




As T.S. Eliot said, “For last year’s words belong to last year’s language and next year’s words await another voice. And to make an end is to make a beginning.”
This issue of Irish Pharmacy News features our End of Year Reviews from a number of representative pharmacy bodies, noting the achievements made in 2022 despite challenges and the opportunites that lie before us for 2023.



For the HPRA, this past year has seen significant progress in several key areas of focus as they continued their work in response to the pandemic in combination with ongoing activities to ensure the successful implementation of new regulations and management of medicines shortages (page 16). Meanwhile, thew Pharmaceutical Society of Ireland, on page 18, highlights the challenges and opportunities of regulating during a time of increased demand for primary care and service. “As expected, it has been a busy year. Our role in the public interest is expansive and the Council has set several areas for strategic development and improvement. We are entering the final year of our Corporate Strategy 2021- 2023 and we strive to meet the commitments within it so that the PSI can respond efficiently and effectively within our sphere of regulation,” she says.
Rachel Hamilton, APPEL Operations Manager discusses their successes on page 22. “This year saw more students than ever completing experiential learning placements at different stages of their integrated M. Pharm programme. Over the 12 months, 545 pharmacy students completed placements in a variety of practice settings across the country, as well as a small number of 4th-year students completing our first[1]ever international placements in academic institutions in Spain, Sweden, and Switzerland.”
The Irish Pharmacy Union believes that 2023 will be the European Year of Skills. “This is apt when you consider the most pressing priorities the pharmacy sector will face next year,” says President Dermot Twomey writing on page 26. “The biggest concern is a growing shortage of pharmacists working in the sector. The impact of this shortage is increasingly apparent with research by the IPU showing it is now taking an average of five months to fill a pharmacy position.”
We also feature our annual Dynamic 100 in this issue. Beginning with the eight tremendous finalists for The People’s Pharmacist Award (won by John Tallon as detailed on page 12), we have the Top 20 Industry Innovators and the Pharmacy Retail Giants. It is fair to say the dedication, passion and innovation behind the profession is never lacking.
Finally, may I take this opportunity on behalf of the entire IPN team to wish all our readers and supporters a very Merry Christmas, and a prosperous New Year. See you in 2023!
The Irish Pharmacy Union (IPU) has welcomed the call from the National Women’s Council of Ireland (NWCI) for pharmacists to be able to supply oral contraception without a prescription, which would support women accessing these services.
Maher, Community Pharmacist and Chair of the IPU’s PCC Committeeconsultation without first having to get a prescription from a GP.
Community Pharmacist and Chair of the IPU’s PCC Committee, Kathy Maher, explained the benefits of expanding access to contraception. “With over 1,900 pharmacies nationwide and over 85% of the population living within 5km of their nearest one, pharmacies provide a straightforward and convenient point of access to many treatments and services. Just as women have the choice over what form of contraception they wish to use; they should also be given the choice of where they can access it.
could simply access contraception from their local pharmacy.”
“This is a service that is freely accessible in over 100 countries around the world. This includes in the USA, Canada, New Zealand and our nearest neighbour the UK. The WHO is supportive of pharmacists supplying contraception without a prescription. The medicines used in contraceptive care are well tolerated and can be managed through a structured pharmacist consultation, as demonstrated in these models of care.”
“This is about providing appropriate choice. Research has shown that almost half of Irish women would prefer to access contraception direct from a pharmacy. For those women, this increased accessibility should be an option.”
How the pharmacy profession around the world responded to the COVID-19 pandemic is detailed in a new report published by the International Pharmaceutical Federation (FIP), 1,000 days since the World Health Organisation declared the new coronavirus a Public Health Emergency of International Concern.
“The global response of pharmacy to the pandemic: The contribution of the profession to COVID-19” documents the actions taken by FIP since 30 January 2020 to date, describing how the federation supported international solidarity, advocated the value of pharmacy on pandemic response, gathered evidence of impact, and adapted its ways of working.
The response by community pharmacists across Ireland are included in the report.
According to ‘Every Woman: Towards Reproductive Health, Choice and Care for All’ a new NCWI report launched on 25 October. The NWCI recommended the “Use of our pharmacist network as well as our GPs could help to ensure we reach all women and people who menstruate and enable equal access to reproductive healthcare.” The IPU has consistently called for increasing access to contraception by allowing pharmacists to supply these medicines following a
“The introduction in September of the Free Contraception Service for 17-25 year olds and the planned expansion of a scheme removed the barrier of cost for those requiring contraception and is extremely welcome. However, as the ‘Report of the Working Group on Access to Contraception’ identified back in 2019, access to local services was a significant barrier to women utilising contraception. With GP waiting lists growing this is a needless bottleneck for women when they
“Pharmacists are experienced at providing contraceptive care services”, Ms Maher explained.
Concluding, Kathy Maher said, “Emergency hormonal contraception (the morning-after pill) has been available without prescription in pharmacies since 2011. Enabling pharmacists to supply other forms of contraception without prescription will allow pharmacists to provide follow on reproductive care for these patients.”
People are encouraged to report suspected side effects of medicines as part of the global #MedSafetyWeek social media campaign.
Launched by the Health Products Regulatory Authority (HPRA), the seventh annual #MedSafetyWeek ran until mid November to encourage everyone to report suspected side effects of medicines. This year’s global campaign involves medicines regulators from 82 countries and focuses on the key role of every healthcare professional, patient, and carer who reports a suspected side effect and contributes to using medicines safely.
All medicines may cause side effects in some patients, so there are steps in place to continuously monitor their safety after they are placed on the market. The purpose of safety monitoring is
to gain more information about known side effects and find out about new ones. Regulators operate systems to detect and analyse those side effects and prevent harm to future patients.
The HPRA collects, organises, and investigates reports of suspected side effects. Reports of side effects have helped identify several safety issues which were not previously recognised as being linked to a particular medicine until the HPRA received information from reports.
By reporting suspected side effects to the HPRA, consumers and pharmacists are actively participating in identifying emerging safety issues, so that
the HPRA can take action when necessary and protect patients from harm.
Sinead Curran, Director of Human Products Monitoring at the HPRA, says, “Every report made by a patient, a healthcare professional, or a carer plays a key role in gaining more knowledge about the benefits and risks of medicines in clinical use and allows action to be taken to minimise risks.

“Reporting suspected side effects to the HPRA helps to improve the safety of medicines for all patients and, in some cases, can result in better tailored prescribing advice, which can improve patient outcomes.”
The report also presents the findings of a sample of FIP member organisations on their pandemic activities to February 2022, with information coming from 42 countries and territories.
The responses of these national professional organisations included: developing and updating of clinical guidance, public education programmes, providing education for health professionals, facilitating supplies of medicines and personal protective equipment, supporting diagnostic testing and vaccinations in pharmacies, science and research, and progressing changes in law.
The aim of this compilation is to both learn from each other about how the profession responded to COVID-19 and to inform future pandemic preparedness, response and recovery.
Organisations’ main challenges during the pandemic, how some of these have been overcome, and their leadership strategies are also shared in the new report.
“FIP has been tracking, collating and monitoring pharmacy’s response to the pandemic since the start of the pandemic.
“We offer this report to the entire profession in order to share best practices and lessons from our members worldwide so that we are better prepared for future pandemics and recovery and to build our sustainable approach to future preparedness,’’ said FIP CEO Catherine Duggan.
A major research programme with a focus on improving support for women during and after cancer treatment that is funded by the Irish Cancer Society has been extended to Galway, the charity has announced.
The first programme of its kind in the country, the Irish Cancer Society Women’s Health Initiative supports a range of studies including the LYSA (Linking You to Advice and Support) trial led by cancer researchers in University College Cork.
A team of researchers at the University of Galway and University Hospital Galway led by Consultant Surgeon Prof Aoife Lowery are now bringing the LYSA trial to the west of Ireland, where it will be trialled by selected participants who have undergone cancer treatment at the hospital.
The initiative had up to now only been available through hospitals in Dublin and Cork.
It explores a new model of linking women into symptom management supports for a range of issues that can continue to have severe impacts even after completing cancer treatment such as pain, premature menopause and psychological distress.
The innovative initiative aims to test the effectiveness of special psychological and dietary supports for women to manage their symptoms through both hospital-based clinics and purpose-built online platforms.
Along with providing active support for women’s posttreatment symptoms, the Galway arm of the Women’s Health Initiative will have a novel project whereby participants’ heart health will be monitored and tested for side-effects associated with their treatment.
Irish Cancer Society Acting Head of Research Dr Claire Kilty said, “We are delighted to be able to make innovative cancer research projects available to women across different parts of the country including Galway, so that more women can potentially benefit from the great progress being made in this space.”
The PHOENIX group has completed the acquisition of parts of McKesson Europe, following the clearance by competition authorities of the full scope of the transaction.
The transaction has now completed. In line with the purchase agreement between McKesson and PHOENIX of July 2021, the transaction includes the McKesson companies in Ireland namely the United Drug, LloydsPharmacy, Median Healthcare and TCP Homecare business entities in addition to companies in Belgium, France, Italy, Portugal, and Slovenia, the European headquarters, a shared service centre in Lithuania, German company Recucare GmbH, and the minority stake in the Brocacef Groep joint venture in the Netherlands.
The acquisition sees the PHOENIX group enter new healthcare markets in Ireland, Portugal, Belgium, and Slovenia, while expanding its existing activities in France and Italy.
As a result of this acquisition, the PHOENIX group now has unique geographical coverage throughout Europe, with a presence in 29 countries, 224 wholesale and pre-wholesale distribution centres, more than 45,000 employees, 17,000 partner pharmacies and over 3,200 of its own pharmacies.
This acquisition is the largest in the history of the PHOENIX group and cements its position as Europe’s leading healthcare provider for the sector.
“With this successful transaction, we are completing the largest acquisition in the company’s history. This crucial step also sets the course for the future.” says Sven Seidel, Chief Executive Officer of the PHOENIX group, adding: “Having a broader geographical and operational
base brings us even closer to our customers and patients.”
Paul Reilly, Chief Executive Officer of McKesson Ireland, stated, “We are excited to join the PHOENIX group, Europe’s leading pharmaceutical wholesaler, pharmacy retailer and healthcare service provider. This announcement will further strengthen our leadership position in Ireland and facilitate greater access to additional products, services, and expertise for the Irish market.
Our number one priority is to continue to serve our customers and patients providing safe, effective, and future-orientated healthcare solutions that make a difference to customers and patients’ lives every day.”
Minister for Health, Stephen Donnelly TD; Chief Medical Officer, Professor Breda Smyth and Chief Nursing Officer, Rachel Kenna recently kicked off flu vaccine season by visiting the Boots Pharmacy store on Lower Baggot Street to receive their annual flu vaccination, highlighting the importance of flu vaccination this year.
This follows on from the launch of a HSE nationwide campaign, led by Minister Donnelly, encouraging all those who are eligible to ensure that vaccinations for both flu and Covid-19 are up to date, so as to be best protected this winter.
The 2022/3 Seasonal Flu Vaccine is now available in approximately 1,300 pharmacies nationwide. With the COVID-19 public health and social distancing restrictions in place over the previous two flu seasons, there was a lower incidence of flu circulating in the community. Pharmacists are advising that this year’s flu season is likely to be harsher and advise early vaccination. Vaccination is free of charge to all those in atrisk groups, medical card holders and children aged between 2-17.
Sharon Foley, Secretary General of the Irish Pharmacy Union said, “Pharmacists have been administering flu vaccines since 2011. The pharmacy profession demonstrated during the COVID-19 pandemic how important a role it can play in national vaccine campaigns
Minister for Health, Stephen Donnelly TD receives his flu vaccine from Susan O’Dwyer, Superintendent Pharmacist, Boots Ireland

by increasing accessibility and vaccination uptake. We were delighted to welcome Minister Donnelly along with the CMO and CNO into a pharmacy today to join the very many Irish citizens who have availed of the convenience
Minister for Health Stephen Donnelly visits Boots Pharmacy Dublin
of being vaccinated in their local pharmacy this year. Pharmacies have administered over 1.2 million vaccines under the COVID-19 and Flu programmes and pharmacists are available to offer advice or to answer queries you may have.”

Medicines for Ireland (MFI) have highlighted the growing risk of medicines shortages as inflation, energy and transport costs continue to rise, and global supply chain disruptions persist.
Padraic O’Brien, MFI’s Chairpersonfor companies supplying generic medicines to Irish hospitals and pharmacies. Additionally, in some cases, our reimbursement prices for certain medicines are too low compared to other EU countries and price adjustments in Ireland are historically downward only. This adds to unsustainable market conditions for suppliers.”
Scientists from Trinity have unearthed a secret that may explain why some people are able to resist viral infections, having screened the immune systems of women exposed to hepatitis C (HCV) through contaminated anti-D transfusions given over 40 years ago in Ireland.
Commenting on MFI’s Pre-Budget Submission, Chairperson Padraic O’Brien said, “The greater use of generic medicines in Ireland helps patients access high quality treatments at considerably lower costs, it also produces significant savings for the State”.

A recent MFI member’s survey found that 91% of MFI members experienced increased costs associated with import and/or manufacturing of pharmaceutical and medical products for the Irish market in 2022. While all MFI member companies envisage increases in transportation costs over the next 12 to 24 months.
According to the Health Products Regulatory Authority (HPRA) website there are currently 186 medicines in short supply in Ireland. Without intervention this situation has the potential to significantly worsen.
The Medicines for Ireland, Pre-Budget Submission 2023 sets out recommendations to tackle and prevent any potential shortages. These include enhancement of national pricing and procurement policies to help mitigate the risk of medicine shortages; exploring the possibility of the establishment of a National Medicines Reserve; and the extension of the 0% VAT rate that currently applies to certain oral medicines and non-
“However, skyrocketing inflation, supply chain disturbances and soaring energy costs have created unforeseen challenges for the generic medicines sector across Europe including Ireland, resulting in thousands of generic medicines disappearing from the market”.
“According to our sister association Medicines for Europe, several countries around the EU are experiences shortages, for example Romania has seen 2000 medicines disappear from the market and in Belgium one in five medicines available last year, are now no longer available.”
He added, “As a small market Ireland is more likely to be badly impacted by inflationary pressure and as costs continue to rise, market conditions will

Expanding on the pre-budget submission, O’Brien said, “MFI’s aim is to deliver industry insights and extend our expertise to help improve the development of medicinal pricing and procurement policies in Ireland. We believe it is time for us to revisit our work with Government and the HSE on the Framework Agreement on Supply and Pricing and develop improvements to mitigate against supply risks”.
“Our main focus is to help Government ensure market conditions in Ireland remain sustainable in order to retain and secure access to reliable and affordable treatment for Irish patients. MFI members are willing to work directly with Government to help tackle this serious issue and prevent potential medicines shortages.” concluded Mr O’Brien.
Between 1977-79 in Ireland, several thousand women were exposed to the hepatitis C virus through contaminated anti-D, which is a medication made using plasma from donated blood and given to Rhesus negative women who are pregnant with a Rhesus positive foetus. The medication prevents the development of antibodies that could be dangerous in subsequent pregnancies. Some of the anti-D used during the 1977-79 period was contaminated with hepatitis C.
From this outbreak, three groups of people were identifiable: those who were chronically infected; those who cleared the infection with an antibody response; and those who appeared protected against infection without making antibodies against hepatitis C.
Cliona O’Farrelly, Professor of Comparative Immunology in Trinity’s School of Biochemistry and Immunology said, “We hypothesised that women who seemed to resist HCV infection must have an enhanced innate immune response, which is the ancient part of the immune system that acts as a first line of defence.
“To test this we needed to make contact with women exposed to the virus over forty years ago and ask them to help us by allowing us to study their immune systems to hunt for scientific clues that would explain their differing responses.

Visit
“After a nationwide campaign over 100 women came forward and we have gained some unique and important insights. That so many women – many of whom have lived with medical complications for a long time – were willing to help is testament to how much people want to engage with science and help pursue research with the potential to make genuine, positive impacts on society.”
Name of product: VIAGRA CONNECT 50 mg film-coated tablets Active ingredient: sildenafil. Supply classification: Pharmacy only. Indications: For erectile dysfunction in adult men. Dosage and Method of use: Adults: one 50 mg tablet taken with water approx. one hour before sexual activity. The maximum dosing frequency is once per day. the onset of activity may be delayed if taken with food. Patients should be advised that they may need to take Viagra Connect a number of times on different occasions (max of one 50 mg tablet per day), before they can achieve a penile erection satisfactory for sexual activity. If patients are still not able to achieve a sufficient penile erection they should be advised to consult a doctor. Elderly: no dosage adjustments required (≥65 years old). Renal Impairment: No dosage adjustments for patients with mild to moderate renal impairment. Dosage adjustments required for those with severe renal impairment, see SmPC. Hepatic Impairment: Dosage adjustments required for those with mild-moderate hepatic impairment, see SmPC. Viagra Connect in contraindicated for patients with severe hepatic impairment (see contraindications). Use in patients taking other medicinal products: individuals receiving concomitant treatment with CYP3A4 inhibitors must be advised to consult their doctor before taking Viagra Connect, dosing adjustments may be required, see SmPC. Patients receiving alpha blocker treatment should be stabilised on therapy prior to initiating sildenafil treatment and must be advised to consult their doctor before taking Viagra Connect as dosing adjustments may be required, see SmPC. Side Effects: The most commonly reported adverse reactions in clinical studies among sildenafil treated patients were headache, flushing, dyspepsia, nasal congestion, dizziness, nausea, hot flush, visual disturbance, cyanopsia and vision blurred. For full list of side effects see SmPC section 4.8. Warnings and Precautions: Erectile dysfunction can be associated with a number of contributing conditions, e.g. hypertension, diabetes mellitus, hypercholesterolaemia or cardiovascular disease. As a result, all men with erectile dysfunction should be advised to consult their doctor within 6 months for a clinical review of potential underlying conditions and risk factors associated with erectile dysfunction (ED). If symptoms of ED have not improved after taking Viagra Connect on several consecutive occasions, or if their erectile dysfunction worsens, the patient should be advised to consult their doctor. Cardiovascular risk factors: Since there is a degree of cardiac risk associated with sexual activity, the cardiovascular status of men should be considered prior to initiation of therapy. Agents for the treatment of erectile dysfunction, including sildenafil, are not recommended to be used by those men who with light or moderate physical activity, such as walking briskly for 20 minutes or climbing 2 flights of stairs, feel very breathless or experience chest pain. For a list of patients who are considered at low cardiovascular risk from sexual activity see SmPC. Patients previously diagnosed with the following must be advised to consult with their doctor before resuming sexual activity: uncontrolled hypertension, moderate to severe valvular disease, left ventricular dysfunction, hypertrophic obstructive and other cardiomyopathies, or significant arrhythmias. Sildenafil has vasodilator properties, resulting in mild and transient decreases in blood pressure. Patients with increased susceptibility to vasodilators include those with left ventricular outflow obstruction (e.g. aortic stenosis), or those with the rare syndrome of multiple system atrophy manifesting as severely impaired autonomic control of blood pressure. Priapism: Patients who have conditions which may predispose them to priapism (such as sickle cell anaemia, multiple myeloma or leukaemia), should consult a doctor before using agents for the treatment of erectile dysfunction, including sildenafil. Prolonged erections and priapism have been occasionally reported with sildenafil in postmarketing experience. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. Concomitant use with other treatments for erectile dysfunction is not recommended. Effects on vision: Patients should be advised that in the event of any sudden visual defect, they should stop taking Viagra Connect and consult a physician immediately. Concomitant use with CYP3A4 inhibitors: patients should be advised to consult a doctor before taking Viagra Connect as a 25 mg tablet may be more suitable for them. Concomitant use with alpha-blockers: Caution is advised when sildenafil is administered to patients taking an alpha-blocker, as the co-administration may lead to symptomatic hypotension in a few susceptible individuals. This is most likely to occur within 4 hours post sildenafil dosing. In order to minimise the potential for developing postural hypotension, patients should be hemodynamically stable on alpha-blocker therapy prior to initiating sildenafil treatment. Thus, patients taking alpha blockers should be advised to consult their doctor before taking Viagra Connect. Treatment should be stopped if symptoms of postural hypotension occur, and patients should seek advice from their doctor on what to do. Effect on bleeding: the use of sildenafil is not recommended in those patients with history of bleeding disorders or active peptic ulceration, and should only be administered after consultation with a doctor. Hepatic impairment: Patients with hepatic or renal impairment must be advised to consult their doctor before taking Viagra Connect., since a 25 mg tablet may be more suitable for them. Lactose: The film coating of the tablet contains lactose. Viagra Connect should not be administered to men with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per tablet. Patients on low sodium diets can be informed that this medicinal product is essentially ‘sodium-free’. Use with alcohol: Drinking excessive alcohol can temporarily reduce a man’s ability to get an erection. Men should be advised not to drink large amounts of alcohol before sexual activity. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. co-administration with nitric oxide donors (such as amyl nitrite, nitrates, ritonavir guanylate cyclase stimulators, such as riociguatis contraindicated. Agents for the treatment of erectile dysfunction, including sildenafil, should not be used by those men for whom sexual activity may be inadvisable, and these patients should be referred to their doctor. This includes patients with severe cardiovascular disorders such as a recent (6 months) acute myocardial infarction (AMI) or stroke, unstable angina or severe cardiac failure. Sildenafil should not be used in patients with severe hepatic impairment, hypotension (blood pressure < 90/50 mmHg) and known hereditary degenerative retinal disorders such as retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases). Sildenafil is contraindicated in patients who have loss of vision in one eye because of non-arteritic anterior ischaemic optic neuropathy (NAION), regardless of whether this episode was in connection or not with previous PDE5 inhibitor exposure. Viagra Connect should not be used in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie’s disease). Viagra Connect is not indicated for use by women. The product is not intended for men without erectile dysfunction. This product is not intended for men under 18 years of age. Legal Category: Product not subject to medical prescription. Marketing Authorisation Number: PA23055/016/001. Marketing Authorisation Holder: Upjohn EESV, Rivium Westlaan 142, 2909 LD Capelle aan den IJssel, Netherlands. For further information on this medicine please contact: Medical Information on 1800 633 363. For queries regarding product availability please contact: Viatris, Newenham Court, Northern Cross, Malahide Road, Dublin 17. Phone number: +353 1 871 1600.

Reporting of adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important.It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to reportany suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse reactions/ events should also be reported to the marketing authorisation holder at email address: pv.ireland@viatris.com or phone 0044(0)8001218267. www.viagraconnect.ie

Perrigo recently held a PharmaFuture event at the Royal College of Surgeons in Ireland hosted by the RCSI Pharmacy Students Society.

Paul Hatton, National Field Sales Controller with Perrigo presented alongside two amazing speakers in Oonagh O’Hagan and Jess Redden. Paul commented, “Oonagh’s story roadmapping her route to owning her first Meagher’s Pharmacy Group was truly inspiring - not to mention the growth since then! Jess delivered a really insightful talk on her journey from Pharmacy Student to practicing Pharmacist and highlighted some super tools she used to help keep her grounded along the way.”
The event had a fantastic turnout with over 50 students in attendance.
The HSE National Clinical Programme for Stroke marked World Stroke Day by launching the new National Stroke Strategy 2022-2027.
Stroke is the second leading cause of death in middle to higher income countries and the leading cause of acquired neurological disability in Ireland. Approximately 5,800 adults were admitted to hospitals with a stroke in 2020. This figure does not include the estimated 1,500-2,000 people admitted or evaluated urgently for a suspected / threatened stroke or a transient ischaemic attack (TIA).
As part of their social values, the team at Edia Healthcare believes that everyone together can do more towards a better future.
That is why they have recently launched their “Giving back to the Community” programme. Through this venture, a percentage of each Edia Healthcare machine sale will go to a community charity, club, fund and so on, chosen by the Pharmacy itself.

Pictured is the team at Reen’s Life Pharmacy ‘giving back’ to their local community.
Phrassa Pharmaceutical is a new company with a familiar feel. Owen McKoen and Martin O’Connell are the owners and have decided to launch this company as a wholly owned Irish entity. It is based in Dunboyne.

Liam O’Donovan, Geraldine O’Leary and Brian Woods joined the team in September. All have worked together previously in Meda, and then Mylan and will be familiar to many pharmacists throughout the country. Their addition will be of enormous benefit to the company and the impetus for further development.
Phrassa launched their first product “Lutein + Vit D” in July for the treatment of AMD (Age Related Macular Degeneration Disease). The formula is the latest update from the ARED’s2 body who guide the treatment for this disease. With the addition of Vitamin D,
Homotaurine and Turmeric this product is the latest step in the treatment of AMD.
This has been followed up with “CYD” Check Your Drink which is the first novel test for drink spiking. This is an issue of which most people are aware, as drink spiking is growing at an alarming rate.
CYD helps protect, control and maintain the safety of our social life for people in Ireland.
Phrassa also provide the Boson Antigen tests which were integral in their start-up phase.
Owen tells IPN, “Our fourth product will be a menopause self-
test which will assist with women going through perimenopause and menopause. Self-testing is an important addition to health care in Ireland.
“Our goal is to focus on the pharmacy OTC sector initially. We are also looking to launch some products in the GUT Health area as we believe that the IBS and Functional dyspepsia patient is being overlooked.”
Professor Rónán Collins, Clinical Lead for the HSE National Clinical Programme for Stroke said, “Stroke is a major cause of mortality and morbidity in our population and a major cost to our health service when outcomes are poor. Much improvement in services and outcomes has occurred since the inception of the National Clinical Programme for Stroke, but the nature of our changing demography, development of new stroke treatments and technologies, and the need for healthcare staff and public engagement on the issue of stroke are a significant challenge over the next decade.
The programme has prioritised an ambitious but realistic strategy to improve resourcing of stroke services and seeks commitment from government for a structured implementation, review and a ‘next steps for stroke’ strategy, to commence in 2026 with the aim of full realisation of the Stroke Action Plan for Europe by 2031.”
To meet the challenge of a predicted 59% increase in the total number of strokes in Ireland four pillars of the new National Stroke Strategy were identified which focus on:
1. Stroke Prevention
2. Acute Care and Cure
3. Rehabilitation and Restoration to Living
4. Education and Research
To view the National Stroke Strategy document, see https:// www.hse.ie/eng/about/who/ cspd/ncps/stroke/
Research by RCSI University of Medicine and Health Sciences has discovered a new role for the blood clotting protein, von Willebrand Factor (VWF), that could lead to the development of new treatments for patients with inflammatory and blood clotting disorders.
Published in Nature Communications, the research finds that VWF plays an important role in regulating immune responses at sites of blood vessel injury. This means that the protein has a newly discovered role in repairing damaged blood vessels in addition to its role in blood clotting.
Deficiency in VWF is called ‘von Willebrand Disease’ and occurs in about 1 in 1,000 people in Ireland. People with this condition have increased risk of serious heavy bleeding. In contrast, people with high levels of the protein in their blood are at risk of developing serious blood clots. For example, very high VWF levels have been implicated in the unusual blood clots seen in the lungs of patients with severe COVID-19.
This research shows, for the first time, that VWF not only regulates blood clotting at the site of damage but also triggers local immune responses. Understanding this new biological role for VWF in regulating inflammatory responses may offer the opportunity to develop entirely new treatment options for patients with inflammatory and blood clotting disorders, such as von Willebrand Disease, deep vein thrombosis and myocardial infarction.
Lead author of the research, Professor James O’Donnell, Director of the Irish Centre for Vascular Biology at RCSI School of Pharmacy and Biomolecular Sciences, said: “This research now helps us to further understand the role that VWF plays in linking blood coagulation and inflammation and thereby paves the way for the development of new treatments.”
United Drug were delighted to have taken home the award for the COVID-19 Crisis Response category at the Pharma Industry Awards.
“We are extremely proud of our teams and the fantastic work that was done by all United Drug employees. Their tireless efforts ensured that vital medications, vaccines, and services could continue to be provided and maintained to the Irish Public throughout the entire Pandemic.”
Paul Reilly, Managing Director of United Drug
This award acknowledges the rapid response to the requirements for the storing, processing, and distribution through supply chain excellence of the Covid19 vaccinations at ultra-low temperatures. The professionalism and the company’s response to the physical demands required were praised by the judges who felt that the complex and challenging task
was met with great success but also inspiring teamwork.
This award acknowledges but most importantly gives recognition to the team’s personal commitment, tireless efforts, and attention to detail over the past 24 months. Through this, they helped to save lives, and there is no better achievement! Well done to all involved.
Accord Healthcare took home four awards including Company of the Year at the Global Generics and Biosimilars Awards, held in Frankfurt, Germany on November 2nd.

The company also won the Biosimilar Initiative of the Year, Value Added Medicine Initiative of the Year and Regulatory Achievement of the Year.
Speaking after the success, Paul Tredwell, EVP, EMENA says: “Year after year we are continuously recognised at these prestigious awards and that a is testament to our people, our culture and our drive to make it better and the innovations we invest in.
“To be named Company of the Year, take the award for Biosimilar Initiative of the Year, Value Added Medicine Initiative of the Year, and Regulatory Achievement of the Year is a real accomplishment for
everyone here at Accord and a real achievement to be awarded four in a highly competitive ceremony.” Also pictured are members of the Accord Healthcare Ireland Team who have filled shoe boxes for the Team Hope Christmas shoebox appeal.
Team Hope’s Christmas Shoebox Appeal is an annual campaign that delivers gifts straight into the hands of children affected by poverty. Often these shoebox gifts are the only gift that a child will receive at Christmas and the joy that they bring is incredible. Since 2010, they have delivered over 2.1 million Shoebox gifts to Vulnerable Children across the world.




Huge congratulations to team totalhealth who completed the Dublin City Marathon on Sunday 30th October. The team weren’t deterred by the poor weather, and all completed the race in good times.

The support from customers, friends and family was acknowledged by the runners and they were immensely thankful for the generous contributions to their chosen charities.
The team collected over ¤5,000 for the Mayo Roscommon Hospice and the Kevin Bell Repatriation Fund.
Sheena Mitchell - Milltown totalhealth Pharmacy
Alan Mitchell - Milltown totalhealth Pharmacy
Emma Malone - Malone’s totalhealth Pharmacy
Cathy Flynn - Ballybunion totalhealth Pharmacy
Nial Tully - Tully’s totalhealth Pharmacy, Castlerea
Michelle Flynn - Tully’s totalhealth Pharmacy, Castlerea
A wide range of opportunities for pharmacists to provide cardiovascular disease (CVD) services are described in a new practice support handbook published by the International Pharmaceutical Federation (FIP).
According to the World Health Organisation, deaths from CVDs currently represent 32% of all deaths globally, with over 75% of these occurring in low- and middle-income countries. With deaths from CVDs projected to increase from 18.9 million in 2020 to 32.3 million by 2030, demand for cardiovascular prevention, care and treatments continues to grow. “Cardiovascular diseases: A handbook for pharmacists” covers prevention, care and management roles, including: health promotion and education;
early detection; triage and referral; interprofessional collaborative practice; disease management (including medication adherence); treatment optimisation; and helping to shape public policies. In particular, it contains a chapter on practice-based research.
“Care goes beyond medicines use and optimising effectiveness and safety. Pharmacists can also be involved in practicebased research to evaluate the impact of CVD services. This new handbook contains several
evidence-based interventions by pharmacists around the world that have led to positive health and economic outcomes for patients, which readers will find valuable and inspirational,” said Inês Nunes da Cunha, FIP practice development and transformation projects manager and lead author of the handbook. “It is important that pharmacists are able to meet needs, expanding and consolidating their CVD services and roles with the appropriate knowledge and skills as defined in
reference guide for professional development in cardiovascular diseases’ [also published by FIP today]. It is also essential that pharmacy professional organisations at global, regional and national levels support practitioners in implementing and providing services in this area,” she said.
The new handbook has been developed in collaboration with the World Heart Federation (WHF) and the European Society of Clinical Pharmacy.


First aid emergencies can happen anytime, anywhere. Be prepared for everything that the festive season brings by making sure your Medicare First Aid Kit is stocked up and ready. Our First Aid Kits have everything you need to treat common injuries.

The Medicare First Aid Pocket Kit is packed with all the essentials needed for any kind of emergency, especially when you are on-the-go. Contains 26 First Aid items to clean, treat and protect - Ideal for little mishaps. Wishing you and yours a healthy and happy Christmas from all at Fleming Medical.

So, whether you burn yourself while cooking the Christmas turkey or get a paper cut while wrapping gifts, have a Medicare First Aid Kit ready. Scan our QR code to browse our Medicare Range of First Aid Kits and First Aid Supplies.
John Tallon has been awarded the title of The People’s Pharmacist for 2022 following a nationwide voting campaign that saw over 16,000 votes and a reach of over half a million!
John Tallon, Bergin’s Pharmacy Newbridge
John was presented with his award by Dave Barrett, Haleon County Manager, Mayor Suzanne Doyle and Managing Director of IPN Communications, Natalie Maginnis.
It was, with unparalleled gratitude to Irish pharmacists, that members of the public received vital medicines, health and wellbeing advice and that the vulnerable and those in need were protected and kept safe.
Now more than ever before, it has become clear to everyone that pharmacists are one of the most critical members of Ireland’s health service family, embedded in the heart of every community.
Through this Award, we are enabling the public to have a voice in recognising the unwavering support and spirit that makes pharmacists the backbone of our health service in every community across Ireland.
John Tallon is Pharmacist at Bergin’s Pharmacy in Newbridge. John has become recognisable during the past number of years throughout his community due to his professionalism, compassion and dedicated hard work.
Bergins is an Independent Community Pharmacy in the heart of Newbridge, serving the Newbridge area and wider Kildare towns for over 25 years with all their healthcare, beauty and fragrance needs.
In every corner of Ireland, whilst the Covid-19 pandemic closed GP surgeries and hospital capacity overflowed, pharmacists stepped up.
Pharmacy doors remained firmly open during an unprecedented challenging time. Indeed, many patients turned to their community pharmacist for consultations for more serious conditions which are usually dealt with by GPs.
In association with Panadol, Irish Pharmacy News launched the search to find The People’s Pharmacist 2022 earlier this year; giving the Irish public the opportunity to nominate and vote for, their local pharmacist who has gone above and beyond.
The People’s Pharmacist Award seeks nominations from across the country, giving patients the opportunity to recognise and salute their local pharmacist.
Through this Award, we are enabling the public to have a voice in recognising the unwavering support and spirit that makes pharmacists the backbone of our health service in every community across Ireland.
From over 700 nominations we had eight extremely deserving finalists and after a nationwide voting campaign, John Tallon of Bergin’s Pharmacy in Newbridge was awarded this prestigious title.

