CIBINQO is indicated for the treatment of moderate-to-severe atopic dermatitis (AD) in adults who are candidates for systemic therapy.
Legal Category : S1A. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
IN THIS ISSUE:
NEWS: Consultants Contract ‘Missed Opporunity Page 7
RESEARCH: Gaps in Understanding of Vitamin D Page 10
FEATURE: What’s new in Ovarian Cancer Page 11
CPD: Multiple Myeloma Page 31
MUSCULOSKELETAL FOCUS: : Preventing Silent Killers ‘Fractures’ Page 38
MUSCULOSKELETAL FOCUS: Ehlers Danos Syndrome Page 46
PHARMACY: Smoking Cessation in Cancer Patients Page 74
HOSPITAL
HPN May 2023 Issue 108 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only © 2022 Pfizer Inc. All rights reserved. July 2022. PP-CIB-IRL-0036
PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
CLINICALLY MEANINGFUL OVERALL SURVIVAL BENEFIT1*
In women with s/gBRCAm advanced ovarian cancer following response to first-line platinum-based chemotherapy
7-YEAR FOLLOW-UP OF THE SOLO-1 TRIAL EVALUATING LYNPARZA AS MAINTENANCE THERAPY IN NEWLY DIAGNOSED BRCAm ADVANCED OVARIAN CANCER SHOWED:


~2 out of 3 patients alive at 7 years
67% with LYNPARZA vs. 46.5% with placebo (HR=0.55; 95% CI: 0.40-0.76; P=0.0004)*1
~1-in-2 patients yet to receive a subsequent treatment at 7 years 45.3% of LYNPARZA vs. 20.6% with placebo (HR=0.37; 95% CI: 0.28-0.48)1
Consistent safety profile
With the primary analysis at the 7-year follow-up, with no new safety signals1,2
For expert opinion on what this data means for clinical practice please scan this QR code.
*7-years OS follow-up data are not statistically significant. P value of <0.0001
declare statistical significance.1
was required to
For additional information, refer to the Summary of Product Characteristics.
ABRIDGED PRESCRIBING INFORMATION
LYNPARZA® (olaparib) 150mg & 100mg FILM-COATED TABLETS
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Ovarian Cancer: As monotherapy for the maintenance treatment of adult patients with advanced (FIGO stages III and IV) BRCA1/2-mutated (germline and/or somatic) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy. As monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. Lynparza in combination with bevacizumab: As maintenance treatment of adult patients with advanced (FIGO stages III and IV) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy in combination with bevacizumab and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either a BRCA1/2 mutation and/or genomic instability. Breast Cancer: As monotherapy or in combination with endocrine therapy for the adjuvant treatment of adult patients with germline BRCA1/2-mutations who have HER2-negative, high risk early breast cancer previously treated with neoadjuvant or adjuvant chemotherapy. As monotherapy for the treatment of adult patients with germline BRCA1/2-mutations, who have HER2 negative locally advanced or metastatic breast cancer. Patients should have previously been treated with an anthracycline and a taxane in the (neo)adjuvant or metastatic setting unless patients were not suitable for these treatments. Patients with hormone receptor (HR)-positive breast cancer should also have progressed on or after prior endocrine therapy, or be considered unsuitable for endocrine therapy. Adenocarcinoma of the pancreas: As monotherapy for the maintenance treatment of adult patients with germline BRCA1/2-mutations who have metastatic adenocarcinoma of the pancreas and have not progressed after a minimum of 16 weeks of platinum treatment within a first-line chemotherapy regimen. Prostate Cancer: As monotherapy for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) and BRCA1/2-mutations (germline and/or somatic) who have progressed following prior therapy that included a new hormonal agent. In combination with abiraterone and prednisone or prednisolone for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated.
Presentation: 150mg and 100mg olaparib film-coated tablets.
Dosage and Administration: Treatment should be initiated and supervised by a physician experienced in the use of anticancer therapies. Patient selection: First-line maintenance treatment of BRCAmutated advanced ovarian cancer: Before Lynparza treatment is initiated for first-line maintenance treatment of high-grade epithelial ovarian cancer (EOC), fallopian tube cancer (FTC) or primary peritoneal cancer (PPC), patients must have confirmation of deleterious or suspected deleterious germline and/ or somatic mutations in the breast cancer susceptibility genes (BRCA) 1 or 2 using a validated test. Maintenance treatment of platinum-sensitive relapsed ovarian cancer: There is no requirement for BRCA1/2 testing prior to using Lynparza for the monotherapy maintenance treatment of relapsed EOC, FTC or PPC who are in a complete or partial response to platinum-based therapy. First-line maintenance treatment of HRD positive advanced ovarian cancer in combination with bevacizumab: Before Lynparza with bevacizumab treatment is initiated for the first-line maintenance treatment of EOC, FTC or PPC, patients must have confirmation of either deleterious or suspected deleterious BRCA1/2 mutation and/or genomic instability determined using a validated test. Adjuvant treatment of germline BRCAmutated high risk early breast cancer: Before Lynparza treatment is initiated for adjuvant treatment of HER2 negative high risk early breast cancer, patients must have confirmation of deleterious or suspected deleterious gBRCA1/2 mutation using a validated test. Monotherapy treatment of gBRCA1/2-mutated HER2-negative metastatic breast cancer: For germline breast cancer susceptibility genes (gBRCA1/2) mutated human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer, patients must have confirmation of deleterious or suspected deleterious gBRCA1/2 mutation before Lynparza treatment is initiated. gBRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. First-line maintenance treatment of gBRCA-mutated metastatic adenocarcinoma of the pancreas: For first-line maintenance treatment of germline BRCA1/2-mutated metastatic adenocarcinoma of the pancreas, patients must have confirmation of a deleterious or suspected deleterious gBRCA1/2 mutation before Lynparza treatment is initiated. gBRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. Monotherapy treatment of BRCA1/2-mutated metastatic castration-resistant prostate cancer: For BRCA1/2-mutated metastatic castration-resistant prostate cancer (mCRPC), patients must have confirmation of a deleterious or suspected deleterious BRCA1/2 mutation (using either tumour or blood sample) before Lynparza treatment is initiated. BRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. Treatment of mCRPC in combination with abiraterone and prednisone or prednisolone: No genomic testing is required prior to using Lynparza in combination with abiraterone and prednisone or prednisolone for the treatment of patients with mCRPC.
Genetic counselling for patients tested for mutations in BRCA1/2 genes should be performed. Posology: Recommended dose in monotherapy or in combination with bevacizumab for ovarian cancer or in combination with abiraterone and prednisone or prednisolone for prostate cancer or endocrine therapy is 300mg (two 150mg tablets) twice daily, equivalent to a total daily dose of 600mg. The 100mg tablet is available for dose reduction.
Tablets should be swallowed whole and not chewed, crushed, dissolved or divided and may be taken without regard to meals. Lynparza monotherapy: Patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy should start Lynparza treatment no later than 8 weeks after completion of their final dose of platinum-containing regimen. Lynparza in combination with bevacizumab: When Lynparza is used in combination with bevacizumab for the first-line maintenance treatment of high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer following completion of first-line platinumbased therapy with bevacizumab, the dose of bevacizumab is 15 mg/kg once every 3 weeks. Lynparza in combination with endocrine therapy: Please refer to the full product information of the endocrine therapy combination partner(s) (aromatase inhibitor/anti-oestrogen agent and/or LHRH) for the recommended posology. Lynparza in combination with abiraterone and prednisone or prednisolone: When Lynparza is used in combination with abiraterone for the treatment of patients with mCRPC, the dose of abiraterone is 1000 mg orally once daily. Abiraterone should be given with prednisone or prednisolone 5 mg orally twice daily. Please refer to the full product information for abiraterone. Duration of treatment: First-line maintenance treatment of BRCA-mutated advanced ovarian cancer: Patients can continue treatment until radiological disease progression, unacceptable toxicity or for up to 2 years if there is no radiological evidence of disease after 2 years of treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating physician can derive further benefit from continuous treatment, can be treated beyond 2 years. Maintenance treatment of platinum-sensitive relapsed ovarian cancer: For patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer, it is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. First-line maintenance treatment of HRD positive advanced ovarian cancer in combination with bevacizumab: Patients can continue treatment with Lynparza until radiological disease progression, unacceptable toxicity or for up to 2 years if there is no radiological evidence of disease after 2 years of treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating physician can derive further benefit from continuous Lynparza treatment, can be treated beyond 2 years. Adjuvant treatment of germline BRCA-mutated high risk early breast cancer: It is recommended that patients are treated for up to 1 year, or until disease recurrence, or unacceptable toxicity, whichever occurs first. Monotherapy treatment of gBRCA1/2-mutated HER2-negative metastatic breast cancer: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. There are no efficacy or safety data on maintenance retreatment with Lynparza following first or subsequent relapse in ovarian cancer patients or on retreatment of breast cancer patients. First-line maintenance treatment of gBRCAmutated metastatic adenocarcinoma of the pancreas: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. Monotherapy treatment of BRCA1/2mutated metastatic castration-resistant prostate cancer: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. Medical castration with luteinising hormone releasing hormone (LHRH) analogue should be continued during treatment in patients not surgically castrated. Treatment of mCRPC in combination with abiraterone and prednisone or prednisolone: It is recommended that treatment be continued until progression of the underlying disease or unacceptable
toxicity when Lynparza is used in combination with abiraterone and prednisone or prednisolone. Treatment with a gonadotropin-releasing hormone (GnRH) analogue should be continued during treatment in all patients, or patients should have had prior bilateral orchiectomy. Please refer to the product information for abiraterone. There are no efficacy or safety data on retreatment with Lynparza in prostate cancer patients. If a patient misses a dose of Lynparza, they should take their next normal dose at its scheduled time. Dose adjustments: Treatment interruption to manage adverse reactions such as nausea, vomiting, diarrhoea, anaemia and dose reduction can be considered. Recommended dose reduction is to 250mg (one 150mg tablet and one 100mg tablet) twice daily, equivalent to a total daily dose of 500mg. If further dose reduction is required, then reduction to 200mg (two 100mg tablets) twice daily, equivalent to a total daily dose of 400mg is recommended. Concomitant use of strong or moderate CYP3A inhibitors is not recommended and alternative agents should be considered. If a strong CYP3A inhibitor must be co-administered, recommended dose reduction is to 100mg (one 100mg tablet) twice daily, equivalent to a total daily dose of 200mg. If a moderate CYP3A inhibitor must be co-administered, recommended dose reduction is to 150mg (one 150mg tablet) twice daily, equivalent to a total daily dose of 300mg. Elderly: No adjustment in starting dose is required. Renal impairment: For patients with moderate renal impairment (creatinine clearance 31 to 50 ml/min) the recommended dose is 200mg (two 100mg tablets) twice daily, equivalent to a total daily dose of 400mg. Lynparza can be administered in patients with mild renal impairment (creatinine clearance 51 to 80 ml/min) with no dose adjustment. No studies have been conducted in patients with severe renal impairment or end-stage renal disease (creatinine clearance ≤ 30 ml/min) and Lynparza is not recommended for use. It may only be used in patients with severe renal impairment if the benefit outweighs the potential risk with careful monitoring of renal function and adverse events. Hepatic impairment: Can be administered in patients with mild or moderate hepatic impairment (Child-Pugh A or B) with no dose adjustment. Not recommended in patients with severe hepatic impairment (Child-Pugh C). Contraindications: Hypersensitivity to the active substance or to any of the excipients. Breast-feeding during treatment and for 1 month after the last dose.
Warnings and Precautions: Haematological toxicity: Treatment should not be started in patients until they have recovered from haematological toxicity caused by previous anticancer therapy (haemoglobin, platelet and neutrophil levels should be ≤CTCAE grade 1). Baseline testing followed by monthly monitoring of complete blood counts is recommended for first 12 months of treatment and periodically thereafter. Treatment should be interrupted and appropriate haematological testing should be initiated if patient develops severe haematological toxicity or blood transfusion dependence. Myelodysplastic syndrome/ Acute Myeloid Leukaemia (MDS/AML): If MDS/AML is suspected, the patient should be referred to a haematologist for further investigations, including bone marrow analysis and blood sampling for cytogenetics. If, following investigation for prolonged haematological toxicity, MDS/AML is confirmed, Lynparza should be discontinued and the patient treated appropriately. Venous Thromboembolic Events: Monitor patients for clinical signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Patients with a prior history of VTE may be more at risk of a further occurrence and should be monitored appropriately. Pneumonitis: Interrupt Lynparza treatment and promptly investigate as appropriate. Discontinue Lynparza if pneumonitis is confirmed and treat patient appropriately. Embryofoetal toxicity: Lynparza could cause foetal harm when administered to a pregnant woman. Pregnancy/contraception: Lynparza should not be used during pregnancy. Women of childbearing potential must use two forms of reliable contraception, before starting Lynparza, during therapy and 6 months after receiving the last dose. Two highly effective and complementary forms of contraception are recommended. Male patients and their female partners of childbearing potential should use reliable contraception during therapy and for 3 months after receiving the last dose. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per 100 mg or 150 mg tablet, that is to say essentially “sodium-free”.
Drug Interactions: The recommended Lynparza monotherapy dose is not suitable for combination with myelosuppressive anticancer medicinal products. Caution and close monitoring if vaccines or immunosuppressant agents are co-administered. Effect of other drugs on Lynparza: Strong CYP3A inhibitors (e.g. itraconazole, telithromycin, clarithromycin, protease inhibitors boosted with ritonavir or cobicistat, boceprevir, telaprevir) or moderate CYP3A inhibitors (e.g. erythromycin, diltiazem, fluconazole, verapamil) are not recommended. If co-administered, the dose of Lynparza should be reduced. It is also not recommended to consume grapefruit juice. Strong CYP3A inducers (e.g. phenytoin, rifampicin, rifapentine, carbamazepine, nevirapine, phenobarbital, and St John’s Wort) are not recommended with Lynparza as the efficacy of Lynparza could be substantially reduced. The magnitude of the effect of moderate to strong inducers (e.g. efavirenz, rifabutin) on olaparib exposure is not established, therefore the co-administration of Lynparza with these medicinal products is also not recommended. Effect of Lynparza on other drugs: Caution and appropriate clinical monitoring is recommended when sensitive CYP3A substrates or substrates with a narrow therapeutic margin (e.g. simvastatin, cisapride, cyclosporine, ergot alkaloids, fentanyl, pimozide, sirolimus, tacrolimus and quetiapine) or P-gp substrates (e.g. simvastatin, pravastatin, dabigatran, digoxin and colchicine) are combined with Lynparza. Lynparza may reduce efficacy of hormonal contraceptives. Lynparza may increase the exposure to substrates of BCRP (e.g. methotrexate, rosuvastatin), OATP1B1 (e.g. bosentan, glibenclamide, repaglinide, statins and valsartan), OCT1, MATE1, MATE2K (e.g. metformin), OCT2 (e.g. serum creatinine), OAT3 (e.g. furosemide and methotrexate). Caution if co-administered with any statin.
Pregnancy and Lactation: Women of childbearing potential should not become pregnant while on Lynparza and not be pregnant at the beginning of treatment. A pregnancy test should be performed prior to treatment and considered regularly throughout treatment. The efficacy of some hormonal contraceptives may be reduced if co-administered with Lynparza. Therefore, an additional non-hormonal contraceptive method should be considered during treatment. For women with hormone dependent cancer, two non-hormonal contraceptives should be considered. Lynparza could cause foetal harm when administered to a pregnant woman Lynparza is contraindicated during breast-feeding and for 1 month after receiving last dose. Male patients must use a condom during therapy and for 3 months after receiving last dose when having sexual intercourse with a pregnant woman or with a woman of childbearing potential. Female partners of male patients must also use highly effective contraception. Male patients should not donate sperm during therapy and for 3 months after treatment.
Ability to Drive and Use Machines: Asthenia, fatigue and dizziness have been reported and patients who experience these symptoms should observe caution when driving or using machines.
Undesirable Events: Consult SmPC for full list of side effects. Very common: Anaemia, neutropenia, leukopenia, nausea, vomiting, diarrhoea, dyspepsia, dysgeusia, decreased appetite, fatigue (including asthenia), headache, dizziness, cough, dyspnoea. Common: Lymphopenia, thrombocytopenia, stomatitis, upper abdominal pain, rash, blood creatinine increased, venous thromboembolism. Uncommon: Myelodysplastic syndrome/Acute myeloid leukaemia, hypersensitivity, dermatitis, mean cell volume increased. Rare: Angioedema, erythema nodosum.
Legal Category: Product subject to prescription which may not be renewed (A)
Marketing Authorisation Number: EU/1/14/959/002-003 (100mg tablets); EU/1/14/959/004-005 (150mg tablets).
Marketing Authorisation Holder: AstraZeneca AB, SE151 85 Södertälje, Sweden. Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 7100.

LYNPARZA is a trade mark of the AstraZeneca group of companies.
Date of API preparation: 12/2022
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
Veeva ID: IE-4543
References: 1. DiSilvestro P, et al. J Clin Oncol. 2022 @Epub ahead of print. doi: 10.1200/JCO.22.01549. 2. Moore K, et al. N Engl J Med. 2018;379(26):2495-2505. VEEVA ID: IE- 4503 | Date of Preparation February 2023
NOW RINVOQ® (upadacitinib)
FOR MODERATE-TO-SEVERE ULCERATIVE COLITIS1
*RINVOQ® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1

Full Summary of Product Characteristics is available at medicines.ie
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
INDICATED
AbbVie® is a registered trademark of AbbVie Inc. RINVOQ® and its design are registered trademarks of AbbVie Inc. REFERENCES: 1. RINVOQ® summary of product characteristics, available at www.medicines.ie. JAK: Janus kinase; UC: ulcerative colitis. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. Legal Classification: POM(S1A). IE-RNQG-220007 | August 2022
Contents Foreword
Report highlights priorities of those affected by neurological diseases P6 Consultants call for clarification on hospital bed-building plan P8 Study shows gaps in understanding of Vitamin D P10 Making a Splash at EAHP 2023 P14
Significant potential for digital technology to improve asthma control P16
Sepsis: A Guide for Pharmacists P24
REGULARS
Feature: Bowel Cancer
Awareness P22
CPD: Multiple Myeloma P31
Musculoskeletal Focus: Giant Cell Arteritis P44
Musculoskeletal Focus: Fracture
Liaison Service P51
Musculoskeletal Focus: Osteoarthritis P54
Clinical R&D: P80
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd


Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
editorial@hospitalprofessionalnews.ie
Editor
In one of our lead news stories this month, the Irish Hospital Consultants Association (IHCA) has called on the Minister for Health Stephen Donnelly to clarify plans announced recently, which are aimed at rapidly delivering up to 1,500 new hospital beds across the country in 2023 and 2024.



As it stands, Ireland has one of the lowest numbers of acute hospital beds in the EU, 40% below the EU27 average of 4.83 per 1,000 population and one third the number in some European countries. The IHCA has previously warned that some 5,000 hospital beds are needed by 2030 alongside a total of at least an additional 2,000 permanent Consultants to treat patients in a timely manner, bring down unacceptable waiting lists and address population/demand changes.
Turn to page 8 to read more.
In clinical research news, A new study has highlighted how little we know about the vitamin D status of ethnic minorities in Ireland. The study identifies groups most at risk of vitamin D deficiency and has highlighted gaps that exist in our understanding of why people are getting tested for vitamin D (a practice the researchers believe is often unnecessary and wasteful) along with how little we know about the vitamin D status of our ethnic minorities.
The crowds were back enjoying the many sessions and connecting at the exhibition area. The focus for the exhibitors this year seems to be very much around technology and robotics in the areas of drug compounding. These technological advances come with a high cost. However, they have to potential to increase efficiency and add safety for the hospital pharmacy team. There was a notable absence from big pharma at the 2023 confer The Fresenius Kabi corporate team designed a conference booth which was sizeable and interactive. This allowed for plenty of visitors to come and visit and learn about our growing portfolio.
Conor Sadlier – Sales Manager Fresenius Kabi
Helena Scully, Mercers Glanbia Bone Research Fellow, MISA Institute, St James’s Hospital/ School of Medicine Trinity College and lead author of the study said, “Our research indicates that those most at risk have inadequate vitamin D intake, dark skin types and avoid sun exposure.”
The full article is available on page 10.
I was delighted to get the opportunity to travel with Fresenius Kabi to the 2023 EAHP Congress. It was my first time attending the event and it surpassed all my expectations. The exhibition hall was impressive with representation from many companies and there was a wide variety of posters to browse and read. The programme itself was excellent and I thoroughly enjoyed the clinical lectures
(Safe medication use in patients with cirrhosis and expanding role of hospital pharmacists in renal transplant patients) which were co presented by a pharmacist and physician. And finally, it was lovely to network and meet new and old Irish faces from the world of hospital pharmacy, industry and academia! Thank you, Fresenius Kabi

Ciara O Riordan, Senior Pharmacist, Cork University Hospital.
ACCOUNTS
Fiona Bothwell cs.ipn@btconnect.com
SALES EXECUTIVE
It was a great opportunity to see the current involvement of pharmacists in hospitals across Europe and to view the initiatives they have recently undertaken. enjoyed attending the Good Practice Initiative presentations and have already had the chance to share ideas with colleagues in Galway. I also attended a very interesting presentation on TPN compounding where the issue of cyber-security was raised. As our health system was without IT systems for three weeks in 2020 following a cyberattack, this was an important reminder to have back-up systems in place. The conference was also a great opportunity to network with colleagues from the Irish hospitals and to share ideas. Treasa Ni Ici Dispensary Manager, Pharmacy Department University Hospital Galway
Amy Evans - amy@ipn.ie
CONTRIBUTORS
Clodagh McCarthy | Aoife McNamara
Aoife Fleming | Sinead Horgan
Lorna Quigley | Denise McCarthy
Dr Roisin McAvera | Rachel Flynn
Michele O’Brien | Dr Anna Witkowska
Dr John Stack | Robert Harrington
Laura Durcan | Kenny Franks
Peter Boyd | Dr Clodagh Toomey
Professor Siobhan Glavey
Theresa Lowry-Lehnen
Dr Brona Dinneen
Professor Finbar O’Shea
Professor Patricia Fitzpatrick
DESIGN DIRECTOR
Ian Stoddart Design
Sepsis claims more lives than lung cancer, and more than breast cancer, bowel cancer and HIV/ AIDs combined. On page 24, Lorna Quigley, Programme Manager with the National Clinical Programme for Sepsis, along with colleagues, provides an indepth guide for pharmacists around sepsis. This article includes an overview as to the epidemiology of sepsis, screening, management and highlights key issues for hospital pharmacists. Our Special Focus for May is on Musculoskeletal Conditions. We have some excellent clinical content around this area, including Dr Anna Witkowska and Dr John Stack from the Mater Private Hospital on ‘Advances in Giant Cell Arteritis’ and Robert Harrington and Laura Durcan from Beaumont Hospital who discuss ‘Demystifying Ehlers Danlos Syndrome’ amongst many other great contributors.
I hope you enjoy the issue.
5 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 May Issue Issue 108 6
Fresenius Kabi Ireland plus delegates make a splash in Lisbon at EAHP 2023
The 27th EAHP Congress 2023 was another great learning and networking opportunity.
14 10 16 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
National Strategy for Genetics
Nuala Ryan, FutureNeuro PPI Panel member, Professor Gianpiero Cavalleri, Deputy Director of SFI FutureNeuro Research Centre and Professor of Human Genetics, School of Pharmacy and Biomolecular Sciences, RCSI and Deirdre McNamara, Director of Strategic Programmes, Office of the Chief Clinical Officer, Health Service Executive
improve diagnosis, provide better treatments and potentially allow preventative actions to take place.”
Key recommendations of the report include:
• Building consultative, collaborative engagement between the National Genetics and Genomics Office and the neurological community.
• Ensuring equity of access for all people living with a neurological condition to an appropriately resourced national genomics service.
A report published recently highlights the priorities and needs of patients affected by neurological disease, as well as clinicians and researchers, to inform the implementation of the HSE’s National Strategy for Accelerating Genetic and Genomic Medicine in Ireland. The report captures dialogue from a multistakeholder consultation led by the SFI FutureNeuro Centre and RCSI University of Medicine and Health Sciences PPI Ignite Network in
partnership with IPPOSI (Irish Platform for Patient Organisations, Science and Industry).

There are over 800,000 people in Ireland living with a neurological condition, which presents a significant impact on health and quality of life. The ‘Deliberative Dialogue on Genomics in Neurological Healthcare and Research’ report launched today sets out recommendations aimed at ensuring the upcoming implementation of the Irish national
genetic and genomic service incorporates the perspective of people living with a neurological condition, families, clinical and research stakeholders.
Nuala Ryan, FutureNeuro PPI Panel member, said: “The Deliberative Dialogue process provides a forum to ensure genuine collaboration across all groups from policy makers, patient advocates, the Health Service Executive (HSE) and research community to help shape the implementation of genomic medicine in our health service towards the needs of our citizens – this will include helping to
Recognising Pharmacy ‘Integral to Health’
• Developing secure data infrastructure to enable timely diagnosis.
• Improving research ethics processes to facilitate impactful research.
• Raising awareness of the value of genomics to healthcare through targeted education and public awareness campaigns.
The Deliberative Dialogue event and report were funded by SFI FutureNeuro Centre and RCSI PPI Ignite (via the Health Research Board and the Irish Research Council).

FIP CEO Catherine Duggan was among 40 invited leaders, including ministers, leaders of international financing institutions, bilateral and multilateral agencies, private sector investors and health worker organisations, contributing to a roundtable on aligning, stimulating and sustaining health workforce investments for health for all at the 5th Global Forum on Human Resources for Health recently.
“Whilst it is important for all health professions to have contributed to the unprecedented needs during the COVID-19 pandemic, it is even more important to understand the need to prepare all professions for such emergencies, with investment and remuneration ahead of time (including now as we all face the impact of the pandemic) and the future, so we manage the gap in the health workforce and the health needs we face globally. “For pharmacy, we need to be routinely recognised as integral to health care by health ministers and finance ministers alike so that access to medicines is safe and equitable for all,” Dr Duggan told policy leaders and decision makers.
Outcomes of the closed discussion were presented at a live broadcast. It was agreed that the health workforce must be recognised as a human capital investment and that the world must now shift to strategic long-term investment and financing. Proposed investment opportunities generated in this roundtable will inform a WHO-convened health investment forum in June 2023 and the United Nations General Assembly’s High-Level Meeting on Universal Health Coverage in September.
6 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Click here to register in digital edition
Consultant Contract: Missed Opportunity
The Irish Medical Organisation (IMO) has criticised the new consultant contract being introduced by Government.
Professor Matthew Sadlier, Chairman of the Consultant Committee of the IMO said that the Government has missed an opportunity to create an attractive common contract that would appeal to all consultants (existing and new).

Speaking at the IMO AGM in Killarney, Professor Sadlier said that feedback from IMO Consultants around the country had identified concerns on issues such as rostering, work locations and the requirement for consultants to provide locum support on top of their own clinical responsibilities.
Professor Sadlier said, “Many consultants do currently work in different locations and do work weekends and evenings and the issue with this contract is to ensure that there are reasonable agreements on how these matters will be dealt with in the future.”
Professor Sadlier said that the lukewarm response to the new contract did not bode well for the urgent need to recruit consultants; “with over 900 posts that we need to fill, the HSE has chosen to go to the market with this contract.
Professor Matthew Sadlier, President, IMO
We are concerned that it may not bode well for their plans to increase urgently needed recruitment.”
Professor Sadlier said that the IMO would monitor the response to the new contract closely over the coming months. He said; “we can’t afford to lose another decade. If this contract runs into difficulties, we will be looking for urgent action to address the shortcomings so that patients are not forced to lose more years while the HSE avoids fixing the mess it has made.”
The Consultant Committee of the IMO also heard from Dr Peadar Gilligan, Head of the Emergency Department at Beaumont Hospital and a former President of the IMO
who spoke on the crowding issue at Emergency Departments (ED). Dr Gilligan said that the lack of available hospital beds was a direct factor in overcrowding in Emergency Departments (ED). He said that a requirement of an admission to hospital was the major predictor of a protracted stay in an ED and the length of time which each patient spends in the ED is the major contributor to crowding.
Dr. Gilligan warned that the problem would get worse. He cited ESRI figures which project a need for between 4,000 and 6,300 additional beds in public and private hospital between 2015 and 2030; with up to 5,600 of these project to be required in public hospitals.
EAHP's 27th Congress: what really matters to patients!
In the vibrant Portuguese capital, the European Association of Hospital Pharmacists (EAHP) brought together approximately 3000 hospital pharmacists from 65 different countries for the 27th edition of its Annual Congress. Over the past three days, attendees expanded their knowledge by joining countless sessions and workshops, and explored the exhibition area to learn more about the latest technologies and network.
EAHP’s Scientific Committee filled the congress programme with seminars, workshops and interactive sessions that aimed at helping hospital pharmacists with translating evidence that is rooted in research into frontline healthcare practice. The keynotes touched on the opportunities for personalised medicine, improvements in the communication of risks and benefits to patients, and patient involvement in pharmacy practice research. EAHP’s team welcomed numerous participants at the EAHP booth to talk about the European Statements of Hospital Pharmacy, the self-assessment tool and the Association’s advocacy work. In one targeted session, students and young professionals had the opportunity to quiz five hospital pharmacists about their career choices. In addition, they got to meet with representatives of the European Pharmaceutical Students' Association (EPSA) during a networking dinner. Three of EAHP’s Special Interest Groups focused on eliminating harm, automation and medication errors in intensive care units showcased their work.
EAHP's poster walk, a unique Congress feature, brought together participants and poster authors to discuss the latest research developments. The Scientific Committee awarded the 1st prize for poster submissions to Shannon Nally for the abstract and poster titled ‘Evaluating the potential clinical and economic impact of chemotherapy prescribing by pharmacists at a university teaching hospital'. The 2023 Good Practice Initiative (GIP) award went to Sophia Hannou for the work on the ‘Opiods room for horror - An interactive learning to improve safety of drug administration'.
EAHP’s 27th Congress also celebrated innovation and excellence with several other awards focused on Statement implementation, students and contributions to EAHP. Among the awards and distinctions given, EAHP’s Board of Directors awarded the Statement Implementation Award to Zora Ćetković for her work on ‘Software tool development for reconstitution and administration of parenteral antibiotics in hospitals - An international project’. This award acknowledges contributions with a specific emphasis on implementing the European Statements of Hospital Pharmacy. Lastly, the 2023 EAHP-EPSA Student Science Award went to Usman Arshad for his abstract ‘Evaluation of antimicrobial-loaded calcium sulfate composites for the management of resistant Gram-negative diabetic foot osteomyelitis’.
The “EAHP Board of Directors Professional Excellence Award”, which recognises an individual who has made outstanding contributions to EAHP and the hospital pharmacy profession, went to Dr. Roberto Frontini. As former EAHP President that shaped the European Statements of Hospital Pharmacy and now retired hospital pharmacist that is still active as a member of the Pharmacovigilance Risk Assessment Committee at the European Medicines Agency, he has shaped the hospital pharmacy profession for decades to come.
Hospital pharmacists are cordially invited to EAHP’s 28th Congress taking place from 20 to 22 March 2024 in Bordeaux, France.
7 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
News
“We can’t afford to lose another decade. If this contract runs into difficulties, we will be looking for urgent action to address the shortcomings so that patients are not forced to lose more years while the HSE avoids fixing the mess it has made”
Clarification needed says Consultants
Without a whole-of-service plan to build and adequately staff extra hospital beds, which would include filling the 900 permanent Consultant posts currently not filled as needed, any plans and efforts to cut waiting lists and tackle the rising numbers of people on trolleys may fall at the first hurdle.
IHCA President Prof Robert Landers said: “We welcome the Minister for Health’s initiative which attempts to start addressing the serious bed and consultant shortages in our public hospitals. But Government must take a whole-of-service approach combined with credible timebound approaches if it is serious in its efforts to expedite the delivery of extra hospital capacity.
the delivery of much needed extra capacity across our health service, at a time when almost 900,000 people are on some form of waiting list and the number of patients on trolleys has reached record levels. However, the Association says it is imperative that there is a whole-of-service approach to alleviating these longrunning, severe deficits.
The Consultants have called on the Minister to work with health service management and consultant representative bodies to put in place a clear staffing plan in parallel to the proposed bed-build programme, which could potentially see blocks of 50 to 150 beds constructed at up to 15 hospital sites.
Capacity shortages
down unacceptable waiting lists and address population/demand changes. To meet this target would require opening more than 700 beds per year over the next 7 years and appointing around an additional 300 permanent Consultants on an annual basis. Just 196 additional approved permanent Consultant posts were filled last year, despite there being around 900 approved permanent post that are not filled as needed. Furthermore, the Government only plans to deliver 209 additional inpatient beds in 2023 and has already missed its target for the end of 2022 by around 260 beds that are yet to open. Just 157 additional public hospital beds were opened last year.
“These beds are long overdue and stem from the 2018 Capacity Review and two subsequent National Development Plans for 2018-2027 and 2021-2023. However, we know these plans are outdated and bed projection figures are underestimated. While the 1,500 rapid build beds are badly needed, we also believe the Minister for Health must plan now to deliver 5,000 beds by 2030.
“This additional capacity is urgently and desperately required across our public hospital system, but the opening of a fully functioning hospital bed requires more than just bricks and mortar.
The Irish Hospital Consultants Association (IHCA) has called on the Minister for Health Stephen Donnelly to clarify plans announced recently, which are aimed at rapidly delivering up to 1,500 new hospital beds across the country in 2023 and 2024.

The IHCA acknowledged the Minister’s efforts to expediate
As it stands, Ireland has one of the lowest numbers of acute hospital beds in the EU, 40% below the EU27 average of 4.83 per 1,000 population and one third the number in some European countries. Bed capacity has in fact decreased on a population basis from 3.03 beds in 2008 to just 2.68 beds in 2022. Ireland also has one of the highest hospital bed occupancy rates in the developed world.
The IHCA has previously warned that some 5,000 hospital beds are needed by 2030 alongside a total of at least an additional 2,000 permanent Consultants to treat patients in a timely manner, bring
The IHCA says it is concerned that the HSE Capital Plan for 2023 also released recently does not make provisions to reflect the ambitious ¤1 billion rapid build capacity plan, nor has it allocated any specific funding for the proposed three new elective hospitals in Cork, Dublin and Galway or for the five suggested surgical hubs nationally.
Staffing plans
While Government has made some plans to increase capacity in the immediate term, Consultants say the Minister must in parallel prioritise a plan to fully resource and staff new beds, noting that the delivery of basic infrastructure alone is not enough to achieve the change required to provide timely care to patients.
“To ensure this new initiative is successful, Government must work in parallel with hospital management and consultant representative bodies to ensure that a clear, time-bound and fully funded staffing plan is put in place, so that if and when these new rapid build beds come on stream, there are the Consultants, doctors, nurses, porters and others needed to ensure we are providing care to patients in those beds from day one.
“It is critical that we get this plan in place now, in order to attract and retain the Consultants and healthcare professionals required to staff these beds so we are not left in a scenario, as seen in the past, where hospital beds lie vacant due to staffing shortages.”
8 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor
Rob Landers, President, IHCA
“This additional capacity is urgently and desperately required across our public hospital system, but the opening of a fully functioning hospital bed requires more than just bricks and mortar”
Consultants seek clarification on Government’s proposed ¤1 billion ‘accelerated hospital bed-building plan’
Sleep Disorders more likely to lead to Stroke
A global study into causes of stroke, co-led from University of Galway, has identified that sleep problems - including getting too much or too little sleep, taking long naps, snoring, snorting and sleep apnoea - can increase the likelihood of having a stroke.
The research was published in the medical journal of the American Academy of Neurology
Dr Christine McCarthy, Stroke and Geriatric Medicine Physician, PhD researcher with the College of Medicine, Nursing and Health Sciences at University of Galway and lead author said, “Not only do our results suggest that individual sleep problems may increase a person’s risk of stroke but having more than five of these symptoms may lead to five times the risk of stroke compared to those who do not have any sleep problems. Our results suggest that sleep problems should be an area of focus for stroke prevention."
The international study involved 4,496 people, including 2,238 people who had a stroke who were matched to 2,258 people who did not have a stroke. The average age of participants was 62.
Participants were asked about their sleep behaviours including how many hours of sleep they got, sleep quality, napping,
Dr Christine McCarthy, Stroke and Geriatric Medicine Physician, PhD researcher with the College of Medicine, Nursing and Health Sciences at University of Galway

snoring, snorting, and breathing problems during sleep.
The study found that breathing problems during sleep, including snoring, snorting, and sleep apnoea were significantly associated with stroke. Participants who reported sleep apnoea and snorting were nearly three times more likely to have a stroke, while people who snored were nearly twice more likely to have a stroke than those who did not.
People who slept for more than nine hours or people who slept
less than five hours were more likely to have a stroke than people who slept an average number of hours.
Following extensive adjusting for other factors that could affect the risk of stroke such as smoking, physical activity, depression, and alcohol consumption, the results remained similar.
Professor Martin O’Donnell, Professor of Neurovascular Medicine at University of Galway and Consultant Stroke Physician at Galway University Hospitals, co-led the international
Review of National Sexual Health Strategy
HIV Ireland has welcomed the publication of the review of the National Sexual Health Strategy as timely, noting the continued reported rise in rates of HIV and STIs in Ireland. The review was commissioned by the Department of Health in advance of the preparation of a new National Sexual Health strategy for Ireland.
Speaking following the publication of the review by Minster of State with responsibility for Public Health, Wellbeing and the National Drugs Strategy, Hildegarde Naughton TD, Executive Director of HIV Ireland Mr Stephen O’Hare said “The review paves the way for the development of the longoverdue update to the National Sexual Health Strategy following the expiry of the previous strategy in 2020.
Real and substantial efforts must be made to ensure the voices of service users, community organisations, and services providers are included at every stage of the strategy development process.”
Reflecting on the 32 recommendations contained in the review Mr O’Hare said, “We are pleased to see so many of the concerns raised by community organisations and sexual health services reflected in the proposals for the development of a new strategy. These include a proposed model of care for HIV and STI services; increased access and availability of PrEP to prevent HIV; improvements in relationship and sexuality education for children and young people; addressing issues relating to chemsex; and improvements to abortion and reproductive services,” he added.
The review, which was undertaken by the independent consultancy firm Crowe, found that of 56 priority actions identified in the previous strategy, progress had been made on the implementation of 49 actions (87%).
The review also examined strategies in three other EU member states, France, Spain and the Netherlands, and consulted with key stakeholders from clinical, community and representative organisations including HIV Ireland and others prior to formulating its recommendations.
“The emphasis must now be on developing a comprehensive, evidence-based, and culturally appropriate National Sexual Health Strategy,” continued Mr O’Hare, “incorporating best practices, ensuring adequate resources,
INTERSTROKE study with Prof Salim Yusuf, McMaster University, Canada. He added, “Globally, one in four people over age 25 will have a stroke in their lifetime. We know from our INTERSTROKE study research that many strokes could be prevented, and this study has been designed to inform approaches to populationlevel prevention. We now need to factor in sleep problems as one of the associations with stroke, and the need for targeted research evaluating interventions design to prevent, and improve, sleep impairments.”
and a responsive approach to the evolving landscape of sexuality and sexual health service provision in Ireland.”
“This should include alignment with existing policy commitments, including the global Fast Track Cities initiative, to combat rising rates of HIV and end new HIV transmission and HIV-related stigma by 2030.” he added.
The Health Protection Surveillance Centre has reported a continuation of the rise in newly notified cases of HIV and STIs, following the steady rise witnessed in 2022. By the end of week 11 (18 March 2023) there had been a reported 91 percent increase in notified cases of HIV on the same period last year, with significant increases in chlamydia (53%) and gonorrhoea (187%) also reported.
9 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
News
Gaps in Understanding of Vitamin D
The study provides the most up to date findings on vitamin D intake and factors affecting vitamin D status of adults in Ireland, and has been published in the British Journal of Nutrition.

The study identifies groups most at risk of vitamin D deficiency and has highlighted gaps that exist in our understanding of why people are getting tested for vitamin D (a practice the researchers believe is often unnecessary and wasteful) along with how little we know about the vitamin D status of our ethnic minorities.
Vitamin D is needed for absorption of calcium and for normal bone and immune health. Vitamin D deficiency can result in softening of the bones and increase the risk of osteoporosis. Vitamin D is made by our skin after sunlight exposure, though in Ireland none can be made from about Halloween to St Patrick’s day, increasing the risk of deficiency. Of concern, food sources of vitamin D are very limited and not commonly eaten; these include oily fish, egg yolk and specific fortified foods such as fortified milk and breakfast cereals.
Currently 300,000 people in Ireland have osteoporosis (according to the Irish Osteoporosis Society), ensuring adequate vitamin D intake in early life can decrease the risk of osteoporosis/ fractures in later life.
The study investigated 383 participants, with a mean age of 56.0 years.
Key Findings
• The majority of adults (81%) not taking supplements did not meet the recommended daily allowance (RDA) for vitamin D and this was the biggest risk factor for deficiency.
• Those with dark skin are nearly 4 times more likely to be vitamin D deficient.
• Participants with low vitamin D intakes, darker skin and who avoid the sun are most at risk of deficiency.
• Knowledge of recommended intakes was poor amongst participants yet there were high levels of inappropriate vitamin D testing. Half of those tested were unaware of the recommended daily allowance for vitamin D.
• Better awareness of vitamin D and need for adequate intakes should be promoted.
• Over a third (40%) of adults had their vitamin D levels tested for non-clinical reasons.
In a previous study, the research team have found that approximately ¤60k was wasted per year in a Dublin hospital in unnecessary testing https://doi. org/10.1515/cclm-2021-0607. This places unnecessary burdens on laboratory and staff resources.
In the published study, it was found that over a third (40%) of adults had their vitamin D levels tested for no valid clinical reason with most being carried out as part a 'routine' clinic check. Only a minority (12%) were aware of the correct recommended daily amount and nearly half had no knowledge of any recommendations. While a minority (30%) felt they were familiar with vitamin D, the majority (86%) did still recognise it as important for bone health and 66% for immunity.
Researchers state that those ‘most at risk’ including adults with dark skin or with very poor intakes (those who don't eat fortified foods
or supplements) and with little sun exposure may need more vitamin D to optimise their levels than recommended by the Food Safety Authority of Ireland. Findings highlight the need for promotion of better awareness of vitamin D and of foods /fortified products that contain vitamin D and or supplements to improve status. They believe that fortification of staple food groups could also help address this issue and has been successful in other countries such as Finland.
Helena Scully, Mercers Glanbia Bone Research Fellow, MISA Institute, St James’s Hospital/ School of Medicine Trinity College and lead author of the study said, “We know that people living in Ireland are at-risk of vitamin D deficiency, particularly in the winter when we don’t get much sun. Our research indicates that those most at risk have inadequate vitamin D intake, dark skin types and avoid sun exposure. Choosing foods such as milk and cereal products with added vitamin D, and taking a supplement (15 micrograms or 600units per day), particularly in the winter can help prevent low vitamin D levels.”
Dr Eamon Laird, Research Fellow Trinity College Dublin and University of Limerick and co-author added, “This study highlights the gaps we have in why people are getting tested and how little we know about vitamin D and nutrition status of our ethnic minorities. There should be a real research focus now with proper funding to investigate these groups as the long term benefits in terms of reduced chronic disease burden is high.”
Dr Kevin McCarroll, Consultant Physician at St James's Hospital, and Clinical Senior Lecturer at Trinity and co-author said, "The study shows that dietary vitamin D intake is inadequate in the majority of adults and is big risk factor for deficiency. Improving the consumption of vitamin D rich / fortified products and taking supplements, especially in the Winter is advisable. This research also shows a high level of inappropriate vitamin D testing whereas greater emphasis instead should be placed on increasing vitamin D intakes.”
You can read the full paper: Vitamin D: determinants of status, indications for testing and knowledge in a convenience sample of Irish adults, in the British Journal of Nutrition here: https:// bit.ly/3JYgaYq
Dr Eamon Laird, Research Fellow Trinity College Dublin and University of Limerick and co-author

10 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Helena Scully, Mercers Glanbia Bone Research Fellow
A new study has highlighted how little we know about the vitamin D status of ethnic minorities in Ireland
Ovarian Cancer
What’s New in Ovarian Cancer
Ireland has one of the highest death rates in Europe from ovarian cancer; almost 300 women die from the disease each year making it the leading cause of death in gynaecological cancers in Ireland. Over 75% of patients here present with late-stage disease, partly due to the vagueness of symptoms and the similarity with other conditions such as irritable bowel syndrome.
The
BEAT campaign
Following the success of their 2022 campaign (winner in the Inspiring Collaborations category at the World Ovarian Cancer Coalition Impact Awards), the Irish Network for Gynaecological Oncology (INGO), together with medical professionals and patients around Ireland will work to raise awareness of ovarian cancer in conjunction with the World Ovarian Cancer Coalition this May. The focus again this year, will be on an information campaign revolving around the BEAT acronym (Bloating, Eating, Abdominal Pain, and Toilet Habits).
• Bloating that is persistent and doesn’t come and go
• Eating less and feeling full more quickly
• Abdominal and pelvic pain you feel most days
• Toilet changes in urination or bowel habits
Women across Ireland are being asked to know the signs and symptoms of ovarian cancer and to go to their GP if they are experiencing symptoms for three weeks or more. Early diagnosis can significantly improve survival. 83% of patients diagnosed with stage one ovarian cancer are alive 5 years after diagnosis whereas only 16% of patients diagnosed with stage four ovarian cancer are alive 5 years after diagnosis.
Over 400 women present with ovarian cancer annually and most cases occur in women over 50 years of age. Worryingly, research commissioned by the INGO in 2022, highlighted that 79% of women in Ireland are not confident they would notice a symptom of ovarian cancer. And a common misconception among women is that cervical cancer screening (smear tests) also screens for ovarian cancer. This is not the case; there is no screening programme for ovarian cancer in Ireland.
In addition to knowing the signs and symptoms of the disease, when it comes to ovarian cancer, knowing your risk factors is equally important.
• Reproductive history: If you have no children or have your first child after the age of 35, your risk of developing ovarian cancer is higher. Breastfeeding may lower a woman’s risk of developing ovarian cancer. Taking the oral contraceptive pill may also reduce a woman’s risk of developing ovarian cancer. Research has shown that the longer you take the pill, the more your risk is thought to come down.
• Age: Ovarian cancer usually affects women who have been through the menopause.
• Family history of cancer: If you have two or more close relatives (mother, sister or daughter) who have had ovarian cancer or breast cancer, you may be at a higher risk of the disease. Having relatives with ovarian cancer does not necessarily mean that you have a faulty inherited gene in
Written by Aoife McNamara, Education and Engagement Manager, Irish Cancer Society

the family. Faulty genes can lead to ovarian cancer in a very small number of women - about 5-10%. These include the genes BRCA1 and BRCA2. There is also a very small risk of ovarian cancer if your family have the genetic mutation known as Lynch Syndrome.
Irish collaborations
The Irish Network for Gynaecological Oncology comprises over 30 of Ireland’s foremost gynaecological cancer campaigners, researchers and patient advocates. The aim of the group is to raise awareness of gynaecological cancers across the Island of Ireland. The group are part of an international effort for two major awareness events annually; World Ovarian Cancer Day on May 8th and World Gynaecological Oncology Day on September 20th. Another significant collaboration among Irish clinicians and researchers is the Women’s Health Initiative. One of the larger programmes funded by the Irish Cancer Society. This is a national effort with studies based in Cork, Galway and Dublin, aimed at developing services and tools to identify and manage symptoms and side-effects for women which have resulted from their cancer and cancer treatments, including ovarian cancer. The overall goal of the project is improving quality of life. These pilot studies are being monitored for effectiveness based on patient experiences and outcomes, and it is hoped that after the initial pilot scheme, it will lead to the rollout of a national programme for gynaecological cancer survivors. There have already been several outputs from this research, to support ovarian cancer patients and their families directly.
Firstly, the development of ‘diagnosis imaging cards’. As many people affected by cancer will be aware of, trying to retain all the information about their cancer at time of diagnosis can be difficult.
Given the complexities of the cancer, and of the female anatomy, it can be hard to understand everything, and even harder to relay this information to family and loved ones afterwards. These diagnosis images provide helpful diagrams and information for the patient to take away with them. The response to these cards has been overwhelmingly positive; such a small change can have a big impact on improving the patient experience.
The second output has been the development of a tailored online resource for women affected by ovarian cancer. The platform is called this is go, a site dedicated to gynaecological malignancies. This website allows patients to create a profile containing their details, such as their diagnosis, and provides customised, up-todate information specific to their needs. As misinformation relating to cancer is widespread, it can be extremely difficult to access accurate online information, and understanding what is relevant to the Irish context can be challenging. This resource makes this significantly easier for patients, providing them with a trusted resource. There are also sections for healthcare professionals, ensuring a standardised approach to care across the country. Finally, there are plans to expand the website to include a section for family members to access information tailored to them, which will include information about cancer and caring for your loved one. Currently, the following sections are live on the site: ovarian, cervical, uterine, vulval, vaginal, BRCA, Lynch Syndrome, and a site dedicated to healthcare professionals. For more, see: www.thisisGO.ie
11 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
"These pilot studies are being monitored for effectiveness based on patient experiences and outcomes, and it is hoped that after the initial pilot scheme, it will lead to the rollout of a national programme for gynaecological cancer survivors"
New All-Island Hospital Remit
Jim Daly, CEO, Private Hospitals Association

The Private Hospitals Association (PHA) has announced it is to extend its remit to become a cross-border representative organisation for the independently funded hospital sector throughout the island of Ireland.
PHA says the addition of Kingsbridge Healthcare Group’s
private hospitals located in Belfast and in Ballykelly, County Derry marks a significant milestone for the Association, and it will now advocate for high-quality private healthcare delivery both north and south.
The PHA has declared itself as the representative body for the independently funded hospital sector on the island of Ireland. Its twenty member hospitals play a significant role in the delivery of acute and mental health services, and account for almost one-third of the acute hospitals on the island of Ireland.
The PHA says is committed to promoting the interests of all its members and in confirming its new all-island status, will seek greater coordination of healthcare delivery and better outcomes for patients on either side of the border. The addition of new members into the PHA membership will enable improved collaboration with healthcare providers in Northern Ireland as well as the sharing of best practices.
The importance of an all-island approach to healthcare is widely recognised. It allows for greater cooperation and sharing of resources across the island, resulting in better healthcare outcomes for the population of the island of Ireland as a whole. The PHA believes that by working together, healthcare providers on both sides of the border can
improve healthcare delivery and deliver increased access to healthcare services.
Commenting on the development, Jim Daly, CEO of the PHA said, "We are delighted to announce the Private Hospitals Association is now an all-island entity with the addition of 2 new private hospitals in Belfast and Derry to our membership. It is an important milestone for our Association on this the 35th year since our establishment back in 1988. We look forward to much greater collaboration on an all-island basis and adding further critical mass to our sector - which ultimately will lead to even better outcomes for our patients. A whole-of system approach to healthcare delivery is critical to improved patient health and we look forward to the active participation of our new colleagues in Northern Ireland in achieving this important goal.”
St James’s Launch Myindici Patient Portal
At the launch of the new Myindici app for patients with haemophillia are Edel Richards, NCC Staff Nurse, Niamh Larkin, Advanced Nurse Practitioner (ANP) in Haemophillia, Professor Niamh O'Connell, Consultant Haematologist and National Haemophillia Director, and Cathy Bergin, NCC Data Analyst.

has long been at the forefront of the development of electronic patient record systems in Ireland. With a national system in place since 2006, and a smartphone app for monitoring and reporting of home treatment since 2010, the haemophilia service in Ireland is consistently recognised as world–class.
Marking World Haemophilia Day, the National Coagulation Centre at St James’s Hospital, Dublin has launched the Myindici Patient Portal, a fully interactive app for people with bleeding disorders. Developed in collaboration with patients and the Irish Haemophilia Society, the app is a significant development for patients with inherited blood disorders in Ireland and will assist them in the management of their lifelong conditions and care.
In a novel development for Ireland, the patient portal incorporates a secure messaging system which will allow patients with bleeding disorders to communicate on non-urgent matters with their healthcare team. The app provides users with access to key parts of their healthcare records, information on their diagnosis, curated educational resources, contact details for haemophilia centres nationwide and other patient information.
Commenting on the benefits of the patient portal for those with bleeding disorders in Ireland, Professor Niamh O’Connell, Consultant Haematologist at the National Coagulation Centre in St James’s Hospital, said: “Access to real-time healthcare information is critical to delivering a high quality and safe service for people with bleeding disorders. Empowering people to become partners in their healthcare by facilitating secure access to their own records is a key driver for patient safety, education and timely care delivery.”
Based at St James’s Hospital, the National Coagulation Centre
The Myindici Patient Portal is a product of the second phase of the Haemophilia Lighthouse Project, funded by eHealth Ireland. Since the beginning of the project in 2016, a new electronic health record for people with bleeding disorders has been implemented and phase two, the launch of the patient portal, is now complete.
Welcoming the patient portal on behalf of fellow haemophilia patients, Brian O’Mahony, Chief Executive of the Irish Haemophilia Society, said: “The patient portal is an exciting development in the care of bleeding disorders in Ireland and will allow patients to take control of their complex conditions. Irish Haemophilia Society members have been involved in the development of the patient portal since its inception and our members are confident that the app will improve their overall care experience.”
12 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Photo credit: Anthony Edwards, Clinical Photographer, St James's Hospital
Development of an Innovative Mucolytic for the Treatment of Lung Diseases
Aer Therapeutics, a joint University College Dublin (UCD) and University of California, San Francisco (UCSF) biopharmaceutical spin-out company developing novel inhaled treatments for muco-obstructive lung diseases, today announced the closing of a $36 million Series A financing round.
Funding was received from a syndicate of premier life science industry investors, including, Canaan, OrbiMed, and Hatteras Venture Partners.
Proceeds from the financing will be used to advance the development of AER-01, the company's novel inhaled small molecule mucolytic drug designed to liquefy mucus plugs in the lungs of patients with chronic obstructive pulmonary disease (COPD). Aer Therapeutics plans to initiate a first-in-human Phase 1 clinical trial of AER-01 in mid-2023.
Aer Therapeutics, headquartered in Raleigh, North Carolina, was cofounded by Professor John Fahy, UCSF, who is originally from Dublin, and Professor Stefan Oscarson, UCD School of Chemistry.



