THE INDEPENDENT VOICE OF PHARMACY
In this issue:
NEWS: Pharmacy Acquisition gets Green Light Page 4
REPORT: Medicines Shortages reaching Critical Level Page 10
MEDICINES: Mapping the Way for Pharmacy Adherence Page 14
FEATURE: Early Stages of Age Related Macular Degeneration Page 32

CPD: Insulin Prescribing Page 41
www.theapharma.ie
AWARDS: OTC & Retail Pharmacy Product Awards Page 46
TEAM TRAINING: Suppository Pain Relief Page 82
FINANCE:
Finance Act 2023
Pension Changes Page 88
February 2023 Volume 15 Issue 2
PHARMACYNEWSIRELAND.COM
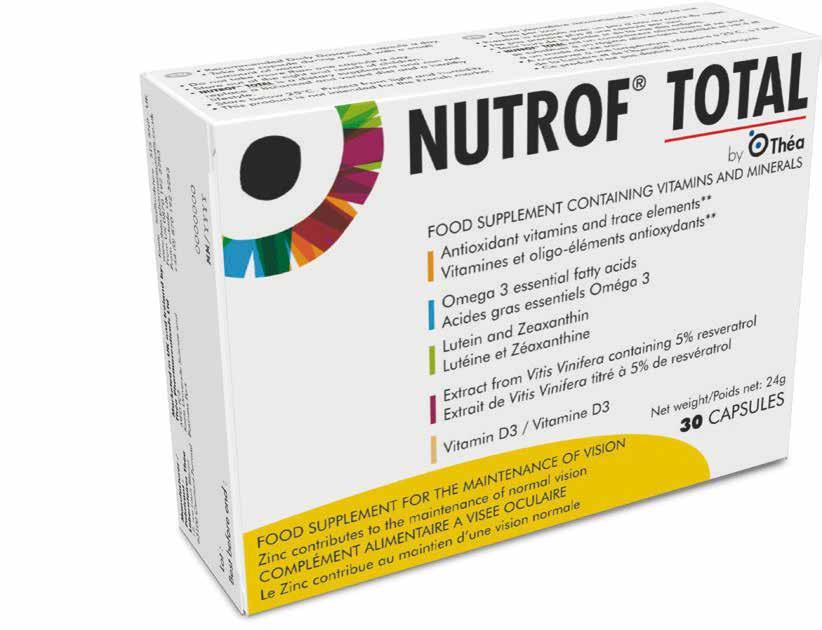
Feed Your Eyes with Nutrof Total Europe’s No1 Eye Health Multivitamin
Each capsule contains: Resveratrol Lutein & Zeaxanthin High intake of Omega-3 Vitamin D3 Vitamins C & E Zinc & Copper Selenium TP23001 This Publication is for Healthcare Professionals Only
THE COMPLETE FORMULA FOR EYE HEALTH & VISION IN JUST 1 CAP PER DAY!
Powerful anti-inflammatory pain relief

For muscle and joints when associated with strains and sprains.
BNEW RAND
CONTAINS
DICLOFENAC TRIPLE EFFEC
Please refer to the Summary of Product Characteristics (SmPC) for full details of Prescribing Information.
Motusol Max (diclofenac sodium) 2% w/w gel Abbreviated Prescribing Information. Presentation: White to almost white, homogeneous gel. 1g of gel contains diclofenac as 23.2mg diclofenac diethylamine corresponding to 20mg of diclofenac sodium. Indications: Local symptomatic treatment of pain in acute strains, sprains or contusions following blunt trauma. For short term treatment only. Dosage and administration: For cutaneous use. Apply to affected parts of the body thinly and gently rub into skin. Wash hands after application unless area to be treated. Allow gel to dry on skin before applying bandages. Adults and adolescents aged 14 years and over: depending on the size of the affected site, apply a cherry to walnut size quantity 2 times a day (preferably morning and evening). Should not be used for longer than 1 week without medical advice. Children: no data in children and adolescents under 14 years of age. Elderly: no dosage adjustment required. Monitor patient carefully. Renal and hepatic impairment: no dosage adjustment required. Contraindications: Hypersensitivity to active substances or excipients; patients with a history of hypersensitivity reactions such as asthma, bronchospasmus, urticaria, acute rhinitis in response to acetylsalicylic acid or non- steroidal anti-inflammatory drugs (NSAIDs); open injuries, inflammations or infections of the skin as well as on eczema or mucous membranes; in the last trimester of pregnancy; in children and adolescents under 14 years of age. Precautions and warnings: Systemic undesirable effects cannot be excluded if applied on larger areas of skin over a prolonged period of time. Must only be applied to intact, not diseased or injured skin. Must not come into contact with eyes and oral mucous membranes. Must not be taken orally. May be used with non-occlusive bandages, but not with airtight occlusive dressing. Consult doctor if symptoms worsen or do not improve after 3-5 days. Patients suffering from asthma, hay fever, swelling of nasal mucous membranes (so called nasal polyps) ) or chronic obstructive pulmonary disease, chronic


respiratory infections (particularly associated with hay fever-like symptoms), and patients with hypersensitivity to painkillers and anti-rheumatic medicinal products of all kinds are rather at risk to asthma attacks (so called analgesic intolerance / analgesic asthma), to local skin or mucous membrane swelling (so-called quincke edema) or to urticaria than other patients when treated with Motusol Max. In these patients, Motusol Max may only be used under certain precautions (emergency preparedness) and direct medical supervision. The same applies for patients who are also allergic to other substances e.g. with skin reactions, itching or urticaria. Discontinue treatment if skin rash occurs. Photosensitivity can occur with the appearance of skin reactions after exposition to sunlight. Avoid children coming into contact to the skin areas where the gel has been applied. Contains butylhydroxytoluene which may cause local skin reactions or irritation to the eyes and mucous membranes. Contains fragrance with benzyl alcohol (0.15mg/g), citral, citronellol, coumarin, eugenol, farnesol, geraniol, d-limonene and linalool which may cause allergic reactions. In addition, benzyl alcohol may cause mild local irritation. Interactions: None known. Pregnancy and lactation: Should not be used during first and second trimester unless clearly necessary. Contraindicated during third trimester. Should only be used during breast-feeding under advice from a healthcare professional and should not be applied on the breasts, nor elsewhere on large areas of skin for a prolonged period of time. Effects on ability to drive and use machines: No or negligible influence. Adverse reactions: Hypersensitivity, angioedema, dermatitis bullous, Common: dermatitis (including contact dermatitis), skin rash, erythema, eczema, pruritus. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: Wash skin with water where applied. If ingested, apply general therapeutic measures normally adopted to treat poisoning with non-steroidal anti-inflammatory medicinal products. Gastric lavage and use of activated charcoal should be considered. Legal category: Pharmacy. Marketing Authorisation Number: PA1986/093/002. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00036. Date of Preparation: June 2021
Job Code: Dic-IE-00009. Date of Preparation: November 2022


T
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Uniphar completes acquisition of Sam McCauley Pharmacy chain Pharmacist starts petition to enhance professional role

Medicines shortages reaching a critical level, warnings
HealthBeacon mapping the way for new pharmacy adherence service
Phoenix Group celebrate Awards
Mulligans Pharmacy breath new life into Post Office
Spotting the early stages of AMD
Foreword
Amidst record levels of overcrowding in Irish hospitals, the community pharmacy sector could provide solutions to free up capacity elsewhere in the health system.


Speaking as health authorities continue to warn that the crisis in our health system is set to continue, President of the IPU Dermot Twomey said, “The pharmacy sector is, as it always is, ready to step up to do more to support patients and provide care in our communities. Ireland’s 1,900 pharmacies are located in practically every community. They are easily accessible and should be among the first line of defence for our health system. But we need to be empowered to do so by the relevant authorities.”
Turn to page 5 for the full story.
The Medicines Shortage Index, prepared by Azure Pharmaceuticals, has found that there are 224 medicines currently unavailable, an increase of 12 medicines in seven days (at 11 January). Among the additional medicines to go out-of-stock in the past weeks are Phenytoin which is used to treat epilepsy.
Analysis by the group found that 40% of the medicines currently out of stock are provided to the Irish market by a single supply. Furthermore, the survey has shown that medicine manufacturers, including companies producing medicines domestically, are getting paid up to four times as much for their products abroad than in Ireland.
The EU is preparing to stockpile drugs and oblige manufacturers to guarantee supplies in efforts to tackle the ongoing medicines shortages.
PUBLISHER:
IPN Communications
Clifton House, Fitzwilliam Street Lower, Dublin 2 00353 (01) 6690562
MANAGING DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
Kelly Jo Eastwood: 00353 (87)737 6308 kelly-jo@ipn.ie
ADVERTISING EXECUTIVE
Amy Evans: amy@ipn.ie
EDITORIAL/ EVENTS & MARKETING EXECUTIVE
Aoife Hunter: aoife@ipn.ie

CONTRIBUTORS
Karina Oganezova
Professor Connail McCrory
Dr Deborah Galvin
Konstantina Danai Karagkiozeli
Dr Matthew O’Riordan
Mr Mark Cahill
Paddy McGeoghegan
Valerie O’Neill
Colm Moore
Dr Elaine McCarthy
Diana Hogan-Murphy
DESIGN DIRECTOR


Ian Stoddart Design
OTC & Retail Pharmacy Product Awards – The Finalists PHARMACYNEWSIRELAND.COM
The European Commission will also try to reduce reliance on China and increase domestic production capacity, the commission told the Financial Times — which reported the news. In a written response to the Greek government, EU health commissioner Stella Kyriakides outlined the plan. The commission will intervene to ensure “strategic autonomy” in basic medicines through a “systemic industrial policy”.
You can read more about this in our Report starting on page 10 of this issue.
This issue also carries the finalists for the 2023 OTC and Retail Pharmacy Product Awards. These Awards are the only industry awards that specifically recognises and rewards the companies and their products within the OTC market.

The OTC and Retail Pharmacy Product Awards act as a celebration and showcase of product innovation, marketing and value to its customer. Originally launched in 2008, they are designed to recognise product development and innovation in the pharmacy sector.
2023 & Retail Pharmacy Product Awards 46
Turn to page 46 for the full coverage.
Irish Pharmacy News is circulated to all independent, multiple Pharmacists and academics in Ireland.
All rights reserved by Irish Pharmacy News. All material published in Irish Pharmacy News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd. has taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
Regulars
FEATURE: TOPICAL TARGETING OF PAIN P36
CPD: INSULIN PRESCRIBING P41
TEAM TRAINING: SUPPOSITORIES P82
FEATURE: MEDICINES IN THE ELDERLY P86
FINANCE: FINANCE ACT 2023 P88
TEAM TRAINING: COUGH P69
CLINICAL PR: P96
3 PHARMACYNEWSIRELAND.COM 10
IRISH PHARMACY NEWS 4 28
@Irish_PharmNews IrishPharmacyNews Contents
Green Light for Pharmacy Acquisition
Uniphar has been given the go-ahead to complete the acquisition of the Sam McCauley pharmacy chain.
Medicines Stakeholder Meeting
The Minister for Health Stephen Donnelly has welcomed a multistakeholder meeting to discuss ongoing efforts to minimise the impact of medicines shortages currently being experienced in Ireland and across the EU.

The Competition and Consumer Protection Commission (CCPC) has cleared, subject to a number of legally binding commitments, the proposed acquisition by Uniphar PLC (Uniphar) of sole control of LXV Remedies (M/22/049). LXV Remedies is the parent company of Sam McCauley Chemists Limited (Sam McCauley). Sam McCauley owns a group of 37 retail pharmacies located across Leinster, Munster and Ulster.
The proposed acquisition was notified to the CCPC in September 2022. The CCPC subsequently opened a Phase 1 investigation to establish whether the proposed transaction would result in a substantial lessening of competition for goods or services in the State.
During the investigation, the CCPC identified a number of potential concerns about competition should the proposed acquisition take place. These potential concerns related to the retail pharmacy sector in Counties Kildare, Meath and Wexford.
To address these potential concerns, Uniphar proposed to make a number of binding commitments to the CCPC.
These include a commitment that Uniphar will divest three specific pharmacies, as going concerns, to a suitable purchaser or purchasers (who will be subject to CCPC approval). The three pharmacies are:
• McHugh’s Allcare Pharmacy, Athy, County Kildare
• Hickey’s Pharmacy, Abbey Road Medical Centre, Navan, County Meath
• McCauley Health and Beauty Pharmacy, Bunclody, County Wexford

Uniphar has also committed not to carry out any act which may reasonably be expected to have a significant adverse impact on the value, management
or competitiveness of the pharmacies before their sale, and not to acquire the pharmacies back for a period of time following their sale. To ensure compliance with these commitments, an independent monitoring trustee will be appointed.
Following the conclusion of its Phase 1 investigation and having taken into account the commitments given by Uniphar, the CCPC has determined that the proposed acquisition will not substantially lessen competition and, therefore, the proposed acquisition can be put into effect. The commitments form part of the basis of the CCPC’s determination to clear the proposed transaction, and so are legally binding on Uniphar.
The PMI’s Annual Pharma Summit will be held on March 30th, 2023 at Croke Park
Our theme for the day is “Partnering to Improve Human Health” and we will be exploring this theme throughout the day from four vantage points: Technology, Government, Cross Company and Intra Company. The day will be highly interactive with a mix of keynote speakers and panel discussions with plenty of opportunities to catch up with industry colleagues and make new connections.
Further details regarding speakers will be revealed over the coming weeks.

The meeting between industry, health sector and patient representatives, officials from the Department of Health and the Health Products Regulatory Authority took place last month. Minister Donnelly said, “At my request the Department of Health today held a multistakeholder event to listen to the experiences of all parties, to update on work ongoing to manage this situation and to discuss any further potential actions deemed appropriate during this period. I look forward to hearing the outcome of this meeting and supporting my officials in ensuring all suitable actions are progressed.
“I wish to acknowledge the ongoing work by all stakeholders in the management of medicines shortages at present. The Medicines Shortages Framework, operated by the Health Product Regulatory Authority (HPRA) on behalf of my department, aims to prevent, where possible, and mitigate the impact of medicines shortages when they occur through timely communication with stakeholders. The current situation is being coordinated by the HPRA through this framework in close collaboration with my department, the HSE, industry and healthcare professionals.”
The Minister added: “I would particularly like to commend the valuable contribution being made by our prescribers and pharmacists who are working closely together in ensuring best outcomes for patients in accessing medicines at this time.”
Due to the high levels of respiratory illnesses in the community in recent weeks, a significant increase in demand for medicines used to treat seasonal conditions such as colds and flus has been observed.
The HSE has established an Incident Management Team (IMT) to address shortages of antibiotics and has issued several memos to support prescribers and pharmacists at this time.
PHARMACYNEWSIRELAND.COM 4 News
Ger Rabbette, CEO, Uniphar Group
Sam McCauley Group which is now acquired by Uniphar
Medicine
Shortages
The Health Products Regulatory Authority has been notified of a shortage of the following products:
• Augmentin 875mg/125mg Film Coated Tablets - PA1077/019/005
• Bisolvon 4mg/5ml Oral Solution
- PA23180/015/001
• Caverject 10mcg Powder and Solvent for Solution for InjectionPA0822/115/002
• Dalmane 30mg Hard Capsules
- PA2010/039/002
• Dermovate 0.05% w/w CreamPA1077/005/001
• Dermovate 0.05%w/w Ointment - PA1077/005/002
• Genotropin MiniQuick 0.6 mg Powder and Solvent for Solution for Injection - PA0822/128/005
• Klacid IV 500mg Powder for Concentrate for Solution for Infusion - PA2010/004/003
• Lercanidipine Clonmel 10mg Film Coated TabletsPA0126/187/001
• Lercanidipine Clonmel 20mg Film Coated TabletsPA0126/187/002
• Lopid 300mg CapsulesPA0822/014/001
• Minims Chloramphenicol 0.5% Eye Drops - PA22698/006/001
• Opticrom allergy 2% w/v Eye Drops - PA23180/010/002
• OxyContin 10mg
Prolonged Release TabletsPA1688/005/002
• Parnate 10mg TabletPA1142/035/001
• Pazenir 5mg/ml Powder for Dispersion for InfusionEU/1/18/1317/001
• Solpadeine Capsules (32 pack) - PA1186/011/002
• Solpadeine Soluble Tablets (12 and 60 pack) - PA1186/011/001
• Tamoxifen 20mg TabletsPA0577/207/001
• Zirtek 10mg Film Coated tablets - PA0891/008/002
The following shortages have been resolved and supply has resumed to the Irish market:
• Adrenaline (Epinephrine)
1:1,000 Solution for InjectionPA0073/035/001
• Dalacin T Topical Lotion
10mg/ml Cutaneous EmulsionPA0822/121/001
• Dioralyte Blackcurrant Powder for Oral SolutionPA23180/006/001
Pharmacy ‘Consistently Overlooked’
Amidst record levels of overcrowding in Irish hospitals, the community pharmacy sector could provide solutions to free up capacity elsewhere in the health system, according to the Irish Pharmacy Union (IPU).
Speaking as health authorities continue to warn that the crisis in our health system is set to continue, President of the IPU Dermot Twomey said, “The pharmacy sector is, as it always is, ready to step up to do more to support patients and provide care in our communities. Ireland’s 1,900 pharmacies are located in practically every community. They are easily accessible and should be among the first line of defence for our health system. But we need to be empowered to do so by the relevant authorities.”
“Last week the public were advised to avoid A&Es by the HSE who recommended they visit pharmacies and GPs instead. Pharmacies are of course well placed to provide care and health advice to those who need it. However, we cannot understand why the HSE is directing patients into pharmacies while consistently doing nothing to increase the range of clinical services we can provide.
“There is huge potential in community pharmacy and much more the sector can do to deliver on the Sláintecare vision of one universal health service for all, providing the right care, in the right place at the right time in turn relieving pressure on the healthcare system.”
Mr Twomey cited several examples of services he claims pharmacies could start offering almost immediately, which would free up capacity elsewhere in the health system. “If properly resourced and if allowed to do so, there is a range of new clinical services which we could deliver. A key priority amongst these is the development and roll-out of a national Community Pharmacybased Triage Programme including a Minor Ailment Scheme, use of Emergency Medicines, and the treatment of Minor Injuries. A lot of these services are available in other jurisdictions and would potentially
eliminate thousands of needless GP and A&E visits each month.”
“With an increase in the number of GP only medical cards on the way this year increasing pressure on an already overworked sector, the HSE needs to actively put in place plans to manage their capacity. Providing greater opportunities for pharmacies to provide patient care would benefit patients and the health system alike.”
In conclusion Mr Twomey criticised health authorities for sleepwalking into the current crisis and ignoring obvious solutions. “The surge in viral infections across the country in recent weeks was predictable and predicted. It is no longer acceptable to explain away the scenes in our hospitals as the result of some extraordinary event. We must do everything practical to enhance the availability and accessibility of patient care. This must include the involvement of the pharmacy sector.”
Pharmacist – ‘Embarrassed to be Irish’
Disappointed at a lack of action from government to advance the role of pharmacy in Ireland, Milltown totalhealth Pharmacist, Sheena Mitchell has initiated a petition, calling for an enhanced role of community pharmacists.

Sheena, who launched Wonderbaba ten years ago to enable parents in Ireland to access safe healthcare information online, launched the petition to support her ambition of the introduction of Patient Group Directives and Minor Ailment’s Scheme or any form of advanced pharmacy services in community pharmacies across the Republic of Ireland.
Within the petition, which has been signed by almost 3,000 at the time of going to press, she has included her letter written to An Taoiseach Leo Varadkar and Health Minister Stephen Donnelly on Dec 16th 2022.
She says, “It’s not often I’m embarrassed to be Irish, but as a pharmacist working within the confines of the Irish system today, I am. I spent five years in university developing my medical expertise in pharmacy. Despite this training my skills and those of my pharmacy colleagues, are being chronically underutilised in an Irish healthcare system at breaking point.
“Pharmacists want to help, we are qualified, our range of services has so much potential to significantly improve access to patient healthcare. What is more, this model of patient care is already working successfully in Scotland and further afield. Every day I see patients suffering who cannot access a doctor due to the extreme pressure that the healthcare system is under. Together we can make a big impact to improve this situation.”
Sheena has asked for urgent action on both the implementation of a Minor Ailments Scheme and the introduction of Patient Group Directives (PGDs).
She adds, “Pharmacists go into the profession because they are passionate about health and their communities.
“There is no legislation to support the supply by pharmacists of
prescription medicines without receipt of a prescription. By law we can administer certain medications in certain circumstances, however the administration and the supply of routine treatment are two very different things.
You can read more about this by visiting www.change.org
PHARMACYNEWSIRELAND.COM 5
News
Sheena Mitchell, Pharmacist, Milltown totalhealth
Interested in helping future pharmacists?
APPEL (Affiliation for Pharmacy Practice Experiential Learning) is now seeking expressions of interest from pharmacists in community and hospital pharmacy settings who would like to facilitate an experiential learning placement for a final-year pharmacy student in 2024.
In the 5th-year of their M.Pharm programme, students solidify their learning by completing an eight-month placement in a patient-facing setting before becoming eligible to sit the Professional Registration Examination (PRE) and enter the PSI register.
The advantages of facilitating an APPEL placement include:
• Continuing Professional Development - APPEL Trainer Training can contribute to
pharmacists CPD, as can the experience of facilitating a placement.
• Development of your talent pipeline - many students will look to start their career in the organisations or practice settings where they undertook their placements
• Engagement - participating in the APPEL programme provides you with the opportunity to increase awareness of your pharmacy/organisation.
Promising Research in Eye Diseases
APPEL training and events provide fantastic networking opportunities.
The next 5th-year placements will run from the 2nd of January to the 23rd of August 2024. The deadline to confirm your interest in offering placements is the 29th of March 2023. You can do this by clicking on the link below: https://forms. gle/C9j4KHmGYFVZGzox7.
If you have any questions or want further information, please contact us at ops@appel.ie.
Life Pharmacy have the Last Word
Life Pharmacy, one of Ireland’s leading pharmacy retail brands, is the new official sponsor of The Last Word with Matt Cooper show on Today FM every Monday to Friday (4:30M-7PM). The sponsorship, which was secured by Media Central, will see Life Pharmacy become an integral part of The Last Word show through stings, promotions and cross station digital display.
Mairead Reen, Chairperson of Life Pharmacy, Matt Cooper, The Last Word host on Today FM and Caroline Burton, Head of Retail Marketing, Uniphar

in Life Pharmacy and The Last Word with Matt Cooper, delivering national reach across Ireland with a dynamic sponsorship pushing seasonal messaging at key times across 2023 for Life Pharmacy. We’re really looking forward to working with Life Pharmacy and Focus Advertising to activate the sponsorship across the year.”
The award-winning Last Word with Matt Cooper show on Today FM now boasts a drive time audience of 164,000 weekly listeners.
Commenting on the sponsorship Caroline Burton, Head of Retail Marketing Uniphar said, “ Life Pharmacy is delighted to partner with the Last Word and Matt Cooper in 2023. With 95 pharmacies nationwide it was important to find a partnership that matched this national reach and Today FM’s Last Word delivers a truly national audience. The Last Word tracks the pulse of Irish life and is a great fit for a
brand that puts the Irish family at the centre of their consumer commitment.”
Mairead Reen, Chairperson of Life Pharmacy added: “The Last Word focuses on the health of the nation, what could be a better fit for Life Pharmacy?”
Ross McDonnell, Sponsorship Director of Media Central said: “It’s been brilliant to bring together two long standing Irish brands
Life Pharmacy is one of Ireland’s leading pharmacy retail brands with over 95 locations nationwide. Established in 2009 Life Pharmacy is an association of independently owned pharmacies, supported by Uniphar, who are grounded in their local communities. Our qualified health professionals truly understand how to offer expert and individual care to our customers. We’re here to build life-long relationships with the people in our communities. In so doing, we help them to live better.
Researchers from Trinity have discovered that a potential new gene therapeutic approach may also be effective in treating patients living with other eye diseases in the future.
The work details how the gene therapy (ophNdi1) boosts mitochondrial performance in retinal ganglion cells, the cells dysfunctional in diseases such as glaucoma.
The results are consistent with those observed, and previously published by the same group, showing benefit in age related macular degeneration (AMD) models and highlights the potential value of ophNdi1 for multiple eye diseases.
“Because a loss of retinal ganglion cells leads to sight loss in many conditions including inherited optic neuropathies and glaucoma, we are excited that this potential therapeutic approach could provide benefit to many patients in the future,” said Dr Naomi Chadderton, first author of the research article and a scientist in Trinity’s School of Genetics and Microbiology.
“Our study shows that ophNdi1 is protective in three models of mitochondrial dysfunction. Notably, the optimisation of the therapy, which is outlined in the study, allows for use of a lower therapeutic dose.”
Mitochondria are known as the “powerhouses” of the cell because they manage the production of energy, however researchers had previously discovered that their performance decreases in the retina of people with eye diseases. This link to a deterioration in sight led them to investigate the potential of therapies to rescue struggling mitochondria.
Professor Jane Farrar, senior author of the research article and Professor in Trinity’s School of Genetics and Microbiology, said, “Our work provides clear evidence supporting using this novel gene therapeutic approach for multiple eye disorders. It also suggests that the ophNdi1 therapeutic platform targeting mitochondrial dysfunction could have applications for other devastating conditions beyond the eye in which mitochondrial dysfunction is in play.”
PHARMACYNEWSIRELAND.COM 6 News
LIVE THE WAY


 ROBBIE HENSHAW
Irish International Rugby Player
ROBBIE HENSHAW
Irish International Rugby Player
Body Clock linked to Lung Conditions
Researchers at RCSI University of Medicine and Health Sciences have discovered how disruption of the circadian clock can lead to increased inflammation in the lungs, which may have negative effects on chronic lung diseases and respiratory infections.

usual function to recruit other immune cells to the lungs. More cells than are needed are brought to the lungs, resulting in damaging inflammation.
patterns in that they are more likely to experience more severe respiratory infections.
This study pinpoints lung fibroblasts as a potential target in the treatment of lung diseases that display this kind of inflammation, a change in approach from most current therapeutic options which focus on easing symptoms rather than treating the cause.
The paper, published in The FASEB Journal, looked at the molecular clock in fibroblasts, a cell type which is abundant in the lung. When the regular rhythm of the fibroblast clock was
lost, it resulted in an increased inflammatory response and therefore, worse symptoms.
This could have implications for individuals such as shift workers or people with erratic sleeping/eating
Lead author Shannon Cox, School of Pharmacy and Biomolecular Sciences at RCSI, said: “This study links together previous knowledge to confirm the importance of lung fibroblasts in inflammation and highlights the negative consequences of disruption to our circadian clock, such as poor night-time sleep, in lung disease. We can apply this understanding when developing therapies and interventions for patients.”
Disturbing the circadian clock in lung fibroblasts impacts their
The majority of this study was supported through funding provided to Professor Annie Curtis by the Science Foundation Ireland Career Development Award (CDA) programme and by the Irish Research Council through a Laureate Award.
Further support was provided by National Heart, Lung, and Blood Institute grant, a Boehringer Ingelheim Fonds travel grant and an RCSI School of Postgraduate Studies International Secondment Award.
United Drug Webinar Series returns for 2023
United Drug were excited to welcome customers back to their first webinar of 2023, following receipt of their award for best Patient Education Programme – Pharmaceutical in December.
In January, they we were joined by Larry Ryan, Director of B&A Research and Insights, Oonagh O’Hagan, Managing Director of Meagher’s Pharmacy Group, and Denis O’Driscoll, Superintendent Pharmacist of LloydsPharmacy Ireland to discuss some of the key trends at play and likely to have influence over how shoppers approach pharmacy in the year ahead.

The panel discussion brought insights to pharmacists into consumer shopping trends by examining various conflicting dynamics including trust, value for money, the drift online, the importance of shopping locally, the green/sustainability agenda, and the many other aspects and vectors that may have a bearing on consumer needs and a pharmacy’s ability to address them.
Larry Ryan from B&A drew on published work undertaken for the IPU and others as well as B&A’s ongoing review of local and global trends and themes. With this, the aim was to give a series of
practical takeaway learnings and action points appropriate to the Pharmacy and their staff.
Discussion points from the night included:
1. Services: How Pharmacies can expand their services given the current challenges at hand.
2. Economy: Given the current economic climate, how can pharmacies address customer price sensitivity but at the same time, be mindful not to devalue the pharmacy’s service with too much focus on discount and promotions.
3. Online: How can pharmacies incorporate online sales into their business.
4. Sustainability: Sustainability remains a key focus for the pharmacy customer for the future.
CPD Learning
United Drug would like to remind pharmacists that the content and materials from our webinars can be
included in your ePortfolio for CPD learning. You are welcome to use the content we provide for your reflective practice to identify new learnings or how you can improve your knowledge, skills, behaviours, or attitudes within the pharmacy.
If you would like to recap on any previous Knowledge Hub webinars, or for further information, please email udwmarketing@united-drug.com
PHARMACYNEWSIRELAND.COM 8 News
Professor Annie Curtis
Looking good just comes naturally
For overall radiance of healthy skin
Sona BeautéActive Skin Complex is specially formulated to maintain the radiance, elasticity and overall wellbeing of your skin. Each capsule contains a unique combination of nutrients Collagen and Hyaluronic Acid.


Collagen is effective in maintaining the elasticity, tone and texture of your skin.

Hyaluronic Acid helps keep your skin moisturised. Also helps the tissue regeneration process involved in healing your skin, essential for a healthy complexion and overall radiance.
Also availableSona BeautéActive Skin Hair Nails Complex
Order direct from Sona on PH: 01 451 5087. Also available from wholesalers, United Drug and Uniphar.
Regularly ingesting Collagen and Hyaluronic Acid can support overall skin health and delay the effects of aging.
35 YEARS OlD 45 YEARS OlD 55 YEARS OlD
Ir I sh v I tam I ns
Medicine Shortages Reaching EU Level and ‘Critical’ Point
Medicines shortages are not a new phenomenon. Certainly since the advent of Covid-19, Brexit and the Northern Ireland protocol, the issue is becoming more critical.
to maximise returns through supplying higher price markets.
Azure is currently not supplying any of the above medicines to the Irish market.
governments are paying twice as much to manufacturers than the comparative prices that the Irish government/industry agreement allows. Some countries are paying up to four times more than Ireland, giving rise to serious shortages for patients here as manufacturers choose
The UK government and the majority of the 27 EU member state governments have taken specific measures in response to the escalating medicine shortage issue. This includes, changes to medicine pricing rules, stockpiling of key products, the introduction
The mainstream media has been increasingly reporting on the situation during the start of this new year, as a severe shortage of OTC cough syrups for adults and children has alarmed parents and caregivers. Sprays for sore throats, dissolvable paracetamol powder and soluble aspirin are also widely unavailable.

The Medicines Shortage Index, prepared by Azure Pharmaceuticals, has found that there are 224 medicines currently unavailable, an increase of 12 medicines in seven days (at 11 January). Among the additional medicines to go out-of-stock in the past week are Phenytoin which is used to treat epilepsy.
There is a major shortage of over-the-counter cough syrups for adults and children. Sprays for sore throats, dissolvable paracetamol powder and soluble aspirin are also widely unavailable.
Antibiotics used to treat bacterial and respiratory infections, including Amoxicillin, Penicillin and Cefalexin, are also in scarce supply.
Analysis by the group found that 40% of the medicines currently out of stock are provided to the Irish market by a single supply.
Furthermore, the survey has shown that medicine manufacturers, including companies producing medicines domestically, are getting paid up to four times as much for their products abroad than in Ireland.

The authors of the Medicine Shortages Index, also identified that medicine shortages have further worsened.
Azure has analysed the average prices paid for 10 essential mainstream medicines by Irish, UK and European governments.
Its analysis shows that, on average, the UK and EU member
PHARMACYNEWSIRELAND.COM 10 Report
Medicine Strength Pack Size IE € UK € EU Average € Treatment For Paracetamol 500 mg tablet 100 pack 1.73 3.05 7.35 Pain Amoxicillin 500mg capsule 100 pack 16.15 31.75 24.46 Infection Lorazepam 1g tablet 100 pack 3.77 7.63 11.43 Anixety Prednisolone 5mg tablet 98 pack 3.06 4.2 7.49 Inflammation/Asthma Nitrofurantoin 100mg Caps 30 pack 6.73 13.03 11.91 Urinary tract infection Amisulpride 200mg 60 pack 31.68 39.08 60.99 Schizophrenia Clonazepam 0.5mg 100 pack 4.33 35.46 13.59 Epilepsy Co-Amoxiclav 125/31mg/5ml 100 pack 1.38 6.25 4.12 Infection Tamoxifen 20mg tabs 30 pack 4.58 3.16 9.11 Breast Cancer Ipratropium Nebules 250mg/ml x 2ml 20 pack 4.5 7.46 7.95 COPD
Sandra Gannon, General Manager, Azure Pharmaceuticals
of an export ban of key drugs, and provision of additional powers to pharmacists. To date, the Department of Health is yet to meaningfully respond to this deepening challenge.
Commenting, Sandra Gannon, General Manager, Azure Pharmaceuticals said:
“In less than a decade, we have gone full circle on what we pay for mainstream medicines with Ireland now paying substantially less than neighbouring countries for a range of medicines. As a result, manufacturers are choosing to supply their medicines to those countries who will pay better prices. This in turn gives rise to growing medicines shortages and
discontinuations here with patients unable to source the medicines they need. We are paying the price for not paying the price.
“The government appears to be at best misinformed and at worst, in denial about the root cause of this worsening problem. Changing legislation to give extra powers to pharmacists should form a key part of a package of solutions, but that alone will not resolve matters. The price we pay, and a medicines pricing agreement that is no longer fit-for-purpose, is at the heart of this issue.
“The HPRA has a co-ordinating role to manage shortages but is can only respond with regulatory measures, as it acknowledged
itself in a statement this week. This issue requires a mix of actions by the Department of Health. To date, that has been lacking.”
40% of the medicines out of stock this month in Ireland have just a single supplier, leaving pharmacists without licensed alternatives for patients. It also leaves Ireland out of sync with the rest of Europe, with a recently published European Commission report showing the EU wide singled-sourced average standing at 25%.

