Legal Category: Product subject to prescription which may be renewed (B). Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Further product information available on request from:
AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00.
FORXIGA is a trademark of the AstraZeneca group of companies.
IN THIS ISSUE:
NEWS: Consultants vote to reject Contract
Page 7
CONFERENCE: Irish Society for Clinical Nutrition & Metabolism
Page 9
MEDICINES: Degrading the development and delivery of new medicines
Page 15
FEATURE: Parkinson’s Disease
Page 17
CPD: Breast Conserving Therapy
Page 31
CARDIOLOGY
FOCUS: Statin induced LDL cholesterol reduction
Page 41
CARDIOLOGY
FOCUS: Unmet needs in cardiovascular disease
Page 47
HPN April 2023 Issue 107 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
Veeva ID: IE-4697 Date of Prep: February 2023
Lynparza is indicated as monotherapy for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) and BRCA1/2-mutations (germline and/or somatic) who have progressed following prior therapy that included a new hormonal agent.1

In the PROfound trial, there was a statistically significant improvement in BICR assessed rPFS and a nominally statistically significant improvement in the final OS analysis for olaparib vs the investigators choice of NHA arm in BRCA1/2m (germline and/or somatic) patients.1,2*
*Gene level analysis was exploratory.2 The primary endpoint of the PROfound trial was rPFS in Cohort A (patients with BRCA 1/2 or ATM mutations).1
Please see SPC for further information.
This information is intended for healthcare professionals only.
ATM, Ataxia Telangiectasia Mutated gene; BRCA, breast cancer gene; BICR, blinded independent central review; mCRPC, metastatic castration-resistant prostate cancer; NHA, new hormonal agent; OS, overall survival; rPFS, radiological progression free survival; SPC, summary of product characteristics.
References
1. Lynparza (olaparib) 100mg and 150mg film-coated tablets. Summary of product characteristics. https://www.medicines.ie/medicines/lynparza-100-mg-and-150-mg-film-coated-tablets-35034/spc
2. de Bono J et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102.
150mg lm coated tablets
Abridged Prescribing Information
LYNPARZA® (olaparib) 150mg & 100mg FILM-COATED TABLETS
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Ovarian Cancer: As monotherapy for the maintenance treatment of adult patients with advanced (FIGO stages III and IV) BRCA1/2-mutated (germline and/or somatic) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy. As monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. Lynparza in combination with bevacizumab: As maintenance treatment of adult patients with advanced (FIGO stages III and IV) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy in combination with bevacizumab and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either a BRCA1/2 mutation and/or genomic instability. Breast Cancer: As monotherapy or in combination with endocrine therapy for the adjuvant treatment of adult patients with germline BRCA1/2-mutations who have HER2-negative, high risk early breast cancer previously treated with neoadjuvant or adjuvant chemotherapy. As monotherapy for the treatment of adult patients with germline BRCA1/2-mutations, who have HER2 negative locally advanced or metastatic breast cancer. Patients should have previously been treated with an anthracycline and a taxane in the (neo)adjuvant or metastatic setting unless patients were not suitable for these treatments. Patients with hormone receptor (HR)-positive breast cancer should also have progressed on or after prior endocrine therapy, or be considered unsuitable for endocrine therapy. Adenocarcinoma of the pancreas: As monotherapy for the maintenance treatment of adult patients with germline BRCA1/2-mutations who have metastatic adenocarcinoma of the pancreas and have not progressed after a minimum of 16 weeks of platinum treatment within a first-line chemotherapy regimen. Prostate Cancer: As monotherapy for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) and BRCA1/2-mutations (germline and/or somatic) who have progressed following prior therapy that included a new hormonal agent. In combination with abiraterone and prednisone or prednisolone for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated.
Presentation: 150mg and 100mg olaparib film-coated tablets.
Dosage and Administration: Treatment should be initiated and supervised by a physician experienced in the use of anticancer therapies. Patient selection: First-line maintenance treatment of BRCA-mutated advanced ovarian cancer: Before Lynparza treatment is initiated for first-line maintenance treatment of high-grade epithelial ovarian cancer (EOC), fallopian tube cancer (FTC) or primary peritoneal cancer (PPC), patients must have confirmation of deleterious or suspected deleterious germline and/or somatic mutations in the breast cancer susceptibility genes (BRCA) 1 or 2 using a validated test. Maintenance treatment of platinum-sensitive relapsed ovarian cancer: There is no requirement for BRCA1/2 testing prior to using Lynparza for the monotherapy maintenance treatment of relapsed EOC, FTC or PPC who are in a complete or partial response to platinum-based therapy. First-line maintenance treatment of HRD positive advanced ovarian cancer in combination with bevacizumab: Before Lynparza with bevacizumab treatment is initiated for the first-line maintenance treatment of EOC, FTC or PPC, patients must have confirmation of either deleterious or suspected deleterious BRCA1/2 mutation and/or genomic instability determined using a validated test. Adjuvant treatment of germline BRCA-mutated high risk early breast cancer: Before Lynparza treatment is initiated for adjuvant treatment of HER2 negative high risk early breast cancer, patients must have confirmation of deleterious or suspected deleterious gBRCA1/2 mutation using a validated test. Monotherapy treatment of gBRCA1/2-mutated HER2negative metastatic breast cancer: For germline breast cancer susceptibility genes (gBRCA1/2) mutated human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer, patients must have confirmation of deleterious or suspected deleterious gBRCA1/2 mutation before Lynparza treatment is initiated. gBRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. First-line maintenance treatment of gBRCA-mutated metastatic adenocarcinoma of the pancreas: For first-line maintenance treatment of germline BRCA1/2-mutated metastatic adenocarcinoma of the pancreas, patients must have confirmation of a deleterious or suspected deleterious gBRCA1/2 mutation before Lynparza treatment is initiated. gBRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. Monotherapy treatment of BRCA1/2-mutated metastatic castration-resistant prostate cancer: For BRCA1/2-mutated metastatic castration-resistant prostate cancer (mCRPC), patients must have confirmation of a deleterious or suspected deleterious BRCA1/2 mutation (using either tumour or blood sample) before Lynparza treatment is initiated. BRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. Treatment of mCRPC in combination with abiraterone and prednisone or prednisolone: No genomic testing is required prior to using Lynparza in combination with abiraterone and prednisone or prednisolone for the treatment of patients with mCRPC. Genetic counselling for patients tested for mutations in BRCA1/2 genes should be performed Posology: Recommended dose in monotherapy or in combination with bevacizumab for ovarian cancer or in combination with abiraterone and prednisone or prednisolone for prostate cancer or endocrine therapy is 300mg (two 150mg tablets) twice daily, equivalent to a total daily dose of 600mg. The 100mg tablet is available for dose reduction. Tablets should be swallowed whole and not chewed, crushed, dissolved or divided and may be taken without regard to meals. Lynparza monotherapy: Patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy should start Lynparza treatment no later than 8 weeks after completion of their final dose of platinum-containing regimen. Lynparza in combination with bevacizumab: When Lynparza is used in combination with bevacizumab for the first-line maintenance treatment of high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer following completion of first-line platinum-based therapy with bevacizumab, the dose of bevacizumab is 15 mg/kg once every 3 weeks. Lynparza in combination with endocrine therapy: Please refer to the full product information of the endocrine therapy combination partner(s) (aromatase inhibitor/anti-oestrogen agent and/or LHRH) for the recommended posology. Lynparza in combination with abiraterone and prednisone or prednisolone: When Lynparza is used in combination with abiraterone for the treatment of patients with mCRPC, the dose of abiraterone is 1000 mg orally once daily. Abiraterone should be given with prednisone or prednisolone 5 mg orally twice daily. Please refer to the full product information for abiraterone. Duration of treatment: First-line maintenance treatment of BRCA-mutated advanced ovarian cancer: Patients can continue treatment until radiological disease progression, unacceptable toxicity or for up to 2 years if there is no radiological evidence of disease after 2 years of treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating physician can derive further benefit from continuous treatment, can be treated beyond 2 years. Maintenance treatment of platinum-sensitive relapsed ovarian cancer: For patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer, it is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. First-line maintenance treatment of HRD positive advanced ovarian cancer in combination with bevacizumab: Patients can continue treatment with Lynparza until radiological disease progression, unacceptable toxicity or for up to 2 years if there is no radiological evidence of disease after 2 years of treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating physician can derive further benefit from continuous Lynparza treatment, can be treated beyond 2 years. Adjuvant treatment of germline BRCA-mutated high risk early breast cancer: It is recommended that patients are treated for up to 1 year, or until disease recurrence, or unacceptable toxicity, whichever occurs first. Monotherapy treatment of gBRCA1/2-mutated HER2negative metastatic breast cancer: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. There are no efficacy or safety data on maintenance retreatment with Lynparza following first or subsequent relapse in ovarian cancer patients or on retreatment of breast cancer patients. First-line maintenance treatment of gBRCA-mutated metastatic adenocarcinoma of the pancreas: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. Monotherapy treatment of BRCA1/2-mutated metastatic castration-resistant prostate cancer: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. Medical castration with luteinising hormone releasing hormone (LHRH) analogue should be continued during treatment in patients not surgically castrated. Treatment of mCRPC in combination with abiraterone and prednisone or prednisolone: It is recommended that treatment be
©2023 AstraZeneca. All rights reserved.
PromoMats ID: IE-4172 | Date of preparation: March 2023
continued until progression of the underlying disease or unacceptable toxicity when Lynparza is used in combination with abiraterone and prednisone or prednisolone. Treatment with a gonadotropin-releasing hormone (GnRH) analogue should be continued during treatment in all patients, or patients should have had prior bilateral orchiectomy. Please refer to the product information for abiraterone. There are no efficacy or safety data on retreatment with Lynparza in prostate cancer patients. If a patient misses a dose of Lynparza, they should take their next normal dose at its scheduled time. Dose adjustments: Treatment interruption to manage adverse reactions such as nausea, vomiting, diarrhoea, anaemia and dose reduction can be considered. Recommended dose reduction is to 250mg (one 150mg tablet and one 100mg tablet) twice daily, equivalent to a total daily dose of 500mg. If further dose reduction is required, then reduction to 200mg (two 100mg tablets) twice daily, equivalent to a total daily dose of 400mg is recommended. Concomitant use of strong or moderate CYP3A inhibitors is not recommended and alternative agents should be considered If a strong CYP3A inhibitor must be co-administered, recommended dose reduction is to 100mg (one 100mg tablet) twice daily, equivalent to a total daily dose of 200mg. If a moderate CYP3A inhibitor must be co-administered, recommended dose reduction is to 150mg (one 150mg tablet) twice daily, equivalent to a total daily dose of 300mg. Elderly: No adjustment in starting dose is required. Renal impairment: For patients with moderate renal impairment (creatinine clearance 31 to 50 ml/min) the recommended dose is 200mg (two 100mg tablets) twice daily, equivalent to a total daily dose of 400mg. Lynparza can be administered in patients with mild renal impairment (creatinine clearance 51 to 80 ml/min) with no dose adjustment. No studies have been conducted in patients with severe renal impairment or end-stage renal disease (creatinine clearance ≤ 30 ml/min) and Lynparza is not recommended for use. It may only be used in patients with severe renal impairment if the benefit outweighs the potential risk with careful monitoring of renal function and adverse events. Hepatic impairment: Can be administered in patients with mild or moderate hepatic impairment (Child-Pugh A or B) with no dose adjustment. Not recommended in patients with severe hepatic impairment (Child-Pugh C).
Contraindications: Hypersensitivity to the active substance or to any of the excipients. Breast-feeding during treatment and for 1 month after the last dose.
Warnings and Precautions: Haematological toxicity: Treatment should not be started in patients until they have recovered from haematological toxicity caused by previous anticancer therapy (haemoglobin, platelet and neutrophil levels should be ≤CTCAE grade 1). Baseline testing followed by monthly monitoring of complete blood counts is recommended for first 12 months of treatment and periodically thereafter. Treatment should be interrupted and appropriate haematological testing should be initiated if patient develops severe haematological toxicity or blood transfusion dependence. Myelodysplastic syndrome/ Acute Myeloid Leukaemia (MDS/AML): If MDS/AML is suspected, the patient should be referred to a haematologist for further investigations, including bone marrow analysis and blood sampling for cytogenetics. If, following investigation for prolonged haematological toxicity, MDS/AML is confirmed, Lynparza should be discontinued and the patient treated appropriately. Venous Thromboembolic
Events: Monitor patients for clinical signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Patients with a prior history of VTE may be more at risk of a further occurrence and should be monitored appropriately. Pneumonitis: Interrupt Lynparza treatment and promptly investigate as appropriate. Discontinue Lynparza if pneumonitis is confirmed and treat patient appropriately. Embryofoetal toxicity: Lynparza could cause foetal harm when administered to a pregnant woman. Pregnancy/contraception: Lynparza should not be used during pregnancy. Women of childbearing potential must use two forms of reliable contraception, before starting Lynparza, during therapy and 6 months after receiving the last dose. Two highly effective and complementary forms of contraception are recommended. Male patients and their female partners of childbearing potential should use reliable contraception during therapy and for 3 months after receiving the last dose. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per 100 mg or 150 mg tablet, that is to say essentially “sodium-free”.
Drug Interactions: The recommended Lynparza monotherapy dose is not suitable for combination with myelosuppressive anticancer medicinal products. Caution and close monitoring if vaccines or immunosuppressant agents are co-administered. Effect of other drugs on Lynparza: Strong CYP3A inhibitors (e.g. itraconazole, telithromycin, clarithromycin, protease inhibitors boosted with ritonavir or cobicistat, boceprevir, telaprevir) or moderate CYP3A inhibitors (e.g. erythromycin, diltiazem, fluconazole, verapamil) are not recommended. If co-administered, the dose of Lynparza should be reduced. It is also not recommended to consume grapefruit juice. Strong CYP3A inducers (e.g. phenytoin, rifampicin, rifapentine, carbamazepine, nevirapine, phenobarbital, and St John’s Wort) are not recommended with Lynparza as the efficacy of Lynparza could be substantially reduced. The magnitude of the effect of moderate to strong inducers (e.g. efavirenz, rifabutin) on olaparib exposure is not established, therefore the co-administration of Lynparza with these medicinal products is also not recommended. Effect of Lynparza on other drugs: Caution and appropriate clinical monitoring is recommended when sensitive CYP3A substrates or substrates with a narrow therapeutic margin (e.g. simvastatin, cisapride, cyclosporine, ergot alkaloids, fentanyl, pimozide, sirolimus, tacrolimus and quetiapine) or P-gp substrates (e.g. simvastatin, pravastatin, dabigatran, digoxin and colchicine) are combined with Lynparza. Lynparza may reduce efficacy of hormonal contraceptives. Lynparza may increase the exposure to substrates of BCRP (e.g. methotrexate, rosuvastatin), OATP1B1 (e.g. bosentan, glibenclamide, repaglinide, statins and valsartan), OCT1, MATE1, MATE2K (e.g. metformin), OCT2 (e.g. serum creatinine), OAT3 (e.g. furosemide and methotrexate). Caution if co-administered with any statin.
Pregnancy and Lactation: Women of childbearing potential should not become pregnant while on Lynparza and not be pregnant at the beginning of treatment. A pregnancy test should be performed prior to treatment and considered regularly throughout treatment. The efficacy of some hormonal contraceptives may be reduced if co-administered with Lynparza. Therefore, an additional non-hormonal contraceptive method should be considered during treatment. For women with hormone dependent cancer, two non-hormonal contraceptives should be considered. Lynparza could cause foetal harm when administered to a pregnant woman Lynparza is contraindicated during breast-feeding and for 1 month after receiving last dose. Male patients must use a condom during therapy and for 3 months after receiving last dose when having sexual intercourse with a pregnant woman or with a woman of childbearing potential. Female partners of male patients must also use highly effective contraception. Male patients should not donate sperm during therapy and for 3 months after treatment.
Ability to Drive and Use Machines: Asthenia, fatigue and dizziness have been reported and patients who experience these symptoms should observe caution when driving or using machines.

Undesirable Events: Consult SmPC for full list of side effects. Very common: Anaemia, neutropenia, leukopenia, nausea, vomiting, diarrhoea, dyspepsia, dysgeusia, decreased appetite, fatigue (including asthenia), headache, dizziness, cough, dyspnoea. Common: Lymphopenia, thrombocytopenia, stomatitis, upper abdominal pain, rash, blood creatinine increased, venous thromboembolism. Uncommon: Myelodysplastic syndrome/Acute myeloid leukaemia, hypersensitivity, dermatitis, mean cell volume increased. Rare: Angioedema, erythema nodosum.
Legal Category: Product subject to prescription which may not be renewed (A)
Marketing Authorisation Number: EU/1/14/959/002-003 (100mg tablets); EU/1/14/959/004-005 (150mg tablets).
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 7100. LYNPARZA is a trade mark of the AstraZeneca group of companies.
Veeva ID: IE-4543
Date of API preparation: 12/2022
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
* Patients with moderate-to-severe COPD not adequately treated by a combination of ICS/LABA, or LAMA/LABA.1

** A worsening of symptoms or a history of exacerbations treated with antibiotics or oral corticosteroids in the past 12 months.
ICS, inhaled corticosteroid; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain1
References: 1. TRELEGY Ellipta SmPC, 2022. Available at www.medicines.ie. Accessed October 2022. 2. Lipson DA et al. Am J Respir Crit Care Med 2017; 196:438–446. 3. Halpin DMG;ERJ Open Research;2021;7;1-11.
Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) Prescribing information. Please consult the full Summary of Product Characteristics (SmPC) before prescribing Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg), umeclidinium bromide (UMEC) 62.5 micrograms and vilanterol as trifenatate (VI) 25 mcg provides a delivered dose of 92 mcg FF, 55 mcg UMEC and 22 mcg VI. Indications: Maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long-acting ß2-agonist (LABA) or a combination of a LABA and a long acting muscarinic antagonist. Dosage and administration: One inhalation once daily at the same time each day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate & magnesium stearate). Precautions: Paradoxical bronchospasm, unstable or life-threatening cardiovascular disease or heart rhythm abnormalities, convulsive disorders or thyrotoxicosis, pulmonary tuberculosis or patients with chronic or untreated infections, narrow-angle glaucoma, urinary retention, hypokalaemia, patients predisposed to low levels of serum potassium, diabetes mellitus. In patients with moderate to severe hepatic impairment patients should be monitored for systemic corticosteroid-related adverse reactions. Eye symptoms such as blurred vision may be due to underlying serious conditions such as cataract, glaucoma or central serous chorioretinopathy (CSCR); consider referral to ophthalmologist. Increased incidence of pneumonia has been observed in patients with COPD receiving inhaled corticosteroids. Risk factors for pneumonia include: current smokers, old age, patients with a history of prior pneumonia, patients with a low body mass index and severe COPD. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take Trelegy. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Systemic effects: Systemic effects of ICSs may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Interactions with other medicinal products: Caution should be exercised with concurrent use of ß-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products), hypokalaemic treatments or non-potassium-sparing diuretics. Co-administration with other long-acting muscarinic antagonists or long acting ß2-adrenergic agonists is not recommended. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, arthralgia, back pain. Uncommon (≥1/1,000 to <1/100): viral respiratory tract infection, dysgeusia, vision blurred, glaucoma, eye pain, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, dysphonia, dry mouth, fractures. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and rash, intraocular pressure increased. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA No. [EU/1/17/1236/002]. Legal category: POM B. Last date of revision: July 2022. Code: PI-6725. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.



CAN TOO*1,2
IT KEEPS GOING SO THEY
is a 31% significant increase in the odds of patients on TRELEGY Ellipta improving their health status/HRQoL compared with those on non-Ellipta Multiple Inhaler Triple therapy (MITT)3 Striving for a life uninterrupted For COPD patients on treatment with ICS/ LABA and at risk of exacerbation**1
There
Find out more here: www.trelegy.ie or request a visit from a GSK representative ©2022 GSK Group of Companies or its licensor Trademarks are owned by or licensed to the GSK Group of Companies PM-IE-FVU-ADVT-220007 | December 2022 TRELEGY Ellipta was developed in collaboration with Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to
GlaxoSmithKline on 1800 244 255.
Medical Council President calls for increased equity in the medical profession P6
Name, blame and shame ethos is not a solution for health service P8
Gaps in care for intestinal failure P9
No proposals for appointment of a Chief Pharmaceutical Officer P11
IPHA hosts Annual Conference 2023 P15

REGULARS
Feature: Chronic lymphocytic leukaemia P24
CPD: Breast Conserving Therapy P31
Focus: Depression and Stroke P43
P44
Foreword
Editor
One of our lead news stories this issue looks at the Irish Hospital Consultants Association ‘Care can’t Wait’ campaign. Airline Captain Niall Downey, a former Cardiothoracic Surgeon, has said the Government should implement an aviation style safety model when it comes to healthcare. Speaking in a New Video as part of the campaign, Captain Downey says a ‘name, blame, shame, retrain’ culture within our health service is placing extra strain on healthcare workers who are already facing immense pressure.
The IHCA has previously highlighted that stress levels among Consultants in recent years has been amplified by deteriorating working conditions and increased recruitment challenges. Consultant and medical staff shortages and a lack of hospital capacity means that Consultants cannot work to the best of their ability, increasing the potential for error. You can read more about this on page 8.
In other, worrying news for pharmacy, it is understood there are currently no ‘active proposals’ to develop a Chief Pharmaceutical Officer role in the Department of Health, Minister for Health Stephen Donnelly has confirmed. Deputy Róisín Shortall recently asked the Minister if he intends to create such a post during a recent Dáil Éireann Debate.
P50
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd


Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
editorial@hospitalprofessionalnews.ie
ACCOUNTS
Fiona Bothwell cs.ipn@btconnect.com
SALES EXECUTIVE
Amy Evans - amy@ipn.ie
CONTRIBUTORS
Aisling M Ryan | Emilie McCormack
Michael Click | Karl Sweeney
Aoife Lowery | Dr Max Waters
Dr Ronan Cusack | Dr Samer Arnous

Dr Cormac O’Connor | Prof Tom Kiernan
Theresa Lowry-Lehnen | Dr Paula Byrne

Ms. Nada Akl | Professor Ian Graham
Dr Lisa Brandon | Dr Usama Boles
Gaston L. Cluzel | Noel M. Caplice
Chloe Carpenter | Umer Salati
Fionnuala Ní Áinle | Andrew Yates
Paul Nolan | Professor Robert Byrne
Dr Sean Fitzgerald | Dr Robert Varley
Professor Richard J Farrell

Diana Angelika Olszewska
Professor Andrew Maree
Dr Kumaren Ramsamy
DESIGN DIRECTOR
Ian Stoddart Design
“The potential role that pharmacy may play in the development and implementation of future healthcare reform, especially in regard to the aims and vision of Sláintecare, is under consideration,” he said. To answer the specific question, at this time currently there are no active proposals to develop a Chief Pharmaceutical Officer role in the Department of Health.” There is more about this on page 11.
IPHA held their annual conference recently and we have coverage on pages 15-16 on the key issues discussed. Proposals from the European Commission to weaken intellectual property rights will degrade the development and delivery of innovative new medicines for patients in Europe, according to the Association. IPHA is urging the Government and other EU member states to request the Commission to amend its proposals and to back science, jobs and innovation by supporting the protection of the IP rights that underpin R&D investment and the scaled-up manufacturing of innovative new medicines in Ireland and Europe.
Our special focus this month is on Cardiology and we have some excellent clinical articles starting on page 36. I hope you enjoy the issue.
5 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 April Issue Issue 107 6
Contents
Cardiology
Cardiology
Cardiac
Cardiology
Aortic Stenosis
Clinical R&D:
15 8 43 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
Focus:
Rehabilitation
Focus:
P80
More Needs to be done to Support Women in Medicine
This International Women’s Day, the President of the Medical Council calls for increased equity in the medical profession.
Nearly 30 years ago women only made up 30% of the medical workforce in Ireland. 10 years ago, this grew to 41% and today women make up 46% of the doctors in Ireland. Growth in the number of women registered as doctors continues, with women making up 52% of doctors in the 20- to 35-year-old age group.
As we move towards 50/50 gender balance in medicine, we must acknowledge that this is not enough; it is not true equity because we do not see it reflected in senior appointments and leadership roles.
Speaking on International Women’s Day President of the Medical Council, Dr Suzanne Crowe said, “As a medical student in the summer of 1990 I was one of many to believe that Ireland would become a major force in international football and women would conquer the medical profession, but unfortunately neither came to pass.”
“Though the numbers of women entering medicine rises each year, this increase is slow to be seen in appointments at a senior level and across different specialties, there is still inequity in terms of training opportunities and career progression. While female doctors should expect equal treatment with their male counterparts, employers and training bodies must also
take into account the necessity to allow a work-life balance for all” continued Dr Crowe.
Dr Crowe further stated that “As a woman working in medicine and holding a leadership role, I would be hoping for more equitable supports for women in medicine. We need more flexibility, it needs to be more family friendly and more accommodating to the challenges facing women in medicine, and others with caring responsibilities. These changes would not just support female doctors, but all doctors, and most importantly the patients they care for.
“While I am President of the Medical Council, I am also trying to maintain a work-life balance of being a mother, whilst also working
Hospital Pharmacy Shortages Survey
as a paediatric intensivist. We need to recognise the individual needs of those within the workforce that may be juggling a career while also being a mother, carer, spouse.”
“The unequal impact of caring responsibilities on women can represent an obstacle to career progression and explains why we do not have as many women in senior leadership roles.
“There’s a brave new world continuing to emerge, that will hopefully improve equity across the spectrum of the medical profession. All bodies and agencies involved in healthcare delivery, policy, and advocacy need to be more cognisant of the importance of equity and equality, diversity, inclusion and work-life balance in their work.”
EAHP launches 2023 Shortage Survey focused on medicinal products and medical devices. For more than a decade the European Association of Hospital Pharmacists (EAHP), has been raising awareness about the problem of medicine shortage and its impact on patients in particular in the hospital environment. Five surveys were conducted by EAHP in 2013, 2014, 2018, 2019 and 2020. The results of these surveys have provided an overview of the severity of the problem as well as its impact on overall patient care.
The 2023 Shortage Survey seeks to investigate some of the reasons for shortages of medicines and medical devices in hospitals as well as the impact on patients. In addition, it aims at collecting solutions and best practices currently applied at the local, regional and national levels. The survey targets hospital pharmacists, nurses, physicians, other healthcare professionals and patients. This survey uses the definition for a ‘medicine shortage’ provided by the European Medicines Agency (EMA): ‘A shortage of a medicinal product for human or veterinary use occurs when supply does not meet demand at a national level’.
The survey will be closed at midnight on 30 April 2023. Visit www.surveymonkey.com/EAHP2023ShortageSurvey
6 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
President of the Medical Council, Dr Suzanne Crowe
Consultants vote to reject Contract
Doctors in the Irish Medical Organisation (IMO) have voted to reject a new contract for hospital consultants with a salary of up to ¤257,193 which came into force last month.
The new contract, which will be offered to all doctors applying for jobs from now on, will mean they will only work in public hospitals, although they can still practice in private hospitals in their own time.
The 37-hour-a-week contract is aimed at filling 900 vacancies, providing more senior specialist cover in hospitals in the evenings and on Saturdays and is seen as the start of removing private practice from public hospitals, although this will take many years.
The result of a ballot by the trade union shows that 57% of current contract holders have indicated they will not switch to the new contract.
Also 64% of NCHD members say they will not take up the new contract.
The IMO said that 59% of consultants currently working overseas say they will not return to Ireland to take up the contract.
The key reasons cited for the rejection of the new offer are: no limitation on the number of evenings and Saturdays a consultant will be required to work; the ability of the HSE to change
the work location of consultants without agreement and a lack of trust in Government that there will be sufficient medical and other staff on weekend and evening shifts to ensure safety.

Professor Matthew Sadlier, Chairman of the IMO Consultant Committee said: “The Government took the unilateral decision to conclude negotiations on a new contract and to present a final document on a take-it or leave-it basis without the agreement of the IMO. While we accept there was progress on some issues, we were very clear at the negotiations that a lack of safeguards around rostering, location and sufficient
New CEO for College of Physicians
number of medical and other staff would make the terms unworkable in practice and create huge uncertainty and inequity. The Irish health services desperately needs more consultants and while we hope this contract will achieve that, there are valid concerns amongst doctors. Our concern is that was a missed opportunity by Government to agree a contract that would both encourage existing consultants to transition to and which would be attractive to much needed new consultants. We are now almost certainly looking at a period of great uncertainty and potentially an ongoing recruitment crisis.”
The Royal College of Physicians of Ireland has appointed Audrey Houlihan as Chief Executive Officer who will take up the new role on 26 June 2023.
Welcoming the new CEO, President Mary Horgan said, “I am delighted to welcome Audrey as the new CEO of the Royal College of Physicians of Ireland (RCPI). Audrey has a strong track record in healthcare and will bring great expertise to RCPI. Through her leadership as CEO, she will support the growth of the college and its commitment to our Staff, Trainees, Members, Fellows and Learners. We are all looking forward to working with her.”
Incoming CEO Audrey Houlihan said: “I am very privileged to have been selected by RCPI to be the CEO of such a vital organisation and look forward to leading the team as I take up this new role.”
Audrey, who is the first female CEO to be appointed to this role at the college, joins RCPI from Our Lady’s Hospice and Care Services (OLHCS) where she is CEO. Audrey holds over 20 years’ experience in executive and senior leadership role within the not-for-profit sector.
During her tenure, she was appointed to several national committees and Director roles that provide advisory services related to policy, strategy and service development matters.

7 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
News
Name, Blame, Shame is not Solution
Captain Downey believes the implementation of adequate safety models with appropriate staffing and hospital capacity levels would inevitably lead to better outcomes for patients and change the culture found within the health service, so that medical professionals are no longer “pilloried” for highlighting an error or issue.
Airline Captain Niall Downey, a former Cardiothoracic Surgeon, has said the Government should implement an aviation style safety model when it comes to healthcare.

Speaking in a New Video as part of the Irish Hospital Consultant Association’s Care Can’t Wait campaign, Captain Downey says a ‘name, blame, shame, retrain’ culture within our health service is placing extra strain on healthcare workers who are already facing immense pressure.
Burnout
The IHCA has previously highlighted that stress levels among Consultants in recent years has been amplified by deteriorating working conditions and increased recruitment challenges. Consultant and medical staff shortages and a lack of hospital capacity means that Consultants cannot work to the best of their ability, increasing the potential for error. This in turn can lead to reduced healthcare
outcomes and care for patients and an even more stressful working environment for staff.
Figures at the end of last year showed that 8 in every 10 Consultants screened positive for burnout, which is exacerbating concerns in our hospitals for both medical professionals and their patients.
Captain Downey says Consultants and other healthcare workers should feel safe in identifying risks and where mistakes have been made, in order to develop a system where health service management can assess “what went wrong, not who went wrong”.
“In aviation we assume we are going to get it wrong, and all our systems are designed around that. We expect error, we don’t blame the individual for that. We have reporting systems where we can speak up without the fear of disciplinary action or dismissal. It’s called a Just Culture”.
Invite for Grant Applications
The MPS Foundation - a global not-for-profit research initiative that aims to shape the future of patient safety – has launched its second grant programme.
Medical Protection Society (MPS) launched The MPS Foundation last year and its first research grant programme received over 150 expressions of interest. 10 applicants were successful in securing funding for their research.
The Foundation is now inviting new research grant applications from both MPS members and non-
members. It will invest in research and analysis with a key focus on patient safety and the wellbeing of healthcare professionals and teams, both medical and dental, and applications will again be considered from the jurisdictions where MPS has members.
Research projects supported by The MPS Foundation need to be academically robust and evidence-based. Available funding will range from EUR 5,694 to EUR 227,767 - the equivalent to £5,000 to £200,000 (subject to currency fluctuations) - depending
Staffing Crisis
Speaking on the staffing deficit across the health service and its implications for safety, Captain Downey said it is inevitable that “mistakes will be made” when our hospitals and mental health services are being run without the necessary levels of staff, as those in the service are consistently overworked.
Over 900 permanent Consultant posts nationally remain either vacant or filled on a temporary or agency basis. This is due to decades of underinvestment, staff and bed shortages, and unsustainable working conditions that are driving our highly trained medical specialists abroad.
Not only does the extent of vacancies and hospital capacity deficits seriously undermine the provision of care to patients and the development of acute hospital and mental health services, but it is also a false economy. The cost to the HSE of managing and settling claims via the State Claims Agency has increased from ¤96m in 2015 to ¤435m in 2022 – more than a four-fold increase over the past 8 years (+¤339m or +350%).
He said it is now imperative that a system is put in place to allow health staff to “put their hand up” and highlight a problem when it arises in order to implement the changes needed to deliver safer models of care.
“In aviation we use a system called avoid, trap, mitigate.
“For example, at 800 feet the plane will tell us if the landing gear isn’t down. It is now too late to correct it, but we can put on full power and climb away. So we haven’t resolved the problem, but we’ve mitigated it in that we haven’t crashed the plane and we can come back and have another go. We could get that sort of mentality within the health service and reduce the blame game.
“In aviation it’s taken for granted that I’ll put my hand up and not worry about losing my job or being sued. Errors will happen, we have to legislate for them not blame people for them.”
With the crisis in our health service growing, latest figures show more than 885,000 people remain on some form of NTPF hospital waiting list2, a further 250,000 are awaiting urgent CTs, MRIs or ultrasounds3, while almost 11,300 admitted patients were treated on trolleys in January alone this year and a record 121,000 patients in 2022.
on the scale, focus and duration of the proposal.
The funding focusses on five main areas:
o the impact of human factors on patient safety, outcomes and risk
o the impact of processes and delivery models on patient safety, outcomes and risk
o the personal and professional wellbeing and development of clinicians
o the impact of digital integration and technology on patient safety, outcomes and risk.
o the evaluation of the effectiveness of teaching and learning innovations and the impact upon patient care, safety, outcomes and risk.
Healthcare professionals can register their interest by visiting www.thempsfoundation.org and entering their details via the online grant portal. Calls for expressions of interest close on 5 May 2023.
8 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Former Cardiothoracic Surgeon Captain Niall Downey
Conference
Experts call out Government on gap in care for Intestinal Failure
Experts at an international conference on nutrition have said lives are being lost – and called out the Government on the lack of a specialist service for Intestinal Failure.
Speakers at the conference on nutrition and health in Dublin, hosted by the Irish Society for Clinical Nutrition and Metabolism (IrSPEN), said the situation was a ‘national disgrace’ – and called for urgent approval of a dedicated adult Intestinal Failure (IF) Unit in line with health services across Europe.
IrSPEN President and Consultant Gastrointestinal Surgeon Professor John Reynolds said there is ‘no excuse’ for not taking action, as it would end suffering and deaths that are avoidable.
“Despite a decade of campaigning to establish a dedicated service for adult Intestinal Failure patients, with promises from Government Ministers and meetings with HSE Executives, Ireland continues to lack a needed specialist service.
“The case for establishing a dedicated Unit at St. James’s Hospital has been accepted by policy makers, and has the support of all the expert groups and professionals.
“A detailed proposal, was originally submitted to Government and the health services in 2017, then resubmitted last year and remains under consideration. We know that this service is literally a matter of life or death and it must be prioritised.
“The costed budget for such a Unit is ¤6m per annum, but a significant portion of this cost would be offset by the service not being needed elsewhere in the health service. This proposal was supported by the then Minister for Health Simon Harris in 2017, but never followed through,” he said.
Each year approximately 100 adults in Ireland develop severe acute Intestinal Failure (IF) with a requirement for highly specialised care.
IF occurs when a patient’s intestine cannot digest food and absorb fluids. Patients must be fed artificially via liquid nutrition through a catheter or needle inserted into a central vein in the chest. The treatment is lifesaving, but the risk of serious
President of the European Society for Clinical Nutrition and Metabolism (ESPEN) Professor Rocco Barazzoni

complications is high if not managed extremely carefully, resulting in emergency hospital admissions, prolonged hospital stay and death.
Also speaking at the conference, the President of the European Society for Clinical Nutrition and Metabolism (ESPEN) Professor Rocco Barazzoni said Ireland was an outlier in its lack of a specialised service to treat Intestinal Failure. “IF patients face high morbidity and mortality risks – and Ireland should be taking steps to provide a dedicated national centre.”
Consultant Gastroenterologist at St. James’s Hospital Dr Cara Dunne said avoidable deaths are happening and must end.
“Much of the medical expertise needed is already in place at St. James’ hospital and what’s now
needed is additional funding to develop this into a specialised Unit that is dedicated to these patients.
“Research from the NHS in the UK has equated under-capacity within Intestinal Failure care with 150 avoidable deaths each year. This would equate to 12 avoidable deaths per year in Ireland.
Currently, Northern Ireland has a 12-bed IF unit for 1.6 million – in contrast there is no dedicated Unit in this country for five million,” Dr. Dunne said.
The theme of today’s conference is on the role of muscle in aging and disease and titled Fit to Function: a new focus on muscle and aging and disease.
Addressing delegates, Professor John Reynolds also said that

there is need for better public and professional awareness on the role of muscle in well-being as people get older, particularly during cancer care.
“Current medical research clearly links the maintenance of muscle mass and function during cancer care with improved outcomes. Losing weight and losing muscle mass are common problems in cancer – affecting at least a third of patients, and has the potential to make treatment less effective and increases the risks of complications.
“A modern goal in cancer care is to minimise weight change during treatment and to preserve as best we can muscle mass and function,” he said.
9 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Professor John Reynolds said lives are being lost
“Despite a decade of campaigning to establish a dedicated service for adult Intestinal Failure patients, with promises from Government Ministers and meetings with HSE Executives, Ireland continues to lack a needed specialist service”
Calls to Prioritise Care for Dementia
A call has been made for the government to prioritise the setting up of the long-promised Commission on Care. The call came during a presentation to an all Oireachtas Committee by clinical staff, persons living with dementia and family members of service users attending Saint Joseph’s Shankill, all of whom are urging the government to revisit Ireland’s approach to the care of people living with dementia. They group is calling for a more patient-centred policy approach to improve the quality of life, social inclusion and dignity of people living with dementia, their caregivers and family members.
Saint Joseph’s Shankill which is the lead Irish partner in the pan-European ‘Dementia Right’ initiative, was invited to Leinster House by Senator Fiona O’Loughlin, Chair of the All-Party Oireachtas Group on Dementia, to present their case to both political and sectoral colleagues.
The Dementia Right Project commenced in 2020 and is a European project with partners in Portugal, Spain, Greece, and
Turkey. The project has four key objectives:
• Improving the quality of life, social inclusion and dignity of people living with dementia, their caregivers and family members;
• Developing a new approach to dementia that is human rights-based, with effective guidelines for implementing and supervising the care provided in health/care facilities to people with dementia regarding their rights;
• Improving the professional competencies of health and social care professionals, social educators and other carers to revamp their care skills and their approach to dementia;
• Making care centres more inclusive and efficient to cope with the diverse needs of people living with dementia.
The team at Saint Joseph’s are positively committed to changing the way people living with dementia are treated and cared for and say national policy must now
be reviewed and modernised to support more progressive models of care.
Siobhan Grant, Fundraising Manager with St Joseph’s Shankill explains why -
“The long-awaited Commission on Care promised under the Programme for Government, can play an important role in commencing a national discussion on dementia and this should be expedited. The conversation is changing as new models of care emerge and the state needs to wake up to this reality.
“Saint Joseph’s Shankill itself introduced significant changes some years ago with the new Butterfly model of care, that uses a person-centred model of care focusing on the social, emotional, psychological, and physical needs of each individual, in as-real-a-home environment as possible. It also seeks to put human rights at its core. Throughout the implementation of this innovative approach over the past decade, the Centre has witnessed significant clinical
New Appointment for Global Surgery
improvements (See Below) across a range of care metrics amongst its sixty-one residents. This more individualised approach to care encourages questioning and innovation, empowering people to make changes, and accepting what those changes bring for the better.
“It is incredibly challenging to inspire change in culture and equally challenging to maintain that change. The team at Saint Josephs have drawn on their own experience of culture and systems changes to inform the Dementia Right Project, by sharing how they value each person using the service, and their own uniqueness.” concludes Ms Grant. The Dementia Right Project seeks to influence people at all levels; from social and healthcare professionals to family members of people living with dementia, to politicians, law professional and the media at large. Inspiring policymakers to support a better future for people living with dementia, where their rights and preferences are always respected and taken into consideration.
Professor Juan Carlos Puyana has been appointed O’Brien Chair of Global Surgery at RCSI University of Medicine and Health Sciences. Professor Puyana will lead the university’s Institute of Global Surgery in its work to improve access to high-quality, essential surgical care for underserved populations.

Professor Puyana joins RCSI from the University of Pittsburgh where he was Director of Global HealthSurgery. He is a renowned trauma/ acute care surgeon and a global health educator.
Professor Puyana is an expert in developing international capacitybuilding programmes in trauma,
trauma registries, and trauma datasets in global surgery. He has established reciprocal mentoring collaborations with several universities and trauma centres from Latin America and Kenya.
He has been the Principal Investigator on six global health research and training grant programmes on capacity building, medical informatics, and eHealth. These grants have supported short- and long-term educational training programmes for health
professionals for several Latin American countries.
During his time with the PanAmerican Trauma Society, he was an influential leader actively promoting international collaborations and educational programs in the areas of trauma ultrasound, disaster and emergency response and trauma courses across Latin America.
In 2017, Professor Puyana was appointed to the Global Health Committee of the National Academy of Sciences (NAS), and he co-authored the publication “Global Health and the Future Role of the United States. As a Global Health trauma surgeon, Professor Puyana has worked in underserved emergency rooms and low-resource public hospitals in numerous Latin American cities. Every year, an estimated 140 million people who needed surgical procedures to save their lives or to prevent long-term disability did not get them and an estimated 81 million people who receive surgical care are impoverished by its costs.
10 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Juan Carlos Puyana
No ‘Proposals’ for Chief Pharmacy Officer
There are currently no ‘active proposals’ to develop a Chief Pharmaceutical Officer role in the Department of Health, Minister for Health Stephen Donnelly has confirmed.
Deputy Róisín Shortall recently asked the Minister if he intends to create such a post during a recent Dáil Éireann Debate.

Answering, he stated, “The Department of Health recognises the key role of Pharmacy in the Community, and the ongoing significant contribution made by this sector to patients and the public, particularly during the pandemic.
“The potential role that pharmacy may play in the development and implementation of future healthcare reform, especially in regard to the aims and vision of Sláintecare, is under consideration. The Department is open to exploring any evidence based appropriately governed services, delivered by appropriately trained professionals which will support this aim.
“In this regard a number of proposals and ways to progress the potential extension of the scope of practice of community pharmacists in the short, medium and longer term are currently being considered.
MSc in Clinical Pharmacy at UCC
To answer the specific question, at this time currently there are no active proposals to develop a Chief Pharmaceutical Officer role in the Department of Health.
“The Department has professionally qualified pharmacists among its staff. Furthermore a number of units within the Department have responsibility for advancing policy in key areas of our health system. They are cognisant of potential deliverables that can be realised
by and through pharmacists and pharmacy services, to deliver patient centred care in line with the vision of Sláintecare.”
Applications are invited for this two-year (part-time) distance learning Master’s Degree offered by the School of Pharmacy, University College Cork course, commencing in September 2023.
The course is structured to provide specialist training to enable pharmacists working in hospital and community pharmacies to extend their professional role within the evolving clinical healthcare system.
The course will promote a greater understanding of the major pharmacotherapeutic issues of various disease states in order to develop a greater understanding of the particular needs of patients with these diseases.
Graduates will gain the skills needed to become leaders in clinical pharmacy services, such as critical appraisal of drug therapies, rational drug use (including pharmacoeconomic evaluation), medication safety management, research project management, presentation skills and report writing.
The flexible, online learning format allows students to remain employed throughout the course. Students will be able to interact with each other and the experienced medical and pharmacy practitioners via webinars, video conferencing, and online discussion boards.
Applicants must hold a primary pharmacy degree and, ideally, have a minimum of one-year practical experience. Applicants must be registered as a pharmacist with the professional accreditation authority in the country where they practice. Visit MSc Clinical Pharmacy online at www.ucc.ie/en/ckx03 to learn more about the course.
Closing date for applications: 30th June 2023
Applicants must apply online at www.ucc.ie/apply. Full details of the application procedure are available on this website. For further information please contact Dr Suzanne McCarthy (Interim Programme Director, S.McCarthy@ucc.ie) School of Pharmacy, University College Cork, Cork, Ireland.
World Pharmacists Day 2023
“Pharmacists strengthening health systems” will be the theme of World Pharmacists Day on 25 September, FIP announced recently.

At a time when health systems around the world are recovering from the COVID-19 crisis and general consensus is that urgent action is needed if health services are to meet future needs, FIP’s 2023 World Pharmacists Day campaign presents opportunities to increase awareness of pharmacists as an intelligent solution.
The campaign is led by FIP every year, with the theme chosen by the FIP Council,
and provides an opportunity to promote the pharmacy profession. “Beyond doubt, pharmacy is essential to a strong healthcare system and COVID-19 has proven this. But there is a wide range of further needs that pharmacy can provide for to make health systems stronger. Our message is: Let pharmacies do more.
“I encourage colleagues across the globe to prepare to join me in this year’s campaign to celebrate our profession’s achievements in supporting our societies, but also to make its value and further potential in improving health more known,” said FIP president Dominique Jordan.
11 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
News
Deputy Roisin Shortall
New Targets for Managing Diabetes

 Edward Gregg, Head of RCSI’s School of Population Health
Edward Gregg, Head of RCSI’s School of Population Health
economies. Yet we know that the worst outcomes from diabetes can be prevented and that many interventions are cost-effective and feasible to implement. However, there are enormous global gaps in their delivery that the efforts of the Compact can alter.
“Type 2 diabetes can be delayed or prevented through intensive lifestyle interventions and medication for individuals at high risk, and population-wide changes to dietary quality, physical activity levels and prevalence of obesity. For people with diagnosed diabetes, delivery of essential medications and management of glycaemia and cardiometabolic risk factors, alongside early screening for complications via well organised care can reduce acute and chronic complications and extend life.”
A new report for the World Health Organisation has identified five core national targets for UN member states aimed at reducing diabetes risk and ensuring that people with diabetes have equitable access to comprehensive and affordable care and prevention.
The lead author on the report for WHO Global Diabetes Compact was Professor Edward Gregg, Head of RCSI’s School of Population Health. Its recommended targets, published in The Lancet, are:
1. Of all people with diabetes, at least 80% have been clinically diagnosed;
2. For people with diagnosed diabetes, 80% have glycated haemoglobin (HbA1c) concentrations below 8·0% (63·9 mmol/mol), an important biomarker for diabetes;
3. 80% of those with diabetes have blood pressure lower than 140/90 mm Hg;
4. At least 60% of those with diabetes who are 40 years or older are receiving therapy with statins;
5. Each person with type 1 diabetes has continuous access to insulin, blood glucose meters, and test strips.
Recent global estimates indicate that 537 million adults have diabetes, of whom 80% live in low-income and middle-income countries (LMICs). The global impact and cost of diabetes are expected to grow considerably, disproportionately affecting the most disadvantaged populations.
Population-based studies show that, at present, the delivery of evidence-based care for people with diabetes is suboptimal even in well-resourced health systems.
Many countries have high proportions of their populations with undiagnosed diabetes and many go without timely care for extended periods.
In high-income countries, the current proportions of people who meet recommended targets for risk factors, such as glycated haemoglobin or blood pressure, ranges from 50% to 70%.
The situation is worse in LMICs with just half of the people with a diagnosis of diabetes having good glycaemic control, and about one in four having good blood pressure control.
Professor Edward Gregg, Head of the School of Population Health, RCSI and lead-author on the report said: “Diabetes is one of the world’s most challenging public health issues due to its high and growing prevalence, and the impact it has on individuals, health systems and national
According to Professor Gregg, “While these goals are ambitious, their achievement can reduce the number of people living with diabetes and greatly improve the outcomes and quality of life of people who are diagnosed with the condition. We hope the WHO Global Diabetes Compact serves as a helpful framework for countries to take action and reduce the burden of diabetes globally.”
The Global Disease Compact was assembled by the WHO to identify potential metrics across four domains (structural, systems or policy level, processes of care, biomarkers and behaviours, and long-term health events and outcomes) and three risk tiers (diagnosed diabetes, high risk for diabetes, and whole population), and prioritised metrics according to their health importance, modifiability, data availability and the degree to which they represent areas of global inequality.
12 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
in digital version
Eimear joins IPHA
The Irish Pharmaceutical Healthcare Association (IPHA) is pleased to announce the appointment of Eimear O’Leary, PhD as Director of Communications and Advocacy.

