In
NEWS: Further Acquisitions for McCabes Pharmacy Group
Page 4
MEDICINES: Positive Steps to Faster Medicines Access Page 8
FEATURE: Irritable Bowel Syndrome
Page 14
CPD: Management of Atopic Dermatitis
Page 41
AWARDS: Irish Pharmacy Awards 2023: Winners

Page 46
RESEARCH: New Survey on Impact of Skin Conditions

Page 78
TEAM TRAINING: Warts & Verrucae

Page 92

June 2023 Volume 15 Issue 6
PHARMACYNEWSIRELAND.COM is available exclusively from www.uniphar.ie (01) 468 7501 A DIVISION OF linkup@uniphar.ie This Publication is for Healthcare Professionals Only
this issue:




with Lactobacillus Rhamnosus GG Oral Electrolyte Solution For Children & Adults MyPro DiaCare is a Zeon Healthcare Ltd. brand t. +353 (0) 44 939 6188 e. info@pharmed.ie Contact your Pharmed Territory Manager to place your order: Elaine: 086 130 1374 | Helen: 086 130 1835 | Máire: 086 602 0567 Reduce lifetime of diarrhoea by over one day NEW 6 SACHET PACK AVAILABLE Quick, effective hydration for ages 3+ years
Foreword
Page 5: Pharmacies at ‘Tipping Point’
Page 6: Pharmacy being ‘stifled’ by Government

Page 11: CommCare host Ambassador Day for Pharmacy

Page 12: Medicines
Shortage Index at 247
Page 18: Managing Diabetes in the Summer
Page 27: ‘Grin and bear it’ menopausal women told
Page 29: Skinside Out event held in Dublin
Page 46: All the winners from the 2023 Irish Pharmacy Awards

PUBLISHER:
IPN Communications
Ireland Ltd.


Clifton House, Fitzwilliam Street
Lower, Dublin 2 00353 (01) 6690562
MANAGING DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood: 00353 (87)737 6308 kelly-jo@ipn.ie
ADVERTISING EXECUTIVE
Amy Evans: amy@ipn.ie
EDITORIAL/ EVENTS & MARKETING EXECUTIVE
Aoife Hunter: aoife@ipn.ie
CONTRIBUTORS
Professor Humphrey O’Connor
Jennifer Kehinde
Claire O’Donnell
AnnMarie Grealish
Orlaith Gavin
Dr Ranbir Kaulsay
Dr Paula Byrne
Theresa Lowry-Lehnen
Dr Cathal O’Connor
Dr Fardod Kelly
Aine Toher
Sarah Mohamed
DESIGN DIRECTOR
Ian Stoddart Design




Irish Pharmacy News is circulated to all independent, multiple Pharmacists and academics in Ireland.



All rights reserved by Irish Pharmacy News. All material published in Irish Pharmacy News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd. has taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
In one of our lead news stories this issue, we detail how community pharmacies are now at a ‘tipping point’ and require a restoration in fees to protect the viability of many pharmacies around the country and to maintain key services to patients.
The representative body, the Irish Pharmacy Union, has called for the Minister for Health to immediately begin substantive talks ahead of a statutory review of pharmacy fees in June. Since 2009, the amount paid to pharmacies per medicine dispensed has reduced by 19%, while the costs for dispensing medications on behalf of the state have increased by 23%. This is not sustainable and will soon reach the stage where it will cost more to dispense an item than what is paid by the state.
Turn to page 5 to read the full story.
Meanwhile, on page 12 we discuss the news that medicine shortages in Ireland continue to persist with 247 different medicines used by Irish patients currently out-of-stock, as a new trend affecting supply develops, according to the latest Medicine Shortage Index.
The latest figures show the number of medicine shortages in Ireland up an additional 19 medicines in short supply since the end of last month, and a 38% increase since the Index began in October. Of the 247 medicines currently unavailable, 13 are listed on the World Medical Organisation’s (WHO) ‘critical medicines’ list.
On Saturday, 27th May over 650 professionals from community pharmacy and their teams, alongside leading members of the pharmaceutical industry gathered at the Clayton Hotel Dublin for the annual Irish Pharmacy Awards.

These Awards have been firmly established as the highlight of the year in community pharmacy as was evident from the outstanding and un-rivalled number of entries comparative to previous years. Our esteemed judging panel had no easy task and while there can only be one winner, each and every one of our finalists deserve applause for their dedication and commitment to not only their profession, but to their customers.

Thanks must also go to our Award Sponsor Stakeholders, without whom the night simply wouldn’t happen. It was a fantastic night, enjoyed by all. Turn to page 46 for full details on all the winners and make sure you catch our upcoming issues for full coverage of their winning projects and entries.
We at the IPN offices are looking forward to 2024 already!
I hope you enjoy the issue.
Regulars
CPD: ATOPIC DERMATITIS P41
FEATURE: DERMATOLOGY P80
FEATURE: VTE DURING PREGNANCY P86
TEAM TRAINING: WARTS & VERRUCAE P92
CLINICAL PR: P96
3 PHARMACYNEWSIRELAND.COM 11
IRISH PHARMACY NEWS 5 29
PHARMACYNEWSIRELAND.COM @Irish_PharmNews IrishPharmacyNews Contents 55
McCabes Pharmacy further Acqusitions
McCabes Pharmacy Group have recently announced the successful acquisition of three new pharmacies from in Dublin, Stanley’s Mountview Blanchardstown, Ellis Pharmacy, Woodstown & Ellis Pharmacy, Springfield, Tallaght.
to Adamstown, Lucan later this year. As well as operating Ireland’s largest online pharmacy.”
Healthy Workplace Website
Minister of State with responsibility for Public Health, Wellbeing and the National Drugs Strategy, Hildegarde Naughton, has welcomed the launch of the Healthy Workplace website.
A company statement said, “McCabes Pharmacy have deeply admired these pharmacies and teams over the years and are truly honoured that the operators of these fine businesses have entrusted McCabes Pharmacy to nurture them from here.

“McCabes Pharmacy have been serving Irish communities for over 40 years, and this move marks a significant milestone in the accelerated growth of the company, bringing the total number of pharmacies to 32 with a new pharmacy opening coming
All Ireland Pharmacy Conference
The 10th All Ireland Pharmacy Conference (AIPC) will be held at Ballymascanlon House Hotel, Dundalk on 6th and 7th November 2023. The theme for this year’s conference is ‘Creating Momentum & Building Capacity’

This conference is jointly coordinated by the Northern Ireland Centre for Pharmacy Learning and Development and the Irish Institute of Pharmacy. Its focus is to share good practice in pharmaceutical care and practice development across the primary and secondary care sectors.
CEO Sharen McCabe welcomes these pharmacy additions adding, “These newly acquired pharmacies have a long-standing reputation for excellence in patient care, we are delighted to welcome the teams to McCabes Pharmacy and look forward to bringing an extended suite of healthcare services to these local communities.”
McCabes Pharmacy employs just under 500 colleagues and following this acquisition will operate 32 stores across Dublin, Dundalk, Kildare, Wicklow, Wexford, Tipperary, and Limerick.
Developed under the National Framework for Healthy Workplaces, the new site is intended to be a one-stop-shop that helps organisations to make their workplace a Healthy Workplace.
It features interactive tools to facilitate the delivery and evaluation of health and wellbeing initiatives in workplaces of any size.
It is designed to cater for the needs of public and private organisations and is targeted towards members of staff from Wellness/Health Promotion, Human Resources, Occupational Health, Health & Safety, decisionmakers in senior management, and employees hoping to champion a Healthy Workplace. Welcoming the launch, Minister Naughton said, “The benefits of a happy and healthy workforce are multi-faceted, for the individual and for society.
Pharmacists, technicians and qualified assistants are encouraged to exchange ideas for pharmaceutical service development in the Republic of Ireland and Northern Ireland.
On the evening of Monday 6th November, the conference dinner will take place at 8.00pm and provides an opportunity to network with colleagues. The main conference proceedings will start at 9.30am on Tuesday 7th November and will involve parallel oral sessions and poster presentations.
Visit www.allirelandpharmacyconference.com for full details.
Handbook for Pharmacists
On World No Tobacco Day recently (May 31st), the International Pharmaceutical Federation (FIP) published a new handbook for pharmacists to support tobacco cessation and treat tobacco dependence.
The handbook has been developed in collaboration with an international group of experts from various organisations, including the World Health Organization. The handbook outlines the latest evidence-based practices, techniques and strategies for pharmacists to help people on the often-challenging journey to a tobacco-free life and remaining abstinent. It includes guidance on how to conduct patient assessments, design treatment plans, support behavioural change, select appropriate medicines to manage withdrawal symptoms
and monitor progress, as well as covering other aspects such as health promotion, patient education and referral, and interprofessional collaboration.
“The information contained in this handbook is practicable and tailored to the needs of pharmacists. Using this new resource from FIP, pharmacists can contribute to improving public health and reducing the large burden of tobacco use on our healthcare systems,” said Dr Inês Nunes da Cunha, FIP practice development and transformation
projects manager, and a co-author of the handbook.
Tobacco cessation is one of the most cost-effective and high impact interventions to reduce the prevalence of non-communicable diseases (NCDs), according to the World Health Organization.1,2 The new handbook is accompanied by a publication describing the knowledge and skills required for the delivery of pharmacist-led interventions to support tobacco cessation in addition to other modifiable NCD risk factors such as physical inactivity, unhealthy diet and harmful use of alcohol.
“With more than two million people employed in Ireland, organisations that use healthyworkplace.ie have enormous potential to influence population health, as the measures they implement will directly impact on the physical, mental, economic and social wellbeing of workers.
“I’d like to thank the website developers and congratulate all the stakeholders who contributed to its development.
“It’s a wonderful resource and I look forward to seeing how organisations use it to build healthier workplace environments.”
Head of Healthy Ireland Tom James said, “The launch of the Healthy Workplace website is a key milestone in the implementation of the National Framework for Healthy Workplaces.
“It provides resources and support to workplaces which are open and ready to engage with the wellbeing agenda and this is essential if we are to improve the health of the whole population.”
The Healthy Workplace website is a priority under the National Framework for Healthy Workplaces.
PHARMACYNEWSIRELAND.COM 4
News
New
Campaign targets Pharmacy
The Irish Heart Foundation has launched a new campaign asking adults, particularly those aged over-50, to have their blood pressure checked with their GP or local pharmacy.
The charity’s ‘Before Damage is Done’ campaign aims to drive awareness of the link between high blood pressure and heart disease and stroke.
The ‘silent killer’ affects two out of three over-50s – but because it is symptomless, half don’t know they have it.
Dr Angie Brown, Consultant Cardiologist and Medical Director with the Irish Heart Foundation, said thousands of people unwittingly go about their daily lives with high blood pressure.
“The only way of finding out is to get it checked with your GP or local pharmacy as you will, most likely, have no symptoms,” she said.
“It is one of the most important risk factors for heart disease and stroke but there are many factors that affect your risk.”
At the campaign launch, which coincided with May Measurement Month, Dr Brown said, “High blood pressure is one of the few conditions that people have the power to successfully manage – but only if they know they have it.
“Once detected, it is easily managed. Medication may be needed in addition to lifestyle changes such as increasing physical activity, quitting smoking, and embracing a balanced diet which limits salt intake. These changes can have a huge impact.”
Core Research for the Irish Heart Foundation shows that while many people know that high blood pressure is a risk factor leading to the development of cardiovascular disease (such as heart disease and stroke), there is a lack of awareness of other serious outcomes – such as dementia, kidney disease and some forms of blindness.
Pharmacies at ‘Tipping Point’
A consistent failure to invest in pharmacy services will have serious consequences for community healthcare if not urgently addressed, warned the Irish Pharmacy Union (IPU).

The Association said that community pharmacies are now at a ‘tipping point’ and require a restoration in fees to protect the viability of many pharmacies around the country and to maintain key services to patients. They called for the Minister for Health to immediately begin substantive talks ahead of a statutory review of pharmacy fees in June.
Since 2009, the amount paid to pharmacies per medicine dispensed has reduced by 19%, while the costs for dispensing medications on behalf of the state have increased by 23%. This is not sustainable and will soon reach the stage where it will cost more to dispense an item than what is paid by the state. In the same period the revenue to pharmacies for state schemes has reduced by 29% while costs have increased dramatically and are continuing to increase. Pharmacies are in fact in certain circumstances subsidising the state for implementing schemes on behalf of patients and this goodwill cannot continue.
Minister for Health, Stephen Donnelly TD is obliged, under the Public Service Pay and Pensions Act 2017, to review pharmacy fees before the end of June this year. Community Pharmacists are calling for a modest increase in dispensing fees to ¤6.50 per item representing an increase of just 50 cents on the rates paid in 2009.
According to Kathy Maher, the Chair of the IPU’s Pharmacy Contractors Committee, “This
restoration and recalibration would be fair and just when looking at other sectors, and those working within healthcare. Despite repeated calls for engagement, the Minister for Health, Department of Health and HSE have left us in the dark. We expect and demand, as a matter of basic respect, that the Minister will engage with the sector in the coming weeks.”
Ms Maher said that the lack of adequate funding and treatment of pharmacists has been manifestly unfair, unjust and downright discriminatory when compared with other professions. “In recent times we have seen civil servants receive an average of 12% pay increases, other healthcare professionals such dentists, nurses, consultants and even hospital pharmacists receive an average of 14% increase. Since the 2019 GP agreement, the additional annual expenditure provided to GPs has increased by ¤211m.”
It should be noted said Ms Maher that pharmacy fees were cut under the Financial Emergency Measures in the Public Interest Legislation in 2009. “But the emergency is long over, and we still have not seen any restoration in comparison to other healthcare sectors. Community pharmacists have been roundly ignored, despite assurances and we are now expected to do more work for the state, for less money, while baring higher costs – this is simply unsustainable.”
The convenience and accessibility of the pharmacy network is one
of the “shining lights of our health system,” according to Ms Maher. “At the same time as the new HSE boss, Bernard Gloster, is calling on hospitals to function more efficiently at weekends, the continued underfunding of pharmacies will lead to weekend closures and reduced hours, particularly in rural areas. The knock-on problems if people cannot receive their medications on weekends will be immense, with knock-on impacts on the whole healthcare system.”
“Pharmacy closures, particularly in rural locations, are becoming increasingly likely. Many pharmacies are fast approaching a tipping point, particularly smaller pharmacies in rural locations that provide an invaluable service to the communities. We must do everything we can to prevent closures which would have an irreversible impact on local areas particularly those areas that have seen a decline in their GP and other healthcare services.”
Concluding Kathy Maher said, “Minister Stephen Donnelly has a decision to make in the coming weeks. We are at a crossroads and community pharmacists’ patience is running out. He can choose to engage with the pharmacy profession, in a meaningful way, treating Irish pharmacists with the same fairness and equity that other professions receive, or he may choose to look the other way, showing disregard for pharmacy and the public. The decision is his.”
PHARMACYNEWSIRELAND.COM 5
News
Kathy Maher, Chair of the IPU’s Pharmacy Contractors Committee
Government Inaction ‘Stifling’ Community Pharmacy
Consistent inaction from government and a failure to support community healthcare through Irish pharmacies is ‘stifling’ community pharmacy, is likely to lead to serious consequences and exasperate the growing shortage of pharmacists.
 Dermot Twomey, President, Irish Pharmacy Union
Dermot Twomey, President, Irish Pharmacy Union

what their largest customer will be paying in just a few weeks’ time. We are calling for him to immediately engage with us in a meaningful way.”
Dementia Model of Care
The HSE Enhanced Community Care Programme has launched a new Model of Care for Dementia to set out care pathways to ensure people living with dementia are at the centre of care practices and service design.
This warning was issued by Irish Pharmacy Union (IPU) President Dermot Twomey when he addresses the organisation’s AGM last month.
Speaking to pharmacists from across the country Mr Twomey said, “Irish people view their pharmacist as their first port of call for their healthcare needs. While the profession continues to expand the vital role it plays for patients, it is continuously taken for granted by the health authorities and the Minister.”
Mr Twomey outlined how pharmacies, who perform a range of services on behalf of the state, are close to breaking point due to years of underfunding and significant increasing levels of administration under the community drugs schemes. “We
have not had an increase in our dispensing fees in nearly 15 years. Since 2009, the amount paid to pharmacies for each medication we dispense has reduced by 19%, while the costs incurred for the provision of this service have increased by 23%.”
“Something has to give as we no longer have a functioning, viable business model and our hands are tied, as we have no ability to pass on these rising costs to patients as reimbursement prices are set by the state with the manufacturers of medicines.”
Under the terms of the Public Service Pay and Pensions Act 2017, Minister for Health Stephen Donnelly must review the rates payable to community pharmacy contractors before the end of June this year. Despite this crucial deadline Mr Twomey said that the Minister and the Department of Health are maintaining complete ‘radio silence.’
“We are now less than two months for the statutory review process to be completed. It is disappointing and distressing that we continue to be completely ignored by the Minister and his officials. Every pharmacy in the sector, which supports the employment of 31,000, has no indication of
A shortage of qualified pharmacists in Ireland is also remains a major concern Mr Twomey revealed. “With the increase in administrative demands placed on community pharmacists by the HSE and the continual lack of investment by the state it is becoming harder and harder to attract and retain qualified pharmacists in our practices. We welcome that the number of pharmacy student places available in Ireland is under review. However, improvements to the process for recognition of qualifications and registering of pharmacists from outside the EU with the pharmacy regulator the PSI has not progressed at anything close to an acceptable pace.”
“There is also a need to address the rapidly increasing bureaucracy, red-tape and the increased administrative load imposed on the sector. Pharmacists are healthcare professionals not administrators and every minute spent on form filling is one less minute spent with our patients. It is a huge frustration that is leading to pharmacists leaving the profession and further exacerbating pharmacist shortages.”
Concluding IPU President Dermot Twomey said, “The health authorities in this country are expecting pharmacists to do more work for the state, for less money, while continuing to bare higher costs. This will not be allowed to continue. We are asking for a viable fee and to be treated with the respect other healthcare professionals enjoy.”
With over 64,000 people currently living with dementia in Ireland, it is a life changing condition. This Model of Care provides an integrated framework to bring together a wide range of services for people living with Dementia and is under pinned by the following:
• people living with dementia are at the centre of considerations relating to service design and recommendations related to care practices
• they receive timely and equitable access to assessment, diagnosis and post-diagnostic support regardless of the location of a service, the type of dementia they have, their age, their ethnicity, any other disability or co-morbidity, or their gender.
The Dementia Model of Care builds upon the work of the National Dementia Strategy (2014), the HSE Corporate Plan 2021-2024 and has been developed within the context of Sláintecare (2020–2023) and the health reform agenda, where delivering the right care, in the right place, at the right time, given by the right team.
Dr Colm Henry, Chief Clinical Officer (CCO), HSE, said, “The Model of Care for Dementia provides a clear pathway for people with dementia and their carers. It will enable the person living with dementia greater accessibility to services, have a streamlined pathway of care from initial concerns of memory loss right through to diagnosis, and post-diagnostic supports, while all the time keeping the person with dementia at the centre of everything. The Model of Care also sets out supports for family carers and supporters, who are critical in supporting people living with dementia.”
PHARMACYNEWSIRELAND.COM 6 News
Click Here to Register Digital Version Only
TREAT EARLY.1 DELAY DISEASE PROGRESSION.1 EXTEND LIFE.1
Use ERLEADA® earlier and keep other
treatment options for later stages1
ERLEADA® (apalutamide) is indicated for the treatment of adult men with:2
• non-metastatic castration-resistant prostate cancer (nmCRPC) who are at high risk* of developing metastatic disease
• metastatic hormone-sensitive prostate cancer (mHSPC) in combination with ADT
Visit the Janssen Medical Cloud to learn more about ERLEADA®
ERLEADA®▼ 60 mg film-coated tablets PRESCRIBING INFORMATION. ACTIVE
INGREDIENT(S): Apalutamide. Please refer to Summary of Product Characteristics (SmPC) before prescribing. INDICATION(S): Treatment of adult men: with non metastatic castration resistant prostate cancer (nm CRPC), at high risk of developing metastatic disease; with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (ADT). DOSAGE & ADMINISTRATION: Adults: 240 mg (four 60 mg tablets) single daily dose, swallowed whole with or without food. Continue medical castration with gonadotropin releasing hormone analogue (GnRHa) throughout in patients not surgically castrated. Elderly: No dose adjustment. Renal impairment: Mild to moderate - no dose adjustment. Severe - not studied; caution advised. If treating, monitor patients for side effects, reduce dose if required. Hepatic impairment: Baseline mild or moderate (Child Pugh Class A and B) - no dose adjustment. Severe - not recommended.
CONTRAINDICATIONS: Hypersensitivity to active substance or any excipient. Women who are or may become pregnant. SPECIAL WARNINGS & PRECAUTIONS: Seizure: History of seizures or other predisposing factors - not recommended. Discontinue permanently if seizure develops during treatment. Risk of seizure may be increased in patients receiving concomitant medicinal products that lower the seizure threshold. Ischaemic heart disease and ischaemic cerebrovascular disorders occurred, including events leading to death; monitor for signs and symptoms of ischaemic heart disease AND ischaemic cerebrovascular disorders, optimise management of risk factors: hypertension, diabetes, dyslipidaemia. Falls and fractures: Reported; evaluate patients for risk before starting Erleada; continue monitoring and manage fractures, consider use of bone-targeted agents. Concomitant use with other medicinal products: Potent enzyme inducer; may lead to loss of efficacy of concomitant medication. Review concomitant medication and refer to SmPC for further guidance. Avoid co-administration with warfarin and coumarin-like anticoagulants. If coadministered with an anticoagulant metabolised by CYP2C9 (e.g. warfarin or acenocoumarol), conduct additional International Normalised Ratio (INR) monitoring. Recent cardiovascular disease: Monitor patients with clinically significant cardiovascular disease for risk factors (e.g. hypercholesterolaemia, hypertriglyceridaemia, other cardio-metabolic disorders) as safety in patients with clinically significant cardiovascular disease in the past 6 months has not been established. Androgen deprivation therapy may prolong QT interval: Assess benefit-risk including potential for Torsade de pointes prior to initiating Erleada in patients with a history or risk factors for QT prolongation and those receiving concomitant medicinal products that might prolong QT interval. Severe Cutaneous Adverse Reactions (SCARs): Including drug reaction with eosinophilia and systemic symptoms (DRESS) and Stevens Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Advise patients of signs
and symptoms suggestive of DRESS or SJS/TEN If these symptoms are observed, withdraw Erleada immediately and patients to seek immediate medical consultation. Do not restart Erleada in patients who have experienced DRESS or SJS/TEN while taking Erleada at any time and consider an alternative treatment. Effects on ability to drive and use machines: Seizures reported; potential risk for driving or operating machines. SIDE EFFECTS: Refer to SmPC for other side effects. Very common: Hot flush, hypertension, diarrhoea, skin rash, fracture, arthralgia, fatigue, weight decreased, fall, decreased appetite. Common: Hypothyroidism, hypercholesterolaemia, hypertriglyceridaemia, dysgeusia, ischaemic heart disease, ischaemic cerebrovascular disorders, pruritus, alopecia, muscle spasm. Other side effects: Seizure, QT prolongation, DRESS, SJS/TEN. LEGAL CATEGORY: Prescription only medicines. PRESENTATIONS, PACK SIZES, MARKETING AUTHORISATION NUMBER(S): Blister pack, 112, EU/1/18/1342/001. MARKETING AUTHORISATION HOLDER: JANSSENCILAG INTERNATIONAL NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. FURTHER INFORMATION IS AVAILABLE FROM: Janssen Sciences Ireland UC, Barnahely, Ringaskiddy, IRL – Co. Cork P43 FA46. Prescribing information last revised: December 2022.
Adverse events should be reported. ▼ This medicinal product is subject to additional monitoring and it is therefore important to report any suspected adverse events related to this medicinal product. Healthcare professionals are asked to report any suspected adverse events via: HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse events should also be reported to, Janssen Sciences Ireland UC on 1 800 709 122 or at dsafety@its.jnj.com.
*High risk is defined as patients with a PSA doubling time of ≤10 months.1 ADT = androgen deprivation therapy
References: 1. Smith MR, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021;79(1):150–158. 2. ERLEADA® (apalutamide). Summary of Product Characteristics. Available from: www.medicines.ie
Date of preparation: January 2023 | CP-361339

Positive Step to Faster Medicines Access
The decision by Minister for Health, Stephen Donnelly T.D. to publish the Mazars Report has been welcomed by the Irish Pharmaceutical Healthcare Association as a positive step towards providing Irish patients with faster access to life-changing new medicines.
as a valuable opportunity for key stakeholders, including industry and patient groups, to contribute to improved patient care and health outcomes.
Working Group, to present its own reform proposals and to hear the view of other stakeholders. It stated, “We will put forward ideas in good faith and will also listen to what is asked of us. Our members are very conscious of industry’s responsibilities to improve the process in a collaborative way.
be pathfinders for this new approach in 2023 and 2024.
• Advocate for an increase in the HSE’s decision authority table thresholds and more generally for a well-structured commercial framework, as exists in other countries.”
IPHA, which represents the major international biopharmaceutical companies in Ireland, commended the Minister’s decision to publish the report and to establish a Working Group to review and improve the reimbursement system for new medicines.

The organisation recognised that the initiative is the first of its kind since the passage of the Health (Pricing and Supply of Medical Goods) Act 2013, describing it
IPHA said the Working Group’s role in reviewing and improving the reimbursement process was especially important considering the many new advances in science and the potential to raise standards of patient care through faster access to newer and better medicines and treatments.
It applauded the Minister’s decision to act immediately to enhance transparency in the reimbursement system through the introduction of a tracker system and indicative timelines, stating that it would result in better planning and management of applications for medicine reimbursement.
IPHA said it welcomed the opportunity to engage in the
Child and Youth Mental Health Lead
We will:
• Bring forward proposals to support the further development of national medicines policy.
• Present the case for an increase in personnel and IT resourcing for the HSE’s Corporate Pharmaceutical Unit, the HSE Drugs Group and the National CPE.
• Explore ways for earlier reimbursement for certain treatments, such as early access schemes, as is done in other countries. IPHA companies are ready with some rare disease, cancer and end-of-life treatments that can
IPHA said there were “many other practical steps that can be taken to improve the processes that fully respect the HSE’s obligations under the 2013 Health Act. We will study the Mazars Report carefully and provide any observations arising from it to the Minister.”
Michael O’Connell, IPHA President said: “Over the past three budgets, the Government has allocated almost ¤100 million to new medicines. The announcement today is a further indication of the Minister’s continued commitment to ensure that patients have faster access to new, innovative medicines. I thank the Minister for driving this reform and bringing all stakeholders together in doing so”.
Minister for Mental Health and Older People Mary Butler welcomed the opening of the recruitment process for the post of Child and Youth Mental Health Lead in the HSE. This key new role will provide leadership, operational oversight, and delegated management of all service delivery across child and youth mental health services across the country. They will also be responsible for managing and coordinating service planning activities, partnership and capacity building, the development of service plans, and setting of service standards right across child and youth mental health services in Ireland.The post holder will report to the HSE National Director for Community Operations and will be supported by a dedicated team for which funding has been provided.
This role, and the wider Child and Youth Mental Health office, will result in improved links with the National Clinical Advisor Group Lead Mental Health, which in turn will support the development of current and future youth mental healthrelated National Clinical Programmes.
Minister Butler stated, “The progression of this role has been a key priority for me over the past year, and it will play a crucial part in ensuring that integrated mental health services for young people have a more centralised and evidence-based focus within the HSE. “We will see the completed reviews and audits arising from the Maskey Report, along with the Final Report of the Mental Health Commission on CAMHS later this year, which together will give us real time data never available before to support this new post. Importantly, there will be new support staff to underpin this welcome initiative. I will ensure that the HSE also progresses as quickly as possible the new post of National Clinical Lead for Youth Mental Health as announced in the Dáil over the last week.”
Pharmacy Role in Hygiene
Advice on hygiene given by pharmacies has widened from oral care to infection control measures, according to a report by the FIP Global Pharmaceutical Observatory (GPO) published last month.
A literature review found educating people on hygiene measures such as handwashing and disinfection to be a novel community pharmacy service during the pandemic. The FIP GPO also conducted a cross-country survey through five FIP member organisations (n=60). More than half of the respondents reported that avoidance of respiratory, viral, communicable, and food and water borne infections were the most common “germ concerns” expressed to
them by the public. The study also looked into current and future learning needs of pharmacy teams.
The findings from the literature review showed that the concept of providing hygiene care advice has been differently shaped preand post-COVID times. Before the COVID-19 pandemic, the area of focus was limited to oral hygiene. All studies reviewed in the literature reinforced the vital role of community pharmacists as the
public’s first choice of healthcare provider in mitigating the spread of the disease as well as their prominent contribution to overall emergency management.
There was also an unequivocal need to support, train and educate community pharmacists and their teams to improve their knowledge because the concept of increasing personal hygiene awareness is a focus area of community pharmacists in developed and
developing countries.
The report states, “As evidenced throughout this report, community pharmacists and their teams can play a leading role in providing reliable recommendations and educating patients on health hygiene, engaging and empowering patients in self-care, and minimising the spread of contagious diseases, ultimately contributing to improving our communities’ overall health.”
PHARMACYNEWSIRELAND.COM 8 News
IPHA President Michael O’Connell
Cetrine Allergy 10mg Film-Coated Tablets available in packs of 7s and 30s. Always read the leaflet.


ABBREVIATED PRESCRIBING INFORMATION
Product Name: Cetrine Allergy 10mg lm-coated tablets & 1 mg/ml oral solution. Composition(s): Each tablet contains 10 mg cetirizine dihydrochloride. One ml of the oral solution contains 1 mg cetirizine dihydrochloride. Description(s): White, oblong lm-coated tablets, scored on one side. Can be divided into equal halves. Clear, colourless liquid with banana avour.
Indication(s): Tablets: Adults and paediatric patients 6 years and above. Oral solution: Adults and children 2 years and above. Relief of nasal and ocular symptoms of seasonal and perennial allergic rhinitis (hay fever); relief of symptoms of chronic idiopathic urticaria.

Dosage: Tablets: Adults, elderly and children aged 12 years and over: 10 mg once daily. Children from 6 years to 12 years: 5 mg (half a tablet) twice daily. Moderate renal insu ciency (creatinine clearance CrCl 30-49 ml/min): 5 mg once daily. Severe renal insu ciency (creatinine clearance ≤30 ml/min): 5 mg once every 2 days. Children under 6 years: Not recommended. Oral solution: Children aged from 2 to 6 years: 2.5 mg twice daily (2.5 ml oral solution twice daily (half a measuring spoon twice daily)). Children aged from 6 to 12 years: 5 mg twice daily (5 ml oral solution (a full measuring spoon twice daily)). Adults and adolescents over 12 years of age: 10 mg once daily (10 ml oral solution (2 full measuring spoons)). Not recommended in children aged less than 2 years. Moderate renal insu ciency (creatinine clearance CrCl 30-49 ml/min): 5 mg once daily. Severe renal insu ciency (creatinine clearance ≤30 ml/min): 5 mg once every 2 days. In paediatric patients su ering from renal impairment: Adjust dose on an individual basis taking into account the renal clearance of the patient, his age and his body weight. Contraindications: History of hypersensitivity to the active substance, to any of the excipients, piperazine derivatives or hydroxyzine. Severe renal impairment < 10 ml/min creatinine clearance.
Warnings and Precautions for Use: Cetirizine may increase risk of urinary retention, therefore caution in patients with predisposition factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia). Caution in epileptic patients and patients at risk of convulsions. Discontinue use of cetirizine three days before allergy testing. Pruritis and/or urticaria may occur when cetirizine is stopped, even if the symptoms were not present before treatment initiation. In some cases, the symptoms may be intense and may require treatment to be restarted. The symptoms should resolve when the treatment is restarted. Tablets contain lactose. Oral solution contains sorbitol, propylene glycol, sodium (essentially ‘sodium free’), methyl - & propyl-parahydroxybenzoate.
Interactions: Caution is advised when taken concomitantly with alcohol or other CNS depressants. Cetirizine does not potentiate the e ect of alcohol (0.5 g/l blood levels). The extent of absorption of cetirizine is not reduced with food, although the rate of absorption is decreased.

Pregnancy and Lactation: Caution during pregnancy and breast-feeding.
Ability to Drive and Use Machinery: Usually non-sedative, patients should take their response to the product into account. In sensitive patients, concurrent use with alcohol or other CNS depressants may cause additional reductions in alertness and impairment of performance.
Undesirable E ects: Cetirizine at the recommended dosage has minor adverse e ects on the CNS, including somnolence, fatigue, dizziness and headache. In some cases, paradoxical CNS stimulation has been reported. Although cetirizine is a selective antagonist of peripheral H1-receptors and is relatively free of anticholinergic activity, isolated cases of micturition di culty, eye accommodation disorders and dry mouth have been reported. Instances of abnormal hepatic function with elevated hepatic enzymes accompanied by elevated bilirubin have been reported which resolves on discontinuation of the drug. Uncommon: Agitation, diarrhoea, pruritus, rash, asthenia, malaise, paraesthesia. See SPC for all adverse reactions.
Marketing Authorisation Holder: Rowex Ltd, Bantry, Co. Cork.
Marketing Authorisation Number: PA0711/075/002-003. Further information and SPC are available from: Rowex Ltd., Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417
E-mail: rowex@rowa-pharma.ie

Legal Category: Not subject to medical prescription.
Date of Preparation: March 2021
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing Rowex pv@rowa-pharma.ie

Date of preparation: (04-22) CCF: 24955 Supply status: Supply through pharmacies only.
FAST RELIEF OF ALLERGIES 24hr
Brexit Update for Medicines
On 26 February 2023, the EU and the UK reached political agreement in principle on the Windsor framework. The parties to the agreement are committed to take “the necessary steps to translate the joint solutions into legally binding instruments and to implement these swiftly and in good faith”.
While the agreement covers trade generally, this update only focuses on human medicines.
The Commission has published a draft regulation regarding the proposal on medicines, to reflect the agreement in principle. Its main purpose is to facilitate the supply of medicines from Great Britain (GB) to Northern Ireland (NI) and to address concerns that were raised around this linked to the implementation of the NI protocol. Of significance in the regulation:
• Prescription medicines in NI will not be permitted to carry the safety features required under the Falsified Medicines Directive (FMD).
• New novel medicines authorised by the EU (centralised medicines) will not be permitted on the NI market, i.e., only those authorised by the UK authorities will be permitted on the NI market.
• All medicines on the NI market must have the words “UK only” on the packaging.
• The provisions do not apply to veterinary medicines.
• The new provisions are due to be implemented from 1 January 2025. This date depends on when the UK provides written guarantees to the European Commission on the protection of the integrity of the EU internal market.
Implications for Marketing Authorisation Holders (MAHs) supplying medicines to the Irish (IE) market
The Windsor agreement has implications for Marketing Authorisation Holders supplying
Risk of ‘Iron Overload’
both the IE and NI markets. In particular, companies with joint packs between GB/IE or NI/IE need to consider what it means for their products.
1. Prohibition of the use of the safety feature on products placed on the NI market.
The Windsor agreement prohibits safety features appearing on the outer packaging of medicines placed on the NI market. This will, in effect, stop joint packs between NI/GB and IE.
The expiry on 31 December 2024 of the existing exemption for having to decommission product going to the UK, was already going to impact joint packs. However, it was expected that joint packs with NI would still be possible in 2025. Split batches where only the IE/NI portion was uploaded to the repository should also have been feasible.
MAHs who previously used joint GB/IE or NI/IE packs should now consider separating those packs in advance of this Windsor agreement legislation implementation.
2. Special wording required for medicines placed on the NI market.
Medicines placed on the NI market must carry the wording “UK Only”. This provision also impacts the ability of MAHs to have a joint pack with IE and NI/GB particularly for OTC medicines which are not required to carry the safety features.
3. Prohibition on putting EU centralised products on the NI market
The proposal prohibits placing medicines authorised by the Commission (known as centralised medicines), on the market in
Adrian Dunne Pharmacy
Raising Vital Funds
The pharmacy team at Adrian Dunne Pharmacy Group have recently raised a staggering amount of money for charity.

All staff across the group came together with the support of their customers and raised a remarkable ¤7,500 for the Irish Red Cross Turkey and Syria Earthquake Appeal.
Said a pharmacy group spokesperson, “These much needed funds raised over the last couple of months will be very much appreciated and we thank you for your generous support.”
Pictured is Managing Director Adrian Dunne with the pharmacy team.
Northern Ireland. These medicines must be separately authorised by the UK authorities for the NI market. They must also carry the words “UK only” and not carry the safety features.
Currently many centralised products are using joint labels for the UK/NI and IE market. These marketing authorisation holders must now start planning for separate packs for the IE market. Joint packs (including where necessary multi-lingual packs) with other EU/EEA MSs remain possible. Expiry of the existing exemptions in December 2024
As MAHs will be aware, updates to EU legislation were implemented in 2022 allowing certain exemptions for a period of 3 years.
In summary they allowed:
• Continued batch release in GB,
• Continued QC testing in GB without the need for retesting on importation into the EU,
• Importation of an authorised medicine from GB by a wholesaler rather than the holder of a manufacturer’s authorisation,
• An exemption from the requirement under the Falsified Medicines Directive to decommission joint packs going to the UK market.
Some of these exemptions were notifiable to the HPRA and onwards to the Commission.
MAHs are reminded that these exemptions only apply until the end of 2024. The HPRA will write to MAHs availing of these exemptions asking them to outline their plans in respect of these medicines.
Despite being Ireland’s most common genetic condition, with one in 83 people at risk of developing haemochromatosis and one in five people carrying the gene, it might come as a surprise to learn that haemochromatosis – or ‘iron overload’ - is also one of the lesser-known conditions amongst the Irish population.
Haemochromatosis is more common in Ireland than anywhere else in the world, giving it the nickname ‘the Celtic Gene’ as one in five people carry one copy of the gene and one in every 83 Irish people carry two copies of the gene, predisposing them to develop iron overload. Recent research also shows that up to 1 in 10 people in Northern Ireland are at risk of having genetic Haemochromatosis.
Early diagnosis is vital and if left untreated, iron overload can lead to organ damage or even premature death. The Irish Haemochromatosis Association estimates there are at least 20,000 undiagnosed cases of haemochromatosis in Ireland.
To mark World Haemochromatosis Awareness Week, which took place from 1st – 7th June 2023, the Irish Haemochromatosis Association aims to raise awareness of the condition and is urging people to join the association in calling for a National Strategy for Haemochromatosis. The Association is also asking people to ‘Get Checked for Haemochromatosis’ to highlight the symptoms in order to save lives.
This year will see several City and County Councils throughout the country supporting the campaign and joining the wider, international initiative to ‘Light Up Red’, lighting up several iconic public buildings during World Haemochromatosis Awareness Week.
The Irish Haemochromatosis Association has this year teamed up with Tomás O’Leary. Tomás has family members living with haemochromatosis and is helping to raise awareness of the common condition, in the hope that someday everyone in Ireland will be aware of the symptoms.
PHARMACYNEWSIRELAND.COM 10
News
CommCare Pharma Ambassador Day 2023
CommCare Pharma hosted their annual Ambassador Day in the Limerick Strand Hotel on May 17th where they welcomed their Pharmacy Ambassadors from totalhealth & Haven Pharmacies around the country.


There was a full agenda with informative presentations from our Support Office team, product training from our Suppliers and engaging breakout & feedback sessions. The attendees were also delighted to hear guest speaker, Sally Foran @Irishbeautyfairy, speak about her experience in promoting people & products on social media.
One of the highlights of the day was a Supplier Expo where some of CommCare’s key SuppliersClonmel Healthcare, Cosmetic Active, Fleming Medical, Haleon, Kenvue, Reckitt and Scope Healthcare – showcased their products, provided training materials and engaged with the Ambassadors.





CommCare Ambassadors are an important link between the Support Office and the pharmacy teams as they relay important information and training to the instore teams.
CommCare was delighted to be able to update their Ambassadors and listen to their feedback and look forward to the next meeting. CommCare would like to thank their Support Office team for their involvement on the day and especially thank the Suppliers for their participation and support.

PHARMACYNEWSIRELAND.COM 11
CommCare Ambassadors attending Supplier training
Paula Gallagher CommCare Group Training & Retail Business Manager with lucky raffle winner Michelle Melia from Haven Pharmacy Brennan’s Cavan
Bobby Fleming, Niamh Fitzroy and Paul Kennedy of Fleming Medical at the Supplier expo
Rose Tierney from Cosmetic Active
Ann Marie Curran from Kenvue
Clonmel Healthcare’s stand at the Supplier Expo
Conor Budds and Erica Dunne from Reckitt Benckiser
News
Karina McCarthy, Laoise Fitzgerald and Mary Sydenham from Scope Healthcare
Medicine Shortages Index at 247
Medicine shortages in Ireland continue to persist with 247 different medicines used by Irish patients currently out-of-stock, as a new trend affecting supply develops, according to the latest Medicine Shortage Index.
used for the treatment of high blood pressure.
Many antibiotics like Amoxicillin and Penicillin and commonly used over-the-counter medicines like Benylin ™ and Dioralyte ™ are still difficult for patients to source.
The Medicine Shortage Index, prepared by industry experts, Azure Pharmaceuticals, analyses data made publicly available by the Health Products Regulatory Authority (HPRA).
Medicine shortages will continue grow incrementally unless political will is shown in Ireland to take measures, like those carried out by other EU nations, to meaningfully tackle the issue, Sandra Gannon, Azure CEO, said:
The latest figures show the number of medicine shortages in Ireland up an additional 19 medicines in short supply since the end of last month, and a 38% increase since the Index began in October.
Of the 247 medicines currently unavailable, 13 are listed on the World Medical Organisation’s (WHO) ‘critical medicines’ list.
The latest shortages analysis indicates a new trend of medicines that are stored or delivered using plastic components now increasingly in short supply. These medicines include nasal sprays, inhalers for the treatment of asthma and 11 different eye drop products. Other medicines still in short supply across multiple suppliers in the past week include those that treat epilepsy, and medicines
Other European countries have already taken specific policy measures to date in response to the escalating medicine shortage issue. Portugal, the UK, Germany, and Switzerland have all taken a range of price related policy measures in response to the problem, including price increases for lower priced medicines.
While, Sweden, Denmark and Malta, which all use tenders to set reimbursement prices, have all experienced price increases due to lack of supply of core medicines. To date, the Irish Department of Health is yet to meaningfully respond to this deepening challenge.

“One of the means we have to protect our domestic supply of stock, to prevent these important medicines from running out, is through pricing. Other European countries have already recognised this fact and taken measures to mitigate against situations where their stocks run out. For example, Portugal recently raised its pricing by up to 5% for cheap medicines.
“Weaknesses in the supply chain alone highlight the imperative of revisiting the pricing framework for medicines to protect supply of stock and protect Irish patients.”
Commenting on the perception that medicine shortages are a
result of exceptional circumstances and are a one-off situation, Ms Gannon pointed to the level of EU activity on the topic, as well as the focus of the European Medicines regulator (EMA) on medicine shortages as evidence that this problem is not going away without serious intervention and planning.
“There’s an awareness in other European countries that market related factors need to be tackled. Medicines shortages are not just winter specific, and shortages are not only occurring as a result of exceptional circumstances. There are systemic factors that need resolution.
“Each patient has different needs and reducing the problem down to exceptional circumstances alone diminishes the quality of life impact that each patient experiences with their illness.”
Medicine shortages experts from Europe and Ireland recently said in Dublin, at a panel discussion examining medicine shortages, that the situation will continue to worsen without the political will to implement measures that put the needs of patients first.
Doctors encouraged to train in Ireland
Irish postgraduate medical colleges were in Dubai recently to discuss the large range of medical training opportunities in Ireland for doctors from the United Arab Emirates and the wider Gulf region. A new website www.postgraduatemedicaltraining.ie was launched by Her Excellency Ambassador Alison Milton, Irish Ambassador to the UAE, detailing Ireland’s clinical postgraduate training opportunities for doctors from across the GCC. It also shares information on relocation support and benefits of training in Ireland.
The clinical residency and fellowship programmes provided by Irish medical colleges are internationally recognised and fully certified. They offer handson supervised training, as well as opportunities for clinical research.
Doctors from the UAE will have the opportunity to move to Ireland and learn from leading experts to develop the medical, communication, management
and interpersonal skills required to provide world-class care and enhance the clinical environments in which they operate.
“The Royal College of Physicians of Ireland has strong links with the United Arab Emirates, partnering with healthcare organisations in the UAE to provide postgraduate medical training opportunities since 2013. These partnerships
are highly valued by the College,” Professor Mary Horgan, President of the Royal College of Physicians of Ireland said.
“Medical graduates from the United Arab Emirates make significant contributions to our medical teams. Graduates report a very positive experience and, upon returning home to the UAE, they are in a strong position to
advance their medical careers and contribute to the development of healthcare in the UAE. There are a growing number of fantastic opportunities for doctors from the UAE to come to Ireland for transformative postgraduate medical training across many specialties and this new website is a comprehensive resource for doctors to explore what’s available,” Prof Horgan said.
PHARMACYNEWSIRELAND.COM 12 News
“There’s an awareness in other European countries that market related factors need to be tackled. Medicines shortages are not just winter specific, and shortages are not only occurring as a result of exceptional circumstances. There are systemic factors that need resolution”
Bio-Kult Boosted is a scientifically developed, extra strength multi-strain formulation containing 14 friendly live bacterial cultures, proven to survive the high acidity of the stomach.

