Helping Patients With Lymphoma Live Not Only Longer, But Better
Researcher Profile: Gita Thanarajasingam, MD



Helping Patients With Lymphoma Live Not Only Longer, But Better
Researcher Profile: Gita Thanarajasingam, MD


Pulse is a publication of the Lymphoma Research Foundation, providing the latest updates on the Foundation and its focus on lymphoma research, awareness, and education.
The Lymphoma Research Foundation is devoted to funding innovative lymphoma research and serving the lymphoma community through a comprehensive series of education programs, outreach initiatives, and patient services.
Steven Eichberg
Chair, Board of Directors
Ann S. LaCasce, MD, MMSc Chair, Scientific Advisory Board
Meghan Gutierrez
Chief Executive Officer
Editor Nichole Musumeci
Associate Editor
Paige Butler
Editorial Board
Micah Banner
Victor Gonzalez





National Headquarters
Wall Street Plaza
88 Pine Street, Suite 2400
New York, NY 10005
212 349 2910 lymphoma.org
Helpline
800 500 9976
helpline@lymphoma.org
Nick Guercio
Kyle Haines
Fatima Hameed
David Meehan-Romero
Eva Migliore
Laura Pedraza
Jessica Sharrow
Sarah Quinlan
Laura Wallenstein

The Lymphoma Research Foundation’s YouTube channel offers a wide variety of educational videos to help the lymphoma community learn about lymphoma. These videos provide disease-specific information as well as education regarding diagnosis, treatment options, clinical trials, and other resources for people dealing with a lymphoma diagnosis. Visit YouTube.com/c/LymphomaResearch to watch and subscribe.
Dr. Gita Thanarajasingam, a hematologist at Mayo Clinic, Rochester, is pioneering a patient-centered approach to lymphoma treatment. With a global upbringing and a deep passion for medicine, she has dedicated her career to improving treatment tolerability and understanding the full patient experience.
The Foundation returned to the American Society of Hematology (ASH) Annual Meeting and Exposition with more than 264 Foundation-affiliated scientists contributing to an abstract presented at the conference.
Longtime keyboardist for The Cure, Roger O’Donnell, shares his personal battle with lymphoma, from initial denial to navigating an 11-month chemotherapy journey. Back on stage, he finds healing in music, using his voice to inspire others facing similar challenges.



At the Lymphoma Research Foundation, we are working every day toward a better future for those touched by lymphoma and the promise of a world without this disease. Nowhere is this promise better realized than in the ever-expanding landscape of blood cancer research, where the scientific minds supported by the Foundation continue to forge new ground and break down barriers.
In this issue of Pulse, we highlight some of the latest advances in this space as we share updates from the 2024 American Society of Hematology (ASH) Annual Meeting. Over 264 Foundation-affiliated scientists, including members of the Foundation’s Scientific Advisory Board (SAB) and Foundation grantees, contributed to research presented at the conference, considered the premier event in hematology. The new ideas shared at the meeting represent the exciting first steps toward new breakthroughs, new treatments, and new possibilities for people with lymphoma.
You’ll also have the opportunity to meet Dr. Gita Thanarajasingam, whose path to medicine began in high school in Brazil, where she worked as an interpreter at a medical clinic in the Amazon, translating between English and Portuguese. Now, as a hematologist at the Mayo Clinic in Rochester, Minnesota, she is doing a different type of translation as she helps bridge the gap between treatment decisions and patient experience. Her work focused on patient-centric care makes it possible for lymphoma patients to live not just longer, but better.
Finally, when Roger O’Donnell, longtime keyboardist of the legendary alternative rock band The Cure, originally began experiencing concerning symptoms, he brushed them off until finishing a 2022 world tour. The last thing he expected was to be diagnosed with lymphoma. He shares his experience coping with the anxieties and challenges of treatment and how he carries his experience with lymphoma with him as he returns to the stage in this issue’s “Story of Hope.”
There is plenty of hard work to be done toward our mission of eradicating this disease — but the future has never been brighter. Thank you for continuing to be a partner in that hard work, and for bringing us closer each day to our shared vision of a world without lymphoma.
Sincerely yours,
Meghan Gutierrez Chief Executive Officer
The Lymphoma Research Foundation recognizes that engagement in public policy is critical to achieving its mission of realizing the promise of science to eradicate lymphoma and serve the community touched by this disease. The Foundation is committed to advocating on behalf of the entire lymphoma community in support of this mission, working collaboratively with policymakers, regulators, the medical community, patient advocates and other stakeholders to advance efforts that will improve the lives of people with lymphoma.
The Foundation supports policy measures which will increase federal funding for lymphoma research and ensure patient access to high quality cancer care and improved anti-cancer treatments. As an extension of these efforts, the Foundation seeks the integration of the lymphoma community’s perspective throughout the policymaking process and urges that lymphoma patients, survivors and their care partners be appointed to relevant federal panels, committees, and commissions.
The Lymphoma Research Foundation supports increasing federal investment in lymphoma research and drug development. These efforts include:
• Funding for the National Institutes of Health and the National Cancer Institute, including research intended to better understand every subtype of the disease, as well as support for the nation’s clinical trials enterprise with a focus on patient-centered outcomes and health equity.
• Funding for the Food and Drug Administration, to ensure efficient review of new cancer therapies and integration of the patient voice in drug development and review activities.
• Increased support for research and regulations that aid in the development of new therapies for rare lymphoma subtypes and underserved patient populations.
• Increased support for research activity to understand the needs of underserved patient populations, including those with cancer and those who are cancer survivors.
The Lymphoma Research Foundation endorses policies which seek to increase access to comprehensive lymphoma treatment and cancer survivorship care, including:
• Legislation that ensures access to adequate and affordable health insurance coverage for people with lymphoma.
• Payment and delivery reform efforts that assist health care providers in improving the quality of cancer care in all communities in the United States.
• A health care system that collects real world data to support access to therapies and quality improvement.
• Coverage standards in public and private health insurance systems to protect access to all approved anticancer regimens including, but not limited to oral and intravenous drugs, injections, cellular and gene therapy, surgery, radiation and transplantation.
• Implementation of policies which support the development of new and improved therapies and clinical trials for rare diseases.
• Policies intended to address the consistent drug shortages in the United States which threaten access to life-saving lymphoma treatments.
The Lymphoma Research Foundation endorses policies which recognizes the increased number of cancer survivors in the United States and the needs of people living with and after a lymphoma diagnosis. These efforts include:
• Legislation which prioritizes the study of cancer survivorship and the needs of cancer survivors.
• Legislation which protects access to quality health insurance for anyone with a preexisting condition.
• Health care payment and delivery reforms that will incentivize delivery of quality survivorship care for patients across the cancer care continuum, inclusive of education and provider reimbursement for cancer care planning.



$190,000
On November 8, 2024, the inaugural Emerging Philanthropists Gala brought together New York’s leading young philanthropists to celebrate the latest advancements made in lymphoma research, patient care, and the dedicated individuals and institutions who help make our work possible. Together, the group raised more than $190,000 to support the Foundation’s mission. The event at the Bowery Hotel featured music by Stevie Guttman, gourmet bites and cocktails from local partners, a silent auction, and much more. The next Emerging Philanthropists Gala is planned for September 2025.
On November 22, 2024, Foundation supporters joined us at TPC Potomac at Avenel Farm for our Maryland Partners in Purpose, A Scientific Social. The event, held at one of Washington, D.C.’s premier golf courses, offered attendees the opportunity to learn more about the Foundation’s critical research portfolio while enjoying cocktails and a seated dinner. Guests raised over $50,000 in support of the lymphoma community while celebrating some our most dedicated donor partners and area advocates.
Our community of supporters makes our work possible — and they showcased their continued generosity and commitment on Giving Tuesday 2024. Giving Tuesday is a global day of giving that takes place annually on the Tuesday following Thanksgiving. This year, Foundation donors raised more than $33,000 to support the Foundation’s lifesaving mission of supporting patients, survivors, care partners, and researchers.

