested stakeholder attendance and to improve public access in a safe environment
The objectives of CCORC are:
• Dissemination of research findings on medical cannabis use, efficacy, safety, and other relevant outcomes
• Provide a venue for clinical and research educational opportunities related to medical cannabis
• Foster research collaboration, and stakeholder engagement, between Consortium member institutions and beyond
The CCORC website is open for registration at (http://ccorc.mmjoutcomes.org/registration/)
2021 ANNUAL REPORT | 31
CCORC is made possible by the hard work of two committees, the CCORC Organizing committee and the Scientific Program Committee, which has been recruited from all Consortium member institutions. Both committees have made great strides in conference planning and have met all preset milestones to-date.
CANNABIS CLINICAL OUTCOMES RESEARCH CONFERENCE (CCORC) PLANNING AND PROGRESS TIMELINE
The Preliminary Agenda for CCORC involves keynote addresses by three national level experts in the field of cannabis research, three panel discussions, as well as poster presentations and an exhibitor hall. CCORC has been approved to offer CME credits to attending physicians.
Preliminary CCORC Agenda
APRIL 8, 2021
12-12:30pm Live Day 1 Welcome address from Consortium Leadership
12:30-1pm Live Keynote (Speaker — Dr. Collins): Cannabis Science vs. Policies: Reconciling the Disconnect
1-1:30pm Static Poster Session: Theme #1
1:30-2:00pm Live Keynote (Speaker — Dr. Abrams): Clinical Trials of Cannabis in Cancer and Sickle Cell Pain: ‘Not as Easy as It Looks!
2:00-3:30pm Live Panel: Research and Regulatory Barriers
3:00-3:30pm Static Poster Session: Theme #2
Continuous Combo Exhibition Hall, Forums, and poster breakout rooms
APRIL 9, 2021
12-12:30pm Live Day 2 Welcome from Consortium Leadership Speaker: Government official (in progress)
12:30-1pm Live Keynote (Speaker — Dr. Cooper): Controlled Human Studies Investigating Cannabis Constituents for Pain: A Translational Perspective
1-1:30pm Static Poster Session: Theme #3 1:30-2:30pm Live Panel: Oral Presentations (top abstracts/hot topics)
2:30-3pm Static Poster Session: Theme #4
3-4pm Live Closing; Board Reception
Continuous Static Exhibition Hall and Forums
32 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
NOVEMBER 2020 DECEMBER JANUARY 2021 FEBRUARY MARCH APRIL
We have confirmed three eminent keynote speakers who have all served on the National Academy of Science panel that synthesized the evidence on marijuana effectiveness and safety in 2018.

Dr. Ziva Coopers’ research focuses on preclinical and clinical studies on the behavioral and physiologic effects of psychoactive drugs that are of significant public health relevance, including cannabis and opioids. Her current research, funded by NIDA and industry contracts, involves understanding the neurobiological, pharmacological, and behavioral variables that influence both the abuse liability and therapeutic potential of cannabinoids (cannabis, cannabinoid receptor agonists, and cannabidiol) and opioids.
Dr. Donald Abrams has conducted numerous clinical trials investigating complementary therapies in patients with HIV, including therapeutic touch, traditional Chinese medicine interventions, medical marijuana, medicinal mushrooms, and distant healing.
Dr. Lorraine Collins’ research interests include cognitive and behavioral approaches to the conceptualization, prevention and treatment of addictive behaviors, particularly among emerging and young adults.
Provider Survey
In June 2020, the Consortium launched its statewide Provider Survey to gather providers’ need for research and training. The survey was pilot-tested at the Annual Medical Marijuana Provider (AMMPA) Meeting in Orlando, FL, in October 2019 before it was disseminated across all providers who are licensed to certify patients for use of MMJ (n=1662) in Florida.
A total of 116 physicians completed this survey. Most providers (82%) practice both traditional medicine and licensed patients for MMJ use, and the majority (83%) have maintained a MMJ practice for more than one year.

2021 ANNUAL REPORT | 33
Results from the Medical Marijuana Physician Survey on Priorities for Research and Training
Providers ranked research on drug-drug interactions, on components of MMJ and the human endocannabinoid system as top priorities (Table below). Three of these topics (marked with asterisk) were new inputs provided by the physician survey that helped develop the research priorities for the Consortium 2021 research grants program.
Top conditions as focus for research included pain, anxiety and reduction of other medications or substance use. Insomnia and sleep disorders (marked with asterisk) were also listed as one of the top medical conditions and added as new input to the consortium’s research priorities. Top training priorities mirrored the expressed research priorities.
The Consortium continues to update its website (https://mmjoutcomes.org/) to disseminate information to researchers, member institutions, physicians/providers, patients, and public. The website provides a comprehensive and interactive hub for the grants program, research updates, and Consortium news. Since its launch in October 2019, more than 2,971 web-users have visited the new website.
The Consortium has started to expand the website utility for patients and providers with two new initiatives: development of patient and provider information sheets and a new series
“Evidence
both discussed in more detail in the following Evidence Core section
34 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Website
PROVIDER SURVEY RESULTS INDICATING PRIORITIES FOR RESEARCH AND TRAINING TOP 5 RESEARCH TOPICS Research on drug interactions with medical marijuana and prescription drugs 54% *Research on comparing different components of medical marijuana (e.g. different terpenes) 48% *Research on human endocannabinoid system 46% Research on different potency levels of THC products (e.g. >10% vs <10% THC) 41% *Research on serious side effects (e.g., psychosis, hyperemesis, falls, accidents) 41% TOP 5 MEDICAL CONDITIONS/SYMPTOMS Chronic pain 59% Anxiety 57% Reduction of other medication (e.g. opioids) or substance use 48% *Insomnia & sleep disorders 42% PTSD 38% TOP 5 TRAINING TOPICS Drug – drug interactions with medical marijuana 72% Evidence for management of specific medical conditions or symptoms 71% Information about how to best recommend doses and ratios for patients 69% Strategies to help patients reduce their use of opioids or other drugs 68% Advantages and disadvantages of specific modes of delivery (e.g. vape, tincture) 67%
in context,”
.
The website also serves as a central location to support collaboration between physicians/ providers, researchers, and patients through various channels, including a secure portal for patients to sign up for participation in research and a collaborative space for researchers to network with other researchers and practicing physicians/providers.
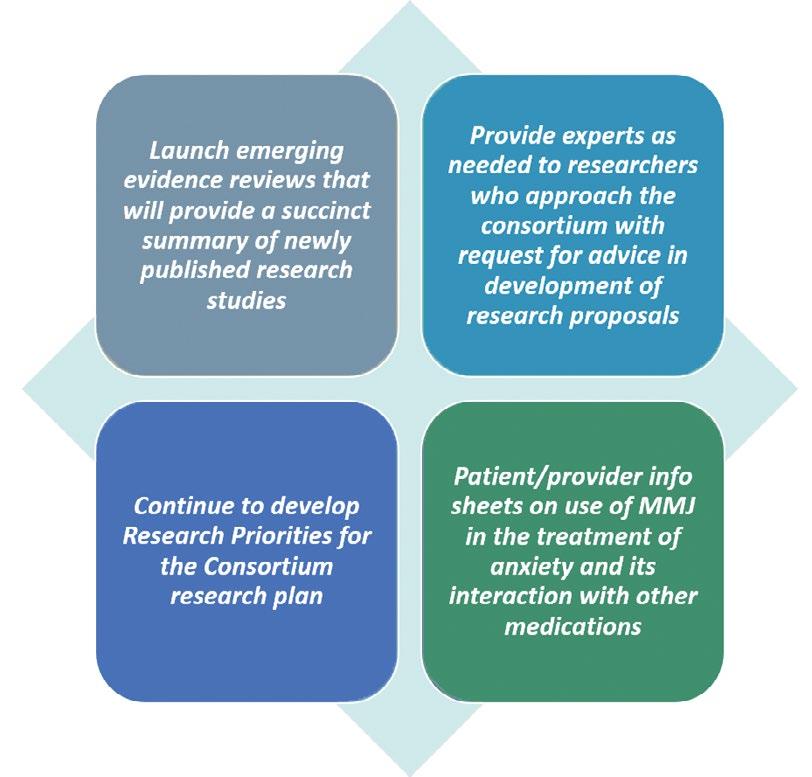
Newsletter
As part of the communication and outreach plan, the Consortium distributes quarterly newsletters via email to researchers, physicians/providers, and individuals interested in MMJ.
The newsletter includes updates on Consortium activities and ongoing research. For recognition, the Consortium branded the newsletter as MEDICAMENT, which stands for MEDICAl Marijuana rEsearch NewsleTter. A total of 472 individuals have signed up for the newsletter as of December 2020.