Available from your local wholesaler
It can be swallowed whole, sprinkled over food or mixed in a drink and it doesn’t need to be refrigerated.
✓ Includes vitamin B12

✓ Backed by clinical research

✓ Everyday use
Bio-Kult is suitable for vegetarians
✓ Suitable to use with antibiotics
SCOPE1E 199
/biokultirl @ biokult_irl scopehealthcare.ie
Irritable Bowel Syndrome
A personalised approach to Irritable Bowel Syndrome
Professor Humphrey J. O’Connor MD FRCP AGAF, Trinity Academic Gastroenterology Group, Trinity Centre for Health Sciences, Tallaght University Hospital, Tallaght, Dublin 24 email: oconnor_hj@hotmail.com

Introduction
When you think about it, the gut is a marvellous machine! Simply eating food automatically sets the machine in motion and all being well we are blissfully unaware of digestion without pain, wind, or noise. In the lower gut, a feeling of rectal fullness disappears once defaecation is complete. The whole process of digestion critically depends on normal gut-brain contact with continuous crosstalk between the automatic nervous system within the gut, the Enteric Nervous System (ENS), and the Central Nervous System (CNS). If for whatever reason, at brain or gut level, that crosstalk between the ENS and CNS becomes disturbed or dysregulated the end result can be the development of a Disorder of Gut Brain Interaction (DGBI). Irritable Bowel Syndrome (IBS) is the commonist DGBI and perhaps the most troublesome. IBS is defined as a syndrome of recurring abdominal pain at least one day per week in the last three months related to defaecation and associated with a change in bowel habit. Other symptoms of IBS can include excess wind, bloating and a feeling of incomplete rectal emptying.
IBS is very common affecting about 1 in 10 people, causes a significant negative impact on quality of life, social life and work productivity, and is associated with increased medical costs and healthcare utilisation. For unknown reasons, IBS is far commoner in women than men (7 of 10 patients) and women also bear the brunt of the condition with more severe symptoms, more comorbid somatic symptoms and increased need for second line medications. IBS is arbitrarily defined by the predominant disturbance in bowel habit. About 10 to 20% of patients have IBS Diarrhoea, 10 to 20%
have IBS Constipation, and the majority of patients are IBS Mixed. The IBS subtype has an important bearing on the choice of treatment options. Nearly 40% of primary care and gastroenterologist visits can be attributed to IBS. Longterm, about a third of patients with IBS improve and symptoms disappear, symptoms remain unchanged in 30 to 50%, but can worsen in about 20%.
Diagnosis
It is important to remember that the majority of IBS patients are diagnosed and managed in primary care. The diagnosis of IBS is primarily based on patients having appropriate symptoms of abdominal pain and altered bowel habit and normal results in a limited number of tests. One of the dilemmas faced by IBS patients is their multiple symptoms, feeling so unwell, and yet all tests are normal. There is no specific test or biomarker for IBS but at the same time doctors and patients do not want to miss a serious or organic disease masquerading as IBS, particularly cancer. Basic tests can be summarised as the four C’s, Complete or full blood count, C reactive protein, Coeliac serology (tTg antibody) and faecal Calprotectin. Thyroid function tests are often added to this list. This set of tests will help exclude inflammatory bowel disease, microscopic colitis, coeliac disease and hyper or hypothyroidism.
The need for invasive tests such as colonoscopy or scans is driven by the presence of “red flag symptoms” such as night waking, weight loss or non-hemorrhoidal rectal bleeding, and patient age. Current guidelines use an age cut-off of 45 or 50 years for colonoscopy but factors such as family history of colorectal cancer or patient worries and wishes should be respected. Another recent concern is the inexplicable and genuine rise in young-onset colorectal cancer, particularly in women, and this phenomenon might also lower the age threshold for colonoscopy.
Long-term follow up of patients positively diagnosed with IBS is reassuring with only rare
development of organic disease. Hence, although symptoms might persist and even change over the years, repeat testing should be avoided. Extensive and fruitless investigations only serve to undermine patients confidence in the diagnosis of IBS and in their healthcare provider. The fact that IBS does not increase the risk of colorectal cancer should also be emphasised.
Treatment
It is in the treatment of IBS that the word “Personalised” really becomes important. A good doctor-patient relationship is important in all aspects of clinical medicine but is paramount in the management of IBS. Dedicated time is needed in clinic to appreciate what are the patients’ predominant symptoms, concerns and preferences, previous treatments, and possible psychological comorbidities. Equally, time is needed to educate and explain to patients in plain language and diagrams the nature of IBS as a brain-gut disorder and the role of factors such as lifestyle, diet, stress, and gut bacteria etc. For many patients, education and the reassurance of normal test results as outlined above, coupled with lifestyle and dietary interventions are enough to ease symptoms and improve quality of life. Increased exercise, healthy eating habits and reduced intake of alcohol, caffeine, fat and spicy or gas-producing foods have all been shown to have beneficial effects on IBS symptoms.

If medication is needed for mild to moderate IBS, the choice is largely driven by the predominant symptoms. Antispasmodics including mebeverine or alverine, the anticholinergic hyoscine, or peppermint oil have all been shown to have some impact on the pain of IBS and can be used as necessary. Water soluble fibre such as ispaghula or the osmotic laxative polyethylene glycol can help constipation. Loperamide is an effective antidiarrhoeal agent but does not improve overall IBS symptoms and again can be used as necessary.
Dietary Therapy
In a recently published study from the Netherlands on treatment preferences, IBS patients showed a strong preference for dietary intervention (48%) compared with pharmacological therapy (29%) or psychotherapy (23%). Since its introduction from Melbourne Australia over a decade ago, low FODMAP diet (LFD) has become the diet of choice worldwide in the management of IBS. LFD works on the premise that Fermentable Oligosaccharides, Disaccharides, Monosaccharides And Polyols are responsible for IBS symptoms by, among other things, creating an osmotic load in the small bowel and increasing colonic gas production. The upshot of at least ten clinical trials suggests LFD can give effective symptom relief in about 50 - 70% of IBS patients and all IBS subtypes can show response with IBS Diarrhoea most favourable. In
PHARMACYNEWSIRELAND.COM 14



Available at Meaghers and selected pharmacies and health food stores. www.meaghers.ie
Irritable Bowel Syndrome
practice, patients firstly eliminate FODMAPs for an arbitrary 2-6 weeks to assess response. If symptom response is good foods are gradually reintroduced to ultimately personalise the diet to the minimum numbers of FODMAPs that need to be avoided. Personalised advice from a dietitian and providing written instructions have been shown to increase compliance and success. LFD comes with a number of caveats. We have very little longterm data on the effects of LFD and concerns have been raised about its nutritional adequacy and the reduction in fibre intake. We are also only beginning to assess combination treatments, for instance, LFD with prebiotics or probiotics, or LFD with psychological treatments.
Probiotics and Antibiotics
The human microbiome, the microorganisms that inhabit the gut, has received enormous attention in recent years with the realisation that a “good” microbiome is essential for health whereas a “disturbed” microbiome might contribute to ill health, including IBS. The fact that by-products of microbiome metabolism might actually interact with the ENS and play a role in brain-gut crosstalk has added further interest. Our colleagues at University College Cork have been to the forefront of this cutting edge research. By putting live microorganisms into the gut, probiotics are designed to improve the microbiome. At least 50 clinical trials have tested the effects of probiotics in IBS. On balance, probiotics have been shown to improve global symptoms as well as bloating and excess wind. However, no solid recommendation can be given at this time on what particular strain or formulation of probiotic is best, or the subtype of IBS most likely to respond.
Ironically, antibiotics which might diminish the microbiome have also shown efficacy in IBS. Clinical trials of Rifaximin, an oral broadspectrum minimally absorbed antibiotic, have shown good effect particularly in non-constipated IBS. The course of Rifaximin lasts two weeks and can be repeated.
Moderate to Severe IBS
About 20% of IBS patients have really troublesome symptoms poorly responsive to first line treatments and dietary changes and will usually be attending a gastroenterology clinic. Added to their bothersome IBS symptoms many of these patients will have other gastrointestinal symptoms, extra intestinal symptoms apparently emanating from several organ systems, and psychological problems (Figure). In clinical practice, the most commonly reported extraintestinal problems include chronic fatigue, fibromyalgia, poor sleep, low back pain, chronic pelvic pain, dysuria, and dyspareunia (Table 1). A history of anxiety, depression or panic disorder is common enough in this group of patients but the extraintestinal symptoms can occur in the absence of mood disorder. On direct questioning some patients will give a history of unresolved childhood abuse. In this setting IBS is aptly described as a BioPsychoSocial disorder disorder and difficult to judge whether treatment should be directed to the brain or gut or both.

Gut
With greater understanding of the ENS over the past decade, the pharmaceutical industry has developed targeted oral treatments that act at gut level to help difficult IBS constipation or diarrhoea. Prucalopride (Resolor), linaclotide, plecanatide and lubiprostone are all prosecretory agents which have been shown to ease the symptoms of IBS Constipation. At present prucalopride is the only one of these medications available on prescription and perhaps the availability of the other agents is
delayed for pharmacoeconomic reasons with linaclotide available on a named patient basis. Eluxadoline, a opioid receptor agonist, has proven efficacy in the management of IBS diarrhoea but again is not available on prescription in Ireland.
In difficult IBS Diarrhoea, it is worth remembering idiopathic bile acid diarrhoea (BAD) as a possible contributor to urgent watery stools and a real risk of faecal incontinence. In idiopathic BAD, increased bile synthesis seems to overpower absorptive capacity in the ileum, with consequent excess colonic bile acids which increase colonic motility, water secretion and mucosal permeability. Diagnostic tests are available for BAD but sometimes an easier alternative is a treatment trial of a bile acid binder medication such as cholestyramine or colesevelam. A favourable response to these medications is tantamount to a positive diagnostic test.
Brain
Faced with difficult poorly responsive IBS, gastroenterologists have increasingly turned to treatment with low dose psychotropic agents, originally developed to treat psychological or psychiatric disorders. Before prescribing, open discussion is needed to reassure the patient that he or she is not being stigmatised with IBS as a psychiatric disorder and to explain potential medication benefits and risks. Sedation is the most common side effect but unwanted weight gain should also be mentioned. Low dose amitriptyline or nortriptyline are particularly useful in the management of IBS diarrhoea or IBS mixed. 10mg at bedtime also helps poor sleep and responders usually feel clinical benefit within a week or two. Nortriptyline may be less sedating than amitriptyline. Patients should be asked to promptly report side effects and a planned follow up contact at two weeks is very beneficial for a good trusted doctor-patient relationship in the long-term. If a
patient has not responded to 10mg of amitriptyline or nortriptyline after two weeks and has not had any side effects, continue 10mg for longer rather than increase the dose as cumulative benefit can occur over time. Increased dose potentially invites more side effects but on occasion the dose can escalate to 50mg daily. If a patient is doing well, treatment should continue for six to twelve months on the premise that a longer course might reduce the chance of symptom recurrence when the medication is slowly withdrawn. Tricyclic antidepressants tend to be avoided in patients with IBS constipation and a treatment trial of a low dose selective serotonin reuptake inhibitor, escitalopram at 5 to 10mg, is an alternative choice in that setting. Other related agents include mirtazapine 15mg at night for IBS Diarrhoea and functional dyspepsia and low dose duloxetine 30mg for abdominal pain and IBS Constipation.
Psychotherapy
A variety of different psychological treatments are effective in the management of IBS including cognitive behavioural therapy, gut-directed hypnotherapy, relaxation therapy, multicomponent psychological therapy and dynamic psychotherapy. Younger patients more often prefer psychotherapy than older patients and at least one study has shown gut-directed hypnotherapy worked best in young female IBS patients with associated anxiety. The practical difficulty in psychological therapies is limited availability, high costs, and the difficulty of weekly appointments. Some of those difficulties may be overcome by mobile Apps such as Nerva which delivers a self-administered gut-directed hypnotherapy programme at home. In an ideal world, the clinic setting for difficult IBS would combine the services of a gastroenterologist, a specialist dietitian, and gastrointestinal psychotherapist.
Conclusions
IBS is a common, often troublesome DGBI that creates a multiplicity of symptoms both within and outside the gut. IBS disimproves quality of life and increases healthcare seeking. The burden of IBS falls very much on women more than men. Management of IBS calls for a personalised doctor-patient relationship that can deliver empathy, explanation, a measured diagnostic workup, and effective safe therapy mindful of the patients condition and preferences. In essence, optimum care of IBS patients requires an effective holistic approach that truly combines the science and art of medicine.
References on request
PHARMACYNEWSIRELAND.COM 16
Table 1










Trust your gut. Trust precision science. Over 20 years of pioneering research ALFLOREX® with Bifidobacterium longum 35624 ® Scan here for more info Learn more about the science behind the unique 35624 culture – visit www.precisionbiotics.science
Managing Diabetes in the Summer

Summertime can bring extra challenges for people with diabetes. Research suggests that hot weather can lead to health issues for people with diabetes, making them more sensitive to high temperatures and humidity. Sitting in the sun for long periods can affect diabetes because sufferers are not being very active, making blood sugar levels higher than usual. On the flipside, for those who take insulin to treat their diabetes it will be will be absorbed more quickly from the injection site in warm weather, and this increases the risk of hypos. If pharmacy teams can advise diabetes patients to be careful about about managing their condition, then there’s no reason they can’t have fun in the sun like anybody else.
Below are some top tips to help your patients stay sun-safe this summer.
TOP TIPS PHARMACY STAFF SHOULD PROVIDE
Keep meters and test strips away from the sun
Extremes of temperature can also affect blood glucose meter and
test strips. Fore those who use these, advise them to keep theirr meter and test strips as close to normal room temperature as possible and out of direct sunlight, but don’t refrigerate them as cold temperatures can also lead to misleading results.
Store insulin properly
Those who take insulin to treat their diabetes, should keep a close eye on how they store it. If their blood sugar levels are consistently higher than expected, it’s worth considering whether their insulin could have been damaged in the sun. Insulin, in the hot weather especially, is best kept in the fridge or a cool bag (taking care that it does not freeze).
When damaged by heat, clear insulin generally becomes cloudy and cloudy insulin becomes grainy and sticks in the side of the glass. Insulin that has been exposed to bright sunlight sometimes has a brownish colour. Do not use insulin that looks like this.
Stay hydrated
Those who are being active or just relaxing, everyone knows that hot weather will make you sweat. This is your body’s natural way of
cooling down, but you’ll need to replace the fluids.
Drinking water or sugar-free soft drinks will help diabetics to stay hydrated. Advise them to carry drinks with them and make sure they have regular sips. Becoming dehydrated increases the risk of hyperosmolar hyperglycemic syndrome (HHS) or Diabetic ketoacidosis (DKA).
Diabetic Foot Care
Diabetes can cause nerve damage and blood vessel disease in the feet. This may cause skin and tissue breakdown, which can develop into non-healing wounds (ulcers), which are at risk of infection. This may even result in limb amputation. Structural deformity leaves bony prominences exposed to increased external pressure on the skin, leaving it at risk of being damaged.
Identification of patients at risk of diabetic foot disease allows early intervention of preventative measures to be taken, and thus reduces the risk of further complications.
As the number of type 2 diabetes (T2D) cases continues to escalate each year, pharmacists are likely
to encounter patients inquiring about proper diabetic foot care. Foot problems are very common in patients with T2D, accounting for a significant portion of diabetesrelated complications and health care costs. Pharmacists are in a pivotal position to educate patients with a new diagnosis of diabetes about their care.
Pharmacists should remind patients about the importance of routine diabetic foot care to prevent or delay complications, such as diabetic foot ulcers and amputations. Patients with diabetes, especially those with poorly controlled disease, are more susceptible to skin-related complications; therefore, patients should be reminded that many dermatologic conditions can be either prevented or effectively treated if identified early.
Conducting a daily skin inspection and adhering to daily skin care, especially foot care, is imperative for all patients with diabetes. Pharmacists should seize every opportunity to stress the importance of maintaining tight glycaemic control and remind patients how proper and routine foot care is critical to decreasing the incidence of foot ulcers and amputations. It is estimated that nearly 85% of amputations are preventable with education and early intervention.
The primary goal of diabetic foot care is prevention of diabetesrelated complications, such as changes in the skin (dryness and itching) and foot ulcers, which are often attributed to vascular disease, neuropathy, and relative immunosuppression.
Strategies for preventing foot problems include patient education, patient involvement and adherence, maintenance of tight glycaemic control, and daily care and inspections of the skin, feet, and nails.
Several OTC dermatologic products are marketed specifically for foot care in patients with diabetes. Prior to recommending any of these products, pharmacists should encourage patients with certain signs or symptoms to seek immediate medical care to avoid further complications. Examples of diabetic foot care products include antimicrobial lotions, skin moisturisers, and antifungal and callus treatments.
PHARMACYNEWSIRELAND.COM 18 Diabetes
Enjoying the sun is one of the things many people look forward to in the summer. But for those with diabetes, it can be harder to manage blood glucose (sugar) levels in the hot weather.
LET HER SHINE IN THE SUN


P R OT ECTI O N W I T H O U T C O M P R O M I S E
KEEP HER PROTECTED
P20 Kids SPF50+ is water resistant for up to 3 hours, allowing for more time to enjoy the sun and outdoors knowing that your kids have the best durable sun protection.
Facts
yourself at P20.com
WE
Riemann
Sun
Enlighten
Pharmacist Ticket to Travel Health

Pharmacy has utilised the changes in legislation since 2000 to increase the range and supply function of services such as travel health to travellers. With the number of travellers leaving Ireland and trying new destinations there is an increasing need for more travel health provision.
Rates of international travel are increasing annually, with particular growth observed in travel to Southeast Asia and to emerging economies. While all patients traveling across geographic regions are recommended to receive a pre-travel consultation to consider their individual risks, many do not, or receive care and recommendations that are not consistent with current evidencebased guidelines.
As experts in medicines, and given the largely preventive nature of most travel health recommendations, pharmacists are well suited to help address this need. Pharmacists possess a high degree of knowledge and confidence with more commonly observed travel health topics in community practice such as travellers’ diarrhoea.
Pharmacists providing travel advice are also reminded to consider non-infectious risks of illness and injury abroad and to advise those presenting in the pharmacy on strategies to minimise these risks in addition to providing drug and vaccine recommendations.
International Travel
The United Nations World Tourism Organisation has reported a steady rise in international travel. In fact, 2016 marked the seventh consecutive year of above-average international arrivals, reaching 1.2 billion. This is expected to continue to increase at a rate of 3.3% annually through 2030.
Survey research suggests that 22–64% of travellers experience some degree of health impairment while traveling.
Travellers’ diarrhoea is most common, affecting 30–80% of travelers, depending on the destination, with malaria and vaccine-preventable infections significantly less common.
Important information to obtain from those seeking medical travel advice includes the travel destination, reason for travel (such as work, or leisure), duration of travel, itinerary, and any specific health concerns.
Pharmacists may also be instrumental in helping patients locate a travel-medicine clinic.
Common Ailments
Diarrhoea is one of the most common symptoms experienced during travel. Travellers’ diarrhoea is defined as passing 3 or more loose/watery bowel motions in 24 hours. It may be accompanied by any of the following symptoms; fever, tummy cramps, urgent need to pass bowel motion, nausea or vomiting.
Most cases occur in the first week of travel and are mild; i.e. diarrhoea is the only symptom and it does not disrupt normal activities. On average, symptoms last for 3-5 days and most cases resolve without any specific treatment. When travellers’ diarrhoea is associated with additional symptoms and this leads to an interruption of normal activities, it is classed as moderate to severe.
Travellers’ diarrhoea can be caused by many different organisms including bacteria, such as E.coli and Salmonella, parasites such as Giardia, and viruses such as norovirus. All these organisms are spread through eating/ drinking contaminated food/water or contact between the mouth and contaminated hands, cups, plates etc.
Loose bowel movements can also result from a change in diet including, for example, spicy or oily foods.
The priority in treatment is preventing dehydration, especially in young children.
• Clear fluids such as diluted fruit juices or oral rehydration solutions (purchased as packeted oral rehydration salts) should be drunk liberally.
• All rehydrating drinks must be prepared with safe water.
Antidiarrhoeal Agents can help, particularly with associated colicky pains.
If > 6 diarrhoea stools are passed in 24 hours and cause incapacitation, or there is blood or mucous in the stool, or marked vomiting, fever, pain, bleeding, medical attention must be sought. Intravenous fluids may be needed to prevent dehydration.
Malaria is a serious disease that can be life-threatening. It can affect anyone who travels to a region where infected mosquitoes are found.
The burden of malaria is felt most strongly in sub-Saharan Africa, where numerous initiatives are aiming to improve disease prevention or treatment. Despite reductions in the number of people affected by malaria each year, progress is threatened by the rapid spread of resistance to insecticides and antimalarial drugs
But malaria shouldn’t ruin anyone’s trip abroad. Mosquito bite prevention is an important way to reduce the risk of getting malaria; however, for many destinations, travellers are also advised to take malaria chemoprophylaxis.
The initial symptoms of malaria resemble flu symptoms, such as vomiting, fever, headache and shivers/chills. Other symptoms, such as diarrhoea, shivering, general malaise, and body aches can also occur. In some cases, flu-like symptoms can be mild or diarrhoea alone may be present – this can make it difficult to diagnose the disease correctly.
Malaria can develop within seven days of being bitten by an infected mosquito. However, P. falciparum malaria can take up to three months to develop, while other types of malaria can lie dormant for up to a year. A patient with malaria may seek overthe-counter cold or flu remedies. If this occurs within one year (but especially in the first three months) of their return from a malaria-endemic country, then malaria should be considered.
The risk of serious malaria will also be increased for some individuals, for example those who are very young, elderly, pregnant, or who have had a splenectomy. Each travel consultation by a pharmacist is unique and requires individualised advice.
Effective bite prevention is the first line of defence against malaria. Stopping bites before they occur also reduces the traveller’s risk of contracting other diseases transmitted via mosquitoes.
Travel thrombosis is now a recognised condition. It can affect all travellers, whether it be by air, sea or road, and seems to be
20 | PHARMACYNEWSIRELAND.COM
Around two million Irish people will go on a sun holiday this year, according to the Irish Travel Agents Association.
REPLENISH with
3 Electrolytes in 1 Hydration Solution
For Adults & Children*
Ideal for
Travel Sport Hot Weather
Available in pharmacies.


*Children aged 2+ years.
Food supplement, not to be used as a substitute for a balanced diet. Always read the label. Clonmel Healthcare Ltd.
Date prepared: March 2023. 2023/ADV/ELE/027H
related to long periods of immobility. There is little evidence to suggest that flying is any more dangerous than taking a long sea voyage or traveling by train, bus or car. A pharmacist can assess the risk of travel thrombosis. These risks clearly relate to increasing age of patients, a past history of deep vein thrombosis of whatever cause, a co-existing medical illness, recent surgery, recent accident and, in particular, immobilisation in a plaster cast.
Any traveller with any of these conditions is clearly at increased risk. Pharmacists can identify those patients who are at no additional risk, those with some risk factors and those with considerable risk factors. The pharmacist is then in a position to provide advice.
All passengers should be aware that sitting down for long periods of time results in the stagnation of blood flow through the legs, which may precipitate thrombus formation. Clots usually start in the small veins in the leg and progress into the larger veins. The process might start during travel and can then continue, often during the holiday period, with further extension occurring during the return journey. For this reason, travellers are more likely to experience problems on the return journey.
Before traveling passengers should be encouraged to exercise (walking). After sitting for long periods further exercise can be taken, which may involve walking or carrying out active exercises while seated. The avoidance of drinking too much alcohol combined with drinking plenty of water is advisable.
Offering Travel Advice
Ann-Marie Horan is a pharmacist at Fortfield Pharmacy, Dublin and a member of the IPU’s Executive committee. Last year, the IPU published a checklist for use by all community pharmacies in helping to advise those travelling.
She says “The days before departing for a holiday can be very hectic. That is why we have published this handy checklist so nothing important gets left behind.”
In terms of offering advice to customers, Ann-Marie suggests the following.
“The most important thing to remember is essential medicines. Individuals should bring at least enough for the duration of their trip, and always bring a copy of their prescription in case they need something while away. Be aware of the storage conditions of their medications, some will need to be refrigerated. Offer tips on how best to transport and store these medications.
“Depending on where a person is travelling to, and their own language skills, they may find it challenging to communicate with a pharmacy abroad. That is why we would always recommend bringing a supply of common medicines including antihistamines, pain relievers and treatments for upset stomachs.”
Of course it is not all about medicines, you should also ensure your store is properly category managed with the full range of sun creams and insect repellents, as well as travel sized toiletries.
Tips to provide:
• Medication: Make sure to bring an adequate supply of prescription medication on holidays. Always carry some medication in hand luggage in case checked-in luggage is delayed or goes missing.
• Sun: In warmer climates, stay out of the sun between 11am and 3pm. Protect with a high sun protection factor (SPF) with UVA protection, along with a hat, sunglasses and t-shirt. Apply a complete sun block to children and reapply frequently, especially after swimming.
• Sunburn: Those with sunburn should stay out of the sun for a few days until the sunburn dies down. Drink plenty of water. Advise on appropriate creams and painkillers to relieve symptoms and bring down a temperature. Severe cases of sunburn may require special burn cream and burn dressings.
• Mixing Alcohol and the Sun: Be careful when drinking alcohol in hot weather as it can cause dehydration. Those who have taken alcohol should avoid swimming or engaging in sporting activities.
• Insect bites: Bring insect repellent to protect from insect bites. Those who get bitten should be advised to wash the area with soap and water, and apply a cool compress or calamine lotion to help cool down the affected area. Finally, apply some antiseptic cream to avoid infection.
Business Opportunities
Alongside giving the appropriate advice, pharmacies can also capitalise on the business opportunity that the travel health category offers. Staff can help customers assemble a travel health kit that is appropriate for the area they will be visiting.
If a customer comes into the pharmacy asking for insect repellent, this gives
pharmacists, and pharmacy staff, the opportunity to ask where they are travelling to and then you can discuss what other items they may need to purchase, such as anti-diarrhoea or oral rehydration treatments.
Depending on their destination, the following items can be useful in a travel health kit:
• Sunscreen
• After-sun lotion
• Oral rehydration sachets
• Anti-diarrhoea tablets
• Laxatives
• Lip balms (with SPF)
• Tissues/wet wipes/alcohol-based hand sanitiser
• Contraception
• Antihistamines
• Motion sickness tablets
• Painkillers such as paracetamol or ibuprofen
• Insect repellent and bite cream
• Remedies for indigestion or overindulgence.
Pharmacists should also suggest that customers take a first aid kit, whether it is pre-prepared or comprises individual items. Again, the recommended contents will depend on the person’s destination.
For example, a basic kit containing antiseptic cream, plasters and antiseptic wipes will often suffice when travelling on a European holiday or to developed countries, but if someone is going on a more adventurous trip, such as trekking through the Amazon, they will need a more comprehensive kit containing items such as sterilised syringes, sutures and clean needles.

22 | PHARMACYNEWSIRELAND.COM
soft capsules



















Paralief 500 mg soft capsules, each capsule contains 500 mg of paracetamol. Pharmaceutical Form: Capsules, soft. Indications: For the short-term treatment of headache, toothache, muscle ache, lumbago, fever and pain with flu and colds. Dosage and administration: Adults and adolescents older than 15 years (> 55 kg body weight): 1 or 2 capsules (500-1,000 mg) at a time, with a maximum of 6 capsules (3,000 mg) within 24 hours. Adults and adolescents older than 15 years (≤ 55 kg body weight): The effective daily dose should not exceed 60 mg/kg/day (up to 2 g/day). Dose adjustment may be required in patients with renal or hepatic impairment. Refer to the SmPC for full dosage guidance. Method of administration: Oral. Swallow capsule with sufficient water. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and precautions: Long-term or frequent use is not advised. In young people who are treated with 60 mg/kg/day of paracetamol, combination with another antipyretic is not allowed except where there is a lack of efficacy. After the long-term use (> 3 months) of analgesics with intake every other day or more frequently, headache can occur or become worse. In these cases, the use of analgesics must be stopped in consultation with a doctor. The patient should be advised to avoid the simultaneous use of this medicinal product with others containing paracetamol, such as flu or cold medicinal products. If another medicine containing paracetamol is administered, the maximum paracetamol dose of 3,000 mg per day should not be exceeded, taking into account the content of all the medicines used by the patient. Patients diagnosed with impaired liver or kidney function, should seek medical advice before taking this medicinal product. The taking of several daily doses at once can cause severe damage to the liver. Immediate medical help must be sought even if the patient feels well because of the risk of irreversible damage to the liver. Caution is required when administering paracetamol to patients with moderate to severe renal insufficiency, mild to moderate hepatic insufficiency (incl. Gilbert’s syndrome), severe hepatic insufficiency (Child-Pugh > 9), acute hepatitis, the concomitant administration of medicinal products which have an influence on hepatic function, glucose-6-phosphate dehydrogenase deficiency, haemolytic anaemia, alcohol abuse, dehydration and chronic malnutrition. The risk of an overdose is greater in patients with non-cirrhotic alcoholic liver conditions. Caution is required in the case of chronic alcoholism. The daily dose may not exceed 2 grams in this case. No alcohol may be used during treatment with paracetamol. In the case of a high fever, symptoms of secondary infection or the persistence of symptoms, it will be necessary to reconsider the treatment. Caution is required in the case of asthmatic patients who are sensitive to acetylsalicylic acid as mild bronchospasms have been reported as a cross-reaction after the use of paracetamol. Cases of hepatic impairment or liver failure have been reported in patients with glutathione depletion, such as patients with severe malnutrition, anorexia or a low body mass index, or patients who chronically consume too much alcohol. In patients with a condition of glutathione depletion such as sepsis, the use of paracetamol may increase the risk of metabolic acidosis. Caution is advised if paracetamol is administered concomitantly with flucloxacillin due to increased risk of high anion gap metabolic acidosis (HAGMA). Close monitoring, including measurement of urinary 5-oxoproline, is recommended. Paracetamol can have an influence on the urine test with wolfram phosphoric acid as well as the blood sugar test with glucose oxidase peroxidase. Patients with hereditary fructose intolerance (HFI) should not take/be given this medicinal product. Interactions: Paracetamol is metabolised in the liver and can consequently enter into interactions with other medicinal products which follow the same metabolic route or which are able to inhibit or induce the route. In the case of chronic alcohol abuse and the use of drugs, which induce hepatic enzymes such as barbiturates and tricyclic antidepressants, an overdose of paracetamol can take a more severe course as a result of the increased and more rapid formation of toxic metabolites. Caution is required with the concomitant intake of enzymeinducing drugs. In the case of concomitant treatment with probenecid, the dose of paracetamol must be reduced. Paracetamol can cause an increase in the half-life of chloramphenicol. The rate of absorption of paracetamol can be increased by metoclopramide or domperidone and absorption can be reduced by colestyramine. The anticoagulatory effect of warfarin and other coumarins can increase during the long-term, regular use of paracetamol with an increase in the risk of bleeding as a result. With the concomitant chronic use of paracetamol and zidovudine, neutropenia often occurs. Salicylamide can prolong the half-life of paracetamol. Isoniazid ensures a reduction in the clearance of paracetamol, which possibly increases the activity and/or toxicity of paracetamol by preventing metabolism in the liver. The concomitant intake of paracetamol with lamotrigine ensures a reduction in the bioavailability of lamotrigine. Caution should be taken when paracetamol is used concomitantly with flucloxacillin. Fertility, pregnancy and lactation: If clinically needed, paracetamol can be used during pregnancy however, it should be used at the lowest effective dose for the shortest possible time and at the lowest possible frequency. Paralief can be used in therapeutic doses by women who breast-feed. There are no available data on the effect of paracetamol on fertility. Undesirable effects: Agranulocytosis (after long-term use), thrombocytopenia, thrombocytopenic purpura, leukopenia, haemolytic anaemia, Allergies (exclusive of angioedema), Depression, confusion, hallucinations,Tremor, headache,Visual abnormalities, Oedema, Bleeding, abdominal pain, diarrhoea, nausea, vomiting,Abnormal hepatic function, hepatic failure, hepatic necrosis, jaundice, Hepatotoxicity, Sterile pyuria. Refer to the SmPC for a full list of undesirable effects. Adverse reactions should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Pack size 20 soft capsules. A copy of the Summary of Product Characteristics is available upon request or go to www.clonmelhealthcare.ie Marketing authorisation holder: Clonmel Healthcare Ltd. Waterford Road, Clonmel, Co. Tipperary Marketing authorisation number: PA126/020/7. Supply through pharmacies only. Date last revised: August 2022.

2023/ADV/PAR/028H Date Prepared: March 2023
Vimovo® 1 tablet, 2

active ingredients...
Sequential release of:
• Esomeprazole (in the stomach) for gastroprotection; followed by


• Naproxen (in the small intestine) for relief of inflammation and pain associated with osteoarthritis.1-5


Scan QR code to view VIMOVO MODE OF ACTION VIDEO
1 tablet twice daily at least 30 minutes prior to food intake.2
Swallow whole with water. Do not split, chew or crush.2
Esomeprazole 20mg Enteric coating Naproxen 500mg
Vimovo® is indicated in adults for the symptomatic treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, in patients who are at risk for developing non-steroidal anti-inflammatory drug (NSAID)-associated gastric and/or duodenal ulcers and where treatment with lower doses of naproxen or of other NSAIDs is not considered sufficient.

RECEIVE INFORMATION
FROM GRÜNENTHAL
2 RECEIVE A CALL FROM A MEDICAL REPRESENTATIVE
Reference: 1. Goldstein JL, Hochberg MC, Fort JG et al. Clinical trial: the incidence of NSAID-associated endoscopic gastric ulcers in patients treated with PN 400 (naproxen plus esomeprazole magnesium) vs. enteric-coated naproxen alone. Aliment Pharmacol Ther. 2010; 32(3):401-413. 2. Vimovo Summary of Product Characteristics. 3. Wang-Smith L, Fort J, Zhang Y et al. Pharmacokinetics and relative bioavailability of a fixed-dose combination of enteric-coated naproxen and non-enteric-coated esomeprazole magnesium. J Clin Pharmacol. 2012 May; 52(5):670-80. 4. Hochberg MC, Fort JG, Svensson O et al. Fixeddose combination of enteric-coated naproxen and immediate-release esomeprazole has comparable efficacy to celecoxib for knee osteoarthritis: two randomized trials. Curr Med Res Opin. 2011; 27(6):1243-1253. 5. Moore RA, Derry S, Simon LS et al. Nonsteroidal antiinflammatory drugs, gastroprotection, and benefit-risk. Pain Pract. 2014; 14(4):378-39. VIMOVO 500mg/20mg modified-release tablets (naproxen, esomeprazole magnesium trihydrate) Prescribing Information. Refer to the Summary of Product Characteristics (SmPC) before prescribing. Presentation: Modified-release tablet (oval, biconvex, yellow, marked 500/20 in black ink) containing enteric-coated naproxen (500 mg) and film-coated esomeprazole (20 mg). Indication: In adults for the symptomatic treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, in patients who are at risk for developing nonsteroidal anti-inflammatory drug (NSAID)-associated gastric and/or duodenal ulcers and where treatment with lower doses of naproxen or of other NSAIDs is not considered sufficient. Dosage and method of administration: Adults: Oral use. Swallowed whole (not split, chewed or crushed) with water. Recommended to take at least 30 minutes prior to food intake. One tablet twice daily. Use lowest effective dose for shortest duration possible. Patients not treated with NSAID previously, or when 1000mg/day of naproxen is not considered appropriate, alternative therapeutic treatment with lower strength of naproxen or of other NSAIDS as nonfixed combination should be utilised. Review at regular intervals and discontinue if no benefit or if worsening. Not intended for relief of acute pain conditions. Flares of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis may be treated. Renal / Hepatic impairment: Use cautiously in mild to moderate renal/hepatic impairment and monitor closely. Consider reduction in total daily naproxen dose. Elderly (>65 years): older people are at risk of serious consequences of adverse reactions. Paediatric population: Safety and efficacy not established in 0 to 18 years. Contra-indications: Hypersensitivity to ingredients, history of asthma, urticaria or allergic-type reactions induced by administration of acetylsalicylic acid or other NSAIDs, 20th week of pregnancy onward, no breast-feeding if you are taking VIMOVO, severe heart failure, severe renal impairment, severe hepatic impairment (Child-Pugh C), active peptic ulceration, gastrointestinal (GI) bleeding, cerebrovascular bleeding or other bleeding disorders, must not be used concomitantly with atazanavir and nelfinavir. Special Warnings and precautions: Avoid concomitant NSAIDs use. Can be used with low dose acetylsalicylic acid. Undesirable effects may be minimized by using lowest effective dose for the shortest duration necessary. Assess at meaningful intervals to prevent over-treatment. Risk-factors to develop NSAID related GI complications include high age, concomitant use of anticoagulants, corticosteroids, other NSAIDs including low-dose acetylsalicylic acid, debilitating cardiovascular disease, Helicobacter pylori infection, and a history of gastric and/or duodenal ulcers and upper GI bleeding. In Inducible porphyries and systemic lupus erythematosus and mixed connective tissue disease use only after rigorous benefit-risk ratio. Contains very low levels of methyl and propyl parahydroxybenzoate. Patients on long-term treatment should be kept under regular surveillance. Elderly: Naproxen: Older people have an increased frequency of adverse reactions especially GI bleeding, and perforation, which may be fatal. Gastrointestinal effects: Naproxen: GI bleeding, ulceration or perforation, which can be fatal with or without warning symptoms or a previous history of serious GI events. Risk is higher with increasing NSAID doses, patients with history of ulcers and in older people. Start with low dose and consider combination therapy with protective agents. Caution in patients receiving NSAIDs with concomitant medications which could increase risk of ulceration or bleeding. Withdraw treatment if GI bleeding or ulceration. Caution in patients with a history of GI disease. Esomeprazole: In the presence of any alarm symptom and when gastric ulcer is suspected, malignancy should be excluded. Dyspepsia could still occur. May lead to increased risk of GI infections. May reduce absorption of vitamin B12 due to hypo- or achlorhydria. Cardiovascular and cerebrovascular effects: Naproxen: Appropriate monitoring and advice for patients with history of hypertension and/or mild to moderate congestive heart failure. The use of coxibs and some NSAIDs may be associated with a small increased risk of arterial thrombotic events. Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease, and/or cerebrovascular disease should only be treated with naproxen after careful consideration. Consideration should be made before initiating longer-term treatment of patients with risk factors for cardiovascular events. Renal effects: Naproxen: Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. Patients at greatest risk of this are those with impaired renal function, hypovolemia, heart failure, liver dysfunction, salt depletion, those taking diuretics, angiotensin converting enzyme (ACE) inhibitors, or angiotensin II receptor antagonists and older people. Use with great caution in patients with impaired renal function and monitoring of serum creatinine and/or creatinine clearance is advised. Contraindicated in patients with baseline creatinine clearance less than 30 ml/min. Assess renal function before and during therapy in patients with compromised renal blood flow. Hepatic effects: Borderline elevations of liver tests may occur, due to hypersensitivity rather than toxicity. Rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some with fatal outcomes have been reported. Hepatorenal syndrome: Use of NASAIDs may be associated with acute renal failure in patients with hepato-cirrhosis. These patients frequently also have concomitant coagulopathy related to inadequate synthesis of clotting factors. Antiplatelet effects associated with naproxen could further increase risk of severe bleeding. Haematological effects: Naproxen: Careful observation when administered to patients who have coagulation disorders or are receiving drug therapy that interferes with haemostasis. Increased risk of bleeding if given to patients with high risk of bleeding and those on full anti-coagulation therapy. Naproxen decreases platelet aggregation and prolongs bleeding time. If active and clinically significant bleeding from any source occurs, withdraw treatment. Eye effects: Naproxen: recommend ophthalmic examination if any change or disturbance in vision occurs. Dermatological effects: Naproxen: Discontinue at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity. Esomeprazole: associated with very infrequent cases of subacute cutaneous lupus erythematosus (SCLE). If lesions occurs and if accompanied by arthralgia, seek medical help promptly and consider stopping treatment. SCLE after previous treatment with a PPI may increase the risk of SCLE with other PPIs. Anaphylactic (anaphylactoid) reactions: Naproxen: anaphylactic (anaphylactoid) reactions may occur in patients with and without a history of hypersensitivity or exposure to acetylsalicylic acid, other NSAIDs or naproxen-containing products. They may also occur in individuals with a history of angio-oedema, bronchospastic reactivity, rhinitis and nasal polyps. Pre-existing asthma: Naproxen: Use with caution and should not be administered to patients with aspirin-sensitive asthma. Inflammation:Naproxen: may reduce fever and other signs of inflammation thereby diminishing their utility as diagnostic signs. Female fertility: not recommended in women attempting to conceive. Combination with other medicinal products: Must not be used with atazanavir. Esomeprazole is a CYP2C19 inhibitor. When starting or ending treatment, the potential for interactions with drugs metabolised through CYP2C19 should be considered. Concomitant use with clopidogrel should be discouraged. Hypomagnesaemia: Severe hypomagnesaemia has been reported in patients treated with Proton Pump Inhibitors (PPIs). Consider measuring magnesium levels before starting PPI treatment and periodically during treatment in patients expected to be on prolonged treatment or those on concomitant digoxin or drugs that may cause hypomagnesaemia. Bone fracture: PPIs, if used in high doses and over long durations (>1 year), may increase the risk of hip, wrist and spine fracture, predominantly in older people or in presence of other recognised risk factors. Patients at risk of osteoporosis should receive care according to current clinical guidelines and have an adequate intake of vitamin D and calcium. Interference with laboratory tests: Increased Chromogranin A (CgA) level may interfere with investigations for neuroendocrine tumours. To avoid this interference, treatment should be stopped for at least 5 days before CgA measurements. Fertility: VIMOVO may make it more difficult to become pregnant. Interactions: Antiretroviralagents: Concomitant use of atazanavir and nelfinavir with esomeprazole is not recommended and concomitant administration with Vimovo is contraindicated. Concomitant use with precaution: Other analgesics including cyclooxygenase-2 selective inhibitors: Concomitant use with other NSAIDs except for lowdose acetylsalicylic acid (≤ 325 mg/day), is not recommended. Acetylsalicylic acid: Can be administered with low dose acetylsalicylic acid (≤ 325 mg/day). In clinical trials, Vimovo in combination with low-dose acetylsalicylic acid did not have increased occurrence of gastric ulcers. However, concurrent use may still increase risk of serious adverse events. Clinical pharmacodynamic data suggests concomitant naproxen usage for more than one day