Join the Lymphoma Research Foundation at the iconic La Gorce Country Club and put your golf skills to the test at one of the most exclusive private golf clubs in the U S , designed by Jack Nicklaus, while being a catalyst in finding cures Golfers will enjoy premium gift bags, breakfast and lunch, course refreshments, a cocktail reception, heavy hors d’oeuvres, and prizes.
Monday, May 5, 2025
La Gorce Country Club, 5685 Alton Road, Miami Beach, FL 33140
Event Chairs
Leigh & Cory Olson
Benefit Committee
Walter Andrews | Laurie & Steve Davis | Barbara Freundlich | Christopher Gorelik
Patricia Kaine | Leah & John Leonard, MD | Ann Singer | Sonya Verny
Pricing Tiers
$8,000 Foursomes | $5,000-$100,000 Sponsorships
Please RSVP by Monday, April 21, 2025
You may visit us online at lymphoma.org/FloridaGolf to RVSP For more information, please contact Fatima Hameed at events@lymphoma org
Thank You to Our Sponsors
Presenting Sponsors
Leigh & Cory Olson
Platinum Sponsors
Laura & Lloyd Blankfein
Patricia Kaine
Hole Sponsors
Gold Sponsor
C F il

Walter Andrews | Jean & Steven Eichberg | Barbara Freundlich
Prince Family Foundation | Kristi & Liam Hendriks
Chike Springer Foundation | Robert Werner



When Joanne Downing first noticed a lump in her neck while playing golf one sunny Florida day in 2001, her first inclination was to dismiss it. However, her friend and golf partner — a former nurse — strongly encouraged her to see a doctor as soon as possible. Joanne made an appointment a few days later, and her doctor ordered an MRI to see what was going on.
“I’m lying there on the table and my doctor came in and said, ‘We have your results,’ putting them up on a screen,” said Downing. “My eyes immediately went to these white spots all over my body. I thought it might be ovarian cancer, but he said, ‘No, look at the white spots. Every white spot is a lymph node.’”
After going back to Georgia to meet with her primary care physician, Joanne was referred to an oncologist, who diagnosed her with stage four follicular lymphoma (FL). She underwent treatment for six months and then another six months of maintenance, which successfully kept her lymphoma in remission for 11 years.
In 2012, however, Joanne’s lymphoma returned. Not being one to sit still for very long, Joanne dove into her new treatment, motivated by a strong desire to be there for her family.
“I have always been an energetic person, and I like doing a lot of things, and I was like, ‘Let’s get this show on the road!’” said Joanne. “I’m an only child, and we adopted our two children, so it’s always been very important to me to be with our children and grandchildren. That was one of the reasons I was determined to get better, because I really wanted to spend time with and get to know my grandkids. That gave me the energy to keep going.”
Joanne underwent a variety of treatments before being treated with rituximab (Rituxan), which proved effective for her. Today, she undergoes maintenance every two to three months and full body scans every six months to make sure that her lymphoma is under control. Her husband, George, has been by her side every step of the way.
“I refuse to give up,” said Joanne. “I’m a fighter, so I keep on trying. You just never know. I’ve found most things in life are that way. If you want to succeed, you need to work for it. My thinking has always been, ‘Let’s get on with the things that we have to do, and let’s enjoy what we’re doing.’ I don’t want to think about it — I want to DO things, so that’s what I did.”
Today, at 83, Joanne and George have two grown children, two grandchildren, and a great-grandchild on the way, along with two lovingly spoiled dogs — Sweet Pepper and Cutie Pie. They split their time among their homes in Florida, Georgia, and North Carolina.

“I don’t play golf or tennis anymore, but I have learned to play mahjong, which I enjoy,” she said. “And of course, I do a lot of reading and enjoy spoiling our two dogs and being there for my family. My granddaughter is now pregnant with her first child, and I can’t wait to hold my greatgrandbaby. That’s definitely a motivator for me.”
Joanne may no longer be on the tennis court or golf course, but she’s still driven to succeed.
“I always set these tasks for myself, and I do them because they’re on my list,” she said. “I’m going to accomplish what I want to do. If I’ve got 13 things I want to do today, I’m going to get as many of them done as I can. That’s just the way I live my life, and it works for me.”
So what’s her secret? Joanne says that she and George are both die-hard optimists who don’t dwell on the negative.
“We look at the good side, and we look at what things can be accomplished. We focus on things that are going to happen, and we don’t dwell on things that have happened,” she said. “We’ve always believed that life is good, and you can always work on it to make it better.”
We’ve always believed that life is good, and you can always work on it to make it better.
A big part of making their lives better is that Joanne and George regularly donate to the Lymphoma Research Foundation. The couple recently made their largest-ever contribution in support of the Foundation’s research program, challenging others to do the same.
“I won’t give money just to give money. I want to give to a Foundation that is trying to help people with my type of lymphoma,” she said. “We wanted to find an organization that understands lymphoma and its impact on those diagnosed. After doing a lot of research, we decided to support the Lymphoma Research Foundation because we believe in the work they’re doing.”
Joanne and George are particularly impressed by the Foundation’s unwavering commitment to finding a cure.
“It’s important to know that someone is out there looking for a cure, that somebody cares,” said Joanne. “We were interested in research being done on my type of lymphoma, and it was very important to us to get to know some of the doctors doing the research. Through the Foundation, we’ve been able to hear about what research is in process, what’s worked, what hasn’t worked, and what might work for me. What we hope for — what we all hope for — is finding a cure.”
Having now lived almost 22 years with lymphoma, Joanne is clear that the key to hope lies in conducting more research into potential cures and improving each patient’s quality of life. She remains very hopeful and looks forward to what’s next.
“I’m just glad that that I’ve had such a wonderful oncologist and that I’ve had a great support system with my family,” said Joanne. “And I’m grateful that I’ve been able to spend almost 22 years working on this and I’m still going strong, so now I’m waiting for my first great-grandbaby to come. So, there’s hope. There’s always hope.”

Through the Foundation, we’ve been able to hear about what research is in process, what’s worked, what hasn’t worked, and what might work for me. What we hope for — what we all hope for — is finding a cure.
Joanne and George have committed to funding research for the Lymphoma Research Foundation and are challenging others to do the same. The Downings will match every gift made to the Foundation’s research efforts, up to a total of $50,000.
Visit giving.lymphoma.org/research to accept the challenge and support lifesaving research.
The Casino, Chicago, IL MAY 29, 2025 | 6:30 PM - 9:30 PM

The Lymphoma Research Foundation is delighted to host Partners in Purpose on May 29, 2025, at The Casino in Chicago, IL This event will showcase the many facets of the Foundation’s critical mission in one event and highlight our most important asset: our Partners in Purpose
A bou t t he Ev ent

Over 300 guests are expected to attend the event, which will bring together key leaders from the lymphoma community for an evening of celebration and tribute. Attendees will include patients, survivors, care partners, the Foundation’s esteemed Scientific Advisory Board members, Foundation research grantees, pharmaceutical and biotech industry partners, and many local businesses who champion our work.
W hat t o Ex pect
The evening will feature live music, premium drinks and hors d'oeuvres, a silent auction, an awards presentation, and an update on Foundation-funded research, programming, and patient services
R S V P T oday
To RSVP online, please visit lymphoma.org/PartnersinPurpose

For more information, contact Sara Lewandowski, Manager of Event Fundraising, at slewandowski@lymphoma.org or by phone at (646) 465-9127.





As the daughter of a Malaysian diplomat and an international environmental lawyer, Gita Thanarajasingam, MD, a hematologist at Mayo Clinic in Rochester, Minnesota, had a truly global upbringing. From Kuala Lumpur to New Delhi and New York, to graduating from high school in Brasil, her international experiences have helped shape her unique perspective on helping others — one that would eventually lead her to revolutionize how we think about lymphoma treatment.
Dr. Thanarajasingam’s path to medicine began during high school in Brazil, where she worked as an informal interpreter at a medical clinic in the Amazon region.
“I am the child of two people who dedicated their lives to making the world a better place,” she said. “Neither of my parents were physicians, but they emphasized the endeavor to make things better for the people around you, and medicine was a natural connection to that.”
“After I’d been there about two years, my Portuguese was passable enough that I was useful for translating between international physician volunteers and local site staff and patients,” she said. “That’s when I realized that medicine is a universal language that encompasses a truly meaningful way to help people.”
That early experience set her on a path through Yale University, medical school and fellowship at Mayo Clinic, residency at the Brigham and Women’s Hospital at Harvard University, and eventually to her current role as a lymphoma specialist and researcher at Mayo Clinic in Minnesota. She found her true calling while in medical school.
“I fell in love with hematology during my second year of medical school,” she said. “The blood is just so complex, and it’s so important. I remember the hematology course director Dr. Alexandra Wolanskyj-Spinner, who evolved into a crucial mentor to me, showing the first picture of a blood smear I had seen. It looked like art to me. It was so beautiful and so powerful, and I became inspired by hematology, which felt like a natural fit for me.”
Today, Dr. Thanarajasingam lives in Rochester, Minnesota with her husband, Ben, who is an emergency physician, their 11-year-old daughter, Kiran, and 7 year-old son, Arjun. Her research focuses on an often-overlooked aspect of cancer treatment: treatment tolerability for the patient. While much attention is rightfully paid to treatment effectiveness, she identified a critical gap in understanding the nuances of a patient’s journey while undergoing treatment.
Her typical workdays are spread among seeing patients with a variety of subtypes of lymphoma and in a variety of different stages of treatment in Mayo Clinic’s outpatient clinic. She also takes care of lymphoma patients who are hospitalized for treatment or diseaserelated symptoms. Simultaneously, she juggles clinical responsibilities with research time, collaborating with her mentors and peers locally, nationally, and internationally to further her research program in measuring cancer treatment tolerability.
“When we think about what matters to patients, of course treatments need to be effective and put the disease into remission,” she said. “However, if you’ve ever been the friend or family member who walks the journey with a patient who has lymphoma or another cancer, you know that it’s not just about the efficacy — it’s also about the safety and the tolerability of the treatment — how a patient feels and functions on and after cancer treatment.”
Dr. Thanarajasingam’s focus on the patient experience has become increasingly important as new treatment options emerge.