Professor Fahy's laboratory at UCSF developed AER-01 with Professor Oscarson's glycochemistry laboratory at UCD in collaboration with Professor Anne Marie Healy's pharmaceutical technology laboratory at Trinity College Dublin. The combined expertise of these laboratories in mucus biology, glycochemistry, inhaled drug formulation, drug delivery, and lung imaging, supported by a development grant from the National Institutes of Health, underlies the novel AER-01 technology.
"We are excited to introduce Aer Therapeutics as a company dedicated to delivering a therapeutic solution to patients with COPD who have severe airway obstruction caused by mucus plugs. Our scientific founders led the pioneering research that uncovered mucus plugs as a key mechanism of disease in COPD, and their laboratories worked together to discover AER-01 as a novel mucolytic treatment. Aer will continue to leverage this expertise in the development of AER-01
and other therapeutic candidates for the treatment of mucoobstructive lung diseases," said Jim Shaffer, President and CEO, Aer Therapeutics.
It is estimated that approximately five million COPD patients in the United States have a mucus plug-high disease subtype. Conventional COPD treatments such as bronchodilators and supplemental oxygen do not treat the airway obstruction caused by mucus plugs.
Recent advances in the understanding of mucus plug biology and novel methods of
quantifying mucus plugs using computed tomography (CT) have created opportunities to advance drug development intended to eliminate mucus plugs. Aer Therapeutics is seizing upon these new opportunities to advance AER-01 for COPD.
"Studies using CT lung scans confirm that mucus plugs are highly prevalent in COPD patients and those with a high mucus plug burden have lower lung function, increased frequency of exacerbations, diminished quality of life, and increased risk of allcause mortality. These findings provide a basis to specifically
treat and remove mucus plugs as a strategy to improve lung health for COPD patients," said John Fahy, M.D., M.Sc., Professor of Medicine at UCSF and founder of Aer Therapeutics.
"COPD is a complex disease and one-size-fits-all treatment approaches are not likely to work. The use of CT imaging in the clinical development for AER-01 will help ensure that treatment is targeted to those patients most likely to benefit from an effective mucolytic."
Professor Stefan Oscarson, UCD School of Chemistry and cofounder, Aer Therapeutics said, "Today’s announcement is a hugely significant milestone for the AER Therapeutics team. It is exciting times for me, when after decades of academic research involving drug and vaccine development with colleagues at UCD, UCSF and TCD, to see a lead drug candidate moving into human clinical trials. The AER-01 mucolytic drug has the potential to meet a wide range of clinical need and to make a difference to many lives.”
As part of the Series A financing, the company has expanded its board of directors to include new appointees: Tim Shannon, M.D., general partner at Canaan; Rishi Gupta, J.D., partner at OrbiMed; Christy Shaffer, Ph.D., general partner at Hatteras Venture Partners; and, Thomas Mathers, CEO at Allievex. They join current board members, Jim Shaffer and Dr Fahy.
13 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Professor Stefan Oscarson, UCD School of Chemistry
News
Professor John Fahy, Co-founder, UCSF Jim Shaffer, President and CEO, Aer Therapeutics
“Studies using CT lung scans confirm that mucus plugs are highly prevalent in COPD patients and those with a high mucus plug burden have lower lung function, increased frequency of exacerbations, diminished quality of life, and increased risk of all-cause mortality. These findings provide a basis to specifically treat and remove mucus plugs as a strategy to improve lung health for COPD patients”
The 27th EAHP Congress 2023 was another great learning and networking opportunity. The crowds were back enjoying the many sessions and connecting at the exhibition area. The focus for the exhibitors this year seems to be very much around technology and robotics in the areas of drug compounding. These technological advances come with a high cost. However, they have to potential to increase efficiency and add safety for the hospital pharmacy team. There was a notable absence from big pharma at the 2023 conference.
The Fresenius Kabi corporate team designed a conference booth which was sizeable and interactive. This allowed for plenty of visitors to come and visit and learn about our growing portfolio.
 Conor Sadlier – Sales Manager Fresenius Kabi
Conor Sadlier – Sales Manager Fresenius Kabi
I was delighted to get the opportunity to travel with Fresenius Kabi to the 2023 EAHP Congress. It was my first time attending the event and it surpassed all my expectations. The exhibition hall was impressive with representation from many companies and there was a w ide variety of posters to browse and read. The programme itself was excellent and I thoroughly enjoyed the clinical lectures (Safe medication use in patients with cirrhosis and the expanding role of hospital pharmacists in renal transplant patients) which were co-presented by a pharmacist and physician. And finally, it was lovely to network and meet new and old Irish faces from the world of hospital pharmacy, industry and academia! Thank you, Fresenius Kabi.
Ciara O Riordan, Senior Pharmacist, Cork University Hospital.
It was a great opportunity to see the current involvement of pharmacists in hospitals across Europe and to view the initiatives they have recently undertaken. I enjoyed attending the Good Practice Initiative presentations and have already had the chance to share ideas with colleagues in Galway. I also attended a very interesting presentation on TPN compounding where the issue of cyber -security was raised. As our health system was without IT systems for three weeks in 2020 following a cyberattack, this was an important reminder to have back-up systems in place. The conference was also a great opportunity to network with colleagues from the Irish hospitals and to share ideas.
Treasa Ni Ici Dispensary Manager, Pharmacy Department, University Hospital Galway
Fresenius Kabi Ireland plus delegates make a splash in Lisbon at EAHP 2023
Noradrenaline 1mg/ml concentrate for solution for infusion.
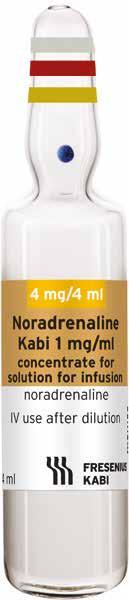
ABBREVIATED PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request. Noradrenaline (Norepinephrine) Kabi 1mg/ml concentrate for solution for infusion. Active ingredient: 1ml concentrate for solution for infusion contains 1mg noradrenaline (norepinephrine) base equivalent to 2mg noradrenaline (norepinephrine) tartrate. Contains 3.4mg sodium per ml. Indications: In adults for use as an emergency measure in the restoration of blood pressure in cases of acute hypotension. Posology and method of administration: When diluted as recommended, each litre contains 40mg noradrenaline base equivalent to 80mg noradrenaline tartrate. If dilutions other than 40mg per litre are used, check infusion rate calculation carefully before starting treatment. Initial rate of infusion – 10-20ml/hour (0.16-0.32ml/min); equivalent to 0.4-0.8mg/hour noradrenaline base. Lower initial infusion rate of 5ml/hour (0.08ml/min); equivalent to 0.2mg/hour noradrenaline base may be preferred. Titration of dose – Once infusion has been established, titrate dose in steps of 0.05-0.1mcg/kg/min of noradrenaline base according to pressor effect observed (see SmPC for details). Continue infusion until adequate blood pressure and tissue perfusion maintained without therapy. Carefully monitor patient. Should only be administered by healthcare professionals familiar with use of noradrenaline and with appropriate monitoring facilities. Avoid abrupt infusion withdrawal; reduce infusion gradually. No experience in treatment of hepatic or renal impairment. Dose selection for an elderly patient should be cautious, starting at the low end of the dosing range. Safety and efficacy in patients less than 18 years old has not been established. Method of administration – Intravenous use only after dilution. Infuse at a controlled rate using either syringe pump, infusion pump or drip counter. Administer via a central venous catheter. If not using a central venous catheter administer into a large vein whenever possible, particularly an antecubital vein to minimize risk of ischemic necrosis. Avoid catheter tie-in technique if possible. Contraindications: Hypersensitivity to the active substance or to any of the excipients, hypotension due to blood volume deficit. Do not use with cyclopropane and halothane anaesthetics as this may cause serious cardiac arrhythmias including ventricular fibrillation. Special warnings and precautions for use: Do not use undiluted. Contraindicated in patients who are hypotensive from blood volume deficits except as an emergency measure to maintain coronary and cerebral artery perfusion until blood volume replacement therapy can be completed. Should only be used in conjunction with appropriate blood volume replacement. If whole blood or blood plasma is indicated to increase blood volume, administer separately (e.g. use Y-tubing, individual containers). Prolonged administration may result in plasma volume depletion which should be continuously corrected by appropriate fluid and electrolyte replacement therapy. Frequently check blood pressure and rate of flow to avoid hypertension. Particular caution in patients with coronary, mesenteric or peripheral vascular thrombosis, hypotension following myocardial infarction, angina (particularly Prinzmetal’s variant angina), diabetes, hypertension or hyperthyroidism, major left ventricular dysfunction associated with acute hypotension (supportive therapy should be initiated simultaneously with diagnostic evaluation; reserve noradrenaline for patients with cardiogenic shock and refractory hypotension, in particular those without elevated systemic vascular resistance), liver failure, severe renal dysfunction,
ischemic heart diseases and elevated intracranial pressure. Reduce dose if heart rhythm disorders occur during treatment. Cardiac arrhythmias may arise when used in conjunction with cardiac sensitizing agents and may be more likely in patients with hypoxia or hypercarbia. Elderly patients may be especially sensitive to the effects of noradrenaline. Not recommended in children. Where indicated, appropriate replacement therapy of blood or fluid together with adoption of the supine position with elevation of the legs, must be instituted and maintained prior to and/or during therapy with noradrenaline. During infusion, record blood pressure every two minutes from the time the administration started until the desired blood pressure is obtained and then every five minutes thereafter if infusion continued. Constantly watch flow rate and never leave patient unattended. Hypertension may eventually lead to acute pulmonary oedema, arrhythmia or cardiac arrest. Caution in patients receiving pressor amines with chloroform, enflurane or other halogenated anaesthetics (may cause serious cardiac arrhythmias) or any other cardiac sensitising agent or in patients who exhibit profound hypoxia or hypercarbia. Extreme caution in patients receiving monoamine oxidase inhibitors or within 14 days of cessation of such therapy and in patients receiving tricyclic antidepressants, adrenergic- serotoninergic drugs or linezolid. Overdoses or conventional doses in hypersensitive persons may cause severe hypertension with violent headache, photophobia, stabbing retrosternal pain, pallor, intense sweating and vomiting. The infusion site should be checked frequently for free flow. Care should be taken to avoid extravasation of noradrenaline tartrate into the tissues, as local necrosis might ensue due to the vasoconstrictive action of the drug (see SmPC for antidote for extravasation ischaemia). Blanching along the course of the infused vein warrants consideration of changing infusion site at intervals. Avoid administration via the veins of the leg in elderly patients or in those suffering from occlusive vascular diseases. Noradrenaline interacts with other medicinal products; some combinations are inadvisable or require additional precautions and close medical supervision – see SmPC. Undesirable effects: Anxiety, insomnia, confusion, weakness, psychotic state, transient headache, tremor, acute glaucoma, bradycardia, arrhythmia, electrocardiogram change, tachycardia, cardiogenic shock, stress cardiomyopathy, palpitations, increase in the contractility of the cardiac muscle resulting from the beta- adrenergic effect on the heart (inotrope and chronotrope), acute cardiac insufficiency, hypertension, peripheral ischaemia including gangrene of the extremities, plasma volume depletion with prolonged use, dyspnoea, respiratory insufficiency or difficulty, nausea, vomiting, paleness, scarification of the skin, bluish skin colour, hot flushes or skin redness, skin rash, hives or itching, retention of urine, extravasation, necrosis at injection site. Legal Category: POM Marketing Authorisation Number: PA2059/073/001
Marketing Authorisation
Holder: Fresenius Kabi Deutschland GmbH; Else-Kröner Straße 1, 61352 Bad Homburg v.d.Höhe, Germany Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance.GB@Fresenius-Kabi.com. Date of Preparation: January 2022 FPI-0041
Website: www.fresenius-kabi.com/ie/
Email: FK-enquiries.ireland@fresenius-kabi.com

Phone: +353 (0)1 841 3030
Date of Prep: March 2022
Job Code: IE-IVF-2200006
Kabi Limited
3B Fingal Bay Balbriggan, Co. Dublin Ireland
Your patients, your profession, our passion. Fresenius
Fresenius Kabi Ireland Unit
Improving Asthma Management
study, led by the INCA Research Team, involved more than 200 patients with severe or difficult-to-control asthma at 10 centres across Ireland, Northern Ireland and England. Half of participants (the ‘control’ group) were monitored using traditional methods while the remaining participants (the ‘active’ group) had their inhaler use monitored by a device called an INCA.
which is one of the challenging distinctions that respiratory physicians often need to make.
“This means that the digital monitoring technology can help doctors decide with more confidence which patients would benefit from a step up to biologic drugs, as opposed to continuing high-dose inhaled corticosteroid therapy alone.
A new international study led by RCSI University of Medicine and Health Sciences has revealed significant potential for digital technology to improve asthma control for patients.
The study, published in The Lancet Respiratory Medicine, examined the effectiveness of combining information from digital device in a digital platform to effectively manage uncontrolled asthma.
The findings, which on average led to a healthcare cost reduction of ¤3,000 per patient, demonstrate the future potential of digital medicine in the management of many chronic diseases.
Asthma is one of the most prevalent chronic respiratory conditions, affecting hundreds of millions of people worldwide. Steroid medicines can effectively treat and manage asthma, if delivered into the airways efficiently and in a timely manner using an inhaler.
About one-in-10 people with asthma find they cannot control their asthma symptoms with inhalers, however, often experiencing severe attacks or needing stronger medication. It is thought that in some of these cases, patients may not be using the inhalers effectively. To assess this, doctors typically have had to rely on patients’ own reports about their inhaler use and symptoms to adjust medications. Now, novel technologies developed by the INCA Research Team at RCSI can deliver and monitor asthma care in a completely digital manner.
Fewer side effects
The Inhaler Adherence in Severe Unstable Asthma (INCA-SUn)
Co-developed by RCSI and Trinity College Dublin, the INCA device provides an objective assessment of how patients use their inhalers, by measuring acoustic or soundwave signals from the inhaler. The data is provided to the patient’s healthcare provider through a digital clinical decision platform which advises the best treatment, which may include educating the patient on effective inhaler technique. The study revealed that patients assigned to the ‘active’ group improved their medication adherence and were less likely to require corticosteroids, thus avoiding potential side effects. Fewer were switched to more expensive biologic medications, with a larger proportion of patients having their medication levels reduced while still controlling their symptoms effectively.
Important distinctions
INCA Lead Investigator Professor Richard Costello, described how the 32-week study showed that the INCA devices can help to differentiate between people who have severe asthma and those who have difficult-to-treat asthma,
Tackling post-pandemic Wait Lists
Physician Associates can make a significant impact in tackling waiting lists and improving access for patients in the Irish healthcare system, according to the Director of Physician Associate Studies at RCSI University of Medicine and Health Sciences.
Speaking ahead of the WHO Global Forum on Human Resources for Health, Professor Lisa Alexander said that greater numbers of Physician Associates and a regulatory framework for the profession would improve both patient care and working conditions across the system.
Physical Associates are trained as generalists to support clinicians
in delivering patient care in areas such as chronic disease management, rapid assessment respiratory units, virtual clinics and mental health.
The programme to train Physician Associates in Ireland was developed by RCSI following successful global implementation of similar programmes in countries such as the UK, USA, the Netherlands and Africa, These programmes have demonstrated an improvement in the flow of patients through health systems and in chronic disease management as well as overall cost savings and an increase in patient and staff satisfaction in these countries.
Over 60 Physician Associates have graduated from the RCSI Masters programme to date and are working in the Irish health system, with the majority working in hospital or specialised care in the Dublin region. Research on this model of care in Ireland has revealed enhanced patient satisfaction and continuity of care and a high level of job satisfaction amongst the practicing Physician Associates.
Professor Alexander said: ‘The impactful role that Physician Associates have had on a number of international health systems became evident during the COVID-19 pandemic. They addressed backlogs across areas including specialist care,
“In this trial, which we believe to be the first of its kind, we have brought a completely digital approach to medication management and we believe that this is a substantial step towards improving the outcomes and economic burden in patients with severe and difficult-to-control symptoms,” said Professor Costello, who is a Professor at RCSI Department of Medicine and a Consultant Physician at Beaumont Hospital.
The INCA SUn (Inhaler Adherence in Severe Unstable Asthma) study was carried out though the RCSI Clinical Research Centre (RCSICRC) and was funded by the Health Research Board, Medical Research Council, INTEREG Europe and an investigatorinitiated project grant from GlaxoSmithKline.
With the support of Enterprise Ireland, Professor Costello has created an RCSI spin-out company called Phyxiom, which is translating the learnings from INCA-SUn into an AI platform that aims to transform the clinical outcomes of patients with uncontrolled asthma.

preventative screening and GP care, amongst others. In Ireland, they have the potential to make a significant impact on population health in the post pandemic health system by helping to address occupational shortages in areas such as general practice and reducing the backlog that was made worse during the pandemic by improving the flow of patients through the health system.”
“We need to see an increase in the numbers graduating as Physician Associates and introduce regulation for the profession so that they can work to their full potential across all sectors of across the health system”, added Professor Alexander.
16 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Richard Costello, Professor at RCSI Department of Medicine and a Consultant Physician at Beaumont Hospital
Potential for a Multi-disciplinary Public Health Workforce
Developing a multidisciplinary public health workforce in Ireland, with career pathways for public health professionals from medical and non-medical backgrounds, is an important consideration for health service reform and planning for current and future public health threats.
That’s according to a new discussion paper, commissioned by the Institute of Public Health (IPH), which explores potential options for a multidisciplinary model of public health in Ireland. The discussion paper was commissioned in the wake of the 2018 Crowe Howarth report, which set out a new vision for public health in Ireland and has paved the way for the reform of existing structures.
The COVID-19 pandemic placed public health at the centre of public policy and this new paper further builds on plans to reform and strengthen the current model of public health in line with the Essential Public Health Functions (EPHFs) - identified by the World Health Organization (WHO) as a minimum requirement for delivering resilient and sustainable public health systems.
In 2021, IPH established an Advisory Group, chaired by Professor Ivan Perry, and commissioned Crowe, a consultancy firm, to develop this discussion paper.
This new report explores options to develop a multidisciplinary public health workforce, including the potential to develop and enhance employment and training paths for public health professionals from both medical and non-medical backgrounds. This report examines public health systems in nine other countries - Wales, Australia, Canada, Finland, Israel, the Netherlands, New Zealand, Slovenia, and Switzerland - and also sought the views of stakeholders already working within existing structures in Ireland, as well as insights from international experts in the public health field.
The discussion paper highlighted the following key messages:
• Stakeholders in Ireland identified a problem of “trapped talent” with skilled graduates and practitioners unable to enter career pathways in public health beyond the routes available for medical graduates. This has resulted in “lost
talent”, with staff moving to other disciplines or working in public health abroad.
• The paper highlighted the success of the Welsh multidisciplinary public health model, which has been supported by modernised public health legislation and strategy, integration with local government, and a registration system for a wide range of public health practitioners.
• International leaders and academic experts in the field of public health said a multidisciplinary workforce could be developed through a strategic roadmap, interdisciplinary competency frameworks for skills development, a registration system for public health professionals, as well as a regulatory body, accreditation, and professional development.
The discussion paper concludes that, to be prepared to address current and future challenges, the public health workforce will need sufficient breadth of skills, diversity and capacity to meet these demands, to address the socioeconomic and environmental determinants of health, and to work across sectors, borders,
professional disciplines, agencies, and specialities.
Dr Jenny Mack, consultant in public health medicine at the Institute of Public Health, said: “Like many other countries, the public health system in Ireland is experiencing unprecedented demands. Current and future public health challenges are highly complex and include the burden of chronic disease linked to unhealthy food environments, physical inactivity, tobacco, and alcohol; the need to address the social determinants of health, such as housing, isolation, and poverty; and the pressing issues of climate change, biodiversity loss, and the risk of further pandemics.
“Responding to these challenges will require a ‘Health in All Policies’ approach and modernised public health legislation focused on prevention and wellbeing, but also a strong, concerted effort across many public health disciplines and specialities. It is promising to see multidisciplinary public health workforces gaining recognition internationally, and this will be an important component of public health service reform; a reform which needs to consider the prevention of disease and health inequalities as a national priority”.
Making safe antimicrobial prescribing a reality for final year medical students in University of Galway using interprofessional simulation-based education
1School of Medicine, College of Medicine Nursing and Health Sciences, University of
2Pharmacy Department, Galway University Hospitals
3The Irish Centre for Applied Patient Safety and Simulation, University of Galway
The teaching of safe antimicrobial prescribing is a key component of medical school curricula. The new intern curriculum states that interns should be able to ‘prescribe and monitor’ (EPA 5)1 and ‘recognise and manage the deteriorating patient’ (EPA 6)2. There is a clear gap between what is being taught in the undergraduate programme and what is expected in practice. Interprofessional simulation-based education (SBE) can address this gap.
SBE forms a critical part of the preparedness for clinical practice (PCP) learning block in the penultimate year of the
University of Galway’s medical curriculum. Students participate in 12 simulated scenarios of which one is a neutropenic sepsis presentation and deterioration. The scenario was co-created with input from medicine, nursing, and pharmacy and the scenario is run and co-debriefed by this interprofessional team. The scenario design supports the ‘stop and go’ method of debriefing and is divided into three parts, each with a specific focus: identification, management including prescribing, and discharge planning.
The scenario was delivered to 220 final year medical students
in University of Galway between January and March 2023. Tasks included requesting laboratory investigations and imaging, contacting and speaking with microbiology and infectious diseases teams, and inpatient antimicrobial prescribing and monitoring. Interprofessional co-debriefing was by a medical consultant and chief 2 medication safety pharmacist with a focus on safe prescribing. Feedback from faculty and learners was very positive.
SBE bridges the gap between classroom learning and clinical practice. Students benefit from ‘doing’ actions rather than
simply observing in a simulated environment. Interprofessional education is a strategic goal of all healthcare education organisations to improve patient safety. Simulation, content co-creation, and co-debriefing are methods to develop valuable interprofessional subject matter expert contribution into a curriculum.
1. Entrustable Professional Activity
2. Entrustable Professional Activity
5 “Prescribe and Monitor Drugs and Fluids
6 “ Recognise and Manage the Acutely Unwell/Deteriorating Patient”
17 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 News
Diana Hogan-Murphy1,2, Cara Egan1,3, Ambyr Reid1,3, Conall Dennedy1, John Given2, Andrea Holmes1, Dara Byrne1,3
Galway
NOW BVBM FOR 20MG ADALIMUMAB
WHAT’S BEHIND AMGEVITA®
AMGEVITA®
HOW AND WHEN
AMGEVITA® is injected a specially designed


PRESCRIBE AMGEVITA®
AMGEVITA® (adalimumab) Brief Prescribing Information
Please refer to the Summary of Product Characteristics (SmPC) before prescribing Pharmaceutical Form: Single dose pre-filled syringe contains 20 mg adalimumab in 0.4 mL (50 mg/mL) solution, 40 mg adalimumab in 0.8 mL (50 mg/mL) solution or single dose pre-filled pen (SureClick®) contains 40 mg adalimumab in 0.8 mL (50 mg/mL) solution. Indications and Dosage: please refer to SmPC for full information. For subcutaneous injection. Treatment should be initiated and supervised by specialist physicians experienced in conditions for which AMGEVITA® is indicated. Ophthalmologists should consult with an appropriate specialist before starting treatment. Give patients a Patient Reminder Card. After training in injection technique, patients may self-inject with medical follow-up as necessary. Optimise other concomitant therapies (e.g. corticosteroids and or/immunomodulatory agents). Rheumatoid arthritis (RA), adults: In combination with methotrexate (MTX) for: 1. Moderate to severe, active RA with inadequate response to disease-modifying antirheumatic drugs (DMARDs) including MTX; 2. Severe, active and progressive RA not previously treated with MTX. Can be given as monotherapy if intolerance to or when continued treatment with MTX is inappropriate.
Dosage: 40 mg every other week (EOW). Continue concomitant MTX. In monotherapy, patients may require 40 mg every week or 80 mg EOW if there is a decrease in clinical response. Reconsider treatment beyond 12 weeks if no clinical response. Consider need for dose interruption, e.g. before surgery or if serious infection occurs. Polyarticular juvenile idiopathic arthritis (pJIA), paediatrics ≥ 2 years: In combination with MTX for active pJIA with inadequate response to one or more DMARDs. Can be given as monotherapy if intolerance to or when continued treatment with MTX is inappropriate.
Dosage: 10 kg to < 30 kg: 20 mg EOW. ≥ 30 kg: 40 mg EOW. Reconsider treatment beyond 12 weeks if no clinical response. Enthesitis-related arthritis (ERA), paediatrics, ≥ 6 years: Active ERA with inadequate response to or intolerance to conventional therapy. Dosage: 15 kg to < 30 kg: 20 mg EOW.
≥ 30 kg: 40 mg EOW. Ankylosing spondylitis (AS), adults: Severe active AS with inadequate response to conventional therapy. Axial spondyloarthritis without radiographic evidence of AS (nr-axSpA), adults: Severe nr-axSpA with objective signs of inflammation (elevated CRP and/or MRI), and an inadequate response to or intolerance to nonsteroidal anti-inflammatory drugs. Psoriatic arthritis (PsA), adults: Active and progressive PsA with inadequate response to DMARDs. Dosage (AS, nr-axSpA, PsA): 40 mg EOW. Reconsider treatment beyond 12 weeks if no clinical response. Psoriasis, adults: Moderate to severe chronic plaque psoriasis in candidates for systemic therapy. Dosage: 80 mg at Week 0, followed by 40 mg EOW from Week 1. Reconsider treatment beyond 16 weeks if no clinical response. Refer to SmPC. Paediatric Plaque Psoriasis, ≥ 4 years: Severe chronic plaque psoriasis with inadequate response to or if topical therapy and phototherapies are inappropriate. Dosage: 15 kg to < 30
You should inject in see diagram below.
The medicine travels that are inflamed and
BY BRAND NAME
Your doctor, pharmacist you or your parent/carer injection. It’s important correctly for it to work.
kg: 20 mg followed by 20 mg EOW starting one week after initial dose. ≥ 30 kg: 40 mg then 40 mg EOW starting one week after initial dose. Reconsider treatment beyond 16 weeks if no clinical response. Safety has been assessed in paediatric patients with plaque psoriasis for a mean of 13 months. Hidradenitis suppurativa (HS), adults and adolescents ≥ 12 years: Active moderate to severe HS (acne inversa) with inadequate response to conventional systemic therapy. Dosage, adult: 160 mg at Day 1, followed by 80 mg at Day 15. At day 29 continue with 40 mg every week or 80 mg EOW. Reintroduction after treatment interruption: 40 mg every week or 80 mg EOW.
Dosage: adolescent (≥ 30 kg): 80 mg at week 0 then 40 mg EOW starting at week 1. If response is inadequate, consider increasing to 40 mg every week or 80 mg EOW. Dosage, adult and adolescent: Antibiotics may be continued if necessary. Use concomitant topical antiseptic wash on a daily basis. Reconsider treatment beyond 12 weeks if no improvement. Periodically evaluate the benefit and risk of continued treatment. Crohn’s disease (CD), adults: Moderately to severely active CD with no response despite a full and adequate course of, intolerance to or contraindication for a corticosteroid and/ or an immunosuppressant therapy. Dosage: Induction: 80 mg at Week 0, then 40 mg at Week 2. For a more rapid response: 160 mg at Week 0, then 80 mg at Week 2; risk of adverse events higher during rapid induction. Maintenance: 40 mg dose EOW. Corticosteroids may be tapered in accordance with clinical guidelines. If decrease in clinical response, can increase to 40 mg every week or 80 mg EOW. If no response by Week 4 there may be benefit from continued therapy to week 12. Reconsider treatment beyond 12 weeks if no clinical response. Paediatric CD, ≥ 6 years: Moderately to severely active CD with inadequate response to, intolerance to or contraindication to conventional therapy including primary nutrition therapy and a corticosteroid and/or an immunomodulator. Dosage: < 40 kg: Induction: 40 mg at week 0 then 20 mg at week 2. For a more rapid response: 80 mg at week 0 then 40 mg at week 2; risk of adverse events higher during rapid induction. Maintenance starting at week 4: 20 mg dose EOW. If insufficient response, consider 20 mg every week. Dosage: ≥ 40 kg: Induction: 80 mg at Week 0 then 40 mg at Week 2. For a more rapid response: 160 mg at Week 0, then 80 mg at Week 2; risk of adverse events higher during rapid induction. Maintenance starting at week 4: 40 mg dose EOW. If insufficient response, consider 40 mg every week or 80 mg EOW. Reconsider treatment beyond 12 weeks if no clinical response. Ulcerative colitis (UC), adults: Moderately to severely active UC with inadequate response to, intolerance to or contraindication for conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA). Dosage: Induction: 160 mg at week 0 and 80 mg at week 2. Maintenance: 40 mg EOW. Corticosteroids may be tapered in accordance with clinical guidelines. If insufficient response, consider increasing to 40 mg every week or 80 mg EOW. Do not continue treatment beyond 8 weeks if no
clinical response. Paediatric UC, ≥6 years: Moderately to severely active UC with an inadequate response to conventional therapy including corticosteroids and/or 6-MP or AZA, or who are intolerant to or have contraindications.
Dosage: < 40 kg: Induction: 80 mg at Week 0 and 40 mg at Week 2. Maintenance starting at week 4: 40mg EOW. Dosage: ≥ 40 kg: Induction: 160 mg Week 0 and 80 mg Week 2. Maintenance starting at week 4: 80mg EOW. Patients reaching 18 years should continue their prescribed maintenance dose. Reconsider treatment beyond 8 weeks if no clinical response. No relevant use in children <6 years. Uveitis, adults: Non-infectious intermediate, posterior and panuveitis with inadequate response to corticosteroids, in patients in need of corticosteroid-sparing, or in whom corticosteroid treatment is inappropriate. Dosage: 80 mg at Week 0 and 40 mg EOW from Week 1. There is limited experience in initiation of treatment with adalimumab alone. Treatment can be initiated in combination with corticosteroids and/or with other non-biologic immunomodulatory agents. Concomitant corticosteroids may be tapered starting two weeks after initiating treatment with AMGEVITA® Evaluate on a yearly basis the benefit and risk of continued treatment. Paediatric uveitis, ≥ 2years: Chronic non-infectious anterior uveitis with inadequate response or intolerance to conventional therapy, or in whom conventional therapy is inappropriate. In paediatric uveitis, there is no experience in the treatment with AMGEVITA® without concomitant treatment with methotrexate.
You may have the injection at home. You can give parent/carer can do injection is given every doctor may change works for you.
You need to follow healthcare team and if you are not feeling
Dosage: < 30 kg: 20 mg EOW in combination with MTX.
For full and detailed administer your AMGEVITA®, instructions provided in
Dosage: ≥ 30 kg: 40 mg EOW in combination with MTX. A loading dose of 40 mg (< 30 kg) or 80 mg (≥ 30 kg) may be administered 1 week in advance of maintenance therapy. No clinical data on use of loading dose in patients < 6 years. Evaluate benefit and risk of continued treatment on a yearly basis. Contraindications: Hypersensitivity to the active substance or to any excipients; Active tuberculosis (TB) or other severe infections such as sepsis and opportunistic infections; Moderate to severe heart failure (NYHA class III/ IV). Warnings and Precautions: Clearly record the name and batch number.
Infections: Patients taking TNF-antagonists are more susceptible to serious infections, especially if impaired lung function. Monitor for infections, before, during and for 4 months after treatment. Do not initiate treatment during an active infection, until infection is controlled. Consider risk/benefit prior to treatment in patients exposed to TB or who have travelled in areas of high risk of TB or endemic mycoses. Evaluate new infections and monitor closely. Stop treatment if new serious infection or sepsis and treat. Exercise caution in patients with a history of recurring infections or who are predisposed to infections, including the use of concomitant immunosuppressive medications.
Serious Infections: Consult SmPC for details. Serious infections, including those associated with hospitalisation or death, were reported in patients receiving treatment. TB: Reactivation and new onset TB, both pulmonary and extra-pulmonary (disseminated). Screen all patients before therapy initiation
® awarded best value biosimilar medicine in a citrate free formulation of adalimumab by the Medicines Management Programme BVBM AWA R DED AWA R DED
funam tca u r ing excellence supply reliability consist e n t qytilau gniunitnoc noitavonni Amgen has >40 YEARS BIOLOGICS EXPERIENCE Amgevita®
best value biologic medicine in a citrate free formulation of adalimumab and is the only BVBM awarded for 20mg adalimumab.
CITRATE FREE
Awarded
6
AMGEVITA® AND ME
MAKES THE DIFFERENCE
WHEN IS IT GIVEN?
injected under the skin (a subcutaneous injection) with designed pen called SureClick® or using a pre-filled syringe.


in the tummy, or the tops of your legs (thighs), below. travels into your bloodstream to work on the areas and causing your symptoms.

Information for Healthcare Professionals
pharmacist or nurse will show parent/carer how to give the important the injection is given work.

injection in hospital or give it to yourself or your do this for you. Usually, the every 2 weeks, but your change this to best suit what
the advice of your and ask to speak to them feeling well.
detailed instructions on how to AMGEVITA®, please read the provided in the product carton.
for active or inactive (latent) TB. Perform detailed medical assessment and appropriate screening tests for TB in all patients and results recorded on patient reminder card. If latent TB is suspected, consult physician with appropriate expertise Despite prophylaxis, TB reactivation has occurred on adalimumab. If active TB is diagnosed, do not initiate AMGEVITA® treatment. Other opportunistic infections: Opportunistic infections were observed in patients receiving adalimumab. Stop AMGEVITA® in patients with signs and symptoms of such infections. Consult with physician with appropriate expertise for diagnosis and administration of empiric antifungal therapy. Hepatitis B reactivation: Reactivation of HBV has occurred in chronic carriers; some cases were fatal. Test patients for HBV infection before initiating treatment. HBV carriers should consult a specialist physician and be closely monitored for reactivation of HBV infection throughout therapy and for several months after treatment. If reactivation occurs, stop treatment and initiate appropriate antiviral and supportive treatment. Neurological events: Caution in patients with pre-existing or recent-onset central or peripheral nervous system demyelinating disorders. Consider discontinuation if any of these develop. Perform neurologic evaluation in patients with non-infectious intermediate uveitis prior to initiation of treatment and regularly during treatment. Allergic reactions: Reports of serious allergic reactions including anaphylaxis. For serious allergic or anaphylactic reaction, stop AMGEVITA® immediately and initiate appropriate therapy. Dry natural rubber: The needle cover of the pen (SureClick®) is made from dry natural rubber (a derivative of latex), which may cause allergic reactions. Malignancies and lymphoproliferative disorders: A possible risk of malignancy, some fatal, including lymphomas and leukaemia, cannot be excluded (see SmPC). Examine all patients, especially those with a medical history of extensive immuno-suppressant therapy or psoriasis patients with a history of PUVA treatment, for non-melanoma skin cancer prior to and during treatment. Melanoma and Merkel cell carcinoma have also been reported. Caution in COPD patients, and in patients with increased risk for malignancy due to heavy smoking. Consider the potential risk with the combination of AZA or 6-MP and AMGEVITA® (hepatosplenic T-cell lymphoma has occurred). Caution in patients with a history of malignancy. Risk of developing dysplasia or colon cancer unknown. Screen patients with UC, history of dysplasia or colon carcinoma, for dysplasia before and during treatment. Haematologic reactions: Adverse events of the haematologic system, including medically significant cytopenia, have been reported. Advise patients to seek immediate medical attention if signs and symptoms of blood dyscrasias develop. Vaccinations: Patients may receive concurrent vaccinations, except for live vaccines. Bring paediatric patients up to date with all immunisations prior to initiating treatment. Congestive heart failure: See contraindications. Caution in mild heart failure (NYHA class I/II). Discontinue treatment if new or worsening symptoms of congestive heart failure.
Autoimmune processes: Autoimmune antibodies may form. Stop treatment if development of a lupus-like syndrome with positive antibodies against doublestranded DNA. Surgery: Consider the long half-life of AMGEVITA® for planned surgical procedures. Monitor closely for infections. There is limited safety experience in patients undergoing arthroplasty or surgical procedures. Elderly patients: Serious infections were higher in patients over 65, some of which were fatal. Consider risk of infections in these patients. Interactions: Antibody formation was lower when AMGEVITA® was given together with MTX rather than as monotherapy. Combination of AMGEVITA® with other biologic DMARDs (e.g. anakinra and abatacept) or other TNF-antagonists is not recommended. Fertility, pregnancy and lactation Only use during pregnancy if clearly needed. Women of childbearing age to consider use of adequate contraception during and for at least 5 months after the last treatment. Administration of live vaccines (e.g. BCG) to infants exposed to AMGEVITA ® in utero is not recommended for 5 months following the mother’s last injection during pregnancy. AMGEVITA ® can be used during breastfeeding. Effects on ability to drive and use machines: AMGEVITA® may have a minor influence on the ability to drive and use machines. Adverse Reactions: Very common ≥ 1/10: Respiratory tract infections, leukopenia, anaemia, lipids increased, headache, abdominal pain, nausea and vomiting, elevated liver enzymes, rash, musculoskeletal pain, injection site reaction. Common ≥ 1/100 to < 1/10: Systemic infections, intestinal infections, skin and soft tissue infections, ear infections, oral infections , reproductive tract infections , urinary tract infections, fungal infections, joint infections, skin cancer excluding melanoma, benign neoplasm, leucocytosis, thrombocytopenia, hypersensitivity, allergies, hypokalaemia, uric acid increased, blood sodium abnormal, hypocalcaemia, hyperglycaemia, hypophosphataemia, dehydration, mood alterations , anxiety, insomnia, paraesthesia, migraine, nerve root compression, visual impairment, conjunctivitis, blepharitis, eye swelling, vertigo, tachycardia, hypertension, flushing, haematoma, asthma, dyspnoea, cough, GI haemorrhage, dyspepsia, gastroesophageal reflux disease, Sicca syndrome, worsening or new onset of psoriasis , urticaria, bruising , dermatitis, onychoclasis, hyperhidrosis, alopecia, pruritus, muscle spasms , renal impairment, haematuria, chest pain, oedema, pyrexia, coagulation and bleeding disorders, autoantibody test positive , blood lactate dehydrogenase increased, impaired healing. Serious, including fatal, adverse reactions have been reported including infections/sepsis, TB, opportunistic infections, allergic reactions, HBV reactivation and malignancies. Serious haematological, neurological and autoimmune reactions have also been reported. These include rare reports of pancytopenia, aplastic anaemia, central and peripheral demyelinating events and reports of lupus, lupusrelated conditions and Stevens-Johnson syndrome. Other less common and rarely reported adverse reactions are listed in the SmPC. Pharmaceutical
Precautions: Store in a refrigerator (2°C – 8°C). Do not freeze. Keep in the outer carton in order to protect from light. Out of the refrigerator, may be stored up to 25°C for up to 14 days. Legal Category: POM. Presentation and Marketing Authorisation Number. AMGEVITA® 20 mg solution for injection in pre-filled syringe: EU/1/16/1164/001 – 1 pack: cost AMGEVITA® 40 mg solution for injection in pre-filled syringe: EU/1/16/1164/003 – 2 pack AMGEVITA® 40 mg solution for injection in pre-filled pen: EU/1/16/1164/007 – 2 pack. Marketing Authorisation Holder: Amgen Europe B.V. Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. AMGEVITA® is a registered trademark of Amgen Inc. Date of PI preparation: December 2021 (Ref:IE-AMB-1121-00005)
Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse reactions/events should also be reported to Amgen Limited on +44 (0) 1223 436441 or Freephone 1800 535 160.
1. BVBM Amgevita Product Information Sheet. https://www.hse. ie/eng/about/who/cspd/ncps/medicines-management/bestvalue-medicines/best-value-biological-medicines/bvb-medicineamgevita-product-information-sheet.pdf. Accessed January 2022.
2. Oireachtas.ie, Debates, December 2020, Written Answer by Minister for Health. https://www.oireachtas.ie/en/debates/ question/2020-12-10/373/. Accessed January 2022.
3. Medicines Management Programme Best-Value Biological Medicines: Adalimumab 20 mg solution for injection. https://www.hse.ie/eng/ about/who/cspd/ncps/medicines-management/best-value-medicines/ best-value-biological-medicines/mmp-report-bvb-medicineadalimumab-20mg-march-2021.pdf. January 2022.
4. AMGEVITA® (adalimumab) summary of product characteristics. IE-AMB-0122-00003 Date of preparation: January 2022
RIGHT LEFT FRONT A B C
The Medicine Management Programme recommends AMGEVITA® as a citrate-free formulation of adalimumab.
AMGEVITA® 40mg SureClick® Pre-filled pen AMGEVITA® 20mg Pre-filled syringe AMGEVITA® 40mg Pre-filled syringe
Prescribing AMGEVITA® has lead to significant savings for the health service, in the order of millions of euros1,2,3
Recognising World Hypertension Day
Written by Clodagh McCarthy, Health Information Manager, Irish Heart Foundation

blood pressure through regular checks and small changes to their lifestyle, if needed.
Fieldwork for this research will start in 2024 and results are anticipated to coincide with the final year of the three-year campaign.
Hypertension (high blood pressure) often goes unnoticed but is among the leading modifiable risk factors for cardiovascular disease and premature death worldwide. The prevalence, consequences, disease risk and associated mortality of hypertension place a considerable burden not only on individuals and but on health service resources.
Despite being the leading modifiable risk factor for cardiovascular disease and premature death, research has shown that Ireland has one of the lowest rates of awareness, treatment and control of hypertension among 12 highincome countries. Indeed, a review of The Irish Longitudinal Study on Ageing (TILDA) in the Journal of Public Health in 2016 reported that 64% of over-50s had high blood pressure and, worryingly, 45% of those were unaware of their condition. Of those with high blood pressure, 59% were taking medication to reduce it, but almost half of those did not actually have their blood pressure controlled to normal levels.
World Hypertension Day is on 17 May. This year the Irish Heart Foundation is collaborating with the Irish College of General Practitioners (ICGP) and the Irish Pharmacy Union (IPU) on a campaign to raise awareness of the causes and impact of hypertension and motivate people to get their blood pressure checked regularly.
The Irish Heart Foundation’s campaign will remind the public that hypertension is a major risk factor for cardiovascular disease (including stroke and heart attack), cognitive decline (including dementia) and kidney disease. It will also inform people of common modifiable risk factors, namely a diet high in salt but low in fruit and vegetables, overweight, high alcohol intake and low physical activity.
Research shows there are significant benefits to hypertension treatment. A major systematic review in the Lancet found that in the populations studied, every 10mmHg reduction in blood pressure resulted in:
• a 17% reduction for coronary heart disease
• a 27% reduction for stroke
• a 28% reduction for heart failure
• a significant 13% reduction in all-cause mortality.
These striking figures point not only to the need for, but also the enormous benefit of, a campaign to help people take control of their
Predicting the ADHD Risk
Targeted at undiagnosed adults aged 50 and over, the campaign will run for three years. It will comprise:
• radio and online ads to encourage people to get their blood pressure checked
• a range of health information on understanding and managing hypertension on irishheart.ie
• posters and flyers, which are being informed by focus groups with underserved communities and will be distributed to community organisations, pharmacies and health centres nationwide.
Another significant element of the campaign will be the availability of free blood pressure checks through the Irish Heart Foundation’s mobile health unit (MHU). The MHU, staffed by nurses, conducts about 10,000 opportunistic blood pressure checks a year in mainly disadvantaged, hard to reach community settings such as men’s sheds, family resource centres and Traveller groups around Ireland. In a three-year period up to 2019, almost one in three (31.6%) health check participants identified with high blood pressure were unaware of having this condition before attending the MHU.
An additional arm of the campaign will be a partnership with The Irish Longitudinal Study on Ageing (TILDA) to assess long-term awareness and behaviour change in relation to hypertension in TILDA’s next wave of research.
Primary care is central to managing hypertension, with multiple opportunities to test and adjust treatment recommendations. Currently, initial blood pressure management and treatment decisions most frequently take place in general practice (involving GPs and wider practice staff), while wider primary care (in particular pharmacy) plays a role supporting adherence to medicines. Recently, there has been a major push to transform healthcare delivery with a focus on achieving better integrated, coordinated, high-value care. The campaign is timely given the recent publication of the HSE’s Second Report of the Structured Chronic Disease Management Programme, which demonstrated reduction in mean population blood pressure readings for those in the programme.
For hypertension in particular, high quality care requires patient awareness of preventive care, regular blood pressure screening, effective communication between healthcare providers and patients and active self-management by patients. The upcoming campaign, led by the Irish Heart Foundation and supported by the ICGP and IPU, presents an opportunity for NGO advocacy, medical expertise and health service engagement to join forces for long-term changes in awareness, behaviours and positive health outcomes.
For more information see www.irishheart.ie
Information available at birth may help to identify children with higher likelihood of developing ADHD, according to new research from RCSI University of Medicine and Health Sciences.
The study, published in Development and Psychopathology, examined data from almost 10,000 children in the United States, showing information about pregnancy and birth may help to help predict the extent of ADHD symptoms in childhood.
The Adolescent Brain Cognitive Development (ABCD) study is an ongoing study of children in the US, born between 2005 and 2009. Children were enrolled to the study at age 9–10 and their parents were asked about aspects of the pregnancy and birth, as well as their child’s current mental health.
The RCSI researchers identified 40 factors that would typically be known by birth, including the sex of the baby, the age of the parents, any complications during the pregnancy or delivery, and the baby’s exposure in the womb to factors such as cigarette smoke. Using machine learning and statistical techniques, the researchers found that 17 of the 40 factors were particularly good at predicting the number of ADHD symptoms in childhood. Co-lead researcher, Dr Niamh Dooley from the RCSI Department of Psychiatry, explained that few studies to date have looked at how prenatal and birth information could be useful in predicting ADHD: “We know that certain events during our time in the womb can have long-lasting consequences for our health. But not many studies have tried to quantify just how useful prenatal information could be to predicting childhood ADHD symptoms. We focused on readily available information about pregnancies and births, the kind that would be in antenatal records. This ensures our results can be compared to other studies using medical records and that they are relevant to public health.
“The other key element of this study was acknowledging the contribution of social, economic, and demographic factors to maternal and child health. For instance, prenatal information did not predict ADHD symptoms equally across the sexes, family income brackets, or racial/ethnic groups,” Dr Dooley said.
20 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Hypertension
The first and only treatment indicated to reduce:
• all-cause mortality
• frequency of CV-related hospitalisations in patients with wild-type or hereditary ATTR-CM.1
ATTR-CM=transthyretin amyloid cardiomyopathy; CV=cardiovascular.
References:
2. VYNDAQEL Summary of Product Characteristics. Vyndaqelq 61 mg soft capsules (tafamidis) Prescribing Information: Before prescribing Vyndaqel please refer to the full Summary of Product Characteristics. Presentation: Vyndaqel 61 mg soft capsules. Each soft capsule contains 61 mg tafamidis. Uses: Vyndaqel is indicated for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). Dosage: Treatment should be initiated under the supervision of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. When there is a suspicion in patients presenting with specific medical history or signs of heart failure or cardiomyopathy, etiologic diagnosis must be done by a physician knowledgeable in the management of amyloidosis or cardiomyopathy to confirm ATTR-CM and exclude AL amyloidosis before starting Vyndaqel, using appropriate assessment tools such as: bone scintigraphy and blood/urine assessment, and/or histological assessment by biopsy, and transthyretin (TTR) genotyping to characterise as wild-type or hereditary. The recommended dose is one capsule of Vyndaqel 61 mg (tafamidis) orally once daily. Vyndaqel 61 mg (tafamidis) corresponds to 80 mg tafamidis meglumine, tafamidis and tafamidis meglumine are not interchangeable on a per mg basis. Vyndaqel should be started as early as possible in the disease course when the clinical benefit on disease progression could be more evident. Conversely, when amyoid-related cardiac damage is more advanced, such as in NYHA Class III, the decision to start or maintain treatment should be taken at the discretion of a physician knowledgeable in the management of patients with amyloidosis or cardiomyopathy. There are limited clinical data in patients with NYHA Class IV. If vomiting occurs after dosing, and the intact Vyndaqel capsule is identified, then an additional dose of Vyndaqel should be administered if possible. If no capsule is identified, then no additional dose is necessary, with resumption of dosing the next day as usual. There are no recommended dosage adjustments for elderly patients or patients with renal or mild and moderate hepatic impairment. Limited data are available in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min). Tafamidis has not been studied in patients with severe hepatic impairment and caution is recommended. There is no relevant use of tafamidis in the paediatric population. Method of Administration: The soft capsules should be swallowed whole and not crushed or cut. Vyndaqel may be taken with or without food. Contra-indications: Hypersensitivity to the active substance or to any of the excipients as listed in section 6.1 of SPC. Warnings and Precautions: Contraceptive measures should be used by women of childbearing potential during treatment with tafamidis and for one month after stopping treatment. Tafamidis should be added to the standard of care for the treatment of patients with transthyretin amyloidosis. Physicians should monitor patients and continue to assess the need for other therapy, including the need for organ transplantation, as part of this standard of care. As there are no data available regarding the use of tafamidis in organ transplantation, tafamidis should be discontinued in patients who undergo organ transplantation. Increase in liver function tests and decrease in thyroxine may occur. This medicinal product contains no more than 44 mg sorbitol in each capsule. Sorbitol is a source of fructose. The additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of
1. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016.
sorbitol (or fructose) should be taken into account. The content of sorbitol in medicinal products for oral use may affect the bioavailability of other medicinal products for oral use administered concomitantly. Pregnancy and Lactation: Tafamidis is not recommended during pregnancy and in women of childbearing potential not using contraception.Available data in animals have shown excretion of tafamidis in milk.A risk to the newborns/infants cannot be excluded. Vyndaqel should not be used during breastfeeding. Interactions: In a clinical study in healthy volunteers, 20 mg tafamidis meglumine did not induce or inhibit the cytochrome P450 enzyme CYP3A4. In vitro tafamidis inhibits the efflux transporter BCRP (breast cancer resistant protein) at the 61 mg/day tafamidis dose with IC50=1.16 μM and may cause drug-drug interactions at clinically relevant concentrations with substrates of this transporter (e.g. methotrexate, rosuvastatin, imatinib). In a clinical study in healthy participants, the exposure of the BCRP substrate rosuvastatin increased approximately 2-fold following multiple doses of Page 2 of 2 2020-0065522 61 mg tafamidis daily dosing. Likewise, tafamidis inhibits the uptake transporters OAT1 and OAT3 (organic anion transporters) with IC50=2.9 μM and IC50=2.36 μM,respectively,and may cause drug-drug interactions at clinically relevant concentrations with substrates of these transporters (e.g. non-steroidal anti-inflammatory drugs, bumetanide, furosemide, lamivudine, methotrexate, oseltamivir, tenofovir, ganciclovir, adefovir, cidofovir, zidovudine, zalcitabine). Based on in vitro data, the maximal predicted changes in AUC of OAT1 and OAT3 substrates were determined to be less than 1.25 for the tafamidis 61 mg dose, therefore, inhibition of OAT1 or OAT3 transporters by tafamidis is not expected to result in clinically significant interactions. No interaction studies have been performed evaluating the effect of other medicinal products on tafamidis. Undesirable Effects: The following adverse events were reported more often in 176 ATTR-CM patients treated with tafamidis meglumine 80 mg compared to placebo: flatulence [8 patients (4.5%) versus 3 patients (1.7%)] and liver function test increased [6 patients (3.4%) versus 2 patients (1.1%)]. A causal relationship has not been established. Safety data for tafamidis 61 mg are not available as this formulation was not evaluated in the double-blind, placebo-controlled, randomised phase 3 study. Legal category: S1A. Marketing Authorisation Numbers: EU/1/11/717/003– 61mg (30 capsules). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at EUMEDINFO@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500. Last revised: 04/2021 q This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Ref: VY 61MG 2_0
Further information available upon request.
PP-VYN-IRL-0159. Date of Preparation: September 2022. © 2022 Pfizer Inc. All rights reserved. Rare Disease ATTR-CMIS LIFE-THREATENING1
ORAL VYNDAQEL 61MG SOFT CAPSULES
Indicated for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). 2
Bowel Cancer
Bowel Cancer Awareness at the Irish Cancer Society
Written by Aoife McNamara, Education and Engagement Manager, Irish Cancer Society

is available on 1800 200 700 for anyone with a cancer-related query or concern.
The research landscape
April is Bowel Cancer Awareness month and as part of our national awareness campaign, the Irish Cancer Society (the Society) is urging people to be aware of the signs and symptoms of the disease. According to the National Cancer Registry Ireland, nearly 2,600 people in Ireland are diagnosed with bowel cancer each year. Bowel cancer is one of the most common cancers in Ireland; it is the second most common cancer amongst men, and the third most common amongst women. As with all cancers, the earlier you are diagnosed, the more treatment options available to you and the better your chance of cure. Most recent official data shows that 483 fewer cases of bowel cancers were registered in the first year of the pandemic, compared to what was expected. Clinicians are now seeing more advanced diagnoses, patients that possibly delayed going to their GP during the pandemic.
Early detection is Vital
Due to the embarrassing nature of the signs and symptoms of the disease, delayed diagnosis has unfortunately been a long-standing issue. We are urging the public to go to their GP, if they notice any changes in their bowel habit. Changes including;
• A lasting change in your bowel habit – going more often, looser motions or constipation.
• Blood in your poo or bleeding from your back passage.
• Pain or discomfort in your tummy area or back passage.
• Trapped wind or fullness in your tummy.
• A lump in your tummy area or rectum.
• Feeling you have not emptied your bowel fully after going to the toilet.
• Unexplained weight loss.
• Feeling tired and breathless due to anaemia (fewer red blood cells).
We have developed a bowel online checker tool that can be accessed on www.Cancer.ie/ Bowel This tool is designed to help the public assess their bowel health. It can generate a report which can then be brought to their GP and be used as a prompt for a conversation about bowel health.
Bowel cancer is primarily a disease of older people, but the landscape is changing as more and more younger people present with symptoms. It is vital that everyone is bowel aware and acts on signs and symptoms. Separate to symptom awareness, another key part of early detection is engaging in screening. During April, BowelScreen, Ireland’s national bowel cancer screening programme, is reminding people to complete the simple at-home test that could save their life. BowelScreen offers a free athome test to people aged from 60-69. The test involves taking a small sample of your stool (poo) using a Faecal Immunochemical Test or FIT kit that is sent to your home, and returned to BowelScreen in the post. It’s done in the privacy of your own bathroom. More information is available at: https://www2.hse.ie/ conditions/bowel-screening/
There are risk factors that increase your chance of developing bowel cancer, age, family history of bowel cancer and inherited bowel conditions, obesity, poor diet and excess alcohol. The Irish Cancer Society Freephone Support Line

Through the support of the public and our donors, the Irish Cancer Society is the largest voluntary funder of cancer research in Ireland and we are committed to improving the lives of those impacted by cancer in Ireland through research. In the arena of bowel cancer, we currently fund two exciting studies. Rebecca O’Brien, Irish Cancer Society PhD Researcher of the Year 2023, is a final year PhD Scholar based in Trinity College Dublin. Rebecca’s PhD project investigates the role of immune proteins, called complement proteins, in bowel cancer. Unfortunately, many bowel cancers do not respond to current treatments, and the exact reasons behind this resistance are poorly understood. Rebecca is
particularly interested in how these immune proteins and immune cells may affect treatment response in bowel cancer. In addition, Rebecca’s research also looks at whether the amount of these proteins in the blood of bowel cancer patient may help to predict resistance to treatment.
Dr Grainne O’Kane, is a medical oncologist with a wealth of experience in gastrointestinal cancer research, based in Trinity St. James’s Cancer Institute. One of the areas of focus of Grainne’s research is bowel cancer. Grainne aims to improve treatment options for bowel cancer patients in Ireland, including providing world class treatment options. Grainne’s research aims to increase the availability of novel clinical trials to patients with bowel cancer in Ireland, to ensure each patient has access to world-class treatment options.
Do you have bloating or trapped wind?
Do you have a lump or pain
in your tummy or back passage?
Does your bowel still feel full
after you go to the toilet?
Are you going to the toilet more?
Do you have diarrhoea or constipation?
Have you lost weight without trying?
Is there blood in your poo?
Is it darker than usual?
22 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Call our Support Line on Freephone 1800 200 700 or email supportline@irishcancer.ie Bowel
know what to look for
Cancer
RCPI Academy Holds Inaugural Meeting at No. 6 Kildare Street
The Royal College of Physicians held its Inaugural Meeting of the RCPI Academy.