The Consortium has published four issues of MEDICAMENT, one every quarter, available at https://mmjoutcomes.org/newsletter.
EVIDENCE CORE
The evidence core focuses on the synthesis and dissemination of scientific evidence for researchers, providers and patients. Evidence core activities include publication of Evidence in Context, a new series, geared at MMJ physicians that will be launched in the next issue of the Consortium Journal, Medical Cannabis & Cannabinoids. This series scans medical literature and highlights new and clinically relevant findings along with brief commentaries on quality and clinical relevance. No such resource is currently available specifically for MMJ.
The Evidence core will also launch a new collection of patient/provider info sheets with initial focus on two important topics: use of MMJ for treatment of anxiety, and interactions of MMJ with prescription drugs. Both topics are included in the Consortium research priorities and represent need for information expressed by providers and patients. Two versions of these info sheets will be developed, including a version using lay language for consumers and a version for providers that is similarly brief, but uses clinical terminology and provides references to the relevant primary literature for further study as desired.
EVIDENCE CORE ACTIVITIES
The purpose of these info sheets is to provide unbiased, evidence-based and up-to-date information about important topics on MMJ clinical outcomes in a format that is clear and concise for all members of the public. The info sheets will be accessible on the Consortium website and disseminated by the Consortium in collaboration with the Florida A&M University (FAMU)’s Medical Marijuana Education and Research Initiative (MMERI).
The Evidence core maintains a national scientific expert panel for input on research priorities and to provide as needed scientific expertise for Consortium researchers.
2021 ANNUAL REPORT | 35
Scientific Expert Panel Members
Participant Institution
Cannabis-Related Expertise
Jeffrey Cassisi University of Central Florida* Cognition and Pain
John Markowitz University of Florida* Drug-Drug Interactions (Kinetics)
Ellen Zimmermann University of Florida* Gastroenterology
Youn Ok Lee~ Research Triangle Institute Vaping, Smoking, other administration routes
Aimee McRae-Clark Medical Univ. of South Carolina Cannabis Use Disorder
Tory Spindle~ Johns Hopkins Routes of Administration
Denise Vidot University of Miami* HIV/AIDS, Use Patterns
Kathleen Egan Moffitt Cancer Center Cancer
Sean Hennessey~ University of Pennsylvania Drug-Drug Interactions (Epidemiology)
Jodi Gilman Mass General/Harvard Medical Cognition and Addiction
Linda Simoni-Wastila University of Maryland Marijuana Use Patterns, Policy
Patricia Green-Powell Florida A&M University* Use Patterns, Stakeholder Engagement
Maija Reblin Moffit Cancer Center Palliative Care
~ has provided invited FDA or congressional expert testimonial; * Consortium member institution Research Priorities for 2021-2022
The Evidence core conducted a systematic literature review in the previous fiscal year to provide an assessment of the status and especially key gaps in evidence on the safety and effectiveness of MMJ. Development of the Consortium research priorities was based on the literature review findings, input from the scientific expert panel and review of the statutory mandate defining the Consortium responsibilities.
For this new fiscal year, the Evidence core build further upon the 2020-2021 research priorities with fresh inputs from the state-wide physician survey and NIH areas of programmatic interest to define the Consortium research priorities for 2021-2022.
2021-22 RESEARCH PRIORITIES
1. Clinical Outcomes of Medical Marijuana use: with particular emphasis on
a. chronic pain
b. anxiety/PTSD
c. symptomatic treatment of cancer
d. insomnia and sleep disorders
e. reduction of other medication (e.g., opioids) or substance use
f. effects on the developing brain
g. effects during pregnancy and breastfeeding
h. research on serious side effects including, psychosis, hyperemesis, falls, accidents, and cardiovascular events
2. Route of Administration: effect of dosing and routes of medical marijuana use on efficacy and safety; of particular interest are studies that evaluate effects of smoking and vaping
3. Interactions of Medical Marijuana with other drugs/medications:
a. with particular focus on medications that are commonly used by patients who seek medical marijuana treatment
b. impact of polysubstance, including interactions with alcohol, tobacco, benzodiazepines, and prescription and nonprescription opioids
4. Epidemiology research to study trends for cannabis use and cannabis use disorder (CUD), including new products, patterns of use, and reasons for use in different populations, including the impact of COVID-19
5. Research on the human endocannabinoid system
6. Measuring components of medical marijuana/cannabis (including hemp and hemp product) and contrast their clinical outcomes:
a. comparing different components of medical marijuana (e.g. different terpenes)
b. research on different potency levels of THC products (e.g. >10% vs <10% THC) standards for measuring cannabis (including hemp and hemp product) dose, intoxication, and impairment
36 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Scientific Journal of the Consortium
The Consortium signed an agreement with a Karger journal “Medical Cannabis and Cannabinoids” in August 2020, to serve as the consortium’s official journal.
Two consortium board members, Drs. Goodin and Brown, have joined the editorial board and lead the launch of the “Evidence in Context” series published in the journal and on the Consortium website. Both of these publication platforms are fully accessible to all members of the public, as the journal uses an open-access publishing format.
To date, the journal has accepted two articles within this series for forthcoming publication. One introduces and promotes the Consortium to a global audience of cannabis researchers and clinicians while the other provides a critical commentary regarding the risk of bias in clinical trials that have studied the health effects of MMJ in humans.

