CIBINQO is indicated for the treatment of moderate-to-severe atopic dermatitis (AD) in adults who are candidates for systemic therapy.
Legal Category : S1A. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
IN THIS ISSUE:
NEWS: 26% of Generic Medicines have ‘Disappeared’
Page 7
FEATURE: Diabetic Retinopathy
Page 14
MEDICINES: The Windsor Agreement and implications for medicines
Page 18
CPD: Atopic Dermatitis
Page 31
DERMATOLOGY
FOCUS: : Patient initiated follow-up
Page 38
DERMATOLOGY
FOCUS: Atopic Dermatitis

Page 40
UROLOGY FOCUS: Prostate Cancer
Page 57
HOSPITAL
NEWS
Hospital Professional Publication HPN June 2023 Issue 109 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only © 2022 Pfizer Inc. All rights reserved. July 2022. PP-CIB-IRL-0036
PROFESSIONAL
IRELAND Ireland’s Dedicated
NOW HSE REIMBURSED IN IRELAND!
Reimbursement is restricted to use as a subsequent line of therapy following treatment with a lower cost biological disease modifying anti-rheumatic drug (bDMARD).
• In phase III studies BIMZELX demonstrated superiority vs ustekinumab (BE VIVID; p<0.0001), placebo (BE READY; p<0.0001) and adalimumab (BE SURE; p< 0.001) in achieving the co-primary endpoints PASI 90 and IGA 0/1 at week 16 with 85% (273/321), 90.8% (317/349) and 86.2% (275/319) of patients achieving PASI 90 at Week 16. At Week 4, 76.9% (247/321), 75.9% (265/349) and 76.5% (244/319) of patients achieved the secondary endpoint of PASI 75.1
• In the BE BRIGHT open label extension study, 62.7% (620/989) of patients achieved PASI 100 at Week 16 (non-responder imputation [NRI]). Of these patients, 84.4% (147/174) of patients randomised to 8 week dosing maintained PASI 100 at Week 148.2
Challenge expectations in psoriasis1,2

Visit Bimzelx.co.uk to discover more
This site contains promotional information on UCB products.
To hear about future UCB projects, educational events and to receive the latest information from UCB, please scan the QR code set your digital preferences.
BIMZELX is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.1
Prescribing Information and Adverse Event can be found below.
Use this QR code to access Bimzelx.co.uk This site contains promotional information on UCB products.
Note: The most frequently reported adverse reactions with BIMZELX are: upper respiratory tract infections (14.5%) and oral candidiasis (7.3%).1 Other common adverse events include: Tinea infection, ear infection, Herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, dermatitis and eczema, acne, injection site reaction and fatigue.
PRESCRIBING INFORMATION
(Please consult the Summary of Product Characteristics (SmPC) before prescribing)
BIMZELX® ▼(Bimekizumab)
Active Ingredient: Bimekizumab – solution for injection in prefilled syringe or pre-filled pen: 160 mg of bimekizumab in 1 mL of solution (160mg/mL) Indications: Moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Dosage and Administration: Should be initiated and supervised by a physician experienced in the diagnosis and treatment of plaque psoriasis. Recommended dose: 320 mg (given as two subcutaneous injections of 160 mg each) at week 0, 4, 8, 12, 16 and every 8 weeks thereafter. For some patients with a body weight ≥ 120 kg who did not achieve complete skin clearance at week 16, 320 mg every 4 weeks after week 16 may further improve treatment response. Consider discontinuing if no improvement by 16 weeks of treatment. Renal or hepatic impairment: No dose adjustment needed. Elderly: No dose adjustment needed. Administer by subcutaneous injection to thigh, abdomen or upper arm. Rotate injection sites and do not inject into psoriatic plaques or skin that is tender, bruised, erythematous or indurated. Do not shake pre-filled syringe or pre-filled pen. Patients may be trained to self-inject.
Contraindications: Hypersensitivity to bimekizumab or any excipient; Clinically important active infections (e.g. active tuberculosis). Warnings and Precautions: Record name and batch number of administered product. Infection: Bimekizumab may increase the risk of infections e.g. upper respiratory tract infections, oral candidiasis. Caution when considering use in patients with a chronic infection or a history of recurrent infection. Must not be initiated if any clinically important active infection until infection resolves or is adequately treated. Advise patients to seek medical advice if signs or symptoms suggestive of an infection occur. If a clinically important infection develops or is not responding to standard therapy,
carefully monitor and do not administer bimekizumab until infection resolves. TB: Evaluate for TB infection prior to initiating bimekizumab – do not give if active TB. While on bimekizumab, monitor for signs and symptoms of active TB. Consider anti-TB therapy prior to bimekizumab initiation if past history of latent or active TB in whom adequate treatment course cannot be confirmed. Inflammatory bowel disease: Bimekizumab is not recommended in patients with inflammatory bowel disease. Cases of new or exacerbations of inflammatory bowel disease have been reported. If inflammatory bowel disease signs/symptoms develop or patient experiences exacerbation of pre-existing inflammatory bowel disease, discontinue bimekizumab and initiate medical management. Hypersensitivity: Serious hypersensitivity reactions including anaphylactic reactions have been observed with IL-17 inhibitors. If a serious hypersensitivity reaction occurs, discontinue immediately and treat. Vaccinations: Complete all age appropriate immunisations prior to bimekizumab initiation. Do not give live vaccines to bimekizumab patients. Patients may receive inactivated or nonlive vaccinations. Interactions: A clinically relevant effect on CYP450 substrates with a narrow therapeutic index in which the dose is individually adjusted e.g. warfarin, cannot be excluded. Therapeutic monitoring should be considered. Fertility, pregnancy and lactation: Women of child-bearing potential should use an effective method of contraception during treatment and for at least 17 weeks after treatment. Avoid use of bimekizumab during pregnancy and breastfeeding. Discontinue breastfeeding or discontinue bimekizumab during breastfeeding. It is unknown whether bimekizumab is excreted in human milk, hence a risk to the newborn/infant cannot be excluded. No data available on human fertility. Driving and use of machines: No or negligible influence on ability to drive and use machines. Adverse Effects: Refer to SmPC for full information. Very Common (≥ 1/10): upper respiratory tract
References: 1. BIMZELX (bimekizumab) Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/ product/12834/smpc. Accessed April 2023. 2. Strober B et al. Poster P1491 presented at the European Academy of Dermatology and Venereology (EADV) meeting, September 7–10 2022; Milan, Italy.
IE-P-BK-PSO-2300066 Date of preparation: April 2023. © UCB Biopharma SRL, 2023. All rights reserved. BIMZELX® is a registered trademark of the UCB Group of Companies.
infection; Common (≥ 1/100 to < 1/10): oral candidiasis, tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis; headache, dermatitis and eczema, acne, injection site reactions, fatigue; Uncommon (≥ 1/1,000 to < 1/100): mucosal and cutaneous candidiasis (including oesophageal candidiasis), conjunctivitis, neutropenia, inflammatory bowel disease. Storage precautions: Store in a refrigerator (2ºC – 8ºC), do not freeze. Keep in outer carton to protect from light. Bimzelx can be kept at up to 25ºC for a single period of maximum 25 days with protection from light. Product should be discarded after this period or by the expiry date, whichever occurs first.
Legal Category: POM
Marketing Authorisation Numbers: EU/1/21/1575/002 (2 x 1
Pre-filled Syringes), EU/1/21/1575/006 (2 x 1 Pre-filled Pens)
Marketing Authorisation Holder: UCB Pharma S.A., Allée de la Recherche 60, B-1070 Brussels, Belgium. Further information is available from: UCB (Pharma) Ireland Ltd, United Drug House, Magna Drive, Magna Business Park, City West Road, Dublin 24, Ireland.
Tel: 1800-930075 Email: ucbcares.ie@ucb.com
Date of Revision: April 2022 (IE-P-BK-PSO-2200034)
Bimzelx is a registered trademark.
▼This medicinal product is subject to additional monitoring. This will allow quick identification ofnew safety information. Healthcare professionals are asked to report any suspected adversereactions.
Reporting forms and information can be found at www.hpra.ie/homepage/about-us/report-an-issue Adverse events should also be reported to UCB (Pharma) Ireland Ltd. Email: UCBCares.IE@ucb.com
THIS ADVERT CONTAINS PROMOTIONAL CONTENT FROM UCB AND IS INTENDED FOR HCPS IN IRELAND
Contents Foreword
Hospital pharmacy gives first impressions on revision of General Pharmaceutical Legislation P4

New Theatre opens at St James’s Hospital P5

New Medicines Working Group must Deliver Change P6
On average - 26% of all generic medicines have disappeared P7
New research on cancer-killing benefits of obesity treatment P9
Revolutionising Cerebral Palsy Care P78
REGULARS
Feature: VTE during Pregnancy P20
CPD: Atopic Dermatitis P31
Dermatology Focus: Cutaneous Malignant Melanoma P37
Dermatology Focus: Skin Cancer Prevalence P44
Urology Focus: Overactive Bladder P61
Clinical R&D: P80
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd


Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis
n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
editorial@hospitalprofessionalnews.ie
Editor
I one of our lead news stories this month, it is revealed that Ireland is once again one of the slowest countries in Western Europe to reimburse and make available new innovative medicines to patients. This is according to figures gathered by data analysts IQVIA for EFPIA, the European pharmaceutical industry body.
The survey of 37 European countries, including 27 in the European Union, covers the full four years between 2018 and 2021 analysing 168 innovative medicines authorised for use by the European Medicines Agency (EMA).
Oliver O’Connor, Chief Executive of the Irish Pharmaceutical Healthcare Association, said, “Over the past three budgets, the Government has allocated almost ¤100 million to new medicines. The figures released today show how urgent it is now to improve the reimbursement system so that new medicines are available to patients and their doctors faster.”
Turn to page 6 for the full story.
In other medicines news, on page 7, we detail how across Europe - on average - 26% of all generic medicines have disappeared. Unsustainable pricing policies are one of the leading drivers of shortages, says Medicines for Europe. Affordable generic medicines available on the market just 10 years ago are disappearing and supply is too consolidated, according to new data shared. This rapid decline and consolidation, higher in certain therapy areas such as cancer care and antibiotics, risks creating more shortages and threatens vital access for patients.
The data showed that the whole generic medicines supply chain is under heavy pressure, with prices being pushed down to the limit of their sustainability. Strikingly, medicines supply consolidation is not just at the active ingredient level but also medicines, thereby creating risks of shortages and reducing access.
ACCOUNTS
Fiona Bothwell cs.ipn@btconnect.com
SALES EXECUTIVE
Amy Evans - amy@ipn.ie

CONTRIBUTORS
Áine Toher
Sarah Mohamed
Dr Cathal O’Connor

Claire Quigley
Eoin.R.Storan
Anna Wolinska
Claire Doyle
Dr Emma Porter
Dr Rebecca Helen
Kevin O’Hagan
James Powell
Helen Forristal
Charles O’Connor
Gregory Nason
Mohammed Hegazy
David Galvin
Theresa Lowry-Lehnen
Dr Fardod Kelly
DESIGN DIRECTOR
Ian Stoddart Design
Áine Toher, Senior Pharmacist and Sarah Mohamed, Pharmacy Intern, both with The National Maternity Hospital give a clinical overview of Low Molecular Weight Heparin for treatment and prophylaxis of Venous Thromboembolism (VTE) during Pregnancy. Failure to recognise and/or treat personal or pregnancy specific risk factors has been identified as a significant contributing factor to maternal mortality and morbidity from VTE in pregnancy, they say. You can read more about this on page 20.
Our clinical special focus this issue is on Dermatology and Urology; these two therapeutic areas highlighting a number of excellent contributed articles across headings such as ‘Dermatology mycology diagnostics in Ireland’ and ‘Prostate cancer in Ireland.’
I hope you enjoy the issue.
3 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023 June Issue Issue 109 5
7 78 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
Fostering Cancer Care in Ireland
Pictured at the official announcement of the partnership were, left to right; Averil Power, CEO, Irish Cancer Society, Dr Linda Doyle, Provost and President of Trinity College, Professor John Kennedy, Medical Director, TJSCI, Professor Maeve Lowery, Academic Director, TSJCI and Noel Gorman, Interim CEO, St James's Hospital
I’m also glad to see that the experience of patients will be included in approaches to research, clinical trials, clinical care and education.”
The Irish Cancer Society has announced a new partnership with Trinity College Dublin and St James’s Hospital designed to develop an outstanding programme of cancer treatment and care for patients in Ireland.

The five-year collaboration will see the Society invest ¤4.5million in several specific exemplar programmes aimed at delivering a new model of cancer care for patients in Ireland.
By investing in the Trinity St James’s Cancer Institute (TSJCI) the Irish Cancer Society aims to accelerate the translation of cancer research into new treatments and better support for patients.
The partnership will integrate Irish Cancer Society services into the
hospital pathway and enhance the patient experience by ensuring better collaboration with patients across research, cancer clinical trials, clinical care, and education.
Dr Linda Doyle, Provost and President of Trinity College Dublin, said, “I want to thank the Irish Cancer Society for this investment and I’m looking forward to a fantastic partnership.
‘We have huge ambitions for cancer research in Trinity and for the Trinity St James Cancer Institute. Today’s announcement is a step towards realising those ambitions.
I particularly welcome the partnership’s goal to accelerate the translation of research into new treatments and better supports for patients.’
Mr Noel Gorman, Interim CEO, St James’s Hospital said, “The challenges posed by cancer in Ireland are such that extensive targeted investment is required to bring about the improvements in facilities, treatment, research and education that will ensure the best outcomes for our patients in the decades to come. This major investment by the ICS in TSJCI programmes for younger patients with gastrointestinal cancers is a statement of intent by both institutions to build a comprehensive collaboration which will address in a multidisciplinary fashion the myriad problems faced by patients with cancer.”
Prof John Kennedy, Medical Director, Trinity St James’s Cancer Institute said: “Today, the Irish
Hospital Pharmacy give First Impressions
The European Commission has finally released the longawaited proposal for the revision of the General Pharmaceutical Legislation, comprised of a draft Regulation and a draft Directive to improve the delivery and availability of medicines for human use in the European Union (EU).
The European Association of Hospital Pharmacists (EAHP) and its members welcome the publication that also marks the start of the negotiation process for this crucial piece of EU legislation. Despite the draft Regulation and the draft Directive being very comprehensive, further amendments should be considered by the legislators, especially those:
• Guaranteeing a very high level of patient safety across Europe
• Combatting antimicrobial resistance and fostering prudent use of antibiotics
• Ensuring accessibility and addressing the root causes of medicine shortages to adequately prevent and control them
• Upholding the high level of pharmacovigilance in Europe
• Enhancing the safety of the health workforce and the environment
Reflecting on the content of the proposal EAHP President
András Süle stressed that "More ambitious measures are needed to enhance patients' access to high-quality and affordable medicinal products across the European Union. Unmet medical needs and access to medicines should be better addressed by utilising the unique compounding skills of hospital pharmacists and their expertise in pharmacology targeting the deconstruction and execution of therapies and in
medicines procurement preserving the financial sustainability of healthcare systems. In addition to advancing innovation, patient safety should be put at the centre of the legislation. Seamless transitions between the interfaces of different health settings need to be considered during the implementation of the proposal to ensure that the patient care started in hospitals can be continued in the community."
Linked to the serious threat of antibiotic resistance that jeopardises the effectiveness of standard treatments EAHP Vice-President Darija Kuruc Poje highlighted that "Arrangements need to be made to ensure that essential antibiotics are maintained on the entire European market including an increase of European production sites to lower dependency on international markets. The subscription models proposed in the UK and Sweden are an interesting approach for
Cancer Society (ICS) announced it is investing ¤4.5m to fund innovative cancer research and care at the Trinity St James’s Cancer Institute (TSJCI). The focus is on pioneering programmes that addresses an unmet need identified by the ICS and TSJCI, specifically for the increasing numbers of younger patients with gastrointestinal cancer. It fits within a Comprehensive Cancer Care model, linking medicine and science, bringing relevant research to the patient, focussing a dedicated multidisciplinary team around an unmet need, and supporting clinicians to devote their time to both patient care and research.”
Professor Maeve Lowery, Academic Director, Trinity St James’s Cancer Institute added, “At TSJCI we work to integrate scientific discovery with patient care to pioneer new ways to prevent, detect and treat cancer. The urgency of this is evidenced by a concerning rise in the incidence of gastrointestinal cancers among patients under fifty years of age. This significant investment by ICS will accelerate the delivery of innovative and comprehensive cancer services to Irish patients and facilitate a long- term partnership based on a shared mission to deliver the major improvements in cancer care that our patients deserve. “
guaranteeing availability. There are however some questions that remain. Due to national particularities, criteria for the establishment of the subscription model, which factor in national needs, must be defined. In the interest of patient care, also, reimbursement of treatment options outside the subscription model would need to be ensured. In addition, to safeguard the availability of antibiotics, also stewardship programmes need to be supported in order to encourage prudent use of them by healthcare professionals and patients."
EAHP has released an Opinion on the Revision of the General Pharmaceutical Legislation that outlines specific concerns and urges the legislators to ameliorate the proposal in the fields of patient safety, antimicrobial resistance, accessibility of medicines, including medicine shortages, pharmacovigilance and the environment.
4 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
New Launch at St James’s Hospital

A lecture theatre dedicated to the memory of Professor Davis Coakley, Founding Director of the Mercer’s Institute for Successful Ageing, has been launched in MISA.
Davis Coakley, who died in September 2022 after a short illness, is one of Ireland’s best known physicians and medical historians.
He held a personal Chair in Medical Gerontology in Trinity College Dublin, awarded in 1996; the first such academic appointment in the country. He went on to establish the first Department of Medical Gerontology, home to many of Ireland’s PhD, MD and Master graduates in the speciality. He pioneered many national policy and service developments benefitting ageing in Ireland over a 40 year period.
Having led negotiations with the American charity, Atlantic Philanthropies and their founder,
Professor J Bernard Walsh, Mary Coakley and Professor Rose
Anne Kenny
Chuck Feeney, he secured a large philanthropic award enabling Ireland to command a leadership role in research in ageing and in a new purpose built institute for successful ageing at St James’s Hospital, known as the Mercer’s Institute for Successful Ageing (MISA). MISA was launched by President Michael D Higgins in 2016.
In addition to publishing over 150 original scientific papers, he published 18 books on medical science and on historical and literary subjects. Two of his medical books were deemed “pioneering volumes in their field”. His beautifully illustrated book “The Anatomy Lesson; Art and Medicine” informed a successful exhibition of Irish anatomical art at the National Gallery. His “Irish
Child and Youth Mental Health Lead
Masters of Medicine” collated the important historical contributions to medicine of 42 Irish medical practitioners and “The Importance of Being Irish; Oscar Wilde” –was the first ever biography to explore how Wilde’s formative years in Ireland had a significant impact on his life and writings, a concept which evolved from
Davis’ extensive knowledge of the medical cultural milieu in which Oscar spent his early years.
It is almost impossible to do justice to the essence of this great scholar and clinician, to his remarkable interpersonal skills and his ever present wit and roguish sense of humour.
Minister for Mental Health and Older People Mary Butler welcomed the opening of the recruitment process for the post of Child and Youth Mental Health Lead in the HSE. This key new role will provide leadership, operational oversight, and delegated management of all service delivery across child and youth mental health services across the country. They will also be responsible for managing and coordinating service planning activities, partnership and capacity building, the development of service plans, and setting of service standards right across child and youth mental health services in Ireland.The post holder will report to the HSE National Director for Community Operations and will be supported by a dedicated team for which funding has been provided.
This role, and the wider Child and Youth Mental Health office, will result in improved links with the National Clinical Advisor Group Lead Mental Health, which in turn will support the development of current and future youth mental healthrelated National Clinical Programmes.
Minister Butler stated, “The progression of this role has been a key priority for me over the past year, and it will play a crucial part in ensuring that integrated mental health services for young people have a more centralised and evidence-based focus within the HSE. “We will see the completed reviews and audits arising from the Maskey Report, along with the Final Report of the Mental Health Commission on CAMHS later this year, which together will give us real time data never available before to support this new post. Importantly, there will be new support staff to underpin this welcome initiative. I will ensure that the HSE also progresses as quickly as possible the new post of National Clinical Lead for Youth Mental Health as announced in the Dáil over the last week.”
Calls for Increased Collaboration
The Private Hospitals Association (PHA) has said it stands ready once again to assist the HSE in helping to reduce growing public hospital waiting lists.
PHA says it is fully committed to collaborating with the government and the HSE in order to alleviate the burden on the public system and to enhance access to timely and quality scheduled care for all citizens.
The announcement comes on back of confirmation that over 85,000 hospital appointments or procedures alone, had to be
cancelled in the first four months of 2023.
CEO of the PHA, Jim Daly, says a joint approach between public and private can bring about a more tangible solution to these challenges.
“After having seen some hopeful signs of progress in this area of late, it is regrettable to once again see public hospital waiting numbers moving upward again in April, and at a time when we traditionally should be making substantive inroads against these lists. The high number of cancelled procedures for each month this year highlights how
underlying structural challenges such as insufficient bed capacity now has an continuously negative impact on full hospital service provision across the public system. The 20 member hospitals across the island of Ireland are at all times ready to work hand-in-hand with the HSE to address this crisis, and provide added capacity as required for the patients most in need of important medical procedures.
"Based both north and south of the border, our member hospitals offer available theatre and bed capacity and clinical expertise which can be quickly called upon to help
reduce waiting times and improve patient outcomes. Our hospitals employ highly skilled healthcare professionals who are ready to contribute their resources and expertise to this collective effort.”
Mr Daly added that private hospitals are committed to optimising all resources such as operating theatres, diagnostics and specialist consultations to assist patients on public waiting lists. By sharing this capacity through collaboration with the HSE, it would help to significantly reduce the backlog of cases and improve waiting times.
5 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
News
New Medicines Working Group must Deliver Change
can make these medicines available faster to patients, as quickly as 102 days, while still managing budgets and doing value for money assessments.
Ireland is once again one of the slowest countries in Western Europe to reimburse and make available new innovative medicines to patients, according to figures gathered by data analysts IQVIA for EFPIA, the European pharmaceutical industry body.

The survey of 37 European countries, including 27 in the European Union, covers the full four years between 2018 and 2021 analysing 168 innovative medicines authorised for use by the European Medicines Agency (EMA). Key points this year are:
• While it takes on average 517 days, post EMA authorisation, to make a new medicine
routinely available across European countries, in Ireland it takes 567 days.
• This time has lengthened in Ireland since 2020: up almost 100 days from 477 three years ago. This is despite the welcome allocation of new funds for new medicines by the Government.
• The number of days it takes for new cancer medicines to be made available to patients has increased to 673 post EMA authorisation for Ireland. This wait of nearly two years is far too long for cancer patients as availability of new medicines is a recognised contributor to Ireland’s improved survival rates in cancer. Many other countries
• Only six of 37 European countries take longer than Ireland to reimburse orphan medicines for rare diseases, of which 95% do not have a recognised medical treatment. It currently takes on average 877 days from EMA market authorisation of orphan medicines for them to become available to Irish patients. The number of days taken depends both on the timing of companies’ applications and the decision-making processes of health authorities. In Ireland, the figures suggest that between 25% and 30% of the timing is attributable to IPHA member companies’ timing of applications for the reimbursement of a medicine to the HSE after the product has been granted EMA market authorisation. The bulk of the timing is taken by the State’s assessment and decision-making process, which typically includes price negotiations with companies.
Longer timelines to availability mean a lower standard of care than could be available for Irish patients, and thus poorer patient outcomes than otherwise could be achieved. The experience of other countries demonstrates that improved partnerships between health authorities and pharmaceutical companies are possible and should become a policy priority in Ireland.
IPHA is keen to work with Government and State bodies to improve the process. We have

welcomed the publication by Minister Donnelly of the Mazars Report in February, and we are looking forward to engaging with the Department of Health Working Group, established to reform the reimbursement system and to report back to the Minister within six months.
Oliver O’Connor, Chief Executive of the Irish Pharmaceutical Healthcare Association, said, “Over the past three budgets, the Government has allocated almost ¤100 million to new medicines. The figures released today show how urgent it is now to improve the reimbursement system so that new medicines are available to patients and their doctors faster. Process reform has to go alongside new funding. That’s why we have welcomed the Minister’s new Working Group to build out recommendations from and beyond the Mazars report. We have welcomed the recommendations on transparency already approved by the Minister and look forward to working on further improvements.
“The lengthening timelines demonstrate that there is a significant job to be done to make innovative medicines available faster to Irish patients. In particular, there is a serious issue with the timelines in relation to orphan medicines and medicines for cancer care. We have six months to agree new practical steps and then start implementing. IPHA member companies are ready to collaborate with all stakeholders through the Working Group to ensure this happens sooner rather than later.”
6 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Click Here to Register Digital Version Only
26% of Generic Medicines have ‘Disappeared’
Medicines for Europe Director General Adrian

Across Europe - on average26% of all generic medicines have disappeared. Unsustainable pricing policies are one of the leading drivers of shortages, says Medicines for Europe.
Affordable generic medicines available on the market just 10 years ago are disappearing and supply is too consolidated, according to new data shared recently. This rapid decline and consolidation, higher in certain therapy areas such as cancer care and antibiotics, risks creating more shortages and threatens vital access for patients.
The data showed that the whole generic medicines supply chain is under heavy pressure, with prices being pushed down to the limit of their sustainability. Strikingly, medicines supply consolidation is not just at the active ingredient level but also medicines, thereby creating risks of shortages and reducing access.
The system is broken and must be fixed.
Key Data:
• Of all the generic medicines available 10 years ago, 26% of generic medicines, 33% of antibiotics and 40% of cancer medicines have disappeared from European markets, on average.
• More than two-thirds (69%) of EU generic medicines on the market now have just one or two suppliers. If one manufacturer has a problem, there will likely be a medicine shortage causing disruption and possible harm for health care practitioners and for patients.
• 20 years ago, Europe used to make about half of the ingredients needed to make its medicines – now it’s down to about a fifth.
Policymakers at the national level need to change their policy approach to pricing of medicines with:
• Dynamic market rules to maintain more suppliers active in the market.
• Multi-criteria, multi winner tenders, beyond lowest price only.
• An inflation-linked-pricing adjustment mechanism.
• Open dialogue between suppliers and the medicines agency to rapidly address supply bottlenecks.
At the EU level, the focus needs to be on a streamlined policy framework with:
• Regulatory optimisation and digitalisation, including security of supply provisions in the revision of the pharmaceutical legislation.
• Security of supply safeguarded in all policies including in environmental legislation.
• Legal guidance on multi-winner and multi-criteria tenders in procurement which is requested by both industry, procurers, hospitals, and healthcare practitioners.
• An industrial policy that supports medicines manufacturing in Europe notably with efficient and competitive funding mechanisms.
Philippe Drechsle, Chair of Medicines for Europe manufacturing committee and Executive Committee member (Teva Pharmaceuticals Europe), said “This research made a health check of Europe’s essential medicine cabinet. We found increasingly empty shelves and a limited variety of essential medicines. Many of these now have just one manufacturer.
“The solutions are there to be implemented both at European and national levels. In the case of pricing, national laws tend to award drug suppliers based on the lowest generic price alone –and ignore important issues like sustainability, which swiftly leads to a less resilient industry, less robust supply chains and worse patient outcomes. This needs to be fixed. The EU has its role to play by adopting legislation that considers the impact of any new additional requirement on medicines availability across Europe. Our regulatory system is too costly, and the environmental obligations should deliver a winwin for the planet and patients’ access to medicines”.
Medicines for Europe Director General Adrian van den Hoven adds, “Antibiotic shortages this winter were a sad example of the policy gaps that make the supply of essential medicines so fragile. For years, generic medicine policies have been based on the lowest price only, with restrictive tender or reference pricing rules. These policies have fuelled market consolidation which make it difficult to respond to sudden demand surges. We are also working with the EU to improve disease forecasting in Europe to better plan manufacturing ahead of infectious seasons. These issues must be addressed in the revision of the EU pharmaceutical legislation.”
7 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
News
van den Hoven
“For years, generic medicine policies have been based on the lowest price only, with restrictive tender or reference pricing rules. These policies have fuelled market consolidation which make it difficult to respond to sudden demand surges. These issues must be addressed in the revision of the EU pharmaceutical legislation”
Boosting Lung Cancer Screening in Ireland and Beyond
The first pilot programmes will be held in 10 EU countries (Croatia, Estonia, France, Germany, Hungary, Poland, Czech Republic, Ireland, Italy, Spain and Greece). Many of these serve remote communities far from a hospital. So SOLACE will help provide transportation or mobile screening units.
Marie-Pierre Revel, from Assistance Publique des Hôpitaux de Paris (APHP), describes how a programme in France is planning to use “media coverage, including local newspapers and flyers, and sending women invitations for lung cancer screening along with their annual invitations er with invitation letters for breast cancer screening.”
A pioneering EU4Health project – under Europe’s Beating Cancer Plan – is launching a programme to expand lung cancer screening in countries throughout Europe.
This new initiative, called SOLACE, will break down barriers to screening so that people across all social and economic groups can access it.
Almost 2,700 people are diagnosed with lung cancer each year in Ireland. The 5-year survival rate tells you what percent of people live at least 5 years after the cancer is found. Presently it’s approximated at 24%.Not surprisingly, lung cancer has the lowest 5-year survival rate of the other most common cancers: only 24%, versus prostate at 99%; breast at 89%; and colorectal at 65%.
Lung cancer is the fourth most common cancer in Ireland after prostate, breast and colorectal.
More than a quarter million people in Europe die from lung cancer each year, according to the European Network of Cancer Registries. That's more than from any other cancer. However, like any other cancer, the disease is more treatable when caught early.
Low-Dose Computed Tomography (LDCT) is a safe, simple, and effective way of screening for lung cancer. Multiple trials in the USA and Europe have shown that LDCT can reduce deaths from lung cancer by 20%. The new EU-funded project will support Member States by providing a personalised toolbox to national and regional centres to facilitate lung-cancer screening programmes, with a particular focus on groups that are at higher risk due to health inequalities.
“This project will provide a comprehensive guideline for initiating lung cancer screening programmes with state-of-the-

art quality and high participation rates. Typical mistakes can then be avoided, and life expectancy increased,” says Hans-Ulrich Kauczor, the project's scientific coordinator.
Many individuals at high risk are currently not accessing lung cancer screening – often because of historical inequalities that exist with marginalised and vulnerable communities. Smoking can also be more prevalent in these groups, further compounding their risk of lung cancer.
Ivica Belina, a member of the European Lung Foundation Lung Cancer Patient Advisory Group, said, “One of the main problems with lung cancer is stigma. We need a change how we relate to people suffering from lung cancer, from a societal point of view. The SOLACE project can really change that and lift the stigma for patients.”
This three-year project is coordinated by the European Institute for Biomedical Imaging Research (EIBIR) and will be implemented by the SOLACE consortium under the scientific leadership of experts appointed by the European Society of Radiology and the European Respiratory Society. Patient-led organisations, European Lung Foundation, and Lung Cancer Europe will provide patient input.
The SOLACE consortium includes 33 entities, including academic and research institutions, university hospitals, national health authorities, organisations and associations representing patients and health care professionals. The European Cancer Organisation is supporting the project’s dissemination, and policy impact. In the long term, the project will provide valuable insights on how best to implement a cost-effective screening programme for lung cancer and the best techniques for reaching out to those groups that are at particularly high risk.

8 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
See ALK+ aNSCLC, Think Brain Metastases, Choose ALUNBRIG (brigatinib)
FIRST-LINE ESMO RECOMMENDATION2
NOW APPROVED FOR ONCE-DAILY FIRST-LINE TREATMENT OF ALK+ aNSCLC3

A lunbr ig is indic ate d as monother apy for the treatment of adul t patient s wi th A L K+ aNS CLC previously: 3
- not treate d wi th an A L K inhibi tor, or, - previously treate d wi th cr izotinib
ALK, anaplastic lymphoma kinase; aNSCLC, advanced non-small cell lung cancer; ESMO, European Society for Medical Oncology.
References:
1. Camidge DR, et al. J Thorac Oncol 2021;16:2091-2108;
2. Planchard
D, et al. Annals Oncol. 2018;29(S4): iv192–237 - Updated version published 15 September 2020. Available at: https://tinyurl.com/3853nnnu. Accessed June 2022.
3. Alunbrig (brigatinib) Summary of Product Characteristics. Available at: https://www.medicines.ie/medicines/alunbrig-34655/ Accessed June 2022.
ALUNBRIG®t(brigatinib) PRESCRIBING INFORMATION FOR REPUBLIC OF IRELAND
Refer to the Summary of Product Characteristics (SmPC) before prescribing
Presentation: Brigatinib 180 mg, 90 mg and 30 mg film-coated tablets. Indications: As monotherapy for the treatment of adult patients with anaplastic lymphoma kinase (ALK)positive advanced non-small cell lung cancer (NSCLC) previously not treated with an ALK inhibitor. As monotherapy for the treatment of adult patients with ALK-positive advanced NSCLC previously treated with crizotinib. Dosage and administration: Recommended starting dose is 90 mg once daily for the first 7 days, then 180 mg once daily. If ALUNBRIG is interrupted for 14 days or longer for reasons other than adverse reactions, treatment should be resumed at 90 mg once daily for 7 days before increasing to the previously tolerated dose. If a dose is missed or vomiting occurs after taking a dose, an additional dose should not be administered and the next dose should be taken at the scheduled time. Treatment should continue as long as clinical benefit is observed. Dosing interruption and/or dose reduction may be required. Refer to SmPC for full dose adjustments. Paediatric population: No data are available. Elderly patients: Dose adjustment is not required. No available data on patients aged > 85 years. Hepatic impairment: A reduced starting dose of 60 mg once daily for the first 7 days, then 120 mg once daily is recommended for patients with severe hepatic impairment (ChildPugh class C). Renal impairment: A reduced starting dose of 60 mg once daily for the first 7 days, then 90 mg once daily is recommended for patients with severe renal impairment (eGFR <30 mL/min). Patients with severe renal impairment should be closely monitored for new or worsening respiratory symptoms (e.g., dyspnoea, cough, etc.) particularly in the first week. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: Refer to SmPC for recommended dose modifications. Pulmonary adverse reactions: Most occurred within the first 7 days of treatment, but they can occur later in treatment. Patients should be monitored for new or worsening respiratory symptoms (e.g., dyspnoea, cough, etc.), particularly in the first week of treatment. If interstitial lung disease/pneumonitis is suspected, the dose of ALUNBRIG should be withheld, and the patient evaluated for other causes of symptoms (e.g., pulmonary embolism, tumour progression, and infectious pneumonia). Dose modification may be required. Hypertension: Heart rate and blood pressure should be monitored regularly. Hypertension should be treated according to standard guidelines. Heart rate should be monitored more frequently in patients if concomitant use of a medicinal product known to cause bradycardia cannot be avoided. Withhold ALUNBRIG in patients with severe hypertension (≥ Grade 3) until hypertension has recovered to Grade 1 or to baseline. Modify dose accordingly. Bradycardia: Caution should be exercised when administering ALUNBRIG in combination with other agents known to cause bradycardia. If symptomatic bradycardia occurs, treatment with ALUNBRIG should be withheld and concomitant medicinal products known to cause bradycardia should be evaluated. Upon recovery, the dose should be modified according to SmPC. In case of life-threatening bradycardia, if no contributing concomitant medication is identified or in case of recurrence, treatment with ALUNBRIG should be discontinued. Visual disturbance: Advise
patients to report any visual symptoms. Consider ophthalmologic evaluation/dose reduction for new or worsening severe symptoms. Creatine phosphokinase (CPK) elevation: Patients should be advised to report any unexplained muscle pain, tenderness, or weakness. CPK levels should be monitored regularly during ALUNBRIG treatment. Based on the severity of the CPK elevation, and if associated with muscle pain or weakness, treatment with ALUNBRIG should be withheld, and the dose modified. Elevations of pancreatic enzymes: Lipase and amylase should be monitored regularly during treatment with ALUNBRIG. Based on the severity of the laboratory abnormalities, treatment with ALUNBRIG should be withheld, and the dose modified. Hepatotoxicity: Liver function, including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin should be assessed prior to the initiation of ALUNBRIG and then every 2 weeks during the first 3 months of treatment. Thereafter, monitoring should be performed periodically. Based on the severity of the laboratory abnormalities, treatment should be withheld, and the dose modified. Hyperglycaemia: Fasting serum glucose should be assessed prior to initiation of ALUNBRIG and monitored periodically thereafter. Antihyperglycaemic medications should be initiated or optimised as needed. If adequate hyperglycaemic control cannot be achieved with optimal medical management, ALUNBRIG should be withheld until adequate hyperglycaemic control is achieved; upon recovery, dose reduce ALUNBRIG as per the SmPC or permanent discontinuation may be considered. Photosensitivity and photodermatosis: Photosensitivity to sunlight has occurred in patients treated with ALUNBRIG. Patients should be advised to avoid prolonged sun exposure while taking ALUNBRIG and for at least 5 days after discontinuation of treatment. When outdoors, advise patients to wear a hat and protective clothing and to use a broad-spectrum Ultraviolet A/B sunscreen and lip balm (SPF≥30). For severe photosensitivity reactions (≥Grade 3) withhold ALUNBRIG until recovery to baseline. The dose should be modified accordingly. Lactose: ALUNBRIG contains lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take ALUNBRIG. Sodium: ALUNBRIG is essentially ‘sodium-free’ containing less than 1 mmol sodium (23 mg) per tablet. Interactions: Avoid use with strong CYP3A inhibitors. Avoid use with strong and moderate CYP3A inducers. Please refer to SmPC for further information and guidance for situations where use cannot be avoided. Grapefruit or grapefruit juice should be avoided. Coadministration of ALUNBRIG with CYP3A substrates with a narrow therapeutic index should be avoided as ALUNBRIG may reduce their effectiveness. Coadministration of ALUNBRIG with substrates of P-glycoprotein, breast cancer resistance protein, organic cation transporter 1, multidrug and toxin extrusion protein (MATE) 1 and 2K may increase their plasma concentrations. Patients should be closely monitored when ALUNBRIG is co-administered with substrates of these transporters with a narrow therapeutic index. Fertility, pregnancy and lactation: Women of reproductive potential should be advised not to become pregnant and to use effective non-hormonal
contraception during treatment with ALUNBRIG and for at least 4 months following the final dose. Men should be advised not to father a child during ALUNBRIG treatment. Men with female partners of reproductive potential should be advised to use effective contraception during and for at least 3 months after the last ALUNBRIG treatment. No clinical data on the use of ALUNBRIG in pregnant women. ALUNBRIG should not be used during pregnancy unless the clinical condition of the mother requires treatment. Breastfeeding should be stopped during treatment with ALUNBRIG. No human data are available on the effect of ALUNBRIG on fertility. Undesirable effects: Very common (≥1/10): Pneumonia, upper respiratory tract infection, anaemia, lymphocyte count decreased, APTT increased, white blood cell count decreased, neutrophil count decreased, hyperglycaemia, hyperinsulinaemia, hypophosphataemia, hypomagnesaemia, hypercalcaemia, hyponatraemia, hypokalaemia, decreased appetite, headache, peripheral neuropathy, dizziness, visual disturbance, hypertension, cough, dyspnoea, lipase increased, diarrhoea, amylase increased, nausea, vomiting, abdominal pain, constipation, stomatitis, AST increased, ALT increased, alkaline phosphatase increased, rash, pruritus, blood CPK increased, myalgia, arthralgia, blood creatinine increased, fatigue, oedema, pyrexia. Common (≥1/100 to <1/10): Decreased platelet count, insomnia, memory impairment, dysgeusia, bradycardia, electrocardiogram QT prolonged, tachycardia, palpitations, pneumonitis, dry mouth, dyspepsia, flatulence, blood lactate dehydrogenase increased, hyperbilirubinaemia, dry skin, photosensitivity reaction, musculoskeletal chest pain, pain in extremity, musculoskeletal stiffness, non-cardiac chest pain, chest discomfort, pain, blood cholesterol increased, weight decreased. Other serious undesirable effects: Uncommon: (≥1/1,000 to <1/100): Pancreatitis. Refer to the SmPC for details on full side effect profile and interactions. Legal classification: POM. Marketing authorisation (MA) numbers: EU/1/18/1264/011, EU/1/18/1264/008, EU/1/18/1264/010, EU/1/18/1264/012. Name and address of MA holder: Takeda Pharma A/S, Delta Park 45, 2665 Vallensbaek Strand, Denmark. Additional information is available on request at: medinfoemea@takeda.com. PI approval code: pi-01842. Date of Preparation: January 2022.
tThis medicinal product is subject to additional monitoring.
Adverse Events should be reported to the Pharmacovigilance Unit at the Health Products Regulatory Authority. Reporting forms and information can be found at: www.hpra.ie. Adverse events should also be reported to Takeda at: AE.GBR-IRL@takeda.com
1
C-APROM/IE/ALUN/0051. Date of preparation: June 2022 Takeda Oncology, and ALUNBRIG are registered trademarks of Takeda Pharmaceutical Company Limited. Other trademarks are the property of their respective owners. ©2022 Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. All rights reserved.
Bed Shortages will ‘Worsen’ – says IMO
The Irish Medical Organisation (IMO) has warned that investment in additional hospital beds is not keeping pace with population growth and that the current dire situation will worsen unless rapid action is taken.
The warning comes after it emerged that over 700 patients around the country were waiting on a hospital bed today (Tuesday).
It also described recent revelations that just 162 new beds would be added to the Irish public hospital system this year as “shocking”.
The IMO said that being unable to move admitted patients to a ward bed in a timely manner is leading to poorer health outcomes for
patients and intolerable conditions for those trying to treat them in an appropriate setting.
It added that, although senior decision-makers were working and available on weekends, discharge pathways and support services are not in place to enable patients to be transferred to appropriate care. Speaking today, Professor Matthew Sadlier, Chair of the Consultant Committee of the IMO, said, “There is an acute need for 5,000 additional beds if we are to provide adequate care for the population. The dire shortage of beds adds to overcrowding in hospitals and EDs, longer waiting lists and poorer patient outcomes.
“It is unacceptable that over 700 patients are currently waiting on a hospital bed after the May bank holiday and emphasises that this is a year-round crisis.
“The idea that just 162 additional beds will be added to the stock this year is shocking and confirms that a long-standing bed shortage will get worse this year not better.”
The IMO cited international figures which highlight how far behind Ireland is in terms of bed capacity compared to other European countries. The average in Europe is 3.87 acute beds per 1,000 people whereas in Ireland the figure is just 2.7 beds per 1,000 of the population.
Mater Private Network invests ¤3m
The IMO has previously called for the use of modular builds to provide additional capacity at hospitals which are under particular pressure.
Professor Sadlier said: “We have to go beyond tinkering with the beds issue. We need urgent action to alleviate crisis points and we need a commitment to a meaningful increase in the number of additional – not replacement –beds. This crisis will persist until we have sufficient beds and doctors to meet the needs of growth in our population and address the complexity of care required.”
Mater Private Network continues to pave the way for the future of healthcare through consistent investment in leading technology across its network of facilities here in Ireland.
Today, Mater Private Network celebrates the commissioning of a new CT scanner in its Radiology Department on Eccles St. This new state-of-the-art equipment, produced by Siemens, is a core element of a significant radiology investment programme to the value of ¤3 million.

Until now, Mater Private Network’s diagnostic imaging services in Dublin has been performing 300-350 CT scans per week; this new CT scanner will deliver a two-fold capacity increase for CT scanning, with waiting lists virtually eliminated as a result. Patients will also benefit from the cutting-edge technology, which gives excellent image quality and reduces the radiation dose to the lowest possible level. With the opening hours for radiology services now extended into the late evening and running seven days a week across
the three Mater Private Network Dublin facilities, this also helps to address the challenge of long waiting lists that exists nationally, and greatly improves access for patients for diagnostic imaging. This transformational project has been supported by a robust, and highly successful recruitment campaign which has seen the Mater Private Network Radiology team increase by 30% and bringing a range of international expertise across the Dublin facilities.
Celebrating the commissioning of the new CT scanner, Prof. Peter J MacMahon, Director of Radiology at Mater Private Network in Dublin, commented, “I am particularly excited about this development and what it means for our patients. Thanks to this new CT scanner, we will be able to diagnose and treat more patients than ever before with our multidisciplinary team of highly skilled of professionals. This is a fantastic step forward for Mater Private Network. We are committed to staying at the forefront of medical technology so that we can continue to deliver the best possible care to those who trust us with their health”.
Bernard Bonroy, Radiology Services Manager, Mater Private Network, has said, “We are proud to be able to offer an enhanced service from our Radiology Department here in Dublin.
Recruiting the right team to ensure we could provide this enhanced service was our north star when working on this project, and I am delighted to share that this has led to a 30% increase in radiography staff across our Dublin sites. Our team at Eccles St. is best in class and we strive to ensure seamless patient care for those who pass through our doors”.
10 JUNE 2023 • HPN |
Report
HOSPITALPROFESSIONALNEWS.IE
Back row - Bernard Bonroy, Professor Peter MacMahon, Dr. Daragh Murphy, Front row - Ciara Newell, Bahia Raffie, Theresa Gleeson, Deirdre Lynch photographed with the new CT Scanner at Mater Private Network Radiology Department, Eccles St., Dublin
New research reveals cancer-killing benefits of popular obesity treatment
Maynooth University’s Kathleen Londsdale Institute for Human Health Research has just published ground-breaking research into the benefits of the popular obesity treatment drug, GLP-1. Previous research has found that people with obesity are at a greater risk of developing cancer, in part due to their anticancer immune cell -- better known as the ‘Natural Killer (NK)’ cell -- being rendered useless due to their disease.
New Health Research Board (HRB) funded research carried out by Dr Andrew Hogan and his team in the Londsdale Health Institute at Maynooth University, has found that the popular, and gold-standard pharmacological treatment for obesity, Glucagonlike peptide (GLP-1) analogues, can actually restore the NK cell function in the body including its ability to kill cancerous cells.
Currently, one in four Irish adults are living with obesity, a disease linked to up to 40% of all cancers.
The research, published in Obesity, the Obesity Society’s Research Journal, one of the world’s leading peer-reviewed obesity journals, also shows that the restored cancer-killing effect of the NK cells is independent of the GLP-1’s main weight loss function so it appears the treatment is directly kick-starting the NK cells’ engine.
Dr Andy Hogan
Dr Andrew E. Hogan, Associate Professor & Principal Investigator, Lonsdale Human Health Institute in Maynooth University discussed the findings stating, “My team and I are very excited by these new findings in relation to the effects of the GLP-1 treatment on people with obesity and it appears to result in real tangible benefits for those currently on the drug.
“While these findings will understandably be welcomed by those living with obesity and looking for safe and effective treatments, it must be noted that unfortunately, these treatments are not fully covered by the Government’s Drug Payment Scheme. With the findings of this research, it’s more important than ever that the HSE work with the Government to ensure the benefits of this treatment become available to as many individuals as possible and as soon as possible.
“Secondly, given the recent spike in popularity related to the benefits of the GLP-1 treatment with global and high-profile celebrities commenting on its success, global demand has increased and resulted in a worldwide shortage of the drug.
“Again, I hope this is something that is brought under control to ensure as many people as possible living with obesity can start their own treatment of this beneficial drug.”
Conor de Barra, PhD student in immunology at Maynooth University and Irish Research Council Scholar, who led the work in Dr Hogan’s lab on this particular research said: “People with obesity can develop a variety of health problems like type 2 diabetes, sleep apnoea and cancer. These can have very negative impacts on their quality of life. This research and other promising findings on improvements in cardiovascular health after GLP-1 therapy indicate
Honorary Fellowship for Professor Clarke
Professor Graeme Clark AC – pioneer of the bionic ear, or multi-channel cochlear implant –has been awarded an Honorary Fellowship of the Royal College of Surgeons in Ireland (RCSI).

The Honorary Fellowship of RCSI is the highest distinction the College bestows, recognising outstanding clinical achievements and humanitarians that include Nelson Mandela, Mother Teresa of Calcutta and President Jimmy Carter.
The multichannel implant developed by the team led by Professor Clark, in association with the Australian firm Cochlear Limited, is the world’s first device to restore a human sense and allow severely-to-profoundly deaf people to understand speech. It has been the world leader for 40
years and has the dominant share of the one million people now implanted in over 120 countries. Professor Clark’s achievements have been recognised globally. His numerous prestigious awards include Australia’s highest civil honor, a Companion of the Order of Australia, for services to medicine in 2004, and Senior Australian of the Year in 2001 and many international honors, including the Lasker DeBakey Award, considered the American Nobel in Clinical Medical Research.
He is a Laureate Professor at the University of Melbourne and a member of The Graeme Clark Institute for Biomedical Engineering, which was named in his honour and seeks to transform healthcare with biomedical engineering.

potential benefits in addition to weight-loss.”
Prof Donal O’Shea, HSE National Lead for Obesity & Principal Investigator, added, “We are finally reaching the point where medical treatments for the disease of obesity are being shown to prevent the complications of having obesity. The current findings represent very positive news for people living with obesity on GLP-1 therapy and suggest the benefits of this family of treatments may extend to a reduction in cancer risk.”
Dr Hogan presented these findings at the 30th European Congress on Obesity, which was held in Dublin on 20th of May.
11 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
News
Professor Graeme Clark AC pictured with Professor Laura Viani, RCSI President
Diabetes during Pregnancy in HIV on the Rise
at the British HIV Association conference recently in Gateshead. The study also found that the proportion of women diagnosed with high blood pressure during pregnancy has not risen.
Blood sugar levels can rise during pregnancy, leading to the development of diabetes. Women are at higher risk of gestational diabetes if they are overweight or have obesity, have a South Asian, Black or African Caribbean or Middle Eastern background, or if a close family member has had diabetes. Having HIV does not raise the risk of developing gestational diabetes, but protease inhibitorbased antiretroviral treatment may raise the risk if it is started early in pregnancy.
lower risk of developing high blood pressure during pregnancy than women without HIV.
The UK study looked at the prevalence of diabetes and high blood pressure during pregnancy in women with HIV in the United Kingdom and Ireland between January 2010 and December 2020. The study also looked at the relationship between gestational diabetes and hypertension and adverse pregnancy outcomes including stillbirth, pre-term birth and low birth weight.
The study evaluated 10,401 pregnancies in 8998 women with HIV; 1104 pregnancies were excluded from the analysis either because HIV was diagnosed during pregnancy or because delivery occurred prior to 24 weeks (either miscarriage or very pre-term birth). The annual number of pregnancies in women with HIV halved between 2010 and 2020, from just under 1300 a year to approximately 600 a year in 2020.

history of diabetes, or belonged to a minority ethnic group with a high prevalence of diabetes.
Gestational diabetes was diagnosed during 554 pregnancies in 503 women; in 511 cases this was defined as gestational diabetes.
High blood pressure was diagnosed during 511 pregnancies in 458 women. In three out of four cases (383 pregnancies), high blood pressure was associated with a diagnosis of pre-eclampsia, a serious complication of pregnancy. Pre-eclampsia is usually detected by the presence of high levels of protein in the urine and high blood pressure. Pre-eclampsia affects transfer of oxygen across the placenta, affecting foetal growth and raising the risk of stillbirth and premature birth. In severe cases, preeclampsia may lead to fits or liver or kidney failure in the mother.
The frequency of diabetes during pregnancy is rising in women with HIV in Ireland and the UK, in line with trends in the rest of the population, Laurette Bukasa of University College London reported
A recently published study of pregnancies in South Africa’s Western Cape province found that women with HIV on antiretroviral treatment before conceiving had a
Women were screened for gestational diabetes between weeks 24 and 28 of pregnancy if they had a body mass index of 30 kg/m2 or above, a previous baby with a high birth weight, a family
The prevalence of high blood pressure did not change significantly between 2010 and 2020 (3.9% in 2010, 5.8% in 2020). Both diabetes and high blood pressure were diagnosed during 46 pregnancies in 43 women.
Latest Figures on Sexually Transmitted Infections
There has been “at least” a 100% increase in teenagers getting diagnosed with STIs this year, a consultant in sexual health has said.
Figures from the HSE have revealed that 783 teenagers in Ireland have been diagnosed with sexually transmitted infections (STIs) in 2023.
The figures included two children who are under 14 years of age.
The most common STI in Ireland this year was chlamydia with 4,311 cases, followed by gonorrhoea with 2,326 cases.
The news come after it was revealed earlier this year that HIV rates more than doubled in Ireland over the last year, according to statistics published by the Health Protection Surveillance Centre (HPSC).
The body responsible for tracking rates of sexually transmitted infections (STIs) has reported that for the first 51 weeks of the year, cases across nine disease types had risen by 56 per cent.
The upward trend follows concerns that Covid-19 restrictions had hampered testing services and the ability of people to present themselves for testing.
Consultant of sexual health and HIV care in genitourinary medicine at St. James Hospital Dr
Aisling Loy said the data “definitely is alarming.”
“It's preliminary data so it gets cleaned up at the end of the year … before it's cleaned up it still is indicative that we are seeing a big rise,” she said.
Dr Loy said last year’s cleaned-up data revealed a total of 950 STI diagnoses among teenagers. “We're already on track to see maybe at least a 100% increase this year,” she said. “We’re a third way through the year and we’re at 700.
“Even when you take out some duplicate cases … there will be a slight drop, but not a huge drop.”
Dr Loy said that within the group of 15- to 19-year-olds, gonorrhoea diagnoses are driving the numbers.
“Traditionally in that age category, it was chlamydia, but actually that had gone down 6% in females, I think around 9% in young men,” she said.
“There's been a huge increase in gonorrhoea in that group.
“Traditionally we thought of these as being sexually transmitted by say penis to vagina, and that it would be traditional sexual routes of transmission.
56 Recommendations for ‘More Inclusive’ Ireland
Fine Gael LGBTQ+ launched Building on Progress: An Inclusive Ireland, a policy document designed to guide officials toward developing a more equal society. It makes 56 recommendations across seven government departments, namely Education, Equality, Foreign Affairs, Health, Housing & Local Government, Justice and Sport.
In the publication, the group outlines over 20 pressing issues that LGBTQ+ people face in Ireland today. These include HIV transmissions, transgender healthcare, hate crimes, safety in schools, conversion therapy, surrogacy, participation in sport, global rights, homelessness and more.
The document was created independently by Fine Gael LGBTQ+, a voluntary organisation made up of party members, staff and Oireachtas officials. While it has been submitted to Fine Gael for consideration, it has not been officially adopted as policy.
On Wednesday, May 3, parliamentary members of the party held a briefing to have a “constructive and positive conversation” on LGBTQ+ issues.
12 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE HIV News

Diabetic Retinopathy
Diabetic Retinopathy in Ireland
“By identifying TRPV2 as a key protein involved in diabetesrelated vision loss, we have a new target and opportunity to develop treatments that halt the advancement of diabetic retinopathy.”
The study was funded by the Biotechnology and Biological Sciences Research Council and the Department for the Economy Postgraduate Studentship scheme. Diabetic retinopathy risk factors:
• Blood sugar levels
The control of blood sugar levels is of immense importance. Lower blood sugar levels can delay the onset and slow the progression of diabetic retinopathy
• Blood pressure
Diabetic retinopathy is a common complication of diabetes and occurs when high blood sugar levels damage the cells at the back of the eye, known as the retina. There are no current treatments that prevent the advancement of diabetic retinopathy from its early to late stages, beyond the careful management of diabetes itself. As a result, a significant proportion of people with diabetes still progress to the vision-threatening complications of the disease.
As the number of people with diabetes continues to increase globally, there is an urgent need for new treatment strategies, particularly those that target the early stages of the disease to prevent vision loss.
Diabetic retinopathy is the most common cause of blindness in working age individuals' in Ireland, at the present time. It is estimated that there are approximately 190,000 people in Ireland with diabetes and 10 per cent of them are at risk of sight threatening retinopathy.
Diabetic RetinaScreen commenced its national population-based diabetic retinopathy screening programme at the end of February 2013 and this is being introduced on a phased basis to ensure quality and safety.
Diabetic RetinaScreen will deliver great benefits to men and women with diabetes in Ireland who are at risk of sight threatening retinopathy, enhancing quality of
life and preserving sight for longer. Of the population screened and treated, it is expected that six per cent will be prevented from going blind within a year of treatment and 34 per cent within ten years of treatment.
The test looks for early warning signs of diabetic retinopathy, a complication of diabetes caused by high blood glucose (sugar) levels damaging the back of the eye (retina). If left undiagnosed and untreated diabetic retinopathy may lead to blindness. However, if diabetic eye disease is found early, treatment can reduce or prevent damage to your sight.
Diabetic RetinaScreen Programme Manager, Helen Kavanagh says, “If left undiagnosed and untreated diabetic retinopathy can deprive people of their sight, but it usually takes many years to reach that stage. This free eye screening test offers people with diabetes an opportunity to detect problems early, which can lead to more successful treatment and better outcomes. The longer you have diabetes the higher the risk, that’s why we’re encouraging everyone aged 12 years and older to be registered with Diabetic RetinaScreen today.”
Latest Research
Last year, researchers at Queen’s University in Belfast uncovered a key process that contributes to vision loss and blindness in those with diabetes.
The retina demands a high oxygen and nutrient supply to function properly. This is met by an elaborate network of blood vessels that maintain a constant flow of blood even during daily fluctuations in blood and eye pressure. The ability of the blood vessels to maintain blood flow at a steady level is called blood flow autoregulation. The disruption of this process is one of the earliest effects of diabetes in the retina.
The breakthrough made by researchers at Queen’s University Belfast pinpoints the cause of these early changes to the retina. The study, has discovered that the loss of blood flow autoregulation during diabetes is caused by the disruption of a protein called TRPV2. Furthermore, they show that disruption of blood flow autoregulation even in the absence of diabetes causes damage closely resembling that seen in diabetic retinopathy. The research team are hopeful that these findings will be used to inform the development of new treatments that preserve vision in people with diabetes.
Professor Tim Curtis, Deputy Director at the Wellcome-Wolfson Institute for Experimental Medicine at Queen’s and corresponding author, explains, “We are excited about the new insights that this study provides, which explain how the retina is damaged during the early stages of diabetes.
High blood pressure damages blood vessels, raising the chances for eye problems. Effective control of blood pressure reduces the risk of retinopathy progression and visual acuity deterioration
• Duration of diabetes
The risk of diabetic retinopathy developing or progressing increases over time. After 15 years, 80 per cent of Type 1 patients will have diabetic retinopathy. After 19 years, up to 84 per cent of patients with Type 2 diabetes will have diabetic retinopathy
• Blood lipid levels (cholesterol and triglycerides)
Elevated blood lipid levels can lead to greater accumulation of exudates, protein deposits that leak into the retina. This condition is associated with a higher risk of moderate visual loss
• Pregnancy
Women who have diabetes and become pregnant have an increased risk of developing retinopathy. If they already have diabetic retinopathy, it may progress
Symptoms
1. Gradually worsening vision
2. Sudden vision loss
3. Shapes floating in your field of vision (floaters)
4. Blurred or patchy vision
5. Eye pain or redness
14 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
HIT BACK A

