HOSPITAL PROFESSIONAL NEWS IRELAND
Ireland’s Dedicated Hospital Professional Publication
Legal Category: Product subject to prescription which may be renewed (B).
Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Further product information available on request from:
AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00.
FORXIGA is a trademark of the AstraZeneca group of companies.
IN THIS ISSUE:
NEWS: Expeditated Electronic Health Record Delivery Page 5
CONFERENCE: IPHA host Annual Conference Page 6
RESEARCH: SFI Discover Programme Funding Page 32
CPD: High Risk Medical Devices Page 37
CARDIOLOGY
FOCUS: Cardiac Rehabilitation Page 44
CARDIOLOGY
FOCUS:
Subclinical Atrial Fibrillation Page 50
ASK THE EXPERT: Dr Cormac Mullins Page 72
HPN April 2024 Issue 119 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
Veeva ID: IE-4697 Date of Prep: February 2023
HOSPITAL PROFESSIONAL NEWS IRELAND
Ireland’s Dedicated Hospital Professional Publication
Call for papers: make your contribution to Hospital Professional News
Articles
Research Papers
Reviews
Programme Descriptions
Reports
Case Reports
Letters to Editor
Support fellow hospital professionals as well as aspiring junior professionals and early-year hospital pharmacists
Practice reports share innovations on any area of practice, including delivering clinical services, pharmacy administration, or new approaches to inform and engage with patients
Perspective articles focus on a specific field or discipline and discuss current advances or future directions, and may include original data as well as expert insight and opinions
Contact: Kelly Jo Eastwood at: kelly-jo@ipn.ie or Danielle Norton - danielle@hospitalprofessionalnews.ie







www.hospitalprofessionalnews.ie





Contents
St Vincent’s University Hospital acknowledges Rare Diseases Day P4
Challenging wait times for new medicines, IPHA Conference hears P6
Irish Medical Council publishes its Medical Workforce Intelligence Report P8
111,000 added to the Waiting Lists, says IHCA P10
Irish cancer experts meet with US Congressional Caucus P11
New Chair of Tallaght University Hospital Board P26
REGULARS
Feature: Management and Treatment of Parkinson’s Disease
CPD: High Risk Medical Devices
Cardiology Focus:



Aortic Dissection P56 Cardiology Focus:
Medicines P58 Cardiology

Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL danielle@hospitalprofessionalnews.ie
ACCOUNTS
Fiona Bothwell cs.ipn@btconnect.com
SALES EXECUTIVE
Avril Boyd
avril@hospitalprofessionalnews.ie
SALES & TRAINING MANAGER
Sibongile Mude s.mude@hospitalprofessionalnews.ie
CONTRIBUTORS
Dr Cormac Mullins
Alison Cahill
Sadhbh Ni Cheallaigh
Jonathan Gallagher
Professor Alan Soo
Tara Byrne
Paul Nolan
Deirdre O’Reilly
Dr Rory Durand
Dr JJ Coughlan
Professor Robert A. Byrne
Theresa Lowry-Lehnen
Lisa Wynne
Gráinne Henry
DESIGN DIRECTOR
Ian Stoddart Design


Foreword
Editor
A Professor of Innovation at Maynooth University says that Ireland is in a prime position to develop the most advanced electronic health record (EHR) system in the world if the appropriate strategy and funding is forthcoming from Government.
On page 5, we detail how Professor Martin Curley, speaking in a new video as part of the Irish Hospital Consultants Association’s (IHCA) Care Can’t Wait campaign, urges the Government to expedite the roll out of electronic health records and other digital systems by establishing an independent digital health agency which would oversee their deployment.
Ireland is currently 15 years behind the majority of developed countries when it comes to digital health capabilities. However, Prof Curley says Ireland could leapfrog other countries and bypass an ‘entire cycle of investment’ if Government embraces the latest innovations.
In other news, the Irish Pharmaceutical Healthcare Association (IPHA) has released new data indicating continuing lengthy timelines of two years or more for the reimbursement of new life-enhancing medicines which can be of vital benefit to Irish patients. IPHA believes these timelines can, and must be, improved significantly. Patients deserve to know that they can, through their clinicians, access the best medicines available to treat cancers, obesity, rare diseases and many others.
The challenge that industry and the HSE can address together is to improve the following issues that their research has established. The data was released during their 2024 Annual Conference. You can read more about that on page 6.
On page 72 we speak to Dr Cormac Mullins, one of the keynote speakers at the recent Irish Pain Society’s Annual Scientific Meeting, where he provided an update on spinal cord stimulation, emphasising the importance of appropriate patient selection. Dr Mullins also provided a useful critique of the very limited placebo-controlled RCT evidence base that currently exists for spinal cord stimulation, the large placebo response, and the need for more high quality RCTs in this area.
Our Special Focus for April is on Cardiovascular Health and we have some excellent contributed articles including Deirdre Reilly, Cardiac Rehabilitation Coordinator Roscommon University Hospital, Saolta Healthcare Group & President Irish Association of Cardiac Rehabilitation discussing cardiac rehabilitation; Paul Nolan, Clinical Lecturer Atlantic Technological University, Sligo & Cardiac Physiologist, Galway University Hospital who gives an overview of subclinical atrial fibrillation and Professor Alan Soo alongside Tara Byrne who highlight acute aortic dissection – a ‘real’ cardiothoracic emergency.
I hope you enjoy the issue.
3 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 April Issue Issue119 4
Acute
Optimising
Focus: Women and CVD
Clinical R&D:
11 6 26 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
P62
P80
Lack of Medical Workforce Planning
The Irish Medical Organisation (IMO) has said that the Medical Council’s Medical Workforce Intelligence Consolidated Report 2022 highlights the lack of workforce planning to ensure we have enough doctors to meet the demands of an increasing population.
The Medical Workforce Intelligence Consolidated Report was released last month (further coverage on page 8).
The report outlines the major, sustained shortfall of doctors across medical specialties, including consultants, GPs and non-consultant hospital doctors (NCHDs). This shortfall is having a significant negative effect on patient care and doctor welfare in Ireland.
Professor Matthew Sadlier, Chair of IMO Consultant Committee said, “Our health system is caught in a vicious circle, whereby we do not have enough doctors to meet the ever-increasing demand, leading to more pressure and burnout for the doctors we do have working in Ireland. This in turn leads to poorer patient outcomes and increased doctor attrition. Unfortunately, this report highlights the fact that we do not have a proper plan in place to reverse this trend. Ireland has one of the lowest ratios of consultants to population ratios in the OECD, an ageing GP population of which many are close to retirement, and an NCHD cohort that is regularly asked to work in contravention to the European Working Time Directive. For example, IMO research shows that more than 77% of NCHDs routinely work
more than 48 hours a week, which is both illegal and unsafe, leading to a widespread risk of burnout and stress.
The Report shows that 3,008 doctors registered with the Medical Council for the first time in 2022, and 1,341 doctors voluntarily withdrew their services from the register.
“This report backs up what the IMO has been saying for many years; namely, that our long working hours, poor work/life balance and stressful working conditions are driving doctors away from Ireland and to countries support them and properly value their contribution.”
Of the doctors who registered with the Medical Council for the first time in 2022, 71% had an international basic medical qualification (BMQ), while just 29%
SVUH commemorates Rare Disease Day

As the lead clinical site for the European Reference Networks (ERNs) for rare lung, rare bone, and rare neuroendocrine cancers, SVUH provides invaluable support to patients across Ireland. We are delighted to be in a position to commence recruitment for this important position.
As the world commemorated Rare Disease Day, St. Vincent’s University Hospital (SVUH) stands at the forefront of rare disease care and research, proudly announcing the successful
funding for a Rare Disease Research Coordinator under the Rare Disease Research Catalyst consortium grant from the Health Research Board (HRB). This significant achievement not only
L-R – Prof Dermot O’Toole, National Clinical Lead for Neuroendocrine Tumours; Prof Rachel Crowley, consultant endocrinologist at SVUH and Prof Cormac McCarthy, Consultant Respiratory Physician at SVUH & Associate Professor of Medicine at UCD
reaffirms SVUH’s unwavering commitment to rare disease care but also highlights the critical importance of this day in raising awareness and support for those affected by rare diseases.
The Rare Disease Clinical Trial Network (CTN) Conference, a visionary initiative pioneered by Professor Rachel Crowley, Consultant Endocrinologist at St. Vincent’s University Hospital and Clinical Professor at University College Dublin (UCD), alongside Professor Cormac McCarthy, Consultant Respiratory Physician at SVUH and Associate Professor of Medicine at UCD, is currently underway in Dublin. This critical assembly serves as a nexus for global experts, researchers, and advocates to delve into the forefront of rare disease clinical research, fostering collaboration and innovation.
had an Irish BMQ. “We are utterly reliant on international medical graduates to plug the gaps but, predictably, we are not doing nearly enough to support them by offering meaningful career and training pathways,” said Prof Sadlier.
Professor Sadlier said that much more needs to be done not only to recruit sufficient numbers of doctors, particularly in the context of expected retirements but to improve the working environment so that they are enabled and supported to deliver care in a safe way. Of the 23,108 doctors retained on the Medical Council’s register in 2022, 21% were aged 55 or above. “This report highlights the crisis in our medical workforce and is all the more ironic given the fact we have an ongoing recruitment freeze for NCHDs not in training posts which is having a detrimental effect on the service.”
Professor Rachel Crowley said, “St. Vincent’s University Hospital is celebrating Rare Disease Day 2024 by highlighting its unparalleled contribution to rare disease care in Ireland. As the lead clinical site for the European Reference Networks (ERNs) for rare lung, rare bone, and rare neuroendocrine cancers, SVUH provides invaluable support to patients across Ireland. We are delighted to be in a position to commence recruitment for this important position, which we hope to have a suitable candidate in place for Q2 2024. We are hugely grateful to the HRB and UCD for all their support.”
The Rare Disease Research Catalyst Consortium award, a substantial ¤3 million grant awarded by the HRB to Professor Rachel Crowley, as lead, in collaboration with UCD, will enable the appointment of a dedicated Rare Disease Research Coordinator at SVUH’s Clinical Research Centre site with UCD. This visionary role will navigate ethical considerations, data protection impacts, and research obstacles, serving as a catalyst to bolster research activity and foster the influx of clinical trials and interventions in Ireland.
4 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Consultants Urge Expeditated Delivery of Electronic Health Records
A Professor of Innovation at Maynooth University says that Ireland is in a prime position to develop the most advanced electronic health record (EHR) system in the world if the appropriate strategy and funding is forthcoming from Government.
Speaking in a new video as part of the Irish Hospital Consultants Association’s (IHCA) Care Can’t Wait campaign, Professor Martin Curley urged the Government to expedite the roll out of electronic health records and other digital systems by establishing an independent digital health agency which would oversee their deployment.
Ireland is currently 15 years behind the majority of developed countries when it comes to digital health capabilities. However, Prof Curley says Ireland could leapfrog other countries and bypass an ‘entire cycle of investment’ if Government embraces the latest innovations.
Professor Curley says this can be done by adopting the most up to date technology which would allow every person in the country to possess a personal EHR on their phone. The target under the ‘EU
Digital Decade’ policy is that 100% of EU citizens will have access to their own electronic health records in just six years’ time.
The Minister for Health Stephen Donnelly recently estimated that a regional deployment of EHRs is likely to cost between ¤200m and ¤300m for each of the six new Health Regions – or up to ¤1.8bn nationally. The recently published HSE National Service Plan allocated just ¤155m in capital funding to its eHealth Division in 2024, together with a ¤259m operational budget and ¤55m for various cyber security measures.
“Technology has advanced so much, and this will allow us to build a health service where the patient is at the centre. This would be a leapfrog strategy, potentially avoiding a whole cycle of investment and implementation, to create a world leading digital
Continuing Pharmacy Education
For the first time, the European Council for Pharmacy Education Accreditation (ECPhA) as the only Pan-European entity for accreditation of continuing pharmacy education and continuing professional development programs, joined the European Union of Medical Specialists Conference on CME/ CPD in Europe.
Said a representative, “As founding members of ECPhA, The European Association of Hospital Pharmacists - EAHP and European Society of Clinical Pharmacy (ESCP) are immensely proud and grateful for the support given by the UEMS throughout the years of building ECPhA.”
Nenad Milijkovic, President-Elect at EAHP said, “I would also like to extend my personal gratitude to the UEMS President Vassilios Papalois, and the Chair of the Partner Session Marc H.M. Hermans for joining forces with pharmacists and emphasizing the importance of high quality education of healthcare professionals for the benefit of our patients.
“ECPhA team Aida Batista, Derek Stewart, Anna Olearova and Gonzalo Marzal Lopez will continue to expand ECPhA's reach and move forward with accrediting upcoming continuing education events in pharmacy.”

health system in Ireland and more importantly better health outcomes for all,” he said.
“In Ireland we have 21st century clinicians and often 21st century
medicines and equipment, but we have Dickensian style medical records and Victorian style Health systems. This has to change. It literally is sometimes a matter of life or death.”

5 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
News
Professor Martin Curley
Challenging of Wait Times for New Medicines

The Irish Pharmaceutical Healthcare Association (IPHA) has released new data indicating continuing lengthy timelines of two years or more for the reimbursement of new lifeenhancing medicines which can be of vital benefit to Irish patients.
IPHA believes these timelines can, and must be, improved significantly. Patients deserve to know that they can, through their clinicians, access the best medicines available to treat cancers, obesity, rare diseases and many others. The challenge that industry and the HSE can address together is to improve the following issues that their research has established:
• Of the 23 medicines of IPHA member companies made available to public patients during 2023, the average time to availability from application to the HSE was 729 days, or approximately two years.
• For medicines, which the HSE and National Centre for Pharmacoeconomics required a Health Technology Assessment, the average time was over 1,000 days to reach patients.
• For cancer medicines the average time was 651 days and for orphan medicines the timeframe was 759 days.
• It takes an average of one year after a health need has been recognised by the HSE Drugs Group to implement a ‘managed access protocol’ before patients can access these medicines.
• While a robust clinical and economic evaluation is needed,
the Irish reimbursement system can have approximately twenty to thirty separate steps, depending on the medicine concerned.
• The HSE Drugs Group meets on average once a month, for two hours, and considers all medicines with a budget impact. The equivalent group in England and Wales delegates their work across four committees and meet for eight hours once a month.
• It takes approximately 3-4 months for a medicine to be considered at the HSE Drugs Group meeting after a price is agreed. It takes approximately six months from a final pricing offer being proposed and accepted to the medicine being made available to patients. There is clear ‘dead time’ in this process.
In most cases, clinicians are well aware of the medicines that are pending. They wish to prescribe them to patients in the public service, as they build on and improve existing standards of care and health outcomes. However, an increasing consequence of the lengthy timelines is that some private patients can avail of cancer treatments under health insurance, immediately on the granting of a licence by the European Commission. Public patients, typically wait two years after this point before accessing the same medicine.
IPHA believes timelines can be reduced by bringing resourcing of the evaluation system up to international standards, enhancing the capacity of the HSE Drugs
Group, eliminating process ‘dead time’, and developing more efficient engagement between pharmaceutical companies and the HSE and NCPE. IPHA members are keen to work closely with these agencies to deliver improvements. Last year, Minister Donnelly announced the establishment of a working group on the reimbursement system following the publication of a report by the firm Mazars.
Speaking ahead of the Annual IPHA Conference in Dublin, Michael O’Connell, IPHA President said, “IPHA’s goal is to ensure a continuous flow of life-enhancing medicines and vaccines for Irish patients in a faster and fairer manner. We welcome the ¤20 million allocated to new medicines last December and the ¤10 million to be found in efficiency savings. This funding will benefit 4,000 Irish patients across a number of different therapy areas.
“IPHA is willing to work with the State towards finding efficiency savings to ease the burden on the overall health budget. We have signified our commitment to realise savings through the IPHA Agreement where we have already delivered more than ¤400 million in savings to the HSE in its first two years. We also look forward to engaging with the newly established Medicines Sustainability Taskforce.
“Patients need the system to work better; they deserve fundamental reform of the process. We stand ready to collaborate with all stakeholders to ensure that meaningful reform is achieved. We look forward to the outcome of the Mazars Working Group. We appreciated the Minister’s invitation to provide input to the Working Group and are very keen now to see a range of actions for urgent implementation.”
Oliver O’Connor IPHA Chief Executive said, “Reducing the lengthy timelines to reimbursement will improve standards of care and outcomes for patients in Ireland.
“Industry and the State can partner much better to improve this. It is very much a shared challenge. No-one wants a public-private divide in access to medicines to grow.
“The specialist staff operating the system have faced muchincreased workloads from new medicines developments and applications; they’ve been under resourced for the challenges. We hope the Minister will follow up on his funding announcements with the approval of much needed staff in the pricing and reimbursement system. These highly specialised experts are as important to the health of the nation as frontline workers.
“Ireland’s health care, health outcomes for patients and the health status of the population have all improved. We can and should aspire to be as good as any health system in Europe. Where there are gaps, such as in timely access to medicines, we can fix them, by collaborative, purposeful work together.
“Ultimately, we can deliver a system where patients in Ireland will receive the best of care and treatment, with the right medicine at the right time.”

6 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE Conference
IPHA President Michael O'Connell


12th Annual Scientific Meeting 2024 Thursday 2nd May 2024 UCD O’Reilly Hall, Dublin - In Person Only(No virtual option) REGISTER NOW! Advance Registration Essential To register, please visit: www.conferencediary.ie Please enter registration code: IMF24 For further information, please contact: Fiona Enright - CCIS The Conference Company fiona@ccis.ie | 061 475 908
Workforce Intelligence Report Published

On International Women’s Day, the Medical Council published its 2022 Workforce Intelligence report. The report analyses and presents data provided by doctors on the Medical Council’s medical register (the ‘Register’). Its data reveals the growing number of female doctors registering with the Medical Council for the first time. It also provides insights from doctors as to why they chose to withdraw from the Register in 2022.
This includes doctors who have registered for the first time or retained registration with the Medical Council, and those who have voluntarily withdrawn from the Register throughout 2022.
The number of doctors on the Register has been increasing year on year, with the number of young female doctors having risen steadily over the past decade.
This year’s International Women’s Day theme is #Inspire Inclusion, and encourages everyone to
Dr Suzanne Crowe, President, Irish Medical Council
recognise the unique perspectives and contributions of women from all walks of life. It calls on those involved to break down barriers, challenge stereotypes, and create environments where all women are valued and respected.
2022 Key Highlights
First Time Registered Doctors:
o 3,008 doctors registered with the Medical Council for the first time in 2022, compared with 2,605 in the previous year, representing a 15% increase, between 2021 and 2022.
o Over half (52.6%) of doctors registering for the first time in 2022 were male, and 47.4% were female.
o Notably, the ratio of females to males is greater in the youngest cohort of doctors, aged 24 and younger (45% male vs 55% female).
Pharmacy Research Grant
The FIP Foundation for Education and Research (FIP Foundation) and the Board of Pharmacy Specialties (BPS) have announced that applications for their collaborative research grant opportunity for non-USA-based investigators researching the impact of pharmacist board certification are now open. Visit the FIP Foundation webpage for the electronic application link. Applications are due 1 May 2024.
BPS, in collaboration with the FIP Foundation, is sponsoring one research grant of up to EUR 5,000 to support researchers outside
of the USA who are interested in conducting research related to the impact of pharmacist board certification. To be eligible, research projects must be focused on the impact of pharmacist board certification on specialisation and advanced practice through the following research aims:
• Patients and/or outcomes (e.g., adherence, quality of life, patient satisfaction, health literacy);
• Healthcare systems (e.g., medication safety, cost avoidance, drug-related
o Furthermore, 71.2% of doctors registering for the first time obtained their qualifications abroad.
Doctors Retaining Registration:
o 18,839 (81.5%) doctors who renewed their registration with the Medical Council were clinically active in Ireland, all or some of the time in 2022.
o In 2022, 46.8% of clinically active doctors in Ireland were female, while 53.2% were male. The distribution of males and females was split more evenly in younger cohorts, whereas the majority of older doctors are male. Over half (51.7%) of clinically active doctors working in Ireland were on the Specialist Division of the Register, 16.3% of doctors were on the Trainee Specialist Division; and 31.2% were on the General Division of the Register.
o In 2022, over a quarter of clinically active doctors working in Ireland self-reported working more than 48 hours a week on average.
In 2022, 1,341 doctors, of whom 593 were female, voluntarily withdrew their registration with the Medical Council. Among doctors who completed the voluntary withdrawal survey, 45.9% offered detailed explanations on the reasons for withdrawing their registration. A number of workrelated issues were cited, including limited career progression and training opportunities, poor working conditions, personal or family reasons, plans to practise
abroad, and other reasons such as registration requirements and the emergency response to COVID-19 coming to an end.
President of the Medical Council, Dr Suzanne Crowe said, “As the world collectively celebrates International Women's Day, I’m glad to see a rising number of women in medicine in Ireland, particularly those aged 24 and under. In 1994, women made up just 30% of the medical Register. Now, 30 years later, we are nearly at a 50/50 split male to female, with the numbers of female doctors in the younger age cohorts outpacing male doctors."
“Over the years, there has been a significant increase in the representation of women within the medical profession, reflecting a positive shift towards greater gender diversity and inclusivity in healthcare.
“One of the key elements of Inspire Inclusion is promoting diversity in leadership and decision-making positions. Women, especially those belonging to underrepresented groups, continue to face barriers when seeking leadership roles.
“Today, I’m also reflecting on the contributions of the women who tirelessly advocate for patients and vulnerable people in Ireland. Today, we celebrate their dedication, compassion, and commitment to making a positive difference in the lives of others. Women can bring a unique understanding of women’s health issues, paving the way for change in medicine, and helping other patients to feel safe and heard.”
problems, admissions/ readmissions, emergency department visits, institutional credentialing and privileging);
• Interprofessional collaboration (e.g., recognition, perception, increased efficiency in providing care); and/or,
• Pharmacist employment, professional development, and/or wellbeing (e.g., job satisfaction, retention, career advancement).
The grant awardee will be recognised during the 82nd FIP
World Congress of Pharmacy and Pharmaceutical Sciences in September 2024 and through FIP Foundation and BPS online publications. The final research report will be suitable for publication in the International Pharmacy Journal.
Questions about the research grant can be directed to the FIP Foundation at foundation@fip.org.
The Board of Pharmacy Specialties (BPS) was established in 1976 as an autonomous division of the American Pharmacists Association (APhA).
8 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Uncovering Secret to Appetite Control
“I’m hopeful our research can have a big impact. Metformin is the most prescribed drug for type-2 diabetes, and it’s very safe and well tolerated. How metformin affects appetite was not known, but this work shows that its influence on Lac-Phe is a key part of its hunger suppressing impact.”
In a ground-breaking study, just published in leading international journal Nature Metabolism, scientists from Trinity and Princeton and Harvard Medical School
share newly uncovered secrets to natural appetite control, which offers promise in the battle against obesity and type-2 diabetes.
The cost of managing diabetes represents approximately 9% of EU Member States’ healthcare budgets – totalling ¤149 billion in 2019.
The scientists report new insights into how the widely used diabetes drug metformin benefits patients with type-2 diabetes. Metformin is described by some as a “wonder drug” even though we still do not know exactly how it works.
This study shows that metformin increases the amount of an appetite suppressing factor called Lactoyl-Phenylalanine (Lac-Phe), identified in 2022 as a natural appetite suppressant, and which is known to be raised by vigorous exercise.
The scientists probed data from other studies involving large numbers of patients, to conclusively demonstrate that LacPhe levels rise after individuals
David Finlay, Associate Professor in Immunometabolism, Trinity College Dublin
take metformin. This work opens a new avenue for developing targeted anti-obesity treatments.
Barry Scott, first author of the research, PhD Candidate in Trinity’s School of Biochemistry and Immunology, based in the Trinity Biomedical Sciences Institute (TBSI) said, “I’m hopeful our research can have a big impact. Metformin is the most prescribed drug for type-2 diabetes, and it’s very safe and well tolerated. How metformin affects appetite was not known, but this work shows that its influence on Lac-Phe is a key part of its hunger suppressing impact.”
David Finlay, Associate Professor in Immunometabolism, Trinity, who co-supervised the work with Professor Lydia Lynch, Princeton
School of Pharmacy at SPHeRE 2024
Staff and students of the School of Pharmacy at University College Cork were actively participating at the SPHeRE (Structured population health, policy and health services research education) conference in RCSI recently.
Dr Edel Burton, SPHeRe Scholar and Lecturer in Clinical Pharmacy presented the following:
Stroke/Transient Ischaemic attack (TIA) survivors and caregivers' perspectives of acute stroke care during the COVID-19 pandemic in Ireland: a qualitative descriptive study. (Oral presentation)
Angiotensin receptor blocker and angiotensin-converting-enzyme inhibitors prescription frequencies following pharmacovigilance concerns during COVID-19 in Ireland and the United Kingdom. (Poster presentation)

and Harvard Medical School, said: “Our study shows that the type of food you eat matters. For instance, eating sugar-rich date fruits caused an immediate and large surge in Lac-Phe, for example, whereas drinking a sugar-rich drink did not. This could help explain why liquid calories can drive obesity.”

Fourth year UCC pharmacy student Órla O’Donohue presented a research poster on work undertaken while on placement with Pfizer Healthcare alongside co-authors Abaigeal Jackson, Duilia Bruno, and Maura Kinahan.
The title of this poster was : “Real World Evidence Use in Medicines Regulation: What does the future hold for Ireland?”.
Edel Burton, Eilis O'Reilly, Meadhbh Cosgrove, Mark Coughlan, Lorna English, Prajakta Meshram, Margaret Bermingham. Dr Aoife Fleming presented on a research study collaboration with Dr Margaret Bermingham and MPharm students Hannah O'Flaherty and Maeve Smith: A cross-sectional survey of pharmacists' views on the provision of regular hormonal contraception in community pharmacy practice in Ireland. (Oral presentation).
A review of the draft Irish Health Information Bill, the European Health Dataspace legislation, and publications on US and EU real world evidence use was undertaken by Orla and her coauthors. It was noted that Ireland has much work to do to implement the Bill to bring the jurisdiction in line with the standards the EU have set for the collection and accessibility of health data in its Member States. Both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) continue to examine the use and standardisation of Real World
Evidence (RWE) use in regulatory decision-making. The EMA have identified areas for improvement before RWE can be used in their decision-making, while the FDA continues to use RWE in the contexts of label expansion and post-approval safety studies as they investigate methods of standardisation for data collection and generation.
This area of RWE use is set to evolve rapidly within the next decade, and it will be interesting to see the clinical, academic, and regulatory impact of the upcoming changes.
9 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 News
Órla O'Donohue, Dr Aoife Fleming, Dr Edel Burton
Lessons
111,000 added to Waiting Lists
Consultants warn Action Plan for 2024 is unlikely to achieve significant cuts in waiting lists without fast-tracking increased capacity

More than 111,000 people have been added to hospital waiting lists in the past four years, despite the Government spending a staggering ¤1.2 billion over the same period on initiatives aimed at reducing patient wait times.
The new analysis comes as the latest National Treatment Purchase Fund (NTPF) figures released reveal that 889,200 people were on some form of hospital waiting list at the end of February. This is an increase of over 111,000 people compared with the number waiting for care in February 2020.
The Irish Hospital Consultants Association (IHCA) said the significant increase in waiting lists compared with pre-pandemic levels comes despite the Government allocating ¤1.233 billion since 2020 to cut waiting lists and wait times for treatment.
The IHCA said that while a further ¤407 million is understood to have been allocated for the Waiting List Action Plan for 2024, due to be unveiled shortly, this is unlikely to achieve a significant reduction in waiting lists unless the opening of planned additional hospital capacity is fast-tracked by the Government.
The vast sums of money spent over the past four years has resulted in increased activity, but this has been offset by higherthan-expected levels of patient demand, with additions to the waiting lists in 2023 8% higher than projected, 12% higher than in 2022 and almost 23% higher than in 2019.
With confirmation that an additional 260,000 people were awaiting diagnostic scans at the end of the year, the total number of people
“The impact that the increased presentations to Emergency Departments and the resulting cancellation of surgical procedures is having on patients is clear evidence of the urgent need for this additional capacity”
on hospital waiting lists is currently estimated at over 1.1 million.
The Association has reiterated its position that the cancellation of surgery cannot become the ‘goto solution’ or default response to ongoing lack of capacity and overcrowding at our acute public hospitals. The total number of hospital cancellations is expected to exceed 260,000 in 2023 when full-year figures are released.
Consultants are concerned that, due to the pressures faced in the system, many of those who require treatment are having their scheduled appointments cancelled because they are described as ‘non-urgent’ cases. However, their conditions will only become more serious and difficult to treat the longer they are left waiting – often in pain, suffering and facing the psychological distress of not knowing when they will be able to receive care.
Commenting on the waiting list figures, IHCA President Professor Rob Landers, said, “The Government needs to fast-track the opening of the promised 1,500 additional rapid build hospital beds across 15 acute public hospital sites this year and avoid deferring their delivery any further.
“The impact that the increased presentations to Emergency
Departments and the resulting cancellation of surgical procedures is having on patients is clear evidence of the urgent need for this additional capacity.
“The opening of the promised six surgical hubs and the long awaited four elective hospitals must also be accelerated in order to provide the extra capacity that is required to make significant inroads into achieving the Government’s waiting list reduction targets. Without this additional capacity coming on steam, there is little prospect of the waiting list coming under control anytime soon.”
Key Points:
• Over 889,000 people on some form of NTPF waiting list at the end of February 2024; an increase of 111,000 in the past four years;
• ¤1.2bn spent on waiting list initiatives since 2020, with further ¤407m pledged for Action Plan in 2024 due to be unveiled;
• Hospital cancellations expected to reach 260,000 in 2023 when full-year figures are released;
• With additional 260,000 awaiting diagnostic scans, the total number of people on hospital waiting lists is over 1.1 million
10 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE Pharmacy
Case Type Waiting Lists at end Feb 2020 Waiting Lists at end Feb 2024 Increase over past 4 years Outpatients 558,554 578,595 20,041 Inpatient/Day Cases 66,705 86,625 19,920 GI Scopes 22,705 24,315 1,610 Other Planned & Suspended lists 130,035 199,678 69,643 TOTALS 777,999 889,213 111,214
Irish Cancer Experts meet with US Congressional Caucus
A delegation of leading cancer specialists from across the island of Ireland has met with and briefed the influential US Congressional Cancer Caucus on how a quarter of a century of transatlantic collaboration has delivered significant impacts in cancer research and care on the island of Ireland.
The Irish delegation was made up of leading participants in the AllIsland Cancer Research Institute (AICRI) including Professor Jarushka Naidoo, Professor of Medical Oncology at RCSI and Consultant Medical Oncologist at Beaumont RCSI Cancer Centre. They met with the members of the US Congress on Capitol Hill in Washington DC today as part of this week’s Ireland-Northern Ireland-US engagement to mark St Patrick’s Week.
The island of Ireland delegation was jointly led by Professor William Gallagher (University College Dublin, Ireland), Professor Mark Lawler (Queen’s University Belfast, Northern Ireland), and Ciaran Briscoe (CEO, North East Cancer Research and Education Trust, Ireland) and included Professor Naidoo.
Members of Congress were briefed on the impact of the Ireland-Northern Ireland –US National Cancer Institute Cancer Consortium, which celebrates its 25th anniversary this year. The consortium, established in 1999 following the Good Friday Agreement, has helped foster significant collaboration both between scientists and health professionals on the island of Ireland and with their counterparts in the United States.
L-R: Prof. William Gallagher (University College Dublin and Co-Lead, AICRI); Quinn Ritchie (Legislative Director for US Congressman Mike Kelly, Co-Chair, US Congressional Cancer Caucus); Prof. Jarushka Naidoo (Beaumont RCSI Cancer Centre and AICRI Principal Investigator); Prof. Mark Lawler (Queen's University Belfast and Co-Lead, AICRI); Jessica Burnell (Legislative Director of the Office of the 26th District of New York); and Ciaran Briscoe (CEO, North East Cancer Research and Education Trust and AICRI Director of Development)
The Congressional Caucus heard how this partnership has significantly increased both the quality and quantity of research across the island of Ireland, contributing to saving thousands of lives and enhancing the quality of life of cancer survivors on our island.
The delegation highlighted the progress that has been made through this unrivalled tripartite approach and how it has acted as a springboard for the development of an All-Island Cancer Research Institute (AICRI). This unique collaboration of ten universities across the island of Ireland, along with other key stakeholders, is dedicated to delivering high quality cancer research and innovation in order to ensure state-of-the art cancer care for all.
AICRI is bringing together the combined strengths of cancer researchers across the island of Ireland to tackle cancer, linking with the United States and other international colleagues in

Europe. Its mission is to provide an overarching framework for cancer research across the island of Ireland, from discovery to implementation, for the benefit of cancer patients and wider society.
Unique opportunity
Congressman Mike Kelly, CoChair of the Congressional Cancer Caucus, commented on the event:
“I am delighted on behalf of the Congressional Cancer Caucus to welcome representatives of the All-Island Cancer Research Institute, north and south to Capitol Hill and hear how their collective work has impacted both nationally and internationally. This event represents a unique opportunity to learn from each other and to strengthen our transatlantic linkages to help deliver better outcomes for cancer patients here in the US and on the island of Ireland.”
One of the areas where US/allisland collaboration could really make a difference is in lung cancer, where outcomes are poor on both
sides of the Atlantic. Professor Naidoo said: “Later this month, we mark 20 years since the introduction of the smoking ban in the workplace in Ireland, the first country in the world to introduce this key public health intervention.
“In this moment, we recognise the urgent need to raise the bar for patients with lung cancer, the cancer responsible for the greatest cancer-related mortality in the US, Ireland and worldwide for more than 50 years. Importantly, this need is now matched by tremendous progress in novel targeted and immunotherapies for this disease, as well as the unrealised potential of early detection.
“Clinical progress in this area has been particularly aided by Irish investigators, whose work and leadership roles have continued global impact. We are now at a critical inflection point, in which strategic investment in lung cancer will allow us to realise the true potential of these advances.”
Major Enhancements to Women’s Health Services
Sligo University Hospital (SUH) recently welcomed Minister for Health Stephen Donnelly, TD to formally launch two significant enhancements in gynaecology and maternity services at the hospital.
The new service in SUH is part of a national plan to improve health outcomes for women through a new model of care. The clinic operates a “see and treat” model which means that women who need gynaecological care can be assessed, treated and discharged on the same day all within this “one stop” setting.
The establishment of the new ambulatory clinic in SUH is
reducing the need for women to have multiple gynaecology appointments for a single episode of care and this in turn is having a positive impact on waiting lists and in-patient bed usage at the hospital, with waiting lists for outpatient appointments reduced by over 50%.
Women are referred to the clinic by their GP and have access to same day diagnostics, investigations, treatments and minor procedures under a team of consultants and specialist nurses. The new unit provides both diagnostic and therapeutic procedures for common gynaecological
conditions including hysteroscopies, ultrasound scans, biopsies and management of intrauterine devices.
Speaking at the official opening Minister Donnelly said, “As Minister I’ve made developing better women’s health services a priority during the lifetime of this Government. The unprecedented levels of funding in women’s health services are now delivering for women. I know that this clinic is a positive, on-theground improvement, that is already making a real difference to the women of Sligo and the Northwest, reducing waiting times and improving access to services.”
Minister Donnelly also visited the Seomra Suaimhnis / Serenity Room which is a wonderful new labour and delivery suite developed by the maternity service in SUH with the support of the National Women and Infants Programme (NWIP).
The suite is designed around a “home from home” approach, the décor and atmosphere aim to create a relaxed and less clinical environment for women to give birth in. Women also have access to a heated birthing pool in the suite which can help with pain relief during their labour.
11 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 News
Menopause Hub App Launched

Women will soon have the power to tackle devastating menopause symptoms at the touch of a button through a ground-breaking Irish app.
The Menopause Hub App, the first of its kind in this country, includes the latest information and research about the hormonal changes endured by women in their 40s and 50s.
They can also use the mobile app to monitor their own menopausal and perimenopausal symptoms and receive medication reminders.
Launched by award-winning specialists, The Menopause
Hub, the app is the brainchild of the clinic’s CEO and founder, Loretta Dignam.
She opened her first clinic in 2019 after finding out for herself the lack of help available to menopausal and perimenopausal women – and said the data gathered by the app can be used by researchers investigating future treatments.
“The aim is that the data collected will provide a valuable insight into the effectiveness of current therapies, such as HRT and/or cognitive behavioural therapies, and this knowledge can also be used by clinicians when prescribing treatments.
Zendra Health CEO, Thomas Coleman, left, and Menopause Hub CEO, Loretta Dignam, with the Menopause Hub App, which will be available to download later this year. Credit: Peter Doyle / Media Consult
“Combining the latest technology with up-to-date data about female health will not only improve outcomes for women today, but will also improve the range and impact of menopause treatments available to future generations,” said Ms Dignam.
“There is a myriad of menopausal symptoms which women in their 40s and 50s experience in varying degrees, including insomnia, hot flushes, anxiety, depression, and brain fog, to name but a few.
“Every women’s menopause will be vastly different. Some will sail through it, while others will struggle to such an extent that they feel they have to give up their careers or their long-term relationships break down.
“It is, therefore, vital that we gather as much clinical data as we can to help medical researchers develop future treatments. For far too long,
the impact menopause was having on women’s lives was ignored. But women are now demanding –and deserve – more help with symptoms.”
The app was developed by medical technology company Zendra Health, which is helping over 80 healthcare services and organisations across the US, UK and Ireland digitalise their care pathways.
CEO, Thomas Coleman, said that the app was co-designed by women going through menopause and clinical experts.
“Hundreds of thousands of lives across Ireland, male and female, are impacted by menopause,” said Mr Coleman, who co-founded the Dún Laoghaire-based tech firm with his twin brother, David. “So we were delighted to develop this streamlined digital care solution for The Menopause Hub. Loretta is one of the thought leaders in this area and we were guided not only by her expertise but by the expertise of her medical team and by the experiences of their clients.”
The Menopause Hub App is available to download from March. See themenopausehub.ie for more details.
New European Microbiome Centres Consortium