NOTABLE OUTCOMES OF THE CONSORTIUM RESEARCH PROGRAM
Over the only 1.5 years of its existence, grant awardees and other Consortium researchers have put the Consortium resources to good use: our research outcomes, generated over such a short time period, include 7 published manuscripts in peer-reviewed journals, one patent and 6 extramural grant applications and a total of 41 trainees who have worked on Consortium-funded research.
2021 ANNUAL REPORT | 37
CONSORTIUM BIBLIOGRAPHY
Publications
1. Brown J. , Costales B., van Boemmel-Wegmann S., Goodin A. , Segal R., Winterstein A. Characteristics of Older Adults Who Were Early Adopters of Medical Cannabis in the Florida Medical Marijuana Use Registry. J Clin Med. 2020 Apr 18;9(4):1166. doi: 10.3390/jcm9041166. PMID: 32325769; PMCID: PMC7230351.
2. Costales B., van Boemmel-Wegmann S., Winterstein A. & Segal S. (2021) Clinical Conditions and Prescription Drug Utilization among Early Medical Marijuana Registrants in Florida, Journal of Psychoactive Drugs, DOI: 10.1080/02791072.2020.1864069
3. Brown J., Winterstein A. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J Clin Med. 2019 Jul 8;8(7):989. doi: 10.3390/jcm8070989. PMID: 31288397; PMCID: PMC6678684.
4. Brown J., Goodin A.. The prevalence of drivers under the influence of medical cannabis must be considered within proper context. Res Social Adm Pharm. 2019 Nov;15(11):1372-1373. doi: 10.1016/j.sapharm.2019.01.015. Epub 2019 Jan 28. PMID: 30709730.
5. Roussos-Ross K., Dukharan V., Goodin, A. Is In-Utero Marijuana Exposure Associated With Childhood Developmental Delay? [20K], Obstetrics & Gynecology: May 2020Volume 135 – Issue – p 118S-119S doi: 10.1097/01.AOG.0000664476.72398.7c
6. Alipour H., Sarayani A., Winterstein A. Letter by Alipour Haris et al Regarding Article, “Marijuana Use Among Young Adults (18-44 Years of Age) and Risk of Stroke: A Behavioral Risk Factor Surveillance System Survey Analysis.” Stroke. 2020 May;51(5):e91. doi: 10.1161/STROKEAHA.120.029273. Epub 2020 Apr 7. PMID: 32252600.
7. Rosenthal M., Pipitone N. Demographics, Perceptions, and Use of Medical Marijuana among Patients in Florida. Med Cannabis Cannabinoids 2020. Doi. 10.1159/000512342
Conference Abstracts
1. Jugl S., Keshwani S., Adkins L., Heldermon C.D., Winterstein A., and Goodin A. A systematic review of evidence for cannabis and cannabinoids as adjuvant therapy in palliative and supportive oncology care. Journal of Clinical Oncology 2020 38:15_suppl, 12091-12091
2. Sajdeya R., Jean-Jacques J., Shavers A., Wang Y., Pipitone N., Rosenthal M., Winterstein A., Cook R. Information sources and training needs on medical marijuana: preliminary results from a state-wide provider survey. Poster presented virtually at the 4th annual scientific meeting of Research Society on Marijuana (RSMj), July 24, 2020.
3. Jugl S., Okpeku A., Costales B., Morris E., Alipour-Harris G., Hincapie-Castillo J., Stetten N., Sajdeya R., Keshwani S., Joseph V., Zhang Y., Shen Y. Adkins L., Winterstein A., Goodin A. A mapping literature review of medical cannabis clinical outcomes and quality of evidence in approved conditions in the United States from 2016 to 2019. Poster presented virtually at the 4th annual scientific meeting of Research Society on Marijuana (RSMj), July 24, 2020.
4. Sachdeva M. Role of Cannabinoids for Breast and Lung Cancer Cell lines, presented at the FAMU Pharmacy Clinical Symposium Mar 6, 2020
5. Wilkerson J. Oral Presentation Presenter. Axially-Chiral Cannabinols as Novel Therapeutics for Neuropathic Pain. UF SPAR (Pain and Addiction Research) Conference, Gainesville, FL December 2019
6. May C., Silver C., Huffstetler C., Cochran B., Maykut N., and Fadool D.A. Does CBD (Cannabidiol), the Non-Psychoactive Ingredient of Cannabis, Mitigate Anxiety in Mice? Florida Undergraduate Research Conference (FURC), hosted Virtually by Florida State University, Feb. 26-27, 2021.
38 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
7. Azari H., Koopaei N., Nouri M.Z., Hall J., Denslow N., Schmittgen T., Reynolds B. Hemp derived extracellular vesicles (evs): a potential anti-glioma therapy. Poster presented at the Society for neuro-oncology (SNO) annual meeting (November 2020)
8. Wang Y., Jacques J., Li Z., Sibille K.T., Cook R. Health outcomes among adults initiating medical marijuana for chronic pain: A 3-month prospective study incorporating ecological momentary assessment (EMA). American Geriatrics Society 2021 Virtual Annual Scientific Meeting.
9. Wilkerson, J. Cannabinols and Terpenes as Novel Therapeutics for Neuropathic Pain, Ireland ICRS (International Cannabinoid Research Society) Annual Conference July 2020 abstract accepted, but meeting canceled due to COVID-19.
Interviews
Wilkerson J. How Cannabis-Based Therapeutics Could Help Fight COVID Inflammation (Medscape, 2020) https://www.medscape.com/viewarticle/940265
Talks
1. Sachdeva M. Investigation on the role of Cannabinoids in Cancer therapeutics, Clinical Symposium organized by the College of Pharmacy FAMU, Mar 6, 2020
2. Winterstein A., Goodin A., Cook R. UF eye opener discovery breakfast about Medical Marijuana. March 11, 2020.
3. Goodin A. and Brown J. “What to know about CBD and Marijuana” at the Alachua County Senior Recreation Center, PrimeTime Institute’s Educational Program Series, in collaboration with UF Health, Nov 12, 2020.
4. Cook R. “Medical Marijuana for Cancer and Cancer-related symptoms,” as part of the Cancer educational wellness series. July 22, 2020
5. Brown J. “Drug-drug and drug-disease interaction potential of cannabis” at Innovatix/ Premier Lunch N Learn Webinar July 15, 2020
6. Brown J. “Drug-drug and drug-disease interaction potential of cannabis” at the American College Health Association Annual Meeting in Chicago in May, 2020.
7. Cook R. and Wang, Y. Discussed medical marijuana terminology, shared evidence review findings from the Consortium, and highlighted ongoing research at the University of Florida and across the state through the Consortium, at the Research and Medical Cannabinoids CME in Ocala February 29th, 2020.
Extramural Grant Submitted:
1. Brown J. submitted a research grant for $1.7M to National Institute of Aging in September 2020, titled “Cannabis use and adverse drug events in older adults,” which is currently under review.
2. Goodin A. submitted a research grant for $629k to the National Institute on Drug Abuse in October 2020, which is currently under review. This project aims to examine maternal and neonatal outcomes resulting from polysubstance use, including cannabis, among pregnant women who are in treatment for opioid use disorder.
3. Wang Y. submitted a research grant for $3.6M to National Institute of Aging in June 2020, titled “Real-Time and Long-Term Effects of Medical Marijuana on Older Adults: A Prospective Cohort Study.” The proposal was scored at 34% at the initial submission. A resubmission is planned for March 2021.