RINVOQ® (UPADACITINIB) IS NOW INDICATED IN
ULCERATIVE COLITIS
PRESCRIBING INFORMATION (PI). RINVOQ®▼ (upadacitinib) 15 mg prolonged release tablets; 30mg prolonged-release tablets; 45 mg prolonged-release tablets. Refer to Summary of Product Characteristics (SmPC) for full prescribing information. PRESENTATION: Each tablet contains upadacitinib hemihydrate, equivalent to 15mg of upadacitinib in the 15mg tablet, 30mg in the 30mg tablet and 45mg in the 45mg tablet. INDICATION: Treatment of moderate to severe active rheumatoid arthritis (RA) and active psoriatic arthritis (PsA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate. Treatment of active nonradiographic axial spondyloarthritis (nr-axSpA) in adult patients with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI), who have responded inadequately to nonsteroidal anti-inflammatory drugs (NSAIDs). Treatment of active ankylosing spondylitis (AS) in adult patients who have responded inadequately to conventional therapy.
Treatment of moderate to severe atopic dermatitis (AD) in adults and adolescents 12 years and older who are candidates for systemic therapy. Treatment of adult patients with moderately to severely active ulcerative colitis (UC) or moderately to severely active Crohn’s disease (CD) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent. DOSAGE AND ADMINISTRATION: Intended for use under guidance and supervision of a physician experienced in diagnosis and treatment of conditions for which upadacitinib is indicated. Dosage: RA, PsA, AS, and nr-axSpA: Recommended oral dose is 15mg once daily. Consideration should be given to discontinuing treatment in patients with AS and nr-axSpA who have shown no clinical response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks. AD: Adults: Recommended oral dose is 15 mg or 30 mg once daily based on individual patient presentation (see SmPC for details). A dose of 15 mg is recommended for patients at higher risk of venous thromboembolism (VTE), major adverse cardiovascular events (MACE) and malignancy. The lowest effective dose to maintain response should be used. For patients ≥ 65 years of age, the recommended dose is 15mg once daily. Adolescents from 12 to 17 years weighing at least 30 kg: Recommended oral dose is 15mg once daily. Consideration should be given to discontinuing upadacitinib treatment in any patient who shows no evidence of therapeutic benefit after 12 weeks of treatment. UC: Induction; Recommended oral dose is 45mg once daily for 8 weeks. For patients who do not achieve therapeutic benefit by week 8, 45mg once daily may be continued for an additional 8 weeks. Discontinue treatment in patients with no evidence of therapeutic benefit by week 16. Maintenance; Recommended oral dose is 15mg or 30mg once daily based on individual patient presentation (see SmPC for details). A dose of 15 mg is recommended for patients at higher risk of VTE, MACE and malignancy. The lowest effective dose to maintain response should be used. Patients ≥ 65 years of age: recommended dose is 15 mg once daily. In patients who have responded to treatment with upadacitinib, corticosteroids may be reduced and/or discontinued in accordance with standard of care. For patients with UC receiving strong inhibitors of cytochrome P450 (CYP) 3A4 the recommended induction dose is 30 mg once daily and maintenance dose is 15 mg once daily. CD: Induction; Recommended oral dose is 45mg once daily for 12 weeks. For patients who have not achieved adequate therapeutic benefit after the initial 12-week induction, 30mg once daily may be considered. For these patients, upadacitinib should be discontinued if there is no evidence of therapeutic benefit after 24 weeks of treatment. Maintenance; Recommended oral dose is 15mg or 30mg once daily based on individual patient presentation (see SmPC for details). A
dose of 15mg once daily is recommended for patients at higher risk of VTE, MACE and malignancy. The lowest effective dose to maintain response should be used. Patients ≥ 65 years of age: recommended dose is 15 mg once daily. In patients who have responded to treatment with upadacitinib, corticosteroids may be reduced and/or discontinued in accordance with standard of care. For patients with CD receiving strong inhibitors of cytochrome P450 (CYP) 3A4 the recommended induction dose is 30 mg once daily and maintenance dose is 15 mg once daily. (see SmPC for more details). Special Populations: Elderly: Upadacitinib should only be used in patients ≥65 years if no suitable treatment alternatives are available. For AD patients ≥ 65 years of age, a dose higher than 15mg once daily is not recommended. For RA, PsA, AS and nr-axSpA there are limited data for patients 75 years of age and older. For UC and CD maintenance therapy, a dose higher than 15 mg once daily is not recommended in patients ≥ 65 years of age. For UC and CD patients ≥ 75 years of age the safety and efficacy of upadacitinib has not been established. Renal: No dose adjustment required in mild-moderate renal impairment. Patients with severe renal impairment should use upadacitinib with caution (See SmPC for dose adjustments). Upadacitinib has not been studied in subjects with end stage renal disease and is therefore not recommended for use in these patients. Hepatic impairment: No dose adjustment is required in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. Should not be used in patients with severe (Child Pugh C) hepatic impairment. Paediatric Population: Safety and efficacy in RA, AS, PsA, nr-axSpA, UC and CD in children and adolescents aged 0 to 18 years and in AD in children under 12 years of age has not been established. No clinical exposure data are available in AD adolescents < 40 kg. CONTRAINDICATIONS: Hypersensitivity to active substance or excipients. Active tuberculosis (TB) or active serious infections. Severe hepatic impairment. Pregnancy. SPECIAL WARNINGS AND PRECAUTIONS: See SmPC for full details.
Upadacitinib should only be used if no suitable treatment alternatives are available in patients: 65 years of age and older; with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers) or with malignancy risk factors (e.g. current malignancy or history of malignancy).
Immunosuppression: Combination use, with immunosuppressants e.g. azathioprine, 6-mercaptopurine, ciclosporin, tacrolimus, and biologic DMARDs or other JAK inhibitors has not been evaluated in clinical studies and is not recommended as the risk of additive immunosuppression cannot be excluded. Serious infections: Serious and fatal infections have been reported. Caution when treating patients ≥65 years. Frequently reported – pneumonia and cellulitis. Bacterial meningitis and sepsis have been reported. Opportunistic infections reported – TB, multidermatomal herpes zoster, oral/oesophageal candidiasis, and cryptococcosis. Do not initiate treatment in patients with active, serious infection, including localised infections. Consider the risk/benefit of treatment in patients with: chronic or recurrent infection, history of serious or opportunistic infection, those exposed to TB, those who have resided or travelled in areas of endemic TB or endemic mycoses, those with underlying conditions that may pre-dispose patients to infection, those with diabetes. Closely monitor patients for signs and symptoms of infection during and after treatment. Interrupt treatment if the patient develops a serious/opportunistic infection. If infection is controlled, resume treatment following risk/benefit consideration. A higher rate of serious infections was observed with upadacitinib 30mg compared to upadacitinib 15mg. Tuberculosis: Pre-screen patients for active or latent TB. RINVOQ should not be given to patients with active TB.
GAINST IBD*
ADULTS WITH MODERATELY TO SEVERELY ACTIVE


& CROHN’S DISEASE1
Consider anti-TB therapy in patients with previously untreated latent TB or in patients with risk factors for TB infection. Consult with a physician with experience in TB therapy to assess whether initiating anti-TB therapy is appropriate for an individual patient. Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy. Viral reactivation: Cases of herpes virus reactivation have been reported e.g. herpes zoster. If herpes zoster is reactivated, consider interruption of upadacitinib therapy until the episode resolves. Screen for viral hepatitis and monitor regularly for reactivation before starting and during therapy with upadacitinib. Patients, positive for hepatitis C antibody/hepatitis C virus RNA and hepatitis B surface antigen/hepatitis B virus DNA were excluded from clinical studies. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted. Vaccination: Use of live, attenuated vaccines during or immediately prior to therapy is not recommended. It is recommended that patients be brought up to date with all immunisations, including prophylactic zoster vaccinations prior to starting therapy, in agreement with current immunisation guidelines. Malignancy: Lymphoma, NMSCs and other malignancies have been reported in patients receiving JAK inhibitors, including upadacitinib. There is a higher rate of malignancies and NMSCs with upadacitinib 30mg compared to 15mg. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer. See SmPC for full details. Haematological abnormalities: Do not start therapy if Absolute Neutrophil Count <1 x 109 cells/L, Absolute Lymphocyte Count <0.5 x 109 cells/L and Hb <8 g/dL. Monitor patients and temporarily stop therapy if abnormal haematological parameters as specified are detected during routine patient management. Gastrointestinal perforations: Events of diverticulitis and gastrointestinal perforations have been reported. Use with caution in patients who may be at risk for gastrointestinal perforation (e.g., patients with diverticular disease, a history of diverticulitis, or who are taking nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or opioids). Patients with active Crohn’s disease are at increased risk for developing intestinal perforation. Patients presenting with new onset abdominal signs and symptoms should be evaluated promptly for early identification of diverticulitis or gastrointestinal perforation. Major adverse cardiovascular events: Events of MACE were observed in clinical studies of upadacitinib (See SmPC for full details). Lipids: Monitor total cholesterol, lowdensity lipoprotein (LDL) and high-density lipoprotein (HDL) at 12 weeks and thereafter according to international guidelines for hyperlipidaemia. Elevated LDL in clinical trials decreased to pre-treatment levels in response to statin therapy, although evidence is limited. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Hepatic transaminase elevations: Monitor liver enzymes at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. In such cases therapy should be interrupted until this diagnosis is excluded. Venous thromboembolism: Deep vein thrombosis (DVT) and pulmonary embolism (PE) were observed in clinical trials for upadacitinib. Use therapy with caution in patients with known VTE risk factors other than cardiovascular or malignancy risk factors. Risk factors include previous VTE, patients undergoing major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, and inherited coagulation disorder. Patients should be re-evaluated periodically during upadacitinib treatment to assess for changes in VTE risk and treatment should be discontinued in patients with suspected VTE. Hypersensitivity: Anaphylaxis and angioedema have been reported in patients receiving upadacitinib. Discontinue treatment and institute appropriate therapy if significant hypersensitivity
occurs. INTERACTIONS: Upadacitinib is metabolised mainly by CYP3A4 and plasma exposures may be affected by CYP3A4 inhibitors or inducers. Upadacitinib 15 mg once daily should be used with caution in patients receiving chronic treatment with strong CYP3A4 inhibitors. Upadacitinib 30 mg once daily dose is not recommended for patients with atopic dermatitis receiving chronic treatment with strong CYP3A4 inhibitors. For patients with ulcerative colitis or Crohn’s disease using strong CYP3A4 inhibitors, the recommended induction dose is 30 mg once daily and the recommended maintenance dose is 15 mg once daily (see Dosage and Administration). Avoid food and drink containing grapefruit during treatment with upadacitinib. See SmPC for full details. FERTILITY, PREGNANCY AND LACTATION: Upadacitinib is contraindicated during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment and for 4 weeks following the final dose of upadacitinib. Female paediatric patients and/or their parents/caregivers should be informed about the need to contact the treating physician once the patient experiences menarche while taking upadacitinib. Upadacitinib should not be used during breast-feeding. The effect of upadacitinib on human fertility has not been evaluated. ADVERSE REACTIONS: Refer to SmPC for full details on adverse reactions. Very common adverse reactions (≥1/10): upper respiratory tract infections, acne. Common adverse reactions (≥1/100 to <1/10): bronchitis, herpes zoster, herpes simplex, folliculitis, influenza, urinary tract infection, pneumonia, non-melanoma skin cancer, anaemia, neutropaenia, lymphopaenia, urticaria, hypercholesterolaemia, hyperlipidaemia, cough, abdominal pain, nausea, rash, fatigue, pyrexia, increased blood CPK/ALT/AST, increased weight and headache.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie.
LEGAL CLASSIFICATION: POM (S1A). MARKETING AUTHORISATION NUMBER/ PRESENTATION: EU/1/19/1404/001: RINVOQ 15 mg prolonged-release tablets in cartons of 28 tablets; EU/1/19/1404/006: RINVOQ 30 mg prolonged-release tablets in cartons of 28 tablets; EU/1/19/1404/010: RINVOQ 45 mg prolonged-release tablets in cartons of 28 tablets. MARKETING AUTHORISATION HOLDER: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. DATE OF REVISION: April 2023. PI-1404-010.
* IBD, Inflammatory Bowel Disease
1. RINVOQ® Summary of Product Characteristics. Available at: www.medicines.ie.
IE-RNQG-230015 | May 2023
Brexit Update for Medicines

• All medicines on the NI market must have the words “UK only” on the packaging.
• The provisions do not apply to veterinary medicines.
• The new provisions are due to be implemented from 1 January 2025. This date depends on when the UK provides written guarantees to the European Commission on the protection of the integrity of the EU internal market.
medicines which are not required to carry the safety features.
3. Prohibition on putting EU centralised products on the NI market
On 26 February 2023, the EU and the UK reached political agreement in principle on the Windsor framework. The parties to the agreement are committed to take “the necessary steps to translate the joint solutions into legally binding instruments and to implement these swiftly and in good faith”. While the agreement covers trade generally, this update only focuses on human medicines. The Commission has published a draft regulation regarding the proposal on medicines, to reflect the agreement in principle. Its main purpose is to facilitate the supply of medicines from Great Britain (GB) to Northern Ireland (NI) and to address concerns that were raised around this linked to the implementation of the NI protocol.
Of significance in the regulation:
• Prescription medicines in NI will not be permitted to carry the safety features required under the Falsified Medicines Directive (FMD).
• New novel medicines authorised by the EU (centralised medicines) will not be permitted on the NI market, i.e., only those authorised by the UK authorities will be permitted on the NI market.
Implications
for Marketing Authorisation Holders (MAHs) supplying medicines to the Irish (IE) market
The Windsor agreement has implications for Marketing Authorisation Holders supplying both the IE and NI markets. In particular, companies with joint packs between GB/IE or NI/IE need to consider what it means for their products.
1. Prohibition of the use of the safety feature on products placed on the NI market.
The Windsor agreement prohibits safety features appearing on the outer packaging of medicines placed on the NI market. This will, in effect, stop joint packs between NI/GB and IE.
The expiry on 31 December 2024 of the existing exemption for having to decommission product going to the UK, was already going to impact joint packs. However, it was expected that joint packs with NI would still be possible in 2025. Split batches where only the IE/NI portion was uploaded to the repository should also have been feasible.
MAHs who previously used joint GB/IE or NI/ IE packs should now consider separating those packs in advance of this Windsor agreement legislation implementation.
2. Special wording required for medicines placed on the NI market.
Medicines placed on the NI market must carry the wording “UK Only”. This provision also impacts the ability of MAHs to have a joint pack with IE and NI/GB particularly for OTC
New Blood-based Cancer Screening Test
The proposal prohibits placing medicines authorised by the Commission (known as centralised medicines), on the market in Northern Ireland. These medicines must be separately authorised by the UK authorities for the NI market. They must also carry the words “UK only” and not carry the safety features. Currently many centralised products are using joint labels for the UK/NI and IE market. These marketing authorisation holders must now start planning for separate packs for the IE market. Joint packs (including where necessary multi-lingual packs) with other EU/ EEA MSs remain possible.
Expiry of the existing exemptions in December 2024
As MAHs will be aware, updates to EU legislation were implemented in 2022 allowing certain exemptions for a period of 3 years. In summary they allowed:
• Continued batch release in GB,
• Continued QC testing in GB without the need for retesting on importation into the EU,
• Importation of an authorised medicine from GB by a wholesaler rather than the holder of a manufacturer’s authorisation,
• An exemption from the requirement under the Falsified Medicines Directive to decommission joint packs going to the UK market.
Some of these exemptions were notifiable to the HPRA and onwards to the Commission.
MAHs are reminded that these exemptions only apply until the end of 2024. The HPRA will write to MAHs availing of these exemptions asking them to outline their plans in respect of these medicines.
Certior Health (“Certior”) is pleased to announce that it has partnered with Datar Cancer Genetics ("Datar") a leading cancer research company focussed on early detection of cancers. Trucheck™ is an innovative cancer screening test that requires a safe, simple and quick blood draw, allowing for early detection of cancer by detecting Circulating Tumour Cells. Trucheck™ intelli can distinguish 70 types of solid tumours which account for 81% of all cancer cases and ~84% of all cancer-related deaths in Europe.*
Dr Vineet Datta, Executive Director at Datar Cancer Genetics, said: “We are delighted to partner with Certior. This collaboration will provide advanced cancer genomics to the patient community, and we will work closely with Certior’s medical team to support better-informed clinical decision-making. Trucheck™ is a CE-certified cancer screening solution offered for single and multi-organ cancer screening. We believe the solution will encourage more men and women to participate in cancer screening for a safer future. Working with Certior will bring this screening service one step closer to the general public."
Maggie Malone, Chief Executive Officer at Certior Health, said: "The development of Trucheck™ cancer screening test is a significant milestone in the fight against cancer through a novel process of utilising CTCs to identify cancers in both early and advanced stages, with validation from more than 40,000 individuals. We are excited to work with Datar and to add the Trucheck cancer screening solution to our portfolio of health and wellness tests offered to ensure our customers get the best opportunity to take preventative measures to look after their health."
Established in 2020, Certior Health’s initial focus has been providing testing solutions to combat the Covid 19 pandemic. Having delivered over 500,000 direct-to-consumer and corporate tests the company enjoyed significant success in customer acquisition achieving a best-in-class 4.7 Trustpilot rating for service delivery. Certior Health is now an ISO 15189 accredited lab whose mission is to empower and inspire customers to have greater control over their health, in order to live a longer and more vital life.
For more information about Certior Health and cancer screening visit certior.com.
18 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
ABBREVIATED PRESCRIBING INFORMATION – IRELAND
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Apexxnar®▼ suspension for injection in pre-filled syringe
Pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed)

Presentation: Each 0.5 mL dose of Apexxnar contains 2.2 micrograms of each of the following polysaccharide serotypes: 1, 3, 4, 5, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F and 4.4 micrograms of polysaccharide serotype 6B. Each polysaccharide is conjugated to the CRM197 carrier protein and adsorbed on aluminium phosphate. 1 dose (0.5 mL) contains approximately 51 µg CRM197 carrier protein and 0.125 mg aluminium.
Indications: Active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older. Apexxnar should be used in accordance with o cial recommendations taking into consideration the risk of invasive disease and pneumonia in di erent age groups, underlying comorbidities as well as the variability of serotype epidemiology in di erent geographical areas.
Dosage and Administration: For intramuscular injection. Individuals ≥ 18 years of age and older: One single dose. The need for revaccination with a subsequent dose of Apexxnar has not been established. Based on the clinical experience with Prevenar 13, if the use of 23-valent pneumococcal polysaccharide vaccine is considered appropriate, Apexxnar should be given first. Special populations: There are no data with Apexxnar in special populations. Limited experience from clinical studies with Prevenar 13 are available in adults at higher risk of pneumococcal infection either immunocompromised individuals or following bone marrow transplantation. Based on these data the following posology was recommended for Prevenar 13: Individuals at higher risk of pneumococcal infection (e.g., individuals with sickle cell disease or HIV infection), including those previously vaccinated with 1 or more doses of PPSV23, were recommended to receive at least 1 dose of Prevenar 13. In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunisation series with Prevenar 13 consisted of 4 doses of 0.5 mL each. The primary series consisted of 3 doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose was recommended 6 months after the third dose.
Contra-indications: Hypersensitivity to the active substances, to any of the excipients, or to diphtheria toxoid.

Warnings and Precautions: Do not administer intravascularly. Appropriate medical treatment and supervision must be available in case of anaphylaxis. Vaccination should be postponed in individuals su ering from acute severe febrile illness. However, the presence of a minor infection, such as a cold, should not result in the deferral of vaccination. It should not be given to individuals with thrombocytopenia or a bleeding disorder that would contraindicate intramuscular injection. The risk of bleeding in patients with coagulation disorders needs to be carefully evaluated before intramuscular administration of any vaccine, and subcutaneous administration should be considered if the potential benefit clearly outweighs the risks. Apexxnar will only protect against Streptococcus pneumoniae serotypes included in the vaccine and will not protect against other microorganisms that cause invasive disease and pneumonia. As with any vaccine, Apexxnar may not protect all individuals receiving the vaccine from pneumococcal disease. Safety and immunogenicity data on Apexxnar are not available for individuals in immunocompromised groups. Vaccination should be considered on an individual basis. Individuals with impaired immune responsiveness, whether due to the use of immuno-suppressive therapy, a genetic
defect, human immunodeficiency virus (HIV) infection, or other causes, may have reduced antibody response to active immunisation. The clinical relevance of this is unknown. In adults across all studied age groups, formal non-inferiority criteria were met although numerically lower geometric mean titres were observed with Apexxnar for most of the serotypes compared to Prevenar 13, however the clinical relevance of this observation for immunocompromised individuals is unknown.
Drug Interactions: Apexxnar may be administered concomitantly with seasonal influenza vaccine (QIV; surface antigen, inactivated, adjuvanted). In subjects with underlying conditions associated with a high risk of developing life-threatening pneumococcal disease, consideration may be given to separating administrations of QIV and Apexxnar (e.g., by approximately 4 weeks). Apexxnar can be administered concomitantly with COVID-19 mRNA vaccine (nucleoside modified).
Fertility, Pregnancy & Lactation: There are no data from the use of Apexxnar in pregnant women. Animal studies do not indicate direct or indirect harmful e ects with respect to reproductive toxicity. Administration of Apexxnar in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and foetus. It is unknown whether Apexxnar is excreted in human milk. Side E ects: As Apexxnar contains the same 13 serotype-specific capsular polysaccharide conjugates and the same vaccine excipients as Prevenar 13, the adverse reactions already identified for Prevenar 13 have been adopted for Apexxnar. In clinical trials, the safety profile of Apexxnar was similar to that of Prevenar 13. No new adverse reactions were identified as compared to Prevenar 13. When Apexxnar was administered to adults aged ≥ 65 years together with the third (booster) dose of a COVID-19 mRNA vaccine (nucleoside modified), the tolerability profile generally resembled that of the COVID-19 mRNA vaccine (nucleoside modified) administered alone. Very common (≥ 1/10): Headache, joint pain, muscle pain, vaccination-site pain/tenderness, fatigue. Common (≥ 1/100 to < 1/10): Vaccination-site induration/ swelling, vaccination-site erythema, pyrexia. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity reaction including face oedema, dyspnoea, bronchospasm, diarrhoea, nausea, vomiting, rash, angioedema, vaccination-site pruritus, lymphadenopathy, vaccination-site urticaria, chills. The following adverse reactions have been spontaneously reported during the postmarketing use of Prevenar 13, which may also occur with Apexxnar. These events were reported voluntarily from a population of uncertain size, so it is not possible to reliably estimate their frequency or to establish, for all events, a causal relationship to vaccine exposure: Anaphylactic/anaphylactoid reaction, including shock, erythema multiforme, vaccination-site dermatitis. Additional information in special populations in studies with Prevenar 13: Adults with HIV infection have similar frequencies of adverse reactions, except that pyrexia and vomiting were very common and nausea common. Adults with an HSCT have similar frequencies of adverse reactions, except that pyrexia, vomiting, and diarrhoea were very common.

For full prescribing information see the Summary of Product Characteristics.
Legal Category: S1A. Package Quantities: Pack of 1 single-dose pre-filled syringe (with separate needle).

Marketing Authorisation Numbers: EU/1/21/1612/002. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500.
Last revised: 12/2022.
Ref: PE 3_0.
vaccination and chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2017;12:3457-3468.
For more information about APEXXNAR® scan the QR code

PP-PNR-IRL-0027 | Date of Preparation: May 2023
APEXXNAR® is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.1
▼
1.
Summary
2. Froes F,
N,
F.
older.
References
APEXXNAR.
of Product Characteristics.
Roche
Blasi
Pneumococcal
Designed to deliver long-lasting immunity.1,2
Helps protect against the 20 Streptococcus pneumoniae serotypes included in the vaccine.1
pregnancy as LMWH is a large molecule and does not cross the placenta. Tinzaparin (Innohep ) and enoxaparin (Clexane®) are the LMWHs most frequently used during pregnancy in Ireland.
Thrombosis
Direct oral anticoagulant agents (DOACs) are not routinely recommended for use in pregnancy or breastfeeding due to limited human safety data. Warfarin should be avoided during pregnancy as it can cause birth defects e.g. fetal warfarin syndrome, however it may sometimes be used in patients with mechanical heart valves. At daily doses lower than 12 mg, warfarin can be prescribed during breastfeeding due to very low levels in breastmilk.
Low Molecular Weight Heparin for treatment and prophylaxis of Venous Thromboembolism (VTE) during Pregnancy
Written
Treatment dose:
Table 1: Suggested treatment doses for antenatal and postnatal LMWH Enoxaparin
Introduction:
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are two components of a single condition called venous thromboembolism (VTE). In pregnancy although VTE is rare (1-2 per 1,000 pregnancies), it remains a leading cause of maternal death, as well as a source of maternal morbidity.
Risk factors for VTE can change during pregnancy, delivery and the postnatal period. A VTE can occur any time in all three trimesters of pregnancy, but the highest risk is in the 6 weeks immediately after birth.

Personal history of VTE is the biggest risk factor for pregnancy associated VTE. Other antenatal risk factors include: medical co-morbidities, increased BMI, advanced age,
HSE & IOG (2013) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management
HSE & IOG (2016) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management
The dose of LMWH should be rounded up or down to a measurable dose for administration.
Enoxaparin dose calculation:
Antenatal weight 62 kg
Dose = 1 mg/kg every 12 hours = 62 mg (rounded to 60 mg)
Use 60 mg syringe of enoxaparin sodium per 0.6 mL.
parity, smoking status, assisted conception and varicose veins. Postnatal risk factors also consist of caesarian delivery (with greater risk associated with emergency caesarean delivery), prolonged hospital admission, postpartum haemorrhage and systemic infection.
Postnatal weight 71 kg
admission to hospital, upon any change in clinical status and essentially in the postpartum period. Women who are at a high risk for VTE and/or treated for VTE in pregnancy should be managed by a combined haematology and obstetric multidisciplinary service.
frequently used during pregnancy in Ireland.
Dose = 1.5 mg/kg every 12 hours = 106.5 mg (rounded to 105 mg)
Use 120 mg enoxaparin sodium in 0.8 mL syringe, discard 0.1 mL and administer 0.7 mL (105 mg)
Failure to recognise and/or treat personal or pregnancy specific risk factors has been identified as a significant contributing factor to maternal mortality and morbidity from VTE in pregnancy. For this reason and due to the increasing prevalence of risk factors in the pregnant and postpartum populations, all pregnant patients should undergo a documented assessment of risk factors for VTE in early pregnancy or prepregnancy. Risk assessment should be repeated on each
The 2013 Irish National Clinical Guideline on VTE thromboprophylaxis in pregnancy issued a rapid risk assessment tool for VTE in pregnancy and this tool or a variation thereof is used in most Irish maternity hospitals and units.
Low Molecular Weight Heparin (LMWH) is the treatment and prophylaxis of choice for VTE during pregnancy as LMWH is a large molecule and does not cross the placenta. Tinzaparin (Innohep®) and enoxaparin (Clexane®) are the LMWHs most
Direct oral anticoagulant agents (DOACs) are not routinely recommended for use in pregnancy or breastfeeding due to limited human safety data. Warfarin should be avoided during pregnancy as it can cause birth defects e.g. fetal warfarin syndrome, however it may sometimes be used in patients with mechanical heart valves. At daily doses lower than 12 mg, warfarin can be prescribed during breastfeeding due to very low levels in breastmilk.
Enoxaparin pre-filled syringes are not available for all required doses. Table 2 below shows the available syringe sizes and the volume to be administered to obtain the required dose
Treatment dose
(see table 1 above):
The dose of LMWH should be rounded up or down to a measurable dose for administration.

20 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Áine Toher, Senior Pharmacist, The National Maternity Hospital,
by
Table 2: Enoxaparin (Clexane®) dose administration
Table 1: Suggested treatment doses for antenatal and postnatal LMWH
Tinzaparin Antenatal 1 mg/kg every 12 hours 175 units/kg every 24 hours Postnatal 1.5 mg/kg every 24 hours 175 units/kg every 24 hours
Enoxaparin Dose Enoxaparin (Clexane®) syringe size Volume to administer 40 mg 40 mg/0.4 mL 0.4 mL 50 mg 60 mg/0.6 mL 0.5 mL 60 mg 60 mg/0.6 mL 0.6 mL 70 mg 80 mg/0.8 mL 0.7 mL 80 mg 80 mg/0.8 mL 0.8 mL 90 mg 100 mg/1 mL 0.9 mL 100 mg 100 mg/1 mL 1 mL 105 mg 120 mg/0.8 mL 0.7 mL 120 mg 120 mg/0.8 mL 0.8 mL 135 mg 150 mg/1 mL 0.9 mL 150 mg 150 mg/1 mL 1 mL *Colour-coded to match colour of enoxaparin (Clexane®) pre-filled syringe and packaging Tinzaparin dose calculation: Weight (antenatal or postnatal) 78 kg *Colour-coded to match colour of enoxaparin (Clexane®) pre-filled syringe and packaging Sarah
Table 2: Enoxaparin (Clexane®) dose administration
Mohamed, Pharmacy Intern, The National Maternity Hospital
IMFINZI is reimbursed for the treatment of locally advanced, unresectable non-small cell lung cancer (NSCLC) in adults whose tumours express PD-L1 on ≥1% of tumour cells and whose disease has not progressed following platinum-based chemoradiation therapy.1

50 mg/mL concentrate for solution for infusion
In a post-hoc subgroup analysis of the PACIFIC study, WITH IMFINZI, the median overall survival (OS) at 5 years, was 63.1 months versus 29.6 months for the placebo arm (HR: 0. 61; 95% CI, 0.44-0.85)1*
*Patients were retrospectively tested for PD-L1 expression on tumour cell using the Ventana PD-L1 (SP263) IHC assay, where available. 63% of patients provided a tissue sample of sufficient quality and quantity to determine PD-L1 expression and 37% were unknown. This exploratory post-hoc 5-year OS analysis was conducted at ~5 years after last patient was randomised and was not powered to show statistical significance.
Primary analysis in the ITT population
Median OS†: Not reached vs 28.7 months with placebo (HR=0.68; 99.73% CI, 0.47-0.997); P=0.00252
Median PFS‡: 16.8 vs 5.6 months with placebo (HR=0.52; 95% CI, 0.42-0.65; P<0.0001)1
The primary 2-year OS analysis was conducted after 299 deaths for 42% maturity (61% of targeted events) with a median follow-up of 25.2 months. Reduction in the risk of death vs placebo was 32% (95% CI, 0.53-0.87).
Median OS was NR with IMFINZI (95% CI, 34.7-NR) vs 28.7 months with placebo (95% CI, 22.9-NR).1,2 ‡Measured based on RECIST v1.1 criteria by BICR. The primary PFS analysis was conducted after 371 events (81% of targeted 458 events) with a median follow-up of 14.5 months. Reduction in the risk of progression or death vs placebo was 48% (HR=0.52; 95% CI, 0.42-0.65). Median PFS was 16.8 months with IMFINZI (95% CI, 13.0-18.1) vs 5.6 months with placebo (95% CI, 4.6-7.8). 3
SAFETY INFORMATION
The most frequent any grade (>10%) adverse reactions were cough/productive cough (21.5%), diarrhoea (16.3%), rash (16.0%), pyrexia (13.8%), upper respiratory tract infections (13.5%), abdominal pain (12.7%), pruritus (10.8%), and hypothyroidism (10.1%)1
See SmPC for recommended treatment modifications for IMFINZI and management considerations.
ABBREVIATED PRESCRIBING INFORMATION
IMFINZI® (durvalumab) 50mg/ml Concentrate for Solution for Infusion
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Non-Small Cell Lung Cancer: • IMFINZI as monotherapy is indicated for the treatment of locally advanced, unresectable non-small cell lung cancer (NSCLC) in adults whose tumours express PD-L1 on ≥ 1% of tumour cells and whose disease has not progressed following platinum-based chemoradiation therapy. • IMFINZI in combination with tremelimumab and platinum-based chemotherapy is indicated for the first-line treatment of adults with metastatic NSCLC with no sensitising EGFR mutations or ALK positive mutations. Small Cell Lung Cancer: IMFINZI in combination with etoposide and either carboplatin or cisplatin is indicated for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC). Biliary Tract Cancer: IMFINZI in combination with gemcitabine and cisplatin is indicated for the first-line treatment of adults with unresectable or metastatic biliary tract cancer (BTC). Hepatocellular Carcinoma: IMFINZI in combination with tremelimumab is indicated for the first line treatment of adults with advanced or unresectable hepatocellular carcinoma (HCC). Presentation: Each ml of concentrate for solution for infusion contains 50mg durvalumab. Dosage and administration: Treatment must be initiated and supervised by a physician experienced in the treatment of cancer. IMFINZI is administered as an intravenous infusion over 1 hour. Refer to ‘Method of administration’ in section 4.2 of SmPC. Patients with locally advanced NSCLC should be evaluated for treatment based on the tumour expression of PD-L1 confirmed by a validated test Monotherapy: For locally advanced NSCLC, the recommended IMFINZI dose is 10 mg/kg every 2 weeks or 1 500 mg every 4 weeks, until disease progression, unacceptable toxicity, or a maximum of 12 months. Combination therapy: For metastatic NSCLC, the recommended IMFINZI dose during platinum chemotherapy is 1 500 mg in combination with tremelimumab 75 mg and platinum-based chemotherapy every 3 weeks (21 days) for 4 cycles (12 weeks). For metastatic NSCLC, the recommended IMFINZI dose for post-platinum chemotherapy is 1 500 mg every 4 weeks as monotherapy and histology-based pemetrexed maintenance therapy every 4 weeks. A fifth dose of tremelimumab 75 mg should be given at week 16 alongside IMFINZI until disease progression or unacceptable toxicity. For ES-SCLC, the recommended IMFINZI dose in combination with chemotherapy is 1 500mg, every 3 weeks (21 days) for 4 cycles, followed by 1 500 mg every 4 weeks as monotherapy, until disease progression or unacceptable toxicity. For BTC, the recommended IMFINZI dose in combination with chemotherapy is 1 500 mg, every 3 weeks (21 days) up to 8 cycles, followed by 1 500 mg every 4 weeks as monotherapy until disease progression or unacceptable toxicity. For HCC, the recommended IMFINZI dose is 1 500 mg administered in combination with 300 mg tremelimumab as a single dose at Cycle 1/Day 1, followed by IMFINZI as monotherapy every 4 weeks until disease progression or unacceptable toxicity. Please consult Section 4.2 in the SmPC for more information. Dose escalation or reduction is not recommended. Treatment withholding or discontinuation may be required based on individual safety and tolerability. Suspected immune-mediated adverse reactions: Adequate evaluation should be performed to confirm aetiology or exclude alternate aetiologies. Based on the severity of the adverse reaction, IMFINZI and/or tremelimumab should be withheld and corticosteroids administered. Guidelines for the management of immune mediated adverse reactions are described in Table 2, Section 4.2 in the SmPC. Increasing dose of corticosteroids and/or using additional systemic immunosuppressants should be considered if there is worsening or no improvement. Upon improvement to ≤ Grade 1, corticosteroid taper should be initiated and continued over at least 1 month. After withhold, IMFINZI and/or tremelimumab can be resumed within 12 weeks if the adverse reactions improved to ≤ Grade 1 and the corticosteroid dose has been reduced to ≤ 10 mg prednisone or equivalent per day. IMFINZI and tremelimumab should be permanently discontinued for recurrent Grade 3 (severe) immune-mediated adverse reactions and for any Grade 4 (life-threatening) immune-mediated adverse reactions, except for endocrinopathies that are controlled with replacement hormones. Special populations: Elderly: No dose adjustment is required for elderly patients (≥ 65 years of age). Renal/Hepatic impairment: No dose adjustment is recommended in patients with mild or moderate renal or hepatic impairment. Data from patients with severe renal or hepatic impairment are too limited to draw conclusions on this population.
Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: Traceability: In order to improve the traceability of biological medicinal products, the tradename and the batch number of the administered product should be clearly recorded. Immune-mediated pneumonitis: Monitor for signs and symptoms of pneumonitis or radiation pneumonitis. Suspected pneumonitis should be confirmed with radiographic imaging and other infectious and disease-related aetiologies excluded. Immune-mediated hepatitis: Monitor alanine aminotransferase, aspartate aminotransferase, total bilirubin, and alkaline phosphatase levels prior to initiation of treatment and prior to each subsequent infusion. Additional monitoring is to be considered based on clinical evaluation. Immune-mediated colitis: Monitor for signs and symptoms of colitis/diarrhoea and intestinal perforation. Immune-mediated hypothyroidism, hyperthyroidism and thyroiditis: Monitor for abnormal thyroid function tests prior to and periodically during treatment and as indicated based on clinical evaluation. Immune-mediated adrenal insufficiency: Monitor for clinical signs and symptoms of adrenal insufficiency. Immune-mediated type 1 diabetes mellitus: Monitor for clinical signs and symptoms of type 1 diabetes mellitus including diabetic ketoacidosis. Immune-mediated hypophysitis/hypopituitarism: Monitor for clinical signs and symptoms of hypophysitis or hypopituitarism. Immune-mediated nephritis: Monitor for abnormal renal function tests prior to and periodically during treatment with IMFINZI. Immune-mediated rash: Events of Stevens-Johnson Syndrome or toxic
epidermal necrolysis have been reported in patients treated with PD-1 inhibitors. Monitor for signs and symptoms of rash or dermatitis (including pemphigoid). Immune-mediated myocarditis: Monitor for signs and symptoms of immune-mediated myocarditis. Immune-mediated pancreatitis: Monitor for signs and symptoms of immune-mediated pancreatitis. Other immune-mediated adverse reactions: Myasthenia gravis, myelitis transverse, myositis, polymyositis, meningitis, encephalitis, Guillain-Barre syndrome, immune thrombocytopenia, and cystitis noninfective have been observed. Monitor for signs and symptoms. All immune-mediated adverse events should be managed as recommended in Section 4.2 of the SmPC. Infusion-related reactions: Monitor for signs and symptoms of infusion related reactions. Consult section 4.2 of SmPC for management recommendations. Cholangitis and biliary tract infections: Closely monitor patients with BTC (especially those with biliary stents) for development of cholangitis or biliary tract infections before initiation of treatment and, regularly, thereafter. Metastatic NSCLC: Limited data are available in elderly patients (≥ 75 years). Careful consideration of the potential benefit/risk of this regimen on an individual basis is recommended. Patients excluded from clinical studies: Patients with: a baseline ECOG performance score ≥ 2; active or prior documented autoimmune disease within 2 years of initiation of the study; a history of immunodeficiency; a history of severe immune-mediated adverse reactions; medical conditions that required systemic immunosuppression, except physiological dose of systemic corticosteroids (≤ 10 mg/day prednisone or equivalent); uncontrolled intercurrent illnesses; active tuberculosis or hepatitis B or C or HIV infection or patients receiving live attenuated vaccine within 30 days before or after the start of IMFINZI. Durvalumab should be used with caution in these populations after careful consideration of the potential benefit/risk on an individual basis. The safety of concurrent prophylactic cranial irradiation (PCI) with IMFINZI in patients with ES-SCLC is unknown. Drug Interactions: The use of systemic corticosteroids or immunosuppressants before starting durvalumab, except physiological dose of systemic corticosteroids (≤ 10 mg/day prednisone or equivalent), is not recommended because of their potential interference with the pharmacodynamic activity and efficacy of durvalumab. However, systemic corticosteroids or other immunosuppressants can be used after starting durvalumab to treat immune-related adverse reactions. PK drug-drug interaction between durvalumab and chemotherapy was assessed and showed concomitant treatment did not impact the PK of etoposide, carboplatin or cisplatin. Additionally, based on population PK analysis, concomitant chemotherapy treatment did not meaningfully impact the PK of durvalumab. PK drug-drug interactions between durvalumab in combination with tremelimumab and platinum-based chemotherapy were assessed and showed no clinically meaningful PK interactions between tremelimumab, durvalumab or any of the chemotherapy drugs involved in the concomitant treatment. Pregnancy and Lactation: Durvalumab may cause foetal harm when administered to a pregnant woman and is not recommended during pregnancy and in women of childbearing potential not using effective contraception during treatment and for at least 3 months after the last dose. A decision must be made whether to discontinue breast-feeding or to discontinue or abstain from durvalumab therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. Undesirable events: Consult SmPC for full list of side-effects Adverse reactions included below are associated with IMFINZI as monotherapy, and IMFINZI in combination with chemotherapy, and IMFINZI in combination with tremelimumab and platinum-based chemotherapy, and IMFINZI in combination with tremelimumab. For more details refer to tables 3 and 4 in section 4.8 of SmPC. Very common (≥ 1/10; any grades): Upper respiratory tract infections, anaemia, leukopenia, neutropenia, thrombocytopenia, hypothyroidism, decreased appetite, cough/productive cough, diarrhoea, abdominal pain, constipation, nausea, vomiting, rash, alopecia, pruritus, arthralgia, pyrexia, fatigue, oedema peripheral, aspartate aminotransferase increased or alanine aminotransferase increased, pneumonia. Common (≥ 1/100 to < 1/10; any grades): Influenza, oral candidiasis, dental and oral soft tissue infections, febrile neutropenia, pancytopenia, adrenal insufficiency, hyperthyroidism, thyroiditis, hypopituitarism or hypophysitis, neuropathy peripheral, pneumonitis, dysphonia, stomatitis, amylase increased, lipase increased, colitis, pancreatitis, hepatitis, night sweats, dermatitis, myalgia, , blood creatinine increased, dysuria, infusion related reaction. Uncommon (≥ 1/1 000 to < 1/100; any grades): Interstitial lung disease, psoriasis, myositis, nephritis, immune thrombocytopenia, diabetes insipidus, type 1 diabetes mellitus, encephalitis, myasthenia gravis, meningitis, pemphigoid, polymyositis, cystitis noninfective. Rare (≥ 1/10 000 to < 1/1 000; any grades): Myocarditis. Not known: Noninfective encephalitis, Guillain-Barré syndrome, myelitis transverse, intestinal perforation, large intestine perforation. Legal Category: Product subject to prescription which may not be renewed (A) Marketing Authorisation
Number: EU/1/18/1322/001; EU/1/18/1322/002 Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden. Further information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15 Tel: +353 1 6097100. IMFINZI is a registered trade mark of the AstraZeneca group of companies.
Date of API Preparation: 05/2023 Veeva ID: IE-4946

Adverse events should be reported directly to: HPRA Pharmacovigilance. Website: www.hpra.ie. Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899 References: 1. Imfinzi-Ireland Summary of Product Characteristics. May 2023. 2. Antonia SJ, et al. N Engl J Med 2018;379:2342−2350. 3. Antonia SJ, et al. N Engl J Med 2017;377:1919-1929. IMFINZI is a trademark of the AstraZeneca group of companies. ©2023 AstraZeneca. All rights reserved. Veeva ID: IE-4726 | DOP: May 2023
Thrombosis
Enoxaparin dose calculation:
Antenatal weight 62 kg
Dose = 1 mg/kg every 12 hours = 62 mg (rounded to 60 mg)
Use 60 mg syringe of enoxaparin sodium per 0.6 mL.
Postnatal weight 71 kg
Dose = 1.5 mg/kg every 24 hours = 106.5 mg (rounded to 105 mg)
Use 120 mg enoxaparin sodium in 0.8 mL syringe, discard 0.1 mL and administer 0.7 mL (105 mg)
Enoxaparin pre-filled syringes are not available for all required doses. Table 2 (on page 20) shows the available syringe sizes and the volume to be administered to obtain the required dose.
Tinzaparin dose calculation:
Weight (antenatal or postnatal)
78 kg
Dose = 78 x 175 IU = 13,650 IU (rounded to 14,000 IU)
Use tinzaparin 14,000 in 0.7 mL prefilled syringe.
Tinzaparin pre-filled syringes are not available for all required doses. Table 3 below shows the available syringe sizes and the volume to be administered to obtain the required dose.
Table 4: Suggested thromboprophylactic doses for antenatal and postnatal LMWH
HSE & IOG (2016) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium
HSE & IOG (2013) Clinical Practice Guideline Venous Thromboprophylaxis in Pregnancy and adapted from RCOG (2015) Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium
Many maternity hospitals follow the doses suggested above, but in some hospitals the weight bands are further divided so prescriptions for tinzaparin 6000 IU and 8000 IU once daily are common.
It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring.
Commencement and prophylaxis duration with LMWH can vary depending on individual VTE risk assessment, if LMWH is required during pregnancy it should be continued for up to 6 weeks postnatally.
Commencement and prophylaxis duration with LMWH can vary depending on individual VTE risk assessment, if LMWH is required during pregnancy it should be continued for up to 6 weeks postnatally.
Prophylaxis: (see Table 4 above)
Many maternity hospitals follow the doses suggested above, but in some hospitals the weight bands are further divided so prescriptions for tinzaparin 6000 IU and 8000 IU once daily are common.
Enoxaparin (Clexane®) syringes are available in the strengths stated in the table above. However, tinzaparin (Innohep®) syringes are not available in all the doses stated above so the
patient will have to be shown how to expel some of the injection from a syringe of a strength closest to their required dose as per Table 3. It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring
Enoxaparin (Clexane®) syringes are available in the strengths stated in the table above. However, tinzaparin (Innohep®) syringes are not available in all the doses stated above so the patient will have to be shown how to expel some of the injection from a syringe of a strength closest to their required dose as per Table 3. It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring
Contraindications to LMWH use in pregnancy and the puerperium:
Contraindications to LMWH use in pregnancy and the puerperium:
Contraindications to LMWH use include known bleeding disorders (e.g. haemophilia, von Willebrand’s disease), thrombocytopenia, acute stroke, active antenatal or postpartum bleeding and those with an increased risk of major haemorrhage (e.g. placenta praevia). As well as uncontrolled hypertension, severe liver or renal disease and recent surgery to the eye or nervous system. Women with a previous or current allergic reaction to LMWH should always be offered an alternative form of prophylaxis. It is recommended that any woman with a contraindication to LMWH should be provided with appropriately sized anti-embolism stockings (AES) with a calf pressure of 1415 mmHg.
Contraindications to LMWH use include known bleeding disorders (e.g. haemophilia, von Willebrand’s disease), thrombocytopenia, acute stroke, active antenatal or postpartum bleeding and those with an increased risk of major haemorrhage (e.g. placenta praevia). As well as uncontrolled hypertension, severe liver or renal disease and recent surgery to the eye or nervous system. Women with a previous or current allergic reaction to LMWH should always be offered an alternative form of prophylaxis. It is recommended that any woman with a contraindication to LMWH should be provided with appropriately sized anti-embolism stockings (AES) with a calf pressure of 14-15 mmHg.
LMWH should be avoided if a lumbar puncture, epidural or spinal anaesthesia is expected within the following 12 hours, or if such regional techniques have taken place within the previous 4 hours. Pregnant patients receiving antenatal LMWH should be educated on the symptoms of labour and instructed not to inject any further LMWH once labour symptoms or vaginal bleeding are observed.
LMWH should be avoided if a lumbar puncture, epidural or spinal anaesthesia is expected within the following 12 to 24 hours, or if such regional techniques have taken place within the previous 4 hours. Pregnant patients receiving antenatal LMWH should be educated on the symptoms of labour and instructed not to inject any further LMWH once labour symptoms or vaginal bleeding are observed.
*Colour-coded to match colour of tinzaparin (Innohep®) pre-filled syringe and packaging
*Colour-coded to match colour of tinzaparin (Innohep®) pre-filled syringe and packaging
It is important that the patient receives the same strength prefilled syringe on each occasion to avoid the chance of a medication or administration error from occurring.
22 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Table 3: Tinzaparin
Prescribed Dose of Tinzaparin Anti-Factor Xa units Tinzaparin (Innohep®) Pre-filled Syringe Volume to inject via subcutaneous injection 6000 8000 IU/0.4 mL 0.3 mL 7000 8000 IU/0.4 mL 0.35 mL 8000 8000 IU/0.4 mL 0.4 mL 9000 10000 IU/0.5 mL 0.45 mL 10000 10000 IU/0.5 mL 0.5 mL 11000 12000 IU/0.6 mL 0.55 mL 12000 12000 IU/0.6 mL 0.6 mL 13000 14000 IU/0.7 mL 0.65 mL 14000 14000 IU/0.7 mL 0.7 mL 15000 16000 IU/0.8 mL 0.75 mL 16000 16000 IU/0.8 mL 0.8 mL 17000 18000 IU/0.9 mL 0.85 mL 18000 18000 IU/0.9 mL 0.9 mL
(Innohep®) dose administration
Table 3: Tinzaparin (Innohep®) dose administration
Weight (kg) Tinzaparin (75 IU/kg/day) Enoxaparin <50 3500 IU once daily 20 mg once daily 50 - 90 4500 IU once daily 40 mg once daily 91 - 130 7000 IU once daily 60 mg once daily 131 - 170 9000 IU once daily 80 mg once daily >170 75 IU/kg once daily 0.6 mg/kg once daily
Table 4: Suggested thromboprophylactic doses for antenatal and postnatal LMWH
Statins and Cholesterol
Statins are one of the most commonly prescribed drugs in Ireland, about one in three adults over-50 take them. Globally, it is estimated that sales of statins were worth $1 trillion by 2020. Rise in statins use
Although these cholesterollowering drugs were originally licensed to prevent the recurrence of cardiovascular disease (CVD) (secondary prevention), they are now also prescribed for those with no history of CVD (primary prevention). In Ireland, over one half of men and almost three-quarters of women who take statins, do so for primary prevention.
Over time, clinical guidelines have expanded eligibility for statins in line with changes to the recommended levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C). By 2016 the level of LDL-C considered acceptable was just over half of that recommended in 1998 according to European guidelines. Such guidelines are underpinned by the theory that the lower the LDL-C, the better. To investigate this, and to estimate the absolute benefit of statins, myself and my colleagues undertook a systematic review of statin trials, which was published in JAMA Internal Medicine in March 2022.
Systematic review findings
We decided to focus on specific ‘hard’ outcomes; all-cause death, myocardial infarction (MI) and stroke rather than ‘major cardiovascular events’ or ‘major
vascular events’, which are ‘composite’ outcomes that are commonly reported in statin trials and reviews. We decided this because composite outcomes are fraught with problems; they can be inconsistently defined and poorly reported. In addition, composites can be heavily weighted by less serious outcomes such as angina or revascularisations (the frequency of which can depend on opinions or preferences of doctors) rather than more objective outcomes such as MI and stroke.
Firstly, we found that while statins were associated with reductions in all-cause death, MI and stroke, these reductions were modest, especially when reported as absolute risk reductions. The absolute risk reduction for dying from any cause are 0.8%, for heart attack, 1.3%, and for stroke, 0.4%, whereas their relative risk reductions are 9%, 29% and 14% respectively. Research has shown that doctors are more likely to prescribe a drug, and patients to agree to take them, when benefits are described in relative terms, which sound more impressive. Thus, it is important that both patients and their prescribers are aware of the absolute risk reduction before deciding on this, or any medical intervention. It is also important that a person’s baseline risk of CVD is understood in this context. An obese, 65-yearold man who smokes is likely to have a higher baseline risk than a slim, non-smoking woman, even if both have elevated cholesterol levels. The potential benefits for a low-risk person are clearly smaller than for someone at high risk.
Secondly, we failed to demonstrate a linear relationship between the level by which LDL-C was reduced from taking statins and reduction in all-cause death, MI and stroke. Indeed, some of the trials with the greatest degree of LDL-C reduction (AURORA, CORONA) reported no clinical or survival benefit.
It is also important to consider the possibility of harm from any prescribed medicine. This is especially true for low-risk people whose potential for benefitting from statins is small. There is vigorous debate about the harms of statins. One influential systematic review estimated that reported that the risk treating 10,000 patients with statins for 5 years could result in 5 cases of myopathy, 50 to 100 new cases of diabetes, and 5 to 10 cases of haemorrhagic stroke. However, observational studies have estimated much larger rates of muscle pain, (although these types of studies are considered lower certainty evidence compared with randomised controlled trials). Some commentators have dismissed muscle symptoms from statins as a ‘nocebo’ effect. However, the research supporting the nocebo theory is not strong, and the rate of genuine sideeffects remains unknown.
Conclusion
It seems surprising that for such a commonly used medicine there are large gaps in our
Menopausal Women told ‘Grin and Bear it’
A doctor at one of Ireland’s leading women’s health clinics claims menopausal women are being told to ‘grin and bear it’ by some GPs.
Dr Talisa Chennells treats women from all over Ireland for symptoms linked to the so-called change of life at the Menopause Hub in Dublin. Her clients, she said, seek help for a vast range of symptoms including fatigue, lack of sleep, anxiety, depression and brain fog. However, many of them had previously been told by a GP not to worry about the menopause as it would “pass in a few years”. Some had even been offered anti-depressants. “That is such

an old fashioned attitude,” said Dr Chennells, who worked as a GP after graduating in medicine.
“Women struggling with menopausal symptoms do not need to suffer in silence anymore. There are a number of treatment options available to them, including hormone replacement therapy (HRT), non-hormonal medicines, herbal treatments, and psychological counselling.
“If you are a woman in your 40s and you’re concerned that your doctor is not really listening to you, then please get in touch,” she said.
Dr Chennells, who is a member of the British Menopause Society,

said some of her former colleagues in general practice remain suspicious of HRT.
“A lot of the women I see say their family doctor was reluctant to give them HRT,” said Dr Chennells, who is originally from Johannesburg, South Africa.
“They’re told their symptoms won’t last, that HRT can cause problems, and that they should just ‘grin and bear it’ until the menopause passes. A number of clients said they had even been offered anti-depressants.
“There are so many misconceptions about HRT and that’s why many doctors don’t prescribe it. But HRT
understanding. This is, as we discovered when conducting our systematic review, due to several problems. Firstly, statin trials are highly heterogeneous; they differ from each other clinically and statistically. Thus, reviews are comparing apples with oranges. Secondly, there have been ongoing problems accessing raw data from clinical trials that would enable independent scrutiny. As noted, the use of composite outcomes and reporting benefits as relative risk reductions are problematic. So too, are clinicians’ and patients’ perceptions of those benefits.
To conclude, we need to be clear with patients about the limitations of our knowledge of these drugs so as to enable informed, shared decision-making. Ideally, we need new statin trials or access to all data from existing ones. However, as statins are now off-patent, the likelihood of new trials is low, and debate rumbles on about access to data from existing trials.
can be a game changer, and even though there is a percentage of women for whom it is not suitable, breast cancer patients, for example, recent studies show the benefits outweigh the risks for most others.”
23 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
News
Dr. Paula Byrne, Senior post-doctoral researcher, University of Galway
Dr Talisa Chennells
azelastine hydrochloride / uticasone propionate
Treating allergic rhinitis patients all year round
Fast and effective treatment of allergic rhinitis symptoms1
2019 ARIA Guideline recommends: a combination therapy for moderate/severe allergic rhinitis patients uncontrolled on INAH or INS monotherapy
in real life6
Well tolerated2,7
INS - Intranasal steroid INAH - Intranasal antihistamine AZ - Azelastine FP - Fluticasone Propionate ARIA - Allergic Rhinitis and its Impact on Asthma
ABBREVIATED PRESCRIBING INFORMATION:
Dymista (azelastine hydrochloride / fluticasone propionate) 137 micrograms / 50 micrograms per actuation, Nasal Spray, Suspension.
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
√Onset of action within 30 minutes in clinical practice1
Nasal & ocular symptom relief 1,2,3
Suitable for long-term use4
Combination approach leads to clinical improvement days earlier than with FP or AZ alone5
Indications, Dosage and Posology: Relief of symptoms of moderate to severe seasonal and perennial allergic rhinitis if monotherapy with either intranasal antihistamine or glucocorticoid is not considered sufficient. Posology, For full therapeutic benefit regular usage is essential. Contact with the eyes should be avoided. Adults and adolescents (12 years and older), One actuation in each nostril twice daily (morning and evening). Children below 12 years, Dymista Nasal Spray is not recommended for use in children below 12 years of age as safety and efficacy has not been established in this age group. Elderly, No dose adjustment is required in this population. Renal and hepatic impairment There are no data in patients with renal and hepatic impairment.
Duration of treatment: Dymista Nasal Spray is suitable for long-term use. The duration of treatment should correspond to the period of allergenic exposure. Method of administration: Dymista Nasal Spray is for nasal use only.
Instruction for use Preparing the spray: The bottle should be shaken gently before use for about 5 seconds by tilting it upwards and downwards and the protective cap be removed afterwards. Prior to first use Dymista Nasal Spray must be primed by pressing down and releasing the pump 6 times. If Dymista Nasal Spray has not been used for more than 7 days it must be reprimed once by pressing down and releasing the pump. Using the spray: The bottle should be shaken gently before use for about 5 seconds by tilting it upwards and downwards and the protective cap be removed afterwards. After blowing the nose the suspension is to be sprayed once into each nostril keeping the head tilted downward (see figure in section 4.2 of the SmPC). After use the spray tip is to be wiped and the protective cap to be replaced. Presentation: Nasal Spray, suspension.
Contraindications: Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 of the SmPC.
Warnings and precautions: During post-marketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression. Therefore, concomitant use of fluticasone propionate and ritonavir should be avoided, unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side-effects (see section 4.5 of the SmPC). Systemic effects of nasal corticosteroids may occur, particularly when prescribed at high doses for prolonged periods. These effects are much less likely to occur than with oral corticosteroids and may vary in individual patients and between different corticosteroid preparations. Potential systemic effects may include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, cataract, glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children). Dymista Nasal Spray undergoes extensive first-pass metabolism, therefore the systemic exposure of intranasal fluticasone propionate in patients with severe liver disease is likely to be increased. This may result in a higher frequency of systemic adverse events. Caution is advised when treating these patients. Treatment with higher than recommended doses of nasal corticosteroids may result in clinically significant adrenal suppression. If there is evidence for higher than recommended doses being used, then additional systemic corticosteroid cover should be considered during periods of stress or elective surgery. In general, the dose of intranasal fluticasone formulations should be reduced to the lowest dose at which effective control of the symptoms of rhinitis is maintained. Higher doses than the recommended one (see section 4.2 of the SmPC) have not been tested for Dymista. As with all intranasal corticosteroids, the total systemic burden of corticosteroids should be considered whenever other forms of corticosteroid treatment are prescribed concurrently. Growth retardation has been reported in children receiving nasal corticosteroids at licensed doses. Since Dymista is also given to adolescents, it is recommended that the growth of adolescents receiving prolonged treatment with nasal corticosteroids is regularly monitored, too. If growth is slowed, therapy should be reviewed with the aim of reducing the dose of nasal corticosteroid if possible, to the lowest dose at which effective control of symptoms is maintained. Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids. Close monitoring is warranted in patients with a change in vision or with a history of increased ocular pressure, glaucoma and/or cataracts. If there is any reason to believe that adrenal function is impaired, care must be taken when transferring patients from systemic steroid treatment to Dymista Nasal Spray. In patients who have tuberculosis, any type of untreated infection, or have had a recent surgical operation or injury to the nose or mouth, the possible benefits of the treatment with Dymista Nasal Spray should be weighed against possible risk. Infections of the nasal airways should be treated with antibacterial or antimycotical therapy, but do not constitute a specific contraindication to treatment with Dymista Nasal Spray. Dymista contains benzalkonium chloride. Long term use may cause oedema of the nasal mucosa. Interactions with other medicinal products and other forms of interactions: Fluticasone propionate Under normal circumstances, low plasma concentrations of fluticasone propionate are achieved after intranasal dosing, due to extensive first pass metabolism and high systemic clearance mediated by cytochrome P450 3A4 in the gut and liver. Hence, clinically significant drug interactions mediated by fluticasone propionate are unlikely. A drug interaction study in healthy subjects has shown that ritonavir (a highly potent cytochrome P450 3A4 inhibitor) can greatly increase fluticasone propionate plasma concentrations, resulting in markedly reduced serum cortisol concentrations. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving intranasal or inhaled fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects. Co-treatment with other CYP 3A4 inhibitors, including cobicistat-containing products is also expected to increase the risk of systemic side-effects. The combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side-effects, in which case patients should be monitored for systemic corticosteroid side-effects. Studies have shown that other inhibitors of cytochrome P450 3A4 produce negligible (erythromycin) and minor (ketoconazole) increases in systemic exposure to fluticasone propionate without notable reductions in serum cortisol concentrations. Nevertheless, care is advised when co-administering potent cytochrome P450 3A4 inhibitors (e.g. ketoconazole), as there is potential for increased systemic exposure to fluticasone propionate. Azelastine hydrochloride No specific interaction studies with azelastine hydrochloride nasal spray have been performed. Interaction studies at high oral doses have been performed. However, they bear no relevance to azelastine nasal spray as given recommended nasal doses result in much lower systemic exposure. Nevertheless, care should be taken when administering azelastine hydrochloride in patients taking concurrent sedative or central nervous medications because sedative effect may be enhanced. Alcohol may also enhance this effect (see section 4.7 of the SmPC). Fertility, pregnancy and lactation: Fertility: There are only limited data with regard to fertility (see section 5.3 of the SmPC). Pregnancy: There are no or limited amount of data from the use of azelastine hydrochloride and fluticasone propionate in pregnant women. Therefore, Dymista Nasal Spray should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus (see section 5.3 of the SmPC) Lactation: It is unknown whether nasally administered azelastine hydrochloride/metabolites or fluticasone propionate/metabolites are excreted in human breast milk. Dymista Nasal Spray should be used during lactation only if the potential benefit justifies the potential risk to the newborns/infant. Undesirable effects: Very common (≥1/10): Epistaxis Common (>1/100, <1/10): Headache, dysgeusia (unpleasant taste), unpleasant smell For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC. Reporting of adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie Adverse reactions/events should also be reported to the marketing authorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267. Legal Category: Product subject to prescription which may be renewed (B)
3. Hampel FC, et al. Double-blind placebo-controlled study of azelastine and uticazone in a single nasal spray delivery device. Ann Allergy Asthma Immunol 2010; (105): 168-173 4. Dymista® Summary of Product Characteristics 5. Scadding GK, et al. BSACI guideline for the diagnosis and management of allergic rhinitis (Revised Edition 2017. First edition 2007). Clin Exp Allergy 2017. 47(7): 856-889. 6. Klimek L et al. Effectiveness of MP20-02 for the treatment of allergic rhinitis in real-life: Results from a non interventional study. Allergy Asthma Proc. 2015; 36:40-47 7. Berger WE, et al. Long-term, Randomized Safety Study of MP29-02 (a Novel Intranasal Formulation of Azelastine Hydrochloride and Fluticazone Propionate in an Advanced Delivery System) in Subjects With Chronic Rhinitis. J Allergy Clin Immunol Pract. 2014; 2:179-85. 8. Bousquet J, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2019
February
References: Job code: DYM-2022-0027 Date of Preparation:
2022 Viatris.com
1. Meltzer E et al. MP29-02 (a novel intranasal formulation of azelastine hydrochloride and uticasone propionate) in the treatment of seasonal allergic rhinitis: A randomized, double-blind, placebo-controlled trial of ef cacy and safety. Allergy Asthma Proc. 2012; 33(4): 324-32. 2. Carr W, et al. A novel intranasal therapy of azelastine with uticasone for the treatment of allergic rhinitis, J Allergy Clin Immunol 2012 May; 129(5): 1282-9
Marketing Authorisation Number: PA2010/059/001 Marketing Authorisation Holder: Mylan IRE Healthcare Limited, Unit 35/36, Grange Parade, Baldoyle Industrial Estate,
Full Prescribing Information available on request from: Viatris, Dublin 17. Phone
8322250. Date of revision of Abbreviated Prescribing Information: 10 Nov 2021 Reference Number: IE-AbPI-Dymista-v003
Dublin 13, Ireland
01
Crohn’s and Colitis Ireland launch Symptom Checker
Ahead of World IBD Day this Friday May 19, and against this backdrop of delayed diagnosis, Crohn’s and Colitis Ireland has launched a new Symptom Checker (www. crohnscolitis.ie/symptomchecker). The tool forms part of its “Poo Taboo” campaign which aims to lift the lid on some of the stigma around IBD symptoms and the importance of people not being too shy to get checked out.
IBD covers a number of conditions in which the digestive tract becomes inflamed, swollen and ulcerated – the two most common conditions being Crohn’s Disease and ulcerative colitis. It is thought that at least 40,000 people are living with IBD in Ireland, with most being diagnosed between 15 and 35 years, and then later in life, between 50 and 70 years. However, Crohn’s and Colitis Ireland believes that many more people remain undiagnosed.
While ulcerative colitis affects the large intestine only, Crohn’s disease can occur anywhere along the digestive tract, and the inflammation can be much deeper, even perforating the bowel. Common symptoms include diarrhoea or loose stools, bleeding from the bottom, fever, fatigue, anaemia, weight loss, cramps and abdominal pain. With ulcerative colitis, there can also be a feeling of being unable to completely empty the bowel.
Don’t wait to get seen… Consultant gastroenterologist, Professor Barbara Ryan, continues, “I have known from my own clinical practice for some time that too many people delay getting seen to. They may put off getting checked out and having their symptoms investigated, or sometimes IBD, particularly Crohn's disease, can be difficult to diagnose. For others, the path to diagnosis is much smoother and quicker, once they start to undergo investigation. Thankfully, once the diagnosis is made, we now have an excellent and ever-expanding array of treatments available, and people can experience a rapid improvement in symptoms.

