
THE MEDICAL PRODUCT SUPPORT NETWORK





MedWrench is your online community to ask questions and share information about specific medical equipment you work with every day.




















PRESIDENT John M. Krieg
VICE PRESIDENT Jayme McKelvey OF BUSINESS DEVELOPMENT
ART DEPARTMENT Karlee Gower
DIGITAL MARKETING Kennedy Krieg
ACCOUNTING Diane Costea
ADMINISTRATIVE Joanna Manjarrez ASSISTANT
DATA ENTRY Emma Vitkovitsky
THE FACE OF Ben Calibrating
MEDWRENCH is a product focused support network where medical professionals, purchasing administrators, manufacturers, dealers and industry experts can provide opinions, share ideas, and gather relevant information on medical technology and equipment.

By Bryant Hawkins
You’re not just solving problems: you’re shaping FUTURES.

Icame across this 30-second short on Instagram, and it hit me like a lightning bolt. It made me stop and think about everything we’re up against as healthcare technology management professionals, and I realized I had to share this with you. Because you need to hear this. This is going to elevate your mindset, shift your energy, and put you on another level entirely.
Listen closely: those challenges you’re facing, those relentless obstacles. They’re not random. They’re a signal. They exist because they see your potential. Those struggles? They’re testing you. They’ve already done the math on your skills, your courage, your resolve and they know something you need to believe: “You are the Solution.”
Maybe you’ve been questioning your path. Maybe you’ve wondered if what you do really matters. Maybe you’ve felt like the weight of everything is too much. But let me tell you something, loud and clear, you are not here by accident. Every piece of you: every strength, every failure, every lesson, has built you for this. You have what it takes, and it’s time to embrace that. From this day forward, you’re going to walk taller, talk with purpose, and remind yourself daily: “I am the answer.”
Think about what you’ve witnessed. The broken systems, the inefficiencies that slow down progress, the teams that feel undervalued. Those aren’t just problems, they’re opportunities. They’re challenges waiting for you to step in, rise up, and create change. You’re not just here to maintain the status quo, you’re here to innovate, inspire and elevate.
Picture this: a ventilator goes down in the middle of a busy day. The clinical staff is panicking, patients are at risk, and time is of the essence. And then, you step in. You diagnose the issue, fix the problem, and restore functionality. But what you’ve done is so much more than just a repair, you’ve enabled the clinical team to do what they do best. You’ve saved time, lives, and confidence. “You are the answer” in moments like this, where precision and urgency collide.
professionals often feels like two different languages. Misunderstandings create tension, but “you are the one” who can change that. By stepping up, facilitating conversations, and educating teams on the importance of HTM professionals, you foster collaboration. You’re not just fixing equipment: you’re fixing relationships, ensuring everyone works together to achieve the same goal. Better patient care. “You are the answer!”
The HTM workforce is aging, and the next generation is still finding its way. That’s where you come in. By mentoring new technicians, advocating for the profession, and sharing your experiences, you’re planting seeds that will grow into the future of this industry. You’re not just solving problems today; you’re building the leaders of tomorrow. That legacy is priceless. “You are the answer!”
This isn’t just about solving technical problems. It’s about creating shifts professionally and personally. If doubt, negativity or frustration have crept into your environment, “you are the one who will break that cycle.” You are not here to simply get by. You are here to lead a movement, to turn obstacles into opportunities, to transform the ordinary into

So, right now, I want you to declare it: “I am the answer.” Say it. Feel it. Own it. And let me tell you, only a few people will truly grasp the power of those words. But the ones who do? They’ll be the ones who lead, the ones who innovate, the ones who elevate this industry into something greater than ever before. “You are one of those people.”
From today on, you’re not just fixing equipment: you’re fixing standards. You’re not just solving problems: you’re shaping futures. Every time you walk into a room, every time you solve a problem no one else could, every time you share your knowledge with someone else, you’re proving it: “I am the answer.”
And if someone asks you why you believe in yourself so strongly, you tell them: “Because I’m the one who’s going to change this industry, one step, one process, one breakthrough at a time.”
Communication between clinical staff and HTM
You’re not just in HTM. You’re transforming HTM. “You are the answer.” Now, go out there and show the world.






