John has also made great efforts in his work with the homeless community, making sure no-one misses out, he has dedicated a huge part of his time to assisting this sector.
“I am a customer of Bergin’s Pharmacy for 30 plus years and Johns care and knowledge has saved my life,” noted his nominee.
“John is a never-ending professional with a true kindness and understanding of complex medical issues. I have witnessed first-hand his dedication to the homeless and less fortunate. He is shy, non-assuming and has
a true vocation to serve his community. We would be lost without him in Newbridge and the surrounding areas.”
Always greeting every customer with a smile and a warm welcome, John has stepped up over and beyond during this pandemic and
has been a friend, a counsellor and a medical advisor to so many throughout his pharmacy’s area.
John said on being nominated, “I feel incredibly honoured to be even nominated for the People’s Pharmacist Award and delighted to be shortlisted. I was pleasantly


surprised to receive such a nomination and the recognition of the entire Pharmacy team at Bergin’s Pharmacy.
“We always try and give 100% to all our customers every day and that they feel that they are our priority at all times.
“I am very proud of all our team and the service they provide to each customer every day and receiving the nomination is fantastic acknowledgement of all the hard work.”
IPU President Dermot Twomey warned, “Antibiotics have been one of the most significant developments in the history of medicine and have saved countless lives in the past hundred years. We must protect their potential, but the growing prevalence of antibiotic overuse poses a risk with the growth of antibiotic resistance and the growing number of highly resistant superbugs (bacteria that are hard to kill with antibiotics), of major concern.”
“Antibiotic resistance will present a major issue for both Irish and global healthcare systems if we continue to overuse antibiotics for ailments of which it is not appropriate to do so. We can already see the negative effects of antibiotic overuse in play today. Antibiotic resistant infections such as MRSA and C. diff have presented a huge challenge to the global health community, and there is certainly a fear that new strains of bacteria may emerge that cannot be treated by existing antibiotics.
“We all have a responsibility to attempt to prevent these new strains of bacteria emerging and that is why this year’s theme of “preventing antimicrobial resistance together” is so relevant. The key message is that antibiotics should only ever be taken when a patient actually requires them to treat a specific bacterial infection. For many common conditions, such as the flu, coughs, colds, vomiting and diarrhoea, antibiotics have no impact. Similarly, COVID-19, which is a viral
infection will not be impacted by antibiotic treatments. Instead, those suffering from these ailments should speak to their pharmacist or GP for remedies to treat the symptoms.”
In conclusion, Mr Twomey said that European Antibiotic Awareness Day offers a valuable focus for us all on this critical healthcare issue, and he outlined a series of pieces of advice that pharmacists all over the country will be continuing to emphasise to patients this week.
Antibiotic overuse is becoming an increasing concern and a growing threat to Irish and global health according to the Irish Pharmacy Union (IPU). Marking European Antibiotic Awareness Day (18 November) and the start of the World Health Organisations Antimicrobial Awareness Week, the IPU has urged everyone to respect antibiotics and to ensure they are only ever used when absolutely necessary to support the global effort to preserve their effectiveness.
Active Iron’s Better Days. Period. campaign aims to prompt conversations around periods and change the narrative about symptoms, like menstrual fatigue, which 64% admitted having. The campaign highlights that 64% of women have had period issues disregarded by friends, family and healthcare professionals
A new study by Active Iron1 revealed that 41% of women are advised ‘not to worry and just get on with it’, in relation to period symptoms, while nearly half, 48%, are told they will be fine in a few days.
Shockingly, 64% of women have had their symptoms disregarded by friends, family and even healthcare professionals, indicating the extent to which society considers women should just put up with ill-health and suffering when menstruating.
The survey of 1,000 women, conducted in September on behalf of the Irish supplement brand, confirmed that 74% of women experience stomach cramps, while 64% have menstrual fatigue, which were two of the most common period symptoms.
The multitude of advice survey respondents had been given ranged from the helpful and positive, to the bizarre and potentially damaging. It included taking pain-relief (85%), staying hydrated (48%), taking a bath (47%), binging on chocolate (18%), avoiding swimming (16%), having an orgasm (11%) and eating nothing (2%).
In addition, 52% of the panel were advised to keep active. But, with menstrual fatigue holding them back, the advice was conflicting.
The lack of understanding and open discussion about periods led the Cork-based makers of Active Iron to launch an education initiative. The Better Days. Period. campaign aims to
empower women to have conversations about the impact of period symptoms, while also taking action to address them.
Claire Lynch explains; “Periods are a completely natural and normal part of a woman’s life, yet, for years, menstruation has been a taboo subject that is not discussed openly enough.”
Recent campaign events saw presenter Lucy Kennedy; pharmacist and content creator, Jess Redden; medical doctor and Miss Ireland 2022, Ivanna McMahon and many more discuss the need to address the impacts of periods on women’s health and wellbeing.


“For example, menstrual fatigue is one of the most common symptoms experienced. This can be caused by low iron, as a result of blood loss, and is easily remedied.”
Dr Ivanna McMahon, explains that, on average, women lose around 80ml of blood during their period, which will contain up to 250mg of iron.
“This is perfectly normal. But accepting we should feel bad during a period is not. Blood loss means iron loss, which means fatigue. However, lifestyle and diet changes, and taking a supplement like Active Iron, will help sustain energy levels throughout a woman’s cycle.”
The medical professional says it is important not to suffer in silence, but to actively seek solutions and advice, especially for recurring symptoms affecting quality of life.
“There is a straightforward solution to the vast majority of period woes, so a chat with your pharmacist or GP is the place to start. A gynaecologist will also help sort troubling symptoms. But the main thing is not to accept feeling awful as the norm,” Ivanna McMahon says.
For those suffering with consistent, frequent symptoms it is important to get your levels checked by your doctor Pharmacist Jess Redden says.
The connection between iron loss and fatigue was mostly missed by the women surveyed. 64% didn’t consider tiredness and lack of concentration was due to loss of iron, and only 13% were ever advised to take an iron supplement.
Many iron formulations can be hard to take due to their side effects, which means there can still be a reluctance to take it, despite proven advantages, Claire Lynch says.
“Active Iron is different. Its ground breaking formula is clinically proven to increase iron and energy levels, and to help avoid side effects like stomach upset2”, the campaign manager says.
Active Iron’s latest product Active Iron Advance is clinically proven to increase iron levels by 94% in 6 weeks.
For more information on managing period symptoms, and the role iron can play to support energy and alleviate menstrual fatigue, visit www.activeiron.com.

exceed 4 tablets in 24 hours. Do not give to children under 12 years.

Minimum dosing interval: 4 hours. Contraindications: Hypersensitivity to paracetamol, caffeine or any ingredients. Precautions: Avoid concurrent use with other paracetamol-containing products. Diagnosed liver of kidney impairment. Patients on concomitant treatment with drugs that induce hepatic enzymes. Patients with depleted glutathione levels or chronic alcoholism or sepsis. Avoid excessive caffeine intake. Caution in those with hereditary sugar intolerance or on a low sodium diet. Should not be used in pregnancy or lactation without medical advice. Caution, due to paracetamol, if administered with flucloxacillin due to increased risk of high anion gap metabolic acidosis. Do not exceed the stated dose. Prolonged use except under medical supervision may be harmful. If high fever, or signs of secondary infection occur or if symptoms persist for longer than 3 days, consult your doctor. Side effects: See SPC for full details. All very rare: Thrombocytopenia, hypersensitivity reactions including anaphylaxis and skin rash, angioedema, Stevens-Johnson syndrome, Toxic Epidermal Necrolysis, bronchospasm, hepatic dysfunction. Frequency unknown: Nervousness, dizziness. When combined with dietary caffeine intake, higher doses of caffeine may increase potential for caffeine related adverse events such as insomnia, restlessness, anxiety, irritability, headaches, GI disturbances and palpitations. Overdose: Immediate medical advice should be sought in the event of an overdose, even if symptoms of overdose are not present. Legal Category: Supply through pharmacy only. MA Number: PA 678/39/10. MA Holder: GlaxoSmithKline Consumer Healthcare (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24. Additional information is available upon request. Text prepared: September 2022. Contains paracetamol. Always read the label/leaflet.


Trade marks are owned by or licensed to the Haleon group of companies. PM-IE-PAN-21-00017.


Central to the success of all these initiatives continues to be close collaborative relationships with all our partners across the broader healthcare system. Indeed, maintaining and establishing new collaborative partnerships with key stakeholders will continue to be a primary focus for the organisation into 2023 as we look to enhance and protect public health through the effective regulation of health products.
There is no doubt that 2022 was a much more positive and optimistic time for our country following the challenges presented by the COVID-19 pandemic during the previous almost two-year period. While there are currently no COVID-19 restrictions in Ireland, public health advice, combined with safe and effective vaccines, continue to help protect our communities. As we enter a new phase in our response to COVID-19, it is important we remain vigilant given the potential for future waves of infections driven by different subvariants. From a regulatory perspective, there was considerable focus on the authorisation of adapted bivalent vaccines to help protect against severe disease and hospitalisation in response to the Omicron variant. Experts from across our organisation, in collaboration with colleagues throughout Europe, conducted rigorous assessments to ensure that newly authorised adapted vaccines continue to demonstrate extremely high levels of safety, effectiveness and quality. In
addition to the authorisation of COVID-19 related therapies and vaccines, regulators from across the globe are currently undertaking a period of reflection to understand how best to retain and implement some of the extraordinary regulatory agilities introduced during the pandemic to support the accelerated development and authorisation of medicines. Indeed, the HPRA continue to engage with colleagues across Europe to ensure efficiencies in the regulation of medicines are retained, wherever possible.
The past year was also a time of significant progress regarding the implementation of new legislative frameworks that will positively affect human health for many years. The new Medical Devices Regulation (MDR) and In-Vitro Diagnostics Regulation (IVDR), for example, will ultimately strengthen existing regulatory systems for medical devices and diagnostics across Europe. The regulations aim to provide, among other things, clearer requirements on clinical data for medical devices and in-vitro diagnostics (IVDs), more specific product requirements and enhanced provisions for market surveillance and performance assessment. Despite the clear and obvious benefits for patients and healthcare providers associated with the MDR and IVDR – in terms of an enhanced focus on clinical safety and innovation support – there are challenges that will need to be addressed before fully realising the system-strengthening opportunities provided by each regulation. Specifically, the coordinated and consistent application and operation of both regulations is critical to deliver for patients and ensure their success. The HPRA, in collaboration with colleagues from other European agencies, are providing leadership, influence and strategic direction to enhance cooperation and coordination within the EU regulatory system to deliver the successful implementation of MDR and IVDR.
We also saw the new Clinical Trials Regulation (CTR) coming into effect in January of this year. Like the MDR and IVDR, the CTR aims to maintain high standards of public transparency and safety for clinical trial participants, while at the same time demonstrating the EU offers an attractive and favourable environment for conducting clinical research on a large scale. The CTR enables sponsors to submit one application via a single online platform, known as the Clinical Trials Information System (CTIS), for approval to run a clinical trial in several European countries. In this way, it makes it more efficient to carry out such multinational trials. Following “golive” in January, the majority of trials submitted to the CTIS have been authorised (+75 trials), with many more currently under evaluation. Despite the relative success of CTIS, submission numbers remain lower than expected at this time and users have experienced technical issues with the platform resulting in the delayed approval of applications. The European Medicines Agency (EMA), together with national regulatory authorities across Europe, including the HPRA, are working collaboratively to resolve high-priority technical issues associated with the CTIS. The goal for all involved is to enable the timely, effective, and efficient authorisation of clinical trials that are so important to facilitating patient access to innovative medicines.
Indeed, a primary focus throughout 2022 was ensuring the continued availability of medicines to meet patient need through our role in coordinating the management of medicines shortages. The HPRA has recently conducted a review of national data associated with the underlying root causes of shortages from the previous twoyear period. This is with a view to establishing the next phase of our multistakeholder response to shortage management in Ireland. Key stakeholders, including healthcare professionals and pharmaceutical companies, were consulted on this review, with recommendations on ways to help prevent and mitigate the effect of
medicines shortages on patients due for publication in the coming year. This represents an exciting new phase in our approach to managing a multistakeholder approach and ensuring the continued availability of critical medicines on the Irish market to meet patient needs.
Finally, from a personal perspective, it was a great honour to be appointed to lead the EMA’s Management Board. What has been evident throughout the years of the pandemic is that EMA must remain agile, responsive, and ready to show leadership and solidarity in the context of significant developments in the operating environment. As chair, my focus will be to continue and further enhance the strong partnership between the EMA, national agencies and the European Commission. It is imperative that the EMA continues to deliver the highest standards of public health protection while maintaining its global reputation as a modern, progressive, and transparent regulator. This will be achieved through the continued close cooperation and partnership approach of the broader European medicines regulatory network.
My role as chair of the EMA Management Board only serves to complements other HPRA staff holding prominent positions of influence at European level. Despite being a medium sized agency in the context of Europe, we continue to provide leadership on a range of important regulatory issues impacting public health.
As we look forward to 2023, I am excited to build on the achievements of the organisation throughout the year as we continue to work in partnership with colleagues from across the national public health system. This is in addition to our well-established commitment to the European, and increasingly global, regulatory network. While we have set ambitious goals and objectives for the coming year, I believe the HPRA will continue to excel in health product regulation through science, collaboration, and innovation.
This year has seen significant progress in several key areas of focus for the HPRA as we continued our work in response to the pandemic in combination with ongoing activities to ensure the successful implementation of new regulations and management of medicines shortages.





I started the year by taking the opportunity to thank registered pharmacists, pharmacy owners and pharmacy teams for their ongoing hard work and commitment in the face of COVID-19 and the participation of many pharmacies in the national vaccination programme. This work continues at the tail-end of the year, and I again extend the PSI Council’s acknowledgement for the important work being undertaken across the country despite various challenges.
While society largely shed masks and other protections as the year went on, healthcare settings remain places where infection prevention and control measures are a necessary high priority for patient and staff safety. Pharmacists are keenly aware of the many people who remain affected, at-risk and isolated due to their vulnerabilities to COVID-19 and other illnesses. The PSI is cognisant of the extensive efforts of pharmacists to remain available to support their patients with appropriate care and treatment at all times.
PSI’s work and engagement with the Department of Health, HSE, other regulators, Schools of Pharmacy and representative bodies has continued, with our
regulatory role being but one part of the broad healthcare system. Pharmacy involvement in vaccination availability, training and management were very much on the agenda throughout the year, with flu and COVID-19 vaccinations being a vital part of this winter’s protections for the public. The PSI has continued to approve all required new vaccination training programmes for pharmacists and has made available information and guidance to our registrants on a range of topics to support their practices and compliance with legislative provisions. Assessing compliance continues through visits to pharmacies, with many more in person visits this year than was possible over the previous two years.
We acknowledge that the availability of pharmacist staff and the associated workforce challenges is a live issue, and it is something that the PSI is actively exploring. In line with our strategic commitment, we are examining the emerging risks to the future pharmacy workforce, so that pharmacy may continue to play a full and active role in the development of an integrated healthcare system. The PSI is very grateful to pharmacists and pharmacy students who have offered to participate in focus groups to feed into this work, and to other pharmacists who applied to be part of the project’s Working Group. This is a complex issue which requires to be addressed collaboratively with all relevant stakeholders.

The PSI has commissioned external support with expertise in workforce planning and is consulting with a wide range of stakeholders, including members of the profession, with the intention of setting out a series of recommended actions for consideration by the PSI Council. The project will run throughout 2023.
The PSI is observing a return to pre-COVID-19 registration
applications with the continued year on year increase to the Register of Pharmacists. Through October to December, the PSI is in the peak time for continued registrations and the first-time registration of new national Masters in Pharmacy graduates. The numbers on the Register of Pharmacists are expected to be over 7,000 for the first time in the new year. This is of interest in the context of pharmacist availability and the aforementioned project.
The new registrant portal for PSI applicants and registrants has demonstrated significant value to the organisation since implemented at the end of last year. It has generally streamlined the application process and improved processing times. PSI staff are committed to continuing to improve and evolve digital offerings in the interest of efficiency for all and in line with the Government’s and PSI’s ‘digital first’ policy.
Along with general increases in registration numbers, queries and applications to the existing Third Country Qualification Recognition (TCQR) route have notably increased this year. Work remains ongoing in relation to our revision of this route for pharmacists who have qualified in a Non-EU/ EEA country. It is the intention to streamline the overall process for the benefit of applicants and to improve internal efficiency.
The Council has approved a policy and an approach for the roll-out of a new TCQR process and we hope to consult on the different aspects of the proposed changes soon. The process is being designed with a more holistic perspective. It will remain a multi-stage process, but depending on an applicant’s qualification, training, and experience, the applicant will move through it differently. Ultimately, our focus must be to ensure that there are fair and robust qualification recognition and registration systems in place
that facilitate the entry of suitably qualified pharmacists to the Irish pharmacy workforce.
In Spring, like many others, the PSI turned its attention to the impacts and challenges brought by the Russian invasion of Ukraine. An internal staff group was initially established to ensure the PSI’s readiness for the range of issues that might be related to the movement of Ukrainians to Ireland, and the PSI remained in close contact with the Department of Health and the HSE about professional registration and medicines availability. The Council extended a waived TCQR application fee to Ukrainian pharmacists coming to Ireland under Temporary Protection Status, as is the case for applicants with refugee status. Staff have been assisting a small number of Ukrainian pharmacist applicants with their requests to enter the Register of Pharmacists.
As expected, it has been a busy year. Our role in the public interest is expansive and the Council has set several areas for strategic development and improvement. We are entering the final year of our Corporate Strategy 20212023 and we strive to meet the commitments within it so that the PSI can respond efficiently and effectively within our sphere of regulation.
I would like to thank the members of the PSI Council for their dedication and support again this year, and to thank all PSI staff for their hard work as we seek to keep pace with ongoing change and our range of commitments. In particular, I would like to thank Dr Lorraine Horgan who has guided the PSI as Interim Registrar throughout this year.
While we advance some key projects in 2023, I look forward to engaging broadly with PSI’s registrants so that we can continue to ensure the delivery of safe, quality pharmacy services, and to assure public trust in our valued profession.
PSI President Muireann Ní Shúilleabháin, in our annual IPN review series, writes of the challenges and opportunities of regulating during a time of increased demand for pharmacy care and service.























decades. This performance has been backed by sensible and stable Government policy, with IDA Ireland leading the effort to attract foreign direct investment.
clinical trials. The old approach of siloed policymaking focused on innovation, manufacturing and healthcare sustainability does not work.
In recent months, tax receipts from some of the country’s most important industrial sectors - biopharmaceuticals and technology, among themdemonstrate the importance of foreign direct investment. In our industry, many of the world’s largest players have commercial, manufacturing and, sometimes, research operations here, employing tens of thousands of people and generating billions in value-added for the economy. These companies’ productivity outputs are helping the Government to respond to cost-ofliving challenges and, before that, to the pandemic. The medicines they make are helping people, in Ireland and globally, to live better for longer. This impact is keenly felt in communities and in families. What medicines innovators do matters economically, socially and in healthcare terms.
It would be a mistake, though, to believe that the continued growth of the biopharmaceutical industry is inevitable. Like other industries, we are buffeted by international policy developments and geopolitical events. The global economic outlook is uncertain, especially with inflation, war in Europe and the ongoing impact of Covid-19.
Product life cycles, industry consolidation patterns, the draw of emerging markets, skills readiness and sub-optimal speeds of adoption of new medicines in the health services combine to create headwinds that could decelerate the pace at which the industry scales in Ireland into the future.
Ireland needs to improve our attractiveness for clinical trials, build our scientific research base,
and position as a destination for production and supply chain investments in ATMPs, including cell and gene therapies, as well as their reimbursement for patients. It makes no sense that Ireland has scale when it comes to the biopharmaceutical manufacturing footprint but not speed when it comes to reimbursing new medicines in the health system. The Government has invested close to ¤100 million in new medicines in the past three Budgets. But an inefficient reimbursement process means we are still among the slowest countries in western Europe to make them available to patients.
The way to improve all this is through structured dialogue between policymakers, in Dublin and Brussels, and our industry. We can work on shared solutions. Together, we should identify how to yield growth opportunities for the country, as well as de-risking an environment that could make a loss of competitiveness or a diminution in standards of care entirely possible.
A recent study by consultants Charles River Associates for EFPIA, the industry’s representative organisation in Europe, urges steps to boost competitiveness so that Europe is less likely to lose out to the US and Asia for new investments in biopharmaceutical research and in production. The study, called ‘Factors Affecting the Location of Biopharmaceutical Investments and Implications for European Policy Priorities’, illustrates our industry’s declining competitiveness in Europe, with the global share of research and development investments, clinical trials and manufacturing output all waning. The study finds that a key driver of most new investments is the location and performance of existing research and development or manufacturing footprint.
That is positive for Ireland. Recent investments in production sites across the country are based, in part, on an already-established performance culture built over
But the study reveals trends all of us should heed. In 2002, the US spent $2 billion more than Europe on research and development. Today, that figure is $20 billion - a rise of 1000%. Of the total research and development investments made in the US, Europe, China and Japan, only 31% occurs in Europe. This has declined steadily from 41% in 2001. China has grown its share from 1% to 8%.
Clinical trial activity for ATMPs is twice as high in the US and almost three times as high in China than in Europe. Cell and gene therapies are usually onetime treatments that can add months, sometimes years, to a patient’s life, replacing a lifetime of treatment. In cancer, haemophilia, SMA Type 1 and ocular diseases, cell and gene therapies can be game-changers. The number of trials for these treatments conducted in the US and AsiaPacific region grew by 70% and 67%, respectively, between 2014 and 2021 while Europe remained stagnant. Europe accounted for a 19.3% share of global clinical trials activity in 2020, a drop of 6.3%, compared with a 25.6% average over the past 10 years. ATMPs are the therapies of the future, with 804 next-generation biotherapeutics, including cell and gene therapies and mRNA technology, in a global pipeline of over 8,000 new medicines. The US has half the world’s ATMP manufacturing facilities. Asia is fast becoming the most competitive region for attracting ATMP clinical trials (255 in 2021), with Europe in decline (89 in 2021). For Europe to compete more effectively, it must recognise the complexity of these new technologies and build the interconnected ecosystem needed to develop them. We have argued for Ireland to aspire to be a centre for ATMP supply chain and production, and to adopt a national policy for adopting ATMPs affordably and fast in the health system. The EU should take a more proactive role in fostering the growth of emerging ATMP clusters, providing support across the ecosystem of research and development, manufacturing and
The study finds that global research and development is expected to grow at a rate of 4.2% annually to $233 billion in 2026. The problem is that it is moving out of the EU. While world-leading hubs like Boston, San Francisco and the UK’s ‘Golden Triangle’ get significant policy focus and strategic funding, European research funding is more uniform across countries. The countries with the highest EU research spend per population are not the centres of innovation. This is a weak strategy. The European Commission should consider more strategic allocation of resources to foster the growth of world-leading research centres.
The European Commission is reviewing the operating environment for medicines innovation as part of the EU Pharmaceutical Strategy. A legislative proposal is due to be published early next year. Our Government, and our MEPs, should adopt a position that backs innovation and supportive intellectual property rights. That means making Europe more competitive for jobs and investments in biopharmaceuticals, accelerating patients’ access to affordable medicines, investing in research for unmet medical needs and making regulatory pathways more agile and efficient. It does not mean curtailing intellectual property rights or linking them to medicines access.
Our industry’s local brand purpose is ‘innovate for life’. Although it is the name of our flagship, multiaward-winning film-led digital campaign, it is much more than a slogan. It means our work changes lives for the better - and that innovation is a life-long endeavour. Covid-19 has shown the value of partnership. It should not take a crisis for us to work together.
Closer collaboration between industry and policy leaders, especially when healthcare outcomes and investments are at stake, makes sense. We should build a permanent bridge, through regular strategic dialogue, that cements the interdependency between citizens, policymakers and industry.
Written by Bernard Mallee, Director of Communications and Advocacy at the Irish Pharmaceutical Healthcare Association
Medicines innovators’ impact is keenly felt but we must safeguard the gains and tackle the gaps
•

 by Rachel Hamilton, APPEL Operations Manager
by Rachel Hamilton, APPEL Operations Manager

assessment process and a special session was held to support any placements taking place in a hybrid working environment. Help and support were only a phone call or email away too as the team assisted with queries from students, Trainers, placement providers, and other stakeholders.
This year saw more students than ever completing experiential learning placements at different stages of their integrated M. Pharm programme. Over the 12 months, 545 pharmacy students completed placements in a variety of practice settings across the country, as
well as a small number of 4th-year students completing our firstever international placements in academic institutions in Spain, Sweden, and Switzerland.
We were also delighted to welcome many new placement providers to the programme as the value of offering APPEL placements has continued to be felt by pharmacies, hospital pharmacy departments, and other organisations.
APPEL’s team of Practice Educators and operations staff were on hand throughout the year to support both students and professionals involved in APPEL placements. Online clinics were organised to assist Trainers with the competency
The team also took on a number of quality improvement projects with APPEL Trainer Training being updated, new features being added to the website, new modules of training being developed, flexibility being added to the placement timetable, and the Standards for APPEL Experiential Learning Placements being overhauled to a new and more user-friendly format. A Strategic Advisory Group was also formed to share expert insight and advice for the future of APPEL.
The APPEL Network (APPEL’s webinar series) continued in 2022 with 4 new topics being covered – communication skills, a PRE information session, using your APPEL experience for CPD, and effective leadership. APPEL welcomed experts from the field to deliver webinars on these topics that were of particular interest to our Trainers. You can watch recordings of these webinars and more on the APPEL website here.
In October, the Future of pharmacIE event returned for its second year. This online careers event was a great success in 2021 and it was even bigger and better in 2022. Nearly 400 pharmacy students and professionals signed up to the event where they could build their professional network,


connect with peers, and learn more about different career opportunities in pharmacy. The event included a panel discussion with pharmacists in a diverse range of practice areas and welcomed back past students who were now supervising placements as registered pharmacists. APPEL was also honoured to welcome Ms. Emer Cooke (Executive Director of the EMA) to give a keynote speech on her career to date and take part in a discussion with Director and National Coordinator of APPEL, Ms. Joanne Kissane.
As the year draws to a close, we are so grateful to all our stakeholders for their ongoing engagement and support. We look forward to working together to help pharmacy students on their journey to practice in 2023!
If you would like to learn more about the APPEL programme, please check out our website (www.appel.ie) or contact us at ops@appel.ie.
Screenshot
1st panel discussion – Main image Ruth McCarthy (UCC Practice Educator for APPEL), from top; Leonie Clarke (IMVO), Fergus Nugent (Mater Private Cork), Rory O’Donnell (TotalHealth Group), Fionnual King (AHDMP), Noor Bajalan (Mater Private Dublin), Tomás Conefrey (Conefrey’s Careplus Pharmacy).
“This year saw more students than ever completing experiential learning placements at different stages of their integrated M. Pharm programme”Written APPEL would like to thank the pharmacy profession for their continued support of our work in 2022.

Eklira® Genuair® 322 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and a green dosage button. Each delivered dose contains 375 µg aclidinium bromide equivalent to 322 µg of aclidinium. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 322 micrograms aclidinium twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents.
























Contraindications: Hypersensitivity to aclidinium bromide or to any of the excipients. Warnings and Precautions: Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Use with caution in patients with symptomatic prostatic hyperplasia or bladder-neck obstruction or with narrow-angle glaucoma. Do not use in patients with rare hereditary problems of galactose intolerance, total lactose de ciency or glucose-galactose malabsorption.


Interactions: Do not administer with other anticholinergic-containing medicinal products. No other interactions expected. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Risk to newborns/infants cannot be excluded. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Side-e ects: Common (1-10%): Sinusitis, nasopharyngitis, headache, cough, diarrhoea, nausea. Uncommon (0.1-1%): Dizziness, blurred vision, tachycardia, palpitations, dysphonia, dry mouth, stomatitis, rash, pruritus, urinary retention. Rare (0.01-0.1%): hypersensitivity. Not known: angioedema, anaphylactic reaction. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/12/778/002 Marketing
Maintenance bronchodilator
symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation
Recommended dose is one inhalation of 340 µg/12 µg
Patients should be instructed on how
administer
correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and Precautions: Do not use in asthma. Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Discontinue if increases in pulse rate, blood pressure or changes in ECG occur. Use with caution in patients with a history of or known prolongation of the QTc interval or treated with products a ecting the QTc interval. Use with caution in patients with severe cardiovascular disorders, convulsive disorders, thyrotoxicosis and phaeochromocytoma. Hypokalaemia may occur, is usually transient and supplementation not needed. In patients with severe COPD, hypokalaemia may be potentiated by hypoxia and concomitant treatment. Use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Do not use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase de ciency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic and/or long-acting β2-adrenergic agonist containing medicinal products. Caution in use with methylxanthine derivatives, steroids, non-potassium-sparing diuretics, β-adrenergic blockers or medicinal products known to prolong the QTc interval. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Sidee ects: Common (1-10%): Nasopharyngitis, urinary tract infection, sinusitis tooth abscess, insomnia, anxiety, headache, dizziness, tremor, cough, diarrhoea, nausea, dry mouth, myalgia, muscle spasms, peripheral oedema, increased blood creatine phosphokinase. Uncommon (0.1- 1%): Hypokalaemia, hyperglycaemia, agitation, dysgeusia, blurred vision, tachycardia, electrocardiogram QTc prolonged, palpitations, angina pectoris, dysphonia, throat irritation, stomatitis, rash, pruritus, urinary retention, increased blood pressure. Rare (0.01-0.1%): Hypersensitivity, bronchospasm, including paradoxical. Not known: anaphylactic reaction, angioedema. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/14/963/001 Marketing Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: October 2019
David Delaney, Head of Policy and Market Access for Europe with Viatris and Chair of the EU Affairs Committee
across the island of Ireland, and throughout the EU.
“There are challenges upcoming to patients’ access to medicines in Northern Ireland and beyond. Time is running out to fix this. Thankfully, the European Parliament will shortly consider legislative changes to help the situation. But, we haven’t seen the proposals yet. The UK and Irish Governments and their regulators have worked tirelessly on related solutions. Let’s redouble all our efforts.
associated with increased usage of generic medicines.
Chairperson for Medicines for Ireland, Padraic O’Brien said “Every time a generic medicine is dispensed, it represents a cost saving to the State and an opportunity for additional funding to be channelled to other areas of the healthcare system. Now more than ever a focus on quality and value is crucial for the State and the patients who rely on timely access to affordable medicines.”
It has been a busy year for the team at Medicines for Ireland as the representative body has worked to safeguard access to medicines, advancing medicines repurposing, ensured uninterrupted supply of medicine between Ireland and the UK and carried out research into the generics market.
We spoke to David Delaney, Head of Policy and Market Access for Europe with Viatris and Chair of the EU Affairs Committee, Medicines for Ireland about the key challenges with regards to medicines access and equality.

David leads advocacy at the European Parliament and Trade Committees for MFI and as a Board member of Medicines for Europe. David helps manage the MFI relationship with Ireland’s Department of Health, other Government Departments, regulators and media on key industry related matters.
Towards the end of last year, David was an invited live speaker at Ireland’s National Parliament Seanad (Senate) Special Committee on the Withdrawal of the UK from the EU.
The industry group has warned that imminent regulatory requirements threaten medicine supplies from Britain to Northern Ireland.
The Brexit agreement for Northern Ireland, designed to avoid a hard
border on the island of Ireland, leaves Northern Ireland within the EU’s regulatory system for pharmaceuticals.
The Northern Ireland Protocol states that the “marketing authorisation holder” – the company or other legal entity authorised to market a product –for any medicines sold in Northern Ireland must be in the EU, the European Economic Area or Northern Ireland but not in Britain.
It is this requirement which is forcing medicines manufacturers to invest in reorganising regulatory filings, packaging and licences on bespoke boxes of medicines for Northern Ireland to service a market in European teams that would be like making special arrangements in the Republic for medicines being sold in Mayo.
David commented, “Before the power cut issues in Dublin today impacting Parliament, we planned to focus on urgently needed solutions to ensure stable patient access to medicines in Northern Ireland and beyond.
“Many thanks to the Committee Cathaoirleach (Chairperson) Senator Lisa Chambers for the invitation to speak and at the new date in October, to express the views and suggested solutions of Medicines for Ireland and Medicines for Europe who represent companies who supply the majority of medicines daily
“With many political representatives engaging with the Committee from Northern Ireland, EU and the U.S. such as Congressman Richard Neal and EU Commissioner Mairead McGuinness, the expert analysis of solutions and issues for the island of Ireland is a great service and legacy from this Committee. We look forward to playing our role too.”
In May of this year, David met with the UK Department of Health and Social Care’s Noah Thorold. David met with Noah in London representing Medicines for Europe – whose member companies make and research & develop the majority of medicines used daily in Northern Ireland and across Europe.
“Stakeholders continue work to ensure continued stable patient access to medicines in Northern Ireland, in the context of the Northern Ireland Protocol and beyond,” he said.
The following month, in June, MFI reported that generic medicines now account for 57% of the volume of the total prescription market in Ireland. According to findings commissioned by MFI and compiled by IQVIA, Over the past ten years the volume of generic medicines across Ireland has risen by 24%, this is due to effective Government policies and legislative changes regarding procurement and supply, as well as consistent activity for MFI members to promote the advantages
“As an organisation MFI are proud that our membership will supply over 60 million packs of prescription medicines to Irish patients this year. This equates to an average of 2 packs of medicines every second.”
They also published a new report new report focused on the benefits of using VAMs in an EU context. ‘Value Added Medicines: Advancing Medicine Repurposing in the EU’ builds on the 2021 published report ‘Discussion Document on the Contribution of Value Added Medicines (VAMs) in Ireland’. It has been produced in conjunction with sister organisation, Medicines for Europe.
Value Added Medicines (VAMs) are medicines based on known molecules that address healthcare needs and deliver relevant improvements for patients and healthcare professionals. VAMs are key drivers for access to medicines and are increasing patient quality of life for chronic diseases, while offering significant benefits to the healthcare community.
VAMs offer a wide range of benefits from ensuring better adherence and compliance, to keeping healthcare costs down by reducing the need for patients to be moved to expensive next line therapies. The importance of using VAMs to address unmet medical needs in a timely and cost-effective manner has been highlighted during the Covid 19 pandemic.
It has been a busy year for the team at Medicines for Ireland as the representative body has worked to safeguard access to medicines, advancing medicines repurposing, ensured uninterrupted supply of medicine between Ireland and the UK and carried out research into the generics market.


On the evening of Friday 21 January Taoiseach Micheál Martin stood on the steps of Government buildings, as he had many times before, to make an announcement regarding COVID-19 regulations. This time to the nation’s collective relief it was to confirm the removal of the majority of the restrictions that had dictated our daily lives for much of the previous two years.
Written by Dermot Twomey, President, Irish Pharmacy Union
We also welcome the moves towards electronic prescribing; while the limitations of Healthmail are well established, so too is its potential. 2023 should be a year where these COVID-19 lessons, many of them positive, are learned to improve how care is delivered in our communities.
For pharmacists too it brought collective relief, providing our sector with a welcome return to ‘patient care as normal.’ Community pharmacists across the length and breadth of Ireland went beyond for their patients over the previous two years. We kept our doors open, provided deliveries and worked hard to fulfil our duty of getting medicines to those who needed them.
2022 therefore can be characterised as a year in which we returned to providing the full range of patient care services that our sector is so adept at delivering. Although there was one vital pandemic function, administering COVID-19 vaccinations, that has continued throughout the year.
In the first ten months of 2022 pharmacies have administered no less than 520,000 COVID-19 vaccines. The role of pharmacy at the centre and forefront of developing community immunity through vaccinations is now here to stay. It is our hope that we build upon this in future years and that the expertise of pharmacists is utilised to provide communities with vaccines for other conditions such as monkeypox and HPV as well as routine travel vaccines.
There were many other changes, often unseen by patients, brought in during COVID-19 that we hope can be learned from. Emergency measures such as allowing 9-month emergency supply of CD medicines have been hugely successful. Where a pharmacist has determined in conjunction with a patient that it is appropriate, there is no reason to discontinue this practice.
A major milestone was reached in 2022 with the implementation of free contraception for young women and its planned expansion announced in the recent Budget. Removing the barrier of cost for those requiring contraception is extremely welcome and pharmacists are proud to be playing a central role in the dispensing of contraception. However, we continue to call for pharmacists to be able to supply oral contraception without a prescription directly from a pharmacy to increase the accessibility of these safe medicines for patients.
Pharmacists are experienced in providing contraceptive care services through emergency hormonal contraception. Oral contraception is freely accessible in pharmacies, over the counter, in over 100 countries around the world. The WHO is supportive of pharmacists supplying contraception without a prescription. With GP waiting lists growing this is a needless bottleneck for women who should be given the choice of where to access their contraception.
A standout achievement of 2022 for community pharmacies is the launch of Safe Pharmacy. This new programme offers any person who is experiencing domestic abuse or coercive control a safe and secure location to seek support. Launched in July the initiative now has over 1,000 participating pharmacies. The designated Safe Pharmacies have trained staff and will provide access to a phone in a private consultation room and contact details for local support services.
Pharmacies have always been an important part of their community, and now with Safe Pharmacy, they can play a crucial role in supporting the safety and wellbeing of some of the most vulnerable in our society. 2023 will be the European Year of Skills – which is apt when you consider the most pressing priorities the pharmacy sector will face next year. The biggest concern is a growing shortage of pharmacists working in the sector. The impact of this shortage is increasingly apparent with research by the IPU showing it is now taking an average of five months to fill a pharmacy position.
We need a concerted set of actions by the government to address the chronic lack of university places for pharmacists in this country. Immediate steps also need to be taken to make it easier for non-EU pharmacists to work in Ireland. There is also much that could be done in tackling red tape that adds nothing to patient care but reduces efficiency and brings frustrations that in turn impacts retention. Also, linked to skills is the everpresent desire of pharmacists across the country to have their unique skillset utilised to the greatest effect. Community pharmacy is the standout success story of Irish healthcare. It is convenient, easy to access and affordable. Over half the population lives within one km of a pharmacy, with 85% living within five km. As a result, with 1.5 million visits to pharmacies each week, they are the most accessed part of our health service. Yet pharmacies are ready willing and waiting to do more for our patients.
Critical to maintaining our high level of services is the delivery of a fair fee for the items we dispense. Pharmacies are doing a lot more work for a lot less income while experiencing dramatically increasing costs. On average the current fee for dispensing a medicine is ¤4.74 per item. It was ¤6.00 an item in 2009. What we are proposing is a flat fee of ¤6.50 per item. The role of pharmacies as a key part of community primary care health must be recognised by government through the
provision of a fair dispensing fee for pharmacists.
The New Year marks the effective halfway point of this government’s term in office. The Programme for Government makes clear commitments to expanding the role of pharmacists and it is my profound hope that 2023 is the year in which meaningful action is taken to achieve this.
If properly resourced there is a range of new clinical services which we believe should be introduced by pharmacies and could be implemented quickly which would be a great support to the national health service. A key priority amongst these is the development and roll-out of a national Community Pharmacy-based Triage Programme including a Minor Ailment Scheme, use of Emergency Medicines, and Minor Injuries.
There is huge potential in community pharmacy and much more the sector can do to deliver on the Sláintecare vision of one universal health service for all, providing the right care, in the right place at the right time. Despite this community pharmacy is often not recognised in policy decisions or in service developments and there is currently no vision for the future of pharmaceutical care for patients in Ireland. What we urgently require is the immediate recruitment of a Chief Pharmaceutical Officer (CPO) who would be tasked with developing a new vision to realise the full potential of the community pharmacy sector, which could deliver meaningful and rapid change to benefit patients.
Pharmacy has done so much to support our communities and deliver excellent care throughout the pandemic and throughout 2022. I am so immensely proud to work as part of this truly unique sector and as President of the Irish Pharmacy Union immensely proud of the dedication shown by each and every one of my colleagues. I look forward to 2023 with optimism that the huge potential of our sector can be utilised and that our position at the centre of providing excellent patient care in the heart of Irish communities will be enhanced long into the future.