consecutively may inhibit the effect of low-dose acetylsalicylic acid on platelet activity and this inhibition may persist for up to several days after stopping naproxen. Clinical relevance of this interaction is not known. Tacrolimus: A reinforced monitoring of tacrolimus concentrations as well as renal function should be performed and dose of tacrolimus adjusted if needed. Ciclosporin: caution when coadministered because of increased risk of nephrotoxicity. Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazides in some patients. Observe patient closely for signs of renal failure as well as to assure diuretic efficacy. SSRIs: Concomitant use of NSAIDs, including COX-2 selective inhibitors, and SSRIs increases the risk of GI bleeding. Corticosteroids: Increased risk of GI bleeding when combined with NSADIs including COX-2 selective inhibitors. Caution when NSAIDS are concomitantly administered with corticosteroids. ACE-inhibitors/Angiotensin II receptor antagonists: caution when combined with NSAIDs in patients who are older, volume-depleted, or with renal impaired renal function as NSAID may diminish antihypertensive effect and increase risk of renal impairment associated with ACE-inhibitor or angiotensin II receptor antagonists. Digoxin: NSAIDs may increase plasma cardiac glycoside levels when co-administered with cardiac glycosides such as digoxin. Lithium: NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. Observe carefully for lithium toxicity. Methotrexate: esomeprazole and naproxen could enhance the toxicity of methotrexate. Caution when administered concomitantly with methotrexate. In high-dose methotrexate administration a temporary withdrawal of Vimovo is recommended. Sulphonylureas, Hydantoins: Naproxen is highly bound to plasma albumin; thus a theoretical potential for interaction with other albumin-bound drugs (sulphonylureas, hydantoins). When coadministered patients should be observed for adjustment of dose. Clopidogrel: Concomitant use should be discouraged due to inconsistent data on clinical implications of a PK/PD interaction of esomeprazole in terms of major cardiovascular events reported from observational and clinical studies. Anti-coagulants and thrombocyte aggregation inhibitors: NSAIDs may enhance the effects of oral anti-coagulants, heparins and thrombocyte aggregation inhibitors. Close monitoring when initiating and ending treatment with warfarin or other coumarine derivatives. Beta receptor-blockers: Naproxen and other NSAIDs can reduce the antihypertensive effect of propranolol and other beta-blockers. Probenecid: Probenecid given concurrently increases naproxen anion plasma levels and extends its plasma half-life significantly. Drugs with gastric pH-dependent absorption: gastric acid suppression during treatment with esomeprazole, and other PPIs might decrease or increase the absorption of drugs with a gastric pH dependent absorption. The absorption of drugs such as ketoconazole, itraconazole, posaconazole and erlotinib can decrease while the absorption of drugs such as digoxin can increase. Concomitant use with posaconazole and erlotinib should be avoided. Other Information Concerning Drug Interactions: Studies evaluating concomitant administration of esomeprazole and either naproxen or rofecoxib did not identify any clinically relevant interaction. Concomitant administration of cholestyramine can delay absorption of naproxen. In healthy volunteers, concomitant administration esomeprazole resulted in an increase in area under the plasma concentration-time curve and prolonged elimination halflife but no significant increase in peak plasma levels of cisapride. Esomeprazole has been shown to have no clinically relevant effects on the pharmacokinetics of amoxicillin and quinidine. Esomeprazole inhibits CYP2C19, the major esomeprazole metabolising enzyme. Esomeprazole is also metabolised by CYP3A4. Drugs known to induce CYP2C19 or CYP3A4 or both (such as rifampicin and St. John's Wort) may lead to decreased esomeprazole serum levels. Omeprazole and esomeprazole act as inhibitors of CYP2C19. Animal data indicate that NSAIDs can increase the risk of convulsions associated with quinolone antibiotics. Patients taking quinolones may have an increased risk of developing convulsions. Drug/LaboratoryTest Interaction: Naproxen may decrease platelet aggregation and prolong bleeding time. Naproxen may result in increased urinary values for 17-ketogenic steroids. Suggested therapy with naproxen be temporarily discontinued 72 hours before adrenal function tests are performed if the Porter-Silber test is to be used. Naproxen may interfere with some urinary assays of 5-hydroxy indoleacetic acid (5HIAA). Pregnancy and lactation: You should not use VIMOVO during the first 6 months of pregnancy unless absolutely necessary and advised by your doctor. From the 20th week of pregnancy onward, VIMOVO use may cause oligohydramnios resulting from foetal renal dysfunction. This may occur shortly after treatment initiation and is usually reversible upon discontinuation. Antenatal monitoring for oligohydramnios and ductus arteriosus constriction should be considered after exposure to VIMOVO for several days from gestational week 20 onward. Do not breast-feed if you are taking VIMOVO. Refer to full SmPC for further information. Undesirable effects: Reported from Vimovo clinical trials: Very common (≥1/10): dyspepsia. Common (≥1/100to <1/10): dizziness, headache, taste disturbance, hypertension, abdominal pain, constipation, diarrhea, esophagitis, flatulence, gastric/duodenal ulcers, gastritis, nausea, vomiting, skin rashes, arthralgia, oedema. Otherimportantundesirable effects: paraesthesia, syncope, arrhythmia, GI bleeding, stomatitis, urticaria, (Uncommon (≥ 1/1,000 to < 1/100), diverticulitis, hypersensitivity reactions, myocardial infarction, tachycardia, rectal bleeding, renal failure, (Rare (≥ 1/10,000 to < 1/1,000). Naproxen: adverse experiences reported during trial and through postmarketing reports: Common (≥1/100 to <1/10): diverticulitis, depression, insomnia, dizziness, drowsiness, headache, lightheadedness, vertigo, visual disturbances, tinnitus, hearing disturbances, palpitations, dyspnea, dyspepsia, abdominal pain, nausea, vomiting, diarrhoea, constipation, heartburn, peptic ulcers, stomatitis, pruritus, ecchymoses, purpura, skin rashes, fatigue, oedema, sweating, thirst. Other important undesirable effects: sepsis, agranulocytosis, eosinophilia, granulocytopenia, Pancytopenia, thrombocytopenia, anaphylactic reaction, anaphylactoid reactions, hypersensitivity reactions, coma, convulsions, optic neuritis, paresthesia, syncope, corneal opacity, papilloedema, arrhythmia, myocardial infarction, tachycardia, eosinophilic pneumonitis, pneumonia, respiratory depression, gastric/duodenal ulcers, gastrointestinal bleeding and/or perforation, pancreatitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn's disease), rectal bleeding, cholestasis, hepatitis, jaundice, liver failure, exanthema, urticaria, bullous reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis, erythema multiforme, systemic lupus erythematosus, pseudoporphyria, nephrotic syndrome, renal failure, renal papillary necrosis, tubular necrosis, Uncommon (≥ 1/1,000 to < 1/100), Rare (≥ 1/10,000 to < 1/1,000). Esomeprazole: adverse drug reactions identified or suspected in clinical trial for enteri-coated esomeprazole and/or post marketing. None were found to be doserelated: Common (≥1/100 to <1/10): headache, abdominal pain, diarrhea, flatulence, nausea/ vomiting, constipation, fundic gland polyps (benign). Other important undesirable effects: paraesthesia, pruritus, urticaria, fracture of the hip, wrist or spine (Uncommon (≥ 1/1,000 to < 1/100). leukopenia, thrombocytopenia, hypersensitivity reactions e.g. fever, angioedema and anaphylactic reaction/shock, depression, paraesthesia, syncope, arrhythmia, gastrointestinal bleeding, stomatitis, pruritus, urticaria, (Rare (≥ 1/10,000 to < 1/1,000)). agranulocytosis, pancytopenia, hepatic failure, hepatic encephalopathy in patients with pre-existing liver disease, erythema multiforme, Interstitial nephritis, Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) (Very rare (< 1/10,000)). Overdose: Effects of overdose expected to reflect the effects of naproxen overdose. Significant naproxen overdosage may be characterized by lethargy, dizziness, drowsiness, epigastric pain, abdominal discomfort, heartburn, indigestion, nausea, transient alterations in liver function, hypoprothrombinemia, renal dysfunction, metabolic acidosis, apnea, disorientation or vomiting. GI bleeding can occur. Hypertension, acute renal failure, respiratory depression, and coma may occur, but rare. Anaphylactoid reactions have been reported. A few patients have experienced convulsions. It is not known what dose of the drug would be life-threatening. The symptoms in connection with esomeprazole overdose are transient. Patients should be managed by symptomatic and supportive care following a NSAID overdose, particularly with respect to GI effects and renal damage. Haemodialysis does not decrease the plasma concentration of naproxen because of the high degree of protein binding. Emesis and/or activated charcoal and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose. Forced diuresis, alkalinization of urine or hemoperfusion may not be useful. Esomeprazole is not readily dialyzable. There are no specific antidotes for naproxen or esomeprazole. Prescribers should consult the SmPC in relation to all adverse reactions. Legal classification: POM, CD (Part VIIIA Category C). Marketing Authorisation numbers, pack sizes and basic NHS cost: PA 2242/014/001, 60 packs. Marketing Authorisation Holder: Grünenthal Pharma Ltd 4045 Kingswood Road Citywest Business Park Citywest Co. Dublin Ireland. Date of preparation: April 2023. Job bag: M-VMO-IE-04-23-0006

Adverse events should be reported. Reporting forms and information can be found at www.medicines.ie/adverse-event-reporting. Adverse events should also be reported to Grünenthal Ltd. Free phone: +44 (0)870 351 8960.
M-VMO-IE-04-23-0005 – April 2023
Prenatal Breastfeeding Education
The effectiveness of prenatal breastfeeding education on breastfeeding uptake postpartum: A systematic review
Written by Jennifer Kehindea, Claire O’Donnella and AnnMarie Grealishb aDepartment of Nursing and Midwifery, University of Limerick, Limerick V94 T9PX, Ireland bFlorence Nightingale Faculty of Nursing, Midwifery & Palliative Care, King’s College



London, United Kingdom
an increase in maternal breastfeeding selfefficacy among mothers who participated in breastfeeding educational programs during prenatal care.

Background
The decision to breastfeed is influenced by physiological, psychological, and emotional factors. However, the importance of equipping mothers with the necessary knowledge for successful breastfeeding practice cannot be ruled out. Studies suggest that the decline in global breastfeeding rate can be linked to lack of adequate breastfeeding education during prenatal stage. Therefore, this review aims to determine the effectiveness of prenatal breastfeeding education on breastfeeding uptake postpartum.
A systematic review of the studies identified by electronic database search (Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medline, Psych INFO, and Sociological Abstracts and Applied Social Sciences Index and Abstracts (ASSIA) published between 2014 - 2021.
A total of 14 studies met the inclusion criteria. Results showed an increase in breastfeeding uptake, breastfeeding knowledge, increase in positive attitude to breastfeeding and
Breastfeeding is the best practice for child health, development, and nutrition (Chong, 2015). According to the world health organization (WHO) and UNICEF’s breastfeeding advocacy initiative, breastfeeding should be initiated within an hour of birth and continued for six months, without any supplements, liquids, or foods except liquid medications (WHO and UNICEF, 2015). Breastfeeding avails the newborn of the best start in life as it facilitates growth and development, deepens the bond between mother and newborn, and enhances the wellbeing of the newborn (Peñacoba and Catala, 2019). Colostrum produced immediately after childbirth contains nutrients and leucocytes strengthening the immunity of the newborn (Brecchia, 2016).
Breast milk contains all food nutrients in correct proportion (Martin et al., 2016). In addition to these nutrients, it contains immune cells, stem cells, omega-3 fatty acids as well as
26 | PHARMACYNEWSIRELAND.COM
Jennifer Kehinde
Claire O’Donnell AnnMarie Grealish
alpha-linolenic acid which cannot be found in baby formulas or artificial milk (Boquien, 2018; Belfort et al., 2016; Gidrewicz and Fenton, 2014). Alpha-linolenic acid has been found to have beneficial effects on the infant’s neurological and retinal development (Birch et al., 1993; Khor et al., 2020; Innis, 2014).
Breastfeeding is an important protective factor against childhood obesity as well as reduces the incidence of chronic diseases in advanced stages of adulthood (Yan et al., 2014; Giugliani et al., 2015). Early breastfeeding initiation and an extended period of breastfeeding are linked to quicker return of the uterus to its pre-pregnant state (involution), reduced risk of breast cancer as well as cancer of the ovaries (Luan et al., 2013; Chowdhury et al., 2015).
Significant economic impacts of breastfeeding on health service systems includes decrease in extra health care costs for respiratory syncytial virus commonly found in non-breastfed infants (Quesada et al., 2020). Remarkably, breastfeeding has been linked to a decrease in yearly expenses for private and government insurers in treating diseases preventable by breastfeeding (Santacruz-Salas et al., 2018). To maximize the health and economic benefits of breastfeeding, society must support breastfeeding protection and promotion (Shin, 2010; Rollins et al., 2016). Breastfeeding support and promotion unarguably aid in safeguarding human and environmental health by reducing environmental harm associated with carbon emissions generated from baby formular manufacturing and transportation (Dadhich et al., 2021). Therefore, breastfeeding support
and education are key to improving human nutrition and health in the face of global health challenges (Victora et al., 2016a).
Regardless of the continuous increase of awareness on breastfeeding over the last decade, breastfeeding rates remain at minimal levels in most countries of the world (Victora et al., 2016b; Prentice, 2022). To this effect, the World Health Assembly has developed strategies to increase the rate of breastfeeding and indeed exclusive breastfeeding to at least 50% by the year 2025 (McGuire, 2015). Statistics as recorded in the WHO’s global breastfeeding scorecard shows that only 44% of newborn babies are breastfed within one hour after birth as compared to the WHO’s aim of improving breastfeeding initiation rates to 70% globally (WHO, 2019).
Data from the World Health organization shows that exclusive breastfeeding rates for babies below the age of six months is currently at 40% with only 23 countries achieving at least 60% of newborn babies being exclusively breastfed (WHO, 2021). These low rates are mostly prevalent in the Americas and Europe as only 6% of countries in the Americas record an exclusive breastfeeding rate above 60% (WHO, 2021) and 25% in the entire European region (Theurich et al., 2019).
However, despite all the improvements recognized and anticipated with the adoption of breastfeeding as the best form of feeding for newborns, breastfeeding initiation and exclusivity rate is yet to be scaled up to a
substantial level globally (Buturović et al., 2017; Edwards et al., 2021). Global decline in breastfeeding rates have been linked to difficulties experienced by mothers while trying to initiate breastfeeding (Gianni et al., 2019).
The WHO has identified the importance of breastfeeding education, emphasizing this as the core element of health promotion during prenatal visits (Coffman, 2019). Breastfeeding education and support delivered by qualified and skilled healthcare professionals positively influences women’s attitudes toward breastfeeding initiation (Lumbiganon et al., 2016; White, 2020; Tanaka and Horiuchi, 2021; Iliadou et al., 2018; Mulcahy et al., 2011). Therefore, the aim of this review is to determine the effectiveness of prenatal breastfeeding education on breastfeeding uptake postpartum.
Conclusion
The study authors conclude that, “Prenatal breastfeeding education increases women’s knowledge of breastfeeding. Mothers who are knowledgeable about breastfeeding and hold a positive approach towards breastfeeding have the tendency to initiate breastfeeding and continue for a lengthened period. Findings demonstrates a general correlation between prenatal breastfeeding education and increased breastfeeding uptake postpartum. The high level of positive breastfeeding outcome inherent in all the studies can be attributed to prenatal breastfeeding education.”
Menopausal Women told ‘Grin and Bear it’
A doctor at one of Ireland’s leading women’s health clinics claims menopausal women are being told to ‘grin and bear it’ by some GPs.
Dr Talisa Chennells treats women from all over Ireland for symptoms linked to the socalled change of life at the Menopause Hub in Dublin. Her clients, she said, seek help for a vast range of symptoms including fatigue, lack of sleep, anxiety, depression and brain fog. However, many of them had previously been told by a GP not to worry about the menopause as it would “pass in a few years”. Some had even been offered antidepressants. “That is such an old fashioned attitude,” said Dr Chennells, who worked as a GP after graduating in medicine.
“Women struggling with menopausal symptoms do not need to suffer in silence anymore. There are a number of treatment options available to them, including hormone replacement therapy (HRT), nonhormonal medicines, herbal treatments, and psychological counselling.
“If you are a woman in your 40s and you’re concerned that your doctor is not really listening to you, then please get in touch,” she said.
Dr Chennells, who is a member of the British Menopause Society, said some of her former colleagues in general practice remain suspicious of HRT – even though recent research shows it can be safely prescribed to suitable patients.
“A lot of the women I see say their family doctor was reluctant to give them HRT,”
said Dr Chennells, who is originally from Johannesburg, South Africa.
“They’re told their symptoms won’t last, that HRT can cause problems, and that they should just ‘grin and bear it’ until the menopause passes. A number of clients said they had even been offered anti-depressants.
“There are so many misconceptions about HRT and that’s why many doctors don’t prescribe it. But HRT can be a game changer, and even though there is a percentage of women for whom it is not suitable, breast cancer patients, for example, recent studies show the benefits outweigh the risks for most others.”
Dr Chennells, 35, said she moved from her native South Africa to Dublin with her husband and three children to join the multi-disciplinary team at the Menopause Hub – Ireland’s firstever clinic solely devoted to helping menopausal women overcome symptoms– because she is passionate about women’s health.

And she added that some women in Ireland are not getting the help they require when they reach their mid-40s, when the perimenopause begins.
“We are here to help, and we understand that every woman’s menopausal journey is as individual as they are,” Dr Chennells added.
The Menopause Hub has two treatment centres – one in Mount Merrion, south Co Dublin, and one in Santry, north Dublin, where Dr Chennells is based.
Last month, the clinic announced plans to open a third centre in Cork city due to overwhelming demand.
For more details, visit themenopausehub.ie
PHARMACYNEWSIRELAND.COM | 27
Dr Talisa Chennells
Preparing People for Living Longer
Our ageing population: how we can prepare for living longer and healthier lives
Written by Orlaith Gavan, Country
up 30% of the population yet accounted for 56% of total health spending.iv
To help ensure people are aging healthily and that we support the wellbeing of society today and in the future, it is vital to understand and address the impacts of aging. Pfizer continues to look holistically at age-related illnesses, including opportunities to support longevity and healthy aging to improve overall quality of life.
Medical Director, Pfizer Healthcare Ireland

pandemic is the importance of collaboration and our collective ability to move science forward. Forging partnerships, like we did with BioNTech years ago was instrumental in us being able to immediately start working on a vaccine in early 2020.
healthcare, disease prevention and health promotion to maintain or improve quality of life during the life course.
We’re actively collaborating whilst looking for new opportunities that may help us advance the science in healthy ageing.
The average life expectancy is now 73 years – representing an increase of nearly six years since 2000 –and all over the globe people are living longer.i While this extended life expectancy is generally positive, the natural consequences of aging include an increased risk of developing chronic, debilitating health conditions that can drastically impact quality of life.
Diseases most often associated with aging, including cancer, diabetes, kidney and heart disease, have now surpassed infectious diseases as the leading causes of death, in addition to accounting for the majority of healthcare costs in the developed world. These diseases may also accelerate the aging process, which can contribute to further decline in functionality and reduced quality of life.
Through our legacy, leadership and purpose to deliver breakthroughs that change patients’ lives, Pfizer is committed to addressing agerelated illnesses by supporting healthy aging and enabling longer, healthier lives.
Age-related illnesses not only reduce quality of life but disproportionately impact medical spending, underscoring an oftenoverlooked global health challenge that puts a strain on individuals and the broader healthcare community.
Over the past 150 years, life expectancy has increased dramatically, but for some, not all the years gained are healthy.ii For example, it is estimated that up to 20% of people’s lives are spent in late-life morbidity, suffering from at least one chronic illness.iii
Aging populations account for a disproportionate amount of their respective country’s medical expenses.
As an example in 2019, U.S. residents over the age of 55 made
As scientific innovations continue to make our world healthier, it is vital that we also consider how to extend and maintain that health even as people age. Over the next decade, we expect the landscape to shift dramatically and place more emphasis on healthy aging, including more extensive research related to longevity.v
Partnerships are key to continued exploration and achieving breakthroughs. One thing we have learned from the
In 2018, our discussions with BioNTech were exploratory –we discussed the possibility of mRNA technology for developing vaccines for cancer based on personalised medicine principles. Thanks to this early collaboration we saved significant time in contracting – what could have easily taken months took days. When it comes to ageing healthy, the promotion of health literacy is hugely important. Health literacy is linked to literacy and entails people’s knowledge, motivation and competencies to access, understand, appraise, and apply health information in order to make judgments and take decisions in everyday life concerning
Pfizer Healthcare Ireland have just announced the launch of a Competitive Grant Program, which aims to promote and benefit health literacy in Ireland. Through this Independent Medical Education Request for Proposals, our objective is to support projects that improve health literacy through awareness and education. Health literacy is hugely important in ensuring that people can understand, appraise, and apply health information in order to make informed health-related decisions.
To find out more details on the application process and to apply please click on IGLC RFP Template Revised 08-30-2017 (pfizer.com):

PHARMACYNEWSIRELAND.COM 28 Advertorial PP-UNP-IRL-0493
National Skin Cancer Prevention Plan
Minister for Public Health, Wellbeing and the National Drugs Strategy, Hildegarde Naughton has launched the National Skin Cancer Prevention Plan 2023-2026, in the Department of Health.
Skin cancer* is the most common cancer in Ireland. According to the National Cancer Registry, annual average incidence during 20182020 was 12,668 cases per year. This figure has more than doubled since 1994 and is projected to more than double again by 2045, to 33,204 cases.
However, the majority of skin cancers could be prevented; ultraviolet radiation (UV) exposure, emitted naturally in sunlight or from artificial sources for example sunbeds, is the main risk factor.
Prevention efforts
The National Skin Cancer Prevention Plan 2023-2026 was developed by the National Cancer Control Programme (NCCP) in partnership with Healthy Ireland, Department of Health and was completed in consultation with
healthcare professionals, cancer charities and national organisations representing priority groups.
The new plan 2023-2026 aims to build on the work of the first National Skin Cancer Prevention Plan 2019-2022, to increase awareness and improve adoption of skin cancer prevention behaviours, to tackle Ireland’s high rates of skin cancer.
Launching the new plan
Minister Naughton said, “Skin cancer is the most commonly diagnosed cancer in Ireland, but it is also largely preventable and we can all significantly reduce our risk by adopting practical skin protection behaviours.”
Dr Triona McCarthy, Consultant in Public Health Medicine, National Cancer Control Programme,
added, “It is so important for physical and mental health to enjoy time outdoors but we should do so while also protecting skin from UV radiation to reduce the risk of our most common cancer. The best ways to protect skin are to cover up with long sleeves, a sunhat, sunglasses and use sunscreen; limit time in the sun when UV radiation is strongest, typically between the hours of 11am and 3pm, from April to September in Ireland; and never use a sunbed.”
Maintaining momentum
The rising incidence of skin cancer is an important public health issue. The Irish Skin Foundation (ISF) welcomes the publication of the new National Skin Cancer Prevention Plan 2023-2026.
David McMahon, CEO, ISF comments, “It’s been very
Skinside Out Event in Dublin
encouraging to see Government, Department of Health, NCCP and Healthy Ireland support for skin cancer prevention, come together with charities and NGOs working in the area in recent years.
“This new national prevention plan is how the ISF see’s public health policy deliver on the ground and for the future health of everyone living in Ireland.
“I see the previous plan, this latest plan, and hopefully continued commitment through other initiatives, as crucial to changing attitudes and behaviours. Skin cancers are largely preventable, public health agencies have a key role to play and this plan is evidence that there is real commitment to taking on that challenge.”
Just last month, the Irish Skin Foundation hosted SkinSideOut, their skin health information and exhibition event in O’Reilly Hall, UCD, Dublin.


SkinSideOut is an information and exhibition event for people affected by a wide range of chronic inflammatory skin conditions in Ireland.
This year’s event included panel discussions on Eczema and Psoriasis moderated by Virgin Media News anchor Claire Brock; talks on Acne, Rosacea and Skin

Cancer Prevention; and “How to” sessions delivered by Dermatology ANPs on Emollient Therapy, HS Wound Care and Scalp Psoriasis. Attendees had the opportunity to meet with skin care brands with exhibition stands on the day and discuss product ranges for different skin problems.
Over 350 people registered for the event, with “How to” sessions on Eczema and Psoriasis selling out in advance!
Following each talk ISF dermatology Helpline nurses were on hand to provide free, up-to-date guidance to attendees on skin conditions.
PHARMACYNEWSIRELAND.COM 29 News
Claire Brock, Moderator; Glenn Kenneally, Patient Advocate; Paul Herriot, Patient Advocate; Prof Alan Irvine, Consultant Dermatologist
Jill Clarke, Communications and Events Manager, Irish Skin Foundation; Dr Rupert Barry, Consultant Dermatologist, St. James’s Hospital; Carmel Blake, Clinical Helpline Manager, Irish Skin Foundation
Claire Brock, Moderator; Jill Clarke, Communications and Events Manager, Irish Skin Foundation; Prof Anne-Marie Tobin, Consultant Dermatologist, Tallaght University Hospital
Dramatic increases in Hay fever expected
3 years after COVID started in Ireland, the last thing that we need is another medical problem, this one however, can be managed very well in most cases. Thankfully, this time we are talking about seasonal Allergic Rhinoconjunctivitis or Hayfever.
Written by Dr Ranbir Kaulsay MD, FACAAI, MSc Allergy (U.K)
 MB BCh BAO LRCP & SI (Irl), Dip Allergy (UK)
MB BCh BAO LRCP & SI (Irl), Dip Allergy (UK)
Fellow American College of Allergy, Asthma and Immunology Consultant Medical Allergist
Bon Secours Consultants Clinic and Beacon Allergy Clinic www.dublinallergy.com dublinallergy@gmail.com
However, it has been globally recognised that with worldwide climate changes, the pollen season has been getting longer and more severe throughout the whole world.
That certainly sounds like a Medical Apocalypse indeed. It is undisputed that Climate Change and Global warming are a reality.
with allergic skin conditions find that their eczema and other skin related conditions also flare up during this time.
Ireland, Our CO2 and Pollen Problem
There are many unproven items such as hay fever bands available and some patients swear as to their effectiveness , although there is no scientific basis to their effectiveness.
The impact of such a change is wide and far – reaching and has even had an impact on our weather patterns, and as far as Ireland is concerned, we have experienced milder winters and very pleasant summers over the last few years with some counties experiencing an increase in natural phenomena such as flooding unfortunately and some unfortunate countries suffering from periods of extended drought. However generally it has resulted in much more pleasant weather in Ireland with longer and stronger pollen levels.
However, all is not rosy, as we have seen a Marked increase in CO2, temperature which has led to a much longer and more intense pollen season. There has also been a marked increase in temperature recently which has caused high levels of tree pollen to surge, and this has caused what is known as a ‘pollen bomb’ effect to occur. Dangerous increase in pollen levels have been noted to cause a lot of health concerns especially in patients who are asthmatic. Unfortunately, up to 80% of patients with asthma also have coexisting hay fever and this may have detrimental effects on their health with sudden reductions in their lung function and an increase in bronchial hyper – Indeed this summer, every pharmacy in the country will be inundated by walk-in and prescription patients looking for symptomatic relief for their symptoms of allergic rhino conjunctivitis and asthma. Unsurprisingly many patients with allergic skin conditions find that their eczema and other skin related conditions also flare up during this time.
urban rather than rural areas also cause pollen to increase in areas where there shouldn’t be too high tree or grass pollen levels such as in cities. As we are all aware CO2 levels continue to rise especially in our cities which is why which is why there has been a global effort to try to reduce climate change. Nonetheless, an increase in CO2 in general would be bad news for both the climate and will lead to an increase in our already increasing trend of Hay Fever.
Tips from Asthma Society of Ireland on how to survive hayfever season:
• Talk to doctor or pharmacist NOW about taking medication to prevent / reduce symptoms. Don’t wait until you feel unwell.
• Keep an eye daily on our pollen tracker on asthma.ie
• Keep windows closed in your bedroom at night
• Keep windows and doors closed when the pollen count is high
• Stay indoors as much as possible on high pollen days
• Stay away from grassy areas, especially when grass is freshly cut
These are the usual tips that one would receive about practical suggestions, but very few of these work with a significant effect especially during times of high pollen surges or high pollen seasons. It is imperative to plan ahead of time for patients who know that they suffer from hay fever so that optimum control can be attained early and well –especially for children and young adults sitting important exams during the summer months like the Junior – Cert and Leaving Cert, not to mention all other summer University final examinations. An international study has shown that school children who suffer from hay fever are more likely to experience a noticeable drop in exam performance. (ISAC study 1995/2007)
Increased carbon dioxide levels which are found to be high in urban rather than rural areas also cause pollen to increase in areas where there shouldn't be too high tree or grass pollen levels such as in cities. As we are all aware CO2 levels continue to rise especially in our cities which is why which is why there has been a global effort to try to reduce climate change.
• Put Vaseline around your nostrils to trap pollen. Newer products containing a barrier and anti-inflammatory preparations like ECTOIN such as nasal sprays, eye drops and even some creams have been quite effective in reducing pollen induced allergy.
Unfortunately, it has been shown that most patients are unsatisfied with their current control of allergic rhinitis and many patients find themselves on multiple medications to control the disease process. It was found that the most common medications used was a combination of antihistamines and intranasal corticosteroids, still with less than adequate control.
However, we have had some bizarre and unusual changes in our predicated weather patterns and these in turn have caused some changes in our flora and fauna.
Ireland, Our CO2 and Pollen Problem

Nonetheless, an increase in CO2 in general would be bad news for both the climate and will lead to an increase in our already increasing trend of Hay Fever.
Increased carbon dioxide levels which are found to be high in
• Wear wraparound sunglasses to stop pollen getting into your eyes
• Shower, wash your hair and change your clothes if you have been outside for an extended period
• Avoid drying clothes outdoors, or shake them outdoors before bringing them in
• Minimise your contact with pets who have been outdoors and are likely to be carrying pollen
• Consider a purifier with a built-in air quality sensor to remove allergens and pollutants from the air. There are numerous brands available and not all have been rigorously tested.
Current guidelines for the treatment of allergic rhinitis based on the current ARIA (Allergic Rhinitis and its Impact on Asthma) place intranasal corticosteroids as the first line of therapy for the treatment of the disease. Second generation antihistamines may be required as may ocular mastcell stabilisers (Cromogylates etc.). New combination intranasal antihistamine / corticosteroid sprays have proven effective in moderate to severe allergic rhinitis and several studies including a recent local study has found this an effective treatment
Diagnosis of Allergy
The definitive evidence of allergy, after appropriate history and examination should be done by either Specific IgE measurements or by skin prick testing. There is little value of Total IgE measurements and no role for
PHARMACYNEWSIRELAND.COM 30 Allergies
Tips from Asthma Society of Ireland on how to survive hayfever season:









SAME SUPER FORMULA NEW TROPICAL TASTE 1 super supplement with 26 active ingredients including 11 vitamins, 7 minerals and 6 amino acids, all in 1 daily sachet GO TROPICAL THIS SUMMER Telephone: 091 769 803 | Email: customerservice@reviveactive.com or contact your local area representative. † Source: Euromonitor Passport report on Consumer Health National Statistics. Date: September 2022 150mg CoQ10 3,000mg L-Arginine Energy Immune System Heart Fatigue Also available in orange and mango flavour †
non-medical tests such as Kinesiology, VEGA testing or food intolerance testing for rhinitis.


Medical Care
The management of allergic rhinitis consists of 4 major categories of treatment, (1) environmental control measures and allergen avoidance, (2) pharmacological management, and (3) immunotherapy (4) surgery.
Environmental control measures and allergen avoidance involve both the avoidance of known allergens (substances to which the patient has IgE-mediated hypersensitivity) and avoidance of nonspecific, or irritant, triggers. Consider environmental control measures, when practical, in all cases of allergic rhinitis. This reinforces the need for proper and accurate allergy testing.
Pollens and outdoor moulds
Because of their widespread
can be difficult to avoid. Reduction of outdoor exposure during the season in which a pollen is present can be somewhat helpful. However, it is not ideal to restrict the movement of patients when the weather becomes better, nor is it possible in most cases.
In general, tree pollens are present in the spring, grass pollens from the late spring through summer (April to August), and weed pollens from late summer through autumn, but exceptions to these seasonal patterns exist. Specific area specific pollens such as ragweed and rape seed may also be of importance in certain parts of Ireland. Certain Pollen counts tend to be higher on dry, sunny, windy days. Outdoor exposure can be limited during this time, but this may not be reliable because pollen counts can also be influenced by a number of other factors. Keeping the windows and doors of the
Immunotherapy
possible during the pollen season (with air conditioning, if necessary, on recirculating mode) can be helpful. Taking a shower after outdoor exposure can be helpful by removing pollen that is stuck to the hair and skin.
Despite these measures, patients who are allergic to pollens usually continue to be symptomatic during the pollen season and usually require some other form of management. As with pollens, avoidance of outdoor/seasonal moulds may be difficult. Symptomatic therapy with newer generation antihistamines uo to three times the recommended doses at most, nasal corticosteroids and ideally immunotherapy for more severe cases may be necessary.
Treatment:
Antihistamines are the first line of treatment for most allergy symptoms and these medications have been in existence for over 60 years but have evolved slowly.
Older antihistamines such as Piriton have significant side effects such as drowsiness and also have strong anti-cholinergic effects. Eg – Dry mouth etc and unfortunately these are still being used as first line antihistamines today.
Loratidine, Ceterizine and Fexofenadine are newer antihistamines and these have evolved into Levoceterizine and Desloratidine which are more effective and have fewer side effects. These preparations are available over the counter in some pharmacies with Fexofenedine still needing a prescription.
One of the best and my personal new favourites is Bilastine or Drynol as it is called here because of its effectiveness, safety and non-drowsy property. As this is one of the newest antihistamines, it
is still available only on prescription but is highly effective.
Nasal sprays
These nasal sprays remain the main treatments for Rhinitis and is divided into
1) Decongestant sprays – Very effective over the counter medications but should never be used for more than five days continuously.
2) Intranasal Corticosteroids –Very effective in Treating all the symptoms of rhinitis and can be used safely for prolonged treatment regimes.
3) Nasal saline washes and moisturisers – effective in washing out mucus and allergens and should be recommended.
4) Newer combination antihistamines and steroid therapy- extremely effective preparation available on prescription (Dymista & Ryaltris)) and has been proven to be effective for all types of rhinitis. Sublingual (meaning ‘under the tongue) Immunotherapy
Sublingual immunotherapy (SLIT) is currently increasing in use, particularly in Europe because of its safety and efficacy and the fact that it is licenced and reimbursable for some patients.
We have had two sublingual immunotherapy treatments for grass pollen registered and available to us (by GMS and on the Drugs Payment Scheme) since 2008.
Oralair (6-grass pollen extract) and Grazax (Timothy Grass extract) has been used by me for patients with severe grass pollen allergy since 2008 where standard antihistamine and other medicines fail to provide relief). We have many patients on or having completed three years of treatment.
It is then effective in reducing hay fever for at least 5 -10 years after completion of treatment and as such may be considered to have a disease modifying effect (which means that some patients on it do not progress to asthma and other allergy illnesses).
Nonetheless, control of seasonal rhinitis and allergies in general has improved significantly, with many safe and effective treatments and options now available to our patients, young or old.
References
The Lancet Planetary Health
Volume 3, Issue 3, March 2019, Pages e124-e131
PHARMACYNEWSIRELAND.COM 32 Allergies
(Clearly
pollens and size of response to a variety of airborne or food allergens –
Pollens and outdoor moulds Because of their widespread presence in the outdoor air, pollens can be difficult to (Clearly
size of response to a variety of airborne or food allergens –
Pollens and outdoor moulds Because of their widespread presence in the outdoor air, pollens can be difficult to (Clearly
Moderate/ severe intermittent Mild persistent Moderate/ severe persistent Mild intermittent Intranasal corticosteroid Local cromone Oral or local non-sedative H1-blocker Intranasal decongestant (<10 days) or oral decongestant Allergen and irritant avoidance
Current treatment recommendations as per ARIA (Allergic Rhinitis and Its Impact on Asthma) Guidelines 2010
visible on the patients forearm are the exact
visible in 15 minutes)
visible on the patients forearm are the exact pollens and
visible in 15 minutes)
visible on the patients forearm are the exact pollens and size of response to a variety of airborne or food allergens – visible in 15 minutes)

























































T +44 800 270 0253 E info@scopeeyecare.com W www.fusion-allergy.com Drug free Complete approach to allergy Preservative free SCOPEUK0491 Feel the shield of Ectoin and Fusion Allergy. A complete range to soothe, treat and prevent allergy symptoms.
Statins and Cholesterol
Statins are one of the most commonly prescribed drugs in Ireland, about
adults over-50 take them. Globally, it is estimated that sales of statins were
according to European guidelines. Such guidelines are underpinned by the theory that the lower the LDL-C, the better. To investigate this, and to estimate the absolute benefit of statins, myself and my colleagues undertook a systematic review of statin trials, which was published in JAMA Internal Medicine in March 2022.
Systematic review findings
Rise in statins use
Although these cholesterollowering drugs were originally licensed to prevent the recurrence of cardiovascular disease (CVD) (secondary prevention), they are now also prescribed for those with no history of CVD (primary prevention). In Ireland, over one half of men and almost three-quarters of women who take statins, do so for primary prevention.
Over time, clinical guidelines have expanded eligibility for statins in line with changes to the recommended levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C). By 2016 the level of LDL-C considered acceptable was just over half of that recommended in 1998
We decided to focus on specific ‘hard’ outcomes; all-cause death, myocardial infarction (MI) and stroke rather than ‘major cardiovascular events’ or ‘major vascular events’, which are ‘composite’ outcomes that are commonly reported in statin trials and reviews. We decided this because composite outcomes are fraught with problems; they can be inconsistently defined and poorly reported. In addition, composites can be heavily weighted by less serious outcomes such as angina or revascularisations (the frequency of which can depend on opinions or preferences of doctors) rather than more objective outcomes such as MI and stroke. Firstly, we found that while statins were associated with reductions in all-cause death, MI and stroke, these reductions were modest, especially when reported as absolute risk reductions. The absolute risk reduction for dying from any cause are 0.8%, for heart attack, 1.3%, and for stroke, 0.4%, whereas their relative risk
reductions are 9%, 29% and 14% respectively. Research has shown that doctors are more likely to prescribe a drug, and patients to agree to take them, when benefits are described in relative terms, which sound more impressive. Thus, it is important that both patients and their prescribers are aware of the absolute risk reduction before deciding on this, or any medical intervention. It is also important that a person’s baseline risk of CVD is understood in this context. An obese, 65-yearold man who smokes is likely to have a higher baseline risk than a slim, non-smoking woman, even if both have elevated cholesterol levels. The potential benefits for a low-risk person are clearly smaller than for someone at high risk.
Secondly, we failed to demonstrate a linear relationship between the level by which LDL-C was reduced from taking statins and reduction in all-cause death, MI and stroke. Indeed, some of the trials with the greatest degree of LDL-C reduction (AURORA, CORONA) reported no clinical or survival benefit.
It is also important to consider the possibility of harm from any prescribed medicine. This is especially true for low-risk people whose potential for benefitting from statins is small. There is vigorous debate about the harms of statins. One influential systematic review estimated that reported that the risk treating 10,000 patients with statins for 5 years could result in 5 cases of myopathy, 50 to 100 new cases of diabetes, and 5 to 10
Pharmacist-Led Vaccination Services

cases of haemorrhagic stroke. However, observational studies have estimated much larger rates of muscle pain, (although these types of studies are considered lower certainty evidence compared with randomised controlled trials). Some commentators have dismissed muscle symptoms from statins as a ‘nocebo’ effect. However, the research supporting the nocebo theory is not strong, and the rate of genuine sideeffects remains unknown.
Conclusion
It seems surprising that for such a commonly used medicine there are large gaps in our understanding. This is, as we discovered when conducting our systematic review, due to several problems. Firstly, statin trials are highly heterogeneous; they differ from each other clinically and statistically. Thus, reviews are comparing apples with oranges. Secondly, there have been ongoing problems accessing raw data from clinical trials that would enable independent scrutiny. As noted, the use of composite outcomes and reporting benefits as relative risk reductions are problematic. So too, are clinicians’ and patients’ perceptions of those benefits.
To conclude, we need to be clear with patients about the limitations of our knowledge of these drugs so as to enable informed, shared decision-making. Ideally, we need new statin trials or access to all data from existing ones. However, as statins are now off-patent, the likelihood of new trials is low, and debate rumbles on about access to data from existing trials.
Recently, during World Immunization Week, the International Pharmaceutical Federation (FIP) offers a new resource to help countries self-assess readiness of their regulations to enable pharmacist-delivered vaccination services and, where needed, to implement such regulations.
Developed by FIP’s Regulators Advisory Group, it covers broad pharmacist-led roles related to vaccination from supply chain management to administration and prescribing. “This new resource from FIP is based on the experiences of professional regulators in countries where pharmacyled vaccination has been successfully introduced and regulated. With it, we aim to support regulators, policymakers and national organisations in developing strategies or enabling regulatory frameworks in countries where the pharmacy workforce has a limited role in vaccination strategies so that it can contribute further to the global imperative of improving immunisation coverage,” said Mr Brett Simmonds, group chair.
The “Pharmacy-led vaccination services: Regulatory self-assessment and implementation tool” aims to help identify strengths and areas for improvement in order to inform vaccination policy and planning efforts.
Any advocacy strategy to achieve an expanded role for the pharmacy workforce in vaccination services at country level needs to be grounded in an in-depth understanding of the needs at country level, an awareness of the requirements, resources (workforce, infrastructure, financial, etc), support systems, and stakeholders in order to design a successful and meaningful strategy, the authors say.
The International Pharmaceutical Federation (FIP) is the global federation of national associations of pharmacists and pharmaceutical scientists, and is in official relations with the World Health Organization. Through our 152 member organisations, we represent over four million practitioners and scientists around the world. Our vision is a world where everyone benefits from access to safe, effective, quality and affordable medicines and pharmaceutical care. www.fip.org
PHARMACYNEWSIRELAND.COM 34 Statins
Dr. Paula Byrne, Senior post-doctoral researcher, University of Galway
one in three
worth $1 trillion by 2020.
Clinically Proven Accuracy



The Medicare LifeSense® A5 Blood Pressure Monitor’s large screen and buttons makes it easy to use and the perfect option for individuals with limited eyesight.


1800 307777 FLEMINGMEDICAL.IE
Extra Large Buttons & Colour Screen Irregular Heartbeat Detection Ideal for Consultation Room & Home Use Large Cuff Size 22 - 42cm Validated By: Scan the QR code to the Training Platform NEW Online Blood Pressure Monitor Training Course FLEMING MEDICAL ACADEMY
Providing ‘Scope’ for Innovation and Excellence
Founded in 2009 by Tom and John Freyne, Scope is a market leading family-run eyecare and healthcare distribution and development company, operating across the UK, Ireland, and USA and 10 other international markets.
Tom Freyne, CEO, Scope

For the first time in scope’s story, they have benchmarked themselves against their commitment to their people, patients, and planet. “We are proud of the impact we are having through our continued investment and growth in social and environmental programmes. With that in mind, we have produced our first annual impact report to enable our customers and business partners to understand our impact. We know we have a long way to go, but just reflecting on what everyone in Scope has done here was a nice moment”
Tom Freyne, CEO Scope.
Scope recognises that they have a responsibility to their customers, their charities, their people and everyone in their communities. “It makes us proud to think that we act with the levels of responsibility that we should. We know that we have a lot more to do, however our progress and commitment gives us energy for the future”.
Tom Freyne, CEO Scope
Since the CSR department was established in 2019, Scope has tried to make a significant difference across its three pillars: people, patients, and planet.
Scope’s partnerships with children’s sight loss agencies allow them to give back within the eyecare sector. To date, Scope has raised and donated over £150,000 to good causes globally.
Scope employees regularly support local communities and causes close to their hearts through Scope’s global volunteer drive and the Scope Community Fund. The company’s employees have dedicated over 700 working hours to help 16 charities across 4 countries through their global volunteer drive so far. These days inspire such great energy and pride across the teams and it’s something that the company encourages a lot.
Alongside Scope’s portfolio of award winning eyecare products, Scope employees are proactively working to support patients at a local and global level to mind and manage their conditions in line with medical guidelines and compliance. To date, the business has connected with over 3000 Dry Eye Patients globally through their non-commercial International Dry Eye Well-Being Seminars. They have delivered 7 sessions in partnership with 9 leading healthcare professionals. These sessions focus on providing practical support and advice for patients around; Dry Eye Disease, understanding the treatments, what role nutrition play and many more topics.
“Our hope is that Scope is not just a job to pay the bills, we are here to energise each other as a team to find that additional meaning from what we do. We believe the work we are doing matters. We can improve the world one tiny bit every day by changing one person’s quality of life with our products and services. We are on a long-term ambitious journey here at Scope and we never stop trying to move forward and improve” Tom Freyne, CEO Scope.
Scope is also tenacious about how they can build on their work to preserve the planet for this generation and future generations as the business continues to grow. The health of our planet has a direct impact on everyone’s health and well-being.
Taking a three-pronged approach, Scope is proactively investing in waste management, the journey to net zero, and in fostering a culture of sustainability across the business from operations to new product development.
As part of its waste management commitment, Scope has moved its OPTASE TTO Blephawipe wipe to a fully biodegradable format while maintaining the quality and effectiveness of the wipe for patients.