“While the results of the recent survey undertaken on behalf of Crohn’s and Colitis Ireland are not surprising to me, they are nonetheless still very concerning. It found that while four in ten would go to see a doctor immediately if
they saw blood in the toilet bowl, an equal number would delay getting seen to.
“We want people to stop ignoring the key signs of what can be a serious disease, to overcome any embarrassment that they may have, and to seek medical advice. With our newly-developed symptom checker, people can, in the comfort and privacy of their own homes, answer a series of questions. In less than a minute, they will know if they need to see a GP for further examination.”
The symptom checker asks questions such as: whether an individual has seen blood in their poo more than once; if symptoms are present such as diarrhoea, needing to have a poo urgently or waking up in the night to poo; and whether a person is experiencing unexplained weight loss, abdominal pain, fatigue and fissures that don’t heal, or abscesses that keep coming back. Based on the results, they will then receive advice as to next steps.
Our Survey Says…
The survey to determine awareness of IBD among the population was conducted by Amárach research in March of this year. It found that:
• Awareness: most people (94%) have heard of IBD, Crohn’s disease or ulcerative colitis. Specifically, 79% had heard of Crohn’s disease while 43% had heard of ulcerative colitis.
• Symptoms: most also had a knowledge of some of the symptoms with four in five (83%) able to identify cramps or abdominal pain, 79% diarrhoea or loose stools, 70% blood in stool/toilet bowl/toilet paper, and 67% a feeling of being unable to completely empty the bowel.
• Taking action: however, when it came to acting on symptoms, two in five (40%) would do nothing or adopt a “wait-andsee” approach. This includes
5% of the overall sample who would be too embarrassed to seek advice, hoping that the symptoms would go away. One in ten (9%) would consult “Dr Google” before deciding what to do, while 7% would seek advice from a family member or trusted friend before deciding next steps. Of the overall sample, less than half (41%) would immediately seek advice from a GP or healthcare professional.
The “Poo Taboo” campaign is supported by the Irish College of General Practitioners.
IBD & YOU – SEVEN THINGS YOU SHOULD KNOW…
1. What is IBD? Inflammatory Bowel Disease (IBD) is an umbrella term for inflammatory conditions of the gastrointestinal (GI) tract including ulcerative colitis and Crohn’s disease. It is a chronic and complex condition with periods when it is both active and inactive. Ulcerative colitis is limited to the large bowel and rectum, while Crohn’s disease can occur anywhere from the mouth to the anus.
2. What causes IBD? The cause of IBD is not fully understood, but may relate to a combination of genetic, gut bacteria and environmental factors.
3. What are the symptoms of IBD? Symptoms of IBD include diarrhoea, rectal bleeding, urgency to use the toilet, mouth ulcers, weight loss, fatigue, anaemia and abdominal pain and cramping. Crohn’s disease is often associated with anal problems such as fissures, tags and abscesses. IBD can also affect
other parts of the body, particularly the joints, skin, eyes and liver.
4. What’s the difference between IBD and IBS? IBD and IBS are two different gastrointestinal disorders. While they can have some similar symptoms, they are not the same condition and require very different treatments. IBD refers to inflammatory disease including Crohn’s disease and ulcerative colitis. IBS is a syndrome consisting of a group of symptoms that occur together, including abdominal pain, changes in bowel movements, which may be diarrhoea or constipation, or both, and excessive gas and bloating.
5. Will I spend the rest of my life running to the bathroom? No. Once a diagnosis and treatment plan are in place, the aim of treatment is to reduce symptoms, obtain remission and improve quality of life.
6. How can diet help? Malnutrition can affect up to 85% of people with IBD and so a well-balanced and healthy diet is important. This should incorporate protein which is key to muscle growth, good fats –known as unsaturated fats – which have health-promoting properties, and carbohydrates which are an important source of energy and B-vitamins. Of course, every person with IBD is different, and so too will be their dietary plan.
7. Is there a cure? There is currently no cure for IBD, however, treatment can help stabilise the disease and provide a return to a normal quality of life. Treatment can involve medications, surgery, and adopting new diet, exercise and lifestyle routines.
25 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
Crohn’s Disease
Consultant gastroenterologist, Professor Barbara Ryan
Multiple Sclerosis Association between speech rate measures and cognitive function in people with relapsing and progressive multiple sclerosis
Multiple sclerosis (MS) is a debilitating autoimmune condition of the central nervous system and a most common non-traumatic form of neurological disability in young adults.1 MS affects diverse functional systems, including sensory, motor, and cognitive impairments. Cognitive impairment is a well-established symptom of MS with deficits observed in between 40% and 65% of people with MS (pwMS) and may present at the earliest stages of the disease.2
Reduced information processing speed and efficiency are among the most common forms of cognitive dysfunction in MS.2–4 Deficits in executive functioning, often encompassing processes such as planning, organisation, reasoning, and attention are also commonly reported.2,3,5 Indeed, such cognitive domains have been utilised as disability metrics in MS clinical trials, due to the frequency and impact of cognitive dysfunction in this population.6 7
Additionally, speech abnormalities and language impairments are almost as pervasive as cognitive symptoms, with up to 50% of MS patients experiencing some form of speech disorder.8,9 Although speech and communicative ability are tightly linked with interpersonal relationships, work status, and quality of life,10 impairments in these domains are among the least well-described manifestation of MS. Speech-motor conditions such as dysarthria are characterised by unclear articulation, for example, slurred, monotone or breathy speech, primarily resulting from muscle weakness. Conversely, speech and language production relies on multifaceted cognitive abilities involving message generation, word-finding, sentence structure as well as articulation,11 and are the focus of this research.
Difficulty with word-finding has been reported as the primary cognitive complaint among pwMS and shown to discriminate pwMS
from healthy controls,12 while slowed articulation rate has repeatedly been linked with a decline in information processing speed.13–15 Swets et al.16 reported the involvement of working memory in sentence planning, suggesting that sentence planning ability is limited by working memory capacity. Therefore, abnormalities or deficits in cognition may be reflected in the prosody of speech.13–15 While cognitive systems are a welldocumented area of concern for pwMS, few studies explore the effect of cognitive impairment on speech and concerning the clinical management of multisystem conditions such as MS, consideration should be given to the interaction of deficits in different domains.
While measures of speech timing, such as speech rate and pause characteristics, have been linked with cognitive processing demands, the speech domain measured, and the method used to elicit speech may impact findings. Spontaneous speech has been reported to be more cognitively demanding than read speech due to the added dimension of planning, recalling content and monitoring.17,18 Many studies in MS, however, employ reading passages such as the Grandfather Passage13,19,20 or utilise speech prompts based on topics familiar to participants (e.g. ‘discuss your last holiday/ favourite hobby’13 14). Like reading, familiar topics may require less cognitive engagement in the form of planning, idea generation and long-/short-term recall, due to frequent discussion, or rely on specific cognitive domains such as memory. Therefore, using unfamiliar images to probe extemporaneous speech generation may engage cognitive domains not employed by familiar topics.
A multitude of cognitive assessments, as well as reading and spontaneous speech tasks, were employed to investigate the relationship between cognition
and speech in pwMS. It was hypothesised that self-generated spontaneous speech would result in longer, more frequent pauses, faster articulation and slower speech rates when compared to free speech and that these differences would be magnified in pwMS. It was also hypothesized that self-generated speech would require greater cognitive demand, as made apparent by cognitive variables explaining more variance in rate measures of spontaneous speech compared to read speech.
METHODS
Participants
Forty-five pwMS were recruited through the outpatient MS clinic. Twenty-one had a diagnosis of relapsing MS (RMS), and 24 had progressive MS (PMS). Inclusion criteria were age 18 to 65 years, a diagnosis of MS as per the 2017 McDonald criteria21 and MS phenotype based on the Lubin criteria.22 For those in the RMS group, an expanded disability severity scale (EDSS) score <3.5 was required, or 3.5 to 6.5 for the PMS category. An EDSS score of 6.5 was used as a cutoff due to advanced symptom severity, which precluded people from completing aspects of the study.23 At time of assessment, the MS cohort had a disease duration of 12.3 ± 10.3 years and 86% were taking diseasemodifying treatments (DMTs). None had undergone speech therapy. Twenty-five age- and sexmatched controls were recruited also. Participants were excluded from the study if they had severe visual or cognitive impairment, neurodevelopmental disabilities impeding their capacity to provide informed consent, any evidence of clinically discernible dysarthria or experienced traumatic brain injury, uncontrolled neuropsychiatric, active malignancy or active MS relapse. Controls were also excluded if they had a history of neurological disease. All participants had normal or corrected vision and no participants reported difficulty
viewing test stimuli at any stage. This study was approved by the Research Ethics Committee of the St. Vincent’s Healthcare Group, Dublin. Written informed consent was obtained from all participants.
Procedure
Clinical and cognitive assessments were administered over two 1-h sessions, with a 10-min break to reduce fatigue. A clinician retrospectively evaluated EDSS scores based on the most recent clinic letter within 3 months of participation in this study. Participants also completed the Hospital and Anxiety Depression Scale (HADS). All assessments were administered by a clinician or assistant psychologist and overseen by an experienced clinical neuropsychologist.
Cognitive battery
A cognitive battery was administered, assessing general intellectual ability (Wechsler Abbreviated Scale of Intelligence – Second Edition (WASI-II) and Vocabulary and Matrix Reasoning subtests24); executive functioning (Delis-Kaplan Executive Function System (D-KEFS) Colour-Word Interference Test (CWIT)25; named colour-word condition); attention (Wechsler Adult Intelligence Scale-IV (WAIS-IV) Forward Digit Span); information processing speed (Symbol Digit Modalities Test (SDMT)26 27); and verbal and visual learning and memory (California Verbal Learning Test-Second Edition (CVLT-II)26; Brief Visuospatial Memory TestRevised (BVMT-R)26). Wordretrieval ability was assessed through Letter and Category Fluency tests (D-KFES28). All tests were administered following standardised procedures.
Speech measures
Participants were provided with a typed script of the ‘Grandfather Passage’29 on a landscapeorientated A4 sheet of white paper using 20-point Times New Roman, black font. Participants were instructed to read the passage aloud at a comfortable
26 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Clodagh O’Keeffe, SiewMei Yap, Laura Davenport, Clodagh Cogley, Fiona Craddock, Alex Kennedy, Niall Tubridy , Céline De Looze , Narin Suleyman , Fiadhnait O’Keeffe, Richard B. Reilly and Christopher McGuigan
Table 1
pace. The spontaneous speech task involved describing ‘The Cookie Theft’ image, from the Boston Diagnostic Aphasia Examination30 presented on a landscape-oriented A4 page. Participants described the image as fully as possible within 15 s. In both cases, the task commenced with the examiner indicating the recording had begun and ended when the participant indicated they had no more to say.
Speech data was recorded using a microphone (GXT 215, Trust International, Netherlands), placed 10 cm from the participant’s mouth. The speech was recorded at 44.1 kHz and quantised at 32 bits using Audacity (Free Software Foundation, Boston, USA). Anonymised recordings were annotated and transcribed using Praat software (Phonetic Sciences, Netherlands31).
Speech, pauses, and syllables were automatically extracted using a Praat script. Pause thresholds were set to 100 ms to distinguish pauses from silent plosives. Alignment of pauses and syllables were manually inspected and adjusted based on auditory and visual examination of spectrograms. Recordings were originally annotated by a neuroscientist, and 10% of files were repeated by a speech linguistic expert to assess interrater reliability. Both were blind to group status. A high degree of reliability was found between experimenters’ measurements. The average measure intraclass correlation coefficient was 0.973 with a 95% confidence interval from 0.822 to 0.953 (p < 0.001).
Global speech timing measures extracted from the speech recordings included speech and articulation rate, the number and duration of silent pauses, as well as the number of dysfluencies and the speech-task duration. Speech rates were calculated by dividing the total number of syllables by the total duration of the speech sample, including pauses. The articulation rate was derived similarly but excluded pause time. Dysfluencies included instances of non-lexical vocalisation or sound hesitation (e.g. um, eh32), false starts, and repetitions.
Statistical analysis
Data analysis was carried out using SPSS-V27.0 (IBM Corp., IBM SPSS Statistics, Armonk, NY). The distribution of variables was assessed through KolmogorovSmirnov tests. Demographic and clinical measures were compared for descriptive purposes and were, therefore, not subject to correction for multiple comparisons.
All the cognitive tests were normalised to z-scores based on the mean and standard deviation of the control group. To reduce the number of variables used to test the hypotheses, composite scores were calculated from mean z-scores for cognitive tests as per Rodgers et al.13 The intellectual ability composite consisted of the Vocabulary and Matrix reasoning subsets of the WASI-II. The memory composite included CVLT-II and BVMT-R z-scores.
Where appropriate, analyses of covariance (ANCOVA) assessed the interaction between three groups and speech tasks on global speech timing measures. Age, sex, years in education and depression were selected as covariates. For variables which violated the assumptions of an ANCOVA, non-parametric analyses were employed. KruskalWallis tests were used to assess differences between groups and Wilcoxon tests explored differences between speech conditions. The resulting p-values of pairwise comparisons have been adjusted to account for multiple comparisons using Bonferroni corrections.
The second hypothesis that cognitive variables would explain variance in speech measures for pwMS was tested using stepwise regression models. To ensure assumptions of the regression analysis were met, the RMS and PMS groups were combined. Speech and articulation rates for spontaneous speech or read speech were set as dependent variables. Demographic characteristics, (age sex, years in education, and depression) were added as a block, and then each cognitive domain (intellectual ability, information
processing speed, memory, attention, executive functioning, and word retrieval) was included using forward stepwise regression where cognitive domains were added in sequential order, with the domain accounting for most variance added first until no further significant domains remained (p > 0.05).
RESULTS
Participant demographics
There was no significant difference between groups in age, gender, handedness, or level of education (p > 0.05). More of the control group were in employment (88%) at the time of recording, compared to pwMS (55%; p = 0.002).
Speech rate measures
Repeated measures ANCOVA were employed to assess articulation rate between the three groups (RMS vs. PMS vs. Control) and across both speech tasks (Read speech vs. Free speech). Controlling for age sex years in education and depression, there was no significant group×speech task interaction effect on articulation rate (F(2,63) = 1.008, p = 0.371, η2 = 0.03). However, there was a significant group effect (F(2,36) = 5.197, p = 0.008, η2 = 0.142). Pairwise comparisons revealed those with PMS have slower articulation rates than those with RMS (p = 0.04), and controls (p = 0.009). Similarly, there was no group × speech task interaction (F(2,63) = 0.259, p = 0.772, η2 = 0.008) for speech rate but the group effect (F(2630 = 4.99, p = 0.01, η2 = 0.137), revealed that speech rate was again reduced in those with PMS compared to RMS (p = 0.024), and the control group (p = 0.017).
Although pause duration remained consistent across tasks, the number of pauses recorded by each group was greater during the reading passage compared to spontaneous speech. Only the PMS group did not exhibit a greater number of dysfluencies during the spontaneous speech task. See Table 1 for a summary of the results.
Kruskal-Wallis H-test revealed that speech-task duration differed between groups for the spontaneous speech task (H(2) = 8.265, p = 0.016 η2 = 0.09), where those with RMS had significantly longer speech-task durations compared to those with PMS. However, for the reading passage, those with PMS has significantly longer speechtask duration compared to both controls (p < 0.001) and the RMS group (p = 0.045). The number of pauses differed between groups for both spontaneous speech passage (H(2) = 12.455, p = 0.002, η2 = 0.15) and read speech passage (H(2) = 9.740, p = 0.008, η2 = 0.12), such that those with PMS exhibited a significantly greater number of pauses than the control group for both tasks (spontaneous speech task: p = 0.001, read speech passage: p = 0.009). The frequency of dysfluencies and pause duration were comparable between groups for both tasks (p > 0.05). See Figure 1 (page 28) for a summary.
Regression
Age, gender, and depression were not significant contributors to any model.
Figure 1. Speech measures by task. Brackets indicate differences between tasks, bars indicate differences between groups. * Denotes significance to the level of p < 0.05, ** signifies p < 0.01, ***indicates significance to the level of p < 0.001.
The stepwise regression model for the self-generated, spontaneous speech task revealed that information processing speed accounted for a substantial proportion of the variance in speech rate (30%). Information processing speed was again retained in the model for articulation rate explaining 18% of the variance observed during spontaneous speech.
For the read speech passage, information processing speed accounted for 30% of the variance
27 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
File duration No. of dysfluencies No. of pauses Pauses duration Relapsing multiple sclerosis (MS) Z = −3.945, p < 0.001 Z = −3.397, p = 0.001 Z = −2.659, p = 0.008 Z = −1.721, p = 0.08 Progressive MS Z = −4.268, p < 0.001 Z = −0.364, p = 0.716 Z = −2.810, p = 0.005 Z = −1.829, p = 0.06 Control group Z = −4.372, p < 0.001 Z = −3.186, p = 0.001 Z = −3.690, p < 0.001 Z = −2.273, p = 0.023 Significant p-values are highlighted in bold.
Multiple Sclerosis
of speech rate while executive functioning (CWIT) accounted for a further 10%, bringing the total variance accounted for to 40%. Again, information processing explained 26% of the variance in the articulation rate of pwMS. Intellectual ability composites, attention, executive functioning, and memory composites provided an insignificant contribution to variance.
Discussion
This study aimed to compare speech measures of read and spontaneous speech and to examine the relationship between speech and cognition in those with MS. The hypothesis that all speakers would demonstrate slower speech rates, faster articulation rates and increased duration of pauses during the spontaneous speech was not supported. However, findings do support the hypothesis that differences in speech rates were magnified in pwMS, specifically within the PMS cohort. Regression models for predicting speech rate from cognitive abilities also added further evidence of the interdependence of cognitive functioning and speech-motor performance in MS.

As hypothesised, those with PMS consistently exhibited a
greater number of pauses and slower speech rates during both speech tasks compared to the other cohorts. Such differences suggest that additional time is needed for cognitive-linguistic processing such as planning and formulating ideas.20 Similarly, articulation rates were notably reduced in the PMS cohort which may reflect compensatory mechanisms to maintain speech intelligibility when reading or speaking aloud.20 This, combined with the increased number of pauses, could also be reflective of greater overall cognitive impairment in those with PMS or offset slowed neural transmission of information. Speech-motor conditions such as dysarthria may also impact timings of speech production. While those with clinically noticeable dysarthria were excluded form participation in this study, some participants
may have experienced subtle sub-clinical dysarthric symptoms. More thorough assessments of dysarthria may be necessary in future studies to rule out the potential impact of subtle oralmotor involvement.
Contrary to the hypothesis and previous reports,20 each group exhibited a greater number and duration of pauses during the reading task than spontaneous speech. This may be due to the pause type. The syntactically complex reading passage contains improbable punctuation resulting in numerous grammatical pauses. However, due to the less formal structure of spontaneous speech, grammatical and ungrammatical pauses were not directly compared in this study. The regression analysis did not support the hypothesis that self-generated, spontaneous
28 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1. Speech measures by task. Brackets indicate differences between tasks, bars indicate differences between groups. * Denotes significance to the level of p < 0.05, ** signifies p < 0.01, ***indicates significance to the level of p < 0.001.
speech requires greater cognitive demand than that of read speech. Information processing was, however, found to contribute significantly (18%–30%) to the variance in speech rate measures for both read and spontaneous speech. This is broadly in line with the results of Rodgers et al.13 and Benedict et al.,4 who also report a link between information processing and measures of read and spontaneous speech. Similarly, a large-scale study carried out by Friedova et al.14 linked reduced information processing speed with a slower articulation rate in those with MS. Therefore, our results reaffirm the role of information processing in speech generation.
However, contrary to previous studies,13 memory composites added a negligible variance beyond that of information processing, during self-generated speech. A possible explanation for this is the use of the Boston Cookie Theft Image as a visual prompt to evoke speech. Perhaps the availability of the image throughout the task minimised demand on memory retrieval, instead concentrating cognitive resources on processing the image. The use of images to elicit speech provides a focus for participants, reducing ambiguity around the topic while also mitigating the use of memory. Notably, executive functioning contributed significantly to the variance of read speech for pwMS. Combined, executive functioning and information processing explained 40% of the variance observed in read speech. Executive functioning encompasses processes such as attentional control and working memory and inhibition. Attentional control is needed to attend to relevant literacy information and suppresses irrelevant information while flexibly shifting between both,33 while working memory is involved in storing and processing information34 and so, executive functioning has long been associated with phonological awareness and literacy.34 The engagement of information processing and executive functioning and the extent to which these cognitive domains explain variance in read speech, therefore, advocate for the reconsideration of the assumption that spontaneous speech necessitates greater cognitive-linguistic demand than read speech.
Such findings further highlight the importance of assessing
information processing speed, executive functions and speech production as part of clinical monitoring in MS and that interventions targeting information processing speed may have a positive impact on speech production. Similarly, speech tasks grant non-intrusive, ecologically valid methods of assessing speech production, while picture description and reading tasks provide consistent stimuli for longitudinal studies, to monitor changes in speech or cognition across disease duration. Due to the ease of administration and richness of the data obtained, speech measures easily translate to clinical settings or familiar environments to monitor disease progression or treatment endpoints.
While this study benefited from the use of well-validated speech and cognitive measures, results must be interpreted with consideration of the constraints in which they were obtained. Although frequently employed, the administration of the Grandfather passage is rarely described in great detail. Font, size, spacing, delivery method, and participant ability may influence readability,35 yet are rarely described in the literature. Without standardised administration, results must be compared with caution. Similarly, while the use of images to prompt speech has advantages, this method may rely on visual information processing specifically, which this study does examine. Differences in visual information processing may have
underpinned or supplemented the effects of information processing speed on individual differences in speech timing due to varying times needed to process the image before planning and articulating the description. Pre-clinical dysarthric articulation may not have been detected during this study nor was there an assessment of respiratory problems which have previously been reported to affect speech in those with MS.36

Another important issue to note is the potential effect of DMTs. As mentioned 86% of participants have been prescribed DMTs including fingolimod (22%), natalizumab (13%), rituximab (11%), peginterferon beta-1a (4%) dimethyl fumarate (7%), ocrelizumab (9%), glatiramer acetate (9%), cladribine (9%), and interferon beta-1a (2%). DMTs may affect cognition by reducing the progression of brain atrophy and relapse rates.37 38 Emerging research described early treatment with fingolimod as providing a cognitive advantage in those with RMS by allowing adaptive neuroplastic changes and providing anti-inflammation effects38 while Mattioli et al.37 reported that natalizumabaided executive functioning and memory improvements through preservation of parahippocampal and frontal grey matter. However, there remains insufficient evidence of the efficacy of DMTs in the treatment of cognitive impairments in pwMS and no medication has been approved, specifically for these symptoms.39
Future research should, where possible, consider the potential effects of respiratory issues on speech timing. Furthermore, sentence intelligibility scores may shed light on the influence of dysarthria on speech measures. Future research should also employ a combination of stimuli to elicit self-generated speech (e.g. familiar topics and conversational speech), as these may engage different cognitive processes, and provide a more sensitive delineation of the cognitive measures associated with speech.
Conclusion
The present study found that speech production is contingent on the cognitive ability of the speaker. Although the analysis did not support the hypothesis that read speech is less cognitively demanding than free speech, differences emerged in speech timing patterns of pwMS that are linked with information processing speed and executive functioning. Therefore, the relationship between speech and cognition in MS may provide a foundation for the introduction of speech-based technologies as a method of cognitive assessment and monitoring in pwMS. This study also reaffirms that reading passages and image prompts are easily administered tools to elicit speech and can provide an ecologically valid method of indirectly assessing cognition, whether in a clinical or familiar environment.
29 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
FIRST-LINE TAGRISSOTM showed GROUNDBREAKING EFFICACY
delivering statistically significant and clinically meaningful OS;

38.6 vs 31.8 months median OS vs.
Adapted from: TAGRISSO. Summary of Product Characteristics. gefitinib and erlotinib
The most commonly reported adverse drug reactions (ADRs) were diarrhoea (47%), rash (45%), paronychia (33%), dry skin (32%), and stomatitis (24%). Grade 3 and Grade 4 adverse reactions across the studies were 10% and 0.1%, respectively. In patients treated with TAGRISSO 80 mg once daily, dose reductions due to adverse reactions occurred in 3.4% of the patients. Discontinuation due to adverse reactions was 4.8%.
IN THE FLAURA STUDY
Progression-free survival was the primary endpoint. Overall survival was a prespecified secondary endpoint.1
TAGRISSO AS MONOTHERAPY IS INDICATED FOR:
• the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.†
• the first-line treatment of adult patients with locally advanced or metastatic NSCLC with activating EGFR mutations.
• the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
†This indication is not currently reimbursed in Ireland.
TAGRISSO® (osimertinib). Summary of Product Characteristics. 2022
TAGRISSO® (osimertinib) 40mg and 80mg FILM-COATED TABLETS. Consult Summary of Product Characteristics (SmPC) before prescribing. Indication: TAGRISSO as monotherapy is indicated for: the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations; the first-line treatment of adult patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC) with activating EGFR mutations and the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC. Presentation: 40mg and 80mg osimertinib (as mesylate) film-coated tablets. Dosage and Administration: Treatment should be initiated by a physician experienced in the use of anticancer therapies. When considering the use of TAGRISSO, EGFR mutation status (in tumour specimens for adjuvant treatment and tumour or plasma specimens for locally advanced or metastatic setting) should be determined using a validated test method. The recommended dose is 80mg once a day, taken with or without food at the same time each day. Patients in the adjuvant setting should receive treatment until disease recurrence or unacceptable toxicity. Treatment duration for more than 3 years was not studied. Patients with locally advanced or metastatic lung cancer should receive treatment until disease progression or unacceptable toxicity. If a dose is missed, the dose should be made up unless the next dose is due within 12 hours. Should be swallowed whole with water and it should not be crushed, split or chewed. If patients are unable to swallow, the tablet may be dispersed in water (non-carbonated). No other liquids should be used. The dispersion can also be administered through a nasogastric tube. Refer to SmPC for full instructions. Dose adjustments: If required to manage safety and tolerability, then dose should be reduced to 40mg taken once daily. Special populations: Hepatic impairment: No dose adjustments are necessary in patients with mild hepatic impairment (Child Pugh A) (total bilirubin ≤upper limit of normal (ULN) and aspartate aminotransferase (AST) >ULN or total bilirubin >1.0 to 1.5x ULN and any AST) or moderate hepatic impairment (Child Pugh B) (total bilirubin between 1.5 to 3 times ULN and any AST). Use in patients with severe hepatic impairment is not recommended. Renal impairment: No dose adjustments are necessary in patients with mild, moderate, or severe renal impairment. Caution should be exercised when treating patients with severe and end stage renal impairment. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Should not be used with St. John’s Wort. Warnings and Precautions: Interstitial Lung Disease (ILD): Patients with acute onset and/or unexplained worsening of pulmonary symptoms (dyspnoea, cough, fever) should have their treatment interrupted while investigations are performed to exclude ILD. If ILD is diagnosed, TAGRISSO should be discontinued and appropriate treatment initiated as necessary. Reintroduction should be considered only after careful consideration of the individual patient’s benefits and risk. Stevens-Johnson syndrome (SJS): Before initiating treatment, patients should be advised of signs and symptoms of SJS. If suggestive, TAGRISSO should be interrupted or discontinued immediately. QTc interval prolongation: May lead to an increased risk for ventricular tachyarrhythmias (e.g. torsade de pointes) or sudden death. When possible, TAGRISSO should be avoided in patients with congenital long QT syndrome. Periodic monitoring with electrocardiograms and electrolytes should be considered in patients with congestive heart failure, electrolyte abnormalities, or those who are taking medicinal products that are known to prolong the QTc interval. Treatment should be withheld in patients who develop a QTc interval greater than 500 msec on at least 2 separate ECGs until the QTc interval is less than 481 msec or recovery to baseline if the QTc interval is greater than or equal to 481 msec, then resume TAGRISSO at a reduced dose. TAGRISSO should be permanently discontinued in patients who develop QTc interval prolongation in combination with any of the following: torsade de pointes,
polymorphic ventricular tachycardia, signs/symptoms of serious arrhythmia. Changes in cardiac contractility: In patients with cardiac risk factors and those with conditions that can affect LVEF, cardiac monitoring, including an assessment of LVEF at baseline and during treatment, should be considered. In patients who develop relevant cardiac signs/symptoms during treatment, cardiac monitoring including LVEF assessment should be considered. Keratitis: Patients with signs and symptoms suggestive of keratitis such as acute or worsening: eye inflammation, lacrimation, light sensitivity, blurred vision, eye pain and/or red eye should be referred promptly to an ophthalmology specialist. Aplastic Anaemia: Rare cases of aplastic anaemia, including fatal events, have been reported in association with osimertinib treatment. Before initiating treatment, patients should be advised of signs and symptoms of aplastic anaemia including but not limited to persistent fever, bruising, bleeding, pallor, infection and fatigue. If signs and symptoms suggestive of aplastic anaemia develop, close patient monitoring and drug interruption or discontinuation of osimertinib should be considered. Osimertinib should be discontinued in patients with confirmed aplastic anaemia. Age and body weight: Elderly patients (>65 years) or patients with low body weight (<50 kg) may be at increased risk of developing adverse events of Grade 3 or higher. Close monitoring is recommended. Drug Interactions: Concomitant use of strong CYP3A inducers (e.g. phenytoin, rifampicin and carbamazepine) should be avoided. Moderate CYP3A4 inducers (e.g. bosentan, efavirenz, etravirine, modafinil) should be used with caution, or avoided when possible. Closely monitor patients taking concomitant medicinal products with disposition dependent upon breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) and with narrow therapeutic index. Concomitant use of St. John’s Wort is contraindicated. Pregnancy and Lactation: TAGRISSO should not be used during pregnancy unless the clinical condition of the woman requires treatment with TAGRISSO. Discontinue breast-feeding during treatment with TAGRISSO. Women of childbearing potential should be advised to avoid becoming pregnant if they or their partner are being treated with TAGRISSO. Undesirable Events: Consult SmPC for full list of adverse events. Very common (all grades): Decreased appetite, diarrhoea, stomatitis, rash, dry skin, paronychia, pruritus, platelet count decreased, leucocytes decreased, lymphocytes decreased, neutrophils decreased. Common (all grades): Epistaxis, interstitial lung disease, alopecia, urticaria, palmar-plantar erythrodysaesthesia syndrome, blood creatine phosphokinase increased, left ventricular ejection fraction decreased, blood creatinine increased. Uncommon (all grades): Keratitis, cardiac failure, QTc interval prolongation, erythema multiforme, cutaneous vasculitis. Rare (all grades): Aplastic anaemia, Stevens-Johnson syndrome, myositis. Legal Category: Product subject to prescription which may not be renewed (A). Marketing Authorisation Number: EU/1/16/1086/001 and EU/1/16/1086/002. Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje, Sweden. Further information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 7100. TAGRISSO® is a trade mark of the AstraZeneca group of companies.
Date of API Preparation: 09/2022 Veeva ID: IE-4270
Adverse events should be reported directly to: HPRA Pharmacovigilance. Website: www.hpra.ie. Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899.

©2023 AstraZeneca. All rights reserved. Date of Preparation: May 2023 | Veeva ID: IE-4940
Reference: 1.
FLAURA
(HR: 0.799; 95.05% CI, 0.641 to 0.997; P=0.0462)1
1st-generation EGFR TKIs* in the
study
In the FLAURA Study,
Number at risk 279 245 270 217 276 236 254 204 193 180 166 153 138 123 86 50 17 2 0 277 219 252 182 263 205 239 165 148 138 131 121 110 101 72 40 17 2 0
ABRIDGED PRESCRIBING INFORMATION
CPD CPD
60 Second Summary
Atopic dermatitis (AD), also known as eczema, is the most common skin condition in children (30% prevalence in the first 2 years) and second most common in adolescents and adults (7% prevalence) after acne. The cause of AD relates to a combination of the triad of skin barrier dysfunction, skin dysbiosis, and dysfunctional inflammation. AD is associated with other atopic conditions such as food allergy, asthma, and allergic rhinitis. These associations often lead to patients with AD being treated with ineffective and inappropriate treatments, such as dietary restriction, which have no role in AD.
To deal with the three intertwining causes of AD, a three-pronged approach is necessary: emollients to replenish the skin barrier, topical steroids or calcineurin inhibitors to reduce inflammation, and antiseptic bleach baths to reduce cutaneous dysbiosis. In special scenarios oral antibacterial or antiviral agents may be required.
Patients with AD should NOT be told to use topical steroids ‘sparingly’ or ‘thinly’ and should be advised to treat for an adequate duration. Patients with AD should NOT be treated with oral steroids (outside of expert centres with a therapeutic exit strategy in place) due to the severe side effects and lack of disease modification. Patients with AD should NOT be told to change their washing powder or reduce/eliminate foods from their diet, as AD is not caused by detergents or food allergy.
Patients with persistent or severe AD despite the use of appropriate potent topical therapy should be referred to dermatology for consideration of advanced therapies.
AUTHOR: Dr Cathal O’Connor, Clinical Research Fellow in Dermatology, Irish Clinical Academic Training Programme, Royal College of Physicians of Ireland

1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?

4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. LEO Pharma has no editorial oversight of the CPD programmes included in these modules.
Management of Atopic Dermatitis



Introduction
Atopic dermatitis (AD), also known as eczema, is the most common skin condition in children (up to 30% prevalence in the first 2 years of life and 20% overall) and second most common in adolescents and adults (7% prevalence) after acne. AD has a significant impact on quality of life (QOL), and children with severe AD have worse QOL than children with type 1 diabetes mellitus or treatment-resistant epilepsy. The main burdensome symptoms are itch and sleep disruption (Images 1A-1D). AD is associated with other atopic conditions such as food allergy, asthma, and allergic rhinitis. These associations often lead to patients with AD being treated with ineffective and inappropriate treatments, such as dietary restriction, which have no role in AD. AD is also associated with neuropsychiatric disease such as anxiety, depression, and attentiondeficit hyperactivity disorder.
Cause of AD
The cause of AD relates to a combination of the triad of skin
barrier dysfunction, skin dysbiosis, and dysfunctional inflammation (Image 2 on page 32). Skin barrier dysfunction is genetically inherited, with filaggrin mutations significantly increasing the risk of developing AD. Filaggrin plays
31 CPD 100: ATOPIC DERMATITIS
Continuing Professional Development
1A 1B 1C 1D
Full prescribing information is available on www.medicines.ie Legal category: POM Further information is available from: LEO Pharma, Cashel Road, Dublin 12, Ireland. E-mail: medical-info.ie@leo-pharma.com Marketing authorisation number and holder: EU/1/21/1554/002 This is a promotional advertisement from LEO Pharma intended for IE healthcare professionals. Date of preparation: May 2023 IE/MAT-65962 Reporting of Suspected Adverse Reactions Adverse events should be reported. Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
an important role in the skin's barrier function. It brings together structural proteins in the outermost skin cells to form tight bundles, flattening and strengthening the cells to create a strong barrier. In AD, skin is heavily colonised with Staphylococcus aureus (S. aureus) in lesional and non-lesional skin. A rising proportion of S. aureus in skin microbiome samples predicts a flare, and diversity of nonstaphylococcal species returns once a flare has been treated. A hallmark of AD is Th2-mediated inflammation, with high levels of IL-4 and IL-13 that promote dysfunctional allergic inflammation. With chronic or untreated AD, other immune pathways can become activated. Each component of the triad interacts with the others in a vicious cycle, for example filaggrin deficiency allows antigens to penetrate the stratum corneum and stimulate inflammation, and subsequently IL-4 and IL-13 production leads to reduced filaggrin production. It has recently been shown that dupilumab (a biologic targeting IL-4 and IL-13) not only reduces inflammation but enhances skin barrier function, highlighting the importance of adequately treating inflammation in AD. It is essential to treat each component of the causes of AD adequately to optimise outcomes.
Treatment of AD
Anti-inflammation

The use of anti-inflammatory steroid creams/ointments is essential to reduce inflammation in AD. Early and aggressive use of topical steroids can reduce
the duration of disease, and may also reduce the risk of developing associated problems. The dermatology team in Crumlin have recently shown that reducing inflammation in the skin using steroid creams also reduces systemic inflammation, suggesting that topical steroids can correct the systemic immune dysregulation in AD. For mild AD, hydrocortisone 1% can be dispensed over the counter. However, hydrocortisone 1% is a very weak anti-inflammatory agent, and is not sufficient to treat more severe eczema. For moderate AD, agents such as clobetasone butyrate (Eumovate)

or betamethasone valerate 0.025% (Betnovate RD) can be used. For severe AD, potent agents such as betamethasone valerate 0.1% (Betnovate 0.1%) or mometasone furoate (Elocon) should be used (Image 3). In general it is better to use a more potent topical steroid less frequently than a weaker topical steroid more frequently, for two reasons: systemic absorption is related to the frequency of application, and the burden of treatment with daily topical steroid application is much higher than twice weekly. Topical steroids should be applied liberally (not sparingly!) so that the affected skin is left glistening afterwards. Ointments are preferable to creams as the higher lipid content makes the vehicle more effective. There is no benefit to using topical steroids more than once daily - more frequent use increases the risk of systemic absorption and reduces patient adherence. In general, therapy should be continued once daily for two weeks, then weaned to alternate days for two weeks, then maintained twice weekly (weekend therapy on Saturdays and Sundays is a good option for busy parents/ patients) until the skin has been clear for several months.




Myth 1 Topical steroids should be applied sparingly/thinly and only for short bursts



Topical steroids are a very elegant way to deliver local antiinflammatory therapy, without the potential side effects that systemic therapy can produce. Skin that is inflamed with AD is on fire immunologically. This immune dysregulation needs to be extinguished appropriately to prevent worsening or chronic AD. If topical steroids are stopped too quickly the inflammation can return rapidly. Parents are almost always counselled by pharmacists and non-dermatology doctors about the risk of skin thinning, but this is generally limited to prolonged use of extremely potent topical steroids such as Dermovate (which should only be prescribed for palmoplantar AD). We have recently performed qualitative research interviewing parents of children with severe eczema, and the ‘mixed messages’ about the safety of topical steroids can cause significant upset.
Myth 2 Topical steroids can never be used on broken skin in AD
When skin inflammation is so severe that it has caused skin breakdown, either directly or indirectly from scratching, it is essential to reduce inflammation to avoid further damage. It is safe to apply topical steroids to broken skin.
Image 3
X5 X50 X200 X300 X1,000
32 CPD 100: ATOPIC DERMATITIS
FACE BODY
Image 2
Full prescribing information is available on www.medicines.ie Legal category: POM Further information is available from: LEO Pharma, Cashel Road, Dublin 12, Ireland. E-mail: medical-info.ie@leo-pharma.com Marketing authorisation number and holder: EU/1/21/1554/002 This is a promotional advertisement from LEO Pharma intended for IE healthcare professionals. Date of preparation: May 2023 IE/MAT-65962 Reporting of Suspected Adverse Reactions Adverse events should be reported. Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
Topical tacrolimus, a calcineurin inhibitor, is an alternative to topical steroids. It is a useful antiinflammatory adjunct, particularly in the maintenance phase of AD (rather than the treatment of flares, when it is less effective than potent topical steroids). The only side effect is mild stinging with the first few applications, which usually settles down with ongoing use. Given the lack of steroid, it is particularly useful for sensitive areas such as the eyelids. Topical tacrolimus is much more expensive than topical steroids.
Myth 3 Topical tacrolimus can never be used in babies and only 0.03% can be used in older children
Topical tacrolimus (Protopic) has been used in dermatology for decades, with an overwhelming volume of reassuring safety data. Protopic 0.1% is licensed for patients over 16 years, and Protopic 0.03% is licensed from two to 16 years. Dermatologists usually prescribe the 0.1% formulation for all ages, because of the extensive safety data and the enhanced efficacy. There is extensive anecdotal evidence of safety of Protopic in younger infants, so Protopic 0.1% is often prescribed off-license for infants under two years.
Skin barrier replenishment
Moisturisers (also known as emollients) should be used twice daily or more, and moisturising after a bath (‘soak and seal’) is an excellent way to hydrate the skin. Moisturisers should be applied downwards to avoid blocking or irritating hair follicles. A pump dispenser is useful for preventing bacterial colonisation of the

moisturiser container. Alternatively, a large spoon can be sterilised (using boiling water) and dipped into a tub to avoid transfer of bugs from the parent’s hand to the container (Image 4). Appropriate bathing/showering advice for AD is to avoid soaps or irritant products that produce bubbles, use an emollient (that does not contain sodium laureth sulfate) as a wash, keep the temperature of the bath/shower tepid, and limit the duration of the bath/shower to 10 minutes or less. The skin should be gently patted dry afterwards.
Myth 4 There is a number one brand of emollient for treating AD
There is no particular brand of emollient that is significantly superior than any other for treating AD. One of the key causes of AD is an impaired skin barrier, and every individual has differing levels of various proteins in their skin that retain moisture and protect us from external threats. Every moisturiser has a different mixture of ingredients, so it is best for patients/parents to try several brands to see which leaves the skin most hydrated.
Antimicrobial strategies
For infection-driven flares, the addition of sodium hypochlorite (Milton) is an effective strategy for reducing microbial colonisation, without causing antibiotic resistance. Two capfuls (60mL) can be added to a baby bath (50L) and four capfuls (120mL) can be added to a full bath (100L).
Topical fusidic acid (Fucidin) should be prescribed with caution and other skin infections.
S. aureus resistance to fusidic
acid has reached crisis point, driven by inappropriate use of topical antibiotics. Moreover, the selection for fusidic acid resistant strains also selects for methicillin resistance, creating even more problems with MRSA. If antibiotics are required for infected AD, then oral antibiotics should usually be prescribed, in conjunction with topical antiseptics (eg Milton).
Myth 5 Topical antibiotics are better than oral antibiotics because they do not cause antimicrobial resistance
Topical antibiotics are known to cause localised antimicrobial resistance in the area being treated, but also in cutaneous sites distant to application. One recent study even showed antimicrobial resistance on the skin of close contacts. While oral antibiotics have scope to cause more antimicrobial resistance in an individual patient due to exposure to other flora such as in the gastrointestinal tract, topical antibiotics are usually washed off ‘as is', compared to oral antibiotics which are excreted in urine or egested in faeces as less active or inactive by-products. The introduction of topical antibiotics directly to waste water is a major contributor to antimicrobial resistance globally.
Special scenarios
Bacterial superinfection
As mentioned, S. Aureus is a significant factor in the pathogenesis and flares of AD. While patients with AD are almost always colonised with S. aureus, some patients will develop bacterial superinfection. This manifests as weeping of
clear or purulent fluid, honeycoloured crust, folliculitis, abscess, or cellulitis (Image 5). If there is evidence of bacterial superinfection then oral antibiotics should be prescribed, guided by microbiological cultures (predominantly for resistance information) and local antimicrobial practices. These should be prescribed in conjunction with topical antiseptic measures, which should be instituted as a preventative measure.
Eczema herpeticum
Eczema herpeticum occurs due to infection with herpes simplex virus, and is more common in children. It manifests as clusters of painful and itchy blisters which evolve into punched-out erosions, sometimes
33
Image 4
Full prescribing information is available on www.medicines.ie Legal category: POM Further information is available from: LEO Pharma, Cashel Road, Dublin 12, Ireland. E-mail: medical-info.ie@leo-pharma.com Marketing authorisation number and holder: EU/1/21/1554/002 This
Date of preparation: May 2023 IE/MAT-65962 Reporting of Suspected Adverse Reactions Adverse events should be reported. Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
Image 5
is a promotional advertisement from LEO Pharma intended for IE healthcare professionals.
associated with fever (Image 6). Patients can have inactive AD, so it does not represent a sign of treatment failure. It is helpful to take viral PCR swabs to confirm the diagnosis. Treatment should be immediately started with aciclovir. Oral acyclovir/ valaciclovir is acceptable unless there is concern for ophthalmic or central nervous involvement, or if the patient is unwell. Patients with recurrent eczema herpeticum should consider prophylactic antiviral treatment.
Eczema coxsackium
Eczema coxsackium is a recently described entity caused by coxsackie A6 or A16 in patients
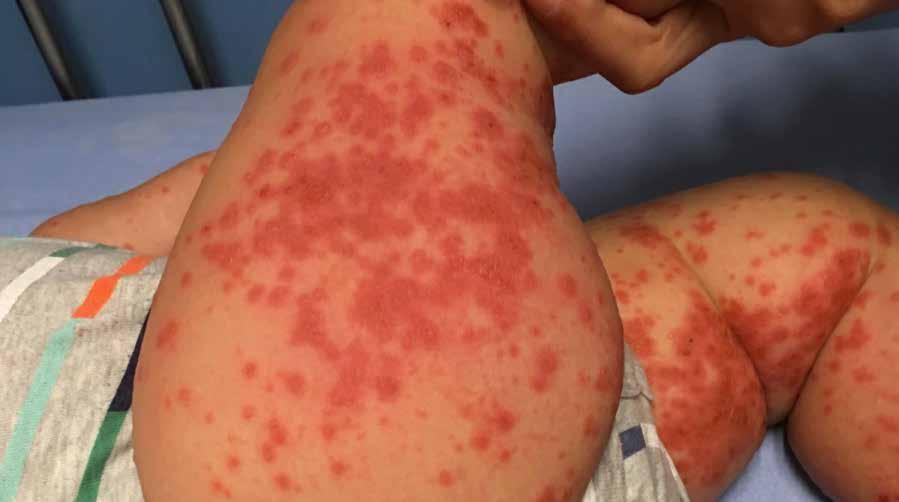

with AD. Clusters usually occur in springtime. It looks very similar to eczema herpeticum but it is not itchy and there is a slightly purpuric hue to the background skin (Image 7). Respiratory viral panels can be sent to confirm enterovirus infection as the PCR testing is difficult to access. Given the similarities to eczema herpeticum it is very reasonable to start treatment with acyclovir or valaciclovir but enteroviruses do not express viral thymidine kinase and therefore do not respond to antiviral treatment.
What NOT to do
Patients with AD should NOT be told to use topical steroids
‘sparingly’ or ‘thinly’ and should be advised to treat for an adequate duration. The consequences of undertreated AD are huge, and the terrible impact on QOL is unnecessary given the safety and efficacy of topical steroids. Patients with AD should NOT be treated with oral steroids (outside of expert centres with a therapeutic exit strategy in place) due to the severe side effects and lack of disease modification. Patients will improve rapidly within a few days of high dose oral steroids but will flare even worse once withdrawn. Given that we now have effective targeted treatments for AD in specialist clinics there is no excuse for
Image
inappropriate prescriptions of oral steroids. Patients with AD should NOT be told to change their washing powder or reduce/ eliminate foods from their diet. AD is absolutely not caused by detergent! In children who have co-morbid AD and food allergy, if an allergenic food is removed from the diet it will not affect the AD but will simply stop immediate IgE-mediated allergic reactions from happening.
When to refer
Patients with persistent or severe AD despite the use of appropriate potent topical therapy should be referred to dermatology for consideration of advanced therapies. Use of 1% hydrocortisone for a few days does not represent a trial of topical steroids: an adequately potent steroid should be prescribed for at least two weeks to assess response. Dermatologists now have access to dupilumab, a biologic drug targeting IL-4 and IL13 which can be life-changing for patients who have lived with severe itch for years or even decades. In addition, other biologic drugs such as tralokinumab (IL-13 inhibitor) and oral janus kinase (JAK) inhibitors such as upadacitinib or abrocitinib are also available. JAK inhibitors have a more rapid onset of action and quickly reduce itch, but there are still some concerns about longterm use, based on studies in other conditions such as rheumatoid arthritis, with patients who represent a very different population to those with AD. Topical JAK inhibitors have been licensed in some countries with significant benefit seen in AD, although cost is currently prohibitive.
Overall it is a very positive time for dermatologists and patients with AD, with effective treatments already available and many more in the pipeline.
34 CPD 100: ATOPIC DERMATITIS
6
Full prescribing information is available on www.medicines.ie Legal category: POM Further information is available from: LEO Pharma, Cashel Road, Dublin 12, Ireland. E-mail: medical-info.ie@leo-pharma.com Marketing authorisation number and holder: EU/1/21/1554/002 This is a promotional advertisement from LEO Pharma intended for IE healthcare professionals. Date of preparation: May 2023 IE/MAT-65962 Reporting of Suspected Adverse Reactions Adverse events should be reported. Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
Image 7
For the treatment of moderate to severe atopic dermatitis in adult patients who are candidates for systemic therapy1
TIME TO PRESS PLAY
The

Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe

Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.ie) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-tosevere atopic dermatitis in adult patients who are candidates for systemic therapy. Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 18 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should be not shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection sites. It is recommended to rotate the
IL, interleukin.

References: 1. Adtralza® SPC. 2. Bieber T. Allergy 2020;75:54–62.
® Registered trademark
injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology. Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: June 2021
Reference number: REF-19238(1)
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie

Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
Date of preparation: February 2022
NEW
is a promotional advertisement from LEO Pharma for IE healthcare professionals. Learn more at www.adtralza.ie
Adtralza®
This
Introducing
first licensed biologic that inhibits IL-13 alone, 1 a
of atopic dermatitis
and symptoms.2
key driver
signs
IE/MAT-54463 V1
information can be found
or
12, Ireland.
Further
in the Summary of Product Characteristics
from: LEO Pharma, Cashel Road, Dublin
E-mail: medical-info.ie@leo-pharma.com
Not an actual patient. For illustrative purposes only.
Discussing reproductive potential with transgender patients in Dermatology clinics

Dear Editor, Transgender people are individuals whose gender identity or gender expression is different from the gender they are assigned at birth. As of 2011, an estimated 0.3% of adults identified as transgender in the United States.1 This is likely reflected globally. Transgender people have their own set of complex medical issues, chief among them are a subset of dermatological issues. As such, as dermatologists, it is important that we not only recognise the specific dermatological needs of these patients, but also the nuances in their treatment and are competent in discussing these issues using appropriate terminology that puts our patients at ease.
We are seeing increasing numbers of patients in our dermatology clinics that identify as Transgender. Some of these patients have had gender affirming procedures. The use of teratogenic medications, while common place in dermatology, can create a nuanced issue when treating patients who identify as transgender, particularly trans-masculine patients (assigned Female at birth, identifies as Male). Discussions regarding reproductive potential have the capacity to enhance gender dysphoria and have previously been identified as

triggering for patients.2
The use of neutral and inclusive terminology is important in creating an environment that is culturally welcoming to all. Making assumptions of gender identity, choice of pronoun or sexual identity can damage the rapport between the clinician and patient and should be avoided.1 It is important that providers use patient centred language, including their name and chosen pronouns, as well as their terms for their sexual orientation, gender identity, sexual behaviour and anatomy.3
A recent article in Pediatric Dermatology recommends at initial and subsequent clinic visits, an open discussion regarding the patients anatomy and pregnancy potential, as well as whether their current or future sexual practices could result in pregnancy ( i.e receptive vaginal penile intercourse), is necessary. This allows providers guide patients on appropriate methods of pregnancy prevention.4 It has also been recommended that counselling regarding pregnancy prevention should focus on the reproductive potential of the patient as opposed to their gender assigned at birth.2
An organ inventory has been recommended. This is a helpful
tool for clinicians to identify patients of reproductive potential, however may also serve as a platform to discuss the need for regular pregnancy testing and/ or contraception. It has been advised to avoid words such as ‘breast’, ‘vagina’ or ‘penis’ and instead use words such as ‘chest’ and ‘genitalia’ to avoid triggering gender dysphoria.3 Transmasculine patients, assigned female at birth, who have a functioning uterus and ovaries, should still be considered as having reproductive potential, even if they are currently receiving testosterone therapy and are currently amenorrhoeic. However transmasculine patients who have undergone hysterectomy and/ or bilateral oophorectomy would not have reproductive potential. It is noteworthy, that the need for regular pregnancy tests may lead to increased gender dysphoria, particularly among transmasculine patients and this may need to be taken into consideration when discussing certain therapeutic
options with patients, such as isotretinoin, where monthly pregnancy tests are required.

Bibliography
1. Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: A systematic review of the literature. J Am Acad Dermatol. 2019;81(2):43847.