4. Wilkerson J. (PI), McMaho L. (Co-I), Sharma A. (Co-I), Leon F. (Co-I) submitted a grant to NCCIH Titled “Terpenes and minor cannabinoids as novel analgesics.”
5. Azari H. and Reynolds B. submitted a grant to NIH Titled “Acidic cannabinoids for the treatment of high-grade glioma.”
6. Azari H. and Reynolds B. submitted a grant to DOD Titled “Cannabinoids Acidic for the treatment of Glioblastoma.”
2021 ANNUAL REPORT | 39
FUTURE PLANS FOR THE CONSORTIUM
SUMMARY OF CONSORTIUM RESEARCH PRIORITIES
Research priorities were set in accordance with the Consortium’s charge per statute, to “… contribute to the body of scientific knowledge on the effects of the medical use of marijuana and inform[s] both policy and medical practice related to the treatment of debilitating medical conditions with marijuana.” As described above, programmatic priority areas were informed by our review of the scientific evidence, national expert panel input, our statewide physician provider survey and NIH areas of programmatic interest. These research priorities will be applied in both the Consortium’s 2021 grants program and will also guide the research projects and proposals directed by Consortium leadership.
RESEARCH PLAN FY 2021-22
Since its inception in July 2019, the Consortium has made great strides towards facilitating and conducting research that will inform clinical care and policy about the medical use of marijuana to improve public health. To build on these initial efforts, the Consortium will continue development of the five original Consortium research program pillars: The Grants program, MEMORY, the Clinical Core, Outreach and Evidence Core Activities. The specific goals and plans for each pillar have been updated and are described below.
The following describes the Consortium Research Plan for fiscal year 2022.
Grants Program
In light of the overwhelming response to the Consortium’s grants program, the board has agreed to continue this effort without major changes in the coming fiscal year, except for the expansion of research priority areas as discussed above. The Consortium staff has implemented several procedural changes to streamline the application process. These include changes in the LOI form to indicate if the proposal was submitted in a previous cycle, inclusion of a translational statement in the research plan, a new environment/resources section, and if applicable description for protection of human subjects, inclusion of Women, Minorities, and Children, vertebrate animals and biohazards.
The 2021 Grants cycle began earlier this cycle, allowing more time for researchers to prepare their submissions and facilitating completion of application reviews by the end of the fiscal year to allow expeditious funding of prioritized proposals, once the Consortium budget for FY 2022 is approved.
The 2021 request for proposals is available at https://mmjoutcomes.org/ research/2021grants/2021rfp/
40 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
MEMORY
Plans for MEMORY development remain unchanged for fiscal year 2022 with the key focus on establishing data sharing processes and procedures with OMMU and AHCA. As envisioned, MEMORY can then serve in two capacities: for controlled studies on MMJ effectiveness and safety and for active surveillance to capture emerging safety issues among MMJ users. The former will employ rigorous study designs including control groups of patients who do not use MMJ but have similar health conditions, while the latter follows MMJ users to capture signals of unexpected adverse events that may be associated with a particular marijuana product.
Clinical Core
Goals for the Clinical Core will include continued expansion of the Consortium infrastructure to support patient recruitment into prospective research studies via its patient contact registry and CARMMA database of collaborating physicians, researchers and industry. The Consortium will launch its patient cohort this summer with the goal to track detail on MMJ use and patient-reported outcomes. The Consortium hopes to establish a patient recruitment mechanism that has proven effective and that can be provided to investigators from all Consortium institutions to accelerate and enhance conduct of clinical outcomes studies. A proven patient recruitment platform is also critical for extramural grant applications (e.g., to NIH) to further expand the consortium’s research program.
The Clinical Core will also continue to work on guidance for investigators on regulatory issues involving use of MMJ in research studies. This will include guidance on DEA licensure and other relevant state and federal regulations.
Outreach
In anticipation of a successful inaugural Cannabis Clinical Outcomes Research Conference (CCORC), we plan to hold the second annual CCORC in spring 2022. We also propose to add a news feed section to our website to inform the community about the latest news and policy changes around MMJ. Other outreach activities through the Consortium website, its quarterly newsletter and participation in scientific conferences will continue.
Evidence Core
We will build upon the two new activities that complement Consortium outreach activities launched by the evidence including publication of the Evidence in context series and patient/provider info sheets. The Consortium considers the emerging evidence reviews and info sheets an important feature to enhance its communication with providers and patients and stimulate interest in MMJ clinical outcomes research.
The board and Consortium staff would like to conclude this report by expressing strong continuing support and enthusiasm to advance the Consortium research program. We are convinced that the Consortium can address an urgent and critical need to inform patients, providers and regulators on the safe and effective use of MMJ, including dose, route and product choice, tailored to other concomitant treatments and patients’ underlying disease conditions. We believe that the medical use of marijuana must be guided by the same scientific evidence that is available for prescription medications and that the safety of MMJ products should be monitored with similar surveillance methods. The Consortium is devoted to building both.
2021 ANNUAL REPORT | 41
APPENDIX A
THE CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH BOARD
WILLIAM (BILL) ANDERSON, PH.D.
Associate Vice President of Research; Florida International University
Associate Vice President William (Bill) Anderson leads initiatives that expand FIU’s efforts in research development for faculty, doctoral students and postdoctoral scholars. Additional areas of leadership focus include research labs, core facilities, research integrity, and laboratory safety, among others. Dr. Anderson joined FIU in 2000 as Assistant Professor and has risen to the rank of Professor.