In today’s healthcare environment, Biomedical Equipment Technicians (BMETs) and Clinical Engineers play a crucial role in ensuring the safety, reliability, and compliance of medical devices. Yet, despite their technical expertise, many biomed teams still rely on spreadsheets, paper records, or siloed systems to manage preventive maintenance (PM), calibrations, repairs, and equipment tracking.
This is where Computerized Maintenance Management System (CMMS) software becomes indispensable. Among the options available, myBC Connect by BC Group International stands out as a particularly effective solution tailored for the needs of the biomedical field.
1. Streamlined Workflows: CMMS software centralizes all service records, PM schedules, and calibration logs into a single digital platform. This makes it significantly easier to assign tasks, monitor performance, and stay on top of regulatory deadlines.
2. Compliance and Audit Readiness: Regulatory bodies such as The Joint Commission (TJC), FDA, and CMS require accurate documentation of equipment maintenance. A CMMS ensures documentation is standardized, time-stamped, and readily accessible for audits.
3. Data-Driven Decision Making: With a CMMS, biomeds can analyze repair histories, failure trends, and PM compliance rates. This empowers departments to shift from reactive to proactive maintenance strategies, ultimately reducing downtime and costs.
4. Asset Management and Traceability: Whether managing a few hundred or thousands of devices, keeping track of serial numbers, warranty statuses, calibration certificates, and usage logs is overwhelming without digital support. CMMS solutions provide real-time visibility into every asset’s lifecycle.
5. Collaboration and Communication: Integrated systems allow seamless communication between biomed teams, IT, procurement, and clinical staff. This reduces miscommunication and accelerates service response times.
WHAT MAKES MYBC CONNECT FROM BC GROUP STAND OUT FROM THE COMPETITION?
While the benefits of CMMS software are clear, not all platforms are created equal. myBC Connect, developed by BC Group International, has emerged as a standout option for biomedical and clinical engineering teams for several key reasons:
1. Purpose-Built for Biomed: myBC Connect is not a generic
maintenance system retrofitted for healthcare and is designed from the ground up with biomedical professionals in mind. That means its features align with the daily workflows, documentation standards, and calibration needs specific to medical device management.

2. Seamless Integration with Test Equipment: As a leading manufacturer of biomedical test equipment, BC Group ensures myBC Connect is optimized to integrate with their testing devices in addition to 3rd party devices. This enables automatic upload of test data and calibration results, eliminating manual data entry and minimizing the risk of human error.
3. Cloud-Based and Accessible Anywhere: myBC Connect is cloudhosted, which means technicians can access it from anywhere using a laptop, tablet, or even mobile device. Whether you’re performing field service or documenting a repair in the shop, real-time access keeps teams agile and connected.
4. Simplified Calibration Tracking: For teams managing both in-house and third-party calibrations, myBC Connect provides a centralized hub for certificates, due dates, and service records. It also links directly to BC Group’s calibration lab, streamlining external service workflows.
5. Scalable for Small Teams or Large Enterprises: Whether you’re a one-person biomed shop or part of a multi-site hospital network, myBC Connect scales with your needs. Customizable dashboards, user permissions, and reporting features make it adaptable to virtually any clinical engineering environment.
As the demands on biomedical teams continue to grow, fueled by increasing device complexity, tighter regulatory scrutiny, and the push for digital transformation, CMMS software is no longer optional. It’s essential.
myBC Connect offers a modern, biomed-centric CMMS solution that bridges the gap between service management and compliance, while also saving time and reducing risk. For healthcare organizations looking to improve efficiency, accountability, and safety, integrating myBC Connect is a smart and forward-thinking investment.
To learn more about the next generation of integration and the benefits that myBC Connect can bring to your organization, visit mybcconnect.com.