Pharmacist Kathy Maher, owner of Haven Pharmacy in Duleek, Co Meathsays that Health Minister Stephen Donnelly must introduce a “serious shortage protocol” that would allow pharmacists to
prescribe an alternative medicine when one a GP has prescribed is out of stock.
She explained, “Some of those antibiotics aren’t available, particularly in medicine in liquid form.
“For me to treat that [patient], then I need to go back to the prescriber, have the prescription changed to a different antibiotic and then go back and dispense it.
“You can imagine the delay that brings. If it is the same day, it’s fine. I can go back to the doctor, it’ll take a couple of hours. If it’s a Friday evening or Saturday, because it was an on-call service, it’s really hard.
PHARMACYNEWSIRELAND.COM 11
“It could be days before I get an answer back.
“What we are calling on is for the Minister for Health to introduce a serious shortage protocol that allows pharmacists to use their clinical skills to switch from one medicine to another when a product isn’t available.
“It means patients will have treatment promptly. If a patient with a significant infection can’t access the antibiotic that they need and ends up in A&E, that could be prevented.”
Medicines for Ireland (MFI) are also urging Government to heed recent warnings from GPs and pharmacists nationwide on the growing risk of medicines shortages as inflation, energy and transport costs continue to rise, and global supply chain disruptions persist.
Medicines for Ireland members are the suppliers of the majority of medicine in Ireland to the HSE and patients directly and played a pivotal role in a new Framework Agreement on the supply and pricing of non-originator, generic, biosimilar, and hybrid medicines, announced by Government last year.
Commenting on increasing medicine shortages, Medicines for Ireland Chairperson, Padraic O’Brien has said “In Ireland and throughout Europe, soaring energy costs, inflation and supply chain disturbances have contributed to thousands of generic medicines disappearing from the European and Irish market.”
“MFI members are willing to work directly with Government to help tackle this serious issue and prevent potential medicines shortages. Our aim is to deliver industry insights and extend our expertise to help improve the development of medicinal pricing and procurement policies in Ireland and safeguard the supply of medicines to Ireland.”
According to the Health Products Regulatory Authority (HPRA) website there are currently 187 medicines in short supply in Ireland. Without intervention this situation has the potential to significantly worsen.
Mr O’Brien added, “As a small market Ireland is more likely to be badly impacted by inflationary pressure and as costs continue to rise, market conditions will become increasingly unviable for companies supplying generic medicines to Irish hospitals and pharmacies. Additionally, in some cases, our reimbursement prices for certain medicines are too low compared to other EU countries”.
“Price adjustments in Ireland are historically downward only, where other European countries employ flexible pricing mechanisms that allows reimbursement prices to rise for medicines that are in short supply. Ireland does not have such a mechanism and is therefore further disadvantaged.”
A recent MFI members survey found that 91% of MFI members experienced increased costs associated with import and/or manufacturing of pharmaceutical and medical products for the Irish market in 2022. While all MFI member companies envisage increases in transportation costs over the next 12 to 24 months.
“Our main focus is to help Government ensure market conditions in Ireland remain sustainable in order to retain and secure access to reliable and affordable treatment for Irish patients. We believe it is time for us to revisit our work with Government and the HSE on the Framework Agreement on Supply and Pricing and develop improvements to mitigate against supply risks.” concluded Mr O’Brien.
The Healthcare Products Regulatory Authority said in a statement, “Due to a combination of factors, including the level of respiratory illnesses in the community, a significant increase in demand for medicines used to treat seasonal conditions such as colds and flus has been observed over recent weeks. In some cases, this demand has been 2-3 times the normal level seen during the same period in previous years. From discussions with suppliers and regulators in other countries, the Health Products Regulatory Authority (HPRA) understands that similar trends have been observed in other European countries who have experienced significant increases in demand.
“The HPRA has been engaging with all stakeholders, including suppliers, with a view to ensuring a coordinated response to this increased demand. The key focus at all times has been to ensure that suitable medicines remain available to treat all patients.
“In the case of medicines used most often in Ireland, there are typically multiple forms, strengths, brands, and generic medicines available from various sources. Where some individual medicines are in short supply, alternative options such as alternative strengths, brands, and generic medicines remain available to ensure continuity of treatment. In some cases where the medicine initially prescribed for the patient is unavailable, patients may be switched to a suitable therapeutic
alternative following appropriate consultation with a healthcare professional. This approach is also consistent with national antimicrobial prescribing guidelines. “Suitable medicines continue to be available to treat respiratory illnesses and their symptoms in both adults and children. Taking into account the wide range of available medicines to treat respiratory illnesses, there is no need for healthcare professionals to order extra quantities of medicines, or for doctors to issue additional prescriptions. Similarly, patients and the general public are asked not to seek supplies of medicines over and above their normal requirements. Doing so will disrupt existing stock levels and hamper the supply of medicines for others.
“Further to the HPRA’s regular and ongoing engagement with industry, we have been informed that in a number of instances suppliers have increased production and sourced additional stock to respond to this recent increase in demand. Although the HPRA has no role in procuring medicines, we continue to engage with the suppliers to obtain updates and remains open to expediting regulatory procedures to enable supply of additional stock, where possible.
“The HPRA fulfils a coordinating role in Ireland’s response to managing medicine shortages when they occur. In each case, the HPRA works with relevant stakeholders as necessary, to coordinate an effective approach to the management of a confirmed product shortage.”
The EU is preparing to stockpile drugs and oblige manufacturers to guarantee supplies in efforts to tackle the on-going medicines shortages.
The European Commission will also try to reduce reliance on China and increase domestic production capacity, the commission told the Financial Times — which reported the news. In a written response to the Greek government, EU health commissioner Stella Kyriakides outlined the plan.
The commission will intervene to ensure “strategic autonomy” in basic medicines through a “systemic industrial policy”.
It plans to propose legislation to “secure access to medicines for all patients in need and to avoid any market disruption of medicines”.
This would require “stronger obligations for supply, earlier notification of shortages and withdrawals and enhanced transparency of stocks”, the commission said.
This follows moves by the Greek health minister last week to bring in changes to address shortages there of inhalers and antibiotics. Greece is putting an export ban in place, but the Financial Times reports this is not being considered by the EU for now.
Greece is also listing alternatives to drugs which are in short supply, as is done in Britain.
This is something the Irish Pharmacy Union has repeatedly called for under the title of a “Serious Shortage Protocol” including calls made in early December when shortages became apparent.
Ms Kyriakides said in a written statement the commission was suspending some regulations and working with EU companies to increase capacity.
“Discussions with industry have already taken place and they are aware that they must rapidly step up production of these medicines,” she said.
Responding to the crisis, the International Generic and Biosimilar Medicines Association (IGBA), a Brussels-based industry group, told the FT that doctors are prescribing antibiotics more often.
It called on governments to share more information about their disease forecasts and loosen trade restrictions to allow drugs and raw materials to move more freely to countries with shortages.
They should also force wholesalers, pharmacies and hospitals to stop stockpiling antibiotics, according to the IGBA.
PHARMACYNEWSIRELAND.COM 12 Report



















































Pharmacy only. Suitable for Adults over 18 years. Food supplements are not intended to be a substitute for a balanced and varied diet and / or a healthy lifestyle. Always read the label. Distributed in Ireland by Clonmel Healthcare Ltd. 2023/ADV/PHA/001H Vitamins C, B1, B2, B3, B5, B6, B7, B12, iron, copper and manganese, each contributing to normal energy yielding metabolism IMMUNITY SUPPORT MENTAL PERFORMANCE ENERGY SUPPORT HIGH in 11 vitamins & minerals each contributing to support energy release + Ginseng Keep fully charged with VITALITY11
HEALTHBEACON ‘MAPS’ the way forward for new pharmacy adherence service
A new opportunity for pharmacy practices to improve patient persistence to injectable medications
The Adherence Problem
Medication non-adherence is one of the largest problems facing healthcare today, with 1 in 2 patients being non-adherent to prescribed treatments.1 The impact on health can be significant, with non-adherence estimated to contribute to the premature deaths of nearly 200,000 Europeans annually.2 In addition to the human cost, the financial implications also add up, with poor and non-adherence costing EU governments an estimated ¤125 billion each year.2
The Irish population is no different, and such global medication adherence issues are reflected in the Irish setting; It is estimated that up to 30% of patients in Ireland do not adhere to medication regimens that may be curative or relieve symptoms, and 30% to 40% fail to follow preventative treatment regimens.3 Patient non-adherence not only leads to significant costs for healthcare systems,it can also have a negative impact on the efficacy of treatments, patient well-being and lead to unnecessary and excessive use of healthcare resources.4,5
HealthBeacon partners with Irish pharmacies to improve persistence
Dublin-based HealthBeacon plc, aims to improve patients’ adherence and persistence by building smart tools for managing medications and has developed the leading at-home, digital

To start this process, HealthBeacon recently partnered with a number of Irish pharmacies for a proof-of-concept study to demonstrate the potential of the HealthBeacon Injection Care Management System (ICMS) to improve patients’ persistence to self-administered injectable medications Pharmacy-based persistence data (Medication Possession Ratio, MPR) was compared to persisten data electronically collected by the HealthBeacon ICMS The preliminary results were positive; 70% of HealthBeacon patients remained on therapy at the end of month 12, compared to 46% of pharmacy-based patients at the end of one year. Demonstrating a relative improvement of 52%, this early data is promising It is evident that t opportunity for pharmacies to partner with HealthBeacon to drive medication persistence, whilst addressing the missing dispensing revenue across
with 8 peer reviewed studies to date. With use of the system, improvements of up to 26% in adherence across certain therapeutic areas has been reported.5 To date, HealthBeacon has deployed over 11,000 devices, tracking over 600,000 injection events, across 17 countries.
eager to partner with innovative pharmacies as early adopters for this exciting new program for community pharmacy.
In September 2022, HealthBeacon partnered with APhA (the American Pharmacy Association) to establish a Fee for Service model, where pharmacists in the US are reimbursed for their engagement with patients around adherence. HealthBeacon is now seeking to replicate this in Ireland, with the aim of establishing a Fee for Service model for pharmacies here.
platform for self-administered injectable treatments (http://www. healthbeacon.com/). HealthBeacon is an evidence-based solution,
HealthBeacon is partnering with community pharmacies in Ireland to introduce their Medication Adherence Pharmacy Services (MAPS). Pharmacies can offer this evidence-based solution to patients, to help optimise their adherence and avoid missed prescription refills. For patients, many of these treatments are vital for their health outcomes, disease control and overall well-being, therefore supporting and maintaining optimal adherence is a priority for all.
Improvement in persistence for patients
Improvement in persistence for patients
To start this process, HealthBeacon recently partnered with a number of Irish pharmacies for a proof-of-concept study to demonstrate the potential of the HealthBeacon Injection Care Management System (ICMS™) to improve patients’ persistence to self-administered injectable medications. Pharmacy-based persistence data (Medication Possession Ratio, MPR) was compared to persistence data electronically collected by the HealthBeacon ICMS. The
n: number of patients at month 1
n: number of patients at month 1
Pharmacy Persistence Measure: Medical Possession Ratio. Note: Patient considered non-persistent if there is no record of dispensing for 3 consecutive months.
Pharmacy Persistence Measure: Medical Possession Ratio. Note: Patient considered non-persistent if there is no record of dispensing for 3 consecutive months. HealthBeacon Persistence Measure: Length of time between 1st drop in HealthBeacon device and treatment end date, a patient is considered “Active” during this period.



Note: Assumes active patients are persisting on device and therefore medication.
HealthBeacon Persistence Measure: Length of time between 1st drop in HealthBeacon device and treatment end date, a patient is considered “Active” during this period. Note: Assumes active patients are persisting on device and therefore medication.
Persistence %: Total Patients persisted in the given month number/Total Patients in the month number 1.
Persistence %: Total Patients persisted in the given month number/Total Patients in the month number 1.
Note:
• All data is associated with patients based in Ireland
• Assumes that Patients within the Pharmacy Data cohort did not have access to the HealthBeacon ICMS
• For the Pharmacy data in cases where the patient does not dispense the drug frequently, only the most recent occurrence of their 3 consecutive months not being dispensed is considered
• Final results were not analysed for statistical significance. Future studies may report different results.
PHARMACYNEWSIRELAND.COM 14 2
100% 98% 90% 80% 70% 100% 100% 61% 53% 46% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 1 2 3 4 5 6 7 8 9 10 11 12 Persistence % Persistence(HB) % Persistence (Pharmacy Data) 52 1% (n = 4000) (n = 914) Month
HealthBeacon
The MAPS service provides patients with access to the Smart Sharps Bin and its companion app for easy injection management
preliminary results were positive; 70% of HealthBeacon patients remained on therapy at the end of month 12, compared to 46% of pharmacy-based patients at the end of one year. Demonstrating a relative improvement of 52%, this early data is promising . It is evident that there is a significant opportunity for pharmacies to partner with HealthBeacon to drive improved medication persistence, whilst addressing the missing dispensing revenue across participating pharmacies .
HealthBeacon is eager to partner with innovative pharmacies as early adopters for this exciting new program for community pharmacy.
About the HealthBeacon Platform
The HealthBeacon platform integrates a Smart Sharps Bin, an FDA cleared, digitally connected sharps container, to track each time a used injection is disposed of, with a comprehensive patient support platform, to accurately record compliance to therapy in the home-setting. With no extra work required on behalf of the patient, but by the simple means of disposing of their sharps waste, their adherence and persistence to therapy over time can be captured and calculated by the Smart Sharps Bin. This real-time adherence data is integrated into a Companion App, data dashboards for pharmacies to review patients’ compliance remotely, and HealthBeacon’s patient services platform to enable real time intervention and follow-up for missed doses, and drive higher adherence to therapy. If adopted in the Irish pharmacy setting, these services will be accessible to the patient through their local pharmacy team.

HealthBeacon Medication Adherence Pharmacy Services (MAPS) – Sustainable, Scalable and Profitable
Where a pharmacy service is not underpinned by a technology, a key challenge is the lack of efficient means to track patients’ progress and systematically follow-up. In many cases, this has rendered services unsustainable given the competing priorities for the pharmacists’ time. For this reason, tailored services are starting to utilise patient tools, such as pillboxes, medication cards, calendars, medication refill synchronization and enhanced follow-up. HealthBeacon’s MAPS aims to help pharmacies optimise their dispensing revenue and provide a ‘fee for service’ model. The service aims to ensure the pharmacy service succeeds, as it:
The act of disposing of used injections becomes an important adherence data point, enabling pharmacists to efficiently identify patients at-risk of non persistence to therapy
1 Enables the pharmacy teams to manage by exception: At the click of a button, those patients with poor adherence scores are identified, enabling pharmacists to spend their time on maximum-impact interventions.
2. Augments the pharmacist’s capacity: HealthBeacon’s patient support team is used for back-end pharmacy support, following a pre-agreed patient support and out-reach cadence . When non-adherence is identified, the HealthBeacon team contacts patients to support them to improve their adherence. All engagement details are shared with the pharmacy team.
3. Provides improved patient insights for the pharmacy team: The pharmacy team has access to a report with patients’ adherence scores and information on any missed doses. This enables fully informed conversations with the patient when they visit the pharmacy.
4. Has a positive impact on dispensing revenue: Improving adherence reduces missed doses and optimises dispensing revenue.
5. Can be continuously evaluated: Using HealthBeacon, patients’ adherence and persistence over time, and any impact on dispensing revenue, can be continuously evaluated, simply and effectively, at the click of a button.
To offer this service to patients, what is involved for the pharmacy team?
1. The Pharmacy team will be given access to HealthBeacon’s training program for pharmacies; pharmacy colleagues who have completed the training will receive a certificate of completion.
2. The pharmacy team will identify appropriate patients for participation; they will consult with each patient, providing a demo of the HealthBeacon product and securing patient consent.
3. The pharmacy team will have access to patients’ adherence reports through HealthBeacon’s online portal and can liaise directly with the HealthBeacon team.
4. The pharmacy will participate in a simple data sharing and analysis process to identify the levels of patient adherence and persistence. This will help measure the positive impact of the HealthBeacon ICMS on patients’ adherence and persistence over time, help pharmacies define the return on investment, and demonstrate the sustainability of the Fee for Service model.
How to participate in this service?
All pharmacies who are interested in offering this innovative service for their patients or are interested in learning more can contact pharmacy@healthbeacon.com.
1. The Pharmacy team will be given access to HealthBeacon’s training program for pharmacies; pharmacy colleagues who have completed the training will receive a certificate of completion.
2. The pharmacy team will identify appropriate patients for participation; they will consult with each patient, providing a demo of the HealthBeacon product and securing patient consent.
3. The pharmacy team will have access to patients’ adherence reports through HealthBeacon’s online portal and can liaise directly with the HealthBeacon team.
4. The pharmacy will participate in a simple data sharing and analysis process to identify the levels of patient adherence and persistence. This will help measure the positive impact of the HealthBeacon ICMS on patients’ adherence and persistence over time, help pharmacies define the return on investment, and demonstrate the sustainability of the Fee for Service model.
Summary
of Key Benefits of MAPS (Medication Adherence Pharmacy Service) programme:
• Reduces non-adherence to medication and ensure refill intervals are achieved as prescribed
• Reduces loss of revenue for pharmacy
PHARMACYNEWSIRELAND.COM 15
“The community pharmacy setting is the ideal location for patients to access innovative pharmacist-delivered medication adherence support and tools,” says Jim Joyce, HealthBeacon CEO. “Today half of all patients fail to utilize their medications as prescribed, costing healthcare systems billions in avoidable costs and costing patients the opportunity to improve their health. Together we have developed a best-in-class tech and service offering to address this challenge and we are delighted to be working with pharmacists in Ireland to make this a reality.”
This year, HealthBeacon will be launching HB Wave, their smart tool for managing pill medication. Pharmacists can register early interest now with details below
Case Study: Oonagh O’Hagan, CEO, Meagher’s Pharmacy Group
Meagher’s Pharmacy Group is one of the first pharmacy groups in Ireland to offer the HealthBeacon product and service to its patients. Oonagh O’Hagan, CEO of Meagher’s pharmacy group, talks to IPN about the importance of offering an adherence service:

• Enables pharmacists to focus on the key interventions required for specific patients
• Frees up time within the pharmacy
• Builds further relationship with patients and supports them effectively
• Solidifies pharmacies position as the go-to healthcare resource for leading on patient adherence
References
1. Sabaté E, ed. Adherence to LongTerm Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. Available from: https://apps.who.int/iris/bitstream/ handle/10665/42682/9241545992. pdf
2. Williams A, Manias E, Walker R. Interventions to improve medication adherence in people with multiple chronic conditions: a systematic review. J Adv Nurs. 2008 Jul;63(2): 132-43. https://pubmed.ncbi.nlm.nih. gov/18537843/
3. Dr Sabah Al-Lawati, PhD. A REPORT ON Patient NonAdherence IN IRELAND. https:// www.drugsandalcohol.ie/21614/1/ Adherence_Report_Final.pdf
4. Pharmaceutical Research and Manufacturers of America Improving Prescription Prescription Medicine Adherence is key to better health care: http://phrma-docs.phrma. org/sites/default/files/pdf/PhRMA_ Improving%20Medication%20 Adherence_Issue%20Brief.pdf
5. Gwadry-Sridhar, F. H., Manias, E., Zhang, Y., Roy, A., Yu-Isenberg, K., Hughes, D. A., & Nichol, M. B. (2009). A framework for planning and critiquing medication compliance and persistence research using prospective study designs. Clinical therapeutics, 31(2), 421–435. https://doi.org/10.1016/j. clinthera.2009.02.021 https:// pubmed.ncbi.nlm.nih.gov/19302915/
6. Sarhan S, McWhinney S, Shah A, Kelly L. The HealthBeacon Injection Care Management System shows improved adherence to gastrological, dermatological and rheumatological treatments. Int J Clin Pharmacy. 44, 276-229. 2021. Available from: https://doi.org/10.1007/s11096-02101373-5

Why is this a suitable service to offer in community pharmacies?
Most patients receive their medicines from their local community pharmacy which positions the community pharmacist as the default healthcare professional to impact adherence. As pharmacists, we are the most accessible and most consulted healthcare professionals and are ideally placed to tackle this huge problem of non-adherence. We already have a track record in driving adherence through programs involving Medicines Use Reviews, patient education, assessments, and surveys, helping patients make informed decisions about their medicines and supporting them in adhering to their prescribed therapy. This service is simply taking this pharmacy support to the next level, introducing a technology which will help us to track and manage the patient’s progress effectively.
What is it about the HealthBeacon service that sets it apart?

Introducing a service to help improve adherence is a win for everyone – it’s a win first and foremost for the patient and it is also a win for pharmacies as patients are not missing doses,
and for the broader healthcare system in terms of reducing downstream costs; many of these injectables medicines are high consequence medicines in terms of importance for patient outcomes as well as the high cost.
We’re delighted at Meagher’s to be partnering with HealthBeacon because we will be implementing a service that is sustainable. Because there is a technology involved, it takes the heavy lifting out of tracking the improvement in adherence. This new level of patient insight is so valuable - at any point in time, you can see which specific patients are getting 100% as their adherence score but also those who need a little extra support. This helps us to best manage our time within the pharmacy. The technology is excellent because it is multifunctional – it serves not only to give reminders to the patient and track their adherence but also as a safe disposal unit to remove the sharps from the home – once the SmartSharpsBin is full, it is collected from the patients home at no extra cost to the pharmacy or the patient.
PHARMACYNEWSIRELAND.COM 16 HealthBeacon
Oonagh O’Hagan, CEO, Meagher’s Pharmacy Group with Leonora O’Brien, Health Beacon
28 PACK NOW AVAILABLE. 28 PACK NOW AVAILABLE
CCF: 24971 Date of preparation: (09-22)
ABBREVIATED PRESCRIBING INFORMATION
Product Name: Emazole Control 20 mg Gastro-Resistant Tablets

Composition: Each tablet contains 20 mg esomeprazole (as magnesium dihydrate).
Description: Light pink oval lm coated tablet.
Indication(s): Proton Pump Inhibitor (PPI): Short-term treatment of re ux symptoms (e.g. heartburn and acid regurgitation) in adults. or crush. Disperse in half a glass of non-carbonated water if di culty in swallowing. Stir until tablets disintegrate, drink liquid with pellets immediately or within 15 min, or administer through a gastric tube. Do not chew or crush pellets. Adults: The recommended dose is 20 mg esomeprazole (one tablet) per day. achieve improvement of symptoms. Duration of treatment is up to 2 weeks. Once complete relief of symptoms has occurred, treatment should be discontinued. If no symptom relief is obtained within 2 weeks of continuous treatment, the patient should be instructed to consult a doctor. recommended. No relevant use in this group in the indication: “short-term treatment of re ux symptoms (e.g., heartburn and acid regurgitation)”. Severe impaired renal function: Caution.
Severe liver impairment: 20 mg max daily dose.
Contraindications: Hypersensitivity to esomeprazole, substituted benzimidazoles or any of the excipients. Not with nel navir.
Contact a physician if symptoms change in character. In the presence of any alarm symptom (e.g. signi cant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with esomeprazole may alleviate symptoms and delay diagnosis. Treatment with proton pump inhibitors (PPIs) may lead to a slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter and in hospitalised patients, also possibly Clostridium di cile. Patients should consult their doctor before taking this medicinal product if they are due to have an endoscopy or urea breath test. Absorption of vitamin B12 may be reduced due to hypo- or achlorhydria. Not recommended for long-term use as the following may also occur: Hypomagnesaemia; Risk of fracture. Consider stopping Emazole Control in cases of Subacute cutaneous lupus erythematosus (SCLE) accompanied by arthralgia. Interference with laboratory tests: Increased Chromogranin A (CgA) level may interfere with investigations for neuroendocrine tumours. To avoid this interference, Emazole Control treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of PPI treatment. Contains glucose and sucrose.

Interactions: E ect of esomeprazole on other drugs: Co-administration with atazanavir is not recommended. If the combination of atazanavir with a PPI is judged unavoidable, close clinical monitoring is recommended in combination with an increase in the dose of atazanavir to 400 mg with 100 mg of ritonavir; esomeprazole 20 mg should not be exceeded. Esomeprazole is a CYP2C19 inhibitor. When starting or ending treatment with esomeprazole, the potential for interactions with drugs metabolised through CYP2C19 should be considered. Serum levels of cilostazol, cisapride, tacrolimus, methotrexate may be increased. An interaction is observed between clopidogrel and esomeprazole, but the clinical relevance is uncertain. As a precaution, concomitant use of esomeprazole and clopidogrel should be discouraged. Gastric acid suppression by PPIs increase or decrease absorption of drugs with pH dependent absorption (decreased absorption of ketoconazole, itraconazole); esomeprazole inhibits CYP2C19 metabolising enzyme and could increase plasma concentrations of diazepam, citalopram, imipramine, clomipramine, phenytoin (monitor plasma levels of phenytoin), etc. resulting in need of a dose reduction; monitor INR when given with warfarin or similar. Caution as absorption of digoxin can increase. E ect of other drugs on esomeprazole: CYP2C19 and CYP3A4 inhibitors (clarithromycin, voriconazole) may increase the esomeprazole exposure. Dose adjustment not regularly required, except in severe hepatic impairment and long-term use. CYP2C19 and/or CYP3A4 inducers (rifampicin and St. John’s wort) may lead to decreased esomeprazole serum levels by increasing the esomeprazole metabolism.
Pregnancy and Lactation: Caution in pregnancy due to lack of clinical data. No studies in lactating women, therefore, not recommended during breast-feeding. Ability to Drive and Use Machinery: Minor in uence on the ability to drive or use machines. Adverse reactions such as dizziness (uncommon) and blurred vision (rare) have been reported. If a ected, patients should not drive or use machines. Undesirable E ects: Common: Headache, abdominal pain, constipation, diarrhoea, atulence, nausea/vomiting, fundic gland polyps (benign). Uncommon: Peripheral oedema, insomnia, dizziness, paraesthesia, somnolence, vertigo, dry mouth, increased liver enzymes, dermatitis, pruritis, rash, urticaria, fracture of the hip, wrist or spine. For other side e ects refer to the SPC.
Marketing Authorisation Holder: IQ Pharmatek Ltd., Gurtna eur, Old Waterford Road, Clonmel, Co. Tipperary. Marketing Authorisation Number: PA 22777/001/001. Further information and SPC are available from: Rowex Ltd, Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417. E-mail: rowex@rowa-pharma.ie
Legal Category: Not subject to medical prescription.
Date of Preparation: September 2019
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing medsafety@hpra.ie or by emailing Rowex pv@rowa-pharma.ie
Through Pharmacy
Supply
Only
Oceans of Success
Ocean Healthcare celebrate 20-year Anniversary in Business
Ocean Healthcare have recently celebrated a milestone, notching up 20 years in pharmacy in Ireland! We recently spoke to Managing Director, Graham Stafford for an insight into the background of this dynamic business and to learn more about future opportunities.
CELEBRATING 20 years
Ocean Healthcare is a dynamic privately owned business.
Established in 2003, Graham and his team offer sales, marketing and distribution solutions to healthcare brand owners within the pharmacy, grocery, and dental channels in Ireland.


“We manage some major international healthcare brands, offering a range of solutions to brand owners,” he says. “From total brand management to sales and national account management, we can tailor a strategy to meet the commercial requirements. Our experienced commercial team have a proven track record building brands and repositioning stagnating brands for growth.”
With established long-standing relationships with key players in the retail trade Ocean Healthcare supply over 2,500 accounts throughout the north and south of Ireland either directly or indirectly via wholesale.
Graham established the business following a return to Ireland from Australia, where he worked for a healthcare distributor. He explains, “Once the Irish market
was deregulated in 2002, it was clear that the pharmacy sector was going to expand quickly.
“I was aware that this would result in more pharmacies and the consolidation of groups. At this point I foresaw – and grabbed - an opportunity; knowing healthcare brand owners would need their brands represented.
“Our vision was to build a leading independently owned full-service distribution business with a dynamic approach to sales, marketing and customer service.”
Graham continues, “We started with a very small but good portfolio of brands including Sudocrem. The first new product we ever introduced to the Irish market was Aloclair, for the treatment of mouth ulcers.”
Twenty years later and Aloclair is still part of Ocean Healthcare’s portfolio.
So what is it that Graham thinks sets Ocean apart from their competitors? The secret to success, he believes, is working with a select portfolio of brands whilst focusing on maximising them to the utmost.
“We focus on maximising the brands within our business via a 360 approach to sales and marketing. We have built an experienced commercial team,” he notes.
“We are also working in partnership with our pharmacy customers to maximise our joint
business. We pride ourselves on excellent service, operating on next day delivery for orders received by 1pm.
“Looking back on the last twenty years, I am immensely grateful that we have had the opportunity to build a great team, work with great brands and with fantastic pharmacy partners. We have had some great wins over the years and now work with an excellent portfolio including Bio-Oil, E45, TePe, Proceive, Foster Grant. Of course behind all successful achievements lie many challenges. For Graham and the team at Ocean Healthcare, this has predominantly lay in the recruitment sector.
“Recruitment and retention of staff in a buoyant market has definitely been a challenge over the last 12 months,” he told us.
“Supply chain issues have also been challenging, but we have increased our resources in this area to ensure continuity of supply of our products.”
With 20 years now under their belt, what is next for this thriving healthcare business? Graham adds, “We are continuing to evolve as a business, we get better at what we do every year! We have invested heavily in recent years in marketing and training managers to help drive sales of our brands. We want to continue building a successful independent Irish owned business.”
For any Employer looking to grow their team, what is one piece of advice Graham would give?
“Have a robust recruitment, onboarding, training, and development process.
PHARMACYNEWSIRELAND.COM 18
Corporate Profile
Graham Stafford, Ocean Healthcare
“We focus on maximising the brands within our business via a 360 approach to sales and marketing. We have built an experienced commercial team…..
We are also working in partnership with our pharmacy customers to maximise our joint business…”
Ocean Healthcare Team
Cold and Flu Film-Coated Tablets contain:



A triple combination of antihistamine, analgesic and stimulant


Caffeine strengthens the analgesic and antipyretic effects of Paracetamol*

A therapy for the management of the symptoms of upper respiratory tract infections







Please consult the summary of product characteristics or contact the marketing authorisation holder for full product information. Name of product: Ilvico Cold and Flu film-coated tablets. Active Ingredient: Paracetamol 325 mg, Caffeine 30 mg, Brompheniramine maleate 3 mg. Supply classification: For supply through pharmacies only. Name and address of the marketing authorisation holder: P&G Health Germany, GmbH, Sulzbacher Strasse 40, 65824 Schwalbach am Taunus, Germany Licence Number: PA22703/001/001. Indication: For the relief of symptoms associated with the common cold, influenza and upper respiratory tract infections. Method of administration: For oral use. Adults and children older than 12 years: One or two tablets taken orally with water three times daily and the maximum dose of 6 tablets daily should not be exceeded. Not recommended for children under the age of 12 years. Please refer to the SmPC for posology instructions for the elderly or those with renal or hepatic impairment. Potential Side effects: thrombocytopenia, leucopenia, agranulocytosis, pancytopenia, haemolytic-anaemia, palpitations, arrhythmia, ventricular depression, bradycardia, hypertention, hypotension, transient vasodepression, sedation, drowsiness, ataxia, headache, restlessness, insomnia, somnolence, impairment of cognition, tremor, dizziness, mydriasis, blurred vision, dry eyes, bronchospasms, dyspnoea, nausea, dry mouth, fatigue, increased level of aminotransferase, jaundice, urinary retention, urinary hesitation, erectile dysfunction, skin reactions, quinke’s oedema, sweating, acute generalised exanthematous pustulosis (AGEP), stevens-johnson syndrome (SJS), toxic epidermal necrolysis (TEN). Special warnings and precautions: Paracetamol should be administered with caution under the following circumstances: hepatic impairment; chronic alcoholism; renal impairment (GFR≤50ml/min); gilbert’s Syndrome (familial non-haemolytic jaundice); concomitant treatment with medicinal products affecting hepatic function; glucose-6-phosphate dehydrogenase deficiency; haemolytic anaemia; glutathione deficiency; dehydration; chronic malnutrition; weight less than 50kg; the elderly. Contraindications: Must not be used if patient: has hypersensitivity to the active substances or to any of the excipients listed in section 6.1; has the presence of narrow-angle glaucoma; has brain damage or epilepsy; is less than 12 years old; has tachyarrhythmias; has peptic ulcers; has severe renal impairment; has severe hepatic impairment (including viral hepatitis); has haemophilia; has vesical neck obstruction, symptomatic prostatic hypertrophy or urinary retention. Reviewed: November 2021.
Further information is available on request from PRL on +353 1 257 4650 or pgsales@prl.ie
 *Summary of Product Characteristics, Ilvico Cold and Flu film-coated tablets.
P&G Health Germany GmbH, Sulzbacher Strasse 40, 65824, Schwalbach am Taunus, Germany
*Summary of Product Characteristics, Ilvico Cold and Flu film-coated tablets.
P&G Health Germany GmbH, Sulzbacher Strasse 40, 65824, Schwalbach am Taunus, Germany
Contour Next Blood Glucose Meters: Right at your fingertips!
Irish Pharmacy News, in partnership with Ascensia Diabetes Care conducted a survey last month where we asked our pharmacist readers questions on how they stock up on blood glucose meters, the frequency of visits from people with diabetes and their recommendations.
• Second-Chance® sampling allows 60 seconds to apply more blood to the same strip if the first sample was insufficient, reducing test strip wastage
FAQs
Is there any training available to pharmacy?
Do the meters come with warranty?
What are Contour Next Meter Key Features?


