
ANNUAL REPORT | 2023-2024
February 2024
Prepared by the Consortium for Medical Marijuana Clinical Outcomes Research
For more information about the Consortium visit: www.mmjoutcomes.org
For questions or comments, contact:
Almut G. Winterstein, RPh, PhD, FISPE
Director, Consortium for Medical Marijuana Clinical Outcomes Research
Email: mmj.outcomes@cop.ufl.edu
Phone: 352-273-6984
TABLE OF CONTENTS EXECUTIVE SUMMARY 3 CONSORTIUM RESEARCH PROGRAM ..........................................................................3 Grants Program 4 MEMORY 4 Clinical Core 4 Evidence Core 5 Outreach ................................................................................................................... 6 CONSORTIUM RESEARCH PLAN 2024-2025 6 INTRODUCTION .............................................................................................................. 8 CONSORTIUM LEADERSHIP AND ADMINISTRATIVE STRUCTURE ............................. 10 BOARD MEETINGS 11 CONSORTIUM ACTIVITIES IN FY 2024 ......................................................................... 12 GRANTS PROGRAM 13 Summary of Findings of the Completed Research Studies of the 2019, 2020, 2021 And 2022 Grant Cycles 14 New Studies from the 2023 Grant Cycle .............................................................. 21 Highlights of Research Conducted by Consortium Grant Awardees 23 MEdical Marijuana clinical Outcomes RepositorY (MEMORY) 29 MEMORY Development – Progress Towards Milestones ................................... 30 Highlights of First MEMORY Analyses 30 CLINICAL CORE ........................................................................................................... 35 MMJ Contact Registry 35 Medical Marijuana & Me (M3) 36 CARMMA ................................................................................................................ 39 EVIDENCE CORE 39 Evidence in Context Series ................................................................................... 39 Evidence Synthesis for Food and Drug Administration (FDA) 40 Collaboration with Office of Medical Marijuana Use (OMMU) at Florida Department of Health (FL DoH): Consumer/Patient and Clinician Informational Resources ....................................................................... 41 Draft Patient and Consumer Informational Resources 42 OUTREACH .................................................................................................................. 43 Website 43 Newsletter 43 Researcher Spotlight Series ................................................................................. 44 Cannabis Clinical Outcomes Research Conference (CCORC) 44
SUMMARY OF CONSORTIUM RESEARCH PRODUCTIVITY.......................................... 45 BIBLIOGRAPHY – PEER-REVIEWED PUBLICATIONS 46 EXTRAMURAL GRANTS SUBMITTED BY CONSORTIUM FACULTY AND GRANT AWARDEES 51 MEDIA COVERAGE 52 PATENTS AND INDs .................................................................................................... 52 CONSORTIUM RESEARCH PLAN 2024-2025 53 GRANTS PROGRAM .................................................................................................... 53 MEMORY 55 CLINICAL CORE 55 EVIDENCE CORE ......................................................................................................... 55 OUTREACH 55 APPENDICES ................................................................................................................. 56 APPENDIX A: THE CONSORTIUM FOR MMJ CLINICAL OUTCOMES RESEARCH BOARD ..................................................................................................... 56 APPENDIX B: CORE FACULTY, STAFF, AND TRAINEES OF THE CONSORTIUM FOR MMJ CLINICAL OUTCOMES RESEARCH .......................... 60 APPENDIX C: REVIEWERS OF THE 2023 RESEARCH GRANTS PROGRAM 65 APPENDIX D: SUMMARY BROCHURE FOR THE CANNABIS CLINICAL OUTCOMES RESEARCH CONFERENCE (CCORC) 2023 66
EXECUTIVE SUMMARY
Since the passing of the Compassionate Use Act in 2014, Florida citizens have access to marijuana and marijuana products for the treatment of certain debilitating conditions. While medical marijuana (MMJ) may improve health outcomes, evidence to support its effectiveness for the treatment of most conditions is scarce and there are significant safety concerns related to cognitive effects, risk of accidents, interactions with other medications, psychosis, and addiction. Moreover, cannabis products vary in terms of components and modes of administration, but there is limited research to elude which plant types, doses and delivery methods provide the optimal risk-benefit. This substantial need for research stands in stark contrast to the available infrastructure to support such work and to the rapid uptake of MMJ. As of Jan 5, 2024, the number of licensed MMJ users in Florida is 867,072 as compared to merely 54,251 in Jan 5, 2018. The number of physicians licensed to treat their patients with MMJ has grown from 883 to 2,748 over the same period. By the end of 2023, Florida dispensaries supplied 332 kg Tetrahydrocannabinol (THC) in non-smokable MMJ weekly, and 604 kg THC in smokable MMJ, assuming a content of 20% THC in smokable MMJ products.
The following report details activities of the Consortium for Medical Marijuana Clinical Outcomes Research (Consortium) completed during the fifth year of its existence (July 2023 – Feb 1, 2024). The first four annual reports were submitted on February 15th each year, from 2020-2023, and are available at mmjoutcomes.org/our-consortium/annualreport/. An overview of Consortium activities was provided to the Healthcare Regulation Subcommittee of the Florida House of Representatives on Feb 15, 2023, and most recently on Dec 13, 2023, and is available for review on the Florida channel: myfloridahouse.gov/VideoPlayer. aspx?eventID=9190 (Dr. Winterstein’s presentation begins at 1:07:30). The presentation includes an overview of the Consortium’s research programs and updates on data regarding certified MMJ patient demographics, average THC dosing, and the most common reported health conditions cited for MMJ use.
CONSORTIUM RESEARCH PROGRAM
The Consortium research program rests on five pillars aimed at supporting the Consortium mission to foster MMJ clinical outcomes research including: A Grants Program, a unique research data repository known as the MEdical Marijuana clinical Outcomes RepositorY (MEMORY), a Clinical Core, an Evidence Core and an Outreach Program. Following is a brief overview of the purpose and accomplishments for each pillar.
2024 ANNUAL REPORT | 3
Grants Program
Launched in September 2019, the Consortium grants program has since completed five award cycles and initiated its sixth cycle with releasing its Request for Proposals (RFP). During the first five grant cycles, 115 research proposals were received and >$3M were awarded to 46 researchers from 7 Consortium member institutions. Research outcomes and health conditions addressed in these proposals include neuropathy and pain of various origins, anxiety, cancer, PTSD, assessment of drug interactions, pattern and motivation for MMJ use, access to MMJ, adverse drug events, improving opioid use disorder, a safety & efficacy trial for anxiety, and cannabis smoke effects on human lung enzymes.
Over these years the grant awardees and Consortium researchers have generated important evidence, resulting in 57 peer-reviewed publications, 1 book chapter and 2 filed patents, 2 IND approvals from FDA, and 34 new extramural grant applications, 10 of which have been awarded. In addition, UF Core Faculty has secured federal funding for cannabis research addressing cannabis outcomes from the National Institutes of Health (NIH) and the Food and Drug Administration (FDA). Noteworthy, 93 trainees including 22 from under-represented minorities have been involved in the funded research grants and two new courses have been developed, one of which is approved by the state university system, supporting the development of research capacity in cannabis outcomes in the state.
MEMORY
The growing use of MMJ in Florida offers a unique opportunity for real-world evaluations of cannabis clinical outcomes that can help overcome the lack of randomized clinical trials and greatly accelerate the availability of evidence on clinical outcomes. MEMORY has been conceived to establish the infrastructure for real-world MMJ clinical outcomes evaluations similar to those employed by the FDA to evaluate and monitor the risk-benefit of approved prescription medications. To ensure comprehensive longitudinal follow-up and capture of relevant health outcomes, the Consortium is linking the Office of Medical Marijuana Use (OMMU) Medical Marijuana Use Registry (MMUR) with other clinical databases commonly used for outcomes research. The Consortium plans to make a de-identified version of the repository accessible to Consortium researchers, thus providing state-wide infrastructure to assess the benefit-risk of MMJ.
In the past years, the Consortium has obtained IRB approval and established Data User Agreements (DUAs) with the Agency for Healthcare Administration (AHCA), the Centers for Medicaid and Medicare Services (CMS) and the Florida Department of Health (FL DoH) Vital Statistics Office. The DUA with the Department of Health OMMU to access the MMUR was signed early 2023. The MEMORY data science team has completed development of the data architecture and has received the data for the years 2016 through 2023 to facilitate the development of MEMORY. Initial analyses of MMJ usage pattern gleaned from OMMU data are included in this report.
Clinical Core
The Clinical Core was established to complement MEMORY and provide infrastructure support for prospective studies including randomized controlled trials. To facilitate such studies, an MMJ patient contact registry to support recruitment has been rolled out statewide, with a registry portal on the consortium website (mmjoutcomes.org/getinvolved/registry/). As of Jan 12th, 2024, over 1,200 individuals who are using or are planning to use MMJ have registered. In addition, clinical and industry partners interested in collaborations are registered in the online Connect and Advance Research for Medical Marijuana Analysis (CARMMA) database (mmjoutcomes.org/collaborate/carmma/).
4 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
In May 2022, the Consortium launched MMJ and Me (M3), its first large prospective MMJ patient cohort in Florida, and one of the first in the United States. M3 design has been guided by a scientific planning committee consisting of 11 members, including six researchers from consortium member universities, four MMJ physicians, and one MMJ patient representative. Enrollees complete a sequence of surveys about their general health, use, and experiences with MMJ and related health outcomes. Recruitment efforts for M3 ended in May 2023 and follow-ups are estimated to be completed in early spring 2024. As of Jan 12th, 2024, 1,230 patients have been enrolled, and data curation and analysis has commenced for the “current MMJ users” cohort.

BIG THEMES OF THE CONSORTIUM IN 2023
Evidence Core
The evidence core focuses on the synthesis and dissemination of scientific evidence regarding MMJ clinical outcomes for researchers, providers, and community members. Evidence core activities include publication of its “Evidence in Context” series, provision of scientific expertise, and evidence reviews as tailored to scientific, clinical and patient communities.
The Evidence in Context series addresses need for rapid distillation and appraisal of emerging evidence in the form of critical evaluation on new studies or developments in cannabis clinical outcomes research and how this research translates to patient care. This series is available as open access and available to the public within the scientific journal Medical Cannabis and Cannabinoids and on the Consortium website at mmjoutcomes. org/evidence/evidence-in-context/
Several Consortium members, led by Principal Investigator Amie Goodin, contracted with the United States Food and Drug Administration (FDA) in 2023 to support their review of evidence informing the classification status of cannabis as a Schedule I substance in the United States. The FDA’s recommendation, to revise the classification status of cannabis to Schedule III, was published as a letter from the Department of Health and Human Services in January 2024. Consortium researchers participating in this project examined a series of harms outcomes described in studies that examined cannabis use for medical, non-medical, and unknown purposes. Additionally, Consortium researchers examined the effectiveness and safety of cannabis when used as therapeutic intervention for several indications.
2024 ANNUAL REPORT | 5
The Consortium is also collaborating with the Office of Medical Marijuana Use (OMMU) at FL DoH to help disseminate consumer/patient and clinician informational resources that focus on evidence for safety and effectiveness, as well as risks of using MMJ for conditions currently approved for MMJ use in Florida.
Outreach
The Consortium’s outreach activities are directed toward healthcare providers, researchers, patients and industry, to maximize participation in research, and engage all stakeholders. The Consortium continues to enhance its outreach activities via its websites and quarterly newsletter, and plans on expanding its researcher spotlight video series.
The first Cannabis Clinical Outcomes Research Conference (CCORC) was hosted virtually on April 8th and 9th, 2021, and drew over 225 registrants from across 31 U.S. states and five countries. The next two annual CCORC conferences were delivered in a hybrid format (with both in person and virtual options) to promote inclusivity and broad outreach, in May of ’22 & ’23, at the UF Research and Academic Center at Lake Nona, Orlando, FL. These conferences attracted more than 120 attendees each. The CCORC program highlights included keynote addresses by national-level experts in cannabis research, panel discussions, workshops, poster sessions for scientific abstracts selected following peer review, and oral presentations of top scientific abstracts.
The fourth annual CCORC is planned for May 30th–31st, 2024, in Orlando, Florida.
CONSORTIUM RESEARCH PLAN 2024-2025
In the coming year, the Consortium will continue its efforts towards facilitating and conducting research that informs clinical care and policy within the five original Consortium research program pillars: the Grants program, MEMORY, the Clinical Core, the Evidence Core, and Outreach activities.
The Grants program will continue with the Consortium Board approved research priorities to emphasize the focus on clinical research, the impact of MMJ on pain management and opioid use, and an expanded scope on epidemiologic research. MEMORY development will continue at an accelerated pace with MMUR data curation, linkages of databases and development of analytical cohorts, to support studies on MMJ effectiveness and safety, utilization and access. Along with final data curation, access to researchers and publication of initial analyses envisaged for M3, the Clinical Core will continue to support patient recruitment into prospective research studies via its patient contact registry and CARMMA database and guide investigators on regulatory issues involving use of MMJ in research studies. The Evidence Core will complete its evidence review for the FDA and begin publication and dissemination of related findings, as well as continue to publish its Evidence in context series, and survey and evaluate emerging evidence to inform Consortium research priorities. In addition to holding its fourth annual conference CCORC, the Outreach Core will disseminate emerging evidence via the Consortium website, its quarterly newsletter, the Researcher Spotlight Series and participation in scientific conferences.
6 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH

The Consortium board, core faculty and staff look forward to continuing this critical work to support the Florida MMJ program’s primary goal to improve the health of Florida citizens. The Consortium addresses an urgent and critical need in this regard in providing patients, providers and regulators the necessary evidence on the safe and effective use of MMJ.

2024 ANNUAL REPORT | 7
INTRODUCTION
Beginning with the Compassionate Use Act passed in 2014 and followed by several amendments, Florida law allows the use of marijuana for the treatment of certain debilitating conditions. While there is some promise that MMJ may improve certain health outcomes, evidence in support of its effectiveness for treatment of most conditions is incomplete and there are certain safety concerns related to cognitive effects, risk of accidents, interactions with other medications, psychosis, or addiction. Moreover, marijuana products vary in terms of specific components and modes of administration, but little is known about what components, doses and delivery methods provide the optimal riskbenefit. There is a substantial and urgent need to understand how MMJ impacts health to guide both policy and clinical decision-making. However, due to the complex legal restrictions, the research infrastructure to support MMJ evaluations is lagging far behind the rapid uptake of MMJ in the state.
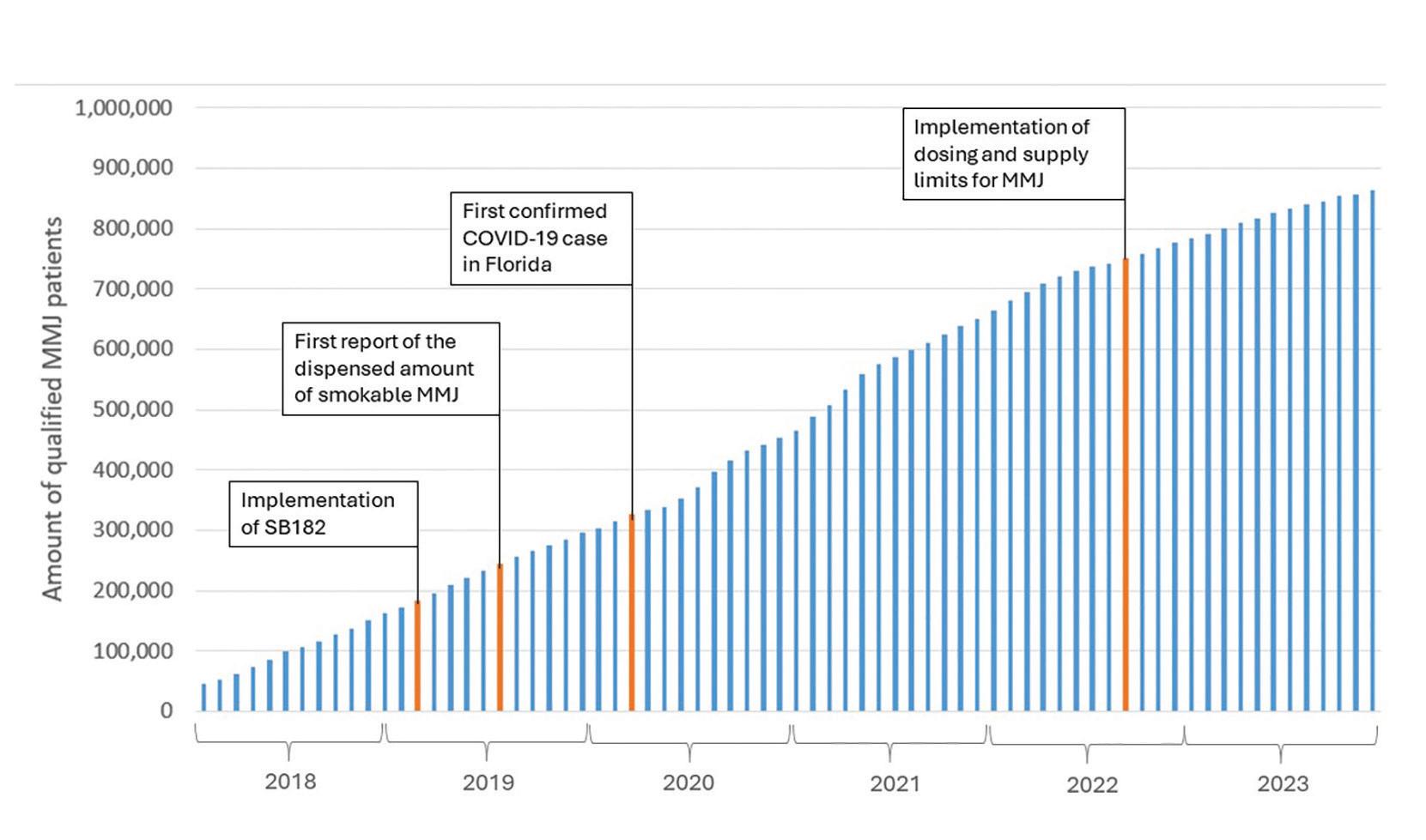
The number of Floridians licensed to use MMJ has continued to increase, with a total of 867,072 (as of Jan 5, 2024) licensed users as compared to 54,251 in Jan 5, 2018.
8 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Growing trend in the number of certified MMJ patients in Florida, 2018 - 2023
To address the need for rigorous evidence on the safety and effectiveness of MMJ for the various patient populations who are seeking certification for use, the state legislature introduced Section 1004.4351, Florida Statutes, to establish the Consortium of Medical Marijuana Clinical Outcomes Research. The statutory-defined mission of the Consortium is to conduct, disseminate and support rigorous scientific research on the clinical outcomes of MMJ use. In July 2019, the Florida State University System Board of Governors, following a competitive request for proposals, designated the University of Florida as the lead university of the Consortium. Eight additional universities have joined the Consortium to date.

Consortium member universities
Consortium responsibilities as defined in its charter and consistent with statute include:
• Conduct rigorous scientific research
• Disseminate research
• Guide statewide policy on ordering and dosing practices for the medical use of marijuana.
The Consortium of MMJ Clinical Outcomes Research presents its fifth Annual Report on its accomplishments in research and outreach and its Research Plan for 2024-2025.
2024 ANNUAL REPORT | 9
CONSORTIUM LEADERSHIP AND ADMINISTRATIVE STRUCTURE
The Consortium of Medical Marijuana Clinical Outcomes Research is open to all public and private universities in Florida. The Consortium is directed by the MMJ Research Board, which is composed of representatives from each participating university. Board members represent a variety of scientific and medical fields as required per statute. Profiles of the Consortium Board members are available in Appendix A.
CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH BOARD BOARD MEMBER INSTITUTION
William Anderson, PhD (Chair) Florida International University
Martha S. Rosenthal, PhD (Vice Chair) Florida Gulf Coast University
Peter Holland, MD Florida Atlantic University
Christopher McCurdy, PhD University of Florida
Jacqueline Sagen, PhD University of Miami
Dinender Singla, PhD University of Central Florida
Eric H. Holmes, PhD Florida State University
Charles Weatherford. PhD Florida A&M University
Max. C. E. Orezzoli, PhD Florida Memorial University
Pursuant to statute, the board appointed Almut G. Winterstein, RPh, PhD, FISPE, Distinguished Professor in Pharmaceutical Outcomes and Policy and Director of the Center for Drug Evaluations and Safety (CoDES) at UF as its director. She is supported by Dr. Robert Cook, MD, MPH, professor of Epidemiology and Internal Medicine and director of the Southern HIV and Alcohol Research Consortium (SHARC) Center for Translational HIV Research. They are supported by Assistant Directors for MEMORY, the Clinical Research Core, and the Evidence Core. Program staff include an Assistant Director for Research Administration, who manages the Consortium operations, a communication expert who leads outreach activities, a research coordinator who assists with participant recruitment, study operations, database management, and teams of analysts and research assistants that support the cores. Taking advantage of its academic base, Consortium leadership involves several trainees in Consortium activities. Profiles of the Consortium leadership and staff are available in the Appendix.
10 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Consortium Administrative Structure
BOARD MEETINGS
Since its inception, the board has met 17 times between August 2019 and Feb 1, 2024. Key accomplishments and decisions at the board meetings in the previous year are summarized below and complete meeting minutes are available on the Consortium website at mmjoutcomes.org/our-consortium/board-meetings/
MAY 19, 2023
• OMMU communications manager discussed strengthening collaboration with the Consortium.
• Dr. Winterstein provided an activities update and highlighted the ongoing Consortium and FDA collaboration to synthesize evidence on clinical risk benefit in support of an ongoing review of marijuana’s current Schedule I classification status.
• Dr. Amie Goodin provided a post-hoc overview of CCORC 2023, and the board offered their congratulations on its success and agreed to continuation of this outreach activity.
JUNE 29, 2023
• The board was informed about the continued funding by the State.
• The board finalized selection of research proposals to be awarded for the 2023 grants funding cycle.
• Dr. Roger Fillingim was recognized for his service to the board at the end of his 4-year term.
OCTOBER 9, 2023
• Dr. Anderson and Dr. Rosenthal agreed to continue as the Board Chair and Vice-Chair respectively, for one more year.
• Dr. Holmes and Dr. Orezzoli will also continue as board members for another term.
• Dr. Christopher McCurdy from UF was introduced as the new board member replacing Dr. Roger Fillingim.
• The board approved the FY24 budget including support for M3, and approved the plans for the fourth conference of the consortium to be held in May 2024 in Orlando.