Eimear previously held the role of Partnerships Manager at the IPHA and succeeds Bernard Mallee who has taken up a global role at Bristol Myers Squibb.
Over the past five years at the IPHA, Eimear has played a lead role in driving the Association’s award-winning communications initiatives and in developing programmes that raised its profile and grew its membership.
She co-delivered the multi-award winning Innovate for Life and Innovate for Vaccines campaigns. The renowned IPHA film series documented the positive impact of innovative medicines on patients’ lives, in science and in communities, achieving over 1.5 million views and 3 million impressions on social media channels.
Eimear led the reform and growth of the IPHA Affiliates Programme, engaging and energising members and affiliate companies through a
successful programme of events. She also spearheaded the launch of the ground-breaking digital Forum Series, providing IPHA audiences with deeper insights into issues affecting the industry and policy development.
She has worked closely with the members of the IPHA Communications and Advocacy Advisory Forum on policy proposals to reform the reimbursement process to deliver faster access to new medicines.
During her time at the IPHA she has worked on developing and promoting medicines.ie.
Eimear began her career in Leinster House where she provided policy, legislative and communications support to Government and Opposition T.D.’s over a fourteen-year period. She also worked in a communications and public affairs role at 360, Finn Partners. She has achieved a PhD and a Masters in Government from University College Cork and has also lectured at conferences and universities.
Oliver O’Connor, IPHA Chief Executive, said: “I am delighted to announce Eimear’s appointment
Eimear O’Leary, Director of Communications and Advocacy, IPHA
as our new Director of Communications and Advocacy. Over the past five years working with the Association, Eimear has developed in-depth knowledge of our industry and the legislative drivers that shape it. Her expertise, insight and initiative are invaluable to us as we work to extend the success of our flagship communications and advocacy initiatives into the future and we wish her well in her new role”.
Eimear O’Leary, said: “Having enjoyed working with the Association and our member
SFI funding to tackle chronic diseases
A world-leading population health scientist, Professor Edward Gregg, Head of the School of Population Health, RCSI University of Medicine and Health Sciences, has been awarded ¤4.3 million in funding from Science Foundation Ireland (SFI) through its prestigious SFI Research Professorship Programme.
The award, which comes as a joint appointment with Imperial College London, will fund a new research centre at RCSI, Converge: Centre for Chronic Disease and Population Health Research. The Centre will transform the way that population-level data and research platforms are deployed in Ireland to make key decisions for the prevention and reduction of the burden of chronic diseases.
The research programme will target diabetes, obesity and other chronic diseases, and links to HSE and Sláintecare action plans. The three key streams to the programme are:
i. developing a novel data ecosystem and undertaking studies to prioritise the current, emerging and future health priorities in chronic disease and morbidity in Ireland and globally;
ii. developing and using new population registries and integrated data sources to quantify the impact of care and disease prevention in the real world, taking both an Irish and global perspective;
iii. developing models to prioritise intervention and risk assessment options for prevention of diabetes, chronic diseases, and multi-morbidity.
This research programme will unite diverse scientific disciplines to drive decisions about the most effective prevention, treatment and risk assessment approaches.
Professor Gregg said: “This research programme will bring together new data and develop novel ways to measure health
impact and determine what approaches work best to reduce the risk of diabetes and other chronic conditions. The global type 2 diabetes pandemic is having devastating effects on individuals, families, health systems and national economies. One-tenth of the world’s adults have diabetes. In Ireland, the number of people living with type 2 diabetes has almost doubled in the past 15 years, accounting for more than ¤550 million per year in direct health care costs.
“We will establish new, smarter disease registries to determine what works best in the real world. This will help public health leaders to prioritise approaches that will change the future risk and damage caused by diabetes and other chronic conditions,” he added.
Director General of Science
Foundation Ireland, Professor Philip Nolan, welcomed the announcement saying: “The SFI Research Professorship
companies over the last five years, I am delighted to be taking up this role, at an exciting time for the industry. I look forward to continuing to tell the industry story in Ireland and working towards enabling faster access to life saving, innovative medicines for patients, who are at the heart of the work we do”.
Programme attracts world-leading researchers to Ireland, building our critical expertise and international reputation for excellence. I am delighted to congratulate and welcome Professor Gregg whose funding will support an additional 13 research positions. His group aims to develop the science and collaborations that will pave the way for innovative population registries for chronic diseases. Such work has valuable potential to improve health outcomes for patients and better inform policy.”
The new centre will recruit a team of cutting-edge researchers, as well as doctoral scholars at the RCSI School of Population Health. Researchers at the centre will collaborate closely with other world-class institutions and researchers, including the World Health Organisation, Imperial College London, the US Centers for Disease Control and Prevention, Maynooth University, the University of Galway and the HSE.
13 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
News
HSE publishes National Service Plan

The HSE today has published the 2023 National Service Plan following approval by the Minister for Health Mr Stephen Donnelly, TD. The National Service Plan (NSP2023) outlines the health and social care services to be provided to the people of Ireland this year. The Government has allocated a budget of ¤21.124bn for this purpose, which is 5.7% above last year’s starting budget. It includes an additional
• ¤240.2m investment in new service developments
• ¤564.5m once off funding for Covid-19 costs
• ¤899.2m to fund increased costs of providing the existing level of service to cover non pay inflation and national pay awards.
The aims of the NSP2023 are to build further on hospital and community services capacity, improve access to services, including women’s healthcare and mental health services, enhance prevention and early intervention work, take forward the work of the Waiting List Task Force, and implement improvements in the security and resilience of critical national infrastructure.
As we work to build back service capacity affected by Covid-19, we will also continue to support Sláintecare reform and finalise design and commencement of initial implementation of the six Regional Health Areas (RHAs) in 2024.
The Minister for Health Stephen Donnelly said, “I am pleased to approve the HSE National Service
Plan for 2023 which reflects the provision by this Government of the largest budget allocation to the HSE in the history of the State.
“This unprecedented level of investment further supports the resilience and preparedness of the health service to increase capacity and permanent staffing levels, to progress the implementation of national strategies and to advance the vision of universal healthcare based on the strategic reform set out in the Programme For Government and Sláintecare. This ongoing reform will ensure the provision of a public health service which provides people with access to high quality, affordable care when they need it.
“I look forward to ongoing collaborative engagement with the HSE Board, CEO and Executive in the implementation of the measures set out in the NSP, which include the ongoing rollout of community initiatives, continued focus on women’s health and the implementation of national strategies and key Sláintecare reforms.”
Speaking about the HSE’s priorities for 2023, Mr Ciarán Devane, HSE Chairman said, “Supporting the delivery of universal healthcare and delivering the reforms outlined in Sláintecare remain the priorities for the HSE. Waiting lists for scheduled care in hospitals and long waits in emergency departments, particularly for older people and those who have more complex needs, remain a concern and a priority for the organisation. Our reform programme continues to seek to address this, as well as addressing waiting times for
mental health and communitybased services, with the ultimate aim of improving the patient / service user experience through innovative initiatives including the ongoing digitisation of our health service.”
Key Priorities for 2023
Hospitals: Additional general acute beds will come on stream in 2023, bringing the total increase since 2020 to 1,179 by end 2023 along with additional critical care beds, bringing the total to 352 by the end of this year. These figures can fluctuate for operational and service reasons. We will continue to deliver safe, timely access to hospital care by implementing a range of measures to ensure better access to unscheduled care, improved cancer care and prevention, and to build on proven waiting list initiatives and ambitious waiting time targets have been set for outpatients.
Community: The Enhanced Community Care Programme (ECC) will continue to roll out nationally to ensure more people can access healthcare in their local community rather than within the acute hospital setting. These measures include the continued development of 96 Community Healthcare Networks, each servicing a population of c.50,000, 30 community specialist teams for older persons and 30 community specialist teams for people living with chronic disease as well as increased access to community diagnostics (up to 240,000 community radiology tests and 266,500 tests across areas such as echocardiography, spirometry and natriuretic peptide blood tests). 23.9million home support
hours will be delivered to 55,910 older persons in 2023.
Mental Health: With an additional budget of ¤14.0m in 2023, we will see an increased number of 12,635 CAMHS referrals, a 17% target increase since 2019.
Disability: We will spend an additional ¤25.0m on disability services including over 70,000 additional hours of personal assistant support to people with disabilities and 1,250 new day services placements for school leavers and graduates of rehabilitative training in line with New Directions policy. We will improve the delivery of a range of specialist communitybased disability services and increase service capacity in the areas of day, respite, multidisciplinary, residential and personal assistant services as well as progressing the delivery of the Assessment of Need process in line with legislative obligations.
Recruitment: Regarding staffing, the HSE will expand the workforce by +6,000 WTEs in 2023 whilst also ensuring we have recruited approximately 10,500 staff to replace those that will retire or leave during the year. The HSE is addressing complex challenges in workforce planning and recruitment while maintaining focus on strengthening the retention of the existing workforce. This includes advancing the Safe Staffing Framework and encouraging and enabling staff to work at the top of their licence. The ultimate aim is to become an ‘employer of choice’ by providing a rewarding and fulfilling workplace for our most valuable asset – our people.
14 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Challenging the Degrading of New Medicines Annual Conference
Proposals from the European Commission to weaken intellectual property rights will degrade the development and delivery of innovative new medicines for patients in Europe, according to the Irish Pharmaceutical Healthcare Association (IPHA).
IPHA, which represents the biopharmaceutical industry in Ireland, set out its position ahead of its Annual Conference, which took place in Dublin last month and the publication of the new EU Pharmaceutical Strategy later the same month.
It is urging the Government and other EU member states to request the Commission to amend its proposals and to back science, jobs and innovation by supporting the protection of the IP rights that underpin R&D investment and the scaled-up manufacturing of innovative new medicines in Ireland and Europe.
The IPHA Conference heard keynote speeches from Minister for Health, Stephen Donnelly, T.D., Tánaiste, Micheál Martin, T.D. and numerous industry experts. It explored the economic and clinical impacts of innovation, the promise of new technologies in the pipeline and the importance of supportive polices.
IPHA has described the imminent publication of the new EU Pharmaceutical Strategy as a key moment for European competitiveness and Europe’s struggle to regain the ground that it has lost to the US and China for cutting-edge science and investments. IPHA highlighted that:
• In the 1990s, half of all new treatments originated in Europe, that figure is now just one in five.
• In 2002 the US spent ¤2bn more than Europe on pharmaceutical research and development, today the US is spending ¤25bn more.
• Employment in the pharmaceutical sector in China has risen by 800% since 2021.
IPHA understands that the Commission has been motivated to overhaul the EU’s legislative framework for the pharmaceutical industry in a bid to improve affordability and reliable access to new medicines for less well-off
assesses innovation pipeline and supportive policies
member states. It believes that specific measures can be taken to assist these states in their national health systems, rather than changing Europe-wide IP rights. It said that the biopharmaceutical industry has already brought forward proposals which set out how improving equity of access across the EU can be achieved by well-chosen, targeted initiatives and co-operative industry agreements. Such targeted measures can have an impact in 2024. By contrast, the proposed changes to IP rights would have the immediate effect of degrading the European environment for long term investment and it would be well into the 2030’s before they
delivered any potential impact on national health systems.
Giving a keynote speech, Minister for Health Stephen Donnelly discussed the future of medicines, investment and the appraisal process.

The future of medicines and the need for partnership
Minister Donnelly stated, “Last year I visited the Texas Medical Centre in Houston. I asked the guy running it what the biggest changes were likely to be to patient care over the next decade. It will come as no surprise to any of you that he referenced personalised medicine – the ability to develop and deliver medicines tailored to individual patients, to identify tiny changes in a patient giving several years warning that they might develop a serious disease, and then intervene to stop it happening.
“Making this a reality, not just for a few wealthy patients, but for everyone, requires a strategic partnership between the State, universities, research institutions and industry. It means ensuring adequate funding from Government is matched with an understanding from industry that there must be value for money in procurement. We need this to protect public funding for new treatments, orphan drugs and initiatives like the new Genetics and Genomics Strategy which I launched in December.”
The appraisal and approval process
The Minister added that he wanted to see improvements in the
transparency of the process and divulged that he has asked the HSE to bring forward proposals in that regard.
“I want to see greater engagement with stakeholders, including patient groups, to ensure that patients are aware of the process and have sight of various steps and timelines,” he said.
“I have asked the HSE to introduce an application tracker on their website, detailing how applications progress. I have also asked the HSE to introduce indicative timelines for a medicine to complete the application/ approval process.”
He added that an Implementation Group is being established between the Department of Health and the HSE to consider and progress the various recommendations contained in the Report as well as other options to improve the process.
“That Group will engage with stakeholders, including patient groups and industry, in seeking to bring about further improvements to the reimbursement process. That Group will also be open to new ideas.”
Future investment
“Of course, an improved and more transparent drug appraisal and approval process only matters if there is funding allocated for new drugs,” said the Minister. “This is something this Government is aware of and has acted on. The combined funding for new drugs in 2019 and 2020 was ¤10m. For 2021 and 2022 it was ¤80m. The full year impact of this ¤80m is
15 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
An Tanaiste Michael Martin TD
IPHA conference
“The Framework Agreements on the Supply and Pricing of Medicines 20212025 are central to the provision of these medicines and the State is committed to on-going investment. Of course, this must deliver real and tangible value for the taxpayer as well as the patient”
¤135m, which is a clear statement of intent from me and Government. The result was 112 new drugs approved, 35 of which are orphan drugs. This year already there are twenty-seven new orphan drugs undergoing pricing and reimbursement assessment.
“The Framework Agreements on the Supply and Pricing of Medicines 2021-2025 are central to the provision of these medicines and the State is committed to ongoing investment.
“Of course, this must deliver real and tangible value for the taxpayer as well as the patient. We have to ensure that the cost of drugs and medicines remains sustainable and that the economic benefits of innovation are realised for all. As you will all appreciate there are growing and extensive demands for enhanced and additional services across the health system, and notwithstanding the unprecedented investment in services by this Government, we must be cognisant that there are finite resources to work with.”
While Ireland’s pharmaceutical industry is one of the biggest producers in Ireland, our country is nonetheless pretty small, he reflected. “As such, partnerships like Beneluxa are critical, particularly when it comes to new medicines for rare diseases. Together we have secured access to Zolgensma for Spinal Muscular Atrophy and work continues with Libmeldy, a treatment for MLD. This is now under joint assessment for reimbursement, and Les Martin and I are in discussions about the process.
“Staying on the international theme, you are all of course aware of The Pharmaceutical Strategy for Europe, published in November 2020. It’s fundamentally about ensuring safe, affordable medicines for all citizens and
patients. It looks to balance this patient-centred approach with protecting the competitiveness and innovative potential of the European pharmaceutical industry.
“I welcome the significant progress that has been made to date including the creation of the Health Emergency Response Authority, the Structured Dialogue initiative and delivery of the Clinical Trials Regulation.
“I know the Commission’s current work on the revision of the general pharmaceutical legislation, and the Orphan and Paediatric frameworks, is of major interest to you all, as it is to Government, and we await the formal publication of the proposal in the coming weeks.
“I expect that the proposal will contain a number of novel policy options to stimulate innovation, including in the area of antimicrobial resistance. These will warrant careful consideration and I know officials in my department will be keen to hear the views of all stakeholders.
“In conclusion, I look forward to continuing to engage with you in the months ahead as we work to ensure that patients in Ireland have timely access to the best medicines, that innovation is fostered and supported and that value for money is secured.”
An Tánaiste, Micheál Martin TD told delegates that as Ireland’s journey has been from one of the poorest countries in Europe, to becoming one of the wealthiest nations in the world, the pharmaceutical industry has been central to this.
“We are now home to nine out of the top ten pharmaceutical companies in the world. We export more than ¤100 billion. More than 42,000 men and women are employed in IDA client companies. We’re home to 90 manufacturing
plants, with 50 of them FDA approved. And more than ¤12 billion has been invested in the industry in the last decade alone.
“It is a phenomenal performance, and I am very pleased to take the chance tonight to acknowledge and pay tribute to it.
“My primary responsibility is to look forward and make the decisions necessary to ensure that Ireland’s story continues to be one of expanding opportunity, better health outcomes for citizens, further sustainable economic growth, social solidarity, and strong, skilled employment.
“And I am confident that your industry will continue to be a big part of all of that,” he said.
Quoting Mark Henry, “The Great Big Miracle of Human Progress comes at a cost” – he went on to add, “Irish public health spending has increased 14-fold over the last 50 years and our state expenditure on pharmaceuticals has doubled in just the last ten years, from ¤1.3bn in 2012, to ¤2.6bn in 2022. It now represents one of the largest components of overall health expenditure and with the growth in demand for high tech and increasingly personalised drugs, the pressure for further increased spending is only going in one direction.
“But our successes as a nation notwithstanding, State resources are finite.
“Together, we need to find ways to enable you to continue innovating and bringing these miraculous products into the world, while at the same time ensuring access to them for as many people as possible while maintaining sustainable public finances. I believe that with good faith and mutual respect among all the parties, we can achieve that.
“Two new Framework Agreements on pricing and supply of medicines were signed in December 2021 with you and with your colleagues in Medicines for Ireland, taking us up to 2025.
These were an important step in reducing prices, improving access for patients and ensuring ongoing State investment in new drugs. The Pharmaceutical Strategy for Europe will be another important dynamic, while my colleague the Minister for Health Stephen Donnelly last week published the Mazars Report. I know Stephen talked to you this morning about what we’re doing with this and that you have welcomed publication of the report.
“I am hopeful that the new Department of Health / HSE Implementation Group that he is establishing to consider and progress the Report’s recommendations will get moving quickly.”
Michael O’Connell, IPHA President said, “The biopharmaceutical industry is Europe’s largest hightech industry. It employs 840,000 people and invests ¤42 billion each year in European R&D. We are calling for Government support to unlock its health, innovation and economic potential so that it can compete on a global scale and reverse a trend of so much investment taking place in the US and China rather than Europe.
“At home, IPHA is intent on working closely with the Government, health authorities and health officials in Ireland to shorten the time that it takes to make new medicines available to patients. We welcome Minister Donnelly’s actions upon the publication of the Mazars Report last week, including the establishment of a Working Group that is open to new ideas, where health authorities, patients’ organisations and industry can work together in new ways to improve the delivery of innovative medicines for patients. IPHA look forward to participating in this Working Group.”
Oliver O’Connor, IPHA Chief Executive added, “We must ensure that EU policies support the innovation and IP rights that are required for the development of new medicines. We need robust IP rights to incentivise investment in the development of innovative new medicines and clinical trials aimed at delivering better outcomes for patients with life-threatening diseases and other serious medical conditions.”

16 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE IPHA conference
Minister for Health Stephen Donnelly
Parkinson’s Disease
World Parkinson’s Day. A reflection on how to move forward in Parkinson’s disease care and research
Written by Diana Angelika Olszewska MD, PhD; Aisling M Ryan, MB, PhD


Affiliations: National Neuroscience Centre, Cork University Hospital, Cork/ University College Cork, Ireland
Corresponding author: diana.angelika.olszewska@gmail.com
We are delighted to contribute an article on Parkinson’s disease to the April issue of the Hospital Professional News Ireland. This is especially as the month of April is dedicated to Parkinson’s disease awareness, and April 11 is designated as World Parkinson’s Day. In this article we highlight different aspects of Parkinson’s disease care. We begin with a historical note, to celebrate the memory of James Parkinson (born on this very day in 1755) and follow with some background information about the disease. We then focus on several special management considerations and conclude with an emphasis on the importance of patients’ education, research, and need for a disease-modifying treatment.
Historical note
While the first descriptions indicative of Parkinson’s disease (PD) can be found both in historic Indian texts dating to 2500 BC and in ancient Chinese texts from 425-221 BC, PD seems to be also described in the non-medical literature: for example, in Shakespeare’s Henry VI: “why dost thou quiver man?” “the palsy and not the fear provokes me”, and other work for example by Leonardo da Vinci who described in his anatomical manuscripts “this appears clearly in paralytics... who move their trembling limbs such as the head or the hands without permission of the soul; which soul with all its power cannot prevent these limbs from trembling.”1-5
However, it was only 206 years ago, in 1817, when James Parkinson (1755-1824), a Londoner, and son of an apothecary and surgeon provided the first comprehensive medical description of the disease. In his Essay on Shaking Palsy comprising five chapters and 66 pages, James Parkinson described six patients (three of whom he observed from a distance) with the disease emphasizing its main characteristics such as insidious onset, slow progression, asymmetry, rest tremor, flexed posture, and festinating gait.
“Involuntary tremulous motion, with lessened muscular power, in parts not in action and even when supported; with a propensity to bend the trunk forward and to pass from a walking to a running pace: the senses and intellects being uninjured” (Parkinson, 1817) 6-7
Fifty years later Jean-Martin Charcot (1825-1893) from Hôpital de la Salpetriere in Paris distinguished an additional feature of bradykinesia in its characteristics:
“their problem relates more to slowness in the execution of movement rather than to real weakness. In spite of tremor, a patient is able to do most things, but he performs them with remarkable slowness . . .” (Charcot, 1887) and renamed the disease as “maladie de Parkinson’s” (Parkinson’s disease).6,8
PD prevalence and diagnosis
PD is the most common cause of parkinsonism (a heterogeneous symptom complex characterized by tremor, bradykinesia, and rigidity), and the second most prevalent neurodegenerative disorder, following Alzheimer’s disease (AD).9
It is estimated that PD affects seven to ten million people worldwide, including 1.2 million of the European population alone. 9 One percent of people over the age of 65, rising to 4% above the age of 85, are affected.10
Approximately 15,000 people in the Republic of Ireland have the disease (Parkinson’s Association Ireland).11 With the aging population, the prevalence of PD is set to double by the year 2030.11 This poses a major socioeconomic burden, as not only direct costs are implicated, but also indirect costs, such as quality of patient’s life, family planning, and consequences of the loss of employment. The annual European cost of brain diseases is estimated at 798 billion euro, while PD alone costs 13.9 billion euro.11-12
PD remains an incurable, progressive condition with only symptomatic treatment available. It
is postulated that multiple factors contribute to its pathogenesis. PD may be triggered by familial and environmental risk factors.11 Most cases are sporadic. Approximately 10% of patients have a family history of PD.12 PD is twice as prevalent in men as in women. It is important to distinguish PD from other forms of parkinsonism such as multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD). However, this poses a significant challenge as the diagnosis of probable PD is clinical, based on the United Kingdom PD Society Brain Bank Clinical Diagnostic Criteria and/or International Movement Disorders Society Criteria, and symptoms between different types of parkinsonism frequently overlap, especially in the early stages when the misdiagnosis rate may be as high as 25% by non-expert secondary care physicians.13-14 While typical PD has an asymmetric, unilateral onset and is frequently accompanied by a typical hand tremor, in atypical parkinsonism the symptoms may be bilateral, and pill-rolling tremor is uncommon. PD is more benign and characterized by a good response to levodopa in comparison to the atypical parkinsonism group where the response to levodopa is poor and progression is much faster. Moreover, red flags for PD such as very early postural instability, or severe autonomic impairment
should be considered as part of the atypical parkinsonism spectrum and are now incorporated into the diagnostic criteria.14 It is also important to differentiate neurodegenerative parkinsonism from drug-induced parkinsonism. While certain drugs such as neuroleptics may either cause drug-induced parkinsonism or unmask neurodegenerative parkinsonism, the former is typically associated with bilateral symptoms. A dopamine transporter (DaT) scan may aid in differentiation between the two, however, it does not help to differentiate between different types of neurodegenerative parkinsonism such as PD, MSA, and PSP.14
Currently, there is no diagnostic test for PD (a biomarker), and a definite diagnosis can only be achieved at a post-mortem examination showing degeneration of dopaminergic neurons in the brain area called substantia nigra pars compacta and build-up of proteinaceous inclusions called Lewy Bodies.15
Selected special considerations:
• Young onset PD
While PD prevalence increases with age, PD can also occur in younger individuals (young onset <50, juvenile onset <21).16 Younger age at onset is more commonly associated with genetic forms of PD. While it is worth considering genetic testing in any patient with
17 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Diana Angelika Olszewska, MD, PhD
Aisling M Ryan, MB, PhD
Parkinson’s Disease
a young onset PD and/or family history of PD, it is also worth noting that genetic test results may be negative. As more genes are being discovered, repeat testing should be considered, particularly as those with specific genetic forms of PD may be candidates for future disease-modifying therapeutic trials. Genetic counseling may be required as the risk of inheritance of several genetic forms of PD is 50%.16
Patients with certain genetic forms, for example, Parkin-PD, may have a more benign disease course than those with an idiopathic PD.16 Patients with Parkin-PD are usually more sensitive to small doses of levodopa/carbidopa and more prone to dyskinesia (involuntary, choreic/dance-like movements), therefore a slow medication titration is warranted (e.g. 50/12.5mg three times daily). While usually preferred to the “OFF” state, dyskinesia may cause embarrassment, and a detailed history should be elicited to assess the patient’s concerns.
Patients with young-onset PD may be at the peak of their professional careers. It is important to minimize the impact of the disease on work and activities of daily living (e.g., tremors on fine motor skills) through careful medication adjustments.
Pregnancy is another important consideration in this group, and while no dedicated clinical trials are addressing this topic, younger individuals may be relatively less affected at the onset of the disease (but not all) and those minimally symptomatic should be withdrawn from all PD medications if possible. Unlike in other conditions, there is no diseasemodifying therapy, and treatment is only symptomatic, therefore, the withdrawal of treatment does not affect the disease course.
In a pregnant patient who is too disabled to be weaned off PD medications, levodopa/carbidopa at the smallest possible doses is considered to be the safest option, but expert opinion should be sought. In fact, the latest review on the topic recommends continuing levodopa/carbidopa throughout the pregnancy as the safest option with less clear data on other available treatment options.17
• An elderly patient with PD
The most important concern in this group surrounds the aspects of daily living and independence. Multidisciplinary approach is an even more vital component of PD management in the elderly than in any other patient group. An occupational therapist can aid in creating a safe home environment, a physiotherapist may aid in fall
prevention and improvement of spatial awareness, and a speech and language therapist in speech exercises and swallowing assessment. Family support is invaluable.
Other special considerations in this group include frailty, polypharmacy, comorbidities, co-existent memory problems, hallucinations, and dementia. Medications should be rationalized as polypharmacy may contribute to falls and result in drug interactions. Importantly, MAOB inhibitors, amantadine, or dopamine agonists should not be used for treatment of PD in the elderly, especially in those with cognitive problems, or dementia (worsening).18 MAOB inhibitors such as rasagiline and selegiline are much less potent as a symptomatic therapy, can contribute to cognitive deterioration (selegiline is metabolized to methamphetamine), and may have serious interactions with other medications. Anticholinergics e.g., trihexyphenidyl should also be avoided in the elderly.18 In case of visual hallucinations, a careful medication history should be elicited (to look for an offending drug). A trial of a low dose of quetiapine can be given along
with practical suggestions such as rearranging the home environment to remove visual cues causing confusion (e.g., removing coats on coat hangers which can look like people)
• Motor and Non-motor symptoms
While motor symptoms of PD such as tremors, slowness, and rigidity are the focus of each clinic visit, and the assessment begins while the patient makes their way into the clinic room, non-motor symptoms may not be as easily recognised. Non-motor symptoms not only contribute to the deterioration of the motor signs/symptoms but are frequently reported by the patients as the primary problem. James Parkinson noted in his “Essay” that the disturbances in sleep and bodily functions associated with bowels, saliva, speech, and swallowing occur in PD.7 The majority of patients with PD suffer from constipation. Daily bowel motion is vital for the appropriate absorption of oral symptomatic therapy. An increase in the dose of oral levodopa/carbidopa in a constipated patient will not provide symptomatic relief until bowel
movement frequency improves. It is common for patients to fear the use of laxatives, and this topic has to be carefully explained. Depression and anxiety are frequent comorbidities. Apathy is very prevalent and needs to be differentiated from the former two.19 While anxiety and depression may require treatment, it is important to note that apathy is part of the condition and has to be recognized in time to avoid overmedicating an apathetic patient with an antidepressant. Drooling of saliva is another nonmotor symptom that may cause major upset for the patient with PD. While oral atropine drops/ scopolamine patches may be useful in younger patients (<65 years old), these should be avoided in the elderly population due to the anticholinergic properties (worsening of cognition, and hallucinations). Three monthly botulinum toxin injections into salivary glands are a good alternative.
the field together toward the discovery of a diagnostic test and the development of a successful disease-modifying therapy in the future.
Non-motor manifestations of PD are presented in table 1. An adequate history must be taken in the clinic to address non-motor symptoms, as their presence may result in the deterioration of motor symptoms/signs of PD.
Table 1
of
(adapted
*in more advanced disease, ** can be due to medication side effects
• Hospitalization
Hospitalization for another cause such as infection, fracture, or a procedure may contribute to
Non-motor
Neuropsychiatric Autonomic Gastrointestinal Sensory Sleep Others
Depression
Orthostatic hypotension*
Anxiety Nocturia, urinary urgency, frequency
Hallucinations, Delusions
Dementia, Cognitive impairment
Obsessive, compulsive behaviors**
Apathy
Siallorhea Pain
Dysphagia, Nausea**
Paresthesia
REM-sleep behavior disorder
Insomnia
Fatigue
Sweating Constipation
Erectile dysfunction*
Olfactory disturbance
Visual blurring
Restless leg syndrome
Vivid dreams
Nocturnal hallucinations
Daytime somnolence
Olszewska DA, Fahn S, Walsh RA, Lynch T. 2016 Neurodegenerative Disorders: A Clinical Guide edited by Orla Hardiman, Colin P. Doherty, Marwa Elamin, Peter Bede, chapter 6
18 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Non-motor manifestations
Parkinson’s disease
from Olszewska et al., 2016)
manifestations of Parkinson’s disease
Table 1 Non-motor manifestations of Parkinson’s disease (adapted from Olszewska et al., 2016) *in more advanced disease, ** can be due to medication side effects
Standard LCIG*
... a reduced levodopa dose can be given
*Levodopa-Carbidopa Intestinal Gel 1. Lecigon Summary of Product Characteristics, 2021.
The same effective and stable plasma levodopa levels are achieved
ABBREVIATED PRESCRIBING INFORMATION. Lecigon 20 mg/ml + 5 mg/ml + 20 mg/ml intestinal gel. 1 ml contains 20 mg levodopa, 5 mg carbidopa monohydrate and 20 mg entacapone. Presentation: Yellow or yellowish-red opaque viscous gel. Indications: Treatment of advanced Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available oral combinations of Parkinson medicinal products have not given satisfactory results. Dosage and administration: Adults/Elderly: Administration by a portable infusion pump directly into the duodenum or upper jejunum via a percutaneous endoscopic gastrostomy(PEG) tube or radiological gastrojejunostony tube. Please consult Summary of Product Characteristics (SmPC) for further information. Only pump Crono LECIG (CE 0476) may be used for the administration of Lecigon. The dose should be titrated to achieve the optimal clinical response in the individual patient, which involves maximising the functional ON-time during the day by minimising the number and duration of OFF episodes (bradykinesia) and minimising ON-time with disabling dyskinesia. Total dose/day of Lecigon is composed of three individually adjusted doses: the morning bolus dose, the continuous maintenance dose, and extra bolus doses. Treatment is usually limited to the patient’s awake period. If medically justified, Lecigon can be administered up to 24 hours/day. The maximum recommended daily dose is 100 ml (2000 mg levodopa, 500 mg carbidopa monohydrate and 2000 mg entacapone. Please consult SmPC for further information. Total morning dose is usually 5–10 ml (100–200 mg levodopa) but not exceeding 15 ml (300 mg levodopa). Continuous maintenance dose is usually 0.7–5.0 ml/hour (15–100 mg levodopa/hour). Extra bolus doses are given if the patient becomes hypokinetic and are normally less than 3ml. An increase in the continuous maintenance dose should be considered if the need for extra doses exceeds 5 doses per day. Please consult SmPC for further information. After initial titration, the morning dose and maintenance dose are fine-tuned over the course of a few weeks. Lecigon is initially given as monotherapy. If needed, other anti-Parkinsonian medicinal products can be taken concurrently. If treatment with other anti-Parkinsonian medicinal products is discontinued or changed, it may be necessary to adjust the doses of Lecigon. Sudden deterioration in response with recurring motor fluctuations may indicate that the tube has dislocated to the stomach. This needs confirmation by X-ray and may require repositioning. Please consult SmPC for further information. The cartridge is for single use only and should not be used for more than 24 hours. Children: There is no relevant indication for use in children and adolescents. Contraindications: Hypersensitivity to the active substances or any of the excipients, narrow-angle glaucoma, severe heart failure, severe cardiac arrhythmia, acute stroke, severe hepatic impairment. Non-selective MAO-inhibitors and selective MAO type A inhibitors are contraindicated and should be discontinued at least two weeks prior to initiating therapy with Lecigon. Conditions in which adrenergics are contraindicated. Previous neuroleptic malignant syndrome (NMS) and/or non-traumatic rhabdomyolysis. Suspected undiagnosed skin lesions or a history of melanoma. Please consult SmPC for further information. Warnings and precautions: Not recommended for the treatment of drug-induced extrapyramidal reactions. Caution in ischaemic heart disease, severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions, past or current psychosis, chronic wide-angle glaucoma, concomitant administration of antipsychotics with dopamine receptor blocking properties or with medicines which may cause orthostatic hypotension. In patients with a history of myocardial infarction who have residual atrial nodal or ventricular arrhythmias, cardiac function should be monitored with particular care during the period of initial dosage adjustments. Monitor all patients for mental changes, depression with suicidal tendencies, and other serious mental changes. Neuroleptic malignant like syndrome with secondary rhabdomyolysis may occur on abrupt dose reduction/discontinuation of Lecigon. Patients should be monitored for the development of impulse control disorders and review of treatment is recommended if such symptoms develop. Patients and caregivers are advised to monitor for melanomas on a regular basis when using Lecigon. Dose may need to be adjusted downwards to avoid levodopa-induced dyskinesia. Periodically evaluate hepatic, haematopoietic, cardiovascular and renal function during extended therapy. Lecigon contains hydrazine, a degradation product of carbidopa that can be genotoxic and possibly carcinogenic. Reported complications for levodopa/carbidopa in clinical studies include bezoar, ileus, implant site erosion/ulcer, intestinal haemorrhage, intestinal ischaemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumoperitoneum and post-operative wound infection. Sudden or gradual worsening of bradykinesia may indicate an obstruction in the tubing system and should be investigated. Weight loss has been associated with the active substances contained in Lecigon, and caregivers should therefore be aware of weight loss. Monitoring of weight is recommended to avoid severe weight loss. Prolonged or persistent diarrhoea that appears during use of entacapone could be a sign of colitis. In case of prolonged or persistent diarrhoea, treatment with the medicinal product should be discontinued and other appropriate medical treatment and investigation considered. Replacement of Lecigon with either levodopa and a DDC inhibitor without entacapone or other dopaminergic therapy may be necessary and should be done slowly. For patients who experience progressive anorexia, asthenia and weight loss within a relatively short period of time, a general medical evaluation including liver function assessment should be considered. Please consult SmPC for further information. Interactions: Antihypertensives, antidepressants, anticholinergics, dopamine receptor antagonists, benzodiazepines, isoniazide, phenytoin, papaverine, sympathicomimetrics , iron, protein-rich diet, amantadine and dopamine agonists (e.g. piribedil) may increase levodopa-related adverse events. Lecigon dose adjustment may be needed when used with these medicines. Lecigon can be taken with MAO type B inhibitors (e.g. selegiline) although serious orthostatic hypotension may occur and the dose of levodopa may need to be reduced. Lecigon may affect metabolism of medicinal products such as S-warfarin and patients should be monitored during initiation with Lecigon therapy when used with this medicine. Please consult SmPC for further information. Pregnancy and lactation: Lecigon is not recommended during pregnancy or in women of childbearing potential not using contraception unless the benefits for the mother outweigh the possible risks to the foetus. It is unknown whether carbidopa and entacapone or their metabolites are excreted in human milk. Breastfeeding should be avoided during treatment with Lecigon. Driving and operation of machinery: Caution; Lecigon can have a major influence on the ability to drive and use machines. Refrain if somnolence and/or sudden sleep episodes occur. Undesirable effects: Weight loss, anxiety, depression, insomnia, dyskinesia, Parkinson’s disease /exacerbation of parkinsonism (e.g. bradykinesia), orthostatic hypotension, nausea, constipation, diarrhoea, pain in muscles and tissues, musculoskeletal pain, chromaturia, fall. Complications of the device and surgery: Postoperative wound infection, abdominal pain, excessive granulation tissue, complication of device insertion, incision site erythema, post-procedural discharge, procedural pain, procedural site reaction. Refer to SmPC for other undesirable effects. Adverse events should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Special precautions for storage: Store in a refrigerator 2°C - 8°C. Do not freeze. Store in the original package in order to protect from light. For storage instructions after first opening of the medicinal product, refer to the summary of product characteristics. Pack size: 7 x 47 ml cartridges. Marketing authorisation holder: LobSor Pharmaceuticals AB, Kålsängsgränd 10 D, SE-753 19 Uppsala, Sweden. Distributed by Clonmel Healthcare Ltd, Clonmel, Co. Tipperary. Marketing authorisation number: PA23144/001/001. Medicinal product subject to medical prescription. A copy of the summary of product characteristics is available upon request or alternatively please go to: www.clonmelhealthcare.ie Last revision date: March 2022. Date of Preparation: October 2022. 2022/ADV/LEC/268H

A combination of three effective Parkinson’s therapies in one convenient gel formulation for intestinal infusion1
Levodopa (20 mg/ml) Carbidopa monohydrate (5 mg/ml) Entacapone (20 mg/ml) Increased levodopa bioavailability with LECIGON®
Parkinson’s Disease
levodopa/carbidopa, others may need much higher doses and the dose should be gradually built up to a total of 1 gram (in divided doses) of levodopa to decide whether the patient is levodopa-responsive or not (a specialist may decide to build it to even higher doses).
• Advanced Therapies
patient’s quality of life, delivery of prognosis, and recruitment into disease-modifying trials. To date, the development of diagnostic tests capable of differentiating PD from similar clinical parkinsonian syndromes has been challenging.
a sudden deterioration in PD. Assessment of both motor and non-motor symptoms is equally important as discussed earlier. Hospitalizations are associated with two main risks:
1. Medications not being given on time or given with meals resulting in increased OFF time and erroneous assessments (a situation when the patient appears to be persistently OFF, the tablet was dispensed, but never actually taken).
2. Medications being abruptly stopped for surgery or when the patient is made nil per os.
Sudden withdrawal of all PD medications may result in a lifethreatening neuroleptic malignant syndrome characterized by fever, altered level of consciousness, severe bradykinesia, and rigidity. Levodopa/carbidopa should be immediately re-started. Morning doses of PD medications should be given pre-surgery and as soon as it is safe to do so after the surgery. Neurology should be consulted for advice regarding the pre- and post-surgical management of complex patients. Patients being made nil per os should be considered on an individual basis. Younger patients without any history of impulsivecompulsive behaviors could be tried on a dopamine agonist patch (rotigotine), while elderly and those with cognitive impairment/ hallucinations should be considered for a nasogastric (NG) tube placement and switched to a levodopa/benserazide dispersible via NG (Madopar Dispersible) (better absorption than the crushed levodopa/carbidopa). The use of the dopamine patch in the elderly may result
in psychosis.18
As a result, antipsychotics, including those contraindicated in PD such as haloperidol, may then be given to counteract the psychosis leading to a chain of undesired events, which could be
avoided by increasing awareness among prescribers. In certain cases, rotigotine patch can be used, but at the lowest possible dose and under close monitoring.
• First treatment conundrum
Levodopa has been the most effective symptomatic treatment for PD for over 60 years, however, its use has been associated with an increased risk of motor complications (MC) in the first five years of treatment (e.g., dyskinesia). 20,21 Dopamine agonists (DA) have a lower MC risk in the early years of therapy and have been considered a good alternative, especially in younger, more prone to MC, patients.22 It has been a frequent practice to use DA to delay levodopa use. However, it is not known if this translates into any benefit later. Not only are DA less effective but they are also associated with significant side effects (sleep attacks, impulsive compulsive disorders, hallucinations, and confusion). In addition, the use of DA does not protect from dyskinesia once the levodopa is added and usually dyskinesia is preferable to patients than the OFF state. This conundrum has recently been investigated by the first author in the only study to date to evaluate the duration between levodopa-first and DA-first treatment and the development of MCs of sufficient severity to warrant consideration of deep brain stimulation (DBS) in 438 patients with PD.23 The duration between the first treatment to the assessment for DBS or DBS surgery itself did not differ between the groups. The results suggested that the development of disabling MCs warranting DBS is independent of the type of first dopaminergic treatment and delaying levodopa may not be indicated.23
It is important to mention that while some patients are sensitive to

Currently available advanced therapies include apomorphine subcutaneous infusion, levodopa/ carbidopa or levodopa/carbidopa/ entacapone intestinal gels and deep brain stimulation (electrodes are placed in either subthalamic nucleus or globus pallidus internus and are connected to an implantable pulse generator located below the clavicle).24-26 DBS parameters can be programmed using a wand to optimize the response. Patients still need to be on symptomatic therapy, but this can be significantly reduced.
Advanced therapies may help reduce unpredictable motor fluctuations by continuous delivery of medication, result in an improvement of OFF symptoms, and help to reduce significant side effect profiles. While DBS used to be considered in more advanced disease, more recently, there has been a shift toward its earlier use. 24-26 While Irish patients have been assessed pre- and post-DBS in Dublin, they still had to travel for surgery itself to the United Kingdom or Northern Ireland until recently when the National DBS Center was established at the Mater Misericoridae University Hospital, Dublin. Advanced therapies are very effective in managing symptoms but are not without possible complications. DBS is a neurosurgical procedure, intestinal gels are delivered through jejunostomy (and require a small procedure), and apomorphine is a dopamine agonist (although very effective as an advanced therapy).
• The role of exercise
In addition to the management of motor and non-motor symptoms, it is important to advise patients about the beneficial role of physical therapy and exercise. Regular exercise positively impacts coordination, spatial awareness, and rigidity in PD.
• Biomarkers and Diseasemodifying trials (research)
Although the research field has been very active, currently, there is no reliable diagnostic biomarker for PD available. As discussed earlier, the diagnosis of probable PD is clinical, heavily relying on the clinician’s skill set. Establishing the correct diagnosis would significantly improve the
Therapy in PD is advancing slowly. So far, all disease-modifying trials to date have failed. This is partially due to the lack of diagnostic biomarkers and the high misdiagnosis rate (and enrollment of misdiagnosed patients into clinical trials). Change of perspective and intense research is vital in developing a diagnostic test for PD.
• Hospital staff and patient education
Education is a crucial step in achieving optimum care for patients.
This applies to nursing staff (medication on time in a busy hospital setting), physicians (assessment of both motor and non-motor symptoms), and patients (improved awareness) alike.
A survey assessing knowledge of PD among 100 PD patients/family members conducted by the first author in one of the Irish hospitals, demonstrated that 30% of patients believed a cure was available, 41% were convinced DBS was curative, and 50% did not know PD could be hereditary. Fifty-one percent of family members mistake apathy for depression (unpublished data). In another survey, PD patients were asked to identify factors worsening their disease. Those with constipation identified constipation as the most important factor (46%), followed by stress, irregular timing of medication, and sleep deprivation; while those without constipation identified stress as a predominant factor (74%), followed by the irregular timing of medication, lack of exercise, and sleep deprivation.28
Conclusion
PD is a complex neurodegenerative condition affecting all ages. The diagnosis remains largely clinical although much work is being done to identify suitable biomarkers. Management of PD requires specialist knowledge of medication and advanced / device-led therapies as well as input from a multidisciplinary team and community rehabilitation facilities. Education, comprehensive patient care, and patient participation in research will help move the field toward discovery of a diagnostic test and development of a successful disease-modifying therapy.
References available on request
20 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
In the treatment of advanced Parkinson’s disease
Because ON TIME is their time1
People in this piece are models, not actual patients.

PRESCRIBING INFORMATION:

Duodopa 20 mg/ml + 5 mg/ml intestinal gel (levodopa/carbidopa)
Refer to Summary of Product Characteristics (SmPC) for full prescribing information.
Presentation: Intestinal gel containing 20mg/ml levodopa and 5mg/ml carbidopa monohydrate.
Indication: Advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results. Dosage and Administration: Adults/Elderly: Administration by portable pump directly into the duodenum or upper jejeunum via a percutaneous endoscopic gastrostomy (PEG) or radiological gastrojejunostomy tube. Initially a nasoduodenal/nasojejunal tube should be considered to determine patient’s response before a PEG-J tube is placed. Where the physician considers this assessment is not necessary, the nasojejunal test phase may be waived and treatment initiated directly with placement of PEG-J. Duodopa is given initially as monotherapy and dose adjusted to optimal response for the individual patient. Total dose/day is composed of three individually adjusted doses: morning bolus, continuous maintenance and extra bolus doses administered over approximately 16 hours. Total morning dose usually 5-10ml (100-200mg levodopa) but not exceeding 15ml (300mg levodopa). Continuous maintenance dose should be between 1-10ml/hr (20200mg levodopa/hr) but usually 2-6ml/hr (40-120mg levodopa/hr). Maximum recommended daily dose is 200ml. Extra bolus doses (if patient becomes hypokinetic during the day) are normally 0.5-2.0ml. Increase maintenance dose if more than 5 extra bolus doses/day are needed. Fine adjustments to the morning bolus, maintenance and extra bolus doses should be made over a few weeks after the initial dose setting. Sudden deterioration in response with recurring motor fluctuations indicates tube may have moved from duodenum/ jejunum into stomach and needs repositioning. Medicine cassettes are for single use only and should not be used for longer than 24 hours, even if some medicinal product remains. Treatment is usually administered during the patient’s awake period. If medically justified, Duodopa may be administered for up to 24 hours. Opened cassettes should not be reused. Children: No relevant use in the paediatric population. Renal/hepatic impairment: No studies on the pharmacokinetics of carbidopa and levodopa in patients with hepatic or renal impairment. Dose titration should be conducted with caution in patients with severe renal and hepatic impairment. Contraindications, Warnings, Precautions etc: Contraindications: Hypersensitivity to ingredients, narrow-angle glaucoma, severe heart failure or cardiac arrhythmia, acute stroke. Conditions where adrenergics are contraindicated (e.g. pheochromocytoma, hyperthyroidism, Cushing’s syndrome). Nonselective MAO-inhibitors and selective MAO type A inhibitors are contraindicated and should be withdrawn at least two weeks before starting Duodopa. Duodopa should not be used in patients with suspicious undiagnosed skin lesions or a history of melanoma. Warnings/Precautions: Not recommended for drug-induced extrapyramidal reactions. Caution in severe pulmonary or cardiovascular disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions, past or current psychosis, chronic wide-angle glaucoma, co-administration with antipsychotics with dopamine receptor blocking properties or with medicines which may cause orthostatic hypotension. In patients with a history of myocardial infarction who have residual nodal or ventricular arrhythmias, cardiac function should be monitored with care during initial dose adjustments. Monitor all patients for development of mental changes, depression with suicidal tendencies and other serious mental changes. Neuroleptic Malignant like Syndrome with secondary rhabdomyolysis has not been reported with Duodopa but may occur on abrupt dose reduction/withdrawal. Periodically evaluate hepatic, haematopoietic, cardiovascular and renal function during extended therapy. Increases in impulse control disorders have been reported and patients should be monitored and reviewed.
Patients and providers are advised to monitor for melanomas on a regular basis when using Duodopa. Ideally, periodic skin examinations should be performed by dermatologists. Dose may need to be adjusted downwards to avoid levodopa induced dyskinesia. Sudden or gradual worsening of bradykinesia may indicate an obstruction in the device and should be investigated. Duodopa contains hydrazine, a degradation product of carbidopa that can be genotoxic and possibly carcinogenic, clinical significance of exposure unknown. Reported complications in clinical studies include abscess, bezoar, ileus, implant site erosion/ulcer, intestinal haemorrhage, intestinal ischaemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumonia (including aspiration pneumonia), pneumoperitoneum, post-operative wound infection and sepsis. Before initiation of treatment, patients and caregivers should be warned of the potential risk of developing Dopamine Dysregulation Syndrome (DDS). Polyneuropathy has been reported in patients treated with levodopa/carbidopa intestinal gel. Before starting therapy evaluate patients for history or signs of polyneuropathy and known risk factors, and periodically thereafter. Drug Interactions: Antihypertensives, tricyclic antidepressants, anticholinergics, dopamine receptor antagonists, benzodiazepines, isoniazide, phenytoin, papaverine, sympathicomimetics, iron, protein-rich diet, COMT inhibitors (e.g. tolcapone, entacapone) amantadine. Duodopa dose adjustment may be needed when used with these drugs. Duodopa can be taken with MAO type B inhibitors (e.g. selegiline) although serious orthostatic hypotension may occur and the dose of levodopa may need to be reduced. Fertility, Pregnancy and Lactation: Limited data relating to the use of levodopa/carbidopa in pregnant women. Duodopa is not recommended during pregnancy. Breast-feeding should be discontinued during treatment with Duodopa. No adverse reactions on fertility have been observed in preclinical studies with carbidopa or levodopa alone. Ability to Drive and Operate Machinery: Caution; Duodopa can have a major influence on the ability to drive and use machines. Refrain from driving/operating machinery if somnolence and/or sudden sleep episodes occur. Side Effects: Very common: Weight decreased, Anxiety, Depression, Insomnia, Dyskinesia, Parkinson’s disease, Orthostatic hypotension, Nausea, Constipation, Fall, Common: Anaemia, Increased weight, Amino acid level increased (Metylmalonic acid increased), Blood homocysteine increased, Decreased appetite, Vitamin B6 & B12 deficiency, Abnormal dreams, Agitation, Confusional state, Hallucination, Impulsive behaviour, Psychotic disorder, Sleep attacks, Sleep disorder, Dizziness, Dystonia, Headache, Hypoaesthesia, On and off phenomenon, Paraesthesia, Polyneuropathy, Somnolence, Syncope, Tremor, Heart rate irregular, Hypertension, Hypotension, Dyspnoea, Oropharyngeal pain, Abdominal distension, Diarrhoea, Dry mouth, Dysgeusia, Dyspepsia, Dysphagia, Flatulence, Vomiting, Dermatitis contact, Hyperhidrosis, Oedema peripheral, Pruritus, Rash, Muscle spasms, Neck pain, Urinary incontinence, Urinary retention, Fatigue, Pain, Asthenia. Device and Procedure Related Adverse Reactions: Very common: Postoperative wound infection, Abdominal pain, Excessive granulation tissue, Complications of device insertion, Incision site erythema, Post procedural discharge, Procedural pain, Procedural site reaction. Common: Incision site cellulitis, Post procedural infection, Abdominal discomfort, Abdominal pain upper, Peritonitis, Pneumoperitoneum, Pneumonia/Aspiration pneumonia, Device dislocation, Device occlusion, Gastrointestinal stoma complication, Incision site pain, Postoperative Ileus, Post procedural complication, Post procedural discomfort, Post procedural haemorrhage. Laboratory values: May change. Prescribers should consult the SmPC for the complete list of reported side effects. HCPs are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Marketing
References: 1. Duodopa® Summary of Product Characteristics, available on www.medicines.ie 2. Nyholm D. The rationale for continuous dopaminergic stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(suppl):S13-S17. doi:10.1016/j.parkreldis.2007.06.005. IE-DUOD-210040. Date of Preparation: January 2023.
Duodopa® is indicated for the treatment of advanced levodoparesponsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results1
Authorisation Number: PA 1824/2/1. Legal Category: POM (S1B). Further information is available from: AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24. Date of Revision: January 2021. PI/2/005 Scan to find out more
You can offer your patients continuous dopaminergic stimulation with Duodopa®1,2
MPOWER Working with Partners
Adam Shanley, Programme Manager of MPOWER
in Ireland, MPOWER adjusted its campaigning to reflect this change in the situation:
liaising with event organizers and venues throughout the city.
The MPOWER Programme at HIV Ireland works with members of the gay, bisexual, and men who have sex with men (MSM) community to design and implement activities to help reduce HIV and other sexually transmitted infections, while also helping to improve the sexual health and well-being of those in its community.
Adam Shanley is Programme Manager of MPOWER and remembers the swift reaction to mpox (monkeypox) in Ireland, well before the first cases were detected in the country.
“When the first cases were notified in the UK and in Portugal, a national crisis management team was convened in Ireland, which included stakeholders from across the health service, including epidemiologists, national and regional public health teams, clinical leads for sexual health and the National Immunization Office. MPOWER was included from the get-go because of its close links to the affected communities, our ability to develop meaningful engagement, and for being able to steer the response from a community perspective.”
Adam believes that trust and collaboration have been major factors in the success of Ireland’s response to mpox, and is proud of the way that authorities view MPOWER as a vital partner in national health responses.
“As a community organization, MPOWER is trusted not only by those most at risk from mpox (men who have sex with men with multiple partners), but our history of successful collaborative working in response to previous disease outbreaks meant that the government and health authorities
already had trust and respect for our expertise when it came to shaping the national response to mpox – also providing the necessary funding to allow us to mount effective responses at key moments in the disease outbreak. Our messaging has also been well tailored, thanks to working closely with another communityled organization, Man2Man, together with the national Health Service Executive.”
Alerting the community to mpox
The first priority of MPOWER at the start of the outbreak was to alert the community to the new disease threat. Calling upon its pool of 50 volunteers, they organized a fullday training course on what was known about mpox, and how to communicate about the risks and ways to prevent it. This formed the basis of their subsequent outreach work.
Everyday throughout May and June 2022 and once a week since then, the volunteers have also helped distribute mpox flyers to every bar, club and sex-onpremises venue they could think of, while gay dating apps, such as Grindr, were also brought on board to carry the organization’s messages on the new health threat.
As Adam explained to us, engaging with sex-on-premises venues was particularly important, as these are often visited by MSM who do not necessarily identify as gay or bisexual, and by a large rural community that travels in to the capital to visit them.
Preparing for Pride Month
With June being Pride Month in Dublin, MPOWER ramped up its efforts and interactions still further,

