Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
of the body thinly and gently rub into skin. Wash hands after application unless area to be treated. Allow gel to dry on skin before applying bandages. Adults and adolescents aged 14 years and over: depending on the size of the affected site, apply a cherry to walnut size quantity 2 times a day (preferably morning and evening). Should not be used for longer than 1 week without medical advice. Children: no data in children and adolescents under 14 years of age. Elderly: no dosage adjustment required. Monitor patient carefully. Renal and hepatic impairment: no dosage adjustment required. Contraindications: Hypersensitivity to active substances or excipients; patients with a history of hypersensitivity reactions such as asthma, bronchospasmus, urticaria, acute rhinitis in response to acetylsalicylic acid or non- steroidal anti-inflammatory drugs (NSAIDs); open injuries, inflammations or infections of the skin as well as on eczema or mucous membranes; in the last trimester of pregnancy; in children and adolescents under 14 years of age. Precautions and warnings: Systemic undesirable effects cannot be excluded if applied on larger areas of skin over a prolonged period of time. Must only be applied to intact, not diseased or injured skin. Must not come into contact with eyes and oral mucous membranes. Must not be taken orally. May be used with non-occlusive bandages, but not with airtight occlusive dressing. Consult doctor if symptoms worsen or do not improve after 3-5 days. Patients suffering from asthma, hay fever, swelling of nasal mucous membranes (so called nasal polyps) ) or chronic obstructive pulmonary disease, chronic

respiratory infections (particularly associated with hay fever-like symptoms), and patients with hypersensitivity to painkillers and anti-rheumatic medicinal products of all kinds are rather at risk to asthma attacks (so called analgesic intolerance / analgesic asthma), to local skin or mucous membrane swelling (so-called quincke edema) or to urticaria than other patients when treated with Motusol Max. In these patients, Motusol Max may only be used under certain precautions (emergency preparedness) and direct medical supervision. The same applies for patients who are also allergic to other substances e.g. with skin reactions, itching or urticaria. Discontinue treatment if skin rash occurs. Photosensitivity can occur with the appearance of skin reactions after exposition to sunlight. Avoid children coming into contact to the skin areas where the gel has been applied. Contains butylhydroxytoluene which may cause local skin reactions or irritation to the eyes and mucous membranes. Contains fragrance with benzyl alcohol (0.15mg/g), citral, citronellol, coumarin, eugenol, farnesol, geraniol, d-limonene and linalool which may cause allergic reactions. In addition, benzyl alcohol may cause mild local irritation. Interactions: None known. Pregnancy and lactation: Should not be used during first and second trimester unless clearly necessary. Contraindicated during third trimester. Should only be used during breast-feeding under advice from a healthcare professional and should not be applied on the breasts, nor elsewhere on large areas of skin for a prolonged period of time. Effects on ability to drive and use machines: No or negligible influence. Adverse reactions: Hypersensitivity, angioedema, dermatitis bullous, Common: dermatitis (including contact dermatitis), skin rash, erythema, eczema, pruritus. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: Wash skin with water where applied. If ingested, apply general therapeutic measures normally adopted to treat poisoning with non-steroidal anti-inflammatory medicinal products. Gastric lavage and use of activated charcoal should be considered. Legal category: Pharmacy. Marketing Authorisation Number: PA1986/093/002. Marketing
Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00036. Date of Preparation: June 2021



Job Code: Dic-IE-00009. Date of Preparation: November 2022

January 2023 Volume 15 Issue 1 In this issue: NEWS: Medicine shortages must be taken seriously Page 4 MEETING: Pharmacy Trends discussed at Future of Pharmacy event Page 11 CPD REVIEW: 2022/2023 ePortfolio Review Page 16 FEATURE: Cough, Cold & Flu Season Page 22 MEDICINES: Supporting patients with medication adherence Page 28 BIOSIMLARS: Biosimilar Medicines and their use in Cancer treatment Page 38 TEAM TRAINING: Sports Injuries Page 98 PHARMACYNEWSIRELAND.COM This Publication is for Healthcare Professionals Only THE INDEPENDENT VOICE OF PHARMACY Powerful anti-inflammatory pain relief For muscle and joints when associated with strains and sprains. BNEW RAND CONTAINS DICLOFENAC TRIPLE EFFEC T Please refer to the Summary of Product Characteristics (SmPC) for full details of Prescribing Information. Motusol Max (diclofenac sodium) 2% w/w gel Abbreviated Prescribing Information. Presentation: White to almost white, homogeneous gel. 1g of gel contains diclofenac as 23.2mg diclofenac diethylamine corresponding to 20mg of diclofenac sodium. Indications: Local symptomatic treatment of pain in acute strains, sprains or contusions following blunt trauma. For short term treatment only. Dosage and administration: For cutaneous use. Apply to affected parts
PAXLOVID is indicated for patients
at high risk of progression to severe COVID191
*
Recommendations in national guidelines may differ from the approved indications.
Key factors that put a patient at high risk for progression to severe COVID-19 include1:
60 years of age and older, regardless of comorbidities
Immunosuppressive disease or treatment
Diabetes
Overweight (BMI >25)
Cardiovascular disease Hypertension
Chronic lung disease (including asthma)
Chronic kidney disease**
Current smoker
Neurodevelopmental disorders Sickle cell disease
Active cancer Medically related technological dependence
PAXLOVID is taken orally, at home, twice daily for 5 days
1
Treatment should begin immediately following a positive COVID-19 test and within 5 days of symptom onset
For more information about PAXLOVID dosing adjustments, including the authorised SmPC, [Scan QR Code]
Learn more about potential drug interactions before treatment begins with the Interaction Finder on www.paxlovideducation.ie [Scan QR Code]
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
* Patients is defined as adults who do not require supplemental oxygen.1
** In patients with moderate renal impairment (eGFR ≥ 30 to < 60 mL/min), the dose of Paxlovid should be reduced to nirmatrelvir/ritonavir 150 mg/100 mg every 12 hours for 5 days to avoid over-exposure (this dose adjustment has not been clinically tested). Paxlovid should not be used in patients with severe renal impairment [eGFR < 30 mL/min, including patients with End Stage Renal Disease (ESRD) under haemodialysis].
Abbreviated Prescribing Information
Paxlovid® (nirmatrelvir/ritonavir) 150 mg + 100 mg film-coated tablets
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions. Please refer to the Summary of Product Characteristics (SmPC) before prescribing Paxlovid. Indications: Treatment of coronavirus disease 2019 (COVID-19) in adults who do not require supplemental oxygen and who are at increased risk for progression to severe COVID 19 (see section 5.1 of the SmPC). Presentation: Each pink film-coated tablet contains 150 mg of nirmatrelvir. Each white film-coated tablet contains 100 mg of ritonavir. Dosage: The recommended dosage is 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) all taken together orally every 12 hours for 5 days. Paxlovid should be administered as soon as possible after a diagnosis of COVID-19 and within 5 days of symptom onset. Completion of the full 5-day treatment course is recommended even if the patient requires hospitalisation due to severe or critical COVID-19. In patients with moderate renal impairment, (eGFR ≥ 30 to < 60 mL/min) the dose of Paxlovid should be reduced to nirmatrelvir/ritonavir 150 mg/100 mg every 12 hours for 5 days. Paxlovid should not be used in patients with severe renal or severe hepatic impairment. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Medicinal products that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions, as well as medicinal products that are potent CYP3A inducers where significantly reduced plasma nirmatrelvir/ritonavir concentrations may be associated with the potential for loss of virologic response and possible resistance. Medicinal products listed below are a guide and not considered a comprehensive list of all possible medicinal products that are contraindicated with Paxlovid: Alpha 1‑adrenoreceptor antagonist: alfuzosin; Analgesics: pethidine, propoxyphene; Antianginal: ranolazine; Anticancer drugs: neratinib, venetoclax; Antiarrhythmics: amiodarone, bepridil, dronedarone, encainide, flecainide, propafenone, quinidine; Antibiotics: fusidic acid, rifampicin; Anticonvulsants: carbamazepine, phenobarbital, phenytoin; Anti gout: colchicine; Antihistamines: astemizole, terfenadine; Antipsychotics/Neuroleptics: lurasidone, pimozide, clozapine, quetiapine; Ergot derivatives: dihydroergotamine, ergonovine, ergotamine, methylergonovine; GI motility agent: cisapride; Herbal products: St. John’s Wort (Hypericum perforatum); Lipid modifying agents: lovastatin, simvastatin, lomitapide; PDE5 inhibitors: avanafil, sildenafil, vardenafil; Sedative/ Hypnotics: clorazepate, diazepam, estazolam, flurazepam, oral midazolam, triazolam. Warnings and Precautions: Risk of serious adverse reactions due to interactions with other medicinal products: Due to effects on CYP3A metabolic pathways, potential for interactions should be considered with other medicinal products prior to and during Paxlovid therapy; concomitant medicinal products should be reviewed during Paxlovid therapy and the patient should be monitored for the adverse reactions associated with the concomitant medicinal products. The risk of interactions with concomitant medications during the 5-day treatment period for Paxlovid should be weighed against the risk of not receiving Paxlovid; please refer to Table 1 in SmPC section 4.5. Severe renal impairment: Paxlovid should not be used in patients with severe renal impairment (eGFR < 30 mL/min, including patients with ESRD under haemodialysis). Severe hepatic impairment: Paxlovid should not be used in patients with severe hepatic impairment. Hepatotoxicity: Caution should be exercised when administering Paxlovid to patients with pre-existing liver diseases, liver enzyme abnormalities or hepatitis. HIV resistance: As nirmatrelvir is coadministered with ritonavir, there may be a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection. Excipients: nirmatrelvir tablets contain lactose, patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine. Contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’. Drug Interactions: Nirmatrelvir and ritonavir are CYP3A substrates; therefore, medicinal products that induce CYP3A may decrease plasma concentrations and reduce therapeutic effect. Paxlovid (nirmatrelvir/ritonavir) is an inhibitor of CYP3A and may increase plasma concentrations of medicinal products that are primarily metabolised by CYP3A. Ritonavir has a high affinity for several cytochrome P450 (CYP) isoforms and may inhibit oxidation with the following ranked order: CYP3A4 > CYP2D6. Ritonavir also has a high affinity for P-glycoprotein (P-gp) and may inhibit this transporter. Ritonavir may induce glucuronidation and oxidation by CYP1A2, CYP2C8, CYP2C9 and CYP2C19 thereby increasing the biotransformation of some medicinal products metabolised by these pathways. As a conservative measure, the drug-drug interactions pertaining to ritonavir used in chronic HIV infection should apply for Paxlovid. Medicinal products listed here are a guide and not considered a comprehensive list of all possible medicinal products that may interact with nirmatrelvir/ritonavir: Amphetamine derivatives: amphetamine; Analgesics: buprenorphine, norbuprenorphine, piroxicam, fentanyl, methadone, morphine; Antiarrhythmics: digoxin; Antiasthmatic: theophylline; Anticancer: afatinib, abemaciclib, apalutamide, ceritinib, dasatinib, nilotinib, vincristine, vinblastine, encorafenib, fostamatinib, ibrutinib, venetoclax (contraindicated in some circumstances); Anticoagulants: rivaroxaban, vorapaxar, warfarin; Anticonvulsants: divalproex, lamotrigine, phenytoin; Antidepressants: amitriptyline, fluoxetine, imipramine, nortriptyline, paroxetine, sertraline, desipramine; Antihistamines: fexofenadine, loratadine; Anti infectives: rifabutin, voriconazole, ketoconazole, itraconazole, erythromycin, atovaquone, bedaquiline, delamanid, clarithromycin, sulfamethoxazole/trimethoprim; Anti HIV: efavirenz, maraviroc, raltegravir, zidovudine; Anti HCV: glecaprevir/pibrentasvir; Antipsychotics: haloperidol, risperidone, thioridazine; β2-agonist (long acting): salmeterol; Calcium channel antagonist: amlodipine, diltiazem, nifedipine; Endothelin antagonists: bosentan, riociguat; HMG Co A reductase: atorvastatin, fluvastatin, pravastatin, rosuvastatin; Hormonal contraceptive: ethinyl estradiol; Immunosuppressants: cyclosporine, tacrolimus, everolimus; PDE5 inhibitors: sildenafil (contraindicated in some circumstances), tadalafil; Sedatives/Hypnotics: parenteral midazolam, alprazolam, buspirone; Sleeping agent: zolpidem; Smoke cessation: buproprion; Steroids: inhaled, injectable or intranasal fluticasone propionate, budesonide, triamcinolone, dexamethasone, prednisolone; Thyroid hormone replacement therapy: levothyroxine. Please refer to Table 1 in SmPC section 4.5 for additional information on interaction with medicinal products / other forms of interaction. Fertility, pregnancy and lactation: Women of childbearing potential should avoid becoming pregnant during treatment with Paxlovid and as a precautionary measure for 7 days after completing Paxlovid. Patients using combined hormonal contraceptives should be advised to use an effective alternative contraceptive method or an additional barrier method of contraception during treatment and until after one complete menstrual cycle after stopping Paxlovid. Paxlovid is not recommended during pregnancy and in women of childbearing potential not using contraception unless the clinical condition requires treatment with Paxlovid. Breast feeding should be discontinued during treatment with Paxlovid and as a precautionary measure for 7 days after completing Paxlovid. Driving and operating machinery: Paxlovid is expected to have no influence on the ability to drive and use machines. Undesirable effects: Common (≥ 1/100 to < 1/10) adverse events reported were dysgeusia, headache, diarrhoea, vomiting and nausea. Uncommon (≥ 1/1,000 to < 1/100) adverse events reported were abdominal pain. Rare (≥ 1/10,000 to < 1/1,000) adverse events reported were malaise. See SmPC section 4.8 for full details.Legal Category: S1A. Package Quantities: 150 mg + 100 mg, 20 + 10 film-coated tablets. Marketing Authorisation Number: EU/1/22/1625/001. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Brussels, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500. Last revised: 11/2022 Ref: PX 4_0
Reference: 1. PAXLOVID™ Summary of Product Characteristics. Pfizer Inc.; October 2022.
© 2022 Pfizer Inc. All rights reserved. PP-PAX-IRL-0096 | November 2022
Foreword


As Hospital Professional News was going to press with the first issue of 2023, the Irish Hospital Consultants Association (IHCA) was expressing dismay that the Government is on course to miss waiting list reduction targets for 2022 by a significant margin.




Minister for Health Stephen Donnelly launched the ¤350 million Waiting List Action Plan for 2022 in February which committed to reducing active waiting lists for acute scheduled care by 18% (more than 132,00) by the end of this year. However, figures show that by the end of November the numbers of those waiting for treatment will have only dropped by a modest 1.6% (fewer than 11,500).
The latest NTPF figures confirm that the number of people on the three main waiting lists decreased from 720,056 at the start of 2022 to 708,590 at the end of November, nowhere near the planned 18% reduction.
Overall, a total of 887,500 people were on some form of hospital waiting list at the end of November, including almost 97,000 children.
When the additional 243,000 people awaiting CTs, MRIs or ultrasounds nationally are added, the total number awaiting hospital care is over 1.1 million – or more than one-fifth of the entire population.
Analysis from the IHCA shows that the HSE is now more than 120,800 outpatient appointments and procedures away from meeting the 18% reduction targets for the end of this year, including 115,100 outpatient appointments and around 5,700 inpatient or day case procedures and GI scopes.
In other news, the National Cancer Registry (NCRI) has published its 2022 Annual Statistical Report: Cancer in Ireland. 1994 – 2020. This report on the status of cancer in Ireland includes updated statistics on cancer incidence, mortality and survival for patients diagnosed in Ireland 1994 – 2020. You can read more about the report findings on page 10 whilst on page 16, Rebecca Parkin, AnneMarie DeFrein and Patricia Heckmann discuss biosimilar medicines and their use in the treatment of cancer.
The authors, from the National Cancer Control Programme state that, “As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer.”
Our Special Focus for January is in the field of Respiratory Care, with some excellent and current clinical updates contributed by leading specialists in this field.
I hope you enjoy the issue.
Regulars
CPD: ATTR AND STROKE P41
ORAL SUPPLEMENTS P47 FINANCE: SEASONAL REFLECTIONS P50 FEATURE: SPORTS PHARMACY P52 FEATURE: SMOKING CESSATION P54 CLINICAL PR: P80
FEATURE:
3 PHARMACYNEWSIRELAND.COM 6 Irish Pharmacy News is circulated to all independent, multiple Pharmacists and academics in Ireland. All rights reserved by Irish Pharmacy News. All material published in Irish Pharmacy News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd. has taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors. IRISH PHARMACY NEWS 5 11 PUBLISHER:
DIRECTOR
EDITOR
ADVERTISING EXECUTIVE
EDITORIAL/ EVENTS & MARKETING EXECUTIVE Aoife
CONTRIBUTORS Damien O’Brien | Rachel Flynn Rebecca Parkin | AnneMarie DeFrein Aisling Geraghty | Laura McBean Sarah Browne | Ciara M. E. Reynolds Patricia Dominguez Castro David Hanlon | Gerard Bury Margaret O’Neill | Sarah Clarke Barbara Clyne | Karen Finnigan Laura McCullagh | Sharon Kennelly Clare A. Corish | Kieran Moore Ahmed W.I. Alanqar | Orla Cooney Faris Soloman Almadi Yousef M. Waly | Ali Al-Sabti Theresa Lowry-Lehnen Mary-Claire Kennedy Patricia Heckmann Professor Ronan Collins Page 5: New PSI Registrar and Chief Officer Page 6: European Research Award for investigating alternatives to antibiotics Page 8: totalhealth Pharmacy celebrate at the Irish Business Awards Page 10: New study on burnout amongst Irish pharmacists Page 11: The latest trends, acquisitions and supply chain issues discussed at Pharmacy event Page 16: ePortfolio Review Updates Page 20: Overprescribing among older people Page 34: Biosimilars in the treatment of Cancer PHARMACYNEWSIRELAND.COM @Irish_PharmNews IrishPharmacyNews Contents 20
IPN Communications Ireland Ltd. Clifton House, Fitzwilliam Street Lower, Dublin 2 00353 (01) 6690562 MANAGING
Natalie Maginnis n-maginnis@btconnect.com
Kelly Jo Eastwood: 00353 (87)737 6308 kelly-jo@ipn.ie
Amy Evans: amy@ipn.ie
Hunter: aoife@ipn.ie
Pharmacy Warning must be taken Seriously
Medicines for Ireland (MFI) are urging Government to heed recent warnings from GPs and pharmacists nationwide on the growing risk of medicines shortages as inflation, energy and transport costs continue to rise, and global supply chain disruptions persist.
Medicines for Ireland members are the suppliers of the majority of medicine in Ireland to the HSE and patients directly and played a pivotal role in a new Framework Agreement on the supply and pricing of non-originator, generic, biosimilar, and hybrid medicines, announced by Government last year.
Commenting on increasing medicine shortages, Medicines for Ireland Chairperson, Padraic O’Brien has said “In Ireland and throughout Europe, soaring energy costs, inflation and supply chain disturbances have contributed to thousands of generic medicines disappearing from the European and Irish market.”
“MFI members are willing to work directly with Government to help tackle this serious issue and prevent potential medicines shortages. Our aim is to deliver industry insights and extend our expertise to help improve the development of medicinal pricing
and procurement policies in Ireland and safeguard the supply of medicines to Ireland.”
According to the Health Products Regulatory Authority (HPRA) website there are currently 187 medicines in short supply in Ireland. Without intervention this situation has the potential to significantly worsen.
Mr O’Brien added, “As a small market Ireland is more likely to be badly impacted by inflationary pressure and as costs continue to rise, market conditions will become increasingly unviable for companies supplying generic medicines to Irish hospitals and pharmacies. Additionally, in some cases, our reimbursement prices for certain medicines are too low compared to other EU countries”.
“Price adjustments in Ireland are historically downward only, where other European countries employ flexible pricing mechanisms that allows reimbursement prices to
rise for medicines that are in short supply. Ireland does not have such a mechanism and is therefore further disadvantaged.”
A recent MFI members survey found that 91% of MFI members experienced increased costs associated with import and/or manufacturing of pharmaceutical and medical products for the Irish market in 2022. While all MFI member companies envisage increases in transportation costs over the next 12 to 24 months.
“Our main focus is to help Government ensure market conditions in Ireland remain sustainable in order to retain and secure access to reliable and affordable treatment for Irish patients. We believe it is time for us to revisit our work with Government and the HSE on the Framework Agreement on Supply and Pricing and develop improvements to mitigate against supply risks.” concluded Mr O’Brien.
The Golden Ticket has been Found
Back in October, Sona Irish Vitamins hid a golden ticket in the packaging of one of their children’s vitamins ranges.
funding vital life-saving equipment and providing essential patient and parental supports, to making ground-breaking, paediatric research possible.
Ethical Dilemmas for Pharmacy
A decision-making framework and its application to help pharmacists deal with different ethical dilemmas in pharmacy practice are presented in a new format of continuing professional development — “FIP CPD Bites” — from the International Pharmaceutical Federation (FIP), launched recently.
“FIP already offers numerous resources to support the profession with CPD, including webinars, digital events, congress sessions and publications such as handbooks and knowledge and skills guides. However, we understand that people have different learning styles and preferences, and the growing demands on pharmacists’ time means that there is a need for concise, engaging, evidence-driven and easily accessible learning materials. ‘FIP CPD Bites’ aims to meet this need through short videos with role-plays, analysis and advice,” said Dr Dalia Bajis, FIP lead for provision and partnerships.
Congratulations to the lucky winner from Dublin, who unwrapped the Sona Golden Ticket and won a tremendous ¤1,000 in cash, just in time for Christmas! The Golden Ticket was hidden inside a Sona Pro10Biotic and purchased at Boots, Blanchardstown.
But that’s not all; when the golden ticket was found, Sona donated an incredible ¤30,000 to Children’s Health Foundation in support of
sick children and their families who attend Children’s Health Ireland (CHI) at Crumlin, Temple Street, Tallaght and Connolly.
Since 2018, Sona has donated over ¤82,000 to Children’s Health Foundation, the charity supporting the life-saving work of Children’s Health Ireland hospitals and urgent care centres. The funds donated by Sona will be used to help sick children across Ireland, from
Ruth Guy (pictured with Sona’s Lena Ryan) was delighted to receive the cheque in the snowy winter wonderland at Children’s Health Ireland at Crumlin and said: “We are quite simply blown away by the incredible support that Sona have shown sick children and their families in Children’s Health Ireland hospitals and urgent care centres in Crumlin, Temple Street, Tallaght and Connolly since 2018. Their commitment to helping sick children is much appreciated. Congratulations to the lucky Golden Ticket winner!”

Lena Ryan from Sona added “We proudly support Children’s Health Foundation and indeed the hospitals and hospital staff working tirelessly helping our sick children. We are delighted to be able to support their work and we thank all our customers across Ireland who help us make this possible.”
“FIP CPD Bites” provides learning support on different topics relevant to practice, science and education in pharmacy. FIP’s first series in this new format comprises seven videos (four available from today) developed by FIP experts on ethics. “As practising pharmacists, we are clinically, ethically and socially accountable for decisions we make regarding the all-important pharmaceutical care of our patients. We often experience ethical dilemmas, where two or more issues could be pulling in opposite directions, making it difficult to decide what direction to take that would be justifiable and in the best interests of the patient. To enable better understanding of our obligations, these CPD bites offer real-life examples in role-play, accompanied by clear, simply articulated analyses and recommended actions to help colleagues make professionally justifiable ethical decisions,” said Dr Betty Chaar, chair, FIP Working Group on Ethics.
The first video in this Ethics in Pharmacy Practice series introduces a framework for decision making on ethical dilemmas.
PHARMACYNEWSIRELAND.COM 4 News
Medicine Shortages
The Health Products Regulatory Authority has been notified of a shortage of the following products:
• Acerycal 10mg/10mg TabletsPA0568/018/004
• Ativan 1mg TabletsPA0822/090/001
• Augmentin Duo Mixed Fruit 400mg/57mg/5ml Powder for Oral Suspension (35 ml presentation)PA1077/019/006
• Cyklokapron 500mg Film Coated TabletsPA2010/055/001
• Golden Eye 0.1% w/v Eye Drops - PA22695/001/001
• Golden Eye 0.15% w/w Eye Ointment - PA22695/001/002
• Inegy 10mg/80mg TabletsPA23198/024/004
• Kalcipos-D forte 500mg/800IU Film Coated TabletsPA2010/040/001
• Levobupivacaine 7.5mg/mL Solution for Injection/InfusionPA2059/009/005
• Lyrica 50mg CapsulesEU/1/04/279/009
• Lyrica 75mg CapsulesEU/1/04/279/012
• Lyrica 100mg CapsulesEU/1/04/279/015
• Methotrexate 2.5mg TabletsPA2315/062/001
• Morphine Sulfate 10mg/ ml Solution for InjectionPA0549/023/001
• Navelbine 30mg Soft Capsules - PA0329/011/002
• Paracetamol 500mg Film Coated Tablets (100 pack)PA2315/065/003
• Sabril 500mg Film Coated Tablets - PA0540/023/001
• Salamol Steri-Neb 2.5mg/2.5ml Nebuliser Solution - PA1986/086/001
• Spasmonal 60mg CapsulesPA2010/043/001
• Tobi 300mg/5ml Nebuliser Solution - PA2010/063/001
• Trandate 200mg Film Coated Tablets - PA1638/006/003
New PSI Registrar and Chief Officer Appointed
The Council of the Pharmaceutical Society of Ireland (PSI), the pharmacy regulator, has announced the appointment of a new Registrar and Chief Officer, Joanne Kissane. The appointment was made following a public recruitment process. Ms Kissane will take up her new role in January 2023.

Registrar and Chief Officer, PSI, Joanne Kissane
Ms Kissane, a pharmacist, has a wealth of knowledge of the pharmacy, education and regulatory sector, and she brings significant leadership, strategy and change management experience to her new role as head of the regulatory body.
She is currently the Director and National Coordinator for APPEL (Affiliation for Pharmacy Practice Experiential Learning), a joint office of the three Schools of Pharmacy in Ireland, which manages the student experiential learning placements for the integrated Masters degree programmes in pharmacy.
She has worked in community pharmacy as a practising pharmacist and, for seven years, was the superintendent pharmacist, followed by the Head of Operational Excellence for the LloydsPharmacy group.
With a keen interest in safe and effective healthcare services, Joanne has been involved with the regulator as a member of the PSI Council since 2015.
She has also been a member of advisory committees tasked
with developing and overseeing strategic work programmes at the regulator, most recently as Chair of its Regulatory and Professional Policy Committee.
Commenting on the appointment, President of the PSI Muireann Ní Shúilleabháin said, “I am delighted to announce Joanne’s appointment as Registrar and Chief Officer. She has an excellent track record and work ethic. Her practical experience of the pharmacy sector, and more latterly in pharmacy education, means she brings a knowledge of stakeholders and the broader healthcare environment, which
Waterford Pharmacist records Christmas Charity Song
Pharmacist Joe Kelly of Kelly’s Pharmacy in Dungarvan, Co Waterford, has written and recorded a charity Christmas single.
All proceeds from his song, ‘Santa, I love you,’ will go towards supporting sick children and their families in CHI hospitals and urgent care centres.

Joe wrote the festive tune as an antidote to the constraints of the pandemic, “It’s very much a children’s take on Christmas, brought to life under the auspices of musical director David Hayes and performed by the David Hennessey stage school in Dungarvan,” he says.
You can donate by visiting: tinyurl.com/justgivingsantailoveyou
will serve to further inform and enhance our approach in fulfilling our regulatory remit to assure public and patient trust in the quality and safety of pharmacy healthcare.”
Reflecting on her appointment, Joanne Kissane said, “I am pleased to continue my involvement with the PSI in taking up this role. The pharmacy regulator has an important contribution to make as part of the broader health system and is an integral component in ensuring pharmacy-led care and treatment are safe and appropriate for patients and the public.”
PHARMACYNEWSIRELAND.COM 5
News
Tackling Antimicrobial Resistance
Professor Paul Ross, Director of APC Microbiome Ireland, a world-leading SFI research centre based at University College Cork, has been awarded a highly prestigious European Research Council Advanced Grant to investigate viable alternatives to antibiotics.
 Keough
Keough
killing drug resistant bacterial while avoiding the collateral damage caused by broad antibiotics to gut microbiota, the collection of beneficial bacteria, viruses and fungi that inhabit intestines.
Professor Ross, who is the first researcher based at UCC to secure an ERC Advanced Award, has received funding of ¤2.3 million for a project entitled BACtheWINNER. The funding gives Europe’s established research leaders the opportunity to lead groundbreaking projects to solve the most pressing social, economic and environmental challenges.
Professor John O’Halloran, President of UCC, said: “I wish to extend the warmest
congratulations to Professor Paul Ross on this significant achievement. This award will enable Paul to advance his pioneering, impactful research in microbiome science, a key area for UCC Futures at UCC. This award will drive momentum for this initiative and provide solutions to one of the most important global challenges of our time.”
The rapid emergence and spread of resistant bacteria is occurring worldwide, endangering
the efficacy of antimicrobials, including antibiotics and antivirals. The Antimicrobial Resistance (AMR) crisis has been attributed to the overuse and misuse of these medications, as well as a lack of new drug development. The discovery and development of new antimicrobial treatments is now urgent.
The BACtheWINNER project will explore the potential of naturally occurring antimicrobial peptides (bacteriocins) produced by bacteria in the human gut and other sites for the development of new antimicrobial therapies. Bacteriocins represent a potential solution to the AMR threat by
HPRA Review of Codeine OTC Medicines
Together with key collaborator Professor Colin Hill, also based at APC Microbiome Ireland at UCC, Professor Ross will lead a team of scientists who aim to develop bacteriocins as effective antimicrobials to predictably edit microbiomes and target pathogens.
Professor Ross said: “I am delighted to receive an ERC Advanced Award to further investigate bacteriocins as alternative therapeutics to antibiotics, in a time when we now appreciate the role of the microbiota in human and animal health. By investigating bacteriocins as potential clinical antimicrobials, we can explore antibiotic alternatives that are target-specific and do not cause collateral damage to microbiomes. Bacteriocins are the most important group of antimicrobial peptides with potentially revolutionary applications in health.”
The HPRA, as the competent authority for determining the method of sale for individual medicines, has commenced a formal regulatory procedure to determine the appropriate method of sale for authorised codeine-containing medicines in Ireland. In general, method of sale refers to the classification of a medicine as either prescription-only or over the counter (OTC). Currently, codeine-containing medicines are available in Ireland OTC through pharmacies and are not subject to medical prescription.
The commencement of this formal procedure is the latest phase in an ongoing HPRA review of the use and classification of these products. This review, which commenced in quarter two of 2022, involves evaluating the available supporting evidence and experience of use for codeinecontaining medicines accrued nationally and from other EU and international markets. The review is also being informed by ongoing engagement with relevant national and international stakeholders.
In recent years, a number of jurisdictions have reviewed the method of sale for codeinecontaining products in response to a growing understanding of the potential impacts of addiction to codeine in low doses. In Ireland, this has been addressed to date by restricting access to
codeine-containing products through pharmacies. While codeine-containing medicines are also similarly available without prescription in some other countries, in certain other jurisdictions they are available solely via a prescription from a doctor.
Current availability and recommendations for use
Over-the-counter codeinecontaining medicines, including those in combination with ibuprofen or with paracetamol, are currently available in Ireland without prescription, under pharmacist supervision. These medicines are used for the short-term treatment of acute, moderate pain that is not relieved by paracetamol or ibuprofen alone. Other codeine containing products
available without prescription in Ireland include a liquid preparation to suppress coughing.
The existing product packaging and patient information leaflet, which accompany codeinecontaining medicines, already contains detailed advice on known warnings and side effects associated with these products. The warnings for codeine-containing medicines in combination with ibuprofen or with paracetamol state that these medicines should be used at the lowest effective dose for the shortest period of time, the duration of treatment should be limited to three days and, if no effective pain relief is achieved, the patient should be advised to seek the opinion of their doctor. The current product information for all codeine-containing
medicines in particular highlights that codeine can cause addiction if taken continuously for more than three days.
The HPRA recommends that patients always take time to read the accompanying patient information leaflet that comes with every medicine and that they follow the dosage and duration of use instructions. Patients should seek further advice from a healthcare professional if they have any queries or concerns about their medicine.
Any suspected adverse reactions associated with the use of medicines, including those containing codeine, may be reported to the HPRA through the available reporting options (www.hpra.ie/report).
PHARMACYNEWSIRELAND.COM 6 News
Professor Paul Ross, Director of APC Microbiome Ireland Pic: Clare
I sh v I tam I ns
The world’s best documented
SONA PRO60BIOTIC is a high potency capsule containing the world’s best documented Bifidobacterium BB-12® , as well as Lactobacillus (LA-5®).


These friendly bacteria colonise the gut and help maintain the natural balance of a body’s intestinal flora, or microbiome. A correct balance of the microbiome is important for the proper digestion and utilisation of nutrients, as well as ensuring a healthy immune system.

SONA have a full range of probiotic products to suit the whole family.






Order direct from Sona on PH: 01 451 5087. Also available from wholesalers, United Drug and Uniphar.
 Sona, serving Irish consumers through Irish pharmacies since 1984
Sona, serving Irish consumers through Irish pharmacies since 1984
RCN: 20042462 | CHY: 13534
Sona proudly Support S
Ir
at the Irish Business Awards
Highest Figure of New HIV Cases
HIV Ireland has called for increased investment in nationwide sexual health services, including personnel and resources, to ensure Ireland can meet its global commitment to end new HIV transmissions. Statistics published weekly by the Health Protection Surveillance Centre indicate that more 750 cases of HIV have been notified in 2022, more than double the number for the same period in 2021. However, despite the increase in notifications, falling rates of new transmissions occurring within Ireland give cause for optimism.
The HPSC records newly notified case as those who have recently acquired the virus together with people already living with the virus and transferring their care to Ireland.
This is the first year of the national recognition scheme GRADAIM Gnó na Éireann, developed by Glór na nGael, in which the work of businesses for the Irish language is celebrated.


Based on the assessment process, which took place last September gold, silver and bronze medals were awarded to the businesses in
three different categories: Signage; Marketing and Branding; Service in Irish.
O’Donnell’s totalhealth pharmacy, Gweedore were delighted to be awarded with several medals for the Services in Irish section.
Following the announcement of their award, the O’Donnell’s totalhealth Pharmacy team took

to Facebook to comment; “Is mór an onóir dúinn é seo a fháil mar chúiteamh ar sheirbhísí trí Ghaeilge” (We are honoured to receive this in return for services through Irish).
Special thanks to Grandaim Gnó na hÉireann for hosting a memorable awards ceremony for Irish Businesses.
Pharma Award for United Drug
United Drug were delighted to have taken home the award for the COVID-19 Crisis Response category at the Pharma Industry Awards.

“We are extremely proud of our teams and the fantastic work that was done by all United Drug employees. Their tireless efforts ensured that vital medications, vaccines, and services could continue to be provided and maintained to the Irish Public throughout the entire Pandemic.”
Paul Reilly, Managing Director of United Drug
This award acknowledges the rapid response to the requirements for the storing, processing, and distribution through supply chain excellence of the Covid19 vaccinations at ultra-low
temperatures. The professionalism and the company’s response to the physical demands required were praised by the judges who felt that the complex and challenging task was met with great success but also inspiring teamwork.
This award acknowledges but most importantly gives recognition to the team’s personal commitment, tireless efforts, and
The team from United Drug
attention to detail over the past 24 months. Through this, they helped to save lives, and there is no better achievement! Well done to all involved.
“Under diagnosis of HIV remains a concern. The European Centre for Disease Prevention and Control has said that an estimated 1 in 8 people living with HIV in the EU/EEA area remain undiagnosed,” said Stephen O’Hare Executive, Director of HIV Ireland.
“In order to decrease the number of undiagnosed cases,” continued Mr O’Hare, “timely access to early testing and subsequent linkage to care is vital.”
This year, UNAIDS has called on the world to unite to end the inequalities that underpin and perpetuate HIV transmission.
The stigma and exclusion faced by people living with HIV and marginalised populations remains a significant barrier to accessing testing, treatment uptake, adherence to medication and seeking support.
HIV-related stigma still persists including among health care professionals. Research conducted this year by Dr Elena Vaughan of NUI Galway and supported by HIV Ireland found that among healthcare workers who were not HIV specialists, 83% claimed knowledge of ‘Undetectable = Untransmittable’ (U=U) and treatment as prevention. However, 40% said they would still be nervous about drawing blood from a person living with HIV leading to unnecessary ‘extra’ precautions, e.g., excessive use of PPE.
PHARMACYNEWSIRELAND.COM 8 News
On Tuesday 15th of November, over 85 businesses attended the City Hall in Dublin to celebrate the Irish Business Awards GRADAIM Gnó na Éireann 2022.
Rory O’Donnell (Chairman of totalhealth) accepted the Awards Sign on behalf of O’Donnell’s totalhealth pharmacy on the day
Name of product: VIAGRA CONNECT 50 mg film-coated tablets Active ingredient: sildenafil. Supply classification: Pharmacy only. Indications: For erectile dysfunction in adult men. Dosage and Method of use: Adults: one 50 mg tablet taken with water approx. one hour before sexual activity. The maximum dosing frequency is once per day. the onset of activity may be delayed if taken with food. Patients should be advised that they may need to take Viagra Connect a number of times on different occasions (max of one 50 mg tablet per day), before they can achieve a penile erection satisfactory for sexual activity. If patients are still not able to achieve a sufficient penile erection they should be advised to consult a doctor. Elderly: no dosage adjustments required (≥65 years old). Renal Impairment: No dosage adjustments for patients with mild to moderate renal impairment. Dosage adjustments required for those with severe renal impairment, see SmPC. Hepatic Impairment: Dosage adjustments required for those with mild-moderate hepatic impairment, see SmPC. Viagra Connect in contraindicated for patients with severe hepatic impairment (see contraindications). Use in patients taking other medicinal products: individuals receiving concomitant treatment with CYP3A4 inhibitors must be advised to consult their doctor before taking Viagra Connect, dosing adjustments may be required, see SmPC. Patients receiving alpha blocker treatment should be stabilised on therapy prior to initiating sildenafil treatment and must be advised to consult their doctor before taking Viagra Connect as dosing adjustments may be required, see SmPC. Side Effects: The most commonly reported adverse reactions in clinical studies among sildenafil treated patients were headache, flushing, dyspepsia, nasal congestion, dizziness, nausea, hot flush, visual disturbance, cyanopsia and vision blurred. For full list of side effects see SmPC section 4.8. Warnings and Precautions: Erectile dysfunction can be associated with a number of contributing conditions, e.g. hypertension, diabetes mellitus, hypercholesterolaemia or cardiovascular disease. As a result, all men with erectile dysfunction should be advised to consult their doctor within 6 months for a clinical review of potential underlying conditions and risk factors associated with erectile dysfunction (ED). If symptoms of ED have not improved after taking Viagra Connect on several consecutive occasions, or if their erectile dysfunction worsens, the patient should be advised to consult their doctor.