• Easy to use blood glucose monitoring system.
• Highly accurate1 test readings.
• Contact Ascensia Diabetes Care by calling Customer Support on freephone +1 800 920111 for more information on how to contact your local representative.
• Lifetime meter warranty excluding consumables. For online meter registrations, visit https://www.diabetes.ascensia.ie/meter-registration/
• smartLIGHT® target range indicator gives instant feedback on blood glucose readings.
How many readings can be stored on device?
• Second-Chance® Sampling technology allows 60 seconds to apply more blood to the same strip.
Does Contour Next have app or online support?
• 800 test memory - store up to 3 months of your results, which is suitable if patient needs maintain a record for the Road Safety Authority.
Is there any training available to pharmacy?
• Free CONTOUR®DIABETES app to support diabetes self-management.
What is the accuracy level of the Contour Next Meter?
• Contact Ascensia by calling Customer Support on freephone +1 800 920111 for more information on how to contact your local representative.
Yes, the Contour® Diabetes app is free to download. Pairing a CONTOUR® connected meter with the CONTOUR®DIABETES app allows readings to be automatically synced. The Contour Diabetes app is associated with improved glycaemic control. It has been demonstrated to reduce the likelihood of hyper- and hypoglycaemic results after 180 days usage.2,3
Does Contour Next have app or online support?
South East - Maria Purcell Ph: 086 8181834 maria.purcell@ascensia.com
There were some interesting findings from the survey and we thank all the pharmacy readers that took the time to complete it. The survey results showed that a large proportion of respondents, at 68% showed a keen interest in diabetes care. Just over half of the respondents, were from the larger ‘Buying Group’ category, followed by 29% from independent retailers.
West - Barbara Muldoon
Ph: 086 0455125 barbara.muldoon@ascensia.com
The survey results also showed that just over half of the participants saw between 20-50 people with diabetes weekly with a majority of 64% having 101-300 people in this cohort on their system.
The CONTOUR®NEXT meter uses the CONTOUR®NEXT blood glucose test strips which have demonstrated to deliver exceptional accuracy1 to help users make better diabetes management decisions.
• Get Smart Alerts - be alerted when blood glucose levels are at a critical high or low and set reminders.
Yes, the Contour® Diabetes app is free to download. Pairing a CONTOUR® connected meter with CONTOUR®DIABETES app allows readings to be automatically synced.
North East - Denise Butler Ph: 087 166 0065 denise.butler@ascensia.com
• Accurate results, compatible with the ISO 15197:2013 industry standard for accuracy.1,4
• My Patterns - receive personalised testing schedules based around daily activities and diabetes management plan.
The Contour Diabetes app is associated with improved glycaemic control. It has been demonstrated to reduce the likelihood of hyper- and hypoglycaemic results after 180 days usage 2,3 .
• Only a tiny sample size of 0.6µl is required.
South West - Mary Gavin Ph: 086 0455136 mary.gavin@ascensia.com
• Second-Chance® sampling allows 60 seconds to apply more blood to the same strip if the first sample was insufficient, reducing test strip wastage. Do the meters come with warranty?
Interestingly, over 71% of participants stated that they didn’t prefer a particular brand over another. Of the 29% that did have a preference, their selection was highly varied. It is important to note that meter features vary and accuracy is an important factor for effective diabetes management.
Although 77% said they would not switch a patient’s meter, reviewing the suitability of a blood glucose meters to suit the specific needs of a patient is a very important aspect of diabetes management.
• Add Events to Readings - record events such as diet, activities and medication to support self management.
• Get Smart Alerts – be alerted when blood glucose levels are at a critical high or low and set reminders.
• My Patterns – receive personalised testing schedules based around daily activities and diabetes management plan.
• Lifetime meter warranty excluding consumables. For online meter registrations, visit https://www.diabetes.ascensia.ie/ meter-registration/
• Test Reminder Plans - learn how different food and activities affect blood glucose readings for optimal test times.
• Share Report with Nurse/Doctor - Easily share readings with HCPs.
• Add Events to Readings – record events such as diet, activities and medication to support self management.
1. Data on File. Ascensia Diabetes Care. N=326. CNext/CTV3 Study. Protocol no. GCA-PRO-2018-006-01.
• Test Reminder Plans – learn how different food and activities affect blood glucose readings for optimal test times.
How many readings can be stored on device?
2. Stuhr A and Pardo S. Impact of Real-World Use of the CONTOUR®DIABETES App on Glycemic Control and Testing Frequency. Poster presented at the Diabetes Technology Meeting (DTM). November 8–10, 2018, North Bethesda, Maryland, USA.
• Share Report with Nurse/Doctor – Easily share readings with HCPs. Our Customer Support team can be contacted by emailing diabetessupport@ascensia.com or calling freephone 1 800 920111 (Mon-Fri 9am to 5pm).
• 800 test memory - store up to 3 months of your results, which is suitable if patient needs to maintain a record for the Road Safety Authority.
3. Pardo S et al. Changes in Blood Glucose Excursions After at Least 180 Days Real-world Use of a New Smartphone Application for Blood Glucose Monitoring. Poster presented at the 12th International Conference on Advanced Technologies & Treatments For Diabetes (ATTD); February 20–23, 2019; Berlin, Germany.
4. International Organization for Standardization. In vitro diagnostic test systems – requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197). International Organization for Standardization, Geneva, Switzerland, 2013. Contact
Feedback from this survey indicated that there exists an appetite for increased training, education for patients on new products as well as updates from professional bodies on the provision of care for diabetes patients.
Please Scan QR to access Contour Next Instructional Video or click here in viewing on line.

PHARMACYNEWSIRELAND.COM 20 Reader
Offer
for meters free of charge and your 2023 wall planner
grainne.oconnor@ascensia.com




*Offer available while stocks last. ** Source: Euromonitor Passport report on Consumer Health National Statistics. Date: September 2022 Food supplements should not be used as a substitute for a varied diet and healthy lifestyle. ** 1 Super Supplement with 26 active ingredients including 11 Vitamins, 7 Minerals & 6 Amino Acids to boost your energy levels, reduce fatigue and support your immunity & heart, all in 1 daily sachet. Energy Immune System Heart 150mg CoQ10 3,000mg L-Arginine IMMUNE SYSTEM ENERGY HEART IMMUNE SYSTEM ENERGY HEARTIMMUNE SYSTEM ENERGY HEART Fatigue Call: 091 769803 | Email: customerservice@reviveactive.com | Web: reviveactive.com 20% EXTRA FREE WHILE STOCKS LAST* THE POWER OF ONE SACHET
Pharmacy Potential to Close Gender Gap
Existence of a gender gap in pain management and the potential for pharmacists to close this gap is highlighted by FIP in a report of a meeting of an international insight board published recently.
FIP Insight Boards are invitation-only meetings of experts and representatives of FIP member organisations to discuss a specific topic and collect country-level insights and best practices on that topic.
An insight board on pain and gender, assembled by FIP in Seville, Spain, in September 2022, was co-chaired by FIP vice president Professor Parisa Aslani. “Research indicates that pain experienced by women is underestimated and undertreated. Alarmingly, in addition to receiving less effective pain relief, evidence suggests that women with pain are more likely to be prescribed antidepressants,” Professor Aslani said. The evidence and insights highlight the existence of gender differences in pain experiences and pain management. A key principle was established: the clinical goal of pain management must be to provide quality, fair and equitable patient-centred treatment. A gender responsive pharmaceutical care approach is required to ensure gender-based differences in physiological pain mechanisms
or psychosocial factors are managed equitably. Pharmacists play a key role in pain management, including medication counselling and referrals, and thus they must be unbiased but also be gender-responsive in pain management during their practice. It should be noted that the views expressed during the insight board are those of the individuals based on their expertise and experience. They do not represent FIP policy or positions, although they may build on existing positions and statements. Reports from FIP insight boards seek to provide qualitative viewpoints and descriptive observations, not generalisable or global or fully evidenced report. These findings can inform further policy development or confirm positions already held but they do not occupy the status of a full FIP report. FIP will use the insights in this report to consider what further support will be required by pharmacists to support evidence-based decision making and appropriate patientcentred care.
The report concludes that, “If pharmacists are to treat their patients equally, they can start
Irish Healthcare Awards
with small steps. Pharmacists and pharmacy students can be educated about bias and the factors unconsciously affecting pharmaceutical services on pain management.
“Creating protocols for pharmacists to manage pain in a gender neutral and responsive way, as well as providing tools to measure and evaluate pharmacists’ interventions, is necessary to approach the problem. Pharmacists also have a responsibility to educate the public on where and how to seek help for pain management, thus further addressing the gender pain gap. This fits closely with the FIP Equity Rx workstream, FIP Development Goal (DG) 10 (equity and equality), alongside access to medicines, devices and services (DG 18), people centred care (DG 15), and advancing integrated services (DG7).”
The insights gathered at the meeting will be used by FIP to develop resources for pharmacists to support evidence-based decision making and appropriate patientcentred care in pain management.
The Irish Businesses, owned by the PHOENIX Group, Europe’s leading healthcare provider, including United Drug, LloydsPharmacy, TCP Homecare and Median Healthcare were delighted to receive some fantastic awards in December.
United Drug were ecstatic to have won the Patient Education Project of the YearPharmaceutical award at the Irish Healthcare Awards in December for their Pharmacy Webinar Series.



United Drug are extremely proud of their teams and the fantastic work that they do. The United Drug Marketing team includes Dervila Mc Garry – Head Of Marketing, Lucia Maria Onofri – Brand and Digital Marketing Manager, and Julie Nicholl-Flood – Senior Digital Marketing Executive along with our Superintendent Pharmacist at LloydsPharmacy Ireland, Denis
O’Driscoll and Clinical Governance Pharmacist Martin Hynes who have been instrumental in delivering their webinar programme to pharmacists nationwide.
The United Drug Distribution team were highly commended at the awards ceremony for their role in delivering the vaccination programme, having been shortlisted for the Ireland’s Covid-19 Vaccinatoin Programme category. The team had previously won an award for their COVID-19 Crisis Response at the Pharma Industry Awards in November.


PHARMACYNEWSIRELAND.COM 22 News
Pictured are the United Drug team, winners of the Patient Education Project of the Year - Pharmaceutical award at the Irish Healthcare Awards
To request more information on Active Iron, please contact your Pharmed Ireland representative or email info@activeiron.com






Or scan:

For more information, please visit www.activeiron.com/activeiron/healthcare-professionals/


Clinically proven twice the
compared
et al.
Acta Haematological, 138: 223-232 Ledwidge et al. Data on file.
absorption
to iron sulfate. Wang
2017,
Solvotrin
Therapeutics, Hoffmann Park, Little Island, Cork T45 YX04
TIME, SAFE, STAY – let’s make it general knowledge!
International Epilepsy Day takes place on the 13th of February and Epilepsy Ireland are hard at work finalising their latest campaign for the day. In this article, Paddy McGeoghegan, Epilepsy Ireland Advocacy & Communications Manager writes about the upcoming day and the three key words of Time, Safe, Stay…

 Written by Paddy McGeoghegan, Advocacy & Communications Manager, Epilepsy Ireland
Written by Paddy McGeoghegan, Advocacy & Communications Manager, Epilepsy Ireland
them is. This could even be your colleagues in a hospital setting – including nurses, doctors, and support staff.
What many people also don’t realise is that up to 70% of people living with epilepsy will go on to become seizure-free when the right treatment for their individual epilepsy is identified. For people within this 70%, epilepsy is sticking to their medication routine, knowing and avoiding their triggers, and thankfully for some, epilepsy can almost become an afterthought for them. For the other 30%, unfortunately the condition will be much more challenging due to uncontrolled seizures and the associated impacts of this.
The reality is that, with almost 1 in 100 people living with epilepsy, epilepsy is far from rare and there are likely people within your close circle who are either living with the condition or somebody close to
International Epilepsy Day is a vital opportunity to improve understanding of epilepsy. At Epilepsy Ireland, we encourage people with epilepsy to share their stories of living with the condition as part of the day in
It was fantastic to once again be approached by the editorial team at Hospital Professional’s News to provide readers with an update on our plans for International Epilepsy Day 2023. As the name suggests, International Epilepsy Day is a global annual event that aims to spread awareness of a condition which affects over 50 million people worldwide.
With preliminary census data released towards the end of last year – coupled with existing prevalence data, we now estimate that there are over 45,000 people living with epilepsy across Ireland today, making it one of the most common neurological conditions in the country.
In our engagements with the public, this number often takes people by surprise. In market research conducted by Amarach Research on the behalf of Epilepsy Ireland towards the end of last year, 80% of over
1,000 respondents totally underestimated the prevalence of epilepsy, suggesting that the public believe epilepsy to be a quite rare condition.
So why is this perception out there? One reason perhaps is that despite the prevalence of epilepsy, there are still longstanding myths and misconceptions around the condition. There can be a misconception that people with epilepsy are not able to do certain things – such as perform in the workplace, drive, play sports or take part in social activities. Too frequently there is a belief that people living with epilepsy need to be treated differently due to their condition. In addition, due to the nature of seizures and how they present, there can be a social stigma attached to the condition for some people. These and other misconceptions can sometimes mean that a person might not necessarily widely disclose that they are living with the condition.
PHARMACYNEWSIRELAND.COM 24 Epilepsy
What many people also don’t realise is that up to 70% of people living with epilepsy will go on to become seizurefree when the right treatment for their individual epilepsy is identified.
LEVETIRACETAM THAME
100MG/ML ORAL SOLUTION

Now added on the List of Interchangeable Medicinal Products
Interchangeable list code: IC0119-171-019
38.55% COST SAVING VS KEPPRA 100MG/ML ORAL SOLUTION1
Cost comparison of Syri Pharma’s Levetiracetam Thame 100mg/ml oral solution and Keppra 100mg/ml oral solution
PRESCRIBING INFORMATION
Levetiracetam Thame 100mg/ml Oral Solution
Presentation: Each ml of oral solution contains 100mg Levetiracetam.
Indication: Monotherapy in the treatment of partial onset seizures in adults and adolescents from 16 years of age with newly diagnosed epilepsy. As adjunctive therapy: In the treatment of partial onset seizures in adults, adolescents, children and infants from 1 month of age with epilepsy, in the treatment of myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy and in the treatment of primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy.
Dosage and administration: Route of administration: Oral. Adults: monotherapy: Initially 250mg to 500mg twice daily. If 250mg given twice daily initially increase to 500mg twice daily after 2 weeks. Dose can be increased to 1500mg twice daily Dose can be increased by 250mg or 500mg twice daily every two weeks depending upon the clinical response Add-on therapy: 500mg twice daily. Can be increased up to 1500mg twice daily. Paediatric population: monotherapy: The safety and efficacy have not been established. Add-on therapy 6 months to 17 years (weighing less than 50kg): 10mg/kg twice daily, can be increased up to 30mg/kg twice daily. Dose changes should not exceed increases or decreases of 10mg/kg twice daily every two weeks. The lowest effective dose should be used Add on therapy infants aged 1 month to less than 6 months; Initial dose 7mg/kg twice daily. Dose can be increased by 7mg/kg twice daily every two weeks to max dose 21mg/kg twice daily. Elderly: Adjustment of the dose is recommended in patients with compromised renal function. Renal impairment: daily dose must be individualized See SmPC. Hepatic impairment: 50% reduction of the daily maintenance dose is recommended when the creatinine clearance is <60ml/min/1.73m2
Contraindications: Hypersensitivity to the active substance or other pyrrolidone derivatives or to any of the excipients.
Precautions and warnings: Renal impairment. Complete blood cells counts if weakness, pyrexia, infections or coagulation disorders reported. Monitor patients for signs of depression and / or suicidal ideation and behaviours.
Monitor for psychotic symptoms, irritability and aggressiveness. Monitor for aggravation of epilepsy. Caution in patients with QTc prolongation, concomitantly treated with drugs affecting QTc interval or in patients with pre-existing cardiac disease or electrolyte disturbance.
Interactions: Methotrexate, macrogol. See SmPC for detailed information. Pregnancy and lactation: can be used during pregnancy, if after careful assessment it is considered clinically needed. Breast-feeding is not recommended.
Driving / using machines; Patients are advised not to drive or use machines until it is established that their ability to perform such activities is not affected. Undesirable effects: Nasopharyngitis, somnolence, headache, anorexia, depression, hostility/aggression, anxiety, insomnia, nervousness/irritability, convulsion, balance disorder, dizziness, lethargy, tremor, vertigo, cough, abdominal pain, diarrhoea, dyspepsia, vomiting, nausea, rash, asthenia/fatigue, thrombocytopenia, leukopenia, suicide attempt, suicidal ideation, psychotic disorder hallucination, confusional state, amnesia, vision disturbance, pancreatitis, hepatic failure, acute kidney injury, infection, pancytopenia, neutropenia, agranulocytosis DRESS, ECG QT prolonged, encephalopathy, Neuroleptic malignant syndrome, hepatic failure, Toxic epidermal necrolysis, Stevens -Johnson Syndrome, erythema multiforme, rhabdomyolysis.
(Please refer to the Summary of Product Characteristics for detailed information)
Legal category: POM
Marketing Authorisation Number: PA22697/012/001
Marketing Authorisation Holder: Syri Pharma Limited t/a Thame Laboratories Floor 0, 1 WML, 1 Windmill Lane, Dublin 2, D02 F206, Ireland. (Distributed by Advanz Pharma Ltd in Ireland)
Date of preparation: Sept 2022
Reference:
1. Data on file Levetiracetam 100mg/ml oral solution June 2020
ADV/LVI/PM/0012 Date of Preparation - December 2022
Product Keppra 100mg/ml oral Syri Pharma's Levetiracetam Thame Cost saving % saving solution IMF June 20201 100mg/ml oral solution Levetiracetam Thame 100mg/ml oral solution € 52.03 € 31.97 € 20.06 38.55% 300ML ORAL SOLUTION WITH 10ML DOSING SYRINGE
order to break down the myths and misconceptions that exist around epilepsy. These lived experiences are an extremely powerful tool in helping people better understand what it is actually like to live with the condition and how to better support people with epilepsy.
Year on year, what is often reported to our service by people with epilepsy and their families is how crucial it is for everyone to know what to do in the event of a person having a seizure. In this regard, the message is very simple, consisting of just three key words – TIME, SAFE, STAY.
The message focuses on three positive ‘actions’ that should be followed, rather than on what not to do, while reducing the perception that seizure first aid is difficult and complex.
So, what do we mean by TIME, SAFE, STAY?
TIME
The first thing you should do is TIME the seizure. This is because if a seizure goes over 5 minutes, an ambulance should be called.
SAFE
Keep the person SAFE during the seizure. If a person is having a convulsive seizure, cushion their head with something soft if possible and remove any harmful objects, e.g., furniture from their
vicinity. NEVER put anything in a person’s mouth or restrain them during a seizure. Be aware that there are also types of seizures where the person does not experience convulsions. Instead, they may “zone out” or stare blankly, become confused or agitated, display behaviours like chewing, smacking their lips, fiddling with their clothes, or wandering aimlessly. In these types of seizure, the person’s awareness of their surroundings is affected, and it is important to gently guide the person
away from any danger. As with convulsive seizures, never restrict the person’s movements. STAY
During the seizure and after it passes, STAY with the person. Often after a seizure, a person with epilepsy will be confused and, in many cases, exhausted. Make sure to stay with them until recovery is complete, explain what has happened and gently reassure them. The person may have experienced an injury if they have fallen, and if this is the case, ensure normal first aid steps are taken.
For last year’s International Epilepsy Day, in our continued promotion of these key words, we presented an eye-catching, tongue in cheek campaign – showing an exaggerated view of how people with epilepsy might be treated differently by the public due to their condition – and an exaggerated view of how seizures might be responded to incorrectly by the public. The campaign featured our incredible volunteers Wayne, Lucy and Lisa donning a suit of armour; being encased in a zorb ball; and wearing a suit of bubble wrap to help drive home the Time, Safe, Stay message.
With over 2.5 million impressions on social media, the campaign certainly grabbed the attention of the public, but equally we know that Time, Safe, Stay and seizure first aid is still some way off becoming general knowledge. This is why we return to the theme of Time, Safe, Stay this year – but with a new angle!


While the involvement of people with epilepsy will still be central to the efforts, this year we’re changing tact and making unsuspecting members of the public the subjects of our campaign as we aim to make Time, Safe, Stay general knowledge!
That is all we can reveal for now, but we’re really excited by what we’re working on and as hospital professionals, we hope you can help assist us in any way you can by sharing this important, potentially life-saving information amongst your colleagues, family and friends. Do join with us in the lead up to #EpilepsyDay to help increase awareness of this incredibly common condition and to help us make seizure first aid general knowledge amongst the public!
Further information on International Epilepsy Day and Seizure first aid –including videos and downloadable posters which can be used in your workplace – can be found on our website, www.epilepsy.ie.
l would also like to remind readers that Epilepsy Ireland is here to support people with epilepsy and their families on their respective journeys with the condition. Our network of Community Resource Officers cover the entire country and if you have a patient who may benefit from their support, please do not hesitate to get in touch with us.
Thank you reading this important piece about seizure first aid and Time, Safe, Stay and keep your eyes and ears peeled for our new campaign launching in the lead up and on International Epilepsy Day on February 13th!
PHARMACYNEWSIRELAND.COM 26 Epilepsy
United Drug Supporting Childline at Christmas
€16,000 Raised to support Childline at Christmas by colleagues and customers of United Drug, LloydsPharmacy, Median Healthcare and TCP Homecare.




The Irish Businesses, owned by the PHOENIX Group, Europe’s leading healthcare provider, include United Drug, LloydsPharmacy, TCP Homecare and Median Healthcare. The group were delighted to announce their official partnership with the ISPCC last September. From here, they formed a group charity committee to roll out their Christmas initiatives and raise much needed funds to support the charity and their overall aim of providing children with the facilities to listen to them, empower them, strengthen their resilience, and enable them to live their best possible lives.
ISPCC was chosen as the new charity partner for the Irish Healthcare Group after a companywide vote and a period of research and consultation with staff. The partnership came into effect at the end of September. This ISPCC partnership further highlights how the group is committed to looking after all customers regardless of age, gender, or location.
Christmas Cards Competition and Donation Days

In 2021, Childline experienced a 28% increase in calls from Children who were “Anxious and Isolated” during the 3-day
Christmas period. It also takes ¤12,000 to run Childline for just one day so the overall goal for the group charity committee was to raise ¤12,000 to support Childline on Christmas Day.
To reach this goal, the group fundraised by selling Christmas Cards designed by the children of staff across the four businesses, United Drug, LloydsPharmacy, TCP Homecare and Median Healthcare. They had a heartening response from the children of their colleagues to the ISPCC Christmas card competition, with 90 entries. Ten fabulous festive designs were transformed into Christmas cards and sold as a pack of 10, priced at ¤10.
In addition to this, they hosted an ISPCC Donation Day in each business consisting of smaller fun events like raffles, Christmas jumper competitions, and a coffee morning.
Smashed their target of ¤12,000
The group were absolutely thrilled to announce that they had smashed their target for the ISPCC Childline Christmas initiative. With a target of ¤12,000, they raised a whopping ¤16,000 to support Childline this Christmas.
CEO Paul Reilly commented, “A special thank you to all our colleagues, customers and suppliers for their generosity, time and fundraising efforts. The Charity
Programme gives our colleagues and customers the chance to support a cause that is close to their hearts and helps to make a difference to the local communities where we work and live.
“Your kindness and generosity helped Childline to continue to provide the safe space where every child and young person who wants to connect with Childline, can and where they can access the support that’s right for them in the way they feel most comfortable, especially at Christmas time.”
Almost 600 calls, texts and online messages were answered by Childline over Christmas
Almost 600 calls, texts and online messages were answered by Childline, over Christmas Eve, Christmas Day, and St Stephens Day. Over 70 volunteers were involved in operating the support line over the Christmas period. The volunteers said that “Many children and young people in Ireland felt lonely, stressed, and upset this Christmas. Their feelings were exacerbated as they saw families and friends celebrating together and it’s not like that for them. They turned to Childline for a listening ear and a supportive voice to hear them.”
On behalf of all the children and young people who Childline supports, the ISPCC thanked the group and everyone who participated and supported the Christmas Campaigns across the four businesses which helped to keep the service there 24 hours a day on Christmas Day.
PHARMACYNEWSIRELAND.COM 27 News
Dervila McGarry, Head of Marketing, McKesson Ireland, John Church, CEO, ISPCC and Paul Reilly, Managing Director of McKesson Ireland
Pharmacy Lease of Life to Post Office
A brand new 5,000-square-foot Pharmacy, Lifestyle store and Café opened recently at the Old Post office in Clonmel town in Tipperary by Mulligan’s Pharmacy Group.

The brand new pharmacy has offered a new lease of life to the disused Old Post office at the top of Gladstone Street, and in the newly transformed space, there is a full pharmacy, a gift gallery, a fragrance counter for ladies and gents, and a beauty hall featuring all of the top Irish and international brands. On the first floor, a brand new Café titled ‘Café 1901’ giving the nod to the building’s history is serving speciality coffee, herbal teas, healthy smoothies and milkshakes plus handmade sourdough pastries and gourmet sandwiches, light bites and a selection of sweet treats.
The new Mulligan’s pharmacy sees 30 people employed at the store with new jobs created both in the pharmacy and at the Café. The Mulligans group already employs 280 people across the Southeast
region with stores across Waterford, Kilkenny and South Tipperary and Dublin.
Commenting on the opening, MD of the group Ronan Mulligan says, “We are delighted to unveil the largest Mulligan’s store in the group here in Clonmel today. Tipperary is a place close to our hearts as it is where my own mother hails from, so today we look forward to seeing all our customers old and new at 32 Gladstone Street.
“We are thrilled to have offered this beautiful old building a new lease of life too, the heritage of the building is something we are celebrating in the new design and we look forward to taking the history of The Old Post Office even more to the forefront of the store over the coming months, with
plans for a heritage feature wall in our sights.”
The new Mulligan’s also hosts a complete range of health and wellbeing products, skincare, makeup, Irish brands, a huge range of men’s and ladies’ fragrances, exclusive cosmetics, plus a brand new gifting area.
The opening is the group’s 20th retail store. Mulligans was first established by Jim and Sheila Mulligan in 1957 when they opened a pharmacy at George’s St in Waterford City. The Irish familyrun business is led by a secondgeneration of the family Ronan and Kate Mulligan.
From today Mulligans Pharmacy Café 1901 at The Old Post Office in Clonmel will be open 7 days per week. For further details, see https://mulliganspharmacy.com/
New research fellowship programme to combat food and health challenges
APC Microbiome Ireland, a world-leading SFI Research Centre based at University College Cork (UCC), has launched the INSPIRE Fellowship programme which seeks to tackle challenges facing global food systems and health.

I am delighted to launch the INSPIRE programme which will support such impactful research.”
The INSPIRE Fellowship will provide experienced researchers with industry placement experience while benefiting from a tailor-made research and career development training plan. INSPIRE fellows will gain a portfolio of transferable skills to enhance their career options and to meet the evolving needs of the academic, NGO and industry sectors.
Professor Paul Ross, Director of APC Microbiome Ireland, added, “The INSPIRE Fellowship reflects APC Microbiome Ireland’s commitment to addressing the UN’s Sustainable Development Goals. We look forward to recruiting 20 postdoctoral researchers who will help create solutions to some of the most pressing global challenges with potential impact in the pharmaceutical, medical and food sectors.”
With funding of ¤2.9 million, INSPIRE (INnovative Sustainable Development InterdisciPlinary Post-Doctoral Research Excellence) is co-funded by the EU Horizon 2020 Marie SklodowskaCurie Actions programme.
INSPIRE will create 20 prestigious postdoctoral research fellowships at APC Microbiome Ireland based at UCC or at one of the APC’s other academic partner institutions.
INSPIRE Fellows will undertake research projects across a variety of areas of microbiome science to address the UN Sustainable Development Goals and create solutions to challenges relating to food and health including anti-microbial resistance, the burden of non-communicable diseases, and the need for sustainable food systems.
Dr Pamela Byrne, Chair of the APC Microbiome Ireland Governance Committee and CEO of the Food Safety Authority of Ireland, said, “Never in our time has sustainability been so important. INSPIRE addresses the huge challenges that we face globally, building a talent pool to ensure that we have skilled scientific researchers equipped and agile to meet the needs we will face in the coming years. APC Microbiome Ireland has a proven record of ground-breaking research targeting the grand challenges of our time.
Professor John Cryan, Vice President for Research & Innovation, said, “As a research-led University that has sustainability at the heart of our strategy and operations, UCC is proud to host the INSPIRE research fellowship programme at APC Microbiome Ireland. INSPIRE is a fantastic programme that will equip a cadre of highcalibre researchers to address the challenges we are facing globally through the development of credible, sustainable, sciencebased technologies within our UCC Futures: Food, Microbiome & Health initiative.”
PHARMACYNEWSIRELAND.COM 28 News
Ronan Mulligan, The Mulligans group Managing Director
Professor John Cryan, Dr Pamela Byrne, Professor Paul Ross, Oonagh Cahalane and Brendan Curran
Quitting Smoking
*Provides significant improvement in quit rate vs patch alone. To verify contact: verify@perrigo.com Lindson N et al. 2019 Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Library. IRE NIQ 2022 55 NiQuitin CLEAR 24 hrs transdermal patches contain nicotine and are indicated for the relief of nicotine withdrawal symptoms including cravings as an aid to smoking cessation. Indicated in adults and adolescents aged 12 years and over. NiQuitin patches should be applied once a day, at the same time each day and preferably soon after waking and worn continuously for 24 hours. Apply a patch to non-hairy clean dry skin surface, a new skin site should be used every day. Therapy should usually begin with NiQuitin 21 mg/24 hrs and reduced according to the following dosing schedule: Step 1: NiQuitin Clear 21 mg/24 hrs transdermal patches first 6 weeks. Step 2: NiQuitin Clear 14 mg/24 hrs transdermal patches next 2 weeks. Step 3: NiQuitin Clear 7 mg/24 hrs transdermal patches last 2 weeks Light smokers (less than 10 cigarettes per day) are recommended to start at Step 2 (14 mg) for 6 weeks and decrease the dose to NiQuitin 7 mg/24 hrs for the final 2 weeks. In some instances (e.g. heavy smokers, those who have relapsed after NRT, or when one NRT product is not enough to control cravings), NiQuitin patches may be used in combination with a nicotine oral format (refer to the package leaflet for dosing guidance). Contraindications: Non-smokers, hypersensitivity, children under 12 years and occasional smokers. Precaution: Supervise use if hospitalised for MI, severe dysrhythmia or CVA, if haemodynamically unstable. Use with caution in patients with active oesophagitis, oral and pharyngeal inflammation, gastritis, peptic ulcers, GI disturbances, susceptible to angioedema, urticaria, renal/hepatic impairment, hyperthyroidism, diabetes, phaeochromocytoma, seizures & epilepsy. Discontinue if severe persistent skin rash. Pregnancy and lactation: Oral formats preferable to patches unless nauseous. Remove patches at bedtime. Side effects: Sleep disorders, abnormal dreams, insomnia, headache, dizziness, nausea, vomiting, application site reactions, nervousness, palpitations, dyspnoea, pharyngitis, cough, dyspepsia, upper abdominal pain, diarrhoea, constipation, dry mouth, sweating, localised pain, urticaria, hypersensitivity, tremor, nervousness, palpitations, tachycardia, contact & allergic dermatitis, photosensitivity, arthralgia, myalgia, asthenia, malaise, influenza-type illness, fatigue, chest or limb pain, pain, seizures and anaphylaxis. Legal classification: GSL: PA 1186/018/004, PA 1186/018/005 & PA 1186/018/006. MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. Date of preparation: 04/2022. RRP (ex. VAT) 7 pack €19.83, 14 pack €35.73. SPC: https://www.medicines.ie/medicines/niquitin-clear-7-mg-24-hours-transdermal-patch-33085/ spc https://www.medicines.ie/medicines/niquitin-clear-14-mg-24-hours-transdermal-patch-33083/spc https://www.medicines.ie/medicines/niquitin-clear-21-mg-24-hours-transdermal-patch-33084/spc

NiQuitin Mini 2mg/4mg Mint Lozenges contain nicotine and are used for the treatment of tobacco dependence by relief of nicotine withdrawal symptoms and cravings. Indicated in adults and adolescents aged 12 years and over NiQuitin Mini 2 mg are suitable for those who smoke 20 cigarettes or less a day. NiQuitin Mini 4 mg are suitable for smokers who smoke more than 20 cigarettes a day. Place a lozenge in the mouth whenever there is an urge to smoke, allow to dissolve completely. Do not chew or swallow whole. In heavy smokers, those who have relapsed after NRT, or when one NRT is not enough to control cravings, NiQuitin Minis may be used in combination with NiQuitin patches (refer to the package leaflet for dosing guidance). Abrupt cessation: Use a lozenge whenever there is an urge to smoke, maximum of 15 lozenges a day. Continue for up to 6 weeks, then gradually reduce lozenge use. Gradual cessation Use lozenges whenever there is an urge to smoke in order to reduce the number of cigarettes smoked for up to 6 weeks, followed by abrupt cessation. Adolescents (12-17 years): Only with advice from a healthcare professional. Should not quit with a combination NRT regimen. Contraindications: Hypersensitivity to nicotine or any of the excipients, children under the age of 12 years and non-smokers. Precaution: Supervised use in dependent smokers with a recent myocardial infarction, unstable or worsening angina pectoris including Prinzmetal’s angina, severe cardiac arrhythmias, uncontrolled hypertensions or recent cerebrovascular accident. Use with caution in those with; stable cardiovascular diseases, diabetes mellitus, susceptibility to angioedema & urticaria, renal/hepatic impairment, phaeochromocytoma & uncontrolled hyperthyroidism, GI disease & seizures. Side effects: Nausea, mouth/throat and tongue irritation, irritability, anxiety, insomnia, sleep disorders, dizziness, headaches, cough, sore throat, vomiting, diarrhoea, upper abdominal pain, GI and oral discomfort, flatulence, hiccups, heartburn, dyspepsia, dry mouth, constipation, ulcerative stomatitis, pharyngitis, pharyngolaryngeal pain, nervousness, depression, palpitations, heart rate increased, dyspnoea, rash, angioedema, pruritus, erythema, hyperhidrosis, urticaria, fatigue, malaise, asthenia, chest pain, anaphylactic reactions, hypersensitivity, tremor, dysgeusia, paresthesia mouth, seizures & epilepsy, dysphagia, eructation, salivary hypersecretion, influenza like illness. Legal classification: GSL: PA 1186/018/017 & PA 1186/018/012 MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. Date of preparation: 12/2022. RRP (ex. VAT) 20 pack €7.55, 60 pack €21.05, 100 pack €28.00. SPC: https://www.medicines.ie/medicines/niquitin-mini-2mg-mint-lozenges-35237/spc https://www.medicines.ie/medicines/niquitin-mini-4mg-mint-lozenges-33091/spc

“Since I quit smoking, I enjoy a healthier me”
Samantha
How to Spot the Early Stages of AMD
Written by: Konstantina Danai Karagkiozeli, MSc, Science Communications Executive at Fighting Blindness (research@fightingblindness.ie ) And the ophthalmologists: Dr Matthew O’Riordan, Royal Victoria Eye and Ear Hospital Research Foundation, Dublin, Ireland - Mr Mark Cahill, Consultant Ophthalmologist, Progressive Vision Research, Dublin, Ireland
Age-related macular degeneration is a painless eye condition often referred to as AMD or macular degeneration. It is a progressive eye disease that affects up to 10% of adults over 65 years of age and is a leading cause of severe vision impairment and blindness in this age group.
window frames or door posts. If they see any distortion or realise sudden changes to their vision it is important to visit their doctor as soon as possible.
Independently of the above, to detect the earliest signs of AMD, it is recommended that everyone has an eye examination every one or two years by an optometrist or an eye doctor. This is particularly encouraged for people over 50 years of age, or who have a family history of AMD or both.
AMD causes the gradual loss of sight due to blurring or loss of central vision however the peripheral vision (vision to the side) is usually not affected. AMD is a chronic disease – it cannot be cured and in many patients sight cannot be restored after it is lost. However certain forms of the disease can be treated. Early detection is important to potentially stop the worsening of the disease and to protect an individual’s sight. Some changes in the vision that could be early signs to spot the development of AMD include:
• Straight lines such as door frames or steps appearing wavy or distorted.
• Smudge, shadow or gaps appearing in the field of vision.
• A glare in bright light or decreased levels of vision in low lighting or darkness.
• Challenges in reading small print, even with glasses, and in recognising colours or find that colours begin to fade.
• Printed words seem very blurry
• Difficulty in recognising or distinguishing faces and may notice everyday activities such as driving or watching TV can become seriously affected over time.