2. Roche D, Murray G, Connolly M, Tobin AM. Transgender issues in dermatology. Clin Exp Dermatol. 2021;46(6):1137-8.
3. Radi R, Gold S, Acosta JP, Barron J, Yeung H. Treating Acne in Transgender Persons Receiving Testosterone: A Practical Guide. Am J Clin Dermatol. 2022;23(2):219-29.
4. Hollingshead N, Hodax JK, Boos MD. Management of acne in transgender and gender diverse youth Part 2: Unique considerations and strategies in medical treatment. Pediatr Dermatol. 2022.
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 36 DERMATOLOGY
Claire Quigley
Written by Claire Quigley1, Eoin.R.Storan1, 2
1Mater Misercordiae University Hospital - 2Chales Institute of Dermatology, UCD
Eoin.R.Storan
Review and Comparison of Body Sites Among Patients with Cutaneous Malignant Melanoma: An Observational Study

 Written by Anna Wolinska,1 Stephanie Bowe,1 Gregg Murray,1 Sinead
Murad1-2
Written by Anna Wolinska,1 Stephanie Bowe,1 Gregg Murray,1 Sinead
Murad1-2
1Department of Dermatology, Our Lady of Lourdes Hospital, Drogheda, Ireland - 2UCD

The incidence of cutaneous malignant melanoma (MM) is increasing worldwide.1-2 Regular self-skin examination can assist in the early detection of MM. However, identifying new or changing lesions on non-visible body sites (NVBS) can be difficult. Often patients are unaware of lesions on NVBS which are identified incidentally during total body examination (TBE) by a Dermatologist. Research investigating MM on NVBS is limited.3 The aim of this study is to compare features of MM on NVBS to visible body sites (VBS) to add data to this patient cohort.
We performed a retrospective chart review identifying patients diagnosed with MM in our institution from 2019–2021. Patients were identified through multidisciplinary meeting records with data drawn from electronic reports and photography.
Clinicopathological features were extracted, analysed, and delineated by year. NVBS was defined as areas including the posterior scalp, ears and neck, posterior shoulder, back, buttocks, posterior thigh, and plantar aspect of the feet. All other sites were considered VBS. Statistical analysis was performed using SPSS software.
A total of 162 patients were included in this study and 45% (n=73) were male. Patients with MM on NVBS were significantly younger (median age=59 years) compared to patients with MM on VBS (median age=66.5 years), (p<0.05). Thirty-seven percent (n=60) of lesions were found on NVBS. Twenty-eight percent (n=17) of MMs on NVBS were identified incidentally on TBE in clinic by a Dermatologist. There was a significantly higher number of patients with melanoma-in-situ on VBS (n=44, 27.1%) compared to NVBS (n=17, 10.6%), (p<0.01). A higher proportion of patients with MM on NVBS had a Breslow thickness (BT) >1mm (n=23,
Anna Wolinska
38.3%), compared to VBS (n=30, 29.4%). Table 1. Fifty-two percent (n=12) of thicker tumours (range: BT 1.1-12mm) were located on the back. Figure 1.
This study has demonstrated that the number of MM diagnosed incidentally following TBE was higher than previously reported by Moran et al.4 Our study focused on a MM specific cohort who potentially may have had lethal skin cancers missed in 28% of patients if TBE was not carried out. Our findings also show that patients are more likely to have non-invasive tumours detected on VBS but more advanced disease on NVBS, further emphasising the importance of TBE. Furthermore, younger patients are more likely to present with MM on NVBS. Although lesion directed assessments such as teledermatology are beneficial to reduce waiting times and allow quicker access for patients, this study suggests that a different strategy may be recommended for high-risk patients who should be enrolled in surveillance programs that encourage regular self-skin examination and periodic TBE by a Consultant Dermatologist. Notably, total body photography (TBP) has been utilised in highrisk populations with limited access to Dermatology centres and has been associated with improved melanoma outcomes through earlier detection of thinner tumours.5-6
This study was limited by its retrospective design on a small number of patients from a single institution. Patient referral rate and attendance may be affected by the COVID-19 pandemic.7
We recommend an increased emphasis on patient education in patients of all ages on the importance of self-examination which, when complemented by physician directed TBE, plays a valuable role in the early detection of MM, particularly on NVBS. This study provides some guidance in designing pathways to target highrisk groups to avoid delays in the diagnosis of MM. Further research to explore trends of MM on NVBS may enable targeted education campaigns in the future.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
Figure 1: Location of MM on NVBS with BT>1mm
DERMATOLOGY: MELANOMA
37
Collins,1 Cliona Feighery,1 Muireann Roche,1 Aizuri
School of Medicine, Dublin, Ireland
Table 1: Clinicopathological features of patients diagnosed with MM 2019 – 2021
5 Table
2019 – 2021 Year 2019 2020 2021 Total Cases (n) 52 61 49 Total Non-Visible Body Sites (n) 23 (44.2%) 15 (65.2%) 8 (34.7%) 56 10 (43.4%) 6 (26%) 7 (30%) 22 (36.1%) 7 (31.8%) 15 (68.1%) 52 3 (13.6%) 10 (45.4%) 9 (40.9%) 15 (30.6%) 8 (53.3%) 7 (46.6%) 61 4 (26.6%) 4 (26.6%) 7 (46.6%) Male (n) Female (n) Median age (years) Breslow Thickness In-situ <1mm >1mm Total Visible Body Sites (n) 29 (55.7%) 8 (27.5%) 21 (72.4%) 72 13 (44.8%) 11 (37.9%) 5 (17.2%) 39 (63.9%) 18 (46.1%) 21 (53.8%) 65 20 (51.2%) 8 (20.5%) 11 (28.2%) 34 (69.3%) 17 (50%) 17 (50%) 67 12 (35.2%) 8 (23.5%) 14 (41.1%) Male (n) Female (n) Median age (years) Breslow Thickness In-situ <1mm >1mm 8.6% - Posterior Upper Limb 17.3% - Posterior thigh 4.3% - Plantar aspect of foot 17.3% - Scalp and Neck 52.1% - Back
Figure 1: Location of MM on NVBS with BT>1mm
1: Clinicopathological features of patients diagnosed with MM
DERMATOLOGY: PATIENT FOLLOW-UP
Patient Initiated Follow-Up in an Irish Dermatology Department: A Pilot Study
 Written
Anna Wolinska,1
Written
Anna Wolinska,1
‘did not attend’ (DNA) in 2021 was 11% (n=823/7093).
Patient initiated follow up (PIFU) is an alternative approach to a traditional, fixed appointment-based system that offers a flexible review for patients when needed. It has successfully been implemented across a range of speciality departments worldwide.1,2 In 2022, the Health Service Executive (HSE) in Ireland announced reforms to introduce ‘Patient Initiated Review’ across the organisation as part of a nationwide strategy to tackle long patient waiting lists.3 Here, we will present our experience of PIFU in an Irish Dermatology Department.
This was a single-centre, prospective pilot study identifying patients who were offered PIFU in a Dermatology department in Ireland between April – June 2021.
Adult and paediatric dermatology patients were identified through electronic clinic reports and data drawn from clinical charts and records. Patients were offered ‘flexible’ review within one year of the original presentation pertaining to the same clinical condition. Patient demographics and clinical

details were extracted and analysed, and patients followed for a 12-month period.
A total of 47 patients were identified, 57% (n=27) were female and median age was 23 years (range; 0-83). Clinical diagnoses included: acne (n=13/47), eczema (n=8/47), infection (n=5/47), non-specific rash (n=4/47), non-melanoma skin cancer (n=3/47), urticaria (n=2/47), hidradenitis suppurativa (n=2/47), actinic keratosis (n=2/47), guttate psoriasis (n=2/47), vasculitis (n=2/47) and other (n=4/47).
Twenty-nine percent (n=14/47) of patients activated PIFU within a 12-month period. Median time until PIFU was 213 days (range; 28-413 days). Forty-two percent (n=6/14) of patients had acne, 28.5% (n=4/14) eczema, 14.2% (n=2/14) actinic keratosis 7% (n=1/14), urticaria and 7% (n=1/14) guttate psoriasis. Seventy percent (n=33) had no further episodes of care during the study interval. The background rate of patients who
World Congress of Dermatology
Previous research has demonstrated the advantage of a flexible approach to follow-up for patients with chronic conditions who require lifelong management in speciality clinics4. Our findings support the view that PIFU has a role in Dermatology for patients with conditions that display periods of quiescence such as that seen in inflammatory dermatoses. Early review allows for appropriate escalation of treatment to avoid potential complications such as infection, hospitalisation, and reduced quality of life.
This study also shows that PIFU can have a positive impact on patient DNA rates. In 2021, the departmental DNA rate was lower than the estimated annual national rate of 13%4. This study demonstrates that PIFU has a useful role in reducing unnecessary attendance of well-controlled patients at OPD clinics, thus reducing the potential for non-attendance.
Study limitations include a small patient number attending a single institution. Our findings may be affected by the HSE Cyber-attack in May 2021 and the COVID-19 pandemic.
This study suggests PIFU is a useful strategy for the management of patients with conditions that display periods of remission to allow for prompt review by a
The 25th World Congress of Dermatology takes place from 3rd – 8th July, 2o23 in Singapore. The World Congress of Dermatology (WCD) is one of the largest international dermatological conferences. It is organised under the auspices of the International League of Dermatological Societies (ILDS).

Dermatologist in the event of an acute deterioration. Further larger studies focusing on client and resource benefits of PIFU can guide speciality specific policies for its use across the HSE in the future.
References
1 Balhorn J, Su’a B, Jin J et al. Changing the routine: a move to patient initiated follow up to improve surgical outpatient clinic. ANZ Journal of Surgery. 2022;92(6):1394-1400.
2 Newton C, Nordin A, Rolland P et al. British Gynaecological Cancer Society recommendations and guidance on patient-initiated follow-up (PIFU). International Journal of Gynecologic Cancer. 2020;30(5):695-700.
3 The Department of Health, Health Service Executive Ireland. 2022 Waiting List Action Plan [Internet]. Hse.ie. 2022 [cited 6 September 2022]. Available from: https://www.hse.ie/eng/ about/who/board-members/ board-meetings/february2022/4-0-1-department-ofhealth-waiting-list-action-planpdf.pdf
4 Khoury L, Møller T, Zachariae C, Skov L. A prospective 52week randomized controlled trial of patient-initiated care consultations for patients with psoriasis. British Journal of Dermatology. 2018.
The first WCD was held in 1889 in Paris. It is now held every 4 years, the most recent one was the 24th WCD, that was staged in June 2019 in Milan, Italy. The WCD aims to promote the science and practice of dermatology, by providing a platform for specialists from around the world to showcase new knowledge, exchange clinical experiences and scientific research, and to build professional and personal networks and collaborations. Over the past 130 years, the WCD has traversed the globe. It has brought together dermatologists and professional societies and has raised the stature and importance of skin health and diseases that affect humankind. Visit www.wcd2023singapore.org for further details.
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 38
Anna Wolinska
by
Gregg Murray,1 Stephanie Bowe,1 Cliona Feighery,1 and Sinead Collins1
1Department of Dermatology, Our Lady of Lourdes Hospital, County Louth, Drogheda, Ireland
DERMATOLOGY: MELANOMA Melanoma of Unknown Primary: A Case Series
Written by Claire Doyle1, Barry O’Sullivan2, Richard E Watchorn1, Karen Eustace1
1Department of Dermatology, Beaumont Hospital, Beaumont Road, Dublin 9, Ireland
2Department of Plastic Surgery, Beaumont Hospital, Beaumont Road, Dublin 9, Ireland

Melanoma of unknown primary (MUP) was originally defined in 1963 by Dasgupta et al. as melanoma in the subcutaneous tissue, lymph nodes or visceral organs without the presence of cutaneous, ocular or mucosal primary.1 The incidence of MUP is reported as between 1 and 8% of all melanomas.2 The median age of presentation of MUP is between 40 and 50 years of age.3 The male to female ratio is approximately 2:1.4 Sixty per cent of MUP presents in the lymph nodes - most frequently axillary lymph nodes, followed by cervical, inguinal and parotid lymph nodes.4 Other sites of presentation include the subcutaneous tissue, lung, gastrointestinal tract and brain.5 The American Joint Committee on Cancer (AJCC) classifies MUP presenting in a lymph node or subcutaneous tissue as stage three and MUP presenting within a visceral organ as stage four.4
Case series
We report our experience in a single tertiary referral centre
in Ireland. A database of all melanomas diagnosed between January 2018 and December 2021 was analysed for MUP. The total number of melanomas diagnosed in that timeframe was 298. Six patients were identified as having MUP with an incidence of 2.01%. The demographics of the patients are detailed in Table 1.
The median age was 63.3 years (range 45–84 years). Fifty per cent (3/6) were male. Fifty per cent (3/6) were female. 16.7% (1/6) presented with a primary dermal metastatic deposit, 66.67% (4/6) presented with isolated lymph node metastases, 0% (0/6) presented with visceral metastases and 16.7% (1/6) presented with isolated brain metastases. The details of the speciality involvement with the patients are detailed in Table 2.
CT to investigate for further metastases. One hundred per cent (6/6) underwent dedicated brain imaging via CT and MRI. One hundred per cent (6/6) underwent surgical resection of their MUP. One hundred per cent (6/6) were reviewed by medical oncology with 83.3% (5/6) commencing treatment with immunotherapy. There were no associated deaths to date. 83.3% (5/6) of the MUP was diagnosed in 2021. 16.67% (1/6) was diagnosed in 2018.
Discussion
Demographics and clinical characteristics
 Claire Doyle
Claire Doyle
Table 1 Demographics and clinical characteristics
One hundred per cent (6/6) were reviewed by dermatology and ophthalmology. Fifty per cent (3/6) were reviewed by ENT. 16.67% (1/6) was referred to gynaecology. 16.67% (1/6) underwent endoscopic assessment for a primary gastrointestinal melanoma. No primary melanoma was identified in any of the patients by any specialist. One hundred per cent (6/6) underwent radiological evaluation via PET-
presentation when compared with a similar period pre-COVID-19.7 Our data indicates an increased rate of MUP presenting after the onset of the COVID-19 pandemic; however, given the low number overall, no conclusions can be drawn. There is no current literature regarding the increased rate of MUP since the COVID-19 pandemic. Further studies may be required to investigate this observation. MUP is rare. Guidelines for the investigation of MUP are currently lacking and are needed to ensure the delivery of consistent evidence-based, care for patients.
Table 1 Demographics and clinical characteristics
The aetiology of MUP is elusive. Possibilities preferred include an unrecognised melanoma, a previously excised melanoma that was misdiagnosed as benign, a primary melanoma that has completely regressed or the de novo malignant transformation of an aberrant melanocyte within a lymph node.4 Recommendations for the evaluation of those with MUP include a full skin examination including subungual and mucosal surfaces by a dermatologist and an ocular examination to exclude primary melanoma. Patients should undergo imaging of the brain, thorax, abdomen and pelvis to assess disease burden. Referral to ENT can be considered to assess for mucosal melanoma of the nasopharynx. Gynaecology referral can be considered for those with inguinal lymphadenopathy.6 Recent studies have shown the impact of the COVID-19 pandemic on the presentation of cutaneous melanoma including an increased Breslow thickness at the time of
1. aWhere available, excluding unknown primary lesions
© The Author(s), under exclusive licence to Royal Academy of Medicine in Ireland 2022
References available on request
Table 2 AJCC clinical stage at initial diagnosis developed metastatic disease
Table 2 AJCC clinical stage at initial diagnosis of patients who developed metastatic disease
From: Metastatic melanoma in the MidWest of Ireland: a retrospective review
From: Metastatic melanoma in the Mid-West of Ireland: a retrospective review
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
39
Table 1 Demographics and clinical characteristics From: Metastatic melanoma in the MidWest of Ireland: a retrospective review
From: Metastatic melanoma in the Mid-West of Ireland: a retrospective review Total patients with metastasis 28 Sex, male (%) 20 (71) Age, years (range) 64.7 (24–90) Site of primary melanoma Number (%) Head and neck 10 (34) Upper limb 4 (14) Lower limb, number 7 (28) Torso, number 3 (10) Unknown primary melanoma 4 (14) Primary melanoma histological characteristicsa Mean Breslow depth, mm (range) 5.5 (0–20) Median Breslow depth, mm 4.3 Ulceration present (%) 7 (25) Mitotic rate, per mm2 (range) 3.6 (0–18) Regression identified (%) 1 (3.5) 1
Metastatic melanoma in the Mid-West of Ireland: a retrospective review patients with metastasis 28 (%) 20 (71) (range) 64.7 (24–90) primary melanoma Number (%) neck 10 (34) 4 (14) number 7 (28) number 3 (10) primary melanoma 4 (14) melanoma histological characteristicsa Breslow depth, mm (range) 5.5 (0–20) Breslow depth, mm 4.3 present (%) 7 (25) rate, per mm2 (range) 3.6 (0–18) identified (%) 1 (3.5)
From: Metastatic melanoma in the Mid-West of Ireland: a retrospective review Total patients with metastasis 28 Sex, male (%) 20 (71) Age, years (range) 64.7 (24–90) Site of primary melanoma Number (%) Head and neck 10 (34) Upper limb 4 (14) Lower limb, number 7 (28) Torso, number 3 (10) Unknown primary melanoma 4 (14) Primary melanoma histological characteristicsa Mean Breslow depth, mm (range) 5.5 (0–20) Median Breslow depth, mm 4.3 Ulceration present (%) 7 (25) Mitotic rate, per mm2 (range) 3.6 (0–18) Regression identified (%) 1 (3.5)
lesions
1. aWhere available, excluding unknown primary
Stage Number of patients (%) Mean time to detection of metastasis after initial investigations, years IV 13 (46%)III (A, B, C) 5 (17%) 1.9 II (A, B, C) 7 (24%) 2.3 I 3 (10%) 6.2
DERMATOLOGY: DERMATITIS
Biologic treatment for atopic dermatitis: new therapies changing the treatment landscape

Atopic dermatitis (AD) is the most common chronic inflammatory skin disease, affecting up to 25% of the paediatric population and up to 7% of adults. The pathophysiology is characterised by epidermal barrier dysfunction and Th2 cytokine mediated inflammation. Moderate to severe AD can significantly affect both the physical and psychological wellbeing of patients, and is associated with anxiety, depression, and sleep disorders. When severe and/or when topical management is ineffective in achieving disease control, until recently practice has been limited to introduction of phototherapy or conventional immunosuppressive therapy. Traditionally used immunosuppressant agents for AD can include ciclosporin, methotrexate, azathioprine and mycophenolate mofetil. Beyond the impact of disease itself on quality of life, there is significant burden associated with treatments - the need for regular topical regimes, financial implications, frequent hospital or clinic visits, potential for side effects and monitoring all play a role in patients’ quality of life.
In recent years, the availability of biologic agents for atopic dermatitis has changed the treatment landscape for those

with severe disease. Dupilumab, a monoclonal antibody blocking interleukin 4 and interleukin 13 via their shared IL-4α subunit, has demonstrated clinical efficacy and safety in treating moderate-tosevere atopic dermatitis. This was approved by the Food and Drug Administration (FDA) in 2017, by the European Medicines Agency (EMA) in 2019, and was first approved for reimbursement by the HSE in 2021 for the treatment of moderate-to-severe AD in those who have failed systemic treatment or for whom it is contraindicated. Tralokinumab, targeting interleukin 13, is another recent addition, with clinical efficacy and tolerability demonstrated in clinical trials. In Ireland, biologic medications for moderate to severe AD may only be prescribed by consultant dermatologists who have agreed to the terms of the relevant access protocol, and require the submission of detailed applications to the HSE Medicines Management Programme for consideration of reimbursement. Clinical trials of dupilumab have shown significant improvement in AD signs and symptoms, including itch, sleep disturbances, anxiety, and depression. Phase III clinical trials demonstrated that dupilumab-treated patients experienced significant
improvement in symptoms and quality of life measures. Patients reported significant improvements in pruritus, sleep disturbance, anxiety, and depression compared to placebo-treated patients. Long-term open-label studies have also confirmed the effectiveness, safety, and tolerability of dupilumab up to four years. In a study published in the British Journal of Dermatology, patients treated with dupilumab reported significant improvements in quality of life and mental health measures. The study found that patients experienced significant reductions in anxiety, depression, and sleep disturbance, which corresponded to their clinical response to dupilumab treatment. A Danish study found excellent long-term efficacy with 2-year drug survival at 86%, though importantly highlighted the prevalence of conjunctivitis in 25% of patients, and difficulty treating head-andneck atopic dermatitis as well as facial erythema secondary to dupilumab treatment.
Oral janus kinase (JAK) inhibitors such as upadacitinib and abrocitinib are other recent additions to treatment options for moderate-to-severe AD in adults who are candidates for systemic therapy. Upadacitinib, a novel selective JAK1 inhibitor, was approved by the FDA in 2022 and EMA in 2021. Like dupilumab, upadacitinib is licensed for patients with severe AD who failed to respond or are intolerant of conventional systemic treatment and has demonstrated efficacy in improvement of AD severity as well as itch. A head to head trial comparing efficacy and safety of dupilumab and upadacitinib found that improvement with upadacitinib was greater and more rapid in onset, though with increased incidence of serious infection, viral infection including herpes zoster, eczema herpeticum, and laboratory-related adverse events. Conjunctivitis and injection-site reactions were more frequent in those receiving dupilumab.
While these studies have shown promising clinical findings, there is still more to be explored
regarding the long-term psychological effects of treatment with monoclonal antibodies and JAK inhibitors on patients with AD. An international qualitative study evaluated both patient and healthcare provider perspectives on treatment with dupilumab, finding that patients describe dramatic improvements in quality of life, though increased choice and flexibility is sought particularly for a significant minority who experience ocular side effects that may prompt treatment cessation A recent Italian study evaluated the psychological outcomes for patients treated with dupilumab for up to 3 years. Excellent clinical improvements were observed, however there was variability in psychological outcomes irrespective of clinical response noted for certain patient subgroups. Patients who developed atopic dermatitis in childhood were more likely to subjectively report improved psychological outcomes, but younger patients (those under age 30) who developed atopic dermatitis after age 19, particularly females, were less likely to perceive improved psychological benefits even with positive clinical improvement of eczema. These findings support the fostering of a biopsychosocial approach for these patients. Further research is needed to better understand the impact of treatment with biologics and JAK inhibitors on psychological outcomes and to identify factors that may influence treatment response.
As more therapies become available and longer-term studies further inform our practice, the treatment landscape for those with moderate and severe AD continues to evolve. As was experienced with emergence of biologics for psoriasis, the advent of these new therapies marks an exciting turning point for treatment of our patients with severe AD beyond the limits of conventional immunosuppression. References available on request
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 40
Written by Dr Emma Porter, Dermatology Registrar, South Infirmary Victoria University Hospital
DERMATOLOGY: PHOTOPROTECTION
Sunscreens: Exploring the Spectrum
Exposure to solar radiation is hazardous for human health and causes skin cancer, photodermatoses and eye disorders. The main health benefit of ultraviolet radiation (UVR) is vitamin D synthesis, however oral supplementation is a safer alternative. Solar radiation reaching the earth’s surface contains UVB (280-315nm), UVA (315-400nm), visible light (VL: 400-780nm), and infrared radiation (780nm-1mm). UVR accounts for almost 8% of solar radiation (5% UVB, 95% UVA). The light dose received is influenced by altitude, latitude, time of day, weather, season and reflection from surrounding surfaces.
UVR is a class I carcinogen and UV-signature mutations are found in basal cell carcinoma, squamous cell carcinoma and melanoma.1 Immunosuppression significantly increases the risk of cutaneous malignancy. This affects a broad range of patients including those with solid organ transplants, iatrogenic immunosuppression for autoimmune diseases, haematological malignancies and HIV. As such, it is incumbent on all clinicians treating these patients to have an understanding of photoprotection and counsel them appropriately.
Ultraviolet light
UVB consists of higher energy, shorter wavelengths which penetrate the epidermis where they induce sunburn and direct DNA damage.2, 3 Delayed tanning is usually due to UVB exposure. UVB experiences greater atmospheric filtering than UVA due to its shorter wavelengths and varies with the solar zenith angle. In Ireland, UVB levels are negligible during the Winter months. Sun protection factor (SPF) describes a ratio whereby a sunscreen increases the time to burning during sun exposure. As this is a ratio, the difference between SPF 30 and SPF 50 is minimal (97% versus 98% UVB protection).
UVA penetrates to the dermis and causes indirect DNA damage through oxidative stress.2 Ambient UVA does not cause sunburn, although high doses from sunbeds or phototherapy can induce burning. UVA is implicated in the pathogenesis of photoageing and photosensitive dermatoses including PLE and cutaneous
lupus. Compared to UVB, UVA exhibits less variation with latitude and is present year round. There are a number of methods to measure the UVA filtering ability of sunscreens. The Boots star rating system measures UVA protection relative to UVB protection, i.e. 5 stars denotes a 90% UVA-filtering efficacy relative to the SPF. The UVA seal denotes UVA protection that is one third that of the SPF.
Visible light
Approximately half of sunlight is in the visible range. While not considered carcinogenic, VL induces oxidative stress and upregulates metalloproteinases.4 VL causes hyperpigmentation, especially in darker phototypes.
Sunscreens
Organic (chemical) filters absorb UVR and appear transparent, but can cause irritancy. The inorganic (physical) filters zinc oxide and titanium dioxide absorb, reflect and scatter UVR, however can leave a white cast due to the reflection of visible light. Nanosized particles are more cosmetically acceptable, however they are less effective against longer wavelengths. Sunscreens are regulated as cosmetics by the European Commission, whereas they are regulated as over-thecounter drugs by the FDA. As a result, Europe has access to a broader range of UV filters. Phenylene bis-diphenyltriazine (TriAsorB) is a recently approved broad-spectrum filter which is effective across UVB, UVA and blue light wavelengths.5
Tinted sunscreens protect against VL and contain mixtures of iron oxides in different shades to match a variety of skin tones.6 They have been shown to improve melasma when combined with topical hydroquinone.7, 8

Endogenous antioxidants scavenge free radicals induced by oxidative stress. There is evidence that topical vitamin C and E increase the efficacy of broad-spectrum sunscreen, however there is no standardized way to measure their biological effects.9 Polypodium leucotomos extract has been reported to reduce susceptibility to sunburn when taken orally.10 There is currently insufficient evidence to recommend oral antioxidants in photoprotection.
Photoprotection advice
Sunscreen should be used as a last resort after the following measures. All patients should be counselled to wear photoprotective clothing with a tight weave, wear a broadbrimmed hat, seek shade and avoid sun exposure 2 hours either side of solar noon. Sunglasses with UV-protective lenses should also be worn.
An appropriate broad-spectrum sunscreen with SPF ≥ 30 and a high level of UVA protection should be chosen. Children should use paediatric sunscreens with inorganic filters and these can be used from 6 months of age. Sunscreen should be applied to all exposed skin. It should be re-applied every 2 hours or after heavy sweating or swimming. One ounce of sunscreen (approximately a shot glass) is required to cover the whole body. A teaspoon is required to cover the head and neck.
Personalized photoprotection advice should take into account Fitzpatrick skin type (FST), geography, lifestyle, atopic dermatitis, acne-prone skin, rosacea, hyperpigmentation, and photosensitivity.11

Challenges
Despite public health initiatives to improve awareness of sunscreens and sun-safe behaviours, there remains a significant amount of misinformation. A recent survey of over 1000 people in the US found that 62% rated themselves as good or excellent at sun protection, despite 63% reporting a tan and 33% reporting sunburn.12 An Irish survey of 178
immunosuppressed patients with inflammatory bowel disease reported that only 70% wore sunscreen and many did not reapply, wear a hat or seek shade.13 Men are less likely than women to wear sunscreen. Factors that may influence this are that the signs of ageing occur later in men, the increased numbers of sebaceous glands make skin more oily and shaving predisposes facial skin to irritation.14 Photoprotection advice and sunscreen recommendations should be tailored to men with these considerations in mind. While claims about photoprotection are regulated, sunscreens often contain potential allergens and irritants such as fragrances and preservatives. A negative experience can deter patients from wearing sunscreen. Navigating the ingredient list effectively can help identify products unsuitable for sensitive skin, however this requires familiarity with the international nomenclature of cosmetic ingredients (INCI).
In conclusion, our daily interactions with sunlight can have lasting effects in relation to skin cancer, photoageing and dyspigmentation. Reliable information from healthcare professionals is key, especially for the immunosuppressed population. Sunscreen is an imperfect defence against solar radiation and should be used appropriately after implementing other more effective photoprotective measures.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
41
Written by Dr Rebecca Hellen Dr Hellen is a final year Dermatology Specialist Registrar working in St. Vincent’s University Hospital, Dublin
for the right patient, at the right time

• Long-term safety and efficacy profile spanning 5 years in psoriasis (PsO) and psoriatic arthritis (PsA)1,2


• Improved quality of life sustained up to 5 years1,2
• No laboratory pre-screening or ongoing drug-specific monitoring1
• No label warning against use with live vaccines1
• 9-hour half-life, rapid clearance1
OTEZLA® (apremilast) 10mg, 20mg and 30mg film coated-tablets Brief Prescribing Information Refer to the Summary of Product Characteristics (SPC) before prescribing Further information is available upon request
Presentation: 10mg, 20mg and 30mg film coated-tablets.
Indications: Psoriatic arthritis: OTEZLA, alone or in combination with Disease Modifying Antirheumatic Drugs (DMARDs), is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior DMARD therapy. Psoriasis: OTEZLA is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who failed to respond to or who have a contraindication to, or are intolerant to other systemic therapy including ciclosporine, methotrexate or psoralen and ultraviolet-A light (PUVA).



Dosage and administration: Treatment with OTEZLA should be initiated by specialists experienced in the diagnosis and treatment of psoriasis or psoriatic arthritis. The recommended dose of OTEZLA is 30mg twice daily taken orally in the AM and PM, approximately 12 hours apart, with no food restrictions. The film-coated tablets should be swallowed whole. An initial dose titration is required per the following schedule: Day 1: 10mg in the AM; Day 2: 10mg in the AM and 10 mg in the PM; Day 3: 10mg in the AM and 20mg in the PM; Day 4: 20mg in the AM and 20mg
in the PM; Day 5: 20mg in the AM and 30mg in the PM; Day 6 and thereafter: 30mg twice daily in the AM and PM. No re-titration is required after initial titration. If patients miss a dose, the next dose should be taken as soon as possible. If it is close to the time for their next dose, the missed dose should not be taken and the next dose should be taken at the regular time.
Patients with severe renal impairment: The dose of OTEZLA should be reduced to 30mg once daily in patients with severe renal impairment (creatinine clearance of less than 30mL per minute estimated by the Cockcroft-Gault equation). For initial dose titration in this group, it is recommended that OTEZLA is titrated using only the AM doses and the PM doses be skipped. Paediatric population: The safety and efficacy of OTEZLA in children aged 0 to 17 years have not been established. No data is available.
Contraindications: Hypersensitivity to the active substance(s) or to any of the excipients. OTEZLA is contraindicated in pregnancy. Pregnancy should be excluded before treatment can be initiated.


Special warnings and precautions: Diarrhoea, nausea and vomiting: Severe diarrhoea, nausea, and vomiting associated with the use of OTEZLA have been reported. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized.
Patients 65 years of age or older may be at a higher risk of complications. Discontinuation of treatment may be


Limited joint involvement
necessary. Psychiatric disorders: OTEZLA is associated with an increased risk of psychiatric disorders such as insomnia and depression. Instances of suicidal ideation and behaviour, including suicide, have been observed in patients with or without history of depression. The risks and benefits of starting or continuing treatment with OTEZLA should be carefully assessed if patients report previous or existing psychiatric symptoms or if concomitant treatment with other medicinal products likely to cause psychiatric events is intended. Patients and caregivers should be instructed to notify the prescriber of any changes in behaviour or mood and of any suicidal ideation. If patients suffered from new or worsening psychiatric symptoms, or suicidal ideation or suicidal attempt is identified, it is recommended to discontinue treatment with OTEZLA. Severe renal impairment: See dosage and administration section. Underweight patients: OTEZLA may cause weight loss. Patients who are underweight at the start of treatment should have their body weight monitored regularly. In the event of unexplained and clinically significant weight loss, these patients should be evaluated by a medical practitioner and discontinuation of treatment should be considered. Lactose content: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
Interactions: Co-administration of strong cytochrome P450
OTEZLA is an intracellular PDE4 inhibitor with demonstrated efficacy in high-impact areas, which can improve your patient’s quality of life1–7
OTEZLA is the simple oral choice for your adult patients with moderate to severe psoriasis or active psoriatic arthritis1
PsO Plaque psoriasis Enthesitis Dactylitis Nail involvement Skin psoriasis PsA Psoriatic arthritis Palms Scalp Nail Itch Genital
3A4 (CYP3A4) enzyme inducer, rifampicin, resulted in a reduction of systemic exposure of OTEZLA, which may result in a loss of efficacy of OTEZLA. Therefore, the use of strong CYP3A4 enzyme inducers (e.g. rifampicin, phenobarbital, carbamazepine, phenytoin and St. John’s Wort) with OTEZLA is not recommended. In clinical studies, OTEZLA has been administered concomitantly with topical therapy (including corticosteroids, coal tar shampoo and salicylic acid scalp preparations) and UVB phototherapy. OTEZLA can be co-administered with a potent CYP3A4 inhibitor such as ketoconazole, as well as with methotrexate in psoriatic arthritis patients and with oral contraceptives. Pregnancy, lactation and fertility: Women of childbearing potential should use an effective method of contraception to prevent pregnancy during treatment. OTEZLA should not be used during breast-feeding. No fertility data is available in humans.
Undesirable effects: Psychiatric disorders: In clinical studies and post-marketing experience, uncommon cases of suicidal ideation and behaviour, were reported, while completed suicide was reported post-marketing. The most commonly reported adverse reactions with OTEZLA in these indications are gastrointestinal (GI) disorders including diarrhoea (15.7%) and nausea (13.9%). These GI adverse reactions generally occurred within the first 2 weeks of treatment and usually resolved within 4 weeks.
Is now the right time to move your patients on to OTEZLA?

Images depict fictional patients.
Adverse reactions reported in the psoriatic arthritis and/ or psoriasis clinical trial programme and post marketing experience include: very common (≥1/10) diarrhoea*, nausea*; common (≥1/100 to <1/10) bronchitis, upper respiratory tract infection, nasopharyngitis*, decreased appetite*, insomnia, depression, migraine*, tension headache*, headache*, cough, vomiting*, dyspepsia, frequent bowel movements, upper abdominal pain*, gastroesophageal reflux disease, back pain*, fatigue; uncommon (≥1/1,000 to <1/100) hypersensitivity, suicidal ideation and behaviour, gastrointestinal haemorrhage, rash, urticaria, weight loss; not known (cannot be estimated from the available data) angioedema. *At least one of these adverse reactions was reported as serious. Please consult the SPC for a full description of undesirable events.
Pharmaceutical Precautions: Do not store above 30ºC.
Legal category: POM. Presentation and Marketing
Authorisation Numbers: Initiation pack containing 27 film coated tablets (4 x 10mg, 4 x 20mg, 19 x 30mg)EU/1/14/981/001; 30mg film coated tablets in a pack size of 56 tablets - EU/1/14/981/002. Marketing Authorisation
Holder: Amgen Europe B.V. Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. OTEZLA is a trademark owned or licensed by Amgen Inc., its subsidiaries, or affiliates.
Date of preparation: April 2020 (Ref: IE-OTZ-2000019).
Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse events should also be reported to Amgen Limited on +44 (0)1223 436441.
Abbreviations: PDE4, phosphodiesterase-4; PsA, psoriatic arthritis; PsO, psoriasis.
References:
1. OTEZLA (apremilast). Summary of Product Characteristics; 2. Kavanaugh A, et al. Arthritis Res Ther. 2019;21:118; 3. Augustin M, et al. J Eur Assoc Dermatol Venereol. 2021;35:123–134; 4. Wollenhaupt J, et al. Presented at EULAR 2020; 3–6 June 2020; Virtual: Poster FRI0365; 5. Crowley JA, et al. Presented at the 73rd Annual Meeting of the American Academy of Dermatology; 20–24 March 2015; San Francisco, CA: P894; 6. Rich P, et al. J Am Acad Dermatol. 2016;74(1):134–142; 7. Reich K, et al. Dermatol Ther. 2022;12:203–221. © 2022 Amgen Inc. All rights reserved. Amgen Ireland Ltd., 21 Northwood Court, Santry, Dublin 9 IE-OTZ-0822-00010
Date of preparation: August 2022
DERMATOLOGY: SKIN CANCER







Skin Cancer Prevalence in Ireland
Written by Kevin O’Hagan, Cancer Prevention Manager, Irish Cancer Society

Programme (NCCP) study showed that 92% of respondents have experienced sunburn at least once in their lifetime, with many recalling multiple episodes. Almost 50% of respondents experienced sunburn at least once in the past 12 months.
A range of Sun Smart campaign materials are available on the HSE / NCCP website. A second plan has recently been published to build on this progress. Skin cancer prevention is a long-term goal, and the success of this work will depend on ongoing Government commitment to ensure funding and resources are made available to fully implement the plan.
and the use of media to drive change all proved to be successful elements of the program.
Skin Smart: Early Detection



The prevention and treatment of skin cancer was the inspiration behind the formation of the Irish Cancer Society 60 years ago. Prof Austin Darragh was inspired to organise the Society after learning that 100 Irish people were dying each year from nonmelanoma skin cancer, (NMSC) a disease that can be easily treated if found early. Skin cancer is still the most common cancer diagnosed in Ireland, with around 13,000 new cases diagnosed every year (26% of overall cancer cases). Treatment outcomes have significantly improved. Today, NMSC has an almost 100% 5-year survival rate and melanoma skin cancer now has an almost 90% 5-year survival rate.
While survival rates have improved, the number of people being diagnosed with skin cancer in Ireland is rising rapidly. The National Cancer Registry of Ireland (NCRI) projections suggest that the average number of cases diagnosed each year may double between 2015 and 2045. This is due to multiple factors, including demographic change with an ageing population, 75% of the Irish population have a ‘Celtic skin type’ which burns easily and is more prone to skin cancer. The increased frequency of travel to hot countries, the perception that a tan is an indicator of health, or the belief that Irish sun is not strong enough to damage skin, all have also contributed to increasing numbers of skin cancer.
The level of awareness of the danger posed by the Irish sun is a major concern. Research carried out on behalf of the Irish Cancer Society in 2022 revealed that a third of people were ‘not at all worried’ about sunburn in Ireland, even though results from an National Cancer Control
Regarding sun protection behaviours, the Irish Cancer Society survey also found that while just a third would apply sun cream regularly, nearly 1 in 7 would never apply sun cream when in Ireland. This rises to 1 in 5 amongst men. The study also revealed that half of the people surveyed disregard the importance of protecting the head, eyes and face from damaging rays with 53% of those surveyed admitting that they never wear a wide-brimmed hat and 1 in 6 never wearing sunglasses.
Prevention
The former director of the International Agency for Cancer Research, Chris Wild once said, “We cannot treat our way out of cancer”, this certainly applies to skin cancer which is the most preventable of all of the cancers.
The publication of the National Cancer Strategy in 2017 was a significant turning point in the work of skin cancer prevention in Ireland. The strategy recommended the development of a skin cancer prevention plan as a matter of priority (Rec. 3). This recommendation was in response to the fact that incidence rate of melanoma, was increasing by 5% per year in men, and 2.5% per year in women at that time.
Irelands first National Skin Cancer Prevention Plan was developed in 2019 in consultation with a number of stakeholders. This has enabled a national strategic approach to skin cancer prevention.
Examples of outcomes from the implementation of the plan include the development of a SunSmart brand, an annual SunSmart awareness campaign, and resources for schools, employers, and research on the cost of skin cancer in Ireland.
Learnings from other successful international campaigns indicate that a long-term strategic approach is necessary. The SunSmart campaign in Australia was established in 1988 and has proved successful, with melanoma rates declining in younger age groups. SunSmart initiatives include mass-media public education campaigns, a schools and early childhood membership program, workplace education and health professional training. This program has contributed to population wide improvements in sun protection behaviours since the 1980s. The SunSmart program has also been instrumental in achieving a considerable cultural shift towards sun protection norms in pre- and primary school–age children, as well as schools and workplaces adopting a sun protection policy. Strong leadership, legislation including banning sunbeds and workplace health and safety legislation, a critical mass of key advocates from a range of disciplines including clinicians and patients,
Unlike many other cancers, skin cancer is the type of cancer you can see with your own eyes and do something about immediately. Despite the visible nature of skin cancer, a recent survey by the Irish Cancer Society indicated that, one third were, not confident in identifying early signs of Skin Cancer and 1 in 5 never checked their skin for marks at all. It also found that people mostly associate skin cancer signs with moles. While over half of those surveyed said they would seek attention within a matter of days if they noticed a worrying sign with a mole this drops to 4 in 10 for other, less well understood skin cancer signs like lumps, spots and rough, scaly patches on the skin.
The Irish Cancer Society launched the Skin Smart campaign to encourage people to check their skin on a regular basis and also to help understand the different changes to watch out for on their skin. A range of skin cancers images are available to view at www.cancer.ie/skin-cancer
For more information or to order leaflets on skin cancer contact the Irish Cancer Society.
Call our Support line Freephone 1800 200 700 or email supportline@irishcancer.ie
44
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE BESKINSMART Skin changes like spots and sores are common and can be caused by other things. But if you have a spot, mole, or sore that looks unusual or broken skin (ulcer) that doesn’t heal within 4 weeks, always get it checked by your GP. Get any unusual changes checked out. For more information call our Support Line: 1800 200 700 Not sure? yourCallGP! When checking for signs of skin cancer/melanoma, look for the following signs this summer Asymmetric moles: A change in shape: one half is unlike the other. Border of a mole: A change in the edges: they look blurred or jagged. Colour of a mole: A change in the colour or differences within the mole: shades of tan, brown, black, or even white, red or blue. Diameter (width) of a mole: Any change in size. Most melanomas are larger than 6mm (the size of the top of a pencil) and keep growing. Evolving: Melanoma moles often change, or evolve. A B C D E BESKINSMART When checking for signs of non-melanoma skin cancer, look for the following signs this summer Not sure? yourCallGP! Skin changes like spots and sores are common and can be caused by other things. But if you have a spot, mole, or sore that looks unusual or broken skin (ulcer) that doesn’t heal within 4 weeks, always get it checked by your GP. Get any unusual changes checked out. For more information call our Support Line: 1800 200 700 A small lump that is smooth, pearly or waxy A flat, red spot that is scaly, crusty or bleeding A lump that is firm, scaly or has a crusted surface, and may be sore Rough, scaly or irregular patches of skin Ba ce rcinoma B ll acinoma Keatin ng q mousc lca n m Ba ell acnom
DERMATOLOGY: MYCOLOGY DIAGNOSTICS



Dermatology mycology diagnostics in Ireland: National deficits identified in 2022 that are relevant internationally
Written by James Powell1, Emma Porter2, Siobhan Rafferty2, Sinead Field2, Nuala H O’Connell1, Colum P Dunne3
1Department of Microbiology, University Hospital Limerick, Limerick, Ireland; School of Medicine and Centre for Interventions in Infection, Inflammation, and Immunity (4i), University of Limerick, Limerick, Ireland
2Department of Dermatology, University Hospital Limerick, Limerick, Ireland
3School of Medicine and Centre for Interventions in Infection, Inflammation, and Immunity (4i), University of Limerick, Limerick, Ireland
Dermatophyte infections are among the most common global diseases, affecting 25% of the world’s population, with asymptomatic carriage in 30%–70% of adults.1 Moreover, in the last two decades, there has been a dramatic increase in their incidence, due to a range of factors including socioeconomic problems, international travel, immigration from tropical countries and contact with animals, particularly pets.2 The clinical features of dermatophytosis may be mistaken for a wide range of other dermatological diseases including bacterial folliculitis, psoriasis and eczema.3 Many localised uncomplicated fungal skin infections in healthy individuals can be treated effectively by community pharmacists and general practitioners, however, access to accurate pathogen identification is important in moderate to severe disease, complicated or recalcitrant disease; in order to direct treatment appropriately. For example, in tinea capitis, treatment is often commenced based on clinical diagnosis; however, the choice of oral antifungal agent is dependent on the suspected species and subsequent pathogen identification; also, guidelines recommend that the definitive end point for adequate treatment must be the mycological cure, rather than clinical response.4 Infections with anthropophilic species such as Trichophyton violaceum or Trichophyton soudanense have shown a good response to terbinafine, yet zoophilic pathogens such as Microsporum canis have better cure rates with the use of griseofulvin or itraconazole.5 6
Historically there has been a preponderance of zoophilic dermatophytes in our region,7 but a recent epidemiological study demonstrated a shift in
prevalence to predominantly anthropophilic species over a 20-year period.8 In the latter period of this study, mycology testing of skin, hair and nail samples was outsourced, and access was curtailed for patients in primary care settings. Conventional mycological diagnostic methods are time-consuming,3 and when faced with staff shortages in 2016, mycology testing was outsourced to a referral laboratory. Thereafter, requests for fungal testing of skin, hair and nail samples were restricted to consultant dermatologists and other practitioners with specialist training, reducing the number of tests performed.
The shortage of medical laboratory scientists is neither a recent phenomenon nor is it simply a local problem for our laboratory; calls to action to address the shortage began in the 1980s,9, 10 and even prior to the COVID-19 pandemic it was recognised that the number of new medical laboratorians entering the workforce was not keeping up with future demand.11 In a National survey of the United States of America in 2018, vacancy rates in laboratories were ‘considerably higher’ than a similar survey in 2016, and Microbiology Departments were amongst the worst affected with vacancy rates over 10%.12 At the time of writing, in our Microbiology Department we have a vacancy rate of 21%, and this has been an on-going issue for many years.
In the scientific literature, there is an abundance of guidance4, 1315 and recommendations16-27 for the diagnosis of dermatomycoses and onychomycoses. However, little is known of the degree to which laboratories have adopted new technologies such as molecular identification tests and antifungal susceptibility testing of dermatophytes. Ireland has no national mycology reference
laboratory and fungal skin, hair or nail infections are not notifiable diseases, so there is no oversight or co-ordinated approaches to diagnosis and surveillance of these pathogens or their susceptibility to anti-fungal agents. The aim of this study is to perform an evaluation of the dermatological mycology diagnostic service of our hospital and the other hospitals of Ireland, in comparison to similar services internationally, and recognised best practice.
2 METHODS Ethics statement
This study was approved by the Research Ethics Committee of University Limerick Hospital Group, Limerick, Ireland.
Setting
The Department of Clinical Microbiology at University Hospital Limerick (UHL) provides a centralised microbiology service for six acute hospital sites of the region’s hospital group, University of Limerick Hospitals’ Group (ULHG). This service is provided to public and private healthcare facilities in the region including general practice, for a population of circa 400,000 people. Of note, there are no electronic patient
records in this group of hospitals. Previous related research from our institution includes fungal bloodstream infections in our ICU patients,28 an epidemiological analysis of dermatomycoses and onychomycoses in our region over a period spanning 20 years,8 and several reports of multi-resistant organisms detected in our hospitals, many of which resulted in outbreaks.29 30
Data and Analysis
All mycology laboratory test counts from January 2001 to December 2021 were extracted from the Laboratory Information Management System (LIMS, iLab, Dedalus Healthcare, Italy), to provide an historical context to recent trends in the numbers of tests performed. For the period 2011–2021, a data extract of dermatology clinic attendance figures for the hospital was performed from the patient management system (iPMS, Dedalus Healthcare, Italy). Figures for the five-year periods prior to and following July 2016 (when the change to testing methodology was implemented) were recorded. Similarly, a keyword search term count was performed of the patient clinical letters database (Filemaker Pro, Claris International)
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
45
James Powell
Emma Porter
held at the dermatology clinic. The keywords ‘fungal’, ‘tinea’ and ‘onychomycosis’ were searched for in the letters of correspondence sent to general practitioners. The count of letters containing these keywords allowed a crude comparison to be made of the number of patients with these conditions seen in the periods before and after access to diagnostics was restricted.
A survey was performed in all of the 28 public hospital Microbiology laboratories of Ireland to determine how many of those laboratories performed in-house mycology testing of skin, nail and hair samples, and which of them routinely performed polymerase chain reaction (PCR) and/or susceptibility testing of dermatophytes and non-dermatophyte moulds. The respondents were invited to supply test count data if they wished. This survey took the form of an e-mail request in January 2022 and subsequent follow-up of non-responders.
The pharmaceutical suppliers of the main dermatological antifungal agents were contacted by e-mail in January 2022, with follow-up e-mails for nonresponders. The companies were asked for details of the number of their unit sales per product for the Irish state and/or for the Mid-West region of Ireland, especially data from 2011 to 2021 where possible.
DERMATOLOGY: MYCOLOGY DIAGNOSTICS

The companies were Brown and Burk IR Limited, GlaxoSmithKline Consumer Healthcare (Ireland) Limited, Novartis Ireland Limited, Viatris Global Healthcare T/A Mylan Limited, Johnson & Johnson Limited and Janssen Sciences Ireland.
Data were analysed using Microsoft Excel.
3 RESULTS
For the five-year period 2011–2015, the median number of skin, hair and nail specimens for mycology analysis received in our laboratory from general practitioners (GPs) was 855 specimens per annum. For the corresponding period following the restriction of access to this service (2017–2021), the median test count was 35 specimens per annum (i.e., a 96% reduction). The positivity rate (microscopy and/or culture) of these samples increased from 36.5% to 40% across these two periods. The dermatology clinic of our hospital showed an increase from 54 specimens per annum to 117 specimens per annum (117% increase) for the same two time periods and a reduction in the positivity rate from 30% to 27%. See Figure 1 for a chart of specimen requests per requesting location.

Total dermatology clinic attendance figures showed a similar increase over the two time periods. The median annual
attendance for the clinic in the pre-curtailment period was 2320 and the corresponding figure postcurtailment was 4570 attendances (97% increase). This increase was weighted more heavily in favour of paediatric patients (140% increase) rather than adult patients (94% increase). See Figure 2 for a chart of annual attendance figures at the clinic.

The results for the count of letters from the patient letters database of the dermatology clinic with matches for the specific search terms ‘fungal’, ‘tinea’ and ‘onychomycosis’ also showed an increase. The total number of letters per annum in the pre-curtailment period was 65 letters (21 ‘fungal’, 39 ‘tinea’ and 5 ‘onychomycosis’), and there were 127 letters per annum (29, 83 and 15, respectively) in the post-curtailment period – a 95% increase. See Figure 3 for the number of matches for patient letters containing the search terms ‘fungal’, ‘tinea’ and ‘onychomycosis’, as well as the total number of patient letters recorded per annum in the clinic.
In January to March 2022, a survey of the Microbiology laboratories of the public health service system (Health Services Executive) hospitals in the Republic of Ireland revealed that 10 of the twentyeight laboratories continue to perform in-house fungal testing of skin, hair and nail samples. See
Figure 4 for a chart of the results of this survey. Nine laboratories refer their specimens to laboratories in larger hospitals in their region, often as part of a hub-and-spoke service that applies to many of the more specialised microbiology tests. Nine other laboratories refer their samples to a private reference facility for testing. Our laboratory was the only one of the six large (>600 beds) hospitals which did not provide in-house testing of these samples. Medium-sized hospitals were defined for this study as those accommodating 300–600 beds, and small hospitals were those with <300 beds. The bed capacity provides only a very rough estimate of the testing throughput of the laboratories; much of the testing workload comes from community healthcare facilities and general practice, which can vary widely for each hospital depending on their location.
The laboratories were also asked whether they had curtailed access to their fungal testing of skin, hair and nails, and were invited to supply test count data. Two hospitals supplied 10 years of test count figures, one from the south of the country (‘Hospital B’) and one from the east of the country (‘Hospital C’), neither of which has had to restrict access to mycology diagnostic services, see Figure 1 for details. Some laboratories reported that they did not provide microscopy results for some of their users (usually general practitioners), but access to fungal culture testing was only restricted by two laboratories (including ours). The second laboratory introduced this measure as a result of the surge in workload due to the Covid-19 testing. In a follow-up question to the above survey, the respondents were asked whether they had in-house capability for either susceptibility
46
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1: Specimen Request Counts 2001-2021
Figure 2: Dermatology Clinic Attendance Per Annum
testing or PCR testing of dermatophytes. One of the respondents had validated a PCR system but had not yet brought it into routine use, and two other hospitals had trials of systems in progress. As such, at the time of the survey there were no hospitals in Ireland with a dermatophyte PCR system available for routine use. None of the respondents had a susceptibility testing system in use, and since there is no national reference lab facility in Ireland, isolates would need to be sent to the United Kingdom for susceptibility testing if required. In February 2022, the following pharmaceutical companies were contacted for sales data (11 years of data if possible) on their dermatological anti-fungal products, and their responses are included below:
1. Brown and Burk IR Limited (oral griseofulvin): No response.
2. GlaxoSmithKline Consumer Healthcare (Ireland) Limited (topical terbinafine): No data available.
3. Novartis Ireland Limited (oral terbinafine): Data for 2017–2021 supplied.

4. Viatris Global Healthcare T/A Mylan Limited (oral terbinafine): Data for 2018–2021 supplied.
5. Johnson & Johnson (Ireland) Limited (topical miconazole, topical clotrimazole, topical ketoconazole, topical terbinafine): Data for 2017–2021 supplied.
6. Janssen Sciences Ireland (topical miconazole and hydrocortisone): Data for 2017–2021 supplied.

No data prior to 2017 were available, but the data provided for
the period 2017–2021 (excluding Nailderm tablets) showed a 12.5% increase in product sales. The data for 2018–2021 (including Nailderm tablets) showed an 11% increase in product sales. Figure 5 provides a chart of the volume of sales for each of the above products.
LIMITATIONS
Local pharmacy sales data were not available for the study. The Primary Care Reimbursement Service (PCRS) was contacted for antifungal reimbursement claims data. No data were available by the time the study concluded.

5 DISCUSSION
The incidence of fungal skin infections is increasing at an alarming rate worldwide.31 Increased incidence was demonstrated in our region by surrogate measures that were examined in this study: Antifungal sales data and dermatology clinic records of confirmed or suspected infections both show double-digit increases in the last 5 years. Furthermore, the twentyyear records of test requests of skin, hair and nail samples show year-on-year increases right up to the point when access to testing was curtailed. It is evident from our patient letter counts (see Figure 3) that patients with fungal-related disorders represent a very small proportion of cases seen at our dermatology clinics, suggesting the main burden of disease and treatment management is in the community setting by general practitioners and pharmacists with curtailed access to appropriate
mycological investigations. Reports of outbreaks involving dermatophytes are commonplace in the scientific literature; a PubMed search for ‘tinea’ and ‘outbreak’ for 2012–2021 provides 767 results. Tinea unguium or onychomycosis was the most common body site mentioned in the study title (50.3% of those with a site stated in the title, 172/342), followed by tinea capitis (27.8%, n = 95), tinea versicolor/ corporis (7.9%, n = 27) tinea pedis (7.3%, n = 25) and tinea faciei (2.3%, n = 8). Where a geographical region is mentioned in the title (n = 423), regions in Asia were the most common (24.8%, n = 105), followed by Africa (24.3%, n = 103), Europe (20.8%, n = 88), the Middle East (13/7%, n = 58), North and South America (4.7% and 10.2%, respectively) and Oceania (1.4%, n = 6). No recent reports of outbreaks are available from Ireland, although a study from Dublin in 2006 described a disproportionate (85.5%) number of patients of African extraction among their paediatric
tinea capitis patients.32 Fungal outbreaks are not unknown on this island however; in 1948, a cluster of 368 tinea capitis cases were detected.33 Despite this, dermatophyte infections are not listed as a notifiable disease in this country, so there is no obligation to report them. A considerable shift in the epidemiology of dermatophytes has been demonstrated in our region in the last 20 years, with an increasing proportion of anthropophilic species detected from both skin or hair samples and from nail samples,8 and this has been mirrored in many other countries.34-37 The migration of people, children in particular, during wartime has been linked with an increase in dermatomycoses. This has been reported in the former Yugoslavia during the war that took place there in the 1990s, and was previously reported after the second world war, when dermatomycoses spread epidemically.38 At the time of writing, more than 14 million people have fled Ukraine due to the war taking place there,39 many of them women and children. It is important now more than ever that dermatomycoses are monitored and identified to prevent large outbreaks from occurring.
The results of this study demonstrated a dramatic reduction in the processing of specimens for fungal analysis from GPs after curtailment of mycology diagnostic
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
47
Figure 3: Search Terms ResultsDermatology Clinic Patient Letters
Figure 4
services. The corresponding increase in dermatology clinic samples did not fill the gap left by this drop in community specimens, which could be explained in part by patients being treated for fungal infection without appropriate diagnostic confirmation, or being left untreated because of the lack of access to diagnostics. Clinical papers24-26 and dermatology guidelines13 unanimously call for laboratory confirmation of fungal infection before oral treatment of onychomycosis is started. Clinical findings, nail disease pattern and mycological investigations are important in the treatment of onychomycosis (fungal nail disease); in particular topical treatments are most effective in superficial onychomycosis but often ineffective in subungual or dystrophic onychomycosis with prolonged courses of systemic antifungals required to eradicate infection.40 Additionally, diagnosis of onychomycosis can be challenging, with similar clinical features seen in non-dermatophyte nail infections, and non-infectious conditions such as psoriasis, chronic trauma, lichen planus and nail bed malignancies.41 Antifungal medications are known to have multiple potential side effects and drug interactions, so prolonged courses in the absence of dermatophyte confirmation is not advised. Mycological identification not only supports diagnosis, it influences antifungal therapy choice and in select cases provides susceptibility information for recalcitrant infections.13 42 Despite the recommendations for microbiological confirmation, investment in fungal diagnostics in this country has been poor. Just 10 of the twenty-eight laboratories surveyed had in-house mycology testing capabilities for skin, hair and nail samples, and none reported access to in-house PCR or susceptibility testing of dermatophytes.
DERMATOLOGY: MYCOLOGY DIAGNOSTICS

and experience required to identify fungi visually (macroscopically and microscopically).
Antifungal resistance has been called a ‘global public health threat’.43, 44 This is exemplified in India where terbinafine resistant T indotineae are highly prevalent,45 and terbinafine resistance in dermatophytes has also been reported in Iran, Japan, Denmark, Belgium, Finland, Switzerland, Germany, the United States, Canada, Bahrain and Brazil.44 Terbinafine resistance is especially concerning because alternative therapeutic options to treat dermatophytoses are limited. Antifungal resistance is also probably underestimated, since many countries,44 including our own, have not been performing susceptibility testing. Susceptibility testing of dermatophytes isolated from recalcitrant infections is imperative,19, 22, 46, 47 so access to this capability should be a priority for our diagnostic services. This would be most readily achieved by the creation of a mycology reference lab for the country, a resource that no national health service should be without. For such testing, current practice in our institution is the transfer of samples overseas to The Mycology Reference Laboratory in Bristol, United Kingdom. This further compounds costs to the health service and delays timely diagnosis.