The European Microbiome Centres Consortium (EMCC), a network of institutions working to ensure coherence and collaboration among leading experts in the field of human microbiome, launched recently during the final conference of the EU-funded project Human Microbiome Action.
In the past years, several links between changes in the human microbiome and various diseases have been observed, but clear connections are yet unknown. The European Microbiome Centres Consortium, launched through the EU-funded Human Microbiome Action project, aims to unlock these connections and starts a new phase of European research and innovation in the field. "The EMCC is committed to promoting and harmonizing standards for microbiome research, ensuring robust and reliable methodologies
and practices across the research community. We aim to promote regulatory frameworks in Europe, advocating for the acknowledgement of scientific consensus and requirements in the field of microbiome research. This will foster innovative translational developments, potentially transforming preventive nutrition and healthcare." - Joël Doré, scientific coordinator of the Human Microbiome Action project, and member of INRAE, one of the five founding partners. The EMCC is launched by five founding partners across Europe: EMBL (European Molecular Biology Laboratory), UCC (University College Cork), University of Trento, UCPH (University of Copenhagen) and INRAE (Institut National de Recherche pour l’agriculture, l’alimentation et l’environnement). Their aim after the conference will be to open it to broaden the networks to Europe-wide members who wish to contribute to this topic. In addition to its
scientific mission, the EMCC is committed to raising awareness and educating the general public and healthcare professionals about the importance of the human microbiome in health and disease.
Professor Paul Ross, Director of APC Microbiome Ireland, a world leading SFI Research Centre and co-Director of Food Microbiome and Heath Futures programme at University College Cork highlighted that, “the EMCC will provide an essential partnership involving a collection of Institutions and Centres actively involved in microbiome research across the EU. The mission of the EMCC is to provide guidance and advice on how to advance microbiome science to tackle some of the major challenges that exist across the food-pharma interface. In this respect this initiative should accelerate the science-based translation of microbiome research to its main stakeholders including the public, policy-makers, and food & health-based industries.”
12 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Prof Paul Ross, APC, UCC
Muscle Pain
Management of Joint and Muscle Pain
Chronic musculoskeletal pain affects about 30% of the world’s population, leading to disability, reduced quality of life, and substantial costs for healthcare systems.
There are different types of pain including nociceptive, neuropathic, nociplastic, idiopathic, and mixed. Pain and nociception are different phenomena, with the experience of pain varying widely for a given type of nociception.
Since 1986, clinicians have been guided by the WHO pain ladder, although its intended use was for treating cancer pain and has certain limitations. A revised version includes additional steps for non-pharmacological, integrative health therapies; and de-escalation of pain medication. Principles of the WHO pain ladder are still used in strategies for pain management, e.g. oral dosing when possible, around the clock rather than on demand administration, and individualised therapy.
There are many drug classes used in the pharmacological management of joint and muscle pain, including simple analgesics/non steroidal analgesics, opioids, anticonvulsants, antidepressants, musculoskeletal agents, anxiolytics, and DMARDs. Nonpharmacological treatments such as cryotherapy/heat therapy can help with reducing muscle spasm.
Transcutaneous electrical nerve stimulation therapy, acupuncture, exercise, and psychological treatments are also useful add-ons for management of joint and muscle pain.
Introduction
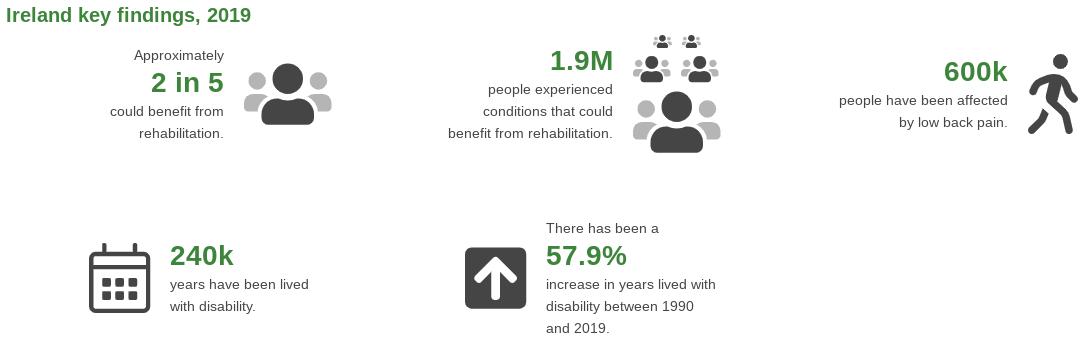
According to the World Health Organisation, 20-33% of the global population has some form of chronic musculoskeletal pain, most commonly low back pain, which is the single leading cause of disability in 160 countries. Musculoskeletal impairments include over 150 diseases or conditions that affect the system, which cause impairments in the muscles, bones, joints, nerves and adjacent connective tissues, and the associated limitations in functioning (temporary or lifelong). It leads to a reduced quality of life due to the inability to enjoy daily
activities or participate in society, increased drug use, high frequency of sick leave; also leading to socioeconomic problems and substantial costs for healthcare systems, especially rehabilitation services. The number of people living with functional limitations of this pain is increasing globally due to population growth and ageing. People with musculoskeletal conditions are also more likely to develop mental health issues.1,2 Increasing age is associated with higher risk of musculoskeletal pain (e.g. knee pain in osteoarthritis). The most common forms are:1
Chronic low back pain
(30-40% of adults)
Neck pain
Osteoarthritis (OA, 43% of people over 65)
Rheumatoid arthritis
Sprained muscles
Bone fracture pain
Gout
Risk factors that have been identified for musculoskeletal pain include:
Smoking
Lower educational status
Sedentary lifestyle
Poor or limited social
 Written by: Donna Cosgrove, MPSI, PhD
Written by: Donna Cosgrove, MPSI, PhD
interactions
Low income
Insomnia or sleep disorders
Anxiety
Depression
Manual labour
Country specific information on musculoskeletal conditions can be found through the World Health Organisation (WHO) Rehabilitation Need Estimator tool (Figure 1).
Joints may be inflamed (arthritis) or painful (arthralgia). Inflammation in the joints often involves warmth, swelling (due to intra-articular fluid/ effusion), and less commonly,
Joint and Muscle Pain
Donna Cosgrove MPSI PhD
13 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Figure 1. WHO Rehabilitation Need Estimator Tool Information for Ireland (3).
Figure 1. WHO Rehabilitation Need Estimator Tool Information for Ireland3
Muscle Pain
erythema. Joint pain can often have an alternative, extra articular source, like a bone or periarticular structures such as tendons, ligaments, bursae or muscles. The synovium and the joint capsule are the major sources of pain within the joint.4
Polyarticular joint pain arising from within the joint can be caused by inflammation (e.g. infection, presence of urate crystals, systemic inflammatory disorders like rheumatoid arthritis). It can also be caused by mechanical disorders like OA or hypermobility syndrome. Peripheral polyarticular arthritis is more likely to be associated with systemic infection or systemic inflammatory disorder than monoarticular arthritis. Causes of acute polyarticular arthritis tend to be due to infection, a flare up of a systemic inflammatory disorder, or gout. Chronic causes include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. OA causes non-inflammatory polyarticular pain in adults.
The Pain Experience
Pain and nociception are different phenomena. Nociception is the physiology of actual or potential tissue damage, whereas pain includes the unpleasant thoughts, emotions, and behaviours that accompany nociception.5 Pain is an “unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”, as defined by the International Association for the Study of Pain (IASP).6 IASP stresses the importance of acknowledging that pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors, such as life experience, and it is important that a person’s report of their pain experience is respected. Pain intensity can vary widely for a given nociception - studies of musculoskeletal injuries have actually found no association between pain intensity and degree of nociception, or injury severity. In fact, variations in pain intensity are accounted for more by measures of psychosocial factors than by measures of pathophysiology. Identifying and addressing psychosocial factors may limit persistent pain.5 Although pain usually serves an adaptive role, it may have adverse effects on function and social and psychological well-being. Pain can be classified into nociceptive, neuropathic,
nociplastic, idiopathic, or mixed type, according to the pathophysiological categories.2
In the ICD 11, there is a separate “parent code” for chronic pain which includes chronic secondary musculoskeletal pain. However, sub diagnoses can fall under multiple parent codes, including one of the pathophysiological categories above.
Nociceptive pain is the most common following tissue injury, and the most commonly implicated in musculoskeletal pain. It can be sharp, throbbing, or aching, is well localised, and has a protective function. It is a normal sensory experience. Somatic nociceptive pain originates from the skin, subcutaneous tissues and muscles. Bone pain originates from the skeleton due to bone fractures or trauma, often associated with tenderness in the overlying tissue. Visceral pain is associated with deep visceral organs, such as in appendicitis. This type of pain is poorly localised, dull, cramping pain; and can also be associated with nausea and vomiting.
Neuropathic (pathological) pain usually occurs as a result of a primary injury that results in dysfunction of the somatosensory nervous system, along the involved neural tissue or structure. It is commonly attributed to sensory changes including hypoesthesia/ hyperesthesia, hypoalgesia/ hyperalgesia, allodynia, or paresthesia. Neuropathic pain is often described as burning, shooting, electric, numbness, or pins and needles.
Mixed pain refers to both a component of continued nociceptive pain with a component of neuropathic pain.
Idiopathic pain is disproportionate to the level of tissue injury, or there is no definite cause identified. Psychological factors can influence idiopathic pain.
Nociplastic pain has recently been added by the IASP as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain”. It may be mistaken for central sensitisation (increased nociceptive responsiveness to stimuli from things that are not typically painful), although pathophysiology has not been fully established. Nociplastic pain is present in many different diseases such as fibromyalgia, complex
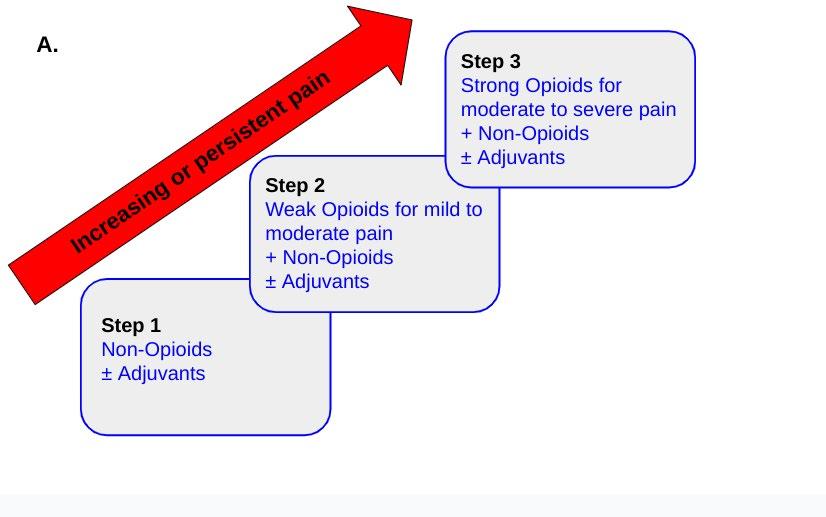
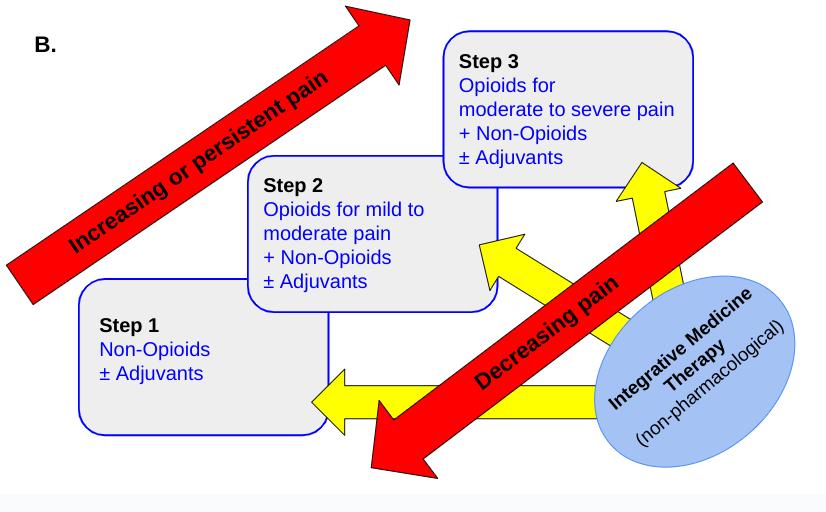
escalation (8). It has been suggested that step 2 ( use of weak opioids) be eliminated, evidence that these offer much for pain control. Instead, reduced doses of strong useful. Another limitation of the original pain ladder was lack of inclusion of approaches into the pain treatment path. Subsequently, a fourth step has been ladder. This integrates non-pharmacological evidence based interventions.
Despite limitations, there are important principles with the WHO pain ladder that can be of a simple strategy for pain management (8):
regional pain syndrome, and irritable bowel syndrome.7
V. Oral dosing of drugs whenever possible.
VI. Around-the-clock rather than on-demand administration.
Evidence Based Recommendations for Musculoskeletal Pain Management
into the pain treatment path. Subsequently, a fourth step has been added to the pain ladder. This integrates non-pharmacological evidence based interventions.
VII. Analgesics prescribed according to pain intensity as evaluated by a pain severity
VIII. Individualised therapy addressing the concerns of the patient (which assumes that standardised dosage in pain treatment.) This is probably the biggest challenge medicine, as the dose must be continuously adapted to the patient, to balance desired and possible side effects.
Pharmacological Pain Management
In terms of pharmacological treatment, healthcare professionals have been guided since 1986 by the WHO pain ladder system (Figure 2), although this pain ladder has been used more widely than its initial intended use (in cancer pain) and has its limitations. The original version was unidirectional (Figure 1A), starting with NSAIDs or paracetamol as step 1, and escalating up to weak (step 2) and strong (step 3) opioids, with or without adjuvants, depending on the patient’s level of pain, with no plan for de-escalation.8 It has been suggested that step 2 ( use of weak opioids) be eliminated, as there is little evidence that these offer much for pain control. Instead, reduced doses of strong opioids may be more useful. Another limitation of the original pain ladder was lack of inclusion of non-pharmacological approaches
Despite limitations, there are important principles with the WHO pain ladder that can be used as part of a simple strategy for pain management (8):
I. Oral dosing of drugs whenever possible.
IX. Proper medication adherence. The updated ladder includes the introduction of integrative medicine therapies and includes down approach for when pain improves.
II. Around-the-clock rather than on-demand administration.
III. Analgesics prescribed according to pain intensity as evaluated by a pain severity scale.
IV. Individualised therapy addressing the concerns of the patient (which assumes that there is no standardised dosage in pain treatment.) This is probably the biggest challenge in pain medicine, as the dose must be continuously adapted to the patient, to balance desired effects and possible side effects.
14 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 2. Original (A) and Revised (B) WHO Pain Ladders
Joint and Donna Cosgrove
Figure 2. Original (A) and Revised (B) WHO Pain Ladders.
Table 1 shows many pharmacological options for pain management in musculoskeletal non opioids, opioids and adjuvants.
Drug Type
Simple analgesics/non steroidal analgesics and antipyretics
Opioids (weak, strong, mixed agonist-antagonist)
Anticonvulsants
Antidepressants
Local anaesthetic
Musculoskeletal agents
Anxiolytics
NMDA receptor antagonist
Alpha 2 agonist
Conventional disease-modifying antirheumatic drugs (DMARDs)
Targeted Synthetic DMARD
Biological DMARDs
Drugs that reduce uric acid
V. Proper medication adherence.
The updated ladder includes the introduction of integrative medicine therapies and includes a step down approach for when pain improves.
Pharmacological Pain Management
Table 1 shows many pharmacological options for pain management in musculoskeletal conditions, non opioids, opioids and adjuvants.
Paracetamol reduces prostaglandin (PG) synthesis from arachidonic acid through
Treatment
Paracetamol
NSAIDs (including non-selective and selective COX-2 inhibitors)
Morphine (strong)
Oxycodone (strong)
Tapentadol (μ-opioid agonist and noradrenaline reuptake inhibitor)
Buprenorphine (partial μ-opioid agonist)
Tramadol (weak, with additional 5HT and NA effects)
Codeine (weak)
Gabapentin
Pregabalin
Carbamazepine
Tricyclic antidepressants
SNRIs
Lidocaine (topical)
Baclofen
Tizanidine
Benzodiazepines
Ketamine
Clonidine
Prednisolone
Hydroxychloroquine
Methotrexate
Leflunomide
Tofacitinib
Drugs that inhibit TNF-α IL-6 inhibitors
Drugs that inhibit of T cell co-stimulation
Drugs that cause B- cell depletion
Colchicine
Allopurinol
Febuxostat
COX 1 and 2 inhibition, and is thought to act centrally. Due to its high tolerability and relative effectiveness, it is commonly used on its own or with NSAIDs. However, research suggests it is ineffective as monotherapy in OA.2 NSAIDs are the mainstay of treatment in OA, and should be considered in chronic lower back pain. Non-specific NSAID use is associated with the potential development of gastrointestinal (GIT), renal, and cardiovascular side effects, and patients should be educated and monitored appropriately.
COX-2 selective inhibitors (e.g. celecoxib, etoricoxib) are as effective as traditional NSAIDs for mild to moderate pain. They have fewer GIT side effects, but long term, an increased risk of cardiovascular effects, which should be taken into account when initiating. COX-2 is found in inflammatory cells, damaged tissue, synovia, endothelium and the CNS. Topical NSAIDs (e.g. diclofenac) should be considered in chronic pain, including knee and hand OA, and those who cannot tolerate oral formulations. Topical NSAIDs should be considered before oral because of the more limited
systemic exposure with topical. Due to the depth of the joint beneath the skin surface in hip OA, in this case topical NSAIDs are unlikely to be beneficial.
Opioids work as agonists at mu, delta and kappa receptors in the brain, spinal cord and periphery. Respiratory depression, sedation, nausea, vomiting and constipation are the main adverse effects associated with most opioids. These are not first line therapy for chronic pain and for short to medium term treatment only in suitable patients.
Anticonvulsants (e.g. gabapentin, pregabalin, carbamazepine) are used to treat pain including neuropathic pain, spinal cord injury, shingles, fibromyalgia, and diabetic nephropathy. Gabapentinoids bind to the alpha 2 delta subunit of the voltage gated calcium channels, reducing ion influx in hyperexcitable states. The mechanism of action of carbamazepine has not been fully elucidated.
Tricyclic antidepressants (e.g. amitriptyline, nortriptyline) demonstrate an analgesic effect separately to their effect as antidepressants, thought to be due to presynaptic reuptake inhibition of serotonin and noradrenaline.
SNRIs (e.g. duloxetine) is used to treat pain from diabetic nephropathy, fibromyalgia, OA, and lower back pain. SSRIs (e.g. fluoxetine) can be considered in fibromyalgia, although trials show that SSRIs are usually not effective in neuropathic pain.
Musculoskeletal agents (e.g. baclofen, tizanidine) are used commonly in pain treatment. Baclofen is used as a skeletal muscle relaxant, and is a GABA agonist. Its mechanism is not fully understood, but it can inhibit synaptic reflexes at the spinal level. Tizanidine is a central alpha 2 adrenergic receptor agonist, causing muscles to relax through increased presynaptic inhibition.
Anxiolytics are often used to help with anxiety associated with acute pain or fluctuations in chronic pain symptoms, but e.g. benzodiazepine treatment should be given short term. If needed for longer periods, appropriate long term treatment e.g. antidepressants should be considered.
Alpha 2 adrenergic agonist activity reduces dorsal horn neuronal activity and inhibits substance P release, leading to analgesia,
15 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Table 1. Pharmacological Pain Management for Musculoskeletal Conditions2,9
Muscle Pain
sedation, and sympatholytic effects. Clonidine and tizanidine (also described above) have been used in chronic pain.
Topical lidocaine patches are used for 12 hours on, 12 hours off, and are useful in treating localised nociceptive pain, neuropathic pain, and post herpetic neuralgia.
Ketamine is a phencyclidine derivative that was developed in the 1960s as an anaesthetic agent. Its analgesic action at subanesthetic doses is thought to be mostly due to N-methyld-aspartate (NMDA) receptor antagonism in the brain and spinal cord.10 NMDA receptors have roles in learning, memory, and synaptic plasticity; but their utility as a target for pain is due to the receptor’s involvement in the amplification of pain signals, development of central sensitisation, and opioid tolerance. The evidence base for use of ketamine in pain is still quite limited. There are at least two ongoing clinical trials including a RCT of paramedic analgesia comparing ketamine and morphine in trauma (PACKMaN), and the Ketamine for Acute Pain after Trauma (KAPT) trial. An RCT comparing oral ketamine vs placebo in cancer-related neuropathic pain reported that ketamine was equivalent to placebo in this cohort, although the authors suggest that there may be subgroups of patients for whom ketamine is helpful, such as those with central sensitisation.11
There are many drugs included under the “conventional synthetic DMARDs” umbrella, for the treatment of RA. Glucocorticoids, e.g. prednisolone, are highly potent anti-inflammatory drugs. They work by general suppression of gene expression.12 Glucocorticoids bind to intracellular glucocorticoid receptors, move to the cell nucleus and reduce gene transcription of inflammatory molecules while promoting transcription of other genes that ultimately reduce inflammation.
The mechanism of hydroxychloroquine is likely through inhibition of lysosomal antigen degradation, ultimately preventing activation of T cells and the following inflammatory responses. Hydroxychloroquine also inhibits the production of RF antibodies, and collagenase/ proteinases which directly cause cartilage breakdown. Ophthalmic toxicity is the most important side effect (4.4 - 19%).
Leflunomide interferes with cell cycle progression by inhibiting the mitochondrial enzyme involved in DNA and RNA synthesis (dihydroorotate dehydrogenase). This action inhibits the production of rapidly dividing cells, e.g. autoimmune T-cells, and the production of antibodies from B cells.13 It is also a tyrosine kinase inhibitor, which means DNA repair, apoptosis, and cell proliferation are affected. The dose-limiting side effects are liver damage, lung disease and immunosuppression.
Methotrexate is an analogue of folic acid that interferes with dihydrofolate reductase.12 This inhibits nucleotide synthesis and purine metabolism. Through this, it produces adenosine, which has direct anti-inflammatory properties. The potential side effects including hair loss, stomatitis, nausea, and hepatotoxicity are caused directly by the disruption of folate metabolism and can be prevented by folic acid supplementation.
Sulfasalazine is a prodrug of 5-ASA, and while the exact mechanism is unknown, it has been shown to have antiinflammatory, immune modulatory, and antibiotic properties. Typical side-effects of sulfasalazine include fatigue, CNS reactions, nausea, abdominal pain (dyspepsia), diarrhoea, hypersensitivity reactions, and with a lower frequency of blood dyscrasias.
Targeted Synthetic DMARDs were developed specifically to disrupt the cytokine-mediated induction of inflammation, i.e. the JAK-STAT pathway.12 Tofacitinib was the first JAK inhibitor approved.
There are four modes of action of current biological DMARDs:12
Inhibition of TNF-α or the TNF receptor: infliximab, certolizumab, adalimumab and golimumab are TNF-α neutralising antibodies. Certolizumab pegol is an anti-TNF-α antibody fragment. Etanercept is a soluble TNF receptor that binds TNF-α
IL-6 receptor antagonism: tocilizumab
Inhibition of T cell co-stimulation by antigen presenting cells: Abatacept is the first of these agents that suppress induction of inflammation upstream of the pro-inflammatory signalling cascade.
Depletion of B cells: Rituximab decreases B cells and diminishes the activation of T cells.
Drugs that reduce uric acid include colchicine, allopurinol and febuxostat. Colchicine prevents granulocyte migration into the inflamed joint, inhibits release of glycoprotein which aggravates inflammation, and binds to the intracellular protein tubulin causing disappearance of microtubules in granulocytes, which are essential for cell function. It also limits formation of IL-1β and IL-18.14 The enzyme xanthine oxidase is responsible for conversion of hypoxanthine and xanthine into urate, and by inhibiting this enzyme, allopurinol and febuxostat prevent formation of urate.
Non-Pharmacological Treatment
A common cause of musculoskeletal pain is muscle spasm. Application of heat or cold reduces muscle shortening, caused either by direct muscle trauma or the underlying neurological or skeletal disease.2 Evidence for many of these modalities is sparse because there are no large clinical trials in this area.
Cryotherapy reduces haemorrhage, vasodilation, local inflammation and oedema production, and pain perception. PRICE (protection, rest, ice, compression, elevation) is an approach often employed for e.g. acute sports injuries, as well as more chronic conditions.
Application of heat causes increased collagen extensibility, blood flow, metabolic rate, and resolution of inflammation in subacute and chronic conditions. Heat application in combination with stretching reduces muscle contraction, joint stiffness, and chronic inflammatory diseases, ultimately leading to pain reduction and increased range and function. Heat raises the pain threshold and reduces muscle excitability.
Transcutaneous electrical nerve stimulation therapy (TENS) is based on the “gate control theory” of pain, where preferential activation of A beta fibres inhibits the transmission of painful impulses. There is support for its use in OA and neuropathic pain, and it has been used to manage postoperative pain, complex regional pain syndrome, phantom limb pain, peripheral nerve injury, and during pregnancy and labour.
Acupuncture is considered to be an invasive procedure due to the insertion of thin metal needles into specific areas of the body, and requires a professional practitioner. There is no evidence that acupuncture is more effective than
NSAIDs in some pain conditions. However, it may be helpful for some types of pain including back or neck pain, knee pain associated with OA, and postoperative pain. It may also help relieve joint pain associated with the use of aromatase inhibitors.
Therapeutic exercise, e.g. passive movements, active assisted exercises, active exercises, stretching, relaxation exercises, can be useful when combined with other pain management modalities. Initial treatment of musculoskeletal pain includes immobilisation, compression, and cryotherapy. Acute muscle injuries may cause contraction or shortening of the muscles as a protective mechanism, so when pain reduces enough, the injured region should be gradually mobilised. If the contraction/ shortening becomes chronic, this causes further pain.
Psychological factors can impact the experience of pain, and can be helped with treatments including cognitive behavioural therapy, explanation, reassurance, stress reduction or counselling.
Conclusion
Joint and muscle pain includes a large variety of conditions with different causes and treatment trajectories that are a significant burden on patients, society and healthcare resources.
Pharmacological treatment is often prescribed and can provide substantial relief. However, depending on the treatment, it may be associated with risks, and not all patients respond adequately. Non pharmacological treatment should be incorporated where appropriate, with patient care involving shared decision making.
References available on request
Donna did her Pharmacy degree in RCSI, and then returned to university to pursue an interest in psychiatric conditions and treatments through a MSc in Neuropharmacology. This led to a PhD investigating the genetics of schizophrenia using data from genome-wide association studies and cognitive test data. This was followed by a postdoctoral research position in a similar area. Donna has worked in hospital, research and community pharmacy settings, and currently works as a community pharmacist in Galway, and as a clinical writer. Donna’s overall aim is to improve patient outcomes through education.
16 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
New technique for Parkinson’s stem cell brain repair brings promise for patients
Neuroscientists at University of Galway have made an exciting discovery that could revolutionise stem cell-based brain repair therapy for Parkinson’s disease.
Parkinson’s is a neurodegenerative condition in which brain cells slowly degenerate and die leading to a progressive deterioration in a person’s ability to control movement. It is estimated that there are 8.5 million people living with the condition worldwide, and 12,000 people in Ireland alone.
Brain repair for Parkinson’s involves replacing the dead cells by transplanting healthy brain cells back into the brain. With recent advancements in regenerative medicine and stem cell technology, “induced stem cells” can now be used as a source of healthy cells.
Induced stem cells are reprogrammed from adult cells, such as skin cells, and can be converted in the laboratory into the appropriate type of brain cell required for repairing the Parkinson’s brain.
However, these skin cellsturned brain cells need to be transplanted into the brain at a very early stage in their conversion, and the vast majority of the cells do not continue to convert -once in the brain - into the mature cells that are required for the therapy to work.
Professor Eilís Dowd, College of Medicine, Nursing and Health Sciences at University of Galway.
Credit Martina Regan
In work funded by The Michael J. Fox Foundation for Parkinson’s Research (MJFF) and Science Foundation Ireland, published this week in the Journal of Neural Engineering, the team in the College of Medicine, Nursing and Health Sciences at University of Galway have shown that transplanting the immature cells in a collagen hydrogel dramatically improves both their survival and maturation in the brain.
Commenting on the research finding, the lead neuroscientist on the project, Professor Eilís Dowd, said, “Our hydrogel nurtures, supports and protects the cells after they are transplanted into the brain, and this dramatically improves their maturation and reparative ability. Ultimately, we hope that continued development of this promising gel will lead to a significant improvement in brain

repair approaches for people living with Parkinson’s.”
The Michael J. Fox Foundation awarded $300,000 to continue the development of the hydrogel.
The new research aims to understand how the immune system in the brain reacts when cells are transplanted alone versus when they are transplanted in combination with the hydrogel.
The research will continue to be led by Professor Dowd, in collaboration with colleagues
from CÚRAM - the Science Foundation Ireland Research Centre for Medical Devices based at University of Galway, the University of Edinburgh and the University of Melbourne.
Professor Dowd’s ongoing research in this field is featured in the short documentary Feats of Modest Valour which won the coveted Scientist Award at the Imagine Science Film Festival in New York, as well as the Professional Documentary Award at the Raw Science Festival in California.
Treatment for Neuroendocrine Tumours now available in Ireland
St. Vincent’s University Hospital (SVUH), supported by the National Cancer Control Programme (NCCP) and the Department of Health, has formally launched a new national treatment service for people with neuroendocrine tumours (NETs).
The treatment involves Peptide Receptor Radionuclide Therapy (PRRT), which delivers precise radiation to cancer cells, minimising damage to surrounding healthy tissue. The treatment has been available in SVUH since October 2023. Before this, all eligible Irish patients had to access the treatment under the HSE Treatment Abroad Scheme. Providing the service in Ireland marks a significant leap forward in cancer care for patients with NETs.
The Department of Health’s National Cancer Strategy 20172026 enabled the NCCP to provide funding to establish this service in Ireland at St. Vincent’s University Hospital.
Professor Risteárd Ó Laoide, National Director, National Cancer Control Programme, HSE, said: “The repatriation of this service is the culmination of more than three years of collaborative work between the NCCP, St. Vincent’s University Hospital and the Department of Health. The availability of this treatment will help to strengthen the network of care for patients with NETs in Ireland. This is all part of the wider collaboration and collective development of services for patients with NETs, including a
shared multidisciplinary team meeting and consistent access to the best available diagnostics, multidisciplinary treatment planning and treatments.”
Spearheaded by the NETs team at SVUH, led by Professor Dermot O’Toole and the Nuclear Medical Team led by Dr. Stephen Skehan, Dr. Nicola Hughes and Dr. Mathilde Colombie, the PRRT for NETs service is expected to grow incrementally over the next two years. This will result in access for all eligible patients to this therapy without the need to travel abroad. St. Vincent’s University Hospital’s comprehensive approach to patient care will ensure that individuals from all corners of Ireland can benefit from this transformative therapy. As the
programme continues to grow, the hospital remains committed to providing accessible and highquality PRRT for patients with NETs to meet the evolving needs of cancer patients nationwide.
Speaking on behalf of the clinical team involved in delivering this service, Professor Dermot O’Toole commented, “The introduction of PRRT for patients with NETs is something that the clinical team here have been striving for over several years. We are delighted that we can now offer this crucial treatment to our patients. I am grateful to everyone involved in the multidisciplinary clinical team that has made this possible and to the National Cancer Control Programme and the Department of Health for their continued support.”
17 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 News
Overactive Bladder
Overactive Bladder: Diagnosis and Treatment

Overactive bladder syndrome is a chronic disabling condition that significantly reduces the quality of life of millions of people worldwide.1 The ICS define overactive bladder as “urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of UTI or other obvious pathology”.2 The definition of overactive bladder (OAB) has undergone changes over time, and currently the most commonly accepted definition is that of the International Consultation on Incontinence Research Society (ICIRS): ‘overactive bladder syndrome is characterised by urinary urgency, with or without urgency urinary incontinence, usually with increased daytime frequency and nocturia, if there is no proven infection or other obvious pathology’.1, 3 OAB can also be associated with detrusor overactivity on urodynamic testing.7 Overactive bladder syndrome affects approximately 350,000 people over the age of 40 in Ireland.4 Although the condition is more common in adults over the age of 40, it can also affect children and young people. The prevalence of OAB increases with age, affecting up to 17% of the population over the age of 18, and 30% of those over 65 years of age.5 OAB can impact significantly on quality of life and is responsible for several other comorbidities, including falls and fractures, depression, and increased risk of admission to hospitals and nursing homes.7
The symptoms of OAB include urgency, frequency, nocturia and urgency incontinence, and many patients experience combinations of these symptoms. Urgency incontinence is more common
Written by:
Theresa Lowry Lehnen: RGN, PG. Dip Coronary Care, RNP, BSc, MSc, PG. Dip. Ed (QTS), M. Ed, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University
in women, and urgency and frequency more common in men.1
The diagnosis of OAB can be challenging due to the lack of a specific diagnostic test and the overlap of symptoms with other lower urinary tract disorders.
Diagnosis
The diagnosis of OAB is based on the presence of urinary urgency, with or without urge incontinence, usually associated with frequency and nocturia, in the absence of urinary tract infection or other obvious pathology. The evaluation of OAB should include a detailed medical history, physical examination, and laboratory tests to exclude other causes of lower urinary tract symptoms, such as urinary tract infection, bladder stones, or malignancy.1 Dipstick urinalysis should be carried out, and a sample may also be sent to the lab to rule out haematuria, infection, and glycosuria, which could indicate the presence of diabetes. Blood tests including levels of creatinine and glycosylated haemoglobin (HbA1C) can also provide additional information.1
A detailed medical history can highlight possible risk factors for OAB. The history should focus on voiding and lower urinary tract symptoms, the severity of symptoms and their impact on the patient’s quality of life. Symptom questionnaires can be used to highlight the impact of the condition on quality of life and to determine whether the patient requires treatment. There are numerous validated questionnaires available, however, they are not widely used.1
Examination should include assessment of urinary, gynaecological, and neurological systems. A focused clinical examination can highlight risk factors and pre-existing conditions and should include an abdominal examination, digital rectal examination of the prostate in males and a vaginal examination in females.1 Abdominal palpation and vaginal examination enables identification of a palpable bladder and prolapse or pelvic mass in females. Symptoms of OAB in men are often attributed to bladder outflow obstruction resulting in misdiagnosis and delays in treatment. A digital rectal exam is imperative to rule out the presence of prostatic hypertrophy, malignancy, and poor rectal tone.7 Neurological causes of OAB can include spinal cord injury, myelodysplasia, or multiple sclerosis. Out ruling neurological causes is important, as these patients may be at risk of upper tract deterioration/renal failure.7
A voiding diary is a useful tool for assessing the frequency and volume of voids, urgency, and episodes of urge incontinence. Uroflowmetry can assess voided volume, flow rate, and post void residual urine volume. Cystoscopy and urodynamics are usually not necessary for the diagnosis of OAB, but may be helpful in selected cases, such as when there is suspicion of bladder outlet obstruction or neurogenic bladder.6
Treatment and Management of OAB
Treatment and management of OAB is based on a stepwise approach, including lifestyle modifications, behavioural therapy, pharmacotherapy, and in selected cases invasive therapy.
First line treatment is lifestyle modification, including bladder training exercises, pelvic floor exercises and fluid management strategies.11 Bladder training exercises involve gradually increasing the time between voiding to increase bladder capacity and reduce urgency.1, 11 Pelvic floor exercises aim to strengthen the pelvic floor muscles, which can improve bladder control and reduce urinary incontinence.8, 11 Fluid management strategies include reducing caffeine and alcohol intake, as these can irritate the bladder and exacerbate OAB symptoms.11 Reduction in fluid intake by 25% may help improve symptoms of OAB but
not UI. Advice on fluid intake should be based on 24-hour fluid intake and urine output measurements. Patients should be advised that fluid intake should be sufficient to avoid thirst and that an abnormally low or high 24-hour urine output should be investigated.11 If overweight, weight loss is encouraged as weight loss has a significant positive effect on leakage. Patients should also be advised to stop smoking if applicable and increase dietary fibre to avoid constipation.7,11
Pharmacological therapy is often required when lifestyle modifications are ineffective or insufficient. Antimuscarinic drugs are the first line pharmacological treatment for OAB. These drugs work by blocking the muscarinic receptors in the bladder, reducing detrusor muscle contractions, and increasing bladder capacity. There are several antimuscarinic drugs available including oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine. The choice of drug depends on the patient’s individual characteristics, including age, co morbidities, and medication interactions. Absolute contraindications to anticholinergic use include urinary retention, gastric retention, uncontrolled narrow-angle glaucoma, and known hypersensitivity to the individual drugs or their ingredients. Elderly patients should be monitored for drug interactions or polypharmacy of drugs with anticholinergic effect i.e., antidepressants, antipsychotics, anxiolytics, as the overall anticholinergic load is associated with confusion, falls, and fractures.7
Anticholinergic drugs and properties7
Mirabegron is a β3-adrenoceptor agonist with a mechanism of action that is distinct from anticholinergics. It has a lower risk of adverse effects compared to antimuscarinic drugs, although it is associated with hypertension and tachycardia in some patients and is considered as a secondline therapy when patients are intolerant to anticholinergics.7, 9
Desmopressin treatment may improve nocturia and urgency episodes. Desmopressin improves quality of sleep by prolonging the period of sleep until the first void and is shown to have an acceptable safety profile in certain patients with overactive bladder syndrome.12
18 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE

Prescribing Information: BETMIGA™ (mirabegron)

50mgs once daily
BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets.
His 14th walk in the park since the day he started BETMIGA1
For full prescribing information, refer to the Summary of Product Characteristics (SPC). Name: BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets. Presentation: Prolongedrelease tablets containing 25 mg or 50 mg mirabegron. Indication: Symptomatic treatment of urgency, increased micturition frequency and/or urgency incontinence as may occur in adult patients with overactive bladder (OAB) syndrome. Posology and administration: The recommended dose is 50 mg orally once daily in adults (including elderly patients). Mirabegron should not be used in paediatrics for OAB. A reduced dose of 25 mg once daily is recommended for special populations (please see the full SPC for information on special populations). The tablet should be taken with liquids, swallowed whole and is not to be chewed, divided, or crushed. The tablet may be taken with or without food. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SPC. Severe uncontrolled hypertension defined as systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 110 mm Hg. Warnings and Precautions: Renal impairment: BETMIGA has not been studied in patients with end stage renal disease (eGFR < 15 ml/min/1.73 m2 or patients requiring haemodialysis) and, therefore, it is not recommended for use in this patient population. Data are limited in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2); based on a pharmacokinetic study (see section 5.2 of the SPC) a dose of 25 mg once daily is recommended in this population. This medicinal product is not recommended for use in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC). Hepatic impairment: BETMIGA has not been studied in patients with severe hepatic impairment (ChildPugh Class C) and, therefore, it is not recommended for use in this patient population. This medicinal product is not recommended for use in patients with moderate hepatic impairment (Child-Pugh B) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC). Hypertension: Mirabegron can increase blood pressure. Blood pressure should be measured at baseline and periodically during treatment with mirabegron, especially in hypertensive patients. Data are limited in patients with stage 2 hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg). Patients with congenital or acquired QT prolongation: BETMIGA, at therapeutic doses, has not demonstrated clinically relevant QT prolongation in clinical studies (see section 5.1 of the SPC). However, since patients with a known history of QT prolongation or patients who are taking medicinal products known to prolong the QT interval were not included in these studies, the effects of mirabegron in these patients is unknown. Caution should be exercised when administering mirabegron in these patients. Patients with bladder outlet obstruction and patients taking antimuscarinics medicinal products for OAB: Urinary retention in patients with bladder outlet obstruction (BOO) and in patients taking antimuscarinic medicinal products for the treatment of OAB has been reported in postmarketing experience in patients taking mirabegron. A
controlled clinical safety study in patients with BOO did not demonstrate increased urinary retention in patients treated with BETMIGA; however, BETMIGA should be administered with caution to patients with clinically significant BOO. BETMIGA should also be administered with caution to patients taking antimuscarinic medicinal products for the treatment of OAB. Interactions: Caution is advised if mirabegron is co-administered with medicinal products with a narrow therapeutic index and significantly metabolised by CYP2D6. Caution is also advised if mirabegron is co-administered with CYP2D6 substrates that are individually dose titrated. In patients with mild to moderate renal impairment or mild hepatic impairment, concomitantly receiving strong CYP3A inhibitors, the recommended dose is 25 mg once daily. For patients who are initiating a combination of mirabegron and digoxin (P-gp substrate), the lowest dose for digoxin should be prescribed initially (see the SPC for full prescribing information). The potential for inhibition of P-gp by mirabegron should be considered when BETMIGA is combined with sensitive P-gp substrates. Increases in mirabegron exposure due to drug-drug interactions may be associated with increases in pulse rate. Pregnancy and lactation: BETMIGA is not recommended in women of childbearing potential not using contraception. This medicinal product is not recommended during pregnancy. BETMIGA should not be administered during breast-feeding. Undesirable effects: Summary of the safety profile: The safety of BETMIGA was evaluated in 8433 adult patients with OAB, of which 5648 received at least one dose of mirabegron in the phase 2/3 clinical program, and 622 patients received BETMIGA for at least 1 year (365 days). In the three 12-week phase 3 double blind, placebo controlled studies, 88% of the patients completed treatment with this medicinal product, and 4% of the patients discontinued due to adverse events. Most adverse reactions were mild to moderate in severity. The most common adverse reactions reported for adult patients treated with BETMIGA 50 mg during the three 12-week phase 3 double blind, placebo controlled studies are tachycardia and urinary tract infections. The frequency of tachycardia was 1.2% in patients receiving BETMIGA 50 mg. Tachycardia led to discontinuation in 0.1% patients receiving BETMIGA 50 mg. The frequency of urinary tract infections was 2.9% in patients receiving BETMIGA 50 mg. Urinary tract infections led to discontinuation in none of the patients receiving BETMIGA 50 mg. Serious adverse reactions included atrial fibrillation (0.2%). Adverse reactions observed during the 1-year (long term) active controlled (muscarinic antagonist) study were similar in type and severity to those observed in the three 12-week phase 3 double blind, placebo controlled studies. Adverse reactions: The following list reflects the adverse reactions observed with mirabegron in adults with OAB in the three 12-week phase 3 double blind, placebo controlled studies. The frequency of adverse reactions is defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000) and not known (cannot be established from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. The adverse events are grouped by MedDRA system organ class. Infections and infestations:
Common: Urinary tract infection, Uncommon: Vaginal infection, Cystitis. Psychiatric disorders: Not known (cannot be estimated from the available data): Insomnia*, Confusional state*. Nervous system disorders: Common: Headache*, Dizziness*. Eye disorders: Rare: Eyelid oedema. Cardiac disorders: Common: Tachycardia, Uncommon: Palpitation, Atrial fibrillation. Vascular disorders: Very rare: Hypertensive crisis*.
Gastrointestinal disorders:
Common: Nausea*, Constipation*, Diarrhoea*, Uncommon: Dyspepsia, Gastritis,
Rare: Lip oedema. Skin and subcutaneous tissue disorders: Uncommon: Urticaria, Rash, Rash macular, Rash papular, Pruritus, Rare: Leukocytoclastic vasculitis, Purpura, Angioedema*. Musculoskeletal and connective tissue disorders: Uncommon: Joint swelling. Renal and urinary disorders: Rare: Urinary retention*. Reproductive system and breast disorders: Uncommon: Vulvovaginal pruritus. Investigations: Uncommon: Blood pressure increased, GGT increased, AST increased, ALT increased. * signifies adverse reactions observed during post-marketing experience. Prescribers should consult the SPC in relation to other adverse reactions. Overdose: Treatment for overdose should be symptomatic and supportive. In the event of overdose, pulse rate, blood pressure, and ECG monitoring is recommended. Basic NHS Cost: Great Britain (GB)/Northern Ireland(NI): BETMIGA 50 mg x 30 = £29, BETMIGA 25 mg x 30 tablets = £29. Ireland (IE): POA. Legal classification: POM. Marketing Authorisation number(s): (GB): PLGB 00166/0415-0416. NI/IE: EU/1/12/809/001-006, EU/1/12/809/008-013, EU/1/12/809/015018. Marketing Authorisation Holder: GB: Astellas Pharma Ltd., 300 Dashwood Lang Road, Bourne Business Park, Addlestone, United Kingdom, KT15 2NX. NI/IE: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of Preparation of Prescribing information: January 2023. Job bag number: MAT-IE-BET-2023-00001. Further information available from: GB/NI: Astellas Pharma Ltd, Medical Information: 0800 783 5018. IE: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. For full prescribing information, please see the Summary of Product Characteristics, which may be found at: GB: www.medicines.org.uk; NI: https://www.emcmedicines.com/en-GB/northernireland/; IE: www.medicines.ie.
United Kingdom (GB/NI)
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018.
Ireland
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com.
Date of preparation: January 2023 References: 1. Freeman R, et al. Current Medical Research and Opinion 2017. https://doi.org/10.1080/03007995.2017.1419170. MAT-IE-BET-2023-00003
DRUG
Oxybutynin
Overactive Bladder
Tolterodine
Trospium
Solifenaci
Darifenacin
Fesoterodine ROUTE Oral, Transdermal patch Transdermal gel Oral Oral Oral Oral Oral
5mg bid-tid 5 or 10mg qd
3.9mg/day, twice weekly 1g (1 sachet) qd
2mg bid 4mg qd
20mg bid
Age >70: 20 mg qd
5 to 10mg qd
7.5 to 15mg qd
4mg, 8mg
https://irishpharmacist.ie/2022/10/05/treatment-and-management-of-oab/
If medications are not effective, intravesical botox injections are another option. Intravesical botulinum toxin A prevents acetylcholine release at the neuromuscular junction, resulting in temporary chemo denervation and muscle relaxation for up to 6 months. The technique places multiple injections under cystoscopic guidance directly into the detrusor. Complete continence can be achieved in 40% - 80% of patients, and bladder capacity improved by 56% for up to 6 months. Maximal benefit is achieved between two and six weeks, maintained over six months. The injections can be repeated when required according to the degree of improvement or relief of symptoms.7
Surgical intervention may be required in severe cases of OAB that are unresponsive to pharmacological therapy. Sacral nerve stimulation (SNS) is a surgical technique that involves implanting a device that delivers electrical impulses to the sacral nerves which regulate bladder function. SNS is associated with risks, including infection, device malformation, and nerve damage, and patients should be fully informed of the benefits and risks before undergoing the procedure. Current indications for sacral nerve stimulation include refractory urge incontinence, refractory urgency and frequency, and idiopathic urinary retention.7,10 More radical
treatments include augmentation cystoplasty and urinary diversion. Augmentation cystoplasty is an operation to enlarge the bladder and allow it to hold more urine, by attaching a piece of the body’s own tissue (usually from the bowel) to the bladder. Urinary diversion involves rerouting the ureters. This allows the bladder to be bypassed and the ureters to be routed directly to the outside of the body allowing urine to be collected in an ostomy bag worn on the abdomen. Urinary diversion should be considered only when conservative treatments have failed, and if sacral nerve stimulation and augmentation cystoplasty are not appropriate or unacceptable to the patient.1, 7
Emerging Therapies
PDE1 and PDE5 inhibitors have demonstrated improvement in urodynamic parameters, however, PDE 5 inhibitors are not currently approved for OAB.7 Percutaneous radiofrequency neurotomy has demonstrated feasibility in patients with neurogenic detrusor overactivity secondary to spinal cord injury, with significant improvement in patient symptoms scores. However, there is currently no evidence that transurethral radiofrequency improves patient-reported symptoms of incontinence.7
Support for people with OAB
Useful information and support for people in Ireland living with
4. Kelly, B. (2022). OAB: Diagnosis and management. The Medical Independent. Available at: https://www. medicalindependent.ie/clinicalnews/genitourinary/oabdiagnosis-and-management/
5. Irwin, D., Milsom, I., Hunskaar, S., et al. (2016). Population based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2016, 50(6): 1306-14.
6. Nambiar, A., Bosch, R., Cruz, F., et al. (2018). EAU guidelines on assessment and non-surgical management of urinary incontinence. EUR Urol. 2018; 73 (4): 596-609.
7. Irish Pharmacist (2022). Treatment and Management of OAB. Available at: https:// irishpharmacist.ie/2022/10/05/ treatment-and-managementof-oab/
8. Dumoulin, C. (2018). Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. The Cochrane Library (2018).
OAB is available at www.oab.ie.13 The Continence Foundation of Ireland (CFI) improves the quality of life for people with incontinence and pelvic floor dysfunction, by raising awareness, and providing information and education.14 More information is available at: http:// www.continence.ie/
References
1. Scarneciu, I., Lupu, S., Bratu, O., Teodorescu, A., Maxim, L., et al. (2021). Overactive bladder: A review and update. Exp Ther Med. 2021 Dec;22(6):1444. doi: 10.3892/ etm.2021.10879. Epub 2021 Oct 14. PMID: 34721686; PMCID: PMC8549091.
2. Haylen, B., de Ridder, D., Freeman, R., Swift, S., Berghmans, B., et al. International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/ International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20. doi: 10.1002/nau.20798. PMID: 19941278.
3. Drake, M. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014; 33:622–624. doi: 10.1002/ nau.22609.
9. Khullar, V. et al. (2017). Efficacy and tolerability of mirabegron compared to antimuscarinic monotherapy or combination therapy for overactive bladdr: a systematic review and network meta-analysis. European urology 71.3 (2017): 347- 356.
10. Herschorn, S., Zimmern, P. (2017). Surgical management of refractory overactive bladder. Nature Reviews Urology 14.5 (2017). 281-289.
11. EEU (2023). EAU Guidelines on Management of NonNeurogenic Female Urinary Tract Symptoms. Available at: https://uroweb.org/guidelines/ non-neurogenic-female-luts/ chapter/disease-management
12. Barakat, B., Franke, K., May, M., Gauger, U., Vögeli. T. Efficacy and safety of desmopressin on frequency and urgency in female patients with overactive bladder and nocturia, current clinical features and outcomes: A systematic review. Asian J Urol. 2022 Jan;9(1):27-34. doi: 10.1016/j.ajur.2021.05.005.
13. oab.ie (2023). Take a break from OAB. Available at: www. oab.ie.
14. CFI (2023). Continence Foundation of Ireland. Available at: http://www. continence.ie/
20 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
DOSE
RECEPTOR SELECTIVITY Non-selective Non-selective Non-selective M3, M1 selective M3 selective Non-selective CNS PENETRATION High High Low High Low High MAIN METABOLISM
Hepatic Hepatic Renal Hepatic Hepatic Hepatic
Global Recognition for Professor Dunne
Leading diabetes researcher and Acting Director of the Institute for Clinical Trials at University of Galway, Professor Fidelma Dunne, has been recognised by the largest global diabetes research and educational organisation in the world, the American Diabetes Association.
Professor Dunne is being honoured for outstanding scientific achievement in the understanding and treatment of diabetes and pregnancy and lifelong dedication to advancing both clinical practice and clinical research in the field.
Professor Dunne said, “I am deeply honoured to receive the Norbert Freinkel Award from the American Diabetes Association. This recognition is a testament to the collaborative efforts of the research team at the University of Galway and the Institute for Clinical Trials and the broader Irish diabetes and endocrinology clinical and research community.
“It reflects not only my own dedication to advancing
knowledge and treatment in the critical intersection of diabetes and pregnancy, but also the patients involved in the trial who place their trust in our team and our clinical research.”
Professor Dunne is Professor of Medicine at University of Galway and Consultant Endocrinologist at Saolta University Health Care Group and becomes the first Irish person to be recognised with the Norbert Freinkel Award from the American Diabetes Association.
The award recognises the value and impact of Professor Dunne’s research into gestational diabetes and healthcare for pregnant women and mothers, as well as a lifetime of contributions, both clinical and research, to the field of diabetes and pregnancy. This work culminated in the EMERGE trial published in JAMA: the Journal of American Medical Association, in 2023, which has alleviated concerns over metformin drug use for mothers with diabetes and their babies.

Professor Fidelma Dunne, Professor of Medicine at University of Galway and Consultant Endocrinologist at Saolta University Health Care Group.
Credit – Martina Regan

President of University of Galway, Professor Ciarán Ó hÓgartaigh added, “Professor Fidelma Dunne’s ground-breaking work in the field of diabetes and pregnancy positions her work here in Galway in an international setting and has significantly contributed to the global understanding of this complex condition. Her leadership
The PMI Pharma Summit 24
and achievements reflect the excellence and commitment to research and clinical advancement at University of Galway.”
As part of the award, Professor Dunne will deliver the Award Lecture at the American Diabetes Association’s Scientific Sessions in Florida in June.
The PMI's Annual Pharma Summit takes place on Thursday 18th April, 2024 in the Croke Park conference centre. The day promises to be a "must-attend" for all those working within the healthcare industry, and those interested in the developments of this dynamic industry.
As part of the Pharma Summit the PMI are planning an engaging panel discussion with industry leaders. Their esteemed panellists are:
Duggan Caitriona, Country Manager with Amgen and Lead of the Market Access Advisory Forum with IPHA, David Delaney, Head of Policy & Market Access with Viatris and Vice-Chair of Medicines for Ireland, Carl Prescott, Head of HTA Consulting with AXIS Healthcare Consulting Ltd and Angela Clayton-Lea, Acting CEO with Cancer Trials Ireland. The discussion will be moderated by Brenda Dooley, CEO with AXIS Healthcare Consulting Ltd
For full details of the event, speakers and to reserve your seat, please visit www.thepmi.com
FIP Approval Membership
The International Pharmaceutical Federation (FIP) is now a member of the Global Alliance Against Chronic Respiratory Diseases, an international network of stakeholders hosted by the World Health Organization (WHO) and with a vision of a world where all people “breathe freely”.
“Approval as a member not only implies an acknowledgement of the role of pharmacists in the prevention and management of
chronic respiratory diseases, but also a recognition of FIP’s work in this area,” said FIP lead for practice development and transformation Gonçalo Sousa Pinto.
One of FIP’s priority programmes focuses on non-communicable diseases (NCDs) and the FIP Practice Transformation Programme on NCDs aims to equip pharmacists with tools and strategic support for developing and implementing
pharmacy services making a sustained positive impact on the prevention, screening, management and treatment optimisation of NCDs, including chronic respiratory diseases. In this area, FIP’s work includes publications like “Chronic respiratory diseases: A handbook for pharmacists” and its accompanying knowledge and skills reference guide, along with several digital events. FIP is also
actively involved in addressing the health impacts of air pollution and promoting tobacco cessation. (Access all resources here: https:// ncd.fip.org/.)
“Membership of this alliance will also give us the opportunity to further disseminate our work on chronic respiratory diseases and tobacco cessation, and to engage in collaboration with other alliance members and the WHO itself,” Mr Sousa Pinto added.
21 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 News
National Cancer Registry Strategic Plan

The Minister for Health Stephen Donnelly T.D. today launched the National Cancer Registry’s (NCRI) Strategic Plan 20242026. The insights provided by cancer registry data are of vital importance as we seek to reduce the impact of cancer, a major health challenge in our community.
The systematic tracking, collation and analysis of cancer cases by the Registry enables better understanding of cancer in Ireland. Registry data helps to plan prevention interventions, improve early detection, and enhance the effectiveness of cancer treatment.
The NCRI’s Strategic Plan sets out five key objectives and outlines the organisation’s continued commitment to work alongside Government and stakeholders to
improve patient outcomes and enhance cancer control.
The Strategic Plan was completed through a significant consultation process with key stakeholders, including Government departments across the health services, academic and research institutions, cancer advocacy groups, patient advocacy groups, the NCRI Board and Advisory Council.
Minister Donnelly also marked the NCRI’s 30th anniversary of cancer data collection and celebrated Ireland’s 30 years of populationbased cancer registration, a significant milestone in the country's efforts to combat cancer. The wealth of data provided by the NCRI has enabled Ireland to benchmark its cancer control efforts against its European peers and inform life-saving policy change and resource allocation.
Joined by NCRI Chairperson, Dr Jerome Coffey, and NCRI Director, Professor Deirdre Murray, Minister Donnelly officially launched the new Strategic Plan and said, “As the National Cancer Registry Ireland marks 30 years of cancer registration in Ireland, it is a good time to reflect on how our attitude to cancer has changed over the decades. Through its regular publications the Registry
Beaumont Hospital Achieves International Re-Certification of CR Centre
shows how the cancer landscape has changed in Ireland and how survival rates have improved. The work of the Registry helps us understand outcomes in cancer care and enables us to make policies that will positively impact cancer patients going forward.”
Speaking at the launch of the new Strategic plan, Professor Deirdre Murray, Director of NCRI said, “The NCRI has always striven to be a dynamic, agile organisation that is responsive to the information needs of policymakers, planners and researchers. In the last 30 years, the environment in which the NCRI operates has seen many changes. I am confident that our new Strategy will ensure that the NCRI is well placed to continue to serve the Irish
public as a trusted source of highquality cancer data”.
Chair of the NCRI Board, Dr Jerome Coffey, welcomed the report and said: “Through collaboration and innovation, the Registry has played its part in Ireland’s efforts to create a more resilient healthcare system that responds to the ever-increasing complexities of cancer detection and treatment. It offers valuable insights that have shaped the development and implementation of Ireland’s national cancer strategies, helped to monitor the impact of primary and secondary prevention programmes as well as providing the key indices for cancer control - incidence, mortality and survival.”

Congratulations to the Cardiac Rehabilitation (CR) Department of Beaumont Hospital who were recently successful in achieving international re-certification (AACVPR) until September 2026.
Beaumont Hospital provides CR to the largest annual volume of patients in Ireland, and is modelled on the Mayo Clinic’s high-performance CR programme. This comprehensive programme of multidisciplinary care provides medically supervised exercise training, education, risk factor modification, stress management and psychological support for patients and their families.
To earn certification, Beaumont Hospital participated in a rigorous application process requiring extensive documentation of their CR programme’s practices and its performance was benchmarked against key performance indictors (KPIs) associated with the highest international standards of CR.
“We’re excited to announce that our CR programme was recently re-certified by the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). This is a huge accomplishment and a testament to our efforts in providing high quality patient care & services,” said Alison Cahill, CR Programme Manager.