If anyone is having any of the above symptoms, we strongly recommend they visit an eye care professional as soon as possible as early detection can help avoid further eyesight damage.
Additionally, a useful test for spotting the early signs of AMD is the Amsler Grid. It is an at-home test where if an individual sees that the lines appear missing or wavy, they may have AMD and should contact their eye doctor immediately. Full instructions on how to use the Amsler Grid can be found on the Fighting Blindness website https://www. fightingblindness.ie/living-with-
sight-loss/eye-conditions/agerelated-macular-degenerationamd/ Please note that even if the grid looks normal, people should still attend regular eye exams for early detection of AMD. Also, frequent self-check-ups can catch the progression of the eye condition while in the early stages of AMD. People can monitor their sight and do a self-check up by closing one eye and look at
General eye check-ups are very important for people with AMD, as these individuals may still be at risk of developing other kinds of eye problems that affect the general population, some of which may be treatable. It is important to note if AMD is left untreated, serious sight loss can occur within 3-6 months so it is highly recommended to act fast in order to save as much as vision is left.
https://www.fightingblindness.ie/living-with-sight-loss/eye-conditions/age-related-maculardegeneration-amd/ Please note that even if the grid looks normal, people should still attend regular eye exams for early detection of AMD.
There are two forms of AMD, Early and Late. Only an eye care professional can tell which form an individual may have. In Early AMD the signs that the retina is being damaged are only visible to an eye care practitioner (optometrist or eye doctor). At this stage the damage does not affect sight and people are unaware of the condition. This highlights the importance of regular eye examinations to detect AMD in its early stages.
Some people progress from Early to Late AMD, where the condition causes loss of vision. There are two main types of Late AMD that can affect sight – Wet AMD and Dry AMD. The Dry form represents around 85-90% of all AMD cases, it causes gradual deterioration in your sight.
Wet AMD is a less common form of AMD but can develop more quickly and cause a rapid loss of vision. Wet AMD occurs when cells within the macula stop working and your body starts growing new blood vessels to fix the problem. The eye doctors may refer to this as neo-vascularisation.

Amsler Grid (Image Source: American Macular Degeneration Foundation)
Amsler Grid (Image Source: American Macular Degeneration Foundation)

Also, frequent self-check-ups can catch the progression of the eye condition while in the early stages of AMD. People can monitor their sight and do a self-check up by closing one eye and look at window frames or door posts. If they see any distortion or realise sudden changes to their vision it is important to visit their doctor as soon as possible.
It is important to note that AMD doesn’t cause a total loss of vision and, also, that it usually starts in one eye affecting the other only at a later stage.

PHARMACYNEWSIRELAND.COM 32 AMD
Konstantina Danai Karagkiozeli MSc
Dr Matthew O’Riordan
Mr Mark Cahill
WITH LUTEIN, MESO-ZEAXANTHIN AND ZEAXANTHIN


MacuShield Original + and MacuShield Original chewable contain B2 and MacuShield Gold contains zinc, both help to maintain normal vision




Visit us at macushield.com * Vitamin B2 helps maintain normal vision. DEC 2022 JB-001916
34 AMD
What tools are available for patients who are experiencing limited sight for the 1st time: tools for their everyday life eg screen readers etc, services available eg for psychological support
Receiving a diagnosis of AMD can be overwhelming, but this is not a journey that anyone has to make alone. There are resources available to provide support for people living with AMD. The eye doctor who gives the initial diagnosis can be an important source of information on the condition, so we recommend contacting them first with questions.
Getting diagnosed with AMD doesn’t mean stopping doing the things a person love; people with AMD can still read or cook or participate in their favourite activities. Here are some tips to help adapt the home when a person has been diagnosed with AMD:
• Colours: bright rather than pastel colours are preferable on the walls, as well as creating contrasts with dark and light tones. Also, items in solid bright colours are preferable rather than patterned or made of transparent material e.g. avoid bottles made of plastic or clear glass. Other way to create contrasts is by choosing different textiles e.g. in bedsheets or towels.
• Lighting: it is highly recommended to make sure there is enough lighting in the home as it can really make a difference. A way to achieve this is using extra lighting when doing specific tasks. For example, when cooking, install under - the - cabinet lights, for reading a lighted magnifier can be used etc.
Furthermore, there are nightlights that turn on automatically when it is dark and motion-sensing stair lights which turn on automatically when the person reaches an individual step.
However, avoiding sun reflections from windows or glares is recommended, so installing shades, curtains or blinds could be beneficial.
• Organisation matters: there are ways that could help make a home safer and more functional, like coloured tapes, sticky notes etc. We suggest using bold labelling when organising clutter, drawer dividers, and bottles of different shapes to differentiate similar looking liquids e.g. water and vinegar.
• Low-vision tools: these include talking clocks, large-button telephones, magnifying glasses (they have hand-held magnifying lenses
or magnifying lenses worn like glasses), tablet computers with voice recognition features and audiobooks.
Aside from buying the above a person can try changing the computer settings, like the font size and contrast. There are lowvision software that magnify text, adjust contrast etc. Moreover, the closed-circuit television systems use video camera to magnify reading material and project it on the screeen.
• Safety: try to keep hallways and staircases clear from things like shoes, umbrellas or anything else that could become an obstacle. Avoid putting furniture in the middle of the rooms and even more the low-level ones like coffee tables. Installing handrails on both sides of the staircase can help a person balance better to prevent accidents.
People with AMD have reported that one of the most difficult parts is when announcing to their family and friends their diagnosis. However, this is only the start and a person can experience many different emotions. Talking about their experiences with a professional or with others who also have the same eye condition can be very beneficial. As such, we encourage people with AMD to seek help from a counsellor or join a support group.
Fighting Blindness offers a free and confidential counselling service (Insight Counselling). For further information please contact insight@fightingblindness.ie or call 01 6746496.
We are also running a mindfulness group every Wednesday which takes place online 11 am-12:15pm. For technology support and guidance, we run online the Dublin-based Technology Exchange Club every Monday 11am-1pm and another Technology Exchange Club, based in Cork every Saturday 11am-1pm. For spouses/partners of somebody affected by sight loss, we run online a Partner’s Peer Support Group on the first Wednesday of the month at 3-4:30pm.
To join or for further information, contact us at 01 674 6496 or email at insightgroups@fightingblindness. ie . All groups run on zoom. We can help with setting people up on zoom or we can assist with any technology queries. We look forward to hearing from you!
How they might control the symptoms – OTC therapies that are available (eye drops / cooling masks etc)
Currently, there are no medical treatments for Dry AMD. Instead, an eye care practitioner will
suggest lifestyle changes and addressing any individual risk factors for AMD. Smoking is an important risk factor for AMD and it is strongly recommended to quit smoking. Other lifestyle recommendations include a healthy diet consisting of green leafy vegetable, fresh fruit and oily fish, and regular exercise. These changes may slow the progression of AMD. They are also part of a healthy lifestyle and will benefit general health too.
Studies have shown that a combination of vitamins and antioxidants may help reduce the risk of progression of Early AMD to late-stage AMD. The recommendations include supplements containing vitamins C and E, zinc, copper, lutein and zeaxanthin. These supplements are available overthe-counter. Remember that these supplements are not a cure for AMD and to find out more about what supplements that may work best for a person, it is advised they speak with an eye doctor. Research efforts are on-going to develop therapies to prevent or treat dry AMD. Injections of a drug into the eye, given by an eye doctor, may soon be available to treat the advanced form of dry AMD. If approved, this would be the first available treatment for late-stage dry AMD.
Fighting Blindness partly funds exciting research in Trinity College Dublin labs that may lead to new treatments. A lab recently announced the development of a promising new gene therapy. Another lab is investigating if changes to the body’s circadian rhythm may lead to leaky blood vessels at the back of the eye and cause dry AMD. You can find those publications in the list with resources at the end of the article, together with a news piece announcing the recent awarding of a grant for the above research.
Wet AMD on the other hand, can be treated if diagnosed early.
Treatment for Wet AMD involves a series of injections into the eye of drugs called anti-VEGF (anti-vascular endothelial growth factor). In Wet AMD, new abnormal blood vessels grow due to high production of the growth factor VEGF. These blood vessels are faulty and may leak fluid or blood into the macula. The treatment works by blocking VEGF and therefore reducing the growth of new blood vessels. However, if the damage has been left for a long time and scarring has already taken place, these treatments may not work well. An eye doctor will determine if a person is suitable for this treatment and discuss the most suitable treatment plan.
There soon may be other ways to deliver anti-VEGF drugs to the eye. A surgical operation could be performed to implant a drug reservoir filled with anti-VEGF in the eye. The reservoir would slowly release the drug over several months and reduce the need for regular injections. However, the device would stay in the eye and still need to be refilled with antiVEGF drugs. These devices are not yet ready for use.
There are alternative methods that are under development (not available yet) that could be less invasive, more user-friendly and self-administering. One of them are eye drops, but it is difficult to ensure that enough drug will reach the macula which is at the back of the eye. As a result, it is not currently possible to treat AMD with eye drops. Another potential treatment could be oral tablets. However, it is important to note that this method is not as targeted as eye injections, where the drug stays concentrated in the eye so other parts of the body are not affected.
As previously mentioned, it is important for people with AMD to have regular eye tests with an eye care professional. For people with AMD in one eye, the healthy eye will be at a greater risk of AMD. Regular monitoring may, therefore, allow for earlier detection of AMD changes or progression to wet AMD, and result in faster access to treatment.
Maximising the remaining vision that an individual has is a crucial step to take, and as discussed above there are many low vision aids such as telescopic and magnifying lenses which may be of benefit. The wide range of assistive technologies for people with visual impairments provides plenty of choice for users at all stages of sight loss and this technology has also removed many barriers to education and employment.
There are a number of useful websites which have information on AMD:
• AMD.ie – information on age related macular degeneration in Ireland.
• NCBI (National Council for the Blind in Ireland) provides support and services for people living with sight loss in Ireland.
• Irish Guide Dogs for the blind helps individuals and their families to achieve improved mobility and independence.
• The Irish College of Ophthalmologists eye doctor website
• Retina International also has an international AMD toolkit on their website
References available on request
PHARMACYNEWSIRELAND.COM







































✓ 100% Preservative Free
Contact lens compatible
Can be used for up to 6 months from opening
Use as part of the 3-step Heat, Cleanse, Hydrate Dry Eye regimen The N umber 1 Eye Care Product in Ireland * Do your patients suffer with dry eyes at night? SCOPEIE188 Freephone 1800 816 005 | info@scopeeyecare.com | www.scopeeyecare.com 0.2% Sodium Hyaluronate *Based on 2022 IQVIA ranking Looking to grow your eye care category? Find out more about the Scope Pharmacy Partnership Program Try HYLO NIGHT ® for night-time management of Dry Eye symptoms, giving your patients 24-hour relief Scan me
✓
✓
✓
Topical Targeting of Pain



Pain is an unpleasant sensory emotional experience associated with actual or potential tissue damage, as defined by the World Health Organization.1 Chronic pain can have a detrimental effect on quality of life and daily activities and is one of the main causes of physical disability in the United States and Europe affecting more than 30% of people worldwide.2,3
Topical as route of administration
Topical analgesic regimes provide pain relief without the burden of the systemic side effects seen with other routes of delivery. They are a targeted therapy that act peripherally on nociceptor pathways at the location of the pain.4 Reduced systemic absorption results in low plasma levels of the therapeutic drug leading to a reduced risk of systemic side effects such as respiratory depression, sedation, nausea and delirium.4,5,6,7,8 The use of oral opioid-based analgesics has resulted in high rates of abuse and addiction, with thousands dying from overdoses.4 Topically administered opioids are beneficial in this regard, having a lower risk of addiction and diversion compared to oral preparations.9
The method of preparation is important to ensure adequate application of the active component. The major methods of achieving this are by causing the active drug to be a solute in a stable preparation most composed of alcohol, sorbitol and stearate for stability. An example of this is capsaicin cream and NSAID preparations. Steroid preparations such as budesonide are stable in paraffin wax solvents as are immunosuppressive preparations such as tacrolimus. The advantage of the paraffin wax solvents is stickiness to the skin as opposed to the alcohol preparations which are easily rubbed off by clothing. The patch method of application is more complex involving an aqueous base in the case of lignocaine with preservatives such as methylparaben and propylparaben. The matrix fentanyl membrane transdermal patch is contained in silicone with a rate-controlling membrane which maintains constant serum fentanyl concentrations throughout its application, proving to be highly important due to fentanyl’s action as a potent respiratory depressant.10
Local Anaesthetics
The lidocaine 5% medicated plaster is used as a second line treatment for peripheral neuropathic pain as well as postoperative pain management.5,11,12 Additionally capsaicin, an

active component of chili peppers, is used as a topical analgesic.11,13 Capsaicin binds to nociceptors in skin that are responsible for burning, itching, or prickling; constant or high exposure to capsaicin can result in desensitization of these receptors, resulting in these nerve terminals unable to conduct an action potential.13 Low dose topical capsaicin creams have not been shown to be effective in neuropathic pain due to its burning sensation, often limiting the number of times it can be applied. Due to this, a high concentration capsaicin cutaneous patch (HCCP) has been introduced to allow a one-time application and was shown to be non-inferior to oral pregabalin in an open label randomized trial.13
Opioids
Buprenorphine is used for chronic arthritic pain in frail patients and fentanyl, which is a strong opioid, is traditionally used for cancer pain relief. Buprenorphine is a semi-synthetic partial µ-opioid receptor agonist and κ- and δ-opioid receptor antagonist that is accompanied with less respiratory depression and sedation. Fentanyl is a synthetic µ-opioid receptor agonist with fast receptor association and dissociation characteristics but is more potent and addictive. Both agents are available as transdermal formulations and penetrate the skin effectively due to their low molecular mass, high lipid solubility and high potency. The fentanyl
36 | PHARMACYNEWSIRELAND.COM
Karina Oganezova, Trinity College Dublin
Written by
Professor Connail McCrory, Trinity College Dublin
Dr Deborah Galvin, Trinity College Dublin


www.prl ie pgsales@prl ie +353 1 257 4650 PRL SALES ULC, Greenogue Business Park, Rathcoole, Co. Dublin MORE than a Multivitamin... NOW with OMEGA-3 for heart1, brain2 and vision3
patch is the treatment of choice for patients with cancer pain in metastatic disease who are unable to swallow or have poor compliance.14
Steroids and NSAIDs
Topical corticosteroids are safe and inexpensive agents commonly used for skin conditions; however, studies have published the effectiveness of these agents as a treatment for osteoarthritis. Although the European League Against Rheumatism suggests NSAID topical therapy as first line choice, there is evidence of inflammation being a potential treatment target in hand osteoarthritis, which could be effectively treated by glucocorticoids.15 Topical NSAID’s like diclofenac and ketoprofen, capsaicin cream, and civamide cream are also used for treatment of osteoarthritis.16
Neuropathic Pain
Peripheral neuropathic pain in conditions such as: post-traumatic neuralgia, post-herpetic neuralgia, painful diabetic neuropathy, and HIV-related neuropathy are indications for topical analgesia.1,11 Surgically-induced neuropathic pain affects 2-10% of individuals following surgery, and is characterized by sensory loss followed by hypersensitivity to pain, resistance to medication, and more likely to become chronic.13 This pain can vary based on surgical factors; for example, about half of patients with chronic pain postthoracotomy are believed to have neuropathic components.13 Although the recommended first line treatments for these conditions are oral medicines such as tricyclic antidepressants, anticonvulsants, and SSRI’s, topical agents may be more beneficial due to its localized activity and minimal systemic effects.1,11
Other conditions
Eczema is a type of dermatitis that causes dry, itchy and inflamed skin. Low-potency
corticosteroids such as hydrocortisone and moderate-potency corticosteroids such as methylprednisolone are topical treatments used for management of eczema.17
Psoriasis is a chronic inflammatory autoimmune skin condition in which the immune system regards normal skin cells as an infection, producing dry, red plaques on the skin. Although it is incurable, there are treatments that manage the condition. One of these treatments is protopic 0.1% ointment, also known as tacrolimus, which is an immunosuppressant licensed for atopic dermatitis. It is often prescribed off-label to treat psoriasis on the face or genitals, due to its ability to suppress the immune system, thereby stopping the hyperproduction of skin cells.18
Complications with use
Although the use of topical agents is accompanied by many advantages in comparison to oral agents, there are several complications that need to be addressed. Using patches may require the need to apply adhesive tapes or bandages to secure the patch into place and this may be problematic for the treatment of distal painful neuropathy such as in HIV or diabetic neuropathy involving the feet.1 Topical formulations such as creams, ointments, and gels have removed this burden, however these lack the accuracy of dosing.1 Although topical analgesics are thought to exert analgesia by interacting with peripheral opioid receptors,
Effect of Deprivation on Cancer
a degree of systemic absorption has been demonstrated. Some studies have observed significant analgesia, however, it was noted to have occurred within 60 minutes of topical application, therefore not deemed appropriate to deal with wound related breakthrough pain.19 Furthermore, studies have shown topical administration of NSAIDs penetrate through the layers of skin and can potentially lead to systemic absorption. This is especially seen with methyl salicylate which is lipophilic and readily penetrates the skin when applied as a topical agent, and is hydrolysed to salicylic acid in the tissues.2 This can result in the tissues becoming saturated leading to chronic salicylate toxicity, which may cause vomiting, confusion, and hyperventilation leading to respiratory alkalosis.
Conclusion
In conclusion, topical analgesics provide a localized treatment for disorders such as chronic neuropathic pain, chronic arthritic pain, cancer pain, post-surgical pain, wound healing, and diabetic or HIV-associated neuropathy. Due to their ability to bypass the systemic circulation and gastrointestinal system, these agents provide relief without the adverse effects associated with oral analgesics, such as addiction and drug diversion, nausea, vomiting, constipation, drowsiness, and headaches. Overall, they are well tolerated. References
The National Cancer Registry Ireland (NCRI) has just published a new report entitled: Cancer inequalities in Ireland by deprivation, 2004-2018. This report measures differences in cancer incidence, five-year survival and stage at presentation between populations living in the most and least deprived areas in Ireland for the diagnosis period 2014-2018. Comparative information is also provided for earlier periods (2004-2008 and 2009-2013).
Key findings include:
• Overall, a 7% higher age-standardised incidence for males and a 5% higher incidence for females living in most deprived areas compared to those living in the least deprived areas in 2014-2018
• Overall, lower five-year cancer survival rates in patients from the most deprived areas compared to those in the least deprived areas. Those in the most deprived areas had a 28% higher mortality risk due to cancer within five years of cancer diagnosis compared to those in the least deprived areas, having adjusted for age, gender, and cancer type.
• Differences in the types of cancers diagnosed in the most and least deprived areas, with a higher incidence of stomach,
lung and cervical cancer in people living in most deprived areas, while those living in least deprived areas show a higher incidence of breast, prostate, and melanoma and nonmelanoma skin cancer.
• People living in the most deprived areas had a higher risk of late-stage presentation for breast and prostate cancers than those living in least deprived areas. No disparities in stage of presentation were found for lung or colorectal cancers when comparing the least and most deprived groups.
A range of potential factors may contribute to such disparities, including differences in general health, exposure to particular risk factors, health-seeking behaviour (influencing early detection), access to healthcare, or other factors that may be linked to socioeconomic or geographic factors.
Chair of the NCRI Board Dr Jerome Coffey, said: “These important data underline the known links between socioeconomic deprivation and cancer incidence and survival, with no major reductions in disparities between groups over the time periods examined. Prevention, screening and early diagnosis are major elements of the National Cancer Strategy 2017-2026 and will have to remain as priorities in subsequent strategies.”
NCRI co-author Dr Niamh Bambury added, “This report provides the most up-to-date, reliable information on the effect of deprivation on cancer incidence and five-year survival. In the health field, advancement of policy initiatives, including Sláintecare and Healthy Ireland, are needed to help address the root causes of these disparities.”
38 | PHARMACYNEWSIRELAND.COM
on request
available
News
IREL AND’S No.1
Sl eep A Id BRAND CAN H E LP YOU GET A GOOD NIGHT’S SLEEP

*Based on IQVIA sales data MAT 07/2022.
CLINICALLY PROVEN
Nytol One-A-Night 50 mg Tablets contains diphenhydramine hydrochloride. A symptomatic aid to the relief of temporary sleep disturbance in adults. Adults: One tablet to be taken 20 minutes before going to bed, or as directed by a physician. Do not exceed the maximum dose of one tablet in 24 hours. Elderly patients or patients with liver or kidney problems should consult their doctor before taking this medicine. Children under 18 years: Not recommended. The product should not be taken for more than 7 days without consulting a doctor. Contraindications: Hypersensitivity to the active substance or to any of the excipients, stenosing peptic ulcer, pyloroduodenal obstruction, phaeochromocytoma, known acquired or congenital QT interval prolongation, known risk factors for QT interval prolongation. Special warnings and precautions: Pregnancy/lactation, renal and hepatic impairment, myasthenia gravis, epilepsy or seizure disorders, narrow-angle glaucoma, prostatic hypertrophy, urinary retention, asthma, bronchitis, COPD. Patients should be advised to promptly report any cardiac symptoms. Tolerance and / or dependence may develop with continuous use. Do not take for more than 7 consecutive nights without consulting a doctor. Should not be used in patients currently receiving MAO inhibitors (MAOI) or patients who have received treatment with MAOIs within the last two weeks Use in the elderly should be avoided. Avoid concomitant use of alcohol or other antihistamine-containing preparations. Do not drive or operate machines. Cases of abuse and dependence were reported in adolescents or young adults for recreational use and/or in patients with psychiatric dis-orders and/or history of abuse disorders. Contains lactose. May suppress the cutaneous histamine response to allergen extracts and should be stopped several days before skin testing. Interactions: Alcohol, CNS depressants, MAO inhibitors, anticholinergic drugs (e.g. atropine, tricyclic antidepressants), metoprolol and venlafaxine, CYP2D6 inhibitors, Class Ia and Class III anti-arrhythmics. Side effects: Dry mouth, fatigue, sedation, drowsiness, disturbance in attention, unsteadiness, dizziness, thrombocytopenia, hypersensitivity reactions, confusion, paradoxical excitation, convulsions, headache, paraesthesia, dyskinesias, blurred vision, tachycardia, palpitations, thickening of bronchial secretions, gastrointestinal disturbance, muscle twitching, urinary difficulty, urinary retention. Legal classification: P. PA1186/016/001. MAH: Chefaro Ireland DAC. The Sharp Building. Hogan Place. Dublin 2. Ireland. Date of preparation: 07/2022. RRP (ex. VAT): 20s €9.00. SPC: https://www.medicines.ie/medicines/nytol-one-a-night-50-mg-tablets-34889/smpc IRE NYT

2022 18
LET

Blood Glucose monitoring System (IDEAL FOR TYPE 1 & 2 PATIENTS).

SYSTEM ACCURACY
99.33% of 4SURE SMART DUO test results are within ± 0.83 mmol/L (low glucose levels) or ± 15% (high glucose levels) of laboratory reference method test results. 4















TO ORDER YOUR FREE METER CONTACT: 1800 26 26 26



SMART
Blood Glucose & ß-Ketone monitoring System (IDEAL FOR TYPE 1 PATIENTS).





























Suitable for Gestational
Additional Feature Additional Feature


Suitable for gestational patients³ Ketone β Affordable blood glucose and -ketone test strips



Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Always read the instructions before use. Date prepared: October 2019. 2019/ADV/4SU/113H. 1. Haematocrit range 0-70% (blood glucose), 10-70% ( -ketone) 2. For driving with diabetes, follow DVLA guidelines 3. Follow NICE guidelines “Diabetes in pregnancy: management from preconception to the postnatal period” 4. Assessment of System Accuracy, Intermediate Measurement Precision, and Measurement Repeatability of a Blood Glucose Monitoring System Based on ISO 15197. Jendrike N, et al., J Diabetes Sci Technol. 2018 Dec 14.
Meal Pre AC Post PC 0-70% HCT Suitable for drivers Pre and post meal markers Suitable for drivers² Wide haematocrit range¹ Bluetooth connectivity
DIABETES CARE
US
YOU
DIABETES
HELP
MANAGE
CPD
60 Second Summary
Diabetes mellitus (DM) is a heterogeneous complex metabolic condition characterised by hyperglycaemia with a degenerative potential resulting from changes in the production, secretion and/or inability of insulin to adequately exercise its effects.
DM is a leading cause of death globally and described as the most challenging health problem in the 21st century, driven primarily by rising levels of obesity and an ageing population.
This audit was conducted over one day in March 2022. The audit was approved by the local Clinical Audit Committee, piloted on two inpatients, and communicated to all data collectors prior to commencement. Generated data were anonymous and securely stored. Independent analysis was conducted by three researchers to confirm reliability of results.
This study identified 17% of inpatients had DM on audit day in GUH of which 9% were insulin dependent and 55% of all inpatients with DM were treated with insulin. This is similar to the most recently published National Health Service (NHS) National Diabetes Inpatient Audit (NaDIA) England 2019 report which found 18% of all inpatients in 188 NHS hospitals had documented DM. Whilst the majority of inpatients were prescribed a meal time supplement, it was only documented clearly by nurses for approximately one in every three inpatients potentiating administration errors and significant patient harm.
Future quality improvement interventions for consideration to optimise patient care include implementing a dedicated insulin safety team that comprises a broad membership to facilitate sustainability and spread of interventions to improve insulin prescribing and administration practice and electronic prescribing as a part of the ongoing implementation of a new pharmacy system.
AUTHORS: D. Hogan-Murphy1, L. Reddington2, M.C. Dennedy2,3, L. Egan3,4, J. Okiro2, J. Given1, S. Donnellan1, M.R. Salehmohamed2
1. Department of Pharmacy, Galway University Hospitals, Galway, Ireland
2. Department of Endocrinology, Diabetes and Metabolism, Galway University Hospitals, Galway, Ireland
3. Department of Pharmacology and Therapeutics, School of Medicine, National University of Ireland, Galway, Ireland
4. Department of Gastroenterology, Galway University Hospitals, Galway, Ireland
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result? Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by IPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Insulin prescribing, administration, and glucose monitoring trends in a hospital setting
ABSTRACT
Aims: Insulin is a high-alert critical medication which can cause significant patient harm when used inappropriately. The aim of this study was to conduct a prospective audit on insulin prescribing, administration, and glucose monitoring trends in Galway University Hospitals.

Methods: This audit was conducted over one day in March 2022. The audit was approved by the local Clinical Audit Committee, piloted on two inpatients, and communicated to all data collectors prior to commencement. Generated data were anonymous and securely stored. Independent analysis was conducted by three researchers to confirm reliability of results.
Results: Four hundred and fifty-four inpatients were reviewed of which 17% [75] had diabetes and 9% [41] were prescribed insulin. The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [37]. In total, 95% [235] insulin brand names and 89% [220] dose units were clearly prescribed, 84% [208] administration times were clearly specified by a prescriber, 87% [214] orders were signed, 58% [25] prescribers clearly documented their registration number/bleep/name at least once for contact purposes, 35% [30] meal time supplements were documented clearly by a nurse, 70% [202] administrations were double checked by a second person, 53% [142] administration times were documented by a nurse, and 26% [10] of inpatients were administered insulin by a nurse when not prescribed.
Conclusion: Results will assist in developing quality improvement initiatives to optimise patient care.
Introduction
Diabetes mellitus (DM) is a heterogeneous complex metabolic condition characterised by hyperglycaemia with a degenerative potential resulting from changes in the production, secretion and/or inability of insulin to adequately exercise its effects1 The most common classifications include Type 1 DM and Type 2 DM, the latter accounting for more than 90% of all cases2. Type 2 DM is characterised by insulin resistance and a relative deficiency of insulin secretion which progressively worsens over time3,4 Type 1 DM results in an absolute deficiency in beta-cell function with autoimmune destruction
of beta-cells a common origin5
Current estimates suggest more than half a billion adults live with DM worldwide, a rise of 16% since previous estimates in 20196. This is predicted to escalate to almost 800 million by 20456. In Ireland, in the absence of a national DM registry, the current approximate projection is 5.6%7
DM is a leading cause of death globally8 and described as the most challenging health problem in the 21st century9,10 driven primarily by rising levels of obesity and an ageing population9,11. A systematic review and meta-analysis on the epidemiology of DM and its complications amongst adults in Ireland found variables from
7–25% for retinopathy; 3–32% for neuropathy; and 3-5% for nephropathy12. The economic burden also plays heavily with Ireland ranked 7 th in the world for DM related health expenditure per person7, and as high as ¤1.4 billion annually with costs mostly associated with hospitalisations and treatment of complications6 Insulin is a high-alert medicine used in the treatment of DM which bears a heightened risk of causing significant patient harm. With limited Irish data availability and few local incidents reported, anecdotal evidence suggests insulin accounts for a substantial number of medication errors13,14. A review by the States
41 CPD: Diabetes
Continuing Professional Development
CPD
Claims Agency of over 20,000 medication incidents reported by Irish acute hospitals in 2017 and 2018 found insulin was the fourth most commonly implicated therapeutic subgroup15, many comprising omissions leading to hyperglycaemia and inaccurate dosing leading to hyperglycaemia and hypoglycaemia16
Insulin has been identified as a significant medication safety concern in Galway University Hospitals (GUH). The aim of this study was to conduct a prospective audit on insulin prescribing, administration, and glucose monitoring trends in GUH in order to identify and develop agreed quality improvement initiatives to enhance patient care.
Methods
A prospective audit on insulin prescribing, administration, and glucose monitoring was conducted over one day in March 2022 on 24 wards in GUH. GUH comprises University Hospital Galway (UHG), a Model 4 public hospital, and Merlin Park University Hospital (MPUH), a Model 2 public hospital, and provides a comprehensive range of services to emergency and elective patients within the Saolta University Healthcare Group in the West of Ireland.
Inclusion criteria comprised inpatients prescribed/administered insulin in UHG and MPUH for the previous 72 hours until 9am on the morning of audit. Exclusion criteria comprised non-admitted patients, Day Wards, Emergency Department, Acute Medical Unit, Short Stay Unit, Emergency Surgical Unit, Critical Care including Post Anaesthetic Care Unit, Maternity Department, and Psychiatry Department. Content of the audit protocol and tool was informed by the research objective, local practices, and existing evidence-based international and national literature. The audit tool
Overall insulin error rate per inpatient drug record
The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [n=41] as presented in Table 1.
Table 1: Overall insulin error rate per inpatient drug record
was piloted on a medical ward in UHG with two random inpatients prescribed and administered insulin. Minor amendments were made to its content and the pilot was excluded from data analysis. The audit was led by two lead researchers and conducted by 29 data collectors comprising endocrine consultant and nonconsultant hospital doctors, diabetes nurse specialists, and pharmacists. The audit tool was guided by the protocol which was communicated to all data collectors prior to the audit via video conferencing and faceto-face meetings and emails. This audit was conducted in accordance with the HSE Code of Governance (2021) and HSE Healthcare Audit Quality Assurance
Results
Nineteen inpatients [n=37; 51%] were prescribed the same insulin as pre-admission, four inpatients [n=37; 11%] were not prescribed the same insulin as pre-admission, and 14 inpatients [n=37; 38%] were not on insulin pre-admission and were either prescribed a meal time supplement [10 inpatients; n=37; 27%] or were newly prescribed insulin on admission [4 inpatients; n=37; 11%]
and Verification Standards (2019) and was approved by the GUH Clinical Audit Committee prior to commencement. All audit forms were anonymous and securely stored in a locked cabinet and all generated data were securely stored on an encrypted password protected work computer. Any audit records will be destroyed after full dissemination of audit findings. Independent analysis was conducted by the two primary researchers and a specialist registrar in endocrinology to confirm reliability of results. This process involved independently inputting content of paper audit forms into excel, analysing data, and comparing results. No significant discrepancies were identified.
General participation and prevalence
A total of 247 insulin doses were prescribed of which 235 orders [95%] had the insulin name clearly documented Two hundred and twenty [89%] insulin dose units were clearly prescribed, 208 [84%] administration times were clearly specified by a prescriber, and 214 [87%] orders were signed by a prescriber (Figure 2).
In total, 454 inpatients were reviewed of which 41 [9%] were prescribed insulin and included in the audit. The number of inpatients using an Insulin and Glucose Monitoring Record was 117 [26%] of which 75 inpatients [17%] had a documented history of DM (Figure 1). This equates to 55% of all inpatients with DM were treated with insulin. Patient specialty comprised medical [27, 66%], surgical [12, 29%], and paediatric [2, 5%].
Overall insulin error rate per inpatient drug record
DM/Insulin Prevalence GUH
Number of inpatients documented with DM
Number of inpatients on Insulin
Number of inpatients using an Insulin and Glucose Monitoring Record
Number of inpatients excluded at time of data collection
Number of inpatients on ward
The overall insulin error rate with one or more errors comprising prescribing and/or inpatient drug record was 90% [n=41] as presented in Table 1. Table 1: Overall insulin error rate per inpatient drug record
42 CPD: Diabetes
Error description Error type Insulin brand incorrect 0% Prescribing error 80% Insulin error 90% Insulin name not clearly prescribed 5% Insulin dose units not clearly prescribed 11% Insulin administration times not clearly specified by the prescriber 16% Insulin orders not signed by the prescriber 13% Prescriber MCRN/bleep/name unclear for contact purposes 42% Meal time supplement documented incorrectly 65% Insulin administration not double checked by a 2nd person 30% Administration error 89% Insulin administration times not documented 47% Insulin administered when not prescribed 26%
Insulin prescribing patterns
Table 1 Overall insulin error rate per inpatient drug record
Figure 1: DM/Insulin prevalence GUH
Figure 1: DM/Insulin prevalence GUH Overall insulin error rate per
inpatient drug record
508 54 117 41 75
Insulin Prescribing Patterns
What number of insulin orders have been signed by the prescriber?
What number of insulin administration times are clearly specified by the prescriber?
What number of insulin dose units are clearly prescribed?
What number of insulin orders have the name clearly prescribed?
What number of insulin doses have been prescribed?
2: Insulin prescribing patterns
The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [n=41] as presented in Table 1.
Insulin prescribing patterns
person. Administration times were documented for 142 doses [n=270; 53%] as illustrated in Figure 3. Ten inpatients [n=39; 26%] were administered insulin by a nurse when not prescribed.
Glucose management
Twenty-five prescribers [n=43; 58%] clearly documented their medical council registration number (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and 25 inpatients [61%] had the meal time supplement signed by a prescriber. The meal time supplement was documented clearly 30 times out of a total of 86 entries [35%]
Insulin administration patterns
Nineteen inpatients [n=37; 51%] were prescribed the same insulin as pre-admission, four inpatients [n=37; 11%] were not prescribed the same insulin as pre-admission, and 14 inpatients [n=37; 38%] were not on insulin pre-admission and were either prescribed a meal time supplement [10 inpatients; n=37; 27%] or were newly prescribed insulin on admission [4 inpatients; n=37; 11%].
Insulin Administration Patterns
A total of 247 insulin doses were prescribed of which 235 orders [95%] had the insulin name clearly documented. Two hundred and twenty [89%] insulin dose units were clearly prescribed, 208 [84%] administration times were clearly specified by a prescriber, and 214 [87%] orders were signed by a prescriber (Figure 2).
What number of doses with administration times are documented?
The GUH DM team reviewed/was contacted to review 21 inpatients insulin drug record [55%, n=38]. Twenty-five inpatients [61%; n=41] had changes to their insulin regimen made during their inpatient stay. This included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin and/or a meal time supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; median 3; range 1-6]. Three inpatients had their insulin omitted after an episode of hypoglycaemia (blood glucose <4 mmol/L).
Discussion
What number of doses are double checked by an independent second person?
All inpatients not prescribed the same insulin as pre-admission were altered by the DM team during admission and were therefore appropriately changed. The remaining inpatients were not on insulin pre-admission and were either prescribed a meal time supplement or were newly prescribed insulin on admission. The overall insulin error rate comprising both prescribing [80%] and administration [89%] per inpatient drug record was 90%. This is significantly higher than the NaDIA 2019 report which found 18% of inpatient drug records had one or more insulin errors17. This figure is also higher than findings from an analysis of the National Reporting and Learning
Two hundred and eighty-seven doses were administered of which 202 [70%] were double checked by an independent second person. Administration times were documented for 142 doses [n=270; 53%] as illustrated in Figure 3 Ten inpatients [n=39; 26%] were administered insulin by a nurse when not prescribed.
System database of patient safety incidents concerning insulin reported from NHS providers in England and Wales over a six year period which found 61% of incidents occurred at the administration stage and 17% at the prescribing stage19 The most common medication error types were wrong dose, strength, or frequency followed by omitted or delayed insulin19 Most prescriptions in this audit had the insulin name, dose, and administration times clearly documented and signed by the prescriber which are positive findings. However, less than three out of five prescribers clearly documented their MCRN/ bleep/name on the insulin drug record at least once for contact purposes leading to possible time delays and patient care issues if communication is required. Whilst the majority of inpatients were prescribed a meal time supplement, it was only documented clearly by nurses for approximately one in every three inpatients potentiating administration errors and significant patient harm. Almost one in three insulin doses were also not double checked by an independent second person and almost half administration times were not documented. Insulin is a highalert medication which requires a two-person check of both dose
What number of doses have been administered?
Figure 3: Insulin administration patterns
This study identified 17% of inpatients had DM on audit day in GUH of which 9% were insulin dependent and 55% of all inpatients with DM were treated with insulin. This is similar to the most recently published National Health Service (NHS) National Diabetes Inpatient Audit (NaDIA) England 2019 report which found 18% of all inpatients in 188 NHS hospitals had documented DM17
Glucose management
Twenty-five prescribers [n=43; 58%] clearly documented their medical council registration number (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and 25 inpatients [61%] had the meal time supplement signed by a prescriber. The meal time supplement was documented clearly 30 times out of a total of 86 entries [35%].
Insulin administration patterns
The NaDIA England and Wales 2015 report identified a lower prevalence of 6% of all inpatients in 206 NHS hospitals were insulin dependent and 36% of inpatients with DM were treated with insulin18
The GUH DM team reviewed/was contacted to review 21 inpatient five inpatients [61%; n=41] had changes to their insulin regimen made during their included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin and/or a meal time supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; their insulin omitted after an episode of hypoglycaemia (blood glucose <4 mmol/L).
Two hundred and eighty-seven doses were administered of which 202 [70%] were double checked by an independent second
The majority of inpatients in this audit were medical and prescribed the same insulin as pre-admission.
43
Figure
247 235 220 208 214
202 142
Figure 2: Insulin prescribing patterns
preparation and administration at the bedside as well as precise administration timing to minimise hypoglycaemia, hyperglycaemia, wide glycaemic excursions, and diabetic ketoacidosis.
More than one in four inpatients were administered insulin by a nurse when not prescribed. A nurse may only administer a non-prescribed medication in a situation that requires immediate intervention in lifethreatening situations and there is no immediate access to an appropriate prescriber. All insulin doses should be prescribed prior to nurse administration.
The GUH DM team reviewed or was contacted to review more than half of the inpatients prescribed insulin. More than three out of five inpatients had changes to their insulin regimen made during their inpatient stay including brand of insulin, the initiation of insulin, and dose changes. Three inpatients had their insulin omitted after an episode of hypoglycaemia. This is lower than a study evaluating insulin information on discharge summaries in a United Kingdom (UK) hospital which found 33 out of 42 patients [79%] had changes made including the initiation and/or discontinuation of insulin therapy, insulin dose changes, and insulin preparation/brand20
Based on audit results as well as evidence from local insulin error reporting, local practices, and best practice, some of the current interventions implemented specific to insulin in GUH with support from hospital management and
Insulin Prescribing Patterns
Future quality improvement interventions for consideration to optimise patient care include implementing a dedicated insulin safety team that comprises a broad membership to facilitate sustainability and spread of interventions to improve insulin prescribing and administration practice; electronic prescribing as a part of the ongoing implementation of a new pharmacy system; an insulin inpatient and discharge checklist; and promotion of an annual national audit on insulin prescribing, administration, and glucose monitoring trends similar to the UK. Once interventions are in situ it is anticipated that results of a reaudit in GUH will be favourable.
Declaration
of Conflicts of Interest
The authors report no conflict of interest.
Corresponding Author: Dr Diana Hogan-Murphy
Department of Pharmacy University Hospital Galway
Insulin administration patterns
medication safety committees which are transferrable to other hospitals include an updated inpatient Insulin and Glucose Monitoring Drug Record; recruitment of a clinical pharmacist with a special interest in diabetes; implementation of mandatory e-learning for nurses and doctors on insulin prescribing and administration; updated/newly approved policies, procedures, protocols, and guidelines; piloting of a self-administration
policy; continuous education and training to patients as well as medical, nursing, and pharmacy undergraduate students and employees in GUH; expansion of a medication safety portal inclusive of educational videos; use of hospital screens and social media to disseminate prudent information; and further promotion of error reporting. No challenges were encountered as all key stakeholders were involved from the onset.
Galway
E-Mail: diana.hogan-murphy@hse.ie
Twenty-five prescribers [n=43; 58%] clearly documented their medical council (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and had the meal time supplement signed by a prescriber. The meal time supplement was 30 times out of a total of 86 entries [35%].
References available on request Figure 3: Insulin administration patterns
Two hundred and eighty-seven doses were administered of which 202 [70%] were double independent second person. Administration times were documented for 142 doses illustrated in Figure 3 Ten inpatients [n=39; 26%] were administered insulin by a nurse
Insulin Administration Patterns
What number of doses with administration times are documented?
What number of doses are double checked by an independent second person?
What number of doses have been administered?
Glucose management
The GUH DM team reviewed/was contacted to review 21 inpatients insulin drug record five inpatients [61%; n=41] had changes to their insulin regimen made during their included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; median 3; range 1-6].
44 CPD: Diabetes
Figure 2: Insulin prescribing patterns
Figure 3: Insulin administration patterns
235 220 208 214
287 202 142
Dandruff?
What dandruff?
Capasal Therapeutic Shampoo