“Here in Ireland, we’re a small, but very well connected community, ” Adam tells us. “During Dublin Pride, bars and clubs in the city were more than happy to help us and to offer their spaces. We also had great access to all the Pride events, including the massive 3-day Block Party, being able to make use of the big screens they put up during this event and the Pride March to display our mpox messaging.”
Crucially, MPOWER was given access to the email addresses of all those who attended the ticketed events, which then allowed them to follow up with more tailored mpox information.
Promoting informed choices around mpox
Following Pride, the next stage in MPOWER’s communication campaigning focused not only on ensuring that MSM had all the information they needed on mpox, but also on encouraging them to make the right choices, as “agents of their own sex lives”, as Adam explains:
“We wanted to say at this stage in the outbreak ‘look, this [mpox] isn’t going anywhere for now, so here are the symptoms to look out for and here is where you can get tested’. We’ve learned from decades of messaging to our community that telling someone not to do something simply isn’t the right approach. Instead, we needed to promote informed choices around questions, such as ‘what does safer sex look like during the mpox outbreak?’, ‘how can you assess your level of risk?’, ‘what is it that you feel comfortable doing right now?’, ‘could you reduce your sexual partners or perhaps create a ‘sex bubble’?’’
This was reflected in a campaign that was rolled out through posters and leaflets, as well as online, and as Adam is proud to relate, seemed to have a real impact on the MSM community, given the positive feedback they received.
Encouraging vaccination
As mpox vaccines became widely available to the MSM community
“We put out a call to the community that highlighted the criteria for those eligible for vaccination, to allow people to identify themselves as in need of the vaccine – so those with multiple sexual partners, that go to sex-on-premises venues, for instance – to make sure they were first in line to get it.”
Adam explained the aim of the campaign was to get community buy-in for vaccination, while promoting the fact that only those at most risk should come forward for it, given that supplies were limited. This was particularly important given that MPOWER had been working with health authorities to ensure there was a “no questions asked” policy when people arrived at vaccination centres, to avoid feelings of stigma and so as not to deter people from coming forward to get their vaccine.
Next steps in the mpox response
Following intense demand before Christmas, there has been a marked drop in those wanting to get vaccinated, possibly as a result of a sharp decline in mpox cases both in Ireland and across the WHO European Region. MPOWER is now working with epidemiologists and the operations teams in the health service to analyse how the current situation has led to changes in perceptions of mpox and how people behave, to shape the next stage in their campaigning.
Despite there having been no new mpox cases in Ireland for several weeks, Adam believes that now is not the time to be complacent and that there are still groups of MSM out there who remain vulnerable to mpox infection.
“In Ireland, we have a large, vibrant Latin American population, and although MPOWER has 2 Spanish and Portuguese speaking outreach workers, and we have produced communications around mpox in both languages, fewer people from that community are coming forward for vaccination than we had expected. We need to investigate why that is and deal with those barriers. The fact that the online booking system is only available in English or Irish may be one of these, for instance.”
22 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
HIV News

Low CD49d expression in newly diagnosed chronic lymphocytic leukaemia may be associated with high-risk features and reduced treatment-free-intervals
Chronic lymphocytic leukaemia (CLL) is the most common form of adult leukaemia diagnosed in the western world and is characterised by a heterogenous clinical course; up to a third of patients are never treated while high-risk subtypes are chemo-resistant and require expensive targeted therapy.1-3 This heterogeneity is related to the IgVH status as well as mutations of TP53, NOTCH1, SF3B1, BIRC3 and ATM genes.4 Furthermore, the complex interplay between these genes and adhesion molecules has been recognised as a mechanism for establishing niche microenvironments leading to high-risk CLL.5-9
NOTCH1 mutation is associated with upregulation of the integrin molecule CD49d.10 CD49d is the α4 heterodimer of the α4β1 integrin molecule and plays a critical role in leucocyte trafficking, activation and survival through upregulation of BCL-2.11 CD49d expression is detectable by flow cytometry in 35–40% of CLL cases and is clinically associated with bulky lymphadenopathy, reduced treatment-free intervals
(TFI) and reduced overall survival (OS) times.12-14
Using a fluorochrome labelled anti-CD49d monoclonal antibody the standard cut-off for positivity in flow cytometry is ≥30%.15 Subpopulations of CD49d+ CLL cells detectable below the 30% cut-off are identified by distinctive ‘bimodal’ patterns on scatter plot analysis and these small CD49+ subpopulations are reported to have the same prognostic implications as cases with high levels of CD49d expression levels of ≥30%.12
Up to a third of patients with CLL are never treated and do not require the detailed prognostic/treatment defining profiles including FISH, IgVH and TP53 mutational status recommended in iwCLL guidelines prior to initiating treatment.16 Nevertheless, predicting clinical outcomes at diagnosis in a cost effective, robust manner using flow cytometry would enable appropriate follow-up and facilitate accurate patient discussions regarding prognosis.
This multi-centre cross-sectional study aims to define the TFI, clinical and molecular features of newly diagnosed CD49d+ CLL. We aim to compare the findings of those with CD49d expression at the standard level of positivity (≥30%) which we have called ‘HiCD49d’ to cases that express low levels of CD49d (≥10 and <30%) and bimodal distribution patterns which we refer to as ‘LoCD49d’.
METHODS
Study design
Newly diagnosed patients with CLL were recruited from the Trinity St. James's Cancer Institute, University Hospital Limerick and the Midlands Regional Hospital, Tullamore as part of a CLL epidemiology study between October 2017 and September 2018.17 Ethics approval was obtained from institutional ethics committees and informed consent sought for clinical data, CD49d immunophenotyping, mutational analysis (TP53, NOTCH1) and biobanking. Consecutive newly diagnosed
cases of CLL were identified by the central flow cytometry laboratory and were included in the study. Cytogenetic analysis was not performed at diagnosis.
Laboratory characterisation
2.2.1 Immunophenotyping
Performed by the regional flow cytometry service using a 3-laser, 8-colour BD FACS CANTO II flow cytometer with BD Biosciences and eBiosciences fluorochrome labelled monoclonal antibodies to CD19, CD22, CD79b, CD23, CD5, FMC7, SmIg, CD38 and CD49d and the modified Matutes scoring system identified CLL with a ≥4 score.18 CD49d expression of ≥30% were classified as ‘HiCD49d’, CD49d expression between ≥10% and <30% triggered a scatterplot review for bimodal peaks and were referred to as ‘LoCD49d’. ‘CD49d+’ referred to both HiCD49d and LoCD49d. CD49d expression levels of <10% or between ≥10% and <30% with no evidence of bimodality were classified as CD49d
Molecular analysis
TP53 and NOTCH1 mutation analysis was performed on all patients and IgVH analysis on CD49d+ patients. TP53 was analysed using the ThermoFisher TP53 community panel and sequenced on the ThermoFisher S5 next generation sequencer (NGS). All pathogenic variants with >5% variant allelic frequency were reported, as per European Research Initiative on CLL guidelines.19 NOTCH1 analysis was performed by PCR and reported as mutated if >10% mutant alleles were detected. IgVH mutational analysis was performed using the Invivoscribe IgVH Somatic Hypermutation assay kit v.2.0. A Bidirectional Sanger sequencing was performed. Consensus sequences were input into the IMGT/V-Quest database (http:// www.imgt.org/IMGT_vquest) to determine mutational status.
24 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Leukaemia
Written by Smyth Elizabeth, Kelly Aidan, O' Brien David, Waldron Deirdre, Brophy Sarah, Atkinson Emer, Perera Kanthi, Gerard M. Crotty, Walsh Aileen, Connolly Michelle, Clifford Ruth, O'Leary Hilary, Khan Ashique, Christopher L. Bacon, Smyth Emily, Anthony M. McElligott, Quinn Fiona, Vandenberghe Elisabeth, Waldron Carmel
• a These were included separately in the NOTCH1 and TP53 figures.
• b N/A – result not available.
• a These were included separately in the NOTCH1 and TP53 figures.
• b N/A – result not available.
Patient follow-up
Patient charts were reviewed on the 1st of April 2021, 42 months after study initiation to determine if and when treatment was commenced.
Statistics
Chi-square and Fisher's exact tests were used to determine an association between CD49d status and treatment requirement at 42 months, CD38 expression and NOTCH1 and TP53 mutational status. Data was checked for normality based on visual interpretation of histograms and Q–Q plot. For analysis of differences in time to treatment between groups, data was not normally distributed therefore Mann–Whitney test was applied. p values < .05 were considered significant.
RESULTS
Immunophenotype
Eighty-five newly diagnosed CLL case (modified CLL Matutes scores of ≥4) were included. In total 33/85 (38.8%) were CD49d+ comprising of; HiCD49d: 23/33 (69.7%) and LoCD49d: 10/33 (30.3%). 52/85 (61.2%) were CD49d . Ten cases expressed CD49d between ≥10% and <30% and on review of the scatterplots, all 10 had bimodal distribution patterns. 20/85 (23.5%) were CD38+, co-expression of CD38 and CD49d accounted for 11/20 (55%) (CD38+/HiCD49D: 9/11; CD38+/LoCD49D: 2/11) and 9/52 (17.3%) were CD49d (p = .87). See Tables 1 and 2 for complete immunophenotypic data.
Molecular results
NOTCH1 analysis was performed on 81/85 (95.3%) cases of whom 7/81 (8.6%) were mutated, all 7 were CD49d+ (HiCD49d: 3/7; LoCD49d: 4/7) (p ≤ .01). IgVH results were available on 29/33 of the CD49d+ group, of whom 21/29 (72.4%) were unmutated and 8/29 (27.6%) were mutated. TP53 mutational analysis was performed on 83/85 (97.6%) of whom 7/83 (8.4%) were mutated; 4/7 (57.1%) were CD49d+ (HiCD49d:2/7; LoCD49d:2/7) and 3/7 (42.8%) were CD49d (p = .461).
Treatment initiated at 42 months
At Forty-two months, 18/85 (21.2%) patients had commenced treatment of whom 10/33 (30.3%) were CD49d+ compared to 8/52 (15.4%) CD49d patients (p = .209). CD49d+ patients had a
shorter median TFI of 11 months, compared to 21.5 months for CD49d patients (p = .722). See Table 2.
DISCUSSION
Expression of CD49d at the standard positivity threshold of ≥30% has been established by numerous studies as an independent risk factor for an aggressive disease course in CLL, with patients shown to have shorter treatment free and overall survival times compared to those with CD49d negative disease.13, 15, 20, 21 Moreover, recent studies have suggested that the prognostic power of CD49d is preserved at levels of expression below the standard 30% cut-off.12 This study concurs with the prognostic value of CD49d at both conventional and low levels of expression.12 There were no significant differences
25 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Variables Total cohort CD49d+ HiCD49d LoCD49d CD49d− N = 85 N = 33 N = 23 N = 10 N = 52 Median age in years (range) 70 (43–88) 70 (47–88) 68 (47–84) 72.5 (56–88) 70 (43–88) Male:female ratio 1.7:1 2.7:1 3.6:1 1.5:1 1.3:1 Binet stages A/B/C 72/11/2 24/8/1 16/7/0 8/1/1 48/3/1 CD38 positive (%) 20 (23.5) 11 (33.3) 9 (39.1) 2(20) 9 (17.3) IgVH mutated (%) 8/29 (27.6) 8/29 (27.6) 6/21 (28.6) 2/8 (25) N/Ab IgVH unmutated (%) 21/29 (72.4) 21/29 (72.4) 15/21 (71.4) 6/8 (75) N/Ab NOTCH1 mutated (%) 7/81 (8.6) 7/31 (22.6) 3/22(13.6) 4/9 (44.4) 0/50 (0) TP53 mutated (%) 7/83 (8.4) 4/33 (12.1) 2/23 (8.7) 2/10 (20) 3/50 (6) aDual TP53 and NOTCH1 mutations (%) 2/81 (2.5) 2/33 (6.1) 0 2/10 (20) 0 Treatment at 42 months (%) 18/85 (21.2) 10/33 (30.3) 6/23 (26.1) 4/10 (40) 8/52 (15.4)
TABLE 1. Demographics, Binet stages, CD38 expression and mutational status at diagnosis and numbers treated at 42 months
Leukaemia
TABLE 2. Characteristics of those requiring treatment within 42 months of diagnosis
• a Included separately in the NOTCH1 and TP53 numbers. One of these two cases displayed unmutated IgVH, the other, did not have IgVH analysis performed (sample not available).
• a Included separately in the NOTCH1 and TP53 numbers. One of these two cases displayed unmutated IgVH, the other, did not have IgVH analysis performed (sample not available).
found between the HiCD49d and LoCD49d groups with respect to age at diagnosis, gender and highrisk features. The CD49d+ patients displayed clinically aggressive disease with 30% requiring treatment within 42 months compared to 15% of the CD49d group. The median TFI in the CD49d+ group was 11 months compared to 21.5 months in the CD49d group. The LoCD49d patients did have some unique features including a lower level of Binet B disease (suggesting early CLL diagnosis) more mutated NOTCH1 (see paragraph 2) and a shorter TFI which may reflect the small sample size.
Molecular results
LoCD49d subgroups), whereas none of the CD49d patients had a NOTCH1 mutation. The association between NOTCH1 and CD49d expression has been reported with the conventional CD49d expression levels of ≥30% but not in those expressing CD49d between 10 and 30%.9 Although CD49d expression is not a surrogate marker for NOTCH1 mutations they appear to be related. In vitro studies have demonstrated that mutated NOTCH1 appears to upregulate CD49d through the NFkB pathway.9
72.4%. An association between with HiCD49d expression and unmutated IgVH has been previously reported although the underlying pathophysiology remains to be elucidated.13, 23, 25
CD49+ group compared to 40.9% of the CD38 /CD49d+ group suggesting that CD49d is the more useful prognostic indicator.
NOTCH1 analysis was performed on 81/85 (95.3%) cases of whom 7/81 (8.6%) were mutated, all 7 were CD49d+ (HiCD49d: 3/7; LoCD49d: 4/7) (p ≤ .01). IgVH results were available on 29/33 of the CD49d+ group, of whom 21/29 (72.4%) were unmutated and 8/29 (27.6%) were mutated. TP53 mutational analysis was performed on 83/85 (97.6%) of whom 7/83 (8.4%) were mutated; 4/7 (57.1%) were CD49d+ (HiCD49d:2/7; LoCD49d:2/7) and 3/7 (42.8%) were CD49d− (p = .461).
A NOTCH1 mutation was found in 8% of the cohort which is in keeping with the incidence of NOTCH1 mutated cases in newly diagnosed CLL22, 29; interestingly all of the NOTCH1 mutated cases expressed CD49d (in both Hi and
Treatment initiated at 42 months
Unmutated IgVH is found in up to 40% of CLL patients at diagnosis and is a highly predictive, stable, prognostic factor for aggressive CLL but is not performed routinely because of assay complexity and cost.23, 24 The incidence of unmutated IgVH in our series of CD49d+ patients (Hi and loCD49d subgroups) was
CD38 is widely used as a prognostic marker in diagnostic CLL panels and its dual expression with HiCD49d has been reported in 20% of CLL cases.26, 30 CD38+/ CD49d+ cases represented 33% of our CD49d+ series which was higher than the CD38+/ CD49d cohort of 17.3%, which may be accounted for by sample size.26 Though CD38 and CD49d are biologically synergistic, they identify different patient populations with 15% of patients being reported as CD38 / CD49d+ in the literature, the relative merit and interaction of both markers' warrants further study.27,28 Treatment was started at 42 months in 9.1% of the CD38+/
At Forty-two months, 18/85 (21.2%) patients had commenced treatment of whom 10/33 (30.3%) were CD49d+ compared to 8/52 (15.4%) CD49d− patients (p = .209). CD49d+
In conclusion, this study suggests that LoCD49d expression in newly diagnosed CLL patients identifies high-risk disease, displaying increased frequencies of mutated NOTCH1 and unmutated IgVH. Forty percent of the LoCD49d group required treatment within 42 months and had a median TFI of 11 months, similar to those with conventional high levels of CD49d expression. Identifying the LoCD49d group as a high-risk population at diagnosis would help personalise patient education and follow up plans. The outcomes of newly diagnosed CLL patients with LoCD49d expression warrants further large-scale studies to confirm our findings.
26 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
TABLE 2. Characteristics of those requiring treatment within 42 months of diagnosis
Variables CD49d+ HiCD49d LoCD49d CD49d–N = 10 N = 6 N = 4 N = 8 Median age in years, (range) 61 (56–77) 59 (56–66) 71 (56–77) 73 (64–77) Male:female ratio 7:3 5:1 1:1 1:1 Binet A/B/C 5/4/1 3/3/0 2/1/1 5/2/1 CD38+ (%) 1 (10) 1 (16.7) 0 2 (25) IgVH mutated 1 (10) 1 (16.7) 0 0 IgVH unmutated 9 (90) 5 (83.3) 4 (100) 3 (37.5) Mutated NOTCH1 (%) 2 (20) 0 2 (50) 0 Mutated TP53 (%) 4 (40) 2 (33.3) 2 (50) 2 (25) aDual TP53 and NOTCH1 mutations (%) 2 (20) 0 2 (50) 0 Median TFI (months) 11 14.5 11 21.5
Preventing violence against healthcare workers
Pinpoint’s P2 staff attack systems reduce the risk of harm to HSE staff
Last year, figures from the Health Service Executive revealed more than 12 health staff were assaulted every day by patients. The report found that 5,800 HSE professionals were physically abused while at work over the course of 19 months. Consequently, 143 employees made use of the HSE’s Serious Physical Assault Scheme, which entitles staff to six months' leave after an assault.
Violence in the healthcare sector is well documented, with a 2019 NHS staff survey indicating 15% of workers experienced physical abuse from members of the public each year. The Coronavirus pandemic, staff shortages and long delays have only exacerbated the situation.
The persistent threat of abuse in the workplace is associated with poor employee mental health, low morale, reduced job motivation,
and ultimately absenteeism and resignation from the sector. Indeed, the Irish Nurses and Midwives Organisation (INMO) has reported "evidence of premature retirement and resignations from the health service amongst our members following assaults".
More needs to be done to tackle the abuse of healthcare professionals to ensure the wellbeing of staff and quality of care delivered to patients.
P2 by Pinpoint

Pinpoint’s award-winning safety systems have been protecting employees at risk of violence in the workplace for over 30 years. Designed by healthcare workers to meet the specific needs of the sector, Pinpoint’s products are easy-to-use, robust and highly efficient, giving staff the confidence to carry out their caring duties without fear of harm.
And with powerful communication capabilities, the Pinpoint P2 System relays the exact location and nature of an alarm activation to a response team in under 85 milliseconds.

All employees are given individual Personal Infrared Transmitters (PITs), discreet devices that can be worn on the individual or concealed in an ID badge holder. The PITs have two call levels, Assistance and Emergency, which correspond to the severity of the incident in question and can be activated by pressing the respective button on the device. For example, if a patient was to begin showing hostile behaviour, an employee may raise the assistance call by pressing a single button on the back of the Badge PIT. However, if the patient became verbally abusive and threatening, the employee would press the larger button on the PIT
to highlight an emergency. This information is automatically sent to key displays and communication devices, exhibiting the exact location and type of call to response teams.
The responders are therefore able to immediately attend the scene to de-escalate the situation and provide necessary support.
The efficiency of this process prevents assaults on health staff through quick intervention and has even been shown to reduce aggressive behaviour from patients. With the Pinpoint P2 System in place, employees feel reassured of their safety which, in turn, increases staff morale, motivation and productivity, ultimately improving the treatment outcomes of patients.

https://www.pinpoint.ltd.uk/

P2 BY PINPOINT AVAILABLE
27 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 PINPOINT’S AWARD-WINNING STAFF SAFETY SYSTEMS PROVIDE YOU WITH THE SAFEST ENVIRONMENT FOR YOUR STAFF. pinpointlimited www.pinpointlimited.com @PinpointLimited High performance, effective and simple to use staff safety systems that enable your employees to call for assistance or emergency in any situation. Lee Smith, head of Cove Healthcare: “I’ve worked with all manner of safety systems, but none of them come anywhere near Pinpoint.”
NOW Advertorial
Alzheimers News
Alzheimer Association Lunch Debate
The Alzheimer Europe lunch debate of 21 March 2023, focusing on “Dealing with behavioural and psychological symptoms (BPSD) in dementia care”, was a hybrid event attended by MEPs and other representatives of the European Parliament, the European Commission, Health Ministries and other national policymakers, industry partners, researchers, members of the European Dementia Carers Working Group (EDCWG) and Alzheimer Europe member organisations, Board and staff. There were 111 participants in total, with 78 of these attending in person and the remaining 33 joining online. The event was hosted by Sirpa Pietikäinen MEP (Finland), Chairperson of the European
Alzheimer’s Alliance (EAA) and Deirdre Clune MEP (Ireland), ViceChairperson of the EAA.

Ms Pietikäinen opened the session with a warm welcome to all delegates both in Brussels and online. She began by highlighting that over 90% of people with dementia experience BPSD, making this a vast issue and one which can be hugely stressful for carers and others close to the person, as well as for medical professionals and of course for the person experiencing the symptoms. She drew attention to the importance of first understanding the root cause of a problem, before responding accordingly, "as we would do for
a blind person, we should do the same for a person with a brain illness". She also stated that she was proud and delighted to host this lunch debate and introduced the first speaker, Sube Banerjee, Professor of dementia and Executive Dean of the Faculty of Health: Medicine, Dentistry and Human Sciences, University of Plymouth, United Kingdom. Sube Banerjee discussed the impact of agitation and aggression on the well-being of people with dementia and their carers. His talk aimed to put in context some of the issues of BPSD and of qualityof-life for people with dementia. He echoed what Ms Pietikäinen said about how common BPSD are, with 90% of people with dementia experiencing them at some point during their illness. Focusing particularly on agitation, he stated that it was important that dementia should not be the first explanation that we attribute this to. First, we should consider unmet needs, response to a stimulus in the environment, psychosocial needs, responses to the approach of carers, nurses, or other residents. From Prof. Banerjee’s perspective, pharmacological solutions are not the answer, in most situations, as they can make things much worse and rarely solve matters. He shared results from studies
which backed up this assertion and said that we should reduce the use of antipsychotics (which can work to an extent, but lead to increased mortality rates in people with dementia) and of other drugs. Careful assessment, imaginative care planning, information, support, problem solving and working with families is the way forward, he stated. Funding needs to be in place and policymakers and healthcare providers need to be informed and convinced that change is needed. We cannot solve the existing problem overnight, but "there is no rationale for doing nothing", he concluded.
Jacqueline Hoogendam, Coordinator for international affairs on dementia, long-term care and ageing, Ministry of Health, Welfare and Sport, Netherlands gave a talk revolving around improving crisis and care co-ordination, drawing on the recommendations of “Act on Dementia”, the EU Joint Action on Dementia. She agreed with the previous speaker that the situation is not easy to resolve quickly and noted that a multidisciplinary approach is needed. Medication may be needed in some cases, she said, but should not be the first or only choice. There are many differences in BPSD and its causes, she said, listing meaningful activity, feeling respected, informed and listened to, as the main contributing factors to helping prevent these crises.
Meanwhile, the Alzheimer Society of Ireland (ASI) has announced it is to be accredited as a 2023 Triple Lock member by Charities Institute Ireland (CII). Members of CII are given the Triple Lock Standard for exhibiting excellent practices in governance, fundraising, and financial reporting. This certification further assures The ASI’s dedication to excellence, transparency, and accountability to donors and stakeholders.
Commenting on the news, The ASI's Head of Fundraising, Mairead Dillon, said: “We are delighted to have been awarded the Triple Lock status by the Charities Institute Ireland (CII). This demonstrates our commitment to ensuring our donors and supporters can have trust and confidence in ASI and the work that we do to support those living with dementia in our communities.”
28 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Deirdre Clune MEP (Ireland) along with members of the Alzheimer Europe
“Sirpa Pietikäinen MEP drew attention to the importance of first understanding the root cause of a problem, before responding accordingly, "as we would do for a blind person, we should do the same for a person with a brain illness"
DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN
Rebuild bone before it breaks again—with Movymia®1
THE MEDICINES MANAGEMENT PROGRAMME RECOMMENDS
MOVYMIA® AS A BEST VALUE MEDICINE FOR TERIPARATIDE
RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,3,*

EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,4
AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage5,6
RE-USABLE: One high quality reuseable pen for the entire treatment period1
MOVYMIA 20 MICROGRAMS/80 MICROLITERS SOLUTION FOR INJECTION
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teriparatide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance. Website: www.hpra.ie Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject toedical prescription. Date last revised: January 2022.
1. Movymia® SmPC. 2. Best Value Medicines https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/best-value-medicines/teriparatide/ Accessed February 2023.
3. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf
4. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 5. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 6. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo®
Date prepared: March 2023. 2023/ADV/MOV/061H
2
REFERENCES:
KISQALI—the
NCCN RECOMMENDED
National Comprehensive Cancer Network® (NCCN®) now recognizes ribociclib (KISQALI®) as the only CDK4/6 inhibitor in combination with an AI designated as a Category 1 Preferred regimen in first-line HR+/HER2- mBC4,5
KISQALI is not indicated for concomitant use with tamoxifen 4
1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival results from the phase III MONALEESA-2 trial of postmenopausal patients with HR+/HER2− advanced breast cancer treated with endocrine therapy ± ribociclib. Presented at: European Society of Medical Oncology; September 16-21, 2021.
2. Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316.

3. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514-524.
4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.2.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Published February 7, 2023. Accessed February 10, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
5. Kisqali Summary of Product Characteristics. www.medicines.ie; access March 2023.
ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Kisqali (ribociclib) 200 mg film-coated tablets
Presentation: Film-coated tablets (FCT) containing 200 mg of ribociclib and 0.344 mg soya lecithin.
Indications: Kisqali is indicated for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy
In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinising hormone-releasing hormone (LHRH) agonist.
Dosage and administration:
Adults: The recommended dose is 600 mg (3 x 200 mg FCT) taken orally, once daily for 21 consecutive days followed by 7 days off treatment, resulting in a complete cycle of 28 days.
Kisqali should be used together with 2.5 mg letrozole or another aromatase inhibitor or with 500 mg fulvestrant.
When Kisqali is used in combination with an aromatase inhibitor, the aromatase inhibitor should be taken orally once daily continuously throughout the 28-day cycle. Please refer to the Summary of Product Characteristics (SmPC) of the aromatase inhibitor for additional details.
When Kisqali is used in combination with fulvestrant, fulvestrant is administered intramuscularly on days 1, 15 and 29, and once monthly thereafter. Please refer to the SmPC of fulvestrant for additional details.
Treatment of pre- and perimenopausal women with the approved Kisqali combinations should also include an LHRH agonist in accordance with local clinical practice
Management of severe or intolerable adverse reactions (ARs) may require temporary dose interruption, reduction or discontinuation of Kisqali. Please see section 4.2 of SmPC for recommended dose modification guidelines.
Kisqali can be taken with or without food (see section 4.5).The tablets should be swallowed whole and should not be chewed, crushed or split prior to swallowing.
Special populations: ♦Renal impairment: Mild or moderate: No dose adjustment is necessary.







Severe: A starting dose of 200 mg is recommended in patients with severe renal impairment. Kisqali has not been studied in breast cancer patients with severe renal impairment. Caution should be used in patients with severe renal impairment with close monitoring for signs of toxicity. ♦Hepatic impairment: Mild: No dose adjustment is necessary. Moderate or severe: Dose adjustment is required, and the starting dose of 400 mg once daily is recommended.
♦Elderly (>65 years): No dose adjustment is required. ♦Pediatrics(<18 years): Safety and efficacy have not been established.
Contraindications: Hypersensitivity to the active substance or to peanut, soya or any of the excipients.
Warnings/Precautions: ♦Neutropenia was most frequently reported AR A complete blood count (CBC) should be performed before initiating treatment. CBC should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. Febrile neutropenia was reported in 1.4% of patients exposed to Kisqali in the phase III clinical studies. Patients should be instructed to report any fever promptly. Based on the severity of the neutropenia, Kisqali may require dose interruption, reduction, or discontinuation as described in Table 5 (see section 4.2).
♦Hepatobiliary toxicity - increases in transaminases have been reported. Liver function tests (LFTs) should be performed before initiating treatment. LFTs should be monitored every 2 weeks
for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. If grade ≥2 abnormalities are noted, more frequent monitoring is recommended. Recommendations for patients who have elevated AST/ALT grade ≥ 3 at baseline have not been established. Based on the severity of transaminase elevations, Kisqali may require dose interruption, reduction, or discontinuation as described in Table 5 (see section 4.2).
♦QT interval prolongation has been reported with Kisqali. The use of Kisqali should be avoided in patients who have already or who are at significant risk of developing QTc prolongation.
The ECG should be assessed prior to initiation of treatment. Treatment with Kisqali should be initiated only in patients with QTcF values <450 msec. The ECG should be repeated at approximately Day 14 of the first cycle and at the beginning of the second cycle, then as clinically indicated. In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended Appropriate monitoring of serum electrolytes (including potassium, calcium, phosphorous, and magnesium) should be performed prior to initiation of treatment, at the beginning of the first 6 cycles, and then as clinically indicated. Any abnormality should be corrected before the start of Kisqali treatment. Based on the observed QT prolongation during treatment, Kisqali may require dose interruption, reduction, or discontinuation as described in Table 5 (see section 4.2).
Based on the E2301 study QTcF interval data, Kisqali is not recommended for use in combination with tamoxifen ♦Critical visceral disease. The efficacy and safety of ribociclib have not been studied in patients with critical visceral disease. ♦Severe cutaneous reactions
Toxic epidermal necrolysis (TEN) has been reported with Kisqali treatment. If signs and symptoms suggestive of severe cutaneous reactions (e.g. progressive widespread skin rash often with blisters or mucosal lesions) appear, Kisqali should be discontinued immediately. ♦Interstitial lung disease/pneumonitis ILD/pneumonitis has been reported with CDK4/6 inhibitors including Kisqali.
Patients should be monitored for pulmonary symptoms indicative of ILD/pneumonitis which may include hypoxia, cough and dyspnoea and dose modifications should be managed in accordance with Table 5 (see section 4.2) Based on the severity of the ILD/pneumonitis, which may be fatal, Kisqali may require dose interruption, reduction or discontinuation as described in Table 5 (see section 4.2).
♦Blood creatinine increase ribociclib may cause blood creatinine increase – if this occurs it is recommended that further assessment of the renal function be performed to exclude renal impairment.
♦CYP3A4 substrates.ribociclib may interact with medicinal products which are metabolised via CYP3A4, which may lead to increased serum concentrations of CYP3A4 substrates (see section 4.5). Caution is recommended in case of concomitant use with sensitive CYP3A4 substrates with a narrow therapeutic index and the SmPC of the other product should be consulted for the recommendations regarding co-administration with CYP3A4 inhibitors
Pregnancy, Fertility and Lacation
♦Pregnancy: Pregnancy status should be verified prior to starting treatment as Kisqali can cause foetal harm when administered to a pregnant woman.
♦Women of childbearing potential who are receiving Kisqali should use effective contraception (e.g. double-barrier contraception) during therapy and for at least 21 days after stopping treatment with Kisqali. ♦Breast-feeding: Patients receiving Kisqali should not breast-feed for at least 21 days after the last dose. ♦Fertility: There are no clinical data available regarding effects of ribociclib on fertility. Based on animal studies, ribociclib may impair fertility in males of reproductive potential.
♦Effects on ability to drive and use machines Patients should be advised to be cautious when
driving or using machines in case they experience fatigue, dizziness or vertigo during treatment with Kisqali.
Interactions: ♦Concomitant use of strong CYP3A4 inhibitors should be avoided, including, but not limited to, clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir, ritonavir, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, verapamil, and voriconazole. Alternative concomitant medicinal products with less potential to inhibit CYP3A4 should be considered. Patients should be monitored for ARs. If concomitant use of a strong CYP3A4 inhibitor cannot be avoided, the dose of Kisqali should be reduced (see section 4.2 of SmPC). ♦Grapefruit or grapefruit juice should be avoided. ♦Concomitant use of strong CYP3A4 inducers should be avoided, including, but not limited to, phenytoin, rifampicin, carbamazepine and St John’s Wort (Hypericum perforatum). An alternative medicinal product with no or minimal potential to induce CYP3A4 should be considered. ♦Caution is recommended when Kisqali is administered with sensitive CYP3A4 substrates with narrow therapeutic index (including, but not limited to, alfentanil, ciclosporin, everolimus, fentanyl, sirolimus, and tacrolimus), and their dose may need to be reduced. ♦Concomitant administration of Kisqali at the 600 mg dose with the following CYP3A4 substrates should be avoided: alfuzosin, amiodarone, cisapride, pimozide, quinidine, ergotamine, dihydroergotamine, quetiapine, lovastatin, simvastatin, sildenafil, midazolam, triazolam. ♦Caution and monitoring for toxicity are advised during concomitant treatment with sensitive substrates of drug transporters P-gp, BCRP, OATP1B1/1B3, OCT1, OCT2, MATE1 and BSEP which exhibit a narrow therapeutic index, including but not limited to digoxin, pitavastatin, pravastatin, rosuvastatin and metformin. ♦Co-administration of Kisqali with medicinal products with known potential to prolong the QT interval should be avoided such as antiarrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol) and other medicinal products known to prolong the QT interval including, but not limited to, chloroquine, halofantrine, clarithromycin, ciprofloxacin, levofloxacin, azithromycin, haloperidol, methadone, moxifloxacin, bepridil, pimozide and intravenous ondansetron. Kisqali is not recommended for use in combination with tamoxifen.
Adverse reactions: ♦Very common: Infections, neutropenia, leukopenia, anaemia lymphopenia, decreased appetite, headache, dizziness, dyspnoea, cough, nausea, diarrhoea, vomiting, constipation, stomatitis, abdominal pain, dyspepsia alopecia, rash, pruritus, back pain, fatigue, peripheral oedema, asthenia, pyrexia, abnormal liver function tests. ♦Common:, thrombocytopenia, febrile neutropenia, hypocalcaemia, hypokalaemia, hypophosphataemia, vertigo, lacrimation increased, dry eye, syncope, dysgeusia, hepatotoxicity, erythema, dry skin, vitiligo, dry mouth, oropharyngeal pain, blood creatinine increased, electrocardiogram QT prolonged, interstitial lung disease (ILD)/pneumonitis. ♦Please refer to SmPC for a full list of adverse reactions.
Legal Category: POM
Pack sizes: Unit packs containing 21, 42 or 63 FCTs. Not all pack sizes may be marketed.
Marketing Authorisation Holder: Novartis Europharm Limited


Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Marketing Authorisation Numbers: EU/1/17/1221/003 & 005.
Full prescribing information is available on request from Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Dublin 4. Tel: 01 2601255 or at www.medicines.ie
Prescribing information last revised: January 2023
Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 2080 612.
February 2023 | IE253121-1
2023 Novartis
©
A9N6
Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4, D04
only CDK4/6 inhibitor with statistically signi cant overall survival across all 3 phase III trials1–3
2023 UPDATE
CPD CPD
60 Second Summary
Breast conserving therapy (BCT), which encompasses breast conservation surgery (BCS) with adjuvant radiation therapy, has been the standard of care for the majority of newly diagnosed early breast cancer for the last four decades. Outcomes of BCT have been repeatedly validated as an oncologically safe locoregional treatment option in early breast cancer with similar long term survival, reduced psychological morbidity and less medical and surgical complications when compared to mastectomy.
The profile of breast cancers being managed has also evolved with the introduction of breast cancer screening programmes, with an increase in the detection of non-palpable breast lesions. The proportion of small (<2cm) tumours has increased from 36% to 68% of detected breast cancers, with a concurrent rise in DCIS diagnosis, necessitating novel localisation techniques.
The evolution of oncoplastic surgical techniques, combined with a shift towards less conservative margin requirements, has expanded the indications for breast conserving surgery, facilitating this approach in larger tumours.
While adjuvant radiotherapy has up to recently been routinely recommended for all cases of breast conserving surgery, for early breast cancers emerging evidence supports de-escalation in dose fractionation and omission in low risk patients. As deescalation in breast cancer care and personalized cancer care is likely to shape future trends, it is anticipated that the burden of cancer treatment will be reduced, at least for those with early stage breast cancer.
AUTHORS: Emilie McCormack1, Michael Click2, Karl Sweeney1, Aoife Lowery3
1Department of Surgery, Clinical Sciences Institute, University Hospital Galway, Galway, Ireland
2Department of Radiology, University Hospital Galway, Galway, Ireland
3Discipline of Surgery, School of Medicine, University of Galway, Galway Ireland
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author. Novartis has no editorial oversight of the CPD programmes included in these modules.

Recent Advances in Breast Conserving Therapy


Introduction
Breast conserving therapy (BCT), which encompasses breast conservation surgery (BCS) with adjuvant radiation therapy, has been the standard of care for the majority of newly diagnosed early breast cancer for the last four decades. Outcomes of BCT have been repeatedly validated as an oncologically safe locoregional treatment option in early breast cancer with similar long term survival,1-4 reduced psychological morbidity5 and less medical and surgical complications when compared to mastectomy.6
.Diagnostic and therapeutic advances have dramatically improved breast cancer outcomes, now approaching >98% survival at 5 years for those diagnosed at stage I with favourable clinical and molecular features.7 Additionally the improved understanding of tumour biology and standard use of targeted adjuvant therapies, such as anti-oestrogen and antiHer2 therapy, has contributed to these improved outcomes.
Surgery is the mainstay of curative treatment for breast cancer and the evolution of oncoplastic surgical techniques, combined with a shift towards less conservative margin requirements, has expanded the indications for breast conserving surgery, facilitating this approach in larger tumours8 and reducing the requirement for mastectomy to achieve locoregional control (76.6% vs 22.4%).9 The
Early Breast Cancer Trialists
Collaborative Group demonstrated adjuvant radiotherapy to be an essential component of successful BCT to reduce both recurrence and mortality.10 While adjuvant radiotherapy has up to recently been routinely recommended for all cases of breast conserving surgery, for early breast cancers emerging evidence supports de-escalation in dose fractionation and omission in low risk patients.11, 12
The profile of breast cancers being managed has also evolved with the introduction of breast cancer screening programmes,

Full prescribing information is available on www.medicines.ie
Legal category: POM
Further information is available from: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4. Marketing Authorisation Numbers: EU/1/17/1221/003 & 005
and enhancement of diagnostic strategies for breast cancer which have facilitated an increase in the detection of non-palpable breast lesions which necessitate localisation strategies.13, 14
In this review we will summarize the recent advances in early breast cancer diagnosis, localization and BCS and discuss the evidence that has contributed to a de-escalation in locoregional treatment for some breast cancers – and will discuss ongoing clinical trials that have the potential to further evolve the treatment landscape.
Breast Cancer Detection
Breast cancer can either be symptomatic or screen detected at presentation. BreastCheck, the national breast screening programme in Ireland, invites women aged 50-69 years of age to participate with the aim of diagnosis at an early stage where contemporary multimodal therapy will be curative. Patients are screened with digital mammography
31 CPD 98: BREAST CANCER
Emilie McCormack
Continuing Professional Development
March 2023 | IE269061-1
suspected adverse reactions of the medical product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medical product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 208 0612.
Reporting
Karl Sweeney Aoife Lowery
(sensitivity 85%, specificity 90%15) and if mammographic abnormalities are detected, are recalled for further radiological assessment. Assessment has recently incorporated the use of digital breast tomosynthesis (DBT) to aid in cancer diagnosis along with digital mammography and ultrasound. DBT captures multiple Xray projections across an arc that are reconstructed into a stacked image. This enables a three-dimensional view of the parenchyma with more detailed lesion characterisation. DBT is associated with an increased cancer detection rate from 1.2 to 4.6 per 1000 examinations, compared to digital mammography.16 It is particularly advantageous for assessing dense breast tissue and may reduce false recalls.17


With the enhancement in breast cancer detection due to screening and improved diagnostics there has been a subsequent rise in impalpable tumours.13 The proportion of small (<2cm) tumours has increased with screening, from 36% to 68% of detected breast cancers with a concurrent rise in DCIS diagnosis.14
Tumour localisation techniques

The increasing detection of impalpable breast cancers has mandated the evolution of tumour localisation techniques as a surgical adjunct to aid in accurate identification of the tumour intraoperatively. Accurate preoperative
tumour localisation techniques are important to inform optimal surgical approach and technique to achieve clear surgical margins reducing need for reintervention after BCS and decreased locoregional recurrence.18
Wire guided tumour localisation is the most broadly used technique for localisation of impalpable tumours. This approach involves the radiologist inserting a soft wire with the tip anchored in the targeted lesion under image guidance, large tumours can be bracketed, with two wires aiming to delineate the extent of the lesion Fig 1a +1b. There are a number of limitations to wire guided localisation which include risk of wire dislodgement, increased patient discomfort and difficulties in resource management. The localising wire is required to be inserted on the morning of surgery, which may lead to operating theatre delays, and is labour intensive requiring a radiologist performed procedure which is additional to the diagnostic biopsy. These limitations have prompted the development of novel methods of tumour localisation, some of which have recently been adopted into clinical practice.13, 18, 19 Radioactive seeds were introduced in the early 2000’s as an alternative to wire localisation with the primary aim of reducing the risk of wire dislodgement and therefore improving intra-operative localisation accuracy. The seeds can be inserted radiologically at the time of diagnostic biopsy for
radiologically suspicious lesions or ahead of the day of surgery, which can improve theatre flow and patient experience,13 Fig 2a+2b. There is also an improvement in cosmetic outcomes as this approach decouples wire insertion location and surgical incision.13 Magnetic-marker localization (ML) involves the magnetic tracing of a non-ferromagnetic marker coil using a magnetic localizer intraoperatively, with radiofrequency-guided localisation radar reflectors and anchor guided localisation having been introduced as other novel methods.18
Operator dependence in estimating the extent of the lesion at the time of surgery based on imaging increases the risk of positive resection margins.13 Novel surgeon driven localisation techniques have been developed to address this challenge; including intraoperative ultrasound, Radioactive occult lesion localization (ROLL), the intratumoral injection of Technetium99 under ultrasound-guidance with use of a handheld gamma probe to localize the extent of the tumour. Indocyanine green fluorescence works similarly with the use of photodynamic eye to demonstrate the full extent of the tumour. Cryoassisted localisation, which involves the insertion of a cryoprobe under ultrasound guidance in the operating room to create an ice ball around the lesion, has been described as a technique to make the lesion palpable therefore
aiding resection.20, 21 Intraoperative ultrasound can be performed for real time guidance and can be performed by the surgeon or a radiologist, supine magnetic resonance imaging which uses realtime magnetic resonance imaging18 can be used similarly. Table one summaries the different localisation techniques with advantages and disadvantages described.
Davey et al a performed a network meta-analysis of randomised controlled trials comparing localisation techniques in non-palpable breast lesions with 24 RCTS and 4236 breasts.18 The primary outcome measure was margin positivity rate, with secondary outcomes including reoperation rates, duration of operation (minutes), complication rate, cost, specimen characteristics and disease recurrence when clear margins were achieved. Cryo-assisted localisation followed by wire guided localisation had the highest rates of margin positivity of 28.2% and 20.1% respectively with reoperation rates of 18.9% and 17.3%. Intraoperative ultrasound guidance had significantly lower margin positivity and reoperation rates when compared to wire guidance alone, 5.4% and 4.8% respectively.18 The MELODY (Methods for Localization of Different types of breast lesions) (EUBREAST 4) Study is a multicentre prospective cohort trial, currently recruiting, to evaluate different image-guided methods for localization of non-palpable malignant breast lesions that will investigate their safety, clinical effectiveness, with a focus on patient, surgeon, and radiologist preference. NCT 05559411.22
Surgical Excision Margins
Positive resection margins after breast conserving surgery are associated with increased risk of local recurrence,23 as such effective breast cancer surgery requires complete tumor removal and most national and international guidelines recommend reoperation, either in form of re-excision or mastectomy, until clear margins have been reached.24
Fig 1a and 1b: Bracketed wire localisation of lesion for excision.
32 CPD 98: BREAST CANCER
1a, craniocaudal
1b mediolateral
March 2023 | IE269061-1 Reporting
adverse reactions
the medical product
to Novartis and the HPRA. It
continued monitoring of the benefit/risk profile of the medical product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 208 0612. Full prescribing information is available on www.medicines.ie Legal category: POM Further information is available from: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4. Marketing Authorisation Numbers: EU/1/17/1221/003 & 005 A B
view,
view
suspected
of
is important
allows
Re-operation rates vary widely, with population-based studies reporting a range of 15-35%, and the requirement for further surgery can result in increased patient anxiety, delays to adjuvant treatment, poor cosmetic outcome and increased complication rates and costs.25, 26 Therefore, re-operation rate has been included as a quality indicator of breast cancer management in several countries.27
Margin status is typically measured by the pathologist as distance from the tumour to the inked margin. This is classed as involved if there are tumour cells at the inked margin – the edge of the resected specimen.28
There has been a relative paucity of high-level evidence and consensus in relation to specific margin width and its relation to locoregional recurrence with some discrepancy in international guidelines.29-31 Acceptable margin width from early trials varied significantly, being variably described as either as ‘’positive’’ or ‘’negative’’ for gross tumour at specimen edge without classification as to the width1 with others recommending at least 10mm for DCIS.32, 33 The first consensus was published in 2014 by the Society of Surgical Oncology and the American Society for Radiation Oncology to define what margin width minimizes the risk of ipsilateral breast tumour recurrence (IBTR). Recommendations defined a
positive margin – ink on invasive cancer or DCIS as having at least a twofold risk of IBTR but concluded that wider margins than “no ink on tumour” did not significantly lower risk.34 Recommendations included in the NCCP 2015 guidelines on breast cancer management are; for DCIS treated with BCS and adjuvant radiotherapy (RTX), a minimum radial margin of 2mm is acceptable and for tumour not to be touching ink for invasive cancers.29 The most recent recommendations from the National Comprehensive Cancer Network (NCCN) are the same but it is to be noted this is based on data from patients who received whole breast radiation therapy and in the omission or de-escalation of adjuvant radiotherapy the optimal margin width is unknown.30 2018 NICE guidelines on breast cancer management (NG101) however have advised offering re-excision for margins <2mm for invasive cancer and DCIS. Molecular subtype has not been shown to have an impact on margin status and therefore this does not need additional consideration for surgical decision making in relation to margin width.35 However, it is to be noted that triple negative and Her 2 expressing. tumours have an increased local regional recurrence risk compared to luminal tumours.36
A recent meta-analysis comprising 112,140 patients over 41 years with early invasive breast cancer (stage I- III) demonstrated a tumour on ink, or positive margin, distal

recurrence risk of 25.4% and local recurrence of 15.9% compared to negative margin(>2mm) risks of 7.4% and 3.9% respectively as well as overall lower survival rates. Close margins (0.01mm to <2mm) had similar findings in terms of recurrence, the effects of which were not attenuated by the usage of chemotherapy and the authors recommended margins of at least 1mm for both invasive cancer and DCIS and recommended a change to international guidelines based on their findings.28
Margin status and the requirement for clear margins is an important discussion point with patients during their initial consultation for BCS due to the potential 20 -25% reoperation rate caused by compromised margins.37 Recommendations to reduce reoperation rates include specimen orientation on 3 sides, intraoperative specimen radiograph review, the incorporation of oncoplastic techniques to allow a larger specimen size with good cosmesis, cavity shaves and consideration for intra-operative pathologic assessment.38
There are several new proposed techniques and models incorporating technology and machine learning for predicting margin positivity and for pathological assessment. One such study developed a nomogram based on initial diagnosis and surgical factors for predicting positivity in DCIS to
Full prescribing information is available on www.medicines.ie
Legal category: POM
Further information is available from: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4. Marketing Authorisation Numbers: EU/1/17/1221/003 & 005
for
aid clinicians in decision making regarding margins re-excision vs mastectomy with positive success.39 Intraoperative 3D specimen imaging and handheld devices for intraoperative margin assessment have shown early promising results.40
Surgical Technique – the evolution of Oncoplastic Breast Conserving Surgery

The aim of successful BCS is adequate tumour resection with clear margins with an oncologically safe and satisfactory cosmetic outcome for both locoregional control and patient satisfaction.5, 28 Patient factors and tumour factors have to be taken into consideration when planning operative technique as well as patient preference with cosmetic outcome largely relating to the tumour resection size as a percentage of whole breast volume.41 There are some absolute and relative contraindications for BCT including inflammatory BC, metastatic disease, previous breast irradiation, strong family history or BRCA mutation carriers.31
A recent Cochrane review encompassing data from 178,813 patients demonstrated the safety of ‘oncoplastic’ BCS when compared to traditional BCS in terms of both local recurrence and disease free survival.8 Oncoplastic surgery aims to provide optimum effectiveness of surgical resection with plastic surgery reconstruction techniques based on two different principles of volume displacement and volume replacement with the aim of conserving breast shape and size. Oncoplastic techniques allow for excision of large tumours with wide margins with satisfactory cosmetic appearances and oncological outcomes.8 The choice of incision will depend on the tumour size, location, and reconstruction technique, as well avoiding scar visibility in clothing. Cosmetic incisions should be circum-areolar, along langers lines or along maximal lines of tension. Reducing excess skin, to account for the loss of volume, may also be considered.8


Volume displacement techniques include Mastopexy or mammoplasty: Local parenchymal
Reporting suspected adverse reactions of the medical product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medical product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by
208
33
March 2023 | IE269061-1
Fig 2a and 2b: Radiofrequency tag localisation of lesion
excision. 2a, craniocaudal view, 2b mediolateral view
calling 01
0612.
A B
flaps are of importance in both reconstructing the shape of the breast but also in covering the pectoralis to avoid skin tethering caused by radiotherapy. Commonly radial or purse string mastopexy is performed with other variations existing such as racquet handle or batwing mastopexy with improved long term cosmetic outcomes.8, 42 Contralateral breast reduction mastopexy aids surgeons operating on patients with large tumours (>5cm) in large breasts where there may be a struggle to maintain symmetry with simple mastopexy. In this situation the patient undergoes bilateral reduction type surgery through Wise-pattern incisions with the tumour bracketed preoperatively under radiological guidance.43 An important operative step when planning oncoplastic BCT is to clip the cavity at the time of surgery to allow proper targeting for radiotherapy as the volume displacement and incision can make this more challenging.30
Volume replacement techniques include rotational and free flaps with several described in the literature most commonly chest wall perforator flaps.8 The use of implants may be considered, particularly in patients with a smaller cup size and proportionally larger tumours. The best results are obtained with bilateral breast implants, with a smaller implant on the contralateral side.44 Other delayed techniques include autologous fat grafts into defects that can become more apparent with healing and scar formation.45
Radiotherapy

The Early Breast Cancer Trialists Collaborative Group provided evidence demonstrating that patients who had undergone BCS and were treated with adjuvant radiation therapy (RTX) had a 10-year absolute risk reduction of 15.7% for any recurrence, locoregional or distant, and an absolute decrease in breast cancer mortality by 3.8% at 15 years in all comers.10 They did identify that the reduction in recurrence or benefit of radiotherapy varied with age, tumour size, tumour grade, ER status and tamoxifen use10 with recent evidence supporting a shift towards de-escalation for certain subsets of patients in practice in relation to low-risk patients. Recent changes in practice include that patients over the age of 70
with T1 tumours who are ER positive should be considered for partial breast radiation or omission of RTX if treated with endocrine therapy.30 RTX in older women however, where risk of locoregional recurrence may impact quality-oflife, is still a preferred treatment and whole breast radiation therapy plus a boost to the tumour bed is recommended in high grade tumours including tumours >/= T3 or T2 tumours with high risk factors including high grade tumours, extensive lymphovascular invasion or absence of oestrogen or progesterone receptor expression.29, 46 Omission of RTX in T1 AND T2 ER positive tumours in women over 65, is associated with increased local recurrence compared to whole breast RTX (9.5% vs 0.9%) but had no impact in overall survival or distal recurrence.11
Hypofractionation has improved patient convenience and compliance and reduced associated toxicities. Radiation dermatitis is the most common early complication of RTX, with regional therapy a risk factor for late onset lymphoedema as well as cardiac and pulmonary complications.46 Hypofractionation, a higher dose of radiation (>2Gy) delivered in fewer fractions has mature data supporting improved locoregional control, overall survival and cosmetic outcomes with reduced tissue toxicity compared to traditional and has now been recommended as first line therapy.12
The FAST FORWARD trial has demonstrated the non-inferiority of an ultra-hypofractionation regimen (26 Gy in 5 fractions of 5.2 Gy over 1 week versus standard hypofractionation of 40 Gy in 15 fractions over 3 weeks) for local control of the conserved breast or chest wall.47 The TARGIOT-IORT trial, demonstrated noninferiority of intraoperative radiotherapy to traditional external beam RTX with local recurrence rates at five years of 2.11%and 0.95% respectively for patients with IDC <3.5cm size. cN0-N1.48 Despite the proposed benefit of accelerated partial breast radiotherapy over whole breast radiation in limiting the risk of acute toxicities, at metaanalysis of over 16000 patients generally >60 years of age with T1N0 G1 or G2 or DCIS, overall partial breast radiation yielded inferior effectiveness and higher
risk of ipsilateral recurrence but this risk was attenuated when the partial breast radiotherapy was given by external beam radiotherapy with CT planning.49 There are several trials currently evaluating stratification of low risk patients to examine the potential omission of radiotherapy. The PRECISION (Profiling Early Breast Cancer for Radiotherapy Omission) trial is a non-randomized phase II trial (Dana Farber Cancer InstituteNCT02653755), evaluating the omission of whole breast RTX after BCS in breast cancer patients (aged 50–75) deemed at favourable-risk and receiving adjuvant endocrine therapy. The LUMINA study, a multicentric single-arm prospective cohort trial (Ontario Clinical Oncology Group-OCOG), aims to evaluate recurrence risk in postmenopausal women with Luminal A breast cancer as determined by IHC treated with BCS, hormonal therapy and the omission of RTX. Personalisation of adjuvant therapy to incorporate tumour and patient factors will be a critical component of decision-making relating adjuvant radiotherapy following breast conserving surgery going forward.
Future directions in BCT
The future of BCT lies in personalisation and precision, ensuring that each patient has the optimal treatment paradigm based on both tumour and patient factors, incorporating de-escalation of treatment where appropriate.
Technology such liquid biopsy, tumour genomics and molecular profiling may assess targets to improve diagnosis, selection for targeted therapies and disease recurrence.40, 50 Gene expression profiling, such as the Oncotype DXTM score, has established over the last decade that the molecular profile of breast cancer can provide information on recurrence rates with oestrogen receptive, node negative disease.51 The TAILORx trial changed practice that routine adjuvant chemotherapy should be prescribed for all these patients, demonstrating non inferiority of endocrine therapy to chemoendocrine therapy for patients with low to midrange scores (0-25) potentially sparing up to 85% of women
with early breast cancer from adjuvant chemotherapy.52
Surgery has been targeted as another area for potential de-escalation. DCIS, the nonobligative precursor for invasive carcinoma (20-30% progression), is under current guidelines treated similarly to invasive cancer with surgery, radiotherapy and endocrine therapy at considerable cost and morbidity risk considering that for patients with low-risk DCIS they may never develop invasive cancer, thereby possibly representing overtreatment. Active surveillance is routine in other low risk cancers such as prostate cancer but only about 3% of patients with DCIS undergo active surveillance.53 COMET is a phase III randomised trial to determine the risks and benefits of guideline comparative care compared to those of active surveillance for low-risk DCIS (over 40 years of age, Grade I/II DCIS without an invasive component on biopsy, hormone receptive positive, HER 2 negative without a physical mass) LORIS, a phase III trial ran out of the UK has been similarly designed to allow for comparison has finished recruitment and is expected to finish analysis in 2029.53, 54 Even for early stage breast cancers there has been a move to de-escalate surgical intervention with vacuum-assisted excision directed by radiologists, a method currently used to excise certain B3 lesions.55 The SMALL trial has recently finished phase 3 recruitment and this study aims to assess the effectiveness of vacuum-assisted excision in the treatment of screen detected grade one luminal A breast cancers that are radiologically node negative.56
As de-escalation in breast cancer care and personalized cancer care is likely to shape future trends, it is anticipated that the burden of cancer treatment will be reduced, at least for those with early stage breast cancer. It is imperative that this evolution takes place with multidisciplinary collaboration and de-escalation in each oncology discipline is undertaken with consideration of how it may interact with evolving practice in the other disciplines, ensuring that optimal oncologic outcomes are maintained.14, 53
References available on request
34 CPD 98: BREAST CANCER
March 2023 | IE269061-1 Reporting suspected adverse reactions of the medical product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medical product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 208 0612. Full prescribing information is available on www.medicines.ie Legal category: POM Further information is available from: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4. Marketing Authorisation Numbers: EU/1/17/1221/003 & 005
Clonmel Healthcare’s Movymia® (Teriparatide) is recommended as a Best Value Medicine
Effective from 1st March 2023 Movymia® is recommended as a Best Value Medicine for Teriparatide on the High Tech Arrangement.1
Implementation of this recommendation will lead to significant savings for the health service.1
MOVYMIA 20 MICROGRAMS/80 MICROLITERS SOLUTION FOR INJECTION
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breastfeeding. Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teripa¬ratide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of child¬bearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Adverse reactions should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Pack size: 1. A copy of the Summary of Product Characteristics is available upon request or go to www.clonmelhealthcare.ie. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject to medical prescription. Date last revised: January 2022.
Movymia® was launched on the Irish market in 2019, providing increased access for patients to advanced medicines. In February 2023 Clonmel Healthcare received confirmation from the HSE Medicines Management Programme that Movymia has been recommended as a Best Value Medicine for Teriparatide. Movymia® is indicated for the treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Please refer to the Summary of Product Characteristics for further information. Movymia® is administered via a reusable, multi-dosage injection device;
the “Movymia® Pen”. The pen is designed to be used for the entire duration of the treatment.
The recent launch of Movymia® increases access for patients, physicians and pharmacists to affordable medicines. Clonmel Healthcare continues to grow and strengthen its portfolio of Biosimilar medicines.