22 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Minister Donnelly launched the NCRI Strategic Plan
Professor Deirdre Murray with Minister Stephen Donnelly and NCRI Chairperson, Dr Jerome Coffey
Parkinson’s Disease
Management and Current Treatment of Parkinson’s Disease
Parkinson's Disease is a progressive neurological condition. It is second most common neurodegenerative disease after Alzheimer’s Disease. However, experts believe we are facing a Parkinson’s Pandemic due to an ageing population, improvements in diagnosis, and advancements in research. Parkinson’s is the fastest growing neurodegenerative disorder.1
In 2020 experts estimated that globally 9.4 million people are living with Parkinson’s Disease (PD). Experts believe the prevalence of Parkinson’s has double in the last 25 years. The rise in PD prevalence calls for attention to the increasing individual and societal burden and the pressing need for measures to address and impact this challenging disease.2
What do we think? There are many myths associated with Parkinson’s disease:
• It is the disease of an older person.
• Parkinson’s is just a tremor.
• Parkinson’s only affects movement.
• Everyone is the same.
What do we know?
In Ireland it is estimated that 15,000- 18,000 people are living with Parkinson’s. A diagnosis of Parkinson’s is more commonly made in a person over the age of 65 years. However, there are approximately 1800 people diagnosed with early or young onset Parkinson’s in Ireland. These are those under the age of 65 and can range from 21 years old and older. Juvenile Parkinson’s can affect any person under the age of 21. Parkinson’s affects slightly more men than women.
Parkinson’s disease is a loss of a neurochemical called Dopamine. As we age, we lose dopamine but with Parkinson’s it is lost at a faster rate. Dopamine is responsible for smooth movement and controlled movement. It also plays a role in sleep, motivation, cognition and mood. Hence the potential symptoms some may experience. The exact cause of PD remains unknown. It is believed that a combination of age, genetics and environmental factors play a role.
Diagnosis & Symptoms
There is no definitive blood test or scan that can diagnose Parkinson’s. Therefore, diagnosis may be based on clinical assessment, history of symptoms including genetic history, a DaTscan (imaging of brain’s dopamine) and finally a response to medication prescribed. PD
research surrounding genetics & diagnosis is rapidly evolving, in 2023 researchers had a significant breakthrough in the development of a tool that can reveal key pathology in PD- a biomarker evident in CSF.3
The three initial symptoms evident in a person with Parkinson’s may include a tremor- usually unilateral . However, 70% will display the symptoms of a tremor, 30 % of those won’t. Bradykinesia (slowness in movement)- a change in pace of walk for example, and thirdly rigidity for example smaller amplitude in arm swing. There are some who will experience symptoms for years before a diagnosis is given. These are referred to as prodromal symptoms. It is estimated that by the stage symptoms emerge, the person has lost 60-70% of dopamine supply.
Motor symptoms associated with PD are sometimes the tip of the iceberg. Parkinson’s may present 40 potential symptoms both motor and non-motor. Examples of motor issues include: tremor, rigidity, facial masking, tremor, shuffle and balance. Examples of non- motor issues include: bowel & bladder issues, sleep, speech, hypotension, anxiety, apathy, mild cognitive changes. A Parkinson’s Ireland members survey(2022), showed that sleep was the top issues experienced by 70% of participants, followed by motor issues and thirdly speech/ oral issues- 58%. The differentiation between men & women is a newer area of research being explored with variation in symptoms, progression and potentially treatment between the sexes. Richelle Flanagan is currently carrying out a worlds first study in collaboration with UCC on the hormonal impact on women with Parkinson’s.
Treatment
There is no cure for Parkinson’s Disease. Research is ongoing into potential disease modifying treatments that may delay its progression. Medication, to manage symptoms, may be prescribed by a physician and this can optimise symptoms and improve quality of life.
Advancements in treatments
Written by Lisa Wynne, Parkinson’s Nurse Specialist, Parkinson’s Ireland
have been in the area of complex therapies such as drug device delivery systems and deep brain stimulation (DBS). These may be considered in advanced Parkinson’s when oral medication is no longer effectively managing symptoms. The national DBS service was launched in 2021 in Ireland with dual site location in the Dublin Neurological Institute, Mater Hospital & Beaumont Hospital. Another significant milestone for those in Ireland who may be eligible for treatment.
Living effectively with Parkinson's disease requires not only taking medication but also learning about the disease, the treatment, and the necessary tools. Promoting self-awareness in turn encourages

self-management. The WHO describes self-management as a critical component to delivery of care “countries where ageing populations and the growing burden of non-communicable disease means that there is ever greater demand for health services.”4
Live well with PD- KEES toolkit. At Parkinson’s Ireland we have developed the KEES toolkit to assist in self-management and efficacy when living

23 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Parkinson’s Disease
with Parkinson’s. The toolkit focuses on the below areas and forms the basis of our ‘Newly Diagnosed Programme’. In 2023 the Parkinson’s nurse team established a programme which takes place virtually, over a period of 4 weeks and includes a follow up with participants for up to 20 weeks. The structure of this programme is to educate, increase self-management and advocacy in a small group setting to assist those living with PD. This course takes place quarterly and is facilitated by our Parkinson’s nurse specialists . Those interested can contact our national office to be placed on the waiting list.
Knowledge - Information from reliable sources and at an appropriate level suitable. Knowledge promotes self-efficacy of a chronic illness, to improve ownership of the condition and maintain person centred care.
Education - The aim of this knowledge is to educate on medication, optimise symptom
control and improve adherence to prescribed medication.
Four crucial aspects to optimise PD medication are:
1. Consistent Timing. Meds on Time, Every Time!
2. Advice to be taken with/ without food
3. Avoiding Constipation
4. Hydration
Medication alongside complimentary therapies are key pieces to the symptom management jigsaw. Hence a personalised and holistic approach and care plan.
Exercise - Exercise is essential in the management of motor and non-motor symptoms. An emphasis should be placed on the psychological benefits for PD. It is becoming increasingly evident, in research, that exercise may have the potential to slow the progression of Parkinson’s
Disease. Regular, high level exercise and when maintained showed strong evidence of slower deterioration in those with PD. This was evaluated using several assessment tools.5 Further research is needed into the specific role of exercise and disease modification.
Support - Recognise and Reach Out.
Participating in research opportunities is a positive step and an opportunity to contribute to the future pathway of Parkinson’s. Support not only includes the network of health professionals but also support & education for family members and care partners. Parkinson’s Ireland facilitate this through our local branch network and daily zoom schedule, alongside our national support line & PD nurse support.
Worth considering “would it be beneficial to refer your patient to other members of the MDT- SLT/ physiotherapy/ OT/ dietician?”
Hospitalisation of a person with PD
Parkinson’s medication is timesensitive and a delay by even 30 minutes can have serious implications for some patients. Delaying the administration of medication have an impact on not only movement but speech, swallowing and anxiety levels. Missed doses or withdrawal of medication may lead to delayed recovery and significant deterioration of a person with Parkinson’s. In 2022 the UK Parkinson’s Audit states”58% of people with Parkinson’s admitted to hospital do not receive their medication on time every time.”7 In addition to timing it is important to be aware of medication that is contraindicated in Parkinson’s such as haloperidol, metoclopramide and others.
Through various research, it is evident that those with PD have an increased hospitalisation rate in comparison to controls. Yes, those with PD may be at an increased
24 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
risk of falls but there are multiple motor & non-motor factors that may increase hospitalisation. The younger the age of onset and length of disease duration increases the risk of admission.6 A review of studies in the UK showed the mean length of hospital stay for PD patients was found to be longer than for controls in a study involving 367 PD patients and 246 emergency admissions in the UK (21.3 vs. 17.8 days). Meanwhile, a longitudinal prospective study from the UK, which included PD and controls over a 12-year period, showed similar overnight durations (10 days for PD, 11.4 days for controls), but lower survival rates for PD. 8 This paper highlights the need for education for all health disciplines, guidelines & protocols for management of PD during a hospitalisation.
Health professional education is essential for managing Parkinson's disease (PD) in order to prevent further burden on our healthcare system and to reduce the amount
of time a PD patient must stay in the hospital or to perhaps avoid a hospital presentation to begin with.
Conclusion
To conclude, a Parkinson’s pandemic is evident. Projected numbers are expected to double by 2040. Parkinson’s is the fastest growing neurological disorder in the world. PD is stigmatised, something that networks all around the world are collaborating to eradicate. As our population continues to age, we need to increase our awareness of this disease. If the incidence of Parkinson’s is rising our health professionals need to be equipped with sufficient knowledge and education in order to provide the best care for those with PD.
Parkinson's disease presents difficulties for both individuals with the condition and medical professionals for a variety of reasons, such as accessibility, clear diagnostic standards, the wide range of symptoms, an individual's reaction to treatment, and variation of the disease's progression. Each individual’s case should be treated accordingly.
"Seven million people, seven million variants of Parkinson's. In everyone, the disease manifests itself differently."
Bas Bloem1
Parkinson’s Ireland
Parkinson’s Ireland is the national charity that supports, educates and connects those living with Parkinson’s, family members and health professionals. We provide services through our 15 branches and also through our national office based in Dublin. The support line is available to anyone trying to navigate Parkinson’s at any stage.
Parkinson’s Ireland provides a Parkinson’s nurse specialist service which includes phone support, virtual meetings one to one or group and nationwide talks for members and health professionals. We offer a call back service for our dietician Richelle Flanagan for those with questions specific to diet, it’s role in PD and living well.
Our national office also provides online classes which our members can avail of focusing on various aspects of Parkinson’s including mindfulness, psychological wellbeing, singing, yoga & physiotherapy classes. Educational leaflets and booklets are available online or on request from our national office. If you would like to find out more please visit www. parkinsons.ie or call 1800 359 359.
At Parkinson’s Ireland, we currently receive no core funding from the Irish state, which means we

rely heavily on fundraising and donations to ensure our services are kept running to support our members and volunteers. If you would like to fundraise or donate contact national office.
References:
1 Bloem, B. R., Okun, M. S., Klein, C. Parkinson's disease. The Lancet. 2020. Vol: 10291, pp.2284–2303. 2021
2 Maserejian, N., Vinikoor-Imler, L., Dilley, A. Estimation of the 2020 Global Population of Parkinson’s Disease (PD), [abstract]. 2020. Mov Disord. ; 35.
3 Siderowf, A., Concha-Marambio, L., Lafontant D., Farris. C., Ma. Y., Ureni. P. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross sectional study. 2023. The Lancet. Vol: 22. Issue 5. Pp 407-417. https://doi.org/10.1016/ S1474-4422(23)00109-6 .
4 World Health Organization. WHO global strategy on peoplecentred and integrated health services. Geneva: World Health Organization. https://iris.who.int/ handle/10665/155002. 2015
5 Tsukita, K., Sakamaki-Tsukita, H., Takahashi, R. Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With
Early Parkinson Disease. 2022. Neurology. 98(8):e859-e871.
6 Okunoye, O., Horsfall, L., Marston, L., Walters, K., & Schrag, A. (2022). Rate of Hospitalizations and Underlying Reasons Among People with Parkinson's Disease: Population-Based Cohort Study in UK Primary Care. Journal of Parkinson's disease, 12(1), 411–420. https://doi.org/10.3233/ JPD-212874
7 Parkinson’s Excellence Network. UK Parkinson’s Audit 2022https://www.parkinsons.org.uk/ professionals/ukparkinsons-audittransforming-care
8 Aminoff, M. J., Christine, C. W., Friedman, J. H., Chou, K. L., Lyons, K. E., Pahwa, R., Bloem, B., et al & National Parkinson Foundation Working Group on Hospitalization in Parkinson's Disease (2011). Management of the hospitalized patient with Parkinson's disease: current state of the field and need for guidelines. Parkinsonism & related disorders, 17(3), 139–145. https://doi.org/10.1016/j. parkreldis.2010.11.009
Recommended additional reading: Parkinson’s disease in adults (nice.org.uk)
Time critical medication and Get It On Time campaign resources | Parkinson's UK (parkinsons.org.uk)
25 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Increase in Health Workforce
Increases in the healthcare workforce and in healthcare activity have been highlighted, with the publication of the latest data tables on non-monetary healthcare statistics.
The data shows:
• the number of practising nurses in Ireland for 2023 was 72,543, an increase of 7% from 2022
• the number of practicing dentists in Ireland for 2023 stood at 2,466, an increase of 5.8% from 2022
• the number of practising caring personnel in 2023 was 27,995, an increase of 2.9% on 2022
• cataract surgery was the most common surgical procedure in
Ireland in 2022, with a total of 39,347 procedures, compared with 33,348 procedures in 2021
• there were 12,830 hip replacement procedures in 2022, compared with 11,159 procedures in 2021
The recently published data has also revealed that Ireland continues to attract healthcare workers from around the world, with India, the United Kingdom, and the Philippines the top 3 countries providing foreign trained nurses in 2023. More than half of all practicing nurses here obtained their first nursing qualification outside of Ireland.
Minister Stephen Donnelly said, "Over the lifetime of this
government, the HSE has hired well over 26,000 extra staff, a 22% increase. In tandem with these, we are examining how we structure our healthcare services to ensure that resources are used effectively, and the productivity of the health service is maximised.
"Compiling these national healthcare statistics is an important element in these efforts as we improve and expand access to the right care in the right place at the right time, in order to strengthen the quality of care and ensure best outcomes for patients."
This is the second year the Department of Health has complied and published the
annual National Healthcare Statistics data as part of the NonMonetary Health Care Statistics questionnaire, administered jointly by Eurostat, the OECD and the World Health Organisation (WHO). This data allows researchers and the public timely access to national information and provides a better understanding of Ireland’s data on a range of health topics and how it compares with other countries. The information is hosted on gov.ie, providing a centralised place for the public to access data and its metadata. It is also available on data.gov.ie in line with the government’s commitment to the European Union Open Data Directive.
New Chair of Tallaght University Hospital Board
 Professor Anne-Marie Brady, new Chair of the Tallaght University Hospital Board
Professor Anne-Marie Brady, new Chair of the Tallaght University Hospital Board
Professor Anne-Marie Brady has been appointed the new Chair of the Tallaght University Hospital Board, taking over from the outgoing Chair Mr. Liam Dowdall. Professor Brady has served on the Hospital Board since 2018 and was until recently the Vice Chair of the Board. She had also previously served as Chair of the Quality, Safety & Risk Management Committee.
Commenting on her appointment as Chair, Professor Brady said, “I would like to acknowledge the superb stewardship of Liam Dowdall during his tenure as Chair of the Hospital. During his tenure, there were many exciting developments but there were also some of the Hospitals greatest challenges including the COVID-19 pandemic and the cyberattack on the healthcare system. It is a great privilege to take on this role and I look forward to working with the Board, management team, and staff of the Hospital in delivering on the ambitious plans of the Hospital, developing our services further into the community.”
CEO of TUH Lucy Nugent said: “I warmly welcome the appointment of Professor Brady, her experience and leadership have greatly benefitted the board of the Hospital and I have no doubt her passion for healthcare and its development will benefit the Hospital, our patients,
staff, and community. I and the management team look forward to working with her.”
Professor Anne-Marie Brady is Chair of Nursing & Chronic Illness in the School of Nursing & Midwifery and Director of Trinity Centre for Practice & Healthcare Innovation at Trinity College Dublin. She previously served as Head of School (2017-20); Director of the Trinity Centre for Practice & Healthcare Innovation (201417) and as Director of Teaching & Learning (Postgraduate) (20102014). She is a Registered General Nurse and Registered Nurse Tutor with over 35 years of clinical practice, management, education, and research experience in healthcare innovation.
Before joining Trinity College Dublin, Professor Brady gained extensive international experience working in healthcare systems in Ireland, the United Kingdom, and the USA. Due to her clinical background, academic leadership, and governance experience, Professor Brady has gained a significant understanding of health service delivery issues in both the Irish and international contexts. Her research work is focused on the development of and innovation of health systems and workforce development in healthcare. She has conducted research studies in collaboration with health service providers to examine issues around chronic illness, patientrelated outcomes, unmet needs, workload measurement, healthcare innovation, and role development among healthcare workers.
26 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Recent Product Launches
Retail
As the world’s leading provider of generic medicines, Teva understands the importance of making quality medications accessible and affordable.

Hospital





Our scientists are continuously developing medicines, that aim to increase access to treatments that can improve patients’ health and save healthcare providers millions.




Freephone:
Prescription Only
Teva Pharmaceuticals Ireland, Digital Office Centre Swords, Suite 101 - 103, Balheary Demesne, Balheary Road, Swords, Co Dublin, K67E5AO, Ireland.
1800 - 201 700 | Email: info@teva.ie
Medicines Further information is available on request or in the SmPC. Product Information also available on the HPRA website. Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to Teva UK Limited on +44 (0) 207 540 7117 or medinfo@tevauk.com Date of Preparation: January 2024 | Job Code: GEN-IE-00056 Visit teva.ie for more information
Crisis Advice
Finding Your Way after Sexual Violence
A guide to options and supports after rape, sexual assault and other forms of sexual violence in Ireland

If you have experienced sexual violence, it can be hard to know where to turn for trusted information and support. From our 45 years of supporting those affected by sexual violence, Dublin Rape Crisis Centre knows it is vital to have easily accessible information and support when you need it. With that in mind, we created Finding Your Way after Sexual Violence, an online guide that aims to support victims and survivors on this difficult journey. It offers information on how to access a Sexual Assault Treatment Unit (SATU), report to the Gardaí, or navigate the criminal courts and legal processes. The guide also describes supports available to survivors, such as the free and confidential 24-hour National Rape Crisis Helpline 1800 778888.
Primarily aimed at survivors of sexual violence, the guide is trauma-informed and uses text, animations, audio clips and direct quotes to explain how the various processes may make a survivor feel, as well as how and where to seek support following sexual violence. However, in each section of the guide, there is also information designed for those seeking to know more about how to support another person.
Finding Your Way after Sexual Violence features a diverse range of personal experiences shared by victims and survivors, in audio and written format. We have included testimonials from medical staff and volunteers working in SATUs, from An Garda Siochana, and from those working and volunteering in the courts and legal system. Their voices provide reassurance that there are empathetic, professional and dedicated people available who want to help and guide victims and survivors, and who understand that the journey is
Written by Gráinne Henry, Dublin Rape Crisis Centre
unique for each person, as are their needs.
Empowering victims and survivors of sexual violence to make choices that are best for them is key to our work. Sexual violence was forced on them, so it is vitally important that we do not take away their decision-making powers or pressure them into any actions or choices.
“Whatever path someone takes after experiencing sexual violence, it can feel so isolating, and having this resource can make a huge difference. The language is easy to understand, and the emotional support that Rape Crisis Centres provide is there. It's a step in the right direction, and it's comforting to know that information and support is available when you need it” - a survivor of sexual violence speaking about the guide.
Sexual Violence in Ireland
As medical professionals working in a hospital, Finding Your Way after Sexual Violence may be useful to you in understanding how victims and survivors of sexual violence may be feeling. In particular, the guide provides an insight into what happens in a SATU and is a great referral point for any patients who would like more information on supports and options available to them. Survivors are often worried about being believed or about what happens next. They have been through an incredibly traumatic experience. With 1072 attendees across SATUs nationwide in 20221, it is important to understand the additional impact of trauma when working with patients who have experienced sexual violence. There was a substantial increase in DRCC’s accompaniment support work in 2022 which provided emotional support to 291 survivors at the Sexual Assault Treatment Unit in Dublin – an increase of 153% over 2021 - along with 233 of their family and friends.2
Reflecting on the SATU figures, it is important to note that SATU is a service for those who have experienced very recent rape or physical sexual assault. The reality is that very many people in Ireland have been harmed by sexual violence. Data from the Central Statistics Office shows that 52% of women and 28% of men reported experiencing sexual violence in their life.3 The National Rape Crisis Helpline had 18,400 contacts in 20222 from people of all genders and ages who had a wide variety of experiences.
Working with survivors of sexual violence
When providing care for someone who has experienced sexual violence, it is important to listen to how they are feeling and to give them space. People have very individual responses to the trauma of rape and sexual assault and the long-term effects also vary widely. Because of this, it is important to respect each person’s way of coping. These impacts may occur many years after a rape or assault as well as in the immediate aftermath. Everyone’s reaction to experiencing sexual violence is different: they may want to talk or be completely silent. They might be blaming themselves or feeling angry, upset or numb. It’s important to remember that there’s no set way to react to sexual violence; everyone is different. Engaging in medical care can be triggering for some survivors. Their autonomy was taken from them when they were assaulted so it is important to allow them to have personal agency. Try to be conscious of this when providing care. If you need to touch a patient, explain to them where you will be touching them, when you will do it, and why it is necessary. Allow them the space to ask questions and try not to rush them. Let them know the options available to them, including the National 24-Hour Helpline (1800 77 8888). You can also direct them to this Finding Your Way guide, where they can find out more about their options.
“Time after time, a survivor will say how much it helps them to meet someone who reassured them that they are believed” - SATU volunteer.
Vicarious Trauma
Encountering the stories of victims of rape or child sexual abuse is stressful and traumatic. In the course of your work, you may hear or read details of abuse, and of the pain, sometimes lifelong, experienced as a consequence. As you witness the stories and the impact of trauma, you may experience a traumatic response yourself. It is worth reflecting on how working with trauma may be impacting you. Here are some ways you can look after yourself and avoid experiencing vicarious or secondary trauma:
• Clearly define your role and responsibilities and its boundaries. You are responsible for fulfilling your limited role in a caring and professional manner.
• If a patient discloses to you that they have experienced sexual violence, you can tell them about the National Helpline where they can get specialised support and information that is free and confidential. You are not responsible for the patient’s experiences or distress or for solving the patient’s problems or meeting all of their needs or concerns.
• When encountering trauma, notice what happens for you, in your thoughts, images, emotions, and body.
• Ground yourself by noticing yourself sitting in the chair, the floor under your feet; take some deep breaths and try to loosen your body. You could be sitting in a tense pose for a couple of hours if you are not aware. Take regular breaks, even for a few moments.
• Allow time for debriefing between one patient and the next. Take a few moments to register how you are feeling to let go of and shake out the session. Stretch and loosen your body, which may be holding some tension and trauma.
• Outside of work, provide yourself with as many opportunities as possible to enjoy activities which are fun and make a conscious decision to link in often with the people in your own life. Help yourself to maintain balance by taking part in activities that give you joy and pleasure.
28 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE

• Remember that you can call the National 24-hour Helpline 1800 77 8888 or use the webchat at drcc.ie if you have been affected by sexual violence in any way, including from working with patients.
• This can be an overwhelming experience for you. If you need support for yourself, the 24-Hour National Helpline is available for you to call on 1800 77 8888. The helpline is free and confidential and is open to everyone who has been affected by sexual violence, including those supporting victims and survivors. We can provide guidance to you on how to support those who have experienced sexual violence.
It can be daunting, and we are here to listen.
Finding Your Way after Sexual Violence is for anyone in Ireland affected by sexual violence, including victims and survivors, their supporters and others engaged in these systems. It is not just a guide for those who have experienced sexual violence directly; it aims to help all of us to understand our role in supporting and understanding victims and survivors.
Finding Your Way after Sexual Violence is available on DRCC’s website at www.drcc.ie/fyw
If you would like flyers about Finding Your Way after Sexual Violence, the National Rape
Crisis Helpline or any of DRCC’s services, please email communications@rcc.ie
For anyone impacted by sexual violence, at any time, the National 24-hour Rape Crisis Helpline offers free, confidential support at 1800 778888
References
1. Sexual Assault Treatment Unit, Annual Report 2022, 2023.
2. Dublin Rape Crisis Centre, Annual Report 2022, 2023.
3. Central Statistics Office, Sexual Violence Survey 2022, 2023.
DRCC operates the National 24-hour Helpline 1800 778888 to support anyone affected by sexual violence in any part of the country.
A webchat support service is available online at drcc.ie Mon-Fri, 10am-5pm, except holidays.
A Helpline Interpreting Service is available for those who do not speak English.
For those who are deaf or hard of hearing, we provide a text service, operating Mon-Fri from 8am to 6:30pm, at 086-8238443.

29 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Ground-breaking Insights into Diabetes

Diabetes Ireland have launched the results of the first-ever survey highlighting the lived experience of people with diabetes in Ireland. For further information on “Accessing and Using Diabetes Health Services Survey 2023.”
An anonymous online survey of adults with diabetes or parents/ carers of children with diabetes was conducted between 16 January and 5 February 2023 via social media and standard online communication to ask about experiences of living with diabetes in Ireland. 517 people completed all sections of the survey: 230 adults with type 1 diabetes, 155 with type 2 diabetes and 12 with other types of diabetes, and 120 parents/carers.
Among adults, 37% reported they were already living with diabetesrelated complications and comorbidities. Most commonly these were hypertension (high blood pressure – 23%), diabetes retinopathy (23%), hypothyroidism (17%) and mental health-related issues (14%). During their lifespan living with diabetes, both adults & children living with type 1 diabetes had experienced severe complications: 40% had experienced DKA, mainly at the time of diagnosis, which should be avoidable if diabetes symptoms were recognised early; and 45% of adults experienced at least one episode of severe hypoglycaemia – a critically low glucose level, which usually comes with unconsciousness and requires third party help and possible hospitalisation.
In terms of their ongoing care, the majority of respondents were cared for in the public system but 20% of people with type 1 diabetes and 46% of those with type 2 diabetes pay privately for diabetes care. Only 13% of adult respondents had no review
Dr Kate Gajewska
appointment with their healthcare provider in 2022 with a further 29% having only one appointment. However, all children with type 1 diabetes had at least two or more appointments in the same period, with 67% of them having three appointments or more.
Overall, respondents expressed satisfaction with their diabetes care but many highlighted the lack of time given to them by their diabetes healthcare provider and felt the appointments were rushed.
The vast majority of respondents (88%) are under the HSE LongTerm Illness Scheme, giving them access to free diabetes-related medications and technologies plus free high blood pressure and high cholesterol medications.
Interestingly, when comparing Dublin with the Rest of the country, there were a number of noticeable differences in people’s daily experience of living with diabetes. More often, people with type 1 diabetes pay privately for their diabetes care if they live outside of Dublin (19% vs. 10%). Among people with type 2 diabetes, those from outside of Dublin were usually receiving their care in general practices (75% vs. 55%). People from Dublin more often were treated in outpatient diabetes hospital clinics (27% vs. 20%) or attended diabetes clinics privately (13% vs. 5%).
Among adults living with type 1 diabetes, those living outside Dublin were more often diagnosed with diabetes-related complications or comorbidities (41% vs. 35%). Among people with type 2 diabetes, those living outside Dublin more often had increased occurrence of diabetes-related complications or comorbidities (48% vs. 20%), mainly hypertension (40% vs. 18%) and mental health-related issues (24% vs. 15%).
Overall, the rates of technology usage (CGM) were very high and there were no differences between Dublin and the rest of the country with insulin pump usage also being similar. However, in openended responses, many people referenced very long waiting lists and difficulties in accessing diabetes-related technology, mainly insulin pump therapy, as well as difficulties in accessing specialist multidisciplinary team members (i.e. dietitians, podiatrists, psychologists etc.).
Generally, people rated their health and well-being as good, but one-third assessed it as fair, poor or very poor. 75% of respondents reported that they do not discuss mental health & wellbeing as part of their diabetes care with half welcoming an opportunity to do so.
Dr Kate Gajewska, Research & Advocacy Manager, Diabetes Ireland who led the research and undertook the survey analysis, said: “We regularly hear from people with diabetes about difficulties in accessing diabetes care services and new technologies across the country. However, we had no real data on this, so we decided to undertake this survey and provide an opportunity for people with diabetes to share their experience of accessing services and living daily with the condition. This survey is the first of its kind in Ireland, and in the absence of a National Diabetes Register and Clinical Audit, it provides very detailed information about experiencing diabetes care by those affected by diabetes in Ireland.”
Alongside the release of the survey findings, Diabetes Ireland is also launching its Pre-Budget Submission 2024 calling on the Minister for Health to set up a task force similar to the Cancer Strategy Taskforce to develop a 10-year National Diabetes Strategy to improve and standardise the delivery of care, access to diabetes services to improve the quality of life of more than 300,000 people living with diabetes in Ireland.
Cormac Devlin TD, Chairperson of the Cross Parliamentary Group on Diabetes said: “The economic burden of diabetes on the Irish healthcare system is now a major challenge for the government and the Health Service Executive.
The high cost of diabetes is mainly caused by the treatment of complications, many of which could be avoided with earlier detection, greater awareness of symptoms, better access to newer treatments and diabetes technologies, multidisciplinary teams, including psychologists, and regular diabetes review appointments. Despite the positive ongoing work of the HSE and Sláintecare, there are still many gaps in current diabetes services, as highlighted by this survey, that need to be tackled strategically in order to provide optimum diabetes care to everyone in need.
Therefore, to improve diabetes care, we need to collectively act now and decide what future care for people with diabetes will look like. “The Cross Parliamentary Group on Diabetes agree that it is time to set up a diabetes task force of relevant stakeholders to develop a 10-year National Diabetes Strategy that provides vision, leadership, direction, goals and priorities, as well as identifying and securing the future funding required to provide optimum care for every person living with diabetes in Ireland. We know this will take some time but we need to be very forwardthinking in our approach to dealing with diabetes long into the future and the immediate setting up of a force will help us achieve it.” – added Mr Devlin.
Diabetes Ireland, alongside the HSE National Clinical Programme, is also calling for additional funding of ¤5m to enable more equitable access to continuous glucose monitoring (CGM) for people with type 1 diabetes. Professor Derek O’Keeffe, National Clinical Programme for Diabetes Lead said: “In line with international best practice, the National Clinical Programme for Diabetes is requesting a broadening of the criteria for the provision of a form of CGM to all individuals living with Type 1 diabetes in Ireland. Bearing in mind that many people already use CGM in Ireland, securing ¤5 million by the Government in the Budget 2024 will facilitate more equitable access and reduce disparities for those living with type 1 diabetes who have not availed of this technology yet, will help them improve their diabetes management, outcomes and quality of life.”

30 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE Diabetes
FOR THE TREATMENT OF DIABETES MELLITUS IN ADULTS, ADOLESCENTS AND CHILDREN FROM THE AGE OF 6 YEARS1

• Help your patients find balance between HbA1c reduction and hypoglycaemic risk 1-7
• Offers flexibility † (+/- 3 hours) in dosing time1,8,9

From the start,1 there to help your patients on their basal insulin journey1 from 6 years
Toujeo® DoubleStar™ prefilled pen is recommended for patients requiring at least 20 units per day.1
† Toujeo® is available in easy-to-use pens,1,8,9 to be administered once daily at any time of the day, preferably at the same time every day.1 When needed, patients can administer Toujeo® up to 3 hours before or after their usual time of administration. Flexible dosing time was evaluated in two randomised, open-label clinical studies, in patients with T2DM.1
Prescribing Information: Toujeo (insulin glargine 300 units/ml) Please refer to Summary of Product Characteristics (SmPC) before prescribing. Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection. Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapidacting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit-to-unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%). Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti-hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed, or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number, then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients. Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an unaffected area has been reported to result in hypoglycaemia. Blood
Intended for Healthcare Professionals in the Republic of Ireland only.
glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar prefilled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There are no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Prescribers should consult the SmPC in relation to other adverse reactions. Legal Category: POM. Marketing Authorisation Number: SoloStar 5 pen pack: EU/1/00/133/035; DoubleStar 5 pen pack: EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2022. MAT-IE-2200355 (V1.0) Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
Abbreviations: HbA1c, Hemoglobin A1c; PD, Pharmacodynamics; PK, Pharmacokinetics; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus.
References: 1. Toujeo® Summary of Product Characteristics July 2020. 2. Home PD, et al. Diabetes Care 2015;38(12):2217-2225. 3. Matsuhisa M, et al. Diabetes Obes Metab 2016;18(4):375-383. 4. Danne T, et al. Diabetes Care 2020; 43(7):1512–1519. 5. Riddle MC, et al. Diabetes Care 2014;37:2755-2762. 6. Yki-Jarvinen H, et al. Diabetes Care 2014;37:3235-3243. 7. Bolli GB, et al. Diabetes Obes Metab 2015;17:386-394. 8. Singh R, et al. Eur Endocrinol 2018;14:47-51. 9. Pohlmeier H, et al. J Diabetes Sci Technol 2017;11;263-269.
MAT-IE-2200486 (v1.0) | December 2022
SFI Discover Programme Research

Two RCSI public engagement projects have received funding under the ¤5 million SFI Discover programme, which is aimed at encouraging understanding of science, technology, engineering and mathematics (STEM).
RCSI’s Department of Obstetrics and Gynaecology in collaboration with the Rotunda Hospital Dublin has been awarded ¤287,000 to run a second edition of 'Debunking the Myths: The Science Behind Our Sexual Health'. The nationwide education programme returns in 2024 with more exciting workshops, online campaigns and new resources for teachers.
The team is committed to equipping teenagers with essential lifelong health literacy skills, empowering them to navigate the complex landscapes of sexual and reproductive health with confidence into their adult years. The new iteration of the programme will also offer free resources for secondary school teachers, a dedicated workshop for parents, and deepen discussions with teens on taboo topics such as menstruation, STIs and sexual health for LGBT+ people, addressing unique health needs, and fostering a supportive and inclusive environment.
As part of the programme the team will kick off a series of social media campaigns where healthcare experts will be answering questions from the students of past workshops, addressing important topics like contraception and tackling misinformation prevalent online.
Professor Fergal Malone, programme lead and Chairman
of the Department of Obstetrics and Gynaecology at RCSI School of Medicine said: “This second round of funding marks a very exciting time for us all at RCSI Department of Obstetrics and Gynaecology and we look forward to engaging with many more students, teachers and parents over the next two years. This is the only health programme that gives teenagers the opportunity to discuss questions in relation to their sexual health with a team of medical experts. Young people are more online than ever, and we need to ensure that the information they receive is factual, relevant, and most of all, safe.”
Epilepsy and concussion
FutureNeuro, the SFI Research Centre for Chronic and Rare Neurological Diseases based at RCSI, has been awarded ¤88,360 for the My Moving Brain: M'Inchinn Gluiseachta (MIG) project which will kick-start a national campaign to grow education and public engagement in brain health, epilepsy and concussion in sport throughout Ireland.
The project will be led by FutureNeuro in collaboration with local sports partnerships representing sports organisations from different disciplines, Epilepsy Ireland, the national charity representing members of the epilepsy community; and the Neurological Association of Ireland, representing members with neurological diseases.
The collaboration will establish the importance of sport for people with epilepsy and address the prevention and management of concussion in sport.
Professor Fergal Malone, 'Debunking the Myths' Programme Lead and Chairman of the Department of Obstetrics and Gynaecology, RCSI School of Medicine
Dr Omar Mamad, RCSI Research Fellow, SFI FutureNeuro Research Centre and My Moving Brain co-lead said: "This project is a testament to our commitment to fostering knowledge and engagement. We are excited to collaborate with our partners to make a meaningful impact on brain health awareness, not just in the sporting community but for the broader public. Through education and collaboration, we aspire to create a safer and more informed environment for all."
In announcing the funding, Minister for Further and Higher Education, Research, Innovation and Science
Simon Harris TD said: “These initiatives, involving the general public and our communities across primary, secondary and third-level education, are essential to fostering curiosity about science, technology, engineering and maths.
“This investment will help to broaden participation in STEM –both geographically and amongst less represented voices – and inspire all generations to deepen their understanding of what learnings, studies and careers in these fields entails. In turn, the next generation, in particular, will be better engaged and empowered to share their ideas and solutions to societal challenges.”
Professor Fergal O’Brien, Deputy Vice-Chancellor for Research and Innovation at RCSI, said: “Public engagement is key priority for RCSI, and we encourage our researchers to leverage their expertise and insights to promote great public awareness of and engagement with the science of health. Congratulations to the team at the Department of Obstetrics and Gynaecology and FutureNeuro for their success in receiving this SFI funding.”
In line with SFI’s 2025 Strategy – Shaping Our Future, the SFI Discover Programme Call aims to empower and inspire deep public engagement and the programme is a key part of SFI education and public engagement activity.


32 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE News
FOR THE TREATMENT OF DIABETES MELLITUS IN ADULTS, ADOLESCENTS AND CHILDREN FROM THE AGE OF 6 YEARS1

• Help your patients find balance between HbA1c reduction and hypoglycaemic risk 1-7
• Offers flexibility † (+/- 3 hours) in dosing time1,8,9

From the start,1 there to help your patients on their basal insulin journey1 from 6 years

Toujeo® DoubleStar™ prefilled pen is recommended for patients requiring at least 20 units per day.1
† Toujeo® is available in easy-to-use pens,1,8,9 to be administered once daily at any time of the day, preferably at the same time every day.1 When needed, patients can administer Toujeo® up to 3 hours before or after their usual time of administration. Flexible dosing time was evaluated in two randomised, open-label clinical studies, in patients with T2DM.1
Prescribing Information: Toujeo (insulin glargine 300 units/ml) Please refer to Summary of Product Characteristics (SmPC) before prescribing. Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection. Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapidacting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit-to-unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%). Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti-hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed, or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number, then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients. Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an unaffected area has been reported to result in hypoglycaemia. Blood
Intended for Healthcare Professionals in the Republic of Ireland only.
glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar prefilled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There are no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Prescribers should consult the SmPC in relation to other adverse reactions. Legal Category: POM. Marketing Authorisation Number: SoloStar 5 pen pack: EU/1/00/133/035; DoubleStar 5 pen pack: EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2022. MAT-IE-2200355 (V1.0) Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
Abbreviations: HbA1c, Hemoglobin A1c; PD, Pharmacodynamics; PK, Pharmacokinetics; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus.
References: 1. Toujeo® Summary of Product Characteristics July 2020. 2. Home PD, et al. Diabetes Care 2015;38(12):2217-2225. 3. Matsuhisa M, et al. Diabetes Obes Metab 2016;18(4):375-383. 4. Danne T, et al. Diabetes Care 2020; 43(7):1512–1519. 5. Riddle MC, et al. Diabetes Care 2014;37:2755-2762. 6. Yki-Jarvinen H, et al. Diabetes Care 2014;37:3235-3243. 7. Bolli GB, et al. Diabetes Obes Metab 2015;17:386-394. 8. Singh R, et al. Eur Endocrinol 2018;14:47-51. 9. Pohlmeier H, et al. J Diabetes Sci Technol 2017;11;263-269.
MAT-IE-2200486 (v1.0) | December 2022
Edge Enhancement Optimization in Flexible Endoscopic Images to the Perception of Ear, Nose and Throat Professionals
Written by G. Geleijnse MSc, L. L. Veder MD, M. M. Hakkesteegt PhD, H. H. W. de Gier MD, B. Rieger PhD, R. M. Metselaar MD, PhD
Flexible endoscopes are essential to examine nose, throat, and upper airway.1 Digital endoscopes have gradually replaced fiber optic endoscopes because of much better image quality.2-6 Digital endoscopes are connected to a video processor that applies various operations to enhance the image without perceivable delay for the observer. One of those operations is edge enhancement and its effect on an in vivo image of the larynx is illustrated in Figure 1. This operation makes the image sharper and sharpness is strongly correlated with the perceived image quality by Ear, Nose and Throat (ENT) professionals.7 Although edge enhancement is applied by all vendors, the literature on edge enhancement in ENT is limited to two articles. Kawaida et al reported that in their experience image quality was improved when structure enhancement, that is, a form of edge enhancement was applied.8 Kawaida et al later showed that edge enhancement also seems to improve diagnostic accuracy: applying structure enhancement refined the diagnosis in 2 out of 15 patients.9

by the vendors, we can only measure the effects on images that are processed by the video processors.
The Laryngoscope, Volume: 134, Issue: 2, Pages: 842-847, First published: 17 August 2023, DOI: (10.1002/lary.30981)
reproduced) or diagnostics.11 15
The purpose of this study was to (1) objectively quantify the level of edge enhancement uniformly from test images, (2) measure the effect of edge enhancement on sharpness and noise, and (3) study the influence of edge enhancement on image quality perceived by ENT professionals.
Materials and Methods
on sharpness and noise, and
(3) study the influence of edge enhancement on image quality perceived by ENT professionals.
operational in our outpatient clinic and readily available.
Materials and Methods
Edge enhancement or sharpening is a known technique to sharpen edges by adding an undershoot on the darker side of an edge and an overshoot on the brighter side.1013 In fact, this operation does not introduce new information or increases the resolution of the image, but increases the step in brightness of edges. This operation probably works so well, because it mimics the biological process of retinal lateral inhibition in the visual system.14 A major drawback of edge enhancement is that the operation cannot discern edges from noise and therefore enhances both.
Edge enhancement can be applied using various methods that determine the resulting image and can be optimized for different purposes, such as aesthetics and fidelity (i.e., the degree of exactness with which reality is
Edge enhancement is commonly applied in digital ENT endoscopes, but the specific method and parameters are not disclosed, and the units to express the level of edge enhancement are arbitrary and differ.13 Literature to substantiate the default settings has not been found and could not be provided by the manufacturers. Because we do not know the methods and parameters applied by the vendors, we can only measure the effects on images that are processed by the video processors.
The Laryngoscope, Volume: 134, Issue: 2, Pages: 842-847, First published: 17 August 2023, DOI: (10.1002/lary.30981)
To study the effect of edge enhancement, we used three endoscopes. Because the purpose was to study image quality metrics and not to compare the types of endoscopes, we refer to endoscope A, B, and C throughout the manuscript and disclose the specific type of endoscope and video processor here once for sake of reproducibility of the study: (A) Olympus ENF-V4 connected to a CV-170, (B) Pentax VNL9-CP connected to a VIVIDEO CP-1000, and (C) Xion HD connected to a Matrix P Spectar. These systems were selected because they were operational in our outpatient clinic and readily available.
The purpose of this study was to
(1) objectively quantify the level of edge enhancement uniformly from test images, (2) measure the effect of edge enhancement
To study the effect of edge enhancement, we used three endoscopes. Because the purpose was to study image quality metrics and not to compare the types of endoscopes, we refer to endoscope A, B, and C throughout the manuscript and disclose the specific type of endoscope and video processor here once for sake of reproducibility of the study:
(A) Olympus ENF-V4 connected to a CV-170, (B) Pentax VNL9-CP connected to a VIVIDEO CP-1000, and (C) Xion HD connected to a Matrix P Spectar. These systems were selected because they were
The user interfaces of the video processors have different names for the option to adjust the level of edge enhancement. System A and C have a numerical value, but system B has a wedge without numerical value. Therefore, we used a ruler on the display, to systematically vary the level of edge enhancement from 0% to 100% in nine steps of 12.5%. The exact levels of edge enhancement that are used in this study for in vitro and in vivo measurements are listed in Table I
The user interfaces of the video processors have different names for the option to adjust the level of edge enhancement. System A and C have a numerical value, but system B has a wedge without numerical value. Therefore, we used a ruler on the display, to systematically vary the level of edge enhancement from 0% to 100% in nine steps of 12.5%. The exact levels of edge enhancement that are used in this study for in vitro and in vivo measurements are listed in Table I
To genuinely capture images, the endoscope was connected to a video processor and the DVI-D video output to the display was split using a Blackbox 1x2 DVI-D
To genuinely capture images, the endoscope was connected to a video processor and the DVI-D video output to the display was split using a Blackbox
DVI
splitter. One output was connected to the surgical display for the ENT
34 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE Endoscopy
Figure 1: (Left) Example image of a larynx recorded without edge enhancement. (Mid) Edge enhancement applied. (Right) Edge enhancement applied to a level at which the operation becomes objectionable
TABLE I. Term Used for Edge Enhancement and Studied Levels of Edge Enhancement Per Endoscope.
Endoscope Enhancement Studied Settings
A0;
A Structure enhancement
A1; … A8
0;
B Edge enhancement
12,5; … 100%
C Contrast–edge sharpness 0; 3; 5; 8; 10; 12; 15; 17; 20
1x2
D
TABLE I. Term Used for Edge Enhancement and Studied Levels of Edge Enhancement Per Endoscope
Edge Enhancement Optimization in Flexible Endoscopic Images to the Perception of Ear, Nose and Throat Professionals
splitter. One output was connected to the surgical display for the ENT specialist, and the other output was connected to an Epiphan DVI2USB3 frame grabber. Images were stored as uncompressed 24bit BMP files.
In vitro measurements
Direct comparison of edge enhancement settings between video processors is impossible due to the arbitrary units provided by the manufacturers. Therefore, we measured the levels of edge enhancement in vitro by capturing images of the Rez Checker Target Nano Matte at 3.0 cm distance. Image Science associates developed this test chart specifically to narrow illumination geometries, notably those used in endoscopic imaging.13, 16 The endoscope was positioned and fixated in a setup, and images were captured at the levels of edge enhancement in Table I. Measurements were performed using slanted edges and gray patches. The level of edge enhancement was determined by subtracting the step response of an image without edge enhancement from the other eight images with edge enhancement and measuring the resulting peak-to-peak differences. These differences were then normalized by dividing by the step size, that is, a value of 1 indicates that the edge height/step is doubled by the processing. Sharpness was
characterized by observing the normalized modulation transfer function (MTF) and computing the spatial frequency at 50% MTF. The standard deviation of the image noise was measured on the gray patches and computed as the square root from the weighted sum of variances of the luminance (Y) and the chrominance channels red (R), green (G), and blue (B) of the pixel values.17
Pairwise Comparison
In Vivo Image Acquisition
To study the effect of edge enhancement in vivo, three different types of endoscopes were used to image the larynx of a single healthy volunteer. The endoscopes were introduced in the nose through the nasal cavities as it is the common practice for this medical examination. The ENT specialist pointed the endoscope at the larynx and steadied the view of the endoscope. For each studied level of edge enhancement (nine levels per type of endoscope), at least two images were captured. The acquired images were immediately quality checked by two ENT professionals, and the examination was repeated for the levels with unsatisfactory images. The best image was selected with respect to positioning, anatomy, and lack of motion blur. The selected images were included in the series for pairwise comparison. The protocol
was reviewed by the accredited Medical Ethical Review Committee Erasmus MC and considered not to be subject to the Medical Research Involving Human Subjects Act (MEC 2022-0268).
In Vivo Image Pairwise Comparison
One image was selected per studied level of edge enhancement (n = 9) and used for a forced pairwise comparison, resulting in a series of (n2 n)/2 = (92–9)/2 = 36 test pairs of images to be compared by ENT professionals.17 Thirty-nine ENT professionals participated in this study: 16 ENT specialists, 16 ENT residents, 2 physician assistants, 3 speech therapists, and 2 researchers. All the participants had more than 6 months of relevant experience at the outpatient clinic. Three series of images with edge enhancement applied by the three included endoscopes were compared separately. The images were displayed on a color-calibrated diagnostic display (EIZO RadiForce MX315W 4096x2160) with their native resolution by a custom made Matlab program. The program randomized the sequence for each observer to prevent any form of learning effect. The side at which the images were presented was also randomized between left and right to prevent any selection bias. The resolution was checked by counting the pixels using the “print-screen” function. The characteristics sharpness,
image noise, and color fidelity were verified by comparison of print screens of the in vitro test images to the original test images. The monitor was calibrated to sRGB color space with a color temperature of 6500 K and gamma of 2.2, and the luminance ranged from 0.5 to 300 cd/m2 The observers were asked to select the image with the best image quality characteristics for diagnostic purposes and neglect the influence of illumination, position, and viewing angle. The test series were preceded by a smaller training series (n = 4) to make the participants familiar with the assignment and user interface.
Pairwise Comparison Analysis
Pairwise comparison is an easy task for the participants, but the analysis is more challenging. We are not aware of pairwise comparison testing applied in the field of endoscopy, but camera industries have developed a solid theoretical basis for analyzing such data.17-21 The goal is to use the votes of the participants to determine the perceived difference of image quality on a psychometric scale. The unit of this scale is the just noticeable difference (JND). One JND is defined as the difference in image quality, where 50% of the observers perceive a difference and vote consistently, whereas the other 50% do not perceive a difference in image quality and will choose randomly.

35 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Figure 2: (A) Sharpness versus the level of edge enhancement. Sharpness increases linearly when edge enhancement is applied. (B) Image noise versus the level of edge enhancement. Noise increases linearly when edge enhancement is increased
The Laryngoscope, Volume: 134, Issue: 2, Pages: 842-847, First published: 17 August 2023, DOI: (10.1002/lary.30981)
Edge Enhancement Optimization in Flexible Endoscopic Images to the Perception of Ear, Nose and Throat Professionals
Endoscopy
One half of the second group will randomly vote the same as the first group, resulting in 75% voting for the image with better image quality, versus 25% for the image with lesser image quality. Hence, +1 JND corresponds to a probability of 75%, 0 JND corresponds to 50%, and 1 JND corresponds to 25%.
Using the JND as a unit provides an intuitive and meaningful measure to evaluate quality differences. We followed the guide of PérezOrtiz and Mantiuk,19 to find the differences on the psychometric scale between the nine levels of edge enhancement per endoscope.
Results
In Vitro Measurements
The measured levels of edge enhancement, sharpness (MTF50), and image noise using the in vitro images are shown in Figure 2A, B. The range of available amount of edge enhancement is similar for endoscope A (0–1.12) and B (0–1.24), but endoscope C has a smaller range (0–0.83).
Sharpness and noise both increased as the level of edge enhancement was increased.
Endoscope C has a larger sharpness and is able to capture smaller details compared with the other endoscopes (Figure 2A). For endoscopes B and C, the horizontal sharpness is approximately equal to the vertical sharpness; however, endoscope A applies a remarkable lower amount of edge enhancement vertically compared with horizontally.
Endoscope A has slightly higher levels of noise compared with B and C has the lowest noise levels (Figure 2B).
Pairwise Comparison
The pairwise comparison of in vivo images was completed by 39 ENT professionals. We found no differences in votes between the specialists and residents.
A third-order polynomial is fitted as a trend line through the quality scores of each endoscope. The trends steeply increase when edge enhancement is switched on for all endoscopes. The trend of endoscope C stabilizes at the level of edge enhancement of 0.7 at a peak of 7 JND. According the Thurstone V model, this means that more than 99.99% of ENT professionals will perceive a difference between the optimum setting and the image without edge enhancement and vote consistently for this optimum.
Endoscope A and B reach an optimum at the levels of edge enhancement 0.75 and 0.90 with peak values of 3.8 JND (99.5%) and 4.5 JND (99.8%), respectively.
The general optimal level of edge enhancement is estimated
between 0.7 and 0.9. This corresponds to setting A5–A6 for endoscope A, 50%–75% for endoscope B, and 15 to 20 for endoscope C.
One image of endoscope B was excluded from the analysis at setting 62.5% as this image had superior positioning and illumination compared with the adjacent settings yielding a quality score deviation of 2 JND above trendline. This indicates that the differences between image quality as a result of edge enhancement are relatively small and other factors like positioning and illumination start playing a role.
Discussion
In this study, we quantified the level of edge enhancement from test images, measured the effect of edge enhancement on sharpness and image noise, and found the optimal setting by pairwise comparison for three different types of flexible ENT endoscopes.
Edge enhancement has a major impact on sharpness, noise, and the image quality as perceived by ENT professionals. We found optima of the trend lines at levels of edge enhancement between 0.7 and 0.9. Although the trend optima are in a relatively small range, the peak of the quality scores is relatively wide spread (Figure 3). For example, the difference in quality scores between the best five images of endoscope C varies by less than 1 JND, meaning that less than 50% of ENT professionals perceive a difference and vote consistently. This relatively wide peak is due to differences between observer preferences. When looking at the data of individual participants across different endoscopes, some observers prefer higher levels of edge enhancement whereas others tend to prefer more subtle levels. Even though individual preferences are present, the optimal setting is certainly not below a level of edge enhancement of 0.5 as smaller details will be perceived as too vague. Levels above 1.0 yield objectionably large edge enhancement artifacts and levels of noise. We think that users can select their setting of preference within the range of 0.5–1.0, without affecting their diagnostic accuracy, because the difference in image quality is within one JND.
In our experience, video processors can be distributed with suboptimal default settings and those settings cannot be motivated upon request. ENT professionals who are not using edge enhancement yet, can improve their endoscopic image
quality by using this feature that is readily available. We encourage ENT professionals to test the effect of edge enhancement for themselves and we recommend to read the manual or contact the local vendor for support when adjusting the edge enhancement settings. Edge enhancement becomes objectionable when either the artifact or image noise becomes too large. The artifact is easily identified at the vocal cords as it is a straight wellilluminated anatomical structure against the dark background of the trachea. The bright line along the edge of the vocal cord and the darker line along the other side of the edge are physically absent, but are image artifacts produced by edge enhancement. When edge enhancement is applied too strong, it may obscure details for example near the sinus of Morgagni. Blood vessel demarcation on the mucosa is facilitated with higher levels of edge enhancement. Image noise is present in the entire image and will be increased by edge enhancement as well. Image noise will become objectionable first in the darker subglottic areas compared with well-illuminated areas such as the vocal folds and ventricular folds. When observing live images or videos, observers will notice that noise has a dynamic behavior resulting in moving noise.
Edge enhancement should be taken into account when comparing image quality of endoscopes for procurement. In our previous study, 30 ENT professionals compared in vivo images of one larynx captured using the settings as recommended by the vendors. Twenty-eight observers preferred endoscope B (edge enhancement setting 50%) over endoscope A (structure enhancement setting A1).7 From the results presented above, however, we know that both systems have similar sharpness and noise characteristics. The results of the previous study would have been different, if structure enhancement of endoscope A was set to a higher value.
Edge enhancement can improve diagnostic accuracy by enhancing surface irregularities of laryngeal lesions, so they are depicted more clearly as described by Kawaida et al, who found differences in clinical diagnosis between edge enhancement switched off (A0) and on (A4 or A8).8, 9 Later, Scholman et al showed that the difference in overall sensitivity between the fiber optic endoscope and the high-definition endoscope they compared was 47.2% versus 59.7% when reviewed by experienced ENT specialists5 or 61% versus 66.3% when reviewed by a more heterogeneous group of ENT professionals.6 We think that the key difference between these endoscopes is sharpness, that is, the level of details that can be recorded. In our previous studies, we measured the sharpness of a fiber optic endoscope and several high-definition endoscopes. High-definition endoscopes have a better sharpness compared with fiber optic endoscopes, and sharpness can be improved by edge enhancement applied in the video processor.7 13 This may not be relevant for relatively large deviations, but will certainly improve visibility of pathologies with finer structures. This remains to be proven yet.
A limitation of this study is that we did not include images with pathology, and it might be possible that participants selected images that were more appealing, although they were explicitly asked to select the image with better image quality for diagnostic purposes. Therefore, our future work will be to study the relationship between sharpness and diagnostic accuracy.
Conclusion
Edge enhancement has a major impact on sharpness, image noise, and the resulting perceived image quality. This feature is readily available. We conclude that ENT professionals benefit from this video processing and should verify if their equipment is optimally configured.
References available on request
36 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
60 Second Summary
Progress in medical device innovation and manufacturing has contributed to the significant decline in death and disability from common medical conditions such as acute coronary syndrome and stroke and improved the quality of life of patients suffering from chronic conditions such as degenerative joint disease. Medical device development and manufacturing accounts for an increasing proportion of overall economic activity in Ireland. In parallel the regulation of medical devices in the European Union (EU) has undergone significant change in recent years.
Widely-reported cases of adverse outcome in significant numbers of patients treated with certain groups of high-risk implantable medical devices over the last 15 years – including breast implants, metal-onmetal hip implants, and vaginal meshes – has focused attention on deficiencies in the regulatory processes for medical devices and led to new legislation for regulation of the industry in the EU.
A recent systematic review of the evidence for high-risk medical devices approved in Europe shows significant deficiencies in the transparency and quality of evidence for selected devices used in cardiovascular medicine, orthopaedics and diabetes mellitus.
The new EU Medical Device Regulation (MDR) came into force in 2021 and represents a root-and-branch revision of all aspects of processes for the approval of medical devices and for the surveillance and safety monitoring of high-risk devices following approval. This article aims to summarise in plain language the key developments and current state-of-the-art in medical regulation for clinicians in practice.
AUTHORS:
Dr Rory Durand, Dr JJ Coughlan, Professor Robert A. Byrne
Affiliations: Dept. of Cardiology and Cardiovascular Research Institute (CVRI) Dublin, Mater Private Network and RCSI University of Medicine and Health Sciences, Dublin, Ireland.
The authors acknowledgment funding to RCSI University from the European Union Horizon 2020 project, Grant Agreement 965246.