There’s a lot of fancy things we can do to measure how patients feel and function, but perhaps one of the best is simply asking them to report it directly.
“We’re living in an era now where there has been a great deal of progress in novel treatments and personalized medicine, so we are often fortunate to have several different treatment options for patients,” she said. “What’s the biology of the tumor? What are the mutations that your lymphoma expresses? What’s its gene expression profile? How do we expect this cancer to behave? It’s no longer a question of, ‘Do I have something to treat a patient?’ but often it is, ‘Which of the options is best to treat the specific patient in front of me?”
Dr. Thanarajasingam’s research has helped pioneer new ways of incorporating patient-generated data into treatment decisions. This includes everything from traditional patient surveys (patient-reported outcomes) to the cutting-edge use of wearable devices to measure physical function during treatment. Even with all the technological innovation taking place in the field, Dr. Thanarajasingam emphasizes the value of simply asking patients about their experiences.
“There’s a lot of fancy things we can do to measure how patients feel and function,” she reflects. “But perhaps one of the best is simply asking them to report it directly.”
This patient-centered approach has led to important insights and a deeper understanding of patients’ journeys with lymphoma. She cites the case of a 21-year-old woman with Hodgkin lymphoma who went through multiple rounds of treatment.
“Years later, when I invited my patient to speak to the medical students I was teaching, I learned something that was shocking to me,” said Dr. Thanarajasingam. “She asked the students, ‘Do you know what the worst part of my treatment was?’ I was thinking she was going to say hearing the diagnosis or going through the stem cell transplant. Instead, she shared, ‘When I couldn’t figure out if I would have the money to do an egg retrieval to preserve my fertility before my stem cell transplant.’”
Such revelations drive home the importance of understanding multiple aspects of the patient experience, beyond medical outcomes. It’s this type of insight that has helped shape Dr. Thanarajasingam’s approach to both research and clinical care.
Her work has gained recognition from the U.S. Food and Drug Administration (FDA), which has increasingly highlighted the importance of patient-reported outcomes reflecting treatment side effects and impacts on physical function in cancer clinical trials. This shift represents a broader change in how the medical community thinks about cancer treatment — moving from a purely clinical focus to one that takes the full patient experience into consideration.
Looking ahead, Dr. Thanarajasingam sees both challenges and opportunities in lymphoma research. While celebrating the “incredible growth in the amount of treatment options we have for patients,” she acknowledges there’s still work to be done.
“There are several high-priority areas where we want to see more improvements, including better treatments and cures, such as with aggressive T-cell lymphomas, central nervous system lymphomas, aggressive B-cell lymphomas after CAR-T cell therapy, among others,” she said. “And in parallel, we want to see patients in these challenging situations feeling and functioning better while we treat their disease and after.”
Dr. Thanarajasingam’s relationship with the Lymphoma Research Foundation began when she was selected as a Scholar through the Foundation’s Lymphoma Scientific Research Mentoring Program (LSRMP), a pivotal moment in her career.
“I first came to the Foundation very earnestly with a project involving analyzing and reporting side effects of treatment differently,” she said. “The mentoring program furnished access to internationally recognized lymphoma clinical investigators who served as mentors and gave me confidence in my ideas. While most of my fellow
participants focused on clinical trials, my LSRMP mentors helped affirm the relevance of my interest in measuring side effects that patients experience.”
That early support has evolved into a long-term relationship. Today, Dr. Thanarajasingam serves on multiple Foundation committees, including as faculty for the very mentoring program that helped shape her career. She’s also actively involved in the Foundation’s Adolescent and Young Adult (AYA) scientific workshops, serves on the Survivorship Working Group, and is on the Steering Committee for the Foundation’s National Virtual Lymphoma Rounds Program. This summer, she will be honored at the Foundation’s Lymphoma Walk in Minnesota and looks forward to fundraising in that effort.
“The Foundation helped me build a network of people who can support and mentor me in my research. As a result of the strong base it helped me build at an early stage in my career, I have received grants from the U.S. National Cancer Institute and FDA, among others, to fund my research program, and I am thrilled when I can then bring that expertise back to the Foundation.”
Dr. Thanarajasingam particularly values how the Foundation supports innovative approaches to research.
“The Foundation is very good at tuning into out-of-thebox thinking, which is why we see this organization push forward so much progress. It also goes beyond the research,” she said. “I had an 82-year-old patient diagnosed with an aggressive B-cell lymphoma who was hospitalized as a result of her symptoms and just about to start treatment. Our hospital team gave her a little purple tote bag that the Foundation had sent for distribution to our patients. She opened up the enclosed blanket and socks with gratitude for the comfort they provided. In addition to the ways Mayo Clinic has supported her during her diagnosis and starting treatment, she was extraordinarily touched by this gesture from the Foundation. It is just one example that reflects how patient-centered the organization is.”
Looking to the future, Dr. Thanarajasingam sees the Foundation’s role becoming increasingly vital in advancing lymphoma research and patient care.
“The Foundation does two things: It helps advance cures and improve treatments, and it supports our patients in that journey. That’s what we need, now more than ever,” she said. “It’s about patients with lymphoma not only living longer but living better.”
The Foundation is very good at tuning into out-of-the-box thinking, which is why we see this organization push forward so much progress.

Whether you are newly diagnosed, want detailed information about your lymphoma subtype, are looking for ongoing support, or are seeking help with long-term survivorship, we are here to help.
3/27 - Update on Diffuse Large B-Cell Lymphoma Webinar
4/2 - Lymphoma Talk: Miami
4/10 - Fertility and Family Building for Lymphoma Patients Webinar
4/17 - Pregunte al doctor sobre el linfoma
4/19 - Living with Lymphoma
4/30 - Lymphoma Talk: New York City
5/7 - Lymphoma Talk: Chicago
Visit lymphoma.org/programs to register and learn more.