MALDI-TOF MS (matrix-assisted laser desorption/ionisation timeof-flight mass spectrometry) instrumentation has been reported to be capable of identifying dermatophytes,23 25 although not yet to the same level of accuracy achieved by conventional methods. The availability of these instruments in most modern microbiology laboratories may mean that in the future the identification of fungal pathogens may not need to be a laborious and subjective methodology, and should make it easier for smaller laboratories to implement mycology testing without the specialised knowledge
Nucleic acid amplification tests have replaced many of the conventional diagnostic techniques of the microbiology laboratory, and mycology testing is no exception. The poor sensitivity of microscopy and culture, particularly after the onset of empirical treatment21 and the long turnaround time for culture results give PCR testing a distinct advantage over traditional methods. There is an abundance of published research available evaluating dermatophyte PCR systems, but consensus has yet to be achieved on their applicability. The earliest publications48 49 described PCR as a supplement to culture for assisting organism identification; later publications5 give a more prominent role to PCR but still suggest that classical methods ‘are still warranted for training purposes and when encountering specific diagnostic problems’. More recently however we see a publication50 suggesting that PCR can ‘replace microscopy and culture for routine dermatophyte diagnosis’, but another author51 regarding the same PCR platform says that direct microscopy ‘remains relevant’ for these specimens. The Netherlands National Healthcare Institute report a higher predictive value for the PCR test over direct microscopy and culture, and they recommend that it should therefore replace traditional diagnostics in routine care.20 Many in-house PCR systems have been developed, some even achieving ISO 15189 accreditation,52 but the most straightforward process for introducing a PCR system is via a ‘CE-IVD’ marked commercial kit; some commercial kits are available that have not been fully evaluated (‘research use only’), these should not be used for routine diagnosis. There are four ‘CE-IVD’ marked dermatomycosis PCR platforms from three manufacturers available for use in Ireland currently: ‘Dermagenius® 2.0’ and ‘Dermagenius® 3.0’ (Pathonostics®), ‘EUROArray Dermatomycosis’ (EUROImmun) and ‘Dermatophytes and Other Fungi 12-Well’ (AusDiagnostics). All four platforms have been described previously.21, 44, 50, 51, 53-57 Other studies have shown that the widespread use of overthe-counter antifungals may
be promoting resistance, most notably to the azole drugs, which can mean that oropharyngeal, vaginal or even systemic yeast infections may need to be treated with less desirable alternatives such as amphotericin B with possible complications and renal toxicity.58 The increased use of immunosuppressive therapy means that invasive fungal infections are an emerging problem worldwide, and the incidence of azole resistance is increasing.59 60 Our data show significant use of topical azole creams and powders in this country; over 700,000units purchased by a population of 5 million people in 2021.
Dermatological mycology testing has not been prioritised in many laboratories around the world, including our own, yet there is growing international evidence of increased incidence of infections and resistance to anti-fungal agents. In Ireland, we have a growing population and increasing immigration, yet the testing capacity of our laboratories are being curtailed, susceptibility testing of dermatophytes is not being performed and new technologies have not been adopted. Furthermore, there is suboptimal epidemiological tracking of organisms and their antifungal susceptibilities, and there is no national oversight. Dermatological fungal infections are commonly misconceived as a cosmetic problem, but left untreated they can cause pain, physical impairment, increased risk of infections such as cellulitis and osteomyelitis in immunocompromised or diabetic patients, and a significant negative impact on quality of life.18 Recently, the WHO published a list of fungal priority pathogens causing systemic invasive infections, in this report, they suggest that future reports will include those causing dermatomycoses, highlighting the economic and health impact of the same. This study serves to highlight the need for improvement of current national practices in dermatological mycology testing, and proposes practical steps toward improving them.
References available on request
Corresponding author: Colum Dunne - Colum.dunne@ul.ie
For the full article please visit: https://doi.org/10.1111/myc.13549 and to see a companion paper please visit: https://doi. org/10.1111/myc.13473
48
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 5: Dermatological Anti-Fungal Consumption
National Skin Cancer Prevention Plan
Minister for Public Health, Wellbeing and the National Drugs Strategy, Hildegarde Naughton has launched the National Skin Cancer Prevention Plan 2023-2026, in the Department of Health.
Skin cancer* is the most common cancer in Ireland. According to the National Cancer Registry, annual average incidence during 20182020 was 12,668 cases per year. This figure has more than doubled since 1994 and is projected to more than double again by 2045, to 33,204 cases.
However, the majority of skin cancers could be prevented; ultraviolet radiation (UV) exposure, emitted naturally in sunlight or from artificial sources for example sunbeds, is the main risk factor.
Prevention efforts
The National Skin Cancer Prevention Plan 2023-2026 was
developed by the National Cancer Control Programme (NCCP) in partnership with Healthy Ireland, Department of Health and was completed in consultation with healthcare professionals, cancer charities and national organisations representing priority groups.

The new plan 2023-2026 aims to build on the work of the first National Skin Cancer Prevention Plan 2019-2022, to increase awareness and improve adoption of skin cancer prevention behaviours, to tackle Ireland’s high rates of skin cancer.
Launching the new plan
Minister Naughton said, “Skin cancer is the most commonly diagnosed cancer in Ireland, but it is also largely preventable and we can all significantly reduce our risk by adopting practical skin protection behaviours.”
Skinside Out Event in Dublin
Dr Triona McCarthy, Consultant in Public Health Medicine, National Cancer Control Programme, added, “It is so important for physical and mental health to enjoy time outdoors but we should do so while also protecting skin from UV radiation to reduce the risk of our most common cancer. The best ways to protect skin are to cover up with long sleeves, a sunhat, sunglasses and use sunscreen; limit time in the sun when UV radiation is strongest, typically between the hours of 11am and 3pm, from April to September in Ireland; and never use a sunbed.”
Maintaining momentum
The rising incidence of skin cancer is an important public health issue. The Irish Skin Foundation (ISF) welcomes the publication of the new National Skin Cancer Prevention Plan 2023-2026.
David McMahon, CEO, ISF comments, “It’s been very encouraging to see Government, Department of Health, NCCP and Healthy Ireland support for skin cancer prevention, come together with charities and NGOs working in the area in recent years.
“This new national prevention plan is how the ISF see’s public health policy deliver on the ground and for the future health of everyone living in Ireland.
“I see the previous plan, this latest plan, and hopefully continued commitment through other initiatives, as crucial to changing attitudes and behaviours. Skin cancers are largely preventable, public health agencies have a key role to play and this plan is evidence that there is real commitment to taking on that challenge.”
Just last month, the Irish Skin Foundation hosted SkinSideOut, their skin health information and exhibition event in O’Reilly Hall, UCD, Dublin.

SkinSideOut is an information and exhibition event for people affected by a wide range of chronic inflammatory skin conditions in Ireland.
This year’s event included panel discussions on Eczema and Psoriasis moderated by Virgin Media News anchor Claire Brock; talks on Acne, Rosacea and Skin Cancer Prevention; and “How to” sessions delivered by Dermatology

ANPs on Emollient Therapy, HS Wound Care and Scalp Psoriasis. Attendees had the opportunity to meet with skin care brands with exhibition stands on the day and discuss product ranges for different skin problems.
Jill Clarke, Communications and Events Manager, Irish Skin Foundation; Dr Rupert Barry, Consultant Dermatologist, St. James’s Hospital; Carmel Blake, Clinical Helpline Manager, Irish Skin Foundation

Over 350 people registered for the event, with “How to” sessions on Eczema and Psoriasis selling out in advance!
Following each talk ISF dermatology Helpline nurses were on hand to provide free, up-todate guidance to attendees on skin conditions.
Claire Brock, Moderator; Glenn Kenneally, Patient Advocate; Paul Herriot, Patient Advocate; Prof Alan Irvine, Consultant Dermatologist
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023 49 DERMATOLOGY FOCUS NEWS
Claire Brock, Moderator; Jill Clarke, Communications and Events Manager, Irish Skin Foundation; Prof Anne-Marie Tobin, Consultant Dermatologist, Tallaght University Hospital
UROLOGY: KIDNEY STONES
‘Like a ticking time bomb’: A qualitative study exploring the illness experiences of adults with kidney stone disease
Written by Emma Ní Néill1, Helen L. Richards1, Derek Hennessey2, Dónal G. Fortune1


KSD can be a fatal condition and in these cases mortality is usually related to sepsis developing in an obstructed kidney due to a ureteric stone (Kum et al., 2016).
Kidney stone disease (KSD) is a urological condition characterized by the development of calcifications inside the collecting system of the kidney. Stones formed in the kidney can cause pain and infections locally but can move into the ureter and obstruct the kidney causing

ureter colic (Parmar, 2004). Renal and ureteric colic are the specific pains associated with KSD and are considered one of the most severe physical pains a human can experience. KSD aetiology is multifactorial for the majority of patients, however, a subset of patients have a
metabolic, congenital or genetic cause (Howles & Thakker, 2020; Parmar, 2004). Patients with KSD experience additional burdensome symptoms such as fever, nausea, haematuria and dysuria (Frassetto & Kohlstadt, 2011), which significantly impact patients' quality of life (Raja et al., 2020).
The current lifetime prevalence of KSD is 14%. This varies globally with research reporting estimates of 5%–9% in Europe, 7%–13% in America (Sorokin et al., 2017) and 1%–19.1% in Asia (Liu et al., 2018). Current rates indicate the prevalence of KSD has risen over the past four decades (Thongprayoon et al., 2020). Historically, KSD was thought to be twice as common in males than females and typically affecting those between 30 and 55 years of age (Parmar, 2004). However, these gender differences are now narrowing, with young females at a higher risk of developing KSD than previous generations (Gillams et al., 2021). Whilst the reasons for KSD causality are complex, the incidence and prevalence of KSD is regarded as an emerging public health concern with implications for the provision of health care resources (Hill et al., 2021). This is in part due to the fiscal cost of treating KSD which is estimated to exceed the combined cost of bladder and prostate cancer in the UK alone (Geraghty et al., 2020). Moreover, patients have a high likelihood of experiencing recurrent stone episodes with estimated recurrence rates of 35%–52% reported at 5–10 years following their first episode (Khan et al., 2016; Uribarri et al., 1989) illustrating the potentially chronic nature of this urological condition and the likely need for future medical intervention.
Whilst effective treatments exist, there are no permanent cures for KSD. Dietary and lifestyle modifications are the cornerstone of improving KSD prognosis by reducing the risk of recurrence (Boarin et al., 2018; Pearle et al., 2014) and, importantly, are evidenced as the most effective measures for preventing KSD


JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 50
Emma Ní Néill
1Department of Psychology, University of Limerick, Limerick, Ireland Health Research Institute, University of Limerick, Limerick, Ireland
2Urology Department, Mercy University Hospital, Cork, Ireland
Derek Hennessey
Dónal G. Fortune
onset. Although these preventative strategies may be perceived as relatively straightforward, adherence to increasing daily fluid intake is low amongst patients (Khambati et al., 2017; van Drongelen et al., 1998).
Patient perspectives are likely to be important in expanding our understanding of the engagement with prevention strategies within the context of illness experience of KSD. Whilst motivation to reduce recurrence is reported to be high (McCauley et al., 2012), perceptions of barriers to increased fluid intake include forgetting to drink and lack of thirst (Streeper et al., 2019). Interestingly, disease-related factors including multiple recurrent stones have not been associated with higher fluid intake (Tarplin et al., 2016). Gaining an understanding of processes that influence this behaviour may identify targets for intervention to reduce this behaviour–intention gap, potentially lessening the individual and health care cost of KSD.
An emerging body of research suggests that KSD is associated with psychological distress (Angell et al., 2012; Chung et al., 2012; Lien et al., 2015) due to stonerelated factors (Angell et al., 2012) including burdensome physical symptoms (Diniz et al., 2007; Kalaitzi et al., 2006), and urological treatment (Lien et al., 2015). KSD populations have a higher risk of experiencing depression within one year following diagnosis (Chung et al., 2012), increasing to 50% for anxiety and 26% for depression during ten years of follow-up compared with nonKSD populations (Lien et al., 2015). Recent studies have begun to examine the physical and psychosocial challenges of KSD from a qualitative perspective (Raja et al., 2020). These impacts tend to be characterized by frequent and painful urinary symptoms, gastrointestinal symptoms, sleep disturbances, work impacts (Ragab et al., 2020; Tran et al., 2018), impact on relationships (Raja et al., 2020) and treatmentrelated anxiety (Kelly & Kelly, 2019; Ragab et al., 2020). Whilst psychological distress experienced by patients may be explained in part by the multi-dimensional consequences of KSD, patients also report a fear of recurrence of KS (Nouri et al., 2021), anxiety about the future (Raja et al., 2020) and uncertainty of the condition (Ragab et al., 2020). Despite these findings, how patients make sense of their illness experience of KSD remains poorly understood.
The Common-Sense Model of Illness Self-Regulation (CSM-SR; Leventhal et al., 1980, 2016) is one of a number of frameworks
that have attempted to formalize the idea that how people think and feel about their illness may impact health outcomes. Illness representations are dynamic processes, whereby people continuously use and integrate information from a variety of sources to formulate a dual cognitive and emotional representation to make sense of their illness and define targets for coping (Leventhal et al., 1998). Cognitive illness representations are key beliefs people hold about the cause, identity, timeline, curability/ controllability and consequences of their illness (Leventhal et al., 1980). Simultaneously, people create emotional representations of their conditions incorporating their affective responses such as worry or anger (Leventhal et al., 2016). These illness representations inform patients' subsequent coping strategies and other mitigation behaviours such as action plans in an attempt to manage their illness (Leventhal et al., 2016). Importantly, research suggests that illness representations are modifiable through intervention by targeting unhelpful illness beliefs (Chilcot & Moss-Morris, 2013; Jones et al., 2016; Vollmann et al., 2021), which may give rise to improved patient treatment adherence (Pereira et al., 2019). To date, no studies have explored the CSM-SR in individuals with KSD.
This study aimed to address this gap in understanding by using a CSM-SR informed lens to examine the illness experiences of adults living with and receiving treatment for KSD. Increasing our understanding of patients' experiences of KSD may help to identify barriers to engagement in self-management behaviours. Additionally, the acquisition of a theoretically informed understanding of individuals' illness representations may enable health care professionals (HCPs) to provide more tailored patient-centred care through understanding the breadth of illness representation experiences that could be amenable to psychologically informed intervention for patients.
METHOD Design
This qualitative study utilized the CSM-SR (Leventhal et al., 1980, 2016) to provide the theoretical framework for analysis and to inform the study's semi-structured interview schedule (see Supporting Information). A qualitative approach was chosen to enable
a deeper exploration (Iphofen & Tolich, 2018) of illness experiences that could not be achieved through quantitative methods alone (Gelo et al., 2008). Individual interviews were selected as it was considered it would enable the researcher to explore personal illness experiences in broader detail (Guest et al., 2017). It was also considered that participants may be willing to share more personal experiences via this format than in a group setting. Ethical approval was received from the Clinical Research Ethics Committee of the Cork Teaching Hospitals (reference number: ECM4(b)120121).
Participants
Participants were recruited from an outpatient kidney stone service between September 2021 and December 2021. The diagnosis of kidney stone disease was made by a senior urological surgeon by history, clinical examination and confirmed by non-contrast computerized tomography of the kidney ureters and bladder. All patients in this study had experienced one or more episodes of renal or ureteric colic, recurrent urinary tract infection, urinary sepsis, emergency kidney surgery or deterioration in kidney function due to their kidney stones. Due to the severity of these symptoms/ complications, all patients were offered surgical intervention with the intention of complete stone clearance. Observation was not offered unless there were specific individual patient factors that would prevent surgery, for example, not fit for anaesthetic. Individuals were eligible to participate if they met the following criteria: (1) had a diagnosis of kidney stones; (2) had sufficient English to engage in the interview; and (3) were over 18 years of age. Forty-two consecutive individuals were invited to participate in this study. Of these, one individual did not consent at the time of recruitment; 7 individuals did not respond to telephone contact; and 1 individual withdrew consent. Thus, the final sample comprised of 33 individuals with KSD. Prior to recruitment a provisional lower and upper limit of participants was estimated (Braun & Clarke, 2019), based on the relatively broad study aims, and the crosscase approach to analysis as outlined for achieving ‘information power’, (i.e., whether the data held adequate information to generate novel understandings), in qualitative studies (Braun & Clarke, 2021; Malterud et al., 2016).
We arrived at a final sample of 33 participants through considerations of the quality of the dialogue, including richness of data, within interviews from ongoing analysis of transcripts (Braun & Clarke, 2019) and pragmatic considerations including a higher than expected number of individuals recruited at the final clinic.
Procedure
All participants provided written and verbal consent to partake in the study and for the interviews to be audio-recorded. All interviews were conducted by the first author (ENN), a Psychologist in Clinical Training with academic knowledge but no lived experience of KSD. There was no personal or professional relationship between the participants and the interviewer. Interviews were conducted via telephone (n = 29) or video call (n = 4) in line with national COVID-19 restrictions in place at the time of data collection (September 2021 to December 2021). Interviews were recorded on a Dictaphone with audio recording properties. Interviews took place within 1 week of the person with KSD being recruited. Interviews were transcribed verbatim and were cross-checked for accuracy and then deleted from the recording device.
Data analysis
Reflexive thematic analysis (Braun & Clarke, 2006, 2021) was chosen as the method for analysis as it allowed the researchers to identify patterns of meaning across the data set, whilst simultaneously drawing on theoretically informed interpretations of meaning from the CSM-SR (Leventhal et al., 1980, 2016). The fifteen-point checklist for thematic analysis (Braun & Clarke, 2013, 2021) was used to ensure rigour.
Analysis was conducted within a critical realist paradigm whereby participants' narratives provided the researcher with access to a mediated reflection of participant's reality (Braun & Clarke, 2021). Whilst we drew on a specific theoretical model, we also acknowledged that data could extend beyond domains of the chosen model (Fletcher, 2017) to generate new understanding.
Data analysis was conducted by the first author (ENN) by hand and followed the six-phase process of thematic analysis (Braun &
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
51
UROLOGY: KIDNEY STONES
TABLE 1. Participant characteristics
Participant characteristics
Gender
Male, n (%)
Female, n (%)
Age (years), mean ± SD
Nationality
Irish, n (%)
Other, n (%)
Relationship status
Single, n (%)
In a relationship, n (%)
Married, n (%)
Separated/divorced, n (%)
Widow/Widower, n (%)
Highest level of Education
Secondary education (e.g., junior or leaving certificate), n (%)
Further education (e.g., Diploma, QQI level 5/6), n (%)
Higher education (e.g., Bachelor/Master/Doctoral degree), n (%)
Employment status
Employed, n (%)
Retired/other, n (%)
Psychological/Psychiatric difficulties n (%)
Other physical health condition
Kidney stone episodes
First time (≤1)
Recurrent (≥1)
Currently experiencing symptoms/KSD episode
Clarke, 2021; Clarke & Braun, 2013). First, familiarization with the data occurred through immersion in the data set and iterations of reading interviews. Coding of data was completed primarily at a semantic (explicit) level, but latent (implicit) meanings were also considered. Importantly, analysis was an iterative process, with the researcher moving backwards and forwards between stages as thinking changed (Braun & Clarke, 2021). ENN maintained a reflexive journal along with field notes to consider their own bias during analysis. Finally, categorized codes were reviewed and refined
into final semantic and latent themes (Braun & Clarke, 2021). In the second stage of the analysis, these themes were mapped onto the domains of the CSM-SR. ENN undertook the initial mapping, which was then discussed with the senior researchers in a transparent attempt to reach a consensus on the mapping. Participant quotations are embedded throughout the analysis to illustrate the patterns of meaning generated by the researcher. Participants are referred to by their assigned code, and any potentially identifiable information has been anonymised or removed.
Adults with KSD (n = 33)
making sense of KSD, (2) normality paused, (3) the psychological burden of KSD, (4) the tensions of managing KSD and (5) improving understanding of KSD.
Making sense of KSD
This theme encompasses participant's narratives describing their attempts to make sense of initial symptoms and diagnosis, commensurate with the CSM-SR illness identity domain as well as participant's beliefs about the causes, coherence and timeline of KSD, aligning with corresponding CSM-SR domains.
I've something wrong with me
Many participants reflected on the accumulation of symptoms, including symptoms of colic, urinary retention, diarrhoea, nausea, vomiting, haematuria, fatigue and fever. Many participants anticipated that the severity of these symptoms indicated the presence of a serious health event:
it was scary like….you cannot imagine being in that much pain unless it's very serious. (P12)
Both male and female participants likened their experience of pain to that of giving birth, which many used as the closest layperson's experiential point of reference: this pain is worse than having a baby! I was talking to a few women who gave birth, and they had kidney stones after – and they said it was a worse pain than pregnancy. (P1)
Disbelief then relief
RESULTS
Individual interviews were conducted with the final sample size of thirty-three adults aged between 22 and 85 (mean age = 55.90 ± 12.94; 19 males, 14 females; see Table 1). The duration of interviews ranged from 17 to 63 min (mean 43 min). If participants self-reported they had experienced more than one stone episode in their lifetime, they were categorized as recurrent stone formers for the purpose of this study (see Table 1).
Five themes and eleven subthemes were generated from analysis: (1)
On learning they had kidney stones, some participant's accounts were permeated with a sense of disbelief at a KSD diagnosis, suggesting an illness identity mismatch between the severity of the symptoms they experienced, and the kidney stone label assigned to them:

when they said kidney stones and I was like kidney stones? I thought it was a big laugh like. (P14)
Following confirmation of KS, participants spoke of a sense of relief and used downward health comparisons to illustrate their relief that they had not been diagnosed with what they perceived to be a more serious health condition:
Grappling with unknowns
There were discrepancies between participants' accounts on the timeline dimension and causes
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Yes, n (%) No, n (%) 19 (57.6) 14 (42.4) 55.90 ± 12.74 29 (87.9) 4 (12.1) 1 (3.0) 2 (6.1) 27 (81.8) 1 (3.0) 2 (6.1) 14 (42.4) 4 (12.1) 15 (45.5) 21(63) 12 (36.4) 2 (6.1) 18 (56.5) 12 (36.4) 21 (63.4) 12 (36.4) 21 (63.6)
of the condition. Participants often struggled with the clinical ambiguity of the condition's timeline and referred to information and data received from HCPs to manage this unknown:
he [Doctor] said there is a 50% chance of recurrence of kidney stones….that the statistic was about 50%. (P17)
For other participants who experienced recurrent stone episodes, KSD was conceptualized as a lifelong condition, with a chronic course:
Normality paused
This theme linked chiefly with the CSM-SR consequences domain and represented participants' narratives of how normality was compromised by symptoms of KSD across work, family, relationships and social domains. Participants spoke of the impact on their intimate relationships:
I wanted to be intimate with him, because I like being intimate with him. But I could not…. and then he was afraid he was going to hurt me. (P25)
Some participants spoke of their decision to leave their employment as frequent sick leave was incompatible with some positions: The psychological burden of KSD
This theme and subthemes principally represented participants' emotional representations of KSD. In addition, ideas about identity, timeline, consequences and illness coherence had common currency across participants narratives.
KSD and the road to distress
The majority of participants described the psychological
impact associated with the experience of KSD symptoms, often characterized by low mood and feelings of sadness:
you felt kind of down in yourself, cos you kind of know that you are able to do all these things, but you know, you cannot do them because you are not well - which is not great. (P10)
A small number of participants recounted ruminating on the intensity of the pain, which gave rise to extreme psychological distress and suicidal ideation: [because] of the pain.…just, you know, end it all….just sick of it all. That's how I'd describe it anyways, bad thoughts were going through my head. (P1)
For some, ruminating on not achieving desired outcomes, such as the removal of all KS, or achieving this within desired timelines perpetuated feelings of distress:
it [KSD] plays with my head….I was worrying too much, and I was constantly aggravated by doctors not doing enough in the kidneys….it just led me down that road eventually. (P13)
Self-consciousness and embarrassment
Both male and female participants spoke of the negative impact of urinary symptoms when in public. Some participants described feeling self-conscious about urinary symptoms and took actions to conceal these symptoms to avoid evaluation from others: the show of blood is alien to a man so….And I mean you would not use a public urinal….why? Because there could be blood –and you are not going to stand – well, you'd go into the WC rather than the urinal, but it's just those things. (P33)
These symptoms and their consequences were sources of concern and embarrassment for participants as symptoms required constant planning for adverse events which perpetuated feelings of anxiety:
Fear of KS recurrence
This subtheme highlighted participants' accounts of a constant state of vigilance to manage the possibility of another symptomatic kidney stone episode. Participants' monitoring of potential physiological changes in their body that made sense as part of KSD, and the cognitive appraisal of these symptoms, reinforced this response even when symptom-free:
It's always a bit of a worry when I am working…. you sort of get in tune with your body and how it feels. But sometimes it's sort of, false alarmed. If something does not feel right for a second or two you are like, oh no - I'm going to have to think about talking to my boss about potentially being out for a day or two to get surgery done. (P28)
The memory of the severity and intensity of colic they experienced was a key factor maintaining participants fear of a recurrent KS episode:
The tensions of managing KSD
This theme linked to the CSM-SR domains of personal and treatment control. Findings also suggested the importance of social contextual factors, such as patient–partner dyads, in managing KSD.
Self-management and its challenges
Participants reported that making dietary changes and increasing fluid helped imbue a sense of control. Yet, across participants' narratives those who perceived KSD to be caused by biological factors tended to express more complex beliefs about personal control:
even before kidney stones, I would have drank 2 litres of water a day you know…. it does not make a difference to me - because it is a hereditary thing. (P29)

Participants named factors such as low motivation and time management as psychosocial challenges to engaging in selfmanagement. For some, the adherence to modifications for KSD waned in subsequent months following resolution of KS episodes, and possibly became de-prioritized by participants over time:
The supportive role of significant others
Many participants perceived that their partners used their personal understanding of KSD to support them with the management of the impacts of the condition. This was described as an important factor that helped participants adjust to the reality of managing treatment: like knowing going into the surgery that he [partner] is staying and dropping me off and collecting me and at home looking after me…. it's nice that someone is there for you. For even the small things, like, just to heat the soup for you, if you want soup, you know. (P25)
Partners also provided important reminders and cues to participants that supported adherence to selfmanagement strategies:
Control is in the doctors' hands Participants had received a variety of treatments for KSD that varied in success. For many, pharmacological management was viewed as the only effective means of managing symptoms such as colic:
Across many narratives, successful treatment was viewed as ‘the light at the end of the tunnel’ (P17). This view was reinforced by participants' perceptions of the doctors' confidence in the effectiveness of the procedures: he [surgeon] told me “I go in, I take them away, they'll be gone”. Sure, if I get a pain like that again I just go back and he can do it again if it comes to it. (P17)
However, many others described their turmoil at prolonged and multiple unsuccessful procedures with seemingly no medical resolution in sight. Yet, even during those times, participants continued to place a high external locus of control on doctors to manage KSD:
Improving understanding of KSD
This theme linked with the CSMSR domain of illness coherence and captured participants desire for more knowledge and the importance of improved doctor communication to bolster understanding of KSD.
The need for knowledge on KSD
Across narratives, participants knowledge of KSD varied, but it was evident that participants desired more information about the condition:
If somebody could just spend 5 minutes saying, this is what's
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
53
happened to you….this is reasonably common, what we generally advise for people who have had kidney stones is that you do x, y and z….it may come again – I mean, 5 or 10 minutes with somebody who is well informed on these would have been helpful I think. (P23)
Participant's narratives also described how providing awareness and information campaigns on KSD would benefit the general population from a prevention perspective and increase the visibility of the condition:
HCP-patient communication and health literacy
Although many instances of effective doctor-patient communication were described, some patients' perceived inadequate communication at times which gave rise to feelings of frustration:
the way it [KS treatment] was discussed with me, I thought I'd be going down once every two or three weeks getting it done.… Instead my first kidney stones – it took three and a half years to get rid of. (P10)
Some participants spoke about how problems in communication gave rise to confusion as to the next steps in their care pathway: Whilst it may have been assumed by HCPs that participants understood surgical procedures, it was clear from participants' accounts that this understanding varied. Some described their alarm after procedures, such as an ureteroscopy, when realizing that KS were located using a flexible scope, typically inserted through the penis or urethra, and subsequently broken down using a laser:
when I woke up and I did not have any scars or anything, I was going how in the name of God did they do this like? and then I found out - now it wasn't an embarrassing thing…. but I was going, okay, well, it would have been nice to know they were going accessing this way. Because I expected to have a scar or something. (P19)
DISCUSSION
To the best of our knowledge, this was the first study to examine the experiences of patients with KSD which utilized the CSM-SR model as a lens to understand
their experiences. We gleaned a better understanding of how people cognitively and emotionally represent KSD with generated themes broadly mapping on to domains of the CSM-SR model (Leventhal et al., 1980, 2016). Findings also provided evidence for how participants make sense of KSD that extended beyond the domains of the original CSMSR model, with patient partner relationships also identified as an important social contextual factor (DeLongis & Morstead, 2019) in one of the themes.
The CSM-SR model proposes that individuals are active problem solvers in managing and appraising threats to their health (Leventhal et al., 2016) and our findings captured how participants appraised the accumulation of severe symptoms, with an acute onset, to indicate a severe health event (Theme 1). Once KSD was confirmed, many participants spoke of the initial relief brought by diagnosis, potentially drawing on their initial understanding of the implications of KS as typically non-life-threatening and utilized downward comparisons with other health conditions (Wills, 1981). Downward comparisons are typically viewed as a cognitive strategy used by patients with chronic illnesses more generally to regulate their anxiety following diagnosis by assuming that others, who are perceived to have more serious health diagnoses, experience greater consequences (Arigo et al., 2014). Although KSD diagnosis brought a sense of initial relief for many, participants also described grappling with the more ambiguous characteristics of the disease including potential causes and the unpredictable timeline of KSD. Given that the CSM-SR proposes that individuals make assessments on how much personal control they have over the condition based on their perception of timeline and causes (Cameron & Leventhal, 2003) the perceived lack of certainty on these facets of KSD may have presented challenges in participants' adjustment to the condition.
A wide range of multi-dimensional impacts to everyday life as a result of KSD were reflected in the theme ‘Normality paused’ (Theme 2). Participants experienced significant psychological burdens associated with KSD (Angell et al., 2012; Chung et al., 2012; Diniz et al., 2007; Lien et al., 2015). Generally, participants narratives mapped onto timeline and consequences representations
UROLOGY: KIDNEY STONES
when describing these burdens. These impacts were seen to curtail intimate relationships and participants described waiting for life to start again once symptoms as a sequalae of kidney stones had resolved.

Participants narratives outlined factors that may further amplify the psychological burdens of KSD for patients (Theme 3). The experience of anxiety for participants with KSD has been documented in previous studies (Ragab et al., 2020; Raja et al., 2020), but our findings provide insight into specific behaviours potentially engaged in (e.g., avoiding public urinals) to avoid urinary symptoms being perceived negatively by others and to manage their affective response of self-consciousness and embarrassment. Furthermore, painful symptoms were associated with experiences of low mood, with some participants experiencing extreme psychological distress, including suicidal ideation. Many KSD patients perceived that pain could only be relieved by pharmacological means (e.g., morphine). It is possible that participants subsequently appraised their personal coping strategies to manage this severe pain (colic) as being ineffective, giving rise to or exacerbating their experience of psychological distress in line with the CSM-SR model (Leventhal et al., 1980, 2016).
Many participants described the fear of KS recurrence, even when symptom-free. Fear of recurrence is well articulated in conditions such as cancer (Durazo & Cameron, 2019) and stroke (Townend et al., 2006). Whilst Nouri et al. (2021) documented participants apprehension relating to potential recurrence, our study described needing to be constantly vigilant and on guard for symptoms that may alert them to a recurrent episode. This experience is likely associated with anxiety and worry within this representation of recurrence for patients in this study.
Participants had varied perspectives of personal and treatment control in managing KSD (Theme 4). Participants who held stronger causal perceptions about KSD resulting from ‘fixed’ factors (e.g., genetics), tended to question the perceived effectiveness of self-management strategies. Whilst these strategies, which include dietary modifications and increased fluid intake, were perceived as straightforward recommendations, many of these participants described
their adherence to such recommendations as challenging. Additionally, patients' key perceptions of the consequences and timeline of KSD were also demonstrated to change over time. This was illustrated by participants reporting that adherence to selfmanagement waned over time as the immediate response to KSD faded, in terms of assessment and treatment of the acute episode, providing insights into adherence barriers for patients in this study. Similarly, recent research suggests that adherence remains low at follow-up, despite initial increases in such fluid intake behaviour (Wright et al., 2022). Patients' perspectives, elicited in this study regarding the reduced need for sustained self-management provides important information that emphasizes the need for health care providers to continuously emphasize patients' agency in the management of their KSD.
Our findings on the role of partners in providing emotional and practical support in managing KSD highlights the influential role of social contextual factors in how people make sense of illness that extends beyond the domains of the original CSM-SR model (DeLongis & Morstead, 2019). This is an important finding, as typically, patients and partners share information and discuss illness, thus developing pathways of shared meaning making of illness which in turn guide behaviours including coping (Berg & Upchurch, 2007). Patients' narratives also demonstrated that their representations of KSD within this theme also utilized their partner's representations of KSD as a source of support and guidance, in line with other research suggesting that partner's representations can facilitate better patient adaption to illness (Karademas et al., 2019). For example, the narratives provided by participants in the subtheme, ‘the supportive role of significant others’ showed a dynamic interplay whereby the significant other reminded their partner with KSD of the need to adhere to specific dietary constraints due to the partners perceptions of the cause and likely consequences of not adhering to dietary recommendations. As such, the illness perceptions of key family members may be important in promoting optimal adjustment and adherence to KSD selfmanagement strategies.
Overall, there was a clear desire from participants for an improved
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
understanding of KSD, aligning with the domain of illness coherence (Theme 5). Some participants reported they did not receive adequate information about KSD or self-management. Suboptimal HCP-patient communication may further compound low levels of illness coherence. Illness coherence is an important factor in the creation of an internally consistent personal illness model, and lower levels of illness coherence may lead to poorer adjustment to the condition (Hagger & Orbell, 2021). In this regard, the need for increasing the understanding of KSD at a patient level, and through informational campaigns at a public health level had common currency in the experiences of participants. This finding is commensurate with studies calling for better informational campaigns to increase awareness in relation to urological conditions more generally (Gagnon et al., 2009; Zhao & Anger, 2021).
Clinical implications and directions for future research
This study extends our understanding of how patients cognitively and emotionally represent KSD with several implications for clinical practice. HCPs may find it beneficial to consider routinely assessing for psychological distress, including suicidal ideation, particularly
during acute KS episodes. Access to timely psychological care where appropriate would also be helpful. Secondly, our results suggest the important role of HCP's gaining a better of understanding of how patients make sense of KSD which may aid identification of unhelpful beliefs as targets for intervention. There is a growing body of evidence of interventions informed by the CSM-SR which have been shown to be effective at targeting illness perceptions (Fortune et al., 2004; Petrie et al., 2012), and improving illness coherence (Vollmann et al., 2021). As such, there may be a role for application of the CSM-SR in the design of specific interventions targeting modifiable illness perceptions in KSD and which may improve patient outcomes and adjustment to the condition.
Our findings highlight participants' challenges in adhering to self-management strategies, particularly in the long term where adherence behaviour may become less automatic over time. Research has highlighted the role that automatic processes, such as automatic coping responses (Henderson et al., 2009) and implicit attitudes (Chevance et al., 2019), may play in long-term adherence to self-management and the significance of targeting treatment-
related habits in interventions (Orbell & Phillips, 2019; Phillips et al., 2016). Importantly recent research suggests that a digital fluid intake intervention (Conroy et al., 2020), shows promise in supporting habit formation. The implementation of digital tools may also reduce the burden on key family members, such as partners who we have reported may share responsibility for aspects of disease management. Given that as few as 13% of patients source information on prevention strategies independently, providing tailored individual counselling on KSD with this patient group may be important (Tarplin et al., 2016). Indeed the communication of self-management strategies and exploration of patients' specific illness representations that may act as barriers to sustained behaviour change may have additional relevance for services. There is significant heterogeneity in the clinical characteristics, disease histories and experiences of patients with KSD and it is possible that such factors influenced individual's experiences. Future research may benefit from exploring the lived experience of these demographic and clinical differences, for example, single episode stone formers versus recurrent stone formers, variation
in stone compositions, time since diagnosis and age, in a comparative manner to better understand the variability of experiences within this population in terms of disease burden.
CONCLUSION
This study is the first to utilize the CSM-SR to explore how KSD patients think and feel about their condition and has examined salient cognitive and emotional representations within a social or interpersonal context that underpins how these patients make sense of KSD. Importantly, we identified factors reinforcing patients' experiences that may amplify, or ameliorate, the potential burden of KSD for individuals. From a clinical perspective, routine assessment of psychological impact in individuals with KSD and timely access to psychological care would be helpful. In addition, HCPs may benefit from gaining a better understanding of patients KSD-specific illness representations, which may prove helpful in managing both the impacts and outcomes, for this common, complex and hugely under-researched condition.
Novel Test for Prostate Cancer Management
OncoAssure have announced that results of its clinical study validating OncoAssure Prostate, a novel test for prostate cancer management, have been published in European Urology Focus, a peer-reviewed and open access journal. OncoAssure Prostate is a new test which provides an improved estimation of the risk of aggressive prostate cancer, allowing optimal treatment selection for patients.
The Irish medical diagnostics company also announced today that this novel cancer test, which addresses an unmet need in prostate cancer management, is now available for commercialisation.

Prostate cancer is the second most common cancer in men, after non-melanoma skin cancer,
and the second leading cause of cancer death in men after lung cancer. Approximately 70% of prostate cancer patients have slow growing cancer and can safely leave their disease untreated while undergoing active surveillance; while 30% have an aggressive cancer with a high potential for recurrence, which require aggressive treatments which are often life changing.
Current approaches do not accurately identify whether a patient has aggressive or low-risk prostate cancer; thus overtreatment is a major issue as many patients undergo unwarranted radical treatments leading to severe complications including infection, incontinence, erectile dysfunction and depression.
OncoAssure Prostate measures a novel set of ‘master driver’ genes linked to the progression of Prostate Cancer. This molecular data is combined with existing clinical parameters to provide a comprehensive and accurate assessment of a patient’s risk for aggressive prostate cancer. As the test uses a concise panel of genes (four prognostic and two reference genes), it provides a more costeffective solution, which can be run in-house in hospital pathology laboratories using standard RTPCR equipment with faster turnaround time and avoids the need to send clinical samples to external testing laboratories.
OncoAssure’s Chief Scientific Officer and co-founder Professor William Gallagher, said, “We are very pleased to demonstrate
that our novel discovery and development process has enabled us to create a best-in-class highly accurate Prostate Cancer test.
This would not have been possible without the collaboration and co-operation of a number of parties within the clinical and pathology environment in Ireland and Sweden, including Professor William Watson (UCD and Lead of the Prostate Cancer Research Consortium) and Professor Anders Bjartell (a prominent urologist at Lund University, Sweden). Thanks also to the background support of the Irish Cancer Society and Science Foundation Ireland in the biobanking and translational cancer space that underpinned this exciting work.”
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
55
News
Longitudinal Epigenome-Wide Analysis of Kidney Transplant Recipients
Pretransplant and Posttransplant
1Centre for Public Health, Queen’s University Belfast, Belfast, Northern Ireland, UK
Introduction
Kidney transplantation remains the gold standard of treatment for end-stage renal disease (ESRD), with improved patient outcomes compared with dialysis. Epigenome-Wide Association Analysis (EWAS) of DNA methylation may identify markers that contribute to an individual’s risk of adverse transplant outcomes, yet only a limited number of EWAS have been conducted in kidney transplant recipients. This EWAS aimed to interrogate the methylation profile of a kidney transplant recipient cohort with minimal posttransplant complications, exploring differences in samples pretransplant and posttransplant.
Methods
We compared differentially methylated cytosine-phosphateguanine sites (dmCpGs) in samples derived from peripheral blood mononuclear cells of the same kidney transplant recipients, collected both pretransplant and posttransplant (N = 154), using the Infinium MethylationEPIC microarray (Illumina, San Diego, CA). Recipients received kidneys from deceased donors and had a mean of 17 years of follow-up.
Transitional care models in adolescent kidney transplant recipientsa systematic review


Dermot Michael Wildes1, Caoimhe S Costigan1, Mairead Kinlough1, Joan Flynn1, Niamh Dolan1, 2, Michael Riordan1,2,3, Clodagh Sweeney1,2, Maria Stack1,2, Mary Waldron1,2, Orla Walsh1,2, Kathleen M Gorman1,4, Atif Awan1,2,4
1The Department of Paediatric Nephrology & Transplantation, Children's Health Ireland at Temple St, Dublin
Results
Five top-ranked dmCpGs were significantly different at false discovery rate (FDR) adjusted P ≤ 9 × 10 8; cg23597162 within JAZF1, cg25187293 within BTNL8, cg17944885, located between ZNF788P and ZNF625ZNF20, cg14655917 located between ASB4 and PDK4 and cg09839120 located between GIMAP6 and EIF2AP3.
Conclusion
Five dmCpGs were identified at the generally accepted EWAS critical significance level of FDR adjusted P (PFDRadj) ≤ 9 × 10 8, including cg23597162 (within JAZF1) and cg17944885, which have prior associations with chronic kidney disease (CKD). Comparing individuals with no evidence of posttransplant complications (N = 105) demonstrated that 693,555 CpGs (89.57%) did not display any significant difference in methylation (PFDRadj ≥ 0.05), thereby this study establishes an important reference for future epigenetic studies that seek to identify markers of posttransplant complications.
2The Department of Paediatric Nephrology, Children's Health Ireland at Crumlin, Dublin
3The Department of Paediatrics and Child Health, Royal College of Surgeons in Ireland, Dublin
4School of Medicine and Medical Science, University College Dublin, Dublin
ABSTRACT
Background
Adolescence is a time of significant change for patients, guardians and clinicians. The paediatrician must ensure patients develop the necessary skills and knowledge required to transition and to function as an independent entity, with autonomy over their own care. The transfer from paediatric to adult care carries an increased risk of graft-related complications attributable to a multitude of reasons, particularly nonadherence to immunosuppressive medicines and poor attendance at scheduled appointments. This systematic review was conducted to ascertain the transitional care models available to clinicians caring for kidney transplant recipients and to compare the approach in each respective case.
Methods
A systematic review was performed, in a methodology outlined by the PRISMA guidelines. OVID MEDLINE and EMBASE databases were searched for studies that outlined valid, replicable models pertaining to transitional care of paediatric kidney transplant recipients between 1946 and Quarter 3 of 2021. The reference lists of selected articles were also perused for further eligible studies and experts in the field were consulted for further eligible articles. Two investigators assessed all studies for eligibility
and independently performed data extraction. Any discrepancies were settled by consensus.
Results
A total of 1121 abstracts were identified, which was reduced to 1029 upon removal of duplicates. A total of 51 articles were deemed appropriate for full-text review and critical appraisal. A total of 12 articles that described models for transition pertaining to kidney transplant patients were included in qualitative synthesis. Every paper utilized a different transition model. All but one model included a physician and nurse at minimum in the transition process. The involvement of adult nephrologists, medical social work, psychology and psychiatry was variable. The mean age for the initiation of transition was 13.4 years (range: 10–17.5 years). The mean age at transfer to adult services was 18.3 years (range: 16–20.5 years).
Conclusions
Despite the well-established need for good transitional care for paediatric solid-organ transplant recipients, models tailored specifically for kidney transplant recipients are lacking. Further research and validation studies are required to ascertain the best method of providing effective transitional care to these patients. Transitional care should become a standardized process for adolescents and young adults with kidney transplants.
56 UROLOGY FOCUS
Laura J. Smyth1 , Katie R. Kerr1 , Jill Kilner1, Áine E. McGill1, Alexander P. Maxwell1, Amy Jayne McKnight1
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
UROLOGY: PROSTATE CANCER Management of Prostate Cancer in Ireland
In 2022 3,941 people were diagnosed with prostate cancer, making it the most common cancer in Ireland excluding nonmelanoma skin cancer. Worldwide, more than 1 million men are diagnosed with prostate cancer each year and more than 300,000 die of the disease.
Startling statistics show that 1 in 6 men will be diagnosed with prostate cancer during their lifetime, 1 in 8 up to 75 years of age. However, when detected early, prostate cancer has very promising five-year survival rates of 93%.
But like many other forms of cancer, these survival rates are all dependent on when the cancer is detected, and we know that early detection saves lives and improves survival outcomes. The Marie Keating Foundation encourages men to be more open about their health and to speak to their GP about a PSA test when they turn 50, or 45 with a family history of prostate cancer.

Urology Nurse Specialist and Director of Nursing Services at the Marie Keating Foundation, Helen Forristal explains: “Men typically get a bad rap for not speaking about their health, but when resources and supports are put in place, we see that this is not the case. We have yearly Prostate Awareness campaigns urging men to Stand Up For Their Prostate and become more prostate health aware, we provide a host of resources on our website including our Marie Keating Foundation Talks Cancer podcast where we dedicated a whole series to Prostate cancer. We hope that by initiating more conversations we will incite more men to take action. Our message is a simple one, if you are 50, ask your GP to consider checking your PSA levels. If you are 45 with a family history of prostate or breast cancer, have that conversation. It could save your life.”
A PSA or Prostate-Specific Antigen test is a simple blood test that monitors possible changes in your prostate which may need further investigations and follow up. By having regular PSA levels checked as instructed by your GP or Urologist, it can help to detect prostate problems or cancer in its earliest stages, even if you are experiencing no symptoms and
please do remember prostate cancer may have no symptoms or signs at all.
More information on the Prostate and Prostate Cancer
What the prostate is
The prostate is a gland found only in those born as males. In younger men, it is about the size of a walnut, but it can be much larger in older men. The prostate is found below the bladder. It surrounds the first part of the water pipe (urethra), which carries urine from the bladder to the penis. The same tube also carries semen, and the function of the prostate is to thicken the semen for ejaculatory purposes. The prostate gland is divided into 2 lobes, to the left and the right of a central groove.
What the prostate does
The role of the prostate is to make some of the fluid that protects and nourishes sperm cells in semen, making the semen more liquid. The growth and function of the prostate depends on the male sex hormone testosterone, which is produced in the testes. Some treatments for prostate cancer work by lowering the levels of testosterone as prostate cancer is a hormone dependent tumour.
Prostate cancer
Several types of cells are found in the prostate, but almost all prostate cancers develop from the gland cells (the cells that make the prostate fluid that is added to the semen).
Prostate Cancer Facts:
• Prostate cancer is the most common male cancer in Ireland.
• Many men with early prostate cancer have no symptoms at all.
• Because you may not have symptoms, if you are a man over the age of 45 it is really important to talk to your doctor about PSA testing if you have a family history of Prostate or Breast cancer. When you reach 50 irrespective of family history you should talk to your GP about the PSA blood test. It is important to note that the PSA blood test is not a screening tool as it is neither sensitive or specific enough to determine a definite cancer diagnosis and may be elevated because the prostate has simply become
enlarged and is producing more PSA. It is also important to note that activities such as cycling and sexual activity can stimulate the prostate to produce more PSA, so important not to embrace such activity the night before an impending test.
• There has been a significant increase in the number of men diagnosed in recent years due to the increasing use of the PSA blood test together with a Digital Rectal Examination (DRE).
• Prostate cancer responds well to treatment and, if detected early, it can be treated successfully. When prostate cancer is detected early, over 93% of men have a really good five-year survival rate.

* If your father or brother has had prostate cancer, you are twice as likely to get prostate cancer at some point in your life.
We encourage men to take an active role in their health, and to speak to their GP if they have any concerns or worries about a change in their bodies. Prostate cancer is very treatable when caught early.
Prostate Cancer’s early warning signs (note that there may not be any) can include:
• frequency passing urine
• getting up a night-time to go to the toilet
• pain on passing urine,
• difficulty passing urine
• blood in your urine
• hesitancy when you go to the toilet
• urgency about getting to the toilet
• your flow has become weak or intermittent
Other factors to consider would be blood in the semen, back pain, unexplained tiredness and weight loss.
Know your risk factors too.
The risk of developing prostate cancer increases with:
• Age - men over 50 years
• Family history - You are two and a half times more likely to get prostate cancer if your father or brother had it.
• Inherited genes - BRCA gene mutation especially BRCA 2 and Lynch Syndrome
• Race - African Americans at greater risk
• Being overweight or obese
Having a risk factor doesn’t mean that you will definitely develop prostate cancer.
• If you have any of the discussed symptoms, visit your G.P.
• Tests your G.P. may do include:
A blood test called a PSA test.
Digital Rectal Examination (DRE) of your prostate
• Based on the results, your G.P. may refer you to one of the HSE 8 Rapid Access Prostate Clinics (RAPC) where you will be reviewed by a Specialist called a Urologist
• Urologist may repeat the above tests and may take a biopsy of your prostate gland – TRUS biopsy/Transperineal biopsy.
• Further tests may also be required and may include one or more of the following: MRI/ Multiparametric MRI (mpMRI)/ CT scan/Pet-CT scan
57 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
Written by Helen Forristal, Director of Nursing Services, Marie Keating Foundation
UROLOGY: BLADDER CANCER
An Overview of the Management of Non-Muscle Invasive Bladder Cancer (NMIBC)
Bladder cancer is the 10th most common cancer globally and carries the 13th highest cancer related mortality.1 Bladder cancer is the 4th most commonly diagnosed cancer in men and the 10th most commonly diagnosed cancer in women in the US.2
Bladder cancer is four times more common in men than women. The risk factors for bladder cancer can be separated into unavoidable and avoidable. Unavoidable risk factors include aforementioned gender, age and genetics. Bladder cancer is predominantly a disease of older adults, with 90% of cases diagnosed over 55 and 80% over 65 in the United States (US). Cancer syndromes like Lynch syndrome and Cowden’s syndrome can predispose to bladder cancer. Mutations in growth factor receptor FGFR and p53 are also implicated in bladder cancer carcinogenesis.1
Smoking is the most significant and avoidable risk factor for bladder cancer accounting for 50-65% of new cases each year.3 One study found that smoking cessation reduced the risk of bladder cancer by approximately 40% within 1-4 years, with a complete return to baseline by 20 years.4 The importance of smoking cessation is highlighted in an Irish review on the incidence of bladder cancer. This retrospective review found the cases per 100,000 drop from approximately 24 and 9 to 13 and 4 in men and women respectively,
from the year 1996-2015, in line with trends in smoking cessation.5 The second most common avoidable risk factor for bladder cancer is occupational exposure, which accounts for about 18% of bladder cancer cases. One meta-analysis found red meat and processed meat to increase bladder cancer risk by 17% and 10%, respectively, while another meta-analysis found a 22% increase for processed meats and no statistically significant increase for red meat.6, 7 Preobesity and obesity increased the risk of bladder cancer in one meta-analysis by 7% and 10%, respectively.8
With regards to survival rates from bladder cancer, according to the American cancer society, there is a 96% 5-year survival for in situ disease, 69% for localised disease (confined to the bladder), 37% for regional disease (spread to nearby structures or lymph nodes) and 6% for metastatic disease.9 These figures highlight the importance of early detection and management.
The main presentation of bladder cancer is haematuria (blood in the urine). Haematuria is classified as visible (VH) or non-visible (NVH), while NVH is further classified as symptomatic NVH and asymptomatic NVH. The investigation of haematuria is risk stratified- patients with VH >45 years or patients with persistent NVH >60 years being deemed
high risk. Haematuria investigation includes flexible cystoscopy for direct bladder visualisation, upper tract imaging with renal ultrasound or CT and urine for cytology.10


The Model of Care for Urology document recommended haematuria should have a direct access pathway through onestop haematuria clinics.11 These clinics could potentially be led by advanced nurse practitioners.12 At flexible cystoscopy, any abnormality within the bladder can be pictured, and if this abnormality looks suspicious for urothelial cancer, the patient is booked for an urgent transurethral resection of bladder tumour (TURBT). The principle of this procedure is to get a tissue sample that gives an accurate representation of the layers of the bladder, including the urothelium, lamina propria and muscularis propria (muscle). TURBT can be diagnostic but also therapeutic. Getting a good tissue sample ensures the histopathologist can demarcate whether the sample of tissue is in situ – stage pTis (confined to urothelium- but flat), pTa: non-invasive (confined to urothelium but often papillary growing in finger-like projections toward the hollow centre of the bladder), pT1 (invading lamina propria), pT2 (invading muscle), pT3 (invading peri-vesical fat) or pT4 (invading surrounding structures like prostate, uterus or vagina). The majority (95%) of
the specimens will be transitional cell carcinomas (TCC).2 Bladder cancer is then categorised as nonmuscle invasive (up to 80%) and muscle invasive based on depth of invasion. These should also be reported with grades 1-3, with grade 1 being the least anaplastic, grade 3 being the most and grade 2 being somewhere in the middle. The patient’s risk of disease progression can be calculated as low, intermediate, high or very high using the EAU non-muscle invasive bladder cancer (NMIBC) Risk calculator https://www.nmibc. net/. This calculator also gives the patient’s probability of progression and 95% confidence interval at 1, 5 and 10 years. This risk is calculated based upon tumor size, grade, multifocality, presence of carcinoma in situ (CIS), age of patient and whether it is a primary or recurrent tumor.
Management of low-risk bladder cancer


If a bladder cancer is suspected or known to be low or intermediate risk, a patient should receive a single postoperative instillation of intravesical chemotherapy, e.g., mitomycin C, within 24 hours of TURBT. The patient should then be enrolled in standard low-grade flexible cystoscopy followup, including a check flexible cystoscopy at 3 & 12 months postoperatively and annually thereafter until five years. Urine for cytology is not recommended for low-risk bladder cancer follow-

JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 58
Charles O’Connor
Written by Charles O’Connor, Mohammed Hegazy, David Galvin, Gregory Nason Department of Urology, Mater Misericordiae University Hospital, Dublin
Mohammed Hegazy David Galvin
Gregory Nason
up. At the 5-year mark, recurrence is unlikely and follow up can be decided between clinician and patient, but some patients like to continue surveillance for peace of mind. If the patient has a recurrence during follow-up, a repeat TURBT should be carried out and their risk of progression re-calculated. If there is a low volume, low-grade recurrence, this patient should maintain in the low-risk category, provided recurrence was not in less than one year; however, if there is a high-volume, low-grade recurrence, this patient would be switched to the intermediate-risk group.
Management of Intermediate risk bladder cancer
One option for these patients is intravesical chemotherapy which has fewer side effects than BCG therapy. The optimal dosing schedule and duration for intravesical chemotherapy is still unknown.13 These patients can also be offered an induction course of Bacillus Calmette-Guerin (BCG). Induction BCG is a six successive weekly course of 1 hour BCG instillations. If patients do not respond to this, they can be offered a re-induction. If the patient responds to either of these inductions, they can be enrolled in maintenance BCG for one year, which comprises BCG given once a week for three successive weeks at 3-, 6- and 12-months post-induction. After this, they can continue surveillance according to the high-grade, flexible cystoscopy follow up schedule, which is every three months for two years, then every six months for three years
and annually thereafter. As these patients are intermediate risk there may be more leniency with this schedule. Urine for cytology should be checked at each flexible cystoscopy, similar to high-risk patients. If an intermediaterisk bladder cancer does not respond to 2 inductions of BCG or maintenance BCG, they should be offered a radical cystectomy, if fit. If patients are unfit, they should be enrolled in a clinical trial; if no trial is available, they should have intravesical chemotherapy if none has already been given. If an intermediate-risk patient recurs in less than a year after one-year maintenance BCG, they are deemed BCG refractory and they should be offered cystectomy; if they recur over a year after one-year maintenance BCG, they may be offered a re-induction of BCG. If a patient recurs after chemotherapy, they may still be offered BCG therapy as intravesical chemotherapy has no effect on BCG instillations.14
Management of high-risk bladder cancer
Patients with pT1 and highgrade pTa tumours should have a re-resection within six weeks of initial TURBT. Patients with pT1 tumours, any high-grade tumour or CIS are deemed high risk for progression. If a patient is pT1, high risk according to the EAU risk calculator and do not respond to induction BCG; they should be offered radical cystectomy. If they are unfit, they should be enrolled
in a clinical trial and intravesical chemotherapy if unavailable. Other high-risk patients at a lower stage than pT1 may be offered a trial of re-induction before progressing in the same manner. If high-risk patients respond to induction or re-induction BCG, they may be enrolled in maintenance BCG for three years.15
Management of very high risk bladder cancer
A subset of patients with NMIBC are deemed very high risk- These patients include very high-risk pT1 disease, lymphovascular invasion & variant histology, e.g., plasmacytoid. These patients should be considered for up front radical cystectomy.