They are also rolling out right now a free recycling service across 50 pharmacies in London. The aim of this initiative is to remove barriers to recycling for customers. 24 products from across the scope portfolio can be recycled in this scheme. The recycled materials are repurposed and used to create materials for the construction industry. Once the pilot concludes Scope will evaluate the impact of the initiative and consider an
additional investment and wider roll out of the programme.
People from across the company are also collaborating to put the structures in place to measure the business’s Scope 1 and 2 carbon emissions. This will allow Scope to measure their impact more effectively across the value chain, while also giving them the insights they need to map out our journey to net zero over the next few years.
Scope is establishing these programmes across the entire business so that all departments are involved and leading on the initiative together.
Scope’s first annual Impact Report takes the form of an environmental social governance report. It outlines the local and global issues the business is working to mitigate and benchmarks the business’s progress in relation to the United Nations Sustainable Development Goals.
The company is keen to hear feedback and work with pharmacy in Ireland to deliver on their goals and encourages members to reach out to discuss any of the content here.

PHARMACYNEWSIRELAND.COM 36 News
“Sustainability and company ambition have to work together for future planning”


TTO BlephaWipe NOW Biodegradable Open the door to a greener future. T 1800 816 005 | E info@scopeeyecare.com | W scopeeyecare.com | Part of the OPTASE family.
Erectile Dysfunction
Management and Treatment of Erectile Dysfunction
Erectile dysfunction (ED) is a common condition occurring in males over 40 years of age, although it can occur earlier. It is estimated that at least 150 million men globally have ED. It is difficult to obtain accurate values for the true prevalence of erectile dysfunction however, as many patients fail to seek medical attention, and many clinicians are reluctant to ask patients about their sexual health.
tissue, corporal endothelium, or tunica albuginea. It is closely related to cardiovascular disease, diabetes mellitus, hyperlipidaemia, hypertension, and endothelial dysfunction,” she explains.
We recently spoke to Theresa Lowry Lehnen, RGN, GPN, RNP, BSc, MSc, M. Ed, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University to understand more about this condition and its impact on males in Ireland.
ED can have a substantial negative impact on a man’s quality of life, Theresa reflects. She says, “Erectile dysfunction is the inability to achieve or maintain an erection for satisfactory sexual performance, and affects a considerable proportion of men at least occasionally. It is often treatable, however, if left untreated, ED can be a source of severe emotional stress for both the man and their partner.”

Theresa notes that erectile dysfunction is often an under recognised, yet important, cardiovascular risk factor. She says, “Owing to its strong association with metabolic syndrome and cardiovascular disease, cardiac assessment is warranted in men with symptoms of ED.”
Aetiology of Erectile Dysfunction
Although most men will experience periodic episodes of erectile dysfunction, it tends to become more frequent with advancing age. “Many factors can contribute to sexual dysfunction in older men, including physical and psychological conditions, comorbidities and polypharmacy,” she adds. “Aspects of an ageing man’s lifestyle behaviour and
androgen deficiency, most often decreasing testosterone levels, can affect sexual function.
“Studies have shown that the percentage of men who engage in some form of sexual activity, decreases from 73% in men aged 57–64 years to 26% for men aged 75–85 years. The aetiology for this decline in male sexual activity is multifactorial, and is in part related to female partners menopause at approximately 52 years of age, leading to a significant decline in female libido and desire to engage in sexual activity.”
While ED is associated with ageing, many studies and largescale surveys have concluded that ED is a major health concern among young men.
Theresa adds, “One study in 2013 reported that 1: 4 men seeking medical help for erectile dysfunction in the real-life setting, is < 40 years of age. Another study in 2016 concluded that 22.1% of men < 40 years of age had low (<21) Sexual Health Inventory for Men (SHIM) scores.
“In the past, erectile dysfunction was almost always considered a psychogenic disorder. However, evidence now suggests that more than 80% of cases have an organic aetiology. While most patients with ED have organic disease, some do have a primary psychological cause, particularly younger men. Even when ED is organic in nature, there are almost always psychological consequences regarding relationship issues, cultural norms and expectations, loss of self-esteem, shame, and anxiety and depression related to sexual performance.”
Erectile dysfunction is multidimensional in nature, and Theresa says it can be broadly divided into endocrine and nonendocrine causes.
“The condition can be caused by any disease process which affects penile arteries, nerves, hormone levels, smooth muscle
“Erectile dysfunction and vascular disease are thought to be linked at the level of the endothelium. Endothelial dysfunction, results in the inability of smooth muscle cells lining the arterioles to relax and prevents vasodilatation. The endothelial cell is known to affect vascular tone and impact the process of atherosclerosis and impacting ED, CVD and peripheral vascular disease. Cardiovascular disease and hypertension are very significant risk factors for erectile dysfunction.”
Besides cardiovascular disease, there are strong correlations between ED and hyperlipidaemia, diabetes, hypogonadism, obesity, smoking, alcoholism, benign prostatic hyperplasia (BPH) with lower urinary symptoms (LUTS), depression, and premature ejaculation. Diabetes is a common aetiology of sexual dysfunction, because it can affect both the blood vessels and the nerves that supply the penis. Men with diabetes are four times more likely to experience erectile dysfunction, and on average, experience it 15 years earlier than men without diabetes.
“Obesity is also correlated to the development of several types of dysfunction, including a decrease in sex drive and an increase in episodes of ED,” she continues. “Neurogenic erectile dysfunction is caused by a deficit in nerve signalling to the corpora cavernosa. Such deficits can be secondary to spinal cord injury, multiple sclerosis, Parkinson disease, lumbar disc disease, traumatic brain injury, radical pelvic surgery and diabetes. Men being treated for prostate cancer with treatments such as radical prostatectomy, radiation therapy or the use of lutenising hormonereleasing hormone (LHRH) agonists and antagonists often experience ED.”
Numerous medications are listed with erectile dysfunction and/ or a decreased libido as a side effect. Drugs that can cause ED include hydrochlorothiazide’s and beta-
blocking agents. Medications used to treat depression, particularly the SSRIs such as citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline, can also contribute to ED. The severity of erectile dysfunction is often described as mild, moderate or severe according to the five-item International Index of Erectile Function (IIEF-5) questionnaire, with a score of 1–7 indicating severe, 8–11 moderate, 12–16 mild–moderate, 17–21 mild and 22–25 no erectile dysfunction.
The International Index of Erectile Function (IIEF-5)
Questionnaire
The IIEF is a multidimensional validated questionnaire with 15 questions in the five domains of sexual function (erectile and orgasmic functions, sexual desire, satisfaction with intercourse and overall sexual satisfaction), and there is also an abbreviated format of five questions in the Sexual Health Inventory for Men (SHIM).
Investigations and Diagnosis
Theresa continues, “A thorough medical history, detailed sexual history, and physical examination are required before commencing treatment or further investigations. It is important to distinguish between psychological and organic causes of ED, as well as to ensure that the patient has erectile dysfunction and not another disorder. History that points towards a psychological aetiology include, sudden onset of erectile dysfunction especially if it is related to a new partner or a major life-changing event, situational ED, normal erections with masturbation or a different partner, presence of morning erections and high daily variability in erectile rigidity.
“The main differential diagnosis for erectile dysfunction is hypogonadism, loss of libido, depression with low mood, and other psychological conditions. It may also be the first manifestation of diabetes or cardiovascular disease as well as depression. It is important to differentiate between true erectile dysfunction and other sexual disorders such as premature ejaculation, and this is usually assessed by obtaining a good sexual history.
PHARMACYNEWSIRELAND.COM
38
An interview with Theresa Lowry- Lehnen (PhD), CNS, GPN, RNP, South East Technological University
The end of erectile problems? Touch
wood.
Sidena. Your new firm favourite.
Sidena 50mg Tablets are now available over the counter in a 4 and 8 pack.

ABBREVIATED PRESCRIBING INFORMATION
Product Name: Sidena 50 mg Tablets.
Composition: Each tablet contains, 50 mg sildena l (as citrate) .
Description: Light blue, round, slightly dotted tablets. Cross breaking notch on one side and marked ‘50’ on the other side. Can be divided into equal quarters. (Only two quarters of the 50 mg is covered by posology).
Indication(s): Treatment of men with erectile dysfunction, which is the inability to achieve or maintain a penile erection su cient for satisfactory sexual performance.
Dosage: Adults and elderly: 50 mg taken as needed approximately one hour before sexual activity. Dose may be decreased to 25 mg. Max dose: 50mg once daily. Impaired renal and hepatic function: Sildena l clearance is reduced in hepatic and severe renal impairment. Consider a dose of 25 mg. Dose may be increased step-wise to 50 mg if tolerated. Children and adolescents below 18 years of age: Contraindicated. Use in patients using other medicines: Starting dose of 25 mg with CYP3A4 inhibitors (not advised to use with ritonavir). To minimise postural hypotension in patients receiving and alpha-blocker, stabilise patient rst on the alpha blocker and use a starting dose of 25 mg sildena l.
Contraindications: Hypersensitivity to sildena l or any of the excipients. Concomitant with ritonavir, nitric oxide donors or nitrates in any form, guanylate cyclase stimulators e.g. riociguat. In patients that sexual activity is inadvisable (e.g. severe cardiovascular disorders such as a recent (6 months) acute myocardial infarction (AMI) or stroke, unstable angina or severe cardiac failure). Refer these patients to a doctor. Patients with loss of vision in one eye due to NAION. Known hereditary degenerative retinal disorders. Severe hepatic impairment. Hypotension. Anatomical deformation of the penis. Women. Not intended if no erectile dysfunction.
Warnings and Precautions for Use: First diagnose erectile dysfunction and determine potential underlying causes (e.g. hypertension, diabetes mellitus, hypercholesterolaemia or cardiovascular disease), before considering pharmacological treatment. Consider the cardiovascular status of patients, since there is a degree of cardiac risk associated with sexual activity. Serious cardiovascular events, including myocardial infarction, unstable angina, sudden cardiac death, ventricular arrhythmia, cerebrovascular haemorrhage, transient ischaemic attack, hypertension and hypotension have been reported post-marketing in temporal association with the use of sildena l. Most, but not all, of these patients had pre-existing cardiovascular risk factors. Sildena l has vasodilator properties, resulting in mild and transient decreases in blood pressure. Caution: Patients with anatomical deformation of the penis (such as angulation, cavernosal brosis or Peyronie’s disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell anaemia, multiple myeloma or leukaemia). Advise patients that in case of priapism, prolonged erections (longer than 4 hours) or sudden visual defect, they should stop taking sildena l and consult a physician immediately. Administer to patients with bleeding disorders or active peptic ulceration only after careful bene t-risk assessment, as there is no safety information available. Interactions: Inhibitors of the cytochrome P450 (CYP) isoforms 3A4 (major route) and 2C9 (minor route) isoenzymes such as CYP3A4 inhibitors: Itraconazole, ketoconazole, erythromycin, cimetidine, HIV protease inhibitor saquinavir: May reduce sildena l clearance and increase sildena l plasma levels. Consider a starting dose of 25 mg. Strong CYP3A4 inducers e.g. rifampicin may increase sildena l clearance and decrease sildena l plasma concentrations. Grapefruit juice: May give rise to modest increases in plasma levels of sildena l. Nicorandil (Hybrid of potassium channel activator and nitrate): Due to the nitrate component it has the potential to have serious interaction with sildena l. Sildena l potentiates the hypotensive e ect of nitrates. Alpha blocker: Concomitant administration of sildena l may lead to symptomatic hypotension in a few susceptible individuals. Patients should be hemodynamically stable on alpha-blocker therapy prior to initiating sildena l treatment. Sildena l potentiates the antiaggregatory e ect of sodium nitroprusside in vitro. Not recommended in patients with a history of bleeding disorders or active peptic ulceration. Not recommended to use with other pulmonary arterial hypertension treatment containing sildena l.
Ability to Drive and Use Machinery: Minor in uence, dizziness and altered vision were reported. Patients should be aware of how they react to sildena l before driving or using machinery. Undesirable E ects: Very common: Headache. Common: Dizziness, visual disorders, visual colour distortion, vision blurred, ushing, hot ush, nasal congestion, nausea, dyspepsia. See SPC for more adverse e ects.
Marketing Authorisation Holder: Rowex Ltd, Bantry, Co. Cork. Marketing Authorisation Number: PA 0711/170/002. Further information and SPC are available from: Rowex Ltd., Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417
E-mail: rowex@rowa-pharma.ie
Legal Category: Not subject to medical prescription.
Date of Preparation: Aug 2022
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing Rowex pv@rowa-pharma.ie
Date of preparation: (10-22) CCF: 25156 Supply status: Supply through pharmacies only.
40 Erectile Dysfunction

“A complete medication list including supplements should be checked with the patient. ED can be a result of prescription or other medications. Prescription drugs that can cause ED include, antidepressants especially SSRIs, cimetidine, ketoconazole, spironolactone, sympathetic blockers, thiazide diuretics, and other antihypertensives. ACE inhibitors and calcium channel blockers are the least likely to cause ED. Beta-blockers are only a minor contributor, while alpha-blockers can improve erectile function.”
Vascular risk factors such as hypertension and diabetes and lifestyle factors such as smoking, activity level, alcohol intake, and the use of any recreational drugs should be assessed, Theresa adds. A full general and cardiovascular examination should be undertaken, as erectile dysfunction can be the first symptom of underlying vascular disease. Peripheral pulses should be checked and blood pressure measured. The genitalia should be carefully inspected for hypogonadism, signs of infection, the presence of penile fibrosis or plaques, and phimosis.
Theresa continues, “The role of testosterone replacement therapy (TRT) as a potential to improve erectile function in ED remains an issue for clinicians who are comfortable treating androgen deficiency. Androgens are known to have a significant impact on the function of the smooth musculature within the corpus spongiosum.
“Testosterone supplementation is more effective as a treatment for low libido than for ED. For most men with both ED and hypogonadism, oral PDE5 inhibitors alone are recommended as the initial therapy. Testosterone supplementation is reasonable in men with proven hypogonadism and ED who have already failed PDE5 inhibitor therapy or who also have low libido. Hypogonadal patients with borderline erectile rigidity are most likely to benefit from testosterone supplementation.
“Testosterone replacement therapy may cause increased levels of haemoglobin or haematocrit which is associated with an increased risk of heart attack, stroke and blood clots. Testosterone treatment can also cause an enlarged prostate or other prostate disorders. During TRT treatment, the prostate specific antigen (PSA) will be measured to monitor for any changes and this is particularly important in men over 45 years of age.
“As a result of using testosterone replacement, natural production of testosterone may be reduced. This may lead to a reduction in sperm production and fertility. Other side effects of TRT include: weight gain, increased appetite, hot flushes, acne, depression, restlessness, irritability, aggression, tiredness, general weakness and excessive sweating. Lifestyle modifications are considered first-line therapy for ED, and men should be encouraged to make the necessary changes to benefit both their sexual function and their overall health.
“PDE5 inhibitors are highly effective and have an overall success rate of up to 76%. PDE5 inhibitors are contraindicated in patients taking nitrates, but otherwise are safe and effective. When PDE5 inhibitors are co-administered with nitrates, pronounced systemic vasodilation and severe hypotension can occur.
“PDE5 inhibitors and α-adrenergic receptor blockers, often used for treatment of BPH, need to be taken at least 4 hours apart. Among second-line therapies,
external vacuum devices (VCDs) are a good, non-surgical option for patients with ED. VCDs are clear plastic chambers placed over the penis, tightened against the lower abdomen with a mechanism to create a vacuum inside the chamber. This directs blood into the penis. If an adequate erection occurs inside the chamber, the patient slips a small constriction band off the end of the VCD and onto the base of the penis. An erection beyond 30 minutes is not recommended. While cumbersome, these devices are considered safe.
“Other second-line therapy includes the use of either intracavernosal injection (ICI) or intraurethral suppositories (IUS). A small needle is used to inject the ICI medication into the lateral aspect of the penis through a small-gauge needle. These vasoactive agents include prostaglandin E1, papaverine and phentolamine and sometimes atropine, which work alone or in combination to elicit an erection. Response is dose related, usually occurs within 10– 15 minutes, and does not require stimulation. A concern with ICI use is priapism, and if this occurs the patient will need to seek urgent medical attention. Bruising can also occur, due to it being an injected medication. The intraurethral suppository consists of a tiny pellet of prostaglandin E1 inserted into the urethral meatus. Response is dose related, and onset usually occurs within 10–15 minutes. Patients need to be trained on the technique of the IUS before use, and should be advised that pain or burning may occur with this medication.
“In men who fail to respond to first or second-line therapy, or who are not interested in conservative therapies, penile prosthesis implantation is available. Penile implants include malleable and inflatable devices, although most implants used are of the inflatable variety. Adverse effects including malfunction and infection are rare, and patient satisfaction is high.”
Outlook
Theresa told us that future Therapies for ED Clinical studies in gene therapy are looking towards replacing proteins that may not be functioning properly in the penile tissue of men with erectile dysfunction.
She says, “Replacement of these proteins may result in improvement in ED. Experimental animal models have demonstrated improvement in erectile function with gene therapy. Human studies may demonstrate success with this therapy in the future, however, gene therapy in humans is controversial, and can take a long time for regulatory approval and public acceptance.
“Stem cell studies may also provide advancements in the treatment of ED in the future. The mechanism of action of stem cells is to generate angiogenesis with subsequent increase in cavernosal smooth muscle cells within the corporal bodies.
“The clinical studies published to date provide encouraging results, with improvement of sexual function reported with no side effects. Although pioneering, stem cell studies to date are small scale, with a short follow up period, various aetiologies of ED and without a control group. Melanocortin activators are drugs that act through the central nervous system, and have been shown in animal studies to produce an erection. Initial studies in humans suggest that the drug (PT-141) can be effective if given intranasally in men with psychological rather than physical causes, and mild to moderate ED.
“Larger studies are necessary, however, to demonstrate the safety and overall effectiveness of these drugs. Another potential new treatment for ED, is penile low intensity shock wave lithotripsy. This consists of 1500 shocks twice a week for 3–6 weeks. The purpose is to stimulate neovascularisation to the corporal bodies with improvement in penile blood flow and endothelial function. The use of low-intensity shock wave lithotripsy may convert PDE5 inhibitor nonresponders to responders.”
PHARMACYNEWSIRELAND.COM
CPD
CPD
60 Second Summary
Atopic dermatitis (AD), also known as eczema, is the most common skin condition in children (30% prevalence in the first 2 years) and second most common in adolescents and adults (7% prevalence) after acne. The cause of AD relates to a combination of the triad of skin barrier dysfunction, skin dysbiosis, and dysfunctional inflammation. AD is associated with other atopic conditions such as food allergy, asthma, and allergic rhinitis. These associations often lead to patients with AD being treated with ineffective and inappropriate treatments, such as dietary restriction, which have no role in AD.
To deal with the three intertwining causes of AD, a three-pronged approach is necessary: emollients to replenish the skin barrier, topical steroids or calcineurin inhibitors to reduce inflammation, and antiseptic bleach baths to reduce cutaneous dysbiosis. In special scenarios oral antibacterial or antiviral agents may be required.
Patients with AD should NOT be told to use topical steroids ‘sparingly’ or ‘thinly’ and should be advised to treat for an adequate duration. Patients with AD should NOT be treated with oral steroids (outside of expert centres with a therapeutic exit strategy in place) due to the severe side effects and lack of disease modification. Patients with AD should NOT be told to change their washing powder or reduce/eliminate foods from their diet, as AD is not caused by detergents or food allergy.
Patients with persistent or severe AD despite the use of appropriate potent topical therapy should be referred to dermatology for consideration of advanced therapies.
AUTHOR: Dr Cathal O’Connor, Clinical Research Fellow in Dermatology, Irish Clinical Academic Training Programme, Royal College of Physicians of Ireland

1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result? Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
Management of Atopic Dermatitis


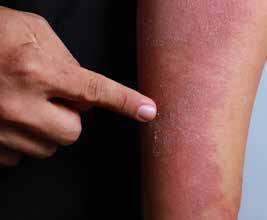
Introduction
Atopic dermatitis (AD), also known as eczema, is the most common skin condition in children (up to 30% prevalence in the first 2 years of life and 20% overall) and second most common in adolescents and adults (7% prevalence) after acne. AD has a significant impact on quality of life (QOL), and children with severe AD have worse QOL than children with type 1 diabetes mellitus or treatment-resistant epilepsy. The main burdensome symptoms are itch and sleep disruption (Images 1A-1D). AD is associated with other atopic conditions such as food allergy, asthma, and allergic rhinitis. These associations often lead to patients with AD being treated with ineffective and inappropriate treatments, such as dietary restriction, which have no role in AD. AD is also associated with neuropsychiatric disease such as anxiety, depression, and attentiondeficit hyperactivity disorder.

Cause of AD
The cause of AD relates to a combination of the triad of skin
barrier dysfunction, skin dysbiosis, and dysfunctional inflammation (Image 2 on page 32). Skin barrier dysfunction is genetically inherited, with filaggrin mutations significantly increasing the risk of developing AD. Filaggrin plays
41 CPD: Atopic Dermatitis
Continuing Professional Development
4 previous steps, log and record your findings. Published by IPN. Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
1A 1B 1C 1D
an important role in the skin's barrier function. It brings together structural proteins in the outermost skin cells to form tight bundles, flattening and strengthening the cells to create a strong barrier. In AD, skin is heavily colonised with Staphylococcus aureus (S. aureus) in lesional and non-lesional skin. A rising proportion of S. aureus in skin microbiome samples predicts a flare, and diversity of nonstaphylococcal species returns once a flare has been treated. A hallmark of AD is Th2-mediated inflammation, with high levels of IL-4 and IL-13 that promote dysfunctional allergic inflammation. With chronic or untreated AD, other immune pathways can become activated. Each component of the triad interacts with the others in a vicious cycle, for example filaggrin deficiency allows antigens to penetrate the stratum corneum and stimulate inflammation, and subsequently IL-4 and IL-13 production leads to reduced filaggrin production. It has recently been shown that dupilumab (a biologic targeting IL-4 and IL-13) not only reduces inflammation but enhances skin barrier function, highlighting the importance of adequately treating inflammation in AD. It is essential to treat each component of the causes of AD adequately to optimise outcomes.
Treatment of AD
Anti-inflammation

The use of anti-inflammatory steroid creams/ointments is essential to reduce inflammation in AD. Early and aggressive use of topical steroids can reduce
the duration of disease, and may also reduce the risk of developing associated problems. The dermatology team in Crumlin have recently shown that reducing inflammation in the skin using steroid creams also reduces systemic inflammation, suggesting that topical steroids can correct the systemic immune dysregulation in AD. For mild AD, hydrocortisone 1% can be dispensed over the counter. However, hydrocortisone 1% is a very weak anti-inflammatory agent, and is not sufficient to treat more severe eczema. For moderate AD, agents such as clobetasone butyrate (Eumovate)

or betamethasone valerate 0.025% (Betnovate RD) can be used. For severe AD, potent agents such as betamethasone valerate 0.1% (Betnovate 0.1%) or mometasone furoate (Elocon) should be used (Image 3). In general it is better to use a more potent topical steroid less frequently than a weaker topical steroid more frequently, for two reasons: systemic absorption is related to the frequency of application, and the burden of treatment with daily topical steroid application is much higher than twice weekly. Topical steroids should be applied liberally (not sparingly!) so that the affected skin is left glistening afterwards. Ointments are preferable to creams as the higher lipid content makes the vehicle more effective. There is no benefit to using topical steroids more than once daily - more frequent use increases the risk of systemic absorption and reduces patient adherence. In general, therapy should be continued once daily for two weeks, then weaned to alternate days for two weeks, then maintained twice weekly (weekend therapy on Saturdays and Sundays is a good option for busy parents/ patients) until the skin has been clear for several months.




Myth 1 Topical steroids should be applied sparingly/thinly and only for short bursts



Topical steroids are a very elegant way to deliver local antiinflammatory therapy, without the potential side effects that systemic therapy can produce. Skin that is inflamed with AD is on fire immunologically. This immune dysregulation needs to be extinguished appropriately to prevent worsening or chronic AD. If topical steroids are stopped too quickly the inflammation can return rapidly. Parents are almost always counselled by pharmacists and non-dermatology doctors about the risk of skin thinning, but this is generally limited to prolonged use of extremely potent topical steroids such as Dermovate (which should only be prescribed for palmoplantar AD). We have recently performed qualitative research interviewing parents of children with severe eczema, and the ‘mixed messages’ about the safety of topical steroids can cause significant upset.
Myth 2 Topical steroids can never be used on broken skin in AD
When skin inflammation is so severe that it has caused skin breakdown, either directly or indirectly from scratching, it is essential to reduce inflammation to avoid further damage. It is safe to apply topical steroids to broken skin.
Image 3
42 CPD: Atopic
Dermatitis
X5
X50 X200 X300 X1,000
FACE
BODY
Image 2
Topical tacrolimus, a calcineurin inhibitor, is an alternative to topical steroids. It is a useful antiinflammatory adjunct, particularly in the maintenance phase of AD (rather than the treatment of flares, when it is less effective than potent topical steroids). The only side effect is mild stinging with the first few applications, which usually settles down with ongoing use. Given the lack of steroid, it is particularly useful for sensitive areas such as the eyelids. Topical tacrolimus is much more expensive than topical steroids.
Myth 3 Topical tacrolimus can never be used in babies and only 0.03% can be used in older children
Topical tacrolimus (Protopic) has been used in dermatology for decades, with an overwhelming volume of reassuring safety data. Protopic 0.1% is licensed for patients over 16 years, and Protopic 0.03% is licensed from two to 16 years. Dermatologists usually prescribe the 0.1% formulation for all ages, because of the extensive safety data and the enhanced efficacy. There is extensive anecdotal evidence of safety of Protopic in younger infants, so Protopic 0.1% is often prescribed off-license for infants under two years.

Skin barrier replenishment
Moisturisers (also known as emollients) should be used twice daily or more, and moisturising after a bath (‘soak and seal’) is an excellent way to hydrate the skin. Moisturisers should be applied downwards to avoid blocking or irritating hair follicles. A pump dispenser is useful for preventing bacterial colonisation of the

moisturiser container. Alternatively, a large spoon can be sterilised (using boiling water) and dipped into a tub to avoid transfer of bugs from the parent’s hand to the container (Image 4). Appropriate bathing/showering advice for AD is to avoid soaps or irritant products that produce bubbles, use an emollient (that does not contain sodium laureth sulfate) as a wash, keep the temperature of the bath/shower tepid, and limit the duration of the bath/shower to 10 minutes or less. The skin should be gently patted dry afterwards.
Myth 4 There is a number one brand of emollient for treating AD
There is no particular brand of emollient that is significantly superior than any other for treating AD. One of the key causes of AD is an impaired skin barrier, and every individual has differing levels of various proteins in their skin that retain moisture and protect us from external threats. Every moisturiser has a different mixture of ingredients, so it is best for patients/parents to try several brands to see which leaves the skin most hydrated.
Antimicrobial strategies
For infection-driven flares, the addition of sodium hypochlorite (Milton) is an effective strategy for reducing microbial colonisation, without causing antibiotic resistance. Two capfuls (60mL) can be added to a baby bath (50L) and four capfuls (120mL) can be added to a full bath (100L).
Topical fusidic acid (Fucidin) should be prescribed with caution and other skin infections.
S. aureus resistance to fusidic
acid has reached crisis point, driven by inappropriate use of topical antibiotics. Moreover, the selection for fusidic acid resistant strains also selects for methicillin resistance, creating even more problems with MRSA. If antibiotics are required for infected AD, then oral antibiotics should usually be prescribed, in conjunction with topical antiseptics (eg Milton).
Myth 5 Topical antibiotics are better than oral antibiotics because they do not cause antimicrobial resistance
Topical antibiotics are known to cause localised antimicrobial resistance in the area being treated, but also in cutaneous sites distant to application. One recent study even showed antimicrobial resistance on the skin of close contacts. While oral antibiotics have scope to cause more antimicrobial resistance in an individual patient due to exposure to other flora such as in the gastrointestinal tract, topical antibiotics are usually washed off ‘as is', compared to oral antibiotics which are excreted in urine or egested in faeces as less active or inactive by-products. The introduction of topical antibiotics directly to waste water is a major contributor to antimicrobial resistance globally.
Special scenarios
Bacterial superinfection
As mentioned, S. Aureus is a significant factor in the pathogenesis and flares of AD. While patients with AD are almost always colonised with S. aureus, some patients will develop bacterial superinfection. This manifests as weeping of
clear or purulent fluid, honeycoloured crust, folliculitis, abscess, or cellulitis (Image 5). If there is evidence of bacterial superinfection then oral antibiotics should be prescribed, guided by microbiological cultures (predominantly for resistance information) and local antimicrobial practices. These should be prescribed in conjunction with topical antiseptic measures, which should be instituted as a preventative measure.
Eczema herpeticum
Eczema herpeticum occurs due to infection with herpes simplex virus, and is more common in children. It manifests as clusters of painful and itchy blisters which evolve into punched-out erosions, sometimes
43
Image 4
Image 5
associated with fever (Image 6). Patients can have inactive AD, so it does not represent a sign of treatment failure. It is helpful to take viral PCR swabs to confirm the diagnosis. Treatment should be immediately started with aciclovir. Oral acyclovir/ valaciclovir is acceptable unless there is concern for ophthalmic or central nervous involvement, or if the patient is unwell. Patients with recurrent eczema herpeticum should consider prophylactic antiviral treatment.
Eczema coxsackium
Eczema coxsackium is a recently described entity caused by coxsackie A6 or A16 in patients


with AD. Clusters usually occur in springtime. It looks very similar to eczema herpeticum but it is not itchy and there is a slightly purpuric hue to the background skin (Image 7). Respiratory viral panels can be sent to confirm enterovirus infection as the PCR testing is difficult to access. Given the similarities to eczema herpeticum it is very reasonable to start treatment with acyclovir or valaciclovir but enteroviruses do not express viral thymidine kinase and therefore do not respond to antiviral treatment.
What NOT to do
Patients with AD should NOT be told to use topical steroids
‘sparingly’ or ‘thinly’ and should be advised to treat for an adequate duration. The consequences of undertreated AD are huge, and the terrible impact on QOL is unnecessary given the safety and efficacy of topical steroids. Patients with AD should NOT be treated with oral steroids (outside of expert centres with a therapeutic exit strategy in place) due to the severe side effects and lack of disease modification. Patients will improve rapidly within a few days of high dose oral steroids but will flare even worse once withdrawn. Given that we now have effective targeted treatments for AD in specialist clinics there is no excuse for
Image
inappropriate prescriptions of oral steroids. Patients with AD should NOT be told to change their washing powder or reduce/ eliminate foods from their diet. AD is absolutely not caused by detergent! In children who have co-morbid AD and food allergy, if an allergenic food is removed from the diet it will not affect the AD but will simply stop immediate IgE-mediated allergic reactions from happening.
When to refer
Patients with persistent or severe AD despite the use of appropriate potent topical therapy should be referred to dermatology for consideration of advanced therapies. Use of 1% hydrocortisone for a few days does not represent a trial of topical steroids: an adequately potent steroid should be prescribed for at least two weeks to assess response. Dermatologists now have access to dupilumab, a biologic drug targeting IL-4 and IL13 which can be life-changing for patients who have lived with severe itch for years or even decades. In addition, other biologic drugs such as tralokinumab (IL-13 inhibitor) and oral janus kinase (JAK) inhibitors such as upadacitinib or abrocitinib are also available. JAK inhibitors have a more rapid onset of action and quickly reduce itch, but there are still some concerns about longterm use, based on studies in other conditions such as rheumatoid arthritis, with patients who represent a very different population to those with AD. Topical JAK inhibitors have been licensed in some countries with significant benefit seen in AD, although cost is currently prohibitive.
Image 7
44 CPD: Atopic
Dermatitis
Overall it is a very positive time for dermatologists and patients with AD, with effective treatments already available and many more in the pipeline. 6
For the treatment of moderate to severe atopic dermatitis in adult patients who are candidates for systemic therapy1
TIME TO PRESS PLAY
The


Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe

Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.ie) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-tosevere atopic dermatitis in adult patients who are candidates for systemic therapy. Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 18 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should be not shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection sites. It is recommended to rotate the
IL, interleukin.
References: 1. Adtralza® SPC. 2. Bieber T. Allergy 2020;75:54–62.
® Registered trademark
injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology. Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: June 2021
Reference number: REF-19238(1)
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie

Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
Date of preparation: February 2022
NEW
is a promotional advertisement from LEO Pharma for IE healthcare professionals. Learn more at www.adtralza.ie
Adtralza®
This
Introducing
first licensed biologic that inhibits IL-13 alone, 1 a
of atopic dermatitis
and symptoms.2
key driver
signs
IE/MAT-54463 V1
information can be found
or from:
Road,
12, Ireland.
Further
in the Summary of Product Characteristics
LEO Pharma, Cashel
Dublin
E-mail: medical-info.ie@leo-pharma.com
Not an actual patient. For illustrative purposes only.
The Irish Pharmacy
Awards 2023
Excellence. Impact. Innovation
Irish Pharmacy Awards 2023





On Saturday, 27th May 2023 over 650 of community pharmacy’s leading individuals, teams and pharma representatives gathered at the Clayton Hotel, Dublin for the biggest night in the pharmacy calendar.



To the public, pharmacy is the most accessible face of the healthcare service. Pharmacy teams play a pivotal role as a community and health asset across Ireland, striving for excellence in public health care and driving delivery of services focused on prevention, health improvement and protection of their local communities.
Much of this work goes unnoticed but the Irish Pharmacy Awards provide the opportunity to honour and applaud these inspirational and deserving professionals and teams. It is through their work that pharmacy is positioned, at the forefront of healthcare, improving the lives of people across the country.





The Irish Pharmacy Awards are brought to you by Irish Pharmacy News, Ireland’s best and most influential independent community pharmacy magazine. These Awards recognise the achievements of individuals and teams working in the community pharmacy sector; their dedication and innovation which positions the profession at the forefront of healthcare, improving the lives of people across the country.
They represent a unique and high-profile opportunity to celebrate the outstanding accomplishments of Ireland’s pharmacy professionals and aim to recognise outstanding examples of high standards, best practice, innovation and excellence.
The Awards recognise outstanding examples of high standards, best practice, innovation and excellence. It has been, and continues to be, the goal of Irish Pharmacy News to recognise the exact added value of the contribution made by community pharmacists and their teams.

The sentiment has been echoed by some of the leading healthcare companies here in Ireland and their support is evident in the most positive way possible with their sponsorship of each of our Awards.
Furthermore, acknowledgement must be given to our prestigious judging panel. The shortlisting process this year was extremely difficult – with some strong entries failing even to make it as finalists – but it is important to highlight that it’s not just about winners and finalists. Everyone who entered this year’s Awards deserves commendation.
14 Awards were given out on the night, and a further extra special recognition Award was presented to Mr Martin Gallagher of Clonmel Healthcare who was recognised with the Professional Contribution Award.
46 | PHARMACYNEWSIRELAND.COM
Awards
Awards
Winner Winner
TouchStore Community Pharmacy Technician of the Year Award 2023









It is evident that pharmacy technicians are playing an increasingly important supporting role as pharmacists are increasingly spending more time with patient consultations and engaging local stakeholders.
The shift in emphasis from dispensing to healthcare provision has meant that the wider pharmacy team has to pull together –pharmacy technicians capture the essence of this in everything that they do.
It was Adrian Dunne Balbriggan Pharmacy Technician Dominika Wikowicz that scooped the title on the night.


Dominika Wikowicz is a Senior Technician in Adrian Dunne Pharmacy Balbriggan. She qualified as an EU Pharmacy Technician in Poland and joined Adrian Dunne group in 2019. In this short period of time she has become the most valuable part of the team.
She is hard working and she is totally committed to her job. Her gentle personality has won the hearts of everybody around her in the Pharmacy. The main way Dominika has enhanced the role of the Pharmacy Technician is through her commitment and attention to detail when it comes to stock accuracy. She has achieved the highest live-stock accuracy score in the company.
Speaking after collecting her trophy, Dominika said, “I am speechless at having been awarded this title. I was honoured to have been nominated by my colleagues and to be included with all the finalists here tonight. Over the last number of years, especially during Covid, everyone in pharmacy worked that bit harder and went that extra mile for their patients and customers.
2023 The Irish Pharmacy
2023 The Pharmacy
PHARMACYNEWSIRELAND.COM | 47
“To be recognised for my work gives me great pride and is a real motivating factor in always wanting to do as much as I can do and be the best I can be.”
Sam Farag, Marketing & Communications, TouchStore with Dominika Witowicz, Adrian Dunne Pharmacy, Balbriggan and Simon Racklyeft, Sales Manager, TouchStore
Simon Racklyeft, Sales Manager, TouchStore with winner of the TouchStore Community Pharmacy Technician of the Year Award, Dominika Witowicz, Adrian Dunne Pharmacy Balbriggan
Awards
Winner Winner
The United Drug Business Development (Independent) Award serves to recognise those who have displayed success in terms of sales, training, recruitment, customer service, product development or other areas of business development.









The Award for 2023 was won by Higgins Pharmacy, Sligo.

The initial key challenge for Higgins Pharmacy was to define their own identity and objectives in order to become known to the Sligo people as kind and trusted professionals, like the Higgins family, who had started the business.
The team had a goal to maintain and then grow the business, whilst facing another challenge of having never run a business without support. One of the most important issues for the owners was to ensure a great team, which they feel is core to running a successful pharmacy business.
Deirdre Butler, Pharmacist and Owner, Higgins Pharmacy Sligo with Jean Tomkins, Business Development & Relationship Manager, United Drug
All of the business initiatives which they have started have all impacted greatly on the business and the community they serve. Being accepted onto the ‘Going for Growth’ and ‘Empower’ programmes have really helped in driving our business forward. Their mentorship programmes highlighted areas for improvements and forced the owners to look at areas needing attention.
Deirdre Butler, Owner and Pharmacist said, “We are delighted to win this Award. We have all worked extremely hard in pharmacy, especially during the pandemic and in recent times and to have this recognition is a real validation that we are doing something right.


“I believe that community pharmacy has proven to be the mainstay of healthcare in Ireland and we are proud to be in this profession and doing what we do for patients and for all our customers.
“Everyone here tonight are winners in my eyes. To even be shortlisted for these Awards is an honour as the standard of work and innovation throughout the island in pharmacy is of such a high standard, this is really the icing on the cake for us.”
48 | PHARMACYNEWSIRELAND.COM
United Drug Business Development (Independent) Award 2023 2023 The Irish Pharmacy
Ann Butler and Deirdre Butler, Higgins Pharmacy Sligo and winners of the United Drug Business Development (Independent) Award with Jean Tomkins, Business Development & Relationship Manager, United Drug




Ireland’s No. 1 Provider of Ostomy Products & Services Customer Care Over 20,000 calls handled annually from our customer care team. Complimentary Cutting Service Cutting up to 10,000 bags per week. Training Partnership Ostomy Excellence – we provide a complimentary education programme. Complimentary Wipes and Bags For Pharmacists to offer their patients. Expanding Range We continue to expand our range as Ireland’s leading stockist for Ostomy & Urology products. Contact our Ostomy Care team for further information Telephone: 01 463 2300
Winner Winner
Théa Pharma OTC Counter Assistant of the Year Award 2023










Edel Cullen of Lannon Late Night Pharmacy, Sligo saw off five other finalists to win the title of the Théa Pharma OTC Counter Assistant of the Year 2023.
Country Manager, Théa Pharma Ireland Diarmuid Gavin with winner of the Thea Pharma OTC Counter Assistant of the Year Award, Edel Cullen, Lannon Late Night Pharmacy, Sligo
Edel has had a considerable impact on her pharmacy team. She is familiar with all regular patients and customers and understands their needs and requirements.
Country Manager, Théa Pharma Ireland Diarmuid Gavin with winner of the Thea Pharma OTC Counter Assistant of the Year Award, Edel Cullen, Lannon Late Night Pharmacy, Sligo and Awards Compere, Marty Whelan


As the pharmacy skincare expert Edel has taken on the promotion of events within the store, identifying brands for promotion and organising these events herself. Edel also liaises with suppliers to identify new products that may do well and introduces them into the pharmacy as a trial. Edel has great communication skills and a warm, friendly demeanour that puts patients at ease and has an ability to listen actively and empathise with people.
Her positive attitude and willingness to help have contributed to a positive working environment within the Pharmacy. Edel told us after accepting her Award, “I am speechless and those who know me, know that I am rarely speechless. This Award means the world to me. I love what I do, I love my team and I love my customers. I have to say I put my heart and soul into everything I do for the betterment of our community and to be recognised with this Award is truly so rewarding.
“I was shocked t have been nominated but never in a million years did I think I would take home the title. I would urge anyone thinking of entering these Awards to go for it; me standing here with this trophy just goes to show what can be achieved.”
2023 The Irish Pharmacy 50 | PHARMACYNEWSIRELAND.COM
Awards



www.theapharma.ie Europe’s No 1 dry eye drops Hydrates & Lubricates Repairs & Protects Lasts for up to 4 hours Clinically Proven1 to treat symptoms of dry eyes Available on the GMS Action 2 1 Pinto-Bonilla JC et al. Ther. Clin Risk Manag 2015:11;595-603 2 IQVIA MAT September 2022 Ireland Dry Eyes
Awards
Winner Winner
Alliance Online Pharmacy Retailer of the Year Award 2023










Winning their first Award at the Irish Pharmacy Awards 2023 was Meaghers Pharmacy Group. The team accepted the Alliance Online Pharmacy Retailer of the Year title.


Meaghers Pharmacy is a well-known Irish pharmacy chain with over 11 locations across the country. In addition to their physical stores, they also have an online store that offers a wide range of health and wellness products, including skincare, vitamins, supplements, beauty products, baby care, and more. The pharmacy team support various health needs such as immune support, energy, and joint health and lead the way in areas such as gut health, pain management, and specialised skincare, with a range of innovative products and knowledge that support patient/customer care.
Alongside beauty, lifestyle, and healthcare products, we also offer customers, access to our team of pharmacists, a prescription delivery service, and online sales of OTC medicines. We’re dedicated to providing the same personalised, excellent customer service to our online customers as we do to our in-store customers.
Darren Hardiman, E-commerce and Marketing Director with Meaghers Pharmacy Group commented, “Meaghers Pharmacy Group is a family business, and so to us it is really important that we serve our customers as best we can. We have a really strong relationship with our customers in-store and so winning the Award for Alliance Online Retailer of the Year and gaining recognition for our online offering is really key for us as we continue to grow that side of the business.
“We are delighted and proud to win this Award. Despite being a relatively small team, we have an incredibly large team effort in everything we do and this effort is showcased day in and day out to make sure whatever services we carry out are successful and ultimately, benefit our customers.”
2023
52 | PHARMACYNEWSIRELAND.COM
The Irish Pharmacy
Adrian Curley, Commercial Lead ROI, Alliance Pharmaceuticals Ireland with Oonagh O’Hagan, Managing Director, Eimear McLaughlin, Ecommerce Manager, Andy Murphy and Darren Hardiman, Head of Operations, Marketing and Ecommerce, all from Meaghers Pharmacy Group with Awards Compere Marty Whelan
Winners of the Alliance Online Pharmacy Retailer of the Year Award Meaghers Pharmacy Group Oonagh O’Hagan, Managing Director, Eimear McLaughlin, Ecommerce Manager, Andy Murphy and Darren Hardiman, Head of Operations, Marketing and Ecommerce pictured with Adrian Curley, Commercial Lead ROI, Alliance Pharmaceuticals Ireland
Awards

Winner Winner
BOI Payment Acceptance (BOIPA) Innovation & Service Development (Chain) Award 2023







Meaghers Pharmacy Group celebrated winning two awards on the night, including the BOI Payment Acceptance (BOIPA) Innovation & Service Development Award 2023.