1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
What doctors need to know about new processes for the regulatory approval of high risk medical devices
Introduction
Medical device development and manufacturing accounts for an increasing proportion of overall economic activity in Ireland.
Some estimates suggest that approximately one in every 100 to 120 workers in Ireland is employed in the medical device industry.
The number of workers in the medical device industry in Ireland per capita is many times higher than the European Union (EU) average and about three times as high as the EU country with the next highest ratio. In addition, for certain high-risk medical devices Ireland dominates global manufacturing capacity, with 80% of all coronary stents worldwide being manufactured in Ireland.
While progress in medical device innovation and manufacturing has contributed to the significant decline in death and disability from common medical conditions such as acute coronary syndrome, stroke and heart valve disease and improved the quality of life of millions of patients suffering from degenerative joint disease, some
controversy has surrounded the processes for approval of medical devices in Europe in recent years.1 Cases involving patient harm due to implantation of substandard breast implants, hip on hip implants and vaginal meshes have received widespread coverage in the lay media and has focused the attention of the medical and political communities at a national and European level (see Table 1). In 2018 a worldwide investigation by journalists released information from the so-called 'Implant Files' detailed a catalogue of problems with certain medical devices, which had resulted in adverse outcomes for patients. This resulted in coordinated front page headlines in newspapers around the world on 26th November that year (see Figure 1).
Partly as a response to these issues, as well as the realisation that historical processes, which had developed somewhat organically over recent decades, had become outdated, a review of the approval and certification process in Europe was initiated
as far back as 2008. This led to the drafting of new legislation and following negotiation and tripartite dialogue between the European Commission, European Parliament and member states represented via the Council of the European Union, the new Medical Device Regulation (MDR) was officially published in 2017.
In contrast to the older processes which were known as the Medical Device Directives (MDD) and had to be adopted individually by member states into law, the new processes were enshrined as a regulation – the MDR – which in common with other regulations, such as the general data protection regulation (GDPR), automatically becomes law in the individual member states of the EU. Due to the coronavirus pandemic, the regulation came into force in May 2021, one year later than had been planned at the time of publication of the legislation.
While the MDR has officially come into law in EU member states, the community finds itself now in a transition phase. This means that devices already approved under
37
CPD 106: MEDICAL DEVICES CPD Continuing Professional Development CPD
Dr Rory Durand
Dr JJ Coughlan Professor Robert A. Byrne
The case of drug eluting stents (2002-2007)
Drug-eluting stents dramatically reduced stent failure due to restenosis, the most common reason for need for re-intervention in the stenosed segment, when introduced into practice following CE mark approval in 2002. After two to three years of clinical experience a small increase in rare stent thrombosis many months and sometimes years after implantation started to become noticeable. This issue was addressed by iterative technological developments and second generation devices with more biocompatible coating polymers were not affected by this issue.
The PIP breast implant scandal (2001-2011)
Probably the best known example of adverse medical device effects, this case arguably owes more to fraud than regulatory failures per se – Poly Implant Protheses (PIP), the manufacturer of a widely used implant, substituting industrial grade silicone for medical grade silicon in its manufacturing.
The case of metal on metal hip implants ca 2010
Originally developed to have enhanced durability, metal on metal hip implants were found to have higher rates of implant failure due to loosening, local adverse tissue effects due to increased microparticulate debris and possible remote side effects due to release of metal ions into the bloodstream.
The case of vaginal mashes (2008-2018)
Poor outcomes in patients treated with transvaginal meshes for stress urinary incontinence and pelvic organ prolapse were attributed to clinical evidence deficiencies where a series of novel devices claimed equivalence to predicate devices without robust evidence. This led to performance creep with overall inferior outcomes with newer generation devices.
The case of bioresorbable coronary stents (2011-2017)
First-in-class self-degrading coronary stents (so-called bioresrobable stents or scaffolds) received CE mark approval in 2011 following publication of results from singe arm studies in selected low-risk populations. After a number of years of clinical use registry studies began to report slightly higher rates of a low frequency adverse event known as stent thrombosis. In the United States randomized clinical trial evidence was required for premarket approval by the FDA. Despite this higher evidential burden, following publication of a large-scale pivotal clinical trial in 2015 approval was granted in 2016. In the face of higher rates of stent thrombosis from additional independent studies and manufacturer trials in 2017 the manufacturer withdrew the device from the market.
the older MDD can remain on the market until the end of the transition phase. However, new devices must be approved under the MDR pathways. Because of some difficulties encountered in the implementation of MDR (see below), the European Commission has agreed to extend the transition phase for the highest risk medical devices for a number of years, up until 2029 for certain classes of devices.
Overview of the regulatory landscape for medical devices in Europe
While the introduction of MDR brought in a number of important changes in the approval of medical devices, some key elements of the approval process remained unchanged. The central steps involved in the process – known as a conformity assessment – are shown in Figure 2. As the name suggests this process evolved

as a legacy from the historical needs of device manufacturers to demonstrate adherence to common standards in the development and manufacture of their devices. Accordingly, the processes were often developed without direct input from practising clinicians or academia. This accounts in some respects for the lack of prominence given to assessment of clinical evaluation data under the MDD and the knock on effect that often high-risk medical devices were approved for use in Europe with a lesser quantity and quality of clinical trial data than was the case in other jurisdictions, such as the United States.
In simple terms, in Europe the manufacturer of a medical device is required to make a submission to a designated company known
as a notified body; the notified body will review the submission dossier and has the power to authorise the device, granting a CE certificate of conformity and facilitating market access in the European Union and other countries aligned with the EU regulatory system. The code designating the name of the notified body that approved the device is usually available on the package of the device directly under the CE mark. The work of the notified bodies is overseen by national competent authorities, the regulatory authorities in each individual member state. In Ireland's case this is the Health Products Regulatory Authority (HPRA). Competent authorities coordinate activities related to medical devices amongst their members in each EU country through a competent authority for medical devices coordination group. At a European Commission level, legislative and guidance roles as well as coordination of medical device regulation lies within the department of the directorate general for health (DG Sante).
Did you know
The four-digit code designating the name of the notified body that approved the device is available on the package of the device directly under the CE mark.
Medical device classification according to risk
The degree of clinical evidence that is required by a notified body in order for CE certification to be granted depends on the risk category of the device. High risk medical devices must have supportive data available from clinical investigations to accompany their application. Interestingly, the general burden of proof as part of a conformity assessment in Europe was that the device should be safe and perform technically as intended. Considerations in relation to efficacy were not central to the process. This differs from approaches used in the United States, where high risk medical devices seeking approval would be expected to have evidence supporting both safety and efficacy. A new concept introduced under EU MDR is that devices
38 CPD 106: MEDICAL DEVICES
Figure 1. Coordinated international newspaper headlines from 26th November 2018 on the release of the so-called Implant Files Front page reproduction of the Irish Times and the Sueddeutche Zeitung in Germany.
Table 1. Case examples due to medical device class failures
Figures and Figure Legends
Figure 1 Coordinated interna�onal newspaper headlines from 26th November 2018 on the release of the so-called Implant Files
Front page reproduc�on of the Irish Times and the Sueddeutche Zeitung in Germany.
should now be required to show evidence of clinical benefit, which may be interpreted to mean both safety and efficacy.
The system of rules that are used to classify any medical device into one of three risk categories is somewhat complicated to understand. In simple terms, however, devices are classified based on whether they are invasive or not, whether they are in contact with important body systems, such as circulatory or central nervous system, and whether or not the contact is transient, short-lived or permanent (as in the case of implants).
Examples of medical devices in each class are shown in the Table 2. The most commonly used highrisk medical devices in clinical practice are coronary stents, heart valves, pacemakers, and hip and knee implants. In recent years medical implants used for glucose monitoring and management of diabetes mellitus have also become common.
Problems with transparency and quality of clinical evidence for high-risk medical devices
We recently completed work as part of a European collaboration of stakeholders involved in medical device regulation, known as CORE-MD.2 This consortium was led by representatives of doctors professional societies, and also comprised representation from the European Commission, national regulatory authorities, notified bodies, patient organisations and public health authorities. Further details on the work of this project can be found at the website www.core-md.eu.
As part of this work, we surveyed the landscape of clinical evidence for high-risk medical devices approved for use in Europe, assessing the quality and transparency of evidence across three medical specialties, cardiovascular medicine, orthopaedics, and diabetes. Two reports have been published in the peer-reviewed literature.
For cardiovascular devices seven groups of Class III devices were predefined, encompassing 71 long term implantable devices marketed
Device class Descrip�on
Class I Low risk Non-electronic stethoscopes
Class IIa
Low to medium risk
Single use scalpels and single use scalpel blades


Class IIb Medium to high risk Indwelling urinary catheter for long term use
Transcatheter aor�c valve replacement device Note. Images sourced from biorender.com
Class III High risk
in the EU between the year 2000 and 2023.3 The identified groups were bioresorbable scaffolds, left atrium appendage closure devices, transcatheter aortic valve implantation systems, subcutaneous ICDs, surgical aortic and mitral valves, transcatheter mitral valve repair or replacement devices and leadless pacemakers.
The most commonly used devices, coronary stents, were not included as we had previously provided a detailed report on this area.4,5 Of the 308 identified studies, most were non-randomised (81%), 51% without a pre-registered study protocol and 61% without sufficient information on event adjudication. No randomised trial was performed prior to CE mark approval for any device.
A second systematic review was performed to give an overview of clinical investigations regarding hip and knee arthroplasty implants published in peer-reviewed journals before entry into force of the MDR.6 A random selection of 30 devices from the Orthopaedic
Data Evaluation Panel (ODEP) and registry reports from European countries were included between years 1995 and 2021. There were no clinical investigations published in peer reviewed literature for any of the selected devices prior to CE marking and on average, only 5 publications within 20 years of CE marking.
In aggregate the CORE-MD led systematic review of the literature for high risk cardiovascular, orthopaedic and diabetes mellitus devices emphasised major issues with the quality and transparency of evidence in these domains. There was a high degree of variability between quality of clinical evidence from published studies across the same device classes, different device classes and between different medical fields. The quality tended to be limited by small sample size and non-randomised studies without a comparator group or subgroup analyses. Usually publication occurred after CE marking.

What are the key changes introduced by the Medical Device Regulation
Some of the deficiencies in quality and transparency of clinical evidence for high-risk medical devices identified by the systematic reviews discussed above are addressed by the change in legislation under MDR. At a high level, the aim of the medical device regulation was to improve and further standardise processes for the evaluation of medical devices with the goals of balancing access to novel treatments and fostering of innovation against enhancing safety for patients and overall confidence in the regulatory system.
The key changes introduced by MDR may be summarized as follows:
• Stricter pre-market control of high-risk devices
3
• Strengthening of the requirements for clinical investigations of high risk medical devices prior to their approval
39
Table 2. Examples of medical devices in each class
Table 2. Examples of medical devices in each class
Example
Note. Images sourced from biorender.com
medical
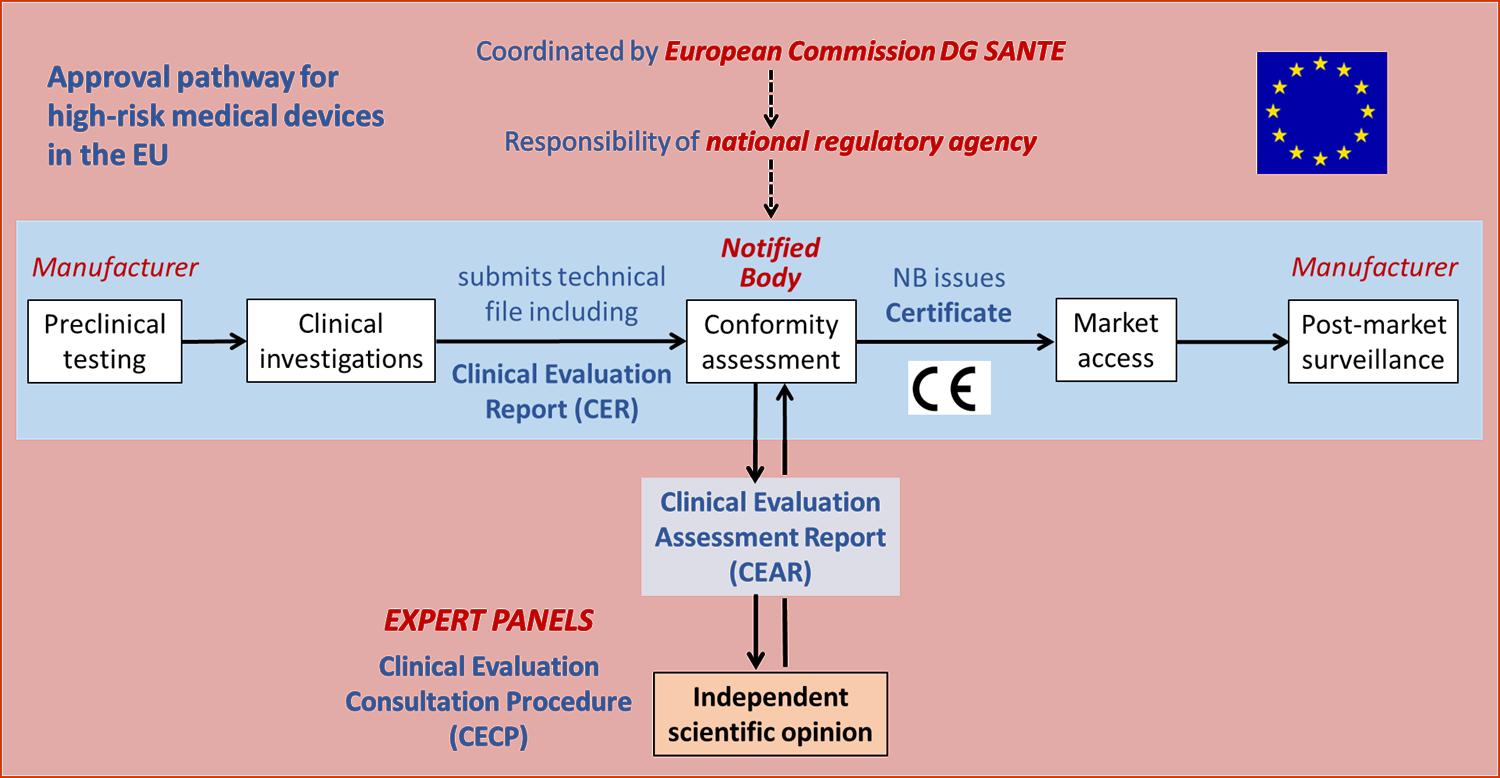
For description of the process see article text
For descrip�on of the process see ar�cle text.
• Establishment of expert panels of clinicians to review clinical evaluation assessment reports of notified bodies
• Enhanced scrutiny and monitoring of the work of notified bodies with requirement for redesignation of each notified body
• Increasing transparency by the development of a central EU database containing information on approved medical devices
• Enhancement of requirement for manufacturers for dedicated post-approval surveillance programmes for certain medical devices
• Improvements in safety reporting and notification of adverse device related events
• Improved device traceability system based on Unique Device Identification (UDI)
Fall out form the introduction of medical devices
The introduction of the new regulation has not been without controversy, and a number of problems in the implementation of the regulation have become apparent.
Firstly, only a small proportion of devices approved under MDD have received recertification under MDR. This has led to concerns that certain devices or groups of devices may disappear from the market, reducing treatment options for patients. The reasons for this are multifactorial but delays in recertification of notified bodies under the new more stringent regulations has played a role. Currently around 45 notified bodies have been redesignated successfully, whereas in the past there were more than 80 in operation.
Secondly, many device manufacturers have claimed that the new processes are overly burdensome in terms of paperwork and that in some cases the amount of clinical data that is required to support an application is unclear. One important development going forward would be the facilitation of an early dialogue process to enable manufacturers to receive direct feedback from regulators and notified bodies in relation to specifics of study design and size for the particular device in question. Such processes would probably result in improved predictability of
clinical investigation programs for manufacturers of high risk medical devices, while ensuring that the enhanced safety of patiences, that is the focus of MDR, is not compromised.
Thirdly, it has become clear that specific measures will need to be introduced for certain types of high-risk medical devices used to treat rare or orphan conditions which are frequently encountered in the treatment of paediatric patients. It is extremely challenging to conduct clinical trials in these settings and alternative approaches to clinical evaluation may be necessary in these cases.
What are a doctors’ responsibilities for safety reporting and vigilance Medical practitioners continue to play an important role in the regulation of high-risk medical devices. Clinicians may be involved in some or all of clinical audit, national registry reports or dedicated post-marketing surveillance studies. In Ireland adverse device events should be reported immediately to the responsible local hospital medical device or quality and safety committee, the device
manufacturer and the HPRA
(https://www.hpra.ie/homepage/ about-us/report-an-issue).
In recent years concerns about device performance in cardiovascular medicine were frequently first brought to light by individual clinicians or groups of clinicians using devices in daily practice (see case examples) and reporting results of case series or registries at specialty meetings and in the peer-reviewed literature.
Conclusions
The regulation of medical devices has undergone significant change in recent years. The publication of the Medical Device Regulation in 2017 represented the outcome of a root and branch review of the processes in Europe. The regulation became law in 2021 and introduces enhanced scrutiny of the regulation system and higher standards for clinical evidence which is particularly relevant for high-risk medical devices. Doctors continue to play a critical role in medical device vigilance, postmarket surveillance and safety event reporting.
References available on request
40 CPD 106: MEDICAL DEVICES
Figure 2. Overview of the approval pathway for high-risk medical devices in Europe
Figure 2. Overview of the approval pathway for high-risk
devices in Europe
UCC Researchers awarded Prestigious Funding

Understanding the role of the gut microbiome in the early postnatal brain function and development of newborns is amongst the University College Cork (UCC) projects to receive funding in the Marie Skłodowska-Curie Actions (MCSA) Postdoctoral Fellowship awards 2023.
Led by Dr Lorena Morales García, from APC Microbiome Ireland (APC), a world leading SFI Research Centre in the study of the microbiome, and the Department of Anatomy and Neuroscience in UCC, the AVALON project will investigate the changes in newborn brain development due to an altered early life microbiota as a result of C-section birth. This surgical intervention is lifesaving, and is commonly performed. Therefore, understanding the effect of C-section on the brain of the newborns is an urgent societal need.
Dr Morales García is one of 1,249 postdoctoral researchers announced by the European Commission in the results of the 2023 call for Marie SkłodowskaCurie Actions (MSCA) Postdoctoral Fellowships. MSCA Postdoctoral Fellowships aim to foster excellence through training and mobility and to equip researchers with new skills and competences to identify solutions to current and future challenges.
Among the new UCC research projects funded a combined ¤890,000 by the MSCA Postdoctoral Fellowship awards are:
Dr Vincent Oliviero (Macroscopic Quantum Matter Group, School of Physics)
Project Title: Sub-Nanoscale Observation of Magnetic Monopole Noise (SNOMAN)
Funding Amount: ¤199,695
The magnetic version of the electron, the Dirac magnetic monopole, although predicted to exist, has yet to be discovered. Recently, the search for emergent magnetic monopoles in quantum materials has focused on spinice compounds, which host magnetic monopoles as emergent quasiparticles. Even though emergent monopoles have been studied using the toolbox of quantum matter research, the long-awaited monopole detection is still lacking because of the limited low spatial resolution probes and temperature range of the experiments. The SNOMAN project is a plan to develop a novel concept called Scanned Spin-Noise Microscope and provide a cutting-edge instrument for realising a Sub-Nanoscale Observation of magnetic monopole Noise. The microscope will be designed to operate in a cryogenfree dilution refrigerator to slow down the monopole dynamics and realise the long-awaited single monopole detection.
Dr Jamie Darby (School of Biological Environmental and Earth Sciences)
Project Title: Biologging to inform the Prevention of Seabird bYcatch (BioPSy)
Funding Amount: ¤274,426
As the global footprint of fisheries increases to match market demands, interactions between seabirds and vessels become more frequent leading to scavenging opportunities for many species, but also increase bycatch risk. Many seabird populations are in rapid decline, and bycatch in fishing gears has been identified as a leading cause. BioPSy is a global project, where the candidate will develop skills pertinent to seabird bycatch
prevention in New Zealand, a country with an internationally recognised track record in applied conservation and seabird bycatch management. On the return phase, these skills will be applied to areas of Europe with severe seabird bycatch issues and little to no legislated corrective measures. Cutting-edge analyses will be developed and applied to seabird biologging data to design effective seabird bycatch prevention measures (SBPMs). BioPSy relies on stakeholder engagement for experimental design, with engagement skills and implementation focal point of the program.
Dr Vilas Patil (MicroNano Systems Centre, Tyndall National Institute)
Project Title: Gated Twisted 2D Superconductor Devices for Quantum Technologies (G-TScon2D)
Funding Amount: ¤215,534
G-TScon2D delves into the exciting realm of superconducting (SC) twistronics, where the manipulation of the twist angle between the layers has opened new avenues for investigating unconventional superconductivity, quantum transport, and emergent phenomena. A significant research gap exists in understanding and harnessing the unique properties of twisted 2D SC materials. G-TScon2D will bridge the scientific gap through a correlated experimental and simulation approach. Its technological advantage lies behind the inherent SC nature of the planned materials, which is expected to lead to higher operating temperature, a critical condition in quantum computing application.
Dr Lorena Morales García (APC Microbiome Ireland, Department of Anatomy and Neuroscience)
Project Title: Investigating the consequences of birth by Caesarean Section in hypothalamic and Amygdala neurovascular development (AVALON)
Funding Amount: ¤199,695
Early postnatal development is a vulnerable period during which environmental factors significantly influence brain development.
Among these factors, the gut microbiota has been found to impact brain function and development. However, there is a lack of mechanistic understanding regarding how changes in early-life microbiota composition can lead to brain dysfunction. AVALON aims to investigate the interactions between neonatal microbiota and neurovascular development in the hypothalamus and amygdala at molecular and cellular levels. I will utilize a C-section mouse model to disrupt early-life microbiota and assess its impact on neurovascular development through in-vivo tracer imaging and integrated multi-omics analysis. Additionally, AVALON will use a reporter mouse line to trace amygdala and hypothalamic neuronal populations. To ensure broad awareness of AVALON's outcomes, a dissemination strategy will be implemented, targeting scientific, public, and industry audiences. The strategy will aim to underscore the critical role of perinatal microbiota in shaping postnatal brain development.
Congratulating the four recipients from University College Cork on their awards, Professor John F. Cryan, UCC Vice President for Research and Innovation said: "Congratulations to these early-career researchers in receiving Marie Curie awards, in key areas which will address critical scientific, health, and environmental challenges. These awards will provide important support to these emerging researchers, enabling them to develop their track record and transition to become independent research leaders."
41 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Dr Farzan Gity (supervisor of MSCA awardee Dr Vilas Patil), Dr Vincent Oliviero, Dr Lorena Morales García, Dr Jamie Darby
News


Cardiology Focus Heart & Stroke
How heart and stroke patients are becoming Champions with the Irish Heart Foundation

“Developing Patient Champions, is an attempt to redress this, to empower, upskill, support & harness the patient experience and their own skills, to achieve better recognition, better representation, better conditions, better advocacy power and more equality in decision making, policy formation, policy design, implementation, evaluation and outcomes so that CVD patients can be better served in our society”
As healthcare professionals, the opportunity to see patients thrive after diagnosis is no doubt rewarding. But while medical therapies have advanced in the area of cardiovascular health, and are allowing patients diagnosed with life long, or life-limiting conditions, to live longer and sometimes with a better quality of life from a medical perspective, increasingly many heart and stroke patients have been reporting to the Irish Heart Foundation that there is a need for far more than just medical therapies, if they are to feel that they can really thrive in their lives post-diagnosis.
Discussions with both heart patients and stroke survivors in the Irish Heart Foundation Patient Support Services, reflected that in addition to medical management of their condition, many patients, particularly of working age, who had professional skills, but were no longer able to use them in a career setting due to their diagnosis, felt redundant, hopeless and lost postdiagnosis. In addition, with many reporting mental health challenges in coping with the isolation and loss of the life and lifestyle they knew as a result of their illness, and admitting to struggling with the reality of the implications
of their diagnosis, there was an emerging need being witnessed by the organisation that a more holistic approach to patient health and wellbeing post diagnosis was required, if these patients were to thrive.
Since Covid 19, online patient service options in the Irish Heart Foundation grew, as did accessibility to these from all parts of Ireland. As a result, more patients have accessed, or have been referred into our services by members of the medical profession. Referrals are often necessary for patients who need support, largely due to the lack of patient support services in their geographical communities. The growth in patients into the service saw an expanse in the Irish Heart Foundation Patient Support Service offerings to include the addition of counselling, mindfulness, wellbeing, back to work training, exercise classes, and a host of other ancillary supports, as well as it’s highly used Nurse line.
Inevitably this increase in patient numbers would result in a greater awareness by the organisation in the scale of patient
need at community level post hospital discharge, and with this knowledge came an increasing need for patient advocacy.
One such patient advocacy issue that occurred around this time was in relation to its heart failure patient community, regarding NIACs decision to offer two different levels of vaccine prioritisation to this population in Ireland based on age, which left the under 70s age group living with heart failure in Ireland at a lower level of vaccine priority, and therefore at greater risk of contracting Covid 19, not only because of the extra time period they had to wait to receive the vaccine, but also because many of this age group were cosharing households with younger cohorts who had returned to schools and colleges and therefore exposed them to further risk.
The campaign was a team effort between the organisation and the patients, and included media interviews at national and local level, conducted jointly by both organisation representatives and patients, lots of letter writing to Dept of Health, HSE and NIAC, lots of research and of course, lobbying politicians at both
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
42
Irish Heart Foundation Patient Champion graduates with Pauline O’Shea, Advocacy Campaign Manager, Irish Heart Foundation


patient representation in both the organisation and in healthcare areas who seek heart or stroke patient participation with regards to policy co-design, feedback, or evaluations in the area of cardiovascular health. In addition, ongoing peer and organisation support is offered, via frequent online meetings and campaign opportunities.
The programme is free, online and accessible from anywhere in the Ireland, so logistically a possibility for patients who may have challenges travelling due to the limitations of their diagnosis, something which patients reported had frequently been overlooked by way of their being able to get involved in social or work opportunities at community level post diagnosis.
From an organisation perspective, having the patient in the room, is proving to be a very useful tool in advocacy. Already our first graduates have gone on to advocate in many ways, not just for themselves but for other heart and stroke survivors e.g. at an Oireachtas Health Committee on cardiovascular funding, in media awareness interviews in relation to their conditions, and they have recently lobbied hard to their local TD’s about the post Budget ‘24 announcement that the national stroke strategy would not be funded, which has helped the charity’s national call for a reversal of this decision.
“Developing Patient Champions, is an attempt to redress this, to empower, upskill, support & harness the patient experience and their own skills, to achieve better recognition, better representation, better conditions, better advocacy power and more equality in decision making, policy formation, policy design, implementation, evaluation and outcomes so that CVD patients can be better served in our society.
“Patient Champions is about helping develop a patient movement in cardiovascular healthcare in Ireland, that when combined with the Irish Heart Foundation can become a powerful force for good in the cardiovascular landscape.”
And what do the patients themselves who have gone through the programme say?
One Patient Champion graduate, Michael, a heart patient living in Cavan says… “I certainly would recommend that others join the programme and get involved. I believe the whole experience can benefit you personally, but you can also give a lot more to other people who do not have a voice.”

national level by the organisation and by patient’s at local TD level in their individual constituencies, resulting in multiple references to the issue being made in the Dail. The experience led to a belief by the organisation that embracing patients into patient advocacy campaigns going forward would improve the efficacy of patient advocacy efforts.
It was decided that the organisation should embark on designing a specific advocacy programme for patients that would recruit, train and support them in the area of advocacy, communications and lobbying, as well as offer them ongoing inclusivity in the patient advocacy agenda of the organisation.
Therefore, in early 2023, the Irish Heart Foundation, launched its advocacy programme for patients, which it called Patient Champions. In line with the organisation’s
mission statement, to defend, empower and care for those affected by heart disease and stroke, the programme was to offer upskilling, ongoing support, opportunities and training in the area of advocacy, as well as peer advocacy support amongst the patient recruits themselves. In this way, it would offer opportunities for heart and stroke patients to not only feel empowered in their own lives, to be able to use their own skills and learn new skills, but to hopefully find purpose again. It would also introduce patients from all over the country to likeminded patients, journeying on a similar path, thereby providing a social support component which could help reduce the level of isolation felt by many.
The programme includes training in the area of media, digital marketing and politics, as well as opportunities for
And the Irish Heart Foundation’s Patient Champions’ programme is being viewed with interest at a European level, with the European Heart Network seeking its inclusion on the agenda for its annual workshop this May ‘24 in Amsterdam, to discuss the possibility of its take up by other heart foundation members in Europe.
Perhaps it is also worth mentioning that the programme itself is spearheaded by a heart patient, who works in the Irish Heart Foundation’s Patient Advocacy team.
Pauline O’Shea, Advocacy Campaign Manager with the Irish Heart Foundation explains;
“I think patients in today’s world, feel like women did 100 years ago… invisible, unequal, lowly ranked, not at the table, more spoken about than to, overlooked, and often left feeling diminished.
Another Patient Champion graduate, Meaghan, a stroke survivor living in Kerry says, “It’s been really helpful for me to get that formal training, to just gain more confidence, so that I can take on those different opportunities. So, I absolutely would recommend joining.”
If you know, or work with heart of stroke patients whom you think would enjoy joining the Patient Champions programme, please ask them to contact Pauline O’Shea on 087 381 0726, or they can apply directly online by emailing ihfvolunteer@ irishheart.ie with subject heading ‘Patient Champions’.
For anyone who would like to know more on this programme, including to watch a video with some of the Irish Heart Foundation’s recent Patient Champion graduates, please visit https://irishheart.ie/patientchampions/
For all other Irish Heart Foundation Patient Support Service offerings for heart and stroke patients, please go to https://irishheart.ie/ support-for-you/heart-supportservices/ or call the Nurse Support Line on 01 668 5001
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 43


Cardiology Focus Cardiac Rehab
Cardiac Rehabilitation: What Makes it Effective?

Cardiovascular disease (CVD) is the leading cause of morbidity, mortality and disability globally imposing a significant burden on individuals, families, and healthcare systems1. Each year in Ireland, over 4000 deaths are attributed to ischaemic heart disease.
The growing population of those living with chronic CVD, heart failure and valvular heart disease are at a higher risk of secondary events, therefore it is essential that these patients receive effective, evidence-based secondary prevention, leading to optimization of cardiovascular health, improved functional capacity and enhanced quality of life.
Written by Deirdre O'Reilly, Cardiac Rehabilitation Coordinator Roscommon University Hospital, Saolta Healthcare Group & President Irish Association of Cardiac Rehabilitation

For over 50 years cardiac rehabilitation (CR) is established as the proven cornerstone of optimal CVD management by reducing the risk of cardiovascular mortality and hospital readmissions, delivering improvements in patient outcomes and providing cost-effective care in these populations. 2,3,4,5
CR is a medically supervised, systematic, multidisciplinary programme offering internationally agreed core components across the continuum of care from the acute inpatient to longer term outpatient phase. 6
The key components which underpin the effectiveness of CR include supervised, guideline-
directed exercise training, patient education, behaviour modification, psychosocial management and comprehensive risk factor management. 6,7
Fully comprehensive CR, recommended by clinical practice guidelines internationally, requires these core components to achieve reductions in mortality and morbidity.8
Timely access is also critical and prompt CR enrolment is associated with both improved participation and outcomes. For example, CR participation is reduced by 1% for every one-day delay in enrolment9 , whereas early enrolment is linked with a 67%

greater improvement in exercise capacity among patients enrolled in a programme within 15 days after hospital discharge compared to patients enrolled 30 or more days following discharge. 10,11
Individualized CR Programme:
A comprehensive evaluation and assessment of clinical status, CVD history, guideline- directed medication therapy, cardiovascular risk factors, dietary habits, body composition, exercise habits and capacity, psychological health and quality of life forms the basis of an individually tailored CR programme. Although risk of an adverse event is low 12 there is agreement across CR guidelines that all patients entering CR should be stratified according to risk for the occurrence of adverse events during exercise and for risk of progression of atherosclerotic disease. 6,7,13
Exercise Training:
Exercise training is central to CR due to the robust evidence base demonstrating its role in reducing mortality and morbidity.14,15,16,17,18,19,20,21 Cardiorespiratory endurance training is recommended as the foundation of most exercise prescriptions for adults with CVD as it represents the most effective way to increase cardiorespiratory fitness which reduces mortality risk. 22 The aim of the exercise prescription is to achieve a safe but therapeutic level of exercise training and progression of exercise intensity. Establishing safe and effective exercise programming is rooted in physiological responses to exercise not solely on subjective indicators.
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
44
The first and only treatment indicated to reduce: • all-cause mortality
•
frequency of CV-related hospitalisations in patients with wild-type or hereditary ATTR-CM.1
ORAL VYNDAQEL
61MG SOFT CAPSULES
Indicated for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). 2
www.vyndaqel.ie
ATTR-CM=transthyretin amyloid cardiomyopathy; CV=cardiovascular.
References: 1. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. 2. VYNDAQEL Summary of Product Characteristics.
Vyndaqel▼ 61 mg soft capsules (tafamidis) Prescribing Information: Before prescribing Vyndaqel please refer to the full Summary of Product Characteristics.
Presentation: Vyndaqel 61 mg soft capsules. Each soft capsule contains 61 mg tafamidis. Uses: Vyndaqel is indicated for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). Dosage: Treatment should be initiated under the supervision of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. When there is a suspicion in patients presenting with specific medical history or signs of heart failure or cardiomyopathy, etiologic diagnosis must be done by a physician knowledgeable in the management of amyloidosis or cardiomyopathy to confirm ATTR-CM and exclude AL amyloidosis before starting Vyndaqel, using appropriate assessment tools such as: bone scintigraphy and blood/urine assessment, and/or histological assessment by biopsy, and transthyretin (TTR) genotyping to characterise as wild-type or hereditary. The recommended dose is one capsule of Vyndaqel 61 mg (tafamidis) orally once daily. Vyndaqel 61 mg (tafamidis) corresponds to 80 mg tafamidis meglumine, tafamidis and tafamidis meglumine are not interchangeable on a per mg basis. Vyndaqel should be started as early as possible in the disease course when the clinical benefit on disease progression could be more evident. Conversely, when amyloid-related cardiac damage is more advanced, such as in NYHA Class III, the decision to start or maintain treatment should be taken at the discretion of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. There are limited clinical data in patients with NYHA Class IV. If vomiting occurs after dosing, and the intact Vyndaqel capsule is identified, then an additional dose of Vyndaqel should be administered if possible. If no capsule is identified, then no additional dose is necessary, with resumption of dosing the next day as usual. There are no recommended dosage adjustments for elderly patients or patients with renal or mild and moderate hepatic impairment. Limited data are available in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min). Tafamidis has not been studied in patients with severe hepatic impairment and caution is recommended. There is no relevant use of tafamidis in the paediatric population. Method of Administration: The soft capsules should be swallowed whole and not crushed or cut. Vyndaqel may be taken with or without food.Contra-indications: Hypersensitivity to the active substance or to any of the excipients as listed in section 6.1 of SPC. Warnings and Precautions: Contraceptive measures should be used by women of childbearing potential during treatment with tafamidis and for one month after stopping treatment. Tafamidis should be added to the standard of care for the treatment of patients with transthyretin amyloidosis. Physicians should monitor patients and continue to assess the need for other therapy, including the need for organ transplantation, as part of this standard of care. As there are no data available regarding the use of tafamidis in organ transplantation, tafamidis should be discontinued in patients who undergo organ transplantation. Increase in liver function tests and decrease in thyroxine may occur. This medicinal product contains no more than 44 mg sorbitol in each capsule. Sorbitol is a source of fructose. The additive effect of concomitantly administered products containing
sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account. The content of sorbitol in medicinal products for oral use may affect the bioavailability of other medicinal products for oral use administered concomitantly. Pregnancy and Lactation: Tafamidis is not recommended during pregnancy and in women of childbearing potential not using contraception. Available data in animals have shown excretion of tafamidis in milk. A risk to the newborns/infants cannot be excluded. Vyndaqel should not be used during breastfeeding. Interactions: In a clinical study in healthy volunteers, 20 mg tafamidis meglumine did not induce or inhibit the cytochrome P450 enzyme CYP3A4. In vitro tafamidis inhibits the efflux transporter BCRP (breast cancer resistant protein) at the 61 mg/ day tafamidis dose with IC50=1.16 μM and may cause drug-drug interactions at clinically relevant concentrations with substrates of this transporter (e.g. methotrexate, rosuvastatin, imatinib). In a clinical study in healthy participants, the exposure of the BCRP substrate rosuvastatin increased approximately 2-fold following multiple doses of 61 mg tafamidis daily dosing. Likewise, tafamidis inhibits the uptake transporters OAT1 and OAT3 (organic anion transporters) with IC50=2.9 μM and IC50=2.36 μM, respectively, and may cause drug-drug interactions at clinically relevant concentrations with substrates of these transporters (e.g. non-steroidal anti-inflammatory drugs, bumetanide, furosemide, lamivudine, methotrexate, oseltamivir, tenofovir, ganciclovir, adefovir, cidofovir, zidovudine, zalcitabine). Based on in vitro data, the maximal predicted changes in AUC of OAT1 and OAT3 substrates were determined to be less than 1.25 for the tafamidis 61 mg dose, therefore, inhibition of OAT1 or OAT3 transporters by tafamidis is not expected to result in clinically significant interactions. No interaction studies have been performed evaluating the effect of other medicinal products on tafamidis. Undesirable Effects: The following adverse events were reported more often in 176 ATTR-CM patients treated with tafamidis meglumine 80 mg compared to placebo: flatulence [8 patients (4.5%) versus 3 patients (1.7%)] and liver function test increased [6 patients (3.4%) versus 2 patients (1.1%)]. A causal relationship has not been established. Safety data for tafamidis 61 mg are available from its open-label long-term extension study. Adverse reactions from cumulative clinical data in ATTR-CM participants: Common (> 1/100 to < 1/10) Diarrhoea, rash, pruritus. Legal category: S1A. Marketing Authorisation Numbers: EU/1/11/717/003–61mg (30 capsules). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500.
Last revised: 02/2023
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Ref: VY 61MG 3_0
PP-VYN-IRL-0206. Date of Preparation: May 2023. © 2022 Pfizer Inc. All rights reserved. ATTR-CMIS LIFE-THREATENING1
Further information available upon request.


43. Ghisi GLM, Rouleau F, Ross MK, Dufour-Doiron M, Belliveau SL, Brideau JR, Aultman C, Thomas S, Colella T, Oh P. Effectiveness of an Education Intervention Among Cardiac Rehabilitation Patients in Canada: A Multi-Site Study. CJC Open. 2020 Mar 4;2(4):214221. doi: 10.1016/j.cjco.2020.02.008. PMID: 32695971; PMCID: PMC7365818.
Cardiology Focus Cardiac Rehab
44. Medina-Inojosa JR, Grace SL, Supervia M, Stokin G, Bonikowske AR, Thomas R, LopezJimenez F. Dose of Cardiac Rehabilitation to Reduce Mortality and Morbidity: A Population-Based Study. J Am Heart Assoc. 2021 Oct 19;10(20):e021356. doi: 10.1161/JAHA.120.021356. Epub 2021 Oct 6. PMID: 34612055; PMCID: PMC8751887.
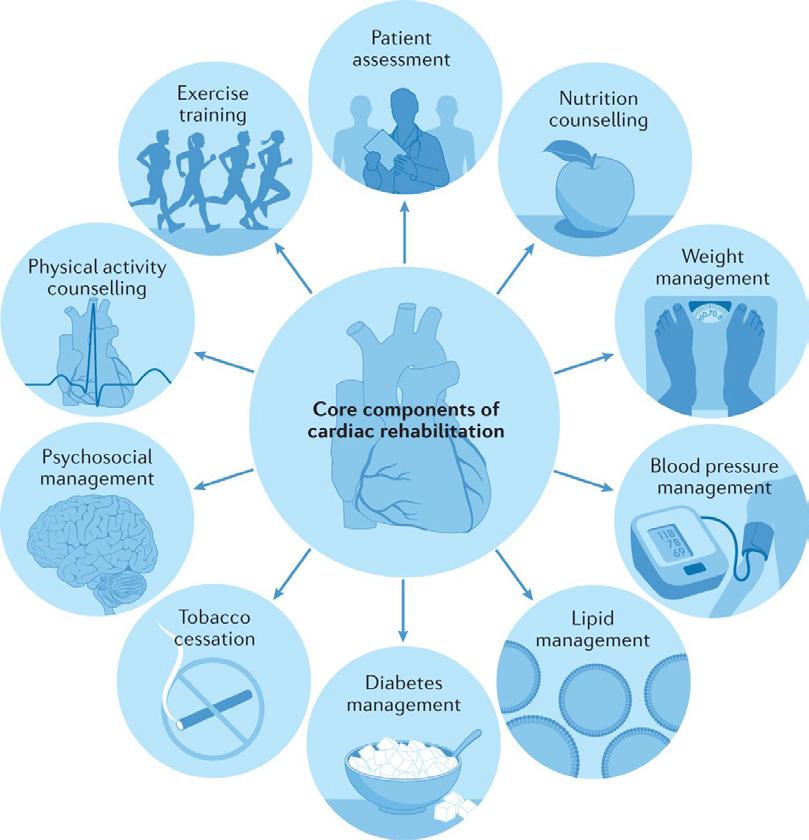
International consensus recommends an initial exercise prescription based on a functional capacity test which is symptom limited to assess exercise capacity, heart rate, blood pressure, occurrence of arrhythmias, and ECG response. Cardiopulmonary exercise testing is recognised as the gold–standard using either a bicycle ergometer or a graded treadmill exercise test, and conducted on regular medications.23,24,6
Exercise prescription is based on FITT -VP principles: Frequency, Intensity, duration (Time), Type Volume and Progression.25
CR clinical guidelines widely recommend 2-4 supervised exercise sessions per week complemented by unsupervised home sessions. 6,23,24,26,27 Intensity may vary between 40-80% of heart rate reserve (HRR)
depending on the patient’s profile and their stage of progression through the programme.
Perceived exertion should be in the moderate to moderate – to – high intensity range with higher-intensity interventions have been demonstrated to be safe and lead to superior outcomes. 28 Most CR guidelines specify up to 60mins per exercise session incorporating warm-up, cool down and resistance training. 6
Functional capacity (measured in Metabolic Equivalents - METs) achieved at CR completion is a stronger predictor of all-cause mortality than METs achieved at CR entry. 29 Progression of each patient’s exercise prescription is required to achieve the necessary cardiovascular adaptations leading to mortality benefit.22
Direct staff supervision should occur during exercise training until
a safe exercise response has been demonstrated, with a variety of modalities employed to monitor patients including continuous ECG monitoring. After an initial period this monitoring may decrease appropriately if there is no evidence of abnormal ECG or haemodynamic findings, abnormal signs and symptoms or intolerance of exercise. 6, 7
Comprehensive CVD Risk Factor Management:
CR programmes incorporate secondary CVD prevention and comprehensive risk factor management based on the clinical assessment at programme onset. Evidence based interventions are implemented to optimise risk factor control in alignment with patient-centred goals. In line with the principle of Making Every Contact Count 30, risk factor management encompasses the
following aspects:
• Lifestyle modifications
• Smoking Cessation
• Nutritional Counselling
• Weight management
• Hypertension Management
• Lipid Management
• Diabetes Management
• Psychosocial Care
• Medication Management: (i.e. reconciliation of prescription, treatment optimisation and / adherence)
• Clinical Management: Patients with cardiac conditions have a broad spectrum of risk of developing arrhythmias that is not easily discernible, and CR represents an ideal opportunity to recognise and manage silent ischaemia 31 and arrhythmia
• Continuity of Care: bridging cardiovascular care ensuring continuity of appropriate secondary prevention management.
Psychosocial Management: The psychological impact of heart disease is considerable, and psychological distress is highly prevalent in patients with CVD. Anxiety, depression and insomnia disorder affect approximately one-third of people with CVD, and up to one in four cardiac patients experience clinically significant levels of posttraumatic stress (PTSD)32,33,34,35. As a minimum, CR patients should be screened for clinically significant psychological distress as this is linked to increased future cardiac events and mortality, poorer quality of life, increased suicide risk, greater healthcare costs and poorer longterm psychological adjustment 32 However, many cardiac patients (e.g. survivors of sudden cardiac arrest, SCAD) also present with unique and complex psychological needs that are best delivered by an experienced clinician (e.g. devicerelated shock anxiety, PTSD) 36,37 Systematic reviews demonstrate that the psychological component drives the benefits achieved by CR, and that psychological interventions not only improve psychological distress and quality of life, but also reduce cardiac events and hospitalizations 4, 38,39,40 When fully integrated with CR, these interventions are also highly cost effective (e.g. group sessions) and deliver an incremental benefit on hard endpoints 41A cardiac psychologist working as an integral
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
46


part of the MDT will also provide expertise in weight management, psychosexual counselling, insomnia treatment, medication adherence, family support (e.g. caregiver burden in heart failure), maintaining lifestyle changes (e.g. exercise, smoking cessation) and enhanced communication with the cardiology team.
Patient Education and SelfManagement:
Education empowers patients to better understand their condition and associated treatment, and to learn effective self-management strategies. This enables them to make informed decisions and actively participate in their recovery process. The benefit of educational interventions delivered during CR is wellestablished 42,43 and shown to reduce mortality and morbidity 8 Effective educational interventions in CR ultimately foster selfefficacy and long-term adherence to healthy lifestyle behaviours.
CR Adherence and Exercise ‘Dose’:
The number of CR exercise sessions attended is inversely
related to major cardiovascular adverse events (MACE). This benefit appears to be linear, with greater risk reduction associated with higher doses, and no upper threshold. For example, , the risk of MACE is significantly lower both for patients completing a median of ≥12 sessions versus less, and those attending CR on average >2 sessions per week. Similarly, a continuous increase in attendance of 1 session is significantly associated with a 1-2% reduction in MACE risk with no ceiling to benefit. 44
In conclusion, for CR to be effective a multi-component and multi-disciplinary programme of care is required. While exercise training remains a central aspect of CR with strong evidence supporting its benefit, other components such as education and particularly psychosocial management are essential.8
The ongoing challenge is the implantation of this high-quality model in different settings, ensuring all eligible patients can access CR in a timely way.
References available on requust
Cardiology News
Heart disease research challenges ‘one size fits all’ aspirin guidelines
Heart disease researchers have identified a group of patients in whom international guidelines on aspirin use for heart health may not apply.
In a study published in the renowned medical journal Circulation, the findings of a review of data from three clinical trials challenge current best practice for use of the drug for primary prevention of heart disease or strokeotherwise known as atherosclerotic cardiovascular disease.
The research examined the results from clinical trials involving more than 47,000 patients in 10 countries, including the US, the UK and Australia, which were published in 2018.
The analysis focused on findings for a subgroup of 7,222 patients who were already taking aspirin before the three trials commenced. Those studied were at increased risk for cardiovascular disease and were taking aspirin to prevent the first occurrence of a heart attack or stroke.

The data showed a higher risk of heart disease or stroke – 12.5% versus 10.4% - for patients who were on aspirin before the trials and who then stopped, compared to those who stayed on the drug.
Analyses also found no significant statistical difference in the risk for major bleeding between the two groups of patients.
The research was led by Professor J. William McEvoy, Established Professor of Preventive Cardiology at University of Galway and Consultant Cardiologist at Saolta University Health Care Group, in collaboration with researchers in University of Tasmania and Monash University, Melbourne.
Professor McEvoy said, “We challenged the notion that aspirin discontinuation is a one-size-fitsall approach.”
The research team noted results from observational studies which suggest a 28% higher risk of heart disease or stroke among adults who were prescribed aspirin to reduce the risk for a first
heart attack or stroke, but who subsequently chose to stop taking the aspirin without being told to do so by their doctor.
Based in large part on three major clinical trials published in 2018, international guidelines no longer recommend the routine use of aspirin to prevent the first occurrence of heart attack or stroke. Importantly, aspirin remains recommended for high-risk adults who have already had a heart disease or stroke event, to reduce the risk of a second event.
The move away from primary prevention aspirin in recent guidelines is motivated by the increased risk of major bleeding seen with this common medication in the three trials, albeit major bleeding is relatively uncommon on aspirin and was most obvious only among trial participants who were started on aspirin during the trial, rather than those who were previously taking aspirin safely. These trials primarily tested the effect of starting aspirin among
adults who have not previously been treated with the drug to reduce the risk of atherosclerotic cardiovascular disease. Less is known about what to do in the common scenario of adults who are already safely taking aspirin for primary prevention.
He added, “Our findings of the benefit of aspirin in reducing heart disease or stroke without an excess risk of bleeding in some patients could be due to the fact that adults already taking aspirin without a prior bleeding problem are inherently lower risk for a future bleeding problem from the medication. Therefore, they seem to get more of the benefits of aspirin with less of the risks.
“These results are hypothesisgenerating, but at present are the best available data. Until further evidence becomes available, it seems reasonable that persons already safely treated with lowdose aspirin for primary prevention may continue to do so, unless new risk factors for aspirin-related bleeding develop.”
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 47


Cardiology Focus Abstracts
Towards a greater understanding of reduced response to aspirin in children with congenital heart disease post-cardiac surgery using immature platelet fraction
Written by Irene E. Regana, e, g, Dermot Coxb, Sean T. Kelleherc, Beatrice Nolana, Kathryn Shawd, Owen P. Smitha and Colin J. McMahonc, e, f, g
aDepartment of Coagulation/Haematology, Children's Health Ireland at Crumlin, Dublin, Ireland
bSchool of Pharmacy and Biomolecular Sciences, Royal College of Surgeons Ireland, Dublin, Ireland
cDepartment of Paediatric Cardiology, Children's Health Ireland at Crumlin, Dublin, Ireland
dDepartment of Paediatric Pharmacy, Children's Health Ireland at Crumlin, Dublin, Ireland
eSchool of Medicine, University College Dublin, Belfield, Dublin 4, Ireland
fSchool of Health Professions Education (SHE), Maastricht University, Maastricht, Netherlands
gNational Children's Research Centre, Children's Health Ireland, Dublin, Ireland
Highlights
• Inadequate response to aspirin occurs in approximately 38 % of paediatric patients undergoing specific high-risk congenital cardiac procedures.
• An elevated platelet turnover as measured by IPF may render aspirin less effective in patients that are recently post-surgery.
• This patient cohort has an increased risk for thromboembolic events.
• Greater surveillance in paediatric patients post-cardiac surgery on aspirin monotherapy is warranted.
Objective
A high platelet turnover rate may produce a population of platelets that confers an inadequate response to aspirin. We aimed to investigate the relationship between residual platelet aggregation and platelet turnover in paediatric cardiology patients on aspirin monotherapy by evaluating the fraction of immature platelets as a marker for turnover and secondly to test the predictive value of the immature platelet fraction (IPF) to classify patients as responsive or non-responsive to aspirin.
Methods
Sixty patients divided into two age categories (≤90 days, >90 days of age) were included in this prospective observational study.
Distinct Inflammatory Phenotypes Are Associated With Subclinical and Clinical Cardiovascular Disease in People With Human Immunodeficiency Virus
Written by Padraig McGettrick, Willard Tinago, Julie O’Brien, Sarah Miles, Leo Lawler, Alejandro Garcia-Leon, Niall Mahon, John Lambert, Gerard Sheehan, Alan Landay, Caroline A Sabin, Aoife G Cotter, Patrick W G Mallon, for the HIV Understanding the Pathology of Comorbid Disease in HIV-Infected Individuals With Coronary Artery Disease (UPBEAT) Study Group and the All Ireland Infectious Diseases (AIID) Cohort Study
Abstract
Patients were then stratified into tertiles using their IPF level. Platelet studies included thromboelastography with platelet mapping (TEGPM).
Results
The overall incidence of ‘inadequate response to aspirin’ was 38 % in our patient cohort recently post-cardiac surgery a consequence that warrants further study. The frequency of inadequate response to aspirin was higher in the upper tertile of IPF when compared to the lower tertile, (88 %) versus (4 %) respectively (p < 0.05). The ‘cut off’ for IPF was determined to be 3.9 % with a sensitivity of 95.7 %, and a specificity of 92.9 % (area under the curve of 0.955 [CI 0.896–1.014, p < 0.05]).
Conclusion
This study demonstrates that inadequate response to aspirin occurs in approximately 38 % of patients undergoing specific high-risk congenital cardiac procedures using the dosing practice of a national centre. This study supports the hypothesis that an elevated platelet turnover may result in aspirin being less effective in patients who are recently post cardiac surgery. These data are of direct translational relevance.
Despite inflammation being implicated in cardiovascular disease (CVD) in people with human immunodeficiency virus (PWH), considerable heterogeneity within populations of PWH exists. Stratifying CVD risk based on inflammatory phenotype could play an important role. Using principal component analyses and unsupervised hierarchical clustering, we examined 38 biomarkers to identify inflammatory phenotypes in 2 independent cohorts of PWH. We identified 3 distinct inflammatory clusters present in both cohorts that were associated with altered risk of both subclinical CVD (cohort 1) and prevalent clinical CVD (cohort 2) after adjusting for CVD risk factors. These data support precision medicine approaches to enhance CVD risk assessment in PWH.
Previous studies have reported inflammatory phenotypes in cohorts of treated PWH similar
to those identified in our analysis [10]; however none have been compared to subclinical and clinical CVD. In an analysis of the Pharmacokinetic and Clinical Observations in People Over Fifty (POPPY) cohort, the systemically inflamed/gut epithelial dysfunction grouping was associated with higher estimated CVD risk, again supporting our findings highlighting this phenotype as a particularly high-risk group.
Our study has limitations. Although independent cohorts, both analyses were cross-sectional, limiting causality determination and assessment of associations over time. The relatively low prevalence of CVD reduced precision of association estimates despite incorporating propensity score matching to reduce the need for extensive adjustments. Biomarker analysis was conducted on cryopreserved rather than fresh samples; however, quality assurance and control measures were strictly observed to minimize cell death and protein degradation. Finally, although we explored the impact of variables on inflammatory cluster composition, there remains the possibility of unmeasured confounding.
In conclusion, this study has identified clinically relevant inflammatory phenotypes in 2 independent cohorts of people with HIV that are associated with both subclinical CAD and prevalent CVD events. These results provide valuable insights into distinct inflammatory pathways contributing to pathogenesis of CAD in people with HIV and provide evidence for a precision medicine–based approach to enhance CVD risk prediction and future CVD preventive strategies.
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
48
Striving for a life uninterrupted


IT KEEPS GOING SO THEY CAN TOO*1,2
For COPD patients on treatment with ICS/LABA or LAMA/LABA and at risk of an exacerbation**1
TRELEGY Ellipta shows superiority vs. non-Ellipta Multiple Inhaler Triple Therapy in a randomized real-world trial in everyday practice3
* Patients with moderate-to-severe COPD not adequately treated by a combination of ICS/LABA, or LAMA/LABA.1
** A worsening of symptoms or a history of exacerbations treated with antibiotics or oral corticosteroids in the past 12 months.
ICS, inhaled corticosteroid; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist
Safety information
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: headache, nasopharyngitis, influenza, upper respiratory tract infection, pneumonia, back pain, rhinitis, cough, pharyngitis and arthralgia.1
In common with other corticosteroid-containing medicines, there is an increased risk of pneumonia in patients with COPD receiving TRELEGY Ellipta.1
TRELEGY Ellipta should be used with caution in patients with unstable or life-threatening cardiovascular disease.1
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain.1
References: 1. TRELEGY Ellipta SmPC, 2023. Available at www.medicines.ie. Accessed June 2023. 2. Lipson DA et al. Am J Respir Crit Care Med 2017; 196:438–446. 3. Halpin DMG;ERJ Open Research;2021;7;1-11.
Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) Prescribing information. Please consult the full Summary of Product Characteristics (SmPC) before prescribing.

or request a visit from a GSK representative
Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg), umeclidinium bromide (UMEC) 62.5 micrograms and vilanterol as trifenatate (VI) 25 mcg provides a delivered dose of 92 mcg FF, 55 mcg UMEC and 22 mcg VI. Indications: Maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long-acting ß2-agonist (LABA) or a combination of a LABA and a long acting muscarinic antagonist. Dosage and administration: One inhalation once daily at the same time each day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate & magnesium stearate). Precautions: Paradoxical bronchospasm, unstable or life-threatening cardiovascular disease or heart rhythm abnormalities, convulsive disorders or thyrotoxicosis, pulmonary tuberculosis or patients with chronic or untreated infections, narrow-angle glaucoma, urinary retention, risk factors for urinary retention, hypokalaemia, patients predisposed to low levels of serum potassium, diabetes mellitus. In patients with moderate to severe hepatic impairment patients should be monitored for systemic corticosteroid-related adverse reactions. Eye symptoms such as blurred vision may be due to underlying serious conditions such as cataract, glaucoma or central serous chorioretinopathy (CSCR); consider referral to ophthalmologist. Increased incidence of pneumonia has been observed in patients with COPD receiving inhaled corticosteroids. Risk factors for pneumonia include: current smokers, old age, patients with a history of prior pneumonia, patients with a low body mass index and severe COPD. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take Trelegy. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Systemic effects: Systemic effects of ICSs may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Interactions with other medicinal products: Caution should be exercised with concurrent use of ß-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products), hypokalaemic treatments or non-potassium-sparing diuretics. Co-administration with other long-acting muscarinic antagonists or long acting ß2-adrenergic agonists is not recommended. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, arthralgia, back pain. Uncommon (≥1/1,000 to <1/100): viral respiratory tract infection, dysgeusia, vision blurred, glaucoma, eye pain, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, dysphonia, dry mouth, fractures. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and rash, intraocular pressure increased, urinary retention, dysuria. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA No. [EU/1/17/1236/002]. Legal category: POM B. Last date of revision: February 2023. Code: PI-6725. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.
Adverse
reported directly to the Health Products Regulatory Authority (HPRA)
their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
Find out more here: www.trelegy.ie ©2023 GSK Group of Companies or its licensor Trademarks are owned by or licensed to the GSK Group of Companies PM-IE-FVU-ADVT-230001 | June 2023 TRELEGY Ellipta was developed in collaboration with
events should be
on


Subclinical Atrial
Cardiology Focus
Atrial Fibrillation
Fibrillation
– where are we now?