Intravesical immunotherapy with BCG
BCG is the gold standard bladder instillation for reducing the risk of recurrence and progression in bladder cancer. Simply put, BCG works by three steps:
1. Infection of the urothelium,
2. Induction of an immune response and 3. Inducing an anti-neoplastic effect. Infection of the urothelium promotes immune response by both TH1-cytokines (cell-mediated acquired immune response) and TH2-cytokines (humoral immune response) which synergistically provide anti-neoplastic activity.16
A systematic review and metaanalysis by Chou et al. from 2017
concluded that while several forms of intravesical therapy decreased the risk of recurrence versus TURBT alone, BCG was the only therapy to decrease the risk of progression versus TURBT alone. This review also found that BCG maintenance regimens were associated with a decreased risk of recurrence vs intravesical chemotherapy; the strength of evidence was low. The rate of adverse events was higher with BCG vs intravesical chemotherapy, including haematuria, granulomatous cystitis, dysuria and fever; strength of evidence was low.17
Intravesical chemotherapy
The most common form of intravesical chemotherapy used in Ireland is Mitomycin C (MMC). Other examples of intravesical chemotherapy include gemcitabine, epirubicin, valrubicin, doxorubicin, thiotepa, interferon, apaziquone, paclitaxel and docetaxel. Different chemotherapy agents have different mechanisms of action, benefits and risks. For example, an alkylating agent, MMC, has a low molecular weight, meaning better bladder wall penetration with increased risk of allergic skin reaction and possible systemic absorption. In contrast, the anthracyclines, e.g., epirubicin and doxorubicin, have high molecular weight and
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
59
Figure 1. Flow diagram of NMIBC with most common interventions in each risk group
NMIBC low-risk post-op MMC intermediate -risk post-op MMC BCG/Chemo high-risk BCG very high-risk Cystectomy
Figure 1. Flow diagram of NMIBC with most common interventions in each risk group
lower systemic absorption.18
All chemotherapy agents have the general risks of haematuria, dysuria and frequency, but to a lesser extent than BCG. The role of these, as mentioned previously, is to be used either as a single dose postoperatively in low and intermediate-risk bladder cancer, as possible adjuvant treatment in intermediate-risk bladder cancer and when BCG fails.
Patients with BCG unresponsive tumours who are not radical cystectomy candidates
This subgroup of patients should be enrolled in a clinical trial if available. If no trial is available, they may be offered intravesical chemotherapy. A Recent randomised controlled trial looked at the effect of MMC with radiofrequency-induced thermochemotherapy effect (RITE).
Patients who recurred after at least one induction course of BCG patients were randomised to MMC with RITE vs control second line therapy- BCG, MMC & MMC and electromotive drug administration. This study found higher diseasefree survival for MMC with RITE in non-CIS papillary tumours (53% vs 24%), suggesting patients with papillary tumours may benefit from this.19 A 2021 phase 2 clinical trial in the Lancet examined the effect
of pembrolizumab monotherapy (200mg intravenously every 3 weeks for up to 24 months) in high risk NMIBC patients unresponsive to BCG. This initially showed promising results with 39 (41%) of 96 patients showing complete response at 3 months. However, only 11% of the initial cohort remained in remission. This means pembrolizumab monotherapy delayed cystectomy in a small number of patients. Given 95% of this patient population were eligible for, but declined cystectomy, it was not considered a sensible treatment option, but may be beneficial for patients who are unfit for cystectomy.20
The financial cost of bladder cancer
Bladder cancer is the most expensive cancer from a health economic perspective given the frequent surveillance schedules, the intravesical treatments and the reoperation rates due to risk of recurrence and progression. As a result, it is key to establish the risk category of the patient accurately at diagnosis and ensure they receive optimal treatment.21
Challenges in the management of bladder cancer
One of the biggest challenges faced with the management of
UROLOGY: BLADDER CANCER
bladder cancer is the lack of supply of BCG and the lack of resources to enrol patients in maintenance BCG. This problem started in 2017 when one of the two suppliers of BCG, Sanofi Pasteur, shut down production. Merck Millipore remains the sole producer of BCG for the US, Canada and 70 countries worldwide. This underproduction means that supply cannot keep up with demand despite production being increased by 100%. This BCG shortage encouraged the use of alternative treatments like intravesical chemotherapy, particularly in intermediate-risk bladder cancer, where there may be less of a distinction regarding inferiority vs BCG. The advice was also given during BCG shortage to curtail maintenance BCG if patients had already received it for a year or two. The priority for usage of BCG was to get high risk, first diagnosis patients, through their induction and 3-month doses of BCG.22 It was also suggested that patients could be considered for early radical cystectomy which is a significant undertaking. While this shortage is not felt as strongly now, there were occasions over the preceding year or two where BCG was unavailable. This hold on the delivery of maintenance and sometimes induction BCG meant that the resources of staffing, hospital beds or outpatient rooms
Enhancing Bowel Cancer Treatment
Dr Aideen Ryan, Associate Professor in Tumour Immunology at University of Galway’s College of Medicine, Nursing and Health Sciences

Credit - Andrew Downes, XPOSURE
diagnosed cases of bowel cancer every year, with limited treatment options for patients at advanced disease stage.
molecules enhances the immune response in tumours that have high levels of these cells.”
were redirected elsewhere in some hospitals. In a situation where BCG is now becoming again more accessible, this reorganisation to put in place effective BCG induction delivery is a challenge, with the delivery of maintenance BCG being a more significant challenge. Nonetheless, these efforts to re-establish BCG delivery are rightly well supported.
Conclusion
NMIBC is common. Patients often present with VH. Diagnosis is made by means of a cystoscopy and TURBT. From a urologist’s perspective, the crucial part of NMIBC management is the risk stratification of patients. Once the risk is calculated, the next part of management is following the correct treatment algorithm for a patient based on risk category and to engage in multi-disciplinary decision making if necessary. The wider hospital team can also help manage patients with bladder cancer; this may include aiding with resources for effective and timely BCG delivery.
Smoking is the key risk factor for bladder cancer that cannot be overlooked or disregarded, and in this regard, there is a duty on patients to manage NMIBC- to help prevent it from occurring or recurring.
References available on request
Researchers at University of Galway studying cell interactions in bowel cancer have identified innovative strategies to enhance how the body and drug treatments fight the disease.
Colorectal, also known as bowel, cancer is a leading cause of death globally with increasing incidence in developing countries and in younger people. In Ireland alone, there are more than 2,500 newly

Aideen Ryan, Associate Professor in Tumour Immunology at University of Galway’s College of Medicine, Nursing and Health Sciences, said, “Unfortunately, a high proportion of colorectal cancer patients do not respond to immunotherapy. We have identified sugar coated molecules with sialic acid, called sialoglycans, that are present on cells in tumours, known as stromal cells. These are associated with poor responses to immunotherapy. Targeting these
The research was carried out by University of Galway in collaboration with VUB, Belgium; Palleon Pharmaceuticals, Boston, USA; CÚRAM, the SFI Research Centre based at University of Galway; Glasgow Beatson Institute for Cancer Research; Queen’s University Belfast.
The researchers studied a previously unknown mechanism of stromal cell immunosuppression. It occurs as sugar coated molecules expressed on the stromal cell surface binds to specific protein receptors expressed on the surface of immune T-cells.
The sugars - sialic acids (or sialoglycans) – bind to receptors
called Siglecs. The Siglecs stop the cancer killing T cells from working. The research showed that stromal cells, when exposed to inflammatory molecules released by bowel cancer cells, express increased amounts of the sialoglycans - on their surface. It also showed that T cells could be re-activated by using specific drugs to disrupt the binding between the cells.
The researchers tested the findings using stromal cells isolated from bowel cancer patient biopsies and got the same results, confirming that targeting the binding of sialic acid/Siglecs may represent an innovative strategy to enhance anti-tumour immunity in immunosuppressive tumour microenvironments.
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 60
News
UROLOGY: OVERACTIVE BLADDER
The overactive bladder complex: management and case studies

The idiopathic overactive bladder (OAB) is a clinically-diagnosed symptom complex consisting of frequency, urgency and nocturia with or without urge associated urinary continence. This complex assumes no underlying cause as a source of bladder dysfunction. The alternate term of detrusor overactivity on the other hand is a urodynamic diagnosis based on cystometrography (CMG) – previously termed bladder instability – which is characterised by an unprovoked rise in detrusor pressure during the filling phase of a CMG, and is often associated with a clinical feeling of urgency in a normally sensate individual.
There have been challenges in determining what is defined by normal, and the International Continence Society (ICS) now suggests that clinical deterioration is what is perceived by the patient, and leads them to seek medical help. In addition to ‘normal’ voiding patterns during the day, the ICS has qualified this as a “sudden and compelling desire to pass urine, which cannot be deferred”, and may lead to urinary incontinence.1 Epidemiological studies have found that the prevalence of this condition is rising, and now affects approximately 16% of European and American populations.2
Diagnosis and management are important in minimising OAB’s impact on activities of daily living. The burden of OAB is even heavier in the older population.
The symptoms can be defined as the following:
Urgency – sudden, compelling desire to void that is difficult to defer
Daytime frequency – the need to frequently urinate during the day
Nocturia – waking up at night to void
Urgency urinary incontinence may be present but is not necessary for a diagnosis of OAB.
Overactive bladder will become an increasingly prevalent problem as populations age. It is associated with additional health risks in older patients, particularly with falls and fractures. It is associated with increased mortality and may be a predictor of institutionalisation (especially in the presence of urinary incontinence).
Older patients are less likely to discuss their OAB symptoms with their healthcare provider, and are more likely to be untreated or undertreated. OAB may interfere with social and occupational activities.
Assessment
We need to tailor investigations to suit the individual, their environment, lifestyle, age, health, expectations, and the mode of treatment we wish to prescribe.
A 20-year-old female with OAB may be particularly bothered by troublesome nocturia as she needs to wake early and work a full day, or by nocturnal enuresis which can affect new relationships. On the other hand, an 80-year-old man might be most upset if he cannot visit friends/relatives without the psychosocial embarrassment of urge associated incontinence.
It is very important however, to exclude urinary tract infections and other associated differential diagnoses before applying a label of an overactive bladder (see Table 1). An important differential for reversible causes of urinary incontinence can be remembered by the mnemonic ‘DIAPPERS’:
Delirium
Infection
Atrophic vaginitis/urethritis (females)
Pharmaceuticals
Psychological problems
Excess urine output
Reduced mobility
Stool impaction.
Urological
UTI
Urethral syndrome
Detrusor overactivity
Bladder tumour
Bladder calculus
Gynaecological
Cystocele
Previous pelvic surgery
Genital
Urethritis
Vulvo-vaginitis
Urethral caruncle
Medical
Upper motor neurone lesion
Impaired renal function

Congestive heart failure
General
Excessive intake
Pregnancy
It is important that urinary tract infections be identified and adequately treated prior to the undertaking of further investigations or prescribing pharmacological treatment for OAB. To this end, it is important to consider the fastidious organisms mycoplasma hominis, ureaplasma
Urethral diverticulum
Small capacity bladder
Interstitial cystitis
Radiation cystitis/fibrosis
Chronic residual
Pelvic mass (fibroids)
Atrophy
Herpes
Warts
Diabetes mellitus
Diabetes insipidus
Anxiety Habit
urealyticum, and chlamydia trachomatis, which are commonly found in both urine and vaginal discharge. As these organisms are fastidious, they must be specifically cultured, and an index of suspicion is required. Treatment for these is low-dose tetracycline (doxycycline) for three months,
61 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
Dr Fardod O'Kelly, Clinical Fellow in Paediatric Urological Surgery, RCSI
We need to tailor investigations to suit the individual, their environment, lifestyle, age, health, expectations, and the preferred mode of treatment
Table 1. Differential diagnosis of OAB
compared to a standard seven to 10 day course of a broad spectrum antibiotic for bacterial organisms.
Frequency-volume chart
The frequency-volume chart, also known as a bladder or voiding diary, is a valuable tool in assessing OAB symptoms; it identifies voiding patterns and the balance between diurnal and nocturnal urinary frequency. They identify those who are drinking a lot of fluid through the day. If the patient’s symptoms are not explained by the voiding diary, one might need to undergo urodynamic investigations, defined as measurements to quantify the ability of the bladder to store and expel urine.
NICE guidelines recommend use of a diary for a minimum of three days to include a combination of work and leisure days.3
Uroflowmetry
Uroflowmetry is the most simple of the urodynamic tests; it is not invasive and is physiological. In normal individuals, the maximum urinary flow rate should be in the region of > 15mL/s for a voided volume of > 150mL. As a rule, a low flow rate implies voiding difficulties, due either to a hypotonic detrusor or to an obstructed outlet, which cannot be differentiated without further assessment. The importance of uroflowmetry in OAB lies in the tendency of the antimuscarinic agents to impair voiding function. Thus, to avoid the possibility of retention, one needs to know the patient’s pre-treatment flow rate as well as postvoid residual urine volume.
UROLOGY: OVERACTIVE BLADDER
CASE STUDY 1
PK is a 70-year-old male who until about a year ago remained quite active. His background history includes an ST-elevation myocardial infarction (seven years ago), and an open radical retropubic prostatectomy two years ago.
Sensitive subjects can be difficult to discuss. As you enquire about his health and how he is recovering since his surgery, you specifically ask if he has any troubles with his bladder. He mentions that these days he has to make sure that wherever he goes there will be a bathroom. As you reassure PK that this is a common concern and that there are ways to treat it, he begins to open up about how the problem has been affecting him. You learn that he has become increasingly restricted in his activity because of his urinary frequency and urgency. He also has occasional urinary incontinence. He is concerned and upset that he can no longer visit his grandchildren because long excursions from home cause him tremendous anxiety about whether he will be able to reach a bathroom as required. Even short trips away from home for such tasks as going into town to shop for necessities are becoming problematic, and he increasingly relies on his wife to do these things. He is beginning to feel isolated and depressed.
PK’s weight is 78kg and his blood pressure is 121/82. His medications include levothyroxine 50mcg once daily, rosuvastatin 10mg once daily, low-dose aspirin 75mg once daily, amlodipine 5mg once daily, pantoprazole magnesium 40mg once daily, metoprolol SR 50mg twice daily, ramipril 5mg twice daily, and furosemide 40mg twice daily.
There are a number of conditions that can contribute to OAB symptoms, and it is important to consider these during initial workup (see Table 2). In this case, it is important to note that diuretics, especially rapidacting agents, cause a rapid increase in bladder volume, which may precipitate urgency and detrusor overactivity. Anticholinergic agents, opioids and calcium-channel blockers decrease bladder contractility and may cause urinary retention with a decreased functional bladder capacity. Cholinesterase inhibitors could contribute to detrusor overactivity by increasing acetylcholine levels, and should be suspected in patients in whom symptoms develop after initiation of treatment.
Based on your recommendation following a frank discussion relating to his ideas, concerns and expectations, you have made some adjustments to his medication regimens, as well as some lifestyle changes, including reducing his caffeine intake.
Behavioural intervention
urinary incontinence in patients > 65 years is associated with a twofold increased relative risk of impaired functioning in activities of daily living. You recommend to PK that he begin pharmaceutical treatment, however he wants to know about what is available and how these work.

Pharmacological treatment
Three classes of treatment are indicated in the management of OAB:
Clinical guidelines recommend first-line pharmacotherapy with antimuscarinic agents, which have been in use for 30 years
A newer treatment option is a selective beta 3 adrenoceptor agonist, mirabegron
Botulinum toxin type A was approved for intradetrusor injections for the secondline treatment of OAB in adult patients who have an inadequate response to or are intolerant of antimuscarinic medication.
Antimuscarinics
Neurological conditions
Systemic conditions
Medication
Multiple sclerosis, spinal cord injury, neurodegenerative disease
Congestive heart failure, diabetes mellitus, constipation
Diuretics, anticholinergics, opioids, calcium channel blockers, cholinesterase inhibitors, alphaagonists, antihistamines
Dietary/lifestyle
Caffeine/alcohol intake, impaired mobility, excessive fluid intake
Behavioural intervention improves central control, based on the idea that if people can learn to become continent in infancy, they can re-learn it in adult life. This approach avoids the morbidity and mortality associated with surgery, as well as the sideeffects of drugs. Bladder ‘drills’ were first described between 1978 and 1980, and following the exclusion of any other relevant pathology, the patient is given an achievable target time for using the toilet, before which they may not do so. The time target is set low enough in order to allow the patient to wait for long enough without wetting. After that goal is met, the target time is increased. The patient maintains a normal fluid intake and keeps their own fluid-balance chart. This works better with continued supervision and encouragement, and exercise compliance rates tend to decrease at home. The relapse rate is high. Other interventions include a reduction in caffeine, weight loss and evening fluid intake.
Despite these drug changes and behavioural interventions, PK has continued to have persistent symptoms and occasional urgency incontinence after three months of good compliance. You are concerned about his lack of progress. Evidence has shown that
A major meta-analysis of trials of antimuscarinic agents for OAB found antimuscarinics efficacious compared to placebo.4 Differences in the relative efficacy of the agents was not apparent, except that some results showed statistical significance in favour of the higher dosage of fesoterodine and solifenacin over placebo and lower dose antimuscarinics.
Options for antimuscarinic treatment include darifenacin extended release tablets, 7.5mg or 15mg; fesoterodine extended-release tablets, 4mg or 8mg; oxybutynin, 5mg regular (immediate release) or extended release tablets, 5mg, 10mg, twice weekly dosing, 36mg (3.9mg/day system); solifenacin succinate tablet, 5mg or 10mg; tolterodine regular or extended release capsules, 2mg or 4mg; and trospium chloride coated tablet, 20mg.
Antimuscarinics differ in their frequency of administration, receptor selectivity and specificity, binding affinity, and other characteristics. The primary sites of activity are the M2 and M3 receptors of the bladder, which mediate contraction. Selectivity for muscarinic receptors is the main reason for variation in side effects across the antimuscarinic agents. Action on muscarinic
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 62
Table 2. Conditions and medications that may contribute to OAB symptoms
50mgs once daily
BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets.
Prescribing Information: BETMIGA™ (mirabegron)
For full prescribing information, refer to the Summary of Product Characteristics (SPC). Name: BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets. Presentation: Prolongedrelease tablets containing 25 mg or 50 mg mirabegron. Indication: Symptomatic treatment of urgency, increased micturition frequency and/or urgency incontinence as may occur in adult patients with overactive bladder (OAB) syndrome. Posology and administration: The recommended dose is 50 mg orally once daily in adults (including elderly patients). Mirabegron should not be used in paediatrics for OAB. A reduced dose of 25 mg once daily is recommended for special populations (please see the full SPC for information on special populations). The tablet should be taken with liquids, swallowed whole and is not to be chewed, divided, or crushed. The tablet may be taken with or without food. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SPC. Severe uncontrolled hypertension defined as systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 110 mm Hg. Warnings and Precautions: Renal impairment: BETMIGA has not been studied in patients with end stage renal disease (eGFR < 15 ml/min/1.73 m2 or patients requiring haemodialysis) and, therefore, it is not recommended for use in this patient population. Data are limited in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2); based on a pharmacokinetic study (see section 5.2 of the SPC) a dose of 25 mg once daily is recommended in this population. This medicinal product is not recommended for use in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC).
Hepatic impairment: BETMIGA has not been studied in patients with severe hepatic impairment (ChildPugh Class C) and, therefore, it is not recommended for use in this patient population. This medicinal product is not recommended for use in patients with moderate hepatic impairment (Child-Pugh B) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC). Hypertension: Mirabegron can increase blood pressure. Blood pressure should be measured at baseline and periodically during treatment with mirabegron, especially in hypertensive patients. Data are limited in patients with stage 2 hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg). Patients with congenital or acquired QT prolongation: BETMIGA, at therapeutic doses, has not demonstrated clinically relevant QT prolongation in clinical studies (see section 5.1 of the SPC). However, since patients with a known history of QT prolongation or patients who are taking medicinal products known to prolong the QT interval were not included in these studies, the effects of mirabegron in these patients is unknown.
Caution should be exercised when administering mirabegron in these patients. Patients with bladder outlet obstruction and patients taking antimuscarinics medicinal products for OAB: Urinary retention in patients with bladder outlet obstruction (BOO) and in patients taking antimuscarinic medicinal products for the treatment of OAB has been reported in postmarketing experience in patients taking mirabegron. A
controlled clinical safety study in patients with BOO did not demonstrate increased urinary retention in patients treated with BETMIGA; however, BETMIGA should be administered with caution to patients with clinically significant BOO. BETMIGA should also be administered with caution to patients taking antimuscarinic medicinal products for the treatment of OAB. Interactions: Caution is advised if mirabegron is co-administered with medicinal products with a narrow therapeutic index and significantly metabolised by CYP2D6. Caution is also advised if mirabegron is co-administered with CYP2D6 substrates that are individually dose titrated. In patients with mild to moderate renal impairment or mild hepatic impairment, concomitantly receiving strong CYP3A inhibitors, the recommended dose is 25 mg once daily. For patients who are initiating a combination of mirabegron and digoxin (P-gp substrate), the lowest dose for digoxin should be prescribed initially (see the SPC for full prescribing information). The potential for inhibition of P-gp by mirabegron should be considered when BETMIGA is combined with sensitive P-gp substrates. Increases in mirabegron exposure due to drug-drug interactions may be associated with increases in pulse rate. Pregnancy and lactation: BETMIGA is not recommended in women of childbearing potential not using contraception. This medicinal product is not recommended during pregnancy. BETMIGA should not be administered during breast-feeding. Undesirable effects: Summary of the safety profile: The safety of BETMIGA was evaluated in 8433 adult patients with OAB, of which 5648 received at least one dose of mirabegron in the phase 2/3 clinical program, and 622 patients received BETMIGA for at least 1 year (365 days). In the three 12-week phase 3 double blind, placebo controlled studies, 88% of the patients completed treatment with this medicinal product, and 4% of the patients discontinued due to adverse events. Most adverse reactions were mild to moderate in severity. The most common adverse reactions reported for adult patients treated with BETMIGA 50 mg during the three 12-week phase 3 double blind, placebo controlled studies are tachycardia and urinary tract infections. The frequency of tachycardia was 1.2% in patients receiving BETMIGA 50 mg. Tachycardia led to discontinuation in 0.1% patients receiving BETMIGA 50 mg. The frequency of urinary tract infections was 2.9% in patients receiving BETMIGA 50 mg. Urinary tract infections led to discontinuation in none of the patients receiving BETMIGA 50 mg. Serious adverse reactions included atrial fibrillation (0.2%). Adverse reactions observed during the 1-year (long term) active controlled (muscarinic antagonist) study were similar in type and severity to those observed in the three 12-week phase 3 double blind, placebo controlled studies. Adverse reactions: The following list reflects the adverse reactions observed with mirabegron in adults with OAB in the three 12-week phase 3 double blind, placebo controlled studies. The frequency of adverse reactions is defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000) and not known (cannot be established from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. The adverse events are grouped by MedDRA system organ class. Infections and infestations:
Common: Urinary tract infection, Uncommon: Vaginal infection, Cystitis. Psychiatric disorders: Not known (cannot be estimated from the available data): Insomnia*, Confusional state*. Nervous system disorders:
Common: Headache*, Dizziness*. Eye disorders: Rare: Eyelid oedema. Cardiac disorders: Common: Tachycardia, Uncommon: Palpitation, Atrial fibrillation. Vascular disorders: Very rare: Hypertensive crisis*. Gastrointestinal disorders: Common: Nausea*, Constipation*, Diarrhoea*, Uncommon: Dyspepsia, Gastritis, Rare: Lip oedema. Skin and subcutaneous tissue disorders: Uncommon: Urticaria, Rash, Rash macular, Rash papular, Pruritus, Rare: Leukocytoclastic vasculitis, Purpura, Angioedema*. Musculoskeletal and connective tissue disorders: Uncommon: Joint swelling. Renal and urinary disorders: Rare: Urinary retention*. Reproductive system and breast disorders: Uncommon: Vulvovaginal pruritus. Investigations: Uncommon: Blood pressure increased, GGT increased, AST increased, ALT increased. * signifies adverse reactions observed during post-marketing experience. Prescribers should consult the SPC in relation to other adverse reactions. Overdose: Treatment for overdose should be symptomatic and supportive. In the event of overdose, pulse rate, blood pressure, and ECG monitoring is recommended. Basic NHS Cost: Great Britain (GB)/Northern Ireland(NI): BETMIGA 50 mg x 30 = £29, BETMIGA 25 mg x 30 tablets = £29. Ireland (IE): POA. Legal classification: POM. Marketing Authorisation number(s): (GB): PLGB 00166/0415-0416. NI/IE: EU/1/12/809/001-006, EU/1/12/809/008-013, EU/1/12/809/015018. Marketing Authorisation Holder: GB: Astellas Pharma Ltd., 300 Dashwood Lang Road, Bourne Business Park, Addlestone, United Kingdom, KT15 2NX. NI/IE: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of Preparation of Prescribing information: January 2023. Job bag number: MAT-IE-BET-2023-00001. Further information available from: GB/NI: Astellas Pharma Ltd, Medical Information: 0800 783 5018. IE: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. For full prescribing information, please see the Summary of Product Characteristics, which may be found at: GB: www.medicines.org.uk; NI: https://www.emcmedicines.com/en-GB/northernireland/; IE: www.medicines.ie.
United Kingdom (GB/NI)

Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018. Ireland
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com.

Date of preparation: January 2023 References: 1. Freeman R, et al. Current Medical Research and Opinion 2017. https://doi.org/10.1080/03007995.2017.1419170. MAT-IE-BET-2023-00003
His 14th walk in the park since the day he started BETMIGA1
receptors in the body beyond the detrusor muscle leads to side effects. The side effect profile of each antimuscarinic differs with possible adverse effects that can, depending on the agent, include dry mouth, constipation, headache, blurred vision, pruritus, tachycardia, somnolence and impaired cognition.
Mirabegron (beta 3 adrenoceptor agonist)
Mirabegron is an oral, once-daily, selective beta-3 adrenoceptor agonist that has shown similar efficacy to antimuscarinics.
Mirabegron seems to act on the storage phase of bladder function to facilitate smooth muscle relaxation, for increased bladder capacity and lengthened interval between voiding.
Mirabegron extended-release tablets are available in 25mg and 50mg. Mirabegron is generally well tolerated; the most commonly reported adverse reactions (> 3% of patients taking mirabegron 50mg/day) were hypertension, urinary tract infection, headache and nasopharyngitis. The incidence of dry mouth and other side effects was similar in rate to placebo.
Botulinum toxin
Botulinum injections are now indicated for the treatment of OAB when there has been inadequate response to antimuscarinic
UROLOGY: OVERACTIVE BLADDER

indicate that she still has clinical evidence of urgency incontinence, and her symptoms of urgency and frequency with leakage match the definition of OAB (wet). Following a balanced review, and a frank risk/benefit discussion, you have recommenced her treatment cycle. Conservative therapy: This includes fluid adjustment, with an encouraged fluid intake of between six and eight cups with a balanced intake throughout the day but decrease evening intake due to nocturia. She should continue to eliminate caffeine and check for other bladder irritants. One also needs to encourage weight loss, as it may improve urgency symptoms
therapy. Botulinum toxin exerts its effect by cleaving key proteins required for nerve activation. First, the toxin binds specifically to nerves which use the neurotransmitter acetylcholine. Once bound to the nerve terminal, the neuron takes up the toxin into a vesicle. As the vesicle moves further into the cell, it acidifies, activating a portion of the toxin which triggers it to push across the vesicle membrane and into the cell cytoplasm. Once inside the cytoplasm, the toxin cleaves SNARE proteins preventing the cell from releasing vesicles of neurotransmitter. This stops nerve signalling, leading to paralysis. Clostridium botulinum type A, a neurotoxin, is injected into the detrusor muscle via a flexible or rigid cystoscope. The needle is inserted into the detrusor and 20 injections are delivered. Clinical improvement may occur within two weeks.
Patients may be considered for reinjection when the clinical effect of the previous injection has diminished, but no sooner than three months from the prior bladder injection. The most common adverse reactions with botulinum injections in the first 12 weeks post-op include urinary tract infections (26%), dysuria (11%), bacteriuria (8%), urinary retention (6%), increased residual urine volume (3%), and pollakiuria (daytime frequency) (2%).
CASE STUDY 2
SP is a 54-year-old female with a five-year history of urinary incontinence. She works as a zoo-keeper which is a very active job, and is distressed with the impact this is having on her work and social life. She experiences frequency every 30-60 minutes during the day and wakes up twice at night to urinate. She gets a sudden urge to void and this is associated with an involuntary leakage of moderate amounts of urine, especially at night. Her urgency is triggered by running water and cold temperatures. She had a hysterectomy at age 45. Her BMI is 29.5kg/m2 (overweight) but she is otherwise healthy, and does not experience any urinary tract infections. Following an initial consultation with her primary care physician, she has restricted her fluid intake (especially after 6pm), has given up caffeine to good effect, and no longer drinks alcohol. Her bladder diary shows a low fluid intake (one cup of water in the early morning and two cups in the evening). She voids 10 times during the day. Her leakage is both diurnal and nocturnal and usually happens on the way to the bathroom. As a result, she has resorted to wearing two to three incontinence pads per day.
SP returns to the office with a completed bladder diary for a further clinical review. These
Behavioural therapy: This includes pelvic floor muscle training, urge suppression and bladder retraining, which will progressively increase sphincter tone, as well as urge suppression and pelvic muscle contractions. Biofeedback training given by a clinical nurse specialist can also help identify voiding issues, and can curtail storage lower urinary tract symptoms
Medication: As described above, if conservative therapies are unsuccessful, a trial of antimuscarinic (anticholinergic) medication (eg. oxybutynin, tolterodine, darifenacin, solifenacin or trospium) should be instigated. A combination of drug and behavioural therapy might act synergistically to improve her symptoms. If incontinence persists, she may be able to use a product suitable for her absorbency requirement. It might be an extra absorbent pad, undergarment or protective underwear. Further discussion should then be had regarding more invasive treatments.
CASE STUDY 3
AD is a 12-year-old male who had closure of a T10 myelomeningocoele as a neonate. He is ambulant with walking aids, and is on long-term bowel management in the form of laxatives. He also complains of recurrent MSU-confirmed UTIs, and urge-associated urinary incontinence.
In the paediatric population, much of the symptomatology is difficult to elicit directly from patients, and one must rely to an extent on the history presented by a parent/guardian. The definitions remain broadly similar to the adult population,

JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 64
however, children broadly fall into four categories when dealing with voiding dysfunction:
Voiding dysfunction with or without constipation (bladder bowel dysfunction)
Neurogenic bladder
Congenital structural anomalies (ectopic ureter leading to continual incontinence)
Idiopathic OAB.
By far, the most common of these is the first category of voiding dysfunction with/ without constipation. However, the most feared is the accurate diagnosis, and management of the neurogenic bladder. Broadly speaking, congenital neurogenic bladders can be categorised into open or occult neural tube defects (NTD), sacral agenesis, spinal cord lesions and cerebral palsy.
Open NTDs can be subcategorised into (lipo)myelomeningocoele (exposed meninges and neural tissue +/- lipoma of the cord), and meningocoele (exposed meninges only). These are thought to affect 1/1,000 births, and rates vary in different geographic regions with access to prenatal ultrasonographic scanning and differing legislation determining termination. They affect the lumbosacral/sacral regions of the cord in approximately 67% of patients, and are usually closed as a neonate. Interestingly, the
severity of symptom complex cannot be predicted as easily as that of a spinal cord injury, however, as a rule of thumb, the higher the lesion, the more likely the degree of severity. Occult NTDs and spinal cord tethering (due to lipomeningocoele, intradural lipoma, dermoid cysts/ sinuses, tight filum terminale) are thought to affect 1/4,000 births, and often don’t present clinically until patients demonstrate a failure to achieve continence.
Urodynamic studies may demonstrate lower tract dysfunction such as large capacity, poorly emptying bladders with associated poor sphincter functions. Neurosurgical correction has been shown to improve outcomes in this cohort, however, there is a paucity of prospective data in this cohort. Sacral agenesis (associated with maternal diabetes mellitus) can be associated with a wide range of neurologic deficits associated with voiding dysfunction, and just over one-third will demonstrate evidence of poor compliance and increased bladder filling pressures on urodynamic studies.
Management of AD’s condition is quite different from the storage lower urinary tract symptoms described in the adult cases outlined above and, following a careful initial history, physical exam, voiding and stooling diaries,

as well as a baseline ultrasound of the abdomen/pelvis, it is also important to involve initial urodynamic studies and longterm follow up for vesicoureteric reflux, incontinence and upper tract deterioration. Those patients with associated detrusor-sphincter dyssinergia (DSD) are more likely to develop poor bladder compliance and worsening upper tracts. Bowel management is also especially important in this cohort. In adolescence and adulthood, management of erections, sexual function and fertility prove a challenging but importance facet to their care.
Urinary incontinence in women
In 2004, the International Consultation on Incontinence (ICI) devised an algorithm that summarised the proposed initial management of urinary incontinence in women.5 This includes an initial history and symptom assessment taking into account co-morbid conditions, lifestyle choices and medications, and following which patients are divided into categories of stress urinary incontinence, mixed urinary incontinence, urgeassociated urinary incontinence and/or OAB symptoms. After careful clinical evaluation with physical examination (including
UCD Researcher receives ERC Funding
Dr Philip Cardiff, Associate Professor at UCD School of Mechanical and Materials Engineering, has received a European Research Council (ERC) Consolidator grant of ¤2 million for his 5-year project XenoSim. With the support of this award, Dr Cardiff will develop advanced computational techniques that can provide unprecedented insights into the cutting-edge realm of pigto-human heart transplants.
Dr Cardiff said: “We stand on the threshold of a groundbreaking medical era where pig-to-human heart transplants are becoming a reality. From an engineering standpoint, pig hearts share similarities with their human counterparts in terms of ‘pump design’; however, their distinct size, shape, and functional
characteristics introduce important differences that can impact their performance within the human body.
“With the support of this ERC Consolidator grant, we aim to unlock invaluable insights into these differences by developing advanced biomechanical computational models. This pioneering research promises to offer not only unprecedented insights into the cutting-edge realm of cardiac xenotransplantation but also to establish pioneering computational techniques with significant implications for a wide range of scientific disciplines.”
The Project
‘XenoSim: Providing Computational Insights into
Cardiac Xenotransplantation’, aims to address these challenges by providing fundamental clinical insights into the nascent field of cardiac xenotransplantation through the development and application of novel high-resolution, higher-order, multiphysics simulation methods. Tremendous progress has been made in biomedical imaging, nonetheless, a multitude of physical phenomena relevant to xenotransplantation are not available for experimental observation.
In-silico studies are uniquely placed to provide insights into the haemodynamic disruption caused by replacing a human heart with an anatomically dissimilar one. XenoSim is targeting the establishment of the first family of porcine cardiac xenotransplant
a bimanual pelvic exam in women), a presumed diagnosis is made based on initial tests such uroflowmetry. Those with OAB go on to bladder retraining and antimuscarinic drugs. Only when these simple measures fail does one offer specialised management.
Conclusion
In those presenting with the symptom complex of frequency, urgency and nocturia with or without urge associated incontinence, one needs to exclude other pathology including UTIs, before diagnosing OAB. Social circumstances and the lifestyle and expectations of the individual patient need to be taken into account in order to tailor appropriate investigations.
Behavioural therapy and lifestyle interventions should be used as initial treatment for most patients, but there is a high risk of relapse. Compliance with antimuscarinic therapy relates to efficacy, but there is significantly reduced compliance over time. Botulinum toxin can be used in those who don’t respond to or who cannot tolerate pharmacological treatment.
models that can provide clinically significant insights into the haemodynamic compatibility of porcine donor hearts, the impact of surgical approach, and the consequence of pathologies.
To provide these novel insights requires new coupled simulation approaches. Accordingly, the project aims to create a new class of monolithic finite volume fluid-electrosolid interaction methods, which can provide predictions in clinically relevant timescales through the exploitation of hybrid CPU-GPU systems. XenoSim will establish the new field of computational cardiac xenotransplantation. Furthermore, the novel numerical methods established by XENOSIM are expected to impact a broad range of fields well beyond the project end.
65 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
News
UROLOGY: CKD
Real-Life Anemia Management Among Patients with Non-DialysisDependent Chronic Kidney Disease in Three European Countries
1Saarland University Medical Center, Homburg, Germany; 2Astellas Pharma Europe Ltd., Addlestone, UK; 3RTI Health Solutions, Manchester, UK; 4Astellas Pharma España S.A., Madrid, Spain; 5Hospital Universitario Puerta de Hierro, Madrid, Spain
Anemia is a common complication of chronic kidney disease (CKD). Its prevalence in non-dialysisdependent (NDD) patients with CKD stages 3a–5 (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73m2 1) is 40–80%, increasing with CKD stage.2,3 CKD progression is one of the most frequently identified risk factors for the development of anemia.4–6 Furthermore, anemia is associated with increased risk of major adverse cardiac events, all-cause and cardiovascular mortality, hospitalization, and CKD progression.4,5 Treatments for anemia include iron therapy and erythropoiesis-stimulating agents (ESAs).7,8

Iron deficiency is frequently observed in patients with NDDCKD, affecting approximately half of this population, and rates remain broadly constant across CKD stages.2 Moreover, iron supplementation is vital to ensure adequate iron stores for erythropoiesis and has been shown to improve response to ESA treatment.7 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend the correction of any iron deficiency in patients with NDD-CKD anemia, prior to ESA initiation, and that ESAs only be initiated in patients with hemoglobin (Hb) <10 g/ dL.7 It is also recommended that other potential causes of anemia, such as chronic inflammation, be investigated before considering ESA therapy, and to evaluate iron status at least every 3 months during ESA treatment.7
Evidence to date suggests that management of patients with anemia of NDD-CKD is suboptimal. Results from the CKDopps study showed that among NDD-CKD patients with Hb <10 g/dL, 34% in France and 30% in Germany did not receive either iron or ESA treatment in the 3 months following Hb measurement.9 In a follow-up
analysis of CKDopps, only 28% of patients with Hb <10 g/dL in Brazil, Germany, and the USA received ESA treatment within 12 months.10 Another study in Italy found that 40% of ESA-eligible patients did not receive ESA treatment.11 To gain further clarity, we aimed to document real-world treatment and management of patients with NDD-CKD stages 3b–5 who received ESA therapy in Germany, Spain, and the UK. Given the differences between countries in reimbursement policies, which make evaluation of combined data difficult, this study presents data per country.
METHODS Ethics
All procedures were performed in accordance with the ethical standards of the relevant institutional review boards (IRBs) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. RTI International’s IRB (Research Triangle Park, North Carolina) determined that this study met all criteria for exemption from ethical considerations (IRB ID: STUDY00020563). In Germany and Spain, the study protocol and its amendments were approved by the Ärztekammer des Saarlandes (ID: 132/19) and the Hospital Universitario Puerta de Hierro Majadahonda (ID: ASTESA-2019-01), respectively. Under UK Health Research Authority guidelines,12 this study was not considered to be research and was thus exempt from National Health Service Research Ethics Committee review.
Informed consent was not sought due to the nature of the study: all retrieved data were anonymous and were collected as part of routine diagnosis and treatment. Medical professionals abstracting the data were healthcare
professionals (HCPs) who had legitimate access to the medical records, and the review had no effect on patient care.
Study Design and Participants
This was a non-interventional, retrospective cohort study of medical records in Germany, Spain, and the UK. A customized data collection form was completed by HCPs using patient medical records.Participating HCPs were nephrologists, consultants, associate specialists, and specialist nurses who were eligible if they had 3–30 years of experience, were treating at least 10 patients per year for CKDrelated anemia, and were actively involved in decision making for the management of these patients. A convenience sampling approach was used to recruit HCPs. For patient selection, a quasi-random method was applied by asking HCPs to select records for patients whose last name began with a randomly generated letter, to reduce the chance of bias.
Eligible patients were adults (≥18 years) with CKD stages 3b–5,1 who initiated ESA treatment for anemia between January 01, 2015 and December 31, 2015 (inclusive). Patients had to have received treatment for CKD continuously in the same clinic following their initial diagnosis, to maximize the probability of a complete data record. Anemia was defined as a record of Hb concentration <13.0 g/dL in males and <12.0 g/dL in females. Patients were excluded if they had received dialysis, a transplant of any kind prior to or upon initiation of ESA treatment, a diagnosis of endstage renal disease (ESRD), or had participated in an interventional clinical trial upon initiation of or during ESA treatment. The date of first ESA initiation following anemia diagnosis was designated as the index date (Supplementary Figure 1). Absolute iron deficiency
was defined as serum ferritin ≤100 ng/mL and transferrin saturation (TSAT) ≤20%.13
Data Extraction and Statistical Analyses
Sociodemographic characteristics and clinical history at the time of ESA initiation were collected. ESA treatment patterns; concomitant intravenous (IV) and oral iron therapy; clinical indicators of response to treatment (Hb level, C-reactive protein [CRP] level, ferritin level, TSAT, and eGFR); and blood transfusions were extracted from the index date until the earliest of the following: 24 months after initiation, the last available medical record, dialysis, ESRD diagnosis, kidney transplant, or death (Supplementary Figure 1). For clinical indicators of response to treatment, physicians were asked to provide the number of measurements from ESA initiation and the dates of each measurement; dates were then aligned according to time since ESA initiation. Data on the incidence of dialysis, ESRD, kidney transplant, and death were collected from ESA initiation until the date of medical record abstraction (Supplementary Figure 1).
Methoxy polyethylene glycolepoetin beta (MPG-EPO; Mircera®) treatment patterns were analyzed separately to those of other darbepoetin alfa due to differences in treatment schedules; the exception was the analysis of the association of patient characteristics or ESA type/dose with time to dialysis or time to ESRD (see below), in which MPG- EPO was grouped with darbepoetin alfa due to the small sample size of the MPGEPO-treated patient group.Patient characteristics and outcomes were described for the total population, for each country, by inflammation and diabetes status, and by ESA dose group. Inflammation status
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 66
Written by Danilo Fliser1, Maria Mata Lorenzo2, Katherine Houghton3, Claire Ainsworth3, Martin Blogg2, Elena González de Antona Sánchez4 and Jose Portoles5
due to incomplete documentation in the original medial record, and/or CKD progression to dialysis, ESRD, transplant, or death. Our findings are similar to those from the international CKDopps study, in which <50% of patients with an index Hb level <10 g/dL had a Hb measurement in the subsequent 3 months.2
Figure 1 Concomitant iron therapy for (A) The initial ESA course, by country and ESA type, (B) The initial ESA course, by ESA dose level group, (C) The initial ESA course, by inflammation subgroup, and (D) By ESA treatment course. (A) Patients could receive either IV and/or oral iron. Percentages are calculated based on the number of patients receiving each ESA formulation, by each country. ESA, erythropoiesis-stimulating agent; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta. (B) ESA weekly dose groups were defined as follows: Group 1, <5000 IU short-acting/<5000 IU darbepoetin alfa/<1800 IU MPG-EPO; Group 2, ≥5000 to ≤8000 IU short-acting /≥5000 to <8000 IU darbepoetin alfa/≥1800 to <3000 IU MPG-EPO; Group 3, >8000 to ≤16,000 IU short-acting ≥8000 to ≤16,000 IU darbepoetin alfa/≥3000 to <5400 IU MPG-EPO; Group 4, >16,000 IU short-acting/>16,000 IU darbepoetin alfa/≥5400 IU MPG-EPO Patients included in the dosing range subgroups remained within that dosing range for the duration of the study period; patients who transitioned between dosing range subgroups during the study period were included in the “increased dosing transition” and “decreased dosing transition” subgroups, as appropriate. Betweengroup differences were assessed using the χ2 test. ESA, erythropoiesis-stimulating agent; IU, international units; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta. (C) Inflammation status was assessed using all CRP data during the 24-month observation period following ESA initiation: no inflammation (CRP consistently recorded at <5 mg/L), fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions), or consistent inflammation (CRP consistently recorded at ≥5 mg/L).
Abbreviations: CRP, C-reactive protein; ESA, erythropoiesis-stimulating agent; IU, international units; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta.
Patients in the overall cohort were approximately evenly distributed between inflammation subgroups (Table 1). Prior to ESA initiation, 39.9% of patients had received no iron therapy—this proportion was numerically higher in Spain compared with Germany and the UK (Table 1). The mean length of time between anemia diagnosis and ESA initiation was over 8 months, and the mean Hb level at ESA initiation was 9.8 g/dL (range 5–13 g/dL) (Table 1).At baseline, 162 (19.1%) patients had absolute iron deficiency (Germany n=31 [14.7%], Spain n=98 [22.8%], and UK n=33 [15.9%]). Of these patients, five had unknown or no documented treatment for NDD-CKD-related anemia prior to ESA therapy (Germany n=3 [9.7%], Spain n=1 [1.0%], and UK n=1 [3.0%]), and 57 (35.2%) received no iron therapy prior to ESA therapy (Germany n=3 [9.7%], Spain n=41 [41.8%], and UK n=13 [39.4%]).
ESA Treatment
1 Concomitant iron therapy for (A) The initial ESA course, by country and ESA type, (B) The initial ESA course, by ESA dose level group, (C) The initial ESA course, inflammation subgroup, and (D) By ESA treatment course. (A) Patients could receive either IV and/or oral iron. Percentages are calculated based on the number of receiving each ESA formulation, by each country. ESA, erythropoiesis-stimulating agent; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta. (B) weekly dose groups were defined as follows: Group 1, <5000 IU short-acting/<5000 IU darbepoetin alfa/<1800 IU MPG-EPO; Group 2, ≥5000 to ≤8000 IU short-acting to <8000 IU darbepoetin alfa/≥1800 to <3000 IU MPG-EPO; Group 3, >8000 to ≤16,000 IU short-acting ≥8000 to ≤16,000 IU darbepoetin alfa/≥3000 to <5400 IU MPG-EPO; Group 4, >16,000 IU short-acting/>16,000 IU darbepoetin alfa/≥5400 IU MPG-EPO Patients included in the dosing range subgroups remained within that dosing for the duration of the study period; patients who transitioned between dosing range subgroups during the study period were included in the “increased dosing transition” and “decreased dosing transition” subgroups, as appropriate. Between-group differences were assessed using the χ 2 test. ESA, erythropoiesis-stimulating agent; international units; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta. (C) Inflammation status was assessed using all CRP data during the 24-month observation period following ESA initiation: no inflammation (CRP consistently recorded at <5 mg/L), fluctuating inflammation (CRP levels varied between <5 mg/L and across testing occasions), or consistent inflammation (CRP consistently recorded at ≥5 mg/L).
IU MPG-EPO; Group 3, >8000 to ≤16,000 IU short-acting/≥8000 to ≤16,000 IU darbepoetin alfa/≥3000 to <5400 IU MPGEPO; Group 4, >16,000 IU shortacting>16,000 IU darbepoetin alfa/≥5400 IU MPG-EPO. Patients included in the dosing range subgroups remained within that dosing range for the duration of the study period; patients who transitioned between dose groups during the study period were categorized into “increasing dosing transition” or “decreasing dosing transition” groups, as appropriate, for analysis. Data were summarized descriptively. Cox proportional hazards regression models were used to estimate time to dialysis and time to ESRD (separately), with selected patient demographics, patient baseline characteristics, and ESA treatment type/dose included as predictors (Supplementary Table 1). For the Cox analysis, patients receiving stable ESA doses were included in three dose groupings: Group 1, Group 2, and Groups 3+4 combined (see above); due to sample size, Groups 3 and 4 were combined into one group and patients in the transitioning dose groups were not included. The significance of interactions was assessed using the Wald test, and p values <0.05 were considered significant. No imputation methods were applied to missing data.
RESULTS
Patient Characteristics
Abbreviations: CRP, C-reactive protein; ESA, erythropoiesis-stimulating agent; IU, international units; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta.
was determined by a patient’s documented CRP data from ESA initiation up to 24 months after initiation: consistently inflamed (CRP consistently recorded at ≥5 mg/L), never inflamed (CRP consistently recorded at <5 mg/L), or fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions). For the purposes of this study, target Hb level was
defined as 10–12 g/dL. Subgroup allocation by ESA dose level was dependent on reported weekly dosing levels throughout the study period. ESA weekly dose groups were defined as follows: Group 1, <5000 international units (IU) short-acting/<5000 IU darbepoetin alfa/<1800 IU MPGEPO; Group 2, ≥5000 to ≤8000 IU short-acting/≥5000 to <8000 IU darbepoetin alfa/≥1800 to <3000
KDIGO guidelines recommend a target Hb level of 10 to ≤11.5 g/dL for ESA treatment, while noting that higher Hb targets (11.5 to ≤13 g/dL) may be beneficial for individual patients.7 For the purposes of this study, patients were considered to have met the target Hb level if they had a documented Hb measurement between 10 and 12 g/dL (the
A total of 848 medical records were abstracted: 211 from Germany, 430 from Spain, and 207 from the UK. On average, patients had had CKD-related anemia for over 5 years (Table 1).

During their initial ESA treatment, most patients (67.0%) received darbepoetin alfa; 23.7% and 9.3% received short- acting ESAs and MPG-EPO, respectively (Supplementary Figure 2). This pattern was similar between countries. The majority of patients received their ESA treatment via subcutaneous administration and at home (Table 2). Most patients (776/848, 91.5%) received just one ESA agent continuously from initiation until either treatment discontinuation or the end of the observation period (Table 2; Supplementary Figure 2). Approximately one-third of patients discontinued ESA treatment within 2 years of initiation (Table 2). The primary reason for discontinuation


HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
67 consider the missing data to be evidence that Hb levels
ESA
rather
It
9 Figure 1 Continued. International Journal of Nephrology and Renovascular Disease 2023:16 //doi.org/10.2147/IJNRD.S401598 Press 123
were not measured close to
initiation for these patients,
than indicative of incomplete data extraction.
is also recommended to monitor Hb concentration every 3 months, yet only 65% had an Hb measurement observed at 3 months after ESA initiation, and this proportion continued to decline up to 15 months. The reasons for the missing data are uncertain and may be
https://doi.org/10.2147/IJNRD.S401598 DovePress International Journal of Nephrology and Renovascular Disease 2023:16 et al
Dovepress
UROLOGY: CKD
Notes: aThe electronic data collection form allowed selection of multiple options for primary practice, and the total percentage may thus be greater than 100. bAssessed using all CRP measurements noted during the 24-month observation period following ESA initiation: no inflammation (CRP consistently recorded at <5 mg/L), fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions), or consistent inflammation (CRP consistently recorded at ≥5 mg/L). cNo CRP measurements noted during the 24-month observation period following ESA initiation.
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; IV, intravenous; NDD, non-dialysis dependent; Q, quartile; SD, standard deviation.
or treatment switch was attainment of Hb target levels.The mean ESA dose varied between countries (Table 2).
During initial ESA therapy, 312 patients (36.8%) had a dose reduction, and 306 (36.1%) had a dose increase (patients could experience both an increase and a reduction) (Table 2). Inflammation level tended to be associated with ESA dose; however, the range of doses recorded was wide for all ESA types and inflammation subgroups (Table 3). Duration of ESA treatment also appeared similar across inflammation subgroups (mean 38.7–42.4 months) (Table 3).
Concomitant Iron Therapy
Fliser et al
1 (Continued).
at
Dovepress
During initial ESA therapy, concomitant IV iron therapy was prescribed for 35.6% of the study population and was more common in the UK than in Germany or Spain (Figure 1A). The overall proportion of patients receiving concomitant oral iron therapy was 41.7% but was much lower in the UK compared with Germany or Spain; UK patients were notably more likely to receive IV rather than oral iron (Figure 1A). In the 162 patients with absolute iron deficiency, concomitant iron therapy during initial ESA therapy was prescribed to 81 (50.0%) patients, with the highest proportional prescriptions in the UK (n=23, 69.7%), compared with Germany (n=18, 58.1%) and Spain (n=40, 40.8%).The highest ESA dose group was associated with lower usage of concomitant iron therapy than the other ESA dose groups (Figure 1B). The proportion of patients receiving concomitant IV or oral iron therapy did not differ significantly between inflammation subgroups (Figure 1C). Notably, the proportion of patients receiving concomitant iron therapy decreased from initial to subsequent ESA therapy courses (Figure 1D). However, few patients (n=72) had more than one ESA therapy course (Supplementary Figure 2), and the number of patients with information available also decreased over this period.
Blood Transfusions
Within 24 months of ESA initiation, 16.4% (87/848) of patients required a blood transfusion; this proportion was notably higher in Spain than in the UK and Germany (Figure 3). Patients with consistent or fluctuating inflammation more commonly had blood transfusions than patients with no inflammation (Supplementary
Notes: aThe electronic data collection form allowed selection of multiple options for primary practice, and the total percentage may thus be greater than 100. bAssessed using all CRP measurements noted during the 24-month observation period following ESA initiation: no inflammation (CRP consistently recorded at <5 mg/L), fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions), or consistent inflammation (CRP consistently recorded at ≥5 mg/L). cNo CRP measurements noted during the 24-month observation period following ESA initiation.
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; IV, intravenous; NDD, non-dialysis dependent; Q, quartile; SD, standard deviation.

JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 68
Table 1 Patient Sociodemographic and Clinical Characteristics All (N = 848) Germany (N = 211) Spain (N = 430) UK (N = 207) Male, n (%) 491 (57.9) 134 (63.5) 232 (54.0) 125 (60.4) Age, years, mean ± SD 66.3 ± 15.7 62.4 ± 15.0 69.2 ± 15.4 64.1 ± 15.8 BMI, kg/m2, mean ± SD 26.9 ± 4.5 26.6 ± 4.6 26.8 ± 4.1 27.3 ± 5.0 White ethnicity, n (%) 766 (90.3) 195 (92.4) 417 (97.0) 154 (74.4) Primary practice settinga, n (%) Academic or teaching hospital 596 (70.3) 80 (37.9) 347 (80.7) 169 (81.6) Public hospital 258 (30.4) 27 (12.8) 126 (29.3) 105 (50.7) Private hospital 13 (1.5) 4 (1.9) 9 (2.1) 0 Voluntary charitable hospital 6 (0.7) 6 (2.8) 0 0 Public office-based facility 56 (6.6) 0 56 (13.0) 0 Private office-based practice 130 (15.3) 116 (55.0) 14 (3.3) 0 Dialysis center 9 (1.1) 8 (3.8) 1 (0.2) 0 Inflammation subgroupb No inflammation 188 (22.2) 67 (31.8) 87 (20.2) 34 (16.4) Fluctuating inflammation 225 (26.5) 57 (27.0) 117 (27.2) 51 (24.6) Consistent inflammation 211 (24.9) 50 (23.7) 91 (21.2) 70 (33.8) Missingc 224 (26.4) 37 (17.5) 135 (31.4) 52 (25.1) CCI category score at baseline Mild (1–2) 41 (4.8) 11 (5.2) 18 (4.2) 12 (5.8) Moderate (3–4) 146 (17.2) 39 (18.5) 60 (14.0) 47 (22.7) Severe (≥5) 661 (77.9) 161 (76.3) 352 (81.9) 148 (71.5) Diabetes, n (%) 322 (38.0) 67 (31.8) 176 (40.9) 79 (38.2) Hypertension, n (%) 625 (73.7) 164 (77.7) 341 (79.3) 120 (58.0) Cancer, n (%) 75 (8.8) 11 (5.2) 49 (11.4) 15 (7.2) Years since CKD diagnosis, mean ± SD 7.9 ± 4.3 8.4 ± 5.0 7.9 ± 4.2 7.3 ± 3.6 Years since NDD-CKD-related anemia diagnosis, mean ± SD 5.2 ± 1.6 5.1 ± 1.3 5.2 ± 1.8 5.1 ± 1.4 CKD stage at NDD-CKD-related anemia diagnosis 3b 343 (40.4) 99 (46.9) 186 (43.3) 58 (28.0) 4 445 (52.5) 95 (45.0) 224 (52.1) 126 (60.9) 5 60 (7.1) 17 (8.1) 20 (4.7) 23 (11.1) Prior treatment for NDD-CKD-related anemia Oral iron therapy 332 (39.2) 102 (48.3) 179 (41.6) 51 (24.6) IV iron therapy 206 (24.3) 37 (17.5) 57 (13.3) 112 (54.1) (Continued) International Journal of Nephrology and Renovascular Disease 2023:16 https://doi.org/10.2147/IJNRD.S401598 DovePress 119 Dovepress Fliser et al Table
All (N = 848) Germany (N = 211) Spain (N = 430) UK (N = 207) No iron therapy 338 (39.9) 70 (33.2) 196 (45.6) 72 (34.8) Blood transfusion 3 (0.4) 1 (0.5) 2 (0.5) 0 Folic acid 7 (0.8) 5 (2.4) 2 (0.5) 0 Vitamin B12 3 (0.4) 2 (0.9) 1 (0.2) 0 Unknown 43 (5.1) 18 (8.5) 21 (4.9) 4 (1.9) Months between anemia diagnosis and ESA initiation Mean ± SD 8.4 ± 19.2 8.4 ± 14.4 9.6 ± 21.6 8.4 ± 16.8 Median (Q1–Q3) 2.4 (0–8.4) 3.6 (1.2–8.4) 2.4 (0–8.4) 2.4 (1.2–8.4) Hb level, g/dL, at ESA initiation N 774 202 378 194 Mean ± SD 9.8 ± 1.0 9.4 ± 1.0 10.0 ± 1.0 9.7 ± 0.8 Missing, n (%) 74 (8.7) 9 (4.3) 52 (12.1) 13 (6.3) eGFR, mL/min/1.73m2
ESA initiation n 715 160 365 190 Mean ± SD 28.0 ± 10.4 29.3 ± 10.2 27.5 ± 9.1 28.1 ± 12.6 Missing, n (%) 133 (15.7) 51 (24.2) 65 (15.1) 17 (8.2)
EVRENZO™ is like a breath of fresh air, in symptomatic anaemia of CKD