Meaghers Pharmacy are continually evolving to deliver innovative services at the core of their community. The pharmacy team are continuously working on new ideas and services that they can bring to their pharmacies to improve the level of care which is provided to each and every one of their patients.
Medication non-adherence is a widespread issue leading to reduced quality of life in patients, increased hospital admissions, premature deaths and increasing healthcare costs. In 2022, Managing Director Oonagh O’Hagan and Superintendent Pharmacist, Elaine Lillis, wanted to highlight the key role pharmacists can play in tackling this issue and so began discussions to identify a new service that they could offer in the pharmacies to set about doing this.
Speaking on behalf of the Group, Managing Director Oonagh O’Hagan said, “At Meaghers Pharmacy group we are always thriving to push forward and the Irish Pharmacy Awards give us the opportunity to benchmark ourselves against the best in class.

“There was some really stiff competition within this category, from the entrants to the finalists and we are honoured to have been recognised but it’s amazing to have actually collect and win this Award as I believe it really shows the level of innovation within Meaghers Pharmacy Group.
“I must say this was a real team effort and a huge shout of thanks goes to Elaine Lillis, our Superintendent Pharmacist and her colleague Grace Grimes as they and their team came up with the idea, worked with stakeholders to implement the idea and launched the partnership with HealthBeacon to drive compliance in community pharmacy.”
PHARMACYNEWSIRELAND.COM | 53
2023
The Irish Pharmacy
Elaine Lillis, Superintendent Pharmacist, Oonagh O’Hagan, Managing Director and Grace Grimes, Supervising Pharmacist, all from Meaghers Pharmacy Group with Barry Gray, Marketing Director, BOI Payment Acceptance
Grace Grimes, Supervising Pharmacist and Elaine Lillis, Superintendent Pharmacist, Meaghers Pharmacy Group, winners of the BOI Payment Acceptance (BOIPA) Innovation & Service Development (Chain) Award with Barry Gray, Marketing Director, BOI Payment Acceptance
Winner Winner
Uniphar Category Development of the Year Award 2023



The CarePlus Pharmacy Network scooped the Uniphar Category Development Award for 2023





their second Award of the evening.
Winners of the Uniphar Category Development Award for the CarePlus Pharmacy Network Women’s Health initiative, Laura Downey, Buyer, Axium Buying Group and Breege McTigue, Director of Customer Engagement, Navi Group with Brian Keogh, Commercial Business Unit Manager, Uniphar


Breege McTigue, Director of Customer Engagement, Navi Group, Laura Downey, Buyer, Axium Buying Group and Brian Keogh, Commercial Business Unit Manager, Uniphar
–
They won this category for their Women’s Health initiative rolled out across all their stores.
Navi identified an opportunity in the market to educate female customers on hormonal health and how pharmacies can assist in identifying their specific nutritional needs based on their age and current symptoms.
By arming the pharmacy staff with up-to-date knowledge on women’s health and specific products available, CarePlus pharmacies became an important destination for women’s to get advice and to improve their lives. The key areas focused on were periods, perimenopause and menopausecovering the full female customer base.
Breege McTigue, Director of Customer Engagement with Navi Group commented, “Our pharmacy teams on the ground are really central to everything we do and so we are continuously looking at the best ways to educate and engage with them across all our products and across all our categories in community pharmacy.
“We are thrilled to have won this Award as it shows the work being put into this being rewarded and recognised. Customers in pharmacy are looking for a solution to a problem, whatever that may be and our CarePlus pharmacists and pharmacy teams are perfectly positioned to solve that with them.”
Laura Downey, Category Manager with Navi Group added, “Winning this Award is fantastic for us and provides a great platform for generating positivity around category development; particularly for us with the women’s health category as we have been doing so much work in this area for our teams.
“Taking this Award shows that we are going in the right direction with that.”


2023 The Irish Pharmacy 54 | PHARMACYNEWSIRELAND.COM
Awards
Winner Winner
Haleon Self-Care Award 2023





















North Road Pharmacy in Drogheda celebrated winning the Haleon Self-Care Award 2023.

The team at North Road Pharmacy have focused in the last 2 years on achieving a healthy lifestyle through exercise, healthy eating and taking positives steps in self-care, whether that is taking vitamins and supplements, taking up a sport or seeking advice from the local GP if struggling with life in general. This has led patients and customers awareness to alternative ranges they currently stock. The alternative route has been a key focus moving forward and the introduction of a Sports and wellbeing department has been instrumental in the growth of the other areas in the front of shop sales overall as proven from the positive feedback from customers which translated into strong growth in terms of sales in these particular areas.
North Road Pharmacy is now recognised and has the reputation for taking care of all their customers and patients and continually strive to be the best in health care in the local pharmacy community.








Siobhan Taylor, Owner and Managing Director said on winning the accolade, “I am delighted to accept the Haleon Self-Care Award on behalf of the whole team from North Road Pharmacy. Each and every member of the team work diligently and with a shared goal of providing the best service and care for our customers and for the community. Self-Care and the promotion of self-care is something we feel very passionate about and so to receive this recognition is the best feeling.

“The entire night has been a fantastic celebration, we have had so much fun spending time with our team and with our wider colleagues but receiving this trophy just tops it all off.
“Our pharmacy is at the heart of the community, playing a critical role in the care and wellbeing of every individual and we can’t wait to bring this Award home and will be proud to show what we have achieved working together.”
Introducing...
MGK9299 Haleon Trade Ads.pdf 1 10/08/2022 14:58
Haleon, formerly part of GSK
2023 The Irish Pharmacy PHARMACYNEWSIRELAND.COM | 55
Awards
Maria McClafferty, Pharmacy Channel Controller, Haleon with winners of the Haleon Self-Care Award Suzanne O’Reilly, Pharmacy Manager and Siobhan Taylor, Owner and Managing Director, North Road Pharmacy
Winner Winner
Easolief DUO Business Development (Chain) Award 2023








Winning their first Award of the night was CarePlus Pharmacy Network and the Easolief DUO Business Development (Chain) Award for their Baby Club project.
The introduction of the CarePlus Baby Club has tackled numerous challenges, including brand loyalty and brand differentiation. The creation of the Baby Club, from the free gift to the monthly exclusive offers and e-newsletters, to baby events, to the Care+Conversations podcast and content on social media and website, has provided a safe space and support network for parents within communities to feel as though there is support, not only in their local CarePlus Pharmacy, but beyond that and online.
The CarePlus Pharmacy Network team are continually learning and progressing all the time from customer and member feedback, putting the customer first, while also adapting to market change and sustainability initiatives.

Winners of the Easolief DUO Business Development (Chain) Award CarePlus Pharmacy Network’s James Turner, Business Development Manager, CarePlus Pharmacy & Axium Buying Group, Siofra Donnellan, Brand Manager, Navi Group and Samantha Doundoulakis, Sales & Marketing Manager, Clonmel Healthcare


James Turner commented on behalf of CarePlus Pharmacy Network, “CarePlus Network relaunched our baby category and baby club earlier this year with a whole new motivation behind it and so it is fantastic to receive this Award tonight in recognition of the work that has gone into that.
“The entire team has put a lot of hard work and thought into this project and really worked towards creating better outcomes for parents and for our communities that rely on the baby category.
“When you put that level of effort into work on a daily basis it can feel ‘normal’ and it sometimes is easy to forget the impact you are making to people’s lives and so these Awards have really brought to the fore the excellence and dedication being carried out and the benefits felt across communities in Ireland.”

2023 The Irish Pharmacy 56 | PHARMACYNEWSIRELAND.COM
Awards
James Turner, Business Development Manager, CarePlus Pharmacy & Axium Buying Group, Siofra Donnellan, Brand Manager, Navi Group and Samantha Doundoulakis, Sales & Marketing Manager, Clonmel Healthcare
DUAL ACTION
References: 1. Merry A, et al. AFT-MX-1, a prospective parallel group, double-blind comparison of the analgesic effect of a combination of paracetamol and ibuprofen, paracetamol alone, or ibuprofen alone in patients with post-operative pain. Department of Anaesthesiology, University of Auckland, New Zealand 2008. *compared with the same daily dose of standard paracetamol or ibuprofen alone.





Easolief DUO 500 mg/150 mg film-coated tablets Each tablet contains paracetamol 500 mg and ibuprofen 150 mg.

Presentation: White, capsule shaped tablet with breakline on one side and plain on the other side. Indications: Shortterm symptomatic treatment of mild to moderate pain. Dosage: Adults/elderly: The usual dosage is one to two tablets taken every six hours up to a maximum of six tablets in 24 hours. Children: Easolief DUO is contraindicated in children under 18 years. Contraindications: Severe heart failure, known hypersensitivity to paracetamol, ibuprofen, other NSAIDs or to any of the excipients, active alcoholism, asthma, urticaria, or allergic-type reactions after taking acetylsalicylic acid or other NSAIDs, history of gastrointestinal bleeding or perforation related to previous NSAID therapy, active or history of recurrent peptic ulceration/haemorrhage, severe hepatic failure or severe renal failure, cerebrovascular or other active bleeding, blood-formation disturbances, during the third trimester of pregnancy. Warnings and precautions: This medicine is for short term use and is not recommended for use beyond 3 days. Clinical studies suggest that use of ibuprofen, particularly at a high dose may be associated with a small increased risk of arterial thrombotic events. Patients with uncontrolled hypertension, congestive heart failure, established ischaemic heart disease, peripheral arterial disease and/or cerebrovascular disease should only be treated with ibuprofen after careful consideration and high doses should be avoided. Careful consideration should be exercised before initiating long-term treatment of patients with risk factors for cardiovascular events. The use of paracetamol at higher than recommended doses can lead to hepatotoxicity, hepatic failure and death. Patients with impaired liver function or a history of liver disease or who are on long term ibuprofen or paracetamol therapy should have hepatic function monitored at regular intervals. Severe hepatic reactions, including jaundice and cases of fatal hepatitis, though rare, have been reported with ibuprofen. Paracetamol can be used in patients with chronic renal disease without dosage adjustment. There is minimal risk of paracetamol toxicity in patients with moderate to severe renal failure. Caution should be used when initiating treatment with ibuprofen in patients with dehydration. The use of an ACE inhibiting drug, an anti-inflammatory drug and thiazide diuretic at the same time increases
the risk of renal impairment. Blood dyscrasias have been rarely reported. Patients on long-term therapy with ibuprofen should have regular haematological monitoring. Like other NSAIDs, ibuprofen can inhibit platelet aggregation. GI bleeding, ulceration or perforation, which can be fatal, has been reported with all NSAIDs at anytime during treatment. Combination therapy with protective agents (e.g. misoprostol or proton pump inhibitors) should be considered. Use with concomitant NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided. NSAIDs may lead to onset of new hypertension or worsening of pre-existing hypertension and patients taking antihypertensive medicines with NSAIDs may have an impaired anti-hypertensive response. Fluid retention and oedema have been observed in some patients taking NSAIDs. NSAIDs may very rarely cause serious cutaneous adverse events such as exfoliative dermatitis, toxic epidermal necrolysis and Stevens-Johnson syndrome. Acute generalised exanthematous pustulosis (AGEP) has been reported in relation to ibuprofencontaining products. Products containing ibuprofen should not be administered to patients with acetylsalicylic acid sensitive asthma and should be used with caution in patients with pre-existing asthma. Adverse ophthalmological effects have been observed with NSAIDs. For products containing ibuprofen aseptic meningitis has been reported only rarely. NSAIDs may mask symptoms of infection and fever. In order to avoid exacerbation of disease or adrenal insufficiency, patients who have been on prolonged corticosteroid therapy should have their therapy tapered slowly rather than discontinued abruptly when products containing ibuprofen are added to the treatment program. Interactions: Warfarin, medicines to treat epilepsy, chloramphenicol, probenecid, zidovudine, medicines used to treat tuberculosis such as isoniazid, acetylsalicylic acid, other NSAIDs, medicines to treat high blood pressure or other heart conditions, diuretics, lithium, methotrexate, corticosteroids. Fertility, pregnancy and lactation: Easolief DUO is contraindicated during the third trimester of pregnancy. Driving and operation of machinery: Dizziness, drowsiness, fatigue and visual disturbances are possible after taking NSAIDs. If affected patients should not drive or operate machinery. Undesirable effects: Dizziness, headache, nervousness, tinnitus, oedema, fluid retention, abdominal pain, diarrhoea, dyspepsia, nausea, stomach discomfort, vomiting, flatulence, constipation, slight gastrointestinal blood loss, rash, pruritus, alanine aminotransferase increased, gamma-glutamyltransferase increased, abnormal liver function tests, blood creatinine increased and blood urea increased. Refer to Summary of Product Characteristics for other adverse effects. Adverse reactions should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Pack size: 24 tablets. Marketing authorisation holder: Clonmel Healthcare Ltd. Marketing authorisation number: PA0126/294/1. Supply through pharmacies only. Date last revised: June 2022. Date of preparation: July 2022. 2022/ADV/EAS/197H.
NOW AVAILABLE TO ORDER Call our freephone order line on 1800 26 26 26
analgesic brand that is clinically proven to provide 30% more effective pain relief for your patients1* IN ONE TABLET
An
Winner Winner
Point of Care Testing Pharmacy of the Year Award 2023










Mark’s Pharmacy, Ardee landed the title of Point of Care Testing Award for 2023, securing the top spot against three other finalists.
Mark’s community pharmacy team exemplifies exceptional team spirit and dedication to enhancing patient care at all levels. Working together efficiently and effectively, they create a collaborative and supportive environment, fostering trust and respect among team members and empowering each individual to contribute their unique expertise towards shared goals.
Innovation and forward-thinking are central to their operations. The team unites around common objectives. This focused and unified approach to healthcare initiatives has led to numerous successful projects, showcasing our excellence in quality, innovation, productivity, and prevention.
One example of these collaborative efforts is the establishment of point-of-care testing services, such as diabetes screening, to promote prevention, early detection, and disease management. This initiative demonstrates their ability to work together to improve patient access to care through effective and rapid technology.
Pharmacist and Owner Mark McPhillips told us this award was a team effort. “As an independent pharmacy we are delighted to have won this Award; the team always have a great night. This is a unique opportunity for us to not only come together as a team but to join with our colleagues across Ireland in celebrating the innovation and hard work that is ongoing.
“What is truly really important about the Irish Pharmacy Awards is that they put pharmacists and their teams in a position whereby we can represent our profession on a national level and in a position to advance where we, as pharmacists, can ultimately be.”

2023 The Irish Pharmacy 58 | PHARMACYNEWSIRELAND.COM
Awards
Linda Callaghan, Jennifer Trainer, Mark McPhillips, Pharmacist and Owner, Gemma McCormick, Irene Caffery and Tara McKenna from Mark’s Pharmacy, Ardee, winners of the Point of Care Testing Award
Linda Callaghan, Jennifer Trainer, Mark McPhillips, Pharmacist and Owner, Gemma McCormick, Irene Caffery and Tara McKenna from Mark’s Pharmacy, Ardee, winners of the Point of Care Testing Award with Kelly Jo Eastwood, Editorial Director, IPN Communications
Awards
Tom has a proven track record of consistently delivering high-quality services, fostering a positive working environment for all and contributing to the wider pharmacy industry. He is always looking for new ways of improving and developing the way he works. Outside of his incredibly busy job, Tom is involved with the Pharmacy Contractors Committee with the IPU and the Workforce task group. He is part of the IPU IT steering group. Tom is constantly striving for improvement in pharmacy in Ireland and being directly involved with these committees and steering groups will shape the community pharmacy of the future.
Over the past 12 months, one of Tom’s main focusses has been the re-introduction and improvement of health services provided in pharmacy post pandemic and to launch new and innovative services across the pharmacy network. Under Tom’s skillful guidance, updated SOP’s and training, Health Services such as Blood pressure, Cholesterol and glucose testing have all been successfully reintroduced into all 115 Hickey’s, Allcare and Cara pharmacies.
Working from feedback from his pharmacies, Tom has implemented a pre-prepared system for Healthmail. He has collaborated with the dispensing software provider to utilise and transform the software to best empower the community pharmacist.
After accepting his Award from National Field Sales Controller with Perrigo, Paul Hatton, Tom commented, “I work with a team of approximately 140 pharmacists and my job really is to guide them to best help and assist their patients and so for me, to receive this Award of Perrigo Superintendent Pharmacist of the Year makes me feel really humbled in the company of such excellent fellow nominees, but proud of the job we do.


“All pharmacists work incredibly hard and whilst we do get recognition from our customers and patients – and we do get a ‘Thank You’ quite often, it’s also really super to get the same acknowledgement from your peers.
“Often we work alone in our dispensaries and therefore nights like tonight give us an opportunity to get together and spend time with team members and professional colleagues and to celebrate pharmacy as an industry.”


Winner Winner
Perrigo Superintendent Pharmacist of the Year 2023








PHARMACYNEWSIRELAND.COM | 59
2023 The Irish Pharmacy
Tom Concannon, Hickey’s/Allcare/Life/Cara Pharmacies won the prestigious Perrigo Superintendent Pharmacist of the Year 2023 Award.
Paul Hatton, National Field Sales Controller, Perrigo with Tom Concannon, Hickey’s/Allcare/ Life/Cara Pharmacies – winner of the Perrigo Superintendent Pharmacist of the Year Award
Paul Hatton, National Field Sales Controller, Perrigo with Tom Concannon, Hickey’s/Allcare/Life/ Cara Pharmacies – winner of the Perrigo Superintendent Pharmacist of the Year Award and Awards compere Marty Whelan
Winner Winner
Reckitt Community Pharmacist of the Year Award 2023






As is the case every year, eight deserving finalists battled it out for the crown of Reckitt Community Pharmacist of the Year 2023.
The title went to John O’Shaughnessy, of Woods CarePlus Pharmacy, Mullingar.

John O`Shaughnessy has been a senior pharmacist for five years in Woods Pharmacy – a Keanes’ CarePlus store –on Mullingar’s main street. He is fully invested in the Keane group’s values in relation to the importance of patient wellbeing, and accordingly leaves no stone unturned to ensure that patients receive the best possible care. During the covid years, he introduced antigen testing, followed by covid vaccinations, showing courage at a time of great uncertainty.
John is totally selfless by nature, and puts others before himself, no matter if they are a patient or a staff member. He may be doing 100 jobs at once - but will drop them all to help anyone with anything, whether that be needing help with a prescription - or taking out the bins from the dispensary.
John told us, “To be the winner of the Reckitt Community Pharmacist of the Year is wonderful not only for me, but for my team. We have worked really, really hard to stay at the forefront of healthcare. Covid caused a whole knockdown across the health service and community pharmacy just got on with it and ploughed through it. We are vaccinating, delivering patient focused services and doing everything we can to ensure patients receive un-interrupted care but that also, they get the same type of care they were getting before Covid took place. Our team in particular are well trained for this.
“The work we have done in the last couple of years in particular has all been around patient care and for the healthcare profession as a whole. We are at the front line and we are happy to be at the front line. Pharmacists are the link between patients and doctors and we never closed our doors during Covid.

“The Irish Pharmacy Awards showcase an extremely high standard of excellence; they recognise the efforts of pharmacists and pharmacies nation-wide and they recognise that we have gone through a massive period of turmoil but collectively, we will et through it together. The standards these Awards ask of us are so high, they have become the standards that we hope to continually abide by.”




60 | PHARMACYNEWSIRELAND.COM
2023 The Irish Pharmacy
Awards
John O’Shaughnessy, Woods CarePlus Pharmacy, Mullingar – winner of the Reckitt Community Pharmacist of the Year Award with Adam Lee, Country Manager, Ireland with Reckitt and Awards compere Marty Whelan
John O’Shaughnessy, Woods CarePlus Pharmacy, Mullingar – winner of the Reckitt Community Pharmacist of the Year Award with Adam Lee, Country Manager, Ireland with Reckitt
Awards
Winner Winner
JPA Brenson Lawlor Young Community Pharmacist of the Year
The JPA Brenson Lawlor Young Community Pharmacist of the Year Award recognises rising talent – those individuals who despite being in the early stages of their pharmacy careers are already demonstrating that they can make a difference to the pharmacy profession and the companies for whom they work and the communities they serve.
The Award for 2023 was won by Daniel McCormack, McCauley Health & Beauty Pharmacy, Dungarvan.

Daniel’s proactive approach to administering Covid-19 vaccinations and providing flu vaccinations demonstrates his dedication to improving the health and well-being of his community. By offering weekend clinics for children’s flu appointments, he is accommodating busy parents who may have difficulty attending during the week.
Furthermore, Daniel’s free blood pressure monitoring service shows his commitment to preventing and managing chronic diseases such as hypertension. Overall, Daniel’s actions show his commitment to providing excellent healthcare services to his community and his willingness to go above and beyond to meet their needs. His efforts will undoubtedly have a positive impact on the health and well-being of those he serves.
Commenting after receiving his Award, Daniel stated, “I honestly wasn’t expecting a nomination, I wasn’t expecting to be shortlisted as a finalist and to be standing here now with the JPA Brenson Lawlor Young Community Pharmacist of the Year Award is simply amazing.

“The team we have right now is phenomenal and couldn’t be any better. Everyone puts so much work and effort in and without their help, the help of the wider pharmacy staff and without the help and support of our local community I wouldn’t be standing here now.


“As pharmacists working in the community every day it can be easy to get overlooked as a profession and so these Awards present us with the platform to get together and celebrate achievements across the industry. It makes each of us very proud to be in the job we are in.”







2023 The
Pharmacy
Irish
Jason Bradshaw, Partner, JPA Brenson Lawlor with Daniel McCormack, McCauley Health & Beauty Pharmacy, Dungarvan - winner of the JPA Brenson Lawlor Young Community Pharmacist of the Year Award and Julieanne Moore, McCauley Health & Beauty Pharmacy
PHARMACYNEWSIRELAND.COM | 61
Jason Bradshaw, Partner, JPA Brenson Lawlor with Daniel McCormack, McCauley Health & Beauty Pharmacy, Dungarvanwinner of the JPA Brenson Lawlor Young Community Pharmacist of the Year Award
Awards
Professional Contribution Award






Winner Winner
In an Irish Pharmacy Awards first, an additional, surprise Award, was presented on the night to a very deserving albeit in the dark recipient.



Martin Gallagher, the former Director of Marketing and Business Development with Clonmel Healthcare was awarded the Professional Contribution Award on the night – a one-off accolade designed with him solely in mind.

Earlier this year Martin announced his retirement after working within the industry for over 40 years and as a key stakeholder sponsor of the Awards and avid supporter of everything they stood for from their inception, we simply could not let the occasion pass un-marked.

The relationship between the pharmacy profession and the pharmaceutical industry is a crucial one; as both work together to ensure patients receive the best possible care and towards improve patient outcomes.
Saying a few words about Martin’s contribution to the industry as a whole and his legacy, was Barry Fitzpatrick, Director of Sales with Clonmel Healthcare.
Barry Fitzpatrick, Director of Sales, Clonmel Healthcare presents the Professional Contribution Award to Martin Gallagher, former Director of Marketing & Business Development, Clonmel Healthcare
Martin Gallagher said, “Receiving this Award was something I totally wasn’t expecting to happen tonight but it was extraordinary to have done so. To be awarded this amongst my peers and among the people I served and Clonmel Healthcare has served over so many years is a wonderful surprise and I feel immensely honoured.
“Having just retired after 45 years in the industry, I have witnessed first hand how pharmacy has passed its way through quite a number of gates over that period of time. I have also attended the Irish Pharmacy Awards since their inception and I have enjoyed watching them grow and reflect in turn the growth in pharmacy. IPN has essentially built on the excellence of pharmacy and these awards play a very important annually with regards to recognising the progression of the profession of pharmacy.”
62 | PHARMACYNEWSIRELAND.COM
Martin Gallagher, recipient of the Professional Contribution Award
2023
Irish
The
Pharmacy
Awards
Winner Winner
McLernons Independent Community Pharmacy of the Year Award 2023



A brand new Award for 2023 was the McLernons Independent Community Pharmacy of the Year, one of the most hotly contested this year.
The Award was won by The Pharmacy Hub, Tallaght.








Pharmacy Hub has collaborated on a number of projects and initiatives within the community. They recently offered a comprehensive health check for a local business and completed a seasonal flu campaign in local schools. The major annual collaboration is Tallaght Health Awareness Month, which is every October.
Pharmacy Hub opened in 2018 with a vision of creating an independent pharmacy providing a personal customer focus. The team has since then built from 4 to 10 anad continue to look forward, embrace change and embed deep roots in their community.
Pharmacy Hub is driven by passion, hard work, dedication, exceptional customer service and innovative thinking. Pharmacy Hub has been recognised and awarded for its attributes within the local community and comprises of a proud team built on traditional core values, considering themselves as a ‘pharmacy family’ within the Hub.
Michelle O’Hagan, Pharmacist and Owner said, “I can speak on behalf of the whole team when I say we feel honoured to receive the McLernons Independent Pharmacy of the Year Award. We have been so are excited and motived about even just being shortlisted as finalists and everyone in our community has got behind us.
“As everyone is aware, the last few years have been incredibly challenging for community pharmacy but we have all stepped up. Winning this Award has just brought us to the next level of excellence, as the Irish Pharmacy Awards showcase the prestige associated with this profession at a national level.”

2023
The Irish Pharmacy
The team from The Pharmacy Hub, Tallaght with Robin Hanna, Sales Director, McLernons
PHARMACYNEWSIRELAND.COM | 63
Michelle O’Hagan, Pharmacy Hub Owner/Superintendent Pharmacist along with Robin Hanna, Sales Director, McLernons
Winner Winner
Viatris Community Pharmacy Team of the Year Award 2023
Once again, eight finalists were in contention for the Viatris Community Pharmacy Team of the Year Award.

The key to any successful pharmacy is Teamwork and this award recognises the power and potential of a focused and unified approach to healthcare initiatives. This Award is targeted at the community pharmacy team that demonstrates the best combination of team spirit and enhancement of patient care at all levels. Mark’s Pharmacy, Ardee were delighted to win the title for 2023, making it their second Award of the evening.
The diverse skillset and responsibilities of each team member at Mark’s Pharmacy contributed to the successful execution of the vaccination service project and the ongoing growth and success of the business. Through collaboration, communication, and a shared commitment to excellence, the team consistently delivers exceptional patient care and service to their community.
Winners of Viatris Community Pharmacy Team of the Year Award from Mark’s Pharmacy, Ardee with Mark McPhillips, Pharmacist & Owner (centre) and Melissa Fisher, General Manager, Viatris (3rd left)


This community pharmacy team excels in team spirit and enhancing patient care at all levels. They consistently collaborate and support each other, fostering an environment of trust and respect that allows each member to contribute their unique skills towards shared goals.
Pharmacist and owner, Mark McPhillips said, “To win this Award has been outstanding. The team at our pharmacy, Mark’s Pharmacy, has made the difference; that difference being the support and work of the people around me.
Winning the Viatris Community Pharmacy Team of the Year has been eleven years in the making, and it is all to do with the people that are around me.
“I am truly overwhelmed and cannot wait to get this trophy home to the pharmacy store.”








64 | PHARMACYNEWSIRELAND.COM
2023 The Irish Pharmacy
Awards
Winners of Viatris Community Pharmacy Team of the Year Award from Mark’s Pharmacy, Ardee with Mark McPhillips, Pharmacist & Owner (centre) and Melissa Fisher, General Manager, Viatris (2nd right) and Awards compere Marty Whelan
ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing Viagra Connect (sildenafil) 50 mg film-coated tablets Indications, Dosage and Administration: Indications: For erectile dysfunction in adult men. Dosage and Method of use: Adults: one 50 mg tablet taken with water approx. one hour before sexual activity. The maximum dosing frequency is once per day. The onset of activity may be delayed if taken with food. Patients should be advised that they may need to take Viagra Connect a number of times on different occasions (max of one 50 mg tablet per day), before they can achieve a penile erection satisfactory for sexual activity. If patients are still not able to achieve a sufficient penile erection they should be advised to consult a doctor. Elderly: no dosage adjustments required (≥ 65 years old). Renal Impairment: No dosage adjustments for patients with mild to moderate renal impairment. Dosage adjustments required for those with severe renal impairment, see SmPC. Hepatic Impairment: Dosage adjustments required for those with mild-moderate hepatic impairment, see SmPC. Viagra Connect is contraindicated for patients with severe hepatic impairment (see contraindications). Presentation: Blue, rounded diamond-shaped film-coated tablets measuring 11.2 mm x 8.1 mm, marked “PFIZER” on one side and “V50” on the other. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Co-administration with nitric oxide donors (such as amyl nitrite), nitrates, ritonavir, guanylate cyclase stimulators (such as riociguat) is contraindicated. Agents for the treatment of erectile dysfunction, including sildenafil, should not be used by those men for whom sexual activity may be inadvisable, and these patients should be referred to their doctor. This includes patients with severe cardiovascular disorders such as a recent (6 months) acute myocardial infarction (AMI) or stroke, unstable angina or severe cardiac failure. Sildenafil should not be used in patients with severe hepatic impairment, hypotension (blood pressure < 90/50 mmHg) and known hereditary degenerative retinal disorders such as retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases). Sildenafil is contraindicated in patients who have loss of vision in one eye because of non-arteritic anterior ischaemic optic neuropathy (NAION), regardless of whether this episode was in connection or not with previous PDE5 inhibitor exposure. Viagra Connect should not be used in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie's disease). Viagra Connect is not indicated for use by women. The product is not intended for men without erectile dysfunction. This product is not intended for men under 18 years of age. Warnings and precautions: Erectile dysfunction can be associated with a number of contributing conditions, e.g. hypertension, diabetes mellitus, hypercholesterolaemia or cardiovascular disease. As a result, all men with erectile dysfunction should be advised to consult their doctor within 6 months for a clinical review of potential underlying conditions and risk factors associated with erectile dysfunction (ED). If symptoms of ED have not improved after taking Viagra Connect on several consecutive occasions, or if their erectile dysfunction worsens, the patient should be advised to consult their doctor. Cardiovascular risk factors: Since there is a degree of cardiac risk associated with sexual activity, the cardiovascular status of men should be considered prior to initiation of therapy. Agents for the treatment of erectile dysfunction, including sildenafil, are not recommended to be used by those men who with light or moderate physical activity, such as walking briskly for 20 minutes or climbing 2 flights of stairs, feel very breathless or experience chest pain. For a list of patients who are considered at low cardiovascular risk from sexual activity see SmPC. Patients previously diagnosed with the following must be advised to consult with their doctor before resuming sexual activity: uncontrolled hypertension, moderate to severe valvular disease, left ventricular dysfunction, hypertrophic obstructive and other cardiomyopathies, or significant arrhythmias. Sildenafil has vasodilator properties, resulting in mild and transient decreases in blood pressure. Patients with increased susceptibility to vasodilators include those with left ventricular outflow obstruction (e.g. aortic stenosis), or those with the rare syndrome of multiple system atrophy manifesting as severely impaired autonomic control of blood pressure. Priapism: Patients who have conditions which may predispose them to priapism (such as sickle cell anaemia, multiple myeloma or leukaemia), should consult a doctor before using agents for the treatment of erectile dysfunction, including sildenafil. Prolonged erections and priapism have been occasionally reported with sildenafil in post-marketing experience. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. Concomitant use with other treatments for erectile dysfunction is not recommended. Effects on vision: Patients should be advised that in the event of any sudden visual defect, they should stop taking Viagra Connect and consult a physician immediately. Concomitant use with CYP3A4 inhibitors: patients should be advised to consult a doctor before taking Viagra Connect as a 25 mg tablet may be more suitable for them. Concomitant use with alpha-blockers: Caution is advised when sildenafil is administered to patients taking an alpha-blocker, as the co-administration may lead to symptomatic hypotension in a few susceptible individuals. This is most likely to occur within 4 hours post sildenafil dosing. In order to minimise the potential for developing postural hypotension, patients should be hemodynamically stable on alpha-blocker therapy prior to initiating sildenafil treatment. Thus, patients taking alpha blockers should be advised to consult their doctor before taking Viagra Connect. Treatment should be stopped if symptoms of postural hypotension occur, and patients should seek advice from their doctor on what to do. Effect on bleeding: the use of sildenafil is not recommended in those patients with history of bleeding disorders or active peptic ulceration, and should only be administered after consultation with a doctor. Hepatic impairment: Patients with hepatic or renal impairment must be advised to consult their doctor before taking Viagra Connect, since a 25 mg tablet may be more suitable for them. Lactose: The film coating of the tablet contains lactose. Viagra Connect should not be administered to men with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per tablet. Patients on low sodium diets can be informed that this medicinal product is essentially ‘sodium-free’. Use with alcohol: Drinking excessive alcohol can temporarily reduce a man's ability to get an erection. Men should be advised not to drink large amounts of alcohol before sexual activity. Interactions with other medicinal products and other forms of interaction: Individuals receiving concomitant treatment with CYP3A4 inhibitors must be advised to consult their doctor before taking Viagra Connect, dosing adjustments may be required, see SmPC. Patients receiving alpha blocker treatment should be stabilised on therapy prior to initiating sildenafil treatment and must be advised to consult their doctor before taking Viagra Connect as dosing adjustments may be required, see SmPC. Caution when sildenafil is initiated in patients treated with sacubitril/valsartan, see SmPC. Fertility, pregnancy and lactation: There was no effect on sperm motility or morphology after single 100 mg oral doses of sildenafil in healthy volunteers. Viagra Connectis not indicated for use by women. Undesirable effects: Very common (≥1/10): headache. Common (>1/100, <1/10): dizziness, visual colour distortions, visual disturbance, vison blurred, flushing, hot flush, nasal congestion, nausea, dyspepsia. For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC. Legal Category: Not subject to medical prescription. Supply through pharmacies only. Marketing Authorisation Number: PA23055/016/001 Marketing Authorisation Holder: Upjohn EESV, Rivium Westlaan 142, 2909 LD Capelle aan den

Reporting of adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse reactions/events should also be reported to the marketing autorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267.
www.viagraconnect.ie For more information please contact Viatris or refer to the SmPC for full prescribing information HELPS YOU GET AND KEEP AN ERECTION VIAC-2023-0099. DOP: May 2023
17. Phone 01 8322250 Date of Revision of Abbreviated Prescribing Information: 03 Feb 2023
IJssel, Netherlands. Full prescribing information available on request from: Viatris, Dublin
Reference Number: IE-AbPI-ViagraConnect-v003
VIATRIS
Helps erection
Irish Pharmacy AwardsThe Best of Pharmacy Awards

Over 650 pharmacy and industry professionals were in attendance as winners across 14 categories were announced at the annual Irish Pharmacy Awards.





Each year Irish Pharmacy News, Ireland’s only independent monthly publication for community pharmacy, celebrates and acknowledges the achievements of teams and individuals within the pharmacy profession.
The twelfth annual Irish Pharmacy Awards took place on Saturday, May 27th 2023. Hosted by Marty Whelan, the awards ceremony took place in the Clayton Hotel, Burlington Road, Dublin. The Irish Pharmacy Awards recognise the achievements of individuals and teams working in the community pharmacy sector; their dedication and innovation which positions the profession at the forefront of healthcare, improving the lives of people across the country.
Pictured are some of those who attended.
2023
The Irish Pharmacy
1 2 3 4 5 6
1. Barbara Kelly and Aislinn Lee, Excel Recruitment 2. The team from Rye Pharmacy, Leixlip 3. The pharmacy team from Adrian Dunne Pharmacy Group 4. Senan Blake, Thèa Pharma and Angela Hanlon, Foynes Pharmacy 5. The pharmacy team from TCP Home Care 6. Fionan Lynagh and Kyle McCartney, Meaghers Pharmacy Group





 1: Ciara Maher, Ciara Mockler and Mary Theresa Mockler from Mockler’s Pharmacy, Thurles 2. Stuart Brown, Stephen Duncan and team from PharmaConex 3. Nial Tully, Tully totalhealth Pharmacy with Sheena Mitchell, Milltown totalhealth Pharmacy with Vincent Tully, Ross Tully and Maura Tully 4. Vanessa Keena, Ewa Borowska, and Marianna Magiera, Cunningham’s Pharmacy Athlone 5. Avril Farrell and Leanne Walsh, Boots Ireland
1: Ciara Maher, Ciara Mockler and Mary Theresa Mockler from Mockler’s Pharmacy, Thurles 2. Stuart Brown, Stephen Duncan and team from PharmaConex 3. Nial Tully, Tully totalhealth Pharmacy with Sheena Mitchell, Milltown totalhealth Pharmacy with Vincent Tully, Ross Tully and Maura Tully 4. Vanessa Keena, Ewa Borowska, and Marianna Magiera, Cunningham’s Pharmacy Athlone 5. Avril Farrell and Leanne Walsh, Boots Ireland
2 1 3 4 6 5
6. The team from Eurosales International






2 1 3 4 6 5
2023 The Irish Pharmacy
1. Enjoying the Irish Pharmacy Awards 2023 2. Fathimah Kara, Hawwa Kara and the team from Reidy’s Pharmacy, Rathcoole 3. The pharmacy team from Chemist Warehouse 4. Giovanni Bonina, Leva Bonina, Giuseppe Di Gregorio and Alessandra Pastore, Village Green Pharmacy 5. Veronica Carragher, Jean Tomkins, United Drug with guest at the Irish Pharmacy Awards 6. Michelle O’Hagan with her team from The Pharmacy Hub Tallaght
Awards





 1. The pharmacy team from Dunville Pharmacy, Dublin 2. Oonagh O’Hagan, Managing Director, Meaghers Pharmacy Group and guest
1. The pharmacy team from Dunville Pharmacy, Dublin 2. Oonagh O’Hagan, Managing Director, Meaghers Pharmacy Group and guest
1 2 4 3 5 6
3. The team from MedPharm & Med Doc 4. Enjoying the Irish Pharmacy Awards 2023 5. The team from Uniphar 6. Aoife Connaughton, Iris and Jayme O’Neill, Hickeys Pharmacy with Sarah and Michaela Harte, Life Pharmacy






2 1 3 4 6 5 Awards 2023 The Irish Pharmacy
1. All smiles at the 2023 Irish Pharmacy Awards 2. Diarmuid Gavin, Country Manager and his team from Thea Pharma 3. Robin Hanna, Sales Director and the team from McLernon Computers 4. The pharmacy team from McCauley Health & Beauty Pharmacy 5. Pictured at the Irish Pharmacy Awards 6. The team from BOI Payment Acceptance





 1. The team from Viatris 2. Jessica Edwards, Jennifer Hilton, Elizabeth and Paul Hatton, Perrigo 3. The pharmacy team from McCabes Pharmacy Group
1. The team from Viatris 2. Jessica Edwards, Jennifer Hilton, Elizabeth and Paul Hatton, Perrigo 3. The pharmacy team from McCabes Pharmacy Group
1 2 4 3 5 6
4. Michelle Dempsy and Sinead Dempsey, Ballisodare Pharmacy, Sligo 5. The pharmacy team from Hickey’s Pharmacy Johnstown 6. Feilim Henry and Aaron Carlyle, Chemist Connect
Urology: Overactive Bladder
The overactive bladder complex: management and

case studies
Assessment
We need to tailor investigations to suit the individual, their environment, lifestyle, age, health, expectations, and the mode of treatment we wish to prescribe. A 20-year-old female with OAB may be particularly bothered by troublesome nocturia as she needs to wake early and work a full day, or by nocturnal enuresis which can affect new relationships. On the other hand, an 80-year-old man might be most upset if he cannot visit friends/relatives without the psychosocial embarrassment of urge associated incontinence.
Delirium
Infection
Atrophic vaginitis/urethritis (females)
Pharmaceuticals
Psychological problems
Excess urine output
Reduced mobility
Stool impaction.
The idiopathic overactive bladder (OAB) is a clinically-diagnosed symptom complex consisting of frequency, urgency and nocturia with or without urge associated urinary continence. This complex assumes no underlying cause as a source of bladder dysfunction. The alternate term of detrusor overactivity on the other hand is a urodynamic diagnosis based on cystometrography (CMG) – previously termed bladder instability – which is characterised by an unprovoked rise in detrusor pressure during the filling phase of a CMG, and is often associated with a clinical feeling of urgency in a normally sensate individual.
There have been challenges in determining what is defined by normal, and the International Continence Society (ICS) now suggests that clinical deterioration is what is perceived by the patient, and leads them to seek medical help. In addition to ‘normal’ voiding patterns during the day, the ICS has qualified this as a “sudden and compelling desire to pass urine, which cannot be deferred”, and may lead to urinary incontinence.1 Epidemiological studies have found that the prevalence of this condition is rising, and now affects approximately 16% of European and American populations.2
Diagnosis and management are important in minimising OAB’s impact on activities of daily living. The burden of OAB is even heavier in the older population.
The symptoms can be defined as the following:
Urgency – sudden, compelling desire to void that is difficult to defer
Daytime frequency – the need to frequently urinate during the day
Nocturia – waking up at night to void
Urgency urinary incontinence may be present but is not necessary for a diagnosis of OAB.
Overactive bladder will become an increasingly prevalent problem as populations age. It is associated with additional health risks in older patients, particularly with falls and fractures. It is associated with increased mortality and may be a predictor of institutionalisation (especially in the presence of urinary incontinence).
Older patients are less likely to discuss their OAB symptoms with their healthcare provider, and are more likely to be untreated or undertreated. OAB may interfere with social and occupational activities.
It is very important however, to exclude urinary tract infections and other associated differential diagnoses before applying a label of an overactive bladder (see Table 1). An important differential for reversible causes of urinary incontinence can be remembered by the mnemonic ‘DIAPPERS’:
It is important that urinary tract infections be identified and adequately treated prior to the undertaking of further investigations or prescribing pharmacological treatment for OAB. To this end, it is important to consider the fastidious organisms mycoplasma hominis, ureaplasma urealyticum, and chlamydia trachomatis, which are commonly found in both urine and vaginal discharge. As these organisms are fastidious, they must be specifically cultured, and an index
Urological
UTI
Urethral syndrome
Detrusor overactivity
Bladder tumour
Bladder calculus
Gynaecological
Cystocele
Previous pelvic surgery
Genital
Urethritis
Vulvo-vaginitis
Urethral caruncle
Medical
Upper motor neurone lesion
Impaired renal function
Congestive heart failure
General
Excessive intake
Pregnancy
Urethral diverticulum
Small capacity bladder
Interstitial cystitis
Radiation cystitis/fibrosis
Chronic residual
Pelvic mass (fibroids)
Atrophy
Herpes
Warts
Diabetes mellitus
Diabetes insipidus
Anxiety Habit
PHARMACYNEWSIRELAND.COM 72
Dr Fardod O'Kelly, Clinical Fellow in Paediatric Urological Surgery, RCSI
Table 1. Differential diagnosis of OAB
We need to tailor investigations to suit the individual, their environment, lifestyle, age, health, expectations, and the preferred mode of treatment
50mgs once daily
BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets.
Her 10th shopping trip since the day she started BETMIGA1
Prescribing Information: BETMIGA™ (mirabegron)
For full prescribing information, refer to the Summary of Product Characteristics (SPC). Name: BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets. Presentation: Prolongedrelease tablets containing 25 mg or 50 mg mirabegron. Indication: Symptomatic treatment of urgency, increased micturition frequency and/or urgency incontinence as may occur in adult patients with overactive bladder (OAB) syndrome. Posology and administration: The recommended dose is 50 mg orally once daily in adults (including elderly patients). Mirabegron should not be used in paediatrics for OAB. A reduced dose of 25 mg once daily is recommended for special populations (please see the full SPC for information on special populations). The tablet should be taken with liquids, swallowed whole and is not to be chewed, divided, or crushed. The tablet may be taken with or without food. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SPC. Severe uncontrolled hypertension defined as systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 110 mm Hg. Warnings and Precautions: Renal impairment: BETMIGA has not been studied in patients with end stage renal disease (eGFR < 15 ml/min/1.73 m2 or patients requiring haemodialysis) and, therefore, it is not recommended for use in this patient population. Data are limited in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2); based on a pharmacokinetic study (see section 5.2 of the SPC) a dose of 25 mg once daily is recommended in this population. This medicinal product is not recommended for use in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC).
Hepatic impairment: BETMIGA has not been studied in patients with severe hepatic impairment (ChildPugh Class C) and, therefore, it is not recommended for use in this patient population. This medicinal product is not recommended for use in patients with moderate hepatic impairment (Child-Pugh B) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC). Hypertension: Mirabegron can increase blood pressure. Blood pressure should be measured at baseline and periodically during treatment with mirabegron, especially in hypertensive patients. Data are limited in patients with stage 2 hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg). Patients with congenital or acquired QT prolongation: BETMIGA, at therapeutic doses, has not demonstrated clinically relevant QT prolongation in clinical studies (see section 5.1 of the SPC). However, since patients with a known history of QT prolongation or patients who are taking medicinal products known to prolong the QT interval were not included in these studies, the effects of mirabegron in these patients is unknown.
Caution should be exercised when administering mirabegron in these patients. Patients with bladder outlet obstruction and patients taking antimuscarinics medicinal products for OAB: Urinary retention in patients with bladder outlet obstruction (BOO) and in patients taking antimuscarinic medicinal products for the treatment of OAB has been reported in postmarketing experience in patients taking mirabegron. A
controlled clinical safety study in patients with BOO did not demonstrate increased urinary retention in patients treated with BETMIGA; however, BETMIGA should be administered with caution to patients with clinically significant BOO. BETMIGA should also be administered with caution to patients taking antimuscarinic medicinal products for the treatment of OAB. Interactions: Caution is advised if mirabegron is co-administered with medicinal products with a narrow therapeutic index and significantly metabolised by CYP2D6. Caution is also advised if mirabegron is co-administered with CYP2D6 substrates that are individually dose titrated. In patients with mild to moderate renal impairment or mild hepatic impairment, concomitantly receiving strong CYP3A inhibitors, the recommended dose is 25 mg once daily. For patients who are initiating a combination of mirabegron and digoxin (P-gp substrate), the lowest dose for digoxin should be prescribed initially (see the SPC for full prescribing information). The potential for inhibition of P-gp by mirabegron should be considered when BETMIGA is combined with sensitive P-gp substrates. Increases in mirabegron exposure due to drug-drug interactions may be associated with increases in pulse rate. Pregnancy and lactation: BETMIGA is not recommended in women of childbearing potential not using contraception. This medicinal product is not recommended during pregnancy. BETMIGA should not be administered during breast-feeding. Undesirable effects: Summary of the safety profile: The safety of BETMIGA was evaluated in 8433 adult patients with OAB, of which 5648 received at least one dose of mirabegron in the phase 2/3 clinical program, and 622 patients received BETMIGA for at least 1 year (365 days). In the three 12-week phase 3 double blind, placebo controlled studies, 88% of the patients completed treatment with this medicinal product, and 4% of the patients discontinued due to adverse events. Most adverse reactions were mild to moderate in severity. The most common adverse reactions reported for adult patients treated with BETMIGA 50 mg during the three 12-week phase 3 double blind, placebo controlled studies are tachycardia and urinary tract infections. The frequency of tachycardia was 1.2% in patients receiving BETMIGA 50 mg. Tachycardia led to discontinuation in 0.1% patients receiving BETMIGA 50 mg. The frequency of urinary tract infections was 2.9% in patients receiving BETMIGA 50 mg. Urinary tract infections led to discontinuation in none of the patients receiving BETMIGA 50 mg. Serious adverse reactions included atrial fibrillation (0.2%). Adverse reactions observed during the 1-year (long term) active controlled (muscarinic antagonist) study were similar in type and severity to those observed in the three 12-week phase 3 double blind, placebo controlled studies. Adverse reactions: The following list reflects the adverse reactions observed with mirabegron in adults with OAB in the three 12-week phase 3 double blind, placebo controlled studies. The frequency of adverse reactions is defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000) and not known (cannot be established from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. The adverse events are grouped by MedDRA system organ class. Infections and infestations:
Common: Urinary tract infection, Uncommon: Vaginal infection, Cystitis. Psychiatric disorders: Not known (cannot be estimated from the available data): Insomnia*, Confusional state*. Nervous system disorders:
Common: Headache*, Dizziness*. Eye disorders: Rare: Eyelid oedema. Cardiac disorders: Common: Tachycardia, Uncommon: Palpitation, Atrial fibrillation. Vascular disorders: Very rare: Hypertensive crisis*. Gastrointestinal disorders: Common: Nausea*, Constipation*, Diarrhoea*, Uncommon: Dyspepsia, Gastritis, Rare: Lip oedema. Skin and subcutaneous tissue disorders: Uncommon: Urticaria, Rash, Rash macular, Rash papular, Pruritus, Rare: Leukocytoclastic vasculitis, Purpura, Angioedema*. Musculoskeletal and connective tissue disorders: Uncommon: Joint swelling. Renal and urinary disorders: Rare: Urinary retention*. Reproductive system and breast disorders: Uncommon: Vulvovaginal pruritus. Investigations: Uncommon: Blood pressure increased, GGT increased, AST increased, ALT increased. * signifies adverse reactions observed during post-marketing experience. Prescribers should consult the SPC in relation to other adverse reactions. Overdose: Treatment for overdose should be symptomatic and supportive. In the event of overdose, pulse rate, blood pressure, and ECG monitoring is recommended. Basic NHS Cost: Great Britain (GB)/Northern Ireland(NI): BETMIGA 50 mg x 30 = £29, BETMIGA 25 mg x 30 tablets = £29. Ireland (IE): POA. Legal classification: POM. Marketing Authorisation number(s): (GB): PLGB 00166/0415-0416. NI/IE: EU/1/12/809/001-006, EU/1/12/809/008-013, EU/1/12/809/015018. Marketing Authorisation Holder: GB: Astellas Pharma Ltd., 300 Dashwood Lang Road, Bourne Business Park, Addlestone, United Kingdom, KT15 2NX. NI/IE: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of Preparation of Prescribing information: January 2023. Job bag number: MAT-IE-BET-2023-00001. Further information available from: GB/NI: Astellas Pharma Ltd, Medical Information: 0800 783 5018. IE: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. For full prescribing information, please see the Summary of Product Characteristics, which may be found at: GB: www.medicines.org.uk; NI: https://www.emcmedicines.com/en-GB/northernireland/; IE: www.medicines.ie.
United Kingdom (GB/NI)

Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018. Ireland
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com.