More than 70 years ago, the right to repair movement emerged in response to manufacturers placing barriers that prevented consumers and independent technicians from servicing products they had legally purchased. These restrictions continue to block timely, cost-effective repairs, limiting options for do-it-yourselfers and professionals alike.
WHY THE RIGHT TO REPAIR MATTERS FOR MEDICAL DEVICES
At its core, the right-to-repair issue asks: Why can’t a product’s owner modify, maintain, or upgrade their own equipment? Why must consumers buy a replacement instead of repairing what they already own?
The pushback has come largely from consumer advocacy organizations, independent service organizations (ISOs), and technicians. Without legislation, original equipment manufacturers (OEMs) are under no obligation to share service manuals, parts, or diagnostic tools—resources necessary for effective medical device servicing.
Consider auto repair. A home mechanic with a consumer-grade OBD-II scanner may still lack access to proprietary data required to diagnose a problem—information locked behind OEM tools. This same dynamic plays out in agriculture and healthcare. Farmers can’t fix tractors, and healthcare technology management (HTM) professionals often face delays when trying to service critical medical equipment.
MEDICAL RIGHT TO REPAIR: LEGISLATIVE CHALLENGES AND OPPORTUNITIES
Organizations representing ISOs and consumer rights have brought right-to-repair issues before state legislatures and Congress. Progress has been incremental, with small legislative wins in a few states—but the movement remains far from reaching its goals in the medical sector.
“The code to fighting for medical right to repair hasn’t been cracked,” said Gay Gordon-Byrne, executive director of The Repair Association. “We’ve seen movement only in a few states like Illinois and Delaware. Legislative leaders avoid fights they think they’ll lose—it’s a Catch-22.”
Thousands of biomedical technicians are affected by the limitations imposed by OEMs. The stakes are high—modern healthcare
equipment is increasingly complex, and restricted access can delay life-saving repairs.
Nathan Proctor, senior director at U.S. PIRG’s Right to Repair Campaign, referenced discussions from the 2024 MD Expo in Las Vegas: “Hospital-based biomeds say access to repair materials should be a condition of bidding on new equipment.”
Yet many in-house biomeds may not feel the urgency. Perry Kirwan, executive at Sutter Health’s eQuip-Center for Clinical Technology Management, says the challenge lies in mobilization.
“Biomeds tend to be introverted. There’s a sense that this won’t affect them directly,” Kirwan said. “But associations offer strength in numbers and provide a support system to drive engagement.”
ASSOCIATIONS AND STRATEGIC PARTNERSHIPS
Kirwan suggested state HTM associations should align with larger organizations like the American Hospital Association (AHA), which has more lobbying power. He also emphasized the potential of the American College of Healthcare Executives (ACHE) as a platform for educating C-suite leaders about the implications of restricted repair access on affordability and patient care.
“State laws favoring right to repair might be attainable while the federal process drags on,” Kirwan said.
HTM professionals at all levels can help drive change. GordonByrne says: “Biomeds can provide facts, but we need legislators who are willing to listen. A crisis like the ventilator shortage during COVID helped before—but that momentum has faded.”
Robert Kerwin, general counsel for the International Association of Medical Equipment Remarketers and Servicers (IAMERS), said that HTM professionals should demand service access during procurement.
“The 2024 FDA Remanufacturing Guidance outlines what’s needed to service equipment,” Kerwin said. “When OEMs use tactics like tying upgrades to long-term service contracts, let IAMERS know. That’s a competition issue.”
Proctor cautioned that not all hospitals have purchasing power to sway manufacturers. “Voting with your dollars doesn’t work well in highly consolidated markets,” he said.

Mike Busdicker, senior director of clinical engineering at Intermountain Health, emphasized educating HTM caregivers and organizational leadership. “Every organization has a legal counsel or government relations team. They need to understand what’s at stake.”
Busdicker said state associations must build local political relationships and unify HTM professionals. “Even with ISOs in the mix, we need to work together.”
CALLING ALL HTM PROFESSIONALS: UNITE FOR THE RIGHT TO REPAIR
Hospital leadership plays a pivotal role in influencing OEM behavior. “If hospital admins want right to repair, it’s far more likely to happen,” GordonByrne said. “Large hospital systems have the leverage of the purchase order.”
She also encouraged tapping into public sentiment. “Legislators respond to voters. If we break down how lack of repair options increases health costs, we can drive pressure through constituents.”
Proctor noted that 2024 saw progress in other sectors: “We passed three new laws—one in Oregon for consumer repair, another in Colorado for electronics, and one in California for wheelchairs. But medical equipment remains a challenge.”
Busdicker concluded, “HTM leaders must understand how this impacts patient care, safety, and costs. Education is the first step. Give biomeds the tools they need to take action.”
SHARE YOUR STORY: HELP AMPLIFY THE MOVEMENT
Gordon-Byrne called for HTM professionals to share repair challenges via The Repair Association’s anonymous complaint collector. These stories help rally public support and attract media attention.
Scan the QR code or click here to help amplify the movement!


Get ready for an adventure! AAMI eXchange attendees who take on MedWrench’s Scavenger Hunt have the chance to win over $2,400 in prizes


HERE’S HOW TO PLAY:
• Grab your Scavenger Hunt card at the MedWrench booth 2944 or from any of our awesome participating sponsors.
• Complete the hunt and turn in your finished card at the MedWrench booth before June 22nd at 2:00 PM CT.
• You must be present for the prize drawing at the MedWrench booth to be one of our lucky winners!

Get ready for an adventure! AAMI eXchange attendees who take on MedWrench’s Scavenger Hunt have the chance to win over $2,400 in prizes

• AAMI #2719
• AAMI #2719
• ALCO Sales and Service Co. #2529
• ALCO Sales and Service Co. #2529
• Block Imaging #2414
• Block Imaging #2414
• FSI #2913
• FSI #2913
HERE’S HOW TO PLAY:
• Innovatus Imaging #2402
• Innovatus Imaging #2402
• Integrity Biomedical Services, LLC #2432
• Integrity Biomedical Services, LLC #2432
• Interlight #3045
• Interlight #3045
• MedWrench #2944
• MedWrench #2944
• MultiMedical Systems, LLC #2737
• MultiMedical Systems, LLC #2737
• PartsSource #2837
• PartsSource #2837
• PM Biomedical #2531
• PM Biomedical #2531