The meeting brought together over 46 retired or semi-retired consultants across a variety of different specialties, sharing years of experience.
The aim of the group is to assemble these retired members to share their wealth of knowledge, skills and expertise working in healthcare and pass it on in several ways, including through the mentorship of earlystage consultants, examinations, education, college representation and volunteer work.
The Academy is also for those who are interested in connecting and engaging with other Members and Fellows.
Prof Trevor Duffy, Director of Healthcare Leadership at RCPI, highlighted the importance of such a group, sharing its key message: Medicine is an everchanging landscape where one can continue life-long learning, which certainly does not stop after retirement.
Following Prof Duffy’s address, the group heard from four speakers, who all shared the unique and inspiring ways they are spending their retirement:

Prof Frank Murray shared his experience with the EQUALS Initiative, which seeks to improve healthcare in lowincome countries by working in
partnership with less developed countries and addressing the huge inequality that exists in health globally through:
1. Sourcing and donating good quality equipment which becomes freely available from Irish hospitals to hospitals in Zambia, in response to local need
2. Supporting the training of biomedical engineers
3. Supporting the development of postgraduate medical training for specialist physicians
4. The initiative began in 2013 when Prof Murray received a request from an Irish doctor in Zambia asking to help source an ultrasound machine for Monze Mission Hospital. From here, RCPI and the HSE established the EQUALS Initiative.
5. RCPI is calling for other retired doctors to get involved and join the academy.
6. If you are interested or would like to receive communications about upcoming Academy events, please contact fionamckenna@rcpi.ie
Novel Gene Therapy for Glaucoma
Experts are projecting an increase in those affected to over 110 million by 2040. While topical eye-drops are critical in preventing disease progression, up to 10% of patients become treatment resistant, putting them at risk of permanent vision loss.
The main clinical risk factor for glaucoma is elevated intraocular pressure; dangerous increases in pressure in the globe of the eye can lead to serious damage to the optic nerve head, which transmits light signals to the brain to allow us to see. This elevated pressure is caused by a build-up of unwanted proteins causing a blockage in drainage channels that, over time, can cause fluid to accumulate and pressure to increase.
Here, the team at the Smurfit Institute of Genetics,
in collaboration with the biotechnology company Exhaura Ltd, have shown that a gene therapy-based approach can decrease intraocular pressure in pre-clinical models of glaucoma. A single injection of a viral vector – essentially a virus the scientists have hijacked with the purpose of using it to deliver specific instructions to cells in the body –can increase the flow of aqueous fluid from the front of the eye and thereby decrease pressure in the eye. The key instructions are for cells to produce an enzyme matrix (metalloproteinase-3, or MMP-3) that helps kick this process into gear.
“This exciting project allowed us to bridge the gap between academia and industry and work very closely with a gene therapy company to
develop a cutting edge therapy that we believe holds immense promise for patients in the future,” said Professor Matthew Campbell, Professor in Genetics at Trinity. Importantly, the work used multiple models of disease as well as making use of donor human eyes to screen the therapeutic efficacy of the gene therapy approach. This makes the impressive results all the more promising.
From rare to common diseases
Gene therapies have seen a dramatic advance in recent years, with multiple drugs now approved by both the FDA and EMA. However, to date, all of the approved gene therapies are for the treatment of rare or ultra-rare conditions. As our understanding of the underlying mechanism of common diseases is now
becoming more evolved, the concept of using gene therapy for common diseases is now possible.
Dr Jeffrey O’Callaghan, Postdoctoral research fellow at Trinity and first author of the study, added, “Our novel approach to treating glaucoma using gene therapy is the culmination of over seven years of research. We are now hopeful that this therapy will pave the way to the development of treatments for other forms of blinding eye diseases.”
A multidisciplinary team of geneticists, ophthalmologists and translational biologists undertook the study. The close collaboration with Exhaura Ltd also allowed the team to direct all experimental outputs towards the translation of the findings to a regulatory focused clinical programme.
23 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
News
Professor Frank Murray shared his experience with EQUALS Initiative. (via Bobby Studios)
Professor Trevor Duffy welcomes attendees to the RCPI Academy Inaugural Meeting. (via Bobby Studios)
Sepsis: a guide for pharmacists
 Written by Fleming A1, Horgan S2, Quigley L3, McCarthy D.4
Written by Fleming A1, Horgan S2, Quigley L3, McCarthy D.4
1 Lecturer in Clinical Pharmacy, School of Pharmacy, University College Cork



2 Sepsis ADoN South/South Western Hospital Group HSE
3 Programme Manager, National Clinical Programme for Sepsis
4 Sepsis CNM3 South/South Western Hospital Group HSE
Sepsis claims more lives than lung cancer, and more than breast cancer, bowel cancer and HIV/ AIDs combined.
Sepsis is a time dependent medical emergency where early recognition and prompt treatment is fundamental to increased survival rates and optimal outcome for survivors of sepsis and septic shock. Sepsis is defined as life threatening organ dysfunction caused by a dysregulated host response to infection (Singer et al., 2016). The majority of sepsis is community acquired and approximately 70-80% of patients with sepsis present to the Emergency Department. Sepsis can affect anyone at any age however, 90% of adults and 70% of children who develop sepsis have underlying risk factors (CDC, 2016). Lack of knowledge and awareness can result in late patient presentations and delayed treatment. The infection can occur at any site and be caused by any pathogen – the most common site affected being the respiratory tract followed by the abdomen, then urinary tract. Pregnancy increases vulnerability especially to viral illnesses, while in late pregnancy the genito-urinary tract is the most common site.
Epidemiology
According to the World Health Organisation The Global report on the Epidemiology and Burden of Sepsis 2020, 20% of global deaths are attributed to sepsis (WHO 2020). There were 11,294 cases of sepsis documented in Ireland
in 2020 with an average hospital sepsis-associated mortality rate of 20.1%, 26.5% of which were admitted to a critical care area (HSE 2022). The average crude mortality rate of these patients admitted to a critical care area was 30.8%.
Whilst sepsis incidence increases with age in adults (Figure 1), mortality peaks at the extremes of age. Age > 75years is considered high risk for sepsis mortality, with >20% crude mortality rate. With ageing, co-morbidities are accumulated and the immune system gradually deteriorates leading to increases in both incidence and mortality.
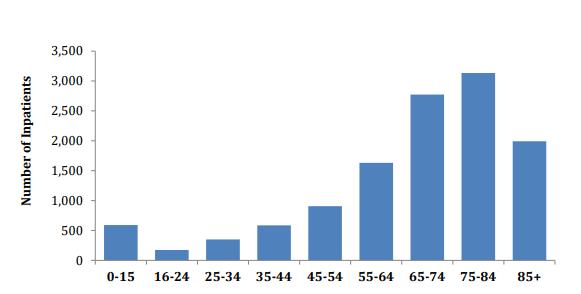
Sepsis Pathway:
The pathology of sepsis involves a complex pathway whereby the normal response to infection is not sufficient to eliminate the local infection. The inflammatory cascade is ramped up and the
signs and symptoms of the Systemic Inflammatory Response (SIRS) manifest i.e. high or low temperature, tachypnoea and tachycardia. The mechanism by which organ dysfunction is triggered is poorly understood. If this process is interrupted with medical intervention (i.e. resuscitation, source control, intravenous antimicrobial therapy) and the infection got under control there can be a rapid return of organ function. Later recognition makes the condition more difficult to treat. A hypo-inflammatory response can then ensue, leading to persistence of infection, secondary infection, worsening organ dysfunction and potentially death.
Screening for Sepsis
Screening for sepsis using a screening tool, as developed by the National Sepsis Programme,
allows for prompt identification of patients with a high-risk presentation e.g. clinically apparent acute organ dysfunction such as acute confusion, respiratory failure or a purpuric rash AND patients who are high risk because of their medical history, e.g. on chemotherapy or having chronic co-morbidities or additional factors such as frailty, age ≥75 and recent trauma or surgery (Figure 2). A further clinical decision support tool, known as the Sepsis Six Bundle, provides guidance on treatment and escalation of care if deterioration.

Community pharmacists are one of the first points of contacts when people present unwell. Therefore, all healthcare professionals need to ask “Could this be sepsis?”. Knowledge of the signs and symptoms of sepsis, prompt diagnosis and early treatment has been shown to improve patient outcomes (Department of Health, 2021).
How to recognise Sepsis

While the above description presents a picture of a critical illness that requires secondary care admission and medical attention, it is vitally important that all healthcare professionals in all settings are aware of and educated on sepsis. Table 1 below highlights underlying conditions that increase the risk of sepsis in adults and children.
As sepsis is often hard to diagnose in the early stages, it is very important to watch for the signs of sepsis which might develop if
24 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Sepsis
Aoife Fleming
Sinead Horgan Lorna Quigley
Denise McCarthy
Figure 1. The number of inpatients with a diagnosis of sepsis by age group 2020. (Includes maternity and paediatrics) National Sepsis Outcome Report, Ireland 2020.
Sepsis Pathway:
The pathology of sepsis involves a complex pathway whereby the normal response to infection is not
Figure 1. The number of inpatients with a diagnosis of sepsis by age group 2020. (Includes maternity and paediatrics) National Sepsis Outcome Report, Ireland 2020.
MAKE TIME
for more moments that matter Extend overall survival for patients in 3rd line mCRC1
trifluridine/tipiracil
Lonsurf® (Trifluridine/ tipiracil): Abbreviated Prescribing Information: Please refer to the Summary of Product Characteristics before prescribing COMPOSITION*: Lonsurf 15 mg/6.14 mg: film-coated tablet containing 15 mg trifluridine and 6.14 mg tipiracil (as hydrochloride). Lonsurf 20 mg/8.19 mg: film-coated tablet containing 20 mg trifluridine and 8.19 mg tipiracil (as hydrochloride). INDICATION*: As monotherapy for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents. As monotherapy for the treatment of adult patients with metastatic gastric cancer including adenocarcinoma of the gastroesophageal junction, who have been previously treated with at least two prior systemic treatment regimens for advanced disease. DOSAGE AND ADMINISTRATION*: Recommended starting dose: 35 mg/m2/dose taken orally twice daily on Days 1 to 5 and Days 8 to 12 of each 28-day cycle, within 1 hour after completion of the morning and evening meals (20mg/m2/dose for patients with severe renal impairment). Dosage calculated according to body surface area, not exceeding 80 mg/dose. Possible dosing adjustments based on individual safety and tolerability: permitted dose reductions to a minimum dose of 20 mg/m2 twice daily (15mg/m2/dose for patients with severe renal impairment), dose escalation not permitted after a dose reduction. CONTRAINDICATIONS*: Hypersensitivity to the active substances or to any of the excipients. WARNINGS *: Bone marrow suppression: Complete blood cell counts must be obtained prior to initiation of therapy, prior to each cycle and as needed. Treatment must not be started if absolute neutrophil count < 1.5 x 109/L, if platelet counts < 75 x 109/L, or if unresolved Grade 3 or 4 nonhaematological clinically relevant toxicity. Patient should be monitored closely for infections, appropriate measures should be administered as clinically indicated. Gastrointestinal toxicity: anti-emetic, anti-diarrhoeal and other measures should be administered as clinically indicated, dose modifications should be applied as necessary. Renal impairment: not recommended if end-stage renal disease. Patients with renal impairment should be monitored closely; patients with moderate or severe renal impairment should be more frequently monitored for haematological toxicities. Hepatic impairment: not recommended if baseline moderate or severe hepatic impairment. Proteinuria: monitoring by dipstick urinalysis recommended prior to starting and during therapy. Excipients: contain lactose. INTERACTIONS*: Precautions: medicinal products that interact with nucleoside transporters CNT1, ENT1 and ENT2, inhibitors of OCT2 or MATE1, human thymidine kinase substrates (e.g. zidovudine), hormonal contraceptives. FERTILITY*. PREGNANCY AND BREASTFEEDING*: Not recommended. CONTRACEPTION*: For women and men, highly effective contraceptive measures must be used during treatment and for 6 months after stopping treatment. DRIVE & USE MACHINES*: Fatigue, dizziness or malaise may occur. UNDESIRABLE EFFECTS*: Very common: Neutropenia, leukopenia, anaemia, thrombocytopenia, decreased appetite, diarrhoea, nausea, vomiting, fatigue. Common: Lower respiratory tract infection, febrile neutropenia, lymphopenia, hypoalbuminaemia, dysgeusia, neuropathy peripheral, dyspnoea, abdominal pain, constipation, stomatitis, oral disorder, hyperbilirubinaemia, Palmar-plantarerythrodysaesthesia syndrome, rash, alopecia, pruritus, dry skin, proteinuria, pyrexia, oedema, mucosal inflammation, malaise, hepatic enzyme increased, blood alkaline phosphatase increased, weight decreased. Uncommon: Septic shock, enteritis infectious, lung infection, biliary tract infection, influenza, urinary tract infection, gingivitis, herpes zoster, tinea pedis, candida infection, bacterial infection, infection, neutropenic sepsis, upper respiratory tract infection, conjunctivitis, cancer pain, pancytopenia, granulocytopenia, monocytopenia, erythropenia, leukocytosis, monocytosis, dehydration, hyperglycaemia, hyperkalaemia, hypokalaemia, hypophosphataemia, hypernatraemia, hyponatraemia, hypocalcaemia, gout, anxiety, insomnia, neurotoxicity, dysaesthesia, hyperaesthesia, hypoaesthesia, syncope, paraesthesia, burning sensation, lethargy, dizziness, headache, visual acuity reduced, vision blurred, diplopia, cataract, dry eye, vertigo, ear discomfort, angina pectoris, arrhythmia, palpitations, embolism, hypertension, hypotension, flushing, pulmonary embolism, pleural effusion, rhinorrhoea, dysphonia, oropharyngeal pain, epistaxis, cough, enterocolitis haemorrhagic, gastrointestinal haemorrhage, pancreatitis acute, ascites, ileus, subileus, colitis, gastritis, reflux gastritis, oesophagitis, impaired gastric emptying, abdominal distension, anal inflammation, mouth ulceration, dyspepsia, gastrooesophageal reflux disease, proctalgia, buccal polyp, gingival bleeding, glossitis, periodontal disease, tooth disorder, retching, flatulence, breath odour, hepatotoxicity, biliary dilatation, skin exfoliation, urticaria, photosensitivity reaction, erythema, acne, hyperhidrosis, blister, nail disorder, joint swelling, arthralgia, bone pain, myalgia, musculoskeletal pain, muscular weakness, muscle spasms, pain in extremity, renal failure, cystitis noninfective, micturition disorder, haematuria, leukocyturia, menstrual disorder, general physical health deterioration, pain, feeling of body temperature change, xerosis, discomfort, blood creatinine increased, electrocardiogram QT prolonged, international normalised ratio increased, activated partial thromboplastin time prolonged, blood urea increased, blood lactate dehydrogenase increased, protein total decreased, C-reactive protein increased, haematocrit decreased. Post-marketing experience: interstitial lung disease. OVERDOSE* PROPERTIES*: Trifluridine is an antineoplastic thymidine-based nucleoside analogue and tipiracil hydrochloride is a thymidine phosphorylase (TPase) inhibitor. Following uptake into cancer cells, trifluridine, is phosphorylated by thymidine kinase, further metabolised in cells to a deoxyribonucleic acid DNA substrate, and incorporated directly into DNA, preventing cell proliferation. However, trifluridine is rapidly degraded by TPase and readily metabolised by a first-pass effect following oral administration, hence the inclusion of the TPase inhibitor, tipiracil hydrochloride.PRESENTATION* Pack of 20 or 60 film-coated tablets. Marketing Authorisation Holder LES LABORATOIRES SERVIER, 50 rue Carnot, 92284 Suresnes cedex France. www.servier.com Marketing Authorisation: EU/1/16/1096/001-006. Legal Classification for Supply: POM. Further information available from: Servier Laboratories (Ireland) Ltd., Second Floor, 19 Lr. George’s Street, Dun Laoghaire, Co. Dublin A96 ER84, Ireland, Tel (01) 6638110, www.servier.ie
Date of last revision of text: January 2021 (date of last approved SmPC: December 2020)

*For complete information, please refer to the Summary of Product Characteristics available on medicines.ie.
Date of preparation of item January 2023. 2223c1LNPressAd A4

and therapy rationalisation then depends on the patient’s clinical response.
By 3 hours from time zero, the patient’s status should be reviewed and care escalated if they are failing to stabilise or deteriorating. By 6 hours of ongoing review and treatment, patients who have failed to stabilise may require pressors and/or critical care input and may now be suffering from septic shock. The latest National Sepsis Outcome Report 2020 is available at: https://www.hse.ie/eng/about/ who/cspd/ncps/sepsis/resources/ national-sepsis-report-2020.pdf. This report presents data on the burden of sepsis in the acute settings, in terms of the number of cases and associated mortality, and compares the data to previous years.
The role of the pharmacist
the infection is aggressive or is not responding to the initial treatment. Signs and symptoms of sepsis may vary, patients with infection who are deteriorating despite usual care or who develop any signs of acute organ dysfunction (Table 2) need to be reviewed urgently by a doctor. Sepsis is only diagnosed if infection is suspected or proven to be the cause of these signs.
1. Recognition and escalation to medical review
How to recognise Sepsis
Table 2. Signs of new onset organ dysfunction in adults, and sepsis in paediatric patients.
2. Diagnosis and initiation of treatment
healthcare professionals. The National Sepsis Programme provides education aids, posters, presentations and material all available and supported by its website www.hse.ie/sepsis. Sepsis education modules have also been developed and are available on HSELand, the Irish health service national online learning and development portal. It is recommended that all relevant Healthcare Professionals undertake the sepsis eLearning and that it is refreshed on a 3 yearly basis.
Community pharmacists are one of the first points of contacts when people present unwell. Therefore, all healthcare professionals need to ask “Could this be sepsis?”. Knowledge of the signs and symptoms of sepsis, prompt diagnosis and early treatment has been shown to improve patient outcomes (Department of Health, 2021).
Advice is often sought from pharmacists in both the community and hospital settings, with community pharmacists often being the first port of call for patients when they feel unwell. Pharmacists can support sepsis management and improved patient quality of care in the following ways;
1. BE AWARE OF THE RISK FACTORS:
• See Table 1
Management of Sepsis
While the above description presents a picture of a critical illness that requires secondary care admission and medical attention, it is vitally important that all healthcare professionals in all settings are aware of and educated on sepsis. Table 1 below highlights underlying conditions that increase the risk of sepsis in adults and children.
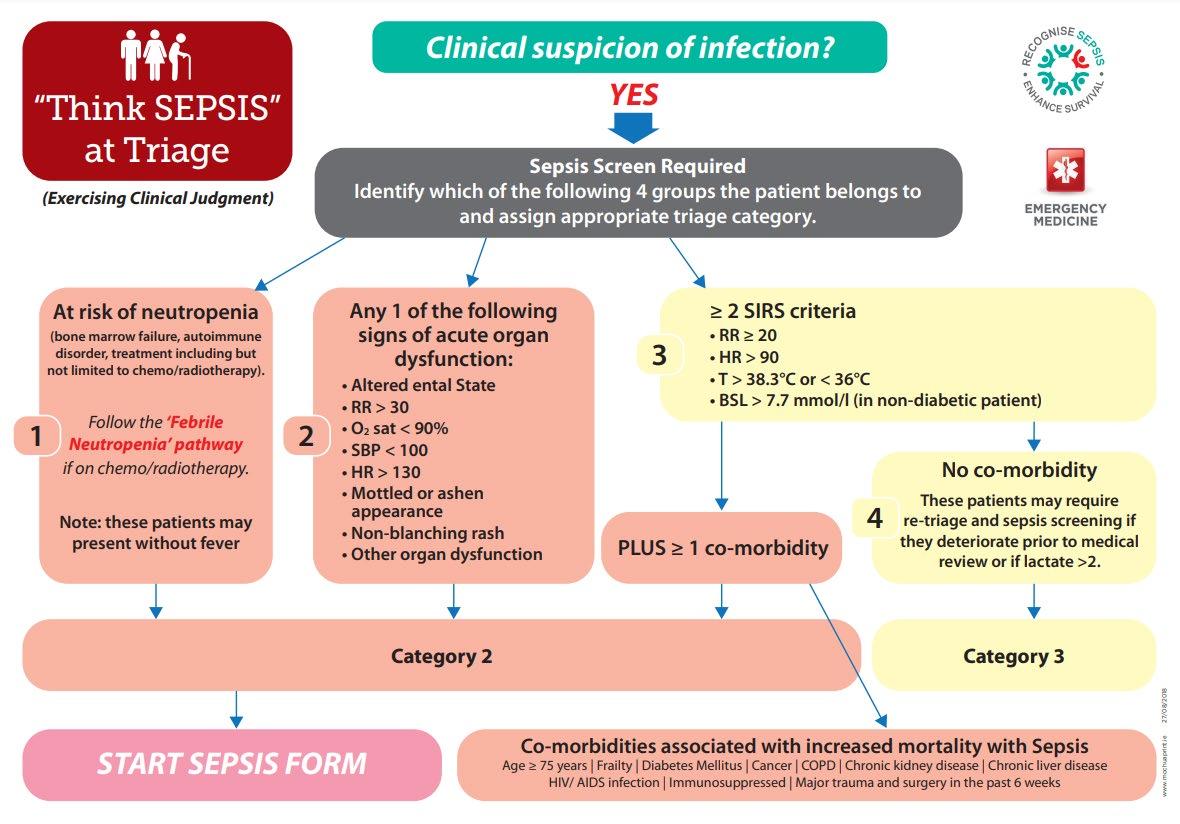
The National Clinical Guideline No.26 Sepsis Management was updated and published in November 2021 by the National Clinical Effectiveness Group (DoH 2021), to standardise management of sepsis in the acute settings. This guideline is pivotal in raising education and awareness of sepsis among medical and nursing professionals as well as among practicing
In terms of active management of sepsis cases two time-dependent processes have been identified as essential for optimal patient care:
1. Recognition and escalation to medical review
2. Diagnosis and initiation of treatment
(time zero), the second time-dependent step is started which is the “Sepsis 6” 1 hour Bundle (Figure 3). The rapid management of sepsis is critical, each hour of delay to antimicrobial administration has been associated with an average decrease in survival of 7.6% in a retrospective cohort study by Kumar et al (2006). Empiric antimicrobial prescribing should be based on locally approved guidelines, the patient’s history of colonisation/infection with antimicrobial resistant organisms, allergy status and the site of infection as determined clinically. Antimicrobials should be reviewed after 24-48 hours by a senior clinician and based on microbiological culture results and clinical response, as per the ‘Start Smart, Then Focus campaign’. (https://www.hpsc. ie/a-z/microbiologyantimicrobialresistance/carebundles/antibioticcarebundle/File,14119,en.pdf.).
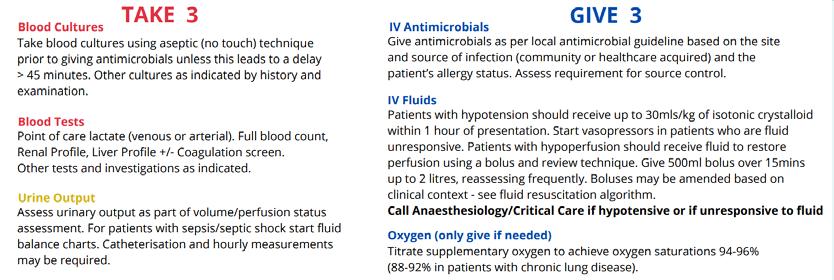
Once a diagnosis of sepsis is made, supported by medical review (time zero), the second timedependent step is started which is the “Sepsis 6” 1 hour Bundle (Figure 3). The rapid management of sepsis is critical, each hour of delay to antimicrobial administration has been associated with an average decrease in survival of 7.6% in a retrospective cohort study by Kumar et al (2006). Empiric antimicrobial prescribing should be based on locally approved guidelines, the patient’s history of colonisation/infection with antimicrobial resistant organisms, allergy status and the site of infection as determined clinically. Antimicrobials should be reviewed after 24-48 hours by a senior clinician and based on microbiological culture results and clinical response, as per the ‘Start Smart, Then Focus campaign’.
(https://www.hpsc.ie/az/microbiologyantimicrobialresistance/carebundles/antibioticcarebundle/File,14119,en.pdf.)
Once a diagnosis of sepsis is made, supported by medical review
Blood cultures are often negative (in up to 60% of sepsis cases)
• For Community pharmacists, refer patients for urgent medical attention if;
any of the alarm signals (Table 2)
patient has deteriorated while being treated for a systemic infection
patient is unwell without a clear cause
patient presents with deteriorating infection and also immunocompromised
2. SUPPORT PREVENTATIVE MEASURES:
• Infection Prevention and Control: Engage in appropriate infection prevention and control measures – See https://www. hse.ie/eng/about/who/nqpsd/ nirp/ncec-ipc-guideline-2022for-consultation.pdf.
26 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Sepsis
Figure 2 Sepsis Triage Screening Tool
Figure 2 Sepsis Triage Screening Tool
Figure 3. Sepsis 6, I hour Bundle; Take 3 and Give 3. Blood cultures are often negative (in up to 60% of sepsis cases) and therapy rationalisation then depends on the patient’s clinical response.
Figure 3. Sepsis 6, I hour Bundle; Take 3 and Give 3
Table 1. Risk factors for sepsis in adults, paediatrics and maternity Patient group
Risk factors
Adults Age ≥ 75 years
Frailty
Diabetes
Chronic obstructive pulmonary disease
Cancer
Chronic kidney disease
Chronic liver disease
Recent surgery, or wounds/injuries from accidents
HIV/ AIDS
On immunosuppressant medications - Chemotherapy/radiotherapy
- Anti-rejection medications
- Biological medications
- Corticosteroids - Methotrexate
Paediatrics Immunocompromised (follow national haematology/oncology guidelines for children with cancer)
Age ≤3 months
Chronic disease
Recent surgery
Break in skin (including chickenpox)
Indwelling line/device
Signs of infection in a wound (including chickenpox)
Incomplete vaccination record
Maternity Pregnancy Related:
Cerclage
Pre-term/prolonged rupture of membranes
Retained products
History pelvic infection
Group A Strep. infection in close contact
Recent amniocentesis
Non Pregnancy Related:
Age > 35 years
Minority ethnic group
Vulnerable socio-economic background
Obesity
Diabetes, including gestational diabetes
Recent surgery
Symptoms of infection in the past week
Immunocompromised e.g. Systemic Lupus
Chronic renal / liver / heart failure
• Vaccination: Encourage and provide vaccination services to ensure that at risk patients are protected from seasonal influenza infection, Covid-19 and pneumococcal pneumonia. Other vaccines are also available for a fee, e.g. chickenpox, shingles. Refer to https://www.hse.ie/eng/health/ immunisation/pubinfo/adult/ pneumo/ for more information. Encourage parents to ensure children are up-to-date with their primary immunisation schedule. https://www.hse.ie/eng/health/ immunisation/pubinfo/.
antimicrobials in all patients and limiting the emergence of antimicrobial resistance. The PAMS-Net has a bank of online resources, discussion fora and links to recorded and upcoming webinars to support appropriate antimicrobial stewardship.
For community pharmacists:
Refer to The HSE Guidelines for Antimicrobial Prescribing in Primary Care (www. antibioticprescribing.ie) when a patient is prescribed an antimicrobial.
Ensure patients are aware of the importance of not missing doses of antimicrobials, and finishing the course.
Stress to patient to contact GP/Pharmacy if troubling side effects to antimicrobial, as alternative may be available.
Stress to patient to re-present to GP/ED if not responding to treatment.
Provide advice when a patient presents with a self-limiting or viral infection and direct them to www.undertheweather.ie.
Manage the expectation of an antimicrobial for a likely viral illness or self-limiting infection, even if the service user may need to see the doctor if unwell.
Remind patients that any unused antimicrobials should be returned to the pharmacy for safe disposal.
For hospital pharmacists:
As sepsis is often hard to diagnose in the early stages, it is very important to watch for the signs of sepsis which might develop if the infection is aggressive or is not responding to the initial treatment. Signs and symptoms of sepsis may vary, patients with infection who are deteriorating despite usual care or who develop any signs of acute organ dysfunction (Table 2) need to be reviewed urgently by a doctor. Sepsis is only diagnosed if infection is suspected or proven to be the cause of these signs.
• Smoking Cessation: See National Clinical Guideline on Smoking Cessation at https://www.gov.ie/en/ publication/4828b-stopsmoking/. Cigarette smoking
has been associated with an increase in the risk of death from sepsis (HR=1.62; 95% CI: 1.11–2.37, p=0.01) (Zhang et al., 2022).
• Management of chronic conditions: Ensuring patients are complying with medications to treat chronic conditions, such as diabetes or kidney disease, so that their condition is well managed. This will reduce the risk of developing sepsis (CDC).
• Appropriate treatment of infection: Engage in active Antimicrobial Stewardship.
HSE AMRIC have developed an e-learning module on ‘Antimicrobial Stewardship in Practise’, available on HSEland. They have also developed Guidance on Antimicrobial Stewardship for all healthcare
workers, available at https:// www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ antibicrobial-stewardship-audittools/hse-amric-antimicrobialstewardship-guidance-forall-healthcare-settings-v1published-august-2022.pdf.
• The Irish Institute of Pharmacy have an online CPD programme Antimicrobial Stewardship for Community Pharmacists to outline the concepts of antimicrobial resistance and appropriate use of antimicrobials.
• The Pharmacist Antimicrobial Stewardship Network (PAMSNet), accessed via the IIOP portal, supports pharmacists across all sectors to work towards the common goal of promoting responsible use of
Ensure the patient is prescribed and administered the right antimicrobial therapy for infection or sepsis, in relation to choice, dose, route and duration as guided by local antimicrobial guidelines and specialist input.
Optimisation of antimicrobial prescribing based on microbiological sampling results.
Consider early use of source control – removal of infection devices, catheters, appropriate management of surgery, wound management procedures.
Review of the antimicrobial therapy according to the Start Smart then Focus care bundle at 24-48 hours, and on an ongoing basis. https://www.hse.ie/eng/ services/publications/clinicalstrategy-and-programmes/startsmart-stay-focused.pdf
If on review, sepsis or infection is not diagnosed, stop antibiotics as appropriate.
27 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Table 1. Risk factors for sepsis in adults, paediatrics and maternity
Table 2. Signs of new onset organ dysfunction in adults, and sepsis in paediatric patients. Adult – Signs of new onset organ dysfunction
Central Nervous System Agitation, confusion, drowsiness
Severe leg pain, unable to weight bear in adolescents
Patients with disabilities may not be functioning at usual level
Respiratory system
Cardiovascular system
Respiratory rate > 30 breaths per minute
Unable to finish sentences in one breath
Cyanosis/hypoxia
Heart rate > 130 beats per minute
Systolic blood pressure < 90 or > 40mmHg drop from usual level.
Unable to palpate radial pulse (by someone who usually can)
Sustained dizziness when upright (so unable to sit up comfortably)
Very pale, cold peripheries with prolonged central capillary refill
Venous lactate ≥ 2mmol/l
Renal system
No urinary output for > 12 hours and no urge to urinate
Very small amounts of very dark urine
In the patient with an indwelling urinary catheter if the urinary output is being measured hourly, a urinary output of < 0.5mls/kg/hr for a minimum of 2 hours
Coagulation system Non-blanching rash – this is a new rash that does not disappear when pressed on by glass; it can be petechial or blotchy.
Beware Campbell de Morgan spots/angiomas, which are nonblanching but are not acute
gov.ie/pdf/?file=https://assets. gov.ie/197784/2e897cca-3b574753-b420-c397474dd171. pdf#page=null
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016;353:i1585 doi:10.1136/bmj.i1585
Centre for Disease Control. CDC Vital Signs. Making Healthcare Safer. Think Sepsis. Time Matters 2016 [Online]. Available: https:// www.cdc.gov/vitalsigns/sepsis/ infographic.html
Health Service Executive, 2022. National Sepsis Outcome Report 2020. Available from: https://www. hse.ie/eng/about/who/cspd/ncps/ sepsis/resources/national-sepsisreport-2020.pdf
Paediatric – Signs of sepsis
Inappropriate tachycardia (if caused by a fever, pain or fear it should resolve with paracetamol and/or reassurance)
Inappropriate Tachypnoea (if caused by fear or exercise it will settle with reassurance)
Altered mental status (irritability, lethargy, floppiness, difficult to rouse)
Altered functional status
Reduced peripheral perfusion/ prolonged central capillary refill (cold extremities, pallor or cyanosis of extremities, oedema, oliguria)
Non-blanching rash
Health Service Executive. Guidelines for antimicrobial prescribing in primary care. Available from: https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/ McPherson D, Griffiths C, Williams M, Baker A, Klodawski A, Jacobson B, Donaldson L. Sepsisassociated mortality in England: an analysis of multiple cause of death data from 2001 to 2010 BMJ Open 2013;3:8.
Management of Sepsis
Evaluate the route of administration and promote early intravenous to oral switch where possible.
Consider patient factors such as interactions, allergies, renal/hepatic impairment, other conditions, age, recent antimicrobial use, or known infection or colonisation with AMR organisms, as they may influence the choice of agent
Liaise promptly with pharmacy procurement when alerted to supply issues in relation to antimicrobials that may cause delays in administration.
how to get better from common infections without the use of antibiotics.
• www.antibioticprescribing.ie for information on infections where antimicrobials have been prescribed.
• Information on vaccination and schedules is available here: https://www.hse.ie/eng/health/ immunisation/pubinfo/
Where appropriate, seek specialist advice from clinical microbiology, infectious diseases, or antimicrobial pharmacist.
The National Clinical Guideline No.26 Sepsis Management was updated and published in November 2021 by the National Clinical Effectiveness Group (DoH 2021), to standardise management of sepsis in the acute settings. This guideline is pivotal in raising education and awareness of sepsis among medical and nursing professionals as well as among practicing healthcare professionals. The National Sepsis Programme provides education aids, posters, presentations and material all available and supported by its website www.hse.ie/sepsis Sepsis education modules have also been developed and are available on HSELand, the Irish health service national online learning and development portal. It is recommended that all relevant Healthcare Professionals undertake the sepsis eLearning and that it is refreshed on a 3 yearly basis.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):80110. doi: 10.1001/jama.2016.0287. PMID: 26903338.
3. PROVIDE INFORMATION:
• Resources on sepsis, including patient information leaflets, are available at: https://www.hse. ie/eng/about/who/cspd/ncps/ sepsis/resources/
• www.undertheweather.ie, a HSE/ICGP/IPU collaboration that provides information on
• Consider the need for Infection Prevention and Control awareness in the long term care facility setting in collaboration with nursing and medical colleagues. (Hyperlink to https://www.hpsc.ie/a-z/ respiratory/coronavirus/novelcoronavirus/guidance/ infectionpreventionandcontrolguidance/ residentialcarefacilities/
Conclusion:
Pharmacists can play a key role in both preventing a patient developing sepsis and improving patient outcomes should it develop. In the community setting, pharmacists can help by educating themselves on sepsis,
and referring patients for urgent assessment if the patient has signs and symptoms. In the hospital setting, pharmacists can ensure optimal antimicrobial stewardship, and compliance with the National Clinical Guideline No 26 in Sepsis Management. Preventative measures in both settings, as well as providing information and education as appropriate will also help to both reduce the burden of sepsis on healthcare in Ireland and improve patient health outcomes. The National Sepsis Programme has developed sepsis patient information leaflets to raise public awareness of the signs and symptoms of sepsis and deterioration which are available on https://www.hse.ie/eng/about/ who/cspd/ncps/sepsis/resources/. The National Programme also strongly recommends that patients with risk factors be vaccinated for preventable infections as a measure to reduce sepsis incidence.
World Health Organisation. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. Geneva: World Health Organisation; 2020. CC BY-NC-SA
3.0 IGC. Available from: https:// www.who.int/publications/i/ item/9789240010789
In terms of active management of sepsis cases two time-dependent processes have been identified as essential for optimal patient care:
References
Department of Health (2021). Sepsis management for adults (including maternity). NCEC National Clinical Guideline No. 26. Available at: https://www.
HSE AMRIC AMS Guidance for all Healthcare settings, available at :https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/antibicrobial-stewardship-audit-tools/ hse-amric-antimicrobial-stewardship-guidance-for-all-healthcare-settings-v1-publishedaugust-2022.pdf.Zhang, N., Liu, Y., Yang, C., Zeng, P., Gong, T., Tao, L., & Li, X. (2022). Association between smoking and risk of death in patients with sepsis: A systematic review and meta-analysis. Tobacco induced diseases, 20.
28 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Sepsis
Table 1. Risk factors for sepsis in adults, paediatrics and maternity

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
More time to live the life they want
+ VTd offers newly-diagnosed transplant-eligible patients:










A 45% increase in rates of MRD-negativity* and a 53% reduction in risk of disease progression or death vs. VTd alone





20 mg/ml Concentrate for Solution for Infusion and 1 800 mg Solution for Injection PRESCRIBING INFORMATION
Daratumumab

Please refer to Summary of Product Characteristics (SmPC)
Newly diagnosed multiple myeloma: in combination with lenalidomide/dexamethasone or bortezomib/melphalan/ prednisone in adults, ineligible for autologous stem cell transplant; in combination with bortezomib, thalidomide and dexamethasone in adults, eligible for autologous stem cell transplant. Relapsed/ Refractory multiple myeloma: Monotherapy for adults whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who have demonstrated disease progression on last therapy. In combination with lenalidomide/dexamethasone or bortezomib/dexamethasone in adults who have received ≥ one Darzalex SC: in combination with pomalidomide and dexamethasone for the treatment of adult patients with multiple myeloma who have received one prior therapy containing a proteasome inhibitor and lenalidomide and were lenalidomiderefractory, or who have received at least two prior therapies that included lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or after the last therapy. in combination with cyclophosphamide, bortezomib and dexamethasone for the treatment of adult patients with newly diagnosed systemic light chain (AL) amyloidosis.
DOSAGE & ADMINISTRATION: Administration by healthcare professional where resuscitation facilities are available, intravenous (IV) infusion or subcutaneous (SC) injection. injection, resuscitation facilities required only for first dose.


16 mg/kg body weight. Dilute with sodium chloride 0.9% solution for injection and administer by IV infusion using incremental escalation of infusion rate, only if previous infusion well-tolerated. inject 15 mL (1 800 mg) Darzalex solution for SC injection into the subcutaneous tissue of approximately 7.5 cm to the right or left of the navel over approximately 3-5 minutes according to dosing schedule. Patients > 120 kg, flat-dose 1 800 mg SC, efficacy not establish no dose adjustments based on body weight recommended. Darzalex solution for SC injection should never be injected into areas where the skin is red, bruised, tender, hard or areas where there are scars, rotate injection site. During treatment with Darzalex SC injection, do not administer other medicinal products for subcutaneous use at the same site as Darzalex. Check the vial labels to ensure that the appropriate formulation (IV or SC formulation) and dose is being given as prescribed. For dose and schedule of medicinal products administered with DARZALEX, refer to SmPC 4.2 and the corresponding SmPC for other products. Administer pre- and post- injection medicinal products to reduce the Recommended concomitant medications for management of infusion/injection-related reactions medicinal products to all patients 1-3 hours prior to every infusion (corticosteroid, pre-medications can be given orally from the first dose. When dexamethasone is background-regimen specific corticosteroid, this dose will serve
More time to live the life they want

as pre-medication on infusion days. If dexamethasone given on infusion day, do not take additional background regimen specific Post- IV infusion/SC injection medicinal products should be administered to reduce the risk of SC injections: patient experiences no major IRRs after the first three SC injections, post-injection corticosteroids (excluding any background regimen corticosteroids) may be discontinued. Consider short/long acting bronchodilators and inhaled corticosteroids in patients with history IV Infusion: severity IRRs, interrupt Darzalex immediately and manage symptoms. Re-starting Darzalex IV infusion: reduce infusion rate (refer to SmPC); Grade 4 IRRs (or third occurrence of Grade 3) – permanently discontinue. For haematological toxicity dose delay may be required to allow recovery of blood cell counts. No dose reductions of Darzalex recommended. Consider anti-viral prophylaxis for Elderly/Renal impairment/Hepatic
Hypersensitivity to active substance









SPECIAL WARNINGS & PRECAUTIONS: can cause serious IRRs including anaphylactic reactions. Majority occurred following first IV infusion/SC injection. Fatal outcomes have been reported with IV infusion: monitor for IRRs throughout the IV infusion, continue monitoring post-IV infusion until symptoms resolve. For , median time to onset of IRRs was 3.7 hours following injection, monitor IRRs especially in the first and second SC in interrupt Darzalex for any severity IRRs. Institute medical management/supportive treatment as needed. For both IV and SC Darzalex if an anaphylactic reaction or life-threatening (Grade 4) IRR occurs, initiate appropriate emergency resuscitation immediately and discontinue Darzalex immediately and permanently. Neutropenia/Thrombocytopenia: Darzalex may increase neutropenia and thrombocytopenia induced by background therapy; monitor for infections & periodic complete blood cell counts (refer to relevant SmPCs); consider supportive care. Antiglobulin Test (Indirect Coombs Test): Daratumumab binds to CD38; may mask detection of antibodies to minor antigens; ABO and Rh blood typing not impacted. Interference may occur up to 6 months post-treatment. Type and screen patients prior to starting daratumumab; consider phenotyping; red blood cell genotyping not affected by daratumumab. Inform blood transfusion centres when appropriate. If emergency transfusion required, give noncross-matched ABO/RhD-compatible RBCs. Hepatitis B virus (HBV) Fatal cases reported in patients treated with Darzalex. Perform HBV screening before initiation of treatment. Suspend treatment in patients who develop reactivation of HBV while on Darzalex. Patient’s with body weight >120 kg receiving SC injection potential for reduced efficacy. SC contains sorbitol.
SIDE EFFECTS: Very common: upper respiratory tract infection, anaemia, neutropenia, thrombocytopenia, lymphopenia, leukopenia, decreased appetite, peripheral sensory neuropathy, paraesthesia, headache, hypertension, cough, dyspnoea, nausea, diarrhoea, constipation,




vomiting, back pain, muscle spasms, fatigue, pyrexia, oedema peripheral, asthenia. insomnia, arthralgia, rash. urinary tract infection, influenza, sepsis, cytomegalovirus infe hypogammaglobulinemia, hyperglycaemia, hypocalcaemia, dehydration, fainting, atrial fibrillation, pulmonary oedema, pancreatitis, chills. dizziness, musculoskeletal chest pain, pruritus, injection site reactions. Other side effects: (uncommon), anaphylactic reaction (rare).
Refer to SmPC for other side effects.
LEGAL CATEGORY: Prescription only medicine

PRESENTATIONS, PACK SIZES, MARKETING AUTHORISATION NUMBER(S) PRESENTATIONS

5 ml vial (100mg daratumumab)
20 ml vial (400mg daratumumab)
15 ml vial (1 800 mg daratumumab)
MARKETING AUTHORISATION HOLDER: Janssen-Cilag International NV Turnhoutseweg 30, B-2340 Beerse, Belgium

FURTHER INFORMATION IS AVAILABLE FROM: Janssen Sciences Ireland UC, Barnahely, Ringaskiddy, IRL – Co. Cork P43 FA46
Prescribing information last revised: January 2022







Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse events via: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to, Janssen Sciences Ireland UC on 1800 709 122 or at dsafety@its.jnj.com.



*DVTd: 64% vs. VTd: 44% (p<0.0001)
†HR 0.47 (95% CI: 0.33–0.67; p<0.0001)
CI, confidence interval; DVTd, DARZALEX , Velcade dexamethasone; MRD, minimal residual disease; VTd, Velcade thalidomide, dexamethasone.
References:
1. Moreau P, 2019;394(10192):29–38.x
DARZALEX® + VTd offers newly-diagnosed
CPD CPD
60 Second Summary
Multiple Myeloma (MM) is a haematological malignancy characterised by clonal expansion of malignant plasma cells in the bone marrow. These plasma cells secrete a monoclonal immunoglobulin (Ig), often known as M-protein, which can lead to organ dysfunction, anaemia, renal impairment and bone fractures. MM patients can present with insidious or more obvious symptoms depending on the main site of disease. Bone marrow failure, due to build up of MM cells, can present as non specific fatigue, weight loss, shortness of breath or in some cases bleeding and bruising (less typical).
The main clinical features of MM are known as CRAB features (hypercalcaemia, renal insufficiency, anaemia and bone lesions).
MM is not treated with a single agent but rather with combinational therapy. Standard of care therapy for MM now consists of a proteasome inhibitor in combination with an immunomodulatory agent. Treatment at diagnosis is focused on getting the patient into a deep remission – measured by a reduction in serum M-protein, normalisation of the light chain ratio and reduction in the percentage of bone marrow plasma cells.
In the last two decades, the introduction of new therapies such as proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies, has undoubtedly improved patient outcome. In Ireland, the 5-year survival rate is now around 53%, doubling since the early 1990s. However, in reality, patient prognosis varies widely for each individual and this is largely because of tumour heterogeneity i.e. differences in genetic makeup each myeloma. In other words, MM is not a single disease entity but rather many different types.


1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?


4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the


4 previous steps, log and record your findings. Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Multiple Myeloma: Disease Overview and the Role of Genomics
Introduction
Multiple Myeloma (MM) is a haematological malignancy characterised by clonal expansion of malignant plasma cells in the bone marrow. These plasma cells secrete a monoclonal immunoglobulin (Ig), often known as M-protein, which can lead to organ dysfunction, anaemia, renal impairment and bone fractures. MM affects around 400 people in Ireland each year, making it the second most common blood cancer. The median age of diagnosis is 66-70, however many patients are much younger at diagnosis making the disease less likely to be suspected. MM can arise from asymptomatic pre-malignant stages known as Monoclonal Gammopathy of Undetermined Significance (MGUS) and Smouldering MM (SMM) at a rate of 1% and 10% per year respectively. Although there are many new therapies available for MM, it remains incurable as eventually most patients will relapse or stop
responding to treatment. As MM is a very heterogeneous disease, it can be challenging to detect initially and may not be suspected in patients under the age of 50. Prognosis varies widely depending on clinical features, disease stage and genetic subgroup. Understanding myeloma genomics has undoubtedly improved prognostic biomarkers. However, this has been slower to translate into targeted therapies compared to other cancers, and in 2023 most patients are treated with similar combination regimens initially, regardless of disease specific features. The availability of new biomarkers of disease aggression and prognosis in the near future will likely result in a movement away from this “one-size-fits-all” approach to a more individualised treatment plan.
Clinical Presentation and Diagnosis
MM patients can present with insidious or more obvious symptoms depending on the main