Date of preparation: January 2023 References: 1. Freeman R, et al. Current Medical Research and Opinion 2017. https://doi.org/10.1080/03007995.2017.1419170. MAT-IE-BET-2023-00002
Urology: Overactive Bladder
Neurological conditions
Systemic conditions
Medication
Multiple sclerosis, spinal cord injury, neurodegenerative disease
Congestive heart failure, diabetes mellitus, constipation
Diuretics, anticholinergics, opioids, calcium channel blockers, cholinesterase inhibitors, alphaagonists, antihistamines
Dietary/lifestyle
Caffeine/alcohol intake, impaired mobility, excessive fluid intake
of suspicion is required. Treatment for these is low-dose tetracycline (doxycycline) for three months, compared to a standard seven to 10 day course of a broad spectrum antibiotic for bacterial organisms.
Frequency-volume chart
The frequency-volume chart, also known as a bladder or voiding diary, is a valuable tool in assessing OAB symptoms; it identifies voiding patterns and the balance between diurnal and nocturnal urinary frequency. They identify those who are drinking a lot of fluid through the day. If the patient’s symptoms are not explained by the voiding diary, one might need to undergo urodynamic investigations, defined as measurements to quantify the ability of the bladder to store and expel urine.
NICE guidelines recommend use of a diary for a minimum of three days to include a combination of work and leisure days.3
Uroflowmetry
Uroflowmetry is the most simple of the urodynamic tests; it is not invasive and is physiological. In normal individuals, the maximum urinary flow rate should be in the region of > 15mL/s for a voided volume of > 150mL. As a rule, a low flow rate implies voiding difficulties, due either to a hypotonic detrusor or to an obstructed outlet, which cannot be differentiated without further assessment. The importance of uroflowmetry in OAB lies in the tendency of the antimuscarinic agents to impair voiding function. Thus, to avoid the possibility of retention, one needs to know the patient’s pre-treatment flow rate as well as postvoid residual urine volume.
CASE STUDY 1
PK is a 70-year-old male who until about a year ago remained quite active. His background history includes an ST-elevation myocardial infarction (seven years ago), and an open radical retropubic prostatectomy two years ago.
Sensitive subjects can be difficult to discuss. As you enquire about his health and how he is recovering since his surgery, you specifically ask if he has any troubles with his bladder. He mentions that these days he has to make sure that wherever he goes there will be a bathroom. As you reassure PK that this is a common concern and that there are ways to treat it, he begins to open up about how the problem has been affecting him. You learn that he has become increasingly restricted in his activity because of his urinary frequency and urgency. He also has occasional urinary incontinence. He is concerned and upset that he can no longer visit his grandchildren because long excursions from home cause him tremendous anxiety about whether he will be able to reach a bathroom as required. Even short trips away from home for such tasks as going into town to shop for necessities are becoming problematic, and he increasingly relies on his wife to do these things. He is beginning to feel isolated and depressed.
PK’s weight is 78kg and his blood pressure is 121/82. His medications include levothyroxine 50mcg once daily, rosuvastatin 10mg once daily, low-dose aspirin 75mg once daily, amlodipine 5mg once daily, pantoprazole magnesium 40mg once daily, metoprolol SR 50mg twice daily, ramipril 5mg twice daily, and furosemide 40mg twice daily.
There are a number of conditions that can contribute to OAB symptoms, and it is important to consider these during initial workup (see Table 2). In this case, it is important to note that diuretics, especially rapidacting agents, cause a rapid increase in bladder volume, which may precipitate urgency and detrusor overactivity. Anticholinergic agents, opioids and calcium-channel blockers decrease bladder contractility and may cause urinary retention with a decreased functional bladder capacity. Cholinesterase inhibitors could contribute to detrusor overactivity by increasing acetylcholine levels, and should be suspected in patients in whom symptoms develop after initiation of treatment.
Based on your recommendation following a frank discussion relating to his ideas, concerns and expectations, you have made some adjustments to his medication regimens, as well as some lifestyle changes, including reducing his caffeine intake.
Behavioural intervention
Behavioural intervention improves central control, based on the idea that if people can learn to become continent in infancy, they can relearn it in adult life. This approach avoids the morbidity and mortality associated with surgery, as well as the side-effects of drugs. Bladder ‘drills’ were first described between 1978 and 1980, and following the exclusion of any other relevant pathology, the patient is given an achievable target time for using the toilet, before which they may not do so. The time target is set low enough in order to allow the patient to wait for long enough without wetting. After that goal is met, the target time is increased. The patient maintains a normal fluid intake and keeps their own fluid-balance chart. This works better with continued supervision and encouragement, and exercise compliance rates tend to decrease at home. The relapse rate is high. Other interventions include a reduction in caffeine, weight loss and evening fluid intake.
Despite these drug changes and behavioural interventions, PK has continued to have persistent symptoms and occasional urgency incontinence after three months of good compliance. You are concerned about his lack of progress. Evidence has shown that urinary incontinence in patients > 65 years is associated with a twofold increased relative risk of
impaired functioning in activities of daily living. You recommend to PK that he begin pharmaceutical treatment, however he wants to know about what is available and how these work.
Pharmacological treatment
Three classes of treatment are indicated in the management of OAB:
Clinical guidelines recommend first-line pharmacotherapy with antimuscarinic agents, which have been in use for 30 years
A newer treatment option is a selective beta 3 adrenoceptor agonist, mirabegron
Botulinum toxin type A was approved for intradetrusor injections for the secondline treatment of OAB in adult patients who have an inadequate response to or are intolerant of antimuscarinic medication.
Antimuscarinics
A major meta-analysis of trials of antimuscarinic agents for OAB found antimuscarinics efficacious compared to placebo.4 Differences in the relative efficacy of the agents was not apparent, except that some results showed statistical significance in favour of the higher dosage of fesoterodine and solifenacin over placebo and lower dose antimuscarinics.
Options for antimuscarinic treatment include darifenacin extended release tablets, 7.5mg or 15mg; fesoterodine extended-release tablets, 4mg or 8mg; oxybutynin, 5mg regular (immediate release) or extended release tablets, 5mg, 10mg, twice weekly dosing, 36mg (3.9mg/day system); solifenacin succinate tablet, 5mg or 10mg; tolterodine regular or extended release capsules, 2mg or 4mg; and trospium chloride coated tablet, 20mg.
Antimuscarinics differ in their frequency of administration, receptor selectivity and specificity, binding affinity, and other characteristics. The primary sites of activity are the M2 and M3 receptors of the bladder, which mediate contraction. Selectivity for muscarinic receptors is the main reason for variation in side effects across the antimuscarinic agents. Action on muscarinic receptors in the body beyond the detrusor muscle leads to side effects. The side effect profile of each antimuscarinic differs with possible adverse effects that
PHARMACYNEWSIRELAND.COM 74
Table 2. Conditions and medications that may contribute to OAB symptoms







= than sanitary pads 4x drier Result of product demonstration consisting of pouring 4x 10 ml liquid to 1 Always Unnoticeable Protection for Incontinence Normal & 1 Al ways Ultra Long pad. 20 minutes from finishing last pour, plotting paper pressed on both pads, resulting in measuring the level of dryness. for SENSITIVE BLADDER www.prl.ie pgsales@prl.ie +353 1 257 4650 PRL SALES ULC, Greenogue Business Park, Rathcoole, Co. Dublin Logistics Solutions In-Market Solutions Integrated Services
Urology: Overactive Bladder

can, depending on the agent, include dry mouth, constipation, headache, blurred vision, pruritus, tachycardia, somnolence and impaired cognition.
Mirabegron (beta 3 adrenoceptor agonist)
Mirabegron is an oral, once-daily, selective beta-3 adrenoceptor agonist that has shown similar efficacy to antimuscarinics. Mirabegron seems to act on the storage phase of bladder function to facilitate smooth muscle relaxation, for increased bladder capacity and lengthened interval between voiding.
Mirabegron extended-release tablets are available in 25mg and 50mg. Mirabegron is generally well tolerated; the most commonly reported adverse reactions (> 3% of patients taking mirabegron 50mg/day) were hypertension, urinary tract infection, headache and nasopharyngitis. The incidence of dry mouth and other side effects was similar in rate to placebo.
Botulinum toxin
Botulinum injections are now indicated for the treatment of OAB when there has been inadequate response to antimuscarinic therapy. Botulinum toxin exerts its effect by cleaving key proteins required for nerve activation.
First, the toxin binds specifically to nerves which use the neurotransmitter acetylcholine.
Once bound to the nerve terminal, the neuron takes up the toxin into a vesicle. As the vesicle moves further into the cell, it acidifies, activating a portion of the toxin which triggers it to push across the vesicle membrane and into the cell cytoplasm. Once inside the cytoplasm, the toxin cleaves SNARE proteins preventing the cell from releasing vesicles of neurotransmitter. This stops nerve signalling, leading to paralysis.
Clostridium botulinum type A, a neurotoxin, is injected into the detrusor muscle via a flexible or rigid cystoscope. The needle is inserted into the detrusor and 20 injections are delivered. Clinical improvement may occur within two weeks.
Patients may be considered for reinjection when the clinical effect of the previous injection has diminished, but no sooner than three months from the prior bladder injection. The most common adverse reactions with botulinum injections in the first 12 weeks post-op include urinary tract infections (26%), dysuria (11%), bacteriuria (8%), urinary retention (6%), increased residual urine volume (3%), and pollakiuria (daytime frequency) (2%).
CASE STUDY 2
SP is a 54-year-old female with a five-year history of urinary incontinence. She works as a zoo-keeper which is a very active job, and is distressed with the
impact this is having on her work and social life. She experiences frequency every 30-60 minutes during the day and wakes up twice at night to urinate. She gets a sudden urge to void and this is associated with an involuntary leakage of moderate amounts of urine, especially at night. Her urgency is triggered by running water and cold temperatures. She had a hysterectomy at age 45. Her BMI is 29.5kg/m2 (overweight) but she is otherwise healthy, and does not experience any urinary tract infections. Following an initial consultation with her primary care physician, she has restricted her fluid intake (especially after 6pm), has given up caffeine to good effect, and no longer drinks alcohol. Her bladder diary shows a low fluid intake (one cup of water in the early morning and two cups in the evening). She voids 10 times during the day. Her leakage is both diurnal and nocturnal and usually happens on the way to the bathroom. As a result, she has resorted to wearing two to three incontinence pads per day.
SP returns to the office with a completed bladder diary for a further clinical review. These indicate that she still has clinical evidence of urgency incontinence, and her symptoms of urgency and frequency with leakage match the definition of OAB (wet). Following a balanced review, and a frank risk/benefit discussion, you have recommenced her treatment cycle.
Conservative therapy: This includes fluid adjustment, with an encouraged fluid intake of between six and eight cups with a balanced intake throughout the day but decrease evening intake due to nocturia. She should continue to eliminate caffeine and check for other bladder irritants. One also needs to encourage weight loss, as it may improve urgency symptoms
Behavioural therapy: This includes pelvic floor muscle training, urge suppression and bladder retraining, which will progressively increase sphincter tone, as well as urge suppression and pelvic muscle contractions. Biofeedback training given by a clinical nurse specialist can also help identify voiding issues, and can curtail storage lower urinary tract symptoms
Medication: As described above, if conservative therapies are unsuccessful, a trial of antimuscarinic (anticholinergic) medication (eg. oxybutynin, tolterodine, darifenacin, solifenacin or trospium) should be instigated. A combination of drug and behavioural therapy might act synergistically to improve her symptoms. If incontinence persists, she may be able to use a product suitable for her absorbency requirement. It might be an extra absorbent pad, undergarment or protective underwear. Further discussion should then be had regarding more invasive treatments.
CASE STUDY 3
AD is a 12-year-old male who had closure of a T10 myelomeningocoele as a neonate. He is ambulant with walking aids, and is on long-term bowel management in the form of laxatives. He also complains of recurrent MSU-confirmed UTIs, and urge-associated urinary incontinence.
In the paediatric population, much of the symptomatology is difficult to elicit directly from patients, and one must rely to an extent on the history presented by a parent/guardian. The definitions remain broadly similar to the adult population, however, children broadly fall into four categories when dealing with voiding dysfunction:
Voiding dysfunction with or without constipation (bladder bowel dysfunction)
Neurogenic bladder
Congenital structural anomalies
PHARMACYNEWSIRELAND.COM 76
(ectopic ureter leading to continual incontinence)
Idiopathic OAB.
By far, the most common of these is the first category of voiding dysfunction with/ without constipation. However, the most feared is the accurate diagnosis, and management of the neurogenic bladder. Broadly speaking, congenital neurogenic bladders can be categorised into open or occult neural tube defects (NTD), sacral agenesis, spinal cord lesions and cerebral palsy.
Open NTDs can be subcategorised into (lipo)myelomeningocoele (exposed meninges and neural tissue +/- lipoma of the cord), and meningocoele (exposed meninges only). These are thought to affect 1/1,000 births, and rates vary in different geographic regions with access to prenatal ultrasonographic scanning and differing legislation determining termination. They affect the lumbosacral/sacral regions of the cord in approximately 67% of patients, and are usually closed as a neonate. Interestingly, the severity of symptom complex cannot be predicted as easily as that of a spinal cord injury, however, as a rule of thumb, the
higher the lesion, the more likely the degree of severity. Occult NTDs and spinal cord tethering (due to lipomeningocoele, intradural lipoma, dermoid cysts/ sinuses, tight filum terminale) are thought to affect 1/4,000 births, and often don’t present clinically until patients demonstrate a failure to achieve continence.
Urodynamic studies may demonstrate lower tract dysfunction such as large capacity, poorly emptying bladders with associated poor sphincter functions. Neurosurgical correction has been shown to improve outcomes in this cohort, however, there is a paucity of prospective data in this cohort. Sacral agenesis (associated with maternal diabetes mellitus) can be associated with a wide range of neurologic deficits associated with voiding dysfunction, and just over one-third will demonstrate evidence of poor compliance and increased bladder filling pressures on urodynamic studies.
Management of AD’s condition is quite different from the storage lower urinary tract symptoms described in the adult cases outlined above and, following a careful initial history, physical exam, voiding and stooling diaries,
as well as a baseline ultrasound of the abdomen/pelvis, it is also important to involve initial urodynamic studies and longterm follow up for vesicoureteric reflux, incontinence and upper tract deterioration. Those patients with associated detrusor-sphincter dyssinergia (DSD) are more likely to develop poor bladder compliance and worsening upper tracts. Bowel management is also especially important in this cohort. In adolescence and adulthood, management of erections, sexual function and fertility prove a challenging but importance facet to their care.
Urinary incontinence in women
In 2004, the International Consultation on Incontinence (ICI) devised an algorithm that summarised the proposed initial management of urinary incontinence in women.5 This includes an initial history and symptom assessment taking into account co-morbid conditions, lifestyle choices and medications, and following which patients are divided into categories of stress urinary incontinence, mixed urinary incontinence, urgeassociated urinary incontinence and/or OAB symptoms. After careful clinical evaluation with
All Ireland Chromatin Consortium
Researchers from Trinity recently attended the first in-person conference of the All-Ireland Chromatin Consortium (AICC), which was established in 2021 to promote scientific exchange and collaborations between chromatin researchers on the island of Ireland, particularly in the aftermath of the COVID-19 pandemic and Brexit.
The AICC has grown into a network of several hundred scientists with a common focus of investigating epigenetics and chromatin regulation in development and disease.
Researchers from institutes across Ireland presented their work on topics ranging from stem cell development to neurodevelopmental disorders, and cancer biology. Darragh Nimmo, a PhD candidate from Trinity, was awarded best research poster for work investigating noncoding driver mutations in breast cancer.

Professor Haruhiko Koseki, from Japan’s renowned RIKEN research institute, delivered a keynote lecture on the latest developmental genetics research from his lab. Professor Koseki was introduced by Trinity’s Professor Adrian Bracken as “an incredibly collaborative scientist who has contributed to the most important progress in chromatin biology in recent decades.”
Special guest Dr Sara Miller, editor of leading scientific journal, Molecular Cell also presented
at the conference, advising on the process of publishing research in high-impact journals.
Professor Bracken, from Trinity’s School of Genetics and Microbiology, said of the conference: “It was an enormous success, showcasing the world-class research being carried out in Ireland. This first meeting provides an ideal springboard for the future of the AICC as we expand our consortium and gain international recognition.”
Dr Eric Conway, Assistant Professor in UCD and PhD graduate of Trinity College Dublin, organised this first conference of the AICC, along with Dr Elaine Dunleavy, senior lecturer in the University of Galway. Dr Conway said “Initiatives like the AICC are critical for maintaining strong research connections across the island, especially in a post-Brexit Ireland. We are hopeful that this network will lead to future research funding, healthcare innovations and breakthroughs in the field of chromatin biology.”
physical examination (including a bimanual pelvic exam in women), a presumed diagnosis is made based on initial tests such uroflowmetry. Those with OAB go on to bladder retraining and antimuscarinic drugs. Only when these simple measures fail does one offer specialised management.
Conclusion
In those presenting with the symptom complex of frequency, urgency and nocturia with or without urge associated incontinence, one needs to exclude other pathology including UTIs, before diagnosing OAB.
Social circumstances and the lifestyle and expectations of the individual patient need to be taken into account in order to tailor appropriate investigations.
Behavioural therapy and lifestyle interventions should be used as initial treatment for most patients, but there is a high risk of relapse.
Compliance with antimuscarinic therapy relates to efficacy, but there is significantly reduced compliance over time. Botulinum toxin can be used in those who don’t respond to or who cannot tolerate pharmacological treatment.
PHARMACYNEWSIRELAND.COM 77
News
Winners of the AICC best research talk and posters awards: (left to right) Dr Michael Rainey (University of Galway), Darragh Nimmo (Trinity College Dublin), Ellen Finnegan (RCSI)
New Survey reveals Impact of Skin Conditions

Citrine Healthcare Ltd are an Irish owned company that provide targeted skincare products for chronic skin conditions. They recently conducted a research survey that revealed the impact of skin conditions on people’s wellbeing and confidence .
Over two thirds of population suffer or have suffered with a skin condition
Most common skin conditions that affect people in Ireland are Acne, Rosacea and Dermatitis (Eczema)
63% reported feeling self-conscious in public because of a skin condition
50% feel their appearance holds them back due to feeling self-conscious
concerns can’t be overstated, as it helps to create a defence system that prevents against acne, boosts skin resilience, and contributes to a positive overall well-being.
“OTC products provide a convenient and affordable option for people experiencing mild to moderate acne, often allowing individuals to take control of their skincare journey without the need for a dermatologist’s intervention,” added O’Leary.
Citrine Healthcare offer a range of 4 OTC products to help manage the symptoms of acne prone skin, that are available at pharmacies nationwide.
Acne is the second most common skin condition after Dermatitis, according to the research. Almost 40% of people in Ireland say they have suffered with acne at some point in their lives, according to new skincare research published by Citrine to mark the beginning of Acne Awareness Month of June 1st. New research finds 2 in 5 people in Ireland have suffered with acne at some point in their lives.
The research, conducted by Citrine Healthcare and involving a sample of one thousand people across the country, uncovers the impact of acne on both the physical and emotional well-being of those affected. Findings reveal that 59% of both men and women surveyed said they were worried about people judging them because of how they look or were embarrassed about how they look.
The research found that men are more affected by acne than women, although women are generally more willing to discuss and share their experiences with the skin condition.
In the treatment of acne, overthe-counter (OTC) products play a pivotal role in the with six in ten people that used OTC medications finding them effective, the research found.
Acne Awareness Month
Acne Awareness Month runs throughout June and aims to
promote solutions and treatment options available for people dealing with acne, as well as empower people to seek professional help and make informed decisions about their skincare.
Sarah O’Leary, Director at Citrine Healthcare, emphasised the importance of increased awareness, education, and support for people affected by acne, as it can profoundly impact an individual’s self-confidence.
“The research findings underscore the importance of a proactive approach to skincare. Skin health is not just about achieving flawless complexions; it’s about fostering self-care and self-esteem.
“By encouraging open conversations about acne, we can create a supportive environment where individuals seek professional help and find the right treatment options.
During the month of June people are being advised that numerous measures can be taken to alleviate the main symptoms of acne.
“A good skincare routine is the foundation of healthy skin. By following a consistent and personalised skin routine, we can help to minimise the occurrence of acne and empower individuals to feel confident in their own skin.
“Embracing a skincare routine tailored to our skin type and
Clinical evidence shows that a daily skincare routine is essential to managing symptoms, therefore the range contains 2 products for daily use- Triboclense Cleansing Gel to help cleanse and hydrate and Ceboderm cream to be used as a daily mosituriser with SPF 30. In addition, Citrine recognises that different skin has different needs, and offer 2 other products in the range to help alleviate the symptoms. First is Triacine Control cream to help reduce blackheads and whiteheads and the next is Benoxor Clear cream that helps reduce the appearance of spots and blemishes.
Visit your local pharmacy for more information or visit www.citrinehealthcare.com

PHARMACYNEWSIRELAND.COM 78 Research
CLEARLY...
...different skin has different needs.
CLEARLY...
...different skin has different needs.
Cleanse, moisturise and manage acne prone skin.
Cleanse, moisturise and manage acne prone
















































CLEARLY...
CLEARLY...















...different skin has different needs.
...different skin has different needs.

Cleanse, moisturise and manage acne prone skin.
Cleanse, moisturise and manage acne prone skin.


manage acne prone skin.


1 %00 I R I S H ENWO D
1 %00 I R I S H ENWO D
H OWNE D 1 %00 I R I
Tel: 01 445 7206 | www.citrinehealthcare.com Protect the skin you’re in
1 00% I R I S H OWNE D 1 %00 I R I S H ENWO D
Tel:
1 00% I R I S H OWNE D 1 %00 I R I S H ENWO D
Tel: 01 445 7206 | www.citrinehealthcare.com Protect the skin
Dermatology: Common Presentations in Pharmacy

a population-based approach involving 44 689 participants (21 887 (48.97%) men and 22 802 (51.03%) women) was carried out and published in 2022.
54% of the Irish population is affected by skin problems annually, and up to 33% could benefit from medical care at any one time. We spoke with Theresa Lowry Lehnen: RGN, PG. Dip Coronary Care, RNP, BSc, MSc, PG. Dip. Ed (QTS), M. Ed, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University to find out more about the most common dermatology presentations in the primary care setting.

An estimated 15-20% of GP consultations relate specifically to dermatology. In Ireland this represents between 712,500 and 950,000 GP consultations for dermatological conditions each year.
Many skin conditions can be managed in primary care and approximately 65,000 referrals occur annually to specialist dermatology departments for more complex forms of skin disease. Some of the most common skin diseases are increasing in frequency, with over 230 skin cancer related deaths in Ireland annually. Approximately 50% of referrals to Dermatology are for skin cancer. The impact of skin diseases on quality of life can be profound, and many noncancerous inflammatory skin diseases are chronic in nature.
Theresa notes, “More than 2000 dermatological entities are listed in The International Classification of Disease (ICD 11), including rare or novel skin diseases, however, a small number account for most of the disease burden. These include inflammatory conditions,
such as eczema, psoriasis, acne, and rosacea; skin cancers; autoimmune conditions, such as lupus and vitiligo; and hereditary diseases.
“No complete data on the prevalence of skin diseases across European countries are available,” she adds. To estimate the prevalence of the most frequent skin conditions or diseases in 27 European countries (24 EU countries, plus Norway, Switzerland, and the United Kingdom (NEUKS)), a study using
The aim of this study was to evaluate the prevalence of the most common dermatological diseases and conditions of adult patients across Europe. Theresa continues, “43.35% of the NEUKS adult population reported having had at least one dermatological disease or condition. The projection in the total population of the 27 countries included in the study resulted in 185 103 774 individuals affected by at least one dermatological condition or disease and estimates that more than 94 million Europeans complain of uncomfortable skin sensations like itch, burning, or dryness.”
The most frequent conditions, she says, are fungal skin infections (8.9%), acne (5.4%), and atopic dermatitis or eczema (5.5%). Alopecia, acne, eczema, and rosacea are more common in women, and psoriasis in men. Acne affects mainly young adults, while psoriasis was more frequent in respondents older than 25 years.
“Timely and accurate diagnosis is key to determining the most effective management approaches for dermatological conditions,” she continues. “Depending on the severity of presentation and stage of disease, management ranges from prevention and self-management approaches to a variety of topical and oral medications, steroid injections, biologics, surgical interventions, and treatments such as phototherapy and chemotherapy. There is also a significant role for psychological and social supports to reduce the impact and burden of disease.
“Long waiting lists for dermatology services exist nationally. Historically a significant part of dermatology OPD workload consisted of benign lesions which require no treatment or cosmetic problems. Examined by the National Clinical Programme for Dermatology (RCPI/ HSE) exclusion criteria for GP referral to dermatology services unless there is diagnostic uncertainty now includes: viral warts and verrucae; molluscum; seborrhoeic warts/keratoses; skin tags; dermatofibromas; spider naevi; epidermal cysts; sebaceous
prescribing/conditions-and-treatments/skin-soft-tissue/dermatophyte-infection-of-theskin/dermatophyte-skin.html
prescribing/conditions-and-treatments/skin-soft-tissue/dermatophyte-infection-of-theskin/dermatophyte-skin.html
Ringworm on the face or scalp may also cause patchy hair loss


https://www2.hse.ie/condition s/ringworm/
https://www2.hse.ie/condition s/ringworm/
https://www2.hse.ie/conditions/athletes-foot/
https://www2.hse.ie/conditions/athletes-foot/
Eczema

PHARMACYNEWSIRELAND.COM 80 Dermatology
Dermatological conditions affect between 30% and 70% of people worldwide and are the most frequent reason for consultation in general practice.
An interview with Theresa Lowry- Lehnen (PhD), CNS, GPN, RNP, South East Technological University
Athlete’s Foot - Itchy white patches between your toes
Infected Eczema Treatment Drug
1st choice option
Flucloxacillin See flucloxacillin dosing table 500mg every 6 hours
7 days Avoid in for children penicillin allergy
To optimise absorption, take on an empty stomach (either 1 hour before food or 2 hours after).
2nd choice options
Cefalexin See cefalexin dosing table 500mg every 12 hours
7 days Cephalosporins for children should not be used in severe penicillin allergy.
Clarithromycin See clarithromycin dosing table for 250mg every 12 hours
7 days Macrolides should children (can be increased if be used with necessaryin severe caution in infection to 500mg pregnancy. every 12 hours) Clarithromycin suitable only in 2nd and 3rd trimester in pregnancy.
1st choice option in pregnant, penicillin allergic patients
Clindamycin 300mg every 6 hours
Topical Treatment (Reserve for localised infections)
Fusidic Acid Topically to affected areas every 8 hours
7 days Caution: risk of C. difficile.
5-7 days
https://www.hse.ie/eng/services/list/2/gp/antibiotic-prescribing/conditions-and-treatments/skin-soft-tissue/eczema/
cysts; lipomas; tattoos; xanthelasma; physiological male balding; and melasma.”
Common Dermatological Presentations in Primary Care Rashes
Rashes and minor skin conditions are very common and can affect people of all ages. They can be troublesome in adults and distressing when they occur in babies and young children.
Theresa says, “Referral to a dermatologist may be important for making difficult diagnoses and selecting certain treatments, however, many rashes are self-limiting, and most can be diagnosed and treated in primary care. Referral to dermatology should be used for the highest scope of practice because the workforce is limited, and specialty care is costly.”
Fungal Infection: Ringworm
Ringworm is a common contagious fungal infection caused by dermatophytes and is easily
spread following contact with an infected person through skin to skin contact, sharing towels, clothing and bed linen. Pets such as cats and dogs can also transmit the infection to humans.
“Classification is generally by the site of the body affected. The rash appears as a circular lesion with a raised outer rim and paler centre,” she says. “The most common infections in prepubertal children are tinea corporis and tinea capitis, whilst tinea cruris, tinea pedis, and fungal nail infections such as onychomycosis are more frequently seen in adolescents and adults.
“Treatment varies with the site affected. For skin infections topical treatment is the first line and some products can be purchased over the counter. Scalp ringworm (tinea capitis) is usually treated for a longer duration (2-4 weeks) with terbinafine. Both the affected person and family members are advised to use an antifungal shampoo (Ketoconazole) twice weekly for two weeks.
Onychomycosis requires a longer course of oral medication before effect is achieved.”
Fungal Infection: Athletes foot
Athlete’s foot, or tinea pedis, is an infection of the skin and feet that can be caused by a variety of different fungi. Although tinea pedis can affect any portion of the foot, the infection most often affects the space between the toes. If it is not treated, it can spread to the toenails and cause a fungal nail infection. Information on the treatment of dermatophyte and other fungal infections of the skin is available at:
7https://www.hse.ie/eng/services/ list/2/gp/antibiotic- prescribing/ conditions-and-treatments/skinsoft-tissue/dermatophyte-infectionof-the-skin/dermatophyte-skin.html
Eczema
“Eczema, frequently called dermatitis is an inflammatory skin condition occurring in all age ranges from babies through to older adults” Theresa notes.
“There are different types of eczema, atopic being the most common, which follows a relapsing and recurring course. Diagnosis is made on examination and the patient typically presents with an acutely inflamed, red, sometimes blistered and weeping patches of skin. Although the rash can occur anywhere, common sites are the flexures of the elbows and backs of the knees.”
Eczema Herpeticum is a dermatological emergency, warranting same day referral or contact with the local dermatology department. Treatment is with acyclovir. She adds, “In infected eczema, swelling and a golden crust suggest probable staphylococcal infection. Swabs are not indicated unless treatment failure or atypical species is suspected. Restoring the barrier with appropriate topical steroids and emollients may reduce bacterial superinfection and lessen anti-microbial requirements.
“Topical antibiotics should be used for a limited period of < 2 weeks
PHARMACYNEWSIRELAND.COM 81
Dose Duration
Children’s Dose Adult
Notes
https://www.hse.ie/eng/services/list/2/gp/antibiotic -prescribing/conditions-andtreatments/skin-soft-tissue/eczema/
• phototherapy – Exposes the skin to certain types of ultraviolet light
• systemic – oral and injected medications that work throughout the entire body Different types of treatment are often used in combination. Referral to a dermatologist may occur if the symptoms are severe.
more erosive form where painful ulcers occur which becoming cancerous (1 in 100 patients) over a period spontaneously without treatment. When itching is severe needed. A potent steroid cream e.g. Betnovate can be of symptoms, aiming to reduce once improvement steroids can be prescribed (20mg daily for 2 to 6 weeks). with a topical steroid but referral to secondary care severe or response to treatment is inadequate. 4
Psoriasis
https://www.nhs.uk/conditions/atopic -eczema/symptoms/
because of bacterial resistance. They should not be co-prescribed with oral antibiotics for the same reason. Using antibiotics, or adding them to steroids, in eczema encourages resistance and does not improve healing unless there are visible signs of infection. Bleach baths may reduce the bacterial load on the skin and contribute to reduced numbers of flares. It is recommended as a maintenance antimicrobial measure once or twice a week. During infective flares it may cause stinging.”
Information on the treatment of eczema is available at: https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ conditions-andtreatments/skinsoft-tissue/eczema/
Psoriasis causes patches of skin that are dry, red and covered in silver scales. Plaque psoriasis (psoriasis vulgaris) is the most common form accounting for 80 to 90% of cases. The scales appear on the elbows, knees, scalp and lower back, but they can appear anywhere on the body. The plaques can be itchy or sore, or both. In severe cases, the skin around the joints may crack and bleed.