Helen Ma, MD, MS, is the assistant professor in the Division of Hematology and Oncology in the Department of Medicine at the University of California, Irvine.
When did you become interested in the study of medicine and in lymphoma specifically?
I first became interested in medicine when I saw the potential of our daily choices in maintaining good health. Many diseases can be prevented with a balanced diet and exercise, especially when our basic needs are met. I was interested in primary care but was drawn to the meaningful relationships and goals of care conversations surrounding the management of patients with cancer. Lymphoma, in particular, captured my interest because my mother was diagnosed with a rare and aggressive subtype. It became personal as I saw firsthand how important access to treatments and clinical trials could be, particularly for patients like my mother who had exhausted treatment options. This motivated me to explore ways to make a meaningful impact in improving outcomes for all lymphoma patients.
At what point in your career did you receive funding from the Lymphoma Research Foundation? What kind of grant(s) did you receive?
I was fortunate to receive my first grant from the Foundation early in my career when I was establishing myself as a lymphoma researcher. The Foundation’s Lymphoma Scientific Research Mentoring Program (LSRMP) connected me to many lymphoma experts who were wonderful and supportive mentors. They helped connect me to people and ideas that propelled my research ideas and proposals forward. This grant was pivotal in shaping my career trajectory, providing the resources and mentorship to explore research interests related to military exposures and the development of lymphomas.
What scientific project did you pursue as part of your research grant(s), and how did the Foundation support your interest in working with veterans?
As part of my Lymphoma Research Foundation grant, I investigated the association between military exposures and the development of lymphoma. The Lymphoma Research Foundation’s support was not just financial; it connected me with a network of researchers and mentors who shared my passion for making a real difference in patients’ lives. When I began working with veterans, particularly those at risk for lymphoma due to military exposures, the Foundation’s encouragement helped me align my research focus with the unique needs of the veteran population, which often faces different challenges in lymphoma diagnosis and treatment.
How has the treatment landscape for lymphoma/CLL changed since you first started conducting your research?
The landscape has shifted and is still constantly changing. I was able to publish an analysis on first-line treatments of chronic lymphocytic leukemia (CLL) within the national U.S. Veterans Administration (VA) healthcare system. In the beginning, treatments were largely centered around chemotherapy, with limited options for patients who relapsed or were refractory to standard therapies. Over time, thanks to clinical trials and advancements in targeted therapy, many patients with CLL have access to more effective and less toxic treatments. These breakthroughs have improved not only survival rates but also the quality of life for many patients.
The Foundation’s Lymphoma Scientific Research Mentoring Program (LSRMP) connected me to many lymphoma experts who were wonderful and supportive mentors.
Was the support and grant funding you received from the Foundation vital to advancing/ dedicating your career to studying lymphoma?
Absolutely. The Foundation’s funding not only was essential for my early research but also provided the confidence and opportunity to dedicate myself fully to lymphoma research. It allowed me to explore ideas that might not have been possible without this support while also fostering connections with experts in the field. The Foundation’s commitment to advancing lymphoma research inspired me to stay focused on improving patient outcomes, especially for underserved groups like veterans.
How has care for veterans evolved since you started working at the VA Long Beach Healthcare System in Long Beach?
Care for veterans has evolved in several meaningful ways. There’s been a significant increase in access to specialized care, with more personalized treatment plans that take into account the unique medical histories and exposures many veterans have. In particular, the VA system has been moving toward a more integrated, multidisciplinary approach to care, where oncologists and other specialists work together to address not only lymphoma but also any comorbid conditions common in veterans. This holistic approach has made a noticeable difference in patient outcomes and overall well-being.
How has your involvement with the Foundation continued since becoming a Foundation Scholar?
During and after the LSRMP grant period ended, I have been involved with the Foundation. It has been a privilege to continue collaborating with them through lymphoma rounds, various research initiatives, and workshops. The Foundation’s ongoing support has been invaluable in my career, and I’m grateful for the opportunity to contribute back to the community however possible.
Why is the Foundation’s mission and focus on lymphoma-specific research and programming important? In other words: How would the lymphoma community be impacted if there was no Lymphoma Research Foundation?
The Foundation plays a critical role in advancing our understanding of lymphoma and improving outcomes for researchers and patients. Without their dedicated focus on lymphoma-specific research and programming, progress in treatment development would likely be slower, and patients would miss out on the support services, education, and advocacy that the Foundation provides. The Foundation’s work fills a unique gap, providing not only funding for innovative research but also raising awareness and ensuring that lymphoma patients and their families have access to the information and care they need.
What research or projects are you currently pursuing that you would like to share with our readers?
Currently, I am focused on investigating how military exposures may impact the development in lymphoma. Using the Million Veteran Program, I am studying how genetic and environmental factors might contribute to the development of lymphoma in veterans, with the hope of identifying new biomarkers for early detection and more effective treatments. I am also involved with the SWOG Cancer Research Network as the VA liaison in the lymphoma committee so cutting-edge clinical trials are available to our veterans.
What are you most excited about in the field of lymphoma research today? Why?
What excites me most is the potential of combining novel targeted therapies to create more personalized, less toxic treatments for lymphoma. The idea that we are moving toward a future where patients no longer have to endure harsh chemotherapy regimens is incredibly inspiring. Additionally, the advances in understanding the molecular and genetic landscape of lymphoma have opened up new avenues for targeted therapies, giving hope to patients who previously had limited options. The pace of innovation in the field is faster than ever, and it’s a truly exciting time to be involved in lymphoma research.

Sunday, April 27, 2025
The Comus Inn, Dickerson, MD
Features Three Routes: 15 Mile, 30 Mile and 50 Mile Courses
Join us on Sunday, April 27, 2025, as we ride toward fulfilling our mission to realize the promise of science to eradicate lymphoma and serve the community touched by this disease lymphoma.org/ResearchRide
The 18th Annual Lymphoma Research Ride is a non-competitive cycling event based at the Comus Inn in Dickerson, MD. The ride combines stunning scenery on a challenging course through vineyards and farmlands to create an unforgettable riding experience and a chance to make a powerful impact. Survivors, caregivers, families, friends, and corporate teams ride in honor and memory of those whose lives have been touched by lymphoma and chronic lymphocytic leukemia (CLL).
This event is fully supported by 100 Volunteers and staff who provide the following:
Morning kick-off including buffet breakfast and entertainment | Rest stops with water, electrolyte solutions, and snacks | Sweep vehicles to pick you up along the route if needed
Medical support | Bike mechanics support
Celebrate after the ride with a gourmet lunch buffet and a selection of alcoholic beverages.

For more information, please contact Danielle Romain, Associate Director of Events, at (646) 465-9106 or dromain@lymphoma org
The Lymphoma Research Foundation returned to the American Society of Hematology (ASH) Annual Meeting and Exposition with 264 Foundation-affiliated scientists, including past and present members of the Foundation’s world-leading Scientific Advisory Board (SAB) and former and current grantees, contributing to an abstract presented at the conference.
Widely regarded as the premier event in malignant and non-malignant hematology, the ASH meeting provides a critical forum for leading hematologists/oncologists to present their findings to over 20,000 of their peers. Among the distinguished scientists attending, more than 80 Foundation-affiliated scientists presented at the 2024 ASH Annual Meeting.
In addition to the Foundation’s representation through presentations, the Foundation’s researchers contributed to 743 abstracts across all hematology and 698 lymphomarelated abstracts. 100 percent of all Foundation scholars — participants in the Foundation’s Lymphoma Scientific Research Mentoring Program (LSRMP) — authored at least one abstract at ASH.

Chimeric antigen receptor (CAR) T-cell therapies have become a standard treatment option for many patients with relapsed or refractory mantle cell lymphoma (MCL). While these therapies are considered highly effective in this setting, many patients still relapse, and there is not much known about outcomes after disease progression post-CAR T-cell therapy. Investigators therefore analyzed patient records from nine different institutions in the United States to better understand treatment patterns and outcomes following disease progression after CD19directed CAR T-cell therapy. This research was presented by Foundation research grantee Zachary D. EpsteinPeterson, MD, of Memorial Sloan Kettering Cancer Center.
The analysis included data from 104 patients who experienced disease progression after brexucabtagene autoleucel (88%) or lisocabtagene maraleucel (12%). Among these patients, 71% had a complete response to initial CAR T-cell therapy, and 13% had a partial response; 15% had no response. The median time from infusion to progression was six months.
After disease progression, 78 patients received a different systemic anti-lymphoma therapy. The most commonly used therapies were pirtobrutinib, chemoimmunotherapy, venetoclax, bispecific antibodies, and small molecule combinations. The overall response rate across all systemic therapies immediately post-CAR T-cell therapy was 42%, with the highest response rates seen with bispecific antibodies (60%) and small molecule combinations (50%).
With a median 15.2 months of follow-up, the median progression-free interval among these patients was 2.3 months and median overall survival was 5.4 months. Survival outcomes were worse in patients with TP53 tumor mutations, older adults (≥65 years of age), and those with no initial response to CAR T-cell therapy.
This study also included contributions from Foundation SAB members Kami J. Maddocks, MD, of The Ohio State University; Gilles Salles, MD, PhD, of Memorial Sloan Kettering Cancer Center; Andrew D. Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center; and Foundation grantees Elise A. Chong, MD, of the University of Pennsylvania; Andrew Ip, MD, MSc, of Hackensack Meridian Health; Caron A. Jacobson, MD, MMSc, of
Dana-Farber Cancer Institute; Manali K. Kamdar, MD, of University of Colorado; Reid W. Merryman, MD, of Dana-Farber Cancer Institute; Javier Munoz, MD, of Mayo Clinic, Phoenix; Peter A. Riedell, MD, of University of Chicago; Christine E. Ryan, MD, of Dana-Farber Cancer Institute; Roni Shouval, MD, PhD, of Memorial Sloan Kettering Cancer Institute; Yucai Wang, MD, PhD, of Mayo Clinic, Rochester; Samuel Yamshon, MD, of Weill Cornell Medicine; Lia Palomba, MD, of Memorial Sloan Kettering Cancer Center; Preetesh Jain, MD, MBBS, PhD, DM, of The University of Texas MD Anderson Cancer Center; and Anita Kumar, MD, of Memorial Sloan Kettering Cancer Center.
Loncastuximab tesirine (loncastuximab) is an antibodydrug conjugate used to treat certain types of B-cell lymphomas. The phase I study of loncastuximab showed promise in a small group of patients with relapsed/ refractory follicular lymphoma (FL) as well, but further studies in this population have been lacking. Investigators therefore led a phase II study to evaluate the efficacy and safety of loncastuximab in combination with rituximab for patients with relapsed/refractory FL. Results from this study were presented by Foundation grantee Juan Alderuccio, MD, of Sylvester Comprehensive Cancer Center.