Cardiovascular risk factors: Since there is a degree of cardiac risk associated with sexual activity, the cardiovascular status of men should be considered prior to initiation of therapy. Agents for the treatment of erectile dysfunction, including sildenafil, are not recommended to be used by those men who with light or moderate physical activity, such as walking briskly for 20 minutes or climbing 2 flights of stairs, feel very breathless or experience chest pain. For a list of patients who are considered at low cardiovascular risk from sexual activity see SmPC. Patients previously diagnosed with the following must be advised to consult with their doctor before resuming sexual activity: uncontrolled hypertension, moderate to severe valvular disease, left ventricular dysfunction, hypertrophic obstructive and other cardiomyopathies, or significant arrhythmias. Sildenafil has vasodilator properties, resulting in mild and transient decreases in blood pressure. Patients with increased susceptibility to vasodilators include those with left ventricular outflow obstruction (e.g. aortic stenosis), or those with the rare syndrome of multiple system atrophy manifesting as severely impaired autonomic control of blood pressure. Priapism: Patients who have conditions which may predispose them to priapism (such as sickle cell anaemia, multiple myeloma or leukaemia), should consult a doctor before using agents for the treatment of erectile dysfunction, including sildenafil. Prolonged erections and priapism have been occasionally reported with sildenafil in postmarketing experience. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. Concomitant use with other treatments for erectile dysfunction is not recommended. Effects on vision: Patients should be advised that in the event of any sudden visual defect, they should stop taking Viagra Connect and consult a physician immediately. Concomitant use with CYP3A4 inhibitors: patients should be advised to consult a doctor before taking Viagra Connect as a 25 mg tablet may be more suitable for them. Concomitant use with alpha-blockers: Caution is advised when sildenafil is administered to patients taking an alpha-blocker, as the co-administration may lead to symptomatic hypotension in a few susceptible individuals. This is most likely to occur within 4 hours post sildenafil dosing. In order to minimise the potential for developing postural hypotension, patients should be hemodynamically stable on alpha-blocker therapy prior to initiating sildenafil treatment. Thus, patients taking alpha blockers should be advised to consult their doctor before taking Viagra Connect. Treatment should be stopped if symptoms of postural hypotension occur, and patients should seek advice from their doctor on what to do. Effect on bleeding: the use of sildenafil is not recommended in those patients with history of bleeding disorders or active peptic ulceration, and should only be administered after consultation with a doctor. Hepatic impairment: Patients with hepatic or renal impairment must be advised to consult their doctor before taking Viagra Connect., since a 25 mg tablet may be more suitable for them. Lactose: The film coating of the tablet contains lactose. Viagra Connect should not be administered to men with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per tablet. Patients on low sodium diets can be informed that this medicinal product is essentially ‘sodium-free’. Use with alcohol: Drinking excessive alcohol can temporarily reduce a man’s ability to get an erection. Men should be advised not to drink large amounts of alcohol before sexual activity. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. co-administration with nitric oxide donors (such as amyl nitrite, nitrates, ritonavir guanylate cyclase stimulators, such as riociguatis contraindicated. Agents for the treatment of erectile dysfunction, including sildenafil, should not be used by those men for whom sexual activity may be inadvisable, and these patients should be referred to their doctor. This includes patients with severe cardiovascular disorders such as a recent (6 months) acute myocardial infarction (AMI) or stroke, unstable angina or severe cardiac failure. Sildenafil should not be used in patients with severe hepatic impairment, hypotension (blood pressure < 90/50 mmHg) and known hereditary degenerative retinal disorders such as retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases). Sildenafil is contraindicated in patients who have loss of vision in one eye because of non-arteritic anterior ischaemic optic neuropathy (NAION), regardless of whether this episode was in connection or not with previous PDE5 inhibitor exposure. Viagra Connect should not be used in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie’s disease). Viagra Connect is not indicated for use by women. The product is not intended for men without erectile dysfunction. This product is not intended for men under 18 years of age. Legal Category: Product not subject to medical prescription. Marketing Authorisation Number: PA23055/016/001. Marketing Authorisation Holder: Upjohn EESV, Rivium Westlaan 142, 2909 LD Capelle aan den IJssel, Netherlands. For further information on this medicine please contact: Medical Information on 1800 633 363. For queries regarding product availability please contact: Viatris, Newenham Court, Northern Cross, Malahide Road, Dublin 17. Phone number: +353 1 871 1600.
Reporting of adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important.It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to reportany suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse reactions/ events should also be reported to the marketing authorisation holder at email address: pv.ireland@viatris.com or phone 0044(0)8001218267. www.viagraconnect.ie
For more information please contact Viatris or refer to the SmPC for full prescribing information HELPS YOU GET AND KEEP AN ERECTION Date: 04/2021. Ref: VC2_0 Viagra Connect (sildenafil) 50 mg film-coated tablets Abbreviated Prescribing information VIAC-2022-0322. DOP: November 2022 50 mg tablets Helps you get keep an erection ©
Pharmacy Burnout shows Prevalence
A new report has been published which found that over half of pharmacists experience burnout, with higher prevalence since Covid-19, with retention and patient care implications.
The report is the Masters Thesis investigating Burnout Syndrome in Pharmacists completed by Dublin Pharmacist Jodie Dee alongside co-authors Nabaa Dhuhaibawi and Professor John Hayden from the Royal College of Surgeons Ireland.
Burnout is a term used to describe the psychological response to work related stress, presenting as emotional exhaustion, increased levels of depersonalisation and cynicism and reduced feeling of personal accomplishment or efficacy.
Burnout prevalence data were extracted from 19 articles involving 11,306 pharmacist participants across eight countries. More than half (51%) of pharmacists were experiencing burnout.
Associated risk factors included longer working hours, less
professional experience, high patient and prescription volumes, excessive workload and poor work/life balance. The COVID-19 pandemic has negatively impacted pharmacist burnout and resilience. Involvement in education and training and access to burnout management resources were associated with lower rates of burnout, but burnout intervention effectiveness is unknown.
“More than half of pharmacists surveyed were defined as experiencing burnout, with prevalence estimates ranging from 5 to 75%, and in the last three years this has increased and plateaued at almost 60%. While prevalence estimates are limited by assessment instrument and study design challenges, these results give a picture of a significant workforce wellbeing problem
within the pharmacy profession,” say the authors.
The authors conclude by stating, “Approximately half of pharmacists are experiencing burnout globally which has the potential to negatively impact patient care. There is a clear increase in the amount of research investigating burnout among pharmacists in the last five years. There is a need for longitudinal studies to account for any transient contributors, like COVID-19. Burnout awareness and management techniques should be addressed within healthcare organisations should provide wellness programmes and support to those at risk of and/or experiencing symptoms of burnout, as well as continuously evaluate their effectiveness and how its organisational structure and work culture affects burnout.”
Mulligans Launch Flagship Store
The 4,000 square foot pharmacy and lifestyle store at Kilbarry is a luxurious flagship Mulligans Store offering a holistic, health and lifestyle range of producats and services. The store also sees the opening of Mulligans very first robot pharmacy service with an outdoor digital kiosk for customers offering a complete ‘click and collect’ service for all products that can be found at Mulligan’s, as the kiosk is fully serviced by robot technology.
Vitamin D and Prediabetes
Researchers from TILDA have released new research, published in the journal eClinicalMedicine, showing that vitamin D deficiency was associated with a 62% increased likelihood of developing prediabetes over 4-years.
Vitamin D is known to play an important role in bone and muscle health, while evidence is accumulating that supports vitamin D having many extramusculoskeletal effects, such as in diabetes. However, in Ireland, having low levels of vitamin D is common, with 1 in 8 adults aged 50 or older having deficient levels. Prediabetes is the stage where blood sugar levels are higher than they should be, but not so high as to have diabetes. The majority will go on to develop diabetes.
Key findings:
• those who had low levels of vitamin D were 62% more likely to develop prediabetes at 4-year follow-up compared to those with higher levels of vitamin D
• each unit increase in body mass index increasing the likelihood of new prediabetes by 7%
• smokers twice as likely to develop prediabetes over a 4-year period compared to non-smokers
Davy Fitzgerald and a number of the Waterford Senior Hurling team joined Mulligans at Kilbarry to officially open the new look store. Speaking following the event Managing Director Ronan Mulligan stated, “Huge thank you again to our staff who put in an incredible amount of effort for this (staff on the shop floor, pharmacists, management, warehouse and all in-between), our new café Frost & Co (Ferrybank), our suppliers,
shop fitters, construction & all involved! Also, to Ann Power for the wonderful work put in to make this day a success! We have a great team & we’re very excited for the future.”
Mulligans Pharmacy group now has 20 stores across Waterford, Kilkenny and South Tipperary and Dublin and was first established by Jim and Sheila Mulligan in 1957 when they opened a pharmacy at George’s St in Waterford City.

This is another innovative step for the family-run pharmacy group having previously opened Ireland’s first drive-thru pharmacy in 2014 and Ireland’s first full-service department store pharmacy in 2021 at Arnott’s.
The high-tech service at Mulligans also manages customer collection deadlines and can keep customers and even carers informed ahead of repeat prescription pick-ups via the new Mulligan’s prescription App which is about to be launched. Advanced technology can be integrated fully with the pharmacy e-commerce website enabling the purchase of non-medicine products out of hours for pick up.
Dr Kevin McCarthy, first author, said, “This study supports the role vitamin D may play in diabetes. Those with the lowest levels of vitamin D were far more likely to have higher blood sugar levels within 4 years. We know most people with prediabetes go on to develop diabetes, and all the health problems that diabetes can bring, so it is important that we address modifiable risk factors to reduce the likelihood of people developing diabetes. The results also highlight the importance of maintaining a healthy weight and not smoking, both of which also have numerous other health benefits.”
PHARMACYNEWSIRELAND.COM 10 News
Mulligans Pharmacy Group Director Ronan Mulligan
Mulligans Pharmacy Group recently launched their new flagship store in Kilbarry Centre, Old Tramore Road, Waterford.
Pharmacy Meeting
Future of Pharmacy
A range of issues were on the agenda, including supply chain disruption, mergers, acquisitions and pharmacy technology. With Matt Cooper serving in the role as MC, delegates heard a number of panel sessions which really delved into the core of what is current, and what the opportunities and challenges are ahead.
Stuart Fitzgerald, Managing Partner with Fitzgerald Power commented, “As Ireland’s leading accountancy firm to the pharmacy sector, we want to bring together great minds from the community pharmacy world to discuss the challenges and opportunities present in the industry today. As supply chain, labour and regulatory challenges mount,
pharmacists may find themselves at a professional crossroads. Our event aims to share sector knowledge and provide insight to help sector stakeholders respond to these challenges.”
Irish Medicines Supply Chain
Dermot Ryan, Managing Director, Supply Chain & Retail and Global Operations Lead with Uniphar joined Paul Reilly, Managing Director for the Phoenix Group to look at Ireland’s supply chain.

In outlining the dynamic between Ireland’s two largest wholesalers, Dermot stated, “There is a huge inter-alliance between us that is often missed. We are his biggest customer and us, his.”
Sharen McCabe, Managing Director, McCabes Pharmacy Group with Daragh Connolly, CommCare Pharma and Matt Cooper
Paul highlighted some of the biggest concerns, with the two wholesalers facing huge costs of their own stating, “We are effectively caught between the HSE and the manufacturers and, unfortunately we can’t even get the HSE to sit at the table with us to discuss the growing cost factors or the price of medicines. This is discussed between the manufacturers and the HSE, we aren’t even in the room.
“The model today in Ireland is broken, and I am not sure how we can fix it. The manufacturer and supplier relationship is as fragile as it has ever been.
“Last month for every 100 packs we ordered, we got 71 and that says – something is happening,” with Dermot adding, “It has been a tough 18 months and I believe it’s only going to get tougher. The challenge we face is that we can’t put prices up. We have written to and tried to meet with the key people from the HSE but have had no response.”
United Drug have recently been acquired by the Phoenix Group and Paul says this is a very positive development. “Phoenix Group are the largest wholesaler in Europe and they will help us to increase scale,” he reflects.
Caoimhe McAuley, Superintendent Pharmacist, Boots Ireland and Daragh Connolly, CommCare Pharma
Oonagh O’Hagan, Managing Director, Meaghers Pharmacy Group and John Carroll, Founder & CEO, Navi Group




PHARMACYNEWSIRELAND.COM 11
Fitzgerald Power recently brought together an excellent panel of speakers for the Future of Pharmacy event held in Teeling’s Distillery in Dublin.
Dermot Ryan, Managing Director, Supply Chain & Retail and Global Operations Lead with Uniphar and Paul Reilly, Managing Director for the Phoenix Group and MC Matt Cooper
Sharen McCabe, Managing Director, McCabes Pharmacy Group
Pharmacy Meeting
Mergers and Acquisitions
Mergers and acquisitions happen in the community pharmacy industry every day, yet there is still so much to learn. Chatting about this was Stuart Fitzgerald and Sharen McCabe from McCabes Pharmacy Group.
For the next 6 months Stuart sees a lot of activity in this area, stating that the pipeline of vendors is very strong. “We have never had more activity on the sales side. It is really instructive that McCabes Pharmacy Group have brought on board a Head of Acquisitions, it shows an appetite for the market. If you look at the organised buyers at the top of the market such a Uniphar and United Drug, I do expect to see a continuation of activity.”
McCabes Pharmacy Group has grown steadily over the last 40 years to their current position within Ireland’s pharmacy landscape. They have recently completed a number of pharmacy acquisitions. They currently have 28 stores and Sharen says, “There are just under 2000 pharmacies and in terms of having meaningful market share we would like to get to having over 100 stores. We have recently appointed a Director of Acquisition and we believe or moment now is to accelerate that. We want to grow sustainably but we do want to increase out scale.”
Stuart added, “The age-profile of those selling is interesting. It is no longer just pharmacists reaching retirement age that are looking towards selling. Pharmacists today are quite jaded; the Covid period has pharmacists jaded, the current labour issues have pharmacists jaded and we are seeing operators come to the market who are maybe 10 years early to the market and that could be due to the pressures being faced today.”
In turning attention to the current labour and recruitment crisis within the industry, Stuart said this is
of the leading issues resulting in sales of pharmacy business before retirement. In 2021 the number one issue bringing people to the market was Covid burn out. In 2022 the number one issue is labour. The locum market is unsustainable in my view.”
Sharen agreed with this sentiment adding that for pharmacies wanting to sell, having a longstanding and happy team can be one of the key factors to drive up the value of your business.
International Pharmacy Trends
Caoimhe McAuley, Superintendent Pharmacist with Boots Ireland joined Daragh Connolly from CommCare Pharma to discuss international pharmacy trends.

Taking another look at post-Covid burnout, Daragh noted how significant it has become. “We are here to talk about the future of pharmacy but we can’t have a conversation about burnout and post-Covid burnout in particular without looking at the broader context of the society we work in.
“What I know from my international experience is that throughout the world, people are feeling the burnout in their profession. Nurses are striking, many professions are striking. You can se that people are choosing what is important to them in their life and in their lifestyle, and there has been an acceleration with that because of how we had to change how we live.
“In pharmacy, we are no different to any other profession that is feeling the pressure. I fear the repercussion from that could be in the quality of the service we can deliver to the people we serve. I don’t want that to suffer because of the burnout and the regulatory forces being placed upon us.”
Caoimhe added, “Reflecting on pharmacy, we are hearing about the 60 hour weeks, we already
Caoimhe McAuley, Superintendent Pharmacist, Boots Ireland and Daragh Connolly, CommCare Pharma with Matt Cooper
have people in practice who have gone through an incredibly difficult few years and then we have the younger generation who are starting to go through their pharmacy programmes, they have seen a pharmacy environment that probably feels a little bit different from what they expected.”
Caoimhe said “The utilisation of community pharmacy has progressed significantly but Ireland is still behind. We could be doing a lot more work, more satisfying work.”
Daragh added, “The most recent IPSOS Poll has shown that 97% of the general public trust their pharmacist. That is a huge number, that is un-precedented in other countries around the world. We can and should be doing more.”
Darah commented on the lack of a Chief Pharmaceutical Officer for Ireland saying, “If you are not at the table - you are on the menu. If we had a Chief Pharmaceutical Officer at the table they could help to be a problem solver for many of the issues facing community pharmacists today.
“We need to make pharmacy a great place to work. Peoples’ priorities have changed, not everyone wants to work a 40+ hour week so we need to look at this and take it into account.”
In looking at the sometimes conflicting relationship between pharmacists and GPs, he added, “Pharmacists and GPs are part of a healthcare continuum. But what is often overlooked is that the person at the centre of that continuum is the person needing care. I am not an advocate for pharmacy, I am a huge advocate for people getting the appropriate and timely access to care.”
Pharmacy Technology
Oonagh O’Hagan, Managing Director of Meaghers Pharmacy Group joined John Carroll, Founder and CEO of Navi Group, on stage to discuss the future of pharmacy technology.

When it comes to pharmacy management systems, Oonagh discussed how she employs some of the new technologies available.
“In any setting where you can employ technology to take the burden away from our biggest asset – our people – I think it’s a good thing to do. We have a rule, which is that if something is being done repetitively, and there is a digital way to do it, we will embrace that; from back of house to front of house.
“Certainly that is not something we typically signed up to do when any of us entered the profession of pharmacy and that is where we currently have a disjoint in community pharmacy at the moment. Pharmacists want to be patient-facing, they want to use their skills, that is what we signed up to do. What we are trying to do is embrace technology and use it as an enabler to take that burden away.”
Discussing the development of a digital health record system in Ireland, John added, “The development and roll-out of the digital health record will be an inflection point in terms of IT, not just in pharmacy but he wider health industry. It is really badly needed at the moment.”
Noting the timeframe for rollout, John added that while there are digital health records in neighbouring countries already, for us in Ireland it is a matter of getting it actioned. There is a lot of excellent work happening there under the leadership of Professor Martin Curley.
Matt Cooper, MC for the event pointed out that there are currently many information systems being developed in parallel but that are not linking with each other. “That is the biggest issue in IT in pharmacy at the moment and across the health service in general,” John agreed.
PHARMACYNEWSIRELAND.COM 12
Paul Reilly, Managing Director for the Phoenix Group
1. Stuart Fitzgerald, Managing Partner, Fitzgerald Power with guests


2. Dermot Ryan, Managing Director, Supply Chain & Retail and Global Operations Lead, Uniphar with guest 3. John Carroll, Founder, CEO, Navi Group and Oonagh O’Hagan, Managing Director, Meaghers Pharmacy Group 4. Patrick McCormack, CEO, Cara Pharmacy Group and Robert Keane, Keanes CarePlus Pharmacy Group 5. Ciaran Foley, Niall Mackey and Frances Keenan, AIB
6. Adrian Dunne, Managing Director, Adrian Dunne Pharmacy Group and Robin Hanna, Sales Director, McLernons


7. Fergus McCauley, Pharmacy Key Account Manager, IQVIA with Jim Curran, Irish Pharmacy Union


8. Grainne Henson, Head of Health Sector, Bank of Ireland, Patrick McCormack, CEO, Cara Pharmacy Group, Cormac Tobin and Noel Winters, Partner, Fitzgerald Power


9. Sharen McCabe, Managing Director, McCabes Pharmacy Group and Daragh Connolly, Chairman, CommCare Pharma Group
10. Rory O’Donnell, O’Donnell’s totalhealth Pharmacy with Filipe Infant, HMR
11. Claire Fitzell, Head of Professional Services, Irish Pharmacy Union with John Arnold, CEO, CommCare Pharma



PHARMACYNEWSIRELAND.COM 13 6
1 2 4 3 5 7 8 9 10 11
Pharmacy Supports ‘Safe’ Initiative
Ireland’s pharmacies have supported International Day for the Elimination of Violence against Women. The Irish Pharmacy Union (IPU) has said that there are now over 1,000 pharmacies signed up to its ‘Safe Pharmacy’ initiative which provides victims of domestic abuse and coercive control a safe place to access support.

Safe Pharmacy was launched in July as a partnership between the Irish Pharmacy Union (IPU), Safe Ireland, An Garda Síochána and the HSE. The participating pharmacies have specially trained staff who will assist anyone who requests it with access to a phone in a private consultation room and contact details for local support services. The goal is to facilitate victims to make contact with a family member, local specialist domestic violence services or An Garda Síochána to seek further help.
Outlining the benefits of the scheme Ruth Garahy a pharmacist and member of the IPU’s Community Pharmacy Committee said, “We know that sadly there are many women and girls who are victims of violence around the world. Here in Ireland this often comes in the form of domestic abuse. Knowing where to go for help can be a big challenge for people in abusive situations and through Safe Pharmacy, we have created over 1,000 locations across the country where support is now available.
“We want anyone to know that in Safe Pharmacies they will find
compassion, respect and support. They will be able to access whatever help they need, in the privacy of a consultation room. If you need help, or even if you think you may need help your nearest Safe Pharmacy is there for you.”
All Safe Pharmacies will display the purple Safe Pharmacy logo near the entrances. Anyone seeking support in a participating pharmacy should ask to speak to a pharmacist in the consultation room, and this will be facilitated.
Safeguarding Trust between Pharmacy and Patients
PGEU, the European organization representing community pharmacists, has published a position paper on the European Commission proposal for a European Health Data Space (EHDS) asking for stronger safeguards to maintain the bond of trust between health care professionals and patients.
The EHDS will support community pharmacists in providing better services and treatments to patients while promoting the safe use of medicines, but it is important that the new legislation doesn’t put excessive burden on healthcare professionals with time-consuming obligations that can potentially compromise the provision of care.
It is also necessary to increase legal certainty by clarifying the uses of health data, further developing strong anonymization standards for secondary uses, and considering ethical duties of healthcare professionals set out in national regulations. PGEU also believes that Article 168 of the Treaty regarding public health should be added as legal basis because the main objective of this
legislation should be to improve public health and quality of care and it should not focus only on the internal market perspective.
PGEU also recommends reconsidering some parts of the impact assessment accompanying the proposal due to the significant consequences it will have on national health care systems. PGEU also asks for the provisions on the re-use of data to be amended to guarantee a higher protection of fundamental rights and effective control mechanisms for citizens. In this regard the proposal has some loopholes to be addressed, such as the possibility for data access applications to be automatically approved if data access bodies do not review the application in two months.
PGEU President Roberto Tobia stated, “Optimal data generation, gathering and interoperability can improve treatments for patients and foster personalized advice. From this perspective, we expect that the European Health Data Space will improve quality of care and promote medical research. Enhanced interoperability will also contribute to unleashing the possibilities of e-prescriptions and support interprofessional collaboration and seamless care. However, to fully exploit its potential, it is vital to ensure that the EHDS legislation is fully in line with the EU General Data Protection Regulation, that it respects Member States competence on health care delivery and takes into account ethical professional duties.”
Vaccine Research
New research by RCSI University of Medicine and Health Sciences has provided new insights into the mechanism behind how our circadian 24hour body clock influences our immune response to vaccines, depending on the time of day.
The paper examined the changes taking place in the mitochondria of a key immune cell involved in the vaccine response and could help improve the design and timing of administration of future vaccines to maximise effectiveness.
It had been previously found that humans mount a greater response to certain vaccines depending on the time of day at which the vaccine is administered, however the reason behind this wasn’t clearly understood. This research has uncovered that our circadian clock is changing the shape of mitochondria within dendritic cells.
Research author Professor Annie Curtis, School of Pharmacy and Biomolecular Sciences at RCSI, said, “Our discovery has shed light on a crucial aspect of our body’s response to vaccination and highlights the importance of circadian rhythms in immunity. We can apply this understanding in vaccine development to ensure we receive the maximum benefits from vaccination.”
The circadian clock within dendritic cells is controlling whether mitochondria form one of two shapes either long strings, ‘networked’, or broken into small punctate pieces. It is within the networked formation that vaccination is most effective as dendritic cells have a better ability break up the vaccine into small pieces for interaction with our immune cells (T cells). Within the study, researchers used an approach to induce the networked phase which could have implications in vaccine design allowing us to optimise our immune response, irrespective of time of day.
Dr Mariana Cervantes-Silva and Dr Richard Carroll, lead authors on the paper, commented, “We are excited to uncover this new link to our immune system and vaccine responses.”
PHARMACYNEWSIRELAND.COM 14 News






















Ir I sh v I tam I ns Ir I sh v I tam I ns the sunshine vitamin for every body Boost your family’s health with a daily dose of Vitamin D VITAMIN D Oireachtas Health Committee recommends: Every adult in Ireland should start taking Vitamin D supplements.** Vitamin D3 Deficiency rates in irelanD peOple aged Over 80 64% peOple in nursing HOmes 67% peOple aged 18-39 47% peOple aged 50-59 35% CHildren aged 1-4 25% *Recommendations on www.hse.ie SONA have a full range of Vitamin D3 products to suit the whole family. Hse recommends: Children 0-4 years should be given Vitamin D supplements.* **Oireachtas Health Committee’s report Apr 7 2021 Order direct from sona on pH: 01 451 5087. a lso available from wholesalers, united drug and uniphar. Sona proudly Support S RCN: 20042462 | CHY: 13534 Ir I sh v I tam I ns
2022/2023 ePortfolio Review
In October 2022, the Irish Institute of Pharmacy (IIOP) sent an email to approximately 1300 pharmacists who have been selected for the 2022/23 ePortfolio Review. The IIOP also sent a reminder email to pharmacists in early December. In January 2023, these pharmacists will be required to submit evidence of their Continuing Professional Development (CPD) to the IIOP via their ePortfolio.
Mary-Claire Kennedy, ePortfolio Review Project Lead
on their ePortfolio. This sign will remain in place until the submission period is closed (29 January) and will not change when you have submitted cycles.
When the first submission period closes this will change from Pending to Active. This means that the review process is happening and is not specific to a particular pharmacist. The Active sign will remain in place until the ePortfolio Review process is complete in early May.
Pharmacists must have their current, active email address registered on their IIOP profile in order to ensure they receive communications from the IIOP about the 2022/23 ePortfolio Review. Should you need help in accessing the website, retrieving your password or editing your details contact the IIOP at info@iiop.ie.
How does the ePortfolio Review process work?
The ePortfolio Review process incorporates two elements:
o A random sample of ePortfolio extracts submitted in the first submission period that do meet the System Based Standards
o All ePortfolio extracts submitted in the second submission period
How will I know which cycles I should submit as part of my ePortfolio Review?
Here, Mary-Claire Kennedy, ePortfolio Review Project Lead, gives an overview of the process. We talk about the key dates, the process and where you can get more support.
So Mary-Claire, how will pharmacists know whether they are selected for the 2022/2023 ePortfolio Review?
In addition to the email communications that pharmacists have received from the IIOP, at this point selected pharmacists will also notice a Pending sign
If I’ve been selected, when will have I have to submit my ePortfolio extract?
On Monday 9 January 2023 an email will be sent from the ePortfolio System (info@iiop.ie) to your IIOP registered email address inviting you to submit CPD cycles from your ePortfolio for review.
You will have until Sunday 29 January to submit your extract. You may submit cycles at any stage during the three-week submission window, and you may make more than one submission.
• A System Based ReviewEach extract (i.e. the cycles you choose to submit) will be automatically reviewed against the System Based Standards which are pre-set within the IIOP ePortfolio system.
• A proportion of the ePortfolio extracts submitted will be reviewed against the Review Standards. This will include;
o All ePortfolio extracts submitted in the first submission period that do not meet the System Based Standards
To help pharmacists ensure the cycles they submit as part of their ePortfolio extract meet the System Based Standards the IIOP developed the Ready Reckoner tool. The Ready Reckoner will be available to pharmacists selected for ePortfolio Review when the submission period opens in January.


Pharmacists can use the Ready Reckoner in two ways;
• Before submissionPharmacists can use the ‘Check readiness’ function before submission to identify cycles to submit which collectively meet all of the System Based Standards.
• After Submission - The Ready Reckoner automatically appears when one or more cycles are submitted for review and uses green and red lights to show progress against the System Based Standards.
In the event that one of the standards has not been met, a red light and a warning icon are shown, and pharmacists can click on the warning icon for an overview of the action they need to take to meet this standard.
I can’t find the “Ready Reckoner” on the IIOP website, where is it?
The Ready Reckoner will only become available to pharmacists included in the 2022/23 ePortfolio Review when the submission period opens on Monday 9 January. It can be found within the Completed Cycles section of the ePortfolio.
I have undertaken CPD but not yet had the opportunity to record it in my IIOP ePortfolio. Can I
PHARMACYNEWSIRELAND.COM 16
ePortfolio
backdate these records to reflect when the work was completed?
Yes, you can backdate cycles to reflect the date the CPD was undertaken. Simply choose the relevant date when entering the ‘Cycle Start Date’. You can find further information on recording your CPD retrospectively in the How to meet the cycle from previous four years standard support resource which can be found on the 2022/23 ePortfolio Review Support page on the IIOP website.
Since I began using the IIOP ePortfolio, I have been recording entries in my ePortfolio but I haven’t completed all of my CPD cycles. I have been selected for the 2022/23 ePortfolio Review, if I completed a cycle now will it count as CPD from the year I created it?
Yes, it will count from the date it was created. We understand that people work in different ways, and the IIOP ePortfolio has been built to reflect this. While some people may like to complete a cycle and finish it right away other people may like to work on them over time.
Do I have to complete the Core Competency Self-Assessment Tool (CCSAT)?
The 2022/23 ePortfolio Review Standards require that pharmacists submit at least one CPD cycle which has originated from their self-assessment against the Core Competency Framework for Pharmacists in the current year (i.e. 2022 to the end of the submission period in January 2023).
For this 2022/23 ePortfolio Review there are two ways that you can demonstrate evidence of this selfassessment;
• By selecting the tick box under the Self-Appraisal stage of the relevant cycle ‘Completing self-assessment against the Core Competency Framework’
• By undertaking a CCSAT selfassessment, exporting the suggested cycle topics into your ePortfolio then completing and submitting at least one cycle for review
I completed the CCSAT in December 2021, and completed a CPD cycle based on the outcome in 2022. Can I submit this as a cycle which originated from my self-assessment against the PSI Core Competency Framework for assessment in the 2022/23 ePortfolio Review?
The 2022/23 ePortfolio Review Standards require that you submit one cycle created following selfassessment against the PSI’s Core Competency Framework in the
current year, therefore the cycle submitted to meet this standard must have a creation date in 2022 to the end of the submission period in January 2023. This is in line with the legislative requirement to regularly self-assess against the Core Competency Framework. A cycle created in 2021 will not meet this standard, however, you may wish to submit this cycle as evidence of one of the cycles that you created in the previous four years.
I have completed some of the CPD courses available through Irish Pharmacy News (IPN) and I have also completed IIOP online courses, are there other activities that I could record in my ePortfolio?
Many pharmacists are surprised to realise the breadth of what counts as CPD. Traditionally, many pharmacists focus their CPD on their clinical expertise. The PSI Core Competency Framework indicates that pharmacists must be competent across a range of domains. It’s important to aim to keep upskilled across all domains – not just those domains relating to medicines. This requires engaging in a breadth of learning.
Consequently, there are many different types of activities that can be recorded in your ePortfolio as CPD. Completing the IPN CPD modules, for example, is a very valuable means of undertaking CPD. Other examples of activities that can be recorded in your ePortfolio as CPD include:
• Attending a live learning course
• Reading an article
• Having a discussion with a colleague
• Attending a conference
• Researching a new drug that has been prescribed for a patient
It’s important to remember that recording any learning activity may be appropriate as long as you can demonstrate how it has contributed to your professional development.
It may be helpful to refer to the 2022/23 ePortfolio Review Standards to see types of criteria within the scope of the Review.
How much information should I record in my CPD cycles?
There is no right or wrong answer to this. Each pharmacist will have their own style; some choose to use bullet point information, while others prefer to be more detailed. What’s important, however, is that you sufficiently outline what you have learned and how that learning has contributed to your understanding, benefited your practice, improved
patient outcomes or advanced your continuing professional development. You will find some sample CPD cycles on the IIOP website, in IIOP newsletters or by attending ePortfolio Review information events. You should write in a way that works for you and allows you to reflect on what you have done.
What happens if my ePortfolio extract does not meet the 2022/23 ePortfolio Review Standards?
The ePortfolio Review enables pharmacists to demonstrate evidence of appropriate and ongoing engagement with CPD, in the interest of outcomes-focused professional development. All pharmacists’ ePortfolio extracts are reviewed against the specific standards, mapped to the legislative requirements, which have been set for that year’s ePortfolio Review.
All pharmacists who submit their cycles within the submission period in January 2023 will be provided with feedback on their ePortfolio extract, should one or more of the standards not be met in the first instance. They will then have an opportunity to resubmit cycles based on this feedback to meet the standards.
In the event that a pharmacist does not meet the standards at the end of their first ePortfolio Review process, they are automatically entered into the following year’s ePortfolio Review. The PSI is not informed at this stage. However, if at the end of the second ePortfolio Review the pharmacist does not meet the standard or does not reengage with this process in year 2, the IIOP is obliged under the terms of the ePortfolio Review Policy to refer the pharmacist to the PSI.
Will the PSI be aware of how I perform in the ePortfolio Review process?
The IIOP undertakes the ePortfolio Review process to enable pharmacists to demonstrate evidence of CPD, in line with the legislation. Whilst the PSI is responsible for selecting pharmacists for ePortfolio Review, the IIOP undertakes the review process itself, at arm’s length from the PSI. The IIOP will not share any aspect of a pharmacist’s ePortfolio with the PSI.
In the event that the IIOP cannot ascertain that a pharmacist is meeting his or her CPD obligations (i.e. if the standard is not met at the end of two years ePortfolio Review, or if the pharmacists fails to submit an ePortfolio extract for review within the timeframe provided) then the IIOP has a statutory obligation to refer the pharmacist to the PSI.
No information from your cycles is communicated to the PSI.
When I was selected for ePortfolio Review, I didn’t apply for an exemption due to extenuating circumstances but my circumstances have now changed and I will be unable to submit my ePortfolio. Is it too late to apply for an exemption?
The PSI manages the selection process for ePortfolio Review including all applications for exemptions from ePortfolio Review under its Extenuating Circumstances process. If circumstances apply to you which would have an impact on your ability to submit an extract from your ePortfolio, you should contact the PSI. The PSI’s Extenuating Circumstances Policy and the relevant application form are available on the PSI website. All applications submitted will be managed by the PSI on a confidential case by case basis.
Where can I get more information?
The ePortfolio Review Support Page can be accessed via the IIOP homepage and is the main source of information relating to the 2022/23 ePortfolio
Review including information on communication from the IIOP, timelines and ePortfolio Review Information Events. The ePortfolio Review support resources are also hosted on this page.
You may also wish to attend an Information Event. The IIOP hosted a series of Information Events from September 2022 until January 2023 to support pharmacists selected for the 2022/23 ePortfolio Review. All events are facilitated by a Peer Support Pharmacist and will specifically focus on the key requirements of the 2022/23 ePortfolio Review so that pharmacists will know exactly what to expect at each point in the process. Pharmacists who took part in previous ePortfolio Review processes consistently highlight the IIOP Information Events as a key source of support in their preparation. They felt more confident in participating in ePortfolio Review and had a clear understanding of what the standards were and how to access support if needed, following attending an event. There are two information events scheduled on 10 and 24 January 2023. You can book via the IIOP website, under the Courses & Events tab. There is also a recorded version of the information event available to view in your own time on the IIOP website.
The IIOP team are always happy to hear from you with any queries you may have.
PHARMACYNEWSIRELAND.COM 17
Covid Concerns Continue to Impact Access to Health
New research from Pfizer Healthcare Ireland confirms that lingering COVID-19 concerns are continuing to impact access to healthcare, which may have a longer-term impact on people’s health due to missed diagnoses. Of those surveyed, over a third of all adults are in some way worried about the possibility of a missed diagnosis due to the impact of COVID-19. The research also reveals that almost one third (32%) of people with an underlying condition are nervous about being in a hospital setting this winter.
Deb Mangone, Country Manager, Pfizer Healthcare Ireland, and Rachel Morrogh, Director of Advocacy & External Affairs, Irish Cancer Society Picture Conor McCabe Photography

have missed a medical diagnosis - we strongly encourage people to attend healthcare appointments, particularly if they have been putting this off and ensure they take preventative action to stay well this winter.”
The research, undertaken as part of the annual Pfizer Health and Science Index, asked respondents about their direct experience of COVID-19, their general health, and attitudes to science. Of the respondents who have had COVID-19 at least once, 80% had it in 2022, with the highest incidence occurring between January and April 2022 (45%). The research also revealed that 15% of people with an existing health condition, who tested positive for COVID-19, feel that their health has deteriorated as a result, with this figure increasing to one in five people (21%) amongst those aged 18-24.
In addition to the COVID-19 pandemic, this year’s Pfizer Index also asked the general public about the cost-of-living and its potential impact on access to healthcare: three in 10 adults say they are less likely to go to the doctor this winter specifically due to the cost-of-living crisis, with younger age groups aged 18-24 (49%) more likely to put off a doctor’s visit because of these concerns. Worryingly, a fifth of all respondents confirmed they have already put off a trip to the doctor this year due to cost-of-living concerns.
While people in Ireland are generally quite positive about their health, scoring themselves 6.9 out of 10, the annual Pfizer Index has captured a gradual decline in this positive sentiment over a number of years. On average, Irish adults are concerned about developing a number of illnesses as they age (more than 4 are mentioned on average), with cancer, heart disease, arthritis and Alzheimer’s topping the list of concerns. More than half the adult population (56%), and two-thirds of women, are concerned about developing a form of cancer.
Looking at attitudes to science, with 40% of respondents studying science up to leaving certificate, and 20% at third level, more than three quarters (77%) believe there should be more focus on science subjects in primary school. There is general acknowledgement that STEM qualifications boost employability (56%), but equally a growth in the perspective that STEM courses necessitate high leaving certificate points and are difficult third-level courses to get into (51%). Four in ten (41%) of younger adults (aged 18-24) feel that Ireland has more STEM opportunities in comparison to other countries.
Commenting on the research, Deb Mangone, Country Manager, Pfizer Healthcare Ireland, said, “The research results showcase the impact of COVID-19 which is still being felt by the public and the health service. It’s really important for people to book their appointment for their booster this winter and if you get COVID-19, and are higher risk, it is especially important that you take action straight away and not ignore concerns. A worrying statistic from the Index shows that 36% of people are concerned they may
Rachel Morrogh, Director of Advocacy and External Affairs, Irish Cancer Society, added, “While we are very concerned to see a third of people say that they are considering putting off a healthcare appointment this year due to the cost-of-living, we are unfortunately not surprised by this. We are yet to see the true impact of COVID-19 in terms of delayed cancer diagnoses, and with cost-of-living concerns adding yet another barrier to healthcare access, a lot of people who should be going to the doctor and getting an early diagnosis may not go, further escalating the scale of this issue. We implore anyone who needs to make an appointment with their healthcare provider to do so without delay. Please put your health first.”
Chris Macey, Head of Advocacy, Irish Heart Foundation, said, “The research shows that close to half (45%) of people who suffer from heart disease state it has a moderate to severe impact on them while 44% of those with high/ low blood pressure also report a moderate to severe impact.
Irish adults also continue to rate developing heart disease as a major future health concern. It’s important to emphasise that up to 80% of cardiovascular disease is preventable. Through actions such as controlling blood pressure and cholesterol levels, maintaining a healthy weight, eating a healthy diet, getting regular exercise, limiting alcohol intake, and not smoking, we can all minimise our risk of heart disease and stroke. As we age, taking proactive lifestyle steps under the advice of a qualified healthcare provider can help us all address the significant concerns highlighted in this research. We encourage anyone with health concerns to make an appointment with their healthcare provider today.”
PHARMACYNEWSIRELAND.COM 18
News
“While we are very concerned to see a third of people say that they are considering putting off a healthcare appointment this year due to the cost-of-living, we are unfortunately not surprised by this”
PRESCRIBING
Presentation: Each ml of oral solution contains 100mg Levetiracetam.
Indication: Monotherapy in the treatment of partial onset seizures in adults and adolescents from 16 years of age with newly diagnosed epilepsy. As adjunctive therapy: In the treatment of partial onset seizures in adults, adolescents, children and infants from 1 month of age with epilepsy, in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy and in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy.