site of disease. Bone marrow failure, due to build up of MM cells, can present as non specific fatigue, weight loss, shortness of breath or in some cases bleeding and bruising (less typical).
Patients with vertebral tumours (plasmacytomas) or bone disease can present with back pain which can be subtle initially but typically progresses rapidly within weeks to months to radiculopathy, severe pain and mobility restriction. Hip pain can also be a presenting feature for some patients and if undetected, can progress to pathological fractures. As these symptoms are common in the general population, MM is often not initially suspected. Raising awareness of these presentations is needed to prevent disability and morbidity for patients.
The main clinical features of MM are known as CRAB features (hypercalcaemia, renal insufficiency, anaemia and bone lesions). Anaemia is not always present and can range from a normocytic mild anaemia to
31 CPD 99: MULTIPLE MYELOMA
Continuing Professional Development
AUTHORS: Dr Roisin McAvera (Postdoctoral researcher) and Professor Siobhan Glavey (consultant haematologist & clinician scientist), Royal College of Surgeons in Ireland & Beaumont Hospital Dublin.
Dr Roisin McAvera
Professor Siobhan Glavey
severe anaeamia and cytopenias. To screen for MM, serum or urine
M-protein is measured – it is essential to check serum protein electrophoresis (SPEP) and serum free light chains (SFLC) as the initial blood based screen as 10% of cases will secrete light chains only and therefore could go undetected with SPEP alone.
Urinary screening for Bence Jones
Protein is also recommended as part of the diagnostic criteria but is more commonly performed by a haematolologist or nephrologist during secondary work up. Bone marrow biopsy is the diagnostic test which assesses for percentage plasma cells and . Bone lesions will also be assessed using methods such as X-rays, positron emission tomography (PET) scans, magnetic resonance imaging (MRI) and computerized tomography (CT) scans. Table 1 shows the diagnostic criteria of MM.
Current Staging Systems
level of lactate dehydrogenase (LDH) and genetic risk factors assessed by fluorescence in situ hybridization (FISH), specifically high-risk cytogenetic abnormalities t(4;14), t(14;16) or del(17p). The R-ISS criteria are shown in Table 2. The incorporation of genetic risk factors was an important step for MM, and since then research has continued to uncover important genetic factors.
Treatment
(cyclophosphamide, bortezomib, dexamethasone), Rd (lenalidomide, dexamethasone) or most recently D-VTD (daratumumab, bortezomib, thalidomide, dexamethasone).
bortezomib and dexamethasone (VD), CyBorD/cyclophosphamide, VD (bortezomib and dexamethasone), daratumumab monotherapy, pomalidomide and dexamethasone, Kd (carfilzomib and dexamethasone), KRd (carfilzomib, lenalidomide and dexamethasone), DVd (daratumumab, bortezomib and dexamethasone), IRD (ixazomib, lenalidomide and dexamethasone), and lenalidomide and dexamethasone.
In 2005, the International Myeloma Working Group (IMWG) introduced the International Staging System (ISS) to stratify patients into three prognostic groups. The ISS does this based on the clinical levels of serum β2 microglobulin and serum albumin. In 2015, this was revised (R-ISS) to include the
Multiple Myeloma
MM is not treated with a single agent but rather with combinational therapy. Standard of care therapy for MM now consists of a proteasome inhibitor in combination with an immunomodulatory agent. Treatment at diagnosis is focused on getting the patient into a deep remission – measured by a reduction in serum M-protein, normalisation of the light chain ratio and reduction in the percentage of bone marrow plasma cells. Initial treatment in Ireland at present consists of one of the following National Cancer Control Programme (NCCP) approved regimens; RVD (bortezomib, lenalidomide, dexamethasone), VMP (bortezomib, melphalan, prednisone), CyBorD
Both criteria must be met:
Treatment choice is largely guided by patient “fitness” in terms of frailty and co-morbidities, age is a factor, but biological age is more important than numerical age. MM patients can be widely classified as either eligible for an autologous stem cell transplant (ASCT), or ineligible for an ASCT, and if eligible, will receive this following induction therapy. Patients over 75 years old are generally not eligible due to the higher risk of toxicity and the availability of effective treatments for these patients. Unfortunately, most patients eventually relapse and require further treatment once symptomatic. The choice of treatment at relapse will depend on which regimen the patient received as their previous line(s) of therapy and their initial response, cytogenetics, aggressiveness of the relapse, and again the patient’s fitness and quality of life. In Ireland, there are several NCCP approved regimens for treatment of relapsed and refractory myeloma; RVD (bortezomib, lenalidomide and dexamethasone),
Excitingly, there are many new therapies in trial for MM, most focused on the relapsed/ refractory settings. Venetoclax, a BCL-2 inhibitor, shows promise for patients with t(11;14) and if approved will be the first targeted therapy for MM. Additionally, many new therapies targeting the bone marrow microenvironment are on the horizon. These include chimeric antigen receptor (CAR) T-cells, bispecific T cell engagers (BiTEs) and novel monoclonal antibodies.
Pathogenesis – Genetic Abnormalities
tomography (PET) scans, magnetic resonance imaging (MRI) and computerized tomography (CT) scans Table 1 shows the diagnostic criteria of MM.
MM progression is driven by the accumulation of genetic abnormalities, as well as interplay between malignant plasma
• Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or extramedullary plasmacytoma
• Any one of the following myeloma defining events:
1. End-organdamage (CRAB):
• Hypercalcemia: serum calcium >0.25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2.75 mmol/L (>11 mg/dL)
• Renal insufficiency: creatinine clearance <40 mL per minute or serum creatinine >177 μmol/L (>2 mg/dL)
• Anaemia: hemoglobin value of >2 g/dL below the lower limit of normal, or a hemoglobin value <10 g/dL
• Bone lesions: one or more osteolytic lesions on skeletal radiography, CT or PET-CT
2. Clonal bone marrow plasma cell percentage ≥ 60%
3. Involved:uninvolved serum free light chain (FLC) ratio ≥ 100 (involved free light chain level must be ≥100 mg/L)
4. >1 focal lesions on magnetic resonance imaging (MRI) studies (at least 5 mm in size)
Table 1. IMWG Diagnostic Criteria for Multiple Myeloma
Current Staging Systems
In 2005, the International Myeloma Working Group (IMWG) introduced the International Staging System (ISS) to stratify patients into three prognostic groups. The ISS does this based on the clinical levels of serum β2 microglobulin and serum albumin. In 2015, this was revised (R-ISS) to include the level of lactate dehydrogenase (LDH) and genetic risk factors assessed by fluorescence in situ hybridization (FISH), specifically high-risk cytogenetic abnormalities t(4;14), t(14;16) or del(17p). The R-ISS criteria are shown in Table 2 The incorporation of genetic risk factors was an important step for
32 CPD 99: MULTIPLE MYELOMA
Table 1. IMWG Diagnostic Criteria for Multiple Myeloma
System (ISS) to stratify patients into three prognostic groups. The ISS does this based on the clinical levels of serum β2 microglobulin and serum albumin. In 2015, this was revised (R-ISS) to include the level of lactate dehydrogenase (LDH) and genetic risk factors assessed by fluorescence in situ hybridization (FISH), specifically high-risk cytogenetic abnormalities t(4;14), t(14;16) or del(17p). The R-ISS criteria are shown in Table 2 The incorporation of genetic risk factors was an important step for MM, and since then research has continued to uncover important genetic factors.
Stage I
Stage II
Stage III
cells and the bone marrow microenvironment. Generally, disease-initiating primary genetic events will occur at pre-malignant MGUS and SMM stages and can broadly be categorised into two groups – hyperdiploid and non-hyperdiploid. Hyperdiploidy refers to extra chromosomal copies, usually trisomies, and tend to affect the odd number chromosomes 3, 5, 7, 9, 11, 15, 19 and 21. Non-hyperdiploid MM is characterised by IGH gene translocations (chromosome 14q32), most commonly t(4;14), t(6;14), t(11;14), t(14;16), and t(14;20). These result from errors during B-cell differentiation where the parts of the IGH gene rearrange.
As the disease progresses to MM, secondary genetic events accumulate. These are commonly copy number variations such as del(17p), gain(1q) and del(13q), or gene mutations in cell signalling pathways such as RAS and NF-κB. As progression continues, the process of clonal evolution may occur whereby the mutations driving the disease change over time as certain clonal cell populations are selected for, either naturally or because of treatment. In rare cases, MM may progress to a very aggressive form known as plasma cell leukaemia (PCL) which occurs when clonal plasma cells circulate in peripheral blood.
Patient Prognosis
In the last two decades, the introduction of new therapies such as proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies, has undoubtedly improved patient outcome. In Ireland, the 5-year
All of the following:
• Serum albumin ≥3.5 gm/dL
• Serum beta-2-microglobulin <3.5 mg/L
• No high-risk cytogenetics
• Normal serum LDH level
Not fitting Stage I or III
Both of the following:
• Serum beta-2-microglobulin >5.5 mg/L
• High-risk cytogenetics [t(4;14), t(14;16), or del(17p)] or elevated serum lactate dehydrogenase level
survival rate is now around 53%, doubling since the early 1990s. However, in reality, patient prognosis varies widely for each individual and this is largely because of tumour heterogeneity i.e. differences in genetic make-up each myeloma. In other words, MM is not a single disease entity but rather many different types.
As a result, patients may be described as either ‘standard-risk’ or ‘high-risk’. Generally, standardrisk patients have a median survival of 7-10 years whilst high-risk patients have a much poorer prognosis of only 3 years.1 Scientific research has aimed to find ways to stratify patients accordingly but cytogenetic abnormalities determined by FISH remain the most common method. According to the Mayo Clinic Risk Stratification for Multiple Myeloma (mSMART), presence of trisomies, t(11;14) or t(6;14) confer standard-risk, whilst presence of t(4;14), t(14;16), t(14;20), del(17p) or gain(1q) is considered high-risk. Moreover, prognosis worsens even more with the presence of two high-risk factors (double hit myeloma), and again if three or more are present (triple hit myeloma). Unfortunately, across different clinical sites, there is often variation in which abnormalities are tested for, or the FISH probes used for detection. As research advances, more prognostic methods are continually being uncovered, such as the use of gene expression profiling for riskstratification, and the presence of minimal residual disease.
Gene Expression Profiling
Genomic testing in the form of gene expression profiling
(GEP) has become increasingly popular, with the identification of many gene signatures that can accurately identify molecular subgroups of MM patients and predict prognosis. To date, two gene signatures have been commercialised – the MyPRS® which detects a 70-gene signature known as UAMS-70, and the MMProfiler™ which detects a 92-gene signature known as EMC92/SKY92. Both are commonly incorporated into ongoing clinical trials and have proved to successfully identify high-risk patients. However, the MMProfiler™ is currently the only GEP test with a CE-IVD (in vitro diagnostic) approval meaning it meets European standards to be used in a clinical setting. The MMprofiler™ works by determining the expression of 92 genes in myeloma plasma cells from patient bone marrow. Dependent on the way these genes are collectively expressed, a score is calculated and anything equal or above 0.827 is considered high-risk with a predicted survival of less than 2 years.2 Several studies have shown that using MMProfiler™ can overcome the need for FISH and in fact provide additional information often missed by FISH. The MMProfiler™ is already used in private healthcare settings in the UK, and in several NHS clinical trials. We are currently investigating its use here in Ireland and hope that it will lead to its routine diagnostic use. Several trials are also investigating whether it can predict treatment response, with preliminary results showing it can aid clinical decision making to escalate or deescalate treatment (PROMMIS trial, NCT02911571).
Minimal Residual Disease
Minimal residual disease (MRD) refers to the low level of cancerous cells that may persist even in patients who have shown a ‘complete response’ to treatment (reduction of M-protein and less than 5% bone marrow plasma cells). MRD is not detected using conventional morphological and serological tests and therefore tests that are more sensitive have been developed. The IMWG recommend that MRD tests reach a sensitivity level of 10-5, meaning that one malignant cell can be detected among 100,000 healthy cells, but recent detection methods report sensitivities of 106, and even 10-7.
Extensive studies show that MRD positivity is associated with inferior progression free survival and overall survival compared to MRD negativity, and in some cases may even overcome cytogenetic risk factors. For example, patients presenting with highrisk cytogenetics (4;14), t(14;16), or del(17p) who reached MRD negativity had a better outcome than standard-risk patients who were MRD positive.3 Alternatively, high-risk patients who are MRD positive exhibited the worst prognosis, highlighting the prognostic potential of assessing both factors in combination.
Unfortunately, the most common methods used for MRD detection also lack sensitivity and standardisation. Multiparameter flow cytometry is most common, which works by immunophenotyping cells by detecting and quantifying cell-surface proteins associated with malignant MM cells. Like
33
Table 2. Revised International Staging System
Table 2. Revised International Staging System
with FISH, this method can vary between clinical sites, with discrepancies in markers used, different antibodies for detection, and varying analysis methods to name a few. Great efforts have been made to standardise flow cytometry, and now next generation flow (NGF) has been approved by the IMWG. NGF was developed by the EuroFlow consortium and comprises of an eight-colour flow assay that allows the simultaneous analysis of up to 10 million cells. However, there are still limitations associated with NGF, the main one being the need for fresh samples and immediate processing. As a result, molecular genomic-based MRD tests are an attractive alternative, since DNA can be preserved and stored for later analysis.
Next-generation sequencing (NGS) techniques have been developed for MRD detection. These look for clonal DNA sequences within immunoglobulin genes, usually the IGH gene, which occur during a process known as V(D) J recombination.4 This is the rearrangement of DNA regions known as Variable (V), Diversity (D) and Joining (J) regions which is necessary to ensure production of antibodies. However, in MM this process is dysregulated resulting in clonal DNA rearrangements, and production of M-protein instead. There are two commercialised, standardised tests designed to detect these rearrangements
– Adaptive’s ClonoSEQ® and Invivoscribe’s LymphoTrack®. Both of these detect the clonal diseasedriving sequence at diagnosis, and determine if it remains in follow-up samples. The main
limitation here is that a diagnostic sample is required, which is not always available. Moreover, to ensure cost effectiveness it is better to run large numbers of samples simultaneously, which may not always be feasible. Despite these, ClonoSEQ® is the only NGS method FDA approved and reports a sensitivity of up to 10-7. However, it is not commercially available which limits its use in many centres due to expense. On the other hand, LymphoTrack® can be performed in laboratories in-house and thus is a more feasible choice for most researchers. As research continues into the benefits of using MRD as a prognostic test, the hope is that it may spare patients from particularly toxic, aggressive therapies where the risk of relapse outweighs any potential benefit.
Spatial Heterogeneity
As mentioned, the accumulation of MM cells in the bone marrow results in focal lesions, which can be visualised using imaging techniques such as PET scans. Bone marrow biopsies taken for testing are usually from the posterior iliac crest meaning any genetic tests performed are from one specific location and give us a ‘snapshot’ rather than the whole picture. However, recent research has shown that myeloma heterogeneity can occur across different focal lesions within the same patient, in other words, spatial heterogeneity. For example, a patient may present as standardrisk at both cytogenetic or GEP level of iliac crest bone marrow, but high-risk when the same tests are performed on plasma cells
from a focal lesion elsewhere.5 Therefore, to understand exactly how each individual’s disease is behaving it may be beneficial to perform genomic sequencing at several disease sites. Of course, however, bone marrow biopsies are very invasive and so in reality this would be difficult to introduce routinely. Both the number and size of focal lesions also correlates with disease severity, and so it is advised that imaging is also used for MRD monitoring. As we learn more about spatial heterogeneity, this may also become a consideration when monitoring MRD using NGS methods, as V(D) J rearrangements may also differ between lesions.
Extramedullary disease
Around 20% of patients will present with extramedullary disease (EMD), which occurs when MM cells infiltrate other organ systems, becoming independent of the bone marrow microenvironment. This is a particularly aggressive form of MM and largely unresponsive to current therapies, and may present at diagnosis or at relapse. Moreover, EMD is a poor prognostic factor with an overall survival rate of only 6 months.6 EMD is associated with both high-risk cytogenetics and high-risk MM as defined by GEP-70. Moreover, these patients rarely present with standard-risk abnormality t(11;14). More recently, a link between EMD and highrisk chromosome 1 abnormalities has also been reported.7 Very few studies have investigated the genomic landscape of EMD, but those that have revealed mutations in tumour-driving RAS signalling pathways. With these early studies already suggesting a high prevalence of various high-risk markers in EMD, it is necessary that we learn more about the genomic landscape of EMD to improve outcomes for this poor prognostic group.
Future Implications
MM outcome is continually improving due to discovery of novel therapies and understanding of disease biology. By using the genomic tools such as those we have discussed, we hope that in the future this will ultimately translate into more targeted therapies for each MM patient.
Using NGS tools to predict prognosis such as GEP and MRD detection will enable us to better characterise how genetic subgroups perform over time and respond to therapies. Moreover, understanding how gene signatures drive disease biology may uncover novel therapeutic targets. Future research should also aim to understand the role of spatial heterogeneity and EMD in patient prognosis.
References
1. Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(8):1086107.
2. Kuiper R, Broyl A, de Knegt Y, van Vliet MH, van Beers EH, van der Holt B, et al. A gene expression signature for high-risk multiple myeloma. Leukemia. 2012;26(11):2406-13.
3. Ha J, Lee H, Shin S, Cho H, Chung H, Jang JE, et al. Ig Gene Clonality Analysis Using Next-Generation Sequencing for Improved Minimal Residual Disease Detection with Significant Prognostic Value in Multiple Myeloma Patients. J Mol Diagn. 2022;24(1):48-56.
4. Deng X, Zhang M, Zhou J, Xiao M. Next-generation sequencing for MRD monitoring in B-lineage malignancies: from bench to bedside. Exp Hematol Oncol. 2022;11(1):50.
5. Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8(1):268.
6. Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34(1):1-20.
7. Kriegova E, Fillerova R, Minarik J, Savara J, Manakova J, Petrackova A, et al. Wholegenome optical mapping of bone-marrow myeloma cells reveals association of extramedullary multiple myeloma with chromosome 1 abnormalities. Sci Rep. 2021;11(1):14671.
34 CPD 99: MULTIPLE MYELOMA
Promoting Awareness of Health
HIQA publishes resources to promote awareness of advocacy in health and social care services
An advocate is a person who protects and promotes people’s human rights, while also respecting their autonomy, privacy, dignity, values, preferences and diversity. Staff in health and social care settings have an important role to play in promoting advocacy services and supporting people to understand and access different types of advocacy.
Advocacy supports person-centred care by placing the person at the centre of any decision-making about their lives and their care. At HIQA, we are committed to promoting person-centred care and supporting staff to uphold a human rights based approach to care. HIQA has now produced a new set of resources on advocacy to help staff incorporate it into their day-to-day work.
All national standards published by HIQA have a focus on advocacy, particularly the National Standards for Adult Safeguarding and the Guidance on a Human RightsBased Approach in Health and Social Care. The new online learning course, booklet and educational video on advocacy will help health and social care staff implement national standards and support people using services to have their human rights met.
These learning resources were developed in response to a recommendation of the COVID-19 Nursing Homes Expert Panel Report. The report highlighted the ongoing need for advocacy services for nursing home residents, their families and friends during the pandemic.
Several respondents to the Expert Panel emphasised the need to create a new narrative of care for older people incorporating the language of inclusion and empowerment. The final report recommended that HIQA ‘continue to highlight and promote independent advocacy services to residents’, and that the rights of residents – in terms of dignity, freedom, choice and equality –need to be respected and at the forefront of policy.
Yet, knowledge of advocacy is still low in health and social care services. The first National Nursing Home Experience Survey, published in November 2022, found that there was limited awareness of advocacy organisations and how to access advocacy supports among both nursing home residents and their relatives and friends.
In particular, most nursing home residents (77%) said that they did not know how to access advocacy services or organisations that could help them to express their views and wishes, and to help them to assert their rights.
The survey found that 28.9% of relatives and friends said that they did not know how to contact organisations that can support their relative or friend to express their views and wishes, and help them to assert their rights.
HIQA’s resources on The Fundamentals of Advocacy in health and social care are designed to improve knowledge and understanding of advocacy
Written by Rachel Flynn, Director of Health Information and Standards, HIQA
and to support people to have their human rights met.
The resources will help health and social care staff understand their role in advocacy, which includes listening to people and supporting them to have their voices heard, supporting them to have their will and preferences met, and respecting their autonomy and confidentiality.
HIQA recommends that all health and social care staff complete the one hour online course, read the booklet and watch the video to improve their knowledge and understanding of advocacy and to help them put national standards into practice in their day-to-day work.
We hope that these resources will help ensure that people using health and social care services have their human rights met at all times.
The new resources are available on the HIQA website, and the online
learning course is also available on HSeLanD.
At HIQA, we are committed to promoting a person’s human rights, through supporting staff to uphold a human rights based approach to care. If you wish to learn more about advocacy in national standards, you can find the National Standards for Adult Safeguarding and the Guidance on a Human Rights-Based Approach in Health and Social Care on the HIQA website. You can also visit HSeLanD to access our online learning courses; National Standards for Adult Safeguarding, and Applying a Human Rightsbased Approach in Health and Social Care.
Abolition of In-Patient Charges
Abolition of In-Patient Charges

Minister for Health Stephen Donnelly has announced that, with effect from Monday 17 April 2023, public in-patient charges have been abolished in all public hospitals.
Minister for Health Stephen Donnelly has announced that, with effect from Monday 17 April 2023, public in-patient charges have been abolished in all public hospitals.
The Health (Amendment) Act 2023 removes the acute public in-patient charge of ¤80 per day, up to a maximum of ¤800 in a year (including day-case charges) for people accessing care as a public patient in all public hospitals. Following the enactment of the legislation, the Minister for Health signed the Commencement Order providing for the removal of charges from today.
The Health (Amendment) Act 2023 removes the acute public in-patient charge of ¤80 per day, up to a maximum of ¤800 in a year (including day-case charges) for people accessing care as a public patient in all public hospitals. Following the enactment of the legislation, the Minister for Health signed the Commencement Order providing for the removal of charges from today.
Minister Donnelly said, “I am delighted to announce that from today, people will no longer be charged when accessing public inpatient care in our public hospitals.
Minister Donnelly said, "I am delighted to announce that from today, people will no longer be charged when accessing public inpatient care in our public hospitals.
“This legislation removes the existing financial burden of public inpatient charges when accessing care in a public hospital. The removal of these charges, announced as part of Budget 2023, is an important step towards reducing the healthcare costs of patients and families.
“This legislation removes the existing financial burden of public inpatient charges when accessing care in a public hospital. The removal of these charges, announced as part of Budget 2023, is an important step towards reducing the healthcare costs of patients and families.
“This measure builds on the abolition of public in-patient charges for children, which I introduced last year, and is another significant step in ensuring that people have access to affordable healthcare services.”
“This measure builds on the abolition of public in-patient charges for children, which I introduced last year, and is another significant step in ensuring that people have access to affordable healthcare services."
35 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 News
Cardiovascular Risk and Screening in Inflammatory Rheumatic Disease
implications has been increasingly recognised, with ongoing research focusing on further understanding the pathogenesis of CVD in IRDs, and also into the development of guidelines and screening tools for clinicians.
While cardiovascular disease (CVD) remains the main cause of death worldwide, CVD morbidity and mortality is higher in patients with inflammatory rheumatic diseases (IRDs) when compared to the general population.1-2 Although the relationship between inflammation and increased cardiovascular risk in rheumatic diseases has been widely accepted, the exact pathogenesis remains unclear and does not appear to be explained by the presence of co-existing traditional cardiovascular risk factors alone.3 CVD is noted to have an increased occurrence across a majority of IRDs and inflammation itself can represent an independent risk factor for development of CVD along with, lifestyle factors in patients with a chronic disabling diseases, such as physical inactivity and certain treatments, namely corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDS).4-7
CVD can be divided into atherosclerotic events such as ischemic heart disease, stroke and peripheral artery disease and non-atherosclerotic events including venous thrombosis, heart failure, myocardial and valvular involvement, and arrhythmias. It is now established that atherosclerosis is not solely due
to lipid accumulation within the arterial wall, but also as a result of chronic inflammation in response to vascular injury.8 An inflammatory process is seen to prompt the early stages of atherosclerotic development, with both innate and adaptive immune responses playing a key part in this process.9 Previous studies suggest, that an increase of certain inflammatory cytokines can be linked with an increased risk of developing cardiovascular diseases, and identification of the key immune cells in this process can potentially lead to new targeted treatment for CVD in the future.10-12
Current Screening Recommendations
Patients diagnosed with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), Axial Spondyloarthrits (AxSpA), Juvenile Idiopathic Arthritis (JIA) and gout all have an increased risk of developing CVD. This risk is partly due to systemic inflammation but there is also a noted increase in the prevalence of traditional cardiovascular risk factors in this patient group, including hypertension and dyslipidaemia.13-15 Over the last decade, the scale of these clinical

In 2010 and with an update in 2015/2016, the European League against Rheumatism (EULAR) recommendations for cardiovascular risk management in patients with (RA), ankylosing spondylitis and PsA were published. This recommended that risk prediction models should be adapted for patients with RA by a 1.5 multiplication factor.16 Following European Society of Cardiology (ESC) guidelines, the EULAR task force recommended to perform CVD risk assessment every 5 years for patients found to be at low-tomoderate cardiovascular risk. Screening should be performed more often in patients deemed to have intermediate risk. Patients at high to very high cardiovascular risk will require treatment for noted cardiovascular risks, according to national guidelines. Unfortunately, many IRDs patient’s risks remain underestimated as current risk prediction model, that have been developed and used for CVD risk assessment (e.g. SCORE, and Framingham) are not validated in external cohorts including IRDs patients. These risk assessment models were shown to underestimate risk in these patients and there is still ongoing debate on whether the use of the 1.5 multiplication factor accurately reclassifies the correct patients from intermediate risk into being high risk of CVD and that this method may also be underestimating patient’s risk of cardiovascular events.17-19 In 2021 ESC guidelines published the new SCORE2 algorithm to estimates an individual’s 10-year risk of fatal and non-fatal CVD events in presumed healthy people

between the ages of 40–69 years, with risk factors that have been untreated or have been stable for several years.20 A recent cross sectional multicentre study from Yagensky and Schirmer, looking at cardiovascular risks and risk stratification in inflammatory joint diseases showed, that using the new SCORE2 algorithm, led to the reclassification of patients to a higher risk group in 30% compared to only 5% when using the original SCORE algorithm. They suggest further longitudinal data could determine whether the new SCORE2 algorithm may be an alternative risk calculator to the 1.5 multiplicators in RA and other inflammatory diseases currently recommended.21
There are fewer studies investigating CVD in other multisystem autoimmune rheumatic diseases, including systemic sclerosis, Sjogren’s syndrome, idiopathic inflammatory myositis, systemic vasculitis and Bechet’s, however recent studies have shown, an association with increased risk of premature CVD in these patients. The EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including SLE and antiphospholipid syndrome were published in 2022, which emphasise the need for a more tailored therapeutic approach in these patients that strikes a balance between the treatment of the underlying IRD and the patient’s individual cardiovascular risks.22 They encourage regular screening and assessment using standard cardiovascular prediction tools, given the lack of validated rheumatic diseasesspecific tools and management of modifiable risk factors similar to those used in the general population and focus on adequate disease control. It is important to note, that each IRD carries its own disease characteristics
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 36
MUSCULOSKELETAL: RHEUMATIC DISEASE
Written by Dr Brona Dinneen & Professor Finbar O’Shea, Consultant Rheumatologist, St James’s Hospital, Dublin. Clinical Lead for Rheumatology, St James’s Hospital, Dublin Clinical Associate Professor, School of Medicine, Trinity College Dublin National Speciality Director (Rheumatology), RCPI, Dublin
Professor Finbar O’Shea
such as manifestations and symptomatology, age of onset, treatment etc. and it could be argued that grouping all IRDs together to discuss their role in the development and progression of CVD as oversimplifying a complex subject matter. Although these guidelines focus mainly on the management of cardiovascular risk factors over risk stratification, they emphasise the need for, increased awareness of the higher risk of CVD, and need for screening in people with IRDs amongst both clinicians and patients.
Therapeutics
When discussing cardiovascular risk in patients with IRDs, it is important to discuss the changing landscape of treatments in rheumatic diseases. With more treatment options becoming increasingly available, treatment can now be tailored based on individual patient risk factors and preferences. While certain drugs used to treat rheumatological conditions can increase the risk of developing CVD when used for prolonged periods, such as NSAIDS and corticosteroids, newer steroid sparing, disease modifying treatment can reduce CVD risk by reducing inflammation as seen with methotrexate and Tumour Necrosis Factor alpha (TNF-alpha) inhibitors.23 Certain medications can also be seen to be cardio protective such as hydroxychloroquine in RA und SLE patients and colchicine in gout patients.24-26 There has however been some controversy with
newer medication, and their role in cardiovascular events. Janus kinase inhibitors (JAKi) indicated in the treatment of RA, PsA, juvenile idiopathic arthritis and AxSpA have recently come under scrutiny for concerns of increased risk of major cardiovascular events, VTE and death due to any cause, when compared with TNF-alpha inhibitors. Following the US Food and Drug Administration (FDA) addition of a ‘black box warning’ on all currently approved JAKi, the European Medicines Agency (EMA) has recommended measures to minimise the risk of serious side effects including the risk of cardiovascular events. These include that, these medicines should be used in the following patients only if no suitable treatment alternatives are available: those aged 65 years or above and those at increased risk of major cardiovascular problems. It is therefore essential that patients are assessed and screened appropriately for CVD, prior to commencing these medications.
What the Future Holds
In recent years, there has been an increase in research into methods to identify patients with subclinical atherosclerosis, with the goal of identifying those patients with a higher risk to developing CVD earlier. This has focused on identifying possible biomarkers and the use of non-invasive imaging. Carotid ultrasound has been utilized to identify patients
with subclinical atherosclerosis disease by detecting the presence of carotid plaque and evaluating carotid intima media wall thickness (cIMT). Carotid ultrasound for CVD screening was included in the EULAR recommendations for CVD risk management in patients with disorders 2015/2016 update, as carotid plaques are associated with future ACS in patients with RA.27 There is currently however, an absence of evidence for routine screening of the carotid arteries and the 2021 ESC guidelines on CVD prevention do not recommend the systematic use of cIMT thickness to improve risk stratification as there is currently a lack of methodological standardization. The future role for the use of carotid ultrasound in CVD risk stratification is promising, with newer studies showing increased cIMT can act as a predictor of cardiovascular events, as well as a marker of subclinical atherosclerosis.28-30 It has been used increasingly in IRDS research as an alternative assessment method for identifying subclinical atherosclerosis and more recently as an endpoint, with studies showing notable improvement in cIMT, once RA disease activity had been controlled with treatment.31-33 With the current inconsistency in cut-off values and standardization, it is likely that in the future, carotid ultrasound results should be combined as part of a comprehensive
cardiovascular assessment to better predict CVD risk.

Studies have shown, the prognosis of premature CVD is poorer when coexisting IRDs are present and as this affects a younger population, there are substantial risks of long-term disabilities and health issues causing further strain on the healthcare system.34-35 It is therefore paramount to develop new screening tools to identify those IRDs patients at higher risk of developing CVD, and therefore utilise the most appropriate diagnostic and therapeutic measures that focus on reducing inflammation and atherosclerosis, limiting CVD development.
Identification and validation of modified CVD risk prediction tools, CVD risk biomarkers and vascular imaging in IRDs patients would aid this practical clinical issue.
While there are ongoing developments into CVD risk stratification in IRDs, it is important patients are educated on the risks and fully supported with possible lifestyle modifications. Clinicians should perform regular CVD risk assessment along with management of cardiovascular risk factors and cautious prescription of medications including NSAIDS, JAKi and minimal dosages of corticosteroids.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 37
Osteoporosis – Preventing the Silent Killers “Fractures”
Written by Michele O'Brien, CEO, The Irish Osteoporosis Society

The disease Osteoporosis is commonly considered to affect just “Old” women, and many people do not appear to be aware that this disease is preventable and treatable in most people. Women over 65 are the highest risk group for fractures, however it affects all age groups, including our young. Only 19% of people with Osteoporosis are diagnosed, which is why everyone should be checking to see if they have any of the 200 causes of bone loss.
Her DXA results show she now has marked Osteopenia in L1 and L3, Osteoporosis at L2 and severe bone loss at L4.
Osteoporosis – Preventing the Silent Killers “Fractures”
Written by Michele O'Brien CEO
Thankfully she was diagnosed, investigated and put on a treatment plan. You can see from the graph named “AP spine: L2-L4 (L3) (BMD) % change vs Previous”, that in two years she has significantly improved her bone health.
Research shows that most fractures occur in the moderate to marked Osteopenia range, which is a T score of -1.5 to -2.49. It was explained to her the consequences of her lifestyle choices and the effects of what vertebral fractures can do to a person’s quality of life. No one wants to end up disfigured with the possibility of a Dowager’s hump which is not reversable. The individual reduced the amount of exercise she was doing to “normal levels”, which resulted in her periods returning, which assisted with her improvement of bone health, as her oestrogen levels returned to normal. She also learned some stress coping techniques that she could use at her office desk, as she had previously increased her running times in an attempt to decrease her stress levels.
than just targeting the highest risk group for bone loss.

NOTE Depo Provera has been proven to cause bone loss, therefore unless a young person is very high risk to become pregnant, the Irish Osteoporosis Society (IOS) would recommend a different form of contraceptive.
Women lose bone leading up to the menopause, going through the menopause and the most significant bone loss occurs in the 10 years post menopause. In general, we can only assume that the more risk factors for bone loss a person has, the higher the bone loss they will experience.
An example of this is the DXA scan below (figure 1). It shows the results of a 21 year old female who was diagnosed with severe bone loss at 19 in her spine.
The Irish Osteoporosis Society
The fact that two of her vertebrae had been eliminated from her DXA scan results, shows that even the young can develop Osteoarthritis, which is why L1 and L3 vertebrae were eliminated. Osteoarthritis can give false higher readings falsely showing the bones are healthier than they are.
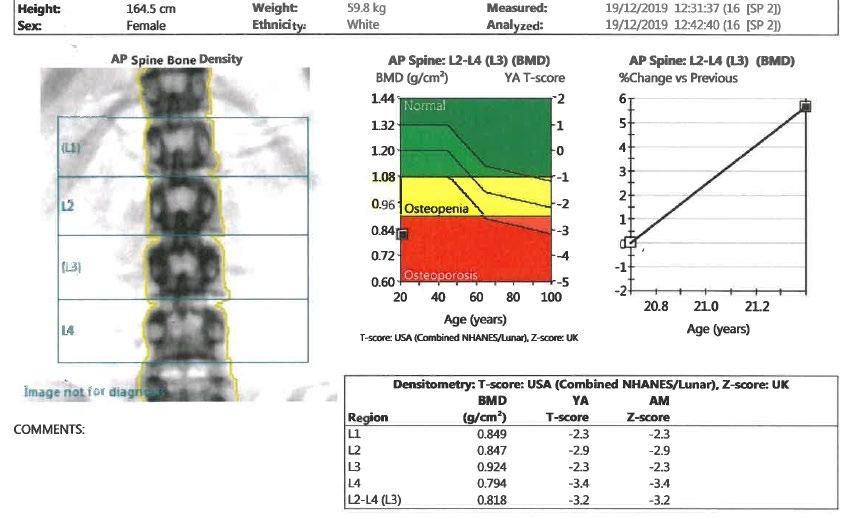
The disease Osteoporosis is commonly considered to affect just “Old” women, and many people do not appear to be aware that this disease is preventable and treatable in most people Women over 65 are the highest risk group for fractures, however it affects all age groups, including our young. Only 19% of people with Osteoporosis are diagnosed, which is why everyone should be checking to see if they have any of the 200 causes of bone loss.
Figure 1
This person’s causes of bone loss was due to overexercising, which caused irregular periods and she also had high cortisol levels due to a stressful job. By early intervention occurring the person so far has been able to improve her bone health and prevent vertebral fractures from occurring.
After the menopause, the ovaries stop producing oestrogen and the adrenal glands produce the male hormone testosterone, which is then converted into oestrogen by the peripheral fat. When a person is underweight, they do not have enough peripheral fat to assist with this process. Oestrogen is necessary to help protect bone and to also help in the formation of Vitamin D and absorption of calcium. When Vitamin D levels are low, bone loss occurs which increases the person risk of fractures.
1 in 7 women will get breast cancer and there is a breast screening service available. In an ideal world it would be wonderful if DXA scanning would become part of an overall health plan tied into a peri menopause screening, as 1 in 2 women aged 50 will break a bone from Osteoporosis in their lifetime.
The lesson to be learned is that prevention of breaking bones is much cheaper and less painful
An example of this is the DXA scan below It shows the results of a 21 year old female who was diagnosed with severe bone loss at 19 in her spine.
Some people are of the belief that DXA scans place a person at high risk of radiation exposure, if you have heard this, it is not accurate. A DXA scan exposes a patient to 10% radiation of a regular Chest X-ray. A person flying from Dublin to New York will be exposed to more radiation than a DXA scan. DXA scanning is what the IOS, the Osteoporosis national experts recommend for the screening, diagnosing and monitoring of bone loss.
Do you want to be the person or a loved one of yours who ends up with multiple fractures which could have been prevented, as research shows that most fractures are preventable?
The Irish Osteoporosis Society believe that focusing on over 65’s is playing “Russian roulette” with people’s lives, especially women’s lives. On the top of page 40 (figure 2) shows why all women should be screened for bone loss. The results also show that when a person has been diagnosed with Osteopenia, which is the early stages of bone loss, they MUST be monitored. The reason monitoring is so important is because bone loss is silent, NO one feels bone loss. There are no signs or symptoms of bone loss and logically all disease’s should be monitored and the DXA scan results show the
Her DXA results show she now has marked Osteopenia in L1 and L3, Osteoporosis at L2 and severe bone loss at L4
Thankfully she was diagnosed, investigated and put on a treatment plan You can see from
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 38
MUSCULOSKELETAL: OSTEOPOROSIS
and the DXA scan results show the woman’s bone health in her spine had declined by 40% in ten years and 23% in her hips
Could these fractures have been prevented? No one can say 100% yes. However logically, if she had been regularly monitored and when the initial bone loss occurred in 2018 her causes of bone loss had been investigated and addressed, there is a very high chance, the vertebral fractures could have been prevented.
The woman’s DXA scan results below show that she had a DXA scan done in 2012.
Her DXA results (aged 60) from 18 06 2018 show her bone density in her spine had declined 22.8% from her previous DXA scan which was done 6 years prior on 02 08 2012 (aged 54)
Her latest DXA scan results (aged 64) on 15 08 2022 show her bone density in her spine had declined by 17.2% since 18 06 2018, in 4 years
Monitoring of bone health is essential, it is common sense. If a person had a heart attack, what cardiologist do you know would say “Come back in 5 years for a check up”. There are those who believe repeat DXA scans should be every 5 years. This woman is a prime example of why if a person has bone loss, they need to be regularly monitored.
woman’s bone health in her spine had declined by 40% in ten years and 23% in her hips.

The woman’s DXA scan results below (figure 3) show that she had a DXA scan done in 2012.
Her DXA results (aged 60) from 18 06 2018 show her bone density in her spine had declined 22.8% from her previous DXA scan which was done 6 years prior on 02 08 2012 (aged 54).
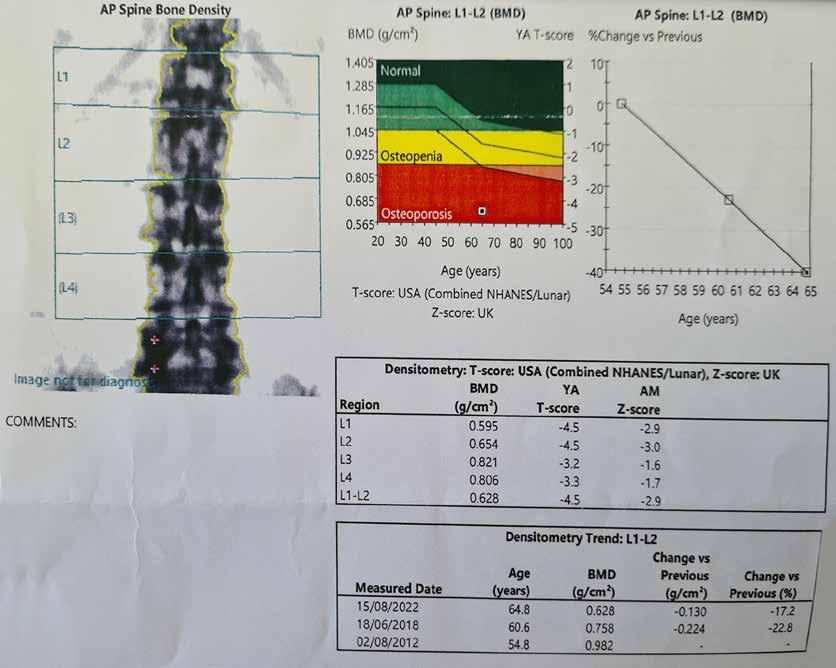
Her latest DXA scan results (aged 64) on 15 08 2022 show her bone density in her spine had declined by 17.2% since 18 06 2018, in 4 years.
The graph in the image on the top of page 41 (figure 4) shows how her BMD (Bone mineral Density) plummeted from age 54 to 64.
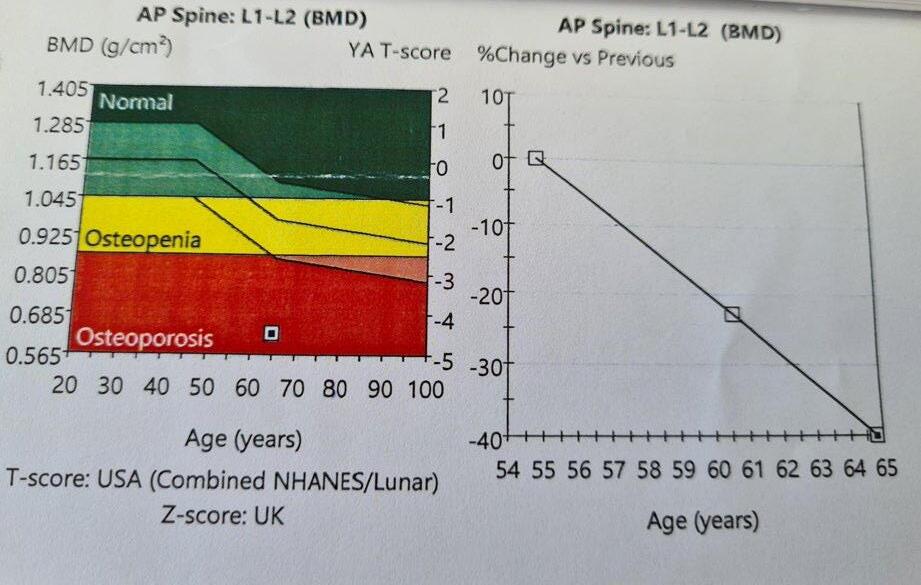
Her DXA results from 18 06 2018 show her bone density in her hips had declined by 13.3% in 6 years.
Her latest DXA scan results in the image below on 15 08 2022 show
her bone density in her hips had declined by a further 9.7% in four years.
The most significant results were from the LVA, which is a Lateral Vertebral Assessment which is a DXA side view of a person’s spine. This woman went through the menopause at 51.
The DXA LVA report at the bottom of page 41 (figure 5) shows she now has a T 5 Moderate Biconcavity fracture at T5 and T9 and a severe Biconcavity fracture at L1.
Osteoporosis is not like most other diseases because there are no signs or symptoms prior to someone fracturing. Most people are taking treatments to supress symptoms or slow down the progression of other diseases, Osteoporosis treatments have been proven to reduce fracture risk. It is essential to ensure a person is responding to their treatment and if a decline occurs switching a treatment should not be the first option. Investigating and addressing why their DXA scan results have declined or similar to the case above, investigating why they have fractured is crucial to help keep the person independent.
Cancer and Osteoporosis
Anyone who is going to receive chemotherapy or radiation for cancer should have a DXA scan done prior to the initiation of either treatment. If positive or negative for bone loss they should be put on a bone health plan, as both cause significant bone loss. If the person is negative for bone loss prior to the treatment, they need to be placed on an Osteoporosis treatment to prevent bone loss occurring. For a person to fight cancer to stay alive and end up with multiple fractures is not acceptable. Ireland is not a third world country and for any of you or your customers who have had either treatment or are on Medications like Arimidex or aromatase inhibitors, they need to be informed to get a DXA scan done.
HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 39
Figure 2
Figure 3
The graph in the image below shows how her BMD (Bone mineral Density) plummeted from age 54 to 64
Her DXA results from 18 06 2018 show her bone density in her hips had declined by 13.3% in 6 years.
Diabetes and Osteoporosis
There are two types of Diabetes Mellitus (DM). Type 1 which is a genetic disorder and people are usually diagnosed when they are young due to an insulin deficiency. Type 2 DM usually develops due to a poor dietary intake, and this usually develops over time. People with Type 1 and 2 DM are at risk of fractures due to Osteoporosis.
Menopause and Osteoporosis

When a woman goes through the menopause, it is very important it is not assumed that this is the only cause of her bone loss, as there are approx. 200 other causes of bone loss.
MUSCULOSKELETAL: OSTEOPOROSIS
• No stretches or exercise that put stress on the bones in your back such as yoga.
• No lifting of children or pets in and out of a cot, car etc
• Raised garden beds are ideal for those who love gardening
Most people are not aware that fractures should be taken far more seriously, due to the secondary complications which can be life threatening, which are a blood clot, pneumonia or infection. 20% of those who fracture their hip aged 60+ will pass away within 6 to 12 months and 50% will lose their independence. Vertebral fractures significantly impact on activities of daily living, such as washing, dressing and walking.
Menopause and Bone Health Tips
• HRT should only be given to those who go through an early menopause before age 45, who do not have contraindications, and those who have menopausal symptoms.
• HRT should not be prescribed just to protect bone health.
• For those who are going through an early menopause HRT should consist of oestrogen and progesterone. Oestrogen only should not be prescribed unless they have had a hysterectomy.
• Premenopausal females who have low oestrogen e.g. due to an eating disorder, can be prescribed either the contraceptive pill, i.e, an oestrogen and a progestogen, or HRT, that contains a different type of oestrogen and progestogen but ensure to tell them that it is not a contraceptive.
• Post-menopausal women who have an early menopause before age 45, if they still have a uterus, should be prescribed HRT consisting of oestrogen and progestogen, because otherwise it can increase the risk of cancer of the body of the uterus.
• Post-menopausal women who have an early menopause before age 45, If they have had their uterus removed they can go on oestrogen only.
Severe bone lossA T score of -3.0 or higher on a DXA scan report
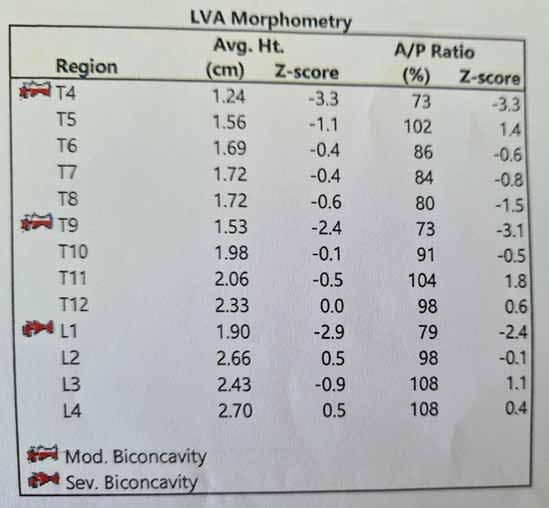
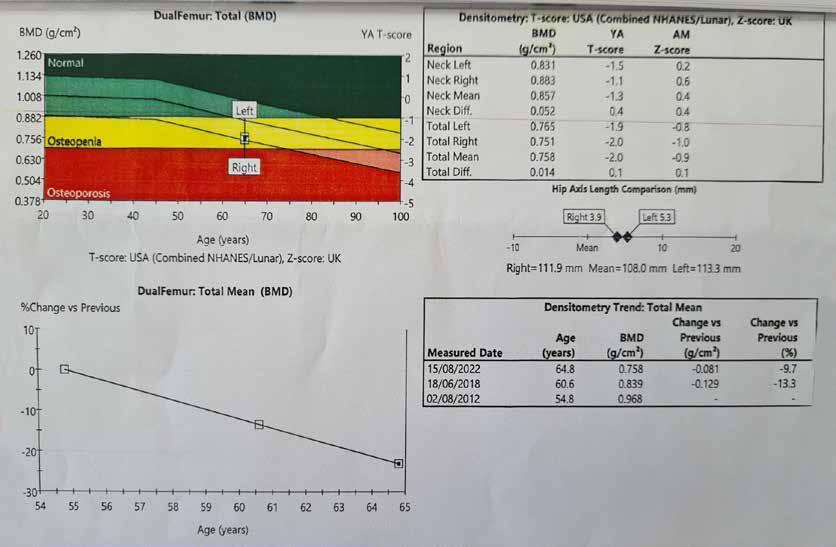
• Avoid when possible, bending forward at your waist with legs straight
• Initially, no lifting anything over 3 pounds
• Put food items in a trolley and only half fill the bags
• Do not use a vacuum, stretch up for items, or twist your body to either side with your feet planted on the ground.
A person can be diagnosed with Osteopenia and/or Osteoporosis. Osteopenia is the precursor to Osteoporosis, and it is essential that the reasons why a person has lost bone is investigated and addressed, not assumed.
NOTE A calcium and vitamin D supplement is not an Osteoporosis treatment. Calcium, vitamin D and first class proteins when ever possible should be from foods.
Men and Osteoporosis
The diagnosis rate for Osteoporosis in men is very low and the IOS believe it is because of the myth that “Osteoporosis only affects old women”. 1 in 4 men over 50 will break a bone from Osteoporosis in their lifetime. More men pass away from Osteoporosis then get prostate cancer, which is why all men should be checking to see if they have risk factors for bone loss.
Exercise and Bone Health
Appropriate weight bearing exercise is very important for those with bone loss, as well as improving muscle strength and balance. A very common stretch done by people is forward flexion which is shown in the image at the top of page 42 (figure 6). The IOS does not recommend this stretch for anyone other than athletes due to the damage it can inflict on a person. This stretch puts excess compression on the front of the body of the vertebrae and strain on the discs between the vertebrae. This stretch also puts excess strain on your sciatic nerve in your back, that if irritated will cause pain to go down your leg.
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 40
Figure 4
Figure 5
DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN
Rebuild bone before it breaks again—with Movymia®1
THE MEDICINES MANAGEMENT PROGRAMME RECOMMENDS
MOVYMIA® AS A BEST VALUE MEDICINE FOR TERIPARATIDE
RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,3,*

EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,4
AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage5,6
RE-USABLE: One high quality reuseable pen for the entire treatment period1
MOVYMIA 20 MICROGRAMS/80 MICROLITERS SOLUTION FOR INJECTION
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teriparatide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance. Website: www.hpra.ie Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject toedical prescription. Date last revised: January 2022.
1. Movymia® SmPC. 2. Best Value Medicines https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/best-value-medicines/teriparatide/ Accessed February 2023.
3. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf
4. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 5. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 6. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo®
Date prepared: March 2023. 2023/ADV/MOV/061H
2
Signs and symptoms of possible undiagnosed Osteoporosis
• A broken bone caused by a trip and fall from a standing position or less, even if on cement or ice.
• Many people believe that it is normal to lose height as you get older, it can be a red flag for undiagnosed vertebral fractures.

• A person’s head protruding forward from their body or their shoulders becoming rounded or a hump developing on their upper back can be sign that vertebral fractures have occurred.
We do not recommend the following for those with bone loss:
The excess pressure on the vertebrae can cause Vertebral fractures. Professor Moira O’Brien who set up the first Osteoporosis clinic in Ireland as well as being the only Osteoporosis consultant in Ireland, has had patients in their 40’s who fractured vertebrae due to this stretch and the IOS have received helpline calls from people who have also fractured from this forward flexion stretch.
depend on the person’s age, if they have broken any bones yet, their DXA scan results, medical history, causes of bone loss, balance, ability and age.
Walking, but the speed and course need to be altered.
Touching your toes while in the sitting position or when standing bending forward at your waist, doing regular sit ups, exercise on trampolines, excessive weights, yoga, horse riding, skiing, twisting your back with your feet on the ground (golf swing), vibrating machines, high impact exercises such as jumping off boxes or heights or pounding your feet when walking. Jogging and running is not recommended for those who have vertebral fractures
Walking up and down stairs works the main muscles for walking, is great for your overall health and helps to build up bone in the hip area. So instead of listening to the adverts on TV or watching your partner switch from one station to another during the breaks, why not walk up and down your stairs a couple of times.
Fall Prevention - Balance and coordination including ensuring your home environment is safe. Example – A good pair of walking shoes instead of wearing slippers, as most do not have good support and no one should wear open back shoes as they are a tripping hazard. Incontinence - There have been significant advances in the treatment for incontinence, which can lead to falls, due to people rushing to the bathroom or slipping on urine after having an accident, please contact the Irish Osteoporosis Society for more details.
• A person’s body shape changing, such as a pot belly developing, an increase in clothes size, or the person not being able to reach previously accessible shelves.
NOTE: Many stretches in Yoga place stress on the bones in the spine, which is why the IOS do not ever recommend yoga for those with and without bone loss.
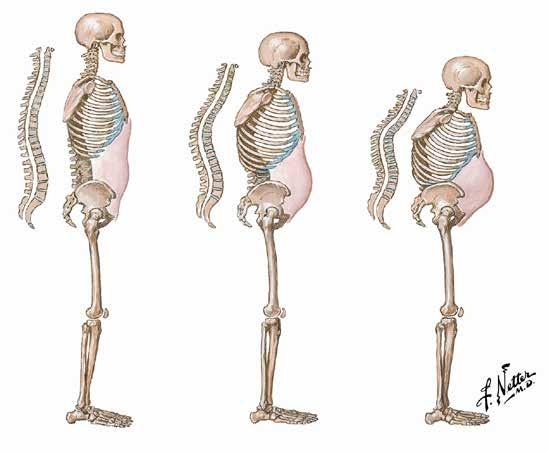
We do not recommend the following for those with bone loss:
Risk Factors for Bone loss
NOTE: The IOS does not recommend anyone with bone loss start an online exercise programme or weights programme, without having been individually assessed by a health care professional, who is up to date on bone loss. Why? To help reduce the risk of people getting hurt. In order for a health professional to put a person with bone loss on a safe and appropriate exercise programme, they must understand DXA scan results. A person can look perfectly fine but have severe bone loss.
• Upper, middle or low back pain should be investigated, and Osteoporosis ruled out, as 75% of people with vertebral fractures have intermittent back pain.
The image below (figure 7) shows the effects of undiagnosed/ untreated bone loss at any age.
Summary
People need the causes of their bone loss investigated and addressed
Walking, but the speed and course need to be altered
When it is raining or very windy outside When the weather is very wet and windy, why not stand in front of your favourite TV programme and march on the spot while watching it or while listening to your favourite radio programme or walk around your home, including going up and down your stairs, as every step counts to help keep your independence.
Family history of Osteoporosis or broken bones (as 81% not diagnosed), menopause/ hysterectomy, corticosteroids, Chemotherapy, Radiation, Arimidex, Aromatase inhibitors, Physiological or psychological stress, RA, Protein pump inhibitors, eating disorders & over exercisers, low vitamin D levels, many medications for anxiety and depression.
We DO recommend the following exercises/activities for those with bone loss, but they may not be appropriate for everyone. It will depend on the person’s age, if they have broken any bones yet, their DXA scan results, medical history, causes of bone loss, balance, ability and age.
People need to prevent falls by making their home safe
People need to ensure they are doing appropriate exercise
People need to ensure their bone health is monitored, as bone loss is silent!
Touching your toes while in the sitting position or when standing bending forward at your waist, doing regular sit ups, exercise on trampolines, excessive weights, yoga, horse riding, skiing, twisting your back with your feet on the ground (golf swing), vibrating machines, high impact exercises such as jumping off boxes or heights or pounding your feet when walking. Jogging and running is not recommended for those who have vertebral fractures.
Dancing, as long as you have good balance, and you are not being swung around.
Walking up and down stairs works the main muscles for walking, is great for your overall health and helps to build up bone in the hip area. So instead of listening to the adverts on TV or watching your partner switch from one station to another during the breaks, why not walk up and down your stairs a couple of times.
NOTE: The IOS does not recommend anyone with bone loss start an online exercise programme or weights programme, without having been individually assessed by a health care professional, who is up to date on bone loss. Why? To help reduce the risk of people getting hurt. In order for a health professional to put a person with bone loss on a safe and appropriate exercise programme, they must understand DXA scan results. A person can look perfectly fine but have severe bone loss.
When it is raining or very windy outside When the weather is very wet and windy, why not stand in front of your favourite TV programme and march on the spot while watching it or while listening to your favourite radio programme or walk around your home, including going up and down your stairs, as every step counts to help keep your independence
Dancing, as long as you have good balance, and you are not being swung around
We DO recommend the following exercises/activities for those with bone loss, but they may not be appropriate for everyone. It will

Strength and conditioning programme, to include core stability. Senior citizens should start out actively exercising their limbs without weights. Light weights should be slowly introduced, as rushing to increase weights and resistance, can place a senior citizen at risk of muscle and joint soreness, which could turn them off exercising, and place those with bone loss at risk of possible fractures.
For those who had previously lifted weights, they should start with low weights and gradually increase.
Exercise in water can help build up muscle strength and endurance, without putting excess stress on joints.
MUSCULOSKELETAL: OSTEOPOROSIS MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 42
Figure 6
Figure 7
Lead your patients to stronger bones with Prolia®1-4

6,7,8,9*
* Prolia® should not be stopped without considering alternative treatment in order to prevent rapid bone mineral density loss and a potential rebound in vertebral fracture risk.6
PROLIA® (denosumab) Brief Prescribing Information
Please refer to the Summary of Product Characteristics (SmPC) before prescribing Prolia. Pharmaceutical Form: Pre-filled syringe with automatic needle guard containing 60 mg of denosumab in 1 ml solution for injection for single use only. Contains sorbitol (E420). Indication: Treatment of osteoporosis in postmenopausal women at increased risk of fractures. Dosage and Administration: 60 mg Prolia administered as a subcutaneous injection once every 6 months. Patients must be supplemented with calcium and vitamin D. No dosage adjustment required in patients with renal impairment. Prolia should not be used in children aged < 18 years because of safety concerns of serious hypercalcaemia. Give Prolia patients the package leaflet and patient reminder card Re-evaluate the need for continued treatment periodically based on the benefits and potential risks of denosumab on an individual patient basis, particularly after 5 or more years of use. Contraindications: Hypocalcaemia or hypersensitivity to the active substance or to any of the product excipients. Special Warnings and Precautions: Traceability: Clearly record the name and batch number of administered product to improve traceability of biological products. Hypocalcaemia: Identify patients at risk for hypocalcaemia Hypocalcaemia must be corrected by adequate intake of calcium and vitamin D before initiation of therapy. Clinical monitoring of calcium levels is recommended before each dose and, in patients predisposed to hypocalcaemia, within 2 weeks after the initial dose. Measure calcium levels if suspected symptoms of hypocalcaemia occur. Renal Impairment: Patients with severe renal impairment (creatinine clearance < 30 ml/min) or receiving dialysis are at greater risk of developing hypocalcaemia. Skin infections: Patients receiving Prolia may develop skin infections (predominantly cellulitis) requiring hospitalisation and if symptoms develop then they should contact a health care professional immediately. Osteonecrosis of the jaw (ONJ): ONJ has been reported rarely with Prolia 60 mg every 6 months. Delay treatment in patients with unhealed open soft tissue lesions in the mouth. A dental examination with preventative dentistry and an individual benefit:risk assessment is recommended prior to treatment with Prolia in patients with concomitant risk factors. Refer to the SmPC for risk factors for ONJ. Patients should be encouraged to maintain good oral hygiene, receive routine dental check-ups and immediately report oral symptoms during treatment with Prolia. While on treatment, invasive dental procedures should be performed only after careful consideration and avoided in close proximity to Prolia administration. The management plan of patients who develop ONJ should be set up in close collaboration between the treating physician and a dentist or oral surgeon with expertise in ONJ. Osteonecrosis of the external auditory canal: Osteonecrosis of the external auditory canal has been reported with Prolia. Refer to the SmPC for risk factors. Atypical femoral fracture (AFF): AFF has been reported in patients receiving Prolia. Discontinuation of Prolia therapy in patients suspected to have AFF should be considered pending evaluation of the patient based on an individual benefit risk assessment. Long-term antiresorptive treatment: Long-term antiresorptive treatment may contribute to an increased risk for adverse outcomes such as ONJ and AFF due to significant suppression of bone remodelling.
Concomitant medication: Patients being treated with Prolia should not be treated concomitantly with other denosumab containing medicinal products. Warnings for Excipients: Patients with rare hereditary problems of fructose intolerance should not use Prolia. Interactions: Prolia did not affect the pharmacokinetics of midazolam, which is metabolized by cytochrome P450 3A4 (CYP3A4). There are no clinical data on the co-administration of denosumab
and hormone replacement therapy (HRT), however the potential for pharmacodynamic interactions would be considered low. Pharmacokinetics and pharmacodynamics of Prolia were not altered by previous alendronate therapy. Fertility, pregnancy and lactation: There are no adequate data on the use of Prolia in pregnant women and it is not recommended for use in these patients. It is unknown whether denosumab is excreted in human milk. A risk/ benefit decision should be made in patients who are breast feeding. Animal studies have indicated that the absence of RANKL during pregnancy may interfere with maturation of the mammary gland leading to impaired lactation post-partum. No data are available on the effect of Prolia on human fertility. Undesirable Effects: The following adverse reactions have been reported: Very common (≥ 1/10) pain in extremity, musculoskeletal pain (including severe cases). Common (≥ 1/100 to < 1/10) urinary tract infection, upper respiratory tract infection, sciatica, constipation, abdominal discomfort, rash, alopecia and eczema. Uncommon (≥ 1/1000 to < 1/100): Cellulitis, ear infection and lichenoid drug eruptions. Rare (≥ 1/10,000 to < 1/1,000): Osteonecrosis of the jaw, hypocalcaemia (including severe symptomatic hypocalcaemia and fatal cases), atypical femoral fractures, and hypersensitivity (including rash, urticaria, facial swelling, erythema and anaphylactic reactions). Very rare (< 1/10,000): Hypersensitivity vasculitis. Please consult the Summary of Product Characteristics for a full description of undesirable effects. Pharmaceutical Precautions: Prolia must not be mixed with other medicinal products. Store at 2°C to 8°C (in a refrigerator). Prolia may be exposed to room temperature (up to 25°C) for a maximum single period of up to 30 days in its original container. Once removed from the refrigerator Prolia must be used within this 30 day period. Do not freeze. Keep in outer carton to protect from light. Legal Category: POM. Presentation and Marketing Authorisation Number: Prolia 60 mg: Pack of 1 pre-filled syringe with automatic needle guard; EU/1/10/618/003. Price in Republic of Ireland is available on request. Marketing Authorisation Holder: Amgen Europe B.V., Minervum 7061, NL-4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. Prolia is a registered trademark of Amgen Inc. Date of PI preparation: May 2022 (Ref: IE-PRO-0322-00006) Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse reactions/events should also be reported to Amgen Limited on +44 (0)1223 436441 or Freephone 1800 535 160.
References:
1. Prolia® [denosumab], Summary of Product Characteristics.
2. Cummings SR et al, N. Eng. J Med 2009;361:756-765.
3. Holzer G et al, J Bone Miner Res 2009;24(3):468-74.
4. Poole K et al; J Bone Miner Res 012; 27 (suppl1):S44 abstract 1122.
5. Bone HG, et al; Lancet Diabetes Endocrinol. 2017;5:513-23;3-23.
6. Tsourdi E, et al. Bone. 2017;105:11–7.
7. Hernlund E, et al. Arch Osteoporos. 2013;8:136;
8. Kanis JA, et al. Osteoporos Int. 2019;30:3–44;
9. Brown JBMR 2013 Vol28 pp746-752.
Amgen Ireland 21 Northwood Court, Santry, Dublin 9, Ireland. © 2022 Amgen Inc. All rights reserved. IE-PRO-0522-00002. Date of preparation: June 2022 10 YEARS DATA5
Osteoporosis is a serious ongoing condition and ongoing treatment is required.
Advances in Giant Cell Arteritis
Written by Dr Anna Witkowska, Rheumatology Registrar, Department of Rheumatology Mater Misericordiae University