The study included 39 adults from a single institution who had previously received at least one line of systemic therapy for FL (range from one to six). Patients ranged in age from 47 to 89 years and consisted primarily of those with advanced (82%) and high-risk (61.5%) disease. Most patients (56.5%) received first-line R-CHOP.
Among 35 patients with available response data, the overall response rate at 12 weeks was 97.1%. A complete metabolic response, as observed using PET/CT scan, was observed in 68.6% of participants, and a partial metabolic response was observed in 28.6%. All complete metabolic responses were maintained, and 40% of the partial metabolic responses observed improved to a complete response at week 21. The 12-month progressionfree survival rate was 94.2%, and the overall survival rate was 93.3%. The overall survival rate was maintained up to 18 months, and more than 90% of patients remained progression-free at 18 months.
The most common adverse events included neutropenia, elevations in liver enzymes, anemia, maulopapular rash, and fatigue. Fluid accumulation was also reported but was mostly mild and was treatable with diuretics. Five cases of severe neutropenia were reported. No treatmentrelated deaths occurred during the trial.
The authors concluded that the results of the study demonstrate “dramatic” activity for loncastuximab plus rituximab in patients with high-risk relapsed/refractory lymphoma and note that a multicenter expansion study is currently in progress.
This study also included contributions from former Foundation SAB member Izidore Lossos, MD, of Sylvester Comprehensive Cancer Center, and Craig H. Moskowitz, MD, of Sylvester Comprehensive Cancer Center; and Foundation former grantee Jonathan H. Schatz, MD, of University of Miami.
Combination therapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, known as R-CHOP, is a standard first-line treatment option for patients with diffuse large B-cell lymphoma (DLBCL). Durable remissions are observed in about 60% of patients who receive R-CHOP, and recent efforts have focused on new combinations that can help deepen remissions and extend progression-free survival.
Several advancements in DLBCL treatment have been made in recent years, including the approval of the PolaR-CHP, an R-CHOP derivative that includes the addition of the antibody-drug conjugate polatuzumab vedotin. A novel CD20-directed bispecific antibody known as glofitamab has also been approved for use in relapsed/ refractory DLBCL for patients who have received at least two other prior lines of therapy. In preliminary trials, use of glofitamab in combination with the Pola-R-CHP regimen has shown promise in the first-line treatment setting for DLBCL, including for those with high-risk disease.
To expand upon these results, Foundation SAB member Ranjana H. Advani, MD, of Stanford Cancer Institute presented the design of a new phase III study, SKYGLO, which will evaluate the efficacy and safety of glofitamab plus Pola-R-CHP versus Pola-R-CHP alone in patients with previously untreated CD20-positive LBCL.
The SKYGLO study, which began enrollment in September 2023, will include an anticipated 1130 adults (up to 80 years of age) from up to 260 centers from around the world. Participants will be randomized to receive either glofitamab plus Pola-R-CHP or Pola-R-CHP alone for
8 cycles of 21 days each. The primary endpoint of the study will be progression-free survival, defined as the time from randomization to the first occurrence of disease progression, relapse, or death. Other endpoints will include safety, response rates, and overall survival. Survival rates will also be analyzed based on risk status to assess the efficacy in patients with high-risk disease.
This study also included contributions from former Foundation SAB member Brad Kahl, MD, of Washington University Medical School and Foundation SAB member and grantee Alex Herrera, MD, of City of Hope.
As treatment options for classic Hodgkin lymphoma (cHL) have improved, minimizing late effects while maximizing treatment efficacy has become a critical goal in early stages of treatment. Investigators therefore examined whether the incorporation of brentuximab vedotin (BV) and nivolumab in the treatment of early-stage cHL could improve progression-free survival without the use of radiotherapy. Results from this phase II study were presented by Ann S. LaCasce, MD at Dana-Farber Cancer Institute, Chair of the Foundation’s SAB.
The study included 153 patients with a median age of 31 years (range, 18-73 years). Nearly two-thirds of patients were classified as having unfavorable disease, and one-third had bulky disease at study entry. All participants received initial ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine therapy). After two cycles, participants underwent PET/CT to determine the next round of treatment.
Among patients with non-bulky PET2-negative disease after initial ABVD, the 18- and 24-month progression-free survival rates were 100%. Patients in this group received either two cycles of additional ABVD plus consolidation nivolumab (three months) or BV plus nivolumab administered every three weeks. Among PET2-negative patients with bulky disease, progression-free survival was 97% at 18 months and 86% at 24 months; patients in this group also received two additional cycles of ABVD plus consolidation nivolumab. Patients with PET2-positive disease received 4 cycles of BV-AVD plus consolidation nivolumab and had 18- and 24-month progressionfree survival rates of 81% and 76%. No patients in any treatment arm received radiotherapy, and no deaths were reported in any treatment group.
The investigators concluded that the incorporation of nivolumab consolidation therapy, with or without BV, provides good disease control in cHL, with a manageable safety profile. They noted that the results of this study support the ongoing AHOD2131 study exploring the incorporation of BV plus nivolumab in early-stage cHL. This study also included contributions from Foundation SAB members Lisa Giulino Roth, MD, of Weill Cornell Medicine; Kristie Blum, MD, of Winship Cancer Institute of Emory University; Sonali M. Smith, MD, of University of Chicago; and Alex Herrera, MD, of City of Hope; and former SAB member Jane N. Winter, MD, of Robert H. Lurie Comprehensive Cancer Center.
Use of fixed-duration Bruton tyrosine kinase inhibitor (BTKi) therapy in treatment-naïve chronic lymphocytic leukemia (CLL) is complicated by the limited efficacy and safety concerns observed with first-generation BTKis. The safety and efficacy of second-generation BTKis in this setting has yet to be established, though; thus the phase III AMPLIFY study aimed to assess the use of fixedduration acalabrutinib plus venetoclax (with or without obinutuzimab) vs chemoimmunotherapy in adults with treatment-naïve CLL. Interim results from this study were presented by Foundation SAB member Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute.
The study included 867 patients (median age, 61 years) who were randomized to receive either acalabrutinib plus venetoclax (AV), acalabrutinib/venetoclax plus obinutuzumab (AVO), or the investigator’s choice of chemoimmunotherapy.
The overall response rate with AV and AVO was 92.8% and 92.7%, respectively, compared with 75.2% with chemoimmunotherapy. At a median 41 months of follow-up, progression-free survival with chemoimmunotherapy was 35% with AV and 58% with AVO. Overall survival benefits were also observed with AV vs chemoimmunotherapy. Serious adverse events occurred in 24.7% of participants in the AV group, 38.4% in the AVO group, and 27.4% in the chemoimmunotherapy group.
The investigators concluded that the second-generation BTKi fixed-dose AV and AVO combination regimens provided deep and durable responses with a manageable safety profile in first-line treatment of CLL. Final results from the study are pending.