2024 ANNUAL REPORT | 11
CONSORTIUM ACTIVITIES IN FY 2024
Central to the Consortium is its mission to foster clinical outcomes research on MMJ across the state. Five pillars constitute the Consortium Research Program: A Grants Program, the MEdical Marijuana clinical Outcomes RepositorY (MEMORY), a Clinical Core, an Evidence Core and Outreach. Consistent with its charter, the Consortium has engaged scientists with relevant research programs to participate in the Consortium and foster research collaborations to accelerate the development of evidence on MMJ clinical outcomes. The following sections provide a description of each of the Consortium functions and a detailed progress report.
12 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Consortium Structure and Functions
GRANTS PROGRAM
Each year, the Consortium offers a Research Grants Program, open to all members of the Consortium and teaching nursing homes as stipulated by statute. The research focus of the grants program is prioritized based on statutory guidance and the annual Consortium research plan to ensure optimal fund utilization. Applications are reviewed by external reviewers, recruited from out-of-state, using NIH review criteria. Final grant awards are made by the MMJ Research Board based on study quality, impact and relevance to the Consortium research priorities. Calls for proposals are disseminated by each board member within their university and through the Consortium website, newsletter, and listservs. We have completed the 2019, 2020, 2021 and 2022 grants program cycles, are mid-way through the 2023 grants program cycle and have launched the 2024 grants cycle.
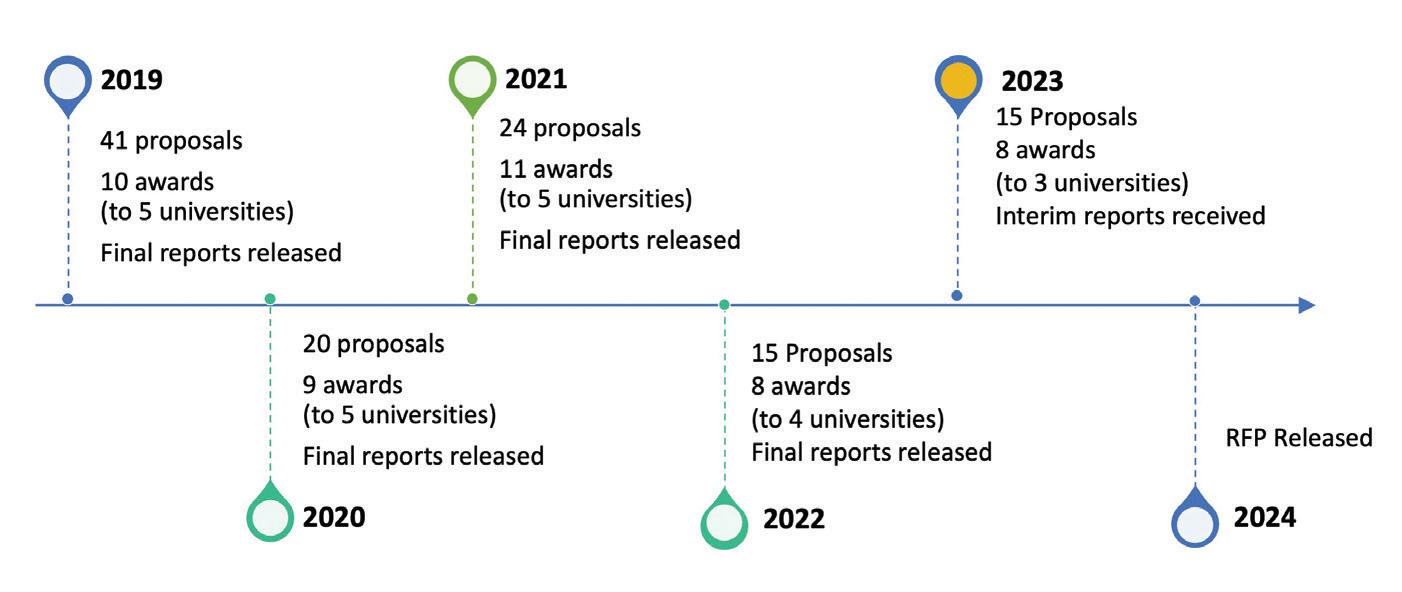
Between 2019 and 2023, the Consortium Grants Program has received 115 research proposals and after review, awarded 46 of these to researchers of 7 of its member institutions. These awards, totaling more than $3 million, cover a broad range of focus areas consistent with Consortium research priorities.

2024 ANNUAL REPORT | 13
Summary of Findings of the Completed Research Studies of the 2019, 2020, 2021 and 2022 Grant Cycles
2019 GRANT AWARDEES
PRINCIPAL INVESTIGATOR & STUDY TITLE
Paul Borsa, PhD
University of Florida
Efficacy of a controlled short-term trial of CBD ingestion on reducing symptomatic response and facilitating recovery after induced muscle injury
Helen Bramlett, PhD
University of Miami
Therapeutic dosing of a cannabinoid (CBD) after mild and moderate brain injury for translation to the clinic
Joshua Brown, PharmD, MS, PhD
University of Florida
Characterizing community and physician-level factors associated with medical marijuana prescriber registration and patient access
Andrea Cippitelli, PhD
Florida Atlantic University
Cannabidiol: A potential treatment for migraine- like pain, negative emotion and Photophobia
Gregory Manus, PhD
Florida Golf Coast University
Rapid identification and quantification of heavy metals and microplastics in CBD oil
Mandip Singh Sachdeva, PhD
Florida Agricultural & Mechanical University
Hyaluronic acid functionalized, Cannabidiol-loaded Mesenchymal Stem Cells (MSC)-Derived Exosomes for Drug Resistant Cancers
SUMMARY OF RESEARCH OUTCOMES PROVIDED BY GRANTEES*
After securing an IND* for their investigational product from FDA, final data collection is ongoing to answer if CBD is effective in reducing symptomatic pain and anxiety and facilitating functional recovery in muscle injury and if therapeutic effects are dose-dependent.
Research indicates that oral consumption of CBD may have reduced inflammation, protected vulnerable brain regions, and reversed certain memory and sensorimotor deficits that are observed after brain injury
This project created a dynamic data visualization tool and linkable database to cross-reference cannabis-licensed physician practices, cannabis dispensary locations, and community-level and physician-level indicators of access and health.
Published findings* reveal that CBD, injected after calcitonin gene-related peptide (CGRP), reversed CGRP-evoked allodynia. CBD also reduced spontaneous pain traits induced by CGRP administration in female mice. CBD blocked CGRP-induced anxiety in male mice, but failed in providing protection from CGRP-induced photophobia in females. Findings demonstrate efficacy of CBD in preventing episodic and chronic migrainelike states. CBD also shows potential as an abortive agent for treating migraine attacks and headache-related conditions such as spontaneous pain and anxiety.
Twenty-five different CBD oil samples from 15 different vendors across the country were examined for presence of heavy metals. Analysis revealed absence of heavy metals in the samples within instrument detection limits.
Published findings* indicate that CBD and THCV appear to counteract histone modifications and their subsequent effects on doxorubicin, resulting in chemo-sensitization against MDAMB-231 resistant cancers.
14 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Jacqueline Sagen, MBA, PhD
University of Miami
Evaluation of medical marijuana for the treatment of chronic spinal cord injury pain using a rat central neuropathic pain model
Krishna Vaddiparti, MSW, MPE, PhD
University of Florida
A feasibility study of real-time monitoring of posttraumatic stress disorder related sleep disturbances and other symptoms among patients on medical marijuana
Jenny L Wilkerson, PhD
University of Florida
Marijuana-derived terpenes for the treatment of chemotherapyinduced pain
Ali M Yurasek, PhD
University of Florida
The Relationship between State Medical Marijuana Laws, Substance Use and Mental Health Disorder Diagnoses, and Associated Health Care Costs
PRINCIPAL INVESTIGATOR & STUDY TITLE
Hassan Azari, PhD
University of Florida
Hemp derived extracellular vesicles (EVs) for the treatment of glioblastoma
Lisa Eckel, PhD
Florida State University
Cannabinoid medication for treatment of a pre-clinical model of anorexia nervosa
Published findings* suggest that CBD and β-caryophyllene (BCP) co-administration may provide a safe and effective treatment option for the management of chronic SCI pain.
Debra Fadool, PhD
Florida State University
Mechanisms of Action for Cannabidiol in a Mouse Model of Anxiety
Published results* show improvements in PTSD symptom severity and sleep disturbances among MMJ users, in the context of a small, single-arm pilot study of 15 participants assessed at baseline, 30- and 70-days post MMJ start.
This research revealed that each of the examined terpenes reversed pain-related behavior in paclitaxel-treated mice. Cannabis-based terpenes possess a pharmacological profile that may yield new efficacious analgesics.
Preliminary analyses from a health claims data set indicate that states with MMJ laws by 2012 had higher proportions of individuals with disorders related to opioid use, cannabis use, alcohol use, trauma, depression, and psychosis, and significantly higher healthcare costs across the disorders examined in these states. Similar result patterns were observed for data collected in 2018, in which 34 states had passed the MMJ laws.
2020 GRANT AWARDEES
SUMMARY OF RESEARCH OUTCOMES
PROVIDED BY GRANTEES*
Patent filed* research showed that naturally available hempEVs can effectively hamper tumor cell proliferation and invasion in tissue culture. Importantly, hemp EVs could be effectively delivered via the intranasal route into the brain tumor bed. Hemp EV therapy significantly reduced tumor growth and increased overall survival in tumor bearing animals.
Research revealed that female rats receiving THC lost less body weight and displayed increased survivability in the activity-based anorexia (ABA) paradigm, relative to vehicletreated rats. Rather than stimulating appetite, THC decreased locomotor activity and metabolic energy expenditure, resulting in attenuated weight loss in rats. These studies offer a translational model for the development of new cannabinoidbased pharmacotherapies, for AN.
Data generated from these studies helped obtain extramural Grants funding*. Data demonstrates that acutely administered CBD decreases obsessive compulsive-like behaviors in male mice but has no effect in female mice. Whereas wildtype female mice are responsive to a low dose of 10 mg/kg CBD and unresponsive to a high dose (20 mg/kg), males require a higher dose to significantly reduce anxiety-like behaviors. Interestingly, in the Kv1.3-/- mice that are naturally more anxious, acutely administered CBD was anxiogenic (anxiety generating or producing).
2024 ANNUAL REPORT | 15
Jason Ford, PhD
University of Central Florida
Patterns, Motives, and Risks Associated with Marijuana Use: A Comparison of Medical Marijuana Patients and Non-Patient Marijuana Users in Florida
John Markowitz, PhD
University of Florida
An Assessment of the Drug Interaction Potential Between Oral Cannabidiol (Epidiolex®) and the CES1 Substrate Methylphenidate in Healthy Volunteers
David Newman, PhD
Florida Atlantic University
Assessing and Supporting Effective and Safe Use of Medical Marijuana for Older Adults with Chronic Pain
Mandip Sachdeva, PhD
Florida Agricultural & Mechanical University
Preclinical evaluation of exosomal cannabinoid formulations in chemotherapy induced peripheral Neuropathy
Douglas Storace, PhD
Florida State University
The influence of cannabinoid receptors on olfactory function
The Florida Young Adult Cannabis Study (FYACS) a study of young adult cannabis users aged 18 to 34, in Florida, revealed detail on several health-related conditions compared to nonpatient users. Patient users were more likely to be diagnosed with PTSD and report avoiding situations, having dreams, or feeling guilty related to PTSD. Patient users were also more likely to test positive and be hospitalized for COVID-19, as well as having access to medical care or medications being limited to the COVID-19 pandemic. However, non-patient users reported that the COVID-19 pandemic had a worse impact on their mental health (e.g., anxiety, depression, loneliness) than patient users.
This published* open-label, placebo-controlled, crossover study in 12 healthy subjects assessed the influence of CBD (administered as Epidiolex®) on the disposition of the known CES1 substrate methylphenidate (MPH; Ritalin®). An increase was noted in area under the plasma concentration curve (AUCtotal) of MPH in 8 of the 12 subjects. However, when the mean MPH values for all 12 subjects were examined with and without CBD concurrent use, the two conditions were approximately bioequivalent i.e., suggesting a significant interaction did not occur.
This explanatory sequential mixed method study recruited 131 MMJ participants. Findings indicate some side effects, the largest being increased appetite (22.3%), followed by lethargy (14.0%), elevated mood changes (12.4%), lack of concentration (11.6%) and dizziness (9.1%). After MMJ use, pain decreased an average of 52.7%.
Published results* report the effects of CBD exosomes in PTX induced neuropathic mice. CBD and CBD-exosome treatments significantly displayed improvement in neurobehavior of paclitaxel induced neuropathic animals. Thermal and mechanical sensitivity was considerably reduced after 6 weeks in PTX induced neuropathic animals when compared to control animals.
Studies revealed that the infusion of WIN 55212 (CB1 receptor agonist) directly to the olfactory bulb caused a decrease in sensitivity, while an intraperitoneal injection caused no change in sensitivity. The infusion of a CB1 receptor antagonist directly into the olfactory bulb had no effect on sensitivity. These results challenge the current theory regarding the effect of cannabinoids on olfactory perception in mice and highlights some potential similarities with humans.
Roberto Vincis, PhD
Florida State University
Endocannabinoid mechanism in the neural processing of foodpredicting sensory cues
The researchers have completed proof-of-concept experiments to investigate the role of cannabinoid modulation of the mouse Insular Cortex (IC), a cortical area known to process motivationally salient stimuli associated with food reward cues and driving food-oriented behaviors.
16 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
PRINCIPAL INVESTIGATOR & STUDY TITLE
Jennifer Attonito, PhD Florida Atlantic University
Acceptance of and access to medical marijuana and CBD as a palliative care and hospice treatments for nursing home patients
Joshua Brown, PharmD, PhD, MS
University of Florida
Characterizing adverse drug events reports involving cannabis and cannabinoids
2021 GRANT AWARDEES
SUMMARY OF RESEARCH OUTCOMES PROVIDED BY GRANTEES*
Researchers surveyed 126 clinicians and interviewed 25 patients in nursing homes and assisted living facilities. Respondents expressed major barriers to utilization existed, such as lack of education/training and clinical guidelines. Some participants considered MMJ and recreational MJ use to be similar. Patients interviewed reported that they have not been offered MMJ by their providers and are unfamiliar with the procedures for obtaining a MMJ card.
Upon evaluation of over 15,000 adverse event reports from FAERS (FDA Adverse Event Reporting System) including cannabis or cannabinoids used medically/recreationally, over 500 adverse drug events (“reactions”, ADEs) were represented among 200 different product names. Prevalent reactions were neuropsychiatric (19%), cardiovascular (14%), infections (12%), and sedation (5%). The most common medications that were concomitantly used by patients with reactions included gabapentin (19%), pantoprazole (19%), lorazepam (18%), amitriptyline (13%), and duloxetine (13%). Opioids were also commonly included as co-medications (e.g., fentanyl (6%), morphine (7%), and tramadol (12%)).
Jenny Wilkerson, PhD and Joshua Brown, PharmD, MS, PhD
University of Florida
Translational examination of the pharmacological interactions of medical marijuana with neuropathic pain analgesics in both young and older adults
Andrea Cippitelli, PhD Florida Atlantic University
Investigating cannabidiol antiheadache actions through PPAR signaling
Lisa Eckel, PhD
Florida State University
Cannabinoid modulation of neuroinflammation in a pre-clinical animal model of anorexia nervosa
Results from these studies show that in both young and aged female mice, when compared to their age matched male cohort, THC, morphine, and gabapentin have small but significant increased potency to attenuate chemotherapy induced peripheral neuropathy (CIPN)-related behaviors. Likewise, in younger mice of both sexes’ THC, morphine, and gabapentin have increased potency to attenuate CIPN-related behaviors. Confirming the original hypothesis that age and sex both likely play important roles in the use of MMJ and other analgesics for pain.
The effects of the pharmacological blockade of PPAR-γ with GW9662 (PPAR antagonist), in reversing the anti-allodynic effects of CBD were tested in male and female mice. The antimigraine effects of CBD, in particular its anti-allodynic effects, are likely not mediated by the activation of the isoform γ. Further studies are needed to validate early findings on a new role for PPARγ on migraine and other headache disorders.
Research revealed that THC treatment slowed the weight loss associated with activity-based anorexia, in a pre-clinical animal model of anorexia, and this was mediated primarily by a decrease in exercise, rather than an improvement in appetite. Further rats with activity-based anorexia develop inflammation in brain areas that control food intake and regulate body weight, and THC can attenuate this neuroinflammatory response. This pre-clinical study offers a translational model for the development of new cannabinoid-based pharmacotherapies, including the use of MMJ.
2024 ANNUAL REPORT | 17
Mariola Edelmann, PhD
University of Florida
The role of endocannabinoids and cannabinoids in the clearance of bacterial infections and macrophage polarization
Debra Fadool, PhD
Florida State University
Early Developmental Mechanisms of Action for Cannabidiol (CBD) in a Mouse Model of Anxiety
Upon testing multiple cannabinoid (CB) compounds in murine bone marrow-derived macrophages, for ability to suppress TNF-alpha, upon infection with Salmonella typhimurium, where THC-mimicking WIN 55,212-2 completely reduced proinflammatory cytokines during Salmonella infection. To validate the data, we infected RAW264.7 macrophages with S. typhimurium, followed by exposure to several concentrations of WIN55,212-2, and this CB completely blocked proinflammatory TNF-alpha responses. We evaluated the effect of CBs on bacterial clearance, where WIN55,212-2 increased bacterial clearance. However, there was no direct suppression of Salmonella’s growth by WIN 55,212-2. These results suggest that CBs decreases inflammation and promote pro-phagocytic functions during Salmonella infection.
Using a newly found mouse model having trait anxiety and attention deficits (Kv1.3-/- mice) researchers observed that when the offspring were raised to adults, CBD exposure in utero turned out to be anxiolytic in females unlike in males. However, both sexes in mice exhibit increased obsessive compulsive-like (OCD) behaviors, and this behavior was not lessoned by cross-fostering in the males. While ADHD behaviors were not affected by in utero CBD exposure, female mice had a reduction in long-term object memory. Developmental changes in the brain were also observed in response to CBD exposure. Of note, fetal exposure to CBD significantly decreased survival of the pups.
Simone Marini, PhD
University of Florida
CBD-induced biomarkers of inflammation reduction in people living with HIV at the single cell level
Mandip Sachdeva, PhD Florida Agricultural & Mechanical University
Evaluation of Minor Cannabinoids loaded Exosomes in Chronic Diabetic Neuropathy
Jacqueline Sagen, PhD, MBA
University of Miami
Alleviation of phantom limb pain in a rat model by treatment with components of Cannabis
Ellen Zimmermann, MD
University of Florida
The Effect of Delta-9tetrahydrocannabinol (THC) on Intestinal Inflammation and Fibrosis in Experimental Crohn’s disease
Published results* suggest that a group of genes is under-expressed after CBD treatment in myeloid cells, including monocytes and granulocytes. Both monocytes and granulocytes play a pivotal role in the inflammatory process, and the great majority of genes emerging from the analysis play a well-known pro-inflammatory role. As chronic inflammation is a condition common to a plethora of different diseases, this study is a first step towards the understanding of how CBD may be used for anti-inflammatory therapeutic strategies for PLWH.
Results demonstrate the role of phytocannabinoids, CBD, Tetrahydrocannabivarin (THCV), and cannabigerol (CBG) in alleviating diabetic induced neuropathic pain via various antiinflammatory and mitochondrial pathways.
Research reveals that a low dose of THC together with a synergistic combination of CBD, and terpene β-caryophyllene (BCP), can significantly block the onset of phantom limb pain symptoms. This combination can also significantly attenuate further progression of phantom limb pain severity once it has begun.
Using a well-established animal model of inflammatory bowel disease (IBD) to test the effectiveness of THC-treatment, it was observed that THC treatment had mixed efficacy with little to no effect on hematocrit and colon length, but altered gene expression consistent with previous data in human cells. Surprisingly, THC was found to have a suppressive effect on normal weight gain in healthy mice, and exacerbated colitisassociated weight loss in treated mice. This surprising result raises new questions and potentially new research paths.
18 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
PRINCIPAL INVESTIGATOR & STUDY TITLE
Naueen Chaudhry, MD
University of Florida
Cannabinoid use in young adults with Crohn’s Disease
Rodrigo Cristofoletti, PhD
University of Florida
Quantitative assessment of complex drug-drug interaction networks involving medical cannabis products in special populations
2022 GRANT AWARDEES
SUMMARY OF RESEARCH OUTCOMES PROVIDED BY GRANTEES*
Using a mixed methods approach, researchers found distinct differences in patterns of MJ use of college-aged students, varying by age, gender, and duration of disease diagnosis.
A forward stepwise, model-based approach recommended by the FDA guidance was used to assess the magnitude of CYP-mediated DDIs precipitated by CBD and Δ9-THC. The basic model showed a DDI potential of CBD with all major CYP enzymes, whereas, for Δ9-THC, DDI risk was detected with CYPs 1A1, 1A2, and 2C9. Metabolic pathways sensitive to DDI by cannabinoids were further investigated using the static mechanistic model, which showed that CBD has a high potential to precipitate severe to moderate DDIs on major CYPmediated metabolic pathways, including CYP3A and CYP2C19. On the contrary, Δ9-THC showed a moderate risk for DDIs. These CBD-mediated DDIs will be translated from in vitro to in vivo scenarios using physiological-based pharmacokinetic (PBPK) modeling. Independent PBPK models are currently being developed.
Robert Dvorak, PhD
University of Central Florida
Event-level changes in psychiatric and physical symptoms following medicinal cannabis use in older adults
Debra Fadool, PhD
Florida State University
Long-term molecular, metabolic and behavioral consequences of perinatal exposure to cannabidiol (CBD) — a safety and efficacy study
Varan Govind, PhD
University of Miami
Evaluation of immunomodulatory effects of chronic medicinal marijuana use and its routes of administration (smoking versus vaping) on the cerebral metabolism, morphology, dopamine (via neuromelanin MRI), and neural circuits of the whole-brain, and pain in young adults living with- and without-HIV
A national sample of older adults (n = 106) with medical conditions approved for treatment using MMJ showed on MMJ use days, there was a drop in all symptoms immediately after use. This was most robust for subjective pain and weaker though still significant for negative affect, trauma symptoms, and nausea. Negative affect, pain, and nausea evidenced momentary negative reinforcement associations with subjective cannabis intoxication levels. These negative reinforcement parameters were associated with adverse cannabis use outcomes.
Researchers observed that administration of CBD in utero results in reduced pup survival, accelerated growth rate, decreased anxiety, increased obsessive compulsive behavior especially in males, and decreased object memory as adults. Additional experiments are measuring if there are long-term changes in gene expression that could moderate the observed changes in behavior and brain development detected in adults that were exposed to CBD in utero
This study will generate a whole-brain imaging database containing different brain data types (i.e., dopamine using neuromelanin as proxy, cerebral metabolites, macroscopic and microscopic cerebral tissue structural integrity, etc.) from 128 participants, together with quantitative blood markers data, pain and behavioral data, and demographical data. Protocols have been developed and this database is anticipated to provide novel data on the effects of cannabis smoking on the CNS.
2024 ANNUAL REPORT | 19
Lori Knackstedt, PhD
University of Florida
A translational animal model to study neurobehavioral consequences of THC and oxycodone polysubstance use
Walter Steigleman, MD University of Florida
Randomized, controlled crossover comparison of cannabidiol to oral opioid for postoperative photorefractive keratectomy pain control
Eva Widerstrom-Noga, PhD
University of Miami
Effects of cannabidiol on resting state EEG and neuropathic pain severity in people with spinal cord injury
Experiments using a rodent model of sequential oxycodone and cannabis intake, to investigate the effects of opioidcannabis polysubstance use on opioid-seeking, anxiety, considering the route of cannabis administration, suggest that THC reduces motivation to seek oxycodone when the effort exerted to obtain the drug is high, and also offers a protective ‘anti-relapse’ effect after a period abstinence. These effects are specific for oxycodone and don’t alter sucrose seeking, nor do they seem to be related to withdrawal symptoms at the time of oxycodone self-administration. These are significant findings as they suggest that offering supervised/managed access to MMJ to active opioid users has the potential to reduce opioid craving.
IND approval was obtained* (167819 for a full spectrum CBD product) for a CBD-based gummy with less than 0.3% THC. A substantial fraction of patients who develop addiction to opioid medications start with a legitimate prescription for postoperative pain. This study will evaluate if an oral CBD product can offer similar pain relief to current standard therapy with an opioid medication. Several patients have been evaluated and meet enrollment criteria. Treatments are underway.
Published results* of a survey study of 227 participants report that 87.9% of participants noted that cannabis reduced their neuropathic pain intensity by more than 30%, and 92.3% reported that cannabis helped them to better deal with their neuropathic pain. Most participants (83.3%) reported substituting their pain medications with cannabis, with the most substituted medication categories being opioids (47.0%), gabapentinoids (42.8%) and over-the-counter pain medications (42.2%). These preliminary results suggest that cannabis and cannabinoids may be effective in reducing neuropathic pain intensity among those with SCI and may help to reduce the need for certain pain medications. The study team has secured a new CBD source and are currently in the processes of resubmitting an updated IND to the FDA with the new formulation.
*Cited in Consortium bibliography and outputs on pages 46-52.
20 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
New Studies from the 2023 Grant Cycle
In July 2023, the Consortium awarded six new grants to researchers as part of their fifth research grants cycle. Additionally, two Level 2 grant awards were made to Dr. Rodrigo Cristofoletti and Dr. Varan Govind for a second year of funding. The new 2023 awardees, their affiliations, along with the project narratives and anticipated impact of their proposed research, are presented below.