Written by Dr David Crosby, Consultant Obstetrician & Gynaecologist, Subspecialist in Reproductive Medicine, Surgery & Genetics, Clinical Lead Fertility Hub, Head of Department of Reproductive Medicine, The National Maternity Hospital, Dublin, Assistant Clinical Professor, University College Dublin
Dr Andrew Downey, Clinical Research Fellow and Specialist Registrar, The National Maternity Hospital & Merrion Fertility Clinic, Dublin

Ms. Michelle Barry, Clinical Nurse Specialist, Fertility Hub, The National Maternity Hospital, Dublin
Ms Catherine Dunne, Administrative Lead, Fertility Hub, The National Maternity Hospital, Dublin
Dr Sorca O’Brien, Clinical Aspire Fellow in Fertility, The National Maternity Hospital and Merrion Fertility Clinic
week and avoiding episodes of intoxication can improve fertility. Those who smoke should be informed that this is likely to reduce their fertility. In addition, passive smoking is likely to affect their chance of conceiving. There is currently no consistent evidence of an association between consumption of caffeinated beverages and fertility problems.
based practice to assist patients to navigate their fertility journey.
Our hub accepts direct consultant and GP referrals from within the Ireland East Hospital Group for patients who meet the following criteria as defined by NWIHP:
Infertility is the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person’s capacity to reproduce either as an individual or with his or her partner. The World Health Organisation (WHO) has characterised infertility as a disease affecting 48 million couples and 186 million individuals globally. Over 80% of couples in the general population will conceive within one year if the woman is aged under 40 years. Of those who do not conceive in the first year, about half will do so in the second year (cumulative pregnancy rate over 90%).
The Health Service Executive (HSE) has estimated that one in six heterosexual couples will experience infertility in Ireland.
The causes of infertility can be due to problems with the female and/ or male reproductive systems. Female causes can relate to abnormal ovulation (polycystic ovarian syndrome, premature ovarian failure, hypothalamic or pituitary disease), fallopian tube damage (pelvic surgery or infection) and uterine disorders (endometriosis, polyps or fibroids, congenital disease). Male causes can relate to reproductive tract
obstruction (trauma, infection), testicular failure (chemotherapy), genetic disorders (cystic fibrosis), hormonal disorders (hypothalamic, pituitary) and medications (steroid use). In approximately one quarter of all cases, the cause is unknown or ‘unexplained’.
It is important that people who are concerned about their fertility know that both female and male fertility decline with age. Couples should be informed that they should have sexual intercourse every two to three days to optimise the chance of pregnancy. Lifestyle factors may negatively impact fertility and should be optimised for anyone trying or planning to try to conceive prior to any investigations or treatment.
Drinking no more than one to two units of alcohol once or twice per
Time to conception can be delayed in people with a body mass index (BMI) of 30kg/m2 or over. Group programmes involving exercise and diet are the most optimal ways to achieve weight loss which may also improve ovulation in women.
Women intending to become pregnant should be informed that dietary supplementation with folic acid before conception and up to 12 weeks gestation reduces the risk of having a baby with neural tube defects. Some patients require 5mg folic acid as they are higher risk (e.g epilepsy or BMI ≥30kg/m2). Women should ensure their cervical cancer screening is up to date, that they have been vaccinated against rubella if they are susceptible and that sexually transmitted disease is performed if applicable.
Regional fertility hubs within the six hospital groups are being rolled out across Ireland. These hubs allow for investigation and management of a large proportion of fertility patients.
The National Maternity Hospital is delighted to announce the launch of its new Ireland East Fertility Hub supported by funding from the National Women and Infants Health Programme (NWIHP). Our vision is to continually strive to provide high quality care that places our patients at the centre of their management. Our approach is to promote a culture of evidence
If they have been unable to achieve a pregnancy after 6 months of regular unprotected sexual intercourse and are 36 years of age and older
If they have been unable to achieve a pregnancy after 12 months of regular unprotected sexual intercourse and are under 36 years of age
If there is a known clinical cause of infertility or a history of predisposing factors for infertility i.e. endometriosis, previous fertility treatment to conceive, not having periods or very few periods per year, pelvic inflammatory disease or undescended testes.
Investigations prior to referral can be extremely helpful in the early identification of fertility problems. Women with regular monthly menstrual cycles should be informed that they are likely to be ovulating. Women should be offered a blood test to measure serum progesterone in the midluteal phase of their cycle (day 21 of a 28-day cycle) to confirm ovulation. Women with irregular menstrual cycles should be offered a blood tests to measure serum gonadotrophins (Follicular stimulating hormone (FSH) and Luteinising Hormone (LH)). If polycystic ovarian syndrome (PCOS) is suspected, a hormone profile including FSH and LH, testosterone, sex hormone binding globulin, and free testosterone index levels should be assessed. Women should have thyroid function tests performed and optimised if there is
“The National Maternity Hospital is delighted to announce the launch of its new Ireland East Fertility Hub supported by funding from the National Women and Infants Health Programme (NWIHP)”Dr David Crosby
Colours are important, they can signal what’s going on.

With the colour range indicator, it is easy to see if you are below, within or above your range.

The meter provides Insights, Guidance and cheers you on, straight from the meter screen.

OneTouch Verio Reflect® meter: inspired by nature.
Google Play and Google Play logo are trademarks of Google LLC.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth SIG. Inc,
Other trademarks and trade names are those of their respective owners.
© 2021-2022 LifeScan IP Holdings, LLC. GL-VRF-2100078 (22-004)
presence of subclinical or clinical thyroid dysfunction.
Once a referral has been accepted, a fertility hub pack is posted to the patient/s for their completion and following completion of further investigations, an initial consultation is offered to ensure that all the necessary investigations have been completed and to advise on an individualised management plan. The range of services provided by our hub include investigative support to GPs (semen analysis and pelvic ultrasound), and secondary level investigations including testing of tubal patency, hysteroscopy, laparoscopy, fertility related surgeries, ovulation induction and follicle tracking.
There are a number of quantitative measures that can be performed as an assessment of ovarian reserve. Serum Anti-Müllerian hormone (AMH) testing may identify women with a lower ovarian reserve than expected for age. These women can be provided with fertility management options in a timely manner. AMH testing can also be a predictor for ovarian response in in vitro fertilization (IVF). AMH testing can be offered through GP practices or through our hub. Another measure of ovarian reserve is the antral follicle count
(AFC), which can be assessed by transvaginal ultrasound. Transvaginal scanning can also assess the endometrium for the presence of polyps or fibroids.
Fallopian tubal patency can be checked by hysterosalpingography (HSG: pelvic x-ray with dye injected through cervix) or hysterosalpingo-Contrast Sonography (HyCoSy: pelvic ultrasound with dye injected through cervix). If there is a suspicion of tubal damage (endometriosis or pelvic inflammatory disease), a diagnostic laparoscopy and dye test can be performed as it allows for a both diagnosis and treatment. A diagnostic or operative hysteroscopy can be performed if endometrial pathology, such as fibroids and polyps, is suspected. These services are provided on site at the National Maternity Hospital.
Men experiencing fertility problems should have a semen analysis performed assessing the sperm concentration, count, morphology, motility, and volume as defined by the WHO. Repeat samples or further investigation and work up may be required if the first result is abnormal.

Ovulation induction with oral and injectable ovulation agents and
follicle tracking is offered through our service to patients with suspected anovulation. Letrozole, an aromatase inhibitor, stops oestrogen synthesis resulting in increased ovarian stimulation via the pituitary gland. Other second line oral medications that are used include Clomid and Tamoxifen. Injectable ovulation induction agents, including FSH, LH and human menopausal gonadotropin (hMG) are available. It is necessary that ultrasound follicle tracking is performed to monitor follicle growth, ensure that the response is optimal and to significantly reduce the risk of multiple pregnancy. Metformin can be used in anovulatory women with PCOS as an adjunctive therapy, with evidence for women with good evidence of restoration of ovulation in women, particularly with BMI of 30kg/m2 or over. If a male sperm factor is identified, adverse lifestyle measures should be addressed and treatment of the underlying disorder may be required.
Some patients may need to be referred for further treatment with assisted reproduction such as Intrauterine Insemination (IUI), In Vitro Fertilisation (IVF), or Intracytoplasmic Sperm Injection (ICSI). IUI involves a semen sample being produced and then placed into a woman’s uterus
around the time of ovulation after laboratory preparation. In IVF, the eggs are removed from the ovary before ovulation and fertilised with sperm in a laboratory with the embryo transferred to the uterus a few days later. ICSI is a specialised form of IVF where a single sperm is directly injected into the egg to allow fertilisation. These treatments require referral to a specialist fertility clinic after initial workup and management in the regional fertility hubs. We are continuously auditing these referral rates to ensure that we provide NWIHP with the most up to date information to facilitate planning for publicly funded assisted reproduction.
Infertility is a common disorder that affects many individuals and couples. The newly established regional fertility hubs provide evidence based fertility work up and management. Through the National Maternity Hospital fertility hub and with its network of regional hubs, we will continue to advocate for the equitable evolution of comprehensive public fertility services, including IVF, in Ireland in the future.
For more information visit https://www.nmh.ie/
CCF: 24971 Date of preparation: (09-22)
Product Name: Emazole Control 20 mg Gastro-Resistant Tablets

Composition: Each tablet contains 20 mg esomeprazole (as magnesium dihydrate).
Description: Light pink oval lm coated tablet.
Indication(s): Proton Pump Inhibitor (PPI): Short-term treatment of re ux symptoms (e.g. heartburn and acid regurgitation) in adults. or crush. Disperse in half a glass of non-carbonated water if di culty in swallowing. Stir until tablets disintegrate, drink liquid with pellets immediately or within 15 min, or administer through a gastric tube. Do not chew or crush pellets. Adults: The recommended dose is 20 mg esomeprazole (one tablet) per day. achieve improvement of symptoms. Duration of treatment is up to 2 weeks. Once complete relief of symptoms has occurred, treatment should be discontinued. If no symptom relief is obtained within 2 weeks of continuous treatment, the patient should be instructed to consult a doctor. recommended. No relevant use in this group in the indication: “short-term treatment of re ux symptoms (e.g., heartburn and acid regurgitation)”. Severe impaired renal function: Caution. Severe liver impairment: 20 mg max daily dose.

Contraindications: Hypersensitivity to esomeprazole, substituted benzimidazoles or any of the excipients. Not with nel navir. Contact a physician if symptoms change in character. In the presence of any alarm symptom (e.g. signi cant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with esomeprazole may alleviate symptoms and delay diagnosis. Treatment with proton pump inhibitors (PPIs) may lead to a slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter and in hospitalised patients, also possibly Clostridium di cile. Patients should consult their doctor before taking this medicinal product if they are due to have an endoscopy or urea breath test. Absorption of vitamin B12 may be reduced due to hypo- or achlorhydria. Not recommended for long-term use as the following may also occur: Hypomagnesaemia; Risk of fracture. Consider stopping Emazole Control in cases of Subacute cutaneous lupus erythematosus (SCLE) accompanied by arthralgia. Interference with laboratory tests: Increased Chromogranin A (CgA) level may interfere with investigations for neuroendo crine tumours. To avoid this interference, Emazole Control treatment should be stopped for at least 5 days before CgA measurements. If CgA and gastrin levels have not returned to reference range after initial measurement, measurements should be repeated 14 days after cessation of PPI treatment. Contains glucose and sucrose. Interactions: E ect of esomeprazole on other drugs: Co-administration with atazanavir is not recommended. If the combination of atazanavir with a PPI is judged unavoidable, close clinical monitoring is recommended in combination with an increase in the dose of atazanavir to 400 mg with 100 mg of ritonavir; esomeprazole 20 mg should not be exceeded. Esomeprazole is a CYP2C19 inhibitor. When starting or ending treatment with esomeprazole, the potential for interactions with drugs metabolised through CYP2C19 should be considered. Serum levels of cilostazol, cisapride, tacrolimus, methotrexate may be increased. An interaction is observed between clopidogrel and esomeprazole, but the clinical relevance is uncertain. As a precaution, concomitant use of esomeprazole and clopidogrel should be discouraged. Gastric acid suppression by PPIs increase or decrease absorption of drugs with pH dependent absorption (decreased absorption of ketoconazole, itraconazole); esomeprazole inhibits CYP2C19 metabolising enzyme and could increase plasma concentrations of diazepam, citalopram, imipramine, clomipramine, phenytoin (monitor plasma levels of phenytoin), etc. resulting in need of a dose reduction; monitor INR when given with warfarin or similar. Caution as absorption of digoxin can increase. E ect of other drugs on esomeprazole: CYP2C19 and CYP3A4 inhibitors (clarithromycin, voriconazole) may increase the esomeprazole exposure. Dose adjustment not regularly required, except in severe hepatic impairment and long-term use. CYP2C19 and/or CYP3A4 inducers (rifampicin and St. John’s wort) may lead to decreased esomeprazole serum levels by increasing the esomeprazole metabolism.
Pregnancy and Lactation: Caution in pregnancy due to lack of clinical data. No studies in lactating women, therefore, not recommended during breast-feeding. Ability to Drive and Use Machinery: Minor in uence on the ability to drive or use machines. Adverse reactions such as dizziness (uncommon) and blurred vision (rare) have been reported. If a ected, patients should not drive or use machines. Undesirable E ects: Common: Headache, abdominal pain, constipation, diarrhoea, atulence, nausea/vomiting, fundic gland polyps (benign). Uncommon: Peripheral oedema, insomnia, dizziness, paraesthesia, somnolence, vertigo, dry mouth, increased liver enzymes, dermatitis, pruritis, rash, urticaria, fracture of the hip, wrist or spine. For other side e ects refer to the SPC.
Marketing Authorisation Holder: IQ Pharmatek Ltd., Gurtna eur, Old Waterford Road, Clonmel, Co. Tipperary. Marketing Authorisation Number: PA 22777/001/001. Further information and SPC are available from: Rowex Ltd, Bantry, Co. Cork. Freephone: 1800 304 400 Fax: 027 50417. E-mail: rowex@rowa-pharma.ie
Legal Category: Not subject to medical prescription.
Date of Preparation: September 2019
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by emailing medsafety@hpra.ie or by emailing Rowex pv@rowa-pharma.ie
p y g g p

Each quarter, Fitzgerald Power publishes our Pharmacy Pulse report, an analysis of the key trends in the Irish Community Pharmacy sector. In it, we discuss the current forces shaping the market, such as medicine shortages and labour cost inflation. In our 2022 Q1 edition, we noted strong community pharmacy item performance, as the prescription market grew in unit terms by 4.65%, according to HMR Ireland. According to IQVIA
operators The locum market is making it very difficult to recruit permanent staff as locum pharmacists can earn more in locuming three days per week than they can in full time employment Some of the hourly locum rates being paid are eye watering and completely unsustainable. For this reason, the profile of vendors (pharmacists disposing of pharmacies) is getting younger and younger Labour resourcing and the impact on owner operator work life balance is the number one reason that pharmacists are considering selling their businesses This shortage of pharmacists comes at a time when the demands on pharmacies have never been higher Describing the shortages as “chronic,” IPU President Mr Dermot Twomey recently said: “If we can’t increase the supply of qualified pharmacists, the sector will be unable to keep up with demand and patient care will suffer Pharmacies are striving to maintain services to patients and the public, but reduced hours and temporary closures cannot be ruled out ”
Each quarter, Fitzgerald Power publishes our Pharmacy Pulse report, an analysis of the key trends in the Irish Community Pharmacy sector. In it, we discuss the current forces shaping the market, such as medicine shortages and labour cost inflation. In our 2022 Q1 edition, we noted strong community pharmacy item performance, as the prescription market grew in unit terms by 4.65%, according to HMR Ireland. According to IQVIA the top five major OTC classes by value were; respiratory, pain relief, digestive, VMS and tonics & skin treatment. Similarly, our Q2 edition reported that the prescription market continued to grow, expanding in unit terms by 3.92%, again according to HMR Ireland. The top five major OTC classes remained largely unchanged in the second three months of the year.
market right now
At the time of writing this article our Q3 Pharmacy Pulse report was being drafted, however, based on PSI data, we estimate the following opening and closures so far this year:
Similarly, our Q2 edition reported that the prescription market continued to grow, expanding in unit terms by 3.92%, again according to HMR Ireland. The top five major OTC classes remained largely unchanged in the second three months of the year. key trends in the Irish Community Pharmacy sector.
At the time of writing this article our Q3 Pharmacy Pulse report was being drafted, however based on PSI data, we estimate the following opening and closures so far this year:
This is an interesting development as the number of pharmacies has always tended to grow yearon-year since deregulation. Many of the pharmacies that have closed are greenfield units, i.e. new pharmacies that have opened in the last 5 years.
Source: PSI (note: this data runs from 1st December 2021 due to the available PSI data)
Drug shortages have given rise to real fear among both medical professionals and patients alike Medicine shortages and reduced availability represent an increasing issue across the EU and the globe, amplified by the pandemic It may have a significant impact on patient care by causing medicine rationing and the delay of critical treatments Due to medicine shortages, patients may need to use less effective alternatives and they could risk using medication incorrectly Just last month, Kathy Maher, Chair of the PCC Committee of the Irish Pharmacy Union (IPU) told Newstalk Breakfast that one pharmacist could not give a palliative care patient their medicine in solid form due to a lack of supplies This is creating huge pressure on pharmacists who are managing patient concerns at the community level. Often patients don’t understand the vagaries of the medicine supply chain system and sometimes pharmacists are wrongly blamed for not having the medicine in stock The EMA has published a guide for patients and healthcare professionals’ organisations with key principles and examples of good practices to support them in preventing and managing shortages of medicines While a secondary concern to patient safety, shortages in the medicine supply chain is having an impact on pharmacy procurement margins We are starting to see the full impact of drug price reductions in the State reimbursed market. Given the size of our population, our peripheral geographical location on the edge of Europe and our drug pricing structure, Ireland is a less compelling market for medicine manufacturers than some of our European neighbors
This is an interesting development as the number of pharmacies has always tended to grow year-on-year since deregulation. Many of the pharmacies that have closed are greenfield units, i.e. new pharmacies that have opened in the last 5 years.
As part of a piece of research we recently carried out for the IPU we analysed PSI and PCRS data to identify the types of pharmacies that had closed. Of the 38 voluntary cancellations noted by the PSI during 2019 and 2020, we were able to identify 76.3% (29 units) of these pharmacies on the PCRS payments to pharmacies list (we focused on 2019 and 2020 as the published PCRS data only ran to 2020 at the time of our research). We estimated more than 50% of these pharmacies (15 units) were greenfield sites, with most closing within 5 years of opening.
Pharmacists were our first clients and our founder’s family business
This suggests the following: it is difficult for new pharmacies to reach profitability and unviable pharmacies have a life cycle of at least 3 – 5 years before they are closed.
Pharmacy is more than a sector to us it’s our DNA
The de-regulated nature of the Irish pharmacy market encourages bullish entrepreneurs to take risks and open new units. The evidence suggests that there is often little commercial rationale for these new units, particularly since the advent of Healthmail. Where they do survive, they merely capture a portion of the local market putting increased pressure on incumbents and in some cases forcing their closure.
Today we are the leading financial advisor to the Irish community pharmacy sector and the leading pharmacy sales brokerage firm in Ireland.
Our exclusive pharmacy team are not just financial experts in this market but are passionate advocates and creative thought leaders helping to drive the sector forward
As part of a piece of research we recently carried out for the IPU we analysed PSI and PCRS data to identify the types of pharmacies that had closed. Of the 38 voluntary cancellations noted by the PSI during 2019 and 2020, we were able to identify 76.3% (29 units) of these pharmacies on the PCRS payments to pharmacies list (we focused on 2019 and 2020 as the published PCRS data only ran to 2020 at the time of our research). We estimated more than 50% of these pharmacies (15 units) were greenfield sites, with most closing within 5 years of opening. very
The biggest issue in the market right now
We noted the following Transaction activity (pharmacies
We noted the following transaction activity (pharmacies bought and sold) in the 9 months to 30th September 2022:
30th September
needs, wants and desires of those working in the sector to understand what can be done to make things better



Each quarter, Fitzgerald Power publishes our Pharmacy Pulse report, an analysis of the key trends in the Irish Community Pharmacy sector. In it, we discuss the current forces shaping the market, such as medicine shortages and labour cost inflation
A survey conducted by the Irish Pharmacy Union (IPU) has shown that Irish pharmacies are suffering and even considering closure due to a chronic lack of qualified pharmacists available to work in community pharmacies. This, together with ongoing absences due to COVID-19, is resulting in some pharmacies being required to reduce hours, or in some cases close for certain days as they struggle to cope with staff shortages. This is affecting hundreds of pharmacies across the country, creating huge pressure and stress for owner-operators. The locum market is making it very difficult to recruit permanent staff as locum pharmacists can earn more in locuming three days per week than they can in full-time employment. Some of the hourly locum rates being paid are eye-watering and completely unsustainable. For this reason, the profile of vendors (pharmacists disposing of pharmacies) is getting younger and younger. Labour resourcing and the impact on owner operator work-life balance is the number one reason that pharmacists are considering selling their businesses. This shortage of pharmacists comes at a time when the demands on pharmacies have never been higher. Describing the shortages as “chronic,” IPU President Mr. Dermot Twomey recently said: “If we can’t increase the supply of qualified pharmacists, the sector will be unable to keep up with demand and patient care will suffer. Pharmacies are striving to maintain services to patients and the public, but reduced hours and temporary closures cannot be ruled out.”
In our 2022 Q1 edition, we noted strong community pharmacy item performance, as the prescription market grew in unit terms by 4 65%, according to HMR Ireland According to IQVIA the top five major OTC classes by value were; respiratory, pain relief, digestive, VMS and tonics & skin treatment
Similarly, our Q2 edition reported that the prescription market continued to grow, expanding in unit terms by 3 92%, again according to HMR Ireland The top five major OTC classes remained largely unchanged in the second three months of the year key trends in the Irish Community Pharmacy sector.
At the time of writing this article our Q3 Pharmacy Pulse report was being drafted, however based on PSI data, we estimate the following opening and closures so far this year:
New Openings 1st December 2021 1st October 2022
Closures between 1st December 2021 1st October 2022
Drug shortages have given rise to real fear among both medical professionals and patients alike. Medicine shortages and reduced availability represent an increasing issue across the EU and the globe, amplified by the pandemic. It may have a significant impact on patient care by causing medicine rationing and the delay of critical treatments. Due to medicine shortages, patients may need to use less effective alternatives and they could risk using medication incorrectly. Just last month, Kathy Maher, Chair of the PCC Committee of the Irish Pharmacy Union (IPU) told Newstalk Breakfast that one pharmacist could not give a palliative care patient their medicine in solid form due to a lack of supplies.
Net Closures between 1st December 2021 1st October 2022
Source: PSI (note: this data runs from 1st December 2021 due to the available PSI data)
12 14 (2)
This is creating huge pressure on pharmacists who are managing patient concerns at the community level. Often patients don’t understand the vagaries of the medicine supply chain system and sometimes pharmacists are wrongly blamed for not having the medicine in stock. The EMA has published a guide for patients and healthcare professionals’ organisations with key principles and examples of good practices to support them in preventing and managing shortages of medicines.
This is an interesting development as the number of pharmacies has always tended to grow year on year since deregulation. Many of the pharmacies that have closed are greenfield units, i e new pharmacies that have opened in the last 5 years
This analysis does not include the Uniphar / McCauley transaction, which is subject to Competition and Consumer Protection Commission approval, according to the Irish Times Transaction data for the first three quarters of 2022 is interesting. The vast majority (95.8%) of units sold were either independent or symbol group pharmacies and many of these were high volume businesses. The quality of the pharmacy stock either on the market or coming to the market remains very strong and valuations have performed well so far this year.
This analysis does not include the Uniphar / McCauley transaction, which is subject to Competition and Consumer Protection Commission approval, according to the Irish Times.
As part of a piece of research we recently carried out for the IPU we analysed PSI and PCRS data to identify the types of pharmacies that had closed Of the 38 voluntary cancellations noted by the PSI during 2019 and 2020, we were able to identify 76 3% (29 units) of these pharmacies on the PCRS payments to pharmacies list (we focused on 2019 and 2020 as the published PCRS data only ran to 2020 at the time of our research) We estimated more than 50% of these pharmacies (15 units) were greenfield sites, with most closing within 5 years of opening.
Transaction data for the first three quarters of 2022 is interesting. The vast majority (95.8%) of units sold were either independent or symbol group pharmacies and many of these were high volume businesses. The quality of the pharmacy stock either on the market or coming to the market remains very strong and valuations have performed well so far this year.
While a secondary concern to patient safety, shortages in the medicine supply chain is having an impact on pharmacy procurement margins. We are starting to see the full impact of drug price reductions in the State reimbursed market. Given the size of our population, our peripheral geographical location on the edge of Europe and our drug pricing structure, Ireland is a less compelling market for medicine manufacturers than some of our European neighbors.
Pharmacists were our first clients and our founder’s family business. Pharmacy is more than a sector to us – it’s our DNA. Today we are the leading financial advisor to the Irish community pharmacy sector and the leading pharmacy sales brokerage firm in Ireland. Our exclusive pharmacy team are not just financial experts in this market but are passionate advocates and creative thought leaders helping to drive the sector forward.
This suggests the following: it is difficult for new pharmacies to reach profitability and unviable pharmacies have a life cycle of at least 3 5 years before they are closed
The de regulated nature of the Irish pharmacy marke urs to take risks and open new units. The evidence sugges ercial rationale for these new units, particularly since the a do survive, they merely capture a portion of the local ma on incumbents and in some cases forcing their closure
A new click-and-collect locker service is to be offered to Irish pharmacies, allowing customers to pick up medicine prescriptions conveniently without having to queue.
Brought to you by OOHPod, Pharmacy Pickup lockers can be placed inside pharmacies or shopping centres and contain refrigerated, temperaturecontrolled compartments, allowing medicine to be stored at the optimal temperature.
Pharmacy Pickup, which is a partnership between Irish smart locker service OOHPod and Finnish pharmacy digitalisation firm Remomedi, says the lockers will reduce in-store queuing and waiting times for prescriptions.

The lockers will also provide a more efficient customer experience, the possibility of after-hours pickup and free up pharmacists and pharmacy staff’s time so they can perform more value-added tasks.
Customers will receive a notification when their order is ready for collection from the locker. They can then go to the locker location and collect their prescription in a matter of seconds, by typing in a code or scanning a QR code to open the locker.
OOHPod CEO John Tuohy said the lockers will provide more convenience for patients working or juggling family and other commitments, as they will be able to collect their medicines quickly and easily.
“There is the added benefit of knowing your prescription will be ready and you won’t have to wait in any line to collect it. The lockers allow for real-time management and monitoring of deliveries, communications and temperatures, with one easy-touse web view of all operations. All prescription collections are tracked and medicine that is not collected is flagged.”

Once a customer receives a notification saying their prescription is ready, they will have 5 days to pick it up. However this time frame can be customised, per unit, to suit the business needs of the pharmacy, according to Mr. Tuohy.
Kari Paukkeri, founder and CEO of Remomedi said, “We are delighted to work with Oohpod to commercialize our innovative parcel delivery technology for pharmacies and health care. Our solutions, developed with some of the leading Finnish and Scandinavian pharmacies and hospitals will enable delivery of medicines and other health products effectively, economically and with very little complexity to consumers”
The company is projecting to have 100 units across Ireland before the end of 2023.


SMA® Anti-Reflux For the dietary management of reflux and regurgitation


SMA LF® Lactose Free For babies with lactose intolerance
Did you know that feeding issues, such as colic, constipation, reflux & regurgitation occur in up to 55% of all infants in the first 6 months of life?1

SMA® Comfort For the dietary management of colic and constipation

SMA High Energy® For medically identified high-energy needs
REFERENCES: 1. Iacono G. et al. Gastrointestinal symptoms in infancy: a population-based prospective study. Dig Liv Dis 2005;37(6):432–438.
IMPORTANT NOTICE: The World Health Organisation (WHO) has recommended that pregnant women and new mothers be informed on the benefits and superiority of breastfeeding – in particular the fact that it provides the best nutrition and protection from illness for babies. Mothers should be given guidance on the preparation for, and maintenance of, lactation, with special emphasis on the importance of a well-balanced diet both during pregnancy and after delivery. Unnecessary introduction of partial bottle-feeding or other foods and drinks should be discouraged since it will have a negative effect on breastfeeding. Similarly, mothers should be warned of the difficulty of reversing a decision not to breastfeed. Before advising a mother to use an infant formula, she should be advised of the social and financial implications of her decision: for example, if a baby is exclusively bottle-fed, more than one can (400 g) per week will be needed, so the family circumstances and costs should be kept in mind. Mothers should be reminded that breast milk is not only the best, but also the most economical food for babies. If a decision to use an infant formula is taken, it is important to give instructions on correct preparation methods, emphasising that unboiled water, unsterilised bottles or incorrect dilution can all lead to illness. •SMA LF® is a lactose-free milk based formula for babies and young children who are intolerant to lactose or sucrose, or who are experiencing symptoms such as diarrhoea, tummy ache or wind caused by temporary lactose intolerance. It is suitable as the sole source of nutrition up to 6 months of age, and in conjunction with solid food up to 18 months of age. The following products must be used under medical supervision •SMA® Anti-Reflux is a special formula intended for the dietary management of bottle-fed babies when significant reflux (regurgitation) is a problem. It is suitable as the sole source of nutrition up to 6 months of age, and in conjunction with solid food up to 12 months of age. If the baby’s reflux does not improve within 2 weeks of starting SMA® Anti-Reflux, or if the baby fails to thrive, the family doctor should be consulted. •SMA High Energy® is a milk based formula for the dietary management of babies and young children with medically determined high energy requirements as identified by a healthcare professional. It is suitable as the sole source of nutrition up to 6 months of age, and in conjunction with solid food up to 18 months of age. SMA High Energy® is not intended for use with preterm babies, for whom fortified breast milk or a low birthweight formula such as SMA Gold Prem® 1 is more appropriate. •SMA® Comfort is a special formula intended for the dietary management of bottle-fed babies with colic and constipation. It is suitable as the sole source of nutrition up to 6 months of age, and in conjunction with solid food up to 12 months of age.
DENWHS016-2; NLNW031; DENWHSP122; DSHL003-1 ZRI912/05/21
In healthy adults, blood pressure can be maintained within the normal range throughout a range of positions. If the cardiovascular system and neural reflexes are unable to maintain blood pressure on standing from a seated or supine position, the resulting abnormal drop in blood pressure is called orthostatic hypotension (OH). The consensus definition for OH is a sustained drop in blood pressure of ≥20 mmHg in systolic blood pressure (SBP) or ≥10 mm Hg in diastolic blood pressure (DBP) within 3 minutes of standing up.
Professor Rose Anne Kenny, TILDAOH40 was independently associated with recurrent (RR = 1.30, 95% CI = 1.02,1.65), injurious (RR = 1.43, 95% CI = 1.13,1.79) and unexplained falls (RR = 1.55, 95% CI = 1.13,2.13). Sustained OH was associated with injurious (RR = 1.55, 95% CI = 1.18,2.05) and unexplained falls (RR = 1.63, 95% CI = 1.06,2.50).
Below we have summarised the main highlights of this study.
Orthostatic hypotension (OH) often co-exists with hypertension. As increasing age affects baroreflex sensitivity, it loses its ability to reduce blood pressure when lying down.
Therefore, supine hypertension may be an important indicator of baroreflex function.
This study examined (i) the association between OH and future falls in community-dwelling older adults and (ii) if these associations persist in those with co-existing OH and baseline hypertension, measured supine and seated.
OH and co-existing hypertension was associated with all falls outcomes but effect sizes were consistently larger with seated versus supine hypertension. OH, particularly when co-existing with hypertension, was independently associated with increased risk of future falls. Stronger effect sizes were observed with seated versus supine hypertension. This supports previous findings and highlights the importance of assessing orthostatic blood pressure behaviour in older adults at risk of falls and with hypertension.
Observed associations may reflect underlying comorbidities, reduced cerebral perfusion or presence of white matter hyperintensities.
Not surprisingly, OH is common in older adults, but the prevalence varies widely depending on the population and setting examined. Research has also shown significant heterogeneity in fall risk factors even in older adults, therefore it is important to understand how risk factors may interact or co-exist with each other. One of the most common comorbidities associated with OH is hypertension. This is often linked to severe autonomic dysfunction and reduced ability of the baroreflex to reduce blood pressure when lying down.
Professor Rose Anne Kenny, The Irish Longitude Study on Ageing

(TILDA) along with colleagues Dr Orna A. Donoghue, also from TILDA, Dr Matthew O’Connell, Department of Population Health Sciences, King’s College London and Dr Robert Bourke, Mercer’s Institute for Successful Ageing, St James’s Hospital Dublin, recently published a study which aimed to examine he association between OH and future occurrence of recurrent, injurious and unexplained falls and syncope over four year follow-up in communitydwelling adults aged 65 years and older. It also aimed to examine these associations in those with co-existing OH and baseline hypertension, measured in both supine and seated positions.
Data from 1500 communitydwelling adults aged ≥65 years from The Irish Longitudinal Study on Ageing (TILDA) were used. Continuous beat-to-beat blood pressure was measured using digital photoplethysmography during an active stand procedure with OH defined as a drop in systolic blood pressure (SBP) ≥20 mmHg and/or ≥10 mm Hg in diastolic blood pressure (DBP) within 3 minutes of standing. OH at 40 seconds (OH40) was used as a marker of impaired early stabilisation and OH sustained over the second minute (sustained OH) was used to indicate a more persistent deficit, similar to traditional OH definitions.
Seated and supine hypertension were defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg. Modified Poisson models were used to estimate relative risk of falls (recurrent, injurious, unexplained) and syncope occurring over four year follow-up.
In conclusion, the authors state, “OH, particularly when coexisting with hypertension, was independently associated with an increased risk of future falls, but not syncope, in community-dwelling adults aged 65 years and older.
“Stronger effect sizes were observed when hypertension was measured seated although effects were also observed with supine hypertension. There were also associations observed between OH40 and unexplained falls and between sustained OH and injurious falls in normotensives.
“The current analysis provides further support to highlight the importance of including a structured cardiovascular assessment within a multifactorial fall prevention assessment to reduce the risk of adverse outcomes associated with falls such as injury, fracture, reduced independence, premature nursing home admission and death.”
Is orthostatic hypotension and co-existing supine and seated hypertension associated with future falls in community-dwelling older adults?
“The current analysis provides further support to highlight the importance of including a structured cardiovascular assessment within a multifactorial fall prevention assessment to reduce the risk of adverse outcomes associated with falls such as injury, fracture, reduced independence, premature nursing home admission and death.”