Patients with CLL and small lymphocytic lymphoma (SLL) have previously been shown to be at increased risk for developing other malignancies relative to the general population. Chemoimmunotherapy has historically been the mainstay of treatment for CLL and SLL, though, which is also known to be associated with increased risks for other cancers. As newer therapies — including targeted therapies and monoclonal antibodies — have become the standard of care, investigators sought to compare the incidence of other cancers in patients treated with newer therapies or chemoimmunotherapy or patients managed with active surveillance.
This analysis, presented by Foundation grantee Helen Ma, MD, of the University of California, Irvine, examined records from 5244 patients with CLL or SLL in the Department of U.S. Veteran Affairs Central Cancer Registry (VACCR) who were diagnosed during or after 2016. Twothirds of patients were managed with active surveillance; 25% and 4% were managed with targeted therapies or single-agent monoclonal antibodies, respectively, and 6% with first-line chemoimmunotherapy. The median followup was 3.19 years, during which time 10% of patients were diagnosed with another form of cancer.
After adjusting for age at diagnosis, race, and tobacco and alcohol use, first-line treatment with chemoimmunotherapy was associated with a 2.4-fold increased risk for other cancers compared with those on active surveillance. No increase was seen, though, in the risk for other cancers with either targeted therapies or monoclonal antibodies when compared with active surveillance.
The authors noted that similar analyses are ongoing at other centers across the country.
The treatment for relapsed/refractory mantle cell lymphoma (MCL) has been revolutionized by the emergence of novel therapies such as BTKis. While these agents have shown promise in clinical trials, it is unclear how these therapies are used in the real-world setting. Investigators therefore analyzed de-identified electronic health records from the United States Flatiron Health database to assess clinical outcomes and treatment patterns in patients with relapsed/refractory MCL. Results from this study were presented by Foundation Mantle Cell Lymphoma Consortium (MCLC) Executive Committee member Tycel Phillips, MD, of City of Hope.
The analysis included results from 1377 patients with relapsed/refractory MCL who had received at least two lines of therapy. Treatments were classified into two groups: novel therapies (including BTKis, BCL2 inhibitors, lenalidomide, bortezomib, and chimeric antigen receptor (CAR) T-cell therapies) and chemoimmunotherapy (any chemoimmunotherapy plus an anti-CD20 antibody). The most common second-line treatment was BTKis (acalabrutinib, ibrutinib, and zanubrutinib), along with bendamustine-rituximab and R-CHOP. Other therapies used in later lines of therapy included brexucabtagene autoleucel.
In the second-line setting, 70% of patients received a novel therapy, and 30% received chemoimmunotherapy. Median time to next treatment was longer with novel therapies (11.9 months) than with chemoimmunotherapy (9.9 months). Median overall survival, however, was longer with chemoimmunotherapy (43.0 months) compared with novel therapy (35.6 months). Similar trends were observed in the third-line treatment setting with regard to time to next treatment; however, overall survival trends were reversed.
The researchers concluded that the use of novel therapies in the second line and beyond was associated with a trend toward improved time to next treatment and overall survival among most patients. They acknowledged, however, that there were notable differences in results observed based on racial or ethnic status. For instance, though time to next treatment was higher with novel second-line therapies than with chemoimmunotherapy in non-Latinx White, Black, and Asian patients, this trend was reversed in Latinx patients. Likewise, while White and Latinx patients had higher median overall survival with chemoimmunotherapy than with novel therapies, the opposite was seen in Black and Asian patients. The researchers noted that further research is needed to understand these differences in real-world treatment patterns and outcomes.
This study also included contributions from Foundation grantees Jia Ruan, MD, of Weill Cornell Medicine; Anita Kumar, MD, of Memorial Sloan Kettering Cancer Center; Yucai Wang, MD, PhD, of Mayo Clinic, Rochester; and Manali Kamdar, MD, MBBS, of University of Colorado.
Late effects from non-Hodgkin lymphoma (NHL) treatment can lead to decreased fertility, which may be of particular concern for adolescents and young adults. Utilization of fertility counseling is often challenging, though, due to time and cost restraints. Investigators therefore sought to understand the frequency and
predictors of fertility counseling and preservation in patients with NHL, using data from the Lymphoma Epidemiology of Outcomes (LEO) cohort study, which included patients who were newly diagnosed with NHL from 2015 to 2020 at eight academic medical centers in the United States.
To understand trends in fertility counseling and preservation, a questionnaire was administered to the LEO cohort at three years of follow-up. The questionnaire contained one question on whether their healthcare providers discussed the impact of lymphoma treatment on fertility and one question on whether providers discussed the process of fertility preservation.
Survey results from 77 patients ages 18 to 50 years were included in the analysis, of which 46% were female and 53% were male. The median age at diagnosis was 40 years, and 47% of participants were in the 18- to 39-year age range. Approximately 6% of female and 12.2% of male patients underwent fertility preservation after lymphoma diagnosis.
Compared with male patients, female patients were nearly twice as likely to receive fertility counseling at the time of diagnosis; 72% of females and 59% of males recalled having these discussions. Younger patients (18 to 39 years) were more than 10 times as likely as older patients (40 to 50 years) to have received fertility counseling. When accounting for confounding factors, younger age and use of alkylating therapy were found to be significant predictors of whether fertility counseling was performed.
The researchers concluded that their results emphasize the importance of ensuring fertility counseling for all patients of childbearing age so all patients have the opportunity to address family-planning concerns prior to treatment.
This study also included contributions from Foundation SAB members Thomas M. Habermann, MD, of Mayo Clinic, Rochester; Jonathan W. Friedberg, MD, of Wilmot Cancer Institute; Peter Martin, MD, of Weill Cornell Medicine; Christopher R. Flowers, MD, MS, of The University of Texas MD Anderson Cancer Center; James Cerhan, MD, PhD, of Mayo Clinic, Rochester; and former Foundation SAB members Brian K. Link, MD, of University of Iowa; Izidore S. Lossos, MD, of Sylvester Comprehensive Cancer Center; Brad Kahl, MD, of Washington University; and former Foundation grantees Carla Casulo, MD, of Wilmot Cancer Institute; Matthew J. Maurer, DSc, of Mayo Clinic, Rochester; Jonathon B. Cohen, MD, MS, of Winship Cancer Institute; Dai Chihara, MD, PhD, of National Institute of Health; and Allison C. Rosenthal, DO, of Mayo Clinic, Phoenix.
Lymphoma Rounds offers a series of accredited CME activities that provide a forum for healthcare professionals to meet regularly and address issues specific to the diagnosis and treatment of lymphoma patients.
Participants share best practices and learn the latest information on the treatment and management of lymphoma through interactive case studies.