• Grab your Scavenger Hunt card at the MedWrench booth 2944 or from any of our awesome participating sponsors.
• Prescott’s #2423
• Prescott’s #2423
• Pronk Technologies #2612
• Pronk Technologies #2612
• QRS Solutions/Datrend Systems, Inc. #2629
• QRS Solutions/Datrend Systems, Inc. #2629
• Complete the hunt and turn in your finished card at the MedWrench booth before June 22nd at 2:00 PM CT.
• Rigel Medical #2441
• Rigel Medical #2441
• Sage Services Group #3029


• Sage Services Group #3029
• You must be present for the prize drawing at the MedWrench booth to be one of our lucky winners!
• TechNation #2845
• TechNation #2845
• United Infusion #2523
• United Infusion #2523
• USOC Medical #2409
• USOC Medical #2409
*Based on the live AAMI eXchange floorplan as of March 31st
*Based on the live AAMI eXchange floorplan as of March 31st
June 20-23, 2025 New Orleans, LA






Hospitals across the United States face a persistent and costly challenge that often flies under the radar: locating misplaced or lost medical equipment. From IV pumps to portable monitors, even the most essential tools can vanish within a hospital’s complex network of departments, floors, and storage area –impacting both patient care and the bottom line.
Studies estimate that nurses and hospital staff spend as much as 30 minutes per shift searching for equipment. The wasted time adds up fast. According to a 2022 report by the Association for the Advancement of Medical Instrumentation (AAMI), hospitals can spend tens of thousands of dollars annually in labor costs alone tied to equipment retrieval. The same report found that some health systems over-order devices simply because they can’t reliably track or retrieve them.
The hidden costs extend beyond time. When vital equipment can’t be located quickly, it may delay patient care, lead to scheduling conflicts in operating rooms, or force departments to rent substitute devices – sometimes at steep daily rates. In emergency situations, the inability to locate the right device can have life-threatening consequences.
Portable and mobile medical devices are particularly vulnerable to misplacement. These include everything from thermometers and pulse oximeters to crash cart accessories and barcode scanners. Even when hospitals use digital tracking systems like RFID or barcode inventory management, human factors such as failure to scan, misplaced carts, or equipment left in non-designated areas can thwart those systems.
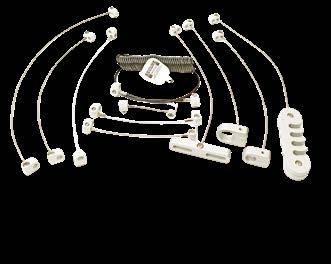
While some facilities invest in complex and expensive asset-tracking software, others are finding success with a far simpler solution: tethering cables.
Tethering devices with durable, tamper-resistant cables offers a cost-effective method to prevent equipment from being moved beyond its intended area. This approach not only secures the item in place but also helps ensure staff can locate it when needed.
Secure Mount, a Michigan-based company headquartered in Jackson, specializes in manufacturing high-strength tethering solutions for medical and industrial environments. The company’s products include coiled and straight steel security cables designed to keep small- to medium-sized equipment firmly attached to workstations, carts, or exam room furniture.
Secure Mount’s cables are made of aircraft-grade stainless steel and come with locking mechanisms that resist tampering and accidental release. The company’s solutions are used in hospitals, clinics, and urgent care centers nationwide, tethering everything from stethoscopes to barcode scanners and defibrillator pads.
In 2023, a 350-bed hospital in Ohio implemented a tethering program using Secure Mount products after an internal audit revealed they were replacing nearly $18,000 worth of small devices each year – mostly due to loss or misplacement.
After installing tethering cables on 125 pieces of high-use mobile equipment, the hospital reported a 70% drop in replacement costs and a noticeable reduction in staff time spent tracking down items. The facilities team also noted improved compliance with infection control protocols, since tethers encouraged equipment to remain in its assigned cleaning zone.
Experts note that tethering works best as part of a broader asset management strategy. While cables can keep high-use items in place, pairing them with digital check-out logs, RFID tags, or inventory audits helps further reduce loss and optimize utilization rates.
As hospitals continue to face staffing shortages, budget constraints, and increasing patient loads, solutions that are both low-cost and highly effective are gaining traction. Tethering is not glamorous, but it works – and that’s often enough.
Whether it’s a $600 vital signs monitor or a $60 thermometer, misplacing medical equipment can snowball into real costs. For facilities operating on tight margins, preventing those losses can translate into meaningful savings – not just in dollars, but in improved workflows and better patient outcomes.
As the old saying goes, time is money – and in hospitals, both are too valuable to waste chasing lost equipment. With simple tools like tethering cables from companies such as Secure Mount, healthcare providers have a practical way to secure the devices they rely on most.
MedWrench is an online resource for medical equipment service professionals to engage with their peers about medical equipment repairs, source parts and to locate service companies. The following are examples of how the MedWrench community members help each other in the website’s forums.