Uniphar has launched its Unity for Hope challenge to raise funds for seven cancer charities across Ireland, the UK, the USA and Australia. The Unity for Hope challenge promotes fitness through fundraising by aiming to achieve 15,000,000 steps or wheel rotations for cyclists in one week. This year, the weeklong challenge ran from Monday 24th October to Sunday 30th October.
Uniphar CEO, Ger Rabbette, with television presenter, Anna Daly at the Uniphar Global Headquarters at Citywest Business Park
Ger hopes to beat last year’s target and encouraged everyone involved to “do whatever activity you can over next week and raise as much money as you can.”
As part of this challenge, there are exciting fitness activities including walking, running, cycling, swimming and yoga taking place across all Uniphar Group companies. Some Uniphar employees even participated in the Dublin Marathon in October.
The seven great charities to benefit from the Unity for Hope challenge include St. Francis Hospice Dublin, The Irish Cancer Society, Cancer Focus Northern Ireland, Teenage Cancer Trust, Childhood Cancer International, The American Cancer Society and Cancer Council Australia.

Speaking on the launch of the Unity for Hope challenge, Uniphar’s CEO, Ger Rabbette, said, “We’re really excited, for the third year now, to be running this Group-wide initiative, Unity for Hope, and this
great cancer charities. We have big targets to hit, and we want to encourage everyone to get out and be active next week. I want to thank everyone that was involved and wish them the very best in
The fundraising target of the challenge is ¤100,000 - ¤200,000 to be raised by employees, with Uniphar promising to match all funds raised up to a further
From its beginnings as an indigenous Irish company, established over 50 years ago by a small group of Irish pharmacists, Uniphar has been transformed into a diversified international healthcare services provider. Today, the Group partners with over 200 multinational pharmaceutical and med-tech manufacturers across three divisions and provides its services into 160 countries around the globe. Uniphar also employ over 2,600 people worldwide through their operations.
APPEL (Affiliation for Pharmacy Practice Experiential Learning) is now seeking expressions of interest from pharmacists in community and hospital pharmacy settings who would like to facilitate an experiential learning placement for a 4th-year pharmacy student in 2023. These placements will run from the 28th August to the 15th December 2023.
Experiential learning placements are a key part of the integrated pharmacy masters (M.Pharm). In their 4th year, students can undertake placements in both patient-facing (i.e. community and hospital pharmacy) and nonpatient-facing settings (including in the pharmaceutical industry and in role-emerging practice).

Pharmacists who have previously facilitated APPEL placements have found the experience enjoyable
and rewarding. In surveys, 90% of pharmacists said they would recommend facilitating a placement to other pharmacists. The advantages of facilitating an APPEL placement include:
• Continuing Professional Development - APPEL Trainer Training can contribute to pharmacists CPD, as can the experience of facilitating a placement.
• Development of your talent pipeline - many students will look to start their career in the organisations or practice settings where they undertook their placements
• Engagement - participating in the APPEL programme provides you with the opportunity to increase awareness of your pharmacy/ organisation. APPEL training
and events provide fantastic networking opportunities.

The deadline to confirm your interest in offering placements is the 11th November 2022. You can do this by clicking on the link below: https://forms.gle/ asBEd93CwDvm72gJ6.
If you have any questions or want further information, please email ops@appel.ie.


Difflam Spray (benzydamine hydrochloride), 0.15% w/v, Oromucosal Spray
Difflam Oral Rinse (benzydamine hydrochloride) 0.15% w/v, gargle
Please refer to Summary of Product Characteristics (SmPC) before prescribing Indications, Dosage and Administration: Difflam Spray is used as an adjunct in the symptomatic relief of painful inflammatory conditions of the throat and mouth. Difflam Oral Rinse is a locally acting analgesic and anti-inflammatory treatment for the relief of painful inflammatory conditions of the mouth and throat including: Traumatic Conditions: Pharyngitis following tonsillectomy or the use of a naso-gastric tube; Inflammatory Conditions: Pharyngitis, aphthous ulcers and oral ulceration due to radiation therapy; Dentistry: For use after dental operations. Benzydamine exerts an anti-inflammatory and analgesic action by stabilising the cellular membrane and inhibiting prostaglandin synthesis. Difflam Spray For oromucosal administration. Adults and Elderly: 4 to 8 puffs, 1½-3 hourly. Children (6-12): 4 puffs, 1½-3 hourly. Children under 6: One puff to be administered per 4 kg body weight, up to a maximum of 4 puffs, 1½-3 hourly. Because of the small amount of drug applied, elderly patients can receive the same dose as adults. The spray should be directed onto the affected area. Uninterrupted treatment should not exceed seven days, except under medical supervision. Difflam Oral Rinse Rinse or gargle with 15 ml (approximately 1 tablespoonful) every 1.5 to 3 hours as required for pain relief. Not suitable for children aged 12 years or under. The solution should be expelled from the mouth after use. Difflam Oral Rinse should generally be used undiluted, but if 'stinging' occurs the rinse may be diluted with water. Uninterrupted treatment should not exceed seven days, except under medical supervision. Elderly: No special dosage recommendations are made for elderly patients. Presentation: Difflam Spray: Oromucosal Spray Difflam Oral Rinse: Gargle. Contraindications: Use in patient with a known hypersensitivity (e.g. bronchospasm, rhinitis, urticaria) to Difflam Spray, Difflam Oral Rinse, benzydamine hydrochloride, or to any of the other ingredients listed in the SmPC for Difflam Spray or Difflam Oral Rinse. Warnings and precautions: Benzydamine use is not advisable in patients with hypersensitivity to acetylsalicylic acid or other NSAIDs. Bronchospasm may be precipitated in patients suffering from or with a previous history of bronchial asthma. Caution should be exercised in these patients. Difflam Spray Avoid contact with the eyes. If the condition is aggravated or not improved use should cease. Difflam Spray contains: • 14 mg of alcohol (ethanol) in each puff. The small amount of alcohol in this medicine will not have any noticeable effects. • less than 1 mmol sodium (23 mg) per dose of 8 puffs, that is to say essentially ‘sodium-free’. • methyl parahydroxybenzoate (E218) which may cause allergic reactions (possibly delayed) • mint flavour with benzyl alcohol, cinnamyl alcohol, citral, citronellol, eugenol, geraniol, isoeugenol, limonene and linalool. These substances may cause allergic reactions. Difflam Oral Rinse Avoid contact with eyes. Difflam Oral rinse is for oromucosal use only and should not be swallowed. Difflam Oral Rinse contains: • 1126 mg of alcohol (ethanol) in each 15 ml dose. The amount in 15 ml of this medicine is equivalent to less than 30 ml beer or 12 ml wine. The small amount of alcohol in this medicine will not have any noticeable effects. • methyl parahydroxy benzoate (E218) which may cause allergic reactions (possibly delayed) • mint flavour with benzyl alcohol, cinnamyl alcohol, citral, citronellol, eugenol, geraniol, isoeugenol, limonene and linalool. Benzyl alcohol, cinnamyl alcohol, citral, citronellol, eugenol, geraniol, isoeugenol, limonene and linalool may cause allergic reactions • less than 1 mmol sodium (23 mg) per 15 ml dose, that is to say essentially ‘sodium- free’. Interactions with other medicinal products and other forms of interaction: There are no known interactions with other medicinal products. Fertility, pregnancy and lactation: Difflam Spray and Difflam Oral Rinse should not be used in pregnancy or lactation unless considered essential by the physician. There is no evidence of a teratogenic effect in animal studies. Undesirable effects: Uncommon (>1/1000, <1/100): Oral numbness (hypoesthesia) and a stinging feeling in the mouth (oral pain). The stinging has been reported to disappear upon continuation of the treatment, however if it persists it is recommended that treatment be discontinued. For details of rare and very rarely reported adverse events see SmPC. Reporting of adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL – Dublin 2; Tel: +353 1 6764971; Fax: + 353 1 6762517. Website: www.hpra.ie. E-mail: medsafety@hpra.ie. Adverse reactions/events should also be reported to the marketing autorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267.
Legal Category: Product not subject to medical prescription Marketing Authorisation Number: PA2010/030/002-003
Marketing Authorisation Holder: Mylan IRE Healthcare Limited, Unit 35/36, Grange Parade, Baldoyle Industrial Estate, Dublin 13, Ireland Full prescribing information available on request from: Viatris, Dublin 17. Phone 01 8322250 Date of Revision of Abbreviated Prescribing Information: 29 March 2021 Reference Number: IE-AbPI-Difflam-Spray+OR-002
References: 1. DifflamTM Spray Summary of Product Characteristics. 2. Agarwal A et al. An evaluation of aspirin and benzydamine hydrochloride gargle for attenuating postoperative sore throat:a prospective sore throat: a prospective,randomized, single-blind study. Anesth Analg 2006; 103(4): 1001-1003. Viatris, Newenham House, Northern Cross, Malahide Road, Dublin 17. www.difflam.ie www.viatris.ie

The majority of simple tonsillitis and pharyngitis treatment can be carried out at a GP level or by over the counter medications such as paracetamol and NSAIDs. In the case of uncomplicated tonsillitis/pharyngitis, a pragmatic expectant policy is recommended. Patients who attend the ED are advised to avoid antibiotics and return to their GP if symptoms persist for a few days.2
Tonsillar exudate
Tender anterior cervical lymphadenopathy or lymphadenitis
History of fever (over 38°C)
Absence of cough
 Written by Conor Prendergast, Emergency Medicine Physician, Trinity College Dublin
Written by Conor Prendergast, Emergency Medicine Physician, Trinity College Dublin
Patients with an acute sore throat are a common presentation to Emergency Departments in Ireland. A recent single centred respective study based in an Emergency Department (ED) in Dublin, found there was over 650 attendances annually, accounting for just below 2% of annual attendances to the department.1
Consequently this cohort of patients accounts for a significant proportion of the workload upon emergency services, and the provision of appropriate emergency care to them is vital.
These patients are usually diagnosed as either tonsillitis or pharyngitis. These diagnoses account for the vast majority of presentations. Both illnesses tend to be viral in origin, with Group A Beta Haemolytic streptococcus representing the most serious bacterial cause. Severe but rare diagnoses such as epiglottitis, retropharyngeal abscess and peritonsillar abscess/quinsy should be also considered. Clinical signs that can help differentiate benign causes from severe causes include significant systemic upset, stridor, the inability to swallow salvia, severe neck stiffness and the patient holding a tripod position.
There is an over tendency to prescribe antibiotics with one UK study finding that 64% of primary care patients are prescribed antibiotics for a sore throat.3 This is despite most evidence displaying that the majority of sore throats are secondary to a viral infection. 85% to 95% of throat infections in adults and children under the age of 5 are caused by viruses. While viruses account for 70% of throat infections in those aged 5 to 15 years.4 This over prescribing of antibiotics has numerous potential consequences including unnecessary exposure to antibiotic side effects and antibiotic resistance. Certain studies have in fact shown that treatment of sore throats with antibiotics only provides mild symptomatic relief, though they do reduce complications such as rheumatic fever. However they have no effect on incidence, in countries such as Ireland, where this disease is not common.5
To help differentiate between viral and bacterial causes there is a number of decision aid tools. Two of these are recommended by NICE, which are the FeverPAIN and CENTOR.6 FeverPAIN scores from zero to five with each of the following scoring one point:
Fever over 38°C.
Purulence (pharyngeal/tonsillar exudate)
A score of less than or equal to 1 only has a 7% of being caused by streptococcus.6 Both tools help reduce the prescription of unnecessary antibiotics by giving the clinician confidence that with a low score, the likelihood of a streptococcus infection is low.
The antibiotic of choice for when one is required is Phenoxymethylpenicillin four times a day for ten days. If the patient is allergic to penicillin the second line option is clarithromycin.7 Antibiotics such as amoxicillin carry the risk of causing maculopapular exanthems in patients diagnosed with infectious mononucleosis and therefore should be avoided in patients presenting with sore throats.7 Other treatments that can be considered include paracetamol and NSAIDs. These medications can also be given rectally, which may be of benefit in patients with odynophagia. Topical mouthwashes or sprays, for example Benzydramine, may also provide symptomatic relief. When patients attend the ED, a commonly used treatment is Dexamethasone to treat severe odynophagia or stridor by reducing soft tissue swelling.
Attend rapidly (3 days or less)
Severely Inflamed tonsils
No cough or coryza
A score of 0-1 has a 13-18% chance of isolating streptococcus.6 The CENTOR scores zero to four with each of the following scoring one point:
One diagnosis to always consider, especially in younger patients presenting with a sore throat is infectious mononucleosis. This can be tested by a Monospot being sent. Rostegaard et al in 2019 found that the cumulative risk of infectious mononucleosis prior to turning 30 years old was 13.3% for males and 22.4% for females.8 Consequently it is a very prevalent infection and carries with it, other side effects that need to be acknowledged. One such effect is splenomegaly which is of particular importance in sport playing children and young adults. Splenomegaly occurs as a consequence of lymphocytic infiltration enlarging the spleen below the level of the ribcage, leaving it susceptible to be
ruptured.9 Patients with confirmed infectious mononucleosis should be advised to rest from contact sports and heavy lifting for at least one month, and return to the E if they develop sudden onset abdominal pain.10
Another complication of bacterial tonsillitis to consider is a peritonsillar abscess or quinsy. This is a unilateral swelling of pus behind the tonsil, where the swelling typically crosses the midline of the mouth. Patient symptoms may include a “hot potato voice” and trismus.11 These patients need to be treated by an ENT surgeon and may need either needle aspiration or incision and drainage.12
Finally, epiglottis thankfully is now a less rare complications since the introduction of the Haemophilus influenzae b vaccine. However this a life threatening emergency and should be considered if the following
clinical symptoms are present: stridor, muffled voice, rapid clinical course and a history of diabetes.13 These patients will need urgent review by ENT and anaesthetics, and may benefit from treatments such as dexamethasone and adrenaline nebulisers.

To conclude, sore throats are a common presentation to the ED. The majority of these presentations are viral in origin and require symptomatic treatment and do not benefit from antibiotic treatment. Through the use of decision aid tools such as the FeverPAIN and CENTOR a clinician can isolate sore throat presentations that are more likely bacterial in origin. Certain diagnoses such as infectious mononucleosis, epiglottis and peritonsillar abscess need to be carefully considered in this cohort.
References available on request
Listening down, speaking up and whistleblowing in healthcare were among the key issues addressed at the second annual MSc in Human Factors in Patient Safety Academy, hosted by RCSI in September.
The conference, organised by RCSI in collaboration with the Human Factors in Patient Safety Alumni Academy, brought together an inter-professional community of patient safety advocates discuss culture in healthcare. It was chaired by Dr Dara O’Keeffe, Simulation Lead in Postgraduate Surgical Education at RCSI. Professor Eva Doherty, Director of Human Factors in Patient Safety at RCSI, said: “The theme for the event was the importance of ‘listening down’ or psychological safety in promoting a just and safe culture in healthcare.”
The conference heard from Martin Bromiley OBE, Founder Clinical Human Factors Group, UK. He said that “to see so many health professionals engaged in the science of safety and human factors was both inspiring and heartening.”
“We all make mistakes, but we can reduce the danger and mitigate against those threats and errors by developing/supporting systems that make it easy to get it right and hard to get it wrong. It is important to encourage and train people to speak up, but it’s more important to encourage those in leadership positions to listen to their team, to seek ideas before stating a preference, and to thank those who do speak up. For leaders asking open questions and demonstrating understanding is a key to optimising solutions.
“To move forward in safety, we all need to build relationships below, across and above us. The quality of those relationships helps bring people together for a coming purpose of making healthcare safer for everyone.”
Mr Peter Duffy, Consultant Urologist in the UK provided attendees with an insight into the challenges and outcomes involved with his own
Dr Dara O’Keeffe, Simulation Lead in Postgraduate Surgical Education, RCSI Surgical Affairs (centre) with keynotes Martin Bromiley OBE, Founder Clinical Human Factors Group, UK and Mr Peter Duffy, Consultant Urologist, UK


whistleblowing case in healthcare. Mr Duffy, said: “I’m really grateful to the Royal College of Surgeons in Ireland for their invitation to the Human Factors in Patient Safety conference.
“I’ll continue to do what I can to promote candour and safeguarding in healthcare, whilst providing audiences with the “reality checks” that are necessary in order to be able to speak out safely without compromising their own employment or future prospects.”
The keynotes were followed by a discussion session regarding the Irish context. A number of points were raised in the discussion including the recognition that there is a lot of improvement still required and the concern that there is a mistaken belief that a just, open culture currently exists in Ireland.
The Human Factors in Patient Safety Academy was established in 2021 and consists of Alumni
and current scholars of the RCSI Postgraduate Diploma/MSc in Human Factors in Patient Safety. The Academy supports graduates to enable the development of safer patient care through human factors knowledge and skills.
Led by Professor Oscar Traynor and Dr Eva Doherty, RCSI established a mandatory human factors curriculum to improve the communication and interpersonal skills of surgical trainees and developed a robust process for competence assessment and performance appraisal. RCSI is now internationally recognised as a leading centre for human factors and patient safety in healthcare.
Mr Peter Duffy, Consultant Urologist, UK Martin Bromiley, OBE, Founder Clinical Human Factors Group and Martin Bromiley OBE, Founder Clinical Human Factors Group



DOVATO is indicated for the treatment of HIV-1 in adults and adolescents above 12 years weighing at least 40 kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.
Dovato (dolutegravir 50mg/lamivudine 300mg) tablets See Summary of Product Characteristics (SmPC) before prescribing. Presentation: Film-coated tablet containing dolutegravir sodium equivalent to 50 mg dolutegravir and 300 mg lamivudine debossed with “SV-137” on one face. Indication: HIV-1 in adults & adolescents above 12 years of age weighing >40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine. Dosing: One tablet once daily with or without food. Use an additional 50mg tablet of dolutegravir approximately 12 hours after the dose of Dovato when co-administered with efavirenz, nevirapine, tipranavir/ritonavir, etravirine (without boosted PI), carbamazepine, oxcarbazepine, phenytoin, phenobarbital, St John’s Wort or rifampicin. Elderly: Limited data in 65+ yrs. Renal impairment: Not recommended in patients with creatinine clearance < 30 mL/min. For patients with a sustained creatinine clearance between 30 and 49 mL/min see SmPC section 4.4. Hepatic impairment: Caution in severe hepatic impairment (Child-Pugh grade C). Contraindications: Hypersensitivity to any ingredient. Co-administration with substrates of OCT-2 with narrow therapeutic windows, such as fampridine. Special warnings/precautions: Risk of hypersensitivity reactions. Discontinue dolutegravir and other suspect agents immediately. Risks of osteonecrosis, immune reactivation syndrome. Monitor LFTs in Hepatitis B/C co-infection and ensure effective Hepatitis B therapy. Caution with metformin: monitor renal function and consider metformin dose adjustment. Use with etravirine requires boosted PI or increased dose of dolutegravir. Use with Mg/Al-containing antacids requires dosage separation. Use with calcium, multivitamins or iron also requires dosage separation if not taken at the same time with food. Use with cladribine or emtricitabine not recommended. When possible, avoid chronic co-administration of sorbitol or other osmotic acting alcohols (see SmPC

section 4.5). If unavoidable, consider more frequent viral load monitoring. Fertility, pregnancy and lactation: Human fertility - no data; animal fertility - studies indicate no effects. Women of childbearing potential (WOCBP) should be counselled about the potential risk of neural tube defects including consideration of effective contraceptive measures. If a woman plans pregnancy, the benefits and the risks of continuing treatment should be discussed with the patient. The safety and efficacy of a duel regime has not been studied in pregnancy. If a pregnancy is confirmed in the first trimester while on Dovato, the benefits and risks of continuing Dovato versus switching to another antiretroviral regimen should be discussed with the patient taking the gestational age and the critical time period of neural tube defect development into account (see SmPC section 4.6). There have been reports of mitochondrial dysfunction in HIV-negative infants exposed in utero and/or post-natally to nucleoside analogues. Do not breast-feed. Side effects: See SmPC for full details. Headache, GI disturbance, insomnia, abnormal dreams, depression, anxiety, dizziness, somnolence, rash, pruritus, alopecia, fatigue, arthralgia, myalgia, hypersensitivity, completed suicide, suicidal ideation or suicide attempt, panic attack, hepatitis, blood dyscrasias, acute hepatic failure, pancreatitis, angioedema, rhabdomyolysis, lactic acidosis, peripheral neuropathy. Elevations of bilirubin, ALT, AST and CPK. MA Nr: EU/1/19/1370/001. MA holder: ViiV Healthcare BV, Van Asch van Wijckstraat 55H, 3811 LP Amersfoort, Netherlands. Legal Category: POM A. Date of preparation of API: February 2022. Code: PI-6305. Further information available from GlaxoSmithKline, 12 Riverwalk, Citywest, Business Campus, Dublin 24. Tel: 01-4955000.

Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
*From November 2020 through October 2021, over 39 markets and territories with data sources available.
References: 1. Data on file. Regimen market share growth November 2020-October 2021. REF-146400. ViiV Healthcare group of companies. Research Triangle Park, NC. 2. Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naïve adults with HIV-1 infection. AIDS. 2022;36(1): 39-48. doi:10.1097/QAD.0000000000003070
3. Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine (DTG/3TC) versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with HIV-1: results through week 144 from the phase 3, non-inferiority TANGO randomized trial. Clin Infect Dis. 2022;ciac036 and suppl 1-18. doi:10.1093/cid/ciac036


DOVATO is owned by or licensed to the ViiV Healthcare group of companies.
©2022 ViiV Healthcare group of companies or its licensor.
For your patients living with HIV, make DOVATO a part of their healthy future.
Virological suppression is the first step to achieving
Continuing Professional Development 1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice? 2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area. 3. PLAN If I have identified a
The number of HIV diagnoses in Ireland is alarming with approximately 8 people diagnosed with HIV every week. This is despite the introduction of several initiatives to tackle the spread of the disease.

Human Immunodeficiency virus (HIV) is a disease of major public health significance in Ireland and across the world. It is the causative agent of acquired immune deficiency syndrome (AIDS) which causes millions of deaths every year.
HIV is found in all bodily fluids but is only present in infectious quantities in genital fluids (vaginal fluids, semen and moisture in the rectum), blood and breast milk. HIV is not infectious in saliva, urine, faeces or tears.
When a person is infected with HIV, the virus replicates in the CD4 T cells within a few days. This stage of HIV infection is referred to as sero-conversion and is characterised by a strong immune response as the body tries to fight the infection. In the days to weeks following initial HIV exposure, individuals experience flu like symptoms such as headaches, fever, sore throat and a rash. At this stage, the viral load is extremely high, and there is a high risk of disease transmission.
Stigma around HIV stems back to its original discovery in gay men in the 1980s where HIV was incorrectly referred to as ‘gay cancer’ or the ‘gay plague’. These misconceptions around the disease are still present today and have an enormous impact on those living with HIV.

The ultimate goal of HIV treatment is to achieve viral suppression, where HIV is undetectable in the blood. Treatment of HIV includes ART, the management of opportunistic infections or malignancies, the management of ‘non-HIV related’ co-morbidities and symptom control.
knowledge gap - will this article satisfy those needs - or will more reading be required? 4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result? Have I identified further learning needs? 5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the

4 previous steps, log and record your findings.
Published by IPN. Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
The number of HIV diagnoses in Ireland is alarming with approximately 8 people diagnosed with HIV every week.1 This is despite the introduction of several initiatives to tackle the spread of the disease. There is also an overwhelming amount of disinformation around HIV. With over 2 million visits to the community pharmacy each month in Ireland, community pharmacists are well placed to dispel myths around HIV and play a pivotal role in both the prevention of HIV transmission as well as the provision of care for those living with HIV.2 This can be achieved by community pharmacists normalising conversations about HIV and sexually transmitted diseases and providing information on how to get tested. If an individual is diagnosed with HIV their treatment is provided through hospital-based services, however community pharmacists can continue to provide advice particularly on potential drug interactions and harm reduction methods.

This article aims to support community pharmacists by providing an overview of the HIV virus including its transmission, testing methods and treatment.
Human Immunodeficiency virus (HIV) is a disease of major public health significance in Ireland and across the world. It is the causative agent of acquired immune
deficiency syndrome (AIDS) which causes millions of deaths every year. HIV disproportionately affects certain population groups, including people who inject drugs (PWIDs), men who have sex with men (MSM) and sex workers, due to the way in which it is transmitted. Over 6,000 people are estimated to be living with HIV in Ireland and it is thought that about 15% of these people do not know yet that they have HIV.3
HIV is a retrovirus which attacks the CD4 T lymphocytes (CD4 T cells) of the immune system. CD4 T cells coordinate the immune response by stimulating other immune cells to fight infection. The stages of disease progression from when an individual is infected with HIV to the development of AIDS is described in stages. In the 1980s, a diagnosis of HIV infection ultimately advanced to AIDS however due to the development of antiretroviral treatment (ART) this is no longer the case. Treatment with ART prevents disease progression such that a person on life-long ART has a life expectancy similar to that of the general population.4
When a person is infected with HIV, the virus replicates in the CD4 T cells within a few days. This stage of HIV infection is referred to as sero-conversion and is characterised by a strong immune response as the body tries to fight the infection. In the days to weeks
following initial HIV exposure, individuals experience flu like symptoms such as headaches, fever, sore throat and a rash. At this stage, the viral load is extremely high, and there is a high risk of disease transmission.
Following the sero-conversion stage, a latency period occurs where there are no signs or symptoms of the disease. This latency period may last for several years and without being tested, individuals may not be aware of their infection. During this period, as the HIV viral load increases, the number of CD4 T cells is slowly depleting. ART prevents an increase in the HIV viral load and prevents the disease progressing past the latency phase. Measuring the CD4 T cell count estimates the level of immunosuppression as a result of HIV. This measurement is the number of CD4 T cells in a sample of the individual’s blood. The normal range can vary between 500 and 1500 cells/mm3.
At a CD4 T cell count below 350 cells/mm3, individuals can become symptomatic as a result of opportunistic infections. Initially symptoms may be mild such as fatigue, weight loss, mouth ulcers and thrush but as the disease progresses the symptoms worsen. AIDS is typically diagnosed by a CD4 T cell count below 200 cells/ mm3 accompanied with a high viral load and opportunistic infections and cancers known as AIDSdefining illnesses.
Figure 1: Concentration of HIV and CD4 T cells in the body following HIV infection during the seroconversion phase, through the latency period and finally progressing to AIDS

condoms, health promotion and risk reduction education, preexposure prophylaxis (PrEP), post exposure prophylaxis following sexual exposure (PEPSE) and treatment as prevention (TasP). Signposting individuals to these services may empower them to engage with them.
Free condoms are available from sexual health clinics, sexual health organisations (e.g., HIV Ireland) and many third level colleges. www.sexualwellbeing. ie is an excellent resource to refer individuals to for support around their sexual health and wellbeing.
HIV is found in all bodily fluids but is only present in infectious quantities in genital fluids (vaginal fluids, semen and moisture in the rectum), blood and breast milk. HIV is not infectious in saliva, urine, faeces or tears.
HIV is found in all bodily fluids but is only present in infectious quantities in genital fluids (vaginal fluids, semen and moisture in the rectum), blood and breast milk. HIV is not infectious in saliva, urine, faeces or tears.
This is a common misunderstanding in the general population. 70% of respondents to a study conducted by HIV Ireland in 2017, believed that HIV can be transmitted through a bite and 24% believed it can be transmitted by kissing.5 This highlights a need for continued public education around this topic as these inaccurate beliefs are harmful and can add to stigma around HIV.
In 2018 in Ireland, MSM accounted for 56% (n=293) of HIV diagnoses in Ireland; 3% of new diagnoses were among PWID and heterosexual transmission accounted for 31% of HIV diagnoses.7 Access to HIV testing and treatment is free for everyone in Ireland, however everyone may not know how to avail of these services. Having an understanding of health disparities and initiating conversations and interventions in the community pharmacy can help to address this shortcoming.
This is a common misunderstanding in the general population. 70% of respondents to a study conducted by HIV Ireland in 2017, believed that HIV can be transmitted through a bite and 24% believed it can be transmitted by kissing (5). This highlights a need for continued public education around this topic as these inaccurate beliefs are harmful and can add to stigma around HIV.
HIV cannot be transmitted through coughing, sneezing, spitting, kissing, hugging, shaking hands, animal or human bites. Furthermore, if an individual with HIV is taking lifelong ART and they have an undetectable HIV viral load, they cannot pass on the virus. In the same 2017 report, only 19% of respondents correctly reported this.5 The global awareness campaign “Undetectable=Untransmittable” or “U=U” aims to eliminate HIV stigma and discrimination.6
The main ways HIV is transmitted is through unprotected anal, vaginal and oral sex. HIV transmission also occurs: through the sharing of unsterilised injecting equipment which is infected with HIV due to being previously used by someone who is HIV positive during pregnancy, childbirth or breastfeeding, from the HIVpositive parent who is not taking HIV treatment to their baby through receiving donated blood or organs infected with HIV, or injections with unsterilised needles carrying the virus in countries with inadequate screening and infection control procedures. Not every act of unprotected sex with an HIV-positive person results in HIV transmission. In addition to the type of sex, other factors can affect the risk that an exposure to HIV leads to infection. These include the presence of STIs, a high viral load, a man being uncircumcised, a woman menstruating, other bleeding and activities that can cause tearing and inflammation.
HIV is tested for as part of routine STI screening in Ireland. HSE public STI screening services are almost exclusively based in hospitals and community clinics around Ireland and are free of charge and confidential. A full list can be found on www.sexualwellbeing.ie. Private STI screening is also available such as through GPs and private clinics.
HIV cannot be transmitted through coughing, sneezing, spitting, kissing, hugging, shaking hands, animal or human bites. Furthermore, if an individual with HIV is taking lifelong ART and they have an undetectable HIV viral load, they cannot pass on the virus. In the same 2017 report, only 19% of respondents correctly reported this (5). The global awareness campaign Undetectable=Untransmittable” or “U=U” aims to eliminate HIV stigma and discrimination (6).
Stigma around HIV stems back to its original discovery in gay men in the 1980s where HIV was incorrectly referred to as ‘gay cancer’ or the ‘gay plague’. These misconceptions around the disease are still present today and have an enormous impact on those living with HIV. Negative attitudes towards HIV can lead to individuals feeling isolated and unsupported, which in turn can have a significant impact on their physical and mental health. Initiating conversations in the pharmacy and tackling misbeliefs through education has the potential to reduce this stigma.
Effective strategies to prevent sexual acquisition of HIV include the use of HIV testing,
SH:24 is a free online sexual health service, delivered in partnership with the HSE in Ireland and the NHS in the UK. Sexually transmitted infection (STI) test kits can be ordered at www.sh24.ie and are posted to a person’s home along with instructions and a return envelope to send the completed kit back to the lab. Results are received by text message within 72 hours of the sample arriving at the lab. This service is anonymous and non-judgemental and anyone with a positive result will be contacted by the SH:24 team to organise free assessment and treatment.
When providing advice to individuals on HIV testing it is important to consider the ‘window period’. The window period is the term used to describe the time between someone getting infected with HIV and developing a positive HIV test which can be
Figure 2: The awareness campaign “Undetectable=Untransmittable” or “U=U” highlights that an individual with an undetectable HIV viral load cannot transmit the virus
up to 12 weeks.8 Repeat testing after the ‘window period’ is often advised after an initial negative test. There are several types of HIV tests: antibody tests, antigen/ antibody tests and nucleic acid tests. No HIV test can detect HIV immediately after exposure due to the window period, which varies depending on the test.
to reduce the risk of HIV infection. Generic PrEP became available in Ireland in December 2017 increasing its affordability.9 A full list of approved PrEP services can be found at www.sexualwellbeing. ie. PrEP is available for free in Ireland to HIV negative individuals who are aged 17 years or older, have a PPSN and fall into one of the following categories:
where the person living with HIV has initiated antiretroviral therapy but has not yet achieved virological suppression.
Antibody tests: This tests for HIV antibodies which are produced after exposure but can take up to 12 weeks to develop. Antibody tests are usually a venous blood sample, a finger stick blood sample or an oral fluid sample.
Antigen/antibody tests: The p24 HIV antigen is present in high levels in newly infected individuals so can be useful for early detection of HIV. Once p24 antibodies are produced, the p24 antigen may become undetectable so a combined antigen/antibody test is more useful for diagnosing HIV. The estimated window period is 18-45 days.
Nucleic acid test (NAT): This test is for the virus itself in the blood, it is also known as a HIV viral load test. It can detect HIV sooner than other types of tests. The window period is estimated at 10-33 days.
PrEP
PrEP is the pre-emptive use of oral ART by HIV-negative individuals
1: Men who have sex with men or transgender women who have sex with men who are:
where the person living with HIV has loss of virological control on antiretroviral therapy and the risk of HIV transmission has been deemed by a consultant physician specialising in HIV Medicine to be substantial and warrant PrEP for the HIVnegative partner
before having sex. Event-based dosage is not recommended for women (including transgender women); transgender men having vaginal sex; men having vaginal sex or anal sex with women and people who are hepatitis B surface antigen positive.
Sexually active with likelihood of remaining sexually active in the next 3 months AND one of the following:
reported condomless anal sex with at least two partners over the last 6 months
episode of documented or reported acute STI over the last 12 months
documented or reported use of HIV PEPSE over the last 12 months
reported engagement in chemsex (the use of drugs as part of one’s sex life) over the last 6 months
2. Individuals having condomless sex with a HIV positive person who is not stably suppressed on antiretroviral therapy, specifically:
where the person living with HIV is not on antiretroviral therapy
3: Other heterosexual men, heterosexual women considered by a senior clinician specialising in HIV Medicine to be at substantial risk for sexual acquisition of HIV.
There are some situations where PrEP is not recommended including in individuals unwilling to attend for follow up, those with significant renal impairment at baseline and those who report sub-optimal adherence with continued significant risk for sexual acquisition of HIV due to the risk of antiretroviral resistance. PrEP prescriptions are usually for three months at a time and individuals must commit to regular follow up appointments.

Event based dosage of PrEP has been shown to be effective for men who have anal sex with other men and can be considered where sex is infrequent (less than 2 times per week) and where the individual is able to allow adequate time for taking the first dose
PEP (post-exposure prophylaxis) is a short course of ART that can be taken to prevent HIV infection within 72 hours of exposure to the virus. The first dose of PEP should be given as soon as possible, ideally within 2 hours of exposure. PEP is available through STI clinics and emergency departments; the latter should be reserved for when it is not possible to get PEP from a local STI clinic within 72 hours. A full course of PEP is 28 days however emergency departments often only supply starter packs for three days and refer the person for further assessment for continued treatment.10 PEP is free in Ireland however, individuals who attend the emergency department without a referral letter, unless exempt, will be charged ¤100. Often the community pharmacy is the first port of call for health advice especially at the weekend. Providing information and signposting individuals to the appropriate services will enable individuals to obtain PEP in a timely manner. A full list of clinics and emergency departments providing PEP can be found at www.sexualwellbeing.ie Figure 3:
Pharmacy based needle exchange is a service that has the potential to dramatically impact the spread of HIV and other blood borne viruses (BBVs). In 2012, across 63 pharmacies offering needle exchange, 33 had transactions where they provided injection equipment packs along with information leaflets on safer injecting advice and harm reduction.11
A national voluntary programme of HIV antenatal screening has been in place in Ireland since 1999. As part of this programme, it is recommended that HIV testing be offered to all women who attend for antenatal services. In 2019, Nine women were newly diagnosed with HIV at their antenatal screen.12
The ultimate goal of HIV treatment is to achieve viral suppression, where HIV is undetectable in the blood. Treatment of HIV includes ART, the management of opportunistic infections or malignancies, the management of ‘non-HIV related’ co-morbidities and symptom control.
In July 2017, the HSE adopted the position that all individuals living with HIV attending HIV services in Ireland are to be offered ART free of charge and informed of the benefits of ART in improving their personal health and reducing HIV infectiousness.
ART is one of the fastest evolving areas of medicine and has transformed a HIV diagnosis from inevitably progressing to AIDS to a manageable disease that one
can live a relatively normal life with. ART is not a cure for HIV, it stops the virus from reproducing in the body and can reduce the viral load to undetectable levels. Once ART is started and the individual adheres to the treatment regime, the virus can become undetectable within 6 months. Adherence is incredibly important, and the community pharmacist can provide support to facilitate this.
Achieving viral suppression improves health outcomes for individuals living with HIV, allowing them to have an improved quality of life and live longer. Viral suppression prevents the spread of HIV as individuals who have an undetectable viral load have effectively no risk of transmitting HIV to their sexual partners
Commonly known as triple therapy, a combination of three antiretroviral agents, selected on the basis of treatment history and resistance tests, should usually be prescribed to increase efficacy and reduce the development of drug-resistant virus. Treatmentnaïve individuals should start with a non-nucleoside reverse transcriptase inhibitors (NNRTIs) backbone in combination with a third agent regardless of their CD4 T cell count. Combination medications such as Truvada (emtricitabine and tenofovir disoproxil), Descovy (emtricitabine and tenofovir alafenamide fumarate) and Biktarvy (bictegravir sodium, emtricitabine and tenofovir alafenamide fumarate) can aid compliance.