Schedule
Lymphoma
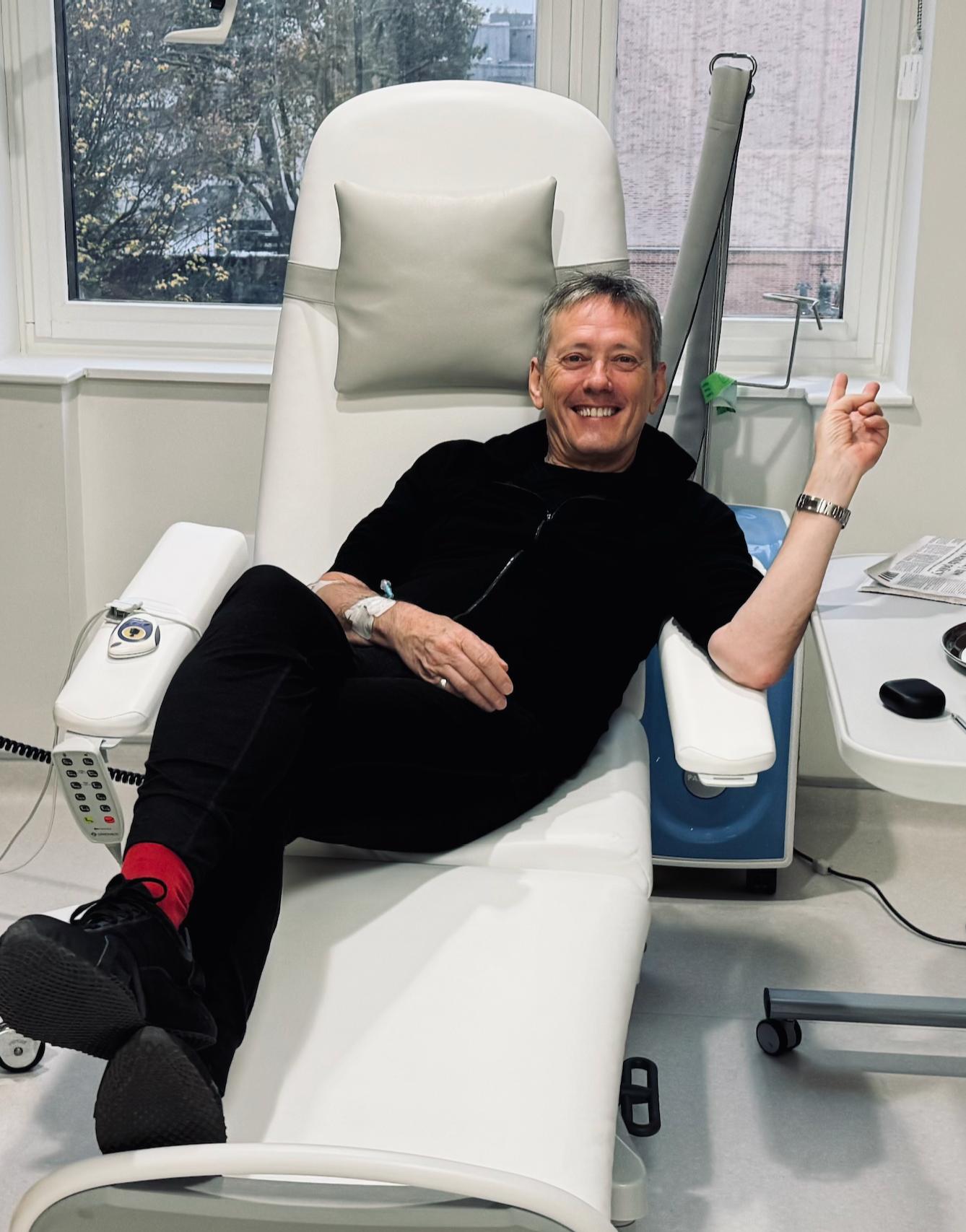
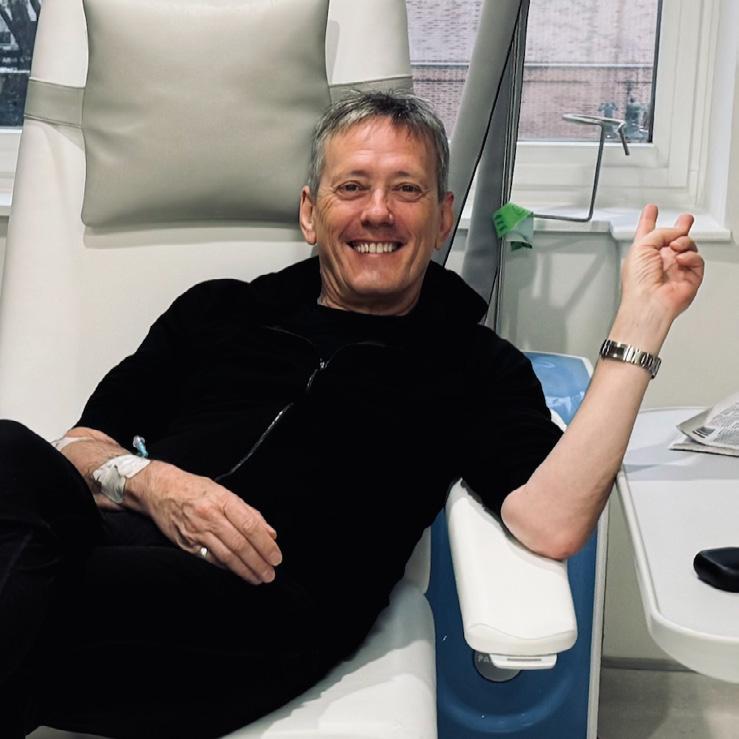
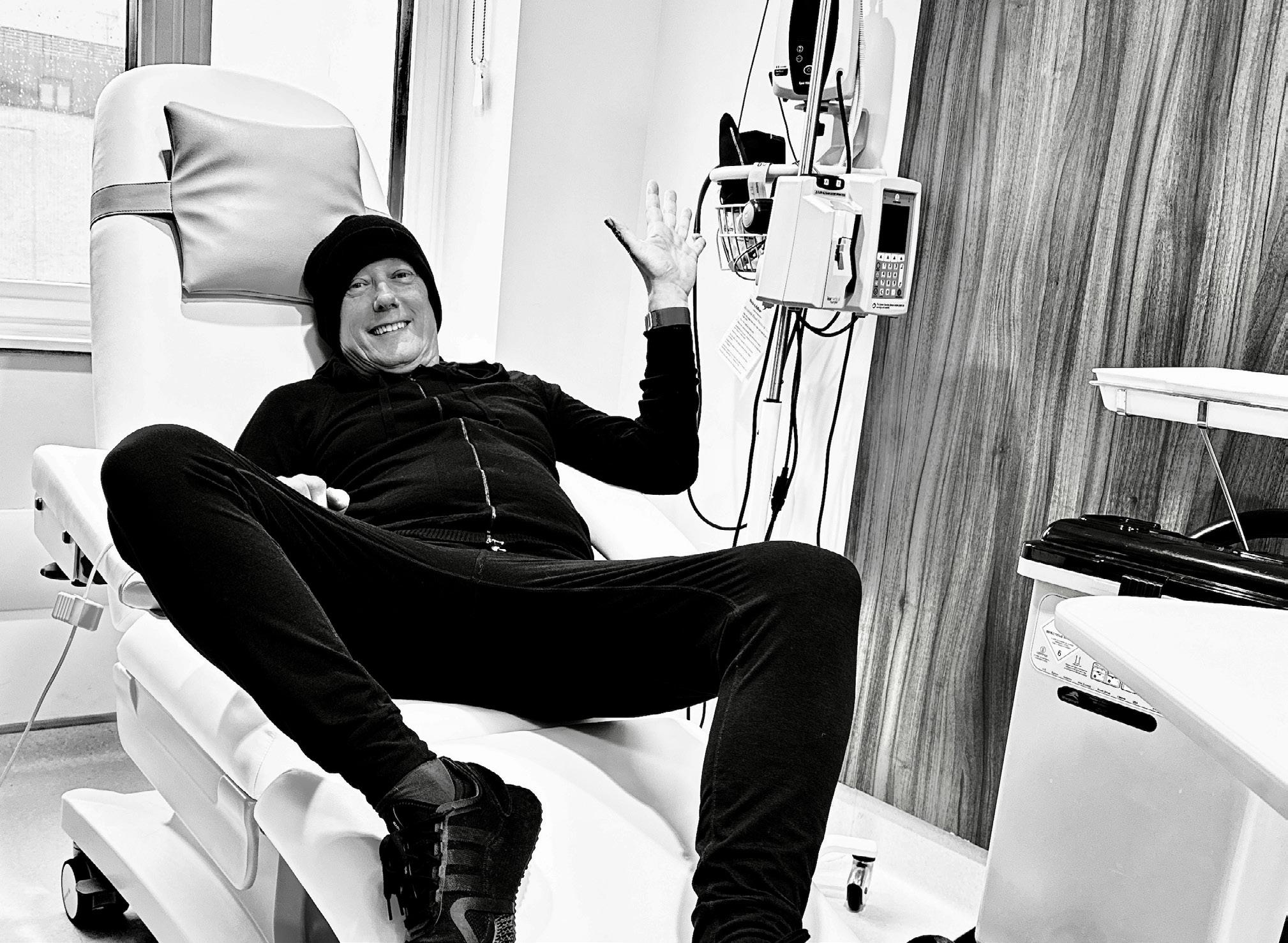
For Roger O’Donnell, longtime keyboardist of the legendary alternative rock band The Cure, music has always been about forging his own path.
“Our band has made a successful career out of turning left when everybody else turned right,” he said. “We’ve always done things our own way.”
A professional musician since 1976, having also played with The Psychedelic Furs and Thompson Twins, O’Donnell joined The Cure in 1987. While on a jam-packed world tour with the band in 2022, he began noticing concerning symptoms, which he originally tried to rationalize away.
”I put off going to the doctor until I finished the tour,” he admits. “I was in denial because when I was growing up, cancer was always considered ‘the bogeyman’ or the mad axe murderer who knocks at the door.”
After the tour and before a planned trip to Italy, O’Donnell visited his General Practitioner (GP), who suggested he get a scan, but didn’t seem overly alarmed. After returning from Italy and performing a show in Chicago, O’Donnell’s instincts told him to investigate further. He scheduled an ultrasound at a local clinic. That ultrasound appointment marked a turning point.
“The nurse said to me, ‘You want me to talk you through it, or should I wait until the end?’” O’Donnell remembers. “I was like, ‘Sure, no problem — I think we both know what it is’ — still rock solid in my denial.”
But when the nurse fell silent during the procedure and quickly left the room to contact O’Donell’s GP, reality began to sink in.
“At that moment, I entered what I can only describe as a different zone … where you’re not really sure what’s going on. You’re certainly not inhabiting your body anymore,” he said.
The process moved swiftly from there. Within 10 days of his initial ultrasound, O’Donnell had undergone the necessary tests and received his official lymphoma diagnosis. His medical knowledge, which he gained from supporting an ex-partner through her journey with Hodgkin lymphoma years before, helped him better navigate the road ahead. Treatment began in November 2022, and O’Donnell wasted no time in reading up on his disease.
“I was very proactive with my research, reviewing every research paper I could get my hands on,” O’Donnell said. “I’m pretty thorough, to the point that if the plumber comes and fixes something in my house, I want to know what he does. If there’s something wrong with my car, I want to know exactly what they’re doing. I don’t want them to say, ‘Okay, we fixed it fine’ — I want the details.”
He worked closely with his medical team to determine the best treatment approach, even presenting them with research papers he’d come across that suggested modified treatment protocols that might reduce long-term effects while maintaining efficacy. Even so, the 11-month chemotherapy treatment regimen proved challenging.
“After the first treatment, and come the morning of the second one, I didn’t want to go,” he admitted.
During his third treatment, he found himself questioning whether he could continue.