Giant cell arteritis (GCA) is an immune mediated inflammatory disease of the large vessels, most commonly affecting cranial arteries but the aorta and its branches can also be involved. The inflammation initiates in the inner layers of the artery, eventually progressing to transmural inflammation, vessel occlusion and then resultant ischaemia. Patients can present with inflammatory and/ or ischaemic symptoms.
GCA is the most common systemic vasculitis among adults. Prevalence increases with age, and it is highest in people with Northern European ancestry, ranging from 18 to 29 cases for 100,000 people over the age of 50.1 It is estimated that the occurrence is likely to increase with ageing population, and it is projected that over 3 million people will be affected by GCA by 2050.2 Whilst the prevalence is highest among patients of northern European ancestry, it is important to note that patients of any ethnic background can be affected.
The most serious complication of GCA is irreversible visual loss due to anterior ischaemic optic neuropathy caused by choroidal
blood vessel inflammation and ischaemia. It occurs in 15-20% of patients with GCA.3 This risk can be reduced to as low as 9% by prompt treatment (high dose corticosteroid) initiation.4 Other less common ischaemic complications include: cerebrovascular infarcts, cranial nerve palsies, infarction of tongue and scalp necrosis. The standard therapy is high dose glucocorticoids which can result in considerable toxicity especially in this often frail and elderly cohort. Thankfully there have been recent advances in therapeutics which can reduce the cumulative burden of glucocorticoids and are discussed in more detail below.
Diagnostic challenges

GCA is a challenging medical diagnosis. Clinical symptoms vary. It is often associated with a new onset headache, usually around the temples however frontal or occipital headaches can also be presenting features. Headache in itself is a non-specific symptom and other more common causes of headache such as migraine, tension headache, cervicogenic headache should be considered when evaluating patients. On
physical exam, the temporal artery can be thickened and tender to palpation. Other less well recognized but much more GCA- specific features include: visual changes such as blurred vision, diplopia or visual loss (either transient or permanent). Jaw claudication is an important sign of GCA that can be easily missed. It is characterized by pain or discomfort in the jaw on chewing and is caused by impaired blood flow to the pterygoid and masseter muscles in the jaw secondary to vessel wall inflammation leading to ischaemia and pain. Ischaemic in nature, it typically radiates down the jaw on chewing. This can be easily confused with pain caused by TMJ pathology, which is usually more localized to the TMJ and is not typically associated with chewing. 40-60% of GCA patients will have symptoms of polymyalgia rheumatica (PMR) which include morning stiffness and pain affecting the shoulder and hip girdle.5 Indeed, GCA and PMR are considered to be on the spectrum of the same disease process, with PMR considered by some as a vascular inflammation limited to the adventitia. In patients with PMR who have suboptimal response to steroids, constitutional symptoms or markedly increased inflammatory markers, GCA in the form of aortitis should be considered. Subclinical GCA can be detected in patients with PMR on vascular imaging such as ultrasound, PET-CT, MRA or CTA. To aid with clinical diagnosis, the Southend GCA probability score has been validated as a risk assessment tool for suspected GCA. The cumulative, weighted score is based on several risk factors for GCA, such as age, presence of headache, ischaemic signs/ symptoms or visual changes. It can help stratify patients into low, moderate and high probability risk of having GCA (when used correctly by professionals familiar with diagnosing GCA).6
Advances in imaging

Different imaging techniques can be used to aid the diagnostic process. Temporal artery ultrasound (TAU) is used in many centres and its diagnostic value has been studied extensively. TAU is used to detect 'halo sign', a non-compressible hypoechoic ring artery lumen that represents vessel oedema resulting from inflammation. [Fig 1] Its sensitivity was found to be 54% with 81% specificity in TABUL study, in which TAU was compared with the gold standard temporal artery biopsy (TAB).7 It is worth mentioning that halo sign may only last for 2-3 days8 after commencing glucocorticoid therapy, thus it should not be delayed. Ultrasound is also used for detection of subacromial and subdeltal bursitis in PMR and it is included in the ACR-EULAR classification criteria for PMR. Another diagnostic tool in GCA work up is FDG-PET. Vascular inflammation is associated with increased glucose metabolism which can be detected by PET CT. This imaging modality can be used to identify aortitis in patients with marked systemic symptoms, treatment resistant disease or constitutional symptoms such weight loss or night sweats where other pathologies ( e.g. infection, malignancy) also need to be excluded.9 In PMR, increased tracer uptake is found in hip and shoulder girdle.10
High resolution MRI was found to have sensitivity of 88% and specificity of 75%.11 It detects soft tissue oedema of the wall of the blood vessels and it is helpful in assessing potential GCA-related vessel stenosis or aneurysm. It is, however, not routinely available in every centre and thus has a limited clinical utility at present.
As previously mentioned, temporal artery biopsy is the gold standard in GCA diagnosis. A positive histological result is undoubtedly the most definite diagnostic criterion. When performed by
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 44 MUSCULOSKELETAL: ARTERITIS
Dr John Stack
Hospital & Dr John Stack, Consultant Rheumatologist, Mater Private Hospital
Dr Anna Witkowska
Cross-sectional
an experienced surgeon it is a relatively low risk procedure. Potential risks include infection and, rarely, potential damage to the branches of facial nerve resulting in eyebrow droop. Sensitivity of TABs is centre dependent and is often low. Potential reasons for this include presence of 'skipped lesions', meaning that the result can be indeed negative if the inflammation involves part of the vessel that was missed during biopsy. Delayed access to TAB can also result in lower diagnostic yield.
In summary, clinical diagnosis of GCA should be considered based on clinical signs and symptoms together with different imaging techniques. Temporal artery ultrasound is considered to be the first line diagnostic tool as it is non-invasive, fast and costeffective. If in clinical doubt, TAU can be followed by temporal artery biopsy.
As TAU images are affected after initiating treatment, development of rapid diagnostic pathways with access to
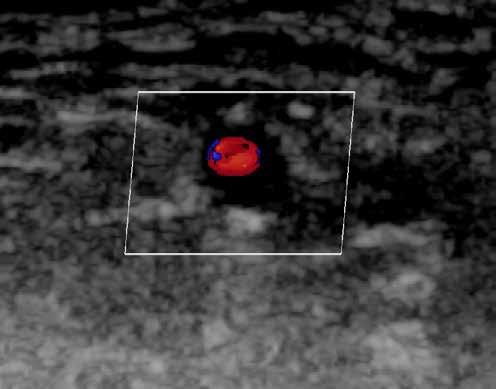
NEWS
ultrasound is desirable. This has shown reduction in inpatient hospitalizations, visual loss and healthcare associated costs.
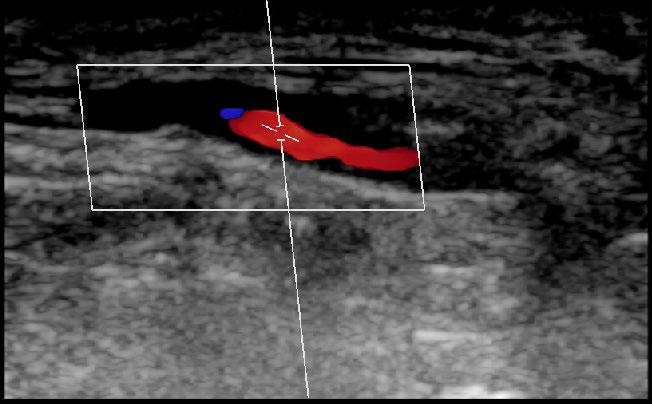
Advances in therapy
As long-term glucocorticoid treatment is associated with an array of complications, especially in an older population, there is a need for new glucocorticoidsparing therapies that are safer yet effective in treatment of GCA.
Tocilizumab, an anti-IL6 receptor monoclonal antibody, showed promising results in randomizedcontrolled trials in treatment for GCA as well as PMR. It was approved for treatment of GCA in 2017, and its use is associated with higher remission rate and shorter period of glucocorticoid use. It is a self-administered subcutaneous injection given every 1 to 2 weeks or, alternatively, it can be given as an IV infusion.
In the GiACTA study, 251 patients were randomly assigned to subcutaneous tocilizumab every week or every
New Wing opens at Mater Hospital
other week, combined with 26-week prednisone taper, or placebo combined with 26 or 52 prednisone taper. Sustained remission after 52 weeks was found in 56% of patients with weekly tocilizumab group and 53% of patients that received tocilizumab every other week on 26-week prednisone taper, as compared to 14% in the placebo group with 26-week prednisone taper and 18% of placebo group with 52-week prednisone taper [12]. Thus, tocilizumab was shown to be more effective than placebo and patients could safely taper steroids over 26 weeks. Some preliminary studies have also shown that use of TOC can protect against visual loss.
Sirukumab another anti IL-6 monoclonal antibody that has similarly shown lower incidence of flares when compared to a placebo in a multi-centre, double-blind trial of 161 patients with GCA.13
Finally a recent phase 2 study showed that Mavrililumab (antigranulocyte-macrophage colonystimulating factor (GM-CSF)) plus

26-week prednisone taper was superior to placebo plus 26 week prednisone taper in a doubleblind, placebo controlled trial involving 72 patients.14 This has laid the ground work for a phase 3 study, the results of which will be eagerly awaited.
In summary, following many years of little progress in terms of diagnostics and targeted therapies the past decade has seen significant advances in terms of diagnosing and treating GCA. Furthermore there is hope of more advanced therapies becoming available in the near future. These advances will help to reduce the frequency of complications of the disease such as blindness whilst also reducing the steroid burden and associated toxicities for these patients.
References available on request
The Taoiseach Leo Varadkar and the Minister for Health Stephen Donnelly have officially opened a new nine storey building at the Mater Misericordiae University Hospital in Dublin. The new wing comprises 98 new beds, including 16 new intensive care unit beds and a new home for the National Isolation Unit, which is based at the Mater.
This new building, to be called “The Rock Wing”, has been designed, built and delivered on budget in two years under emergency legislation enacted during the first part of the Covid pandemic. In the early part of the response to Covid-19, the lack of bed capacity, particularly intensive care facilities and appropriate single bedded rooms, was identified as an issue facing many acute hospitals. The wing cost approximately ¤103 million to build and it is estimated that because of the fast-track approach adopted under the emergency legislation, this resulted in a saving of some ¤40 million for the taxpayer.
The new building at the Mater will cater for an additional 5,000 patients annually in world class facilities.
Speaking at today's event, Taoiseach Leo Varadkar said: “Projects like these turn the vision of Sláintecare into reality. We all know we need to fundamentally change the way we provide healthcare if we are to meet the ever-increasing needs of our growing and ageing population, not to mention the wide array of new treatments becoming available. Investment in healthcare infrastructure, like this new facility, is a big part of achieving what we want to achieve and the budget for new healthcare infrastructure now exceeds ¤1bn a year.”
Fig 1
HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 45
and longitudinal vascular ultrasound of a patient with GCA demonstrating concentric ring of vessel wall oedema- ‘Halo sign’
Fig 1: Cross-sectional and longitudinal vascular ultrasound of a patient with GCA demonstrating concentric ring of vessel wall oedema- ‘Halo sign’
Demystifying Ehlers Danlos Syndrome
Written by: Robert Harrington MB BCh BAO, MRCPI, Department of Rheumatology, Beaumont Hospital, Dublin, Ireland
Laura Durcan MD FRCPI, Department of Rheumatology, Beaumont Hospital, Dublin, Ireland
muscular pain, fatigue and often palpitations, dizziness, presyncope and symptoms suggestive of postural orthostatic tachycardia syndrome (POTS).

Writen by Robert Harrington

History
Ehlers Danlos syndrome (EDS) is a group of inheritable collagen disorders which present with a constellation of clinical features including skin fragility, hyperextensibility, brittle blood vessels, atrophic scarring, and joint hypermobility. While hypermobility disorders were recognised by Hippocrates, the eponymous syndrome was first described by Danish dermatologist Edvard Ehlers and French dermatologist Alexandre Danlos in Paris in 1936.1
Overview
Today 13 subtypes of EDS are recognised. The combined prevalence of all EDS variants is estimated at 1 in 5,000 with the hypermobility variant the most common at approximately 1 in 10,000.2, 3 Given EDS is a collagen disorder, involvement of blood vessels, heart valves, internal organs, bone and even sclerae, may present as childhood bleeding diathesis, varicose veins in early adulthood, aneurysmal rupture, cardiac failure, uterine rupture during pregnancy, gastrointestinal dysmotility and perforation, spontaneous pneumothoraces, severe scoliosis and even ocular complications. Fortunately, these are all rare with most catastrophic outcomes occurring in those with the vascular EDS (vDS) variant.
Department of Rheumatology, Beaumont Hospital, Dublin, Ireland. Laura Durcan MD FRCPI

Department of Rheumatology, Beaumont Hospital, Dublin, Ireland.
History
While hEDS tends to display an autosomal dominant (AD) inheritance pattern there is no known associated monogenic cause. This cohort are hypermobile by definition and therein lies the controversy as 10-30% of the general population meet the required hypermobility Beighton score of 5 or more for diagnosis.5 If this degree of “hypermobility” is coupled with a history of chronic widespread pain and a family history of hEDS, then it becomes all too easy for this diagnostic label to be assigned.
pseudotumours (calcified haematomas), spheroids and poor post-surgical incision site healing, life expectancy is still unaffected. In paediatrics, easy bruising, hypotonia and delayed motor developmental milestones with a family history of hypermobility should raise suspicion of this disorder. Despite similarities to the hEDS variant, the distinguishing feature of cEDS is the pronounced skin involvement.
Classical-Like EDS
The Most Common Variants Hypermobile EDS
Classical EDS
Ehlers Danlos syndrome (EDS) is a group of inheritable collagen disorders which present with a constella�on of clinical features including skin fragility, hyperextensibility, britle blood vessels, atrophic scarring, and joint hypermobility. While hypermobility disorders were recognised by Hippocrates, the eponymous syndrome was first described by Danish dermatologist Edvard Ehlers and French dermatologist Alexandre Danlos in Paris in 1936. [1]
The diagnosis of hypermobile EDS (hEDS) remains particularly controversial and divisive amongst physicians. It was previously known as EDS type 3 and was historically characterised by frequent dislocations or subluxations of large and small joints alike, along with smooth, soft, velvet like skin with a marked predisposition to easy bruising. There is less of a tendency towards hyper-elasticity of the skin. Some affected individuals tend to report chronic
Overview
Classical EDS (cEDS) has historically accounted for 80% of EDS cases reported in the literature.6 It displays AD inheritance pattern with mutations typically affecting the production of type V collagen (COL5A1, COL5A2). These patients display hypermobility with joint dislocations, increased skin elasticity with a soft velvet like quality and a predisposition to atrophic scaring. While cEDS patients may develop hernias, uterine complications, molluscoid
Classical-like EDS (cLEDS) displays an autosomal recessive (AR) pattern with a mutation in the TNXB gene on chromosome 6 causing a tenascin X deficiency. Given the role of tensascin X in the function of healthy type 1 collagen, patients with this mutation also display hypermobility, skin hyperextensibility and easy bruising. The differentiating hallmark in cLEDS as opposed to cEDS is the absence of atrophic papyraceous scars. These patients may also develop mild muscle weakness, atrophy of hand and foot muscles, lower limb oedema and cavitary prolapse.
Vascular EDS
Vascular EDS (vEDS) displays an AD inheritance pattern, and unlike the classical or
Today 13 subtypes of EDS are recognised. The combined prevalence of all EDS variants is es�mated at 1 in 5,000 with the hypermobility variant the most common at approximately 1 in 10,000. [2][3] Given EDS is a collagen disorder, involvement of blood vessels, heart valves, internal organs, bone and even sclerae, may present as childhood bleeding diathesis, varicose veins in early adulthood, aneurysmal rupture, cardiac failure, uterine rupture during pregnancy, gastrointes�nal dysmo�lity and perfora�on, spontaneous pneumothoraces, severe scoliosis and even ocular complica�ons. Fortunately, these are all rare with most catastrophic outcomes occurring in those with the vascular EDS (vDS) variant.
EDS Variant Inheritance Patern Associated Gene
1 Classical (cEDS) AD COL5A1, COL5A2, COL1A1
2 Classical-like (cLEDS) AR TNXB
3 Cardiac-Valvular (cvEDS) AR COL1A2
The 2017 Interna�onal Classifica�on of the Ehlers-Danlos syndromes [4]
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 46 MUSCULOSKELETAL:
DANLOS
EHLERS
Robert Harrington
Laura Durcan MD FRCPI
Demys�fying Ehlers Danlos Syndrome
4 Vascular (vEDS) AD COL3A1, COL1A1
Arthrochalasia
5 Hypermobile (hEDS) AD None iden�fied 6
(aEDS) AD COL1A1, COL1A2 7 Dermatosparaxis (dEDS) AR ADAMTS2 8 Kyphoscolio�c (kEDS) AR PLOD1, FKBP14
Myopathic
9 Britle Cornea syndrome (BCS) AR ZNF469, PRDM5 10 Spondylodysplas�c (spEDS) AR B4GALT7, B3GALT6, SLC39A13 11 Musculocontractural (mcEDS) AR CHST14, DSE 12
(mEDS) AD/AR COL12A1 13 Periodontal (pEDS) AD C1R, C1S
The Most Common Variants Hypermobile EDS
The 2017 International Classification of the Ehlers-Danlos syndromes4
appearance of the eyes all the more prominent. As a result, when the classical facial as in the majority of cases, vEDS may remain undiagnosed in those with de novo catastrophic acute presenta�on.
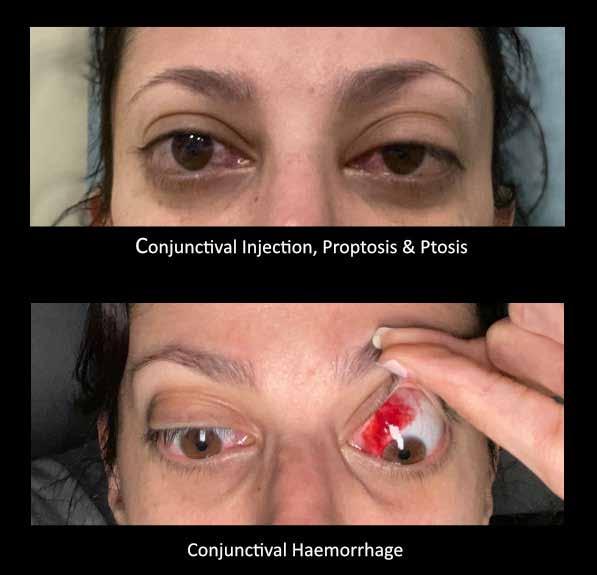

classical-like variants, it is associated with premature mortality with a historical mean survival of 51 years owing largely to arterial or visceral rupture.7 vEDS occurs due to a mutation affecting type 3 collagen (COL3A1). For every 10 cases of classical EDS, there exists 1 case of vascular EDS in the general population. This makes vEDS is exceptionally rare at approximately 1 case per 200,000 people.
Eyes of Vascular EDS
While it displays an AD inheritance pattern, 50% of cases occur as a result of a de novo mutation. Younger individuals are protected from aneurysm formation until the end of puberty when elastin production drops off dramatically. Skin may appear translucent making veins very noticeable. vEDS patients tend to display faster skin aging particularly where skin is thinner over the hands and feet. Significantly, there is no increased skin laxity like in other forms of EDS. Hypermobility is only present in the fingers and there may be a predisposition to easy bruising.
appearance of the eyes all the more prominent. As a result, when the classical facial features are absent as in the majority of cases, vEDS may remain undiagnosed in those with de novo mutations until catastrophic acute presentation.
The Red Flag Red Eyes of Vascular EDS
in pa�ents in their teens and twen�es should be considered in those that develop spontaneous pneumothoraces, bowel rupture or varicose veins. Ul�mately 80% will age of 40 with a vascular event. Commonly aor�c aneurysms and caro�dfistulae develop over �me. In the later such cases, pa�ents may present with swelling, conjunc�val injec�on or haemorrhage, pulsa�ng proptosis of the ocular globe palsy, all of which are due to arterialisa�on of blood flow from the caro�d artery sinus and the superior ophthalmic vein. The risk of aor�c aneurysmal rupture is yet opera�ve interven�on has historically conferred a 1 in 3 risk of death within opera�ve period. There is also a high risk of uterine rupture in vEDS with pregnancy 5% mortality risk to the mother.
This is the case of patient A, a woman in her early 30s who was originally investigated for a collagen disorder in her 20s after severe bleeding and poor wound healing post varicose vein stripping. She looked different to other family members on account of her large eyes, slender facial features and propensity to easy bruising. Ms. A was diagnosed with vascular Ehlers Danlos after specialist review and genetic testing ex domo.
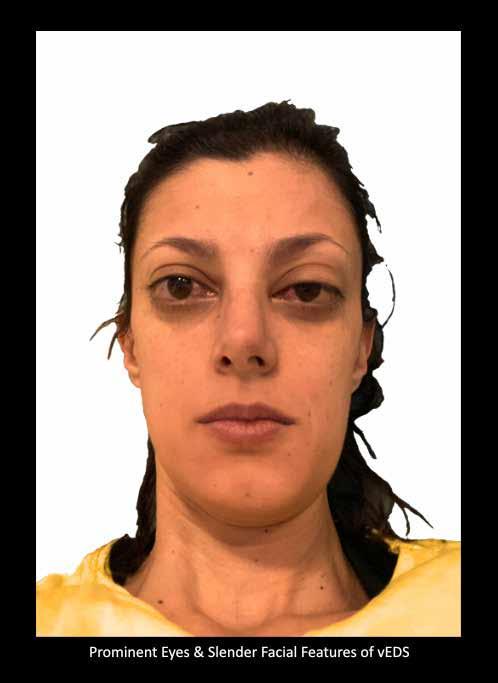
dexamethasone was commenced with partial recovery to date.
What the Rheumatologist Needs to Know
Benign Hypermobily or hEDS?
pa�ent A, a woman in her early 30s who was originally inves�gated for a collagen a�er severe bleeding and poor wound healing post varicose vein stripping. She other family members on account of her large eyes, slender facial features and bruising. Ms. A was diagnosed with vascular Ehlers Danlos a�er specialist review ex domo.
Only 30% of vEDS patients have the classical EDS facies of prominent eyes, thin lips and nose, along with a lean hollowed
New cases of vEDS in patients in their teens and twenties should be considered in those that develop recurrent spontaneous pneumothoraces, bowel rupture or varicose veins. Ultimately 80% will present before the age of 40 with a vascular event. Commonly aortic aneurysms and carotid-cavernous sinus fistulae develop over time. In the latter such cases, patients may present with progressive swelling, conjunctival injection or haemorrhage, pulsating proptosis of the ocular globe along with 6th nerve palsy, all of which are due to arterialisation of blood flow from the carotid artery into the cavernous sinus and the superior ophthalmic vein. The risk of aortic aneurysmal rupture is also extremely high yet operative intervention has historically conferred a 1 in 3 risk of death within the 2 week post operative period. There is also a high risk
In recent months, Ms. A began to develop a whooshing sound and diplopia with exertional Valsalva while weight training. She presented with progressive swelling, pain and protuberance of the left eye. Examination revealed chemosis, marked conjunctival injection and pulsating proptosis of the left globe with evident mild bilateral 6th nerve palsies. Visual acuity was preserved at 6/5 with normal optic nerve and disc.
In recent months, Ms. A began to develop a whooshing sound and diplopia with exer�onal while weight training. She presented with progressive swelling, pain and protuberance eye. Examina�on revealed chemosis, marked conjunc�val injec�on and pulsa�ng proptosis globe with evident mild bilateral 6th nerve palsies. Visual acuity was preserved at 6/5 with op�c nerve and disc. Neuroimaging with CT intracranial angiogram and MRA demonstrated caro�d-cavernous fistulae with engorgement of both superior ophthalmic veins, le� greater right. Ms. A proceeded to endovascular emboliza�on of the le� caro�d-cavernous fistula developed a complete le� 6th nerve palsy and a complete le� 3rd nerve palsy in the post period. Treatment with high dose dexamethasone was commenced with par�al recovery
Prominent Eyes & Slender Facial Features of vEDS
Neuroimaging with CT intracranial angiogram and MRA demonstrated bilateral carotid-cavernous fistulae with engorgement of both superior ophthalmic veins, left greater than right. Ms. A proceeded to endovascular embolization of the left carotid-cavernous fistula but developed a complete left 6th nerve palsy and a complete left 3rd nerve palsy in the post-operative period. Treatment with high dose
Many suspected EDS cases referred to tertiary care centres will either be the hEDS variant or simply benign joint hypermobility syndrome (JHS) which most often does not display increased elasticity of the skin. Skin hyperextensibility is defined as a capacity to stretch the skin 3cm at the neck, elbows and knees and 1.5cm at the distal forearm or dorsum of the hand. After hEDS, the most common variants encountered by the adult rheumatologist will be the classical and classical-like variants, followed then by vascular EDS. Everything else is either exceptionally rare or diagnosed in the first 3 years of life on account of known family history, congenital hip dislocations, easy bruising, profound muscle hypotonia and missed motor developmental milestones.
In recent months, Ms. A began to develop a whooshing sound and diplopia with exer�onal while weight training. She presented with progressive swelling, pain and protuberance eye. Examina�on revealed chemosis, marked conjunc�val injec�on and pulsa�ng proptosis globe with evident mild bilateral 6th nerve palsies. Visual acuity was preserved at 6/5 with op�c nerve and disc. Neuroimaging with CT intracranial angiogram and MRA demonstrated caro�d-cavernous fistulae with engorgement of both superior ophthalmic veins, le� greater right. Ms. A proceeded to endovascular emboliza�on of the le� caro�d-cavernous fistula developed a complete le� 6th nerve palsy and a complete le� 3rd nerve palsy in the post period. Treatment with high dose dexamethasone was commenced with par�al recovery
When trying to differentiate between hEDS and JHS, there is undoubtedly a significant overlap of symptomatology with other chronic pain syndromes such as fibromyalgia. Both hEDS

HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 47
the Rheumatologist Needs to Know
Hypermobily or hEDS?
and JHS are associated with chronic headaches, fatigue, pain amplification, depression, palpitations and dizziness. So much so that some experts will argue that hEDS and JHS are one and the same, differing only in terms of severity. Indeed, the diagnostic label of hEDS itself remains highly controversial given the highly malleable diagnostic criteria.
MUSCULOSKELETAL: EHLERS DANLOS
family history and genetic testing. LDS classically presents with the characteristic triad of bifid uvula or cleft palate, hypertelorism and aortic aneurysm. Importantly, while LDS also displays AD inheritance, 75% of cases arise from de novo mutations. vEDS and LDS can usually be differentiated between by evident increased ocular spacing and abnormalities of the arch of the palate and the uvula. Cutis laxa tends to be detected earlier in life on account of the dramatic lack of elasticity of the skin, though the condition can be acquired either due to certain drug exposures or autoimmune and neoplastic causes. The differentiating clinical finding is the slow manner in which skin distension in these patients returns to its original form.
useful in the middle-aged patient with multiple arterial aneurysms without alternative explanation. Fortunately, EDS genetic panels are becoming more inexpensive over time, but still add little that would change management in most cases seen in the adult rheumatology outpatient setting. Is this really vascular EDS or something else?
suspected EDS cases referred to ter�ary care centres will either be the hEDS variant or simply joint hypermobility syndrome (JHS) which most o�en does not display increased elas�city of skin. Skin hyperextensibility is defined as a capacity to stretch the skin 3cm at the neck, elbows knees and 1.5cm at the distal forearm or dorsum of the hand. A�er hEDS, the most common variants encountered by the adult rheumatologist will be the classical and classical like variants, followed then by vascular EDS. Everything else is either excep�onally rare or diagnosed in the first 3 of life on account of known family history, congenital hip disloca�ons, easy bruising, profound hypotonia and missed motor developmental milestones.
A Reasonable Investigatory Approach to the Hypermobile Patient
trying to differen�ate between hEDS and JHS, there is undoubtedly a significant overlap of symptomatology with other chronic pain syndromes such as fibromyalgia. Both hEDS and JHS are associated with chronic headaches, fa�gue, pain amplifica�on, depression, palpita�ons and dizziness. So much so that some experts will argue that hEDS and JHS are one and the same, differing terms of severity. Indeed, the diagnos�c label of hEDS itself remains highly controversial given highly malleable diagnos�c criteria.
After exploring patient and family history, a thorough examination of the integumentary system should be performed assessing skin distensibility over dorsum of hand, elbow, neck and knees making note of both skin quality, translucency, veins and any evident scarring. This should be followed by assessment of hypermobility formally using the Beighton criteria. In small children, motor development should be assessed along with generalised muscle tone. Easy bruising should be investigated with laboratory tests for FBC, coagulation profile and clotting factors to outrule bleeding disorders. Baseline transthoracic echocardiogram should be performed in children under the age of 10.8
then CT of aorta, aortic arch and intracranial angiogram should be performed to outrule arterial aneursyms of the aorta, carotid and vertebral arteries, circle of Willis or the abdominal and renal arterial vasculature. If there is a concern with radiation exposure the corresponding MRA sequences may be requested. Crucially, the rheumatologist should be aware that pneumothoraces and bowel perforation may occur in teenage vascular EDS patients long before arterial aneurysms begin to form as they are generally protected from catastrophic arterial events until elastin production drops off dramatically in the 3rd decade of life.
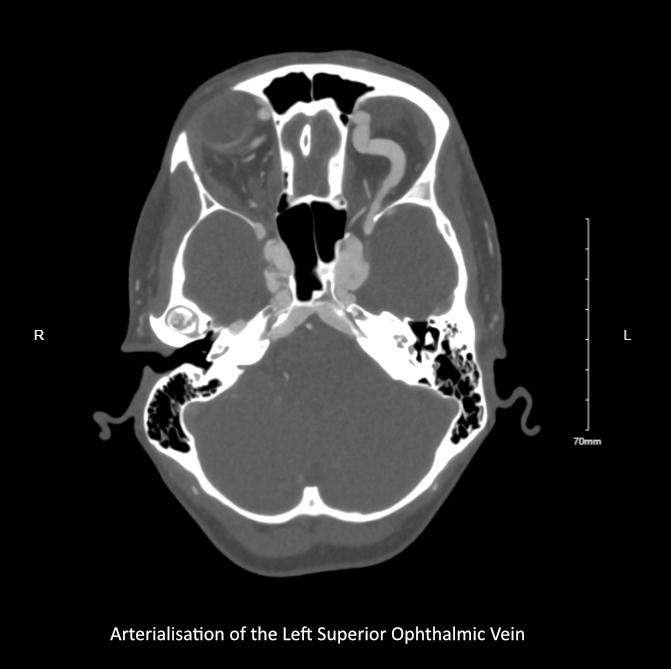
Lastly, there are also a few important distinctions to make between EDS and Osteogenesis Imperfecta (OI) type 1. While also hypermobile, OI patients tend to experience multiple fractures over the course of their lifetime. Non-deforming OI patients, like vascular EDS, may present with complications of multiple arterial aneurysms. In the absence of genetic testing, OI patients differ insofar as they may also present with blue sclerae, dental issues and sensorineural deafness. Furthermore, while the OI patient may be hypermobile, the vascular EDS patient is not in the general sense save for the fingers.
Managing Vascular EDS
If there is a family history of vascular EDS or a history of unexplained recurrent pneumothoraces or bowel perforation in a young patient,

Most of the time in adult rheumatology, genetic testing will not add much additional benefit given most of the cases encountered will either be the hypermobile variant, for which there is no one gene association, or the classical or classical-like variants which do not affect life expectancy. Rarer forms of EDS arrive with a diagnosis from either family history or genetic testing early in life as an infant. Genetic testing may be useful in the young patient with recurrent pneumothoraces and/or spontaneous bowel perforation without family history of vascular EDS. This may either be confirmatory of vEDS before aneurysms form, or may allay such concerns for the future. Testing for the COL3A1 mutation may also be
Reasonable Inves�gatory Approach to the Hypermobile Pa�ent exploring pa�ent and family history, a thorough examina�on of the integumentary system be performed assessing skin distensibility over dorsum of hand, elbow, neck and knees note of both skin quality, translucency, veins and any evident scarring. This should be followed by assessment of hypermobility formally using the Beighton criteria. In small children, development should be assessed along with generalised muscle tone. Easy bruising should be
Recurrent pneumothoraces, spontaneous bowel rupture, varicose veins with or without atrophic scarring in early adulthood should raise suspicion of cEDS, cLEDS and vEDS. The key distinguishing feature being that patients affected by the vascular variant do not display skin hyperelasticity and are only hypermobile in the fingers. There exists an important differential for the hypermobile patient with evidence of aortic root involvement on echocardiogram that all rheumatologists should be aware of. This differential includes the vascular EDS variant, Marfan syndrome, Loeys-Dietz syndrome (LDS) and Cutis Laxa. Interestingly vEDS, Marfan’s and Loeys-Dietz may all display pectus abnormalities and both vEDS and Marfan’s may display a high arched palate. Marfan’s can be distinguished clinically from vEDS easily by its characteristic phenotype of tall stature, arm span to height ratio, long thin extremities and arachnodactyly, as well as by
It is strongly recommended that vEDS patients wear an identifying medical alert bracelet as well as an information card as the morbidity and mortality of the condition are not widely appreciated by all in the medical community. Medical attention should be promptly sought if any unexplained severe pain develops. Sudden onset chest, back or abdominal pain should be considered a medical emergency until proven otherwise as it may indicate dissection or even rupture of aorta or perforation of the bowel or solid organ. As operative intervention is fraught with risk, surgery should only proceed if there is a serious life-threatening event such as arterial rupture or perforation of the bowel or solid organ. Any other intervention should ideally be discussed in detail with careful planning and risk mitigation. Input from vascular, plastics and GI surgical specialties are strongly advised if surgery is elective.
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 48
Arterialisation of the Left Superior Ophthalmic Vein
Close control of systolic blood pressure over time is thought to potentially delay or offset aneurysm development and arterial emergencies. The antihypertensive agent of choice is usually a beta-blocker with celiprolol showing some promise in small studies to date.9 Celiprolol is available in Europe but to the best of the authors’ knowledge is still not approved in the US for vEDS, despite being a medication first introduced in the 1980s. Alternatively, atenolol and propranolol may be reasonable choices for beta-blockade. If a second anti-hypertensive is needed, angiotensin receptor blockers (ARBs) are likely the next most beneficial agents to consider. Given the high risk of bowel perforation, vEDS patients may benefit from stool softeners and
bulk laxatives rather than stimulant laxatives which may actually increase the risk of perforation.
Mechanistically, inhibition of matrix metalloproteinase activity with tetracyclines remains appealing but there is still a lack of strong real-world evidence demonstrating the delay of aneurysm formation and reduction in mortality. Critically, fluoroquinolone antibiotics should be avoided entirely given their deleterious effect on collagen. The same is true in other connective tissue disorders such as Marfan syndrome and Loeys-Dietz syndrome.
All pregnant women with vEDS should be followed in a high-risk obstetric clinic. Ideally, careful
family planning would include genetic counselling and discussion of the very real pregnancy associated mortality risk to the mother. While the risk of uterine rupture confers a 5% risk of death to the expectant mother, 50% of those deaths occur with the first pregnancy. Any potential benefit of scheduled caesarean section remains unclear. In certain parts of the world, pre-implantation genetic diagnosis and the selection of unaffected embryos for implantation is a possibility to ensure an unaffected healthy child.
vEDS patients should avoid contact sports, overhead weight lifting and any activity involving
Strategic Roadmap on Bone Health
The World Health Organization (WHO) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) signed an agreement to develop a strategic roadmap on bone health and ageing and advocate for a public health strategy to prevent fractures among older people. The collaboration entails a strategic roadmap of research and publication deliverables, in recognition of the fact that osteoporosis is a major contributor to the Global Burden of Disease.
The collaboration agreement, signed on February 23, 2023 at WHO Headquarters in Geneva, Switzerland, is an important springboard for osteoporosis, a disease which continues to suffer from under-prioritization in healthcare agendas worldwide.

Although globally an estimated one in three women and one in five men aged over 50 years will sustain a potentially lifethreatening fragility fracture in their remaining lifetimes, the majority of high-risk individuals remain undiagnosed and untreated. Furthermore, systematic post-fracture care for secondary fracture prevention is
not available to the vast majority of fracture patients who are at high risk of recurring fractures.
Within the framework of the fiveyear collaboration, commencing in the first quarter of 2023, WHO, in collaboration with the International Osteoporosis Foundation (IOF) and the WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Ageing at the University of Liège, Belgium will commence on a broad range of research. This will include, among other research objectives, collecting data on the prevalence and incidence of osteoporosis and fractures for countries and regions around the world, and reviewing evidence on interventions for preventing fractures in older persons to inform guideline development and evidenceinformed decision-making. In return, WHO has committed to generating a range of important publications, including global health estimates on prevalence and incidence, as well as commentary on interventions for preventing fractures.
Together, the impactful data and influential WHO publications are expected to put osteoporosis on the global map, thereby helping to drive much-needed policy action.

prolonged Valsalva. Light weights, gentle jogging and low impact exercise such as cycling and swimming are all more appropriate ways of maintaining cardiovascular fitness and general health.
Acknowledgements:
Beaumont rheumatology department would like to thank our vascular EDS patient for kindly giving permission to use her case history, imaging and clinical photographs for this review article and other educational purposes.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 49
News
MUSCULOSKELETAL: FRACTURES
Updates in Ireland’s Fracture Liaison Service
Written by Professor Frances Dockery, Fracture Liaison Service Database Clinical lead, Consultant Geriatrician, Beaumont Hospital
of future falls and more serious fractures such as those of the hip or spine.
The aim of a Fracture Liaison Service (FLS) is to identify people at risk of fracture, investigate when required, and initiate appropriate bone strengthening medication as indicated. A fracture resulting from a simple fall is known as a “fragility fracture”, and usually indicates the presence of osteoporosis. Fractures as a result of osteoporosis are one
of the leading healthcare costs across Europe and numbers are rising steeply. A recent Europewide scientific report on fragility fractures predicts that Ireland will see an increase in cases of ~ 58%, the highest of all EU countries because of ageing demographics (Scope 2021, Kanis). These fragility fractures cause great distress and disability for over 30,000 people annually in Ireland, and can impact survival. Despite highly effective treatments being available to manage osteoporosis and thus reduce the risk of fracture, only a minority receive these.
FLS provides a system for assessing people aged 50 years and over who sustain a fragility fracture, to diagnose osteoporosis and identify those most at risk
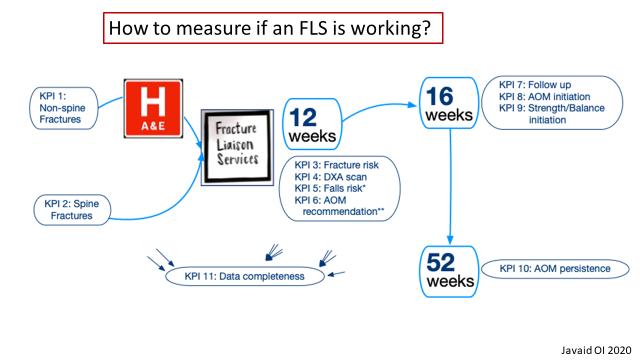
FLS uses a consultant led multidisciplinary team approach and is an integrated service between acute and primary care settings. If properly resourced, it has the potential to reduce the number of people with acute, debilitating fractures admitted to the hospital setting. This aligns closely with both Sláintecare and Healthy Ireland principles. Ireland is only the second country globally to develop a national FLS database, with the aim of improving care for these people who sustain a first non-hip fragility fracture.
Up to 50% of people who sustain a major fracture (e.g. a hip fracture) would have had a previous nonhip “warning fracture”. Most of these people do not get screened, or treated at that point, for osteoporosis. An assessment of their risk of falling, which might help prevent future fractures, is also seldom carried out at the time of their initial fracture.
Many recent publications in the scientific literature have outlined the benefits that FLS provide, both in reducing the person’s risk of recurrent fractures, and for

society as a whole in reducing the costs associated with caring for people who lose their ability to live independently as a result of these fractures.
FLS has been adopted globally as real-world data has demonstrated that this model of care has proven benefits in reducing future fracture numbers when investment is made in a high-quality service. Currently, Europe spends an estimated (¤37 billion on managing fragility fractures (¤400 million in Ireland), of which just ¤2 billion is spent on prevention.
The National Fracture Liaison Service (FLS) Steering Group welcomes the funding of eight advanced nurse practitioner (ANP) posts by the HSE during 2022. These nurses will play a critical role in patient care by proactively identifying and managing the care of patients presenting with fractures. They will ensure screening for, and treatment of osteoporosis and falls risk, with patient education to enable selfmanagement of their bone health. The FLS ANP’s also monitor treated patients for the year ahead to ensure tolerability and adherence with prescribed medication given the very low treatment rates postfracture presently.

MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 50 In October 2022 the first Irish FLS – DB Report was published. This report analysed the secondary preventative management of fragility fractures received by over 2,000
While these ANP posts are welcomed, it is imperative that national implementation with full resourcing occurs in the next two years. This will enable each FLS to address rising fracture numbers, create value for the health service and deliver significant benefits to patients who would otherwise go on to sustain additional fragility fractures.
The Irish Fracture Liaison Service Database (FLS – DB) was established in 2020 by the National Fracture Liaison Services steering committee under the governance of the National Clinical Programme for Trauma and Orthopaedic Surgery. The FLS – DB is aligned with the International Osteoporosis Foundation’s “Capture the Fracture” initiative which aims to improve access for people to comprehensive Fracture Liaison Services globally.

In October 2022 the first Irish FLS – DB Report was published. This report analysed the secondary preventative management of fragility fractures received by
over 2,000 patients (~ 26% of the expected number) during 2021 in eight out of the sixteen hospitals in Ireland which currently manage patients presenting with fractures. The low proportion of patients identified and treated demonstrates the gaps in service provision within Ireland in adequately managing these patients, in line with international best practice standards.
Mr Paddy Kenny, Joint National Clinical Lead for the National Clinical Programme for Trauma and Orthopaedic Surgery said “The development of a Fracture Liaison Services Database is a key enabler in implementing recommendation 15 of the 2018 HSE report - “A Trauma System for Ireland”. Having Irish data will facilitate policy makers to plan service delivery more effectively and efficiently into the future. I commend the national clinical leads in promoting the work that this group has completed to date and welcome last year’s appointment of eight advanced

nurse practitioners for fracture liaison services nationally.”
Prof Frances Dockery, joint clinical lead for the Fracture Liaison Service Database said: “This report provides a benchmark for future service developments in Ireland. The current lack of investment in a national Fracture Liaison Service needs to be addressed as a matter of urgency so that all 16 hospitals that manage patients with fractures in Ireland can establish a sustainable FLS, which will contribute data to the National FLS Database to monitor the standards of care their patients receive”.
Mr Aaron Glynn, joint clinical lead for the Fracture Liaison Service Database, noted that “Fracture Liaison Services should be considered essential ‘secondary prevention’ services, and as such is an enabler in improving patient health and wellbeing.”
The second report of the FLS – Database will be published in October 2023. To find out more about this initiative please contact fls@rcsi.ie
The second report of the FLS – Database will be published in October 2023. To find out more about this initiative please contact fls@rcsi.ie
Prof Frances Dockery, joint clinical lead for the Fracture Liaison Service said: “This report provides a benchmark for future service developments
The current lack of investment in a national Fracture Liaison Service addressed as a matter of urgency so that all 16 hospitals that manage fractures in Ireland can establish a sustainable FLS, which will contribute National FLS Database to monitor the standards of care their patients
Mr Aaron Glynn, joint clinical lead for the Fracture Liaison Service Database, noted that “Fracture Liaison Services should be considered essential ‘secondary prevention’ services, and as such is an enabler in improving patient health and wellbeing.”
51 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Mr Aaron Glynn, Fracture Liaison Service Database Clinical lead, Consultant Orthopaedic Surgeon, Our Lady of Lourdes Hospital Drogheda, Navan General Hospital
Exercise and Education: First line treatment for hip and knee osteoarthritis
Written by: Dr Clodagh Toomey, School of Allied Health, University of Limerick
OA should be viewed as a chronic condition, where prevention and early comprehensive care models are the accepted norm, as is the case with other chronic diseases. A paradigm shift from tertiary to evidence-based secondary prevention strategies is required, in order to limit progression and minimise the health consequences of the disease.
Clinical Guidelines: Exercise, Education and Weight Loss as First Line of Treatment
Osteoarthritis Burden
Osteoarthritis (OA) is a leading cause of disability among older adults and affects upwards of one in eight adults. Due to population ageing along with other factors, this figure is expected to double within one generation. It is a progressive chronic disease characterised by joint pain, stiffness and swelling, which results in significant mobility limitations and poor quality of life in an individual. Irish data from the TILDA study suggests that patients with OA make significant more use of GP and outpatient services than those without OA, at an annual cost of €13.6 million. The highest proportion of direct costs are attributed to orthopaedic surgery and in-patient hospital stays, with smaller proportions accounted for by medications, physician visits, other health professional visits and diagnostic procedures. Substantial indirect costs include productivity losses from absenteeism, presenteeism (disease-related loss in productivity that occurs even when the person is at work), premature death and early retirement (increased social welfare costs and reduced tax revenue).
Current Management
Despite being a typical chronic disease characterised by long duration, current management practices for OA globally are best described as reactive and palliative. Too much focus is on eventual end-stage joint replacement surgery and painrelieving medication strategies in the interim, at a significant cost

to society and the individual. Ireland has substantial orthopaedic waiting list times and the lowest number of rheumatology specialists (consultants and physiotherapists) per capita in the European Union. As of the end of February 2023, over 65,000 patients across Ireland were waiting for an orthopaedic outpatient appointment, with 20% of these waiting over a year. Yet, even in the absence of a clear complication, total joint replacement does not always provide relief: up to 30% of total knee replacement patients remain dissatisfied with their surgical result after one year. With the projected growth and cost of this disease, the sustainability of this health-care model is questioned.
What do the clinical guidelines (i.e. OARSI, NICE, ACR and EULAR) say and what is the evidence for each type of treatment? At the bottom of the treatment pyramid (see Figure), every person seeking care for joint pain should be offered the core treatments of education, exercise and weight management (if needed). Metaanalysis shows that we can be confident that land-based exercise therapy effectively reduces pain in patients with knee OA compared to no or minimal intervention. Treatment effect is independent of radiographic severity and pain level with little to no adverse effects so can safely be offered to all patients. The evidence has not shown that one type of exercise is better than another. However, we do know that the exercise has a better effect if supervised by a
physiotherapist, and a minimum of 2-3 times weekly is necessary to get the effective dose. It is generally recommended, based on the literature, that 3-5kg, or losing 5% of your baseline weight will relieve pain and improve function for people with OA who are overweight. In 2020, a metaanalysis including 22 studies and 3602 patients with OA looked at weight loss interventions versus minimal care. Weight loss was observed to be effective for moderate pain relief in hip and knee OA but is best combined with exercise and education for optimal results in pain and function.
Clinical Guidelines: Adjuncts as Second Line of Treatment
If the first line of treatment doesn’t lead to a satisfactory outcome, it can be complimented with adjunct treatments such as pain medications, TENS, manual therapy, braces, shoe insoles, other aids, and in some instances injections. However, these strategies cannot address the range of factors contributing to pain and disability. For example, the only way to improve your strength is to complete an exercise program. Second-line treatments usually only incur short-term benefits, some are high risk and almost all are more costly. The most common types

MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 52
MUSCULOSKELETAL: OSTEOARTHRITIS
Osteoarthritis Treatment Pyramid
of pharmacological pain relief used by people with OA are 1 – paracetamol, 2- Nonsteroidal anti-inflammatories (NSAIDS) and 3- opioids. According to the latest OARSI 2019 guidelines, paracetamol is conditionally not recommended (at Level 4A and 4B), and this is a recommendation that many are not yet aware of. A 2021 meta-analysis found that found that topical NSAIDs are more effective than paracetamol, but not more effective than oral NSAIDs to improve function. However, it noted that topical NSAIDs are safer than paracetamol and oral NSAIDs due to a lower association with all-cause mortality, with cardiovascular disease and with gastrointestinal bleeding. All guidelines strongly recommend against the use of opioids due to low effect and high risk of adverse events. Intraarticular glucocorticoid injections may be considered for the medical treatment of knee OA but only in a timely manner, considering they have no benefit after 6 weeks, and repeated injections of cortisone over 2 years leads to more rapid cartilage loss. Other adjuncts such as TENS, manual therapy, braces, shoe insoles, dietary supplements, other aids may have a positive effect that is short-term and not much more than a placebo effect. However, most have no side effects. Secondline treatments should never replace the core recommended treatments of exercise, education and weight loss.
Clinical Guidelines: Surgery as Third Line of Treatment

And at the top of the treatment pyramid, for those with very severe symptoms (10-15%), surgery may be considered. This should be a last resort, since surgery is associated with adverse effects, such as joint infection and blood clots that can be life threatening. About 20% experience one adverse event and rates of dissatisfaction in the knee are up to 30%. Joint replacement surgery is most effective when indicated. On the other hand, arthroscopic knee surgery has no better effect than a sham or placebo and should not be offered.
GLA:D Ireland – An Example of an Evidence-based Management Programme

GLA:D (Good Life with osteoArthritis Denmark) is a non-profit initiative that has addressed a gap in osteoarthritis care by training and supporting physiotherapists to implement guidelines for hip and knee OA (exercise and education) in clinical practice. Since 2013, the programme has trained over 6,000 clinicians and over 100,000 patients have benefitted from the programme across 4 continents. Research data from the GLA:D programme tells us that patients can expect decreased pain and have improved function and quality of life up to one year after taking the programme. The programme includes 12 supervised

neuromuscular group exercise sessions, twice per week, and 2 education sessions, with inclinic or online delivery options. To support research related to the programme, the GLA:D Ireland National Register collects physiotherapist- and patientreported outcomes electronically at 3- and 12-months post programme delivery. Initial funding for the GLA:D Ireland programme was granted by a Health Research Board Emerging Investigator Award. There are over 70 GLA:D trained physiotherapists in Ireland currently and we plan to continue delivering 2-3 courses per year for physiotherapists across public and private health sectors. If you are interested in finding out more about GLA:D, join our mailing list by emailing gladireland@ul.ie or take a look at our website: www.gladireland.ie.
Dr Clodagh Toomey is a Physiotherapist and Research Fellow at the School of Allied Health, University of Limerick. Clodagh completed her PhD at the University of Limerick in 2014 and a Postdoctoral Fellowship in 2017 at the Sport Injury Prevention Research Centre, University of Calgary, where she also holds an adjunct position. Her clinical and research experience has focused on prevention and management of musculoskeletal disease across the lifespan. Most recently, she was a recipient of a Health Research Board Emerging Investigator Award, which is investigating how to optimise implementation of clinical guidelines for osteoarthritis in practice. Clodagh is the research lead on the Good Living with osteoArthritis Denmark (GLA:D®) initiative in Ireland.

Figure: Osteoarthritis Treatment Pyramid
HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 53
Figure: GLA:D Ireland Logo
Figure: GLA:D Ireland Course for Physiotherapists
Figure: GLA:D Ireland Course for Physiotherapists
Figure: GLA:D Ireland Course for Physiotherapists
Management of Osteoporosis in Ireland

low-grade inflammation found in osteoarthritis contributes to disease development and progression. Theresa notes that during OA progression, the entire synovial joint, including cartilage, subchondral bone, and synovium, are involved in the inflammation process.
symptoms is joint pain, stiffness and locomotor restriction. Patients can also present with muscle weakness and problems with balance.
Osteoarthritis (OA) is a chronic degenerative joint disorder characterised by cartilage loss. It is a leading cause of pain and disability, affecting 3.3 to 3.6% of the global population and is the most common form of arthritis worldwide.
As osteoarthritis affects approximately 400, 000 Irish people and is the most common joint disease in Ireland, we recently spoke to Theresa Lowry Lehnen, RGN, GPN, RNP, BSc, MSc, M. Ed, PhD and Clinical Nurse Specialist and Associate Lecturer South East Technological University to learn more.