In the community pharmacy it is important to be aware of drug interactions with ART for HIV. The University of Liverpool drug interaction checker (www.hiv-druginteractions.org) is
a useful tool which can be used to check for drug interactions. Some ART drugs are substrates of certain CYP enzymes and so inducers and inhibitors of these enzymes can affect their concentrations. When counselling individuals on over the counter and prescription items, it is vital to check if they are on any other medication. For individuals taking triple therapy, drug interactions should be ruled out to ensure treatment efficacy is not affected. Opportunistic infections occur in individuals with HIV when their immune system is compromised. Examples are pneumocystis carinii pneumonia (PCP) and tuberculosis (TB). Cotrimoxazole is a commonly used antibiotic to prevent opportunistic infections in individuals with HIV.
In Ireland, HIV care is provided in nine hospitals. Adult care is provided in Cork (Cork University Hospital), Dublin (Beaumont Hospital, Mater Misericordiae University Hospital, St. James’s Hospital, St. Vincent’s University Hospital), Galway (Galway University Hospital) and Limerick (Limerick University Hospital). Paediatric HIV care is provided in Dublin at Temple St. University Hospital and Our Lady’s Children’s Hospital.
The role of the community pharmacist is ever evolving and there is potential for involvement in services such as HIV testing, like our counterparts in the USA and Canada.13 Using a holistic approach to support individuals living with HIV, the community pharmacist can have a positive impact on their life and treatment outcomes.
References available on request
5 questions and answers for the readers to ensure they understand the article:
1. Q: What are the main stages following infection with HIV.
A: (1) Sero-conversion (2) Latency period (3) AIDS
2. Q: What is a CD4 T cell count?
A: This measurement is the number of CD4 T cells in a sample of an individual’s blood. The normal range can vary between 500 and 1500 cells/ mm3.
3. Q: What is the main way in which HIV is transmitted?
A: The main ways HIV is transmitted is through unprotected anal, vaginal and oral sex.
4. Q: Where can an individual access PEP?
A: PEP is available through STI clinics and emergency departments; the latter should be reserved for when it is not possible to get PEP from a local STI clinic within 72 hours.
5. Q: What are the constituents of a HIV ART treatment regime for treatment-naïve individuals?
A: Treatment-naïve individuals should start with a non-nucleoside reverse transcriptase inhibitors (NNRTIs) backbone in combination with a third agent regardless of their CD4 T cell count.
*To verify contact verify@perrigo.com Solpa-Extra 500mg/65mg Soluble Tablets contain paracetamol and caffeine. For the treatment of mild to moderate pain. Adults and children over 16 years: 1-2 tablets dissolved in water every 4-6 hours. Max 8 tablets a day. Children 12-15 years: 1 tablet disolved in water every 4-6 hours. Max 4 tablets a day. Not suitable for children under 12 years. Contraindications: Hypersensitivity to the ingredients. Precautions: Particular caution needed under certain circumstances, such as renal or hepatic impairment, chronic alcoholism and malnutrition or dehydration. Precautions needed in asthmatic patients sensitive to acetylsalicylic acid, patients on a controlled sodium diet and with rare hereditary problems of fructose intolerance. Patients should be advised not to take other paracetamol containing products concurrently. Pregnancy and lactation: Not recommended during pregnancy and breastfeeding. Side effects: Rare: allergies. Very rare: thrombocytopenia, anaphylaxis, bronchospasm, hepatic dysfunction, cutaneous hypersentitivity reactions. Unknown: nervousness, dizziness. Further information is available in the SmPC. PA 1186/017/001. P. MAH: Chefaro Ireland DAC, The Sharp Building, Hogan Place, Dublin 2, Ireland. Date of preparation: July 2020. Legal Class P – Pharmacy only. IRE/SOL1/2022/02

encompass lifestyle modifications, use of over-the-counter (OTC) medications and highlight when it is appropriate to consult their general practitioner (GP).8 With this in mind, pharmacists should regularly remind themselves of red flag symptoms which should prompt urgent referral to the GP: Unintentional weight loss Anorexia
Dyshagia or odynophagia
Age 55 or over with new onset dyspepsia

Epigastric mass Management
1. Non-pharmacological management

anti-inflammatory (NSAIDs) but can also be caused by corticosteroids, bisphosphonates or iron supplements amongst others.1 However, evidence supporting the use of lifestyle and dietary modifications for symptomatic relief is lacking meaning medical therapy is the mainstay of treatment.2

The term dyspepsia refers to a complex of symptoms arising from the upper gastrointestinal tract (GIT) presenting as epigastric pain or discomfort. It is a common condition affecting 20-40% of the world’s population at some point in their lives.1 Affected individuals may complain of postprandial fullness, early satiety, bloating, belching or nausea.2,3 Dyspepsia has an extensive differential diagnosis. It may occur secondary to organic causes such as Helicobacter pylori infection, peptic ulcer disease (rarely), gastrointestinal malignancies, gastroesophageal reflux disease or may be medication induced. However, up to 80% of patients with dyspepsia will have no explanation for their symptoms at endoscopy; known as functional or idiopathic dyspepsia. The global prevalence of functional dyspepsia is estimated between 5 and 11%.4
While the pathophysiology of functional dyspepsia is not well understood, several mechanisms have been suggested. Disturbances in gastrointestinal motility, impaired gastric accommodation, increased visceral
sensitivity, abnormal gut-brain axis and gut microbiota dysbiosis have all been linked to the occurrence of functional dyspepsia. The latter hypothesis is supported by research demonstrating an increased likelihood of dyspepsia following infective gastroenteritis.5-7
Community pharmacists often provide the first line of care for patients with dyspeptic symptoms. According to NICE guidelines, pharmacists should offer initial and ongoing help to patients presenting with symptoms of dyspepsia. Advice should
Simple lifestyle measures for dyspeptic symptoms include weight loss if appropriate, eating smaller meals, cutting down on caffeine and high fat foods, avoiding late meals, raising the bedhead and adhering to alcohol consumption guidelines. This may also provide an opportunistic consultation for smoking cessation which may also improve symptoms. It may also be appropriate to carry out a medication review to identify any medications that might be causing or worsening symptoms, with one of the most common offenders being nonsteroidal
A short interview with the patient can identify the most appropriate course of action. Pharmacists should ask the patient to describe their symptoms in terms of the nature, frequency and severity along with careful screening for the presence of red flag symptoms as mentioned previously. Proton pump inhibitors (PPIs) omeprazole and esomeprazole are available OTC. PPIs inhibit gastric H,K-ATPase thereby inhibiting gastric acid secretion. Due to their short halflife, it takes about 48-72 hours to reach steady state inhibition of acid secretion.9 Alternatively, H2-receptor antagonists such as famotidine are also available OTC. This class decreases gastric acid secretion by reversibly binding to histamine H2 receptors on gastric parietal cells thereby reducing acid release. They have a quick onset of action of approximately 60 minutes and provide relief for 4-10 hours.10 However, while
combination should not be administered to patients with a history of hypersensitivity to other H2-receptor antagonists. Special warnings and precautions for use. Warnings: Patients with renal or hepatic impairment should consult a physician before using Pepcid Duo Chewable Tablets. In case of rena failure, monitoring of serum magnesium and calcium should be undertaken. Pepcid Duo is contraindicated in patients with severe renal failure. As some serious underlying conditions can have symptoms in common with simple indigestion, it is recommended patients seek medical advice in case of: indigestion symptoms accompanied by unintentional weight loss, dif culty swallowing, persistent abdominal discomfort, heartburn occurring for the rst time or if these symptoms have recently changed. Patients with pre-existing known hypercalcaemia, hypermagnesaemia, hypophosphataemia, hypercalcuria, a history of renal calculi or nephrocalcinosis should consult a physician before using famotidine/antacid combination. As this product contains lactose, glucose and benzyl alcohol: Patients with rare hereditary problems of galactose intolerance, total lactase de ciency or glucose-galactose malabsorption should not take this medicine. Patients with rare glucose-galactose malabsorption should not take this medicine. This medicine contains 0.000238 mg benzyl alcohol in each tablet which is equivalent to 0.000129 mg/g. Benzyl alcohol may cause allergic reactions. High volumes of benzyl alcoho should be used with caution and only if necessary, especially in subjects pregnant or breastfeeding, or with liver or kidney impairment because of the risk of accumulation and toxicity (metabolic acidosis). With long term use, especially during concomitant treatment with other calcium products and/or Vitamin D products, there is a risk of hypercalcaemia with subsequent kidney function impairment. Patients should stop use and consult a physician if new symptoms develop or if they experience dysphagia (dif culty swallowing) odynophagia (pain on swallowing), sever vomiting, melaena (black stools), choking or chest pain. Precautions for use: If symptoms persist after 2 weeks of continuous treatment or get worse, an etiologic survey must be done and the conduct of the treatment should be re-evaluated. Undesirable effects: Immune system disorders Not known Hypersensitivity, Anaphylactic reaction. Nervous system disorders Common Headache Uncommon Nervousness, Paresthesia, Dizziness Not known Somnolence. Gastrointestinal disorders Uncommon Abdominal discomfort and pain Abdominal distension Nausea Diarrhoea Flatulence Dyspepsia Eructation Dysgeusia Oropharyngeal discomfort and pain Dry mouth, thirst Vomiting Not known Abdominal pain upper. Skin and subcutaneous tissue disorders Not known Pruritus Rash Urticaria Angioedema. General disorders and administration site conditions Not known Asthenia, fatigue. Other side effects noted in isolated reports with higher dosages of famotidine in principle cannot be excluded. There have been very rare reports of: Cutaneous: as with other H2 antagonists, severe skin reactions (toxic epidermal necrolysis). Hypersensitivity reactions: bronchospasm. Hepatic disorders including hepatic cholestasis and such as raised laboratory values for transaminases, gamma-GT, alkaline phosphatase and bilirubin. Neurological disorders such as hallucinations: disorientation, confusion and insomnia, epileptic seizures, drowsiness and agitation and depression related states. These have been reported to be reversible on stopping medication. Blood disorders such as thrombocytopenia, leucopenia, agranulocytosis and pancytopenia. Musculoskeletal disorders, such as muscle cramps. Others such as impotence, reduced libido, breast tension. Alopecia Malaise. The following side effects are generally attributed to antacids containing calcium and magnesium salts: change in stool frequency and consistence, bloating and fullness. MAH: Johnson & Johnson (Ireland) Limited, Airton Road, Tallaght, Dublin 24, Ireland. MA Number: PA 330/47/1. Date of revision: April 2022. Product not subject to medical prescription, supply through pharmacies only. Full prescribing information available upon request form Johnson & Johnson (Ireland) Ltd.


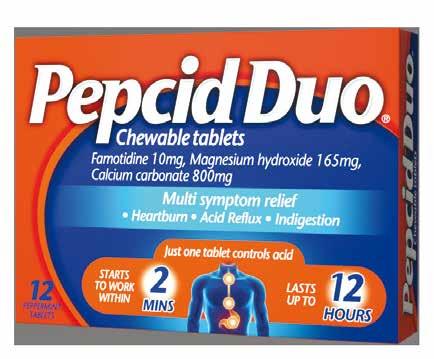
these medications can provide relief of symptoms, their OTC indication is for short term use only. Patients who are regularly purchasing these medications should be advised to attend their GP for further investigation.
What is Gastro-oesophageal reflux disease?
Gastro-oesophageal reflux disease (GORD) is a condition that develops when reflux of stomach contents into the oesophagus causes symptoms and/or complications.11, 12
GORD classically presents as heartburn and regurgitation. Heartburn or pyrosis is generally described as retrosternal burning postprandially while regurgitation the upward movement of gastric contents into the mouth or hypopharynx.13 Other symptoms may include chest pain, a feeling of fullness in the throat, hoarseness or a chronic cough.14
The pathophysiology underlying the development of GORD is multifactorial, comprising both physiological and pathological factors. Transient lower oesophageal sphincter (LOS) relaxations, reduced LOS pressure, hiatal hernias, impaired oesophageal acid clearance and delayed gastric emptying are other factors identified in the literature.14,15 Obesity is a known
risk factor for GORD, potentially due to increased intragastric pressure.16, 17 Cigarette smoking is also a risk factor; it reduces pressure across the LOS and increases the time taken to clear acid from the oesophagus.18 Despite an association between certain foods and GORD, evidence linking specific foods with GORD is lacking. Common triggers however, may include fatty foods, spicy foods, coffee, alcohol and fizzy drinks.19-22
Similar to dyspepsia, the presence of ALARM or red flag symptoms should prompt urgent referral to the GP to rule out possible underlying malignancy.
Non-pharmacological measures should include weight loss where appropriate; weight loss has strong evidence for efficacy and has been shown to have a dose-dependent association with reduction of symptoms.11, 23 Other approaches include elevation of the head of the bed and avoiding large meals close to bedtime.24 Fatty foods, caffeine and alcohol may exacerbate GORD as previously mentioned and should be minimised if known to trigger symptoms. Further, smoking cessation is also recommended.12
Antacids are commonly used self-prescribed medications that
provide symptomatic relief from the symptoms associated with GORD. They consist of calcium carbonate, sodium bicarbonate and aluminium or magnesium salts in various compounds and combinations. Once dissolved in the stomach, they partially neutralise gastric acid, thus raising gastric pH. This effect also inhibits pepsin activity, thereby reducing its proteolytic activity.25 They are available in both liquid and chewable preparations. Some antacids contain alginate, which have a unique mechanism of action - in the presence of acid, they precipitate into a gel forming a raft that localises to the acid pocket in the stomach.26 Antacids are best taken with food or after meals when symptoms are most likely to occur. For patients taking other medications, it is important to remind patients to avoid taking other medications within 2-4 hours of taking an antacid due to decreased absorption by chelation or adsorption of other drugs.25

It is important to counsel patients that while antacids can provide relief from symptoms, they do not treat the underlying cause and long-term use is not recommended as it may mask disease.
Acute gastroenteritis is defined as diarrhoeal disease that has a rapid onset lasting less than two weeks in duration. It may be accompanied by vomiting, abdominal pain or fever.27, 28 Most cases of acute gastroenteritis are viral with norovirus being the most
common offender. Norovirus is also known as the “winter vomiting bug” due to its pronounced peak during the winter months.29 Other common viral pathogens include rotavirus, enteric adenovirus and astrovirus.30, 31 Non-viral causes may include bacteria such as Staphylococcus aureus, Campylobacter jejuni, Shigella spp, Salmonella spp and Escherichia coli. Less commonly, parasites such as Giardia and Cryptosporidium can cause acute gastroenteritis. In light of the current global pandemic, gastrointestinal manifestations of COVID-19 should not be overlooked as a differential diagnosis. Characteristics suggesting viral etiology include an intermediate incubation period (24-60hours), a short duration of symptoms (12-60 hours) and a high frequency of vomiting.32 In terms of duration, Norovirus usually lasts ~2 days, rotavirus lasts between 3-8 days while Campylobacter and Salmonella last approximately 2-7 days.33
For most individuals, acute viral gastroenteritis is self-limiting and can be treated at home with supportive measures. Maintaining adequate fluid hydration is the mainstay for the management of acute gastroenteritis and can be achieved with water, sport drinks and oral rehydration solutions which are available OTC. Evidence supporting restriction of certain foods during acute gastroenteritis is weak and so patients should be encouraged to eat as tolerated.34 Anti-emetics such as domperidone may be appropriate for those experiencing nausea and vomiting and pharmacists should refer to the Pharmaceutical society of Ireland (PSI) guidance on the safe and appropriate sale of domperidone taking care to screen for any contraindications to its use.35 The use of anti-diarrhoeal agents such as Loperamide is generally not recommended unless a patient needs to shorten the length of time the diarrhoea lasts. Alarm symptoms and signs identify those who may need referral to the GP or local emergency department and include:
Severe abdominal pain
Severe volume depletion/ dehydration
Aged 65 or older
Comorbidities e.g. immunocompromised
Prolonged symptoms
Recent hospitalisation or antibiotic use in the previous 3-6 months
References available on request


A cup of coffee and a deeper conversation about it made us more aware of the increased need for automation in the pharmacy environment.
The pandemic has changed the way that users see the pharmacy going from mainly a place to collect prescriptions and to buy some OTC products, to the perfect go to health point before seeing the GP.
From vaccines to travel clinics, monitored dose to drug usage reviews, skin clinics to ear wax removal; Covid has pushed pharmacy to be the main health point within local communities.
Unfortunately, the pandemic has had other unforeseen effects in local business - the worst of them being staff shortages. How would a pharmacy be able to offer all these services with the quality they strive to achieve if they can’t get enough staff to even cover daily tasks?
That’s where Pharmacy Automation and companies like Edia Healthcare have offered help to Irish pharmacies. Our experience in the field and our products help to reduce the pressure by cutting down the time required to carry out some daily time-consuming tasks. Who wouldn’t like to prepare their MDS blister pack in less than half the time it is currently taking?
Pharmacy automation has been carrying some stigmas over the years that we would like to dismantle by answering to some
of the most common questions that we at Edia Healthcare face everyday.
Is Pharmacy automation only for big chains?
No. Of the 20 machines that Edia Healthcare look after, 14 of them are in independent pharmacies.
Do you need to do a lot of MDS packs a month to install a machine?
Most of our customers were packing 60 to 80 patients per month when they purchased the machine.
Isn’t Pharmacy automation too expensive?
Our smallest range of automated MDS blister packing or MDS pouch packing starts at ¤55,000 for a machine capable of packing up to 100 patients per week. And a system of Pouch packing machine + Checking camera for ¤90,000
But we still have to manually check all of the blisters.
Our smallest pouch checking solution is powered by AI, can check up to 3 pouches/second and will decrease the pharmacist checking time by 70%
It’s a waste of time to have to do a double entry of the data
We write our own software to run our machines and we can integrate with any PMR software in the market. We currently have integrated with the two main PMR software providers in Ireland, eliminating double entry and minimizing errors.
But you are based in the north and we can’t wait for hours when we need help
We have 2 technical support teams, one in Belfast and one in Limerick. Our maintenance contract stipulates that in case of a machine breakdown we will be on site within 4 hours in any corner of this island. If we don’t fulfill it, we won’t charge the maintenance fee for that month.
“Our purchase of the Edia machine was our first venture into automation in the Pharmacy and there was a certain amount of trepidation associated with it.
“The installation, set up and training was far more seamless than I could have hoped for. Fran and his team were on hand at all times to guide us through the process.
We are the sister company of the internationally known Farmadosis Group and most of our products are designed and built in Spain which makes them more affordable. The fact that we are manufacturers- means that we know our machines inside out and in the eventuality of any issue we have the complete know-how. Although we could tell you all of the benefits of our machines, our customers do a much better job!!
The Edia machine has helped streamline our dispensary. By taking the blister packs out of the day-to-day work the entire process flows much more efficiently. We plan ahead and roster cover for the days we intend to do a batch of blister packs .
“The blister packs themselves are very similar to what we had been using previously . They are easy to manage for both Pharmacists and patients and very simple to reseal if there are changes in a patient’s medicines.”
At the beginning of 2022 one of our customers said to us, “Covid has changed the world in general and the old pharmacy model.”
The Group were crowned ‘Company of the Year’ at the annual Irish Business Times Awards whilst earlier this year the team welcome back colleagues as they reopened their Head Office in Citywest.

The office has undergone a significant renovation to create an amazing working environment for the entire workforce that encourages collaboration and teamwork. “It was great to see our colleagues meet face to face again and catch up over a cup of coffee. Special thanks to our Chairman Maurice Pratt and CEO Ger Rabbette for officially reopening the office,” said a Uniphar spokesperson.
Meanwhile, with war raging on in Ukraine and thousands of displaced Ukrainians still having to adapt to life in Ireland, Uniphar did its bit to help by handing over a cheque for ¤20,000 to Wexford Volunteer Centre to assist with their Ukrainian effort.
In May they demonstrated their support of community pharmacists across Ireland by supporting the Uniphar Category Development Award, which was won at the Irish Pharmacy Awards by McCabes Pharmacy Group.




Through analysis of external market trends, alongside internal data analysis, McCabes Pharmacy were inspired to further invest in the vitamins category across the business and enhance consumer experience. Operating a consumer centric strategy with innovation at the heart of this allows McCabes to continually thrive and stay ahead of the market curve as well as competitors.
Uniphar’s own CEO Ger Rabbette was recognised in the Business & Finance Media Group Top 100 CEO Index. An industry veteran, Ger joined Uniphar from Celesio, where he was Managing Director
of Movianto and Head of Celesio Manufacturing Solutions. He is a Chartered Accountant by training and has held a range of senior positions in supply chain with Cahill May Roberts and the wider Celesio Group. He was appointed Chief Executive Officer in 2010.
October 2022 saw the Uniphar Connect Awards – celebrating access again in Cork for the Munster colleagues. The winners on the night included:
Customer Experience Award
Hickey’s Pharmacy Mayfield, Cork
Vaccinations Award
Hogan’s Life Pharmacy, Limerick
Outstanding Contribution Award
Hickey’s Pharmacy, Ballincollig
Length of Service Award
Glencora Harbour Allcare Pharmacy, Glin





It is that time of year again when everyone is coming down with a cold or the flu. You do everything you can to keep your children healthy, but they still get sick.
“friendly” bacteria, or probiotics, play a significant role in a child’s development, helping to support the child’s immune system and protect against infections.
Children are born with their intestinal tracts sterile. They acquire some probiotics from the mother during passage through the birth canal and more while being breastfed. Children born via Caesarean section and or are bottle fed do not get any and are at a higher risk of developing gastrointestinal problems and having reduced immunity in general as a result.
Even if you are careful about handwashing and keeping your home clean, there are some things that just cannot be avoided – like exposure to other children, handling and sharing toys or even food.
A daily probiotic supplement can help support your child’s immune system and provide better overall health. Probiotics are beneficial bacteria that line the gut and support digestion, absorption of nutrients, and production of vitamins.
What are probiotics and why are they important for children’s health?
Probiotics are live bacteria that are like the ones found naturally in the gut. They are often taken as a supplement to improve gut health. Probiotics are found in some foods and supplements. They are often called “good” or “friendly” bacteria because they can help us maintain a healthy balance of necessary bacteria in the gut where much of our immunity resides.
This is important for children’s health because a child’s gut health is not only important for their immunity but can also impact their overall health and well-being.
Probiotics can help promote a healthy digestive system, strengthen their immune system, and even improve mood and cognitive function.
Studies have shown that probiotics can help reduce the severity and duration of certain types of
infections, such as rotavirus and diarrhoea. They can also help prevent the development of some allergies and eczema.
For these reasons, it is important for parents to ensure their children’s health is enhanced by getting enough probiotics through diet or supplements.
How do probiotics help support immunity in children?
The human gastrointestinal tract is home to a complex and diverse ecosystem of microorganisms, many of which are essential for good health. These so-called
While probiotics are also found naturally in some foods and can also be taken as supplements. Some studies have shown that early introduction of probiotics may be particularly beneficial for children, helping to reduce the risk of gastrointestinal problems and other infections.
For example, one study found that children who took probiotics were less likely to experience diarrhoea. Probiotics may also help reduce the duration of respiratory infections and improve symptoms of allergies and eczema. As more research is conducted, it is becoming increasingly clear that probiotics offer a range of benefits for children’s health.
The child’s gut microbiome is constantly changing and exposure to new probiotic strains can help to promote a healthy balance. Probiotics can help to reduce the risk of gastrointestinal infections, allergies, and eczema.
They may also help to improve cognitive function and emotional wellbeing. While more research is needed, probiotics offer a promising approach to supporting children’s health.


SONA BabyBiotic is suitable for babies, from birth.

Providing five billion Bifidobacterium (BB-12®) in each daily dose. BB-12®

has been tested in more than 300 clinical trials with proven safety and efficacy. BB-12® has many documented benefits, including lowering the frequency of colic.
SONA Pro10Biotic Kiddie is a citrus flavoured chewable tablet containing ten billion per tablet of one of the best documented Lactobacillus strains, L. casei 431®. These friendly bacteria colonise the gut and help maintain a favourable intestinal flora. A correct balance of intestinal flora is important for the proper digestion and utilisation of nutrients and a healthy immune system. Suitable for children ages 3+.
Contraception is the act of preventing pregnancy. Although hormonal contraceptives and IUDs are highly effective at preventing pregnancy, they do not protect against sexually transmitted infections (STIs). We spoke to Theresa Lowry Lehnen, Clinical Nurse Practitioner and Associate Lecturer South East Technological University to find out more about the differing choices currently available and what pharmacy teams need to be aware of.
There are many different types of contraception available. Theresa says, “Choosing the right method is a personal decision and finding what works best for the individual. Contraceptive counselling with a health professional is a marked contributor to the successful use of contraception. When considering contraceptive methods counselling should include efficiency, safety, acceptability and availability including accessibility and affordability. Screening for pregnancy is important prior to prescribing contraception.”


Theresa explains that combined hormonal contraceptives (CHCs) containing synthetic oestrogen and progesterone are among the most commonly prescribed and wellresearched types of contraceptive medication in use. “CHCs can be delivered through a pill, patch,
or vaginal ring. In CHCs, both progestins and oestrogen inhibit the hypothalamic–pituitary–ovarian axis, which controls the reproductive cycle,” she told us.
“Progestins prevent pregnancy by inhibiting the luteinizing hormone (LH) surge, suppressing ovulation, thickening the cervical mucus, lowering fallopian tube motility, and causing the endometrium to become atrophic. Oestrogens prevent pregnancy by suppressing follicle-stimulating hormone (FSH) production, which prevents the development of a dominant follicle. Progesterone is responsible for most contraceptive action and side effects, and the addition of oestrogen helps prevent irregular or unscheduled bleeding.”

The combined pill is more than 99% effective with perfect use and 91% effective with ‘typical use’. “Advantages are that it does not interrupt sex, often reduces bleeding and period pain and may help with premenstrual
An interview with Theresa LowryLehnen (PhD), CNS, GPN, RNP, South East Technological University
symptoms. Disadvantages are that it can be less effective if a pill is missed, there is vomiting or severe diarrhoea, or by taking certain medication. It is not suitable for smokers over 35 years of age, or those who are obese. It may not be suitable for women who are breastfeeding and medical advice should be obtained,” she adds.
The CHC transdermal patch is a thin adhesive square about 4-5 cms wide, containing synthetic oestrogen and progesterone. It can be placed on the stomach, upper arm, buttock, or back, and must be completely attached to the skin to be effective. The patch is replaced every week for three weeks. During the fourth week no patch is worn and a withdrawal bleed occurs. It is over 99% effective with perfect use, and 91% effective if not always used correctly.
Theresa adds, “Effectiveness may be reduced by taking certain medications. It is not suitable for people who are obese, and smokers over the age of 35. It may not be suitable for women who are breastfeeding, and medical advice should be sought.”
The CHC vaginal ring (NuvaRing) is a clear, flexible ring about two inches in diameter containing synthetic oestrogen and progesterone that is placed in the vagina for 21 days, and removed for seven days to allow for withdrawal bleeding. It is replaced monthly. Users can place the ring in the vaginal canal themselves.
“As with the patch, the less frequent applications can be appealing and can lead to increased adherence. The ring’s internal placement ensures the steady delivery of hormones, which allows for lower serum concentrations than occur with either the patch or pills. As a result, the ring generally has milder side effects than seen with other CHC delivery methods. Some users may experience increased vaginal irritation and discharge. There is also some evidence of reduced vaginal dryness, which may appeal to perimenopausal women and others who tend to experience such dryness,” Theresa adds. It is
mycontraception.ie (2022) year old’s can now get free contraception in Ireland. The free scheme is to be extended September 2023. The free contraception scheme covers the full cost of prescription
mycontraception.ie (2022)
17 25 year old’s can now get free contraception in Ireland. The free scheme is to be extended in September 2023. The free contraception scheme covers the full cost of prescription
for more than 24 hours , or the same patch is left on the skin for more than
but there is no limit to the number of implants that can be fitted.
over 99 % effective with perfect use, and 91% effective if not always used correctly.
“Advantages are that it can be self-inserted, does not interrupt sex, and stays in place for 21 days before removal. Its effectiveness may be reduced by taking certain medications, and it is not suitable for people who are obese, and smokers over 35 years of age. It may also not be suitable if breastfeeding, and medical advice should be sought for same.”
Ring users may have concerns about their risk for pregnancy if the ring is removed intentionally or accidentally. The ring can be removed for up to three hours without diminishing its contraceptive effect.
Progesterone only pill - POP Progestogen-only pills (POP) are generally made with first-generation progestins, and dosage amounts are substantially lower than those found in any CHC. POPs must be taken at the same time every day. They are used continuously, with no hormone-free interval.
“Progestogen-only pills are considered safe in many clinical scenarios where CHCs are contraindicated,” Theresa says but adds, “Progestogen-only pills however, have a higher failure rate when not taken at the same time every day, because effective drug levels are only maintained in the bloodstream for 22 hours. The progestogen-only pill may be commenced at any time. Another method of contraception should be used if vaginal intercourse takes place during the first 48 hours of pill use. Protection will begin after two days.
“There are two types of progestogen-only pills available in Ireland, Noriday and Cerazette, which differ in the way they work and instructions for what to do if a pill is missed. If a pill is forgotten,
it should be taken as soon as remembered, and the next one at the correct time. This may mean taking two pills in one day. If the Noriday pill is taken more than 3 hours late, the woman is not protected. She should continue to take the pill as soon as possible and will be protected again after 2 days of taking the pill normally. Until then, using condoms or abstinence from sex is required.
compliance. LARC needs to be inserted and removed by a specially trained doctor or nurse. Most are more than 99% effective. An exception is the injection which can have lower efficacy. LARC can be used by most women of any age, including those who cannot use contraception containing oestrogen; experience side effects with oestrogen such as nausea or breast tenderness; have migraines; smoke; have never had a baby; are breastfeeding or have recently had a baby; have recently had a termination of pregnancy; are overweight; have diabetes or epilepsy; living with HIV; or have inflammatory bowel disease.”
reduced by taking certain medications. It is not suitable for people who are obese, and over the age of 35. It may not be suitable for women who are breastfeeding , and medical advice should be sought . 2, 3 It is a very effective method when used correctly, however, pregnancy can occur if an error is made using the patch, especially if it becomes loose or falls off for more than 24 hours , or the same patch is left on the skin for more than one week.5
Implant (Implanon)
Removal may leave a small scar. The implant is not affected by common antibiotics, but is affected by enzyme-inducing medication such as HIV treatments or epilepsy medication. An implant can be inserted 3 weeks (21 days) after giving birth. If the implant is put in, on or before day 21 the woman will be protected from pregnancy immediately. If the implant is put in later than day 21 an extra method of contraception is required for 7 days. The implant can also be used immediately after a miscarriage or termination of pregnancy. There is no evidence that there is a delay in return to fertility after the removal of an implant. Over 90% of women will have returned to a normal cycle within 4 – 6 weeks.

