hemangioma – Bożena Romanowska-Dixon, Barbara Jakubowska
Choroidal osteoma – Bożena Romanowska-Dixon, Barbara Jakubowska ........................
Sclero-choroidal calcifications – Bożena Romanowska-Dixon, Barbara Jakubowska ...........................................................................................................................
pigmented tumors – Bożena Romanowska-Dixon, Barbara Jakubowska, Izabella Karska-Basta
tumor size, extraocular extension and chromosomal abnormalities in the tumor tissue (primarily monosomy of chromosome 3 and abnormality of chromosomes 6 and 8) [170].
Diagnosis of choroidal melanoma
Choroidal tumors are identified and distinguished almost entirely (in 98% cases) on the basis of ophthalmoscopy (funduscope, Volk lens) and ultrasound US examination (10 MHz US and 35.50 MHz UBM). Recently DRI-OCT evaluation has proved useful in certain situations when a reliable diagnosis cannot be established. This evaluation helps to identify the actual nature of the lesion under review or to confirm an initial diagnosis. Additional data is provided by angiography (indocyanine and fluorescein angiography), autofluorescence and photography in red-free light [31, 103–107].
Characteristic features, diagnostic aspects and differentiation of malignant and benign intraocular tumors are necessary to make diagnosis. Choroidal melanoma may be recognized and differentially diagnosed using simple and readily available diagnostic methods [1–3].
The basic ocular examination comprising a measurement of visual acuity and intraocular pressure, slitlamp examination of the anterior segment of the eye and dilated-pupil ophthalmoscopy helps to identify whether a tumor is present and if so, what its nature is. Making an appropriate diagnosis requires knowledge of morphological properties and location of the tumor in the ocular fundus, which is typical for some lesions, an ultrasound scan and characteristic manifestation of choroidal tumors in the DRI OCT image.
History of the present illness also plays an important role. In most cases, medical history is not characteristic but helps to initially guide the diagnostic process. A sudden loss of vision in a diabetic or hypertensive patient may suggest a hemorrhage into the vitreous chamber, a gradual deterioration of visual acuity in a patient aged 60 and more and a sudden loss of vision may indicate a subretinal hemorrhage secondary to AMD while a defect in the visual field is suggestive of a retinal detachment (including a secondary retinal detachment related to a choroidal tumor). Medical history of a systemic diseases (including tumors in the patient’s family) may also be helpful in establishing a diagnosis.
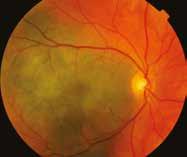
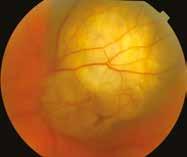
There is a close correlation between the pigmentation of a choroidal melanoma and the type of melanin. Tumors of light colors contain more pheomelanin while intensively pigmented tumors (black, russet) contain almost exclusively eumelanin. Sometimes a single tumor displays a range of pigmentation. Tumors are usually clearly demarcated from the surrounding tissue, and the retina at the base of large tumors may be detached and, above the tumor, lifted. Tumor enlargement may be accompanied by a hemorrhage on the surface or at its base, and sometimes by a hemorrhage in the vitreous body.
The most important examination is A and B-scan ultrasonography US (10 MHz US), which helps to establish an accurate diagnosis and measure the tumor, assess the surrounding structures, and identify whether sclera is not ruptured and extraocular extension of melanoma UM is present [108].
An ultrasound scan also helps to precisely measure the thickness and width of tumor base in a longitudinal and parallel presentation, the parameters which are important for therapy planning and assessment of its effectiveness.
Choroidal melanoma, due to its homogeneous histological structure, shows a regular internal pattern and a low internal reflectivity in an ultrasonographic examination. Pulsating vasculature may be seen at the base of the tumor. A-scan shows a characteristic low reflectivity of the tumor with a high initial peak from the retina. Tumors may appear flat, dome-shaped or mushroom-shaped. Some melanomas have an irregular shape. Mushroom-shape, typical of large choroidal melanomas, is caused by a tumor enlarging above the retina after Bruch’s membrane is ruptured.