Introduction
Atrial Fibrillation (AF) is the most prevalent cardiac arrhythmia in the world with an estimated 50 million people worldwide living with the rhythm in 2020.1 It is expected that, by 2060 in the EU, over 14 million aged 65 years and over will have an AF diagnosis and that the lifetime of risk of someone a of EU descent aged over 55 years of developing AF in their lifetime is 37%, i.e. one in three.2 Whilst there are many aetiologies for atrial fibrillation, including valve disease, hypertension, heart failure with reduced or preserved ejection fraction (HFrEF or HFpEF), the rhythm is associated with a significant increased risk of mortality and morbidity. AF is associated with a 1.5-3.5fold increased risk of all-cause mortality, a 5-fold increased risk of stroke with AF accounting for 20-30% of all ischaemic strokes and an annual hospitalisation rate of 10-40%.2
The overall management of atrial fibrillation can be neatly summarised by either the SOS or ABC mnemonic quoted in the American or European Guidelines respectively.1,2 In the SOS nomenclature
• S – assessment and treatment of Stroke risk
• O – Optimisation of all modifiable risk factors
• S – Symptom management with either a rhythm or rate control strategy
The European ABC scheme emphasises similar points
• A – Avoid stroke
• B – Better symptom control
• C – Cardiovascular risk factor and concomitant disease optimisation
Prescribing of oral anticoagulation in patients who meet the threshold using the CHA2DS2-VASc score
Written by Paul Nolan, Clinical Lecturer Atlantic Technological University, Sligo & Cardiac Physiologist, Galway University Hospital
aims to reduce stroke risk with modifiable risk factors including obesity, smoking, alcohol consumption, increased exercise as well as good control of diabetes and hypertension.
Clinical AF vs Subclinical AF vs Device Detected AF
Clinical atrial fibrillation is that which is documented on 12-lead ECG, or an ECG rhythm strip of ≥30 seconds in either a symptomatic or asymptomatic patient. Often patients present for ECG due to symptoms, such as palpitations or dyspnoea, associated with their atrial fibrillation.
Subclinical atrial fibrillation can be defined as atrial fibrillation identified in a patient, with no previous history or ECG evidence of atrial fibrillation. The detection of this subclinical atrial fibrillation includes documentation by wearables and implanted cardiac devices. Because some wearables and consumer targeted single lead ECG devices, such as the Kardia device and app, can provide ECG evidence of atrial fibrillation with strips of 30s, the distinction between clinical and subclinical AF is becoming blurred.
Implanted devices such as pacemakers or implantable cardioverter defibrillators (ICDs) have advanced diagnostic features that can automatically record atrial high-rate episodes (AHREs) and such events are sometimes referred to as device detected atrial fibrillation but are also referred to as subclinical AF. For us to diagnose device detected AF from AHREs it is important that the stored device tracings are
inspected to ensure they are true events and not artefactual.
Smartwatches and handheld ECG devices
There are number of handheld ECG devices available, typically marketed as patient owned devices, but they are also used by healthcare professionals in a clinical environment. These devices typically record a live single channel ECG trace, which then can be stored on a digital device, such as a phone or tablet, via an accompanying app. Some of these devices will give an interpretation of the rhythm, such as “possible arial fibrillation”, some with sensitivities of 98% and specificity of 97%.3
Many smartwatches or fitness trackers collect heart rate data and can flag up possible atrial fibrillation. These devices do not use ECG based technology but rather photoplethysmography (PPG). This is a clinically wellestablished technology, used in pulse oximeters. PPG detects pulse rate based on changes in the reflection of light, emitted from the back of the smartwatch, by the skin. The amount of light reflected changes in proportion to the about of blood under the skin, which changes with each pulse, thus the device can detect both heart rate and assess regularity. Some higher end smart watches such as the Apple or Samsung watch allow recording of single lead ECG by incorporating small electrodes in the back and side of the watch.
PPG based technology is easily integrated into devices and underpins apps, such as Fibricheck, which use a camera phone to check for possible atrial fibrillation. While this technology works well at rest there is some evidence that when subjects are ambulatory that motion artefact reduces the sensitivity and specificity.4 Further affecting the sensitivity for detection of AF, some older PPG based devices do not continuously assess rhythm regularity but sample for a minute every two hours, thus regularity is only checked for 12 minutes in a 24-hour window.
The incidence of irregular rhythm/ suspected AF alerts in smart watches is low. The Apple Heart Study monitored 419297 patients over 8 months and
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
50
irregular rhythm notifications were identified in only 0.52% of subjects. 450 of these patients had 7-day ECG based monitoring with 34% demonstrating clinical AF.5 It should be noted that with handheld ECG asymptomatic paroxysmal atrial fibrillation can be missed and with wearable technology, pick up rates will be dependent on how long the device is worn.
European guidance6 on the use of digital devices recommends that if PPG based monitoring is indicative of AF, an ECG-based method should be used to confirm the diagnosis of AF. If ECG evidence of atrial fibrillation is obtained, then the patient should be managed using the SOS or ABC approach outlined above. Whilst the European guidance states that PPG-based or ECG-based devices are preferred to pulse palpation for AF screening, this is contradicted by NICE guidance from 2019 which states there is not enough evidence to recommend the routine use of single lead ECG devices to detect AF in patients with an irregular pulse.7
Patient owned devices have the potential to increase the detection of atrial fibrillation, although the reported incidence has been low in populations using them, and prompt them to seek medical advice. The true clinical consequence of these asymptomatic episodes, particularly if paroxysmal, are not fully clear. The main randomised
trial which over 7000 participants, with no history of AF recorded twice daily handheld ECG over a 14-day period demonstrated a small 4% relative risk reduction in the primary combined endpoint of ischaemic or haemorrhagic stroke, systemic embolism, bleeding leading to hospitalisation, and all-cause death, over a median follow-up of 6.9 years, in those where AF was documented, and anticoagulation was commenced.18
Device detected atrial fibrillation
There is over 15 years of data demonstrating that implanted cardiac devices record AHREs or subclinical atrial fibrillation. However, many of these trials used different rate and duration cut-offs in their definitions. What we do know is that subclinical AF is common in patients ≥65 years, with the ASSERT II trial showing an incidence of an incidence rate of 34.4% per person-year for episodes >5mins.8 This study, using implanted loop recorders, also showed that the rate of detection for episodes ≥30 minutes was 21.8%/y, ≥6 hours was 7.1%/y and ≥24 hours, 2.7%/y.
With subclinical AF being common and of varying duration the question is what burden of device detected atrial fibrillation increases the risk of stroke. The TRENDS Study,9 which looked at AHREs in over 3000 pacemaker or ICD patients with and without a prior history of AF, demonstrated that a daily AF burden of ≥ 5.5hrs was associated with 2.2-fold

increased risk of stroke (95%
CI 0.96-5.05, p=0.06), although this was not quite statistically significant. It is interesting to note that this increased risk of stroke is less that the 5-fold increased risk of clinical AF.
Data like this supported the original European Heart Rhythm consensus in 201710 recommending oral anticoagulation in patients with a CHA2DS2-VASc ≥2 and a daily AHRE duration of ≥5.5hrs. However, a later analysis of the original ASSERT Trial data,11 demonstrated that subclinical AF duration >24 h was associated with a significantly increased risk of subsequent stroke or systemic embolism. The 2023 ACC/AHA/ ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation1 take the approach of combining AHRE daily burden and CHA2DS2-VASc in a decision spectrum. They recommend oral anticoagulation if the CHA2DS2-VASc ≥2 for men and ≥3 for women, the AHRE burden is ≥23.5 and AF is confirmed of the certainty is high. This left a large cohort with either shorter durations and high CHA2DS2-VASc scores or longer durations but lower scores. It was hoped, at the time of publication of these guidelines, that two, soon to be reported, randomised clinical trials, NOAH-AFNET 6 and ARTESIA, investigating the efficacy and safety of oral anticoagulation for device detected AF would inform this gap.
NOAH-AFNET 6 and ARTESIA
NOAH-AFNET 612 enrolled 2536 patient, aged over 65, with device detected AHREs >170bpm for >6 mins and at least one other risk factor for stroke. Patients were randomised to either edoxaban or placebo with patients in the placebo arm receiving aspirin if indicated. The primary outcome was a composite of CV death, stroke or systemic embolism with a safety outcome of death from any cause or major bleed.
The mean age was 78, with median AHRE durations of 2.8 hours and CHA2DS2-VASc scores of 4. There was no statistically significant difference in the primary efficacy outcome between the oral anticoagulation and placebo arm. Whilst there was a 35% relative risk reduction in a secondary end point of ischaemic stroke or
systolic embolism in the group receiving anticoagulation, this did not reach statistical significance (CI 39%-107%). There was an increased risk the primary safety outcome being met with anticoagulation, HR 1.31 (95% CI 1.02-1.67) with 2.1-fold increased risk of major bleeding. This led to the trial being terminated early due to safety concerns and futility and led the authors to conclude, that despite the relatively high-risk population, that the rate of stroke was lower than similar populations and it was appropriate to withhold anticoagulation.
ARTESIA13 enrolled patients over 55 years of age with a CHA2DS2VASc scores ≥3 or patients with a history of stroke over 75 years and were randomised to apixaban or placebo. The placebo in this case was aspirin as this was commonly used at the time of the trial design. Patients had to have device detected AHREs ≥6 mins but unlike NOAH-AFNET 6 episodes >24 hours were removed from the trial medication and commenced on indicated anticoagulation.
The mean age of 76.8 across the 4012 patients enrolled was similar to NOAH-AFNET 6, with a similar stroke risk, mean CHA2DS2VASc score of 3.9. Apixaban significantly reduced the risk of stroke or systemic embolism with a HR of 0.63 (CI 0.45-0.88) as well as ischaemic stroke. There was no increase in haemorrhagic stroke, death from any cause or cardiovascular death. Consistent with the finding from NOAHAFNET 6 there was an increase in major bleeding with a HR of 1.36 (CI 1.01-1.82) but no increase in fatal bleeds. ARTESIA measured stroke severity using a modified Rankin scale with anticoagulation giving a 49% relative risk reduction in fatal or disabling stroke (CI 29-88%).
Both NOAH-AFNET 6 and ARTESIA found an increased risk of major bleeds in patients who are anticoagulated for device detected AHREs. However, they have divergent results in terms of benefit with ARTESIA demonstrating a 37% relative risk reduction in stroke or systolic embolism, but NOAH-AFNET 6 showed no reduction its primary end point. There were several differences in the trials, for example the inclusion of cardiovascular death as part of primary end point of NOAH-AFNET 6 potentially diluted a signal for stroke reduction as only 10% of CV deaths were likely to be due to stroke.14 Indeed, a significant limitation of NOAH-AFNET 6 was the low event rate, combined

HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 51
Typical patient owned ECG based recording device and app


Cardiology Focus
Atrial Fibrillation
with the early termination of the trial resulted in the trial being underpowered to detect a small benefit in stroke reduction.
Another important finding across both studies is that 1 in 5 of patients with device detected AF went on to develop either episodes >24hr or clinical atrial fibrillation. Whilst a sub study of NOAH-AFNET 6 did investigate the benefit of oral anticoagulation in patients with AHREs >24hrs, there was no benefit, although the low event rates are a limitation with only 2 strokes per patient year in each group.15 Interestingly, 29.3% of patients with AHREs >24 hours developed clinical AF. So, at a minimum, device detected AF is a predictor of the development of clinical atrial fibrillation and episodes >24 hours are a stronger predictor.
A meta-analysis of the two trials,16 over 6,500 patients, which was published last year demonstrated that oral anticoagulation reduced the risk of ischaemic stroke,
relative risk 0.68 (CI 0.5-0.92). It also reduced a composite of cardiovascular death, all cause stroke, peripheral arterial embolism, myocardial infarction, or pulmonary embolism with a relative risk of 0.85 (CI 0.73-1.0). Not surprisingly this benefit was at a cost of an increased risk of major bleeding, relative risk 1.62 (CI 1.05-2.5).
As healthcare professionals we may perceive the stroke reduction versus increased bleeding risk as an equipoise, almost like comparing apples and apples. However, the patient perspective is different and the literature suggests they compare them as apples and oranges. A metaanalysis of patient perspectives on oral anticoagulation showed that patients were willing to accept higher bleeding risks if a certain threshold in stroke risk reduction could be reached and that the preferences of patients may differ from the perspective of clinicians.17 The fact that, in ARTESIA, major bleeds were managed conservatively, including transfusion, in 90% of cases but there was a 47% relative risk reduction in disabling or fatal stroke is an important element
to consider when using this new data to make decisions around anticoagulation of device detected AF.14
Conclusion
Prevalence of clinical atrial fibrillation is increasing and there is clear guideline on appropriate management. The increase in patient owned devices and implanted cardiac devices, such as pacemakers, has increased our detection of subclinical atrial fibrillation and there are remaining questions about how to best manage these.
The incidence of AF amongst patient with smart watches appear to be low and if AF is flagged through PPG technology, this must be confirmed with ECG based monitoring. If a patient owned device flags up an AF alert and can record a confirmatory ECG strip of 30s, then this meets the threshold for ECG evidence, particularly if reviewed by a clinician. There does remain a question about how much AF meets a threshold for anticoagulation.
For device detected AF, the two randomised trials tell us that the incidence of stroke is lower than
for clinical AF at 1-1.2% per patient year, however in ARTESIA
43% of the stroke in the placebo arm were fatal or disabling.
Anticoagulation reduces the risk of ischaemic stroke across both trials and the risk of fatal or disabling stroke significantly. The bleeding risk was significantly increased but 90% were managed without need for surgical intervention.
For device detected AF a 1-1.2% per year stroke rate is the threshold for anticoagulation on the CHA2DS2-VASc score, although it should be noted that this was developed based on clinical AF. Some commentators state that the results tell us that there should be a clear discussion with patients regarding the potential benefits and risks of anticoagulation.14 If a decision is made not to commence anticoagulation, then given that one in five patients will develop episodes >24hrs or clinical AF, there should be careful monitoring for further episodes or the development of clinical atrial fibrillation. This is a task ideally suited to remote monitoring, where alerts can be set for various AHRE daily burdens.
References available on request
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
52
Example of a stored episode of atrial fibrillation detected by an implanted Cardiac Resynchronisation Device (CRT-D)


ONOURS Hospital Professional 2024
The Hospital Professional Honours will take place on Saturday, 14th September 2024 in the Radisson Blu Hotel, Dublin. Entries are now open across a number of categories including:
Haematology Project of the Year
Young Hospital Pharmacist of the Year
Galapagos Biotech Ltd, an Alfasigma company, Multidisciplinary Team of the Year
Fresenius Kabi Innovation in Aseptic Compounding
Medisource Hospital Pharmacy Technician of the Year
Grünenthal Advancing the Standard of Care in Pain Management
Athlone Pharmaceuticals Hospital Pharmacy Team of the Year
Excellence in Respiratory
Viatris Excellence in Cardiovascular Initiative
Pharmasource Hospital Pharmacist of the Year
MSD Excellence in Oncology Initiative
MSL Healthcare Innovation and Service Development
Excellence in Patient Safety
GSK ViiV Infectious Diseases Project of Year
Consultant-Led Team of the Year
To Enter:
For an entry form or for further information please contact Danielle Norton at: danielle@hospitalprofessionalnews.ie
Entry Deadline: Friday, April 26th, 2024
Hospital Professional Honours – Saturday 14th September, 2024 – Radisson Blu Dublin











Cardiology Focus Conference
Heart patients set to receive treatment tailored to their genetic and health information
An innovative project using artificial intelligence (AI) to personalise therapies for patients with cardiovascular disease has kicked off at a meeting in Utrecht, the Netherlands.
The NextGen project has received ¤7.6 million from the EU’s Horizon Europe programme and will be delivered by a 21-member consortium, including the European Society of Cardiology (ESC).
Cardiovascular diseases (CVDs) are the leading cause of death globally, accounting for nearly 18 million fatalities every year.
In the EU, CVDs are responsible for approximately one in three deaths.3 CVD comes with a high price, and is estimated to cost the EU ¤282 billion annually, equivalent to 2% of Europe’s GDP.4 CVD also takes its toll on individuals, often leading to disability, absence from work, premature retirement, and absenteeism.
Personalised medicine, whereby prevention and treatment of disease is tailored to an individual’s unique genetic make-up and health information, holds promise for shifting the dial on the burden of CVD. Now is the time to harness the potential of individualised treatment. Genetic information is more readily available than ever before as the cost of laboratory analysis continues to fall, and cutting edge AI techniques make it possible to combine vast amounts of data in record time.
NextGen will capitalise on these trends by bringing together clinical research organisations, universities, small and mediumsized enterprises (SMEs), and professional associations to integrate multiple sources of data on individual people. This work is complex due to data privacy and governance requirements, the presence of multiple standards across Europe, varying formats of data, and the sheer volume of information.
The first step will be to map out the initiatives already underway to ensure that the project is truly ground-breaking and meets an unmet need. Consortium members will then develop novel tools to merge different types of data in a secure way that upholds individual privacy and allows

the information to be used in research. The effectiveness of the methods for removing current barriers to data integration in CVD will be demonstrated in real-world pilot studies.
The work will complement the 1+ Million Genomes initiative, which aims to enable secure access to genomics and clinical data across Europe, and the European Health Data Space, a European Commission governance framework for the safe and secure exchange, use and reuse of health data.
Consortium member Professor Panos Deloukas of Queen Mary University of London, UK, said: “This is a tremendous opportunity and a challenge we have in building the right toolbox that will allow [us] to unite CVD patient data across Europe and implement precision medicine to improve cardiovascular healthcare.”
Project co-ordinator Professor Pim van der Harst of University Medical Center Utrecht, the Netherlands, said: “No two people are exactly the same, and so it makes sense that each person needs a slightly different strategy to optimise their health. Personalised medicine is therefore the way forward for preventing heart disease, speeding up diagnosis, and monitoring and treating
The trial is re-evaluating the role of ICD implantation in post-myocardial infarction patients in the context of contemporary medical treatment and will provide vital new information to optimally guide therapy and address this serious health issue.”
people with CVD. To develop individualised therapies, we need to compile as much information as possible about individuals, and that’s where NextGen comes in. The unique picture we generate will then form the basis for improving cardiovascular health and wellbeing.”
Results of AFFECT-EU Project
People at risk should be tested for atrial fibrillation every time they attend a health appointment, according to results of the AFFECTEU project, which is holding its final event today in Brussels, Belgium. Patients at high risk of the disorder, such as those with heart failure or prior stroke, should be invited for a screening test.

Scientific coordinator Professor Renate Schnabel of the University Hospital Hamburg-Eppendorf, Germany said: “Screening for atrial fibrillation can identify undiagnosed atrial fibrillation so that the condition can be managed according to guidelines, including starting anticoagulation medication to prevent strokes. AFFECT-EU has concluded that opportunistic screening, where at-risk groups are screened when they contact the healthcare system, plus targeting patients at particular risk, may be a productive and cost-effective way to implement screening across Europe.”
Atrial fibrillation is the most common heart rhythm disorder globally.1 The number of adults aged 55 years and older living with the condition in the European Union is expected to more than double from 8.8 million in 2010 to 17.9 million by 2060.2 People with atrial fibrillation are up to five times more likely to have a stroke than their healthy peers.1 The disorder often has no symptoms and remains undiagnosed until a stroke occurs.3,4
The four-year, EU-funded AFFECT-EU project brought together healthcare professionals, patient representatives, payers, and industry in a consortium of 26 partners, including the European Society of Cardiology (ESC), to define a feasible atrial fibrillation screening strategy for healthcare systems across Europe, with the ultimate aim of preventing subsequent strokes and premature death.
The ability of atrial fibrillation screening to reduce strokes was
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
54
Professor Panos Deloukas of Queen Mary University of London
Professor Panos Deloukas of Queen Mary University of London
identified by the consortium in a contemporary meta-analysis in 35,836 participants.5 A further study by the consortium in 11 European countries found that there were no national screening programmes, and most atrial fibrillation was detected in patients with symptoms.6 But in a survey conducted by the group in 18 European countries, GPs said screening for atrial fibrillation was nearly as important as screening for common cancers.7
A subsequent analysis by project members demonstrated that screening result in savings of stroke-related costs regardless of the method (e.g. opportunistic or targeted). Project members then developed a budget impact analysis calculator that can be used by health regulators and payers to estimate the financial impact of implementing a screening programme over a fiveyear period.
Regarding who is at elevated risk of atrial fibrillation or stroke and should therefore be screened, studies by consortium members identified that the following are risk factors: increasing age, obesity, high blood pressure, and high blood levels of N-terminal pro B-type natriuretic peptide (NT-proBNP), which is commonly tested to diagnose heart failure.8-13
Project manager Daniel Engler of the University Hospital HamburgEppendorf, Germany said: “People with atrial fibrillation are more
likely to be severely disabled or die from a stroke or heart failure than those without atrial fibrillation,14 making prevention an imperative to reduce morbidity and maintain a high quality of life.
AFFECT-EU has paved the way for well-implemented atrial fibrillation screening programmes to increase the number of new diagnoses, leading to guideline-adherent care, thereby reducing stroke risk and atrial fibrillation disease burden.”
Trial to Prevent Sudden Death after a Heart Attack
The first clinical trial to challenge the routine implantation of a defibrillator in myocardial infarction survivors with heart failure has enrolled its first patient. The PROFID EHRA trial is part of the EU-funded PROFID project, which aims to personalise the prevention of sudden cardiac death after myocardial infarction and involves a consortium of 21 multidisciplinary partners including the European Society of Cardiology (ESC).
Sudden cardiac death is a major public health problem responsible for approximately one in five fatalities in Europe. Most sudden cardiac deaths occur in myocardial infarction survivors. To prevent these deaths, patients whose heart pumps less well than it should following a heart attack currently receive an implantable cardioverter defibrillator (ICD). However, modern drug treatments have been shown to lower the risk

of sudden death in these patients, thereby reducing the need for lifesaving ICD shocks.1-5
Dr. Nikolaos Dagres, chief investigator of the trial, said: “The PROFID EHRA trial is set to influence clinical practice around the world by closing a huge evidence gap that has existed for the past 20 years. The trial is re-evaluating the role of ICD implantation in post-myocardial infarction patients in the context of contemporary medical treatment and will provide vital new information to optimally guide therapy and address this serious health issue.”
The study will test whether in post-myocardial infarction patients with symptomatic heart failure and reduced left ventricular ejection fraction (35% or less) drug treatment alone is not inferior to drug treatment plus an ICD for preventing sudden death in heart attack survivors with heart failure and a reduced pump function.
Professor Gerhard Hindricks, chief investigator of the trial, said: “PROFID EHRA is a groundbreaking study that could change the prevention of sudden cardiac death in clinical practice. Currently, many patients who receive an ICD never need one, while some who could benefit miss out. This trial will provide novel, randomised evidence on which patients should receive a defibrillator, and which patients can be spared an unnecessary procedure which
typically requires an overnight stay in hospital and may lead to complications or unintended shocks from the device.”
The trial will recruit some 3,595 patients from 180 hospitals in 13 countries – namely Austria, Belgium, Czechia, Denmark, France, Germany, Hungary, Israel, Poland, Spain, Sweden, the Netherlands, and the UK. The first patient was enrolled from the Heart Centre Segeberger Kliniken in Germany.
Participants are being randomly allocated to 1) optimal medical therapy alone or 2) optimal medical therapy plus ICD implantation. Participants will be followed up for around 2.5 years for the primary outcome of all-cause death. The investigators will also examine the impact of the two treatment strategies on death from cardiovascular causes, sudden cardiac death, hospital readmissions for cardiovascular causes, length of stay in hospital, quality of life, and cost effectiveness. The study is due to last for approximately 49 months, with results expected in early 2027.
Professor Jose L. Merino, EHRA president and national coordinator of the PROFID EHRA trial in Spain, said: “The PROFID EHRA trial is set to redefine the use of ICDs in myocardial infarction survivors, and is therefore a very important scientific study for the European Heart Rhythm Association (EHRA) and for clinical practice globally.”


HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 55


Cardiology Focus
Aortic Dissection
Acute Aortic Dissection- A “Real” Cardiothoracic Emergency
Written by Professor Alan Soo, Cardiothoracic Surgeon, University Hospital Galway and Tara Byrne, Advanced Nurse Practitioner, Cardiothoracic department, Adjunct Clinical Lecturer, Department of nursing University of Limerick Faculty of Education and Health Sciences
Acute Aortic Dissection- A “Real” Cardiothoracic Emergency.

Introduction
Introduction

at the branches of the heart whereas type B dissection involves the lower aorta.4
Written by Dr Alan Soo, Cardiothoracic Surgeon, University Hospital Galway and Tara Byrne, Advanced Nurse Practitioner, Adjunct Clinical Lecturer in the Department of Nursing Studies & Midwifery, Faculty of Education and Health Sciences, University of Limerick
Risk Factors
International studies across Europe, North and South America and Asia indicate the age of risk to vary from 40- 75 years with favour towards increases occurrence in males. There is evidence to suggest that China has the youngest age associated with aortic dissection and this is correlated to being approximately 10-15 years younger than other areas;5 however, this is noted to be approximate as often cases are diagnosed during post mortem.
King George II suffered a collapse and died from a type A aortic dissection with pericardial tamponade, the first incidence of aortic dissection ever documented in the literature
King George II suffered a collapse and died from a type A aortic dissection with pericardial tamponade, the first incidence of aortic dissection ever documented in the literature.
Aortic dissection involving the ascending and descending aorta is associated with high morbidity and mortality rate.
of the clinical characteristics of aortic dissection as a timely referral for cardiothoracic intervention could potentially be a matter of life or death.
Pathophysiology
Genetic disorders such as connective tissue diseases that decrease the structural stability of the aorta wall, such as Marfan syndrome and Ehlers-Danlos syndrome predispose the aorta to dissection and account for 20% of cases.6 Genetic testing and screening are available but require investment and improvement.5
chest pain, which radiates from one to the other with no evidence of an acute cardiac event on ECG. On occasions there may be a recent history of strenuous exercise or the use of recreational drugs which is followed by sudden pain, in this instance, dissection should be included in the initial differentials.6 Along with strokelike symptoms such as abrupt speech difficulties, sight loss, and weakness on one side of the body, individuals may also feel dyspnoea, loss of consciousness, and shortness of breath. The symptoms can vary depending on the level of the dissection and the associated branch that is potentially mal perfused due to the presence of a false lumen. It should be understood that aortic dissection can sometimes happen without any signs.4
Diagnostics
Aortic dissection involving the ascending and descending aorta is associated with high morbidity and mortality rate. The aortic dissection charitably trusts states that aortic dissection claims the lives of approximately 2,000 persons in the UK and Ireland annually; in contrast, car accidents claim the lives of 1,870 people. The incidence of aortic dissection has risen sharply in the older adult population and is reported to be 8.6/100,000 in ages 60 to 80 years and 32/100,000 for people aged over 80 years(1) The statistics also indicate that physicians correctly suspect a dissection in as few as 15%-43% of cases who proceed to have a verified ascending aortic dissection(2), if left untreated the mortality rate is as high as 50% in the first 48 hours of symptom onset. Physicians must be aware of the clinical characteristics of aortic dissection as a timely referral for cardiothoracic intervention could potentially be a matter of life or death.
The aortic dissection charitably trusts states that aortic dissection claims the lives of approximately 2,000 persons in the UK and Ireland annually; in contrast, car accidents claim the lives of 1,870 people. The incidence of aortic dissection has risen sharply in the older adult population and is reported to be 8.6/100,000 in ages 60 to 80 years and 32/100,000 for people aged over 80 years.1 The statistics also indicate that physicians correctly suspect a dissection in as few as 15%-43% of cases who proceed to have a verified ascending aortic dissection,2 if left untreated the mortality rate is as high as 50% in the first 48 hours of symptom onset. Physicians must be aware

33% are misdiagnoised.


The aorta is the largest artery in the body, which facilitates the transfer of oxygenated blood from the left ventricle to all the organs in the body via the renal arteries, iliac arteries, inferior mesenteric artery, superior mesenteric artery, and celiac artery.3 Three layers make up the aorta wall: the thick, muscular-elastic tunica media, the outer fibrous tunica adventitia, and the thin tunica intima, which faces the bloodstream. Aortic dissection is a potentially fatal disorder that arises from a rupture in the aortic intimal layer, leading to haemorrhage within the aortic wall and eventual layer separation and complete dissection. The types of dissection vary characterised by the level of the dissection within the aorta itself. Aortic dissection is classified into type A or type B. Type A aortic dissection occurs when the tear in the aorta occurs at the ascending part of the aorta

2000 people per year loose their life in the UK and Ireland
Modifiable risk factors for aortic dissection as a result of prolonged stress on the aorta wall include longstanding hypertension, obesity, smoking, dyslipidaemia, substance abuse such as cocaine use, treatment of infections such as syphilis and TB.6
Other associated risks of dissection include trauma from fall or car accident and there are some associated iatrogenic factors such as cross-clamping, side clamping, graft anastomosis and patch aortaplasty6,7 or the presence of a developed aortic aneurysm.
Signs and Symptoms
Unfortunately, presentations are diverse hence the difficulty in achieving a timely diagnosis. The most common symptoms are sudden onset of severe back or
While there are no biomarkers as such to aid the diagnosis of an acute dissection there is some evidence to support the use of a D-Dimer. Aorta dissection is said to be associated with increased levels of soluble elastin fragments (sELAF), tenascin-C, smooth muscle myosin heavy chain (sm-MHC), and fibrinogen/ fibrin degradation products, which results in a raised D-Dimer level.8, 9, 10 Theoretically, because the false lumen is exposed in thoracic aorta dissection, the D-dimer would be higher, as the endothelialization of the false lumen would prevent a substantial increase in D-dimer following chronic thoracic aortic dissection, however, there are no confirmed reference ranges.
The use of diagnostic imaging is the most beneficial to clinicians in the diagnosis of aortic dissection and it guides the decision re classification, location of dissection and ultimately




11% of all maternal deaths


10 lives per week could be saved with timely diagnosis


APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
56
Professor Alan Soo
Tara Byrne

the level of urgency required. Currently, transthoracic echo (TTE), transoesophageal echo (TOE), magnetic resonance imaging (MRI), and CT are the primary diagnostic imaging modalities used. The primary reasons for using contrast-enhanced CT angiography are its accessibility, speed, and non-invasiveness.6, 11

Management6
Pulmonary embolus
Patient with chest pain
Blood biomarkers, ECG
Urgent CT scan
Triple rule out CT if intermediate pretest probability of CAD
Negative initial imaging, high clinical suspicion –add TTE
Aortic dissection
Stanford type A
Stanford type B
Acute coronary syndrome
Complications
• Aortic rupture
• End-organ ischaemia
• Continuing pain and hypertension despite full medical therapy
• Early false lumen expansion
• Large single entry
The conservative and surgical management of an acute aorta dissection is identified based on the classification and severity of the dissection. Initial management should aim to obtain blood pressure control and limit fluctuations in pressure with the use of intravenous beta blockers. Medications should be adjusted to maintain a systolic pressure of 100-120mm with a heart rate of 60- 80 beats per minute.6 Type A aortic dissections with the presence of a non-thrombosed false lumen should be considered for emergency surgery. The goal of prompt surgical intervention is to cut off the entry into the false lumen and restore the aortic true lumen using a synthetic interposition graft, either with or without coronary artery re-implantation; however, the primary focus of the surgery is to save a life, therefore, the procedure selection requires an experienced judgement of the anatomical requirements.12
Conclusion
Despite advancements in medicine, acute aortic dissection is a complex life-threatening medical emergency with a recently confirmed mortality rate of 51.4%.13 A high clinical suspicion is imperative to ensure timely identification, investigation and treatment. Clinicians must consider the possibility of dissection in patients who present with chest pain and also be aware that in certain instances aortic dissection may be present with no symptoms. It may soon be possible to anticipate and, to some extent, prevent acute aortic dissection due to advances in our understanding of genetic risk and propensity.
References available on request
Open surgery after initial risk assessment
Uncomplicated: Medical treatment
Complicated: Endovascular treatment
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 57
4 | Page
Management (6)


Cardiology Focus Optimising Care
Optimising Medicines in Cardiovascular Care: A Multidisciplinary Approach
Written by Alison Cahill, RNP, Clinical Nurse Manager II (Cardiac Rehabilitation), Beaumont HospitalSadhbh Ni Cheallaigh, Senior Pharmacist (Cardiology), Beaumont Hospital and Jonathan Gallagher, Senior Psychologist, Cardiology (Cardiac
Rehabilitation), Beaumont Hospital
Medication Adherence in Cardiovascular Disease (CVD)
Medication adherence is a complex multifactorial process, with problems arising when either (a) the medical team fail to start or intensify guidelinerecommended treatment (therapeutic inertia); or (b) when the patient’s actual intake of medication is sub-optimal.1
Although patient adherence to prescribed medication can vary and wane over time,2 adherence to cardiovascular medications is crucial for risk reduction following a cardiac event, and medication non-adherence is consistently associated with poorer clinical outcomes and subsequent re-hospitalizations.3,4
Despite the therapeutic benefits, many patients do not persistently adhere to their prescribed cardiovascular medications.5
A recent large meta-analysis estimated that approximately one third of patients after a cardiac event are nonadherent, irrespective of the cardiac medication prescribed.6
Similarly, the European Society of Cardiology (ESC) state that adherence to medication remains sub-optimal, ranging from 50% in primary prevention to
66% in secondary prevention.7 Additionally, 9% of atherosclerotic cardiovascular disease (ASCVD) events in Europe are estimated to occur as a result of sub-optimal medication adherence.7
Cardiac Rehabilitation (CR)
Cardiac rehabilitation (CR) has been identified as an opportune environment to optimise pharmacological therapy & improve long term outcomes in cardiac patients. This model of care for secondary prevention is recognised by the ESC as a Class 1A recommendation to improve outcomes for patients with ASCVD and heart failure.8 CR is primarily delivered in an outpatient setting by a multi-disciplinary team (MDT) of healthcare professionals coordinated by specialist nurses. Several CR core components have been identified which favourably impact mortality and morbidity,9 including patient assessment, blood pressure, weight, diabetes and lipid management, tobacco cessation, psychosocial care, physical activity and nutritional counselling.9 Each of these components may also necessitate the prescription of medications for cardiovascular protection and disease prevention.

CR: an ideal setting to optimise treatment adherence
Due to its systematic process of care delivery, collaborative team approach and emphasis on continuity of care, CR is viewed as the ideal setting to optimise adherence in patients with CVD. For example, a recent crosssectional study of medication adherence in patients with CVD identified that two factorspositive beliefs about medication and the ability to refill medicationswere independently predictive of greater cardiac medication adherence, and the authors concluded that cardiac nurses were ideal candidates to deliver such a service.10 Registered Nurse
Prescribers (RNPs) in particular can greatly enhance service provision. In a review of the role of nurse prescribers, Wilson and colleagues identified this practice as safe, cost-effective, and necessary, resulting in “health system and healthcare efficacy gains” and patients receiving “assured, timely and rapid access to needed care”.11 Clinical pharmacist intervention is also strongly linked with improved cardiac outcomes through drug optimisation, avoidance of adverse events, medication reconciliation and patient education.12
Beaumont Hospital’s CR Programme
Our dedicated multi-disciplinary team - comprising of specialist cardiac nurses, nurse prescriber, dietitian, pharmacist and smoking cessation specialist - provides comprehensive care to individuals affected by heart disease. Additionally, the psychologist’s role is fully integrated within the CR programme to provide another facet of comprehensive cardiac care. As well as providing psychological care, the psychologist’s remit extends to targeting ‘normal’ psychological processes underpinning health behaviour change and treatment adherence during CR. Integrated
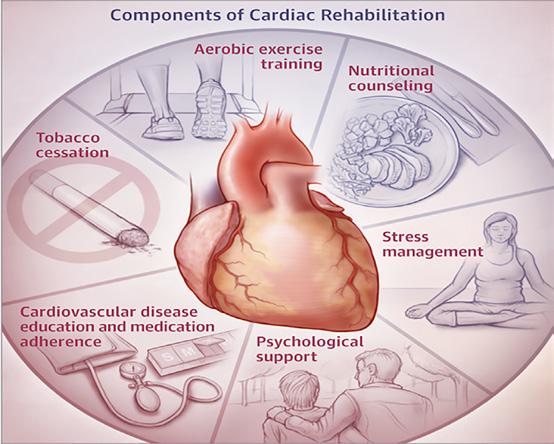
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
58
care is ensured by seamless cooperation between MDT members, and the Medical Director (consultant cardiologist) supervises the quality of care provided by the CR team.
CR patients typically attend a 10-week supervised exercise circuit programme (30 exercise sessions) in conjunction with 1-2 group education/self-management sessions per week delivered by either a nurse, dietitian, pharmacist or psychologist. Close monitoring of blood pressure, lipids and glycaemic status is also conducted throughout CR. All patients have their medications reviewed during CR and approximately one in three have their medications adjusted during the programme.13 Interestingly, exercise attendance during CR has been shown to serve as a ‘carrier’ for other important health behaviours. Similar to the dose-response relationship between exercise and mortality,14 greater exercise adherence in CR is associated with greater improvements in (objectively measured) adherence to cardiovascular medications.15,16
The Registered Nurse Prescriber (RNP) in CR
Legislation giving prescriptive authority to nurses and midwives in Ireland was introduced in 2007. Registration is awarded by the regulatory body of the Nursing and Midwifery Board of Ireland (NMBI) when candidates meet the required criteria. The practice standards and guidelines for Nurses and Midwives with Prescriptive Authority from the NMBI (2019) outline the legislation requirements and provide the foundation on which to build an appropriate scope of practice whilst maintaining professional competency.
During CR, risk factor assessment is conducted for each participant with optimal medical treatment as an end goal. This presents the nurse prescriber with optimal conditions to ensure patients are receiving the best possible medical therapy for their diagnosis. The National Institute for Health and Care Excellence (NICE) guidelines for medicines outline the importance of patient involvement in decisionmaking and how to support patients who are prescribed medicines.17 Non-adherence to medications can result in deteriorating health, limited response to medications that are taken and cost ramifications. NICE outlines the duty of the prescriber to help patients by implementing key principles
such as adopting a “no-blame” approach, involving patients in decision making, reviewing medications & communicating between healthcare professionals.
For the RNP in a CR setting these principles are easily applied and continuity of care facilitates review in a timely manner. Accordingly, a recent study by Thomson and colleagues found that by addressing medication adherence early in CR the odds of treatment persistence at 6 months increased by 13.5 times.18
Working within a multi-disciplinary service is particularly conducive to safe and effective prescribing.
For example, a review by Peterson and colleagues concluded that for patients with heart failure, reduced hospitalizations may be the result of an MDT disease management programme, whereby increased access to the team by patients resulted in high quality, evidencebased care through education, close follow-up and medication titration as required.19
In addition to the MDT members outlined above, the wider CR community also incorporates Advanced Nurse Practitioners (ANPs) in a variety of related roles such as chest pain management, heart failure, and arrhythmia management. This facilitates excellent clinical leadership, and the support of the MDT enhances collaborative practice, governance, and risk management.
For the new Nurse Prescriber, the responsibility of prescribing for patients can be daunting. Safe prescribing for the patient whilst maintaining professional standards is of paramount importance. To optimise the safety of prescribing in CR certain elements and practices should be integral to the scope of practice. Establishing the correct environment is important. For clinical decision making, a review of patient medical notes, an open discussion with the patient and a physical examination need to be conducted. Appropriate space that allows for confidentiality is also a requirement. Evaluation and interpretation of test results prior to prescribing will ensure appropriate prescriptive authority, and this may even include the discontinuing of medications for a patient. The scope of practice is determined through consultation and agreement with the CR Medical Director (Consultant Cardiologist).
As Beaumont Hospital’s rehabilitation unit is located within an acute hospital this information is often readily to hand for the team. Thus the practice standard

of managing the medication cycle appropriately is easy to implement. As an out-patient department there are no drugs stored within this setting and the prescription of medication can be clearly separated from administration as patients are given the prescription to fill at home.
For the RNP, evaluation of prescribing practice through the medium of audit is a requirement in this setting, and forms part of the scope of practice framework document. National audits are also available for use co-ordinated by the Office of the Nursing and Midwifery Services Director (ONMSD). Auditing allows for the review of prescriber practices and can help identify areas of treatment below the necessary requirements for best practice. For example, if an audit of practice revealed that most RNP prescriptions are for lipid-lowering statin therapy as patients are not at desired targets, this may prompt a review of the current practices of the medical team’s prescribing of these drugs at discharge. Audit also allows the RNP to review prescribing practices and minimise risk, for example, by determining if the writing of the prescription meets the practice standards of the NMBI.20
The Pharmacist in CR
The day-to-day work of the clinical pharmacist assigned to Cardiology in Beaumont Hospital includes working in the Coronary Care Unit (CCU). Here patients are cared for at the very start of their journey, having just experienced a very serious and potentially lifechanging event. By contrast, the weekly Cardiac Rehab pharmacy sessions occur in a more positive environment where patients are at the other side of their journey.