Triple Action Therapy
Salicylic acid removes scales, distilled coal tar relieves itching, with coconut oil to soften and moisturise the scalp






Recommend Capasal Therapeutic Shampoo
Prescribing information
Capasal™ Therapeutic Shampoo
Salicylic acid 0.5% w/w, coconut oil 1.0% w/w, distilled coal tar 1.0% w/w.
Uses: As a shampoo in the treatment of dry, scaly scalp conditions such as seborrhoeic eczema, seborrhoeic dermatitis, pityriasis capitis, psoriasis and cradle cap in children. It may also be used to remove previous scalp applications.
Directions: Adults, children and the elderly: Use as a shampoo, once or twice weekly until the condition improves. Therea er, occasional use may be necessary. Wet the hair thoroughly. Massage a small amount of the shampoo into the scalp, leaving on for a few minutes. Remove as much lather as possible with the hands, before rinsing out thoroughly under running water. Repeat if necessary.
Contra-indications, warnings, side e ects etc: Please refer to SPC for full details before prescribing. Do not use if sensitive to any of the ingredients. If there is no improvement a er 4 weeks, or the condition is aggravated, discontinue treatment. Keep away from the eyes. Keep out of the reach of children. Use in pregnancy: avoid use during rst trimester.
Package quantities, trade prices and MA number: 100ml bottle €3.48, 250ml bottle €6.96, PA23128/008/001.

Legal category: Supply through pharmacy only.
Further information is available from: Dermal Laboratories (Ireland) Ltd, Head O ce Tatmore Place, Gosmore, Hitchin, Herts, SG4 7QR, UK.

Date of preparation: February 2021. ‘Capasal’ is a trademark.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Dermal.

CAPPR 027 /I/JAN 23
Best Baby Skincare Product
Irish parents increasingly invest in their children’s health focusing on using preventative measures to strengthen their children’s immune systems and overall health, according to the latest market research.

Product innovation, attractive packaging, impact to the customer, unique selling point, and manufacturer support are all important if a baby product is to be successful in an already saturated marketplace.
Consumers are very much concerned not only about what they feed to their baby but are also equally concerned about what they are applying on their baby’s skin. People prefer products which are chemical free and full of natural/organic ingredients for their children. Millennial parents are much more concerned about the ingredients that are being used in the baby skin care products.

The Finalists in this Category are:
Attitude Baby Leaves Hair & Body Wash


Attitude is a Canada-based company that develops natural personal care products to promote healthy living. We believe in living consciously. In creating and using products that are kinder to our bodies and our planet. We believe in being the change we want to see in the World. Attitude Baby Leaves 2 in 1 Shampoo & Body Wash along with all the Attitude products are
• Crafted with natural, hypoallergenic, dermatologically-tested and EWG VERIFIED™ ingredients.
• Adhering to highest quality and ecoconscious standards.
• 100% vegan, cruelty-free, and certified by PETA and ECOLOGO.
46 | PHARMACYNEWSIRELAND.COM
2023 & Retail Pharmacy Product Awards
Elave Sensitive Baby Bath


Elave Sensitive Baby Bath is certified ECOCERT Organic. ECOCERT is an international, independent certification body that sets strict requirements for products and their manufacturing chain to guarantee ingredients are derived from renewable resources and products are manufactured using environmentally friendly processes. Containing Organic Aloe Barbadensis Leaf Juice, famous for its ability to soften and heal with naturally antimicrobial and antiinflammatory properties while offering skin-penetrating hydration, it is known to reduce irritation, redness, and soothe the skin. Also containing naturally derived mild surfactants – cleansing agents that lower the surface tension of water so that it can penetrate more fully allowing two substances that normally do not mix to become dissolved or dispersed in one another, to separate dirt and oil from the skin. Their foaming action helps water get under dirt and oil on skin, allowing the water to carry the dirt away. The cleansers in our Elave Sensitive Baby Bath are sulfate-free and biodegradable offering a natural alternative to harsh chemicals.
Eucerin Aquaphor Soothing Skin Balm


Eucerin’s Aquaphor Soothing Skin Balm is a water-free ointment that soothes the skin and is clinically proven for use on all of your dry skin needs, from extremely dry to chapped, chafed or cracked skin. Fragrance- and preservative-free, and formulated with only seven ingredients, dermatological studies show it is extremely tolerable and gentle enough to be used on highly sensitive skin as well as on babies. Eucerin Aquaphor Soothing Skin Balm protects the skin by creating a breathable protective barrier, allowing oxygen and water vapour to circulate to and from the skin, allowing skin to ‘breathe’ and strengthen its natural barrier function. With this protective barrier in place, the skin’s regeneration process is accelerated.
Uriage Peri Oral
Baby’s 1st skincare - 1st Peri-Oral care Soothing & repairing care for irritations around the mouth

Its formula, including the natural complex [Uriage Thermal Water + Organic Edelweiss] combined with the patented TLR2-Regul, soothes irritations, helps to reduce redness and protects baby’s delicate skin for a long time.

Enriched with Copper and Zinc, 1st Peri-Oral care purifies, repairs and restores comfort and elasticity to baby’s skin. Patented technology TLR2-Regul and Aloe Vera soothes irritations and Copper and Zinc cleanses the mouth contour area to limit bacterial flora. NATURAL ORIGIN FORMULA, Eco Friendly, tube and packaging 100% recyclable
- 94% natural origin ingredients
- Locally sourced complex The Alps 100% natural
47
Best Baby and Children’s Supplement
Vitamins, minerals and supplements (VMS) have always been an important category in the pharmacy.
Growing children, especially those who don’t eat a varied diet, sometimes don’t get enough vitamins A and C. It can also be difficult to get enough vitamin D through food alone for them.
Children’s vitamins, alongside a healthy balanced diet, can help to make sure babies and children are getting everything they need to prevent vitamin deficiencies and stay healthy.

The Finalists in this Category are:
Minami DHA+EPA Liquid Kids

Minami DHA+EPA Liquid Kids provides ultra-pure, highly concentrated omega-3 and vitamin D3 for the maintenance of a healthy brain, vision, strong bones and optimal immune function. This liquid omega-3 formula comes in a delicious natural orange and berry flavour, which you can easily dose and stir into the food or drinks for you and your children or take directly from the spoon.
• EPA and DHA are proven to help maintain a healthy heart function

• DHA is proven to contribute to normal brain function and vision
• Vitamin D3 is needed for normal growth and development of bone in children
• Vitamin D3 contributes to the normal function of the immune system in children

MINAMI® is one of the highestconcentrated Omega-3s available. Our fish oils come from small fish species that aren’t endangered, sourced from southern waters of the Pacific Ocean. We’re proud to have been certified by Friend of the Sea for our work.
Wellbaby Multivitamin Liquid

Carefully balanced supplement for babies & infants From the makers of Pregnacare, Wellbaby Multi-vitamin Liquid is ideal for babies and young children from 6 months to 4 years to provide a carefully balanced, comprehensive range of 14 essential vitamins and minerals. Wellbaby Multivitamin Liquid with Swiss Alpine malt has a delicious taste your little one will love. Wellbaby Multi-vitamin Liquid formula includes vitamins A, C and D. Wellbaby Multi-vitamin Liquid provides an ideal nutritional safeguard during a time when babies and infants have a high nutrient requirement in relation to body size, to support their rapid physical growth and development of bones, muscles, blood and the brain. Key nutrients include iron, which contributes to normal cognitive development, and 10 µg vitamin D, which is needed for normal growth and development of bone in children. Vitamin D also contributes to their normal immune system function.

48 | PHARMACYNEWSIRELAND.COM 2023 & Retail
Product
Pharmacy
Awards
AYA Kids Liquid Multi suitable

for 3+
Live the AYA way with AYA Kids Liquid Multi. This contains a spectrum of ingredients to support the normal functioning of the immune system, such as vitamins A, C, D, B6, B12, zinc along with pantothenic acid. The liquid formulation of AYA Kids Liquid Multi supports optimum absorption of the nutrients.

Optibac Probiotics Babies & Children




Optibac Probiotics Babies & Children is a highly researched, expert formula designed especially for use from birth to twelve years of age. It contains 3 of the most scientifically proven probiotic strains for babies and children: Lactobacillus rhamnosus HN001, L. rhamnosus GG – the most scientifically studied strain in the world - and Bifidobacterium breve M-16V® which has been used in over 140 baby care units across the globe. Collectively, these world-renowned strains have been trialled in over 5,000 children worldwide. Along with added vitamin D and prebiotic FOS fibres, this specialist supplement offers all-round support for digestion, immunity, and skin health in children of all ages, in a natural, sugar-free format.
Eskimo-3 Kids
Eskimo-3 Kids provides optimised levels of Omega-3 fatty acids EPA and DHA, Omega-6 and Omega-9, contributing to normal brain function, vision and heart health. The formula is packed with omega-3 DHA to support brain function, concentration and vision, while omega-6 helps to keep your mood more balanced.
Eskimo-3 Kids also contains Vitamin D which supports healthy teeth, bones and muscles, as well as a strong immune system, all important for growing children Eskimo-3 kids is available in either liquid (tutti frutti or orange flavour) or as an orange chewable. The delicious orange or tutti frutti liquid can be taken directly off the spoon or added to smoothies, yoghurt, juice or food for an extra brain boost. The Eskimo-3 Kids chewables are a tasty orange flavour which children absolutely love. Eskimo3.ie
PHARMACYNEWSIRELAND.COM | 49
Best Baby and Children’s Product
Baby care and child-specific products have an important role to play in pharmacy particularly in Ireland as birth rates here are currently higher than the EU average. Last year, Ireland’s birth rate increased by just over 16% to 16,131 in the first quarter of the year when compared with the same period in 2021.

The baby and children’s OTC product category has grown in value for the first time in several years, rising by 2.8% in value to £676m in 2020. Parents have acted more cautiously as a result of the COVID-19 pandemic, demonstrating stockpiling, bulk-buying and increased hygiene behaviours, which have driven higher spend across all three segments.

The Finalists in this Category are:
Matchstick Monkey

Matchstick Monkey was born when new mum, Katie Windridge couldn’t find anything to ease her baby, Minnie’s teething pain. At just one-month-old, Minnie’s pain was constant. She wouldn’t settle and no one was getting much sleep. Minnie refused every teething toy, and Katie’s fingers hurt from being bitten when she tried to apply teething gel. After nursing yet another bleeding finger, Katie realised there had to be a better solution. Inspired by her daughters love of monkeys, the original designs for Matchstick Monkey were created. You can soothe your baby’s sore gums as well as care for their new teeth. Soft, safe teethers with a variety of textures. Ergonomically designed to help your baby hold from a young age. More than just a teething Toy. Apply teething gels or granules to the bumps and help your baby massage them into the source of their teething pain.

2023 & Retail Pharmacy Product Awards
50 | PHARMACYNEWSIRELAND.COM
Tonstix Honey Jelly Pops
Tonstix Honey Jelly Pops for Childrens Sore Throats & Coughs are an all natural product formulated to melt in the mouth which facilitates lubrication of irritated throats caused by allergies, infections, and viruses.


With added Vitamin C and Zinc, it gives instant comfort to unsettled children. Their unique stick person shape makes them the perfect size for little hands to hold.
Tonstix comes with a set of fun stickers to cheer children up with a moment of fun and can help them communicate how they are feeling, which is often challenging particularly for frustrated toddlers when they are unwell.
Klearvol Capsules
Klearvol capsules provide a natural remedy for parents who have trouble getting their children to relax into a soothing sleep. Klearvol contains natural essential oils of pine, thymol and menthol to deliver soothing vapours for inhalation. When placed on a handkerchief close by, Klearvol can help comfort and relieve symptoms of colds such as congestion and stuffy nose in babies and children. Klearvol’s essential oils work via the olfactory system and the skin. Via the Nose, the scent molecules in the essential oils travel from the olfactory nerves directly to the brain and impact the emotional center of the brain. Suitable for children from 3 months upwards.


Eucerin Aquaphor Soothing Skin Balm

Eucerin’s Aquaphor Soothing Skin Balm is a water-free ointment that soothes the skin and is clinically proven for use on all of your dry skin needs, from extremely dry to chapped, chafed or cracked skin. Fragrance- and preservative-free, and formulated with only seven ingredients, dermatological studies show it is extremely tolerable and gentle enough to be used on highly sensitive skin as well as on babies. Eucerin Aquaphor Soothing Skin Balm protects the skin by creating a breathable protective barrier, allowing oxygen and water vapour to circulate to and from the skin, allowing skin to ‘breathe’ and strengthen its natural barrier function. With this protective barrier in place, the skin’s regeneration process is accelerated.

PHARMACYNEWSIRELAND.COM | 51
Best Pain Relief Product
Overall, adult analgesics recorded a sharper increase in demand due to the pandemic. Consumers increased their consumption of OTC analgesics in order to prepare themselves for the lockdown which occurred in Ireland, as consumers bought pain medications to treat themselves and those in their household at home instead of going to the doctor.
The Covid-19 pandemic caused an initial increase in demand for OTC analgesics particularly for acetaminophen. However, the massive effect of stockpiling that affected countries such as the UK was prevented as the Irish Pharmacy Union (IPU) warned members of the public not to stockpile medicine amid the initial panic advising that there were adequate stocks of medicine for people throughout the delay phase of Covid-19 precautions and that, as per Health Service Executive (HSE) rules, people were allowed to have one month’s supply of any medicines that they require.

The Finalists in this Category are:
Solpa-Extra
Solpa-Extra Soluble Tablets are used for the treatment of mild to moderate pain. It’s double action formula delivers fast acting pain relief from headache and migraine, backache, musculoskeletal pain, toothache, menstrual pain, neuralgia, sore throat, and for the relief of fever, aches, and pains of cold and flu. Solpa-Extra is available from Pharmacy only.
Solpa-Extra Soluble Tablets gets to work two times faster than standard paracetamol alone*

Solpa-Extra Soluble Tablets contain Paracetamol 500g and Caffeine 65mg. For the treatment of mild to moderate pain. Always read the leaflet. IRE/ SOL1/2022/01
*based on absorption data. To verify contact verify@perrigo.com

2023 & Retail Pharmacy Product Awards 52 | PHARMACYNEWSIRELAND.COM
Calpol
Calpol is Ireland’s #1 Paediatric Pain Brand (based on IMS units, IMS/Nielsen Value), trusted by HCPs for over 45 years. It is specially formulated for children and gets to work on fever in 15 minutes. It is suitable from 2 months plus, gentle on tummies (can be used with an upset stomach) and kids love the taste.
Calpol Infant range is available in Original and Sugar Free Formulations in 60ml and 140ml size. Dosing syringe is included and is strawberry flavoured.


Calpol Six Plus Suspension range is available in Original (orange flavour) and Sugar Free (strawberry flavour) in 60ml and 140ml sizes and is suitable from age 6. Calpol Six Plus Fastmelts are a strawberry flavour tablet that melt in the mouth – no water needed.
Motusol Max

Sprains and strains are common, painful injuries that affect muscles and ligaments reducing the ability to do daily activities. Teva Ireland have launched Motusol Max, the first alternative 2% diclofenac gel (P). Motusol Max provides up to 12 hours of pain relief, enabling patients to treat pain at the source, reduce swelling and restore movement in the comfort of their home. It also has an easy-grip cap to open & close. Teva is committed to give patients access to affordable medicines and provide more options to pharmacists. Motusol Max helps patients take control of their pain and treat it effectively.
Panadol
We are very proud that Panadol has been made in Ireland since 1981. The 700 GSK employees at our site in Dungarvan, Co. Waterford play a vital role in Panadol’s global success, which is now exported to over 70 countries worldwide and the site produces over 6.5 billion tablets annually.
Panadol Extra Soluble is indicated against 5 types of tough pain, only available in pharmacy and contains a second active ingredient (caffeine) that amplifies the analgesic effect of paracetamol, making it 30% more powerful than standard paracetamol tablets.


PHARMACYNEWSIRELAND.COM | 53
Best Irish Pharmacy Brand
In an increasingly borderless world, many consumers are favouring Irish pharmacy brand products. Research has shown that a brand’s country of origin can be as important as other purchasing criteria such as price and quality with many Irish pharmacy shoppers wanting to support local businesses, finding local more trustworthy and more attuned to their needs and wants.

With OTC Products available from global companies also, Irish brands must work hard to establish themselves in order to get ahead of the competition.

The Finalists in this Category are:
Active Iron
Irish brand Active Iron is an iron supplement with a ground-breaking new method of delivering iron. Active Iron is clinically proven to increase iron levels by 94% whilst helping to avoid the common side effects of iron.


Women living with low iron levels is a global problem affecting 1 in 4 women, with monthly periods being the leading cause of iron loss worldwide. For this reason, Active Iron are focused on supporting women’s health and supplement needs across Ireland through their campaign ‘Better Days. Period’. Active Iron is now recognised as a leading iron supplement in Irish pharmacy as they continue to put Irish women’s health needs at the heart of their brand campaigns.
Revive Active
Revive Active is a super supplement that stands out with a unique formulation that contains 11 vitamins, 7 minerals, 6 amino acids and is enriched with 150mg of CoQ10 and 3,000mg L-Arginine, to help you get the most from your busy, active lifestyle. Each powdered sachet is packed with 26 active ingredients working in synergy to support your energy levels, immune system, the heart and circulatory system and reduce fatigue.

Formulated for adults who are looking for a comprehensive supplement and delivered in an easily absorbed powdered sachet, it’s the convenient way to start each day.

2023
& Retail Pharmacy Product Awards
54 | PHARMACYNEWSIRELAND.COM
AYA Ultimate One a Day

Live the AYA way with AYA Ultimate One A Day. This is a complete solution to boost the performance of the mind and body. A potent powerhouse packed with more than 40 vital vitamins and minerals, this flagship product was designed by scientists to meet the complex daily demands of modern life and the human body. This is the perfect supplement for those who want a quick and effective way of meeting their vitamin needs with minimum fuss.

Can help to support:
Stress & Hectic Lifestyle
Energy
Detox & Cell Protection
Immunity
Restricted diets - e.g. Vegetarian, Gluten sensitivity, Calorie-restricted diet
¤14.95
Panadol
We are very proud that Panadol has been made in Ireland since 1981. The 700 GSK employees at our site in Dungarvan, Co. Waterford play a vital role in Panadol’s global success, which is now exported to over 70 countries worldwide and the site produces over 6.5 billion tablets annually.
Panadol Extra Soluble is indicated against 5 types of tough pain, only available in pharmacy and contains a second active ingredient (caffeine) that amplifies the analgesic effect of paracetamol, making it 30% more powerful than standard paracetamol tablets.


MyPro DiaCare
Introducing the new size MyPro DiaCare
6 Duocam Dual Sachet Pack. A unique oral electrolyte solution which contributes to electrolyte balance, normal digestion and absorption of water. DiaCare is a food supplement for children and adults suffering from diarrhoea, fever or other conditions which can cause loss of body fluids.

It is a scientifically balanced blend of glucose electrolyte, minerals and probiotic fibres (Lactobacillus Rhamnosus GG).

DiaCare contains:
• Magnesium which contributes to electrolyte balance.
• Chloride which contributes to normal digestion by production of hydrochloric acid in the stomach.
• Carbohydrate electrolyte solution which enhances the absorption of water during physical exercise.
DiaCare’s unique dual chambered sachet allows for TWO independent benefits:
- Contains electrolytes which tackle the problem of dehydration.
- Contains a probiotic which helps to swiftly restore the gut to normal.
PHARMACYNEWSIRELAND.COM | 55
Best Natural Product
More and more consumers are in search of natural solutions to their ailments, including both homeopathic remedies and supplements supportive of a healthier lifestyle.

Driven by consumer interest in all things natural, the market for natural products available over-the-counter has increased over the last number of years. Consumers are purchasing these for both prevention and immune boosting properties as well as to help aid healing when they are sick.

The Finalists in this Category are:
King Hair & Beauty Gold Dust Dry Shampoo


Gold Dust Dry Shampoo is a weightless and intelligent formula that is kind to both hair and scalp. Quick and easy to apply ensuring oils are banished in record time, but with lasting effects. Small and discrete but BIG on results.
A quick dusting revives the hair, adding body, and leaving your locks feeling clean and fresh to help maintain your crowning glory. The formula features a host of beneficial and unique ingredients, such as Corn Starch which is a fantastic, natural alternative to talc. Camellia Japomica Extract moisturises the hair and gives it a healthy shine, along with Orchird Extract which helps to repair damaged hair and promotes moisture retention, keeping your hair feeling moisturised.
2023 & Retail Pharmacy Product Awards
56 | PHARMACYNEWSIRELAND.COM
Jordan Green Clean Gentle Whitening Toothpaste


Jordan Green Clean Gentle Whitening toothpaste was created with you and the planet in mind! This toothpaste has proven to give whiter teeth in 2 weeks with daily use in a clinical study. With the environment in mind, Jordan have carefully chosen ingredients that are readily biodegradable in nature, with 98% from natural origin and 100% Vegan. It contains fluoride and xylitol to provide the best protection against cavities. Activated charcoal is added as a natural colourant and the toothpaste has a fresh cool mint flavour. The tube contains up to 50% recycled plastic, originating from food containers that consumers have recycled.
Jordan Green Clean Oral Care provides a wide range of products for Adults, Juniors, Kids and Baby to offer the most sustainable solutions across the oral care category from the first tooth.
Dr Hauschka. Rose

Day Cream
A true classic , celebrity favourite and the hero of the Dr. Hauschka range. Rose Cream was the product on which Dr. Hauschka and Elisabeth Sigmund funded their WALA natural skin care company in 1967.

Rose Day Cream captures the strength and softness of the rose to nourish and protect normal, dry and sensitive skin. Especially beneficial for skin prone to redness, irritation and couperose. The rich, luxurious formulation harmonises oil and moisture content, gently guiding the skin to a state of comfort, balance and radiance.
• Precious extracts of rose petals and wild rose hips nurture and balance the skin
• Shea butter, rose petal wax and avocado oil protect and help retain moisture
• Extracts of marsh mallow and St. John’s wort soothe redness, hydrate and fortify
• Used as directed, Rose Day Cream lasts approximately 3 months
Tonstix Honey Jelly Pops
Tonstix Honey Jelly Pops for Childrens Sore Throats & Coughs are an all natural product formulated to melt in the mouth which facilitates lubrication of irritated throats caused by allergies, infections, and viruses.


With added Vitamin C and Zinc, it gives instant comfort to unsettled children. Their unique stick person shape makes them the perfect size for little hands to hold.
Tonstix comes with a set of fun stickers to cheer children up with a moment of fun and can help them communicate how they are feeling, which is often challenging particularly for frustrated toddlers when they are unwell.
PHARMACYNEWSIRELAND.COM | 57
Best Oral Care Product
The lockdown and remote working enabled Irish consumers to re-evaluate their hygiene routines and make improvements. Unlike showering, oral hygiene has stood to benefit considerably from this.

Social media has progressively steered today’s consumer to be highly conscious of their appearance. This trend was solidified by remote working regulations, which meant that the Irish population turned to video calls far more than previously.
Although new product development was significantly stymied in 2021 as a result of the financial impact of the pandemic, the forecast period is set to experience further innovations, potentially leading to an increase in unit prices.

The Finalists in this Category are:
Sonisk Sonic Toothbrush

We are Sonisk. A pioneering UK healthcare brand aiming to put the best oral care technology within everyone’s reach. We’re inspired by travel, and designing products to suit modern life. We believe in minimal fuss and the ultimate care for a refreshing feeling.
No day is ever the same at our London HQ which, is just how we like it. To take on the big guys in the oral care world, we’re thinking and doing things differently and move at pace to make our mark. Our mantra is very much aligned with the Scandinavian way of life. Did you know our name comes from the Norwegian word for sonic?
Scandinavia in particular, is where health is taken very seriously, fresh air and outdoor space are respected, and the fjords and mountains take your breath away. From the minimal and spacious design, to the soft tonal colours derived from Scandinavia’s surroundings, our brand keep it’s Scandi visuals at our core.
2023 &
Retail Pharmacy Product Awards
58 | PHARMACYNEWSIRELAND.COM
Polished London’s Hydro XP Dental Capsule


Polished London’s Hydro XP Dental Capsule offers a fast and convenient way to clean and sterilise dental aligners, retainers, dentures and mouthguards by using the latest in advanced dental cleaning technology and without the use of chemicals.
Delivering a superior clean using a combination of high-frequency Sonic waves and powerful Ultraviolet Type-C light in just five minutes, the Hydro Dental Capsule will save you valuable time and effort, leaving you confident that your oral appliances are hygienic and ready to use.
TePe EasyPick

TePe EasyPick is the perfect alternative to regular toothpicks. TePe EasyPick is a patented simple and easy to use pick to remove food deposits between your teeth. They are an easy and efficient way to clean the hard-to-reach areas between your teeth.

A firm but flexible core, covered in a soft silicon coating, and a non-slip grip ensure effortless and efficient cleaning. The durable plastic is what makes them superior to wooden picks.
TePe EasyPick are an on-the-go product. Overlapping conical sizes to fit all interdental spaces. The unique lamellae allows for a wide contact area with the approximal surfaces.
Jordan Green Clean Gentle Whitening Toothpaste


Jordan Green Clean Gentle Whitening toothpaste was created with you and the planet in mind! This toothpaste has proven to give whiter teeth in 2 weeks with daily use in a clinical study. With the environment in mind, Jordan have carefully chosen ingredients that are readily biodegradable in nature, with 98% from natural origin and 100% Vegan. It contains fluoride and xylitol to provide the best protection against cavities. Activated charcoal is added as a natural colourant and the toothpaste has a fresh cool mint flavour. The tube contains up to 50% recycled plastic, originating from food containers that consumers have recycled.
Jordan Green Clean Oral Care provides a wide range of products for Adults, Juniors, Kids and Baby to offer the most sustainable solutions across the oral care category from the first tooth.
PHARMACYNEWSIRELAND.COM | 59
Best Skincare Product
Many consumers in Ireland have educated themselves about skin care to discover and experiment. For example, research suggests that consumers are now more willing to try new beauty products compared to pre-pandemic years. Many Irish consumers experienced increased aggravation and inflammation of their skin, highlighting the link between lifestyle factors such as stress, poor diet, pollution and skin care. The category is therefore seen as a tool for self-care and well-being management.


The move to ingredients-led skin care was driven by the closure of dermatologists and other specialists during lockdown. The accessibility of online education and the prevalence of skin care advice on social media has given Irish consumers a wealth of information for those looking for salon professional quality skin at home.
The Finalists in this Category are:
NEOSTRATA 15% Vitamin C + PHA Serum


Introducing a unique powerhouse face serum featuring a blend of clinically proven antioxidants and exfoliating Polyhydroxy Acid for brighter, more radiant skin in just one week.
This lightweight and easy-to-absorb formula glides on smoothly and gives skin a boost of L-Ascorbic Acid (Pure Vitamin C), Feverfew Extract, Green Tea and Gluconolactone (Polyhydroxy Acid) to provide skin with antioxidant protection from external aggressors and oxidative stress, helping to diminish visible skin discolouration and even skin tone.
Polyhydroxy Acid helps to gently exfoliate the skins surface, allowing the Pure Vitamin C to absorb into fresh, renewed surface layers of skin. You’ll “C” the visible difference in your skin in no time with this daily brightening concentrate.
2023 &
Retail Pharmacy Product Awards
60 | PHARMACYNEWSIRELAND.COM
KELO-COTE®
KELO-COTE® Our patent-protected, clinically proven, advanced formula silicone treatment, helps improve the appearance of raised scars (hypertrophic and keloid scars).