Martin Gallagher, Director of Marketing / Biosimilars Division said “Since launching this product in 2019 we remain steadfastly committed to bringing a comprehensive range of Biosimilar medicines to the market, thus offering therapeutic options to the prescribing physicians. We are delighted in doing so to contribute to the ongoing savings to the Irish Healthcare system, as well as supporting patient needs”.
1 Best Value Medicines https:// www.hse.ie/eng/about/who/cspd/ ncps/medicines-management/ best-value-medicines/teriparatide/ Accessed February 2023.

35 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 Clinical News
Martin Gallagher, Director of Marketing / Biosimilars Division, Clonmel Healthcare
CARDIOLOGY: TAVI
TAVI updates: complex valve-in-valve cases
Written by Dr Max Waters, Dr Ronan Cusack, Dr Samer Arnous, Dr Cormac O’Connor, Prof Tom Kiernan


3.
4.
2. Vascular access site
The first TAVI was performed in 2002, in France, by Professor Alain Cribier, since then the technology has rapidly expanded. Currently 2021 European Society for Cardiology guidelines give a class 1 recommendation, for the use of TAVI, in patients >75years old or who are high-risk for surgery (based on risk scores: STS-PROM or EUROSCORE). Valve-in-valve TAVI represents a niche percutaneous approach for patients with severe symptomatic aortic stenosis/regurgitation, secondary to degeneration of a previous surgically implanted bioprosthetic valve. Heart team approach to these cases involving, interventional cardiologists, non-interventional cardiologists and cardiothoracic surgeons is international best practise. At these meetings; clinical, anatomical and procedural factors are evaluated to choose the correct valve and approach for each patient. We describe a case below to highlight this concept.

Case Example:
A 71-year-old gentleman had a history of bioprosthetic surgical aortic valve and CABG 12 years prior, presented with NYHA class IV heart failure symptoms. Transthoracic echocardiogram revealed severe left ventricular dysfunction with severe intravalvular aortic regurgitation. Transoesophageal echocardiography (TOE) demonstrated the mechanism of severe aortic regurgitation as degenerative prolapse of the bioprosthetic aortic valve leaflet (Image 1).

During hospitalisation his clinical status deteriorated, he developed fast atrial fibrillation, cardiorenal syndrome, which progressed to cardiogenic shock, requiring haemodialysis and inotropes to
stabilise. Following heart team discussion, a surgical redooperation was considered too high risk, given his co-morbidities with worsening clinic status.



Preoperative CT TAVI was performed, with CT reconstruction of the valvular prosthesis, showed a 21mm magna ease valve (Edwards lifescience) with an inner diameter of 18.4mm (image 3). The coronary ostium was located a safe distance 12mm from the aortic annulus indicating a low risk of coronary obstruction. Using the ViV (valve-in-valve) app, preprocedure calculations estimated 18 atmospheres of balloon pressure to adequately expand the valve using an Edwards Sapien 3 (21mm balloon expandable TAVI). The TAVI was implanted via right common femoral artery approach without complication.

After deployment of the TAVI, there was immediate improvement in blood pressure, and clinical status. Following improvement in clinical status, dialysis was weaned when urine output improved, avoiding long-term dialysis. Follow-up transthoracic echocardiogram post procedure, revealed a well seated valve with no AS/AR, subsequently the patient was discharged home.

TAVI Planning and Imaging
Key to the procedure is ECGgated-CT aorta, which is the gold standard for pre-procedure planning. CT TAVI evaluates:

1. Annular sizing and valve morphology
2. Vascular access site
3. Coronary artery anatomy, including coronary height and risk of obstruction
4. Ascending aortic root angulation and implantation angle
3. Coronary artery anatomy, including coronary height and risk of obstruction
4. Ascending aortic root angulation and implantation angle
2. Vascular access site
3. Coronary artery anatomy, including coronary height and risk of obstruction
and calcification are crucial in determining preprocedural vascular risk, given the TAVI sheath insertion profile is 22fr – 26fr (8.4 – 9.2mm).
In valve-in-valve TAVI planning, the annulus diameter is used for valve sizing, and the landing zone is determined to prevent coronary obstruction. These measurements are used to assess feasibility by comparing them with a TAVI valve-in-valve app which gives information on aortic valve implants from the last 20 years. This guides operators on valve size and the amount of pressure needed to expand the valve within the old bioprosthesis.
4. Ascending aortic root angulation and implantation angle
In valve-in-valve TAVI planning, the annulus diameter is used for valve sizing, and the landing zone is determined to prevent coronary obstruction. These measurements are used to assess feasibility by comparing them with a TAVI valve-in-valve app which gives information on aortic valve implants from the last 20 years. This guides operators on valve size and the amount of pressure needed to expand the valve within the old bioprosthesis. Following this the access point is chosen, most commonly this is the femoral artery as it is the lowest risk for a vascular complication, a key driver of mortality in early TAVI trials. Detailed measurement of vessel diameter, tortuosity and calcification are crucial in determining preprocedural vascular risk, given the TAVI sheath insertion profile is 22fr –26fr (8.4 – 9.2mm).
In valve-in-valve TAVI planning, the annulus diameter is used for valve sizing, and the landing zone is determined to prevent coronary obstruction. These measurements are used to assess feasibility by comparing them with a TAVI valve-in-valve app which gives information on aortic valve implants from the last 20 years. This guides operators on valve size and the amount of pressure needed to expand the valve within the old bioprosthesis. Following this the access point is chosen, most commonly this is the femoral artery as it is the lowest risk for a vascular complication, a key driver of mortality in early TAVI trials. Detailed measurement of vessel diameter, tortuosity and calcification are crucial in determining preprocedural vascular risk, given the TAVI sheath insertion profile is 22fr –26fr (8.4 – 9.2mm).
Conclusion
Conclusion
In valve-in-valve TAVI planning, the annulus diameter is used for valve sizing, and the landing zone is determined to prevent coronary obstruction. These measurements are used to assess feasibility by comparing them with a TAVI valve-in-valve app which gives information on aortic valve implants from the last 20 years. This guides operators on valve size and the amount of pressure needed to expand the valve within the old bioprosthesis. Following this the access point is chosen, most commonly this is the femoral artery as it is the lowest risk for a vascular complication, a key driver of mortality in early TAVI trials. Detailed measurement of vessel diameter, tortuosity and calcification are crucial in determining preprocedural vascular risk, given the TAVI sheath insertion profile is 22fr –26fr (8.4 – 9.2mm).
Conclusion
Conclusion
Following this the access point is chosen, most commonly this is the femoral artery as it is the lowest risk for a vascular complication, a key driver of mortality in early TAVI trials. Detailed measurement of vessel diameter, tortuosity
Valve-in-valve TAVI is a niche procedure with increasing volume. Given the prevalence of aortic valve disease and improvements in both surgical/percutaneous options, it is expected that more patient will outlive their valvular prothesis Careful patient selection at heart team discussions and shared decision making with patients on lifetime risks, are important to fully realise the potential of both surgical and percutaneous valve interventions.
Valve-in-valve TAVI is a niche procedure with increasing volume. Given the prevalence of aortic valve disease and improvements in both surgical/percutaneous options, it is expected that more patient will outlive their valvular prothesis Careful patient selection at heart team discussions and shared decision making with patients on lifetime risks, are important to fully realise the potential of both surgical and percutaneous valve interventions.
Valve-in-valve TAVI is a niche procedure with increasing volume. Given the prevalence of aortic valve disease and improvements in both surgical/percutaneous options, it is expected that more patient will outlive their valvular prothesis. Careful patient selection at heart team discussions and shared decision making with patients on lifetime risks, are important to fully realise the potential of both surgical and percutaneous valve interventions.
Valve-in-valve TAVI is a niche procedure with increasing volume. Given the prevalence of aortic valve disease and improvements in both surgical/percutaneous options, it is expected that more patient will outlive their valvular prothesis Careful patient selection at heart team discussions and shared decision making with patients on lifetime risks, are important to fully realise the potential of both surgical and percutaneous valve interventions.
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 36
Dr Ronan Cusack
Dr Max Waters Dr Samer Arnous Dr Cormac O’Connor Professor Tom Kiernan
2. Vascular access site
Coronary artery anatomy, including coronary height and risk of obstruction
Ascending aortic root angulation and implantation angle
Image 2: CT TAVI coronal view of left ventricle with aorta and bioprosthetic valve
Image 1: TOE showing severe AR
Image 4: CT TAVI of femoral artery and abdominal aorta
Image 3: Bioprosthetic annular diameter on CT TAVI
Image 1: TOE showing severe AR
Image with aorta and
Image 1: TOE showing severe AR
Image 4: CT TAVI of femoral artery and abdominal aorta
Image 3: Bioprosthetic annular diameter on CT TAVI Image 3: Bioprosthetic annular diameter on CT TAVI
Image 2: CT TAVI coronal view of left ventricle with aorta and bioprosthetic valve
Image 4: CT TAVI of femoral artery and abdominal aorta
Image 2: CT TAVI coronal view of left ventricle with aorta and bioprosthetic valve
Image 1: TOE showing severe AR
Image 4: CT TAVI of femoral artery and abdominal aorta
Image 3: Bioprosthetic annular diameter on CT TAVI
CARDIOLOGY: HYPERLIPIDAEMIA Focus on Hyperlipidaemia
Hyperlipidaemia is among the leading risk factors associated with cardiovascular disease. Hyperlipidaemia is characterised by an increase in one or more of the plasma lipids, including cholesterol, cholesterol esters, triglycerides, phospholipids and or plasma lipoproteins including very low-density and low-density lipoprotein along with reduced high-density lipoprotein levels. We recently spoke to Theresa Lowry Lehnen, Clinical Nurse Specialist and Associate Lecturer South East Technological University who told us more about this common condition.
Hyperlipidaemia, in particular elevated LDL cholesterol (lowdensity lipoprotein), is one of the most prevalent risk factors contributing to atherosclerosis and consequent vascular disease. In contrast, high-density lipoprotein (HDL) cholesterol assists in regulating cholesterol levels to prevent imbalances.
Theresa tells us, “Atherosclerosis is a pathologic process characterised by the accumulation of lipids, cholesterol and calcium and the development of fibrous plaques within the walls of large and medium arteries. Hypercholesterolemia and hypertriglyceridemia are the main cause of atherosclerosis which is strongly related to ischemic heart disease (IHD).”
Atherosclerosis often remains asymptomatic until plaque stenosis reaches 70 to 80% of the vessel's diameter. It originates after underlying endothelial damage occurs, which stems from loss of nitric oxide within the endothelium. This process leads to increased inflammation directly around the dysfunctional site, permitting the accumulation of lipids within the innermost layer of the endothelial wall. Theresa adds, “The lipids are then engulfed by macrophages, leading to the establishment of "foam cells" or debris. Cholesterol build-up within the foam cells causes subsequent mitochondrial dysfunction, apoptosis, and necrosis of the underlying tissues. Smooth muscle cells encapsulate the pack of foam cells which produces a fibrotic plaque that inhibits underlying lipids from being destroyed.”
Cholesterol is naturally produced
within the body and can also be obtained from animal fats in food. Cholesterol is absent in plant based foods, however, plant products such as flax seeds may contain cholesterol-like compounds called phytosterols which are beneficial in the body.
She adds, “Cholesterol is an essential substance in the body necessary for the production of cell structures and plasma membranes, energy expenditure and bile formation and it is important for the synthesis of steroids, hormones, vitamin D and other substances. Adults typically synthesise about 1 g of cholesterol per day and the total body content is approximately 35g. The liver is the primary organ that synthesises cholesterol. About 20–25% of total daily cholesterol production occurs in the liver. It is also synthesised to a smaller extent in the adrenal glands, intestines and reproductive organs. The predominant route of cholesterol elimination from the body is by excretion into the bile. Cholesterol from cells is transported from the plasma membranes of peripheral cells to the liver HDLmediated process termed reverse cholesterol transport.”
Noting that the major dietary sources of cholesterol include cheese, egg yolks, beef, pork, poultry, and shrimp, Theresa explains that saturated and tran saturated fats in food raise blood cholesterol.
“Saturated fats are present in full fat dairy products, animal fats, and several types of oil and chocolate. Tran saturated fats are present in hydrogenated oils which are found in many fast and snack foods and fried or baked products.”
Hyperlipidaemia can be subdivided into two broad categories: primary (familial) or secondary (acquired).
Familial hypercholesterolaemia (FH) is an inherited genetic defect that may lead to early development of atherosclerosis and IHD. Homozygous FH is rare affecting 1:160,000 people, while Heterozygous FH is more common affecting approximately 1:250 people.
She continues, “Familial combined hyperlipidaemia may occur as an interaction of

multiple genes and the person’s environment, and occurs in 1:100 to 1:200 people. Secondary hyperlipidaemia accounts for up to 40% of hyperlipidaemia cases and typically originates from an alternate aetiology, such as an unhealthy diet, medications including amiodarone, and glucocorticoids, hypothyroidism, uncontrolled diabetes, and/or a poor lifestyle.
“For most patients, hyperlipidaemia is polygenic and influenced by factors such as obesity, saturated fat intake and the cholesterol content within the person's diet. Another mechanism involves elevated levels of "apo B-100" lipoproteins within the plasma, which may lead to atherosclerotic disease, even when the patient has no other risk factors. There is often a combination of genetic and environmental factors involved that contribute to a person's risk of developing hyperlipidaemia and cardiovascular disease.”
Diagnosis
The diagnosis of Hyperlipidaemia is often an incidental finding following a routine screening by a patients’ General Practitioner or General Practice Nurse conducting a cardiovascular risk assessment.
“Regularly, patients presenting with underlying hyperlipidaemia remain asymptomatic, therefore obtaining a thorough history is essential. It is important to assess a patient’s family history
of cardiovascular disease, hyperlipidaemia, and/or familial hypercholesterolemia, the patient’s diet and exercise habits, tobacco, alcohol or drug use, the presence of coronary artery disease, risk factors or history of CAD; and/or symptoms of peripheral arterial disease or angina.

“A focused physical exam is very important including accurate blood pressure measurements, observing the patients skin for xanthomas, listening for carotid and femoral bruits for evidence of stenosis, listening for an S4 heart sound, and palpating for intact peripheral pulses in all four extremities,” Theresa continues. “Bloods including a lipid profile (fasting) will be carried out which routinely includes LDL, HDL, triglycerides and total cholesterol. Other blood tests may include LFTs, HbA1c and TFTs. Urinalysis can be collected to screen for albuminuria.”
Treatment and Management
The treatment of hyperlipidaemia involves both primary and secondary prevention. Primary prevention concerns people with high cholesterol at risk of CVD with comorbidities such as high blood pressure, type 1 and 2 diabetes or kidney disease , who do not yet have established CVD. Secondary prevention involves treating individuals with established CVD. The European Society of Cardiology and European Atherosclerosis Society (ESC/ EAS) 2019 guidelines for the
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 37
An interview with Theresa LowryLehnen (PhD), CNS, GPN, RNP, South East Technological University
management of dyslipidaemia outline the risk categories and patient groups in each of these categories (figure 1), and intervention strategies for primary and secondary prevention as a function of total cardiovascular risk and untreated low-density lipoprotein cholesterol levels (figure 2).
“Initial treatment modalities are focused on diet and lifestyle modification, with the addition of lipid-lowering medications if needed. Patients with mild hyperlipidaemias and low CVD risk should focus on a low fat, low carbohydrate diet, and moderate to high-intensity physical activity. Heart health diets are low in saturated and trans fats, sodium, added sugars and refined grains.”
Weight loss and regular exercise do not result in significant
CARDIOLOGY: HYPERLIPIDAEMIA
LDL reduction, however, they play an important part in good cardiovascular health and are associated with reduced triglyceride levels. Theresa notes, “Smoking cessation, lowering blood pressure and losing weight are beneficial to lowering vascular disease risk. For patients at moderate to high CVD risk, the above lifestyle modification applies and lipid-lowering "statin" medications should be added. There is a clear and proven benefit to statin therapy for the vast majority of patients from low risk to high risk, therefore, these drugs side effects and costs should be weighed against the individual patient's potential benefit from taking the medication.”
The ESC/ EAS 2019 guidelines for the management of dyslipidaemia outline target plasma LDL

cholesterol levels according to the patients risk level (figure 3)
“Statins are associated with a 30-60% reduction in LDL cholesterol, a reduction of 2040% in triglyceride levels and an increase in HDL cholesterol levels of approximately 3%. Statins are the first-line pharmacological intervention for abnormal lipid profiles. They work by inhibition of the enzyme HMG-CoA reductase, which is involved in the production of mevalonic acid in the cholesterol biosynthesis pathway.
“By preventing the endogenous production of cholesterol, the expression of LDL receptors in liver cells is up-regulated, enhancing the clearance of the circulating LDL-C particles from the blood. Five statins are marketed for use in Ireland; Atorvastatin, Fluvastatin, Pravastatin, Rosuvastatin and
Simvastatin. Atorvastatin is the preferred statin for the treatment of hypercholesterolaemia and prevention of cardiovascular events under the HSE Medicine Management Programme (MMP) guidance.

“The usual starting dose of Atorvastatin is 10mg once daily, and the dose can be adjusted at intervals of 4 weeks or more to achieve the target LDL cholesterol level. In secondary prevention, 30mgs daily should be initiated after diagnosis.
“Prior to commencing cholesterol lowering therapy, baseline cholesterol, LDL and HDL and triglycerides must be measured. TFTs should also be checked and hypothyroidism treated if present before commencing cholesterol reducing medication. LFTs should be measured and statins
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 38
Figure 1: Risk categories and patient groups in each of these categories
ESC/EAS (2019). Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk

should not be commenced if liver transaminases are ≥ 3 times the upper normal limit.”
Theresa adds, “Ezetimibe, co-administered with an HMGCoA reductase inhibitor (statin) is indicated as an adjunctive therapy to diet for use in patients with primary (heterozygous familial and non-familial) hypercholesterolaemia who are not appropriately controlled with a statin alone. PCSK9 inhibitors, Repatha (evolocumab)
and Praluent (alirocumab) are monoclonal antibodies administered subcutaneously which have shown an LDL cholesterol reducing effect of up to 60% when used alone and up to 70% when used in conjunction with a statin.
“They can lower triglycerides by over 25%. PCSK9 inhibitors are associated with reductions in the risk of heart attack and stroke. Repatha and Praluent are both indicated for use in adults over

the age of 18 and Repartha is indicated for use in patients over the age of 12.”
Patient education and information regarding lifestyle modification and pharmacological therapy is the key to success for improved cholesterol control and to prevent complications. Theresa adds that weight management, proper diet, increased physical activity and smoking cessation are important factors to address, to decrease cardiovascular risk.
If pharmacological therapy is required, discussion of the risks and benefits of each medication should be explained to the patient before initiation.
“Primary care clinicians and pharmacist have a duty to educate the patient on medication compliance, side effects, interactions, and overall risks versus the benefits of the medications prescribed. The patient must also understand the potential risks related to
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 39
Figure 2: Strategies for primary and secondary prevention as a function of total cardiovascular risk and untreated low-density lipoprotein cholesterol levels
CARDIOLOGY: HYPERLIPIDAEMIA

not taking the medication and what alternatives they may have as treatment options. Statin medication complications include myopathy, renal injury, arthralgia, extremity pains, nausea, myalgia, elevated liver enzymes/ hepatotoxicity, diarrhoea, and rhabdomyolysis. Up to 5 to 20% of patients taking a statin medication report experiencing a muscle-related intolerance. A lower dose of the statin should be tried or a transition to another lipid-lowering medication such as ezetimibe or a PCSK9 inhibitor such as evolocumab or alirocumab attempted.
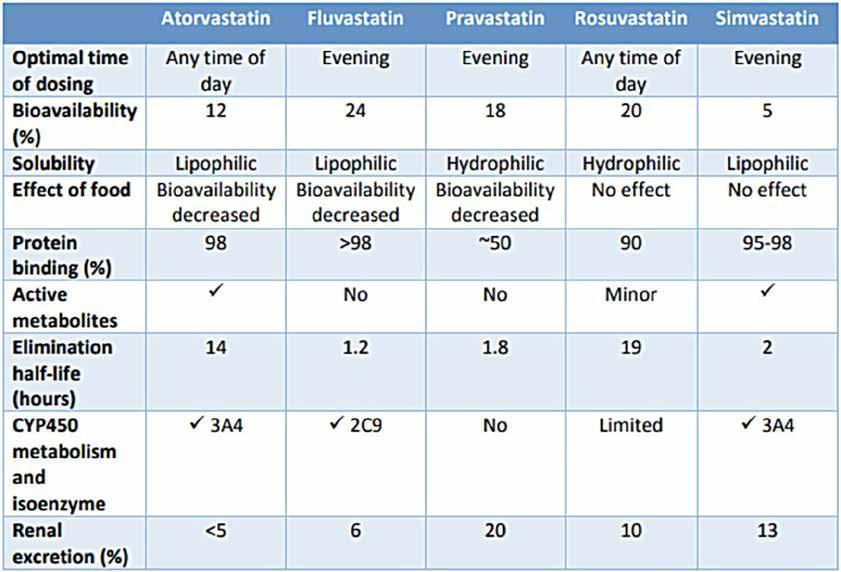
“Based on a 20 year follow up of the "West of Scotland Coronary Prevention Study", patients who received statin therapy for five years demonstrated improved survival rates and a clinically significant reduction in cardiovascular disease over the 20 year period. This data and numerous other studies and clinical trials have shown a significant cardiovascular risk reduction when statin therapy is used appropriately.”
She concludes, “Patient should be monitored regularly in practice to prevent hyperlipidaemia progression and improve patient outcomes. Patients receiving lipid lowering medications should be reviewed after three months of initiating treatment and then at least once yearly. In those receiving statin therapy, liver function needs to be assessed three months after commencing therapy and again at 12 months. Patients should be monitored for medication compliance, tolerance, adherence and adverse effects. If hyperlipidaemia is left untreated or undertreated, the condition is progressive and can lead to severe cardiovascular disease, which can be fatal.”

APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 40
Figure 3: Target LDL-cholesterol level according to CVD risk profile
ESC/EAS (2019). Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk
Figure 4: Pharmacokinetic properties of statins HSE (2020). Medicines Management Programme Preferred Drugs. Statin monotherapy for the treatment of hypercholesterolemia and prevention of cardiovascular events in adults.
Association of statin induced LDL cholesterol reduction and cardiovascular outcomes
Background
Statins are one the world’s most commonly prescribed drugs and by 2020, global sales were estimated to have reached approximately $1trillion. They were first approved for patients at high-risk of cardiovascular disease in 1987. Their approval and subsequent use was on the basis that the accumulation of low-density-lipoprotein-cholesterol (LDL-C) in blood vessels walls is a causative factor in the development of atherosclerosis and hence, cardiovascular disease (CVD). Since the 1980s, iterative clinical practice guidelines for the prevention of CVD have tended to expand the proportion of patients who are potentially eligible for statin treatment. Doctors are now encouraged to aggressively lower LDL-C both in patients both with and without a history of CVD and, in Ireland, about one in three adults over the age of 50 take statins.
The expansion in statin utilisation has been partly driven by assumption that the lower a patients LDL-C, the better. For example, the 2018 American Heart Association guidelines note that LDL-C is the ‘primary cause of atherosclerosis’ and promote ‘the general principle that the lower the better for LDL-C’. This loglinear relationship between LDL-C and cardiovascular outcomes has been reported in a series of meta-analyses by the Cholesterol Treatment Trialist’s (CTT) Collaboration and others.
Limitations of current analyses
While such analyses are highly influential, particularly in informing clinical guidelines, they are subject to a number of serious limitations. Firstly, the studies by the CTT are based on individual patient data which are inaccessible to independent researchers.
Secondly, the relative risk reduction (RRR) from taking statins is often described in terms of reductions in a ‘composite outcome’. A composite outcome is a combination of a number of cardiovascular outcomes which is given a title, for example, ‘major vascular events’. However, reported RRRs in composite outcomes may be
associated with reductions in potentially subjective outcomes, such as revascularisation or hospitalisation, the frequency of which may depend on opinions or preferences of the attending doctor, rather than arguably more objective outcomes such as all-cause mortality, myocardial infarction [MI], or stroke. Reporting composite outcomes may give misleading impressions of the effect of treatment.
Thirdly, reporting RRRs in cardiovascular outcomes without reporting the associated absolute risk reductions (ARRs) can be misleading. RRRs can inflate the importance of a clinical intervention and may exaggerate trivial associations.
What we did
To facilitate shared-decisionmaking between patients and their clinicians, we undertook a reanalysis of the seminal statin trials. We decided to focus only on the ‘hard’ clinical outcomes of all-cause death, MI and stroke, (reporting absolute as well as relative risk reductions) and to explore the association between the degree of LDL-C lowering from statins and these clinical outcomes.
We undertook a systematic review and meta-analysis of statin trials and published our paper in JAMA Internal Medicine in March 2022.
What we found
'We included 21 statin trials in our analysis. We appraised the quality of these studies and found them to be largely at low risk of bias.
We conducted a meta-analysis on 19 of the 21 trials that reported data on all-cause mortality and 18 trials reporting data on MI and stroke. The ARR was 0.8% for all-cause mortality, 1.3% for MI, and 0.4% for stroke. The RRR for all-cause mortality was 9%, 29% for MI, and 14% for stroke for the groups randomized to receive statin therapy compared with placebo or usual care.
We undertook meta-regressions to explore the potential mediating association of LDL-C reduction with relative and absolute treatment effects. Our findings were inconclusive, indicating very little, if any, association between
Written by Dr Paula Byrne, Senior post-doctoral researcher, University of Galway

the magnitude of LDL-C reduction and size of the treatment effect.
Discussion
Our results suggested a benefit from taking statins in all clinical outcomes. However, this benefit was modest, particularly when considering ARR rather than RRR. Reporting only RRR has been described as ‘the first sin against transparent reporting’ as it may increase people’s willingness to receive a treatment, advise treatment, and pay to prevent the risk compared with ARR or other methods for communicating risk. Some patients who experience harms might choose to discontinue their use of the drug if they were counselled about the absolute degree of risk and benefit. However, some studies report that doctors tend to ‘succumb to the lure of relative risk reports’ and recommend treatment more on the basis of relative rather than absolute risk reductions.
In addition, the degree of benefit for an individual depends on that person’s baseline risk. The online
algorithm QRisk, for example, calculates a person’s 10-year risk of heart attack or stroke based on factors such as blood pressure, sex, age, smoking status among others. For example, an overweight 65-year-old man who smokes, has high blood pressure and total cholesterol may be at high risk of cardiovascular disease, compared with a 45-year-old, non-smoking woman with slightly raised cholesterol and blood pressure and no other risk factors. If a doctor were to assess their risk of dying in the next ten years, the estimated risk for the man might be 38%, for example, whereas the woman’s risk might be only 1.4%. Now consider the impact of taking statins for both. According to our study, statins would reduce the relative risk of dying by 9%. In absolute terms, the man would reduce his risk from 38% to 34.6%, and the woman from 1.4% to 1.3%.
For all patients, but particularly those at low risk of CVD, the decision to take statins should include consideration of potential benefits and harms and there has

HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 41
CARDIOLOGY: STATINS
“Our results suggested a benefit from taking statins in all clinical outcomes. However, this benefit was modest, particularly when considering ARR rather than RRR”
been vigorous debate about the extent of harms from statins. For example, another CTT analysis reported that the risk treating 10000 patients with statins for 5 years could result in 5 cases of myopathy, 50 to 100 new cases of diabetes, and 5 to 10 cases of haemorrhagic stroke. However, observational studies have estimated much larger rates of muscle pain, (although these types of studies are considered lower certainty evidence compared with randomised controlled trials (RCTs)). For example, Buettner et. al reported that 22% of those taking statins in their study reported musculo-skeletal pain in at least one anatomical region in the previous 30 days compared with 16.7% of those who did not use a statin. In addition, some have argued that the CTT definition may be a high bar for diagnosing muscle symptoms among statin users who may simply define myopathy as any muscle symptom. More recently, researchers from the University of Sheffield undertook a study of people who had discontinued statins because of pain. This was a blinded N-of-
CARDIOLOGY: STATINS

1 study design. An N-of-1 is a randomised trial of an individual patient designed to determine the best course of action for an individual patient. Participants were randomised to a sequence of six double-blinded treatment periods during which they received either statin or placebo daily. The study found that fewer muscle symptoms were reported during the period when people were on statins than on placebo. However, there are limitations to this study design including the potential carry-over of statin related adverse effects from the statin period into the placebo period and whether aggregation of a number of N-of-1 studies is methodologically valid. Thus, there is still considerable uncertainty regarding muscle symptoms in statin users. High-quality RCTs designed specifically to measured adverse effects would be required, the results of which should be open to independent scrutiny.
In our analyses we considered the association between LDL-C reduction and individual ‘hard’ outcomes of all-cause mortality,
MI, and stroke rather than focusing on the composite outcomes reported in other meta-analyses. We did so because of the inherent problems associated with composite outcomes including the fact that they can be inconsistently defined and inadequately reported. In addition, it can be difficult to quantify the components within a composite. Mora et al. for example, examined the outcome ‘total CVD events’ in a statin trial called JUPITER and found that women had a significant reduction in revascularisations and unstable angina, but not in other components of the composite outcome, including stroke.
Patients’ and prescribers’ decision making may be influenced by seemingly impressive reductions in a composite outcome even though larger treatment effects may be associated with the less clinically important components.
Current clinical guidelines for prevention of CVD assume a log-linear relationship as I noted already. In contrast, our metaregression analysis yielded inconsistent results, thus we could neither prove nor disprove an association between the magnitude of LDL-C reduction and the size of treatment effect.
Limitations
In our analysis, we reported significant clinical heterogeneity among the included trials. The trials differed in terms of the type and dose of statins, compliance with treatment, base-line levels of LDL-C, population characteristics, study duration, control treatment and definition of outcomes.
We also identified statistical heterogeneity. As a result of these limitations our calculations of ARR and RRR must be interpreted with caution, as do those of all prior analyses based on these trials. These issues have received little of no attention in prior meta-analyses of statins, many of which form the basis for clinical guidelines.
In addition, there has been some recent debate as to whether LDL-C is the most appropriate metric for measuring atherogenic risk; instead the concentration of Apoprotein B (ApoB) may be more reliable. However, LDL-C was the metric used in the design of the 21 statin trials we analysed, whereas few reported ApoB levels. In addition, ApoB is not routinely measured by doctors or used to inform decisions to prescribe statins.
Conclusion and future research
Our study found modest reductions in all-cause death, MI and stroke associated with statin use in terms of ARR. We argued that patients and their clinicians need to be clear on the absolute reduction in clinically important outcomes in order to make informed decisions about statins. In addition, we failed to demonstrate a linear relationship between the degree of LDL-C reduction and these clinical outcomes. Thus, our findings have implications for the individual patient and their doctors, for the development of future clinical guidelines and for policy makers and payers considering the opportunity cost of statins.
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 42
STATIN
lipid-lowering drug
“…..there is still considerable uncertainty regarding muscle symptoms in statin users. High-quality RCTS designed specifically to measured adverse effects would be required, the results of which should be open to independent scrutiny”
Link between Depression and Stroke
Researchers at University of Galway have found that people with symptoms of depression were more likely to suffer an acute stroke and have a worse recovery afterwards.
The findings come from a new INTERSTROKE study, published in Neurology®, the medical journal of the American Academy of Neurology.

INTERSTROKE is a global study of 26,877 adults with an average age of 62, across 32 countries including participants in Europe, Asia, North and South America, the Middle East and Africa. Participants with stroke were matched to controls who had not suffered a stroke, but were similar in age, gender, racial or ethnic identity.
Dr Robert P Murphy was the study author and consultant Stroke Physician and researcher at the College of Medicine, Nursing and Health Sciences at University of Galway.

Dr Murphy said, “Depression affects people around the world and can have a wide range of impacts across a person’s life. Our study provides a broad picture of depression and its link to risk of stroke by looking at a number of factors including participants’ symptoms, life choices, and antidepressant use. Our results show depressive symptoms were linked to increased stroke risk and the risk was similar across different age groups and around the world.”
Dr Robert P Murphy
The INTERSTROKE study found:
• Of study participants, 18% of those who had a stroke had symptoms of depression compared to 14% of controls who did not have a stroke.
• After adjusting for age, sex, education, physical activity and other lifestyle factors, people with depressive symptoms before stroke had a 46% increased risk of stroke compared to those with no depressive symptoms.
• The more symptoms of depression participants had, the higher their risk of stroke.
• Participants who reported five or more depressive symptoms had a 54% higher risk of stroke than those with no symptoms.
• Those who reported three to four depressive symptoms and those who reported one or two symptoms of depression had 58% and 35% higher risk, respectively.
• While people with symptoms of depression were not more likely to have more severe strokes, they were more likely to have worse functional outcomes one month after the stroke than those without depressive symptoms.
Professor Martin O’Donnell, Professor of Neurovascular Medicine at University of Galway and Consultant Stroke Physician at Galway University Hospitals, co-led the international INTERSTROKE study in partnership with Professor Salim Yusuf from the Population Health Research Institute at McMaster University, Canada.
Professor O’Donnell added, “The goal of INTERSTROKE
is to better understand the importance of risk factors for stroke in different regions of the world and impact of stroke. In the INTERSTROKE study we have previously examined the roles of hypertension, alcohol, lipids and psychosocial stress as global determinants of stroke risk. The current analysis provides deeper insights into the association of depressive symptoms with stroke risk, reporting an increased risk. These analyses suggest that effective identification and management of depression may also be associated with reduce stroke risk, although the observational nature of the study does not permit definitive conclusions.”
Neurorehabilitation teams required for Stroke patients
The Neurological Alliance of Ireland (NAI), of which the Irish Heart Foundation is a member, held a briefing event last month at Leinster House, for Oireachtas members, where a fresh appeal was made for the implementation of the full-scale community neurorehabilitation teams promised by Government in 2019, for the 800,000 people in Ireland living with neurological conditions, many of whom have experienced stroke.
Currently, there are only two community neurorehabilitation teams in Ireland, despite nine teams being recommended, one
for each CHO around the country, in the Implementation Framework (2019-2021) for the National Neurorehabilitation Strategy. This means that only 15% of neurological patients in Ireland have access to teams that provide vital care to support their recovery. The neurorehabilitation teams in the community would provide crucial, specialist therapy supports, including physiotherapy, speech and language, and occupational therapy, as well as neuropsychology for adults with neurological conditions living in the community.
For people living with acquired conditions such as stroke and brain injury, these teams allow them to leave hospitals more quickly to continue their recovery at home, reducing the need for longer-term care while receiving necessary care close to where they live.
Dr Rachael Doyle, Consultant in Geriatric Medicine at St. Vincent’s University Hospital, and Chair of the Irish Heart Foundation Stroke Council was one of a panel of four medical consultants in attendance, advocating for these centres at the event. Irish Heart
Foundation Advocacy Campaign Manager, Pauline O’Shea and stroke patient advocate, Fiona Bardon, represented the organisation’s stroke patients on the day.
The event concluded with politicians, including Social Democrats TD, Roisin Shorthall and Sinn Fein TD, Louise O’Reilly agreeing that supporting this endeavour and implementing these centres as soon as possible was vital.
More information on the Patients Deserve Better campaign can be viewed here.
#PatientsDeserveBetter
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 43 CARDIOLOGY: NEWS
CARDIOLOGY: REHABILITATION

The Role of the Dietitian in Cardiac Rehabilitation
beliefs should also be taken into account. Thus, clinicians should incorporate a shared decisionmaking strategy to find sustainable and healthy alternatives that patients will be able to adhere to.
Cardiac Rehabilitation and Nutrition
Cardiac rehabilitation (CR) is a continuous process of care which is commenced in hospital. It is a supervised programme which helps in the improvement of cardiovascular health and is proven to be effective in the secondary prevention of cardiovascular disease. Whilst supervised exercise is an essential part of CR, this programme is a multicomponent model of care (as seen in figure 1) which encompasses different

aspects such as nutrition and psychological counselling.

Nutrition counselling is a core component of the prevention of non-communicable diseases including cardiovascular disease. Recent evidence also highlights the importance of implementing a team-based approach to delivering dietary interventions, with the dietitian’s educational input supplemented by other members of the multidisciplinary team (MDT). For example, the reinforcing of key messages by other CR staff may increase the likelihood of adopting new dietary habits. Furthermore, while we should strive to deliver care in accordance with current clinical practice guidelines, it is equally important to communicate nutritional information in a way that
Nutrition and Cardiovascular Health
Cardiovascular disease (CVD) is the leading cause of death in Western countries, representing almost 30% of all deaths worldwide. Over the past decades, there have been a number of high-quality studies evidencing a strong association between the effectiveness of healthy dietary patterns and lifestyles for the prevention of CVD. A diet with a poor quality has been linked with increased risk of cardiovascular disease morbidity and mortality. In contrast, a heart-healthy diet includes maximising the consumption of vegetables, fruits, oily fish, wholegrains, and legumes and reducing the consumption of saturated fats, red meats, and refined carbohydrates. Due to interactions between different food groups and nutrients, dietary recommendations for the prevention of CVD should be primarily focused on healthy dietary patterns rather than single food groups or nutrients.
The Mediterranean and the DASH diets in particular have been associated with a lower risk of mortality from CHD and CVD. These dietary patterns have been linked with a reduction in blood pressure, LDL cholesterol levels and overall cardiovascular risk. However, it is important to note that there are different types of diets, and patients’ own needs/
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 44
Written by Ms. Nada Akl, staff-grade dietitian working in Beaumont Hospital covering the areas of cardiology, endocrinology and stroke cover
Figure 1: Components of cardiac rehabilitation
is easily accessible to patients and takes into account varying degrees of health literacy. Therefore, the delivery of effective dietetic interventions as an integral part of the CR programme is essential.
A recent systematic review conducted in 2023 demonstrated significant changes in dietary intake outcomes after completion of cardiac rehabilitation, but also cautioned that there are few high-quality studies that can reliably inform clinical practice. For example, many interventions were reported in a manner that does not allow replication in routine care. To advance the area of nutrition in CR, we need more proven interventions that effectively improve diet quality.
Beaumont Hospital
As part of a comprehensive 10week CR programme in Beaumont Hospital, patients participate in an interactive group session delivered by the dietitian, which is informed by the patients’ blood test results (all patients have their bloods checked pre- and post-CR). Educational content is delivered in a discursive manner and patients are encouraged to play an active role in this process. Several topics are discussed including explanations of medical terms patients often encounter and their association with the diet (e.g. LDL and HDL cholesterol, triglycerides and hypertension). Different food groups are also examined whilst giving patients the opportunity to ask any questions throughout the session. This allows for confusing and/or seemingly contradictory advice
around nutrition to be clarified. While the dietitian’s role also includes a focus on behavioural change, this may be supported by other members of the MDT including the psychologist, nurse and pharmacist (e.g. weight management, relapse prevention and relevant drug interactions). Food labelling is also explained to patients. A guiding principle is that the path to healthy eating starts from what we buy and therefore, by understanding nutritional information on products, this allows patients to have sufficient knowledge to make informed decisions about their food choices. Accordingly, patients are empowered to achieve better self-management of their health condition.
The recent Consensus Statement (2023) from the European Association of Preventive Cardiology (EAPC) has highlighted recommendations for healthcare professionals for the promotion of effective nutritional counselling in cardiac rehabilitation. Firstly, a detailed assessment of the patient’s dietary habits should be undertaken (in practice, this can be challenging as busy caseloads often preclude dietitians having the capacity to deliver individual sessions). Secondly, practitioners should encourage and support behavioural changes that will enable adherence to a healthier diet. Finally, there should be good communication with members of the MDT as we should all collaborate to support the patient’s needs.
EAPC have further recommended that nutritional information should be individualised to each patient, making sure they are involved in the treatment decision process. Practitioners should also adopt a flexible dietary approach, taking into account patient preferences and beliefs. It is important to note that, currently, there is a knowledge gap as there are no studies examining the effectiveness of different methods used in CR (group vs individual sessions) on the maintenance and adherence of healthy dietary habits. Therefore, there is no recommended approach and more studies need to be done in this area to assess the most impactful intervention(s).
Improving Quality of Service
Patient satisfaction with care is routinely assessed during CR and there is typically very positive feedback from patients regarding dietetic input specifically, despite significant resource constraints. It is nonetheless important to continuously improve the quality of service delivered, and for that reason, a process is already underway in Beaumont to carry out a clinical audit of the current nutritional service in CR. This will help to determine the impact of the intervention on increasing patient’s knowledge around nutrition and heart disease and may identify potential gaps in current service provision. This will enable us to further improve our service and
enhance the delivery of high-quality care to this patient population.
Conclusion
In conclusion, the role of a dietitian in cardiac rehabilitation is a fundamental aspect of this programme of care. Evidence has shown that a heart-healthy diet is essential to follow to reduce the risk of cardiovascular disease, and that specialist input from a dietitian facilitates the delivery of evidenced-based nutritional advice in a way that is easily accessible to patients. A multi-disciplinary approach is also key for the delivery of best practice care to our patients.
Ideally, additional resourcing would permit further nutritional input to be provided during CR (e.g. additional group sessions and/or one to-one consultations), however, due to time constraints and increasingly busy caseloads, dietitians are limited in their capacity to deliver more extensive input. In addition, continuous audits should be carried out to ensure that we are not only effective in the delivery of our nutritional advice but that patients are satisfied with the information delivered. This will increase the probability of successful long-term dietary change and thus, improved cardiovascular health.
References available on request
Cardiology News

Kidney Health and Heart Health go ‘Hand-in-Hand’
The Irish Kidney Association (IKA) and the National Renal Office (NRO), together with the Irish Heart Foundation and Croí Heart and Stroke Charity, are joining forces for a campaign to raise awareness about the close link between Heart Disease and Chronic Kidney Disease. This campaign will mark World Kidney Day which was celebrated on Thursday, 9th March 2023, and will include a free public webinar titled ‘Kidney and Heart Health Go Hand in Hand’
Heart health and kidney health are closely related, as the heart pumps oxygen-rich blood to all parts of the body including the kidneys, which in turn control
blood pressure, and clean the blood (removing waste products). Chronic Kidney Disease (CKD) can cause heart disease and vice versa, making it important to understand the relationship between these two conditions and ways to reduce the risks associated with them.
Known as the ‘hidden disease’, 1 in 8 people have Chronic Kidney Disease (CKD), with many unaware they have it. While only 1 in 1,000 people will reach End Stage Kidney Disease (ESKD) for which the only treatment is conservative care[1], dialysis or a kidney transplant, those with
any of the five stages* of CKD are at risk of developing Heart Disease. Conservative estimates are that at least 50% of those with CKD Stages 4 or 5 have cardiac disease, and at least 50% of those with heart failure have CKD.
Diabetes and High Blood Pressure are leading causes of both Chronic Kidney Disease (CKD) and Heart Disease. Even without these conditions, CKD can increase the risk of heart problems, and heart disease can lead to kidney problems. CKD is often associated with Anaemia, which reduces the blood's ability to carry oxygen, putting extra strain on the heart.
To reduce the risk of both kidney disease and heart disease, it is important to follow a healthy diet, be physically active, and stay engaged with your own healthcare. Knowing your numbers, such as blood pressure, blood sugar, and weight, can help detect any changes that may indicate a problem. Regular check-ups with your doctor, attending specialists’ appointments when scheduled, and knowing when to seek urgent medical attention are also important. Understanding your own medications and ensuring your doctor and pharmacist are aware of all of the medications you take to avoid side effects are also key.
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 45
COMBINING POWER AND CONFIDENCE AGAINST
LDL-C*
High intensity statin single pill combination for LDL-C reduction1
Therapeutic Indications:
Primary Hypercholesterolaemia/Homozygous Familial Hypercholesterolaemia (HoFH) - Suvezen is indicated as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia in adult patients who are not appropriately controlled with statin alone, who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products. Prevention of Cardiovascular Events - Suvezen is indicated as substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently, at the same dose level as in the fixed dose combination, but as separate products to reduce the risk of cardiovascular events in patients with coronary heart disease (CHD) and a history of acute coronary syndrome (ACS).
Prescribing Information: Suvezen (rosuvastatin/ ezetimibe) film-coated tablets