OA presents with joint pain and loss of function, however, the disease is widely variable and can present from an asymptomatic incidental finding to a permanently disabling disorder.
Theresa tells us, “Osteoarthritis is a heterogeneous disease caused by multiple factors. Risk factors for developing OA include increasing age, female gender, obesity, anatomical factors, muscle weakness and joint injury, particularly from occupational and sports activities. The prevalence and incidence of OA increase with age with a majority of individuals over the age of 65 affected.”
The occurrence of osteoarthritis is progressively rising due to increased life expectancy and population aging together with a rise in obesity. Excess
weight compounds the problem by putting extra strain on damaged joints. Theresa adds, “Osteoarthritis is more common and often more severe in women, especially in the knees and hands. It often starts after the menopause. Certain occupations can place excessive loads on the joints resulting in OA in later years. Occupations with repetitive knee bending can result in knee OA, while heavy manual labour may predispose to hip osteoarthritis.
“Although complex, the genetic contribution to OA is significant. The roles of genes and signalling pathways in OA pathogenesis have been demonstrated by ex vivo studies using tissues derived from OA patients and in vivo studies using surgically induced OA animal models and genetic mouse models. Nodal osteoarthritis is one common form of OA that runs strongly in families and particularly affects the hands of middleaged women. In other common forms of osteoarthritis, heredity plays a small part compared with obesity, ageing and joint injury. There are some very rare forms of osteoarthritis that start at a young age and run in families and these are linked with single genes that affect collagen which is an essential component of cartilage.”
Osteoarthritis is a disease of the entire joint and recognised as a low grade inflammatory disease involving cartilage degradation, bone remodelling, osteophytes and synovitis. The chronic
Osteoarthritis can be classified into 2 categories: primary osteoarthritis and secondary osteoarthritis. Theresa explains, “Primary osteoarthritis is the most common subset and is diagnosed in the absence of predisposing trauma or disease. Primary OA can be localised or generalised, the latter more commonly found in postmenopausal women, with development of Heberden’s nodes. Secondary osteoarthritis occurs with a pre-existing joint abnormality. Predisposing conditions include trauma or injury, congenital joint disorders, inflammatory arthritis, avascular necrosis, infectious arthritis, Paget disease, osteopetrosis, osteochondritis dissecans, metabolic disorders, hemoglobinopathy, Ehlers-Danlos syndrome or Marfan syndrome.
“Healthy cartilage is smooth, strong and flexible. It absorbs the stresses put on a joint and allows the bones to move smoothly over each other. In osteoarthritis, the cartilage becomes pitted, brittle and thin, and over time, can wear out completely. When the cartilage deteriorates, the bone underneath thickens and broadens out. Over time, the synovial fluid, which functions as a lubricant, can deteriorate in quality and the synovial membrane that makes the fluid can become inflamed and swollen. These processes, combined with thinning cartilage, affect the smooth movement of the joint and contribute to pain, stiffness and joint swelling. Bony spurs can develop around the joint, causing it to change shape. At the same time, the joint capsule becomes thicker and the amount of synovial fluid can increase, often causing the joint to swell, stiffen and become painful to move.”
Symptoms
The presentation and progression of osteoarthritis varies greatly from person to person. The triad of
Pain is usually related to activity and resolves with rest. For patients in whom the disease progresses, pain is more continuous and affects activities of daily living, eventually causing severe limitations in function. Patients may also experience bony swelling, joint deformity and instability.
“Osteoarthritis typically affects proximal and distal interphalangeal joints, first carpometacarpal joints, hips, knees, first metatarsophalangeal joints and joints of the lower cervical and lumbar spine and can be mono or poly articular in presentation,” she continues. “Shoulder and elbow joints are also susceptible to osteoarthritis although this is much rarer. Joints can be at different stages of disease progression. Typical exam findings include bony enlargement, crepitus, effusions, and a limited range of motions. Tenderness may be present at joint lines, and there may be pain upon passive motion. Classic physical exam findings in hand osteoarthritis include Heberden’s nodes, Bouchard’s nodes and “squaring” at the base of the thumb.”
Diagnosis
A thorough history and physical examination with a focused musculoskeletal exam should be carried out. Differential diagnosis can include rheumatoid arthritis, psoriatic arthritis, crystalline arthritis, hemochromatosis, bursitis, avascular necrosis, tendinitis, radiculopathy, among other soft tissue abnormalities.
“There are no specific blood tests for diagnosing osteoarthritis, however, blood tests including FBC, ESR, rheumatoid factor and Anti-nuclear antibodies can be ordered to rule out inflammatory arthritis.
“X-rays of the affected joint can show findings consistent with OA, such as marginal osteophytes, joint space narrowing, subchondral sclerosis, and cysts, however,
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 54 MUSCULOSKELETAL: OSTEOARTHRITIS
An interview with Theresa Lowry Lehnen, RGN, GPN, RNP, BSc, MSc, M. Ed, PhD Clinical Nurse Specialist and Associate Lecturer South East Technological University
The early stages of osteoarthritis
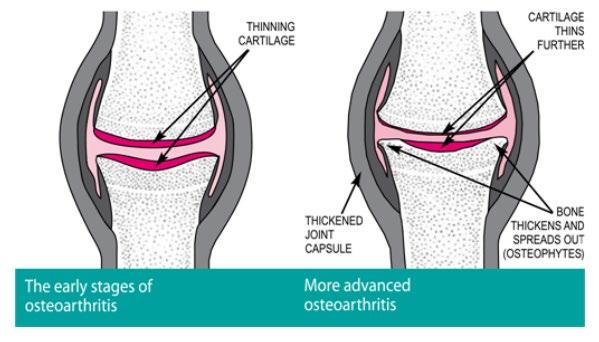
“There are also some risks involved with glucocorticoid joint injections including; Injury to the joint tissues, mainly with repeated injections; thinning of cartilage; weakening of the ligaments of the joint; inflammation in the joint caused by a corticosteroid that has crystallised; irritation of the nerves; infection; and whitening or thinning of the skin at the injection site.”
Non-pharmacologic therapy includes avoidance of activities that exacerbate pain, exercise to improve strength, weight loss if applicable, wearing suitable footwear and occupational therapy for unloading joints.

“Malalignment of joints should be corrected via mechanical means such as realignment knee brace or orthotics. Weight loss is an important intervention in patients who are overweight and obese. Regular exercise that keeps a person active, builds up muscle and strengthens the joints usually helps to improve symptoms. Patients should however, be advised to avoid exercise that puts strain on joints and forces them to bear an excessive load, such as running and weight training. Activities such as swimming and cycling, where strain on joints is more controlled is advisable.”
Theresa adds that in addition to lifestyle changes and pharmacology, patients may benefit from a number of supportive treatments that can help reduce pain and make everyday tasks easier.
https://www.arthritisireland.ie/osteoarthritis
https://www.arthritisireland.ie/osteoarthritis
“Healthy cartilage is smooth, strong and flexible. It absorbs the stresses put on a joint and allows the bones to move smoothly over each other. In osteoarthritis, the cartilage becomes pitted, brittle and thin, and over time, can wear out completely. When the cartilage deteriorates, the bone underneath thickens and broadens out. Over time, the synovial fluid, which functions as a lubricant, can deteriorate in quality and the synovial membrane that makes the fluid can become inflamed and swollen. These processes, combined with thinning cartilage, affect the smooth movement of the joint and contribute to pain, stiffness and joint swelling. Bony spurs can develop around the joint, causing it to change shape. At the same
radiographic findings do not correlate to the severity of condition and may not be present early in the disease. MRI is not routinely indicated for OA workup, however, it can detect osteoarthritis at earlier stages than normal radiographs. Ultrasound can also identify synovial inflammation, effusion, and osteophytes which can be related to osteoarthritis.”
and symptomatic progression of osteoarthritis, Theresa says. Management of the disease involves both pharmacological and non-pharmacological therapies.
prescribed for osteoarthritis of the hands or knees, if topical NSAIDs have not been effective in easing pain. Capsaicin cream works by blocking the nerves that transmit pain in the treated area.
“Healthy cartilage is smooth, strong and flexible. It absorbs the stresses put on a joint and allows the bones to move smoothly over each other. In osteoarthritis, the cartilage becomes pitted, brittle and thin, and over time, can wear out completely. When the cartilage deteriorates, the bone underneath thickens and broadens out. Over time, the synovial fluid, which functions as a lubricant, can deteriorate in quality and the synovial membrane that makes the fluid can become inflamed and swollen. These processes, combined with thinning cartilage, affect the smooth movement of the joint and contribute to pain, stiffness and joint swelling. Bony spurs can develop around the joint, causing it to change shape. At the same
Treatments
Current therapeutic options are aimed at keeping the associated pain, inflammation, and degeneration of synovial joint tissues manageable in order to minimise the structural
“Pharmacotherapy involves oral, topical, and/or intra-articular options. Paracetamol and oral NSAIDs are usually the initial choice of pharmacological treatment. Topical NSAIDs are less effective than their oral counterparts but have fewer gastrointestinal and other systemic side effects. A proton pump inhibitor (PPI) may also be prescribed at the same time as oral NSAIDs to reduce the risk of damage to the stomach lining. Capsaicin cream may also be
https://www.arthritisireland.ie/osteoarthritis
“Intra-articular joint injections can be an effective treatment for osteoarthritis. Glucocorticoid injections have a variable response however, and there is ongoing controversy regarding repeated injections. Although corticosteroid injections can ease osteoarthritis symptoms, they have limitations. They cannot repair damaged cartilage or slow the progression of osteoarthritis and relief is only temporary.
“Healthy cartilage is smooth, strong and flexible. It absorbs the stresses put on a joint and allows the bones to move smoothly over each other. In osteoarthritis, the cartilage becomes pitted, brittle and thin, and over time, can wear out completely. When the cartilage deteriorates, the bone underneath thickens and broadens out. Over time, the synovial fluid, which functions as a lubricant, can deteriorate in quality and the synovial membrane that makes the fluid can become inflamed and swollen. These processes, combined with thinning cartilage, affect the smooth movement of the joint and contribute to pain, stiffness and joint swelling. Bony spurs can develop around the joint, causing it to change shape. At the same
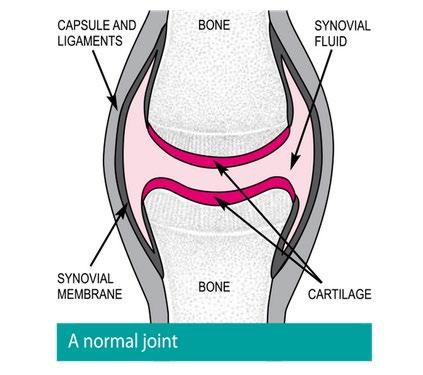


She highlights, “Not using joints can cause muscles to waste and increase the stiffness caused by osteoarthritis. Manual therapy is a technique where a physiotherapist uses their hands to stretch, mobilise and massage the body tissues to keep a patients joints supple and flexible. Applying hot or cold packs to the affected joints can also help relieve the pain and symptoms of osteoarthritis in some people. Transcutaneous electrical nerve stimulation (TENS), if advised by a doctor or health care professional may help ease the pain caused by osteoarthritis, by numbing the nerve endings in the spinal cord which control pain.
“For osteoarthritis in the lower limbs, such as hips, knees or feet, special footwear or insoles for shoes may be helpful. Footwear with shock-absorbing soles can
HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 55
More advanced osteoarthritis THICKENED JOINT CAPSULE BONE THICKENS SPREADS OUT (OSTEOPHYTES) CARTILAGE THINS FURTHER
https://www.arthritisireland.ie/osteoarthritis
BONE
help relieve some of the pressure on the leg joints when walking, and special insoles may help spread weight more evenly. Leg braces and supports also work in the same way. For osteoarthritis in the hip or knee that affects mobility, the use of a walking aid, such as a stick or cane may be beneficial. Occupational therapists can provide help and advice about using assistive devices in the home or workplace.
“Osteoarthritis can affect any joint in the body, but the most common areas affected are the knees, hips and small joints in the hands. For patients with knee or hip osteoarthritis for whom pharmacological and nonpharmacological treatments have failed, joint replacement surgery/ arthroplasty is the next option. Failure rates for both knee and hip replacements are quite low, and joint replacement can provide pain relief and increased functionality. The timing of surgery is important. Poor functional status and considerable muscle weakness may not lead to improved postoperative functional status versus those undergoing surgery earlier in the disease course.
MUSCULOSKELETAL: OSTEOARTHRITIS
Most patients who undergo joint replacement tend to have a good prognosis with success rates of over 80%. However, most prosthetic joints wear out in 10 to 15 years, and repeat surgery is required.1 A newer type of joint replacement surgery called resurfacing uses only metal components and may be more suitable for younger patients.
“If a joint replacement is not a suitable option, an operation to fuse the joint in a permanent position known as an arthrodesis, may be carried out. The joint will be stronger and less painful, however, the patient will no longer be able to move it. For patients with osteoarthritis of the knees but who are unsuitable for knee replacement surgery, an osteotomy may be performed. This involves adding or removing a small section of bone either above or below the knee joint. This helps realign the knee so weight is no longer focused on the damaged part of the knee. An osteotomy can relieve symptoms of osteoarthritis, although the patient may still need knee replacement surgery in the future.”
Outlook
The prognosis for osteoarthritis patients depends on the joint

involved, how many joints are affected, and the severity of the disease. Theresa notes that while there is currently no cure for osteoarthritis, and all available treatments are directed towards reducing symptoms, significant research has been carried out and progress made in recent years.
“The molecular mechanisms of osteoarthritis initiation and progression is still not fully understood,” she adds. “The last decade has seen significant advances however, in understanding the processes that contribute to osteoarthritis and over the coming years these new insights will hopefully lead to better treatment options. Further research and a better understanding of the molecular mechanisms could accelerate the development of novel therapeutic strategies for osteoarthritis.
“Traditional osteoarthritis management approaches often result in varying treatments, evidence practice gaps and delayed treatment due to over demand and long waiting lists. Despite considerable societal, economic and the personal burden associated with osteoarthritis, the condition is often overlooked in health care strategic plans for chronic disease management.
A shift is needed to promote evidence based management of osteoarthritis and address the underutilisation of core recommended treatments and over reliance on surgery and pharmacological agents.”
Model of Care. ENACt Project Osteoarthritis does not feature alongside other chronic diseases such as asthma or diabetes and there is currently no model of care (MoC) for osteoarthritis in primary care in Ireland. The ENACt research project (2020- 2024), led by researchers at the RCSI hopes to change that, and is in the process of developing a model of care for osteoarthritis in primary care to deliver best practice nationwide. The ENACt project is developing a model of care for OA specifically tailored to the Irish healthcare system, with the goal of implementing and evaluating it on a large scale national level. The research to be carried out in three work packages over four years, aims to identify current primary care management of OA in Ireland and develop a model of care focusing on core interventions, education, exercise and weight loss.
References available on request
New report highlights services for people with neurological conditions
A new report putting a spotlight on the community services available nationwide for people with neurological conditions was launched by Minister for Disability Anne Rabbitte.
The Neuro-Mapping Project Report describes the range of community services across the country that people with neurological conditions can access from voluntary organisations, and identifies a number of joint service initiatives between voluntary organisations and the HSE.
It also shows that there are over 60 community-based voluntary organisations supporting those people, providing a wide variety of services in response to their needs.
The report highlights that the vast majority of the 800,000 people living with neurological conditions across Ireland, live within the community.
The report was a joint partnership between the Health Service Executive (HSE), the Disability Federation of Ireland (DFI) and the Neurological Alliance of Ireland (NAI), funded by the Strengthening Disability Fund.
The three key outputs from the Neuro-Mapping project are:
• The development of a unique service mapping template which identifies services across Ireland, categorising them together with agreed and standardised service descriptors.
• Baseline Mapping of all services across each Community Healthcare Organisation (CHO), showing the range and scale of neuro-rehabilitation supports provided in the community by voluntary organisations.
• Documented good practice examples of joint service initiatives between voluntary sector providers and statutory services provided by the HSE.
MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 56
News
Pictured at the launch of the Neuro-Mapping Project Report are Minister for Disability Anne Rabbitte; Deirdre Murphy, HSE; Magdalene Rogers, Neurological Alliance of Ireland; and John Dolan, CEO Disability Federation of Ireland. Pic: John McElroy
Arthritis Ireland’s Clinic Referral Programme
The purpose of Arthritis Ireland’s Clinic Referral Programme is to provide a direct route from hospital clinic to Arthritis Ireland, allowing the newly diagnosed and vulnerable to begin the journey to patient empowerment and effective self-management. For those that have been living with arthritis for a longer period and are experiencing a flare or difficult time, it can be extremely helpful to link in with Arthritis Ireland’s services and supports.
I really appreciate how quickly everyone reacted when the diagnosis was made, after a couple of tough years. It's been really good to know there is support available. It's been brilliant and very professional. I’m much more positive and confident, and I'll manage things going forward. I would absolutely recommend this programme to others who are newly diagnosed, and I hope it can be brought to all the clinics – patient referred from rheumatology clinic. Anecdotally, we know that people are lost on diagnosis, so the principal of the programme is fantastic – helpline volunteer
It was understood that the newly diagnosed were a cohort of our community that could be approached earlier in their journey with arthritis and that direct referral into Arthritis Ireland rather than handing out our contact details and leaving it up to the patient, was the best course of action.
The programme was first piloted in five hospital centres to see that this method was successful; for patients, clinics, the Arthritis Ireland Services Support Officer (SSO) and volunteers.
One rheumatology ANP involved in the pilot said “the referral process could not have been easier. That’s why (Arthritis Ireland) got the referrals, 10/10 there. We definitely felt supported to be able to tell patients that they would get the call from (Arthritis Ireland) to discuss any general queries. It was great to know that patients were being made aware of the support services available out there.”
Two of the standout comments from the participants emphasised that the referral calls reduced isolation, gave information and empowered them to be more confident about their situation.
“I am isolated, alone, with no carer and I can’t go anywhere so I really appreciate these calls as they show I’m not the only one like this.”
Arthritis Ireland Clinic Referral Programme

“I found it helpful and informative. It did sort of make it real, so I put the information pack to one side for a couple of days and then came back to them. I actually didn’t know whether I wanted the call or not at the beginning. I was afraid of the unknown and what it would be like. However, when it was over, it made me look at things differently and accept things more. I let the information come to me and felt much better afterwards. So much so that when we arranged the follow-up call, I was actually looking forward to chatting to (SSO) and telling him how I was doing.”
It can be overwhelming to hear about all the various ways arthritis can impact us at the very beginning. What we have learned to do is evolve the service so that we continue to emphasise the role of the helpline and peer-to-peer services. The patients are made aware of all the supports available, but reminded that they may not need them all immediately.
Written by Peter Boyd, Services Support Officer, Arthritis Ireland
In mid-2021 a scoping exercise was carried out to assess the need for a new service for the newly diagnosed. Extensive research was undertaken into how the programme would look if it took place.
The National Rheumatoid Arthritis Society (NRAS) in the UK, had set-up a similar programme and numerous meetings were held between both organisations and within Arthritis Ireland to ensure the programme was fit for purpose. Collaboration across the Arthritis Ireland team ensured the collateral, promotion and forms were in place to roll the programme out in Autumn 2021. The pilot project involved five clinics providing geographical spread while maintaining accurate recording of all data and timely responses to all referrals.
As we were going through lockdowns, individual zoom meetings were held with each clinic to brief them on the programme and its operation. Consultant rheumatologists, advanced nurse practitioners, clinical nurse specialists and clinical specialist physiotherapists attended.
The service launched on October 4th, 2021, emphasising supported ‘self’ management – whereby the individual patient takes control of the management of their condition but is supported by Arthritis Ireland at every step of their journey.
The pilot had 60 referrals and there were 39 successful or completed calls (65%) out of 60. When the service was opened up to all newly diagnosed patients in all clinics across the country in
2022, successful or completed calls were 50%.

Throughout 2022, the Services Support Officer visited rheumatology clinics across Ireland to inform staff of the benefits of the Referral Programme. It is important to stress at all times that this programme runs parallel to the medical treatment provided by the clinic and offers the time and space to discuss lifestyle and day-to-day issues brought up by life with arthritis.
In late 2022, the Arthritis Ireland helpline underwent further changes and upgrades when it attained the Helplines Partnership Standard and accreditation. This gives further confidence to all callers and participants in safeguarding and privacy.
With all public clinics in Ireland now referring any patient that they believe would benefit from specialised and individualised support from the Referral Programme, there has been a 33% increase in the number of referrals and completed calls are running at 70%.

Every single patient that is referred via the Referral Programme is spoken to by the Arthritis Ireland Helpline. As we move further into 2023 the Arthritis Ireland Clinic Referral Programme will seek more and more referrals from rheumatology clinics throughout the country and anyone with interest in the programme should contact:
Peter Boyd – Arthritis Ireland Services Support Officer pboyd@arthritisireland.ie

57 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 MUSCULOSKELETAL: ARTHRITIS
What is the Clinic Referral Programme? • A personally tailored information and support service. • A direct route to Arthritis Ireland’s services. • An enhanced service, introducing self-management for anyone living with arthritis. How does it work? • You provide consent to being referred directly to Arthritis Ireland. • Arthritis Ireland will arrange a convenient time to talk. • The conversation can cover but is not limited to: – Diagnosis – Family – Employment – Fatigue – Anxiety – Relationships – Pain • Your medical treatment will continue with your clinic as normal. • Lifestyle support and self-management information is provided by Arthritis Ireland.
Medicine Shortages Issue raised in Dáil
to medicines for patients in Ireland over the next 4 years.
“Negotiations towards these new Agreements with industry began in May 2021, against a backdrop of a growing annual drugs budget, up from ¤1.95bn in 2016 to almost ¤2.25bn by 2020.”
The Minister outlined the multiannual agreements with the Irish Pharmaceutical Healthcare Association (IPHA) and Medicines for Ireland (MFI) which he said ‘represent an important step in facilitating access to innovative new medicines for patients.’
of alternative suppliers for that medicine, the potential alternative therapeutic options, the clinical need for the product, the potential budget impact, and European pricing.
“The HSE also takes an active role at the time of notification, in engaging with manufacturers/ suppliers intending to discontinue priority medicines due to the lack of commercial viability of those products. In many of the cases

where this arises as a scenario, the commercial decision relates to global discontinuations and is outside of the control of the HSE i.e., cannot be addressed through pricing increases at a national level. The Agreements are the product of extensive collaboration between the State, HSE, and Industry. They remain current and appropriate for their purpose since their signing in December 2021.”
Update on Shortages
Concerns around Ireland’s growing medicine shortage crisis were voiced recently during a Dáil Éireann Debate
Deputy Holly Cairns TD asked the Minister for Health, Stephen Donnelly about the steps he is taking to address concerns in relation to the ongoing issue of medicine shortages.
Responding, Minister Donnelly stated that medicine supply issues and their prevention are not unique to Ireland.
He said, “Similar increases in demand and medicines supply issues have been observed in the UK, throughout the EU and across the world at present. In the case of medicines used most often in Ireland, there are typically multiple alternatives available from various sources that remain available to ensure continuity of treatment. Of the products currently in short supply, alternative treatments are available for every single product.
“Management of medicine supply issues necessitates a multi-faceted, multi-stakeholder response to ensure patient safety, continuity of care and protection of public health. To that end, at the request of the Department of Health, the Health Products Regulatory Authority (HPRA) has assumed a coordinating function in Ireland’s response to managing medicine shortages when they occur.
“Two new Framework Agreements on Pricing and Supply of Medicines 2021-2025 were signed in December 2021. These Agreements provide stability to the medicines reimbursement market for the State and for Industry, thus facilitating sustainable and affordable access
He added, “There are a number of clauses set out in both agreements in detail, which provide clear pricing rules for applicant companies, that have been agreed between the State and main Pharmaceutical Industry representative groups in Ireland as appropriate and intended to provide a predictable environment for Marketing Authorisation Holders (MAHs) to supply their products (and prevent shortages). These are grossly summarised as:
1. The pricing application form for new medicines is based on a basket of 14 countries (13 EU Member States and the UK). Pricing is permitted to be no more than the average price based on the pricing in these 14 countries.
2. The pricing of non-patented medicines (i.e., generic, biosimilar or hybrid medicine marketed in Ireland) is based on the originator medicine at a specific time point.
“Under the terms of the agreement, there is scope for the Department of Health, the HSE, and industry to continue to maintain their engagement throughout the lifetime of the agreement on all matters pertaining to the agreement.
“As of 16th March 2023, there are 25 applications from manufacturers/suppliers currently under review by the HSE, for a pricing increase request related to a claim of increased cost of goods, making continuity of supply to the Irish market and Irish patients unsustainable.
In the case of such pricing increase requests the time taken to adjudicate on these is highly variable (on a case-by-case basis) and dependent on a number of factors, that the HSE is required to consider including the number
This is the HPRA’s latest weekly update on medicines shortages. The HPRA has been notified of a shortage of the following products:
Product
Azarga 10mg/ml + 5mg/ml Eye Drop Suspension
EU/1/08/482/001
Novartis Europharm Limited
Chloromycetin 0.5% w/v Redidrops Eye Drops
PA1142/021/001
Amdipharm Limited
Clarithromycin 250mg Film Coated Tablets
PA2315/136/003
Accord Healthcare Ireland Ltd.
Clarithromycin 250mg Film Coated Tablets
PA2050/002/001
Sun Pharmaceutical Industries Europe B.V.
Creon 25000 Gastro-Resistant Capsules
PA2010/008/002
Mylan IRE Healthcare Limited
Exforge HCT 160/12.5/10mg Film Coated Tablet
EU/1/09/569/014
Novartis Europharm Limited
Exforge HCT 160/25/5mg Film Coated Tablets
EU/1/09/569/026
Novartis Europharm Limited
Imvaggis 0.03 mg Pessary
PA1054/005/001
Laboratoires Besins International
Ketalar 10mg/ml Solution for Injection/Infusion
PA0822/013/001
Pfizer Healthcare Ireland
Ketalar 50mg/ml Solution for Injection/Infusion
PA0822/013/002
Pfizer Healthcare Ireland
Senokot 7.5mg Tablets
PA0979/016/002
Reckitt Benckiser Ireland Ltd
Synarel 200mcg/dose Nasal Spray Solution
PA0822/138/001
Pfizer Healthcare Ireland
Zanidip 20mg Film Coated Tablets
PA0812/001/002
Recordati Industria Chimica e Farmaceutica SpA
Active Ingredient(s)
Timolol & brinzolamide
Chloramphenicol
Clarithromycin
Clarithromycin
Pancreas powder
Amlodipine, hydrochlorothiazide &valsartan
Amlodipine, hydrochlorothiazide &valsartan
Estriol
Ketamine
Ketamine
Senna
Nafarelin
Lercanidipine
58 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
News

The solution to all your sourcing pains For more information please contact: Free Phone 1800 440 440 I PharmaSource@uniphar.ie I www.uniphar.ie
New endoscopic tools in inflammatory bowel disease
Written by Olga Maria Nardone, Rosanna Cannatelli, Subrata Ghosh, Marietta Iacucci
In the last decade, short- and long-term treatment targets in inflammatory bowel diseases (IBD) evolved, shifting towards objective measures of disease activity.1, 2 Hence, the role of endoscopy is becoming more and more relevant. In this context, new advanced endoscopic techniques are now available and can provide a more comprehensive endoscopic and histological assessment. Virtual electronic chromoendoscopy (VCE), endocytoscope, and confocal laser endomicroscopy (CLE) converge towards a deeper ultra-structural characterisation of the mucosa, reducing the gap with histology, which is increasingly considered a measure of remission depth and favourable outcome.2, 3
We provide an overview of the evolution of endoscopic armamentarium to assess the grade of inflammation, healing and detect/characterise dysplasia in IBD with an additional focus on how the implementation of Artificial Intelligence (AI) might revolutionise clinical management of IBD toward personalised medicine no longer considered hype but reality.
THROUGH THE EYES OF VIRTUAL ELECTRONIC CHROMOENDOSCOPY
VCE is widely available in most endoscopic units and enhances the mucosal and vascular intestinal architecture. Mainly, this helps differentiate between patchy and mild inflammation versus endoscopic remission, a key therapeutic goal in IBD patients. Whilst narrow-band imaging (NBI, Olympus Japan) uses optical filters for a narrow band of blue and green light; optical enhancement iSCAN (iSCAN-OE, Pentax, Japan), flexible imaging colour enhancement and LASEREO system (FICE, Fujinon), which included the linked-colour imaging (LCI), and blue-laser imaging (BLI) use a post-processing digital software algorithm to recreate virtual images.4 Recently, new software was released from Olympus, the EVIS X1, with two new modes to define better Texture and Colour Enhancement Imaging (TXI) which improves the structure and brightness of the endoscopic images and Red Dichromatic Imaging (RDI) with
the purpose to enhance the blood vessels and bleeding.5
Currently, the application of VCE in IBD ranges from the assessment of the inflammation to the detection, characterisation and therapeutic management of colonic lesions during surveillance colonoscopies.
Endoscopic assessment of endo-histologic remission
Several new scores have been developed by using VCE platforms. Paddington International Virtual Chromoendoscopy Score (PICaSSO)6, 7 graded mucosal (including elongated crypts, scars, micro erosions, ulcers) and vascular changes (such as sparse vessels, a vessel with dilation or crowded and bleeding) and ranged from 0 to 15 (Figure 1). In a large multicentre international study, endoscopic remission was defined by a value equal to or less than 3, and PICaSSO showed a strong correlation with five histological scores, namely Robarts Histological Index (RHI), Nancy Histological Index (NHI), Villanacci Simple Score, Geboes Score and Extent, Chronicity, Activity and Plus Score (ECAP) with Pearson's correlation between 0.77 and 0.79. Furthermore, it has also shown very good interobserver variability with K agreement of 0.88 and PICaSSO <3 predicted good longterm outcomes at 6 and 12 months with a hazard ratio (HR) of 0.19 and 0.22, respectively.8 It was initially developed and validated on the i-scan platform, then reproduced on the other endoscopic platforms currently available showing an intraclass correlation coefficient of 0.825. Moreover, the correlation between PICaSSO assessed by NBI and BLI/LCI showed a good correlation with RHI (0.83 and 0.63, respectively) and with NHI (0.79 and 0.65, respectively).9 Notably, it has been demonstrated that endoscopic remission measured with VCE PICaSSO reflected composite endoscopic-histologic remission, increasingly explored as an ultimate endpoint in Ulcerative Colitis (UC) for predicting specified clinical outcomes at 12 months.10
Regarding other platforms, NBI combined with magnification distinguished a three-categories score. BV-H (honeycomb-like blood vessels) and BV-BB (blood
vessels shaped like bare branches) were associated with endoscopic healing, whilst BV-V (blood vessels shaped like vines) was linked to histological activity.11 Moreover, mucosal vascular pattern (MPV) graded by NBI as obscure expressed more acute cell infiltration and depletion of globet cell compared to clear MPV (26% vs. 0% and 32% vs. 5%, respectively).12
Similarly, LCI was used to develop three categories of scores such as LCI-A (no redness), LCI-B (redness with visible vessels) and LCI-C (redness without visible vessels), which showed a strong correlation with the histopathology Matts score.13
A recent meta-analysis compared the correlations between endoscopy and histologic disease activity scores across several endoscope technologies and found no significant difference among them. However, VCE was more accurate in predicting histological remission than White Light Endoscopy (WLE).14
As a more recent VCE, the new Dual Red Imaging (DRI) is a new tool that uses the wavelengths 600 and 630 nm and strongly correlates with Mayo Endoscopic Score (MES). DRI maintained remission at 2 years of follow-up.15
To date, PICaSSO is the only validated and reproduced score that all endoscopic platforms can use.
Regarding CD, the current approach for scoring endoscopic activity is the Simple Endoscopic Score (SES-CD),16 despite several limitations. Therefore, recently, a new scoring tool named the modified multiplier of the SES-CD (MM-SES-CD) has been developed, including the evolution of treatment endpoints into the definition of endoscopic remission.17 This can predict the achievement of endoscopic remission while on active therapy by considering each parameter's prognostic value. Hence, it is more accurate than the original SES-CD scoring approach for predicting endoscopic remission (comparison of area under the curves on the testing cohort for MM-SES-CD vs. original SES-CD p = 0.0052).
These implicate that this score can identify patients with a low baseline probability for endoscopic remission with standard therapies in whom treat-to-target monitoring with biomarkers, therapeutic drug concentrations and repeated endoscopy may be most beneficial.
• Surveillance colonoscopy
• a) for detection of colonic lesions
The most recent European Society of Gastrointestinal Endoscopy guidelines18 suggested performing surveillance colonoscopy with Dye Chromoendoscopy (DCE) or VCE after appropriate training.
A multicentre study on 188 patients showed no differences between VCE (iscan) and HDWLE in detecting dysplasia during surveillance colonoscopies (14.9% vs. 24.2%, respectively, p = 0.14) with a similar withdrawal time.19 Similarly, another prospective randomised study conducted on 129 IBD patients showed no difference between DCE and VCE (iscan), with detection of 17.9% versus 11.3% (p = 0.2). However, in this case, the withdrawal time was significantly higher in the DCE group (p < 0.001) compared to the VCE group.20 A randomised controlled trial (RCT) on 48 patients who underwent both VCE and DCE confirmed these data and included patients' preferences for VCE.21 More recently, a metaanalysis on 11 RCT (1328 patients) showed no difference between VCE, DCE and HD-WLE in the per-patient analysis; however, in the per-dysplasia analysis, VCE was inferior to HD-WLE (RR 0.62) and not inferior to DCE (RR 0.72).22 A possible explanation of this can be related to the fact that more lesions can be found once dysplasia is detected in a patient. However, there is still an ongoing debate about the best technique for surveillance colonoscopy. Previous studies showed that DCE did not increase dysplasia detection compared with WLE with targeted and random biopsies.23, 24 In recent times, high definition (HD) endoscopy and VCE have become widely available,
60 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Bowel Disease
and hence DCE, WLE, NBI, and VCE with targeted biopsy sampling are all considered acceptable modalities for surveillance when using HD colonoscope.25
• b) Characterisation of the colonic lesion
Surveillance for Colorectal Endoscopic Neoplasia
Detection and Management in Inflammatory Bowel Disease
Patients: International Consensus
Recommendation consensus has introduced the modified Paris classification, taking into account the morphology (polypoid or nonpolypoid), borders and ulcerations to characterise colonic lesions associated with IBD.26 However, the surface was not considered. However, The Kudo pit pattern, assessed by DCE or VCE, strongly correlated with histology in predicting dysplasia (73% vs. 71% respectively).27, 28
The Frankfurt Advanced Chromoendoscopic IBD LEsions (FACILE) classification developed with DCE and VCE, considered as the morphology of nonpolypoid lesion (OR 3.13), irregular vessel architecture (OR 3.49), signs of inflammation within the lesion (OR 2.42) and irregular surface pattern (OR 8.89) as predictors of dysplasia.29
Recently, European Crohn's and Colitis organization (ECCO) topical review on the endoscopic report introduced the new ‘5S’ features, which include Site, Size (using biopsy forceps as reference standard), Shape (polypoid, non-polypoid, or lateral spreading tumour, distinct or indistinct borders, presence of ulcers), Surface (Kudo pit pattern or FACILE classification) and Surrounding (mucosal activity, colitis area/ non-colitis area, or other lesions in surrounding area).30
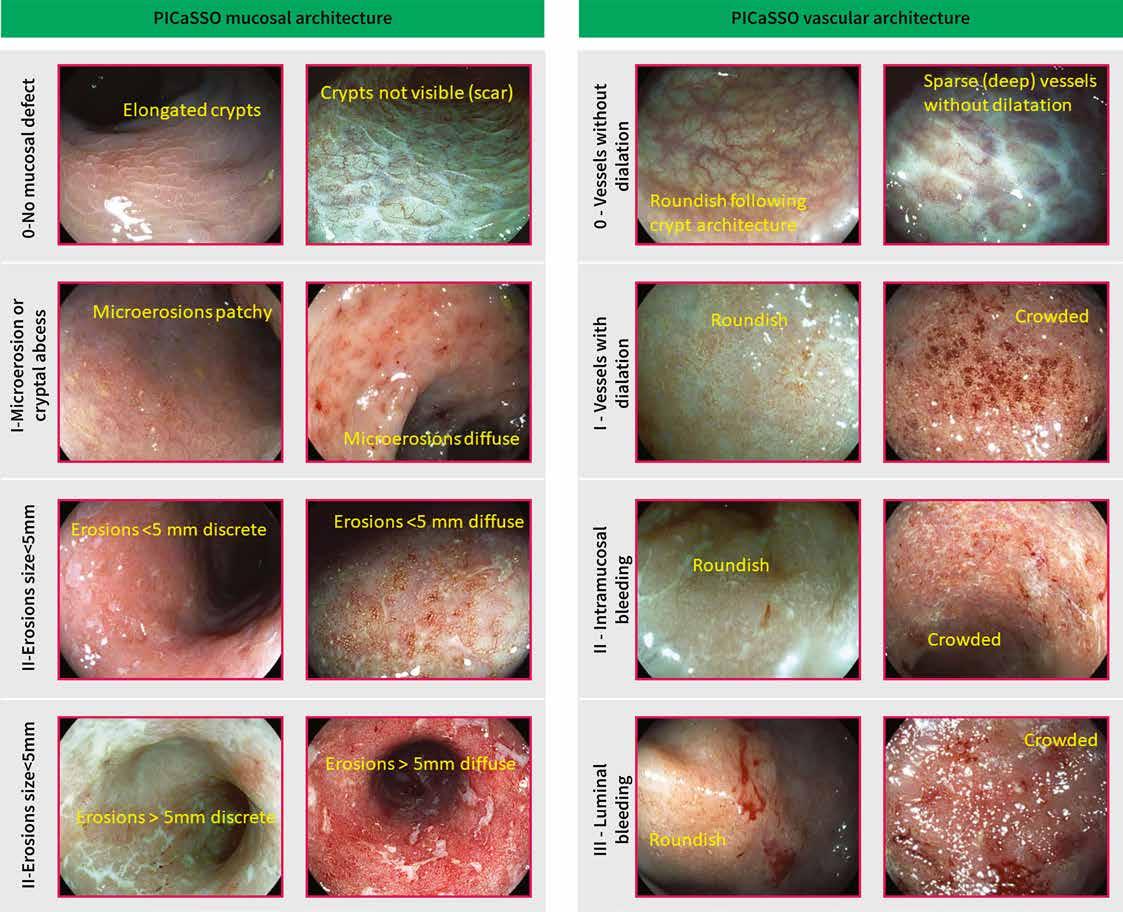
Moreover, the Japan NBI Expert Team (JNET) classification is based on vessels and surface patterns and ranges from types 1, 2A, 2B to 3, showing prediction of dysplasia and submucosal invasion.31 It has been used in IBD patients in a small study on 19 UC patients with UC-associated neoplasms, and the JNET type 2A had a low positive predictive value (PPV, 50.0%) and a high negative predictive value (NPV; 94.7%). However, the inter-observer and intra-observer agreements among experts were fair (0.401 and 0.387, respectively).32
Furthermore, the Kudo classification assessed by FICE on 205 colonic lesions predicted histology with 91% sensitivity and 76% specificity.33
VCE is widely available and can be easily used by pushing ‘in realtime’ the button on the handpiece
of the scope. The newly VCE validated PICaSSO score can assess inflammation accurately and has made endoscopy closer to histology. In addition, VCE can be adopted as a surveillance colonoscopy technique after adequate training for detection and characterisation of dysplasia associated with IBD, enhancing the morphology, borders, and surface of the lesion and guiding therapy with organ sparing.
CONFOCAL LASER ENDOMICROSCOPY
CLE is a highly innovative endoscopic technique as it provides new insight into several gastrointestinal diseases. It was introduced in 2006 to provide ‘in vivo histology’ with very high magnification, and resolution of the images of the mucosal layer based on a cellular and
61 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Figure 1
Bowel Disease
subcellular level after applying a systemic fluorescent agent (i.e., fluorescein sodium) injected intravenously before imaging. This system is based on a probe down the accessory channel of an endoscope.
Mauna Kea Technologies, Paris, France (Figure 2). In IBD, CLE was used for structural and functional assessment of the intestinal epithelium, characterisation and classification of inflammatory activity and mucosal healing (MH) in active disease, dysplasia detection and molecular imaging for precision medicine.3, 34, 35 The assessment of MH in IBD was a further application of CLE since it could accurately distinguish between patch/mild inflammation and MH.
Hundorfean36 developed a MH score by using endomicroscopic scoring system (eMHs). This showed high sensitivity, specificity, and accuracy values (100% with 95% confidence interval [CI] of 15.81%–100%; 93.75% with 95% CI of 69.77%–99.84%, and 94.44%, respectively) and a good correlation with the histological Gupta score (rs = 0.82, P < 0.0001) and the endoscopic Mayo subscore (MES) (rs = 0.81%, P < 0.0001).
The ability of CLE to predict disease relapse and clinical outcome was first assessed by Kiesslich et al.37 They observed local epithelial barrier defects with increased cell shedding with fluorescein leakage in IBD patients with subsequent relapse 12 months, indicating that CLE can relapse or define a stable disease when the barrier function is intact. Karstensen et al.38 evaluated confocal features in response to various treatment regimens
(anti-tumor necrosis factor (TNF), thiopurines, steroids, etc.) in patients with UC using the probe-based CLE system and correlated colonic CLE appearances with histopathology and macroscopic appearance before and after the intensification of the therapy 6–8 weeks later. Fluorescein leakage, microerosions, tortuosity of the crypts, distortion of the crypt openings, inflammatory infiltrates and decreased crypt density were frequently present in active UC as opposed to inactive UC and controls. Interestingly, a decline in histopathology score after medical treatment escalation correlated with diminished crypt tortuosity, distortion of crypt openings, and decreased crypt density.
Buda et al.39 composed an outcome score by probe confocal laser endomicroscopy (pCLE), combining fluorescence and crypt diameter (p < 0.01), able to predict disease flare during a 12-month follow-up period in patients affected by long-standing UC. Pericrypt fluorescence >3100 pixels and a crypt diameter >90 μm increased the probability of disease relapse significantly.
Regarding CD, Tontini et al. demonstrated that CD endomicroscopy findings were predictors of the need for therapy escalation and progression of disease with transmural damage and complication such as strictures or perianal diseases within 1 year of follow-up.40
However, in a recent study,41 pCLE did not add significant advantages in respect of VCE, giving rise to the idea that the new HD-VCE scopes used by well-trained IBD endoscopists are equal to pCLE in assessing the disease. However, future studies are required.
Confocal laser endomicroscopy and molecular imaging
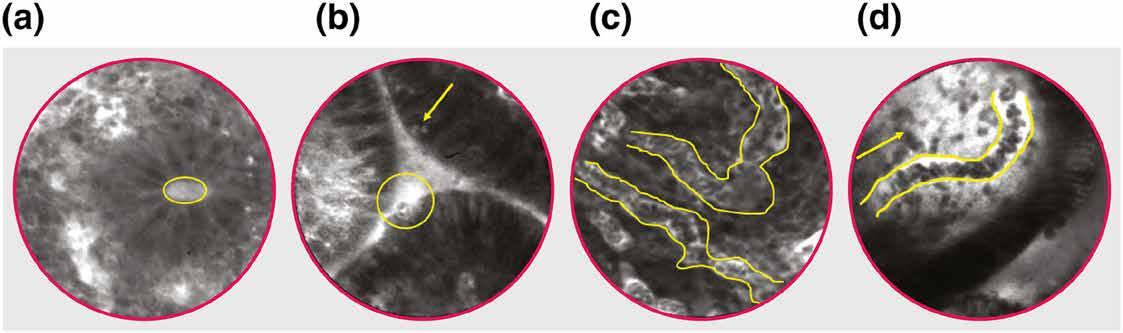
The additional application of molecular endoscopy in IBD allows topical application of labelled probes, mainly antibodies, against specific target structures expressed in the tissue to predict response or failure to biological therapies. This leads to individualised and personalised IBD therapy.
The first molecular target of interest in IBD was TNF. A phase II clinical trial investigated the impact of membrane-bound TNF (mTNF) binding by a fluorescent-labelled adalimumab anti-TNF antibody visualised by CLE ‘in vivo’ endoscopy on clinical outcome.
Patients with a higher number of cells mTNF positive have a higher probability of clinical relapse compared to patients with lower cells mTNF positive.42
Similarly, Rath et al. analysed the ex vivo topical administration of fluorescein-labelled antiadhesion molecule antibody fluorescein isothiocyanate (FITC) labelled α4β7 (vedolizumab) in CD patients.43
Patients with positive α4β7–expressing mucosa cells before vedolizumab induction were considered responders to new vedolizumab therapy as opposed to non-responders' patients in whom no positive α4β7–expressing cells were observed during prior ex vivo examination.
Preliminary data presented at ECCO 2021 have investigated the predictors of response to biologics in 29 IBD patients using computerised image analysis of pCLE in vivo and the binding of fluorescent-labelled biologics ex vivo. Vessel tortuosity was the only parameter that was significantly altered (reduced) after treatment (p < 0.05) in all patients. Additionally,
in UC, treatment significantly reduced fluorescein leakage through the colonic mucosa (p < 0.05), whereas, in CD patients, it reduced crypt area, eccentricity, and inter-crypt distance (p < 0.05).
Targeted biopsies were further taken for FITC-labelled infliximab and anti-integrin-α4β7. The endoscopic procedure was repeated at weeks 12–14 to assess therapeutic response. Ex vivo, higher mucosal binding to the biological agent pre-treatment was associated with a higher likelihood of response to the treatment.
Interestingly, the magnitude of this prediction of response was greater in UC (area under the ROC curve (AUROC) 83%, accuracy 77%, PPV 89%, NPV 50%) compared to CD (AUROC 58%, accuracy 64%, PPV 40%, NPV 78%). Noteworthy genes predictive of response were identified. A panel including ACTN1, CXCL6, LAMA4, EMILIN1, CRIP2, CXCL13 and MAPKAPK2 involved in pathways such as inflammation, chemotaxis, TGFsignalling and extracellular matrix showed good prediction of antiTNF response (AUROC > 0.7).44 However, despite the potential advantages, molecular imaging of CLE is far from having widespread clinical use as it requires expert endoscopists, specialised equipment and needs further validation and increases the cost. Nevertheless, it provides the basis for a new era of precisely tailored medicine.
ENDOCYTOSCOPY
Endocytoscopy (ECS; CF- Y-00581 prototype, Olympus Japan) is a recent high-ultra magnification endoscopic technique that provides in vivo microscopic imaging during endoscopy, with ultra-high magnification ranging from 450-fold to 1400-fold.
62 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 2
After the application on the mucosa of absorptive agents, such as methylene blue, toluidine blue or cresyl violet, alongside a mucolytic agent (N-acetylcysteine) which allows better penetration of the contrast agent, EC allows looking at cells and nuclei of mucosal surfaces by producing an image close to histology3 (Figure 3).
Several studies have shown that ECS is a reliable technique to assess endoscopic and histological remission with a high concordance between EC and histology (100%). Of note, EC could differentiate precisely inflammatory cells, such as neutrophilic, basophilic, eosinophilic granulocytes and lymphocytes getting closer to histology.
The significant advantage is the reduced need for biopsy specimens. Indeed, biopsies can assess only a limited area, whereas ECS is an optical diagnosis tool which can sample a wider area in vivo of the colonic mucosa.
Bessho et al.45 developed the first endocytosocpy score (ECSS), which evaluated the shape and the distance between crypts and the visibility of microvessels. This score showed a strong correlation with Matts's histopathological score
and a substantial correlation with Geboes histopathological score.
A further score by Ueda46 et al. is correlated with MES for mild and moderate UC patients. Deformed pits with distorted crypt lumen with the irregular arrangement or disruptive or disappeared pits were considered active disease features. Therefore, patients with these characteristics had more relapsed in the follow-up period.
Nakazato et al.44 developed an ECS score (ECSS), which strongly correlated with histological severity. Subsequently, the same authors investigated if ECS can distinguish patients in histological remission from patients with histologically active disease among patients in endoscopic remission.45 Notable, the ECSS score had good diagnostic accuracy, with a sensitivity of 77% (95% CI, 59–89), specificity of 97% (95% CI, 83–99), and accuracy of 86% (95% CI, 75–93) to predict histological remission in patients with UC.
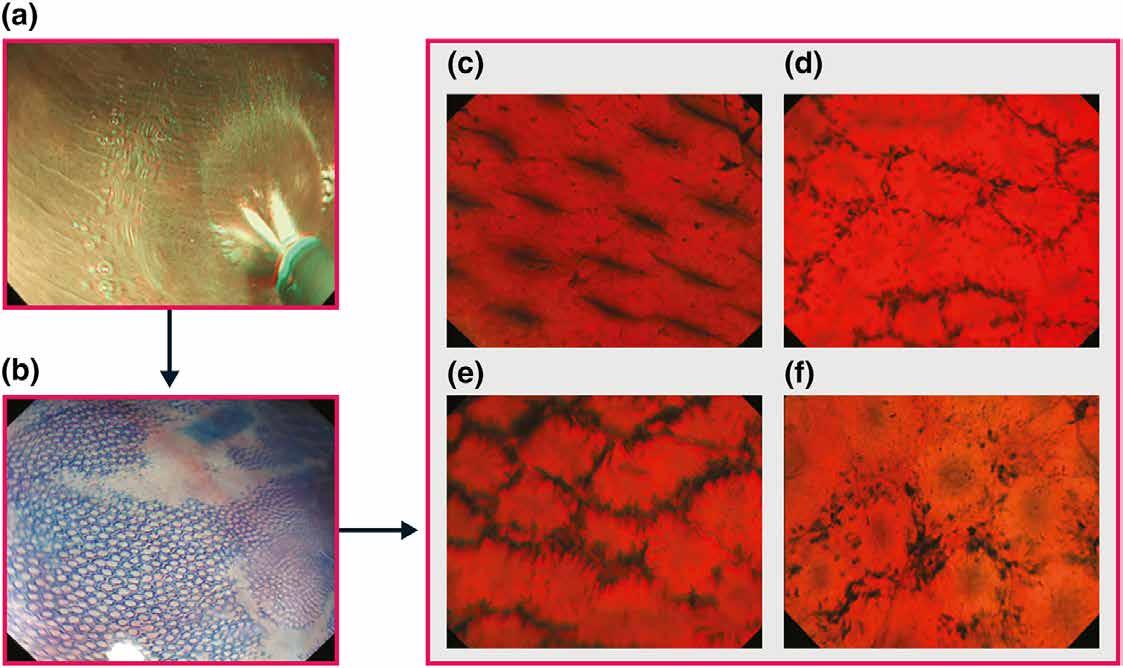
Recently we assessed the correlation between endocytoscopy and histology in a prospective study including 29 UC patients.47 An endocytoscopy scoring system (ECSS) was developed based on Nakazato et
al. score by including endoscopic findings representative of disease activity (infiltration of the cell). Importantly we found that endocytoscopy features such as crypt architecture, distance between crypts, cellular infiltration, and visibility of microvessels were strongly correlated with RHI (r = 0.89; 95% CI, 0.51–0.98) and NHI (r = 0.86; 95% CI, 0.42–0.98) but correlated poorly with MES (r = 0.28; 95% CI, 0.27–0.70).
Furthermore, RNA sequencing and bioinformatics analysis were performed to define differentially expressed genes/pathways in healing and nonhealing samples and their correlation with endoscopic scores defined by ultra-high magnification with histology scores. We identified genes relevant to TGF-β signalling such as TGFBR2, PDZK1IP1, USP2, and YOD1 and macrophage recruitment into tissues such as RNASET2, neutrophil and plasma cell function RNF4 and PIM2, and tumour suppressor genes human homolog of Drosophila headcase (HECA) and BIN3. These were shared by MES and ECSS-defined healing and histological healing.48
In an innovative study by Maeda et al.49 a computer-aided diagnosis (CAD) based on an endocytoscopy system was used to predict
persistent histologic activity and long-term clinical prognoses. CAD revealed good performance measures in terms of sensitivity, specificity, and accuracy of 74% (95% CI: 65%–81%), 97% (95% CI: 95%–99%), and 91% (95% CI: 83%–95%) respectively.
Despite the encouraging results, EC requires dedicated training to achieve good competence before its implementation in clinical practice. However, high costs represent a limitation for its use in routine clinical practice in the management of IBD patients.
ARTIFICIAL
INTELLIGENCE IN IBD: HYPE OR REALITY?
The use of AI-assisted endoscopy in IBD is a rapidly evolving area of research with promising results and additional benefits for more precise endoscopic diagnosis.
Given the significant heterogeneity in presentation, disease course, and treatment response in IBD, AI represents a step towards an objective assessment of the disease.
AI's potential applications in IBD include diagnosis, identifying mucosal disease activity assessment, predicting response to therapy/recurrence/
63 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Figure 3
Bowel Disease
complications/hospitalisations, and detecting dysplasia.
The studies on AI-IBD patients published so far are primarily focused on the assessment of inflammation versus remission, mainly using machine learning algorithms based on frames and videos of colonoscopies. To objectively evaluate healing or disease progression, Bossuyt et al. built an algorithm called red density based on an evaluation of the redness map and vascular pattern recognition which correlated with endoscopic and histological disease activity in a cohort of 29 UC patients and control.50
A further study by Stidham et al. demonstrated the ability of deep learning techniques to distinguish disease in remission versus moderate/severe disease using MES with AUROC of 0.97 and agreement to human reviewer scores, κ = 0.86.51
Subsequently, Takenaka et al., in a prospective study based on a deep convolutional neural network (CNN) construct on 40,758 images validated in 875 UC
patients, developed a system that predicted endoscopic remission with 90% accuracy, κ = 0.80 and histological remission with 93% accuracy, κ = 0.86.52
Due to high interobserver variability in endoscopy, we expect that AI results could be accurately reproduced, leading to standardising the assessment of disease activity. Furthermore, it can significantly contribute to the accuracy, precision, and reproducibility of central reading in clinical trials. In this context, Gottlieb et al. compare the performance of a recurrent neural network model with a human reader score. Importantly this system produced accurate Mayo and ulcerative colitis endosopic index of severity (UCEIS) scores with agreement/reproducibility of κ = 0.84 and 0.85, respectively.53
Furthermore, we have recently developed the first VCE AI system to accurately distinguish in realtime endoscopic activity and remission in UC colonoscopy videos of both WLE and VCE. A total of 1090 endoscopic videos (638,287 frames) from 283
patients came from the PICaSSO multicenter study were used to develop a CNN to distinguish ER/ activity and predict HR/activity. This AI-based CAD system detected endoscopic remission/ activity (PICaSSO ≤ 3) in VCE videos with a sensibility of 79%, specificity of 95%, and the AUROC 0.94. It is worthy to note that it also predicted histologic activity/ remission and the occurrence of adverse clinical outcomes.54
Finally, a recent study using a set of 614 biopsies from 307 patients with UC enrolled on a prospective multicentre study used a novel deep learning strategy based on a CNN architecture to detect neutrophils, calculate the PICaSSO Histologic Remission Index (PHRI) and identify active from quiescent UC. Importantly this AI algorithm accurately predicted histological remission and differentiated active from quiescent UC with 78% sensitivity, 91.7% specificity and 86% accuracy.55
CONCLUSIONS
In recent years several innovative and necessary steps have
been taken in the endoscopic assessment of IBD, including prediction of histology, treatment response, and molecular labelling (Figure 4).
Advances in VCE have led to a focus on microscopic details no longer invisible to the human eye, thereby reducing the gap with histology and increasing detection and characterisation of dysplasia associated with IBD. AI systems support clinicians in interpreting and standardising findings such as grading inflammation, detecting adenomatous polyps, predicting histology and thereby clinical outcomes.
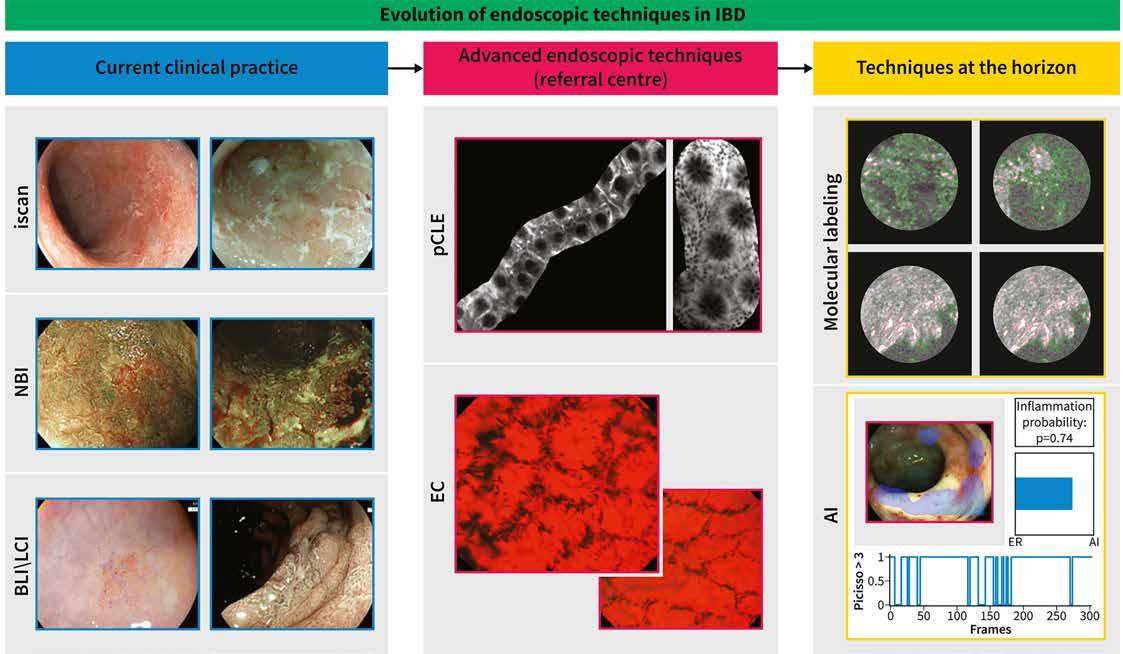
However, the road to IBD precision medicine is still challenging. In the near future bioinformatics tools and integration of multi-omics including faecal metagenomic, serum metabolomic and proteomic profiles will revolutionise IBD management and shed the light on a new fascinating and promising target to achieve: molecular healing and drive precision medicine.
References available on request
64 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 3
Major disparities in patient-reported adherence compared to objective assessment of adherence using mass spectrometry: A prospective study in a tertiary-referral hypertension clinic
Written by James M. G. Curneen, Louise Rabbitt, Darragh Browne, Darragh F. O'Donoghue, Yousef Alansari, Brendan Harhen, Ailbhe Ní Ghríofa, John Ferguson, John William McEvoy,
Hypertension, blood pressure
≥140 mmHg systolic and ≥90 mmHg diastolic, represents the greatest burden of noncommunicable disease-associated morbidity and mortality globally, with a worldwide adult prevalence of disease estimated at 31% and affecting 1.39 billion individuals.1, 2 Internationally, blood pressure remains uncontrolled in approximately 63% of all diagnosed hypertensive patients in high-income western countries.3 Several factors contribute to poor blood pressure control, including undiagnosed or unrecognised secondary hypertension, socalled treatment-resistant hypertension and nonadherence to antihypertensive therapy.4, 5 Recent data from a primary care cohort have estimated that true treatmentresistant hypertension occurs with less frequency than previously described, attributing a high proportion of treatment resistance to antihypertensive nonadherence.6 In the current study, we have investigated the prevalence of incomplete adherence to antihypertensive therapy within the setting of a difficult and young hypertension clinic.
It is widely recognised that antihypertensive medication adherence is central to effective management of hypertension and the sustained control of blood pressure.7 Specifically, adherence is related to reduced frequency of clinic visits, rational and prudent prescription, and lower incidence of adverse clinical consequences of uncontrolled high blood pressure.8, 9 However, there remain several challenges to medication adherence within this setting. Many antihypertensives carry mild adverse effects which many patients find unacceptable.10 Patients do not often see the direct and immediate benefits of taking antihypertensive medications given the usually asymptomatic and chronic nature of disease. Yet a catastrophic event such as stroke or myocardial infarction is often followed by prolonged periods of improved adherence and blood pressure control.11
Measurement of adherence to medication is fraught and presents a clinical challenge.12 This is further complicated by the detailed taxonomy of adherence. Several methodologies, such as pill counting and self-reported adherence, have typically been associated with underestimation of the extent of the challenge.12 Directly observed therapy represents a costly option to assess adherence and is challenged by the cost of hospital admission, the availability of elective facilities and the artificiality of the environment in which the patient is observed.13 Of course this approach can also damage the essential relationship of trust between doctor and patient.14 In countries with large, detailed health databases, a more accurate estimation of adherence can be made by assessing the percentage of days covered by prescriptions, the medical possession ratio or the new prescription medication gap.15 Regrettably, detailed, consistent and integrated data collection is not always undertaken in the context of health and wellbeing, and therefore these parameters of medication adherence remain difficult to measure.
In recent years, with the advent of high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS), it has become possible to measure antihypertensives or their metabolites within the urine or blood of patients.6, 16 These assays represent an accurate, point-intime estimation of antihypertensive medication adherence. Previous studies using this methodology have measured the prevalence of medication nonadherence in a so-called treatment-resistant cohort of patients in primary care and tertiary care between 24% and 68%.6, 16, 17 To our knowledge, a comparison of patient-reported antihypertensive adherence with objective evidence using mass spectrometry spot urinalysis has never been performed in any tertiary referral clinic setting. In our study, we aimed to undertake this assessment
within the setting of a difficult and young hypertension clinic using a self-reported questionnaire immediately followed by urine collection for measurement for the presence of medically reconciled prescribed antihypertensives or their metabolites.
METHODS
Ethical approval for this study was granted by the research ethics committees at Galway University Hospitals (GUH) Ref GUH: C.A. 1773. This study was conducted in accordance with the ethical principles as set out by the World Medical Association Declaration of Helsinki. Written informed consent was obtained from each participant prior to study initiation.
Study design
This was a prospective observational single-centre cohort study carried out between July 2017 and August 2018 at University College Hospital, Galway, Ireland. Patients aged ≥18 years old on one or more antihypertensive medications and who were attending the tertiary hypertension clinic were eligible for inclusion and were recruited at a routine clinic visit without prior knowledge of the study. This clinic receives referrals from both primary and secondary care clinicians for patients with hypertension that is poorly controlled despite three antihypertensives or if a secondary cause for their hypertension is suspected. This was an unselected group of patients that were recruited at the time of presentation to clinic. Many of the participants had undergone several changes in their antihypertensives in the community or within the clinic prior to the study. Study participants were required to complete a self-reported assessment of antihypertensive adherence and to provide a spot sample of urine for the presence of antihypertensive medications and/ or their respective metabolites. The samples were collected at the point of presentation to the clinic, between 08:30 and 10:30. Patients in general are advised to
take their medications as normal on the day of clinic. Patients were not informed in advance that adherence testing would be undertaken on the day of clinic. The adherence phases of initiation and persistence in continuing antihypertensive therapy were assessed in combination. Antihypertensive adherence was determined by two different methods. First, each participant completed a self-reported assessment of antihypertensive adherence, including their list of medications, using a questionnaire based on the validated Brief Medication Questionnaire (this questionnaire screens patientreported adherence and barriers to adherence identified by patients). Second, analysis of urine provided by participants on the day of their clinic visit for the qualitative analysis of the presence of each antihypertensive and/or their respective metabolites was carried out using LC-MS/ MS. Subjects who demonstrated both self-reported and LC-MS/ MS evidence of antihypertensive medication adherence of all reported antihypertensives were characterised as adherent, whilst patients who reported either nonadherence or adherence without spectrometry evidence of adherence were classified as nonadherent. The participant information leaflet and questionnaire are available on request.
Data collection
Baseline clinical demographics including age, sex, body mass index (BMI), blood pressure measurements in mmHg (systolic [SBP] and diastolic [DBP] using an automated oscillometric device [Omron] after participants had been seated quietly for 5 minutes) and a patient-reported list of antihypertensive medications were recorded at the time of the clinic visit, including when the patient takes their medication. For patients who were unable to fill in the questionnaire due to, for example, visual impairment, a member of staff would assist them. Spot samples of
65 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Hypertension
David Lappin, David P. Finn, Paula M. O'Shea, Michael Conall Dennedy
Hypertension
participants' urine were collected and delivered to the laboratory at University College Hospital Galway at room temperature and were subsequently directed to the Clinical Research Facility, Galway where they were stored within 7 hours of collection at 80 °C pending analysis by LC-MS/MS.
Analytical methods
Parameters for qualitative detection of the antihypertensive drugs or their metabolites are presented along with the specific multiple-reaction monitoring (MRM) parameters and retention time windows (see Supporting Information Tables S1-S3).
These were determined using pharmaceutical-grade targets pulverised by pestle and diluted as stock solutions in methanol. LCMS/MS analysis was performed using an Agilent 1290 Infinity
ultra-high performance liquid chromatograph and a 6460 triple quadrupole mass spectrometer with electrospray ionisation. The system was controlled by Agilent Mass Hunter software. Targets were detected by scheduled MRM transitions, triggered by target retention time(s). For sample preparation, a simple “dilute-andshoot” approach was adopted where urine samples (20 μL) were diluted 10-fold in Milliporegrade water and subsequently 1 μL of sample injected on the column. Samples were run twice, first in positive ion mode and thereafter in negative ion mode. A total of 28 antihypertensive drugs or their metabolites were screened for, most in positive ion mode but some in negative ion mode. Unit resolution was maintained in positive ion mode, but the quadrupole resolution was
widened in negative ion mode to increase sensitivity.
The samples were separated by gradient high-performance liquid chromatography using water (solvent A) and acetonitrile (solvent B) binary gradients containing 0.1% (v/v) formic acid and an Agilent SB C18 RRHD column with dimensions of 50 mm length, 2.1 mm diameter and particle size 2 μm. The flow rates and chromatographic gradient programme parameters utilised are displayed in Supporting Information Tables S1-S3. The total run time including a 4-minute re-equilibration run after each sample was 15 minutes.
An internal standard (sulfameter) was also included. The assay detected the presence or absence of antihypertensive drugs or metabolites. Neither concentration
of drug nor metabolite were measured. Therefore, adherence was based on the presence or absence of drug rather than on a concentration threshold. This assay was not configured to detect labetalol, celiprolol, metoprolol, piretanide, nifedipine and minoxidil, and therefore objective adherence for these particular medications could not be assessed.
Statistical analyses
Patients were initially classified as either nonadherent (selfreported or objective or both) or adherent. Subgroup analysis was performed on participants based on both their self-reported adherence and mass spectrometry results. Data were analysed using SPSS version 20 (IBM Corp., Armonk, NY, USA). NonGaussian data were represented as a as median (interquartile range). Permutation testing was used to determine categorical factors that might be related to adherence status, adjusted for age and sex. Percentages and cross-tabulations with adherence were also reported. Post data collection, a power calculation was derived to determine the minimum levels of association that could be detected with the sample of 73 individuals. This calculation showed that relative risks of at least 3.2 between objectively measured adherence and a two-level baseline factor (ie, people with the baseline factor were 3.2 times more adherent to their medications) could be detected with 80% power at a significance level of 5%. This is assuming an overall probability of medication adherence of 27% (as estimated from our database) and that the prevalence of the baseline factor in the database is 50% (an example being gender), implying that the relative risk of 3.2 implies proportions adherent of 13% and 42% among the two levels of the baseline factor.
RESULTS
A total of 73 participants were enrolled, following application of eligibility criteria (Figure 1). The summary statistics of participants compare adherent (both selfreported antihypertensive medication adherence and objective adherence based on mass spectrometry) and nonadherent individuals (self-
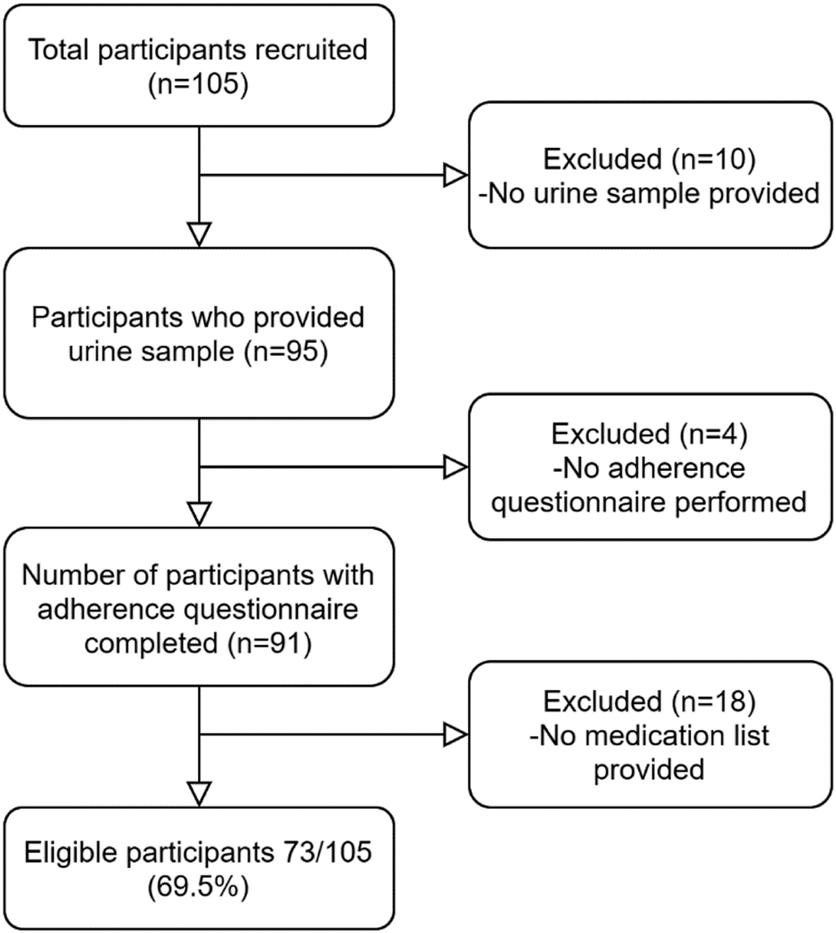
66 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1
reported nonadherence or self-reported adherence with no objective adherence on mass spectrometry). The phases of medication adherence are presented in combination.
Of the 73 participants, 16 (21.9%) recalled taking more than three antihypertensive therapies (95% confidence interval [CI] 0.1-0.3). The median number of antihypertensive medications was 2 (25-75% interquartile range [IQR] 2-3). Calcium-channel blockers (dihydropyridine and nondihydropyridine) were the most reported antihypertensive, with nearly 80% (95% CI 0.7-0.9) of participants reporting their usage, followed by angiotensin II receptor blockers (ARBs) and alpha-blockers. A total of 72.6% (95% CI 0.6-0.8) of participants were not adherent to their antihypertensive medications. While no subgroups demonstrated statistically significant associations with adherence after a correction for multiple testing, there were nominally significant associations between adherence groups and angiotensin-converting enzyme (ACE) inhibitors (0.023) and mineralocorticoid receptor antagonist (0.018) use, and also between adherence groups and antihypertensive single pill combination usage (0.031).
As outlined in Table 1, 55 of the total participants (75.3%, 95% CI 0.6-0.8) report antihypertensive medication adherence. Following mass spectrometry of urine results, only 20 of these (36.4%, 95% CI 0.2-0.5) demonstrated objective medication adherence. Only 18 subjects (24.7%, 95% CI 0.2-0.4) stated that they were nonadherent; of these, eight were confirmed to be fully nonadherent by spectrometry results. There was no statistical significance between groups of objectively nonadherent and adherent individuals. Adherence, both self-reported and using mass spectrometry, was demonstrated in 27.4% of participants (95% CI 0.2-0.4).
Further analysis of antihypertensive medication adherence is displayed in Figure 2, where participants are divided into three distinct groups:
1. Self-reported nonadherence
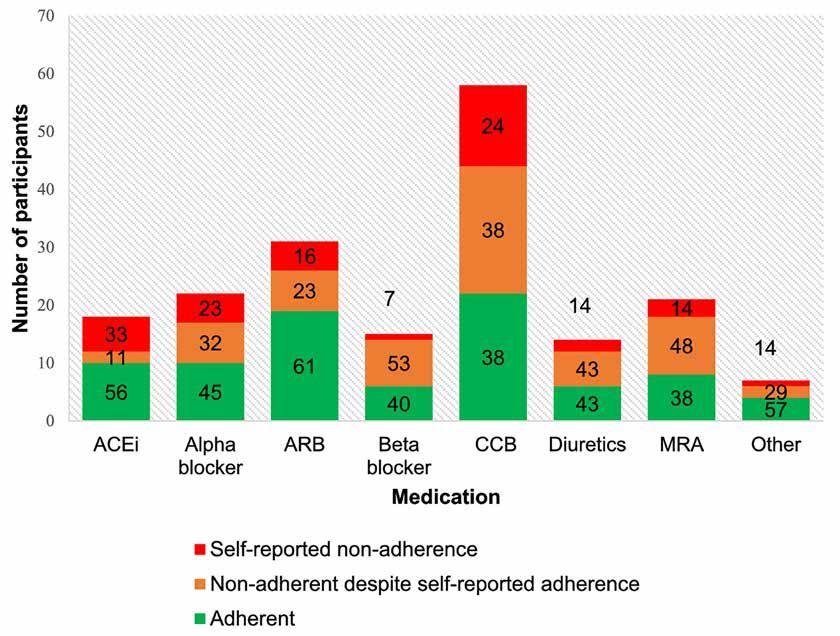
2. Self-reported adherence but objective nonadherence, based on mass spectrometry
3. Self-reported and objective adherence
Greatest medication adherence was achieved with ARBs (61%), with calcium-channel blockers and mineralocorticoid antagonists demonstrating least adherence (38%). Participants reported the greatest degree of self-reported nonadherence with ACE inhibitor medications, while urinary mass
spectrometry demonstrated least adherence with beta-blocker (53%) and mineralocorticoid antagonist (48%) therapy despite patient-reported adherence. There was no difference in participantreported side-effects between the two groups.
DISCUSSION
Nonadherence to medication represents one of the most significant causes of apparent treatment-resistant hypertension.18 Approximately half of patients who are prescribed an antihypertensive medication become nonadherent within the first year.19-21 Improvements in medication adherence that lead to improved clinical outcomes could result in significant reductions
in healthcare costs.22 In patients with difficult hypertension attending our hypertension clinic, over 70% were nonadherent to antihypertensive medications, yet approximately three-quarters self-reported adherence. Objective analysis, using urinary tandem mass spectrometry, demonstrated that two-thirds of participants who were nonadherent would therefore not have been identified by self-reporting alone. These results demonstrate a marked disparity between self-reported antihypertensive medication adherence when compared to objective evidence of adherence in patients attending a tertiary referral centre. Interestingly, in patients satisfying objective evidence of adherence, many selfreported occasional or frequent nonadherence. This finding is novel and confirms the importance of multimodality testing of medication adherence using both objective and subjective means in the setting of hypertension. Patients are more likely to take their antihypertensives immediately in advance of their clinic appointment and therefore urine analysis alone
67 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Nonadherent participants, LCMS/MS Adherent participants, LCMS/MS P value Nonadherent: selfreported 8 (18.6) 10 (33.3) .176 Adherent: self-reported 35 (81.4) 20 (66.7) Total 43 (58.9) 30 (41.1)
•
LC-MS/MS, liquid chromatography tandem mass spectrometry.
LC-MS/MS, liquid chromatography tandem mass spectrometry
TABLE 1. Comparison of self-reported medication adherence and mass spectrometry evidence of adherence
Figure 2
Hypertension
would miss a proportion of nonadherent patients.23, 24 This study prospectively evaluated a geographic catchment area of urban and rural dwellers with a participant profile representative of the regional demographic. The participation rate was high and the study was designed to minimise behavioural interference through spot urine sampling and testing without prior knowledge. Consequently, our data demonstrate higher levels of nonadherence when compared to similar studies in a tertiary setting.
A previous study by Tomaszewski et al also using LC-MS/MS to determine antihypertensive adherence in a tertiary referral hypertension clinic had much lower rates of partial adherence or nonadherence than those reported herein.16 This may be explained by demographic differences, as well as the methodology for medication reconciliation in this study (patient recall vs clinic letter). In another tertiary centre study, Jung et al demonstrated a nonadherence rate of 53%. This study selected patients with uncontrolled hypertension on four agents within a less clinically diverse population and excluded participants following drug optimisation and investigation for secondary hypertension.25 Our study investigated all patients attending clinic in a nonselected manner and used a combined approach to evaluation of nonadherence using both self-
reporting and objective urine analysis for antihypertensives and their metabolites. It is in fact likely that rates of variable adherence and nonadherence are even higher than those reflected in our current data. As the LC-MS/MS analysis only establishes the presence or absence of antihypertensives or their metabolites and not the concentration, this study accurately identifies nonadherence through initiation and discontinuation but does not detect nonadherence in the context of implementation. Therefore, while we describe a high level of nonadherence amongst our study sample, this limitation may indeed have underestimated overall nonadherence in this regard.
Calcium-channel blockers represented the most frequent medication class for which nonadherence was demonstrated. Almost two-fifths of subjects who have been prescribed a calciumchannel blocker failed to exhibit objective evidence of adherence on mass spectrometry, despite reporting adherence. Greater rates of adherence were found for other antihypertensives. Hayes and colleagues examined the feasibility of establishing nonadherence to medication in primary care and demonstrated that 74% of patients were fully adherent to treatment, 24% were partially adherent and 2% were fully nonadherent. Moreover, these authors found no significant associations
between the adherence status of participants and patient characteristics, number or type of medications used, morbidity or blood pressure.6 It is not necessarily surprising that patients attending a tertiary hypertension clinic have poorer adherence rates when compared to patients in the primary care setting given the referral bias of these patients for poorer control. We highlight the importance of screening for and addressing nonadherence at the first clinic visit in the tertiary setting as part of the investigational armamentarium.
Adherence was higher in patients taking single-pill combination antihypertensive preparations. These findings, albeit with a small number of participants in this cohort, favour the use of single-pill these therapies where possible and are consistent with previous evidence and international guidelines.26-28
A clear recommendation arising from this study is that medication adherence should be assessed and managed at the point of entry to all tertiary hypertension clinics. Nonetheless, careful consideration must be given to the modality of analysis. LC-MS/ MS is limited by cost, availability of effective validated assays and the required expertise for analysis. Moreover, single-sample analysis cannot reflect the temporality of adherence, representing a snapshot in time only. The assay used in this study is capable of
identifying the presence of up to 28 antihypertensives across a wide range of antihypertensive classes in a rapid and reproducible manner. Nonetheless, in our clinical practice we do not yet routinely screen patients for medication adherence using this methodology for reasons of convenience and cost. Patient self-reporting alone clearly underestimates the prevalence of nonadherence and is often difficult to address due to the potential for confrontation if not addressed with sufficient sensitivity.29
While there is marked variability in documented antihypertensive nonadherence in the literature between individual studies and across patient populations, it is clear that this remains one of the greatest challenges to adequate blood pressure control.29 A combination of optimising clinician-patient communication through self-reporting of nonadherence as well as the use of noninvasive objective evidence (mass spectrometry urinalysis) offers the potential to enhance more accurate assessment of adherence rates in a hypertension clinic compared to the single use of either modality alone Further exploration of intervention in this context is warranted to best assess how the difficult challenge of poor adherence rates among patients taking antihypertensives can be addressed in the future. References available on request
Dr Katalin Karikó awarded UCC Honorary Doctorate
Doctorate during a special ceremony at UCC on Wednesday (26th April) during what is World Immunisation Week.
University College Cork (UCC) will award an Honorary Doctorate to Dr Katalin Karikó for her pioneering mRNA research, which led to the BioNTech/Pfizer and Moderna vaccines against COVID-19.