EVRENZO mimics the body’s natural response to low oxygen conditions, such as those experienced at high altitude.1 By stimulating a coordinated erythropoietic response, oral EVRENZO increases endogenous production of erythropoietin and improves iron bioavailability – ultimately leading to increased haemoglobin production and increased red blood cell production.1 All of which could reduce the complexity of current anaemia management.2
EVRENZO is indicated for treatment of adult patients with symptomatic anaemia associated with chronic kidney disease (CKD)1
Prescribing Information
EVRENZO™▼ (roxadustat) film-coated tablets
Name: EVRENZO 20 mg film-coated tablets, EVRENZO 50 mg film-coated tablets, EVRENZO 70 mg film-coated tablets, EVRENZO 100 mg film-coated tablets, EVRENZO 150 mg film-coated tablets.
Presentation: Film-coated tablets containing 20 mg, 50 mg, 70 mg, 100 mg or 150 mg roxadustat. Indications: Treatment of adult patients with symptomatic anaemia associated with chronic kidney disease (CKD). Posology and Administration:Treatment should be initiated by a physician experienced in the management of anaemia. All other causes of anaemia should be evaluated prior to initiating therapy with EVRENZO and when increasing the dose. EVRENZO must be taken orally three times per week and not on consecutive days. The tablets are taken orally with/without food, swallowed whole and should not be chewed, broken or crushed. EVRENZO can be taken before or after dialysis (see SPC section 5.2). Individualise dose to achieve and maintain target haemoglobin (Hb) levels of 10–12 g/dL. Treatment should not continue beyond 24 weeks if a clinically meaningful increase in Hb levels is not achieved. Starting dose: Ensure adequate iron stores prior to initiation. Patients not currently/previously treated with an erythropoiesis-stimulating agent (ESA): Recommended starting dose: Patients <100kg: 70 mg three times weekly. Patients ≥100kg: 100mg three times weekly. Patients converting from an ESA: : Patients on ESA treatment can be converted to roxadustat. Dialysis patients stable on ESA: only consider conversion if clinically valid reasons exist. Non-dialysis patients stable on ESA: conversion not studied, only consider on benefit-risk to patient. The recommended starting dose is based on the average prescribed ESA dose in the 4 weeks before conversion. The first roxadustat dose should replace the next scheduled ESA dose. See Table 1. in the SPC. Maximum recommended dose: Patients not on dialysis do not exceed a roxadustat dose of 3 mg/kg body weight or 300 mg three times weekly, whichever is lower. Patients on dialysis do not exceed a roxadustat dose of 3 mg/kg body weight or 400 mg three times weekly, whichever is lower. Dose adjustments and Hb monitoring: The individualised maintenance dose ranges from 20 mg to 400 mg three times per week (400 mg only for CKD patients on dialysis). Monitor Hb every 2 weeks until a level of 10-12 g/dL is reached and stabilised, then every 4 weeks or as clinically indicated. The dose of roxadustat can be adjusted stepwise up or down from the starting dose 4 weeks after treatment start, then every 4 weeks except if the Hb increases by >2 g/dL, in which case the dose should be reduced by one step immediately. When adjusting the dose, consider the current Hb level and the recent rate of change in Hb level over the past 4 weeks, and follow the dose adjustment steps in Table 2 in SPC section 4.2. If dose reduction is required for a patient on the lowest dose, reduce the dose frequency to twice a week. If further dose reduction is needed, the frequency may be reduced to once weekly. Maintenance dose: After stabilisation of target Hb levels, monitor Hb levels regularly and follow dose adjustment rules. Consider alternative explanations in patients with inadequate Hb response (see SPC section 4.2). Patients starting dialysis while on roxadustat treatment: No specific dose adjustments required. Follow normal dose adjustment rules. Concomitant roxadustat treatment with inducers or inhibitors: When initiating/discontinuing concomitant treatment with strong inhibitors or inducers of CYP2C8, or inhibitors of UGT1A9, monitor Hb levels routinely and follow dose adjustment rules. Missed dose: If there is >1 day until the next dose, the missed dose must be taken as soon as possible. If ≤ one day remains before the next dose, skip the missed dose. Then resume the regular dosing schedule. Elderly: No adjustment of starting dose (see SPC section 5.2). Patients with hepatic impairment: Mild hepatic impairment: No adjustment of starting dose. Moderate hepatic impairment: Caution is recommended. Reduce starting dose by half or to the level closest to half the starting dose. Severe hepatic impairment: Not recommended (see SPC sections 4.4 & 5.2). Paediatric population: No data are available in patients < 18 years of age. Contraindications: EVRENZO is contraindicated in the following conditions: Hypersensitivity to the active substance, peanut, soya, or to any of the excipients listed in section 6.1 of the SPC; Third trimester of pregnancy (see sections 4.4 & 4.6 of the SPC); Breastfeeding (see section 4.6 of the SPC). Warnings and precautions: Cardiovascular and mortality risk: Overall, the cardiovascular and mortality risk for treatment with roxadustat has been estimated to be comparable to the cardiovascular and mortality risk for ESA therapy based on data from direct comparison of both therapies (see SPC section 5.1). Since, for patients with anaemia associated with CKD and not on dialysis, this risk could not be estimated with sufficient confidence versus placebo, a decision to treat these patients with roxadustat should be based on similar considerations that would be applied before treating with an ESA. Further, several contributing factors have been identified that may impose this risk, including treatment non responsiveness, and converting stable ESA treated dialysis patients (see SPC sections 4.2 and 5.1). In the case of non-responsiveness, treatment with roxadustat should not be continued beyond 24 weeks after the start of treatment (see SPC section 4.2). Conversion of dialysis patients otherwise stable on ESA treatment is only to be considered when there is a valid clinical reason (see SPC section 4.2). For stable ESA treated patients with anaemia associated with CKD and not on dialysis, this risk could not be estimated as these patients have not been studied. A decision to treat these patients with roxadustat should be based on a benefit risk consideration for the individual patient. Thrombotic vascular events: The reported risk of thrombotic vascular events (TVEs) should be carefully weighed against the benefits to be derived from treatment with roxadustat particularly in patients with pre existing risk factors for TVE, including obesity and prior history of TVEs (e.g., deep vein thrombosis [DVT] and pulmonary embolism [PE]). Deep vein thrombosis was reported as common and pulmonary embolism as uncommon amongst the patients in clinical studies. The majority of DVT and PE events were serious. Vascular access thrombosis (VAT) was reported as very common amongst the CKD patients on dialysis in clinical studies (see SPC section 4.8). In CKD patients on dialysis, rates of VAT in roxadustat treated patients were highest in the first 12 weeks following initiation of treatment, at Hb values more than 12 g/dL and in the setting of Hb rise of more than 2 g/dL over 4 weeks. It is recommended to monitor Hb levels and adjust the dose using the dose adjustment rules (see Table 2) to avoid Hb levels of more than 12 g/dL and Hb rise of more than 2 g/dL over 4 weeks. Patients with signs and symptoms of TVEs should be promptly evaluated and treated according to standard of care. The decision to interrupt or discontinue treatment should be based on a benefit risk consideration for the individual patient. Seizures: Seizures were reported as common amongst the patients in clinical studies receiving roxadustat (see SPC section 4.8). Roxadustat should be used with caution in patients with a history of seizures (convulsions or fits), epilepsy or medical conditions associated with a predisposition to seizure activity such as central nervous system (CNS) infections. The decision to interrupt or discontinue treatment should be based on a benefit risk consideration of the individual patient. Serious infections: The most commonly reported serious infections were pneumonia and urinary tract infections. Patients with signs and symptoms of an infection should be promptly evaluated and treated according to standard of care. Sepsis: Sepsis was one of the most commonly reported serious infections and included fatal events. Patients with signs and symptoms of sepsis (e.g., an infection that spreads throughout the body with low blood pressure and the potential for organ failure) should be promptly evaluated and treated according to standard of care. Secondary hypothyroidism: Cases of secondary hypothyroidism have been reported with the use of roxadustat (see SPC section 4.8). These reactions were reversible upon roxadustat withdrawal. Monitoring of thyroid function is recommended as clinically indicated. Inadequate response to therapy: Inadequate response to therapy with roxadustat should prompt a search for causative factors. Nutrient deficiencies should be corrected. Intercurrent infections, occult blood loss, haemolysis, severe aluminium toxicity, underlying haematologic diseases or bone marrow fibrosis may also compromise the erythropoietic response. A reticulocyte count should be considered as part of the evaluation. If typical causes of non-response are excluded, and the patient has reticulocytopenia, an examination of the bone marrow should be considered. In the absence of an addressable cause for an inadequate response to therapy, Evrenzo should not be continued beyond 24 weeks of therapy. Hepatic impairment: Caution is warranted when roxadustat is administered to patients with moderate hepatic impairment (Child Pugh class B). Evrenzo is not recommended for use in patients with severe hepatic impairment (Child Pugh class C) (see SPC section 5.2). Pregnancy and contraception: Roxadustat should not be initiated in women planning on becoming pregnant, during pregnancy or when anaemia associated with CKD is diagnosed during pregnancy. In such cases, alternative therapy should be started, if appropriate. If pregnancy occurs while roxadustat is being administered, treatment should be discontinued and alternative treatment started, if appropriate. Women of childbearing potential must use highly effective contraception during treatment and for at least one week after the last dose of EVRENZO (see SPC sections 4.3 and 4.6). Misuse: Misuse may lead to an excessive increase in packed cell volume. This may be associated with life threatening complications
of the cardiovascular system. Excipients: EVRENZO contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose galactose malabsorption should not take this medicinal product. EVRENZO contains Allura Red AC aluminium lake (see SPC section 6.1) which may cause allergic reactions. EVRENZO contains traces of soya lecithin. Patients who are allergic to peanut or soya, should not use this medicinal product. Effects on ability to drive and use machines: Roxadustat has minor influence on the ability to drive and use machines. Caution should be exercised when driving or using machines. Interactions: Effect of other medicinal products on roxadustat: Phosphate binders and other products containing multivalent cations: Roxadustat should be taken >1 hour after administration of phosphate binders or other medicinal products or supplements containing multivalent cations (not lanthanum carbonate) (see SPC section 4.2). Modifiers of CYP2C8 or UGT1A9 activity: Monitor Hb levels when initiating/ discontinuing concomitant treatment with gemfibrozil, probenecid, other strong inhibitors/inducers of CYP2C8 or other strong inhibitors of UGT1A9. Adjust the dose of roxadustat following dose adjustment rules based on Hb monitoring. (see SPC section 4.2). Effect of roxadustat on other medicinal products OATP1B1 or BCRP Substrates: Co administration of roxadustat with simvastatin in healthy subjects increased the AUC and C max of simvastatin and simvastatin acid. The concentrations of simvastatin and simvastatin acid also increased when simvastatin was administered 2 hours before or 4 or 10 hours after roxadustat. Co administration of roxadustat with rosuvastatin increased the AUC and C max of rosuvastatin. Co administration of 200 mg of roxadustat with atorvastatin increased the AUC and Cmax of atorvastatin. Interactions are also expected with other statins. Monitor for adverse reactions associated with statins and for the need of statin dose reduction. Roxadustat may increase the plasma exposure of other medicinal products that are substrates of BCRP or OATP1B1. Monitor for possible adverse reactions of co administered medicinal products and adjust dose accordingly. See SPC. Roxadustat and ESAs: It is not recommended to combine administration. Pregnancy and lactation: There are no data on the use of roxadustat in pregnant women. Roxadustat is contraindicated in the third trimester of pregnancy and is not recommended during the first and second trimester. If pregnancy occurs during EVRENZO treatment, discontinue EVRENZO and switch to an alternative if appropriate. EVRENZO is contraindicated during breast-feeding. Fertility: The potential effects of roxadustat on male fertility in humans are unknown. At a maternally toxic dose, increased embryonic loss was observed. Women of childbearing potential must use highly effective contraception during treatment and for at least one week after the last dose. Undesirable effects: Summary of the safety profile: The safety of EVRENZO was evaluated in 3542 non dialysis dependent (NDD) and 3353 dialysis dependent (DD) patients with anaemia and CKD who have received at least one dose of roxadustat. The most frequent (≥10%) adverse reactions associated with roxadustat are hypertension (13.9%), vascular access thrombosis (12.8%), diarrhoea (11.8%), peripheral oedema (11.7%), hyperkalaemia (10.9%) and nausea (10.2%). The most frequent (≥1%) serious adverse reactions associated with roxadustat were sepsis (3.4%), hyperkalaemia (2.5%), hypertension (1.4%) and deep vein thrombosis (1.2%). List of adverse reactions: Adverse reactions observed during clinical studies and/or in post-marketing experience are listed in this section by frequency category and MedDRA system organ class. Frequency categories are defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data). Infections and infestations: : Common: Sepsis. Endocrine disorders: Not known: Secondary hypothyroidism. Metabolism and nutrition disorders: Very common: Hyperkalaemia. Psychiatric disorders: Common: Insomnia. Nervous system disorders: Common: Seizures, headache. Vascular disorders: Very common: Hypertension, vascular access thrombosis (VAT)1, Common: Deep vein thrombosis (DVT). Gastrointestinal disorders: Very common: Nausea, diarrhoea, Common: Constipation, vomiting. Skin and subcutaneous tissue disorders: Not known: Dermatitis Exfoliative Generalised (DEG). Hepatobiliary disorders: Uncommon: Hyperbilirubinaemia. Respiratory, thoracic, mediastinal disorders: Uncommon: Pulmonary embolism. General disorders and administration site conditions: Very common: Peripheral oedema. Investigations: Not known: Blood thyroid stimulating hormone (TSH) decreased.1This adverse reaction is associated with CKD patients who were on dialysis while receiving roxadustat. Description of selected adverse reactions. Thrombotic vascular events: In CKD patients not on dialysis, DVT events were uncommon, occurring in 1.0% (0.6 patients with events per 100 patient years of exposure) in the roxadustat group, and 0.2% (0.2 patients with events per 100 patient years of exposure) in the placebo group. In CKD patients on dialysis, DVT events occurred in 1.3% (0.8 patients with events per 100 patient years of exposure) in the roxadustat group and 0.3% (0.1 patients with events per 100 patient years of exposure) in the ESA group (see SPC section 4.4). In CKD patients not on dialysis, pulmonary embolism was observed in 0.4% (0.2 patients with events per 100 patient years of exposure) in the roxadustat group, compared to 0.2% (0.1 patients with events per 100 patient years of exposure) in the placebo group. In CKD patients on dialysis, pulmonary embolism was observed in 0.6% (0.3 patients with events per 100 patient years of exposure) in the roxadustat group, compared to 0.5% (0.3 patients with events per 100 patient years of exposure) in the ESA group (see SPC section 4.4). In CKD patients on dialysis, vascular access thrombosis was observed in 12.8% (7.6 patients with events per 100 patient years of exposure) in the roxadustat group, compared to 10.2% (5.4 patients with events per 100 patient years of exposure) in the ESA group (see SPC section 4.4). Seizures: : In CKD patients not on dialysis, seizures occurred in 1.1% (0.6 patients with events per 100 patient years of exposure) in the roxadustat group, and 0.2% (0.2 patients with events per 100 patient years of exposure) in the placebo group (see SPC section 4.4). In CKD patients on dialysis, seizures occurred in 2.0% (1.2 patients with events per 100 patient years of exposure) in the roxadustat group, and 1.6% (0.8 patients with events per 100 patient years of exposure) in the ESA group (see SPC section 4.4). Sepsis: In CKD patients not on dialysis, sepsis was observed in 2.1% (1.3 patients with events per 100 patient years of exposure) in the roxadustat group, compared to 0.4% (0.3 patients with events per 100 patient years of exposure) in the placebo group. In patients on dialysis, sepsis was observed in 3.4% (2.0 patients with events per 100 patient years of exposure) in the roxadustat group, compared to 3.4% (1.8 patients with events per 100 patient years of exposure) in the ESA group (see SPC section 4.4). Skin reactions: : Dermatitis exfoliative generalised, part of severe cutaneous adverse reactions (SCARs), has been reported during postmarketing surveillance and has shown an association with roxadustat treatment (frequency not known). Prescribers should consult the full summary of product characteristics in relation to other adverse reactions. Overdose: Single supratherapeutic doses of roxadustat 5 mg/kg (up to 510 mg) in healthy subjects were associated with a transient increase in heart rate, an increased frequency of mild to moderate musculoskeletal pain, headaches, sinus tachycardia, and less commonly, low blood pressure (all non serious). Roxadustat overdose can elevate Hb levels above the desired level; manage with discontinuation or reduction of roxadustat dosage and careful monitoring and treatment as clinically indicated. Roxadustat and its metabolites are not significantly removed by haemodialysis. Package Quantities, Basic NHS cost: EVRENZO (12 pack tablets): United Kingdom (UK): 20 mg = £59.24, 50 mg = £148.11, 70 mg = £207.35, 100 mg = £296.21, 150 mg = £444.32. Ireland (IE): POA. Legal
Classification: UK: POM. Ireland POM/S1A Product licence numbers: Great Britain (GB): PLGB 00166/0427-0431. Northern Ireland (NI)/ IE: EU/1/21/1574/001-005. Marketing Authorisation Holder: GB: Astellas Pharma Ltd., 300 Dashwood Lang Road, Bourne Business Park, Addlestone, United Kingdom, KT15 2NX. NI/IE: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of Preparation of Prescribing Information: February 2023. Document number: MAT-IE-EVZ-2023-00002 Further information available from: UK: Astellas Pharma Ltd., Medical Information: 0800 783 5018. IE: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. For full prescribing information, please see the SPCs which may be found at: GB: www.medicines.org.uk; NI: https://www.emcmedicines. com/en-gb/northernireland/; IE: www.medicines.ie.
United Kingdom Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/ yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018.
Ireland Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com.
▼ This
is
to additional
CKD,
chronic kidney disease. 1.EVRENZO SMPC. 2. Sanghani NS, Haase VH. Adv Chronic Kidney Dis 2019; 26:253–266.
MAT-IE-EVZ-2023-00007 | March 2023
medicinal product
subject
monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Fliser et al
UROLOGY: CKD

Abbreviations: ESA, erythropoiesis-stimulating agent; IU, international units; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta; Q, quartile; SD, standard deviation.
measurement upon ESA initiation, despite the guideline recommendations. As these patients did have Hb measurements at later dates, we consider the missing data to be evidence that Hb levels were not measured close to ESA initiation for these patients, rather than indicative of incomplete data extraction.
It is also recommended to monitor Hb concentration every 3 months, yet only 65% had an Hb measurement observed at 3 months after ESA initiation, and this proportion continued to decline up to 15 months. The reasons for the missing data are uncertain and may be due to incomplete documentation in the original medial record, and/or CKD progression to dialysis, ESRD, transplant, or death. Our findings are similar to those from the international CKDopps study, in which <50% of patients with an index Hb level <10 g/ dL had a Hb measurement in the subsequent 3 months.
Figure 3A). Higher ESA doses were also more commonly associated with blood transfusions (Supplementary Figure 3B).
CKD Progression and Deaths
Other Clinical Indicators
KDIGO guidelines recommend a target Hb level of 10 to ≤11.5 g/dL for ESA treatment, while noting that higher Hb targets (11.5 to ≤13 g/ dL) may be beneficial for individual patients.7 For the purposes of this study, patients were considered to have met the target Hb level if they had a documented Hb measurement between 10 and 12 g/dL (the higher target level allowed for variability in the guideline recommendations and potential laboratory errors). Within 2 years, the majority of patients achieved this target. However, it is uncertain how many patients maintained their target level: by 12 months after target achievement, <50% of patients had a documented Hb level within the target range, and there was a substantial proportion with missing data.
following target achievement increased over time (at 6 months: 13.2%, 97/735; at 12 months: 22.9%, 168/735). For these patients, it is unknown whether the Hb target was maintained.
From ESA initiation until the abstraction date, rates of dialysis and ESRD were 19.3% and 24.6%, respectively, and were similar between countries (Figure 3). The expected time to both dialysis (p=0.002) and ESRD (p=0.020) significantly increased with each year increase in age (Supplementary Table 1). Patient sex, country, time to ESA initiation, and Charlson comorbidity index at baseline were not significantly associated with time to either dialysis or ESRD (Supplementary Table 1). The expected time to both dialysis and ESRD significantly increased with each unit increase in baseline eGFR (p<0.001 for both outcomes); similarly, lower CKD stage at the time of anemia diagnosis was significantly associated with longer time to dialysis (stage 4 versus stage 5, p=0.003), but not ESRD (Supplementary Table 1). Neither the ESA type first initiated nor ESA weekly dose group was significantly associated with time to dialysis or ESRD (Supplementary
Table 1).The rate of kidney transplant was low overall (4.8%; 41/848) and was similar between countries (Figure 3). The rate of death (8.8% overall; 75/848) was also similar between countries (Figure 3). Of all 75 patients who had died at the time of data abstraction, 18 had received a short-acting ESA, 53 had received darbepoetin alfa, and 4 had received MPG-EPO, for their initial ESA treatment. No differences in the rate of death were observed as a function of inflammation or ESA dose group (Supplementary Figure 3A and B).
Ferritin and TSAT levels tended to increase over the 24 months following ESA initiation, while eGFR decreased (Table 4). After a median TSAT of 20% at ESA initiation, levels increased above 20% for the remainder of the observation period (Table 4). Similar to Hb, ferritin, TSAT, and eGFR data were not documented for many patients,
Discussion
https://doi.org/10.2147/IJNRD.S401598
This study provides insights into real-life management of patients with NDD-CKD anemia, including ESA treatment and concomitant iron therapy, in Germany, Spain, and the UK. Across the three countries, the mean and median Hb level at ESA initiation was 9.8 g/dL, which is in accordance with KDIGO guidelines for anemia treatment in patients with NDD- CKD.7 However, the Hb level at initiation ranged from 5 to 13 g/dL, indicating that some patients were initiated on ESAs despite having a Hb level above 10.0 g/dL. In addition, 9% of patients did not have a recorded Hb
Prior to ESA initiation, approximately 60% of patients had received either IV or oral iron therapy; this could indicate that iron deficiency had been explicitly investigated and corrected in line with KDIGO guidelines. However, ferritin and TSAT measure-ments were missing at ESA initiation for 15% and 33% of patients, respectively, so it is not possible to determine whether iron therapy was indicated for these patients. Moreover, only approximately half of patients had documented ferritin and TSAT measurements in the months following ESA initiation, and only 36% and 42% of patients received concomitant IV and/or oral iron therapy, respectively. After ESA initiation, patients did not remain iron deficient over the 2-year observation period: TSAT levels were consistently >20% and ferritin >100 ng/mL, based on available measurements. In the CKDopps study, 26–77% of patients with NDD-CKD stages 3a–5, depending on country and CKD stage, had no TSAT or ferritin measurements within 3 months of their index Hb measurement,9 and only 29–43% of patients with Hb <10 g/dL received iron therapy in the 3 months following their index
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 70
Table 2 ESA Treatment Course All N=848 Germany N=211 Spain N=430 UK N=207 ESA therapy duration in months, mean ± SD 41.2 ± 18.2 42.9 ± 17.0 40.2 ± 18.5 41.6 ± 18.9 Weekly dose, IU Short-acting ESAs Mean ± SD 5176.0 ± 7026.4 6124.2 ± 4104.8 4752.1 ± 8812.5 4856.1 ± 3927.7 Median (Q1–Q3) 3237.5 (2333.3–6000.0) 4892.7 (3000.0–8500.0) 2923.6 (1850.0–5012.5) 3000.0 (2366.7–6750.0) Darbepoetin alfa Mean ± SD 6220.0 ± 14,300.0 6280.0 ± 4280.0 6580.0 ± 19,680.0 5520.0 ± 4920.0 Median (Q1–Q3) 4000.0 (2680.0–6000.0) 5000.0 (3000.0–8000.0) 4000.0 (2340.0–6000.0) 40,000.0 (2920.0–6000.0) MPG-EPO 4440 ± 3460 3860 ± 2100 5020 ± 4680 4840 ± 1440 Mean ± SD 1332.0 ± 1038.0 1158.0 ± 630.0 1506.0 ± 1404.0 1452.0 ± 432.0 Median (Q1–Q3) 1032.0 (690.0–1500.0) 1032.0 (690.0–1380.0) 948.0 (690.0–1596.0) 1650.0 (1116.0–1800.6) Dose change during initial ESA therapy, n (%) Decrease 312 (36.8) 75 (35.5) 170 (39.5) 67 (32.4) Increase 306 (36.1) 62 (29.4) 165 (38.4) 79 (38.2) Switched treatment, n (%) 72 (8.5) 25 (11.8) 40 (9.3) 7 (3.4) Discontinuation within 2 years of initiation, n (%) 281 (33.1) 66 (31.3) 163 (37.9) 52 (25.1) Mode of administration, n (%) Short-acting ESAs N=216 N=64 N=116 N=36 Subcutaneous 188 (87.0) 51 (79.7) 107 (92.2) 30 (83.3) IV 29 (13.4) 13 (20.3) 9 (7.8) 7 (19.4) Darbepoetin alfa N=577 N=121 N=289 N=167 Subcutaneous 551 (95.5) 118 (97.5) 282 (97.6) 151 (90.4) IV 26 (4.5) 3 (2.5) 7 (2.4) 16 (9.6) MPG-EPO N=87 N=41 N= 38 N=8 Subcutaneous 87 (100.0) 41 (100.0) 38 (100.0) 8 (100.0) Administration setting Home Short-acting 166 (76.9) 46 (71.9) 92 (79.3) 28 (77.8) Long-acting 498 (86.3) 97 (80.2) 254 (87.9) 147 (88.0) MPG-EPO 66 (75.9) 38 (92.7) 27 (71.1) 1 (12.5) Hospital-based clinic Short-acting 46 (21.3) 13 (20.3) 24 (20.7) 9 (25.0) Long-acting 67 (11.6) 13 (10.7) 35 (12.1) 19 (11.4) MPG-EPO 18 (20.7) 0 11 (28.9) 7 (87.5) Care home Short-acting 1 (0.5) 1 (1.6) 0 0 Long-acting 2 (0.3) 0 1 (0.3) 1 (0.6) MPG-EPO 0 0 0 0 Dialysis center Short-acting 4 (1.9) 4 (6.3) 0 0 Long-acting 0 0 0 0 MPG-EPO 0 0 0 0 Private office Short-acting 0 0 0 0 Long-acting 13 (2.3) 13 (10.7) 0 0 MPG-EPO 3 (3.4) 3 (7.3) 0 0 Abbreviations: ESA, erythropoiesis-stimulating agent; IU, international units; IV, intravenous; MPG-EPO, methoxy polyethylene glycol-epoetin beta; Q, quartile; SD, standard deviation. International Journal of Nephrology and Renovascular Disease 2023:16
DovePress 121 Dovepress
Table 3 ESA Dose and Duration by Inflammation Subgroup
Notes: Inflammation status was assessed using all CRP data during the 24-month observation period following ESA initiation: no inflammation (CRP consistently recorded at <5 mg/L), fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions), or consistent inflammation (CRP consistently recorded at ≥5 mg/L).
Abbreviations: ESA, erythropoiesis-stimulating agent; IU, international units; MPG-EPO, methoxy polyethylene glycol-epoetin beta; SD, standard deviation.
Notes: Inflammation status was assessed using all CRP data during the 24-month observation period following ESA initiation: no inflammation (CRP consistently recorded at <5 mg/L), fluctuating inflammation (CRP levels varied between <5 mg/L and ≥5 mg/L across testing occasions), or consistent inflammation (CRP consistently recorded at ≥5 mg/L).
especially after ESA initiation (Table 4). Additionally, one-quarter of all patients had no documented CRP measurements for the entire observation period (Table 1).
Blood Transfusions
Within 24 months of ESA initiation, 16.4% (87/848) of patients required a blood transfusion; this proportion was notably higher in Spain than in the UK and Germany (Figure 3). Patients with consistent or fluctuating inflammation more commonly had blood transfusions than patients with no inflammation (Supplementary Figure 3A). Higher ESA doses were also more commonly associated with blood transfusions (Supplementary Figure 3B).
CKD Progression and Deaths

Hb measurement.9 Other research in Italy found that a majority of patients with iron deficiency (TSAT ≤20% and ferritin ≤100 ng/mL) did not receive iron therapy,11 while a study of nephrology centers in Ireland reported that only 14.1% of patients with iron deficiency received iron therapy.14 Despite availability of global (KDIGO) guidelines, there were differences in the management of these patients between countries. There was a substantially higher rate of blood transfusions in Spain than in Germany or the UK, which may be linked to the lower use of IV iron in Spain compared with the other countries. Conversely, in the UK, there was a markedly higher use of IV versus oral iron, whereas the reverse was seen in the other two countries. The suboptimal management of anemia and iron deficiency, and between-country differences in management, may reflect variability between KDIGO and national or local guidelines, or uncertainty as to their application.8,14 Another underlying cause may be lack of resources or training,15
or country-specific medical administration practices.A notable finding of our study was the tendency for inflammation to be associated with higher ESA doses, although this was not consistent across all ESA types. This may indicate resistance to ESAs in patients with inflammation, either because of infection16 or because other possible causes of anemia were not sufficiently investigated. This finding is consistent with a study of patients with CKD in Sweden, which found a clear relationship between CRP levels and ESA dose.3 Alternatively, functional iron deficiency could be a consequence of inflammatory status, since inflammation upregulates hepcidin, causing iron to be trapped within cells and making it unavailable for erythropoiesis.3 Moreover, our data show that a numerically higher proportion of patients with either consistent or fluctuating inflammation received a blood transfusion than those with no inflammation. However, due to the nature of this study, statistical analyses were not conducted for these relationships
From ESA initiation until the abstraction date, rates of dialysis and ESRD were 19.3% and 24.6%, respectively, and were similar between countries (Figure 3). The expected time to both dialysis (p=0.002) and ESRD (p=0.020) significantly increased with each year increase in age (Supplementary Table 1). Patient sex, country, time to ESA initiation, and Charlson comorbidity index at baseline were not significantly associated with time to either dialysis or ESRD (Supplementary Table 1). The expected time to both dialysis and ESRD significantly increased with each unit increase in baseline eGFR (p<0.001 for both outcomes); similarly, lower CKD stage at the time of anemia diagnosis was significantly associated with longer time to dialysis (stage 4 versus stage 5, p=0.003), but not ESRD (Supplementary Table 1). Neither the ESA type first initiated nor ESA weekly dose group was significantly associated with time to dialysis or ESRD (Supplementary Table 1).
The rate of kidney transplant was low overall (4.8%; 41/848) and was similar between countries (Figure 3). The rate of death (8.8% overall; 75/848) was also similar between countries (Figure 3). Of all 75 patients who had died at the time of data abstraction, 18 had received a short-acting ESA, 53 had received darbepoetin alfa, and 4 had received MPG-EPO, for their initial ESA treatment. No differences in the rate of death were observed as a function of inflammation or ESA dose group (Supplementary Figure 3A and B).
and no firm conclusions can be drawn. CRP levels were unusually high (mean 13.1 mg/L, median 5.0 mg/L)—it is not certain whether this is due to poor data recording or if it is reflective of the real- life situation. Some patients may have had intercurrent bacterial infection or the numbers may reflect a negative selection bias, whereby HCPs were more likely to monitor and record CRP data for patients whose levels were abnormal. Strengths of this study include its 2-year observation period, which enabled longitudinal follow-up of patient outcomes from ESA treatment. This study was of a retrospective, observational, noninterventional design, in which centers were not selected on the basis of their compliance with clinical best practice. We, therefore, consider it representative of typical clinical practice
Abbreviations: CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESA, erythropoiesisstimulating agent; Hb, hemoglobin; Q, quartile; SD, standard deviation; TSAT, transferrin saturation.
HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023 71
No Inflammation (N=188) Fluctuating Inflammation (N=255) Consistent Inflammation (N=211) Overall duration of ESA therapy (months), mean ± SD 38.7 ± 19.4 41.4 ± 18.4 42.4 ± 17.9 ESA dose Short-acting ESAs n 43 62 60 Weekly dose, IU Mean ± SD 4797.7 ± 4062.5 5079.5 ± 3776.9 4972.7 ± 4092.1 Median (Q1–Q3) 3174.6 (2000.0–6000.0) 3583.3 (2500.0–6000.0) 3366.7 (2416.7–6375.0) Darbepoetin alfa n 127 153 132 Weekly dose, IU Mean ± SD 5240.0 ± 4140.0 5720.0 ± 8580.0 5900.0 ± 5740.0 Median (Q1–Q3) 4000.0 (2680.0–6200.0) 4000.0 (2500.0–6000.0) 4000.0 (3000.0–6000.0) MPG-EPO n 25 17 24 Weekly dose, IU Mean ± SD 1302.0 ± 552.0 1158.0 ± 672.0 1992.0 ± 1608.0 Median (Q1–Q3) 1224.0 (1032.0–1380.0) 828.0 (690.0–1500.0) 1620.0 (690.0–2700.0)
Abbreviations:
IU,
MPG-EPO, methoxy polyethylene glycol-epoetin beta; SD, standard deviation. https://doi.org/10.2147/IJNRD.S401598 DovePress International Journal of Nephrology and Renovascular Disease 2023:16 122 Fliser et al
ESA, erythropoiesis-stimulating agent;
international units;
Dovepress
Parameter ESA Initiation, N=848 3 Months, N=843 6 Months, N=835 9 Months, N=824 12 Months, N=822 15 Months, N=809 18 Months, N=796 21 Months, N=791 24 Months, N=780 Hb (g/dL) n 774 545 540 470 493 396 386 354 375 Mean ± SD 9.8 ± 1.0 10.8 ± 1.2 11.0 ± 1.1 11.1 ± 1.1 11.2 ± 1.0 11.3 ± 1.0 11.3 ± 1.0 11.3 ± 1.0 11.4 ± 0.9 Median (Q1–Q3) 9.8 (9.1–10.4) 10.8 (10.0–11.5) 11.0 (10.3–11.7) 11.1 (10.5–11.8) 11.1 (10.6–11.8) 11.2 (10.7–11.9) 11.2 (10.7–11.9) 11.2 (10.7–12.0) 11.4 (10.9–12.0) Missing, n (%) 74 (8.7) 298 (35.3) 295 (35.3) 354 (43.0) 329 (40.0) 413 (51.1) 410 (51.5) 437 (55.2) 405 (51.9) CRP, mg/L n 549 335 331 271 283 240 232 193 222 Mean ± SD 13.1 ± 20.8 10.5 (16.4) 11.6 (19.0) 9.1 (15.0) 10.2 (18.0) 10.8 (17.8) 9.7 (15.8) 9.4 (17.7) 12.3 (19.9) Median (Q1–Q3) 5.0 (2.1–10.0) 4.3 (2.0–9.0) 4.7 (2.0–9.0) 4.0 (1.9–8.0) 4.2 (2.0–8.0) 5.0 (2.0–9.0) 4.5 (2.0–8.0) 4.0 (1.4–7.0) 4.0 (1.5–8.0) Missing, n (%) 299 (35.3) 508 (60.3) 504 (60.4) 553 (67.1) 539 (65.6) 569 (70.3) 564 (70.9) 598 (75.6) 558 (71.5) Ferritin, ng/mL n 725 445 437 377 391 314 316 281 302 Mean ± SD 167.3 ± 155.3 197.0 ± 153.8 205.6 ± 165.4 209.5 ± 147.7 198.7 ± 147.6 202.2 ± 130.7 212.4 ± 151.0 219.9 ± 150.0 205.7 ± 136.8 Median (Q1–Q3) 119.0 (60.0–220.0) 160.0 (90.0–250.0) 158.0 (96.0–266.0) 185.0 (100.0–280.0) 179.0 (99.0–250.0) 189.0 (102.0–270.0) 178.5 (100.0–296.0) 189.0 (110.0–299.0) 180.0 (110.0–270.0) Missing, n (%) 123 (14.5) 398 (47.2) 398 (47.7) 447 (54.2) 431 (52.4) 495 (61.2) 480 (60.3) 510 (64.5) 478 (61.3) TSAT, % n 572 352 346 303 312 270 253 230 244 Mean ± SD 22.1 ± 12.7 25.1 ± 14.2 25.1 ± 13.9 25.8 ± 14.0 25.6 ± 14.8 25.9 ± 13.7 27.3 ± 16.5 25.7 ± 14.3 25.8 ± 13.6 Median (Q1–Q3) 20.0 (15.0–26.0) 22.0 (18.0–28.5) 22.0 (18.0–28.0) 23.0 (19.0–28.0) 22.0 (18.8–28.0) 23.0 (19.0–28.0) 22.3 (19.3–29.0) 22.0 (19.0–27.0) 23.0 (20.0–28.0) Missing, n (%) 276 (32.5) 491 (58.2) 489 (58.6) 521 (63.2) 510 (62.0) 539 (66.6) 543 (68.2) 561 (70.9) 536 (68.7) eGFR, mL/min/1.73 m2 n 715 488 486 424 436 359 350 315 337 Mean ± SD 28.0 ± 10.4 26.3 ± 9.3 26.1 ± 9.3 25.3 ± 9.4 26.1 ± 9.3 24.4 ± 8.7 24.8 ± 9.1 24.2 ± 9.5 25.3 ± 9.2 Median (Q1–Q3) 27.0 (20.5–35.0) 26.0 (19.0–32.4) 25.0 (20.0–32.0) 25.0 (19.0–31.0) 25.0 (19.2–32.0) 24.0 (18.0–30.0) 24.0 (18.0–30.0) 23.0 (17.0–30.0) 24.6 (19.0–32.0) Missing, n (%) 133 (15.7) 355 (42.1) 349 (41.8) 400 (48.5) 386 (47.0) 450 (55.6) 446 (56.0) 476 (60.2) 443 (56.8) Abbreviations: CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; Q, quartile; SD, standard deviation; TSAT, transferrin saturation. International Journal of Nephrology and Renovascular Disease 2023:16 https: //doi.org/10.2147/IJNRD.S401598 Dove press
Table 4 Clinical Indicators of Response to Treatment Following ESA Initiation
Dovepress
UROLOGY: CKD
Figure 2 Achievement
Hb target levels within 24 months, by ESA type. Proportions are based on the number of patients with observed Hb data, for each ESA formulation group.
Abbreviations: ESA, erythropoiesisstimulating agent; Hb, hemoglobin; MPG-EPO, methoxy polyethylene glycolepoetin beta.
Abbreviations
Abbreviations
in the participating countries. However, the retrospective design and dependence on medical records are also limitations, as it was not possible to follow up on the reasons underlying the observed data. The dependence on medical records may have led to the absence of some patient
data, since records require that treating physicians are sufficiently motivated to provide complete information. We also acknowledge that relatively modest numbers of patients from each country were included. In addition, with the exception of the incidence data for dialysis, ESRD, kidney
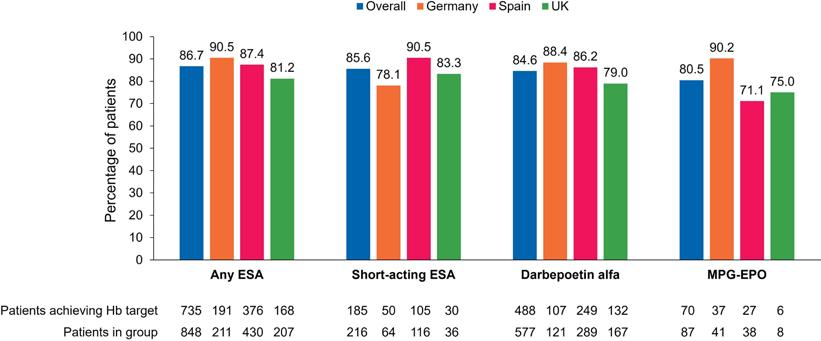

transplant, and death, the last possible date for the 24-month follow-up was December 31, 2017; consequently, the data analyzed here are a minimum of four years old and some changes in clinical practice may have occurred in the intervening period. The study was designed to be descriptive and no
hypothesis testing was conducted. Lastly, the convenience sampling strategy for recruiting HCPs and high levels of missing data restrict the conclusions that can be drawn; caution should be taken when extrapolating these findings to other patient populations. References available on request
Percentages are calculated based on the number of patients for whom a response was received on the CRF, including those for whom “don’t know” was stated. Data on blood transfusions were extracted from ESA initiation until the earliest of the following: 24 months after initiation, the last available medical record, ESRD, transplantation, dialysis, or death. Incidence of dialysis, ESRD, kidney transplant and death were documented from
Figure 3 Blood transfusions, CKD progression, and deaths, by country. aPercentages are calculated based on the number of patients for whom a response was received on the CRF, including those for whom “don’t know” was stated. Data on blood transfusions were extracted from ESA initiation until the earliest of the following: 24 months after initiation, the last available medical record, ESRD, transplantation, dialysis, or death. Incidence of dialysis, ESRD, kidney transplant and death were documented from ESA initiation until the date of abstraction.

Abbreviations: CKD, chronic kidney disease; CRF, chronic renal failure; ESA, erythropoiesis-stimulating agent; ESRD, end-stage renal disease
higher target level allowed for variability in the guideline recommendations and potential laboratory errors). Within 2 years, the majority of patients achieved this target. However, it is uncertain how many patients maintained their target level: by 12 months after target achievement, <50% of patients had a documented Hb level within the target range, and there was a substantial proportion with missing data.
transfusions were extracted from ESA initiation until the earliest of the following: 24 months after initiation, the last available medical record, ESRD, transplantation, dialysis, or death. Incidence of dialysis, ESRD, kidney transplant and death were documented from ESA initiation until the date of abstraction.
Abbreviations: CKD, chronic kidney disease; CRF, chronic renal failure; ESA, erythropoiesis-stimulating agent; ESRD, end-stage renal disease.
Health Capital Plan
Prior to ESA initiation, approximately 60% of patients had received either IV or oral iron therapy; this could indicate that iron deficiency had been explicitly investigated and corrected in line with KDIGO guidelines. However, ferritin and TSAT measurements were missing at ESA initiation for 15% and 33% of patients, respectively, so it is not possible to determine whether iron therapy was indicated for these patients. Moreover, only approximately half of patients had documented ferritin and TSAT
higher target level allowed for variability in the guideline recommendations and potential laboratory errors). Within 2 years, the majority of patients achieved this target. However, it is uncertain how many patients maintained their target level: by 12 months after target achievement, <50% of patients had a documented Hb level within the target range, and there was a substantial proportion with missing data.
Minister for Health Stephen Donnelly has announced the publication of the Health Service Executive’s (HSE’s) Capital Plan for 2023.
https://doi.org/10.2147/IJNRD.S401598
The 2023 Capital Plan reiterates the government’s commitment to investing in our health service. This plan will support the delivery of strategic reform and a move towards better care in the community and builds on measures required due to COVID-19.
The health capital funding available in 2023 for the construction and equipping of healthcare facilities is ¤967 million, with a further ¤50 million provided for capital infrastructure resulting from COVID-19 actions and an additional ¤10 million from income generated in 2022, totalling ¤1.027 billion.
https://doi.org/10.2147/IJNRD.S401598
The overarching vision and long-term policy direction for Ireland’s healthcare system is the achievement of universal healthcare set out in the Programme for
Government and as reflected in Ministerial priorities for 2023.This investment will enable the HSE to progress government priority projects, including the completion of the New Children's Hospital, advancement of the National Maternity Hospital, progressing Sláintecare initiatives to bring about the delivery of care closer to home, maintaining investment in minor capital initiatives, the delivery of the equipment and ambulance replacement programmes and
Prior to ESA initiation, approximately 60% of patients had received either IV or oral iron therapy; this could indicate that iron deficiency had been explicitly investigated and corrected in line with KDIGO guidelines. However, ferritin and TSAT measurements were missing at ESA initiation for 15% and 33% of patients, respectively, so it is not possible to determine whether iron therapy was indicated for these patients. Moreover, only approximately half of patients had documented ferritin and TSAT
to progress the Infrastructure and Decarbonisation Strategy and Implementation roadmap in partnership with Sustainable Energy Authority of Ireland.
This Capital Plan includes investment to facilitate reorienting the model of care away from acute hospitals and towards primary and community settings and addressing capacity and infrastructural deficits that exist in the health and social care services.
News
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 72
Figure 2 Achievement of Hb target levels within 24 months, by ESA type. Proportions are based on the number of patients with observed Hb data, for each ESA formulation group.
: ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; MPG-EPO, methoxy polyethylene glycol-epoetin beta.
DovePress International Journal of Nephrology and Renovascular Disease 2023:16 126 Fliser et al
of
Figure 2 Achievement of Hb target levels within 24 months, by ESA type. Proportions are based on the number of patients with observed Hb data, for each ESA formulation group.
: ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; MPG-EPO, methoxy polyethylene glycol-epoetin beta.
Figure 3 Blood transfusions, CKD progression, and deaths, by country. aPercentages are calculated based on the number of patients for whom a response was received on the CRF, including those for whom “don’t know” was stated. Data on blood
DovePress International Journal of Nephrology and Renovascular Disease 2023:16 126 Fliser et al Dovepress
UROLOGY: ERECTILE DYSFUNCTION Management and Treatment of Erectile Dysfunction
Erectile dysfunction (ED) is a common condition occurring in males over 40 years of age, although it can occur earlier. It is estimated that at least 150 million men globally have ED. It is difficult to obtain accurate values for the true prevalence of erectile dysfunction however, as many patients fail to seek medical attention, and many clinicians are reluctant to ask patients about their sexual health.
We recently spoke to Theresa Lowry Lehnen, RGN, GPN, RNP, BSc, MSc, M. Ed, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University to understand more about this condition and its impact on males in Ireland.
ED can have a substantial negative impact on a man’s quality of life, Theresa reflects. She says, “Erectile dysfunction is the inability to achieve or maintain an erection for satisfactory sexual performance, and affects a considerable proportion of men at least occasionally. It is often treatable, however, if left untreated, ED can be a source of severe emotional stress for both the man and their partner.”

Theresa notes that erectile dysfunction is often an under recognised, yet important, cardiovascular risk factor. She says, “Owing to its strong association with metabolic syndrome and cardiovascular disease, cardiac assessment is warranted in men with symptoms of ED.”

Aetiology of Erectile Dysfunction
Although most men will experience periodic episodes of erectile dysfunction, it tends to become more frequent with advancing age.
“Many factors can contribute to sexual dysfunction in older men, including physical and psychological conditions, comorbidities and polypharmacy,” she adds. “Aspects of an ageing man’s lifestyle behaviour and androgen deficiency, most often decreasing testosterone levels, can affect sexual function.
“Studies have shown that the percentage of men who engage in some form of sexual activity,
decreases from 73% in men aged 57–64 years to 26% for men aged 75–85 years. The aetiology for this decline in male sexual activity is multifactorial, and is in part related to female partners menopause at approximately 52 years of age, leading to a significant decline in female libido and desire to engage in sexual activity.”
While ED is associated with ageing, many studies and largescale surveys have concluded that ED is a major health concern among young men.
Theresa adds, “One study in 2013 reported that 1: 4 men seeking medical help for erectile dysfunction in the real-life setting, is < 40 years of age. Another study in 2016 concluded that 22.1% of men < 40 years of age had low (<21) Sexual Health Inventory for Men (SHIM) scores.
“In the past, erectile dysfunction was almost always considered a psychogenic disorder. However, evidence now suggests that more than 80% of cases have an organic aetiology. While most patients with ED have organic disease, some do have a primary psychological cause, particularly younger men. Even when ED is organic in nature, there are almost always psychological consequences regarding relationship issues, cultural norms and expectations, loss of self-esteem, shame, and anxiety and depression related to sexual performance.”
Erectile dysfunction is multidimensional in nature, and Theresa says it can be broadly divided into endocrine and nonendocrine causes.
“The condition can be caused by any disease process which affects penile arteries, nerves, hormone levels, smooth muscle tissue, corporal endothelium, or tunica albuginea. It is closely related to cardiovascular disease, diabetes mellitus, hyperlipidaemia, hypertension, and endothelial dysfunction,” she explains.
“Erectile dysfunction and vascular disease are thought to be linked at the level of the endothelium. Endothelial dysfunction, results in the inability of smooth muscle cells lining the arterioles to relax
and prevents vasodilatation. The endothelial cell is known to affect vascular tone and impact the process of atherosclerosis and impacting ED, CVD and peripheral vascular disease. Cardiovascular disease and hypertension are very significant risk factors for erectile dysfunction.”
Besides cardiovascular disease, there are strong correlations between ED and hyperlipidaemia, diabetes, hypogonadism, obesity, smoking, alcoholism, benign prostatic hyperplasia (BPH) with lower urinary symptoms (LUTS), depression, and premature ejaculation. Diabetes is a common aetiology of sexual dysfunction, because it can affect both the blood vessels and the nerves that supply the penis. Men with diabetes are four times more likely to experience erectile dysfunction, and on average, experience it 15 years earlier than men without diabetes.
“Obesity is also correlated to the development of several types of dysfunction, including a decrease in sex drive and an increase in episodes of ED,” she continues. “Neurogenic erectile dysfunction is caused by a deficit in nerve signalling to the corpora cavernosa. Such deficits can be secondary to spinal cord injury, multiple sclerosis, Parkinson disease, lumbar disc disease, traumatic brain injury, radical pelvic surgery and diabetes. Men being treated for prostate cancer with treatments such as
radical prostatectomy, radiation therapy or the use of lutenising hormonereleasing hormone (LHRH) agonists and antagonists often experience ED.”
Numerous medications are listed with erectile dysfunction and/ or a decreased libido as a side effect. Drugs that can cause ED include hydrochlorothiazide’s and betablocking agents. Medications used to treat depression, particularly the SSRIs such as citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline, can also contribute to ED. The severity of erectile dysfunction is often described as mild, moderate or severe according to the five-item International Index of Erectile Function (IIEF-5) questionnaire, with a score of 1–7 indicating severe, 8–11 moderate, 12–16 mild–moderate, 17–21 mild and 22–25 no erectile dysfunction.
The International Index of Erectile Function (IIEF-5)
Questionnaire
The IIEF is a multidimensional validated questionnaire with 15 questions in the five domains of sexual function (erectile and orgasmic functions, sexual desire, satisfaction with intercourse and overall sexual satisfaction), and there is also an abbreviated format of five questions in the Sexual Health Inventory for Men (SHIM).
Investigations and Diagnosis
Theresa continues, “A thorough medical history, detailed sexual
73 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
An interview with Theresa Lowry- Lehnen (PhD), CNS, GPN, RNP, South East Technological University
history, and physical examination are required before commencing treatment or further investigations. It is important to distinguish between psychological and organic causes of ED, as well as to ensure that the patient has erectile dysfunction and not another disorder. History that points towards a psychological aetiology include, sudden onset of erectile dysfunction especially if it is related to a new partner or a major life-changing event, situational ED, normal erections with masturbation or a different partner, presence of morning erections and high daily variability in erectile rigidity.
“The main differential diagnosis for erectile dysfunction is hypogonadism, loss of libido, depression with low mood, and other psychological conditions. It may also be the first manifestation of diabetes or cardiovascular disease as well as depression.
It is important to differentiate between true erectile dysfunction and other sexual disorders such as premature ejaculation, and this is usually assessed by obtaining a good sexual history.
“A complete medication list including supplements should be checked with the patient. ED can be a result of prescription or other medications. Prescription drugs that can cause ED include, antidepressants especially SSRIs, cimetidine, ketoconazole, spironolactone, sympathetic
UROLOGY: ERECTILE DYSFUNCTION


blockers, thiazide diuretics, and other antihypertensives. ACE inhibitors and calcium channel blockers are the least likely to cause ED. Beta-blockers are only a minor contributor, while alpha-blockers can improve erectile function.”
Vascular risk factors such as hypertension and diabetes and lifestyle factors such as smoking, activity level, alcohol intake, and the use of any recreational drugs should be assessed, Theresa adds. A full general and cardiovascular examination should be undertaken, as erectile dysfunction can be the first symptom of underlying vascular disease. Peripheral pulses should be checked and blood pressure measured. The genitalia should be carefully inspected for hypogonadism, signs of infection, the presence of penile fibrosis or plaques, and phimosis.
Theresa continues, “The role of testosterone replacement therapy (TRT) as a potential to improve erectile function in ED remains an issue for clinicians who are comfortable treating androgen deficiency. Androgens are known to have a significant impact on the function of the smooth musculature within the corpus spongiosum.
“Testosterone supplementation is more effective as a treatment for low libido than for ED. For most men with both ED and hypogonadism, oral PDE5 inhibitors alone are recommended as the initial therapy. Testosterone supplementation is reasonable in men with proven hypogonadism and ED who have already failed PDE5 inhibitor therapy or who also have low libido. Hypogonadal patients with borderline erectile rigidity are most likely to benefit from testosterone supplementation.
“Testosterone replacement therapy may cause increased levels of haemoglobin or haematocrit which is associated with an increased risk of heart attack, stroke and blood clots. Testosterone treatment can also cause an enlarged prostate or other prostate disorders. During TRT treatment, the prostate specific antigen (PSA) will be measured to monitor for any changes and this is particularly important in men over 45 years of age.
“As a result of using testosterone replacement, natural production of testosterone may be reduced. This may lead to a reduction in sperm production and fertility. Other side effects of TRT include: weight gain, increased appetite, hot flushes, acne, depression, restlessness, irritability, aggression, tiredness, general weakness and excessive sweating. Lifestyle modifications are considered first-line therapy for ED, and men should be encouraged to make the necessary changes to benefit both their sexual function and their overall health.
“PDE5 inhibitors are highly effective and have an overall success rate of up to 76%. PDE5 inhibitors are contraindicated in patients taking nitrates, but otherwise are safe and effective. When PDE5 inhibitors are co-administered with nitrates, pronounced systemic vasodilation and severe hypotension can occur.
“PDE5 inhibitors and α-adrenergic receptor blockers, often used
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
74
ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing
Viagra Connect (sildenafil) 50 mg film-coated tablets Indications, Dosage and Administration: Indications: For erectile dysfunction in adult men. Dosage and Method of use: Adults: one 50 mg tablet taken with water approx. one hour before sexual activity. The maximum dosing frequency is once per day. The onset of activity may be delayed if taken with food. Patients should be advised that they may need to take Viagra Connect a number of times on different occasions (max of one 50 mg tablet per day), before they can achieve a penile erection satisfactory for sexual activity. If patients are still not able to achieve a sufficient penile erection they should be advised to consult a doctor. Elderly: no dosage adjustments required (≥ 65 years old). Renal Impairment: No dosage adjustments for patients with mild to moderate renal impairment. Dosage adjustments required for those with severe renal impairment, see SmPC. Hepatic Impairment: Dosage adjustments required for those with mild-moderate hepatic impairment, see SmPC. Viagra Connect is contraindicated for patients with severe hepatic impairment (see contraindications). Presentation: Blue, rounded diamond-shaped film-coated tablets measuring 11.2 mm x 8.1 mm, marked “PFIZER” on one side and “V50” on the other. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Co-administration with nitric oxide donors (such as amyl nitrite), nitrates, ritonavir, guanylate cyclase stimulators (such as riociguat) is contraindicated. Agents for the treatment of erectile dysfunction, including sildenafil, should not be used by those men for whom sexual activity may be inadvisable, and these patients should be referred to their doctor. This includes patients with severe cardiovascular disorders such as a recent (6 months) acute myocardial infarction (AMI) or stroke, unstable angina or severe cardiac failure. Sildenafil should not be used in patients with severe hepatic impairment, hypotension (blood pressure < 90/50 mmHg) and known hereditary degenerative retinal disorders such as retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases). Sildenafil is contraindicated in patients who have loss of vision in one eye because of non-arteritic anterior ischaemic optic neuropathy (NAION), regardless of whether this episode was in connection or not with previous PDE5 inhibitor exposure. Viagra Connect should not be used in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie's disease). Viagra Connect is not indicated for use by women. The product is not intended for men without erectile dysfunction. This product is not intended for men under 18 years of age. Warnings and precautions: Erectile dysfunction can be associated with a number of contributing conditions, e.g. hypertension, diabetes mellitus, hypercholesterolaemia or cardiovascular disease. As a result, all men with erectile dysfunction should be advised to consult their doctor within 6 months for a clinical review of potential underlying conditions and risk factors associated with erectile dysfunction (ED). If symptoms of ED have not improved after taking Viagra Connect on several consecutive occasions, or if their erectile dysfunction worsens, the patient should be advised to consult their doctor. Cardiovascular risk factors: Since there is a degree of cardiac risk associated with sexual activity, the cardiovascular status of men should be considered prior to initiation of therapy. Agents for the treatment of erectile dysfunction, including sildenafil, are not recommended to be used by those men who with light or moderate physical activity, such as walking briskly for 20 minutes or climbing 2 flights of stairs, feel very breathless or experience chest pain. For a list of patients who are considered at low cardiovascular risk from sexual activity see SmPC. Patients previously diagnosed with the following must be advised to consult with their doctor before resuming sexual activity: uncontrolled hypertension, moderate to severe valvular disease, left ventricular dysfunction, hypertrophic obstructive and other cardiomyopathies, or significant arrhythmias. Sildenafil has vasodilator properties, resulting in mild and transient decreases in blood pressure. Patients with increased susceptibility to vasodilators include those with left ventricular outflow obstruction (e.g. aortic stenosis), or those with the rare syndrome of multiple system atrophy manifesting as severely impaired autonomic control of blood pressure. Priapism: Patients who have conditions which may predispose them to priapism (such as sickle cell anaemia, multiple myeloma or leukaemia), should consult a doctor before using agents for the treatment of erectile dysfunction, including sildenafil. Prolonged erections and priapism have been occasionally reported with sildenafil in post-marketing experience. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. Concomitant use with other treatments for erectile dysfunction is not recommended. Effects on vision: Patients should be advised that in the event of any sudden visual defect, they should stop taking Viagra Connect and consult a physician immediately. Concomitant use with CYP3A4 inhibitors: patients should be advised to consult a doctor before taking Viagra Connect as a 25 mg tablet may be more suitable for them. Concomitant use with alpha-blockers: Caution is advised when sildenafil is administered to patients taking an alpha-blocker, as the co-administration may lead to symptomatic hypotension in a few susceptible individuals. This is most likely to occur within 4 hours post sildenafil dosing. In order to minimise the potential for developing postural hypotension, patients should be hemodynamically stable on alpha-blocker therapy prior to initiating sildenafil treatment. Thus, patients taking alpha blockers should be advised to consult their doctor before taking Viagra Connect. Treatment should be stopped if symptoms of postural hypotension occur, and patients should seek advice from their doctor on what to do. Effect on bleeding: the use of sildenafil is not recommended in those patients with history of bleeding disorders or active peptic ulceration, and should only be administered after consultation with a doctor. Hepatic impairment: Patients with hepatic or renal impairment must be advised to consult their doctor before taking Viagra Connect, since a 25 mg tablet may be more suitable for them. Lactose: The film coating of the tablet contains lactose. Viagra Connect should not be administered to men with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per tablet. Patients on low sodium diets can be informed that this medicinal product is essentially ‘sodium-free’. Use with alcohol: Drinking excessive alcohol can temporarily reduce a man's ability to get an erection. Men should be advised not to drink large amounts of alcohol before sexual activity. Interactions with other medicinal products and other forms of interaction: Individuals receiving concomitant treatment with CYP3A4 inhibitors must be advised to consult their doctor before taking Viagra Connect, dosing adjustments may be required, see SmPC. Patients receiving alpha blocker treatment should be stabilised on therapy prior to initiating sildenafil treatment and must be advised to consult their doctor before taking Viagra Connect as dosing adjustments may be required, see SmPC. Caution when sildenafil is initiated in patients treated with sacubitril/valsartan, see SmPC. Fertility, pregnancy and lactation: There was no effect on sperm motility or morphology after single 100 mg oral doses of sildenafil in healthy volunteers. Viagra Connectis not indicated for use by women. Undesirable effects: Very common (≥1/10): headache. Common (>1/100, <1/10): dizziness, visual colour distortions, visual disturbance, vison blurred, flushing, hot flush, nasal congestion, nausea, dyspepsia. For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC. Legal Category: Not subject to medical prescription. Supply through pharmacies only. Marketing Authorisation Number: PA23055/016/001 Marketing Authorisation Holder:

Reporting of adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse reactions/events should also be reported to the marketing autorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267.
www.viagraconnect.ie For more information please contact Viatris or refer to the SmPC for full prescribing information HELPS YOU GET AND KEEP AN ERECTION VIAC-2023-0099. DOP: May 2023
IJssel, Netherlands. Full prescribing information available on request from: Viatris, Dublin 17. Phone 01 8322250 Date of Revision of Abbreviated Prescribing Information: 03 Feb 2023
IE-AbPI-ViagraConnect-v003
Upjohn EESV, Rivium Westlaan 142, 2909 LD Capelle aan den
Reference Number:
VIATRIS
Helps erection
for treatment of BPH, need to be taken at least 4 hours apart. Among second-line therapies, external vacuum devices (VCDs) are a good, non-surgical option for patients with ED. VCDs are clear plastic chambers placed over the penis, tightened against the lower abdomen with a mechanism to create a vacuum inside the chamber. This directs blood into the penis. If an adequate erection occurs inside the chamber, the patient slips a small constriction band off the end of the VCD and onto the base of the penis. An erection beyond 30 minutes is not recommended. While cumbersome, these devices are considered safe.
“Other second-line therapy includes the use of either intracavernosal injection (ICI) or intraurethral suppositories (IUS). A small needle is used to inject the ICI medication into the lateral aspect of the penis through a small-gauge needle. These vasoactive agents include prostaglandin E1, papaverine and
UROLOGY: ERECTILE DYSFUNCTION

malfunction and infection are rare, and patient satisfaction is high.”
phentolamine and sometimes atropine, which work alone or in combination to elicit an erection. Response is dose related, usually occurs within 10– 15 minutes, and does not require stimulation. A concern with ICI use is priapism, and if this occurs the patient will need to seek urgent medical attention. Bruising can also occur, due to it being an injected medication. The intraurethral suppository consists of a tiny pellet of prostaglandin E1 inserted into the urethral meatus. Response is dose related, and onset usually occurs within 10–15 minutes. Patients need to be trained on the technique of the IUS before use, and should be advised that pain or burning may occur with this medication.
“In men who fail to respond to first or second-line therapy, or who are not interested in conservative therapies, penile prosthesis implantation is available. Penile implants include malleable and inflatable devices, although most implants used are of the inflatable variety. Adverse effects including
Outlook
Theresa told us that future Therapies for ED Clinical studies in gene therapy are looking towards replacing proteins that may not be functioning properly in the penile tissue of men with erectile dysfunction.
She says, “Replacement of these proteins may result in improvement in ED. Experimental animal models have demonstrated improvement in erectile function with gene therapy. Human studies may demonstrate success with this therapy in the future, however, gene therapy in humans is controversial, and can take a long time for regulatory approval and public acceptance.
“Stem cell studies may also provide advancements in the treatment of ED in the future. The mechanism of action of stem cells is to generate angiogenesis with subsequent increase in cavernosal smooth muscle cells within the corporal bodies.
New Model of Care for Dementia in Ireland
The HSE Enhanced Community Care Programme has launched a new Model of Care for Dementia to set out care pathways to ensure people living with dementia are at the centre of care practices and service design.
With over 64,000 people currently living with dementia in Ireland, it is a life changing condition.
Dr Sean O’ Dowd, Consultant Neurologist, Clinical Lead, National Dementia Office, HSE, added, “As a clinician, it gives me great pleasure to be part of the launch for the Dementia Model of Care. We are all aware of the growing prevalence of dementia in Ireland, so the delivery of this document is timely. The Dementia Model of Care will provide an integrated framework to bring together a wide range of services into a coherent pathway for people living with dementia. There is no doubt that the Model of Care for Dementia in Ireland is an ambitious document that seeks to place Ireland to the fore internationally
in our approach to brain health, cognitive impairment and dementia. With this Model of Care and its associated service developments, it means that the people of Ireland will have timely access to exemplar memory services that will deliver best practice in assessment, communicating a diagnosis, personalised care planning and post-diagnostic supports.”
The Dementia Model of Care builds upon the work of the National Dementia Strategy (2014), the HSE Corporate Plan 20212024 and has been developed within the context of Sláintecare (2020–2023) and the health reform agenda, where delivering the right care, in the right place, at the right time, given by the right team.
The Dementia Model of Care provides for a diagnostic model utilising three levels of assessment:
• Level 1: Primary Care GP delivered assessment is considered Level 1, this may include support and
information from any of the Enhanced Community Care (ECC) programme services; CHN/Primary Care Teams, Community Specialist Teams for older people, Community Specialist Teams for chronic disease and Community Intervention Teams (CIT). The decision on appropriateness of referral to Memory Assessment and Support Service, Regional Specialist Memory Clinic or the National Intellectual Disability Memory Service is at the discretion of the primary care physician.
• Level 2: Memory Assessment and Support Service: People 65 years or over with a typical and clear presentation of dementia will predominantly be assessed and supported in a Level 2 Memory Assessment and Support Service (MASS) (or/other specialist service). If a diagnosis is communicated, the person with dementia will be offered relevant post diagnostic supports in their geographical
“The clinical studies published to date provide encouraging results, with improvement of sexual function reported with no side effects. Although pioneering, stem cell studies to date are small scale, with a short follow up period, various aetiologies of ED and without a control group. Melanocortin activators are drugs that act through the central nervous system, and have been shown in animal studies to produce an erection. Initial studies in humans suggest that the drug (PT-141) can be effective if given intranasally in men with psychological rather than physical causes, and mild to moderate ED. “Larger studies are necessary, however, to demonstrate the safety and overall effectiveness of these drugs. Another potential new treatment for ED, is penile low intensity shock wave lithotripsy. This consists of 1500 shocks twice a week for 3–6 weeks. The purpose is to stimulate neovascularisation to the corporal bodies with improvement in penile blood flow and endothelial function. The use of low-intensity shock wave lithotripsy may convert PDE5 inhibitor nonresponders to responders.”
area. As of May 2023; nine MASS’s have been funded to date, located in the Donegal, Sligo, Cavan/Monaghan, Mullingar, Limerick, Kerry, Cork, Wexford and Waterford. Funding for additional MASS sites will be sought in future NSP estimates bids.
• Level 3: Regional Specialist Memory Clinic (RSMC): People 65 years or under with a suspected dementia or those with atypical or unclear presentations that require a more detailed assessment will predominantly be assessed and supported in a Level 3: Regional Specialist Memory Clinic (RSMC). However, they may utilise the post diagnostic support services that are local to where the person with dementia lives. As of April 2023; four RSMC’s have been funded to date, they are located in the Mercy University Hospital, Cork, Galway University Hospital, Tallaght University Hospital and St James’s Hospital, Dublin.
JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
76
News
First Institute for Clinical Trials Launched
The University of Galway has announced the establishment of Ireland’s first Institute for Clinical Trials.
The new institute will transform the clinical research landscape by creating an environment where scientific advances are translated into improved care for patients. The Institute for Clinical Trials will transform lives by ensuring patients get access to the latest medicines and treatments in a timely way.
Through its ambitious programme of research excellence, the Institute will position Ireland at the forefront of clinical and biomedical discovery.
University of Galway President Professor Ciarán Ó hÓgartaigh said, “As a university for the public good, led by our values, including excellence and openness, the establishment of the Institute for Clinical Trials will chart new paths in research for the benefit of the health and well-being of people at their most vulnerable time, in Ireland and internationally. This is a shining example of our ambition and
a manifestation of University of Galway looking beyond the horizon, forging breakthroughs in science and in research, in the world and for the world."
Director of the Institute, Professor Peter Doran, outlined the ambition for the Institute, said, “Research is critical to the healthcare ecosystem. Patients who attend hospitals that are research active have better outcomes, due to both increased access to early lifesaving treatments, and through the culture that pervades when research and inquiry are at the core of the health systems. By increasing clinical research activity, which is at the centre of the institute ambition, we will drive outcomes for patients.
“We also know that indigenous companies, particularly in the medtech sector, struggle to conduct clinical evaluations in Ireland, which is essential for market access. We are setting in motion a strategy to address the barriers which limit the conduct of clinical trials in Ireland.”
Cancer Matters Budget Submission
A major component of the Institute’s activity will be to improve how trials are done, integrating innovative methodologies, with enhanced technologies and better molecular analysis to create the trial of the future and position Ireland as a leader in clinical trials.
Professor Doran continued: “The cross-sectoral activities of the Institute for Clinical Trials will be
nationally distinctive, will align with Ireland’s regional development strategies and will enhance economic competitiveness by attracting investment, jobs and talent, in addition to its core mission of improving the health of the population.”