Q: My ultrasound channel is showing up as unavailable. I wanted to try and buy a new wand to determine if it is the wand or the unit. But I can’t find one anywhere. Looks like the part number is 77501A
A: These are no longer available new. Chattanooga stopped producing these many years ago. Having them repaired or rebuilt is the only option.
A: Usually that message means the cable is broken. Happens all the time. Replacing the cable requires some soldering that can be a bit of a pain. You almost need a butane torch to get the ground off. Anyway, I usually buy parts from https:// mrchatt.com/ for that generation. He doesn’t have it on the website but Ken has 5cm wands on hand. Not associated with the site, just a customer. Good Luck!
A: Hi Doc Nev, it seems like your issue may stem from a broken cable or a faulty ultrasound head. If you’re unable to source a new wand, consider having it repaired or rebuilted.
Q: Hi guys, in our cardiac ICU, we have 7 suction machines, and we have the same problem. When the machine is turned on, the push button comes blue, it makes a sound like there is no power, when I check the outlet for 12v for the motor, it is not present. if somebody has experience with this kind of problem, please, if you can help me
A: Hi, first are you a biomed or a user? When you say you check the outlet, do you have the unit apart, are you
checking the wall outlet or are you checking where the power cord plugs into the device? If you are checking the motor for 12v, perhaps your power supply is bad and you’re not getting the 12v, or you’re not plugged into a working wall outlet and therefore not getting the 120v. If your power supply is putting out 12v but it’s not showing at your motor, then perhaps you have a bad power switch or cabling. If there is a control board in between, perhaps it is not sending a signal to the pump.
A: I believe maquet owns that thing, I can’t find anything more than a user manual unfortunately. If you’re getting 12vdc to the motor. Then your motor is bad, if not, the pcb. Not really going to do board level repairs so, save yourself time, play with the power supply later. Let us know what you find.
A: Ok so you’ve got a bad pcb. We don’t do component level for a reason, liability. For fun, sure play with every component till you find it, for insurance reasons, just pull the entire board and replace it.
Q: We have an old Pelton and Crane that used to work well despite infrequent use/cleaning/servicing. Lately it has started to take 30 minutes to reach the ‘green zone’ for temp and PSI, and will often go over the maximum values of the green zone. Then the door latch is super hard to open, but a bit better after we cleaned the whole autoclave thoroughly. Any idea if this is something that we could repair ourselves? Once it is hot, it still works ok, but the
drying process while on ‘vent’ also takes about 10 minutes rather than the advertised 3-5 min in the manual. We might be looking for a replacement if we cannot fix ourselves, as we have gotten quotes of around $600 with no guarantee that it can be fixed from repair services. Many thanks for any insight!
A: Hi, the issue with the unit heating up slowly and it being an inconsistent temperature is most commonly due to a bad/old heating element. The PSI going over the safe zone could be due to a variety of reasons, with the most common being a faulty door seal, or faulty pressure relief valve in my experience. Unless you have someone who is incredibly handy or has experience working on autoclaves or even home appliances (somewhat similar to dishwashers), this may be a tall task. The most difficult step may be finding parts for a unit of this age. I would be more than happy to assist further or my company also sells used and new autoclaves too.
A: Are you saying that the vent process takes 10 minutes or that drying everything takes 10 minutes? The OCR and OCM have a multi part bellows assembly that may be worn out. RPI has the rebuild kit. The bellows is supposed to let steam out until around 200F then close but it it’s work out it may not be closing until much later or just not sealing at all. That lets all the water boil away and the temps can then spike.
A: The Pelton Crane is too old, to invest you money and time.
A: The Pelton OCM was built between 1966 &1980. Most rugged autoclave ever built!! But, keeping this model running will be quite challenging. Temp fluctuations: The oldest model used a mechanical thermostat, (evident by a 12” long slender metal tube laying in the center of the chamber). If that’s what you have, this unit is pre 1972. Those thermostats can fluctuate greater than 5’ as they age. Solution: replace/upgrade to an electronic thermostat (repair shop). If it has an electronic thermostat, (evident by a cooling block (heat sink) sticking out the back side of the unit, there could be corrosion on the PC board. Or, the temp sensor is going bad. Again, only an experienced repair shop can help you.Slow heating: If the heat eventually goes up to the sterilizing range and can stay there - at least a half hour, the valves are ok. A bit of warning: if the wiring is covered with a soft cottony insulation - that is asbestos, and you should just retire the unit.
A: From what information you’ve given, it sounds like your bellows is failing or vent valve isn’t allowing all of the air to escape.
A: Also check the voltage of the sockets if is to low, sometime happened from power line.
A: Buy a new or 2nd hand unit, sediment and heat issue will cost you more money & time than is reasonable.
Q: I have a vip6 that displays level sense errors when attempting to start a clean cycle. Process completes with no errors. When inspecting sensors I find paraffin buildup around sensors. Temps are within ranges. Any ideas?
A: It could be bad level sensors, but the front heater on the retort can fail and it will cause these errors. The retort may feel hot to touch and it will not give an heater errors, you can ohm out the heaters to see if they are out of range.
A: Clean the sensors, for heaven’s sake. You don’t want any build-up on or around the level sensors. Each level sensor has a vertical slot on one side or the other. You need a brush that will get into that slot and clean it thoroughly.
A: It’s the front heaters. We had to source the heater units ourselves to replace as sakura will only replace the entire retort. My company also can provide repair service to do this but if you aren’t in one of the handful of states we have employees at, it can be a little more costly (but doubtful it will cost as much as sakura would for replacing the entire retort and heaters)