PI: LISA ECKEL, PHD
Florida State University
Study Title: Cannabinoid Treatment for Reducing Chemotherapy-Induced Cachexia and Neuropathic Pain in a Pre-Clinical Rodent Model
Project Narrative: Cachexia is a common side effect in advanced cancer patients receiving chemotherapy. Our research will examine the effects of multiple cannabinoids, including cannabidiol (CBD) and cannabigerol (CBG), in alleviating anorexia, loss of adipose and lean tissue mass, inflammation, and allodynia in a rodent model of cisplatin-induced cachexia.
Anticipated Impact: Cannabinoid based treatments, including CBD and CGD offer a novel therapeutic option for chemotherapy-induced cachexia and represent a viable alternative to opioids for chronic, neuropathic pain management with less dependence liability. Outcomes of the proposed studies have significant implications for informing medical practice related to treating cachexia and chronic neuropathic pain.

PI: JENNIFER HU, PHD
University of Miami
Study Title: Impacts of Medical Marijuana Use on Inflammasome Activation and Breast Cancer Clinical Outcomes
Project Narrative: A recent survey reported that 42% of breast cancer patients used MMJ to manage treatment-related symptoms. Although MMJ is generally considered safe and well-tolerated in cancer patients, there are potential adverse effects and conflicting reports of interactions with cancer therapies on clinical outcomes. We propose a prospective cohort
study of a diverse breast cancer population (50% minorities) to assess the impacts of MMJ on clinical outcomes and quality of life.
Anticipated Impact: Our research will bridge critical scientific knowledge gaps regarding the benefits and harms of MMJ in breast cancer patients during and after treatments, whether inflammasome or inflammatory biomarkers mediate the benefits and harms of MMJ in breast cancer therapies and other medications used, and the impact of MMJ characteristics on the benefits and harms and biomarkers.
 PI: HABIBEH KHOSHBOUEI, PHD, PHARMD
PI: HABIBEH KHOSHBOUEI, PHD, PHARMD
University of Florida
Study Title: Development of Cannabinoid Treatments to Ameliorate Methamphetamine Use Disorder
Project Narrative: Increasing efforts are made to develop treatments for methamphetamine use disorder. Endocannabinoid type 1 receptor (CB1R) agonists, such as THC, have been demonstrated to elevate basal dopamine levels. This may alleviate methamphetamine withdrawal symptoms by counteracting the low dopamine state that occurs during abstinence. Although interactions between cannabinoid receptors and increased dopamine transmission are reported, their properties for methamphetamine relapse have been scarcely investigated. This study assesses the hypothesis that in freely behaving mice, THC reduces methamphetamine regulation of ventral tegmental area (VTA) dopamine neurons and striatal dopamine transmission.
Anticipated Impact: Findings from this work are expected to provide evidence on whether and how cannabinoid treatments may be used to treat methamphetamine use disorder.
2024 ANNUAL REPORT | 21
 PI: CATALINA LOPEZ-QUINTERO, MD, PHD
PI: CATALINA LOPEZ-QUINTERO, MD, PHD
University of Florida
Study Title: A Pilot Double-Blind Placebo-Controlled, Randomized, Safety, Efficacy, and Acceptability Trial of a HempDerived Cannabidiol Extract for the Treatment of Anxiety
Project Narrative: Animal and small-scale human studies have demonstrated CBD’s anxiolytic, antidepressant, panicolytic, and anticompulsive actions, however, more research is needed to investigate the mechanisms of action and determine whether these products represent a safe and effective option for the treatment of anxiety. We will conduct a 1:1 randomized, double-blind, 4-week pilot clinical trial to investigate the dosing, safety, tolerability, efficacy, and acceptability of a hemp-derived CBD extract compared with placebo among 30 study participants (15 per treatment group).
Anticipated Impact: There is a pressing need to elucidate the mechanisms of action for CBD and other minor cannabinoid products and determine their potential as a therapeutic resource for anxiety treatment. CBD may offer an alternative option for the approximately 30% of individuals with anxiety who are unresponsive to conventional treatments.
 PI: JOHN S. MARKOWITZ, PHARMD
PI: JOHN S. MARKOWITZ, PHARMD
University of Florida
Study Title: The Influence of Cannabis Smoke Condensate on Drug Metabolizing Enzymes in the Human Lung
Project Narrative: The lungs are exposed to thousands of compounds in MMJ smoke. Importantly, the lung contains many of the same drug-metabolizing enzymes as the liver, and their disruption is anticipated to impair the metabolism of inhaled therapeutics and the normal physiological functioning of lung drug-metabolizing enzymes (DMEs) in detoxification from
environmental exposures. The influence of smoked MMJ on drug metabolism occurring in the lung is essentially unknown. Current regulatory and legal constraints highly limit research studies of smoked cannabis. We are incorporating a highly novel in vitro approach to assessing this issue via the production and use of cannabis smoke condensate (CSC) as a highly representative mixture to assess the influence of MMJ smoking on DMEs utilizing human lung microsomes. Furthermore, we will assess the influence of CSC on the metabolism of the commonly used inhaled corticosteroid for asthma, beclomethasone.
Anticipated Impact: Study results could have direct implications for presently marketed inhaled therapeutics. Results from these studies will provide the first information, and perhaps guidance on the influence of smoked medical cannabis on drug metabolism, and help guide the selection of MMJ formulations and administration routes (e.g. oral vs smoking) in individual patients.
 PI: NIALL MURPHY, PHD
PI: NIALL MURPHY, PHD
University of Florida
Study Title: Comparative evaluation of cannabinoids and opioids for treating chronic pain in aged subjects
Project Narrative: Treating chronic pain in the elderly is challenging due to a poor understanding of how advanced age affects physiology and drug action. In this project, we will test the analgesic potency of marijuana constituents in aged animals, seeking evidence that CBD particularly may present itself as a safer alternative to other analgesics, e.g., opioids, for treating pain in the elderly.
Anticipated Impact: Finding safe and efficacious analgesics for treating pain in the elderly is a high priority, particularly given the rise in aged populations. Results from the current study aim to address this need by seeking evidence to support using marijuana constituents as an alternative and safer pain treatment for the elderly.
22 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Highlights of Research Conducted by Consortium Grant Awardees
Over the past five years, the Consortium has supported researchers from its member universities in conducting research on studying clinical outcomes of MMJ use. As part of our annual report each year we feature several of the impactful studies concluded in the previous year as described below.
Can cannabis constituents other than Δ9-tetrahydrocannabinol (THC) help reduce pain?
The most frequently reported use of MMJ is for pain relief. Analgesic properties have been attributed to several active ingredients, including THC. THC has a more severe side effect profile than other active components such as CBD and β-caryophyllene (BCP). Consortium researchers evaluated the analgesic potential of CBD and BCP individually, and in combination in a rat spinal cord injury (SCI) clip compression chronic pain model. A single, un-bending filament is applied with increasing force until a paw withdrawal response is elicited. The force at which this response occurs is recorded automatically by the apparatus and is designated as the paw withdrawal threshold (PWT). Individually, both phytocannabinoids produced dose-dependent reduction in tactile and cold hypersensitivity in male and female rats with SCI. When co-administered at fixed ratios based on individual A50s (drug concentration needed for half-maximal effect), CBD and BCP produced enhanced dose-dependent reduction in allodynic responses with synergistic effects observed for cold hypersensitivity in both sexes and additive effects for tactile hypersensitivity in males. Antinociceptive effects of both individual and combined treatment were generally less robust in females than males. CBD:BCP co-administration also partially reduced morphineseeking behavior in a conditioned place preference (CPP) test. Minimal cannabinoidergic side effects were observed with high doses of the combination.

Time course of Cannabidiol (CBD) and β-caryophyllene (BCP) in combination on spinal cord injury (SCI) pain responses in males and female rats.
Time plots of the effect of (A) CBD plus BCP on mechanical Paw Withdrawal Threshold (PWT) in males; (B) CBD plus BCP on PWT in females; (C) CBD plus BCP on acetone (cold) responses in males; (D) CBD plus BCP on acetone responses in females (n = 5 per treatment group). All doses and dose ratios were determined from previous individual dose-response analysis as described in the published manuscript. *, **, ***, **** denote p < 0.05, 0.01, 0.001 and 0.0001 compared to vehicle for each treatment group.
2024 ANNUAL REPORT | 23
Results suggest that CBD and BCP co-administration may provide an effective treatment option for the management of chronic SCI pain.
This work was completed by 2019 Consortium grants awardee Jacqueline Sagen, PhD, University of Miami and published in the journal PLosOne. (Eeswara A, Pacheco-Spiewak A, Jergova S, Sagen J. Combined non-psychoactive Cannabis components cannabidiol and β-caryophyllene reduce chronic pain via CB1 interaction in a rat spinal cord injury model. PLoS One. 2023 Mar 13;18(3):e0282920. doi: 10.1371/journal.pone.0282920)
Is medical marijuana effective in reducing common symptom clusters on older adults?
Increasingly, older adults are turning to cannabis to address medical issues. MMJ may affect a wide range of symptoms particularly relevant to older adults, but research specific to this population is lacking. This study looked at four different symptom clusters: negative affect, trauma symptoms, nausea, and pain leading up to MMJ use and in the hours after MMJ use at the daily level and to examine the extent to which the effects of MMJ on these symptoms may be associated with adverse cannabis-related outcomes.

Trajectories of outcomes in the hours prior to and following MMJ use
Note. B = unstandardized coefficient with random variance component; SE = Standard Error; Cohen’s d uses the fixed effect Use slope divided by the Standard Error of the fixed effect multiplied by the square-root of the sample. *95% Bayesian Credibility Intervals do not include 0.
A national sample of older adults (n = 106) licensed to use MMJ in Florida was recruited by Consortium researchers through social media. Once enrolled, participants received text messages to complete 6 daily assessments of symptoms for 15 days. Participants provided 5,522 event-level assessments across 1,106 MC use days. These ecological momentary assessments (EMA) were used to examine trajectories of four symptom clusters prior to and post MMJ initiation and momentary associations between subjective intoxication and
24 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
current symptoms. These associations were used to predict cannabis-related problems and cannabis use disorder (CUD) symptoms. “Cannabis-related Problems” were assessed using the Brief Marijuana Adult Consequences Scale (B-MACQ). The B-MACQ consists of 22 problems individuals may experience subsequent to cannabis use (e.g., driving while intoxicated, neglecting responsibilities, physical fights, etc.). “Cannabis Use Disorder Symptoms” was assessed using the Cannabis Use Disorder Identification Test – Revised (CUDIT-R). The CUDIT-R is used to assess Cannabis Use Disorder symptoms.
Results indicated that MMJ offers multiple therapeutic effects on every symptom cluster examined, but these benefits come with a cost. The cost was especially heavy for psychiatric symptom clusters; the only clusters associated with CUD symptoms. This may represent a general tendency toward comorbid psychopathology among individuals who are already experiencing psychiatric issues. In contrast, physical symptom clusters were associated with cannabis-related problems, but not CUD symptoms. This may indicate small changes in use patterns (e.g., harm reduction approaches and/or psychoeducation) may allow individuals to reap the therapeutic effects on while avoiding the costs.
Coefficients of fixed effects predicting adverse cannabis outcomes
Note. Coefficients are standardized. *95% Bayesian Credibility Intervals do not include 0, suggesting a significant effect
These data suggest that marijuana may be effective in reducing common symptom clusters. However, the negative reinforcing effect may complicate therapeutic use by increasing problematic use.
This work was completed by 2022 Consortium grants awardee Robert Dvorak, PhD, University of Central Florida.
2024 ANNUAL REPORT | 25
Cannabis Related Problems Cannabis Use Disorder Symptoms β 95% BCI β 95% BCI Negative Affect Pre-use Slope -0.154 -0.436 to 0.146 -0.107 -0.399 to 0.186 Post-use Slope 0.042 -0.189 to 0.257 -0.019 -0.260 to 0.202 Use Effect 0.027 -0.180 to 0.237 -0.084 -0.295 to 0.133 Reinforcement Effect -0.218* -0.415 to -0.015 -0.289* -0.480 to -0.081 Trauma Symptoms Pre-use Slope 0.092 -0.488 to 0.568 0.158 -0.429 to 0.681 Post-use Slope -0.147 -0.421 to 0.135 -0.317* -0.587 to -0.008 Use Effect 0.135 -0.130 to 0.373 0.004 -0.247 to 0.262 Reinforcement Effect 0.055 -0.172 to 0.273 0.031 -0.194 to 0.251 Pain Symptoms Pre-use Slope 0.076 -0.322 to 0.437 0.107 -0.326 to 0.496 Post-use Slope -0.083 -0.317 to 0.165 -0.140 -0.382 to 0.134 Use Effect 0.110 -0.121 to 0.345 -0.021 -0.258 to 0.216 Reinforcement Effect -0.261* -0.460 to -0.032 -0.109 -0.329 to 0.121 Nausea Symptoms Pre-use Slope -0.133 -0.577 to 0.400 -0.206 -0.678 to 0.352 Post-use Slope -0.211 -0.489 to 0.088 -0.227 -0.541 to 0.072 Use Effect 0.154 -0.071 to 0.384 0.139 -0.097 to 0.367 Reinforcement Effect -0.257* -0.451 to -0.040 -0.134 -0.350 to 0.088
Can cannabidiol (CBD) help relieve chemotherapy related pain?
In cancer patients, chronic paclitaxel (PTX) treatment causes significant pain, limiting its use in chemotherapy. The neuroprotective potential of synthetic CBD and CBD formulated in extracellular vesicles (CBD-EVs) isolated from human umbilical cord derived mesenchymal stem cells was investigated in C57BL/6J mice with PTX-induced neuropathic pain (PIPN) by Consortium researchers. In terms of decreasing mechanical and thermal hypersensitivity, CBD-EVs treatment was superior to EVs treatment or CBD treatment alone (p < 0.001). CBD and CBD-EVs have potent mitoprotective effects in neuronal cells via activating serotonin (also known as 5-HT) 1A receptor (5HT1A) receptors and the AMP-activated protein kinase (AMPK) pathway. CBD was shown to depend on AMPK activation in improving mitochondrial function and biogenesis against PIPN in vitro and in vivo.
The primary finding of this study is that CBD-EVs prepared from the sonication method have shown potential in reducing mechanical and thermal pain sensitivities, which are superior to CBD alone against PIPN in mice.
This work was completed by 2019, and 2020 Consortium grants awardee Mandip Sachdeva, PhD, from Florida Agricultural and Mechanical University, and published in the journal Pharmaceutics.
Kalvala AK, Bagde A, Arthur P, Kulkarni T, Bhattacharya S, Surapaneni S, Patel NK, Nimma R, Gebeyehu A, Kommineni N, Meckes DG Jr, Sun L, Banjara B, Mosley-Kellum K, Dinh TC, Singh M Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics. 2023 Feb 7;15(2):554. doi: 10.3390/pharmaceutics15020554
Cannabis and cannabinoids may be effective in reducing neuropathic SCI pain. Approximately 60% of individuals with a spinal cord injury (SCI) experience neuropathic pain, which often persists despite the use of various pharmacological treatments. The primary objective of the study conducted by Consortium researchers was to investigate the perceived effects of cannabis on neuropathic pain among those who were currently or had previously used these approaches. Additionally, the study aimed to determine if common pain medications are substituted by cannabis. Participants (N = 342) were recruited from existing opt-in listserv sources within the United States. Of those, 227 met the inclusion criteria and were enrolled in the study. The participants took part in an anonymous online survey regarding past and current use of cannabis and their perceived effects on neuropathic pain, including the use of pain medication. Those in the sample reported average neuropathic pain intensity scores over the past week of 6.8 ± 2.1 (0 to 10 scale), reflecting a high moderate to severe level of pain. Additionally, 87.9% noted that cannabis reduced their neuropathic pain intensity by more than 30%, and 92.3% reported that cannabis helped them to better deal with their neuropathic pain symptoms.
Most participants with a history of cannabis/cannabinoid use, accounting for 93.5% of the sample (n = 186), reported experiencing at least some benefit from cannabis, either by decreasing neuropathic pain intensity, unpleasantness or interference with activities, mood, and sleep (See A in Figure). Overall, 71.9% of participants reported cannabis and cannabinoids improving their global well-being “much” (n = 73) or “very much” (n = 68) (See B in Figure). Among the participants who experienced reduced neuropathic pain intensity due to cannabis and cannabinoid use, 87.9% reported a decrease of more than 30% (n = 110), with 18.2% reporting more than a 75% reduction (n = 24) (See C in Figure).
26 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
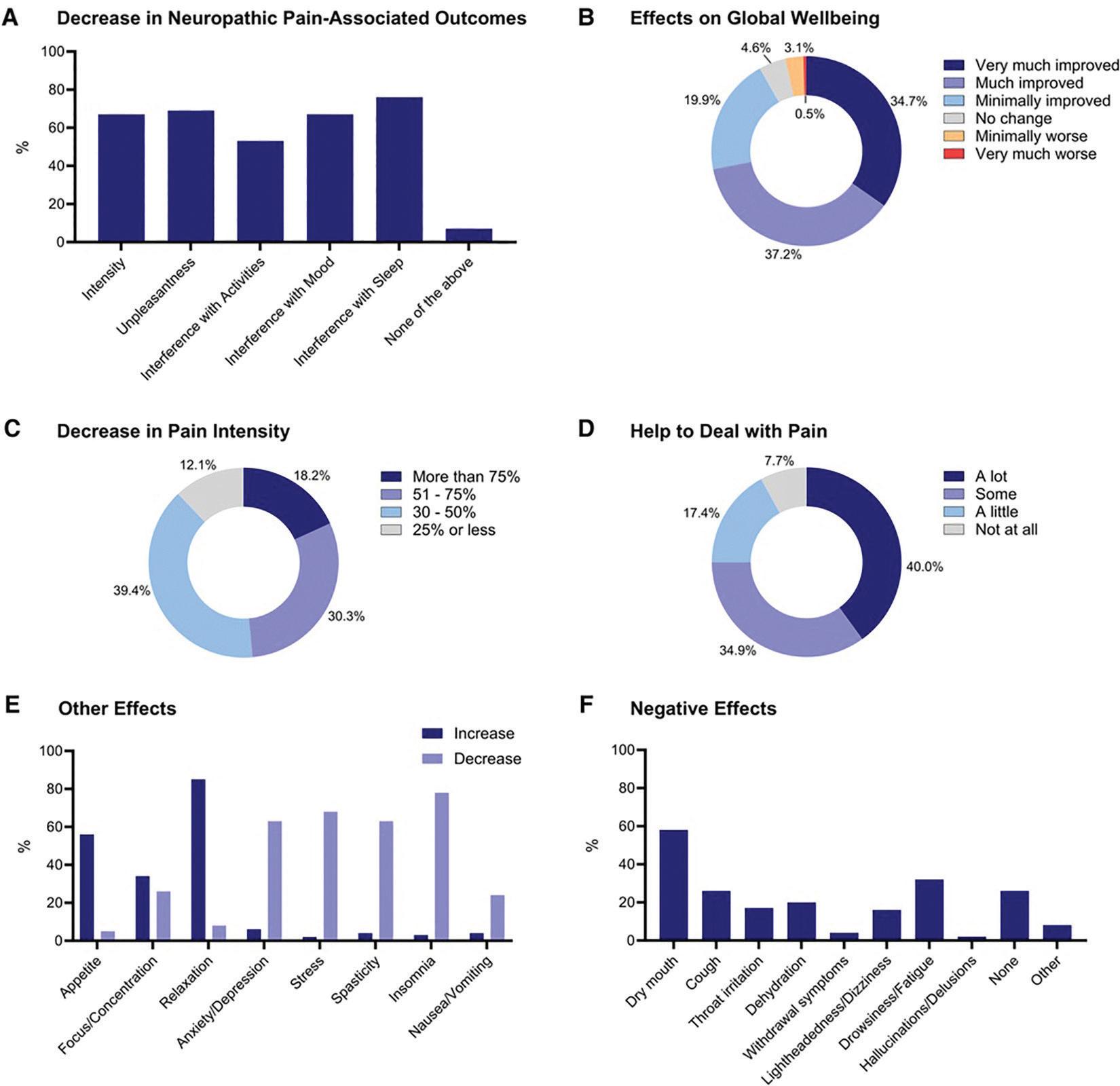
Decrease in neuropathic pain associated outcomes (A), effects on global well-being (B), decrease in pain intensity (C), help to deal with pain (D) other effects (E) and negative effects (F) associated with Cannabis and Cannabinoid use. Responses are reported as percentages (%).
Furthermore, 40.0% (n = 78) stated that cannabis and cannabinoids did help them deal with their neuropathic pain “a lot” (See D in Figure). Additionally, participants reported the use of cannabis and cannabinoids decreased their stress (68.4%, n = 134), anxiety and depression (62.8%, n = 123), spasticity (62.8%, n = 123), insomnia (77.6%, n = 152), and feelings of nausea and vomiting (24.5%, n = 48) while increasing their relaxation (85.2%, n = 167), as well as appetite (56.1%, n = 110) and focus and concentration (33.7%, n = 66) (See E in Figure). Negative effects from cannabis and cannabinoids were reported mostly as dry mouth (58.2%, n = 114), drowsiness (31.6%, n = 62), and cough (26.0%, n = 51) (See F in Figure). In addition, (25.5%, n = 50) of the participants reported experiencing no negative side effects from cannabis and cannabinoids.
Most participants (83.3%) also reported substituting their pain medications with cannabis, with the most substituted medication categories being opioids (47.0%), gabapentinoids (42.8%) and over-the-counter pain medications (42.2%).
2024 ANNUAL REPORT | 27
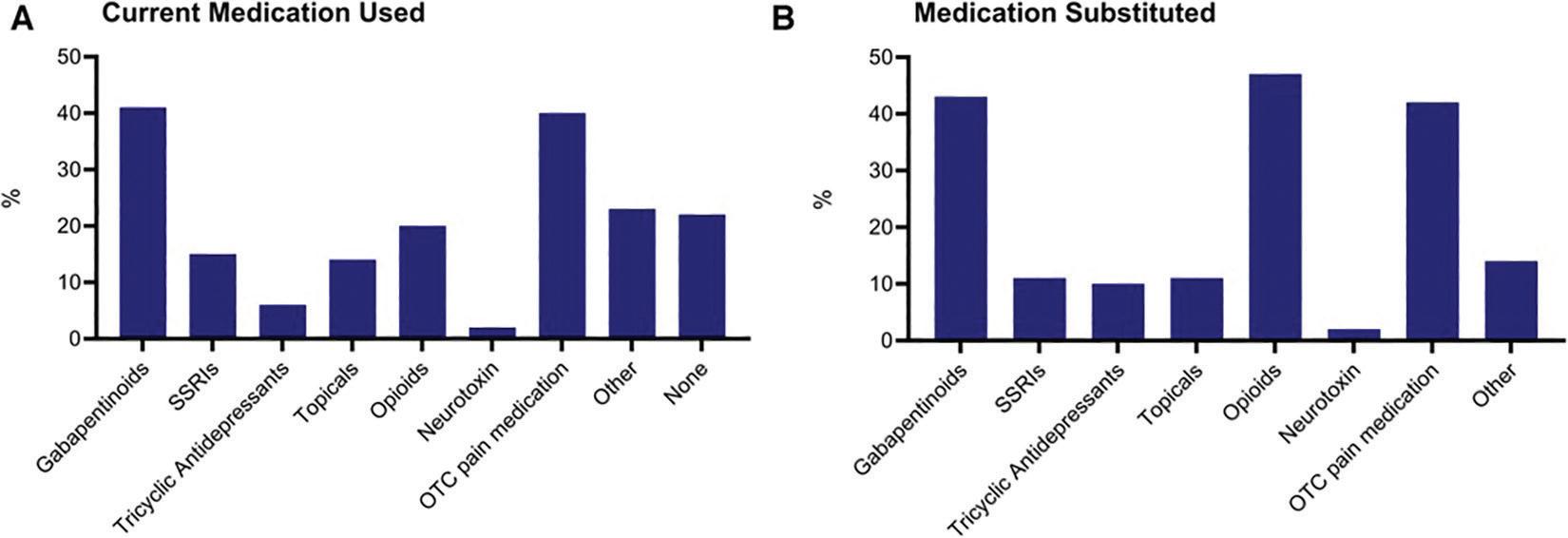
Medication use and substitution. Medication categories currently used (A) and currently substituted with cannabis and cannabinoids (B) SSRI: selective serotonin reuptake inhibitor; OTC: over-thecounter
These preliminary results suggest that cannabis and cannabinoids may be effective in reducing neuropathic pain among those with SCI and may help to limit the need for certain pain medications.
This work was completed by 2022 Consortium grants awardee Eva Widerström-Noga, PhD, from University of Miami, and published in the journal Frontiers in Pain Research
Kinnunen K, Robayo LE, Cherup NP, Frank SI, Widerström-Noga E. A preliminary study evaluating self-reported effects of cannabis and cannabinoids on neuropathic pain and pain medication use in people with spinal cord injury. (Front Pain Res. 2023 Dec 21; 4:1297223. doi: 10.3389/ fpain.2023.1297223)