Administratively, he has served as Chair of the Department of Earth & Environment and Associate Dean of Faculty in the College of Arts, Sciences & Education where he most recently served as the Vice Dean.
He received a doctorate of Natural Sciences from the Swiss Federal Institute of Technology (ETH-Zentrum), a M.S. in Geology from Syracuse University and a B.A. in Geology from the University of Kansas. A stable isotope biogeochemist, Dr. Anderson’s research focuses on biogeochemical cycling of carbon and nitrogen in marine systems, as well as the oxygen, carbon, and nitrogen isotopic signatures in organic material. His research has been published in top tier journals; he has presented in national and international conferences; and he has received funding from the NSF, the American Chemical Society, and the U.S. Department of the Interior, among others.
W. DALTON DIETRICH, PH.D.
Scientific Director, The Miami Project to Cure Paralysis; Senior Associate Dean for Discovery Science; Co-Director, Institute for Neural Engineering; Associate Director, Miami CTSI; Professor of Neurological Surgery, Neurology, Biomedical Engineering and Cell Biology; Leonard M. Miller School of Medicine; University of Miami

Dr. W. Dalton Dietrich is Scientific Director at The Miami Project to Cure Paralysis and the Kinetic Concepts Distinguished Chair in Neurosurgery at the University of Miami Miller School of Medicine. He received his Ph.D. in Anatomy from the Medical College of Virginia in 1979 and completed a postdoctoral fellowship in the Department of Pharmacology at Washington University, St. Louis, MO, 1981. In 1981, Dr. Dietrich joined the Department of Neurology at the University of Miami, with a joint appointment in Cell Biology and Anatomy, and in 1993 attained the rank of Professor. Dr. Dietrich served as ViceChairman for Basic Science in the Department of Neurology and in 1997 and accepted the position of Scientific Director of The Miami Project to Cure Paralysis. Dr. Dietrich also serves as the Senior Associate Dean for Discovery Science, Co-Director of the Institute for Neural Engineering and Associate Director of the Miami Clinical and Translational Science Institute (CTSI) at the University of Miami.
Dr. Dietrich’s laboratory is focused on clarifying the pathophysiology of brain and spinal cord injury with the ultimate goal of developing new therapies to protect and enhance recovery of function. Over the last 35 years, Dr. Dietrich and colleagues have studied the cellular and molecular injury mechanism underlying various neurological disorders including stroke, cardiac arrest, traumatic brain and spinal cord injury. In terms of neuroprotection, he and his colleagues provided the initial preclinical data indicating that small differences in the temperature of the brain and spinal cord critically determine whether neurons die
42 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
following neurological injury. Most recently, Dr. Dietrich and colleagues have investigated the importance of abnormal inflammasome activation in the brain and spinal cord after injury. These studies have uncovered a new therapeutic target for modifying the immediate immune response to injury. Finally, Dr. Dietrich and colleagues are using novel cellular and drug treatments to promote reparative process and functional recovery after brain and SCI. He is currently the Sponsor of first-in-man FDA approved clinical trials testing the safety of human Schwann cell transplants in people with severe spinal cord and peripheral nerve injuries.
Dr. Dietrich has published over 400 refereed journal articles, 60 book chapters and 4 books. His published work has been cited over 35,000 times. He has been listed by the Institute of Scientific Information as a “Highly Cited Researcher,” placing him in the top 0.5% of all scientists based on the impact his research has made on other scientists. Dr. Dietrich has been a thesis/dissertation advisor to 9 graduate students and has trained over 40 postdoctoral fellows and visiting scholars. His research programs are supported by the NIH, Department of Defense, State of Florida and The Miami Project to Cure Paralysis. He serves on study sections for NIH, Department of Defense and several state brain and spinal cord injury research programs. He is currently Editor-In-Chief of the Journal Therapeutic Hypothermia & Temperature Management and Deputy Editor of the Journal of Neurotrauma. He is a co-founder of two start-up biotechnology companies, InflamaCORE, LLC and Aceso Therapeutics, LLC to help move new discoveries to the clinic.
ROGER B. FILLINGIM, PH.D.
Distinguished Professor; Director, University of Florida, Pain Research and Intervention Center of Excellence (PRICE); University of Florida
Roger B. Fillingim, Ph.D., a Clinical Psychologist, is Distinguished Professor in the University of Florida (UF) College of Dentistry and Director of the UF Pain Research & Intervention Center of Excellence. Dr. Fillingim maintains an active research program investigating individual differences in pain.
He has been continuously NIH-funded since 1994, and his current grants include a MERIT Award from the National Institute on Aging, which investigates biological and psychosocial factors contributing to ethnic group differences in osteoarthritis pain. He also serves as Director of the UF Center for Advancing Minority Pain and Aging Science. He has published more than 300 scientific articles and is a frequent speaker at national and international conferences. He served as President of the American Pain Society from 2012-2014, served as Co-Chair of the Federal Pain Research Strategy Disparities Workgroup, and is currently a member of the US Department of Health and Human Services Interagency Pain Research Coordinating Committee. He has received several awards, including a University of Florida Term Professorship, as well as the Fordyce Clinical Investigator Award and the Distinguished Service Award, both from the American Pain Society.

DANIEL C. FLYNN, PH.D.