KELO-COTE® gel helps soften and flatten raised scars as well as helping to relieve itching, discomfort and reduce redness in a range of scars. KELO-COTE® 15g gel is waterproof and flexible, transparent and odourless, can be used under make up and is suitable for sensitive skin.

KELO-COTE® revenues have grown threefold since the acquisition from Sinclair Pharma in 2015.
Sold in more than 65 countries, this performance has been driven by our planned marketing support, both at global and local level and expanded engagement with G-Scars, promoting best practice in scar management globally.
Topical silicone therapies like KELO-COTE® have been used by plastic surgeons and other healthcare professionals to treat hypertrophic and keloid scars since the early 1980’s1 (with KELO-COTE® formulation established in 1998).
Elave Daily Defence SPF 45


Elave Daily Skin Defence SPF45 is an Irish Made SPF infused moisturiser that is ideal for daily use, at home and on the go. Highly suitable for those with dry skin, eczema, psoriasis, dermatitis, rosacea, and sensitive skin, thanks to its extremely low irritancy profile.
Elave Daily Skin Defence SPF45 No.108 contains a unique combination of oil-free emollients to hydrate the skin, together with high UVA & UVB protection. The Invisible Zinc is absorbed quickly and the Vitamins C, B5 & E anti-oxidant enriched formula helps protect skin and repair environmental skin damage with its unique combination of physical and chemical sunscreens.
Dr Hauschka Cleansing Balm


With its innovative gel-to-milk texture, the new Cleansing Balm now complements Dr. Hauschka’s facial cleansing range. Its application is quite simple: When mixed with water, the balm forms a light milk that thoroughly removes impurities and make-up, leaving the skin feeling smooth and refreshed.
Dr. Hauschka Cleansing Balm is suitable for all skin conditions. The main ingredient which acts as a cleansing substance is Rügen chalk, a component long-known precisely for its cleansing effect. Its large surface area absorbs impurities and excess sebum, as well as a fine-grained structure thanks to which it can be gently washed off.
The Cleansing Balm also contains organic apricot kernel oil which nourishes the skin and makes it supple, while extracts of anthyllis, birch and calendula give it a revitalising boost. The result is a mild formulation which helps preserve the skin’s own oil-moisture balance and prepares your skin perfectly for the next care step.
PHARMACYNEWSIRELAND.COM | 61
Best Pregnancy/Fertility Product
Community pharmacy is often the starting point for many when it comes to fertility and pregnancy. Infertility is a public health concern and as such, utilisation of pregnancy and fertility services is prevalent, and the role of the pharmacist and the wider pharmacy team. Pharmacists can assist customers by providing education on infertility causes, referrals, nonpharmacologic and pharmacologic management options, and the OTC options. For those trying for a baby, there are some essentials that can really help to support them.
The Finalists in this Category are:
Pregnacare Plus



Pregnacare Plus even greater nutritional care for mother and baby Pregnacare Plus dual pack provides the original fully comprehensive multivitamin tablet, which delivers the essential nutrients during pregnancy and breast-feeding, plus an additional Omega-3 capsule to provide a rich source of docosahexaenoic acid (DHA) which contributes to normal brain and eye development of the foetus.
Pregnacare Plus has been specially developed so that mothers-to-be can safeguard their extra dietary requirements, without confusion about which supplements to take.

2023 &
Retail Pharmacy Product Awards
62 | PHARMACYNEWSIRELAND.COM
Proceive® Women
Proceive® Women is a scientifically formulated pre-conception supplement designed to support the nutritional needs of women when trying for a baby.


Developed by fertility experts using evidence-based ingredients proven to support the nutritional needs of the female reproductive system at optimum dosage levels. With no fillers, binders or preservatives, Proceive® Women offers pure, active nutrition. Each dose contains 28 vitamins, minerals and amino acids, including 400ug folic acid in the superior L-Methylfolate form, to support the processes involved in egg production, ovulation, hormone regulation and more. Guaranteed Irish, Proceive® Women offers high-strength, tailored nutrition when trying for a baby.
Arkopharma Cys-Control FORT
From the global leaders in natural alternatives, comes the formulation of Arkopharma Cys-Control FORT 10 sachet pack.

Used to treat and prevent the recurrence of urinary tract infections.
• Safe to take during Pregnancy and Breastfeeding
• Compliment to antibiotics
• New 10 sachet pack size
• 5 day treatment course
• Intensive formula
Cys-Control FORT is a complete and effective 100% natural treatment for recurring UTI’s due to the complementary action of Cranberry and D-Mannose on E.Coli.
In addition, the anti-bacterial properties of Heather promote the “auto flush” phenomenon.
Medicare femSense® Ovulation Tracker


The Medicare femSense® Ovulation Tracker is an innovative smart patch and app that helps woman detect ovulation during their fertile window. The first-of-its-kind medically certified smart patch continuously measures, records and analyses body temperature data. Your data is then transferred from the patch to the app when scanned using NFC (contactless). Precise measurements and an intelligent algorithm mean that Medicare femSense® helps detect the tiny increase in body temperature caused by ovulation.
The app notifies you of the days you are most likely to get pregnant. Furthermore, the femSense app will measure and confirm when ovulation has occurred. When compared to other traditional tracking methods, femSense offers benefits such as no more peeing on sticks, being discreet, hormone free and skin friendly.
PHARMACYNEWSIRELAND.COM | 63
Best Women’s Product
Health issues and care strategies for women’s health is far more widereaching than just pregnancy and gynaecology. Research has shown that increased global focus on women’s health disorders and an overall aging female population are helping to drive growth of the women’s health products over-thecounter.

Today’s women are much more well informed with consumers leaning towards self-care and self-medication and thus the outlook has been witnessing a dramatic transformation in recent years.
The Finalists in this Category are:
Active Iron Women
Irish brand Active Iron is an iron supplement with a ground-breaking new method of delivering iron. Active Iron is clinically proven to increase iron levels by 94% whilst helping to avoid the common side effects of iron.


Women living with low iron levels is a global problem affecting 1 in 4 women, with monthly periods being the leading cause of iron loss worldwide. For this reason, Active Iron’s team of scientists at Trinity College Dublin have developed a combination product specially designed for women. Active Iron for Women contains two components: non-constipating Active Iron and a multivitamin specially formulated for use with Active Iron to optimise absorption. The product aims to support iron levels and fight ongoing menstrual fatigue, which 64% of women admit having.
Meno Active
Meno Active is a super supplement with a scientifically formulated blend of premium ingredients, for women to take during their menopausal journey. Formulated after 2 years of research and development it contains 31 active ingredients, including 4 plant extracts, 12 vitamins, 7 minerals, 4 digestive enzymes, 3 strains of live friendly bacteria equating to 9,000 CFU’s per sachet and 350mg Omega 3 DHA, to support hormonal activity, brain function, nervous system and energy.


Meno Active is manufactured in Ireland and it is delivered in a single daily powdered sachet and single capsule that can be taken with or after food. It is suitable for vegetarians and it is gluten free.

64 | PHARMACYNEWSIRELAND.COM
2022 & Retail Pharmacy Product Awards
Utipro® Plus AF
Utipro® Plus AF is the latest innovation in the management of UTIs.


Utipro Plus has a unique dual mode of action working simultaneously in the bladder and intestine to control and prevent urinary tract infections for uncomplicated and recurring UTIs.
Utipro Plus contains a combination of natural ingredients, Xyloglucan-Gelose & Hibiscus and Propolis. Xyloglucan-Gelose creates a plant based protective layer over the surface of the intestinal mucosa to reduce the growth of bacteria that cause UTIs while Hibiscus and Propolis acidify the urine to stop bacterial growth, giving fast symptom relief
An oral capsule, available OTC. Information at www.utipro.ie
Optibac Probiotics For Women


Optibac Probiotics For Women is recognised as one of the best probiotics for intimate health. It is Optibac’s best reviewed supplement, being highly recommended by complementary healthcare practitioners, top UK gynaecologists, and consumers alike. This specialist supplement is designed specifically for the support of vaginal and urinary tract. For Women contains highly researched strains of friendly probiotic bacteria, which are scientifically proven to reach the intimate area and complement the natural vaginal flora.
Optibac Probiotics For Women is the most researched probiotic supplement for vaginal health. It contains a worldremowned combination of probiotic strains, Lactobacillus rhamnosus GR-1® & Lactobacillus reuteri RC-14®, which have been clinically trialled in thousands of women around the world. Featured in over 30 clinical studies, they are the most researched probiotic strains for uro-genital health. The unique formula has been designed particularly for those who suffer with thrush, cystitis, or bacterial vaginosis (BV) infections.
Regelle
Did you know?
• 75% of women suffering from symptoms of vaginal dryness DO NOT volunteer the information to their healthcare professional.
• Up to 40% post-menopausal women experience vaginal dryness.
• 10% were too embarrassed to discuss the problem at all with their doctors. Regelle is a water-based intravaginal gel that is clinically proven to provide soothing, continuous moisture, giving relief from symptoms of vaginal dryness, atrophy, itching, irritation and discomfort. Regelle is a NON-hormonal preparation and can be used in conjunction with systemic hormone replacement therapy (HRT). Regular use of Regelle, once every 3 days, can help maintain vaginal health and comfort by replenishing essential vaginal moisture before and after menopause. Regelle is also completely safe to use by women who are taking systemic Hormone Replacement Therapy (HRT).


PHARMACYNEWSIRELAND.COM | 65
Best Beauty Product
The beauty industry is fast-moving and highly saturated. Understanding changing consumer opinions and the behavioural indicators behind global trends is integral to securing the success of any company or brand within the space.
Last year, consumer spending on colour cosmetics is starting to increase, regaining the value lost over the previous years. In 2022, 53% of global consumers claim to prefer a natural or no make-up style, seeking to achieve a clean, fresh, and healthy look with the make-up products they use every day. Originating in K-beauty, dewy and glassy skin has been a key trend, prompting the adoption of “your skin but better” products. As attitudes change, the definition of beauty is becoming more blended with wellness, and as such, beauty brands are under increasing pressure to promote skin health. The Finalists in this Category are:


King Hair & Beauty Gold Dust Dry Shampoo


Gold Dust Dry Shampoo is a weightless and intelligent formula that is kind to both hair and scalp. Quick and easy to apply ensuring oils are banished in record time, but with lasting effects. Small and discrete but BIG on results.
A quick dusting revives the hair, adding body, and leaving your locks feeling clean and fresh to help maintain your crowning glory. The formula features a host of beneficial and unique ingredients, such as Corn Starch which is a fantastic, natural alternative to talc. Camellia Japonica Extract moisturises the hair and gives it a healthy shine, along with Orchird Extract which helps to repair damaged hair and promotes moisture retention, keeping your hair feeling moisturised.
2023 & Retail Pharmacy Product Awards 66 | PHARMACYNEWSIRELAND.COM
Dr Hauschka Translucent Bronzing Tint
Dr. Hauschka’s Translucent Bronzing Tint is ideal for use alone or with all Dr. Hauschka moisturizers. This liquid mineral tint offers a sun-kissed glow while gently blending and softening the appearance of blemishes and imperfections. Skin looks healthy, fresh, even-toned and radiant.


• Witch hazel, olive oil, beeswax and mineral pigments protect and nurture while evening out skin tone.
• This is the natural answer to fake tan from Dr.Hauschka.
• For all skin conditions
Usage: Mix Translucent Bronzing Tint with a day cream (we recommend Quince Day Cream) in your palm then apply to your face and neck, including décolleté, if desired. The colour intensity depends on the amount of tint added. Reapplying several times intensifies the effect.
Vegetarian
Vegan
Embryolisse Lait-Crème Concentré

Viral on TikTok, this cult classic has stayed around for a reason!
Invented in 1950, for years Lait-Crème Concentré was the best-kept backstage beauty secret. Today, it is still a favourite among dermatologists and professional make-up artists. The Lait-Crème Concentré is a truly versatile, multipurpose cream. It can be used as a primer, moisturizer, treatment mask and make up remover. Thanks to a blend of ingredients from natural origin rich in essential fatty acids and vitamins, it delivers nutrients to the skin, retains moisture, helps protect against external aggression and helps to accelerate cellular renewal for a more youthful complexion.
Nuxe Huile Prodigieuse® Gold


The golden shimmering version of NUXE’s iconic Huile Prodigieuse® nourishes, softens and illuminates the skin and hair thanks to its fine mineral-origin pearly particles. The dry oil has a unique nongreasy texture and a captivating scent with notes of Orange Blossom, Magnolia and Vanilla. With 98% natural origin ingredients, the formula is enriched with Vitamin E and contains a unique blend of 7 precious botanical oils with complementary properties: Macadamia, Sweet Almond, Hazelnut, Camelia, Tsubaki, Argan, and Borage.
On the skin, the unique blend of oils contains anti-ageing and antioxidant properties, while repairing and reducing the appearance of stretch marks.
On the hair, the oils will nourish, repair, strengthen and condition. They will restore natural elasticity and shine, while protecting against split ends and heat damage. Committed to women’s beauty and the environment, it truly embodies NUXE’s pillars - Nature, Science and Hedonism - making it a pioneering brand in natural-origin cosmetology.
PHARMACYNEWSIRELAND.COM | 67
√
√
Best Cough, Cold and Flu Product

According to the most recent research, the outlook for cold, flu and cough remedies over the forecast period is favourable, with most categories projected to expand consistently in volume terms. Overall demand should remain buoyant in the short term as life in Ireland continues to return to normal in the wake of the pandemic and people are more exposed to pathogens and environmental pollutants that cause common or seasonal respiratory illnesses and allergies.


The category has doubled in growth YTD, albeit from a low base in 2021 due to lockdown. +6% versus 2020. Cough medicines have experienced the largest demand followed by cold remedies and decongestants.
Bronchostop
Bronchostop Cough Syrup and Pastilles are used for the relief of ANY cough based on traditional use.
If consumers don’t know what type of cough they have or if their cough changes from one to another, Bronchostop takes the hassle out of finding a solution. Bronchostop is traditionally used for the relief of any cough such as chesty coughs and dry, tickly, irritating coughs and phlegm.

Buttercup Bronchostop Berry Flavour Cough Pastilles contain thyme herb extract. Buttercup Bronchostop Cough Syrup and Buttercup Bronchostop Berry Flavour Cough Pastilles are traditional herbal medicinal products used for the relief of coughs, such as chesty, dry, tickly, irritating coughs and catarrh, exclusively based upon long-standing use as traditional herbal remedies.
Always read the leaflet. IRE/BRO/2022/44
2023 & Retail Pharmacy Product Awards
68 | PHARMACYNEWSIRELAND.COM
VIVIO® Junior Cough Syrup



VIVIO® Junior Cough Syrup is a 100% natural and preservative free cough syrup, specifically formulated for Children 1 year+ for both dry and congested cough.
VIVIO® Junior Cough Syrup provides a sensation of calm, hydration and protection of the upper respiratory tract, modulating the cough without suppressing it.
The exclusive formula has a soothing and delicious taste thanks to the natural honey and strawberry.
The added marshmallow extract reinforces the soothing and calming properties of the syrup due to its natural mucilage properties.
Calpol Vapour Plug & Nightlight

Colds disturbing your family’s sleep? Calpol has a range of products to treat the symptoms of a cold, not just the fever. For those night time sniffles…try Calpol Vapour Plug & Nightlight for a comfortable night’s sleep.
Calpol Vapour Plug & Nightlight is an electrical plug-in device suitable from 3 months plus. The device comes with 3 refill pads and further ones can be purchased in packs of 5 or 10. Refill pads emit a blend of Aromatic Oils, including lavender, chamomile, menthol, camphor and eucalyptus.

The plug and refills help to clear and ease breathing and provides up to 8 hours night-time comfort relief. The nightlight emits a soft orange light to help comfort your child and aid a more restful sleep, also helping to guide you in the room so that you don’t disturb your sleeping child.
Olbas Oil
Olbas Oil’s powerful natural decongestant vapours helps relieve bronchial and nasal congestion and hay fever by inhalation. Placing a handkerchief with some drops of the oil onto your pillowcase at night provides soothing night-time congestion relief to aid restful sleep. For a more intense Olbas experience, place a few drops of Olbas Oil in a bowl of hot water, lean over and cover head with a towel. This clears even the most stubborn congestion. Olbas Oil is suitable for adults and children as young as 3 months.

PHARMACYNEWSIRELAND.COM | 69
Best Digestive Product
Digestive remedies continue to experience stable demand due to a common diet that is high in sugar, fats, refined carbohydrates and low in fibre. As more consumers opt for fast food options and maintain poor diets, they will continue to reach for products that offer them fast relief from the resulting symptoms of heartburn and indigestion.

Before the pandemic, the digestive health sector benefited from consumer preferences shifting towards preventative health remedies. Digestive remedies benefited further from a small boost as stomach upset and diarrhoea were declared symptoms of Covid-19 and consumers bought preventative products.
IBS, which is characterised by abdominal pain, excess gas and diarrhoea or constipation, is becoming a common gastrointestinal disorder in Ireland. The condition is predominantly seen in young and middle-aged females as women are twice as likely to experience IBS as men.

The Finalists in this Category are:
Alflorex® Dual Action
Alflorex® Dual Action is a unique combination of two PrecisionBiotic® cultures (35624® and 1714 - Serenitas® A clinical study confirmed the combination of the 35624® and 1714Serenitas® strains were synergistic. This product was developed in line with Rome IV criteria, which now defines Irritable Bowel Syndrome (IBS) as a ‘disorder of gut-brain interactions’. Two individual strains, two different modes of action both targeting key mechanisms in IBS together, this specific combination of strains targets the gut-brain axis and helps manage both the gut and psychological symptoms associated with Irritable Bowel Syndrome (IBS).
Alflorex® Dual Action also contains selected B vitamins, vitamin B6 and pantothenic acid, to reduce tiredness and fatigue* and contains calcium to support normal digestion*


Bio-Kult Advanced
THE NATURAL POWER TARGETING THE DIGESTIVE SYSTEM
Bio-Kult Advanced is a unique multistrain live bacteria supplement with 14 strains of live bacteria. Its naturally powerful formulation targets the digestive tract.

Bio-kult is backed by clinical research and has been proven to survive the high acidity of the stomach, does not need to be refrigerated, is suitable to be taken alongside antibiotics, when travelling, for everyday use as part of a healthy diet, when pregnant and can be taken by children. (we would always suggest consulting your doctor or healthcare practitioner before taking any food supplement).

2023 & Retail Pharmacy Product Awards 70 | PHARMACYNEWSIRELAND.COM
Optibac Probiotics Every Day EXTRA


Optibac Probiotics Every Day EXTRA is an advanced digestive supplement containing 20 billion live probiotic cultures, proven to reach the gut alive and complement the natural gut bacteria. Expertly formulated for those looking for an extra strength, high quality probiotic, either as a daily probiotic, or at certain times, such as after a course of antibiotics. This comprehensive formula contains four extensively researched probiotic strains which naturally occur in the digestive tract, and target both the small (Lactobacillus) & large (Bifidobacterium) intestines. The probiotic strain combination includes Lactobacillus acidophilus NCFM®, the most researched acidophilus strain in the world, clinically trialled and shown to support immune health, and overall digestion. Despite its powerful strength, this highly researched supplement is safe and suitable for use from the age of 4 years, including pregnant women and older people, in one convenient daily dose. The prebiotic-free formula is particularly suitable for lowFODMAP diets and those looking for a probiotic without added prebiotics, such as IBS sufferers.
Nexium Control

Nexium Control is a non-prescription medication for the short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. When your stomach reacts badly to something (food, stress etc) it can create too much acid. This can rise up into your throat causing pain and swelling. This is called heartburn or acid reflux. Nexium Control works by limiting the amount of acid your stomach produces, helping prevent heartburn. It does this without affecting your ability to digest food and drink.
With Nexium Control you could enjoy life without heartburn. Nexium Control offers 24 hour protection from heartburn and acid reflux, it is clinically proven to relive night-time heartburn for a good night’s sleep and helps restore your life to how it should be heartburn free.
Alflorex®
Alflorex® is the leading probiotic for IBS, a condition that affects up to 1 in 7 people in Ireland every day. This probiotic is taken daily to manage and prevent recurrence of IBS symptoms. It costs approx. ¤1/day and reduces ALL of the symptoms of IBS including:


abdominal pain
bloating & gas
unpredictable bowel movements like alternating diarrhoea and constipation
The unique 35624® culture in Alflorex is a naturally occurring bacterial strain found in the human gut. This Bifidobacterium longum culture is part of the family of bacteria given by mother to baby at birth. The main long-term aim for Alflorex is to strive to have all IBS patients in Ireland take Alflorex for 1 month. The product conversion score is more than 80% (continued usage) and with a high percentage of this group needing direction and treatment we believe Alflorex can change their lives.
PHARMACYNEWSIRELAND.COM | 71
Best VMS Product
Increasing numbers of people are taking a proactive approach to their health and the wellbeing trend is sweeping the nation. Keeping up with consumers or even getting a bit ahead of their desires is the name of the game. In the ever-changing and extremely fickle vitamins, minerals and supplements (VMS) category, it is even more vital to stay on pace or ahead of the curve with those visiting their local pharmacy.
According to latest market research reports, vitamins sales are expected to continue growing as consumers increasingly look towards preventative measures over cures. A growing number of consumers are expected to monitor their health through blood tests, which will result in greater awareness of vitamin and other deficiencies.


The Finalists in this Category are:
O.R.S Hydration Tablets
Fast, Proven Hydration
Clinova’s innovative O.R.S soluble hydration tablets are based on the standard oral rehydration salts formula. Containing a balanced combination of glucose, salts and electrolytes.
The soluble tablets replenish water and salts, thereby helping to maintain a proper fluid balance. Perfect for before, during or after exercise.
Drop, dissolve, drink!
O.R.S is used by:
• People who lose excess fluid
• Professional and recreational athletes
• Visitors to hot/tropical countries
• People who are dehydrated due to alcohol consumption
• People who work in hot climates
O.R.S Sport contains added Vitamin D and Magnesium.


2023 & Retail Pharmacy Product Awards
72 | PHARMACYNEWSIRELAND.COM
Formulated and developed by the winner of the prestigious 2022 ‘Pharmacist of the Year’ award, Laura Dowling, fabÜ is a unique range of premium-grade botanical and mushroom extracts blended with vitamins and minerals.
fabÜ‘s scientifically targeted supplement formulations take the guessing game out of pulling together long lists of endless - and often generic - ingredients. The result is a product range combining just 6-8 powerful, key ingredients tailored specifically to your wellbeing needsthanks to Laura’s incredible fascination with the wonderful powers of plants, herbs and mushrooms from across the world. fabÜ demonstrates perfectly how nature and science can work seamlessly together in absolute harmony.
fabÜ SHROOMS IMMUNE, fabÜ R&R RELAX and fabÜ MENO & PERI are available in pharmacies nationwide. The product reviews on www.fabuwellness. com are nothing short of fabulous!


Zest Active
Zest Active is a super supplement scientifically formulated with 25 active ingredients, all carefully selected to support energy, the brain, muscle function and the immune system.


Zest Active is unique due to its powdered formula, high bioavailability and its combination of vitamins, minerals, amino acids and beta glucans. It is free from fillers, binders, preservatives, artificial colours, caffeine and other stimulants.
It’s the perfect accompaniment for fitness enthusiasts, students, young professionals and those trying to balance it all.
AYA Ultimate One a Day

Live the AYA way with AYA Ultimate One A Day. This is a complete solution to boost the performance of the mind and body. A potent powerhouse packed with more than 40 vital vitamins and minerals, this flagship product was designed by scientists to meet the complex daily demands of modern life and the human body. This is the perfect supplement for those who want a quick and effective way of meeting their vitamin needs with minimum fuss.

Can help to support:
Stress & Hectic Lifestyle
Energy
Detox & Cell Protection
Immunity
Restricted diets - e.g. Vegetarian, Gluten sensitivity, Calorie-restricted diet
PHARMACYNEWSIRELAND.COM | 73
fabÜ
Best Eye Care Product
In addition to the rapidly ageing population of the most developed consumer health markets, a rising incidence and prevalence of allergies, more exposure to digital screens, pollution, lifestyle diseases and side effects of common medications are leading to more diagnosed cases of dry eye, allergy eyes and aged-related macular degeneration (AMD).

Consistently strong growth is expected within OTC Eye Care market in the future. Screen time is having a negative impact on consumers’ eyes, which often dry out and become sore. Innovation is likely to also fuel growth over the coming years.
The Finalists in this Category are:
HYLO-FORTE®
HYLO-FORTE® is an eye drop containing 0.2% sodium hyaluronate that is used for the intensive and therapeutic lubrication of the ocular surface in more severe and persistent dry eye sensation.

HYLO-FORTE® is contact lens compatible and it’s preservative and phosphate free formula means it can be used as often as required. Delivered via the patented COMOD® delivery system, HYLO-FORTE® releases one drop per pump and has a guaranteed 300 drops per bottle. HYLO-FORTE® can also be used for up to 6 months from first opening, making it economical and cost effective.
2023 &
Retail Pharmacy Product Awards
74 | PHARMACYNEWSIRELAND.COM
Thealoz Duo
Thealoz Duo is Europe’s No 1 dry eye drop solution created exclusively by Europe’s No 1 Ophthalmology Group, Laboratoires Théa. Thealoz Duo is widely acclaimed for its innovative, synergistic formulation since it’s the only dry eye drop in the pharmacy shelf that combines the fixed, combination action of 2 active ingredients: 0.15% Hyaluronic acid which hydrates and lubricates the eyes and 3% Trehalose which protects and repairs them. Thealoz Duo is 100% preservative free and clinically proven to treat symptoms of dry, tired, red, stingy eyes. It is suitable for all dry eye sufferers including contact lens wearers.


MACU-SAVE® GOLD+
Contains all 3 macular carotenoids which are essential for macular health:
• 10mg Lutein
• 10mg Meso-Zeaxanthin
• 2mg Zeaxanthin
• Plus, Vitamin B2, Vitamin C, Vitamin E, Copper & Zinc
Normal Vision
Zinc and Vitamin B2 help to maintain normal vision. Copper and Vitamin C contribute to the normal functioning of the nervous system. Vitamin C and E contribute to the protection of cells from oxidative stress.
MACU-SAVE® GOLD+ capsules do not contain beta carotene and are sugar-free.
Therefore, it can be taken by ex-smokers and/or smokers. It is suitable for diabetics, gluten-free, yeast-free, and lactose-free, and contains no artificial preservatives or sweeteners.
MacuShield™ Original Chewable
MacuShield™ Original Chewable is a food supplement for eye health, which contains Vitamin B2 to help maintain normal vision. In addition, it contains all three macular carotenoids - Lutein, Meso-zeaxanthin and Zeaxanthin.


MacuShield™ Original Chewable is a onea-day, bilberry flavoured chewable tablet, which offers an easy-to-take alternative for those who don’t like swallowing capsules. All MacuShield™ products contains 10mg of lutein, 10mg of mesozeaxanthin and 2mg of zeaxanthin per day. These nutrients are found at the back of the eye, in the macula, where they form the macular pigment.
The macular carotenoids in MacuShield™ Original Chewable are obtained from marigolds (Tagetes Erecta), specifically grown for this purpose.
The MacuShield™ brand began with a single product in 2006, the range of products has increased and revenues have grown threefold since our acquisition in 2015. Through our continuous HCP, trade and consumer marketing we endeavour to empower people to proactively look after their eye health.
PHARMACYNEWSIRELAND.COM | 75
Best Sleep/Stress Relief product
More than 70% of consumers suffer from sleep disorders or stress, especially during recent times. This is worrying considering long-term lack of sleep can be associated with increased risk of obesity, stroke, heart disease, depression and anxiety.

According to the most recent OTC market data, the growing number of patient base suffering from respiratory issues, cardiovascular illnesses, and Obstructive Sleep Apnea (OSA), coupled with the rising occurrence of insomnia, are the primary factors that are likely to accelerate the industry demand across the globe.

The Finalists in this Category are:
Nytol
Nytol is Ireland’s #1 sleep aid brand*. Nytol is available in pharmacy only to help consumers get a good night’s sleep. Nytol One A Night 50mg Tablets are used to relieve temporary sleeping difficulties in adults.

Nytol can help with all round sleep cycle support; helping consumers to drift off gently, improve quality of sleep and wake up feeling refreshed.
Nytol is the only clinically proven sleep aid available without prescription from your pharmacist.
Nytol One-A-Night 50mg tablets contains diphenhydramine hydrochloride. An aid to the relief of temporary sleep disturbances in adults. For further information, ask your doctor or pharmacist. Always read the leaflet. IRE/NYT/2022/29
*Based on IQVIA sales data Oct 2022.

2023 &
Pharmacy Product Awards 76 | PHARMACYNEWSIRELAND.COM
Retail
Zenflore®
Zenflore® is the world’s first and only supplement harnessing the unique 1714-Serenitas® bacterial culture. Combining the unique 1714-Serenitas® culture with specially selected vitamins, Zenflore® provides support for mind and body through life’s daily challenges.


Aids Mental Performance
Reduces Fatigue.
Improves Coping
The special 1714-Serenitas® culture in Zenflore has been clinically studied in partnership with scientists and clinicians from one of the world’s leading centres of research on the human microbiome. Zenflore’ s special formulation is stable at room temperature and does not need to be refrigerated.
Kalms Night
Kalms Night is a traditional herbal remedy to help promote a refreshing natural night’s sleep – without causing drowsiness the next day. Kalms Night contain herbs traditionally used for their sedative action which may be helpful in the case of sleep disorders. Kalms Night active ingredient is Valerian Root. Kalms Night One-A-Night is in a single tablet form making it simple and convenient to take. Intended for use for temporary relief of sleep disturbances, Kalms night can be taken for two to four weeks and they can help to leave you feeling refreshed and energetic the following morning.


Rescue Night
Containing the 5 original Bach flower essences used in Rescue Remedy, Rescue Night also contains White Chestnut, an essence traditionally taken to help with racing thoughts. One of the biggest challenges that people who don’t sleep properly face is actually getting to sleep. Rescue Night provides emotional support to calm the mind before bed, allowing for a natural night’s sleep that won’t leave you feeling groggy in the morning.


PHARMACYNEWSIRELAND.COM | 77
Best Launch of the Year
When launching a new product there are several steps to success that are crucial for any business, taking time and careful planning. These include understanding your target audience and how to reach them effectively, looking at what makes your product innovative or different and unifying the product team across all channels, whether it’s marketing, social media or sales.
The finalists in this category have demonstrated that consistency is key when launching a new product within the OTC market.
The Finalists in this Category are:
Sprains and strains are common, painful injuries that affect muscles and ligaments reducing the ability to do daily activities. Teva Ireland have launched Motusol™ Max, the first alternative 2% diclofenac gel (P). Motusol™ Max provides up to 12 hours of pain relief, enabling patients to treat pain at the source, reduce swelling and restore movement in the comfort of their home. It also has an easy-grip cap to open & close. Teva is committed to give patients access to affordable medicines and provide more options to pharmacists. Motusol™ Max helps patients take control of their pain and treat it effectively.



Alflorex® Dual Action
Alflorex® Dual Action is a unique combination of two PrecisionBiotic® cultures (35624® and 1714 - Serenitas® A clinical study confirmed the combination of the 35624® and 1714Serenitas® strains were synergistic. This product was developed in line with Rome IV criteria, which now defines Irritable Bowel Syndrome (IBS) as a ‘disorder of gut-brain interactions’. Two individual strains, two different modes of action both targeting key mechanisms in IBS together, this specific combination of strains targets the gut-brain axis and helps manage both the gut and psychological symptoms associated with Irritable Bowel Syndrome (IBS).
Alflorex® Dual Action also contains selected B vitamins, vitamin B6 and pantothenic acid, to reduce tiredness and fatigue* and contains calcium to support normal digestion*


2023 &
78 | PHARMACYNEWSIRELAND.COM
Retail Pharmacy Product Awards
Max
Motusol™
Formulated and developed by the winner of the prestigious 2022 ‘Pharmacist of the Year’ award, Laura Dowling, fabÜ is a unique range of premium-grade botanical and mushroom extracts blended with vitamins and minerals.
fabÜ‘s scientifically targeted supplement formulations take the guessing game out of pulling together long lists of endless - and often generic - ingredients. The result is a product range combining just 6-8 powerful, key ingredients tailored specifically to your wellbeing needsthanks to Laura’s incredible fascination with the wonderful powers of plants, herbs and mushrooms from across the world. fabÜ demonstrates perfectly how nature and science can work seamlessly together in absolute harmony.
fabÜ SHROOMS IMMUNE, fabÜ R&R RELAX and fabÜ MENO & PERI are available in pharmacies nationwide. The product reviews on www.fabuwellness. com are nothing short of fabulous!


Tonstix Honey Jelly Pops
Tonstix Honey Jelly Pops for Childrens Sore Throats & Coughs are an all natural product formulated to melt in the mouth which facilitates lubrication of irritated throats caused by allergies, infections, and viruses.


With added Vitamin C and Zinc, it gives instant comfort to unsettled children. Their unique stick person shape makes them the perfect size for little hands to hold.
Tonstix comes with a set of fun stickers to cheer children up with a moment of fun and can help them communicate how they are feeling, which is often challenging particularly for frustrated toddlers when they are unwell.
Immune Teen
Active Immune Teen was created with your immune and energy health in mind. It was created by Pharmacists who wanted to create a supplement which offered adequate immune and energy support for teenagers, without including unnecessary ingredients. It is Vegan friendly and Gluten Free.