A and B-scan ultrasonography is a valuable examination informing a differential diagnosis between uveal melanoma and nevi, choroidal metastases, choroidal melanoma, choroidal osteoma and a variety of other benign and malignant tumors. Choroidal hemangioma has multiple, small vascular caverns which form a regular structure and show a high internal reflectivity in an ultrasound US examination. Choroidal hemangiomas show a significantly higher reflectivity than uveal melanoma and higher reflectivity than metastatic tumors. Choroidal metastases have an irregular, chaotic arrangement of cells and varying distances between them which is shown as an irregular internal structure and a variable internal reflectivity in an ultrasound scan. Choroidal osteoma shows a very high reflectivity (resembling that of osseous tissue);
a B-scan presents an intensive shadow in the orbit. When an extensive extraocular invasion has been identified by means of ultrasonography US (10 MHz US), magnetic resonance imaging of the orbits is helpful for assessing its size. Orbital MRI is also recommended after enucleation of eyes with choroidal melanoma involving implantation of an orbital implant.
In cases which appear challenging for a diagnostician, additional methods may prove useful, including:
SS-OCT (showing the choroid), transillumination (diaphanoscopy) helpful in diagnosing ciliary body tumors, infrared photography, autofluorescence and indocyanine angiography. The most critical issue for therapy planning and the patient’s future is to distinguish a choroidal melanoma from choroidal metastases and benign tumors, choroidal hemangioma. At present, the ability to obtain SS-OCT images of choroidal lesions often helps to diagnose tumors of unclear origin.
Figure 6.185. Choroidal melanomas with different degree of pigmentation.
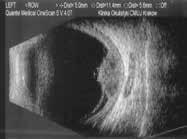
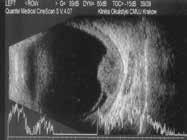
Figure 6.186. Dome-shaped choroidal melanoma in 10 MHz US (green frames). Medium level internal reflectivity of the tumor and secondary retinal detachment in presentation (yellow frame).
6.185
6.186
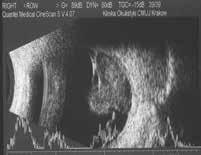
Figure 6.187. Mushroom-shaped choroidal melanoma in 10 MHz US. Initially dome-shaped tumor (white arrows), disruption of the Bruch’s membrane (blue arrows).
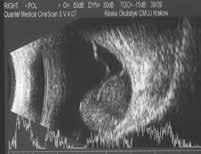
Figure 6.188. Mushroom-shaped choroidal melanoma with secondary retinal detachment in 10 MHz US. Low internal reflectivity at the tumor base (white arrow) and medium internal reflectivity at the tumor apex (blue arrow).
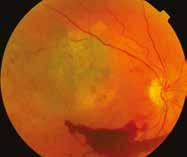
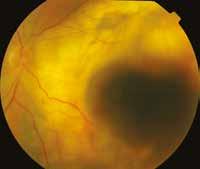
Figure 6.189. Diffuse choroidal melanoma located in the posterior pole with extraocular extension: a – color photograph; b – 10 MHz US – diffuse flat tumor with extraocular extension (yellow arrows).
6.189
6.187
6.188
Ultrabiomicroscopy (UBM)
When diagnosing neoplasms of the anterior segment of the eye (iris and/or ciliary body), high-frequency ultrasonography US (35 and 50 Mz), called ultrabiomicroscopy (UBM), is very useful and conclusive. It provides a high-resolution image, which makes it possible to obtain a detailed image of a tumor, and paths of extension through the sclera and surrounding structures, and to measure the tumor.
A differential diagnosis of intraocular tumors by means of ultrasonography with a 35-50 MHz probe should take into account:
y tumors of iris and ciliary body;
y tumors of peripheral choroid;
y cysts of iris pigment epithelium and ciliary body.
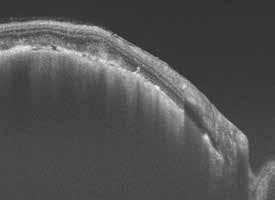
Figure 6.190. Small peripapillary choroidal melanoma: a – infrared image; b, c – SS-OCT images.
Indocyanine angiography (ICGA)
ICGA is much superior to fluorescein angiography in imaging the vasculature of a choroidal melanoma. This is attributable to the different light absorption properties of these two dyes. In addition, indocyanine is absorbed by serum proteins and does not leak through choriocapillaris fenestrations, thus helping to visualize the vasculature. ICGA is considered to be a supplementary examination in a differential diagnosis of a small melanoma. Maximum fluorescence of vessels is achieved after ca. 18 minutes from an intravenous dye administration.