From free subscriptions, to free webinars, to free admission to conferences... we have you covered!
From free subscriptions, to free webinars, to free admission to conferences... we have you covered!


2



1

1 eNews: Industry news, exclusive promotions, spotlights on HTM professionals,
2 webinarwednesday.live
print magazine and online community: The leading publication for the biomed & HTM industry 1technation.com
3


3

Webinar Wednesday: Live webinars, live tools of the trade demos, on-demand webinars, and podcast. Our series is 11 years strong and eligible for 1 CE from the ACI. eNews: Industry news, exclusive promotions, spotlights on HTM professionals, and more.











































































































































































n a significant effort to support the professional development of biomedical technicians and managers in Puerto Rico, Boyd Campbell and Greg Johnson of Southeastern Biomedical recently traveled to San Juan to present a comprehensive training session. Given that Puerto Rico lacks a formal biomedical association, this initiative provided valuable opportunities for both education and networking in a field that is essential to the healthcare industry.











































The training program, designed by Southeastern Biomedical, went beyond the typical curriculum found in biomedical schools. The focus was on areas that technicians and managers don’t often get the chance to learn in their formal education but are crucial in real-world applications. Among the topics covered were:































• Calibration Weight Classifications: Understanding the standards and classifications used to ensure accurate calibration of biomedical equipment.





Scale Testing Best Practices: Effective methods for testing and maintaining medical scales to ensure precision.

























• Temperature Measurement Devices: An overview of various temperature measurement tools and their specific applications in the biomedical field.












Reading Test Device Specifications: Guidance on how to interpret and use device specifications effectively.


• Infant Incubator Testing: Best practices for testing and maintaining infant incubators, ensuring they meet safety and health standards.











These hands-on, real-world topics were chosen to enhance the skill sets of technicians and ensure they are well-equipped to handle the diverse challenges in the field.






























The event wasn’t just about training – it also provided a valuable opportunity for attendees to network with one another, building connections that are essential for ongoing professional growth and support. The social hour allowed participants to engage with peers and share insights, fostering a sense of community and collaboration among biomedical professionals in Puerto Rico.




















Additionally, the attendees were able to explore some of the latest cutting-edge testing devices from Fluke Biomedical, a leader in biomedical instrumentation. This gave the attendees a firsthand look at the innovations shaping the future of biomedical testing and device maintenance.


































By bringing this training to Puerto Rico, Southeastern Biomedical not only provided valuable educational resources but also helped bridge the gap for professionals in a region without a dedicated biomedical association. This initiative serves as a reminder of the importance of continuous learning and professional development in a field that directly impacts patient care and safety.



