Dosage and administration: Route of administration: Oral. Adults: monotherapy: Initially 250mg to 500mg twice daily. If 250mg given twice daily initially increase to 500mg twice daily after 2 weeks. Dose can be increased to 1500mg twice daily Dose can be increased by 250mg or 500mg twice daily every two weeks depending upon the clinical response Add-on therapy: 500mg twice daily. Can be increased up to 1500mg twice daily. Paediatric population: monotherapy: The safety and efficacy have not been established. Add-on therapy 6 months to 17 years (weighing less than 50kg): 10mg/kg twice daily, can be increased up to 30mg/kg twice daily. Dose changes should not exceed increases or decreases of 10mg/kg twice daily every two weeks. The lowest effective dose should be used Add on therapy infants aged 1 month to less than 6 months; Initial dose 7mg/kg twice daily. Dose can be increased by 7mg/kg twice daily every two weeks to max dose 21mg/kg twice daily. Elderly: Adjustment of the dose is recommended in patients with compromised renal function. Renal impairment: daily dose must be individualized See SmPC. Hepatic impairment: 50% reduction of the daily maintenance dose is recommended when the creatinine clearance is <60ml/min/1.73m2
Contraindications: Hypersensitivity to the active substance or other pyrrolidone derivatives or to any of the excipients. Precautions and warnings: Renal impairment. Complete blood cells counts if weakness, pyrexia, infections or coagulation disorders reported. Monitor patients for signs of depression and / or suicidal ideation and behaviours.
Monitor for psychotic symptoms, irritability and aggressiveness. Monitor for aggravation of epilepsy. Caution in patients with QTc prolongation, concomitantly treated with drugs affecting QTc interval or in patients with pre-existing cardiac disease or electrolyte disturbance.
Interactions: Methotrexate, macrogol. See SmPC for detailed information. Pregnancy and lactation: can be used during pregnancy, if after careful assessment it is considered clinically needed. Breast-feeding is not recommended.
Driving / using machines; Patients are advised not to drive or use machines until it is established that their ability to perform such activities is not affected. Undesirable effects: Nasopharyngitis, somnolence, headache, anorexia, depression, hostility/aggression, anxiety, insomnia, nervousness/irritability, convulsion, balance disorder, dizziness, lethargy, tremor, vertigo, cough, abdominal pain, diarrhoea, dyspepsia, vomiting, nausea, rash, asthenia/fatigue, thrombocytopenia, leukopenia, suicide attempt, suicidal ideation, psychotic disorder hallucination, confusional state, amnesia, vision disturbance, pancreatitis, hepatic failure, acute kidney injury, infection, pancytopenia, neutropenia, agranulocytosis DRESS, ECG QT prolonged, encephalopathy, Neuroleptic malignant syndrome, hepatic failure, Toxic epidermal necrolysis, Stevens -Johnson Syndrome, erythema multiforme, rhabdomyolysis.
(Please refer to the Summary of Product Characteristics for detailed information)
Legal category: POM
Marketing Authorisation Number: PA22697/012/001
Marketing Authorisation Holder: Syri Pharma Limited t/a Thame Laboratories Floor 0, 1 WML, 1 Windmill Lane, Dublin 2, D02 F206, Ireland. (Distributed by Advanz Pharma Ltd in Ireland)

Date of preparation: Sept 2022
Reference:
1. Data on file Levetiracetam 100mg/ml oral solution June 2020
ADV/LVI/PM/0012 Date of Preparation - December 2022 LEVETIRACETAM THAME 100MG/ML ORAL SOLUTION Now added on the List of Interchangeable Medicinal Products Interchangeable list code: IC0119-171-019 38.55% COST SAVING VS KEPPRA 100MG/ML ORAL SOLUTION1 Cost comparison of Syri Pharma’s Levetiracetam Thame 100mg/ml oral solution and Keppra 100mg/ml oral solution Product Keppra 100mg/ml oral Syri Pharma's Levetiracetam Thame Cost saving % saving solution IMF June 20201 100mg/ml oral solution Levetiracetam Thame 100mg/ml oral solution € 52.03 € 31.97 € 20.06 38.55% 300ML ORAL SOLUTION WITH 10ML DOSING SYRINGE
Thame
Oral
INFORMATION Levetiracetam
100mg/ml
Solution
Overprescribing among Older People: TILDA Study

International evidence shows that people approaching end of life (EOL) have high prevalence of polypharmacy, including overprescribing. Overprescribing may have adverse side effects for mental and physical health and represents wasteful spending. Little is known about prescribing near EOL in Ireland.
The study authors comprised of Soraya Matthews, Dr Frank Moriarty, Dr Mark Ward, Dr Anne Nolan, Professor Charles Nomand, Professor Rose Anne Kenny and Peter May. They aimed to describe the prevalence of two undesirable outcomes, and to identify factors associated with these outcomes: potentially questionable prescribing, and potentially inadequate prescribing, in the last year of life (LYOL). They used The Irish Longitudinal Study on Ageing, a biennial nationally representative dataset on people aged 50+ in Ireland. Below we summarise their findings.
In a nationally representative sample of 525 older people with high mortality risk in Ireland, 401 (76%) had potentially inadequate and 294 (56%) potentially questionable medications. Prevalence of these prescribing patterns among those in LYOL was 62% and 38% respectively. One factor was significantly associated with potentially inadequate medications in LYOL: male (OR 4.40, p = .004) Three factors were associated with potentially
questionable medications in LYOL: male (OR 3.37, p = .002); three or more activities of daily living (ADLs) (OR 3.97, p = .003); and outpatient hospital visits (OR 1.03, p = .02).
Comparatively lower prevalence of the specified prescribing patterns in LYOL is encouraging to the extent that they may indicate a level of deprescribing is occurring as people approach end of life. However, approximately 30,000 people die annually in Ireland (the majority of them over the age of 50), and our data suggest that a large proportion of these are at risk of inappropriate polypharmacy.
Tackling high prevalence of overprescribing near end of life requires ex ante identification of those at greatest risk. Multivariable regression results indicate that male sex is the strongest predictor of both undesirable outcomes. The odds of men receiving potentially inadequate medications were 4.4 times higher (than the odds of women receiving potentially inadequate medications), after controlling for other important predictors of prescribing. Similarly, the odds of men receiving potentially questionable medications were 3.4 times higher (than the odds of women receiving questionable medications), after controlling for the same predictors. Three or more ADL difficulties and outpatient engagements were also associated with potentially questionable medications, but neither cancer
nor cardiovascular disease were associated with outcome.
Examining the prevalence of potentially inadequate and questionable medications among male and female participants, differences were noted across medications used in the prevention and treatment of cardiovascular disease (antiplatelet and anticoagulant drugs, lipid-lowering drugs), with higher prevalence among males, and to a lesser extent, higher prevalence of medication for osteoporosis among females. These patterns are reflective of the patterns of prescribing observed in middle-aged and older adults more generally, with noted undertreatment of women compared to men given the same level of cardiovascular morbidity, while older men with similar risk of fracture to women are less likely to be treated for osteoporosis. This suggests that being of high mortality risk does little to reduce the disparity in prescribing between sexes. There were also high rates of medical or GP visit card eligibility (based on income) among those with questionable or inadequate medications, potentially reflecting an impact of higher burden and severity of co-morbidities, or greater access to care.
Much of the existing literature on inappropriate prescribing among people with limited life expectancy has focussed on
use of preventive medications, where the likely benefits will not be realised within a patient’s life expectancy. Most studies have focussed on people with a specific life-limiting condition, most often cancer. The most often cited preventive medications prescribed inappropriately were lipid-lowering drugs and antihypertensives, similar to the findings of our study, as well as antidiabetic drugs, which had a relatively low prevalence in the current study. Often such medications are only stopped in the last month of life. This underlines the importance of reviewing and discussing the need and benefit of such long-term medications to align treatment with patients’ goals, in general but particularly among people with life-limiting illness. It is also worth noting that while overprescribing is the focus of much research on optimal medication use near end of life, under prescribing is also an important issue, where use of symptom-relieving medications that enhance quality of life may be insufficient. Care for such patients should focus not just on reducing medications, but ensure therapy maximises quality and quantity of life and is aligned with patient priorities.
Ireland is ranked fourth worldwide for end-of-life care, meaning that there is relatively high per capita palliative care provision at the population level. Our paper highlights that even in a setting with well-established palliative care services, the risk of inappropriate prescribing among people with limited life expectancy in significant. However, these deficiencies in patient experience must be understood in the context of other challenges; previous research in TILDA demonstrated strong evidence for undermanagement of illness symptoms near end of life, whereas overprescribing is a form of overtreatment.
Thousands of older people die annually in Ireland with potentially inappropriate or questionable prescribing patterns. Gender differences for these outcomes are very large. Further work is needed to identify and reduce overprescribing near EOL in Ireland, particularly among men.
PHARMACYNEWSIRELAND.COM 20
Researchers from the Irish Longitudinal Study on Ageing (TILDA) have recently published a study which looked at overprescribing among older people near end of life in Ireland.
Professor Rose Anne Kenny, TILDA
Overprescribing





preparation: November 2022
For further information, please speak to your local Star OUTiCO representative: West Gary Lydon: 085 8716855 Munster Ger Allen: 087 2958103 Leinster Toby Kavanagh: 087 9262991 Product Name Legal Classification Star Reference EAN Code License Number MA Holder HYDROXOCOBALAMIN 1MG/ML SOLUTION FOR INJECTION POM 789525 9008732009132 PA 1770/002/001 G.L. Pharma GmbH, Schlossplatz 1, 8502 Lannach, Austria Additional information available on request. Hydroxocobalamin 1mg/ml Solution for Injection (vitamin B12) AVAILABLE NOW from
Date of
Ref: IE2022/014/00
Feeling the Chill: Cough, Cold and Flu
Written by Damien O’Brien, Pharmacist, Adrian Dunne Pharmacy Baldoyle

the more sudden onset of fluwhich usually appears within a few hours. Cold symptoms are usually in the upper respiratory tract and include blocked or runny nose, sneezing, cough, sore throat, loss of taste and smell, as well as fever and cough.4 Symptoms of flu include a sudden onset of fever, fatigue, body aches, difficulty sleeping, loss of appetite, dry cough and nausea.5 Both viruses can be unpredictable but symptoms generally start to resolve after one week.
inhalation with Zanamivir (Relenza). These are both antivirals of the neuraminidase inhibitor class, that have activity against both influenza A and B. Previously healthy patients with no underlying condition generally do not need antiviral therapy, unless the clinician feels the patient is at risk of developing complications. Pregnant women and patients who are deemed to be at risk of complications should be commenced on oseltamivir as soon as possible, even without laboratory confirmation of diagnosis.
Cough is a part of the body’s immune defence that is used to clear the airways of mucus and other irritants. Cough is a symptom and most often is caused by cold or flu. However, a number of other factors can cause cough include smoking, allergies, bronchitis, post nasal drip or acid reflux. Coughing is useful in clearing phlegm from the lungs, where it could otherwise cause infections such as pneumonia.2
Vaccination is the single most effective way to protect against flu. Unfortunately, there is no vaccination against the common cold. Flu vaccines are safe and effective, and are available through pharmacies nationwide. Pharmacists have been administering flu vaccines in the community since 2011.
Due to reducing antibodies and different flu strains each year, this vaccine is annual and is available between October and April. The effectiveness can vary from year to year, but it generally reduces the risk of getting flu by 40-60% and can also reduce severity of symptoms. The flu vaccine is recommended and is available for free for many patients, including those who are aged 65 years and older, aged 2-17 years, pregnant or have certain medical conditions. A full list of those eligible is available on the HSE website. Side effects are generally mild and can include soreness or swelling around the injection site, fever, aches and fatigue. Patients can
treat these symptoms with rest and paracetamol. More serious side effects are rare and should be reported to the HPRA.3
Although cold and flu share similarities as both are contagious respiratory illnesses, they differ in many ways. Flu is caused by influenza viruses, while colds can be caused by a variety of viruses including coronaviruses, parainfluenza and rhinoviruses.1 With regards to symptoms, cold and flu can also be similar but flu tends to be more severe than cold. The onset of cold is generally gradual, compared to
Flu is generally self-limiting in healthy individuals. For certain individuals, flu and complications of flu can lead to serious illness. The most common complications are pneumonia and bronchitis. Patients that are aged 65 years or older, pregnant or have a long term medical condition are at an increased risk of complications from flu. As patients with asthma, COPD and heart failure are at high risk of complications, there may be a need to refer to a medical doctor for further treatment. Pharmacists should also be aware that patients may need to be referred on to a medical doctor if their symptoms don’t improve after 7 days or they have a weakened immune system due to HIV or chemotherapy.6
Cold and flu are generally treated based on symptoms. Cold or flu can’t be fully diagnosed based on symptoms alone, but laboratory tests are rarely used. They are generally treated with over the counter medications that are used to provide symptomatic relief. Paracetamol and ibuprofen are both useful to treat aches and pains, as well as to reduce fever.6 Pseudoephedrine is a decongestant that provides symptomatic relief for congestion of the upper respiratory tract.7 Brompheniramine and diphenhydramine are both first generation antihistamines that can be used to treat symptoms of cold and flu including runny nose, sneezing and itchy/watering eyes.8, 9 Pharmacists should also counsel patients to get plenty of rest and sleep, keep warm, avoid smoking and to drink plenty of water.6
Flu can also be treated orally with Oseltamivir (Tamiflu) and by
Efficacy of oseltamivir has been demonstrated when initiated within two days of onset of symptoms, with evidence suggesting that treatment can reduce the risk of mortality if commenced within five days of onset of symptoms. Zanamivir is not currently licensed in Ireland, but may be used as an unlicensed product to treat oseltamivir resistant influenza.10
Coughs generally will generally improve after a number of days. Patients should be counselled to drink plenty of fluids and get adequate rest. As with cold and flu, paracetamol and ibuprofen can be used to treat associated pain if appropriate.4
Cough syrups are available over the counter, with Benylin, Exputex and Bronchostop among the most common. Exputex is a mucolytic agent used to treat coughs characterised by excess mucus.11 Bronchostop is a traditional herbal medicinal that is used to provide symptomatic relief for a variety of coughs, exclusively based on long standing use.12 A wide range of Benylin cough bottles are licensed to treat a range of different types of coughs from 2 years plus.13
However, a hot lemon with honey drink is similarly effective as over the counter cough medicines. If a cough is caused by a cold or flu, antibiotics will not help to alleviate a patient’s symptoms. However, pharmacists may need to refer a patient for further medical treatment if the cough lasts for more than three weeks, the patient is coughing up blood or the patient is at risk of complications.4
References available on request
PHARMACYNEWSIRELAND.COM 22
Cough, Cold
Flu
and
Pharmacists should also be aware that patients may need to be referred on to a medical doctor if their symptoms don’t improve after 7 days or they have a weakened immune system due to HIV or chemotherapy
Cough, cold and flu can often wreak havoc during the winter months, both due to the symptoms they cause to patients and the difficulty in differentiating between them. Both flu and the common cold are contagious respiratory illnesses caused by viruses and tend to be more widespread in winter months.1


www.prl ie pgsales@prl ie +353 1 257 4650 PRL SALES ULC, Greenogue Business Park, Rathcoole, Co. Dublin MORE than a Multivitamin... NOW with OMEGA-3 for heart1, brain2 and vision3
Educate Parents as RSV Cases Increase
Findings from a recently conducted survey have shown the need to educate parents about respiratory syncytial virus (RSV), as cases increased throughout 2022.

“Unfortunately, some babies become quite unwell with more serious symptoms such as rapid breathing, refusing food and liquids as well as a persistent high temperature of 38°C or above, even after administering paracetamol and ibuprofen, and it is a common cause of hospital admission. If any parent or caregiver is concerned that a child’s symptoms are worsening they should contact their GP and if the infant experiences any difficulty breathing they should seek medical advice immediately by calling the emergency services.
“The same precautions to prevent the spread of illnesses such as Covid-19 or flu can be applied with RSV. Encouraging children to cough into their elbow or tissue as well as frequent hand washing with soap and warm water or using hand sanitiser can also minimise the spread of infection.”
The survey was conducted among 319 parents in Ireland, to understand their level of awareness and knowledge of RSV. There are several respiratory illnesses which may affect babies. It was carried out by Sanofi.
Respiratory syncytial virus (RSV) is a common virus that can cause infection in the lower part of the respiratory system. These are known as lower respiratory tract infections (LRTIs). The survey results revealed that over half (53%) of Irish parents had heard of RSV but surprisingly their overall understanding of the disease was low.
When asked how their knowledge of the disease compared with other common childhood illnesses only one in five (20%) of parents surveyed had a good level of knowledge of RSV in comparison to diseases such as the common cold (67%), influenza (53%) and pneumonia (42%).

RSV is a major cause of severe respiratory illness among children under 2 years of age. It is also the most common cause of hospital admissions due to acute respiratory illness in young children, as well as being the most common cause of bronchiolitis (inflammation of the small airways in the lung) in infants.
Approximately 90% of children are infected with RSV by 2 years of age, with RSV being the leading cause of LRTIs in babies.
Dr Maire Finn, GP at The Ennis Family Medical Centre highlights the importance of being RSV aware saying, “As we enter into the winter season it is important for parents and caregivers, particularly those with small children, to be aware of the risks of common illnesses and respiratory infections including RSV, which is a major cause of respiratory illness in small children. Most babies have minor symptoms that resolve quickly but even then it can cause distress and worry for parents.
In Ireland, the RSV season is defined by the HSE as starting at the beginning of October, and ending in late February (Calendar Week 40 – Week 8). RSV has been a notifiable disease in Ireland since January 2012 and RSV activity in Ireland monitored by the HSEHealth Protection Surveillance Centre (HPSC).
Precautionary steps can also be taken to help protect infants. These include:
Frequent, careful hand washing is the most important measure in preventing the spread of RSV
Coughing into your sleeve
Keeping symptomatic siblings and other people away.
For the latest HSE guidance on all respiratory tract infections, visit: https:// www.hpsc.ie/a-z/respiratory/ respiratorysyncytialvirus/factsheet/
PHARMACYNEWSIRELAND.COM 24 Cough, Cold and Flu


For the treatment of chesty and phlegmy coughs Sugar free Gluten free Suitable for vegans Contains thyme liquid extract Children from 12 years and adults: 15ml, 4 times a day Medithyme Cough Syrup contains liquid extract from Thymus vulgaris L., herba (Thyme herb). Contains: ethanol, maltitol (E965) and benzoate salt (E211). For oral short-term use only. Contraindications: Hypersensitivity to thyme or to other members of the Lamiaceae (mint) family, or to any of the excipients. For supply through general sale. A copy of the summary of product characteristics is available upon request. TR 126/319/001. TR Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co. Tipperary. Date prepared: November 2022. 2022/ADV/MED/301H. *Medithyme Cough Syrup is a traditional herbal medicinal product, for use in case of productive cough associated with cold, exclusively based on longstanding use. Traditional herbal medicinal product 180ml pack size
Pharmacist Impact on Sore Throat Management
A new resource from the International Pharmaceutical Federation (FIP) to aid community pharmacists in supporting people to manage a sore throat has been published. Intended as a “quick reference guide for pharmacists”, it covers causes, triage, and pharmacological and non-pharmacological treatments, and it provides links to further resources. “Sore throat is a common condition that is usually selflimiting. Pharmacists can support its management through a range of products and services, thereby avoiding unnecessary visits to general practitioners,” said Rúben Viegas, FIP practice development and transformation projects coordinator, and author of the guide.
Sore throat can be defined as a painful, dry or scratchy feeling in the throat. It is generally a selflimiting condition that usually has no significant long-term harmful effects on health. Throat pain is one of the most commonly encountered symptoms in the community pharmacy, especially during winter, and accounts for a high number of GP visits each year, therefore placing a considerable burden on the health systems.
Causes of sore throat can vary and can include viral infections, environmental factors, allergies and smoking. It is less commonly caused by bacteria. The most common occurrence of bacterial infection causing sore throat is group A streptococcal throat infections or commonly referred to as “strep throat”.
Viral infections are frequently accompanied by cough, runny nose, hoarseness and sometimes conjunctivitis. In contrast, bacterial infections (strep throat) include symptoms such as fever, white patches on the throat (tonsillar exudates which indicate the presence of bacteria) and generally high levels of local inflammation.
Other factors that might cause a sore throat include allergies, postnasal drip, dryness caused by external factors or irritants, irritation from excessive voice use, or inflammation due to reflux disease. Exposure to tobacco or chemical irritants, a weakened immune system, and recurrent upper respiratory infections can put an individual at higher risk of developing a sore throat.
The routine use of antibiotics for sore throat is not recommended. Sore throat is one of the most common upper respiratory tract infections where inappropriate and unjustified antibiotics use occurs. There are increasingly more studies on the use of rapid tests to differentiate between viral and bacterial throat infections to guide antibiotics prescription and minimise the misuse of antibiotics for infections caused by viruses or other microbial agents.
Signs and symptoms
Symptoms of sore throat include:
• Pain or a scratchy sensation in the throat;
• Pain that worsens with swallowing or talking (odynophagia);
• Difficulty swallowing (dysphagia);
• Sore, swollen glands in the neck or jaw;
• Swollen, red tonsils; and
• A hoarse or muffled voice.
Red flag symptoms include:
• A sore throat that is severe or lasts longer than a week;
• Difficulty and painful swallowing (odynophagia or dysphagia), with difficulty in breathing or opening the mouth;

• Joint pain, earache or swelling of the neck;
• Fever higher than 38.3ºC (101ºF) with or without shaking, chills and night sweats;
• Frequently recurring sore throats; and
• Hoarseness lasting more than two weeks.
The presence of any red flag symptoms should lead the pharmacist to refer the patient to the healthcare system where the patient can see a doctor and get an adequate follow-up and treatment if needed.
Triage Community pharmacies can play an important role in screening for bacterial infections through the use of rapid tests. These tests help distinguish between cases that can be managed with non-prescription (over the counter) medicines and those that require referral for further examination and a potential prescription for Empowering selfcare: A handbook for pharmacists| p21 antibiotics.
Offering these types of screening tests can aid in antimicrobial stewardship efforts within the community while relieving the pressure placed on health systems
by frequent consultations for sore throat. In countries and territories without accessible point-of-care diagnostic testing or screening tools, the tendency for antibiotics to be prescribed is higher than in countries and territories that possess such capacity.
As an example, in the UK, there is a community pharmacybased screening and treatment service using point-of-care testing. This type of service supports the mitigation of antimicrobial resistance by reducing inappropriate antibiotics use. In comparison, in instances without access to such tests, several tools, such as the Centor or McIsaac scores, can support pharmacists in screening patients for streptococcal throat infections
In addition to the use of these tests, pharmacists play an important role in accurately collecting patient data and screening for red flag symptoms in their patient assessments. Specific questions that pharmacists may ask patients to guide their recommendations on the most appropriate treatment for sore throat include:
• Do you have any visible red or white dots in your throat?
• Is cough present? Dry or productive? What colour is your sputum?
• Do you have fever? For how long? What is the average reading? How did you measure it?
• Does it hurt when you swallow? Are you able to adequately eat or drink?
• Do you have any known allergies?
• Do you feel any other symptoms, such as acid reflux? The decision to refer the patient or recommend interventions to manage symptoms should be considered on a case-by-case basis after careful patient assessment.
Pharmacological management
As sore throat is a self-limiting condition, advising patients to get plenty of rest is helpful to allow the immune system to fight off the infection. The use of nonprescription (over the counter) medicines can also relieve symptoms associated with sore throat in most cases and prevent unnecessary visits to the doctor.
Pharmacological options usually available in the pharmacy are generally aimed at reducing local pain and inflammation. These can include anti-inflammatory and analgesic agents, lozenges, sprays, drops and mouthwashes.
Concomitant cough may also increase irritation and worsen the sore throat. In these cases, medicines to reduce cough may be also useful. Moreover, postnasal drip is frequent in conditions like rhinitis or sinusitis and may cause further irritation in the throat. Post nasal drip is often associated with cough, which can also increase the symptoms of sore throat. Gastric reflux and patient allergies can also lead throat irritation. With regard to allergies, non-prescription (over the counter) antihistamines may be recommended to help reduce symptoms and support management of the sore throat.
PHARMACYNEWSIRELAND.COM 26 Cough, Cold and Flu
1


Associated with Common Cold, Bronchostop Junior is a MD. 2 Based on Traditional use, Bronchostop Adult is a THR. * Based on combined IQVIA Data - June 2022. Buttercup Bronchostop Cough Syrup contains thyme herb extract and marshmallow root extract. A traditional herbal medicinal product for the relief of coughs, such as chesty coughs and dry, tickly, irritating coughs and catarrh, exclusively based upon long-standing use as a traditional remedy. Adults and children over 12 years: 15ml every 4 hours, 4 times per day. Max 6 doses (90ml) per day. Max dose should not be exceeded. To be taken 30 to 60 minutes before or after intake of other medicines. Not recommended for children under 12 years. To be administered undiluted or diluted in water or warm tea. Seek medical advice if symptoms persist after 7 days or if dyspnoea, fever or purulent sputum occurs. Contraindications: Hypersensitivity to marshmallow root, thyme, to other members of the Lamiaceae family or to any of the excipients. Warnings and precautions: Asthmatics and atopic patients should consult a doctor before using the medicine. Contains E218 and E216 that may cause allergic reactions (possibly delayed). Patients with HFI, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take themedicine. The additive effect of concomitantly administered products containing fructose (or sorbitol) and dietary intake of fructose (or sorbitol) should be considered. Pregnancy and lactation: Not recommended. Side effects: pruritus, rash, urticaria, angioedema, anaphylactic reaction, oral mucosal blistering, abdominal pain, diarrhoea, nausea, vomiting, dyspnoea, exacerbation of asthma. Legal classification: GSL. TR 2006/001/001.TR Holder: Kwizda Pharma GmbH, Effingergasse 21, A-1160 Vienna, Austria. RRP (ex VAT): €4.99 Date of preparation: 05/2022. SPC: http://www.medicines.ie/medicine/16380/SPC/Buttercup+Bronchostop+Cough+Syrup/ Bronchostop Junior is a medical device according to Directive 93/42/EEC, used to relieve any cough (dry & chesty) associated with a cold for children from 1 year. Children under 3 years of age should consult with a doctor to exclude more serious diseases being present. Use in children under 1 year of age is not recommended. Children aged 1 year and above: 5ml up to 3 times daily. Children 2 to 3 years: 5ml up to 4 times daily. Children 4 to 5 years: 7.5ml up to 4 times daily. Children 6 to 11 years: 15 ml up to 4 times daily. Always read the Instructions for Use. Date of preparation: 05/2022. Date of preparation: August 2022. IRE/BRO/2022/37


TO RELIEVE ANY COUGH1,2
COUGH BRAND*
IRELANDS No.1
HealthBeacon Share the Vision

HealthBeacon recently held a Vision event in Dublin, collaborating on supporting patients with their adherence to medicines and ensuring optimisation of health outcomes. The team also showcased their new smart for managing pill medicines – the HB Wave.





Meaghers Pharmacy Group will be one of the first pharmacies in Ireland to support patients by giving them access to HealthBeacon’s technologies and systematic adherence support. HealthBeacon’s Leonora O’Brien commented, “Thanks so much to Oonagh O’Hagan and Superintendent pharmacist Elaine Lillis for being such great leaders and innovators and for always pushing the boundaries of possibility in pharmacy practice.
“Medication non-adherence one of the most serious issues in healthcare, contributing to the premature deaths of nearly 200,000 Europeans and costing EU governments an estimated ¤125 billion annually. HealthBeacon are helping solve this issue, on a global scale. It has been great to be able to share the Vision for the key role of pharmacists will play in this adherence service.”
Next month’s issue of Irish Pharmacy News will feature more details about this exciting new technology.
PHARMACYNEWSIRELAND.COM 28 Vision Event
Leonora O’Brien, Expert Channel Lead Advisor - International Pharmacy Markets, HealthBeacon
From left to right: Jim Joyce, Leonora O’Brien, Elaine Lillis (Superintendent Meagher’s Pharmacy Group), Laura Dowling, Oonagh O’Hagan and Natalie Maginnis
Laura Dowling, ‘The Fabulous Pharmacist’
Oonagh O’Hagan, MD Meagher’s Pharmacy Group
Jim Joyce - CEO and Co-Founder HealthBeacon Plc
Kieran Daly - CTO and Co-Founder of HealthBeacon gives demo of the “HB Wave” for adherence to pill medication
Recommendations on Electronic Sharing
HIQA publishes recommendations to support electronic sharing of information in older persons’ services
In November 2022, the Health Information and Quality Authority (HIQA) published a set of recommendations to enable the electronic sharing of health information in older persons’ services. Read more to find out all you need to know.
Background
In common with many other countries, Ireland faces the challenge of an ageing population and it is expected that people aged 65 years or more will account for 50% of all healthcare activity by 2031. An older person typically receives care from a wide range of health and social care professionals across both acute and community settings. However, the health information of an older person is usually held across a number of IT systems or in paper records, making it difficult for health and social care professionals to easily access and share this information across all settings. This means that it is often difficult to get a full picture of the care needed for an older person. Additionally, the high number of different services an older person experiences, and the increasingly complex nature of older persons care, are challenges to the safe and effective sharing of health information in older persons’ services.
The need for change
These challenges in older persons’ services were intensified by the COVID-19 pandemic.
In August 2020, the COVID-19 Nursing Homes Expert Panel recommended the introduction of an integrated IT system to support the effective sharing of health information for older persons.
The recommendation from the Expert Panel looked for the safe and effective sharing of information along the care journey, which includes numerous settings, such as: residential care settings (including public and private nursing homes): day care settings; home support services (including public and private providers); GP practice management systems; and discharge planning in acute settings.
HIQA’s involvement
The Department of Health asked HIQA to explore how the capabilities recommended by the Expert Panel could be progressed and implemented. HIQA worked with stakeholders across health and social care, and ICT, to develop Recommendations on the
Written by Rachel Flynn, Director of Health Information and Standards, HIQA

ICT Enablement of Older Persons Services. The recommendations support the safe and effective electronic sharing of health information for older persons (and for other populations) across both public and private sectors, and are aligned to national eHealth goals under Sláintecare.
The recommendations also provide an analysis of the current situation nationally and international best practice. Informed by this evidence, they outline measures that would ensure that the capabilities requested by the Expert Panel are provided in line with national priorities.
Recommendations
The recommendations are:
Strategy and governance: A national health information strategy, with identified priorities and associated funding, is needed to provide strategic direction across the entire health information system. A clear overall strategy for ICT enablement of older persons services is also needed, to ensure that the capabilities outlined by the Expert Panel are delivered. This should be developed by the appropriate governance structure(s).
Vision and roadmap: A shared vision must be developed, in collaboration with all stakeholders, with older persons being a critical stakeholder group. It is essential that stakeholders are fully engaged throughout the programme to ensure acceptance and adoption.
Standardised sharing of information: A suite of national standards are fundamental for the effective sharing of information and should include minimum datasets that are based on the actual needs of users. This will support the safe and effective sharing of appropriate information along the older person’s care journey, with those authorised to provide care having the information they need when they need it.
User engagement: All systems should meet basic user-centred principles and users should have the targeted training and broader digital education they need, such as through broad national initiatives. This will ensure that users have confidence in, and are ready to adopt, the systems that are developed, which is a critical point of failure in some eHealth projects.
Next steps
To progress these recommendations, HIQA will develop a comprehensive suite of national standards to help facilitate the digital enablement of older persons services and the health information system in general for all populations.
Health information generates large volumes of data every day, and health and social care professionals spend a significant amount of their time handling and collecting it, and looking for and storing it. The current ICT infrastructure in Ireland’s health and social care services is highly fragmented with major gaps and silos of information, preventing the safe and effective transfer of health information. These recommendations aim to support the digital enablement of older persons services to ensure that high quality health information is easily available to all health and social care professionals providing care to older people at all stages along their patient journey. The Recommendations on the ICT Enablement of Older Persons Services can be read in full on www.hiqa.ie.
PHARMACYNEWSIRELAND.COM 29 HIQA Report
Pharmacy Management of Sleep
Sleep is characterised by altered consciousness and reduced reaction to stimuli, as well as reduced sensory and muscle activity. This helps to restore body systems - which are vital in cognition, mood, memory, metabolism and immune response, amongst others.1 The average adult requires 7 to 9 hours sleep per day.2 Benefits of adequate sleep can include improved mood, reduced stress, reduced risk of sickness, maintenance of a healthy weight and a lower risk for serious health problems.3
Unfortunately, many issues can arise with sleep from a variety of different sources. Insomnia is the most commonly diagnosed sleep disorder and is defined as the inability to fall asleep or stay asleep, which results in reduced functioning the following day. Insomnia is generally multifactorial and can be related to physiological issues, medication use, drug use, concomitant disease and poor sleep hygiene.4 Other sleep disorders include narcolepsy, a condition where an individual can’t control when they sleep,5 and sleep apnoea, a condition where the muscles and soft tissue in the throat relax and collapse which can cause a total blockage of the airway.6 Pharmacists are ideally placed in the community to recognise insomnia in patients and make interventions or refer the patient if necessary.
Numerous medical conditions are associated with an increased risk of insomnia including, but not limited to, menopause, pain, cancer, dementia, Parkinson’s disease, asthma and heart disease. Mental health disorders are among the most common conditions that lead to disruption of sleep. Anxiety, depression and post-traumatic stress disorder are examples of mental health conditions that can cause insomnia.7 Pharmacists
can recognise these issues when counselling these patients, provide advice on how to improve sleep quality or refer to GP, mental health services or cognitive behaviour therapy as appropriate.
Medication can have both a positive and negative impact on sleep quality. Certain drugs can be used to treat insomnia directly. Drugs can also be used to improve the medical conditions mentioned above, which can alleviate insomnia associated with these conditions. However, it’s important to consider that certain medications can also have a negative effect on sleep quality. Beta blockers, alpha blockers, SSRIs, corticosteroids
and loop diuretics are among the medications associated with reduced sleep quality. Pharmacists should have a consultation with patients or conduct a medication review to ascertain if certain medications are harming a patient’s sleep quality. For example, counselling a patient to take corticosteroids as a single dose in the morning can reduce wakefulness at night or taking diuretics in the early part of the day can reduce urinary urgency at night, which in turn can improve sleep.8 Pharmacists should also counsel patients on lifestyle advice that may reduce the need for certain medication or consult with the patient and doctor about possible alternative medication that may effectively treat the condition, without the associated adverse effect on sleep.
Lifestyle factors can greatly contribute to poorer sleep quality and insomnia. A number of habits can lead to deterioration of sleep quality including drinking alcohol or caffeine, eating large meals before bed, smoking cigarettes, shift work, sleeping during the day or looking at blue light screens before bed.2 Pharmacists are ideally placed to identify these lifestyle factors and counsel patients on how to change behaviours to reduce their negative impact on sleep. Motivational interviewing can be an effective technique to provide direction for change and to strengthen motivation.
The use of melatonin as an aid to sleep can be considered controversial in Ireland. Melatonin is a natural product that is associated with treatment of insomnia. It is available over the counter and as a food supplement in certain countries, such as the USA. However, it is not available without prescription in Ireland. It is also not licensed for use in Ireland and must be supplied on foot of a valid prescription as an exempt medicinal product. It is also currently not reimbursable under HSE schemes. Pharmacists should be aware of the medical use, adverse effects, pharmacology,
interactions etc. of melatonin due to the increasing number of prescriptions observed in recent years and the anecdotal evidence of patients obtaining medication online without a prescription.9
Nytol has been licensed as an OTC product in Ireland in recent years as a symptomatic aid to the relief of temporary sleep disturbance in adults. The active ingredient is Diphenhydramine, a sedating antihistamine at the H1 receptor. The recommended dosage is 50mg, taken 20 minutes before going to bed. Nytol should be used for the shortest possible duration - more than 7 consecutive days without physician supervision is not recommended.
Diphenhydramine is associated with prolongation of the QT interval and therefore pharmacists should recognise patients may have other risk factors for QT interval prolongation. Appropriate screening is recommended due to the contraindications and cautions in using this product.10
Lifestyle changes and certain medications can be useful in providing relief for sleep disturbance - however, there are certain instances where patients should be referred for medical review. Patients should be referred to a GP or a mental health professional if they feel like they are finding it hard to cope as a result of their sleep issue or if their sleep issue is having a negative impact on their dayto-day life. Pharmacists should also refer if they suspect there is a possibility of an undiagnosed medical condition causing sleep disturbance. If the sleep problem has been persisting for weeks without improvement or getting worse and if improving sleeping habits has failed, the patient should also be referred to a GP. Therapy or talking to a professional can be a source of help - referral to cognitive behavioural therapy can help change thoughts and behaviours that reduce sleep quality.4
References available upon request

PHARMACYNEWSIRELAND.COM 30 Sleep
Pharmacists are ideally placed to identify these lifestyle factors and counsel patients on how to change behaviours to reduce their negative impact on sleep. Motivational interviewing can be an effective technique to provide direction for change and to strengthen motivation.
Written by Damien O’Brien, Pharmacist, Adrian Dunne Pharmacy Baldoyle
In response to stress, the body’s nervous system is activated due to the sudden release of hormones.
This is known as the fight or f light response, which evolved as a survival mechanism, enabling people and other mammals to react quickly to life-threatening situations as your body becomes ‘ready’ and in a ‘state of alert.’The released hormones increases heartbeat and respiration rate in order to provide the energy and oxygen to the body that will be needed to fuel a rapid response rate.
This can have a positive effect on your performance as it can help you to prepare and focus, however if this state of alert becomes prolonged or permanent, your performance abilities become restricted.
The viscious circle
Modern life can be stressful and demanding, as we struggle to find a balance between the needs of the family, pressures from work and our own health and well-being. Neuro Balance may help you to find your inner balance.

The lack of a regular balance between tension and relaxation may have effects on your cardiovascular system, immune system and digestion. Your nervous system suffers from prolonged stress too, and constant stimuli can lead to nervous restlessness. The roundabout of thoughts just doesn’t stop and it can become difficult to relax at night which results in beginning the next day already exhausted.
Simple tips to help yourself
Taking a daily timeout may release the present state of stress. Taking a relaxing bath without access to your phone and social media can help you switch off and unwind.
Take exercise - whatever type you choose, be it swimming, cycling or walking, it’s a perfect outlet in order to release the stress caused by accumulated energy.
Being out in the fresh air can have many benefits, both physically and mentally. If you cannot get out, even sitting by the window can help.
Try relaxation techniques such as Yoga, Tai Chi, Qigong, meditation and special breathing techniques, which can help you to relax and restore balance.
Distributed in Ireland by: The Natural Medicine Company, Burgage, Blessington, Co. Wicklow Tel: 045-865575 email: sales@naturalmedicine.ie www.naturalmedicine.ie
A bedtime routine can help the body and mind to wind down and prepare you for sleep. Writing a diary may enable you to leave your thoughts of the day behind.
Floradix Neuro Balance, with its contained ashwagandha root and herbal extracts of passion flower, lavender, lemon balm and rosemary, may help you to find your inner balance.

What is Ashwagandha?
Ashwagandha is an evergreen shrub, also known as Indian ginseng or winter cherry, that is found in India and parts of Africa. The plant, particularly its root powder has been used for hundreds of years for medicinal purposes and to help improve the body’s resistance to stress.
Floradix
Available
in Pharmacies Nationwide. www.floradix.ie
Lack
Restless
Exhaustion
Nervous tension
of recreation
sleep



Biological medicines: overview
Biosimilar medicines and their use in the treatment of cancer
cell systems and recombinant DNA technology. The EU legislation imposes strict requirements for the manufacture of all medicines:
Written by: Rebecca Parkin, AnneMarie DeFrein and Patricia Heckmann, National Cancer Control Programme
Introduction
Biological medicines (‘biologicals’) contain active substances from a biological source, such as living cells or organisms. Biological medicines are well established in clinical practice and in many cases they are indispensable for the treatment of serious and chronic conditions such as diabetes, autoimmune diseases and cancers.
to develop and agree a national guidance document on the use of biosimilars in the treatment of cancer.6

� EU manufacturers must hold a manufacturer’s license and are legally obliged to comply with Good Manufacturing Practice (GMP), the agreed standards to obtain a medicine with proven quality.
used in the same way as the reference biological and in accordance with its Summary of Product Characteristics (SmPC).6, 7
Key features of biological medicines
This article aims to outline the background to the development and approval of biosimilars, with a focus on their role in the treatment of cancer.
Most biological medicines in current clinical use contain active substances made of proteins. These can differ in size and structural complexity, from simple proteins like insulin or growth hormone to more complex ones such as coagulation factors or monoclonal antibodies (figure 1).
What are biological medicines and biosimilars?