28 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
MEdical Marijuana clinical Outcomes RepositorY (MEMORY)

The growing uptake of MMJ in Florida offers a unique opportunity for real-world evaluations of MMJ outcomes that can help overcome the slow availability of randomized clinical trials and provide timely evidence on MMJ risk-benefit.
MEMORY has been conceived to establish the infrastructure for real-world MMJ clinical outcomes evaluations similar to those employed by the FDA to evaluate and monitor the riskbenefit of prescription medications after marketing.
MEMORY will support:
• controlled studies on MMJ effectiveness and safety
• active surveillance to capture emerging safety issues involving individual products or generalized effects among MMJ users
• MMJ utilization studies on MMJ access and utilization pattern across Floridians
To ensure comprehensive longitudinal follow-up to capture relevant health outcomes and availability of control groups, the Consortium is linking the Office of Medical Marijuana Use (OMMU) Medical Marijuana Use Registry (MMUR), with other clinical databases commonly used for outcomes research to create a robust research-ready repository. The established and planned linkages will optimize detail on MMJ use (type, dose, route, from the MMUR) and detail on patient health history, other treatments and outcomes (from linked clinical encounter data), and facilitate controlled longitudinal studies on safety and effectiveness outcomes. Importantly, via linkage to other clinical databases including pharmacy dispensing records, we will be able to establish control groups to facilitate comparisons of outcomes among patients who have initiated MMJ and patients with similar conditions and health history who are relying on conventional therapeutic approaches alone.
The Consortium plans to make a de-identified version of the repository available to Consortium researchers, thus providing state-wide infrastructure for real-world clinical outcomes research.
2024 ANNUAL REPORT | 29
MEMORY Development — Progress Towards Core Milestones
MEMORY development involves several milestones including:
• Regulatory approvals
• Data acquisition, curation and linkage, resulting in a well-documented longitudinal database of patients who initiated MMJ and adequate control groups who have not (yet) initiated MMJ,
• Provision of adequate study cohort data for researchers along with analytical support, and
• Policies and procedures to access and use the data.
Regulatory approvals for the access to identifiable claims data from the Agency for Healthcare Administration (AHCA) and the Centers for Medicare and Medicaid Services (CMS) and to vital statistics data from the Department of Health Vital Statistics Offices have been obtained. On January 30, 2023, the Consortium received approval by the Department of Health OMMU to access the MMUR and the Florida Department of Health Institutional Review Board approved MEMORY in August 2023.
The MEMORY data science team has started data curation and development of analytical cohorts and linked OMMU and Medicaid claims data. Initial analyses of user demographics and utilization pattern are summarized below.
Highlights of First MEMORY Analyses
User demographics
Between January 2018 to June 2022, a total of 946,975 patients were registered to use MMJ for a medical condition, with a slightly larger proportion of patients identifying as male. Age distributions of these newly licensed patients showed two peaks: the first around 30 years for both female and male patients and the second at around 60 years for female and 65 years for male patients. Children made very small contributions to the MMJ user population.

30 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Age at time of registry by gender between January 2018 – June 2022 (n=number of patients)
From July 2021 to June 2022, persons identifying as male purchased more MMJ across both non-smokable marijuana (e.g., edibles) and smokable marijuana (e.g., flower) than those identifying as female. Noticeably, when examining age groups, those between 25 and 34 years of age represented the largest proportion among the MMJ user populations, followed closely by those aged 35 to 44 years. This trend was evident between both smokable and non-smokable marijuana.
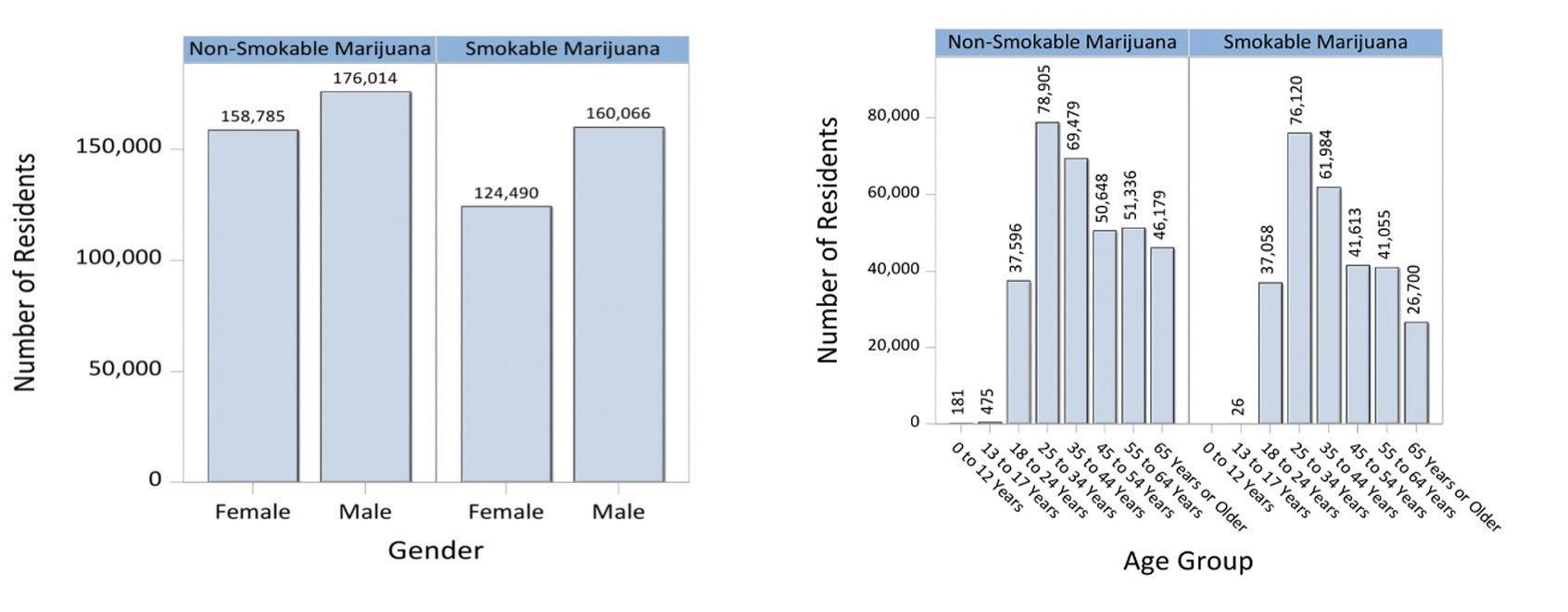
Product type by gender/age among residents with certifications and ≥1 purchase between July 2021 – June 2022 (n=360,115)
Dose and duration of medical marijuana use
To estimate daily doses of MMJ products, we analyzed dispensing records of MMJ products that are collected in the MMUR. Patients were followed from July 2021 to June 2022. Dispensing records were categorized as being associated with MMJ that could be taken by mouth (e.g., edibles, sublingual tinctures, capsules) or inhaled (e.g., flower, vapes). Within these categories, the amount of THC purchased within each order was calculated and divided by the time between the first purchase and the end of the order. If a new order followed within 30 days, time was extended until the end of that order and new purchased THC amounts were added to the originally calculated amount.
Because the THC content of smokable products (flower) is not available in the MMUR data, two alternative assumptions were made. Assuming a 15% THC content in purchased flower, the average daily dose among users who purchased products between July 2021 to June 2022 was about 175mg, whereas the dose increased to about 250mg when a 25% THC content was assumed. These assumed concentrations are consistent with observed values in studies of THC concentration in purchased MMJ (Cash et al., PLOSOne 2020*). This assumption was not necessary to make with regard to orally-administered products (e.g., edibles, capsules), as the THC content is directly recorded in the MMUR. The average daily dose of oral products was smaller than the dose of inhaled products at about 30mg. Average daily doses decreased with older age for products that could be inhaled, but were largely stable with regard to orally-administered products.
*Cash MC, Cunnane K, Fan C, Romero-Sandoval EA (2020) Mapping cannabis potency in medical and recreational programs in the United States. PLoS ONE 15(3): e0230167. https://doi.org/10.1371/journal. pone.0230167
2024 ANNUAL REPORT | 31
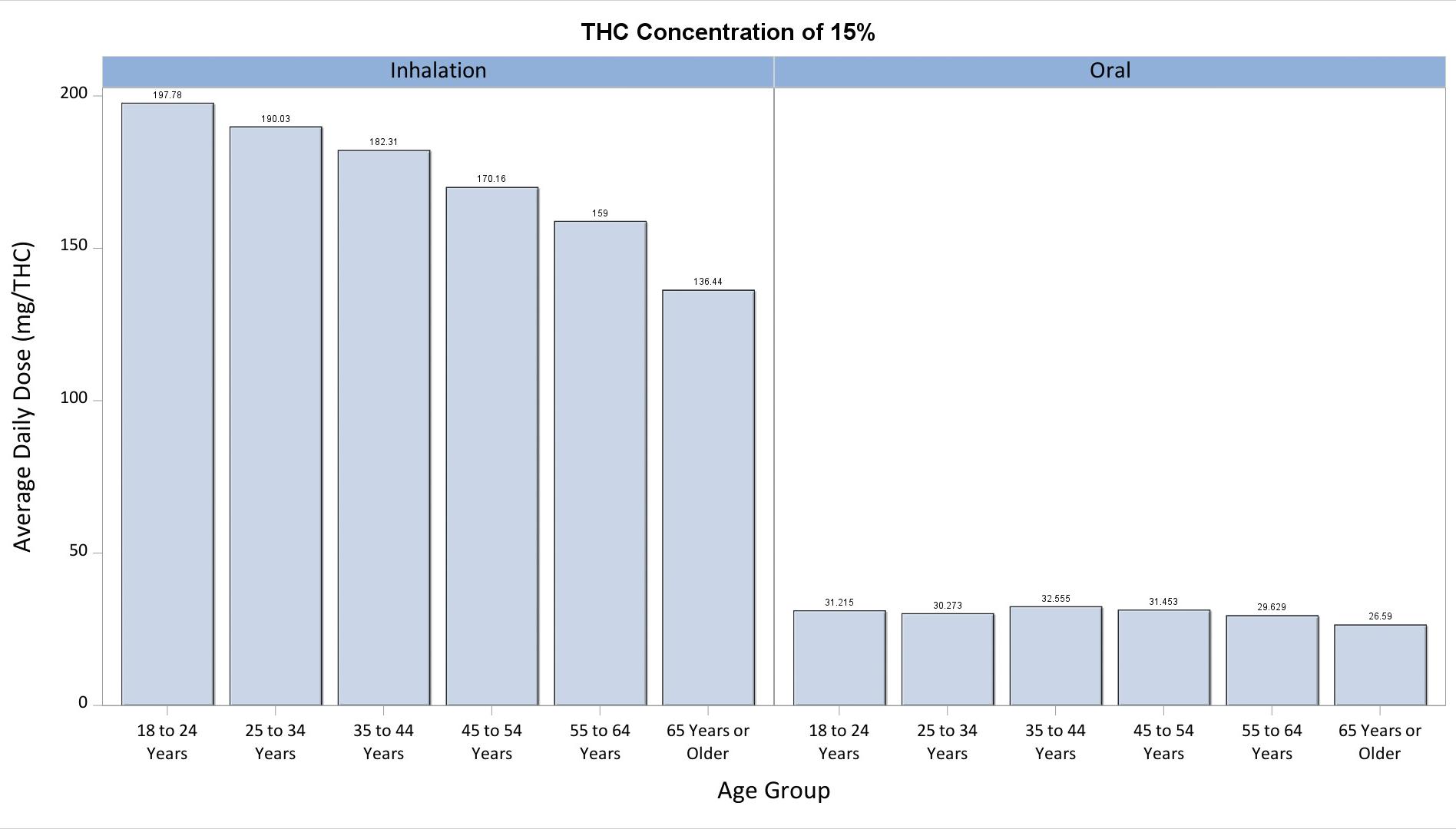