Choroidal melanomas display a heterogeneous fluorescence (hypo-, iso- or hyperfluorescence) depending on the intensity of tumor pigmentation. Choroidal metastases present a homogenous diffuse hyperfluorescence with a late isofluorescence. Choroidal hemangio-
RETINOBLASTOMA
Joanna KOBYLARZ, Bożena ROMANOWSKA-DIXON
Introduction
Retinoblastoma, even though it accounts for only ca. 3% of neoplasms recognized in patients below the age of 15 years, is the most common primary malignant ocular tumor in children. According to published data, each year around 7,000–8,000 children are born with a retinoblastoma, and ca. 3,000–3,500 die because of this tumor. Prognosis depends on the standard of, and access to medical care, which is why mortality rates are much lower in developed countries, relative to underdeveloped countries. In developed countries, the tumor is usually recognized in 7–10% at neonatal age, that is in the first 4 weeks of life in full-term children, and before week 44 of gestational age in preterm children [11–13].
The clinical manifestation and the course of the disease varies; retinoblastoma may be familial or sporadic, solitary or multiple, occur unilaterally or bilaterally and develop synchronically, or metachronically. In advanced stages, retinoblastoma may extend to the orbit, and metastasize to the CNS and other remote organs. Based on this criterion, there are two types of retinoblastoma: intraocular and extraocular. Extraocular retinoblastoma is associated with a higher mortality rate relative to the intraocular type. Retinoblastoma of the CNS (so-called trilateral retinoblastoma) usually occurs in the pineal gland and is associated with a particularly poor prognosis – most children die because of the disease or its complications. The chief reason for extraocular extension of retinoblastoma is late presentation to the ophthalmologist, and thus late initiation of treatment. The sooner retinoblastoma is recognized and treated, the greater the chances for not only the child’s survival but also for salvaging the eye or even useful vision [13, 14].
Pathogenesis
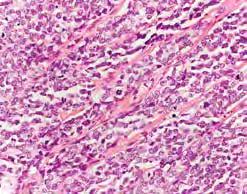
Retinoblastoma is a neuroectodermal tumor, which develops from the primitive cells of the retina. Well-differentiated tumors are characterized by the presence of Flexner–Wintersteiner rosettes and fleurettes with small necrotic
areas and few mitotic figures. Poorly-differentiated retinoblastomas do not show these features, have a very high mitotic index, and may demonstrate anaplastic cellular features (fig. 8.42).
Retinoblastoma is a genetic anomaly. It develops as a result of a genetic defect – a mutation of the oncogene RB1, one of the tumor suppressor genes on chromosome 13 in locus 13q14. For retinoblastoma to arise, both alleles of RB1 gene need to be affected by mutation, which was described by Knudson in his theory. New research also demonstrates alterations in the expression of other oncogenes and suppressor genes. Retinoblastoma shows an autosomal dominant pattern of inheritance with a 90–95% penetration. Familial cases in which a single allele is inherited represent 40% of retinoblastomas. The remaining 60% are sporadic cases in which both alleles are mutated at the time of individual development. Patients with the inherited type are at a risk of developing retinoblastoma at a third site (tumors in both eyes and the third tumor in the pineal gland region or in the parasellar region), 13q syndrome (multifocal retinoblastoma and multiple phenotype abnormalities) and other primary neoplasms (sarcoma, urothelial tumors, lung carcinoma, leukemia, melanoma and breast carcinoma) [13–15].
Symptoms
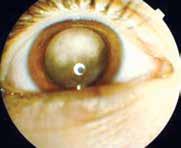
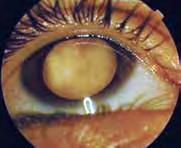
Symptoms related to retinoblastoma depend on the size and location of the tumor(s) at the time of diagnosis. In the initial stage, small tumor(s), particularly those located at the retinal periphery can be asymptomatic. A symptom which is most often reported by children presenting for treatment is a white pupil (‘leukocoria’ or cat eye). It is a white reflex from the ocular fundus caused by the tumor showing through the pupil (fig. 8.43), which can be seen in slit lamp photographs (photoleukocoria) or even in family photographs when a flash is used. Leukocoria can be observed when the disease is advanced and the tumor(s) occupies/occupy a significant portion of the eye ball [13, 14, 16, 17].
Figure 8.42. Retinoblastoma – histopathological examination: a – Flexner Wintersteiner rosette (black arrow); b – mitosis (blue arrow).