Please refer to the Summary of Product Characteristics (SmPC) for full prescribing details.
Presentations: Suvezen 10mg/10mg, 20mg/10mg and 40mg/10mg: Each film-coated tablet contains 10mg; 20mg or 40mg of rosuvastatin (as rosuvastatin calcium) respectively, and 10mg ezetimibe. Indication: Primary Hypercholesterolaemia/Homozygous Familial Hypercholesterolaemia (HoFH) - Suvezen is indicated as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia in adult patients who are not appropriately controlled with statin alone, who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products. Prevention of Cardiovascular Events - Suvezen is indicated as substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently, at the same dose level as in the fixed dose combination, but as separate products to reduce the risk of cardiovascular events in patients with coronary heart disease (CHD) and a history of acute coronary syndrome (ACS). Dosage and Administration: The patient should be on and continue, an appropriate lipid-lowering diet, during treatment with Suvezen. Suvezen is not suitable for initial therapy. When Suvezen is indicated for patients not controlled by statin alone, the dose of Suvezen should be individualized according to the target lipid levels and the patient’s response. When Suvezen is indicated for patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate product, treatment initiation or dose adjustment if necessary should only be done with the monocomponents and after setting the appropriate doses the switch to the fixed dose combination of the appropriate strength is possible. Patient should use the strength corresponding to their previous treatment. The recommended dose is one Suvezen tablet daily. To be administered at any time of the day, with or without food. The tablet should be swallowed whole with a drink of water. If co-administered with bile acid sequestrant (BAS), administration of Suvezen should occur either ≥2 hours before or ≥4 hours after administration of a BAS. Special populations: Paediatric (<18 years): Safety and efficacy has not been established. Elderly (>70 years): Starting dose of 5 mg rosuvastatin is recommended. The combination is not suitable for initial therapy. Hepatic impairment: Mild: No dosage adjustment is required. Moderate/Severe: Treatment with Suvezen is not recommended. Renal impairment: Mild: No dose adjustment is necessary. Moderate (creatinine clearance <60 ml/min): The recommended start dose is rosuvastatin 5mg. Race: The recommended start dose is rosuvastatin 5 mg for patients of Asian ancestry due to increased systemic exposure. The fixed dose combination is not suitable for initial therapy. Monocomponent preparations should be used to start the treatment or to modify the dose. Suvezen 40 mg/10 mg tablets are contraindicated in these patients. Genetic polymorphisms: In patients who are known to have specific types of genetic polymorphisms that can lead to increased rosuvastatin exposure, a lower daily dose of Suvezen is recommended. Dosage in patients with pre-disposing factors to myopathy: The recommended start dose is rosuvastatin 5mg in patients with pre-disposing factors to myopathy. Suvezen 40 mg/10 mg tablets are contraindicated in some of these patients. Concomitant therapy: The risk of myopathy (including rhabdomyolysis) is increased when Suvezen is administered concomitantly with certain medicinal products that may increase the plasma concentration of rosuvastatin (e.g. ciclosporin and certain protease inhibitors including combinations of ritonavir with atazanavir, lopinavir, and/or tipranavir). Whenever possible, alternative medications should be considered, and, if necessary, consider temporarily discontinuing Suvezen therapy. In situations where co-administration of these medicinal products with Suvezen is unavoidable, the benefit and the risk of concurrent treatment and rosuvastatin dosing adjustments should be carefully considered. Contraindications: Hypersensitivity to the active substances or excipients. Pregnancy, breast-feeding and in women of childbearing potential not using appropriate contraceptive measures. Active liver disease or any serum transaminase elevations which are unexplained, persistent or exceeding 3x the upper limit of normal (ULN). Severe renal impairment (creatinine clearance <30ml/min); myopathy or receiving concomitant ciclosporin. 40mg/10mg dose contraindicated in patients with predisposing factors for myopathy/rhabdomyolysis; such factors include: Moderate renal impairment (creatinine clearance <60ml/min), hypothyroidism, personal or family history of hereditary muscular disorders, previous history of muscular toxicity with another HMG-CoA reductase inhibitor or fibrate, alcohol abuse, situations where an increase in plasma levels of rosuvastatin may occur, Asian patients, concomitant use of fibrates.
Precautions and Warnings: Skeletal muscle effects: have been reported in rosuvastatin-treated patients with all doses and in particular with doses >20mg. As with other HMG-CoA reductase inhibitors, reporting rate for rhabdomyolysis is associated with use at doses >40mg. Post-marketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. If myopathy is suspected based on muscle symptoms or is confirmed by a creatine phosphokinase (CPK) level, Suvezen and any of these other agents that the patient is taking concomitantly should be immediately discontinued. All patients starting therapy with Suvezen should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness, particularly if associated with malaise or fever. Creatine kinase (CK) measurement: CK should not be measured following strenuous exercise or in the presence of a plausible alternative cause of CK increase. If CK levels are significantly elevated at baseline (>5x ULN) a confirmatory test should be carried out within 5 – 7 days. If the repeat test confirms a baseline CK >5x ULN, treatment should not be started. Patients with predisposing factors for myopathy/rhabdomyolysis: Caution should be exercised in these patients. Risk: benefit of treatment should be considered and clinical monitoring is recommended. CK levels should be measured in these patients. Therapy should be discontinued if CK levels are markedly elevated (>5x ULN) or if muscular symptoms are severe and cause daily discomfort. If symptoms resolve and CK levels return to normal, then consideration should be given to re-introducing treatment at the lowest dose. Immune-mediated necrotising myopathy (IMNM): Clinically characterised by proximal muscle weakness and elevated serum CK, has been reported very rarely during or after treatment with statins, including rosuvastatin, despite discontinuation of statin treatment. In clinical trials an increase in the incidence of myositis and myopathy has been seen in patients receiving other HMG-CoA reductase inhibitors together with fibric acid derivatives. Suvezen should not be used in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g. sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disorders; or uncontrolled seizures). Liver effects: In controlled co- administration trials in patients receiving ezetimibe with statin, consecutive transaminase elevations ≥3x ULN have been observed. It is recommended that liver function tests be carried out prior to, and 3 months following, the initiation of treatment. Rosuvastatin should be discontinued or the dose reduced if the level of serum transaminases is >3x ULN. The reporting rate for serious events is higher at the 40mg dose. In patients
with secondary hypercholesterolaemia caused by hypothyroidism or nephrotic syndrome, the underlying disease should be treated prior to initiating therapy with rosuvastatin. Liver disease and alcohol: As with other HMG-CoA reductase inhibitors, rosuvastatin should be used with caution in patients who consume excessive quantities of alcohol and/or have a history of liver disease. Renal effects: Proteinuria has been observed in patients treated with higher doses of rosuvastatin and was transient or intermittent in most cases. Proteinuria has not been shown to be predictive of acute or progressive renal disease. An assessment of renal function should be considered during routine follow-up of patients treated with a dose of 40mg. Diabetes mellitus: Some evidence suggests that statins raise blood glucose and in some patients, at high risk of future diabetes, may produce a level of hyperglycaemia where formal diabetes care is appropriate. This risk, however, is outweighed by the reduction in vascular risk with statins and therefore should not be a reason for stopping statin treatment. Patients at risk (fasting glucose 5.6 – 6.9mmol/l, BMI >30kg/m2, raised triglycerides, hypertension) should be monitored both clinically and biochemically according to national guidelines. Interstitial lung disease: Exceptional cases have been reported with some statins, especially with long term therapy. Presenting features can include dyspnoea, non-productive cough and deterioration in general health (fatigue, weight loss and fever). If it is suspected, statin therapy should be discontinued. Severe cutaneous adverse reactions: Stevens-Johnson syndrome (SJS) and drug reaction with eosinophilia and systemic symptoms (DRESS), which could be life-threatening or fatal, have been reported with rosuvastatin. If signs and symptoms suggestive of this reaction appears, Suvezen must be discontinued immediately and an alternative treatment should be considered. Treatment with Suvezen must not be restarted at any time. Protease inhibitors: Increased systemic exposure to rosuvastatin has been observed in subjects receiving rosuvastatin concomitantly with various protease inhibitors in combination with ritonavir. Consideration should be given both to the benefit of lipid lowering by use of Suvezen in HIV patients receiving protease inhibitors and the potential for increased rosuvastatin plasma concentrations when initiating and up titrating rosuvastatin doses in patients treated with protease inhibitors. The concomitant use with certain protease inhibitors is not recommended unless the dose of rosuvastatin is adjusted. Fibrates: The safety and efficacy of ezetimibe administered with fibrates have not been established. If cholelithiasis is suspected in a patient receiving Suvezen and fenofibrate, gallbladder investigations are indicated and therapy should be discontinued. Anticoagulants: If Suvezen is added to warfarin, another coumarin anticoagulant, or fluindione, the International Normalised Ratio (INR) should be appropriately monitored. Fusidic acid: Suvezen must not be co-administered with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid treatment. In patients where the use of systemic fusidic acid is considered essential, statin treatment should be discontinued throughout the duration of fusidic acid treatment. There have been reports of rhabdomyolysis (including some fatalities) in patients receiving fusidic acid and statins in combination. The patient should be advised to seek medical advice immediately if they experience any symptoms of muscle weakness, pain or tenderness. Statin therapy may be re- introduced seven days after the last dose of fusidic acid. In exceptional circumstances, where prolonged systemic fusidic acid is needed, e.g., for the treatment of severe infections, the need for coadministration of Suvezen and fusidic acid should only be considered on a case by case basis and under close medical supervision. Suvezen contains lactose and sodium: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine. This medicine is essentially ‘sodium-free’. Interactions: Contraindicated combinations: Ciclosporin. Not- recommended combinations: Fibrates and other lipid-lowering products, protease inhibitors, transporter protein inhibitors and fusidic acid. Other possible interactions: Cytochrome P450 enzymes, antacids, colestyramine, anticoagulants, Vitamin K antagonists, clopidogrel, ticagrelor, erythromycin, oral contraceptive/ hormone replacement therapy. When co- administering rosuvastatin with other medicinal products known to increase exposure to rosuvastatin, doses should be adjusted (see SmPC for full details). The maximum daily dose should be adjusted so that the expected rosuvastatin exposure would not likely exceed that of a 40mg daily dose of rosuvastatin taken without interacting medicinal products. Fertility, Pregnancy and Breastfeeding: No clinical data are available on the use of ezetimibe during pregnancy. Potential risk from inhibition of HMG-CoA reductase outweighs the advantage of treatment during pregnancy. If a patient becomes pregnant during use of Suvezen, treatment should be discontinued immediately. Animal studies have shown excretion of medicinal product through breast milk. However, there are no data in humans. No clinical trial data on the effects of fertility in humans. Adverse Reactions: Adverse drug reactions previously reported with one of the individual components (ezetimibe or rosuvastatin) may be potential undesirable effects with Suvezen. Common: diabetes mellitus, headache, dizziness, constipation, nausea, abdominal pain, diarrhoea, flatulence, myalgia, ALT and/or AST increased, asthenia and fatigue. Uncommon: decreased appetite, paraesthesia, hot flush, hypertension, cough, dyspepsia, gastroesophageal reflux disease, nausea, dry mouth, gastritis, pruritus, rash, urticaria, arthralgia, muscle spasms, neck pain, back pain, muscular weakness, pain in extremity, ALT and/or AST increased, blood CPK increased, gamma-glutamyltransferase increased, liver function test abnormal, chest pain, pain, asthenia, oedema peripheral. Rare: thrombocytopenia, hypersensitivity reactions including angioedema, pancreatitis, increased hepatic transaminases, myopathy (including myositis), rhabdomyolysis, lupus-like syndrome and muscle rupture. Very rare: polyneuropathy, memory loss, jaundice, hepatitis, arthralgia, haematuria, gynaecomastia. Not known: thrombocytopenia, hypersensitivity (including rash, urticaria, anaphylaxis and angioedema), depression, peripheral neuropathy, sleep disturbances (including insomnia and nightmares), dizziness, paraesthesia, cough, dyspnoea, diarrhoea, pancreatitis, constipation, hepatitis, cholelithiasis, cholecystitis, Stevens Johnson syndrome, erythema multiforme, drug reaction with eosinophilia and systemic symptoms, immune-mediated necrotising myopathy, tendon disorders (sometimes complicated by rupture), myalgia, myopathy/rhabdomyolysis, oedema, asthenia. Prescribers should consult the SmPC in relation to other adverse reactions. Legal Category: POM. Marketing Authorisation Numbers: 10mg/10mg: PA0540/193/001; 20mg/10mg: PA0540/193/002; 40mg/10mg: PA0540/193/003. Marketing Authorisation Holder: Sanofi-Aventis Ireland Ltd. T/A SANOFI, Citywest Business Campus, Dublin 24, Ireland. Further information is available from: Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com
Date of Preparation: February 2023 (MAT-IE-2300051 v1.0)
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com

20 mg /10 mg
Rosuvastatin + Ezetimibe 10 mg /10 mg
Rosuvastatin + Ezetimibe 40 mg /10 mg
Rosuvastatin + Ezetimibe
MAT-IE-2101261 (v5.0) | March 2023 * LDL-C: Low-density lipoprotein Cholesterol. Reference: 1. Suvezen Summary of Product Characteristics.
CARDIOLOGY: PREVENTION
The biggest unmet needs in cardiovascular disease prevention in 2023
“The primary determinants of disease are mainly economic and social, and therefore its remedies must be economic and social. Medicine and politics cannot and should not be kept apart” (Geoffrey Rose)
Disclaimer: This paper is based on an invited review that has been submitted to the journal 'Cardiology'
Abstract
1. While the major causes of atherosclerotic cardiovascular disease are known, clinical audits continue to show inadequate risk factor control, even in the highest risk subjects.
2. More effective risk estimation methods may help, and advances in this field are outlined.

3. There exist excellent Guidelines on cardiovascular disease prevention, but their very length and complexity may limit their use. Other factors inhibiting guideline implementation are explored.
4. While new medications continue to be developed, the real challenges to effective CVD prevention are societal and political. Both nationally and at European levels, cohesive, integrated strategies with defined responsibilities and accountability are needed, together with empowerment of people to understand the concept of risk and what they can do about it.
5. There are profound health inequalities between and within countries that need to be addressed.
Introduction
The major causes of atherosclerotic cardiovascular disease (CVD) are known. There has been some progress with regards to control of tobacco smoking, hypertension and hyperlipidaemia in recent decades. Despite the fact that control of these unequivocally reduces risk, clinical audits such as EuroAspire and SURF1,2 continue to show inadequate risk factor recording and control. Levels of overweight and consequent diabetes continue to increase.
The great Geoffrey Rose taught us that a small reduction in risk at population level will have a larger impact on population health than a more intense intervention applied to a small number of very high-risk persons, even though such individuals should be identified as they may gain much as individual people.3 This has led to a widespread realisation that population and high-risk strategies are complementary and not competitive. Rose also stated that “The primary determinants of disease are mainly economic and social, and therefore its remedies must be economic and social. Medicine and politics cannot and should not be kept apart”.3
These considerations have informed World Health Organisation (WHO),4 the American Heart Association5 and the European Alliance for Cardiovascular Health (EACH)
report "A European Cardiovascular Health Plan: The need and the ambition".6 This far-reaching report provides a comprehensive road map for prevention and clearly calls for the European Commission to take more responsibility.

In this paper we look at advances in cardiovascular risk estimation, comment on some
issues with guidelines and their implementation, consider the lack of commitment at societal and political level and address the issue of over-medicalisation and the need to facilitate personal responsibility for health. We also comment on the striking inequalities in CVD risk and mortality between and within countries.
Written by Professor Ian M Graham MD, Professor of Cardiovascular Medicine, Trinity College Dublin
It is not the purpose of this review to examine drug treatments which are amply dealt with elsewhere except to note the more widespread availability of generic

HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 47
statins, the fact that newer therapies can achieve LDL goals in most persons7 albeit at high cost. Newer therapies to reduce Lp(a) hold promise but with, to date, no evidence of an impact on CVD endpoints.
Although this paper focusses on Europe, it is hoped that the principles may have universal applicability.
Risk estimation
This topic was reviewed recently in a paper describing the European Society of Cardiology's (ESC) Cardiovascular Risk Collaboration.8 Guidelines on CVD prevention recommend the use some form of risk calculator because the occurrence of CVD is usually the result of several interacting risk factors, and the clinical estimation of these effects is uncertain. The current ESC prevention Guidelines9 use SCORE210 and SCORE2-OP11 as
their recommended risk estimation systems for people below and above 70 years of age. Like other systems, these are limited by the facts that they start at the age of inception of the cohorts from which they are derived (usually age 40- therefore after many years of exposure to risk) and, strictly speaking, apply to populations rather than individuals.
A thoughtful editorial by Navar and colleagues,12 based on a paper by Mortensen et al,13 notes some of the limitations of SCORE as used in the 2021 ESC Guidelines on prevention [9], including the impact of regional variations in risk factors, risk and statin usage. Although SCORE is indeed re-calibrated for different risk regions, this may not have fully adjusted for these issues. More importantly, the intervention thresholds for recommending statins may have a profound impact on statin usage. Using these new recommendations,
CARDIOLOGY: REHABILITATION


Mortensen and colleagues13 estimated the proportion of people free of diabetes or kidney disease in Denmark now eligible for statin therapy for primary prevention. As Navar notes, "the results are alarming: under the 2019 ESC guideline, 20% of the population received a class 1 recommendation for a statin, and under the 2021 guideline, this decreased to 4%. Including class II recommendations, the proportion of individuals recommended for a statin dropped from 56% to 19%". The thresholds used for recommending statins in the 2021 Guidelines need review.
As noted by Brian Ference in his contribution to,8 current risk estimation systems are dominated by age which is likely not a risk factor as such but rather a measure of exposure time. The future may lie in developing an exposure-time lifetime risk model based on years of exposure to risk factors. Mendelian randomisation
studies may suggest that the rate of rise in LDL cholesterol and blood pressure from birth on may be causally determined by polymorphisms. Using causal estimates of the cumulative effect of lipids, systolic blood pressure and other factors that cause atherosclerosis may have the potential to permit individualised true lifetime risk at a much earlier age than is currently possible. In this approach, risk would be expressed not as 10-year risk but in terms of the combined effects of years of exposure to individual risk factors, with adjustment later lifestyle factors such as diet and smoking. It will likely require judicious use of artificial intelligence in its application. Much work needs to be done to see if the potential of this approach can be realised. These concepts have been outlined in a paper reporting the individual and combined effects of genetically determined levels of blood pressure and LDLcholesterol on lifetime risk.14
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 48
Polygenic scores have to date shown only a modest effect on risk estimation. This may be in part because, as above, the rate of rise in LDL-cholesterol and blood pressure is determined by polymorphisms and these effects may be greater than the independent effects of polygenic scores (B. Ference, personal communication). Guidelines and their implementation
Many Guidelines for the prevention of CVD exist, and yet risk factor control remains poor, even in the highest risk subjects.1,2 Perhaps Konrad Lorenz (1903-1969) understood the reasons"Said is not heard, heard is not understood, understood is not agreed upon, agreed is not applied, applied is not at all maintained"
(Brought to our attention by Prof Ulrich Keil)
The European Association of Preventive Cardiology has devoted considerable time to this issue.15
Their work re-emphasises the findings of earlier studies such as one published by the ESC:16
Barriers to guideline usage include:
• Lack of physician time
• Insufficient training in both prevention and behaviour change
• Lack of remuneration (physicians are generally re-imbursed for treating the sick, not promoting health).
• Lack of clarity (guidelines too detailed, too complicated).
• Unhelpful health policies, including a lack of a focussed health strategy and remuneration issues as above.
• Patient difficulties with adhering to advice- more a comment on our communication skills than a criticism.
Of these, the length and complexity of current guidelines may be a major factor impeding their widespread use. The 2021 ESC Guidelines on CVD prevention9 run to no less than 111 pages and 837 references. Fortunately, pocket guidelines, Apps and slide sets are available through www.escardio.org. Simple communication tools such as the American Heart Association
'Life's Essential 8'5 may simplify messages both for health professionals and the public. Factors which may increase guideline usage include:
• Simple, clear, credible national guidelines.
• Sufficient time.
• Appropriate use of Apps and social media.
• An effective multidisciplinary implementation strategy.
• Facilitatory government policy
- Defined prevention strategy-
- Reimbursement for health professionals
- Public awareness and education from school on.
Societal and political commitment
The factors that impact on Guideline usage may also inform more effective preventive efforts. Additional factors that may militate against prevention are the overmedicalisation of prevention and the lack of a pan-European legal framework for health.
Do we, as healthcare workers, try to over-control prevention rather than fostering knowledge and skills in the public? The basic facts regarding cardiovascular risk and how to make choices about its components should surely be integrated into school curricula. "Integrated' implies more than an add on module - it might include, for example, the relationships between the tobacco and food industries and health as well as knowledge of risk factors and the principles of behaviour change.
In 2011, Morgan, Burke and McGee17 undertook a benchmarking survey regarding the implementation of prevention in 13 European countries. In general, it was impossible to find any one person with responsibility for coordinated national policy with regards to prevention.
The same pertains at EU level - no one person with responsibility for prevention exists. Furthermore, there is no pan-European legal framework for health promotion. The EU may offer advice but, unlike food safety, has no legal basis for enforcing action. Attempts by bodies such as
the ESC and European Heart network (EHN) to lobby the EU and European Parliament tend to be met with well-meaning words but little effective action- what has been termed 'Implementation Deficiency Disorder'.
As long ago as 2007, the ESC, in partnership with the World Health Organisation (WHO) and EHN published the European Heart Health Charter [18] to address some of these issues. This was presented to the EU and virtually all countries in Europe signed up to the Charter, but little seemed to change. There was a lack of a follow-up strategy to ask each country to be accountable for implementation efforts. The Charter has now been updated and the implementation strategy is under discussion.
In the meantime, the EACH report6 makes widespread and logical recommendations that are essentially complementary to the European Heart Health Charter, including the following:
"Proposed Key components of a future CVH Plan"
Horizontal, cross-cutting actions:
• A European Cardiovascular Health Data Knowledge Centre

• A European Cardiovascular Health Observatory
• The Co-Creation of National CVH Action Plans
• Creating an incubator and progressive policy environment for digital transformation in CVH
Vertical actions:
• Primary prevention to decrease premature mortality and morbidity at population level
• Secondary prevention through screening for early detection and precision diagnosis - A European Cardiovascular Health Check
• Early intervention, access to care and optimal treatment
• Rehabilitation
• Quality of life and other psychosocial outcomes across the spectrum of cardiovascular diseases
A CVH Mission – comprising a new research and innovation
agenda in the framework of HORIZON Europe"
Regarding implementation, detailed proposals are made for the consideration of the European Commission. But, as defined above, until such time as Europe can approach a cohesive, integrated approach to prevention with defined responsibilities, implementation will prove very challenging. It behoves us, as health professionals to lobby for this, individually and through our professional bodies.
Health inequalities
The principles defined thus far may be reasonable, but there is another dimension. There is a West-East gradient in CVD mortality, with a higher mortality in Eastern countries that may lack the resources to combat the problem. And inequalities exist within countries- the socially deprived have higher levels of risk factors and the cliche that 'the poor die young' is true. These issues are clearly defined in the EACH report [6]. Women in particular are less likely to have risk assessments or to be offered appropriate help. Any national or European strategy needs a specific plan to address these issues.
In conclusion, we know the causes of CVD and the interventions that reduce risk. Current information and monitoring systems are insufficient for the needs of a coordinated prevention strategy. Above all, integrated and accountable plans at National and European level are required and it is time for both individual countries and the European Union to commit to responding to their responsibilities if Europe's major cause of death is to be addressed.
Acknowledgement:
I am grateful to Cardiology for inviting this review.
Conflict of interest statement: None
Funding sources: None
Author contributions: Sole author
Correspondence address: Woodvale, Rocky Valley Drive, Kilmacanogue, Co. Wicklow A98 EV18 Ireland
email: ian@grahams.net
Telephone: +353 87 2622946
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 49
CARDIOLOGY: AORTIC STENOSIS
An Update on Aortic Stenosis: Pathophysiology, Progression, Diagnosis and Current treatment options
Written by
Aortic stenosis
Aortic stenosis (AS) is the second most common valvular abnormality. Incidence increases with age and it is present in up to 2% of 60 year olds and 10% of 80 year olds. The disease process comprises fibrocalcification of the valve that results in orifice narrowing and LV outflow obstruction and is referred to as calcific aortic valve disease (CAVD). Aortic valve stenosis causes increased LV pressure, compensatory LV hypertrophy and affects cardiac output. Symptoms comprise a “classic triad” of shortness of beath, chest pain or syncope. Previously AS was considered a “degenerative process” that could not be modified however recent histological assessment confirms that AS is an active process of oxidised lipids and infiltrating inflammatory cells, perhaps opening pathways to future preventative therapies.
Untreated symptomatic AS has a two-year mortality of up to 50%. To date no pharmacological treatment has been shown to slow disease progression. Therefore, treatment options for severe AS are limited to valve replacement either surgically or percutaneously with a transcatheter system.

Although effective treatments, they do expose the patient to peri-procedural risk, possible need for anticoagulation (metallic valves) or risk related to bioprosthetic valve degeneration.

Pathophysiology and progression
Aortic stenosis was previously considered a degenerative valve process; however in-vitro studies indicate that this view may be oversimplistic. It is now thought that there is an initiation phase followed by a propagation phase as valve disease progresses from aortic sclerosis, through mild, moderate and severe AS. This involves a complex network of cellular and molecular mechanisms. The initiation phase appears to start with endothelial damage as a result of mechanical forces and shear stress across the valve.
This facilitates lipid infiltration and propagates an inflammatory response. The process then progresses to the propagation phase. During this phase valve interstitial cells (VICs), a fibroblastlike cell of the spongiosa layer, convert to osteoblast-like cells and secrete calcium.
Levels of macrocalcification of CAVD vary by gender.
Females have a lower burden
of calcification that is reflected in a lower Agatson scores on CT imaging (1200 for females with severe AS versus 2000 for males). Conversely, post-operative stenosed aortic valves explanted from females have higher levels of fibrosis. Diseased aortic valves also have evidence of angiotensin converting enzyme (ACE) colocalising with apolipoprotein B in the extracellular matrix, and myofibroblasts in the fibrosa have shown evidence of Angiotensin Type-1 (AT-1) receptors, possibly implicating the RAAS system in disease propagation.
Risk factors for AS mirror those for coronary atherosclerosis. These include age, being male, hypertension, CKD, hypercholesterolaemia, smoking, diabetes mellitus and established vascular disease. Valvular specific risk factors include congenitally abnormal leaflets e.g. bicuspid valve (BAV), or previous rheumatic heart disease. Shear stress is particularly relevant when leaflets are abnormal. Patients with BAVs typically develop severe AS one to two decades earlier than those with tricuspid aortic valves.
Patients may also have genetic predisposition to BAV and general AS. A Swedish registry study identified NOTCH1 mutations as a risk factor for BAV.

Aortic Stenosis Diagnosis
Aortic stenosis is usually diagnosed clinically through detection of an ejection systolic murmur. This is heard loudest in the right sided second intercostal space, radiates to the carotid arteries and is confirmed by transthoracic echocardiogram (TTE). Typical echo findings of severe aortic stenosis include restricted movement of calcified leaflets, a transvalvular velocity > 4m/s, mean pressure gradient of > 40mmHg, an aortic valve area < 1cm2 and a dimensionless index of <25. Related parameters for assessing severity and treatment include LV ejection fraction (LVEF), degree of aortic regurgitation (AR), assessment of other valvular abnormalities and measurement of right heart pressures.
Low flow low gradient AS occurs when valve gradients are disproportionate to valve area. In these cases severity may be more accurately determined by exercise or dobutamine stress echocardiography (DSE). This helps to distinguish severe versus pseudo-severe AS. Aortic valve area does not increase in true severe AS with increased cardiac output (CO). Transoesophageal echocardiogram or Cardiac CT (CCT) can also help to elucidate severity in uncertain cases. A calcium score of >2,000 for men and >1,200 for women on CCT indicates a high likelihood of severe AS.
Aortic Sclerosis
Aortic sclerosis is the term used for early abnormalities of the aortic valve. These comprise valve thickening and calcification that occurs without outflow tract obstruction or elevation of transvalvular velocity gradient on TTE (<2m/s). Clinically patients may have an ejection systolic murmur on examination, without an effect on the second heart sound.
Aortic sclerosis (AScl) is common, being present in up to 25% of 65-74 year olds and up to 48% of 84 year olds, and is a recognised antecedent of AS. Determinants of progression to AS remain unclear. Studies indicate that only 9% progress to any degree of AS over 5 years.
Aortic sclerosis is a marker of increased cardiovascular risk. Studies show an increased risk of death, MI, ventricular arrhythmia and ventricular systolic dysfunction among patients with aortic sclerosis versus those without. Thus patients with aortic sclerosis may be candidates for more aggressive risk factor management and more systematic follow up.
Tradition cardiac risk factors only predict the presence of AS/AScl, not rate of disease progression. Once valvular damage is sufficient to cause an outflow gradient on TTE, valvular dysfunction progresses roughly at a rate of 0.1-0.3m/sec increase in maximum gradient per year. This translates to approximately 3-10mmHg
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 50
Dr Lisa Brandon, St James Hospital, Dublin
Professor Andrew Maree, St James Hospital, DublinTrinity College, Dublin
Noradrenaline 1mg/ml concentrate for solution for infusion.

ABBREVIATED PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request. Noradrenaline (Norepinephrine) Kabi 1mg/ml concentrate for solution for infusion. Active ingredient: 1ml concentrate for solution for infusion contains 1mg noradrenaline (norepinephrine) base equivalent to 2mg noradrenaline (norepinephrine) tartrate. Contains 3.4mg sodium per ml. Indications: In adults for use as an emergency measure in the restoration of blood pressure in cases of acute hypotension. Posology and method of administration: When diluted as recommended, each litre contains 40mg noradrenaline base equivalent to 80mg noradrenaline tartrate. If dilutions other than 40mg per litre are used, check infusion rate calculation carefully before starting treatment. Initial rate of infusion – 10-20ml/hour (0.16-0.32ml/min); equivalent to 0.4-0.8mg/hour noradrenaline base. Lower initial infusion rate of 5ml/hour (0.08ml/min); equivalent to 0.2mg/hour noradrenaline base may be preferred. Titration of dose – Once infusion has been established, titrate dose in steps of 0.05-0.1mcg/kg/min of noradrenaline base according to pressor effect observed (see SmPC for details). Continue infusion until adequate blood pressure and tissue perfusion maintained without therapy. Carefully monitor patient. Should only be administered by healthcare professionals familiar with use of noradrenaline and with appropriate monitoring facilities. Avoid abrupt infusion withdrawal; reduce infusion gradually. No experience in treatment of hepatic or renal impairment. Dose selection for an elderly patient should be cautious, starting at the low end of the dosing range. Safety and efficacy in patients less than 18 years old has not been established. Method of administration – Intravenous use only after dilution. Infuse at a controlled rate using either syringe pump, infusion pump or drip counter. Administer via a central venous catheter. If not using a central venous catheter administer into a large vein whenever possible, particularly an antecubital vein to minimize risk of ischemic necrosis. Avoid catheter tie-in technique if possible. Contraindications: Hypersensitivity to the active substance or to any of the excipients, hypotension due to blood volume deficit. Do not use with cyclopropane and halothane anaesthetics as this may cause serious cardiac arrhythmias including ventricular fibrillation. Special warnings and precautions for use: Do not use undiluted. Contraindicated in patients who are hypotensive from blood volume deficits except as an emergency measure to maintain coronary and cerebral artery perfusion until blood volume replacement therapy can be completed. Should only be used in conjunction with appropriate blood volume replacement. If whole blood or blood plasma is indicated to increase blood volume, administer separately (e.g. use Y-tubing, individual containers). Prolonged administration may result in plasma volume depletion which should be continuously corrected by appropriate fluid and electrolyte replacement therapy. Frequently check blood pressure and rate of flow to avoid hypertension. Particular caution in patients with coronary, mesenteric or peripheral vascular thrombosis, hypotension following myocardial infarction, angina (particularly Prinzmetal’s variant angina), diabetes, hypertension or hyperthyroidism, major left ventricular dysfunction associated with acute hypotension (supportive therapy should be initiated simultaneously with diagnostic evaluation; reserve noradrenaline for patients with cardiogenic shock and refractory hypotension, in particular those without elevated systemic vascular resistance), liver failure, severe renal dysfunction,
ischemic heart diseases and elevated intracranial pressure. Reduce dose if heart rhythm disorders occur during treatment. Cardiac arrhythmias may arise when used in conjunction with cardiac sensitizing agents and may be more likely in patients with hypoxia or hypercarbia. Elderly patients may be especially sensitive to the effects of noradrenaline. Not recommended in children. Where indicated, appropriate replacement therapy of blood or fluid together with adoption of the supine position with elevation of the legs, must be instituted and maintained prior to and/or during therapy with noradrenaline. During infusion, record blood pressure every two minutes from the time the administration started until the desired blood pressure is obtained and then every five minutes thereafter if infusion continued. Constantly watch flow rate and never leave patient unattended. Hypertension may eventually lead to acute pulmonary oedema, arrhythmia or cardiac arrest. Caution in patients receiving pressor amines with chloroform, enflurane or other halogenated anaesthetics (may cause serious cardiac arrhythmias) or any other cardiac sensitising agent or in patients who exhibit profound hypoxia or hypercarbia. Extreme caution in patients receiving monoamine oxidase inhibitors or within 14 days of cessation of such therapy and in patients receiving tricyclic antidepressants, adrenergic- serotoninergic drugs or linezolid. Overdoses or conventional doses in hypersensitive persons may cause severe hypertension with violent headache, photophobia, stabbing retrosternal pain, pallor, intense sweating and vomiting. The infusion site should be checked frequently for free flow. Care should be taken to avoid extravasation of noradrenaline tartrate into the tissues, as local necrosis might ensue due to the vasoconstrictive action of the drug (see SmPC for antidote for extravasation ischaemia). Blanching along the course of the infused vein warrants consideration of changing infusion site at intervals. Avoid administration via the veins of the leg in elderly patients or in those suffering from occlusive vascular diseases. Noradrenaline interacts with other medicinal products; some combinations are inadvisable or require additional precautions and close medical supervision – see SmPC. Undesirable effects: Anxiety, insomnia, confusion, weakness, psychotic state, transient headache, tremor, acute glaucoma, bradycardia, arrhythmia, electrocardiogram change, tachycardia, cardiogenic shock, stress cardiomyopathy, palpitations, increase in the contractility of the cardiac muscle resulting from the beta- adrenergic effect on the heart (inotrope and chronotrope), acute cardiac insufficiency, hypertension, peripheral ischaemia including gangrene of the extremities, plasma volume depletion with prolonged use, dyspnoea, respiratory insufficiency or difficulty, nausea, vomiting, paleness, scarification of the skin, bluish skin colour, hot flushes or skin redness, skin rash, hives or itching, retention of urine, extravasation, necrosis at injection site. Legal Category: POM Marketing Authorisation Number: PA2059/073/001
Marketing Authorisation
Holder: Fresenius Kabi Deutschland GmbH; Else-Kröner Straße 1, 61352 Bad Homburg v.d.Höhe, Germany Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance.GB@Fresenius-Kabi.com. Date of Preparation: January 2022 FPI-0041
Website: www.fresenius-kabi.com/ie/
Email: FK-enquiries.ireland@fresenius-kabi.com

Phone: +353 (0)1 841 3030
Date of Prep: March 2022
Job Code: IE-IVF-2200006
Kabi Limited
3B Fingal Bay Balbriggan, Co. Dublin Ireland
Your patients, your profession, our passion. Fresenius
Fresenius Kabi Ireland Unit
increase in mean gradient, and drop in aortic valve area of 0.1cm2 per year. There is significant interpatient variability however, and in some patients aortic stenosis may progress more rapidly such as those with renal impairment.
Aortic stenosis and the left ventricle
As AS increases so does left ventricular (LV) afterload and adaptive change in the left ventricle, so called LV remodelling, ensues. LV size, wall thickness, structure and function evolve.
LV wall stress reflects afterload on the LV and results in direct tension on individual myocardial fibres. It is modulated by LV pressure, wall thickness and diameter (law of LaPlace). Increased wall stress leads to myocyte hypertrophy which can occur in a concentric or eccentric pattern. Progressive LVH is a compensatory mechanism that initially improves wall stress and maintains CO until severity of AS precludes further increases of CO and LV changes become maladaptive. Patterns of LVH may differ by gender.
As LVH progresses myocardial oxygen demand increases and coronary perfusion deficits arise. Myocardial hypo perfusion causes subendocardial ischaemia, necrosis and fibrosis and reduced
CARDIOLOGY: AORTIC STENOSIS

(SAVR), or minimally invasively with a transcatheter bioprosthetic aortic valve (TAVR) using either a balloonexpandable or self-expandable valve system.
Surgical risk previously precluded treatment of a significant cohort of patients with severe AS. This unmet need prompted development of TAVR platforms in the early 2000s. Surgical risks are usually calculated with risk models such as the STS-PROM (Society of Thoracic Surgeons predicted risk of mortality) score or EuroScore and classify patients as low, intermediate or high risk for mortality and post-operative complications (arrhythmia, stroke, bleeding, poor wound healing, thrombosis risk and infection). Average length of stay after an uncomplicated SAVR is 7 days in hospital.
diastolic filling time. Fibrosis causes myocardial stiffness, elevated LV end diastolic pressures and impaired diastolic function. This perpetuates a cycle of ischaemia and fibrosis and leads to further decline in LV function. Left ventricular ejection fraction (LVEF) may be maintained at this time, but LV global longitudinal strain (GLS) is affected early in the disease process and correlates with mortality/survival post AVR.
LV wall stress activates neuroendocrine, paracrine and autocrine systems to maintain CO. These include the RAAS, sympathetic nervous system, oxidative pathways and proinflammatory cytokines (TGF-beta1/TNF).
Medical treatment
To date no medication has been shown to slow AS progression. If was postulated that statins would dampen valvular inflammation and thus retard AS progression, but clinical trials have failed to show benefit. PCSK9 inhibitors are currently undergoing similar trials. Studies of ACE inhibitors (LIFE trial), losartan and atenolol have also failed to show benefit.
Interventional treatment
Untreated symptomatic severe AS is associated with a two-
year mortality of up to 50%, however timing of intervention remains contentious. Previous guidelines recommended active surveillance for patients with asymptomatic severe AS, though it is recognised that symptoms can be subjective and may be masked by co-morbidities such as chronic obstructive pulmonary disease (COPD). This makes safe timing for treatment of such patients challenging.
On-going studies attempt to identify asymptomatic AS patients at higher risk for whom surveillance may not be appropriate. Some high-risk parameters have already been incorporated into treatment guidelines. These include depressed LVEF, very high gradient AS (> 5m/s), rapidly increasing gradient (> 0.3m/s per year), abnormal blood pressure response to exercise stress testing, or unexplained elevation of brain natriuretic peptide levels (BNP). Others that remain subject of trials include degree of LVH, left atrial size, LV strain patterns on TTE, autonomic parameters and abnormal biomarker levels such as troponin, fetuin-A, and copeptin.
Current treatment of severe AS focuses on valve replacement, either surgically with a bioprosthetic or metallic valve
TAVR was initially offered to patients at high surgical risk, but recent trials have shown that TAVR is non-inferior to SAVR in patients without a bicuspid valve at low and intermediate surgical risk (PARTNER 3 trial, Evolut low risk trial). Current TAVR related risks include stroke (approx. 1%), femoral access site bleeding, arrhythmia, pacemaker implant (approx. 6%), increased risk of significant aortic regurgitation versus SAVR and death (approx. 1%). Average in hospital length of stay after an uncomplicated TAVR is 2 days.

Thus, the ESC guidelines currently recommend TAVR for patients >75 years of age and those with high surgical risk (STS-PROM/ EuroSCORE II > 8%), SAVR for patients <75 years, those at low surgical risk (STS-PROM/ EuroSCORE II <4%) and those with unfavourable anatomy for TAVR, and either option can be considered for patients with intermediate risk depending on anatomical suitability and patient preference.
Conclusion
Severe aortic stenosis is a common abnormality that is associated with significant mortality and prevalence increases with age. Current treatment options include surgical and transcatheter aortic valve replacement. There is growing evidence for the safety and efficacy of TAVR among a broad patient cohort.
52
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
“You are what you eat”
The gut microbiota and cardiometabolic disease
Written by Gaston L. Cluzel1, Noel M. Caplice2
1PhD Student , APC Microbiome Ireland

2Professor Cardiovascular Sciences, Cardiologist, PI APC Microbiome IrelandUniversity College Cork, Cork University Hospital


Cardiovascular disease (CVD) is the leading cause of mortality worldwide, accounting for a third of total annual death. The combination of obesity related disorders and cardiometabolic disease has now reached epidemic proportions, threatening to overwhelm healthcare services in the developed industrialised world. Current strategies for fighting symptomatic cardiometabolic disease rely heavily on labour intensive surgical procedures or drugs with limited efficacy and potentially hazardous secondary effects. Therefore, new thinking and treatment approaches are urgently required to fight CVD in the midst of the current metabolic syndrome pandemic.
The human gut is inhabited by a rich community of trillions of microorganisms known as the gut microbiota. The presence of these bacteria, yeasts, viruses and protozoa inside the human body is a consequence of millions of years of co-evolution, during which microorganisms have adapted to take advantage of the sheltering environment that is our gut. The gut microbiota as a “super-organism/ organ” has developed strategies so as to be immune-tolerated by our body. The host (our body) enjoys the fruits of these commensal microorganisms since they offer a protection against pathogenic gastrointestinal infections, improve digestion and provide key macro and micronutrients essential for health. However, the gut microbiota must be tightly controlled by our body since changes within this microbial community may initiate health disorders.
Recent research has identified a strong link between the gut microbiota and cardiovascular health, shedding light on a novel and promising field of potential intervention for CVD patients. Indeed, the gut microbiota not only plays a critical role in regulating physiological processes such as digestion, metabolism and immune response but can be considered as a hidden but essential metabolic
organ. For instance, alteration in the gut microbiota, a phenomenon known as “dysbiosis”, has been associated with health disorders such as gastrointestinal disease, metabolic syndrome and systemic inflammation - all of which can contribute to progression of CVD. Therefore, manipulating the gut microbiota could constitute a reliable strategy for fighting cardiometabolic disorders.
The gut microbiota lies at the interface between our diet and our intestine which is the largest human immune interface (6 metres long) with the external environment. It is directly exposed to our daily dietary habits and can change its composition within days of exposure to a poor diet. Moreover the proximity of the gut microbiota to the gut lining means signaling between this organ and the human body is continuous with potential for good and bad outcomes. Certain diets promote a healthy microbiota that in turn benefits our gut health and by extension the general health of the host. For instance, the “Mediterranean” diet - rich in plant-based ingredients, nuts and fibre - is associated with a thriving microbiota rich in beneficial bacterial species. This type of diet has also been shown to promote weight loss and reduces the cardiovascular effects of obesity and metabolic syndrome. On the other hand, a “Westernised” diet rich in fats, sugars and processed ingredients leads to dysbiosis with a microbiota depleted of beneficial bacterial species and is typically associated with obesity and cardiometabolic related disorders.
Beyond diet, certain food components appear to be particularly efficient in shaping the gut microbiota and promoting health. Dietary fibre and certain prebiotics are not digested by the body but instead are fermented by the gut microbiota, promoting the growth of beneficial bacterial species. Initiating their beneficial effect on the microbiota prebiotics also improve host health and may
be a modifier of CVD risk. Indeed, clinical studies have found that prebiotics can promote weight loss, improve lipid profiles, reduce low grade inflammation, and improve insulin sensitivity, all of which are risk factors for CVD. Moreover, reports from preclinical studies suggest that prebiotics may tackle some of the root mechanisms of CVD by preventing cellular and metabolic events that precede full blown CVD.
It is also possible to intervene on the microbiota directly using bacteria or yeast preparations, enriched for chosen beneficial species, to colonise our guts. Probiotics are described as live microorganisms that can provide health benefits when consumed in adequate amounts. Probiotics can also be combined with prebiotics in an attempt to obtain a greater effect than individual components; this is called a synbiotic treatment. Probiotics and synbiotics exhibit numerous health benefits and have been shown to reduce some CVD risk factors in clinical studies. Finally, it is also possible to colonise patient’s microbiota with the microorganisms of a healthy donor. This technique is called faecal microbiota transplantation (FMT)
and its success could reside in the transfer of beneficial microbiota properties from donor to patient. FMT has been most successful in treating gastrointestinal disease driven by clostridium difficle. While this technique is still experimental in other diseases, early studies have shown promising results in reducing inflammation and improving metabolic health, paving the way to clinical trials in the near future.
Although microbiota-targeted strategies show promising results in preventing CVD and other disorders, there is still a vast chasm in our knowledge deficit within this emerging field of research. The gut microbiota is an incredibly diverse environment composed of trillions of organisms with vast numbers of different species interacting at a protein, carbohydrate, complex sugar, amino acid, fatty acid and hormonal level. This complexity makes mechanistic interpretation of microbe-host interactions very difficult to decipher short of reductionist methods on one hand or machine learning tools on the other. Moreover, each human being has a unique microbial signature that evolves constantly throughout our life having been
Gaston Cluzel
53 CARDIOLOGY: GUT HEALTH
Professor Noel Caplice
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
hardwired as early as neonatal life with respect to training of the host’s immune system. Therefore, the major challenge of this field does not reside in the capacity of gut microbes to prevent CVD but rather in our ability to harness their power reliably at the scale of human populations.
In response to this challenge, current biomedical research strives to decipher the core mechanisms that rule the interactions between host and gut bacteria. As discussed above gut bacteria are involved in several biological processes participating in the maintenance of cardiovascular health. Here are some major concepts involved in understanding the interaction between the gut microbiota and cardiovascular organs
Intestinal permeability: The outer cellular layer of our body that is in direct contact with our intestinal content is called the intestinal epithelium. The major role of epithelium is to absorb nutrients while constituting an impermeable barrier to gut microbes. It has been repeatedly observed that patients suffering from cardiometabolic disorders suffer from a derangement of this intestinal barrier, a phenomenon called intestinal permeability. This process is explained by the weakening of the tight connections between epithelial cells as well as reduction in the protective mucous layer that lines the gut epithelium. As a result, gut
CARDIOLOGY: GUT HEALTH

bacteria or surface constituents of bacterial membranes/capsules enter into contact with the deeper cellular layers of the intestine, triggering immune defences and contributing to local tissue inflammation. It is still not clear how this phenomenon is initiated but studies suggest that an altered gut microbiota could more easily escape these defences and breach the gut permeability barrier.
Systemic inflammation: Obesity and cardiometabolic syndrome are associated with elevated levels of pro-inflammatory molecules in the blood, a phenomenon called systemic low grade inflammation (LGI). This effect is likely a direct result of spill over from local activation of inflammation within the gut mucosa. LGI can lead to activation of immune responses in distal organs including the liver, heart and vascular system. Systemic inflammation appears to be central in this interaction between dysbiosis and end organ disease in the case of CVD.
Microbial lipopolysaccharides (LPS): LPS are highly inflammatory bacterial toxins found on the cell walls of gram-negative bacteria. The presence of LPS in the systemic circulation is called endotoxemia, and has been detected in obese subjects especially in the presence of metabolic syndrome, suggesting that bacterial components may cross the human intestinal barrier to reach the circulation. Moreover, “Westernised” diet that promotes gut dysbiosis is also associated with an increased proportion of LPS producers
within the gut microbiome. The immune response to LPS has been shown to contribute to systemic inflammation and activate CVDrelated biological mechanisms in heart tissue and blood vessels.
Microbial products: gut bacteria ferment food components into secondary products. Short-chain fatty acids (SCFAs) for instance are produced from dietary fibre fermentation. SCFAs support intestinal barrier function by promoting epithelial cell growth. The beneficial effects of these molecules also reaches into the cardiovascular system due to their anti-inflammatory and metabolic properties. CVD is associated with reduction in these beneficial SCFAs at an intestinal and systemic level.
The above concepts connect dietary habits to cardiovascular health through their respective ties to the gut microbiota and the immune system. Maintaining a good microbiota through dietary intervention therefore promotes intestinal barrier function protecting against systemic inflammation and in turn, against CVD.
The clinical potential of microbiotatargeted interventions is only emerging and remains to be fully proven in preclinical gain and loss of function studies to nail down specific biological mechanism(s). The precise factors and interactions between microbiota and gut must be identified in order to develop reliable therapeutic tools. So far, several promising cardiovascular targets have been found that may be modulated as targets for gut microbiota intervention.
NLRP3 (NOD-like receptor family, pyrin domain containing 3): The NLRP3 pathway belongs to the innate immune system response. Upon activation, NLRP3 releases pro-inflammatory cytokines that have been shown to promote CVD processes such as cellular hypertrophy and death. NLRP3 activation has been linked to cardiac remodelling in heart failure and atrial fibrillation. NLRP3 activation has been found to be increased following gut microbiota alteration and endotoxemia. Interestingly, the activation of NLRP3 in atrial fibrillation has been attributed to increased circulating LPS originating from age-related microbiota dysbiosis.
TLR4 (Toll-like receptor 4):
TLR4 is a key receptor of the innate immune system for recognizing microbial products such as LPS. Increased TLR4
activation has been shown to contribute to CVD progression such as atherosclerosis, that can be attributed to increased endotoxemia.

TNFR1 (Tumour necrosis factor receptor 1): TNFR1 is a receptor for a major pro-inflammatory cytokine TNF-α. Increased TNFR1 activation has been associated with cardiovascular disorders such as atherosclerosis, heart failure and atrial fibrillation. TNFR1 activation has been shown to increase with systemic inflammation originating from the gut.
Several inflammatory targets appear to be involved in CVD progression following gut microbiota alteration, and all of these factors and their cognate receptors appear to have amplified activity within the end organs implicated in CVD. Therefore, future gut microbiota-targeted intervention should aim at reducing the source of inflammatory signals from the gut that trigger the activation of inflammatory receptors in the cardiovascular system. These future studies will include preclinical models where reductionist approaches can identify necessary and sufficient pathways for microbiota-CVD interaction, machine learning approaches that interpret the complex interplay between diverse microbial constituents in the gut and therapeutic approaches that go beyond current crude live bacterial therapy to druggable products that result from mechanistic mining of the microbe-host interaction in the gut. Finally, insights from latest clinical and pre-clinical research in the field of the gut microbiota point toward a more holistic approach to CVD in general. Cardiovascular disorders in the past half century appear to be manifestations of a more general societal disease state that can be traced back in part to gut microbiota alteration and industrial food production and dietary habits. Therefore, modern clinical engagement with CVD should not only include conventional risk factor modification and therapies but new approaches to diet that incorporate a more sophisticated understanding of the interaction between food, gut microbiota, host intestinal permeability, low grade inflammation and cardiovascular disease progression
As a closing remark, we can note that the concept “We are what we eat” becomes literal when considering this microbial community as a part of us and of our cardiovascular wellbeing.
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 54
CARDIOLOGY:
ATRIAL FIBRILLATION
Innovative technology in Atrial Fibrillation Ablations; A Step to the Future
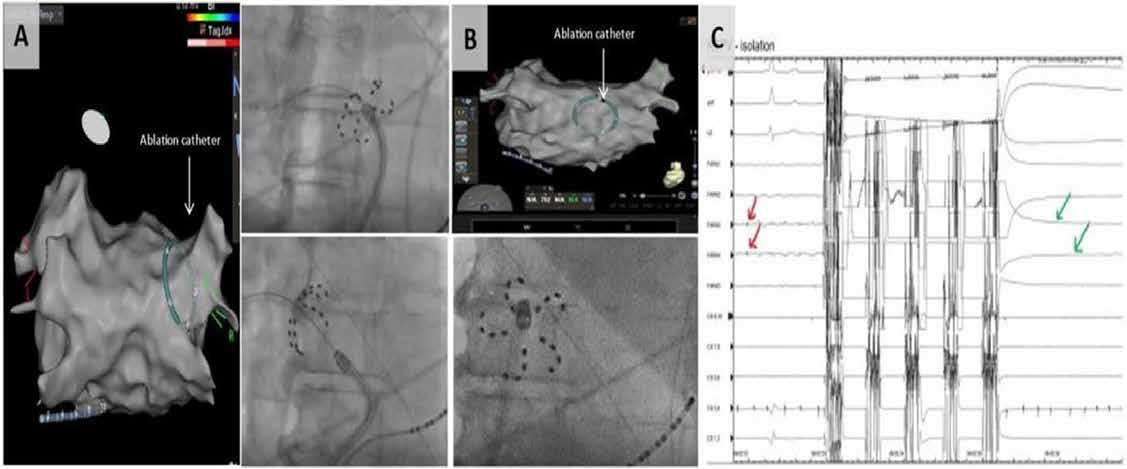
 Written by: Dr Usama Boles, MB, BCH, MSc, PhD, FRCPI, FESC, FHRS, FEHRA - Consultant Cardiologist and Electrophysiologist, Senior Lecturer RCSI Cardiology Department, Tipperary
Written by: Dr Usama Boles, MB, BCH, MSc, PhD, FRCPI, FESC, FHRS, FEHRA - Consultant Cardiologist and Electrophysiologist, Senior Lecturer RCSI Cardiology Department, Tipperary
University Hospital, Clonmel.
Dr Kumaren Ramsamy, BSc (Hons) Med Science; MBChB, MRCP UK - Registrar in Cardiology, Tipperary University Hospital, Clonmel.