“If the Cerazette pill is more than 12 hours late the woman is not protected against pregnancy. Protection against pregnancy will return after 2 days of taking the pill normally. Until then using condoms or abstinence from sex is required.
“It is unlikely that taking the pill during early pregnancy will increase the risk of defects in the foetus. However, although it is rare, the likelihood of ectopic pregnancy is greater if pregnancy occurs while taking the progestogen-only pill.”
ring (NuvaRing) is a clear, flexible ring about two inches in diameter synthetic oestrogen and progesterone that is placed in the vagina for 21 days , and days to allow for withdrawal bleeding . It is replaced monthly. Users can vaginal canal themselves. As with the patch, the less frequent applications and can lead to increased adherence. The ring’s internal placement ensures of hormones, which allows for lower serum conc entrations than occur patch or pills. As a result, the ring generally has milder side effects than seen delivery methods. Some users may experience increased vaginal irritation and also some evidence of re duced vaginal dryness, which may appeal to women and others who tend to experience such dryness. 4 It is over 99 % perfect use, and 91% effective if not always used correctly. Advantages are that inserted, does not interrupt sex, and stays in place for 21 days before removal. may be reduced by taking certain medications, and it is not suitable for obese, and smokers over 35 years of age. It may also not be suitable if medical advice should be sought for same. 2, 3

The hormonal coil also called an IUS, is a small, T-shaped device that is placed in a woman’s uterus. It is made of flexible plastic which contains a slow-releasing hormone called Levonorgestrel. An IUS works by changing the conditions in the uterus and cervix. These changes prevent sperm fertilising an egg and may also prevent a fertilised egg implanting in the womb.
Long acting reversible contraception (LARC) are a group of contraception methods that provide very effective contraception, are long acting and reversible when removed or stopped. They include injections, intrauterine devices- hormonal coil (IUS) and copper coil (IUCD), and subdermal contraceptive implants.
The contraceptive implant is a small flexible plastic rod which is implanted with minor surgery under the skin, in the inner part of the upper arm. Local anaesthetic is used to numb the skin before insertion. The implant slowly releases progesterone hormone and gives contraceptive protection for three years. It can be removed at any time with minor surgery, and it can be used by women of all ages whether they have had children or not. A pregnancy test will be checked before insertion to confirm the woman is not pregnant. The implant is suitable for women who are breast-feeding or who do not tolerate oestrogen. Fertility returns to normal once the implant is removed. In the first 3 – 6 months of use, many women have irregular bleeding. After this most women will have lighter or less frequent periods. Some will have regular monthly periods, and some will not bleed at all.
The CHC vaginal ring (NuvaRing) is a clear, flexible ring about two inches in diameter containing synthetic oestrogen and progesterone that is placed in the vagina for 21 days, and removed for seven days to allow for withdrawal bleeding . It is replaced monthly. Users can place the ring in the vaginal canal themselves. As with the patch, the less frequent applications can be appealing and can lead to increased adherence. The ring’s internal placement ensures the steady delivery of hormones, which allows for lower serum conc entrations than occur with either the patch or pills. As a result, the ring generally has milder side effects than seen with other CHC delivery methods. Some users may experience increased vaginal irritation and discharge. There is also some evidence of re duced vaginal dryness, which may appeal to perimenopausal women and others who tend to experience such dryness. 4 It is over 99 % effective with perfect use, and 91% effective if not always used correctly. Advantages are that it can be self inserted, does not interrupt sex, and stays in place for 21 days before removal. Its effectiveness may be reduced by taking certain medications, and it is not suitable for people who are obese, and smokers over 35 years of age. It may also not be suitable if breastfeeding, and medical advice should be sought for same. 2, 3
Theresa says, “LARC are the most effective reversible methods of contraception because their efficacy is not reliant on patient
The implant will begin to work within one week. if there are no medical problems the implant can be used as a method of contraception until the menopause. Each implant will have to be changed every 3 years,
Theresa explains, “There are three types of IUS available; the Mirena which lasts for eight years, Kyleena which lasts five years, and Jaydess which lasts three years. An IUS can be fitted at any time during the menstrual cycle once it is confirmed that the woman is not pregnant. An internal examination is carried out before the IUS is inserted and the insertion visit takes approximately 15-20 minutes. Insertion can be uncomfortable and some women experience a period-type pain and light bleeding for a few days afterwards. Some women may experience common hormonal side effects such as headaches, breast tenderness and abdominal discomfort. There is a small risk of infection and of expulsion. The risk of perforation is very low when the IUS is fitted by an experienced HCP.
T-frame of Kyleena contains barium sulphate which makes it visible in X-ray examination. The system should be removed no later than by the end of the fifth year. If the woman wishes to continue using the same method, a new system can be inserted immediately following removal of the original system. If pregnancy is not desired, the removal should be carried out within 7 days of the onset of menstruation, provided the woman is experiencing regular menses. After removal of Kyleena, the system should be examined to ensure that it is intact. Elderly patients: Kyleena has not been studied in women over the age of 65 years. There is no indication for the use of Kyleena in postmenopausal women. Paediatric population: Use of this product before menarche is not indicated. Contraindications: Pregnancy; acute or recurrent pelvic inflammatory disease (PID) or conditions associated with increased risk for pelvic infections; acute cervicitis or vaginitis; postpartum endometritis or infected abortion during the past three months; cervical intraepithelial neoplasia until resolved; uterine or cervical malignancy; progestogen-sensitive tumours, e.g. breast cancer; abnormal vaginal bleeding of unknown aetiology; congenital or acquired uterine anomaly including fibroids which would interfere with insertion and/or retention of the IUS (i.e. if they distort the uterine cavity); acute liver disease or liver tumour; hypersensitivity to the active substance or to any of the excipients. Warnings and Precautions: Use with caution after specialist consultation, or consider removal of the system if any of the following conditions exist or arise for the first time: migraine, focal migraine with asymmetrical visual loss or other symptoms indicating transient cerebral ischemia; exceptionally severe headache; jaundice; marked increase in blood pressure; severe arterial disease such as stroke or myocardial infarction. May affect glucose tolerance, monitor the blood glucose concentration in diabetic users. However, there is generally no need to alter the therapeutic regimen in diabetics using levonorgestrel - IUS. Medical examination/consultation: Before insertion, a woman must be informed of the benefits and risks of Kyleena, including the signs and symptoms of perforation and the risk of ectopic pregnancy, see below. A physical examination including pelvic examination, examination of the breasts, and a cervical smear should be performed. Pregnancy and sexually transmitted diseases should be excluded. Genital infections should be successfully treated prior to insertion. The position of the uterus and the size of the uterine cavity should be determined. Fundal positioning of Kyleena is important in order to maximize the efficacy and reduce the risk of expulsion. Insertion and removal may be associated with some pain and bleeding. The procedure may precipitate a vasovagal reaction (e.g. syncope, or a seizure in an epileptic patient). A woman should be re-examined 4 to 6 weeks after insertion to check the threads and ensure that the system is in the correct position. Follow-up visits are recommended once a year thereafter, or more frequently if clinically indicated. Kyleena is not for use as a post-coital contraceptive. The use of Kyleena for the treatment of heavy menstrual bleeding or protection from endometrial hyperplasia during oestrogen replacement therapy has not been established. Ectopic pregnancy: In clinical trials, the overall incidence of ectopic pregnancy with Kyleena was approximately 0.20 per 100 woman-years. Approximately half of the pregnancies that occur during Kyleena use are likely to be ectopic. For women who become pregnant while using Kyleena, the possibility of an ectopic pregnancy must be considered and evaluated. Women with a previous history of ectopic pregnancy, tubal surgery or pelvic infection carry an increased risk of ectopic pregnancy. Because an ectopic pregnancy may impact future fertility the benefits and risks of using Kyleena should be carefully evaluated on an individual basis. Effects on the menstrual bleeding pattern: Effects on the menstrual bleeding pattern are expected in most users of Kyleena. Those alterations are a result of the direct action of levonorgestrel on the endometrium and may not correlate with the ovarian activity. Irregular bleeding and spotting are common in the first months of use. Thereafter, the strong suppression of the endometrium results in the reduction of the duration and volume of menstrual bleeding. Scanty flow frequently develops into oligomenorrhea or amenorrhea. Pregnancy should be considered if menstruation does not occur within six weeks of the onset of previous menstruation. A repeated pregnancy test is not necessary in subjects who remain amenorrheic unless indicated by other signs of pregnancy. Pelvic infection: Pelvic infection has been reported during use of any IUS or IUD. In clinical trials, PID was observed more frequently at the beginning of Kyleena use. Before electing use of Kyleena, patients should be fully evaluated for risk factors associated with pelvic infection (e.g. multiple sexual partners, sexually transmitted infections, prior history of PID). As with other gynaecological or surgical procedures, severe infection or sepsis (including group A streptococcal sepsis) can occur following IUD insertion, although this is extremely rare. If a woman experiences recurrent endometritis or PID or if an acute infection is severe or does not respond to treatment, Kyleena must be removed. Expulsion: In clinical trials with Kyleena, the incidence of expulsion was low (<4% of insertions) and in the same range as reported for other IUDs and IUSs. Symptoms of partial or complete expulsion of Kyleena may include bleeding or pain. However, the system can be expelled from the uterine cavity without the woman noticing it, leading to loss of contraceptive protection. As Kyleena decreases menstrual flow, increase of menstrual flow may be indicative of an expulsion. Risk of expulsion is increased in: Women with history of heavy menstrual bleeding; Women with greater than normal BMI at the time of insertion; this risk increases gradually with increasing BMI. Women should be counselled on possible signs of expulsion and how to check the threads of Kyleena and advised to contact a healthcare professional if the threads cannot be felt. A barrier contraceptive (such as a condom) should be used until the location of Kyleena has been confirmed. Partial expulsion may decrease the effectiveness of Kyleena. A partially expelled Kyleena should be removed. A new system can be inserted at the time of removal, provided pregnancy has been excluded. Perforation: Perforation or penetration of the uterine corpus or cervix by an intrauterine contraceptive may occur, most often during insertion, although it may not be detected until sometime later, and may decrease the effectiveness of Kyleena. In case of a difficult insertion and/or exceptional pain or bleeding during or after insertion, the possibility of perforation should be considered and appropriate steps should be taken, such as physical examination and ultrasound. Such a system must be removed; surgery may be required. Physical examination may not be sufficient to exclude partial perforation. A large prospective comparative noninterventional cohort study in users of other IUDs (N=61,448 women) with a 1-year observational period, showed that both breastfeeding at the time of insertion and insertion up to 36 weeks after giving birth were associated with an increased risk of perforation. Both risk factors were independent of the type of IUD inserted. Extending the observational period to 5 years in a subgroup of this study (N=39009 women inserted with another levonorgestrel- IUS or copper IUD, 73% of these women had information available over the complete 5 years of follow-up), the incidence of perforation detected at any time during the entire 5-year period was 2.0 (95% CI: 1.6-2.5) per 1000 insertions. Breastfeeding at the time of insertion and insertion up to 36 weeks after giving birth were confirmed as risk factors also in the subgroup that were followed up for 5 years. The risk of perforations may be increased in women with fixed retroverted uterus. Re-examination after insertion should follow the guidance given under the heading “Medical examination/consultation” which may be adapted as clinically indicated in women with risk factors for perforation. Lost threads: If the removal threads are not visible at the cervix on follow-up examinations, unnoticed expulsion and pregnancy must be excluded. Ultrasound or, if appropriate, x-ray may be used to ascertain the correct position of Kyleena. Ovarian cysts/enlarged ovarian follicles: Sometimes atresia of the follicle is delayed and folliculogenesis may continue. These enlarged follicles cannot be distinguished clinically from ovarian cysts and have been reported in clinical trials as adverse drug events in approximately 22.2 % of women using Kyleena including ovarian cyst, hemorrhagic ovarian cyst and ruptured ovarian cyst. Should an enlarged follicle fail to resolve spontaneously, continued ultrasound monitoring and other diagnostic/therapeutic measures may be appropriate. Psychiatric disorders: Depressed mood and depression are well-known undesirable effects of hormonal contraceptive use. Depression can be serious and is a well-known risk factor for suicidal behaviour and suicide. Women should be advised to contact their physician in case of mood changes and depressive symptoms, including shortly after initiating the treatment. Interactions: Interactions can occur with medicinal products that induce microsomal enzymes, which can result in increased clearance of sex hormones. Substances known to increase the clearance of levonorgestrel are Phenytoin, barbiturates, primidone, carbamazepine, rifampicin, and possibly also oxcarbazepine, topiramate, felbamate, griseofulvin, and products containing St. John’s wort. The influence of these medicinal products on the efficacy of Kyleena is not known. Many HIV/HCV protease inhibitors and non-nucleoside reverse transcriptase inhibitors when co-administered with sex hormones can have variable effects on the clearance of levonorgestrel (i.e. increase or decrease plasma concentrations of the progestin). Magnetic resonance imaging (MRI): Non-clinical testing has demonstrated that a patient can be scanned safely after placement of Kyleena under the following conditions: Static magnetic field of 3-Tesla or less, maximum spatial gradient magnetic field of 36000-Gauss/cm or less and maximum whole body averaged specific absorption rate (SAR) of 4 W/ kg in the First Level Controlled mode for 15 minutes of continuous scanning. Fertility, pregnancy and lactation: Fertility: The use of a levonorgestrel-releasing intrauterine system does not alter the course of future fertility. Upon removal of the intrauterine system, women return to their normal fertility. Pregnancy: The use of Kyleena during an existing or suspected pregnancy is contraindicated. If the woman becomes pregnant while using Kyleena, the system should be removed as soon as possible, since any intrauterine contraceptive left in situ may increase the risk of abortion and preterm labour. Removal of Kyleena or probing of the uterus may also result in spontaneous abortion. Ectopic pregnancy should be excluded. Clinical experience of the outcomes of pregnancies under Kyleena treatment is limited due to the high contraceptive efficacy. Breast-feeding: A levonorgestrel-releasing IUS does not affect the quantity or quality of breast milk. Small amounts of progestogen (about 0.1 % of the levonorgestrel dose) pass into the breast milk in nursing mothers. Effects on ability to drive and use machines: Kyleena has no known influence on the ability to drive or use machines. Undesirable Effects: Very common: headache, abdominal/pelvic pain, acne/seborrhoea, bleeding changes including increased and decreased menstrual bleeding, spotting, infrequent bleeding and amenorrhoea, ovarian cyst, vulvovaginitis; Common: depressed mood/depression, decreased libido, migraine, dizziness, nausea, alopecia, upper genital tract infection, dysmenorrhea, breast pain/discomfort, device expulsion (complete and partial), genital discharge, increased weight; Uncommon: hirsutism, uterine perforation. With the use of levonorgestrel-IUS, cases of hypersensitivity including rash, urticaria and angiooedema have been reported. Marketing Authorisation Number: PA 1410/081/001. Marketing Authorisation Holder/ Further information available from: Bayer Limited, 1st Floor, The Grange Offices, The Grange. Brewery Road, Stillorgan, Co. Dublin, A94 H2K7. Tel.: (01) 2163300. Classification for sale or supply: prescription only. Date of preparation: 11/2022

Ref: 1. Bayer. Kyleena® Summary of Product Characteristics. * Year 1 Pearl Index: 0.16 (95% CIs 0.02–0.58). 5-years Pearl Index: 0.29 (95% CIs 0.16–0.50). The failure rate was approximately 0.2% at 1 year. The cumulative failure rate was approximately 1.4% at 5 years. available in a 5-year IUS

woman’s uterus. It is made of flexible plastic with a coating of thin copper two soft threads on the end which hang through the opening at the cervix vagina. An IUCD works by preventing sperm from surviving in the cervix, tubes. It may also work by stopping a fertilised egg from implanting in the
IUCD- Copper Coil
effective method of contraception for 5 to 10 years depending on the are different types of IUCDs , the newer devices contain more copper and effective. Older IUCDs have less copper and are less effective. An IUCD time during the menstrual cycle once it is confirmed that the woman is internal examination is carried out before the IUCD is inserted. Insertion can and some women experience a period type pain and light bleeding for a
is inserted. Insertion can be uncomfortable and some women experience a period-type pain and light bleeding for a few days after insertion. There is a slight risk that an IUCD might perforate the uterus or cervix during insertion. This may cause pain but there are often no symptoms. If this happens the IUCD may have to be surgically removed. The risk of perforation is very low when the IUCD is fitted by an experienced HCP. Another contraceptive method will need to be used until then. An IUCD can be used safely while breastfeeding.
work by preventing semen from entering the vagina. A male condom is made from very thin, natural latex rubber which is soft and stretchy. It is closed at one end, with a teat to collect sperm, and fits over an erect penis. A female condom is made of very thin polyurethane plastic. It is closed at one end, and designed to form a loose lining to a woman’s vagina with two flexible rings, one at each end, to keep it in place.
Until then using condoms or abstinence from sex is required. 5 It is that taking the pill during early pregnancy will increase the risk of defects in the lthough it is rare, the likelihood of ectopic pregnancy is greater if pregnancy occurs while taking the progestogen only pill.5
“A check-up is required 4 to 6 weeks after insertion, to check the strings, make sure the device is properly in place and that there are no problems. The woman is taught to feel for the strings, and should check them monthly to make sure the IUS is in place. Normal fertility returns as soon as the IUS is removed. Another IUS can be inserted directly after the old IUS has been removed.”
Emergency contraception is a safe, effective and responsible method of preventing pregnancy when regular contraception has failed, no contraception was used, and in the event of sexual assault. Emergency contraception will usually prevent pregnancy, if taken on time. Emergency contraception also known as post-coital contraception is available in two forms: Emergency contraceptive pill (ECP) progesterone (3-day) and ulipristal (5-day); or insertion of an intrauterine copper device (IUCD) by a trained health care professional, as described above (See IUCD- Copper Coil). The progesterone ECP is available from pharmacies without a prescription or from a doctor or family planning clinic. It must be taken within 72 hours (3 days) of unprotected sex but is most effective the sooner it is taken.
Long acting reversible contraception (LARC) are a group of contraception methods that provide very effective contraception , are long acting and reversible when removed or stopped. They include injections, intrauterine devices hormonal coil (IUS) and copper coil (IUCD), and subdermal contraceptive implants. LARC are the most effective reversible methods of contraception because their efficacy is not reliant on patient compliance.
An IUCD, also known as the IUD, loop or copper coil, is a small T-shaped contraceptive device which is placed in a woman’s uterus. It is made of flexible plastic with a coating of thin copper wire. It has one or two soft threads on the end which hang through the opening at the cervix into the top of the vagina. An IUCD works by preventing sperm from surviving in the cervix, uterus or fallopian tubes. It may also work by stopping a fertilised egg from implanting in the uterus.

The contraceptive injection DepoProvera contains the hormone progestogen. It is effective for 12 weeks. “The hormone is injected into a muscle, initially during the first five days of a woman’s period and further injections are required every 12 weeks. The injection acts primarily by preventing ovulation. Less often it works by thickening cervical mucus to prevent sperm from fertilising an egg or by altering the lining of the uterus, which may prevent implantation of a fertilised egg,” she says. “The contraceptive injection may be associated with weight gain and other side effects such as acne, breast tenderness, bloating and change in mood. For some women, use of DepoProvera may be associated with an increase in weight of up to 2– 3 kg over 1 year.
Advantages are that condoms help prevent the spread of HIV and STIs, are easily accessible, only need to be used when having sex, and have very few or no side effects. Male condoms come in a variety of shapes and sizes. A female condom can be put in any time before sex. Disadvantages are that male condom can sometimes slip off or split. Some people are sensitive to latex, though this is rare. Female condom may be noisy and may slip during sex. Female condoms are not suitable for women who have an infection in their vagina or cervix. Male condoms are 98% effective with perfect use every time, but 82% effective when the method is not used correctly every time.
LARC needs to be inserted and removed by a specially trained doctor or nurse. Most are more than 99% effective. An exception is the injection which ca n have lower efficacy. LARC can be used by most women of any age, including those who cannot use contraception containing oestrogen; experience side effects with oestrogen such as nausea or breast tenderness ; have migraines; smoke; have never had a baby; are breastfeeding or have recently had a baby ; have recently had a termination of pregnancy; are overweight; have diabetes or epilepsy; living with HIV; or have inflammatory bowel disease . Different LARC work in different ways. 6
“The IUCD acts as an effective method of contraception for 5 to 10 years depending on the device used,” says Theresa. “There are different types of IUCDs, the newer devices contain more copper and are more than 99% effective. Older IUCDs have less copper and are less effective.”
An IUCD can be fitted at any time during the menstrual cycle once it is confirmed that the woman is not pregnant. An internal examination is carried out before the IUCD
“Some medical conditions may make the injectable contraceptive unsuitable. These can include breast cancer, unexplained vaginal bleeding, thrombosis, heart attack or stroke, diabetes with complications or for more than 20 years, active liver disease, and risk factors for osteoporosis. As with any injection there is a risk of a small infection at the site of the injection. Prolonged Depo Provera use for more than 3-4 years is associated with a small loss of bone mineral density which is largely recovered when the contraceptive injection is stopped.”
Theresa adds, “The main brand of the ulipristal ECP in Ireland is ellaOne, and is available from pharmacies without a prescription or from a doctor or family planning clinic. It must be taken within 120 hours (5 days) of unprotected sex but is most effective the sooner it is taken. Evidence suggests that the ulipristal ECP (ellaOne) is more effective than the progesterone ECP. Both methods of the ECP work by preventing or delaying ovulation, thereby preventing fertilisation. There are no long-term side effects from using the ECP. It is important to take the ECP as soon as possible after unprotected sex, and the doctor or pharmacist should be informed if the woman is taking any other medication. A small number of medications may reduce the effectiveness of the ECP and the woman may need a different dosage or an IUCD. If vomiting occurs within 3 hours of taking the ECP, it is important to return to the doctor or pharmacist as soon as possible for a second dose or further advice.
Diaphragm: A vaginal diaphragm is a barrier method of contraception which fits in the woman’s vagina. Vaginal diaphragms are domes made of thin, soft silicone with a flexible rim. The diaphragm covers the woman’s cervix and prevents the sperm from reaching the egg. To be effective, diaphragms need to be used with a contraceptive gel to inactivate any sperm that are present. If used according to instructions, latex diaphragms are 92-96% effective when used correctly and with contraceptive gel. It is 71-88% effective if not always used correctly.

The contraceptive implant is a small flexible plastic rod which is implanted with minor surgery under the skin, in the inner part of the upper arm. Local anaesthetic is used to numb the skin before insertion. The implant slowly releases progesterone hormone and gives contraceptive protection for three years. It can be removed at any time with minor surgery, and it can be used by women of all ages whether they have had children or not. A pregnancy test will be checked before insertion to confirm the woman is not pregnant. The implant is suitable for women who are breast feeding or who do not tolerate oestrogen. Fertility returns to normal once the implant is removed. In the first 3 6 months of use, many women have irregular bleeding. After this most women will have lighter or less frequent periods. Some will have regular monthly periods, and some will not bleed at all. 3, 7
Condoms: Condoms are a barrier method of contraception which
Theresa says, “Advantages are that the diaphragm can be put in place anytime before sex, reused after careful washing and drying, and it is a useful method if the woman wants to avoid taking hormonal contraception. Disadvantages are that it can disrupt sex; may not be suitable for people who are sensitive to the chemicals in contraceptive gel; annual checks are required to make sure it still fits; a different size may be needed if more than 3 kgs in weight is added or lost; it must be left in place for at least 6 hours after sex, but not longer than 24 hours; extra spermicide must be used if sex occurs more than once; and it does not protect against STIs.”
Implant
2 years. Do not use as an inhalation for children < 6 years. Precautions: Patient groups with a history of airway disease or pronounced hypersensitivity of the airways / asthma should use with caution or consult doctor before use. Keep out of reach and sight of children. If symptoms persist, consult your doctor. Topical: Do not apply to broken skin, wounds or mucous membranes. Do not swallow or apply directly onto the nostrils, eyes, mouth or face. For external use only. Do not bandage tightly. Do not use with heat wrap. Inhalation: Do not use boiling water to prepare inhalations. Do not heat or re-heat the mixture in a microwave. Undesirable effects: Local effects: Redness, irritation of the skin, irritation of the eyes (by inhalation), allergic dermatitis. Irritations or allergic reactions are usually mild and occur rarely. General disorders and administration site conditions: Burns at application site – frequency not known. Systemic effects: Due to the recommended route of administration; systemic exposure is very low and undesirable effects due to systemic exposure have not been observed. Supply Status: Supply through non-pharmacy outlets and pharmacies. PA Number: PA2294/003/001 PA Holder: WICK Pharma - Zweigniederlassung der Procter & Gamble GmbH, Sulzbacher Str. 40, 65823 Schwalbach am Taunus, Germany. Text prepared: August 2020. Further information available on request.
1. Procter & Gamble. Online Consumer Survey conducted among 313 French cold sufferers in the last 12 months. Data on File 2022. 2. Vicks VapoRub SmPC Theraupetic indications. 3. Santhi N, Ramsey D, Phillipson G, Hull D, Revell VL, Dijk D-J. (2017) Efficacy of a topical aromatic rub (Vicks VapoRub®) on effects on self-reported and actigraphically assessed aspects of sleep in common cold patients. OJRD 7: 83-101. 4. Eccles, R., Jawad, M., Ramsey, D.L. and Hull, J.D. (2015) Efficacy of a Topical Aromatic Rub (Vicks VapoRub®)-Speed of Action of Subjective Nasal Cooling and Relief from Nasal Congestion. Open Journal of Respiratory Diseases, 5, 10-18. 5. Procter & Gamble. Technical Report RPS1147/44. Data on File 2010. * There are no known interactions with other medicines according to usage directions (SmPC 4.5).










Introducing Teva’s NEW brand MotusolTM Max, a powerful anti-inflammatory gel that provides targeted, effective pain relief for sprains, strains, and bruising caused by blunt trauma.
• Rest – Avoid activity for 48-72 hours after injury
• Ice – Every 2-3 hours, apply ice or a bag of frozen vegetables (wrapped in a damp towel) for 15-20 minutes
• Compression – Use an elastic bandage to control swelling. This should only be worn when awake and it should not be tight
• Elevation – Where possible, raise injured area with pillow until swelling subsides
Aside from dispensing prescription medicines, there are many over-the-counter options available for pharmacy patients. Analgesics such as Paracetamol can ease the pain and topical medications such as non-steroidal anti-inflammatory drug (NSAID) gels can be used to reduce swelling in sprains and strains.3-4 MotusolTM Max, containing diclofenac it is an efficient NSAID that reduces inflammation-related discomfort and swelling.2
Why Recommend MotusolTM Max?
3. Helps restores movement by reducing inflammation, MotusolTM gel helps speed up recovery time and restore movement in the affected area
It provides:
• Effective relief from inflammation in joints and muscles when associated with strains and sprains
• All-day pain relief when applied twice daily
• An Easy Open Cap, helping patients suffering from hand strains or sprains to open easily
Patients should be advised most strains and sprains should feel better after 2 weeks but severe ones might take months to get back to normal. If symptoms worsen or do not improve after 3 - 5 days, a doctor should be consulted.
For more information about MotusolTM Max contact your local Teva Representative, call 1800-201-700 or email info@teva.ie email.
Did you know, approximately 1.71 billion people worldwide are estimated to suffer from some form of musculoskeletal pain?1 Motusol’sTM active ingredient, diclofenac, offers direct joint and muscle pain reduction when applied topically.2
Strains and sprains are common soft tissue injuries to the muscles, tendons and ligaments.
• Strains relate to a stretch/tear of muscle fibres typically affecting the foot, hamstring or back muscles3
• Sprains are injuries to the ligaments which connect one bone to another, giving stability and strength to a joint. Ankles, knees, wrists and thumbs are typically affected3
team evaluate if the patient should be sent to a doctor or manage symptoms at home.
During an examination of the injury, observe signs of swelling, skin damage and/or bruising.
• Sprain symptoms usually include pain around the affected joint, tenderness, swelling, bruising, pain on weight-bearing, and limited range of movement
• Strain symptoms can include muscle pain, cramping, and spasm; muscle weakness, inflammation, and/or bruising
How to identify a strain/sprain Pharmacy teams play a key role in providing support and information to enable the self-care process. Knowing the signs of a suspected strain or sprain and how to manage will help your pharmacy
When a strain or sprain has been identified as something that can be treated at home, PRICE is the first self-care step for early intervention. PRICE should be started as soon as possible and continue for 72 hours after injury.
Its triple effect formula works in three ways to provide effective pain relief when applied directly to the source of injury:
• Protection – Protect area from further injury (suitable supportive footwear or wrist support)
Its triple-effect formula works in three ways to provide effective pain relief when applied directly to the source of injury:
1. Targets Pain directly at the source – Unlike tablets, which have to be absorbed via the stomach, MotusolTM Max is applied directly to the affected parts of the body, penetrating the skin to target the inflamed tissues providing effective pain relief right where it’s needed

2. Reduces inflammation – It develops its therapeutic efficacy mainly by inhibiting prostaglandin synthesis by cyclooxygenase 2 (COX-2), reducing inflammatory-related pain and swelling
Full PI can be found on adjacent page
References:
1. https://www.who.int/ newsroom/ factsheets/detail/ musculoskeletal-conditions Assessed September 2022 2. Motusol Max (diclofenac) SMPC 3. Cks.nice.org.uk/topics/sprainsstrains/management Assessed: November 2022 4. Derry S, et al. Cochrane Database Sys Rev 015;6:CD007402
Date of Preparation: November 2022 Job Code: Dic-IE-00011
1. Targets Pain directly at the source Unlike tablets, which have to be absorbed via the stomach, MotusolTM Max is applied directly to the affected parts of the body, penetrating the skin to target the inflamed tissues providing effective pain relief right where it’s needed.

2. Reduces inflammation
It develops its therapeutic efficacy mainly by inhibiting



Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com
Please refer to the Summary of Product Characteristics (SmPC) for full details of Prescribing Information.

Motusol Max (diclofenac sodium) 2% w/w gel Abbreviated Prescribing Information. Presentation: White to almost white, homogeneous gel. 1g of gel contains diclofenac as 23.2mg diclofenac diethylamine corresponding to 20mg of diclofenac sodium. Indications: Local symptomatic treatment of pain in acute strains, sprains or contusions following blunt trauma. For short term treatment only. Dosage and administration: For cutaneous use. Apply to affected parts of the body thinly and gently rub into skin. Wash hands after application unless area to be treated. Allow gel to dry on skin before applying bandages. Adults and adolescents aged 14 years and over: depending on the size of the affected site, apply a cherry to walnut size quantity 2 times a day (preferably morning and evening). Should not be used for longer than 1 week without medical advice. Children: no data in children and adolescents under 14 years of age. Elderly: no dosage adjustment required. Monitor patient carefully. Renal and hepatic impairment: no dosage adjustment required. Contraindications: Hypersensitivity to active substances or excipients; patients with a history of hypersensitivity reactions such as asthma, bronchospasmus, urticaria, acute rhinitis in response to acetylsalicylic acid or non- steroidal anti-inflammatory drugs (NSAIDs); open injuries, inflammations or infections of the skin as well as on eczema or mucous membranes; in the last trimester of pregnancy; in children and adolescents under 14 years of age. Precautions and warnings: Systemic undesirable effects cannot be excluded if applied on larger areas of skin over a prolonged period of time. Must only be applied to intact, not diseased or injured skin. Must not come into contact with eyes and oral mucous membranes. Must not be taken orally. May be used with non-occlusive bandages, but not with airtight occlusive dressing. Consult doctor if symptoms worsen or do not improve after 3-5 days. Patients suffering from asthma, hay fever, swelling of nasal mucous membranes (so called nasal polyps) ) or chronic obstructive pulmonary disease, chronic

respiratory infections (particularly associated with hay fever-like symptoms), and patients with hypersensitivity to painkillers and anti-rheumatic medicinal products of all kinds are rather at risk to asthma attacks (so called analgesic intolerance / analgesic asthma), to local skin or mucous membrane swelling (so-called quincke edema) or to urticaria than other patients when treated with Motusol Max. In these patients, Motusol Max may only be used under certain precautions (emergency preparedness) and direct medical supervision. The same applies for patients who are also allergic to other substances e.g. with skin reactions, itching or urticaria. Discontinue treatment if skin rash occurs. Photosensitivity can occur with the appearance of skin reactions after exposition to sunlight. Avoid children coming into contact to the skin areas where the gel has been applied. Contains butylhydroxytoluene which may cause local skin reactions or irritation to the eyes and mucous membranes. Contains fragrance with benzyl alcohol (0.15mg/g), citral, citronellol, coumarin, eugenol, farnesol, geraniol, d-limonene and linalool which may cause allergic reactions. In addition, benzyl alcohol may cause mild local irritation. Interactions: None known. Pregnancy and lactation: Should not be used during first and second trimester unless clearly necessary. Contraindicated during third trimester. Should only be used during breast-feeding under advice from a healthcare professional and should not be applied on the breasts, nor elsewhere on large areas of skin for a prolonged period of time. Effects on ability to drive and use machines: No or negligible influence. Adverse reactions: Hypersensitivity, angioedema, dermatitis bullous, Common: dermatitis (including contact dermatitis), skin rash, erythema, eczema, pruritus. Consult the Summary of Product Characteristics in relation to other side effects. Overdose: Wash skin with water where applied. If ingested, apply general therapeutic measures normally adopted to treat poisoning with non-steroidal anti-inflammatory medicinal products. Gastric lavage and use of activated charcoal should be considered. Legal category: Pharmacy. Marketing Authorisation Number: PA1986/093/002. Marketing Authorisation Holder: Teva B.V., Swensweg 5, 2031GA Haarlem, Netherlands. Job Code: MED-IE-00036. Date of Preparation: June 2021
Job Code: Dic-IE-00009. Date of Preparation: November 2022

With Autumn truly behind us, many of our patients and customers are beginning to seek advice on managing winter skin issues. While most of us will feel at least some effects of the cold weather on our skin, many skin conditions, such as psoriasis and eczema, also worsen significantly at this time of year. Understanding some of the causes of skin discomfort is important in helping our patients and customers to soothe and prevent irritation.
The skin is comprised of three layers:
• The outermost layer is the epidermis, which provides a waterproof barrier against the elements
• Beneath the epidermis is the dermis, which contains sweat glands, hair follicles, and connective tissue
• The deepest layer is the hypodermis, which is made up of fat and connective tissue. Each layer of the skin is impacted by the winter elements and requires increased care.
As the largest organ in the body, the skin serves multiple functions, but one of the most important is that of protection. The skin acts as a barrier against harmful environmental elements and toxins. Several aspects of winter can impact on the skin’s protective performance. Temperature extremes, changes to diet and activity levels, and colder weather all mean the skin has to work harder.
Exposure to cold temperatures causes the blood vessels in the surface of the skin to narrow, in order to preserve heat. When exposed to warmth, the same blood vessels widen. During winter, when we come in from the cold to warmer temperatures quickly, the blood vessels at the skin’s surface can be put under more pressure due to the sudden increase in blood flow. Additionally, during colder temperatures, the production of lipids that help hydrate and nourish the skin begins to slow down. The production of filaggrin, a protein essential for maintaining barrier function and moisturising of the skin, also slows down in winter.
All of this combined with decreased humidity, exposure to indoor heating and less sunlight leads to dryer, tighter, and paler skin, as well as worsening of existing skin conditions. Complaints can include itching, flaking skin, redness, cracked lips, and dry scalp.
Existing skin conditions can all be affected by the winter elements, and many of our patients suffering from these conditions will seek additional advice in the coming months.
Eczema sufferers can experience more flare-ups during the winter months due to the decrease in humidity and the cold temperatures. Emollients are key in managing increased dryness. They will moisturise dryer skin and should also be used before going outside to prevent worsening symptoms.

Psoriasis symptoms can become activated when the body’s defences are put under pressure. Managing this condition can be much more difficult in winter, due to decreased humidity and reduced sunlight exposure. Psoriasis symptoms can also be brought on by illness, including colds and flus. Sufferers should be advised to increase their emollient usage, dress warmly to avoid temperature drops, and increase their water intake in order to avoid dehydration.
Chilblains present as red patches of blistering and swelling on the skin, resulting from exposure to extreme temperature changes, as blood flow levels fluctuate more rapidly. Sufferers should avoid trying to help their symptoms by rapidly warming up affected areas as this can make symptoms worse. Prevent chilblains by wrapping up warm to
avoid exposure and taking OTC pain relievers if necessary.
Chapped Lips are common in colder weather and can be extremely uncomfortable. This is easily prevented by using a non-perfumed lip balm during the colder months, especially before exposure to cold conditions.
Winter Itch occurs as a result of increased dryness, so the skin becomes itchy and tight, sometimes also with a rash or scaly skin. While the symptoms can present in a similar way to eczema, winter itch is usually only experienced during colder weather. It can be prevented by ensuring increased moisturising with nonperfumed products. Sufferers should avoid wearing wool which can further irritate the skin.
Rosacea can be triggered by cold weather, causing redness as blood vessels become visible on the face. Sufferers should protect themselves against temperature fluctuations by wrapping up warm before going out and avoiding warming up too quickly. Gentle moisturisers will help, but steroid creams should be avoided.
Acne is usually associated with increased oil production, which we tend to think should be more under control during colder weather. But the dryness that winter can cause can also worsen acne, as an increase in the build up of dead skin can lead to clogged pores. Acne sufferers should ensure to be adherent to existing treatments during the winter months
and use appropriate exfoliants to gently get rid of dead skin. While the skin may be uncomfortable or itchy, patients should try their best to avoid touching their skin too much to reduce the risk of bacteria affecting the skin.
Raynaud’s Disease can be drastically impacted by cold temperatures, with the extremities being most affected. Affected patients should be advised to avoid extremes of temperatures, by wrapping up warm and ensuring they never get too cold. If symptoms are severe, a GP should be consulted to avoid infection.
Dry Scalp or Dandruff can be worse during colder weather. Ensure patients are advised against the use of over-perfumed products. Opt for appropriate treatments and avoid over-washing the hair. Most dermatological brands offer shampoos that can keep symptoms at bay and soothe flare-ups.
There are lots of steps your customers and patients can take to reduce the impact winter has on the skin, whether they have a skin condition or not.

Clothing is crucial. Firstly, for protection against the elements. Gloves, scarves, and hats should be worn outside to add a barrier of protection against the elements. Those suffering with uncomfortable skin symptoms should be advised to avoid irritating fabrics such as wool or flannel.
Avoid Extremes in Temperatures changes as much as possible. Not only cold but also heat. Turning up the central heating one or two notches might sound tempting but indoor heating can result in the skin heating up too quickly. Increased temperatures inside can also reduce humidity.
A Humidifier may be beneficial to those experiencing skin discomfort in winter. Indoor heating reduces humidity which can further dry out the skin. Dry skin conditions like eczema can also be soothed by increasing humidity.
Warm Showers or Baths are far kinder to skin that hot. While it may seem comforting to take a hot shower after being out in the cold, this can lead to temperature changes that are too much for the skin, but also to increased dryness. Make sure bathing is as short in duration as necessary. It is also advisable to pat dry after bathing rather than rubbing the skin with your towel. Use gentle, non-perfumed bathing products to avoid stripping the skin of natural oils. If exfoliation is needed, make sure to use gentle products for sensitive skin.
Prepare the Skin for cold exposure by increasing moisturisation. In general, the greasier a product is, the more protection against the cold it will offer. If suffering from skin conditions, always use appropriate products. Lip balm is also advisable during cold temperatures.
Skin Care Products should not necessarily be changed up too much for winter, to avoid any reactions to new products. Instead, advise your customers to change their regime slightly to ensure winter protection. Avoid harsh cleansers or scrubs, opting for gentler versions. Products containing retinol or vitamin C should be eased back during the winter. If using lotions for moisturisation, switch to a
cream. Night-time products will assist in cell-regeneration and improve the skin’s natural barrier abilities.
Don’t forget the Body! It’s not all about the face. We should ensure all-over body care during the winter by using appropriate body moisturisers, particularly after bathing.
Hydration is crucial. We may not be inclined to drink as much water in the cold as during the summer. But the reduced humidity that winter brings means our skin can become dehydrated more easily. Hydrating from within is essential, particularly when our intake of hot drinks like tea and coffee can increase during the colder weather. These drinks cause further dehydration. Opting for herbal teas or water will make a big difference to the skin’s hydration levels.
Sun Protection is still required during the winter, sun or no sun! Although UV rays are weaker during the winter, they are still present. Moisturisers containing an SPF should be recommended, or the use of a facial suncream.
Alcohol intake can increase in the winter, particularly with longer nights and seasonal socialising. However, alcohol not only dehydrates you, it can also worsen conditions like eczema and psoriasis, increasing flareups. People should always be advised to stick with recommended unit intakes, and always avoid binge drinking. Tobacco smoke exposure in pubs or clubs will also irritate dry or uncomfortable skin.
Nutrition is as important to skin health as products. A healthy, balanced diet will ensure the skin gets all the nourishment it requires to function as it should. Vegetables are a crucial source of antioxidants that will further improve the skin’s effectiveness as a barrier to winter elements. Some specific vitamins believed to be important for skin health include:
• Vitamin D, which we primarily get from sunlight exposure. This is more difficult during the winter months, so supplementation is usually advised. Food sources of vitamin D may not be appropriate to provide the body with the levels required.
• Omega 3 is an essential fatty acid that serves many important functions, including skin hydration and ensuring appropriate oil production. Food sources of Omega 3 include oily fish, flaxseeds, walnuts, and chia seeds. Supplementation should be recommended for those who don’t enjoy oily fish.
• Vitamin E has antioxidant effects and helps protect the skin from exposure. While the body usually produces enough through the production of sebum, this can deplete in the winter months. Food sources of Vitamin E include sunflower seeds, almonds, and hazelnuts
• Vitamin C is also an important antioxidant and plays an essential role in collagenproduction. It is particularly essential for the epidermis to act as it should. Vitamin C sources include citrus fruits, broccoli, and spinach.
• Vitamin K is believed to improve the skin’s ability to heal. Good food sources include kale, green beans, cabbage, lettuce, and spinach.
The parent company of Boots is reportedly considering putting its pharmacy chain on the market next year for an estimated £10 billion. Boots has 89 outlets in Ireland employing more than 2,000 people. According to Sky News, the US retail giant, Walgreen Boots Alliance (WBA), is lining up Goldman Sachs advisers to look into a potential sale of the pharmacy.
McCauley, the leading Irish owned retail pharmacy group, announced its exciting partnership with Guaranteed Irish, a business membership network championing local and multinational businesses operating within Ireland. As a proudly Irish-owned business and advocate of shopping local and community pharmacy, McCauley is thrilled to be expanding its ongoing support of Irish based businesses as the first Irish pharmacy to partner with Guaranteed Irish.
Mulligans Pharmacy opened their first store in Dublin with the launch of ‘The Pharmacy by Mulligans,’ a 3,000 square foot pharmacy at Arnotts on Henry Street.