Average Daily dose (mg THC) of inhaled and orally-administered purchased MMJ products by age assuming (A) 15% THC in Flower and (B) 25% THC in Flower
The difference in dosing of orally and inhaled products was confirmed when evaluating the total amount of products sold per the total number of MMJ cardholders in a given month.
From January 2018 through June 2022, the average daily dose of THC dispensed per route of administration was calculated assuming both a 15% and 25% THC content of smokable products. The average daily dose of orally-administered MMJ (e.g., edibles, oral capsules) remained comparatively stable to the average daily dose of inhaled MMJ (e.g., flower, vapes), which saw a precipitous increase in dose following the introduction of Senate Bill 182 in March of 2019, permitting the use of smokable marijuana flower for medical purposes. Hence, increases in daily dose over the time of the FL MMJ program are largely attributed to the legalization of cannabis flower for medical purposes.
32 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
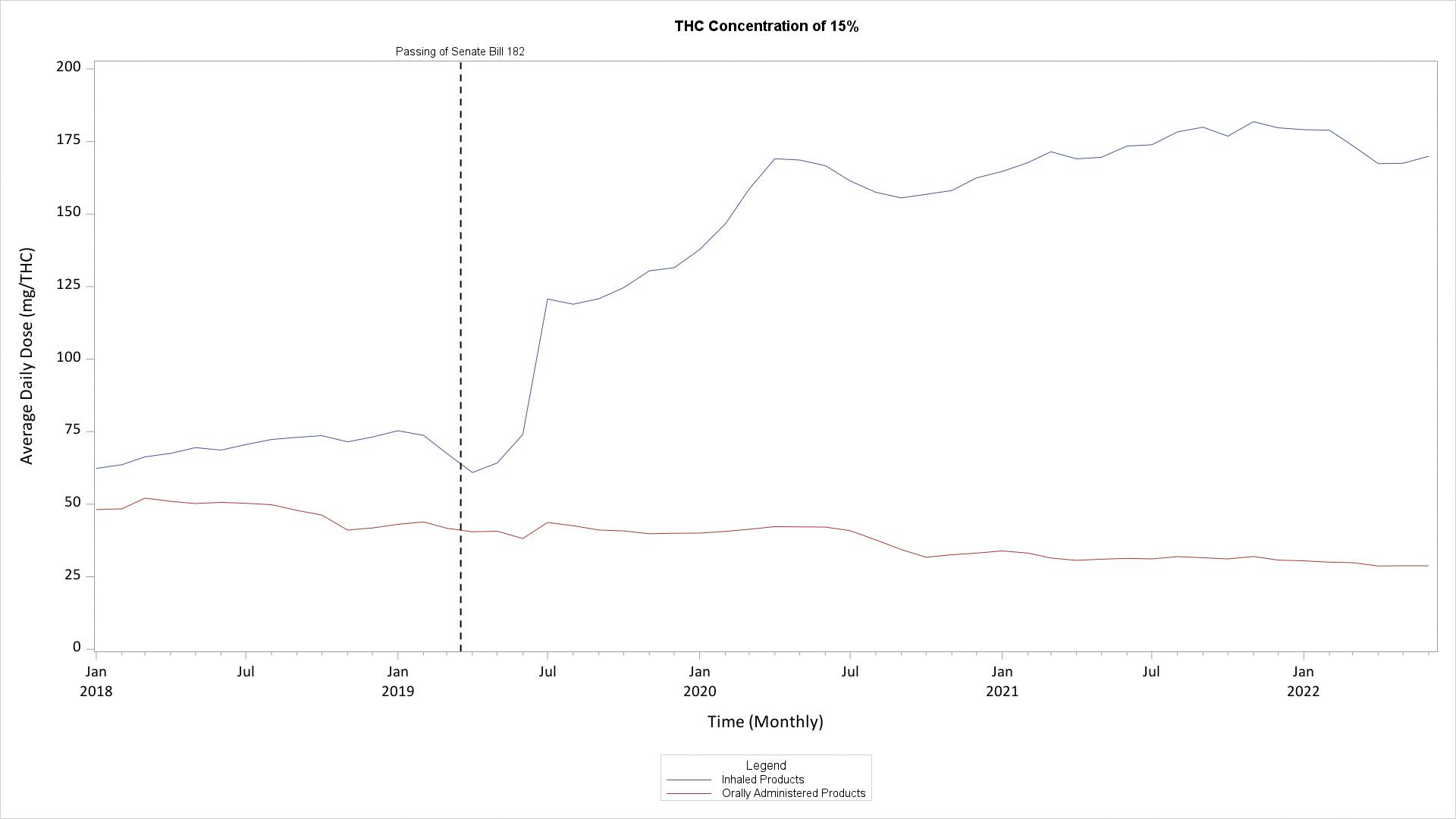
Average Daily Dose of MMJ by Route of Administration Per Month from January 2018 to June 2022, Assuming a 15% THC Concentration in Flower
Distribution of clinical conditions among MMJ users
We accessed data housed in the Medical Marijuana Use Registry (MMUR) and calculated the prevalence of qualifying conditions in MMJ certified patients in Florida in 2020. We allowed individual certified patients to count once for each reported qualifying condition even if multiple certifications were available. Patients could contribute to multiple conditions. The certifications in the MMUR data include checkboxes for each approved medication condition (a) – (j) as well as an umbrella category for other qualifying conditions (k) labeled “Medical conditions of the same kind or class as comparable to those enumerated in paragraphs (a)-(j)”. If (k) is selected one of the approved conditions (a) – (j) to which the other condition is similar in symptoms must be selected as well. For this analysis, we did not explore what these conditions were, but did conduct sensitivity analysis to confirm robustness of our findings after excluding certifications with a qualifying condition of k.
To compare the distribution of qualifying conditions in the MMUR data with relevant disease prevalences in the state, we searched the scientific literature, as well as national reports (e.g., from the Centers for Disease Control and Prevention). If no Florida specific estimates were available, we prioritized population-based prevalence estimates reported for the southern part of the US over national estimates. When the option was available, we prioritized reports that estimated prevalence across the entire population over reports for selected subpopulations, e.g., only those with private health insurance.
2024 ANNUAL REPORT | 33
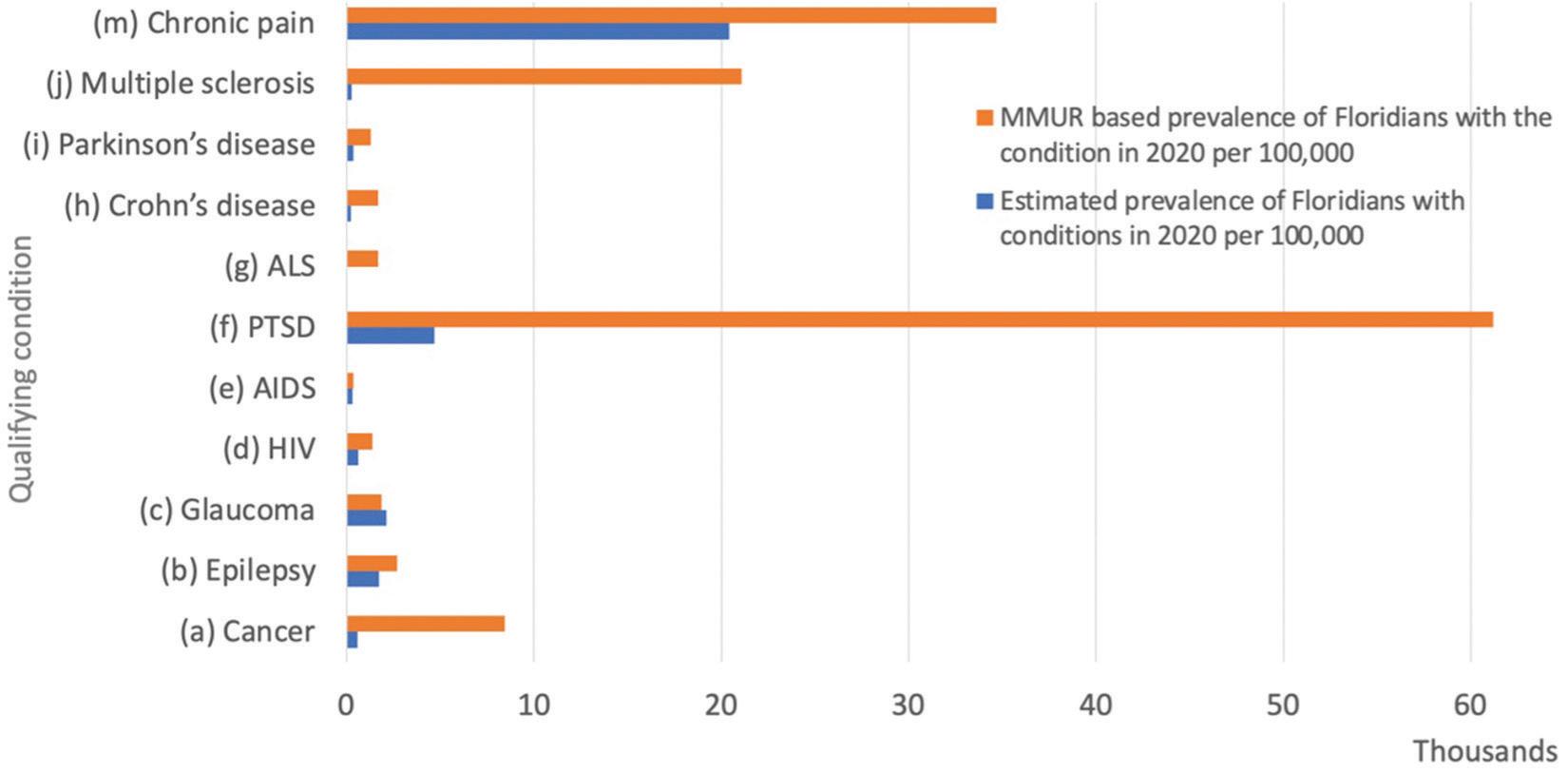
Population-based prevalence of qualifying conditions in Florida (south region or US) compared to the 2020 Medical Marijuana Use Registry (MMUR) Data
In our MMUR cohort of 2020, we identified 405,142 patients with 929,934 qualifying conditions. The most prevalent qualifying conditions among patients certified for MMJ in 2020 were (f) post-traumatic stress disorder (PTSD) (61,190 per 100,000 MMJ certified Floridians), (m) chronic non-malignant pain (34,700 per 100,000), and (j) multiple sclerosis (21,070 per 100,000). The three qualifying conditions with the least representation among certified patients were (e) acquired immune deficiency syndrome (AIDS) (330 per 100,000), (i) Parkinson’s disease (1,280 per 100,000), and (d) human immunodeficiency syndrome (HIV) (1,360 per 100,000). Chronic pain (20,400 per 100,000 Floridians) and PTSD (4,700 per 100,000 Floridians) were also the most prevalent conditions in the general population. However, prevalence estimates of qualifying conditions in the general population were lower than prevalence estimates in the MMUR registry, with the exemption for glaucoma, with a prevalence of 2,110 per 100,000 Floridians vs. 1,830 per 100,000 MMJ certified Floridians. The general difference across prevalence estimates of both population is expected because the presence of at least one condition is required to qualify for MMJ certification. Our analysis indicates that there appears to be no strong correlation between the existence of high-quality evidence in the literature supporting favorable risk-benefit of MMJ for a specific condition, and the frequency of occurrence of that condition among certified patients. For example, while there is some evidence to support the effectiveness of MMJ in the treatment of epilepsy and in pain management, limited evidence exists for its use in ALS or PTSD. It should also be noted that the representation of each condition among MMJ users differed when compared to the population-based prevalence. Comparing the orange and blue bars, we see that the prevalence of glaucoma, epilepsy, and HIV/AIDS in among MMJ certififed Floridians was similar to the overall prevalence in the state, while the prevalence of PTSD, MS or cancer was much higher among MMJ users than in the overall populations.
This work was completed by Consortium core faculty and the Consortium MEMORY data science team.
34 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
CLINICAL CORE
The Clinical Core establishes infrastructure support for prospective studies including randomized controlled trials. With expert guidance from Ms. Sheila Austin, Regulatory Specialist, at the Clinical and Translational Science Institute (CTSI) at UF, we have worked to assist researchers in submitting and obtaining two Investigational New Drug Application (IND) approvals from the Food and Drug Administration (FDA). Collaborators for the Clinical Core span across the state from University of Miami to Florida International University, Florida Atlantic University and Florida State University, and help with patient recruitment, data collection or analysis, clinical research study design, support with regulatory issues (with FDA or DEA), and access to laboratory experts for product analysis. The Clinical Core also provides three specific resources to support clinical outcomes research: its Patient Contact Registry, the Medical Marijuana & Me (M3) patient cohort, and a database of research collaborators (CARMMA).
MMJ Contact Registry
The MMJ contact registry facilitates patient recruitment for future research on MMJ clinical outcomes. The registry was approved by the University of Florida IRB in November 2020. As of January 2024, over 1600 MMJ users across the state are enrolled in the contact registry. Clinics participating in recruitment and the geographic location of enrolled patients is similar to the current geographic distribution of MMJ clinics and dispensaries.

2024 ANNUAL REPORT | 35
Medical Marijuana Patient Contract Registry Recruitment: (A) Clinics assisting in patient recruitment (B) Geographic representation of MMJ registry participants
Medical Marijuana & Me (M3)
In May 2022, the Consortium launched Medical Marijuana & Me (M3) (mmjoutcomes.org/ m3study/), the first large MMJ patient research cohort in Florida, and one of the first in the United States. M3 provides detailed patient-reported data on MMJ utilization and outcomes to enhance our understanding of patient experiences with MMJ.
• collect patient-centered data, focusing on the most common health conditions, to characterize the experiences and clinical outcomes among a diverse and representative group of MMJ users in Florida.
• provide access to data and recruitment infrastructure for consortium researchers to support pilot studies, papers, and grant proposals.
M3 data domains
• support high-quality, impactful research that can inform state policy, clinician practice and patients.
Participants complete a sequence of surveys assessing the M3 data domains including general health, use and experiences with MMJ, and related health outcomes. M3 has recruited a total of 1,230 patients: 598 new MMJ users who are followed over 9 months via consecutive survey waves, and 632 current users who were assessed in one cross-sectional survey.
Recruitment of the current user cohort and data curation are complete. Researchers can request for access to cross-sectional data from M3 and will soon be able to request the longitudinal data of the new user cohort. The data request form is now open for applications at mmjoutcomes.org/m3study/data-request-application/. More information, and a summary of research studies that have been approved, is available at mmjoutcomes.org/m3study/
Following are preliminary analyses of M3 current MMJ user participants. M3 participants representing current MMJ users cover a broad range of ages and racial/ethnic groups.
36 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
SURVEY VARIABLE RESULTS Mean Age (IQR) 45 (35, 48) Sex at Birth Female 63% Male 37% Ethnicity/Race White 75% Black/African American 6% Hispanic 15% Other 5% Education Level Graduate/ professional degree after college 18% Some college or college graduate 65% High school or GED 16% Middle school 1% Elementary school or below 0% VARIABLE RESULTS Employment Status Full-time 46% Part-time 12% Unemployed – looking for work 3% Unemployed – disabled/ unable to work 16% Student 2% Retired 16% Veteran (yes) 10% Health Insurance Private 52% Medicaid 14% Medicare 25% VA coverage 5% None 11% Annual Household Income in USD < $20,000 13% $20,000 - $39,999 21% $40,000 - $59,999 17% $60,000 - $79,999 16% $80,000 - $99,999 8% ≥ $100,000 21%
DEMOGRAPHICS AND BASELINE CHARACTERISTICS OF CURRENT USERS OF MMJ (N=632), IN THE M 3

Percentage of M3 current MMJ users who reported a specific health condition as a main reason for MMJ use (n=632). The bars and corresponding percentages illustrate the proportion of participants who self-reported each health condition as one of the main reasons for using MMJ in the total sample. Abbreviations: ADHD, attention-deficit hyperactivity disorder; MMJ, medical marijuana; PTSD, posttraumatic stress disorder.
The top ten most frequently reported main reasons for using MMJ in the total sample were anxiety (n=383, 60.60%), chronic pain (n=278, 43.99%), depression (n=252, 39.87%), PTSD (n=220, 34.81%), headaches/ migraine (n=134, 21.20%), fibromyalgia (n=67, 10.60%), ADHD (n=59, 9.34%), bipolar disorder (n=53, 8.39%), high blood pressure (n=41, 6.49%), and cancer, (n=18, 2.85%).
We identified and ranked the most frequently self-reported reasons for using MMJ within each self-reported qualifying condition category. Among those certified for PTSD, the most frequently reported main reasons for using MMJ was PTSD (n=155/187, 82.89%), followed by anxiety (n=134/187, 71.66%), depression (n=96/187, 51.34%), insomnia/ sleeping problems (n=83/187, 44.39%), and chronic pain (n=75/187, 40.11%). Among those who reported being certified for a condition other than those listed, the most frequently reported main reasons for using MMJ was anxiety (n=107/175, 61.14%), followed by depression (n=71/175, 40.57%), insomnia/ sleeping problems (n=59/175, 32.71%), and chronic pain (n=52/175, 29.71%), respectively. Among those certified for medical conditions of the same kind or class as or comparable to the others listed, the most frequently reported main reason for using MMJ was anxiety (n=100/140, 71.43%), followed by insomnia/ sleeping problems (n=67/140, 47.86%), depression (n=66/140, 47.14%), and chronic pain (n=61/140, 43.57%), respectively. Finally, among those certified for chronic nonmalignant pain, the most frequently reported main reason for using MMJ was chronic pain (n=138/162, 85.19%), followed by anxiety (n=82/162, 50.62%), insomnia/ sleeping problems (n=66/162, 40.74%), and depression and PTSD (for both, n=46/162, 28.40%).
Overall, self-reported reasons for MMJ use were highly aligned with self-reported qualifying conditions on the MMJ certification. For example, there was an alignment of 81.25% for cancer (n=13/16), 85.19% for chronic pain (n=138/162), 75.00% for glaucoma (n=9/12) among patients reported this qualifying condition for MMJ certification and as main reason for using MMJ. The agreement was 100% for the infrequently reported conditions (epilepsy, multiple sclerosis, and Parkinson’s disease, for which data are not displayed due to privacy considerations (n<10)).
2024 ANNUAL REPORT | 37
HEALTH CONDITION REPORTED AS ONE OF THE MAIN REASONS FOR USING MMJ
M3: Heat map depicting percent agreement between self-reported qualifying conditions and main reasons for MMJ use and ranking of the most frequent main reasons of MMJ use in each qualifying condition category among 632 respondents.
Note: Total column percent’s do not add to 100% as responses are not mutually exclusive. Abbreviations: ADHD, attention-deficit hyperactivity disorder; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; MMJ, medical marijuana; N, number; PTSD, post-traumatic stress disorder.
M3 design has been guided by a scientific planning committee consisting of 11 members, including six researchers from consortium member universities, four MMJ physicians, and one MMJ patient representative.
Denise Vidot, PhD
Michelle Weiner, DO, MPH
The following clinics and practitioners have served as participant recruitment sites for M3: Dr. Bob’s Compassion Clinic, Dr. Justin Davis, MD, Dr. Melanie Bone, MD, CannaMD, DocMJ, Green Health & Wellness, Releafe Now, ReliefMD, and Spine & Wellness Centers of South Florida.
38 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
QUALIFYING CONDITION FOR MMJ CERTIFICATION Cancer (N=16) Chronic nonmalignant pain (N=162) Glaucoma (N=12) HIV/AIDS (N=10) PTSD (N=187) Crohn’s disease (N=10) Medical conditions of he same kind (N=140) Anxiety 31.25% 50.62% 33.33% 60% 71.66% 50% 71.43% Depression 18.75% 28.40% 16.67% 40% 51.34% 40% 47.14% PTSD 12.50% 28.40% 16.67% 30% 82.89% 40% 32.86% ADHD 0% 6.17% 0% 20% 13.90% 20% 12.14% Bipolar Disorder 6.25% 6.79% 0% 10% 12.83% 30% 14.29% Insomnia/Sleeping Problems 25% 40.74% 41.67% 20% 44.39% 30% 47.86% Headaches/Migraines 6.25% 20.99% 16.67% 0% 25.13% 30% 27.86% Fibromyalgia 12.50% 14.81% 16.67% 0% 8.02% 0% 13.57% Chronic Pain 43.75% 85.19% 83.33% 50% 40.11% 60% 43.57% Cancer 81.25% 3.09% 8.33% 0% 0.53% 0% 0.00% High Blood Pressure 0% 9.88% 16.67% 0% 8.02% 10% 7.86% Diabetes 6.25% 4.94% 0% 0% 2.14% 0% 4.29% IBD 0% 1.23% 0% 0% 1.60% 90% 1.43% Epilepsy/Seizures 0% 1.23% 0% 0% 2.14% 10% 2.14% Glaucoma 6.25% 3.70% 75.00% 0% 1.07% 0% 2.86% Other Health Conditions 0% 8.64% 0% 10% 6.42% 10% 13.57%
SCIENTIFIC PLANNING COMMITTEE MEMBERS — M3 (MMJ PATIENT COHORT) PARTICIPANT INSTITUTION ROLE ON PLANNING GROUP Anonymous patient University of Florida MMJ Patient Liaison John Crump, MD Releafe Now Certified MMJ Provider Justin Davis, MD Florida Marijuana Doctors Certified MMJ Provider Jason Ford, PhD University of Central Florida MMJ Researcher Raul Gonzalez, PhD Florida International University MMJ Researcher
Green-Powell, PhD Florida A&M University MMJ Researcher Dushyantha Jayaweera, MD University of Miami MMJ Researcher
Quinonez, DO CannaMD Certified MMJ Provider
Patricia
Jonathon
Florida Gulf
MMJ Researcher
Martha Rosenthal, PhD
Coast University
University
MMJ Researcher
of Miami
Spine
Certified MMJ Provider
Wellness America
CARMMA
The Consortium launched the Connect and Advance Research for Medical Marijuana Analysis (CARMMA) Database to connect researchers, physicians, and industry collaborators with the goal to increase and accelerate MMJ research (mmjoutcomes.org/collaborate). Researchers, physicians, and industry collaborators can add their contact information and connect with other collaborators. As of January 2024, CARMMA has 29 researchers, 13 physicians, and 12 industry collaborators and their information is available to Consortium members.
EVIDENCE CORE
The evidence core focuses on the synthesis and dissemination of scientific evidence for researchers, providers and patients. Evidence core activities include publication of its Evidence in Context series, provision of scientific expertise as needed by Consortium researchers, clinicians, policy-makers and other stakeholders and development of evidence reviews to inform the Consortium research priorities. A new and prominent activity of the Evidence Core that has recently concluded includes the provision of an evidence synthesis on cannabis riskbenefit to the FDA to support the agency’s recommendations for either maintaining or revising the current Schedule I classification for cannabis.
Evidence in Context Series
The evidence base for medical cannabis and cannabinoids continues to evolve rapidly while researchers, healthcare providers, and patient communities remain in need of clear translation of study findings to future or current implications for clinical practice. The “Evidence in Context” series addresses these needs for rapid distillation and appraisal in the form of brief, plain-language commentaries. These articles are available in the scientific journal Medical Cannabis and Cannabinoids and on the Consortium websites and both of these publication platforms are fully accessible to all members of the public, as the journal uses an open-access publishing format. To date, the journal has published seven articles within this series accessible at mmjoutcomes.org/evidence/evidence-in-context/ and linked to in the Bibliography section. No such resource was previously available specifically for MMJ.
2024 ANNUAL REPORT | 39
A summary of the citations of evidence in context publications is listed below.
Evidence Synthesis for Food and Drug Administration (FDA)
In January 2023, members of Consortium leadership received funding from the United States Food and Drug Administration (FDA) for a project titled, “Medical Literature and Data on Cannabis Use.” The purpose of this project was to synthesize the literature to support a comprehensive assessment of the clinical risk-benefit of cannabis.
In December 2022, an administrative order was put forward by President Biden to the Secretary of Health and Human Services and to the Attorney General to trigger the review of marijuana’s current Schedule I classification status. This triggered review responsibilities within both the FDA and the Drug Enforcement Agency (DEA), where the FDA was responsible for contributing to an ‘8 Factor Analysis’ (8FA) for the Scheduling status review of cannabis. The 8FA requires consideration of eight ‘factors’ (contributors to risk or benefit), as outlined within the Controlled Substance Act, to justify classification of any substance. The results from the project led by Consortium researchers were to be used by the FDA as supporting evidence as they drafted their recommendations for either maintaining or revising the current Schedule I classification for cannabis. The FDA’s recommendation, to revise the classification status of cannabis to Schedule III, was published as a letter from the Department of Health and Human Services in January 2024.
The Consortium research team accomplished the assessment of clinical risk-benefit through the conduct of large-scale systematic literature reviews and meta-analyses. Project 1 examined a series of harms outcomes, also known as adverse events, as described in studies that examined cannabis use for medical, nonmedical, and unknown purposes. Project 2 examined the effectiveness of cannabis for the following therapeutic indications: Pain, PTSD, Anxiety Disorders, Epilepsy, Nausea, Anorexia, HIV/AIDS, and Crohn’s Disease.
40 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
EVIDENCE IN CONTEXT Title Year Usage* Climbing the Evidence Pyramid: Dosing Considerations for Medical Cannabis in the Management of Chronic Pain 2023 678 Cannabis Effects on Driving Performance: Clinical Considerations 2023 2,486 Clinical Considerations for Cannabis Use and Cardiovascular Health 2022 1411 Will Cannabis or Cannabinoids Protect You from SARS-CoV-2 Infection or Treat COVID-19? 2022 12,585 Much Ado about Dosing: The Needs and Challenges of Defining a Standardized Cannabis Unit 2021 5,654 Perinatal Cannabis Exposures and Autism Spectrum Disorders 2021 3,561 Evidence in Context: High Risk of Bias in Medical Cannabis and Cannabinoid Clinical Trials Dictates the Need for Cautious Interpretation 2021 3,574 Introducing Commentary Series: Evidence in Context 2021 1,382 *Combination of article views and downloads.
The team consisted of members from the Consortium at the University of Florida, the Harvard Pilgrim-based Sentinel Initiative, and the FDA. The Consortium will be acknowledged during reports to federal lawmakers and Consortium leaders are strongly represented in this high-profile project, as Amie Goodin served as Principal Investigator, Almut Winterstein and Yan Wang as co-Investigators, and Bob Cook as a Subject Matter Expert. This significant effort, which required a rapid turnaround time was also joined by 9 UF graduate students, 3 research interns, and several ad hoc UF pharmacy students, in addition to UF Health Sciences Specialist Librarian Lauren Adkins.
Collaboration with Office of Medical Marijuana Use (OMMU) at Florida Department of Health (FL DoH): Consumer/Patient and Clinician Informational Resources
The Consortium is building a strong collaboration with OMMU (FL DoH), which focuses on the development of consumer patient and clinician information resources on the effectiveness and safety of MMJ and specific conditions. The first focus is on the conditions approved for MMJ use in Florida and informational sheets in lay language will be made available to consumers explaining the evidence currently on safety and effectiveness or lack of it, as well as potential risks. This information will be made available online and DoH will work with the Florida Medical Association (FMA) to disseminate this information further to providers and patients.
2024 ANNUAL REPORT | 41


42 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Draft Patient and Consumer Informational Resources
OUTREACH
The Consortium’s outreach activities are directed towards community members, providers, researchers, and industry, to maximize participation in research and keep these stakeholders abreast of the latest research findings. The Consortium’s outreach platform includes a website, a quarterly newsletter, the Researcher Spotlight video series, and the annual Cannabis Clinical Outcomes Research Conference (CCORC).
Newsletter
As part of the communication and outreach plan, the Consortium distributes a quarterly newsletter via email to researchers, physicians/providers, and individuals interested in MMJ research. The newsletter includes updates on Consortium activities and ongoing research. For recognition, the Consortium branded the newsletter as MEDICAMENT, which stands for MMJ rEsearch NewsleTter. A total of 1204 individuals have signed up for the newsletter as of December 2023.
The Consortium has published 17 issues of MEDICAMENT, one every quarter, available at mmjoutcomes.org/newsletter
Website
The Consortium continues to expand its website (mmjoutcomes.org) to disseminate information to researchers, member institutions, physicians/ providers, patients, and the public. The website provides a comprehensive and interactive hub for the grants program, research updates and Consortium news. The Consortium has optimized the website to be fully mobileresponsive. Further improvements continue to increase website performance across all devices.