Scalp psoriasis occurs on parts of or the whole scalp. It causes red patches of skin covered in thick, silvery-white scales. Some people find scalp psoriasis itchy, while others have no discomfort. In extreme cases, it can cause hair loss, although this is usually only temporary.
Nail Psoriasis: In approximately half of all people with psoriasis, the condition affects the nails, causing them to develop small dents or pits. The nails can become discoloured or grow abnormally,
can become loose and separate from the nail bed, and in severe cases may crumble.
more erosive form where painful ulcers occur which are linked to an approximate 1% risk of becoming cancerous (1 in 100 patients) over a period of 10 years. Resolution can occur spontaneously without treatment. When itching is severe a sedating antihistamine may be needed. A potent steroid cream e.g. Betnovate can be used and the dose tailored to severity of symptoms, aiming to reduce once improvement is seen. In severe cases, systemic oral steroids can be prescribed (20mg daily for 2 to 6 weeks). Oral lichen planus can be treated with a topical steroid but referral to secondary care will be needed when symptoms are severe or response to treatment is inadequate. 4

Guttate psoriasis causes small drop-shaped sores on the chest, arms, legs and scalp. The condition often disappears completely after a few weeks, but some people go on to develop plaque psoriasis. This type of psoriasis sometimes occurs after a streptococcal throat infection and is more common among children and teenagers.
https://www.nhs.uk/conditions/lichen -planus/
Chickenpox
Theresa adds, “Less common types of psoriasis include: Pustular psoriasis, Zumbusch psoriasis, Palmoplantar pustulosis, Acropustulosis and Erythrodermic psoriasis. Treatments depend on the type and severity of psoriasis and the area of skin affected. Treatment often starts with a topical cream applied to the skin, and then stronger treatments if required.”
Treatment falls into 3 categories:
condition caused prevalent in children under the age of ten, with over 90% Chickenpox is generally a mild illness, but can immunocompromised, and can have more serious consequences transmission from person to person by breathing in infected or coughing, or less commonly through contact with days after contracting the infection, but may take longer. of days prior to developing the typically itchy rash, with loss of appetite and fever. Adults generally have more develop lung problems, such as pneumonia, with smokers
Lichen planus is a less well-known rash than eczema or psoriasis, and Theresa highlights that it is more common in adults than children. “It is a non-infectious itchy rash, seen as small shiny, reddish raised papules most commonly on the wrist, ankles, elbows, and lower back although it can develop at any site.
Chickenpox is a highly infectious condition caused by the varicella zoster virus. It is most prevalent in children under the age of ten, with over 90% of cases occurring in this age group. Chickenpox is generally a mild illness, but can be fatal in neonates and the immunocompromised, and can have more serious consequences in adults. Spread occurs by transmission from person to person by breathing in infected respiratory droplets via sneezing or coughing, or less commonly through contact with weep ing spots. The rash develops 10-14 days after contracting the infection, but may take longer. The child is often unwell for a couple of days prior to developing the typically itchy rash, with additional symptoms of headaches, loss of appetite and fever. Adults generally have more serious symptoms, and 5-14% of adults develop lung problems, such as pneumonia, with smokers at a greater risk. 4
Treatment aims to ease symptoms and comprise of ease the itching. Paracetamol may be required recommended as there is an increased risk of soft tissue treatment, ideally to commence within 72 hours of severity For adults’ acyclovir 800 mg is taken five times intervals, during waking hours. Treatment should continue
• topical – creams and ointments applied to the skin
Treatment aims to ease symptoms and comprise of a sedating antihistamine and lotion to ease the itching. Paracetamol may be required if feverish, however ibuprofen is not recommended as there is an increased risk of soft tissue infection. Adults may need antiviral treatment, ideally to commence within 72 hours of onset of the rash to reduce symptom severity For adults’ acyclovir 800 mg is taken five times daily at approximately four-hourly intervals, during waking hours. Treatment should continue for seven days. 4, 10
“Lichen planus occasionally affects the oral cavity and may occur alone or in combination with symptoms at another site. It causes burning or stinging and discomfort in the mouth and on examination the mucosa is covered with painless white streaks. There is a more erosive form where painful ulcers occur which are linked to an approximate 1% risk of becoming cancerous (1 in 100 patients) over a period of 10 years.
“Resolution can occur spontaneously without treatment. When itching is severe a sedating antihistamine may be needed. A potent steroid cream e.g. Betnovate can be used and the dose tailored to severity of symptoms, aiming to reduce once improvement is seen. In severe cases, systemic oral steroids can be prescribed (20mg daily for 2 to 6 weeks). Oral lichen planus can be treated with a topical steroid but referral to secondary care will be needed when symptoms are severe or response to treatment is inadequate.”
treatments/skin-soft-tissue/eczema/
PHARMACYNEWSIRELAND.COM 82 Dermatology
Lichen planus
Information on t he treatment of eczema is available
anywhere on the body. The plaques can be itchy or sore, or both. In severe around the joints may crack and bleed. 9
Atopic Eczema
Plaque Psoriasis
Chickenpox
Chickenpox is a highly infectious condition caused by the varicella zoster virus. It is most prevalent in children under the age of ten, with over 90% of cases occurring in this age group.
Theresa continues, “Chickenpox is generally a mild illness, but can be fatal in neonates and the immunocompromised, and can have more serious consequences in adults. Spread occurs by transmission from person to person by breathing in infected respiratory droplets via sneezing or coughing, or less commonly through contact with weeping spots. The rash develops 1014 days after contracting the infection, but may take longer.
“The child is often unwell for a couple of days prior to developing the typically itchy rash, with additional symptoms of headaches, loss of appetite and fever. Adults generally have more serious symptoms, and 5-14% of adults develop lung problems, such as pneumonia, with smokers at a greater risk. Treatment aims to ease symptoms and comprise of a sedating antihistamine and lotion to ease the itching. Paracetamol may be required if feverish, however ibuprofen is not recommended as there is an increased risk of soft tissue infection.
“Adults may need antiviral treatment, ideally to commence within 72 hours of onset of the rash to reduce symptom severity. For adults’ acyclovir 800 mg is taken five times daily at approximately four-hourly intervals, during waking hours. Treatment should continue for seven days.”
Shingles
Herpes Zoster (HZ) also known as shingles, is a secondary infection that occurs in some individuals as the result of reactivation of the latent varicella zoster virus, usually within a single ganglion. The individual lifetime risk of developing herpes zoster is between 24% and 30%.
“Although herpes zoster can occur at any age, incidence increases with age. Two thirds of cases occur in individuals aged 50 years and older and the risk of developing the disease in those aged 85 years and above is 50%. Once the virus activates, it can lead to a painful, blistery rash,” Theresa warns.
Early symptoms of herpes zoster including headache, fever and malaise are nonspecific, and may
result in an incorrect diagnosis. These symptoms are commonly followed by sensations of burning pain, itching, hyperesthesia, or paraesthesia. Herpes zoster is diagnosed clinically, typically based on history and symptom presentation.
“The treatment of herpes zoster has three major objectives; treatment of the acute viral infection, treatment of the acute pain associated with herpes zoster and prevention of postherpetic neuralgia. Early identification and prompt treatment of HZ with antiviral drugs and analgesics frequently reduces acute rash and pain and may prevent some complications.
“Antiviral drugs have been shown to reduce acute pain and rash severity, accelerate rash resolution and reduce duration of pain. Herpes zoster can be treated with antiviral medications acyclovir, valacyclovir, or famciclovir, most effective when started within 72 hours after the onset of the rash.”
Information on the treatment of shingles is available at:
https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ conditions-andtreatments/skinsoft-tissue/shingles/
Acne
Acne is a chronic inflammatory skin disease and is one of the most common dermatological problems seen in general practice.
Acne usually occurs at puberty or in early adult life, when there is a surge of hormones, and it is more common in males than females. It can present with inflammatory and non-inflammatory lesions mainly on the face but can also occur on the upper arms, trunk, and back. Hypersensitivity to fluctuations in hormones causes the pilosebaceous unit to over produce oil, leading to blocked pores called comedones.
Grade 1: Comedones are of two types, open and closed. Open comedones are due to plugging of the pilosebaceous orifice by sebum on the skin surface. Closed comedones are due to keratin and sebum plugging the pilosebaceous orifice below the skin surface.
Grade 2: Inflammatory lesions present as a small papule with erythema.
Grade 3: Pustules.
Grade 4: Many pustules coalesce to form nodules and cysts. First
line treatment is to tackle the excess oil and comendones. It is advisable not to scrub the skin or use astringents as these may rupture the comedones and promote inflammatory lesions. Acne washes containing salicyclic acid 0.5% - 2% may be helpful, however most people will also need a topical retinoid, or retinoid agent or a combination of agents.
Information on the treatment of acne is available at:
https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ conditions-andtreatments/skinsoft-tissue/acne-vulgaris/
Impetigo
“Impetigo is a common infection of the superficial layers of the epidermis that is highly contagious and most commonly caused by gram-positive bacteria. It usually presents as erythematous plaques with a yellow crust and may be itchy or painful. The lesions are highly contagious and spread easily. Diagnosis is typically based on the symptoms and clinical manifestations alone. Treatment involves topical and/or oral antibiotics and symptomatic care.”
Information on the treatment of impetigo is available at:
https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ conditions-andtreatments/skinsoft-tissue/impetigo
Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) also referred to as acne inversa, is a chronic, relapsing, inflammatory skin condition that typically occurs after puberty, with the average age of onset in the second or third decade of life.
Theresa says, “Patient with HS present with inflammation of hair follicles in the apocrine glandbearing regions; armpits, genital area, groin, inframammary region, perianal region and buttocks that initially manifests as painful nodules or boils and progresses to abscesses, sinus tracts and scarring. The presentation of hidradenitis suppurativa is distinct, although the condition is often not well-recognised in primary care.
“The most troublesome symptom of HS is chronic pain which is reported by almost all patients. The pain associated with HS can be intense and is reported by patients as the most significant factor contributing to impaired quality of life. Early diagnosis is very important
for patients with hidradenitis suppurativa, to ensure the best possible course and prompt disease management. However, HS diagnosis generally occurs after an average 7-year delay, because the early stages are often mistaken for other conditions.”
Information on the treatment of HS is available at:
https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ conditions-andtreatments/ skin-soft-tissue/hidradenitissuppurativa/
Conclusion
Theresa concludes, “Dermatology outpatient referral numbers have increased significantly over the past decade in Ireland with more than 115,000 outpatient visits in 2019. At the end of 2019 there were 48,850 Irish people waiting for a dermatology outpatient’s appointment, representing a 63% increase since December 2015.
“Approximately 50% of referrals to Dermatology are for skin cancer, rates of which are rising rapidly and expected to double between 2020 and 2040. Secondary and tertiary specialist care in Ireland is led by consultant dermatologists, with additional SpR, NCHD, ANP, CNS, RGN and administrative staff. Some surgeries are carried out by plastic surgeons.
“Most work occurs in dedicated dermatology departments in hospitals or through outreach to peripheral centres from these departments. There are 11 Dermatology Departments (Hub) and 16 Peripheral Clinics (Spoke) operating a hub and spoke model (excluding CHI).
“Lengthy waiting lists for dermatology services exist nationally, and many patients with debilitating skin conditions are waiting for prolonged periods. In 2019 the dermatology waiting list was 44,763, with 13,350 patients waiting for more than 12 months for an appointment.
“National Treatment Purchase Fund (NTPF) figures in 2021 highlighted significant increases in outpatient waiting lists on the previous 12 months, including Dermatology (12%, +4,888).
Demand for dermatology appointments have increased, driven largely by our aging population, skin cancer and increased need for the care of moderate-to-severe inflammatory skin disease like psoriasis, eczema, HS, and other disorders.”
PHARMACYNEWSIRELAND.COM 83
Research reveals Impact of Pain
Difficulty using the stairs (66%), getting in and out of the car (57%), carrying groceries (50%) and ability to put on socks (43%) are just some of the everyday issues faced by people living with chronic joint pain before undergoing surgery.
Dr Niall Hogan, Consultant Orthopaedic Surgeon at Blackrock Health Blackrock Clinic

¤2 million in SFI Frontiers

to relieve symptoms and enable them to regain motion.
Dr Niall Hogan, Consultant Orthopaedic Surgeon at Blackrock Health Blackrock Clinic says that if you’re experiencing joint pain, don’t ignore it. “From my experience, most patients wait too long to have a hip or knee replacement. The older someone is, the more likely they have multiple co-existing health conditions, such as high blood pressure or diabetes, and those can complicate recovery, further underlining the importance of earlier intervention.
Minister for Further and Higher Education, Research, Innovation and Science, Simon Harris TD has announced funding of ¤2 million to support four research projects led by RCSI University of Medicine and Health Sciences through Science Foundation Ireland’s Frontiers for the Future programme.
The RCSI projects will facilitate novel research in breast cancer, colorectal cancer, anti-cancer drugs and skeletal injury and disease.
The successful RCSI recipients are:
New research from Blackrock Health has revealed the real-life impact that chronic hip and knee pain has on those living with it and highlights the importance of acting on joint issues through earlier surgical intervention when appropriate.
The research reveals that 60% of those who have had a hip/knee replacement claim they lived with pain for up to three years before undergoing surgery, with just over 83% claiming that their hip/knee pain affected their ability to enjoy major milestones in life including weddings, birthdays, and holidays. Activities of daily life were also significantly impacted on a daily or weekly basis while they waited for a hip or knee replacement:
• 47% could not take part in sports.
• 53% said it impacted their sleep.
• 47% said it affected their ability to drive.
• 43% said it affected their ability to work.
• 47% said it affected their ability to be intimate with their partner.
The research also investigated the moment when a person living with hip and knee pain considered a replacement with one third (32%) claiming they only considered a replacement when they could no longer go about their daily life with ease, indicating that people wait until their condition is at a chronic stage before considering surgical intervention despite 87% of people stating that their mobility improved as a result of same.
The Freedom in Motion campaign has been developed to encourage people living with chronic hip or knee pain to engage with their healthcare professional to assess if and when a surgical joint replacement should be considered
“Lean muscle mass also declines with age, so the physical effort of recovery can seem harder than if the surgery was performed a bit earlier in life. The new Blackrock Health research shows that 87% of people have improved mobility compared with before surgery, enabling them to recover many aspects of life lost previously due to levels of pain experienced. Many patients who undergo surgery have told me that they wished they would have had their surgery earlier so as to regain freedom in motion and enjoy more years with less pain,” he adds.
Blackrock Health is home to one of the most established orthopaedic faculties in Ireland with a team of internationally-recognised consultants. Blackrock Clinic, Galway Clinic and Hermitage Clinic offers convenient locations for a full range of orthopaedic services, including consultation, diagnosis, treatment, and rehabilitation care.
• Dr Oran Kennedy, Department of Anatomy and Regenerative Medicine, is awarded ¤616,775 for the project ‘OsteoLeukins: osteocytes as an important source of interleukins in skeletal injury and disease’
• Dr Jamie O’Sullivan, School of Pharmacy and Biomolecular Sciences, is awarded ¤614,342 for the project ‘Examining the role of von Willebrand Factor as a potential therapeutic target in triple negative breast cancer’
• Dr Darren Griffith, Department of Chemistry, is awarded ¤535,065 for the project ‘Development of Pt-based PROteolysis TArgeting Chimeras (Pt-PROTACs) as molecular probes for Ptbinding proteins and next generation anticancer agents’
• Dr Simon Furney, Department of Physiology and Medical Physics, is awarded ¤302,246 for the project ‘NoCoSMiCC: non-coding somatic mutations in colorectal cancer’
The RCSI researchers were awarded funding under the project stream of the SFI Frontiers for the Future programme which supports high-risk, high-reward research projects that will facilitate highly innovative and novel approaches to research.
Professor Fergal O’Brien, Deputy Vice Chancellor for Research and Innovation at RCSI, said: “RCSI’s success in this year’s SFI Frontiers for the Future programme is a testament to the high-quality, focused health sciences research taking place at the university. Through this programme, our researchers will lead innovative projects that have the potential to bring about vital improvements in diagnostics and therapeutics that will benefit patients in Ireland and globally.”
PHARMACYNEWSIRELAND.COM 84 News


enoxaparin (Clexane®) are the LMWHs most frequently used during pregnancy in Ireland.
Direct oral anticoagulant agents (DOACs) are not routinely recommended for use in pregnancy or breastfeeding due to limited human safety data. Warfarin should be avoided during pregnancy as it can cause birth defects e.g. fetal warfarin syndrome, however it may sometimes be used in patients with mechanical heart valves. At daily doses lower than 12 mg, warfarin can be prescribed during breastfeeding due to very low levels in breastmilk.
Low Molecular Weight Heparin for treatment and prophylaxis of Venous Thromboembolism (VTE) during Pregnancy
Treatment dose:
Written by
Introduction:
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are two components of a single condition called venous thromboembolism (VTE). In pregnancy although VTE is rare (1-2 per 1,000 pregnancies), it remains a leading cause of maternal death, as well as a source of maternal morbidity.
Risk factors for VTE can change during pregnancy, delivery and the postnatal period. A VTE can occur any time in all three trimesters of pregnancy, but the highest risk is in the 6 weeks immediately after birth.

Personal history of VTE is the biggest risk factor for pregnancy associated VTE. Other antenatal risk factors include: medical co-morbidities, increased BMI, advanced age,
Table 1: Suggested treatment doses for antenatal and postnatal LMWH
Table 1: Suggested treatment doses for antenatal and postnatal LMWH
Antenatal 1 mg/kg every 12 hours 175 units/kg every 24 hours
Postnatal 1.5 mg/kg every 24 hours 175 units/kg every 24 hours
HSE & IOG (2016) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management
HSE & IOG (2013) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management
The dose of LMWH should be rounded up or down to a measurable dose for administration.
Enoxaparin dose calculation:
Antenatal weight 62 kg
Dose = 1 mg/kg every 12 hours = 62 mg (rounded to 60 mg)
Use 60 mg syringe of enoxaparin sodium per 0.6 mL.
parity, smoking status, assisted conception and varicose veins. Postnatal risk factors also consist of caesarian delivery (with greater risk associated with emergency caesarean delivery), prolonged hospital admission, postpartum haemorrhage and systemic infection.
Postnatal weight 71 kg
admission to hospital, upon any change in clinical status and essentially in the postpartum period. Women who are at a high risk for VTE and/or treated for VTE in pregnancy should be managed by a combined haematology and obstetric multidisciplinary service.
frequently used during pregnancy in Ireland.
Dose = 1.5 mg/kg every 12 hours = 106.5 mg (rounded to 105 mg)
Use 120 mg enoxaparin sodium in 0.8 mL syringe, discard 0.1 mL and administer 0.7 mL (105 mg)
Failure to recognise and/or treat personal or pregnancy specific risk factors has been identified as a significant contributing factor to maternal mortality and morbidity from VTE in pregnancy. For this reason and due to the increasing prevalence of risk factors in the pregnant and postpartum populations, all pregnant patients should undergo a documented assessment of risk factors for VTE in early pregnancy or prepregnancy. Risk assessment should be repeated on each
The 2013 Irish National Clinical Guideline on VTE thromboprophylaxis in pregnancy issued a rapid risk assessment tool for VTE in pregnancy and this tool or a variation thereof is used in most Irish maternity hospitals and units.
Enoxaparin pre-filled syringes are not available for all required doses. Table 2 below shows the available syringe sizes and the volume to be administered to obtain the required dose
Low Molecular Weight Heparin (LMWH) is the treatment and prophylaxis of choice for VTE during pregnancy as LMWH is a large molecule and does not cross the placenta. Tinzaparin (Innohep®) and enoxaparin (Clexane®) are the LMWHs most
Direct oral anticoagulant agents (DOACs) are not routinely recommended for use in pregnancy or breastfeeding due to limited human safety data. Warfarin should be avoided during pregnancy as it can cause birth defects e.g. fetal warfarin syndrome, however it may sometimes be used in patients with mechanical heart valves. At daily doses lower than 12 mg, warfarin can be prescribed during breastfeeding due to very low levels in breastmilk.
Treatment dose
(see table 1 above):
The dose of LMWH should be rounded up or down to a measurable dose for administration.
*Colour-coded to match colour of enoxaparin (Clexane®) pre-filled syringe and packaging

PHARMACYNEWSIRELAND.COM 86 Thrombosis
Áine Toher, Senior Pharmacist, The National Maternity Hospital,
Table 2: Enoxaparin (Clexane®) dose administration
Enoxaparin Tinzaparin
Enoxaparin Dose Enoxaparin (Clexane®) syringe size Volume to administer 40 mg 40 mg/0.4 mL 0.4 mL 50 mg 60 mg/0.6 mL 0.5 mL 60 mg 60 mg/0.6 mL 0.6 mL 70 mg 80 mg/0.8 mL 0.7 mL 80 mg 80 mg/0.8 mL 0.8 mL 90 mg 100 mg/1 mL 0.9 mL 100 mg 100 mg/1 mL 1 mL 105 mg 120 mg/0.8 mL 0.7 mL 120 mg 120 mg/0.8 mL 0.8 mL 135 mg 150 mg/1 mL 0.9 mL 150 mg 150 mg/1 mL 1 mL
Tinzaparin dose calculation: Weight (antenatal or postnatal) 78 kg Dose = 78 x 175 IU = 13,650 IU (rounded to 14,000 IU)
Table 2: Enoxaparin (Clexane®) dose administration
*Colour-coded to match colour of enoxaparin (Clexane®) pre-filled syringe and packaging Sarah Mohamed,
Pharmacy Intern, The National Maternity Hospital
Floradix
Chapped lips, brittle hair, constant fatigue and palpitations are not on a prospective mum’s wish list. They are, however, some of the tell-tale signs of iron deficiency, which is common amongst pregnant women. Iron is needed to make haemoglobin, the chemical that transports oxygen in the blood. A range of stressful symptoms can indicate a deficiency, but severe cases can lead to anemia, total exhaustion and a weakened immune system. Throughout pregnancy, eating for two also means that your body requires twice the amount of iron to stay healthy.
Keeping levels healthy
A healthy, balanced diet with ironrich foods will provide most of the iron you need during pregnancy. This should include lean meats; pulses such as peas, beans and lentils; fruit such as prunes, apricots and raisins; and dark green leafy vegetables such as spinach.
However, it can be difficult to absorb enough iron from a balanced diet alone for you and your growing baby, particularly if you have a deficiency as you need to eat a lot of iron-rich foods to correct it. This is where Floradix can really help. This high quality liquid supplement contains organic iron gluconate, and its easy absorption into the body is enhanced by vitamins C and B complex, herbal extracts and fruit juice concentrates. This special blend of ingredients mean that it’s easy to digest and the unpleasant side effects of many iron preparations, such as stomach cramps and constipation, are avoided. So it’s no wonder Floradix is recommended by numerous midwives and health care professionals. What’s more, it’s completely free of chemical preservatives, colourings or flavourings, and is also available in a gluten-free form called Floravital.

Helping Absorption
Vitamin C, animo acids, fructose and glucose all help your body absorb more iron from foods and supplements. Other elements of our diet will decrease absorption, such as calcium, milk proteins, black tea, coffee, fibre, vitamin E and phosphates (found in soft drinks). So to maximise the amount of iron you are getting, take an iron supplement before eating.
Warning signs of iron deficiency
• pale skin under eyes and nails
• brittle hair
• cracked skin around mouth
• breathlessness when exercising
• heart palpitations
• rapid pulse
• difficulty concentrating
• cold hands and feet
• increased frequency of infection
88 Thrombosis
Enoxaparin dose calculation:
Antenatal weight 62 kg
Dose = 1 mg/kg every 12 hours = 62 mg (rounded to 60 mg)
Use 60 mg syringe of enoxaparin sodium per 0.6 mL.
Postnatal weight 71 kg
Dose = 1.5 mg/kg every 24 hours = 106.5 mg (rounded to 105 mg)
Use 120 mg enoxaparin sodium in 0.8 mL syringe, discard 0.1 mL and administer 0.7 mL (105 mg)
Enoxaparin pre-filled syringes are not available for all required doses. Table 2 (on page 20) shows the available syringe sizes and the volume to be administered to obtain the required dose.
Tinzaparin dose calculation:
Weight (antenatal or postnatal)
78 kg
Dose = 78 x 175 IU = 13,650 IU (rounded to 14,000 IU)
Use tinzaparin 14,000 in 0.7 mL prefilled syringe.
Tinzaparin pre-filled syringes are not available for all required doses. Table 3 below shows the available syringe sizes and the volume to be administered to obtain the required dose.
Table 4: Suggested thromboprophylactic doses for antenatal and postnatal LMWH
HSE & IOG (2016) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium
HSE & IOG (2013) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium
Many maternity hospitals follow the doses suggested above, but in some hospitals the weight bands are further divided so prescriptions for tinzaparin 6000 IU and 8000 IU once daily are common.
It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring.
Commencement and prophylaxis duration with LMWH can vary depending on individual VTE risk assessment, if LMWH is required during pregnancy it should be continued for up to 6 weeks postnatally.
Commencement and prophylaxis duration with LMWH can vary depending on individual VTE risk assessment, if LMWH is required during pregnancy it should be continued for up to 6 weeks postnatally.
Prophylaxis: (see Table 4 above)
Many maternity hospitals follow the doses suggested above, but in some hospitals the weight bands are further divided so prescriptions for tinzaparin 6000 IU and 8000 IU once daily are common.
Enoxaparin (Clexane®) syringes are available in the strengths stated in the table above. However, tinzaparin (Innohep®) syringes are not available in all the doses stated above so the
patient will have to be shown how to expel some of the injection from a syringe of a strength closest to their required dose as per Table 3. It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring
Enoxaparin (Clexane®) syringes are available in the strengths stated in the table above. However, tinzaparin (Innohep®) syringes are not available in all the doses stated above so the patient will have to be shown how to expel some of the injection from a syringe of a strength closest to their required dose as per Table 3. It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring
Contraindications to LMWH use in pregnancy and the puerperium:
Contraindications to LMWH use in pregnancy and the puerperium:
Contraindications to LMWH use include known bleeding disorders (e.g. haemophilia, von Willebrand’s disease), thrombocytopenia, acute stroke, active antenatal or postpartum bleeding and those with an increased risk of major haemorrhage (e.g. placenta praevia). As well as uncontrolled hypertension, severe liver or renal disease and recent surgery to the eye or nervous system. Women with a previous or current allergic reaction to LMWH should always be offered an alternative form of prophylaxis. It is recommended that any woman with a contraindication to LMWH should be provided with appropriately sized anti-embolism stockings (AES) with a calf pressure of 1415 mmHg.
Contraindications to LMWH use include known bleeding disorders (e.g. haemophilia, von Willebrand’s disease), thrombocytopenia, acute stroke, active antenatal or postpartum bleeding and those with an increased risk of major haemorrhage (e.g. placenta praevia). As well as uncontrolled hypertension, severe liver or renal disease and recent surgery to the eye or nervous system. Women with a previous or current allergic reaction to LMWH should always be offered an alternative form of prophylaxis. It is recommended that any woman with a contraindication to LMWH should be provided with appropriately sized anti-embolism stockings (AES) with a calf pressure of 14-15 mmHg.
LMWH should be avoided if a lumbar puncture, epidural or spinal anaesthesia is expected within the following 12 hours, or if such regional techniques have taken place within the previous 4 hours. Pregnant patients receiving antenatal LMWH should be educated on the symptoms of labour and instructed not to inject any further LMWH once labour symptoms or vaginal bleeding are observed.
LMWH should be avoided if a lumbar puncture, epidural or spinal anaesthesia is expected within the following 12 to 24 hours, or if such regional techniques have taken place within the previous 4 hours. Pregnant patients receiving antenatal LMWH should be educated on the symptoms of labour and instructed not to inject any further LMWH once labour symptoms or vaginal bleeding are observed.
*Colour-coded to match colour of tinzaparin (Innohep®) pre-filled syringe and packaging
*Colour-coded to match colour of tinzaparin (Innohep®) pre-filled syringe and packaging
It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring.
PHARMACYNEWSIRELAND.COM
Prescribed Dose of Tinzaparin Anti-Factor Xa units Tinzaparin (Innohep®) Pre-filled Syringe Volume to inject via subcutaneous injection 6000 8000 IU/0.4 mL 0.3 mL 7000 8000 IU/0.4 mL 0.35 mL 8000 8000 IU/0.4 mL 0.4 mL 9000 10000 IU/0.5 mL 0.45 mL 10000 10000 IU/0.5 mL 0.5 mL 11000 12000 IU/0.6 mL 0.55 mL 12000 12000 IU/0.6 mL 0.6 mL 13000 14000 IU/0.7 mL 0.65 mL 14000 14000 IU/0.7 mL 0.7 mL 15000 16000 IU/0.8 mL 0.75 mL 16000 16000 IU/0.8 mL 0.8 mL 17000 18000 IU/0.9 mL 0.85 mL 18000 18000 IU/0.9 mL 0.9 mL
Table 3: Tinzaparin (Innohep®) dose administration
Table 3: Tinzaparin (Innohep®) dose administration
Weight (kg) Tinzaparin (75 IU/kg/day) Enoxaparin <50 3500 IU once daily 20 mg once daily 50 - 90 4500 IU once daily 40 mg once daily 91 - 130 7000 IU once daily 60 mg once daily 131 - 170 9000 IU once daily 80 mg once daily >170 75 IU/kg once daily 0.6 mg/kg once daily
Table 4: Suggested thromboprophylactic doses for antenatal and postnatal LMWH
The journey to triple protection in type 2 diabetes mellitus1†
INVOKANA® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:
> as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
> in addition to other medicinal products for the treatment of diabetes
For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1 of the Summary of Product Characteristics (SmPC).1
† Improvement in glycaemic control and reduction of cardiovascular and renal morbidity and mortality are integral parts of the treatment of type 2 diabetes.1

1. INVOKANA® Summary of Product Characteristics March 2022.
Invokana (canagliflozin) 100mg & 300mg film-coated tablets
Abbreviated Prescribing Information: Please consult the Summary of Product Characteristics (SmPC) for full prescribing information. Presentation: Canagliflozin 100mg and canagliflozin 300mg, film-coated tablets. Use: Adults with insufficiently controlled type 2diabetes mellitus as an adjunct to diet and exercise: (1) as monotherapy when metformin is considered inappropriate due to intolerance or contraindications, or (2) in addition to other medicinal products for the treatment of diabetes. Dosage and administration: Oral administration. Recommended starting dose is 100mg once daily, preferably taken before the first meal of the day. Tablets should be swallowed whole. In patients needing tighter glycaemic control and tolerating canagliflozin 100mg once daily, dose can be increased to 300mg once daily if eGFR ≥ 60 mL/ min/1.73 m2. [Refer to SmPC for dose adjustment according to eGFR]. Care when increasing dose in patients ≥ 75 years of age, patients with known cardiovascular disease (CVD), other patients for whom the initial canagliflozin-induced diuresis poses risk. Correcting volume depletion prior to initiation of canagliflozin is recommended. Consideration of a lower dose(s) of insulin/insulin secretagogue if using canagliflozin as an add-on therapy is recommended. Elderly (≥ 65 years old): Renal function and risk of volume depletion should be taken into account. Paediatric population: The safety and efficacy of canagliflozin in children under 18 years of age have not yet been established. Contraindications: Hypersensitivity to the active substance or any of the excipients. Warnings and Precautions: [Refer to SmPC for more detail] Renal impairment: Limit dose to 100mg once daily in patients with eGFR < 60 mL/min/1.73 m 2. Regardless of pretreatment eGFR, patients on canagliflozin may experience an initial fall in eGFR that attenuates over time. Monitor renal function prior to and after initiating canagliflozin. Also monitor after initiating concomitant products that may reduce renal function. Patients at risk of volume depletion: Canagliflozin induces osmotic diuresis which may reduce intravascular volume and decrease blood pressure (BP). Caution should be exercised in patients for whom a canagliflozin-induced drop in BP could pose a risk. Advise patients to report symptoms of volume depletion. Canagliflozin is not recommended for use in patients receiving loop diuretics or who are volume depleted. Diabetic ketoacidosis (DKA): Rare cases of DKA, including life-threatening and fatal cases, have been reported in patients treated with SGLT2 inhibitors. Presentation of DKA may be atypical. Risk of DKA appears to be higher in patients with moderately to severely decreased renal function who require insulin. Assess patients immediately if symptoms occur, regardless of blood glucose level. Where DKA is suspected/diagnosed, discontinue canagliflozin immediately. Treatment should be interrupted in patients hospitalised for major surgical procedures or acute serious medical illnesses. Monitor ketones (preferably blood ketones) in these patients. Before initiating canagliflozin, consider factors in patient history that may predispose to DKA. Canagliflozin should not be used for treatment of patients with type 1 diabetes. Lower limb amputations: Before initiating canagliflozin, consider factors in patient history that may increase risk for amputation. Consider careful monitoring of patients with a higher risk for amputation, and counsel patients. Consider stopping canagliflozin in patients who develop events which may precede amputation. Necrotising fasciitis of the perineum: (Fournier’s gangrene): This rare but serious and potentially life-threatening event requires urgent surgical intervention and antibiotic treatment. Advise patients to seek medical attention if they experience a combination of pain, tenderness, erythema, or swelling in the genital or perineal area, with fever or malaise. Urogenital infection or perineal abscess may precede necrotising fasciitis. If Fournier’s gangrene is suspected, discontinue canagliflozin and institute prompt treatment. Elevated haematocrit: Monitor haematocrit levels in patients with an already elevated haematocrit. Elderly (≥ 65 years old):
* If further glycaemic control is needed, the addition of other anti-hyperglycaemic agents should be considered. § Continue dosing until dialysis or renal transplantation.
Elderly patients may be at a greater risk for volume depletion, are more likely to be treated with diuretics, and to have impaired renal function. Genital mycotic infections: Vulvovaginal candidiasis, and balanitis/ balanoposthitis were reported in clinical studies. Urinary tract infections (UTIs): Complicated UTIs including pyelonephritis and urosepsis have been reported. Temporary interruption of canagliflozin should be considered. Cardiac failure: Experience in New York Heart Association (NYHA) class III is limited, with no experience in clinical studies with canagliflozin in NYHA class IV. Urine laboratory assessments: Patients taking canagliflozin will test positive for glucose in their urine. Lactose intolerance: Patients with galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take this product. Interactions: Diuretics: may increase risk of dehydration and hypotension. Insulin and insulin secretagogues: risk of hypoglycaemia; consider lower dose of insulin or insulin secretagogue. Effects of other medicines on Invokana: Enzyme inducers (e.g. St. John’s wort, rifampicin, barbiturates, phenytoin, carbamazepine, ritonavir, efavirenz) may decrease exposure of canagliflozin; monitor glycaemic control. Consider dose increase to 300 mg if administered with UGT enzyme inducer. Cholestyramine may reduce canagliflozin exposure; take canagliflozin at least 1 hour before or 4-6 hours after a bile acid sequestrant. Effects of Invokana on other medicines: Monitor patients on digoxin , other cardiac glycosides, dabigatran Inhibition of Breast Cancer Resistance Protein cannot be excluded; possible increased exposure of drugs transported by BCRP (e.g. rosuvastatin and some anti-cancer agents). Pregnancy and lactation: Do not use canagliflozin during pregnancy or when breast-feeding. Discontinue canagliflozin when pregnancy is detected. Effect on fertility unknown. Effects on ability to drive and use machines: Canagliflozin has no or negligible influence on ability to drive and use machines. However, patients should be alerted to the risk of hypoglycaemia when canagliflozin is used as add-on therapy with insulin/insulin secretagogue, and to the elevated risk of adverse reactions related to volume depletion. Side Effects (SEs): Adverse reactions are based on the pooled analysis of placebo-controlled, clinical studies. Very Common (≥ 1/10): vulvovaginal candidiasis, hypoglycaemia in combination with insulin or sulphonylurea. Common (≥ 1/100, <1/10): balanitis or balanoposthitis, urinary tract infection (pyelonephritis and urosepsis have been reported post-marketing), constipation, thirst, nausea, polyuria or pollakiuria, dyslipidemia, haematocrit increased. Uncommon (≥ 1/1,000 to < 1/100): dehydration, dizziness postural, syncope, hypotension, orthostatic hypotension, photosensitivity, rash, urticaria, bone fracture, renal failure (mainly in the context of volume depletion), blood creatinine increased, blood urea increased, blood potassium increased, blood phosphate increased, lower limb amputations (mainly of the toe and midfoot) especially in patients at high risk for heart disease. For less frequent side effects see SmPC.
Pack sizes: 30 x 1 film-coated tablets. Legal category: POM. Marketing Authorisation number: EU/1/13/884/001-004 (100mg) and EU/1/13/884/005-008 (300mg). Marketing Authorisation holder: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd. Further information is available on request from Janssen Sciences Ireland UC, Barnahely, Ringaskiddy, IRL – Co. Cork P43 FA46 or may be found in the SPC.
Date of preparation: January 2023.
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse events via: HPRA Pharmacovigilance Website: www.hpra.ie. Adverse events should also be reported to Janssen Sciences Ireland UC on 1800 709 122 or at dsafety@its.jnj.com
Initiate with 100 mg. In patients tolerating 100 mg once / day and requiring additional glycaemic control, the dose can be increased to 300 mg. Continue 100 mg for patients already taking Invokana§ Invokana should not be initiated. Use 100 mg. ≥60 From 30 to <60* <30* (with UACR >300 mg/g) Dose adjustment recommendations according to eGFR¹
Date of preparation: March 2023 IR-INV-23-2023
90 Crohn’s Disease
Crohn’s and Colitis Ireland launch Symptom Checker

Ahead of World IBD Day this Friday May 19, and against this backdrop of delayed diagnosis, Crohn’s and Colitis Ireland has launched a new Symptom Checker (www.crohnscolitis.ie/symptomchecker). The tool forms part of its “Poo Taboo” campaign which aims to lift the lid on some of the stigma around IBD symptoms and the importance of people not being too shy to get checked out.
rectum, while Crohn’s disease can occur anywhere from the mouth to the anus.
2. What causes IBD? The cause of IBD is not fully understood, but may relate to a combination of genetic, gut bacteria and environmental factors.
Our Survey Says…
The survey to determine awareness of IBD among the population was conducted by Amárach research in March of this year. It found that:
• Awareness: most people (94%) have heard of IBD, Crohn’s disease or ulcerative colitis. Specifically, 79% had heard of Crohn’s disease while 43% had heard of ulcerative colitis.
3. What are the symptoms of IBD? Symptoms of IBD include diarrhoea, rectal bleeding, urgency to use the toilet, mouth ulcers, weight loss, fatigue, anaemia and abdominal pain and cramping. Crohn’s disease is often associated with anal problems such as fissures, tags and abscesses. IBD can also affect other parts of the body, particularly the joints, skin, eyes and liver.
IBD covers a number of conditions in which the digestive tract becomes inflamed, swollen and ulcerated – the two most common conditions being Crohn’s Disease and ulcerative colitis. It is thought that at least 40,000 people are living with IBD in Ireland, with most being diagnosed between 15 and 35 years, and then later in life, between 50 and 70 years. However, Crohn’s and Colitis Ireland believes that many more people remain undiagnosed. While ulcerative colitis affects the large intestine only, Crohn’s disease can occur anywhere along the digestive tract, and the inflammation can be much deeper, even perforating the bowel. Common symptoms include diarrhoea or loose stools, bleeding from the bottom, fever, fatigue, anaemia, weight loss, cramps and abdominal pain. With ulcerative colitis, there can also be a feeling of being unable to completely empty the bowel.
Don’t wait to get seen… Consultant gastroenterologist, Professor Barbara Ryan, continues, “I have known from my own clinical practice for some time that too many people delay getting seen to. They may put off getting checked out and having their symptoms investigated, or sometimes IBD, particularly Crohn’s disease, can be difficult to diagnose. For others, the path to diagnosis is much smoother and quicker, once they start to undergo
investigation. Thankfully, once the diagnosis is made, we now have an excellent and ever-expanding array of treatments available, and people can experience a rapid improvement in symptoms.
“While the results of the recent survey undertaken on behalf of Crohn’s and Colitis Ireland are not surprising to me, they are nonetheless still very concerning. It found that while four in ten would go to see a doctor immediately if they saw blood in the toilet bowl, an equal number would delay getting seen to.
“We want people to stop ignoring the key signs of what can be a serious disease, to overcome any embarrassment that they may have, and to seek medical advice. With our newly-developed symptom checker, people can, in the comfort and privacy of their own homes, answer a series of questions. In less than a minute, they will know if they need to see a GP for further examination.”
The symptom checker asks questions such as: whether an individual has seen blood in their poo more than once; if symptoms are present such as diarrhoea, needing to have a poo urgently or waking up in the night to poo; and whether a person is experiencing unexplained weight loss, abdominal pain, fatigue and fissures that don’t heal, or abscesses that keep coming back. Based on the results, they will then receive advice as to next steps.
• Symptoms: most also had a knowledge of some of the symptoms with four in five (83%) able to identify cramps or abdominal pain, 79% diarrhoea or loose stools, 70% blood in stool/toilet bowl/toilet paper, and 67% a feeling of being unable to completely empty the bowel.
• Taking action: however, when it came to acting on symptoms, two in five (40%) would do nothing or adopt a “wait-andsee” approach. This includes 5% of the overall sample who would be too embarrassed to seek advice, hoping that the symptoms would go away. One in ten (9%) would consult “Dr Google” before deciding what to do, while 7% would seek advice from a family member or trusted friend before deciding next steps. Of the overall sample, less than half (41%) would immediately seek advice from a GP or healthcare professional.
The “Poo Taboo” campaign is supported by the Irish College of General Practitioners.
IBD & YOU – SEVEN THINGS YOU SHOULD KNOW…
1. What is IBD? Inflammatory Bowel Disease (IBD) is an umbrella term for inflammatory conditions of the gastrointestinal (GI) tract including ulcerative colitis and Crohn’s disease. It is a chronic and complex condition with periods when it is both active and inactive. Ulcerative colitis is limited to the large bowel and
4. What’s the difference between IBD and IBS? IBD and IBS are two different gastrointestinal disorders. While they can have some similar symptoms, they are not the same condition and require very different treatments. IBD refers to inflammatory disease including Crohn’s disease and ulcerative colitis. IBS is a syndrome consisting of a group of symptoms that occur together, including abdominal pain, changes in bowel movements, which may be diarrhoea or constipation, or both, and excessive gas and bloating.
5. Will I spend the rest of my life running to the bathroom? No. Once a diagnosis and treatment plan are in place, the aim of treatment is to reduce symptoms, obtain remission and improve quality of life.
6. How can diet help? Malnutrition can affect up to 85% of people with IBD and so a well-balanced and healthy diet is important. This should incorporate protein which is key to muscle growth, good fats –known as unsaturated fats – which have health-promoting properties, and carbohydrates which are an important source of energy and B-vitamins. Of course, every person with IBD is different, and so too will be their dietary plan.
7. Is there a cure? There is currently no cure for IBD, however, treatment can help stabilise the disease and provide a return to a normal quality of life. Treatment can involve medications, surgery, and adopting new diet, exercise and lifestyle routines.
PHARMACYNEWSIRELAND.COM
Consultant gastroenterologist, Professor Barbara Ryan
Revolutionising CP Care in Ireland
The Cerebral Palsy Foundation (CPF) has announced the launch of a Programme of Excellence to revolutionise the delivery of cerebral palsy care in Ireland. Through the development of three major clinical and research hubs at Trinity, University College Cork, and RCSI University of Medicine and Health Sciences, this first-of-its-kind initiative will establish Ireland as an international leader in cerebral palsy care and research. Children’s Health Ireland (CHI) is an implementation partner in the academic healthcare programme.
Cerebral Palsy is the most common childhood-acquired lifelong physical disability. Many individuals with CP face significant and unnecessary challenges in their daily lives, including problems with movement, speech, and other body systems.
An estimated 150 babies receive a CP diagnosis in Ireland each year, and an estimated 3,000 children and young people and 9,500 adults are living with CP in Ireland. Early intervention and the right care pathways can make a significant difference in the longterm outcomes and quality of life for people living with CP.
Backed by $12.5 million in philanthropic funding pledged from a group of donors including John and Patrick Collison, this fiveyear programme will change the trajectory of people’s lives affected by CP in Ireland.
The Programme of Excellence will advance CP care and research across four major pillars:
Clinical Implementation: implementing the best evidence into clinical practice in healthcare settings.
Research: conducting highquality research programmes and developing an international network of researchers committed to implementation, dissemination, and knowledge transfer.
Dissemination and Education: promoting community education and providing professional education and career pipelines to encourage and develop new clinicians and researchers in the fields of CP clinical care and research.
Advocacy and Policy: identifying and developing stakeholder groups, supporting individuals with CP and their families to drive improved care.
The programme will build on significant strides already made by the CP research community and leading clinicians from around the world. An Expert Steering Committee of cerebral palsy and non-cerebral palsy experts from clinical care, academia, industry, and the CP community will guide the implementation ensuring
a multi-disciplinary and multi-stakeholder approach.
Four priority areas of clinical implementation and research have been identified as priority for the initial phase of the programme:
• Early Detection & Intervention (0-2yrs) led by UCC
• Musculoskeletal and Orthopaedic Care (children and young people) led by Trinity
• Community-Based Motor Management Services (children, young people, and adults) led by UCC, TCD & RCSI
• Adult services and support led by RCSI
These priority areas will be piloted in regions across Ireland. Once systems have been developed and proven they will be used as models to expand care across the country. The Programme will utilise already established networks including In4Kids, a Health Research Board national paediatric clinical trial network. CPF is committed to expanding this programme through further research funding and philanthropic support. Institutional support from Trinity, UCC, and RCSI guarantees its continuation beyond the initial five-year period.
"Our vision is to make Ireland a world leader in the delivery of Cerebral Palsy care," said Rachel Byrne, Executive Director of the Cerebral Palsy Foundation. “We want to create a sustainable continued care model for infants through to adults with CP in Ireland led by expert clinicians and researchers. We will leverage our extensive network and international expertise on best practice to help drive the programme through collaboration. We welcome all partners, including the CP Community, academia, industry, and government to join us on this journey."
Eilísh Hardiman, Chief Executive of Children’s Health Ireland welcomed this Programme:

“Children’s Health Ireland is committed to improving clinical outcomes for infants, children, and young people with Cerebral Palsy. As a partner in paediatric academic health sciences, CHI welcomes collaboration with Trinity and UCC to use this philanthropic funding pledge by the CP Foundation to truly make a difference to healthcare, education, research, and innovation in CP care in Ireland.”
Professor Geraldine Boylan, Director of the INFANT Research Centre at University College Cork, expressed excitement for the programme’s potential impact. “We are thrilled to be part of this programme in Ireland and to work with the Cerebral Palsy Foundation, healthcare & research community, and families to make a significant and positive difference in the lives of people with cerebral palsy. Early intervention is critical for people with CP. The earlier we can diagnose and intervene, the better the outcomes for the child and the family.”
Professor Colin Doherty, Head of School of Medicine at Trinity College Dublin said: "This programme provides a rare and unique opportunity for Ireland to become a world leader in cerebral palsy care and treatment. Trinity is working alongside other partners to make real discovery in the development of new diagnostic techniques, new therapies and new pathways of integrated care
between the hospital and the community and between childhood, adolescence and adulthood for people with cerebral palsy.”
Professor Cathal Kelly, ViceChancellor, RCSI University of Medicine and Health Sciences said: “We are really pleased to be a partner in this important initiative which will have a positive impact on the lives of people with cerebral palsy. As a university wholly focused on health, RCSI is deeply committed to educating the next generation of health care professionals and to research which drives improvements in patient outcomes and quality of life. The RCSI team will now build on the School of Physiotherapy’s track record of leading research and education on cerebral palsy, with a particular focus on adulthood, as part of this new collaboration.”
Professor Eleanor Molloy, Professor of Paediatrics & Child Health, Trinity Institute of Neurosciences (TCIN) said “every individual with cerebral palsy is different so it's very important that we can personalise care plans for each person. Huge work is being done across all aspects of research and patient care and this is being translated into tailored patient pathways and interventions throughout life.”
The Programme will also support the development of a Cerebral Palsy Register in Ireland, enabling the collection of important data to inform research and improve care.
PHARMACYNEWSIRELAND.COM 91
News
Professor Geraldine Boylan, Director of the INFANT Research Centre at University College Cork
Topic Team Training – Warts & Verruca
A community pharmacy environment that fosters teamwork ensures high levels of consumer satisfaction. This series of articles is designed for you to use as a guide to assist your team in focusing on meeting ongoing CPD targets and to identify any training needs in order to keep the knowledge and skills of you and your team up to date.
and make it more comfortable to remove clothing, if this is necessary. Examinations can be performed in the consulting room without the need for any specialised equipment, however on occasions a magnifying glass may be useful. Distribution of warts is generally asymmetric, and lesions are often clustered or may appear in a linear configuration due to scratching.
Treatment Goals
that contain salicylic acid and karaya gum–glycol vehicles that contain salicylic acid. Prior to recommending the use of these products, it is critical that pharmacists determine whether self-treatment is appropriate, first ruling out patients with any contraindications relevant to the use of salicylic acid.
Cryotherapy Products
The below information, considerations and checklist provide support to enable you to run a team training session and identify opportunities for learning within the topic of warts and verrucae.
Warts can look different depending on where they appear on the body and how thick the skin is. There are several different types of warts. The more common types include:
common warts
plantar warts (verrucae)
plane warts
filiform warts
periungual warts
mosaic warts
The appearance of each type of wart will depend on several factors:
where it is located on the body
the strain (type) of HPV that is responsible for the wart
factors such as whether the sufferers has a weakened immune system
whether they have rubbed or knocked the wart
Consider:
Verrucae
Plantar warts are found on the weight bearing areas of the sole and heel. Their appearance is different from the rest of the body owing to constant pressure imparted to the sole of the foot, causing the lesion to be pushed inwards.