Figure 8.43 a–c. Leucocoria – white or creamy reflex from the pupil.
Retinoblastoma must be excluded in every child presenting with leukocoria. In the differential diagnosis of retinoblastoma, one should take into account other possible reasons underlying leukocoria, of which the most common are: primary persistent hyperplastic vitreous body, Coats’ disease, ocular toxocariasis, retinopathy of prematurity, retinal hamartoma, choroidal coloboma, vitreous hemorrhage, astrocytoma, familial exudative vitreoretinopathy, retinal hypoplasia, rhegmatogenous retinal detachment, juvenile retinal dysplasia, medulloepithelioma and congenital cataract [18, 19].
Strabismus is another frequent symptom related to retinoblastoma, usually caused by tumor invasion of the macular region and damage to central vision. In a paper discussing the outcomes of retinoblastoma treatment at our Clinic in 1996–2004, a thorough medical history provided by patients helped to establish that strabismus was the first symptom in 37.5% children presenting with leukocoria [20]. Therefore, every squinting child, regardless of age, should be evaluated by an ophthalmologist to exclude out or confirm retinoblastoma. Other, less common symptoms included: ocular redness and pain mostly caused by a secondary glaucoma, pseudouveitis, nystagmus, unilateral pupil dilation, iris heterochromia, blood or pseudohypopyon in the anterior chamber, and preseptal orbital cellulitis. Exophthalmos secondary to orbital infiltration is the second feature related to advanced retinoblastoma after leukocoria in reports from developing countries. Retinoblastoma may also be discovered by accident, at a routine ophthalmologist examination (e.g. ophthalmic screening in pre-term children and children following obstetric trauma). According to our retrospective studies published in 2009, both eyes were salvaged in all children whose retinoblastoma was detected by accident in the first month after birth [17]. Unfortunately, incidental discovery of retinoblastoma still represents a small proportion of diagnoses. If any of the symptoms presented above occur (particularly the cat-eye white reflex or squinting), parents or foster parents should immediately bring the child to an ophthalmologist. An ophthalmologist who identifies or suspects a retinoblastoma should refer the affected child to a specialized ocular oncology center, which has all the necessary equipment and trained staff experienced with novel treatment modalities [13, 14].
Diagnosis
It is vitally important to obtain a detailed medical history from the parents or foster parents of children with retinoblastoma, in order to establish the occurrence of retinoblastoma in the family or rule out other diseases, which may give rise to similar symptoms. Fundoscopy is the most important evaluation in diagnosing a retinoblastoma. In order to accurately assess the number and location of tumor(s), it is recommended to examine a child with retinoblastoma under general anesthesia. After a thorough examination of the anterior segment of the eye and measurement of intraocular pressure, the ocular fundus must be evaluated with a maximum pupil dilation using Fison binocular ophthalmoscope with a scleral depressor to see the most peripheral sections of the retina up to the ora serrata. It is recommended to produce a photographic documentation of the ocular fundus. A- and B-scan US images of the globe and orbit aid accurate evaluation of the internal features of a tumor, such as calcifications and invasion of the optic nerve, choroid or orbit, and measure the tumor thickness and diameter at base. Computed tomography (CT) may show calcifications within a retinoblastoma. However, due to radiation exposure from CT, which increases the risk of other neoplasms developing at a later time, the use of this examination technique should be limited, particularly in children with retinoblastoma originating from germinal mutations. At present, MRI is the preferred imaging method as it is superior in showing soft tissue, safer and more useful, particularly in detecting extraocular extensions of the tumor, invasion of the optic nerve, and evaluation of the brain. Angiography, OCT, ultrabiomicroscopy (UBM) may be helpful in establishing the final diagnosis as part of a differential diagnosis process. Children with retinoblastoma suspected of developing metastases need to undergo bone marrow puncture, examination of the cerebrospinal fluid, bone scintigraphy and positron emission tomography (PET) scans [13, 14, 16].
It is hoped that fluid biopsy and genetic testing of eyes with retinoblastoma may prove useful in future, but further observations need to be made before their prognostic value is confirmed [13, 21, 22].
Figure 8.44. Differential diagnosis of retinoblastoma and Coats disease.
Figure 8.45. Retinoblastoma – tumor located in anterior chamber of the eye (a), iris neovascularization and secondary glaucoma (b).