O’Donnell maintained a positive attitude throughout treatment, though he acknowledges it wasn’t always easy.
“I just wanted to get through the treatment. I wanted to get it done. I wanted it over with,” he said. His partner, Mimi, stood by him through every step of what he calls “the nightmare of the last year,” providing crucial support. The medical team and caregivers he worked closely with also became like family.
“I can’t stress enough how supportive my partner and my medical team were,” O’Donnell said. “I’ve always been the one that makes everyone laugh, so it was very difficult for me to take on the role of being the patient instead of the person who cares.”
O’Donnell completed his treatment in July 2023, but the emotional journey continues. He recently started therapy to process what he went through, recognizing signs of PTSD from the experience.
“I’m still coming to terms with the fact that I went through it,” he explained. “I’m not scared of what’s to come … it’s just trying to work out how I got through it in the first place.”
Just months after completing treatment, O’Donnell was back on stage with The Cure for a worldwide broadcast celebrating the band’s latest album release.
“I was standing on stage playing and thinking, ‘At this time last year, I was in treatment … I had no hair, and I felt like the worst I’ve ever felt in my entire life,’” he recalls. “It was at that moment that I realized that I could use this experience to help others going through something similar.”

After O’Donnell posted about his lymphoma diagnosis on Twitter (now X), the Lymphoma Research Foundation’s Board of Directors Chair Steven Eichberg reached out to O’Donnell’s agent to offer support. O’Donnell liked what he learned about the organization.
“Not only does the Foundation encourage and fund important lymphoma research, but it offers support for people going through the disease as a patient or caregiver,” he notes. “It’s a very holistic approach.”
To those newly diagnosed with lymphoma, O’Donnell offers heartfelt encouragement:
Not only does the Foundation encourage and fund important lymphoma research, but it offers support for people going through the disease as a patient or caregiver.
“You’re not alone. There are a lot of people going through it at the same time, and there are a lot of people working toward making you better,” he said. “Nothing is ever as scary or as horrible as you think it is. Your imagination is your worst enemy … nothing was ever as bad as I thought it was going to be.”
Music continues to be a source of healing. During a recent performance of “Plainsong,” a beloved classic of The Cure, O’Donnell experienced a profound realization about just how far he’d come in the last year. The song, which he describes as “a big, powerful string orchestral sound,” helped him fully grasp that he had made it through his treatment to the other side.
Looking ahead, O’Donnell remains focused on his music while gradually accepting how his experience has shaped him. “I don’t want people to think of me differently,” he said, though he acknowledges that accepting and processing his journey is part of the process of moving forward.
“The part that sticks with me is that throughout my treatment, my amazing caregivers and I actually had fun together, and I still go back and see the chemo nurses from time to time,” he said. “They genuinely love helping people, and we just laugh and laugh. Some of my care team have even said they want to attend future Cure concerts.”
Today, O’Donnell splits his time between London and Devon, England. As The Cure prepares for future tours and performances, O’Donnell continues to embrace creating and performing music. His journey with lymphoma has added another layer to his story, one that resonates with hope and resilience.
You’re not alone. There are a lot of people going through it at the same time, and there are a lot of people working toward making you better.
The Lymphoma Research Foundation’s volunteer Scientific Advisory Board, comprising 45 world-renowned lymphoma experts, guides the Foundation’s research activities, seeking out the most innovative and promising lymphoma research projects for support.
Ann S. Lacasce, MD, MMSC Chair
Harvard Medical School Dana-Farber Cancer Institute
Andrew M. Evens, DO, MSc, FACP Chair-Elect
Rutgers Cancer Institute of New Jersey
Ranjana Advani, MD Stanford University School of Medicine
Ash A. Alizadeh, MD, PhD
Stanford University School of Medicine
Stephen Ansell, MD, PhD Mayo Clinic, Rochester
Kristie A. Blum, MD
Winship Cancer Institute of Emory University
Jennifer Brown, MD Dana-Farber Cancer Institute
Sharon Castellino, MD, MSc Emory University School of Medicine
James Cerhan, MD, PhD Mayo Clinic, Rochester
Ethel Cesarman, MD, PhD NewYork-Presbyterian Hospital Weill Cornell Medicine
Sandeep Dave, MD, MS Duke University
Kieron M. Dunleavy, MD Lombardi Cancer Center of Georgetown University
Kojo S.J. Elenitoba-Johnson, MD Memorial Sloan Kettering Cancer Center
Todd Fehniger, MD, PhD Washington University School of Medicine
Christopher R. Flowers, MD, MS The University of Texas MD Anderson Cancer Center
Jonathan W. Friedberg, MD, MMSc University of Rochester
James P. Wilmot Cancer Institute
Leo I. Gordon, MD, FACP Past Chair, 2015-2017
Robert H. Lurie Comprehensive Cancer Center of Northwestern University
Michael Green, PhD, MD
The University of Texas MD Anderson Cancer Center
Thomas M. Habermann, MD Past Chair, 2017-2019 Mayo Clinic, Rochester
Alex Herrera, MD City of Hope Medical Center
Steven M. Horwitz, MD Memorial Sloan Kettering Cancer Center
Eric D. Hsi, MD Mayo Clinic, Rochester
Kara Kelly, MD
Roswell Park Comprehensive Cancer Center University at Buffalo Jacobs School of Medicine
John P. Leonard, MD
Past Chair, 2012-2015
NewYork-Presbyterian Hospital Weill Cornell Medicine
Kami Maddocks, MD Ohio State University Comprehensive Cancer Center
Peter Martin, MD Weill Cornell Medicine
Ari Melnick, MD
NewYork-Presbyterian Hospital Weill Cornell Medicine
Lindsay Morton, PhD National Cancer Institute
Markus Müschen, MD, PhD Yale School of Medicine
Teresa Palomero, PhD Institute for Cancer Genetics, Columbia University
Laura Pasqualucci, MD
Institute for Cancer Genetics, Columbia University
Barbara Pro, MD Columbia University
Lisa Rimsza, MD Mayo Clinic, Arizona

Lisa Roth, MD
Weill Cornell Medicine
Gilles Salles, MD, PhD Memorial Sloan Kettering Cancer Center
Kerry J. Savage, MD British Columbia Cancer
David Scott, MBChB, PhD
BC Cancer, Vancouver
Laurie Sehn, MD, MPH BC Cancer, Vancouver
Margaret Shipp, MD
Harvard Medical School
Dana-Farber Cancer Institute
Sonali M. Smith, MD Immediate Past Chair, 2021-2023
The University of Chicago
Eduardo M. Sotomayor, MD
Tampa General Hospital
Christian Steidl, MD
BC Cancer, Vancouver
John M. Timmerman, MD
UCLA Jonsson Comprehensive Cancer Center
Hans-Guido Wendel, MD Memorial Sloan Kettering Cancer Center
Andrew D. Zelenetz, MD, PhD Past Chair, 2019-2021 Memorial Sloan Kettering Cancer Center
Morton Coleman, MD
NewYork-Presbyterian Hospital Weill Cornell Medicine
Brian Link, MD University of Iowa
Kanti R. Rai, MD
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell
Michael E. Williams, MD, ScM University of Virginia Cancer Center
3.27 Update on Diffuse Large B-Cell Lymphoma Webinar
4.2 Lymphoma Talk: Miami 4.10 Fertility and Family Building for Lymphoma Patients Webinar
4.17
Pregunte al doctor sobre el linfoma
4.19 Living with Lymphoma 4.27
2025 South Florida Golf Invitational 5.7 Lymphoma Talk: Chicago 6.7 2025 Minnesota Lymphoma Walk 6.14
2025 New York Lymphoma Walk 7.27
2025 Chicago Lymphoma Walk



To learn more about Planned Giving, c Jessica Sharrow at jsharrow@lymph or visit lymphoma.org/legacy. Wall
When you include the Lymphoma R Foundation in your estate plan, you in the most promising research that greatest potential to dramatically im lives of those impacted by lymphom