Background and Scope of problem:
Atrial fibrillation (AF) is a common cardiac rhythm disturbance, and its prevalence increases with advancing age. Put simply, AF is an uncoordinated atrial electric activation that results in ineffective atrial contraction and hence reduced stroke volume and subsequently reduced cardiac output. It affects a substantial proportion of the population, approximately 37.5 million people worldwide. In Ireland, AF affects around 11% of adults aged over 65 years and up to 25% at the age over 80 years. It is undeniably a cause of disability, imposing additional burden on the healthcare system.
Reducing Atrial Fibrillation Burden:
The attention, in contemporary medical authorities, has therefore
been focused on reducing AF burden through early screening of target groups, diagnosing, treating AF and avoiding its complications thereof - stroke, heart failure and dementia. The range of treatment options include lifestyle changes, such as, increasing physical activity, weight loss, smoking and alcohol avoidance and oral anticoagulation. Rhythm strategy can be achieved by antiarrhythmic drugs, cardioversion and/ or Pulmonary Vein Isolation (PVI) ablation. Guidelines (either European or American) recommend catheter ablation in symptomatic patients who are refractory to drugs, with heart failure and likewise younger group of patients with high exercise endurance.
Rhythm strategy should be discussed between arrhythmia specialists and patients ahead of rate control strategies as the clear benefit in all aspects is quite well
established- it improves functional capacity and quality of life, at the same time reducing the long-term risks of stroke, morbidities and hospital admissions in patients with AF. It reduces the need for anti-arrhythmic drugs, which often
Fig 1: A) PFA multi-electrode catheter in 3 D map around Right Superior Pulmonary Vein on the left panel while Right panel is X Ray of the same configuration. B) Application of PFA on the posterior wall, in some cases with Persistent AF as an additional strategy to the procedures. C) Red arrows show Pulmonary veins potential pre application, then 5 pulses of ablation for 2 seconds at 2000mV and finally green arrows revealed complete resolution and isolation of the pulmonary veins potentials.
come with a wide range of side effects, namely thyroid dysfunction or lung fibrosis. In addition, patients with low to moderate stroke risk might be allowed to discontinue blood thinners and hence minimising the accumulated risk of bleeding.
Advances in Technology of AF ablation:
The advances made in ablative technology, namely pulsed field ablation and high power with short
Dr Usama Boles
55
Dr Kumaren Ramsamy
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Dr Usama Boles
Dr Kumaren Ramsamy
duration radio-frequency ablations created a paradigm shift in the current management of AF.
A) Pulsed field ablation (PFA):
Is a non-thermal ablative modality that is highly safe as it is tissue selective. Antral application of PFA around the pulmonary vein areas are guided by X-rays and / or 3 D mapping and then energy is transmitted through multi-electrode catheters. Each vein requires 4 applications of 2 seconds each. The scar tissue formed, blocks the electric signals from the veins, thereby isolating the pulmonary veins (Fig. 1). The mechanism of action lies in creating pores within the cell membranes, leading to
CARDIOLOGY: ATRIAL FIBRILLATION

B) High power- Short duration
Radiofrequency ablation:
irreversible apoptosis of the antral endocardial cells- the term called electroporation. Importantly, various tissues have specifically characteristic threshold field strengths that induce necrosis. Myocardiocytes have the lowest threshold values of any tissue. This myocardial sensitivity limits collateral damage of non-target tissue such as the oesophagus and phrenic nerve and hence avoids complications. PFA also spares the extra cellular matrix, preventing disruption of tissue planes and minimises the risk of pulmonary vein stenosis. Recent studies comparing RFA and PVI with PFA confirm superiority in terms of outcome, durability of lesions, as well as safety.
The conventional approach of using radio-frequency (RF) energy, of a thermal nature, had its own limitations. In recent years, the novelty of high power (90 watts) and short duration (4 seconds) meant that the ablation phase could be reduced to only 5 mins and all procedure time to 25 mins – much reduced from former procedural times of around 180 mins in the near past. This means that more procedures can be performed in the Cath lab and subsequently drive the lower cost of the procedure. Furthermore, the high safety profile of the newer technology would encourage performing it in elderly group of symptomatic patients, who in the past were excluded due to limitations of previous rhythm strategies.
The Future for AF treatment:
Catheter ablation remains a minimally invasive procedure with overall complication risks less than 1%. In clinical practice, however, it is not uncommon to find that the knowledge regarding treatment options, amongst patients with AF, to be suboptimal. There are at times the fear or misconceptions about newer technology, that


can be a hindrance. That said, more attention needs to be paid towards improving the perception of emerging technology within the community and clinicians to be better advocate for their patients. Rhythm strategy in AF management must emphasise on the new technology that would reduce complications rate and increase procedure safety, reduce procedure time and hence rapid recovery, reduce cath lab time, increase volume of performed procedures, reduce waiting lists, reduce the overall cost, increase opportunities for training, minimise the related budgets to drugs, hospital admissions and related complications.
The future is ever so promising in this ablation era, with innovative technology unfolding and evidence backed up by valid trials. From low voltage myocardium guided ablation, to epicardial or ganglion ablation, it will take some well-trained Electrophysiologist equipped with the new technology to promote and uplift the current management strategies of AF and maintaining the international standard.
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 56
Fig 2: Example of high power – short duration RF of PVI procedure. Dotted red points represent effective RF lesions around the pulmonary veins. Middle of screen, the bale green circle with red circle indicates the accuracy of the catheter tip and contact with endocardial tissue to ensure accuracy to delivery effective RF lesions.
CARDIOLOGY: HI PEITHO

Intermediate-high risk pulmonary embolism: addressing a knowledge gap through multinational collaboration
 Written
Chloe
Written
Chloe

Case Study
A 78-year-old male with a background medical history of hypertension presented to the emergency department with pleuritic chest pain and a 30-second episode of collapse. Further investigations revealed elevated d-dimer (>20 µg/mL), troponin (232 ng/L), and BNP (2644 ng/L) levels. A CT pulmonary angiogram (CTPA) showed extensive bilateral pulmonary emboli in the main pulmonary arteries, extending into the lobar and segmental pulmonary branches. The CTPA also revealed evidence of heart strain with a RV/LV ratio >1.0. On the basis of clinical and imaging parameters, the patient was categorised as having an intermediate-high risk pulmonary embolism.

Introduction
Venous thromboembolism, comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading cause of death in developed countries.1 PE can be lethal through a spiral of events culminating in right ventricular strain, right ventricular failure, systemic hypotension and cardiac failure.2 Assessment of PE severity is crucial when determining appropriate treatment
options. High risk pulmonary embolism is defined by current European Society of Cardiology (ESC) guidelines by cardiac arrest or haemodynamic instability/ shock and is associated with very high mortality.2, 3 Patients with intermediate risk pulmonary embolism do not fulfil criteria for high-risk PE but may be very unwell, with right ventricular strain, elevated biomarkers of myocardial injury and a high “PE severity index”, which predicts early mortality.2
In particular, patients with severe (intermediate-high risk) pulmonary embolism according to ESC criteria may deteriorate rapidly. This situation is a clinical emergency. Optimal management of these patients remains an urgent knowledge gap and is now being addressed in a landmark randomized controlled trial (RCT) comparing ultrasoundfacilitated, catheter-directed thrombolysis to anticoagulation for acute intermediate-high risk pulmonary embolism.4 This trial, the “higher-risk pulmonary embolism thrombolysis (HiPEITHO) study” (ClinicalTrials. gov Identifier: NCT04790370) has been designed to address this important gap in clinical evidence by comparing the clinical benefits
of the ultrasound-facilitated local delivery of a thrombolytic agent and anticoagulation with those of anticoagulation alone in patients with intermediate high-risk PE at a higher estimated risk of early decompensation based on clinical parameters at presentation. The EkoSonicTM Endovascular device (Boston Scientific) will be utilized in the HI PEITHO RCT, employing ultrasound energy intended for the ultrasound-facilitated, controlled, and selective infusion of physicianspecified fluids, including thrombolytics, into the vasculature for the treatment of PE.
The primary objective of the study is to assess whether ultrasoundfacilitated, catheter directed, thrombolysis and anticoagulation are associated with a significant reduction in the composite outcome of PE-related mortality, cardiorespiratory decompensation or collapse, or non-fatal symptomatic and objectively confirmed recurrence of PE compared to anticoagulation alone within seven days of randomization. Additional objectives are to contribute further evidence to the existing data on the treatment and outcomes of acute, intermediatehigh risk PE and to provide controlled data comparing a catheter-based intervention to the

standard of care that is currently recommended in the guidelines.
Study Design
The trial is a post-market, randomized, controlled, adaptive, open-label, multicentre parallel group trial with blinded adjudication of the primary composite outcome. Subjects will be randomized 1:1 to treatment with ultrasound-facilitated catheter-directed thrombolysis (USCDT) and anticoagulation or anticoagulation alone. The study is unblinded to Investigators and subjects, but adjudication of the outcome measure and other safety outcomes will be completed by a blinded Clinical Events Committee.
Initially, 406 subjects are planned to be enrolled at up to 65 sites in the US and Europe. There will be a planned interim analysis after 50% are enrolled, with the potential to enrol up to 544 subjects. The follow up period for each subject is 12 months.
Treatment Groups
Test Arm: Assignment to the USCDT will include both treatment with the USCDT procedure and treatment with anticoagulation. The USCDT procedure will entail delivery of alteplase using the EkoSonicTM Endovascular
Fionnuala Ní Áinle
57
Andrew Yates
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
by:
Carpenter, Umer Salati, Fionnuala Ní Áinle and Andrew Yates
Chloe Carpenter
Umer Salati
serve.
CARDIOLOGY: HI PEITHO
National Principal Investigator) and Galway University Hospital (under the leadership of Dr Gerry O’Sullivan). The Hi-PEITHO study will, for the first time, address a crucial knowledge gap that has been deemed to be of extremely high priority by the patients that we serve. With clinically important endpoints, the highquality study design, led by global leaders in the field of PE care and intervention, will be delivered by teams of multidisciplinary partners in both hospitals. The data will shape future clinical guidelines by providing high-quality data to guide the management of some of our sickest PE patients.
References
1. Barco, S., et al., Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med, 2020. 8(3): p. 277-287.
2. Konstantinides, S.V., et al., 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J, 2019.

3. Barco, S., et al., Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and metaanalysis. Eur Heart J, 2019. 40(11): p. 902-910.

4. Klok, F.A., et al., Ultrasoundfacilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediatehigh-risk pulmonary embolism: Rationale and design of the HI-PEITHO study. Am Heart J, 2022. 251: p. 43-53.
System. Treatment must be initiated as soon as practical but no more than 6 hours after confirmation of diagnosis of intermediate-high risk PE. It is strongly recommended that it begins within 2 hours of randomization. Assignment to the experimental USCDT arm will also include initiation or continuation of anticoagulation therapy i.e. low-
molecular weight heparin (LMWH) or unfractionated heparin (UFH).
Control Arm: Assignment to the anticoagulation-only control arm will include either initiation or continuation of anticoagulation therapy i.e., low-molecular weight heparin (LMWH) or unfractionated heparin (UFH). Therapeutic anticoagulation is the current
standard of care in the treatment of acute intermediate-high risk PE.
The Irish Network for VTE Research is privileged to participate in this potentially practice-defining study and two sites have been selected in Ireland: The Mater Misericordiae University Hospital (under the leadership of Dr Umer Salati,
With clinically important endpoints, the high quality study design, led by global leaders in the field of PE care and intervention, will be delivered by teams of multidisciplinary partners in both
The data will shape future clinical guidelines by providing high-quality data to guide the
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 58
Figure 1: Overview of key features of Hi-PEITHO study design
Figure 1: Overview of key features of Hi-PEITHO study design

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
CARDIOLOGY: PREGNANCY
Cardiovascular Disease in Pregnancy

Cardiovascular disease remains a major cause of both morbidity and mortality during pregnancy and in the peripartum period, despite major advances in the care of such patients. The concept of the cardio-obstetric team has received renewed attention and importance in the 2018 European Society of Cardiology guidelines. We wish to present the following case which demonstrated the utility of multidisciplinary care in complex cardiac patients in terms of achieving excellent maternal and foetal outcomes.

A 38-year-old (G2P1) refugee from Eastern Europe was referred to our cardiology service regarding
management of high-risk cardiovascular disease during her pregnancy. Her cardiovascular background was notable for prior history of rheumatic fever, culminating in a mechanical mitral valve replacement at 32 years old. Her background history was also notable for prior venous thromboembolism while taking the combined oral contraceptive pill. Her obstetric history was notable for prior lower segment Caesarean section in 2012 under general anaesthesia prior to her mitral valve replacement. Unfortunately, we were unable to get more specific details regarding prior surgery and transthoracic echocardiogram (TTE) results from her country of birth.
Given the complexity of the case, she was discussed as part of the local high-risk obstetric multidisciplinary team for optimal management of this patient. This included input from Obstetrics,




Cardiology, Cardiothoracic Surgery, Haematology, Anaesthesia, and other allied healthcare staff.
She was categorised as maternal WHO Class III; current guidelines recommend at least monthly assessment as this risk stratification implies significant maternal and foetal risk during pregnancy, but not to a degree in which continuation of the pregnancy is contraindicated provided that relevant local expertise is available. The management of anticoagulation in patients with mechanical prostheses remains challenging; current ESC Guidelines recognise the paucity of high-quality evidence in this area. After meeting with the patient and discussing the potential options regarding management of her anticoagulation during pregnancy,
consensus opinion was to switch to low molecular weight heparin with anti-factor Xa monitoring at least every two weeks and remain on same throughout her pregnancy until time of delivery.
TTE at 12 weeks demonstrated moderate-to-severe aortic regurgitation, preserved left ventricular function, with a wellseated mitral valve prosthesis and a moderate transvalvular gradient of 7mmHg. This was followed by serial studies conducted in the late second and third trimesters, which demonstrated a mild increase in the transvalvular gradient; this was discussed between cardiology and cardiothoracic surgery and was felt

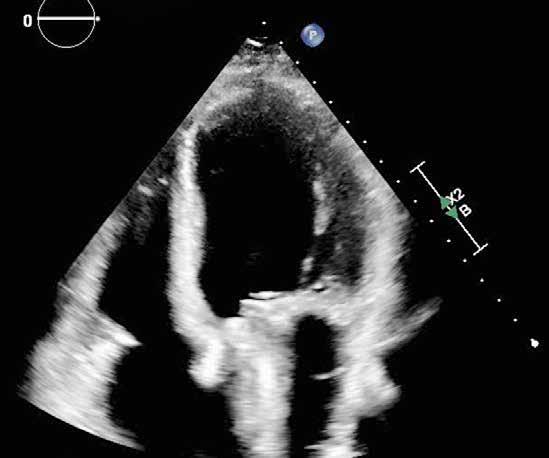
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 60
Dr Ronan Cusack
Written by Dr Max Waters, Dr Ronan Cusack, Dr Samer Arnous, Dr Cormac O’Connor, Prof Tom Kiernan
Dr Max Waters
Dr Samer Arnous
Dr Cormac O’Connor Professor Tom Kiernan
Figure 1. Transthoracic echocardiography (apical four chamber) demonstrating mechanical mitral valve replacement in situ
Figure 2. Transthoracic echocardiography (parasternal long axis view) demonstrating mechanical mitral valve replacement in situ
Allied Health Professionals
Cardiothoracic Surgery
Cardiology

















to be secondary to haemodynamic changes related to the pregnancy, given that mobile leaflets were seen without any concern for valve thrombosis morphologically and an otherwise unchanged appearance on imaging.
Obstetrics
Anaesthesia Haematology
Elective Caesarean section was undertaken after 38 weeks gestation. Epidural anaesthesia with slow titration, with radial arterial line in situ for invasive blood pressure monitoring was undertaken. Low molecular weight heparin was stopped 24 hours prior to delivery, with anticoagulation restarted six hours post-delivery and bridging onto warfarin until a therapeutic INR was reached.
Thankfully, our patient underwent an uncomplicated Caesarean section and delivery of a healthy neonate without complication, and after overnight monitoring in our high dependency unit was felt to be stable enough to transfer back to the maternity hospital. On review in the outpatient cardiology clinic she remains clinically well and asymptomatic from a cardiovascular perspective, though she will require monitoring and follow-up of her native aortic valvular regurgitation and mechanical mitral prosthesis.
members of high risk pregnancy multidisciplinary team for patients with cardiac disease.
Cardiology News

Heart Health and the Menopause
The inaugural National Menopause Summit, hosted by Gráinne Seoige took place in Dublin recently. The Irish Heart Foundation was the Official Wellbeing Partner for the summit, and provided important information and resources to attendees on how to care for their heart health as they move through menopause.
The summit featured a range of speakers and panellists, including Dr Sumi Dunne, Loretta Dignam of The Menopause Hub, GP Dr Máire Finn and, Dietitian Aveen Bannon who discussed the challenges that can accompany perimenopause and menopause. Speakers also discussed how to shift the existing narratives around menopause by debunking commonplace myths and taboos, providing inclusive and factual information, and advocating for change to support women in both their personal and professional lives.
The Irish Heart Foundation’s Director of Health Promotion, Information & Training Janis Morrissey, was among the panellists for the summit’s discussion on health and wellness. Speaking on the panel, Janis outlined how, “medical research has really done women a disservice, as heart disease and stroke has been under-recognised, under-diagnosed, and undertreated in women.”
“So much around women’s health is around checking your breasts, cervical checks, etc., but what about hearts” said Janis , as she was keen to highlight the startling statistics that women are six times more likely to die of heart disease or stroke than breast cancer. She drew attention to the need for women to ‘take stock’ of their current lifestyle and use the self-care and wellbeing journal developed by the Irish Heart Foundation as part of its Her Heart
Matters campaign when they are ready to make a change. Other resources available includes an infographic about heart health and menopause, a menopause and your heart health leaflet and, real women’s stories.
Dr Deirdre Lundy also spoke about how the drop in oestrogen that occurs during menopause can place women at a higher risk of developing heart disease or stroke. In discussing hormone replacement therapy (HRT) and the heart, Dr Lundy drew on the current research, but reminded attendees of the need for more research focused on women’s health. Dr Lundy is a key voice in the Irish Heart Foundation’s Her Heart Matter campaign, and her FAQ about menopause and heart health can be read here.
As well as providing information about how women’s risk of heart disease and stroke increases
with menopause, Janis Morrissey highlighted how symptoms of heart attack can present differently in women, and often are dismissed or unrecognised. She explained how “the symptoms can often be much more subtle,” such as experiencing arm, jaw, neck or upper back pain, extreme fatigue, or nausea and encouraged women to “get help immediately” if something does not feel right.
Empowering women was another theme of the summit, and attendees left the event equipped with best-in-class knowledge and information to navigate the changes and challenges that menopause and perimenopause present.
Addressing the National Menopause Summit generally, keynote speaker Davina McCall described menopause as being, “like a second spring; it’s a time of being reborn and navigating your way through it.”
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 61
Figure 3. Core members of high-risk pregnancy multidisciplinary team f or patients with cardiac disease
Transthoracic echocardiography (parasternal long axis view) demonstrating mechanical mitral valve in situ.
CARDIOLOGY: ECHOCARDIOGRAPHY
Role of Echocardiography in Amyloid Cardiomyopathy
Written by Paul Nolan, Lecturer in Clinical Measurement Physiology & Cardiac Physiologist.


European Association of Cardiovascular Imaging –Certification in Adult Echocardiography.
Introduction
Cardiac Amyloidosis is a rapidly progressive infiltrative cardiomyopathy associated with significant mortality and morbidity. It is characterised by the deposition of amyloid fibrils, misfolded protein deposits, in the myocardium and there are two types described. One, light chain amyloidosis (AL), is
due to myocardial deposition of monoclonal immunoglobulin light chain proteins produced by bone marrow plasma cells and is rare. The second, transthyretin amyloidosis (ATTR), is caused by a transport protein that is synthesized primarily by the liver. ATTR has two sub-types, hereditary or variant (vATTR) and wild-type (wtATTR), with wildtype being the significantly more prevalent of the two.
AL and ATTR have different clinical courses, prognosis, and potential treatments. Cardiac involvement is common in both, occurring in up to 75% of AL cases and it is the dominant clinical feature in ATTR
patients. ATTR has a slower and more varied clinical course with a median survival of around 3-5 years if untreated, compared to the more rapid progression of AL, with an untreated median survival of 6 months.
Previously, treatment for ATTR cardiomyopathy (ATTR-CM) was solid organ transplantation but the new effective treatments and emerging therapies have increased the importance of recognising it at an early stage.

Role of Echocardiography
While the gold standard for diagnosis of the disease is myocardial biopsy, echo plays
a major role in the diagnosis. However, echo by itself is insufficient to make the diagnosis with other investigations required to confirm the presence of cardiac amyloid and differentiate between AL and ATTR. Consensus guidelines recommend it should be combined with electrocardiographic, clinical, biomarker, and other imaging findings to maximize diagnostic accuracy.1 Some proposed scoring systems combine ECG, echo, and biomarker measurements. One group has suggested a combination of troponin T >30ng/L, left ventricular wall thickness >13.6mm and QRS width >120ms had strong positive
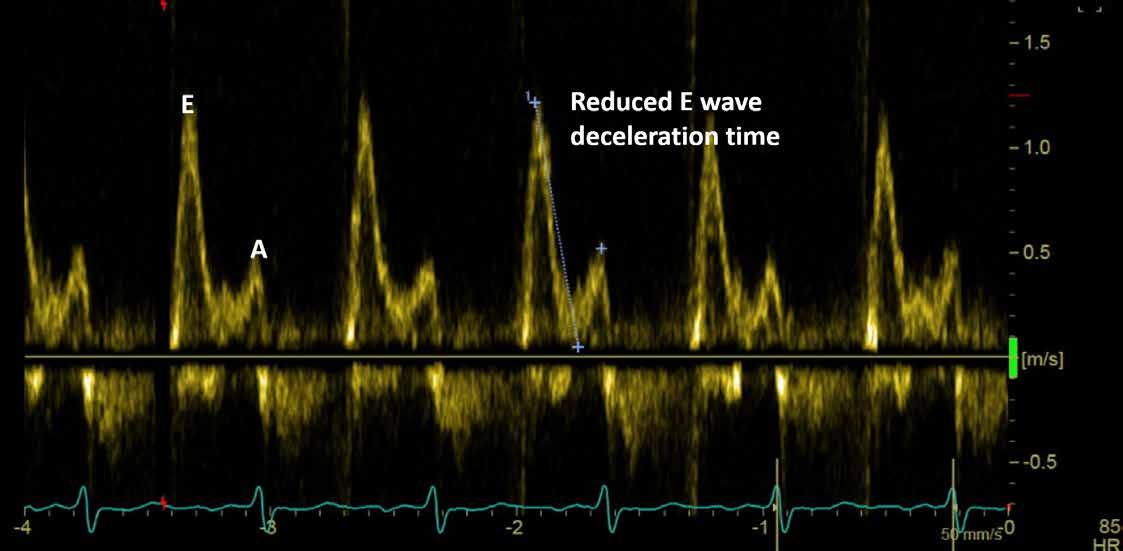
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 62
Fig 1 – Transmitral pulsed wave doppler trace showing reduced E wave deceleration times and raised E:A ratios
and negative predictive value.2 They also highlight the importance of other imaging modalities with potential sequential use of echo, cardiac MRI, and SPECT scanning to aid in the diagnosis and the differentiation between AL and ATTR cardiomyopathies.
Whilst echocardiography is widely available, it does lack the tissue characterisation ability of MRI, so echo relies on the presences of highly suggestive features which include.
• Increased LV and RV wall thickness
• Biatrial enlargement and thickening of the interatrial septum
• Evidence of stage II diastolic dysfunction or worse

• Reduced mitral annular velocities on tissue doppler imaging
• Reduced global longitudinal strain
• Low flow-low gradient aortic stenosis
• Diffuse valve thickening
• Pericardial effusion
One of the challenges with the often-quoted list2 is that these findings are quite non-specific and the typical features, such as a markedly increased wall thickness and a restrictive filling pattern may
not be present until advanced stages of the disease.
Increased wall thickness, or left ventricular hypertrophy (LVH), occurs in other conditions, such as hypertension, hypertrophic cardiomyopathy (HCM) or indeed other infiltrative conditions such as Fabry’s disease. A wall thickness >12mm, without another plausible cause, is utilised and this represents only a mild thickening which may be present in undiagnosed hypertension. LVH is also a key feature of significant aortic stenosis (AS), a very plausible cause, but it has been shown that ATTR cardiomyopathy can be present in 16% of elderly TAVI patients,3 so we need to maintain an appropriate level of suspicion, even in cases of mild LVH or in cases of AS.
Whilst challenging to differentiate LVH due to amyloid from other causes, amyloid cardiomyopathy is associated with small chamber sizes and low stroke volumes, compared to hypertensive heart disease. A relative wall thickness (calculated as twice the posterior wall thickness divided by LV diastolic diameter) of >0.42 can be suggestive of amyloid cardiomyopathy. In true LVH an increase in size of the muscle cells leads to an increase in voltage, i.e., larger amplitude QRS complexes on ECG. However, in amyloid, as the wall thickness is due to infiltration,
there is an ECG-LVH mismatch, with the QRS amplitudes not reflecting the increased wall thickness seen. This mismatch can also raise a suspicion that the increased thickness is due to cardiac amyloid. A key feature of HCM is asymmetrical hypertrophy and we would also expect large QRS amplitudes on ECG. It is proposed that a septal to posterior wall thickness <1.6 combined with a summed QRS amplitude of <30mm could differentiate between hypertrophic and amyloid cardiomyopathies.4 The combination of right and left ventricular hypertrophy is a classic finding seen in cardiac amyloid but not in other cases of left ventricular hypertrophy. The pattern of LVH seen on echo can help differentiate AL from ATTR cardiomyopathy with the former demonstrating concentric pattern of LVH compared to the latter where septal hypertrophy is more likely.
The classic phenotype of amyloid cardiomyopathy is heart failure with preserved ejection fraction (HFpEF) due to the infiltrative process leading to significant diastolic dysfunction. However, this is not an essential element as this may only manifest in more advanced stages of the disease. Patients with cardiac amyloid often have stage II or stage III (a restrictive filling pattern) diastolic dysfunction on echocardiography. This is seen as reduced E wave
– Image showing reduced mitral annular velocities on tissue doppler imaging associated with amyloid cardiomyopathy
deceleration times and raised E:A ratios on transmitral doppler (Fig 1), raised E/e’ measurements, and left atrial (LA) enlargement. Amyloid cardiomyopathy is also associated with reduced measurements on tissue doppler imaging with S’, e’ and a’ all measuring <5cm/s (Fig 2). Echo grading of diastolic dysfunction can be complex and the above measurements, in combination with the velocity of tricuspid regurgitation should be assessed and classified according to current guidelines.5 LA enlargement in diastolic dysfunction is due to atrial remodelling in the setting of raised LA pressures, secondary to raised LV diastolic pressures. We also see atrial dilatation in the setting of atrial fibrillation, which is also commonly associated cardiac amyloid.
The increasingly used tool in echocardiography of global longitudinal strain (GLS) is useful in the diagnosis of amyloid cardiomyopathy. GLS is a unitless measure of longitudinal LV function and can demonstrate a reduction before a frank reduction in ejection fraction can be measured. GLS tracks the echo “speckles” within the myocardium. In an actively contracting segment of myocardium, speckles will move closer to each other. Reflecting this shortening, the rate of shortening is expressed as a negative value, with more negative values indicating more
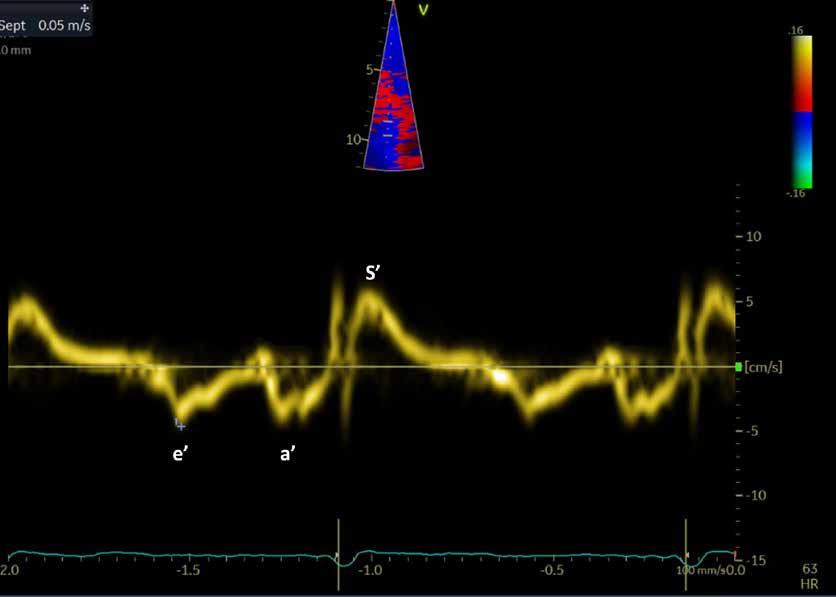
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 63
Fig 2
deformation or shortening. Thus, a segment showing a GLS of -20 is contracting more than a segment with a value of -10. There are software differences amongst vendors resulting in variances in quoted normal values. In general values more negative than -18 to -20 are considered normal, but it is recommended that serial studies on a patient are carried out on the same manufacturer machine as the original study. In a GLS study each of the seventeen echo segments from basal to apical is given a score and an average GLS is also calculated.
In amyloid cardiomyopathy, the GLS “bullseye” plot, can show a classical apical sparing, where basal and mid GLS decreases with normal values in the apex (Fig 3). This pattern is seen in both AL and ATTR cardiomyopathy and is sometimes referred to as having a “cherry on top” appearance, as brighter red values signify greater shortening. The exact mechanism for this is not fully understood but an accepted hypothesis is that the amyloid fibrils are preferentially deposited in the basal and mid segments of the ventricles. Several ratios using GLS, such as average apical to average basal and mid values; septal apical to basal values; and EF to average GLS have been studied.
CARDIOLOGY: ECHOCARDIOGRAPHY
Fig 3 – “Bullseye” Global Longitudinal Strain image, demonstrating classic apical seen in the condition. Note Basal segments are on the outside, apical segments in the centre with brighter red signifying more longitudinal shortening
GLS can be prone to errors in its performance and the apical sparing pattern is not exclusive to amyloid cardiomyopathy, therefore, alone it is insufficient to make the diagnosis.
Where there is echocardiographic evidence or a clinical suspicion of amyloid cardiomyopathy the echo report should explicitly comment on the likelihood of amyloidosis based on the imaging as not suggestive, strongly suggestive, or equivocal for cardiac amyloid.

From an echocardiography point of view AL and ATTR cardiomyopathies demonstrate similar echo findings, with perhaps the only differentiator being the asymmetrical hypertrophy seen more commonly in ATTR. For that reason, those in the field of echocardiography should be aware of some key clinical features that would raise a suspicion of ATTR cardiomyopathy. The most striking is that nearly 50% have bilateral carpal tunnel syndrome.6 They can also present with peripheral and autonomic neuropathies. As the amyloid deposits also affect the cardiac conduction tissue, they can develop arrhythmias and high grade atrio-ventricular block.
Conclusion
Amyloid cardiomyopathy is associated with significant
mortality and morbidity and with new and emerging treatments early recognition is important. Echocardiography plays a central role in this and whilst no one feature is diagnostic, key red-flags should raise a suspicion when you see unexplained LVH, particularly in older patients. We should also remember the association between aortic stenosis and ATTR cardiomyopathy, keeping this dual diagnosis in mind in the presence of other features.
Classic echo findings include left and right ventricular hypertrophy, biatrial enlargement, significant diastolic dysfunction with reduced annular tissue doppler velocities and the apical sparing pattern seen in GLS. One should remember that no single feature is diagnostic for the disease, but rather it is a constellation of findings. Those working in echo should have an index of suspicion for amyloid cardiomyopathy in the setting of HFpEF and/or the echo features described in this article.

About the author
Paul Nolan is a Clinical Lecturer on the Clinical Measurement Physiology programmes in Atlantic Technological University and is a Cardiac Physiologist of over 20 years’ experience. He is a holds certification from
the European Association of Cardiovascular Imaging in Adult Echocardiography.
References
1) Dorbala et al. ASNC/AHA/ ASE/EANM/HFSA/ISA/SCMR/ SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis. Journal of Cardiac Failure Vol. 2019;25(11)
2) Cuddy et al. Practical Points for Echocardiography in Cardiac Amyloidosis. J Am Soc Echo 2022;35(9):A31-40
3) Marume K, Takashio S, Nishi M, et al. Combination of commonly examined parameters is a useful predictor of positive 99 mtc-labeled pyrophosphate scintigraphy findings in elderly patients with suspected transthyretin cardiac amyloidosis. Circ J. 2019;83(8):1698-1708.
4) Castaño A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879-2887
5) Nagueh SF, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314.
6) Connors LH, Sam F, Skinner M, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wildtype transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 64
CARDIOLOGY: ESC 2022
ESC 2022 – What's new in trials and guidelines? Important new clinical trials and guidelines were launched at the ESC Congress 2022 in Barcelona
Written by Professor Robert Byrne, Director of Cardiology, Mater Private Network, Chair of Cardiovascular Research, RCSI and Dr Sean Fitzgerald, Clinical and Research Fellow, Interventional Cardiology, Mater Private Network/CVRI

In terms of clinical practice guidelines, new or updated guidelines were released in four areas relevant to current clinical practice at ESC 2022: cardio-oncology, pulmonary hypertension, cardiovascular assessment of patients undergoing non-cardiac surgery, and ventricular arrhythmias and sudden cardiac death.
The cardio-oncology guidelines have been published for the first time and emphasise the integration of knowledge across cardiology, oncology and haematology with specific emphasis on minimising unnecessary cancer therapy interruptions and cancer therapy-related cardiovascular toxicity across the entire spectrum of cancer care.1
In terms of the updated pulmonary hypertension (PH) guidelines, key messages to note are that the definition of PH has been updated to a mean pulmonary artery pressure > 20 mmHg (from 25 mmHg) with a peripheral vascular resistance (PVR) of > 2 WU and pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg, although the efficacy of pulmonary hypertension therapies at the lower limits of this range is unproven.2
In terms of ventricular arrhythmias and the prevention of sudden cardiac death, when considering ICD therapy benefit, competing risk factors for non-arrhythmic death and the patient’s wishes and quality of life need to be taken into account.3

From the point of view of guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery, it is important to note that clinical examination, patient-reported functional capacity and non-invasive tests represent the cornerstone of pre-operative cardiac assessment, and invasive assessment is not always required.4
Left ventricular dysfunction
Several ground-breaking trials, published simultaneously in the New England Journal of
Medicine, were presented at ESC. REVIVED-BCIS2 was a study on percutaneous revascularisation for ischaemic left ventricular dysfunction.5 It was academia-led and independent from industry funding. The hypothesis of this study was that revascularisation in combination with optimal medical therapy, compared to optimal medical therapy alone, leads to improved event-free survival in patients with severe ischaemic left ventricular dysfunction and evidence of myocardial viability. Of note, there is evidence that in the same setting, albeit without formal viability assessment, that surgical revascularisation improves longterm survival on the basis of the results of the STITCH trial.6
To be included, patients needed to have a left ventricular ejection fraction of less than 35% and have demonstrated evidence of viability in at least four myocardial segments that were amenable to percutaneous revascularisation. Patients also required reasonably extensive evidence of coronary artery disease and this was assessed by a special scoring system, the BCIS Jeopardy score, which is not in routine use outside of the UK. It was a trial of chronic coronary syndrome, so patients with a recent acute coronary syndrome or current unstable presentation were excluded. The overarching result was that there is no difference between PCI and optimal medical therapy in terms of the primary outcome of death from any cause or hospitalisation for heart failure (HF). Specifically, the relative risk was 0.99 (95% CI 0.78-1.27) for patients treated with PCI. Of note, there was a difference in the numbers of spontaneous myocardial infarction (MI) with 18 in the PCI arm versus 33 in the medical therapy arm. Ultimately, when interpreting the
results of the trial, it is important to take the inclusion criteria into account and not generalise the results of this study, taking note in particular that patients with angina were not well represented. Overall, the results of this trial do re-emphasise that the niche for PCI in stable ischaemic coronary artery disease is symptom control, rather than providing a mortality benefit. It would be interesting to determine if the results of the STICH trial still held up if it were run in the present day with excellent medical therapy for HF, in particular the availability of sacubitril-valsartan and SGLT-2 inhibitors.
DOACs vs warfarin in RHD
INVICTUS was a non-inferiority trial which aimed to compare the use of the DOAC (direct oral anticoagulant), rivaroxaban versus vitamin K antagonists in the treatment of rheumatic heart disease in Africa, Asia and Latin America.7 The presence of a metallic valve or likelihood of implantation of a metallic valve in the next six months was an exclusion criterion. The primary endpoint was a composite of stroke, systemic embolism, MI or
death from vascular or unknown causes. In terms of the KaplanMeier curves, we can clearly see a divergence between rivaroxaban and vitamin K antagonists at 54 months, in favour of vitamin K antagonists in a statistically significant manner, although numerically there were more bleeding events in the vitamin K antagonist group compared to the rivaroxaban group. However, this difference in bleeding outcomes was not statistically significant. Thus, vitamin K antagonists remain the anticoagulant of choice in the setting of rheumatic valvular heart disease.
Outcomes
in patients post highrisk PCI
The Post-PCI trial attempted to answer the question of whether routine care or systematic postprocedural functional testing led to differing outcomes for patients post high-risk PCI.8 Patients required at least one high-risk anatomical feature such as a bifurcation or left main lesion for inclusion in the study, or one highrisk clinical feature, eg. diabetes or chronic kidney disease. A total of 1,706 patients were included.
65
Professor Robert Byrne
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Functional testing included nuclear, exercise ECG or exercise stress test. The primary endpoint was a composite of death from any cause, MI or hospitalisation from unstable angina at two years. The key result was that no difference was seen between the functional testing group versus standard of care, with the primary endpoint occurring in 5.5% of the functional testing group compared to 6.0 % of the standard care group. Looking at the individual components of the primary endpoint, there was a numerical but not statistically significant difference in terms of all cause death and MI (with higher event rates in the functional testing arm) and there does not appear to be a clear mechanistic explanation underlying them. Overall, we can conclude that there is no additional benefit to routine functional testing over standard care post-PCI.
Dapagliflozin in heart failure

DELIVER looked at dapagliflozin in HF with mildly reduced or preserved ejection fraction.9 This was a double blind randomised controlled trial and patients were only eligible for randomisation if they had a left ventricular ejection fraction of > 40%, had elevated natriuretic peptides and evidence of structural heart disease. The primary outcome was defined as a composite of either an unplanned hospitalisation for HF, an urgent

visit for HF or cardiovascular death. A significant difference was seen in the dapagliflozin arm compared to the placebo arm, corresponding to a hazard ratio of 0.82 (0.73-0.92), p < 0.001 for dapagliflozin. Adverse events appeared well balanced between the groups. Here, we can conclude that dapagliflozin is one of the few medications that is safe and effective in the difficult-to-treat cohort of patients with mildly reduced or preserved HF. It will be interesting to see further research outlining the differential effect of dapagliflozin as ejection fraction increases, particular in terms of whether a benefit remains in those patients with an EF of > 60%.
Polypill treatment
The SECURE trial looked at the efficacy of a polypill-based strategy, containing aspirin, ramipril and atorvastatin, as compared to usual care, with respect to major cardiovascular outcomes in older patients with recent MI.10 Eligible patients needed to have had a type one MI in the past six months and have a significant risk factor profile as dictated by age > 75 or > 65 with diabetes, mild or moderate kidney dysfunction, previous MI or coronary revascularisation or previous stroke. The hazard ratio for the polypill compared to usual care was 0.76 (95% CI, 0.60-0.96), p = 0.02 for superiority in terms of a primary endpoint of death from
CARDIOLOGY: ESC 2022
cardiovascular causes, non-fatal type one MI, non-fatal ischaemic stroke or urgent revascularisation. Even without consideration of urgent revascularisation, there was a significant difference between groups. While use of a polypill is reasonably common in Ireland, this is not the case in other European countries, and it will be interesting to see if there is an increased uptake internationally on the basis of this trial. Furthermore, the currently available polypill has the weakness that it does not come in a formulation that includes a highintensity statin (eg. atorvastatin 80mg), and it would be interesting to assess the additional difference in outcomes as a consequence of including this higher dose. Overall though, we can conclude that use of a polypill is a relatively simple strategy to improve patient outcomes in this higher-risk cohort, and should be implemented where possible.
Acetazolamide in HF treatment
The ADVOR trial was designed to assess the benefit of acetazolamide in acute decompensated HF with volume overload.11 The aim of the study was to assess if the addition of acetazolamide to standard IV loop diuretic therapy would improve the incidence of successful decongestion. It was a multicentre, parallel-group, double-blind, randomised, placebo-controlled trial in adult patients with AHDF, clinical signs
of volume overload and elevated natriuretic peptide levels. The primary endpoint was successful decongestion – absence of signs of volume overload within three days after randomisation without an indication for escalation of decongestive therapy. Patients needed to be on a stable dose of oral diuretics for at least one month prior to randomisation. Of note, treatment with other medication which acted on the proximal tubule, including SGLT2 inhibitors, was an exclusion criterion. In summary, the risk ratio for successful decongestion within three days after randomisation was 1.46 (95% CI, 1.17-1.82) for acetazolamide vs placebo, with successful decongestion at discharge also being higher (78.8% of acetazolamide vs 62.5% of placebo, RR 1.27, 95% CI 1.13-1.43). In the longer term, there was no significant difference between death from any cause or rehospitalisation for HF during follow-up. Overall, we can report that acetazolamide seems to be helpful in terms of improving decongestion, but at present does not translate into a difference in hard clinical endpoints.
In summary, in the spheres of percutaneous coronary intervention in ischaemic heart disease, HF with reduced ejection fraction and anticoagulation for rheumatic heart, significant new information at ESC 2022 has been made available, which will guide our assessment and management of these challenging cases.
References available on request
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 66
ABBREVIATED PRESCRIBING INFORMATION – IRELAND
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Apexxnar®▼ suspension for injection in pre-filled syringe
Pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed)

Presentation: Each 0.5 mL dose of Apexxnar contains 2.2 micrograms of each of the following polysaccharide serotypes: 1, 3, 4, 5, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F and 4.4 micrograms of polysaccharide serotype 6B. Each polysaccharide is conjugated to the CRM197 carrier protein and adsorbed on aluminium phosphate. 1 dose (0.5 mL) contains approximately 51 µg CRM197 carrier protein and 0.125 mg aluminium.
Indications: Active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older. Apexxnar should be used in accordance with o cial recommendations taking into consideration the risk of invasive disease and pneumonia in di erent age groups, underlying comorbidities as well as the variability of serotype epidemiology in di erent geographical areas.
Dosage and Administration: For intramuscular injection. Individuals ≥ 18 years of age and older: One single dose. The need for revaccination with a subsequent dose of Apexxnar has not been established. Based on the clinical experience with Prevenar 13, if the use of 23-valent pneumococcal polysaccharide vaccine is considered appropriate, Apexxnar should be given first. Special populations: There are no data with Apexxnar in special populations. Limited experience from clinical studies with Prevenar 13 are available in adults at higher risk of pneumococcal infection either immunocompromised individuals or following bone marrow transplantation. Based on these data the following posology was recommended for Prevenar 13: Individuals at higher risk of pneumococcal infection (e.g., individuals with sickle cell disease or HIV infection), including those previously vaccinated with 1 or more doses of PPSV23, were recommended to receive at least 1 dose of Prevenar 13. In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunisation series with Prevenar 13 consisted of 4 doses of 0.5 mL each. The primary series consisted of 3 doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose was recommended 6 months after the third dose.
Contra-indications: Hypersensitivity to the active substances, to any of the excipients, or to diphtheria toxoid.
Warnings and Precautions: Do not administer intravascularly. Appropriate medical treatment and supervision must be available in case of anaphylaxis. Vaccination should be postponed in individuals su ering from acute severe febrile illness. However, the presence of a minor infection, such as a cold, should not result in the deferral of vaccination. It should not be given to individuals with thrombocytopenia or a bleeding disorder that would contraindicate intramuscular injection. The risk of bleeding in patients with coagulation disorders needs to be carefully evaluated before intramuscular administration of any vaccine, and subcutaneous administration should be considered if the potential benefit clearly outweighs the risks. Apexxnar will only protect against Streptococcus pneumoniae serotypes included in the vaccine and will not protect against other microorganisms that cause invasive disease and pneumonia. As with any vaccine, Apexxnar may not protect all individuals receiving the vaccine from pneumococcal disease. Safety and immunogenicity data on Apexxnar are not available for individuals in immunocompromised groups. Vaccination should be considered on an individual basis. Individuals with impaired immune responsiveness, whether due to the use of immuno-suppressive therapy, a genetic
References
APEXXNAR. Summary of Product Characteristics.
defect, human immunodeficiency virus (HIV) infection, or other causes, may have reduced antibody response to active immunisation. The clinical relevance of this is unknown. In adults across all studied age groups, formal non-inferiority criteria were met although numerically lower geometric mean titres were observed with Apexxnar for most of the serotypes compared to Prevenar 13, however the clinical relevance of this observation for immunocompromised individuals is unknown.
Drug Interactions: Apexxnar may be administered concomitantly with seasonal influenza vaccine (QIV; surface antigen, inactivated, adjuvanted). In subjects with underlying conditions associated with a high risk of developing life-threatening pneumococcal disease, consideration may be given to separating administrations of QIV and Apexxnar (e.g., by approximately 4 weeks). Apexxnar can be administered concomitantly with COVID-19 mRNA vaccine (nucleoside modified).
Fertility, Pregnancy & Lactation: There are no data from the use of Apexxnar in pregnant women. Animal studies do not indicate direct or indirect harmful e ects with respect to reproductive toxicity. Administration of Apexxnar in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and foetus. It is unknown whether Apexxnar is excreted in human milk. Side E ects: As Apexxnar contains the same 13 serotype-specific capsular polysaccharide conjugates and the same vaccine excipients as Prevenar 13, the adverse reactions already identified for Prevenar 13 have been adopted for Apexxnar. In clinical trials, the safety profile of Apexxnar was similar to that of Prevenar 13. No new adverse reactions were identified as compared to Prevenar 13. When Apexxnar was administered to adults aged ≥ 65 years together with the third (booster) dose of a COVID-19 mRNA vaccine (nucleoside modified), the tolerability profile generally resembled that of the COVID-19 mRNA vaccine (nucleoside modified) administered alone. Very common (≥ 1/10): Headache, joint pain, muscle pain, vaccination-site pain/tenderness, fatigue. Common (≥ 1/100 to < 1/10): Vaccination-site induration/ swelling, vaccination-site erythema, pyrexia. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity reaction including face oedema, dyspnoea, bronchospasm, diarrhoea, nausea, vomiting, rash, angioedema, vaccination-site pruritus, lymphadenopathy, vaccination-site urticaria, chills. The following adverse reactions have been spontaneously reported during the postmarketing use of Prevenar 13, which may also occur with Apexxnar. These events were reported voluntarily from a population of uncertain size, so it is not possible to reliably estimate their frequency or to establish, for all events, a causal relationship to vaccine exposure: Anaphylactic/anaphylactoid reaction, including shock, erythema multiforme, vaccination-site dermatitis. Additional information in special populations in studies with Prevenar 13: Adults with HIV infection have similar frequencies of adverse reactions, except that pyrexia and vomiting were very common and nausea common. Adults with an HSCT have similar frequencies of adverse reactions, except that pyrexia, vomiting, and diarrhoea were very common.
For full prescribing information see the Summary of Product Characteristics.
Legal Category: S1A. Package Quantities: Pack of 1 single-dose pre-filled syringe (with separate needle).

Marketing Authorisation Numbers: EU/1/21/1612/002. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500.
Last revised: 12/2022.
Ref: PE 3_0.
Froes F, Roche N, Blasi F. Pneumococcal vaccination and chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2017;12:3457-3468.


PP-PNR-IRL-0023 | Date of Preparation: January 2023
APEXXNAR® is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.1
▼
older.
1.
2.
Designed to deliver long-lasting immunity.1,2
Helps protect against the 20 Streptococcus pneumoniae serotypes included in the vaccine.1
CARDIOLOGY: CARDIAC ARRESTS

Documentation of Cardiac Arrests in a Model 4 Emergency Department. A Cardiac arrest proforma in St James’ hospital ED: an audit
Written by Ríona McCarthy, William Blake, Barry Keane, Deirdre Glynn, St James’s Hospital, Dublin
Good record keeping is an essential facet of patient care and clinical practice. Accuracy of documentation in cardiac arrests is of particular importance in establishing a cause for the cardiac arrest, for continuity of patient care and frequently has medicolegal implications, playing a role in coroner’s investigation, complaint and civil proceedings. In pressurised situations requiring immediate and cohesive response from healthcare staff, recalling specific details without prompting can be difficult. St James’s Hospital (SJH) ED, located in Dublin’s South inner city, receives an attendance of over 50,000 patients annually.1
When cardiac arrests present to or occur in the department, the emergency staff are immediately alerted and begin resuscitation. The medical notes are completed by the responsible doctor retrospectively, after return of spontaneous circulation (ROSC) and patient stabilisation or cessation of resuscitation attempts. These notes are then uploaded to an electronic patient record (EPR) system. In Ireland, there is currently no standardised documentation proforma for cardiac arrest. In 2018, a proforma was designed by an SJH ED registrar to improve documentation.