� National regulatory authorities in the EU regularly inspect manufacturing sites for compliance with GMP requirements.
Biomanufacturing strictly regulated
A biological medicine (or biological) is a medicine that contains an active substance made by a biological process or derived from a biological source.7 Biologicals include proteins such as hormones (erythropoietins, insulins and growth hormones) or enzymes that are naturally produced in the human body, and monoclonal antibodies. They may also be blood products, immunological medicinal products and advanced technology products such as gene and cell therapies.8 They vary in size and structural complexity, from simple proteins such as insulin or growth hormone to more complex ones such as coagulation factors or monoclonal antibodies (see Figure 1).
The manufacture of biological medicines tends to be more complex than for chemically-derived molecules. Most biological medicines are made by biotechnology, often using sophisticated
Cancer is recognised as a major health concern. The number of cancers diagnosed annually in Ireland is rising and the significant cost of new drug treatments for cancer, including biological medicines (or biologicals), has been cited as an ongoing challenge.1, 2 In recent years, in addition to their increased use in other diseases, biologicals have become a vital part of treatment for cancer, as both therapeutic and supportive care agents.3, 4 However, the high cost of these treatments places a significant burden on healthcare systems. The expiration of patents for biologicals has allowed the development of biosimilar medicines (biosimilars). A regulatory framework for the approval of biosimilars was established in Europe in 2003, with the USA implementing a framework in 2009.3 Since the introduction of the first biosimilar by the European Medicines Agency (EMA) in 2006, a number of biosimilars have been approved and safely used in the treatment of cancer.5 Their approval has led to increased market competition, resulting in a reduced cost burden and enabling accessibility of treatments to patients with cancer.5 As such, biosimilars represent one of the ways to obtain sustainability of systemic anti-cancer therapy (SACT ) and maximise funding for new medicines. The introduction of biosimilars for the treatment of cancer into the healthcare market in Ireland is welcomed by the National Cancer Control Programme (NCCP). In 2017, the NCCP convened a multidisciplinary working group, involving key stakeholders,
All biologicals have an inherent degree of natural variability due to their biological nature, i.e. no two batches of a product will be the exact same. This is also true for biosimilars, which will never be an exact copy of their biological counterparts because of the complex process needed for their production.5 EMA approval indicates that any differences between a biosimilar and its reference biological will have been shown not to affect safety or effectiveness,5 through a rigorous comparability exercise which is carried out at quality, nonclinical and clinical levels.
� If some manufacturing steps take place outside the EU, then non-EU manufacturers, importers and wholesale distributors are obliged to follow the same strict requirements and are also regularly inspected.
A biosimilar is a biological medicine that is highly similar to another biological (also known as a reference biological medicine) in terms of its quality, safety and efficacy.6, 7 A reference biological medicine is one which already has a marketing authorisation and has been approved for use in patients. Biosimilars contain a version of the active substance of an approved reference biological and should be
Are biosimilars the same as generic medicines?
No, they are not the same. Generics are smallmolecule drugs made from a fixed chemical structure that can be easily replicated, e.g. methotrexate, whereas biosimilars of advanced biologicals are much larger, complex molecules derived from living cells with inherent natural variability, e.g. monoclonal antibodies such
For biological medicines, some of the GMP requirements have been adapted to take into account their specific nature (e.g. use of appropriate aseptic techniques, refrigeration and other storage conditions, stability, transport etc.).
Examples of different protein types in biologicals approved in the EU Adapted from5
Figure 1. Examples of types of proteins in biological medicines approved in the EU
34 | PHARMACYNEWSIRELAND.COM
Monoclonal antibody 150,000 daltons Growth hormone 22,000 daltons Insulin 5,808 daltons
Figure 1
CCF: 24971 Date of preparation: (09-22)
ABBREVIATED PRESCRIBING INFORMATION
Product Name: Emazole Control 20 mg Gastro-Resistant Tablets

Composition: Each tablet contains 20 mg esomeprazole (as magnesium dihydrate).
Description: Light pink oval lm coated tablet.
Indication(s): Proton Pump Inhibitor (PPI): Short-term treatment of re ux symptoms (e.g. heartburn and acid regurgitation) in adults. or crush. Disperse in half a glass of non-carbonated water if di culty in swallowing. Stir until tablets disintegrate, drink liquid with pellets immediately or within 15 min, or administer through a gastric tube. Do not chew or crush pellets. Adults: The recommended dose is 20 mg esomeprazole (one tablet) per day. achieve improvement of symptoms. Duration of treatment is up to 2 weeks. Once complete relief of symptoms has occurred, treatment should be discontinued. If no symptom relief is obtained within 2 weeks of continuous treatment, the patient should be instructed to consult a doctor. recommended. No relevant use in this group in the indication: “short-term treatment of re ux symptoms (e.g., heartburn and acid regurgitation)”. Severe impaired renal function: Caution. Severe liver impairment: 20 mg max daily dose.
Contraindications: Hypersensitivity to esomeprazole, substituted benzimidazoles or any of the excipients. Not with nel navir. Contact a physician if symptoms change in character. In the presence of any alarm symptom (e.g. signi cant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with esomeprazole may alleviate symptoms and delay diagnosis. Treatment with proton pump inhibitors (PPIs) may lead to a slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter and in hospitalised patients, also possibly Clostridium di cile. Patients should consult their doctor before taking this medicinal product if they are due to have an endoscopy or urea breath test. Absorption of vitamin B12 may be reduced due to hypo- or achlorhydria. Not recommended for long-term use as the following may also occur: Hypomagnesaemia; Risk of fracture. Consider stopping Emazole Control in cases of Subacute cutaneous lupus erythematosus (SCLE) accompanied by arthralgia. Interference with laboratory tests: Increased Chromogranin A (CgA) level may interfere with investigations for neuroendocrine tumours. To avoid this interference, Emazole Control treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of PPI treatment. Contains glucose and sucrose. Interactions: E ect of esomeprazole on other drugs: Co-administration with atazanavir is not recommended. If the combination of atazanavir with a PPI is judged unavoidable, close clinical monitoring is recommended in combination with an increase in the dose of atazanavir to 400 mg with 100 mg of ritonavir; esomeprazole 20 mg should not be exceeded. Esomeprazole is a CYP2C19 inhibitor. When starting or ending treatment with esomeprazole, the potential for interactions with drugs metabolised through CYP2C19 should be considered. Serum levels of cilostazol, cisapride, tacrolimus, methotrexate may be increased. An interaction is observed between clopidogrel and esomeprazole, but the clinical relevance is uncertain. As a precaution, concomitant use of esomeprazole and clopidogrel should be discouraged. Gastric acid suppression by PPIs increase or decrease absorption of drugs with pH dependent absorption (decreased absorption of ketoconazole, itraconazole); esomeprazole inhibits CYP2C19 metabolising enzyme and could increase plasma concentrations of diazepam, citalopram, imipramine, clomipramine, phenytoin (monitor plasma levels of phenytoin), etc. resulting in need of a dose reduction; monitor INR when given with warfarin or similar. Caution as absorption of digoxin can increase. E ect of other drugs on esomeprazole: CYP2C19 and CYP3A4 inhibitors (clarithromycin, voriconazole) may increase the esomeprazole exposure. Dose adjustment not regularly required, except in severe hepatic impairment and long-term use. CYP2C19 and/or CYP3A4 inducers (rifampicin and St. John’s wort) may lead to decreased esomeprazole serum levels by increasing the esomeprazole metabolism.

Pregnancy and Lactation: Caution in pregnancy due to lack of clinical data. No studies in lactating women, therefore, not recommended during breast-feeding. Ability to Drive and Use Machinery: Minor in uence on the ability to drive or use machines. Adverse reactions such as dizziness (uncommon) and blurred vision (rare) have been reported. If a ected, patients should not drive or use machines. Undesirable E ects: Common: Headache, abdominal pain, constipation, diarrhoea, atulence, nausea/vomiting, fundic gland polyps (benign). Uncommon: Peripheral oedema, insomnia, dizziness, paraesthesia, somnolence, vertigo, dry mouth, increased liver enzymes, dermatitis, pruritis, rash, urticaria, fracture of the hip, wrist or spine. For other side e ects refer to the SPC.
Marketing Authorisation Holder: IQ Pharmatek Ltd., Gurtna eur, Old Waterford Road, Clonmel, Co. Tipperary. Marketing Authorisation Number: PA 22777/001/001. Further information and SPC are available from: Rowex Ltd, Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417. E-mail: rowex@rowa-pharma.ie
Legal Category: Not subject to medical prescription.
Date of Preparation: September 2019
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing medsafety@hpra.ie or by emailing Rowex pv@rowa-pharma.ie
Supply Through Pharmacy Only 28 PACK NOW AVAILABLE. 28 PACK NOW AVAILABLE
as rituximab (see Figure 2). Because of their biological and variable nature, biosimilars cannot be regarded as generic medicines. As such, it should be remembered that “biosimilar” is a regulatory term, not a scientific one.6
How are biosimilars approved?
Biosimilars are reviewed by the EMA through a centralised procedure, following an application from the pharmaceutical company. The EMA provide a scientific opinion to the European Commission who approve the biosimilar medicine and grant it an EU-wide marketing authorisation. The legal framework and regulatory pathway agreed in the EU outlines that biosimilars must be manufactured to the same quality standards as the reference biological medicine.5, 6 Since 2006, biosimilar versions of several reference biologicals have been approved in the EU and many are available in Ireland. In the Irish setting, after a biosimilar has gained EU approval, the company may submit a reimbursement application to the HSE, in line with the criteria outlined in the Framework Agreement on the Supply and Pricing of Generic, Biosimilar and Hybrid Medicines (2021).10
Extrapolation of indications
For the approval of biosimilars, a process called extrapolation of indications is used. This is where a biosimilar is approved for use in the same indication held by the reference biological medicine, even though it has not been directly studied in a comparative clinical trial.11 For a biosimilar to gain approval, it needs to demonstrate that there are no clinically meaningful differences relative to the reference medicine, in a sensitive patient population. Once comparability has been demonstrated in one indication (i.e. the most sensitive),
Larger biologicals – More complex protein molecules – Produced in living cells – Have immunogenic potential


mAbs -168,000 GCSF 18,800
Molecular mass HGH 22,000 Enoxaparin 4500 [mm] Insulin -3800
Large biologicals – Complex protein molecules – Produced in living cells – Have immunogenic potential
Small-molecule drugs
– Simple, well-characterized chemical structure – Produced by chemical synthesis – Mostly non-immunogenic



confirmative clinical studies are not required for all other approved indications.
Acetylsalicylic acid 180
EPO 30,000
<100,000 Da <10,000 Da -100 Da
This extrapolation of indications, versus a clinical trial, may have led to some initial hesitancy in the adoption of biosimilars. Research in the US has shown that uptake

Figure 2 Molecular weight comparisons: small-molecule drugs versus larger biologicals. Not drawn to scale. Ave: Average; Da: Daltons; EPO: Erythropoietin; mAbs: Monoclonal antibodies
Figure1.Molecularweightcomparisons:small-moleculedrugsversuslargerbiologicals. Notdrawntoscale. Adaptedfrom [13] Ave:Average;Da:Daltons;EPO:Erythropoietin;mAbs:Monoclonalantibodies.


Adapted from9
RPcanimpactpharmacologicactivityandimmunogenicpotentialaswellasclinicalefficacyandsafetyofthe biosimilar,itisimportanttoidentifyandquantifythemearlyintheproductdevelopmentprocess [19–21] Animportantpointtobearinmindduringthedevelopmentofbiosimilarsistheunderstandingofthedifference betweencomparabilityandbiosimilarity.Comparabilityreferstoapostapprovalmanufacturingchangemadetoa biologicproductbytheproduct’smanufacturer.Suchchangestothemanufacturingprocessarerelativelycommon andmayincludeachangeinmanufacturingequipmentorchangesmadetoscale-upyieldortoreduceimpurities, andsoforth.Comparabilityoftheproductsbeforeandafterthemanufacturingchangeshavebeenmadeassuresthat theproducthasretaineditsattributes.Thus,thereisnoexpectationofadverseeffectonthesafetyorefficacyofthe productafterthechange [13].Suchmanufacturingchangesaresuccessfullymadebecausetheproductmanufacturer hasanexhaustiveunderstandingoftheproduct,includingitsdevelopmenthistory.Thisdoesnotimplythatover timethebiologicbecomesabiosimilarofitself.Biosimilarity,ontheotherhand,describestheconceptwhereby amanufacturerdevelopsamoleculetobesimilarinstructureandfunctiontoanexistingproduct,theRP.A biosimilarismanufacturedanewwithoutanypriorknowledgeofthedevelopmentalhistoryoftheRP;forthis reason,abiosimilarcanneverbeanexactreplicaofitsRP.Inthiscase,demonstrationofbiosimilarityisbasedona comprehensivecomparativecharacterizationoftheproposedbiosimilarthatisproducedusinganew/differentcell linedevelopedbyanew/differentmanufacturer.
36 | PHARMACYNEWSIRELAND.COM
www.futuremedicine.com
futuresciencegroup
can be slow initially due to challenges such as policy change and lack of patient and provider education.12 An Irish study carried out in 2017 highlighted concerns held by medical specialists in relation to biosimilars and emphasised the need for educational initiatives.13 However, there is a growing body of evidence to support their effectiveness, with uptake in some countries near 100%.14 Increased uptake may be driven by financial incentives offered to encourage biosimilar prescribing.15
Interchangeability and substitution
In the context of biosimilars and reference biological medicines, it is important to be aware of the terminology for interchangeability and substitution practices in the EU.5
Interchangeability
The EMA defines interchangeability as ‘the possibility of exchanging one medicine for another medicine that is expected to have the same clinical effect. This could mean replacing a reference biological medicine with a biosimilar (or vice versa) or replacing one biosimilar with another’.5 This process must be carried out under the supervision of a physician. Hospital biosimilar policy should detail the process to be followed when changing a patient from one biological medicine to another.6, 7 This may include supplying the patient with dedicated patient information on the use of biosimilars and / or a discussion between the patient and prescribing physician.7
In September 2022, the EMA and the Heads of Medicines Agencies (HMA) issued a joint statement confirming that biosimilars approved in the EU are interchangeable (at prescriber level) with their reference medicine or with an equivalent biosimilar. While already practised in many Member States, this joint position on interchangeability harmonises the EU approach. Decisions regarding substitution at pharmacy-level, however, are managed by individual Member States.16
Substitution (at pharmacy level)
Unlike small-molecule drugs , biological medicines cannot be substituted at pharmacy level. The Health (Pricing and Supply of Medical Goods) Act 2013 specifically excludes biological medicines (including biosimilars) from being substituted at pharmacy level.17
Biosimilars in Ireland
In January 2016, the HSE Medicines Management Programme (HSE-MMP) highlighted the potential for biosimilars to significantly reduce drug expenditure and facilitate greater access to such treatments.18
On the introduction of a biosimilar to the Irish market, the 2021 Framework Agreement on the Supply and Pricing of Medicines provides for an automatic price reduction of 37.14% for patent-expired, non-exclusive biologicals in addition to the agreed rebates.10, 19 Since 2016, the HSE-MMP has supported the appropriate introduction of biosimilars into clinical use in Ireland, including implementing a prescribing initiative, which has resulted in a significant increase in biosimilar uptake in Ireland since 2019.18, 19
In 2018, the MMP commenced the best-value biological (BVB) medicine initiative to evaluate therapeutic areas where there is potential for biosimilars to be introduced. The initiative aims to realise potential efficiencies from the availability of biosimilars, and to work with
subcutaneous formulations available for administration
intravenous form.
Table 1 Biosimilars licensed in Ireland for supportive care agents used in the treatment of cancer
subcutaneous formulations available for administration but a biosimilar is only available in intravenous form.
Table 1 Biosimilars licensed in Ireland for supportive care agents used in the treatment of cancer
Supportive care agent
Cancer treatment indications
Table 1 Biosimilars licensed in Ireland for supportive care agents used in the treatment of cancer
Filgrastim
Date of 1st approval*
Supportive care agent Cancer treatment indications Date of 1st approval*
Filgrastim
Pegfilgrastim
Pegfilgrastim
Epoetin alpha
Epoetin zeta
Epoetin zeta
• Management of neutropenia related to cancer treatment
• Mobilisation of peripheral blood progenitor cells 2008
• Management of neutropenia related to cancer treatment
• Neutropenia related to cancer treatment 2018
• Mobilisation of peripheral blood progenitor cells 2008
Epoetin alpha • Anaemia related to cancer treatment
• Neutropenia related to cancer treatment 2018
• Anaemia related to cancer treatment 2009
• Anaemia related to cancer treatment 2007
• Anaemia related to cancer treatment 2007
Table 2 Biosimilars licensed in Ireland for monoclonal antibodies used in the treatment of cancer Monoclonal antibody Cancer treatment indications
Table 2 Biosimilars licensed in Ireland for monoclonal antibodies used in the treatment of cancer
Date of 1st approval*
Rituximab
Rituximab
Trastuzumab
• Non-Hodgkin's lymphoma and chronic lymphocytic leukaemia
2017
Table 2 Biosimilars licensed in Ireland for monoclonal antibodies used in the treatment of cancer Monoclonal antibody Cancer treatment indications Date of 1st approval*
2017
• HER2-positive metastatic breast cancer
• Non-Hodgkin's lymphoma and chronic lymphocytic leukaemia 2017
Trastuzumab • HER2-positive metastatic breast cancer
• Early HER2-positive breast cancer in combination with chemotherapy
Bevacizumab
• Early HER2-positive breast cancer in combination with chemotherapy
• Metastatic gastric cancer
• Colorectal cancer
• Metastatic gastric cancer
• Unresectable, locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer
2018
2018
2017 Bevacizumab • Colorectal cancer
• Unresectable, locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer
• Advanced or metastatic renal cell cancer
• Advanced or metastatic renal cell cancer
• Recurrent ovarian, fallopian tube or primary peritoneal cancer
• Recurrent ovarian, fallopian tube or primary peritoneal cancer
• Persistent, recurrent or metastatic cancer of the uterine cervix
• Persistent, recurrent or metastatic cancer of the uterine cervix
• Recurrent glioblastoma
*A number of biosimilar products are licensed in Ireland but availability of these products may vary
• Recurrent glioblastoma
*A number of biosimilar products are licensed in Ireland but availability of these products may vary
*A number of biosimilar products are licensed in Ireland but availability of these products may vary
Conc lusion and future directions
Conc lusion and future directions
the HSE and clinicians to increase uptake of identified BVB medicines in Ireland. To date, the MMP has identified BVB medicines for TNF-α inhibitors (adalimumab and etanercept) under the High Tech Arrangement. They have recently initiated a BVB process for granulocyte stimulating factors (G-CSFs) which are used as supportive agents in the treatment of cancer.
Cancer prevalence and the cost of cancer treatment continue to increase globally. The high costs, particularly of biologicals, may present a challenge in ensuring access for patients to optimal treatment. Cost and accessibility barriers can create disparities in treatment for cancer and resulting clinical outcomes (21) Biologicals and their biosimilar counterparts have an integral role to play in the treatment of cancer. As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer (22) The implementation of biosimilars should follow a methodical, staged approach with engagement of all relevant stakeholders (6, 7, 23)
Cancer prevalence and the cost of cancer treatment continue to increase globally. The high costs, particularly of biologicals, may present a challenge in ensuring access for patients to optimal treatment. Cost and accessibility barriers can create disparities in treatment for cancer and resulting clinical outcomes (21). Biologicals and their biosimilar counterparts have an integral role to play in the treatment of cancer. As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer (22). The implementation of biosimilars should follow a methodical, staged approach with engagement of all relevant stakeholders (6, 7, 23)
Biosimilars for the treatment of cancer in Ireland
Biologicals and their biosimilar counterparts have been used in the treatment of cancer for many years and this use will grow as more patents expire. Biosimilars approved for the treatment of cancer are licensed by the EMA for all indications held by the reference product, based on extrapolation of efficacy and safety data.20 There are a number of biosimilars available in Ireland used in the treatment of cancer. These include the supportive care agents G-CSFs, used for the prevention and treatment of chemotherapy-induced neutropenia and epoetins, which can be used for the treatment of chemotherapy-induced anaemia,3 see Table 1 above. Additionally, there are biosimilars of therapeutic agents including monoclonal antibodies (mAbs), see Table 2 above. The availability of different formulations has been a particular challenge to the uptake of some biosimilars used in the treatment of cancer. For example, rituximab and trastuzumab have both intravenous and subcutaneous formulations available for administration but a biosimilar is only available in intravenous form.
Conclusion and future directions
Cancer prevalence and the cost of cancer treatment continue to increase globally.
The high costs, particularly of biologicals, may present a challenge in ensuring access for patients to optimal treatment. Cost and accessibility barriers can create disparities in treatment for cancer and resulting clinical outcomes.21 Biologicals and their biosimilar counterparts have an integral role to play in the treatment of cancer. As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer.22 The implementation of biosimilars should follow a methodical, staged approach with engagement of all relevant stakeholders.6, 7, 23
Several additional biosimilar candidate drugs / medicines are being globally investigated and are at various stages of clinical development and regulatory approval.4 More than 10 years of research has shown that biosimilars are safe and effective.3 There has been a significant increase in the utilisation of biosimilars in Ireland since 2019,19 with a growing body of evidence and experience supporting broader uptake.
The continued development and utilisation of biosimilars is welcomed by the NCCP, the broader HSE and a number of other Irish and European bodies. Optimising the use of biosimilars will facilitate access for patients and support health services to deal with the rising cost of new medicines.
For additional information, please refer to the NCCP Guidance on the use of Biosimilar Medicines in Cancer Treatment document (available on the NCCP website) or email oncologydrugs@cancercontrol.ie.
References available on request
PHARMACYNEWSIRELAND.COM | 37
or
example,
biosimilar is only available in
but a
PMI Annual Charity Lunch

The PMI’s Annual Charity Lunch returned to the Mansion House on Thursday 8th December.



Over 350 members of the pharmaceutical industry attended to celebrate the end of the year and to raise some much needed funds for charity.





Through the event, the PMI & their members raised over ¤9,500 for the chosen charity: Our Lady’s Hospice & Care Services. Audrey Houlihan, CEO with OLH joined the event and gave an overview of their work in the community.
Helena McNamara from United Drug won first prize in the raffle, and immediately donated the signed Leinster rugby shirt to Audrey for OLH to use it for further fundraising. Further photos from the event are available at: www.thepmi.com/gallery
PHARMACYNEWSIRELAND.COM 38
Fiona Dunphy, Commercial Manager with The PMI
PMI Lunch
PMI members & their guests at 2022 Charity Lunch
PMI members & their guests at 2022 Charity Lunch






| For more information please contact your local BR Healthcare representative or our office on 01-8850800.
ABBREVIATED PRESCRIBING INFORMATION
Ozempic®t semaglutide

Please refer to the Summary of Product Characteristics (SmPC) before prescribing. Ozempic® 0.25 mg solution for injection in pre-filled pen. Ozempic® 0.5 mg solution for injection in pre-filled pen. Ozempic® 1 mg solution for injection in pre-filled pen. One ml of solution contains 1.34 mg of semaglutide (human glucagon-like peptide-1 (GLP-1) analogue). Indication: Ozempic® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1 of the Ozempic® SmPC. Posology and administration: Administered once weekly at any time of the day, with or without meals. Injected subcutaneously in the abdomen, thigh or upper arm. Starting dose: 0.25 mg once weekly. After 4 weeks the dose should be increased to 0.5 mg once weekly. After at least 4 weeks with a dose of 0.5 mg once weekly, the dose can be increased to 1 mg once weekly to further improve glycaemic control. When Ozempic® is added to existing metformin and/ or thiazolidinedione therapy or to an SGLT2 inhibitor, the current dose of metformin and/ or thiazolidinedione or SGLT2 inhibitor can be continued unchanged. When Ozempic® is added to a sulfonylurea or insulin, a reduction in dose of sulfonylurea or insulin should be considered to reduce the risk of hypoglycaemia. Blood glucose self-monitoring is necessary to adjust the dose of sulfonylurea and insulin, particularly when Ozempic® is started and insulin is reduced. A stepwise approach to insulin reduction is recommended. Children: No data available. Elderly: No dose adjustment required, therapeutic experience in patients age ≥75 is limited. Renal impairment: No dose adjustment is required for patients with mild, moderate
or severe renal impairment. Experience in patients with severe renal impairment is limited. Not recommended for use in patients with end-stage renal disease. Hepatic impairment: No dose adjustment is required for patients with hepatic impairment. Experience with severe hepatic impairment is limited. Caution should be exercised when treating these patients with semaglutide. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Special warnings and precautions for use: Should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis. Not a substitute for insulin. Diabetic ketoacidosis has been reported in insulin-dependent patients whom had rapid discontinuation or dose reduction of insulin. There is no experience in patients with congestive heart failure NYHA class IV and is therefore not recommended in these patients.
Use of GLP-1 receptor agonists may be associated with gastrointestinal adverse reactions. This should be considered when treating patients with impaired renal function as nausea, vomiting, and diarrhoea may cause dehydration which could cause a deterioration of renal function. Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, semaglutide should be discontinued; if confirmed, semaglutide should not be restarted. Caution should be exercised in patients with a history of pancreatitis. Use of semaglutide in combination with a sulfonylurea or insulin may have an increased risk of hypoglycaemia, therefore consider reducing the dose of sulfonylurea or insulin when initiating treatment with Ozempic®. In patients with diabetic retinopathy treated with insulin and semaglutide, an increased risk of developing diabetic retinopathy complications has been observed. Caution should be exercised when using semaglutide in patients with diabetic retinopathy treated with insulin. These patients should be monitored closely and treated according to clinical guidelines. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy, but other mechanisms cannot be excluded. When semaglutide is used
in combination with a sulfonylurea or insulin, patients should be advised to take precautions to avoid hypoglycaemia while driving and using machines. Fertility, pregnancy and lactation: Women of childbearing potential are recommended to use contraception when treated with semaglutide. Should not be used during pregnancy or breast-feeding. Discontinue at least 2 months before a planned pregnancy. Effect on fertility unknown. Undesirable effects: Very common (≥1/10): Hypoglycaemia when used with insulin or sulfonylurea, nausea, diarrhoea. Common (≥1/100 to <1/10): Hypoglycaemia when used with other oral antidiabetic medications, decreased appetite, dizziness, diabetic retinopathy complications, vomiting, abdominal pain, abdominal distension, constipation, dyspepsia, gastritis, gastrooesophageal reflux disease, eructation, flatulence, cholelithiasis, fatigue, increased lipase, increased amylase, weight decreased. Uncommon (≥1/1,000 to <1/100): Hypersensitivity, dysgeusia, increased heart rate, acute pancreatitis, injection site reactions. Rare (≥1/10,000 to <1/1,000): Anaphylactic reaction. Not known (cannot be estimated from available data): Angioedema. The SmPC should be consulted for a full list of side effects. MA numbers: Ozempic® 0.25 mg pre-filled pen EU/1/17/1251/002. Ozempic® 0.5 mg pre-filled pen EU/1/17/1251/003. Ozempic® 1 mg pre-filled pen EU/1/17/1251/005. Each pre-filled pen delivers 4 doses and includes 4 disposable NovoFine® Plus needles. Legal Category: POM. For complete prescribing information, please refer to the SmPC which is available on www. medicines.ie or by email from infoireland@novonordisk.com or from the Clinical, Medical and Regulatory Department, Novo Nordisk Limited, 1st Floor, Block A, The Crescent Building, Northwood Business Park, Santry, Dublin 9. Date last revised: March 2021.
tAdverse events should be reported to the Health Products Regulatory Authority. Information about adverse event reporting is available at www.hpra.ie. Adverse events should also be reported to Novo Nordisk on Tel: 1850 665 665 or complaintireland@novonordisk.com
versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulinnaive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017;5: 355–66. 7. Sorli C et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251–60. 8. Ahrén B et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5: 341–54. 9. Pratley RE et al. Semaglutide versus dulaglutide once-weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6275-286. 10.Buse JB et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020 Feb; 43(2):487-493.
Ozempic®
t

This
Novo Nordisk Limited, First Floor, Block A, The Crescent Building, Northwood Business Park, Santry, Dublin 9, D09 X8W3, Ireland.
Tel: 01 8629 700, Fax: 01 8629 725, Lo call: 1 850 665665. infoireland@novonordisk.com www.novonordisk.ie
Ozempic® and the Apis bull logo are registered trademarks owned by Novo Nordisk A/S. Date of preparation: October 2021. IE21OZM00098
Once-Weekly Ozempic
Disease1-3 Ozempic® is recommended by the ADA/EASD Consensus Report for people with type 2 diabetes who have established atherosclerotic cardiovascular disease10 References: 1. Ozempic® Summary of Product Characteristics www.medicines.ie 2. Marso SP, et al; Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. (SUSTAIN 6) N Engl J Med. 2 2016;375:1834-1844. 3. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(suppl1):S1-S108. 4. Lingvay I et al, Once weekly Semaglutide vs Canagliflozin in type 2 diabetes (SUSTAIN 8) : a double blind phase 3 randomised control trial: Lancet Diabetes Endocrinol 2019; 7: 834–44. 5. Ahmann AJ et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care 2018;41:258-266. 6. Aroda VR et al. Efficacy and safety of once-weekly semaglutide
Think
for People with Type 2 Diabetes who have Atherosclerotic Cardiovascular
* 26% CV risk reduction in patients with type 2 diabetes and high CV risk, compared to placebo, in addition to standard treatment.
# Major Adverse Cardiovascular Events
Ozempic® provided a significant 26% risk reduction of MACE# in people with type 2 diabetes* and cardiovascular disease.1,2,§
† Results apply to Ozempic® across SUSTAIN trials, which included placebo, sitagliptin, dulaglutide, canagliflozin, exenatide PR and glargine U100.1-9 § p=0.02 for superiority ‡ p<0.05
This includes a 39% relative risk reduction in non-fatal stroke.1,2,‡ Ozempic® also has superior blood glucose and weight-loss efficacy across all head-to-head clinical trials in the SUSTAIN program.1-9,†,‡
is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. of the SmPC.1
This medicinal product is subject to additional monitoring.
will allow quick identification of new safety information.
PR = Prolonged Release; ADA = American Diabetes Association; EASD = European Association for the Study of Diabetes.
SUSTAIN = Semaglutide Unabated Sustainability in treatment of Type 2 Diabetes. *SUSTAIN was the phase 3 clinical trial programme investigating the effects of once weekly semaglutide versus other anti-diabetic agents.
Continuing Professional Development
AUTHORS: Professor Rónán Collins, consultant physician in geriatric and stroke medicine and Associate Clinical Professor in Gerontology Trinity College Dublin.

One in Three !! : A trial Fibrillation and Stroke
Written by
Professor Collins is Clinical lead National Stroke Programme, Member of the British and Irish Stroke Physicians (BIASP), Fellow of European Stroke Organsiation (FESO), Steering group member for EHRA-PATHS and member of the EHRA
60 Second Summary
Atrial Fibrillation (AF) is the commonest cardiac arrhythmia affecting at least 3% of Irish adults over 60 and 6 % over 70. The worldwide prevalence has been rising and is approximately 600 in men and 375 in women per 100,000 population.
While much progress has been made in the last 10 years in particular with collaborative interdisciplinary working and research between cardiologists and stroke physicians, nevertheless there are many unanswered questions and dilemmas in AF.
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result? Have I identified further learning needs?
4 previous steps, log and record your findings.
Published by IPN. Copies can be downloaded from www.irishpharmacytraining.ie
Atrial Fibrillation (AF) is the commonest cardiac arrhythmia affecting at least 3% of Irish adults over 60 and 6 % over 70 [1]. The worldwide prevalence has been rising and is approximately 600 in men and 375 in women per 100,000 population [2]. The lifetime risk of developing atrial fibrillation is almost 1 in 3 and it is estimated there may be as many as 18 million people with AF in the EU by 2060.
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
3. PLAN - If I have identified a


5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
Despite this very significant burden of disease we are still in a relative infancy in our understanding of the pathophysiology of AF as a truly systemic condition and its association with risk of stroke and other illnesses. AF is associated with a 2-3.5 fold increase in mortality, impaired left ventricular dysfunction in 20-30% of cases, a hazard ratio of 1.6 for the development of dementia independent of previous stroke and associated with an impaired quality of life in almost 60% cases and a hospitalisation rate of 10-40% annually [3]
One in Three!! : Atrial Fibrillation and Stroke
While much progress has been made in the last 10 years in particular with collaborative interdisciplinary working and research between cardiologists and stroke physicians, nevertheless there are many unanswered questions and dilemmas in AF.
Screening for AF has huge potential benefits not least in preventing stroke, development of AF associated symptoms or preventing irreversible remodelling of the atrium and associated atrial or ventricular tachycardia associated cardiomyopathy.
In recent years international guidelines have correctly focused more on stroke prevention as the priority in the management of AF. In their “Atrial Fibrillation Better Care (ABC)” approach to AF the latest ESC guidelines on the management of AF stress the ‘A’ -to Avoid stroke / Anticoagulation as the prime issue of concern before addressing ‘B’ – better symptom control and ‘C’ optimisation of cardiovascular comorbidity and other risk factors.’
Both biomarkers and echocardiographic variable have been shown to independently predict risk of stroke and may well improve upon the accuracy of the CHA2DS2-VASC score. Secondary prevention for the AF stroke patient is of prime importance and applies to both stroke due to ischaemia and probably surprisingly also in cases of stroke due intracerebral haemorrhage.
ESC guidelines promote an integrated management to the AF patient and in their ‘circle of ideal integration’ list the importance of access to a many specialty model with the patient at the centre of decision making.
Atrial Fibrillation (AF) is the commonest cardiac arrhythmia affecting at least 3% of Irish adults over 60 and 6 % over 70.1 The worldwide prevalence has been rising and is approximately 600 in men and 375 in women per 100,000 population.2 The lifetime risk of developing atrial fibrillation is almost 1 in 3 and it is estimated there may be as many as 18 million people with AF in the EU by 2060.
unanswered questions and dilemmas in AF.
Much attention has focused on the potential to screen for AF given it fulfils many of the original Wilson and Jungner criteria adopted by the WHO for a disease condition which would be amenable to a screening programme (see table 1.) and more specifically that the condition is common, detectable, associated with serious disease (stroke) which is preventable (with anticoagulation) [4].
Table 1: Original Wilson and Jungner Principles for screening
other illnesses. AF is associated with a 2-3.5 fold increase in mortality, impaired left ventricular dysfunction in 20-30% of cases, a hazard ratio of 1.6 for the development of dementia independent of previous stroke and associated with an impaired quality of life in almost 60% cases and a hospitalisation rate of 10-40% annually.3
Much attention has focused on the potential to screen for AF given it fulfils many of the original Wilson and Jungner criteria adopted by the WHO for a disease condition which would be amenable to a screening programme (see table 1.) and more specifically that the condition is common, detectable, associated with serious disease (stroke) which is preventable (with anticoagulation).4
WILSON AND JUNGNER PRINCIPLES FOR SCREENING (1968)