Vice President for Research; Florida Atlantic University
Daniel C. Flynn, Ph.D., Vice President for Research, oversees research administration at Florida Atlantic University. In addition, he’s responsible for the University’s research institutes and centers as well as leading its economic development and entrepreneurial initiatives, such as FAU Tech Runway, an innovative start-up incubator. He also launched FAU Wave, a successful undergraduate research and entrepreneurship program, as well as the Florida Small Business Development Center at FAU.
2021 ANNUAL REPORT | 43
Prior to joining FAU, Flynn served as Associate Dean for Research in the University of Delaware’s College of Health Sciences where he helped grow the research enterprise by 60 percent, promoted invention disclosures, faculty patent applications and developed undergraduate entrepreneurial and research programs. He served as the founding Associate Dean for Research and Economic Development at The Commonwealth Medical College, a new medical school in Scranton, PA. Flynn spent 17 years at West Virginia University where he served as a Professor and Deputy Director of the Mary Babb Randolph Cancer Center. During his career, he has been awarded more than $40 million in research funding. The National Institutes of Health funded Flynn’s research for more than 20 years. At WVU, he served as Director of a Center of Biomedical Research Excellence for Cancer Cell Biology, developed mentoring programs, organized core facilities and served as coordinator of the M.D./Ph.D. training program.
He has published 72 research articles and has also served on an editorial board, advisory committees and study sections for NIH and the Association of American Medical Colleges. He earned his B.S. at the University of Maryland, College Park, Ph.D. at North Carolina State University and his post-doctoral work at the University of Virginia.
TIMOTHY A. GILBERTSON, PH.D.

Professor of Medicine; University of Central Florida
Timothy A. Gilbertson is a Professor of Medicine in the Department of Internal Medicine at the University of Central Florida, College of Medicine. Prior to his current appointment which he assumed in 2018, he was most recently Professor of Biology and Co-Director of the Neuroscience PhD Program at Utah State University. He received his Ph.D. in Neurobiology from the University of California-Davis in 1991 and his postdoctoral training at Colorado State University from 1991-1993.
From 1993-2000 he was Assistant/Associate Professor at Louisiana State University and the Pennington Biomedical Research Center. He has served as Chairman of the National Institutes of Health Communication Disorders Review Committee at the National Institute of Deafness and other Communication Disorders and as Chairperson of the Health and Scientific Advisory Board and on the Board of Directors of the Institute of Public Health and Water Research (IWPR).
In the past 20 years, he has served as a consultant to or done contract research for numerous companies including Novartis Pharmaceuticals, Kraft Foods, Frito Lay, Purina, Givaudan, Nestlé, and Glaxo Smith Kline. He currently serves as a consultant to PepsiCo Global R&D. From 2007-2011 he served as the Director for the Center for Advanced Nutrition at Utah State University. His research focuses on how the body recognizes and responds to nutrients and how this process is tuned to the underlying nutritional needs of an organism. This has implications ranging from basic mechanisms for taste transduction and the design of taste mimetics to post-ingestive nutrient chemoreception and the control of food intake, dietary-induced obesity and diabetes.
His laboratory was the first to elucidate the mechanisms underlying the taste of both fat and sour and has been the first to show unequivocally that nutrient recognition in the peripheral taste system is modulated by diet and disease. Dr. Gilbertson has generated well in excess of $7 million in extramural funding, published over 70 research articles including in Science, PNAS, The Journal of Neuroscience and Neuron and received the Ajinomoto Award for Outstanding Research in Gustation and the Outstanding Graduate Mentor Award from Utah State University. He has served as advisor and mentor for 25 graduate students, 9 postdoctoral fellows and several visiting professors.
44 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
ERIC H. HOLMES, PH.D.
Assistant Vice President for Research; Florida State University
Eric Holmes has a PhD in Biochemistry from the University of California, Davis. Since 2013 he has been an Assistant Vice President for Research in the FSU Office of the Vice President for Research.
He currently also serves as the Interim Director for the FSU Office of Human Subjects. Prior to joining FSU, he was Director of Research at the University of Hawaii’s John A. Burns School of Medicine.

Dr. Holmes has a long track record of directing NIH-funded research in biochemical oncology. He is an author of approximately 100 research publications and is an inventor on over 30 issued US and foreign patents. Dr. Holmes has also worked in the Biotech industry in development-stage pharmaceutical companies located in the Pacific Northwest focused on antibody therapy and drug delivery technologies, and has designed and managed clinical trials related to the development of these technologies.
CYNTHIA (CINDY) HUGHES HARRIS, PH.D., OTR, FAOTA, FASAHP
Dean, School of Allied Health Sciences; Florida A&M University
Cynthia Hughes Harris, Ph.D., OTR, FAOTA, FASAHP currently serves as the Dean of the School of Allied Health Sciences at Florida A&M University. As the Dean, Dr. Harris is responsible for the oversight and management of graduate programs in physical therapy, occupational therapy, and health administration as well as undergraduate programs in health science, health care management, health informatics and information management and cardiopulmonary science.
Dr. Harris is a graduate of the University of Illinois at both the graduate and undergraduate levels. Currently, Dr. Harris serves as the Research Director for the Medical Marijuana Education and Research Initiative at Florida A&M University.

Her interest in marijuana use began when, as a researcher, she focused on HIV prevention programs for high-risk urban adolescents. She successfully implemented such programs in Chicago public schools as well as schools in the South Bronx section of New York City. Additionally, she has been particularly successful in receiving external funding for the improvement of societal health and the elimination of health disparities in both urban and rural communities of Florida.
Other investigative initiatives addressed the results of the qualitative experiences of minority students in different academic environments. She has served as a Presidential appointee to the Advisory Committee of the White House Conference on Aging as well as the Health Resources Services Administration (HRSA) Advisory Committee on Interdisciplinary Community-Based Linkages.
2021 ANNUAL REPORT | 45
MAX C. E. OREZZOLI, PH.D.
 Assistant Professor of Sociology (Medical); Florida Memorial University
Assistant Professor of Sociology (Medical); Florida Memorial University
Max C. E. Orezzoli, Ph.D. is an Assistant Professor of Sociology (Medical) specializing in health and quantitative analysis at Florida Memorial University. Dr. Orezzoli has 15 years of experience in minority health disparities research focusing on Substance Use Disorder (SUD), including marijuana use, nutrition, and HIV research, and how these areas intersect.
Additionally, Dr. Orezzoli has provided experimental and instrument design, data collection trainings, evaluation, statistical and methodological consulting and assistance to institutions, biomedical, educational, and healthcare organizations.
His expertise and research interests are centered on transdisciplinary and translational public health and Community Based Participatory Research (CBPR) interventions that positively impact the health of underrepresented communities regionally, nationally and internationally.
Dr. Orezzoli is bilingual and fluent in Spanish. He has extensive experience in formative and summative program evaluation using quantitative and qualitative methods, which are regionally and culturally appropriate. He has served as an evaluator on several Substance Abuse and Mental Health Services Administration (SAMHSA) grants addressing SUD in Hispanic, black, and Native American communities.
Moreover, Dr. Orezzoli is well equipped to conduct research involving human participants and, addressing sensitive issues/topics of focus (medical marijuana, diet research, HIV, SUD, violence, etc.) while adhering to all confidentiality and privacy requirements. Dr. Orezzoli serves as the Co-chair of the Institutional Review Board (IRB) at Florida Memorial University.
MARTHA S. ROSENTHAL, PH.D.
Professor of Neuroscience/Physiology; Director of the Cannabis Research, Education, and Workforce initiative; Florida Gulf Coast University
Dr. Martha Rosenthal is a Professor of Neuroscience & Physiology at Florida Gulf Coast University, where she teaches courses in cannabis, drugs and society, neuroscience, human physiology, and human sexuality.
Dr. Rosenthal received her bachelor’s degree in biology from the University of Virginia, her master’s degree in neuropharmacology from Brown University, and her Ph.D. in neuroscience from UCLA. She began her career teaching in the College of Pharmacy at the University of Florida, and then moved to Fort Myers to be one of the founding faculty members of FGCU.
Dr. Rosenthal is the Director of the Cannabis Research, Education, and Workforce initiative (CREW) at FGCU, and runs the cannabis professional certificate program. She is the author of a number of textbooks, including Drugs: Mind, Body, and Society.
Dr. Rosenthal has been honored to receive the Teacher of the Year award at both the University of Florida and at FGCU and to have presented a TED talk about sex and gender.