Today, the U.S. Food and Drug Administration announced its intent to expand the use of unannounced inspections at foreign manufacturing facilities that produce foods, essential medicines, and other medical products intended for American consumers and patients. This change builds upon the agency’s Office of Inspection and Investigations Foreign Unannounced Inspection Pilot program in India and China and aims to ensure that foreign companies will receive the same level of regulatory oversight and scrutiny as domestic companies.
“For too long, foreign companies have enjoyed a double standard—given advanced notice before facility inspections, while American manufacturers are held to rigorous standards with no such warning. That ends today. This is a key step for the FDA as part of a broader strategy to get foreign inspections back on track,” said FDA Commissioner Martin A. Makary, M.D, M.P.H.
In addition, the FDA will evaluate the agency’s policies and practices for improvements to the foreign inspection program to ensure that the FDA is the gold standard for regulatory oversight. These changes will include clarifying policies for FDA investigators to refuse travel accommodations from regulated industry including lodging and transportation arrangements (taxi, limousine, and for-hire vehicle transit), to maintain the integrity of the oversight process.
The FDA conducts approximately 12,000 domestic inspections and 3,000 foreign inspections each year in more than 90 countries. While U.S. manufacturers undergo frequent, unannounced inspections, foreign firms have often had weeks to prepare, undermining the integrity of the oversight process. Despite the advanced warning that foreign firms receive, the FDA still found serious deficiencies more than twice as often than during domestic inspections.
Only in specific programs and cases are the FDA’s domestic inspections pre-announced to assure that appropriate records and personnel will be available during the inspection. But regulated companies do not have the authority to negotiate the day or time of the inspection— nor should foreign companies have the capability to do so either.
With this shift, the FDA is further ensuring that every product entering the U.S. is safe, legitimate, and honestly made. Unannounced inspections will also help expose bad actors—those who falsify records or conceal violations—before they can put American lives at risk. The FDA is authorized to take regulatory action against any firm that seeks to delay, deny, or limit an inspection, or refuses to permit entry for an unannounced drug or device inspection.
“The FDA’s rigorous, science-based global inspections of manufacturing facilities ensure that the food and drug products that enter the U.S. marketplace, and the homes of American consumers, are safe, trusted, and accessible,” said FDA Assistant Commissioner for Inspections and Investigations Michael Rogers. “These inspections provide real-time evidence and insights that are essential for making fact-based regulatory decisions to protect public health.”
The FDA’s global inspections generate real-time intelligence that strengthens enforcement and keeps American families safe. Every inspection goes through a classification assignment process to enable an appropriate regulatory response. Even inspections that yield a “No Action Indicated” provide important regulatory intelligence that strengthens the safety net for American consumers.
This expanded approach marks a new era in FDA enforcement—stronger, smarter, and unapologetically in support the public health and safety of Americans.
The FDA is aware that BD and their subsidiary, C.R. Bard Urology and Critical Care, have issued a letter to affected customers that all lots of certain esophagogastric balloon tamponade tubes have updated use instructions. Users are sometimes unable, or find it difficult, to remove the plastic plugs from the rubber lumen to inflate the gastric and/or esophageal balloons. In some cases, the devices may become damaged during the removal of the plastic plugs and may require a replacement device.
Potential health consequences include delays in diagnosis or in treatment, which may result in the onset or prolongation of hypotension and its potential short- and long-term complications, up to and including death. This issue may also result in additional and unexpected diagnostic and medical interventions to manage the patient’s bleeding. BD has reported two serious injuries and one death associated with this issue.
The FDA has classified this recall as the most serious type. This device may cause serious injury or death if you continue to use it without following the updated instructions.
EAUX, France | DESKi has received FDA clearance for HeartFocus, its AI-powered heart exam software that allows any healthcare professional, even novices, to perform clinical-quality heart scans after just a few hours of training. The FDA also approved DESKi’s Predetermined Change Control Plan (PCCP), paving the way for faster integration with other ultrasound systems and broader patient access.
“Heart disease is the leading global killer, and there’s a critical shortage of cardiologists and sonographers,” said Dr. Bertrand Moal, CEO of DESKi. “HeartFocus helps bridge that gap, enabling providers anywhere to capture precise cardiac images. This clearance accelerates our mission to bring earlier diagnoses and life-saving care to all, including underserved areas.”
HeartFocus’ efficacy was proven in a major international trial with Northwell Health (U.S.) and CHU Bordeaux (France). In a blind review, 100% of scans performed by novices using HeartFocus met diagnostic standards, matching the quality of those by expert sonographers.
“This is a major step forward in community access to echocardiography,” said Dr. Varinder P. Singh of Northwell. “HeartFocus ensures faster diagnosis and treatment.”
Professor Stéphane Lafitte of CHU de Bordeaux added, “This AI breakthrough proves novices can reliably perform echocardiograms, potentially transforming global cardiac care.”
Nanox Imaging LTD, an innovative medical imaging technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Nanox.ARC X, its new multi-source digital tomosynthesis system. The FDA 510(k) clearance was received in less than 30 days from the date of submission and covers the production of tomographic images for general use, including the human musculoskeletal system and pulmonary, intra-abdominal and paranasal sinus indications, adjunctive to conventional radiography on adult patients.
The Nanox.ARC X maintains the Nanox.ARC’s proprietary digital Nanox.SOURCE and advanced tomosynthesis technology with a cold cathode, which enables it to create a more comprehensive, sliced three-dimensional view of the body, enhance visualization with multiple layers of images, and reduce the super-imposition of structures often seen in 2D X-rays. The Nanox.ARC X introduces a fully integrated, single-unit system with a streamlined design that significantly reduces the physical footprint. The system features ‘plug and play’ one-day installation capability, making advanced tomosynthesis technology more accessible to diverse healthcare settings with space constraints.
“The FDA clearance of the Nanox.ARC X marks an important evolution in our imaging technology,” said Erez Meltzer, Chief Executive Officer and Acting Chairman of Nanox. “By integrating our proprietary digital technology in this new imaging system, we’re making it easier for healthcare providers to adopt digital 3D imaging. This milestone advances our mission to expand access to essential medical imaging technology to more patients, regardless of their location. We look forward to introducing further capabilities to the Nanox.ARC X in the future and making them available through remote and immediate upgrades.”
The Nanox.ARC X is designed to be installed in any standard X-ray shielded room with minimal infrastructure requirements. The system operates on standard power (110v/230v 16A) and features a cables-free design for enhanced user and patient safety, ease of cleaning, and maintenance.
Nanox will offer the Nanox.ARC X system, later this year, alongside the current Nanox.ARC, expanding the Company’s product portfolio to meet diverse customer needs and use cases.
Scan the QR code or click here for a full list of FDA alerts.
9
SPONSORED BY