• The condition sought should be an important health problem.
• There should be an accepted treatment for patients with recognized disease.
• Facilities for diagnosis and treatment should be available.
• There should be a recognizable latent or early symptomatic stage.
• There should be a suitable test or examination.
• The test should be acceptable to the population.
• The natural history of the condition, including development from latent to declared disease, should be adequately understood.
• There should be an agreed policy on whom to treat as patients.
• The cost of case-finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole.
• Case-finding should be a continuing process and not a ‘once and for all’ project.
41 CPD: Atrial Fibrillation
CPD
CPD
writing group for Practical guide to anticoagulation with NOACS in non-vavlulat atrial fribrillation.
Table 1: Original Wilson and Jungner Principles for screening
/watches 91-99% 84-100%
Adapted from Hindricks G et al. European Heart Journal 2021;42(5):373–498
• Adapted from Hindricks G et al. European Heart Journal 2021;42(5):373–498
Table 2 Reported reliabilities of methods of AF detection
Stroke and AF
Given those facts the national clinical programmes for stroke and chronic disease management in Ireland have introduced case finding as part of the primary care contract to opportunistically pulse check for AF in an older population. The advent of personal digital devices with the potential to detect irregularities of heart rhythm has expanded this potential to detect AF.
At present despite a growing body of evidence for use of digital devices, there is no evidence to suggest more strokes would be prevented adopting such strategies over opportunistic screening by pulse checking in high- risk groups. An overview of reported specificities and sensitivities of various detection approaches is outlined below in table 2. All suspected AF must be confirmed by a 12 lead ECG or a 30 second single lead strip read by a physician experienced in AF diagnosis.
Sum Zero game: The Low CHA2DS2-VASC patient
Stroke and AF
tool for risk assessment, there are concerns that it performs modestly and has relatively poor validity (C-statistic of 0.64 at best) and that the emphasis should perhaps be on a positive bias for anticoagulation than not. This author still regularly sees cases of stroke due to atrial fibrillation in people with seemingly low risk as indicated by a CHA2DS2-VASC score of 0 or 1 (for female sex) who are on aspirin or nothing rather than an anticoagulant and this approach is not necessarily at odds with current guidelines.
In recent years international guidelines have correctly focused more on stroke prevention as the priority in the management of AF [7]. In their “Atrial Fibrillation Better Care (ABC)” approach to AF the latest ESC guidelines on the management of AF stress the ‘A’ -to Avoid stroke / Anticoagulation as the prime issue of concern before addressing ‘B’ – better symptom control and ‘C’ optimisation of cardiovascular comorbidity and other risk factors.’
A proof of concept study using apples i-phone and watch for example found a positive predictive value 0.84 when a notification was received of an irregular pulse.5 The recently published ‘Smartphonebased screening for atrial fibrillation: a pragmatic randomised clinical trial (eBrave)’ showed a doubling of detection of clinically relevant atrial fibrillation in a cohort of older people who owned a smartphone in both phases of the crossover design.6
disease, obstructive sleep apnoea etc. though these variables are often closely related to the CHA2DS2-VASC risk factors themselves and their inclusion does not seem to improve score predictability. It is unknown as to whether including factors such co-existent malignancy, smoking or obesity would improve reliability. Then as we age, we must recognise that the risk of stroke with AF is a continuum and likely to increase with the duration of AF as the atrium becomes increasingly dilated and dysfunctional with chronic fibrillation.
In addition, many more people now have implantable pacemaker and ICD devices capable of detecting AF if interrogated for same and there is increasing use of implantable recorders in patients with a high index of suspicion for AF. Many such devices pick up brief runs of AF or Atrial High-Rate Episodes (AHREs)that may suggest a risk of developing AF though it is not proven that anticoagulation in such cases would prevent more stroke.
Screening for AF has huge potential benefits not least in preventing stroke, development of AF associated symptoms or preventing irreversible remodelling of the atrium and associated atrial or ventricular tachycardia associated cardiomyopathy. It can also however create unnecessary anxiety, lead to over diagnosis and treatment with anticoagulation or even potentially lead to further invasive investigations that may have associated risks and be unnecessary.
In recent years international guidelines have correctly focused more on stroke prevention as the priority in the management of AF.7 In their “Atrial Fibrillation Better Care (ABC)” approach to AF the latest ESC guidelines on the management of AF stress the ‘A’ -to Avoid stroke / Anticoagulation as the prime issue of concern before addressing ‘B’ – better symptom control and ‘C’ optimisation of cardiovascular comorbidity and other risk factors.’
Sum Zero game: The Low CHA2DS2-VASC
While at higher scores the CHA2DS2-VASC performs modestly it does seem to perform more consistently when the a score is low and the incidence of stroke seems < 1% consistently in those with a score of 0 or 1 (for female sex). However, it is increasingly recognised that some populations with low CHA2DS2VASC scores of 0 or 1 may have appreciably higher risk of stroke than previously thought, (e.g up to 2% risk per annum) and that this risk seems greater in studies of Asian populations and perhaps in other groups too.8,9
For all these reasons it is good practice that people with a low CHA2DS2-VASC score where there may be doubt about initiating anticoagulation, should be referred for a specialist opinion and a thorough assessment of risk. This may include assessment of biomarkers such as D-Dimer, Troponin, NT ProBNP, IL-6 and CRP among others, many of which have been shown to independently improve upon the risk stratification of CHA2DS2-VASC alone.
While the CHA2DS2-VASC score has remained the standard tool for risk assessment, there are concerns that it performs modestly and has relatively poor validity (C-statistic of 0.64 at best) and that the emphasis should perhaps be on a positive bias for anticoagulation than not. This author still regularly
patient
There is no role for aspirin or clopidogrel therapy in the prevention of stroke in AF and indeed it may just increase risk of bleeding without benefit.
While the CHA2DS2-VASC score has remained the standard
A limitation of CHA2DS2-VASC is that it does not include other variables such as chronic kidney
Echocardiographic evaluation of the left atrium to look for dilatation, spontaneous contrast or thrombus in the left atrial appendage, low atrial appendage exit velocity
Suggested best Practice for the CHA2 DS2-VASC = 0 (or 1 for female sex) patient?
• ESC advises no anticoagulation may be reasonable in this population BUT to reassess regularly
• Risk of stroke is < 1% but it is not zero and catastrophic strokes do occur in this population
• Lifestyle advice, weight loss and BP control may reduce burden of Paroxysmal AF
• Seek specialist opinion from an AF service to further assess risk
- specific echocardiography evaluation
- biomarkers e.g Troponin, Pro-BNP, D-Dimer, CRP IL-6 etc
• Discuss in full with the patient, explain and document indicative risk of stroke and risk of bleeding (HAS-BLED score)
• Give your opinion on management when asked – you’re their doctor!
The AF patient with stroke
A third of our strokes in Ireland are associated with AF and AF is a strong predictor of recurrent events
Secondary prevention for the AF stroke patient is of prime importance and applies to both stroke due to ischaemia and probably surprisingly also in cases of stroke due intracerebral haemorrhage.
Post-stroke AF patient with cerebral infarction
Stroke recurrence is an early risk in AF. A significant decision is when to anticoagulate the AF stroke patient after a stroke due to infarction. This is generally a specialist decision made with the benefit of
42 CPD: Atrial Fibrillation
checking in high- risk groups. An overview of reported specificities and sensitivities of various
Smartphone
detection approaches is outlined below in table 2. All suspected AF must be confirmed by a 12 lead ECG or a 30 second single lead strip read by a physician experienced in AF diagnosis. Table 2 Reported reliabilities of methods of AF detection sensitivity specificity Pulse taking 87-97% 70-81% Automated BP monitors 93-100% 86-92% Single lead ECG 94-98% 76-95%
devices
and measurement of Total atrial conduction time (PA-TDI) may also be useful in individual risk stratification.10 MR imaging is another growing area of interest in assessing left atrial function.
Both biomarkers and echocardiographic variable have been shown to independently predict risk of stroke and may well improve upon the accuracy of the CHA2DS2-VASC score. A number of more complex risk scores such as Garfield-AF, Anticoagulation and Risk factors in Atrial Fibrillation (ATRIA) and ABC-stroke have been shown to modestly improve upon CHA2DS2-VASC though a pragmatic balance must also be struck between the time involved to use such tools in clinical practice and added expenses of additional measurements versus accruing benefit. At least one biomarker-based risk stratification tools is currently undergoing evaluation in a RCT (ABC-AF Study NCT 03753490).
While acknowledging the ESC guidelines it is important to remember that, as pointed out in the preamble, that a guideline does not robustly cover every situation where there is a paucity of evidence and is not a substitute for physician-based judgement of their individual patient. In discussing the uncertainty of apparent low risk of stroke with patients it is my personal practice to advise anticoagulation in patients with low CHA2DS2-VASC scores (i.e 0 or 1 for female sex alone) with elevated biomarkers or possibly ‘risky’ echocardiographic measurements. All other patients need to understand that the risk of stroke is not Zero, that the risk of stroke in AF is a continuum and unless there is a high risk of bleeding, that anticoagulation may be the best strategy especially where the risk of bleeding is low. Where a decision is made not to anti-coagulate in the CHA2DS2VASC =0 or 1 (for female sex) patient, this should be reviewed regularly with reassessment of risk factors. Almost 15% of new AF cases will have one new non sex CHA2DS2-VASC risk factor detected at one year the majority of new comorbidities detected within 5 months after diagnosis.11
There is no role for aspirin or clopidogrel therapy in the
prevention of stroke in AF and indeed it may just increase risk of bleeding without benefit.
The AF patient with stroke
A third of our strokes in Ireland are associated with AF and AF is a strong predictor of recurrent events. Secondary prevention for the AF stroke patient is of prime importance and applies to both stroke due to ischaemia and probably surprisingly also in cases of stroke due intracerebral haemorrhage.
Post-stroke AF patient with cerebral infarction
Stroke recurrence is an early risk in AF. A significant decision is when to anticoagulate the AF stroke patient after a stroke due to infarction. This is generally a specialist decision made with the benefit of repeated neuroimaging and assessment of infarct size and the presence or absence of significant haemorrhagic transformation. A traditional role of thumb, largely dictated by concern for haemorrhagic transformation in the era of Warfarin, had been to start anticoagulation in the post-stroke AF patient on the same day -2 days for TIA small infarcts, 4-7 days for moderate sized infarcts and 10-14 days for large volume infarcts. This was always a judgement based
on expert consensus rather than expert science.
More recently the TIMING study published in Circulation,12 showed that it was safe to start anticoagulation with a NOAC in the AF patient with cerebral infarction < 5 days (early) compared to starting > 5 days (delayed). ‘Early’ initiation was not inferior to ‘Delayed’ initiation with a NOAC and though, perhaps not surprisingly, it was associated with less recurrent events numerically, it was not found to be superior. A caveat to the trial was the incidence of intracerebral haemorrhage was very low (only 3 cases and not symptomatic) compared to anticipated 3-4% incidence from previous studies in a warfarin era, and the median NIHSS was only 4 suggesting mainly milder and perhaps lower volume infractions, thought the latter was not detailed. A previous small study initiating rivaroxaban or warfarin within 5 days of infarction showed no difference in rates of recurrence or bleeding on neuroimaging at 4 weeks and the AREST study comparing early initiation with apixaban within 5 days versus delayed initiation with warfarin showed a 2.1 % incidence of haemorrhagic transformation in
the warfarin group versus non with apixaban.13,14
Three further RCTs (OPTIMAS NCT 03759938, ELAN NCT 03148457 and START NCT 03021928) aim to examine the issue of superiority of an early versus delayed strategy to initiation of anticoagulation with a NOAC in the post-stroke AF patient, but the tide is moving to earlier initiation of anticoagulation for AF patients post stroke due to infarction and with a NOAC as the safest option.
The Post stroke AF patient with intracerebral haemorrhage
It seems counter-intuitive and perhaps even dangerous to be considering anticoagulation in the AF patient who has suffered an intracerebral haemorrhage (ICH), but good longitudinal data shows that the AF patient who has suffered a stroke due to ICH does better in terms of recurrent stroke events (both ischemic and haemorrhagic) if started on anticoagulation than not.15,16 A number of caveats need to be stated here, the initiation needs careful risk assessment by a stroke specialist with repeated neuroimaging to ensure the volume of ICH is stable and resolving, that the patient does not have underlying cerebral

43
and symptoms. It is important at each visit that the patients’ CHA2DS2-VASC, HAS-BLED, creatinne clearance as calculated by the Cockroft -Gault equation and the European Heart Rhythm Association (EHRA) Symptom Score (table 3) [18]

Table 3 Modified EHRA symptom score for AF
mEHRA score symptoms description
1 No symtoms 2a Mild
Mild. Daily activity not affected and symptoms not troublesome to the patient 2b Moderate
Moderate. Daily activity not affected but symptoms troublesome to the patient 3 Severe Normal daily activity affected 4 Disabling Normal daily activity discontinued
Adapted from Wynn GJ et al. EP Europace, 2014:16(7):965–972, https://doi.org/10.1093/europace/eut395
Adapted from Wynn GJ et al. EP Europace, 2014:16(7):965–972, https://doi.org/10.1093/europace/eut395
amyloid angiopathy and that underlying anatomical risks such as aneurysm or AV malformation have been adequately addressed and treated and that blood pressure control is good.
(e.g using the lower dose of dabigatran if on higher dose). In general, a period of 4-8 weeks has been suggested with repeated neuroimaging before restarting anticoagulation in the AF patient who has suffered stroke due to ICH.
The frequency of return assessments at an AF service is largely determined by patients’ symptoms and uncontrolled co-morbidities such as hypertension which can increase risk of bleeding, or the presence of troublesome bleeding itself. In all other cases a good rule of thumb is to see the patient a month after initiation of oral anticoagulation and thereafter at monthly intervals suggested by the simple formula of [19]
If the ICH had occurred in the AF patient on an anticoagulant already, then it is important to undertake an assessment of pre-morbid drug adherence; review the time in therapeutic range (TTR) if on warfarin; ensure correct dose of NOAC adjusted for creatinine clearance, age and weight (where indicated); conduct a review of the full prescription for other drugs that increase haemorrhagic risk (e.g concomitant antiplatelet use which may no longer be needed).
Ongoing Management of Atrial Fibrillation
Interval of Routine follow up in months = creatinine clearance (Cockroft Gault) 10
in gerontology and it is intuitive that a properly constructed integrated model of care that includes cardiology, gerontology / strokeneurology , advanced nurse practitioners, clinical pharmacy and clinical nutrition would improve on patient education, medication adherence, better decision making re stroke prevention, AF symptom management, reduced drug errors or unnecessary prescription and reduced burden of AF and symptoms. It is important at each visit that the patients’ CHA2DS2-VASC, HAS-BLED, creatinne clearance as calculated by the Cockroft -Gault equation and the European Heart Rhythm Association (EHRA) Symptom Score (table 3).18
In such cases where the AF patient had been on an oral anticoagulant before the ICH, then consideration should be given to switching from warfarin to a NOAC in all cases (unless patient has true valvular AF* or a mechanical heart valve) as NOACS are associated with a 50% lower risk of intracranial haemorrhage and in vent of iCH outcomes are better on NOACS than Vitamin K antagonists.17 If the patient had been on a NOAC pre-ICH consider switching agent or lowering the dose of anticoagulant where appropriate
ESC guidelines promote an integrated management to the AF patient and in their ‘circle of ideal integration’ list the importance of access to a many speciality model with the patient at the centre of decision making. A criticism personally would be the very obvious glaring omission of geriatric medicine, given that AF is primarily a disease of later life and that many patients with AF have significant comorbidities, polypharmacy, frailty syndromes and may have already had a stroke, be suffering with cognitive impairment and pose some of the more difficult decisions when deciding to initiate anticoagulation and its subsequent management.
While the available research on models of integrated care for AF have had mixed results the components of such care have been heterogenous, none have included a specialist
The frequency of return assessments at an AF service is largely determined by patients’ symptoms and uncontrolled co-morbidities such as hypertension which can increase risk of bleeding, or the presence of troublesome bleeding itself. In all other cases a good rule of thumb is to see the patient a month after initiation of oral anticoagulation and thereafter at monthly intervals suggested by the simple formula of:19 Interval of Routine follow up in months = creatinine clearance (Cockroft Gault)
Many older patients with AF have significant co-morbidities such as cancer, hypertension , dementia, polypharmacy or are trauma patients or awaiting surgery . Management of AF within this context can be challenging as oral anticoagulation can pose a risk . The EHRA-PATHS project funded by Horizon 2020 aims to address the need for a more holistic approach to the older patient with AF through development of pathways of care that trigger both actions and KPIs for the domains of detected / suspected co-morbidity [20]. It is planned to do a RCT on the new proposed pathways of care and you can read more about this important project in the management of AF at https://ehra-paths.eu/
Many older patients with AF have significant co-morbidities such as cancer, hypertension , dementia, polypharmacy or are trauma patients or awaiting surgery . Management of AF within this context can be challenging as oral anticoagulation can pose a risk . The EHRA-PATHS project funded by Horizon 2020 aims to address the need for a more holistic approach to the older patient with AF through development of pathways of care that trigger both actions and KPIs for the domains of detected / suspected co-morbidity.20 It is planned to do a RCT on the new proposed pathways of care and you can read more about this important project in the management of AF at https://ehra-paths.eu/
References available on request
44 CPD: Atrial Fibrillation
Table 3 Modified EHRA symptom score for AF
AF symptom management, reduced drug errors or unnecessary prescription and reduced burden of AF
prevention,


















Improves the symptoms of acute uncomplicated cystitis*
9/10 patients did not require antibiotics with Utipro® Plus*

Reduces the use of antibiotics





*Garcia-Larossa A, Alexe O.Efficacy and safety of a medical device versus placebo in the early treatment of patients with symptoms of a urinary tract infection: a randomised controlled trial . Clin. Microbiol. 5,1 (2016).

AF
utipro®Plus
for the Control and Prevention of Significantly reduces recurring UTIs
www.utipro.ie Available in Pharmacies or online at www.utipro.ie
Disparities in Oral Nutritional Supplement

Usage and Dispensing Patterns across Primary Care in Ireland: ONSPres Project



In Europe, 8.5% of older adults in the community and 17.5% in residential care are at high risk of malnutrition.1 Approximately 140,000 adults in Ireland have disease-related malnutrition.2 Enhancing dietary intake is the first approach for treatment of malnutrition and involves advice to consume foods that are high in energy and protein.3 In circumstances where optimising the diet is not sufficient and malnutrition risk is high, oral nutritional supplements (ONSs) are advised. ONSs are products formulated to provide an energyand protein-dense addition to an individual’s habitual diet without the suppression of appetite or food intake.4 ONSs are commercially manufactured and can be in liquid form, semi-solids, or powders, containing varying concentrations of macronutrients and micronutrients. Patients prescribed ONSs should be reviewed within three months to assess their clinical condition and monitor progress.5,6 However, research in Ireland and the UK indicates that many patients prescribed ONSs do not undergo regular review.7–9
General practitioners (GPs) are frequently the first contact point for community-dwelling individuals at risk of malnutrition and are the primary prescribers of ONSs in Ireland.10 However, GPs often feel unsupported when managing malnutrition and report a lack of evidence-based decision making and confidence around ONSs.11 In the UK, inconsistency and substantial differences in both the identification and management of malnutrition amongst adults in the community
is also an issue.12 ONS usage is influenced by an array of factors, such as variation in healthcare professionals’ approaches, poor monitoring and inefficiencies as patients move between healthcare settings.11,13,14 In the UK’s National Health Service, dietitians can also prescribe ONSs, but prescribing is still influenced by various factors, including local policy and protocol, and patient circumstances.14
Despite research focusing on determinants of malnutrition and methods of management,15 there remains a dearth of knowledge on patterns of ONS usage in the community and characteristics of ONS users. We aimed to investigate the characteristics of patients in receipt of ONSs and describe patterns of ONS dispensing in a large population in Ireland in 2018.
Materials and Methods
Study Design
A retrospective secondary analysis was performed on anonymised dispensed pharmacy claims data in 2018 (from 1 January to 31 December inclusive). The data were obtained from the General Medical Services scheme (GMS), which provides free at the point-ofdelivery general practice care to around 30% of the Irish population with a defined low-income threshold. A database was obtained which contained prescriptions dispensed through the GMS scheme, alongside demographic information for patients and prescribers (GPs).
This information is managed by the Health Service Executive Primary Care and Eligibility Reimbursement Service (PCRS). The study examined all dispensed claims originating from GP practices located in three of Ireland’s nine Community Health Organisations (in the counties of Dublin, Kildare and Wicklow in the Republic of Ireland) which comprise 30% of all dispensed pharmacy claims and 33% of the GMS population nationally (approximately 300,000 people).
Data relating to all non-disease-specific ONSs, in addition to anonymised demographic information for 14,282 patients aged ≥18 years and 700 GPs who had prescribed ONSs, were available for 2018. Data were only available for patients or GPs linked to a dispensed claim on the PCRS system. Ethical approval was obtained from the University College Dublin Human Research Ethics Committee (reference LS-18-50-Corish) and the Irish College of General Practitioners (ICGP) Research Ethics Committee.
The following data were analysed: patient sex, age, residential care status, ONS product and volume (in units) dispensed and cost of ONSs (EUR). The data were further grouped by age category (18-44, 45-64 and ≥65 years, in line with previous research in this population16) and living situation (patient living in residential care or independently). Non-disease-specific ONSs were categorised by protein and energy content. Patients were categorised based on total volume (units) of ONSs dispensed into

PHARMACYNEWSIRELAND.COM | 47
Aisling A. Geraghty
Written by Aisling A. Geraghty1,2, Laura McBean1,2, Sarah Browne1,2, Patricia Dominguez Castro1,2, Ciara M. E. Reynolds1,2, David Hanlon3, Gerard Bury4, Margaret O’Neill5, Sarah Clarke6, Barbara Clyne7, Karen Finnigan8, Laura McCullagh8, Sharon Kennelly3 and Clare A. Corish1,2,*
Laura McBean
Sarah Browne
Clare A. Corish
‘Moderate’ (<75th centile of volume), ‘High’ (75th–89th centile) and ‘Very High’ ONS users (≥90th centile). This identified high users based on annual volumes dispensed over the course of the year and so may not have identified high clinical or daily usage over a shorter period within the year. Costs refer to the individual product cost calculated using publicly available standardised HSE-listed reimbursement price.
Statistical Analysis
The assessment of normality for all variables was performed by visual analyses of histograms. Parametric or non-parametric tests were used as required. A bivariate analysis was used with one-sample t-tests to assess sex differences in the cohort. Mann–Whitney U tests were used to examine sex differences and differences between residential caredwelling and independent-dwelling patients in relation to age, ONS units and cost. Differences between age groups, intakes of ONS categories between groups and ONS user categories were investigated using cross-tabulations and the chi-squared statistical test. Results with a p < 0.05 were considered significant. Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) software version 24.0 (IBM).
Results
Characteristics of Patients on Oral Nutritional Supplements

A total of 14,282 community-dwelling adults was dispensed ONSs. The median age was 76 years with 71% of the cohort aged over 65 years. Females were older with a median age of 80 years, compared to 71 years in males (p < 0.001). In total, 81% of patients dispensed ONSs were living independently and 18.7% were in residential care (p < 0.001). A higher proportion of females in residential care were on ONSs than males (23% vs. 12.6%; p < 0.001).
There were 1027 GPs on the GMS register within the healthcare areas analysed, 68.2% of whom were linked to a dispensed ONS claim (n = 700). Almost one-third (31.8%, n = 327) prescribed no ONS during 2018. Per GP, the median number of patients dispensed ONSs was 13; however, this ranged from 1 to 297 and almost 40% of GPs prescribed ONSs to a patient in residential care.
Characteristics of Oral Nutritional Supplement Dispensing
Very high energy sip feed
High energy, standard protein sip feed
High energy modular
High protein sip feed
High energy semi-solid
Figure 1. Breakdown of oral nutritional supplement products cohort (n = 14,282).
3.3. Oral Nutritional Supplement Dispensing in Residential ing
Textured-modified high energy high protein sip feed
Other
high-energy semi-solid ONS products, which were more common for females (18% vs. 13.8%; p < 0.001). However, when split by age, differences were observed.
More patients within residential care were years (99% of females vs. 65% of males; p < 0.001) ble 3). Residential care patients had higher volumes vs. 112; p < 0.001) and ONSs were also more costly 541, compared to EUR 212 for patients living independently terns of all ONS categories differed based on residential ucts dispensed in each category split by residential
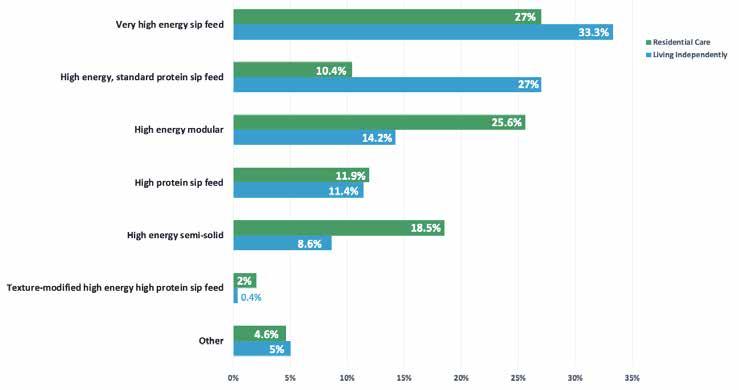
Oral Nutritional Supplement Dispensing in Residential Care Compared to Independent Living
More patients within residential care were female (72% vs. 55% males) and aged ≥65 years (99% of females vs. 65% of males; p < 0.001)
than patients living independently. Residential care patients had higher volumes of ONS dispensed (median, 240 units vs. 112; p < 0.001) and ONSs were also more costly, with the median yearly cost being EUR 541, compared to EUR 212 for patients living independently (p < 0.001). Dispensing patterns of all ONS categories differed based
Figure 1. Breakdown of oral nutritional supplement products dispensed across categories in the full cohort (n = 14,282). Nutrients 2022, 14, x FOR PEER REVIEW 6 of 11

A total of 3640,446 units of ONSs were dispensed to this cohort in 2018. Per patient, this ranged from 1 to 7206 units, with a median of 126 units per patient. Most patients (92.5%) were dispensed under 730 units of ONSs, the equivalent of two units per day over the year. Overall, male patients were dispensed higher volumes of ONSs than females; however, when split by age, this was only seen in males aged <65 years (median, 136 units for males vs. 90 units for females; P<0.001). Specifically, males in the 18–44-year-old category were dispensed more units of ONSs than females in the same age category (median units, 120 (IQR 236) for males and 60 (IQR 146) for females; p < 0.01).
There were seven ONS categories, with the most common being very-high-energy sip feeds (which were dispensed to 45% of the cohort and making up 31.8% of all products. There was no difference in category of product dispensed to males and females, apart from
Figure 2. Bar chart with breakdown of all oral nutritional supplement products dispensed in the cohort from each category to patients in residential care and patients living independently.
Very high energy sip feed
High energy, standard protein sip feed
High energy modular
High protein sip feed
High energy semi-solid Textured-modified high energy high protein sip feed
Other
Residential Care Living Independently
Figure 2. Bar chart with breakdown of all oral nutritional supplement products dispensed in the cohort from each category to patients in residential care and patients living independently
Table 3. Comparison of characteristics and oral nutritional supplement dispensing between patients living in residential care and living independently across a 12-month period.
on residential status. The ONS products dispensed in each category split by residential status are presented in Figure 2.
Characteristics of ‘High’ and ‘Very High’ Oral Nutritional Supplement Users
There were 2152 patients (15.1%) classified as ‘High’ ONS users and 1428 (10%) ‘Very high’ ONS users. The median age of those in the ‘High’ and ‘Very High’ user categories was higher than that of ‘Moderate’ ONS users (p < 0.001). There were more females in each group; however, the proportion of males increased in the ‘High’ and ‘Very High’ users groups (p = 0.04).
Of ‘Very High’ and ‘High’ users, 36.4% and 26.6% were in residential care, respectively, compared to only 14.8% of ‘Moderate’ users (p < 0.001). ‘Moderate’ ONS users had a median 84 units yearly of ONS dispensed (median costs of EUR 153 per patient), ‘High’ ONS users 420 units (EUR 806) and ‘Very High’ ONS users had 892 units (EUR 2402; p < 0.001).
Discussion
Main Findings
In this analysis of 14,282 patients aged over 18 years, clear disparities were identified in ONS usage across patient age groups, between males and females and also between patients living independently in the community and patients in residential care facilities. In particular, higher ONS usage was noted in younger males and among patients in residential care, with further differences in ONS category usage identified among patients in residential care. In this sample of one third of Ireland’s population, two-thirds of GPs had prescribed ONSs at least once during the year, with medians of 20 prescriptions for 13.5 patients, identifying this role as an important issue for general practice.
Comparison with Existing Literature
We identified a higher proportion of female patients on ONSs, similar to previous research in Ireland and internationally reporting ONS users being predominantly female and older.1,8 This may be due to females having higher life expectancies and, as a result, increased frailty and risk of malnutrition. Although more ONS users were female, younger males were dispensed much higher volumes. We found males aged under 44 were dispensed twice the volume of ONSs compared to females, beyond what could be explained by their increased nutritional requirements or treatment of conditions such as Crohn’s Disease. Research carried out in America similarly found that the proportion of males was higher among ONS users in a slightly younger population.17
This raises the question around the use of ONSs in younger populations and factors influencing their prescription. Our group has previously described an association between ONS prescribing in this age group and simultaneous prescribing of psychoactive drugs;16 chronic illness and multimorbidity are common among opiate-dependent patients in Ireland.18 ONS prescribing may be a feature of the management of opiate dependency in Ireland and further research should explore the implications of this finding. Recent qualitative research exploring factors influencing ONS prescribing focused mainly on older adults and

found that ONSs were often prescribed without evidence for their use.11,13 Additional enquiry is needed to understand these patterns and whether they are related to social factors.
Despite the reported effectiveness of ONSs in managing malnutrition in patients in residential care,19 we found that these patients were dispensed twice the volume of ONSs compared to patients living independently. Research carried out on 23,500 patients in residential care across Europe and America found that almost 14% used ONSs and usage was associated with increasing age and functional impairment.20 Similarly, in this population, polypharmacy was associated with long-term usage of ONSs, which may indicate a decline in health.16 Although higher requirements for ONSs in residential care may be explained by the advancing age of patients, which is also reflected by the higher proportions of female patients who have a longer life expectancy and higher disease rates, given that they have access to and assistance with meals and snacks in residential care, this increased need should be mitigated. Unfortunately, data on medication or health status was not available in this analysis, but future analyses should incorporate these to help elucidate the reasons behind increased ONS use in residential care.
While dietary counselling combined with ONSs in care homes have been shown to be highly effective for treating malnutrition,21 the appropriateness of ONS prescribing in this setting remains a concern. Reported ONS usage in residential care varies drastically with a recent publication estimating rates ranging from 1% to 43% across countries.20 Previous research in Ireland indicated that up to onethird of ONS prescriptions in the community were inappropriate for patient needs8 and a recent review of ONS usage within care homes in the UK identified high levels of inappropriate prescribing.7 Similarly, a recent analysis in the United States reported that, although 25% of their cohort of adults in the community were classified as being at high risk of malnutrition, only 11% consumed ONSs.17 This highlights the need for clarification around appropriate prescribing of ONSs, particularly for patients in residential care due to the variations in usage in this setting.
There is limited research on factors influencing prescriber choice of ONS products. However, recent research in Ireland similarly identified a preference for high-protein ONS products with the main factors driving ONS product choice being nutritional value and patient palatability.22 We found that the ONS category did not differ between males and females; however, patient age and residential location did differ with the ONS category dispensed. Previously, in Ireland, the cost of ONSs was reported as a concern for ONS prescribers,11 which may influence the product prescribed. We found that almost one-third of GPs within these community areas did not prescribe ONSs for any patients, potentially also due to cost concerns and lack of standardised screening for malnutrition. This is despite evidence from a recent systematic review indicating that ONS use in the community is near neutral or produces a cost advantage.23 This, alongside differences in ONS products and volumes, suggests that education and support are needed for GPs and healthcare professionals prescribing ONSs.
Strengths and Limitations
This analysis had many strengths, in that it included comprehensive prescribing data for around 300,000 people (30% of the GMS population in Ireland) and identified almost 15,000 ONS users. As the data related to dispensed ONS claims, this removed any reporting or recall bias from patients or healthcare providers relating to ONS usage. However, this analysis was not without limitations. Only data relating to patients or GPs linked to a dispensed ONS claim on the PCRS system were available, so comparisons between non-ONS using patients or non-ONS prescribing GPs were not possible. Limited characteristics and demographic information were available on patients and the background of the users, including the purpose of dispensing, is not known, so future analysis should explore the impact of factors such as socioeconomic status, health status and other medication usage. Additional avenues of exploration which could help elucidate these findings include prevalent pathologies, the degree of malnutrition and ONS effectiveness. As these data related to ONS dispensing claims rather than consumption of ONSs, it was not possible to access appropriate ONS usage and, similarly, prescribing practises could not be evaluated without a review of individual patients, which is highly encouraged in future studies.
Implications for Research and Practice
Particular consideration should be given to younger males and patients in residential care facilities which used higher volumes of ONSs. Of equal concern is the possible ‘undertreatment’ of malnutrition by GPs. Given that 30% of GPs did not prescribe any ONSs, there is a need for consistent approaches whereby evidence-based prescribing is implemented in all settings. Further education around appropriate usage of ONSs, both for patients and healthcare professionals, is vital to ensure both appropriate prescribing and appropriate usage of ONSs, to efficiently prevent and treat malnutrition in the community. Multidisciplinary teams are indispensable in effectively managing malnutrition in our community and adequate support must be provided at all levels of healthcare to ensure successful treatments.
Conclusions
Strong disparities were identified in ONS usage between patient groups in primary care in Ireland. Further research is warranted to elucidate the reasons for high ONS usage in younger males and patients in residential care. Further education and support are required for healthcare professionals working in malnutrition management.
References available on request
A Seasonal Reflection – My 30-year Journey from Salesman to Advisor

As we begin another year, I thought I might write something a little different for this issue, a reflection on the 30-plus years I have worked in the financial services sector. Some of what I have learned about myself and the nature of the role and the evolution of financial advice independent and otherwise.
Written by Kieran Moore QFA, SIA, Director,Moore Wealth Management Ltd

Moore Wealth Management has been advising the Irish Pharmacy Community for over 20 years with extensive knowledge of the sector. They can be contacted at 086-3801868, kieran@mwm.ie or www.mwm.ie
But they should nonetheless be labelled based on their actions, not their job titles. Unless you consider the person who sold you your last car your automotive advisor or think of your local bartender more as your alcohol advisor, please don’t consider the person who sold you financial products without any real financial plan behind it to be a financial advisor, regardless of how nice the building they work from. I recognise this now for what it is because that was once the game I played too, pretend advisor.
ourselves as the white knights taking on the status quo. A lot of our time and marketing was spent focusing our disdain on those in the sector guilty of the little secret of the financial advice game, the lack of transparency around the independence of the direct salespeople prevailing in the market.
For most of that 30-plus years, my job title could have been described as a “financial advisor” or some variant of it. Although the job titles over the years remained similar the focus and nature of what I did and thought I did most certainly did not.
I was a salesman for the first ten years, I truly believed I was a financial advisor at the time. But with honest reflection I was better summed up by the joke “What do you get if you cross an insurance salesman with someone from the mafia? Someone who makes you
an offer you can’t understand “. I see now that I understood little beyond the products I was trained to sell.
Tragically there are still way too many of old me in the financial services sector today. Too many financial advisors are simply product sellers that baffle people with glossy brochures and wellrehearsed sales pitches. The biggest problem is they are hard to spot because they can very often be found behind desks in credible locations such as banks and other financial services organizations.
The second decade of my life in financial services was one marked by learning. Never too late as they say, in two years of working in a new role with actual experts in the sector I learned considerably more than the previous twelve. And there were now courses and exams for those like me who wanted to be a better adviser for clients with internationally recognized qualifications and ongoing development.
Over the next 8 years, I resolved to be as expert as those around me soaking up knowledge like the proverbial sponge. I would now describe my actions at that stage of my career as “advisor adjacent”, I was getting there.
At the time I and many of my expert colleagues did see
It was a serious bone of contention for me then and if I’m honest now, partly because the way the central bank regulates the financial advice sector leaves a lot to be desired. Truly Independent advisors are held to one standard while those working for banks and directly for insurance companies are held to another.
The problem is the consumer is not being informed of the difference and while the central bank seems very comfortable in its role of advisor police it has never embraced the mission of educating the public on the limitations and dangers of getting biased financial advice. Perhaps because this mainly comes from banks…. Who knows?
A very simple example of this is that banks are not required to inform you that the mortgage protection policy they sell you with your mortgage is usually not the best value for money. Because they are not independent
PHARMACYNEWSIRELAND.COM 50 Finance
it’s deemed to be obvious to the consumer that they are selling you the only product they have access to. They are not obliged to inform you that you can get a cheaper version of the product elsewhere and they must still accept it to cover your loan.
In contrast, a regulated independent financial advisor is required under central bank regulations to offer you the best value protection contract available in the market under what is termed ‘fair analyses. Now hands up how many of you knew that only independent brokers are required to act in your best interest while “advisors” from banks work under much less stringent and dare I say, inadequate consumer protection rules?
You are supposed to surmise that their lack of independent status which is well disguised, should naturally imply a bias in their advice. So you get what you get because it’s all they have to give.
Back to me. From my perspective 15 years in I was now much closer to an adviser than a salesman. I now knew that I had non-product knowledge that could help people significantly with their financial well-being. Knowledge about managing tax, business structures, business exit planning and how to set financial goals and build investment portfolios.
I could tell people these things, advise them of them even, and so I did. And then I morphed into the seasoned and experienced financial adviser I always wanted to be but more importantly, the one clients needed.
Much of this last decade since then has been spent building a business that focuses on an advice lead process, highlighting the difference between this and
the product sellers in the market. It is not easy because salespeople are typically good at…you know … sales, while advisers are typically good at... you guessed it …advising.
Salespeople usually work with teams that have marketing experts and large budgets to push their message. Advisors are typically found in smaller independent brokerages, often more forced on existing clients than new ones but we wear that mentality as a badge of honour.
So as another year begins here is a little actual financial advice to consider along with your new year’s resolutions.
Established your maximum revenue allowances for company pension contributions, including those special allowances for back service. If you have them and most of you will, then top up your pensions from surplus company funds and use the valuable corporation tax relief to you.
As central bank rates rise to combat inflation, rethink your strategy for surplus corporate cash. Strange as it may seem banks only remembered to apply recent increases in interest rates to loans not savings.
Inflation is the real enemy right now and there are now deposit alternatives that offer 100% capital protection and pay circa 3% per annum to help offset the erosion of the value of these corporate funds over time. Shop for these alternatives in the broker market they are not available from banks.
Stock markets will remain volatile for the foreseeable future as the combination of the war in Ukraine, inflation management by central banks around the world and the potential for a global slowdown in response to all this dominate market sentiment.

This does not mean you avoid investing, especially if it is for the medium to long term, successful investing is not about timing the bottom of the market it’s about time in the market.
Finally, from one independent small business to another and those working in them can I wish you and your families a Happy and prosperous New Year. I hope you have enjoyed our articles throughout the last year. We will be continuing to do our bit this year to keep you informed with regular articles on financial issues that are relevant to you and your circumstances.
New Director of Innovation
University College Cork (UCC) has appointed Dr Sally Cudmore as its new Director of Innovation. Under Dr Cudmore’s
Dr Sally Cudmore
Dr Cudmore, who held the position of Interim Director since June 2021, is the first woman to be appointed as Director of Innovation at University College Cork.

Welcoming the appointment, Professor John Cryan, Vice President for Research & Innovation, said: said: “I am delighted that Dr Cudmore will lead UCC Innovation at this exciting time for research and innovation in UCC. Through our UCC Futures programme, cutting edge technologies and ideas are being harnessed to tackle global challenges in an ambitious and creative manner. This role will accelerate entrepreneurial activity across campus and as the first female leader of this office, Sally will be a role model to inspire even greater female entrepreneurship participation”.
A science graduate of UCC, Dr Cudmore has over twenty-five years of leadership experience in academia and industry. Following a PhD in Cell Biology from European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, she completed her postdoctoral research at University of California San Francisco (UCSF). She then moved to the pharmaceutical industry where she led the Cell Biology division in Elan Pharmaceuticals research and development. She has an MSc in Technology Management from Smurfit Business School in UCD.
In 2002, Dr Cudmore returned to UCC where she took on the role of General Manager of APC Microbiome Ireland, a worldleading SFI research centre based at UCC, which explores how to harness and manipulate microbes for the benefit of human and planetary health. During her management, APC secured ¤200 million in competitive research funding, collaborated with more than 70 companies from the food, pharma and biotech sectors, trained more than 600 research graduates, spun out 4 companies, and published over 3,000 research articles.
Dr Cudmore co-founded UCC spin-out company, Atlantia Clinical Trials Ltd, which provides clinical trial expertise to the food, beverage and supplements sectors, and served as the (parttime) CEO for its initial years. Atlantia employs 85 people in Cork and Chicago today.
Dr Sally Cudmore commented: “I am excited to take on this role and drive UCC’s ambition to become the innovation university in Ireland. My goal is to grow UCC’s impact even further through an ambitious programme to enhance entrepreneurship amongst students, graduates and staff resulting in an increased number of new companies created. We also aim to develop strategic partnerships with industry, through developing a new Business Engagement Unit that will interface with industry and provide access to the breadth of research, innovation and expertise in UCC.”
PHARMACYNEWSIRELAND.COM 51
leadership, UCC’s research community will advance its efforts to deliver breakthroughs, expertise, and technologies to enhance society and economy.
News
Sports Pharmacy Practice and Education
Inspired by the athletes themselves, their athleticism, and the spirit of competitive sport, pharmacists have become key players as athlete support personnel (ASP). As ASP, pharmacists support appropriate medication selection and provision of medicines management in the healthcare of athletes and pharmacy operations of sporting events. Increasingly recognised within the world of sports as a valuable expertise, the practice is known as “sports pharmacy”.
FIP has teamed up with colleagues from the International Sports Pharmacists Network to publish a global overview of sports pharmacy practice and education, released. The report highlights how pharmacists have been using their pharmacy knowledge and skills to act as key athlete support personnel through pharmaceutical care, doping control and providing services at sporting events. It defines sports pharmacy as a specialty area and outlines the knowledge and skills required to practise it.
“Since the publication of FIP guidelines on ‘The role of the pharmacist in the fight against doping in sport’ in 2014, the work of many individual pharmacists has nurtured sports pharmacy into an expanding specialty. This growth occurred parallel to and often intertwined with growth of the anti-doping movement. Internationally, major stakeholders acknowledge sports pharmacists as key contributors to antidoping and clean sport initiatives. Facets of pharmaceutical care for athletes led by experienced sports pharmacists have become embedded in sports medicine around the world.
The specialty of sports pharmacy is no longer a niche area but is one gaining traction as a recognised speciality on a global scale,” explained Mark Stuart, co-author and a sports pharmacist who has worked at many Olympic Games. In addition to discussing sports pharmacy education and qualifications, the report includes sections on pharmacists’ roles in the anti-doping movement, therapeutic use of prohibited medicines and risk assessment of dietary supplements.
In 1999, the World Anti-Doping Agency (WADA) was formally established to protect athletes by promoting clean sport and embedding the “spirit of sport” on an international level. To achieve this, stakeholders harmonised anti-doping rules and established policies for all sports globally, which primarily focus on
medicines and methods of blood or gene manipulation.
This focus provides a unique opportunity for pharmacists, the experts in therapeutics, to accurately interpret pharmaceutical and pharmacological information and evidence for athletes and the ASP. Through their accessibility and expertise, pharmacists help athletes to avoid inadvertent doping while preserving their health and athleticism.