Her work has been credited with turning the tide on the pandemic, with billions of people around the world now protected from the COVID-19 virus. Dr Karikó will be presented with her Honorary
Dr Katalin Karikó has dedicated her career to achieving a dream that conventional scientific wisdom once deemed implausible. For three decades, her research has focused on the use of messenger RNA (mRNA) - the genetic code that carries DNA instructions to each cell. She was convinced mRNA could be used to make their own medicine. She surmounted challenge after challenge until she prevailed, crucially discovering how to overcome the potentially lethal inflammatory response caused by synthetic mRNA that had precluded its use in humans.
The UCC honour recognises Dr Katriko’s discovery of this revolutionary mRNA technique and her outstanding contributions to science despite facing grant rejections and funding uncertainty. In advance of her honorary conferring Dr Karikó will meet with UCC medicine and health students and researchers.
UCC has a strong heritage in RNA research. In 2021, a team of UCC researchers led by Professor Atkins, together with Swiss collaborators, made a discovery that highlighted a potential drug target against the Covid causative virus. The pioneering work of Professor Caitriona O’Driscoll and Dr Piotr Kowalski are focused on developing innovative solutions to deliver RNA-based therapeutics.
Dr Katalin Karikó said, “I am deeply honoured to learn that
leaders of the University College Cork are awarding me an Honorary Doctorate degree. I am delighted to accept this prestigious recognition and looking forward to visit the University campus, meet the students and professors during my first-time travel to Ireland.”
The challenge for RNA-based researchers at UCC is to develop RNA medicines for chronic diseases where current treatments have researched a therapeutic ceiling and urgent needs exist to develop new innovative and targeted therapies. This goal is the focus of the UCC Futures – Future Medicines initiative launched in 2022 which is recruiting world leading scientists, engineers and clinician investigators at UCC and affiliated hospitals to deliver high-impact, transformational, next generation medicines and medical technologies.
68 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
Dr Katalin Karikó
A systematic review of the outcomes reported in the treatment of uncomplicated urinary tract infection clinical trials
Written by Sinead Duane1, Claire Beecher2, Akke Vellinga3, Andrew W. Murphy3,4, Martin Cormican5, Andrew Smyth6, Patricia Healy7, Michael Moore7, Paul Little7, Declan Devane2,3
1Discipline of Marketing, J. E. Cairnes School of Business and Economics, National University of Ireland Galway Ryan Institute, Environmental, Marine, and Energy Research, Martin Ryan Building, National University of Ireland Galway
2HRB TMRN, College of Medicine, Nursing and Health Sciences, National University of Ireland Galway
3College of Medicine, Nursing and Health Sciences, National University of Ireland Galway
4HRB Primary Care Clinical Trial Network Ireland, College of Medicine, Nursing and Health Sciences, National University of Ireland Galway
5Discipline of Bacteriology, College of Medicine, Nursing and Health Sciences, National University of Ireland Galway
6HRB Clinical Research Facility Galway, National University of Ireland
7Primary Care and Population Sciences, Primary Care and Population Sciences, University of Southampton, Faculty of Medicine
Urinary tract infections (UTIs) are among the most commonly presenting infections in the outpatient setting1 and account for significant morbidity and mortality at both individual and societal levels.2 A UTI is categorized as either complicated or uncomplicated. The most common causative agent in both is the Gram-negative bacteria uropathogenic Escherichia coli (UPEC); however, Gram-positive bacteria and fungi are also implicated.3
An acute, uncomplicated UTI in adults is characterized as the onset of acute cystitis occurring in otherwise healthy individuals without known structural or functional abnormalities of the urinary tract.4 A complicated UTI is associated with a structural or functional abnormality that increases the risk of treatment failure or serious complications.5 The epidemiology of UTIs varies depending on factors such as age and sex.6 The incidence of UTI is higher in females than in males. It is estimated that every woman will have at least one UTI in their lifetime7 and by the age of 24 years one in three women will have had at least one UTI diagnosed by a clinician requiring antibiotic treatment.8
In a bid to assess the most effective treatment intervention for uncomplicated UTI, clinical trials have been, and continue to be, completed internationally. Clinical trials are research studies undertaken to evaluate the safety and efficacy of a medical, surgical or behavioural intervention on human health outcomes. However, the comparison of the outcomes of these clinical trials is complicated by the variation in outcomes
reported, which hampers evidence synthesis,9 limiting the reliability of evidence to guide healthcare decisions. The development and adoption of a core outcome set (COS) addresses this lack of standardization in measuring outcomes, facilitates evidence synthesis and can help reduce reporting biases.10,11 A COS is an agreed minimum set of outcomes that should be reported in, and across, clinical trials of a specific condition.12
To date, a COS on the outcomes reported in trials examining the safety and efficacy of interventions for the treatments for uncomplicated UTI in adults has not been developed. The Core Outcome Set for treatment of Urinary Tract Infections (COSUTI) study seeks to address this knowledge gap. The first step is to review the treatment outcomes currently reported within clinical trials systematically. This systematic review aims to identify a comprehensive list of outcomes currently reported in clinical trials examining the effectiveness of interventions for the treatments for uncomplicated UTI in adults. For this review, uncomplicated UTI is defined as ‘the acute onset of dysuria, frequency, or urgency in healthy male and non-pregnant women without known functional or anatomical abnormalities of the urinary tract’.13 The outcomes reported in trials will be categorized, and any variance discussed to develop a core outcome set.
Methods
The protocol for this systematic review has been published previously as part of the COSUTI study protocol14 and a summary is presented below.
Included in this review were all randomized trials and systematic reviews of randomized trials (with and without meta-analyses) comparing the effectiveness of any interventions for the treatment of uncomplicated UTI or cystitis in healthy adults. Recurrent UTIs and pyelonephritis were excluded as they were beyond the scope of this study. A combination of search terms was used to search for relevant studies between 2007 and 2017 within the Cochrane Database of Systematic Reviews (including CENTRAL, CDSR and DARES), PubMed and Embase. Search terms included: urinary tract infection; UTI; Cystitis, Randomized controlled trial; Controlled clinical trial; randomized; Placebo; randomly; trial. The full search strategy is available by accessing the COSUTI protocol.14
A 10 year period was chosen to ensure that outcomes identified reflected contemporary treatments. Only papers available in English were screened. Two reviewers independently screened the title and abstracts and the subsequent eligible full text. Additional reviewers were consulted if there was uncertainty.
Outcomes were extracted verbatim. Similar outcomes were grouped into domains using a paired comparison process and where required were further categorized into subdomains. This categorization process allowed for similar outcomes to be grouped together under themes. Once this process was complete, each outcome and its domain categorization were verified by the COSUTI team. Patterns of outcome reporting were examined, including the number
of studies and frequency with which outcomes were reported and defined, the time frame of reporting and reproducibility. The PRISMA and COMET11 guidelines will be used to report the conduct and findings of this review.
Results Study selection
Across the four databases, we identified 8743 papers. Once duplicates were removed 6313 were eligible for title and abstract screening. A further 6177 papers were excluded during title and abstract screening. The majority of papers were excluded as they referred to treatments of complicated UTIs, recurrent UTI or children. Trials conducted on animals and other illnesses were also excluded. Once title screening was complete 136 studies were deemed eligible for full text screening. A further 94 were excluded for reasons outlined in Figure 1. Papers were excluded for various reasons including different patient population (n = 23); not available in English (n = 12); duplicate studies (n = 9); reported in abstract form only (n = 23); different study design (n = 7) or setting (n = 14); discussion paper (n = 5); and no outcomes reported (n = 1). Fortyone studies were included in the data extraction process. Figure 1 summarizes the COSUTI PRISMA.
Characteristics of studies
In total, 334 outcomes were reported across 41 papers.15–55 A definition was provided for 73 (22%) of these outcomes. The average number of outcomes reported per trial was eight. The lowest number of outcomes reported was 1, the highest
69 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
UTI
UTI
PRISMA flow diagram.
Issue 2, April 2022, dlac025, https://doi.org/10.1093/jacamr/dlac025
JAC Antimicrob Resist, Volume 4, Issue 2, April 2022, dlac025, https://doi.org/10.1093/jacamr/dlac025
The content of this slide may be subject to copyright: please see the slide notes for details.
copyright: please see the slide notes for details.
being 15, which were reported within three papers.
copyright: please see the slide notes for details.
Once duplicate outcomes were removed, 124 outcomes that demonstrated similar characteristics were categorized under 18 domains.
The variety of domains reflects

the difference across the outcome measures that have been reported in UTI treatment trials. The majority of outcomes were categorized under the clinical cure domain, with outcomes further categorized into eight related subdomains (clinical cure, symptom resolution, symptoms, complications, adverse
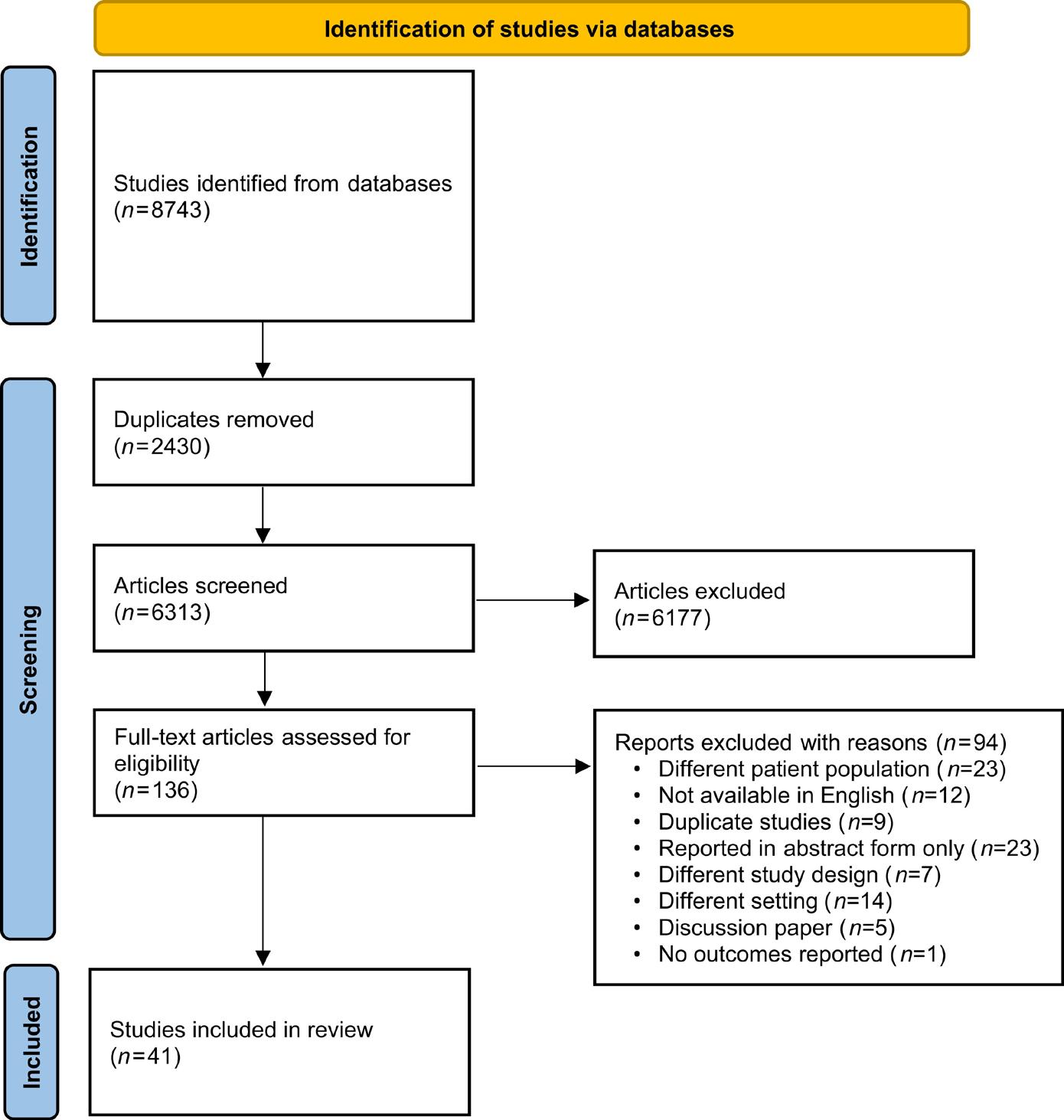
treatment reactions, relapse and failure). Microbiological cure was also identified as a domain, with outcomes further categorized into four subdomains.
Issue 2, April 2022, dlac025, https://doi.org/10.1093/jacamr/dlac025
Outcomes related to antibiotic use included the use of antibiotics and the duration of treatment.
Other domains investigated the patients’ quality of life, satisfaction with treatment, discontinuance, tolerance and antibiotic resistance.
Only 3 out of the 18 domains (antibiotic use, symptoms and failure) were further classified into subdomains, which reflected nuances in what the outcome was measuring.
70 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1. COSUTI PRISMA flow diagram
PRISMA flow diagram.
Table 1.
Clinical cure domain
Clinical cure
Clinical cure
Clinical cure by Day 4 from initiation of treatment
Clinical cure by Day 7 from initiation of treatment
Clinical cure 5–9 days after completion of the treatment
Clinical cure 3 weeks after completion of the treatment
Clinical cure 4–6 weeks after completion of the treatment
Clinical cure by the 30 day follow-up visit
Adverse treatment reactions and complications
The domains adverse treatment reactions (n = 25 outcomes) and complications (n = 17 outcomes) had the most reported outcomes. A range of outcomes were documented within these domains. There were inconsistencies in how adverse treatment reactions and complications were reported in trials, which demonstrates the difficulty in reporting these outcomes. In some cases, each adverse treatment reaction or complication related to treatment was reported as a separate trial outcome.
Quality of life
Quality of life outcomes were not measured across the majority of trials. Five studies included quality of life outcomes (e.g. patient reported quality of life and mental health status), and an additional three included absenteeism from work as outcomes.
Timepoints
Eight of the 18 domains included similar outcomes measured or reported at different points in time. The variety of timepoints reported within outcomes has resulted in an overall increase in the number of outcomes reported. For example,
the relapse domain included outcomes related to relapse and recurrence after the initial resolution of UTI symptoms. Thirteen of 16 outcomes included within this domain reported a timepoint. Reported timepoints ranged from short- (14 days) to long-term (1 year) periods. The outcomes that were not time-bound were temporally broad, e.g. relapse after initial resolution of symptoms, and related to measuring when a relapse happened.
Seven outcomes were categorized as clinical cure (Table 1). Temporally, these outcomes related to clinical cure generally, clinical cure after initiation of treatment, on completion of treatment and at follow-up. This illustrates the need for trialists to evaluate outcomes of treatment at different timepoints. However, the inconsistency and overlap of timepoints related to initiation and completion of treatment may also contribute to difficulties in synthesizing trial findings.
Discussion
This systematic review of clinical trials for the treatment of uncomplicated UTIs demonstrates the substantial differences in how outcomes are measured, with 124 outcomes reported across 18 domains. The acute nature of
UTI episodes contributes to the ambiguity around key domains such as ‘clinical cure’, which in turn also contributes to the inconsistency in associated timepoints of outcome measurement. The optimal time to report many of the outcomes remains unclear due to a lack of definition or justification for the choice of timepoint. There is a need to develop a core outcome set to improve reporting of trial evidence and strengthen evidence synthesis capability in this area. This would also help minimize the measurement and collection of outcomes of less relevance to stakeholders.
The descriptive nature of this review aligns with its purpose to describe outcomes reported currently rather than compare the results of the included trials. The definition of uncomplicated UTI described in this paper differs from the FDA guidance for developing drugs for treatment of uncomplicated UTI as well as the IDSA/ESCMID treatment guidelines for uncomplicated UTI by including male members of the population; this decision was taken as some of the trials investigating treatments of UTI specified adult populations and not exclusively female populations.
Conclusions
As the second most common infection presenting in primary care, UTI treatment contributes significantly to the prescription of antibiotics in primary care, accounting for approximately 15%–20% of antibiotic prescriptions.56 As the fight against antibiotic resistance intensifies, it is important that the search continues for effective, safe alternative treatments to antibiotics for UTI.
The impact of the trials investigating the most effective treatments for UTI that have been completed or are ongoing in this area is limited due to the volume and variation of outcomes currently reported. This systematic review highlights the inconsistencies around which outcomes are most important or at what timepoints they should be reported. This hampers evidence synthesis in this area.
To improve consistency, a standardized set of outcomes should be developed to report trials investigating effective treatments for UTI and evidence synthesis. The next phase of COSUTI will focus on the development of a core outcome set to address this research gap. References available on request
71 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Table 1 - Clinical cure domain
Respiratory Syncytial Virus: An Overview
Respiratory syncytial virus (RSV) is a common, ubiquitous and contagious viral pathogen that infects the respiratory tract of most children by 2 years of age.
We recently spoke to Theresa Lowry Lehnen, Clinical Nurse Practitioner and Associate Lecturer South East Technological University to find out more about the condition.
RSV is an RNA pneumovirus of the Paramyxoviridae family, and humans are the only natural host. Theresa explains, “RSV typically spreads via hands, fomites and the airborne aerosol route. Respiratory syncytial virus spreads from person to person by aerosol droplets through coughing or sneezing, and is also spread through direct contact by touch.”
Respiratory syncytial virus is primarily a childhood infection, however, it may occur at any age and can be most severe in infants under one year of age, the immunocompromised, and in people aged 65 years and older, she says.
“It infects 90% of children within the first 2 years of life and frequently re-infects older children and adults. Respiratory Syncytial Virus infection can present as a variety of clinical syndromes including upper respiratory tract infections, bronchiolitis, pneumonia, exacerbations of asthma and viral-induced wheeze.
“Worldwide, it is estimated that RSV is responsible for approximately 33 million lower respiratory tract illnesses, three million hospitalisations, and up to
199,000 childhood deaths. The majority of deaths are in resourcelimited countries.”
Respiratory Syncytial Virus infection has been a notifiable disease in Ireland since January 2012, and RSV activity in Ireland is monitored by the HSE-Health Protection Surveillance Centre (HPSC). RSV notifications are reported in the HPSC infectious disease weekly report and outbreaks in the weekly outbreak report. According to the HPSC (2022), nearly all children have been infected with RSV at least once, by two years of age.
Theresa adds, “Most cases are not specifically diagnosed as RSV, however, the infection causes 80% of bronchiolitis and 20% of pneumonia cases in young children and RSV is a significant cause of infection and outbreaks in hospitals, neonatal units, day units and nursing homes.
“While most infections cause only mild symptoms, RSV is the most important cause of viral lower respiratory tract infection (LRTI) in infants and children globally, and is responsible for one-third of deaths resulting from acute LRTI in the first year of life. In medium and high resource countries, RSV mortality rate in infants is almost nine times that of influenza.
“Respiratory Syncytial Virus infections occur in a seasonal pattern in temperate climates with epidemics from October to April. Outbreaks typically occur in the winter months with the highest numbers of infections usually reported in December and January every year.
There is only one serotype of RSV, but it is classified into two strains, “A” and “B,” with differences consisting of variation in the structure of several structural membrane proteins, most especially the attachment protein. One of the two major antigenic subgroups of RSV A or B, usually predominates each season. Transmission occurs through contact with aerosolised viral particles generated through sneezing and coughing, or from contaminated surfaces or fomites. She adds, “Largeparticle droplets can survive on contaminated surfaces for up to 6 hours. Handwashing is the most effective infection control procedure. Infected individuals shed RSV for 3 to 8 days but immunocompromised patients with severe infection may shed virus for up to 4 weeks.
“The frequent occurrence of mild or asymptomatic infection in otherwise healthy individuals makes infection control challenging. Incubation Period and Symptoms
The incubation period for RSV is usually 4 - 6 days, but ranges from 2 to 8 days depending on host factors such as the age of the patient and whether it is the patient’s primary infection with RSV.
“After inoculation into the nasopharyngeal or conjunctival mucosa, the virus rapidly spreads into the respiratory tract, where it targets its preferred growth medium, apical ciliated epithelial cells. There it binds to cellular receptors using the RSV-G glycoprotein and uses the RSV-F fusion glycoprotein to fuse with host cell membranes and insert its nucleocapsid into the host cell to begin its intracellular replication.”
Symptoms of RSV include; fever, rhinorrhoea, pharyngitis, nasal congestion, sneezing, coughing which can be croupy or barking in nature, tachypnoea, sore throat, wheeze, decreased appetite and ear infections in children. In very young infants, irritability, decreased activity and breathing difficulties may be the only symptoms of infection. Lower respiratory tract infections, such as pneumonia or pneumonitis, are most likely to occur during a child’s first infection with RSV and may develop in 30-70% of first infection.
Typically, only between 1% and 3% of infected infants require hospitalisation.
“The infectious period lasts from shortly before onset to one week post the onset of symptoms. Most children recover in 8- 15 days,” Theresa notes. “Even after recovery, however, very young infants and children with weakened immune systems can continue to spread the virus for 1-3 weeks. Immunity is incomplete and short-lived.”

Bronchiolitis is an inflammatory process in the small airways of the lungs and is the most common clinical syndrome associated with RSV infection. It typically presents in infants under 1 year of age but may be diagnosed in children up to 2 years old, and is characterised by a short history of low-grade fever, cough, coryza, dyspnoea and reduced feeding. The symptoms usually peak in clinical severity between day 3 and 5 of the illness.
“RSV bronchiolitis presents a significant clinical burden,” Theresa tells us. “In the UK, infection with RSV is responsible for up to 80% of all cases of bronchiolitis, similar to that of 65–70% in the US. In older children, Respiratory Syncytial Virus typically presents as an URTI, viral pneumonia, episodic viral-induced wheeze or an acute exacerbation of asthma.”
Viral pneumonia is a common illness with 5 million cases reported in children annually. A meta-analysis of nine studies involving over 4000 children investigating viruses identified by polymerase chain reaction, found that RSV was the causative organism in 11% of communityacquired pneumonia cases.
Theresa says that diagnosis includes a thorough medical history and a physical exam. “A chest X-ray may be requested and blood and urine tests may be carried out to out-rule a bacterial infection or other conditions. Differential diagnosis can include, asthma, bronchiolitis, influenza, croup, bronchitis and pneumonia.
“Respiratory viral testing may be used in a clinical setting to increase confidence in the diagnosis of a viral, rather than bacterial, cause for respiratory illness. RSV can
72 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
RSV Virus
An interview with Theresa LowryLehnen (PhD), CNS, GPN, RNP, South East Technological University
be detected in nasopharyngeal aspirate, broncho-alveolar lavage, sputum, or swabs from the nose and throat by using real-time PCR, immunofluorescence, ELISA and growth in cell culture. Reverse transcriptase-polymerase chain reaction (RT-PCR) assays are currently the gold standard in RSV testing and are available commercially. These are more sensitive than antigen detection and virus isolation methods,” she adds.
The mainstay of treatment for the vast majority of RSV infections is supportive including rest, fluids and paracetamol, but passive preventive immunisation is available for at-risk children, including premature infants and infants with a history of cardiac, pulmonary, or neuromuscular diseases.
“Those with severe respiratory illness require hospitalisation, oxygen therapy, IV fluids and ventilatory support in the form of a high-flow nasal cannula, CPAP, or intubation, and mechanical ventilation. Ribavirin is the only licensed antiviral medication for the specific treatment of RSV infection but due to drug toxicity, including bone marrow suppression and potential carcinogenicity and teratogenicity and minimal clinical benefit, it has not been recommended for routine clinical use.
“Ribavirin, may be considered for a small number of patients
and treatment of RSV with Ribavirin must be done under the supervision of an infection specialist such as a consultant microbiologist or an infectious disease specialist. Other treatment modalities for bronchiolitis have been tried in the past and have failed to show broad, reproducible efficacy on clinically significant outcomes in RSV and bronchiolitis. Antibiotics are not effective against RSV and it is important that unnecessary antibiotics are discontinued once a diagnosis is confirmed, to avoid adverse drug reactions and antibiotic resistance.
“Infants who are recovering from RSV bronchiolitis can continue to have respiratory symptoms including cough and postbronchiolitis wheeze for several weeks/months. There is no evidence for the use of steroids, montelukast or other medications in preventing these symptoms but the acute episodes often respond to anti-asthma medication.”
Theresa notes that high-risk infants with other co-morbidities may require longer admission and some may even require mechanical ventilation.
However, she adds, “the majority of children with RSV make a full recovery and have an excellent outcome. The majority of children who need hospital admission are usually discharged in several days. Some infants with RSV may develop wheezing but recent
studies do not show an increased risk of asthma. Prevention and patient education is key, and frequent, careful handwashing is the most important measure in preventing the spread of RSV.
“Respiratory etiquette should be properly maintained and people with cold/flu-like symptoms should cover their nose and mouth preferably with a tissue or cough and sneeze into their elbow and wash their hands afterwards for at least 20 seconds or use an alcohol-based rub/gel. Used tissues should be properly disposed of.
“Sharing utensils with persons who have RSV illness should be avoided and cleaning contaminated surfaces such as door handles may help stop the spread of RSV. Parental smoking is a known risk factor for RSV infection in infancy, and parents or carers who smoke should be offered smoking cessation advice and encouraged to stop smoking. Breastfeeding also offers some protection against RSV infection.
“Persons with RSV should not attend crèches, school, work and non-residential institutions until well. It is important to prevent young infants, frail older persons and immunocompromised people coming into contact with individuals with respiratory infection.”
The management of Respiratory Syncytial Virus disease in infants and children is primarily supportive
with antiviral medications reserved for the most vulnerable, she concludes. “The development of a well-tolerated, clinically effective and cost-effective RSV vaccine and therapeutic agent remains a global health priority. It is likely that a licensed RSV vaccine is several years away, however, given the burden of RSV infection and the associated costs globally there is much ongoing research into the development of a well-tolerated and effective vaccine.
“The main target populations for vaccination include infants, school age children, pregnant women and older adults. At least 14 anti-RSV treatment products are undergoing phase I and II clinical trials, of which 5 have included paediatric patients. Novel therapeutic molecules developed to date include, fusion inhibitors, non-fusion inhibitors, polymerase inhibitors, antibodies, nucleoside analogues, small-interfacing RNAs and a benzodiazepine. They have various targets on RSV such as the F protein, RNA polymerase, nucleoprotein and nucleocapsid mRNA. It is hoped that one of these products will become a licensed treatment for RSV infection in children and adults over the coming years.
“The development of a successful treatment or prophylactic agent has the potential to revolutionise the care and outcome for severe RSV infections in the world’s most vulnerable infant population.”
73 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Smoking Cessation for Patients with Cancer –the role of the Pharmacist