TOOLS OF THE TRADE
Save the date for this live webinar. Participation is eligible for 1 CE credit from the ACI.
SPONSORED BY

AEDs Under the Microscope: Why Testing is Non-Negotiable
SPONSORED BY
TOOLS OF THE TRADE Strategic Data Analysis and Planning for HTM Departments
SPONSORED BY
Expert Tips on Endoscope Maintenance with Frank Majerowicz


All webinars, podcast, and product demos are eligible for 1 CE credit from the ACI.
SPONSORED BY

Mastering Precision Gas Flow Measurement and Ventilation Testing
SPONSORED BY

TOOLS OF THE TRADE Exposure Management in Claroty xDome
21-25

Course: The Contrast Injector Service Training Course will teach the service technician the proper operation of contrast injectors, identify the injector components, proper PM and calibrations/calibration verification procedures and troubleshooting as well as the tools and test equipment needed. Each student will be trained on, and have the opportunity to perform, a PM and calibration/calibration verification on the following injector systems (student’s choice):
• ACIST CVi
• Mark 7 Arterion®
• Mark V Plus®
• ProVis®
• Stellant/Stellant Flex®
• Spectris Solaris®
• MRXperion®
• Empower CTA/CTA+
• Envision (manual only)
• Angiomat Illumena™
• CT 9000™ ADV (manual only)
• OptiVantage™
Aug. 4-15

Overview
Principles of Servicing
Diagnostic X-Ray Systems is a skills development program that teaches the new service professional the cognitive skills necessary to understand the X-ray system and its applications in the medical community. The program is divided into six major learning units:
• Introduction to radiography
• Radiation safety
• The production of X-rays
• Formation of the X-ray image
• Image receptor technologies
• PACS troubleshooting basics
The course contains lecture, demonstration, and hands-on training, which teach participants proper operation, radiation safety, image quality assessment, and global understanding of the Xray system.
Aug. 5-18

Course: Basic Principles of CT course is designed for service engineers. Course covers Basic CT principles, Theory, Image reconstruction, image performance, Simplified block diagrams. The course will be conducted in classroom and on fully functional CTsystems.
Qualifications for Admission: Students should have prior service experience, preferably with X-ray devices. They should have a minimum of a two- year degree in electronics or equivalent experience. Students should have basic computer skills and a laptop computer.
Course Overview: Features: Basic CT X-ray & theory principles, history, image generation algorithms, simplified CT system block diagrams, general CT Image Quality Assurance, and generic CT subsystems and identification overview.
Scan the QR code or click here for a full list of continuing education classes.




MedWrenchers have access to tons of industry resources from solving medical device problems to purchasing!

Stay up to date on industry news, events, FDA alerts, and more!




Ask tough medical equipment repair questions & help solve problems by sharing your expertise.


Beers, brats and biomeds with WBA!
JULY 31 - AUG. 1, 2025
MILWAUKEE, WI

Viva Las Vegas! AHRA is heading to Las Vegas, Nevada for their annual conference.
AUGUST 3-6, 2025
LAS VEGAS, NV
Don’t miss New England’s Biggest Health Technology Conference, NESCE 2025 HTM Symposium.
OCTOBER 15-16, 2025
MANCHESTER, NH
Dallas, TX • November 10-12, 2025

Yeehaw! MD Expo is heading to Dallas, TX!
NOVEMBER 10-12, 2025
DALLAS, TX
Join the global radiology community in exploring the future of precision medicine and inclusivity at RSNA 2025: Imaging the Individual.
NOV. 30- DEC. 4, 2025
CHICAGO, IL

FBS is headed to Disney World!
DECEMBER 4-7, 2025
ORLANDO, FL