A unique food supplement with carefully selected vitamins, minerals, Wellmune® to help support your immune and energy system in a powdered tropical flavour sachet which can be mixed with water, juice or a smoothie.
It was created and manufactured in Ireland.
PHARMACYNEWSIRELAND.COM | 79 Pharma Plus Active
fabÜ
Best Immunity Product
A healthy immune system is the first step of attack against outside invaders such as bacteria or viruses. When an immune system is strong, consumers are more likely to be able to ward off sickness.
There are lifestyle factors that can aid a healthy immune system such as getting enough sleep and exercise, but OTC supplements are also available to help strengthen the body’s immune response either by taking them regularly or at the onset of certain symptoms.
The Finalists in this Category are:
Udo’s Choice Super 8 Immune

A high strength microbiotic with 42 Billion friendly live bacteria per capsule + Vitamin C.
Super 8 Immune contains a powerful therapeutic formula of eight specific strains of friendly bacteria specially formulated for supporting flora imbalance or disruption and supporting immune function.
• High Strength: Minimum of 42 billion ‘friendly’ bacteria per capsule with Vitamin C
• Contains 8 strains of bacteria chosen for their value to both the upper and lower bowel health

• Gastric acid resistant strains
• Ideal post-antibiotic
• Refrigerated to preserve ‘live’ bacteria
• High levels of friendly bacteria in the gut will boost the immune system
• Suitable during pregnancy and breastfeeding, and with children age 5 and upwards
2023 & Retail Pharmacy Product Awards 80 | PHARMACYNEWSIRELAND.COM
AYA Ultimate One a Day

Live the AYA way with AYA Ultimate One A Day. This is a complete solution to boost the performance of the mind and body. A potent powerhouse packed with more than 40 vital vitamins and minerals, this flagship product was designed by scientists to meet the complex daily demands of modern life and the human body. This is the perfect supplement for those who want a quick and effective way of meeting their vitamin needs with minimum fuss.

Can help to support:
Stress & Hectic Lifestyle
Energy
Detox & Cell Protection
Immunity
Restricted diets - e.g. Vegetarian, Gluten sensitivity, Calorie-restricted diet
Kelkin Effervescent Vitamin C


Vitamin C has a number of important roles within our body: antioxidant activity to help maintain the body’s cell defences, assisting the formation of collagen needed for healthy skin, bones, teeth and gums.
• Provides 1000mg of Vitamin C
• 20 effervescent tablets
• Orange flavour
• Immunity
Alflorex® Immune
Alflorex® Immune from PrecisonBiotics combines a targeted bacterial culture with extra strength vitamin D to support your immune system*.


It contains the unique PB-VIR® culture, a PrecisionBiotics® strain.
The PB-VIR™ culture is a naturally occurring and safe bacterial strain, belonging to the Bifidobacterium longum species; these are types of bacteria passed from mother to baby at birth.
It was discovered by PrecisionBiotics and has been the focus of research since 2010. In a PrecisionBiotics’ pre-clinical study on influenza 2 , intranasal administration of the Bifidobacterium longum PB-VIR™ strain resulted in a host immune response that controlled viral numbers in the lung at an earlier stage of infection than seen in the control group, preventing any later inflammatory response and thereby reducing the mortality and lung damage associated with influenza.
The results strongly indicate the potential of Bifidobacterium longum PB-VIR™ to help protect against influenza, a severe respiratory viral infection.
PHARMACYNEWSIRELAND.COM | 81
Topic Team Training – Suppository Pain Relief
A community pharmacy environment that fosters teamwork ensured high levels of consumer satisfaction. This series of articles is designed for you to use as guide to assist your team in focusing on meeting ongoing CPD targets and to identify any training needs in order to keep the knowledge and skills of you and your team up to date.
4. Nasal suppositories: The nasal suppositories are also called nasal bougies or Bulgaria. The nasal suppositories are meant for introduction into the nasal cavity. They are usually prepared with a glycerogelatin base.
5. Ear cones: Ear cones are used for insertion into the ear. They are also known as Auraria. They are used rarely. For the preparation of ear cones generally, theobroma oil is used as a base. They are prepared in urethral bougies mold and cut according to size.
The below information, considerations and checklist provides support to enable you to run a team training session and identify opportunities for learning within the topic of Suppository Pain Relief.
Suppositories may not be the most pleasant product you’ll ever advise on. But they can make it easier to take medicine that some can’t swallow or that their stomach or intestines wouldn’t absorb well.
Types of Suppositories
Suppositories have a base made from substances like gelatin or cocoa butter that surrounds the drug. As the warmth of the body melts the outside, the drug slowly releases.
Suppositories and pessaries as drug delivery vehicles are not new dosage forms. Rectal drug delivery is one of the world’s oldest strategies for drug dosing and rectally applied products have existed for hundreds of years. Suppositories have been referenced in the Hebrew Scriptures. Even vaginally applied pessaries are equally as old with documentation reported in Egyptian papyruses. Hippocrates wrote of various acorn-based medicines delivered rectally and vaginally for local pharmacologic effects.
Consider:
Different types of suppositories go into the rectum, vagina, or the urethra. Sometimes they treat the area where they are inserted. Or the medicine absorbs into the blood and travels to other parts of the body.
Regardless of indication, such as constipation, yeast infection, pain relief, viral prevention or antiviral treatment, drug delivery from a suppository, like all topically applied dosage forms, occurs primarily by passive diffusion for both vaginal and rectal administration.
1. Rectal suppositories: These are administered rectally either to treat local conditions such as hemorrhoids or to achieve systemic absorption (sedatives, tranquilizers, and analgesics). Rectal suppositories can be used when the patient is unable to take medication by mouth, or when the patient is unconscious.
2. Vaginal suppositories: These are applied vaginally for a local effect. The vaginal suppositories are larger than the rectal suppositories. They are used for their local action in the vagina.
3. Urethral suppositories: These are cylindrical dosage forms, administered urethral to treat local conditions. They are unusual dosage forms.
The different types of suppositories
What is required for administration
Indications for administering
The administration procedure
Any follow-up actions that may be required
Parenteral and rectal routes of administration are preferred when quick therapeutic response is required. Rectally administered drugs are found to achieve better plasma levels and therapeutic effectiveness when compared with orally or intramuscularly administered drugs of similar dose The primary advantages of suppositories over other dosage forms include reduced first pass metabolism, both topical and systemic effect, accommodates patients who have difficulty swallowing pills, and increased bioavailability of drugs.
Advantages of Suppositories
1. Suppositories can exert a local effect on rectal mucosa.
2. It is used to promote evacuation of the bowel.
3. It avoids any gastrointestinal irritation.
4. Suppositories can be used in unconscious patients (e.g. during fits).
Key Points:
Check your pharmacy team are aware and understand the following key points:
The pharmacy team knows to be sensitive in dealing with customers who may feel vulnerable as a result of poor mental health
The team knows which groups of customers are likely to be at risk of depression and other mental health conditions, such as anxiety
My pharmacy assistants can meet the points in this training checklist.
5. Suppositories can be used for systemic absorption of drugs and to avoid first-pass metabolism.
6. It is useful for babies or old people who cannot swallow oral medication.
7. It is also useful for postoperative people who cannot be administered oral medication.
8. It is a very suitable dosage form for people suffering from severe nausea or vomiting.
Disadvantages of Suppositories
1. Suppositories have a problem with patient acceptability.
2. In some cases, the total amount of the drug must be given. This will be either too irritating or in a greater amount than reasonably can be placed into a suppository.
3. Incomplete absorption may be obtained because the suppository usually promotes evacuation of the bowel.
4. Suppositories are not suitable for patients suffering from diarrhea
Further Resources:
YourMentalHealth.ie
YourMentalHealth Information Line 1800 111 888
Actions:
Ensure efficient sign posting to discreet consultation areas within the pharmacy for further help and advice
Ensure that I know the recommendations for each OTC treatment which impact a customer’s mental health
Do any of us suffer from depression in the pharmacy team? Are some individuals more affected than others? If so, why?
Are we confident about raising issues of depression and mental health with customers?
Can we provide good advice on dealing with depression?
Train the team to meet all the above considerations
82 | PHARMACYNEWSIRELAND.COM




Joining Standards of Excellence & Innovation
The Irish Pharmacy Awards 2023
“There are no secrets to success. It is the result of preparation, hard work and learning from failure”
In launching the eleventh annual Irish Pharmacy Awards, we are seeking to uncover those individuals and teams putting in the groundwork quoted above to the enhancement of community pharmacy throughout Ireland.
The Irish Pharmacy Awards represent a unique and high-profile opportunity to celebrate the excellence and amazing achievements of Ireland’s pharmacy professionals, and reaffirm IPN’s commitment to the core pursuits of pharmacy excellence in health and innovation.
The Awards recognise outstanding examples of high standards, best practice, innovation and excellence.
It has been, and continues to be, the goal of Irish Pharmacy News to recognise the exact added value of the contribution made by community pharmacists and their teams. Through the submissions to these Awards and the examples of innovation that they demonstrate, IPN want to inspire you to think about your role, that of your team and of the community pharmacy profession in which you operate, as a whole.
The 15 Award Categories are inviting entries across a broad spectrum of fields; from innovation and business development, to the rising stars of the profession and the teams and individuals making a difference.
2023 Award Categories:
Haleon Self-Care Award
United Drug Business Development (Independent) Award

Easolief DUO Business Development (Chain) Award
Perrigo Superintendent Pharmacist of the Year

Reckitt Community Pharmacist of the Year

Uniphar Group Category Development of the Year Award

JPA Brenson Lawlor Young Community Pharmacist of the Year Award
Roche Diagnostics Point of Care Testing Pharmacy of the Year Award


EVO Payments Bank of Ireland Innovation & Service Development (Chain) Award

Originalis Community Pharmacy Team of the Year Award
McLernons Independent Pharmacy of the Year Award
Counter Assistant of the Year Award
OTC Retailer of the Year Award
Community Pharmacy Technician of the Year Award
Online Pharmacy Retailer of the Year Award

BOOK YOUR TABLE
Over 650 of Ireland’s pharmacy industry professionals will join us on Saturday, May 27th 2023 at the Clayton Hotel (Burlington Road) Dublin. Don’t miss out on this opportunity to network and celebrate community pharmacy.
This is sure to be a night to remember. Seats and tables are filling up fast. Book your table now to attend Ireland’s premier Pharmacy Awards event.
To request an application pack contact: aoife@ipn.ie?
2023 The Irish Pharmacy
Awards
www.irishpharmacyawards.ie/shop/ Introducing... Haleon, formerly part of GSK

SAVE THE DATE Saturday, May 27th 2023
Medicines Management in Older People
Older adults aged over 60 years account for more than 1 million people in Ireland, equating to 1 in 4 of the adult population.1 Older adults represent a large proportion of pharmacy customers, in particular as increasing age is associated with increasing medical conditions and drug treatments.2, 3 Common medical conditions include hypertension, hypercholesterolemia, diabetes mellitus, cardiovascular disease, chronic lung disease, arthritis or chronic pain.3
Education
Written by Dr Emily Killeen, Specialist Registrar in Age Related Health Care

Regular Medication Reviews
Optimisation of medicines is important for many reasons. Medications have potential side-effects and this risk can be increased in older adults for a multitude of reasons including altered pharmacodynamics/ pharmacokinetics, visual or hearing impairments, cognitive impairment and polypharmacy.

Polypharmacy is described as the prescription of 5 or more regular medications. Polypharmacy is associated with an increased risk of adverse drug reactions, potentially inappropriate medicines and non-adherence.2
Polypharmacy is reported in 33% of older adults and is more likely in adults with lower educational level, poor self-rated health, medical card eligibility and greater morbidity. The Irish Longitudinal Study on Ageing (TILDA) report in 2012 showed that polypharmacy accounts for “over half of the annual costs of prescribing to the entire population over 50 years.”2 Patients with polypharmacy also represent “51% of inpatient hospital visits, 55% of outpatient hospital visits and 41% of GP visits.”2
How do we tackle medication management to support older adults living in Ireland? There are a number of simple measures that can be considered:
The ‘Supporting Prescribing in Older Adults with Multimorbidity in Irish Primary Care’ (SPPiRE) trial published in January 2022 demonstrated that GPled medication review led to a small but significant reduction in the quantity of prescribed medications.4 One limitation, however, is the significant time constraints under which GPs operate. Therefore the responsibility should be spread across the healthcare system, with opportunities to review medications at each patient contact. Medication reviews can be undertaken in community, outpatient or inpatient settings by pharmacists, doctors and advanced nurse practitioners. Review tools that can be utilised include the Anticholinergic Burden Scale,5 Medication Appropriateness Index6 and STOPP-START criteria.7
International research has shown improved health outcomes and medication adherence in patients who are well informed.8 Ideally patients and/or caregivers should be educated about the indications, benefits and risks of prescribed medications. Instructions should be clear, simple and if necessary be provided in written format to optimise understanding and promote health literacy. If required translators or translations should be used to optimise communication.
Package Leaflets can be informative however they can be written in small print with detailed, elaborate instructions and extensive precautions that may be difficult for the consumer to understand. More user-friendly alternatives can be administered by the prescribers or dispensers. These include patient information leaflets, alert cards and reminder cards9 that should be easy to read and in large print without the use of complex language or medical jargon. For example, the HSE website provides clear patient information leaflets on common
medications.10 Reminder systems to augment education and safe medication use include blister packs, medication charts and smartphone apps.11
National Prescribing System
A national prescribing system that could be made accessible to both community and pharmacist healthcare workers would optimise medication safety and adherence. It could also reduce the risk of medication errors including omissions. This would allow for joined-up care for our older adults as they navigate the healthcare system. It could also provide an easily accessible reference system for medication prices, including the availability of generic options.
In conclusion, older adults account for a large proportion of healthcare and medication users. Optimisation of medications through simple measures such as medication reviews and education can improve patient outcomes, promote health literacy and reduce the risk of medication adverse events.
References available on request
PHARMACYNEWSIRELAND.COM 86 Elderly Care
The Pensions Landscape in Ireland is evolving at a rapid pace
Are you getting the right advice for your circumstances ?

Recent changes have significantly altered the Irish Pension Market with








○ Removal of Directors/Company Paid Pensions








○ Introduction of Master Trust Pensions





○ Changes to the PRSA that have made it the optimal choice for certain people
○ Auto Enrolment





























Now more than ever you need to make sure you are looking at all the options available. A comprehensive fi nancial review from a CERTIFIED FINANCIAL PLANNER™ running full cash flow modelling will give you the peace of mind in knowing that your fi nances are working as hard as you are.
What we analyse

1 Suitability of Current Pension
2 Performance
3 Fees – You don’t overpay for stock for your business yet every week we see people overpaying for their pension which has a bigger impact
4 Was you pension factored into an overall cashflow model
Moore Wealth Management Limited trading as Moore Wealth Management is Regulated by the Central Bank of Ireland Reg number 55195 colm@mwm.ie 086 860 39 53 | kieran@mwm.ie 086 380 18 68
Significant Pension Changes in Finance Act 2022 – What you need to know
Signed into law by President Michael D Higgins in early January this year The Finance Act 2022 brought into effect the single biggest change to pension legislation in Ireland for 30 years and this has barely got a mention outside of adviser circles.
Written by Colm Moore, Moore Wealth Management
Colm Moore is a CERTIFIED FINANCIAL PLANNER™ with Moore Wealth Management. They have been advising the pharmacy community for over 20 years. He can be contacted on 086-8603953 or at colm@mwm.ie
The Planning Opportunity
In recent months we wrote how the pensions authority of Ireland in the middle of a pension funding crisis stopped the establishment of company-paid pensions for business owners owing to the implementation of the IORPS II directive from Europe. This was followed by communication from the Central Bank who advised brokers not to set up any pensions for clients between June and December last year unless we were certain we knew what changes the unpublished and unwritten Finance Act would bring!

The establishment of The Master Trust Pension to replace company pensions was warmly welcomed in December and once again we could offer solutions to clients that in reality look very similar to before apart from the removal of surrender penalties and a circa 1 basis point increase in fees to cover the cost of auditing each scheme annually(an IORPs requirement). There was also the promise that legislation governing PRSAs would be updated to make them more attractive and in particular, remove the Benefit in Kind implications for employer contributions.
On publication Section 22 of the Act removes the provision which deemed an employer PRSA
contribution to be an employee PRSA contribution for income tax relief purposes. However, the way they did this also created a significant opportunity that goes against all revenue practice regarding pension funding for the last 30 years.
Previously as you may be aware when a customer set up a pension the adviser runs what is called a revenue maximum funding calculation to determine what they can contribute to a pension based on the following factors
1) Age
2) Selected retirement age
3) Current Salary
4) Service with the employer to date
5) The value of any existing pensions
For a real-world example take a client age 55 who has a pension valued at ¤750,000 and a salary of ¤50,000 with the business sitting on significant cash and is considering retirement. Under current revenue funding rules the maximum that the client could put in his pension is ¤19,666 per annum up to his retirement age of 65.
The significant change to the Finance Act is that none of the above factors(age, salary, service etc) are now factored in. There is no funding calculation for an employer contribution to a PRSA. In the above scenario the individual if they wanted could now fund a PRSA to the maximum tax-efficient pension of ¤2.15m which is of course a huge increase from the current allowance. Under the new rules, this person has gone from a scenario where they could fund ¤196,660 into a pension to one where they can contribute ¤1.4m to a PRSA.
If you change the above scenario to someone who already has the maximum funded pension and has a spouse employed in the business on a nominal salary and who has none or very little pension funding to date they can under the new rules contribute up to ¤2.15m into a PRSA for them.
As you will see there is a significant opportunity here for cash extraction in several different scenarios that we have not seen before. This will be particularly attractive to those who are sitting on cash reserves and
1. Have an underfunded pension and do not run a high salary so cannot contribute any additional funds
2. Have a maxed-out pension but also have a spouse employed on a small salary who previously could only contribute a small amount
3. Those close to business exit looking to extract cash tax efficiently.
There is no consensus as to whether this was the intention of the changes, yet when the initial draft of the Finance Act was announced in Oct 22 this was immediately highlighted as the situation. The view was that when the final draft arrived in early January that this would be amended or removed. However it was not and the Department of Finance know the implications. To get this changed again would require another statutory instrument which means this is likely in place for this year at least. As I mentioned above this is a change from revenue practice and it is the current situation at least for this year and anyone availing of it is following the legislation as written. Chief among any concerns about interpretation of the legislation would be revenues view on the implementation and this needs to be factored into any decision made and be a part of the conversation with your accountant and tax adviser.
There are of course many more variables to factor into someone putting such a lump sum into their pension and clients need to be advised accordingly to ensure this is the right course of action for their circumstances. This is where holistic advice by a CERTIFIED FINANCIAL PLANNER™ can prove invaluable and in conjunction with a lifetime cashflow model there is an opportunity here to look at an earlier and more efficient exit from your business than you thought possible.
Wealth Demographics Report Ireland 2023
One of the benefits of being a CERTIFIED FINANCIAL PLANNER™ is access to research not available outside of the small proportion of advisers in Ireland operating at this level. Recently a report was commissioned by the Financial Planning Standard Board of Ireland entitled Wealth Demographics and the Planning Landscape in Ireland that has provided great insight into the market now and in the future.
PHARMACYNEWSIRELAND.COM 88 Finance
This highlighted the robust shape of the Irish economy along with the fact we had the third highest life expectancy in Europe after Norway and Iceland with an average expectancy of 20.7 years for those retiring at age 65. Those with a more proactive approach and better access to healthcare will skew these statistics on the upside which must be factored into your financial planning and longevity calculations.
The report highlighted the large deficit in pension coverage in Ireland compared to other countries as a percentage of GDP.

Auto Enrolment of which we have expressed concerns about before will as predicted at the time of writing miss the self imposed 2024 deadline. This will help with the deficit when introduced but needs change before it even starts. I don’t think as the owners of small businesses reading this there will be any disappointment that the administrative and extra financial burden this will eventually bring is pushed own the line.
Of course housing came up in the report as an issue and the estimates are that 700,000 new households will be formed between now and 2040 highlighting the task ahead in providing accommodation for those seeking it. One figure in particular jumps out and that is the difference in lifetime wealth between those who can afford to get on the property ladder and those who can’t.
When you factor in a lifetime of paying rent and not having an asset growing in your name the difference is estimated at a staggering ¤1.4m. This will lead to
questions about inequality and a lack of fairness in the situation now that we can quantify the impact. I’m often asked what is the best saving or investment you can make for children to provide for their future. After a good education, the figures show a strong case for making sure they can afford a home. This could be in the form of a loan from the bank of mum and dad or the gift of a cash sum to enable them to afford the deposit which can be an obstacle to those spending a vast portion of nett earnings on ever-increasing rents if they can find accommodation in the first place.
This assistance to your child has the potential to become intergenerational with the hope they could then do the same for their children. How you accumulate these funds needs advice as they could come from accumulated savings, business exit, a targeted savings contract or tax-free lump sums from your pension. Again the services of an adviser who can quantify that you have what you need to enjoy your retirement before you consider this is vital and the decision must be made in the context of your circumstances.
Auto Enrolment of which we have expressed concerns about before will as predicted at the time of writing miss the self imposed 2024 deadline. This will help with the deficit when introduced but needs change before it even starts. I don’t think as the owners of small businesses reading this there will be any disappointment that the administrative and extra financial burden this will eventually bring is pushed own the line.
Of course housing came up in the report as an issue and the estimates are that 700,000 new households will be formed between now and 2040 highlighting the task ahead in providing accommodation for those seeking it. One figure in particular jumps out and that is the difference in lifetime wealth between those who can afford to get on the property ladder and those who can't.
When you factor in a lifetime of paying rent and not having an asset growing in your name the difference is estimated at a staggering €1.4m. This will lead to questions about inequality and a lack of fairness in the situation now that we can quantify the impact. I’m often asked what is the best saving or investment you can make for children to provide for their future. After a good education, the figures show a strong case for making sure they can afford a home. This could be in the form of a loan from the bank of mum and dad or the gift of a cash sum to enable them to afford the deposit which can be an obstacle to those spending a vast portion of nett earnings on ever-increasing rents if they can find accommodation in the first place.
This assistance to your child has the potential to become intergenerational with the hope they could then do the same for their children. How you accumulate these funds needs advice as they could come from accumulated savings, business exit, a targeted savings contract or tax-free lump sums from your pension. Again the services of an adviser who can quantify that you have what you need to enjoy your retirement before you consider this is vital and the decision must be made in the context of your circumstances.
Colm Moore is a CERTIFIED FINANCIAL PLANNER™ with Moore Wealth Management. They have been advising the pharmacy community for over 20 years. He can be contacted on 086-8603953 or at colm@mwm.ie
PHARMACYNEWSIRELAND.COM 89
Country % of GDP 2020 Denmark 229% Netherlands 213% Iceland 207% Canada 180% United States 170% Switzerland 167% Australia 132% UK 127% Sweden 109% Total OECD 100% Ireland 36%
The Irish Ocular Oncology Service
In 2010 a dedicated ocular oncology service was established in Ireland at The Royal Victoria Eye and Ear Hospital (RVEEH) in conjunction with St Luke’s hospital (SLH) in Rathgar. Prior to this all patients were referred to the United Kingdom for management of Uveal Melanoma (UM).
Written by Valerie O’Neill, Ocular Oncology Clinical Nurse Specialist, Royal Victoria

Plaque Brachytherapy
This treatment requires hospital admission for up to a week and includes two surgical procedures with general anaesthesia, insertion, and subsequent removal of the plaque once the radiation dose has been delivered.
There Is no known cause of UM. However, it is more common in those who are fair skinned and have light eye colour.
Treatment Pathways
Nationwide referrals are received by this dedicated oncology clinic, patients are referred with a suspicious naevus or ocular lesion for specialist investigation either via optician’s, another ophthalmologist, or through our own casualty department. A small minority come from the diabetic screening national service.
This is a globe saving treatment option. Plaque Brachytherapy is used for uveal melanomas up to 10mm in thickness. In general ruthenium plaques Ru-106 are used to treat tumours up to 5mm in thickness and iodine plaques I-125 are used to treat tumours between 5-10mm. Plaque brachytherapy is described as a high intensity localised tumour control.
Proton Beam radiation
clinic run by the CNS for all subsequent appointments.
Systemic Surveillance
Despite the availability of multiple treatment options, the survival for uveal melanoma patient has not changed for decades.
Approximately 50% of uveal melanoma patients will develop a metastatic disease within a 5–10year period. All treated patients within the service are referred to a medical oncologist locally for systemic surveillance. The liver is the most common site in the body affected by metastasis of an ocular melanoma and is often associated with poor prognosis.
Support
The multidisciplinary team, delivering this service, is composed of an ocular oncology consultant, a radiation oncologist, a medical ophthalmologist, two residents, Physicists, we work closely with the pathologist and local medical oncologists, a Clinical Nurse Specialist (CNS), a Medical Social Worker (MSW), Eye Clinic Liaison Officer (ECLO) and our administration support.
Uveal Melanoma
Uveal melanoma is the most common primary intraocular malignancy in adults, albeit still a rare form of cancer. Melanoma arises from the pigmented cells (melanocytes) of the uvea. The uvea is divided into three parts termed the iris, ciliary body, and choroid. The uvea is comprised of blood vessels and melanocytes. A malignant melanoma of the uvea originates from these melanocytes, in the choroid, ciliary body or iris.
Uveal melanoma may not cause any signs or symptoms and is commonly found incidentally, detected during routine examination. Symptoms may include decreased or blurry vision.
A diagnosis of uveal melanoma is made based on clinical findings from a dilated fundus examination and multimodal imaging, which consists of colour fundus photography optical coherence topography and B-Scan ultrasound. Treatment pathway decisions are based on tumour thickness and tumour location. These pathways include Brachytherapy for tumours that are less than 10mm, Proton beam radiation for tumours that are small and are close to the optic nerve, Enucleation removal of the globe and its contents for larger tumours, or specific laser treatment for smaller tumours known as transpupillary laser therapy (TTT) or Photodynamic therapy (PDT).
Treatment of uveal melanoma has evolved over the last four decades with eye conserving treatment options such as proton beam radiation and brachytherapy. Despite the therapeutic shift enucleation is still undertaken for larger tumours not amenable to radiation treatment.
The most commonly used care pathways are plaque brachytherapy, which is conducted in SLH, Enucleation which is performed in RVEEH and Proton beam radiation.
This treatment pathway is used for Uveal Melanomas located in the peripapillary or juxtapupillary region, these melanomas are close to the optic nerve. These patients are referred to St Pauls Eye hospital Liverpool and the Clatterbridge cancer care centre. This treatment is used to treat smaller tumours and is highly effective in achieving local control. All proton beam patients are treated in the UK, but all follow up care is completed in RVEEH.
Enucleation
This care pathway treats larger uveal melanomas measuring greater than 10mm in thickness. These larger tumours are not amenable to radiation.
Enucleation is removal of the globe and its contents and insertion of a volume replacing implant, leaving behind the muscles of the eye which support movement of the prosthesis once fitted. This procedure is performed in RVEEH. The patient stays overnight in RVEEH and is discharged with a pressure dressing in place for one week. They return to a nurse led clinic for removal of this dressing one week after surgery.
The patient is seen five weeks later to ensure the sutures are dissolved and they are ok to proceed with the prosthesis fitting. Development of the ocular oncology service over time has given us the scope to set up nurse led clinics which are specifically for the post-operative enucleation patient where these patients are seen in a dedicated
Patient support in the service includes a CNS who can be contacted directly, excellent support from our medical social work department who links with local cancer support services or psycho-oncology supports if needed. We also have direct links with the Irish cancer society and have run the cancer thrive and survive programme (CTS) on site with a view to running this again in the coming year.
The CTS programme focuses on problems common to cancer survivors and teaches coping strategies through action planning, feedback, behaviour modelling, problem solving and decisionmaking techniques. Family members, friends and caregivers are also welcomed to participate. This programme provides information and teaches practical skills on how to manage health care issues. CTS gives people the confidence and motivation they need to manage the challenges of living with a cancer diagnosis and meet people socially with similar needs and experiences.
Uveal melanoma and its treatment can influence the physical and psychological well-being of patients in a way that differs from other cancers. Factors to consider are visual impairment changes in appearance day to day function ocular discomfort and worry regarding disease recurrence. We are committed to excellence in care and to support the UM patient along the care pathway.
PHARMACYNEWSIRELAND.COM 90 Ocular Oncology
Eye and Ear Hospital
JPA Brenson Lawlor Appoints Three New Partners

JPA Brenson Lawlor is the recognised leader in advising community pharmacies in Ireland Growing our professional team ensures we remain your first choice for advising on getting the most out of your pharmacy business. We are delighted to announce a further expansion of the firm with the appointment of three new Partners

Ciara Ferguson becomes Partner, Audit and Business Advisory With a Masters in Accounting from UCD Smurfit, Ciara is also ACA qualified. Ciara completed her training with PwC and has worked with JPA Brenson Lawlor for the past seven years.
John Manning becomes Partner, Audit and Business Advisory. John graduated out of Technological University of Dublin and followed that with an ACA training contract with JPA Brenson Lawlor John joined the firm ten years ago
Deborah Drought becomes Partner, Taxation Services A graduate of South East Technological University Deborah joined JPA Brenson Lawlor ten years ago having started her career working with a number of firms in Edinburgh, Scotland.
Our three newly elected Partners have a wealth of experience working with owner managed businesses specifically in the community pharmacy sector. If you have any business related queries please do not hesitate to contact us.
info@jpabrensonlawlor.ie 01 688 9760 www.jpabrensonlawlor.ie jpabrensonlawlor
Partner group from left to right: Jason Bradshaw, Deborah Drought, Padraic Ferguson, John Manning, Henry Kinch, Ian Lawlor (Managing Partner), Ciara Ferguson, Thomas McDonald, Michael O'Leary
Iron deficiency – who to watch out for and how to help
Iron deficiency is the most common micronutrient deficiency in the world. In contrast to most other nutrient deficiencies, iron deficiency is widespread in both developing countries and in more developed nations such as Ireland. Although widespread from a geographical standpoint, the risk of deficiency is greatest amongst only part of the population in any country, namely women and young children.
include tiredness, weakness, dizziness, gastrointestinal (GI) upset, reduced immune function, a lack of energy and difficulties with concentration.
Most recent estimates suggest that iron deficiency anaemia affects over 1.2 billion people worldwide. It is considered one of the leading contributors to the global burden of disease, but particularly amongst premenopausal women and young children.
important risk factor to consider in this cohort.
Due to the critical role of iron in almost all bodily functions, requirements for iron increase dramatically during periods of rapid growth and development. These key periods of life include pregnancy, lactation, infancy and early childhood.
Perhaps more troublesome again is that European data suggests that almost half of women are iron depleted prior to ever becoming pregnant. The stark reality is that, physiologically, many women are on the back foot in terms of iron levels long before they ever become mothers.
Written
Anaemia is even more common, estimated to affect one third of the global population but iron deficiency is only one such cause of anaemia. This is important to remember, as there are many other nutritional and non-nutritional causes of anaemia so appropriate testing and diagnosis are essential.
Risk factors for iron deficiency

There are three underlying causes of iron deficiency and IDA: an inadequate iron uptake, elevated blood losses and/or increased iron requirements.
Iron is essential for the transport of oxygen (and carbon dioxide) around the body due to the critical role it plays in haemoglobin and red blood cell development. Aside from this key function, iron is critical to most cells and organ systems as it is an essential component of many proteins and enzymes, especially those involved in energy metabolism.
Iron deficiency should be considered along a continuum. The earliest stage is classified as iron depletion, due to a reduction in iron stores in the body, that are mostly in the form of the storage protein ferritin. This depletion can progress into iron deficiency, where reductions in iron transport proteins such as transferrin are also seen. Prolonged depletion and deficiency will lead to iron deficiency anaemia (IDA), characterised by a reduction in the functional capacity of the body, presenting as reduced haemoglobin concentrations.
Symptoms of iron depletion or deficiency are not always apparent as the body uses its iron stores to protect erythropoiesis and haemoglobin production. However, symptoms of a more prolonged deficiency, resulting in IDA can
Issues with iron uptake can be due to a diet lacking in ironrich foods and/or an inability to absorb dietary iron, as is the case in conditions characterised by malabsorption. Inadequate dietary iron intakes have been frequently reported in the Irish population but most commonly amongst toddlers, school-age children and adult women. Individuals following a vegetarian or vegan diet can be at particular risk of poor dietary iron intakes, especially of the highly bioavailable haem iron.
Increased blood losses occur most frequently through menstruation in women of reproductive age. Iron losses during menstruation vary significantly amongst women but a woman typically loses at least 1 mg of iron per cycle or roughly 0.3-0.6 mg of iron per day. Women with heavy or prolonged periods can lose 5-6 times this amount of iron, further increasing their risk of deficiency.
Chronic blood losses from the digestive tract can also put an individual at risk of iron deficiency. While iron deficiency and IDA are considered uncommon amongst men, GI bleeding caused by inflammatory bowel disease, ulcers or certain cancers is an
Iron requirements increase almost 10-fold during pregnancy, with the greatest demand seen during the third trimester to support the needs of both the mother and her growing child. While the body can make remarkable adaptations during pregnancy to increase the rates of iron absorption from the diet, meeting these high requirements remain a challenge for many pregnant women.
Rates of iron deficiency in pregnant women across Europe range from 28% to as high as 85% in some parts. Poor dietary iron intakes and compliance with dietary guidelines are widely reported in this population.
In addition to the unwanted symptoms of tiredness and weakness, IDA during pregnancy can result in adverse pregnancy outcomes including preterm birth and a low-birth-weight baby. Further to this, it also increases the chances that the baby itself will be born iron deficient.
This is important as iron plays a fundamental role in the growth and development of a child’s brain, most of which occurs in the first 1,000 days i.e. during the pregnancy and first 2 years of a child’s life. Iron deficiency during this critical period of development can result in poorer intelligence, low cognition, poor motor skills and behavioural problems that are often long-lasting.
Table 1. How much iron should people consume - Recommended Dietary Allowances from the Food Safety Authority of Ireland (1999)
Adult, 18-64 years (males)
Adult, 18-64 years (females) 14 mg
Adult, 65+ years (males) 10 mg
Adult, 65+ years (females) 9 mg Pregnancy and Lactation
mg
PHARMACYNEWSIRELAND.COM 92 Iron Deficiency
Population Group Daily Iron Requirement Infants, 0-3 months 1.7 mg Infants, 4-6 months 4.3 mg Infants, 7-11 months 7.8 mg Children, 1-3 years 8 mg Children, 4-6 years 9 mg Children, 7-10 years 10 mg
11-14 years (males) 13 mg
14 mg
by Dr Elaine McCarthyLecturer in Nutrition at the School of Food and Nutritional Sciences in University College Cork (UCC) and Lead Investigator at UCC’s internationally renowned INFANT Research Centre
Children,
Children, 11-14 years (females)
Children, 15-17 years (males) 14 mg Children, 15-17 years (females) 14 mg
10 mg
15
taking care of you naturally

Throat Spray creates a resistant film with a barrier effect on the irritated mucosa, ensuring longlasting hydration and relief.
ForTuss Cough

Syrup with pure Manuka honey to help combat dry and productive coughs

Throat Gel
Enriched with honey - a special gel formula useful for throat

irritation, burning or painful swallowing

Distributed in Ireland by:
A functional iron deficiency?
Perhaps less considered or acknowledged is something termed a functional iron deficiency. A functional deficiency can occur despite adequate dietary iron intakes or large stores of iron in the body. However, the use and/ or uptake of this iron is impeded, typically through the action of the iron regulatory hormone, hepcidin. During times of inflammation, hepcidin concentrations are elevated which prevents the body from absorbing iron. While inflammation is commonplace in certain disease states, this phenomenon has also been reported in more low-grade, chronic inflammation such as that seen during obesity. This effect is seen in the pregnant population too, with maternal obesity both prior to and during pregnancy
associated with an increased risk of iron deficiency in the mother and her newborn baby.
This type of functional deficiency or inflammatory induced deficiency has most recently been acknowledged in athletes and physically active individuals. This inflammatory effect, coupled with increased iron losses particularly via higher rates of sweating and GI bleeding, puts athletes and other physically active individuals at risk for iron deficiency.
Strategies to combat the problem
When it comes to iron, the saying “too much of a good thing” rings true. Excess iron is toxic and extremely harmful to the body; therefore, appropriate testing and diagnosis are critical first steps. Once a diagnosis of iron deficiency or IDA is confirmed, the
aim of treatment is to replenish iron stores and if anaemia is present, return haemoglobin concentrations to normal. The first line of treatment involves oral iron supplements, typically in the form of ferrous sulfate, gluconate or fumarate. Multivitamin-mineral preparations usually contain too low a dose of iron to correct a deficiency and may contain other minerals, such as zinc, that can inhibit iron absorption. Iron supplements are available in tablet or liquid form, with controlled or slow-release preparations possible.
Previous treatment guidelines proposed a daily dose of 100200 mg elemental iron divided into multiple doses across the day. Following advancements, particularly in our understanding of hepcidin and its control over
iron absorption, many recent guidelines recommend lower doses (30-60 mg) once daily or even intermittently, every second day. Intermittent supplementation has been shown to be effective in improving iron status but with fewer side effects. Improvements in haemoglobin concentrations should be seen within weeks of effective iron treatment, but it may take 3-6 months of treatment for a complete replenishment of iron stores.
While this form of treatment is effective, compliance with such supplementation strategies is extremely poor in the population.
GI side-effects are widely reported, in up to 75% of users in some studies, and include diarrhoea, constipation, abdominal pain, nausea, flatulence and black stools. A deleterious effect of oral iron supplementation on the gut microbiota has also been proposed. Recent advances in the incorporation of ferrous iron into whey protein microspheres has shown promise for protection against these side effects.