As therapeutics have evolved and advanced, so too has the expert role of the pharmacist in sports evolved and advanced with growing significance; this report aims to define this emerging specialist pharmacy area. Accessibility to accredited continuing professional development in sports pharmacy will improve athletes’ safety and therapeutic outcomes and pharmacists’ value to their communities.
The report also calls for adoption of formalised and quality education on sports pharmacy across international undergraduate curricula. Undergraduate and postgraduate education programmes in sports pharmacy could and should train and equip pharmacists with baseline knowledge and skills to advise and support athletes in making rational choices regarding effective clinical drug use or nutritional supplement options while ensuring compliance with the rules of the World AntiDoping Code (WADC) and sports federations.
Guidance on a framework for sports pharmacy education is presented in this report, along with examples of successful implementation at several pharmacy schools across the globe. This report further seeks to demonstrate the wider impact that pharmacists have within sports and the health care of athletes
based on experts’ experiences and FIP members’ case examples.
To fully embed this into pharmacy practice, it is necessary to have a robust strategy that underpins the delivery of quality continuing education and training to upskill the workforce.
In summary, pharmacists with an interest in sports pharmacy can use this document to self-assess their own learning needs and identify areas where they may require additional education and learning.
Pharmacists involved in delivering education can use the knowledge and skills described within the document as a guide for educational course development. The overall aims of this report are to:
• Highlight potential career paths for pharmacists with a mission to support athletes with safe and effective pharmacy care, while avoiding possible inadvertent doping; and
• Increase awareness of opportunities where pharmacists can gain knowledge and increase their skills within sports pharmacy through reputable quality training, experience and education programmes.
PHARMACYNEWSIRELAND.COM 52 Sports Pharmacy



























































Like your customers, ours have come to rely on the value of expert guidance. With CERTIFIED FINANCIAL PLANNER™ expertise, Moore Wealth Management has given the very best, completely independent advice to pharmacy professionals for over 20 years. Research shows, professionals who’ve taken independent financial advice enjoy a more comfortable retirement with much greater financial security and peace of mind. Scan here for more info Moore Wealth Management Limited trading as Moore Wealth Management is Regulated by the Central Bank of Ireland Reg number 55195 colm@mwm.ie 086 860 39 53 | kieran@mwm.ie 086 380 18 68 We all need trusted advice +300% +173% +75% Savings and investment holdings A 15 year wealth increase An average pension pot increase
Effect of Smoking on the Development of Arthritis and Osteoporosis in the Elderly Population of Ireland: A Cross-Sectional Study
by

Arthritis and osteoporosis are two musculoskeletal disorders that run rampant worldwide. Arthritis is a general term describing the pathological inflammation and swelling of a joint. Several different etiologies of arthritis exist; reactive arthritis, immune-mediated, trauma or wearing with age. Osteoarthritis is the most common subtype of arthritis and is a widespread cause of debilitation and is linked with old age.1
It was estimated that in 2010-2012, 22.7% of all adults in the United States suffered from diagnosed arthritis and the percentage of individuals is on the rise. It is predicted by 2040 that 78.4 million (25.9%) Americans are to suffer from arthritis.2 By 2050, a 310% and 240% increase in the incidence of hip fractures in males and females respectively is expected.3 Osteoporosis, commonly known as “brittle bone disease”, is defined as a condition in which there is a pathological condition with decreased bone density.4

As the density of bone decreases, so does its integrity therefore pathological and stress fractures are common among individuals who suffer from this chronic disease. Safiri et al., provide a comprehensive review and up to date statistics in their analysis of the burden of osteoarthritis. It was estimated that 303.1 million individuals in 2017 suffered from knee and hip joint osteoarthritis.5 In the same review, age standardized as Years Lived with Disability (YLD) had increased by 9.6% from 1990 to 2017.5

This leads to the conclusion that the extent to which osteoarthritis and other joint/bone disease have an effect on the quality of life. In the United States, the financial burden has raised yet another concern; in 2013 nearly $304 billion was attributed to the medical care of osteoarthritis.6 Similarly, it was estimated the total annual economic burden of osteoporosis in Italy equated to $2.64 billion (€2.2 billion).7
Tobacco smoking is indeed harmful and a wide variety of its effects are known however some associations of disease it may be linked to are yet unknown. It is predicted that tobacco smoke has direct and indirect effects on the skeletal system, which ultimately both lead to the imbalance of osteoblastic and osteoclastic function.8 This in turn, decreases bone mass and increases bone resorption leading to skeletal disease.
The question lies: is there a statistical correlation between former smokers and bone/joint disease?
Subjects & Methods
The data used for the analysis was crosssectional data collected from The Irish Longitudinal Study on Ageing (TILDA), wave one (2009-2010).9 The target population was people over the age of 50 and living in residential addresses in the Republic of Ireland, including their spouses or partners of any age. The sample frame used was the RANSAM system which gives each member of the population a non-zero chance of being included in the sample, while reducing
selection bias compared to the initial Personal Public Service (PPS). The study involved participants receiving a number of questions to answer in a written questionnaire form called CAPI which covers a wide variety of topics including smoking, presence of arthritis, bone problems, exercise, age, sex, and BMI. With regards to smoking, participants were asked whether they smoked or not, and for the duration (in years). For a person to count as a smoker in the past, they needed to have smoked for at least a period of 1 year. A new variable was made to categorize the continuous variable, that is, “For how many years [do/did] you smoke altogether?” into groups of none, 1 to 5 years, 6 to 10 years, 11 to 15 years, 11 to 20 years, and 21 years and above. The questions for arthritis or bone disease were asked as part of a general question regarding chronic diseases and the answer was if they had the disease or not. In this analysis, the number of participants included is 8,504. Statistical Analysis The statistical analysis was done for groups meeting certain criteria and the number included in such analysis will be highlighted. The software used to analyze the data for this report is the IBM SPSS Statistics version 27.
Results
First, cross tabulation was conducted between smoking status and the presence of arthritis or osteoporosis as shown in the descriptive figures below (Figures 1 and 2). This included a sample size of 8,504. As it is clear, 26.2% of people who never smoked (for at least a period of 1 year) suffer from arthritis.
On the other hand, people who smoked in the past and current smokers have a prevalence of arthritis of 27.4%. There is a difference of 1.2% in rates of arthritis between smokers and people who have never smoked.


54 | PHARMACYNEWSIRELAND.COM
Ahmed W.I. Alanqar
Written
Ahmed W.I. Alanqar1, Faris Soloman Almadi1*, Yousef M. Waly1, Ali Al-Sabti2 1 Royal College of Surgeons in Ireland-Bahrain. 2University College Dublin *Correspondence Email Address: faris.almadi1@gmail.com
Faris Soloman Almadi Yousef M. Waly Ali Al-Sabti
Figure 1: Smoking Status * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
Percentage (%)
Canadian Journal of Clinical Nutrition, Volume 10, Issue 2, June 2022 ISSN 1927-8942 (Print Edition), ISSN 1927-8950 (Online Edition)
Never smoked Smoked (Current or past)
Figure
However, the results in the crosstabulation of smoking status against arthritis are insignificant as the Chi-Square tests show a P-value greater than 0.05. [X2 (1, N = 8504) = 1.56, P = .212]. In Figure 2, 10.7% of people who never smoked suffer from osteoporosis compared to 8.2% of smokers. The difference was 2.5% and the ChiSquared test showed a pvalue of less than 0.01 which means this result is not due to random chance. [X2 (1, N = 8504) = 15.54, P < .001]. In addition, relative risk was done on both tables.
100.0%
90.0%
80.0%
70.0%
Percentage (%)
60.0%
50.0%
40.0%
30.0%
20.0%
10.0%
The relative risk (RR) for arthritis was 0.956 [95% CI: 0.890-1.026]. The RR for developing osteoporosis from smoking is 1.305 meaning smoking increases the risk to develop osteoporosis was roughly 1.3 times more than non-smokers [95% CI: 1.143-1.489].
In the third and fourth descriptive tables shown below (Table 1 and 2), total years smoked is crosstabulated against arthritis and osteoporosis respectively. Since this analysis includes multiple categories for the variable,
total years smoked, RR was not run in this analysis. In Table 1, arthritis rates drop (26.2%) from non-smokers to 23.3% in the case of people who smoked for a period of 11 to 15 years, but then rise again to 28.6% in people who smoked for more than 21 years.
Overall there is an increase between the nonsmokers and people who smoked for more than 21 years of 2.4%. Chi-Squared analysis was performed and showed no significance in Table 1, P -value = 0.161. [X2 (5, N = 8504) = 7.92, P = 0.161]. The same trend was observed in the analysis of osteoporosis, where there is a drop in the rates in groups who smoked for longer, then a rise again. In Table 2, the rates of disease were 10.7% then it dropped to as low as 6.3% in the group who smoked for 11 to 15 years. The percentage rises back again to 8.5% in the group who smoked for more than 21 years and over.
Overall, there was a 2.2% difference in the prevalence of osteoporosis in people who smoked for more than 21 years compared to people who did not smoke. However, results in Table 2 show a significance as P - value = 0.002 [X2 (5, N = 8504) = 18.81, P = 0.002].
Binominal logistic regression was carried through to assess the effect of confounding variables on the prevalence of arthritis and osteoporosis respectively. The dependent variables were the presence of arthritis or osteoporosis respectively. The binomial logistic regression model contained 7 independent variables: Age, Gender, BMI, Participation in sports or exercise, number of supplements reported taken, the highest level of education completed, and the total years smoked.
89.3% 3327 10.7% 400
91.8% 4384 8.2% 393 0.0%
Not Osteoporosis
Osteoporosis
Never Smoked Smoked (Current or past)
The binomial logistic regression model for Arthritis was statistically significant [X2 (25, N = 5290) = 596.57, P < 0.001]. Between 10.7% (Cox and Snell R square) and 15.6% (Nagelkerke R square) of the variance in arthritis, and correctly classified 74.9% of cases. As can be seen, 6 variables contribute significantly to the prevalence of arthritis: Age, Gender, BMI, Number of reported supplements, the highest level of education completed, smoking for periods of 6 to 10 years and over 20 years. The B values for these variables are positive except for the level of education completed. This indicates that all but the level of education completed increases the risk for arthritis. The two strongest predictors to reporting arthritis are gender (female) and the number of reported supplements of 2 with an odds ratio (OR) of 1.955 and 1.976 respectively. Females report almost twice as many times from suffering from arthritis compared to males.
For binomial logistic regression model of Osteoporosis, it was also statistically significant [X2 (25, N = 5290) = 756.93, P < .001]. Between 13.3% (Cox and Snell R square) and 28.0% (Nagelkerke R square) of variance in osteoporosis, and correctly classified 90.4% of cases. Four variables contributed significantly to the prevalence of osteoporosis: Age, Gender, body mass index (BMI), and number of supplements reported. The B values for age, gender, and the number of supplements reported are positive, while the B value for
Figure 2: Smoking Status * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
Figure 2: Smoking Status * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
PHARMACYNEWSIRELAND.COM | 55
Copyright © 2022 by The Global Science Heritage Publisher (http://www.globalscienceheritage.org). All Rights Reserved. is an open access article distributed under the terms of the Library and Archives/Government of Canada, www.collectionscanada.gc.ca) & The Creative Commons Attribution Non-Commercial License which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Page 50
1: Smoking Status * Has a doctor ever told you that you have any of the following conditions? Crosstabulation 73.8% 2750 26.2% 977 72.6% 3467 27.4% 1310 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% 80.0% Not Arthritis Arthritis
Table 1: Total years smoked * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
NO Arthritis Arthritis Total
Total Years Smoked None Count Percentage 2750 (73.8%) 977 (26.2%) 3727 1 to 5 Yrs Count Percentage 296 (73.1%) 109 (26.9%) 405 6 to 10 Yrs Count Percentage 397 (73.8%) 141 (26.2%) 538 11 to 15 Yrs Count Percentage 316 (76.7%) 96 (23.3%) 412 16 to 20 Yrs Count Percentage 490 (74.2%) 170 (25.8%) 660 21 Yrs and over Count Percentage 1937 (71.4%) 774 (28.6%) 2711 Total Count Percentage 6186 (73.2%) 2267 (26.8%) 8453 (100.0%)
Table 1: Total years smoked * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
associated risk between arthritis and smoking. Similar results were found by Amin et. al, the initial statistical analysis portrayed an insignificant association between smoking and arthritis in 159 men who suffered osteoarthritis.15
However, with further analysis in the study and the inclusion of confounding variables (such as age and BMI), a significant association was observed.15 Despite the significance found one may argue that BMI should not be considered a confounding variable, as mediator for disease.15 As explained by Felson et al., BMI is a main cause of osteoarthritis while smoking has not been a main cause of an increase in BMI (rather a habit seen in individuals who suffer obesity) as a majority of smokers are thinner in stature: taking BMI into consideration (as a confounding) interferes with the integrity of a statistical analysis.16
Yrs: Years
BMI is negative. The strongest predictor here is the female gender, with an odds ratio of 8.337. Females are 8.337 more likely to report suffering from osteoporosis. People who are taking 1 to 3 supplements are also more likely to report they are suffering from osteoporosis. The older the people got, the more they reported suffering from osteoporosis by 1.045 times and a 1-point increase in BMI decreased the rate of osteoporosis by a very minute margin. Smoking showed no significance as P > 0.05.
prevalence of osteoporosis at the time in the same sample was 9.3% indicating a much lower prevalence of osteoporosis than arthritis within the tested population.
Smoking and BMI on Arthritis
Despite the strength of this argument, several studies in the literature show how smoking is associated with a lower BMI.17, 18 The results of this study could be expected due to several different reasons considering the potential confounding variables, including BMI. Smoking was then divided into different ranges (ex. Smoked for 1-5 years) and was measured against arthritis and similarly for osteoporosis.
Canadian Journal of Clinical Nutrition, Volume 10, Issue 2, June 2022 ISSN 1927-8942 (Print Edition), ISSN 1927-8950 (Online Edition)
Copyright © 2022 by The Global Science Heritage Publisher (http://www.globalscienceheritage.org). All Rights Reserved. This is an open access article distributed under the terms of the Library and Archives/Government of Canada, (www.collectionscanada.gc.ca) & The Creative Commons Attribution Non-Commercial License which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Discussion
An association between smoking and musculoskeletal disorders, specifically bone and joint disease, has been observed throughout the literature.8, 10-12 The aim of this study was to observe the extent if any, and a correlation between smoking and osteoporosis or arthritis.
Considering that living a preventative and healthy lifestyle would support and protect musculoskeletal health while counterintuitive habits, such as smoking, would result in a fall in bone and joint disease health. In this study, 8,504 responses from TILDA (The Irish Longitudinal Study on Aging) participants were observed. We found that out of 8,504 individuals, 3,727 were non-smokers while 4,777 were smokers. Nearly 56% of participants in the cross-sectional study were identified as smokers meaning a theoretical increase in the prevalence of bone and joint disease was expected Furthermore, 26.9% of individuals who participated in TILDA suffered from arthritis (osteoarthritis and Rheumatic Arthritis). In a country with similar geography and demographic makeup, the United Kingdom, the prevalence of osteoarthritis and rheumatoid arthritis was 10.7% and 0.67% respectively.13,14
While the data from TILDA is much more representative of a population due to the large sample size and variety in demographics, this value still remains high. However, the
Table 2: Total years smoked * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
In this Irish cohort study, it was observed that 27.4% of smokers and 26.2% of non-smokers suffered from arthritis. This primary analysis may suggest that smoking may increase the risk of arthritis. This difference however, was not statistically significant as the p-value observed was greater than 0.05 [X2 (1, N = 8504) = 1.56, P = 0.212]. Moreover, a RR ratio calculated was also insignificant as the RR<1 with the confidence intervals crossing the value of one, suggesting that there was no
Page 52
Table 2: Total years smoked * Has a doctor ever told you that you have any of the following conditions? Crosstabulation
The results showed a prevalence of arthritis in 26.2% of non-smokers and 23.3% of individuals who smoked for 11-15 years. However, against the common trend, 28.6% of individuals who smoked longer than 21 years suffered arthritis which then appeared to be statistically insignificant (P > 0.05) after conducting a ChiSquared [X2 (5, N = 8504) = 7.92, P = .161]. This investigation called for further detailed analysis in order to count for the confounding variables, a binomial logistic regression was conducted.
NOT Osteoporosis Osteoporosis Total
Total Years Smoked None Count Percentage 3327 (89.3%) 400 (10.7%) 3727 1 to 5 Yrs Count Percentage 374 (92.3%) 31 (7.7%) 405 6 to 10 Yrs Count Percentage 489 (90.9%) 49 (9.1%) 538 11 to 15 Yrs Count Percentage 386 (93.7%) 26 (6.3%) 412 16 to 20 Yrs Count Percentage 609 (92.3%) 51 (7.7%) 660 21 Yrs and over Count Percentage 2481 (91.5%) 230 (8.5%) 2711 Total Count Percentage 7666 (90.7%) 787 (9.3%) 8453 (100.0%)
Yrs: Years
56 | PHARMACYNEWSIRELAND.COM
Page 53
Nicorette QuickMist 1 mg/spray, oromucosal spray, solution. One spray delivers 1 mg nicotine in 0.07 ml solution. 1 ml solution contains 13.6 mg nicotine. Excipient with known effect: Ethanol (less than 100 mg of ethanol/spray). Propylene glycol, Butylated hydroxytoluene. Pharmaceutical form: Oromucosal spray, solution. A clear to weakly opalescent, colourless to yellow solution. Indications: For the treatment of tobacco dependence in adults by relief of nicotine withdrawal symptoms, including cravings, during a quit attempt (or to cut down smoking before stopping completely. Permanent cessation of tobacco use is the eventual objective. Nicorette QuickMist should preferably be used in conjunction with a behavioral support program. Dosage: Subjects should stop smoking completely during the course of treatment with Nicorette QuickMist. Adults and Elderly: The following chart lists the recommended usage schedule for the oromucosal spray during full treatment (Step I) and during tapering (Step II and Step III). Up to 4 sprays per hour may be used. Do not exceed 2 sprays per dosing episode and do not exceed 64 sprays (4 sprays per hour, over 16 hours) in any 24-hour period. Step I: Weeks 1-6: Use 1 or 2 sprays when cigarettes normally would have been smoked or if cravings emerge. If after a single spray cravings are not controlled within a few minutes, a second spray should be used. If 2 sprays are required, future doses may be delivered as 2 consecutive sprays. Most smokers will require 1-2 sprays every 30 minutes to 1 hour. Step II: Weeks 7-9: Start reducing the number of sprays per day. By the end of week 9 subjects should be using HALF the average number of sprays per day that was used in Step I. Step III: Weeks 10-12: Continue reducing the number of sprays per day so that subjects are not using more than 4 sprays per day during week 12. When subjects have reduced to 2-4 sprays per day, oromucosal spray use should be discontinued. To help stay smoke free after Step III, subjects may continue to use the oromucosal spray in situations when they are strongly tempted to smoke. One spray may be used in situations where there is an urge to smoke, with a second spray if one spray does not help within a few minutes. No more than four sprays per day should be used during this period. Regular use of the oromucosal spray beyond 6 months is generally not recommended. Some ex-smokers may need treatment with the oromucosal spray longer to avoid returning to smoking. Any remaining oromucosal spray should be retained to be used in the event of sudden cravings. Gradual cessation through progressive reduction in smoking: For smokers who are not willing or ready to quit abruptly. The oromucosal spray is used between periods of smoking in order to prolong the smoke-free intervals and with the intention to reduce smoking as much as possible. The patient should be aware that an incorrect use of the spray may enhance adverse effects. A cigarette is replaced with one dose (1-2 sprays) and a quit attempt should be made as soon as the smoker feels ready and no later than 12 weeks after start of treatment. If a reduction in cigarette consumption has not been achieved after 6 weeks of treatment, a healthcare professional should be consulted. After quitting smoking, gradually reduce the number of sprays per day. When subjects have reduced to 2-4 sprays per day, oromucosal spray should be discontinued. Regular use of the oromucosal spray beyond 6 months is not recommended. Some ex-smokers may need treatment with the oromucosal spray longer to avoid returning to smoking. Any remaining oromucosal spray should be retained to be used in the event of sudden cravings. Paediatric population: Do not administer this medicine to persons under 18 years of age. There is no experience of treating adolescents under the age of 18 with this medicine. Method of administration: After priming, point the spray nozzle as close to the open mouth as possible. Press firmly the top of the dispenser and release one spray into the mouth, avoiding the lips. Subjects should not inhale while spraying to avoid getting spray into the respiratory tract. For best results, do not swallow for a few seconds after spraying. Subjects should not eat or drink when administering the oromucosal spray. Behavioural therapy advice and support will normally improve the success rate. Contraindications: Hypersensitivity to nicotine or to any of the excipients. Children under the age of 18 years. Those who have never smoked. Special warnings and precautions for use: This medicine should not be used by non-smokers. The benefits of quitting smoking outweigh any risks associated with correctly administered nicotine replacement therapy (NRT). A risk-benefit assessment should be made by an appropriate healthcare professional for patients with the following conditions: Cardiovascular disease: Dependent smokers with a recent myocardial infarction, unstable or worsening angina including Prinzmetal’s angina, severe cardiac arrhythmias, recent cerebrovascular accident and/or who suffer with uncontrolled hypertension should be encouraged to stop smoking with non-pharmacological interventions (such as counselling). If this fails, the oromucosal spray may be considered but as data on safety in this patient group are limited, initiation should only be under close medical supervision. Diabetes Mellitus. Patients with diabetes mellitus should be advised to monitor their blood sugar levels more closely than usual when smoking is stopped and NRT is initiated as reduction in nicotine induced catecholamine release can affect carbohydrate metabolism. Allergic reactions: Susceptibility to angioedema and urticaria. Renal and hepatic impairment: Use with caution in patients with moderate to severe hepatic impairment and/or severe renal impairment as the clearance of nicotine or its metabolites may be decreased with the potential for increased adverse effects. Phaeochromocytoma and uncontrolled hyperthyroidism: Use with caution in patients with uncontrolled hyperthyroidism or phaeochromocytoma as nicotine causes release of catecholamines. Gastrointestinal Disease: Nicotine may exacerbate symptoms in patients suffering from oesophagitis, gastric or peptic ulcers and NRT preparations should be used with caution in these conditions. Paediatric population: Danger in children: Doses of nicotine tolerated by smokers can produce severe toxicity in children that may be fatal. Products containing nicotine should not be left where they may be handled or ingested by children. Transferred dependence: Transferred dependence can occur but is both less harmful and easier to break than smoking dependence. Stopping smoking: Polycyclic aromatic hydrocarbons in tobacco smoke induce the metabolism of drugs metabolised by CYP 1A2 (and possibly by CYP 1A1). When a smoker stops smoking, this may result in slower metabolism and a consequent rise in blood levels of such drugs. This is of potential clinical importance for products with a narrow therapeutic window, e.g. theophylline, tacrine, clozapine and ropinirole. The plasma concentration of other medicinal products metabolised in part by CYP1A2 e.g. imipramine, olanzapine, clomipramine and fluvoxamine may also increase on cessation of smoking, although data to support this are lacking and the possible clinical significance of this effect for these drugs is unknown. Limited data indicate that the metabolism of flecainide and pentazocine may also be induced by smoking. Excipients: This medicine contains about 7 mg of alcohol (ethanol) in each spray which is equivalent to 97 mg/ml. The amount in one spray of this medicine is equivalent to less than 2 ml beer or 1 ml wine. The small amount of alcohol in this medicine will not have any noticeable effects. This medicinal product contains less than 1 mmol sodium (23 mg) per spray, i.e. essentially ‘sodium- free’. This medicine contains 12 mg propylene glycol in each spray which is equivalent to 157 mg/mL. Due to the presence of butylated hydroxytoluene, Nicorette QuickMist may cause local skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes. Care should be taken not to spray the eyes whilst administering the oromucosal spray. Undesirable effects: Effects of smoking cessation: Regardless of the means used, a variety of symptoms are known to be associated with quitting habitual tobacco use. These include emotional or cognitive effects such as dysphoria or depressed mood; insomnia; irritability, frustration or anger; anxiety; difficulty concentrating, and restlessness or impatience. There may also be physical effects such as decreased heart rate; increased appetite or weight gain, dizziness or presyncopal symptoms, cough, constipation, gingival bleeding or apthous ulceration, or nasopharyngitis. In addition, and of clinical significance, nicotine cravings may result in profound urges to smoke. This medicine may cause adverse reactions similar to those associated with nicotine given by other means and these are mainly dose-dependent. Allergic reactions such as angioedema, urticaria or anaphylaxis may occur in susceptible individuals. Local adverse effects of administration are similar to those seen with other orally delivered forms. During the first few days of treatment irritation in the mouth and throat may be experienced, and hiccups are particularly common. Tolerance is normal with continued use. Daily collection of data from trial subjects demonstrated that very commonly occurring adverse events were reported with onset in the first 2-3 weeks of use of the oromucosal spray, and declined thereafter. Adverse reactions with oromucosal nicotine formulations identified from clinical trials and during post-marketing experience are presented below. The frequency category has been estimated from clinical trials for the adverse reactions identified during post-marketing experience. Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1 000 to <1/100); rare (≥1/10 000 to <1/1 000); very rare (<1/10 000); not known (cannot be estimated from the available data).


Common Dysgeusia, paraesthesia Eye disorders Not known Blurred vision, lacrimation increased Cardiac disorders Uncommon Palpitations, tachycardia Not known Atrial fibrillation Vascular disorders Uncommon Flushing, hypertension Respiratory, thoracic and mediastinal disorders Very common: Hiccups, throat irritation Uncommon Bronchospasm, rhinorrea, dysphonia, dyspnoea, nasal congestion, oropharyngeal pain, sneezing, throat tightness Common: cough Gastrointestinal disorders Very common Nausea Common Abdominal pain, dry mouth, diarrhoea, dyspepsia, flatulence, salivary hypersecretion, stomatitis, vomiting Uncommon Eructation, gingival bleeding, glossitis, oral mucosal blistering and exfoliation, paraesthesia oral Rare Dysphagia, hypoaesthesia oral, retching Not known Dry throat, gastrointestinal discomfort, lip pain Skin and subcutaneous tissue disorders Uncommon Hyperhidrosis, pruritus, rash, urticaria Not known Erythema General disorders and administration site conditions Common Burning sensation, fatigue Uncommon Asthenia, chest discomfort and pain, malaise. MAH: Johnson & Johnson (Ireland) Limited, Airton Road, Tallaght, Dublin 24, Ireland. PA Number: PA 330/37/13 Date of revision of text: August 2022. Product not subject to medical prescription. Supply through pharmacy and non-pharmacy outlets. Full prescribing information available upon request. The Nicorette ® brand proudly supporting Marie Keating Foundation’s Your Health, Your Choice campaign and Community Nurses. NICORETTE® contains Nicotine. ALWAYS READ THE LABEL. NICORETTE® is a stop smoking aid. JNTL Consumer Health I (Ireland) Limited will donate €30,000 to The Marie Keating Foundation, Registered Charity Number 20038953, in relation to this charitable campaign. No purchase necessary IE-NI-2200126 TOGETHER SUPPORTING HEALTHY HABITS Join the Challenge
Immune system disorders Common Hypersensitivity Not known Allergic reactions including angioedema and anaphylaxis Psychiatric disorders Uncommon Abnormal dream Nervous system disorders Very common Headache
Confounding’s and Arthritis
Seven variables ranging from age to level of education were included in the regression. The results showed how these variables contributed and their significance in the likelihood of the development (positive B-values) of arthritis, including smoking [X2 (25, N = 5290) = 596.57, P < .001]. One out of the seven variables was insignificant, level of education did not impact the outcome of the results. The greatest confounding variables identified were gender (female OR= 1.955) and supplementation (OR= 1.976).
These findings are consistent with supporting literature as smoking may increase the risk of Rheumatoid Arthritis11,12,19 whether by oxidative stress, inflammation, or apoptosis. Additionally, cigarette smoke condensate has been shown to induce the production of proinflammatory cytokines such as IL-1a, IL-1B, IL-6, IL-8 and TNF-a which in turn further increases the production of these cytokines causing a vicious cycle of inflammatory disease.20 Interestingly, individuals who took a larger number of supplements were expected to have a fortified musculoskeletal system however the results of this study suggested otherwise.
It can be postulated that individuals who were taking supplements over a period of time in order to combat musculoskeletal pathologies or other chronic illnesses leading to a lower quality of life, particularly that the sample population was above the age of 50.
Confoundings and Osteoporosis
Similarly, osteoporosis is a multifactorial disease in which one risk factor may contribute to a collection of other risk factors. Smoking, similar to arthritis, is hypothesized to be a potent risk factor for the development of osteoporosis as seen in the literature.8,10,21 Interestingly, the relative risk for developing
osteoporosis from smoking is 1.305 (95% CI: 1.143 lower and 1.489 upper). In this study, a 2.2% difference was observed between individuals who never smoked and individuals who smoked for 21 years and was seen to be statistically significant having a P-value = 0.002 [X2 (5, N = 8504) = 18.81, P = .002]. In order to further strengthen the results of the statistical analysis, a binomial logistic regression was also conducted to account for the same confounding variables in the arthritis analysis.
The results of this analysis showed that these confounding factors had an impact to the outcome of the results, [X2 (25, N = 5290) = 756.93, P < .001]. Four of the seven variables were significant including age, gender, BMI and the number of supplements taken. Further analysis revealed females, in this data collection, have an odds ratio of 8.337 eluding to how the woman may be susceptible to osteoporosis. On the other hand, smoking was shown as a not significant variable in this analysis.
Supplementation and Osteoporosis
Considering age as well as gender, all individuals in this study were above the age of 50 at the time and the average age of menopause in a similar population demographic is between 50 and 52.22 Postmenopausal women are at the highest risk of developing osteoporosis due to hormonal changes. Estrogen is osteoprotective in the sense of how it regulates and composes several different components of bone synthesis, from decreasing the activity of osteoclasts by downregulation of RANK-L leading to decreased bone resorption to stimulating osteoblastic function.23
Therein, a deficiency in estrogen leads to increased bone resorption explaining why elder women are 8.337 more likely to suffer from osteoporosis. The other major risk factors include increased BMI and, unexpectedly, individuals
who take supplements. Supplementation is essential for individuals at risk for osteoporosis in which the majority of people at risk are postmenopausal women, explaining the significance of this confounding variable.
Future Implications
With the results of this study, preventative measures may be introduced into an aging society in order to minimize the risks of the development of arthritis and osteoporosis. Smoking, occupation, and socioeconomic status are important risk factors in the development of rheumatoid arthritis.24 Public health awareness implementation through the campaigns has shown to be an effective mode to increase smoking cessation, as seen throughout various studies.
In particular, Duke et al. investigated the effect of a statewide campaign on smoking cessation among adults in Florida showing that 66% of smokers attempted to quit.25 In addition, the implementation of campaigns to promote physical exercise amongst the entire population and all age groups may have an effect on the prevalence of either arthritis or osteoporosis, as physical exercise is considered a modifiable risk factor.26 Furthermore, possible supplementation of calcium and other minerals in at risk populations should be considered as a nationwide program. As seen, individuals who were taking supplements in this tested population were at higher risk of developing osteoporosis or arthritis. Since all individuals who participated were above the age of 50, were at risk and suffered these musculoskeletal diseases at the time. Therefore, administrating calcium supplementation to premenopausal women could possibly slow the development of osteoporosis.27 The goal of these programs is to induce a wide primordial prevention strategy rather than a symptomatic secondary or tertiary prevention strategy.
New research Fellowship Programme
Professor John Cryan, Dr Pamela Byrne, Professor Paul Ross, Oonagh Cahalane and Brenda Curran launch the programme
INSPIRE Fellows will undertake research projects across a variety of areas of microbiome science to address the UN Sustainable Development Goals and create solutions to challenges relating to food and health including anti-microbial resistance, the burden of non-communicable diseases, and the need for sustainable food systems.
Food Safety Authority of Ireland, said, “Never in our time has sustainability been so important. INSPIRE addresses the huge challenges that we face globally, building a talent pool to ensure that we have skilled scientific researchers equipped and agile to meet the needs we will face in the coming years. APC Microbiome Ireland has a proven record of ground-breaking research targeting the grand challenges of our time. I am delighted to launch the INSPIRE programme which will support such impactful research.”
With funding of ¤2.9 million, INSPIRE (INnovative Sustainable Development InterdisciPlinary Post-Doctoral Research Excellence) is co-funded by the EU Horizon 2020 Marie Sklodowska-Curie Actions programme. INSPIRE will create 20 prestigious postdoctoral research fellowships at APC Microbiome Ireland based at UCC or at one of the APC’s other academic partner institutions.

The INSPIRE Fellowship will provide experienced researchers with industry placement experience while benefiting from a tailor-made research and career development training plan. INSPIRE fellows will gain a portfolio of transferable skills to enhance their career options and to meet the evolving needs of the academic, NGO and industry sectors.
Dr Pamela Byrne, Chair of the APC Microbiome Ireland Governance Committee and CEO of the
Professor Paul Ross, Director of APC Microbiome Ireland, said, “The INSPIRE Fellowship reflects APC Microbiome Ireland’s commitment to addressing the UN’s Sustainable Development Goals. We look forward to recruiting 20 postdoctoral researchers who will help create solutions to some of the most pressing global challenges with potential impact in the pharmaceutical, medical and food sectors.”
58 | PHARMACYNEWSIRELAND.COM
News
APC Microbiome Ireland, a world-leading SFI Research Centre based at University College Cork (UCC), has launched the INSPIRE Fellowship programme which seeks to tackle challenges facing global food systems and health.
Quitting Smoking
*Provides significant improvement in quit rate vs patch alone. To verify contact: verify@perrigo.com Lindson N et al. 2019 Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Library. IRE NIQ 2022 55 NiQuitin CLEAR 24 hrs transdermal patches contain nicotine and are indicated for the relief of nicotine withdrawal symptoms including cravings as an aid to smoking cessation. Indicated in adults and adolescents aged 12 years and over. NiQuitin patches should be applied once a day, at the same time each day and preferably soon after waking and worn continuously for 24 hours. Apply a patch to non-hairy clean dry skin surface, a new skin site should be used every day. Therapy should usually begin with NiQuitin 21 mg/24 hrs and reduced according to the following dosing schedule: Step 1: NiQuitin Clear 21 mg/24 hrs transdermal patches first 6 weeks. Step 2: NiQuitin Clear 14 mg/24 hrs transdermal patches next 2 weeks. Step 3: NiQuitin Clear 7 mg/24 hrs transdermal patches last 2 weeks Light smokers (less than 10 cigarettes per day) are recommended to start at Step 2 (14 mg) for 6 weeks and decrease the dose to NiQuitin 7 mg/24 hrs for the final 2 weeks. In some instances (e.g. heavy smokers, those who have relapsed after NRT, or when one NRT product is not enough to control cravings), NiQuitin patches may be used in combination with a nicotine oral format (refer to the package leaflet for dosing guidance). Contraindications: Non-smokers, hypersensitivity, children under 12 years and occasional smokers. Precaution: Supervise use if hospitalised for MI, severe dysrhythmia or CVA, if haemodynamically unstable. Use with caution in patients with active oesophagitis, oral and pharyngeal inflammation, gastritis, peptic ulcers, GI disturbances, susceptible to angioedema, urticaria, renal/hepatic impairment, hyperthyroidism, diabetes, phaeochromocytoma, seizures & epilepsy. Discontinue if severe persistent skin rash. Pregnancy and lactation: Oral formats preferable to patches unless nauseous. Remove patches at bedtime. Side effects: Sleep disorders, abnormal dreams, insomnia, headache, dizziness, nausea, vomiting, application site reactions, nervousness, palpitations, dyspnoea, pharyngitis, cough, dyspepsia, upper abdominal pain, diarrhoea, constipation, dry mouth, sweating, localised pain, urticaria, hypersensitivity, tremor, nervousness, palpitations, tachycardia, contact & allergic dermatitis, photosensitivity, arthralgia, myalgia, asthenia, malaise, influenza-type illness, fatigue, chest or limb pain, pain, seizures and anaphylaxis. Legal classification: GSL: PA 1186/018/004, PA 1186/018/005 & PA 1186/018/006. MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. Date of preparation: 04/2022. RRP (ex. VAT) 7 pack €19.83, 14 pack €35.73. SPC: https://www.medicines.ie/medicines/niquitin-clear-7-mg-24-hours-transdermal-patch-33085/ spc https://www.medicines.ie/medicines/niquitin-clear-14-mg-24-hours-transdermal-patch-33083/spc https://www.medicines.ie/medicines/niquitin-clear-21-mg-24-hours-transdermal-patch-33084/spc NiQuitin Mini 2mg/4mg Mint Lozenges contain nicotine and are used for the treatment of tobacco dependence by relief of nicotine withdrawal symptoms and cravings. Indicated in adults and adolescents aged 12 years and over NiQuitin Mini 2 mg are suitable for those who smoke 20 cigarettes or less a day. NiQuitin Mini 4 mg are suitable for smokers who smoke more than 20 cigarettes a day. Place a lozenge in the mouth whenever there is an urge to smoke, allow to dissolve completely. Do not chew or swallow whole. In heavy smokers, those who have relapsed after NRT, or when one NRT is not enough to control cravings, NiQuitin Minis may be used in combination with NiQuitin patches (refer to the package leaflet for dosing guidance). Abrupt cessation: Use a lozenge whenever there is an urge to smoke, maximum of 15 lozenges a day. Continue for up to 6 weeks, then gradually reduce lozenge use. Gradual cessation Use lozenges whenever there is an urge to smoke in order to reduce the number of cigarettes smoked for up to 6 weeks, followed by abrupt cessation. Adolescents (12-17 years): Only with advice from a healthcare professional. Should not quit with a combination NRT regimen. Contraindications: Hypersensitivity to nicotine or any of the excipients, children under the age of 12 years and non-smokers. Precaution: Supervised use in dependent smokers with a recent myocardial infarction, unstable or worsening angina pectoris including Prinzmetal’s angina, severe cardiac arrhythmias, uncontrolled hypertensions or recent cerebrovascular accident. Use with caution in those with; stable cardiovascular diseases, diabetes mellitus, susceptibility to angioedema & urticaria, renal/hepatic impairment, phaeochromocytoma & uncontrolled hyperthyroidism, GI disease & seizures. Side effects: Nausea, mouth/throat and tongue irritation, irritability, anxiety, insomnia, sleep disorders, dizziness, headaches, cough, sore throat, vomiting, diarrhoea, upper abdominal pain, GI and oral discomfort, flatulence, hiccups, heartburn, dyspepsia, dry mouth, constipation, ulcerative stomatitis, pharyngitis, pharyngolaryngeal pain, nervousness, depression, palpitations, heart rate increased, dyspnoea, rash, angioedema, pruritus, erythema, hyperhidrosis, urticaria, fatigue, malaise, asthenia, chest pain, anaphylactic reactions, hypersensitivity, tremor, dysgeusia, paresthesia mouth, seizures & epilepsy, dysphagia, eructation, salivary hypersecretion, influenza like illness. Legal classification: GSL: PA 1186/018/017 & PA 1186/018/012 MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. Date of preparation: 12/2022. RRP (ex. VAT) 20 pack €7.55, 60 pack €21.05, 100 pack €28.00. SPC: https://www.medicines.ie/medicines/niquitin-mini-2mg-mint-lozenges-35237/spc https://www.medicines.ie/medicines/niquitin-mini-4mg-mint-lozenges-33091/spc


“Since I quit smoking, I enjoy a healthier me” Samantha
Lung cancer was the leading cause of cancer death in both sexes during 2018-2020
Cancer in Ireland: Latest Report Published
Over 50% increase in numbers of cancer survivors compared with a decade ago
In males, cancer of the prostate , colorectal (bowel), pancreas and oesophagus were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively. Colorectal (bowel) cancer was the 2nd most common cancer death in males during 2016-2018, but dropped to 3rd behind prostate cancer during 2018-2020.
In females, cancer of the breast, colorectal (bowel), ovary and pancreas were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively.
The National Cancer Registry (NCRI) has published its 2022 Annual Statistical Report: Cancer in Ireland. 1994 – 2020. This report on the status of cancer in Ireland includes updated statistics on cancer incidence, mortality and survival for patients diagnosed in Ireland 1994 – 2020.
The National Cancer Registry (NCRI) has published its 2022 Annual Statistical Report: Cancer in Ireland. 1994 – 2020. This report on the status of cancer in Ireland includes updated statistics on cancer incidence, mortality and survival for patients diagnosed in Ireland 1994 – 2020.
The National Cancer Registry of Ireland is now in its 29th year of data collection, and in the 2022 annual statistical report, they summarise cancer data collected up to diagnosis year 2020. In additional to the more regular reporting of incidence, mortality and survival figures, this year they again provide additional focus on impacts of the COVID-19 pandemic on numbers of cancers diagnosed.
Key findings include:
• Over 50% increase in numbers of cancer survivors compared with a decade ago as, for the first time, the number of patients living after an invasive cancer diagnosis has exceeded the 200,000 mark, equivalent to 1 in 24 people in Ireland. This reflects the ongoing improvement in cancer survival.
• More complete data on the impact of the COVID-19 pandemic on cancer diagnoses indicates that the pandemic resulted in a 10% reduction in cancer diagnoses (based on all cancers) or 11% (based on microscopically verified cancers) in 2020, compared to what was expected that year.
• Median age at diagnosis for all cancer combined (excluding non-melanoma skin cancers) was 69 years in men and 67 years in women, with little change over time. The median age at death for all invasive cancers combined was 74 years in both men and women, an increase compared with the median of 72 years in both men and women during 1994-1998, consistent with improved cancer survival.
• Further evidence of improvements in colorectal cancer control in men, as this cancer drops from 2nd to 3rd most common cause of cancer deaths in men.
Top five most common causes of cancer death during 2018 -2020 Males Females
Annual statistical report 2022
What are the most common cancers causing death?
National Cancer Registry Ireland
Lung cancer was the leading cause of cancer death in both sexes during 2018-2020.
In males, cancer of the prostate , colorectal (bowel), pancreas and oesophagus were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively. Colorectal (bowel) cancer was the 2nd most common cancer death in males during 2016-2018, but dropped to 3rd behind prostate cancer during 2018-2020.