2024 ANNUAL REPORT | 43
Researcher Spotlight Series
The Consortium initiated the Researcher Spotlight Series, a video series showcasing Consortium researchers. The spotlight series intends to disseminate Consortium research and raise awareness about Consortium work and the importance of the scientific process in evaluating MMJ outcomes.
Each video includes an overview of the researcher’s study, methodological approaches, clinical relevance, and current/expected results. The first two spotlight videos featuring Dr. John Markowitz and Dr. Paul Borsa, two Consortium funded researchers, are available online at mmjoutcomes.org/researcher-spotlight/


Cannabis Clinical Outcomes Research Conference (CCORC)
The Consortium for MMJ Clinical Outcomes Research launched its annual Cannabis Clinical Outcomes Research Conference (CCORC) in 2021. This research-centric meeting, open to patients and providers, offers a venue for sharing research findings, disseminating the latest evidence on the health impacts of marijuana, and catalyzing research collaborations both state-wide and nationally. Specific objectives of CCORC are:
• Dissemination of research findings on MMJ use, efficacy, and safety
• Provide a venue for clinical and research educational opportunities related to MMJ
• Foster research collaboration, and stakeholder engagement, between Consortium member institutions and beyond
Adapting to conditions created by COVID-19, the Consortium held its inaugural CCORC conference virtually on April 8th and 9th, 2021. CCORC 2022 and 2023 were held using a hybrid attendance model; the conference was held in person at UF’s satellite campus in Lake Nona, FL with select presentations available to stream online.
The Consortium is planning its fourth Cannabis Clinical Outcomes Research Conference (CCORC) to be held on May 30th and 31st, 2024 in Orlando, Florida. A scientific program committee with participation from consortium member institutions advises the organizing committee on conference themes and scientific content. The CCORC save-the-date announcements and call for abstracts have been widely disseminated.

44 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
SUMMARY OF CONSORTIUM RESEARCH PRODUCTIVITY
Over the 4.5 years of its existence, Consortium core faculty and Consortium grant awardees have generated 57 published manuscripts in peer-reviewed journals and one book chapter. New data generated through Consortium funding has served as the basis for 2 patents and 34 new extramural grant applications, ten of which were awarded. Noteworthy, more than 93 new trainees including 22 from underrepresented minorities have been involved in the funded research grants and two new courses have been developed, one of which is SUS approved, supporting the development of MMJ research capacity in the state.
2
58
conference abstracts
21
invited talks and media interviews
57
peer reviewed publications book chapter
1
New college course developed SUS-approved
Outputs of the Consortium Research Program
patents filed
93
undergraduates, post-doctoral, fellows & sta trained in research (22 from URM)
34
extramural grants submitted; 10 funded
2
INDs obtained
Dynamic data visualization tool and linkable database created
2024 ANNUAL REPORT | 45
BIBLIOGRAPHY — PEER-REVIEWED PUBLICATIONS
1. Algarin AB, Plazarte GN, Sovich KR, Seeger SD, Li Y, Cohen RA, Striley CW, Goldberger BA, Wang Y, Somboonwit C, Ibañez GE, Spencer EC, Cook RL. Marijuana Use and Health Outcomes in Persons Living With HIV: Protocol for the Marijuana Associated Planning and Long-term Effects (MAPLE) Longitudinal Cohort Study. JMIR Res Protoc. 2022 Aug 30;11(8):e37153. doi: 10.2196/37153 . PMID: 36040775; PMCID: PMC9472048.
2. Alipour Haris G, Sarayani A, Winterstein AG. Letter by Alipour Haris et al Regarding Article, “Marijuana Use Among Young Adults (18-44 Years of Age) and Risk of Stroke: A Behavioral Risk Factor Surveillance System Survey Analysis”. Stroke. 2020 May;51(5): e91. doi: 10.1161/STROKEAHA.120.029273 . Epub 2020 Apr 7. PMID: 32252600.
3. Bilbrey JA, Ortiz YT, Felix JS, McMahon LR, Wilkerson JL. Evaluation of the terpenes β-caryophyllene, α-terpineol, and γ-terpinene in the mouse chronic constriction injury model of neuropathic pain: possible cannabinoid receptor involvement. Psychopharmacology (Berl). 2022 May;239(5):1475-1486. doi: 10.1007/s00213-02106031-2 . Epub 2021 Nov 30. PMID: 34846548.
4. Blanton HL, Barnes RC, McHann MC, Bilbrey JA, Wilkerson JL, Guindon J. Sex differences and the endocannabinoid system in pain. Pharmacol Biochem Behav. 2021 Mar; 202:173107. doi: 10.1016/j.pbb.2021.173107 . Epub 2021 Jan 12. PMID: 33444598; PMCID: PMC8216879.
5. Brown JD, Costales B, van Boemmel-Wegmann S, Goodin AJ, Segal R, Winterstein AG. Characteristics of Older Adults Who Were Early Adopters of Medical Cannabis in the Florida MMJ Use Registry. J Clin Med. 2020 Apr 18;9(4):1166. doi: 10.3390/jcm9041166 PMID: 32325769; PMCID: PMC7230351
6. Brown JD, Goodin AJ. High risk of bias in medical cannabis and cannabinoid clinical trials dictates the need for cautious interpretation. Med Cannabis Cannabinoids. 2021; 4:63-66. PMID: 34676351 PMCID: PMC8525149 doi: 10.1159/000514732
7. Brown JD, Goodin AJ. The prevalence of drivers under the influence of medical cannabis must be considered within proper context. Res Social Adm Pharm. 2019 Nov;15(11):1372-1373. doi: 10.1016/j.sapharm.2019.01.015 Epub 2019 Jan 28. PMID: 30709730.
8. Brown JD, Goodin AJ. Will cannabis or cannabinoids protect from SARS-CoV-2 infection or treat COVID-19? Med Cannabis Cannabinoids 2022;5(1):1-4. https://doi. org/10.1159/000522472 PMID: 35702401 PMCID: PMC9149510
9. Brown JD, Rivera KJ, Crespo-Hernandez LY, Doenges MR, Auchey I, Pharm T and AJ Goodin. Natural and Synthetic Cannabinoids: Pharmacology, Uses, and Adverse Drug Events. J Clin Pharmacol. 2021; Aug; 61 Suppl 2:S37-S52. https://doi.org/10.1002/ jcph.1871 . PMID: 34396558.
10. Brown JD, Winterstein AG. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J Clin Med. 2019 Jul 8;8(7):989. doi: 10.3390/jcm8070989 PMID: 31288397; PMCID: PMC6678684
11. Brown JD. Potential Adverse Drug Events with Tetrahydrocannabinol (THC) Due to DrugDrug Interactions. J Clin Med. 2020 Mar 27; 9(4):919. doi: 10.3390/jcm9040919 . PMID: 32230864; PMCID: PMC7231229.
12. Costales B, Babalonis S, Brown JD, Goodin, AJ. Cannabis effects on driving performance: clinical considerations. Med Cannabis Cannabinoids. 2023; 6:8-14. doi: https://doi.org/10.1159/000528714
46 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
13. Costales B, van Boemmel-Wegmann S, Winterstein A, Segal R. Clinical Conditions and Prescription Drug Utilization among Early Medical Marijuana Registrants in Florida. J Psychoactive Drugs. 2021 Jul-Aug;53(3):185-194. doi: 10.1080/02791072.2020.1864069 Epub 2021 Jan 4. PMID: 33393877.
14. Boullosa CC, Vaddiparti K, Lopez-Quintero C, Varma DS, Cook RL. Clinical Decision Making by Medical Marijuana Physicians in Florida: A Qualitative Assessment. Subst Use Misuse. 2022;57(14):2042-2052. doi: 0.1080/10826084.2022.2129995 . Epub 2022 Oct 28. PMID: 36305815.
15. Eeswara A, Pacheco-Spiewak A, Jergova S, Sagen J. Combined non-psychoactive Cannabis components cannabidiol and β-caryophyllene reduce chronic pain via CB1 interaction in a rat spinal cord injury model. PLoS One. 2023 Mar 13;18(3):e0282920. doi: 10.1371/journal.pone.0282920 . PMID: 36913400; PMCID: PMC10010563.
16. Goodin AJ, Wilson DL, Cook RL, Wang Y, Brown J, Winterstein AG: Proceedings of the 2021 Cannabis Clinical Outcomes Research Conference. Med Cannabis Cannabinoids 2021. Sep 10;4(2):143-146 https://doi.org/10.1159/000519037 PMID: 35224433; PMCID: PMC8832197.
17. Goodin AJ, Crum J, Fadool DA, Konin JG, Paikoff S, Sachdeva MS, Singla D, Stiffin R, Vidot DC, Villalba K, Jyot J, Winterstein AG. Abstracts for the 2023 Cannabis Clinical Outcomes Research Conference (CCORC), on behalf of the Scientific Program Committee. Med Cannabis Cannabinoids 20 December 2023; 6 (1): 102–124. https:// doi.org/10.1159/000534044
18. Goodin AJ, Tran PT, McKee S, Sajdeya R, Jyot J, Cook RL, Wang Y, Winterstein AG Proceedings of the 2023 Cannabis Clinical Outcomes Research Conference. Med Cannabis Cannabinoids. 2023 Sep 29;6(1):97-101. doi: 10.1159/000533943 . PMID: 37900895; PMCID: PMC10601943.
19. Goodin AJ, Winterstein AG, Cook R, Wang Y, Brown, JD. Introducing Commentary Series: “Evidence in Context.” Med Cannabis Cannabinoids 2021 Feb 23;4(1):61-62 doi: 10.1159/000512684 PMID: 34676350; PMCID: PMC8525212.
20. Huffstetler CM, Cochran B, May CA, Maykut N, Silver CR, Cedeno C, Franck E, Cox A, Fadool DA. Single cannabidiol administration affects anxiety-, obsessive compulsive-, object memory-, and attention-like behaviors in mice in a sex and concentration dependent manner. Pharmacol Biochem Behav. 2023 Jan; 222:173498. doi: 10.1016/j. pbb.2022.173498 . Epub 2022 Nov 29. PMID: 36455670.
21. Jean-Jacques J, Cook R, Winterstein AG, Goodin A, Brown JD, Jugl S, Wang Y. Priorities for Medical Marijuana Research from the Perspective of Physicians, Dispensary Owners/ Staff, and Patients: A Survey Study. Med Cannabis Cannabinoids. 2021 Aug 2;4(2):107113. doi: 10.1159/000518105 . PMID: 35224430; PMCID: PMC8832249.
22. Jugl S, Goodin AJ, Brown JD. Climbing the Evidence Pyramid: Dosing Considerations for Medical Cannabis in the Management of Chronic Pain. Med Cannabis Cannabinoids. 2023 Apr 26;6(1):41-45. doi: 10.1159/000530251. PMCID: PMC10134049
23. Jugl S, Okpeku A, Costales B, Morris E, J, Alipour-Haris G, Hincapie-Castillo J, M, Stetten NE, Sajdeya R, Keshwani S, Joseph V, Zhang Y, Shen Y, Adkins L, Winterstein AG, Goodin A. A Mapping Literature Review of Medical Cannabis Clinical Outcomes and Quality of Evidence in Approved Conditions in the USA from 2016 to 2019. Med Cannabis Cannabinoids 2021; 4:21-42. doi: 10.1159/000515069 PMID: 34676348; PMCID: PMC8525213.
24. Jugl S, Sajdeya R, Morris EJ, Goodin AJ, Brown JD. Much Ado about Dosing: The Needs and Challenges of Defining a Standardized Cannabis Unit. Med Cannabis Cannabinoids 2021 Jun 17;4(2):121-124. doi: 10.1159/000517154 PMID: 35224432; PMCID: PMC8832202
2024 ANNUAL REPORT | 47
25. Kalvala AK, Bagde A, Arthur P, Kulkarni T, Bhattacharya S, Surapaneni S, Patel NK, Nimma R, Gebeyehu A, Kommineni N, Meckes DG Jr, Sun L, Banjara B, Mosley-Kellum K, Dinh TC, Singh M. Cannabidiol-Loaded Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Paclitaxel-Induced Peripheral Neuropathy. Pharmaceutics. 2023 Feb 7;15(2):554. doi: 10.3390/pharmaceutics15020554 Erratum in: Pharmaceutics. 2023 Aug 25;15(9): PMID: 36839877; PMCID: PMC9964872.
26. Kalvala AK, Nimma R, Bagde A, Surapaneni SK, Patel N, Arthur P, Sun L, Singh R, Kommineni N, Nathani A, Li Y, Singh M. The role of Cannabidiol and tetrahydrocannabivarin to overcome doxorubicin resistance in MDA-MB-231 xenografts in athymic nude mice. Biochimie. 2022 Dec 17;208:19-30. doi: 10.1016/j. biochi.2022.12.008 Epub ahead of print. PMID: 36535544.
27. Kinnunen K, Robayo LE, Cherup NP, Frank SI, Widerström-Noga E. A preliminary study evaluating self-reported effects of cannabis and cannabinoids on neuropathic pain and pain medication use in people with spinal cord injury. Front Pain Res (Lausanne). 2023 Dec 21;4:1297223. doi: 10.3389/fpain.2023.1297223. PMID: 38188193; PMCID: PMC10767995.
28. Kumar Kalvala A, Bagde A, Arthur P, Kumar Surapaneni S, Ramesh N, Nathani A, Singh M. Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-induced neuropathic pain in rodents. Int Immunopharmacol. 2022 Jun; 107:108693. doi: 10.1016/j. intimp.2022.108693 . Epub 2022 Mar 15. PMID: 35303507.
29. Lorenzo, E, McClean, C, Ford, JA. Young Adult Medical Cannabis Patients in Florida: Gender Differences in Characteristics of use, Experiences at Dispensaries, and Association With Health-Related Problems. J Drug Issues, 2023. 0(0). https://doi. org/10.1177/00220426231189486
30. Marini S, Huber A, Cash MN, Salemi M, Cook RL, Borsa P, Mavian CN. Oral Cannabidiol Treatment Is Associated with an Anti-Inflammatory Gene Expression Signature in Myeloid Cells of People Living with HIV. Cannabis Cannabinoid Res. 2024 Jan 22. doi: 10.1089/can.2023.0139 . Epub ahead of print. PMID: 38252549.
31. Markowitz JS, De Faria L, Zhang Q, Melchert PW, Frye RF, Klee BO, Qian Y. The Influence of Cannabidiol on the Pharmacokinetics of Methylphenidate in Healthy Subjects. Med Cannabis Cannabinoids. 2022 Nov 4;5(1):199-206. doi: 10.1159/000527189 . PMID: 36467779; PMCID: PMC9710314.
32. McMahon AN, Varma DS, Fechtel H, Sibille K, Li Z, Cook RL, Wang Y. Perceived effectiveness of medical cannabis among adults with chronic pain: Findings from interview data in a three-month pilot study. Cannabis. 2023 Jul 5;6(2):62-75. doi: 10.26828/cannabis/2023/000149 . PMID: 37484052.
33. Ortiz, YT, McMahon, LR, & Wilkerson, JL Medicinal Cannabis and Central Nervous System Disorders. Front Pharmacol. 2022 Apr 21;13:881810. https://doi.org/10.3389/ fphar.2022.881810 PMID: 35529444; PMCID: PMC9070567.
34. Parisi CE, Wang Y, Varma DS, Vaddiparti K, Ibañez GE, Cruz Carrillo L, Cook RL. Changes in frequency of cannabis use among people with HIV during the COVID-19 pandemic: a multi-methods study to explore the underlying reasons for change. Am J Drug Alcohol Abuse. 2023 Jul 4;49(4):470-480. doi: 10.1080/00952990.2023.2176234 . Epub 2023 Mar 10. PMID: 36898081.
35. Patel N, Kommineni N, Surapaneni SK, Kalvala A, Yaun X, Gebeyehu A, Arthur P, Duke LC, York SB, Bagde A, Meckes DG Jr, Singh M. Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in vitro and in vivo models. Int J Pharm. 2021 Sep 25; 607:120943. doi: 10.1016/j.ijpharm.2021.120943 . Epub 2021 Jul 27. PMID: 34324983; PMCID: PMC8528640.
48 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
36. Rosenthal M., Pipitone N. Demographics, Perceptions, and Use of MMJ among Patients in Florida. Med Cannabis Cannabinoids 2021;.4:13-20. doi: 10.1159/000512342 PMID: 34676347; PMCID: PMC8525215
37. Roussos-Ross K, Dukharan V, Goodin A. Is In-Utero Marijuana Exposure Associated with Childhood Developmental Delay? [20K], Obstetrics & Gynecology: 135(5):p 118S-119S, May 2020. doi: 10.1097/01.AOG.0000664476.72398.7c
38. Sagen J, Konin J, Paikoff S, Ford J, Newman D, Jyot J, Goodin AJ. Abstracts of the 2022 Cannabis Clinical Outcomes Research Conference (CCORC), on behalf of the Scientific Program Committee. Med Cannabis Cannabinoids 2022; 5:142-158. https://doi. org/10.1159/000527081
39. Sajdeya R, Brown JD, Goodin AJ: Perinatal Cannabis Exposures and Autism Spectrum Disorders. Med Cannabis Cannabinoids 2021, 4:67-71. doi: 10.1159/000515871 PMID: 34676352; PMCID: PMC8525188.
40. Sajdeya R, Cook RL. Need to Improve Dose Measurements in Studies of Marijuana Use for Pain. J Acquir Immune Defic Syndr. 2020 Mar 1; 83(3):e23. doi: 10.1097/ QAI.0000000000002238 PMID: 32032280.
41. Sajdeya R, Fechtel HJ, Spandau G, Goodin AJ, Brown JD, Jugl S, Smolinski NE, Winterstein AG, Cook RL, Wang Y. Protocol of a Combined Cohort and CrossSectional Study of Persons Receiving Medical Cannabis in Florida, USA: The Medical Marijuana and Me (M3) Study. Med Cannabis Cannabinoids. 2023 May 9;6(1):46-57. doi: 10.1159/000530052 . PMID: 37261066; PMCID: PMC10228286.
42. Sajdeya R, Goodin AJ, Tighe PJ. Cannabis use assessment and documentation in healthcare: Priorities for closing the gap. Prev Med. Dec; 153:106798. doi: 10.1016/j. ypmed.2021.106798 Epub 2021 Sep 8 PMID: 34506820.
43. Sajdeya R, Joseph V, Stetten N, Ibañez G, Wang Y, Powell L, Somboonwit C, Corsi K, Cook R. Reasons for Marijuana Use and Its Perceived Effectiveness in Therapeutic and Recreational Marijuana Users Among People Living with HIV in Florida. Cannabis. 2021, Volume 4 (1). doi: 10.26828/cannabis/2021.01.002
44. Sajdeya R, Jugl S, Cook RL, Brown JD, Goodin AJ. Clinical considerations for cannabis use and cardiovascular health. Med Cannabis Cannabinoids 2022; Sept 28;5(1):120-127. https://doi.org/10.1159/000526731 PMID: 36467784; PMCID: PMC9710318.
45. Sajdeya R, Mardini MT, Tighe PJ, Ison RL, Bai C, Jugl S Hanzhi G, Zandbiglari K, Adiba FI, Winterstein AG, Pearson TA. Cook RL, Rouhizadeh M. Developing and validating a natural language processing algorithm to extract preoperative cannabis use status documentation from unstructured narrative clinical notes. J Am Med Inform Assoc 2023 Jul 19;30(8):1418-1428. doi: 10.1093/jamia/ocad080 . PMID: 37178155; PMCID: PMC10354766.
46. Sajdeya R, Shavers A, Jean-Jacques J, Costales B, Jugl S, Crump C, Wang Y, Manfio L, Pipitone RN, Rosenthal MS, Winterstein AG, Cook RL. Practice Patterns and Training Needs Among Physicians Certifying Patients for MMJ in Florida. J Prim Care Community Health. 2021 Jan-Dec; 12: 21501327211042790. doi: 10.1177/21501327211042790 PMID: 34452585; PMCID: PMC8404623.
47. Smolinksi N, Sajdeya R, Cook RL, Wang Y, Winterstein AG and AJ Goodin. Proceedings of the 2022 Cannabis Clinical Outcomes Research Conference. Med Cannabis Cannabinoids 2022; 5:138-141. https://doi.org/10.1159/000527080 PMID: 36467782; PMCID: PMC9710315.
48. Sturaro C, Fakhoury B, Targowska-Duda KM, Zribi G, Schoch J, Ruzza C, Calò G, Toll L, Cippitelli A. Preclinical effects of cannabidiol in an experimental model of migraine. Pain. 2023 Nov 1;164(11):2540-2552. doi: 10.1097/j.pain.0000000000002960 . Epub 2023 Jun 9. PMID: 37310430.
2024 ANNUAL REPORT | 49
49. Surapaneni SK, Patel N, Sun L, Kommineni N, Kalvala AK, Gebeyehu A, Arthur P, Duke LC, Nimma R, G Meckes D Jr, Singh M. Anticancer and chemosensitization effects of cannabidiol in 2D and 3D cultures of TNBC: involvement of GADD45α, integrin-α5, -β5, -β1, and autophagy. Drug Deliv Transl Res. 2022 Nov;12(11):2762-2777. doi: 10.1007/ s13346-022-01137-2 . Epub 2022 Feb 25. PMID: 35217991; PMCID: PMC9811521.
50. Vaddiparti K, Liu Y, Bottari S, Boullosa CC, Zhou Z, Wang Y, Williamson J, Cook RL
Improved Post-Traumatic Stress Disorder Symptoms and Related Sleep Disturbances after Initiation of Medical Marijuana Use: Evidence from a Prospective Single Arm Pilot Study. Med Cannabis Cannabinoids. 2023 Nov 13;6(1):160-169. doi: 10.1159/000534710 . PMID: 37965569; PMCID: PMC10642978.
51. Vaddiparti K, Liu Y, Bottari S, Crump C, Zhou Z, Wang Y, Willaimson J, Cook RL
Improved posttraumatic stress disorder symptoms and related sleep disturbances after initiation of medical marijuana use: Evidence from a prospective single arm pilot study. Med Cannabis Cannabinoids. 2023 Nov 13;6(1):160-169. doi: 10.1159/000534710 PMID: 37965569
52. Wang Y, Jean Jacques J, Li Z, Sibille KT, Cook RL. Health Outcomes among Adults
Initiating Medical Cannabis for Chronic Pain: A 3-month Prospective Study Incorporating Ecological Momentary Assessment (EMA). Cannabis. 2021 Oct;4(2):69-83. doi: 10.26828/cannabis/2021.02.006 PMID: 34671723; PMCID: PMC8525881
53. Wang Y, Robinson KR, Fechtel HJ, Hartog A. Medical cannabis use and its impact on health among older adults: Recent research findings and future directions. Current Addiction Reports. 2023. https://link.springer.com/article/10.1007/s40429-02300519-x
54. Wilkerson JL, Alberti LB, Thakur GA, Makriyannis A, Milligan ED. Peripherally administered cannabinoid receptor 2 (CB2R) agonists lose anti-allodynic effects in TRPV1 knockout mice, while intrathecal administration leads to anti-allodynia and reduced GFAP, CCL2 and TRPV1 expression in the dorsal spinal cord and DRG. Brain Res. 2022 Jan 1; 1774:147721. doi: 10.1016/j.brainres.2021.147721 . Epub 2021 Nov 10. PMID: 34774500.
55. Wilkerson JL, Bilbrey JA, Felix JS, Makriyannis A, McMahon LR. Untapped endocannabinoid pharmacological targets: Pipe dream or pipeline? Pharmacol Biochem Behav. 2021 Jul; 206:173192. doi: 10.1016/j.pbb.2021.173192 . Epub 2021 Apr 29. PMID: 33932409.
56. Wilkerson, JL, Alberti, LB, Thakur, GA, Makriyannis, A, Milligan, ED. Peripherally administered cannabinoid receptor 2 (CB2R) agonists lose anti-allodynic effects in TRPV1 knockout mice, while intrathecal administration leads to anti-allodynia and reduced GFAP, CCL2 and TRPV1 expression in the dorsal spinal cord and DRG. Brain Res. 2022 Jan 1; 1774:147721. doi: 0.1016/j.brainres.2021.147721 Epub 2021 Nov 10. PMID: 34774500.
57. Zhang, Q, Melchert, PW, Markowitz, JS. Pharmacokinetic Variability of Oral Cannabidiol and Its Major Metabolites after Short-Term High-Dose Exposure in Healthy Subjects. Med Cannabis Cannabinoids 2024, 7 (1): 1–9. https://doi.org/10.1159/000535726
50 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
EXTRAMURAL GRANTS SUBMITTED BY CONSORTIUM FACULTY AND GRANT AWARDEES
Lead principal investigator, funding agency, title
• Amie Goodin (co-Is: A Winterstein, Y Wang, S Guo), United States Food and Drug Administration via Sentinel Initiative. Medical Literature and Data on Cannabis Use. Funded from January 2023-May 2023 for $328,281.
• Amie Goodin (MPIs: D Roussos-Ross, D Varma, B Goldberger), University of Florida Research Opportunity Seed Fund. Assessing Prevalence, Maternal Perceptions, and Fetal Development Outcomes of Perinatal Marijuana Use. Funded from June 2022-May 2024 for $90,000
• Amie Goodin (sub-PI; Chris Delcher -PI; S Jugl, Co-I), Center for Disease Control (CDC). Beyond Opioids: Policy Evaluation for the Polysubstance Crisis.
• Amie Goodin, NIDA. Medication use trajectories for opioid use disorders among pregnant women and resulting neonatal outcomes
• Andrea Cippitelli (Co-I; PI-Lawrence Toll), Department of Defense (DOD). Investigation into NOP Receptor Agonists for the Treatment of Chronic Migraine. Funded from 06/01/2021-05/31/2024 for $335,000/year
• Andrea Cippitelli, (NIH/NINDS). The involvement of GPR37L1 on a chronic migrainelike state.
• Debra Fadool and Mandip Sachdeva, James and Esther King Foundation. Role of Exosomal Formulations of CBD for Treatment of Cancer-related Peripheral Neuropathy
• Debra Fadool, Council on Research and Creativity (CRC). Does cannabidiol (CBD) really lessen anxiety behavior and is it safe to use during pregnancy, Funded June 2022June 2023 for $25,000
• Debra Fadool, HEERF III (Federal Government). Higher Education Emergency Relief Fund, Funded from Sept 2021-Sept 2022 for $42,091
• Douglas Storace, NIDCD. Defining the role(s) of the olfactory bulb in adaptation
• Douglas Storace, NIDCD. Defining the role(s) of the olfactory bulb in adaptation
• Hassan Azari, DOD. Cannabinoids Acidic for the treatment of Glioblastoma
• Hassan Azari, NIH. Acidic cannabinoids for the treatment of high-grade glioma
• Hassan Azari, The Florida Center for Brain Tumor research (FCBTR). Effects of cannabigerolic acid containing hemp NPs on glioma tumor progression
• Jacqueline Sagen (Co-Is: Stanislava Jergova, Mandip Sachdeva), Department of Defense SCIRP. Harnessing naturally-derived cannabinoid potential in development of neuropathic SCI pain treatments.
• Jacqueline Sagen, NCCIH. Combination analgesic evaluation of minor cannabinoids and terpenes with exercise in chronic spinal cord injury pain
• Jenny Wilkerson, NCCIH. Terpenes and minor cannabinoids as novel analgesics
• Jenny Wilkerson, NIDA. Terpenes and minor cannabinoids as novel analgesics
• Joshua Brown, NIH, Cannabis use and adverse drug events in older adults
• Lori Knackstedt (MPI; Setlow-MPI; Schwendt co-I), NIH/NIDA. Risks and benefits of cannabis-opioid co-use
• Mandip Sachdeva, DDRFA Foundation. Exosomal Delivery of Cannabinoids for the Treatment of DMG/DIPG
• Mandip Sachdeva, James and Esther King Foundation. Role of Exosomal Formulations of CDB for Treatment of Cancer-related Peripheral Neuropathy
• Mandip Sachdeva, NIH. Preclinical evaluation of minor cannabinoids in chemotherapy induced peripheral Neuropathy
2024 ANNUAL REPORT | 51
• Mandip Sachdeva, NIH. Role of Exosomal Formulations of CDB for Treatment of Cancer-related Peripheral Neuropathy, Funded April 2023-March 2027 for $540,000
• Mandip Sachdeva, NIH. Tetrahydrocannabivarin (THCV) and cannabidiol (CBD) combination treatment for paclitaxel induced neuropathy.
• Mariola Edelmann, NIH. The regulatory functions of the endocannabinoid system in the innate immune responses against Gram-negative pathogens
• Nicole Ennis, Sherrilene Classen, NIDA. MMJ Use and Driving Performance: A Test of Psychomotor Functioning in Adults 50 and Older, Funded for $205,662 from 09/15/2020 – 09/14/2022.
• Paul Borsa (Co-Is-Mark Bishop, Abhisheak Sharma, Arkaprava Roy), NCCIH. CBD treatment for musculoskeletal pain.
• Rodrigo Cristofoletti (Co-PI; Asley Brown PI), Emulate. Using Organ-On-Chips to assess the impact of cannabinoids on the SARS-CoV-2 infection in the digestive system
• Rodrigo Cristofoletti (PI), NIDA. Transporter-mediated interactions at the blood-brain barrier: can opioids and cannabinoids affect brain penetration of antiretroviral drugs?
• Simone Marini (Contact PI; MPIs Wang and Sibille), NIH/NIA. Medical Marijuana and chronic musculoskeletal pain in older adults: a multi-level precision medicine analysis
• Yan Wang, (MPIs: Jennifer Hu), NIH/NCI. Assessing benefits and harms of medical cannabis and cannabinoid use in breast cancer patients during and after treatments. Funded 09/2024-08/2028 for $3,264,626
• Yan Wang, NIA. Real-Time and Long-Term Effects of MMJ on Older Adults: A Prospective Cohort Study. Funded from 2/15/2022-11/30/2026 for $2,940,426
• Yan Wang, NIH/NIA. R01 AG071729-01A1S1 Administrative Supplement, Real-time and long-term effects of MMJ on older adults: A prospective cohort study, Funded 02/2022-11/2026 for $375,000
MEDIA COVERAGE
• Dr. Jenny Wilkerson, How Cannabis-Based Therapeutics Could Help Fight COVID Inflammation, Medscape, www.medscape.com/viewarticle/940265
• Dr. Joshua Brown, Can weed protect you from COVID? (slate.com)
• Dr. Joshua Brown, Are THC and CBD actually safe for everyone? Renee Consorte, July 27, 2021. Medium, perma.cc/LCD7-PAHA
• Dr. Almut Winterstein, UF From Florida podcast, What is Florida’s medical marijuana research consortium studying – and what has it found so far?
Book Chapter: Muschett M, Brown JD. Profile of Older Users of Medical Cannabis and Cannabinoids. In: Martin, Patel, & Preedy (editors). Medicinal Usage of Cannabis and Cannabinoids. 1st ed. Elsevier. 2022 (In press).
PATENTS AND IND s
• Hasan Azari, Patent filed: “Cannabinoid containing plant derived extracellular vesicles and therapeutic methods using the same…”
• Mandip Sachdeva, Patent filed: Cannabinoid composition and method of treating diabetic and chemotherapy induced neuropathic pain.
• Paul Borsa, IND approved for investigational product manufactured and processed through a Florida-based CBD company (SunFlora, Inc, St. Petersburg, FL)
• Walter Steigleman, IND 167819 approved for a full spectrum CBD product IND with less than 0.3% THC from SunFlora.
52 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
CONSORTIUM RESEARCH PLAN
2024-2025
Since its inception in July 2019, the Consortium has made great strides towards facilitating and conducting research that informs clinical care and policy about the medical use of marijuana. In the coming year, the Consortium will continue these efforts within the five original Consortium research program pillars: the Grants program, MEMORY, the Clinical Core, the Evidence Core, and outreach activities. The specific goals and plans for each pillar have been updated and are described below.
In the coming year, the Consortium will continue its efforts towards facilitating and conducting research that informs clinical care and policy within the five original Consortium research program pillars: the Grants program, MEMORY, the Clinical Core, the Evidence Core, and outreach activities. Two recent developments will play an important role in the coming year: Receipt of MMUR data will allow development of MEMORY, which will accelerate the ability to generate evidence on MMJ clinical outcomes. The new contract with the FDA has allowed a new focus on evidence synthesis to support regulatory decision making. The 2024-25 Consortium research plan, focuses on the following research priorities:
• Generation of conclusive evidence on clinical outcomes of MMJ use including both risk and benefit
• Evaluations of route and dosing, in particular research on high potency THC
• Research on interactions of MMJ with other drugs/medications
• Epidemiologic studies on utilization pattern and accessibility of MMJ across diverse groups
• Research on the effects of MMJ use in reducing opioid dependency
• Evaluating Components of MMJ/Cannabis
The following describes the Consortium Research Plan for fiscal year 2025.
GRANTS PROGRAM
The Consortium grant funding program was restructured in 2022 to support two studies at $130,000 over a total of two years, where the second-year funding is committed if intermediate research goals for the first year have been accomplished and adequate funding is available. An additional fund for one-year projects as offered previously has been retained. We will continue with these funding structure changes in the coming year.
The Consortium will also continue with the Consortium Board revised research priorities to emphasize the focus on clinical research, the impact of MMJ on pain management and opioid use, and an expanded scope on epidemiologic research.
2024 ANNUAL REPORT | 53
Consortium Research Priorities 2024
1. Clinical Outcomes of MMJ Use: with particular emphasis on human subject research, with priority granted to studies investigating efficacy and safety, for the treatment of qualifying conditions listed for MMJ use in Florida (knowthefactsmmj.com/patients/)
2. Effect of MMJ Use in Reducing Opioid Dependence: human subjects research on the effectiveness of MMJ as an analgesic/adjuvant in pain management and reduction in opioid use.
3. Routes of Administration: effect of dosing and routes of MMJ use on efficacy and safety; of particular interest are studies that evaluate effects of smoking and vaping.
4. Interactions of MMJ with Other Drugs/Medications:
a. with particular focus on medications that are commonly used by patients who seek MMJ treatment
b. impact of polysubstance, including interactions with alcohol, tobacco, benzodiazepines, and prescription and nonprescription opioids
5. Epidemiology Research to study trends for cannabis use and cannabis use disorder (CUD), including new products, patterns of use, and reasons for use in different populations, and medical, sociological and/or economic disparities in the access to and outcomes of MMJ across diverse communities.
6. Evaluating Components of MMJ/Cannabis and contrast their clinical outcomes:
a. comparing different components of MMJ (e.g. different terpenes)
b. research on different potency levels of THC products (e.g. >10% vs <10% THC)
c. standards for measuring cannabis dose, intoxication, and impairment
The Consortium launched its sixth grants cycle in October 2023 with the release of its Request for Proposals with the above research priorities. The intent is to complete the application reviews by the end of the fiscal year to expedite funding of prioritized proposals, upon approval of the FY 2025 Consortium budget.
54 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
MEMORY
With the release of the MMUR data, MEMORY development is underway. This includes data curation, linkage of databases and development of analytical cohorts for access by Consortium researchers. As envisioned, MEMORY will then serve to support studies on MMJ effectiveness and safety, and utilization and access. Consortium core faculty plans to have a first set of safety and effectiveness studies completed by the end of the new fiscal year.
CLINICAL CORE
Goals for the Clinical Core include continued expansion of the Consortium infrastructure to support patient recruitment into prospective research studies via its patient contact registry and CARMMA database of collaborating physicians, researchers, and industry. The Consortium is set to finish follow-up of participants in the M3 study by the spring of 2024, finalize data curation for access by Consortium researchers, and complete a first set of analyses and publications over the coming year. The Consortium hopes that established patient recruitment mechanism that have proven effective for M3 can be provided to investigators from all Consortium institutions to accelerate and enhance conduct of clinical outcomes studies. A proven patient recruitment platform is also critical for extramural grant applications (e.g., to NIH) to further expand the consortium’s research program. The Clinical Core will also continue to work on guidance for investigators on regulatory issues involving use of MMJ in research studies. This will include guidance on DEA licensure and other relevant state and federal regulations.
EVIDENCE CORE
In the coming year, the Evidence Core will have completed its evidence review for the FDA to support the 8-factor analysis requested by Congress and begin publications and other dissemination of related findings. The Consortium will also continue to publish its Evidence in context series, and survey and evaluate emerging evidence to inform the Consortium research priorities.
OUTREACH
Building on the success of our first three Cannabis Clinical Outcomes Research Conference (CCORC), the Consortium plans to hold its fourth annual CCORC in May 2024. Other outreach activities through the Consortium website, its quarterly newsletter and participation in scientific conferences will continue. The Researcher Spotlight Series will be developed further to promote Consortium activities and disseminate research outcomes.
The board and Consortium faculty and staff would like to conclude this report by expressing strong continuing support and enthusiasm to advance the Consortium research program. The Consortium addresses an urgent and critical need to provide patients, providers and regulators the necessary evidence on the safe and effective use of MMJ. The medical use of marijuana must be guided by the same level of scientific evidence that is available for prescription medications and MMJ products should be monitored with similar surveillance methods. The Consortium is devoted to establishing both.
2024 ANNUAL REPORT | 55
APPENDICES
APPENDIX A: THE CONSORTIUM FOR MMJ CLINICAL OUTCOMES RESEARCH BOARD