46 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
APPENDIX B
LEADERSHIP AND STAFF OF THE CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
ALMUT G. WINTERSTEIN, R.PH., PH.D., FISPE Professor & Chair, Pharmaceutical Outcomes & Policy; Dr. Robert and Barbara Crisafi Chair for Pharmaceutical Outcomes & Policy; College of Pharmacy, University of Florida
CONSORTIUM DIRECTOR
Almut Winterstein, R.Ph., Ph.D., FISPE received her pharmacy degree from Friedrich Wilhelm University in Bonn, Germany and her Ph.D. in Pharmacoepidemiology from the Charité Humboldt University in Berlin, Germany.
She holds the position of Professor and Chair in the Department of Pharmaceutical Outcomes and Policy at the College of Pharmacy, and an affiliate appointment in the Department of Epidemiology at the Colleges of Medicine and Public Health and Health Professions, both at the University of Florida.
In 2017, she was named the Dr. Robert and Barbara Crisafi Chair in recognition of her research on evaluating drug safety and effectiveness in real-world populations and on devising ways to improve medication use.
Since joining the UF College of Pharmacy in 2000, Dr. Winterstein has served as principal investigator on more than 25 extramurally funded grants and contracts and published more than 300 manuscripts and conference abstracts. Her research interests have centered on the post-marketing evaluation of drugs in pediatrics and perinatal care, infectious disease and psychiatry and the evaluation and improvement of quality surrounding medication use using real-world data.As an internationally recognized expert in drug safety, she has chaired the Food and Drug Administration’s Drug Safety and Risk Management Advisory Committee from 2012-2018. Recognizing her contributions in pharmacoepidemiology, Dr. Winterstein was inducted as a fellow of the International Society of Pharmacoepidemiology in 2013 and started her term as president-elect of the society in 2018.
Before she became department chair in 2016, Dr. Winterstein served as graduate program director in her department, which included responsibility for a M.S. program for the FDA. She has chaired a total of 21 Ph.D. committees and has served as member on several others in her department, and the Departments of Epidemiology, Biostatistics and Statistics.

2021 ANNUAL REPORT | 47
ROBERT L. COOK, MD, MPH Professor, Epidemiology, Medicine Director, Southern HIV & Alcohol Research Consortium (SHARC) College of Public Health & Health Professions, University of Florida
CONSORTIUM ASSOCIATE DIRECTOR
Robert L. Cook, MD, MPH is a Professor of Epidemiology at the University of Florida, with a joint appointment in the Division of General Internal Medicine.

Over the past 20 years, Dr. Cook’s research has focused on strategies to improve health outcomes related to HIV and sexually transmitted diseases. He is the Director of the Southern HIV Alcohol Research Consortium (SHARC), which supports collaborative research and training related to alcohol and HIV infection across the state of Florida.
Dr. Cook’s research is translational, ranging from basic science to implementation science, and he is currently the PI or MPI of 4 major NIH grants with over $10 million in total research support.
Most recently, Dr. Cook has begun to study the effects of marijuana on HIV-related health and cognition, the systemic connections between the gut microbiome and neuroinflammation, the use of clinical information systems to improve quality of clinical pain management, and the use of real-time monitoring to measure alcohol consumption.
Mentoring is also an important aspect of Dr. Cook’s academic career. He has served as PhD dissertation chair for 8 students, PhD committee member for over 30 students, and mentor for numerous additional trainees, post-docs and junior faculty. When not working, Dr. Cook spends time with his family, plays in a rock-and-roll band, and tries to improve his tennis skills.
AMIE J. GOODIN, PHD, MPP
Assistant Professor, Pharmaceutical Outcomes & Policy; College of Pharmacy, University of Florida
CONSORTIUM LEAD, EVIDENCE
Amie J. Goodin, PhD, MPP is an Assistant Professor within the Department of Pharmaceutical Outcomes and Policy (POP) at the University of Florida.

Dr. Goodin received her Master of Public Policy degree from the University of Kentucky (UK) and completed her Doctor of Philosophy degree at UK’s Martin School of Public Policy, with specialization in pharmaceutical outcomes and an additional Certificate in Informatics. She completed a Postdoctoral Fellowship at University of Florida POP, specializing in pharmacoepidemiology methods while continuing her work in Health Services Research.
Dr. Goodin previously worked at the Institute for Pharmaceutical Outcomes and Policy as well as the Center for the Advancement of Pharmacy Practice, both of which were housed in the UK College of Pharmacy. Currently, Dr. Goodin’s research projects incorporate mixedmethod approaches to assess the impact of policy changes related to treatment access and utilization for Substance Use Disorders, particularly among persons enrolled in Medicaid and pregnant women.
48 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
JOSHUA D. BROWN, PHARMD, PHD, MS Assistant Professor, Pharmaceutical Outcomes & Policy; College of Pharmacy, University of Florida