This represents an ideal time to provide more in-depth education about medication, as patients have had time to come to terms with what has happened and are typically not as overwhelmed as they would have been when all the medications were first introduced.
Cardiology is a fulfilling speciality to work in as a pharmacist, as there are strong evidencebased treatment guidelines and medications that will benefit our patients immensely if used correctly. There are also clearly defined targets for risk factor reduction, such as LDL cholesterol, to help motivate our patients and guide us in their treatment. Having the opportunity to improve adherence through education sessions is particularly rewarding.
Pharmacy educational intervention: In preparation for each pharmacy group education session, each patient's history and medication list are reviewed, and the CR nurses are consulted to determine if there are any particular concerns that patients in the group may have. Each small group session (up to 6 patients) begins with the pharmacist’s introduction, and then inviting the patients to introduce themselves and briefly describe the events leading to them coming to CR. This enables the pharmacist to understand the patient's perception of their condition and to elicit any concerns they might have. Having the patients start off the session themselves helps to open up a discussion rather than taking the form of a formal teaching session or a lecture. Once each participant has had an opportunity to share their experiences, the pharmacist discusses each medication class in turn. Patients receive explanations as to what the

HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 59


medications are, their indication, how they relate to the patient's condition and how they can be of benefit. As with other disciplines in CR, a variety of behavioural change techniques (BCTs) may be employed by the pharmacist, and sometimes creative descriptions and analogies are required to help patients visualise their condition and its associated treatment. Where there is a wide variety of cardiac conditions in the CR group, a larger number of medications require discussion, and so every session is tailored to the specific group in attendance.
As the session progresses, the pharmacist helps each patient in the group understand which medications are relevant to them personally, as not all patients will be on the same medications or have the same cardiac condition. Common side-effects for each drug class are also discussed and patients are encouraged to share anything unusual that they may have experienced. Possible barriers to adherence are discussed and problem-solved collaboratively with patients, and tips to improve adherence are also shared among the group.
Practicalities such as the best time to take medications, misseddose procedures, sick-day rules and planning for travel are also discussed. Written material is provided to patients, including the Irish Heart Foundation medication booklet,21 the HSE ‘My
Cardiology Focus Optimising Care
Medicines List’22 and anticoagulant information booklets, where applicable. This allows patients to refer back to the information received in the group session when needed.
Every effort is made for patients to feel comfortable sharing issues they have regarding adherence, adverse effects and any medication-related concerns they may have. There is also an opportunity to have a private discussion with the pharmacist if the patient would rather not share something in a group setting. This way, any identified problems can be addressed by providing reassurance or advice and/or referring to CR nursing colleagues as needed. A key strength of Beaumont’s CR programme is having an RNP as part of the MDT, which allows for prompt changes to medication to be made as appropriate.
Once the discussion starts, most patient groups will have plenty of questions. It is always gratifying to hear someone say "I'm glad I asked you that" which is heard at least once at every session! It's also rewarding to see that someone's mind has been put at ease by providing an open environment and adequate time to answer their questions. A key objective of the pharmacy session is to try to instil in each patient a sense of ownership over their
medication. It is emphasised that medications will be part of their long-term management and that they can, and should, regularly ask their GP to ensure the dose and choice of each medication is still right for them once they have moved on from hospitalbased care. Patients are also reassured that if they do develop adverse effects in the future, there are actions that can be taken to alleviate these. A change in drug or dose is often possible, so they never need to suffer in silence or be afraid to say they can no longer tolerate a medication. Having this information empowers patients to advocate for themselves and their health long after Cardiac Rehabilitation has finished.
The Role of the Psychologist in Treatment Adherence
A growing evidence base suggests that adherence to cardiovascular treatment is largely a psychological phenomenon influenced by the nature of the event itself, perceptions of illness and beliefs about medicines.23,24 As an example, depression is a strong predictor of non-adherence to CVD medications25 with depressed cardiac patients up to three times more likely to be non-adherent.26-28 Furthermore, it has been estimated that up to 70% of medication non-adherence is intentional.29 Beliefs about medications significantly impact treatment
adherence in cardiac patients, particularly perceptions of personal need for treatment (necessity beliefs) and concerns about the potential adverse consequences of taking medications. Perceived necessity for and concerns about cardiovascular medicines have been independently associated with adherence to statins, beta-blockers and ACEi/ARBs respectively.30-32
Both illness perceptions and treatment beliefs are targeted as part of the Psychosocial Management component of Beaumont Hospital’s CR programme. A selection of strategies are outlined below:
Screening:
• Brief validated screening measures assessing both beliefs about cardiac medication and self-reported adherence are administered to all patients at baseline assessment.33,34
• The screening tools themselves are patient-centred in that their very wording normalizes medication non-adherence, reinforcing a ‘no-blame’ approach to discussing medication concerns during CR.
• Screening results are used to identify patients at risk of medication non-adherence, and this is discussed with the CR nurse and/or pharmacist as appropriate.
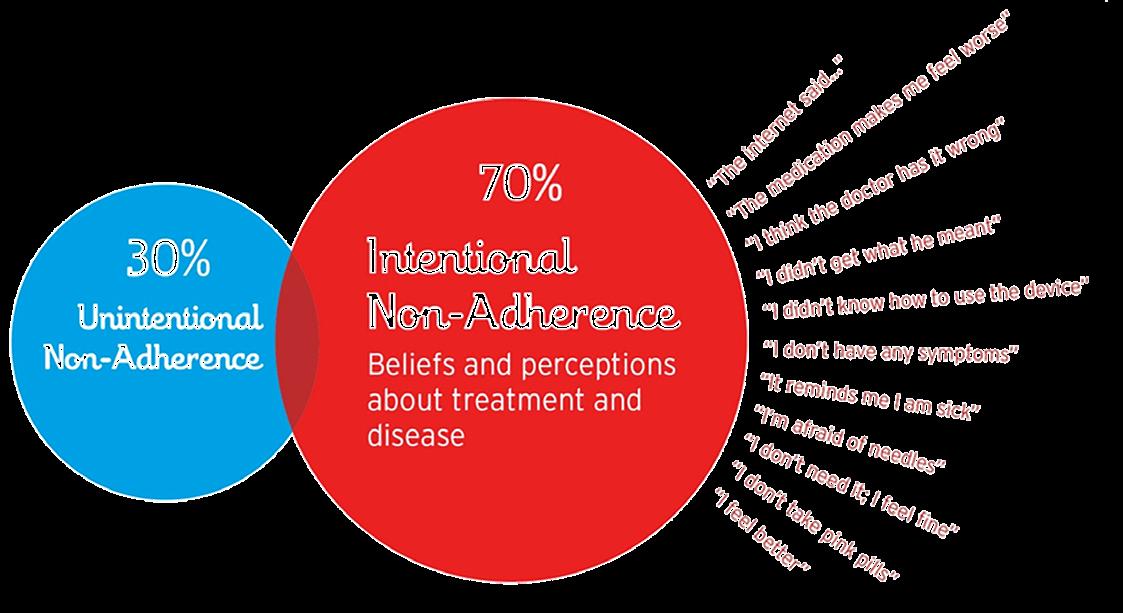
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
60


Psychoeducational Group Sessions (Health Behaviour Change)
• Adaptive beliefs about heart disease which foster medication adherence are also targeted:
o Helping patients to adopt a view of heart disease as primarily caused by modifiable risk factors35
o Adopting a ‘chronic’ (as opposed to ‘acute’) model of illness which is linked with greater treatment adherence36
• The group dynamic in CR is particularly useful to facilitate medication adherence if moderated skilfully. As most patients are adherent, a discussion of adherence in this context can be gently guided so that non-adherers are exposed to important ‘pro-adherence’ feedback from their peers. For example:
o A patient describing how initial side-effects experienced disappeared after a short period.
o A patient describing how they worked successfully with their doctor to address side-effects by changing / reducing the dose of medications.
o A group member attributing their own repeat cardiac event to having suddenly discontinued cardiac medications by themselves.
• During CR patients also learn to psychologically ‘reframe’ adhering to their medications as a form of empowerment (i.e. an action they choose to take whereby they feel more in control of their health) as opposed to evoking feelings of dependence or reminders of a compromised health status.
• Where therapeutic alliance is well established, the ‘nocebo effect’ and symptom attribution
(e.g. overlap with other factors) may be discussed with patients.
• Teaching patients how to ‘google’ effectively (e.g. obtaining accurate information from reputable medical sites).
• Addressing unintentional nonadherence (e.g. forgetting): teaching strategies to help routinise medication taking and increase habit strength (e.g. ‘implementation plans’).
• In individual patient consultations with the psychologist (or other MDT member), motivational interviewing may also be employed with ‘flagged’ at-risk individuals.
Digital Health: Use of Multimedia
• A series of smart screens are utilised in both our CR waiting area and exercise gym to exploit the educational opportunities presented when patients wait between each hourly exercise class and/or during the exercise
sessions themselves (i.e. make every contact count). Thus, medication adherence intervention(s) in CR are further reinforced by a series of motivational audio-visual content directly targeting this topic as part of the CR patienteducation curriculum.
To conclude, interventions that address patient beliefs such as self-reported low necessity and high concerns about medications are likely to improve both adherence and clinical outcomes,37 particularly if depression is targeted concurrently.
38 While strategies for improving adherence are generally recommended to adopt a multifaceted approach, simple one-component interventions have shown similar results when compared to more complex interventions.39 In CR, the challenge is to deliver effective interventions that are seamless, efficient and exploit the synergy of the MDT.
References available on request
Cardiology Abstract
Priorities for developing stroke care in Ireland from the perspectives of stroke survivors, family carers and professionals involved in stroke care: A mixed methods study
Written
by Eithne Sexton1, Karen Fowler1, Anne Hickey1, David J. Williams2,3, Frances Horgan4, Elaine Byrne5, Chris Macey6 ,
Padraic Cuffe6, Suzanne Timmons7 and Kathleen Bennett1
1School of Population Health, RCSI University of Medicine and Health Sciences, Dublin 2Department of Geriatric and Stroke Medicine, RCSI University of Medicine and Health Sciences, Dublin 3Department of Geriatric and Stroke Medicine, Beaumont Hospital, Dublin 4School of Physiotherapy, RCSI University of Medicine and Health Sciences, Dublin 5Centre for Positive Health Science, RCSI University of Medicine and Health Sciences, Dublin 6Irish Heart Foundation 7Centre for Gerontology and Rehabilitation, University College Cork, Cork
Introduction
Increasing numbers of people are living with stroke, due to population ageing and improved survival, leading to a need for evidence to inform future policy decision-making. This study aimed to engage with stakeholders in Ireland to identify priorities for stroke services development.
Methods
A sequential mixed methods design was used. Phase 1 (qualitative) was exploratory, involving initial priority gathering via an online qualitative survey and interviews, with stroke survivors, family/main carers, and
professionals working in stroke care. Framework analysis was used to generate a long-list of improvements to stroke services. Phase 2 involved a quantitative survey, where stakeholders selected five priority improvements from the long-list. Results were discussed in a stakeholder meeting.
Results
In-depth interviews were completed with 18 survivors, 13 carers and 8 professionals, while 80 professionals took part in a qualitative survey (phase 1). Priority areas of care were identified and a long-list
of 45 priority improvements was generated. In phase 2, 34 survivors, 19 family carers and 42 professionals completed a survey. The highest priority improvements (selected by >20% of respondents) were access to specialist neurorehabilitation, ongoing support for life after stroke, recruitment/ retention of specialist staff, improved information and support for health system navigation, and access to specialist acute care. Stroke survivors/carers prioritised exploring ways to improve access for strokes with atypical presentation, while professionals prioritised specialist inpatient rehabilitation and early supported discharge.
Neither group prioritised stroke prevention. Based on discussions in the stakeholder meeting (n = 12), it was decided that support for mental health should also be included as a priority.
Discussion
The development of stroke services benefits from exploring the priorities of those receiving and delivering stroke care. Findings emphasise the need for equitable access to high quality adequately-staffed services, particularly post-discharge, that are easy to navigate, with good communication, and effective information provision.
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 61


Cardiology Focus Women & CVD Shining a Spotlight on Women and CVD

In support of International Women’s Day 2024, THE National Institute for Prevention and Cardiovascular Health (NIPC) hosted a NIPC Series webinar Women and CVD Matters! on Tuesday 5th March.
A panel of experts came together to share their insights on key challenges specific to women’s heart health. Women face many unique health challenges throughout their lives and this webinar explored cardiovascular disease (CVD) risk factors and menopause, lipid management, important messages for CVD prevention and control and the lived experience of SCAD.
Topics included:
• Spotlight on Sex-Specific Risk Factors
• Peri-Menopause, Menopause, HRT and CVD – Your Top Questions Answered
• Is Lipid Management Important in Women and if So When, in Whom and How Best?
• What are the most Important Messages for Women in CVD Prevention and Control?
• Spontaneous Coronary Artery Dissection – What Every Practitioner Should Know?
Below and over the next few pages, we give an overview of some of the keynote talks and interviews which were carried out with Dr Jenni jones, Director of Training and Education for the NIPC.
Dr Jenni Jones speaking Dr Nicola Cochrane, GP & Menopause Specialist, Carrig Clinic and National Maternity Hospital
Should HRT be advocated and if so, when? Is it something we should be actively promoting?
The logic would be the HRT is advocated for women who are experiencing distressing symptoms of menopause. That can be before menopause. Women in perimenopause have often developed so many symptoms that their quality of life is very much obliterated and at that point it is
logical to start HRT even though they’re still having periods. Their periods may be irregular, but they would gain a lot of benefit from starting it when they discover they have symptoms. Most women will ask their doctor.
From the point of view of whether it is suitable for everyone, there are only a very small number of women where we would have some considerable caution about giving it. It is very much the women where they’ve perhaps already had a diagnosis of a hormone receptor cancer, where their oncologist would be concerned that using extra estrogen might reignite that cancer process.
For heart disease, there has been studies and evidence and the information we have now is incredibly encouraging and reassuring.
When or if you have a female in the CCU, with acute coronary syndrome and has been on HRT, should they continue with HRT?
Absolutely, to be honest a woman experiencing a devastating health event such as a heart attack who’s in a coronary care unit, which is a terrifying place to be – the last thing you want to do is take her estrogen away from her and be suddenly thrown into menopause symptoms.
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
62




In the middle of that is creating emotional and physical stress on your body. Its achieving absolutely no benefit as far as health protection and you could argue that its nearly exposing you to increased risk of further cardiac events.
So the logic would be that is they’re on it, let them stay on it. We often meet women who present when they develop really troublesome menopause symptoms, and they already got a diagnosis of heart disease. They have had a previous heart attack; they’ve got coronary artery disease and they’re on treatment from a specialist and they are concerned about where its safe for them to take HRT. After many studies, over many years, it is entirely safe for women who have already got heart disease to take HRT. The benefit and the logic would be to start your HRT when your menopause starts or within 10 years of when your menopause starts. So if its actually much later, if you were then into your sixties and you hadn’t started it before, we would have concerns. You wouldn’t say it is impossible, but you would have concerns that is might then offer some risks.
Is there a best form of HRT?
When we think about heart disease, in parallel with that, the concern would be around risk of blood clots. When we prescribe PHRT, currently we will tend to ask women to use a transdermal product that’s with a patch, gel or spray gel because we have overwhelming evidence now to probe that those are very, very safe and there is no increased risk of blood clot with those products. But a tablet of estrogen can slightly increase your risk of
blood clots and particularly in any women with a heart condition or blood pressure, they would be at a little bit more risk from blood clots. We would prefer to have them on a transdermal, product.
There has been some negative publicity around estrogen, could you talk us through your views on that?
The big study that people would have been first aware of it, was when the American studies from the Women’s Health Institute were published in 2001. This raised overwhelming concern about risk of stroke, heart attack with HRT. But that study was doing using tablet-based HRT and they were offering the medication to women in their sixties. They were working at the beginning with the idea that it was already well recognised that HRT was beneficial to women at normal menopause age. So maybe, it could also be beneficial to older women and the obvious result was no. It can actually be quite dangerous to suddenly introduce a very high done of estrogen to someone in their sixties who’s never used HRT. But we didn’t see what might have happened in that age group if they used a lower dose patch. That study was subsequently done with some of the early estrogen studies done in the UK and in France and Denmark. So we have good data on that now, and it clarified the difference between tablets and patches or gels.
People with familial hypercholesterolemia – perhaps HRT is considered carefully in this group. Any comments on that?
That brings us into a discussion on the imminent new guidelines
from NICE on menopause care, particularly om cardiovascular. They published their draft evidence already and they’re not going to look at new evidence. They have already offered their conclusions on the evidence that they have and said in all cardiovascular disease, that includes families with hypercholesterolemia, that is no recognized from the evidence to date, that it is safe for them to use. But the proviso would probably be that under specialist input from a menopause specialist or GP who has expertise in menopause, know how to prescribe safely, particularly to give them a transdermal estrogen rather than a tablet. Its very good news to see that NICE have concluded that they’re satisfied that transdermal HRT is safe for heart disease and I think it is going to be welcomed by most of those groups who are experiencing conditions like familial hypercholesterolemia, premature heart disease. Are there any new messages from NICE that we haven’t touched on yet?
So some of their guidance extended as well to stroke and they came out with the really clear message that timing is really important, that using transdermal estrogen under the age of 60 or within 10 years of menopause was safe. But that you could see an increased risk of stroke in people who were given HRT over 60 and a really important group to consider carefully when prescribing were women with black ethnicity. We know that quite a lot of women with black ethnicity have hypertensive heart disease and have preexisting heart disease by their genetic dispositions. So they are more vulnerable to complications and certainly you would prefer to give them a transdermal estrogen and not a tablet.
What about Progesterone? How does that fit?
they're only relatively recently starting to look at what role progesterone plays in, um, risk and safety with HRT, um, are preferred at the moment from the knowledge we have based on research is to use a micronized progesterone. Um, and that is sometimes referred to in women, might be familiar with the term a body similar because it's the most closely, um, connected to natural progesterone. Um, the reason that we usually prescribe that as a first line, and that would be EU progestin or Avastin, which is droge, is because it has a very
good record of breast safety and it has a lower side effect profile than some of the other progesterones that sometimes give people almost a feeling of premenstrual, irritability, puffiness, bloating. They just really don't feel good on some of them. Whereas we don't tend to run into that quite so much with micronized. And there's one honourable exception, which we don't yet have licensed in Ireland, but it is available in the uk. Sperone is a tablet based, um, progesterone, it's a fourth generation progesterone. It's based on the diuretic tablet, which what used to be used for high blood pressure spironolactone. So it's very low on progesterone side effects because it actually clears fluid out of your system. So rather than feeling puffy, you feel the opposite. It's very skin friendly for people that are prone to acne. But what the research has also found is that it's very helpful for cholesterol. So it actually lowers cholesterol and that's been welcomed by menopause doctors so that it's now included in British menopause guidance to doctors prescribing because they have sperone only in the UK now it's called Lindy, which is a slightly funny name. Um, but um, we'll see it in Ireland over the next six to 12 months hopefully. And they're offering it as an adequate progesterone to balance any estrogen. Um, and they said that we're, it's not currently licensed for that usage. There's ample evidence to support the safety of it for that method. So that's a very helpful endorsement. Um, and it will be something that will be very welcome as another option.
Is HRT enough to treat your lipids? Is there a belief that is I take HRT, my lipids will be magically improved?
There's quite divided opinion on that. So we would have very, very optimistic ideas that it stops the clock in that estrogen is very protective to, arteries and the lining of arteries, and it minimizes buildup of cholesterol deposits. We know that from a lot of research. Starting your HRT at menopause, if you were at normal population risk, you could be optimistic that it, it's likely to be somewhat helpful at preserving the integrity of your arteries. But if you're in a family where other people in the family have suffered heart disease at a much younger age, maybe in their thirties or in their forties, then we know that it's not enough in itself and that you very definitely may need
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 63
Dr Catherine McGorrian Dr Nicola Cochrane


to use cholesterol medication or other medication under specialist guidance so that the HRT would be a reasonable parallel, um, to manage your menopause symptoms, but your heart health might need a little bit more.
Dr Jenni Jones talks to Dr Catherine McGorrian, Consultant Cardiologist, The Mater Misericordiae University Hospital
Managing Lipids – is it going to be different to men or the same as men? Can younger women need lipid lowering? Catherine what are your views in terms of managing lips in women?
We know when it comes to risk factors, so first of all, of course we know cholesterol and LDL cholesterol as a significant risk factor for coronary artery disease, but also cerebral vascular disease, peripheral arterial disease, and atherosclerotic disease in general. Um, when it comes to women's risks, we know that women, have a lower risk profile up to menopause, and then once they reach menopause at the cholesterol, can actually increase at that point. I know we spoke about this, there's some very nice work coming out of Galway, where we see that the average men, average cholesterol for women actually goes a little bit higher than that of the men, around that time of menopause and there on in. But there are situations where lipid profile, can lead treatment at younger ages even before menopause. Nicola already mentioned, for instance, you know, if a person had a particular family history of coronary artery disease, and I can see somebody in the chat has alluded to familial hypercholesterolemia. There are some situations, where a person can, it doesn't matter which sex they are, can have, a very elevated cholesterol during the life course. And exactly as Nicola says, we know that LDL cholesterol is what we call atherogenic, so it predisposes to atherosclerotic plaque. I suppose it can be, we can be more at risk in one of two ways with regards to our cholesterol burden. Either that our absolute cholesterol burden itself is higher, or that we're ex we're exposed to it over a longer period during the life course. So people with familial hypercholesterolemia, which is a genetic condition, they will have a higher cholesterol for a longer period of life and it doesn't matter if you're a man or a woman. So knowing your family history is really helpful there. And then knowing the other cardiac risk factors as well and how they build into your cholesterol risk.
Cardiology Focus Women & CVD
Am I right in understanding that sort of perimenopause menopause, the time when we have these significant hormonal changes, the time where we accumulate a bit more visceral adipose tissue, you are saying that at that point almost our lipids can overtake men in a sense? Is that what I am understanding correctly?
That's what the data showed actually, and it came out of quite a comprehensive study out of, university Hospital Galway. Whether our cholesterol as women is higher itself or whether that perhaps we are getting it treated less, that wasn't explored. And I did wonder myself if perhaps, as women we're, we're maybe geared to think of, cholesterol as something that, you know, is for the men. But it definitely isn't. So whether we're, you know, perhaps not accessing the, the right care and treatment around it, it's very well established. You know, women suffer atherosclerotic cardiovascular events about 10 years later than men do in the life course. But we live longer, it's really important to be aware of our risk factors, um, and have them treated.
In terms of lipid treatments, therapies, are there any exciting in the future? Any exciting additions to the basket of options in Ireland?
The standard treatment, of course, is starting off with our diet, which is our Mediterranean diet. The big piece is to try and avoid, the trans fats, the saturated fats, the processed meats, and a Mediterranean diet. But it does give us a nice kind of framework and that idea of the more modern food pyramid. Then we have our statins, which are H mg quinzy, a reductase inhibitors, and they're fantastically efficacious. They reduce all cause mortality, reduce stroke, heart attack. They're really our go-to, and of course they can be limited in some people, relating to myopathy, abnormal liver function tests, things like that. A fibrates in particular for hypertriglyceridemia. So that's your biso fate or your fenofibrate is the other one. Up and coming is bemed doic acid, it's a prodrug. It comes in the pathway before the statins. As a pro-drug, essentially, it doesn't have the panoply of side effects in terms of myopathy, things like that. It's broken down in the liver, so it's very promising. Then the other one is the one I always have to try and say carefully in Razan, a little bit like the PCSK nines, which I neglected to mention. But it's a small interfering RNA
substance. It essentially affects the PCSK nine protein, which is a protein that recycles our LDL receptors. So why is that important? You'll know, of course, that we eat cholesterol, we make cholesterol, and it circulates in our bloodstream, particularly the LDL is the one that we always look at, in terms of atherogenic. But there are other ones. There's the VLDL and the IDL as well. But essentially, we circulate our LDL cholesterol, it's atherogenic, and then our LDL receptors suck it out of circulation and send it back to be repackaged. But if we've got a problem with our LDL receptors, we leave our cholesterol circling for longer. And that's specific, for instance, to familial hypercholesterolemia. We've heard already people with heterozygous homozygous, familial, familial hypercholesterolemia will have a problem with their LDL receptors, so their cholesterol will circulate for long. In Rizza and the PCSK nines as well, they stop us recycling our LDL receptors so we have more of them for longer, so we can suck the cholesterol out for longer. So these allow us to reduce our LDL cholesterol burden and its very clear than then reduce cardiovascular risk.
My understanding is that both of those, agents are used in the UK but they are licensed and its really looking at the reimbursement side in Ireland, but it’s looking hopeful that we’re going to have more options in treating people who are perhaps statin intolerant or with conditions like FH, which leads me to that media side, the newspapers saying that statins causing of all sorts of side effects. How common is statin intolerance?
For instance, maybe we've used our risk factor calculator, we've used our score two or our Q risk and we're recommending a statin for somebody, male or female. The first thing people will say to you is, do I have, will I be on this for a life? That's a difficult conversation because you're introducing the idea of a daily medication. Once there's an indication for it, I always try to explain to people the indication doesn't go away. So, if it's indicated for you now, it'll still be indicated for you in 5, 10, 15 years’ time. In terms of the side effect profile, I, my own feel is that I'm seeing fewer and fewer side effects. I think people are less, are more trustful of statins. I mean, realistically, what are the side effects about one person in 10 might get a myopathy. Although the research does show that many
of these myopathies are actually not themselves related to the statin that they may be, there's an element of subjectivity or an element that there's something else causing it. Um, abnormal liver function tests, which are usually tolerated and usually don't require, uh, withdrawal of the statin. I mean, the options are to change to a different type of statin, um, or to change the dose. I do find that sometimes with the high potency statins, people will tell me that they get very lucid dreams with them. I don’t know if anybody else hears that. I hear that a fair bit and I tell them to take them in the morning then, to save themselves from the lucid dreams. But typically, they're well tolerated, and they are such an efficacious medication in terms of all the information we have. They're kind of the building block, aren't they? And then we layer on top the ezetimibe if we need that or move to the PCSK nine if that's indicated. But there's very few people in Ireland at the moment getting PCSK nine inhibitor agents. Perhaps for these newer agents, we'll see, see more of these usages down the line.
What are your views in terms of calculating risk?
Where you talk about personalized medication and med and personalized advice, and that's really at the heart of it because those risk scores, and, you know, our own Professor Graham here in Ireland, you know, was one of the original, creators of those risk scores. They're mathematical modelling. They're based on large pieces of the population. When you, when you build those kind of risk scores, it’s very interesting. We all have predetermined ideas of what we think should go into risk scores. But mathematically, when you put those things in, they don't always have, uh, uh, an improvement around kind of how effective they are calculating risk. So, I think it comes down to the individual. You take what you get from the risk score, but then you must modify it further based on what your own personal risk elements are. And these might be your gestational diabetes, or gestational hypertension. Or indeed, you know, if you've had a history of breast cancer, radiation therapy, to the chest wall, family history, of course it doesn't feature in score. There's a lot of gaps. And, and really that's where your, your personalized your own information must be layered on top of that risk score.
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
64
Cardiology Focus Pulmonary Embolism
Pulmonary Embolism (PE) to Chronic Thromboembolic Pulmonary Disease (CTEPD)

 Written by Joanna Pepke-Zaba1, Luke Howard2, David G. Kiely3,4, Shruti Sweeney5 andMartin Johnson
Written by Joanna Pepke-Zaba1, Luke Howard2, David G. Kiely3,4, Shruti Sweeney5 andMartin Johnson
6,*
1Pulmonary Vascular Diseases Unit, National Pulmonary Hypertension Service, Royal Papworth Hospital, Cambridge
2National Pulmonary Hypertension Service, Hammersmith Hospital, London
3Sheffield Pulmonary Vascular Disease Unit, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield
4Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, NIHR Biomedical Research Centre, Sheffield
5Medical Affairs Department, Janssen-Cilag Ltd., High Wycombe
6Scottish Pulmonary Vascular Unit, Golden Jubilee National Hospital, Glasgow
Chronic thromboembolic pulmonary disease (CTEPD) is a complication of acute pulmonary embolism (PE).1,2 More research has been conducted on CTEPD with pulmonary hypertension (PH) (chronic thromboembolic pulmonary hypertension, CTEPH), while the natural history of CTEPD without PH and how it relates to CTEPH are unclear.3 The cumulative 2-year incidence of CTEPH was recently estimated to be 2.3% in acute PE patients in the prospective, multicentre and observational FOCUS study,4 and the presence of PH confers a poorer prognosis. Left untreated, the historical 5-year survival rate for severe (mean pulmonary artery pressure: >50 mmHg) CTEPH is 10%.5
The modern treatment for CTEPH is multimodal: pulmonary endarterectomy (PEA), balloon pulmonary angioplasty and medical treatment for PH may be used singly or in combination.2 For those with a surgically accessible disease, PEA offers the best prospect of a cure.1 Using an international, prospective registry of CTEPH patients, it was found that the 3-year survival was 89% in operated patients (N = 404) versus 70% in non-operated patients (N = 275),6 and a UK study of 550 CTEPH patients found that the 5-year survival was superior in patients who underwent PEA (83%) versus patients who either declined surgery (53%) or were ineligible for surgery (59%).7 According to the treatment guidelines, the possibility of CTEPH should be considered in all symptomatic patients with breathlessness after 3 months of therapeutic anticoagulation.2,8 Diagnostic algorithms for CTEPH
recommend an echocardiographic assessment of the probability of PH, assessment of lung perfusion (e.g., using ventilation/ perfusion (V/Q) lung scintigraphy) and cardiopulmonary exercise testing (CPET).1,8,9,10 However, CTEPH awareness is low among PE-treating physicians,11,12,13 and CTEPH often goes undiagnosed for over a year,14 negatively impacting patient prognosis, with higher pulmonary artery pressures and an increased risk of death.15
We aimed to understand the level of awareness of CTEPD (with or without PH) among UK physicians and the current practices in the follow-up of patients presenting to hospital with PE. The key takeaways of this study are shown in Box 1.
Materials and Methods
This was a cross-sectional online survey of PE-treating pulmonologists, cardiologists, haematologists and internal
medicine specialists (other hospital departments were excluded) in the UK (other countries excluded), performed between 2 December 2021 and 24 January 2022. Eligible physicians were either Specialty Registrars or Consultants (other job titles excluded); had been working in their specialty for 3–40 years; were based in a district general hospital, teaching hospital, PH centre or PH shared care centre in the UK (other primary work settings excluded); and personally managed ≥10 PE cases in a typical year (those managing fewer than 10 cases were excluded). Participants were advised that their personal information would not be passed to a third party without their permission, that personal data would be destroyed after 2 years and that they had the right to withhold information or withdraw at any time. Participants were also informed that the research was treated in accordance with the European Society for Opinion and
Box 1. Key takeaways from the CONNECT survey of UK PE-treating physicians.
• CTPA is most often used as the first-line of investigation for CTEPD fol-lowing PE despite cardiac echocardiography and V/Q scintigraphy being recommended;
• CTEPH was ranked as the most common cause for persistent breathless-ness post-PE despite it being relatively rare compared to other issues such as deconditioning. This may lead to onward referrals without the appro-priate investigations being conducted;
• Haematologists with an interest in thrombosis should be involved in the PE follow-up pathway;
• Protocols for PE follow-up should highlight the need for a retrospective review of the CTPA from the initial PE assessment;
• Hospital protocols should ensure smooth integration of their PE care pathways and ensure that physicians are aware of their nearest specialist PH centre.
Marketing Research (ESOMAR) and the Market Research Society (MRS)’s Code of Conduct, that all data would be anonymised and that they would not be asked to provide data on an individual patient. Participants were advised that the survey findings were intended to be shared externally, including publication in a relevant peer-reviewed journal. Finally, participants were informed that it would be a requirement that any possible adverse events, product complaints or special reporting situations related to the study sponsor’s products were passed on (anonymously), even if already reported via the Medicines and Healthcare products Regulatory Agency’s (MHRA) ‘Yellow Card’ system, and they were asked if they would still like to proceed.
The survey was conducted by Sermo using the Decipher survey platform (FocusVision) and developed by the authors. It consisted of seven screening questions: two background questions, 10 questions on PE management and 14 questions on experience in evaluating suspected CTEPD (Supplementary Materials). The questions used nomenclature following the European Respiratory Society statement on CTEPH, including the term ‘CTEPD’ rather than ‘chronic thromboembolic disease’ [16]. The questionnaire took approximately 20 min to complete, and respondents were remunerated for their participation in accordance with fair market value rates.
RESULTS
Participants
The target of 175 physicians completing the questionnaire was reached: 50 each from respiratory
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 65


Cardiology Focus Pulmonary Embolism
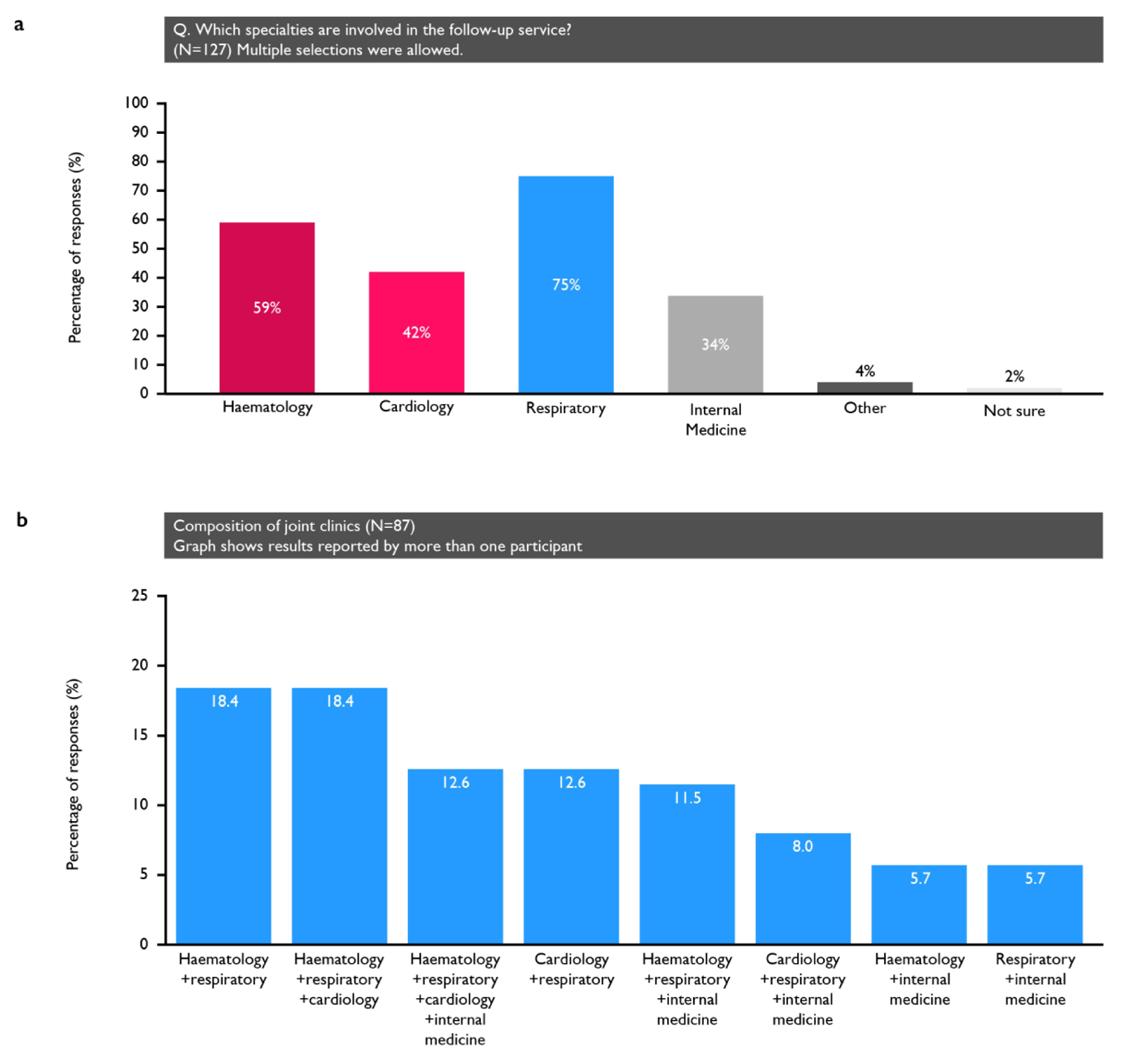
medicine, cardiology and internal medicine, plus 25 from haematology. The response rate of the individuals approached for this study was 11%. The respondents were mainly consultants (88%) based in district general or teaching hospitals (82%)
General Management of PE in the Centres in Which the Respondents Practice
Eighty-nine percent reported that their centre had local guidelines on PE management. The percentage of physicians reporting that their centre had a
PE response team (PERT) ranged from 14% (internal medicine) to 54% (respiratory medicine).
All but two physicians reported access to computed tomography (CT) imaging (two selected “not sure which, if any, [CT] modalities are available”), while—of the nuclear medicine modalities—77% had access to V/Q scanning, 10% Q scan only and 22% singlephoton emission computed tomography (SPECT). For the magnetic resonance imaging (MRI) modalities, 51% of physicians reported access to pulmonary angiography and 30% to lung
perfusion mapping. Thirty-nine (22%) physicians reported access to conventional pulmonary angiography or digital subtraction angiography.
Fifty-one percent of respondents reported a dedicated clinic for the follow-up of all PE patients (including low-risk, outpatientmanaged patients) and 14% ran a clinic for hospital-admitted patients only. The specialties involved in the follow-up service are shown in Figure 1. Of the 127 participants reporting a dedicated clinic, 87 (68.5%) had joint clinics, most commonly comprising
either pulmonologists and haematologists or pulmonologists, haematologists and cardiologists (with 16 participants reporting each; 18%). Additionally, 65% of physicians reported that their clinic had a specialist nurse (12% were not sure).
Figure 1. (a) Specialties involved in those centres that had dedicated clinics; (b) the composition of the clinics that involved multiple specialties.
On average, the physicians’ centres saw 24 acute PE patients each month, with two-thirds
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
66
Figure 1


Discussion
Most participants reported that their centre had local guidelines on PE management, but only half had a protocol for the investigation of CTEPD. Only 65% of physicians reported patients being followed up in a dedicated PE clinic, and only 22% reported that nonresolving breathlessness was investigated for CTEPD in >75% of cases. There was large variation in the follow-up of acute PE patients and in the awareness and approaches to the investigation of suspected CTEPD. The key takeaways are summarised in Box 1.
PE Management
centres have a PERT; studies should also investigate how these PERTs operate and which treatment options they have available.
Use of Imaging Modalities
All except two respondents (who were not sure) reported access to CT scans, and most had access to some form of nuclear imaging. Similarly, the BTS audit of outpatient PE management found that 92% of UK centres had 7-day access to CTPA, while 10% did not have access to nuclear imaging for patients unable to undergo CTPA [17].
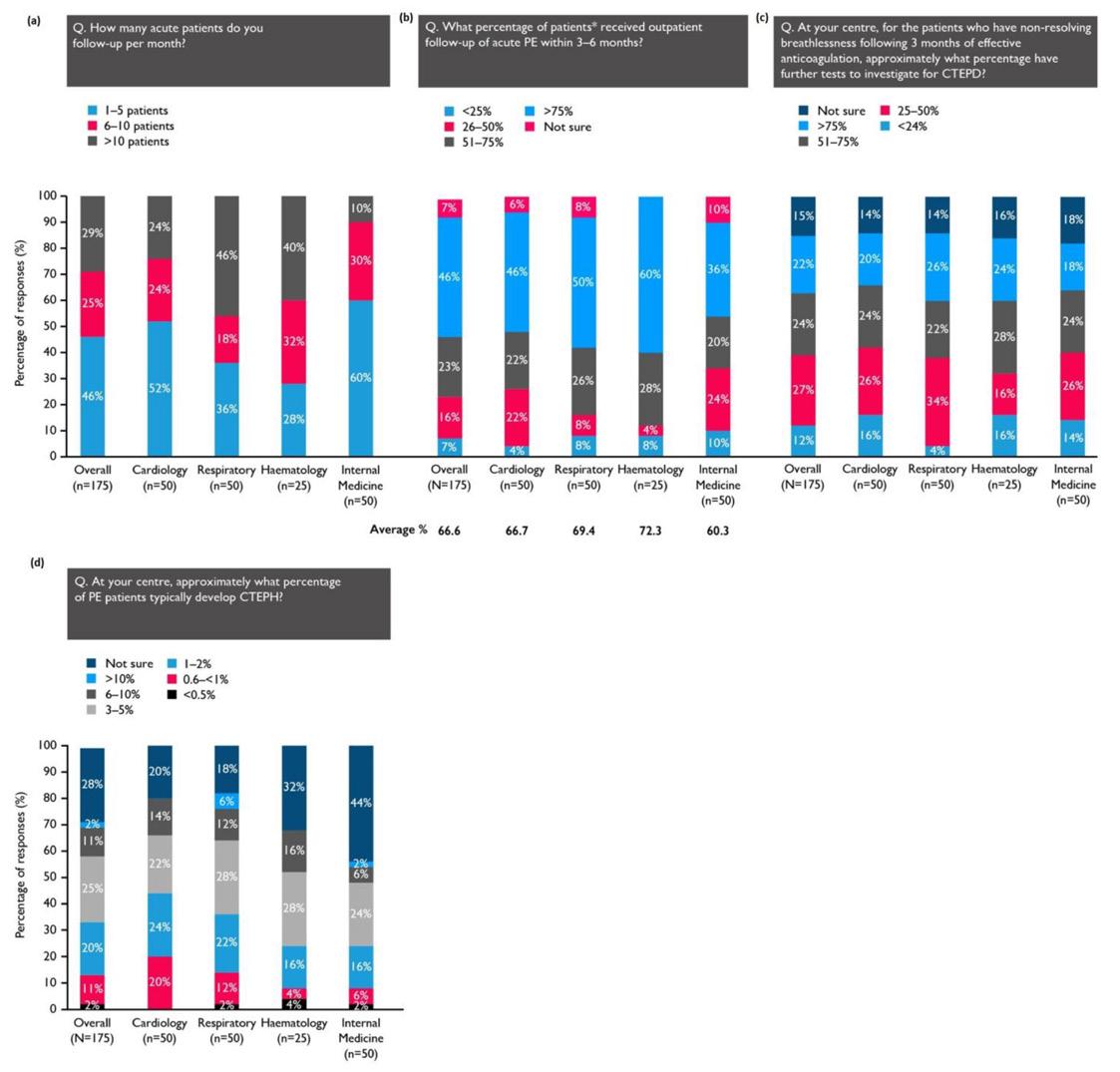
receiving outpatient follow-up within 3–6 months (Figure 2). The patients treated by haematologists and pulmonologists were the most likely to receive a follow-up during this time frame (Figure 2b).
therapeutic anticoagulation who are investigated for CTEPD; (d) the percentage of patients who typically develop CTEPH, according to the respondents.
The local guidelines for the followup of PE patients were followed by 63% of physicians, while 62% followed British Thoracic Society (BTS) guidance, 48% followed National Institute of Health and Care Excellence guidelines, 35% followed European Society of Cardiology (ESC) guidelines and 7% the American College of Chest Physicians guidelines.
It is encouraging that 89% of the respondents considered there to be local guidelines for PE follow-up. This is consistent with the results of the first BTS audit of the outpatient management of PE (audit period: September and October 2021), which demonstrated that 80% of participating UK centres (n = 108) had a formalised outpatient PE pathway [17]. Thirty-eight percent of respondents reported that their centre had a PERT; however, of these respondents, 47% were based in London and the term ‘PERT’ was not defined in our survey. Therefore, further study is needed to confirm how many
The ESC/European Respiratory Society (ERS) guidelines on PE and PH recommend the V/Q scan (or an alternative perfusion imaging modality) for first-line imaging in suspected CTEPH [1,2,8]; however, 10% of respondents only had access to Q scan imaging, although the added value of ventilation imaging when other complementary imaging modalities are available is limited. There has been a gradual move toward the use of SPECT, as it is superior to planar V/Q imaging (Table S6) [16,18]. In our study, 22% of survey participants had access to SPECT. The survey results also show that CTPA and echocardiography were preferred over V/Q scanning as the first
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 67
Figure 2. * Of those managed as outpatients at the physician’s centre. (a) The number of PE patients who are seen by the respondents; (b) the percentage of those that receive a followup within the recommended timeframe; (c) the percentage of patients with persistent dyspnoea after 3 months of
Figure 2


test to investigate for CTEPD (used first by 58%, 29% and 4% of physicians, respectively). The reasons for this were not captured, but it could be due to a greater availability of CT and echocardiography versus V/Q scans or the advantage of being able to look at the heart and lungs with CT imaging (Table S6) [18]. Test suitability may also vary among patients; for example, a V/Q scan may be preferred for young female patients for whom exposure to CTPA radiation is a concern or in cases where the interpretation of the CTPA findings is difficult, such as for patients with a higher cardiac output or where there may be concerns regarding the ability of the patient to cooperate with the breath-holding requirements during the CT imaging. It should also be noted that 80% of physicians always or sometimes used lung function tests (80%) to investigate the possibility of CTEPD, despite these tests not being as discriminatory as the imaging tools. It is important to conduct appropriate tests, and this finding suggests there is room for improvement.
Access to other imaging modalities was higher than expected. This survey could have been completed by more than one respondent from the same centre, thereby falsely inflating these percentages; however, the distribution of respondents across the regions and specialties suggests that the level of this type of ‘duplication’ is not excessive.
Other Tools to Support Post-PE Follow-Up
The use of CTEPH risk scores varied across respondents; however, only one such tool has been validated [19]. The In Shape II validation study of 424 patients found that the tool detected most (10 of 13) CTEPH patients within 4 months following PE [20]. However, when applied to 2256 PE patients in the Computerized Registry of Patients with Venous Thromboembolism (RIETE), its sensitivity was found to be too low (27.3%) to rule out CTEPH [21]. CTEPH risk score calculators may prove particularly useful when resources are limited, but they have some disadvantages. Because they are focused on distinguishing PH from non-PH, they do not help diagnose patients without PH. Also, completing the risk score before deciding on the next steps can require more visits than simply booking a test and the subsequent follow-up appointment at the same visit.
Cardiology Focus
Pulmonary Embolism
In our study, 18% of physicians reported asking their patients to complete QoL questionnaires. The only validated PE-specific QoL tool [22] was developed before quality standards for such instruments were devised and does not fully adhere to the guidelines [23]. The low use reported in this survey is consistent with the authors’ observation that QoL questionnaires tend to be used in clinical trials rather than in clinical practice.
Aspects of Post-PE Follow-Up Needing Improvement
The BTS audit of outpatient PE management demonstrated that 87% of UK centres performed follow-up at 3 months, with this occurring in a dedicated PE clinic in 36% of the centres [17]. In comparison, we found that 65% of the respondents’ centres had a dedicated PE clinic, but only 46% of the physicians reported that more than three-quarters of patients were offered a 3–6-month follow-up. A retrospective study of adults discharged from a UK teaching hospital with a diagnosis of acute PE in the first half of 2019 found that 31% of intermediate–high-risk and 37% of intermediate–low-risk patients were not followed up [24]. The authors hypothesised that dedicated PE clinics with multidisciplinary team meetings could streamline post-PE follow-up and improve outcomes [24].
The reasons for this gap in care are unknown; however, our finding that 74% of respondents routinely consider a diagnosis of CTEPD would suggest that lack of awareness is not predominant. Indeed, the high percentage of respondents rating CTEPD and CTEPH among the most common reasons for persistent symptoms following PE indicates that physicians are mindful of these conditions. Importantly, however, this finding reflects an inaccurate perception of the prevalence of CTEPD and CTEPH. Persistent dyspnoea and/or poor physical performance are relatively common several months after acute PE, but most cases are attributable to muscle deconditioning (ranked as the most likely reason by only 18% of survey respondents) [1,2]. This finding could reflect that physicians’ answers are biased because they are aware the survey is related to CTEPD. The availability of the CT and V/Q scanning modalities reported by the respondents (99% and 90%, respectively) indicates this is not a significant barrier to providing care.
The CLARITY survey found that the most frequent barriers affecting the detection of CTEPH after PE reported by respondents (353 physicians from Europe, Asia–Pacific and the Americas) were low disease awareness among nonexperts (77%), lack of structured follow-up after acute PE (56%), nonspecific presentation of disease (43%), incomplete understanding of the natural history of disease (39%) and lack of clinical guidelines to screen for possible CTEPH (73, 23%) [25]. Moreover, low adherence to guidelines was reported as a barrier to CTEPH diagnosis by approximately one-third of respondents [26]. The variability in the physicians’ practices, as shown in this survey, could support this finding that more comprehensive guidelines on PE follow-up are needed.
The survey pinpointed a specific practice that could be improved: 28% of physicians overall and 40% of internal medicine specialists occasionally or never review the CTPA from the initial PE assessment for signs of CTEPH. The In Shape III study showed that experts’ overall judgement on whether CTPAs at acute PE presentation showed signs of CTEPH achieved a sensitivity of 72% and a specificity of 94% [27]. A subsequent study demonstrated that nonexpert readers could also accurately detect CTEPH based on specific radiological signs according to a scoring form [28]. Therefore, routinely performing closer examination of initial CTPAs could improve detection of CTEPH.
Multidisciplinary Follow-Up of PE
In our survey, two-thirds of dedicated follow-up clinics were jointly run by more than one specialty. The benefits of such multidisciplinary clinics have been described previously [24]. A clear pathway for the follow-up of patients presenting with PE who are already under other specialties and have risk factors for PE (e.g., oncology) will be important [29,30]. In our study, the lower percentage of haematologists than other specialties routinely investigating for CTEPD in patients with thrombophilia likely reflects an understanding that investigations in these cases do not change disease management in many cases and suggests that the involvement of haematologists in clinic reviews could potentially avoid unnecessary investigations. Internal medicine specialists
reported lower rates of follow-up with PE patients and consideration of CTEPD as a diagnosis. One of the BTS quality standards for the outpatient management of PE is that follow-up should be performed by a senior clinician with special interest in PE, as part of a formal pathway [31].
4.6. Referral to PH Centre
Most respondents were aware of the location of their regional PH centre. There are seven adult specialist PH centres in the UK; three are in London and the others are in Newcastle, Glasgow, Cambridge and Sheffield. Hospital protocols should ensure smooth integration of their PE care pathways.
According to the ESC and ERS guidelines for the management of PE and PH, symptomatic patients with persistent mismatched perfusion defects 3 months following acute PE should be referred to a specialist centre after consideration of the results of echocardiography, brain natriuretic peptide (BNP)/Nterminal pro-brain natriuretic peptide (NT-proBNP) and/or CPET investigations [2,8]. However, 42% of respondents in our survey reported that they typically referred (some) breathless patients without performing further tests, and 38% (sometimes) referred patients even if the subsequent test results were normal. A referral before conducting any further tests may be appropriate where there is a high index of suspicion based on original imaging, echocardiographic findings and medical history; however, in the majority of cases, patients would undergo further tests beforehand, as per guideline recommendations [1,2]. In cases in which patients are referred even if tests are normal, access to V/Q scintigraphy may prevent unnecessary referrals or support referrals where appropriate.
Conclusions
Our survey results demonstrated a wide variation in clinical practice in the follow-up of patients presenting with acute PE and in the awareness and approaches to the investigation of suspected CTEPD. This could indicate that more comprehensive and structured guidelines for PE follow-up are needed. These data support the conduct of a national audit to improve our understanding of the barriers to the timely detection of CTEPD.
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
68
Cardiology Focus Heart Failure
Exploring heart failure nurse practitioner outcome measures: a scoping review

 Written by Mary Ryder1, Tara Mannion1,2, Eileen Furlong1, Ethel O’Donoghue1,3, Bronagh Travers1,4, Michael Connolly1,5 and Niamh Lucey1,3
Written by Mary Ryder1, Tara Mannion1,2, Eileen Furlong1, Ethel O’Donoghue1,3, Bronagh Travers1,4, Michael Connolly1,5 and Niamh Lucey1,3
1School of Nursing, Midwifery & Health Systems, University College Dublin, Dublin 2St Claires Integrated Care Centre 3St Vincent’s University Hospital, Dublin 4Tallaght University Hospital, Dublin 5Our Lady’s Hospice and Care Services, Dublin
Clinical guidelines consistently recommend that patients with heart failure (HF) are enrolled in a structured multidisciplinary care programme.1,2 The purpose of multidisciplinary care is to provide a comprehensive plan of care for patients, including self-care, optimizing disease-modifying therapies, ongoing cardiovascular risk factor management, timely follow-up with healthcare professionals, and enrolment in cardiac rehabilitation. The specialist nurse is routinely a member of the multidisciplinary team (MDT) in heart failure. A plethora of nursing literature has demonstrated the positive impact that the specialist heart failure nursing role has had on patients, particularly related to self-care education.3,4
In Ireland, there are two specialist nursing roles in HF, namely Clinical Nurse Specialist (CNS) and Nurse Practitioner (NP).5 The International Council of Nurses (ICN) defines Nurse Practitioner as a registered nurse who has acquired the expert knowledge base, complex decision-making skills, and clinical competencies for expanded practice, the characteristics of which are shaped by the context and/or country in which s/he is credentialed to practice.6 The differences between the roles in Ireland include that the CNS works collaboratively within the MDT to manage complex patients with heart failure, whereas the NP manages an agreed low-risk patient caseload autonomously.7
Nurse-sensitive outcomes, according to Twigg8,9 and colleagues, are patient outcomes that are used as indicators of the quality of care. Diane Doran in 200310 p.p.vii defined nursesensitive outcomes as:
Outcomes that are sensitive to nursing are those that are relevant, based on nursing scope and domain of practice and for which there is empirical evidence linking nursing inputs and interventions to outcomes.
In the context of HF, they include readmission rates, self-care scores, medication optimization, quality of life (QOL) measurements, and functional status measurements.11 The NP’s role and specific effectiveness as a key stakeholder in the multidisciplinary HF team is not clearly defined. Recent research has reported NP outcome indicators for acute and community care settings,12 including cardiovascular care13 but not specifically for HF. Consistent with previous literature on nursing outcomes, patient outcomes have also been identified as NP outcome indicators14,15 including NP heart failure care.13 Patient outcome measures are not exclusive to nursing interventions in HF, and international guidelines acknowledge that multiple members of the MDT are attributed to the delivery of selfcare education.1,2 It is yet to be determined what specific outcome measures are sensitive to NPs in
HF to elucidate their explicit role and contribution within the MDT. This review aims to identify the literature related to NP-sensitive outcomes, determine the outcome measures that relate to the role of the NP in HF management, and explore these measures to understand the NP contribution to multidisciplinary HF team management.
Methods
A scoping review was conducted to identify the key concepts that underpin the field related to NP-sensitive outcomes in heart failure. The review was conducted according to accepted guidelines using the Joanna Briggs Institute (JBI) framework for scoping reviews.16
Eligibility criteria
In this review, participants are Advanced Practice Nurses which
include NPs, the context is heart failure, and the concept explores outcomes (Table 1). Original research reporting on NP-led heart failure interventions that included patient outcomes [QOL/length of stay (LOS)/readmission/titration/ mortality/morbidity] were included. Original research that did not include NPs or those unrelated to HF were excluded. Full-text studies including randomized controlled trials (RCTs), qualitative designs, pre- and post-evaluations using research methods, and surveys published in English, between 2010 and 2022, were considered. The dates were agreed following preliminary searches of the literature where patient and nursing outcome literature emerged.
Search methods
Electronic searches
The first step included a hand search of literature related to nurse-sensitive outcomes in
First term (population)
Second term (concept) Third term (context)
Advance nurse practitioner OR advanced nurse practice OR nurse practitioner OR clinical nurse specialist Heart failure OR cardiac failure OR congestive heart failure OR chronic heart failure
First term (population)
Patient outcomes OR patient-reported outcomes OR patient-reported outcome measure* OR health-related quality of life
Adverse event* OR adverse outcome* OR adverse patient outcome* OR acute decompensated heart failure OR readmission OR hospitalization OR hospitalization OR readmit* OR length of stay
Self-care OR self-management OR self-care behaviour* OR self-care behaviour* OR self-care scale* OR self-care measurement OR self-care score* OR self-care education
Patient satisfaction OR patient experience OR patient attitudes OR patient perceptions
Symptom burden OR comorbidity* OR anxiety OR depression OR anaemia* OR anaemia* OR frail* OR sleep apnoea OR sleep apnoea OR chronic obstructive sleep apnoea OR chronic obstructive sleep apnoea OR chronic lung disease* OR chronic pulmonary disease* OR interstitial lung disease* OR interstitial pulmonary disease* OR diabetes*
Second term (concept) Third term (context)
Medication adjust* OR medication titration* OR drug adjust* OR drug titration* OR medicines adjust* OR medicines titration*
*Boolean phrase OR was used within each term; AND was applied between terms.
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 69
Table 1
Search terms