The General Medical Council’s ethical guidance and the Utstein fields, developed by Allen et al,2 were used in formulating the document. The Utstein fields are a set of guidelines for uniform reporting of cardiac arrest as agreed upon by the international resuscitation community. This resulted in a comprehensive proforma being developed (see figure 1). This study aims to assess the compliance in using this proforma, and its quality.
Methodology
This audit compared the use of the standardised proforma versus freehand documentation over two periods, pre- and post-education. Period A ran from 1st of March
2021 to 31st of July 2021. Period B followed the intervention, running from 1st of September to 31st of December 2021. Records from all triage category 1 patients were examined.3
Patients arriving to the ED in cardiac arrest were included as were patients who arrested in the department. Patients suffering out of hospital cardiac arrest (OOHCA) who achieved return of spontaneous circulation (ROSC) prior to arrival were excluded, as were patients who arrested after leaving the ED under inpatient teams.
The electronic paper records for all included patients were examined for whether a proforma had been completed, or if the events had been recorded on an ED card using freehand record keeping. The completeness of documentation was then assessed for the presence
or absence of data in 30 domains listed in table 5.
This was undertaken retrospectively for period A. Following period A, an education intervention was undertaken. The ED staff were educated formally and informally about proforma use. Formal education was undertaken at NCHD teaching, informing staff about the presence of the proforma, and encouraging staff to complete it after treating a cardiac arrest in the department.
The proforma was also discussed at handover meetings and made more accessible in the resuscitation area, in addition to being uploaded to the SJH ED website. Awareness intervention was undertaken on social media, with NCHD whatsapp groups targeted to encourage use of the proforma. Performance in period B was then re-audited.
The audit was registered through St James’ s hospital electronic audit registration form, it was

approved by the audit supervisor and it was reviewed by the hospital data protection office as per SJH Clinical Audit Policy.
Results
During period A, a total of 228 category 1 patients were examined, 18 of whom fit the inclusion criteria. Of these, the proforma was completed in 5 cases (27.78%). In period B, a total of 175 category 1 patients were examined, 23 of whom fit the inclusion criteria. The proforma was completed in 15 cases (65.22%). Proforma utilisation increased from 27.78% (5/18) in period A to 65.22% (15/23) in period B.
Regarding individual categories of details recorded, documentation was completed to a higher standard when the proforma was used in comparison to freehand record keeping (85.83% vs 64.92%). However, when comparing periods A and B
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 68
Figure 1: Cardiac arrest proforma
Fig 1: Cardiac arrest proforma
proforma use), there was no overall improvement in completeness of documentation following intervention (75.07% vs 75.12%). The majority of the GMC standards were met both with and without the proforma. 100% of the following categories were documented in every case; legible writing, written in black ink, patient name, and date of birth.
Patient MRN was not included in 1 case when using the proforma, during period B. It was included in all other cases of documentation. Doctor name was documented in 100% of cases when using the proforma, in period A and period B. It was documented in 90.47% of cases without proforma use. There was less compliance with filling in doctor rank, and even less so with doctor contact details. However this information was provided significantly more when using the proforma (doctor rank was 95% vs 42.85%, and contact details was 85% vs 0% when using vs not using the proforma). Doctor signature was included to the same extent with and without the proforma (90% vs 90.47%). Date was included in 100% of cases with and without the proforma.
Time was included in the majority of cases, without much difference between proforma and no proforma (95% vs 85.7%). The largest differences were seen in relation to specific details surrounding cardiac arrests. Proforma use was associated with improved documentation when compared to freehand medical note keeping in the following areas; who triggered the arrest call (90% vs 47.6%), time interval to response (85% vs 28.57%), patient circumstances prior to arrest (100% vs 66.67%), primary rhythm (100% vs 76.19%), number of cycles (70% vs 33.33%), shocks given (100% vs 42.86%), IV access (95% vs 42.86%), blood gas (75% vs 47.62%), blood results (80% vs 33.33%), timings (75% vs 52.38%), who made the decision (70% vs 28.57%) and discussion with family (85% vs 42.86%).
Table
of proforma utilisation: Proforma utilisation increased from 27.78% (5/18) in period A to 65.22% (15/23) in period B.
All data (percentage of 30 categories documented): Record keeping was completed to a higher standard when measured against our 30 domains when a proforma was used (85.83% vs 64.92%). However, there was no improvement in record keeping between period A and B (75.19% vs 75.07%).
Table 2 All data (percentage of 30 categories documented): Record keeping was completed to a higher standard when measured against our 30 domains when a proforma was used (85.83% vs 64.92%). However, there was no improvement in record keeping between period A and B (75.19% vs 75.07%).
All data (percentage of 30 categories documented): Record keeping was completed to a higher standard when measured against our 30 domains when a proforma was used (85.83% vs 64.92%). However, there was no improvement in record keeping between period A and B (75.19% vs 75.07%).
Table 3 Professional responsibilities of medical practitioners regarding documentation as per the General Medical Council's (GMC) guidance Good medical practice4: that documents are legible, filled out in black ink and include patient name, MRN, date of birth, scribe signature, record rank, signature and contact details.
Professional responsibilities of medical practitioners regarding documentation as per the General Medical Council's (GMC) guidance Good medical practice [4]: that documents are legible, filled out in black ink and include patient name, MRN, date of birth, scribe signature, record rank, signature and contact details.
Professional responsibilities of medical practitioners regarding documentation as per the General Medical Council's (GMC) guidance Good medical practice [4]: that documents are legible, filled out in black ink and include patient name, MRN, date of birth, scribe signature, record rank, signature and contact details.
The only areas where freehand notes outperformed the proforma documentation were the recording of who was the initial responder(50% vs 71.43%) and ECG results (30% vs 62%). Areas that showed little change whether proforma was used or not were: clinical status on arrival (95% vs 85.7%), airway management (85.71% vs 71.43%), drugs used (85% vs 71.43%), observations (40% vs 42.86%) and outcome (100% vs 90.48%).
Discussion
This audit assessed the compliance of using a standardised cardiac arrest
proforma in SJH ED, and compared completeness of record keeping when using the proforma vs freehand medical note keeping. Proforma use was associated with more complete medical records, correlating with other studies that have shown that the use of pre-printed proformas improve documentation.5 Given the high stakes involved, there are few clinical scenarios in which accurate record keeping is more important.
This audit would support a role for proforma use in other critical events where highly charged situations result in difficulty of total recall after the event. It would also support the development and use of a national proforma to be used in all EDs that receive patients in cardiac arrest.

records apply, when they are written and by whom. This is crucial for both continuity of care and so that a clear record is available in the event of medicolegal proceedings. Both with and without the proforma, the majority of the GMC standards were met.
Cardiac arrest specific information: who triggered the arrest call, who responded, time interval to response, patient circumstances prior to arrest, patient’s clinical status on arrival, primary rhythm, number of cycles, shocks given, IV access, airway management, drugs used, blood gas, blood results, ECG, timings, observations, outcome, who made the decision, discussion with family.
The GMC standards for medical record keeping reflect the importance of establishing the identity of those to whom the
However there was a noticeable disparity in relation to doctor contact details. When not using the proforma, in both period A
HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023 69
Table 2
Table 3
No Proforma Proforma Period A n = 18 72.22% n = 13 27.78% n = 5 Period B n = 23 34.78% n = 8 65.22% n = 15 No Proforma Proforma Total Period A 67.95% n = 13 94.00% n = 5 75.19% n = 18 Period B 60.00% n = 8 83.11% n = 15 75.07% n = 23 Total 64.92% n = 21 85.83% n = 20 75.12% n = 41 Table
2
Table 3
No Proforma Proforma Period A n = 18 72.22% n = 13 27.78% n = 5 Period B n = 23 34.78% n = 8 65.22% n = 15 No Proforma Proforma Total Period A 67.95% n = 13 94.00% n = 5 75.19% n = 18 Period B 60.00% n = 8 83.11% n = 15 75.07% n = 23 Total 64.92% n = 21 85.83% n = 20 75.12% n = 41
1 Comparison
Table 4
No Proforma Proforma Total Period A 86.01% n = 13 96.36% n = 5 88.89% n = 18 Period B 77.27% n = 8 96.36% n = 15 89.72% n = 23 Total 77.27% n = 21 96.36% n = 20 89.36% n = 41 No Proforma Proforma Total Period A 57.49% 92.63% 67.25%
Cardiac arrest specific information: who triggered the arrest call, who responded, time interval to response, patient circumstances prior to arrest, patient’s clinical status on arrival, primary rhythm, number of cycles, shocks given, IV access, airway management, drugs used, blood gas, blood results, ECG, timings, observations, outcome, who made the decision, discussion with family.
CARDIOLOGY: CARDIAC ARRESTS
Table 4 Cardiac arrest specific information: who triggered the arrest call, who responded, time interval to response, patient circumstances prior to arrest, patient’s clinical status on arrival, primary rhythm, number of cycles, shocks given, IV access, airway management, drugs used, blood gas, blood results, ECG, timings, observations, outcome, who made the decision, discussion with family.
was developed, however it highlights the importance of educating staff to fully complete the proforma when using it. While proforma use is associated with improved record keeping and proforma use increased between periods, the overall completeness of record keeping was no better between periods.
and B, doctor contact details were included in 0% of cases. When using the proforma, in period A doctor contact details were included 100% of the time, and in period B they were filled in 80% of the time. The absence of scribe contact details in documentation is concerning, particularly for the potential medicolegal proceedings that could arise from situations with a high fatality. This highlights the importance of educating staff and monitoring this vital component of medical responsibility.
Similarly, in the absence of a proforma, doctor rank is seldom
identified. A more significant difference was seen in cardiac arrest specific details where the proforma significantly outperformed freehand note taking. Clear documentation of cardiac arrest specific details are imperative in establishing the likely cause of the cardiac arrest and instituting potentially lifesaving treatments both intra-arrest and in the period post ROSC.6 Good quality documentation of cardiac arrest specific details is also important in debriefing and teaching following cardiac arrests and could be used as a
quality improvement tool to ensure the highest standard of care is delivered in lifesaving treatment. The most frequently missing data from proformas were ECG results (70%) and observations (60%).

In SJH ED hard copies of ECGs are kept with patients’ paper ED notes and are scanned to EPR once the patient leaves the department. Observations are generally recorded by nursing staff on EPR. This likely accounts for their low rates of documentation on the Cardiac Arrest proforma. This may not be an issue in other hospitals if a national proforma
Table 5 Missing data: the most frequently omitted information from all data collected, comparing the with and without proforma groups.
This is difficult to account for, but may largely be due to the small sample size, in addition to sample size being different between both periods, with only 5 proformas being used in period A vs 15 in period B. This does not allow accurate reflection of the improvement in details included. Further investigation with a larger sample size would be necessary. It also emphasises the necessity of educating new staff when joining the department, in relation to the existence of a proforma specific to cardiac arrests, and further to recommend completing the documentation in its totality. The audit of period A was conducted retrospectively, with clinicians unaware that an audit would be conducted. However, for period B, the staff had received and education session and were aware that an audit was being conducted, introducing an element of observation bias. The study is further limited by the relatively short periods of observation.
Nevertheless, the improvement in proforma utilisation is significant. From this study, it is impossible to associate a proforma with any improved patient outcomes. This would likely require a large-scale randomised control trial that would be impractical and difficult to undertake. However, previous research has demonstrated that poor quality documentation has a negative impact on patient outcomes.7
Conclusion
Accurate documentation is an essential component of patient care, particularly for handover. Structured documents provide a quick and easy overview of events that have occurred. They also ensure complete record keeping for medicolegal purposes and represent a good research and audit tool for quality improvement. This study highlights the value of a standardised template for documentation in cardiac arrest. A national standardised proforma should be considered in the future. Furthermore, educating new and existing ED staff about the cardiac arrest proforma is essential to increase its utilisation.
References available on request
APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 70
Table 4
No Proforma Proforma Total Period A 57.49% n = 13 92.63% n = 5 67.25% n = 18 Period B 50% n = 8 75.44% n = 15 66.59% n = 23 Total 54.64% n = 21 79.74% n = 20 66.88% n = 41
Table 5 Missing data: the most frequently omitted information from all data collected, comparing the with and without proforma groups. Item %missing without proforma (n = 21) %missing proforma (n = 20) %missing all Doctor contact details 100% 15% 59% Observations 57% 60% 59% ECG 38% 70% 54% Who made decision 71% 30% 51% Number of cycles 67% 30% 49% Time interval to response 71% 15% 44% Blood results 67% 20% 44% Blood gas 52% 25% 39% Who responded 29% 50% 39% Timings 48% 25% 37% Discussion with family 57% 15% 37% Doctor rank 57% 5% 32% IV access 57% 5% 32% Who triggered arrest call 52% 10% 32% Shocks given 57% 0% 29% Drugs 29% 15% 22% Airway management 29% 10% 20% Patient circumstances prior to arrest 33% 0% 17% Primary rhythm 24% 0% 12% Patient's clinical status on arrival 14% 5% 10% Time 14% 5% 10% Doctor signature 10% 10% 10% Doctor name 10% 0% 5% Outcome 10% 0% 5% Patient MRN 0% 5% 2% Date 0% 0% 0% Patient DOB 0% 0% 0% Patient name 0% 0% 0% Black ink 0% 0% 0% Legible 0% 0% 0% Discussion This audit assessed the compliance of using a standardised cardiac arrest proforma in SJH ED, and compared completeness of record keeping when using the proforma vs freehand medical note keeping
Multiple Myeloma
HRAS mutation positive multiple myeloma in the type 2 CALR mutation positive essential thrombocythemia: A case report
Written by Krzysztof Lewandowski,
Out of BCR-ABL negative myeloproliferative neoplasm (MPNPh-) patients, 3%–14% display a concomitant monoclonal gammopathy (MGUS).1, 2 The diagnosis of MPN proceeds lymphoproliferative disease (LPD) occurrence in about 50% of patients. The median time between the diagnosis of MPNPh- and LPD was established at 72 months.3 Herein, we present an unusual outcome of low risk essential thrombocythemia (ET), diagnosed in 2008 in a 50-year-old woman. Initially, she was treated with hydroxyurea (HC) 1.5 g/daily orally. Her medical history revealed mitral and aortal valve insufficiency, which was diagnosed in 2018, and the episode of transient ischaemic attack (2018), resulting in foci cerebral ischaemia (MRI). In 2019, due to toxicity, the HC treatment was stopped. The therapy with the pegylated interferonα 2a (Pegasys, PEG-INFα2a) was started in August 2020. The molecular work-up showed no JAK2 V617F and MPL exon 10 mutation and the presence of the type 2 CALR mutationLRG_828t1:c.1154_1155insTTGTC, LRG_828p1:p.(Lys385Asnfs*47)
(VAF 35%). The bone marrow (BM) biopsy performed in 2020 showed the normocellular BM, locally hypercellular, with an erythroid/ granulocytic cell ratio of 1:3, and normal erythroid and granulocytic proliferation index and maturation pattern. The blast cell (CD34+, CD117+) content was determined at 1%–2% of nuclear cells. An increased number of mediumsized and large megakaryocytes (factor VIII+) was found, without the tendency to form clusters (locally forming loose clusters consisting of 4–8 cells). The BM fibrosis (MF) grade according to the European consensus criteria was 0/1.
In September 2021, she experienced a fracture of the L1 vertebrae of the spine which was treated with Th12-L1 stabilization with laminectomy. The PETCT scan revealed multiple osteolytic lesions in the bones.
Immunofixation studies showed the IgG kappa monoclonal protein in the blood at the concentration of 49.6 g/L. The WBS performed in January 2022 documented the fracture of Th9, the presence of an intramedullary tumour in the bottom part of the stern. The MFC of the BM cells showed 65% of abnormal plasma cells. The repeated biopsy documented BM cellularity 60% with about 30% of dispersed plasma cells, locally forming infiltrates. Erythroid cell line content e-cadherin+ was determined at 30%. The granulocytic cell [CD15+, MPO+] line and megakaryocyte (factor VIII+, CD61+) evaluation showed a normal morphology and maturation pattern. MF +1. Retrospective analysis of a biopsy sample collected in 2020 (before PEG-INFα2a treatment initiation) confirmed the presence of a small, abnormal plasma cell population with the same immunophenotypic characteristic (Figure 1).
An NGS analysis of the BM cells was performed using a sample collected in January 2022, before MM treatment initiation. The targeted enrichment approach with a custom-designed gene
panel revealed the presence of the Harvey rat sarcoma (HRAS) gene mutation (chr11:000534282-A > C VAF 14% NM_001130442.1:p. Val14Gly/c.41 T > G) and the wild type of CALR, JAK2 and MPL gene sequence. The analysis of the blood sample collected at the time of PEG-INF2α treatment initiation using the SS did not confirm the presence of the HRAS variant. The HRAS mutations were also undetectable in the DNA obtained from the blood MNC and buccal swab cells collected immediately before MM treatment initiation.

After the diagnosis of MM, the treatment with bortezomib, thalidomide and dexamethasone (VTD) was introduced. After three VTD therapy cycles, a significant reduction of the protein M concentration in the blood from 49.6 g/L to 3.6 g/L was confirmed (Figure 2).
In the presented case, the mechanism of the CALR mutation positive clone disappearance is not clear. It cannot be ruled out that the PEG-INFα2a treatment resulted in HSC exhaustion and CALR mutation positive clone eradication. The mentioned hypothesis is in agreement with the published data confirming that INFα treatment resulted in complete molecular remission (CMR) in 10% of ET patients.4 It
is probable that PEG-INFα targets the type 2 CALR mutation positive haematopoietic stem and progenitor cells preferentially, as was documented by Mosca et al.5
The published data suggest that the CALRins5 (type 2) in comparison to the CALRdel52 (type 1) differentially activate the inositol-requiring enzyme 1α (IRE1α)-X-box-binding protein 1 (XBP1) pathway of the unfolded protein response system to drive malignancy. The up-regulation of the ATF6 chaperone targets specifically in CALRins5 cells suggests that the loss of the chaperone function by CALRins5 may underlie the differential activation of ATF6.6 In 2019, Salati et al. demonstrated that CALR mutations induce increased sensitivity to oxidative stress, leading to the increase of oxidative DNA damage and the down-modulation of the oxidation resistance gene 1 in CALRmutated cells.7 The experimental study of bone marrow cells confirmed the presence of the CALR mutation even in multipotent precursors (MPP) of lymphohematopoiesis.8 Therefore, it is possible that the RE stress due to the CALRins5 in MPP promotes oncogenesis via the abnormal function of IRE1aXBP1 axis, even in MPP. Another probable mechanism responsible
71 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Agata Kopyd�owska, Zuzanna Kandu�a, Bart�omiej Sankowski, Marcin Machnicki, Marta Baranska, Kinga Gwózdz-Ba˛k, Tadeusz Kubicki, Anna P�otka, �ucja Przysiecka, Grzegorz Dworacki, Piotr Koz�owski, Tomasz Stok�osa
Figure 1
Multiple Myeloma
for secondary malignancies development in MPNPh- includes immunosuppressive effects mediated by exon-9-mutated CALR released from the malignant cell, resulting in the inhibition of phagocytosis of dying cancer cells by dendritic cells.9 The above-mentioned data suggest a possible association between the CALR mutation driven malignancies and an increased risk of secondary neoplasms, including MM. In the systematic review done by Marchetti et al., the coexistence of the type 1 CALRdel52 mutation positive ET and MGUS was reported in 3 patients.3 Loscocco et al. described a CALRdel52 mutation positive (VAF 57%) ET case who was diagnosed with smouldering MM 1 year after the diagnosis of ET. Four years later, the progression to post-ET myelofibrosis was documented. After another 2 years, the MGUS evolution to the IgG lambda MM was observed.10
The occurrence of MM is rare in JAK2 mutation positive PV patients and no case of an MM diagnosis preceding an MPN one has been reported. It was shown that 9% of patients with MPN (36.0% of ET) harboured an M-protein. ET-MGUS (IgGκ and IgMκ) was diagnosed in 20% of ET patients, including cases with CALR type 1 and type 2 mutations.11 The frequency of the coexistence of MM with ET was established at 15%.3 The mentioned data are in agreement with a previous report documenting the direct role of calnexin, calreticulin, and tapasin abnormalities in the MGUS progression to MM.12
The presented case is the first reported HRAS Val14Gly/c.41 T > G variant positive haematological malignancy. The HRAS variant detected in our patient had been reported only once in Clinvar (accession number VCV000654373.1) in the case of Costello Syndrome. It is likely pathogenic according to Clinvar and of uncertain significance according to Varsome. The Functional Analysis Through Hidden Markov Models (FATHMMMKL) categorizes this change
as damaging with a 0.99 coding score. Algorithms developed to predict the effect of missense changes on protein structure and function (SIFT, PolyPhen-2, AlignGVGD) all suggest that this variant is likely to be disruptive, but these predictions have not been confirmed by published functional studies (Clinvar).
The presence of NRAS and KRAS mutations was confirmed in 8% and 4% of myeloid malignancies. It was documented that missense mutations at codons 12 and 13 are thought to limit GTPase-activating protein (GAP) interaction with the GTPase site of RAS proteins, preventing their hydrolysis to an inactive state. It was previously documented that mutations of RAS family genes contribute to myelomagenesis, including the transformation from MGUS to MM.13 In MM patients, the presence of the NRAS and KRAS gene mutations was confirmed in 19.5% and 24.2% of patients, respectively.14 The data concerning HRAS mutations in myeloid disorders are limited. The role of HRAS gene mutations in the process of the malignant transformation of plasma cells also remains unknown. In the report of O'Donnell et al., a HRAS mutation [c.181 C > A (p.Q61K)] was identified in 1/67 of patients with MM.15 Frontzek et al.16 confirmed the presence of HRAS mutations in 2% of patients with plasmablastic lymphoma.
One still unsolved mystery is that of the origin of MM cells in patients with MPNPh-. The available data suggest that hydroxyurea or interferonα treatments do not increase the risk of secondary cancers. In the summary of the published case reports and studies prepared by Malhotra et al., it has not yet been definitively shown that these 2 entities arise from a common-ancestor HSPC.1 In 2021, Hui W. et al. identified eight proteins that were found to be dysregulated differently in ET patients with mutated CALRdel52 and those with JAK2V617F mutation. The proteomic analysis showed that 20 proteins were altered in ET with the CALRdel52 mutation: those involved in cell signalling (Erk1/2, PTEN, Raf-B, Rap1, Axin, ERβ, TGF-β), cell cycle (Cdc42, Cdc2, CyclinD1, p27), apoptosis (Bcl-Xl, c-IAP2, NFκB p50, cPKCα, Survivin), transcription factor (eIF4B, SRC-1), adhesion (E-cadherin) and DNA repair (TDP1).17 Similar data, concerning CALR type 2 mutation detected in our patient is not available yet. Therefore, we performed an additional review of The Cancer Genome Atlas (TCGA) database, including datasets of >10,000 samples of 33 cancer types, searching for a possible co-occurrence of the CALR driver mutations and mutations in the RAS family genes (HRAS, NRAS, and KRAS). We did not find any sample with co-occurring CALR type 1 or type 2 mutations and
mutations in the RAS family genes. The only few CALR mutations co-occurring with mutations in the RAS genes seem to be random, they were mostly missense mutations randomly distributed in different positions of the CALR gene, detected mostly in cancers (UCEC and COAD) known to be frequently hypermutated due to different mutagenic processes and aberrations in DNA repair machinery (Table S1). We realize that our hypotheses have considerable limitations, as more studies (including functional studies) are necessary to explore the link between the CALR-driven malignancies and secondary cancer development and origin. For this reason, the precise mechanism responsible for CALRmut-driven oncogenesis remains unknown and is probably multifactorial.
The question is that of the medical significance of the presented data. In our opinion, more attention should be paid to the monitoring of MPN patients for the presence of MGUS, to allow for an early diagnosis of MM and the initiation of an appropriate treatment.10 Still, the study of the genetic aberration profile in MPNPh-patients with coexisting MM is needed to precisely define and attack common molecular target(s) responsible for oncogenesis.18 References available on request

72 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
2
Figure
Irish TB Smartphone Study
Tuberculosis (TB) is the leading cause of death worldwide from an infectious agent, resulting in more deaths than HIV and malaria. Deaths from TB rose for the first time in more than a decade due to Covid 19 (WHO, 2022).
Although TB is treatable, nonadherence to medication leads to ongoing transmission, disease progression and development of drug-resistant strains. Treatment is lengthy, ranging from six months to two years depending on the type and location of TB.
The Health Innovation Hub Ireland (HIHI) Trinity Hub and National TB centre (both based at St James’s Hospital, Dublin) studied 34 patients over 18 months, to investigate the use of technology in contrast to in-person care in Tuberculosis (TB) medication adherence.
16 study participants were on the current Irish standard of careDirect Observed Therapy (DOT) – where a public health nurse must physically witness patients taking medication. 18 participants were randomised to the World Health Organisation (WHO)-recommended UK and US standard of care: Video Observed Therapy (VOT).
VOT allows patients to take TB medications remotely, recording through their smartphone and sending securely to the clinical team. A total of 3,634 videos were viewed during the study period.
Main Findings
Revenue – Health Service
Executive (HSE) financial and
budget implications: Using the current method of Direct Observed Therapy (DOT), the HSE cost is ¤3,362 per patient over six months, compared to ¤300 with Video Observed Therapy (VOT) for same period.
Workflow - HSE resources saved: The time for each public health nurse to carry out DOT in person, using hard copy records for one patient was up to 60 minutes. Using VOT, each patient video took - on average - one minute of the public health nurse’s time at the computer.
Clinical pathway - Patient selfmanagement: Seven participants were transferred from DOT to VOT due to an inability to comply with DOT, citing work hours, or reported undue stress due to DOT. Patient sentiment however, with VOT was extremely positive. VOT empowered continuation of normal work/life routines and allowed patients to take medications at a time convenient to them. Contrastingly, those on DOT had to be available at set times suitable to the public health nurses, which was disempowering and restrictive.
The study also showed that VOT had higher levels of treatment observation in the first two months of treatment and remained consistently high throughout treatment compared to DOT. VOT can facilitate twice daily dosing at no additional cost. For patients on twice daily dosing on DOT, typically the second afternoon dose in the PM could
Health Innovation Hub Ireland
Manger, Dublin, Eimear Galvin

not be facilitated due to time constraints of the public health nurse. As a result, not all doses were observed, which presents a significant health risk.
Health Innovation Hub Ireland Manger, Dublin, Eimear Galvin (Clinical Medicine, School of Medicine, Trinity College) said, “Health Innovation Hub Ireland’s focus is introducing innovative solutions to our health system that meet priority needs of Irish patients. HIHI was thrilled to work with the TB team to deliver this important study, which shows remarkable health system savings, increases treatment access and supports self-management in the community. The need for this kind of remote care and patient selfmanagement is even more obvious now since Covid-19.”
Co- Principal Investigators, TB Clinical Nurse Specialist Lorraine Dolan and Professor Ann Marie McLaughlin (both, national TB Centre) added, “TB remains a public health imperative. We are using World TB day to urge the Irish health system to review TB care in Ireland based on the overwhelming
St Patrick’s Day Medal Award
Taoiseach Leo Varadkar TD has presented the prestigious Science Foundation Ireland (SFI) St Patrick’s Day Science Medal for Academia to UCC graduate Dr Paul K. Whelton, Professor of Global Public Health at Tulane University, New Orleans, recognising his outstanding contributions to research and innovation.
The SFI St Patrick’s Day Science Medal is awarded to distinguished US-based science, engineering or technology leaders with strong Irish connections. It highlights and honours their role in supporting and engaging with the research ecosystem in Ireland and beyond.
A graduate of UCC School of Medicine Class of 1970, Dr Paul K. Whelton, is Show Chwan Professor of Global Public Health at Tulane
University School of Public Health and Tropical Medicine, in New Orleans, and President of the World Hypertension League.
A native of Cork City, Dr Whelton has served as the principal investigator for a number of highprofile research studies that have informed the diagnosis, prevention, and treatment of high blood pressure. He has authored over 550 peer-reviewed publications, and has received numerous awards, including the 2018 American Heart Association Hypertension Council Excellence Award.
Dr Whelton said, “I am deeply honoured to accept the 2023 SFI St. Patrick’s Day Science Medal for my work in the area of high blood pressure, which is a leading cause of death, taking
the lives of 18 million people globally each year. My research in cardiovascular and renal disease epidemiology continues to push for new discovery and improved clinical outcomes for patients. I am very proud to be part of the Irish research diaspora community in the US and to support our Transatlantic partnerships which enable us to further our knowledge, and deliver truly excellent science with global benefits.”
At the celebratory event in Washington DC, Mr Paul R. Daugherty, Group Chief Executive - Technology and Chief Technology Officer at Accenture, was awarded the SFI St Patrick’s Day Science Medal for Industry.
Commending the recipients, Taoiseach Leo Varadkar TD
results of this new study. VOT is hugely cost saving; time saving across staff teams, supports patient self-management and increased TB patient medication observation per day. Due to resource constraints in Ireland, the Health Protection Surveillance Centre advises selective DOT. However, universal observation is most beneficial to stop the transmission chain and resource is not an issue using VOT.” Convinced of the efficacy of VOT in TB care, TB Clinical Nurse Specialist, Lorraine Dolan, approached HSE and Enterprise Ireland partnership, Health Innovation Hub Ireland, in 2019 to collaborate on the first of its kind research study in Irish TB care. The study site was the National TB Centre, St James’s Hospital, in the outpatient department, incorporating the Respiratory Assessment Unit.
said: “Irish research is driving transformational discovery and innovation that is aiding our response to global challenges, particularly in the areas of climate change, digitalisation and health. As set out in Ireland’s Research and Innovation Strategy ‘Impact 2030’, the Irish Government is committed to strengthening Ireland’s position as a global innovation leader.”
“We pay tribute today to the enormous contribution made by STEM talent in the Irish diaspora in the USA. These important international collaborations continue to forge new opportunities for ground-breaking research, improving our ability to adapt to a rapidly changing world and create a better future for all.”
73 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
News
Precision Medications for Irritable Bowel Syndrome with Predominant Constipation (IBS-C)
Written by
Irritable Bowel Syndrome (IBS) is one of the most common gastrointestinal disorders seen in clinical practice. In recent years, significant advances have been made in understanding its complex pathophysiology, with considerable new evidence published concerning its diagnosis, investigation and management. This led to the British Society of Gastroenterology (BSG) publishing

updated IBS clinical practice guidelines in 2021.1 In this review, we will outline the approach to diagnosing and managing IBS with Predominant Constipation (IBS-C).
How
is IBS diagnosed?
IBS is a clinical diagnosis of exclusion. All patients presenting with symptoms of IBS for the first time must be investigated with:

1. Full blood count (FBC)
2. C reactive protein (CRP) or erythrocyte sedimentation rate (ESR)
3. Coeliac serology: Anti-tissue transglutaminase (Anti-TTG) and IgA levels
4. Thyroid function tests, Calcium and Magnesium levels
5. Faecal calprotectin in under 45 year olds, to exclude inflammatory bowel disease
Local and national guidelines for colorectal and ovarian cancer screening should be followed. In Ireland, all patients aged 6070 should participate in Bowel Screen, the national colorectal cancer screening programme and


a home FIT test can be arranged by contacting 1 800 454555.
Colonoscopy is not indicated in IBS without alarm symptoms or signs (summarised in Box 1).1 However, it should be considered in those patients with symptoms suggestive of IBS with diarrhoea who have atypical features and/or relevant risk factors for macroscopic or microscopic colitis (female sex, age ≥50 years, family history of inflammatory bowel disease, coexistent autoimmune disease or inflammatory arthropathy/skin rashes, nocturnal or severe, watery diarrhoea, duration of diarrhoea <12 months, weight loss or use of potential precipitating drugs for microscopic colitis including nonsteroidal anti-inflammatory drugs,

74 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Gastroenterology: IBS
Dr Robert Varley, Department of Gastroenterology, Connolly Hospital, Dublin, Ireland
Figure 1: IBS Diagnostic Criteria
Figure2A: Bristol Stool Chart
Figure 2B: IBS Subtype Graph
Professor Richard J Farrell , Department of Gastroenterology, Connolly Hospital, Dublin, Ireland
aminosalicylates, proton pump inhibitors, statins and SSRIs, etc)
A diagnosis of IBS should be made based on symptoms, the absence of alarm symptoms or signs, and no abnormalities on the above simple blood and stool tests. Referral to gastroenterology is warranted where there is diagnostic doubt, in patients with symptoms that are severe, or refractory to first-line treatments, or on individual patient request.
IBS is diagnosed using the Rome Criteria, last updated as Rome IV in 2016.2 Criteria must be fulfilled for the last 3 months with
symptom onset at least 6 months prior to diagnosis:
Recurrent abdominal pain on average at least 1 day/week in the last 3 months, associated with two or more of the following criteria:

1. Related to defaecation
2. Associated with a change in frequency of stool
3. Associated with a change in form (appearance) of stool
IBS is further subdivided by predominant symptom, summarised in Figures 1 + 2.2 As shown, IBS with Predominant
Constipation (IBS-C) requires over 25% of abnormal bowel movements to be very solid (Bristol stool type 1 and 2) with less than 25% being very loose (Bristol stool type 6 and 7).
How is IBS-C Managed?
The treatment algorithm for IBS is shown in Figure 3, with overall diet and lifestyle advice followed by treatments directed towards predominant symptoms1
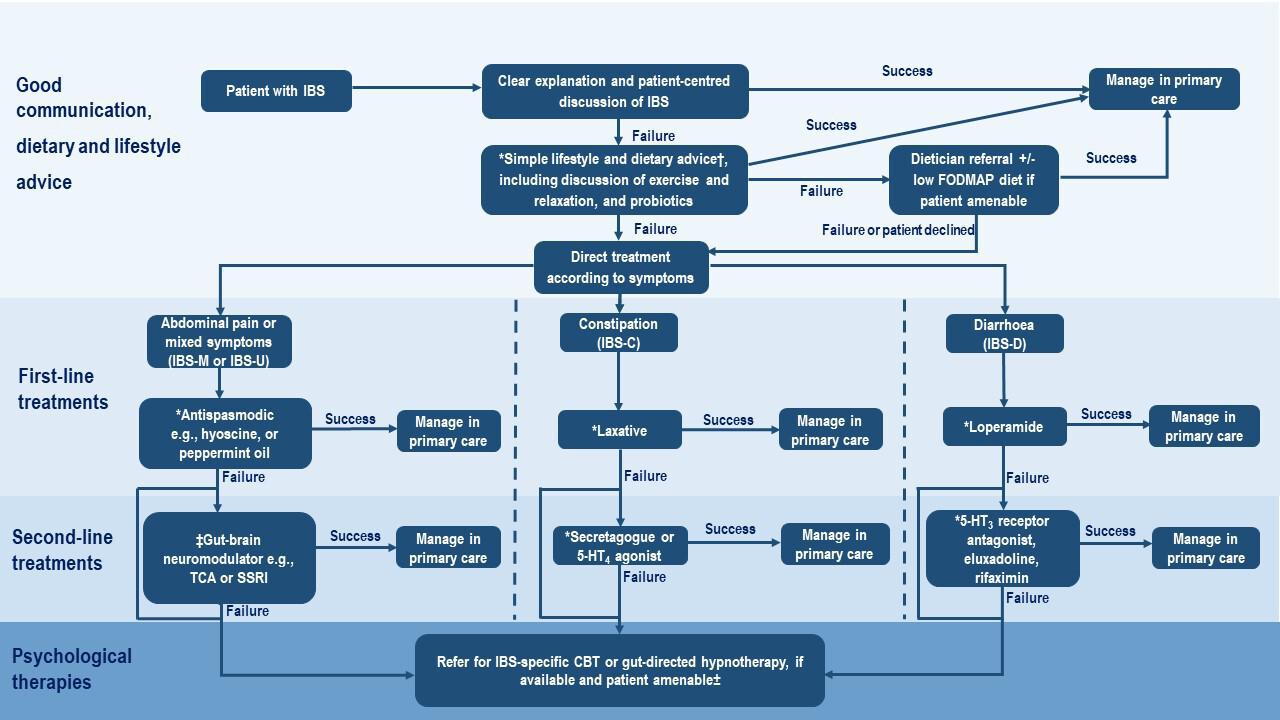
New patients should be given a clear explanation of the diagnosis, pathophysiology, natural history and the most common symptom triggers
IBS should be explained as a disorder of gut-brain interaction, with the impact of diet, stress, cognitive, behavioural and emotional responses to symptoms, as well as postinfective changes
To manage expectations, there should be a realistic discussion regarding the limitations of treatments - while there is no cure, significant improvements in symptoms and quality of life are very achievable
In IBS-C, the treatment target is the normalisation of bowel movement pattern, defined as at least three completed spontaneous bowel movements per week
It is important to exclude excessive use or dependence on opiates as well as iron tablets, calcium containing antacids and some anti-depressants all of which can contribute to chronic constipation.
Treatment should commence with either dietary therapies or first-line drugs, as per patient preference
Second-line drugs are reserved for those not improving with these measures, with many second-line drugs only available in secondary care
The efficacy of a treatment should be evaluated after 3 months, and discontinued if no response with escalation to the next available treatment
1. Fibre and Dietary Therapy:
First line dietary advice should be offered to all patients with IBS. This includes eating regular meals, maintaining adequate nutrition, limiting alcohol and adjusting caffeine and fiber intake (increase if constipation, reduce if diarrhoea), and reducing intake of spicy and fatty foods
Guidelines
75 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Figure 3: Treatment Algorithm for IBS
Gut: first published as 10.1136/gutjnl-2021-324598 on 26 April 2021. Downloaded
Figure 2 Treatment algorithm for IBS. *Review efficacy after 3 months of treatment and discontinue if no response. †As per the National Institute for Health and Care Excellence IBS dietary advice sheet, plus consider ispaghula. ‡TCAs should be first choice, starting at a dose of 10 mg at night, and titrating slowly (eg, by 10 mg/week) according to response and tolerability. Continue for at least 6 months if the patient reports
Box 1: Lower Gastrointestinal alarm symptoms or signs that are referral criteria for suspected colorectal cancer
Gastroenterology: IBS
Adjusting fruit intake is often critical to managing constipation (reducing bland fruit intake such as bananas, applesauce while increasing more colourful fruit intake such as kiwis, prunes, plums, pears, apples, oranges)
For IBS-C and IBS-M, diet and lifestyle measures including drinking plenty of water, regular exercise and a high-fibre diet (up to 30g/day) are recommended
Soluble fibre, such as isphagula (Fybogel), is a bulk-forming laxative and an effective treatment for IBS global symptoms while FybogelMebeverine may be helpful for IBS with constipation or altered bowel habit presents in conjunction with abdominal pain or cramps. This should be commenced at a low dose (3-4g/day) and built up gradually (to 20-30g/day) to avoid bloating
Insoluble fibre (e.g. wheat, bran) should be avoided as it may exacerbate symptoms
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (i.e. the low FODMAPs diet) is an effective second-line dietary therapy for IBS global symptoms and abdominal pain. It is not effective for managing constipation. Its implementation should be supervised by a dietician, with foods reintroduced according to tolerance.
Food elimination diets based on IgG antibodies and a gluten-free diet are not recommended and should never be implemented in advance of checking TTG/IgA coeliac serology tests.
2. Probiotics:
Probiotics significantly improve IBS global symptoms and in particular distention and abdominal pain
These is currently insufficient evidence to recommend a particular species or strain
Patients wishing to try probiotics are advised to take them for up to 12 weeks, and discontinue if no improvement in symptoms
Commonly prescribed probiotics include Alforex, Udos Super 8 and Symprove
3. Laxatives - First-line Drugs for IBS-C:
Laxative doses should be titrated according to symptoms
Both osmotic and stimulant and osmotic laxatives are effective in the treatment of chronic idiopathic constipation but long term use of osmotic laxatives are favoured over stimulant laxatives to minimise laxative abuse or a lazy or laxative bowel.
Osmotic laxatives: lactulose (Duphalac), macrogol (Movicol), polyethylene glycol
Stimulant laxatives: bisacodyl (Dulcolax), senna (Senokot), sodium picosulfate
Only osmotic laxatives have been formally evaluated for IBS-C, with limited efficacy seen - across two RCTs, polyethylene glycol significantly increased bowel movements in one trial with no significant effect on abdominal pain in either trial
4. Second-line Drugs for IBS-C and severe constipation: In patients with severe IBS-C and constipation not improving with laxatives (i.e patients who go 4-5 days or over a week between bowel motions despite taking 3-4 Movicol sachets daily, escalation to second-line treatments should be considered. These include two medication classes 5HT4 agonists and Secretagogues:
Securing Essential Antiibiotics
5HT4 Agonists: These are prokinetic agents, stimulating propulsive gut motility. Examples include:
1) Prucalopride (Resolor): Shown to improve abdominal pain, bloating and frequency of bowel movements in large multiple randomised controlled trials in patients with idiopathic chronic constipation. The safety profile has been encouraging, especially the absence of arrhythmogenic potential. The standard dose is 2mg OD with or without food at any time of the day with some patients reporting increased efficacy up to 4mg daily. To date, there have been no trials specifically for IBS-C
2) Tegaserod: Licenced in the USA for IBS-C in females under 65 years of age with no prior cardiovascular disease history. It is not currently licenced by the EMA
B.Secretagogues: These activate ion channels on the intraluminal surface of enterocytes, causing efflux of ions and water into the lumen, softening stool and accelerating transit. Examples include:
1) Linaclotide (Constella): A guanylate-cyclase c agonist that improves abdominal pain, bloating and frequency of bowel movements. The standard dose is 290µg OD. Across all trials, It is the most efficacious secondline IBS-C treatment but has the highest rate of side-effects, most commonly diarrhoea (over 10%)
2) Lubiprostone (Amitiza): A chloride channel activator that improves abdominal pain, bloating and frequency of bowel movements. The standard dose is 24μg BD. It is efficacious with lower rates of diarrhoea than linaclotide but nausea is common (30%)
3) Other secretagogues: Plecanatide and tenapanor have similar efficacy and safety profiles to linaclotide and lubiprostone. They are licenced for IBS-C in the USA but not currently by the European Medical Agency (EMA)
5. Psychological Therapies:
IBS-specific Cognitive Behavioural Therapy (CBT) and Gut-directed Hypnotherapy are two areas of ongoing research that may be efficacious in global IBS symptoms
These should be considered when symptoms have not improved after 12 months of drug treatment, or earlier if locally available or patient preference
Their role specifically in IBS-C needs further evaluation
As you can see, there are many novel therapeutic options in IBS-C, offering patients many and varied treatments that can significantly improve symptoms as well as quality of life. Furthermore, this remains an area with considerable ongoing research, hopefully providing patients with even more treatment options in the coming years.
References:
1. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome, Vasant DH, et al. Gut 2021; 70: 1214 - 1240. doi: 10.1136/gutjnl-2021-324598
2. https://theromefoundation.org/ rome-iv/rome-iv-criteria/
References:
1. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome, Vasant DH, et al. Gut 2021; 70: 1214 - 1240. doi: 10.1136/gutjnl-2021-324598
2. https://theromefoundation.org/romeiv/rome-iv-criteria/
Recent shortages of essential antibiotics in Europe have renewed the urgency to reform the pricing and industrial infrastructure for these essential medicines. The off-patent sector manufactures and supplies most antibiotics in Europe needed to control bacterial infections. Pricing policies for these antibiotics must urgently be reviewed so that they remain viable to produce and supply.
The AMR industry alliance report titled “Strengthening the Sustainability of the Off-Patent Supply Chain” states that the key requirements to improve the supply of antibiotic medicinesare:
1. New pricing policies that reflect the value of essential antibiotics instead of lowest price only
2. Sustainable tender policies that reward multiple winners, thus reducing shortage risks
3. Improving health forecasting so antibiotic manufacturing can be adapted to meet demand spikes. Medicines for Europe Director General Adrian van den Hoven said, “Antibiotic shortages this winter were a perfect example of the policy gaps that make the supply of antibiotics so fragile. For years, policies have been based on the lowest price only, with restrictive tender rules
“Both policies have fuelled market consolidation making it difficult to respond to sudden demand surges. We also need better disease forecasting in Europe to better plan manufacturing ahead of infectious seasons. These issues must be addressed in the upcoming revision of the EU pharmaceutical legislation to combat AMR.”
Resource hub The AMR industry alliance report titled “Strengthening the Sustainability of the Off-Patent Supply Chain” is available at https://www.amrindustryalliance.org/wp-content/uploads/2023/02/CRA-AMR-Industry-AllianceSustainability-Framework-Report-FINAL.pdf
76 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Conjunctivitis
Conjunctivitis: Infectious and Non-Infectious
Conjunctivitis, also known as red or pink eye, is an eye conditions caused by infection or allergies. We recently spoke to Theresa Lowry Lehnen (PhD), Clinical Nurse Specialist and Associate Lecturer South East Technological University for a further insight into the infectious and non-infectious diagnosis of this very common and prevalent condition.
Conjunctivitis can be acute or chronic and infectious or noninfectious. Viruses and bacteria are the most common infectious causes. Theresa explains, “Non-infectious conjunctivitis includes allergic, toxic, and cicatricial conjunctivitis, as well as inflammation secondary to immune-mediated diseases and neoplastic processes.2 Prevalence of conjunctivitis varies according to the underlying cause, which may be influenced by the patient’s age, as well as the season of the year.”
Viral conjunctivitis is the most common cause of infectious conjunctivitis both overall and in the adult population and is more prevalent in summer. Theresa points out that the signs and symptoms of viral conjunctivitis at presentation are variable - it usually does not require treatment, she says.
“Bacterial conjunctivitis is the second most common cause of infectious conjunctivitis, with most uncomplicated cases resolving in 1 to 2 weeks. Bacterial conjunctivitis is responsible for the majority of cases in children and occurs more frequently in winter. The majority of bacterial conjunctivitis cases are self-limiting and no treatment is necessary in uncomplicated cases. However, conjunctivitis caused by chlamydia or gonorrhoea and conjunctivitis in contact lens wearers should be treated with antibiotics. Allergic conjunctivitis occurs most often in spring and summer. Treatment with antihistamines and mast cell stabilisers usually alleviates the symptoms.”1 Treatment with antihistamines and mast cell stabilizers alleviates the symptoms of allergic conjunctivitis.
Symptoms
The classic symptoms of the three most common types of conjunctivitis are:2
Viral: symptoms of itching and tearing, history of recent upper respiratory tract infection, watery discharge, inferior palpebral
conjunctival follicles, tender pre-auricular lymphadenopathy.
Bacterial: symptoms of redness and foreign body sensation, morning matting of the eyes, white-yellow purulent or mucopurulent discharge, conjunctival papillae, infrequently pre-auricular lymphadenopathy.
Allergic: symptoms of itching or burning, history of allergies/atopy, watery discharge, oedematous eyelids, conjunctival papillae, no pre-auricular lymphadenopathy.
Diagnosis
“A focused history and ocular examination are important for making a diagnosis and appropriate decisions about the treatment and management of conjunctivitis,” says Theresa. Labs and cultures are rarely indicated to confirm the diagnosis of conjunctivitis. Cultures and cytology are usually reserved for cases of recurrent conjunctivitis, those resistant to treatment, suspected gonococcal or chlamydial infection, suspected infectious neonatal conjunctivitis, and adults presenting with severe purulent discharge.1, 2
“Ocular history includes timing of onset, prodromal symptoms, unilateral or bilateral eye involvement, associated symptoms, previous treatment and response, past episodes, type of discharge, and presence of pain, itching, eyelid characteristics, periorbital involvement, vision changes, photophobia, and corneal opacity.2 The ocular exam should focus on visual acuity, extraocular motility, visual fields, discharge type, shape, size and response of pupil, the presence of proptosis, corneal opacity, foreign body assessment, tonometry, and eyelid swelling.2
“Eye discharge type and ocular symptoms can help determine the cause. A purulent or mucopurulent discharge is often due to bacterial conjunctivitis, a watery discharge is more characteristic of viral conjunctivitis and itching is associated with allergic conjunctivitis. However, clinical presentation is often nonspecific and relying on the type of discharge and patient symptoms does not always lead to an accurate diagnosis.1
“Similar to redness and discharge, other common signs and symptoms of conjunctivitis

An interview with Theresa Lowry-

are nonspecific and can make determining the underlying cause more difficult.” For example, she told us, itching is historically correlated with allergic conjunctivitis and while in the context of watery discharge and a history of atopy this is likely the case, one study found that 58% of patients with culture-positive bacterial conjunctivitis also reported itchy eyes.2
Papillae, a nonspecific finding in conjunctivitis can be present in both infectious and noninfectious conjunctivitis. Theresa adds, “Papillae, small elevations usually under the superior tarsal conjunctival, with central vessels are often present in bacterial conjunctivitis, allergic conjunctivitis, and contact lens intolerance. Papillae in chronic allergic conjunctivitis can lead to a cobblestone appearance of the conjunctiva.2 While also non-specific, the presence of follicles, in conjunction with other findings, can help differentiate the aetiology of conjunctivitis. Follicles are small elevated yellow-white lesions found at the junction of the palpebral and bulbar conjunctiva and are a lymphocytic response often present in chlamydial and adenoviral conjunctivitis.2
“Differential diagnosis can include glaucoma; iritis; keratitis; episcleritis; scleritis; pterygium; corneal ulcer; corneal abrasion; corneal foreign body; subconjunctival haemorrhage; blepharitis; hordeolum; chalazion; contact lens over use and dry eye. Other signs and symptoms that can point to diagnosis other than conjunctivitis include localised redness, redness not including the
keeping it open.2 It is important to differentiate associated with severe sight- or life-threatening presentations can often overlap, a systematic should safely out rule acute sight-threatening 7
entire conjunctiva, ciliary flush, elevated intraocular pressure, vision loss, moderate to severe pain, hypopyon, hyphema, pupil asymmetry, decreased pupil response, and trouble opening the eye or keeping it open.2 It is important to differentiate conjunctivitis from other causes of “red eye” associated with severe sight- or life-threatening consequences.” Theresa also notes that while presentations can often overlap, a systematic approach including a thorough history and ocular exam should safely out rule acute sightthreatening diagnoses and identify the likely cause of conjunctivitis.7
Viral Conjunctivitis
Viral Conjunctivitis
Image: creative commons. https://creativecommons.org/ Viruses are responsible for up to 80% “Clinical accuracy in diagnosing viral conjunctivitis confirmation and many cases are misdiagnosed 90% of cases of viral conjunctivitis are caused conjunctival fever and epidemic keratoconjunctivitis, associated with viral conjunctivitis.
Image: creative commons. https:// creativecommons.org/
Viruses are responsible for up to 80% of all cases of acute conjunctivitis. She continues, “Clinical accuracy in diagnosing viral conjunctivitis is less than 50% compared with laboratory confirmation and many cases are misdiagnosed as bacterial conjunctivitis. Between 65% and 90% of cases of viral conjunctivitis are caused by adenoviruses, which can produce pharyngoconjunctival fever and epidemic keratoconjunctivitis, two of the common clinical entities associated with viral conjunctivitis.
“Pharyngo-conjunctival fever (PCF) caused by the presence of fever, pharyngitis, conjunctivitis. Additional ocular surface haemorrhages of the conjunctiva as cytokines and conjunctival vasculature.
“Pharyngo-conjunctival fever (PCF) caused by HAdV types 3, 4 and
“This condition is self-limited, often resolving treatment.6 The most severe ocular keratoconjunctivitis (EKC) which affects lasting and permanent ocular surface changes
77 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Lehnen (PhD), CNS, GPN, RNP, South East Technological University
and less frequently serotype 4 were believed
are recommended, however, topical corticosteroids should be avoided because they potentiate the virus and may cause harm.1
Incubation and communicability are estimated to be 5 to 12 days and 10 to 14 days, respectively.1
Conjunctivitis
“Conjunctivitis caused by the herpes simplex virus is usually unilateral. The discharge is thin and watery, and accompanying vesicular eyelid lesions may occur. Topical and oral antivirals are recommended, however, topical corticosteroids should be avoided because they potentiate the virus and may cause harm.1
7 is usually characterised by the presence of fever, pharyngitis, periauricular lymphadenopathy, and acute follicular conjunctivitis. Additional ocular surface findings include oedema, hyperaemia, and petechial haemorrhages of the conjunctiva as a result of interaction between proinflammatory cytokines and conjunctival vasculature.
“This condition is self-limited, often resolving spontaneously in two–three weeks without any treatment.6 The most severe ocular manifestation of adenoviral infection is epidemic keratoconjunctivitis (EKC) which affects both the conjunctiva and cornea, leaving behind longlasting and permanent ocular surface changes and visual disturbances. Serotypes, and less frequently serotype 4 were believed to be associated with EKC, but more recently, HAdV-D53 and HAdV-D54 have been identified in several outbreaks and thought to be responsible for the majority of EKC cases.6 Ocular manifestations of EKC include conjunctival discharge, follicular conjunctivitis, corneal sub epithelial infiltrates (SEI), corneal scarring, development of conjunctival membranes and pseudo membranes and symblepharon formation.6
“Herpes zoster virus, responsible for shingles, can involve ocular tissue, especially if the first and second branches of the trigeminal nerve are involved. Eyelids are the most common site of ocular involvement, followed by the conjunctiva. Corneal complication and uveitis may occur. Patients with suspected eyelid or eye involvement or those presenting with Hutchinson sign (vesicles at the tip of the nose, which has high correlations with corneal involvement) should be referred for a thorough ophthalmic evaluation. Treatment usually consists of a combination of oral antivirals and topical steroids.”1
with Hutchinson sign (vesicles at the tip of the nose, which has high correlations with corneal involvement) should be referred for a thorough ophthalmic evaluation. Treatment usually consists of a combination of oral antivirals and topical steroids.”1
Discharge is often purulent or mucopurulent, however, patients often present with mild symptoms for weeks to months.
Bacterial Conjunctivitis
Bacterial Conjunctivitis
Bacterial Conjunctivitis
“Herpes zoster virus, responsible for shingles, can involve ocular tissue, especially if the first and second branches of the trigeminal nerve are involved. Eyelids are the most common site of ocular involvement, followed by the conjunctiva. Corneal complication and uveitis may occur. Patients with suspected eyelid or eye involvement or those presenting with Hutchinson sign (vesicles at the tip of the nose, which has high correlations with corneal involvement) should be referred for a thorough ophthalmic evaluation. Treatment usually consists of a combination of oral antivirals and topical steroids.”1
“Chlamydial conjunctivitis is often acquired via oculogenital spread or other intimate contact with infected individuals and in new-borns the eyes can be infected after vaginal delivery by should be investigated.”1, 2, 6
allergic conjunctivitis (SAC) and perennial allergic conjunctivitis (PAC); combined IgE and non-IgE-mediated reactions, including VKC and AKC; and non-IgE-mediated reactions, including giant papillary conjunctivitis (GPC) and contact dermatoconjunctivitis (CDC).6
“Treatment involves avoidance of the offending antigen and the use of saline solution or artificial tears to dilute and remove the allergens. Topical decongestants, antihistamines, mast cell stabilisers, nonsteroidal anti-inflammatory drugs and corticosteroids may be indicated. Steroids must be used judiciously and only when indicated.”1, 7
chlamydial infection should be managed accordingly. Conjunctival hyperemia, discharge, and lymphoid follicle formation are hallmarks of chlamydial Discharge is often purulent or mucopurulent, however, patients often present symptoms for weeks to months.