The Institute will be led from University of Galway’s College of Medicine, Nursing and Health Sciences.
The Irish Cancer Society has launched its ‘Cancer Matters’ Budget 2024 submission, urging Government to invest in cancer prevention and treatment, timely access to diagnostics, and to relieve some of the out-of-pocket costs paid by people affected by cancer. Some of the key asks outlined include:
• Allocating ¤20 million in the National Cancer Strategy to support and develop cancer services
• Abolishing VAT on suncream to ensure that sun protection is more affordable
• Removing car parking charges to remove this extra layer of expense on cancer patients when they are financially vulnerable
• Guaranteeing women treated for cancer post-partum can postpone maternity leave during their treatment
Director of Advocacy Rachel Morrogh said, “Timing is everything when it comes to diagnosing and treating cancer. This is why we call for a protected cancer care pathway to ensure that people can access the diagnostic tests as and when they are needed, and access cancer treatment in a timely manner. Investment in the National Cancer Strategy, and in the vital services, is essential towards ensuring good results for cancer patients during and after cancer.”
New Cancer Research Clinic at Tallaght
An important new Cancer Research Clinic, the first and only one of its kind in Ireland has been established at Tallaght University Hospital (TUH). The new Testicular Survivorship Clinic is conducting research to try and discover new treatments for patients, who have had the disease.
Those centrally involved in this important new clinic at TUH include; Dr M Raheel Khan (Research Registrar) Prof Ray Mc Dermott (Medical Oncologist) and Advanced Nurse Practitioner in Oncology, Patrice Sheehan. The
team started work on the clinic back in September 2022 and saw their first patient in October 2022.
To date, they have seen around 75 patients.
So far medical staff involved in this trial at TUH have met with patients to examine them, take blood tests and get them to fill in a targeted questionnaire. If anything suspicious arises out of this initial screening then further tests, scans and if necessary follow-up treatment can be arranged. The team behind this new development are hoping this clinic will improve
morbidity and mortality for patients with Testicular Cancer. Medical Oncologists around the country are very keen to replicate this model in other centres.
Testicular Cancer is typically diagnosed in a young male population (15 to 35 years) and has a cure rate of 95%. So there is a big cohort of patients who are survivors. Recent studies have shown a very high incidence of second cancers, cardiovascular events and psychological issues, resulting in higher rates of early deaths, suicide, unemployment
and disabilities. To help them and intervene early if these other complications arise, numerous medical studies including this one at TUH are being conducted in the US, UK and Scandinavian countries.
In March 2023, at a stakeholder meeting of Cancer Trial Ireland, it was agreed to establish similar clinics nationwide. A central registry will be maintained in the Trials Unit of TUH. As a result, the TUH Cancer trials team will collect and maintain data on patients from all over Ireland.
77 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
News
Professor Peter Doran
Revolutionising CP Care in Ireland
Professor Geraldine Boylan, Director of the INFANT Research Centre at University College Cork, expressed excitement for the programme’s potential impact. “We are thrilled to be part of this programme in Ireland and to work with the Cerebral Palsy Foundation, healthcare & research community, and families to make a significant and positive difference in the lives of people with cerebral palsy. Early intervention is critical for people with CP. The earlier we can diagnose and intervene, the better the outcomes for the child and the family.”
The Cerebral Palsy Foundation (CPF) has announced the launch of a Programme of Excellence to revolutionise the delivery of cerebral palsy care in Ireland. Through the development of three major clinical and research hubs at Trinity, University College Cork, and RCSI University of Medicine and Health Sciences, this firstof-its-kind initiative will establish Ireland as an international leader in cerebral palsy care and research. Children’s Health Ireland (CHI) is an implementation partner in the academic healthcare programme. Cerebral Palsy is the most common childhood-acquired lifelong physical disability. Many individuals with CP face significant and unnecessary challenges in their daily lives, including problems with movement, speech, and other body systems.
An estimated 150 babies receive a CP diagnosis in Ireland each year, and an estimated 3,000 children and young people and 9,500 adults are living with CP in Ireland. Early intervention and the right care pathways can make a significant difference in the longterm outcomes and quality of life for people living with CP.
Backed by $12.5 million in philanthropic funding pledged from a group of donors including John and Patrick Collison, this fiveyear programme will change the trajectory of people’s lives affected by CP in Ireland.

The Programme of Excellence will advance CP care and research across four major pillars:
Clinical Implementation: implementing the best evidence into clinical practice in healthcare settings.
Research: conducting highquality research programmes and developing an international network of researchers committed to implementation, dissemination, and knowledge transfer.
Dissemination and Education: promoting community education and providing professional education and career pipelines to encourage and develop new clinicians and researchers in the fields of CP clinical care and research.
Advocacy and Policy: identifying and developing stakeholder groups, supporting individuals with CP and their families to drive improved care.
The programme will build on significant strides already made by the CP research community and leading clinicians from around the world. An Expert Steering Committee of cerebral palsy and non-cerebral palsy experts from clinical care, academia, industry, and the CP community will guide the implementation ensuring a multi-disciplinary and multi-stakeholder approach. Four priority areas of clinical implementation and research have been identified as priority for the initial phase of the programme:
• Early Detection & Intervention (0-2yrs) led by UCC
• Musculoskeletal and Orthopaedic Care (children and young people) led by Trinity
• Community-Based Motor Management Services (children, young people, and adults) led by UCC, TCD & RCSI
• Adult services and support led by RCSI
These priority areas will be piloted in regions across Ireland. Once systems have been developed and proven they will be used as models to expand care across the country. The Programme will utilise already established networks including In4Kids, a Health Research Board national paediatric clinical trial network. CPF is committed to expanding this programme through further research funding and philanthropic support. Institutional support from Trinity, UCC, and RCSI guarantees its continuation beyond the initial five-year period.
"Our vision is to make Ireland a world leader in the delivery of Cerebral Palsy care," said Rachel Byrne, Executive Director of the Cerebral Palsy Foundation. “We want to create a sustainable continued care model for infants through to adults with CP in Ireland led by expert clinicians and researchers. We will leverage our extensive network and international expertise on best practice to help drive the programme through collaboration. We welcome all partners, including the CP Community, academia, industry, and government to join us on this journey."
Eilísh Hardiman, Chief Executive of Children’s Health Ireland welcomed this Programme: “Children’s Health Ireland is committed to improving clinical outcomes for infants, children, and young people with Cerebral Palsy. As a partner in paediatric academic health sciences, CHI welcomes collaboration with Trinity and UCC to use this philanthropic funding pledge by the CP Foundation to truly make a difference to healthcare, education, research, and innovation in CP care in Ireland.”
Professor Colin Doherty, Head of School of Medicine at Trinity College Dublin said: "This programme provides a rare and unique opportunity for Ireland to become a world leader in cerebral palsy care and treatment. Trinity is working alongside other partners to make real discovery in the development of new diagnostic techniques, new therapies and new pathways of integrated care between the hospital and the community and between childhood, adolescence and adulthood for people with cerebral palsy.”
Professor Cathal Kelly, ViceChancellor, RCSI University of Medicine and Health Sciences said: “We are really pleased to be a partner in this important initiative which will have a positive impact on the lives of people with cerebral palsy. As a university wholly focused on health, RCSI is deeply committed to educating the next generation of health care professionals and to research which drives improvements in patient outcomes and quality of life. The RCSI team will now build on the School of Physiotherapy’s track record of leading research and education on cerebral palsy, with a particular focus on adulthood, as part of this new collaboration.”
Professor Eleanor Molloy, Professor of Paediatrics & Child Health, Trinity Institute of Neurosciences (TCIN) said “every individual with cerebral palsy is different so it's very important that we can personalise care plans for each person. Huge work is being done across all aspects of research and patient care and this is being translated into tailored patient pathways and interventions throughout life.”
The Programme will also support the development of a Cerebral Palsy Register in Ireland, enabling the collection of important data to inform research and improve care.
78 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Geraldine Boylan, Director of the INFANT Research Centre at University College Cork
CLONMEL HEALTHCARE: NEW LAUNCH – FLAVOUR AND PACK SIZE!
FEEL HYDRATED, STAY RECHARGED WITH ELECTROSAL
Clonmel Healthcare are delighted to announce the launch of Electrosal Orange Flavour AND a brand new 5 pack. The 5 pack is an addition to the existing 10 pack.
Electrosal is an oral hydration solution containing 3 electrolytes in 1. Electrosal can be used for effective hydration to help replace the loss of fluid and body salts due to exercise, sports, hot weather or travel. Electrosal is vegan friendly, gluten free and GMO free
Available in 3 flavours – Orange, Lemon & Raspberry and Blackcurrant.
the perfect solution to achieving the ultimate summer glow while also keeping the skin youthful with their new serum. This fast-drying face serum is a game-changer, combining hydration and skin-smoothing properties to give you a radiant complexion like never before.
only does this invigorating aroma uplift your mood, but the oil also possesses anti-inflammatory properties that help diminish redness and irritation, ensuring a calm and soothing effect on your skin.
With these exceptional active ingredients working harmoniously, TanOrganic’s Anti-Ageing Facial Tan Serum offers unparalleled antiageing benefits that will transform your skincare routine. Say goodbye to dull and ageing skin and embrace a luminous and revitalized complexion with TanOrganic’s ground-breaking formula.
With an impressive 100% score on the Ethical Organization, TanOrganic stands as one of only seven companies globally to achieve this remarkable distinction.
is adding to its portfolio with a new shampoo for daily use; Nizoral® Anti-Dandruff Daily Prevent Shampoo.

Nizoral® Anti-Dandruff Daily Prevent Shampoo provides ongoing relief for dry, itchy and flaky scalps and is clinically proven to provide instant protection from the first wash.
Nizoral® Anti-Dandruff Daily Prevent Shampoo is suitable for sensitive scalps, and all hair types. Please contact Clonmel Healthcare on 01-6204000 if you require any additional information, or visit www.nizoral.ie.
Electrosal is available in two pack size – 5 pack and 10 pack. Electrosal is suitable for adults and children over 2 years.

Please contact Clonmel Healthcare on 01-6204000 if you require any additional information on Electrosal. Electrosal is a food supplement not to be used as a substitute for a balanced diet. Always read the label.
For more information visit www.electrosal.ie
Clonmel Healthcare Ltd., Clonmel, Co. Tipperary.
Date Prepared: May 2023 2023/ ADV/ELE/118H
TANORGANIC INTRODUCES REVOLUTIONARY ANTI-AGEING FACIAL TAN SERUM FOR A

RADIANT AND YOUTHFUL GLOW
This summer season, TanOrganic are thrilled to announce the launch of their latest addition to the collection and a step into the skin health space with their NEW Anti-Ageing Facial Tan Serum (RRP ¤44.99). Recommended by dermatologists, TanOrganic, the leading provider of organic and natural tanning products provides
This breakthrough serum is designed to provide a naturallooking tan while simultaneously delivering powerful anti-ageing benefits, allowing users to embrace their confident and radiant selves during those makeup-free summer days. This revolutionary serum redefines the self-tanning experience. Formulated with over 90% organic ingredients, including the remarkable peptide Matryxl 3000, apple stem cell, hyaluronic acid, orange peel oil, and aloe vera, TanOrganic’s Anti-Ageing Facial Tan Serum provides a comprehensive skincare experience that goes beyond mere tanning.
One of the standout components is Matryxl 3000, a potent peptide renowned for its ability to stimulate collagen production in the skin. Collagen is the key protein responsible for maintaining skin elasticity and firmness, but its levels decline with age, leading to unwanted wrinkles and sagging. By harnessing the power of Matryxl 3000, TanOrganic’s serum effectively reduces the appearance of fine lines and wrinkles, while also improving overall skin texture and tone.
In addition to Matryxl 3000, TanOrganic have incorporated apple stem cell peptides, known for their remarkable capacity to promote skin regeneration and reduce the visibility of wrinkles. These peptides work tirelessly to enhance your skin’s health, resulting in a more youthful and rejuvenated complexion. To elevate your sensory experience, the Anti-Ageing Facial Tan Serum features orange peel oil, providing a refreshing citrus scent. Not
TanOrganic products are COSMOS Vegan Society Certified, Leaping Bunny Approved, Ethically Certified and Guaranteed Irish.
TanOrganic’s Anti-Ageing Facial Tan Serum is available on TanOrganic.com and in Boots, Dunnes Stores, Shaws, and Tesco stores nationwide and leading pharmacies including McCauleys, Meaghers, and McCabes.
Instagram @TanOrganicOffical & Facebook@TanOrganic
For further information/ imagery/ giveaways contact
Joanna Timmons at The Publicity Loft/ Email joanna@publicityloft. com or phone 085 7666 473
About TanOrganic
TanOrganic use only the best, natural and organic ingredients that are free from all synthetics, parabens, and toxins, the world’s first and only eco-certified tan brand enriched with luxurious aloe vera and hyaluronic acid to keep your skin hydrated and give it a natural radiance with the brand removing 1lb of plastic from the ocean for every bottle sold!
As the world’s first and only Ecocertified, Vegan Society, Cruelty-Free, and Ethically Certified self-tanning brand, TanOrganic has established itself as a leader in the industry. The brand’s commitment to using only natural and organic ingredients resonates with conscious consumers who prioritize both their skin health and the environment.
THE SCALP CARE EXPERT
NIZORAL® LAUNCHES
Nizoral Dandruff 20mg/g Shampoo contains 20mg of ketoconazole per each gram of shampoo. Supply through pharmacies only. A copy of the Summary of Product Characteristics is available upon request or go to www.clonmelhealthcare.ie.
Marketing authorisation holder: Clonmel Healthcare Ltd. Waterford Road, Clonmel, Co. Tipperary. Always read the label. Date prepared May 2023. 2022/ADV/ NIZ/310H.
DATE FOR YOUR DIARY –MEDTECH IRELAND 2023
Medical Technology Ireland will bring together manufacturers, academic institutions, entrepreneurs/start-ups, financial institutions and suppliers alike in a combined programme, defining present and future medical device trends, to investigate, discuss, and improve the quality of life and to increase the life expectancy for patients worldwide.
The Medical Technology Ireland Conference is taking place on 20-21 September 2023 in Galway.
NIZORAL®
ANTI-DANDRUFF DAILY PREVENT SHAMPOO
The scalp care expert Nizoral®; known and trusted by Irish customers for treating dandruff with Nizoral Dandruff Shampoo;
A Call for Papers is now open for individuals and organisations that would like to submit a presentation for the Conference programme. Please send your proposal to Tom Burke, tom@harpe.ie
79 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023 Clinical R&D
Clinical R&D
BIOSIMULYTICS ENTERS EXCLUSIVE HPC CLOUD PARTNERSHIP WITH CGG

Biosimulytics, an Irish pharma software company, today announced that it has signed an exclusive partnership agreement with CGG, a global technology and HPC leader headquartered in Paris, France. CGG will provide Biosimulytics, which uses artificial intelligence (AI) technologies to dramatically improve drug development, with a fully customized HPC (high performance computing), AI, and cloud solution to rapidly serve the company’s growing volume of pharmaceutical customers worldwide.
Using CGG’s algorithm and HPC expertise enables Biosimulytics to fully scale its breakthrough pharmatech platform which provides pharmaceutical companies with a powerful predictive simulation capability when developing new drug molecules.
The Biosimulytics technology shortens the time to market and reduces the risks for important developments in the pharmaceutical industry in getting from molecules to medicine (M2M) and is a key enabling technology for the new era of precision medicine. CGG’s custom solution meets the specialist needs of Biosimulytics for a high-performing HPC and AI environment that optimizes its suite of specialized molecular simulation applications from crystal structure prediction (CSP) to structure-based drug design (SBDD).
Biosimulytics can now run applications at least five times faster than its previous public cloud solution, enabling it to dramatically improve its return on investment and rapidly expand its business.
As a European company offering cloud services hosted in the European Union, CGG also offers the full legal protection of EU regulations for the intellectual property of Biosimulytics and its customers.
Biosimulytics, which is headquartered at NovaUCD in Dublin, is a University College Dublin (UCD) spin-out company from the UCD School of Chemical and Bioprocess Engineering that was founded in 2019. Biosimulytics is also an Enterprise Ireland High-Potential Start-Up (HPSU) company and is strongly backed at the European level through EIT Digital and other EU-funded accelerator programmes.
Biosimulytics is working as a Digital R&D solution provider to some of the world’s leading pharmaceutical companies. The
AI drug development market is expected to exceed US$10bn by 2030 growing at a CAGR of 25%.
Peter Doyle, CEO and CoFounder of Biosimulytics, said, “We are delighted to announce this exclusive partnership with CGG since our need for a high-performing HPC and AI environment to optimize our Biosim M2M platform is uniquely met with CGG's customized solution.
The AI drug development space is now at an inflection point and our ambition is to enable the global pharma industry to advance potential molecules to approved medicines quicker and with a much greater probability of success by making AI-powered predictive technology much more accessible and affordable for widespread use by everyone from Big Pharma to small emerging biotech companies. Today’s announcement with CGG is a significant milestone on that journey.”
Agnès Boudot, EVP, HPC & Cloud Solutions, CGG, said, “CGG is a global expert in industrial and customized end-to-end HPC and AI services with over 70 years of experience in pioneering computing solutions. After successfully establishing our cloud services offering in the energy, energy transition and mining sectors, where more than 30 external clients are currently directly accessing and using CGG Cloud solution services for delivering insights into their data, we are pleased to expand into the healthcare and life sciences market by supporting Biosimulytics in using AI- and HPC-based technologies to help unlock new and improved therapies faster and more cost efficiently.
Our experience of hosting and optimizing scientific workflows
running on over 350 petaflops at an industrial scale ensures that we can provide Biosimulytics with a scalable solution to meet their future growth requirements for this complex HPC and AI workflow.”
LETTERKENNY UNIVERSITY HOSPITAL WELCOMES PUBLICATION OF HIQA REPORT FOLLOWING INSPECTION
Letterkenny University Hospital welcomes the publication by Health Information Quality Authority (HIQA) of its report into its inspection of three areas at Letterkenny University Hospital (LUH). An announced inspection was carried out at the hospital on the 16th and 17th of November 2022.
The areas assessed were the Emergency Department, Medical 2 Ward and the Gynaecology Ward. The focus of the inspection was to monitor the National Standards for Safer Better Healthcare. HIQA’s core assessment focused on key standards relating to leadership, governance and management, workforce, person-centred care, safe care and support and effective care.
During this inspection, HIQA looked at 13 National Standards in relation to leadership, governance and management and workforce. Of these, Letterkenny University Hospital was substantially compliant with 3 National Standards, partially compliant with six National Standards and non-compliant with four National Standards.
Sean Murphy Hospital Manager at Letterkenny University Hospital said, “We welcome this report and recognise the important and valuable role of HIQA in promoting safety and quality in the healthcare services. Urgent action has been taken to address the issues identified by HIQA and significant progress has been made by all staff to ensure that we provide our services safely.
“I would like to acknowledge the on-going commitment and dedication of our staff in providing a patient centred approach and we will work together to build on the good practice highlighted in this report.”
Significant work has been carried out on all areas where the hospital was found to be non-compliant or partially compliant. In a number of areas this work had commenced prior to the HIQA Inspection.
In the area of Overall Governance, the revision of the Clinical Handover Policy has been completed.
As part of the implementation of the National Healthcare communication programme,
facilitator training is underway with five members of staff identified to undertake the training.
A programme of work to strengthen governance within Letterkenny University Hospital (LUH) has been identified. The LUH Hospital Executive Board (HEB) and the QPS service, working with the Change Implementation Manager and Saolta Executive will agree and implement changes to the governance structure at LUH to include updated terms of reference; membership; and reporting relationships.
The HIQA concerns regarding the governance of the Respiratory Receiving Unit (RRU) were in the process of being addressed at the time of inspection. Changes were implemented in December 2022 and full management of the service was transferred to the emergency medicine service in January.
The acute medical assessment unit was re-established at the hospital in February with the employment of Consultant AMAU Physician. This marked the cessation of a distinct RRU facility. With changes to Covid pathway guidance, the area is no longer utilised as a red ED stream. The AMAU is under the governance of the medical directorate.
In regards to access to diagnostics, a second CT scanner is now operational at the site with protected slots for AMAU each day. The MRI scanner at the hospital is scheduled for replacement later this year and the new scanner will increase both functionality and speed within the service.
In relation to the risk register internal audit, risk register meetings have increased from quarterly to monthly to ensure the timely implementation of all recommendations. 5 out of the 7 recommendations are fully implemented with the remaining 2 almost fully implemented.
Recruitment remains a top priority for LUH. Since November 2022 the hospital has recruited 140 new staff across all grades. A new electronic employment control tracker has been implemented in April to streamline the recruitment process between LUH and Saolta. LUH HR Department has carried out significant recruitment over the last number of years for the Pharmacy Department at LUH, with an increase of 12 WTE (pharmacists and technicians) approved for LUH in the last two years, however like many hospitals LUH faces significant challenges in recruiting senior pharmacists required to undertake clinical pharmacy roles.
80 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Doyle, CEO and Co-Founder, Biosimulytics
In relation to Safe Care and Support, a further Assistant Director of Nursing was appointed to assist patient flow in February. The Saolta Group Unscheduled Care Lead is working very closely with the patient flow team in LUH ensuring all optimal patient flow processes are in place.
LUH has also received funding to employ four additional Consultant in Emergency Medicine. This will allow the hospital to improve the availability of Senior Clinical Decision makers within the Emergency Department and extend their onsite presence. LUH is seeking to fill of posts on a locum basis pending permanent filling of same. A Consultant has been employed to cover AMAU which has direct responsibility for staff in AMAU under Medical Department governance.
The Pathfinder project commenced in LUH in April 2023 to support admission avoidance by providing assessment and treatment at home. An ambulance liaison person is also now based in the ED to support nursing staff in caring for patients in ambulances. The hospital has also recently appointed a GP Liaison Senior nurse to be the link between GPs throughout Donegal and the LUH Emergency Department.
A Pathway stream has also been developed to fast track patients presenting with minor injuries through the Emergency Department.
LUH are working with HSE Estates colleagues to provide a modular building to relocate non clinical functions in the Emergency Department and convert the non-clinical accommodation into additional patient treatment areas for patients with potential transmissible diseases.
A Patient Advice and Liaison Service (PALS) Coordinator officer has been appointed to ensure patients receive communication and access to advice as required.
The Patient Advice and Liaison Service Coordinator acts as a visible focal point for patients, families and carers and is often the main contact within the hospital. They help to resolve issues for patients and their families and work towards improving the patient experience at any available opportunity. A second PALS officer is currently being recruited.
Under the area of Effective Care and Support actions have also been introduced in response to high levels of C.Difficile. The hospital continues to advertise for antimicrobial pharmacist and Consultant Microbiology
posts. In the interim there is a system in place to manage Infection Prevention and Control (IPC) issues and locums will be employed where possible.
NEW STANDARDS DEVELOPED TO SUPPORT CHILDREN HAVING HEALTHCARE PROCEDURES
Children in Hospital Ireland (CIH Ireland) welcome new international standards to support children and young people as they undergo healthcare procedures.
Lucy Bray, Professor of Child Health Literacy at Edgehill University has worked with an international team of health professionals, academics, children, young people, parents, child rights specialists, psychologists and youth workers to develop a set of standards for children and young people having health care procedures.
Anna Gunning, CEO of Children in Hospital Ireland stated, “For a child, it can be very daunting to visit a hospital and undergo new procedures, and these experiences can have a huge impact on a young person’s physical, emotional and psychological well-being. The child’s interests must come first and it is crucial that they are met with positive experiences. This will build confidence, reduce harm and establish trust. We warmly welcome these standards and look forward to Lucy Bray sharing her research with us here in Ireland.”
Children in Hospital Ireland will host their Annual Lecture with Lucy Bray on May 2nd to explore these standards and learn how parents and healthcare professionals can work together to improve the experiences for children and young people in hospital. Children in Hospital Ireland is a national charity that supports children and families in hospital through play and advocacy. Working for over 50 years in hospitals throughout Ireland, CIH provides play opportunities for children in hospital and seeks to support the rights and improve the welfare of children in hospital.
Professor, Lucy Bray added “We need to do more to make sure every child is supported to have a positive procedural experience and that their short- and long-term best interests are prioritised in all clinical decisions.
We would urge health professionals and organisations to do all they can to familiarise themselves with the standards and we hope that they prompt conversations in practice about how to support children before, during and after their procedure.“
Lucy Bray will be the keynote speaker at CIH’s Annual Lecture on May 2nd at The Albert Lecture Theatre in RCSI Dublin. To attend, please book your place online at: https://www.eventbrite.ie/e/annuallecture-getting-it-right-first-andevery-time-tickets-597713496157
HORIZON THERAPEUTICS PLC RANKS FIRST IN PATIENT CENTRICITY AND INTEGRITY
Horizon Therapeutics plc (Nasdaq: HZNP) today announced it ranked first in patient centricity, integrity, ease of relations and transparency in pricing by patient groups who worked with the company around the world. It ranks second in overall corporate reputation among these same groups. The results are based on PatientView’s annual survey of more than 2,200 patient groups evaluating more than 40 biotechnology and pharmaceutical companies.
Among patient groups familiar, but not working directly with Horizon, the company also ranked first in patient centricity, integrity and ease of relations and third overall in overall corporate reputation.
“Everything we do at Horizon is guided by people living with challenging diseases and the patient groups who serve them,” said Matt Flesch, vice president, product communications and patient advocacy, Horizon.
“We listen to input from patient groups on everything from clinical trial design to disease education initiatives, and work to support programs that have the greatest potential impact. We are constantly evolving our approach to best meet the needs of patient communities, and that is why this positive PatientView report means a great deal to all of us at Horizon.”
For the 2022 PatientView survey, 247 patient groups from around the world claimed familiarity with Horizon, an increase of 67 from 2021. Additionally, 130 groups said they had worked with the company, an increase of 43 from 2021. In each of the 13 indicators by which patient groups assessed corporate reputation, Horizon ranked in the top tier of global pharmaceutical and biotechnology companies
Horizon’s Commitment to Patients and their Advocates
• Held the first Rare Autoimmune Emerging Leaders' Summit, bringing together 26 patient advocate leaders in the rare and autoimmune space to provide important connections and a forum for groups to learn from each other.
• First ever Thyroid Eye Disease (TED) Mobile Exhibit in Atlanta,
Georgia, providing TED resources and information to attendees. This was part of Horizon’s ongoing effort to bring education and awareness to underserved communities.
• Brought together patients, care partners, and patient advocacy leaders to Horizon’s U.S. headquarters to discuss current challenges the NMO community is facing and to brainstorm on novel ideas and opportunities to support the NMO community.
• In Europe, hosted a disease awareness photo exhibition of individuals living with NMOSD at the 17th World Congress on Controversies in Neurology (CONy) Congress; individuals also attended the opening inperson to speak to Congress participants about their journey and experiences of living with NMOSD.
• In Brazil, supported the importance of multidisciplinary care to overcome the social and economic burden of rare autoimmune diseases through disease education initiatives that involved the art of spreading the patients' voice, like the "Fazendo Arte com Gustavo Rosa" art workshops for NMOSD patients and the short film "Atrás dos Meus Olhos", that tells the story of a TED patient.
• Became sole National Presenting Sponsor for Arthritis Foundation’s flagship event, Walk to Cure Arthritis, creating Horizon teams to volunteer and participate at over 70 events.
• Enhanced Horizon’s global, disease-agnostic #RAREis campaign and platforms including:
o Global Advocate Grant: Awarded 30 $5,000 grants supporting the rare disease community by providing financial assistance to global patient advocacy groups working to advance, educate and address the needs of the community.
o Scholarship Fund: In partnership with the EveryLife Foundation for Rare Diseases awards a one-time $5,000 educational scholarship to adults living in the U.S. with rare diseases. 177 scholarships have been awarded to date.
o Adoption Fund: In partnership with Gift of Adoption, provided financial support for 54 children living with rare diseases to be adopted.
81 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
Clinical R&D
NEW STRATEGY AIMS TO PROMOTE, PROTECT AND PROGRESS NURSING AND MIDWIFERY PROFESSIONS
The Nursing and Midwifery Board of Ireland (NMBI) has launched its Statement of Strategy which will run from 2023-2025.

As part of the new strategy, over the next three years, NMBI will focus on protecting, promoting, and progressing the nursing and midwifery professions. These strategic priorities will be supported through partnership and technology.
The strategy reflects the changing healthcare landscape and the need for nurses and midwives to be equipped with the knowledge, skills and resources to provide safe, high-quality care.
Some of the key priorities within the strategy include:
• Developing an online and inperson hub to support nurses and midwives from overseas to apply to join the Register in Ireland, and to adapt to working here.
• Reviewing entry pathways into nursing and midwifery to establish alternative routes, such as graduate-entry nursing.
• Working across health and education sectors to develop a plan to increase undergraduate student placement numbers.
• Implementing a more person-centred fitness to practise process to ensure a compassionate approach.
The strategy was developed following extensive consultation with nurses, midwives, educators and other key stakeholders. It sets out a clear vision and objectives to advance the nursing and midwifery professions in Ireland and enhance patient care.
Minister for Health, Stephen Donnelly TD said: "Nurses and midwives play an integral and important role across Irish
healthcare. I welcome the publication of NMBI’s Statement of Strategy 2023-2025, which acknowledges this key role and sets out an ambitious agenda focussing on how the NMBI, as a modern and progressive regulator, intends to play its leading role in supporting the development of the nursing and midwifery professions over the next three years.”
The Chief Nursing Officer, at the Department of Health, Rachel Kenna said: “I welcome the publication of this statement of strategy, which will help shape the future of our professions in Ireland. As Chief Nursing Officer, I am committed to supporting the implementation of this strategy and to ensuring that nurses and midwives have the resources and support they need to provide the best possible care.”
The President of NMBI, Louise Kavanagh McBride said: “As President of the Nursing and Midwifery Board of Ireland (NMBI) I am delighted to be publishing our new Statement of Strategy 2023-25. This strategy is the result of extensive consultation and collaboration with nurses, midwives, and stakeholders across the country, and it reflects their aspirations for the professions. Today’s publication is grounded in evidence-based practice and international best practice. On behalf of the Board, I would like to thank all stakeholders who took part in the consultation making this strategy possible.”
NMBI CEO, Sheila McClelland said: “We are acutely aware of the challenges facing Ireland’s healthcare settings in recruitment and retention. The objectives outlined in this strategy set out a clear vision and roadmap for the future of these vital professions in Ireland. I believe that this
Chief Nursing Officer at the Department of Health, Rachel Kenna, President of NMBI Louise Kavanagh McBride and NMBI CEO, Sheila McClelland
strategy will not only enhance the professional development and well-being of nurses and midwives, but it will also have a positive impact on patient outcomes and experiences. I look forward to seeing the positive impact it will have on our professions and the wider healthcare system."
A full copy of NMBI’s Statement of Strategy 2023-2025 is available to view online: https:// www.nmbi.ie/NMBI/media/ NMBI/NMBI-Statement-ofStrategy-2023-2025.pdf
SPECIALIST NURSES SHINE THE SPOTLIGHT ON HEART FAILURE AWARENESS
European Heart Failure Awareness Week occurs between 01 to 07 May. “Let’s Bump up the Pump” is a new campaign devised by the Irish Association of Heart Failure Nurses (IAHFN) to raise awareness around heart failure, with a focus on prevention, early detection and also to provide education around how to live well with this condition.
The campaign is supported by the heart and stroke charity Croi who recognise the important role Heart Failure Nurse Specialists play in heart failure care. “Croi is delighted to support the IAHFN in its campaign to raise awareness of heart failure and in particular to raise awareness of the signs and symptoms which are often dismissed or ignored. It is very important that people know the signs and symptoms and that they convey these to their doctor and ask could they have heart failure” says Croi CEO, Neil Johnson. Heart Failure can be an alarming diagnosis, and many do not understand the term. Heart failure is a condition where the heart fails to pump or relax well enough to circulate sufficient blood to meet the body’s needs. As a consequence, affected individuals can complain of breathlessness on exertion or at rest, swollen legs, and tiredness. It is a serious condition, left untreated heart failure is as deadly as cancer but there is good treatment out
there, the key is in early diagnosis and to begin treatment as soon as possible to reduce death and disability from this disease. Attending your GP for a simple blood test called NTproBNP can help support the diagnosis of Heart Failure and support to ‘detect the undetected’.
Heart Failure can happen for a variety of reasons, but is more common as we get older, in people with heart disease and in those with high blood pressure. It is a common condition affecting about 2% of the Irish population, rising to 10% in older age groups. Worryingly, there are about another 2% of the population who are currently undiagnosed, and trends are rising in terms of expected prevalence due to an aging population and better medical treatments. The “Let’s bump up the pump” campaign aims to raise awareness to ensure earlier detection of those who are at risk of heart failure.
Staff from across the Saolta Group in Galway, Mayo and Roscommon and Community Healthcare West (CHW) are working together to roll out the Enhanced Community Care Programme, which provides greater levels of care in the community. This includes the creation of ambulatory hubs to facilitate GPs to refer patients such as those who have or are suspected to have heart failure, for diagnostics and access to expert opinion and support.
Dr Susan Connolly, Consultant Cardiologist at Galway University Hospitals and who is leading out on the Cardiovascular Ambulatory Hub in Galway said, “As part of the new Integrated Care Chronic Disease Management Programme in Saolta/CHW we are focusing
Dr Susan Connolly, Integrated Care Consultant Cardiologist in Cardiovascular Disease at Galway Ambulatory Hub and Niamh Elwood, Integrated Care Clinical Nurse Specialist Cardiovascular Disease, Galway Ambulatory Hub

82 JUNE 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
on detecting heart failure earlier by providing GPs with more rapid access to diagnostics, by providing early access to specialist cardiology opinion and by having a skilled team of cardiovascular specialist nurses to help patients manage their condition and lead a normal life as possible. Don’t delay, if you or someone you know has these symptoms seek help – we are here to do our best for you.”
The “Let’s bump up the pump” campaign wants to bump up awareness of the signs and symptoms of heart failure, to bump up the number of those with an earlier diagnosis, bump up knowledge of living with the condition and ultimately bump up survival for those living with heart failure. This campaign supports the global “25in25” initiative seeking to reduce heart failure deaths by 25% in the next 25 years.
Emer Burke, Advanced Nurse Practitioner with Galway University Hospitals and the Galway Integrated Heart Failure Service advises, “Anyone concerned about symptoms of heart failure please contact your GP for an assessment – Ask about the NTProBNP test. The earlier you get diagnosed, the earlier treatment can begin which can potentially alter the course of this serious disease, with treatment you can live well with heart failure.”
ABBVIE ANNOUNCES
EUROPEAN COMMISSION APPROVAL OF RINVOQ® (UPADACITINIB) FOR THE TREATMENT OF MODERATELY TO SEVERELY ACTIVE CROHN’S DISEASE
AbbVie (NYSE: ABBV) has announced European Commission (EC) approval of upadacitinib, 45 mg [induction dose] and 15 mg and 30 mg [maintenance doses] as the first oral Janus Kinase (JAK) inhibitor for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1-4
“The EC approval of RINVOQ in Crohn’s disease is a significant milestone in offering patients the first and only once-daily oral treatment that can provide endoscopic improvement, and sustained symptom relief, making a difference in their daily lives,” said Thomas Hudson, M.D., senior vice president, research and development, chief scientific officer, AbbVie. “With existing therapies, not all patients are able to achieve adequate disease control to meet their treatment goals2,3, which is why we continue
to embrace the challenge of expanding our IBD portfolio with new treatment options.”
The EC approval is supported by data from two induction studies, U-EXCEED and U-EXCEL, and the U-ENDURE maintenance study.1 Statistical significance was achieved for the co-primary endpoints and key secondary endpoints with upadacitinib 45 mg in the induction studies and upadacitinib 15 mg and 30 mg in the maintenance study compared to placebo.1-4
Co-Primary Endpoint Results from the Phase 3 program include1-4:
• Endoscopic response : In U-EXCEED and U-EXCEL, 35% and 46% of patients treated with upadacitinib 45 mg achieved endoscopic response at week 12, respectively, compared to 4% and 13% of patients receiving placebo.1 In U-ENDURE, 28% and 40% of patients treated with upadacitinib15 mg and 30 mg achieved endoscopic response at week 52, respectively, compared to 7% of patients receiving placebo.1
• Clinical remission : In U-EXCEED and U-EXCEL, 40% and 51% of patients treated with upadacitinib 45 mg achieved clinical remission at 12 weeks, respectively, compared to 14% and 22% of patients receiving placebo.1 Additionally, in U-ENDURE, 36% and 46% patients treated with upadacitinib 15mg and 30mg achieved clinical remission at 52 weeks, respectively, compared to 14% of patients receiving placebo.1
Key Secondary and Additional Endpoints include:
• Corticosteroid-free clinical remission : In U-EXCEED and U-EXCEL, 37% and 44% of patients treated with upadacitinib 45 mg achieved steroid-free remission at week 12, respectively, compared to 7% and 13% of patients receiving placebo. In U-ENDURE, 35% and 45% of patients treated with upadacitinib 15 mg and 30 mg achieved steroid-free remission at week 52, respectively, compared to 14% of patients receiving placebo.1
• Mucosal healing : Additionally, in U-EXCEED and U-EXCEL, 17% and 25% of patients treated with upadacitinib 45mg achieved SES-CD ulcerated surface subscore of 0 at week 12, respectively, compared to 0% and 5% of patients receiving placebo. In U-ENDURE, 13% and 24% of patients treated with upadacitinib 15 mg and 30 mg achieved SES-CD ulcerated
surface subscore of 0 at week 52 compared to 4% of patients receiving placebo (all with nominal p-value<0.001).1
“Crohn’s disease is a burden that can present patients with daily, often uncomfortable challenges,” said Laurent PeyrinBiroulet, M.D., Ph.D., professor of gastroenterology and head of the Inflammatory Bowel Disease group at the Gastroenterology Department, University Hospital of Nancy, France. “These studies demonstrated upadacitinib's ability to achieve key treatment targets, including endoscopic outcomes and symptomatic relief, that are critical for patients and beneficial for long-term care.”
Upadacitinib in Crohn's disease was well tolerated, with no new safety risks observed compared to the known safety profile of upadacitinib.2-4 Similar rates of serious adverse events including serious infections, were observed between patients receiving upadacitinib and placebo.1-4 The most common adverse events included nasopharyngitis, acne and COVID-19 in the upadacitinib treatment group.1-4 Reports of malignancy, major cardiovascular events, venous thromboembolic events and gastrointestinal perforation were infrequently observed (<1.0 Events/100 Patient-Years).2-4
Upadacitinib is approved in the EU for the treatment of adults with radiographic axial spondyloarthritis, non-radiographic axial spondyloarthritis, psoriatic arthritis, rheumatoid arthritis, ulcerative colitis, adults and adolescents with atopic dermatitis and now Crohn’s disease.1,5-9
References available on request
IRISH LIFE HEALTH EXPANDS DIGITAL DOCTOR SERVICE
Irish Life Health today announced the expansion of its Digital Doctor service, introducing next day access to in-person GP appointments at 50 Centric Health centres across Ireland. This service is available on all hospital plans when required post a Digital Doctor consultation.
Irish Life Health moved its Digital Doctor service to Centric Health in June 2022, meaning its customers have had unlimited and timely 24/7 access to Irish-based GPs by phone or video call. Since introducing the enhanced service, Irish Life Health has seen a 32% increase1 in usage of the digital doctor service.
To date, our experience is that over 90% of digital doctor consultations resolve the issue, however for the less than 10% where this
isn’t possible2, a rapid follow up in-person GP appointment is required. This expansion of the service means that when a customer’s issue cannot be resolved through their digital GP appointment, they will be offered next-day access to an in-person appointment with a GP across one of 50 Centric Health clinics nationwide.
This service is of particular importance to patients who aren’t registered with a local GP, as it allows them to access one of 50 GP practices nationwide for inperson care.
In addition, this development in primary healthcare provision will provide timely access to quality care in the community, ensuring that Irish Life Health customers have easy access to the right care closer to home when they need it.
Speaking about the new in-person GP appointments, Ger Davis, Irish Life Health Managing Director said:
“At Irish Life Health, we believe that regular access to highquality primary care is critical for customers in proactively managing their health. GPs are the gatekeepers to the health care system as well as being trusted advisers for patients. We are delighted to launch this marketleading primary care service, integrating our Digital Doctor service supported by rapid inperson consultation follow ups. We have seen a significant increase in take-up of the Digital Doctor service in the last year, reflecting the quality of the service and also the challenges of arranging physical GP appointments. We know from our over 500,000 customers that convenience and timely access to medical advice are key, so the Digital Doctor service and personalised healthcare is a priority for them. However, not all issues can be solved over the phone, and as convenience is a vital part of healthcare, providing our customers access to 50 Centric Health centres nationwide is a natural next step.
This new service enhances our health insurance offering which already gives our customers access to an excellent network of best in class diagnostic and urgent care centres as well as access to health care in the home. For example, our customers have access to specialised nurses treating patients for certain conditions at home, rather than in a hospital setting. In 2022 alone our customers avoided spending the night in hospital on over 4,700 occasions through the use of this service, freeing up hospital beds for other patients in need.”
83 HOSPITALPROFESSIONALNEWS.IE | HPN • JUNE 2023
TAKE IT ON WITH CIBINGO
CIBINQO is a convenient once-daily oral JAK1 inhibitor for moderate-to-severe atopic dermatitis (AD) that o ers1-4


















Significant skin clearance at week 12, with sustained control at week 481-3,5
Rapid itch relief, superior to dupilumab + TCS at week 2 for CIBINQO 200 mg + TCS, with significant results as early as day 41,6

A once-daily pill available in multiple doses that can be used with or without medicated topical therapies so you can tailor treatment to meet the individual needs of your patients1-3,7,8
CONSISTENT SAFETY PROFILE: Rigorously studied in >3500 patients across 7 clinical trials, including one ongoing LTE 9


PRESCRIBING INFORMATION CIBINQO® ▼ (ABROCITINIB)






Please refer to full Summary of Product Characteristics (SmPC) before prescribing Cibinqo®.


Presentation: Film-coated tablets containing 50 mg, 100 mg, or 200 mg abrocitinib. Each tablet contains 1.37 mg, 2.73 mg, and 5.46 mg of lactose monohydrate, respectively. Indications: For the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy. Dosage: Treatment should be initiated and supervised by a healthcare professional experienced in the diagnosis and treatment of atopic dermatitis. Posology: The recommended starting dose is 100 mg or 200 mg once daily based on individual patient characteristics. A starting dose of 100 mg once daily is recommended for patients at higher risk of venous thromboembolism (VTE), major adverse cardiovascular event (MACE) and malignancy. If the patient does not respond adequately to 100 mg once daily, the dose can be increased to 200 mg once daily. A dose of 200 mg once daily may be appropriate for patients who are not at higher risk of VTE, MACE and malignancy with high disease burden or for patients with an inadequate response to 100 mg once daily. Upon disease control, dose should be decreased to 100 mg once daily. If disease control is not maintained after dose reduction, re-treatment with 200 mg once daily can be considered. The lowest e ective dose for maintenance should be considered. Discontinuation of treatment should be considered in patients who show no evidence of therapeutic benefit after 24 weeks. Cibinqo can be used with or without medicated topical therapies for atopic dermatitis. Treatment initiation: TTreatment should not be initiated in patients with a platelet count < 150 × 103/mm3, an absolute lymphocyte count (ALC) < 0.5 × 103/mm3, an absolute neutrophil count (ANC) < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Dose interruption: If a patient develops a serious infection, sepsis or opportunistic infection, dose interruption should be considered until the infection is controlled. Interruption of dosing may be needed for management of laboratory abnormalities. Missed doses: If a dose is missed, patients should be advised to take the dose as soon as possible unless it is less than 12 hours before the next dose, in which case the patient should not take the missed dose. Thereafter, dosing should be resumed at the regular scheduled time. Interactions: In patients receiving dual strong inhibitors of cytochrome CYP2C19 and moderate inhibitors of CYP2C9, or strong inhibitors of CYP2C19 alone (e.g., fluvoxamine, fluconazole, fluoxetine and ticlopidine), the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. Treatment is not recommended concomitantly with moderate or strong inducers of CYP2C19/CYP2C9 enzymes (e.g., rifampicin, apalutamide, efavirenz, enzalutamide, phenytoin). In patients receiving acid reducing agents (e.g. antacids, proton pump inhibitors and H2 receptor antagonists), 200 mg once daily dose of abrocitinib should be considered (see section 4.5). Renal impairment: No dose adjustment is required in patients with mild renal impairment, i.e., estimated glomerular filtration rate (eGFR) of 60 to < 90 mL/min. In patients with moderate (eGFR 30 to < 60 mL/min) renal impairment, the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. In patients with severe (eGFR < 30 mL/min) renal impairment, 50 mg once daily is the recommended starting dose. The maximum daily dose is 100 mg. Cibinqo has not been studied in patients with end-stage renal disease (ESRD) on renal replacement therapy. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Cibinqo must not be used in patients with severe (Child Pugh C) hepatic impairment. Elderly: For patients 65 years of age and older, the recommended dose is 100 mg once daily. Paediatric population: The safety and e cacy of Cibinqo in children under 12 years of age have not yet been established. No data are available. Cibinqo has been studied in adolescents 12 to < 18 years of age. However, because of bone findings in juvenile rats (comparable to a 3 month old human), additional long-term data in growing adolescents is needed to conclude that the benefits outweigh the risks. Method of administration: This medicinal product is to be taken orally once daily with or without food at approximately the same time each day. In patients who experience nausea, taking Cibinqo with food may improve nausea. Tablets should be swallowed whole with water and should not be split, crushed, or chewed because these methods have not been studied in clinical trials. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. Active serious systemic infections, including tuberculosis (TB), severe hepatic impairment, pregnancy, and breast-feeding.
Warnings and Precautions:
Abrocitinib should only be used if no suitable treatment alternatives are available in patients:
• 65 years of age and older;
• patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers);
• patients with malignancy risk factors (e.g. current malignancy or history of malignancy)
Infections/serious infections: Serious infections have been reported in patients receiving Cibinqo. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia. As there is a higher incidence of infections in the elderly and in the diabetic populations in general, caution should be used when treating the elderly and patients with diabetes. In patients 65 years of age and older abrocitinib should only be used if no suitable treatment alternatives are available. Treatment must not be initiated in patients with an active, serious systemic infection. Risks and benefits of treatment prior to initiating Cibinqo should be considered. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with abrocitinib. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing and appropriate antimicrobial therapy should be initiated. The patient should be closely monitored, and therapy should be temporarily interrupted if the patient is not responding to standard therapy. Tuberculosis: Tuberculosis was observed in clinical studies with abrocitinib. Patients should be screened for TB before starting treatment and yearly screening for patients in highly endemic areas for TB should be considered. Abrocitinib must not be given to patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, preventive therapy for latent TB should be started prior to initiation of treatment. Viral reactivation: Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies. The rate of herpes zoster infections was higher in patients who were treated with 200 mg, 65 years of age and older, with a medical history of herpes zoster, with a confirmed ALC < 1 × 103/mm3 prior to the event and patients with severe atopic dermatitis at baseline. If a patient develops herpes zoster, temporary interruption of treatment should be considered until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy and during therapy. Patients with evidence of active hepatitis B or hepatitis C (positive hepatitis C PCR) infection were excluded from clinical studies. Patients who were hepatitis B surface antigen negative, hepatitis B core antibody positive, and hepatitis B surface antibody positive had testing for hepatitis B virus (HBV) DNA. Patients who had HBV DNA above the lower limit of quantification (LLQ) were excluded. Patients who had HBV DNA negative or below LLQ could initiate treatment; such patients had HBV DNA monitored. If HBV DNA is detected, a liver specialist should be consulted. Vaccination: No data are available on the response to vaccination in patients receiving Cibinqo. Use of live, attenuated vaccines should be avoided during or immediately prior to treatment. Prior to initiating treatment with this medicinal product, it is recommended that patients be brought up to date with all immunisations, including prophylactic herpes zoster vaccinations, in agreement with current immunisation guidelines. Venous thromboembolism (VTE): Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a dose dependent higher rate of VTE including deep venous thrombosis (DVT) and pulmonary embolism (PE) was observed with tofacitinib compared to TNF inhibitors. A higher rate of VTE was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients with cardiovascular or malignancy risk factors abrocitinib should only be used if no suitable treatment alternatives are available. In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, abrocitinib should be used with caution. VTE risk
factors other than cardiovascular or malignancy risk factors include previous VTE, patients undergoing major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, inherited coagulation disorder. Patients should be re-evaluated periodically during abrocitinib treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue abrocitinib in patients with suspected VTE, regardless of dose Major adverse cardiovascular events (MACE): Events of MACE have been observed in patients taking abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE), defined as cardiovascular death, non-fatal myocardial infarction (MI) and non-fatal stroke, was observed with tofacitinib compared to TNF inhibitors. Therefore, in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, abrocitinib should only be used if no suitable treatment alternatives are available. Malignancy (excluding non-melanoma skin cancer [NMSC]): Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including abrocitinib. In a large randomized active controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of malignancies, particularly lung cancer, lymphoma and non-melanoma skin cancer (NMSC) was observed with tofacitinib compared to TNF inhibitors. A higher rate of malignancies (excluding non-melanoma skin cancer, NMSC) was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients 65 years of age and older, patients who are current or past long-time smokers, or with other malignancy risk factors (e.g. current malignancy or history of malignancy), abrocitinib should only be used if no suitable treatment alternatives are available. Non-melanoma skin cancer: NMSCs have been reported in patients receiving abrocitinib. Periodic skin examination is recommended for all patients, particularly those who are at increased risk for skin cancer. Haematologic abnormalities: Confirmed ALC < 0.5 × 103/mm3 and platelet count < 50 × 103/mm3 were observed in less than 0.5% of patients in clinical studies. Treatment with abrocitinib should not be initiated in patients with a platelet count < 150 × 103/mm3, an ALC < 0.5 × 103/mm3, an ANC < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Complete blood count should be monitored 4 weeks after initiation of therapy and thereafter according to routine patient management. Lipids: Dose dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The e ect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. Elderly: The safety profile observed in elderly patients was similar to that of the adult population with the following exceptions: a higher proportion of patients 65 years of age and older discontinued from clinical studies and were more likely to have serious adverse reactions compared to younger patients; patients 65 years and older were more likely to develop low platelet and ALC values; the incidence rate of herpes zoster in patients 65 years of age and older was higher than that of younger patients. There are limited data in patients above 75 years of age. Use in patients 65 years of age and older: Considering the increased risk of MACE, malignancies, serious infections, and all-cause mortality in patients 65 years of age and older, as observed in a large randomised study of tofacitinib (another JAK inhibitor), abrocitinib should only be used in these patients if no suitable treatment alternatives are available. Immunosuppressive conditions or medicinal products: Patients with immunodeficiency disorders or a first-degree relative with a hereditary immunodeficiency were excluded from clinical studies and no information on these patients is available. Combination with biologic immunomodulators, potent immunosuppressants such as ciclosporin or other Janus kinase (JAK) inhibitors has not been studied. Their concomitant use with abrocitinib is not recommended as a risk of additive immunosuppression cannot be excluded. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Drug Interactions: Potential for other medicines to a ect pharmacokinetics of abrocitinib: Abrocitinib is metabolised predominantly by CYP2C19 and CYP2C9 enzymes, and to a lesser extent by CYP3A4 and CYP2B6 enzymes, and its active metabolites are renally excreted and are substrates of the organic anion transporter 3 (OAT3). Therefore, exposures of abrocitinib and/or its active metabolites may be a ected by medicinal products that strongly inhibit or induce theses enzymes and transporter. Dose adjustments, as appropriate, may be required. Co-administration with products which increase gastric pH: When abrocitinib 200 mg was administered concomitantly with famotidine 40 mg, an H2-receptor antagonist, abrocitinib active moiety exposures decreased by approximately 35%. The e ect of elevating gastric pH with antacids, or proton pump inhibitors (omeprazole) on the pharmacokinetics of abrocitinib has not been studied and may be similar to that seen with famotidine. The higher 200 mg daily dose should be considered for patients treated concomitantly with products which increase gastric pH, as they may reduce the e cacy of abrocitinib. Potential for Cibinqo to a ect pharmacokinetics of other medicinal products: No clinically significant e ects of Cibinqo were observed in drug interaction studies with oral contraceptives. Caution should be exercised for concomitant use of abrocitinib with dabigatran. Caution should be exercised as the levels of P-gp substrates with a narrow therapeutic index, such as digoxin, may increase. Caution should be exercised when using abrocitinib concomitantly with narrow therapeutic index medicines that are primarily metabolised by CYP2C19 enzyme (e.g. S mephenytoin and clopidogrel). Dose adjustment may be required for other medicines primarily metabolised by CYP2C19 enzyme in accordance with their product information (e.g. citalopram, clobazam, escitalopram and selumetinib). Fertility, pregnancy, and lactation: Women of childbearing potential: Women of reproductive potential should be advised to use e ective contraception during treatment and for 1 month following the final dose of Cibinqo. Pregnancy planning and prevention for females of reproductive potential should be encouraged. Pregnancy: There are no or limited amount of data on the use of abrocitinib in pregnant women. Studies in animals have shown reproductive toxicity. Abrocitinib has been shown to cause embryo-foetal lethality in pregnant rats and rabbits, skeletal variations in the foetuses of pregnant rats and rabbits and to a ect parturition and peri/postnatal development in rats. Cibinqo is contraindicated during pregnancy. Breast-feeding: There are no data on the presence of abrocitinib in human milk, the e ects on the breast fed infant, or the e ects on milk production. Abrocitinib was secreted in milk of lactating rats. A risk to newborns/infants cannot be excluded and Cibinqo is contraindicated during breast feeding. Fertility: Based on the findings in rats, oral administration of Cibinqo may result in temporary reduced fertility in females of reproductive potential. The e ects on female rat fertility were reversible 1 month after cessation of abrocitinib oral administration. Driving and operating machinery: Cibinqo has no e ect on the ability to drive or use machines. Side E ects: The most commonly reported adverse reactions are nausea (15.1%), headache (7.9%), acne (4.8%), herpes simplex (4.2%), blood creatine phosphokinase increased (3.8%), vomiting (3.5%), dizziness (3.4%) and abdominal pain upper (2.2%). The most frequent serious adverse reactions are infections (0.3%) including herpes simplex, herpes zoster, and pneumonia. Refer to SmPC for further information on side e ects. Legal Category: S1A. Marketing Authorisation Numbers: EU/1/21/1593/002 - Cibinqo 50 mg (28 film-coated tablets), EU/1/21/1593/007 - Cibinqo 100 mg (28 film-coated tablets); EU/1/21/1593/012 - Cibinqo 200 mg (28 film-coated tablets). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 467 6500.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Last revised: 03/2023.
Ref: CQ 5_0 IE.
References: 1. Bieber T, Simpson EL, Silverberg JI, et al. N Engl J Med. 2021;384(12):1101-1112. 2. Simpson EL, Sinclair R, Forman S, et al. Lancet. 2020;396(10246):255-266. 3. Silverberg JI, Simpson EL, Thyssen JP, et al. JAMA Dermatol 2020;156(8):863-873. 4. Boeri M, Sutphin J, Hauber B, et al. J Dermatolog Treat. 2020 Nov 2:1-10. doi:10.1080/09546634.1832185. 5. Reich K, Silverberg JI, Papp K, et al. Presented at the Revolutionizing Atopic Dermatitis Virtual Conference; 13 June 2021. 6. Ständer S, Kwatra SG, Silverberg JI, et al. Am J Clin Dermatol. 2023;24(1):97-107. doi: 10.1007/s40257-022-00738-4. 7. Cibinqo Summary of Product Characteristics. 8. Blauvelt A, Silverberg JI, Lynde CW, et al. J Am Acad Dermatol. Published online 17 August 2021. doi: 10.1016/j.jaad.2021.05.075. 9. Simpson EL, Silverberg JI, Nosbaum A, et al. British Journal of Dermatology, 2023;188,supp2. doi: 10.1093/bjd/ljac140.025.
Although some clinical trials included adolescents, please note that CIBINQO is indicated for the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy.
TCS includes low- to medium-potency topical corticosteroids, topical calcineurin inhibitors, or topical phosphodiesterase 4 inhibitors, per protocol guidance in JADE COMPARE.
Nonmedicated topicals were also required.1
AD=atopic dermatitis; JAK=Janus kinase; LTE=long-term extension; TCS=topical corticosteroid. © 2023 Pfizer Inc. All rights reserved. March 2023. PP-CIB-IRL-0103






Whether your patients’ moderate-to-severe AD is FIERCE or TAMER,



































































 Written by Anna Wolinska,1 Stephanie Bowe,1 Gregg Murray,1 Sinead
Murad1-2
Written by Anna Wolinska,1 Stephanie Bowe,1 Gregg Murray,1 Sinead
Murad1-2













































