WILLIAM (BILL) ANDERSON, PHD CHAIR OF THE CONSORTIUM BOARD
Associate Vice President of Research; Florida International University
Associate Vice President William (Bill) Anderson leads initiatives that expand FIU’s efforts in research development for faculty, doctoral students and postdoctoral scholars. Additional areas of leadership focus include research labs, core facilities, research integrity, and laboratory safety, among others. Dr. Anderson joined FIU in 2000 as Assistant Professor and has risen to the rank of Professor. Administratively, he has served as Chair of the Department of Earth & Environment and Associate Dean of Faculty in the College of Arts, Sciences & Education where he most recently served as the Vice Dean.
He received a doctorate of Natural Sciences from the Swiss Federal Institute of Technology (ETH-Zentrum), a M.S. in Geology from Syracuse University and a B.A. in Geology from the University of Kansas. His research has been published in top tier journals; he has presented in national and international conferences; and he has received funding from the NSF, the American Chemical Society, and the U.S. Department of the Interior, among others.

MARTHA S. ROSENTHAL, P H D VICE-CHAIR OF THE CONSORTIUM BOARD
Professor of Neuroscience/Physiology; Director of the Cannabis Research, Education, and Workforce initiative; Florida Gulf Coast University
Dr. Martha Rosenthal is a Professor of Neuroscience & Physiology at Florida Gulf Coast University, where she teaches courses in cannabis, drugs and society, neuroscience, human physiology, and human sexuality. Dr. Rosenthal received her bachelor’s degree in biology from the University of Virginia, her master’s degree in neuropharmacology from Brown University, and her PhD in neuroscience from UCLA. She began her career teaching in the College of Pharmacy at the University of Florida, and then moved to Fort Myers to be one of the founding faculty members of FGCU.
Dr. Rosenthal is the Director of the Cannabis Research, Education, and Workforce initiative (CREW) at FGCU, and runs the cannabis professional certificate program. She is the author of a number of textbooks, including Drugs: Mind, Body, and Society. Dr. Rosenthal has been honored to receive the Teacher of the Year award at both the University of Florida and at FGCU and to have presented a TED talk about sex and gender.
56 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH

CHRISTOPHER R. MCCURDY, BSPH, PHD, FAAPS
Associate Dean for Faculty Development
Professor, Departments of Medicinal Chemistry and Pharmaceutics
The Frank A. Duckworth Eminent Scholar Chair
Director, UF CTSI Translational Drug Development Core College of Pharmacy, University of Florida
Dr. McCurdy serves as Associate Dean for Faculty Development and Professor in the Departments of Medicinal Chemistry and Pharmaceutics; and The Frank A. Duckworth Eminent Scholar Chair in Drug Research and Development at the University of Florida College of Pharmacy. He is also the Director of the UF Translational Drug Development Core. Dr. McCurdy completed his training as a pharmacist at Ohio Northern University and moved on to complete his PhD in medicinal chemistry from the University of Georgia, followed by a three-year postdoctoral fellowship at the University of Minnesota. McCurdy began his academic career in 2001 at the University of Mississippi where he rose to the rank of full professor (with tenure). He moved to the University of Florida in January of 2017. He is a broadly trained pharmaceutical scientist, and pharmacist whose research focuses on the design, synthesis, and development of drugs to treat pain, anxiety, and substance use disorders. He is an internationally recognized expert on kratom (Mitragyna speciosa). He also has developed a PET/MR imaging diagnostic agent for visualizing the origins of chronic neuropathic pain. Phase 1 and 2 human clinical trials are currently underway. He has published more than 200 manuscripts and holds 8 patents. He is currently funded by multiple NIH grants and the Florida Department of Health. Dr. McCurdy serves as a consultant to the US Food and Drug Administration’s Drug Safety and Risk Management Advisory Committee and previously served as President of the American Association of Pharmaceutical Scientists (AAPS).

DINENDER K. SINGLA, PHD, FAHA, FIACS, FAPS
Professor of Medicine, Cardiovascular Division Leader, Florida Hospital Chair in Cardiovascular Science; University of Central Florida
Dr. Dinender Singla is a translational scientist. His team investigates the role of stem cells and its derivative exosomes in anti-cancer drug induced cardiac toxicities and diabetes-induced muscle myopathy and cardiomyopathy.
He is continuously serving to review the grants for various NIH, AHA, ministry of Italian health, and Hong Kong study sections. He is an Academic Editor for PLOS one, Associate Editor for the Canadian Journal of Physiology and Pharmacology, as well as an editorial board member for different journals such as American Journal of Physiology: Heart and Circulatory. He has served as a chair for the TPIG committee and the American Physiology Society, and a general secretary for the North American section of the International Academy of Cardiovascular Sciences. He is President of the International Society for Adaptive Medicine. He is a fellow at the International Academy of Cardiovascular Sciences, American Physiological Society and American Heart Association. Dr. Singla has served as a chair for various scientific sessions throughout the world with experience in organizing scientific conferences. He has published a book on stem cells and is an author/coauthor for more than 100 peer-reviewed papers.
2024 ANNUAL REPORT | 57

ERIC H. HOLMES, PHD
Assistant Vice President for Research, Florida State University
Eric Holmes has a PhD in Biochemistry from the University of California, Davis. Since 2013 he has been an Assistant Vice President for Research in the FSU Office of the Vice President for Research. He currently also serves as the Interim Director for the FSU Office of Human Subjects. Prior to joining FSU, he was Director of Research at the University of Hawaii’s John A. Burns School of Medicine.
Dr. Holmes has a long track record of directing NIH-funded research in biochemical oncology. He is an author of approximately 100 research publications and is an inventor on over 30 issued US and foreign patents. Dr. Holmes has also worked in the Biotech industry in development-stage pharmaceutical companies located in the Pacific Northwest focused on antibody therapy and drug delivery technologies, and has designed and managed clinical trials related to the development of these technologies.

PETER J. HOLLAND, MD, FACOEM, DLFAPA
Director of Clinical Research, Florida Atlantic University
Prior to joining FAU in 2003, Peter Holland founded Boca Raton Psychiatric Group and Boca Raton Medical Research – a clinical trial organization with a national reputation. Since joining FAU, he has been the principal investigator of numerous, double-blind, placebo-controlled clinical trials. He is currently the medical director of the FAU Clinical Research Unit. Dr. Holland is also a founding faculty member of FAU’s Schmidt College of Medicine where he directs medical student education in psychiatry.
Dr. Holland earned his medical degree at the University of South Florida College of Medicine. He completed his internship in Medicine and Psychiatry at the University of South Florida and continued his residency training and fellowship in Psychiatry at the University of North Carolina-Chapel Hill. Dr. Holland is board-certified in Psychiatry with additional qualifications in Geriatric Psychiatry. He is a Distinguished Life Fellow of the American Psychiatric Association and a Fellow of the American College of Occupational and Environmental Medicine.