Pressure of the nerve pain causes considerable pain and patients often complain of pain when walking. Tiny black dots that characterise verrucas are the thrombosed capillaries. This may not be visible until the hardened skin is shed away. They are distinguishable from corns as they have a whitened appearance and remain soft.
Corns appear as hard corns (top of toes) or soft corns (between toes). Callouses appear as flattened, yellow white thickened skin and are common on the balls of the foot. Like warts, they are 1cm in diameter and can occur singly or in crops.
Most pharmacies now have a consultation room. These should be utilised for performing examinations, allowing the patient to feel at ease, reduce embarrassment at the counter
Treatment goals for warts include effective removal that produces no scarring and prevents reoccurrence or spreading to other areas of the body or to another person. Certain patients, including those with diabetes, peripheral vascular disease, and immunodeficiency disorders, as well as those on immunosuppressive agents, should never attempt to use these products unless they have been referred to their GP.
Prior to recommending the use of products for self-treatment of warts, pharmacists should always make sure that self-treatment is appropriate.
Patients should be encouraged to seek medical care therapy if the wart remains after 12 weeks of therapy or if there are signs of infection, swelling, pain, or irritation.
Salicylic Acid Products
Products that can be used for self-treatment of common or plantar warts include plaster/ pad vehicles that contain salicylic acid, collodion-like vehicles
Key Points:
The importance of asking questions to enquire about symptoms
Who suffers from warts and verrucae?
Common causes and signs/symptoms of warts and of verrucae
The areas most affected on the body
The importance of self-care techniques when applying treatment
These are best for warts on hands. Cryotherapy has been used for several years and it used to freeze the wart. OTC products, marketed as home cryotherapy treatments, are available for home use. In offering guidance, patients should be advised to always adhere to the directions provided with these products, since improper use can cause damage to adjacent unaffected areas of the skin. Warts typically fall off 10 days after the application of the cryotherapy agent.
A persistent wart can only be treated 3 times using these products.
Formaldehyde is used for the treatment of verrucae. It is an irritant so is less suitable on hands. However, the thickened layer of skin on feet protects against this irritant. It is a gel formula.
Glutaraldehyde is also used for treatment of verrucae and should be used twice daily.
It is available in 5% and 10% percent strengths. It should not be used for anogenital warts only verrucae. It stains skin brown, although this is reversible.
Actions:
Ensure support staff understand the following key points:
The common presenting symptoms
The characteristics of a good analgesic for OTC use in the treatment of warts and verrucae
The benefits and limitations of OTC medicines
Is the pharmacy team fully trained on the indications and benefits of all products for the treatment of warts and verrucae?
How are wart and verrucae products displayed in the pharmacy?
Do we make the most of the potential for linked sales?
Am I up to date with the latest guidance?
Am I aware which preparations are recommended first-line?
The importance of cryotherapy products and how they work
Which OTC products to recommend
When to refer.
The importance of what outcome a patient presenting with warts or verrucae wants
Lifestyle issues that may impact on flare-ups
When to refer customers to the pharmacist.
92 | PHARMACYNEWSIRELAND.COM
1 Based on the market data, where salicylic acid is considered the most conventional liquid. The efficacy of this product has been demonstrated against salicylic acid. 2 In comparison to cryotherapy.

IRE WRT 2023 03
Diabetes during Pregnancy in HIV on the Rise
The frequency of diabetes during pregnancy is rising in women with HIV in Ireland and the UK, in line with trends in the rest of the population, Laurette Bukasa of University College London reported at the British HIV Association conference recently in Gateshead.
Blood sugar levels can rise during pregnancy, leading to the development of diabetes. Women are at higher risk of gestational diabetes if they are overweight or have obesity, have a South Asian, Black or African Caribbean or Middle Eastern background, or if a close family member has had diabetes. Having HIV does not raise the risk of developing gestational diabetes, but protease inhibitor-based antiretroviral treatment may raise the risk if it is started early in pregnancy.
A recently published study of pregnancies in South Africa’s Western Cape province found that women with HIV on antiretroviral treatment before conceiving had a lower risk of developing high blood pressure during pregnancy than women without HIV.
in women with HIV in the United Kingdom and Ireland between January 2010 and December 2020. The study also looked at the relationship between gestational diabetes and hypertension and adverse pregnancy outcomes including stillbirth, pre-term birth and low birth weight.
The study evaluated 10,401 pregnancies in 8998 women with HIV; 1104 pregnancies were excluded from the analysis either because HIV was diagnosed during pregnancy or because delivery occurred prior to 24 weeks (either miscarriage or very pre-term birth). The annual number of pregnancies in women with HIV halved between 2010 and 2020, from just under 1300 a year to approximately 600 a year in 2020.

Gestational diabetes was diagnosed during 554 pregnancies in 503 women; in 511 cases this was defined as gestational diabetes.
The study also found that the proportion of women diagnosed with high blood pressure during pregnancy has not risen.
The UK study looked at the prevalence of diabetes and high blood pressure during pregnancy
Women were screened for gestational diabetes between weeks 24 and 28 of pregnancy if they had a body mass index of 30 kg/m2 or above, a previous baby with a high birth weight, a family history of diabetes, or belonged to a minority ethnic group with a high prevalence of diabetes.
High blood pressure was diagnosed during 511 pregnancies in 458 women. In three out of four cases (383 pregnancies), high blood pressure was associated with a diagnosis of pre-eclampsia, a serious complication of pregnancy. Pre-eclampsia is usually detected by the presence of high levels of protein in the urine and high blood pressure. Pre-eclampsia affects transfer of oxygen across the placenta, affecting foetal growth and raising the risk of stillbirth and premature birth. In severe cases, pre-eclampsia may lead to fits or liver or kidney failure in the mother. The prevalence of high blood pressure did not change significantly between 2010 and 2020 (3.9% in 2010, 5.8% in 2020). Both diabetes and high blood pressure were diagnosed during 46 pregnancies in 43 women.
Latest Figures on Sexually Transmitted Infections
There has been “at least” a 100% increase in teenagers getting diagnosed with STIs this year, a consultant in sexual health has said.
Figures from the HSE have revealed that 783 teenagers in Ireland have been diagnosed with sexually transmitted infections (STIs) in 2023.
The figures included two children who are under 14 years of age.
The most common STI in Ireland this year was chlamydia with 4,311 cases, followed by gonorrhoea with 2,326 cases.
The news come after it was revealed earlier this year that HIV rates more than doubled in Ireland over the last year, according to statistics published by the Health Protection Surveillance Centre (HPSC).
The body responsible for tracking rates of sexually transmitted infections (STIs) has reported that for the first 51 weeks of the year, cases across nine disease types had risen by 56 per cent.
The upward trend follows concerns that Covid-19 restrictions had hampered testing services and the ability of people to present themselves for testing.
Consultant of sexual health and HIV care in genitourinary medicine at St. James Hospital Dr Aisling Loy said the data “definitely is alarming.”
“It's preliminary data so it gets cleaned up at the end of the year … before it's cleaned up it still is indicative that we are seeing a big rise,” she said.
Dr Loy said last year’s cleaned-up data revealed a total of 950 STI diagnoses among teenagers. “We're already on track to see maybe at least a 100% increase this year,” she said. “We’re a third way through the year and we’re at 700.
“Even when you take out some duplicate cases … there will be a slight drop, but not a huge drop.”
Dr Loy said that within the group of 15- to 19-year-olds, gonorrhoea diagnoses are driving the numbers.
“Traditionally in that age category, it was chlamydia, but actually that had gone down 6% in females, I think around 9% in young men,” she said.
“There's been a huge increase in gonorrhoea in that group.
“Traditionally we thought of these as being sexually transmitted by say penis to vagina, and that it would be traditional sexual routes of transmission.
56 Recommendations for ‘More Inclusive’ Ireland
Fine Gael LGBTQ+ launched Building on Progress: An Inclusive Ireland, a policy document designed to guide officials toward developing a more equal society. It makes 56 recommendations across seven government departments, namely Education, Equality, Foreign Affairs, Health, Housing & Local Government, Justice and Sport.
In the publication, the group outlines over 20 pressing issues that LGBTQ+ people face in Ireland today. These include HIV transmissions, transgender healthcare, hate crimes, safety in schools, conversion therapy, surrogacy, participation in sport, global rights, homelessness and more. The document was created independently by Fine Gael LGBTQ+, a voluntary organisation made up of party members, staff and Oireachtas officials. While it has been submitted to Fine Gael for consideration, it has not been officially adopted as policy.
On Wednesday, May 3, parliamentary members of the party held a briefing to have a “constructive and positive conversation” on LGBTQ+ issues.
PHARMACYNEWSIRELAND.COM 94 HIV News
CLONMEL HEALTHCARE: NEW LAUNCH – FLAVOUR AND PACK SIZE!
FEEL HYDRATED, STAY RECHARGED WITH ELECTROSAL
Clonmel Healthcare are delighted to announce the launch of Electrosal Orange Flavour AND a brand new 5 pack. The 5 pack is an addition to the existing 10 pack. Electrosal is an oral hydration solution containing 3 electrolytes in 1. Electrosal can be used for effective hydration to help replace the loss of fluid and body salts due to exercise, sports, hot weather or travel. Electrosal is vegan friendly, gluten free and GMO free
Available in 3 flavours – Orange, Lemon & Raspberry and Blackcurrant.
the perfect solution to achieving the ultimate summer glow while also keeping the skin youthful with their new serum. This fast-drying face serum is a game-changer, combining hydration and skin-smoothing properties to give you a radiant complexion like never before.
only does this invigorating aroma uplift your mood, but the oil also possesses anti-inflammatory properties that help diminish redness and irritation, ensuring a calm and soothing effect on your skin.
With these exceptional active ingredients working harmoniously, TanOrganic’s Anti-Ageing Facial Tan Serum offers unparalleled antiageing benefits that will transform your skincare routine. Say goodbye to dull and ageing skin and embrace a luminous and revitalized complexion with TanOrganic’s ground-breaking formula. With an impressive 100% score on the Ethical Organization, TanOrganic stands as one of only seven companies globally to achieve this remarkable distinction.
is adding to its portfolio with a new shampoo for daily use; Nizoral® Anti-Dandruff Daily Prevent Shampoo.

Nizoral® Anti-Dandruff Daily Prevent Shampoo provides ongoing relief for dry, itchy and flaky scalps and is clinically proven to provide instant protection from the first wash.
Nizoral® Anti-Dandruff Daily Prevent Shampoo is suitable for sensitive scalps, and all hair types. Please contact Clonmel Healthcare on 01-6204000 if you require any additional information, or visit www.nizoral.ie.
Electrosal is available in two pack size – 5 pack and 10 pack. Electrosal is suitable for adults and children over 2 years.
Please contact Clonmel Healthcare on 01-6204000 if you require any additional information on Electrosal. Electrosal is a food supplement not to be used as a substitute for a balanced diet. Always read the label.
For more information visit www.electrosal.ie
Clonmel Healthcare Ltd., Clonmel, Co. Tipperary.
Date Prepared: May 2023 2023/ ADV/ELE/118H
TANORGANIC INTRODUCES REVOLUTIONARY ANTI-AGEING FACIAL TAN SERUM FOR A RADIANT AND YOUTHFUL GLOW

This summer season, TanOrganic are thrilled to announce the launch of their latest addition to the collection and a step into the skin health space with their NEW Anti-Ageing Facial Tan Serum (RRP ¤44.99). Recommended by dermatologists, TanOrganic, the leading provider of organic and natural tanning products provides
This breakthrough serum is designed to provide a naturallooking tan while simultaneously delivering powerful anti-ageing benefits, allowing users to embrace their confident and radiant selves during those makeup-free summer days. This revolutionary serum redefines the self-tanning experience. Formulated with over 90% organic ingredients, including the remarkable peptide Matryxl 3000, apple stem cell, hyaluronic acid, orange peel oil, and aloe vera, TanOrganic’s Anti-Ageing Facial Tan Serum provides a comprehensive skincare experience that goes beyond mere tanning.
One of the standout components is Matryxl 3000, a potent peptide renowned for its ability to stimulate collagen production in the skin. Collagen is the key protein responsible for maintaining skin elasticity and firmness, but its levels decline with age, leading to unwanted wrinkles and sagging. By harnessing the power of Matryxl 3000, TanOrganic’s serum effectively reduces the appearance of fine lines and wrinkles, while also improving overall skin texture and tone.
In addition to Matryxl 3000, TanOrganic have incorporated apple stem cell peptides, known for their remarkable capacity to promote skin regeneration and reduce the visibility of wrinkles. These peptides work tirelessly to enhance your skin’s health, resulting in a more youthful and rejuvenated complexion. To elevate your sensory experience, the Anti-Ageing Facial Tan Serum features orange peel oil, providing a refreshing citrus scent. Not
TanOrganic products are COSMOS Vegan Society Certified, Leaping Bunny Approved, Ethically Certified and Guaranteed Irish. TanOrganic’s Anti-Ageing Facial Tan Serum is available on TanOrganic.com and in Boots, Dunnes Stores, Shaws, and Tesco stores nationwide and leading pharmacies including McCauleys, Meaghers, and McCabes.
Instagram @TanOrganicOffical & Facebook@TanOrganic
For further information/ imagery/ giveaways contact
Joanna Timmons at The Publicity Loft/ Email joanna@publicityloft. com or phone 085 7666 473
About TanOrganic
TanOrganic use only the best, natural and organic ingredients that are free from all synthetics, parabens, and toxins, the world’s first and only eco-certified tan brand enriched with luxurious aloe vera and hyaluronic acid to keep your skin hydrated and give it a natural radiance with the brand removing 1lb of plastic from the ocean for every bottle sold!
As the world’s first and only Ecocertified, Vegan Society, Cruelty-Free, and Ethically Certified self-tanning brand, TanOrganic has established itself as a leader in the industry. The brand’s commitment to using only natural and organic ingredients resonates with conscious consumers who prioritize both their skin health and the environment.
THE SCALP CARE EXPERT NIZORAL® LAUNCHES
ANTI-DANDRUFF
PREVENT SHAMPOO
The scalp care expert Nizoral®; known and trusted by Irish customers for treating dandruff with Nizoral Dandruff Shampoo;
Nizoral Dandruff 20mg/g Shampoo contains 20mg of ketoconazole per each gram of shampoo. Supply through pharmacies only. A copy of the Summary of Product Characteristics is available upon request or go to www.clonmelhealthcare.ie.
Marketing authorisation holder: Clonmel Healthcare Ltd. Waterford Road, Clonmel, Co. Tipperary. Always read the label. Date prepared May 2023. 2022/ADV/ NIZ/310H.
DATE FOR YOUR DIARY –MEDTECH IRELAND 2023
Medical Technology Ireland will bring together manufacturers, academic institutions, entrepreneurs/start-ups, financial institutions and suppliers alike in a combined programme, defining present and future medical device trends, to investigate, discuss, and improve the quality of life and to increase the life expectancy for patients worldwide.
The Medical Technology Ireland Conference is taking place on 20-21 September 2023 in Galway. A Call for Papers is now open for individuals and organisations that would like to submit a presentation for the Conference programme. Please send your proposal to Tom Burke, tom@harpe.ie
PHARMACYNEWSIRELAND.COM 95 Clinical Profiles
NIZORAL®
DAILY
BIOSIMULYTICS ENTERS EXCLUSIVE HPC CLOUD PARTNERSHIP WITH CGG

Biosimulytics, an Irish pharma software company, today announced that it has signed an exclusive partnership agreement with CGG, a global technology and HPC leader headquartered in Paris, France. CGG will provide Biosimulytics, which uses artificial intelligence (AI) technologies to dramatically improve drug development, with a fully customized HPC (high performance computing), AI, and cloud solution to rapidly serve the company’s growing volume of pharmaceutical customers worldwide.
Using CGG’s algorithm and HPC expertise enables Biosimulytics to fully scale its breakthrough pharmatech platform which provides pharmaceutical companies with a powerful predictive simulation capability when developing new drug molecules.
The Biosimulytics technology shortens the time to market and reduces the risks for important developments in the pharmaceutical industry in getting from molecules to medicine (M2M) and is a key enabling technology for the new era of precision medicine. CGG’s custom solution meets the specialist needs of Biosimulytics for a high-performing HPC and AI environment that optimizes its suite of specialized molecular simulation applications from crystal structure prediction (CSP) to structure-based drug design (SBDD).
Biosimulytics can now run applications at least five times faster than its previous public cloud solution, enabling it to dramatically improve its return on investment and rapidly expand its business.
As a European company offering cloud services hosted in the European Union, CGG also offers the full legal protection of EU regulations for the intellectual property of Biosimulytics and its customers.
Biosimulytics, which is headquartered at NovaUCD in Dublin, is a University College Dublin (UCD) spin-out company from the UCD School of Chemical and Bioprocess Engineering that was founded in 2019. Biosimulytics is also an Enterprise Ireland High-Potential Start-Up (HPSU) company and is strongly backed at the European level through EIT Digital and other EU-funded accelerator programmes.
Biosimulytics is working as a Digital R&D solution provider to some of the world’s leading pharmaceutical companies. The
AI drug development market is expected to exceed US$10bn by 2030 growing at a CAGR of 25%.
Peter Doyle, CEO and CoFounder of Biosimulytics, said, “We are delighted to announce this exclusive partnership with CGG since our need for a high-performing HPC and AI environment to optimize our Biosim M2M platform is uniquely met with CGG's customized solution.
The AI drug development space is now at an inflection point and our ambition is to enable the global pharma industry to advance potential molecules to approved medicines quicker and with a much greater probability of success by making AI-powered predictive technology much more accessible and affordable for widespread use by everyone from Big Pharma to small emerging biotech companies. Today’s announcement with CGG is a significant milestone on that journey.”
Agnès Boudot, EVP, HPC & Cloud Solutions, CGG, said, “CGG is a global expert in industrial and customized end-to-end HPC and AI services with over 70 years of experience in pioneering computing solutions. After successfully establishing our cloud services offering in the energy, energy transition and mining sectors, where more than 30 external clients are currently directly accessing and using CGG Cloud solution services for delivering insights into their data, we are pleased to expand into the healthcare and life sciences market by supporting Biosimulytics in using AI- and HPC-based technologies to help unlock new and improved therapies faster and more cost efficiently.
Our experience of hosting and optimizing scientific workflows
running on over 350 petaflops at an industrial scale ensures that we can provide Biosimulytics with a scalable solution to meet their future growth requirements for this complex HPC and AI workflow.”
LETTERKENNY UNIVERSITY HOSPITAL WELCOMES PUBLICATION OF HIQA REPORT FOLLOWING INSPECTION
Letterkenny University Hospital welcomes the publication by Health Information Quality Authority (HIQA) of its report into its inspection of three areas at Letterkenny University Hospital (LUH). An announced inspection was carried out at the hospital on the 16th and 17th of November 2022.
The areas assessed were the Emergency Department, Medical 2 Ward and the Gynaecology Ward.
The focus of the inspection was to monitor the National Standards for Safer Better Healthcare. HIQA’s core assessment focused on key standards relating to leadership, governance and management, workforce, person-centred care, safe care and support and effective care.
During this inspection, HIQA looked at 13 National Standards in relation to leadership, governance and management and workforce. Of these, Letterkenny University Hospital was substantially compliant with 3 National Standards, partially compliant with six National Standards and non-compliant with four National Standards.
Sean Murphy Hospital Manager at Letterkenny University Hospital said, “We welcome this report and recognise the important and valuable role of HIQA in promoting safety and quality in the healthcare services. Urgent action has been taken to address the issues identified by HIQA and significant progress has been made by all staff to ensure that we provide our services safely.
“I would like to acknowledge the on-going commitment and dedication of our staff in providing a patient centred approach and we will work together to build on the good practice highlighted in this report.”
Significant work has been carried out on all areas where the hospital was found to be non-compliant or partially compliant. In a number of areas this work had commenced prior to the HIQA Inspection.
In the area of Overall Governance, the revision of the Clinical Handover Policy has been completed.
As part of the implementation of the National Healthcare communication programme,
facilitator training is underway with five members of staff identified to undertake the training.
A programme of work to strengthen governance within Letterkenny University Hospital (LUH) has been identified. The LUH Hospital Executive Board (HEB) and the QPS service, working with the Change Implementation Manager and Saolta Executive will agree and implement changes to the governance structure at LUH to include updated terms of reference; membership; and reporting relationships.
The HIQA concerns regarding the governance of the Respiratory Receiving Unit (RRU) were in the process of being addressed at the time of inspection. Changes were implemented in December 2022 and full management of the service was transferred to the emergency medicine service in January.
The acute medical assessment unit was re-established at the hospital in February with the employment of Consultant AMAU Physician. This marked the cessation of a distinct RRU facility. With changes to Covid pathway guidance, the area is no longer utilised as a red ED stream. The AMAU is under the governance of the medical directorate.
In regards to access to diagnostics, a second CT scanner is now operational at the site with protected slots for AMAU each day. The MRI scanner at the hospital is scheduled for replacement later this year and the new scanner will increase both functionality and speed within the service.
In relation to the risk register internal audit, risk register meetings have increased from quarterly to monthly to ensure the timely implementation of all recommendations. 5 out of the 7 recommendations are fully implemented with the remaining 2 almost fully implemented.
Recruitment remains a top priority for LUH. Since November 2022 the hospital has recruited 140 new staff across all grades. A new electronic employment control tracker has been implemented in April to streamline the recruitment process between LUH and Saolta. LUH HR Department has carried out significant recruitment over the last number of years for the Pharmacy Department at LUH, with an increase of 12 WTE (pharmacists and technicians) approved for LUH in the last two years, however like many hospitals LUH faces significant challenges in recruiting senior pharmacists required to undertake clinical pharmacy roles.
PHARMACYNEWSIRELAND.COM 96 Clinical Profiles
Doyle, CEO and Co-Founder, Biosimulytics
In relation to Safe Care and Support, a further Assistant Director of Nursing was appointed to assist patient flow in February. The Saolta Group Unscheduled Care Lead is working very closely with the patient flow team in LUH ensuring all optimal patient flow processes are in place.
LUH has also received funding to employ four additional Consultant in Emergency Medicine. This will allow the hospital to improve the availability of Senior Clinical Decision makers within the Emergency Department and extend their onsite presence. LUH is seeking to fill of posts on a locum basis pending permanent filling of same. A Consultant has been employed to cover AMAU which has direct responsibility for staff in AMAU under Medical Department governance.
The Pathfinder project commenced in LUH in April 2023 to support admission avoidance by providing assessment and treatment at home. An ambulance liaison person is also now based in the ED to support nursing staff in caring for patients in ambulances. The hospital has also recently appointed a GP Liaison Senior nurse to be the link between GPs throughout Donegal and the LUH Emergency Department.
A Pathway stream has also been developed to fast track patients presenting with minor injuries through the Emergency Department.
LUH are working with HSE Estates colleagues to provide a modular building to relocate non clinical functions in the Emergency Department and convert the non-clinical accommodation into additional patient treatment areas for patients with potential transmissible diseases.
A Patient Advice and Liaison Service (PALS) Coordinator officer has been appointed to ensure patients receive communication and access to advice as required. The Patient Advice and Liaison Service Coordinator acts as a visible focal point for patients, families and carers and is often the main contact within the hospital. They help to resolve issues for patients and their families and work towards improving the patient experience at any available opportunity. A second PALS officer is currently being recruited.
Under the area of Effective Care and Support actions have also been introduced in response to high levels of C.Difficile. The hospital continues to advertise for antimicrobial pharmacist and Consultant Microbiology
posts. In the interim there is a system in place to manage Infection Prevention and Control (IPC) issues and locums will be employed where possible.
NEW STANDARDS DEVELOPED TO SUPPORT CHILDREN HAVING HEALTHCARE PROCEDURES
Children in Hospital Ireland (CIH Ireland) welcome new international standards to support children and young people as they undergo healthcare procedures.
Lucy Bray, Professor of Child Health Literacy at Edgehill University has worked with an international team of health professionals, academics, children, young people, parents, child rights specialists, psychologists and youth workers to develop a set of standards for children and young people having health care procedures.
Anna Gunning, CEO of Children in Hospital Ireland stated, “For a child, it can be very daunting to visit a hospital and undergo new procedures, and these experiences can have a huge impact on a young person’s physical, emotional and psychological well-being. The child’s interests must come first and it is crucial that they are met with positive experiences. This will build confidence, reduce harm and establish trust. We warmly welcome these standards and look forward to Lucy Bray sharing her research with us here in Ireland.”
Children in Hospital Ireland will host their Annual Lecture with Lucy Bray on May 2nd to explore these standards and learn how parents and healthcare professionals can work together to improve the experiences for children and young people in hospital. Children in Hospital Ireland is a national charity that supports children and families in hospital through play and advocacy. Working for over 50 years in hospitals throughout Ireland, CIH provides play opportunities for children in hospital and seeks to support the rights and improve the welfare of children in hospital.
Professor, Lucy Bray added “We need to do more to make sure every child is supported to have a positive procedural experience and that their short- and long-term best interests are prioritised in all clinical decisions.
We would urge health professionals and organisations to do all they can to familiarise themselves with the standards and we hope that they prompt conversations in practice about how to support children before, during and after their procedure.“
Lucy Bray will be the keynote speaker at CIH’s Annual Lecture on May 2nd at The Albert Lecture Theatre in RCSI Dublin. To attend, please book your place online at: https://www.eventbrite.ie/e/annuallecture-getting-it-right-first-andevery-time-tickets-597713496157
HORIZON THERAPEUTICS
PLC RANKS FIRST IN PATIENT CENTRICITY AND INTEGRITY
Horizon Therapeutics plc (Nasdaq: HZNP) today announced it ranked first in patient centricity, integrity, ease of relations and transparency in pricing by patient groups who worked with the company around the world. It ranks second in overall corporate reputation among these same groups. The results are based on PatientView’s annual survey of more than 2,200 patient groups evaluating more than 40 biotechnology and pharmaceutical companies. Among patient groups familiar, but not working directly with Horizon, the company also ranked first in patient centricity, integrity and ease of relations and third overall in overall corporate reputation.
“Everything we do at Horizon is guided by people living with challenging diseases and the patient groups who serve them,” said Matt Flesch, vice president, product communications and patient advocacy, Horizon. “We listen to input from patient groups on everything from clinical trial design to disease education initiatives, and work to support programs that have the greatest potential impact. We are constantly evolving our approach to best meet the needs of patient communities, and that is why this positive PatientView report means a great deal to all of us at Horizon.”
For the 2022 PatientView survey, 247 patient groups from around the world claimed familiarity with Horizon, an increase of 67 from 2021. Additionally, 130 groups said they had worked with the company, an increase of 43 from 2021. In each of the 13 indicators by which patient groups assessed corporate reputation, Horizon ranked in the top tier of global pharmaceutical and biotechnology companies
Horizon’s Commitment to Patients and their Advocates
• Held the first Rare Autoimmune Emerging Leaders' Summit, bringing together 26 patient advocate leaders in the rare and autoimmune space to provide important connections and a forum for groups to learn from each other.
• First ever Thyroid Eye Disease (TED) Mobile Exhibit in Atlanta,
Georgia, providing TED resources and information to attendees. This was part of Horizon’s ongoing effort to bring education and awareness to underserved communities.
• Brought together patients, care partners, and patient advocacy leaders to Horizon’s U.S. headquarters to discuss current challenges the NMO community is facing and to brainstorm on novel ideas and opportunities to support the NMO community.
• In Europe, hosted a disease awareness photo exhibition of individuals living with NMOSD at the 17th World Congress on Controversies in Neurology (CONy) Congress; individuals also attended the opening inperson to speak to Congress participants about their journey and experiences of living with NMOSD.
• In Brazil, supported the importance of multidisciplinary care to overcome the social and economic burden of rare autoimmune diseases through disease education initiatives that involved the art of spreading the patients' voice, like the "Fazendo Arte com Gustavo Rosa" art workshops for NMOSD patients and the short film "Atrás dos Meus Olhos", that tells the story of a TED patient.
• Became sole National Presenting Sponsor for Arthritis Foundation’s flagship event, Walk to Cure Arthritis, creating Horizon teams to volunteer and participate at over 70 events.
• Enhanced Horizon’s global, disease-agnostic #RAREis campaign and platforms including:
o Global Advocate Grant: Awarded 30 $5,000 grants supporting the rare disease community by providing financial assistance to global patient advocacy groups working to advance, educate and address the needs of the community.
o Scholarship Fund: In partnership with the EveryLife Foundation for Rare Diseases awards a one-time $5,000 educational scholarship to adults living in the U.S. with rare diseases. 177 scholarships have been awarded to date.
o Adoption Fund: In partnership with Gift of Adoption, provided financial support for 54 children living with rare diseases to be adopted.
PHARMACYNEWSIRELAND.COM 97
NEW STRATEGY AIMS TO PROMOTE, PROTECT AND PROGRESS NURSING AND MIDWIFERY PROFESSIONS
The Nursing and Midwifery Board of Ireland (NMBI) has launched its Statement of Strategy which will run from 2023-2025.

As part of the new strategy, over the next three years, NMBI will focus on protecting, promoting, and progressing the nursing and midwifery professions. These strategic priorities will be supported through partnership and technology.
The strategy reflects the changing healthcare landscape and the need for nurses and midwives to be equipped with the knowledge, skills and resources to provide safe, high-quality care.
Some of the key priorities within the strategy include:
• Developing an online and inperson hub to support nurses and midwives from overseas to apply to join the Register in Ireland, and to adapt to working here.
• Reviewing entry pathways into nursing and midwifery to establish alternative routes, such as graduate-entry nursing.
• Working across health and education sectors to develop a plan to increase undergraduate student placement numbers.
• Implementing a more person-centred fitness to practise process to ensure a compassionate approach.
The strategy was developed following extensive consultation with nurses, midwives, educators and other key stakeholders. It sets out a clear vision and objectives to advance the nursing and midwifery professions in Ireland and enhance patient care.
Minister for Health, Stephen Donnelly TD said: "Nurses and midwives play an integral and important role across Irish
healthcare. I welcome the publication of NMBI’s Statement of Strategy 2023-2025, which acknowledges this key role and sets out an ambitious agenda focussing on how the NMBI, as a modern and progressive regulator, intends to play its leading role in supporting the development of the nursing and midwifery professions over the next three years.”
The Chief Nursing Officer, at the Department of Health, Rachel Kenna said: “I welcome the publication of this statement of strategy, which will help shape the future of our professions in Ireland. As Chief Nursing Officer, I am committed to supporting the implementation of this strategy and to ensuring that nurses and midwives have the resources and support they need to provide the best possible care.”
The President of NMBI, Louise Kavanagh McBride said: “As President of the Nursing and Midwifery Board of Ireland (NMBI) I am delighted to be publishing our new Statement of Strategy 2023-25. This strategy is the result of extensive consultation and collaboration with nurses, midwives, and stakeholders across the country, and it reflects their aspirations for the professions. Today’s publication is grounded in evidence-based practice and international best practice. On behalf of the Board, I would like to thank all stakeholders who took part in the consultation making this strategy possible.”
NMBI CEO, Sheila McClelland said: “We are acutely aware of the challenges facing Ireland’s healthcare settings in recruitment and retention. The objectives outlined in this strategy set out a clear vision and roadmap for the future of these vital professions
in Ireland. I believe that this strategy will not only enhance the professional development and well-being of nurses and midwives, but it will also have a positive impact on patient outcomes and experiences. I look forward to seeing the positive impact it will have on our professions and the wider healthcare system."
A full copy of NMBI’s Statement of Strategy 2023-2025 is available to view online: https:// www.nmbi.ie/NMBI/media/ NMBI/NMBI-Statement-ofStrategy-2023-2025.pdf
SPECIALIST NURSES SHINE THE SPOTLIGHT ON HEART FAILURE AWARENESS
European Heart Failure Awareness Week occurs between 01 to 07 May. “Let’s Bump up the Pump” is a new campaign devised by the Irish Association of Heart Failure Nurses (IAHFN) to raise awareness around heart failure, with a focus on prevention, early detection and also to provide education around how to live well with this condition.
The campaign is supported by the heart and stroke charity Croi who recognise the important role Heart Failure Nurse Specialists play in heart failure care. “Croi is delighted to support the IAHFN in its campaign to raise awareness of heart failure and in particular to raise awareness of the signs and symptoms which are often dismissed or ignored. It is very important that people know the signs and symptoms and that they convey these to their doctor and ask could they have heart failure” says Croi CEO, Neil Johnson.
Heart Failure can be an alarming diagnosis, and many do not understand the term. Heart failure is a condition where the heart fails to pump or relax well enough to circulate sufficient blood to meet the body’s needs. As a consequence, affected individuals can complain of breathlessness on exertion or at rest, swollen legs, and tiredness. It is a serious condition, left untreated heart failure is as deadly as cancer
but there is good treatment out there, the key is in early diagnosis and to begin treatment as soon as possible to reduce death and disability from this disease. Attending your GP for a simple blood test called NTproBNP can help support the diagnosis of Heart Failure and support to ‘detect the undetected’.
Heart Failure can happen for a variety of reasons, but is more common as we get older, in people with heart disease and in those with high blood pressure. It is a common condition affecting about 2% of the Irish population, rising to 10% in older age groups. Worryingly, there are about another 2% of the population who are currently undiagnosed, and trends are rising in terms of expected prevalence due to an aging population and better medical treatments. The “Let’s bump up the pump” campaign aims to raise awareness to ensure earlier detection of those who are at risk of heart failure.
Staff from across the Saolta Group in Galway, Mayo and Roscommon and Community Healthcare West (CHW) are working together to roll out the Enhanced Community Care Programme, which provides greater levels of care in the community. This includes the creation of ambulatory hubs to facilitate GPs to refer patients such as those who have or are suspected to have heart failure, for diagnostics and access to expert opinion and support.
Dr Susan Connolly, Consultant Cardiologist at Galway University Hospitals and who is leading out on the Cardiovascular Ambulatory Hub in Galway said, “As part of the new Integrated Care Chronic Disease Management Programme in Saolta/CHW we are focusing
Dr Susan Connolly, Integrated Care Consultant Cardiologist in Cardiovascular Disease at Galway Ambulatory Hub and Niamh Elwood, Integrated Care Clinical Nurse Specialist Cardiovascular Disease, Galway Ambulatory Hub

PHARMACYNEWSIRELAND.COM 98
Clinical Profiles
Chief Nursing Officer at the Department of Health, Rachel Kenna, President of NMBI Louise Kavanagh McBride and NMBI CEO, Sheila McClelland
on detecting heart failure earlier by providing GPs with more rapid access to diagnostics, by providing early access to specialist cardiology opinion and by having a skilled team of cardiovascular specialist nurses to help patients manage their condition and lead a normal life as possible. Don’t delay, if you or someone you know has these symptoms seek help – we are here to do our best for you.”
The “Let’s bump up the pump” campaign wants to bump up awareness of the signs and symptoms of heart failure, to bump up the number of those with an earlier diagnosis, bump up knowledge of living with the condition and ultimately bump up survival for those living with heart failure. This campaign supports the global “25in25” initiative seeking to reduce heart failure deaths by 25% in the next 25 years.
Emer Burke, Advanced Nurse Practitioner with Galway University Hospitals and the Galway Integrated Heart Failure Service advises, “Anyone concerned about symptoms of heart failure please contact your GP for an assessment – Ask about the NTProBNP test. The earlier you get diagnosed, the earlier treatment can begin which can potentially alter the course of this serious disease, with treatment you can live well with heart failure.”
ABBVIE ANNOUNCES
EUROPEAN COMMISSION
APPROVAL OF RINVOQ® (UPADACITINIB) FOR THE TREATMENT OF MODERATELY TO SEVERELY ACTIVE CROHN’S DISEASE
AbbVie (NYSE: ABBV) has announced European Commission (EC) approval of upadacitinib, 45 mg [induction dose] and 15 mg and 30 mg [maintenance doses] as the first oral Janus Kinase (JAK) inhibitor for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1-4
“The EC approval of RINVOQ in Crohn’s disease is a significant milestone in offering patients the first and only once-daily oral treatment that can provide endoscopic improvement, and sustained symptom relief, making a difference in their daily lives,” said Thomas Hudson, M.D., senior vice president, research and development, chief scientific officer, AbbVie. “With existing therapies, not all patients are able to achieve adequate disease control to meet their treatment goals2,3, which is why we continue to embrace the challenge of
expanding our IBD portfolio with new treatment options.”
The EC approval is supported by data from two induction studies, U-EXCEED and U-EXCEL, and the U-ENDURE maintenance study.1 Statistical significance was achieved for the co-primary endpoints and key secondary endpoints with upadacitinib 45 mg in the induction studies and upadacitinib 15 mg and 30 mg in the maintenance study compared to placebo.1-4
Co-Primary Endpoint Results from the Phase 3 program include1-4:
• Endoscopic response : In U-EXCEED and U-EXCEL, 35% and 46% of patients treated with upadacitinib 45 mg achieved endoscopic response at week 12, respectively, compared to 4% and 13% of patients receiving placebo.1 In U-ENDURE, 28% and 40% of patients treated with upadacitinib15 mg and 30 mg achieved endoscopic response at week 52, respectively, compared to 7% of patients receiving placebo.1
• Clinical remission : In U-EXCEED and U-EXCEL, 40% and 51% of patients treated with upadacitinib 45 mg achieved clinical remission at 12 weeks, respectively, compared to 14% and 22% of patients receiving placebo.1 Additionally, in U-ENDURE, 36% and 46% patients treated with upadacitinib 15mg and 30mg achieved clinical remission at 52 weeks, respectively, compared to 14% of patients receiving placebo.1
Key Secondary and Additional Endpoints include:
• Corticosteroid-free clinical remission : In U-EXCEED and U-EXCEL, 37% and 44% of patients treated with upadacitinib 45 mg achieved steroid-free remission at week 12, respectively, compared to 7% and 13% of patients receiving placebo. In U-ENDURE, 35% and 45% of patients treated with upadacitinib 15 mg and 30 mg achieved steroid-free remission at week 52, respectively, compared to 14% of patients receiving placebo.1
• Mucosal healing : Additionally, in U-EXCEED and U-EXCEL, 17% and 25% of patients treated with upadacitinib 45mg achieved SES-CD ulcerated surface subscore of 0 at week 12, respectively, compared to 0% and 5% of patients receiving placebo. In U-ENDURE, 13% and 24% of patients treated with upadacitinib 15 mg and 30 mg achieved SES-CD ulcerated surface subscore of 0 at week
52 compared to 4% of patients receiving placebo (all with nominal p-value<0.001).1
“Crohn’s disease is a burden that can present patients with daily, often uncomfortable challenges,” said Laurent PeyrinBiroulet, M.D., Ph.D., professor of gastroenterology and head of the Inflammatory Bowel Disease group at the Gastroenterology Department, University Hospital of Nancy, France. “These studies demonstrated upadacitinib's ability to achieve key treatment targets, including endoscopic outcomes and symptomatic relief, that are critical for patients and beneficial for long-term care.”
Upadacitinib in Crohn's disease was well tolerated, with no new safety risks observed compared to the known safety profile of upadacitinib.2-4 Similar rates of serious adverse events including serious infections, were observed between patients receiving upadacitinib and placebo.1-4 The most common adverse events included nasopharyngitis, acne and COVID-19 in the upadacitinib treatment group.1-4 Reports of malignancy, major cardiovascular events, venous thromboembolic events and gastrointestinal perforation were infrequently observed (<1.0 Events/100 Patient-Years).2-4
Upadacitinib is approved in the EU for the treatment of adults with radiographic axial spondyloarthritis, non-radiographic axial spondyloarthritis, psoriatic arthritis, rheumatoid arthritis, ulcerative colitis, adults and adolescents with atopic dermatitis and now Crohn’s disease.1,5-9
References available on request
IRISH LIFE HEALTH EXPANDS DIGITAL DOCTOR SERVICE
Irish Life Health today announced the expansion of its Digital Doctor service, introducing next day access to in-person GP appointments at 50 Centric Health centres across Ireland. This service is available on all hospital plans when required post a Digital Doctor consultation.
Irish Life Health moved its Digital Doctor service to Centric Health in June 2022, meaning its customers have had unlimited and timely 24/7 access to Irish-based GPs by phone or video call. Since introducing the enhanced service, Irish Life Health has seen a 32% increase1 in usage of the digital doctor service.
To date, our experience is that over 90% of digital doctor consultations resolve the issue, however for the less than 10% where this isn’t possible2, a
rapid follow up in-person GP appointment is required. This expansion of the service means that when a customer’s issue cannot be resolved through their digital GP appointment, they will be offered next-day access to an in-person appointment with a GP across one of 50 Centric Health clinics nationwide.
This service is of particular importance to patients who aren’t registered with a local GP, as it allows them to access one of 50 GP practices nationwide for in-person care.
In addition, this development in primary healthcare provision will provide timely access to quality care in the community, ensuring that Irish Life Health customers have easy access to the right care closer to home when they need it.
Speaking about the new in-person GP appointments, Ger Davis, Irish Life Health Managing Director said:
“At Irish Life Health, we believe that regular access to highquality primary care is critical for customers in proactively managing their health. GPs are the gatekeepers to the health care system as well as being trusted advisers for patients. We are delighted to launch this marketleading primary care service, integrating our Digital Doctor service supported by rapid inperson consultation follow ups. We have seen a significant increase in take-up of the Digital Doctor service in the last year, reflecting the quality of the service and also the challenges of arranging physical GP appointments. We know from our over 500,000 customers that convenience and timely access to medical advice are key, so the Digital Doctor service and personalised healthcare is a priority for them. However, not all issues can be solved over the phone, and as convenience is a vital part of healthcare, providing our customers access to 50 Centric Health centres nationwide is a natural next step.
This new service enhances our health insurance offering which already gives our customers access to an excellent network of best in class diagnostic and urgent care centres as well as access to health care in the home. For example, our customers have access to specialised nurses treating patients for certain conditions at home, rather than in a hospital setting. In 2022 alone our customers avoided spending the night in hospital on over 4,700 occasions through the use of this service, freeing up hospital beds for other patients in need.”
PHARMACYNEWSIRELAND.COM 99








































*suitable for children from 6 months & pregnant women DulcoEase Hydrate can be used for up to 28 days, after which the causes of constipation should be investigated by a doctor who will advise on the suitability to continue treatment. Always read the label. Distributed in Ireland by Clonmel Healthcare Ltd. *consultation with a doctor is advised. Pharmacy only brand To place an order, contact your Clonmel Healthcare Area Representative or call our freephone order line on 1800 262626 WORKS WITHIN 24 to 72hrs GENTLE RELIEF GLUTEN LACTOSE & SUGAR FREE WORKS WITHIN 24 to 72hrs GENTLE RELIEF GLUTEN LACTOSE & SUGAR FREE WORKS WITHIN 24 to 72hrs GENTLE RELIEF GLUTEN LACTOSE & SUGAR FREE 2022/ADV/DUL/098H Gentle & effective relief of constipation and irregularity






















 Dermot Twomey, President, Irish Pharmacy Union
Dermot Twomey, President, Irish Pharmacy Union






































































 MB BCh BAO LRCP & SI (Irl), Dip Allergy (UK)
MB BCh BAO LRCP & SI (Irl), Dip Allergy (UK)











































































































































































 1: Ciara Maher, Ciara Mockler and Mary Theresa Mockler from Mockler’s Pharmacy, Thurles 2. Stuart Brown, Stephen Duncan and team from PharmaConex 3. Nial Tully, Tully totalhealth Pharmacy with Sheena Mitchell, Milltown totalhealth Pharmacy with Vincent Tully, Ross Tully and Maura Tully 4. Vanessa Keena, Ewa Borowska, and Marianna Magiera, Cunningham’s Pharmacy Athlone 5. Avril Farrell and Leanne Walsh, Boots Ireland
1: Ciara Maher, Ciara Mockler and Mary Theresa Mockler from Mockler’s Pharmacy, Thurles 2. Stuart Brown, Stephen Duncan and team from PharmaConex 3. Nial Tully, Tully totalhealth Pharmacy with Sheena Mitchell, Milltown totalhealth Pharmacy with Vincent Tully, Ross Tully and Maura Tully 4. Vanessa Keena, Ewa Borowska, and Marianna Magiera, Cunningham’s Pharmacy Athlone 5. Avril Farrell and Leanne Walsh, Boots Ireland











 1. The pharmacy team from Dunville Pharmacy, Dublin 2. Oonagh O’Hagan, Managing Director, Meaghers Pharmacy Group and guest
1. The pharmacy team from Dunville Pharmacy, Dublin 2. Oonagh O’Hagan, Managing Director, Meaghers Pharmacy Group and guest











 1. The team from Viatris 2. Jessica Edwards, Jennifer Hilton, Elizabeth and Paul Hatton, Perrigo 3. The pharmacy team from McCabes Pharmacy Group
1. The team from Viatris 2. Jessica Edwards, Jennifer Hilton, Elizabeth and Paul Hatton, Perrigo 3. The pharmacy team from McCabes Pharmacy Group















































































