CONSORTIUM LEAD, MEMORY
Joshua D. Brown, PharmD, PhD, MS joined the University of Florida in 2016. Dr. Brown has training in clinical pharmacy and pharmacoepidemiology having received PharmD and MS degrees from the University of Arkansas for Medical Sciences and a PhD from the University of Kentucky.
During his graduate training, Dr. Brown focused on research related to medication safety and effectiveness in high-risk populations, especially older adult and geriatric patient groups. He has also conducted research on medical devices, drug-drug interactions, and healthcare policy. As a graduate student, Dr. Brown was recognized as the Pfizer-Humana Research Fellow and received two Young Investigator Awards from the International Society for Thrombosis and Hemostasis.
Dr. Brown’s research program continues to evaluate the effectiveness and safety of medications used in real-world populations. He conducts research focusing on drug-drug interactions with hormonal contraceptives and anticoagulants and evaluates medication safety in older adult populations. His research has been funded by the U.S. Food and Drug Administration, the Bill & Melinda Gates Foundation, and the UF Institute on Aging. Dr. Brown has been recognized as a Claude D. Pepper Scholar in aging research, was invited to the National Academy of Medicine’s Emerging Leaders Symposium, and was awarded an early career recognition by the Academy of Managed Care Pharmacy.
YAN WANG, PHD
Assistant Professor, Epidemiology; College of Public Health & Health Professions, University of Florida
CONSORTIUM LEAD, CLINICAL CORE
Yan Wang, PhD is an Assistant Professor of Epidemiology at the University of Florida. Dr. Wang has training and expertise in both psychology and epidemiology. She received her MS and PhD in Child and Family Studies from Syracuse University in 2013. She joined the Department of Epidemiology as a postdoctoral research associate in 2014, working on NIH funded projects on risk behaviors among rural-to-urban migrants in China. In 2016, she was promoted to Research Assistant Scientist.
With an interdisciplinary perspective, her research focuses on leveraging advanced methodology and new technology (e.g., wearable sensor) to improve health behavior monitoring and intervention. One of her current research projects focuses on improving alcohol use monitoring using a wearable alcohol biosensor and ecological momentary assessment. She is also working on a UF funded pilot project to investigate the real-time and long-term health effects of medical marijuana among patients with chronic pain. Dr. Wang has also worked on a number of NIH funded projects including those on mental health and risk behaviors among rural-to-urban migrants in China, alcohol use and marijuana use among persons living with HIV/AIDS in Florida, and advanced quantum modeling on sexual risk behaviors.
One of her research papers, “Stress and Alcohol Use in Rural Chinese Residents: A Moderated Mediation Model Examining the Roles of Resilience and Negative Emotions” published in the journal Drug and Alcohol Dependence has been recognized by the Matilda White Riley Early Stage Investigator Honor Program, sponsored by the National Institutes of Health Office of Behavioral and Social Sciences Research (NIH/OBSSR).

2021 ANNUAL REPORT | 49
JEEVAN JYOT, PHD, PMP
College of Pharmacy, University of Florida
CONSORTIUM PROGRAM COORDINATOR
Dr. Jyot received her PhD in Microbiology and Molecular Biology from the Institute of Microbial Technology (India) and completed her postdoctoral fellowship and was an Assistant Scientist at Division of Infectious Diseases and Global Medicine, Department of Medicine, University of Florida. In addition she has Project Management Professional credentials.

Dr. Jyot has previously served as Research Program Coordinator at Division of Research Program Development (DRPD) at Office of Research at University of Florida. Currently, Dr. Jyot is part of the Department of Pharmaceutical Outcomes and Policy (POP) at the University of Florida and serves the Medical Marijuana Clinical Outcomes Research Consortium.
ANNA SHAVERS, MPA College of Public Health & Health Professions, University of Florida
CONSORTIUM COMMUNICATIONS SPECIALIST
Anna Shavers, MPA is the Communications Specialist of the Consortium for Medical Marijuana Clinical Outcomes Research.

Anna received her Master of Public Administration with a focus in Public Health Administration at Troy University. Her background includes various roles in marketing, communications, and health outreach initiatives.
Before joining the Consortium for Medical Marijuana Clinical Outcomes Research, Anna served with the Peace Corps as a Community HIV/AIDS Outreach Coordinator in South Africa.

50 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
APPENDIX C LIST OF REVIEWERS OF THE 2020 RESEARCH GRANTS PROGRAM
*reviewers who reviewed more than one proposal
Omayma Alshaarawy Michigan State University
Caroline Arout Columbia University Irving Medical Center
Assistant Professor
Assistant Professor
Mark Beasley University of Alabama School of Public Health Professor
Faye Belgrave Virginia Commonwealth University Professor
Tammy Chung Rutgers University Professor
Kathlene Curtis Oklahoma State University Professor
Natacha De Genna University of Pittsburgh
Nicholas DiPatrizio UC Riverside
Joseph Ditre* Syracuse University
Greg Dussor The University of Texas at Dallas
Sylvia Fitting University of North Carolina
David H.Gorski Wayne State University
Assistant Professor
Assistant Professor
Associate Professor
Associate Professor
Assistant Professor
Associate Professor
George Hasko* Columbia University Professor
Coleen Hegg Michigan State University
Associate Professor
Thomas Heinbockel* Howard University Professor
Sean Hennessy University of Pennsylvania Professor
Michael Hoane Augusta University Professor
Martin Kaczocha Stony Brook University
Assistant Professor
Brian Kelly Purdue University Professor
Santosh Kesari John Wayne Cancer Institute Professor
Kelly Koltyn University of Wisconsin Professor
Kate Lapane University of Massachaussetts Professor
Geoffroy Laumet Michigan State University
Assistant Professor
Michael Mason University of Tennessee Professor
Aimee Mcrae-Clark Medical University of South Carolina Professor
Bryan Miller Clemson University
Associate Professor
Tim Moran Johns Hopkins University Professor
Kelly Naugle Indiana University-Purdue University Indianapolis
Jiangnan Peng Morgan State University
Assistant Professor
Assistant Professor
Kristina Phillips Center for Integrated Health Care Research (CIHR) Research Investigator
Ana Pocivavsek University of Southern Carolina
Rajiv Radhakrishnan Yale University
Stephen Safe Texas A&M University
Peter Shaw University of Califronia San Diego
Assistant Professor
Assistant Professor
Distinguished Professor
Assistant Professor
Keshav Singh University of Alabama Professor
Alex Straiker Indiana University Bloomington
Kelly Young-Wolff Kaiser Permanente Division of Research
Senior Research Scientist
Research Scientist
2021 ANNUAL REPORT | 51
www.mmjoutcomes.org














































 Assistant Professor of Sociology (Medical); Florida Memorial University
Assistant Professor of Sociology (Medical); Florida Memorial University