MAX C. E. OREZZOLI, PHD
Associate Professor of Healthcare, School of Arts & Sciences, Florida Memorial University
Max C. E. Orezzoli, PhD is an Associate Professor of Healthcare in the Department of Health & Natural Sciences at Florida Memorial University. He holds a doctorate in Sociology (Medical) with an emphasis on quantitative analysis from Florida International University. His expertise and research focus are centered on community-based interventions that positively impact the health of underrepresented communities. Dr. Orezzoli has over 15 years of experience in minority health disparities research, including substance use disorder (SUD), marijuana use, nutrition, violence, and HIV research. Additionally, Dr. Orezzoli has provided experimental and instrument design, data collection trainings, evaluation, statistical and methodological consulting and assistance to institutions, biomedical, educational, and healthcare organizations.
58 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Dr. Orezzoli is bilingual and fluent in Spanish. He has extensive experience in formative and summative program evaluation using quantitative and qualitative methods which are regionally and culturally appropriate. Additionally, due to his research/evaluation experience, including SAMHSA projects, Dr. Orezzoli is well equipped to conduct research involving human participants and, addressing sensitive issues/topics of focus (MMJ, diet research, HIV, SUD, violence, etc.) while adhering to all confidentiality and privacy requirements. Dr. Orezzoli served as the Co-chair of the Institutional Review Board (IRB) at Florida Memorial University from 2018-2022.

JACQUELINE SAGEN, PHD
Professor of Neurological Surgery, University of Miami
Dr. Jacqueline Sagen is a Professor of Neurological Surgery at the Miami Project to Cure Paralysis, University of Miami Miller School of Medicine. She received her B.A. in Neuroscience from Northwestern University, PhD in Pharmacology from the University of Illinois College of Medicine in 1984 and an M.B.A. in Entrepreneurship from University of Illinois at Chicago in 1994. She completed a postdoctoral fellowship in 1986 and joined the faculty at University of Illinois College of Medicine in the Department of Anatomy and Cell Biology as Assistant/Associate Professor through 1995. From 1995-1998 she was Associate Director, Pharmacology and Behavioral Research, CytoTherapeutics, Inc and Adjunct Associate Professor of Cellular Technology, Brown University, prior to her current position at University of Miami.
Research in Dr. Sagen’s laboratory over the past 35 years has been focused on exploring novel therapeutic strategies for chronic pain management that have the potential to provide sustained relief on a long-term or permanent basis. As chronic pain syndromes are often resistant to traditional pain interventions and/or limited by untoward side effects and possible analgesic dependence, the long term goal of work in her lab is to identify and develop more potent interventive approaches to improve the quality of life of these patients. A primary initiative in her lab is the generation of gene therapies and cell transplantation that can provide a continually renewable source of pain-reducing substances. The identification of superior alternatives to opioids, such as cannabinoids, is a current focus of her research. She has published over 150 articles and book chapters and holds 6 patents in the field of novel pain therapies. She serves on numerous scientific review panels for NIH, DoD, VA, CIRM, and private foundations, and is faculty representative on the FDP.

CHARLES A. WEATHERFORD, PHD
Vice President for Research, Florida A&M University
Charles Weatherford is the Vice President for Research at Florida A&M University (FAMU). Dr. Weatherford is also the Director of the FAMU Industrial Hemp Pilot Project, Principal Investigator on the FAMU MMJ Education and Research Initiative, Director of the FAMU Center for Plasma Science and Technology, as well as a Professor in the FAMU Department of Physics.
He received his PhD in Atomic and Molecular Physics from Louisiana State University in 1974. Dr. Weatherford is a Fellow of the American Physical Society and a Fellow of the National Society of Back Physicists. Dr. Weatherford has a Patent Disclosure 2018: “Field-Assisted Muon-Catalyzed Fusion”, 224 journal publications, 5 book articles, and 2 books. He was PI or Co-PI on $60.5 million in Research Grants and Contracts. His research interests include Materials and Energy for National Security, Correlation in Many-Body Quantum Chemistry, Laser-Matter Interactions, High-Energy Density Science, Field-Assisted Muon-Catalyzed Fusion, and Computational Science.
2024 ANNUAL REPORT | 59
APPENDIX B: CORE FACULTY, STAFF, AND TRAINEES OF THE CONSORTIUM FOR MMJ CLINICAL OUTCOMES RESEARCH

ALMUT G. WINTERSTEIN, RPH, PHD, FISPE CONSORTIUM DIRECTOR
Distinguished Professor, Pharmaceutical Outcomes & Policy; Dr. Robert and Barbara Crisafi Chair in Medication Safety; College of Pharmacy, Director, Center for Drug Evaluation and Safety, University of Florida
Almut Winterstein is Distinguished Professor in the Department of Pharmaceutical Outcomes and Policy at the College of Pharmacy, the founding Director of the Center for Drug Evaluation and Safety, and affiliate professor in epidemiology at the University of Florida. In 2017, she was named the Dr. Robert and Barbara Crisafi Chair for Medication Safety in recognition of her research on drug safety and on devising ways to improve medication use. Since 2019 she serves as director of the Consortium.
She received her pharmacy degree from Friedrich Wilhelm University in Bonn, Germany and her PhD in Pharmacoepidemiology from Humboldt University in Berlin. Since joining the University of Florida in 2000, Winterstein has served as principal investigator on more than 25 extramurally funded grants and contracts and published more than 400 manuscripts and conference abstracts. Her research interests center on the post-marketing evaluation of drugs in pediatrics and pregnancy, infectious disease and psychiatry and the evaluation and improvement of quality surrounding medication use using real-world data. As expert in drug safety, she has chaired the Food and Drug Administration’s Drug Safety and Risk Management Advisory Committee from 2012-2018. Recognizing her contributions in pharmacoepidemiology, Dr. Winterstein was inducted as a fellow of the International Society of Pharmacoepidemiology in 2013 and served as president of the society from 2019-2020. In 2022 she was inducted in the Academy of Science, Engineering and Medicine in Florida. She has chaired more than two dozen PhD committees and was awarded the UF-wide Dissertation Advisor/Mentoring award for her excellence in graduate training.

ROBERT L. COOK, MD, MPH CONSORTIUM ASSOCIATE DIRECTOR
Professor, Epidemiology, Medicine, Director, Southern HIV & Alcohol Research Consortium (SHARC), College of Public Health & Health Professions,University of Florida
Robert L. Cook, MD, MPH is a Professor of Epidemiology at the University of Florida, with a joint appointment in the Division of General Internal Medicine. Dr. Cook has expertise and experience in a range of research methods, including randomized clinical trials, cohort studies, and qualitative research.
Over the past 20 years, Dr. Cook’s research has focused on strategies to improve health outcomes related to HIV and sexually transmitted diseases. He is the Director of the Southern HIV Alcohol Research Consortium (SHARC), which supports collaborative research and training related to alcohol and HIV infection across the state of Florida.
Dr. Cook has also been engaged in a range of research related to clinical outcomes and MMJ. He recently completed a 5-year NIH-supported project on the effects of marijuana use on clinical outcomes in persons with HIV, including cognitive function and systemic inflammation.
Dr. Cook’s research is translational, ranging from basic science to implementation science, and he is currently the PI or MPI of three major NIH grants and a T32 training program that supports PhD students and postdoctoral scholars.
60 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Mentoring is also an important aspect of Dr. Cook’s academic career. He has served as PhD dissertation chair for 9 students, PhD committee member for over 30 students, and mentor for numerous additional trainees, post-docs and junior faculty.

AMIE J. GOODIN, PHD, MPP CONSORTIUM ASSISTANT DIRECTOR, EVIDENCE
Assistant Professor, Pharmaceutical Outcomes & Policy; College of Pharmacy, University of Florida
Amie J. Goodin, PhD, MPP is an Assistant Professor within the Department of Pharmaceutical Outcomes and Policy (POP) at the University of Florida. Dr. Goodin received her Master of Public Policy degree from the University of Kentucky (UK) and completed her Doctor of Philosophy degree at UK’s Martin School of Public Policy, with specialization in pharmaceutical outcomes and an additional Certificate in Informatics. She completed a Postdoctoral Fellowship at University of Florida POP, specializing in pharmacoepidemiology methods while continuing her work in Pharmaceutical Health Services Research.
She is the lead for Health Services Research track within POP’s graduate program and serves on the advisory board for the Center for Drug Evaluation and Safety (CoDES). She is a Fellow of the Center for Public Health Law Research. Her research evaluates the impact of policy changes on treatment access, utilization, and care quality.

MD MAHMUDUL HASAN, PHD, MSC CONSORTIUM ASSISTANT DIRECTOR, MEMORY
Assistant Professor, Pharmaceutical Outcomes & Policy; Information Systems and Operations Management, College of Pharmacy, University of Florida
Md Mahmudul Hasan, PhD is an assistant professor in the Department of Pharmaceutical Outcomes and Policy (POP) with a joint appointment in the department of Information Systems and Operations Management (ISOM) at the University of Florida. His appointment is a part of the UF’s larger AI initiative. Prior to joining UF, Dr. Hasan worked as an Oak Ridge Institute for Science and Education (ORISE) Postdoctoral Fellow of Drug Safety and Artificial Intelligence Methods in the Center for Drug Evaluation and Research (CDER) at the United States Food and Drug Administration (FDA). Dr. Hasan has completed PhD and worked as a postdoctoral research scientist at the Decision Analytics Lab at Northeastern University, Boston, Massachusetts. He was also an active member of Northeastern’s Center for Health Policy and Healthcare Research.
Dr. Hasan conducts data-driven interdisciplinary research to address complex challenges in public health, contributing to healthcare decision-making, policy, and management. Funded by CDC in collaboration with Massachusetts Department of public health (MDPH), he has collaborated in several interdisciplinary research projects that address critical issues surrounding opioid use disorder and opioid overdose epidemic. His current research lies in the intersection of pharmaceutical outcomes and health service utilization with a focus on substance use and mental health disorder, opioid related adverse drug events, and chronic diseases. His primary research interests include developing (i) fair, trustworthy, and equitable AI-driven and evidence-based Clinical Decision Support Systems (CDSS) to help predict adverse health outcomes; and (ii) prescriptive decision analytic models to improve medication adherence and effectiveness of personalized interventions to better manage the chronic health conditions. From a methodological standpoint, Dr. Hasan leverages data science, in particular AI/machine learning, statistical modeling, and management science techniques.
2024 ANNUAL REPORT | 61

YAN WANG, PHD
CONSORTIUM ASSISTANT DIRECTOR, CLINICAL CORE
Assistant Professor, Epidemiology; College of Public Health & Health Professions, University of Florida
Yan Wang, PhD is an Assistant Professor of Epidemiology at the University of Florida. Her research interests focus on leveraging advanced technologies and methods (e.g., wearable sensors, ecological momentary assessment/EMA) to improve the understanding of etiology and outcomes of substance use (e.g., alcohol, MMJ). Dr. Wang is the PI/MPI of two prospective cohort studies funded by NIH (R01AG071729, PI: Wang; U01CA286810, MPIs: Hu & Wang) which will utilize technology-based assessments to investigate health effects of medical cannabis on older adults with chronic pain and breast cancer patients, respectively. Dr. Wang is currently the PI or Co-I on multiple NIH projects focusing on alcohol use among persons with HIV that involve the use of wrist-worn alcohol biosensor (R01AA030481, MPIs: Cook & Wang; P01AA029547, MPIs: Naar & MacDonald; P01AA029543, MPIs: Cook & Cohen), and she is Co-I on several NIH projects (R01 AA030481, PI: Weafer & Leeman; R21AA029489, PI: Jaffe; R34AA031379, PI: Scaglione) to contribute her expertise on alcohol biosensors or EMA. Additionally, Dr. Wang has also been contributing as Co-I on several NIH or FDA funded projects such as a U24 grant for biomedical data repository (U24 AA029959, PI: Wu & Cook).

JEEVAN JYOT, PHD, PMP
CONSORTIUM ASSISTANT DIRECTOR, RESEARCH ADMINISTRATION
College of Pharmacy, University of Florida
Dr. Jyot received her PhD in Microbiology and Molecular Biology from the Institute of Microbial Technology (India) and completed her postdoctoral fellowship and was an Assistant Scientist at Division of Infectious Diseases and Global Medicine, Department of Medicine, University of Florida. In addition, she has Project Management Professional credentials.
Dr. Jyot has previously served as Research Program Coordinator at Division of Research Program Development (DRPD) at Office of Research at University of Florida. Currently, Dr. Jyot is part of the Department of Pharmaceutical Outcomes and Policy (POP) at the University of Florida and serves the MMJ Clinical Outcomes Research Consortium.

ALLISON VELIZ CONSORTIUM COMMUNICATIONS SPECIALIST
College of Pharmacy, University of Florida
Allison is the Communications Specialist of the Consortium for MMJ Clinical Outcomes Research. Allison attended the University of Florida, earning a Bachelor of Science in Journalism and a Master of Arts in Mass Communication. Before joining the Consortium for MMJ Clinical Outcomes Research, Allison worked for the University of Florida International Center and the University of Miami.
62 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH

SOPHIE MALONEY, RESEARCH COORDINATOR CLINICAL CORE
Research Coordinator, Epidemiology; College of Public Health & Health Professions, University of Florida
Sophie assists with participant recruitment for the contact registry and M3 study, research operations, database management for the M3 dataset and CARMMA, and data analysis. Prior to joining the Consortium, Sophie obtained a Bachelor of the Arts in Sociology from the University of Florida and is currently pursuing her Master of Public Health from Florida International University online. She also previously worked at the Alachua County Department of Health as a case manager for low-income and uninsured residents. Outside her work with the Consortium, Sophie is a research assistant for the Florida Cohort study in the Southern HIV & Alcohol Research Consortium (SHARC).

SHEILA AUSTIN, MS, ACRP-CP REGULATORY SPECIALIST
Clinical and Translational Science Institute, University of Florida
Sheila works with Investigators to negotiate the FDA Investigational New Drug (IND) and Investigational Device Exemption (IDE) submission and approval process. Sheila is also a part of the admin team for UF ClinicalTrials.gov.
CONSORTIUM SUPPORTED GRADUATE STUDENTS AND POST-DOCS



HANZHI GAO, PHD
Postdoctoral Associate, Biostatistics, College of Public Health & Health Professions, University of Florida
Dr. Gao is a postdoctoral associate within the Department of Biostatistics at the University of Florida. He serves as a Data Manager for the M3 study and was integral to survey development.
JUAN PEREZ, MD
Predoctoral Research Assistant, Epidemiology, College of Public Health & Health Professions, University of Florida
Dr. Juan Perez is a predoctoral graduate assistant in the Department of Epidemiology at the University of Florida. He serves as a data analyst and research assistant for the Clinical Core.
JABED AL FAYSAL, MS
Graduate Assistant, Department of Pharmaceutical Outcomes & Policy, College of Pharmacy, University of Florida
Jabed Faysal is a graduate assistant within the Department of Pharmaceutical Outcomes & Policy. He serves as a data analyst for MEMORY studies.
2024 ANNUAL REPORT | 63

MATTHEW MUSCHETT, PHARM.D.
PhD Student, Graduate Research Assistant, Data Analyst III, Department of Pharmaceutical Outcomes and Policy, College of Pharmacy, University of Florida
Matthew Muschett is a third-year PhD student, pharmacist by training, whose research with the Consortium involves the analysis of data obtained from Florida’s Medical Marijuana Use Registry to estimate average daily doses of MMJ in a variety of subpopulations.


PRIYANKA KULKARNI, MPH, B. PHARM.
PhD Student, Graduate Assistant, Pharmaceutical Outcomes and Policy, College of Pharmacy, University of Florida
Priyanka Kulkarni works as a research assistant on the MEMORY project, conducting research and analysis of data to determine the utilization of MMJ and its impacts on medically certified patients in Florida.
RUBA SAJDEYA, MD, PHD
Postdoctoral Research Assistant, Epidemiology, College of Public Health & Health Professions, University of Florida
Dr. Sajdeya is a postdoctoral research assistant within the Department of Epidemiology at the University of Florida. She maintained active participation within the Core throughout her predoctoral training and is a current research assistant.

SEBASTIAN JUGL, MS, RPH
PhD Student, Research Assistant, Evidence Core & MEMORY, Pharmaceutical Outcomes & Policy, College of Pharmacy, University of Florida
Since joining the Consortium in 2019 as a research assistant, Sebastian Jugl has both contributed to and led multiple projects within MEMORY and the Evidence core, enhancing our scientific comprehension of medical marijuana.
64 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
APPENDIX C: REVIEWERS OF THE 2023 RESEARCH GRANTS PROGRAM
*reviewers who reviewed more than one proposal
Amber Kleckner University of Maryland Assistant Professor
Anjie Zhen University of California at Los Angeles Assistant Professor
Aurelio Galli University of Alabama at Birmingham Professor
Barbara Kaplan Mississippi State University Associate Professor
Bradley Alger University of Maryland School of Medicine Professor
Deepika Slawek Albert Einstein College of Medicine Assistant Professor
Dionna Whitney Williams Johns Hopkins Medical Institute Assistant Professor
Dinesh Pashankar Yale University Professor
James White Duke Molecular Physiology Institute Assistant Professor
Jiangnan Peng Morgan State University Assistant Professor
John Streicher University of Arizona Associate Professor
Joseph Ditre Syracuse University Professor
Kabirullah Lutfy Western University of Health Sciences Professor
Kelly Naugle Indiana University-Purdue University Associate Professor
Kevin King University of Washington Professor
Mercy N. Mumba University of Alabama Associate Professor
Michael Mason University of Tennessee Professor
Omayma Alshaarawy* Michigan State University Assistant Professor
Rajiv Radhakrishnan Yale University Assistant Professor
Rhoderik Machekano Elizabeth Glaser Pediatric AIDS Foundation Associate Professor
S. Casey Laizure University of Tennessee Health Science Center Professor
Salameh Keyhani University of California at San Francisco Professor
Steven Kinsey* University of Connecticut Professor
Susan Ferguson University of Washington Associate Professor
Thomas Heinbockel Howard University College of Medicine Professor
Tim Moran John Hopkins University Professor
Tory Spindle Johns Hopkins Medical Institute Associate Professor
Xuan-Zheng Shi University of Texas Medical Branch Professor
2024 ANNUAL REPORT | 65
APPENDIX D: SUMMARY BROCHURE FOR THE CANNABIS CLINICAL OUTCOMES RESEARCH CONFERENCE (CCORC) 2023


















CCORC 2023 OVERVIEW
































































CCORC 2023 was a 1.5 day conference gathering medical marijuana researchers, providers, patients, and industry representatives.

















The conference featured 3 keynote presentations, 8 oral presentations from top abstracts, 3 panel discussions, 2 poster sessions featuring 55 research abstracts selected following peer review, and 2 breakout sessions. Participation in the conference program increased dramatically, boasting a 28% increase in session presenters and a 39% increase in poster presentations. Student representation saw a doubling in registration numbers, increasing their share of the audience from 17% in 2022 to 34% in 2023










CCORC 2023 QUICK FACTS



133

































Disentangling

































THREE EMERGING THEMES FROM THE CONFERENCE





























































66 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH
Medical
and Public Health
Solutions to Address Barriers Faced when Conducting Clinical Cannabis Research Unpacking the Data Behind Cannabis Use, Mental Health, and Cognition 1 55% RESEARCHERS 34% STUDENTS 28% PROVIDERS
Conflicting Evidence in Research Studying the Relationship Between
Cannabis
Seeking
REGISTRANTS
US STATES
POSTERS PRESENTED
SESSION PRESENTERS
9
39
32
CCORC SUMMARY BROCHURE

































































Consortium for









Dr. Robert L. Cook



















University of Florida
















Keynote: Opioid and Cannabinoid Interactions: Abuse Potential and Clinical Implications* Δ
















Dr. Shanna Babalonis
University of Kentucky


Director of the University of Kentucky Cannabis Center
Presentations of Top Abstracts w/ Q&A#

Bassma Eltanameli
University of Florida
Mounika Aare








Moderator: Dr. Rose Stiffin, Florida Memorial University

Kristin Perrucci

Florida A&M University
University of Miami





































Martina Compagno
Florida State University
































































2024 ANNUAL REPORT | 69 4 6 - CCORC 2023 Day 2
Time (EDT) Session Description 8:00-8:15am Day 2 Welcome Address# Δ
PROGRAM AT A GLANCE
Clinical Outcomes Research
Medical Marijuana
8:15-9:30am
9:30-10:30am
10:30-10:45am Exhibitor Hall | Coffee Break Δ=VIRTUAL OPTION AVAILABLE
CCORC SUMMARY BROCHURE



















































































































Dr. Anthony Ferrari | Dr. Jeff Konin Florida International University Dr. Paul Borsa | Sheila Austin University of Florida

Session A: Using a hemp pathway to probe the entourage effect. What other questions can be answered using this regulatory pathway?#












Dr. Mandip Sachdeva Florida A&M University






Session B: Role of Exosomes containing CBD in cancer and chemotherapy induced peripheral neuropathy#









CE Information











































ccorc.mmjoutcomes.org























* May be eligible for CME and CPE credits. Please check ccorc.mmjoutcomes.org/ agenda/cme-credit/ and ccorc.mmjoutcomes.org/agenda/cpe-credit/ for details. The University of Florida College of Medicine designates this live activity for a maximum of 3.75 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. The University of Florida College of Medicine is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The University of Florida is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Each of the Keynote addresses is eligible for 1.25 CPE credits. # Not accredited for CPE


































70 | CONSORTIUM FOR MEDICAL MARIJUANA CLINICAL OUTCOMES RESEARCH 5 CANNABIS CLINICAL OUTCOMES RESEARCH CONFERENCE - 7 Time (EDT) Session Description 10:45-11:30am Note: Sessions A & B Will Be Held Simultaneously
11:30-12:00pm Closing and Awards Ceremony# Δ 12:00-1:00pm Board Meeting and Invited Lunch Reception May 19, 2023
Δ=VIRTUAL OPTION AVAILABLE
www.mmjoutcomes.org














 PI: HABIBEH KHOSHBOUEI, PHD, PHARMD
PI: HABIBEH KHOSHBOUEI, PHD, PHARMD
 PI: CATALINA LOPEZ-QUINTERO, MD, PHD
PI: CATALINA LOPEZ-QUINTERO, MD, PHD
 PI: JOHN S. MARKOWITZ, PHARMD
PI: JOHN S. MARKOWITZ, PHARMD
 PI: NIALL MURPHY, PHD
PI: NIALL MURPHY, PHD













































































































