Edward Murphy, Project Manager with the HSE Tobacco Free Ireland Programme authored a clinical article on the role community pharmacists can play in helping smokers kick the habit.

Paul Neill, Director, Generic Medicines Ireland with Teva Pharmaceuticals, was appointed as Vice Chairperson of Medicines for Ireland.
IPN reported that immediate action was required to ensure that a growing shortage of community pharmacists does not impact upon patients. The Irish Pharmacy Union (IPU) warned that attracting and retaining young community pharmacists has become increasingly difficult throughout the pandemic, which if it continues could impact the accessibility of pharmacy services in future.

Lynch’s totalhealth Pharmacy in Kells celebrated 30 years in business.

We carried a Profile piece on Nigel Moloney, Carrigaline CarePlus Pharmacy who has worked as a hospital pharmacist, in clinical development with one of the world’s healthcare giants, in marketing and working across four retail pharmacies, a wholesaler and providing dedicated healthcare to Cork’s homeless community.
The Minister for Health, Stephen Donnelly announced two new Framework Agreements on Pricing and Supply of Medicines 20212025. The multiannual agreements with the Irish Pharmaceutical Healthcare Association (IPHA) and Medicines for Ireland (MFI) represented an important step in reducing the cost of medicines and improving access to innovative new medicines for patients.
The February issue carried all the Finalists for the 2022 OTC & Retail Pharmacy Product Awards.
Chemist Warehouse opened its fourth store in Ashbourne, Co Meath.
Stacks Pharmacy Group celebrated being 22 years in business. Ade Stack opened her first pharmacy in Skerries, Co Dublin in February 2000.





Cara Pharmacy Group joined the team at Allcare Pharmacy under the Uniphar umbrella.



Ella Sloane from Trinity College Dublin authored an article putting the spotlight on equality in pharmacy and asked, ‘is enough being achieved on equality, diversity and inclusion in higher education and pharmacy?’


Paul Hatton, National Field Sales Controller with Perrigo recently launched the University College Cork Pharmacy Society’s latest competition. Paul called in with Society members to officially launch their digital media competition on ‘Pharmacy Heroes of the Pandemic.’
Boots Ireland became the first pharmacy chain in Ireland to offer HPV vaccinations when their new service launched in 14 pharmacies nationwide.
Anna Daly was announced as the new Brand Ambassador for AYA, the Irish vitamin and supplement brand.

Pharmacy organisations across Europe and indeed the world, called on pharmacists to provide aid to Ukraine. The International Pharmaceutical Federation (FIP), made the request on behalf of its member organisation the All-Ukrainian Pharmaceutical Chamber.
It was revealed that up to 40% of dementias are preventable by highlighting the need to resource early intervention, awareness and new treatments, a special Oireachtas briefing led by The Alzheimer Society of Ireland (ASI) was told. Pharmacists were called on to offer their support to a new campaign highlighting the importance of screening for early detection of Chronic Kidney Disease.
We announced the winners of the OTC & Retail Pharmacy Product Awards, including Voltarol Emugel Extra Strength 2% w/w Gel 100g for Best Launch of 2022.
McCabes Pharmacy Group announced the acquisition of Finnstown Pharmacy in Lucan and Newcastle Pharmacy in South-West Dublin.
Energia announced it will supply United Drug with 100% renewable energy for the next two years.

Four University College Cork (UCC) subjects, including Pharmacy, were ranked in the top 100 globally, with Nursing, Agriculture & Forestry, Pharmacy & Pharmacology, and Law at UCC positioned highly in the latest edition of the QS World University Rankings by Subject.
Grainne Power was appointed as Director of Compliance with the Health Products Regulatory Authority (HPRA).
The Finalists for the 2022 Irish Pharmacy Awards were announced.
The pharmacy team from the National Maternity Hospital authored a clinical article on the role of the multidisciplinary team in supporting women and babies with diabetes.
Sharon Foley was appointed as Secretary General Designate to the Irish Pharmacy Union.


The Pharmaceutical Managers’ Institute held their first Pharma Summit in three years. Speakers included Dr James O’Byrne, Metabolic Physician & Clinical Geneticist, Mater Misericordiae University Hospital.
Dr Maria Donovan and Clara Heinrich from the School of Pharmacy at UCC authored an article on community pharmacists’ attitudes towards deprescribing in primary care in Ireland.

Théa Pharma celebrated its new office opening in Castlebar, Co Mayo. The state-of-the-art offices are 6,000 square feet and boast a modern office layout with bright airy spaces.


Our Corporate Profile with Adam Lee, General Manager for Ireland with Reckitt detailed how the company is supporting healthcare professionals to protect, heal and nurture consumers.
It was revealed that the Pharmaceutical Society of Ireland was undertaking a project, due to run across 2022-’23, ‘Emerging Risks to the Future Pharmacy Workforce’. In 2022, this project was set to “assess emerging risks to the continued availability of a professional pharmacy workforce within community and hospital pharmacy in Ireland.”
The use and learn phase of the Falsified Medicines Directive ended for wholesalers on 9th May 2022 and for pharmacies and hospitals for 30th May 2022.
The updated LloydsPharmacy located at the Primary Care Centre in Bray was unveiled with Minister for Health & Wicklow TD Stephen Donnelly.




We profiled StayWell Pharmacy, part of the Navi group, as they celebrated the opening of two new outlets in Cork city and county.

The Cosmetic Association, after an absence of two years, returned to the RDS in Dublin.
Damien English TD, Minister of State for Business, Employment and Retail, announced changes made by S.I. to the employment permits system which includes adding the occupations of Community Pharmacists to the Critical Skills Occupations List, signalling a recognition of the significant shortage of pharmacists here.
The CEO of the HSE Paul Reid advised the Chairman, the Board of the HSE and the Minister for Health that he would be stepping down from his position.

LloydsPharmacy teamed up with Men’s Sheds to undertake Heart Health Checks and give advice to members.
Meaghers Pharmacy Managing Director Oonagh O’Hagan announced the launch of a new distribution company for Symprove.
This issue featured the annual IQVIA Top 100 over-the-counter pharmacy brands in Ireland.
Community ‘Fabulous’ Pharmacist of the Year Laura Dowling introduced fabÜ, a unique new range of nutritional supplements that simplifies the search and merges the power of nature with the precision of science.
A number of community pharmacists, over 1,400 thus far, came together online to sign a petition to the Minister for Health. The petition relates to the Falsified Medicines Directive and its implementation, which for a huge number of pharmacists, is becoming un-tenable.
It was revealed that out of a list of 1,000 new college places announced recently, not one space was left for pharmacy students, a move which has alarmed the profession.
A new initiative to enable people experiencing domestic abuse and coercive control to receive support in their local pharmacy was launched nationwide. Safe Pharmacy, is being led by the Irish Pharmacy Union (IPU) in partnership with Safe Ireland, An Garda Síochána and the HSE.



We spoke with Dave Barrett, Haleon Country Manager after it was announced that the new company to result from the proposed demerger of Consumer Healthcare from GSK, will be called Haleon.
IPN launched the search to find The People’s Pharmacist 2022, inviting nominations from members of the public across Ireland.


It was revealed that Uniphar is in talks to buy the Sam McCauley Health & Beauty Pharmacy chain.
The CEO of Six RCSI researchers were awarded funding totalling more than ¤2.2 million by the Health Research Board (HRB) under the Investigator-Led Projects (ILP) 2022 scheme.
The upcoming budget must take steps to address the underfunding of Ireland’s community pharmacy sector, the Irish Pharmacy Union (IPU) stated.

The Health Products Regulatory Authority (HPRA) has stated that whilst the volume of detained illegal medicines in the first half of 2022 has fallen significantly compared to the same period in 2021, a substantial amount of product is still being sourced online which poses risks to people’s health.



We profiled case studies of the winners from the 2022 Irish Pharmacy Awards, including Damien O’Brien who scooped the JPA Brenson Lawlor Young Community Pharmacist of the Year Award.
The McGreals Group opened the doors of their new pharmacy in Glenageary shopping centre in County Dublin.
The Irish Pharmacy Union welcomed patient friendly measures announced in Budget 2023. However, the IPU has said with the extension of free GP care to over 400,000 children it is more important than ever to properly resource and utilise the pharmacy sector.
IPHA said the Government’s Budget allocation of ¤18 million for new medicines falls short of the amount needed to optimise patients’ access to new medicines next year and to help Ireland catch up with peer European countries.
McCabes Pharmacy Group announced their new charity partnership with ALONE.

The Finalists were revealed for The People’s Pharmacist 2022. The winner is showcased in this (December) issue on page 12.
The National Cancer Registry (NCRI) has just published a new cancer trends report entitled Breast, cervical and colorectal cancer 19942019: National trends for cancers with population-based screening programmes in Ireland.












































































































In association with Panadol, Irish Pharmacy News launched the search to find The People’s Pharmacist 2022 earlier this year; giving the Irish public the opportunity to nominate and vote for, their local pharmacist who has gone above and beyond. The People’s Pharmacist Award seeks nominations from across the country, giving patients the opportunity to recognise and salute their local pharmacist.


Through this Award, we enabled the public to have a voice in recognising the unwavering support and spirit that makes pharmacists the backbone of our health service in every community across Ireland.
From over 700 nominations we had eight extremely deserving finalists and after a nationwide voting campaign, John Tallon of Bergin’s Pharmacy in Newbridge was awarded this prestigious title.




John has also made great efforts in his work with the homeless community, making sure no-one misses out, he has dedicated a huge part of his time to assisting this sector.





“I am a customer of Bergin’s Pharmacy for 30 plus years and Johns care and knowledge has saved my life,” noted his nominee.


“John is a never-ending professional with a true kindness and understanding of complex medical issues. I have witnessed first-hand his dedication to the homeless and less fortunate. He is shy, non-assuming and has a true vocation to serve his community. We would be lost without him in Newbridge and the surrounding areas.”




Emmett McCann has been described as a healthcare professional at the heart of the community who always goes ‘above and beyond in terms of service, friendliness and support offered to everyone customers.’
One particular customer was suffering from very low self-esteem when their immune system was low. As a result they developed worsening of their psoriasis. Emmett stepped up, took the customer through a private consultation and explained their treatment and management thoroughly, advising on vitamins and supplements and being a huge factor in their recovery.
Victoria Jones has made huge and measurable difference to the lives of many women in her community. Bonnybrook Pharmacy specialise in women’s health including Menopause and Victoria is passionate about these topics.


“Our local pharmacist Victoria Jones so knowledgeable and generous with her time,” said one nominee. “She doesn’t just provide the advice she backs it up with research. It is so refreshing to have someone to talk to about female issues who genuinely cares.”
Syrian refugee Fadi Almasri opened the first pharmacy in Ballon after coming to Ireland with the help of the UN in 2014.In that short period of just over a year, he has endeared himself and his staff to the whole community. “His pharmacy is always very well stocked but importantly the addition of the pharmacy to our village is a very welcome service. Ballon pharmacy has gone above and beyond in helping out the Ukrainian refugees who have been in our local community centre since April. All the staff at the pharmacy are friendly chatty and extremely helpful.”
Rebecca Barry is one of the most passionate committed pharmacists always going the extra mile in her community for her patients. One of her many nominees told us, “Rebecca has embraced many services including vaccinations over the past year and has provided both walk in clinics several times a week as well as appointment booking service. Rebecca is a regular guest speaker on Limerick FM providing insights, tips and advice on a range of health topics and provides talks in her local community sharing her knowledge and expertise from mum and baby events, to the Menopause summit.”


Derek Haughey was nominated by a customer from his local community who has described him as a ‘lifeline’ when it comes to patient care. Not only that, but both he and his team have been described as a pharmacy that not only helps provide knowledge and expertise in abundance, but a warm welcome with an open door; with all team members always happy to take time out of an incredibly pressured and busy day to check in on others and those around them.
so
One customer in particular has been overwhelmed with the guidance and advice she has given during a difficult time. A local couple undergoing IVF treatment needed a lot of support from the pharmacy; when they couldn’t manage the necessary injections and treatment needed, Pippa administered them every evening at 6pm without fail, even on her day off – and some morning up until only recently.
Jennifer Gallagher received an enormous amount of nominations. One in particular that stood out was when she was critical in helping to recently save a patients’ life. When a customer began having a seizure in the pharmacy, Jennifer dropped everything else that was going on, protected the customer, organised and administered a Buccolam pen and stayed with them until they came round and were feeling better.








“She saved my life and I suffered no injuries because of the care Jennifer gave me,” they reflect.

In this section we highlight the Industry32Innovators within the pharmaceutical industry; those with the proven knowledge and expertise within their field who are ensuring that initiatives are borne to the betterment of the pharmacy profession. These individuals, and the professional bodies/companies they represent play a critical role in ensuring pharmacists and their teams are able to seize the opportunities and squarely meet the challenges.
Arnold has had a busy year as CEO of CommCare Pharma to ensure that the members, all independent pharmacists, are best placed to face these challenges. In April 2021, the merger of the totalhealth and Haven Pharmacy Groups was announced. CommCare Pharma members are now benefitting from expanded support structures including improved business intelligence reporting on all areas of their businesses. They also have access to reviews of their performance and professional advice on any area they feel needs attention. CommCare Pharma has also focussed attention on expanded training supports for members and their staff teams.



Colm Baker is an expert in marketing and a qualified pharmacist who is assisting pharmacies in developing their online brands. He has a plethora of marketing skills that he can apply to his knowledge of the pharmaceutical industry. The Social Pharmacist is a pharmacy-specific digital marketing agency launched to help independent pharmacy owners build an online brand they can be proud of. Colm and his team help busy independent pharmacy owners build a consistent, modern, high-quality online brand to boost their retail and online businesses. The Social Pharmacist is now not only helping independent pharmacies in Ireland but also UK, USA, and Canada.
Dave Barrett headed up the formation of new company, Haleon, following the demerger of Consumer Healthcare from GSK. As a standalone company, Haleon (pronounced Hay-Lee-On) will be a new world-leader in consumer healthcare, offering a compelling proposition – to bring deep human understanding together with trusted science – to deliver better everyday health with humanity. Dave leads a team delivering world-leading brands and is a champion of the emergence and growth of the Self-Care OTC market.




Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.



www.azure-pharma.com

In July of 2022 Sarah was appointed as the Operations Director of the IIOP. Sarah has been working in the IIOP since March 2018. In this time, she has led a range of projects including statutory requirements such as ePortfolio Review and Practice Review as well as managing specific projects that focus on the development of pharmacists. In October 2021, the IIOP Mentoring Programme was launched with Sarah as the Mentoring Programme Lead. The programme has gone on to successfully run for a third cohort, connecting another cohort of thriving mentors and mentees. It has gone on to win the EMCC Global Mentoring Award in 2021 and was shortlisted for the IITD Best Coaching & Mentoring Initiative in 2022.


















A pharmacy technician by training, Audrey joined the IIOP in March 2020, just as the country went into lockdown. She led the implementation of a new online format for events for the IIOP during COVID, supporting the establishment and implementation of the IIOP “In Conversation With” webinar series. This series has been maintained post-COVID due to its popularity amongst pharmacists, providing a platform for connection for the profession where contemporaneous pharmacy issues can be discussed. Audrey is instrumental in the Peer Support agenda in the IIOP, and managed the IIOP Peer Support Event in October 2022, a forum which enabled pharmacists to contribute to the future of the peer support agenda for Irish pharmacists.
In the past year, Dr Dalton has been a champion in providing a greater voice to pharmacists in Ireland through his research. He has showcased how the COVID-19 pandemic and other recent pharmacy practice changes have impacted on community pharmacies and the pharmacists themselves. Notably, Kieran has published research highlighting the effect that COVID-19 had on community pharmacy practice and pharmacist wellbeing, as well as how both the introduction of electronic prescription transfer via Healthmail and the Falsified Medicines Directive have affected community pharmacies. Kieran has also continued his ongoing work to help the advancement of the pharmacist profession by publishing his findings on pharmacists’ views of pharmacist prescribing in hospital settings, which he hopes will contribute to enhancing the scope of pharmacist roles in Ireland and improve the delivery of patient care. Additionally, Kieran has recently supervised a pharmacist-led project in an Irish hospital that has demonstrated important findings involving a significant reduction in errors with pharmacist prescribing versus medical prescribing for chemotherapy regimens, associated with cost savings.

Through his leadership David was an embodiment of the core objective of Medicines for Ireland (MFI). 2020 and 2021 have been the most challenging of years for the entire healthcare sector, however behind the scenes, the steps to ensure no interruptions to the supply of medicines have often gone unnoticed.
Throughout Covid, MFI member companies were operating at 100% capacity, with very significant pressures to meet increased demand in Ireland and globally. Through his leadership and his work with colleagues throughout Ireland David Delaney helped ensure that key policy requirements were communicated and implemented to ensure medicines supply remain unaffected by the day-to-day restrictions in place.

The added complexity of Brexit and the increased cost of importing and exporting for businesses in Ireland, placed even greater pressure on an already overstretched sector. Through collaboration and engagement with stakeholders, David led the Medicines for Ireland team through these challenges.

In December 2018, borne out of her own experience of menopause, Loretta set about smashing the taboo and stigma surrounding menopause in Ireland, providing menopause management and care for menopausal women and for driving education and awareness around menopause in the workplace, through establishing a centre of excellence – The Menopause Hub. For World Menopause Day, October 18th, Loretta will open her second clinic in North Dublin to better service the Northeast community of women. To date she has seen well over 6,000 patients in her clinic. In addition, she has been an advocate for menopausal women for the past 3 and a half years, with key stakeholders, such as Government, politicians, the Department of Health, the medical profession, the pharmacy profession, with members bodies like the INMO, media and consumers. Loretta has been particularly vocal about the HRT shortages during 2021 & 2022 which have adversely affected women.
As General Manager of McCabes Pharmacy, Karen has steadfastly stewarded the company through the various opportunities and challenges of the past two years. In particular she has been an advocate for the driving well organised pharmacy teams that are equipped to deliver consistent and authoritative healthcare advice. With responsibility for the day to day running of the business, Karen’s skills have reached from leading out compassionate, caring and high performing teams to devising best in class plans for the Digital Transformation and ecommerce plan at McCabes Pharmacy during 2022. She has been responsible for driving McCabesPharmacy.com into Ireland’s largest OTC website during 2021.






Fleming, Lecturer in Clinical Pharmacy, University College Cork

Aoife is passionate about Interprofessional Learning (IPL) and enhancing the Pharmacy student experience at the School of Pharmacy, University College Cork. She has been instrumental in supporting pharmacy and medicine students IPL sessions in the Mercy University Hospital, to support their clinical practice student experience. Aoife played a key role in ensuring that pharmacy students had an active part in a recent Health Service Executive (HSE)-UCC pilot project to implement a multidisciplinary, IPL session for students in Feb 2022, which received very positive student and staff feedback. She led on the research component of this initiative, in collaboration with colleagues in the College of Medicine and Health UCC. This initiative was nominated in three categories at the Irish Healthcare Centre Awards May 2022 and has promoted and highlighted the importance of multidisciplinary IPL in UCC. Aoife organized and chaired the first All-Ireland Interprofessional Educational Competition in March 2022.

Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com

Robin has been working for McLernons in the pharmacy IT sector for close on 40 years and has been part of the team that has overseen the transformation of Dispensary Management Systems from the early days of very basic recording and pricing to the highly technical cloudbased applications of today. McLernons prides itself on the team of people who develop, install and support its pharmacy IT suite of products, including MPS, MPS Retail and our Head Office programme, with dedicated engineers and trainers assigned on a geographical basis to meet the needs of our customers, supported by a highly trained, professional and friendly Service Desk. This team, lead by directors like Robin across Ireland, are continuously working for Irish pharmacists – not just meeting their IT needs but by anticipating and exceeding them.
Jenny has been the Brand Manager for LloydsPharmacy for almost three years. During this time, she has managed all the customer communications, marketing campaigns and social media for the brand. In the past 12 months this included obtaining over 290 articles in media on a range of topics, over 10million impressions exposure, grew our social media following to over 105,000 followers, attended WellFest the Health and wellbeing event in Dublin and educated this key target audience of the importance of getting regular Blood Pressure checks, Tanita scales measurements and provided a pharmacist consultation on site to support with Health checks and advice. In addition she championed the Men’s Health month through a partnership with Men’s Shed which facilitated Pharmacy teams attending the local Mens Sheds and providing Blood Pressure checks and medical/lifestyle advice.


Earlier this year, Lee-Ann and her team carried out survey research around Breast Cancer, showing that 6 in 10 women in Ireland do not regularly check their breasts for lumps or abnormalities


CarePlus Pharmacy became the first retail pharmacy network in Ireland to join the movement to end period poverty in 2021. The brand’s outlets across the country are now offering free tampons or sanitary pads to women and girls to help ensure no-one has to worry about affording these essential items as they go about their daily lives.
Pharmacy staff already provide support and advice for customers in understanding their menstrual healthcare needs and today’s announcement will supplement that approach. The decision to offer the service was inspired by the steps taken by Lidl Ireland in recent days to provide a similar service.






Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Kelly has over 12 years of experience in finding talent across the Pharmacy Industry. Barbara’s team screens all candidates to ensure they have the education and experience, as well as having a cultural and personality fit for each individual pharmacy. Her knowledge of this sector is unparalleled and her proven successful track record in recruiting Pharmacists speaks for itself. Barbara is always looking for new solutions to fill vacancies. One example of this is sourcing EU Pharmacists by looking at the best talent in the EU and managing their move over to Ireland. The fusion of these skills has enabled our pharmacy desk to merge with the retail division, meaning excel recruitment can service all recruitment needs whether it’s in a chemist or a pharmacy support office.

















Mary-Claire joined the IIOP team this year. Mary-Claire has been a Peer Support Pharmacist with the IIOP since 2013, supporting Practice Review, the delivery of Introduction to CPD and ePortfolio Review webinars. Most recently she has been responsible for hosting and sourcing a range of subject matter experts to feature on the IIOP “In Conversation With…” webinar series. She was awarded her PhD from the School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin in 2015. Mary-Claire has worked as a community pharmacist and also completed a Post-Graduate Diploma in Statistics in Trinity College while completing her PhD research.
As a former pharmacist, Adam understands how important it is that Reckitt provides valuable support to healthcare professionals nationwide. Adam’s pharmacist background is helping him in his current role to the needs of healthcare professionals in Ireland. Adam doesn’t only have a keen mind for the commercial side of his role, his passion for helping people and making a difference in a positive way through healthcare ensures the passion for the work he does shines through. One way Reckitt is driving change with their healthcare professional partners is through Reckitt’s ‘Detail and Train’ focus, running webinars with all of big customers where they share the latest insights that they have.

In her role with AMRIC, Ellen works with the team and collaborates with various stakeholders to promote and support antimicrobial stewardship in all healthcare settings in Ireland. Ellen has been instrumental in the creation and development of the Pharmacist Antimicrobial Stewardship Network (PAMS-Net). This forum is the result of a collaboration between the HSE Antimicrobial Resistance and Infection Control (AMRIC) team and the Irish Institute of Pharmacy. The network aims to support pharmacists across all sectors in their role as antimicrobial stewards. Ellen chairs the PAMS-net working group which brings together pharmacists from a range of professional backgrounds to inform and advise on the continuous development of the network. Ellen was a member of the Antimicrobial Stewardship Guidance Working Group for the recently published HSE Antimicrobial Stewardship Guidance for all healthcare settings.
Louise joined Uniphar 2 years ago as Consumer Business Unit Manager. During her time as Head of Category Management for McKesson UK, Louise built a strong team, grew retail sales in what was a challenging retail environment at the same time as managing an aggressive store refit programme. Over the last two years during Louise’s management the Consumer business has doubled its turnover and profit. Louise has brought an energy and passion for pharmacy retail, customer focus, brilliant basics and cross functional collaboration to the Consumer business. She has fostered a positive work culture within Consumer, focused on building a commercially focused and highly motivated team.



Conor celebrated his 10th year within McCabes Pharmacy this year. Having started as Financial controller in 2012, he swiftly moved to become Finance Director and a member of the McCabe Pharmacy Board of Directors. He has presided over the steady, consistent growth of the McCabe Pharmacy company in recent years and careful stewardship of the company as it embarked on fundraising to secure recent series of acquisitions. He has overseen 3 pharmacy acquisitions in recent months and negotiated entry into Greenfield pharmacy locations.



Cormac recognised there was a need for a really good app for Irish pharmacies to act as a seamless communication tool supporting all aspects of the patient pharmacy journey. As the founder of the fintech solution, VillagePod, he set about designing, developing and then successfully launching the PharmacyConnect platform. This multi-use platform can be developed and redesigned for any independent pharmacy or group to deliver a seamless communication tool for the future of pharmacy/patient relationships in Ireland. From Healthmail to prescription collection, the PharmacyConnect app is an easy-to-use communication tool for ordering, housing patient information, two-way communication, payment and delivery.





Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
McMahon contributes to a range of national agendas, most notably acting as Pharmacist lead on the








undertakes a range of peer-support and expert roles for the Irish Institute of Pharmacy and was part of the development team for the Know Check Ask online training programme (available on www.iiop.ieand HSEland). Earlier this year she successfully completed consultant pharmacist credentialing by the Royal Pharmaceutical Society, which demonstrates her commitment to ongoing professional development and pharmacy leadership. Her current research interests relate to addressing polypharmacy and potentially inappropriate prescribing in frail older people, and reducing medication-related falls risk.

In



David
Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com





Elaine is passionate about motivating people and encouraging a positive team spirit across the group and has implemented many positive initiatives to support staff members both as individuals and as part of their teams. Elaine this year has recently completed her BA in HRM Strategy and Practice and combined with the wealth of knowledge she gained on the shop floor has contributed to, and now leads the company’s HR strategy. “Thank You Thursdays” have become a common event across the group with Elaine organising thank you themes for the team on a regular basis. Recognising that whilst these individual events were important, it was imperative to look at an initiative that supported longer term health and wellbeing. Elaine was dynamic in her approach to this and reviewed many options that she felt would support the pharmacy teams. Elaine has also led the development of a Professional Training Hub which ensures that all newly qualified pharmacists and EU pharmacists and technicians are given the skills and knowledge to hit the ground running when they start a new role within the company.











Adrian is a rising star of the industry with over ten years’ experience across the various different sectors. Since joining United Drug Adrian has overseen a complete overhaul of the Pharmax offering and the subsequent doubling of its membership over the past two years. This has had the effect of significantly changing the landscape of the market giving independent pharmacies freedom of choice and increased value in the buying group sphere. A hands-on operator, Adrian has helped Pharmax members make smarter procurement choices, consequently adding much needed margin to their bottom line in an ever challenging environment. Adrian was a key figure in bringing ARC – United Drug’s claims reconciliation tool – to the market in 2020 and since its introduction it has evolved over the past two years into a crucial everyday work tool for it’s ever growing number of users.


 Lucia Onofri, Brand & Digital Marketing Manager, United Drug
Lucia Onofri, Brand & Digital Marketing Manager, United Drug
In the past 12 months this included 10 Webinar topics, almost 100 supplier Marketing campaigns (Email, Tote Drops, Telesales etc) highlighting new products/offers/tips and advice to Pharmacy sector and broadening the conversation on Social media to highlight important developments, health updates, company and sectoral stories and news. Lucia also played a key role in the redesign and enhancements to the United Drug customer order portals including UDW.ie, Pharmax.ie and launched the United-Drug company corporate site to share news and updates amongst the sector.
Philbin, Chief Antimicrobial Pharmacist, HSEMarie Philbin is the first Chief Antimicrobial Pharmacist to be appointed to the HSE’s Antimicrobial Resistance and Infection Control (AMRIC) team. She was one of the first antimicrobial pharmacists in Ireland, working in Midland Regional Hospital Tullamore for 14 years and was a founding member of the Irish Antimicrobial Pharmacists Group. Her role involves monitoring the consumption of antimicrobials in all setting in Ireland and she is also involved in coordinating the National Annual Acute Hospital Point Prevalence Survey. Marie has been instrumental in the development of the HSE Antimicrobial Stewardship guidance for all healthcare settings and is passionate about coordinating and developing antimicrobial stewardship from a national perspective.
Marie Adrian O’Sullivan, Buying Group Business Development Manager, United DrugHaving joined the Uniphar Supply Chain & Retail team as a Retail Buyer in 2017, Ruth has continued to grow in tandem with the extensive growth that the business has seen. Now working as the Commercial Manager for the retail business, Ruth is responsible for the Front of Shop Commercial strategies for Allcare, Life & Hickeys Groups. A graduate of DCU with a degree in Business through the Irish language, Ruth has worked in Buying roles in the Pharmacy industry for the last 10 years. During this time, she has taken part in many key retail projects including the acquisition and rebranding of Bradleys Pharmacies into Allcare Pharmacies and recently overseen the commercial integration of the Hickeys Pharmacy group into Uniphar.

Ger Rabbette is CEO Uniphar plc, a diversified healthcare services provider, headquartered in Ireland, but operating across 160 countries worldwide. An accountant by training, Ger has spent most of his career in senior roles in the pharmaceutical and healthcare sectors. In 2009, Ger was appointed CEO of Uniphar Group plc and took on the onerous job of saving a large Irish business, which was loss-making and carrying over ¤200M in debt. Today, Uniphar is a great Irish success story with a market capitalisation of ¤1BN and annual revenues in excess of ¤1.9BN. Its 3,000+ employees operate across Europe, the US, MENA and APAC. In 2019, Uniphar plc listed on the Irish & London Stock exchanges and since then has continued to grow both organically and by acquisition, purchasing more than 20 companies worldwide.
United Drug is Ireland’s largest pharmaceutical distribution company and was originally founded by a group of pharmacists in Ballina, Mayo in 1948. In 2022 United Drug became part of the PHOENIX group and is now part of Europe’s leading healthcare provider in the sector. Employing more than 650 staff across operations in Dublin, Limerick and Mayo, UD partner with more than 1,900 pharmacies, hospitals, clinics and primary care centres across Ireland. The business spans across three business units; United Drug Wholesale, United Drug Distribution and United Drug Consumer – all leaders in their category. Earlier this year United Drug made the switch to Renewable Gas (Biomethane) - supplied by Flogas Enterprise - as part of the company’s efforts to become carbon neutral by 2030. The biomethane, which is being sourced from Green Generation in Cush, Co Kildare, will increase incrementally in the coming years.










Dermot joined Uniphar Group 10 years ago as the Financial Director of the Allcare Pharmacy business, taking over as Managing Director of the Retail Services business in 2017. Since then, he has overseen the growth and expansion of the Uniphar Supply Chain & Retail business to become market leader in wholesale to pharmacy in Ireland with a market share of c.52%, a 10-point jump in 5 years, and built a network of 378 symbol and owned community pharmacies, from just 45 in 2017, creating the largest buying group in the country. In March of this year Dermot Ryan was appointed Group Operations Lead, overseeing Uniphar global operations, in addition to continuing to manage Uniphar’s significant Irish operations and retail business. In his new role, Dermot will have responsibility for global supply chain networks, ensuring that Uniphar move towards common technology platforms, build a globally connected distribution and warehousing network, and maximise our service offering to clients.
Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
To all Pharmacists, not only are you trusted health advisors in your communities, but you are also exceptional role models for pharmacy students like Aura.

From all in United Drug, thank you for your relentless efforts and commitment to your patients.
We think you’re amazing, and it’s a privilege to support you.
The Retail60Leaders reflects on those professionals within the retail pharmacy arena who have been, and who continue to be, empowering others moving into the future. The 60 listed on the following pages feature those who act as ambassadors and role models for their peers and colleagues; redrawing the landscape of the future, with the patient at the heart of everything they do. Those who have made this list have displayed motivating behaviour and inspirational leadership affecting the development of retail pharmacy across Ireland.
One of Brian’s key strengths is his ability to build strong relationships with the teams in stores especially the pharmacists and technicians, collaborating closely with them on the ground to support them in their day-to-day operational activities. Brian is currently leading a program on Lean Methodology in McCabes dispensaries to improve efficiencies and customer service across all stores. This program is key to allowing pharmacists to move away from administration work and concentrate on engaging with customers on a one-to-one basis as much as possible to counsel and advise and also to allow them time to concentrate on offering key pharmacy services such as vaccinations and Health Checks. Brian with the support of the operations team and the staff in store have recently completed a number of pilots in specific areas of the dispensary business that not only improve processes but also the experience for the customer giving a dual impact.
Eamonn’s vision of community pharmacist role is to “help people to help themselves” through education and information initiatives. These range from his “Ask the Pharmacist” articles in local and national press, to public health talks to education led initiatives to local schools. Business at Whelehans has grown from 5 staff in 2005 to over 40 staff now in two locations. Offering flu vaccines since 2011, in 2020 Whelehans expanded the service to offer vaccines off site. Whelehans now administers over 1500 flu vaccines annually. Whelehans pharmacists have gone on the road to administer flu vaccines to workplaces and nursing homes all over the midlands and beyond. Whelehans have been supporting the HSE in administering Covid-19 vaccines since June 2021. Since the vaccine roll out began, Whelehans have administered over 3000 Covid-19 vaccines. In February 2022, when approached by the local Ukrainian community in Mullingar, Eamonn and his staff at Whelehans Pharmacy set up a Ukrainian Fundraising Appeal to donate Medical Supplies direct to Ukraine. Through this initiative, and the generosity of customers and the people of Westmeath, Whelehans bought over ¤30,000 worth of the medical supplies between February and May 2022 and sent direct to hospitals and health facilities in Ukraine using a Mullingar based Courier Smullen’s Couriers who are part of the Irish humanitarian effort for Ukraine.
Seamus Burke established his pharmacy in the heart of the market town of Macroom in 2012. His focus as a young Pharmacist and owner has been to devote his time to consistently improving the offer to the customer in-store and online and to being the stand out pharmacy in Macroom for service, advice, opening hours etc.











Seamus is extremely motivated in so many ways with the business and while he has expanded his in-store offering he has also developed an easy to use website and developed an active social media presence. His online store is well stocked with health, beauty, mother and baby and gift product ranges. This dedication to providing excellence in patient and customer service to his customers in-store and online in Macroom has made Haven Pharmacy Burkes a destination pharmacy in Macroom, supporting local jobs in an independent community pharmacy.
Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
2022 was yet another challenging year managing realities of the pandemic for both McCabes Pharmacy patients and pharmacy teams. Lisa pulled together another highly effective COVID-19 and Flu vaccination clinic program across multiple locations and timeframes and ensuring that electronic booking systems were state of the art for patient needs. Under her leadership, McCabes pharmacy become one of the biggest vaccinator companies in the State. She has rolled out best in class regulatory control frameworks to ensure highest standards of clinical governance and engagement of all McCabes pharmacy dispensary teams. Her renowned interest, care and support for all her pharmacists is legendary and had led to McCabes Pharmacy having highest employee retention rates in Ireland.