Alongside any supplementation strategy, the importance of an individual’s daily diet should not be ignored. The absorption of iron is highly influenced by a series of promotors and inhibitors in the diet, so the following advice should be remembered:
• Oral iron supplements are most effective when taken on an empty stomach or at least an hour before meals, and preferably in the morning.
• Iron supplements shouldn’t be taken with milk, antacids or calcium supplements.
• Iron absorption is strongly inhibited by the phytic acid found in whole grains and pulses and the polyphenols found in coffee, teas and chocolate. So avoid that cuppa while taking your supplement!
• Vitamin C or ascorbic acid is a very potent enhancer of iron absorption. If people find it difficult to take supplements on an empty stomach and need to take it with a small meal, including a good source of vitamin C with the meal can help to overcome the inhibitory effect of the meal.
• Don’t forget that many foods are good natural sources of iron or have been fortified with iron, including lean meat and poultry, beans and pulses, nuts and dried fruit, fortified breads and cereals.
PHARMACYNEWSIRELAND.COM 94
Iron Deficiency
Mental Health Commission’s CAMHS review reveals risks to children’s safety
Mental Health Reform is reacting with concern to the Mental Health Commission’s Independent Review of the provision of Child and Adolescent Mental Health Services (CAMHS) in the State.
The report reviews CAMHS in five of the HSE’s nine community healthcare organisations covering Kerry, Cork, Clare, Tipperary, Limerick, Dublin, Wicklow, Carlow, Kilkenny, Waterford and Kildare.
Róisín Clarke, Interim CEO, Mental Health Reform said: “This report is a damning indictment of the deepening crisis in our mental health services. Persistent failures of clinical oversight are putting children’s safety at risk. Every child who uses mental health services has the right to appropriate care and support. Due to unacceptable waiting lists and a substandard level of care, many children are being denied this right.
The Maskey report revealed that a lack of clinical governance in South Kerry CAMHS exposed children to the risk of harm. It is now clear that poor clinical governance is a national issue across the mental health system. The failure to manage risk, recruit
key staff and provide standardised care is resulting in dysfunctional and unsafe mental health services for children and young people.
It is deeply alarming that some CAMHS teams are neglecting to monitor children using antipsychotic medication. Also of serious concern is the lack of follow up care for children. This has left some families without a review appointment, a prescription renewal or advice about their child’s care while on medication. These practices could have grave repercussions for a child’s physical and mental health.






The crisis in CAMHS requires a national coordinated response. The Government must prioritise the recruitment of a Youth Mental Health in the HSE as promised in Budget 2023. This post is essential to ensuring leadership in the improvement of mental health services for children and young people.
There is a fundamental need for greater accountability and oversight across the health service. We are calling for the reinstatement of a National Director for Mental Health in the HSE who would report directly to the CEO of the HSE. There has been no National Director for Mental Health since 2016, despite the current Programme for Government containing a commitment to reinstate the position.

This review highlights once more the need to reform the Mental Health Act, 2001 to ensure that children’s rights are adequately protected when they access mental health services. Due to gaps in the legislation, the existing Act is significantly out of line with international human rights standards. The Mental Health (Amendment) Bill must be progressed this year as a matter of urgency.
Children should be able to rely on our mental health services in their hour of need. We must do all we can to restore trust in the mental health system and ensure that families and children receive the high standard of care they deserve.”
PHARMACYNEWSIRELAND.COM 95 News
Róisín Clarke, Interim CEO, Mental Health Reform
ALZECURE SELECTS CD AND
ENTERS
NEXT DEVELOPMENT PHASE WITH ALZSTATIN ACD680 AGAINST ALZHEIMER’S
AlzeCure Pharma AB (publ) (FN STO: ALZCUR), a pharmaceutical company that develops a broad portfolio of small molecule drug candidates for diseases affecting the central nervous system, with projects in both Alzheimer’s disease and pain, has announced that the company has chosen a candidate drug (CD) and started the preclinical development phase with the company’s preventive and disease-modifying candidate drug Alzstatin® ACD680.
ACD680 is being developed within AlzeCure’s Alzstatin platform, with the aim of developing a preventive and disease-modifying drug for the early treatment of Alzheimer’s disease. In the project, a CD has now been selected which will continue into the preclinical development program, which includes preclinical safety and tolerability studies, as well as formulation work and stability testing.
In Alzheimer’s disease, a protein, amyloid beta (Aβ42), accumulates into larger aggregates, such as plaques, which have a harmful effect on nerve cells and their function. ACD680 is a so-called gamma-secretase modulator (GSM), which constitutes a promising class of small-molecule
Aβ42-lowering anti-amyloidogenic substances for preventive and disease-modifying treatment of Alzheimer’s disease. The GSM thereby affects the production of the very building block of the harmful amyloid aggregates and exhibits several key properties that distinguish it from antibody treatments, including that it can be taken in tablet form, easily crosses the blood-brain barrier and can be produced more cost-effectively.
”With Alzstatin, we want to offer a preventive and disease-modifying treatment against Alzheimer’s in the form of an oral therapy, which is non-invasive for patients. In addition to affecting an important disease mechanism, ACD680 also derives from a new series of molecules that, among other things, are expected to provide benefits from a patent perspective, with a significantly longer patent period,” said Gunnar Nordvall, project leader and Director of Medicinal Chemistry at AlzeCure.
”We are very pleased to have begun preclinical development with ACD680. We hereby build further on the communicated strategy to strengthen the project portfolio with the development
of several candidates in parallel and also demonstrate AlzeCure’s capacity in terms of development and delivery. With the increased interest in the Alzheimer field, we see exciting commercial opportunities for Alzstatin going forward,” said AlzeCure’s CEO Martin Jönsson.
NEW MUMS VIRTUAL WELLBEING HUB INTRODUCED AT UNIVERSITY HOSPITAL GALWAY
The Maternity Department at University Hospital Galway (UHG) has introduced a ‘New Mums Virtual Wellbeing Hub’ which provides online support, advice and signposting on postnatal services available to mothers and their families both in the hospital and the community.

The hub is a collaborative initiative between the Maternity Department, UHG and Community Healthcare West. The team includes a Midwife, Social Worker, Paediatrician, GP, Pharmacist, Public Health Nurse, Dietician, Physiotherapist, Lactation Consultant, Urogynaecology Specialist Midwife and a member of the Perinatal Mental Health Team.
Carmel Connolly, Clinical Nurse Manger 2 and Project Lead
said, “The lack of support and connection, change in identity and unrealistic expectations of parenthood due to the influence of social media has affected maternal wellbeing. Feeling overwhelmed, exhausted and lonely is not uncommon.
“Listening to our mothers concerns led to the design of this new service. Mothers need to connect with other mothers. There is a real fatigue and loneliness after giving birth for many women. They need consistent, evidenced-based information and signposting to services available to them in Galway.
“We launched the hub in November and had 19 couples in attendance. It is so reassuring to see the difference the hub has already created in the lives of mothers and their families. There has already been increased referrals to Physiotherapy, Lactation Consultant and Urogynaecology Specialists. Mothers have connected with these services as a direct result of attending the wellbeing hub.
“The virtual hub is live on the third Tuesday of every month at 11am and booking is available via www.ughmaternity.com.”
One mother who attended the hub said, “It was lovely to be able to connect with other mothers. I was having breastfeeding issues and I knew who to contact after the hub. It is reassuring to see the healthcare team from the hospital and the community working together. It is a fantastic service.”
Another mother said, “I wish this service was available after my first baby. I did not know what was normal. I was so down in myself and was embarrassed to look for help. I picked up lots of skills from the Clinical Psychologist. I am very grateful for the support of this hub.”
KINARUS THERAPEUTICS REPORTS PRECLINICAL DATA IN LUNG FIBROSIS AND DISCLOSES CLINICAL DEVELOPMENT PLAN
Kinarus Therapeutics AG (SIX:KNRS) “Kinarus”, a Swiss clinical-stage biopharmaceutical company has announced preclinical data supporting the potential effectiveness of its lead clinical candidate, KIN001, as an oral treatment for idiopathic pulmonary fibrosis (IPF). Kinarus has developed a protocol for a Phase 2 clinical trial in patients with IPF in consultation with key
From left, Ailish Killilea, Assistant Director of Public Health Nursing, Community Healthcare West; Helen Murphy, Director of Midwifery, University Hospital Galway; Carmel Connolly Project Lead and Clinical Midwife Manager 2, University Hospital Galway; June Barrett Dietitian, University Hospital Galway; Peter Kidd, Pharmacist, University Hospital Galway; Anne Marie Grealish Assistant Director of Midwifery, University Hospital Galway; Eithne Gilligan, Clinical Nurse Manager 2, Community Midwives, University Hospital Galway; Marie Conway, Masters Student, Social Work Department, University Hospital Galway and Mary Moran, Midwife, Parent Education Services, University Hospital Galway
PHARMACYNEWSIRELAND.COM 96
Clinical Profiles
experts, including PD Dr. Katrin Hostettler, Senior Physician in Pulmonology, at the University Hospital of Basel.
In a mouse model of lung injury, KIN001 significantly reduced lung weights and tissue fibrosis score vs. controls. KIN001 was more effective in direct comparison to pirfenidone, a marketed IPF therapy. The combination of KIN001 with pirfenidone demonstrated greater reduction in lung fibrosis, indicating the potential for additional benefit of KIN001 in combination with the current standard of care. In order to understand the mechanisms of action of KIN001, global gene expression changes in lung tissue were measured by the RNAseq method. KIN001 significantly reduced the upregulation of multiple key inflammatory cytokines and chemokines implicated in the pathology of lung fibrosis.
“These preclinical data strongly support the potential of KIN001 to be an effective treatment for IPF and fibrotic disorders of the lung and other organs, both in single or combination therapy.” said Thierry Fumeaux, Chief Medical Officer of Kinarus. “Many patients stop therapy with the currently available drugs, due to side effects and lack of efficacy. Our data demonstrate that KIN001 possesses broad anti-inflammatory and anti-fibrotic properties which increase the probability that a patient may respond to KIN001. Together with our superior safety profile, demonstrated in clinical testing to date, this indicates that KIN001 may be more effective and better tolerated than available drugs. These data also support the potential of KIN001 to reduce severity and long-term organ damage in COVID-19.”
The Phase 2 study in IPF will be a 52-week, double-blind, randomized, placebo-controlled trial evaluating the effect of oral KIN001 on Forced Vital Capacity (FVC) in 80 patients with IPF. This study will enroll patients on current standard of care including Ofev and Esbriet. Patients not currently treated with either drug will also be included since a substantial number of patients end treatment with these two drugs due to safety concerns.
Kinarus is currently conducting a Phase 2 trial of KIN001 to treat ambulatory COVID-19 patients (“KINFAST”) with a positive SARS-CoV-2 test, which is actively recruiting in Switzerland and Germany. The primary endpoint of the study is the reduction in the severity and duration of Covid-19 symptoms.
LONG COVID PATIENT EDUCATION PROGRAMME IN GALWAY UNIVERSITY HOSPITALS WINS NATIONAL HEALTHCARE AWARD
A group patient support programme developed by team members from the Long COVID and post COVID clinics in Galway University Hospitals has won the Irish Medical Times Irish Healthcare Award in the patient education category.

Long COVID is estimated to occur in 10-20% of people infected with the SARS-CoV-2 virus and people can experience a wide range of symptoms including fatigue; brain fog, non-restorative sleep, pain and breathlessness. These symptoms often impact on a person’s quality of life and daily functions with work, education, parenting and self-care all being affected. The patient education programme in GUH is in place since July 2021 and aims to enable patients to manage their personal symptom profile in a supportive and structured environment. The programme uses a Multi-Disciplinary approach to help patients learn more about the condition and manage their symptoms.
The programme covers a range of areas including fatigue management, brain fog, sleep restructuring, post-exertional malaise, pacing, breathing pattern changes and relaxation. 70% of the group participants identified work as a key area of concern,
with household tasks, parenting, self-care and leisure activities also being common problems among many. A problem solving approach is taken, enabling each person to apply the knowledge in a way that is unique to their symptom profile and personal circumstances.
This programme is delivered online, to make it accessible to patients across the West of Ireland, by specially trained occupational therapists and physiotherapists. The programme runs every week for one hour in duration for a total of four weeks. Referrals to the program are accepted via the long COVID clinic
Speaking of the achievement Ciara Breen, Occupational Therapy Manager said; “After an evaluation of the first groups was completed the results showcased significant improvements in the areas targeted in the programme. These included improved fatigue, cognition, function and mood. Patient feedback has been very positive to date, and the group approach also created a firm foundation for the therapeutic relationship to flourish for those with additional one-toone goals afterwards.
“Given the increasing evidence that recovery after COVID-19 infection is prolonged for a significant proportion of people, and has a complicated and overlapping symptom profile, this group programme highlights the value of supported self-management programs for individuals with long COVID and the feasibility of delivery of this education in an online group format.
“This programme would not be possible without the support of our Infectious Diseases and Respiratory consultants, and the
dedication of our MDT in GUH. It is our aim to continue to strive towards improving care and services for patients.”
Chris Kane, General Manager of Galway University Hospitals added, “This award is a recognition of the adaptability of our post COVID and long COVID clinical teams, being able to respond so innovatively to the varied needs of this patient group is a huge achievement. I want to congratulate the team and thank them for continuing to put patient care at the centre of our services.”
FDA ACCEPTS NIRSEVIMAB APPLICATION AS FIRST PROTECTIVE OPTION AGAINST RSV DISEASE FOR ALL INFANTS
The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) has accepted the Biologics License Application (BLA) for nirsevimab for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants entering or during their first RSV season and for children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Nirsevimab is being developed jointly by Sanofi and AstraZeneca and, if approved, would be the first protective option for the broad infant population, including those born healthy, at term or preterm, or with specific health conditions. The FDA has indicated they will work to expedite their review. The Prescription Drug User Fee Act date, the FDA target action date for their decision, is in the third quarter of 2023.
Thomas Triomphe, Executive Vice President, Vaccines, Sanofi said, “This is a landmark file acceptance in the US as it brings us one step closer to offering the first and only broadly protective option against RSV disease designed for all infants. Given the unprecedented number of otherwise healthy infants who have been hospitalized with RSV this year in the US and the recurrent pattern of RSV epidemics year after year, it is our intention to make nirsevimab available, if approved in time, for the 2023/2024 season to help alleviate the burden of RSV on families and the healthcare system.”
RSV is a very contagious virus that can lead to serious respiratory illness, according to the Centers for Disease Control and Prevention (CDC).10 In the US, RSV is the leading cause of hospitalisation for babies under one.11 Any infant can be
PHARMACYNEWSIRELAND.COM 97
Accepting the Irish Healthcare Award on behalf of the GUH Long COVID service patient education team: Catherine O Sullivan, Physiotherapy Manager; Gillian Collins, Clinical Specialist Occupational Therapist and Ciara Breen, Occupational Therapy Manager
hospitalized in their first RSV season: about 75% of infants hospitalized for RSV in the U.S. are born at term, with no underlying conditions.12-14 The current 2022/23 RSV season has placed a particularly high burden on infants and families in the United Stated with the American Academy of Pediatrics (AAP) requesting the White House declare an emergency to support the national response to the alarming surge of pediatric hospitalizations due to RSV and influenza.
Dr William Muller, Associate Professor, Pediatrics, Northwestern University Feinberg School of Medicine and Scientific Director, Clinical and Community Trials, Ann & Robert H. Lurie Children’s Hospital of Chicago, Illinois added, “A substantial burden of disease from RSV affects infants, families, and healthcare providers every year. Effective interventions to prevent RSV are a critical need. This year in the US, we’ve seen first-hand how frightening the impact of this respiratory disease is on our patients and how stressful it is on the healthcare system, highlighting the urgency of addressing this problem.”
The submission was based on results from the Phase 3 MELODY, Phase 2/3 MEDLEY and Phase
2b trials.1-8 Results across the MELODY and Phase 2b trials showed that nirsevimab demonstrated consistent protection of approximately 80%, against medically attended RSV disease with a single dose.1-5
In these trials, nirsevimab helped protect an all-infant population (including healthy term, late preterm, and preterm infants, as well as infants with specific health conditions) against RSV disease requiring medical care, including physician office, urgent care, emergency room visits and hospitalizations, through the duration of the RSV season.1-8 The safety profile of nirsevimab was similar to placebo. Nirsevimab also demonstrated a comparable safety and tolerability profile to palivizumab in the Phase 2/3 MEDLEY trial.7-9
ZENAS BIOPHARMA ANNOUNCES FIRST PATIENT DOSED IN PHASE 3 CLINICAL STUDY OF OBEXELIMAB FOR THE TREATMENT OF IMMUNOGLOBULIN G4RELATED DISEASE (IGG4-RD)
Zenas BioPharma, a global biopharmaceutical company committed to becoming a leader in the development and commercialization of immune-
based therapies, has announced that the first patient has been dosed in the INDIGO Phase 3 registrational study of obexelimab. The INDIGO study will evaluate the clinical efficacy and safety of obexelimab treatment in the prevention of IgG4-related disease (IgG4-RD) flare. Obexelimab is a high-affinity bifunctional antibody that inhibits B-cell lineages by simultaneously binding to CD19 and FcƳRIIB, thereby downregulating B-cell activity in patients with autoimmune diseases associated with autoantibodies, such as IgG4-RD.
“IgG4-RD is a chronic and serious fibroinflammatory condition that can affect nearly any organ system and can have a profound impact on many patients, leading to severe organ damage or death,” said Hua Mu, M.D., Ph.D., Chief Executive Officer at Zenas. “There are no currently approved treatments for patients living with IgG4-RD. Based upon the promising data from a Phase 2 study of obexelimab in IgG4-RD patients, we are excited to continue to evaluate the potential of obexelimab in the INDIGO study.”
About the INDIGO Study
The INDIGO study is a global multicenter, randomized, doubleblind, placebo-controlled study enrolling up to 200 adults with active IgG4-RD signs/ symptoms (i.e., flare) that require steroid therapy. Patients will be randomized in a ratio of 1:1 to receive either obexelimab or placebo, administered as subcutaneous injections.
The primary endpoint of INDIGO is time to first IgG4-RD flare (defined as the reappearance of previous signs/symptoms or appearance of new signs/symptoms of IgG4-RD) that requires initiation of rescue therapy from randomization to Week 52. Safety will be evaluated throughout the study duration.
More information on the INDIGO study (NCT05662241) is available at clinicaltrials.gov.
OVERCROWDING CRISIS RISKS DELAYED CANCER DETECTION
Efforts to catch cancer cases early may be at risk due to concerns among patients and the public about overcrowding in the healthcare system, the Irish Cancer Society has warned.
The Society’s Director of Advocacy Rachel Morrogh said: “We are concerned that people with cancer symptoms are putting off seeking medical care because they do not think it is serious enough amid the ongoing hospital overcrowding crisis, and the call
for only urgent cases to attend Emergency Departments.
“Cancer detection is time-critical: any delays to a cancer diagnosis being picked up can negatively impact patients. Our concern is that people may be delaying seeking medical advice because of the crisis across the health service, and when this is added to wait-times for diagnostic tests for those who do present with symptoms, there may be delayed diagnoses of cancer.
“We already know that around 1 in 10 cancer cases expected to be picked up in 2020 were not, due to the effects of the pandemic, which has continued to be a factor in the last two years.
“People in treatment may also be worried about contacting or attending hospitals if they are feeling unwell, due to fears of being admitted through Emergency Departments. We are very concerned about the chill effect this could have on them getting in touch with their cancer teams.
“Government needs to do much more to ensure that timely and appropriate care is available to everyone who needs it. Patients still face unacceptably long delays, and our health service is woefully under-resourced to meet the pressures that are already emerging from the growing number of people being diagnosed with cancer.
“Lastly, we would urge anyone experiencing symptoms that are unexplained, unusual or persistent, or who is currently undergoing cancer treatment, to please still seek medical advice.
“If that isn’t possible, please contact the Irish Cancer Society to speak to a nurse who can give information, support and advice for free on 1800 200 700.
“Even amid the current difficulties accessing care, nobody should suffer alone at home and we don’t want anyone to be afraid, so please pick up the phone and make contact with us.”
SFI AWARDS ¤1.5M TO RCSI FOR NEW FACILITY TO ADVANCE NEUROLOGICAL RESEARCH
RCSI University of Medicine and Health Sciences has been awarded funding of ¤1.5m for a new facility to support neurological research through the Science Foundation Ireland (SFI) Research Infrastructure Fund.
The RCSI funding was announced by Minister for Further and Higher Education, Research, Innovation and Science, Simon Harris TD, as part of an overall investment of ¤53.3 million for the advancement of high-quality and high-impact research activities in institutions across Ireland.
Professor David Henshall, Professor of Molecular Physiology and Neuroscience at RCSI and Director of the SFI FutureNeuro Research Centre, is leading the new project to establish a Core Medium Throughput Facility at the University. The infrastructure will enable researchers to rapidly screen compounds and gene therapies for effects on the electrical activity of neurons in models of brain disease, establishing advanced drug discovery capabilities.
Commenting on the significance of the facility, Professor Henshall said: “This new facility will provide streamlined screening and data analysis for our scientists, enabling faster research outputs across a number of projects that aim to develop urgently-needed treatments to improve quality of life and outcomes for people living with neurological diseases.
“The investment will help our team to advance scientific research at a faster pace and answer new research questions to identify novel drug targets in epilepsy, autism, multiple sclerosis, pain, neurodegenerative, psychiatric, and motor neuron diseases. It will enhance our attractiveness to industry collaborators and strengthen our ability to win competitive EU and international research funding awards.”
The project is co-funded by RCSI and FutureNeuro with each contributing ¤80,000 towards the facility with technical support to the value of a further ¤35,000 each.
Professor Fergal O’Brien, Deputy Vice-Chancellor for Research and Innovation at RCSI said: “I would like to congratulate Prof. Henshall and everyone involved for securing this important funding through the SFI Research Infrastructure Programme. This project will make more scientific discoveries possible for neurological researchers in RCSI and at our SFI FutureNeuro Research Centre by speeding up drug and biomarker discovery and screening programmes.
“This facility will also have a significant impact on the national research landscape, by providing Irish researchers access to cuttingedge research infrastructure that will enhance high quality research
PHARMACYNEWSIRELAND.COM
98 Clinical Profiles
activities and innovation in areas of strategic importance. I am confident that this enhanced capability will promote and facilitate opportunities for partnerships that will lead to a variety of discoveries and ultimately benefit the health of patients.”
The SFI Research Infrastructure Programme supports the research community in building and sustaining cutting edge infrastructure in order to accomplish high-quality, impactful and innovative research. The programme facilitates broad usage across Ireland and to encourage partnerships and collaboration between different cohorts of researchers in Ireland; for example, between universities, technological universities, institutes of technology, other eligible research bodies, researchers in the Republic of Ireland and Northern Ireland, and between different cohorts of researchers in Ireland.
NEW CROSS-BORDER HEALTH PARTNERSHIP WILL SUPPORT PROCUREMENT OF IRISH MEDTECH SMES IN SECONDARY CARE
Supported by InterTradeIreland’s Synergy initiative, the All-Island MedTech SMEs (AIMS) is being delivered by Health Innovation Hub Ireland (HIHI) and the Health Innovation Research Alliance Northern Ireland (HIRANI). AIMs brings together a wide group including SMEs, academics and healthcare procurement, to capture procurement barriers encountered by MedTech SMEs in the Northern
Irish and Irish public healthcare systems (Health and Social Care (HSC) and Health Service Executive (HSE) respectively). By involving all stakeholders, AIMs will focus on developing a framework to support procurement and improve MedTech adoption in secondary care across both healthcare systems.
HIHI National Director Dr. Tanya Mulcahy said, “We have been working with MedTech SMEs for nearly ten years in HIHI. The size and liquidity of companies means that smaller entities are often precluded from the procurement process. AIMS will look at how we can address barriers such as these so that indigenous MedTech can scale and grow and our health systems can choose from a wider pool of innovation.”
Ultimately, the All-Island MedTech SMEs (AIMS) is bringing together Irish SMEs and procurement to develop and propose a model for innovative procurement for allisland MedTech adoption in Irish health services North and South.
AIMS is funded by InterTradeIreland’s Synergy initiative. A cross-border cluster initiative set up by InterTradeIreland, the crossborder trade and business development body. It aims to scale cross border collaboration among SMEs and other players such as universities, third sector organisations and government agencies using cluster and networking supports.
HIRANI
Programme Manager Dr
Siobhán McGrath said, “We are ambitious with AIMs because we know that there are solutions to ensure that Irish MedTech SMEs can thrive in the indigenous
market. Through creating a network of health stakeholders North and South, capturing common challenges, sharing knowledge in relation to all-island supply chains and procurement channels, we can deliver this.”
START-UP OF THE YEAR AWARD
An early-stage Medtech start-up focused on transforming obesity care has won the 2022 University College Dublin Start-Up of the Year Award.

MetHealth received the award, and its ¤30,000 prize fund, after being declared the overall winner of the 2022 UCD VentureLaunch Accelerator Programme – which aims to equip its participants with the knowledge and skills required to work as part of a team leading a new commercial venture.

The annual Programme, which is run by NovaUCD, aims to support the creation and launch of sustainable and profitable new start-ups emerging from University College Dublin.
As winners of ‘Start-Up of the Year’ MetHealth will receive a prize package worth ¤30,000, which includes a ¤10,000 cash prize sponsored by AMD, a professional service package sponsored by Deloitte and Bryan Maguire Financial Services and incubation space at NovaUCD.
The company is developing a biomarker-based risk-stratification platform and associated digital health solution that can identify patients with complications of obesity, including liver disease.
Members of the MetHealth included Dr Fiona McGillicuddy, who took part in the VentureLaunch programme, and Professor Stephen Pennington, UCD School of Medicine and
UCD Conway Institute; Associate Professor Catherine Mooney, UCD School of Computer Science and UCD Conway Institute, Dr Rachel Byrne, UCD School of Medicine, Dr Anna Antoniadi, UCD School of Computer Science and Aleksandra Dudzik, UCD School of Medicine.
“The global prevalence of obesity is increasing significantly. In the EU alone some 1.2 million people are dying annually due to obesity complications, including from liver disease,” said Dr Fiona McGillicuddy.
“Early identification of those patients with liver inflammation is key but this currently involves painful invasive tests. We are developing a novel biomarkerbased platform technology which can, using a blood sample, stratify patients with obesity who are at high risk of complications of obesity including liver disease.
“This non-invasive test eliminates pain for the patient, is significantly more cost effective than an invasive test, can lead to early interventions for high-risk patients and can aid clinical trial recruitment for new drugs targeting the liver.”
She added: “On behalf of the MetHealth team I am delighted to win the 2022 UCD Start-Up of the Year Award following the completion of the VentureLaunch Accelerator Programme at NovaUCD.
“We currently plan to spin-out of UCD at the end of 2023 when we will be seeking to raise ¤2 million in seed funding to set up the MetHealth laboratory and develop a market ready prototype.”
PHARMACYNEWSIRELAND.COM 99
Professor David Henshall, Professor of Molecular Physiology and Neuroscience at RCSI
Dr Fiona McGillicuddy, MetHealth
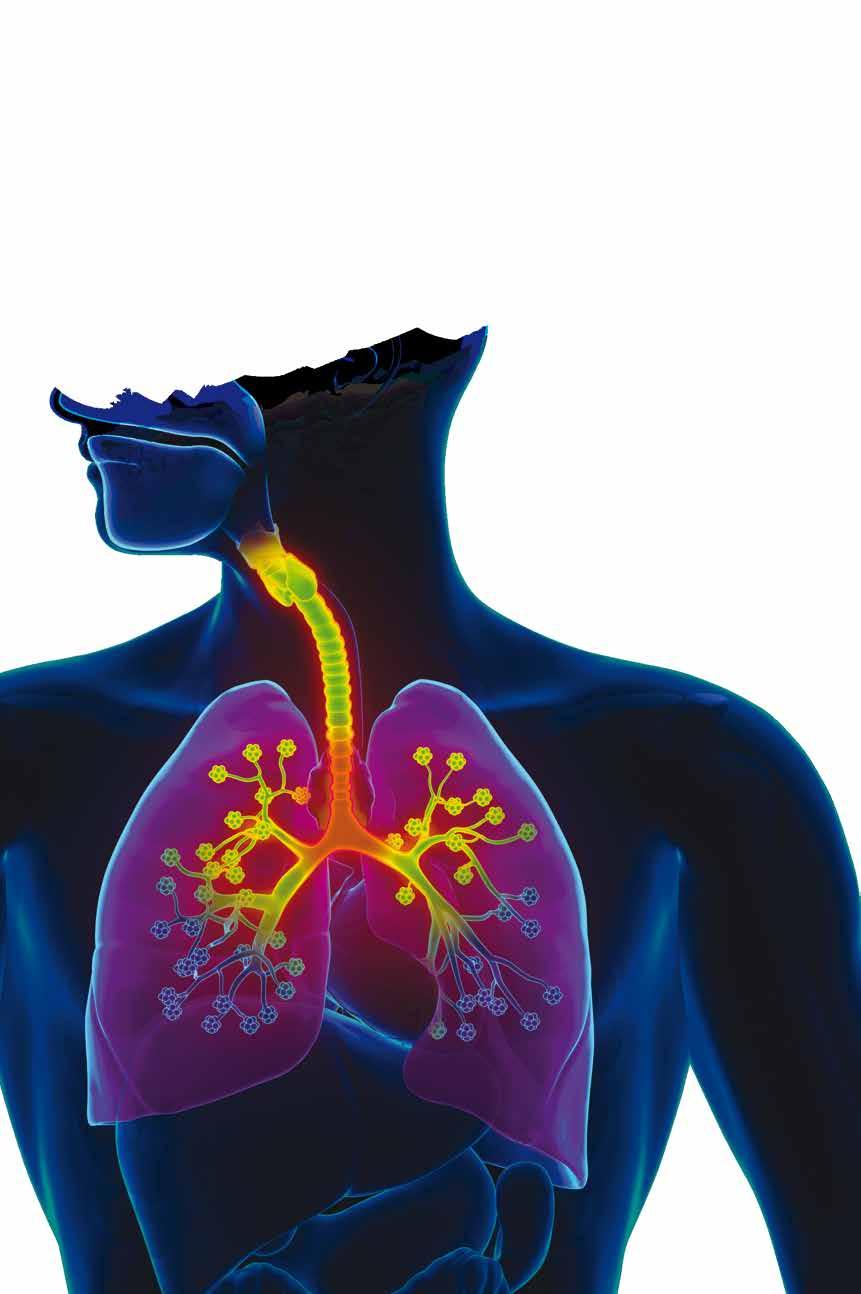

For the treatment of chesty and phlegmy coughs Sugar free Gluten free Suitable for vegans Contains thyme liquid extract
from 12 years and adults: 15ml, 4 times a day Medithyme Cough Syrup contains liquid extract from Thymus vulgaris L., herba (Thyme herb). Contains: ethanol, maltitol (E965) and benzoate salt (E211). For oral short-term use only. Contraindications: Hypersensitivity to thyme or to other members of the Lamiaceae (mint) family, or to any of the excipients. For supply through general sale. A copy of the summary of product characteristics is available upon request. TR 126/319/001. TR Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co. Tipperary. Date prepared: November 2022. 2022/ADV/MED/301H. *Medithyme Cough Syrup is a traditional herbal medicinal product, for use in case of productive cough associated with cold, exclusively based on longstanding use. Traditional herbal medicinal product 180ml pack size
Children



















 ROBBIE HENSHAW
Irish International Rugby Player
ROBBIE HENSHAW
Irish International Rugby Player































































 *Summary of Product Characteristics, Ilvico Cold and Flu film-coated tablets.
P&G Health Germany GmbH, Sulzbacher Strasse 40, 65824, Schwalbach am Taunus, Germany
*Summary of Product Characteristics, Ilvico Cold and Flu film-coated tablets.
P&G Health Germany GmbH, Sulzbacher Strasse 40, 65824, Schwalbach am Taunus, Germany



















 Written by Paddy McGeoghegan, Advocacy & Communications Manager, Epilepsy Ireland
Written by Paddy McGeoghegan, Advocacy & Communications Manager, Epilepsy Ireland


























































































































































































































































































































































