Cardiology Focus Heart Failure

the broad term, NP-sensitive outcomes, cardiovascular nursesensitive outcomes, and patientreported outcome measures. The second step included a clearly defined search strategy to conduct a scoping review of the literature exploring NP-sensitive outcomes in heart failure.
zvad108, https://doi.org/10.1093/eurjcn/zvad108
slide may be subject to copyright: please see the slide notes for details.
Search terms
The team, in consultation with a librarian, reached consensus on agreed search terms which were replicated in each data source (Table 1). The search occurred in April 2022.
Data sources
The following electronic databases were searched: Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost), MEDLINE (PubMed), EMBASE (embase.com), ProQuest Dissertations and Theses Global (ProQuest), PsycINFO (Ovid), and TRIP database (tripdatabase. com). Only articles written in the English language and published
between 2010 and 2022 were included. The search strategy for CINAHL appears in Supplementary material online, File S1. In addition, OpenDOAR, GreyNet, Sigma Nursing Repository, and LENUS (IRL) were screened in May 2022. A grey literature search was carried out to examine international evidence-based practice guidelines for the management of heart failure. These included guidelines produced by the European Society of Cardiology (ESC), Heart Failure Association (HFA), National Institute for Health and Care Excellence (NICE), and the collaborative American Heart Association (AHA)/Heart Failure Society of America (HFSA)/ American College of Cardiology (ACC) guidelines. The websites of the Irish Heart Foundation (IHF), British Heart Foundation (BHF), and Croi were screened in June 2022 for reference to NP outcomes. Finally, an advanced Google search was conducted for documents pertaining to HF NPsensitive outcomes.
Results
Literature search
A total of 414 references were identified from the databases and imported for screening. Sixty-nine duplicates were automatically identified and removed using covidence software (Figure 1). The remaining 345 studies were screened against title and abstract by two members of the research team (M.R. and T.M.) resulting in a further 250 studies that were excluded as they did not meet the inclusion and exclusion criteria. Ninety-five articles were read and assessed for full-text eligibility determined by the inclusion and exclusion criteria, by three members of the research team (T.M., M.C., and E.o.D.). Conflicting decisions were discussed among the reviewers and in consultation with the project lead, and 80 articles were excluded. The primary reasons for exclusion were conference abstract (n = 29) and outcomes unrelated to NPs (n = 28); the
remainder (n = 23) did not meet the inclusion criteria. Fifteen texts were selected for data extraction. A hand search of reference lists of a recent systematic review12 was conducted, and one further article was selected for analysis.
Data extraction
Data extraction was completed by two researchers (M.R. and T.M.), by condensing the headings recommended for JBI scoping review.15 The selected publications are summarized in the data extraction table. All selected articles were critically appraised by M.R. and E.F., using the Crowe Critical Appraisal Tool (CCAT).18 The tool uses a five-point score allocation across eight categories. Scoring was consistent between both reviewers. The lowest score in any category was three out of a possible five for each. All selected articles scored over a total of 30 out of a possible score of 40 and were considered high quality by the reviewers, and they were therefore selected
APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
70
Figure 1 PRISMA search strategy. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools
Eur. J. Cardiovasc. Nurs., zvad108, https://doi.org/10.1093/eurjcn/zvad108
The content of this slide may be subject to copyright: please see the slide notes for details. Cardiovasc. Nurs.,
for further analysis. The data was analysed for frequency of concepts, populations, characteristics, and frequency of outcome measurement tools used consistent with JBI scoping review evidence analysis.15 The data were then presented focusing on the population, context, and concept outlined previously.
Research methods
The stated purpose of all research projects was to improve heart failure patient outcomes by translating evidence-based research into NP-led care. Five RCTs were identified.21,23,26,28,31 One study described a pragmatic trial with ramdomization.32 Four pre- and post-interventional studies were identified,22,24,27,34 with control group used for comparison purposes in three22,27,34 studies. Four studies were described as quality improvement projects, one with a control20 and three using comparison groups for analysis.25,29,30 Two studies used implementation science and reported the evaluation phase of evidence-based practice intervention,20,25 and two were identified as feasibility studies.19,33
Discussion
The review highlights a lack of consistent reporting of nursesensitive outcome measurements to reflect the specific impact of the NP role in heart failure patient management. Sixteen articles were selected for analysis. A similar model of NP personcentred HF care was described in the articles reviewed. A variety of outcome measures were reported to evaluate the models of care implemented. The most common outcome measures reported were readmission rates (specifically 30-day readmission), self-care measurement scales, functional status scores, QOL measurements, and medication optimization outcomes.
International guidelines conclude that holistic, personcentred, multidisciplinary disease management clinics are recommended above heart failure educational programmes alone.1 Heart failure care programmes are associated with reduced hospitalization and mortality, with nurse-led HF care being one established model.35 Largely pioneered in Sweden, nurseled HF care programmes have demonstrated improved survival and reduced risk of hospital readmission.36 Several studies included in this review report NP outcomes where the NP was part of the HF MDT19,21,26,29–31 and in some instances included other specialist
HF nursing roles.23 The unique contribution that the NP has in MDT heart failure management remains unclear. A number of possibilities for NPs in heart failure have been discussed in the literature15 but does not extend to discuss outcome measurement.
The most reported outcome measure in the literature examined was heart failure readmission rate, specifically within 30 days. Heart failure is commonly associated with high readmission rates. This outcome measure was frequently associated with several nursesensitive patient outcomes, as identified in a previous scoping review of hospital nursing factors.37
The 30-day readmission outcome measure has been associated with pharmacist-led transitional care in a meta-analysis of outcome literature.38 The purpose of this benchmark as an outcome indicator in HF is to highlight the significant readmission rate associated with a quarter of heart failure hospitalizations.39 A previous systematic review identifying the benefits of NP-led HF care reported a 30-day readmission rate.40 However, the literature implies this is not a measure related exclusively to NP interventions.
Most studies in this review examined the management of complex HF patients including people with cognitive impairment, New York Heart Association (NYHA) III–IV, and older persons. This is consistent with previous research41 which reported that patients with more severe HF were more likely to be enrolled in nurse-led HF programmes.
The positive outcomes reducing 30-day readmission rates, despite the complex patient profile, can be attributed to the interventions outlined in some of the studies. However, the 30-day readmission rate reduction was not consistently lowered within the studies reviewed and therefore cannot be attributed as an NP-sensitive outcome.
The second most frequently reported NP outcome measure in this review was HF self-care and functional status. Tailored personcentred, self-care education based on scientific evidence education is recommended in international guidelines. The ESC 20211 guidelines acknowledge that the delivery of self-care education improves patient outcomes, irrespective of the heterogeneity in intensity or the personnel who deliver the interventions. Guidelines recommend that selfcare is delivered by a MDT.1,2 The recommended content addresses areas of expertise for multiple team members; therefore, it is

reasonable that teaching self-care is not exclusive to one profession. However, recent research exploring the education for heart failure patients reported that physicians provided little to no education for patients with heart failure; the nurse on the team was identified as the patient educator.42 Self-care was embedded in all the research projects reviewed; however, the outcome score was only reported in five studies.22,23,26,27,32 Self-care measurement scales determine three separate dimensions of self-care including maintenance, management, and confidence.43 Consistent with the research explored in this review, the evidence indicates that confidence measures are associated with good day-today self-care maintenance.43 Inclusion of caregivers in self-care education and using a teachback approach suggest improved outcomes for heart failure patients.44 Caregiver inclusion was referenced in a limited number of studies reviewed, and the methodology of education was not described.20,24,29 Consistent with findings that the benefits of NP-led HF management include an emphasis on patient education,40 this outcome measure cannot be attributed to NP interventions alone.
There was a myriad of tools and inconsistent reporting of functional status, health status, and QOL in heart failure patients across the literature in the review. Whilst there is overlap between tools used in assessing HF functional status, there are important differences.45 For example, the Kansas City Cardiomyopathy Questionnaire (KCCQ) is a specific heart failure tool best used to assess the health status of patients and is in contrast to the 6-min walk test, which should be used to assess functional status and as a prognostic indicator.45 These tools are complimentary but not interchangeable, and this important difference was not addressed in the studies reviewed. A recent concept analysis of the added value of NP roles in healthcare proposes that, when exploring the added value of the role, it is important to capture the skills, competencies, outcomes, and professional activities associated with the role.46 Whilst not specific to heart failure, NPs have demonstrated significant impact on healthcare outcomes related to hospitalization.14 A recent evaluation of NP roles across Ireland47 reported that NP interventions resulted in hospital avoidance in chronic illness.
Additionally, the evidence suggests that NP roles improve access to care and patient self-care.14 A previous review of the literature12 identified that NP-led care improved evidence-based practice and guideline application, thus improving patient outcomes.
Commonly reported NP outcomes in the literature, irrespective of specialist area of practice, include reduced hospital LOS, increased prescription rates of evidencebased pharmacological therapies, and improved patient behaviours.12 These NP outcome measures are consistent with several reported in studies analysed for this review. It is worth mentioning that no outcomes reflect a unique contribution of nursing in the NP role for heart failure management. Standards are important to demonstrate accountability for the quality and safety of care provided by healthcare professionals, including NPs. Standardizing outcome measures provides a benchmark to measure the quality of care provided for heart failure patients and demonstrates the effectiveness of the NP role in HF management.
Internationally, there are inconsistencies related to the regulation, operationalization and integration of NP roles, irrespective of the specialist area of practice.48 Therefore, it is arguably a complex undertaking to develop international standards related to HF NP outcomes. Perhaps, the next step may be to commence work at national levels where the NP roles are consistent and therefore standard outcome measures can be agreed.
Limitations
We acknowledge some limitations in the methods we have used, which may have an impact on the comprehensiveness of our findings. These include not describing all the characteristics of the selected manuscripts and the outcomes described.
Conclusion
This review highlights that the reporting of HF NP outcome indicators is inconsistent and disparate across the literature. Consistent reporting of agreed outcomes in HF would strengthen the evidence of the impact of NP interventions. Standardized NP-specific outcome measures, at national and/or European levels, would serve to highlight the effectiveness of the role in a multidisciplinary HF team but must capture the fundamental aspects of nursing in these measures.
References available on request

HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024 71
Ask The Expert
Ask the Expert: Dr Cormac Mullins

Dr Mullins was one of the keynote speakers at the recent Irish Pain Society’s Annual Scientific Meeting, where he provided an update on spinal cord stimulation, emphasising the importance of appropriate patient selection.
Dr Mullins also provided a useful critique of the very limited placebo-controlled RCT evidence base that currently exists for spinal cord stimulation, the large placebo response, and the need for more high quality RCTs in this area.
We spoke to Dr Mullins to gain further insights into his valuable work and career to date.
Can you give our readers a brief background of your career to date?
I started as a consultant in anaesthesiology and pain medicine at Cork University Hospital and South Infirmary Victoria University Hospital just over a year ago. Before that, I was working as a pain consultant in Guy's and St. Thomas's NHS Trust in London for a year, where I also did my fellowship training. I am from Galway originally and trained in Trinity College Dublin and completed my anaesthesiology training in Ireland. I also did a pain fellowship at St. James's Hospital Dublin before going to London. My fellowship training was in the area of spinal cord stimulation which is an implantable device which can be very successful for treating certain types of chronic pain. I trained at one of the biggest centres in Europe for this form of therapy and am excited to bring this expertise to Ireland.
Tell us a bit more about your current research work
My main area of research is in the area of spinal cord stimulation (SCS) and headache.
Spinal cord stimulation (SCS) is a form of therapeutic neuromodulation. It was defined by the International Neuromodulation Society as “the alteration of the nerve activity through targeted delivery of a stimulus, such as an electrical stimulation or chemical agents, to specific neurological sites in the body”.
I published three papers in the last year on the topic of SCS.1-3 These were interesting papers that looked at certain areas such as MRI conditionality of devices at followup (which is a very important consideration for patients undergoing SCS implantation as MRIs are so accessible these days), migration of SCS leads during trials and combined implantation of SCS with dorsal root ganglion stimulation which can provide additional flexibility and therapeutic alternatives for spinal cord stimulation. I also published a paper on the use of lignocaine infusions as a treatment for primary headache disorders, such as migraine, and trigeminal neuralgia and this was shown to be quite effective in those refractory to other therapies.4 I also have a review paper on neuromodulation options in migraine and other headache disorders coming out soon. This can provide a non-drug alternative for those who have a condition such as migraine and cannot tolerate medication.
Dr Cormac Mullins is a Consultant Pain Specialist and Anaesthesiologist in Cork University Hospital and the South Infirmary Victoria University Hospital, Cork
a key challenge involves moving beyond this to a more integrated and holistic model and taking a person-centred approach to care.
How does Ireland compare with the rest of Europe at present Pain Medicine and Anesthesiology?
What are your hopes for 2024 for Pain Medicine and Anaesthesiology?
My hopes are to deliver effective multidisciplinary pain management strategies to the greatest number of people while tackling the waiting list for chronic pain services in Ireland. We are hoping that we can deliver evidencebased treatment approaches which focus on enhancing selfmanagement for patients with chronic pain in Cork and improve the throughput and quality of care delivered in our service.
Can you outline some of the biggest challenges facing this field for 2024 and the key opportunities?
In Ireland, the biggest challenge is undoubtedly access to multidisciplinary pain management strategies in a timely fashion. The waiting lists in Ireland for pain medicine are far too long and in Cork we are hoping to redesign how we see patients. We are really excited to have a few excellent new additions to our department in Cork in the form of specialist physiotherapists and a clinical psychologist and we need to focus on getting the message out there for those with chronic pain to remain active, continue with usual activities and not let pain take over. Key management principles that are covered in pain-management programmes involve establishing what is important to the individual and continuing to take committed action towards these goals in spite of chronic pain. Unfortunately, there is often an over-reliance on medical approaches to chronic pain and
In my experience, I believe we are only just catching up to the message that chronic pain needs to be treated with a multidisciplinary team of medical and nursing professionals, physiotherapists and psychologists and this is mirrored in chronic pain clinics across Ireland that have insufficient numbers of multidisciplinary staff. We are lucky in Cork to have made recent appointments that will allow us to become a true multidisciplinary pain clinic with clinicians from different specialities working together in the same space and communicating with each other frequently to deliver coordinated, patient-centred and evidencebased care.
I am optimistic about the quality of the pain training programme that exists in Ireland now that will rival any pain medicine training programme in Europe and that this will deliver the highest quality pain medicine consultants in the future.
What would be your personal goals and hopes for this field in the future?
My personal goals include tackling the waiting lists for chronic pain in Ireland, delivering effective evidence-based multidisciplinary to the greatest volume of patients, removing the siloed approach to back pain within medicine and exploring cross-speciality therapeutic approaches. I am hopeful that in a year's time that significant dents will have been made in the waiting times for patients to access care and that the quality of care they will receive will be truly multidisciplinary and evidence-based. I am hopeful that we can deliver advanced pain management modalities to those who will benefit from it, including spinal cord stimulation, and we are keen to re-establish this service in Cork this year.
Some of Dr Mullins published scientific papers include:
A retrospective review of elevated lead impedances in impedance-dependent magnetic resonance-conditional spinal cord stimulation devices
Advances in Spinal cord stimulation (SCS) device
72 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
technology in recent years have led to the development of SCS systems that are magnetic resonance imaging (MRI)conditional, most of which are dependent on normal lead impedances. The objective of this study was to retrospectively analyze the rate of elevated lead impedance in these devices to determine the rate of failure of MRconditional modes.
This was a single-center, retrospective, chart-based review conducted during a five-year period. Patients were included if they had been implanted with an impedance-dependent MR-conditional SCS and had a documented impedance check at least 6 months after implantation. A Kaplan-Meier survival analysis was performed to map the survival of MR-conditionality over time.
There were 363 cases included between 2015 and 2020, which corresponded to a total of 602 SCS leads. Nevro was the most common manufacturer (67.8%), followed by Boston Scientific (22.3%) and Abbott (9.9%). The average overall follow-up time was 2.25 years. Overall, 67 (18.5%) of patients had lead impedances over 10,000 Ω at follow-up with a total of 186 electrode contacts (3.9%). Leads most commonly had either one (40%), two (22%) or three (12%) electrode contacts out of range. Risk of failure of lead impedances increased by 35.4% with each successive year to a peak of 43% of all leads by year 5. Mean overall survival time of normal lead impedances was 4.77 years (CI 4.40-5.13). There was no statistically significant difference in mean overall survival time between Abbott (M = 4.0 years, SD = 1.25), Boston Scientific (M = 4.64 years, SD = 1.75) and Nevro (M = 4.80 years, SD = 3.28), χ2 (2, N = 358) = 1.511, p = 0.47; however, Abbott leads had a greater total number of failed impedance contacts (50/568, 8.8%), in comparison to Nevro (124/3064, 4.0%), χ2 (1, N = 3630) = 23.76, p < 0.00001, at a similar follow-up time.
This retrospective study identified elevated impedances in 18.5% of MR-conditional SCS devices at an average of 2.25 years follow-up resulting in loss of MRconditionality and a mean overall lead survival time of 4.77 years for normal lead impedance.
Radiographic lead migration in percutaneous spinal cord stimulator trials
https://rapm.bmj.com/content/ rapm/early/2023/07/20/rapm2023-104347.full.pdf
Lead migration during spinal cord stimulator (SCS) trials is relatively neglected in the literature and presents a different set of
challenges compared with fully implanted leads. There is no consensus on what constitutes a clinically significant amount of radiographic lead migration during SCS trials. We wished to evaluate the incidence and extent of radiographic lead migration during percutaneous SCS trials, to investigate the risk factors for lead migration and whether this has impacted on trial success.
This prospective observational study of percutaneous SCS trials took place in a tertiary referral center in the UK between April 2021 and January 2022. Radiographs of SCS lead position were taken at baseline and prior to lead removal. Lead migration ≥50% of a vertebral level was deemed significant.
One hundred trials were included comprising 162 leads. Mean migration distance was 0.55 vertebral levels (SD 0.85) or 12.5 mm (SD 18.2) in a caudal direction. Significant radiographic migration occurred in 50% of all leads (81 of 162 leads), at least one lead in 62% of cases and all leads in 44% of cases.
Radiographic lead migration was not found to be associated with reduced trial success. A single lead and mechanical anchors were associated with greater incidence of lead migration.
Radiographic lead migration of approximately half of a vertebral level in a caudal direction can be expected during percutaneous SCS trials and this can be anticipated by siting leads half of a vertebral level higher to accommodate for this. Additional factors should be considered in the setting of radiographic lead migration to determine whether this can be considered clinically significant.
Effectiveness of Combined Dorsal Root Ganglion and Spinal Cord Stimulation: A Retrospective, Single-Centre Case Series for Chronic Focal Neuropathic Pain Effectiveness of Combined Stimulation
This case series retrospectively reviewed the outcomes in patients implanted with combined, synchronous dorsal root ganglion stimulation (DRGS) and spinal cord stimulation (SCS) connected to a single implantable pulse generator (IPG) in a tertiary referral neuromodulation centre in the United Kingdom. Materials and Methods Twenty-six patients underwent a trial of DRGS+SCS for treating focal neuropathic pain between January 2016 and December 2019, with a follow-up in February 2022. A Transgrade approach was employed for DRGS. Patients were provided with three possible stimulation programmes: DRGS-only, SCS-
only, or DRGS+SCS. Patients were assessed for pain intensity, patients’ global impression of change (PGIC), preferred lead(s) and complications. Results Twenty patients were successful and went on for full implantation. The most common diagnosis was Complex Regional Pain Syndrome. After an average of 3.1 years follow-up, one patient was lost to follow-up, and two were non-responders. Of the remaining 17 patients, 16 (94%) continued to report a PGIC of 7. The average pain intensity at Baseline was 8.5 on an NRS scale of 0-10. At the last follow-up, the average NRS reduction overall was 78.9% with no statistical difference between those preferring DRGS+SCS (n = 9), SCS-only (n = 3) and DRGS-only (n = 5). The combination of DRGS+SCS was preferred by 53% at the last follow-up. There were no serious neurological complications. Conclusion This retrospective case series demonstrates the potential effectiveness of combined DRGS+SCS with sustained analgesia observed at an average follow-up of over three years. Implanting combined DRGS+SCS may provide programming flexibility and therapeutic alternatives.
A single infusion of intravenous lidocaine for primary headaches and trigeminal neuralgia: a retrospective analysis https://pubmed.ncbi.nlm.nih. gov/37638187/
Intravenous (IV) lidocaine has been used as a transitional treatment in headache and facial pain conditions, typically as an inpatient infusion over several days, which is costly and may increase the risk of adverse effects. Here we report on our experience using a single one-hour IV lidocaine infusion in an outpatient day-case setting for the management of refractory primary headache disorders with facial pain and trigeminal neuralgia. This is a retrospective, singlecenter analysis on patients with medically refractory headache with facial pain and trigeminal neuralgia who were treated with IV lidocaine between March 2018 and July 2022. Lidocaine 5 mg.kg-1 in 60 mL saline was administered over 1 h, followed by an observation period of 30 min. Patients were considered responders if they reported reduction in pain intensity and/or headache frequency of 50% or greater. Duration of response was defined as short-term (< 2 weeks), medium-term (2-4 weeks) and long-term (> 4 weeks).
Forty infusions were administered to 15 patients with trigeminal autonomic cephalalgias (n = 9), chronic migraine (n = 3) and trigeminal neuralgia (n = 3).
Twelve patients were considered responders (80%), eight of whom were complete responders (100% pain freedom). The average duration of the treatment effect for each participant was 9.5 weeks (range 1-22 weeks). Six out of 15 patients reported mild and selflimiting side effects (40%).
A single infusion of IV lidocaine might be an effective and safe transitional treatment in refractory headache conditions with facial pain and trigeminal neuralgia. The sustained effect of repeated treatment cycles in some patients may suggest a role as long-term preventive therapy in some patients.
1. Mullins CF, Harris S, Pang
D. A retrospective review of elevated lead impedances in impedance-dependent magnetic resonance-conditional spinal cord stimulation devices. Pain Pract. 2024 Feb;24(2):270-277. doi: 10.1111/papr.13301. Epub 2023 Oct 14. PMID: 37837248.
2. Mullins CF, Royds J, Al-Kaisy A. Radiographic lead migration in percutaneous spinal cord stimulator trials. Reg Anesth Pain Med. 2023 Jul 21:rapm2023-104347. doi: 10.1136/ rapm-2023-104347. Epub ahead of print. PMID: 37479237.
3. Mullins CF, Palumbo GJ, Harris S, Al-Kaisy O, Wesley S, Yearwood T, Al-Kaisy A. Effectiveness of combined dorsal root ganglion and spinal cord stimulation: a retrospective, single-centre case series for chronic focal neuropathic pain. Pain Med. 2024 Feb 1;25(2):116124. doi: 10.1093/pm/pnad128. PMID: 37738574.
4. Mullins CF, Fuccaro M, Pang D, Min L, Andreou AP, Lambru G. A single infusion of intravenous lidocaine for primary headaches and trigeminal neuralgia: a retrospective analysis. Front Neurol. 2023 Aug 10;14:1202426. doi: 10.3389/fneur.2023.1202426. PMID: 37638187; PMCID: PMC10448809.
Dr Cormac Mullins is a pain specialist and anaesthesiologist at the South Infirmary Victoria University Hospital, Cork University Hospital and Affidea at the Elysian, Cork.
Public referrals:
Pain Medicine Unit, South Infirmary Victoria University Hospital, Old Blackrock Road, Cork, T12 X23H.
Private referrals:
Affidea Cork, The Elysian, Cork, T12 KTD1
Email: drcmullins@privateclinic.ie Phone: 01-5293476
73 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Empowering people worldwide to live healthier at every stage of life

With 1,600 people working across five sites in Ireland, Viatris provides access to medicines,
develops innovative solutions and improves healthcare for patients.
Viatris
Newenham Court, Malahide Road, Dublin 17, Dublin, Ireland.
Viatris.ie
Job Code: CC-2023-001
Date of Preparation: July 2023
www.viatris.ie

Prostate Cancer
Application and optimization of prostate-specific antigen screening strategy in the diagnosis of prostate cancer: a systematic review
mortalityratesdifferdependingonthegeographicalarea,where Africanmalesfaceahighmorbidity26.6age-standardizedrateper 100000(ASR)andmortality14.6ASR,whileAsianmalesfacea decreasedmorbidity11.5ASRandmortality4.5ASR(2).Overthe pastfewyears,onestudyreportedthatPCascreeningcanreduce thedeathrateofprostatecancerby20%( 3).Therefore,PSA screeninghasmadegreatprogressinthediagnosisofPCa becauseofitsimportance.
PCascreeningisamedicaldiagnosticpracticebasedon cantigen(PSA)testing.PSAisanenzyme producedbytheprostatethatdegradesagelatinoussemen protein,therebyreleasingmotilesperm(4, 5).Whenprostate epithelialcellsaredestroyedbytumors,largeamountsofPSAare releasedintothebloodstream(5).PSAlevelsarealsoelevatedwhen amed,infected,orbenignprostatehyperplasia
, Aimin Tian1, Jizhong Che1, Yandong Miao2,Yuanyuan Liu1, Yangyang Liu1 and
Department of Urology, Yantai Affiliated Hospital of Binzhou Medical University, The Second Clinical Medical College of Binzhou Medical University, Yantai, Shandong, China, 2Department of Oncology, Yantai Affiliated Hospital of Binzhou Medical University, The Second Clinical Medical College of Binzhou Medical University, Yantai,
).Asaresult,ElevatedPSAisnotenoughtomakea nitivediagnosisofprostatecancer(8).However,PSAcanscreen outpotentialPCapatientsfromthepopulationwhoneedfurther
).PSAtestingiscommonlyusedinmiddle-agedand oldermenwithlowerurinarytractsymptoms(LUTS)andin asymptomaticmenatriskforPCa(10).Patientswithelevated PSAoftenrequireprostatemagneticresonanceimaging(MRI)and/ orprostatebiopsyforfurtherdiagnosis(11).
PCa stands as the prevailing form of cancer among males in over half of the nations across the globe.1 The occurrence and mortality rates differ depending on the geographical area, where African males face a high morbidity 26.6 age-standardized rate per 100 000 (ASR) and mortality 14.6 ASR, while Asian males face a decreased morbidity 11.5 ASR and mortality 4.5 ASR.2 Over the past few years, one study reported that PCa screening can reduce the death rate of prostate cancer by 20%.3 Therefore, PSA screening has made great progress in the diagnosis of PCa because of its importance.
ThePSAtestingwasapprovedbytheU.S.FoodandDrug Administration(FDA)in1986forthepurposeofmonitoringthe advancementofPCa.In1994,theFDAapprovedPSAforPCa screeninginasymptomaticmen.Consequently,therewasa cantincreaseintheprevalenceofPCaduringthe1980sand 1990s,primarilyattributedtotheextensiveutilizationofPSA
PCa screening is a medical diagnostic practice based on prostate-specific antigen (PSA) testing. PSA is an enzyme produced by the prostate that
degrades a gelatinous semen protein, thereby releasing motile sperm.4, 5 When prostate epithelial cells are destroyed by tumors, large amounts of PSA are released into the bloodstream.5 PSA levels are also elevated when the prostate is inflamed, infected, or benign prostate hyperplasia6, 7 (Figure 1). As a result, Elevated PSA is not enough to make a definitive diagnosis of prostate cancer.8 However, PSA can screen out potential PCa patients from the population who need further diagnosis.9 PSA testing is commonly used in middle-aged and older men with lower urinary tract symptoms (LUTS) and in asymptomatic men at risk for PCa.10 Patients with elevated PSA often require prostate magnetic resonance imaging (MRI) and/ or prostate biopsy for further diagnosis.11
screening(12).PCascreeningisaimedatasymptomaticmen.The significanceofPCascreeningistodecreasethedeathrateofPCain thescreenedpopulationwhilemaintainingthequalityoflifefor thosebeingscreened(13).TheprimarypurposeofPCascreeningis toenhancetherateofidentifyingPCaandidentifyingPCaatan earlystage,particularlyPCathatisclinicallysignificant.Menwho areingoodphysicalconditionandhavealifeexpectancyexceeding 10yearsshouldundergoPSA-basedPCascreeningeverytwoyears, constitutingthecurrentfocusofthescreeningtargetpopulationfor PCa.ItisimportanttofocusonPCascreeningamonghigh-risk populations,whichincludemalesagedover50,malesagedover45 withafamilialbackgroundofPCa,andmalesagedover45with BRCA2genemutations(14).Thisisparticularlyimportantfor developingpopulationscreeningstrategiesforPCa. WhilethemortalityofPCahasbeendecreasedbyPSA screening(3),researchhasindicatedthat20to60percentof cancersidenti fi edthroughPSAtestingareinstancesof overdiagnosis(15, 16).Thelong-termfatalriskofPCaremains verylow,especiallyindevelopedcountries(17, 18).Currently,there existnotablevariationsintheprevalenceandfatalityratesofPCa acrossnations.Approximately81%ofnewlyreportedcasesinthe UnitedStatesareclassifiedasclinicallylocalizedPCa,whereasthe percentageisonly33%inChina.Theremainingcasesconsistof advancedormetastaticpatients(19).TheextensivePSAscreening inEuropeandtheUnitedStatesislikelyresponsibleforthese findings.TheprevalenceofPCaintheUnitedStatesincreased signi fi cantlystartinginthelate1980sduetothewidespread adoptionofPSA-basedscreening( 20 ).Consequently, implementingPSAscreeningforhigh-riskpopulationsin
The PSA testing was approved by the U.S. Food and Drug Administration (FDA) in 1986 for the purpose of monitoring the advancement of PCa. In 1994, the FDA approved PSA for PCa screening in asymptomatic men. Consequently, there was a significant increase in the prevalence of PCa during the 1980s and 1990s, primarily attributed to the extensive utilization of PSA screening.12 PCa screening is aimed at asymptomatic men. The significance of PCa screening is to decrease the death rate of PCa in the screened population while maintaining the quality of life for those being screened.13 The primary purpose of PCa screening is to enhance the rate of identifying PCa and identifying PCa at an early stage, particularly PCa that is clinically significant. Men who
75 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
10.3389/fonc.2023.1320681
DiagramofthemechanismofPSAreleaseintothebloodcirculation(madebyonlineFigdraw).
Prostate Cancer
are in good physical condition and have a life expectancy exceeding 10 years should undergo PSAbased PCa screening every two years, constituting the current focus of the screening target population for PCa. It is important to focus on PCa screening among high-risk populations, which include males aged over 50, males aged over 45 with a familial background of PCa, and males aged over 45 with BRCA2 gene mutations.14 This is particularly important for developing population screening strategies for PCa.
comprehensiveexaminationofresearchesontheutilizationof PSAscreeningforPCa.The discussionfocusedonthe importanceofitsvalueindiagnosingandtreatingPCa.Besides, anexaminationwasconductedonthefactorscontributingtothe excessivetreatmentofPCa.Relatedstudiesultimatelypresentedthe optimizationplanforPSAscreeningindetectingPCa.Theaimisto providevaluablesuggestionsfortheoptimizationofPCa screeningstrategies.
Materials and methods
2Materialsandmethods
While the mortality of PCa has been decreased by PSA screening,3 research has indicated that 20 to 60 percent of cancers identified through PSA testing are instances of overdiagnosis.15, 16 The long-term fatal risk of PCa remains very low, especially in developed countries.17, 18 Currently, there exist notable variations in the prevalence and fatality rates of PCa across nations. Approximately 81% of newly reported cases in the United States are classified as clinically localized PCa, whereas the percentage is only 33% in China. The remaining cases consist of advanced or metastatic patients.19 The extensive PSA screening in Europe and the United States is likely responsible for these findings. The prevalence of PCa in the United States increased significantly starting in the late 1980s due to the widespread adoption of PSAbased screening.20 Consequently, implementing PSA screening for high-risk populations in developing nations is a crucial approach for the early detection and management of clinically significant PCa. In 2012, the US Preventive Services Task Force (USPSTF) objected to PSAbased PCa screening, stating that the drawbacks of screening outweigh the advantages with reasonable confidence.This review presents a comprehensive examination of researches on the utilization of PSA screening for PCa. The discussion focused on the importance of its value in diagnosing and treating PCa. Besides, an examination was conducted on the factors contributing to the excessive treatment of PCa. Related studies ultimately presented the optimization plan for PSA screening in detecting PCa. The aim is to provide valuable suggestions for the optimization of PCa screening strategies.
This systematic review adhered to the guidelines of the preferred reporting items for systematic reviews (PRISMA). In order to obtain randomized and nonrandomized screening studies on the utilization of PSA screening in PCa diagnosis, we conducted searches in the most pertinent databases (PubMed and Web of Science). Using the Boolean operator, we combined the terms (PCa and PSA screening) OR (PCa screening and PSA). We considered articles that were published in the English language between July 2013 and July 2023. All of the published articles included in this collection were clinical studies that reported on the utilization of PSA screening for the diagnosis of PCa. Reviews, retrospective and cross-sectional studies were not considered. Due to the variation in age, screening techniques, and regional disparities in PCa treatment among the participants in the study, a meta-analysis was
not conducted. Two reviewers evaluated the quality of bias in all the studies included, using the Cochrane risk bias assessment tool. The outcome indicators of PSA screening were determined by utilizing clinical prognostic indicators of patients.
Results
Literature search
Thissystematicreviewadheredtotheguidelinesofthe preferredreportingitemsforsystematicreviews(PRISMA).In ordertoobtainrandomizedandnon-randomizedscreening studiesontheutilizationofPSAscreeninginPCadiagnosis,we conductedsearchesinthemostpertinentdatabases(PubMedand WebofScience).UsingtheBooleanoperator,wecombinedthe terms(PCaandPSAscreening)OR(PCascreeningandPSA).We
tothevariationinage,screeningtechniques,andregional disparitiesinPCatreatmentamongtheparticipantsinthestudy, ameta-analysiswasnotconducted.Tworeviewersevaluatedthe qualityofbiasinallthestudiesincluded,usingtheCochrane riskbiasassessmenttool.TheoutcomeindicatorsofPSA screeningweredeterminedbyutilizingclinicalprognostic indicatorsofpatients.
3Results
trials were included. All studies included people aged 45-85 years. All included studies were conducted in Sweden, Finland, Netherlands, Spain, United States, England, Germany, China, and some European countries. Three of the studies included more than 100,000 people, five more than 50,000 people, and 16 more than 10,000 people.
3.1Literaturesearch
PubMed and Web of Science yielded a total of 339 articles through a comprehensive search The retrieved literatures were then screened, and reviews,276 retrospective studies and crosssectional studies21 were excluded. In the end, a total of 21 pieces of literature were incorporated into the ultimate examination (Figure 2).
Basic characteristics
Main findings
Impact of PSA screening on prognosis of patients with PCa
PubMedandWebofScienceyieldedatotalof339articles throughacomprehensivesearch.Theretrievedliteratureswerethen screened,andreviews(276),ret rospectivestudiesandcrosssectionalstudies(21)wereexcluded.Intheend,atotalof21 piecesofliteraturewereincorporatedintotheultimate examination(Figure2).
The studies included in this review were published between July 2013 and July 2023. Fifteen randomized screening trials and five non-randomized screening
The controversy surrounding the improvement of outcomes for men with PCa through screening persists, as the potential negative effects of excessive testing and unnecessary treatment may surpass the advantages in terms of potential mortality or quality of life.40 Over the past few years, numerous clinical studies have been carried out by researchers, the majority of which have discovered that screening for PCa can greatly decrease
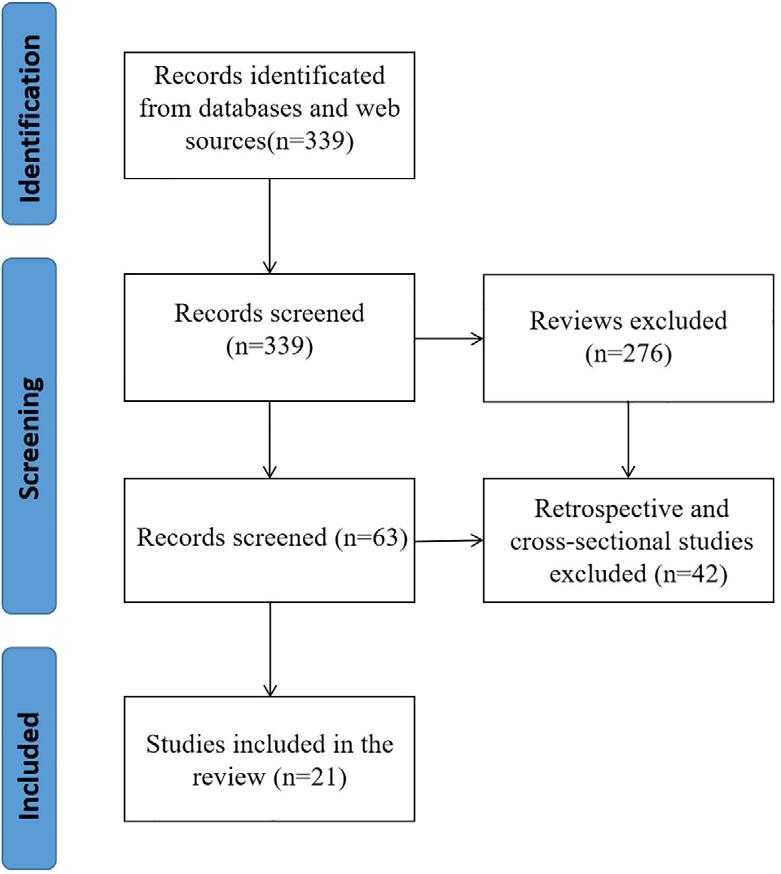
76 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
FIGURE2
Thesystematicreview flowdiagramofselectedstudies.
FIGURE 2
The systematic review flow diagram of selected studies
patient mortality. Additionally, PSA screening has been found to lower the occurrence of PCa metastasis, and early treatment can be initiated through screening cases.24, 25, 28, 32, 34, 40, 41 Nevertheless, certain studies that have conducted extensive monitoring over a prolonged period have discovered that PSA screening does not provide any advantage in terms of survival.23, 26, 30, 33, 35, 36 This is primarily attributed to a decrease in long-term mortality caused by PCa. Therefore, it is particularly important to explore the influencing factors of PSA screening on the prognosis of PCa patients for optimizing PCa screening.
The European Randomized Study of PCa Screening (ERSPC), the largest current study of randomized screening for PCa, found that PSA screening resulted in a significant reduction in PCa mortality after 9 and 11 years of follow-up.21 Due to the variation in the occurrence of PCa across different regions, the treatment methods for it also exhibit substantial differences among these regions. Therefore, the researchers also used the above data to carry out research analysis in each region. J. Hugosson et al.34 used data from eight European countries in the ERSPC trial to determine whether PSA screening reduces PCa mortality up to 16 years. The results of the study that included 182,160 men confirmed that early PSA screening significantly reduced PCa mortality, and the absolute benefit was greater with longer follow-up. This study also confirms that repeated screening may be important in reducing PCa mortality in the population. M. J. Roobol et al.24 evaluated PCaspecific mortality data from the Rotterdam portion of the ERSPC trial. 42,376 men aged 54 to 74 years were randomly assigned to screening and control groups at a frequency of 1 screening every 4 years. After a median follow-up of 12.8 years, PSA-based systematic screening reduced prostatespecific mortality by 32% in the 55-69 year age range. Besides, in order to explore the effects of PCa screening on different age stages, Okhorst et al.25 used part of the Rotterdam data (34,833 men aged 55-69 years) from the ERSPC trial to explore the impact of PCa screening on mortality. The primary endpoint was PCa specific mortality. The results of the study found that PCa screening at the Rotterdam section of the ERSPC reduced the risk of PCa death by 51% compared to men who were not screened for PCa. In addition, J. Hugosson et al.32 randomly assigned 20,000 men aged 50-64 years from the Goteborg
population Registry to receive PSA screening and controls. Men in the screening group were invited to undergo PSA testing every two years until the median age of 69. The study followed for 18 years and found that systematic PSA screening showed a greater PCa mortality benefit among men who began screening at ages 55-59.
Recently, M. Franlund et al.40 analyzed the results of the 22-year follow-up of the Goteborg Randomized PCa screening trial. The results showed that the 22year cumulative PCa mortality was 1.55% in the experimental group and 2.13% in the control group. These studies suggest that PSAbased screening can significantly reduce PCa mortality.
The researchers also attempted to explore the specific mechanisms by which PSA screening leads to reduced mortality in patients. C. Buzzoni et al.28 evaluated the incidence of PCa in study groups according to the risk category at diagnosis to assess the potential impact on PCa mortality. Information on patient groups, centers, T and M stages, Gleason scores, serum PSA at diagnosis, age at randomization, duration of follow-up, and survival status were extracted from the ERSPC database. The results confirmed that in the screening group, the metastatic disease at diagnosis reduced markedly. The results of this study suggest that the reduction in metastatic disease during PSA screening is a major determinant of the reduction in PCa mortality. In addition, S. Neupane et al.41 identified prognostic factors for patients dying from PCa in the Finnish PCa screening trial. The 15-year survival rate was significantly lower in the control group than in the screening group. The study showed that PSA screening led to earlier treatment of cases in the screening group.
However, other similar studies have not found a benefit from PSA screening in reducing mortality in men with PCa. M. Luján et al.26 reported the long-term results of a PCa screening trial conducted in the Mediterranean region. A total of 4,276 men aged 45 to 70 years were randomly assigned to screening and control groups. After 15 years of follow-up, the study failed to demonstrate a benefit of PCa screening in terms of all-cause and PCa-specific mortality. M. Lujan et al.30 also studied all-cause mortality and cancer-specific mortality in the Spanish branch of the ERSPC study. A total of 18,612 men aged 45 to 70 years were randomly assigned to the screening or control group for a median followup of 15.8 years. The study found
no difference in cancer-specific mortality between the two groups. The lower long-term PCa mortality rates found in the above study may be the most important factor contributing to these results. Subsequently, M. Lujan Galan et al.35 provided the latest results of the ERSPC Spanish Research Center follow-up after 21 years. No benefit of PCa screening in terms of overall survival or cancerspecific survival was found in the Spanish study portion of the 21-year follow-up. Similarly, R. M. Martin et al.33 evaluated the effect of a single PSA screening intervention and standardized diagnostic pathways on PCa specific mortality. The Cluster Randomized Trial of PSA Testing for PCa enrolled 419 582 men aged 50 to 69 years. In clinical practices randomized to receive a single PSA screening intervention, there was no significant difference in PCa mortality after a median follow-up of 10 years compared to standard practice without screening. T. P. Kilpelainen et al.23 evaluated mortality outcomes from the Finnish PCa screening trial, the largest component of the ERSPC. The results found that a relatively conservative screening regimen resulted in a small but not statistically significant reduction in PCa-specific mortality at 12 years, at the cost of moderate overdiagnosis. Furthermore, K.Talala et al.36 compared general health-related quality of life (HRQOL) and disease-specific HRQOL in PCa patients with up to 15 years of follow-up in the Finnish population-based Randomized Study of PCa Screening. At 5 to 15 years of follow-up, there were no significant differences in healthrelated quality of life between the PCa screening and control groups.
PSA screening and overdiagnosis of PCa Studies have shown that organized screening reduces PCa mortality, but the effects of opportunistic screening have been largely unknown. R. Arnsrud Godtman et al.27 compared the ability of organized and opportunistic screening to reduce PCa mortality and the risk of overdiagnosis. The Goteborg Screening study has randomly selected 10,000 men since 1995 for a PSA test every 2 years and recommends prostate biopsies for men with PSA≥2.5 ng/ml. The study found that organized screening reduced PCa mortality, but was associated with overdiagnosis. Opportunistic PSA testing had little impact on PCa mortality and led to more overdiagnosis. Besides, S. D. Walter et al.12 estimated overdiagnosis rates using Finnish data from the ERSPC trial. The study defined the
overdiagnosis rate as the relative excess cumulative incidence in the screening group at this time. Studies have shown some overdiagnosis in screening, but the extent is uncertain. In addition, a study from China suggests that early screening is more cost-effective than no screening for high-risk prostate cancer patients.37 Therefore, promoting early screening for high-risk prostate cancer patients is also a valuable strategy.
PCa screening relies on a careful balance of benefits in reducing PCamortality and harms in terms of overdiagnosis and overtreatment. A. J. Vickers et al.16 evaluated the impact of limiting PSA testing based on age and baseline PSA on overdiagnosis. Two independent cohorts (1,577 and 1,197 participants, respectively) were included in the PSA screening group, with a Swiss cohort that had not received PSA screening serving as a control group, and the included cohorts were followed for 25 years. Studies have found that overdiagnosis of PCa is closely related to age and PSA level. Limiting screening of men older than 60 to those with PSA above the median (>1 ng/ml) would critically reduce overdiagnosis. These studies suggest that in order to avoid overdiagnosis, PSA screening strategies need to be optimized, and screening populations need to be selected for appropriate age stages and PSA levels.
Optimization of PSA screening strategy
Most of the available findings show a significant reduction in PCa mortality among men screened in the intervention group. Nevertheless, there are also studies that suggest it can lead to problems such as overdiagnosis. Therefore, it is necessary to optimize PSA screening strategies through existing studies in order to reduce PCa-specific mortality, overdiagnosis and cost, and improve the quality of life of patients.
The optimization of PSA screening strategy first needs to optimize the screening population. E. A. Heijnsdijk et al.29 used a microsimulation model based on data from the ERSPC trial to predict the cost-effectiveness of various screening strategies starting at age 55 with a PSA threshold of 3. The study found that 2 to 3 PCa screenings in the 55-59 age group were cost-effective. Due to the loss of quality-adjusted life-years (QALYs) due to overdiagnosis, screening is less cost-effective in people older than 63 years. Similarly, A. Grenabo Bergdahl et al.22 explored
77 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
screeningontheincidenceofPCaindifferentriskgroups.
ParticipantsintheFinnishtrialscreeninggroup(31,867men)
Prostate Cancer
edbasedonscreeningfrequency.Theresultsshowed thattheincidenceoflowriskPCaincreasedwiththenumberof screeningtimes,whiletheincidenceofintermediateandhighrisk PCawasnotsignificantlyassociatedwiththenumberofscreening times.SinglescreeningshowsnobenefitinPCaincidence,and repeatedscreeningisnecessarytorealizethescreeningadvantage.
the risk of PCa after stopping screening. The study included 20,000 men with an average age of 69 years in the Goteborg area of Sweden who underwent PSA randomized screening. The sthdy found that nine years after PSA testing stopped, the incidence of potentially fatal cancers was comparable to that of men who had not been screened. This study reaffirms that PSA screening can be stopped in patients over 70 years of age. Besides, the researchers explored the appropriate age for PSA screening. S. Carlsson et al.31 evaluated the effect of PSA screening initiated at ages 50 to 54 on PCa mortality. The study found that PSA screening for PCa reduces PCa mortality in men aged 50-54 years, which is comparable to the results of a previously reported randomized study of PCa screening in men aged 55-69
years in Europe in a similar followup. Guidelines may consider whether PSA screening guidelines recommend starting screening no later than age 50-54. A randomized trial (PROBASE) conducted by C. Arsov et al.39 recruited 46 642 men aged 45 years to determine the efficacy of risk-adapted PSA screening starting at age 45 or 50 years. The prevalence of screendetected aggressive PCa was very low among men aged 45 years. Therefore, PSA screening is recommended to start after 50 years old. At present, there is no accepted initial age for PCa screening.
Inthemethodologicalassessmentofthequalityoftheincluded literatures(Figure3),16ofthemwereatmoderateriskofbias,and thequalitywasrelativelyhighduetothelargenumberofincluded studypopulations.In Figure3A,thosethatmeetthestandardare
“ + ” andthosethatfailtomeetthestandardare “” Figure3B statisticalchartoftheproportionofeachiteminthe methodologicalassessment.
4Discussion
Furthermore, the selection of the optimal PSA screening frequency is also particularly important for PCa screening. T. Pakarainen et al.38 explored the effects of participation in screening on the incidence of PCa in different risk
E.Kovacetal.(18)assessedthelong-termriskofanyPCaand clinicallysignificantPCainmenaged55-60yearsbasedonbaseline PSAlevels.TheresultsfoundthatbaselinePSAlevelsinmenaged 55to60yearswereassociatedwithalong-termriskofclinically cantPCa.These findingssuggestthatthefrequencyofrepeat screeningcanbereducedinmenaged55-60yearswithlowbaseline PSAlevels(lessthan2.00ng/mL),andscreeningmaybe discontinuedinmenwithbaselinePSAlevelsbelow1.00ng/mL. TooptimizethefrequencyofPSAscreening,R.Landyetal.(42) assessedthe5-,10-,and15-yearrisksofinvasivecancerandPCarelateddeathamongmenwithbaselinePSAlevelsof0.5ngper milliliterorless,1ngpermilliliterorless,and1.01to2.5ngper
groups. Participants in the Finnish trial screening group (31,867 men) were classified based on screening frequency. The results showed that the incidence of low risk PCa increased with the number of screening times, while the incidence of intermediate and high risk PCa was not significantly associated with the number of screening times. Single screening shows no benefit in PCa incidence, and repeated screening is necessary to realize the screening advantage. E. Kovac et al.18 assessed the long-term risk of any PCa and clinically significant PCa in men aged 55-60 years based on baseline PSA levels. The results found that baseline PSA levels in men aged 55 to 60 years were associated with a long-term risk of clinically significant PCa. These findings suggest that the frequency of repeat screening can be reduced in men aged 55-60 years with low baseline PSA levels (less than 2.00 ng/mL), and screening may be discontinued in men with baseline PSA levels below 1.00 ng/mL. To optimize the frequency of PSA screening, R. Landy et al. (42) assessed the 5 -, 10 -, and 15-
year risks of invasive cancer and PCarelated death among men with baseline PSA levels of 0.5 ng per milliliter or less, 1 ng per milliliter or less, and 1.01 to 2.5 ng per milliliter. The study found that for 45% of men with PSA ≤ 1 ng/mL, a 5-year screening interval may be appropriate. Men ≥65 years of age with PSA ≤ 0.5 ng/mL may consider stopping screening.
Accordingtothe2001updatedguidelinesfromtheAmerican CancerSociety,thereisstilluncertaintyregardingtheoverall effectivenessofPSAscreeninginreducingthelikelihoodofdeath fromPCa.NumerousclinicaltrialsinvestigatingPSAscreening haveconsistentlydemonstratedadecreaseinmortalityassociated withPCa(24, 25, 28, 32, 34, 40, 41).Additionalresearchrevealed thatthedeclineinthespreadofcancertootherpartsofthebodyas aresultofPSAscreeningwastheprimaryfactorinthedecreaseof mortalitycausedbyPCa.Moreover,PSAscreeningfacilitated promptinterventionforindiv idualsinthescreeninggroup.
Assessment for the risk of bias
In the methodological assessment of the quality of the included literatures (Figure 3), 16 of them were at moderate risk of bias, and the quality was relatively high due to the large number of included study populations. In Figure 3A, those that meet the standard are “+” and those that fail to meet the standard are “-”. Figure 3B is a statistical chart of the proportion of each item in the methodological assessment.
Discussion
According to the 2001 updated guidelines from the American Cancer Society, there is still uncertainty regarding the overall effectiveness of PSA screening in
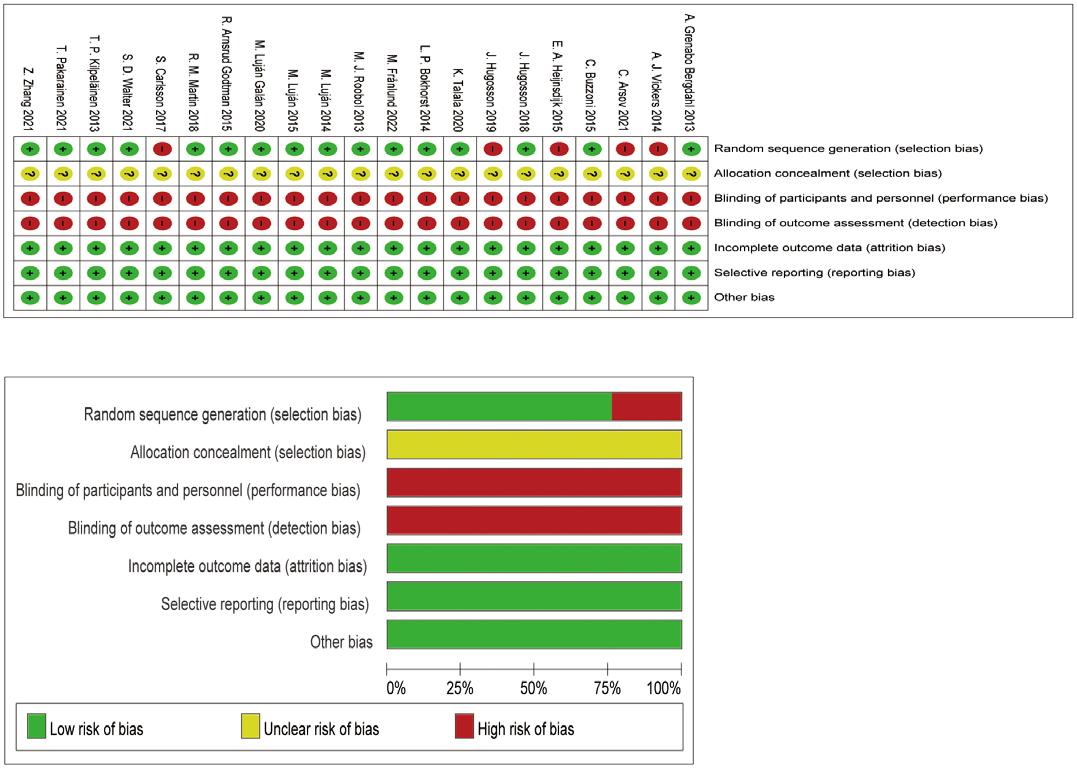
78 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
Pakarainenetal.( 38 )exploredtheeffectsofparticipationin
fi
wereclassi
B A Assessmentfortheriskofbias. (A) showsthequalityoftheincludedliteratureevaluatedbydifferentitems; (B) showstheproportionofquality assessmentitemsintheincludedliterature.
FIGURE 3
Assessment for the risk of bias. (A) shows the quality of the included literature evaluated by different items; (B) shows the proportion of quality assessment items in the included literature
reducing the likelihood of death from PCa. Numerous clinical trials investigating PSA screening have consistently demonstrated a decrease in mortality associated with PCa.24, 25, 28, 32, 34, 40, 41 Additional research revealed that the decline in the spread of cancer to other parts of the body as a result of PSA screening was the primary factor in the decrease of mortality caused by PCa. Moreover, PSA screening facilitated prompt intervention for individuals in the screening group.
Nevertheless, certain randomized experiments have failed to demonstrate a survival advantage of PSA screening in males diagnosed with PCa.26, 30, 35, 43 With the rapid development of PCa treatment modalities, the longterm reduction of PCa mortality will weaken the impact of PSA screening on patient mortality, which may be the reason for the different results of PSA screening related studies.
Hence, the utilization of PSA screening for PCa is a subject of debate. PSA screening can effectively decrease patientspecific mortality in the diagnosis and treatment of PCa. However, some scholars argue that it may also increase the risk of overdiagnosis and overtreatment due to PCa’s slow-growing nature. Due to its comparatively
sluggish progress, PSA screening is capable of identifying certain cancers that may otherwise go undetected during a man’s lifetime.44 However, it is important to note that diagnosing these abnormalities through screening does not effectively decrease mortality rates. The reason for this excessive diagnosis could be the existence of tumor slowdeveloping or inactive growths that can stay without symptoms for numerous years. Harmful consequences may arise as a result of screening in such instances. In addition, the above situation may also be due to regional differences, such as differences in the overall followup results of ERSPC study data and follow-up results in different regions.21, 24, 30, 34, 35 After all, the present therapeutic techniques for PCa are progressing swiftly, and the influence of varying degrees of treatment on the particular fatality rate of examined individuals is substantial.
Numerous studies have verified that the screening of PSA can enhance patients’ prognosis and decrease mortality rates. However, large-scale PSA screening not only leads to overtreatment but also imposes a certain economic burden on social health care. To address this issue, it is necessary to enhance the effectiveness of PSA screening strategies.
Research has indicated that PSA testing for PCa can decrease PCa death rates in males between the ages of 50 and 54, and it is advised to initiate PSA screening by the age of 50 to 54. For patients at high risk of PCa, the age of screening should be appropriately reduced. Given that the predictive advantage of PSA screening for PCa diminishes in males above the age of 70, it is recommended to discontinue PSA screening in individuals aged 70 and above. Individuals between the ages of 55 and 59 should undergo two or three screenings for PCa. The incidence of PCa does not benefit from a single screening, and to achieve the advantage of screening, it is necessary to undergo repeated screenings. Furthermore, men with a PSA level of ≤1 ng/mL may find a 5year screening interval suitable, and for men aged ≥65 with a PSA level of ≤0.5 ng/mL, the option of discontinuing screening could be contemplated. Furthermore, screening can be less frequent for men aged 55-60 years with a baseline PSA level below 2.00 ng/mL.
This study also has limitations, as most of the included studies had a risk of bias score of moderate risk of bias. In addition, a part of the included studies analyzed the data of multi-center studies in different regions or with different follow-up
times, resulting in heterogeneity such as regional differences and different follow-up times in the included studies, so no Metaanalysis was performed. However, the population size of the selected studies is large, so the conclusions obtained through comprehensive analysis are also reliable.
Conclusions
To summarize, the aforementioned studies indicate that PSA screening is effective in reducing mortality specifically related to PCa. The overdiagnosis and overtreatment of PCa occur because of the low long-term specific mortality of PCa, which is due to the inert nature of PCa and advancements in comprehensive treatment technology. Hence, it is crucial to enhance the suitability of PSA screening for specific age groups, modify the screening frequency, and determine the optimal PSA levels. This will aid in the development of a personalized screening program, thereby enhancing the effectiveness of PSA screening in diagnosing PCa. Data availability statement
The original contributions presented in the study are included in the article/ supplementary material. Further inquiries can be directed to the corresponding author.
References available on request
Reshaping the Lives of those with Chronic Kidney Disease
Renal services across the country are transforming the lives of patients with chronic kidney disease, improving quality of life through effective care and treatment that fits around people’s lives.
On World Kidney Day 2024 (Thursday, 14 March 2024), the HSE highlighted how patient health and wellbeing is improving with increased access to home dialysis services.
Last year:
• 100,000 home dialysis therapy treatments were delivered to patients
• over 45,000 hospital visits avoided
• up to 300,000 hours saved of patients’ time
Professor George Mellotte, HSE National Clinical Lead for Renal Services, and Consultant Renal Physician, Tallaght University Hospital says, “There were 5,257 people with chronic kidney disease or failure treated by dialysis or kidney transplantation in Ireland in 2023, an increase of 109 from the previous year. More than 440,000 dialysis treatments took place, with the majority of patients having to travel three times per week to one of 24 dialysis centres across the country for treatment.
each patient on this type of dialysis can avoid 150 hospital visits a year. Our patients tell us it provides great flexibility as they can manage their treatment in the comfort of their own homes, at a time that suits them and their family.”
The number of patients with chronic kidney disease in Ireland is increasing. This is likely related to the ageing population, as well as newer treatment strategies that support people to live longer. Chronic kidney disease is more common as people age, leaving patients vulnerable to other medical complications. A diagnosis is often unexpected as it is a silent disease, but is easily confirmed with simple blood and urine tests.
Nationally, key overall figures for renal services include:
• In 2023, the HSE provided over 440,000 dialysis treatments, 100,000 of which are home dialysis
• The number of patients requiring treatment by dialysis or kidney transplantation in Ireland increased to 5,190 adults and 67 children in 2023
• The number of patients in Ireland with the functioning kidney transplant is 2,755
• The number of patients treated by dialysis now stands at 2,502 (1,461 receive haemodialysis treatment in a HSE hospital based dialysis units, and 736 in HSE contracted dialysis units. 305 patients carry out dialysis in their own home).
“This is why we have developed and funded a modernised care pathway for home dialysis, which can significantly improve the patients’ quality of life, meaning News
79 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Clinical R&D
BEAUMONT HOSPITAL CELEBRATES PATIENT LIVING WITH DONOR KIDNEY TRANSPLANTED 50 YEARS AGO
Staff at Beaumont Hospital had the pleasure of last week welcoming a patient to the Kidney Clinic to celebrate a kidney transplanted 50 years ago.
Christine Kelly and her family were welcomed to Beaumont Hospital last week, 50 years after she received a donor kidney transplant.
Christine developed kidney failure in the early 70s and was receiving haemodialysis prior to her transplant in Jervis St Hospital. In 1974 Christine’s sister Mary donated her kidney which was successfully transplanted by Mr Peter McClean FRCSI on 1st March 1974.
The transplant was carried out at Jervis Street whilst Beaumont Hospital has been responsible for Christine’s care since 1987 when Jervis St Hospital moved to Beaumont.
Despite various health challenges over the years Christine has enjoyed good health and quality of life with thanks to the generosity of her sister.
Speaking to mark the occasion
Professor Peter J Conlon, Lead Clinical Director, Beaumont, said, “We were delighted to welcome Christine and her family to the clinic to celebrate 50 years since her successful kidney transplant.