Bacterial conjunctivitis can be contracted from infected individuals or result from abnormal proliferation of the conjunctival flora.
Images: creative commons. https://creativecommons.org/
Images: creative commons. https://creativecommons.org/
Chronic bacterial conjunctivitis is used to describe any conjunctivitis lasting more than 4 weeks. Staphylococcus aureus, Moraxellalacunata, and enteric bacteria are the most common causes. Ophthalmologic consultation should be sought for management.
Treatment and Management
Bacterial conjunctivitis can be contracted from infected individuals or result from abnormal proliferation of the conjunctival flora.
Lymphadenopathy occurs in up to 50% of viral conjunctivitis cases and is more prevalent in viral conjunctivitis compared with bacterial conjunctivitis. Treatment with antihistamines and mast cell stabilizers alleviates the symptoms of allergic conjunctivitis.
Viral conjunctivitis secondary to adenoviruses is highly contagious and spreads through direct contact example via contaminated fingers, medical instruments, swimming pool water, or personal items.” Incubation and communicability are estimated to be 5 to 12 days and 10 to 14 days, respectively.1
Bacterial conjunctivitis can be contracted from infected individuals or result from abnormal proliferation of the conjunctival flora.
“Contaminated fingers, oculogenital spread, and contaminated fomites are common routes of transmission. Certain conditions such as compromised tear production, disruption of the natural epithelial barrier, abnormality of adnexal structures, trauma, and immunosuppressed status can predispose to bacterial conjunctivitis. Bacterial conjunctivitis is much more common in children than adults, and the pathogens responsible vary depending on the age group. The most common pathogens responsible for bacterial conjunctivitis in adults are staphylococcal species, followed by Streptococcus pneumoniae and Haemophilus influenzae. In children, the condition is often caused by H influenzae, S pneumoniae, and Moraxella catarrhalis The infection usually lasts 7 to 10 days. 1, 2
“Contaminated fingers, oculogenital spread, and contaminated fomites are common routes of transmission. Certain conditions such as compromised tear production, disruption of the natural epithelial barrier, abnormality of adnexal structures, trauma, and immunosuppressed status can predispose to bacterial conjunctivitis. Bacterial conjunctivitis is much more common in children than adults, and the pathogens responsible vary depending on the age group. The most common pathogens responsible for bacterial conjunctivitis in adults are staphylococcal species, followed by Streptococcus pneumoniae and Haemophilus influenzae
Theresa concludes by adding that effective management of conjunctivitis includes timely diagnosis, appropriate differentiation of the various aetiologies, and appropriate treatment.
“Chlamydial conjunctivitis is often acquired via oculogenital spread or other intimate with infected individuals and in new-borns the eyes can be infected after vaginal infected mothers. Treatment with systemic antibiotics such as oral azithromycin doxycycline is efficacious. Patients and their sexual partners must be treated and with gonorrhoea should be investigated.” 1, 2, 6
Signs and symptoms include red eye, purulent or mucopurulent discharge, and chemosis.
“Conjunctivitis caused by the herpes simplex virus is usually unilateral. The discharge is thin and watery, and accompanying vesicular eyelid lesions may occur. Topical and oral antivirals are recommended, however, topical corticosteroids should be avoided because they potentiate the virus and may cause harm.1


“Herpes zoster virus, responsible for shingles, can involve ocular tissue, especially if the first and second branches of the trigeminal nerve are involved. Eyelids are the most common site of ocular involvement, followed by the conjunctiva. Corneal complication and uveitis may occur. Patients with suspected eyelid or eye involvement or those presenting
Theresa adds that incubation and communicability are estimated to be 1 to 7 days and 2 to 7 days, respectively.
Chronic bacterial conjunctivitis is used to describe any conjunctivitis lasting weeks. Staphylococcus aureus, Moraxellalacunata, and enteric bacteria common causes. Ophthalmologic consultation should be sought for management.

In children, the condition is often caused by H influenzae, S pneumoniae, and Moraxella catarrhalis The infection usually lasts 7 to 10 days. 1, 2
“Hyperacute bacterial conjunctivitis presents with decreased vision and a severe copious purulent discharge. There is often accompanying eyelid swelling, eye pain on palpation, and preauricular adenopathy. It is often caused by Neisseria gonorrhoeae and carries a high risk for corneal involvement and subsequent corneal perforation. Treatment for hyperacute conjunctivitis secondary to N gonorrhoea is intramuscular ceftriaxone, and concurrent
Contaminated fingers, oculogenital spread, and contaminated fomites are common routes of transmission. Certain conditions such as compromised tear production, disruption of the natural epithelial barrier, abnormality of adnexal structures, trauma, and immunosuppressed status can predispose to bacterial conjunctivitis. Bacterial conjunctivitis is much more common in children than adults, and the pathogens responsible vary depending on the age group. The most common pathogens responsible for bacterial conjunctivitis in adults are staphylococcal species, followed by Streptococcus pneumoniae and Haemophilus influenzae In children, the condition is often caused by H influenzae, S pneumoniae, and Moraxella catarrhalis The infection usually lasts 7 to 10 days.1, 2
“Hyperacute bacterial conjunctivitis presents with decreased vision and a severe copious purulent discharge. There is often accompanying eyelid swelling, eye pain on palpation, and preauricular adenopathy. It is often caused by Neisseria gonorrhoeae and carries a high risk for corneal involvement and subsequent corneal perforation. Treatment for hyperacute conjunctivitis secondary to N gonorrhoea is intramuscular ceftriaxone, and concurrent chlamydial infection should be managed accordingly. Conjunctival hyperemia, mucopurulent discharge, and lymphoid follicle formation are hallmarks of chlamydial conjunctivitis.
“Hyperacute bacterial conjunctivitis presents with decreased vision and a severe copious purulent discharge. There is often accompanying eyelid swelling, eye pain on palpation, and preauricular adenopathy. It is often caused by Neisseria gonorrhoeae and carries a high risk for corneal involvement and subsequent corneal perforation. Treatment for hyperacute conjunctivitis secondary to N gonorrhoea is intramuscular ceftriaxone, and concurrent
“Bilateral mattering and adherence of the eyelids, lack of itching, and no history of conjunctivitis are strong positive predictors of bacterial conjunctivitis,” she says. “Severe purulent discharge should always be cultured and gonococcal conjunctivitis should be considered. Conjunctivitis not responding to standard antibiotic therapy in sexually active patients warrants a chlamydial evaluation. The possibility of bacterial keratitis is high in contact lens wearers, who should be treated with topical antibiotics and referred to an ophthalmologist. A patient wearing contact lenses should be asked to remove them.”1, 6
Signs and symptoms include red eye, purulent or mucopurulent discharge, and Theresa adds that incubation and communicability are estimated to be 1 to 7 days, respectively.
“Bilateral mattering and adherence of the eyelids, lack of itching, and conjunctivitis are strong positive predictors of bacterial conjunctivitis,” she purulent discharge should always be cultured and gonococcal conjunctivitis considered. Conjunctivitis not responding to standard antibiotic therapy in patients warrants a chlamydial evaluation. The possibility of bacterial keratitis contact lens wearers, who should be treated with topical antibiotics and ophthalmologist. A patient wearing contact lenses should be asked to remove
Allergic conjunctivitis
Allergic conjunctivitis
“Most acute bacterial conjunctivitis infections are self-limiting and do not require topical antibiotics. Acute bacterial conjunctivitis is usually unilateral with yellow-white mucopurulent discharge and symptoms usually resolve within 5-7 days without treatment. If topical antibiotics are considered necessary a delayed prescription for 3 days should be considered to see if symptoms resolve with self-care and without antibiotic eye drops.7 “Antibiotic ointments last longer than drops, however they tend to interfere with vision. Chloramphenicol is not recommended in pregnancy or breastfeeding. In 2021 the Summary of Product Characteristics for Chloromycetin® 0.5% Redi-Drops was updated to contra-indicate use of the drops in children under 2 years. This is due to a risk of toxicity from boron. This excipient is not present in the ointment formulation and the contra-indication applies to the drops only.”7
Image: creative commons. https://creativecommons.org/
Image: creative commons. https:// creativecommons.org/
Allergic conjunctivitis is the inflammatory response of the conjunctiva to allergens such as pollen, animal fur and other environmental antigens. Redness and itching are the most consistent symptoms.2
Allergic conjunctivitis is the inflammatory response of the conjunctiva to allergen pollen, animal fur and other environmental antigens. Redness and itching consistent symptoms. 2
“Ocular allergic conditions can be classified into three main categories: reactions, including seasonal allergic conjunctivitis (SAC) and perennial allergic
“Ocular allergic conditions can be classified into three main categories: IgE-mediated reactions, including seasonal
“Antibiotic drops are indicated for complicated bacterial conjunctivitis, in conjunctivitis caused by gonorrhoea or chlamydia, and in bacterial conjunctivitis in contact lens wearers.5 The recommended treatment for gonococcal conjunctivitis is ceftriaxone 1gm IM, and it is also recommended to treat for concurrent chlamydial infection with 1gm azithromycin orally. The neonatal dosing for gonococcal conjunctivitis is 25 to 50mg/kg ceftriaxone IV/IM with a max dose of 125mg, with 20mg/ kg azithromycin PO once daily for three days.2
78 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
HSE (2021). Conjunctivitis: Antibiotic Prescribing. Available at: https://www.hse.ie/eng/services/ list/2/gp/antibiotic-prescribing/conditions-and-treatments/skin-soft-tissue/conjuncitivitis/ conjuncitivitis-ophthalmology.html

“Viral conjunctivitis due to adenoviruses is usually selflimiting, and treatment should target symptomatic relief including cold compresses and saline solution or artificial tears.2
“Herpes simplex keratitis requires antiviral therapy such as aciclovir or ganciclovir and the patient should be reviewed by an ophthalmologists to monitor for complications.2 Treatment of herpes zoster conjunctivitis includes a
combination of oral antivirals and topical steroids, however, steroids should only be part of therapy in consultation with ophthalmology. Antiviral doses differ from those used for herpes simplex and consist of oral acyclovir 800mg five times a day, oral famciclovir 500mg TDS, or oral valacyclovir 1g TDS for 7 to 10 days.”3, 4

Topical corticosteroids are not recommended for cases of bacterial or viral conjunctivitis,
except for herpes zoster, as they can prolong the condition or potentiate the infection, resulting in complications including corneal damage and blindness.1, 2

“Treatment for allergic conjunctivitis consists of allergen avoidance, artificial tears, cold compresses, and a wide range of topical agents. Topical agents include topical antihistamines alone or in combination with vasoconstrictors, topical mast
cell inhibitors and topical glucocorticoids for refractory symptoms. Oral antihistamines can also be used in moderate to severe cases of allergic conjunctivitis.1, 2 “Any patient with moderate to severe pain, vision loss, corneal involvement, severe purulent discharge, conjunctival scarring, recurrent episodes, lack of response to therapy, or herpes simplex keratitis should receive a prompt referral to an ophthalmologist. In addition, patients requiring steroids, contact lens wearers, and those with photophobia should also be referred to an ophthalmologist.2
“Viral and bacterial conjunctivitis can spread by direct contact and have high transmission rates. Patient education is important to prevent transmission and the importance of hand hygiene should be highlighted.
Caution with steroids/ antibioticsteroid combination drops
“Steroids should be used with caution. Steroid drops or combination drops containing steroids should not be used routinely. Topical steroids are associated with cataract formation and can cause an increase in eye pressure, leading to glaucoma.1 if an undiagnosed corneal ulcer secondary to herpes, bacteria, or fungus is present, steroids can also worsen the condition, leading to corneal erosion and blindness.1
Complications
“Complications of acute conjunctivitis are rare. However, patients who fail to show improvement should be referred to an ophthalmologist for further evaluation. Patients with HZV conjunctivitis are at the highest risk of complications including corneal complications and uveitis, and should always see an ophthalmologist for close re-evaluation. Patients with N. gonorrhoea are also at high risk for corneal involvement and secondary corneal perforation and should be treated appropriately,”2 she concludes.
References available on request
79 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Clinical R&D
ALZECURE ACHIEVES LAST PATIENT LAST VISIT (LPLV) IN ITS PHASE II CLINICAL TRIAL OF THE NON-OPIOID ACD440 IN NEUROPATHIC PAIN
AlzeCure Pharma AB (publ) (FN STO: ALZCUR) a pharmaceutical company that develops a broad portfolio of drug candidates for diseases affecting the central nervous system, with projects in both Alzheimer’s disease and pain, has announced that the Last Patient Last Visit (LPLV) has now been completed in the ongoing phase II clinical trial with ACD440, the lead non-opioid drug candidate in the Painless platform, which is being developed against peripheral neuropathic pain.
”We look forward to seeing the results of this important phase II trial in peripheral neuropathic pain, an area of very high medical need where up to 80% of patients are not responding satisfactorily with their current treatments and where many are still prescribed opioids”, said Märta Segerdahl, project leader and CMO at AlzeCure Pharma.
This double-blind, placebocontrolled, randomized cross-over study, which is carried out in collaboration with LINK Medical Research in Sweden, is aimed at evaluating the efficacy, safety and pharmacokinetics of AlzeCure's lead drug candidate in pain, ACD440. The main results from the study are expected by the summer of 2023 at the latest.
AlzeCure's drug candidate
ACD440 is a TRPV1 antagonist for the local treatment of patients with peripheral neuropathic pain. The discovery of TRPV1 that underlies the project was awarded the Nobel Prize in Physiology or Medicine in 2021. There is thus a strong scientific basis for this biological mechanism, including its relationship to pain signaling. ACD440, which is also the company's leading drug candidate within the Painless platform, completed a positive phase Ib study in 2021. It showed both very good tolerability and safety, but also a potent, long-lasting painrelieving effect of the substance that is applied as a gel to the skin.
The medical need in neuropathic pain is very great, not least to find alternatives to opioids. The neuropathic pain market is the single largest market segment in pain management with annual sales exceeding $11 billion. Up to 80% of patients do not get a satisfactory effect with their current treatments.
"The lack of effective and safe drugs, combined with the fact that upwards of 8% of the adult population suffers from
neuropathic pain, makes clear the great medical need and the enormous potential of our project. ACD440 can become a first-inclass drug and meet a very large patient need, which of cause is a value driver in connection with the outlicensing of the project", said Martin Jönsson, CEO of AlzeCure Pharma.
KENNY FAMILY PRESENTS CHEQUE FOR ¤40,500 TO UNIVERSITY HOSPITAL GALWAY

A very generous donation of ¤40,500 was recently presented to the Intensive Care Unit and the Patient Comfort Fund at University Hospital Galway (UHG) in memory of the late Mary Kenny from Loughrea, Co Galway. In addition, ¤5,000 was donated to Strange Boat Donor Foundation – The National Organ Donor Commemorative Garden (Circle of Life) located in Galway, and ¤2,000 to Quest Brain Injury Services, Galway.
The cheque was presented to the Intensive Care Unit and the Patient Comfort Fund, by John and Isabelle Kenny (husband and daughter of the late Mary
Kenny). The funds were raised through activities which included a GoFundMe page and a coffee morning bonanza at the Willow Café in conjunction with The Woodford Garden Centre. An evening of music was held in Carton House, as well as a raffle and spot prizes sponsored by local businesses. Finally, 20 brave individuals 'Jumped for Mary' by participating in a sky dive.
John and Isabelle Kenny expressed their gratitude and said, “We would like to thank the doctors and nurses who cared for Mary in her final days. The medical and nursing team supported us to make the final decision to entrust Mary to the angels and to donate Mary’s organs, saving three people’s lives. To this dedicated, professional, supportive and compassionate team – from our family to yours we say a heartfelt thank you.”
Gillian Shanahan, Organ Donation Nurse Manager said, “We are forever humbled by organ donors and their families who find the strength in their depths of grief, to give the gift of life. On behalf of
Pictured at the cheque presentation to the Intensive Care Unit and Patient Comfort Fund at University Hospital Galway, from left front row: Bernie Moran; Edel Kelly, Assistant Directive of Nursing; John Kenny; Isabelle Kenny; Gillian Shanahan, Organ Donation Nurse Manager; Emma Gunning; Yvonne Shaw, Clinical Nurse Manager 2, ICU; Angela Burke O’Brien, Clinical Nurse Manager 2, ICU
From left back row: Pauline May, Assistant Director of Nursing /Former Organ Donation Nurse Manager; Pat Kenny; Dr John Bates, Consultant Intensivist; Ann Sheehan, Clinical Facilitator;
myself and the team at University Hospital Galway we would like to express our sincere gratitude to all those involved for their time and efforts in raising this substantial amount. The money will be used to improve the comfort and experience of patients and their families during their stay in the Intensive Care Unit."
NEW DATA WHICH MAY SHOW INDICATOR OF OSTEOPOROSIS
Findings from a recent survey found that one in five (22%) adults have broken or fractured a bone since they turned 40 years of age. Over a third (35%) of these patients reported to have had a fragility fracture.1 Osteoporosis is a condition that causes bones to become weakened which can lead to fragility fractures. A fragility fracture is a broken bone that occurs due to minimal trauma, such as from a fall from standing height or less. Surprisingly, the rate of fragility fractures was high amongst 40-54 year olds with almost one in five (18%) surveyed having sustained a fragility fracture. This level of fractures would generally be expected amongst older age groups.
The research commissioned by Athena Pharmaceuticals surveyed 600 Irish men and women aged 40-90 to understand the occurrence of fragility fractures and awareness of appropriate nutritional supplementation for optimal bone health as people advance in age. The findings revealed a significant lack of awareness and confusion
80 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
regarding the recommended daily allowances (RDA) of both Calcium and Vitamin D. 85% of adults are unaware of the correct amount of Calcium they require daily and 90% are unclear on the correct amount of Vitamin D required for optimal bone health.1
Diet is a major contributor to optimal bone health. The recommended daily allowance of calcium and Vitamin D as defined by the Institute of Medicine and Food Safety Authority of Ireland4 is outlined in table 1. Calcium intake may be achieved by consuming three portions of any of the following: 1 glass (200ml) of milk, 1 carton (125g) yoghurt, 1 bottle (200ml) yoghurt drink, 2 thumbs (25g) of hard or semi hard cheese such as cheddar or edam or 2 thumbs (25g) soft cheese such as brie or camembert. Of those surveyed, over three-quarters (76%) of men and women above 40 years old are not getting the appropriate amounts of calcium in their diet, only consuming <50% of the RDA. Worryingly, 80% of adults who have a history of a fragility fracture are not consuming adequate calcium and alarmingly, 7% report they are consuming none. Vitamin D5 is available from two sources, sunlight exposure during the summer months and diet. Sources include natural foods such as egg yolks and oily fish, and fortified foods such as cheese and milk drinks. Supplements are usually necessary to get the recommended amount. But this survey found that 90% of people are unsure of the daily vitamin D requirements.
Dr Miriam Delaney, Specialist in Osteoporosis, Metabolic Bone Disorders and Calcium Metabolism at the Galway Clinic and Athena Pharmaceuticals Spokesperson, said: “It is important that people build and maintain their ‘bone bank’ throughout their lives. Anyone who has had a fragility fracture over the age of 40, is considered at high risk of osteoporosis and should discuss evaluation and treatment with their doctor. Achieving bone health may include behavioural changes, such as improving diet, engaging in weight-bearing exercise and taking oral supplements of calcium and vitamin D, if required.
“Eating behaviours have changed drastically over the past three decades with our families and children eating very differently to how we, or our parents, would have eaten in the past. We develop our bone bank during our early decades of life and after achieving peak bone mass, we naturally lose bone mass with ageing. Hence it is essential that our diet provides us with sufficient calcium and vitamin D, to minimise bone loss as we
age. People with little or no calcium in their diet are at elevated risk of osteoporosis and fractures in later years. Falling and fracturing the hip or spine, is a major cause of longterm pain, decreased mobility, loss of independence, hospitalisation and death in the elderly.
Individuals at a higher risk of Osteoporosis include those with many underlying illnesses, or taking certain medications such as oral steroids (for asthma or COPD), patients being treated for cancer, those with limited mobility, those in care homes or hospitals, etc and should be recognised as being at risk of weakened bones as a result of their underlying illness and treatment.6
When supplementation is deemed necessary, it is important that people get expert advice from their pharmacist or healthcare provider.” For more information about the risk factors, assessment and treatment of osteoporosis visit https://www.osteoporosis. foundation/educational-hub/ material/patient-resources
2023 TRANSPLANT GAMES
Fourteen Irish transplant recipients will travel to Perth, Australia this month to represent Ireland at the World Transplant Games (15-21 April). This follows a four-year hiatus for the biennial World Transplant Games, as the previous event planned for 2021 had to be cancelled due to the global COVID-19 pandemic. The Irish squad gathered at the ALSAA Sports Complex, Dublin airport on Sunday, 12th March and were joined by family, friends and other

members of the Transplant Team Ireland programme, to receive their official team kit. The guest speaker at the event organised by the Irish Kidney Association was former Olympian triathlete Gavin Noble, the Olympic Federation of Ireland’s Chef de Mission for the Paris 2024 Olympic Games and 2023 European Games.
Ranging in age from 36 right up to 75, the current Transplant Team Ireland panel of 10 inspirational men and 4 women travelling to Perth have all received organ transplants including 2 liver, 2 bone marrow, and 10 kidney. They will be among over 1,200 participants from over 50 countries, all embracing their gift of life and honoring their donors in this celebration of life through sport.
In his talk to Transplant Team Ireland, Gavin Noble shared some of the wisdom he gained from the lead up to, and participation in, the 2012 Olympic Games in London and how he is now invested into helping other athletes be the best that they can be. He said, "Each and every one of you on the Transplant Team are an inspiration as you are living your best life, honouring your donors and representing your country in sport. Sport brings people together as does organ donation".
The Irish Kidney Association is responsible for managing and supporting Transplant Team Ireland’s involvement at the Games and its National Advocacy and Projects Manager Colin White is the Team Manager.
To find out more about the World Transplant Games visit website www.worldtransplantgames.org and to find out more about members of Transplant Team Ireland and to follow their progress at the Games, you can follow the team blog; www.transplantteamireland.ie
SANOFI TO ACQUIRE PROVENTION BIO, ADDING TO PORTFOLIO TZIELD, THE FIRST DISEASE-MODIFYING TREATMENT FOR THE DELAY OF STAGE 3 TYPE 1 DIABETES (T1D) Sanofi and Provention Bio, Inc., a U.S.-based, publicly traded biopharmaceutical company focused on intercepting and preventing immune-mediated diseases including type 1 diabetes (T1D), have entered into an agreement under which Sanofi has agreed to acquire Provention Bio, Inc., for $25.00 per share in cash, representing an equity value of approximately $2.9 billion.
Transplant Team Ireland members pictured at a team gathering in preparation for The World Transplant Games held in Perth, Australia next month. Transplant Team Ireland's participation at the Games is managed by the Irish Kidney Association. Fourteen inspirational Irish men and women who have received liver, kidney and bone marrow transplants will travel to Perth, Australia next month to represent Ireland at the World Transplant Games (15-21 April). Picture Conor McCabe Photography
81 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
Clinical R&D
The transaction adds an innovative, fully owned, first-inclass therapy in type 1 diabetes to Sanofi’s core asset portfolio in General Medicines and further drives its strategic shift toward products with a differentiated profile. TZIELD (teplizumab-mzwv) was approved in the U.S. last year as the first and only therapy to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D. The acquisition is a strategic fit for Sanofi at the intersection of the company’s growth in immunemediated diseases and diseasemodifying therapies in areas of high unmet need, and its expertise in diabetes. Sanofi will continue to utilize its capabilities in diabetes to maximize TZIELD’s potential as a transformative therapy globally and in the U.S., aiming to delay the onset of Stage 3 type 1 diabetes for some of the approximately 65,000 people diagnosed every year1. The purchase builds on an existing co-promotion agreement with Provention Bio that is already delivering TZIELD to patients in need of this immunemediated therapy.
Olivier Charmeil, Executive Vice President, General Medicines, Sanofi said, “The acquisition of Provention Bio builds on Sanofi’s mission to deliver best- and firstin-class medicines and resonates with our purpose of chasing the miracles of science for the benefit of people. By coupling Provention Bio’s transformative innovation with Sanofi’s expertise, we aim to bring life-changing benefits to people at risk of developing Stage 3 type 1 diabetes. Any additional indications, approvals and pipeline assets only serve to further our excitement. Given our existing partnership and complementary work in the diabetes and immunology spaces, we foresee a seamless integration and execution.”
TZIELD is a CD3-directed antibody indicated to delay the onset of Stage 3 T1D in adults and pediatric patients aged 8 years and older with Stage 2 T1D. Stage 3 T1D is associated with significant health risks, including diabetic ketoacidosis, which can be life threatening, and patients who progress to Stage 3 T1D eventually require insulin injections for life.
TZIELD is also in late-stage clinical development for the treatment of pediatric and adolescent patients that are newly diagnosed with clinical T1D (Stage 3). A Phase 3 trial, PROTECT, is currently
underway and top line results are expected in the second half of 2023. Additional opportunities for TZIELD include re-dosing and formulations as well as new therapeutic indications.
Provention Bio also brings certain pipeline assets in early development in immunemediated diseases.
Transaction Terms
Under the terms of the merger agreement, Sanofi will commence a cash tender offer to acquire all outstanding shares of Provention Bio, Inc. for $25.00 per share in cash, reflecting a total equity value of approximately $2.9 billion. The consummation of the tender offer is subject to customary closing conditions, including the tender of a number of shares of Provention Bio, Inc. common stock, that together with shares already owned by Sanofi or its affiliates, represents at least a majority of the outstanding shares of Provention Bio, Inc. common stock, the expiration or termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, and other customary conditions.
If the tender offer is successfully completed, then following the successful completion of the tender offer, a wholly owned subsidiary of Sanofi will merge with and into Provention Bio, Inc., and all of the outstanding Provention Bio, Inc. shares that are not tendered in the tender offer will be converted into the right to receive the same $25.00 per share in cash offered to Provention Bio, Inc. shareholders in the tender offer. Sanofi plans to fund the transaction with available cash resources. Subject to the satisfaction or waiver of customary closing conditions, Sanofi currently expects to complete the acquisition in the second quarter of 2023.
PJT Partners is acting as exclusive financial advisor to Sanofi and Weil, Gotshal & Manges LLP is acting as its legal counsel. BofA Securities, Inc. and Centerview Partners LLC are acting as financial advisors to Provention Bio, Inc. and Ropes & Gray LLP is acting as its legal counsel.
DUPIXENT® (DUPILUMAB)
APPROVED BY EUROPEAN COMMISSION AS FIRST AND ONLY TARGETED MEDICINE FOR CHILDREN AS YOUNG AS SIX MONTHS OLD WITH SEVERE ATOPIC DERMATITIS
The European Commission (EC) has approved Dupixent® (dupilumab) in the European Union (EU) to treat severe atopic dermatitis in children aged 6 months to 5 years old who are candidates for systemic therapy. With this approval, Dupixent is the first and only targeted medicine indicated to treat these young children in Europe and the U.S. Korey Capozza, MPH, Founder and Executive Director of Global Parents for Eczema Research (GPER) ays, “Watching an infant or young child grapple with the debilitating and wide-reaching impacts of severe atopic dermatitis is heartbreaking. I’ve personally witnessed how this chronic skin disease can disrupt the lives of entire families when left uncontrolled. Intervening with effective treatments during infancy and early childhood can help manage the challenging impact this disease has on children and their families during such formative years.”
Atopic dermatitis is a chronic type 2 inflammatory skin disease. Between 85% and 90% of patients first develop symptoms before 5 years of age, which can often continue through adulthood. Symptoms include intense, persistent itch and skin lesions that cover much of the body, resulting in skin dryness, cracking, pain, redness or darkening, crusting and oozing, which can increase the risk of skin infection. Severe atopic dermatitis may also significantly impact the quality of life of young children and their caregivers.
Treatment options in this age group are primarily topical corticosteroids (TCS), which can be associated with safety risks and may impair growth when used long-term.
The approval is based on data from a Phase 3 trial evaluating Dupixent every four weeks (200 mg or 300 mg based on body weight) plus low-potency TCS or TCS alone (placebo) in 162 children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. At 16 weeks, Dupixent improved skin clearance and reduced overall disease severity and itch compared to placebo in the overall enrolled population. In a subset of those with severe atopic dermatitis, patients randomized to Dupixent (n=63) experienced the following compared to placebo (n=62) at 16 weeks:
• 46% of patients achieved 75% or greater improvement in overall disease severity compared to 7% treated with placebo, a coprimary endpoint.
• 14% of patients achieved clear or almost clear skin compared to 2% treated with placebo, a co-primary endpoint.
• 55% average reduction in overall disease severity from baseline compared to 10% with placebo.
• 42% average reduction in itch from baseline compared to a 1% increase with placebo.
Dupixent also improved sleep quality, skin pain and healthrelated quality of life compared to placebo in both the overall and severe populations. Long-term efficacy data showed the clinical benefit at 16 weeks was sustained through 52 weeks.
The most common side effects across indications include injection site reactions, conjunctivitis, conjunctivitis allergic, arthralgia, oral herpes and eosinophilia. The safety results of the 6 months to 5 years old trial were generally consistent with the known safety profile of Dupixent in its approved indications; in the trial, adverse events more commonly observed (≥5%) with Dupixent compared to placebo included eosinophilia and conjunctivitis. The longterm safety profile through 52 weeks was similar to the safety profile observed at 16 weeks, and consistent with what was observed in older patients with atopic dermatitis.
BIOGEN’S DIROXIMEL FUMARATE NOW AVAILABLE IN IRELAND FOR ORAL TREATMENT OF RELAPSINGREMITTING MULTIPLE SCLEROSIS (RRMS)
Biogen IRE has announced that VUMERITY® (diroximel fumarate), an oral fumarate, for the treatment of adults living with active RRMS1 is now available to patients in Ireland following EMA approval. Approximately 9,000 people are currently living with MS in Ireland. It is estimated that 85% are living with RRMS, the most common form of the condition.11
Diroximel fumarate, which is manufactured in Athlone, Ireland, offers those living with RRMS a treatment option with an improved gastrointestinal (GI) tolerability profile and comparable efficacy and safety characteristics as compared with the established treatment dimethyl fumarate (due to bioequivalence).2-5
The new oral fumarate treatment may reduce the severity and frequency of burdensome GI events like nausea, vomiting,
82 APRIL 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Minister Hildegarde Naughton, Minister of State at the Department of Health and at the Department of Children, Equality, Disability, Integration and Youth; Dr Angie Brown, Medical Director, Irish Heart Foundation; Consultant Cardiologist, Dublin; Professor J. William Mc Evoy, Research and Medical Director, National Institute for Prevention and Cardiovascular Health; Professor of Preventive Cardiology, University of Galway; Neil Johnson Acting Chief Executive, National Institute for Prevention and Cardiovascular Health and Chief Executive Croí.
diarrhoea and upper and lower abdominal pain, whilst also offering the convenience and flexibility to be taken with or without food.2-5,10
“The launch of Vumerity enables HCPs in Ireland to offer an alternative at-home oral DMT, empowering people with MS to take control of their care,” said Michael O'Connell, Country Lead Biogen Ireland. “Vumerity provides reduced relapse rate in newly diagnosed RRMS patients and improved GI tolerability profile, which is critical in supporting people to start and stay on treatment.”
“Our mission at MS Ireland is to enable people living with MS to live a life that is not defined by MS, we advocate on behalf of the MS community on any issue which impacts quality of life. We welcome the launch of Vumerity in Ireland as it improves choice while offering convenience, freedom, and flexibility without compromising on efficacy” said Ava Battles, CEO MS Ireland.
Pivotal phase 3 trials (EVOLVEMS-1 and EVOLVE-MS-2), which compared diroximel fumarate with dimethyl fumarate demonstrated similar efficacy safety profiles.3,4 GI events, such as nausea, vomiting, diarrhoea and upper and lower abdominal pain were less severe and lasted fewer days with diroximel fumarate compared with dimethyl fumarate and therefore were less likely to interfere with patients’ daily lives.4,5 In addition, since diroximel fumarate’s launch in the US, real-world evidence has reinforced the GI tolerability profile and confirmed that patient experience demonstrated in clinical trials is consistent with clinical practice. The realworld retrospective analysis of persistence1, 2 and adherence in diroximel fumarate-treated patients (n=263) showed high overall persistence over 12 months (~81.8%) [95% CI, 76.1–86.3], low discontinuation rate due to GI AEs (4.6%, 12/263) and high adherence to therapy (mean PDC 89.7%),1, 3 aligning with expectations based on DRF clinical trials.6
“Vumerity becoming available in Ireland is a step further in our commitment to providing more treatment choices to people with MS,” said Dr. Bronagh Hayden, Medical Lead, Biogen Ireland. “For 25 years, we have led in the research and development of new MS therapies, continually striving to help address the diverse unmet needs that people living with MS may have throughout their lives.”
The launch follows European Union authorisation in December 2021.
GAPS IN CARDIOVASCULAR CARE CONTRIBUTING TO DEATHS
A report launched by the National Institute for Prevention and Cardiovascular Health (www. nipc.ie) and the National CVD Prevention Council has identified gaping holes in Ireland’s cardiovascular healthcare. The report outlines that cardiovascular disease (CVD) kills nearly 9,000 in people in Ireland every year, despite an estimated 80% of premature CVD being preventable.
While there is a myriad of gaps in how the Irish healthcare system detects signs of CVD, notable issues include Ireland having the lowest rate of detection of high blood pressure in Western Europe.
This is particularly worrying when considering that in 75% of those that had a heart attack between 2017-2020, the heart attack was
the first manifestation of CVD. Likewise, more than 1,200 people that suffered an ischaemic stroke in 2020 were found to also have atrial fibrillation (AF), a common type of irregular, usually rapid, heartbeat. However, AF was not identified in 40% of patients until they suffered the stroke.
The report recommends a screening program for familial hypercholesterolaemia (FH), a genetic condition which causes dangerously high cholesterol levels from birth. FH affects around 1 in 200-250 people in Ireland however the majority of this goes undetected. Childhood FH screening programmes are commonplace throughout Europe.
The report also details issues in the treatment and discharge of patients following the detection of CVD. These issues include long waiting times in public hospitals and the lack of tailored discharge plans for patients.
Access to cardiac rehabilitation is paramount to the recovery of those post-cardiac events yet it has significantly declined. A 2017 study previously identified that there was national capacity to meet only 39% of the need for cardiac rehabilitation while in 2021 there was a waiting list of more than 2,800 people, with 40% waiting at least three months following hospital discharge.
The issues in the management of patient discharges are particularly worrying when considering that in the absence of Irish data, the report references a Swedish study which reveals that nearly 20% of people that suffer a heart attack die from a cardiovascular cause or experience a repeat heart attack or stroke within a year.
In response to the urgent needs identified by the report, NIPC and the National CVD Prevention Council is calling on the Government to develop a national strategy to tackle cardiovascular disease (CVD). The previous national strategy expired in 2019 and has not been replaced. The implementation of electronic health records is identified as a critically important key to integration of services required for effective prevention in clinical practice.
The report also outlines the requirement for investment in data collection and analysis to inform the strategy, the need to expand the role of nurses and allied health professionals and to increase access and care to disadvantaged groups.

83 HOSPITALPROFESSIONALNEWS.IE | HPN • APRIL 2023
CIBINQO is a convenient once-daily oral JAK1 inhibitor for moderate-to-severe atopic dermatitis (AD) that o ers1-4


















Significant skin clearance at week 12, with sustained control at week 481-3,5
Rapid itch relief, superior to dupilumab + TCS at week 2 for CIBINQO 200 mg + TCS, with significant results as early as day 41,6

A once-daily pill available in multiple doses that can be used with or without medicated topical therapies so you can tailor treatment to meet the individual needs of your patients1-3,7,8
CONSISTENT SAFETY PROFILE: Rigorously studied in >3500 patients across 7 clinical trials, including one ongoing LTE 9


PRESCRIBING INFORMATION CIBINQO® ▼ (ABROCITINIB)






Please refer to full Summary of Product Characteristics (SmPC) before prescribing Cibinqo®.


Presentation: Film-coated tablets containing 50 mg, 100 mg, or 200 mg abrocitinib. Each tablet contains 1.37 mg, 2.73 mg, and 5.46 mg of lactose monohydrate, respectively. Indications: For the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy. Dosage: Treatment should be initiated and supervised by a healthcare professional experienced in the diagnosis and treatment of atopic dermatitis. Posology: The recommended starting dose is 100 mg or 200 mg once daily based on individual patient characteristics. A starting dose of 100 mg once daily is recommended for patients at higher risk of venous thromboembolism (VTE), major adverse cardiovascular event (MACE) and malignancy. If the patient does not respond adequately to 100 mg once daily, the dose can be increased to 200 mg once daily. A dose of 200 mg once daily may be appropriate for patients who are not at higher risk of VTE, MACE and malignancy with high disease burden or for patients with an inadequate response to 100 mg once daily. Upon disease control, dose should be decreased to 100 mg once daily. If disease control is not maintained after dose reduction, re-treatment with 200 mg once daily can be considered. The lowest e ective dose for maintenance should be considered. Discontinuation of treatment should be considered in patients who show no evidence of therapeutic benefit after 24 weeks. Cibinqo can be used with or without medicated topical therapies for atopic dermatitis. Treatment initiation: TTreatment should not be initiated in patients with a platelet count < 150 × 103/mm3, an absolute lymphocyte count (ALC) < 0.5 × 103/mm3, an absolute neutrophil count (ANC) < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Dose interruption: If a patient develops a serious infection, sepsis or opportunistic infection, dose interruption should be considered until the infection is controlled. Interruption of dosing may be needed for management of laboratory abnormalities. Missed doses: If a dose is missed, patients should be advised to take the dose as soon as possible unless it is less than 12 hours before the next dose, in which case the patient should not take the missed dose. Thereafter, dosing should be resumed at the regular scheduled time. Interactions: In patients receiving dual strong inhibitors of cytochrome CYP2C19 and moderate inhibitors of CYP2C9, or strong inhibitors of CYP2C19 alone (e.g., fluvoxamine, fluconazole, fluoxetine and ticlopidine), the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. Treatment is not recommended concomitantly with moderate or strong inducers of CYP2C19/CYP2C9 enzymes (e.g., rifampicin, apalutamide, efavirenz, enzalutamide, phenytoin). In patients receiving acid reducing agents (e.g. antacids, proton pump inhibitors and H2 receptor antagonists), 200 mg once daily dose of abrocitinib should be considered (see section 4.5). Renal impairment: No dose adjustment is required in patients with mild renal impairment, i.e., estimated glomerular filtration rate (eGFR) of 60 to < 90 mL/min. In patients with moderate (eGFR 30 to < 60 mL/min) renal impairment, the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. In patients with severe (eGFR < 30 mL/min) renal impairment, 50 mg once daily is the recommended starting dose. The maximum daily dose is 100 mg. Cibinqo has not been studied in patients with end-stage renal disease (ESRD) on renal replacement therapy. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Cibinqo must not be used in patients with severe (Child Pugh C) hepatic impairment. Elderly: For patients 65 years of age and older, the recommended dose is 100 mg once daily. Paediatric population: The safety and e cacy of Cibinqo in children under 12 years of age have not yet been established. No data are available. Cibinqo has been studied in adolescents 12 to < 18 years of age. However, because of bone findings in juvenile rats (comparable to a 3 month old human), additional long-term data in growing adolescents is needed to conclude that the benefits outweigh the risks. Method of administration: This medicinal product is to be taken orally once daily with or without food at approximately the same time each day. In patients who experience nausea, taking Cibinqo with food may improve nausea. Tablets should be swallowed whole with water and should not be split, crushed, or chewed because these methods have not been studied in clinical trials. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. Active serious systemic infections, including tuberculosis (TB), severe hepatic impairment, pregnancy, and breast-feeding.
Warnings and Precautions:
Abrocitinib should only be used if no suitable treatment alternatives are available in patients:
• 65 years of age and older;
• patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers);
• patients with malignancy risk factors (e.g. current malignancy or history of malignancy)
Infections/serious infections: Serious infections have been reported in patients receiving Cibinqo. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia. As there is a higher incidence of infections in the elderly and in the diabetic populations in general, caution should be used when treating the elderly and patients with diabetes. In patients 65 years of age and older abrocitinib should only be used if no suitable treatment alternatives are available. Treatment must not be initiated in patients with an active, serious systemic infection. Risks and benefits of treatment prior to initiating Cibinqo should be considered. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with abrocitinib. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing and appropriate antimicrobial therapy should be initiated. The patient should be closely monitored, and therapy should be temporarily interrupted if the patient is not responding to standard therapy. Tuberculosis: Tuberculosis was observed in clinical studies with abrocitinib. Patients should be screened for TB before starting treatment and yearly screening for patients in highly endemic areas for TB should be considered. Abrocitinib must not be given to patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, preventive therapy for latent TB should be started prior to initiation of treatment. Viral reactivation: Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies. The rate of herpes zoster infections was higher in patients who were treated with 200 mg, 65 years of age and older, with a medical history of herpes zoster, with a confirmed ALC < 1 × 103/mm3 prior to the event and patients with severe atopic dermatitis at baseline. If a patient develops herpes zoster, temporary interruption of treatment should be considered until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy and during therapy. Patients with evidence of active hepatitis B or hepatitis C (positive hepatitis C PCR) infection were excluded from clinical studies. Patients who were hepatitis B surface antigen negative, hepatitis B core antibody positive, and hepatitis B surface antibody positive had testing for hepatitis B virus (HBV) DNA. Patients who had HBV DNA above the lower limit of quantification (LLQ) were excluded. Patients who had HBV DNA negative or below LLQ could initiate treatment; such patients had HBV DNA monitored. If HBV DNA is detected, a liver specialist should be consulted. Vaccination: No data are available on the response to vaccination in patients receiving Cibinqo. Use of live, attenuated vaccines should be avoided during or immediately prior to treatment. Prior to initiating treatment with this medicinal product, it is recommended that patients be brought up to date with all immunisations, including prophylactic herpes zoster vaccinations, in agreement with current immunisation guidelines. Venous thromboembolism (VTE): Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a dose dependent higher rate of VTE including deep venous thrombosis (DVT) and pulmonary embolism (PE) was observed with tofacitinib compared to TNF inhibitors. A higher rate of VTE was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients with cardiovascular or malignancy risk factors abrocitinib should only be used if no suitable treatment alternatives are available. In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, abrocitinib should be used with caution. VTE risk
factors other than cardiovascular or malignancy risk factors include previous VTE, patients undergoing major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, inherited coagulation disorder. Patients should be re-evaluated periodically during abrocitinib treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue abrocitinib in patients with suspected VTE, regardless of dose Major adverse cardiovascular events (MACE): Events of MACE have been observed in patients taking abrocitinib. In a large randomized active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE), defined as cardiovascular death, non-fatal myocardial infarction (MI) and non-fatal stroke, was observed with tofacitinib compared to TNF inhibitors. Therefore, in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, abrocitinib should only be used if no suitable treatment alternatives are available. Malignancy (excluding non-melanoma skin cancer [NMSC]): Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including abrocitinib. In a large randomized active controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years and older with at least one additional cardiovascular risk factor, a higher rate of malignancies, particularly lung cancer, lymphoma and non-melanoma skin cancer (NMSC) was observed with tofacitinib compared to TNF inhibitors. A higher rate of malignancies (excluding non-melanoma skin cancer, NMSC) was observed with abrocitinib 200 mg compared to abrocitinib 100 mg. In patients 65 years of age and older, patients who are current or past long-time smokers, or with other malignancy risk factors (e.g. current malignancy or history of malignancy), abrocitinib should only be used if no suitable treatment alternatives are available. Non-melanoma skin cancer: NMSCs have been reported in patients receiving abrocitinib. Periodic skin examination is recommended for all patients, particularly those who are at increased risk for skin cancer. Haematologic abnormalities: Confirmed ALC < 0.5 × 103/mm3 and platelet count < 50 × 103/mm3 were observed in less than 0.5% of patients in clinical studies. Treatment with abrocitinib should not be initiated in patients with a platelet count < 150 × 103/mm3, an ALC < 0.5 × 103/mm3, an ANC < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Complete blood count should be monitored 4 weeks after initiation of therapy and thereafter according to routine patient management. Lipids: Dose dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The e ect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. Elderly: The safety profile observed in elderly patients was similar to that of the adult population with the following exceptions: a higher proportion of patients 65 years of age and older discontinued from clinical studies and were more likely to have serious adverse reactions compared to younger patients; patients 65 years and older were more likely to develop low platelet and ALC values; the incidence rate of herpes zoster in patients 65 years of age and older was higher than that of younger patients. There are limited data in patients above 75 years of age. Use in patients 65 years of age and older: Considering the increased risk of MACE, malignancies, serious infections, and all-cause mortality in patients 65 years of age and older, as observed in a large randomised study of tofacitinib (another JAK inhibitor), abrocitinib should only be used in these patients if no suitable treatment alternatives are available. Immunosuppressive conditions or medicinal products: Patients with immunodeficiency disorders or a first-degree relative with a hereditary immunodeficiency were excluded from clinical studies and no information on these patients is available. Combination with biologic immunomodulators, potent immunosuppressants such as ciclosporin or other Janus kinase (JAK) inhibitors has not been studied. Their concomitant use with abrocitinib is not recommended as a risk of additive immunosuppression cannot be excluded. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Drug Interactions: Potential for other medicines to a ect pharmacokinetics of abrocitinib: Abrocitinib is metabolised predominantly by CYP2C19 and CYP2C9 enzymes, and to a lesser extent by CYP3A4 and CYP2B6 enzymes, and its active metabolites are renally excreted and are substrates of the organic anion transporter 3 (OAT3). Therefore, exposures of abrocitinib and/or its active metabolites may be a ected by medicinal products that strongly inhibit or induce theses enzymes and transporter. Dose adjustments, as appropriate, may be required. Co-administration with products which increase gastric pH: When abrocitinib 200 mg was administered concomitantly with famotidine 40 mg, an H2-receptor antagonist, abrocitinib active moiety exposures decreased by approximately 35%. The e ect of elevating gastric pH with antacids, or proton pump inhibitors (omeprazole) on the pharmacokinetics of abrocitinib has not been studied and may be similar to that seen with famotidine. The higher 200 mg daily dose should be considered for patients treated concomitantly with products which increase gastric pH, as they may reduce the e cacy of abrocitinib. Potential for Cibinqo to a ect pharmacokinetics of other medicinal products: No clinically significant e ects of Cibinqo were observed in drug interaction studies with oral contraceptives. Caution should be exercised for concomitant use of abrocitinib with dabigatran. Caution should be exercised as the levels of P-gp substrates with a narrow therapeutic index, such as digoxin, may increase. Caution should be exercised when using abrocitinib concomitantly with narrow therapeutic index medicines that are primarily metabolised by CYP2C19 enzyme (e.g. S mephenytoin and clopidogrel). Dose adjustment may be required for other medicines primarily metabolised by CYP2C19 enzyme in accordance with their product information (e.g. citalopram, clobazam, escitalopram and selumetinib). Fertility, pregnancy, and lactation: Women of childbearing potential: Women of reproductive potential should be advised to use e ective contraception during treatment and for 1 month following the final dose of Cibinqo. Pregnancy planning and prevention for females of reproductive potential should be encouraged. Pregnancy: There are no or limited amount of data on the use of abrocitinib in pregnant women. Studies in animals have shown reproductive toxicity. Abrocitinib has been shown to cause embryo-foetal lethality in pregnant rats and rabbits, skeletal variations in the foetuses of pregnant rats and rabbits and to a ect parturition and peri/postnatal development in rats. Cibinqo is contraindicated during pregnancy. Breast-feeding: There are no data on the presence of abrocitinib in human milk, the e ects on the breast fed infant, or the e ects on milk production. Abrocitinib was secreted in milk of lactating rats. A risk to newborns/infants cannot be excluded and Cibinqo is contraindicated during breast feeding. Fertility: Based on the findings in rats, oral administration of Cibinqo may result in temporary reduced fertility in females of reproductive potential. The e ects on female rat fertility were reversible 1 month after cessation of abrocitinib oral administration. Driving and operating machinery: Cibinqo has no e ect on the ability to drive or use machines. Side E ects: The most commonly reported adverse reactions are nausea (15.1%), headache (7.9%), acne (4.8%), herpes simplex (4.2%), blood creatine phosphokinase increased (3.8%), vomiting (3.5%), dizziness (3.4%) and abdominal pain upper (2.2%). The most frequent serious adverse reactions are infections (0.3%) including herpes simplex, herpes zoster, and pneumonia. Refer to SmPC for further information on side e ects. Legal Category: S1A. Marketing Authorisation Numbers: EU/1/21/1593/002 - Cibinqo 50 mg (28 film-coated tablets), EU/1/21/1593/007 - Cibinqo 100 mg (28 film-coated tablets); EU/1/21/1593/012 - Cibinqo 200 mg (28 film-coated tablets). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 467 6500.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Last revised: 03/2023.
Ref: CQ 5_0 IE.
References: 1. Bieber T, Simpson EL, Silverberg JI, et al. N Engl J Med. 2021;384(12):1101-1112. 2. Simpson EL, Sinclair R, Forman S, et al. Lancet. 2020;396(10246):255-266. 3. Silverberg JI, Simpson EL, Thyssen JP, et al. JAMA Dermatol 2020;156(8):863-873. 4. Boeri M, Sutphin J, Hauber B, et al. J Dermatolog Treat. 2020 Nov 2:1-10. doi:10.1080/09546634.1832185. 5. Reich K, Silverberg JI, Papp K, et al. Presented at the Revolutionizing Atopic Dermatitis Virtual Conference; 13 June 2021. 6. Ständer S, Kwatra SG, Silverberg JI, et al. Am J Clin Dermatol. 2023;24(1):97-107. doi: 10.1007/s40257-022-00738-4. 7. Cibinqo Summary of Product Characteristics. 8. Blauvelt A, Silverberg JI, Lynde CW, et al. J Am Acad Dermatol. Published online 17 August 2021. doi: 10.1016/j.jaad.2021.05.075. 9. Simpson EL, Silverberg JI, Nosbaum A, et al. British Journal of Dermatology, 2023;188,supp2. doi: 10.1093/bjd/ljac140.025.
Although some clinical trials included adolescents, please note that CIBINQO is indicated for the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy.
TCS includes low- to medium-potency topical corticosteroids, topical calcineurin inhibitors, or topical phosphodiesterase 4 inhibitors, per protocol guidance in JADE COMPARE.
Nonmedicated topicals were also required.1
AD=atopic dermatitis; JAK=Janus kinase; LTE=long-term extension; TCS=topical corticosteroid. © 2023 Pfizer Inc. All rights reserved. March 2023. PP-CIB-IRL-0103






Whether your patients’ moderate-to-severe AD is FIERCE or TAMER,










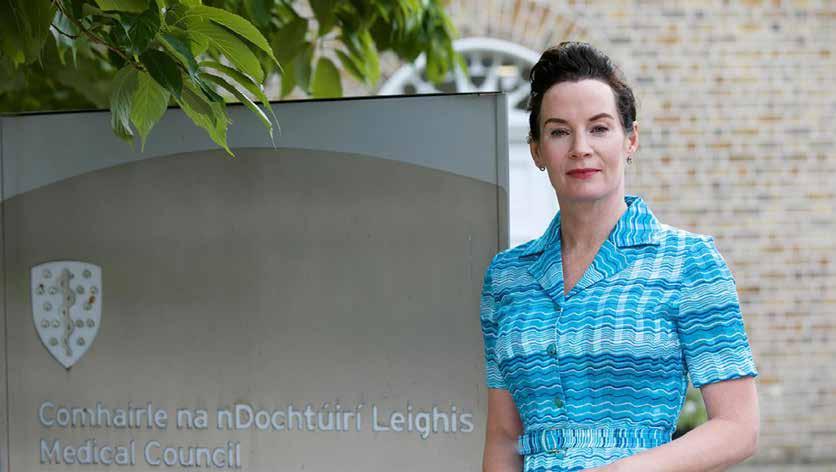









 Edward Gregg, Head of RCSI’s School of Population Health
Edward Gregg, Head of RCSI’s School of Population Health







































































 Written
Chloe
Written
Chloe































