The report shows that lung cancer was the leading cause of cancer death in both sexes during 20182020. In males, cancer of the prostate, colorectal, pancreas and oesophagus were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths respectively. Colorectal cancer was the 2nd most common cancer death in males during 2016-2018 but dropped to 3rd behind prostate cancer during 2018-2020.

How many previously diagnosed cancer patients are still alive?
In females, cancer of the breast, colorectal (bowel), ovary and pancreas were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively.
contained in the National Cancer Strategy 2017-2026.”
About 207,000 cancer patients or former cancer patients were alive in Ireland at the end of 2020 (about 4.2% or 1 in 24 of the Irish population).
The top six most common cancers among survivors were: breast cancer (23% of all cancer survivors), prostate cancer (21%), colorectal (bowel) cancer (11%) and skin melanoma (7%), non-Hodgkin lymphoma (4%) and lung cancer (4%) which together account for 70% of all cancer survivors.
Top five most common causes of cancer death during 2018 -2020 Males Females
These figures exclude non -melanoma skin cancers, which are rarely fatal.
Number of cancer survivors
In females, cancer of the breast, colorectal, ovary and pancreas were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths respectively.
The report also shows, around 207,000 cancer patients or former cancer patients were alive in Ireland at the end of 2020 (about 4.2% or one in 24 of the Irish population).
How many previously diagnosed cancer patients are still alive?
Total=207,365 (100%)
The top six most common cancers among survivors were: breast cancer (23% of all cancer survivors), prostate cancer (21%), colorectal cancer (115) and skin melanoma (7%), non-Hodgkin lymphoma (4%) and lung cancer (4%) which together account for 70% of all cancer survivors.
Director of the National Cancer Registry, Professor Deirdre Murray states, “One notable milestone we report this year is that, by the end of 2020, for the first time, the number of people living after an invasive cancer diagnosis had exceeded the 200,000 mark to reach 207,000. This is equivalent to 4.2% of the population, or about 1 in 24 persons in Ireland, a >50% increase in numbers of cancer survivors compared with one decade ago. This reflects both an increase in the number of people being diagnosed with cancer every year and ongoing improvements in cancer survival, as also reported here.”
The six most common cancers among cancer survivors
The report authors state, “This year’s report is the first in which we have primarily reported age-standardised incidence and mortality rates based on the ‘newer’, 2013 European Standard Population (ESP), while still retaining equivalent figures using the older 1976 standard in the appendices for continuity. Agestandardisation is one of the key methods to control for different age distributions among populations or over time, to help ensure valid comparisons between countries, regions or periods.
About 207,000 cancer patients or former cancer patients were alive in Ireland at the end of 2020 (about 4.2% or 1 in 24 of the Irish population).
The top six most common cancers among survivors were: breast cancer (23% of all cancer survivors), prostate cancer (21%), colorectal (bowel) cancer (11%) and skin melanoma (7%), non-Hodgkin lymphoma (4%) and lung cancer (4%) which together account for 70% of all cancer survivors.

6
These figures exclude non -melanoma skin cancers, which are rarely fatal.
Chair of the NCRI Board Dr Jerome Coffey, welcomed the report and said, “These data tell a clear story of improvements in cancer survival rates over time. Combined with increasing numbers of new cases the report describes the number of people alive and well following diagnosis and treatment. This further underlines the recommendations for service developments
Number of cancer survivors
Total=207,365 (100%)
“This change is necessary to ensure such valid comparisons but one consequence of using a different standard is that the rates based on the 2013 standard are higher than those based on the 1976 standard. This does not imply any ‘real’ change in rates or risk and, in fact, overall rates of cancer incidence (allowing for population growth and ageing) have been stable or falling over the last decade.”
The six most common cancers among cancer survivors

PHARMACYNEWSIRELAND.COM 60 Report
National Cancer Registry Ireland
Annual statistical report 2022
What are the most common cancers causing death?
6
In launching the twelfth annual Irish Pharmacy Awards, we are seeking to uncover those individuals and teams putting in the groundwork quoted above to the enhancement of community pharmacy throughout Ireland.
The Irish Pharmacy Awards represent a unique and high-profile opportunity to celebrate the excellence and amazing achievements of Ireland’s pharmacy professionals, and reaffirm Irish Pharmacy News’s commitment to the core pursuits of pharmacy: excellence in health and innovation.

The Awards recognise outstanding examples of high standards, best practice, innovation and excellence.

It has been, and continues to be, the goal of Irish Pharmacy News to recognise the exact added value of the contribution made by community pharmacists and their teams.
Through the submissions to these Awards and the examples of innovation that they demonstrate, IPN want to inspire you to think about your role, that of your team and of the community pharmacy profession in which you operate, as a whole.
The aim of the Irish Pharmacy Awards is to celebrate, recognise and reward those displaying dedication and commitment. They also create a platform which fosters shared learning and education.
Applications are now open. Further and full information on each available award category will be available in the February issue of IPN.
Irish Pharmacy Awards 2023
May 27th, 2023
Register your interest / apply for the Irish Pharmacy Awards 2023 We will send you a unique link to the online application forms. Contact aoife@ipn.ie / 087 337 9258 Applications are now open!
Saturday,
Clayton Hotel (Burlington Road) Dublin Entries are now Open
Health Living Ireland Survey
Minister of State with responsibility for Public Health, Wellbeing and the National Drugs Strategy, Frank Feighan, and Chief Medical Officer, Professor Breda Smyth, have announced the results of the Healthy Ireland Survey 2022, funded by the Department of Health and conducted by Ipsos.
period poverty. Findings show a significant level of menstrual discomfort experienced by respondents, with 67% having experienced period pain, and with 49% having somewhat and 10% having significantly limited their daily activities on a regular basis due to their symptoms.
The survey, which is run on an annual basis, gives an up-to-date picture of the health of the nation and reports on many lifestyle behaviours, including smoking, alcohol consumption, weight, oral health and utilisation of health services.
The prevalence of smoking has remained the same since last year’s survey. 18% are smokers and 46% of smokers have attempted to quit. The peer-led We Can Quit Programme is being rolled out in 19 community areas across Ireland. The National Stop Smoking Clinical Guideline published in January 2022 defines best practice for care of people who smoke including helping them to quit. In Budget 2023, all
Nicotine Replacement Therapy (NRT) was exempted from VAT. And the Public Health (Tobacco Products & Nicotine Inhaling Products) Bill in development will introduce several restrictions on the sale of smoking products.
Findings on alcohol consumption indicate 67% have consumed alcohol in the previous 6 months and 32% of those would be considered binge drinkers. The Public Health (Alcohol) Act 2018 introduced a number of measures to reduce and delay alcohol consumption including minimum unit pricing, health labelling, regulation of advertising, reduced product visibility and the regulation of sale and supply.
Commenting on the survey findings, the Chief Medical Officer, Professor Breda Smyth, said:
“I would really encourage smokers to visit the HSE’s Quit website for advice and support for quitting smoking. Similarly, the HSE’s Ask About Alcohol website has lots of helpful information and resources for anyone concerned about alcohol and other drugs, including advice for parents on how to raise these topics with their children.”
This year’s survey also included information about menstrual health and period poverty for the first time.
The findings indicate that 24% of women have experienced
Measures to improve menstrual health, part of the Women’s Health Action Plan, include the development of a network of 20 “see-and treat” gynaecology clinics. 12 are open with 8 more in development and Budget 2023 will fund a 21st clinic. 5 menopause clinics in Dublin, Nenagh and Galway have opened and the 6th clinic in Cork is expected to open in 2023. 2 specialist centres for endometriosis care and funding for six interdisciplinary teams across the country to support treatment of endometriosis are being established. The free contraception scheme available since September 2022 made hormonal contraceptive medications and devices, also used to manage period symptoms, free for women aged 17-25, and this scheme is due to be expanded to include women aged up to 30 by the end of 2023.
Other members of this team included Prof Abina Crean, Prof Brendan Griffin, Prof Stephen Byrne, Dr Waleed Faisal, Dr Rachel Moloney, Dr Sonja Vucen, Ms Aine Healy, Ms Noreen Moynihan, Ms Laura Gleeson (postgraduate representative) and Ms Maria Mulrooney (undergraduate representative). Prof Brendan Griffin stated that the award “will encourage us to seek further opportunities to achieve even more success and, in this sense, enable us to excel further as a place where staff and
students can fulfill personal career goals.” Dr Murphy said that “the success of the application is due to the staff and students of the School. Their daily embodiment of the Athena SWAN principles is what drives improvement and delivers impact. Seeing this, I am confident that the best days are ahead of us.” Ms Noreen Moynihan attended the 2022 Athena Awards Ceremony on behalf of the School of Pharmacy at the Atlantic Technological University (ATU) Castlebar on 8 November 2022.

PHARMACYNEWSIRELAND.COM 62
News
Swan Bronze Award for Pharmacy School The School of Pharmacy received their renewed Athena Swan Bronze award. This application was led by
Pharmacy’s
XX Healthy Ireland Summary Report 2022 15-24 25-34 35-44 45-54 55-64 65-74 75+ Obese 64 6 22 8 46 3 34 17 40 1 34 25 33 1 39 27 30 2 39 29 34 2 42 21 37 1 41 22 Overweight Normal weight BMI BY AGE (%) 28% 20% 22% Underweight SMOKING 23% 23% 22% 20% 17% 18% 18% 2015 2016 2017 2018 2019 2021 2022 PREVALENCE OF SMOKING - BY YEAR AND GENDER 13% 44% 42% 13% 54% 33% CHANGES IN DRINKING BEHAVIOUR SINCE THE START OF THE COVID-19 PANDEMIC 2021 2022 Drinking more Drinking has remained the same Drinking less 2021 2022 2018 INCIDENCE OF BINGE DRINKING IN THE POPULATION WEIGHT MANAGEMENT ALCOHOL 02 Healthy Ireland Summary Report 2022 Executive Summary
Athena
Dr Kevin Murphy, chair of the School of
Athena SWAN team.
School Manager Noreen Moynihan, School of Pharmacy, UCC

CONTOUR®NEXT
light for diabetes management *Patients should always consult their healthcare professional before setting or changing any target ranges. 1. CONTOUR®NEXT (connected) BGMS User Guide, Rev. 05/19. 2. Grady M et al. J Diabetes Sci Technol. 2018;12(6):1211-1219. 3. Richardson J et al. Clinical Relevance of Reapplication of Blood Samples During Blood Glucose Testing. Poster presented at the Diabetes Technology Meeting (DTM). November 12-15 2020. © 2021 Ascensia Diabetes Care. All rights reserved. Ascensia, the Ascensia Diabetes Care logo, Contour, Smartlight and Second-Chance are trademarks and/or registered trademarks of Ascensia Diabetes Care Holdings AG.
September 2022. HCPCNEXTad092022v1 To order your FREE CONTOUR®NEXT meters and support materials contact your local territory manager: South East - Maria Purcell Ph: 086 8181834 maria.purcell@ascensia.com North East - Denise Butler Ph: 087 166 0065 denise.butler@ascensia.com West - Barbara Muldoon Ph: 086 0455125 barbara.muldoon@ascensia.com South West - Mary Gavin Ph: 086 0455136 mary.gavin@ascensia.com
is an easy-to-use system that supports diabetes management providing clear, accurate readings you can trust.1
Highly accurate1 readings you can trust to guide your patients
smartLIGHT® feature. Target range indicators have been demonstrated to help improve HbA1c control compared to BGMs without a target range indicator*2
Second-Chance® sampling can reduce the need to re-lance and can help save strips.3
Connected with the CONTOUR®DIABETES app to support self-management To Order 2023 Wall Planners or Contour Next Meters Please Contact Grainne O’Connor grainne.oconnor@ascensia.com
Your guiding
Date of preparation:
CONTOUR®NEXT
•
•
• 60-second
•
New Year = New Online Retail Opportunities
2022 was the year that solidified the idea that eCommerce and omnichannel are the future of retail. Even with the return to bricks and mortar stores, Irish retailers and consumers continued to embrace online shopping, with retail eCommerce sales in Ireland estimated at €7.435 million in 2022 (Statistics, 2022).
by Orla Cooney, CEO, Magico

researching more extensively before spending their hard earned cash. Trust is critical and customers will often visit multiple touch points including your bricks and mortar store, social media, offline media and your online store before making a purchasing decision. Consistency and alignment in branding, messaging and promotions across each channel is crucial.
Be Sustainable
In 2022 retailers started to embrace sustainability, but 2023 will see a much greater emphasis on retailers adopting sustainable practices. Consumer expectations are driving this change, indicating that they are more likely to choose a brand with a clear sustainability commitment.
The online Irish Pharmacy market particularly flourished, and Magico forecast an average uplift of 31% among our Pharmacy clients from 2020 to 2022. We have seen an increase in Total Number of Orders, and the Average Order Value for Pharmacy clients has increased notably, by 6%. Also encouraging, is that many of our Pharmacy retailers have achieved a higher Conversion Rate in 2022.
Looking forward, there is much to be positive about and to help you navigate this dynamic eCommerce environment, we explore the top trends for 2023.
Customer Focus
In 2022, high customer acquisition costs led successful retailers to realise the potential of existing customers. To prosper in 2023, retailers should focus on driving up Customer Lifetime Value (CLTV). CLTV takes into account a number of metrics including Traffic, Conversion rate, Purchase Frequency (PF), AOV (Average Order Value), CRR (Customer Retention Rate), Customer Acquisition Cost (CAC) and Gross Margin. By tracking CLTV, retailers can truly evaluate their success.
Ambitious retailers also understand the benefits of becoming indispensable to their customers. Offer online pharmacy services such as book a vaccination, e-prescriptions, online doctor and online photo printing to meet customer needs, secure repeat custom and increase their purchase frequency. Ultimately, creating a loyalty loop.
As competition increases remember that your customer service will amplify your brand. The customer experience from initial online engagement, to answering pre sales queries, responsive delivery and returns policies, and after care service must be flawless. Can you go above and beyond with personal touches such as personalised packaging and thank you for your order notes? Little surprises to delight your customers!
The question at the forefront of every eCommerce team should not be, how can we improve our website but rather how can we better serve our customers.
We are all Mobile Shopping
Almost half of all online purchases made in Ireland last year were through a mobile device - the second-highest rate in Europe, after the UK. Review not only the visual design and UX elements but also the search capability and ease of checkout on a mobile device. Don’t lose out on impulse sales because you are not mobile optimised.
Think Retail not Online vs Physical
As economic conditions continue to challenge, consumers are
With customers engaging with multiple touchpoints, consider supplying in store tablets so customers can browse your online stock, inform the sales team of your online promotions and always ensure that your online merchandising is as strong as your instore.
Personalisation is essential
Your customers will expect some form of personalisation in 2023. This could be introduced simply through your email marketing, or incorporated into your online store but retailers failing to personalise will lose sales to more savvy competitors.
Personalised promotions based on customer preferences, product recommendations based on past purchases, or browsing habits are all effective personalization strategies.
Personalisation is a fruitful means of staying connected with your customers, by showing how much you understand them.
Stay connected with Chatbots 2023 will see increased interaction with Chatbots. Consumers expect that they can access customer support on demand, and will increasingly expect to engage online with retailers with product queries or for post-sales support. Live and automated chat is the one tool that can bridge that gap and bring that rich customer experience of chatting to the instore team, online.
Keep customers engaged with Video
Video is an extremely effective tool for capturing your target customers’ attention, keeping them engaged with your online store and social media. Use video to showcase new products, provide answers to common problems and advise on topical subjects such as flu season, allergies etc.
Everything from paperless offices to plastic-free packaging, consolidation of shipments and reducing the distance packages travel all contribute to how your target audience perceive your brand. It’s time to start thinking Green, and communicating that or you will fall behind.
Offer Payment Flexibility
Offering more than one payment option can help boost conversion rate. Consider flexible installment payment options for high ticket items. Ensure that online shoppers, in particular mobile shoppers can benefit from faster transactions, without sacrificing security.
Data driven clarity
In an increasingly competitive marketplace, when decisions need to be made quickly, eCommerce teams should examine data to assist with this process and break through stalemates. Access to good data will be pivotal in the success of your eCommerce team. Data is no longer used to merely influence product range but to assess how, and where you can improve the overall customer experience.
How to succeed online in 2023
Going online was the challenge during the pandemic. The challenge now is how to grow your online pharmacy business. You need to know your customer both instore and online and be prepared to continuously adapt, addressing changes in consumer behaviour.
Don’t rely on what worked last year or even last season. In a rapidly changing environment consumers expectations are constantly evolving, so stay in tune with your customer and deliver an experience that keeps them coming back for more.
PHARMACYNEWSIRELAND.COM 64 Advertorial
Written

Functionality tailored to pharmacy industry Existing integrations with top pharmacy software Supporting Ireland’s leading pharmacy retailers Bring your pharmacy online & grow fast ocooney@magico.com | www.magico.com IRELAND ' S PHARMACY ECOMMERCE SPECIALISTS CONTACT
Dr Organic – The Destination Beauty Brand

The Dr Organic brand was founded with a focus on blending clean, organic ingredients to create accessible, cruelty-free and exceptionally high-quality beauty and personal care products for today’s conscious consumer.

Established in 2008, the Dr. Organic brand has grown to become synonymous with quality organic beauty and personal care, and today offers a range of extract-led products designed to offer the ecologically-aware consumer a skin and body care solution which delivers for both people and planet.
Working in harmony with nature, Dr. Organic seeks out the treasures of the natural world to locate and capture the purest and most potent organic extracts, exploring the world of beauty from age-old traditions through to supercharged botanicals, to create breakthrough formulations designed to make the user look and feel incredible.
With a range encompassing over 180 products in 25 extract-led options, from exotic vitamin C-rich Guava to soothing Aloe Vera, nourishing Moroccan Argan Oil to indulgent Rose Otto, there is something for every skin type, hair type and phase.
A brand committed to organic, Dr. Organic products have always contained as much organic content as possible. However, passionate about extending the positive impact that organic has the capacity to have on both the skin, body and hair and on the environment, Dr. Organic has worked with the Soil Association to start certifying every product in its range to the COSMOS Organic standard.
COSMOS is the leading global organic standard for the beauty industry, providing consumers with independent validation and certification for the ingredients within a product. and certification to the COSMOS organic standard means that every product within the Dr. Organic range has passed stringent criteria, utilising only the finest quality organic ingredients to create a superior quality product offering.

What is more, because growing organic ingredients is better for the environment, you know that Dr. Organic products are not only better for your skin, but they are also better for our planet too.
Dr. Organic products are created following a stringent ingredient policy. All are made using the finest natural and organic ingredients, with as much water as possible replaced with soothing, nutrient-rich aloe vera to enhance the benefits of each product.
Products are cruelty-free and all are suitable for vegetarians, with the majority suitable for vegans too (only those containing by-product ingredients such as beeswax and honey are not).
Ranges are also free from SLS, parabens, GMO ingredients, palm oil as a proprietary ingredient, artificial preservatives and petrochemicals such as mineral oils and petroleum.
Furthermore, Dr. Organic is committed to zero to landfill and regularly donates to charitable causes. At their head office site in Swansea, Dr. Organic has a biomass boiler system that uses recycled pellets, and the brand reuses all cardboard such as pallet corners and outer boxes by shredding to use as packing materials.
Packaging & Sustainability

As part of a wider drive for sustainability, Dr. Organic has collaborated with the social enterprise, Ecologi, which works to address climate action worldwide. As part of Dr. Organic’s work with Ecologi, the brand is planting trees around the world to offset carbon emissions for both the business and all of its employees, and also supporting a diverse range of environmental projects from preservation of the Amazon Rainforest in Brazil to support for a methane capture project in India. As part of this work, Dr. Organic is offsetting the carbon emissions for the entire workforce through tree planting, turning the whole Dr Organic team officially climate positive.
Dr. Organic is constantly reviewing and improving processes to ensure that their practices and packaging are as ecologically sound as they can be. Committed to playing an integral part when it comes to plastic waste, the most recent improvement has seen changes to all the shampoo, conditioner and body wash products that have all been moved into ocean-bound plastic, which has been collected from the river courses and areas around the coastline which might otherwise have found its way into the ocean.
Today, Dr. Organic is a destination beauty brand for those looking to care for people and planet.
PHARMACYNEWSIRELAND.COM 66 Advertorial


listed on the World Medical Organisation’s (WHO) ‘critical medicines’ list.

The medicines experiencing shortages are for use across a range of health areas, including infections, pain relief, cancer treatment, seizures, mental health, blood pressure, diabetes and hormone replacement therapy (HRT).
Finding solutions to drug supply shortages
Those of us in healthcare know that challenges come with the job. We also learn that the collective ability to anticipate and address challenges can be patchy. These realities are once again apparent when it comes to the very real challenge that is medicine shortages.
From the second half of 2019, the warning lights on medicine shortages were flashing amber. The Medicines Pricing Agreement agreed in December 2021, failed to adequately anticipate the issue. The reimbursement process failed to evolve and adapt to changing market realities. As supply chains creaked, along came Covid, upending medicine flows and exposing the harsh reality that, when it comes to medicines, Ireland is a small fish swimming among the sharks.
These exposures made us vulnerable, vulnerabilities that are now being exposed. What was flashing amber is now firmly signalling red. Patients, pharmacists and doctors are grappling with the fallout.
Pharmacists are increasingly spending their valuable time trying to find new suppliers for everyday medicines, simultaneously dealing compassionately with patient needs.
Indicators pointing to this problem have been clear.
Supply chain experts, the Resilinc Corporation, have estimated that there was a 74% increase in pharmaceutical supply chain disruption in 2021, and that percentage has been compounded by an even more turbulent 2022.
A recent report by the EU Commission on shortages indicated that 97% of medicine shortages are off-patent medicines, with the average time since patent expiry being 19 years. 52% of these shortages involved generic medicines which indicates that off-patent branded medicines are more of an issue than might be expected. An IQVIA white paper on the topic suggests that as much 24% of all shortages are for single source off-patent (brand or generic) medicines.










The Health Products Regulatory Authority (HPRA) has been pro-active and ahead of many EU regulators by publishing a weekly update and where possible, advising Marketing Authorisation Holders (MAHs) on the available regulatory solutions. The Irish Pharmacy Union is also tracking the numbers.
At surface level, the numbers give a glimpse of the problem. At Azure Pharmaceuticals, we decided to go deeper, publishing in December the Medicine Shortage Index. Our analysis shows that while medicine shortages are a global challenge, Ireland is increasingly an outlier compared to neighbouring countries.
187 different medicines are currently out of stock, including 11 medicines listed on the


GROWING THE AZURE PORTFOLIO IS www.azure-pharma.com
40% of the medicines out of stock in Ireland this month have just a single supplier. The EU wide singled-sourced average is 25%. Such a divergence considerable, reflecting the smaller market size of Ireland and the impact of industry pricing agreements relating to the price required for generic launches of older medicines. This causes a significant challenge for pharmacists to both procure suitable medicines and to deal with patient expectations in a hospital or community setting.
0
35% 38% 38% 41% 43% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45%
When considering medicine vulnerabilities and what the key drivers for shortages are, there are a number of factors. However, two issues contribute to 76% of all shortages according to the European Commission report.
World Medical Organisation’s (WHO) ‘critical medicines’ list. The medicines experiencing shortages are for use across a range of health areas, including infections, pain relief, cancer treatment, seizures, mental health, blood pressure, diabetes and hormone replacement therapy (HRT).
The most common reason cited in the report is manufacturing or quality related issues which can include Active Pharmaceutical Ingredient (API) shortages. API shortages are now of increasing concern. Downward pressure on pricing has forced manufacturers to move to low-cost API sources in Asia, leaving certain APIs fully supplied by one or two manufacturers.
40% of the medicines out of stock in Ireland in December had just a single supplier. The EU wide singled-sourced average is 25%. Such a divergence is considerable, reflecting the smaller market size of Ireland and the impact of industry pricing agreements relating to the price required for generic launches of older medicines. This causes a significant challenge for pharmacists to both procure suitable medicines and to deal with patient expectations in a hospital or community setting.
When considering medicine vulnerabilities and what the key drivers for shortages are, there are a number of factors. However, two issues contribute to 76% of all shortages according to the European Commission report.
The most common reason cited in the report is manufacturing or quality related issues which can include Active Pharmaceutical Ingredient (API) shortages. API shortages are now of increasing concern. Downward pressure on pricing has forced manufacturers to move to low-cost API sources in Asia, leaving certain APIs fully supplied by one or two manufacturers.
A recent study by the German association, Progenerika indicated that there is no EU production capacity for 17% of required APIs and that technical knowledge used in the manufacturing processes may no longer be available in Europe. EU pressure to comply with environmental legislation is also putting pressure on the manufacturing methods for older APIs. The cost to re-invent these processes to current compliance levels is exacerbating the exit of API manufacturers in Europe to less rigorous jurisdictions.
The impact of API consolidation and the move to low-cost environments has been seen most recently with paracetamol shortages globally. In Ireland, over the last six months, prescription packs of paracetamol have been consistently in short supply, as API output cannot match surging demand and price per kg of API has sky-rocketed in the last 12 months.
Closer to home, the race to the bottom is also biting. In the case of paracetamol, 100 paracetamol tablets have a reimbursement price in Ireland of ¤1.73 while the average price for the same medicine across Europe is ¤3.51, a 51% differential. With a global shortage of paracetamol API and the opportunity presented in the free pricing OTC segment which commands 25-30% more per tablet (500mg) than the reimbursed version, it is little wonder that the Irish market has suffered a shortage of more than 6 months’ duration. In times of shortage, medicines go where they get the best price and right now, that is not Ireland.


68 News
A recent study by the German association, Progenerika indicated that there is no EU production capacity for 17% of required APIs and that technical knowledge used in the manufacturing processes may no longer be available in Europe. EU pressure to comply with environmental 20 40 60 80 100 120 140 160 180 200
SKUs out of stock % of SKUs from single source
SKUs out of stock Single source off-patent % from single source
it is little wonder that the Irish market has suffered a shortage of more than 6 months’ duration. . In times of shortage, medicines go where they get the best price and right now, that is not Ireland.
Wholesale pricing for Paracetamol 100 pack:
Brand/Alternative brand name Strength Pack Size IE FIN UK € DK NL PO SWE Average
Paracetamol tabs 500mg 100 1.60 3 2.8 3.67 NA NA 4.57 3.51
Wholesale pricing for Paracetamol 100 pack:





Ireland’s reimbursement climate for older medicines is exacerbating the situation. Our Medicine Shortages Index highlights the reimbursement disparity between Ireland and other EU markets in relation to some of the older medicines that have been in short supply here (see examples below).
The onerous requirement of the 2021 Framework Agreement for all generic medicines to be 60% below the brand, even when the brand price was set in the 1980s, is creating a market that no generic company can viably enter with a second source of supply. The one-way movement of price downwards and lengthy delays in adjudicating on hardship cases is a deterrent for suppliers to remain here and deprives the market of the opportunity to access alternative suppliers.
The over-reliance on Exempt Medicines is masking of the seriousness if the situation. Current estimates indicate that over three million packs of unlicensed medicines are being dispensed in Ireland every year. EMP supply should be used as exceptions rather than the norm – the reimbursement of these
medicines at higher prices undermines the licensing process and disincentivises suppliers, as well as requiring patients to foot the cost of the EMP.
Solutions
The European Medicines Agency (EMA) is now taking a pre-eminent role in attempting to prevent or limit the impact of such shortages, including requiring MAHs to register and inform of impending issues. The HPRA is a part of this effort.
Ireland’s reimbursement climate for older medicines is exacerbating the situation. Our Medicine Shortages Index highlights the reimbursement disparity between Ireland and other EU markets in relation to some of the older medicines that have been in short supply here (see examples below). The onerous requirement of the 2021 Framework Agreement for all generic medicines to be 60% below the brand, even when the brand price was set in the 1980s, is creating a market that no generic company can viably enter with a second source of supply. The one-way movement of price downwards and lengthy delays in adjudicating on hardship cases is a deterrent for suppliers to remain here and deprives the market of the opportunity to access alternative suppliers.
We can and must do more. That starts with an early warning system. We need to better riskmitigate when it comes to medicines because the later we know, the longer the catch-up. Equally, the timelines and functioning of the reimbursement process needs to be reset to reflect the new realities.
Opening the door to alternative sources for medicines can be transformative. We need to actively encourage licensed alternative sources for single-source medicines and revisit the 2021 Framework Agreement, inserting a clause specifically pertaining to older single-source
medicines which have had supply issues to encourage second sources.


In the same way that generic Reference Prices are reduced to the median of EU markets, increasing prices using the same mechanism to the EU average should be deployed.
The IPU and others have rightly made the point that we need to give pharmacists more meaningful powers to respond, just as has been done in the UK and elsewhere.
Finally, manufacturing. The take-away from Covid is the need to broaden our manufacturing base and de-risk our dependency on particular markets. It is longsince time to consider specific measures aimed at encouraging the manufacturing of essential APIs and finished dose pharmaceuticals in Ireland and elsewhere in Europe.
We are paying the price for not paying the price. Medicines will continue to follow the best prices and until Ireland can better compete, shortages will continue. It is time for collective action.
69
MEDICINES
SSURED
The over reliance on Exempt Medicines is masking of the seriousness if the situation. Current estimates indicate that over three million packs of unlicensed medicines are being dispensed in Ireland every year. EMP supply should be used as exceptions rather than the norm – the reimbursement of these medicines at higher prices undermines the licensing process and disincentivises suppliers, as well as requiring Brand/Alternative brand name Strength Pa ck Size IE FIN UK € DK NL PO SWE Average Paracetamol tabs 500mg1001.6 3 2.83.67 NANA 4.573.51 Diazepam tabs 5mg 902.248.943.1410.523.63.333.195.45 Prednisolone tabs 5mg 983.067.443.719.034.95.055.35.90 Tamoxifen tabs 20mg 304.5811.723.5210.3510.88.374.768.25 Alprazolam 0.25mg1002.22 NANA 20.38 6 5.77.9710.01 Spironolactone 25mg1004.3313.247.9812.38 7 6.783.668.51 Doxazosin XL 4mg 283.68 5.887.313.367.6917.367.55 Estradot (Vivelle Dot) 50mcg/24 8 6.378. 214.1313.56 6.5910.62 Estradot (Vivelle Dot) 75mcg/24 8 7.610. 04 17.54 8.1611.91 Evorel (Femsete) 50mcg/24 8 5.21 4. 56 10 6.59 Lenzetto Spray 1.53mg 568.5211.178.1211.7111.41206.311.45 Divigel/Sandrena 0.10% 28 doses 9.3316.846.8812.61 12.11 Oestrogel 0.6g80g 5.811.95.6514.0814.08 11.43 Vagifem 10mcg2410.3211.09 37.2223.2820.72 23.08 Vagirux(Rewellfem/Formyra) 10mcg2410.32 27.7623.2826.96 26 Epipen Jr 0.15mg0.3ml32.2553.2840.3571.2248.4649.6864.7354.62 Anapen 0.15mg0.3ml35.41 40.35 NANA 46.0434.1140.16 Creon Caps 250005016.9625.13 40.265520.7421.05 Creon Caps 1000010016.2922.58 4424.11 30.23 www.azure-pharma.com
MORE
Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
Puressentiel Purifying Spray
PROVEN TO GET RID OF BACTERIA, VIRUSES AND UNPLEASANT ODOURS, 100% NATURALLY
Pure Indoor Air for Immunity and Respiratory Health
Outdoor pollution has rightly received a lot of attention in recent years, but what about indoor pollutants in houses, offices and shops?
A report from the European Lung Foundation identifies more than 900 different compounds found in indoor pollution, with
some irritants occurring at levels two to five times higher than the air outside.
The pandemic has significantly raised awareness of the importance of indoor air quality. Research(2) from Puressentiel, producers of a range of 100% natural, evidence-backed essential oil solutions

Immune and respiratory health – what’s the relationship?
for everyday health and wellness needs – has found that 8 out of 10 people correctly believe air quality in their home can impact their wellbeing, including respiratory health and breathing as well as the health of the immune system –which protects against bacteria, viruses, fungi, and toxins.
Immune health and respiratory health are linked to air quality and the viral and bacterial load in the air we all breathe in.
Some indoor pollutants which affect indoor air are obvious like cooking fumes, insecticides and paints and varnishes etc. Even more insidious, however, are indoor pollutants such as moulds, which are present in 10 to 50% of buildings, including houses, offices and shops, and particularly in homes, and house-dust mites in furniture, carpets and pet hairs. These irritants often go unnoticed until they trigger wheezing and other respiratory symptoms, as well as compromising our immune health. (1)
1. https://europeanlung.org/en/information-hub/keeping-lungs-healthy/indoor-air-pollution/ 2. Perspectus Global; 1,109 consumers questioned; Autumn 2022
Puressentiel Purifying Spray
Puressentiel ranges providing immune health support

people try to minimise poor indoor air quality by opening windows, avoiding drying washing indoors and using natural cleaning products. Cosmetic measures such as burning synthetic candles or air fresheners are a no, no as they add even more pollutants to the indoor air.
Address the root cause by improving air flow, avoiding or minimising use of known sources of indoor pollutants and adopting measures which remove or neutralise indoor pollutants such as moulds, fungi, bacteria, viruses and house dust mites, which have a significant impact on respiratory health.
Puressentiel Air Purifying and Respiratory range ticks all the boxes and provide an evidence-backed natural solution to tackle respiratory and immune health through their anti-inflammatory, antibacterial, anti-viral and anti-fungal properties, with a direct action on the respiratory passages. Plant extracts and essential oils with recognised benefits for respiration and symptom relief include eucalyptus, echinacea, camphor, clove, and menthol.
• Echinacea has long been used to treat cold symptoms and the European Medicines Agency (EMA) concluded that
the most recent clinical trials confirm it “can prevent and improve the symptoms of a cold more rapidly than placebo when taken early.”(3) Vapourised essential oils and plant compounds including eucalyptol, limonene, and terpinene — which feature in Puressentiel’s Purifying & Respiratory Range — significantly reduce levels of airborne coronavirus and other pathogens – all good news for helping to keep our immune health fit.(4)

• A clinical trial of Puressentiel Purifying Air Spray, which contains 41 essential oils involved 53 volunteers with a history of wheezing and other breathing issues and found improvements in two key measures of lung function — forced vital capacity (FVC) and forced expiratory volume (FEV) — in just four weeks(5). Laboratory research found that Puressentiel Purifying Air Spray reduced the number of viable cells of four common bacteria, including staphylococcus and E-coli, by a factor of 100,000 (6) .
Viable cells of candida and asperigillus niger were reduced by a factor of
10,000(7). In another laboratory test, the Puressentiel Purifying Air Spray was shown to kill house-dust mites — a common cause of allergic wheeze and asthma — in less than an hour and have a repellency rate of 97% seven days after spraying.(8) Clinical trials in patients with mild to moderate asthma confirm the Puressentiel Purifying Air Spray is well tolerated and causes no inflammation of the airways. That’s why in France, around 20% of doctors prescribe or recommend Puressentiel Purifying Air Spray.
Overall, essential oils, such as those contained in the Puressentiel Respiratory and Purifying ranges, safely and effectively tackle hazardous indoor pollution whilst helping to protect respiratory and immune health. For more information see: https:// uk.puressentiel.com/collections/respiratory
So, what are the alternatives?
3. https://www.ema.europa.eu/en/medicines/herbal/echinaceae-purpureae-herba 4. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjZvZeUzIL6AhULUcAKHeVvBQsQFnoECAkQAQ&url=https%3A%2F%2Fwww.mdpi.com%2F2079-6382%2F11%2F3%2F393%2Fpdf&usg=AOvVaw11vqESB5l-NfRUaGHfKsPk 5. Data on file (GB cahier aroma page 8&9) 6. Bactericidie PURESSENTIEL 2009 Spray Assainissant. On file 7. Conclusion fongicidie PURESSENTIEL Spray Assainissant On file 6. Data on file BAT PAO 21.12.2015_16h50_PURESSENTIEL_GB.PDF
Many
Nature’s Medicine: Results from latest Re-MIND Study
A new study, titled ‘Re-MIND’, from the Nutrition Research Centre Ireland (NRCI) at South East Technological University (SETU) finds that patients with Alzheimer’s disease who consume a nutritional supplement containing fish oil, carotenoids and vitamin E benefit from targeted nutritional intervention.
Professor John Nolan, Principal Investigator, at the Nutrition Research Centre Ireland
carers) of patients receiving the active intervention.
Professor Nolan adds, “The many scientific discoveries over the last two decades have led us to this point. Our earlier published studies clearly demonstrated that carotenoids are related to cognitive function in the general population across all ages. When combined with omega-3 fish oil we see improved response of the carotenoids in blood, and improvements in cognitive function can be achieved with supplementation of these nutrients.
The authors conclude that this supplement formulation should be considered in the overall management of Alzheimer’s disease.
The clinical trial was led by Professor John Nolan and Dr Rebecca Power of the NRCI at SETU along with Professor Ríona Mulcahy, Consultant Physician in General and Geriatric Medicine at University Hospital Waterford, along with many other expert colleagues.
Professor Nolan is Principal Investigator, at the Nutrition Research Centre. Explaining a bit of his background leading up to this ground-breaking trial, he told Irish Pharmacy News “My work in this field really began 24 years

ago when I started to look at the connection between eye health and nutrition. Specifically within the field of Age-Related Macular Degeneration.
“I discovered that there are certain nutrients called carotenoids, which affect eye health and embarked on a number of trials and further research. Hence, these nutrients are now known as the gold standard in eye care.
“The idea is simple. People who are at risk of AMD have deficiencies in three carotenoids (natural plantbased pigments found in fruits & veg), especially meso-zeaxanthin, the central macular pigment. These pigments act as a protective layer, filtering out harmful blue light and reducing oxidative damage at
the back of the eye. What we’ve discovered is how to rebuild these pigments and by doing so, we can improve vision.
“We’ve learned that the three carotenoids – lutein, zeaxanthin, and meso-zeaxanthin – which form the macular pigment in the eye also play a role in the brain by improving cognitive function, likely due to their antioxidant and antiinflammatory properties. It’s very exciting in terms of what we can do with the right nutrition”
Re-MIND (Memory Investigation with Nutrition for Dementia), a follow-on from a breakthrough study published by the NRCI in 2018, was a double-blind, placebo-controlled, clinical trial researching patients with mild to moderate stage Alzheimer’s disease.
Individuals consuming the nutritional supplement demonstrated statistically significant increases in their blood concentrations of carotenoids (plant-based pigments that give fruits and vegetables their colours), omega-3 fatty acids (the building blocks of our cells), and vitamin E (one of four essential fat-soluble vitamins) after 12 months in comparison to patients receiving a placebo.
The main outcomes of the trial included slower rates of disease progression, and greater improvements in mood and memory (as reported by the
“Then, we observed that these positive effects can also be achieved in individuals with mild cognitive impairment. In 2014, we reported that patients with Alzheimer’s disease have very low levels of carotenoids compared to aged matched controls, so the results of the Re-MIND trial are compelling when all the evidence is considered.”
So what is so unique about this nutritional supplement?
Professor Nolan tells us, “Really the innovation behind this is the synergistic effect, the combination of these particular nutrients and discovering the synergy of using them together. Now, for the first time, patients and their carers can access this treatment in real time.
“It is important therefore, as we move on from the results of this study, that patients have access to this nutritional supplement. It must be pointed out that we can’t leave these patients to operate under their own devices so community pharmacists will play a huge role in helping to get the information out there and to support their customers in making the right management decisions.
“Furthermore, we really need to see major investment in this sector. We need to stop trying to find the ‘silver bullet’ but realise that a change in lifestyle in a way that is meaningful, is the way forward.
“This nutritional supplement is available now, pharmacists should be engaging with the customers and informing them as to its availability and the difference it can make to quality of life. It really is nature’s medicine.”
PHARMACYNEWSIRELAND.COM 72 Brain Health
“It is important therefore, as we move on from the results of this study, that patients have access to this nutritional supplement. It must be pointed out that we can’t leave these patients to operate under their own devices so community pharmacists will play a huge role in helping to get the information out there and to support their customers in making the right management decisions”





Helps to support normal brain function* Unique, patented food supplement with carotenoids, omega-3 fatty acids and vitamin E Clinically tested in 'Memory Investigation with Nutrition for Dementia' Trial at Nutrition Research Centre Ireland, SETU, Waterford All natural ingredients, no side-effects Diabetic friendly and gluten free Approved by Supplement Certified *DHA contributes to the maintenance of normal brain function. The beneficial effect is obtained with a daily intake of 250mg DHA. Food supplements should not be used as a substitute for a varied and healthy diet. Targeted Brain Nutrition. Backed by Science. Available to order from To arrange training call Karen: 087-7637489
STIs, HIV and Effective Screening
Sexually transmitted infections (STIs) are a major public health concern, and an increasing cause of ill health globally. STIs are spread through intimate sexual contact, including unprotected vaginal, anal and oral sex with an infected partner. Even protected sex can be a risk of contracting some sexually transmitted infections.
and Pelvic Inflammatory Disease (PID) in females. PID is associated with an increased risk of tubal factor infertility, ectopic pregnancy and chronic pelvic pain. Diagnosis can be made on first void urine in males and vulvovaginal or endocervical swab (less sensitive) in females. Vulvovaginal swabs can be provider or self-taken. In MSM, as well as first void urine, pharyngeal and rectal sites should also be tested.”
Turning to Hepatitis B, a viral infection that infects the liver and is a major cause of serious liver disease, Theresa notes, “Hepatitis B affects millions of people worldwide. It is vaccine preventable. Hepatitis C is a viral infection that can cause long-term liver disease, such as liver cirrhosis and liver cancer. There is currently no vaccination against hepatitis C. Most cases of hepatitis C are found in people who inject drugs, however, the number of cases of sexually transmitted hepatitis C diagnosed in men who have sex with men (MSM) in Ireland, has increased.
2021 between 01/01/2021 and 21/08/2021, compared to the same time- period in 2020 (349 cases).
She adds, “Human Immunodeficiency Virus (HIV) is a virus that attacks the human immune system and weakens its ability to fight infection and disease. Over 6,000 people are estimated to be living with HIV in Ireland and approximately 15% of people with HIV in Ireland are unaware they have the condition, because they have either not been tested, or developed HIV since their last test.
about sexually transmitted infections and their associated screening.
STIs usually affect the genitals, anus, or mouth, but can also affect other areas of the body. Many STIs have no signs and symptoms and if left untreated have the potential to cause significant morbidity in women and men, including infertility, ectopic pregnancy and other medical conditions, which is why STI screening is so important.
“Some activities pose higher risk than others including unprotected sex with casual partners, multiple partners, with a partner who has taken risks - had unprotected sex with casual partners or with a partner who has injected drugs. Men who have unprotected anal sex with other men (MSM) are also at higher risk,” says Theresa.