Professor Patricia Fitzpatrick and a research team from UCD, the HSE Tobacco Free Ireland Programme, specialist cancer hospitals and patient representatives, conducted a research programme funded by the Irish Cancer Society entitled “Smoking cessation for cancer patients in Ireland: a scoping and feasibility initiative.”
It is well-known that tobacco smoking increases the risk of several cancers. It also has an impact on outcomes for people with cancer who continue to smoke but this is often overlooked. Tobacco smoking is associated with poorer treatment outcomes (including treatment-related complications and toxicities), increased risk of recurrence, the development of second primaries, lower survival, and decreased quality of life among continuing smokers. Notwithstanding the adverse outcomes, a significant number of people who have been diagnosed with cancer continue to smoke.
This research had 6 work packages: a rapid review of smoking cessation interventions in cancer patients; a review of national smoking rates among cancer patients (2014-2018); a national audit of existing smoking cessation services across all adult cancer hospitals; semi-structured interviews with 25 cancer patients who smoke or quit at diagnosis,
semi-structured interviews with 18 cancer healthcare professionals, and a Patient Voice in Cancer Research workshop (www.ucd.ie/ patientvoicecancer) . We selected patients with cancers particularly associated with smoking (head & neck cancer, cervical, lung) and highly prevalent cancers (breast cancer), in addition to other patients attending oncology clinics). The study findings of particular importance for pharmacists are described below. Using Hospital In-Patient Enquiry (HIPE) data we found that during 2014–2017, current smoking on discharge from hospital increased overall in patients with cancer (10.5–11.7%), patients with lung cancer (24.7–27.2%), cervical cancer (11–19.8%) and head and neck cancer (3–12.7%), with small reductions in all in 2018. Smoking rates in patients with breast cancer were stable. These rates are lower than the Irish general population (23–20%) but are increasing or have plateaued, unlike the national figures. It is important to recognise that HIPE data are subject to potential duplicate episodes of care and under-documentation of smoking; patients may also stop smoking in hospital due to a combination of feeling sick and difficulties smoking as an inpatient on smoke-free hospital campuses but revert to smoking on return home. However, trend analysis is useful, as these limitations should be stable.
Qualitative analysis of patient interviews identified key themes as follows: prior to diagnosis, patients were striving but struggling to quit; diagnosis was a shock but also a cue to action with many patients quitting at diagnosis- a reminder of
the “teachable moment”; routine but variable smoking cessation assessment and support was available in hospital; patients felt they needed a well-defined referral system. An important smoking cessation facilitator was support from others (family and healthcare professionals (HCPs)). Patients wanted a sustained, intensive, non-judgmental approach to smoking cessation incorporating pharmacological and behavioural interventions spanning hospital and communitybased settings. Some but not all patients were aware of Nicotine Replacement Therapy (NRT) and other stop smoking medicines like varenicline being free if they have a medical card. Smoking cessation barriers included limited knowledge of smoking cessation support, lack of family support and lack of willpower. Analysis of interviews with Health Care Professionals working in four of nine specialist cancer centres, identified challenges in discussing smoking cessation with patients diagnosed with cancer. While patients expect to be asked about their smoking habit, the timing of the conversation adds a layer of complexity at times when patients are dealing with a new cancer diagnosis, treatment options, disease and treatment-related complications/toxicities. Providing supportive non-judgemental conversations is critical, and pharmacists, using the Make Every Contact Count Framework, specifically Brief Advice and Brief Interventions, are well placed to support patients with cancer on their journey to smoking cessation.
Patients are seeking support to quit. Seizing the teachable moment, hospital pharmacists can support the early dispensing of NRT products to patients admitted to smoke-free campus hospitals, as part of either a smoking management approach or a start of smoking cessation Community pharmacists can assist in several ways. Brief advice from pharmacists takes 30 seconds and can double the chance of someone stopping smoking successfully (3 A’s Model - Ask, Advise and Act). Asking about smoking behaviour sets the stage
for support. People who smoke expect to be asked about tobacco use and are surprised when this is not raised with them. Advise people who smoke about the harm it causes and the benefits of quitting. For people with cancer, these harms and benefits are often overlooked. Finally, act by providing or arranging safe, effective, and clinically sound support to stop smoking. If the person with cancer is accompanied by a family member who also smokes, consider extending your advice, as a smoke free home is supportive to smoking cessation. The best chance of successfully stopping smoking is through using behavioural support and stop smoking medicines. Behaviour support is available free of charge across the country through HSE services where patients can be referred (you can find a service near to you here: https://www2.hse.ie/living-well/ quit-smoking/support-services/).
Varenicline and combination NRT is recommended in National Stop Smoking Guidelines as the best first-line option where suitable and available. Combination NRT is also a good option, using a long-acting patch and a short acting product like a spray, inhaler lozenge or gum. Pharmacists are well positioned to offer support with over-thecounter medicines and will be able to advise and encourage patients, especially those with cancer who may be on other medicines for comorbidities, on the best options. HSE QUIT services are available on QUIT.ie, Freetext QUIT to 50100, and Freephone 1800201203. Pharmacists with Healthlink can make a referral using that system, or else a referral can be made by emailing quit@healthmail.ie, which is a safe and secure way to share information. An easy to use onepage resource has been developed by HSE QUIT for pharmacists: https://www.hse.ie/eng/about/who/ tobaccocontrol/national-clinicalguidelines/supporting-peopleto-stop-smoking-advice-forpharmacists.pdf
For further information or to see a list of published outputs please contact Patricia.Fitzpatrick@ucd.ie References available on request
74 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Pharmacy
Professor Patricia Fitzpatrick
Professor Patricia Fitzpatrick1,2, Dr Paul Kavanagh3, Associate Professor Kate Frazer4, Dr Patricia Fox4, Professor Sinead Brennan5, Dr Ailsa Lyons2, Dr Nancy Bhardwaj1, on behalf of the Research Team
1School of Public Health, Physiotherapy & Sports Science, University College Dublin 2St Vincent’s University Hospital, Dublin 3Health Service Executive Tobacco Free Ireland Programme 4School of Nursing, Midwifery & Health Systems, University College Dublin 5St Luke’s Hospital, Rathgar, Dublin
The impact of the COVID-19 pandemic on the provision of endovascular thrombectomy for stroke: an Irish perspective
1Department of Neuroradiology, Beaumont Hospital, Beaumont, Dublin 9, D09 V2N0, Ireland; Faculty of Radiologists, RCSI, Dublin
2National Thrombectomy Service, Beaumont Hospital, Dublin, Ireland
outcomes were also investigated, including the total number of referrals and patient outcomes. We hypothesised that the total number of AIS patients referred to the thrombectomy centre with LVO, as well as the number of EVTs performed, would reduce following the onset of the pandemic. This would be due to a combination of delayed patient presentation to the hospital, local hospital workflow issues and the logistical challenges of interhospital transfers. We also hypothesised that EVT procedure time metrics would disimprove because of delays associated with COVID infection control measures amongst other factors.
Methods
Novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus disease (COVID-19) have had a huge impact on non-COVID-19-related care in hospitals. To date, there have been over 650 million documented cases and over 6.5 million reported mortalities.1 Literature has demonstrated an association between COVID-19 and acute stroke, with an incidence of 1.4% of those affected with the disease and almost 80% of COVID-19 patients with acute ischaemic stroke (AIS) have been shown to have a large vessel occlusion (LVO) pattern.2 Endovascular therapy (EVT) has been proven to be the gold standard management of LVO strokes.3 Prompt management with EVT is imperative, with small
reductions in treatment delays providing marked improvements in long-term functional outcomes.4,5 The COVID-19 pandemic presented unprecedented challenges to global healthcare. Testing, contact precautions, use of personal protective equipment and infection control procedures may all impede expedient management of patients with AIS secondary to LVO.
The first case of COVID-19 in Ireland was confirmed on 29 February 2020, and over 1.6 million cases and 8000 deaths have since been recorded.1, 6 When the pandemic initially took hold, an unprecedented impact on service provision swept across the nation, with significant delays in almost all non-COVID-19-related care as a nationwide ‘lockdown’
was imposed.7 Challenges included staff redeployment, increased staff sick leave, delays in patient presentation and infection control measures. Spikes in case numbers also threatened to overwhelm the healthcare system and deplete resources. Despite numerous guidelines and recommendations published to advise management considerations with COVID-19, significant concerns were expressed in relation to potential hold up of treatment in AIS with LVO.8,9,10,11
This study aims to assess the impact of COVID-19 on service provision at the primary endovascular stroke centre (ESC) in Ireland. EVT time metrics constitute the primary outcome measure in this study. Numerous secondary
Beaumont Hospital is Ireland’s national neurosciences centre and primary endovascular stroke centre (ESC), providing emergency EVT services for patients with AIS resulting from LVO to the majority of the country’s population. Until early 2021, a national 24-h thrombectomy service was provided solely by Beaumont Hospital with Cork University Hospital providing daytime cover for the majority of the South of the Republic of Ireland. In April 2021, Cork University Hospital commenced 24-h emergency EVT provision.12
In Ireland, patients presenting with suspected stroke outside of the geographical catchment area of an ESC are assessed, imaged and treated with intravenous thrombolysis at the nearest PSC. Candidates for EVT identified on imaging are discussed directly with an ESC regarding suitability for consideration of EVT. Patients were transferred for EVT from 21 sites in 2020 and 20 sites in 2021, in addition to direct presentations to our institution.12,13,14
Suspected stroke cases presenting to PSCs undergo a clinical examination and subsequent radiological investigation including
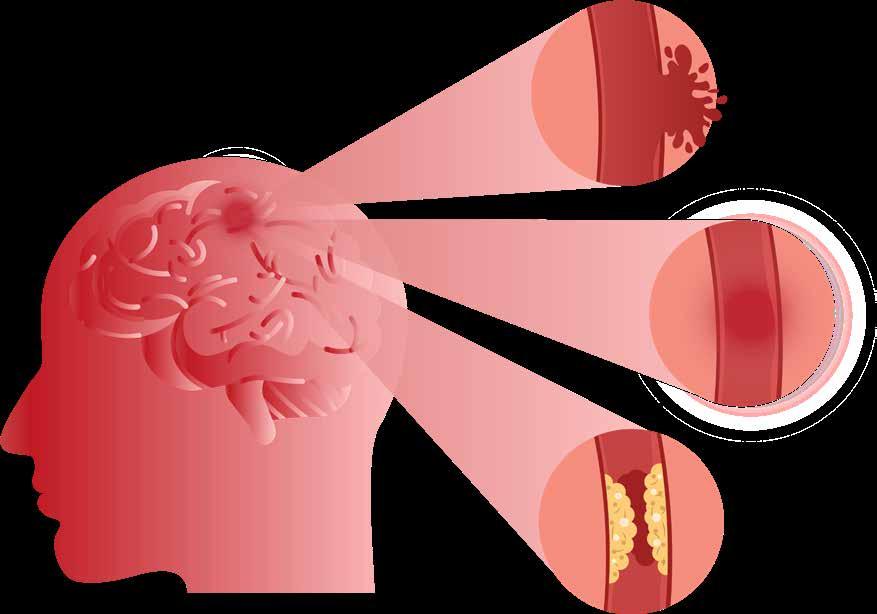
75 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023 Stroke
Written by Conor Brosnan1, David Brennan1, Conor Reid1, Sarah Power1,2, Alan O’Hare1,2, Paul Brennan1,2, John Thornton1,2 & Matthew Crockett1,2
Table 1 Patient demographics
Back to article page Not logged in - 82.8.44.181 Not affiliated © 2023 Springer Nature Switzerland AG. Part of Springer Nature Demographics Total (n = 940) 2019 (n = 306) 2020 (n = 307) 2021 (n = 327) Median age 73 73 72 73 Male:female 482:458 153:153 152:155 177:150 Median arrival NIHSS 15 15 16 14 Median pre-inter vention ASPECTS 9 9 9 9 % thrombolysed 39% (364/938) 42% (128/304) 43% (132/307) 32% (105/327) Occlusion site Multiple occlusions 136 (14%) of patients had > 1 occlusion 57 (18%) of patients had > 1 occlusion 21 (7%) of patients had > 1 occlusion 58 (14%) of patients had > 1 occlusion Total occlusions 1127 373 330 424 ACA 17 3 5 9 Basilar/PCA/vert 79 27 17 35 ICA cervical/carotid T/L 246 100 65 81 MCA (M1 + M2) 785 243 243 299 Total considered for thrombectomy 1107 357 357 393 Thrombectomy candidates transferred from PSC 806 266 257 283 Thrombectomy candidates presenting to ESC 134 40 50 44 Already in ESC* 24 10 7 7 Total number of thrombectomies 941 307 307 327 Unsuitable for thrombectomy 167 51 50 66
.com/article/10.1007/s11845-023-03314-9/tables/1
initial non-contrast CT followed by single or multiphase CT angiography (CTA). In select cases, CT perfusion may be also performed, although this is only available in a few centres in the country. EVT eligibility is based on National Thrombectomy Service guidelines derived from the best evidence.15, 16 Patients are assessed on a case-by-case basis, with general inclusion criteria including all patients with an LVO presenting within 24 h of symptom onset or last known well time, with an ASPECTS score of > 5. After the commencement of intravenous thrombolysis, if appropriate, all cases eligible for EVT are discussed immediately with the interventional neuroradiology service of the ESC. Decisions for transfer are made on a case-bycase basis. Patients suitable for further management are transferred immediately by paramedics, accompanied by medical and nursing staff. Repeat assessment is performed on arrival by the stroke team at the ESC. Repeat imaging may be performed in some instances prior to transfer to the neurointerventional suite for EVT. Numerous measures were taken to mitigate the spread of COVID-19 to healthcare workers providing EVT to patients with AIS and LVO. Necessary efforts were also made to reduce possible delays in providing treatment. Implemented measures followed the most upto-date international guidelines available at the time.8, 17
All patients presenting directly to our centre were tested immediately on arrival for COVID-19. Testing was performed on all patients presenting to external PSCs prior to transfer. Initial reverse transcriptase polymerase chain reaction (RT PCR) testing was replaced by rapid COVID-19 GeneXpert testing. For cases returning a negative result, standard infection precautions were followed. Patients with a positive test and those without a negative result were treated as positive. Our centre houses two neuroangiographic suites, with a dedicated COVID-19 suite in the setting of two available rooms. To expediate management, all procedural equipment for standard EVT was pre-prepared outside both thrombectomy suites in an expectant fashion.
For COVID-19-positive cases, all unnecessary objects were removed from the angiographic suite prior to the procedure, and all surfaces were covered with aseptic drapes. Staff for each procedure were kept to a minimum with a single nurse, radiographer and consultant interventional neuroradiologist (and a single interventional neuroradiology fellow during normal working hours) present. Doors of the suite were taped closed during the procedure. Additional personal protective equipment was used with a double-glove technique, N-95-approved face masks, shoe covers and face shields worn to reduce spread.
Study periods, patient population and outcomes
A retrospective review of the prospectively maintained National Thrombectomy Service database in Beaumont Hospital was performed. All patients with AIS and LVO presenting to our centre or transferred from external sites for thrombectomy between 1 January 2019 and 31 December 2021 were included. Data obtained was broken down into three separate study periods, 1 January 2019 to 31 December 2019, representing the year prior to COVID-19, 1 January 2020 to 31 December 2020, representing the initial impact of COVID-19, and 1 January 2021 to 31 December 2021 to represent continued care and the response to COVID-19.
Baseline demographics were acquired for 2019, 2020 and 2021, including patient age and sex (detailed patient demographics are displayed in Table 1). The number of patients with AIS secondary to LVO and presentation to ESC or external PSC were documented. Degree of neurological deficit (objectively measured by the National Institutes of Health Stroke Scale (NIHSS)) and basic image findings such as Alberta Stroke Programme Early CT score (ASPECTS) and occlusion site were also recorded.
The primary outcome of the study was time of arrival at ESC to time of groin puncture (doorto-puncture time), which was
assessed as a continuous variable. Several other time metrics were also analysed over the study period including median procedure duration and median time from groin puncture to recanalisation, median door-to-imaging time, median door-to-intravenous thrombolysis time and median time of symptom onset/last seen well to presentation, groin puncture and recanalisation. Measures of response to thrombolytic therapy (using the ‘thrombolysis in cerebral infarction (TICI)’ scoring system) and patient functional outcomes (using the ‘modified Rankin scale (mRS)’ scoring system) were also assessed.
TICI scores were recorded by the interventional neuroradiology consultant performing the procedure for all anterior circulation strokes. A second, independent interventional neuroradiologist reviewed imaging and ascribed a TICI score to each case prior to entry into the retrospective database. Any discrepancies were resolved by a third, senior interventional neuroradiologist. Rates of 2b, 2c and 3 are considered good outcomes.18,19,20 Expected rates internationally are 80% for TICI 2b-TICI3 inclusive.12
76 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
1
Stroke Table
Patient demographics
mRS scores were recorded routinely 90 days after presentation. A good functional outcome was taken to be an mRS score of ≤ 2 or the ability to perform basic activities of daily living without a problem.21, 22 Home Irish Journal of Medical Science (1971 -) Article Table 1
From: The impact of the COVID-19 pandemic on the provision of endovascular thrombectomy for stroke: an Irish perspective
Table 2 Time metrics and functional outcomes for those undergoing thrombectomy
From: The impact of the COVID-19 pandemic on the provision of endovascular thrombectomy for stroke: an Irish perspective
Table 2 Time metrics and functional outcomes for those undergoing thrombectomy
Results
Over the course of the study period, a total of 940 patients underwent EVT at Beaumont Hospital. Eight hundred six patients were transferred from PSCs and 158 patients presented directly to Beaumont Hospital. Three hundred seven thrombectomies were performed in 2019 and 2020 and 327 were performed in 2021. The total number of patients transferred for thrombectomy was 357 in 2019 and 2020, with an increase to 393 in 2021. A total of 167 patients were transferred to the ESC but deemed unsuitable for thrombectomy upon arrival over the course of the study period. This included 51 patients in 2019, 50 in 2020 and 66 in 2021. The median age of presentation was 73. An equal number of
males and females presented in 2019. More females presented than males in 2020 (155 and 152 respectively). In 2021, more males presented than females (177 and 150 respectively). The median NIHSS on arrival to ESC was 15 in 2019, 16 in 2020 and 14 in 2021. The median pre-intervention ASPECTS score was 9 in all 3 years examined. Patient demographics are displayed in Table 1.
Most patients managed with EVT had a middle cerebral artery (MCA) occlusion, which accounted for 69% (785/1127) of total occlusions in the study period. Sixty-five percent (243/373) of occlusions were in an MCA distribution in 2019 with 74% (243/330) and 71% (299/424) in the MCA 2020 and 2021. The next most common site of occlusion was the internal carotid artery (ICA), which made up 22% (246/1127) of total occlusions
overall. ICA occlusions accounted for 27% of all occlusions in 2019 (100/373) and 19% in 2020 (65/330) and 2021 (81/424). Seven percent (79/1127) of occlusions were in the posterior circulation, which made up 7% (27/373) in 2019, 5% (17/330) in 2020 and 8% (35/424) in 2021. Anterior cerebral artery (ACA) occlusions comprised 2% (17/1127) of total occlusions (1% (3/373) in 2019, 2% (5/330) in 2020 and 2% (9/424) in 2021. In total, 14% (136/1127) of patients had more than one occlusion. In 2019, 18% (57/373) of patients had over one occlusion; this figure dropped to 7% (21/330) in 2020 and increased again in 2021 to 14% (58/424).
Patient demographics and occlusion site figures were comparable across all three study periods. There was no statistically significant difference in the degree
of the neurological deficit between 2020 and 2021 (mean presenting NIHSS 16 and 14 respectively) and the median pre-intervention ASPECTS score did not vary between either year (9).
Primary outcome
page
The median door-to-groin puncture time increased from 15 min in 2019 to 18 min in 2020 and 2021. For patients transferred to ESC from PSCs, door-to-puncture time was 14 min in 2019 and 15 min in 2020 and 2021. The door-to-puncture time for those presenting directly to ESC was 64 min in 2019 and 65 min in 2020. This figure dropped to 51 min in 2021.
Secondary outcomes
Numerous other time metrics were examined in this study. The median time from symptom onset/ time last seen well to presentation
77 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Back to article
Not logged in - 82.8.44.181 Not affiliated © 2023 Springer Nature Switzerland AG. Part of Springer Nature Outcomes 2019 2020 2021 Symptom onset/last seen well to presentation, median minutes (IQR) ESC 100 (65–362) (n = 30) 165 (68–319) (n = 43) 157 (64–507) (n = 36) Transfers 115 (65–369) (n = 234) 150 (78–380) (n = 215) 154 (***) (n = 240) Overall 115 (65–371) (n = 264) 157 (75–378) (n = 258) 156 (82–461) (n = 276) Door to imaging, median minutes (IQR) ESC 21 (13–25) (n = 30) 20 (15–29) (n = 43) 18 (14–27) (n = 36) Transfers 22 (14–38) (n = 235) 23 (15–34) (n = 216) 25 (30–56) (n = 241) Overall 22 (14–35) (n = 265) 22 (15–34) (n = 259) 24 (15–36) (n = 277) Door to thrombolysis, median minutes (IQR) ESC 44 (23–57) (n = 17) 43 (29–60) (n = 25) 28 (24–37) (n = 16) Transfers 54 (34–84) (n = 105) 50 (32–67) (n = 93) 49 (39–85) (n = 98) Overall 50 (31–81) (n = 122) 48 (31–65) (n = 118) 42 (31–65) (n = 114) Door-to-groin puncture, median minutes (IQR) ESC 64 (51–115) (n = 30) 65 (49–96) (n = 43) 51 (39–85) (n = 37) Transfers 14 (9–28) (n = 265) 15 (9–26) (n = 250) 15 (16–38) (n = 282) Overall 15 (9–32) (n = 295) 18 (10–38) (n = 293) 18 (10–34) (n = 319) Median symptom onset/last seen well to puncture, median minutes (IQR) ESC 192 (119–497) (n = 40) 242 (125–698) (n = 50) 160 (102–555) (n = 44) Transfers 335 (205–558) (n = 264) 376 (244–588) (n = 256) 355 (74–140) (n = 283) Overall 322 (194–552) (n = 305) 349 (216–580) (n = 306) 347 (219–608) (n = 327) Puncture to recanalisation, median minutes (IQR) ESC 26 (16–38) (n = 38) 22 (15–38) (n = 47) 22 (15–44) (n = 43) Transfers 19 (13–28) (n = 250) 19 (12–28) (n = 239) 20 (16:49) (n = 275) Overall 20 (13–29) (n = 288) 19 (13–29) (n = 286) 20 (13–33) (n = 318) Procedure duration, median minutes (IQR) ESC 37 (25–61) (n = 40) 31 (24–52) (n = 50) 37 (18–57) (n = 43) Transfers 31 (18–51) (n = 261) 26 (17–46) (n = 253) 27 (16–36) (n = 281) Overall 32 (18–53) (n = 301) 27 (17–48) (n = 303) 28 (16–50) (n = 324) Symptom onset/last seen well to recanalisation, median minutes (IQR) ESC 218 (141–500) (n = 38) 267 (145–464) (n = 47) 229 (113–603) (n = 43) Transfers 371 (225–573) (n = 249) 409 (267–604) (n = 239) 380 (235–614) (n = 275) Overall 348 (212–567) (n = 287) 375 (246–597) (n = 286) 375 (244–627) (n = 318) TICI 2b/2c/3 recanalisation 91% (257/281) 94% (272/289) 96% (289/302) mRs 90 days post-thrombectomy ≤ 2 = 45% (136/302) ≤ 2 = 45% (105/234) ≤ 2 = 48% (151/315)
Stroke
was seen to increase from 115 min overall in 2019 to 157 min in 2020 and 156 min in 2021. For patients presenting directly to ESC, this figure was 100 min in 2019, increasing to 165 min in 2020 and 157 min in 2021. The median time was higher in those presenting to PSCs at 115 min in 2019 and was recorded to be 150 min in 2020 and 154 min in 2021.
The median time from presentation to commencement of radiological investigation (door-to-imaging time) was 22 min overall in 2019 and 22 min in 2020. This increased to 24 min in 2021. At ESC, the door-to-imaging time reduced from 21 min in 2019 to 20 min and 18 min in 2020 and 2021 respectively. Conversely, the door-to-imaging time increased slightly in patients presenting to other PSCs between the three study periods observed (22 min in 2019, 23 min in 2020 and 25 min in 2021).
Overall, 39% (364/938) of patients received intravenous thrombolysis. Forty-two percent (128/304) received IV thrombolysis in 2019 and 43% (132/307) in 2020. This figure dropped to 32% (105/327) in 2021. The time taken to institute thrombolytic therapy (door-toneedle time) was observed to reduce across the length of the study (50 min overall in 2019, 48 min in 2020 and 42 min in 2021), with shorter door-to-needle times seen in both ESC and other PSCs from year to year (44 min, 43 min and 28 min and 54 min at ESC and 50 min and 49 min at PSCs in 2019, 2020 and 2021 respectively).
The median time from symptom onset/time last seen to groin puncture was observed to increase from 2019 to 2020 and subsequently decrease in 2021. At ESC, the median time rose from 192 min in 2019 to 242 min in 2020 and back to 160 min in 2021. There was less variability in this metric at other PSCs with median time rising from 335 min in 2019 to 376 min in 2020 and 355 min in 2021. Overall timing mirrored trends of individual PSCs at 322 min, 349 min and 347 min in 2019, 2020 and 2021 respectively.
Regarding time metrics directly pertaining to EVT, there was little variability seen for overall procedure time or time from groin puncture to recanalisation. Procedure duration was observed to be 32 min in 2019, 27 min in 2020 and 28 min in 2021 (37 min, 31 min and 37 min in 2019, 2020 and 2021 respectively for those directly presenting to ESC and 31 min, 26 min and 27 min in 2019, 2020 and 2021 for those transferred from other sites). Time from puncture to recanalisation reduced from 20 min in 2019 to 19 min in 2020 and 20 min in 2021
overall (26 min, 22 min and 22 min in those directly presenting to ESC and 19 min, 19 min and 20 min in those transferred from other sites. Regarding the technical success of thrombectomy procedures performed, TICI 2b-3 recanalisation rates were reported in 91% (257/281) of cases in 2019. This figure increased to 94% (272/289) in 2020 and 96% (289/302) in 2021. mRS scores at 90 days post-thrombectomy were used to assess the level of function, with 45% (136/302, 105/234) of patients reported to have a good outcome (mRS ≤ 2) in 2019 and 2020 and 48% (151/315) in 2021 (Table 2).
Discussion
The COVID-19 pandemic presented unprecedented challenges to modern healthcare systems. The time-sensitive nature of EVT for LVO stroke meant that these challenges were exacerbated with issues such as delays in patient presentation to hospital, staff, ambulance and bed shortages and new infection control protocols all having the potential to hinder service provision leading to delayed reperfusion and worse patient outcomes.23
International studies from several countries including China, France, Germany and Spain have shown reductions in the number of EVT cases during the COVID-19 pandemic.23,24,25,26 Reduced case numbers were experienced despite the known association between COVID-19 and ischaemic stroke with LVO, presumably related to delayed hospital presentations and delays in diagnostic and clinical assessment. For example, large multicentre studies performed by Zhoa et al.23 in China and Kerleroux et al.26 in France demonstrated 25.3% and 21% reductions in total thrombectomy cases performed during the COVID-19 pandemic, compared to equal time intervals before it.
The onset of the pandemic did not lead to a reduction in the number of EVTs performed at our institution with stasis in numbers between 2019 and 2020. In the years preceding the pandemic, there had however been a trend for year-on-year increases in EVTs, and the lack of such an increase in 2020 is therefore consistent with a “real world” reduction in numbers. This suggests that our initial hypothesis, that the COVID-19 pandemic would lead to a reduction in the number of EVTs performed, was correct. However, the relative maintenance of EVT numbers in the year after the pandemic onset, as well as the bounce in EVT numbers by 7% in the pandemic’s second year, is a testament to both the robustness
of service provision at our ESC and at the referring hospitals. The increase in the number of EVTs performed in 2021 is all the more impressive given that it coincided with the commencement of a 24/7 thrombectomy service in Cork University Hospital, a factor that would have been expected to lead to reduced referrals to our service. Contrary to our hypothesis that the pandemic would lead to delays in patient treatment, our findings demonstrated no major change to most intrahospital workflow time metrics pertaining to EVT. Median time from door to groin puncture, which constituted the primary outcome measure of this study, only slightly increased from 15 to 18 min between 2019 and 2020. Results are not dissimilar to previously published literature from other countries, including single- and multi-centre studies in Spain and Germany, which actually demonstrated small reductions in door-to-groin puncture times post-COVID-19.24, 25 In fact, for patients that presented directly to our ESC, this metric was observed to reduce by 14 min from 2020 to 2021 from 65 to 51 min. These findings most likely reflect increased efficiency associated with improving familiarisation with COVID-19 protocols.
With regards to the secondary outcome measures of this study, significant increases were observed in the median time from symptom onset/time last seen well to presentation and intervention. These delays in patient presentation are most likely explained by patient reluctance to attend hospital during the pandemic and are most pronounced in the group presenting directly to ESC, with median time to presentation from symptom onset increasing by over an hour between 2019 and 2020 (100 to 165 min). Interestingly, our experience of large delays between symptom onset in patients outside of hospital and presentation appears to be unmatched when compared to several European26 studies. Rudilosso et al.24 in Spain found ‘no difference in prehospital time metrics’ when comparing time from symptom onset to arrival at hospital in patients prior to and during the pandemic. Kerleroux et al.26 in France reported similar findings, with no significant differences in time between symptom onset and imaging in patients with LVO between 2019 and 2020. Interestingly, significant time delays remain in 2021, despite continued relaxation of COVID-19 restrictions, with the median time from symptom onset to presentation being 57 min greater than in 2019 (157 min and 100 min).
The other time metrics which we examined in this study, such as median door-to-imaging time and median door-to-needle time were largely comparable across all study periods, with figures serving to emphasise the consistent and reliably prompt service provision in our ESC, despite the ongoing barriers against this from the COVID-19 pandemic. Finally, this study also evaluated the impact of COVID-19 on procedural and patient functional outcomes following EVT. There was an excellent technical success rate of EVT across the study, with TICI2b-3 reperfusion rates in excess of 90% in all three time periods, improving from 91% in 2019 to 96% in 2021. Reassuringly, despite the marked delays in time from symptom onset to both presentation and institution of endovascular treatment from 2019 to 2020 and 2021, the proportion of treated patients who achieved a good functional outcome remained stable, with an mRS ≤ 2 seen in almost half of the patients managed with EVT in 2019, 2020 and 2021.
Although this is a retrospective study, all data was extracted from a prospectively maintained EVT database and the near complete data capture on almost 1000 patients is a huge strength. Data was only collected and assessed for patients referred to our service and therefore patients presenting to peripheral hospitals with strokes that were not referred to our service were not captured in this study. This means that the true impact of the pandemic on overall stroke numbers in Ireland has not been determined and further investigation is suggested.
Conclusion
This study is the first paper evaluating the effect of the COVID-19 pandemic on EVT service provision in Ireland. Despite the myriad of challenges presented by the pandemic, service provision at the primary Irish ESC, and the referring hospitals, has proven to be robust. Procedural time metrics were maintained whilst the expected reduction in the number of EVTs performed did not materialise, there actually being a significant increase in the number of EVTs performed in the pandemic’s second year. Delays in patient presentation to hospital were identified; however, EVT success rates and patient outcomes did not appear significantly impacted. References available on request
78 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
TREAT EARLY.1 DELAY DISEASE PROGRESSION.1 EXTEND LIFE.1
Use ERLEADA® earlier and keep other
treatment options for later stages1
ERLEADA® (apalutamide) is indicated for the treatment of adult men with:2
• non-metastatic castration-resistant prostate cancer (nmCRPC) who are at high risk* of developing metastatic disease
• metastatic hormone-sensitive prostate cancer (mHSPC) in combination with ADT
Visit the Janssen Medical Cloud to learn more about ERLEADA®
ERLEADA®▼ 60 mg film-coated tablets PRESCRIBING INFORMATION. ACTIVE
INGREDIENT(S): Apalutamide. Please refer to Summary of Product Characteristics (SmPC) before prescribing. INDICATION(S): Treatment of adult men: with non metastatic castration resistant prostate cancer (nm CRPC), at high risk of developing metastatic disease; with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (ADT). DOSAGE & ADMINISTRATION: Adults: 240 mg (four 60 mg tablets) single daily dose, swallowed whole with or without food. Continue medical castration with gonadotropin releasing hormone analogue (GnRHa) throughout in patients not surgically castrated. Elderly: No dose adjustment. Renal impairment: Mild to moderate - no dose adjustment. Severe - not studied; caution advised. If treating, monitor patients for side effects, reduce dose if required. Hepatic impairment: Baseline mild or moderate (Child Pugh Class A and B) - no dose adjustment. Severe - not recommended.
CONTRAINDICATIONS: Hypersensitivity to active substance or any excipient. Women who are or may become pregnant. SPECIAL WARNINGS & PRECAUTIONS: Seizure: History of seizures or other predisposing factors - not recommended. Discontinue permanently if seizure develops during treatment. Risk of seizure may be increased in patients receiving concomitant medicinal products that lower the seizure threshold. Ischaemic heart disease and ischaemic cerebrovascular disorders occurred, including events leading to death; monitor for signs and symptoms of ischaemic heart disease AND ischaemic cerebrovascular disorders, optimise management of risk factors: hypertension, diabetes, dyslipidaemia. Falls and fractures: Reported; evaluate patients for risk before starting Erleada; continue monitoring and manage fractures, consider use of bone-targeted agents. Concomitant use with other medicinal products: Potent enzyme inducer; may lead to loss of efficacy of concomitant medication. Review concomitant medication and refer to SmPC for further guidance. Avoid co-administration with warfarin and coumarin-like anticoagulants. If coadministered with an anticoagulant metabolised by CYP2C9 (e.g. warfarin or acenocoumarol), conduct additional International Normalised Ratio (INR) monitoring. Recent cardiovascular disease: Monitor patients with clinically significant cardiovascular disease for risk factors (e.g. hypercholesterolaemia, hypertriglyceridaemia, other cardio-metabolic disorders) as safety in patients with clinically significant cardiovascular disease in the past 6 months has not been established. Androgen deprivation therapy may prolong QT interval: Assess benefit-risk including potential for Torsade de pointes prior to initiating Erleada in patients with a history or risk factors for QT prolongation and those receiving concomitant medicinal products that might prolong QT interval. Severe Cutaneous Adverse Reactions (SCARs): Including drug reaction with eosinophilia and systemic symptoms (DRESS) and Stevens Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Advise patients of signs
and symptoms suggestive of DRESS or SJS/TEN If these symptoms are observed, withdraw Erleada immediately and patients to seek immediate medical consultation. Do not restart Erleada in patients who have experienced DRESS or SJS/TEN while taking Erleada at any time and consider an alternative treatment. Effects on ability to drive and use machines: Seizures reported; potential risk for driving or operating machines. SIDE EFFECTS: Refer to SmPC for other side effects. Very common: Hot flush, hypertension, diarrhoea, skin rash, fracture, arthralgia, fatigue, weight decreased, fall, decreased appetite. Common: Hypothyroidism, hypercholesterolaemia, hypertriglyceridaemia, dysgeusia, ischaemic heart disease, ischaemic cerebrovascular disorders, pruritus, alopecia, muscle spasm. Other side effects: Seizure, QT prolongation, DRESS, SJS/TEN. LEGAL CATEGORY: Prescription only medicines. PRESENTATIONS, PACK SIZES, MARKETING AUTHORISATION NUMBER(S): Blister pack, 112, EU/1/18/1342/001. MARKETING AUTHORISATION HOLDER: JANSSENCILAG INTERNATIONAL NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. FURTHER INFORMATION IS AVAILABLE FROM: Janssen Sciences Ireland UC, Barnahely, Ringaskiddy, IRL – Co. Cork P43 FA46. Prescribing information last revised: December 2022.
Adverse events should be reported. ▼ This medicinal product is subject to additional monitoring and it is therefore important to report any suspected adverse events related to this medicinal product. Healthcare professionals are asked to report any suspected adverse events via: HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse events should also be reported to, Janssen Sciences Ireland UC on 1 800 709 122 or at dsafety@its.jnj.com.
*High risk is defined as patients with a PSA doubling time of ≤10 months.1 ADT = androgen deprivation therapy
References: 1. Smith MR, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021;79(1):150–158. 2. ERLEADA® (apalutamide). Summary of Product Characteristics. Available from: www.medicines.ie
Date of preparation: January 2023 | CP-361339

Clinical R&D
ERLEADA®(APALUTAMIDE) APPROVED
FOR REIMBURSEMENT IN IRELAND FOR THE TREATMENT OF PATIENTS WITH METASTATIC HORMONE-SENSITIVE PROSTATE CANCER
(MHSPC)
Janssen, the Pharmaceutical Companies of Johnson & Johnson, announced that Erleada®(apalutamide) has been granted reimbursement in Ireland for the treatment of adult men with metastatic hormonesensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (ADT).
Professor Paul Donnellan, Consultant Medical Oncologist, Galway University Hospital, Mayo University Hospital and Galway Clinic said: “For patients with newly-diagnosed metastatic prostate cancer, delaying diseaseprogression and improving survival are paramount. For many years, suppressing androgen production has been the mainstay of treatment. It has been proven that adding an oral agent to this treatment, significantly improves both time to progression and overall survival, without any negative impact on Quality of Life (QoL). This represents a significant advance for all patients with advanced prostate cancer.”
Dr Thorsten Giesecke, General Manager, Commercial Business, Janssen Sciences Ireland UC, said: “Janssen is committed to improving prognosis and outcomes for men across the prostate cancer continuum with a growing portfolio and pipeline of therapeutics spanning all modalities and stages of the disease. Today’s reimbursement of apalutamide for the treatment of mHSPC represents an important, positive step on that journey.”
Data from the Phase III TITAN study assessed the addition of apalutamide to ADT in a broad range of patients with mHSPC, regardless of disease volume, prior treatment with docetaxel or staging at initial diagnosis.1 The dual primary endpoints of the study were overall survival (OS) and radiographic progressionfree survival (rPFS).1,2 Data from the interim analysis2 showed that at 24 months, apalutamide plus ADT significantly improved OS compared to placebo plus ADT with a 33 percent reduction in the risk of death (HR=0.67; 95% CI, 0.51-0.89; p=0.005).2 The interim analysis also revealed that apalutamide plus ADT also significantly improved rPFS compared to placebo plus ADT
with a 52 percent reduction in risk of radiographic progression or death compared to placebo plus ADT (HR=0.48; 95% CI, 0.39-0.60; p<0.001).2
The final analysis of TITAN and its long-term results demonstrate that apalutamide plus ADT consistently provides significant improvements in OS and delays onset of progression.1 The OS rates at 48 months were 65.1% and 51.8% for apalutamide- and placebo-treated patients, respectively.1
The safety profile observed in the TITAN study for apalutamide plus ADT was consistent with that described in previous studies.2,3 In the final analysis, the overall incidence of any treatmentemergent adverse events (TEAEs) was similar between treatment groups and to those reported previously.1 The most common treatment-related AEs (adverse events) related to treatment with apalutamide were rash and fatigue.1 There were no treatmentrelated deaths.1
HSE NATIONAL AMBULANCE SERVICE (NAS) EXPANDS SERVICE
A HSE National Ambulance Service (NAS) led service called Pathfinder, designed to safely keep older people who phone 112/999 in their own home rather than taking them to a hospital
emergency department, has went live in Letterkenny.
Pathfinder improves outcomes for older people by providing safe alternative care at home rather than in hospital and will be provided by National Ambulance Service staff working with colleagues from Letterkenny University Hospital.

Pathfinder has been working as a collaborative service between NAS and Dublin’s Beaumont Hospital Occupational Therapy & Physiotherapy Departments for more than two years and also went live in Limerick, Tallaght and Waterford in October 2022 and last month in Kilkenny and Cork.
Robert Morton, Director of the HSE National Ambulance Service said the expansion of the NAS Pathfinder Model is part of a plan to offer different groups of patients an alternative pathway other than presenting to a busy emergency department.
“The National Ambulance Service is very pleased with the outcomes for individual patients being achieved by this service and we are delighted to be working with Letterkenny University Hospital which has agreed to support the expansion of the Pathfinder model to this part of the country. The NAS Pathfinder model is improving outcomes for older people by
offering safe alternative care pathways for many older people in their own homes who might otherwise have to be conveyed to a busy Emergency Department. Pathfinder is enabling NAS to play an increasing role as an integrator of health service delivery by also connecting 999 patients with the increasing range of community services being developed by the HSE to support the needs of older people.”
Welcoming the introduction of the service to Letterkenny, Sean Murphy, Hospital Manager, Letterkenny University Hospital added: “We are pleased to be able to offer this service to suitable older people in our local communities.
“Through the Pathfinder Model, many older patients can be safely and appropriately managed in their own home rather than being transported to the ED for assessment when they dial 112/999.
“Pathfinder aims to reduce congestion in busy EDs and makes for a better environment for patients and staff on the floor whilst improving overall flow through the ED. The service enables increased ED capacity to care for other patients, by supporting this cohort of complex, frail patients at home,” he added.
JJ McGowan, GM Operations West with NAS said: “Many patients who present to the emergency department have non-urgent care needs that could be treated elsewhere. Overall, Pathfinder has shown that it is a
80 MAY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Gary Gardiner Advanced Paramedic, Eimear McGlynn Senior Occupational Therapist, Aoife O’Donnell Senior Physiotherapist, Shane Connelly, Advanced Paramedic, Rosaleen Greene Clinical Specialist Occupational Therapist, Martin Conlon Advanced Paramedic
safe and acceptable service for older people who dial 999/112 with low acuity complaints.”
The Pathfinder ‘Rapid Response Team’ respond to 999/112 calls for older people (65 years and older) in their homes. The older person is assessed by both an Advanced Paramedic and Occupational Therapist/Physiotherapist. Where safe, the team supports the older person at home rather than transporting them to emergency department, by linking with a wide range of alternative hospital and community services. Pathfinder also operates a ‘Follow-Up Team’ (Physiotherapy & Occupational Therapy) which provides immediate home-based rehabilitation, equipment provision and casemanagement in the subsequent days following a 999/112 call. On average two thirds of patients seen by Pathfinder following a 999 call have remained at home rather than being brought to the Emergency Department.
CAMPAIGN LAUNCHED FOR VITAL REHABILITATION SERVICES FOR BRAIN INJURY SURVIVORS
A new campaign has been launched to improve rehabilitation services for brain injury survivors in Ireland at a World Congress being hosted in Dublin.

Speaking at the 14th World Congress on Brain Injury organised by the International Brain Injury Association (IBIA) in partnership with Acquired Brain Injury Ireland (ABII), ABII CEO and Co-Founder Barbara O’Connell called for an investment of ¤6 million per annum to meet urgent need.
“19,000 people in Ireland acquire a brain injury each year, and many of these end up unnecessarily in nursing homes long-term. This must end. The slogan of our campaign is ‘Don’t Save Me, Then Leave Me –give me a pathway home’.
“The truth is, in Ireland there is no clear pathway for survivors of brain injury. Once they’re discharged from hospital, their future is in the hands of a geographic lottery – the system is under resourced and under-funded. Assessment Teams and specialist brain injury Case Managers can play a key role in addressing these issues at minimal cost to the State.
“Today at the World Congress on brain injury, ABII is calling for ¤2m to establish a nationwide brain injury Case Management service and ¤4m for three National Assessment Teams for young
Launching a new campaign ‘Don’t Save Me, Then Leave Me – give me a pathway home’ for improved rehabilitation services for brain injury in Ireland was brain injury survivors Niamh Cahill, Des Vallely and Lorraine Duffy together with Acquired Brain Injury Ireland CEO, Barbara O’Connell. The new campaign was launched at the recent World Congress on Brain Injury which took place in Ireland. www.abiireland.ie
survivors living inappropriately in nursing homes.
“This would enable survivors to move much more effectively from acute hospital care, through specialist in-patient rehabilitation if needed, and on to communitybased services. Doing so also avoids delayed discharges from acute hospitals, frees up beds in our National Rehabilitation Hospital, reduces the burden of care on families and creates significant cost savings to the State.
“Too many brain injury survivors are left merely to exist, inappropriately placed in hospitals and nursing homes where they do not belong. We have solutions to help survivors rebuild their lives in their own communities – and we need urgent investment to make it happen.
“It is unfathomable that our Government would not take notice as the World Congress takes place in Ireland this week. Following this Congress, ABII will work closely with the Government and the HSE in order to make this happen,” Ms O’Connell said.
Opening the Congress via video link the Minister of State for Disabilities Anne Rabbitte said: “It’s very significant for our
country to be hosting the world’s leading experts in brain injury research and rehabilitation for the next three days. In Ireland, we recognise the vital importance of investing in brain health and brain injury treatment at all stages of the neuro rehabilitation pathway, from acute hospital to home. Timely, individualised rehabilitation has the potential to increase independence, maximise ability and enable survivors to participate as active citizens in our communities.
“That’s why Government has committed to addressing the needs of those impacted by brain injury, including through our National Neuro Rehabilitation Strategy. We already have many of the components of an effective brain injury rehabilitation pathway in place to make Ireland a global exemplar in this field. This is a priority for Government and I look forward to working with those involved in the Congress and the sector over the coming months to implement our plans and realise their shared ambition.”
The Congress brings together brain injury professionals from across the world and many different disciplines. Leading Irish rehabilitation physicians,
neurosurgeons, advocates and multidisciplinary practitioners are among the keynotes, panellists and speakers at the Convention Centre in Dublin from Thursday until Saturday.
GLOBAL PATIENT SAFETY AND WELLBEING RESEARCH INITIATIVE INVITES GRANT APPLICATIONS
The MPS Foundation - a global not-for-profit research initiative that aims to shape the future of patient safety – has launched its second grant programme.
Medical Protection Society (MPS) launched The MPS Foundation last year and its first research grant programme received over 150 expressions of interest. 10 applicants were successful in securing funding for their research. This includes a research project in Ireland which will assess the impact of a tailored educational intervention to improve clinical communication involving adults with age-related hearing loss.
The Foundation is now inviting new research grant applications from both MPS members and nonmembers. It will invest in research and analysis with a key focus on patient safety and the wellbeing of healthcare professionals and teams, both medical and dental, and applications will again be considered from the jurisdictions where MPS has members. This includes Ireland, Hong Kong, Australia, the UK, New Zealand, South Africa, Singapore, Malaysia, the Caribbean and Bermuda.
81 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Clinical R&D
Research projects supported by The MPS Foundation need to be academically robust and evidence-based. Available funding will range from EUR 5,694 to EUR 227,767 - the equivalent to £5,000 to £200,000 (subject to currency fluctuations) - depending on the scale, focus and duration of the proposal.
The funding focusses on five main areas:
the impact of human factors on patient safety, outcomes and risk
the impact of processes and delivery models on patient safety, outcomes and risk
the personal and professional wellbeing and development of clinicians
the impact of digital integration and technology on patient safety, outcomes and risk.
the evaluation of the effectiveness of teaching and learning innovations and the impact upon patient care, safety, outcomes and risk.
Dr Graham Stokes, MPS Foundation Chair, said: “Patient safety and the wellbeing of healthcare professionals and teams are more vital today than ever. Until now, funding for research into both has been limited, but this is all set to change with access to research funding through The MPS Foundation. As a global not-forprofit research initiative, we aim to take non-clinical research in a new direction and transform the future of patient safety.
“Our aim is simple: to create sustainable global change through ambitious research that can be used to develop a knowledge base that improves patient safety, enhances the wellbeing of healthcare teams and reduces risks.
“I am delighted with the progress The MPS Foundation has made and to launch the second grant programme. Our first grant programme saw expressions of interest from all MPS jurisdictions with 10 of those research projects awarded funding. These projects are exciting, diverse and carry great potential.
“All research projects must be academically robust and evidence based. While funding will be available up to £200,000, smaller grants from £5,000 will also be available and I would strongly encourage healthcare professionals to consider applying for support whatever the scale of their proposal.”
Healthcare professionals can register their interest by visiting www.thempsfoundation.org and
entering their details via the online grant portal. Calls for expressions of interest close on 5 May 2023.
The Foundation is part of MPS – the world’s leading protection organisation – which currently supports more than 300,000 doctors, dentists and healthcare professionals, and has almost 130 years of global healthcare experience and expertise.
TWO FITUSIRAN PHASE 3 STUDIES PUBLISHED IN THE LANCET AND THE LANCET HAEMATOLOGY HIGHLIGHT POTENTIAL TO ADDRESS UNMET NEEDS ACROSS ALL TYPES OF HEMOPHILIA
Two studies, published in The Lancet and The Lancet Haematology, evaluating the efficacy and safety of fitusiran, an investigational siRNA therapy for the prophylactic treatment of adults and adolescents with hemophilia A or B, reinforce the potential of this investigational therapy to transform the current standard of care and address unmet needs for all types of hemophilia, regardless of inhibitor status.
Hemophilia A and B are rare congenital lifelong bleeding disorders in which the ability of a person’s blood to clot is impaired, leading to excessive bleeds and spontaneous bleeds into joints that can result in joint damage and chronic pain, and significantly impact quality of life. Fitusiran has the potential to provide prophylaxis for all types of hemophilia, regardless of inhibitor status, with as few as six subcutaneous injections per year.
Dietmar Berger, M.D., Ph.D. Head of Global R&D ad interim and Chief Medical Officer at Sanofi said, “Sanofi is committed to advancing the standard of care for all people with hemophilia through innovative science, providing consistent bleed protection while reducing treatment burden. We are entering a new era in hemophilia where, for the first time, people can choose therapies that meet their personal needs. These published data validate our science and add to a growing body of evidence supporting fitusiran’s potential to transform the treatment landscape. We look forward to sharing additional data on fitusiran later this year.”
Both Phase 3 studies compared once-monthly subcutaneous fitusiran prophylaxis (80mg) with on-demand/episodic use of clotting factor concentrates in the ATLAS-A/B study, and on-demand/ episodic use of bypassing agents in the ATLAS-INH study. Across both clinical studies, prophylactic treatment with fitusiran reduced annualized bleeding rates by
90% (95% CI [84.1%; 93.6%], P <0.0001) compared to the control arms, showing a statistically significant and clinically meaningful improvement in bleeding episodes when compared to on-demand treatments; and showed improvement in quality of life.
In the study ATLAS-INH study published by The Lancet, 66% of participants with inhibitors (25 out of 38) receiving fitusiran 80mg monthly experienced zero bleeding episodes compared to 5% (1 out of 19) receiving an on-demand bypassing agent after nine months of treatment.
The ATLAS A/B study published in The Lancet Haematology showed 51% of participants without inhibitors (40 out of 79) who received fitusiran 80mg monthly prophylaxis experienced zero bleeds compared to 5% (2 out of 40) in the comparator group, receiving on-demand clotting factor concentrates.
Sanofi is currently investigating the efficacy and safety of fitusiran under a revised regimen which includes lower doses and less frequent dosing (as few as six subcutaneous injections per year), maintaining an antithrombin target range of 15-35% in all ongoing studies.
TIZIANA LIFE SCIENCES INITIATES PROGRAM TO DEVELOP INTRANASAL FORALUMAB IN TYPE 1 DIABETES
Tiziana Life Sciences Ltd. (Nasdaq: TLSA) (“Tiziana” or the “Company”), a biotechnology company developing breakthrough immunomodulation therapies via novel routes of drug delivery, has announced that it is initiating a program to develop intranasal foralumab for the treatment or prevention of Type 1 Diabetes.
“With the FDA approval of the humanized anti-CD3 mAb TZIELD™ (teplizumab-mzwv injection) and then its subsequent announced acquisition of Provention Bio by Sanofi for $2.9 billion1, CD3 has emerged as an established and attractive target for Type 1 Diabetes,” commented Gabriele Cerrone, Executive Chairman, Founder and interim Chief Executive Officer of Tiziana. “We believed that having a fully human CD3 antibody may offer benefits to patients across many different chronic disease indications.”
“The potential of not requiring an injection or infusion and having a take-home self-administered nasally delivered therapy would offer additional benefit and convenience to sufferers of Type 1 Diabetes”, stated Matthew W.
Davis, M.D., RPh, Chief Medical Officer of Tiziana. “Although given intranasal, our fully human antiCD3 mAb foralumab has shown to release anti-inflammatory T regulatory cells throughout the body while returning proinflammatory effector T cells to their naïve state.2 We are actively discussing various study designs with our Key Opinion Leaders as well as with an internationally known network of dedicated diabetes physicians. I believe that the data published in PNAS2 shows that the novel immunomodulatory effects of our intranasal fully human anti-CD3 mAb foralumab may have an important role in the diabetes market.”
“MENTAL HEALTH CRISIS
AMONG OLDER PEOPLE NOT BEING TALKED ABOUT”:
ALONE, the charity that supports older people, has called for additional focus on mental health difficulties being experienced by older people as the number of interventions to support mental health carried out by ALONE has increased by almost 300% in the past year.
Speaking at the Joint Oireachtas Sub-Committee on Mental Health, ALONE CEO Seán Moynihan said that in Q4 2022, 29% of the 1,926 older people ALONE assessed for their services identified they had issues relating to their mental health, but over half of these had not attended a GP for support. ALONE also cited research from the Irish Longitudinal Study on Ageing (TILDA) which found that 78% of older adults who have evidence of depression, and 85% who have evidence of anxiety, do not have a doctor’s diagnosis.1
“We do not give older age due consideration as a time where mental health difficulties may emerge for the first time. We do not discuss how getting older is associated with age-specific psychosocial risk factors for mental health difficulties, such as living alone, bereavement, physical illness, disability, and cognitive decline,” he said.
“While many older people enjoy positive mental health, there is evidence to suggest that there is a significant mental health crisis among older people which is not being talked about. This has become all the more evident in the aftermath of COVID-19 restrictions. Increasingly, we are working with older people who have completely cut themselves off from their family, friends, community and life in general due to fears around COVID-19 which have not subsided,” Moynihan continued.
82 MAY 2023 • HPN |
HOSPITALPROFESSIONALNEWS.IE
The organisation has said that specific mental health policy, evidence-based programmes and research for older people must be committed to, funded, and implemented as part of Sharing the Vision, Ireland’s national mental health policy. ALONE has also said that the action plan to combat loneliness and social isolation must be completed, funded and committed to, including funding for Irish research, and older people must be provided with additional supports to reengage with their communities, in collaboration with experts and the community and voluntary sector.
Moynihan continued, “A Dáil debate took place in April two years ago on COVID-19, mental health, and older people. Many positive ideas were discussed, and not progressed. We believe that significant action on mental health difficulties being experienced by older people is urgently overdue.”
“As a country we put significant effort into telling older people to cocoon and stay inside, and offering them support to do so. We have not done the same to support older people to reengage with their communities. We need to identify and implement precision, research-backed interventions for the loneliness and social isolation which is impacting this group, and all other groups affected by loneliness.”
Moynihan concluded, “We have quoted repeatedly the research which shows that loneliness has been shown to have as severe an impact on our health as smoking.2 Why has it not received the same public health response?”
If you, or an older person you know, requires ALONE’s support please call our National Support and Referral Line at 0818 222 024 from 8am to 8pm.
If you are interested in supporting older people by becoming a volunteer, then you can visit alone. ie/volunteer for more information.
1 https://tilda.tcd.ie/publications/ reports/pdf/w1-key-findings-report/ Chapter6.pdf
2 https://journals.sagepub.com/ doi/10.1177/1745691614568352
INTHELIA THERAPEUTICS NAMED AS AWARDEE OF BLUE KNIGHT™ QUICKFIRE CHALLENGE
RCSI spin-out company Inthelia Therapeutics has been named as an awardee of the international BLUE KNIGHT™ QuickFire Challenge: Next-Generation Preparedness Solutions related

to the company’s work on nonantibiotic therapies for sepsis.
Blue Knight is a joint initiative between Johnson & Johnson Innovation LLC and the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response at the U.S. Department of Health and Human Services.
The challenge invited innovators to submit potentially groundbreaking ideas or technologies that aim to enhance preparedness towards future known and unknown disease threats.
Inthelia Therapeutics was recognised for its innovative approach aiming to treat infections by targeting a conserved known host target to which multiple pathogens bind rather than targeting multiple, often evolving, or unknown pathogens. This approach has the potential to uniquely create rapid responses to breakthrough pathogens to better manage or control future pandemics. The award recognises the potential to develop a biomarker-led treatment for early sepsis, a leading cause of hospital deaths. Inthelia’s innovation may also contribute to combating the growing threat of antibiotic resistance.
Inthelia Therapeutics is a spin-out company from RCSI University of Medicine and Health Sciences founded by Professor Steve Kerrigan, RCSI School of Pharmacy and Biomolecular Sciences, and serial entrepreneur, Dr Ivan Coulter.
Professor Steve Kerrigan commented: “Sepsis is a complication caused by the body’s overwhelming and life-threatening response to an infection, which can lead to tissue damage, organ failure, and death. Slow diagnosis and over-dependence on antibiotics1 have resulted in sepsis becoming one of the top three causes of adult hospitalisations and is responsible for half of all hospital deaths globally.”2
“Antibiotics fail in many patients with sepsis, increasing risk of death and antimicrobial resistance. With low success rates, we cannot depend on new antibiotics to solve the growing threats of sepsis and antimicrobial resistance. There is an urgent and significant need for more rapid diagnosis and treatment with novel non-antibiotics or patient targeted therapy in emergency departments.”
“Inthelia Therapeutics is advancing an innovative biomarker-guided, host-targeted therapy towards late-stage clinical trials in early sepsis which has as a goal to have immeasurable impact on human lives especially with the rapid advancement of anti-microbial resistance. We are honoured to be recognised as a Blue Knight QuickFire Challenge awardee for our work to address this significant threat to human health.”
Professor Fergal O’Brien, Deputy Vice Chancellor for Research and
Innovation at RCSI commented: “Congratulations to Professor Steve Kerrigan and the team at Inthelia Therapeutics on their success at the prestigious Blue Knight QuickFire Challenge. This recognition of the work of an RCSI spin-out company on the international stage we feel is a testament to the world-class research at the RCSI School of Pharmacy and Biomolecular Sciences which is being advanced by Inthelia Therapeutics, and will potentially benefit the 49 million people who are impacted by sepsis annually.”
The QuickFire challenge awardees were announced at the 2022 BARDA Industry Day with other awardees being Immunyx, NanoFreeze, and Vaxess Technologies. To help advance their science, the awardees will receive access to the global Johnson & Johnson Innovation – JLABS network through Blue Knight residency with mentorship from experts across the Johnson & Johnson Family of Companies and BARDA. The QuickFire Challenges are managed by Johnson & Johnson Innovation, with the goal to help address today’s greatest healthcare challenges.
RCSI partners with investors and entrepreneurs to support spinout company creation and drive improvements in human health.
83 HOSPITALPROFESSIONALNEWS.IE | HPN • MAY 2023
Co-founders of RCSI spin-out company Inthelia Therapeutics Professor Steve Kerrigan, RCSI School of Pharmacy and Biomolecular Sciences and Dr Ivan Coulter, CEO, Inthelia
INDIC A T E D FOR























Legal Category: S1A. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com















XELJANZ is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent.
XELJANZ in combination with methotrexate (MTX) is indicated for the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs.
XELJANZ in combination with MTX is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an








































inadequate response or who have been intolerant to a prior diseasemodifying antirheumatic drug (DMARD) therapy.

XELJANZ is indicated for the treatment of adult patients with active ankylosing spondylitis (AS) who have responded inadequately to conventional therapy.
XELJANZ is indicated for the treatment of active polyarticular juvenile idiopathic arthritis (rheumatoid factor positive [RF+] or negative [RF-] polyarthritis and extended oligoarthritis), and juvenile psoriatic arthritis (PsA) in patients 2 years of age and older, who have responded inadequately to previous therapy with DMARDs.

PP-XEL-IRL-0822 | Date of preparation: October 2022
| UC | PsA | JIA |
RA
AS























 Conor Sadlier – Sales Manager Fresenius Kabi
Conor Sadlier – Sales Manager Fresenius Kabi














 Written by Fleming A1, Horgan S2, Quigley L3, McCarthy D.4
Written by Fleming A1, Horgan S2, Quigley L3, McCarthy D.4