Nuala also runs two online pharmacy ecommerce websites, glenpharmacy.ie and manly.ie. Nuala qualified as a pharmacist 15 years ago, and opened her own pharmacy over 12 years ago. Over the years the business has evolved greatly and now employs 11 staff across the pharmacy, beauty salon and the running of the two websites. Earlier this year, Nuala’s niche pharmacy website for men, manly.ie, scooped the award for the Online Retailer of the Year for 2022. Nuala is an extremely dedicated, hardworking pharmacist, who is committed to providing excellent service to her patients and their families. She sees the community pharmacist role as one of great importance especially in rural communities and is passionate about not allowing her community to feel disadvantaged in terms of access to health due to location. Nuala is a community activist and is involved in many voluntary associations throughout her communities, not least the local defibrillator group for which she secured funding to purchase 5 extra defibrillators earlier this year.
Tom has overseen a large project to standardise processes across what is the largest network of owned stores. Tom is an IPU committee member and works on a number of steering and working groups within the union. Recognising that community pharmacy has been falling behind other sectors in terms of employee benefits, Tom has been instrumental in ensuring that not only pharmacists, but all staff across the Uniphar store network are eligible for an industry leading pension as part of their enhanced benefit package. Under Tom’s stewardship Uniphar have forged stronger links with the schools of pharmacy and Uniphar now have Teacher Practitioners in each of the schools. Tom is a strong advocate for the scope of services Pharmacist can offer. He is also particularly interested in the role technology can play in enhancing safety and improving the patient experience.

Despite already having already had a large instore vitamins and supplements service, pharmacist Siobhan Costigan and her team wanted to be able to develop an online product, one that was unique to the Irish market. A personalised pharmacist-led product that would encourage buy-in from the customer, improving health through better choice of product and better compliance. The service is provided by an online site called www.MyNutrients.ie. Customers take a quiz and based on their answers a combination of products is recommended. On payment the team sachet pack the products in daily doses and post directly to the customer. To decide on the best brands, strengths and combinations of vitamins and minerals, Siobhan worked closely with a pharmacist who is also a qualified herbalist.

















THE AZURE PORTFOLIO IS www.azure-pharma.com
Siobhan Costigan, Pharmacist/Owner, Costigan’s Pharmacy, Tipperary Nuala Carey, Superintendent Pharmacist/Owner, Glengarriff Pharmacy & Beauty Lounge, CorkIn May 2022 David Dodd Pharmacy won the McLernons Innovation & Service Development (Independent) Award. David consulted with and developed a brief for the development company VillagePod to create a pharmacy app to be a seamless communication tool supporting all aspects of the patient pharmacy journey. From Healthmail to prescription collection, the app development brought a next-generation standard in an easy-to-use communication tool for ordering, housing patient information, two-way communication, payment and delivery. It has cut phone time, customer in store time, removed stress from patients and has received five-star reviews from customers since it was launched.
Deirdre was awarded the title of Community Pharmacy Technician of the Year 2022 at the IPN Awards in May, a worthy reward for her commitment to community pharmacy in the Donegal Gaeltacht. One of the most relied-upon members of staff, Deirdre is regularly described as dedicated, adaptable, reliable, motivated, and hard-working. She has impeccable people skills and is a true team-player. Deirdre joined the pharmacy team at O’Donnell’s totalhealth Pharmacy 25 years ago in 1997 as a trainee OTC assistant. Her passion for community pharmacy was evident from the beginning, and she continued to progress her knowledge and her career.

Kathleen
Commended
earlier this year for the 2022
Counter
of
Year Award. Kathleen has been a well-respected, kind and caring member of the Arva community for over 46 years. She goes out of her way to source items for customers and is always very determined not to let anyone down which in turn means she exhausts every avenue until she succeeds. Kathleen has acted as a mentor and a superb role-model for many young pre-registrations pharmacists who have come through the doors at Foster’s Pharmacy. She has proved to be all of her employers most valuable asset and has served each with the highest degree of loyalty and dedication, this being one of the main reason why the business began to thrive and still thrives today.
Laura Dowling is the 2022 PrecisionBiotics Community Pharmacist of the Year. She is an outstanding, inspirational leader. She is an incredible ambassador for the pharmacy profession in Ireland. Throughout her 20 years in the profession, Laura has bravely sought to progress her career in so many ways, above and beyond the simple ‘day job’ duties as a pharmacist manager. She is a powerful role model and example to young pharmacists seeking to develop their careers in both traditional and non-traditional ways. Also this year Laura launched the fabÜ range of nutritional supplements.










Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Leann has been the driving force behind the overhaul of the Adrian Dunne Baldoyle pharmacy dispensary which has significantly improved the efficiency of the teams’ work and has allowed them to further improve patient care in the pharmacy. Leann has bdination, because I’m with you and should have the dasheen heavily involved with engaging with the local community, especially the most vulnerable that need help in her local community and has been behind the co-ordination of the pharmacy’s delivery service. Leann is an extremely committed and reliable member of the pharmacy team. She is caring, compassionate and will always go out of her way to help a customer and was a finalist for the Community Pharmacy

of the Year Award.
Adam and his team launched a new Fertility Service earlier in 2022. According to the HSE, one in six couple will experience fertility issues here in Ireland. That means approximately eight in 10 couples will successfully conceive within a year. This leaves those struggling to conceive often searching for assistance. Adam has developed a new software which essentially free’s up his pharmacists time in order to help deliver additional services. He upskilled all of the pharmacists in being able to describe the entire fertility journey and everything in between. From counselling patients to providing accurate and up to date fertility advice and integrated that into the pharmacy’s in-house developed software, which allows for them to carry out a review in a coordinated, professional and cohesive manner.








Dr Fisher is a Pharmacist at the newly opened Staywell Ardfallen Pharmacy in Cork. Nigel Moloney has built a high profile in Cork city through his outreach work with the homeless community, and already had two outlets under Navi Group brands - a CarePlus Pharmacy in Carrigaline and a StayWell Pharmacy on Washington Streetwhen he decided the premises at Ardfallen Mall would be another fitting addition to the StayWell Pharmacy group.


Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Jane is in every sense of the word your true community pharmacist. Over the past year, Jane went above and beyond to re-connect with her customers after the pandemic both in person and online. She took the initiative to “bring the pharmacist online” through her much-loved videos on Facebook & Instagram. Jane continues to use her innovative mind to expand the role of the pharmacist & has recently created a facility for customers to book an online consultation with the pharmacist on murphyspharmacy.ie, all from the comfort of their own home. According to Jane “being a pharmacist is all about the personal touch & being there for your customers.
Chemist Warehouse is currently Australia’s largest pharmacy retailer offering discounted prices on health and pharmaceutical products. Colin saw an opportunity to give Irish consumers leading brands and affordable healthcare at the best price and with a high level of customer service. He is passionate about ensuring that his pharmacy’s are progressive and that their pharmacists skillsets are fully utilised to secure better patient outcomes. He leads with professionalism and a passion for retail pharmacy. To date the chain has outlets in Blanchardstown, Henry St., Dun Laoghaire, Ashbourne and Navan employing over 300 staff. The Irish public have really embraced Chemist Warehouse as a one stop shop for all their healthcare and beauty needs and with plans to grow the number of stores in Ireland over the next couple of years.







John recognises that knowledgeable, qualified staff are the cornerstone of his business. He believes that having staff who are competent, educated, and confident will make his pharmacy a patient’s first destination for their healthcare needs.












John’s number one priority is the care of his patients and providing them with excellent customer service, often delivering medication to vulnerable patients after hours. He is passionate about care for the elderly and is delighted to be back visiting the elderly in local nursing homes advising them about medication management and keeping well.
John recently expanded the pharmacy business premises to incorporate a modern Primary Care Centre offering a variety of HSE services, a busy medical practice, a dental surgery, a foot health practitioner and an opticians practice.
COVID-19 obviously necessitated many changes in the way pharmacy teams work. Like every pharmacy team in the country, the Riverstown team worked quickly to adapt to the ever-changing guidelines to ensure patient and staff safety and continuity of service. But Riverstown worked also to ensure patient communication was as effective as possible.
Marina was integral in organising a Covid Response Group for the local area. In response to the homeless crisis, the team at Riverstown, renowned in the totalhealth group for their charity efforts, decided to arrange a fundraiser for the Northwest Simon Community. The team decided to organise a sleep-out on Friday 10th December 2021, staying outside the pharmacy doors all night. After a valiant effort, their fundraising total passed ¤5,000, all of which went directly to the Northwest Simon Community who support over 100 families experiencing hardship and housing uncertainty.
Claire Hickey is a team member who consistently drives innovation. She recently took the very innovative decision to start using enviropharm fully compostable grip seal bags. Her pharmacy was the first pharmacy in Thurles to set the bar for a more sustainable green future. These bags can be sanitised and reused. If someone does not want to reuse the bag they can discarded in the food bin which allows it to be converted into soil or fertiliser. There is a huge amount of waste in the pharmacy world and Claire wanted to combat this and to help Hickey’s pharmacy to become a green more sustainable business.
In his time as a Supervising Pharmacist within the McCauley Group, Ray has trained countless OTC assistants and Technicians in addition to supporting a Pharmacy Intern. Ray development his pharmacy from a branch that offered core dispensing services into the largest vaccinating pharmacy within the McCauley Group. Ray often sacrificed his time off to support other pharmacies and pharmacists that needed guidance and advice. Ray recently stepped into his new role as Pharmacy Services/Vaccination Lead Pharmacist. Ray has worked tirelessly to support pharmacists and pharmacies across the group in delivering pharmacy services and vaccinations. Rays understanding of the challenges that community pharmacists face daily has allowed him to develop new and more efficient ways for pharmacists to deliver services to their patients.
Store Manager, Pat Hogan totalhealth Pharmacy, Galway





When the Galway Community Circus launched “LifeLine-Achieving the Impossible”, Laura was immediately interested.
LifeLine was Europe’s largest highwire spectacle performed over the River Corrib and Claddagh Basin in Galway City. In July of this year, during Galway Arts Festival, LifeLine took place over five hours in the Claddagh Basin. While LifeLine had displays by many professional highwire artists, it also include highwire walks by many community participants to remind us that we can all achieve the impossible. LifeLine uses wire-walking as a practice to improve wellbeing and aspires to reinfuse hope into a landscape that carries great sadness and beauty.


Volunteers spent months being trained in the art of wire-walking in preparation for July 16th. Laura Hogan was delighted to take part and thoroughly enjoyed the experience.



Mark has worked diligently to assist PSI in their improvement of the Third Country Qualification Route. He continues to work hard at PSI Council and as a member of the Performance and Resource Committee at PSI to represent the Pharmacist, Pharmacy as a profession and help in the safe keeping and continued health of the public.

During the troubling times of the Covid19 pandemic, Mark began an Instargam account which later grew to nearly eleven thousand followers where he hoped to open accessibility to the profession and help the public in a time of great uncertainty. Here he continues to educate the public on health, medicine and wellness to improve their patient experience. Mark also feeds into several IPU committees and groups including an IT steering group where IPU aim to assist in improving the Pharmacist experience in Community and in turn feed into chronic issues such as Pharmacist retention in community.
Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Deirbhle is extremely enthusiastic, optimistic and always prioritises patient care, going above and beyond for each patient that comes in to the Pharmacy. She is currently completing an Executive Masters in Business Administration (MBA) at University of Galway, having received two scholarships, to broaden her knowledge, transferable skills and confidence required for innovative and effective management and leadership. She also really enjoys giving back to her Profession by engaging in mentoring with APPEL Pharmacy students and with dispensers/technician students. She is currently embarking on a formal mentoring program with the IIOP. She actively keeps up to date with new emerging products and existing products available on the Irish market through engagement with IPU and IPN initiatives.
Fathimah has made it a priority to educate her team and patients alike on public health guidelines regarding COVID-19 and to provide knowledge on these ever-changing guidelines in a clear and concise manner to make them ‘user-friendly’. She did this through open discussions with her patients, addressing any concerns they may have had and to clarify any myths or misinformation that they may have come across. Once the inclusion of pharmacists as vaccinators in administering COVID-19 vaccines, Fathimah immediately stepped up to the plate and did a self-audit on the existing flu vaccination service she had set up in the pharmacy when she first began working in Reidy’s Pharmacy. She had patients from all over Dublin and the surrounding areas come to receive their vaccine from her, many of whom had heard from word of mouth at how efficient a service she provided and how professional and excellent she was at administering the vaccine.
Emily is a community pharmacist with the McCauley’s pharmacy group, where she was also a Superintendent Pharmacist from 2019-2020. Emily received her Masters of Pharmacy from the University of Brighton, she has since held senior management operational roles for several large pharmacy groups throughout Europe including Ireland, Italy, Belgium, and Czech Republic.



Emily has been a Peer Support Pharmacist for the Irish Institute of Pharmacy since 2017 and is active in the Peer Support agenda by delivering ePortfolio Review information webinars and playing a leading role in facilitating the Peer Support Event in October 2022. Emily is a strong advocate for creating a mentoring culture in pharmacy, in 2021, she supported the implementation of the IIOP Mentoring Programme. She is an active mentor and a member of the Mentoring Steering group.





On top of his current workload as a Supervising Pharmacist at Chemist Connect, Jack has integrated several new seamless services. Being quite a distance from other primary care services, can mean some of local patients have difficulty accessing services they might normally receive from their GP practice. One of the main services recently has been that of vaccinations which Jack has taken up from the beginning, with some house bound patients being offered the service in their own home. He also initiated the EU covid travel digital certificate (antigen test) service, which because of his pharmacys’ access to the border has allowed many patients on NI and ROI to avail of a quality, convenient service and dependable service at a cost much less than other national suppliers. Jack also has used his clinical skills to over parental administration of Prolia and Vitamin B12 injections which were essential to patients when the doctors were closed to these services during the pandemic.




Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
CarePlus Pharmacy, Marketpoint














Aoife is an exceptional pharmacist with an extensive knowledge of both medicines and her patients. Her considerations of all aspects of patient care are extraordinary. She exemplifies how professional practice, patient advocacy and leadership should be performed. Her nominee describes her as an ‘allrounder’ with an astute awareness of the growth of the business.



Agnese always strives for high standards in her work and is constantly thinking of new ways to improve the services offered at her pharmacy. Outside of her daily duties as a pharmacy technician, Agnese has also shown her compassion as a patient advocate. This year, Agnese recognised that many of her pharmacy’s blister pack patients were also collecting ostomy products each month and decided she wanted to learn more about these products so that she would have better understanding of her patient’s needs. On her own initiative, Agnese enrolled in and completed an online course in ostomy care in the community pharmacy setting, and now brings a wealth of knowledge to this area of the pharmacy’s service.
Lillis, Superintendent Pharmacist, Meaghers Pharmacy GroupElaine is the winner of the 2022 Perrigo Superintendent Pharmacist of the Year Award. Elaine has been Superintendent Pharmacist for Meaghers Pharmacy Group since August 2019 and in her time as Superintendent she has championed a number of public health initiatives. Since taking on the role of Superintendent the number of flu vaccines delivered in Meaghers has increased dramatically year on year. The last two years has seen record numbers of patients registering for a flu vaccine at their local Meaghers Pharmacy. Elaine recently teamed up with leading Consultant Hepatologist and Gastroenterologist Professor Suzanne Norris, to run several successful liver scans clinics in a number of Meaghers pharmacies, the country’s only pharmacy-led Diabetes Liver Screen Initiative. The highly ambitious programme is the first trial of its type in Europe.
 Agnese
Agnese
Tara is exceptionally patient focused. She is so natural with customers easily adapting her communicating style to approach any situation. Her nominee says, “I could not speak about Tara’s achievements over the past 12 months without mentioning the significant impact she has had on a local nursing home, for whom she manages their medication. Since joining the Meaghers team, Tara has put a huge focus on improving the current processes and systems in place for our nursing home in an effort to improve patient care and patient safety for both residents and staff. An effort that is frequently

Committed to providing accessible, affordable healthcare in communities nationwide Caoimhe has overseen the implementation and delivery of a broad range of public health initiatives over the last 12 months including ensuring continuity of pharmacy service by guaranteeing that each and every pharmacy in the Boots network remained as open and accessible as possible in spite of strict public health restrictions and associated resourcing challenges; expansion of Covid-19 Testing Services at Boots Ireland to include antigen testing services in addition to PCR testing; promoted increased awareness of menopause and fertility issues through participation in two– Let’s Make Menopause Visible and Let’s Give Fertility Visibility and launched a new HPV Vaccination Service, the first service of its kind in community pharmacy in Ireland.
McCabes Pharmacy Group consistently lead from the front. Announcing another first for Ireland this year, McCabes decided to create a McCabes Pharmacy digital personalised service, creating an intelligent guided selling (IGS) “Help me Choose” journey. They chose the Skincare category first, as choosing skincare products is complex, and everybody’s skin is different, calling it the Skincare Advisor. The Skincare Advisor helped bridge the gap between their in-store and online experience. With health expertise and services being central to their Digital offering, they extended the guided journeys to other categories. This is a first for Irish pharmacy.


Earlier in 2022 McCabes were recognised as a Deloitte Best Managed Company while two of their stores were announced finalists for the Retail Excellence Top 100 Retail Stores in Ireland.







Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
As a pharmacist that gives a word of advice freely with all consultations, Regina not only sells well w.r.t. probiotics, nutrients, supplements, OTC preparations but each sale is carried out with such care for the patient and genuine interest in their wee-being that the sale comes across as anything but pushy. A tutor pharmacist, Regina has trained and encouraged pharmacy and pre-registration students to help them reach milestones on their training journey.


Roz has been a pharmacist at Ryan’s Pharmacy since 2013 and is described as an ‘inspiring professional’ who has brought immeasurable benefits to the pharmacy team. Diligent and dedicated, she has introduced several new innovations within the store in order to help the pharmacy work more efficiently and to the betterment of patient care. Roz has also been instrumental in assisting the pharmacy technicians in developing their role further, and has acted as a mentor to the younger next generation of community pharmacists.
Denise McEvoy has been working in St Gabriel’s pharmacy for over 20 years. She has championed continued learning for the other staff members in the pharmacy and has taken it upon herself to ensure staff continue to learn through 4Front Pharmacy online training. She has dedicated her working life to pharmacy & always goes above and beyond to help our patients and work colleagues. Denise worked tirelessly through COVID & continues to improve the health outcomes of her patients on a daily basis. She can often be found delivering medication to vulnerable patients after she has finished work.

Mark opened his pharmacy in Ardee in 2012. To Mark, trust is such a critical quality in Pharmacy and the health sector and therefore he has taken a very active role in the local community in Ardee. One recent example is Mark’s new Healthy Hub, launching later this year, to proactively improve the health and wellness of the Ardee community. This is a comprehensive, guided 12-week weight-loss programme, with weekly Pharmacy check-ins measuring key health markers. As an experienced Neuro Linguistic Programme Practitioner, Mark uses his training and experience to help clients better understand and deal with their health. Mark has plans to further develop the Pharmacy’s involvement in the community, expanding the Healthy Hub and offering extended services.






Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.


www.azure-pharma.com
 Mark McPhilips, Pharmacist/Owner, Mark’s Staywell Pharmacy, Ardee
Mark McPhilips, Pharmacist/Owner, Mark’s Staywell Pharmacy, Ardee
Sheena’s daily interactions with both new and experienced parents in her pharmacy in Dublin, inspired her to start a family healthcare platform for her community to access out of hours – WonderBaba. ie The most recent podcast addition to her spectrum of advisory platforms has recently taken to the airwaves with its second season focusing on Respiratory Health.
With weekly episodes dropping every Tuesday morning, Sheena educates, informs, and even entertains listeners through her concise WonderBaba Explains podcast episodes. With a format of Real Lives parent interviews, Expert Advice from fellow professionals, and finally Little Voices where the small people in our lives get to have their say. Sheena was a Finalist in the 2022 Irish Pharmacy Awards for the Uniphar Category Development Award.
Nigel Moloney has built a high profile in Cork city through his outreach work with the homeless community, and already had two outlets under Navi Group brands - a CarePlus Pharmacy in Carrigaline and a StayWell Pharmacy on Washington Street - when he decided the premises at Ardfallen Mall would be another fitting addition to the StayWell Pharmacy group. He comments, “In a few short years, StayWell Pharmacy has established a great reputation and image, and offers proven management and work practices, access to national advertising and ongoing support. When you join a group like this, it takes a lot of the guesswork out of your initial setup. Our latest location serves a broad mix of customers, including locals and professionals working nearby.”





Jonathon Morrissey is Chair of the IPU’s Community Pharmacy Committee and in September of this year they joined forces with Saint John of God Hospital Pharmacy and the HSE National Medication Safety Programme in a novel new initiative to launch a new patient Information Booklet on Lithium Therapy.














The joint initiative aims to promote safer lithium therapy and empower patients to engage more with their Healthcare Professional on all aspects of lithium therapy including monitoring and potential side-effects.
In 2022, Mulligans Pharmacy opened their first pharmacy in Dublin with the launch of ‘The Pharmacy by Mulligans’, a 3000 square foot pharmacy at Arnotts on Henry Street. The group has 18 stores across Waterford, Kilkenny, and South Tipperary and was first established by Jim and Sheila Mulligan in 1957 when they opened a pharmacy at George’s St in Waterford City. The family group employs 250 people across the region with an additional 15 jobs being created at the Dublin store.
THE AZURE PORTFOLIO IS www.azure-pharma.com
Padraig has spent many hours explaining the merits of a good night’s sleep and giving people reliable information from leading authors.

One area of key focus is nutrition. Over the past year in particular he has tried to empower diabetic patients using evidence from Dr Sachin Panda on intermittent. He also promotes the well-documented benefits of Omega 3 fish oils in improving outcomes in conjunction with medication for mental health, combined with B vitamins and a dietary source of phospholipids that can prevent the progression of early dementia in older people and help maintain cognitive function.
Of particular focus for Padraig over the past 12 months was engagement with the wider community and not just the pharmacy team and existing clientele. He became a regular contributor to local radio on Southeast radio morning program with Alan Corcoran, covering topics such as the latest research into vaccine development, the current public health measures and why they were so important to prevent Covid-19 and its variants.
Hailing from Cascais in Portugal, Duarte Nunes Da Silva has made Ireland his home-fromhome since starting with McGreals in 2016. In this current role - as the lead pharmacist in McGreals Baltinglass - Duarte has built strong ties in the town with patients and partners alike. Hitting the headlines in Jan 2022, having administered 1,600 Covid vaccines – the most in any one pharmacy at that time – Duarte commented “People trust us locally and were happy to be able to receive their vaccinations in the safe and friendly local environment they are used to.” Duarte is the epitome of the “trusted pharmacist” and credits his strong team and the fantastic community spirit in Baltinglass as key factors in his success and the growth of the pharmacy footprint locally.

Damien won the JPA Brenson Lawlor Young Community Pharmacist of the Year Award 2022. Damien
been the driving force in the promotion of public health services in our community. His commitment to his customers and wealth of knowledge has allowed our pharmacy to flourish and become the main point of contact for health advice in Baldoyle. Damien’s ability to work well under pressure and make crucial decisions while maintaining a calm demeanour has been pivotal. He has administered 1200 vaccinations, managed a large pharmacy whilst still carrying out his duties as a pharmacist and his performance of CPR to a patient in need that resulted in saving the man’s life has been outstanding.







Rory and his Dispensary team are one of the highest vaccinating Pharmacies in the LloydsPharmacy group, (ranked number 3) regularly offering walk in clinics for Antigen testing, COVID19 and Flu Vaccinations. Rory has an exceptional patient centric approach, regularly greeting and chatting to the Patients, providing tips and advice in a friendly, caring yet professional way. He is highly respected by his team and customers in his community and has led to a growth in patient loyalty and repeat custom during the short time he has been in the Pharmacy.
He has been involved in three potentially life saving incidences keeping a calm and reassuring professional manner with the people involved. The Pharmacy store Manager describes Rory as someone who “Never says no -he is always happy to help. Rory is well respected not only in our community but also across our Pharmacy colleagues, Head Office and wider Pharmacist Network.”


Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Rory has been recently re-elected to the role of Vice-President of the PSI Council of Ireland for a 12-month period having previously served as President and Vice-President of the PSI, President of the IPU and President of the Pharmacy Benevolent Fund.

Rory has been a driving force in the successful merger of totalhealth and Haven and serves on the board of the newly formed company, CommCare Pharma, the holding company for the merger of Haven and totalhealth. Rory has been involved in developing support and training materials for pharmacy students doing their placements in Haven and totalhealth pharmacies. e was a driving force behind recent initiatives by CommCare to support innovation among final year TCD pharmacy students. This involved developing a special annual prize for the strongest 5th year project. Rory continues to speak at events such as the APPEL Future Pharmacy seminar and he has lectured on the RCSI Leadership in Pharmacy modules.
Denis currently is the clinical lead for one of the largest pharmacy chain in Ireland, 89 pharmacies and is responsible for management and delivery of quality pharmacy service to the public. His interest in Addition continues as he is voluntary independent chai r for Naloxone Advisory Group for HSE Strategy on Overdose Prevention. In addition to ensuring our teams are trained, prepares and oversees adherence to SOPS etc Denis plays a pivotal and lead role in overseeing the Health and Safety of all Pharmacies in the group, employees and patients during COVID pandemic, ensuring new protocols and processes established. Denis also supported Pharmacy teams administering Vaccines across communities and Head office protecting local communities around Ireland. Denis is also a regular host and presenter on the United Drug Webinars which contribute to Pharmacy CPD points and training development.
Oonagh
Oonagh O’Hagan marks her 21st Year in business leading the Meaghers Pharmacy group from strength to strength. Their strength in OTC offering saw them winning the GSK Self-Care Award 2022. Through adaptation of a detailed strategy to include a digital element, collaboration with other healthcare professionals, expansion of services and supply of innovative products Oonagh aims to offer the Meaghers customer more solutions to their health and wellbeing problems. Oonagh is passionate about the growth, development and success of the Pharmacy sector and indeed of SME’s in Ireland and has established a reputation as a leading voice of the industry demonstrating this with her work in both Trinity College Dublin, RCSI as well as on the Irish Governments Taskforce for SME’s and Entrepreneurship here in Ireland.










A previous nursing graduate, Sandy decided to pursue pharmacy as she felt her expertise was more centred in the medicinal accepts of patient care. Sandy joined the pharmacy team at Moran’s Pharmacy during the Flu vaccination programme of 2021. She took the responsibility to set up the programme and played the major role in the smooth running of the vaccination programme in the local community. Late last year, when a patient fell unconscious outside the pharmacy, Sandy shut the shop and went to his aid. She carried out CPR and undoubtedly her actions saved his life. It is the bravery and presence of mind that sets Sandy apart and shows her excellence. Sandy is truly a pharmacist who goes above and beyond to ensure her patients and customers have the best care and services they need.
Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com

In September of this year, Audrey helped publish a new patient information booklet on lithium therapy, which is also aimed at healthcare professionals. The aim of the booklet is to promote safer lithium therapy and empower patients to engage more with healthcare professionals on all aspects of their treatment, including potential side-effects.






It contains important safety and clinical information, as well as a means of recording essential information on lithium levels and blood test results. Additional information is also provided on different lithium products on the market, as well how to recognise signs of lithium toxicity.










With 5 pharmacies in the Kildare/ Offaly areas, these communities benefit greatly from Shane’s tireless work and genuine concern for those around him. Shane has recruited loyal dedicated staff in each pharmacy which has allowed him to continue to work as a pharmacist himself as well as manage all other pharmacies, with the help of his great staff. Shane continuously moves with the times and has renovated and refurbished every single one of his pharmacies, ensuring that the buildings and interiors are maintained at the high standard that the industry requires, as well as providing a welcoming space for customers and staff. He is always first up to support charities and local teams. When he isn’t working, he’s running Marathons and his energy and motivation is inspirational.
Ana Santos, Pharmacist, Hanly’s Local Pharmacy, New Ross
Ana has established herself in New Ross as an all round community pharmacist. Anna strongly believes in providing a holistic service to patients, which includes so much more than simply issuing medication and provides a stable support network for the New Ross community.


Anna is a friendly and welcoming community pharmacist who always puts the patient and the community at the centre of every healthcare scenario.
Dermot and his team won the 2022 United Drug Business Development (Independent) Award. The pharmacy team have overcome numerous challenges during the last year, including vaccination provision and an increased reliance on community pharmacy by patients which has resulted in the team having to react quickly and decisively to retain and grow the current business. With the support of the pharmacy team and their dedication and hard work, they have been able to survive the obstacles thrown up by the pandemic, grow the business and place it on a sound footing for the future.
Dermot Smyth, Pharmacist/Owner, Slane Pharmacy, Slane Shane Ryan, Pharmacist/Managing Director, RyansThe role of pharmacy and most especially pharmacy technician has evolved over the past number of years. Pharmacy technicians now play a pivotal role in the provision of healthcare as the role of the Pharmacist has become broadened. Tracey has proven to be a huge support to the pharmacists in Tralee utilizing her computer skills and completing all paperwork involved in the rollout of the vaccination service we provide to the community. She has adapted to the current demands of the pharmacy and played a vital role in rearranging the pharmacy in order to increase the space for dispensed prescriptions due to the increase in electronic prescriptions.
Claire won the 2022 Avene Counter Assistant of the Year Award. She has worked as a counter assistant for over 16 years in Enfield, almost 7 of which were with the Keane’s CarePlus Group. Claire became the front of shop supervisor as her result of her high service levels and dedication to the pharmacy. This is evident from her hard work and commitment over the years. As a result of the high service levels, Claire has built a fantastic rapport with the customers within the community. She was served them over the 16 years and has seen babies grow into young adults.
When the Keane’s Group took over from the previous owners and transitioned to the CarePlus model, Claire was instrumental in the change. With the transition, came significant change. Claire spearheaded the change in store layout, products stocked and communicated with suppliers to ensure that the transition was as smooth as possible.











Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Nial Tully, Pharmacist/Owner, Tully’s totalhealth Pharmacy,



Nial believes that community pharmacy should be a place where patients can access health services easily and quickly and for that reason Nial has added a second consultation room and expanded his service offering over the past two years. Having health services available certainly made things easier for the community in a time when it was difficult to stay positive.
Nial and his team offer a “Safe Place” in the pharmacy every year to local children to wait if the Mum’s & Dad’s get delayed on their way to collect them after school or they miss their school bus. This service is greatly appreciated in the town. The children are free to wait safe and warm in the pharmacy and they get to use the pharmacy Wi-Fi until they can be collected.

James Tully has a wonderful ability in understanding what patients are going through and his approach to patients with mental health conditions is outstanding. His team describe him as a team player who treats everybody with the same respect. He initiated the delivery of medicines during the pandemic by firstly delivering them himself but is now structured by a postal service with next day delivery. The role of a pharmacist has evolved in recent years and professionals need to possess certain attributes. It is an everchanging role that demands humility. Character, empathy, knowledge, skill, accuracy and patience are all attributes that James possesses and at the highest standard.
Whitty, Support Pharmacist, McCauley Pharmacy Group
Sadhbh qualified as a pharmacist in November 2021 and took up her role as a Support Pharmacist in one of McCauleys busiest stores in the southeast. Sadhbh displayed a confidence and performance level from the outset that belied her experience. Sadhbh immediately became involved in the vaccination services being offered in the store having completed her training while waiting for her registration. One of Sadhbh’s major achievements over the last year has been supporting the Pharmacy and Store in the transition period between Supervising Pharmacists. Without any prompting, Sadhbh stepped into the leadership role in the pharmacy ensuring that patient care and customer service was unaffected through the change. Sadhbh has worked with the store manager to ensure that appropriate resource has been available to allow the pharmacy to continue to operate safely.
Rob joined the McCauley Pharmacy Group in 2021, taking over as the Supervising Pharmacist Store Manager in one of the groups new acquisition stores. Through his outstanding people leadership skills and patient centred approach, he has not only ensured the continuity of care for his customers but improved this by streamlining the operational set up of the pharmacy, building key relationships with other healthcare professionals in the area and launching new pharmacy services within the store. Rob has launched Flu and Covid-19 Vaccinations, 24 Hour Blood Pressure Monitoring, Lipid and Glucose Testing in his pharmacy over the last 12 months.







Rob is incredibly passionate to share his knowledge and pass on his experience to the next generation of Pharmacists. Rob acts as a Mentor to three other pharmacists to support them in their development. Robs belief that we can achieve more when working together as opposed to in isolation can be seen in his work as a Mentor Pharmacist and his collaborative work with McCauley.

Our purpose is to safeguard the medicine supply chain to ensure that older medicines at risk of going out of stock remain available and affordable.
www.azure-pharma.com
Rob Wood, Supervising Pharmacist Manager, McCauley Pharmacy Group James Tully, McCauleys Health & Beauty Pharmacy, TraleeA community pharmacy environment that fosters teamwork ensures high levels of consumer satisfaction. This series of articles is designed for you to use as a guide to assist your team in focusing on meeting ongoing CPD targets and to identify any training needs in order to keep the knowledge and skills of you and your team up to date.
essential for healthy circulation. ALA can contribute to the production of EPA and DHA in the body but not in amounts enough to be of benefit.
tendency), reduce blood levels of triacylglycerols, prevent atherosclerosis and arrhythmias and reduce blood pressure
The below information, considerations and checklist provide support to enable you to run a team training session and identify opportunities for learning within the topic of Omega 3.
Omega-3 is one of the most widely studied areas of nutrition, with claims of benefits to multiple aspects of health, from AMD to CVD.
Fish oil’s popularity has soared in recent years, propelled by research that suggests consuming omega-3 fatty acids can ward off heart disease. Community pharmacists play an important role in helping patients make informed decisions about fish oil and omega-3 supplementation.
But with hundreds of OTC options available (and labels claiming that the supplements treat everything from bipolar disorder to menstrual cramps) how can pharmacists distinguish between them to help patients make the best choice?
Fish oils are a type of polyunsaturated fatty acids (PUFAs). They are the omega-3 and omega-6 fatty acids.
Regarding heart health, the active ingredients in fish oils are DHA and EPA. The hypothesis is that omega-3s offer protection
Consider:
from cardiovascular disease by reducing the heart’s susceptibility to arrhythmias, decreasing platelet aggregation and lowering blood pressure and triglyceride levels.
Sold by pharmacists since the early years of the 20th century, cod liver oil is one of the oldest and most well-known food supplements. The oil first gained its reputation as an effective preventative against rickets because of its high vitamin D content and it was taken by generations of children, decreasing in popularity only when, in the 1960s, the British government decided to phase it out as a welfare food.
Fish oils are most commonly used in supplements for conditions related to the heart and blood system. They may also be used for many kidney-related problems.
There are eleven types of Omega-3 but the three most referred to are:

• ALA (alpha linolenic acid) which contributes to overall health but is deemed to be of lower importance that other types.
• EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are both deemed to be of most importance to over health,
There is little doubt that n-3 fatty acids have an important role in nutrition. They are significant structural components of the phospholipid membranes of tissues throughout the body, particularly in the brain and retina. Because of their role in cell membranes, n-3 fatty acids are essential for the formation of new tissue and are therefore important for development and growth (eg, during foetal and infant development).
For optimal foetal health, women can be advised to take a DHA dominant Omega-3 oil from the start of their pregnancy.
Long chain n-3 fatty acids (and other fatty acids) are present in breast milk and European Union regulations now allow their addition to infant formulas.
Pharmacy team should be aware of, and understand, the following key points:
• Those who could benefit from an increased intake of n-3 fatty acids. The typical Irish diet contains relatively low amounts of these.
• Fish oil appears to reduce the risk of CHD. It may help to reduce the risk of thrombosis (by increasing bleeding
What types of pain can be considered for management in the pharmacy
Which customers should be referred to the pharmacist
• Fish oil may have a role in various mental disorders, such as depression, schizophrenia and Alzheimer’s disease, but research in this area is in its infancy
How much Omega-3 is required?
• 18 months to 3 years – 1-3 tablespoons
• 4 to 4 years – 2-4 tablespoons
• 7-11 years – 4-6 tablespoons
• 12 years to adult – 140g.
Actions:
Ensure support staff understand the following key points:
The common types of acute pain
The characteristics of a good analgesic for OTC use in the treatment of acute pain
The benefits and limitations of OTC medicines
The importance of what outcome a patient presenting with pain wants
Is the pharmacy team fully trained on the indications and benefits of all products for the treatment of acute pain?
How are pain relief products (oral and topical) displayed in the pharmacy?
Do we make the most of the potential for linked sales (e.g. oral and topical analgesics, food supplements, complementary therapies, support bandages)
Am I up to date with the latest guidance?
Am I aware which preparations are recommended first-line?
The use of the PRICE method
Lifestyle issues that may impact on pain recovery, and tips on improving lifestyle
What approaches are recommended by the World Health Organisation (WHO) analgesic ladder
The role of dietary supplements