Christine’s story demonstrates the success of organ transplantation and gives hope to all patients with kidney failure.
Christine, with thanks to the generosity of her sister in 1974 has lived successfully with her transplant enjoying good health and quality of life. This is a momentous occasion for Christine, her family and Beaumont and Jervis St Hospitals. Her story underpins the importance of organ donation in saving lives.”
Individuals who wish to support organ donation are encouraged to share their decision with their loved ones and to keep reminders of their decision by carrying the organ donor card, permitting Code 115 to be included on their driver’s licence, or having the ‘digital organ donor card’ App on their smartphone.
DAMIEN O’DOWD APPOINTED CHIEF EXECUTIVE OF ST JOHN OF GOD HOSPITAL
Saint John of God Hospital announces the appointment of Mr Damien O’Dowd as the new Chief Executive of the Hospital and its associated services Mental Health First Aid Ireland and St Joseph’s Centre, Shankill.
Mr O’Dowd is a senior healthcare executive and director with over twenty years’ experience at leadership and senior level management level within healthcare organisations and services in both the private and voluntary healthcare sectors.
Damien O’Dowd’s appointment sees his return to St John of God Hospital having commenced his career there in the 1990’s. He has most recently held Senior Consultant and Director roles in Vision Consulting and Virtus Health respectively and was Chief Executive of the Bloomfield Health Service between the years 2011 and 2018.
Damien has an in-depth experience in the design and successful implementation of strategies for healthcare organisations focussed on continuous service improvement. He is highly effective in change management and operational management of busy and complex healthcare organisations with particular experience of planning and delivering risk management, quality assurance & accreditation systems. He is equally recognised for his experience in developing systems of governance at both clinical and corporate levels in healthcare and related organisations and also has extensive experience in regulatory preparedness & compliance.
Damien has a strong business growth and service development

acumen with wide experience of financial management & healthcare funding.
Mr O’Dowd holds a Masters Graduate of the School of Law, Queens University Belfast and an MBA Graduate of the Smurfit Graduate School of Business, University College Dublin. He also lectures in management and leadership at undergraduate and post graduate levels.
Speaking about his new appointment, Damien O’Dowd said: “I am delighted to rejoin St. John of God Hospital as CEO, where I first began my career in mental health over 30 years ago and which remains a leading service provider of mental health care in Ireland. I am privileged to be leading an excellent team of highly experienced healthcare professionals and we will continue to work together to elevate the patient experience across all our associated services. The healthcare landscape is everevolving and this is a time of positive change and renewal within our sector. Going forward, we will continue to prioritise service excellence and the well-being of all our patients and the communities we serve.”
NEW PHASE 2B RESULTS FOR AMLITELIMAB SUPPORT POTENTIAL FOR BESTIN-CLASS MAINTENANCE OF RESPONSE IN ATOPIC DERMATITIS
Positive results from Part 2 of the investigational amlitelimab Phase 2b study STREAM-AD showed sustained improvement of signs and symptoms for 28 weeks in adults with moderate to severe AD who previously responded to amlitelimab and continued treatment. High responder rates were also observed in participants who were taken off amlitelimab. The safety profile was consistent
with Part 1 of the study with amlitelimab being well-tolerated and no new safety concerns identified. These results were presented as part of a latebreaking session at the American Academy of Dermatology (AAD) 2024 Conference in San Diego and support the quarterly (every 12-week) dosing of amlitelimab 250 mg with 500 mg loading dose (LD) now being investigated in a larger Phase 3 clinical program (OCEANA).
Professor Stephan Weidinger, M.D, Ph.D, Director, Professor, Chair of Department of Dermatology and Allergy, University Hospital SchleswigHolstein said, “Despite available treatment options, not all patients with moderate-to-severe atopic dermatitis respond sufficiently to these treatments, and many continue to suffer from skin lesions and symptoms such as persistent itch, which can have a high impact on their day-to-day lives. Results from this part of the study indicate amlitelimab’s potential for durable off-drug efficacy which supports the evaluation of a less frequent every 12-week dosing. This could offer an important benefit in the treatment of AD patients.”
In the second part of the doseranging STREAM-AD study, responders to amlitelimab who achieved a 75% improvement in Eczema Area and Severity Index (EASI-75) score and/or Investigator Global Assessment (IGA) score of 0 or 1 during the 24-week treatment period (Part 1) were re-randomized to explore the maintenance of clinical response over an additional 28-week period with continued amlitelimab treatment or amlitelimab withdrawal. Across all dose arms, patients who continued amlitelimab treatment maintained high EASI-75 and/or IGA 0/1, IGA 0/1, and EASI-75 responder rates through 28 weeks. High responder rates were also demonstrated among patients who were taken off treatment.
In 69.2% of patients with continued treatment with amlitelimab 250 mg Q4W with 500 mg loading dose (LD) vs 58.8% of patients withdrawn from treatment IGA 0/1 and/or EASI-75 response was maintained.
An analysis including pooled dose-arms showed that IGA 0/1 response was maintained in 71.9% of patients with continued treatment vs 57% of patients withdrawn from treatment. In this analysis, EASI-75 response was maintained in 69% of patients with continued treatment vs. 61.6% of patients withdrawn from treatment.
AD-related biomarkers remained reduced at Week 52 in both
80 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
Mr Damien O’Dowd, Chief Executive, Saint John of God Hospital
Professor Peter J Conlon, Lead Clinical Director, Beaumont with Christine Kelly
amlitelimab withdrawn and continuing groups, despite amlitelimab reaching negligible levels in serum. Reduction of TARC, eosinophils, and IL22 observed at Week 24 was maintained during withdrawal as well as in patients continuing treatment to Week 52. These biomarker data suggest the modulation of inflammatory T cells via the blockade of OX40L and durable control of AD after amlitelimab withdrawal.
The aggregated safety profile of amlitelimab in Part 2 of this study was consistent with that of Part 1, with amlitelimab being well-tolerated, and no new safety concerns were identified during the 28-week maintenance/withdrawal period. Overall rates of treatmentemergent adverse events (TEAEs) were 69.8% for continued amlitelimab treatment, 71.9% for the amlitelimab withdrawal-arm and 66.7% for placebo. TEAEs more commonly observed included headache (11.6% amlitelimab continuation, 3.9% amlitelimab withdrawal, 6.7% placebo), upper respiratory tract infection (9.3% amlitelimab continuation, 5.5% amlitelimab withdrawal, 20% placebo). No adverse events such as fever or chills, oral ulcers or conjunctivitis were observed across doses.
Amlitelimab is a fully human non-T cell depleting monoclonal antibody that blocks OX40-Ligand, a key immune regulator, and has the potential to be a first- or bestin-class treatment for a range of immune-mediated diseases and inflammatory disorders, including moderate-to-severe atopic dermatitis (Phase 3), asthma (Phase 2), hidradenitis suppurativa (Phase 2), scleroderma, celiac disease, and alopecia (Phase 2 studies to be initiated in 2024). By targeting OX40-Ligand, amlitelimab aims to restore balance between proinflammatory and regulatory T cells.
Amlitelimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
ABBVIE RECOGNISED AMONG BEST LARGE WORKPLACES IN IRELAND AT 2024 GREAT PLACE TO WORK AWARDS
AbbVie, the global biopharmaceutical company, has been listed as one of the best places to work in Ireland at the 2024 Great Place to Work Awards. It is the eleventh consecutive year that the company has featured on the list.
AbbVie, which employs approximately 2,600 people across six locations in Ireland, was

recipients and the Taoiseach, Leo Varadkar TD. The awards celebrated individuals and partners who have significantly contributed to Vision Ireland's mission, impacting various aspects of everyday life.
Those who were honoured on the evening included:
• Jason Smyth MBE QFA and Karen Byrne, for their remarkable efforts in changing public perceptions through their achievements on "Dancing With The Stars." The duo was lauded for their inspiring performances, highlighting the potential within everyone, regardless of vision impairment.
ranked 13th Best Large Company in Ireland at the awards, and was joined by 36 other companies on this prestigious roll call.
Great Place to Work has previously recognised AbbVie as a Best Workplace for Women for the fifth consecutive year in 2023 and was one of 19 organisations included in the most recent Best Workplaces in Pharma & Healthcare list.
Speaking about this new accolade, Andres Rodrigo, General Manager at AbbVie (Ireland), said:
“Being named as one of Ireland’s top large workplaces again this year is an incredible accomplishment for the company and an important recognition of the high-performing, inclusive culture that we’ve created over the past eleven years.
“This latest recognition reflects the various AbbVie programmes that support meaningful development, employee well-being, diversity and inclusion, as well as long-standing initiatives which help to support and positively impact our local communities,” he added.
Great Place to Work assesses the policies and practices in place in organisations under key areas including employee development, hiring practices, employee wellbeing and social impact. It then benchmarks these against other organisations in other countries.
Employee development continues to be a key priority area for AbbVie and the company has rolled out a number of mentorship programmes, harnessing the capabilities and experience of leaders, graduates and other colleagues in the organisation.
AbbVie also supports key diversity and inclusion projects, including the launch of the company’s Pride, Asian Leader Network, and Ability, employee groups at its sites across Ireland.
AbbVie’s award-winning Week of Possibilities volunteering initiative and the Employee Giving Campaign also provide employees with opportunities to personally improve and impact the communities in which AbbVie people work and live.
Commenting on the awards, CEO at Great Place to Work Ireland, Cathal Divilly, said, “I'm delighted to extend a huge congratulations to all the exceptional companies recognised as a Best Workplace in Ireland 2024. This achievement is not just a milestone but a reflection of their tireless efforts at crafting workplaces where talent not only flourishes, but also feels valued and empowered. I'd like to congratulate these organisations once again on this outstanding achievement, and may this recognition fuel their continued journey towards excellence and innovation,” he added.
VISION IRELAND, THE NEW NAME FOR NCBI, CELEBRATES OUTSTANDING ACHIEVEMENTS AT DÁIL ÉIREANN
Vision Ireland, the new name for NCBI, is thrilled to announce the resounding success of its 2nd Annual Oireachtas Celebration of Achievement, held on Thursday, 29th February 2024.
Hosted in the Dáil member's dining room by Senator Sen Martin Conway, the evening began with a meet & greet involving the award
• Evie Smurfit Baxter, named Youth Advocate of the Year, for her resilience and advocacy in the face of Stargardt’s disease. Her work, including an animation featured in cinemas, demonstrates the unlimited possibilities for those facing visual challenges.
• Jo Scutt, recognised as Vision Ireland Shop Volunteer of the Year, for her decade-long dedication and fundraising creativity, including marathon running and local charity events, significantly benefiting Vision Ireland.
• Peter Ryan, honoured as Fundraiser of the Year, for his extraordinary effort running from Malin Head to Mizen Head, raising over ¤120,000. His journey from visual impairment to finding a new purpose through sport embodies the spirit of determination.
• Campion Insurance, celebrated for its partnership with Vision Ireland, supporting the innovative Vision Van project, showcasing the power of collaboration in making a tangible difference in the community.
The event also extended heartfelt gratitude to Dr Terry Cross OBEfor his generous auction prize donations, which played a crucial role in raising significant funds during the evening. Also in attendance on the evening was last year's award winner Denise Harris. Both Dr. Cross, Denise Harris’s and all who attended ongoing support exemplifies the community's commitment to Vision Ireland's cause.
The Vision Ireland Foundation extends its sincerest thanks to all guests, honorees, and supporters for their part in making the event memorable. The foundation looks forward to continuing its mission and celebrating more achievements in the years to come.
81 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Back Row (L-R): Arindam Guha, AbbVie Westport; Clare O’Donnell, AbbVie Westport; Blayne Walsh, AbbVie North Dublin; Andrew Shaw, AbbVie Citywest; Marco Froehlich, AbbVie North Dublin; Elizabeth Grogan, AbbVie Citywest, Dublin; Gary O’Mahoney, AbbVie Ireland; Geraldine McGroary, AbbVie Ballytivnan, Sligo; Gerard Byrne, AbbVie North Dublin; and Denise Bulman, AbbVie Cork.
Front Row (L-R): Andres Rodrigo, AbbVie Citywest, Dublin; and Rakesh Dontireddy, AbbVie Manorhamilton Road, Sligo.
Clinical R&D
ACCORD HEALTHCARE LAUNCH NEBIVOLOL 5 MG TABLETS
Accord Healthcare is delighted to announce the launch of Nebivolol 5 mg tablets which come in packs of 28 tablets.
This medicine is indicated for:
• Hypertension
• Treatment of essential hypertension.
• Chronic heart failure (CHF)
Treatment of stable mild and moderate chronic heart failure in addition to standard therapies in elderly patients >70 years
Please refer to the Summary of Product Characteristics (SPC) for further information. The SPC will be available at www.hpra.ie and for Healthcare Professionals atwww.accord-healthcare.ie from launch.
Nebivolol will be available from both full-line wholesalers from launch. For further information please contact Accord Healthcare in Cork on 021-461 9040.
DUPIXENT® SBLA ACCEPTED FOR FDA PRIORITY REVIEW FOR TREATMENT OF COPD WITH TYPE 2 INFLAMMATION
The U.S. Food and Drug Administration (FDA) has accepted for Priority Review the supplemental Biologics License Application (sBLA) for Dupixent® (dupilumab) in a sixth potential indication as an add-on maintenance treatment in certain adult patients with uncontrolled chronic obstructive pulmonary disease (COPD). The target action date for the FDA decision is June 27, 2024. Regulatory submissions are also under review in China and the European Union.
The sBLA, as well as other submissions around the world, is supported by data from the Phase 3 COPD clinical research program evaluating the efficacy and safety of Dupixent in adults who were current or former smokers with uncontrolled COPD with evidence of type 2 inflammation (screening blood eosinophils >300 cells/ microliter). All patients were on background maximal standardof-care inhaled therapy (nearly all on triple therapy). The primary endpoint was met in both trials (BOREAS, NOTUS), showing Dupixent significantly reduced
moderate or severe acute COPD exacerbations by 30% and 34% respectively, compared to placebo. In both trials, Dupixent also rapidly and significantly improved lung function compared to placebo, with improvements sustained at 52 weeks.
Safety results in both trials were generally consistent with the known safety profile of Dupixent in its approved indications. Adverse events more commonly observed with Dupixent (≥5%) compared to placebo in either trial were back pain, COVID-19, diarrhea, headache and nasopharyngitis.
Priority Review is granted to regulatory applications seeking approval for therapies that have the potential to provide significant improvements in the treatment, diagnosis or prevention of serious conditions. The potential use of Dupixent in COPD is currently under clinical development, and its safety and efficacy for this indication have not been fully evaluated by any regulatory authority.
FIRST-OF-ITS-KIND ROBOTICASSISTED SPINAL FUSION SURGERIES
Mater Private Network has completed two new types of robotic-assisted spinal fusion surgeries which mark the inaugural use of this cutting-edge technology in the Republic of Ireland.
Since its introduction in 2019, robotic-assisted spinal surgery has transformed surgical practices at Mater Private Network. This innovative approach empowers surgeons to perform minimally invasive procedures with precision and accuracy, offering patients several important benefits, including reduced incision size and greatly improving post-operative discomfort, as well as minimal blood loss and muscle trauma.
In January 2024, a patient (60s) with adult scoliosis presented to the Mater Private Dublin, having previously undergone surgery on their spine to decompress and relieve pressure on the spinal nerves. Unfortunately, all previous surgeries were unsuccessful, and the patient presented with continued and progressive pain in their lower back and legs. This patient had undergone physiotherapy and had received pain relief injections for chronic pain, but neither provided a satisfactory outcome.
In this context, Mr. Deb Roy, Spinal Surgeon at Mater Private Network, took on the case. He performed an extreme lateral interbody fusion with assistance from the latest in roboticassisted surgical technology.
This minimally invasive surgery involved gaining access to the patient’s spine through a small incision on the side. Once the spine and the nerves around the spine were identified, Mr. Roy removed damaged discs and replaced them using an interbody cage with bone graft.
A second case involved a patient (50s) presenting with progressive pain in the lower back and legs. The pain was so severe that the patient was unable to work or sleep, severely impacting their quality of life. Pain management plans had previously been put in place for the patient, to no avail. To treat this case, Mr. Roy, Spinal Surgeon at Mater Private Network, performed a micro-access interpedicular lumbar fusion. Gaining access to the spine through the patient’s back using a small incision, Mr. Roy thereafter decompressed, stabilised and fused the affected area of the spine.
Mr. Roy commented "It is an honour to have conducted these pioneering surgeries with Mater Private Network. These procedures mark a significant milestone in spinal care in the Republic of Ireland and highlight how we can harness the technologies available to us to drive better outcomes for patients."
Committed to the adoption and utilisation of the latest in medical technology, Mater Private Network continues to show its commitment to innovation and patient-centric care, enhancing patient outcomes across its network in Ireland.
To learn more about the services offered by Mater Private Network, visit www.materprivate.ie
MAJOR ENHANCEMENTS TO WOMEN’S HEALTH
Sligo University Hospital (SUH) has welcomed Minister for Health Stephen Donnelly, TD to formally launch two significant enhancements in gynaecology and maternity services at the hospital. Minister Donnelly has cut the ribbon on a new Ambulatory Gynaecology clinic in Sligo University Hospital.
The new service in SUH is part of a national plan to improve health outcomes for women through a new model of care. The clinic operates a “see and treat” model which means that women who need gynaecological care can be assessed, treated and discharged on the same day all within this “one stop” setting.
The establishment of the new ambulatory clinic in SUH is reducing the need for women to have multiple gynaecology
appointments for a single episode of care and this in turn is having a positive impact on waiting lists and in-patient bed usage at the hospital, with waiting lists for outpatient appointments reduced by over 50%.
Women are referred to the clinic by their GP and have access to same day diagnostics, investigations, treatments and minor procedures under a team of consultants and specialist nurses. The new unit provides both diagnostic and therapeutic procedures for common gynaecological conditions including hysteroscopies, ultrasound scans, biopsies and management of intrauterine devices.
Speaking at the official opening Minister Donnelly said, “As Minister I’ve made developing better women’s health services a priority during the lifetime of this Government. The unprecedented levels of funding in women’s health services are now delivering for women. I know that this clinic is a positive, on-the-ground improvement, that is already making a real difference to the women of Sligo and the Northwest, reducing waiting times and improving access to services.”
Minister Donnelly also visited the Seomra Suaimhnis / Serenity Room which is a wonderful new labour and delivery suite developed by the maternity service in SUH with the support of the National Women and Infants Programme (NWIP).
The suite is designed around a “home from home” approach, the décor and atmosphere aim to create a relaxed and less clinical environment for women to give birth in. Women also have access to a heated birthing pool in the suite which can help with pain relief during their labour.
Grainne Mc Cann, Hospital Manager at SUH said, “The opening of the Ambulatory Gynaecology clinic in SUH is a major step forward in the delivery of women centred care in our region. Since opening last year we have seen over 320 women in the clinic and have delivered a fully integrated model of care to these patients.
“There is a huge patient benefit involved in the “see and treat” model, the most important being improved access to specialist care for women who need to avail of it.”
Speaking about the Seomra Suaimhnis / Serenity Room Grainne added, “In SUH we welcome nearly 1,300 babies
82 APRIL 2024 • HPN | HOSPITALPROFESSIONALNEWS.IE
into the world every year, I’m delighted we can now offer some of our mums a beautiful, relaxed and homely space to facilitate a positive birth experience.”
LAYA HEALTHCARE OPENS SECOND LAYA HEALTH AND WELLBEING CLINIC
Laya healthcare have announced the launch of a new Laya Health & Wellbeing Clinic in Swords Business Campus, Swords, Co Dublin. This will increase the nationwide network of Laya Health and Wellbeing Clinics to four and add an additional clinic to the Dublin area making it the fastest growing urgent care network in Ireland.
The North Dublin clinic will offer a range of health and wellbeing services including walk-in urgent care for the treatment of minor injuries and illnesses where patients as young as 12 months+ will be seen within 60 minutes - available to laya healthcare members and non-members. All Laya Health and Wellbeing Clinics have diagnostic imaging onsite continuing laya healthcare’s promise to improve access and ensure the current and future healthcare needs of members. The Laya Health and Wellbeing Clinic in Swords will provide X-Ray, MRI, Ultrasound and Dexa scan.
The investment of over ¤3million in the opening of this brand-new Laya Health and Wellbeing Clinic in North Co. Dublin will result in a new 25,000 sq ft clinic which will treat approximately 30,000 patients annually. As part of the expansion, 25 new jobs will be created including clinical and administrative teams. As with all Laya Health and Wellbeing clinics, it will be open 365 days per year from 10am – 10pm.
Commenting on the major investment and expansion of the Laya Health and Wellbeing clinic network, John Mc Call, Director of Claims and Provider Relations said, “Contributing to the ambition of Slaintecare, we firmly believe in the importance of delivering the right care at the right time, and that is why our clinics are open 365 days per year with consultant-led care delivered within one hour.”
To find out more about the Laya Health and Wellbeing Clinics visit www.layahealthcare.ie
JANSSEN BURSARY MEDAL FOR HEALTH ECONOMICS
At an event hosted by Janssen, the Pharmaceutical Companies of Johnson & Johnson, students and lecturers from University

(Stages II-IIIA) and by 73% (HR 0.27; 95% CI 0.21-0.34) in the overall trial population (Stages IB-IIIA). In an updated analysis in 2022, the median DFS was reached at nearly five and a half years (65.8 months) for both the primary and overall populations treated with osimertinib, compared to 21.9 and 28.1 months in the primary and overall populations, respectively, treated with placebo. Consistent DFS results were seen regardless of prior adjuvant chemotherapy use and across all prespecified subgroups. The safety and tolerability of osimertinib in this trial was consistent with previous trials in the metastatic setting.
of Galway’s Health Economics Masters class met with members of Janssen’s Market Access and Government Affairs team, touring the office and participating in presentations and workshops.
At the event, in recognition of academic excellence in health economics in 2023, Orla Jenkins of HIQA was the recipient of the Janssen Bursary Medal.
Since 2012, Janssen has collaborated with the University of Galway, which has provided Health Economics students with valuable placement opportunities, contributing to their professional growth. In 2022, Janssen launched the WiSTEM2D mentorship programme with the university, designed to nurture future female leaders in healthcare specifically.
WiSTEM2D stands for Women in Science, Technology, Engineering, Math, Manufacturing and Design –the initiative supports women and girls in pursuit of STEM2D studies and careers.
Janssen is proud to support the next generation of talent and to continue their partnership with University of Galway.
TAGRISSO (OSIMERTINIB)
APPROVED IN IRELAND
AstraZeneca’s osimertinib is now reimbursed in Ireland for the adjuvant treatment of adult patients with early-stage (IB, II and IIIA) epidermal growth factor receptormutated (EGFRm) non-small cell lung cancer (NSCLC) after complete tumour resection, from
March 1st. Osimertinib is indicated for EGFRm patients whose tumours have exon 19 deletions or exon 21 (L858R) mutations.
While up to 30% of all patients with NSCLC may be diagnosed early enough to have surgery with curative intent, recurrence is still common in early-stage disease. Historically, nearly half of patients diagnosed in Stage IB, and over three quarters of patients diagnosed in Stage IIIA, have experienced disease recurrence within five years. About a fifth of the world’s lung cancer patients are in the EU and among those with NSCLC, approximately 10 - 15% have tumours with an EGFR mutation.
The approval by the European Commission (May 2021) was based on positive results from the ADAURA Phase III trial in which, osimertinib demonstrated a statistically significant and clinically meaningful improvement in disease-free survival (DFS) in the primary analysis population of patients with Stage II and IIIA EGFRm NSCLC. The trial also showed a statistically significant and clinically meaningful improvement in DFS for osimertinib in the overall trial population, a key secondary endpoint.
In the ADAURA trial, adjuvant treatment with osimertinib reduced the risk of disease recurrence or death by 77% (based on a hazard ratio [HR] of 0.23; 95% confidence interval [CI] 0.18-0.30) in the primary analysis population
In June 2023, the final planned overall survival analysis showed osimertinib reduced the risk of death by 51% compared to placebo in both the primary analysis population (Stages II-IIIA) (21% data maturity, OS hazard ratio [HR] of 0.49; 95.03% confidence interval [CI] 0.33-0.73; p=0.0004), and in the overall trial population (Stages IB-IIIA) (18% data maturity, OS HR of 0.49; 95.03% CI 0.34-0.70; p<0.0001). In the primary analysis population, an estimated 85% of patients treated with osimertinib were alive at five years compared to 73% on placebo. In the overall trial population, an estimated 88% of patients treated with osimertinib were alive at five years compared to 78% on placebo. Median OS was not yet reached in either population or treatment group. Patients on placebo that recurred with metastatic disease had the opportunity to receive osimertinib treatment.
Dr Eynat Dotan, Head of Medical, AstraZeneca, Ireland said: “The ADAURA trial brought the first targeted medicine to patients with early-stage EGFR-mutated non-small cell lung cancer. The exciting overall survival results validate adjuvant osimertinib as the standard of care in this setting and reinforce the importance of early diagnosis and testing for EGFR mutation in lung cancer.”
Osimertinib is now approved to treat early-stage lung cancer in more than fifty countries, including in the US and China, and additional global regulatory reviews are ongoing. Osimertinib is also approved for the 1st-line treatment of patients with locally advanced or metastatic EGFRm NSCLC and for the treatment of locally advanced or metastatic EGFR T790M mutation-positive NSCLC in the EU, the US, Japan, China and many other countries.
83 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2024
Pictured at Janssen’s bursary medal presentation was (L-R) Brita O’Reilly, Director of Market Access & Government Affairs at Janssen Sciences Ireland UC; Professor Brendan Kennelly, Programme Director MSc. Health Economics at University of Galway; and Orla Jenkins, recipient of the Janssen Bursary Medal 2023.


ABRIDGED PRESCRIBING INFORMATION
ABRIDGED PRESCRIBING INFORMATION
BRING PROTECTION TO LIFE1
BRING PROTECTION TO LIFE1
GFR mL/min/1.73m2
GFR mL/min/1.73m2
INITIATE treatment1,2
INITIATE treatment1,2
FORXIGA® (dapagliflozin) 5MG & 10MG FILM-COATED TABLETS.
Consult Summary of Product Characteristics (SmPC) before prescribing.
FORXIGA® (dapagliflozin) 5MG & 10MG FILM-COATED TABLETS.
Consult Summary of Product Characteristics (SmPC) before prescribing.
FORXIGA 10 mg
No titration required except in patients with severe hepatic impairment1
No titration required except in patients with severe hepatic impairment1
Once daily
FORXIGA 10 mg
Once daily
Indications: Adults and children aged 10 years and above: Type 2 diabetes mellitus: For the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise, as monotherapy when metformin is considered inappropriate due to intolerance, or in addition to other medicinal products for the treatment of type 2 diabetes. Adults: Heart Failure: Indicated in adults for the treatment of symptomatic chronic heart failure. Chronic kidney disease: Indicated in adults for the treatment of chronic kidney disease.
Indications: Adults and children aged 10 years and above: Type 2 diabetes mellitus: For the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise, as monotherapy when metformin is considered inappropriate due to intolerance, or in addition to other medicinal products for the treatment of type 2 diabetes. Adults: Heart Failure: Indicated in adults for the treatment of symptomatic chronic heart failure. Chronic kidney disease: Indicated in adults for the treatment of chronic kidney disease.
Presentation: Film-coated tablets. 5mg or 10mg of dapagliflozin (as propanediol monohydrate). Each 5mg tablet contains 25mg of lactose. Each 10mg tablet contains 50mg of lactose.
Presentation: Film-coated tablets. 5mg or 10mg of dapagliflozin (as propanediol monohydrate). Each 5mg tablet contains 25mg of lactose. Each 10mg tablet contains 50mg of lactose.
Dosage and Administration: Adults: Type 2 diabetes mellitus: The recommended dose is 10mg once daily. Consider a lower dose of insulin or insulin secretagogue such as a sulphonylurea when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Children and adolescents: No dose adjustment in children aged 10 years and above. No safety and efficacy data available for children below 10 years of age. Heart Failure: The recommended dose is 10mg once daily. Chronic kidney disease: The recommended dose is 10 mg once daily. Children and adolescents: <18 years: Safety and efficacy not yet established. Elderly: ≥65 years: No dose adjustment is recommended based on age. Renal impairment: No dose adjustment is required based on renal function. Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. If GFR falls below 45 mL/min, additional glucose lowering treatment should be considered in patients with diabetes mellitus if further glycaemic control is needed. Mild or moderate hepatic impairment: No dose adjustment. Severe hepatic impairment: Starting dose of 5mg is recommended, if well tolerated, dose may be increased to 10mg. Method of administration: Forxiga can be taken orally at any time of day with or without food. Tablets should be swallowed whole.
Contraindications: Hypersensitivity to dapagliflozin, or excipients.
Dosage and Administration: Adults: Type 2 diabetes mellitus: The recommended dose is 10mg once daily. Consider a lower dose of insulin or insulin secretagogue such as a sulphonylurea when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Children and adolescents: No dose adjustment in children aged 10 years and above. No safety and efficacy data available for children below 10 years of age. Heart Failure: The recommended dose is 10mg once daily. Chronic kidney disease: The recommended dose is 10 mg once daily. Children and adolescents: <18 years: Safety and efficacy not yet established. Elderly: ≥65 years: No dose adjustment is recommended based on age. Renal impairment: No dose adjustment is required based on renal function. Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. If GFR falls below 45 mL/min, additional glucose lowering treatment should be considered in patients with diabetes mellitus if further glycaemic control is needed. Mild or moderate hepatic impairment: No dose adjustment. Severe hepatic impairment: Starting dose of 5mg is recommended, if well tolerated, dose may be increased to 10mg. Method of administration: Forxiga can be taken orally at any time of day with or without food. Tablets should be swallowed whole.
Contraindications: Hypersensitivity to dapagliflozin, or excipients.
Warnings and Precautions: Dapagliflozin should not be used in patients with type 1 diabetes mellitus. Renal impairment: Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. The glucose lowering efficacy of dapagliflozin is dependent on renal function, and is reduced in patients with GFR < 45 mL/min and is likely absent in patients with severe renal impairment. Hepatic impairment: Exposure is increased in patients with severe hepatic impairment. Use in patients at risk of volume depletion and/or hypotension: Dapagliflozin increases diuresis which may lead to a modest decrease in blood pressure, it may be more pronounced in patients with very high blood glucose concentrations. Exercise caution in patients for whom a dapagliflozin induced drop in blood pressure could pose a risk, such as patients on anti hypertensive therapy with a history of hypotension or elderly patients. Careful monitoring of volume status and electrolytes is recommended in conditions leading to volume depletion, such as acute gastrointestinal illness. In volume depleted patients temporary interruption of dapagliflozin is recommended until volume depletion is corrected. Diabetic ketoacidosis (DKA): Rare cases of DKA, including life-threatening and fatal cases, have been reported in patients treated with SGLT2 inhibitors, including dapagliflozin. In a number of cases, the presentation of the condition was atypical with only moderately increased blood glucose values, below 14mmol/L (250mg/dL). The risk of DKA must be considered in the event of non-specific symptoms such as nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue or sleepiness. Patients should be assessed for ketoacidosis immediately if these symptoms occur, regardless of blood glucose level. In patients where DKA is suspected or diagnosed, dapagliflozin treatment should be stopped immediately. Treatment should be interrupted in patients who are hospitalised for major surgical procedures or acute serious medical illnesses. Monitoring of ketones is recommended in these patients. Measurement of blood ketone levels is preferred to urine. Treatment with dapagliflozin may be restarted when the ketone values are normal and the patient’s condition has stabilised. Before initiating dapagliflozin, factors in patient history that may predispose to ketoacidosis should be considered. Patients who may be at higher risk of DKA include patients with a low beta cell function reserve (e.g. patients with low C peptide or latent autoimmune diabetes in adults (LADA) or patients with a history of pancreatitis), patients with conditions that lead to restricted food intake or severe dehydration, patients for whom insulin doses are reduced and patients with increased insulin requirements due to acute medical illness, surgery or alcohol abuse. SGLT2 inhibitors should be used with caution in these patients. Restarting SGLT2 inhibitor treatment in patients experiencing a DKA while on SGLT2 inhibitor treatment is not recommended, unless another clear precipitating factor is identified and resolved. Dapagliflozin should not be used for treatment of patients with type 1 diabetes mellitus. Necrotising fasciitis of the perineum (Fournier’s gangrene): Postmarketing cases have been reported in female and male patients taking SGLT2 inhibitors. Urgent surgical intervention and antibiotic treatment is required. Advise patients to seek medical attention if they experience a combination of pain, tenderness,
Warnings and Precautions: Dapagliflozin should not be used in patients with type 1 diabetes mellitus. Renal impairment: Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. The glucose lowering efficacy of dapagliflozin is dependent on renal function, and is reduced in patients with GFR < 45 mL/min and is likely absent in patients with severe renal impairment. Hepatic impairment: Exposure is increased in patients with severe hepatic impairment. Use in patients at risk of volume depletion and/or hypotension: Dapagliflozin increases diuresis which may lead to a modest decrease in blood pressure, it may be more pronounced in patients with very high blood glucose concentrations. Exercise caution in patients for whom a dapagliflozin induced drop in blood pressure could pose a risk, such as patients on anti hypertensive therapy with a history of hypotension or elderly patients. Careful monitoring of volume status and electrolytes is recommended in conditions leading to volume depletion, such as acute gastrointestinal illness. In volume depleted patients temporary interruption of dapagliflozin is recommended until volume depletion is corrected. Diabetic ketoacidosis (DKA): Rare cases of DKA, including life-threatening and fatal cases, have been reported in patients treated with SGLT2 inhibitors, including dapagliflozin. In a number of cases, the presentation of the condition was atypical with only moderately increased blood glucose values, below 14mmol/L (250mg/dL). The risk of DKA must be considered in the event of non-specific symptoms such as nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue or sleepiness. Patients should be assessed for ketoacidosis immediately if these symptoms occur, regardless of blood glucose level. In patients where DKA is suspected or diagnosed, dapagliflozin treatment should be stopped immediately. Treatment should be interrupted in patients who are hospitalised for major surgical procedures or acute serious medical illnesses. Monitoring of ketones is recommended in these patients. Measurement of blood ketone levels is preferred to urine. Treatment with dapagliflozin may be restarted when the ketone values are normal and the patient’s condition has stabilised. Before initiating dapagliflozin, factors in patient history that may predispose to ketoacidosis should be considered. Patients who may be at higher risk of DKA include patients with a low beta cell function reserve (e.g. patients with low C peptide or latent autoimmune diabetes in adults (LADA) or patients with a history of pancreatitis), patients with conditions that lead to restricted food intake or severe dehydration, patients for whom insulin doses are reduced and patients with increased insulin requirements due to acute medical illness, surgery or alcohol abuse. SGLT2 inhibitors should be used with caution in these patients. Restarting SGLT2 inhibitor treatment in patients experiencing a DKA while on SGLT2 inhibitor treatment is not recommended, unless another clear precipitating factor is identified and resolved. Dapagliflozin should not be used for treatment of patients with type 1 diabetes mellitus. Necrotising fasciitis of the perineum (Fournier’s gangrene): Postmarketing cases have been reported in female and male patients taking SGLT2 inhibitors. Urgent surgical intervention and antibiotic treatment is required. Advise patients to seek medical attention if they experience a combination of pain, tenderness,
erythema, or swelling in the genital or perineal area, with fever or malaise. Either uro-genital infection or perineal abscess may precede necrotising fasciitis. If suspected, discontinue Forxiga and institute prompt treatment (including antibiotics and surgical debridement). Urinary tract infections: Temporary interruption of dapagliflozin should be considered when treating pyelonephritis or urosepsis. Elderly (≥65 years): No dose adjustment is recommended based on age. Elderly patients are more likely to have impaired renal function, be treated with medicines such as anti-hypertensives or diuretics, and be at a greater risk of volume depletion. Cardiac failure: Experience with dapagliflozin in NYHA class IV is limited. Infiltrative cardiomyopathy: Patients with infiltrative cardiomyopathy have not been studied. Chronic kidney disease: There is no experience with dapagliflozin for the treatment of chronic kidney disease in patients without diabetes who do not have albuminuria. Patients with albuminuria may benefit more from treatment with dapagliflozin. Lower limb amputations: Counsel patients with diabetes on routine preventative foot care as an increase in cases of lower limb amputation (primarily of the toe) has been observed in long term, clinical studies in type 2 diabetes mellitus with SGLT2 inhibitors. Urine laboratory assessments: Patients will test positive for glucose in the urine due to mechanism of action. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take Forxiga.
erythema, or swelling in the genital or perineal area, with fever or malaise. Either uro-genital infection or perineal abscess may precede necrotising fasciitis. If suspected, discontinue Forxiga and institute prompt treatment (including antibiotics and surgical debridement). Urinary tract infections: Temporary interruption of dapagliflozin should be considered when treating pyelonephritis or urosepsis. Elderly (≥65 years): No dose adjustment is recommended based on age. Elderly patients are more likely to have impaired renal function, be treated with medicines such as anti-hypertensives or diuretics, and be at a greater risk of volume depletion. Cardiac failure: Experience with dapagliflozin in NYHA class IV is limited. Infiltrative cardiomyopathy: Patients with infiltrative cardiomyopathy have not been studied. Chronic kidney disease: There is no experience with dapagliflozin for the treatment of chronic kidney disease in patients without diabetes who do not have albuminuria. Patients with albuminuria may benefit more from treatment with dapagliflozin. Lower limb amputations: Counsel patients with diabetes on routine preventative foot care as an increase in cases of lower limb amputation (primarily of the toe) has been observed in long term, clinical studies in type 2 diabetes mellitus with SGLT2 inhibitors. Urine laboratory assessments: Patients will test positive for glucose in the urine due to mechanism of action. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take Forxiga.
Drug Interactions: Diuretics: Dapagliflozin may add to the diuretic effect of thiazide and loop diuretics and may increase the risk of dehydration and hypotension. Insulin and insulin secretagogues: Consider a lower dose of insulin or insulin secretagogue when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Interference with 1,5 AG assay: Monitoring glycaemic control with 1,5-AG assay is not recommended as measurements of 1,5 AG are unreliable in assessing glycaemic control in patients taking SGLT2 inhibitors. Alternative methods should be used. Interference with lithium: Dapagliflozin may increase renal lithium excretion and the blood lithium levels may be decreased. Serum concentration of lithium should be monitored more frequently after dapagliflozin initiation and dose changes. Please refer the patient to the lithium prescribing doctor in order to monitor serum concentration of lithium.
Drug Interactions: Diuretics: Dapagliflozin may add to the diuretic effect of thiazide and loop diuretics and may increase the risk of dehydration and hypotension. Insulin and insulin secretagogues: Consider a lower dose of insulin or insulin secretagogue when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Interference with 1,5 AG assay: Monitoring glycaemic control with 1,5-AG assay is not recommended as measurements of 1,5 AG are unreliable in assessing glycaemic control in patients taking SGLT2 inhibitors. Alternative methods should be used. Interference with lithium: Dapagliflozin may increase renal lithium excretion and the blood lithium levels may be decreased. Serum concentration of lithium should be monitored more frequently after dapagliflozin initiation and dose changes. Please refer the patient to the lithium prescribing doctor in order to monitor serum concentration of lithium.
Pregnancy and Lactation: Not recommended during the second and third trimesters of pregnancy. Treatment should be discontinued when pregnancy is detected. Do not use whilst breast-feeding.
Pregnancy and Lactation: Not recommended during the second and third trimesters of pregnancy. Treatment should be discontinued when pregnancy is detected. Do not use whilst breast-feeding. Ability to Drive and Use Machines: Alert patients on the risk of hypoglycaemia when dapagliflozin is used in combination with a sulphonylurea or insulin.
Ability to Drive and Use Machines: Alert patients on the risk of hypoglycaemia when dapagliflozin is used in combination with a sulphonylurea or insulin.
Undesirable Events: Consult SmPC for full list of side effects. Very common (≥1/10): Hypoglycaemia (when used with SU or insulin). Common (≥1/100 to <1/10): Vulvovaginitis, balanitis and related genital infections, urinary tract infection, dizziness, rash, back pain, dysuria, polyuria, haematocrit increased, creatinine renal clearance decreased during initial treatment, dyslipidaemia. Uncommon (≥1/1,000 to < 1/100): Fungal infection, volume depletion, thirst, constipation, dry mouth, nocturia, vulvovaginal pruritus, pruritus genital, blood creatinine increased during initial treatment, blood urea increased, weight decreased. Rare (≥ 1/10,000 to < 1/1,000): Diabetic ketoacidosis (when used in type 2 diabetes mellitus). Very Rare (< 1/10,000): Angioedema, necrotising fasciitis of the perineum (Fournier’s gangrene), Tubulointerstitial nephritis.
Legal Category: Product subject to prescription which may be renewed (B).
Undesirable Events: Consult SmPC for full list of side effects. Very common (≥1/10): Hypoglycaemia (when used with SU or insulin). Common (≥1/100 to <1/10): Vulvovaginitis, balanitis and related genital infections, urinary tract infection, dizziness, rash, back pain, dysuria, polyuria, haematocrit increased, creatinine renal clearance decreased during initial treatment, dyslipidaemia. Uncommon (≥1/1,000 to < 1/100): Fungal infection, volume depletion, thirst, constipation, dry mouth, nocturia, vulvovaginal pruritus, pruritus genital, blood creatinine increased during initial treatment, blood urea increased, weight decreased. Rare (≥ 1/10,000 to < 1/1,000): Diabetic ketoacidosis (when used in type 2 diabetes mellitus). Very Rare (< 1/10,000): Angioedema, necrotising fasciitis of the perineum (Fournier’s gangrene), Tubulointerstitial nephritis.
Legal Category: Product subject to prescription which may be renewed (B).
Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00. FORXIGA is a trademark of the AstraZeneca group of companies.
Date of API preparation: 02/2023 Veeva ID: IE-4664
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00. FORXIGA is a trademark of the AstraZeneca group of companies.
Date of API preparation: 02/2023 Veeva ID: IE-4664
References:
References:
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
1. FORXIGA 10 mg film-coated tablets. Summary of product characteristics.
1. FORXIGA 10 mg film-coated tablets. Summary of product characteristics.
2. Heerspink HJL et al. N Engl J Med. 2020;383(15):1436–1446.
2. Heerspink HJL et al. N Engl J Med. 2020;383(15):1436–1446.
eGFR = estimated glomerular filtration rate
eGFR = estimated glomerular filtration rate
©AstraZeneca 2022. All Rights Reserved. Veeva ID: IE-4836 Date of Prep: September 2023
©AstraZeneca 2022. All Rights Reserved. Veeva ID: IE-4836 Date of Prep: September 2023































 Written by: Donna Cosgrove, MPSI, PhD
Written by: Donna Cosgrove, MPSI, PhD

















 Professor Anne-Marie Brady, new Chair of the Tallaght University Hospital Board
Professor Anne-Marie Brady, new Chair of the Tallaght University Hospital Board







































































