Chlamydia trachomatis (CT, chlamydia) is the most commonly diagnosed bacterial sexually transmitted infection (STI) in Ireland, and most cases occur in young people under the age of 25. Theresa explains, “Often asymptomatic in both males and females, symptoms in males can include dysuria and a urethral discharge, and in women, vaginal discharge, intermenstrual bleeding and post coital bleeding. Infection can lead to epididymitis in males
Gonorrhoea (Neisseria gonorrhoeae) is the second most common bacterial sexually transmitted infection in Ireland, and is found most frequently in young people under the age of 25 and in men who have sex with men (MSM). Infection may involve the genitals, mouth, or rectum. Infected men may experience pain or burning with urination, discharge from the penis, testicular pain and epididymitis.
Symptoms in women can include vaginal discharge, intermenstrual bleeding, post coital bleeding and PID. Rectal infection can lead to proctitis. Diagnosis can be made on first void urine in males and vulvovaginal or endocervical swab in females. Vulvovaginal swabs can be provider or self-taken. In MSM, pharyngeal and rectal sites should also be tested. Gonorrhoea is a notifiable disease.
Theresa continues, “The Nucleic Acid Amplification Test (NAATs)/ PCR is the gold standard for chlamydia and gonorrhoea testing. The type of test may vary at different laboratories.”
Genital herpes is a viral infection caused by the herpes simplex virus (HSV). There are two types: HSV-1 and HSV-2. “Type 2 is most commonly associated with genital infection. Type 1 has also been found to cause genital infection but is more commonly associated with oral herpes (cold sores). Genital herpes is common in Ireland, and is mostly diagnosed in young women.
“The diagnosis can be made clinically but should be confirmed with a HSV NAAT swab of the lesions to determine if HSV-1 or HSV-2.”
“Many cases have been in MSM who are also infected with HIV.
“Hepatitis C testing (HCV) should be considered part of routine sexual health screening for MSM; people living with HIV; commercial sex workers and people who inject drugs (PWID). Partners should also be considered for HCV testing.
Trichomoniasis (TV) is a sexually transmitted infection (STI) caused by a protozoan-Trichomonas vaginalis. Most cases are found in women, and although it can affect both men and women, infection in men is uncommon. TV can infect the vagina and cervix in women, and the urethra and underneath the foreskin in men.
“In women it usually presents with a vaginal discharge which may be offensive with an associated vulvitis /vaginitis. Men usually present as sexual contacts of women with infection, and may present with symptoms of urethritis including dysuria and urethral discharge. Trichomoniasis is a notifiable disease.”
Syphilis is a sexually transmitted infection caused by a bacterium called Treponema pallidum. There were 611 cases reported in Ireland in 2020. The highest rate in males and females was in the 30-34year age group, 75.6 per 100,000 population and 6.9 per 100,000 population, respectively. Most new cases of syphilis are among men who have sex with men.
While the number of STI notifications decreased in Ireland in 2020, most likely due to the impact of the COVID-19 pandemic, early infectious syphilis (EIS) is on the increase, with a 43% increase (498 cases) reported by HPSC in
“Approximately half of new cases of HIV in Ireland are among men who have sex with men (MSM), the other half being mostly heterosexual men and women, and people who inject drug. HIV is diagnosed with a blood test. HIV testing is available in many health care settings including STI clinics, GPs and student health clinics. Many NGOs offer HIV testing in Ireland, including rapid HIV testing in community venues. HIV is a notifiable disease.”
HIV Prevention: PrEP and PEP/PEPSE Pre-exposure prophylaxis (PrEP) is the use of oral antiretroviral therapy in HIV negative people to reduce the risk of HIV infection. Theresa told us, “PrEP is taken by HIV negative people prophylactically before having sex (pre-exposure) and after sex, to prevent HIV. The window period for HIV is 45 days. PrEP is available free of charge through the HSE to people who meet the clinical eligibility criteria and who are deemed to be at substantial risk of acquiring HIV.
“Post-exposure prophylaxis (PEP) is short-term antiretroviral treatment to reduce the likelihood of HIV infection after potential exposure, either occupationally or through sexual intercourse. PEP may prevent infection with HIV developing. A full course of PEP (two or three antiretroviral HIV drugs) is for 28 days (4 weeks). The medication must be started within 72 hours of possible exposure to HIV infection to be effective. The term PEPSE is sometimes used, and stands for PEP after Sexual Exposure.”
HIV can be treated effectively with antiretroviral medications which stops HIV reproducing in the body. When taken properly, HIV
PHARMACYNEWSIRELAND.COM 74
We spoke with Theresa Lowry Lehnen, RGN, RNP, BSc, MSc, PG. Dip. Ed, M. Ed, PhD Clinical Nurse Practitioner and Associate Lecturer South East Technological University to find out more
HIV
An interview with Theresa Lowry Lehnen (PhD), Clinical Nurse Practitioner and Associate Lecturer South East Technological University
Who is eligible for free PrEP?
HIV negative individuals who are aged 17 years or older, have a PPSN and fall into one of the categories set out below:
1. Men who have sex with men or transgender women who have sex with men who are:
Sexually active with likelihood of remaining sexually active in the next 3 months AND one of the following:
Reported condomless anal sex with at least two partners over the last 6 months
Episode of documented or reported acute STI over the last 12 months
Documented or reported use of HIV post-exposure prophylaxis following sexual exposure (PEPSE) over the last 12 months
Reported engagement in chemsex over the last 6 months
2. Individuals having condomless sex with a HIV positive person who is not suitably suppressed on antiretroviral therapy specifically:
Where the person living with HIV is not on antiretroviral therapy
Where the person living with HIV has initiated antiretroviral therapy but has not yet achieved virological suppression
Where the person living with HIV has loss of virological control on antiretroviral therapy and the risk of HIV transmission has been deemed by a consultant physician specialising in HIV Medicine to be substantial and warrant PrEP for the HIV-negative partner
3. Other heterosexual men, heterosexual women considered by a senior clinician specialising in HIV Medicine to be at substantial risk for sexual acquisition of HIV
treatment reduces the chance of a person living with HIV passing it on to someone else. When a person living with HIV is on treatment and the viral load in the body is ‘undetectable’, HIV cannot be transmitted to sexual partners. This is known as ‘undetectable’ equals ‘un-transmittable’ (U=U).
Current Situation and Outlook
Theresa concludes, “The timeliness of treatment for STIs can have consequences for the individual and increase the likelihood of infecting others. Given the high burden of STIs, and the risk of

medical complications associated with these infections, screening, accurate diagnosis, and timely and appropriate treatment are critical. The testing and treatment of sexual partners is important in preventing the spread of STIs, decreasing the rate of reinfection, and preventing medical complications of asymptomatic infections.
“The continuing rise in STIs annually in Ireland is a worrying trend. The key message from the HSE HPSC is for people to get tested regularly, use condoms for vaginal, oral and anal sex and reduce the number of partners
and overlapping partners. People should get tested for STIs if they have symptoms of an STI, change their sexual partner, have multiple or overlapping partners or their partner has an STI.
“Stigma causes many people to avoid seeking STIrelated services because of negative experiences such as discrimination, indifference, and overt hostility in health care settings. In addition to effective public health infrastructures and the technologies needed for diagnosis and treatment, global STI prevention and control efforts
Table 1: Eligibility for PrEP (HIV Pre Exposure Prophylaxis in Ireland) (HSE Guidance)2
require renewed commitment to systematic programmatic approaches to prevent or reduce STI-related stigma.”
The HSE, SHCPP’s Sexual Health Promotion Training Strategy 2019-2029, directs the activities and funding of the SHCPP, and aims to address the training of professionals enabling them to respond to the sexual health education and information needs of their service users. The strategy enables the integration of sexual health promotion into core support services within health, social care, education, community and youth work so that service users can be supported to make good sexual health choices.
She adds, “Sexual health is an essential component of overall health. Effective STI prevention and control emerges from a holistic, sexual health perspective involving many levels of society and a variety of approaches. An integrated approach is required that involves taking action throughout the entire population and at all levels of the prevention and care continua. Further advancement in technology, specifically the development of rapid, sensitive and specific point-of-care testing, will provide additional tools for STI diagnosis and control. These actions can lead to better prevention, screening, and treatment.”
New Pharmacy Handbook on HIV
On World AIDS Day, the International Pharmaceutical Federation (FIP) has published a new handbook for pharmacists on the prevention, screening and management of HIV.
“While immense progress has been made in terms of prevention, testing, treatment and quality of life for people living with HIV, the virus remains both a major global health threat and burden for individuals, health systems and societies. Concerted efforts by all stakeholders and healthcare providers are essential to control this epidemic, but pharmacists certainly have an important role to play,” said Gonçalo Sousa Pinto, FIP lead for practice development and transformation, and co-editor of the handbook. The handbook
was developed in collaboration with an international advisory group of experts from Brazil, Canada, Malaysia, South Africa, the USA and the International AIDS Society.
Mr Gonçalo Sousa Pinto added that, as with many prevention strategies, pharmacists are ideally placed to support patients with ways to reduce the risk of transmission, including advising on safer sex practices. In some countries, pharmacists are contributing to improved access
to, and supporting the use of pharmacological approaches to prevention such as pre- or postexposure prophylaxis, and also to harm reduction strategies for intravenous drug users.
They are also contributing to screening and testing, helping to identify cases that need to receive treatment and care. A section of “HIV prevention, screening and
management: A handbook for pharmacists” is dedicated to the public health roles for pharmacists in this disease area. These include campaigning to raise community awareness, tackling stigma, preventing sexual violence (which is often associated with HIV and other sexually transmitted infections) and supporting its victims, and advocacy work.
PHARMACYNEWSIRELAND.COM 75
News
Topic Team Training – Sports Injuries
A community pharmacy environment that fosters teamwork ensured high levels of consumer satisfaction. This series of articles is designed for you to use as guide to assist your team in focusing on meeting ongoing CPD targets and to identify any training needs in order to keep the knowledge and skills of you and your team up to date.
Points to consider when taking a history from the patient include:
• Was the patient treated immediately or later after the injury?
• What treatment was received?
• Was the patient able to continue with their activity?
• What was the intensity of the activity leading up to sustaining the injury?
• Has this or any other injury occurred before?
• Is this an acute or chronic injury?
Some of the most common sports injuries include:
• Sprains,
• Strains,
• Cuts and bruises,
• Bone fractures and breaks,
• Tendonitis (inflammation of a tendon),
• Blisters, and
The below information, considerations and checklist provides support to enable you to run a team training session and identify opportunities for learning within the topic of Sports Injuries.
Sport injuries can be acute or chronic. Acute sports injuries occur as a result of a sudden impact or awkward movement. Examples include a cut to the skin or a sprained ankle. Chronic sports injuries develop over time, often due to continual use of the same joints or muscle groups.
Chronic sports injuries can occur due to bad technique or occasionally structural abnormalities, such as an inherited bone or muscle problem. These injuries should be further investigated by a medical professional to determine the cause and to prevent the injury getting worse.
Consider:
• Head injuries.
Whilst the figures regarding sports injury presentation to A&E are of note, community pharmacies will collectively see many more minor sports injuries.
Common risk factors for sports injury include inadequate warm up, fatigue, over intensive training, unsuitable equipment and a changed environment (e.g. very hot weather, poor lighting, or physical contact with another person or equipment).
Use non-steroidal antiinflammatory drugs (NSAIDs) to treat pain, reduce any localised heat, decrease swelling and improve mobility. Oral pain relief normally starts soon after the first dose and the full analgesic effect occurs within a week.
Topical NSAIDs can be applied directly to the site of the injury.
Topically applied NSAIDs penetrate the skin and result in therapeutically significant concentrations in underlying inflamed soft tissues, joints and synovial fluid, probably entering the synovial joint mainly via systemic circulation.
In tendinopathies (painful tendons), inflammation plays a lesser role and so NSAIDs have little influence on healing but they can help with short-term analgesia. If used in the first seven days in sprains, strains or ligament tears NSAIDs can also be used to limit pain and swelling thus increasing the chances of the patient regaining function and returning to activity sooner.
Patients should be advised to take NSAIDs with food. Following that advice, short-term use of NSAIDs is safe but patients should be advised that long-term use increases the risks of systemic side effects (such as gastrointestinal and cardiovascular effects).
Injuries to the head, cervical spine, or the thoracic or abdominal organs should be treated as potentially life-threatening and referred to secondary care urgently. A patient with suspected concussion following a blow to the head, face, neck or where an ‘impulsive’ force may be transmitted to the head, who presents subsequently with changes in behaviour, vomiting, dizziness, headache, double vision or excessive drowsiness requires urgent medical assessment.
Further Resources:
Actions:
Ensure efficient sign posting to discreet consultation areas within the pharmacy for further help and advice
Ensure that I know the recommendations for each OTC treatment which impact a customer’s mental health
The importance
My pharmacy assistants can meet the points in this training checklist.
Do any of us suffer from depression in the pharmacy team? Are some individuals more affected than others? If so, why?
Are we confident about raising issues of depression and mental health with customers?
Can we provide good advice on dealing with depression?
76 | PHARMACYNEWSIRELAND.COM
The different types of sports injuries, causes and symptoms Know how to advise customers on preventative measures to avoid injury Be able to pair the right treatment to the relevant issue
of good
Train the team to meet all the above considerations for everyone When to refer customers to the pharmacist.
physical activity
Key Points:
Check your pharmacy team are aware and understand the following key points:
The pharmacy team knows to be sensitive in dealing with customers who may feel vulnerable as a result of poor mental health
The team knows which groups of customers are likely to be at risk of depression and other mental health conditions, such as anxiety
YourMentalHealth.ie
YourMentalHealth Information Line 1800 111 888
Powerful anti-inflammatory pain relief

For muscle and joints when associated with strains and sprains.
CONTAINS DICLOFENAC TRIPLE EFFEC T


Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Please refer to the Summary of Product Characteristics (SmPC) for full details of Prescribing Information.
Motusol Max (diclofenac sodium) 2% w/w gel Abbreviated Prescribing Information. Presentation: White to almost white, homogeneous gel. 1g of gel contains diclofenac as 23.2mg diclofenac diethylamine corresponding to 20mg of diclofenac sodium. Indications: Local symptomatic treatment of pain in acute strains, sprains or contusions following blunt trauma. For short term treatment only. Dosage and administration: For cutaneous use. Apply to affected parts of the body thinly and gently rub into skin. Wash hands after application unless area to be treated. Allow gel to dry on skin before applying bandages. Adults and adolescents aged 14 years and over: depending on the size of the affected site, apply a cherry to walnut size quantity 2 times a day (preferably morning and evening). Should not be used for longer than 1 week without medical advice. Children: no data in children and adolescents under 14 years of age. Elderly: no dosage adjustment required. Monitor patient carefully. Renal and hepatic impairment: no dosage adjustment required. Contraindications: Hypersensitivity to active substances or excipients; patients with a history of hypersensitivity reactions such as asthma, bronchospasmus, urticaria, acute rhinitis in response to acetylsalicylic acid or non- steroidal anti-inflammatory drugs (NSAIDs); open injuries, inflammations or infections of the skin as well as on eczema or mucous membranes; in the last trimester of pregnancy; in children and adolescents under 14 years of age. Precautions and warnings: Systemic undesirable effects cannot be excluded if applied on larger areas of skin over a prolonged period of time. Must only be applied to intact, not diseased or injured skin. Must not come into contact with eyes and oral mucous membranes. Must not be taken orally. May be used with non-occlusive bandages, but not with airtight occlusive dressing. Consult doctor if symptoms worsen or do not improve after 3-5 days. Patients suffering from asthma, hay fever, swelling of nasal mucous membranes (so called nasal polyps) ) or chronic obstructive pulmonary disease, chronic


respiratory infections (particularly associated with hay fever-like symptoms), and patients with hypersensitivity to painkillers and anti-rheumatic medicinal products of all kinds are rather at risk to asthma attacks (so called analgesic intolerance / analgesic asthma), to local skin or mucous membrane swelling (so-called quincke edema) or to urticaria than other patients when treated with Motusol Max. In these patients, Motusol Max may only be used under certain precautions (emergency preparedness) and direct medical supervision. The same applies for patients who are also allergic to other substances e.g. with skin reactions, itching or urticaria. Discontinue treatment if skin rash occurs. Photosensitivity can occur with the appearance of skin reactions after exposition to sunlight. Avoid children coming into contact to the skin areas where the gel has been applied. Contains butylhydroxytoluene which may cause local skin reactions or irritation to the eyes and mucous membranes. Contains fragrance with benzyl alcohol (0.15mg/g), citral, citronellol, coumarin, eugenol, farnesol, geraniol, d-limonene and linalool which may cause allergic reactions. In addition, benzyl alcohol may cause mild local irritation. Interactions: None known. Pregnancy and lactation: Should not be used during first and second trimester unless clearly necessary. Contraindicated during third trimester. Should only be used during breast-feeding under advice from a healthcare professional and should not be applied on the breasts, nor elsewhere on large areas of skin for a prolonged period of time. Effects on ability to drive and use machines: No or negligible influence. Adverse reactions: Hypersensitivity, angioedema, dermatitis bullous, Common: dermatitis (including contact dermatitis), skin rash, erythema, eczema, pruritus. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: Wash skin with water where applied. If ingested, apply general therapeutic measures normally adopted to treat poisoning with non-steroidal anti-inflammatory medicinal products. Gastric lavage and use of activated charcoal should be considered. Legal category: Pharmacy. Marketing Authorisation Number: PA1986/093/002. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00036. Date of Preparation: June 2021
Job Code: Dic-IE-00009. Date of Preparation: November 2022
BNEW RAND
PA I N R E L I E F




16 HO UR S
UP TO * SELF-HEATING WRAPS FOR LONG LASTING, PAIN RELIEF * When worn for 8 hours. ® Trade Mark Read the instructions for use carefully before use. Do not use for more than 8 hours in a 24 hour period. Date prepared July 2020. 2020/ADV/THE/049HC









Clinical Profiles
3.8M FUNDING TO DEVELOP WEARABLE PATCH FOR TYPE 2 DIABETES
A project to develop a patch worn on the cheek that delivers peptide* treatments for Type 2 Diabetes has been awarded just under ¤3.8 million by the EU’s Horizon Europe RESILIENCE programme.
The ‘BUCCAL-PEP’ project will combine skills to develop a multifunctional biomaterial patch which allows, for the first time ever, delivery of peptide therapies across the cheek (buccal). Existing patches have already been designed for small molecules but these cannot effectively deliver peptide treatments by this route of administration. Type 2 Diabetes patients need the peptide insulin to treat the disease.
Type 2 Diabetes is the chronic disease of focus for the research because patients tend to prefer non-injected drug administration routes over injections, which improves patient compliance. BUCCAL-PEP has the potential to provide an alternative administration route for peptides other than by injections and oral routes, which could benefit the treatment of other conditions such as pain relief and certain cancers.
Prof David Brayden, Professor of Advanced Drug Delivery at University College Dublin, Fellow of UCD Conway Institute for Biomolecular and Biomedical Research, and Funded Investigator at CÚRAM SFI Research Centre for Medical Devices is the coordinator on the grant. He will lead a consortium of seven partners across Ireland, Denmark, Germany, France, the Netherlands, and the UK, including a large pharmaceutical company, Novo Nordisk (Denmark), several SMEs, and academic partners (listed below).
Professor Brayden said, “I am delighted to be the coordinator on this exciting new grant which combines academic and industry partners across the EU. Patients need alternative routes for large molecule delivery over injections as this has an impact over their willingness to adhere to therapy. Buccal administration has particular challenges and our project will attempt to address these using new patch designs.”
UCD Vice-President for Research, Innovation and Impact, Professor Orla Feely said, “We congratulate Prof David Brayden on bringing together this significant consortium project and securing equally significant EU funding. It is a key goal of
Horizon Europe programmes to connect academics, industry and varied stakeholders to produce research, new technologies and outcomes that empower people in ways that truly matter. In this case, the project will support the translation of research into clinical applications that have significant potential to really address the needs of patients living with chronic conditions.”
The patch design uses permeation enhancer, (a substance that boosts penetration), along with multiple biomaterials and a peptide cargo, enabling diffusion of the peptide across the mucosal surface of the cheek for effective delivery of the treatment.
Oral delivery of macromolecules including peptides – such as insulin – is one of the great challenges in pharmaceutical research: only five peptideanalogues are administered by tablets or capsules. Low bioavailability, dosage control, and restrictions in use (e.g. undesirable food interactions) remain key challenges. Buccal delivery has the added benefit of avoiding food effects on the absorption of peptides, a common inconvenient problem found with oral peptide administration.
BOOTS CUSTOMERS AND COLLEAGUES RAISE OVER ¤100,000 FOR THE IRISH CANCER SOCIETY NIGHT NURSES THROUGH THE BOOTS NIGHT WALK

Boots customers and colleagues have raised over ¤100,000 for the
Irish Cancer Society Night Nurse service through the 2022 Boots Night Walk. The annual sponsored 5km walk saw participants and Boots colleagues raise ¤103,516 through fundraising and donations. The Irish Cancer Society Night Nurses provide in-home care through the night, for cancer patients requiring end-of-life care, whilst also providing rest and respite for the patient’s families. The service is provided free of charge, and is an invaluable support to families and their loved ones during what can be a particularly difficult time.
Boots has been a proud supporter of the Irish Cancer Society since 2012, and through the generosity of customers, patients and colleagues has raised over ¤2.6 million, equating to over 7,500 nights of care for the service.
Across Boots stores, 150 Boots Irish Cancer Society Information Pharmacists and over 50 Boots Cancer Beauty Advisors are specially trained and on hand to support cancer patients and their loved ones.
Stephen Watkins, Managing Director of Boots Ireland, says,”We are extremely proud to have raised ¤103,516 this year for the Irish Cancer Society Night Nurse service. Since 2012, our customers, patients and colleagues have supported this special service, providing care and comfort to cancer patients and their loved ones. This ongoing partnership helps further our commitment to care for our customers and patients in the
communities we serve at all stages of their life.”
Averil Power, CEO, Irish Cancer Society, says, “On behalf of everyone at the Irish Cancer Society, I would like to thank Boots Ireland colleagues and customers for their continued support and enthusiasm for the Boots Night Walk, an absolutely wonderful fundraising initiative for the Irish Cancer Society Night Nursing service.
The service allows end-of-life care for cancer patients in the comfort of their own homes, surrounded by their loved ones. With each year, demand for this valuable service grows. Approximately 97% of the Society’s funding comes from donations, so we simply could not provide this crucial service without your generosity. Thank you for enabling us to be there for cancer patients and their families across Ireland, when they need us most.”
FIRST ALL-IRELAND GUIDELINES LAUNCHED FOR PEOPLE LIVING WITH LYMPHOEDEMA
The HSE, together with the Lymphoedema Network Northern Ireland and the Public Health Agency in Northern Ireland, have launched the first ‘All-Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’.
Boots Night Walk for Night Nurses 2022, Martha Ryan, Stephen Watkins, Fionnuala O’Leary and Mary Twohill
PHARMACYNEWSIRELAND.COM 80
¤
More than 45,000 people in Ireland and Northern Ireland live with lymphoedema, an incurable and chronic illness that causes swelling in the body’s tissue. This evidencebased clinical practice guideline has been launched to support clinicians and ultimately improve the quality of life of people living with lymphoedema across Ireland.
These guidelines support the implementation of good lymphoedema care in all settings and improve the experience and safety of people in our care. The guideline will provide healthcare professionals with clear, evidence-based guidance on the diagnosis, assessment and management of patients with all types of lymphoedema.
Developed in collaboration with clinicians, service user representatives, and key stakeholders, including Higher Educations Institutions, across the Republic of Ireland and Northern Ireland, they are a guide for managers and commissioners to support the development and modernisation of pathways of lymphoedema care, education and research.
The ‘All Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’ provide:
• The most up-to-date, evidence based recommendations to support best clinical practice, standardisation of care and improved patient outcomes.
• An in-depth evidence review and recognition of changes in pathways, healthcare practices and advances in technology that support the Sláintecare strategy.
• A progressive way of integrating specialist services and non-specialist services and promoting supported self management in the community.
• A focus on prevention and early detection in areas that are high risk for lymphoedema
• Education requirements for lymphoedema services and service users.
• An implementation and evaluation plan
Speaking about the guidelines, HSE Chief Clinical Officer, Dr Colm Henry said: “The availability of these guidelines will support the implementation of evidence based, standardised lymphoedema care in all health care settings and improve the experience and safety of
people in our care. It will provide guidance to healthcare workers on prevention, early detection, effective treatments and supported self-care to ensure that every patient receives optimal care. The recommended treatment pathways are patient-centred and support the Sláintecare strategy of the right care, in the right place, at the right time.”
Visit hse.ie/lymphoedema to read the ‘All-Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’
PIRFENFAMOTIDOINE CLONMEL
Clonmel Healthcare is delighted to announce the launch of Pirfenidone Clonmel 267mg and 801mg film-coated tablets.

cobas MPXV targets two different regions of the MPXV genome, which are both less prone to mutations than other parts of the genome. This dual-target approach ensures that cobas MPXV will continue to detect the virus even if a mutation occurs in one of the target regions.
“When multiple clusters of monkeypox virus infection were initially reported in countries where the disease is not endemic, Roche was among the first companies to address virus concerns with test kits,” said Thomas Schinecker, CEO of Roche Diagnostics. “In order to meet the testing needs and workflow demands of laboratories as well as expand access to safe and reliable diagnostic solutions, we developed the cobas MPXV on the fully automated and high-throughput cobas 6800/8800 system.”
The high-throughput solution can help individuals get the right results quickly. This is important so that patients are not subjected to unnecessary additional testing or isolation, and will have access to appropriate treatment as soon as possible.
Alongside Olivia Reilly of Clonmel Healthcare is Karl Henry, the newest face of Pharmaton, leading fitness and wellness expert, and Podcast host Karl Henry. Now moved to West Cork, Karl was at Hickey Pharmacy to promote Pharmaton’s latest immunity supplement, Immuno10; built to boost immunity and help you get back to living better faster.
Pharmaton is available over the counter from almost all pharmacies nationwide (RRP: ¤17.95). The recommended daily dose is one film-coated tablet per day in the morning with a glass of water, preferably at breakfast. Immuno10 is suitable for adults 18 years +. Food supplements should not replace a balanced diet or healthy lifestyle. Always read the label.
NEW PROGRAMME FOR HEART HEALTH
The Irish Heart Foundation’s High-Risk Prevention Project (HRPP) in General Practice works with patients from disadvantaged areas at high risk of heart disease and stroke.
Pirfenidone Clonmel is indicated in adults for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF).
Full prescribing information is available on request or alternatively please go to www. clonmel-health.ie. Medicinal product subject to medical prescription.
Please contact Clonmel Healthcare on 01-6204000 if you require any additional information.
PA 126/350/1-2. PA Holder: Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Date prepared: November 2022. 2022/ADV/ PIR/287H.
ROCHE RECEIVES U.S. FDA EMERGENCY USE
AUTHORIZATION FOR ITS HIGH-THROUGHPUT
TEST TO DETECT MONKEYPOX VIRUS
Roche has announced that the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for cobas® MPXV for use on the cobas® 6800/8800 Systems. The test is a real time PCR test for the qualitative detection of DNA from monkeypox virus (MPXV) in lesion swabs collected from individuals suspected of monkeypox infection by their healthcare provider.
Like many viruses, monkeypox cannot be conclusively diagnosed by symptoms alone. This is because many monkeypox symptoms closely resemble those of other rash-producing illnesses such as chickenpox, measles, bacterial skin infections, and even hives or allergies.
PHARMATON PHARMACY VISIT
Pharmaton Ambassador Karl Henry recently madea pharmacy visit to Hickey Pharmacy, Ballincollig, Cork (below).

A new heart health programme by the Irish Heart Foundation has shown to be beneficial to people at high risk of heart disease and stroke.
Launched in January 2021 and supported by the HSE, The Irish Heart Foundation’s High-Risk Prevention Project in General Practice works with patients from disadvantaged areas at high risk of heart disease and stroke and has helped patients make positive changes to their lifestyles and improve their health and wellbeing.
PHARMACYNEWSIRELAND.COM 81
Clinical Profiles
Despite taking place during the COVID-19 pandemic,the programme resulted in measurable positive change and feedback from the majority of participants.
Under the HSE Chronic disease management programme, those at high risk of cardiovascular disease are entitled to one annual GP visit and one practice nurse visit. Such high-risk patients require support to actively manage their risk factors.
This is challenging to achieve through an annual visit, particularly for vulnerable people and people in deprived areas.
The Irish Heart Foundation, therefore, identified that this group of people could benefit from the support of a tailored lifestyle behaviour change programme and developed the high-risk prevention project in general practice to help with this.
Six practices in disadvantaged areas in Dublin (4) and Wexford (2) were involved in the programme.
The HRPP consisted of six weekly one-to-one sessions, where patients were aided in introducing lifestyle changes that they would like to make such as increased physical activity. They were then supported to set goals and overcome barriers to making positive changes to their habits.
Across the six practices 304 people started the programme, and 260 successfully completed the six-week intervention showing a high patient retention rate (86%) despite the challenges of the pandemic., the average age of participants was 58.
In a preliminary review of the project, 72 patients who were followed up at 12 months demonstrated a significant reduction in BMI, waist circumference and overall body weight as well as improved dietary habits, increased physical activity and less sedentary behaviors.
Participants reported that the programme has improved their knowledge of how to better manage their health issues such as blood pressure, weight, and cholesterol. They also highlighted the benefit of the intervention being carried out at their local GP practice.
Practice Nurses who took part in the programme also reported a number of benefits from their experience with one stating, “I had a patient from 1st cohort come into me today for a check and he is doing brilliant, has lost a stone in 8 weeks. Blood pressure hugely improved and overall he feels a new
person. You know its such great motivation to see them embrace it and see the health benefits. He said that no one had ever taken any interest in his health before and found that alone motivational.”
NEW PARTNERSHIP TO INCREASE MENTAL HEALTH SUPPORTS
The HSE has announced a national partnership with digital mental health platform SilverCloud®. The partnership will help address the growing need for access to mental health support in Ireland.
According to research from Maynooth University and Trinity College earlier this year, 42% of Irish adults have a mental disorder. WHO research also indicates that mental health is one of the greatest social and economic challenges of our time, more pronounced now than before the pandemic.
Through this partnership, clinicians can refer to SilverCloud’s evidencebased digital CBT programmes. Active use of the digital platform in Ireland has doubled since the 12-month review of the pilot in April 2022. The service has seen over 10,000 referrals from Ireland based GPs, Primary Care Psychologists, Counselling in Primary Care and Jigsaw as they identify SilverCloud as a potential solution to support their patients.
Minister for Mental Health, Mary Butler TD said, “The announcement of this partnership with the Health Service Executive and digital mental health platform SilverCloud® for the provision of Guided
Digital Cognitive Behavioural Therapy (CBT) is hugely positive. I am passionate about improving access to and enhancing the reach of mental health services for people who require them. This announcement follows a very successful pilot which has seen the service receive over 10,000 referrals. This national rollout will be a key part of Ireland’s mental health strategy in the years ahead making it easier for people to secure the support they need.”
A new report published by SilverCloud, reviews the first 18-month application of the service in Ireland. 89 per cent of people provided access to SilverCloud felt that digital CBT would work for them and their needs prior to commencing a programme. The overall satisfaction rate of the programme was 94% post-use. Patient engagement with the programmes has seen a broad age range between 18-44 years (76%) and a representation across all 26 counties with Dublin, Cork and Galway leading in account volume.
Further results have shown the effectiveness of digital CBT:
• 49% of users with clinical levels of depression (excl. severe) transitioned to recovery.
• 53% of users with clinical levels of anxiety (excl. severe) transitioned to recovery.
• GPs are the source of the highest number of referrals, representing 89% of the total referrals.
• On average users spent 4.5 hours on the platform
“Barriers to access can be caused by finances, fear of stigma, or difficulties related to taking the first step to talk to someone. The SilverCloud digital CBT programmes can be accessed confidentially from one’s own home, therefore, waitlists for appointments and even location in remote areas of the country are no longer impediments.” - Dr Derek Richards, Chief Science Officer of SilverCloud, Amwell. “Additionally, a large body of research has now accumulated to demonstrate the effectiveness of guided digitally delivered CBT for depression and anxiety[4],[5],[6]. Commensurate with this research, the clinical outcomes and patient satisfaction ratings with the national digital CBT service are notable.”
HEALTH RESEARCH CHARITIES IRELAND RESEARCH IMPACT AWARD

Dr Mairéad O’Driscoll, CEO, Health Research Board, Professor Sally Ann Lynch, CHI at Temple Street and Crumlin, and UCD School of Medicine, and Dr Avril Kennan, CEO of Health Research Charities Ireland, pictured at the inaugural Health Research Charities Ireland Research Impact Award 2022, which was presented to Professor Lynch.
The award recognises the vital role of health research charities in funding research and the
Left to right Dr Mairéad O’Driscoll, Professor Sally Ann Lynch and Dr Avril Kennan
PHARMACYNEWSIRELAND.COM 82
contributions of principal investigators, funded through the Health Research Charities Ireland/ Health Research Board Joint Funding Scheme.
Professor Lynch’s work, which was supported by the National Children’s Research Centre and the Children’s Health Foundation, Temple Street, was recognised for its real-world impact and for making a positive difference to patients’ lives. For more information, visit www.hrci.ie/joint-funding-scheme
GLOUP® GOES SUGAR FREE!
Gloup® Swallowing Gel has launched a new sugar free formulation called Gloup Zero® The raspberry flavoured gel can still be used from 2 years of age* to swallow solid medicines, including:

Nutritional Supplements
It works by moistening the mucous membranes in the mouth and throat cavity and allowing the tablets to pass smoothly via the oesophagus to the stomach.
To use put the tablets/ capsules on a tablespoon, add 5ml of Gloup Zero® and swallow. Multiple tablets / capsules can be taken at the same time.
Gloup Zero® is sugar free and is suitable for diabetics.
Please contact Clonmel Healthcare on 01-6204000 if you require any additional information.
*Can be used by anyone over the age of 2 years, who can initiate their own swallow.
Always read the label. Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Date prepared: November 2022. 2022/ ADV/GLO/183H.
UNIPHAR AND FRIENDS OF CROSS CHARITY INITIATIVE
A joint fundraising initiative between Uniphar plc and cancer charity Friends of Cross was marked at Trinity College recently with the handover of a cheque amounting to ¤110,000 to the Trinity St. James’s Cancer Institute. The Provost of Trinity College, Linda Doyle, accepted the cheque on behalf of the Institute, and said it was a wonderful act of

generosity that would greatly assist the important ground-breaking cancer research ongoing at the Trinity St. James’s campus.
Uniphar Chairman, Maurice Pratt and former Ireland rugby legend and Friends of Cross patron, Paul Wallace, were on hand to jointly carry out the official cheque handover to the Provost, which took place beneath the historic Trinity Campanile on the College’s front square.
The monies raised came from the Uniphar plc ‘Unity for Hope’ campaign which was supported by the company’s 2,900 staff members globally, with matched funding by Uniphar plc and from supporters of the Friends of Cross, all of whom ran novel fundraising campaigns throughout the 20-month pandemic lockdown period
The total amount raised came to ¤110,000 and will go towards continued support of life-saving cancer research at the Trinity Translational Medicine Institute, Trinity College Dublin and St. James’s Hospital.
Friends of CROSS has helped raise over ¤1 million for the cancer research charity over the past 12 years, which directly supports the education and training of students in their careers as young researchers. The funds also contribute to the purchase of
Professor John Reynolds, Professor of Surgery and Head of Department at Trinity College Dublin and St James’s Hospital Dublin; Paul Wallace, Patron Friends of Cross; Maurice Pratt, Chairman Uniphar PLC; and Dr Linda Doyle, Provost of Trinity College Dublin
state-of-the-art equipment, used by over 60 researchers in Ireland working across several cancer types including oesophageal cancer, breast cancer, colon cancer and lung cancer.
The Chairman of Uniphar, Maurice Pratt, said, “It is a great honour to present this significant donation to Trinity St James’s Cancer Institute after such a lengthy gap due to the Covid pandemic. Uniphar plc has over 2,900 staff globally, and each person participated in our ‘Unity for Hope’ cancer charity campaign by running micro fundraising initiatives from their homes and office spaces during the pandemic. The money they raised was then matched by Uniphar plc and a very welcome additional contribution was also made by the Friends of Cross. We are delighted to be finally handing the final cheque here to the wonderful team at the Trinity St. James’s Cancer Institute.”
PHARMACYNEWSIRELAND.COM 83
Tablets
Capsules
Vitamins








































*suitable for children from 6 months & pregnant women DulcoEase Hydrate can be used for up to 28 days, after which the causes of constipation should be investigated by a doctor who will advise on the suitability to continue treatment. Always read the label. Distributed in Ireland by Clonmel Healthcare Ltd. *consultation with a doctor is advised. Pharmacy only brand To place an order, contact your Clonmel Healthcare Area Representative or call our freephone order line on 1800 262626 WORKS WITHIN 24 to 72hrs GENTLE RELIEF GLUTEN LACTOSE & SUGAR FREE WORKS WITHIN 24 to 72hrs GENTLE RELIEF GLUTEN LACTOSE & SUGAR FREE WORKS WITHIN 24 to 72hrs GENTLE RELIEF GLUTEN LACTOSE & SUGAR FREE 2022/ADV/DUL/098H Gentle & effective relief of constipation and irregularity












 Keough
Keough









 Sona, serving Irish consumers through Irish pharmacies since 1984
Sona, serving Irish consumers through Irish pharmacies since 1984































































































































































































































































































































